User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Social isolation, loneliness tied to death, MI, stroke: AHA
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Neuropathy drives hypoglycemia cluelessness in T1D
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on researchsquare.com as a preprint that has not yet been peer reviewed.
Key takeaways
- In Japanese adults with type 1 diabetes insulin-pump treatment (continuous subcutaneous insulin infusion) and higher problem-solving perception appear protective against impaired awareness of hypoglycemia (IAH), while diabetic peripheral neuropathy (DPN) is associated with increased risk.
- Diabetes distress and fear of hypoglycemia are common in people with IAH.
Why this matters
- Adults with type 1 diabetes and IAH have a reduced ability to perceive hypoglycemic symptoms and are at risk of severe hypoglycemic events because they are unable to take immediate corrective action.
- This is the first study to identify protective factors and risk factors of IAH in Japanese adults with type 1 diabetes.
- People with IAH may plan to loosen tight glucose management and intentionally omit insulin injection to prevent severe hypoglycemia.
- The information in this report may help improve the management of people with problematic hypoglycemia, the authors suggested. Treatment with an insulin pump and structured education aimed at improving problem-solving skills may be useful interventions for adults with type 1 diabetes and IAH, they suggested.
Study design
- The study involved a cross-sectional analysis of 288 Japanese adults with type 1 diabetes who averaged 50 years old, had diabetes for an average of about 18 years, had an average hemoglobin A1c at baseline of 7.7%, and included about 37% men and 63% women.
- The cohort included 55 people with IAH (19%) and 233 with no impairment of their hypoglycemia awareness, based on their score on the .
Key results
- DPN was significantly more prevalent in the IAH group than in the control group (12.0% vs. 26.5%). A logistic regression analysis showed that the odds ratio for DPN was 2.63-fold higher among people with IAH, compared with those without IAH, but there were no differences in other complications or by HbA1c levels.
- Treatment with continuous subcutaneous insulin therapy (an insulin pump) was significantly less prevalent in the IAH group, compared with those without IAH (23.6% vs 39.5%), with an adjusted odds ratio of 0.48. The two subgroups showed no differences in use of continuous glucose monitoring, used by 56% of the people in each of the two subgroups.
- The two subgroups showed no differences in their healthy lifestyle score, sleep debt, or rates of excessive drinking.
- Mean autonomic symptom scores for both sweating and shaking were significantly reduced in the IAH group, but no between-group differences appeared for palpations or hunger.
- All mean neuroglycopenic symptom scores were significantly lower in those without IAH, including confusion and speech difficulty.
- Scores for measures of diabetes distress and for the worry component of the fear of hypoglycemia were significantly higher in the IAH group, but there were no differences in other psychological measures.
- Higher were significantly associated with decreased IAH risk with a calculated odds ratio of 0.54, but other aspects of hypoglycemia problem-solving such as detection control, goal setting, and strategy evaluation showed no significant links.
Limitations
- The study used a cross-sectional design, which is not suited to making causal inferences.
- The authors characterized DPN as either present or absent. They did not evaluate or analyze the severity of peripheral neuropathy.
- The authors evaluated diabetic cardiac autonomic neuropathy (DCAN) by a person’s coefficient of variation of R-R intervals, and definitive diagnosis of DCAN required at least two positive results on a cardiac autonomic test. More vigorous evaluation using a more definitive assessment of DCAN is needed to relate DCAN and IAH status.
Disclosures
- The study received no commercial funding.
- The authors have disclosed no relevant financial relationships.
This is a summary of a preprint research study, “Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross- sectional analysis of baseline data from the PR-IAH study,” written by researchers at several hospitals in Japan, all affiliated with the National Hospital Organization, on Research Square. The study has not yet been peer reviewed. The full text of the study can be found on researchsquare.com.
A version of this article first appeared on Medscape.com.
Onset and awareness of hypertension varies by race, ethnicity
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.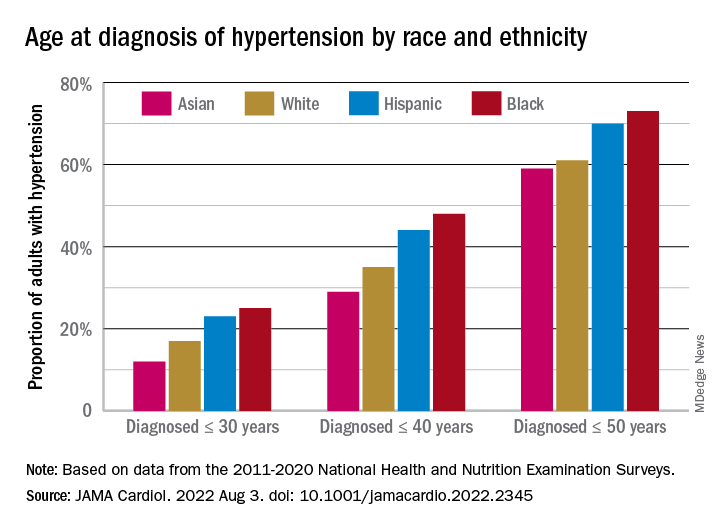
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
FROM JAMA CARDIOLOGY
One in eight COVID patients likely to develop long COVID: Large study
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
‘Staggering’ CVD rise projected in U.S., especially in minorities
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF AMERICAN COLLEGE OF CARDIOLOGY
Why exercise doesn’t help people with long COVID
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
The gut microbes have spoken: All fiber is good fiber
Finding a fiber of good moral fiber
If you’ve ever wandered into the supplement aisle at your local grocery store, you’ve probably noticed an overabundance of fiber supplements that claim to do this for you and benefit that. Since there’s no Food and Drug Administration regulation on fiber supplements, manufacturers are free to (and do) make whatever wild claims they like. And much like choosing which of 500 shows to watch on Netflix, when you’re spoiled for choice, it can be difficult to pick.
Enter a team of molecular geneticists and microbiologists from Duke University. They can’t tell you what show to watch next, but they can tell you which fiber to choose, thanks to their new study. And the answer? Yes.
Well that’s not very helpful, but let us explain. For their study, a group of 28 received three of the main fiber supplements (inulin, dextrin, and galactooligosaccharides) for a week each, followed by a week off of fibers for their gut to return to baseline until they’d received all three. Those who consumed the least fiber at baseline saw the greatest benefit from fiber supplementation, with no appreciable difference between the three types. It was the same story for study participants who already consumed enough fiber; because their guts already hosted a more-optimal microbiome, the type of supplement didn’t matter. The benefits were the same across the board.
In an additional study, the Duke researchers found that gut microbiomes reacted to new fiber within a day, being primed to consume fiber on the first dose and digesting it more quickly on the second fiber dose.
The results, the researchers pointed out, make sense, since the average American only consumes 20%-40% of their daily recommended supply of fiber. Our digestive systems aren’t picky; they just want more, so go out there and choose whatever fiber you’d like. Do that, and then feel free to eat as many double bacon cheeseburgers as you’d like. That is the pinnacle of diet right there. Dietitians literally could not complain about it.
Jarlsberg vs. Camembert: This time it’s skeletal
Fiber is fabulous, of course, but the road to dietary health and wellness fulfillment takes us to many other, equally wondrous places. Hey, look! This next exit is covered with cheese.
All the cheeses are here, from Abbaye de Belloc to Zwitser, and there, right between the jalapeno cheddar and the Jermi tortes you’ll find Jarlsberg, a mild, semisoft, nutty-flavored cheese that comes from Jarlsberg in eastern Norway. A recent study also suggests that Jarlsberg may help to prevent osteopenia and osteoporosis.
A group of Norwegian investigators gathered together 66 healthy women and gave them a daily portion of either Jarlsberg or Camembert for 6 weeks, at which point the Camembert group was switched to Jarlsberg for another 6 weeks.
The research team choose Camembert because of its similarity to Jarlsberg in fat and protein content. Jarlsberg, however, also is rich in vitamin K2, which is important for bone health, and a substance known as DHNA, which “might combat bone thinning and increase bone tissue formation,” they said in a Eurekalert release.
After the first 6 weeks, blood levels of osteocalcin; vitamin K2; and PINP, a peptide involved in bone turnover, were significantly higher in the Jarlsberg group only. All those measures rose significantly after the switch from Camembert to Jarlsberg, while levels of total and LDL cholesterol “fell significantly in the Camembert group after they switched to Jarlsberg,” the team added.
But wait! There’s more! HbA1c fell significantly among those initially eating the Jarlsberg but rose sharply in those eating Camembert. Do you see where this is going? After the Camembert group made the switch to Jarlsberg, their HbA1c levels fell significantly as well.
So it’s not just a cheese thing: The effects are specific to Jarlsberg. Can you guess what we’re having for lunch? Double bacon and fiber Jarlsbergers. Mmm, Jarlsburgers.
Luck be a lady: The mother of twins
It’s widely believed that women who have twins must be more fertile, giving birth to more than one child at a time. Some studies have supported the idea, but more recent work is refuting that claim. In actuality, it might just be more statistics and luck than fertility after all.
Those earlier studies supporting fertility didn’t specify whether the chances of twin births were based on the ability to produce more than one egg at a time or on the number of births that women had overall. Looking at 100,000 preindustrial European births, before contraception was available, researchers from Norway, Germany, France, and the United Kingdom found that the number of total births, twins included, makes all the difference.
“When a woman gives birth several times, the chances increase that at least one of these births will be a twin birth,” investigator Gine Roll Skjærvø of the Norwegian University of Science and Technology said in a written statement.
Since twins occur in 1%-3% of all births, the more births that a woman has, the better her chances of giving birth to twins. The researchers compared it to playing the lottery. You buy enough tickets, eventually your numbers are going to come up. Despite that, however, they found that women who give birth to twins give birth less often than those who don’t have twins. Which raises the idea of sheer luck.
The researchers said that there’s still a lot to uncover in twin births, noting that “uncritically comparing groups of women with and without twins can trick us into believing the opposite of what is really true. These groupings may either hide the effects of twinning and fertility genes where they exist, or vice versa, create the illusion of these if they do not exist.”
For now, this new research claims that it’s basically a lottery. And women who give birth to twins hit the jackpot.
Those with low wages may be earning future memory loss
Not only are low wages detrimental to our souls, hopes, and dreams, but a new study shows that low wages also are linked to quicker memory decline later in life. Sustained low wages not only cause stress and food insecurity in the lives of many, but they also can cause diseases such as depression, obesity, and high blood pressure, which are risk factors for cognitive aging.
The study was conducted using records from the Health and Retirement Study for the years 1992-2016 and focused on 2,879 adults born between 1936 and 1941. The participants were divided into three groups: those who never earned low wages, those who sometimes did, and those who always did.
The investigators found that workers who earned sustained low wages – defined as an hourly wage lower than two-thirds of the federal median wage for the corresponding year – “experienced significantly faster memory decline in older age” than did those who never earned low wages.
There are signs of inflation everywhere we look these days, but many people are not earning higher wages to compensate for the extra expenses. “Increasing the federal minimum wage, for example to $15 per hour, remains a gridlock issue in Congress,” lead author Katrina Kezios of the Columbia University Mailman School of Public Health, said in a statement released by the university.
If only salaries would rise instead of prices for once.
Finding a fiber of good moral fiber
If you’ve ever wandered into the supplement aisle at your local grocery store, you’ve probably noticed an overabundance of fiber supplements that claim to do this for you and benefit that. Since there’s no Food and Drug Administration regulation on fiber supplements, manufacturers are free to (and do) make whatever wild claims they like. And much like choosing which of 500 shows to watch on Netflix, when you’re spoiled for choice, it can be difficult to pick.
Enter a team of molecular geneticists and microbiologists from Duke University. They can’t tell you what show to watch next, but they can tell you which fiber to choose, thanks to their new study. And the answer? Yes.
Well that’s not very helpful, but let us explain. For their study, a group of 28 received three of the main fiber supplements (inulin, dextrin, and galactooligosaccharides) for a week each, followed by a week off of fibers for their gut to return to baseline until they’d received all three. Those who consumed the least fiber at baseline saw the greatest benefit from fiber supplementation, with no appreciable difference between the three types. It was the same story for study participants who already consumed enough fiber; because their guts already hosted a more-optimal microbiome, the type of supplement didn’t matter. The benefits were the same across the board.
In an additional study, the Duke researchers found that gut microbiomes reacted to new fiber within a day, being primed to consume fiber on the first dose and digesting it more quickly on the second fiber dose.
The results, the researchers pointed out, make sense, since the average American only consumes 20%-40% of their daily recommended supply of fiber. Our digestive systems aren’t picky; they just want more, so go out there and choose whatever fiber you’d like. Do that, and then feel free to eat as many double bacon cheeseburgers as you’d like. That is the pinnacle of diet right there. Dietitians literally could not complain about it.
Jarlsberg vs. Camembert: This time it’s skeletal
Fiber is fabulous, of course, but the road to dietary health and wellness fulfillment takes us to many other, equally wondrous places. Hey, look! This next exit is covered with cheese.
All the cheeses are here, from Abbaye de Belloc to Zwitser, and there, right between the jalapeno cheddar and the Jermi tortes you’ll find Jarlsberg, a mild, semisoft, nutty-flavored cheese that comes from Jarlsberg in eastern Norway. A recent study also suggests that Jarlsberg may help to prevent osteopenia and osteoporosis.
A group of Norwegian investigators gathered together 66 healthy women and gave them a daily portion of either Jarlsberg or Camembert for 6 weeks, at which point the Camembert group was switched to Jarlsberg for another 6 weeks.
The research team choose Camembert because of its similarity to Jarlsberg in fat and protein content. Jarlsberg, however, also is rich in vitamin K2, which is important for bone health, and a substance known as DHNA, which “might combat bone thinning and increase bone tissue formation,” they said in a Eurekalert release.
After the first 6 weeks, blood levels of osteocalcin; vitamin K2; and PINP, a peptide involved in bone turnover, were significantly higher in the Jarlsberg group only. All those measures rose significantly after the switch from Camembert to Jarlsberg, while levels of total and LDL cholesterol “fell significantly in the Camembert group after they switched to Jarlsberg,” the team added.
But wait! There’s more! HbA1c fell significantly among those initially eating the Jarlsberg but rose sharply in those eating Camembert. Do you see where this is going? After the Camembert group made the switch to Jarlsberg, their HbA1c levels fell significantly as well.
So it’s not just a cheese thing: The effects are specific to Jarlsberg. Can you guess what we’re having for lunch? Double bacon and fiber Jarlsbergers. Mmm, Jarlsburgers.
Luck be a lady: The mother of twins
It’s widely believed that women who have twins must be more fertile, giving birth to more than one child at a time. Some studies have supported the idea, but more recent work is refuting that claim. In actuality, it might just be more statistics and luck than fertility after all.
Those earlier studies supporting fertility didn’t specify whether the chances of twin births were based on the ability to produce more than one egg at a time or on the number of births that women had overall. Looking at 100,000 preindustrial European births, before contraception was available, researchers from Norway, Germany, France, and the United Kingdom found that the number of total births, twins included, makes all the difference.
“When a woman gives birth several times, the chances increase that at least one of these births will be a twin birth,” investigator Gine Roll Skjærvø of the Norwegian University of Science and Technology said in a written statement.
Since twins occur in 1%-3% of all births, the more births that a woman has, the better her chances of giving birth to twins. The researchers compared it to playing the lottery. You buy enough tickets, eventually your numbers are going to come up. Despite that, however, they found that women who give birth to twins give birth less often than those who don’t have twins. Which raises the idea of sheer luck.
The researchers said that there’s still a lot to uncover in twin births, noting that “uncritically comparing groups of women with and without twins can trick us into believing the opposite of what is really true. These groupings may either hide the effects of twinning and fertility genes where they exist, or vice versa, create the illusion of these if they do not exist.”
For now, this new research claims that it’s basically a lottery. And women who give birth to twins hit the jackpot.
Those with low wages may be earning future memory loss
Not only are low wages detrimental to our souls, hopes, and dreams, but a new study shows that low wages also are linked to quicker memory decline later in life. Sustained low wages not only cause stress and food insecurity in the lives of many, but they also can cause diseases such as depression, obesity, and high blood pressure, which are risk factors for cognitive aging.
The study was conducted using records from the Health and Retirement Study for the years 1992-2016 and focused on 2,879 adults born between 1936 and 1941. The participants were divided into three groups: those who never earned low wages, those who sometimes did, and those who always did.
The investigators found that workers who earned sustained low wages – defined as an hourly wage lower than two-thirds of the federal median wage for the corresponding year – “experienced significantly faster memory decline in older age” than did those who never earned low wages.
There are signs of inflation everywhere we look these days, but many people are not earning higher wages to compensate for the extra expenses. “Increasing the federal minimum wage, for example to $15 per hour, remains a gridlock issue in Congress,” lead author Katrina Kezios of the Columbia University Mailman School of Public Health, said in a statement released by the university.
If only salaries would rise instead of prices for once.
Finding a fiber of good moral fiber
If you’ve ever wandered into the supplement aisle at your local grocery store, you’ve probably noticed an overabundance of fiber supplements that claim to do this for you and benefit that. Since there’s no Food and Drug Administration regulation on fiber supplements, manufacturers are free to (and do) make whatever wild claims they like. And much like choosing which of 500 shows to watch on Netflix, when you’re spoiled for choice, it can be difficult to pick.
Enter a team of molecular geneticists and microbiologists from Duke University. They can’t tell you what show to watch next, but they can tell you which fiber to choose, thanks to their new study. And the answer? Yes.
Well that’s not very helpful, but let us explain. For their study, a group of 28 received three of the main fiber supplements (inulin, dextrin, and galactooligosaccharides) for a week each, followed by a week off of fibers for their gut to return to baseline until they’d received all three. Those who consumed the least fiber at baseline saw the greatest benefit from fiber supplementation, with no appreciable difference between the three types. It was the same story for study participants who already consumed enough fiber; because their guts already hosted a more-optimal microbiome, the type of supplement didn’t matter. The benefits were the same across the board.
In an additional study, the Duke researchers found that gut microbiomes reacted to new fiber within a day, being primed to consume fiber on the first dose and digesting it more quickly on the second fiber dose.
The results, the researchers pointed out, make sense, since the average American only consumes 20%-40% of their daily recommended supply of fiber. Our digestive systems aren’t picky; they just want more, so go out there and choose whatever fiber you’d like. Do that, and then feel free to eat as many double bacon cheeseburgers as you’d like. That is the pinnacle of diet right there. Dietitians literally could not complain about it.
Jarlsberg vs. Camembert: This time it’s skeletal
Fiber is fabulous, of course, but the road to dietary health and wellness fulfillment takes us to many other, equally wondrous places. Hey, look! This next exit is covered with cheese.
All the cheeses are here, from Abbaye de Belloc to Zwitser, and there, right between the jalapeno cheddar and the Jermi tortes you’ll find Jarlsberg, a mild, semisoft, nutty-flavored cheese that comes from Jarlsberg in eastern Norway. A recent study also suggests that Jarlsberg may help to prevent osteopenia and osteoporosis.
A group of Norwegian investigators gathered together 66 healthy women and gave them a daily portion of either Jarlsberg or Camembert for 6 weeks, at which point the Camembert group was switched to Jarlsberg for another 6 weeks.
The research team choose Camembert because of its similarity to Jarlsberg in fat and protein content. Jarlsberg, however, also is rich in vitamin K2, which is important for bone health, and a substance known as DHNA, which “might combat bone thinning and increase bone tissue formation,” they said in a Eurekalert release.
After the first 6 weeks, blood levels of osteocalcin; vitamin K2; and PINP, a peptide involved in bone turnover, were significantly higher in the Jarlsberg group only. All those measures rose significantly after the switch from Camembert to Jarlsberg, while levels of total and LDL cholesterol “fell significantly in the Camembert group after they switched to Jarlsberg,” the team added.
But wait! There’s more! HbA1c fell significantly among those initially eating the Jarlsberg but rose sharply in those eating Camembert. Do you see where this is going? After the Camembert group made the switch to Jarlsberg, their HbA1c levels fell significantly as well.
So it’s not just a cheese thing: The effects are specific to Jarlsberg. Can you guess what we’re having for lunch? Double bacon and fiber Jarlsbergers. Mmm, Jarlsburgers.
Luck be a lady: The mother of twins
It’s widely believed that women who have twins must be more fertile, giving birth to more than one child at a time. Some studies have supported the idea, but more recent work is refuting that claim. In actuality, it might just be more statistics and luck than fertility after all.
Those earlier studies supporting fertility didn’t specify whether the chances of twin births were based on the ability to produce more than one egg at a time or on the number of births that women had overall. Looking at 100,000 preindustrial European births, before contraception was available, researchers from Norway, Germany, France, and the United Kingdom found that the number of total births, twins included, makes all the difference.
“When a woman gives birth several times, the chances increase that at least one of these births will be a twin birth,” investigator Gine Roll Skjærvø of the Norwegian University of Science and Technology said in a written statement.
Since twins occur in 1%-3% of all births, the more births that a woman has, the better her chances of giving birth to twins. The researchers compared it to playing the lottery. You buy enough tickets, eventually your numbers are going to come up. Despite that, however, they found that women who give birth to twins give birth less often than those who don’t have twins. Which raises the idea of sheer luck.
The researchers said that there’s still a lot to uncover in twin births, noting that “uncritically comparing groups of women with and without twins can trick us into believing the opposite of what is really true. These groupings may either hide the effects of twinning and fertility genes where they exist, or vice versa, create the illusion of these if they do not exist.”
For now, this new research claims that it’s basically a lottery. And women who give birth to twins hit the jackpot.
Those with low wages may be earning future memory loss
Not only are low wages detrimental to our souls, hopes, and dreams, but a new study shows that low wages also are linked to quicker memory decline later in life. Sustained low wages not only cause stress and food insecurity in the lives of many, but they also can cause diseases such as depression, obesity, and high blood pressure, which are risk factors for cognitive aging.
The study was conducted using records from the Health and Retirement Study for the years 1992-2016 and focused on 2,879 adults born between 1936 and 1941. The participants were divided into three groups: those who never earned low wages, those who sometimes did, and those who always did.
The investigators found that workers who earned sustained low wages – defined as an hourly wage lower than two-thirds of the federal median wage for the corresponding year – “experienced significantly faster memory decline in older age” than did those who never earned low wages.
There are signs of inflation everywhere we look these days, but many people are not earning higher wages to compensate for the extra expenses. “Increasing the federal minimum wage, for example to $15 per hour, remains a gridlock issue in Congress,” lead author Katrina Kezios of the Columbia University Mailman School of Public Health, said in a statement released by the university.
If only salaries would rise instead of prices for once.
Haven’t had COVID yet? Wanna bet?
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
Long COVID comes in three forms: Study
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
Burnout and stress of today: How do we cope?
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.











