User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
FDA approves canakinumab for gout flares
The U.S. Food and Drug Administration has approved canakinumab (Ilaris) for the treatment of gout flares in adults who cannot be treated with NSAIDs, colchicine, or repeated courses of corticosteroids. The drug is also indicated for people who could not tolerate or had an inadequate response to NSAIDs or colchicine.
The drug, a humanized anti–interleukin-1 beta monoclonal antibody, is the first and only biologic approved in the United States for the treatment of gout flares, according to Novartis. It is administered in a single, subcutaneous injection of 150 mg.
“At Novartis, we are committed to bringing medicines that address high unmet needs to patients. We are proud to receive approval on our eighth indication for Ilaris in the U.S. and provide the first biologic medicine option for people with gout flares to help treat this painful and debilitating condition,” the company said in a statement to this news organization.
Canakinumab was first approved in the United States in 2009 for the treatment of children and adults with cryopyrin-associated periodic syndrome (CAPS). Since then, it has been approved for the treatment of several other autoinflammatory diseases, including Still’s disease and recurrent fever syndromes.
In 2011, an FDA advisory panel voted against the approval of canakinumab to treat acute gout flares refractory to NSAIDs, colchicine, or repeated courses of corticosteroids, while in 2013, the European Medicine Agency approved the drug for this treatment indication.
Since that FDA advisory committee meeting and the FDA’s subsequent rejection letter, “[Novartis] has conducted additional studies in patients with gout flares and other related populations to further characterize the short- and long-term safety of canakinumab supporting the current application. To further support the benefit-risk [profile of the drug], the indication is for a more restricted population than initially proposed in 2011,” the FDA’s Center for Drug Evaluation and Research said in a statement to this news organization. “Given these considerations and the available safety information, the Agency determined that canakinumab, at the recommended dosage, has a favorable risk-benefit profile” in the specified patient population.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved canakinumab (Ilaris) for the treatment of gout flares in adults who cannot be treated with NSAIDs, colchicine, or repeated courses of corticosteroids. The drug is also indicated for people who could not tolerate or had an inadequate response to NSAIDs or colchicine.
The drug, a humanized anti–interleukin-1 beta monoclonal antibody, is the first and only biologic approved in the United States for the treatment of gout flares, according to Novartis. It is administered in a single, subcutaneous injection of 150 mg.
“At Novartis, we are committed to bringing medicines that address high unmet needs to patients. We are proud to receive approval on our eighth indication for Ilaris in the U.S. and provide the first biologic medicine option for people with gout flares to help treat this painful and debilitating condition,” the company said in a statement to this news organization.
Canakinumab was first approved in the United States in 2009 for the treatment of children and adults with cryopyrin-associated periodic syndrome (CAPS). Since then, it has been approved for the treatment of several other autoinflammatory diseases, including Still’s disease and recurrent fever syndromes.
In 2011, an FDA advisory panel voted against the approval of canakinumab to treat acute gout flares refractory to NSAIDs, colchicine, or repeated courses of corticosteroids, while in 2013, the European Medicine Agency approved the drug for this treatment indication.
Since that FDA advisory committee meeting and the FDA’s subsequent rejection letter, “[Novartis] has conducted additional studies in patients with gout flares and other related populations to further characterize the short- and long-term safety of canakinumab supporting the current application. To further support the benefit-risk [profile of the drug], the indication is for a more restricted population than initially proposed in 2011,” the FDA’s Center for Drug Evaluation and Research said in a statement to this news organization. “Given these considerations and the available safety information, the Agency determined that canakinumab, at the recommended dosage, has a favorable risk-benefit profile” in the specified patient population.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved canakinumab (Ilaris) for the treatment of gout flares in adults who cannot be treated with NSAIDs, colchicine, or repeated courses of corticosteroids. The drug is also indicated for people who could not tolerate or had an inadequate response to NSAIDs or colchicine.
The drug, a humanized anti–interleukin-1 beta monoclonal antibody, is the first and only biologic approved in the United States for the treatment of gout flares, according to Novartis. It is administered in a single, subcutaneous injection of 150 mg.
“At Novartis, we are committed to bringing medicines that address high unmet needs to patients. We are proud to receive approval on our eighth indication for Ilaris in the U.S. and provide the first biologic medicine option for people with gout flares to help treat this painful and debilitating condition,” the company said in a statement to this news organization.
Canakinumab was first approved in the United States in 2009 for the treatment of children and adults with cryopyrin-associated periodic syndrome (CAPS). Since then, it has been approved for the treatment of several other autoinflammatory diseases, including Still’s disease and recurrent fever syndromes.
In 2011, an FDA advisory panel voted against the approval of canakinumab to treat acute gout flares refractory to NSAIDs, colchicine, or repeated courses of corticosteroids, while in 2013, the European Medicine Agency approved the drug for this treatment indication.
Since that FDA advisory committee meeting and the FDA’s subsequent rejection letter, “[Novartis] has conducted additional studies in patients with gout flares and other related populations to further characterize the short- and long-term safety of canakinumab supporting the current application. To further support the benefit-risk [profile of the drug], the indication is for a more restricted population than initially proposed in 2011,” the FDA’s Center for Drug Evaluation and Research said in a statement to this news organization. “Given these considerations and the available safety information, the Agency determined that canakinumab, at the recommended dosage, has a favorable risk-benefit profile” in the specified patient population.
A version of this article first appeared on Medscape.com.
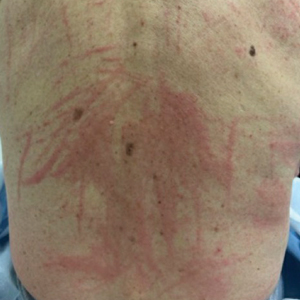
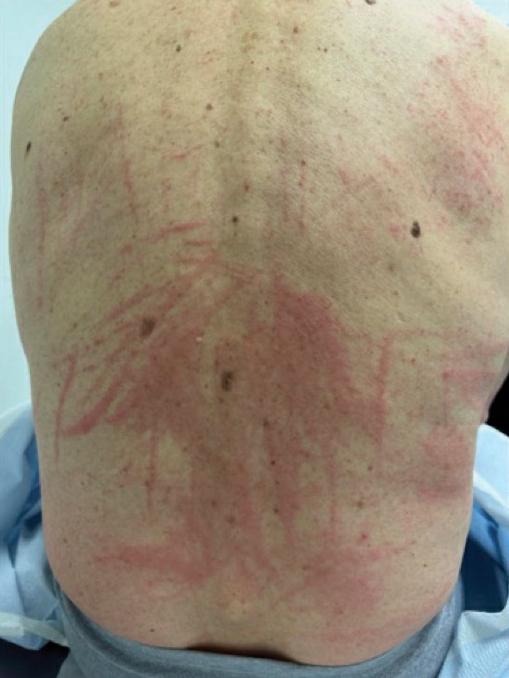
Raised Linear Plaques on the Back
The Diagnosis: Flagellate Dermatitis
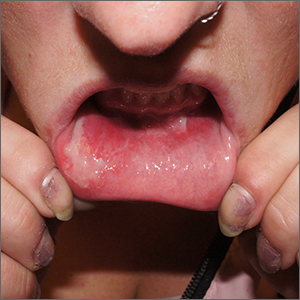
Upon further questioning by dermatology, the patient noted recent ingestion of shiitake mushrooms, which were not a part of his typical diet. Based on the appearance of the rash in the context of ingesting shiitake mushrooms, our patient was diagnosed with flagellate dermatitis. At 6-week followup, the patient’s rash had resolved spontaneously without further intervention.
Flagellate dermatitis usually appears on the torso as linear whiplike streaks.1 The eruption often is pruritic and may be preceded by severe pruritus. Flagellate dermatitis also is a well-documented complication of bleomycin sulfate therapy with an incidence rate of 8% to 66%.2
Other chemotherapeutic causes include peplomycin, bendamustine, docetaxel, cisplatin, and trastuzumab.3 Flagellate dermatitis also is seen in some patients with dermatomyositis.4 A thorough patient history, including medications and dietary habits, is necessary to differentiate flagellate dermatitis from dermatomyositis.
Flagellate dermatitis, also known as shiitake dermatitis, is observed as erythematous flagellate eruptions involving the trunk or extremities that present within 2 hours to 5 days of handling or consuming undercooked or raw shiitake mushrooms (Lentinula edodes),5,6 as was observed in our patient. Lentinan is the polysaccharide component of the shiitake species and is destabilized by heat.6 Ingestion of polysaccharide is associated with dermatitis, particularly in Japan, China, and Korea; however, the consumption of shiitake mushrooms has increased worldwide, and cases increasingly are reported outside of these typical regions. The rash typically resolves spontaneously; therefore, treatment is supportive. However, more severe symptomatic cases may require courses of topical corticosteroids and antihistamines.6
In our case, the differential diagnosis consisted of acute urticaria, cutaneous dermatomyositis, dermatographism, and maculopapular cutaneous mastocytosis. Acute urticaria displays well-circumscribed edematous papules or plaques, and individual lesions last less than 24 hours. Cutaneous dermatomyositis includes additional systemic manifestations such as fatigue, malaise, and myalgia, as well as involvement of the gastrointestinal, respiratory, or cardiac organs. Dermatographism is evoked by stroking or rubbing of the skin, which results in asymptomatic lesions that persist for 15 to 30 minutes. Cases of maculopapular cutaneous mastocytosis more often are seen in children, and the histamine release most often causes gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea, as well as flushing, blushing, pruritus, respiratory difficulty, and malaise.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503.
- Yagoda A, Mukherji B, Young C, et al. Bleomycin, an anti-tumor antibiotic: clinical experience in 274 patients. Ann Intern Med. 1972;77:861-870.
- Cohen PR. Trastuzumab-associated flagellate erythema: report in a woman with metastatic breast cancer and review of antineoplastic therapy-induced flagellate dermatoses. Dermatol Ther (Heidelb). 2015;5:253-264. doi:10.1007/s13555-015-0085-2
- Grynszpan R, Niemeyer-Corbellini JP, Lopes MS, et al. Bleomycininduced flagellate dermatitis. BMJ Case Rep. 2013;2013:bcr2013009764. doi:10.1136/bcr-2013-009764
- Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
The Diagnosis: Flagellate Dermatitis
Upon further questioning by dermatology, the patient noted recent ingestion of shiitake mushrooms, which were not a part of his typical diet. Based on the appearance of the rash in the context of ingesting shiitake mushrooms, our patient was diagnosed with flagellate dermatitis. At 6-week followup, the patient’s rash had resolved spontaneously without further intervention.
Flagellate dermatitis usually appears on the torso as linear whiplike streaks.1 The eruption often is pruritic and may be preceded by severe pruritus. Flagellate dermatitis also is a well-documented complication of bleomycin sulfate therapy with an incidence rate of 8% to 66%.2
Other chemotherapeutic causes include peplomycin, bendamustine, docetaxel, cisplatin, and trastuzumab.3 Flagellate dermatitis also is seen in some patients with dermatomyositis.4 A thorough patient history, including medications and dietary habits, is necessary to differentiate flagellate dermatitis from dermatomyositis.
Flagellate dermatitis, also known as shiitake dermatitis, is observed as erythematous flagellate eruptions involving the trunk or extremities that present within 2 hours to 5 days of handling or consuming undercooked or raw shiitake mushrooms (Lentinula edodes),5,6 as was observed in our patient. Lentinan is the polysaccharide component of the shiitake species and is destabilized by heat.6 Ingestion of polysaccharide is associated with dermatitis, particularly in Japan, China, and Korea; however, the consumption of shiitake mushrooms has increased worldwide, and cases increasingly are reported outside of these typical regions. The rash typically resolves spontaneously; therefore, treatment is supportive. However, more severe symptomatic cases may require courses of topical corticosteroids and antihistamines.6
In our case, the differential diagnosis consisted of acute urticaria, cutaneous dermatomyositis, dermatographism, and maculopapular cutaneous mastocytosis. Acute urticaria displays well-circumscribed edematous papules or plaques, and individual lesions last less than 24 hours. Cutaneous dermatomyositis includes additional systemic manifestations such as fatigue, malaise, and myalgia, as well as involvement of the gastrointestinal, respiratory, or cardiac organs. Dermatographism is evoked by stroking or rubbing of the skin, which results in asymptomatic lesions that persist for 15 to 30 minutes. Cases of maculopapular cutaneous mastocytosis more often are seen in children, and the histamine release most often causes gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea, as well as flushing, blushing, pruritus, respiratory difficulty, and malaise.
The Diagnosis: Flagellate Dermatitis
Upon further questioning by dermatology, the patient noted recent ingestion of shiitake mushrooms, which were not a part of his typical diet. Based on the appearance of the rash in the context of ingesting shiitake mushrooms, our patient was diagnosed with flagellate dermatitis. At 6-week followup, the patient’s rash had resolved spontaneously without further intervention.
Flagellate dermatitis usually appears on the torso as linear whiplike streaks.1 The eruption often is pruritic and may be preceded by severe pruritus. Flagellate dermatitis also is a well-documented complication of bleomycin sulfate therapy with an incidence rate of 8% to 66%.2
Other chemotherapeutic causes include peplomycin, bendamustine, docetaxel, cisplatin, and trastuzumab.3 Flagellate dermatitis also is seen in some patients with dermatomyositis.4 A thorough patient history, including medications and dietary habits, is necessary to differentiate flagellate dermatitis from dermatomyositis.
Flagellate dermatitis, also known as shiitake dermatitis, is observed as erythematous flagellate eruptions involving the trunk or extremities that present within 2 hours to 5 days of handling or consuming undercooked or raw shiitake mushrooms (Lentinula edodes),5,6 as was observed in our patient. Lentinan is the polysaccharide component of the shiitake species and is destabilized by heat.6 Ingestion of polysaccharide is associated with dermatitis, particularly in Japan, China, and Korea; however, the consumption of shiitake mushrooms has increased worldwide, and cases increasingly are reported outside of these typical regions. The rash typically resolves spontaneously; therefore, treatment is supportive. However, more severe symptomatic cases may require courses of topical corticosteroids and antihistamines.6
In our case, the differential diagnosis consisted of acute urticaria, cutaneous dermatomyositis, dermatographism, and maculopapular cutaneous mastocytosis. Acute urticaria displays well-circumscribed edematous papules or plaques, and individual lesions last less than 24 hours. Cutaneous dermatomyositis includes additional systemic manifestations such as fatigue, malaise, and myalgia, as well as involvement of the gastrointestinal, respiratory, or cardiac organs. Dermatographism is evoked by stroking or rubbing of the skin, which results in asymptomatic lesions that persist for 15 to 30 minutes. Cases of maculopapular cutaneous mastocytosis more often are seen in children, and the histamine release most often causes gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea, as well as flushing, blushing, pruritus, respiratory difficulty, and malaise.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503.
- Yagoda A, Mukherji B, Young C, et al. Bleomycin, an anti-tumor antibiotic: clinical experience in 274 patients. Ann Intern Med. 1972;77:861-870.
- Cohen PR. Trastuzumab-associated flagellate erythema: report in a woman with metastatic breast cancer and review of antineoplastic therapy-induced flagellate dermatoses. Dermatol Ther (Heidelb). 2015;5:253-264. doi:10.1007/s13555-015-0085-2
- Grynszpan R, Niemeyer-Corbellini JP, Lopes MS, et al. Bleomycininduced flagellate dermatitis. BMJ Case Rep. 2013;2013:bcr2013009764. doi:10.1136/bcr-2013-009764
- Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503.
- Yagoda A, Mukherji B, Young C, et al. Bleomycin, an anti-tumor antibiotic: clinical experience in 274 patients. Ann Intern Med. 1972;77:861-870.
- Cohen PR. Trastuzumab-associated flagellate erythema: report in a woman with metastatic breast cancer and review of antineoplastic therapy-induced flagellate dermatoses. Dermatol Ther (Heidelb). 2015;5:253-264. doi:10.1007/s13555-015-0085-2
- Grynszpan R, Niemeyer-Corbellini JP, Lopes MS, et al. Bleomycininduced flagellate dermatitis. BMJ Case Rep. 2013;2013:bcr2013009764. doi:10.1136/bcr-2013-009764
- Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
A 77-year-old man with a history of hypertension, hyperlipidemia, and nonmelanoma skin cancer presented to the dermatology clinic for evaluation of a new rash of 2 days’ duration. He trialed a previously prescribed triamcinolone cream 0.1% without improvement. The patient denied any recent travel, as well as fever, nausea, vomiting, or changes in bowel habits. Physical examination revealed diffuse, erythematous, raised, linear plaques on the mid to lower back.
Screening finds high rates of CVD in diabetes, COPD patients
AMSTERDAM – , compared with usual care, in a Dutch study involving more than 1,200 people and 25 primary care practices.
Scaling up this program to larger populations could potentially uncover huge numbers of currently unrecognized people with CVD given the large number of adults with type 2 diabetes plus those with COPD, Amy Groenewegen, MD, said at the annual congress of the European Society of Cardiology.
“I think this screening is ready for routine use, but it could be followed by prospective studies that investigate whether it produces more benefits in patient-centered outcomes,” Dr. Groenewegen said in a press briefing. She stressed that it has not yet been clearly proven that patients with these chronic diseases are better off long term when their CVD is detected sooner using the tested approach.
“We need simple ways to identify relevant patients for additional screening and potential treatment” of CVD, commented Lars Kober, MD, designated discussant at the Congress and a cardiologist and professor at Rigshospitalet, Copenhagen University Hospital.
A ‘very simple’ symptom questionnaire
The study is important because it tested a “very simple” symptom questionnaire as the initial screening phase, yet resulted in a CVD diagnostic rate that was two- to threefold higher than in the control patients managed with usual care, Dr. Kober noted.
The Reviving the Early Diagnosis of CVD (RED-CVD) trial randomized 14 primary care practices in the Netherlands to apply a structured screening protocol to adults with type 2 diabetes or COPD, and another 11 practices that served as controls and provided their patients with usual care.
The study included 624 people in the screening arm and 592 in the usual-care arm. Their average age was about 68 years. In the screening arm, 87% had type 2 diabetes and 20% had COPD, including 6.3% with both. In the usual-care arm, 86% had type 2 diabetes, 21% had COPD, with 7.4% having both.
About a quarter of the study cohort had a history of a CVD diagnosis, but they were included for their potential for developing another form of CVD. The study considered three types of CVD: coronary artery disease, heart failure, and atrial fibrillation.
The CVD screening protocol began with an 11-question survey, completed by patients, that asked about their symptoms. The survey was devised by a research team at the University Medical Center Groningen, the Netherlands, who collaborated on the study.
The second phase for people who had suggestive symptoms was a physical examination, measurement of serum N-terminal pro-brain natriuretic peptide (elevated levels signal incident heart failure), and an ECG. People who continued to show findings consistent with CVD in this phase were then referred on a discretionary basis by the attending physician to a specialist.
More than doubling the CVD diagnosis rate
The screening program produced a total of 50 new CVD diagnoses in the screening cohort (8%) and 18 in the control, usual-care arm (3%), for the study’s primary endpoint. The greatest number of events involved heart failure, followed by coronary disease.
The screening questionnaire identified 70% of the people who completed it with suggestive symptoms, such as shortness of breath, claudication, or palpitations. The follow-up assessments of phase two narrowed the group with possible new CVD down to 44% of the people in this arm, and the participating physicians referred 39% to a specialist.
An analysis that adjusted for several demographic and clinical variables and excluded nonobstructive coronary disease as a new CVD diagnosis showed that the systematic screening approach resulted in 2.4-fold more new diagnoses than usual care, reported Dr. Groenewegen, an epidemiologist at University Medical Center Utrecht, the Netherlands.
RED-CVD received no commercial funding. Dr. Groenewegen disclosed no relevant financial relationships. Dr. Kober has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , compared with usual care, in a Dutch study involving more than 1,200 people and 25 primary care practices.
Scaling up this program to larger populations could potentially uncover huge numbers of currently unrecognized people with CVD given the large number of adults with type 2 diabetes plus those with COPD, Amy Groenewegen, MD, said at the annual congress of the European Society of Cardiology.
“I think this screening is ready for routine use, but it could be followed by prospective studies that investigate whether it produces more benefits in patient-centered outcomes,” Dr. Groenewegen said in a press briefing. She stressed that it has not yet been clearly proven that patients with these chronic diseases are better off long term when their CVD is detected sooner using the tested approach.
“We need simple ways to identify relevant patients for additional screening and potential treatment” of CVD, commented Lars Kober, MD, designated discussant at the Congress and a cardiologist and professor at Rigshospitalet, Copenhagen University Hospital.
A ‘very simple’ symptom questionnaire
The study is important because it tested a “very simple” symptom questionnaire as the initial screening phase, yet resulted in a CVD diagnostic rate that was two- to threefold higher than in the control patients managed with usual care, Dr. Kober noted.
The Reviving the Early Diagnosis of CVD (RED-CVD) trial randomized 14 primary care practices in the Netherlands to apply a structured screening protocol to adults with type 2 diabetes or COPD, and another 11 practices that served as controls and provided their patients with usual care.
The study included 624 people in the screening arm and 592 in the usual-care arm. Their average age was about 68 years. In the screening arm, 87% had type 2 diabetes and 20% had COPD, including 6.3% with both. In the usual-care arm, 86% had type 2 diabetes, 21% had COPD, with 7.4% having both.
About a quarter of the study cohort had a history of a CVD diagnosis, but they were included for their potential for developing another form of CVD. The study considered three types of CVD: coronary artery disease, heart failure, and atrial fibrillation.
The CVD screening protocol began with an 11-question survey, completed by patients, that asked about their symptoms. The survey was devised by a research team at the University Medical Center Groningen, the Netherlands, who collaborated on the study.
The second phase for people who had suggestive symptoms was a physical examination, measurement of serum N-terminal pro-brain natriuretic peptide (elevated levels signal incident heart failure), and an ECG. People who continued to show findings consistent with CVD in this phase were then referred on a discretionary basis by the attending physician to a specialist.
More than doubling the CVD diagnosis rate
The screening program produced a total of 50 new CVD diagnoses in the screening cohort (8%) and 18 in the control, usual-care arm (3%), for the study’s primary endpoint. The greatest number of events involved heart failure, followed by coronary disease.
The screening questionnaire identified 70% of the people who completed it with suggestive symptoms, such as shortness of breath, claudication, or palpitations. The follow-up assessments of phase two narrowed the group with possible new CVD down to 44% of the people in this arm, and the participating physicians referred 39% to a specialist.
An analysis that adjusted for several demographic and clinical variables and excluded nonobstructive coronary disease as a new CVD diagnosis showed that the systematic screening approach resulted in 2.4-fold more new diagnoses than usual care, reported Dr. Groenewegen, an epidemiologist at University Medical Center Utrecht, the Netherlands.
RED-CVD received no commercial funding. Dr. Groenewegen disclosed no relevant financial relationships. Dr. Kober has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , compared with usual care, in a Dutch study involving more than 1,200 people and 25 primary care practices.
Scaling up this program to larger populations could potentially uncover huge numbers of currently unrecognized people with CVD given the large number of adults with type 2 diabetes plus those with COPD, Amy Groenewegen, MD, said at the annual congress of the European Society of Cardiology.
“I think this screening is ready for routine use, but it could be followed by prospective studies that investigate whether it produces more benefits in patient-centered outcomes,” Dr. Groenewegen said in a press briefing. She stressed that it has not yet been clearly proven that patients with these chronic diseases are better off long term when their CVD is detected sooner using the tested approach.
“We need simple ways to identify relevant patients for additional screening and potential treatment” of CVD, commented Lars Kober, MD, designated discussant at the Congress and a cardiologist and professor at Rigshospitalet, Copenhagen University Hospital.
A ‘very simple’ symptom questionnaire
The study is important because it tested a “very simple” symptom questionnaire as the initial screening phase, yet resulted in a CVD diagnostic rate that was two- to threefold higher than in the control patients managed with usual care, Dr. Kober noted.
The Reviving the Early Diagnosis of CVD (RED-CVD) trial randomized 14 primary care practices in the Netherlands to apply a structured screening protocol to adults with type 2 diabetes or COPD, and another 11 practices that served as controls and provided their patients with usual care.
The study included 624 people in the screening arm and 592 in the usual-care arm. Their average age was about 68 years. In the screening arm, 87% had type 2 diabetes and 20% had COPD, including 6.3% with both. In the usual-care arm, 86% had type 2 diabetes, 21% had COPD, with 7.4% having both.
About a quarter of the study cohort had a history of a CVD diagnosis, but they were included for their potential for developing another form of CVD. The study considered three types of CVD: coronary artery disease, heart failure, and atrial fibrillation.
The CVD screening protocol began with an 11-question survey, completed by patients, that asked about their symptoms. The survey was devised by a research team at the University Medical Center Groningen, the Netherlands, who collaborated on the study.
The second phase for people who had suggestive symptoms was a physical examination, measurement of serum N-terminal pro-brain natriuretic peptide (elevated levels signal incident heart failure), and an ECG. People who continued to show findings consistent with CVD in this phase were then referred on a discretionary basis by the attending physician to a specialist.
More than doubling the CVD diagnosis rate
The screening program produced a total of 50 new CVD diagnoses in the screening cohort (8%) and 18 in the control, usual-care arm (3%), for the study’s primary endpoint. The greatest number of events involved heart failure, followed by coronary disease.
The screening questionnaire identified 70% of the people who completed it with suggestive symptoms, such as shortness of breath, claudication, or palpitations. The follow-up assessments of phase two narrowed the group with possible new CVD down to 44% of the people in this arm, and the participating physicians referred 39% to a specialist.
An analysis that adjusted for several demographic and clinical variables and excluded nonobstructive coronary disease as a new CVD diagnosis showed that the systematic screening approach resulted in 2.4-fold more new diagnoses than usual care, reported Dr. Groenewegen, an epidemiologist at University Medical Center Utrecht, the Netherlands.
RED-CVD received no commercial funding. Dr. Groenewegen disclosed no relevant financial relationships. Dr. Kober has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AT ESC CONGRESS 2023
FDA clears new capabilities for diabetes app BlueStar
The latest clearance, announced on Aug. 23, enables the app-based platform to provide bolus insulin dose recommendations that are based on glucose and trend data from a compatible continuous glucose monitoring (CGM) device. On Aug. 15, the FDA cleared the BlueStar to use connected insulin dosing data in personalized bolus insulin dosing recommendations.
“Welldoc is the first company to receive clearance for a CGM-informed bolus calculator specifically designed for adults who manage their diabetes with multiple daily injections of insulin,” according to a company statement.
“With this clearance, Welldoc is filling a significant gap for people who require complex insulin regimens. By connecting directly with CGM data and using both glucose values and trend arrows, the BlueStar solution will provide precise and in-the-moment insulin dosing guidance directly to individuals, helping them reach their glucose targets,” endocrinologist Grazia Aleppo, MD, of Northwestern University, Chicago, said in the statement.
The new features extend the platform’s existing digital diet and lifestyle coaching capabilities. Previous FDA clearances included expansions to use most types of available insulins, including bolus and premixed insulin titration for patients with type 2 diabetes, in September 2021 and for basal insulin adjustment in June 2020.
Dr. Aleppo was a principal investigator in Welldoc’s clinical validation study for BlueStar.
A version of this article first appeared on Medscape.com.
The latest clearance, announced on Aug. 23, enables the app-based platform to provide bolus insulin dose recommendations that are based on glucose and trend data from a compatible continuous glucose monitoring (CGM) device. On Aug. 15, the FDA cleared the BlueStar to use connected insulin dosing data in personalized bolus insulin dosing recommendations.
“Welldoc is the first company to receive clearance for a CGM-informed bolus calculator specifically designed for adults who manage their diabetes with multiple daily injections of insulin,” according to a company statement.
“With this clearance, Welldoc is filling a significant gap for people who require complex insulin regimens. By connecting directly with CGM data and using both glucose values and trend arrows, the BlueStar solution will provide precise and in-the-moment insulin dosing guidance directly to individuals, helping them reach their glucose targets,” endocrinologist Grazia Aleppo, MD, of Northwestern University, Chicago, said in the statement.
The new features extend the platform’s existing digital diet and lifestyle coaching capabilities. Previous FDA clearances included expansions to use most types of available insulins, including bolus and premixed insulin titration for patients with type 2 diabetes, in September 2021 and for basal insulin adjustment in June 2020.
Dr. Aleppo was a principal investigator in Welldoc’s clinical validation study for BlueStar.
A version of this article first appeared on Medscape.com.
The latest clearance, announced on Aug. 23, enables the app-based platform to provide bolus insulin dose recommendations that are based on glucose and trend data from a compatible continuous glucose monitoring (CGM) device. On Aug. 15, the FDA cleared the BlueStar to use connected insulin dosing data in personalized bolus insulin dosing recommendations.
“Welldoc is the first company to receive clearance for a CGM-informed bolus calculator specifically designed for adults who manage their diabetes with multiple daily injections of insulin,” according to a company statement.
“With this clearance, Welldoc is filling a significant gap for people who require complex insulin regimens. By connecting directly with CGM data and using both glucose values and trend arrows, the BlueStar solution will provide precise and in-the-moment insulin dosing guidance directly to individuals, helping them reach their glucose targets,” endocrinologist Grazia Aleppo, MD, of Northwestern University, Chicago, said in the statement.
The new features extend the platform’s existing digital diet and lifestyle coaching capabilities. Previous FDA clearances included expansions to use most types of available insulins, including bolus and premixed insulin titration for patients with type 2 diabetes, in September 2021 and for basal insulin adjustment in June 2020.
Dr. Aleppo was a principal investigator in Welldoc’s clinical validation study for BlueStar.
A version of this article first appeared on Medscape.com.
Even an hour’s walk a week lowers risk in type 2 diabetes
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM DIABETES CARE
More weight loss linked with more benefit in STEP-HFpEF
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
Both too much and not enough sleep raises T2D risk
TOPLINE:
suggests an analysis of a Dutch study.
METHODOLOGY:
- Data on 5,561 participants aged 40–75 years from The Maastricht Study who completed the baseline survey between November 2010 and January 2018 and had full data available were included.
- Sleep duration was assessed as the in-bed time in minutes, using a median of 7 nights’ data from an activPAL3 (PAL Technologies) accelerometer, which is worn on the thigh.
- Glucose metabolism was determined via an oral glucose tolerance test and categorized as prediabetes or type 2 diabetes in line with World Health Organization diagnostic criteria.
- The association between sleep duration and type 2 diabetes was assessed on multivariate logistic regression analysis, taking into account a range of potential confounding factors.
TAKEAWAY:
- The mean age of the participants was 60.1 years, and there was an even split between men and women. In all, 832 had prediabetes and 1,341 type 2 diabetes, and the mean sleep duration was 8.3 hours.
- The results indicated there was a U-shaped relationship between sleep duration and type 2 diabetes, so that both long and short sleep durations increased the risk.
- In the fully adjusted model, a sleep duration of 5 hours was associated with an odds ratio for type 2 diabetes versus 8 hours sleep of 2.9. For a sleep duration of 12 hours, the odds ratio was 1.8.
- The association between sleep duration and diabetes was not significant.
IN PRACTICE:
The results “support the idea that sleep duration could be a relevant risk factor for type 2 diabetes independent of lifestyle risk factors, including diet, physical activity, smoking behavior, and alcohol consumption,” wrote the authors.
“These findings underpin the importance of promoting healthy sleep habits to avoid sleep deprivation,” they added.
STUDY DETAILS:
The research was led by Jeroen D. Albers, MSc, department of social medicine, Maastricht (the Netherlands) University, and published in Sleep Health. It is an analysis of The Maastricht Study.
LIMITATIONS:
The study is limited by its cross-sectional nature, particularly because there are “plausible causal paths between sleep duration and type 2 in both directions,” the authors note. The accelerometer used in the study also cannot reliably distinguish between waking and sleeping time in bed, with the potential for misclassification. Daytime naps were also not included, and long-term changes sleep patterns were not measured. In addition, it was not possible to control for some potential confounding factors.
DISCLOSURES:
The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs, Stichting De Weijerhorst, the Pearl String Initiative Diabetes, the School for Cardiovascular Diseases, the School for Public Health and Primary Care, the School for Nutrition and Translational Research in Metabolism, Stichting Annadal, Health Foundation Limburg, and unrestricted grants from Janssen-Cilag, Novo Nordisk, and Sanofi Aventis Netherlands. One author declares a relationship with Novo Nordisk outside the submitted work. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
TOPLINE:
suggests an analysis of a Dutch study.
METHODOLOGY:
- Data on 5,561 participants aged 40–75 years from The Maastricht Study who completed the baseline survey between November 2010 and January 2018 and had full data available were included.
- Sleep duration was assessed as the in-bed time in minutes, using a median of 7 nights’ data from an activPAL3 (PAL Technologies) accelerometer, which is worn on the thigh.
- Glucose metabolism was determined via an oral glucose tolerance test and categorized as prediabetes or type 2 diabetes in line with World Health Organization diagnostic criteria.
- The association between sleep duration and type 2 diabetes was assessed on multivariate logistic regression analysis, taking into account a range of potential confounding factors.
TAKEAWAY:
- The mean age of the participants was 60.1 years, and there was an even split between men and women. In all, 832 had prediabetes and 1,341 type 2 diabetes, and the mean sleep duration was 8.3 hours.
- The results indicated there was a U-shaped relationship between sleep duration and type 2 diabetes, so that both long and short sleep durations increased the risk.
- In the fully adjusted model, a sleep duration of 5 hours was associated with an odds ratio for type 2 diabetes versus 8 hours sleep of 2.9. For a sleep duration of 12 hours, the odds ratio was 1.8.
- The association between sleep duration and diabetes was not significant.
IN PRACTICE:
The results “support the idea that sleep duration could be a relevant risk factor for type 2 diabetes independent of lifestyle risk factors, including diet, physical activity, smoking behavior, and alcohol consumption,” wrote the authors.
“These findings underpin the importance of promoting healthy sleep habits to avoid sleep deprivation,” they added.
STUDY DETAILS:
The research was led by Jeroen D. Albers, MSc, department of social medicine, Maastricht (the Netherlands) University, and published in Sleep Health. It is an analysis of The Maastricht Study.
LIMITATIONS:
The study is limited by its cross-sectional nature, particularly because there are “plausible causal paths between sleep duration and type 2 in both directions,” the authors note. The accelerometer used in the study also cannot reliably distinguish between waking and sleeping time in bed, with the potential for misclassification. Daytime naps were also not included, and long-term changes sleep patterns were not measured. In addition, it was not possible to control for some potential confounding factors.
DISCLOSURES:
The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs, Stichting De Weijerhorst, the Pearl String Initiative Diabetes, the School for Cardiovascular Diseases, the School for Public Health and Primary Care, the School for Nutrition and Translational Research in Metabolism, Stichting Annadal, Health Foundation Limburg, and unrestricted grants from Janssen-Cilag, Novo Nordisk, and Sanofi Aventis Netherlands. One author declares a relationship with Novo Nordisk outside the submitted work. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
TOPLINE:
suggests an analysis of a Dutch study.
METHODOLOGY:
- Data on 5,561 participants aged 40–75 years from The Maastricht Study who completed the baseline survey between November 2010 and January 2018 and had full data available were included.
- Sleep duration was assessed as the in-bed time in minutes, using a median of 7 nights’ data from an activPAL3 (PAL Technologies) accelerometer, which is worn on the thigh.
- Glucose metabolism was determined via an oral glucose tolerance test and categorized as prediabetes or type 2 diabetes in line with World Health Organization diagnostic criteria.
- The association between sleep duration and type 2 diabetes was assessed on multivariate logistic regression analysis, taking into account a range of potential confounding factors.
TAKEAWAY:
- The mean age of the participants was 60.1 years, and there was an even split between men and women. In all, 832 had prediabetes and 1,341 type 2 diabetes, and the mean sleep duration was 8.3 hours.
- The results indicated there was a U-shaped relationship between sleep duration and type 2 diabetes, so that both long and short sleep durations increased the risk.
- In the fully adjusted model, a sleep duration of 5 hours was associated with an odds ratio for type 2 diabetes versus 8 hours sleep of 2.9. For a sleep duration of 12 hours, the odds ratio was 1.8.
- The association between sleep duration and diabetes was not significant.
IN PRACTICE:
The results “support the idea that sleep duration could be a relevant risk factor for type 2 diabetes independent of lifestyle risk factors, including diet, physical activity, smoking behavior, and alcohol consumption,” wrote the authors.
“These findings underpin the importance of promoting healthy sleep habits to avoid sleep deprivation,” they added.
STUDY DETAILS:
The research was led by Jeroen D. Albers, MSc, department of social medicine, Maastricht (the Netherlands) University, and published in Sleep Health. It is an analysis of The Maastricht Study.
LIMITATIONS:
The study is limited by its cross-sectional nature, particularly because there are “plausible causal paths between sleep duration and type 2 in both directions,” the authors note. The accelerometer used in the study also cannot reliably distinguish between waking and sleeping time in bed, with the potential for misclassification. Daytime naps were also not included, and long-term changes sleep patterns were not measured. In addition, it was not possible to control for some potential confounding factors.
DISCLOSURES:
The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs, Stichting De Weijerhorst, the Pearl String Initiative Diabetes, the School for Cardiovascular Diseases, the School for Public Health and Primary Care, the School for Nutrition and Translational Research in Metabolism, Stichting Annadal, Health Foundation Limburg, and unrestricted grants from Janssen-Cilag, Novo Nordisk, and Sanofi Aventis Netherlands. One author declares a relationship with Novo Nordisk outside the submitted work. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM SLEEP HEALTH
Severe COVID may cause long-term cellular changes: Study
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
The small study, published in Cell and funded by the National Institutes of Health, details how immune cells were analyzed through blood samples collected from 38 patients recovering from severe COVID and other critical illnesses, and from 19 healthy people. Researchers from Weill Cornell Medicine, New York, and The Jackson Laboratory for Genomic Medicine, Farmington, Conn., found through isolating hematopoietic stem cells that people recovering from severe bouts of COVID had changes to their DNA that were passed down to offspring cells.
The research team, led by Steven Josefowicz, PhD, of Weill Cornell’s pathology department, and Duygu Ucar, PhD, associate professor at The Jackson Laboratory for Genomic Medicine, discovered that this chain reaction of stem cell changes caused a boost in the production of monocytes. The authors found that, due to the innate cellular changes from a severe case of COVID, patients in recovery ended up producing a larger amount of inflammatory cytokines, rather than monocytes – distinct from samples collected from healthy patients and those recovering from other critical illnesses.
These changes to patients’ epigenetic landscapes were observed even a year after the initial COVID-19 infection. While the small participant pool meant that the research team could not establish a direct line between these innate changes and any ensuing health outcomes, the research provides us with clues as to why patients continue to struggle with inflammation and long COVID symptoms well after they recover.
While the authors reiterate the study’s limitations and hesitate to make any clear-cut associations between the results and long-term health outcomes, Wolfgang Leitner, PhD, from the NIH’s National Institute of Allergy and Infectious Diseases, predicts that long COVID can, at least in part, be explained by the changes in innate immune responses.
“Ideally, the authors would have had cells from each patient before they got infected, as a comparator, to see what the epigenetic landscape was before COVID changed it,” said Dr. Leitner. “Clear links between the severity of COVID and genetics were discovered already early in the pandemic and this paper should prompt follow-up studies that link mutations in immune genes with the epigenetic changes described here.”
Dr. Leitner said he had some initial predictions about the long-term impact of COVID-19, but he had not anticipated some of what the study’s findings now show.
“Unlike in the case of, for example, influenza, where the lungs go into ‘repair mode’ after the infection has been resolved – which leaves people susceptible to secondary infections for up to several months – this study shows that after severe COVID, the immune system remains in ‘emergency mode’ and in a heightened state of inflammation,” said Dr. Leitner.
“That further aggravates the problem the initial strong inflammation causes: even higher risk of autoimmune disease, but also, cancer.”
Commenting on the findings, Eric Topol, MD, editor-in-chief of Medscape Medical News, said the study presents “evidence that a key line of immune cells are essentially irrevocably, epigenetically altered and activated.
“You do not want to have this [COVID],” he added.
The study also highlights the researchers’ novel approach to isolating hematopoietic stem cells, found largely in bone marrow. This type of research has been limited in the past because of how costly and invasive it can be to analyze cells in bone marrow. But, by isolating and enriching hematopoietic stem cells, the team can decipher the full cellular diversity of the cells’ bone marrow counterparts.
“This revelation opened the doors to study, at single-cell resolution, how stem cells are affected upon infection and vaccination with a simple blood draw,” representatives from the Jackson lab said in a press release.
A version of this article appeared on Medscape.com.
FROM CELL
Inner lip erosions
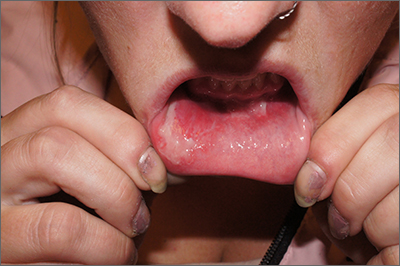
The patient was having a flare of pemphigus vulgaris (PV), a rare and sometimes life-threatening acquired autoimmune blistering disease that affects the skin and/or mucosa. Ashkenazi Jewish patients and patients from Mediterranean and Middle Eastern countries are more likely to be affected.
In PV, acquired autoantibodies target the desmosomes that connect epithelial cells together, weakening the intercellular adhesion. It can affect skin, mucosa, or both. Patients present with fragile bullae or ulcers. The connections between the cells are often so damaged that rubbing on the skin creates a new blister called “Nikolsky sign.” In the mouth, bullae erode rapidly. Look for disease affecting the ocular conjunctiva or sclera, as well. PV can also occasionally affect the nasopharynx and esophagus, usually manifesting as hemoptysis, dysphagia, and nosebleeds with ulcer seen on endoscopy or otolaryngoscopy.
Although PV is often severe (and can warrant hospitalization when significant body surface area is involved), some patients may have few active lesions and can be managed safely as outpatients.
The diagnosis requires 2 biopsies and serum for indirect immunofluorescence. One biopsy (either by punch or shave to the upper dermis) is taken from the edge of a bulla or ulcer. Another biopsy (by punch or shave) is taken from nearby normal-looking skin or mucosa for testing the direct immunofluorescence pattern. In the mucosa, a punch biopsy may be left open or closed with absorbable sutures. A serum sample is taken for indirect immunofluorescence to differentiate pemphigus vulgaris from other forms of pemphigus.1
PV is treated by suppressing the immune system. Focal disease may be treated with super-potent topical steroids, including clobetasol 0.05% ointment. Even in the mouth, topical clobetasol 0.05% may be used off-label twice daily until control is achieved. When topical treatment is used in the mouth, advise patients to apply the clobetasol ointment to a piece of gauze and place the gauze (ointment side down) over affected areas for 20 to 30 minutes twice daily.
Patients with widespread or severe disease should be hospitalized. In severe cases, supportive wound care is provided, and treatment is aimed at immunosuppression. Systemic options include high-dose prednisone 0.5 to 1 mg/kg daily until clear, a steroid-sparing immunosuppressant such as mycophenolate mofetil up to 1000 mg bid, or rituximab in 1 of several regimens.
Three years prior to this patient’s visit, she had been successfully treated for PV with a course of rituximab. To treat the current flare, she was started on prednisone 60 mg/d. In addition, the plan was for her to complete 2 infusions of 1000 mg rituximab 2 weeks apart.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.
1. Didona, D, Schmidt, MF, Maglie, R, et al. Pemphigus and pemphigoids: clinical presentation, diagnosis and therapy. J Dtsch Dermatol Ges. 2023;1-20. doi: 10.1111/ddg.15174

The patient was having a flare of pemphigus vulgaris (PV), a rare and sometimes life-threatening acquired autoimmune blistering disease that affects the skin and/or mucosa. Ashkenazi Jewish patients and patients from Mediterranean and Middle Eastern countries are more likely to be affected.
In PV, acquired autoantibodies target the desmosomes that connect epithelial cells together, weakening the intercellular adhesion. It can affect skin, mucosa, or both. Patients present with fragile bullae or ulcers. The connections between the cells are often so damaged that rubbing on the skin creates a new blister called “Nikolsky sign.” In the mouth, bullae erode rapidly. Look for disease affecting the ocular conjunctiva or sclera, as well. PV can also occasionally affect the nasopharynx and esophagus, usually manifesting as hemoptysis, dysphagia, and nosebleeds with ulcer seen on endoscopy or otolaryngoscopy.
Although PV is often severe (and can warrant hospitalization when significant body surface area is involved), some patients may have few active lesions and can be managed safely as outpatients.
The diagnosis requires 2 biopsies and serum for indirect immunofluorescence. One biopsy (either by punch or shave to the upper dermis) is taken from the edge of a bulla or ulcer. Another biopsy (by punch or shave) is taken from nearby normal-looking skin or mucosa for testing the direct immunofluorescence pattern. In the mucosa, a punch biopsy may be left open or closed with absorbable sutures. A serum sample is taken for indirect immunofluorescence to differentiate pemphigus vulgaris from other forms of pemphigus.1
PV is treated by suppressing the immune system. Focal disease may be treated with super-potent topical steroids, including clobetasol 0.05% ointment. Even in the mouth, topical clobetasol 0.05% may be used off-label twice daily until control is achieved. When topical treatment is used in the mouth, advise patients to apply the clobetasol ointment to a piece of gauze and place the gauze (ointment side down) over affected areas for 20 to 30 minutes twice daily.
Patients with widespread or severe disease should be hospitalized. In severe cases, supportive wound care is provided, and treatment is aimed at immunosuppression. Systemic options include high-dose prednisone 0.5 to 1 mg/kg daily until clear, a steroid-sparing immunosuppressant such as mycophenolate mofetil up to 1000 mg bid, or rituximab in 1 of several regimens.
Three years prior to this patient’s visit, she had been successfully treated for PV with a course of rituximab. To treat the current flare, she was started on prednisone 60 mg/d. In addition, the plan was for her to complete 2 infusions of 1000 mg rituximab 2 weeks apart.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.

The patient was having a flare of pemphigus vulgaris (PV), a rare and sometimes life-threatening acquired autoimmune blistering disease that affects the skin and/or mucosa. Ashkenazi Jewish patients and patients from Mediterranean and Middle Eastern countries are more likely to be affected.
In PV, acquired autoantibodies target the desmosomes that connect epithelial cells together, weakening the intercellular adhesion. It can affect skin, mucosa, or both. Patients present with fragile bullae or ulcers. The connections between the cells are often so damaged that rubbing on the skin creates a new blister called “Nikolsky sign.” In the mouth, bullae erode rapidly. Look for disease affecting the ocular conjunctiva or sclera, as well. PV can also occasionally affect the nasopharynx and esophagus, usually manifesting as hemoptysis, dysphagia, and nosebleeds with ulcer seen on endoscopy or otolaryngoscopy.
Although PV is often severe (and can warrant hospitalization when significant body surface area is involved), some patients may have few active lesions and can be managed safely as outpatients.
The diagnosis requires 2 biopsies and serum for indirect immunofluorescence. One biopsy (either by punch or shave to the upper dermis) is taken from the edge of a bulla or ulcer. Another biopsy (by punch or shave) is taken from nearby normal-looking skin or mucosa for testing the direct immunofluorescence pattern. In the mucosa, a punch biopsy may be left open or closed with absorbable sutures. A serum sample is taken for indirect immunofluorescence to differentiate pemphigus vulgaris from other forms of pemphigus.1
PV is treated by suppressing the immune system. Focal disease may be treated with super-potent topical steroids, including clobetasol 0.05% ointment. Even in the mouth, topical clobetasol 0.05% may be used off-label twice daily until control is achieved. When topical treatment is used in the mouth, advise patients to apply the clobetasol ointment to a piece of gauze and place the gauze (ointment side down) over affected areas for 20 to 30 minutes twice daily.
Patients with widespread or severe disease should be hospitalized. In severe cases, supportive wound care is provided, and treatment is aimed at immunosuppression. Systemic options include high-dose prednisone 0.5 to 1 mg/kg daily until clear, a steroid-sparing immunosuppressant such as mycophenolate mofetil up to 1000 mg bid, or rituximab in 1 of several regimens.
Three years prior to this patient’s visit, she had been successfully treated for PV with a course of rituximab. To treat the current flare, she was started on prednisone 60 mg/d. In addition, the plan was for her to complete 2 infusions of 1000 mg rituximab 2 weeks apart.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.
1. Didona, D, Schmidt, MF, Maglie, R, et al. Pemphigus and pemphigoids: clinical presentation, diagnosis and therapy. J Dtsch Dermatol Ges. 2023;1-20. doi: 10.1111/ddg.15174
1. Didona, D, Schmidt, MF, Maglie, R, et al. Pemphigus and pemphigoids: clinical presentation, diagnosis and therapy. J Dtsch Dermatol Ges. 2023;1-20. doi: 10.1111/ddg.15174
Use of mental health services soared during pandemic
By the end of August 2022, overall use of mental health services was almost 40% higher than before the COVID-19 pandemic, while spending increased by 54%, according to a new study by researchers at the RAND Corporation.
During the early phase of the pandemic, from mid-March to mid-December 2020, before the vaccine was available, in-person visits decreased by 40%, while telehealth visits increased by 1,000%, reported Jonathan H. Cantor, PhD, and colleagues at RAND, and at Castlight Health, a benefit coordination provider, in a paper published online in JAMA Health Forum.
Between December 2020 and August 2022, telehealth visits stayed stable, but in-person visits creeped back up, eventually reaching 80% of prepandemic levels. However, “total utilization was higher than before the pandemic,” Dr. Cantor, a policy researcher at RAND, told this news organization.
“It could be that it’s easier for individuals to receive care via telehealth, but it could also just be that there’s a greater demand or need since the pandemic,” said Dr. Cantor. “We’ll just need more research to actually unpack what’s going on,” he said.
Initial per capita spending increased by about a third and was up overall by more than half. But it’s not clear how much of that is due to utilization or to price of services, said Dr. Cantor. Spending for telehealth services remained stable in the post-vaccine period, while spending on in-person visits returned to prepandemic levels.
Dr. Cantor and his colleagues were not able to determine whether utilization was by new or existing patients, but he said that would be good data to have. “It would be really important to know whether or not folks are initiating care because telehealth is making it easier,” he said.
The authors analyzed about 1.5 million claims for anxiety disorders, major depressive disorder, bipolar disorder, schizophrenia, and posttraumatic stress disorder, out of claims submitted by 7 million commercially insured adults whose self-insured employers used the Castlight benefit.
Dr. Cantor noted that this is just a small subset of the U.S. population. He said he’d like to have data from Medicare and Medicaid to fully assess the impact of the COVID-19 pandemic on mental health and of telehealth visits.
“This is a still-burgeoning field,” he said about telehealth. “We’re still trying to get a handle on how things are operating, given that there’s been so much change so rapidly.”
Meanwhile, 152 major employers responding to a large national survey this summer said that they’ve been grappling with how COVID-19 has affected workers. The employers include 72 Fortune 100 companies and provide health coverage for more than 60 million workers, retirees, and their families.
Seventy-seven percent said they are currently seeing an increase in depression, anxiety, and substance use disorders as a result of the pandemic, according to the Business Group on Health’s survey. That’s up from 44% in 2022.
Going forward, employers will focus on increasing access to mental health services, the survey reported.
“Our survey found that in 2024 and for the near future, employers will be acutely focused on addressing employees’ mental health needs while ensuring access and lowering cost barriers,” Ellen Kelsay, president and CEO of Business Group on Health, said in a statement.
The study was supported by grants from the National Institute of Mental Health and the National Institute on Aging. Coauthor Dena Bravata, MD, a Castlight employee, reported receiving personal fees from Castlight Health during the conduct of the study. Coauthor Christopher M. Whaley, a RAND employee, reported receiving personal fees from Castlight Health outside the submitted work.
A version of this article appeared on Medscape.com.
By the end of August 2022, overall use of mental health services was almost 40% higher than before the COVID-19 pandemic, while spending increased by 54%, according to a new study by researchers at the RAND Corporation.
During the early phase of the pandemic, from mid-March to mid-December 2020, before the vaccine was available, in-person visits decreased by 40%, while telehealth visits increased by 1,000%, reported Jonathan H. Cantor, PhD, and colleagues at RAND, and at Castlight Health, a benefit coordination provider, in a paper published online in JAMA Health Forum.
Between December 2020 and August 2022, telehealth visits stayed stable, but in-person visits creeped back up, eventually reaching 80% of prepandemic levels. However, “total utilization was higher than before the pandemic,” Dr. Cantor, a policy researcher at RAND, told this news organization.
“It could be that it’s easier for individuals to receive care via telehealth, but it could also just be that there’s a greater demand or need since the pandemic,” said Dr. Cantor. “We’ll just need more research to actually unpack what’s going on,” he said.
Initial per capita spending increased by about a third and was up overall by more than half. But it’s not clear how much of that is due to utilization or to price of services, said Dr. Cantor. Spending for telehealth services remained stable in the post-vaccine period, while spending on in-person visits returned to prepandemic levels.
Dr. Cantor and his colleagues were not able to determine whether utilization was by new or existing patients, but he said that would be good data to have. “It would be really important to know whether or not folks are initiating care because telehealth is making it easier,” he said.
The authors analyzed about 1.5 million claims for anxiety disorders, major depressive disorder, bipolar disorder, schizophrenia, and posttraumatic stress disorder, out of claims submitted by 7 million commercially insured adults whose self-insured employers used the Castlight benefit.
Dr. Cantor noted that this is just a small subset of the U.S. population. He said he’d like to have data from Medicare and Medicaid to fully assess the impact of the COVID-19 pandemic on mental health and of telehealth visits.
“This is a still-burgeoning field,” he said about telehealth. “We’re still trying to get a handle on how things are operating, given that there’s been so much change so rapidly.”
Meanwhile, 152 major employers responding to a large national survey this summer said that they’ve been grappling with how COVID-19 has affected workers. The employers include 72 Fortune 100 companies and provide health coverage for more than 60 million workers, retirees, and their families.
Seventy-seven percent said they are currently seeing an increase in depression, anxiety, and substance use disorders as a result of the pandemic, according to the Business Group on Health’s survey. That’s up from 44% in 2022.
Going forward, employers will focus on increasing access to mental health services, the survey reported.
“Our survey found that in 2024 and for the near future, employers will be acutely focused on addressing employees’ mental health needs while ensuring access and lowering cost barriers,” Ellen Kelsay, president and CEO of Business Group on Health, said in a statement.
The study was supported by grants from the National Institute of Mental Health and the National Institute on Aging. Coauthor Dena Bravata, MD, a Castlight employee, reported receiving personal fees from Castlight Health during the conduct of the study. Coauthor Christopher M. Whaley, a RAND employee, reported receiving personal fees from Castlight Health outside the submitted work.
A version of this article appeared on Medscape.com.
By the end of August 2022, overall use of mental health services was almost 40% higher than before the COVID-19 pandemic, while spending increased by 54%, according to a new study by researchers at the RAND Corporation.
During the early phase of the pandemic, from mid-March to mid-December 2020, before the vaccine was available, in-person visits decreased by 40%, while telehealth visits increased by 1,000%, reported Jonathan H. Cantor, PhD, and colleagues at RAND, and at Castlight Health, a benefit coordination provider, in a paper published online in JAMA Health Forum.
Between December 2020 and August 2022, telehealth visits stayed stable, but in-person visits creeped back up, eventually reaching 80% of prepandemic levels. However, “total utilization was higher than before the pandemic,” Dr. Cantor, a policy researcher at RAND, told this news organization.
“It could be that it’s easier for individuals to receive care via telehealth, but it could also just be that there’s a greater demand or need since the pandemic,” said Dr. Cantor. “We’ll just need more research to actually unpack what’s going on,” he said.
Initial per capita spending increased by about a third and was up overall by more than half. But it’s not clear how much of that is due to utilization or to price of services, said Dr. Cantor. Spending for telehealth services remained stable in the post-vaccine period, while spending on in-person visits returned to prepandemic levels.
Dr. Cantor and his colleagues were not able to determine whether utilization was by new or existing patients, but he said that would be good data to have. “It would be really important to know whether or not folks are initiating care because telehealth is making it easier,” he said.
The authors analyzed about 1.5 million claims for anxiety disorders, major depressive disorder, bipolar disorder, schizophrenia, and posttraumatic stress disorder, out of claims submitted by 7 million commercially insured adults whose self-insured employers used the Castlight benefit.
Dr. Cantor noted that this is just a small subset of the U.S. population. He said he’d like to have data from Medicare and Medicaid to fully assess the impact of the COVID-19 pandemic on mental health and of telehealth visits.
“This is a still-burgeoning field,” he said about telehealth. “We’re still trying to get a handle on how things are operating, given that there’s been so much change so rapidly.”
Meanwhile, 152 major employers responding to a large national survey this summer said that they’ve been grappling with how COVID-19 has affected workers. The employers include 72 Fortune 100 companies and provide health coverage for more than 60 million workers, retirees, and their families.
Seventy-seven percent said they are currently seeing an increase in depression, anxiety, and substance use disorders as a result of the pandemic, according to the Business Group on Health’s survey. That’s up from 44% in 2022.
Going forward, employers will focus on increasing access to mental health services, the survey reported.
“Our survey found that in 2024 and for the near future, employers will be acutely focused on addressing employees’ mental health needs while ensuring access and lowering cost barriers,” Ellen Kelsay, president and CEO of Business Group on Health, said in a statement.
The study was supported by grants from the National Institute of Mental Health and the National Institute on Aging. Coauthor Dena Bravata, MD, a Castlight employee, reported receiving personal fees from Castlight Health during the conduct of the study. Coauthor Christopher M. Whaley, a RAND employee, reported receiving personal fees from Castlight Health outside the submitted work.
A version of this article appeared on Medscape.com.