User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Smartphones for children with type 1 diabetes: Cause for concern?
My young patient with type 1 diabetes (T1D) had her cell phone out to provide a share code for her Dexcom clarity app as she was checking into her visit. As my nurse was recording the code, the patient asked him, “Hey, can you add me on Snapchat?”
Her father scrolled through his own Facebook feed in the chair next to her, showing no concern that his daughter was looking to connect with an adult on a social media platform. Meanwhile, we were all grateful that the little girl, who had had a seizure due to hypoglycemia in her preschool and pre–continuous glucose monitoring (CGM) years, had access to the tools harnessed within the sparkly encased phone she held in her small hands. But did anyone in the room fully understand the potential dangers?
We are living in an exhilarating era of diabetes technology, a treatment environment that I couldn’t have dreamed of during my pediatric endocrinology fellowship. T1D is a volatile condition that changes day to day, especially in growing children. A short decade ago, the best CGM available was a bulky device on loan to patients for 3 days at a time. Information was later downloaded in-office to get a better idea of general glucose trends, if insurance would approve its use at all.
Now, we have a variety of very wearable and accurate disposable CGMs accessible to most patients. Every major insulin pump has available closed-loop capabilities. Some patients can dose from apps on their cell phones rather than juggle another device or draw attention to an insulin pump at the cafeteria table.
These developments have been game changers for children and teenagers with diabetes and for their families. When wondering whether an athlete’s dazed appearance on a soccer field was due to hypoglycemia, a parent no longer must demand that a coach pull the player – a quick glance at a smartphone app can verify the blood glucose and change rate. Children can use programs and search engines to quickly verify carbohydrate counts. Life360 and other tracking programs have increased parental feelings of security, especially with young drivers living with a chronic medical condition.
The inevitable outcome of this available technology is that children living with T1D are given cell phones far earlier than are their siblings or peers owing to “necessity.” Parents understandably want a means to stay in close contact with their children in case of a medical emergency. As a physician and mother of young children, I am thankful for the technology that keeps my patients safer and that allows them to fully participate in everything from sports to travel to an uninterrupted night’s sleep.
Smartphone presence in classrooms empowers teachers, students, parents, and school nurses to be aware of glycemic trends and prevent hypoglycemic emergencies. Smartphones have also shown to be a major distraction in that setting, causing many schools to ban their use entirely. Video apps such as YouTube and TikTok can provide a wealth of support and medical information but may also open the door to misinformation and dangerous social contagion, particularly surrounding disordered eating. Informative podcasts such as The Juicebox Podcast and online forums provide incredible support for families, but the constant siren call of a phone in their pockets leads to distracted parents constantly tending to other conversations or responding to ever more demanding employers rather than focusing on face-to-face education sessions.
The Surgeon General recently released a report concerning social media use in children. This eye-opening report revealed that one-third of children admitted to using their cell phones “almost constantly.” Social media use is associated with higher rates of anxiety and depression, especially in teen girls. This is particularly concerning for children with T1D, who are more likely to suffer from these conditions.
Beyond mental health concerns, especially to developing brains, unfettered Internet use increases the risk that children are exposed to predators and harmful content. The online safety monitoring platform Bark shared data from its 2021 surveillance. Bark found that 72% of tweens and 85% of teens were involved in an online bullying situation. Sixty-nine percent of tweens and 91% of teens encountered nudity or sexual content. Ten percent of tweens and 21% of teens encountered predatory behavior.
These alarming finds mirror the prevalence suggested by conversations in my office. I hear reports of my patients sneaking out at night to meet adults they met through social media, having suicidal ideation and attempts after Internet bullying, and sharing earnest belief in bizarre conspiracy theories gleaned from online forums that lead to dangerous health care practices.
Furthermore, time is a finite resource. Teens who are spending an average of 3.5 hours daily on their devices are running out of time to play, study, and grow extracurricular interests. My friend who coaches high school baseball lamented recently the poor athleticism in his recent teams. He theorized that his players had spent their summers on tablets rather than playing catch or climbing trees. The resulting declines in exercise in young people only serve to worsen the childhood obesity epidemic.
What is a concerned parent to do? First, all phones have controls that allow parents to choose which apps are allowed and which are blocked. Caregivers must understand how various social media platforms work. Installing programs such as Bark provides an additional layer of monitoring, though these are no substitute for parental vigilance. Importantly, parents should talk to their children about their concerns regarding social media.
Sadly, I have often noticed that caregivers pity the extra hardships their children endure as the result of T1D and other chronic diseases. Being lax with rules to attempt to compensate for other suffering is far too tempting. The goal is for children and teens living with T1D to have a full and normal childhood, and unrestricted smartphone access and early social media use should not be the goal for any child. For every family, a media use plan is a smart approach. The American Academy of Pediatrics suggests several commonsense steps to use technology wisely, and parents often must address their own relationships with their devices to model healthy engagement.
As health care professionals, we owe it to our patients to discuss the ups and downs of technology with our patients. We can’t ostrich our way through this. We can point our patients and families to supportive groups such as Osprey (Old School Parents Raising Engaged Youth), founded by Ben and Erin Napier from the HGTV show Home Town along with my college friends Taylor and Dr. Catherine Sledge. Wait Until 8th provides information and motivation for parents to make wise choices regarding phone use for their children. The documentary Childhood 2.0 is another compelling resource developed by pediatric emergency physician Dr. Free Hess and her team that summarizes many of these concerns.
In another decade, many of these dangers will be far clearer. As ubiquitous as smartphone misuse is in our society, I remain hopeful that our society will change its behaviors. Just because “everyone else” allows an unhealthy relationship with technology doesn’t mean that we should for our children.
When I was a child, smoking was glamorized in movies and restaurants had dedicated smoking sections. After strong public policy efforts, many geared toward children, smoking is now almost unthinkable. My 8-year-old asked me lately whether a lady smoking a cigarette in the car next to us would have to go to jail. I chose a career in pediatrics because I am an optimist at my very core. We can’t ignore the dangers associated with the wide door opened by mobile devices. We can celebrate the benefits while clearly facing the pitfalls.
Dr. Lilley is director of the pediatric diabetes and lipid program at the Mississippi Center for Advanced Medicine, Madison. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
My young patient with type 1 diabetes (T1D) had her cell phone out to provide a share code for her Dexcom clarity app as she was checking into her visit. As my nurse was recording the code, the patient asked him, “Hey, can you add me on Snapchat?”
Her father scrolled through his own Facebook feed in the chair next to her, showing no concern that his daughter was looking to connect with an adult on a social media platform. Meanwhile, we were all grateful that the little girl, who had had a seizure due to hypoglycemia in her preschool and pre–continuous glucose monitoring (CGM) years, had access to the tools harnessed within the sparkly encased phone she held in her small hands. But did anyone in the room fully understand the potential dangers?
We are living in an exhilarating era of diabetes technology, a treatment environment that I couldn’t have dreamed of during my pediatric endocrinology fellowship. T1D is a volatile condition that changes day to day, especially in growing children. A short decade ago, the best CGM available was a bulky device on loan to patients for 3 days at a time. Information was later downloaded in-office to get a better idea of general glucose trends, if insurance would approve its use at all.
Now, we have a variety of very wearable and accurate disposable CGMs accessible to most patients. Every major insulin pump has available closed-loop capabilities. Some patients can dose from apps on their cell phones rather than juggle another device or draw attention to an insulin pump at the cafeteria table.
These developments have been game changers for children and teenagers with diabetes and for their families. When wondering whether an athlete’s dazed appearance on a soccer field was due to hypoglycemia, a parent no longer must demand that a coach pull the player – a quick glance at a smartphone app can verify the blood glucose and change rate. Children can use programs and search engines to quickly verify carbohydrate counts. Life360 and other tracking programs have increased parental feelings of security, especially with young drivers living with a chronic medical condition.
The inevitable outcome of this available technology is that children living with T1D are given cell phones far earlier than are their siblings or peers owing to “necessity.” Parents understandably want a means to stay in close contact with their children in case of a medical emergency. As a physician and mother of young children, I am thankful for the technology that keeps my patients safer and that allows them to fully participate in everything from sports to travel to an uninterrupted night’s sleep.
Smartphone presence in classrooms empowers teachers, students, parents, and school nurses to be aware of glycemic trends and prevent hypoglycemic emergencies. Smartphones have also shown to be a major distraction in that setting, causing many schools to ban their use entirely. Video apps such as YouTube and TikTok can provide a wealth of support and medical information but may also open the door to misinformation and dangerous social contagion, particularly surrounding disordered eating. Informative podcasts such as The Juicebox Podcast and online forums provide incredible support for families, but the constant siren call of a phone in their pockets leads to distracted parents constantly tending to other conversations or responding to ever more demanding employers rather than focusing on face-to-face education sessions.
The Surgeon General recently released a report concerning social media use in children. This eye-opening report revealed that one-third of children admitted to using their cell phones “almost constantly.” Social media use is associated with higher rates of anxiety and depression, especially in teen girls. This is particularly concerning for children with T1D, who are more likely to suffer from these conditions.
Beyond mental health concerns, especially to developing brains, unfettered Internet use increases the risk that children are exposed to predators and harmful content. The online safety monitoring platform Bark shared data from its 2021 surveillance. Bark found that 72% of tweens and 85% of teens were involved in an online bullying situation. Sixty-nine percent of tweens and 91% of teens encountered nudity or sexual content. Ten percent of tweens and 21% of teens encountered predatory behavior.
These alarming finds mirror the prevalence suggested by conversations in my office. I hear reports of my patients sneaking out at night to meet adults they met through social media, having suicidal ideation and attempts after Internet bullying, and sharing earnest belief in bizarre conspiracy theories gleaned from online forums that lead to dangerous health care practices.
Furthermore, time is a finite resource. Teens who are spending an average of 3.5 hours daily on their devices are running out of time to play, study, and grow extracurricular interests. My friend who coaches high school baseball lamented recently the poor athleticism in his recent teams. He theorized that his players had spent their summers on tablets rather than playing catch or climbing trees. The resulting declines in exercise in young people only serve to worsen the childhood obesity epidemic.
What is a concerned parent to do? First, all phones have controls that allow parents to choose which apps are allowed and which are blocked. Caregivers must understand how various social media platforms work. Installing programs such as Bark provides an additional layer of monitoring, though these are no substitute for parental vigilance. Importantly, parents should talk to their children about their concerns regarding social media.
Sadly, I have often noticed that caregivers pity the extra hardships their children endure as the result of T1D and other chronic diseases. Being lax with rules to attempt to compensate for other suffering is far too tempting. The goal is for children and teens living with T1D to have a full and normal childhood, and unrestricted smartphone access and early social media use should not be the goal for any child. For every family, a media use plan is a smart approach. The American Academy of Pediatrics suggests several commonsense steps to use technology wisely, and parents often must address their own relationships with their devices to model healthy engagement.
As health care professionals, we owe it to our patients to discuss the ups and downs of technology with our patients. We can’t ostrich our way through this. We can point our patients and families to supportive groups such as Osprey (Old School Parents Raising Engaged Youth), founded by Ben and Erin Napier from the HGTV show Home Town along with my college friends Taylor and Dr. Catherine Sledge. Wait Until 8th provides information and motivation for parents to make wise choices regarding phone use for their children. The documentary Childhood 2.0 is another compelling resource developed by pediatric emergency physician Dr. Free Hess and her team that summarizes many of these concerns.
In another decade, many of these dangers will be far clearer. As ubiquitous as smartphone misuse is in our society, I remain hopeful that our society will change its behaviors. Just because “everyone else” allows an unhealthy relationship with technology doesn’t mean that we should for our children.
When I was a child, smoking was glamorized in movies and restaurants had dedicated smoking sections. After strong public policy efforts, many geared toward children, smoking is now almost unthinkable. My 8-year-old asked me lately whether a lady smoking a cigarette in the car next to us would have to go to jail. I chose a career in pediatrics because I am an optimist at my very core. We can’t ignore the dangers associated with the wide door opened by mobile devices. We can celebrate the benefits while clearly facing the pitfalls.
Dr. Lilley is director of the pediatric diabetes and lipid program at the Mississippi Center for Advanced Medicine, Madison. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
My young patient with type 1 diabetes (T1D) had her cell phone out to provide a share code for her Dexcom clarity app as she was checking into her visit. As my nurse was recording the code, the patient asked him, “Hey, can you add me on Snapchat?”
Her father scrolled through his own Facebook feed in the chair next to her, showing no concern that his daughter was looking to connect with an adult on a social media platform. Meanwhile, we were all grateful that the little girl, who had had a seizure due to hypoglycemia in her preschool and pre–continuous glucose monitoring (CGM) years, had access to the tools harnessed within the sparkly encased phone she held in her small hands. But did anyone in the room fully understand the potential dangers?
We are living in an exhilarating era of diabetes technology, a treatment environment that I couldn’t have dreamed of during my pediatric endocrinology fellowship. T1D is a volatile condition that changes day to day, especially in growing children. A short decade ago, the best CGM available was a bulky device on loan to patients for 3 days at a time. Information was later downloaded in-office to get a better idea of general glucose trends, if insurance would approve its use at all.
Now, we have a variety of very wearable and accurate disposable CGMs accessible to most patients. Every major insulin pump has available closed-loop capabilities. Some patients can dose from apps on their cell phones rather than juggle another device or draw attention to an insulin pump at the cafeteria table.
These developments have been game changers for children and teenagers with diabetes and for their families. When wondering whether an athlete’s dazed appearance on a soccer field was due to hypoglycemia, a parent no longer must demand that a coach pull the player – a quick glance at a smartphone app can verify the blood glucose and change rate. Children can use programs and search engines to quickly verify carbohydrate counts. Life360 and other tracking programs have increased parental feelings of security, especially with young drivers living with a chronic medical condition.
The inevitable outcome of this available technology is that children living with T1D are given cell phones far earlier than are their siblings or peers owing to “necessity.” Parents understandably want a means to stay in close contact with their children in case of a medical emergency. As a physician and mother of young children, I am thankful for the technology that keeps my patients safer and that allows them to fully participate in everything from sports to travel to an uninterrupted night’s sleep.
Smartphone presence in classrooms empowers teachers, students, parents, and school nurses to be aware of glycemic trends and prevent hypoglycemic emergencies. Smartphones have also shown to be a major distraction in that setting, causing many schools to ban their use entirely. Video apps such as YouTube and TikTok can provide a wealth of support and medical information but may also open the door to misinformation and dangerous social contagion, particularly surrounding disordered eating. Informative podcasts such as The Juicebox Podcast and online forums provide incredible support for families, but the constant siren call of a phone in their pockets leads to distracted parents constantly tending to other conversations or responding to ever more demanding employers rather than focusing on face-to-face education sessions.
The Surgeon General recently released a report concerning social media use in children. This eye-opening report revealed that one-third of children admitted to using their cell phones “almost constantly.” Social media use is associated with higher rates of anxiety and depression, especially in teen girls. This is particularly concerning for children with T1D, who are more likely to suffer from these conditions.
Beyond mental health concerns, especially to developing brains, unfettered Internet use increases the risk that children are exposed to predators and harmful content. The online safety monitoring platform Bark shared data from its 2021 surveillance. Bark found that 72% of tweens and 85% of teens were involved in an online bullying situation. Sixty-nine percent of tweens and 91% of teens encountered nudity or sexual content. Ten percent of tweens and 21% of teens encountered predatory behavior.
These alarming finds mirror the prevalence suggested by conversations in my office. I hear reports of my patients sneaking out at night to meet adults they met through social media, having suicidal ideation and attempts after Internet bullying, and sharing earnest belief in bizarre conspiracy theories gleaned from online forums that lead to dangerous health care practices.
Furthermore, time is a finite resource. Teens who are spending an average of 3.5 hours daily on their devices are running out of time to play, study, and grow extracurricular interests. My friend who coaches high school baseball lamented recently the poor athleticism in his recent teams. He theorized that his players had spent their summers on tablets rather than playing catch or climbing trees. The resulting declines in exercise in young people only serve to worsen the childhood obesity epidemic.
What is a concerned parent to do? First, all phones have controls that allow parents to choose which apps are allowed and which are blocked. Caregivers must understand how various social media platforms work. Installing programs such as Bark provides an additional layer of monitoring, though these are no substitute for parental vigilance. Importantly, parents should talk to their children about their concerns regarding social media.
Sadly, I have often noticed that caregivers pity the extra hardships their children endure as the result of T1D and other chronic diseases. Being lax with rules to attempt to compensate for other suffering is far too tempting. The goal is for children and teens living with T1D to have a full and normal childhood, and unrestricted smartphone access and early social media use should not be the goal for any child. For every family, a media use plan is a smart approach. The American Academy of Pediatrics suggests several commonsense steps to use technology wisely, and parents often must address their own relationships with their devices to model healthy engagement.
As health care professionals, we owe it to our patients to discuss the ups and downs of technology with our patients. We can’t ostrich our way through this. We can point our patients and families to supportive groups such as Osprey (Old School Parents Raising Engaged Youth), founded by Ben and Erin Napier from the HGTV show Home Town along with my college friends Taylor and Dr. Catherine Sledge. Wait Until 8th provides information and motivation for parents to make wise choices regarding phone use for their children. The documentary Childhood 2.0 is another compelling resource developed by pediatric emergency physician Dr. Free Hess and her team that summarizes many of these concerns.
In another decade, many of these dangers will be far clearer. As ubiquitous as smartphone misuse is in our society, I remain hopeful that our society will change its behaviors. Just because “everyone else” allows an unhealthy relationship with technology doesn’t mean that we should for our children.
When I was a child, smoking was glamorized in movies and restaurants had dedicated smoking sections. After strong public policy efforts, many geared toward children, smoking is now almost unthinkable. My 8-year-old asked me lately whether a lady smoking a cigarette in the car next to us would have to go to jail. I chose a career in pediatrics because I am an optimist at my very core. We can’t ignore the dangers associated with the wide door opened by mobile devices. We can celebrate the benefits while clearly facing the pitfalls.
Dr. Lilley is director of the pediatric diabetes and lipid program at the Mississippi Center for Advanced Medicine, Madison. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When treating scars, ‘rehabilitation’ is the goal, not perfection
SAN DIEGO – , according to Victor Ross, MD.
“A lot of lip service is paid to how to inject the steroid,” Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego, said at the annual Masters of Aesthetics Symposium. “The most important part is the amount and the fastidiousness that you have injecting. You should see the tip of the needle and be very slow. Use a 1 cc syringe.” He used to inject scars with triamcinolone acetate 40 mg/mL, but now he almost always injects 10-20 mg/mL to avoid inducing white streak-like atrophy or hypopigmentation around the treated area.
“When you treat a scar, you treat the features of the scar that make it stand out,” Dr. Ross continued. “If it’s red, you address the hyperemia. If it’s brown, you address the pigment. You want to have a reasonable pathophysiological basis for what you’re doing. Understand how the scar got there and have a reasonable algorithm.” When he counsels patients about clinical outcomes to expect, he emphasizes rehabilitation instead of blemish-free perfection. “It’s not making the scar go away,” he said. “It’s not restoring completely normal skin form and function; it’s a restorative effort to get toward normality. That’s what it’s all about.”
Besides injecting scars with triamcinolone acetate, other scar treatment options include intralesional 5-fluorouracil, oral antihistamines, COX-2 inhibitors, hydrogel sheeting, compression, acoustic wave therapy, photodynamic therapy, radiofrequency, and lasers. “I’m not a big fan of low-level light; it probably does something [to scars], but I’m skeptical,” Dr. Ross said.
In his clinical opinion, most scars respond best to treatments with ablative and nonablative fractional lasers tuned to gentle settings such as an energy level of 20 millijoules at a density of 5%-10%. “Every scar deserves a chance for laser remediation and rehabilitation,” he said. “With radiation scars you want to be particularly gentle. If you have a Mohs scar that has been subsequently treated with radiation, I would lower my settings by half, because I’ve had some scars worsen with settings for red scars after radiation therapy.”
He often uses fractional lasers for stubborn acne scarring. “The hyperemic component you can treat with a vascular laser, then come back [and treat the scarring] with a nonablative fractional laser, or you could use radiofrequency microneedling as well,” Dr. Ross said.
New or innovative scar treatments coming down the pike, he said, include the following: mitomycin C (applied topically, he said that this has worked well for postoperative keloids), tamoxifen, oral methotrexate, imiquimod (which has mixed results to date), platelet-rich plasma, and retinoids.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
SAN DIEGO – , according to Victor Ross, MD.
“A lot of lip service is paid to how to inject the steroid,” Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego, said at the annual Masters of Aesthetics Symposium. “The most important part is the amount and the fastidiousness that you have injecting. You should see the tip of the needle and be very slow. Use a 1 cc syringe.” He used to inject scars with triamcinolone acetate 40 mg/mL, but now he almost always injects 10-20 mg/mL to avoid inducing white streak-like atrophy or hypopigmentation around the treated area.
“When you treat a scar, you treat the features of the scar that make it stand out,” Dr. Ross continued. “If it’s red, you address the hyperemia. If it’s brown, you address the pigment. You want to have a reasonable pathophysiological basis for what you’re doing. Understand how the scar got there and have a reasonable algorithm.” When he counsels patients about clinical outcomes to expect, he emphasizes rehabilitation instead of blemish-free perfection. “It’s not making the scar go away,” he said. “It’s not restoring completely normal skin form and function; it’s a restorative effort to get toward normality. That’s what it’s all about.”
Besides injecting scars with triamcinolone acetate, other scar treatment options include intralesional 5-fluorouracil, oral antihistamines, COX-2 inhibitors, hydrogel sheeting, compression, acoustic wave therapy, photodynamic therapy, radiofrequency, and lasers. “I’m not a big fan of low-level light; it probably does something [to scars], but I’m skeptical,” Dr. Ross said.
In his clinical opinion, most scars respond best to treatments with ablative and nonablative fractional lasers tuned to gentle settings such as an energy level of 20 millijoules at a density of 5%-10%. “Every scar deserves a chance for laser remediation and rehabilitation,” he said. “With radiation scars you want to be particularly gentle. If you have a Mohs scar that has been subsequently treated with radiation, I would lower my settings by half, because I’ve had some scars worsen with settings for red scars after radiation therapy.”
He often uses fractional lasers for stubborn acne scarring. “The hyperemic component you can treat with a vascular laser, then come back [and treat the scarring] with a nonablative fractional laser, or you could use radiofrequency microneedling as well,” Dr. Ross said.
New or innovative scar treatments coming down the pike, he said, include the following: mitomycin C (applied topically, he said that this has worked well for postoperative keloids), tamoxifen, oral methotrexate, imiquimod (which has mixed results to date), platelet-rich plasma, and retinoids.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
SAN DIEGO – , according to Victor Ross, MD.
“A lot of lip service is paid to how to inject the steroid,” Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego, said at the annual Masters of Aesthetics Symposium. “The most important part is the amount and the fastidiousness that you have injecting. You should see the tip of the needle and be very slow. Use a 1 cc syringe.” He used to inject scars with triamcinolone acetate 40 mg/mL, but now he almost always injects 10-20 mg/mL to avoid inducing white streak-like atrophy or hypopigmentation around the treated area.
“When you treat a scar, you treat the features of the scar that make it stand out,” Dr. Ross continued. “If it’s red, you address the hyperemia. If it’s brown, you address the pigment. You want to have a reasonable pathophysiological basis for what you’re doing. Understand how the scar got there and have a reasonable algorithm.” When he counsels patients about clinical outcomes to expect, he emphasizes rehabilitation instead of blemish-free perfection. “It’s not making the scar go away,” he said. “It’s not restoring completely normal skin form and function; it’s a restorative effort to get toward normality. That’s what it’s all about.”
Besides injecting scars with triamcinolone acetate, other scar treatment options include intralesional 5-fluorouracil, oral antihistamines, COX-2 inhibitors, hydrogel sheeting, compression, acoustic wave therapy, photodynamic therapy, radiofrequency, and lasers. “I’m not a big fan of low-level light; it probably does something [to scars], but I’m skeptical,” Dr. Ross said.
In his clinical opinion, most scars respond best to treatments with ablative and nonablative fractional lasers tuned to gentle settings such as an energy level of 20 millijoules at a density of 5%-10%. “Every scar deserves a chance for laser remediation and rehabilitation,” he said. “With radiation scars you want to be particularly gentle. If you have a Mohs scar that has been subsequently treated with radiation, I would lower my settings by half, because I’ve had some scars worsen with settings for red scars after radiation therapy.”
He often uses fractional lasers for stubborn acne scarring. “The hyperemic component you can treat with a vascular laser, then come back [and treat the scarring] with a nonablative fractional laser, or you could use radiofrequency microneedling as well,” Dr. Ross said.
New or innovative scar treatments coming down the pike, he said, include the following: mitomycin C (applied topically, he said that this has worked well for postoperative keloids), tamoxifen, oral methotrexate, imiquimod (which has mixed results to date), platelet-rich plasma, and retinoids.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
FROM MOAS 2023
The case for ‘pleasure hygiene’: Sexual health in patients with chronic illness
A recent study found a significant association between lower sexual frequency and greater all-cause mortality in young and middle-aged people with hypertension. Should primary care physicians be offering a pleasure prescription to the 6 in 10 Americans living with chronic illness?
Ask, don’t tell
First, we need to ask routinely about sexual well-being and pleasure. Without asking patients their views, we do not know the relevance of sex for their quality of life. Unless we ask, we do not know what specific kinds of sexual play are important for a person’s pleasure, nor can we assume how they prioritize their sexual functioning in the context of their medical care. When I began asking my primary care patients about sexual well-being, many more than I expected were quietly holding on to distressing issues. Now, as a sexual medicine specialist, in each sexual function evaluation, I ask three key questions: What are your goals? What does sex mean to you? What kinds of sexual play are important for your (and your partner’s) pleasure?
Chronic disease – with physical symptoms as well as psychological, relational, and cultural components – affects both general and genital physiology. Any disease process that alters vascular, neuroendocrine, or musculoskeletal function is likely to influence sexual function, either directly through the disease process or indirectly through complications or the effect on identity and well-being. In addition, a host of iatrogenic changes to sexual function may accompany effects of treatments.
Managing the effects of chronic illness on sexuality requires resilience and flexibility. A serious injury may require a massive adjustment to sexuality, but progressive disease may require continuous accommodations to sexual changes. The life stage at which the disease occurs also matters. People facing disease early in life encounter challenges (finding willing sexual partners and limited medical guidance regarding their sexual functioning) as well as benefits (they may integrate their disease as part of their sexual life). Those who experience sexual changes related to their illness later in life may face a loss of “normal” sexual function and well-being.
Meanwhile, the partner who is not ill may have their own sexual needs, fears, and worries. Both patients and partners may experience disenfranchised grief – a sense of loss about something one is not culturally permitted to mourn (“I/my partner is alive in the face of this terrible illness; who am I to worry about our/my sexual pleasure?”).
Positive marital relationships influence health through improved survival, improved medical adherence, better quality of life for the patient, and improved life satisfaction. Sexual satisfaction is an important factor in relational satisfaction. Helping our patients with these changes therefore may improve not only sexual health but overall health.
How, then, should we address sexual pleasure in chronic illness care? Here are a few tips:
Focus on pleasure. “Performance” is foul language when it comes to sex. Full attention to sensation and enjoyment, the only sexual “skill” anyone needs, is impossible while trying to perform.
Encourage flexibility and recognize that sex encompasses a wide and varied menu of experiences that change over a lifetime. Sex is everything from kissing and cuddling to the wildest things a mind can imagine. We can help both patients and partners think about the wide variety of ways to meet sexual needs. Balancing acceptance of sexual changes with motivation for improvement also is part of our role.
Address the effects of illness on the patient’s relationship with their body. Illness may alter not only bodily function but also self-esteem and body image. A reorganization of self-concept may occur (“I am no longer a sexual person; I’m a sexually dysfunctional asthmatic/diabetic/etc. and should avoid sexual intimacy”). Examining these self-constructs allows shifts in thoughts and behaviors, leading to improved psychological and sexual well-being. Encourage patients to explore what feels good in this body now. When possible, we can help with referral for corrective surgeries or direction to resources like stoma covers, wigs, scarves, and tattoos.
We offer suggestions for “sleep hygiene”; how about pleasure hygiene?
- Encourage open communication with partner(s) and offer resources to develop communication skills.
- Consider needs for physical and emotional preparation for sexual play: adequate rest, preparing the environment for body fluids, pillows for comfort or aides for positioning, and plenty of lubricant at hand.
- Allow adequate time for sexual play and encourage the ability to adjust or stop and start over – with humor and self-compassion.
- Use sexual aides to enhance pleasure.
- Seek out sexual medicine and sex therapy colleagues when things become tricky.
All bodies, no matter their health or illness state, are capable of pleasure. Hey, pleasure might even save lives!
Dr. Kranz is an clinical assistant professor of obstetrics/gynecology and family medicine, University of Rochester (N.Y.) Medical Center. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent study found a significant association between lower sexual frequency and greater all-cause mortality in young and middle-aged people with hypertension. Should primary care physicians be offering a pleasure prescription to the 6 in 10 Americans living with chronic illness?
Ask, don’t tell
First, we need to ask routinely about sexual well-being and pleasure. Without asking patients their views, we do not know the relevance of sex for their quality of life. Unless we ask, we do not know what specific kinds of sexual play are important for a person’s pleasure, nor can we assume how they prioritize their sexual functioning in the context of their medical care. When I began asking my primary care patients about sexual well-being, many more than I expected were quietly holding on to distressing issues. Now, as a sexual medicine specialist, in each sexual function evaluation, I ask three key questions: What are your goals? What does sex mean to you? What kinds of sexual play are important for your (and your partner’s) pleasure?
Chronic disease – with physical symptoms as well as psychological, relational, and cultural components – affects both general and genital physiology. Any disease process that alters vascular, neuroendocrine, or musculoskeletal function is likely to influence sexual function, either directly through the disease process or indirectly through complications or the effect on identity and well-being. In addition, a host of iatrogenic changes to sexual function may accompany effects of treatments.
Managing the effects of chronic illness on sexuality requires resilience and flexibility. A serious injury may require a massive adjustment to sexuality, but progressive disease may require continuous accommodations to sexual changes. The life stage at which the disease occurs also matters. People facing disease early in life encounter challenges (finding willing sexual partners and limited medical guidance regarding their sexual functioning) as well as benefits (they may integrate their disease as part of their sexual life). Those who experience sexual changes related to their illness later in life may face a loss of “normal” sexual function and well-being.
Meanwhile, the partner who is not ill may have their own sexual needs, fears, and worries. Both patients and partners may experience disenfranchised grief – a sense of loss about something one is not culturally permitted to mourn (“I/my partner is alive in the face of this terrible illness; who am I to worry about our/my sexual pleasure?”).
Positive marital relationships influence health through improved survival, improved medical adherence, better quality of life for the patient, and improved life satisfaction. Sexual satisfaction is an important factor in relational satisfaction. Helping our patients with these changes therefore may improve not only sexual health but overall health.
How, then, should we address sexual pleasure in chronic illness care? Here are a few tips:
Focus on pleasure. “Performance” is foul language when it comes to sex. Full attention to sensation and enjoyment, the only sexual “skill” anyone needs, is impossible while trying to perform.
Encourage flexibility and recognize that sex encompasses a wide and varied menu of experiences that change over a lifetime. Sex is everything from kissing and cuddling to the wildest things a mind can imagine. We can help both patients and partners think about the wide variety of ways to meet sexual needs. Balancing acceptance of sexual changes with motivation for improvement also is part of our role.
Address the effects of illness on the patient’s relationship with their body. Illness may alter not only bodily function but also self-esteem and body image. A reorganization of self-concept may occur (“I am no longer a sexual person; I’m a sexually dysfunctional asthmatic/diabetic/etc. and should avoid sexual intimacy”). Examining these self-constructs allows shifts in thoughts and behaviors, leading to improved psychological and sexual well-being. Encourage patients to explore what feels good in this body now. When possible, we can help with referral for corrective surgeries or direction to resources like stoma covers, wigs, scarves, and tattoos.
We offer suggestions for “sleep hygiene”; how about pleasure hygiene?
- Encourage open communication with partner(s) and offer resources to develop communication skills.
- Consider needs for physical and emotional preparation for sexual play: adequate rest, preparing the environment for body fluids, pillows for comfort or aides for positioning, and plenty of lubricant at hand.
- Allow adequate time for sexual play and encourage the ability to adjust or stop and start over – with humor and self-compassion.
- Use sexual aides to enhance pleasure.
- Seek out sexual medicine and sex therapy colleagues when things become tricky.
All bodies, no matter their health or illness state, are capable of pleasure. Hey, pleasure might even save lives!
Dr. Kranz is an clinical assistant professor of obstetrics/gynecology and family medicine, University of Rochester (N.Y.) Medical Center. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
A recent study found a significant association between lower sexual frequency and greater all-cause mortality in young and middle-aged people with hypertension. Should primary care physicians be offering a pleasure prescription to the 6 in 10 Americans living with chronic illness?
Ask, don’t tell
First, we need to ask routinely about sexual well-being and pleasure. Without asking patients their views, we do not know the relevance of sex for their quality of life. Unless we ask, we do not know what specific kinds of sexual play are important for a person’s pleasure, nor can we assume how they prioritize their sexual functioning in the context of their medical care. When I began asking my primary care patients about sexual well-being, many more than I expected were quietly holding on to distressing issues. Now, as a sexual medicine specialist, in each sexual function evaluation, I ask three key questions: What are your goals? What does sex mean to you? What kinds of sexual play are important for your (and your partner’s) pleasure?
Chronic disease – with physical symptoms as well as psychological, relational, and cultural components – affects both general and genital physiology. Any disease process that alters vascular, neuroendocrine, or musculoskeletal function is likely to influence sexual function, either directly through the disease process or indirectly through complications or the effect on identity and well-being. In addition, a host of iatrogenic changes to sexual function may accompany effects of treatments.
Managing the effects of chronic illness on sexuality requires resilience and flexibility. A serious injury may require a massive adjustment to sexuality, but progressive disease may require continuous accommodations to sexual changes. The life stage at which the disease occurs also matters. People facing disease early in life encounter challenges (finding willing sexual partners and limited medical guidance regarding their sexual functioning) as well as benefits (they may integrate their disease as part of their sexual life). Those who experience sexual changes related to their illness later in life may face a loss of “normal” sexual function and well-being.
Meanwhile, the partner who is not ill may have their own sexual needs, fears, and worries. Both patients and partners may experience disenfranchised grief – a sense of loss about something one is not culturally permitted to mourn (“I/my partner is alive in the face of this terrible illness; who am I to worry about our/my sexual pleasure?”).
Positive marital relationships influence health through improved survival, improved medical adherence, better quality of life for the patient, and improved life satisfaction. Sexual satisfaction is an important factor in relational satisfaction. Helping our patients with these changes therefore may improve not only sexual health but overall health.
How, then, should we address sexual pleasure in chronic illness care? Here are a few tips:
Focus on pleasure. “Performance” is foul language when it comes to sex. Full attention to sensation and enjoyment, the only sexual “skill” anyone needs, is impossible while trying to perform.
Encourage flexibility and recognize that sex encompasses a wide and varied menu of experiences that change over a lifetime. Sex is everything from kissing and cuddling to the wildest things a mind can imagine. We can help both patients and partners think about the wide variety of ways to meet sexual needs. Balancing acceptance of sexual changes with motivation for improvement also is part of our role.
Address the effects of illness on the patient’s relationship with their body. Illness may alter not only bodily function but also self-esteem and body image. A reorganization of self-concept may occur (“I am no longer a sexual person; I’m a sexually dysfunctional asthmatic/diabetic/etc. and should avoid sexual intimacy”). Examining these self-constructs allows shifts in thoughts and behaviors, leading to improved psychological and sexual well-being. Encourage patients to explore what feels good in this body now. When possible, we can help with referral for corrective surgeries or direction to resources like stoma covers, wigs, scarves, and tattoos.
We offer suggestions for “sleep hygiene”; how about pleasure hygiene?
- Encourage open communication with partner(s) and offer resources to develop communication skills.
- Consider needs for physical and emotional preparation for sexual play: adequate rest, preparing the environment for body fluids, pillows for comfort or aides for positioning, and plenty of lubricant at hand.
- Allow adequate time for sexual play and encourage the ability to adjust or stop and start over – with humor and self-compassion.
- Use sexual aides to enhance pleasure.
- Seek out sexual medicine and sex therapy colleagues when things become tricky.
All bodies, no matter their health or illness state, are capable of pleasure. Hey, pleasure might even save lives!
Dr. Kranz is an clinical assistant professor of obstetrics/gynecology and family medicine, University of Rochester (N.Y.) Medical Center. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
How to manage heartburn cost-effectively after PPI failure
TOPLINE:
, matching therapy to phenotype.
METHODOLOGY:
- Researchers compared the cost-effectiveness over 1 year of four strategies for managing patients in whom empirical PPI treatment failed.
- Strategies were PPI optimization without diagnostic testing; endoscopy with PPI optimization to identify erosive reflux disease; endoscopy with PPI discontinuation when no erosive reflux disease was found; and combined endoscopy/ambulatory reflux monitoring and PPI discontinuation as appropriate for the phenotype (i.e., erosive disease, nonerosive disease, or functional heartburn).
- All index testing was assumed to be done while patients were off PPI treatment.
TAKEAWAY:
- PPI optimization without testing cost insurers $3,784 a year and patients $3,128 a year owing to health care expenses and lower work productivity associated with suboptimal symptom relief, resulting in a loss of 40 healthy days over the course of the year.
- Endoscopy with PPI optimization lowered insurer costs by $1,020 a year and patient costs by $1,621 a year, compared with optimization without testing, and added 11 healthy days a year by identifying erosive reflux disease.
- Endoscopy with PPI discontinuation added 11 healthy days a year by identifying patients without erosive reflux disease who did not need PPI therapy.
- Endoscopy with ambulatory reflux monitoring and a trial of PPI discontinuation was the most effective strategy, optimizing phenotype-guided treatment, saving insurers $2,183 and patients $2,396 a year, and adding 22 healthy days a year.
- The findings support recent clinical practice guidelines from the American Gastroenterological Association and the
IN PRACTICE:
“[A]n algorithmic approach to comprehensively stratify erosive and non-erosive reflux disease from functional heartburn combined with a trial of PPI discontinuation for patients without erosive findings provides value to patients and insurers,” the authors wrote.
SOURCE:
Eric D. Shah, MD, MBA, division of gastroenterology and hepatology, Michigan Medicine, Ann Arbor, led the study, which was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
Centers may have limited capacity for routine ambulatory reflux monitoring or may not perform it at all. Single-center and older studies were used for model inputs when no other data were available.
DISCLOSURES:
The study had no specific funding. Dr. Shah is supported by a National Institutes of Health grant and disclosed that he has consulted for Salix, Mahana, Neuraxis, Phathom, Takeda, Ardelyx, Sanofi, and GI Supply. Other coauthors have consulted for pharmaceutical and/or biotech companies.
A version of this article appeared on Medscape.com.
TOPLINE:
, matching therapy to phenotype.
METHODOLOGY:
- Researchers compared the cost-effectiveness over 1 year of four strategies for managing patients in whom empirical PPI treatment failed.
- Strategies were PPI optimization without diagnostic testing; endoscopy with PPI optimization to identify erosive reflux disease; endoscopy with PPI discontinuation when no erosive reflux disease was found; and combined endoscopy/ambulatory reflux monitoring and PPI discontinuation as appropriate for the phenotype (i.e., erosive disease, nonerosive disease, or functional heartburn).
- All index testing was assumed to be done while patients were off PPI treatment.
TAKEAWAY:
- PPI optimization without testing cost insurers $3,784 a year and patients $3,128 a year owing to health care expenses and lower work productivity associated with suboptimal symptom relief, resulting in a loss of 40 healthy days over the course of the year.
- Endoscopy with PPI optimization lowered insurer costs by $1,020 a year and patient costs by $1,621 a year, compared with optimization without testing, and added 11 healthy days a year by identifying erosive reflux disease.
- Endoscopy with PPI discontinuation added 11 healthy days a year by identifying patients without erosive reflux disease who did not need PPI therapy.
- Endoscopy with ambulatory reflux monitoring and a trial of PPI discontinuation was the most effective strategy, optimizing phenotype-guided treatment, saving insurers $2,183 and patients $2,396 a year, and adding 22 healthy days a year.
- The findings support recent clinical practice guidelines from the American Gastroenterological Association and the
IN PRACTICE:
“[A]n algorithmic approach to comprehensively stratify erosive and non-erosive reflux disease from functional heartburn combined with a trial of PPI discontinuation for patients without erosive findings provides value to patients and insurers,” the authors wrote.
SOURCE:
Eric D. Shah, MD, MBA, division of gastroenterology and hepatology, Michigan Medicine, Ann Arbor, led the study, which was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
Centers may have limited capacity for routine ambulatory reflux monitoring or may not perform it at all. Single-center and older studies were used for model inputs when no other data were available.
DISCLOSURES:
The study had no specific funding. Dr. Shah is supported by a National Institutes of Health grant and disclosed that he has consulted for Salix, Mahana, Neuraxis, Phathom, Takeda, Ardelyx, Sanofi, and GI Supply. Other coauthors have consulted for pharmaceutical and/or biotech companies.
A version of this article appeared on Medscape.com.
TOPLINE:
, matching therapy to phenotype.
METHODOLOGY:
- Researchers compared the cost-effectiveness over 1 year of four strategies for managing patients in whom empirical PPI treatment failed.
- Strategies were PPI optimization without diagnostic testing; endoscopy with PPI optimization to identify erosive reflux disease; endoscopy with PPI discontinuation when no erosive reflux disease was found; and combined endoscopy/ambulatory reflux monitoring and PPI discontinuation as appropriate for the phenotype (i.e., erosive disease, nonerosive disease, or functional heartburn).
- All index testing was assumed to be done while patients were off PPI treatment.
TAKEAWAY:
- PPI optimization without testing cost insurers $3,784 a year and patients $3,128 a year owing to health care expenses and lower work productivity associated with suboptimal symptom relief, resulting in a loss of 40 healthy days over the course of the year.
- Endoscopy with PPI optimization lowered insurer costs by $1,020 a year and patient costs by $1,621 a year, compared with optimization without testing, and added 11 healthy days a year by identifying erosive reflux disease.
- Endoscopy with PPI discontinuation added 11 healthy days a year by identifying patients without erosive reflux disease who did not need PPI therapy.
- Endoscopy with ambulatory reflux monitoring and a trial of PPI discontinuation was the most effective strategy, optimizing phenotype-guided treatment, saving insurers $2,183 and patients $2,396 a year, and adding 22 healthy days a year.
- The findings support recent clinical practice guidelines from the American Gastroenterological Association and the
IN PRACTICE:
“[A]n algorithmic approach to comprehensively stratify erosive and non-erosive reflux disease from functional heartburn combined with a trial of PPI discontinuation for patients without erosive findings provides value to patients and insurers,” the authors wrote.
SOURCE:
Eric D. Shah, MD, MBA, division of gastroenterology and hepatology, Michigan Medicine, Ann Arbor, led the study, which was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
Centers may have limited capacity for routine ambulatory reflux monitoring or may not perform it at all. Single-center and older studies were used for model inputs when no other data were available.
DISCLOSURES:
The study had no specific funding. Dr. Shah is supported by a National Institutes of Health grant and disclosed that he has consulted for Salix, Mahana, Neuraxis, Phathom, Takeda, Ardelyx, Sanofi, and GI Supply. Other coauthors have consulted for pharmaceutical and/or biotech companies.
A version of this article appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
COVID booster may transiently raise glucose levels in T1D
TOPLINE:
METHODOLOGY:
- In a single-center prospective cohort study of 21 adults with type 1 diabetes, patients were given a blinded Dexcom G6 Pro continuous glucose monitor (CGM) at the first research clinic visit.
- After 3-4 days, participants received a COVID-19 booster vaccine.
- They returned to the clinic 10 days after the initial visit (5-6 days after booster vaccination) to have the CGM removed and glycemia assessed.
TAKEAWAY:
- Compared with baseline, the mean daily glucose level was significantly increased at day 2 (162.9 mg/dL vs. 172.8 mg/dL; P = .04) and day 3 (173.1 mg/dL; P = .02) post vaccination.
- Glucose excursions at day 0 (173.2 mg/dL; P = .058) and day 1 (173.1 mg/dL; P = .078) didn’t quite reach statistical significance.
- One participant experienced increases in glucose of 36%, 69%, 35%, 26%, 22%, and 19% on days 0-5, respectively, compared with baseline.
- Glucose excursions of at least 25% above baseline occurred in four participants on day 0 and day 1 and in three participants on days 2 and 5.
- Insulin resistance, as measured by Total Daily Insulin Resistance (a metric that integrates daily mean glucose concentration with total daily insulin dose), was also significantly increased from baseline to day 2 post vaccination (7,171 mg/dL vs. 8,070 mg/dL units; P = .03).
- No other measures of glycemia differed significantly, compared with baseline.
- Outcomes didn’t differ significantly by sex, age, or vaccine manufacturer.
IN PRACTICE:
- “To our knowledge this is the first study investigating the effect of the COVID-19 booster vaccine on glycemia specifically in people with type 1 diabetes,” say the authors.
- “Clinicians, pharmacists, and other health care providers may need to counsel people with T1D to be more vigilant with glucose testing and insulin dosing for the first 5 days after vaccination. Most importantly, insulin, required to control glycemia, may need to be transiently increased.”
- “Further studies are warranted to investigate whether other vaccines have similar glycemic effects, and which individuals are at highest risk for profound glucose perturbations post vaccination.”
SOURCE:
The study was conducted by Mihail Zilbermint, MD, of the division of hospital medicine, Johns Hopkins Medicine, Bethesda, Md., and colleagues. It was published in Diabetes Research and Clinical Practice.
LIMITATIONS:
- The sample size was small.
- There were no measurements of inflammatory markers, dietary intake, physical activity, or survey patient symptomatology to adjust for variables that may have influenced glycemic control.
- In the study cohort, glycemia was moderately well controlled at baseline.
DISCLOSURES:
The study was supported by an investigator-initiated study grant from DexCom Inc. Dr. Zilbermint has consulted for EMD Serono.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In a single-center prospective cohort study of 21 adults with type 1 diabetes, patients were given a blinded Dexcom G6 Pro continuous glucose monitor (CGM) at the first research clinic visit.
- After 3-4 days, participants received a COVID-19 booster vaccine.
- They returned to the clinic 10 days after the initial visit (5-6 days after booster vaccination) to have the CGM removed and glycemia assessed.
TAKEAWAY:
- Compared with baseline, the mean daily glucose level was significantly increased at day 2 (162.9 mg/dL vs. 172.8 mg/dL; P = .04) and day 3 (173.1 mg/dL; P = .02) post vaccination.
- Glucose excursions at day 0 (173.2 mg/dL; P = .058) and day 1 (173.1 mg/dL; P = .078) didn’t quite reach statistical significance.
- One participant experienced increases in glucose of 36%, 69%, 35%, 26%, 22%, and 19% on days 0-5, respectively, compared with baseline.
- Glucose excursions of at least 25% above baseline occurred in four participants on day 0 and day 1 and in three participants on days 2 and 5.
- Insulin resistance, as measured by Total Daily Insulin Resistance (a metric that integrates daily mean glucose concentration with total daily insulin dose), was also significantly increased from baseline to day 2 post vaccination (7,171 mg/dL vs. 8,070 mg/dL units; P = .03).
- No other measures of glycemia differed significantly, compared with baseline.
- Outcomes didn’t differ significantly by sex, age, or vaccine manufacturer.
IN PRACTICE:
- “To our knowledge this is the first study investigating the effect of the COVID-19 booster vaccine on glycemia specifically in people with type 1 diabetes,” say the authors.
- “Clinicians, pharmacists, and other health care providers may need to counsel people with T1D to be more vigilant with glucose testing and insulin dosing for the first 5 days after vaccination. Most importantly, insulin, required to control glycemia, may need to be transiently increased.”
- “Further studies are warranted to investigate whether other vaccines have similar glycemic effects, and which individuals are at highest risk for profound glucose perturbations post vaccination.”
SOURCE:
The study was conducted by Mihail Zilbermint, MD, of the division of hospital medicine, Johns Hopkins Medicine, Bethesda, Md., and colleagues. It was published in Diabetes Research and Clinical Practice.
LIMITATIONS:
- The sample size was small.
- There were no measurements of inflammatory markers, dietary intake, physical activity, or survey patient symptomatology to adjust for variables that may have influenced glycemic control.
- In the study cohort, glycemia was moderately well controlled at baseline.
DISCLOSURES:
The study was supported by an investigator-initiated study grant from DexCom Inc. Dr. Zilbermint has consulted for EMD Serono.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In a single-center prospective cohort study of 21 adults with type 1 diabetes, patients were given a blinded Dexcom G6 Pro continuous glucose monitor (CGM) at the first research clinic visit.
- After 3-4 days, participants received a COVID-19 booster vaccine.
- They returned to the clinic 10 days after the initial visit (5-6 days after booster vaccination) to have the CGM removed and glycemia assessed.
TAKEAWAY:
- Compared with baseline, the mean daily glucose level was significantly increased at day 2 (162.9 mg/dL vs. 172.8 mg/dL; P = .04) and day 3 (173.1 mg/dL; P = .02) post vaccination.
- Glucose excursions at day 0 (173.2 mg/dL; P = .058) and day 1 (173.1 mg/dL; P = .078) didn’t quite reach statistical significance.
- One participant experienced increases in glucose of 36%, 69%, 35%, 26%, 22%, and 19% on days 0-5, respectively, compared with baseline.
- Glucose excursions of at least 25% above baseline occurred in four participants on day 0 and day 1 and in three participants on days 2 and 5.
- Insulin resistance, as measured by Total Daily Insulin Resistance (a metric that integrates daily mean glucose concentration with total daily insulin dose), was also significantly increased from baseline to day 2 post vaccination (7,171 mg/dL vs. 8,070 mg/dL units; P = .03).
- No other measures of glycemia differed significantly, compared with baseline.
- Outcomes didn’t differ significantly by sex, age, or vaccine manufacturer.
IN PRACTICE:
- “To our knowledge this is the first study investigating the effect of the COVID-19 booster vaccine on glycemia specifically in people with type 1 diabetes,” say the authors.
- “Clinicians, pharmacists, and other health care providers may need to counsel people with T1D to be more vigilant with glucose testing and insulin dosing for the first 5 days after vaccination. Most importantly, insulin, required to control glycemia, may need to be transiently increased.”
- “Further studies are warranted to investigate whether other vaccines have similar glycemic effects, and which individuals are at highest risk for profound glucose perturbations post vaccination.”
SOURCE:
The study was conducted by Mihail Zilbermint, MD, of the division of hospital medicine, Johns Hopkins Medicine, Bethesda, Md., and colleagues. It was published in Diabetes Research and Clinical Practice.
LIMITATIONS:
- The sample size was small.
- There were no measurements of inflammatory markers, dietary intake, physical activity, or survey patient symptomatology to adjust for variables that may have influenced glycemic control.
- In the study cohort, glycemia was moderately well controlled at baseline.
DISCLOSURES:
The study was supported by an investigator-initiated study grant from DexCom Inc. Dr. Zilbermint has consulted for EMD Serono.
A version of this article first appeared on Medscape.com.
FROM DIABETES RESEARCH AND CLINICAL PRACTICE
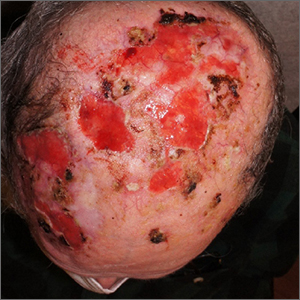
Nonhealing postsurgical scalp ulcers

Two shave biopsies were taken, 1 in the center of a previous SCC site with hyperkeratosis, the other in a site not previously affected by SCC but with the physical features of a pustule. Biopsy results from both sites were consistent with erosive pustular dermatosis, an unusual inflammatory disorder that mimics SCC.
Erosive pustular dermatosis of the scalp is an uncommon dermatitis that usually affects older women but may appear in men and women of all ages. It can mimic many other conditions that can affect the scalp, including seborrheic dermatitis, psoriasis, actinic keratosis, and SCC.
The exact causative mechanism is not understood, and cases may develop spontaneously. Rough papules, pustules, crusts, and ulcers develop and (apart from the pustules) share many features of actinic keratoses, SCCs, and field cancerization. The presence of pustules helps point to the diagnosis.
Triggers include previous surgery or physical trauma, burns, skin or hair grafts, and treatment of actinic keratoses with imiquimod, 5-fluourouracil, or photodynamic therapy. Some autoimmune diseases (including Hashimoto thyroiditis, autoimmune hepatitis, and rheumatoid arthritis) have been linked to disease occurrence and severity.1
Treatment includes potent or super-potent topical steroids such as clobetasol 0.05% ointment. Topical tacrolimus 0.1% ointment and calcipotriene 0.005% cream have been reported as steroid alternatives. Paradoxically, photodynamic therapy, while associated with triggering disease, has also been used therapeutically. Systemic immunomodulators such as cyclosporine 3 mg/kg/d or prednisone 0.5 to 1 mg/kg/d may be needed in severe cases. Antibiotics including topical dapsone 5% gel, systemic dapsone from 50 mg bid to tid, and doxycycline have been helpful due, in part, to their immunomodulatory effects.1,2
This patient was told to apply topical triamcinolone 0.1% ointment around and over ulcers and pustules and to take doxycycline 100 mg twice daily. The patient cleared well after 6 weeks. He continued to apply topical triamcinolone every few days as maintenance therapy.
He had some mild recurrence after discontinuing all topical and oral therapy, so he currently is being maintained on topical clobetasol 0.05% ointment every other day. He comes in for follow-up appointments every 3 months to monitor for control of the erosive pustular dermatosis of the scalp and for skin cancer surveillance.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME
1. Karanfilian KM, Wassef C. Erosive pustular dermatosis of the scalp: causes and treatments. Int J Dermatol. 2021;60:25-32. doi: 10.1111/ijd.14955
2. Sasaki R, Asano Y, Fujimura T. A pediatric case of corticosteroid-resistant erosive pustular dermatosis of scalp-like alopecia treated successfully with oral indomethacin, doxycycline, and topical tacrolimus. J Dermatol. 2022;49: e299-e300. doi: 10.1111/1346-8138.16425

Two shave biopsies were taken, 1 in the center of a previous SCC site with hyperkeratosis, the other in a site not previously affected by SCC but with the physical features of a pustule. Biopsy results from both sites were consistent with erosive pustular dermatosis, an unusual inflammatory disorder that mimics SCC.
Erosive pustular dermatosis of the scalp is an uncommon dermatitis that usually affects older women but may appear in men and women of all ages. It can mimic many other conditions that can affect the scalp, including seborrheic dermatitis, psoriasis, actinic keratosis, and SCC.
The exact causative mechanism is not understood, and cases may develop spontaneously. Rough papules, pustules, crusts, and ulcers develop and (apart from the pustules) share many features of actinic keratoses, SCCs, and field cancerization. The presence of pustules helps point to the diagnosis.
Triggers include previous surgery or physical trauma, burns, skin or hair grafts, and treatment of actinic keratoses with imiquimod, 5-fluourouracil, or photodynamic therapy. Some autoimmune diseases (including Hashimoto thyroiditis, autoimmune hepatitis, and rheumatoid arthritis) have been linked to disease occurrence and severity.1
Treatment includes potent or super-potent topical steroids such as clobetasol 0.05% ointment. Topical tacrolimus 0.1% ointment and calcipotriene 0.005% cream have been reported as steroid alternatives. Paradoxically, photodynamic therapy, while associated with triggering disease, has also been used therapeutically. Systemic immunomodulators such as cyclosporine 3 mg/kg/d or prednisone 0.5 to 1 mg/kg/d may be needed in severe cases. Antibiotics including topical dapsone 5% gel, systemic dapsone from 50 mg bid to tid, and doxycycline have been helpful due, in part, to their immunomodulatory effects.1,2
This patient was told to apply topical triamcinolone 0.1% ointment around and over ulcers and pustules and to take doxycycline 100 mg twice daily. The patient cleared well after 6 weeks. He continued to apply topical triamcinolone every few days as maintenance therapy.
He had some mild recurrence after discontinuing all topical and oral therapy, so he currently is being maintained on topical clobetasol 0.05% ointment every other day. He comes in for follow-up appointments every 3 months to monitor for control of the erosive pustular dermatosis of the scalp and for skin cancer surveillance.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME

Two shave biopsies were taken, 1 in the center of a previous SCC site with hyperkeratosis, the other in a site not previously affected by SCC but with the physical features of a pustule. Biopsy results from both sites were consistent with erosive pustular dermatosis, an unusual inflammatory disorder that mimics SCC.
Erosive pustular dermatosis of the scalp is an uncommon dermatitis that usually affects older women but may appear in men and women of all ages. It can mimic many other conditions that can affect the scalp, including seborrheic dermatitis, psoriasis, actinic keratosis, and SCC.
The exact causative mechanism is not understood, and cases may develop spontaneously. Rough papules, pustules, crusts, and ulcers develop and (apart from the pustules) share many features of actinic keratoses, SCCs, and field cancerization. The presence of pustules helps point to the diagnosis.
Triggers include previous surgery or physical trauma, burns, skin or hair grafts, and treatment of actinic keratoses with imiquimod, 5-fluourouracil, or photodynamic therapy. Some autoimmune diseases (including Hashimoto thyroiditis, autoimmune hepatitis, and rheumatoid arthritis) have been linked to disease occurrence and severity.1
Treatment includes potent or super-potent topical steroids such as clobetasol 0.05% ointment. Topical tacrolimus 0.1% ointment and calcipotriene 0.005% cream have been reported as steroid alternatives. Paradoxically, photodynamic therapy, while associated with triggering disease, has also been used therapeutically. Systemic immunomodulators such as cyclosporine 3 mg/kg/d or prednisone 0.5 to 1 mg/kg/d may be needed in severe cases. Antibiotics including topical dapsone 5% gel, systemic dapsone from 50 mg bid to tid, and doxycycline have been helpful due, in part, to their immunomodulatory effects.1,2
This patient was told to apply topical triamcinolone 0.1% ointment around and over ulcers and pustules and to take doxycycline 100 mg twice daily. The patient cleared well after 6 weeks. He continued to apply topical triamcinolone every few days as maintenance therapy.
He had some mild recurrence after discontinuing all topical and oral therapy, so he currently is being maintained on topical clobetasol 0.05% ointment every other day. He comes in for follow-up appointments every 3 months to monitor for control of the erosive pustular dermatosis of the scalp and for skin cancer surveillance.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME
1. Karanfilian KM, Wassef C. Erosive pustular dermatosis of the scalp: causes and treatments. Int J Dermatol. 2021;60:25-32. doi: 10.1111/ijd.14955
2. Sasaki R, Asano Y, Fujimura T. A pediatric case of corticosteroid-resistant erosive pustular dermatosis of scalp-like alopecia treated successfully with oral indomethacin, doxycycline, and topical tacrolimus. J Dermatol. 2022;49: e299-e300. doi: 10.1111/1346-8138.16425
1. Karanfilian KM, Wassef C. Erosive pustular dermatosis of the scalp: causes and treatments. Int J Dermatol. 2021;60:25-32. doi: 10.1111/ijd.14955
2. Sasaki R, Asano Y, Fujimura T. A pediatric case of corticosteroid-resistant erosive pustular dermatosis of scalp-like alopecia treated successfully with oral indomethacin, doxycycline, and topical tacrolimus. J Dermatol. 2022;49: e299-e300. doi: 10.1111/1346-8138.16425
SGLT2 inhibitors: No benefit or harm in hospitalized COVID-19
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Lead exposure still a global health burden
TOPLINE:
Globally, lead exposure is linked to more than 5.5 million adult cardiovascular deaths in 2019, as well as loss of 765 million intelligence quotient (IQ) points in children younger than 5 years, which cost U.S. $6 trillion in lost productivity, new research suggests.
METHODOLOGY:
- Global lead exposure has declined substantially since leaded gasoline was phased out, but several sources of lead remain, resulting in adverse health and economic effects, particularly in low- and middle-income countries (LMICs).
- Estimates of cardiovascular disease (CVD) deaths from lead exposure have been limited to effects of increased blood pressure, but studies show that lead exposure has cardiovascular impacts through mechanisms other than hypertension.
- Drawing from various sources and studies, researchers estimated global blood lead levels and the impact of lead exposure on CVD mortality in 2019 among adults aged 25 years or older, IQ loss in children younger than 5 years, and the related economic costs.
TAKEAWAY:
- Researchers estimated that there were 5,545,000 (95% confidence interval, 2,305,000-8,271,000) cardiovascular deaths in adults from lead exposure in 2019, with as many as 90.2% of these deaths in LMICs; however, this estimate may be incomplete because it does not include the effect of lead exposure on CVD mortality mediated through hypertension.
- The estimated global IQ loss in children younger than 5 years due to lead exposure was 765 million (95% CI, 443 million-1,098 million) IQ points in 2019, 95.3% of which occurred in LMICs.
- These estimates place lead exposure on a par with ambient particulate matter and household air pollution combined, and ahead of unsafe household drinking water, sanitation, and handwashing, as an environmental risk factor.
- The estimated global cost of lead exposure from CVD mortality and IQ loss combined is U.S. $6.0 trillion (range, $2.6 trillion-9.0 trillion) in 2019, equivalent to 6.9% of the 2019 global gross domestic product.
IN PRACTICE:
Given the magnitude of the estimated health effects of lead exposure, particularly in LMICs, “it is imperative that nationally representative periodic blood lead level measurements be institutionalized,” write the authors, adding that these measurements could be incorporated into existing household surveys.
STUDY DETAILS:
The study was conducted by Bjorn Larsen, PhD, environmental economist and consultant to the World Bank, and Ernesto Sánchez-Triana. It was published online in The Lancet Planetary Health.
LIMITATIONS:
- Global blood lead level estimates may be inaccurate, given that measurements are absent for many countries.
- Certain income projections and income losses are uncertain.
- Because the study does not capture the detrimental effects of lead exposure other than IQ loss and CVD mortality, the estimates of global costs are conservative.
DISCLOSURES:
The study received support from the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Globally, lead exposure is linked to more than 5.5 million adult cardiovascular deaths in 2019, as well as loss of 765 million intelligence quotient (IQ) points in children younger than 5 years, which cost U.S. $6 trillion in lost productivity, new research suggests.
METHODOLOGY:
- Global lead exposure has declined substantially since leaded gasoline was phased out, but several sources of lead remain, resulting in adverse health and economic effects, particularly in low- and middle-income countries (LMICs).
- Estimates of cardiovascular disease (CVD) deaths from lead exposure have been limited to effects of increased blood pressure, but studies show that lead exposure has cardiovascular impacts through mechanisms other than hypertension.
- Drawing from various sources and studies, researchers estimated global blood lead levels and the impact of lead exposure on CVD mortality in 2019 among adults aged 25 years or older, IQ loss in children younger than 5 years, and the related economic costs.
TAKEAWAY:
- Researchers estimated that there were 5,545,000 (95% confidence interval, 2,305,000-8,271,000) cardiovascular deaths in adults from lead exposure in 2019, with as many as 90.2% of these deaths in LMICs; however, this estimate may be incomplete because it does not include the effect of lead exposure on CVD mortality mediated through hypertension.
- The estimated global IQ loss in children younger than 5 years due to lead exposure was 765 million (95% CI, 443 million-1,098 million) IQ points in 2019, 95.3% of which occurred in LMICs.
- These estimates place lead exposure on a par with ambient particulate matter and household air pollution combined, and ahead of unsafe household drinking water, sanitation, and handwashing, as an environmental risk factor.
- The estimated global cost of lead exposure from CVD mortality and IQ loss combined is U.S. $6.0 trillion (range, $2.6 trillion-9.0 trillion) in 2019, equivalent to 6.9% of the 2019 global gross domestic product.
IN PRACTICE:
Given the magnitude of the estimated health effects of lead exposure, particularly in LMICs, “it is imperative that nationally representative periodic blood lead level measurements be institutionalized,” write the authors, adding that these measurements could be incorporated into existing household surveys.
STUDY DETAILS:
The study was conducted by Bjorn Larsen, PhD, environmental economist and consultant to the World Bank, and Ernesto Sánchez-Triana. It was published online in The Lancet Planetary Health.
LIMITATIONS:
- Global blood lead level estimates may be inaccurate, given that measurements are absent for many countries.
- Certain income projections and income losses are uncertain.
- Because the study does not capture the detrimental effects of lead exposure other than IQ loss and CVD mortality, the estimates of global costs are conservative.
DISCLOSURES:
The study received support from the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Globally, lead exposure is linked to more than 5.5 million adult cardiovascular deaths in 2019, as well as loss of 765 million intelligence quotient (IQ) points in children younger than 5 years, which cost U.S. $6 trillion in lost productivity, new research suggests.
METHODOLOGY:
- Global lead exposure has declined substantially since leaded gasoline was phased out, but several sources of lead remain, resulting in adverse health and economic effects, particularly in low- and middle-income countries (LMICs).
- Estimates of cardiovascular disease (CVD) deaths from lead exposure have been limited to effects of increased blood pressure, but studies show that lead exposure has cardiovascular impacts through mechanisms other than hypertension.
- Drawing from various sources and studies, researchers estimated global blood lead levels and the impact of lead exposure on CVD mortality in 2019 among adults aged 25 years or older, IQ loss in children younger than 5 years, and the related economic costs.
TAKEAWAY:
- Researchers estimated that there were 5,545,000 (95% confidence interval, 2,305,000-8,271,000) cardiovascular deaths in adults from lead exposure in 2019, with as many as 90.2% of these deaths in LMICs; however, this estimate may be incomplete because it does not include the effect of lead exposure on CVD mortality mediated through hypertension.
- The estimated global IQ loss in children younger than 5 years due to lead exposure was 765 million (95% CI, 443 million-1,098 million) IQ points in 2019, 95.3% of which occurred in LMICs.
- These estimates place lead exposure on a par with ambient particulate matter and household air pollution combined, and ahead of unsafe household drinking water, sanitation, and handwashing, as an environmental risk factor.
- The estimated global cost of lead exposure from CVD mortality and IQ loss combined is U.S. $6.0 trillion (range, $2.6 trillion-9.0 trillion) in 2019, equivalent to 6.9% of the 2019 global gross domestic product.
IN PRACTICE:
Given the magnitude of the estimated health effects of lead exposure, particularly in LMICs, “it is imperative that nationally representative periodic blood lead level measurements be institutionalized,” write the authors, adding that these measurements could be incorporated into existing household surveys.
STUDY DETAILS:
The study was conducted by Bjorn Larsen, PhD, environmental economist and consultant to the World Bank, and Ernesto Sánchez-Triana. It was published online in The Lancet Planetary Health.
LIMITATIONS:
- Global blood lead level estimates may be inaccurate, given that measurements are absent for many countries.
- Certain income projections and income losses are uncertain.
- Because the study does not capture the detrimental effects of lead exposure other than IQ loss and CVD mortality, the estimates of global costs are conservative.
DISCLOSURES:
The study received support from the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Heart attack deaths static in those with type 1 diabetes
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
FROM EASD 2023
Cold weather may challenge blood pressure control
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2023