User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Attempted suicide in high school America, 2019
according to newly released data from the 2019 Youth Risk Behavior Survey.
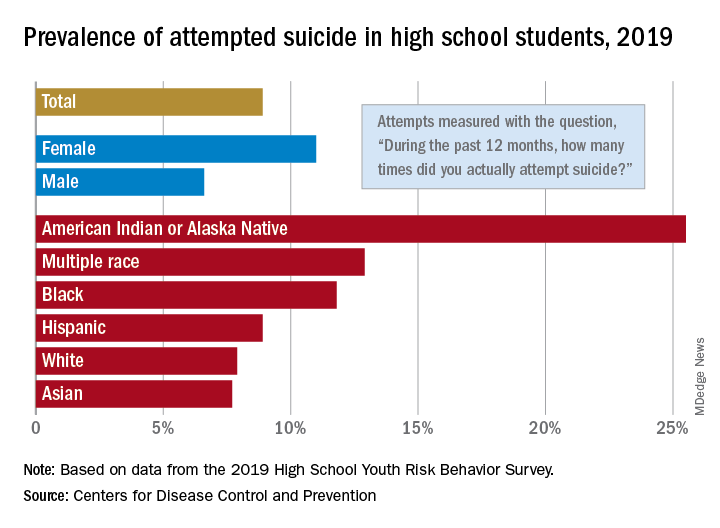
The prevalence of attempted suicide during the previous 12 months was 8.9% among the 13,677 students in grades 9-12 who took the survey last year, but the rate was 25.5% for American Indian/Alaska Native (AI/AN) respondents, almost 2.9 times higher, the YRBS data show.
Respondents with multiple races in their backgrounds, at 12.9%, and African Americans, with a prevalence of 11.8%, also were above the high school average for suicide attempts, while Whites (7.9%) and Asians (7.7%) were under it and Hispanics equaled it, the Centers for Disease Control and Prevention reported.
The number of AI/AN students was insufficient to examine differences by sex, but females in all of the other racial/ethnic groups were more likely than males to have attempted suicide: multiple race (17.8% vs. 7.3%), African American (15.2% vs. 8.5%), Hispanic (11.9% vs. 5.5%), White (9.4% vs. 6.4%), and Asian (8.4% vs. 7.1%), the CDC’s Division of Adolescent and School Health said.
Among all respondents, 11.0% of females had attempted suicide in the 12 months before the survey, a figure that is significantly higher than the 6.6% prevalence in males. Females also were significantly more likely than males to make a plan about how they would attempt suicide (19.9% vs. 11.3%) and to seriously consider an attempt (24.1% vs. 13.3%), CDC investigators said in a separate report.
Significant differences also were seen when looking at sexual identity. Suicide attempts were reported by 6.4% of heterosexuals, 16.1% of those who weren’t sure, and 23.4% of lesbians/gays/bisexuals (LGBs). For serious consideration of suicide, the respective numbers were 14.5%, 30.4%, and 46.8%, they reported (MMWR Supp. 2020 Aug 21;69[1]:47-55).
For nonheterosexuals, however, males were slightly more likely (23.8%) than females (23.6%) to have attempted suicide, but females were more likely to seriously consider it (49.0% vs. 40.4%) and to make a plan (42.4% vs. 33.0%), according to the YRBS data.
“Adolescence … represents a time for expanded identity development, with sexual identity development representing a complex, multidimensional, and often stressful process for youths,” the CDC investigators said in the MMWR. “To address the health differences in suicidal ideation and behaviors observed by student demographics and to decrease these outcomes overall, a comprehensive approach to suicide prevention, including programs, practices, and policies based on the best available evidence, is needed.”
according to newly released data from the 2019 Youth Risk Behavior Survey.

The prevalence of attempted suicide during the previous 12 months was 8.9% among the 13,677 students in grades 9-12 who took the survey last year, but the rate was 25.5% for American Indian/Alaska Native (AI/AN) respondents, almost 2.9 times higher, the YRBS data show.
Respondents with multiple races in their backgrounds, at 12.9%, and African Americans, with a prevalence of 11.8%, also were above the high school average for suicide attempts, while Whites (7.9%) and Asians (7.7%) were under it and Hispanics equaled it, the Centers for Disease Control and Prevention reported.
The number of AI/AN students was insufficient to examine differences by sex, but females in all of the other racial/ethnic groups were more likely than males to have attempted suicide: multiple race (17.8% vs. 7.3%), African American (15.2% vs. 8.5%), Hispanic (11.9% vs. 5.5%), White (9.4% vs. 6.4%), and Asian (8.4% vs. 7.1%), the CDC’s Division of Adolescent and School Health said.
Among all respondents, 11.0% of females had attempted suicide in the 12 months before the survey, a figure that is significantly higher than the 6.6% prevalence in males. Females also were significantly more likely than males to make a plan about how they would attempt suicide (19.9% vs. 11.3%) and to seriously consider an attempt (24.1% vs. 13.3%), CDC investigators said in a separate report.
Significant differences also were seen when looking at sexual identity. Suicide attempts were reported by 6.4% of heterosexuals, 16.1% of those who weren’t sure, and 23.4% of lesbians/gays/bisexuals (LGBs). For serious consideration of suicide, the respective numbers were 14.5%, 30.4%, and 46.8%, they reported (MMWR Supp. 2020 Aug 21;69[1]:47-55).
For nonheterosexuals, however, males were slightly more likely (23.8%) than females (23.6%) to have attempted suicide, but females were more likely to seriously consider it (49.0% vs. 40.4%) and to make a plan (42.4% vs. 33.0%), according to the YRBS data.
“Adolescence … represents a time for expanded identity development, with sexual identity development representing a complex, multidimensional, and often stressful process for youths,” the CDC investigators said in the MMWR. “To address the health differences in suicidal ideation and behaviors observed by student demographics and to decrease these outcomes overall, a comprehensive approach to suicide prevention, including programs, practices, and policies based on the best available evidence, is needed.”
according to newly released data from the 2019 Youth Risk Behavior Survey.

The prevalence of attempted suicide during the previous 12 months was 8.9% among the 13,677 students in grades 9-12 who took the survey last year, but the rate was 25.5% for American Indian/Alaska Native (AI/AN) respondents, almost 2.9 times higher, the YRBS data show.
Respondents with multiple races in their backgrounds, at 12.9%, and African Americans, with a prevalence of 11.8%, also were above the high school average for suicide attempts, while Whites (7.9%) and Asians (7.7%) were under it and Hispanics equaled it, the Centers for Disease Control and Prevention reported.
The number of AI/AN students was insufficient to examine differences by sex, but females in all of the other racial/ethnic groups were more likely than males to have attempted suicide: multiple race (17.8% vs. 7.3%), African American (15.2% vs. 8.5%), Hispanic (11.9% vs. 5.5%), White (9.4% vs. 6.4%), and Asian (8.4% vs. 7.1%), the CDC’s Division of Adolescent and School Health said.
Among all respondents, 11.0% of females had attempted suicide in the 12 months before the survey, a figure that is significantly higher than the 6.6% prevalence in males. Females also were significantly more likely than males to make a plan about how they would attempt suicide (19.9% vs. 11.3%) and to seriously consider an attempt (24.1% vs. 13.3%), CDC investigators said in a separate report.
Significant differences also were seen when looking at sexual identity. Suicide attempts were reported by 6.4% of heterosexuals, 16.1% of those who weren’t sure, and 23.4% of lesbians/gays/bisexuals (LGBs). For serious consideration of suicide, the respective numbers were 14.5%, 30.4%, and 46.8%, they reported (MMWR Supp. 2020 Aug 21;69[1]:47-55).
For nonheterosexuals, however, males were slightly more likely (23.8%) than females (23.6%) to have attempted suicide, but females were more likely to seriously consider it (49.0% vs. 40.4%) and to make a plan (42.4% vs. 33.0%), according to the YRBS data.
“Adolescence … represents a time for expanded identity development, with sexual identity development representing a complex, multidimensional, and often stressful process for youths,” the CDC investigators said in the MMWR. “To address the health differences in suicidal ideation and behaviors observed by student demographics and to decrease these outcomes overall, a comprehensive approach to suicide prevention, including programs, practices, and policies based on the best available evidence, is needed.”
Mitigating psychiatric disorder relapse in pregnancy during pandemic
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
FDA approves point-of-care COVID-19 antigen test

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.
COVID-19 vaccine supply will be limited at first, ACIP says
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
Anorexia may stunt growth in teenage girls
Anorexia nervosa may stunt the growth and impact the future height of teenage girls, according to data from 255 adolescents.
Illness and malnutrition during critical child and adolescent growth periods may limit adult height, but the effect of anorexia nervosa (AN) on growth impairment and adult height has not been well studied, wrote Dalit Modan-Moses, MD, of Chaim Sheba Medical Center, Tel Aviv, and colleagues.
Individuals with AN lose an unhealthy amount of weight on purpose through dieting, sometimes along with excessive exercise, binge eating, and/or purging, and because the condition occurs mainly in adolescents, the subsequent malnutrition may impact growth and adult height, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism, the researchers reviewed data from 255 adolescent girls who were hospitalized for AN at an average age of 15 years. They measured the girls’ height at the time of hospital admission, discharge, and at adulthood. The participants were followed in an outpatient clinic after hospital discharge with biweekly visits for the first 2 months, monthly visits for the next 4 months, and every 3 months until they reached 18 years of age. The average body mass index of the patients at the time of admission was 16 kg/m2 and the average duration of illness was 2 years. Of the 225 patients, 174 had a diagnosis of restrictive type anorexia nervosa and 81 had binge-purge type.
The midparental target height was based on an average of the parents’ heights and subtracting 6.5 cm. The main outcome of adult height was significantly shorter than expected (P = .006) based on midparental target height. Although the patients’ heights increased significantly during hospitalization, from 158 cm to 159 cm (P < .001), “the change in height-SDS [standard deviation scores] was not significant and height-SDS at discharge remained significantly lower compared to the expected in a normal population,” the researchers noted.
Although premorbid height SDS in the study population were similar to normal adolescents, the height-SDS measurements at hospital admission, discharge, and adulthood were significantly lower than expected (–0.36, –0.34, and –0.29, respectively).
Independent predictors of height improvement from hospital admission to adulthood were patient age and bone age at the time of hospital admission, linear growth during hospitalization, and change in luteinizing hormone (LH) during hospitalization, based on a stepwise forward linear regression analysis.
The findings were limited by several factors including the inpatient study population, which may limit the generalizability to patients with less severe illness, as well as incomplete data on LH levels, which were undetectable in 19% of the patients, the researchers noted. However, the study is among the largest to describe growth in female AN patients and included data on linear growth and LH not described in other studies, they said.
“Our study is unique in presenting complete growth data (premorbid, admission, discharge, AH) as well as target height, laboratory results and bone age data in a large cohort of adolescent females with AN,” they wrote.
The findings not only support the need for early intervention in patients with AN and the need for long-term weight gain to achieve catch-up growth, but also may apply to management of malnutrition in adolescents with chronic diseases such as cystic fibrosis and inflammatory bowel disease, they concluded.
“Anorexia nervosa is a prevalent and severe disease with multiple short- and long-term complications. Still, despite the large body of research regarding this disease, data regarding growth patterns and final height of patients was incomplete and inconclusive, Dr. Modan-Moses said in an interview. The findings were not surprising, and were consistent with the results of a previous study the researchers conducted (Modan-Moses D et al. PLoS One. 2012 Sept 18. doi: 10.1371/journal.pone.0045504).
“Our first study was retrospective, and many pertinent parameters influencing growth were not available,” Dr. Modan-Moses noted. “The current study was designed to include a comprehensive evaluation including examination of the patients to document how far advanced in puberty they were, measuring height of parents in order to document the genetic height potential, bone age x-rays of the hand to determine the growth potential at the time of admission to hospitalization, and laboratory tests. This design enabled us to validate the results of our first study so that our findings are now more scientifically grounded,” she said.
“Our findings imply that in many cases there is a considerable delay in the diagnosis of anorexia nervosa, so that by the time of diagnosis significant growth delay has already occurred. Our findings also imply that damage caused by this delay in diagnosis was in part irreversible, even with intensive treatment,” Dr. Modan-Moses emphasized. On a clinical level, the results highlight the “importance of careful monitoring of height and weight by pediatricians, and early detection and early initiation of treatment of anorexia nervosa in adolescents with long-term efforts to improve and accelerate weight gain in order to prevent complications,” she said. “Research is needed to better define factors affecting catch-up growth (that is improved growth with correction of the height deficit observed at the time of admission) and to determine accordingly optimal treatment plans,” Dr. Modan-Moses added.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Modan-Moses D et al. J Clin Endocrinol Metab. 2020 Aug 20. doi: 10.1210/clinem/dgaa510.
Anorexia nervosa may stunt the growth and impact the future height of teenage girls, according to data from 255 adolescents.
Illness and malnutrition during critical child and adolescent growth periods may limit adult height, but the effect of anorexia nervosa (AN) on growth impairment and adult height has not been well studied, wrote Dalit Modan-Moses, MD, of Chaim Sheba Medical Center, Tel Aviv, and colleagues.
Individuals with AN lose an unhealthy amount of weight on purpose through dieting, sometimes along with excessive exercise, binge eating, and/or purging, and because the condition occurs mainly in adolescents, the subsequent malnutrition may impact growth and adult height, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism, the researchers reviewed data from 255 adolescent girls who were hospitalized for AN at an average age of 15 years. They measured the girls’ height at the time of hospital admission, discharge, and at adulthood. The participants were followed in an outpatient clinic after hospital discharge with biweekly visits for the first 2 months, monthly visits for the next 4 months, and every 3 months until they reached 18 years of age. The average body mass index of the patients at the time of admission was 16 kg/m2 and the average duration of illness was 2 years. Of the 225 patients, 174 had a diagnosis of restrictive type anorexia nervosa and 81 had binge-purge type.
The midparental target height was based on an average of the parents’ heights and subtracting 6.5 cm. The main outcome of adult height was significantly shorter than expected (P = .006) based on midparental target height. Although the patients’ heights increased significantly during hospitalization, from 158 cm to 159 cm (P < .001), “the change in height-SDS [standard deviation scores] was not significant and height-SDS at discharge remained significantly lower compared to the expected in a normal population,” the researchers noted.
Although premorbid height SDS in the study population were similar to normal adolescents, the height-SDS measurements at hospital admission, discharge, and adulthood were significantly lower than expected (–0.36, –0.34, and –0.29, respectively).
Independent predictors of height improvement from hospital admission to adulthood were patient age and bone age at the time of hospital admission, linear growth during hospitalization, and change in luteinizing hormone (LH) during hospitalization, based on a stepwise forward linear regression analysis.
The findings were limited by several factors including the inpatient study population, which may limit the generalizability to patients with less severe illness, as well as incomplete data on LH levels, which were undetectable in 19% of the patients, the researchers noted. However, the study is among the largest to describe growth in female AN patients and included data on linear growth and LH not described in other studies, they said.
“Our study is unique in presenting complete growth data (premorbid, admission, discharge, AH) as well as target height, laboratory results and bone age data in a large cohort of adolescent females with AN,” they wrote.
The findings not only support the need for early intervention in patients with AN and the need for long-term weight gain to achieve catch-up growth, but also may apply to management of malnutrition in adolescents with chronic diseases such as cystic fibrosis and inflammatory bowel disease, they concluded.
“Anorexia nervosa is a prevalent and severe disease with multiple short- and long-term complications. Still, despite the large body of research regarding this disease, data regarding growth patterns and final height of patients was incomplete and inconclusive, Dr. Modan-Moses said in an interview. The findings were not surprising, and were consistent with the results of a previous study the researchers conducted (Modan-Moses D et al. PLoS One. 2012 Sept 18. doi: 10.1371/journal.pone.0045504).
“Our first study was retrospective, and many pertinent parameters influencing growth were not available,” Dr. Modan-Moses noted. “The current study was designed to include a comprehensive evaluation including examination of the patients to document how far advanced in puberty they were, measuring height of parents in order to document the genetic height potential, bone age x-rays of the hand to determine the growth potential at the time of admission to hospitalization, and laboratory tests. This design enabled us to validate the results of our first study so that our findings are now more scientifically grounded,” she said.
“Our findings imply that in many cases there is a considerable delay in the diagnosis of anorexia nervosa, so that by the time of diagnosis significant growth delay has already occurred. Our findings also imply that damage caused by this delay in diagnosis was in part irreversible, even with intensive treatment,” Dr. Modan-Moses emphasized. On a clinical level, the results highlight the “importance of careful monitoring of height and weight by pediatricians, and early detection and early initiation of treatment of anorexia nervosa in adolescents with long-term efforts to improve and accelerate weight gain in order to prevent complications,” she said. “Research is needed to better define factors affecting catch-up growth (that is improved growth with correction of the height deficit observed at the time of admission) and to determine accordingly optimal treatment plans,” Dr. Modan-Moses added.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Modan-Moses D et al. J Clin Endocrinol Metab. 2020 Aug 20. doi: 10.1210/clinem/dgaa510.
Anorexia nervosa may stunt the growth and impact the future height of teenage girls, according to data from 255 adolescents.
Illness and malnutrition during critical child and adolescent growth periods may limit adult height, but the effect of anorexia nervosa (AN) on growth impairment and adult height has not been well studied, wrote Dalit Modan-Moses, MD, of Chaim Sheba Medical Center, Tel Aviv, and colleagues.
Individuals with AN lose an unhealthy amount of weight on purpose through dieting, sometimes along with excessive exercise, binge eating, and/or purging, and because the condition occurs mainly in adolescents, the subsequent malnutrition may impact growth and adult height, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism, the researchers reviewed data from 255 adolescent girls who were hospitalized for AN at an average age of 15 years. They measured the girls’ height at the time of hospital admission, discharge, and at adulthood. The participants were followed in an outpatient clinic after hospital discharge with biweekly visits for the first 2 months, monthly visits for the next 4 months, and every 3 months until they reached 18 years of age. The average body mass index of the patients at the time of admission was 16 kg/m2 and the average duration of illness was 2 years. Of the 225 patients, 174 had a diagnosis of restrictive type anorexia nervosa and 81 had binge-purge type.
The midparental target height was based on an average of the parents’ heights and subtracting 6.5 cm. The main outcome of adult height was significantly shorter than expected (P = .006) based on midparental target height. Although the patients’ heights increased significantly during hospitalization, from 158 cm to 159 cm (P < .001), “the change in height-SDS [standard deviation scores] was not significant and height-SDS at discharge remained significantly lower compared to the expected in a normal population,” the researchers noted.
Although premorbid height SDS in the study population were similar to normal adolescents, the height-SDS measurements at hospital admission, discharge, and adulthood were significantly lower than expected (–0.36, –0.34, and –0.29, respectively).
Independent predictors of height improvement from hospital admission to adulthood were patient age and bone age at the time of hospital admission, linear growth during hospitalization, and change in luteinizing hormone (LH) during hospitalization, based on a stepwise forward linear regression analysis.
The findings were limited by several factors including the inpatient study population, which may limit the generalizability to patients with less severe illness, as well as incomplete data on LH levels, which were undetectable in 19% of the patients, the researchers noted. However, the study is among the largest to describe growth in female AN patients and included data on linear growth and LH not described in other studies, they said.
“Our study is unique in presenting complete growth data (premorbid, admission, discharge, AH) as well as target height, laboratory results and bone age data in a large cohort of adolescent females with AN,” they wrote.
The findings not only support the need for early intervention in patients with AN and the need for long-term weight gain to achieve catch-up growth, but also may apply to management of malnutrition in adolescents with chronic diseases such as cystic fibrosis and inflammatory bowel disease, they concluded.
“Anorexia nervosa is a prevalent and severe disease with multiple short- and long-term complications. Still, despite the large body of research regarding this disease, data regarding growth patterns and final height of patients was incomplete and inconclusive, Dr. Modan-Moses said in an interview. The findings were not surprising, and were consistent with the results of a previous study the researchers conducted (Modan-Moses D et al. PLoS One. 2012 Sept 18. doi: 10.1371/journal.pone.0045504).
“Our first study was retrospective, and many pertinent parameters influencing growth were not available,” Dr. Modan-Moses noted. “The current study was designed to include a comprehensive evaluation including examination of the patients to document how far advanced in puberty they were, measuring height of parents in order to document the genetic height potential, bone age x-rays of the hand to determine the growth potential at the time of admission to hospitalization, and laboratory tests. This design enabled us to validate the results of our first study so that our findings are now more scientifically grounded,” she said.
“Our findings imply that in many cases there is a considerable delay in the diagnosis of anorexia nervosa, so that by the time of diagnosis significant growth delay has already occurred. Our findings also imply that damage caused by this delay in diagnosis was in part irreversible, even with intensive treatment,” Dr. Modan-Moses emphasized. On a clinical level, the results highlight the “importance of careful monitoring of height and weight by pediatricians, and early detection and early initiation of treatment of anorexia nervosa in adolescents with long-term efforts to improve and accelerate weight gain in order to prevent complications,” she said. “Research is needed to better define factors affecting catch-up growth (that is improved growth with correction of the height deficit observed at the time of admission) and to determine accordingly optimal treatment plans,” Dr. Modan-Moses added.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Modan-Moses D et al. J Clin Endocrinol Metab. 2020 Aug 20. doi: 10.1210/clinem/dgaa510.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Asymptomatic SARS-CoV-2 infections in kids tied to local rates
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19: ‘Record’ spike in Internet anxiety, panic queries
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Internet searches regarding acute anxiety reached an all-time high between March and May 2020, new research shows.
Investigators used data collected by Google to monitor the daily percentage of all Internet searches originating in the United States that included the terms “anxiety” or “panic” in combination with “attack” between January 2004 and May 2020.
They found an 11% increase in all acute anxiety queries between March 2020, when President Donald Trump first declared the COVID-19 pandemic a national emergency, and May 2020. This translates into approximately 375,000 more searches than expected.
Most of the increase in inquiries occurred when specific developments in COVID-19 were reported.
“We found record levels of people potentially having panic attacks, as reflected by their online queries since early in the pandemic,” lead author John W. Ayers, PhD, associate adjunct professor of medicine, school of health sciences, University of California, San Diego, said in an interview.
“There are two main take-home messages from our research – one is that we need to think about how to address acute anxiety during COVID-19, and the other is that who is also vice chief of innovation, division of infectious diseases and global public health at the University of California, San Diego.
The study was published online August 24 in JAMA Internal Medicine.
Real-time data
“There has been a lot of speculation about collateral consequences of COVID-19, especially in mental health,” Dr. Ayers said.
Most of the research has been conducted via self-report survey, but these types of surveys may miss individuals who do not participate in the surveys or do not seek care, Dr. Ayers added.
“We need a strategy that can measure behavioral health in real time so we can design interventions to meet these needs,” he said.
He explained that he and his colleagues “looked at one case study – panic attacks – because it is the most prevalent form of mental health problem driven by your surroundings, and it is socially contagious, meaning that when someone you know is having a severe acute anxiety or panic attack, you’re more likely to have one yourself.”
The researchers turned to publicly available nonidentifiable data collected via Google Trends – a feature of Google that shows how frequently a given search term is entered into Google’s search engine, relative to the site’s total search volume, over a specific period.
They monitored all searches containing their keywords over a 15-year period (Jan. 1, 2004–May 4, 2020). Search volumes between March 13, 2020 (when the national emergency was declared) and the last date of available data (May 9, 2020) were compared with the expected search volumes that would have been found had COVID-19 not occurred.
Headline-related spikes
Cumulatively, all acute anxiety searches were 11% higher than expected for the 58-day study period (95% confidence interval, 7%-14%). There was a dramatic increase in searches (375,000), or a total of 3.4 million searches, during that period.
Most of these searches took place between March 16, 2020, and April 14, 2020, when searches were cumulatively 17% higher than expected (95% CI, 13%-22%).
Several COVID-19–related milestones took place during that period.
- First imposition of social distancing guidelines occurred (March 16, 2020).
- The United States surpassed China with the most reported COVID-19 cases (March 26, 2020).
- Extension of social distancing guidelines occurred (March 29, 2020).
- The Centers for Disease Control and Prevention recommended use of face masks (April 3, 2020).
- The United States surpassed Italy for having the most COVID-19–related deaths (April 11, 2020).
The largest spike in acute anxiety queries occurred on March 28, 2020, on which date there were 52% more searches than expected. Queries returned to expected levels on April 15, 2020, and have fallen within expected ranges since then.
Dr. Ayers noted that, although other stressors have affected people in the United States, including electoral and economic issues, “the headlines around COVID-19 were driving the anxiety, and those days with dramatic headlines were associated with large spikes in queries.”
“Our messaging surrounding how COVID-19 is reported may need to change to prevent this,” Dr. Ayers said. “Headlines that hit people in the head by reporting how many people died and bury in the article how we can slow the spread may increase anxiety more than headlines reporting strategies that work right up front.”
He noted that media reporting of suicide has begun to change; there have been fewer sensationalized headlines, and there has been an increase in referrals to suicide hotlines. “We need to be thinking about similar strategies when reporting COVID-19,” said Dr. Ayers.
He also suggested tapping existing resources, such as state suicide hotlines, by training staff to assist persons experiencing acute anxiety and panic attacks.
As an example of this model, the authors point to an Illinois-based hotline, Call4Calm, which helps people cope with acute COVID-19 anxiety.
Google queries concerning panic and anxiety do not yield any links to helplines, although OneBox, a Google feature, provides information to people inquiring about suicide and addiction. “This approach could be used to promote resources about anxiety and panic and COVID-19,” Dr. Ayers suggested.
Call to action
Elspeth Cameron Ritchie, MD, chair of the department of psychiatry, Medstar Washington Hospital Center, said the study’s recommendations were “interesting and worth amplifying “ and that “headlines calling for calm” were “a good suggestion.” She was not involved with the study.
“A multifaceted, multimedia approach is needed – not only what’s on Google, but it would be helpful if politicians could acknowledge the anxiety and make available more mental health resources,” she added.
Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington, suggested that it is a “simple option [during patient visits] to incorporate a question as to how your patient is being affected by the pandemic into your standard template, together with questions about sleep, appetite, sexual functioning, etc.”
Dr. Ayers said that the “call to action” that he and his colleagues are making is to use their methodology to “know what mental health needs are in the population” and to use existing frameworks and strategies to address them.
The study was supported by a grant from the University of California office of the president and by intramural support from the division of infectious diseases and the Center for Data Driven Health at the Qualcomm Institute, both with the University of California, San Diego. One coauthor was funded by Bill and Melinda Gates through the Global Good Fund. Dr. Ayers owns equity positions in Directing Medicine, Health Watcher, and Good Analytics, companies that advise on the use of digital data for public health surveillance. Dr. Ritchie reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Convalescent plasma actions spark trial recruitment concerns
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
Nine antihypertensive drugs associated with reduced risk of depression
The risk of depression is elevated in patients with cardiovascular diseases, but several specific antihypertensive therapies are associated with a reduced risk, and none appear to increase the risk, according to a population-based study that evaluated 10 years of data in nearly 4 million subjects.
“As the first study on individual antihypertensives and risk of depression, we found a decreased risk of depression with nine drugs,” reported a collaborative group of investigators from multiple institutions in Denmark where the study was undertaken.
In a study period spanning from 2005 to 2015, risk of a diagnosis of depression was evaluated in patients taking any of 41 antihypertensive therapies in four major categories. These were identified as angiotensin agents (ACE inhibitors or angiotensin II receptor blockers), calcium antagonists, beta-blockers, and diuretics.
Within these groups, agents associated with a reduced risk of depression were: two angiotensin agents, enalapril and ramipril; three calcium antagonists, amlodipine, verapamil, and verapamil combinations; and four beta-blockers, propranolol, atenolol, bisoprolol, and carvedilol. The remaining drugs in these classes and diuretics were not associated with a reduced risk of depression. However, no antihypertensive agent was linked to an increased risk of depression.
All people living in Denmark are assigned a unique personal identification number that permits health information to be tracked across multiple registers. In this study, information was linked for several registries, including the Danish Medical Register on Vital Statistics, the Medicinal Product Statistics, and the Danish Psychiatric Central Register.
Data from a total of 3.75 million patients exposed to antihypertensive therapy during the study period were evaluated. Roughly 1 million of them were exposed to angiotensin drugs and slightly more than a million were exposed to diuretics. For calcium antagonists or beta-blockers, the numbers were approximately 835,000 and 775,000, respectively.
After adjustment for such factors as concomitant somatic diagnoses, sex, age, and employment status, the hazard ratios for depression among drugs associated with protection identified a risk reduction of 10%-25% in most cases when those who had been given 6-10 prescriptions or more than 10 prescriptions were compared with those who received 2 or fewer.
At the level of 10 or more prescriptions, for example, the risk reductions were 17% for ramipril (HR, 0.83; 95% CI, 0.78-0.89), 8% for enalapril (HR, 0.92; 95% CI, 0.88-0.96), 18% for amlodipine (HR, 0.82; 95% CI, 0.79-0.86), 15% for verapamil (HR, 0.85; 95% CI, 0.79-0.83), 28% for propranolol (HR, 0.72; 95% CI, 0.67-0.77), 20% for atenolol (HR, 0.80; 95% CI, 0.74-0.86), 25% for bisoprolol (HR, 0.75; 95% CI, 0.67-0.84), and 16% for carvedilol (HR, 0.84; 95% CI, 0.75-0.95).
For verapamil combinations, the risk reduction was 67% (HR, 0.33; 95% CI, 0.17-0.63), but the investigators cautioned that only 130 individuals were exposed to verapamil combinations, limiting the reliability of this analysis.
Interpreting the findings
A study hypothesis, the observed protective effect against depression, was expected for angiotensin drugs and calcium-channel blockers, but not for beta-blockers, according to the investigators.
“The renin-angiotensin systems is one of the pathways known to modulate inflammation in the central nervous system and seems involved in the regulation of the stress response. Angiotensin agents may also exert anti-inflammatory effects,” the investigators explained. “Dysregulation of intracellular calcium is evident in depression, including receptor-regulated calcium signaling.”
In contrast, beta-blockers have been associated with increased risk of depression in some but not all studies, according to the investigators. They maintained that some clinicians avoid these agents in patients with a history of mood disorders.
In attempting to account for the variability within drug classes regarding protection and lack of protection against depression, the investigators speculated that differences in pharmacologic properties, such as relative lipophilicity or anti-inflammatory effect, might be important.
Despite the large amount of data, William B. White, MD, professor emeritus at the Calhoun Cardiology Center, University of Connecticut, Farmington, is not convinced.
“In observational studies, even those with very large samples sizes, bias and confounding are hard to extricate with controls and propensity-score matching,” Dr. White said. From his perspective, the protective effects of some but not all drugs within a class “give one the impression that the findings are likely random.”
A member of the editorial board of the journal in which this study appeared, Dr. White said he was not involved in the review of the manuscript. Ultimately, he believed that the results are difficult to interpret.
“For example, there is no plausible rationale for why 2 of the 16 ACE inhibitors or angiotensin II receptor blockers or 4 of the 15 beta-blockers or 3 of the 10 calcium-channel blockers would reduce depression while the others in the class would have no effect,” he said.
Despite the investigators’ conclusion that these data should drive drug choice for patients at risk of depression, “I would say the results of this analysis would not lead me to alter clinical practice,” Dr. White added.
According to the principal investigator of the study, Lars Vedel Kessing, MD, DSc, professor of psychiatry at the University of Copenhagen, many variables affect choice of antihypertensive drug. However, the depression risk is elevated in patients with cardiovascular or cerebrovascular disease and hypertension.
When risk of a mood disorder is a concern, use of one of the nine drugs associated with protection from depression should be considered, “especially in patients at increased risk of developing depression, including patients with prior depression or anxiety and patients with a family history of depression,” he and his coinvestigators concluded.
However, Dr. Kessing said in an interview that the data do not help with individual treatment choices. “We do not compare different antihypertensives against each other due to the risk of confounding by indications, so, no, it is not reasonable to consider relative risk among specific agents.”
The authors reported no potential conflicts of interest involving this topic.
SOURCE: Kessing LV et al. Hypertension. 2020 Aug 24. doi: 10.1161/HYPERTENSIONAHA.120.15605.
The risk of depression is elevated in patients with cardiovascular diseases, but several specific antihypertensive therapies are associated with a reduced risk, and none appear to increase the risk, according to a population-based study that evaluated 10 years of data in nearly 4 million subjects.
“As the first study on individual antihypertensives and risk of depression, we found a decreased risk of depression with nine drugs,” reported a collaborative group of investigators from multiple institutions in Denmark where the study was undertaken.
In a study period spanning from 2005 to 2015, risk of a diagnosis of depression was evaluated in patients taking any of 41 antihypertensive therapies in four major categories. These were identified as angiotensin agents (ACE inhibitors or angiotensin II receptor blockers), calcium antagonists, beta-blockers, and diuretics.
Within these groups, agents associated with a reduced risk of depression were: two angiotensin agents, enalapril and ramipril; three calcium antagonists, amlodipine, verapamil, and verapamil combinations; and four beta-blockers, propranolol, atenolol, bisoprolol, and carvedilol. The remaining drugs in these classes and diuretics were not associated with a reduced risk of depression. However, no antihypertensive agent was linked to an increased risk of depression.
All people living in Denmark are assigned a unique personal identification number that permits health information to be tracked across multiple registers. In this study, information was linked for several registries, including the Danish Medical Register on Vital Statistics, the Medicinal Product Statistics, and the Danish Psychiatric Central Register.
Data from a total of 3.75 million patients exposed to antihypertensive therapy during the study period were evaluated. Roughly 1 million of them were exposed to angiotensin drugs and slightly more than a million were exposed to diuretics. For calcium antagonists or beta-blockers, the numbers were approximately 835,000 and 775,000, respectively.
After adjustment for such factors as concomitant somatic diagnoses, sex, age, and employment status, the hazard ratios for depression among drugs associated with protection identified a risk reduction of 10%-25% in most cases when those who had been given 6-10 prescriptions or more than 10 prescriptions were compared with those who received 2 or fewer.
At the level of 10 or more prescriptions, for example, the risk reductions were 17% for ramipril (HR, 0.83; 95% CI, 0.78-0.89), 8% for enalapril (HR, 0.92; 95% CI, 0.88-0.96), 18% for amlodipine (HR, 0.82; 95% CI, 0.79-0.86), 15% for verapamil (HR, 0.85; 95% CI, 0.79-0.83), 28% for propranolol (HR, 0.72; 95% CI, 0.67-0.77), 20% for atenolol (HR, 0.80; 95% CI, 0.74-0.86), 25% for bisoprolol (HR, 0.75; 95% CI, 0.67-0.84), and 16% for carvedilol (HR, 0.84; 95% CI, 0.75-0.95).
For verapamil combinations, the risk reduction was 67% (HR, 0.33; 95% CI, 0.17-0.63), but the investigators cautioned that only 130 individuals were exposed to verapamil combinations, limiting the reliability of this analysis.
Interpreting the findings
A study hypothesis, the observed protective effect against depression, was expected for angiotensin drugs and calcium-channel blockers, but not for beta-blockers, according to the investigators.
“The renin-angiotensin systems is one of the pathways known to modulate inflammation in the central nervous system and seems involved in the regulation of the stress response. Angiotensin agents may also exert anti-inflammatory effects,” the investigators explained. “Dysregulation of intracellular calcium is evident in depression, including receptor-regulated calcium signaling.”
In contrast, beta-blockers have been associated with increased risk of depression in some but not all studies, according to the investigators. They maintained that some clinicians avoid these agents in patients with a history of mood disorders.
In attempting to account for the variability within drug classes regarding protection and lack of protection against depression, the investigators speculated that differences in pharmacologic properties, such as relative lipophilicity or anti-inflammatory effect, might be important.
Despite the large amount of data, William B. White, MD, professor emeritus at the Calhoun Cardiology Center, University of Connecticut, Farmington, is not convinced.
“In observational studies, even those with very large samples sizes, bias and confounding are hard to extricate with controls and propensity-score matching,” Dr. White said. From his perspective, the protective effects of some but not all drugs within a class “give one the impression that the findings are likely random.”
A member of the editorial board of the journal in which this study appeared, Dr. White said he was not involved in the review of the manuscript. Ultimately, he believed that the results are difficult to interpret.
“For example, there is no plausible rationale for why 2 of the 16 ACE inhibitors or angiotensin II receptor blockers or 4 of the 15 beta-blockers or 3 of the 10 calcium-channel blockers would reduce depression while the others in the class would have no effect,” he said.
Despite the investigators’ conclusion that these data should drive drug choice for patients at risk of depression, “I would say the results of this analysis would not lead me to alter clinical practice,” Dr. White added.
According to the principal investigator of the study, Lars Vedel Kessing, MD, DSc, professor of psychiatry at the University of Copenhagen, many variables affect choice of antihypertensive drug. However, the depression risk is elevated in patients with cardiovascular or cerebrovascular disease and hypertension.
When risk of a mood disorder is a concern, use of one of the nine drugs associated with protection from depression should be considered, “especially in patients at increased risk of developing depression, including patients with prior depression or anxiety and patients with a family history of depression,” he and his coinvestigators concluded.
However, Dr. Kessing said in an interview that the data do not help with individual treatment choices. “We do not compare different antihypertensives against each other due to the risk of confounding by indications, so, no, it is not reasonable to consider relative risk among specific agents.”
The authors reported no potential conflicts of interest involving this topic.
SOURCE: Kessing LV et al. Hypertension. 2020 Aug 24. doi: 10.1161/HYPERTENSIONAHA.120.15605.
The risk of depression is elevated in patients with cardiovascular diseases, but several specific antihypertensive therapies are associated with a reduced risk, and none appear to increase the risk, according to a population-based study that evaluated 10 years of data in nearly 4 million subjects.
“As the first study on individual antihypertensives and risk of depression, we found a decreased risk of depression with nine drugs,” reported a collaborative group of investigators from multiple institutions in Denmark where the study was undertaken.
In a study period spanning from 2005 to 2015, risk of a diagnosis of depression was evaluated in patients taking any of 41 antihypertensive therapies in four major categories. These were identified as angiotensin agents (ACE inhibitors or angiotensin II receptor blockers), calcium antagonists, beta-blockers, and diuretics.
Within these groups, agents associated with a reduced risk of depression were: two angiotensin agents, enalapril and ramipril; three calcium antagonists, amlodipine, verapamil, and verapamil combinations; and four beta-blockers, propranolol, atenolol, bisoprolol, and carvedilol. The remaining drugs in these classes and diuretics were not associated with a reduced risk of depression. However, no antihypertensive agent was linked to an increased risk of depression.
All people living in Denmark are assigned a unique personal identification number that permits health information to be tracked across multiple registers. In this study, information was linked for several registries, including the Danish Medical Register on Vital Statistics, the Medicinal Product Statistics, and the Danish Psychiatric Central Register.
Data from a total of 3.75 million patients exposed to antihypertensive therapy during the study period were evaluated. Roughly 1 million of them were exposed to angiotensin drugs and slightly more than a million were exposed to diuretics. For calcium antagonists or beta-blockers, the numbers were approximately 835,000 and 775,000, respectively.
After adjustment for such factors as concomitant somatic diagnoses, sex, age, and employment status, the hazard ratios for depression among drugs associated with protection identified a risk reduction of 10%-25% in most cases when those who had been given 6-10 prescriptions or more than 10 prescriptions were compared with those who received 2 or fewer.
At the level of 10 or more prescriptions, for example, the risk reductions were 17% for ramipril (HR, 0.83; 95% CI, 0.78-0.89), 8% for enalapril (HR, 0.92; 95% CI, 0.88-0.96), 18% for amlodipine (HR, 0.82; 95% CI, 0.79-0.86), 15% for verapamil (HR, 0.85; 95% CI, 0.79-0.83), 28% for propranolol (HR, 0.72; 95% CI, 0.67-0.77), 20% for atenolol (HR, 0.80; 95% CI, 0.74-0.86), 25% for bisoprolol (HR, 0.75; 95% CI, 0.67-0.84), and 16% for carvedilol (HR, 0.84; 95% CI, 0.75-0.95).
For verapamil combinations, the risk reduction was 67% (HR, 0.33; 95% CI, 0.17-0.63), but the investigators cautioned that only 130 individuals were exposed to verapamil combinations, limiting the reliability of this analysis.
Interpreting the findings
A study hypothesis, the observed protective effect against depression, was expected for angiotensin drugs and calcium-channel blockers, but not for beta-blockers, according to the investigators.
“The renin-angiotensin systems is one of the pathways known to modulate inflammation in the central nervous system and seems involved in the regulation of the stress response. Angiotensin agents may also exert anti-inflammatory effects,” the investigators explained. “Dysregulation of intracellular calcium is evident in depression, including receptor-regulated calcium signaling.”
In contrast, beta-blockers have been associated with increased risk of depression in some but not all studies, according to the investigators. They maintained that some clinicians avoid these agents in patients with a history of mood disorders.
In attempting to account for the variability within drug classes regarding protection and lack of protection against depression, the investigators speculated that differences in pharmacologic properties, such as relative lipophilicity or anti-inflammatory effect, might be important.
Despite the large amount of data, William B. White, MD, professor emeritus at the Calhoun Cardiology Center, University of Connecticut, Farmington, is not convinced.
“In observational studies, even those with very large samples sizes, bias and confounding are hard to extricate with controls and propensity-score matching,” Dr. White said. From his perspective, the protective effects of some but not all drugs within a class “give one the impression that the findings are likely random.”
A member of the editorial board of the journal in which this study appeared, Dr. White said he was not involved in the review of the manuscript. Ultimately, he believed that the results are difficult to interpret.
“For example, there is no plausible rationale for why 2 of the 16 ACE inhibitors or angiotensin II receptor blockers or 4 of the 15 beta-blockers or 3 of the 10 calcium-channel blockers would reduce depression while the others in the class would have no effect,” he said.
Despite the investigators’ conclusion that these data should drive drug choice for patients at risk of depression, “I would say the results of this analysis would not lead me to alter clinical practice,” Dr. White added.
According to the principal investigator of the study, Lars Vedel Kessing, MD, DSc, professor of psychiatry at the University of Copenhagen, many variables affect choice of antihypertensive drug. However, the depression risk is elevated in patients with cardiovascular or cerebrovascular disease and hypertension.
When risk of a mood disorder is a concern, use of one of the nine drugs associated with protection from depression should be considered, “especially in patients at increased risk of developing depression, including patients with prior depression or anxiety and patients with a family history of depression,” he and his coinvestigators concluded.
However, Dr. Kessing said in an interview that the data do not help with individual treatment choices. “We do not compare different antihypertensives against each other due to the risk of confounding by indications, so, no, it is not reasonable to consider relative risk among specific agents.”
The authors reported no potential conflicts of interest involving this topic.
SOURCE: Kessing LV et al. Hypertension. 2020 Aug 24. doi: 10.1161/HYPERTENSIONAHA.120.15605.
FROM HYPERTENSION
Prognosis for rural hospitals worsens with pandemic
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.









