User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
New resilience center targets traumatized health care workers
A physician assistant participating in a virtual workshop began to cry, confessing that she felt overwhelmed with guilt because New Yorkers were hailing her as a frontline hero in the pandemic. That was when Joe Ciavarro knew he was in the right place.
“She was saying all the things I could not verbalize because I, too, didn’t feel like I deserved all this praise and thousands of people cheering for us every evening when people were losing jobs, didn’t have money for food, and their loved ones were dying without family at their side,” says Mr. Ciavarro, a PA at Mount Sinai Medical Center in New York.
Mr. Ciavarro, who also manages 170 other PAs on two of Mount Sinai’s campuses in Manhattan, has been on the front lines since COVID-19 first hit; he lost a colleague and friend to suicide in September.
The mental anguish from his job prompted him to sign up for the resilience workshop offered by Mount Sinai’s Center for Stress, Resilience, and Personal Growth. The center – the first of its kind in North America – was launched in June to help health care workers like him cope with the intense psychological pressures they were facing. The weekly workshops became a safe place where Mr. Ciavarro and other staff members could share their darkest fears and learn ways to help them deal with their situation.
“It’s been grueling but we learned how to take care of ourselves so we can take care of our patients,” said Mr. Ciavarro. “This has become like a guided group therapy session on ways to manage and develop resilience. And I feel like my emotions are validated, knowing that others feel the same way.”
Caring for their own
Medical professionals treating patients with COVID-19 are in similar predicaments, and the psychological fallout is enormous: They’re exhausted by the seemingly never-ending patient load and staffing shortages, and haunted by fears for their own safety and that of their families. Studies in China, Canada, and Italy have revealed that a significant number of doctors and nurses in the early days of the pandemic experienced high levels of distress, depression, anxiety, nightmares, and insomnia.
after witnessing the deaths of so many patients who were alone, without family.
But the resilience workshop that Mr. Ciavarro attended offers some hope and is part of a multifaceted program that aims to be a model for other institutions and communities. The Mount Sinai health system already had some programs in place, including centers for 9/11 responders, for spirituality and health, and a wellness program to aid burned-out doctors. But the leadership at Mount Sinai, which includes psychiatrist Dennis Charney, MD, dean of the medical school and a leading expert on PTSD, knew early in the pandemic that emotional and psychological distress would plague health care workers, according to Deborah Marin, MD, director of the new center.
“We decided to quickly put in place a program that we could do virtually, with workshops and apps, that would give access to several services above and beyond what was already going on,” says Dr. Marin, a professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, who also directs their center for spirituality and health.
The key components include a comprehensive screening tool that helps doctors at the center identify which potential participants are most at risk. Participants build personal inventories that detail the intensity of work-related exposures, personal or family stressors that have arisen because of the pandemic, or any mental health conditions or substance abuse problems that may make staff members more vulnerable.
The weekly workshops led by trained staff are designed to give participants the tools to foster resilience and process their experiences. Online apps provide feedback on their progress and engage them with video and other resources around meditation, relaxation, and resilience techniques.
In addition, all 40,000 members of the Mount Sinai staff are eligible for up to 14 one-on-one sessions with psychologists and psychiatrists who specialize in treating trauma.
“That’s highly unusual – to offer this at no cost to everyone,” said Dr. Marin. “We also have a treatment service that is specifically focused on behavioral health care, so people can learn better coping strategies, and we also have social workers to provide coaching.”
While the center doesn’t have specific numbers on how many nurses, physicians, and other staff have participated in treatment, they have trained over 70 peer leaders for their five workshops that home in on the most important factors of resilience.
“We’ve gotten enthusiastic responses from PAs and nurses,” said Craig Katz, MD, an expert in disaster psychiatry at Mount Sinai and a workshop moderator. Physicians have been slower to get on board. “Doctors are a tough nut to crack – it’s largely a culture where they may burn out but don’t want to talk about it. And asking for help is a hard transition for physicians to make.”
How to protect in midst of trauma
In formulating the program’s platform, Mount Sinai experts drew upon their extensive experience aiding 9/11 responders at the World Trade Center (WTC), as well as their system-wide wellness program that aids demoralized and burned-out physicians. While the reach of the pandemic is much broader than 9/11, experts see some commonalities in conditions that emerge after traumatic events, and they also discovered what can help.
“We learned from our WTC experience about what are protective factors – what are the social supports that buffer against depression, anxiety, and PTSD,” said Jonathan DePierro, PhD, clinical director of CSRPG and a psychologist at the Mount Sinai WTC Mental Health Program. “We also learned that people who have more prolonged exposures are at greater risk of developing mental health difficulties.”
The program itself reflects these lessons – and that’s why it’s open to all employees, not just medical professionals. Housekeepers, security staffers, even construction workers are also dealing with their lives being in danger. “That wasn’t in their job description,” said Dr. DePierro. “These people tend to have fewer social and economic resources, make less money and have fewer structural supports, which makes them even more vulnerable.”
Dr. Charney’s strategies on building resilience became a bible of sorts for the workshops, according to Dr. Katz, who authored the training curriculum. Sessions deal with how to build up reservoirs of realistic optimism, keep gratitude journals, find spiritual meaning in their lives, maintain physical wellness and create networks of social support. The workshops are meant to help participants create action plans, to reach out for support in their social networks, and keep the focus on the positives.
The goal is to give demoralized health care workers a renewed sense of competence. “The resilience workshop is a launching point to get people to show up and talk,” said Dr. Katz. “And if we do that, we’ve accomplished a lot just getting people in the door.”
The center will also have a research component to identify what works and what doesn’t so their platform can provide a template for other institutions; Dr. Marin said they’ve gotten inquiries about the program from major hospital systems in Michigan and California. They’ll also conduct longitudinal research to determine what lingering problems persist among healthcare workers over time.
Since the center opened its virtual doors, the curriculum has also been altered in response to feedback from the support staff, many of whom live in the community that surrounds Mount Sinai in northern Manhattan, which is largely lower-income Latinx and Black individuals. Workshop materials have been translated into Spanish and now feature people who reflect a more diverse set of experiences.
“Many of our employees and the population we serve identify as non-White so we’ve been doing outreach with a lot of the local unions,” said Dr. Marin. “Our next step is to take what we’re doing and work with local community organizations.”
A version of this article first appeared on Medscape.com.
A physician assistant participating in a virtual workshop began to cry, confessing that she felt overwhelmed with guilt because New Yorkers were hailing her as a frontline hero in the pandemic. That was when Joe Ciavarro knew he was in the right place.
“She was saying all the things I could not verbalize because I, too, didn’t feel like I deserved all this praise and thousands of people cheering for us every evening when people were losing jobs, didn’t have money for food, and their loved ones were dying without family at their side,” says Mr. Ciavarro, a PA at Mount Sinai Medical Center in New York.
Mr. Ciavarro, who also manages 170 other PAs on two of Mount Sinai’s campuses in Manhattan, has been on the front lines since COVID-19 first hit; he lost a colleague and friend to suicide in September.
The mental anguish from his job prompted him to sign up for the resilience workshop offered by Mount Sinai’s Center for Stress, Resilience, and Personal Growth. The center – the first of its kind in North America – was launched in June to help health care workers like him cope with the intense psychological pressures they were facing. The weekly workshops became a safe place where Mr. Ciavarro and other staff members could share their darkest fears and learn ways to help them deal with their situation.
“It’s been grueling but we learned how to take care of ourselves so we can take care of our patients,” said Mr. Ciavarro. “This has become like a guided group therapy session on ways to manage and develop resilience. And I feel like my emotions are validated, knowing that others feel the same way.”
Caring for their own
Medical professionals treating patients with COVID-19 are in similar predicaments, and the psychological fallout is enormous: They’re exhausted by the seemingly never-ending patient load and staffing shortages, and haunted by fears for their own safety and that of their families. Studies in China, Canada, and Italy have revealed that a significant number of doctors and nurses in the early days of the pandemic experienced high levels of distress, depression, anxiety, nightmares, and insomnia.
after witnessing the deaths of so many patients who were alone, without family.
But the resilience workshop that Mr. Ciavarro attended offers some hope and is part of a multifaceted program that aims to be a model for other institutions and communities. The Mount Sinai health system already had some programs in place, including centers for 9/11 responders, for spirituality and health, and a wellness program to aid burned-out doctors. But the leadership at Mount Sinai, which includes psychiatrist Dennis Charney, MD, dean of the medical school and a leading expert on PTSD, knew early in the pandemic that emotional and psychological distress would plague health care workers, according to Deborah Marin, MD, director of the new center.
“We decided to quickly put in place a program that we could do virtually, with workshops and apps, that would give access to several services above and beyond what was already going on,” says Dr. Marin, a professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, who also directs their center for spirituality and health.
The key components include a comprehensive screening tool that helps doctors at the center identify which potential participants are most at risk. Participants build personal inventories that detail the intensity of work-related exposures, personal or family stressors that have arisen because of the pandemic, or any mental health conditions or substance abuse problems that may make staff members more vulnerable.
The weekly workshops led by trained staff are designed to give participants the tools to foster resilience and process their experiences. Online apps provide feedback on their progress and engage them with video and other resources around meditation, relaxation, and resilience techniques.
In addition, all 40,000 members of the Mount Sinai staff are eligible for up to 14 one-on-one sessions with psychologists and psychiatrists who specialize in treating trauma.
“That’s highly unusual – to offer this at no cost to everyone,” said Dr. Marin. “We also have a treatment service that is specifically focused on behavioral health care, so people can learn better coping strategies, and we also have social workers to provide coaching.”
While the center doesn’t have specific numbers on how many nurses, physicians, and other staff have participated in treatment, they have trained over 70 peer leaders for their five workshops that home in on the most important factors of resilience.
“We’ve gotten enthusiastic responses from PAs and nurses,” said Craig Katz, MD, an expert in disaster psychiatry at Mount Sinai and a workshop moderator. Physicians have been slower to get on board. “Doctors are a tough nut to crack – it’s largely a culture where they may burn out but don’t want to talk about it. And asking for help is a hard transition for physicians to make.”
How to protect in midst of trauma
In formulating the program’s platform, Mount Sinai experts drew upon their extensive experience aiding 9/11 responders at the World Trade Center (WTC), as well as their system-wide wellness program that aids demoralized and burned-out physicians. While the reach of the pandemic is much broader than 9/11, experts see some commonalities in conditions that emerge after traumatic events, and they also discovered what can help.
“We learned from our WTC experience about what are protective factors – what are the social supports that buffer against depression, anxiety, and PTSD,” said Jonathan DePierro, PhD, clinical director of CSRPG and a psychologist at the Mount Sinai WTC Mental Health Program. “We also learned that people who have more prolonged exposures are at greater risk of developing mental health difficulties.”
The program itself reflects these lessons – and that’s why it’s open to all employees, not just medical professionals. Housekeepers, security staffers, even construction workers are also dealing with their lives being in danger. “That wasn’t in their job description,” said Dr. DePierro. “These people tend to have fewer social and economic resources, make less money and have fewer structural supports, which makes them even more vulnerable.”
Dr. Charney’s strategies on building resilience became a bible of sorts for the workshops, according to Dr. Katz, who authored the training curriculum. Sessions deal with how to build up reservoirs of realistic optimism, keep gratitude journals, find spiritual meaning in their lives, maintain physical wellness and create networks of social support. The workshops are meant to help participants create action plans, to reach out for support in their social networks, and keep the focus on the positives.
The goal is to give demoralized health care workers a renewed sense of competence. “The resilience workshop is a launching point to get people to show up and talk,” said Dr. Katz. “And if we do that, we’ve accomplished a lot just getting people in the door.”
The center will also have a research component to identify what works and what doesn’t so their platform can provide a template for other institutions; Dr. Marin said they’ve gotten inquiries about the program from major hospital systems in Michigan and California. They’ll also conduct longitudinal research to determine what lingering problems persist among healthcare workers over time.
Since the center opened its virtual doors, the curriculum has also been altered in response to feedback from the support staff, many of whom live in the community that surrounds Mount Sinai in northern Manhattan, which is largely lower-income Latinx and Black individuals. Workshop materials have been translated into Spanish and now feature people who reflect a more diverse set of experiences.
“Many of our employees and the population we serve identify as non-White so we’ve been doing outreach with a lot of the local unions,” said Dr. Marin. “Our next step is to take what we’re doing and work with local community organizations.”
A version of this article first appeared on Medscape.com.
A physician assistant participating in a virtual workshop began to cry, confessing that she felt overwhelmed with guilt because New Yorkers were hailing her as a frontline hero in the pandemic. That was when Joe Ciavarro knew he was in the right place.
“She was saying all the things I could not verbalize because I, too, didn’t feel like I deserved all this praise and thousands of people cheering for us every evening when people were losing jobs, didn’t have money for food, and their loved ones were dying without family at their side,” says Mr. Ciavarro, a PA at Mount Sinai Medical Center in New York.
Mr. Ciavarro, who also manages 170 other PAs on two of Mount Sinai’s campuses in Manhattan, has been on the front lines since COVID-19 first hit; he lost a colleague and friend to suicide in September.
The mental anguish from his job prompted him to sign up for the resilience workshop offered by Mount Sinai’s Center for Stress, Resilience, and Personal Growth. The center – the first of its kind in North America – was launched in June to help health care workers like him cope with the intense psychological pressures they were facing. The weekly workshops became a safe place where Mr. Ciavarro and other staff members could share their darkest fears and learn ways to help them deal with their situation.
“It’s been grueling but we learned how to take care of ourselves so we can take care of our patients,” said Mr. Ciavarro. “This has become like a guided group therapy session on ways to manage and develop resilience. And I feel like my emotions are validated, knowing that others feel the same way.”
Caring for their own
Medical professionals treating patients with COVID-19 are in similar predicaments, and the psychological fallout is enormous: They’re exhausted by the seemingly never-ending patient load and staffing shortages, and haunted by fears for their own safety and that of their families. Studies in China, Canada, and Italy have revealed that a significant number of doctors and nurses in the early days of the pandemic experienced high levels of distress, depression, anxiety, nightmares, and insomnia.
after witnessing the deaths of so many patients who were alone, without family.
But the resilience workshop that Mr. Ciavarro attended offers some hope and is part of a multifaceted program that aims to be a model for other institutions and communities. The Mount Sinai health system already had some programs in place, including centers for 9/11 responders, for spirituality and health, and a wellness program to aid burned-out doctors. But the leadership at Mount Sinai, which includes psychiatrist Dennis Charney, MD, dean of the medical school and a leading expert on PTSD, knew early in the pandemic that emotional and psychological distress would plague health care workers, according to Deborah Marin, MD, director of the new center.
“We decided to quickly put in place a program that we could do virtually, with workshops and apps, that would give access to several services above and beyond what was already going on,” says Dr. Marin, a professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, who also directs their center for spirituality and health.
The key components include a comprehensive screening tool that helps doctors at the center identify which potential participants are most at risk. Participants build personal inventories that detail the intensity of work-related exposures, personal or family stressors that have arisen because of the pandemic, or any mental health conditions or substance abuse problems that may make staff members more vulnerable.
The weekly workshops led by trained staff are designed to give participants the tools to foster resilience and process their experiences. Online apps provide feedback on their progress and engage them with video and other resources around meditation, relaxation, and resilience techniques.
In addition, all 40,000 members of the Mount Sinai staff are eligible for up to 14 one-on-one sessions with psychologists and psychiatrists who specialize in treating trauma.
“That’s highly unusual – to offer this at no cost to everyone,” said Dr. Marin. “We also have a treatment service that is specifically focused on behavioral health care, so people can learn better coping strategies, and we also have social workers to provide coaching.”
While the center doesn’t have specific numbers on how many nurses, physicians, and other staff have participated in treatment, they have trained over 70 peer leaders for their five workshops that home in on the most important factors of resilience.
“We’ve gotten enthusiastic responses from PAs and nurses,” said Craig Katz, MD, an expert in disaster psychiatry at Mount Sinai and a workshop moderator. Physicians have been slower to get on board. “Doctors are a tough nut to crack – it’s largely a culture where they may burn out but don’t want to talk about it. And asking for help is a hard transition for physicians to make.”
How to protect in midst of trauma
In formulating the program’s platform, Mount Sinai experts drew upon their extensive experience aiding 9/11 responders at the World Trade Center (WTC), as well as their system-wide wellness program that aids demoralized and burned-out physicians. While the reach of the pandemic is much broader than 9/11, experts see some commonalities in conditions that emerge after traumatic events, and they also discovered what can help.
“We learned from our WTC experience about what are protective factors – what are the social supports that buffer against depression, anxiety, and PTSD,” said Jonathan DePierro, PhD, clinical director of CSRPG and a psychologist at the Mount Sinai WTC Mental Health Program. “We also learned that people who have more prolonged exposures are at greater risk of developing mental health difficulties.”
The program itself reflects these lessons – and that’s why it’s open to all employees, not just medical professionals. Housekeepers, security staffers, even construction workers are also dealing with their lives being in danger. “That wasn’t in their job description,” said Dr. DePierro. “These people tend to have fewer social and economic resources, make less money and have fewer structural supports, which makes them even more vulnerable.”
Dr. Charney’s strategies on building resilience became a bible of sorts for the workshops, according to Dr. Katz, who authored the training curriculum. Sessions deal with how to build up reservoirs of realistic optimism, keep gratitude journals, find spiritual meaning in their lives, maintain physical wellness and create networks of social support. The workshops are meant to help participants create action plans, to reach out for support in their social networks, and keep the focus on the positives.
The goal is to give demoralized health care workers a renewed sense of competence. “The resilience workshop is a launching point to get people to show up and talk,” said Dr. Katz. “And if we do that, we’ve accomplished a lot just getting people in the door.”
The center will also have a research component to identify what works and what doesn’t so their platform can provide a template for other institutions; Dr. Marin said they’ve gotten inquiries about the program from major hospital systems in Michigan and California. They’ll also conduct longitudinal research to determine what lingering problems persist among healthcare workers over time.
Since the center opened its virtual doors, the curriculum has also been altered in response to feedback from the support staff, many of whom live in the community that surrounds Mount Sinai in northern Manhattan, which is largely lower-income Latinx and Black individuals. Workshop materials have been translated into Spanish and now feature people who reflect a more diverse set of experiences.
“Many of our employees and the population we serve identify as non-White so we’ve been doing outreach with a lot of the local unions,” said Dr. Marin. “Our next step is to take what we’re doing and work with local community organizations.”
A version of this article first appeared on Medscape.com.
Intense intervention may boost addiction program retention
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
COVID-19 mortality rates declined, but vary by hospital
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
Shared medical appointments may bridge the opioid treatment gap
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘She’s not a real doctor, she’s a psych doctor’
During a particularly hectic day, Janeni Nayagan, MD, a first-year resident in the department of psychiatry and behavioral health at Cooper University Hospital, Camden, N.J., was taken aback – but not surprised – by a patient’s comment.
In the middle of an emergent situation, she overheard a patient say that Dr. Nayagan wasn’t a “real doctor, she’s a psych doctor.”
“When it happened, I wasn’t particularly angry. It was something I knew I would hear eventually because I’d heard of others experiencing it,” Dr. Nayagan said in an interview. “Psychiatry is one of the fields of medicine that is often questioned in terms of legitimacy, and I knew that when I applied for psych residency,” she said.
Nevertheless, she wrote a post about the incident on Twitter, and she discovered she was far from alone. She posted her original tweet at the start of a night shift and was surprised by the number of responses when she opened her account the next day.
So far, Dr. Nayagan’s initial tweet has garnered 86 replies, 960 likes, and 35 retweets. Some clinicians reported similar experiences from both patients and colleagues, and others offered advice on how to handle such slights.
“There were a lot from people within mental health, but I also received responses from pathologists, radiologists, and others. I didn’t realize how much this experience pervaded through medicine, where a certain specialty would be told: You’re not a real doctor. So it was nice having that support,” Dr. Nayagan said.
Dr. Nayagan noted that psychiatrists encounter “specialty bias” to a greater extent than other medical professionals, and it can be a deterrent for medical students when considering psychiatry as a career path.
“It is something that has been around for a while, but it’s surprising that it’s still prevalent in 2020,” Dr. Nayagan said.
‘Busting the myth’
This type of bias is real, agreed Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences and vice chair for education at the University of Minnesota, Minneapolis, and former director of the psychiatry residency program at the school for 8 years.
She said there is “discrimination” toward psychiatrists, but noted that the problem is improving.
“I think we’re better now than 2 years or 5 years or 10 years ago, so we’re heading in the right direction. But ,” Dr. Nelson said in an interview.
“Psychiatrists have the same robust biomedical training as all specialties. They completed medical school and have this additional specialty and in some cases subspecialty training that is comprehensive of biology, psychology, and social components. We call that using a biopsychosocial model,” she said.
However, she noted that, when talking with students about the field of psychiatry, there’s an awareness “that perhaps we aren’t wearing a white coat” or a stethoscope around the neck.
“That gets translated as, You are giving up medicine – you got this medical training, and you’re not using it. And I have to bust that myth and convey that it really is a privilege to be able to integrate these aspects of knowledge and expertise. It’s in no way giving up medicine – we’re practicing medicine every single day,” said Dr. Nelson.
Remnants of stigma remain
Tristan Gorrindo, MD, deputy medical director and chief of the division of education at the American Psychiatric Association, noted “remnants of stigma” still occur.
“In my mind, it’s really a misunderstanding of the relationship between mental health and physical health,” Dr. Gorrindo said in an interview.
“There’s still this notion that holds over from an old belief that the mind and the body are separate. However, the contemporary thinking in most of modern medicine is that mental illness and physical illness are really one and the same, and they influence each other in a very dynamic way all the time,” he said.
“Psychiatrists stand in both worlds. They’re really the bridge to both the psychiatric and physical aspects,” he added.
Dr. Gorrindo agreed with Dr. Nelson that this understanding has become more prevalent during past few decades.
“Within society, it’s become much more acceptable for people to talk about their mental illness and seek treatment. In a way, shedding daylight on this issue has allowed psychiatry to step forward and demonstrate its value,” he said.
“I think over time we’re going to see that stigma or specialty bias become an anachronism that will fall by the wayside as we see psychiatry more broadly integrated and accepted within the entire house of medicine,” said Dr. Gorrindo.
Taking a toll
Although some responders on Twitter advised Dr. Nayagan and other psychiatrists to “educate with a smile” when faced with specialty discrimination, Dr. Nelson noted that it’s important to recognize that experiencing “microaggressions” takes a toll.
“Anytime you’re given a signal that you aren’t really a physician or you’re not doing a real job, whether it’s based on race, gender, ethnicity, or being a psychiatrist, there is a cost. I’d say, know what you’re doing and hold your head up high, but recognize that there’s a cost for which you may need community and support from colleagues,” she said.
“Together, our culture is changing, and the future is bright. But it’s a little bit of an oversimplification to say, ‘Just brush it off.’ We must recognize that there’s a burden that comes from those forms of exclusion,” Dr. Nelson concluded.
A version of this article first appeared on Medscape.com.
During a particularly hectic day, Janeni Nayagan, MD, a first-year resident in the department of psychiatry and behavioral health at Cooper University Hospital, Camden, N.J., was taken aback – but not surprised – by a patient’s comment.
In the middle of an emergent situation, she overheard a patient say that Dr. Nayagan wasn’t a “real doctor, she’s a psych doctor.”
“When it happened, I wasn’t particularly angry. It was something I knew I would hear eventually because I’d heard of others experiencing it,” Dr. Nayagan said in an interview. “Psychiatry is one of the fields of medicine that is often questioned in terms of legitimacy, and I knew that when I applied for psych residency,” she said.
Nevertheless, she wrote a post about the incident on Twitter, and she discovered she was far from alone. She posted her original tweet at the start of a night shift and was surprised by the number of responses when she opened her account the next day.
So far, Dr. Nayagan’s initial tweet has garnered 86 replies, 960 likes, and 35 retweets. Some clinicians reported similar experiences from both patients and colleagues, and others offered advice on how to handle such slights.
“There were a lot from people within mental health, but I also received responses from pathologists, radiologists, and others. I didn’t realize how much this experience pervaded through medicine, where a certain specialty would be told: You’re not a real doctor. So it was nice having that support,” Dr. Nayagan said.
Dr. Nayagan noted that psychiatrists encounter “specialty bias” to a greater extent than other medical professionals, and it can be a deterrent for medical students when considering psychiatry as a career path.
“It is something that has been around for a while, but it’s surprising that it’s still prevalent in 2020,” Dr. Nayagan said.
‘Busting the myth’
This type of bias is real, agreed Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences and vice chair for education at the University of Minnesota, Minneapolis, and former director of the psychiatry residency program at the school for 8 years.
She said there is “discrimination” toward psychiatrists, but noted that the problem is improving.
“I think we’re better now than 2 years or 5 years or 10 years ago, so we’re heading in the right direction. But ,” Dr. Nelson said in an interview.
“Psychiatrists have the same robust biomedical training as all specialties. They completed medical school and have this additional specialty and in some cases subspecialty training that is comprehensive of biology, psychology, and social components. We call that using a biopsychosocial model,” she said.
However, she noted that, when talking with students about the field of psychiatry, there’s an awareness “that perhaps we aren’t wearing a white coat” or a stethoscope around the neck.
“That gets translated as, You are giving up medicine – you got this medical training, and you’re not using it. And I have to bust that myth and convey that it really is a privilege to be able to integrate these aspects of knowledge and expertise. It’s in no way giving up medicine – we’re practicing medicine every single day,” said Dr. Nelson.
Remnants of stigma remain
Tristan Gorrindo, MD, deputy medical director and chief of the division of education at the American Psychiatric Association, noted “remnants of stigma” still occur.
“In my mind, it’s really a misunderstanding of the relationship between mental health and physical health,” Dr. Gorrindo said in an interview.
“There’s still this notion that holds over from an old belief that the mind and the body are separate. However, the contemporary thinking in most of modern medicine is that mental illness and physical illness are really one and the same, and they influence each other in a very dynamic way all the time,” he said.
“Psychiatrists stand in both worlds. They’re really the bridge to both the psychiatric and physical aspects,” he added.
Dr. Gorrindo agreed with Dr. Nelson that this understanding has become more prevalent during past few decades.
“Within society, it’s become much more acceptable for people to talk about their mental illness and seek treatment. In a way, shedding daylight on this issue has allowed psychiatry to step forward and demonstrate its value,” he said.
“I think over time we’re going to see that stigma or specialty bias become an anachronism that will fall by the wayside as we see psychiatry more broadly integrated and accepted within the entire house of medicine,” said Dr. Gorrindo.
Taking a toll
Although some responders on Twitter advised Dr. Nayagan and other psychiatrists to “educate with a smile” when faced with specialty discrimination, Dr. Nelson noted that it’s important to recognize that experiencing “microaggressions” takes a toll.
“Anytime you’re given a signal that you aren’t really a physician or you’re not doing a real job, whether it’s based on race, gender, ethnicity, or being a psychiatrist, there is a cost. I’d say, know what you’re doing and hold your head up high, but recognize that there’s a cost for which you may need community and support from colleagues,” she said.
“Together, our culture is changing, and the future is bright. But it’s a little bit of an oversimplification to say, ‘Just brush it off.’ We must recognize that there’s a burden that comes from those forms of exclusion,” Dr. Nelson concluded.
A version of this article first appeared on Medscape.com.
During a particularly hectic day, Janeni Nayagan, MD, a first-year resident in the department of psychiatry and behavioral health at Cooper University Hospital, Camden, N.J., was taken aback – but not surprised – by a patient’s comment.
In the middle of an emergent situation, she overheard a patient say that Dr. Nayagan wasn’t a “real doctor, she’s a psych doctor.”
“When it happened, I wasn’t particularly angry. It was something I knew I would hear eventually because I’d heard of others experiencing it,” Dr. Nayagan said in an interview. “Psychiatry is one of the fields of medicine that is often questioned in terms of legitimacy, and I knew that when I applied for psych residency,” she said.
Nevertheless, she wrote a post about the incident on Twitter, and she discovered she was far from alone. She posted her original tweet at the start of a night shift and was surprised by the number of responses when she opened her account the next day.
So far, Dr. Nayagan’s initial tweet has garnered 86 replies, 960 likes, and 35 retweets. Some clinicians reported similar experiences from both patients and colleagues, and others offered advice on how to handle such slights.
“There were a lot from people within mental health, but I also received responses from pathologists, radiologists, and others. I didn’t realize how much this experience pervaded through medicine, where a certain specialty would be told: You’re not a real doctor. So it was nice having that support,” Dr. Nayagan said.
Dr. Nayagan noted that psychiatrists encounter “specialty bias” to a greater extent than other medical professionals, and it can be a deterrent for medical students when considering psychiatry as a career path.
“It is something that has been around for a while, but it’s surprising that it’s still prevalent in 2020,” Dr. Nayagan said.
‘Busting the myth’
This type of bias is real, agreed Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences and vice chair for education at the University of Minnesota, Minneapolis, and former director of the psychiatry residency program at the school for 8 years.
She said there is “discrimination” toward psychiatrists, but noted that the problem is improving.
“I think we’re better now than 2 years or 5 years or 10 years ago, so we’re heading in the right direction. But ,” Dr. Nelson said in an interview.
“Psychiatrists have the same robust biomedical training as all specialties. They completed medical school and have this additional specialty and in some cases subspecialty training that is comprehensive of biology, psychology, and social components. We call that using a biopsychosocial model,” she said.
However, she noted that, when talking with students about the field of psychiatry, there’s an awareness “that perhaps we aren’t wearing a white coat” or a stethoscope around the neck.
“That gets translated as, You are giving up medicine – you got this medical training, and you’re not using it. And I have to bust that myth and convey that it really is a privilege to be able to integrate these aspects of knowledge and expertise. It’s in no way giving up medicine – we’re practicing medicine every single day,” said Dr. Nelson.
Remnants of stigma remain
Tristan Gorrindo, MD, deputy medical director and chief of the division of education at the American Psychiatric Association, noted “remnants of stigma” still occur.
“In my mind, it’s really a misunderstanding of the relationship between mental health and physical health,” Dr. Gorrindo said in an interview.
“There’s still this notion that holds over from an old belief that the mind and the body are separate. However, the contemporary thinking in most of modern medicine is that mental illness and physical illness are really one and the same, and they influence each other in a very dynamic way all the time,” he said.
“Psychiatrists stand in both worlds. They’re really the bridge to both the psychiatric and physical aspects,” he added.
Dr. Gorrindo agreed with Dr. Nelson that this understanding has become more prevalent during past few decades.
“Within society, it’s become much more acceptable for people to talk about their mental illness and seek treatment. In a way, shedding daylight on this issue has allowed psychiatry to step forward and demonstrate its value,” he said.
“I think over time we’re going to see that stigma or specialty bias become an anachronism that will fall by the wayside as we see psychiatry more broadly integrated and accepted within the entire house of medicine,” said Dr. Gorrindo.
Taking a toll
Although some responders on Twitter advised Dr. Nayagan and other psychiatrists to “educate with a smile” when faced with specialty discrimination, Dr. Nelson noted that it’s important to recognize that experiencing “microaggressions” takes a toll.
“Anytime you’re given a signal that you aren’t really a physician or you’re not doing a real job, whether it’s based on race, gender, ethnicity, or being a psychiatrist, there is a cost. I’d say, know what you’re doing and hold your head up high, but recognize that there’s a cost for which you may need community and support from colleagues,” she said.
“Together, our culture is changing, and the future is bright. But it’s a little bit of an oversimplification to say, ‘Just brush it off.’ We must recognize that there’s a burden that comes from those forms of exclusion,” Dr. Nelson concluded.
A version of this article first appeared on Medscape.com.
Moderna’s COVID-19 vaccine deemed ‘highly effective,’ but further studies needed
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
Key clinical point: The FDA’s Vaccines and Related Biological Products Advisory Committee regarded Moderna’s COVID-19 vaccine as highly effective with a favorable safety profile, based on interim phase 3 results.
Major finding: The two-dose vaccine regimen had a low frequency of serious adverse events (1.0% each in the mRNA-1273 and placebo arms, respectively) and demonstrated 94.1% (95% CI, 89.3%-96.8%) vaccine efficacy.
Study details: A briefing document summarized interim data and recommendations from the FDA’s VRBPAC on Moderna’s mRNA-1273 COVID-19 vaccine.
Disclosures: The associated phase 3 study was sponsored by ModernaTX.
Source: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
Child abuse visits to EDs declined in 2020, but not admissions
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.
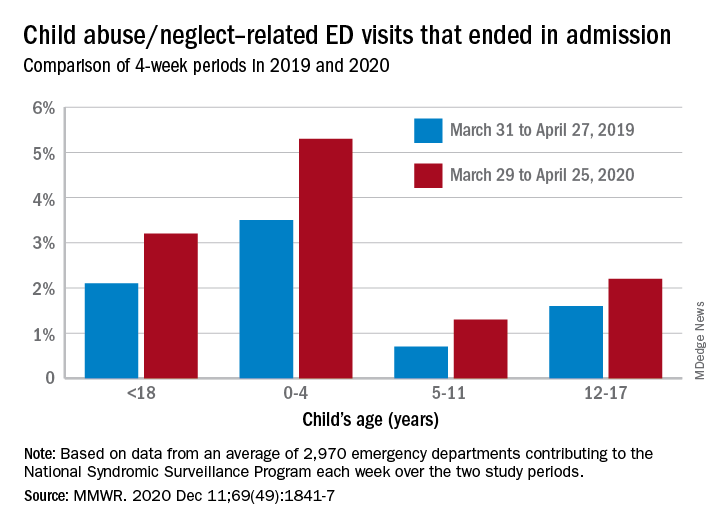
The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.

The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.

The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
FROM MMWR
Call to arms: vaccinating the health workforce of 21 million strong
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
COVID-19 anticoagulation trials ‘paused’ for futility, safety
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Doctors publish paper on COVID-19 protocol; Experts unconvinced
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.