User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Could these old drugs help fight COVID-19 and save lives?
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Unexpected results in new COVID-19 ‘cytokine storm’ data
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
HHS plan to improve rural health focuses on better broadband, telehealth services
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
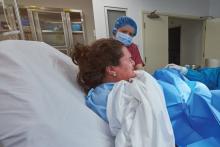
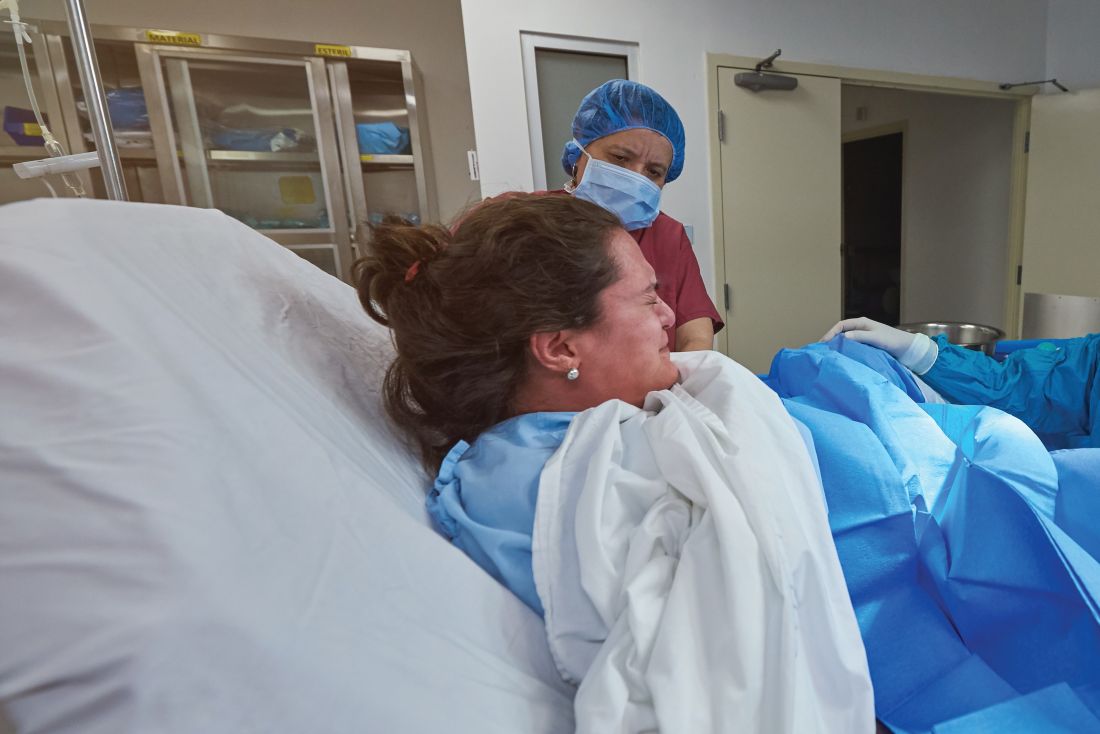
Study: 10% of pregnant women test positive for COVID-19, with most asymptomatic
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
FROM BMJ
‘No mobile phone’ phobia tied to sleep problems in college students
In a study of more than 300 college students, nearly 9 in 10 (89%) were classified as having moderate to severe nomophobia. Greater levels of nomophobia were significantly linked to daytime sleepiness and more behaviors associated with poor sleep hygiene.
“My undergraduate research team came up with the idea for this study,” said study investigator Jennifer Peszka, PhD, professor of psychology at Hendrix College, Conway, Ark. She explained that her students had been looking at the impact of technology use in the 2 hours before bed, and hypothesized that ‘cell phone addiction’ might play a role in sleep problems.
Incidentally, “that group of students were all pretty high on nomophobia themselves so they were really interested in the outcome,” Dr. Peszka said.
The study findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
A likely suspect
The study involved 327 undergraduates (mean age, 19.7 years) recruited from introductory psychology courses and campus newsletters. They completed several questionnaires, including the Nomophobia Questionnaire, the Epworth Sleepiness Scale, and the Sleep Hygiene Index.
Nomophobia was prevalent, with mild, moderate, and severe nomophobia reported by 10%, 83%, and 7% of students, respectively. Only one student reported no nomophobia at all. Dr. Peszka said the fact that 89% of students had moderate or severe nomophobia is “concerning,” given a 2012 study suggesting that 77% of 18- to 24-year-olds had nomophobia. This phobia “very well may be on a rapid rise,” she lamented.
Greater severity of nomophobia was significantly correlated with greater sleepiness measured by both the Epworth Sleepiness Scale (P < .05) and the Associated Features of Poor Sleep Hygiene daytime sleepiness item (P < .05). More severe nomophobia was also related to decreased motivation (a commonly reported symptom of insufficient sleep) and with more maladaptive sleep hygiene behaviors (including using technology during sleep time, long daytime naps, inconsistent wake and bed times, using bed for nonsleep purposes, uncomfortable bed, and bedtime cognitive rumination).
Prior research has shown that smartphones may lead to compulsive “checking” habits, compulsive usage, increased distress, and potentially addictive behaviors. Active phone use at bedtime has also been implicated in disrupted sleep. Nomophobia is likely to be an important consideration when treating sleep disorders and/or making any sleep hygiene recommendations, Dr. Peszka said.
Proliferation of ‘night owls’
Reached for comment, Rajkumar (Raj) Dasgupta, MD, University of Southern California, Los Angeles, said this is a “very timely study with COVID-19. Right now, more than ever, technology is a double-edged sword. I’m a father of three kids and, for now, technology is the only way some kids are going to be socializing and learning.”
Yet a foundation of good sleep hygiene is keeping a nightly sleep routine, said Dr. Dasgupta, who was not involved in the study. “Right now, it seems like all my sleep patients are becoming night owls and sleep time is becoming more and more delayed because there is so much news to keep up with. Also, you may be stressed at night and you may not have the motivation to wake up early in the morning.”
He said it is important to counsel patients to “put technology away at night. That goes for kids and adults.”
Support for the study was provided by Hendrix College Charles Brewer Fund for Psychology. Dr. Peszka and Dr. Dasgupta disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a study of more than 300 college students, nearly 9 in 10 (89%) were classified as having moderate to severe nomophobia. Greater levels of nomophobia were significantly linked to daytime sleepiness and more behaviors associated with poor sleep hygiene.
“My undergraduate research team came up with the idea for this study,” said study investigator Jennifer Peszka, PhD, professor of psychology at Hendrix College, Conway, Ark. She explained that her students had been looking at the impact of technology use in the 2 hours before bed, and hypothesized that ‘cell phone addiction’ might play a role in sleep problems.
Incidentally, “that group of students were all pretty high on nomophobia themselves so they were really interested in the outcome,” Dr. Peszka said.
The study findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
A likely suspect
The study involved 327 undergraduates (mean age, 19.7 years) recruited from introductory psychology courses and campus newsletters. They completed several questionnaires, including the Nomophobia Questionnaire, the Epworth Sleepiness Scale, and the Sleep Hygiene Index.
Nomophobia was prevalent, with mild, moderate, and severe nomophobia reported by 10%, 83%, and 7% of students, respectively. Only one student reported no nomophobia at all. Dr. Peszka said the fact that 89% of students had moderate or severe nomophobia is “concerning,” given a 2012 study suggesting that 77% of 18- to 24-year-olds had nomophobia. This phobia “very well may be on a rapid rise,” she lamented.
Greater severity of nomophobia was significantly correlated with greater sleepiness measured by both the Epworth Sleepiness Scale (P < .05) and the Associated Features of Poor Sleep Hygiene daytime sleepiness item (P < .05). More severe nomophobia was also related to decreased motivation (a commonly reported symptom of insufficient sleep) and with more maladaptive sleep hygiene behaviors (including using technology during sleep time, long daytime naps, inconsistent wake and bed times, using bed for nonsleep purposes, uncomfortable bed, and bedtime cognitive rumination).
Prior research has shown that smartphones may lead to compulsive “checking” habits, compulsive usage, increased distress, and potentially addictive behaviors. Active phone use at bedtime has also been implicated in disrupted sleep. Nomophobia is likely to be an important consideration when treating sleep disorders and/or making any sleep hygiene recommendations, Dr. Peszka said.
Proliferation of ‘night owls’
Reached for comment, Rajkumar (Raj) Dasgupta, MD, University of Southern California, Los Angeles, said this is a “very timely study with COVID-19. Right now, more than ever, technology is a double-edged sword. I’m a father of three kids and, for now, technology is the only way some kids are going to be socializing and learning.”
Yet a foundation of good sleep hygiene is keeping a nightly sleep routine, said Dr. Dasgupta, who was not involved in the study. “Right now, it seems like all my sleep patients are becoming night owls and sleep time is becoming more and more delayed because there is so much news to keep up with. Also, you may be stressed at night and you may not have the motivation to wake up early in the morning.”
He said it is important to counsel patients to “put technology away at night. That goes for kids and adults.”
Support for the study was provided by Hendrix College Charles Brewer Fund for Psychology. Dr. Peszka and Dr. Dasgupta disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a study of more than 300 college students, nearly 9 in 10 (89%) were classified as having moderate to severe nomophobia. Greater levels of nomophobia were significantly linked to daytime sleepiness and more behaviors associated with poor sleep hygiene.
“My undergraduate research team came up with the idea for this study,” said study investigator Jennifer Peszka, PhD, professor of psychology at Hendrix College, Conway, Ark. She explained that her students had been looking at the impact of technology use in the 2 hours before bed, and hypothesized that ‘cell phone addiction’ might play a role in sleep problems.
Incidentally, “that group of students were all pretty high on nomophobia themselves so they were really interested in the outcome,” Dr. Peszka said.
The study findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies.
A likely suspect
The study involved 327 undergraduates (mean age, 19.7 years) recruited from introductory psychology courses and campus newsletters. They completed several questionnaires, including the Nomophobia Questionnaire, the Epworth Sleepiness Scale, and the Sleep Hygiene Index.
Nomophobia was prevalent, with mild, moderate, and severe nomophobia reported by 10%, 83%, and 7% of students, respectively. Only one student reported no nomophobia at all. Dr. Peszka said the fact that 89% of students had moderate or severe nomophobia is “concerning,” given a 2012 study suggesting that 77% of 18- to 24-year-olds had nomophobia. This phobia “very well may be on a rapid rise,” she lamented.
Greater severity of nomophobia was significantly correlated with greater sleepiness measured by both the Epworth Sleepiness Scale (P < .05) and the Associated Features of Poor Sleep Hygiene daytime sleepiness item (P < .05). More severe nomophobia was also related to decreased motivation (a commonly reported symptom of insufficient sleep) and with more maladaptive sleep hygiene behaviors (including using technology during sleep time, long daytime naps, inconsistent wake and bed times, using bed for nonsleep purposes, uncomfortable bed, and bedtime cognitive rumination).
Prior research has shown that smartphones may lead to compulsive “checking” habits, compulsive usage, increased distress, and potentially addictive behaviors. Active phone use at bedtime has also been implicated in disrupted sleep. Nomophobia is likely to be an important consideration when treating sleep disorders and/or making any sleep hygiene recommendations, Dr. Peszka said.
Proliferation of ‘night owls’
Reached for comment, Rajkumar (Raj) Dasgupta, MD, University of Southern California, Los Angeles, said this is a “very timely study with COVID-19. Right now, more than ever, technology is a double-edged sword. I’m a father of three kids and, for now, technology is the only way some kids are going to be socializing and learning.”
Yet a foundation of good sleep hygiene is keeping a nightly sleep routine, said Dr. Dasgupta, who was not involved in the study. “Right now, it seems like all my sleep patients are becoming night owls and sleep time is becoming more and more delayed because there is so much news to keep up with. Also, you may be stressed at night and you may not have the motivation to wake up early in the morning.”
He said it is important to counsel patients to “put technology away at night. That goes for kids and adults.”
Support for the study was provided by Hendrix College Charles Brewer Fund for Psychology. Dr. Peszka and Dr. Dasgupta disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SLEEP 2020
Experts advocate for the elimination of daylight savings time
In the interest of public health and safety, – a recommendation that has garnered strong support from multiple medical and other high-profile organizations.
“Permanent, year-round standard time is the best choice to most closely match our circadian sleep-wake cycle,” M. Adeel Rishi, MD, lead author of the AASM position statement, said in a news release. “Daylight saving time results in more darkness in the morning and more light in the evening, disrupting the body’s natural rhythm,” said Dr. Rishi, of the department of pulmonology, critical care, and sleep medicine, Mayo Clinic, Eau Claire, Wis., and vice chair of the AASM Public Safety Committee.
The position statement was published Aug. 26 in the Journal of Clinical Sleep Medicine to coincide with the virtual annual meeting of the Associated Professional Sleep Societies .
Significant health risks
In the United States, the annual “spring forward” to daylight saving time and “fall back” to standard time is required by law, although under the statute some exceptions are permitted.
There has been intense debate over the last several years about transitioning between standard and daylight saving time. The AASM says there is “an abundance of evidence” to indicate that quick transition from standard time to daylight saving time incurs significant public health and safety risks, including increased risk of heart attack, stroke, mood disorders, and car crashes.
“Although chronic effects of remaining in daylight saving time year-round have not been well-studied, daylight saving time is less aligned with human circadian biology – which, because of the impacts of the delayed natural light/dark cycle on human activity, could result in circadian misalignment, which has been associated in some studies with increased cardiovascular disease risk, metabolic syndrome and other health risks,” the authors wrote.
A recent study also showed an increase in medical errors in the week after switching to daylight saving time.
“Because the adoption of permanent standard time would be beneficial for public health and safety, the AASM will be advocating at the federal level for this legislative change,” said AASM President Kannan Ramar, MBBS, MD, with the Mayo Clinic in Rochester, Minn.
It seems that many Americans are in favor of the change. In July, an AASM survey of roughly 2,000 U.S. adults showed that two-thirds support doing away with the seasonal time change. Only 11% opposed it. In addition, the academy’s 2019 survey showed more than half of adults feel extremely, or somewhat, tired after the springing ahead to daylight saving time.
Strong support
The position statement has been endorsed by 19 organizations, including the American Academy of Cardiovascular Sleep Medicine, American College of Chest Physicians (CHEST), American College of Occupational and Environmental Medicine, National PTA, National Safety Council, Society of Anesthesia and Sleep Medicine, and the Society of Behavioral Sleep Medicine.
Weighing in on the issue, Saul Rothenberg, PhD, from the Sleep Center at Greenwich Hospital, Conn., said the literature on daylight saving time has grown over the past 20 years. He said he was ”humbled” by the research that shows that a “relatively small” misalignment of biological and social clocks has a measurable impact on human health and behavior.
“Because misalignment is associated with negative health and performance outcomes, keeping one set of hours year-round is promoted to minimize misalignment and associated consequences,” he added.
In light of this research, the recommendation to dispense with daylight saving time seems “quite reasonable” from a public health perspective. “I am left with a strengthened view on the importance of regular adequate sleep as a way to enhance health, performance, and quality of life,” he added.
This research had no commercial funding. Dr. Rishi and Dr. Rothenberg have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the interest of public health and safety, – a recommendation that has garnered strong support from multiple medical and other high-profile organizations.
“Permanent, year-round standard time is the best choice to most closely match our circadian sleep-wake cycle,” M. Adeel Rishi, MD, lead author of the AASM position statement, said in a news release. “Daylight saving time results in more darkness in the morning and more light in the evening, disrupting the body’s natural rhythm,” said Dr. Rishi, of the department of pulmonology, critical care, and sleep medicine, Mayo Clinic, Eau Claire, Wis., and vice chair of the AASM Public Safety Committee.
The position statement was published Aug. 26 in the Journal of Clinical Sleep Medicine to coincide with the virtual annual meeting of the Associated Professional Sleep Societies .
Significant health risks
In the United States, the annual “spring forward” to daylight saving time and “fall back” to standard time is required by law, although under the statute some exceptions are permitted.
There has been intense debate over the last several years about transitioning between standard and daylight saving time. The AASM says there is “an abundance of evidence” to indicate that quick transition from standard time to daylight saving time incurs significant public health and safety risks, including increased risk of heart attack, stroke, mood disorders, and car crashes.
“Although chronic effects of remaining in daylight saving time year-round have not been well-studied, daylight saving time is less aligned with human circadian biology – which, because of the impacts of the delayed natural light/dark cycle on human activity, could result in circadian misalignment, which has been associated in some studies with increased cardiovascular disease risk, metabolic syndrome and other health risks,” the authors wrote.
A recent study also showed an increase in medical errors in the week after switching to daylight saving time.
“Because the adoption of permanent standard time would be beneficial for public health and safety, the AASM will be advocating at the federal level for this legislative change,” said AASM President Kannan Ramar, MBBS, MD, with the Mayo Clinic in Rochester, Minn.
It seems that many Americans are in favor of the change. In July, an AASM survey of roughly 2,000 U.S. adults showed that two-thirds support doing away with the seasonal time change. Only 11% opposed it. In addition, the academy’s 2019 survey showed more than half of adults feel extremely, or somewhat, tired after the springing ahead to daylight saving time.
Strong support
The position statement has been endorsed by 19 organizations, including the American Academy of Cardiovascular Sleep Medicine, American College of Chest Physicians (CHEST), American College of Occupational and Environmental Medicine, National PTA, National Safety Council, Society of Anesthesia and Sleep Medicine, and the Society of Behavioral Sleep Medicine.
Weighing in on the issue, Saul Rothenberg, PhD, from the Sleep Center at Greenwich Hospital, Conn., said the literature on daylight saving time has grown over the past 20 years. He said he was ”humbled” by the research that shows that a “relatively small” misalignment of biological and social clocks has a measurable impact on human health and behavior.
“Because misalignment is associated with negative health and performance outcomes, keeping one set of hours year-round is promoted to minimize misalignment and associated consequences,” he added.
In light of this research, the recommendation to dispense with daylight saving time seems “quite reasonable” from a public health perspective. “I am left with a strengthened view on the importance of regular adequate sleep as a way to enhance health, performance, and quality of life,” he added.
This research had no commercial funding. Dr. Rishi and Dr. Rothenberg have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the interest of public health and safety, – a recommendation that has garnered strong support from multiple medical and other high-profile organizations.
“Permanent, year-round standard time is the best choice to most closely match our circadian sleep-wake cycle,” M. Adeel Rishi, MD, lead author of the AASM position statement, said in a news release. “Daylight saving time results in more darkness in the morning and more light in the evening, disrupting the body’s natural rhythm,” said Dr. Rishi, of the department of pulmonology, critical care, and sleep medicine, Mayo Clinic, Eau Claire, Wis., and vice chair of the AASM Public Safety Committee.
The position statement was published Aug. 26 in the Journal of Clinical Sleep Medicine to coincide with the virtual annual meeting of the Associated Professional Sleep Societies .
Significant health risks
In the United States, the annual “spring forward” to daylight saving time and “fall back” to standard time is required by law, although under the statute some exceptions are permitted.
There has been intense debate over the last several years about transitioning between standard and daylight saving time. The AASM says there is “an abundance of evidence” to indicate that quick transition from standard time to daylight saving time incurs significant public health and safety risks, including increased risk of heart attack, stroke, mood disorders, and car crashes.
“Although chronic effects of remaining in daylight saving time year-round have not been well-studied, daylight saving time is less aligned with human circadian biology – which, because of the impacts of the delayed natural light/dark cycle on human activity, could result in circadian misalignment, which has been associated in some studies with increased cardiovascular disease risk, metabolic syndrome and other health risks,” the authors wrote.
A recent study also showed an increase in medical errors in the week after switching to daylight saving time.
“Because the adoption of permanent standard time would be beneficial for public health and safety, the AASM will be advocating at the federal level for this legislative change,” said AASM President Kannan Ramar, MBBS, MD, with the Mayo Clinic in Rochester, Minn.
It seems that many Americans are in favor of the change. In July, an AASM survey of roughly 2,000 U.S. adults showed that two-thirds support doing away with the seasonal time change. Only 11% opposed it. In addition, the academy’s 2019 survey showed more than half of adults feel extremely, or somewhat, tired after the springing ahead to daylight saving time.
Strong support
The position statement has been endorsed by 19 organizations, including the American Academy of Cardiovascular Sleep Medicine, American College of Chest Physicians (CHEST), American College of Occupational and Environmental Medicine, National PTA, National Safety Council, Society of Anesthesia and Sleep Medicine, and the Society of Behavioral Sleep Medicine.
Weighing in on the issue, Saul Rothenberg, PhD, from the Sleep Center at Greenwich Hospital, Conn., said the literature on daylight saving time has grown over the past 20 years. He said he was ”humbled” by the research that shows that a “relatively small” misalignment of biological and social clocks has a measurable impact on human health and behavior.
“Because misalignment is associated with negative health and performance outcomes, keeping one set of hours year-round is promoted to minimize misalignment and associated consequences,” he added.
In light of this research, the recommendation to dispense with daylight saving time seems “quite reasonable” from a public health perspective. “I am left with a strengthened view on the importance of regular adequate sleep as a way to enhance health, performance, and quality of life,” he added.
This research had no commercial funding. Dr. Rishi and Dr. Rothenberg have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SLEEP 2020
Ten ways docs are cutting costs and saving money
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.
New schizophrenia treatment guideline released
The American Psychiatric Association has released a new evidence-based practice guideline for the treatment of schizophrenia.
The guideline focuses on assessment and treatment planning, which are integral to patient-centered care, and includes recommendations regarding pharmacotherapy, with particular focus on clozapine, as well as previously recommended and new psychosocial interventions.
“Our intention was to make recommendations to treat the whole person and take into account their family and other significant people in their lives,” George Keepers, MD, chair of the guideline writing group, said in an interview.
‘State-of-the-art methodology’
Dr. Keepers, professor of psychiatry at Oregon Health and Science University, Portland, explained the rigorous process that informs the current guideline, which was “based not solely on expert consensus but was preceded by an evidence-based review of the literature that was then discussed, digested, and distilled into specific recommendations.”
Many current recommendations are “similar to previous recommendations, but there are a few important differences,” he said.
Two experts in schizophrenia who were not involved in guideline authorship praised it for its usefulness and methodology.
Philip D. Harvey, PhD, Leonard M. Miller Professor of Psychiatry and Behavioral Sciences, University of Miami, said in an interview that the guideline “clarified the typical treatment algorithm from first episode to treatment resistance [which is] very clearly laid out for the first time.”
Christoph Correll, MD, professor of psychiatry and molecular medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the guideline “followed state-of-the-art methodology.”
First steps
The guideline recommends beginning with assessment of the patient and determination of the treatment plan.
Patients should be “treated with an antipsychotic medication and monitored for effectiveness and side effects.” Even after the patient’s symptoms have improved, antipsychotic treatment should continue.
For patients whose symptoms have improved, treatment should continue with the same antipsychotic and should not be switched.
“The problem we’re addressing in this recommendation is that patients are often treated with an effective medication and then forced, by circumstances or their insurance company, to switch to another that may not be effective for them, resulting in unnecessary relapses of the illness,” said Dr. Keepers.
“ and do what’s in the best interest of the patient,” he said.
“The guideline called out that antipsychotics that are effective and tolerated should be continued, without specifying a duration of treatment, thereby indicating indirectly that there is no clear end of the recommendation for ongoing maintenance treatment in individuals with schizophrenia,” said Dr. Correll.
Clozapine underutilized
The guideline highlights the role of clozapine and recommends its use for patients with treatment-resistant schizophrenia and those at risk for suicide. Clozapine is also recommended for patients at “substantial” risk for aggressive behavior, regardless of other treatments.
“Clozapine is underutilized for treatment of schizophrenia in the U.S. and a number of other countries, but it is a really important treatment for patients who don’t respond to other antipsychotic agents,” said Dr. Keepers.
“With this recommendation, we hope that more patients will wind up receiving the medication and benefiting from it,” he added.
In addition, patients should receive treatment with a long-acting injectable antipsychotic “if they prefer such treatment or if they have a history of poor or uncertain adherence” (level of evidence, 2B).
The guideline authors “are recommending long-acting injectable medications for people who want them, not just people with poor prior adherence, which is a critical step,” said Dr. Harvey, director of the division of psychology at the University of Miami.
Managing antipsychotic side effects
The guideline offers recommendations for patients experiencing antipsychotic-induced side effects.
VMAT2s, which represent a “class of drugs that have become available since the last schizophrenia guidelines, are effective in tardive dyskinesia. It is important that patients with tardive dyskinesia have access to these drugs because they do work,” Dr. Keepers said.
Adequate funding needed
Recommended psychosocial interventions include treatment in a specialty care program for patients with schizophrenia who are experiencing a first episode of psychosis, use of cognitive-behavioral therapy for psychosis, psychoeducation, and supported employment services (2B).
“We reviewed very good data showing that patients who receive these services are more likely to be able to be employed and less likely to be rehospitalized or have a relapse,” Dr. Keepers observed.
In addition, patients with schizophrenia should receive assertive community treatment interventions if there is a “history of poor engagement with services leading to frequent relapse or social disruption.”
Family interventions are recommended for patients who have ongoing contact with their families (2B), and patients should also receive interventions “aimed at developing self-management skills and enhancing person-oriented recovery.” They should receive cognitive remediation, social skills training, and supportive psychotherapy.
Dr. Keepers pointed to “major barriers” to providing some of these psychosocial treatments. “They are beyond the scope of someone in an individual private practice situation, so they need to be delivered within the context of treatment programs that are either publicly or privately based,” he said.
“Psychiatrists can and do work closely with community and mental health centers, psychologists, and social workers who can provide these kinds of treatments,” but “many [treatments] require specialized skills and training before they can be offered, and there is a shortage of personnel to deliver them,” he noted.
“Both the national and state governments have not provided adequate funding for treatment of individuals with this condition [schizophrenia],” he added.
Dr. Keepers reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Harvey reports no relevant financial relationships. Dr. Correll disclosed ties to Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association has released a new evidence-based practice guideline for the treatment of schizophrenia.
The guideline focuses on assessment and treatment planning, which are integral to patient-centered care, and includes recommendations regarding pharmacotherapy, with particular focus on clozapine, as well as previously recommended and new psychosocial interventions.
“Our intention was to make recommendations to treat the whole person and take into account their family and other significant people in their lives,” George Keepers, MD, chair of the guideline writing group, said in an interview.
‘State-of-the-art methodology’
Dr. Keepers, professor of psychiatry at Oregon Health and Science University, Portland, explained the rigorous process that informs the current guideline, which was “based not solely on expert consensus but was preceded by an evidence-based review of the literature that was then discussed, digested, and distilled into specific recommendations.”
Many current recommendations are “similar to previous recommendations, but there are a few important differences,” he said.
Two experts in schizophrenia who were not involved in guideline authorship praised it for its usefulness and methodology.
Philip D. Harvey, PhD, Leonard M. Miller Professor of Psychiatry and Behavioral Sciences, University of Miami, said in an interview that the guideline “clarified the typical treatment algorithm from first episode to treatment resistance [which is] very clearly laid out for the first time.”
Christoph Correll, MD, professor of psychiatry and molecular medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the guideline “followed state-of-the-art methodology.”
First steps
The guideline recommends beginning with assessment of the patient and determination of the treatment plan.
Patients should be “treated with an antipsychotic medication and monitored for effectiveness and side effects.” Even after the patient’s symptoms have improved, antipsychotic treatment should continue.
For patients whose symptoms have improved, treatment should continue with the same antipsychotic and should not be switched.
“The problem we’re addressing in this recommendation is that patients are often treated with an effective medication and then forced, by circumstances or their insurance company, to switch to another that may not be effective for them, resulting in unnecessary relapses of the illness,” said Dr. Keepers.
“ and do what’s in the best interest of the patient,” he said.
“The guideline called out that antipsychotics that are effective and tolerated should be continued, without specifying a duration of treatment, thereby indicating indirectly that there is no clear end of the recommendation for ongoing maintenance treatment in individuals with schizophrenia,” said Dr. Correll.
Clozapine underutilized
The guideline highlights the role of clozapine and recommends its use for patients with treatment-resistant schizophrenia and those at risk for suicide. Clozapine is also recommended for patients at “substantial” risk for aggressive behavior, regardless of other treatments.
“Clozapine is underutilized for treatment of schizophrenia in the U.S. and a number of other countries, but it is a really important treatment for patients who don’t respond to other antipsychotic agents,” said Dr. Keepers.
“With this recommendation, we hope that more patients will wind up receiving the medication and benefiting from it,” he added.
In addition, patients should receive treatment with a long-acting injectable antipsychotic “if they prefer such treatment or if they have a history of poor or uncertain adherence” (level of evidence, 2B).
The guideline authors “are recommending long-acting injectable medications for people who want them, not just people with poor prior adherence, which is a critical step,” said Dr. Harvey, director of the division of psychology at the University of Miami.
Managing antipsychotic side effects
The guideline offers recommendations for patients experiencing antipsychotic-induced side effects.
VMAT2s, which represent a “class of drugs that have become available since the last schizophrenia guidelines, are effective in tardive dyskinesia. It is important that patients with tardive dyskinesia have access to these drugs because they do work,” Dr. Keepers said.
Adequate funding needed
Recommended psychosocial interventions include treatment in a specialty care program for patients with schizophrenia who are experiencing a first episode of psychosis, use of cognitive-behavioral therapy for psychosis, psychoeducation, and supported employment services (2B).
“We reviewed very good data showing that patients who receive these services are more likely to be able to be employed and less likely to be rehospitalized or have a relapse,” Dr. Keepers observed.
In addition, patients with schizophrenia should receive assertive community treatment interventions if there is a “history of poor engagement with services leading to frequent relapse or social disruption.”
Family interventions are recommended for patients who have ongoing contact with their families (2B), and patients should also receive interventions “aimed at developing self-management skills and enhancing person-oriented recovery.” They should receive cognitive remediation, social skills training, and supportive psychotherapy.
Dr. Keepers pointed to “major barriers” to providing some of these psychosocial treatments. “They are beyond the scope of someone in an individual private practice situation, so they need to be delivered within the context of treatment programs that are either publicly or privately based,” he said.
“Psychiatrists can and do work closely with community and mental health centers, psychologists, and social workers who can provide these kinds of treatments,” but “many [treatments] require specialized skills and training before they can be offered, and there is a shortage of personnel to deliver them,” he noted.
“Both the national and state governments have not provided adequate funding for treatment of individuals with this condition [schizophrenia],” he added.
Dr. Keepers reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Harvey reports no relevant financial relationships. Dr. Correll disclosed ties to Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association has released a new evidence-based practice guideline for the treatment of schizophrenia.
The guideline focuses on assessment and treatment planning, which are integral to patient-centered care, and includes recommendations regarding pharmacotherapy, with particular focus on clozapine, as well as previously recommended and new psychosocial interventions.
“Our intention was to make recommendations to treat the whole person and take into account their family and other significant people in their lives,” George Keepers, MD, chair of the guideline writing group, said in an interview.
‘State-of-the-art methodology’
Dr. Keepers, professor of psychiatry at Oregon Health and Science University, Portland, explained the rigorous process that informs the current guideline, which was “based not solely on expert consensus but was preceded by an evidence-based review of the literature that was then discussed, digested, and distilled into specific recommendations.”
Many current recommendations are “similar to previous recommendations, but there are a few important differences,” he said.
Two experts in schizophrenia who were not involved in guideline authorship praised it for its usefulness and methodology.
Philip D. Harvey, PhD, Leonard M. Miller Professor of Psychiatry and Behavioral Sciences, University of Miami, said in an interview that the guideline “clarified the typical treatment algorithm from first episode to treatment resistance [which is] very clearly laid out for the first time.”
Christoph Correll, MD, professor of psychiatry and molecular medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., said in an interview that the guideline “followed state-of-the-art methodology.”
First steps
The guideline recommends beginning with assessment of the patient and determination of the treatment plan.
Patients should be “treated with an antipsychotic medication and monitored for effectiveness and side effects.” Even after the patient’s symptoms have improved, antipsychotic treatment should continue.
For patients whose symptoms have improved, treatment should continue with the same antipsychotic and should not be switched.
“The problem we’re addressing in this recommendation is that patients are often treated with an effective medication and then forced, by circumstances or their insurance company, to switch to another that may not be effective for them, resulting in unnecessary relapses of the illness,” said Dr. Keepers.
“ and do what’s in the best interest of the patient,” he said.
“The guideline called out that antipsychotics that are effective and tolerated should be continued, without specifying a duration of treatment, thereby indicating indirectly that there is no clear end of the recommendation for ongoing maintenance treatment in individuals with schizophrenia,” said Dr. Correll.
Clozapine underutilized
The guideline highlights the role of clozapine and recommends its use for patients with treatment-resistant schizophrenia and those at risk for suicide. Clozapine is also recommended for patients at “substantial” risk for aggressive behavior, regardless of other treatments.
“Clozapine is underutilized for treatment of schizophrenia in the U.S. and a number of other countries, but it is a really important treatment for patients who don’t respond to other antipsychotic agents,” said Dr. Keepers.
“With this recommendation, we hope that more patients will wind up receiving the medication and benefiting from it,” he added.
In addition, patients should receive treatment with a long-acting injectable antipsychotic “if they prefer such treatment or if they have a history of poor or uncertain adherence” (level of evidence, 2B).
The guideline authors “are recommending long-acting injectable medications for people who want them, not just people with poor prior adherence, which is a critical step,” said Dr. Harvey, director of the division of psychology at the University of Miami.
Managing antipsychotic side effects
The guideline offers recommendations for patients experiencing antipsychotic-induced side effects.
VMAT2s, which represent a “class of drugs that have become available since the last schizophrenia guidelines, are effective in tardive dyskinesia. It is important that patients with tardive dyskinesia have access to these drugs because they do work,” Dr. Keepers said.
Adequate funding needed
Recommended psychosocial interventions include treatment in a specialty care program for patients with schizophrenia who are experiencing a first episode of psychosis, use of cognitive-behavioral therapy for psychosis, psychoeducation, and supported employment services (2B).
“We reviewed very good data showing that patients who receive these services are more likely to be able to be employed and less likely to be rehospitalized or have a relapse,” Dr. Keepers observed.
In addition, patients with schizophrenia should receive assertive community treatment interventions if there is a “history of poor engagement with services leading to frequent relapse or social disruption.”
Family interventions are recommended for patients who have ongoing contact with their families (2B), and patients should also receive interventions “aimed at developing self-management skills and enhancing person-oriented recovery.” They should receive cognitive remediation, social skills training, and supportive psychotherapy.
Dr. Keepers pointed to “major barriers” to providing some of these psychosocial treatments. “They are beyond the scope of someone in an individual private practice situation, so they need to be delivered within the context of treatment programs that are either publicly or privately based,” he said.
“Psychiatrists can and do work closely with community and mental health centers, psychologists, and social workers who can provide these kinds of treatments,” but “many [treatments] require specialized skills and training before they can be offered, and there is a shortage of personnel to deliver them,” he noted.
“Both the national and state governments have not provided adequate funding for treatment of individuals with this condition [schizophrenia],” he added.
Dr. Keepers reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Harvey reports no relevant financial relationships. Dr. Correll disclosed ties to Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has received grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
A version of this article originally appeared on Medscape.com.
Antidepressant use shows gender, racial disparities
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.
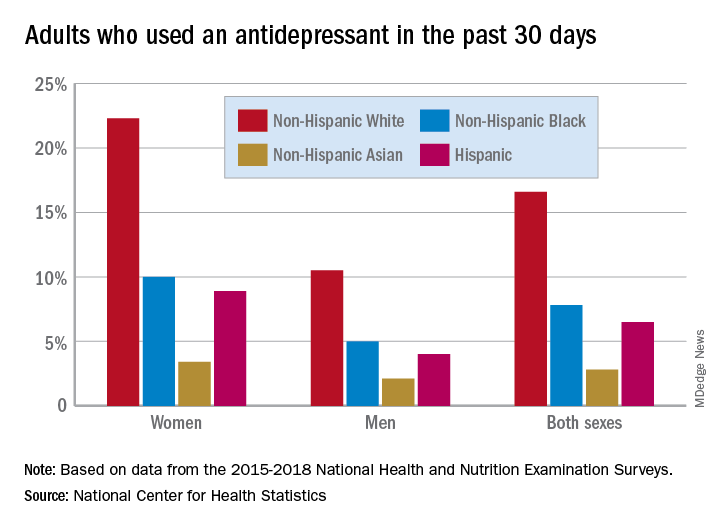
Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.

Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.

Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Asymptomatic children may transmit COVID-19 in communities
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
FROM JAMA PEDIATRICS