User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
No benefit of cannabis on depression in pregnant women with OUD
Cannabis is ineffective at alleviating depression in pregnant women undergoing opioid agonist therapy (OAT), new research shows.
A study of more than 120 pregnant women undergoing treatment of opioid use disorder (OUD) showed that those who used cannabis to alleviate their depressive symptoms while undergoing OAT continued to have high depression scores at the end of opioid treatment.
In addition, depression scores improved for those who abstained from cannabis use after their first positive screen. Interestingly, cannabis use did not affect patient retention in treatment for OUD, the investigators note.
“To our knowledge, this is the first time looking at the impact of cannabis on the specific population of pregnant women with opioid use disorder, who are very vulnerable to depression,” lead author Abigail Richison, MD, University of Arkansas for Medical Sciences, Little Rock, said in an interview.
The findings were presented at the American Academy of Addiction Psychiatry (AAAP) 31st Annual Meeting, which was held online this year because of the COVID-19 pandemic.
A safer alternative?
Data from the National Survey on Drug Use and Health show that perinatal cannabis use increased by 62% between 2002 and 2014. Many women try to ameliorate their depression symptoms by using cannabis in the mistaken belief that it will help their depression, the investigators noted.
In addition, many women consider cannabis safer during pregnancy than prescribed medications for improving mood, said Dr. Richison. She said that cannabis does not alleviate depression and may even worsen it.
Dr. Richison noted that at her center, which has a women’s health program that treats pregnant women with OUDs, she was seeing a lot of patients who reported using cannabis to improve their mood.
“However, it didn’t seem like it was really helping, so I started researching about cannabis and depression,” Dr. Richison said.
“ and can be accused of perinatal substance use. I think it is very important to screen for depression as well as cannabis use in this population,” she added.
To shed some light on the impact of cannabis use by pregnant patients with OUD, the investigators conducted a retrospective chart review of 121 pregnant women with OUD who attended outpatient OAT. All were prescribed buprenorphine.
At each visit, Beck Depression Inventory (BDI) scores were obtained and urine drug screens were administered. The primary outcome was BDI score. Other measures included retention, urinary drug screen results, and antidepressant use.
The women were divided into two groups. The first comprised cannabis users, defined as having more than one urine drug screen that was positive for cannabis (n = 35). The other group comprised nonusers, defined as having urine drug screens that were negative for cannabis (n = 86).
Cannabis users were a little younger (mean age, 27 years) than non–cannabis users (mean age, 29.5 years; P = .006). Most of the participants were White (80.2%). Roughly half were on Medicaid, and most of the other participants had private insurance; a small number of women had no insurance.
Results showed that cannabis users had significantly higher BDI scores than non–cannabis users (mean scores, 16 vs. 9.3; P < .001).
Cannabis use continued to be associated with elevated scores for depression when controlling for opioid misuse and antidepressant use. There were no significant differences in retention or lapse to opioid misuse between the two groups.
More evidence of risk
Commenting on the findings in an interview, Carla Marienfeld, MD, professor of psychiatry at the University of California, San Diego, said there is a growing body of evidence about risks from cannabis use during pregnancy, “a time where we already know the endocannabinoid system is very active in the developing fetus.”
She noted that the current study’s design makes it hard to know whether marijuana use causes worse depression.
However, “it clearly is not associated with helping to improve mood the way people who are using it believe or hope for,” said Dr. Marienfeld, who was not part of the research.
“The risk for harm in terms of worse mood for the pregnant woman or risks for harm to the developing fetus are being better understood with many new studies,” she added.
Yet as more and more states legalize medical marijuana, cannabis use during pregnancy is only going to rise, experts fear.
Cornel Stanciu, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., who was asked for comment, noted that public endorsement for potential benefits of the marijuana plant is at an all-time high.
“To date, 33 states and the District of Columbia have responded by legalizing medical marijuana, with 10 states also having legalized recreational use of marijuana. The current practice is said to be ahead of science, as robust research has been hindered by strict regulations – and most epidemiological studies point toward harmful associations,” Dr. Stanciu said in an interview.
“Given the decreased perception of harm by the general public, women are certainly compelled to seek what they perceive as more natural self-management remedies,” he said.
A harmful habit
Dr. Stanciu cited a recent study conducted in Colorado in which researchers contacted cannabis dispensaries, identified themselves as being pregnant, and asked for guidance in managing pregnancy-related symptoms.
Almost 70% of dispensaries recommended products to treat symptoms, particularly in the vulnerable first trimester; 36% of them also provided reassurance of the safety profile. Very few encouraged a discussion with the physician.
“Consumption of cannabis during pregnancy results in cannabinoid placental crossing and accumulation in the fetal brain, as well as other organs, where it interferes with neurodevelopment and the endocannabinoid system,” he said.
In addition, retrospective studies have shown an association between prenatal cannabis ingestion and anemia in the mothers, low birth weight, greater risk for preterm and stillbirths, and increased need for neonatal ICU admissions.
“Children born to mothers who used cannabis during pregnancy have higher rates of impulsivity, delinquency, learning and memory impairment, as well as executive function deficits. There is also an increased association with proneness to psychosis during middle childhood,” Dr. Stanciu said.
When used during pregnancy, cannabis has been associated with increased anxiety in mothers, as well as increased risk for depressive disorders, incidence of suicidal ideations and behavior, and symptoms of mania and psychosis among those with bipolar and schizophrenia spectrum conditions. Cannabis has also been linked to coingestion of other substances and with alcohol use.
“So cannabis can pose harm, especially when used by those with affective disorders,” Dr. Stanciu said.
The study was funded by the National Institute on Drug Abuse. Dr. Richison, Dr. Marienfeld, and Dr. Stanciu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Cannabis is ineffective at alleviating depression in pregnant women undergoing opioid agonist therapy (OAT), new research shows.
A study of more than 120 pregnant women undergoing treatment of opioid use disorder (OUD) showed that those who used cannabis to alleviate their depressive symptoms while undergoing OAT continued to have high depression scores at the end of opioid treatment.
In addition, depression scores improved for those who abstained from cannabis use after their first positive screen. Interestingly, cannabis use did not affect patient retention in treatment for OUD, the investigators note.
“To our knowledge, this is the first time looking at the impact of cannabis on the specific population of pregnant women with opioid use disorder, who are very vulnerable to depression,” lead author Abigail Richison, MD, University of Arkansas for Medical Sciences, Little Rock, said in an interview.
The findings were presented at the American Academy of Addiction Psychiatry (AAAP) 31st Annual Meeting, which was held online this year because of the COVID-19 pandemic.
A safer alternative?
Data from the National Survey on Drug Use and Health show that perinatal cannabis use increased by 62% between 2002 and 2014. Many women try to ameliorate their depression symptoms by using cannabis in the mistaken belief that it will help their depression, the investigators noted.
In addition, many women consider cannabis safer during pregnancy than prescribed medications for improving mood, said Dr. Richison. She said that cannabis does not alleviate depression and may even worsen it.
Dr. Richison noted that at her center, which has a women’s health program that treats pregnant women with OUDs, she was seeing a lot of patients who reported using cannabis to improve their mood.
“However, it didn’t seem like it was really helping, so I started researching about cannabis and depression,” Dr. Richison said.
“ and can be accused of perinatal substance use. I think it is very important to screen for depression as well as cannabis use in this population,” she added.
To shed some light on the impact of cannabis use by pregnant patients with OUD, the investigators conducted a retrospective chart review of 121 pregnant women with OUD who attended outpatient OAT. All were prescribed buprenorphine.
At each visit, Beck Depression Inventory (BDI) scores were obtained and urine drug screens were administered. The primary outcome was BDI score. Other measures included retention, urinary drug screen results, and antidepressant use.
The women were divided into two groups. The first comprised cannabis users, defined as having more than one urine drug screen that was positive for cannabis (n = 35). The other group comprised nonusers, defined as having urine drug screens that were negative for cannabis (n = 86).
Cannabis users were a little younger (mean age, 27 years) than non–cannabis users (mean age, 29.5 years; P = .006). Most of the participants were White (80.2%). Roughly half were on Medicaid, and most of the other participants had private insurance; a small number of women had no insurance.
Results showed that cannabis users had significantly higher BDI scores than non–cannabis users (mean scores, 16 vs. 9.3; P < .001).
Cannabis use continued to be associated with elevated scores for depression when controlling for opioid misuse and antidepressant use. There were no significant differences in retention or lapse to opioid misuse between the two groups.
More evidence of risk
Commenting on the findings in an interview, Carla Marienfeld, MD, professor of psychiatry at the University of California, San Diego, said there is a growing body of evidence about risks from cannabis use during pregnancy, “a time where we already know the endocannabinoid system is very active in the developing fetus.”
She noted that the current study’s design makes it hard to know whether marijuana use causes worse depression.
However, “it clearly is not associated with helping to improve mood the way people who are using it believe or hope for,” said Dr. Marienfeld, who was not part of the research.
“The risk for harm in terms of worse mood for the pregnant woman or risks for harm to the developing fetus are being better understood with many new studies,” she added.
Yet as more and more states legalize medical marijuana, cannabis use during pregnancy is only going to rise, experts fear.
Cornel Stanciu, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., who was asked for comment, noted that public endorsement for potential benefits of the marijuana plant is at an all-time high.
“To date, 33 states and the District of Columbia have responded by legalizing medical marijuana, with 10 states also having legalized recreational use of marijuana. The current practice is said to be ahead of science, as robust research has been hindered by strict regulations – and most epidemiological studies point toward harmful associations,” Dr. Stanciu said in an interview.
“Given the decreased perception of harm by the general public, women are certainly compelled to seek what they perceive as more natural self-management remedies,” he said.
A harmful habit
Dr. Stanciu cited a recent study conducted in Colorado in which researchers contacted cannabis dispensaries, identified themselves as being pregnant, and asked for guidance in managing pregnancy-related symptoms.
Almost 70% of dispensaries recommended products to treat symptoms, particularly in the vulnerable first trimester; 36% of them also provided reassurance of the safety profile. Very few encouraged a discussion with the physician.
“Consumption of cannabis during pregnancy results in cannabinoid placental crossing and accumulation in the fetal brain, as well as other organs, where it interferes with neurodevelopment and the endocannabinoid system,” he said.
In addition, retrospective studies have shown an association between prenatal cannabis ingestion and anemia in the mothers, low birth weight, greater risk for preterm and stillbirths, and increased need for neonatal ICU admissions.
“Children born to mothers who used cannabis during pregnancy have higher rates of impulsivity, delinquency, learning and memory impairment, as well as executive function deficits. There is also an increased association with proneness to psychosis during middle childhood,” Dr. Stanciu said.
When used during pregnancy, cannabis has been associated with increased anxiety in mothers, as well as increased risk for depressive disorders, incidence of suicidal ideations and behavior, and symptoms of mania and psychosis among those with bipolar and schizophrenia spectrum conditions. Cannabis has also been linked to coingestion of other substances and with alcohol use.
“So cannabis can pose harm, especially when used by those with affective disorders,” Dr. Stanciu said.
The study was funded by the National Institute on Drug Abuse. Dr. Richison, Dr. Marienfeld, and Dr. Stanciu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Cannabis is ineffective at alleviating depression in pregnant women undergoing opioid agonist therapy (OAT), new research shows.
A study of more than 120 pregnant women undergoing treatment of opioid use disorder (OUD) showed that those who used cannabis to alleviate their depressive symptoms while undergoing OAT continued to have high depression scores at the end of opioid treatment.
In addition, depression scores improved for those who abstained from cannabis use after their first positive screen. Interestingly, cannabis use did not affect patient retention in treatment for OUD, the investigators note.
“To our knowledge, this is the first time looking at the impact of cannabis on the specific population of pregnant women with opioid use disorder, who are very vulnerable to depression,” lead author Abigail Richison, MD, University of Arkansas for Medical Sciences, Little Rock, said in an interview.
The findings were presented at the American Academy of Addiction Psychiatry (AAAP) 31st Annual Meeting, which was held online this year because of the COVID-19 pandemic.
A safer alternative?
Data from the National Survey on Drug Use and Health show that perinatal cannabis use increased by 62% between 2002 and 2014. Many women try to ameliorate their depression symptoms by using cannabis in the mistaken belief that it will help their depression, the investigators noted.
In addition, many women consider cannabis safer during pregnancy than prescribed medications for improving mood, said Dr. Richison. She said that cannabis does not alleviate depression and may even worsen it.
Dr. Richison noted that at her center, which has a women’s health program that treats pregnant women with OUDs, she was seeing a lot of patients who reported using cannabis to improve their mood.
“However, it didn’t seem like it was really helping, so I started researching about cannabis and depression,” Dr. Richison said.
“ and can be accused of perinatal substance use. I think it is very important to screen for depression as well as cannabis use in this population,” she added.
To shed some light on the impact of cannabis use by pregnant patients with OUD, the investigators conducted a retrospective chart review of 121 pregnant women with OUD who attended outpatient OAT. All were prescribed buprenorphine.
At each visit, Beck Depression Inventory (BDI) scores were obtained and urine drug screens were administered. The primary outcome was BDI score. Other measures included retention, urinary drug screen results, and antidepressant use.
The women were divided into two groups. The first comprised cannabis users, defined as having more than one urine drug screen that was positive for cannabis (n = 35). The other group comprised nonusers, defined as having urine drug screens that were negative for cannabis (n = 86).
Cannabis users were a little younger (mean age, 27 years) than non–cannabis users (mean age, 29.5 years; P = .006). Most of the participants were White (80.2%). Roughly half were on Medicaid, and most of the other participants had private insurance; a small number of women had no insurance.
Results showed that cannabis users had significantly higher BDI scores than non–cannabis users (mean scores, 16 vs. 9.3; P < .001).
Cannabis use continued to be associated with elevated scores for depression when controlling for opioid misuse and antidepressant use. There were no significant differences in retention or lapse to opioid misuse between the two groups.
More evidence of risk
Commenting on the findings in an interview, Carla Marienfeld, MD, professor of psychiatry at the University of California, San Diego, said there is a growing body of evidence about risks from cannabis use during pregnancy, “a time where we already know the endocannabinoid system is very active in the developing fetus.”
She noted that the current study’s design makes it hard to know whether marijuana use causes worse depression.
However, “it clearly is not associated with helping to improve mood the way people who are using it believe or hope for,” said Dr. Marienfeld, who was not part of the research.
“The risk for harm in terms of worse mood for the pregnant woman or risks for harm to the developing fetus are being better understood with many new studies,” she added.
Yet as more and more states legalize medical marijuana, cannabis use during pregnancy is only going to rise, experts fear.
Cornel Stanciu, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., who was asked for comment, noted that public endorsement for potential benefits of the marijuana plant is at an all-time high.
“To date, 33 states and the District of Columbia have responded by legalizing medical marijuana, with 10 states also having legalized recreational use of marijuana. The current practice is said to be ahead of science, as robust research has been hindered by strict regulations – and most epidemiological studies point toward harmful associations,” Dr. Stanciu said in an interview.
“Given the decreased perception of harm by the general public, women are certainly compelled to seek what they perceive as more natural self-management remedies,” he said.
A harmful habit
Dr. Stanciu cited a recent study conducted in Colorado in which researchers contacted cannabis dispensaries, identified themselves as being pregnant, and asked for guidance in managing pregnancy-related symptoms.
Almost 70% of dispensaries recommended products to treat symptoms, particularly in the vulnerable first trimester; 36% of them also provided reassurance of the safety profile. Very few encouraged a discussion with the physician.
“Consumption of cannabis during pregnancy results in cannabinoid placental crossing and accumulation in the fetal brain, as well as other organs, where it interferes with neurodevelopment and the endocannabinoid system,” he said.
In addition, retrospective studies have shown an association between prenatal cannabis ingestion and anemia in the mothers, low birth weight, greater risk for preterm and stillbirths, and increased need for neonatal ICU admissions.
“Children born to mothers who used cannabis during pregnancy have higher rates of impulsivity, delinquency, learning and memory impairment, as well as executive function deficits. There is also an increased association with proneness to psychosis during middle childhood,” Dr. Stanciu said.
When used during pregnancy, cannabis has been associated with increased anxiety in mothers, as well as increased risk for depressive disorders, incidence of suicidal ideations and behavior, and symptoms of mania and psychosis among those with bipolar and schizophrenia spectrum conditions. Cannabis has also been linked to coingestion of other substances and with alcohol use.
“So cannabis can pose harm, especially when used by those with affective disorders,” Dr. Stanciu said.
The study was funded by the National Institute on Drug Abuse. Dr. Richison, Dr. Marienfeld, and Dr. Stanciu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Give psych patients the COVID vaccination now, experts say
With COVID-19 vaccinations now underway, mental health experts around the world continue to push for patients with serious mental illness (SMI) to be considered a high-priority group for the vaccine.
Research shows that patients with SMI are at increased risk of being infected with SARS-CoV-2 and have higher rates of hospitalization and poor outcomes, Nicola Warren, MBBS, University of Queensland, Brisbane, Australia, and coauthors write in a viewpoint published online Dec. 15 in JAMA Psychiatry
Factors behind the worse outcomes in individuals with SMI include concomitant medications, poorer premorbid general health, physical comorbidity, reduced access to medical care, and environmental and lifestyle factors such as lower socioeconomic status, overcrowding, smoking, and obesity.
“In light of these vulnerabilities, it is important that people with SMI are a priority group to receive a vaccination,” Dr. Warren and colleagues say.
Yet there are challenges at the individual and public health level in getting people with SMI vaccinated against COVID-19, they point out.
Challenges at the individual level include getting people with SMI to recognize the importance of the vaccine and combating negative beliefs about safety and misconceptions that the vaccine itself can make them sick with COVID-19.
Mental health professionals are “uniquely skilled” to deliver vaccine education, “being able to adapt for those with communication difficulties and balance factors influencing decision-making,” Dr. Warren and colleagues write.
, like getting to a vaccination clinic.
Research has shown that running vaccination clinics parallel to mental health services can boost vaccination rates by 25%, the authors note. Therefore, one solution may be to embed vaccination clinics within mental health services, Dr. Warren and colleagues suggest.
Join the chorus
Plans and policies to ensure rapid delivery of the COVID-19 vaccine are “vital,” they conclude. “Mental health clinicians have a key role in advocating for priority access to a COVID-19 vaccination for those with SMI, as well as facilitating its uptake,” they add.
Dr. Warren and her colleagues join a chorus of other mental health care providers who have sounded the alarm on the risks of COVID-19 for patients with SMI and the need to get them vaccinated early.
In a perspective article published last month in World Psychiatry, Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors called for individuals with SMI to have priority status for any COVID-19 vaccine, as reported by this news organization.
Dr. De Hert and colleagues noted that there is an ethical duty to prioritize vaccination for people with SMI given their increased risk of worse outcomes following COVID-19 infection and the structural barriers faced by people with SMI in accessing a vaccine.
Joining the chorus, Benjamin Druss, MD, MPH, from Emory University, Atlanta, Georgia, warned in a JAMA Psychiatry viewpoint in April that the COVID-19 pandemic represents a looming crisis for patients with SMI and the health care systems that serve them.
“Careful planning and execution at multiple levels will be essential for minimizing the adverse outcomes of this pandemic for this vulnerable population,” Dr. Druss wrote.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With COVID-19 vaccinations now underway, mental health experts around the world continue to push for patients with serious mental illness (SMI) to be considered a high-priority group for the vaccine.
Research shows that patients with SMI are at increased risk of being infected with SARS-CoV-2 and have higher rates of hospitalization and poor outcomes, Nicola Warren, MBBS, University of Queensland, Brisbane, Australia, and coauthors write in a viewpoint published online Dec. 15 in JAMA Psychiatry
Factors behind the worse outcomes in individuals with SMI include concomitant medications, poorer premorbid general health, physical comorbidity, reduced access to medical care, and environmental and lifestyle factors such as lower socioeconomic status, overcrowding, smoking, and obesity.
“In light of these vulnerabilities, it is important that people with SMI are a priority group to receive a vaccination,” Dr. Warren and colleagues say.
Yet there are challenges at the individual and public health level in getting people with SMI vaccinated against COVID-19, they point out.
Challenges at the individual level include getting people with SMI to recognize the importance of the vaccine and combating negative beliefs about safety and misconceptions that the vaccine itself can make them sick with COVID-19.
Mental health professionals are “uniquely skilled” to deliver vaccine education, “being able to adapt for those with communication difficulties and balance factors influencing decision-making,” Dr. Warren and colleagues write.
, like getting to a vaccination clinic.
Research has shown that running vaccination clinics parallel to mental health services can boost vaccination rates by 25%, the authors note. Therefore, one solution may be to embed vaccination clinics within mental health services, Dr. Warren and colleagues suggest.
Join the chorus
Plans and policies to ensure rapid delivery of the COVID-19 vaccine are “vital,” they conclude. “Mental health clinicians have a key role in advocating for priority access to a COVID-19 vaccination for those with SMI, as well as facilitating its uptake,” they add.
Dr. Warren and her colleagues join a chorus of other mental health care providers who have sounded the alarm on the risks of COVID-19 for patients with SMI and the need to get them vaccinated early.
In a perspective article published last month in World Psychiatry, Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors called for individuals with SMI to have priority status for any COVID-19 vaccine, as reported by this news organization.
Dr. De Hert and colleagues noted that there is an ethical duty to prioritize vaccination for people with SMI given their increased risk of worse outcomes following COVID-19 infection and the structural barriers faced by people with SMI in accessing a vaccine.
Joining the chorus, Benjamin Druss, MD, MPH, from Emory University, Atlanta, Georgia, warned in a JAMA Psychiatry viewpoint in April that the COVID-19 pandemic represents a looming crisis for patients with SMI and the health care systems that serve them.
“Careful planning and execution at multiple levels will be essential for minimizing the adverse outcomes of this pandemic for this vulnerable population,” Dr. Druss wrote.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With COVID-19 vaccinations now underway, mental health experts around the world continue to push for patients with serious mental illness (SMI) to be considered a high-priority group for the vaccine.
Research shows that patients with SMI are at increased risk of being infected with SARS-CoV-2 and have higher rates of hospitalization and poor outcomes, Nicola Warren, MBBS, University of Queensland, Brisbane, Australia, and coauthors write in a viewpoint published online Dec. 15 in JAMA Psychiatry
Factors behind the worse outcomes in individuals with SMI include concomitant medications, poorer premorbid general health, physical comorbidity, reduced access to medical care, and environmental and lifestyle factors such as lower socioeconomic status, overcrowding, smoking, and obesity.
“In light of these vulnerabilities, it is important that people with SMI are a priority group to receive a vaccination,” Dr. Warren and colleagues say.
Yet there are challenges at the individual and public health level in getting people with SMI vaccinated against COVID-19, they point out.
Challenges at the individual level include getting people with SMI to recognize the importance of the vaccine and combating negative beliefs about safety and misconceptions that the vaccine itself can make them sick with COVID-19.
Mental health professionals are “uniquely skilled” to deliver vaccine education, “being able to adapt for those with communication difficulties and balance factors influencing decision-making,” Dr. Warren and colleagues write.
, like getting to a vaccination clinic.
Research has shown that running vaccination clinics parallel to mental health services can boost vaccination rates by 25%, the authors note. Therefore, one solution may be to embed vaccination clinics within mental health services, Dr. Warren and colleagues suggest.
Join the chorus
Plans and policies to ensure rapid delivery of the COVID-19 vaccine are “vital,” they conclude. “Mental health clinicians have a key role in advocating for priority access to a COVID-19 vaccination for those with SMI, as well as facilitating its uptake,” they add.
Dr. Warren and her colleagues join a chorus of other mental health care providers who have sounded the alarm on the risks of COVID-19 for patients with SMI and the need to get them vaccinated early.
In a perspective article published last month in World Psychiatry, Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors called for individuals with SMI to have priority status for any COVID-19 vaccine, as reported by this news organization.
Dr. De Hert and colleagues noted that there is an ethical duty to prioritize vaccination for people with SMI given their increased risk of worse outcomes following COVID-19 infection and the structural barriers faced by people with SMI in accessing a vaccine.
Joining the chorus, Benjamin Druss, MD, MPH, from Emory University, Atlanta, Georgia, warned in a JAMA Psychiatry viewpoint in April that the COVID-19 pandemic represents a looming crisis for patients with SMI and the health care systems that serve them.
“Careful planning and execution at multiple levels will be essential for minimizing the adverse outcomes of this pandemic for this vulnerable population,” Dr. Druss wrote.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Global experts map the latest in bipolar management
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.
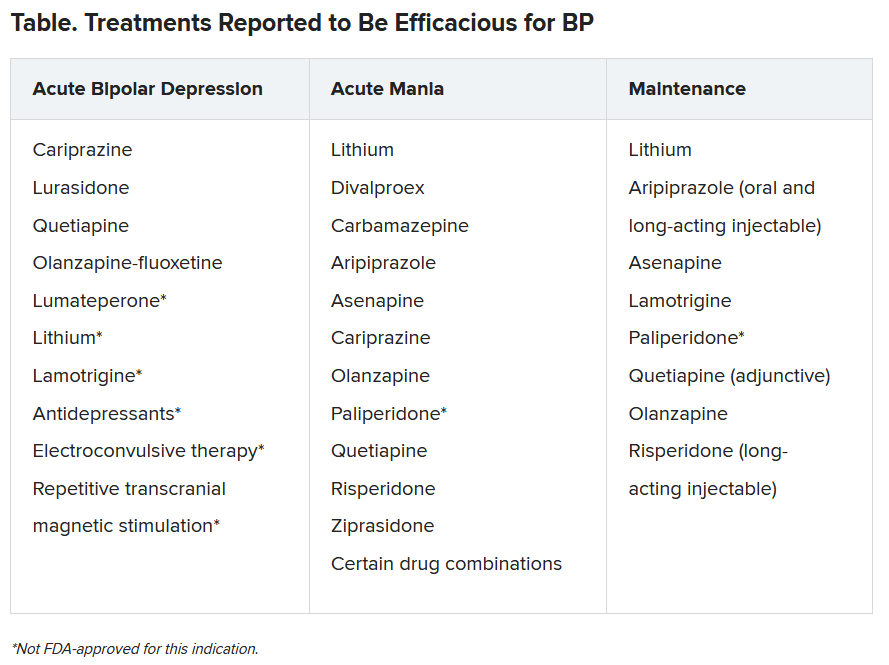
In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.

In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.

In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
COVID-19 variant sparks U.K. travel restrictions
Researchers have detected a highly contagious coronavirus variant in the United Kingdom, leading Prime Minister Boris Johnson to shut down parts of the country and triggering other nations to impose travel and shipping restrictions on England.
Mr. Johnson held a crisis meeting with ministers Monday after Saturday’s shutdown announcement. The prime minister said in a nationally televised address that this coronavirus variant may be “up to 70% more transmissible than the old variant” and was probably responsible for an increase in cases in southeastern England.
“There is still much we don’t know. While we are fairly certain the variant is transmitted more quickly, there is no evidence to suggest that it is more lethal or causes more severe illness. Equally there is no evidence to suggest the vaccine will be any less effective against the new variant,” he said.
Public Health England says it is working to learn as much about the variant as possible. “We know that mortality is a lagging indicator, and we will need to continually monitor this over the coming weeks,” the agency says.
That scientific uncertainty about the variant’s threat shook European nations that were rushing to ship goods to England in advance of a Dec. 31 Brexit deadline. Under Brexit, which is short for “British exit,” the United Kingdom will leave the European Union on Jan. 31, 2020. Until then, the two sides will come up with new trade and security relationships.
European Union members Austria, Belgium, Bulgaria, France, Germany, Ireland, Italy, and the Netherlands announced travel restrictions hours after Johnson’s speech.
Those restrictions created food uncertainty across the U.K., which imports about a quarter of its food from the EU, according to The New York Times. Long lines of trucks heading to ports in the U.K. came to a standstill on major roads such as the M20 near Kent and the Port of Dover.
Outside Europe, Canada, India, Iran, Israel, Hong Kong, Saudi Arabia, and Turkey banned all incoming flights from the U.K. And more bans could come.
The U.S. reaction
The United States has not imposed any new limits on travel with the United Kingdom, although New York Gov. Andrew Cuomo (D) has requested all passengers bound for John F. Kennedy International Airport from the U.K. be tested before boarding and a new travel ban be placed for Europe. He says the federal government must take action now to avoid a crisis situation like the one New York experienced in March and April.
“The United States has a number of flights coming in from the U.K. each day, and we have done absolutely nothing,” Mr. Cuomo said in a statement on the governor’s webpage. “To me, this is reprehensible because this is what happened in the spring. How many times in life do you have to make the same mistake before you learn?”
Leading U.S. health officials have downplayed the dangers of the virus.
“We don’t know that it’s more dangerous, and very importantly, we have not seen a single mutation yet that would make it evade the vaccine,” U.S. Assistant Secretary of Health and Human Services Adm. Brett Giroir, MD, said Sunday on ABC’s This Week with George Stephanopoulos. “I can’t say that won’t happen in the future, but right now it looks like the vaccine will cover everything that we see.”
Dr. Giroir said the HHS and other U.S. government agencies will monitor the variant.
“Viruses mutate,” he said. “We’ve seen almost 4,000 different mutations among this virus. There is no indication that the mutation right now that they’re talking about is overcoming England.”
Where did the variant come from?
Public Health England says the coronavirus variant had existed in the U.K. since September and circulated at very low levels until mid-November.
“The increase in cases linked to the new variant first came to light in late November when PHE was investigating why infection rates in Kent were not falling despite national restrictions. We then discovered a cluster linked to this variant spreading rapidly into London and Essex,” the agency said.
Public Health England says there’s no evidence the new variant is resistant to the Pfizer-BioNTech vaccine, which is now being given across the country to high-priority groups such as health care workers.
An article in The BMJ, a British medical journal, says the variant was first detected by Covid-19 Genomics UK, a consortium that tests the random genetic sequencing of positive COVID-19 samples around the U.K. The variant cases were mostly in the southeast of England.
A University of Birmingham professor said in a Dec. 15 briefing that the variant accounts for 20% of viruses sequenced in Norfolk, 10% in Essex, and 3% in Suffolk. “There are no data to suggest it had been imported from abroad, so it is likely to have evolved in the U.K.,” he said.
The variant is named VUI-202012/01, for the first “variant under investigation” in December 2020, BMJ says. It’s defined by a set of 17 mutations, with the most significant mutation in the spike protein the virus uses to bind to the human ACE2 receptor.
“Changes in this part of spike protein may, in theory, result in the virus becoming more infectious and spreading more easily between people,” the article says.
The European Centre for Disease Prevention and Control says the variant emerged during the time of year when people usually socialize more.
“There is no indication at this point of increased infection severity associated with the new variant,” the agency said. “A few cases with the new variant have to date been reported by Denmark and the Netherlands and, according to media reports, in Belgium.”
Mr. Johnson announced tighter restrictions on England’s hardest-hit areas, such as the southeast and east of England, where new coronavirus cases have continued to rise. And he said people must cut back on their Christmas socializing.
“In England, those living in tier 4 areas should not mix with anyone outside their own household at Christmas, though support bubbles will remain in place for those at particular risk of loneliness or isolation,” he said.
A version of this article first appeared on WebMD.com.
Researchers have detected a highly contagious coronavirus variant in the United Kingdom, leading Prime Minister Boris Johnson to shut down parts of the country and triggering other nations to impose travel and shipping restrictions on England.
Mr. Johnson held a crisis meeting with ministers Monday after Saturday’s shutdown announcement. The prime minister said in a nationally televised address that this coronavirus variant may be “up to 70% more transmissible than the old variant” and was probably responsible for an increase in cases in southeastern England.
“There is still much we don’t know. While we are fairly certain the variant is transmitted more quickly, there is no evidence to suggest that it is more lethal or causes more severe illness. Equally there is no evidence to suggest the vaccine will be any less effective against the new variant,” he said.
Public Health England says it is working to learn as much about the variant as possible. “We know that mortality is a lagging indicator, and we will need to continually monitor this over the coming weeks,” the agency says.
That scientific uncertainty about the variant’s threat shook European nations that were rushing to ship goods to England in advance of a Dec. 31 Brexit deadline. Under Brexit, which is short for “British exit,” the United Kingdom will leave the European Union on Jan. 31, 2020. Until then, the two sides will come up with new trade and security relationships.
European Union members Austria, Belgium, Bulgaria, France, Germany, Ireland, Italy, and the Netherlands announced travel restrictions hours after Johnson’s speech.
Those restrictions created food uncertainty across the U.K., which imports about a quarter of its food from the EU, according to The New York Times. Long lines of trucks heading to ports in the U.K. came to a standstill on major roads such as the M20 near Kent and the Port of Dover.
Outside Europe, Canada, India, Iran, Israel, Hong Kong, Saudi Arabia, and Turkey banned all incoming flights from the U.K. And more bans could come.
The U.S. reaction
The United States has not imposed any new limits on travel with the United Kingdom, although New York Gov. Andrew Cuomo (D) has requested all passengers bound for John F. Kennedy International Airport from the U.K. be tested before boarding and a new travel ban be placed for Europe. He says the federal government must take action now to avoid a crisis situation like the one New York experienced in March and April.
“The United States has a number of flights coming in from the U.K. each day, and we have done absolutely nothing,” Mr. Cuomo said in a statement on the governor’s webpage. “To me, this is reprehensible because this is what happened in the spring. How many times in life do you have to make the same mistake before you learn?”
Leading U.S. health officials have downplayed the dangers of the virus.
“We don’t know that it’s more dangerous, and very importantly, we have not seen a single mutation yet that would make it evade the vaccine,” U.S. Assistant Secretary of Health and Human Services Adm. Brett Giroir, MD, said Sunday on ABC’s This Week with George Stephanopoulos. “I can’t say that won’t happen in the future, but right now it looks like the vaccine will cover everything that we see.”
Dr. Giroir said the HHS and other U.S. government agencies will monitor the variant.
“Viruses mutate,” he said. “We’ve seen almost 4,000 different mutations among this virus. There is no indication that the mutation right now that they’re talking about is overcoming England.”
Where did the variant come from?
Public Health England says the coronavirus variant had existed in the U.K. since September and circulated at very low levels until mid-November.
“The increase in cases linked to the new variant first came to light in late November when PHE was investigating why infection rates in Kent were not falling despite national restrictions. We then discovered a cluster linked to this variant spreading rapidly into London and Essex,” the agency said.
Public Health England says there’s no evidence the new variant is resistant to the Pfizer-BioNTech vaccine, which is now being given across the country to high-priority groups such as health care workers.
An article in The BMJ, a British medical journal, says the variant was first detected by Covid-19 Genomics UK, a consortium that tests the random genetic sequencing of positive COVID-19 samples around the U.K. The variant cases were mostly in the southeast of England.
A University of Birmingham professor said in a Dec. 15 briefing that the variant accounts for 20% of viruses sequenced in Norfolk, 10% in Essex, and 3% in Suffolk. “There are no data to suggest it had been imported from abroad, so it is likely to have evolved in the U.K.,” he said.
The variant is named VUI-202012/01, for the first “variant under investigation” in December 2020, BMJ says. It’s defined by a set of 17 mutations, with the most significant mutation in the spike protein the virus uses to bind to the human ACE2 receptor.
“Changes in this part of spike protein may, in theory, result in the virus becoming more infectious and spreading more easily between people,” the article says.
The European Centre for Disease Prevention and Control says the variant emerged during the time of year when people usually socialize more.
“There is no indication at this point of increased infection severity associated with the new variant,” the agency said. “A few cases with the new variant have to date been reported by Denmark and the Netherlands and, according to media reports, in Belgium.”
Mr. Johnson announced tighter restrictions on England’s hardest-hit areas, such as the southeast and east of England, where new coronavirus cases have continued to rise. And he said people must cut back on their Christmas socializing.
“In England, those living in tier 4 areas should not mix with anyone outside their own household at Christmas, though support bubbles will remain in place for those at particular risk of loneliness or isolation,” he said.
A version of this article first appeared on WebMD.com.
Researchers have detected a highly contagious coronavirus variant in the United Kingdom, leading Prime Minister Boris Johnson to shut down parts of the country and triggering other nations to impose travel and shipping restrictions on England.
Mr. Johnson held a crisis meeting with ministers Monday after Saturday’s shutdown announcement. The prime minister said in a nationally televised address that this coronavirus variant may be “up to 70% more transmissible than the old variant” and was probably responsible for an increase in cases in southeastern England.
“There is still much we don’t know. While we are fairly certain the variant is transmitted more quickly, there is no evidence to suggest that it is more lethal or causes more severe illness. Equally there is no evidence to suggest the vaccine will be any less effective against the new variant,” he said.
Public Health England says it is working to learn as much about the variant as possible. “We know that mortality is a lagging indicator, and we will need to continually monitor this over the coming weeks,” the agency says.
That scientific uncertainty about the variant’s threat shook European nations that were rushing to ship goods to England in advance of a Dec. 31 Brexit deadline. Under Brexit, which is short for “British exit,” the United Kingdom will leave the European Union on Jan. 31, 2020. Until then, the two sides will come up with new trade and security relationships.
European Union members Austria, Belgium, Bulgaria, France, Germany, Ireland, Italy, and the Netherlands announced travel restrictions hours after Johnson’s speech.
Those restrictions created food uncertainty across the U.K., which imports about a quarter of its food from the EU, according to The New York Times. Long lines of trucks heading to ports in the U.K. came to a standstill on major roads such as the M20 near Kent and the Port of Dover.
Outside Europe, Canada, India, Iran, Israel, Hong Kong, Saudi Arabia, and Turkey banned all incoming flights from the U.K. And more bans could come.
The U.S. reaction
The United States has not imposed any new limits on travel with the United Kingdom, although New York Gov. Andrew Cuomo (D) has requested all passengers bound for John F. Kennedy International Airport from the U.K. be tested before boarding and a new travel ban be placed for Europe. He says the federal government must take action now to avoid a crisis situation like the one New York experienced in March and April.
“The United States has a number of flights coming in from the U.K. each day, and we have done absolutely nothing,” Mr. Cuomo said in a statement on the governor’s webpage. “To me, this is reprehensible because this is what happened in the spring. How many times in life do you have to make the same mistake before you learn?”
Leading U.S. health officials have downplayed the dangers of the virus.
“We don’t know that it’s more dangerous, and very importantly, we have not seen a single mutation yet that would make it evade the vaccine,” U.S. Assistant Secretary of Health and Human Services Adm. Brett Giroir, MD, said Sunday on ABC’s This Week with George Stephanopoulos. “I can’t say that won’t happen in the future, but right now it looks like the vaccine will cover everything that we see.”
Dr. Giroir said the HHS and other U.S. government agencies will monitor the variant.
“Viruses mutate,” he said. “We’ve seen almost 4,000 different mutations among this virus. There is no indication that the mutation right now that they’re talking about is overcoming England.”
Where did the variant come from?
Public Health England says the coronavirus variant had existed in the U.K. since September and circulated at very low levels until mid-November.
“The increase in cases linked to the new variant first came to light in late November when PHE was investigating why infection rates in Kent were not falling despite national restrictions. We then discovered a cluster linked to this variant spreading rapidly into London and Essex,” the agency said.
Public Health England says there’s no evidence the new variant is resistant to the Pfizer-BioNTech vaccine, which is now being given across the country to high-priority groups such as health care workers.
An article in The BMJ, a British medical journal, says the variant was first detected by Covid-19 Genomics UK, a consortium that tests the random genetic sequencing of positive COVID-19 samples around the U.K. The variant cases were mostly in the southeast of England.
A University of Birmingham professor said in a Dec. 15 briefing that the variant accounts for 20% of viruses sequenced in Norfolk, 10% in Essex, and 3% in Suffolk. “There are no data to suggest it had been imported from abroad, so it is likely to have evolved in the U.K.,” he said.
The variant is named VUI-202012/01, for the first “variant under investigation” in December 2020, BMJ says. It’s defined by a set of 17 mutations, with the most significant mutation in the spike protein the virus uses to bind to the human ACE2 receptor.
“Changes in this part of spike protein may, in theory, result in the virus becoming more infectious and spreading more easily between people,” the article says.
The European Centre for Disease Prevention and Control says the variant emerged during the time of year when people usually socialize more.
“There is no indication at this point of increased infection severity associated with the new variant,” the agency said. “A few cases with the new variant have to date been reported by Denmark and the Netherlands and, according to media reports, in Belgium.”
Mr. Johnson announced tighter restrictions on England’s hardest-hit areas, such as the southeast and east of England, where new coronavirus cases have continued to rise. And he said people must cut back on their Christmas socializing.
“In England, those living in tier 4 areas should not mix with anyone outside their own household at Christmas, though support bubbles will remain in place for those at particular risk of loneliness or isolation,” he said.
A version of this article first appeared on WebMD.com.
New coalition demands urgent action on COVID-19 mental health crisis
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
COVID-19 ‘far more serious’ than flu, inpatient data confirm
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADHD meds may boost treatment retention in comorbid addiction
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA grants emergency use for Moderna COVID-19 vaccine
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
COVID-19 vaccine found effective but doctors watching for reactions, adverse events
The Pfizer COVID-19 vaccine was shown to be highly effective in a large trial, but clinicians will be waiting and watching for reactions and adverse events in their vaccinated patients.
A two-dose regimen of the BNT162b2 mRNA COVID-19 vaccine was found to be safe and 95% effective in preventing SARS-CoV-2 infection in persons aged 16 years and older, according to an ongoing phase 2/3 trial. Pfizer and BioNTech published safety and efficacy results from the landmark global phase 1/2/3 trial of their COVID-19 vaccine candidate in the New England Journal of Medicine .
“We previously reported phase 1 safety and immunogenicity results from clinical trials of the vaccine candidate BNT162b2,” lead author Fernando P. Polack, MD, of Vanderbilt University, Nashville, Tenn., and colleagues wrote. “This data set and [present] trial results are the basis for an application for emergency-use authorization,” they explained.
The BNT162b2 vaccine trial
Among 43,448 individuals aged 16 years and older, the efficacy, safety, and immunogenicity of the BNT162b2 vaccine candidate was evaluated in a continuous phase 1/2/3 study. Participants were randomly assigned (1:1) to receive two injections of either 30 mcg of BNT162b2 (n = 21,720) or saline placebo (n = 21,728) administered intramuscularly 21 days apart. The safety evaluation, where subjects were monitored 30 minutes post vaccination for acute reactions, was observer blinded.
Eligibility criteria included healthy individuals or those with stable chronic medical conditions, including viral hepatitis B and C, as well as human immunodeficiency virus. Persons with a diagnosis of an immunocompromising condition, those receiving immunosuppressive therapy, and individuals with a medical history of COVID-19 were excluded.
The first primary endpoint was efficacy of BNT162b2 against laboratory-confirmed COVID-19 with onset at least 7 days following the second dose. The primary safety endpoint was local and systemic reactions occurring within 7 days post injection of BNT162b2 or placebo.
Safety
“At the data cutoff date of Oct. 9, a total of 37,706 participants had a median of at least 2 months of safety data available after the second dose and contributed to the main safety data set,” the authors wrote.
Among these participants, 83% were White, 28% were Hispanic or Latinx, and 9% were Black or African American; 49% of subjects were female and the median age was 52 years, with 42% over aged 55 years.
Overall, BNT162b2 had a favorable safety profile. Mild to moderate pain at the injection site within 7 days after the injection was the most frequently reported local reaction (<1% across all age groups reported severe pain). Most local reactions resolved within 1-2 days and no grade 4 reactions were reported.
The investigators reported: “Fever (temperature, ≥38° C) was reported after the second dose by 16% of younger vaccine recipients and by 11% of older recipients. Only 0.2% of vaccine recipients and 0.1% of placebo recipients reported fever (temperature, 38.9-40° C) after the first dose, as compared with 0.8% and 0.1%, respectively, after the second dose.”
BNT162b2 recipients had more injection-site pain than those receiving the placebo. After the first and second doses, younger recipients (under 55 years) had more pain at the injection site (83 vs. 14 and 78 vs. 12 events, respectively), redness (5 vs. 1 and 6 vs. 1), and swelling (6 vs. 0 and 6 vs. 0), compared with placebo recipients.
The same trend was observed for patients aged over 55 years, with vaccine recipients reporting more pain at the injection site (71 vs. 9 and 66 vs. 8 events, respectively), redness (5 vs. 1 and 7 vs. 1), and swelling (7 vs. 1 and 7 vs. 1) than placebo recipients.
Pain was less common overall among vaccine recipients aged over 55 years (71% reported pain after the first dose; 66% post second dose) than among younger vaccine recipients (83% post first dose; 78% post second dose).
The most common systemic events following the second dose were fatigue and headache, which occurred in 59% and 52% of younger vaccine recipients and 51% and 39% of older vaccine recipients, respectively. But fatigue and headache were also reported by participants in the placebo group (23% and 24%, respectively, post second dose, among younger vaccine recipients; 17% and 14% among older recipients).
The incidence of serious adverse events was low and similar in the vaccine (0.6%) and placebo (0.5%) arms. Severe systemic events occurred in 2% or less of vaccine recipients following either dose, except for fatigue (3.8%) and headache (2.0%) post second dose. No deaths were considered to be vaccine or placebo related.
“The safety appears comparable to other vaccines, but the relatively short period of observation, 2 months, and the relatively small number of subjects who have received the vaccine (less than 30,000), compared to the hundreds of millions likely to ultimately receive the vaccine, precludes conclusions regarding the potential for rare long term adverse effects,” David L. Bowton, MD, FCCP, a pulmonologist and professor emeritus of critical care anesthesiology at Wake Forest University, Winston-Salem, N.C., said in an interview. “Clinicians should be aware of the risk of anaphylactic reactions and discuss it with their patients [who have] a history of these reactions.”
Efficacy
Among 36,523 subjects without evidence of existing or prior COVID-19 infection, 8 cases of COVID-19 with onset at least 7 days after the second dose were seen among vaccine recipients and 162 among placebo recipients, corresponding to 95.0% vaccine efficacy (95% credible interval, 90.3%-97.6%).
“Supplemental analyses indicated that vaccine efficacy among subgroups defined by age, sex, race, ethnicity, obesity, and presence of a coexisting condition was generally consistent with that observed in the overall population,” the authors wrote.
Between the first and second doses, 39 cases of COVID-19 were observed among BNT162b2 recipients and 82 cases among placebo recipients, corresponding to 52% vaccine efficacy during the 21-day interval (95% CI, 29.5%-68.4%) suggesting early protection may begin as soon as 12 days after the first injection.
“This is an incredible achievement given that an effective vaccine has never been developed and approved for use in such a short timeframe,” Dr. Bowton explained. “That the vaccine is highly effective in reducing the incidence of symptomatic COVID-19 seems incontrovertible.”
“This vaccine has shockingly amazing efficacy and is well tolerated, and the results are beyond even optimistic projections,” Douglas S. Paauw, MD, of the University of Washington, Seattle, said in an interview.
Questions remain
“It is not yet known if the vaccine prevents asymptomatic infections, with their attendant risk of contagion, as rates of seroconversion of trial participants against betacoronavirus nucleoproteins not included in the vaccine has not been reported,” Dr. Bowton commented.
“Common questions our patients will ask us remain unanswered for now, [including] how long will the protection last, is it safe in pregnant women, and does it prevent asymptomatic infection,” Dr. Paauw explained. “We do not know everything about longer term side effects, but the benefits of this vaccine appear to outweigh the risks of the vaccine.”
The researchers noted these and other limitations in their report, acknowledging that longer follow-up is needed to evaluate long-term safety of the vaccine.
This study was supported by BioNTech and Pfizer. Several authors disclosed financial relationships with Pfizer and other pharmaceutical companies outside the submitted work. Dr. Bowton and Dr. Paauw had no conflicts to disclose.
SOURCE: Polack FP et al. N Engl J Med. 2020 Dec 10. doi: 10.1056/NEJMoa2034577
The Pfizer COVID-19 vaccine was shown to be highly effective in a large trial, but clinicians will be waiting and watching for reactions and adverse events in their vaccinated patients.
A two-dose regimen of the BNT162b2 mRNA COVID-19 vaccine was found to be safe and 95% effective in preventing SARS-CoV-2 infection in persons aged 16 years and older, according to an ongoing phase 2/3 trial. Pfizer and BioNTech published safety and efficacy results from the landmark global phase 1/2/3 trial of their COVID-19 vaccine candidate in the New England Journal of Medicine .
“We previously reported phase 1 safety and immunogenicity results from clinical trials of the vaccine candidate BNT162b2,” lead author Fernando P. Polack, MD, of Vanderbilt University, Nashville, Tenn., and colleagues wrote. “This data set and [present] trial results are the basis for an application for emergency-use authorization,” they explained.
The BNT162b2 vaccine trial
Among 43,448 individuals aged 16 years and older, the efficacy, safety, and immunogenicity of the BNT162b2 vaccine candidate was evaluated in a continuous phase 1/2/3 study. Participants were randomly assigned (1:1) to receive two injections of either 30 mcg of BNT162b2 (n = 21,720) or saline placebo (n = 21,728) administered intramuscularly 21 days apart. The safety evaluation, where subjects were monitored 30 minutes post vaccination for acute reactions, was observer blinded.
Eligibility criteria included healthy individuals or those with stable chronic medical conditions, including viral hepatitis B and C, as well as human immunodeficiency virus. Persons with a diagnosis of an immunocompromising condition, those receiving immunosuppressive therapy, and individuals with a medical history of COVID-19 were excluded.
The first primary endpoint was efficacy of BNT162b2 against laboratory-confirmed COVID-19 with onset at least 7 days following the second dose. The primary safety endpoint was local and systemic reactions occurring within 7 days post injection of BNT162b2 or placebo.
Safety
“At the data cutoff date of Oct. 9, a total of 37,706 participants had a median of at least 2 months of safety data available after the second dose and contributed to the main safety data set,” the authors wrote.
Among these participants, 83% were White, 28% were Hispanic or Latinx, and 9% were Black or African American; 49% of subjects were female and the median age was 52 years, with 42% over aged 55 years.
Overall, BNT162b2 had a favorable safety profile. Mild to moderate pain at the injection site within 7 days after the injection was the most frequently reported local reaction (<1% across all age groups reported severe pain). Most local reactions resolved within 1-2 days and no grade 4 reactions were reported.
The investigators reported: “Fever (temperature, ≥38° C) was reported after the second dose by 16% of younger vaccine recipients and by 11% of older recipients. Only 0.2% of vaccine recipients and 0.1% of placebo recipients reported fever (temperature, 38.9-40° C) after the first dose, as compared with 0.8% and 0.1%, respectively, after the second dose.”
BNT162b2 recipients had more injection-site pain than those receiving the placebo. After the first and second doses, younger recipients (under 55 years) had more pain at the injection site (83 vs. 14 and 78 vs. 12 events, respectively), redness (5 vs. 1 and 6 vs. 1), and swelling (6 vs. 0 and 6 vs. 0), compared with placebo recipients.
The same trend was observed for patients aged over 55 years, with vaccine recipients reporting more pain at the injection site (71 vs. 9 and 66 vs. 8 events, respectively), redness (5 vs. 1 and 7 vs. 1), and swelling (7 vs. 1 and 7 vs. 1) than placebo recipients.
Pain was less common overall among vaccine recipients aged over 55 years (71% reported pain after the first dose; 66% post second dose) than among younger vaccine recipients (83% post first dose; 78% post second dose).
The most common systemic events following the second dose were fatigue and headache, which occurred in 59% and 52% of younger vaccine recipients and 51% and 39% of older vaccine recipients, respectively. But fatigue and headache were also reported by participants in the placebo group (23% and 24%, respectively, post second dose, among younger vaccine recipients; 17% and 14% among older recipients).
The incidence of serious adverse events was low and similar in the vaccine (0.6%) and placebo (0.5%) arms. Severe systemic events occurred in 2% or less of vaccine recipients following either dose, except for fatigue (3.8%) and headache (2.0%) post second dose. No deaths were considered to be vaccine or placebo related.
“The safety appears comparable to other vaccines, but the relatively short period of observation, 2 months, and the relatively small number of subjects who have received the vaccine (less than 30,000), compared to the hundreds of millions likely to ultimately receive the vaccine, precludes conclusions regarding the potential for rare long term adverse effects,” David L. Bowton, MD, FCCP, a pulmonologist and professor emeritus of critical care anesthesiology at Wake Forest University, Winston-Salem, N.C., said in an interview. “Clinicians should be aware of the risk of anaphylactic reactions and discuss it with their patients [who have] a history of these reactions.”
Efficacy
Among 36,523 subjects without evidence of existing or prior COVID-19 infection, 8 cases of COVID-19 with onset at least 7 days after the second dose were seen among vaccine recipients and 162 among placebo recipients, corresponding to 95.0% vaccine efficacy (95% credible interval, 90.3%-97.6%).
“Supplemental analyses indicated that vaccine efficacy among subgroups defined by age, sex, race, ethnicity, obesity, and presence of a coexisting condition was generally consistent with that observed in the overall population,” the authors wrote.
Between the first and second doses, 39 cases of COVID-19 were observed among BNT162b2 recipients and 82 cases among placebo recipients, corresponding to 52% vaccine efficacy during the 21-day interval (95% CI, 29.5%-68.4%) suggesting early protection may begin as soon as 12 days after the first injection.
“This is an incredible achievement given that an effective vaccine has never been developed and approved for use in such a short timeframe,” Dr. Bowton explained. “That the vaccine is highly effective in reducing the incidence of symptomatic COVID-19 seems incontrovertible.”
“This vaccine has shockingly amazing efficacy and is well tolerated, and the results are beyond even optimistic projections,” Douglas S. Paauw, MD, of the University of Washington, Seattle, said in an interview.
Questions remain
“It is not yet known if the vaccine prevents asymptomatic infections, with their attendant risk of contagion, as rates of seroconversion of trial participants against betacoronavirus nucleoproteins not included in the vaccine has not been reported,” Dr. Bowton commented.
“Common questions our patients will ask us remain unanswered for now, [including] how long will the protection last, is it safe in pregnant women, and does it prevent asymptomatic infection,” Dr. Paauw explained. “We do not know everything about longer term side effects, but the benefits of this vaccine appear to outweigh the risks of the vaccine.”
The researchers noted these and other limitations in their report, acknowledging that longer follow-up is needed to evaluate long-term safety of the vaccine.
This study was supported by BioNTech and Pfizer. Several authors disclosed financial relationships with Pfizer and other pharmaceutical companies outside the submitted work. Dr. Bowton and Dr. Paauw had no conflicts to disclose.
SOURCE: Polack FP et al. N Engl J Med. 2020 Dec 10. doi: 10.1056/NEJMoa2034577
The Pfizer COVID-19 vaccine was shown to be highly effective in a large trial, but clinicians will be waiting and watching for reactions and adverse events in their vaccinated patients.
A two-dose regimen of the BNT162b2 mRNA COVID-19 vaccine was found to be safe and 95% effective in preventing SARS-CoV-2 infection in persons aged 16 years and older, according to an ongoing phase 2/3 trial. Pfizer and BioNTech published safety and efficacy results from the landmark global phase 1/2/3 trial of their COVID-19 vaccine candidate in the New England Journal of Medicine .
“We previously reported phase 1 safety and immunogenicity results from clinical trials of the vaccine candidate BNT162b2,” lead author Fernando P. Polack, MD, of Vanderbilt University, Nashville, Tenn., and colleagues wrote. “This data set and [present] trial results are the basis for an application for emergency-use authorization,” they explained.
The BNT162b2 vaccine trial
Among 43,448 individuals aged 16 years and older, the efficacy, safety, and immunogenicity of the BNT162b2 vaccine candidate was evaluated in a continuous phase 1/2/3 study. Participants were randomly assigned (1:1) to receive two injections of either 30 mcg of BNT162b2 (n = 21,720) or saline placebo (n = 21,728) administered intramuscularly 21 days apart. The safety evaluation, where subjects were monitored 30 minutes post vaccination for acute reactions, was observer blinded.
Eligibility criteria included healthy individuals or those with stable chronic medical conditions, including viral hepatitis B and C, as well as human immunodeficiency virus. Persons with a diagnosis of an immunocompromising condition, those receiving immunosuppressive therapy, and individuals with a medical history of COVID-19 were excluded.
The first primary endpoint was efficacy of BNT162b2 against laboratory-confirmed COVID-19 with onset at least 7 days following the second dose. The primary safety endpoint was local and systemic reactions occurring within 7 days post injection of BNT162b2 or placebo.
Safety
“At the data cutoff date of Oct. 9, a total of 37,706 participants had a median of at least 2 months of safety data available after the second dose and contributed to the main safety data set,” the authors wrote.
Among these participants, 83% were White, 28% were Hispanic or Latinx, and 9% were Black or African American; 49% of subjects were female and the median age was 52 years, with 42% over aged 55 years.
Overall, BNT162b2 had a favorable safety profile. Mild to moderate pain at the injection site within 7 days after the injection was the most frequently reported local reaction (<1% across all age groups reported severe pain). Most local reactions resolved within 1-2 days and no grade 4 reactions were reported.
The investigators reported: “Fever (temperature, ≥38° C) was reported after the second dose by 16% of younger vaccine recipients and by 11% of older recipients. Only 0.2% of vaccine recipients and 0.1% of placebo recipients reported fever (temperature, 38.9-40° C) after the first dose, as compared with 0.8% and 0.1%, respectively, after the second dose.”
BNT162b2 recipients had more injection-site pain than those receiving the placebo. After the first and second doses, younger recipients (under 55 years) had more pain at the injection site (83 vs. 14 and 78 vs. 12 events, respectively), redness (5 vs. 1 and 6 vs. 1), and swelling (6 vs. 0 and 6 vs. 0), compared with placebo recipients.
The same trend was observed for patients aged over 55 years, with vaccine recipients reporting more pain at the injection site (71 vs. 9 and 66 vs. 8 events, respectively), redness (5 vs. 1 and 7 vs. 1), and swelling (7 vs. 1 and 7 vs. 1) than placebo recipients.
Pain was less common overall among vaccine recipients aged over 55 years (71% reported pain after the first dose; 66% post second dose) than among younger vaccine recipients (83% post first dose; 78% post second dose).
The most common systemic events following the second dose were fatigue and headache, which occurred in 59% and 52% of younger vaccine recipients and 51% and 39% of older vaccine recipients, respectively. But fatigue and headache were also reported by participants in the placebo group (23% and 24%, respectively, post second dose, among younger vaccine recipients; 17% and 14% among older recipients).
The incidence of serious adverse events was low and similar in the vaccine (0.6%) and placebo (0.5%) arms. Severe systemic events occurred in 2% or less of vaccine recipients following either dose, except for fatigue (3.8%) and headache (2.0%) post second dose. No deaths were considered to be vaccine or placebo related.
“The safety appears comparable to other vaccines, but the relatively short period of observation, 2 months, and the relatively small number of subjects who have received the vaccine (less than 30,000), compared to the hundreds of millions likely to ultimately receive the vaccine, precludes conclusions regarding the potential for rare long term adverse effects,” David L. Bowton, MD, FCCP, a pulmonologist and professor emeritus of critical care anesthesiology at Wake Forest University, Winston-Salem, N.C., said in an interview. “Clinicians should be aware of the risk of anaphylactic reactions and discuss it with their patients [who have] a history of these reactions.”
Efficacy
Among 36,523 subjects without evidence of existing or prior COVID-19 infection, 8 cases of COVID-19 with onset at least 7 days after the second dose were seen among vaccine recipients and 162 among placebo recipients, corresponding to 95.0% vaccine efficacy (95% credible interval, 90.3%-97.6%).
“Supplemental analyses indicated that vaccine efficacy among subgroups defined by age, sex, race, ethnicity, obesity, and presence of a coexisting condition was generally consistent with that observed in the overall population,” the authors wrote.
Between the first and second doses, 39 cases of COVID-19 were observed among BNT162b2 recipients and 82 cases among placebo recipients, corresponding to 52% vaccine efficacy during the 21-day interval (95% CI, 29.5%-68.4%) suggesting early protection may begin as soon as 12 days after the first injection.
“This is an incredible achievement given that an effective vaccine has never been developed and approved for use in such a short timeframe,” Dr. Bowton explained. “That the vaccine is highly effective in reducing the incidence of symptomatic COVID-19 seems incontrovertible.”
“This vaccine has shockingly amazing efficacy and is well tolerated, and the results are beyond even optimistic projections,” Douglas S. Paauw, MD, of the University of Washington, Seattle, said in an interview.
Questions remain
“It is not yet known if the vaccine prevents asymptomatic infections, with their attendant risk of contagion, as rates of seroconversion of trial participants against betacoronavirus nucleoproteins not included in the vaccine has not been reported,” Dr. Bowton commented.
“Common questions our patients will ask us remain unanswered for now, [including] how long will the protection last, is it safe in pregnant women, and does it prevent asymptomatic infection,” Dr. Paauw explained. “We do not know everything about longer term side effects, but the benefits of this vaccine appear to outweigh the risks of the vaccine.”
The researchers noted these and other limitations in their report, acknowledging that longer follow-up is needed to evaluate long-term safety of the vaccine.
This study was supported by BioNTech and Pfizer. Several authors disclosed financial relationships with Pfizer and other pharmaceutical companies outside the submitted work. Dr. Bowton and Dr. Paauw had no conflicts to disclose.
SOURCE: Polack FP et al. N Engl J Med. 2020 Dec 10. doi: 10.1056/NEJMoa2034577
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Should I be afraid of getting COVID again?
Is it over or do I have to brace myself for the possibility of a reinfection? Moreover, could the second time potentially be worse than the first?
I was diagnosed with COVID in March of this year. After spending 10 days in the hospital, and one night in the ICU, it took another 2 months for the air-hunger, headaches, and fatigue to completely resolve. Compared with many other unfortunate victims, I did all right – and I am very grateful for the care I received.
Now, as the surge in cases takes new life, I will be on the front lines taking care of patients. Having had an eventful personal encounter with the virus, I now have a unique vantage point and remain fully committed to paying my fortunate circumstances forward. Although I can’t help but have the same question faced by millions of others: Am I safe now?
It is no surprise that studies have shown health care workers comprising 6% of COVID hospital admissions, with one-third of these admissions being nurses. Recently, we heard that over 900 health care workers at Mayo Clinic had acquired the infection in the first 2 weeks of the ongoing second COVID surge. Are these frontline workers protected? Can they return to work with no fear of a rerun? Or, for that matter, anyone who has been afflicted by COVID – are they now forever immune?
There are no clear answers here. But to understand this a little, let’s quickly revisit some basic principles of immunity.
Innate and adaptive immunity
Simply put, there are two forms of immunity: innate and adaptive. Innate immunity encompasses our body’s natural protective mechanisms that come into play almost immediately. This enables recognition of the virus and activates an immediate antiviral defense and attempt at removal of the infective agent. This, however, does not always do the job. Accordingly, a couple weeks after the initial exposure to the pathogen, adaptive immunity is invoked. Circulating white blood cells within our body recognize the virus and set off an immune response, involving the activation of T and B cells that actively attack the infective agent. It is this T- and B-cell–mediated immunity that should protect one against a second infection with the same agent.
What about herd immunity?
Herd immunity is defined as essentially yielding to the virus and letting it spread naturally in order to develop community-wide immunity. By consequence of a large proportion of the population becoming immune after exposure to the disease, person-to-person spread can potentially be mitigated. This does not confer immunity to the virus at the individual level; rather, it reduces the risk of vulnerable people coming in contact with the pathogen.
Unfortunately, depending on herd immunity as a way to deal with COVID-19 has not worked well, even in well-contained countries like Sweden, where a disproportionate number of their most vulnerable populations have died. It is self-evident that containment strategies with vaccination may be our best way forward to achieve herd immunity. Not surrendering to the virus.
Am I safe from reinfection?
In all honesty, we’re not entirely sure. But it is important to recognize a few points when considering your relative safety.
- The immune system is far from perfect. Not everyone has a robust immune response. And in those who do, the immune response can wane over time, potentially allowing for reinfection. While rare, there have already been some clearly documented reinfections, four that have been confirmed and published; two patients (in Nevada and Ecuador) actually fared worse the second time around.
- The virus can mutate and escape detection by the immune system. One could still be susceptible to reinfection from a different strain. (At least, this remains the case with the influenza virus.) There is some evidence that SARS-CoV-2 does not mutate rapidly, and hence this may not be a problem. But we don’t know for certain, at least as of yet.
- Even a vigorous immune response can be overwhelmed by the virus. It is unclear whether the relative length of time and the amount of virus exposure could undermine a previously primed immune system.
A prior infection and a consequent healthy immunity may help you combat a reinfection but it does not prevent you from harboring or carrying the virus. You may be asymptomatic, but you can still be a carrier and spread the infection. I am a strong advocate for limiting your exposure to others no matter your previous exposure status, in order to limit the spread of the virus.
So, what should I do?
I guess the answer is that you can’t be too careful. Not everyone has had their antibody levels tested, and even if positive, it is unclear how well that affords protection. It is best to presume that you are vulnerable for a reinfection and that you can still carry and spread the virus. This may be the safest approach until we actually achieve herd immunity through vaccination.
Even then, for a period of time, there will remain a sense of uncertainty. So, containment strategies inclusive of distancing and masking will and should remain a way of life at least until mid-2021, when we will be in a better position to reassess the landscape.
The surge is back. As I repay my debt and get back to the front line, I will continue to mask up and practice distancing. I am taking no chances of getting reinfected or being an asymptomatic carrier.
I had COVID, I also have antibodies, and I will be taking the vaccine. I implore you all to do the same.
Jag Singh is a physician, scientist, and professor at Harvard. He is passionate about social issues, leadership, digital health, and medical innovations. You can follow him on Twitter @JagSinghMD.
A version of this article first appeared on Medscape.com.
Is it over or do I have to brace myself for the possibility of a reinfection? Moreover, could the second time potentially be worse than the first?
I was diagnosed with COVID in March of this year. After spending 10 days in the hospital, and one night in the ICU, it took another 2 months for the air-hunger, headaches, and fatigue to completely resolve. Compared with many other unfortunate victims, I did all right – and I am very grateful for the care I received.
Now, as the surge in cases takes new life, I will be on the front lines taking care of patients. Having had an eventful personal encounter with the virus, I now have a unique vantage point and remain fully committed to paying my fortunate circumstances forward. Although I can’t help but have the same question faced by millions of others: Am I safe now?
It is no surprise that studies have shown health care workers comprising 6% of COVID hospital admissions, with one-third of these admissions being nurses. Recently, we heard that over 900 health care workers at Mayo Clinic had acquired the infection in the first 2 weeks of the ongoing second COVID surge. Are these frontline workers protected? Can they return to work with no fear of a rerun? Or, for that matter, anyone who has been afflicted by COVID – are they now forever immune?
There are no clear answers here. But to understand this a little, let’s quickly revisit some basic principles of immunity.
Innate and adaptive immunity
Simply put, there are two forms of immunity: innate and adaptive. Innate immunity encompasses our body’s natural protective mechanisms that come into play almost immediately. This enables recognition of the virus and activates an immediate antiviral defense and attempt at removal of the infective agent. This, however, does not always do the job. Accordingly, a couple weeks after the initial exposure to the pathogen, adaptive immunity is invoked. Circulating white blood cells within our body recognize the virus and set off an immune response, involving the activation of T and B cells that actively attack the infective agent. It is this T- and B-cell–mediated immunity that should protect one against a second infection with the same agent.
What about herd immunity?
Herd immunity is defined as essentially yielding to the virus and letting it spread naturally in order to develop community-wide immunity. By consequence of a large proportion of the population becoming immune after exposure to the disease, person-to-person spread can potentially be mitigated. This does not confer immunity to the virus at the individual level; rather, it reduces the risk of vulnerable people coming in contact with the pathogen.
Unfortunately, depending on herd immunity as a way to deal with COVID-19 has not worked well, even in well-contained countries like Sweden, where a disproportionate number of their most vulnerable populations have died. It is self-evident that containment strategies with vaccination may be our best way forward to achieve herd immunity. Not surrendering to the virus.
Am I safe from reinfection?
In all honesty, we’re not entirely sure. But it is important to recognize a few points when considering your relative safety.
- The immune system is far from perfect. Not everyone has a robust immune response. And in those who do, the immune response can wane over time, potentially allowing for reinfection. While rare, there have already been some clearly documented reinfections, four that have been confirmed and published; two patients (in Nevada and Ecuador) actually fared worse the second time around.
- The virus can mutate and escape detection by the immune system. One could still be susceptible to reinfection from a different strain. (At least, this remains the case with the influenza virus.) There is some evidence that SARS-CoV-2 does not mutate rapidly, and hence this may not be a problem. But we don’t know for certain, at least as of yet.
- Even a vigorous immune response can be overwhelmed by the virus. It is unclear whether the relative length of time and the amount of virus exposure could undermine a previously primed immune system.
A prior infection and a consequent healthy immunity may help you combat a reinfection but it does not prevent you from harboring or carrying the virus. You may be asymptomatic, but you can still be a carrier and spread the infection. I am a strong advocate for limiting your exposure to others no matter your previous exposure status, in order to limit the spread of the virus.
So, what should I do?
I guess the answer is that you can’t be too careful. Not everyone has had their antibody levels tested, and even if positive, it is unclear how well that affords protection. It is best to presume that you are vulnerable for a reinfection and that you can still carry and spread the virus. This may be the safest approach until we actually achieve herd immunity through vaccination.
Even then, for a period of time, there will remain a sense of uncertainty. So, containment strategies inclusive of distancing and masking will and should remain a way of life at least until mid-2021, when we will be in a better position to reassess the landscape.
The surge is back. As I repay my debt and get back to the front line, I will continue to mask up and practice distancing. I am taking no chances of getting reinfected or being an asymptomatic carrier.
I had COVID, I also have antibodies, and I will be taking the vaccine. I implore you all to do the same.
Jag Singh is a physician, scientist, and professor at Harvard. He is passionate about social issues, leadership, digital health, and medical innovations. You can follow him on Twitter @JagSinghMD.
A version of this article first appeared on Medscape.com.
Is it over or do I have to brace myself for the possibility of a reinfection? Moreover, could the second time potentially be worse than the first?
I was diagnosed with COVID in March of this year. After spending 10 days in the hospital, and one night in the ICU, it took another 2 months for the air-hunger, headaches, and fatigue to completely resolve. Compared with many other unfortunate victims, I did all right – and I am very grateful for the care I received.
Now, as the surge in cases takes new life, I will be on the front lines taking care of patients. Having had an eventful personal encounter with the virus, I now have a unique vantage point and remain fully committed to paying my fortunate circumstances forward. Although I can’t help but have the same question faced by millions of others: Am I safe now?
It is no surprise that studies have shown health care workers comprising 6% of COVID hospital admissions, with one-third of these admissions being nurses. Recently, we heard that over 900 health care workers at Mayo Clinic had acquired the infection in the first 2 weeks of the ongoing second COVID surge. Are these frontline workers protected? Can they return to work with no fear of a rerun? Or, for that matter, anyone who has been afflicted by COVID – are they now forever immune?
There are no clear answers here. But to understand this a little, let’s quickly revisit some basic principles of immunity.
Innate and adaptive immunity
Simply put, there are two forms of immunity: innate and adaptive. Innate immunity encompasses our body’s natural protective mechanisms that come into play almost immediately. This enables recognition of the virus and activates an immediate antiviral defense and attempt at removal of the infective agent. This, however, does not always do the job. Accordingly, a couple weeks after the initial exposure to the pathogen, adaptive immunity is invoked. Circulating white blood cells within our body recognize the virus and set off an immune response, involving the activation of T and B cells that actively attack the infective agent. It is this T- and B-cell–mediated immunity that should protect one against a second infection with the same agent.
What about herd immunity?
Herd immunity is defined as essentially yielding to the virus and letting it spread naturally in order to develop community-wide immunity. By consequence of a large proportion of the population becoming immune after exposure to the disease, person-to-person spread can potentially be mitigated. This does not confer immunity to the virus at the individual level; rather, it reduces the risk of vulnerable people coming in contact with the pathogen.
Unfortunately, depending on herd immunity as a way to deal with COVID-19 has not worked well, even in well-contained countries like Sweden, where a disproportionate number of their most vulnerable populations have died. It is self-evident that containment strategies with vaccination may be our best way forward to achieve herd immunity. Not surrendering to the virus.
Am I safe from reinfection?
In all honesty, we’re not entirely sure. But it is important to recognize a few points when considering your relative safety.
- The immune system is far from perfect. Not everyone has a robust immune response. And in those who do, the immune response can wane over time, potentially allowing for reinfection. While rare, there have already been some clearly documented reinfections, four that have been confirmed and published; two patients (in Nevada and Ecuador) actually fared worse the second time around.
- The virus can mutate and escape detection by the immune system. One could still be susceptible to reinfection from a different strain. (At least, this remains the case with the influenza virus.) There is some evidence that SARS-CoV-2 does not mutate rapidly, and hence this may not be a problem. But we don’t know for certain, at least as of yet.
- Even a vigorous immune response can be overwhelmed by the virus. It is unclear whether the relative length of time and the amount of virus exposure could undermine a previously primed immune system.
A prior infection and a consequent healthy immunity may help you combat a reinfection but it does not prevent you from harboring or carrying the virus. You may be asymptomatic, but you can still be a carrier and spread the infection. I am a strong advocate for limiting your exposure to others no matter your previous exposure status, in order to limit the spread of the virus.
So, what should I do?
I guess the answer is that you can’t be too careful. Not everyone has had their antibody levels tested, and even if positive, it is unclear how well that affords protection. It is best to presume that you are vulnerable for a reinfection and that you can still carry and spread the virus. This may be the safest approach until we actually achieve herd immunity through vaccination.
Even then, for a period of time, there will remain a sense of uncertainty. So, containment strategies inclusive of distancing and masking will and should remain a way of life at least until mid-2021, when we will be in a better position to reassess the landscape.
The surge is back. As I repay my debt and get back to the front line, I will continue to mask up and practice distancing. I am taking no chances of getting reinfected or being an asymptomatic carrier.
I had COVID, I also have antibodies, and I will be taking the vaccine. I implore you all to do the same.
Jag Singh is a physician, scientist, and professor at Harvard. He is passionate about social issues, leadership, digital health, and medical innovations. You can follow him on Twitter @JagSinghMD.
A version of this article first appeared on Medscape.com.











