User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Adaptive treatment aids smoking cessation
Smokers who followed an adaptive treatment regimen with drug patches had greater smoking abstinence after 12 weeks than did those who followed a standard regimen, based on data from 188 individuals.
Adaptive pharmacotherapy is a common strategy across many medical conditions, but its use in smoking cessation treatments involving skin patches has not been examined, wrote James M. Davis, MD, of Duke University, Durham, N.C., and colleagues.
In a study published in JAMA Network Open, the researchers reviewed data from 188 adults who sought smoking cessation treatment at a university health system between February 2018 and May 2020. The researchers planned to enroll 300 adults, but enrollment was truncated because of the COVID-19 pandemic.
Participants chose between varenicline or nicotine patches, and then were randomized to an adaptive or standard treatment regimen. All participants started their medication 4 weeks before their target quit smoking day.
A total of 127 participants chose varenicline, with 64 randomized to adaptive treatment and 63 randomized to standard treatment; 61 participants chose nicotine patches, with 31 randomized to adaptive treatment and 30 randomized to standard treatment. Overall, participants smoked a mean of 15.4 cigarettes per day at baseline. The mean age of the participants was 49.1 years; 54% were female, 52% were White, and 48% were Black. Baseline demographics were similar between the groups.
The primary outcome was 30-day continuous abstinence from smoking (biochemically verified) at 12 weeks after each participant’s target quit date.
After 2 weeks (2 weeks before the target quit smoking day), all participants were assessed for treatment response. Those in the adaptive group who were deemed responders, defined as a reduction in daily cigarettes of at least 50%, received placebo bupropion. Those in the adaptive group deemed nonresponders received 150 mg bupropion twice daily in addition to their patch regimen. The standard treatment group also received placebo bupropion.
At 12 weeks after the target quit day, 24% of the adaptive group demonstrated 30-day continuous smoking abstinence, compared with 9% of the standard group (odds ratio, 3.38; P = .004). Smoking abstinence was higher in the adaptive vs. placebo groups for those who used varenicline patches (28% vs. 8%; OR, 4.54) and for those who used nicotine patches (16% vs. 10%; OR, 1.73).
In addition, 7-day smoking abstinence measured at a 2-week postquit day visit was three times higher in the adaptive group compared with the standard treatment group (32% vs. 11%; OR, 3.30).
No incidents of death, life-threatening events, hospitalization, or persistent or significant disability or incapacity related to the study were reported; one death in the varenicline group was attributable to stage 4 cancer.
The findings were limited by several factors including the few or no participants of Alaska Native, American Indian, Hispanic, or Pacific Islander ethnicities, or those who were multiracial. The free medication and modest compensation for study visits further reduce generalizability, the researchers noted. Other limitations included the smaller-than-intended sample size and inability to assess individual components of adaptive treatment, they said.
However, the results support the value of adaptive treatment and suggest that adaptive treatment with precessation varenicline or nicotine patches followed by bupropion for nonresponders is more effective than standard treatment for smoking cessation.
The study was supported by the National Institute on Drug Abuse; the varenicline was provided by Pfizer. Dr. Davis had no financial conflicts to disclose.
Smokers who followed an adaptive treatment regimen with drug patches had greater smoking abstinence after 12 weeks than did those who followed a standard regimen, based on data from 188 individuals.
Adaptive pharmacotherapy is a common strategy across many medical conditions, but its use in smoking cessation treatments involving skin patches has not been examined, wrote James M. Davis, MD, of Duke University, Durham, N.C., and colleagues.
In a study published in JAMA Network Open, the researchers reviewed data from 188 adults who sought smoking cessation treatment at a university health system between February 2018 and May 2020. The researchers planned to enroll 300 adults, but enrollment was truncated because of the COVID-19 pandemic.
Participants chose between varenicline or nicotine patches, and then were randomized to an adaptive or standard treatment regimen. All participants started their medication 4 weeks before their target quit smoking day.
A total of 127 participants chose varenicline, with 64 randomized to adaptive treatment and 63 randomized to standard treatment; 61 participants chose nicotine patches, with 31 randomized to adaptive treatment and 30 randomized to standard treatment. Overall, participants smoked a mean of 15.4 cigarettes per day at baseline. The mean age of the participants was 49.1 years; 54% were female, 52% were White, and 48% were Black. Baseline demographics were similar between the groups.
The primary outcome was 30-day continuous abstinence from smoking (biochemically verified) at 12 weeks after each participant’s target quit date.
After 2 weeks (2 weeks before the target quit smoking day), all participants were assessed for treatment response. Those in the adaptive group who were deemed responders, defined as a reduction in daily cigarettes of at least 50%, received placebo bupropion. Those in the adaptive group deemed nonresponders received 150 mg bupropion twice daily in addition to their patch regimen. The standard treatment group also received placebo bupropion.
At 12 weeks after the target quit day, 24% of the adaptive group demonstrated 30-day continuous smoking abstinence, compared with 9% of the standard group (odds ratio, 3.38; P = .004). Smoking abstinence was higher in the adaptive vs. placebo groups for those who used varenicline patches (28% vs. 8%; OR, 4.54) and for those who used nicotine patches (16% vs. 10%; OR, 1.73).
In addition, 7-day smoking abstinence measured at a 2-week postquit day visit was three times higher in the adaptive group compared with the standard treatment group (32% vs. 11%; OR, 3.30).
No incidents of death, life-threatening events, hospitalization, or persistent or significant disability or incapacity related to the study were reported; one death in the varenicline group was attributable to stage 4 cancer.
The findings were limited by several factors including the few or no participants of Alaska Native, American Indian, Hispanic, or Pacific Islander ethnicities, or those who were multiracial. The free medication and modest compensation for study visits further reduce generalizability, the researchers noted. Other limitations included the smaller-than-intended sample size and inability to assess individual components of adaptive treatment, they said.
However, the results support the value of adaptive treatment and suggest that adaptive treatment with precessation varenicline or nicotine patches followed by bupropion for nonresponders is more effective than standard treatment for smoking cessation.
The study was supported by the National Institute on Drug Abuse; the varenicline was provided by Pfizer. Dr. Davis had no financial conflicts to disclose.
Smokers who followed an adaptive treatment regimen with drug patches had greater smoking abstinence after 12 weeks than did those who followed a standard regimen, based on data from 188 individuals.
Adaptive pharmacotherapy is a common strategy across many medical conditions, but its use in smoking cessation treatments involving skin patches has not been examined, wrote James M. Davis, MD, of Duke University, Durham, N.C., and colleagues.
In a study published in JAMA Network Open, the researchers reviewed data from 188 adults who sought smoking cessation treatment at a university health system between February 2018 and May 2020. The researchers planned to enroll 300 adults, but enrollment was truncated because of the COVID-19 pandemic.
Participants chose between varenicline or nicotine patches, and then were randomized to an adaptive or standard treatment regimen. All participants started their medication 4 weeks before their target quit smoking day.
A total of 127 participants chose varenicline, with 64 randomized to adaptive treatment and 63 randomized to standard treatment; 61 participants chose nicotine patches, with 31 randomized to adaptive treatment and 30 randomized to standard treatment. Overall, participants smoked a mean of 15.4 cigarettes per day at baseline. The mean age of the participants was 49.1 years; 54% were female, 52% were White, and 48% were Black. Baseline demographics were similar between the groups.
The primary outcome was 30-day continuous abstinence from smoking (biochemically verified) at 12 weeks after each participant’s target quit date.
After 2 weeks (2 weeks before the target quit smoking day), all participants were assessed for treatment response. Those in the adaptive group who were deemed responders, defined as a reduction in daily cigarettes of at least 50%, received placebo bupropion. Those in the adaptive group deemed nonresponders received 150 mg bupropion twice daily in addition to their patch regimen. The standard treatment group also received placebo bupropion.
At 12 weeks after the target quit day, 24% of the adaptive group demonstrated 30-day continuous smoking abstinence, compared with 9% of the standard group (odds ratio, 3.38; P = .004). Smoking abstinence was higher in the adaptive vs. placebo groups for those who used varenicline patches (28% vs. 8%; OR, 4.54) and for those who used nicotine patches (16% vs. 10%; OR, 1.73).
In addition, 7-day smoking abstinence measured at a 2-week postquit day visit was three times higher in the adaptive group compared with the standard treatment group (32% vs. 11%; OR, 3.30).
No incidents of death, life-threatening events, hospitalization, or persistent or significant disability or incapacity related to the study were reported; one death in the varenicline group was attributable to stage 4 cancer.
The findings were limited by several factors including the few or no participants of Alaska Native, American Indian, Hispanic, or Pacific Islander ethnicities, or those who were multiracial. The free medication and modest compensation for study visits further reduce generalizability, the researchers noted. Other limitations included the smaller-than-intended sample size and inability to assess individual components of adaptive treatment, they said.
However, the results support the value of adaptive treatment and suggest that adaptive treatment with precessation varenicline or nicotine patches followed by bupropion for nonresponders is more effective than standard treatment for smoking cessation.
The study was supported by the National Institute on Drug Abuse; the varenicline was provided by Pfizer. Dr. Davis had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
When does a bicarb drip make sense?
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
A 70-year-old woman is admitted to the intensive care unit with a pH of 7.1, an acute kidney injury (AKI), and ketonuria. She is volume depleted and her history is consistent with starvation ketosis. This LOL truly is in NAD (that’s little old lady in no acute distress, for those who haven’t read The House of God). She is clinically stable and seemingly unperturbed by the flurry of activity surrounding her admission.
Your resident is concerned by the severity of the acidosis and suggests starting an intravenous bicarbonate drip. The fellow is adamantly against it. He’s been taught that intravenous bicarbonate increases the serum pH but paradoxically causes intracellular acidosis. As the attending you elect to observe fellow autonomy – no bicarb is given. Because any debate on rounds is a “teachable moment,” you decide to review the evidence and physiology behind infusing bicarbonate.
What do the data reveal?
An excellent review published in CHEST in 2000 covers the physiologic effects of bicarbonate, specifically related to lactic acidosis, which our patient didn’t have. Aside from that difference, the review validates the fellow’s opinion. In short, It is unlikely to provoke hemodynamic or respiratory compromise outside the setting of shock or hypercapnia. Intravenous bicarbonate can lead to intracellular acidosis, hypercapnia, hypocalcemia, and a reduction in oxygen delivery via the Bohr effect. The authors concluded that because the benefits are unproven and the negative effects are real, intravenous bicarbonate should not be used to correct a metabolic acidosis.
The CHEST review hardly settles the issue, though. A survey published a few years later found a majority of intensivists and nephrologists used intravenous bicarbonate to treat metabolic acidosis while the Surviving Sepsis Campaign Guidelines for the Management of Sepsis and Septic Shock published in 2017 recommended against bicarbonate for acidosis. It wasn’t until 2018 that we reached the holy grail: a randomized controlled trial.
The BICAR-ICU study randomly assigned patients with a pH of 7.20 or less, PCO2 of 45 mm Hg or less, and sodium bicarbonate concentration of 20 mmol/L or less to receive no bicarbonate versus a sodium bicarbonate drip to maintain a pH greater than 7.30. There’s additional nuance to the trial design and even more detail in the results. To summarize, there was signal for an improvement in renal outcomes across all patients, and those with AKI saw a mortality benefit. Post–BICAR-ICU iterations of the Surviving Sepsis Campaign Guidelines have incorporated these findings by recommending intravenous bicarbonate for patients with sepsis who have AKI and a pH of 7.20 or less.
That’s not to say BICAR-ICU has settled the issue. Although it’s far and away the best we have, there were fewer than 400 total patients in their intention-to-treat analysis. It was open label, with lots of crossover. The primary outcome was negative for the entire population, with only a subgroup (albeit a prespecified one) showing benefit. Finally, the results weren’t stratified by etiology for the metabolic acidosis. There was also evidence of alkalosis and hypocalcemia in the treatment group.
Last but not least in terms of importance, in most cases when bicarbonate is being considered, wouldn’t some form of renal replacement therapy (RRT) be preferred? This point was raised by nephrologists and intensivists when we covered BICAR-ICU in a journal club at my former program. It’s also mentioned in an accompanying editorial. RRT timing is controversial, and a detailed discussion is outside the scope of this piece and beyond the limits of my current knowledge base. But I do know that the A in the A-E-I-O-U acute indications for dialysis pneumonic stands for acidosis.
Our patient had AKI, a pH of 7.20 or less, and a pCO2 well under 45 mm Hg. Does BICAR-ICU support the resident’s inclination to start a drip? Sort of. The majority of patients enrolled in BICAR-ICU were in shock or were recovering from cardiac arrest, so it’s not clear the results can be generalized to our LOL with starvation ketosis. Extrapolating from studies of diabetic ketoacidosis (DKA) seems more appropriate, and here the data are poor but equivocal. Reviews are generally negative but don’t rule out the use of intravenous bicarbonate in certain patients with DKA.
Key takeaways
Our patient survived a 24-hour ICU stay with neither cardiopulmonary decompensation nor a need for RRT. Not sure how she did out of the ICU; presumably she was discharged soon after transfer. As is always the case with anecdotal medicine, the absence of a control prevents assessment of the counterfactual. Is it possible she may have done “better” with intravenous bicarbonate? Seems unlikely to me, though I doubt there would have been demonstrable adverse effects. Perhaps next time the fellow can observe resident autonomy?
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University of the Health Sciences, Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
The surprising way to fight asthma symptoms
Asthma is a sneaky foe.
a professor of nursing at Columbia University and a spokesperson for the Asthma and Allergy Foundation of America.
But that doesn’t mean exercise should be avoided, she said.
Exercise, in fact, is one of the best ways to reduce asthma symptoms. Research over the past 2 decades has shown that physical activity can help improve lung function and boost quality of life for someone with asthma.
As their fitness improves, asthma patients report better sleep, reduced stress, improved weight control, and more days without symptoms. In some cases, they’re able to cut down their medication doses.
Exercise reduces inflammatory cytokines and increases anti-inflammatory cytokines, according to a 2023 review by researchers in the United Kingdom. That could help calm chronic airway inflammation, easing symptoms of asthma.
A few simple guidelines can help patients reap those benefits while staying safe.
Make sure the first steps aren’t the last steps
For someone who’s new to exercise, there’s only one way to begin: Carefully.
The Global Initiative for Asthma recommends twice-weekly cardio and strength training.
“You always start low and slow,” said Spencer Nadolsky, DO, a board-certified obesity and lipid specialist and medical director of Sequence, a comprehensive weight management program.
“Low” means light loads in the weight room. “Slow” means short, easy walks.
Many have been put “through the wringer” when starting out, discouraging them from continuing, Dr. Nadolsky said. “They were too sore, and it felt more like punishment.”
An even bigger concern is triggering an asthma attack. Patients should take steps to lower the risk by carrying their rescue inhalers and keeping up on medications, he added.
“A health care professional should be consulted” before the start of a new activity or ramping up a program, or anytime asthma interferes with a workout, Dr. George said.
Those who exercise outside need to be aware of the air quality, especially at a time when smoke and particulates from a wildfires in Canada can trigger asthma symptoms in people thousands of miles away.
The harder one works, the higher one’s “ventilation” – taking more air into the lungs, and potentially more allergens and pollutants.
Temperature and humidity also become risky at the extremes. Cold, dry air can dehydrate and constrict the airways, making it hard to breathe.
How to choose the best type of exercise
Step 1: Be realistic. People with asthma often have less exercise capacity than those who don’t – understandable when shortness of breath is the default setting.
Second, allow for plenty of time to warm up. A solid warm-up routine – particularly one with a mix of lower- and higher-intensity exercises – may help prevent exercise-induced bronchoconstriction causing shortness of breath and wheezing.
For example, warming up on a treadmill or exercise bike could be mixed with a few short bursts of faster running or cycling, with a couple of minutes of recovery at a slower pace in between.
That concept can be expanded into a full-blown workout.
High-intensity interval training (HIIT) is a promising option for people with asthma. A 2021 study showed that three 20-minute interval workouts per week significantly improved asthma control.
“The benefit of HIIT is that ventilation is able to recover intermittently,” said Carley O’Neill, PhD, an exercise scientist at Acadia University in Nova Scotia and the study’s lead author.
That’s a key difference from conventional cardio, where the constant exertion can evaporate water from the lungs faster than your body can replenish it. “Dehydrating of the airways can, in some, trigger exercise-induced asthma,” Dr. O’Neill said.
HIIT, conversely, allows the airways to recover and rehydrate between exercise bouts.
Another recent study found that people with asthma who did HIIT workouts had fewer breathing problems and felt less fatigued, compared with a matched group who did cardio training at a constant pace. (Both types of cardio led to similar improvements in aerobic fitness.)
Individuals can choose other types of intermittent exercise as well. Strength training, for example, requires relatively short periods of exertion, with plenty of rest in between.
The one choice you don’t want to make
While there are lots of good exercise options for someone with asthma, there’s one clearly bad choice, according to Dr. George: “Avoiding exercise.”
Being inactive puts one at higher risk for obesity and all the health problems that go with it. And allowing one’s fitness level to decline makes it much harder to move when one needs or wants to.
Any choice is better than that one.
A version of this article first appeared on WebMD.com.
Asthma is a sneaky foe.
a professor of nursing at Columbia University and a spokesperson for the Asthma and Allergy Foundation of America.
But that doesn’t mean exercise should be avoided, she said.
Exercise, in fact, is one of the best ways to reduce asthma symptoms. Research over the past 2 decades has shown that physical activity can help improve lung function and boost quality of life for someone with asthma.
As their fitness improves, asthma patients report better sleep, reduced stress, improved weight control, and more days without symptoms. In some cases, they’re able to cut down their medication doses.
Exercise reduces inflammatory cytokines and increases anti-inflammatory cytokines, according to a 2023 review by researchers in the United Kingdom. That could help calm chronic airway inflammation, easing symptoms of asthma.
A few simple guidelines can help patients reap those benefits while staying safe.
Make sure the first steps aren’t the last steps
For someone who’s new to exercise, there’s only one way to begin: Carefully.
The Global Initiative for Asthma recommends twice-weekly cardio and strength training.
“You always start low and slow,” said Spencer Nadolsky, DO, a board-certified obesity and lipid specialist and medical director of Sequence, a comprehensive weight management program.
“Low” means light loads in the weight room. “Slow” means short, easy walks.
Many have been put “through the wringer” when starting out, discouraging them from continuing, Dr. Nadolsky said. “They were too sore, and it felt more like punishment.”
An even bigger concern is triggering an asthma attack. Patients should take steps to lower the risk by carrying their rescue inhalers and keeping up on medications, he added.
“A health care professional should be consulted” before the start of a new activity or ramping up a program, or anytime asthma interferes with a workout, Dr. George said.
Those who exercise outside need to be aware of the air quality, especially at a time when smoke and particulates from a wildfires in Canada can trigger asthma symptoms in people thousands of miles away.
The harder one works, the higher one’s “ventilation” – taking more air into the lungs, and potentially more allergens and pollutants.
Temperature and humidity also become risky at the extremes. Cold, dry air can dehydrate and constrict the airways, making it hard to breathe.
How to choose the best type of exercise
Step 1: Be realistic. People with asthma often have less exercise capacity than those who don’t – understandable when shortness of breath is the default setting.
Second, allow for plenty of time to warm up. A solid warm-up routine – particularly one with a mix of lower- and higher-intensity exercises – may help prevent exercise-induced bronchoconstriction causing shortness of breath and wheezing.
For example, warming up on a treadmill or exercise bike could be mixed with a few short bursts of faster running or cycling, with a couple of minutes of recovery at a slower pace in between.
That concept can be expanded into a full-blown workout.
High-intensity interval training (HIIT) is a promising option for people with asthma. A 2021 study showed that three 20-minute interval workouts per week significantly improved asthma control.
“The benefit of HIIT is that ventilation is able to recover intermittently,” said Carley O’Neill, PhD, an exercise scientist at Acadia University in Nova Scotia and the study’s lead author.
That’s a key difference from conventional cardio, where the constant exertion can evaporate water from the lungs faster than your body can replenish it. “Dehydrating of the airways can, in some, trigger exercise-induced asthma,” Dr. O’Neill said.
HIIT, conversely, allows the airways to recover and rehydrate between exercise bouts.
Another recent study found that people with asthma who did HIIT workouts had fewer breathing problems and felt less fatigued, compared with a matched group who did cardio training at a constant pace. (Both types of cardio led to similar improvements in aerobic fitness.)
Individuals can choose other types of intermittent exercise as well. Strength training, for example, requires relatively short periods of exertion, with plenty of rest in between.
The one choice you don’t want to make
While there are lots of good exercise options for someone with asthma, there’s one clearly bad choice, according to Dr. George: “Avoiding exercise.”
Being inactive puts one at higher risk for obesity and all the health problems that go with it. And allowing one’s fitness level to decline makes it much harder to move when one needs or wants to.
Any choice is better than that one.
A version of this article first appeared on WebMD.com.
Asthma is a sneaky foe.
a professor of nursing at Columbia University and a spokesperson for the Asthma and Allergy Foundation of America.
But that doesn’t mean exercise should be avoided, she said.
Exercise, in fact, is one of the best ways to reduce asthma symptoms. Research over the past 2 decades has shown that physical activity can help improve lung function and boost quality of life for someone with asthma.
As their fitness improves, asthma patients report better sleep, reduced stress, improved weight control, and more days without symptoms. In some cases, they’re able to cut down their medication doses.
Exercise reduces inflammatory cytokines and increases anti-inflammatory cytokines, according to a 2023 review by researchers in the United Kingdom. That could help calm chronic airway inflammation, easing symptoms of asthma.
A few simple guidelines can help patients reap those benefits while staying safe.
Make sure the first steps aren’t the last steps
For someone who’s new to exercise, there’s only one way to begin: Carefully.
The Global Initiative for Asthma recommends twice-weekly cardio and strength training.
“You always start low and slow,” said Spencer Nadolsky, DO, a board-certified obesity and lipid specialist and medical director of Sequence, a comprehensive weight management program.
“Low” means light loads in the weight room. “Slow” means short, easy walks.
Many have been put “through the wringer” when starting out, discouraging them from continuing, Dr. Nadolsky said. “They were too sore, and it felt more like punishment.”
An even bigger concern is triggering an asthma attack. Patients should take steps to lower the risk by carrying their rescue inhalers and keeping up on medications, he added.
“A health care professional should be consulted” before the start of a new activity or ramping up a program, or anytime asthma interferes with a workout, Dr. George said.
Those who exercise outside need to be aware of the air quality, especially at a time when smoke and particulates from a wildfires in Canada can trigger asthma symptoms in people thousands of miles away.
The harder one works, the higher one’s “ventilation” – taking more air into the lungs, and potentially more allergens and pollutants.
Temperature and humidity also become risky at the extremes. Cold, dry air can dehydrate and constrict the airways, making it hard to breathe.
How to choose the best type of exercise
Step 1: Be realistic. People with asthma often have less exercise capacity than those who don’t – understandable when shortness of breath is the default setting.
Second, allow for plenty of time to warm up. A solid warm-up routine – particularly one with a mix of lower- and higher-intensity exercises – may help prevent exercise-induced bronchoconstriction causing shortness of breath and wheezing.
For example, warming up on a treadmill or exercise bike could be mixed with a few short bursts of faster running or cycling, with a couple of minutes of recovery at a slower pace in between.
That concept can be expanded into a full-blown workout.
High-intensity interval training (HIIT) is a promising option for people with asthma. A 2021 study showed that three 20-minute interval workouts per week significantly improved asthma control.
“The benefit of HIIT is that ventilation is able to recover intermittently,” said Carley O’Neill, PhD, an exercise scientist at Acadia University in Nova Scotia and the study’s lead author.
That’s a key difference from conventional cardio, where the constant exertion can evaporate water from the lungs faster than your body can replenish it. “Dehydrating of the airways can, in some, trigger exercise-induced asthma,” Dr. O’Neill said.
HIIT, conversely, allows the airways to recover and rehydrate between exercise bouts.
Another recent study found that people with asthma who did HIIT workouts had fewer breathing problems and felt less fatigued, compared with a matched group who did cardio training at a constant pace. (Both types of cardio led to similar improvements in aerobic fitness.)
Individuals can choose other types of intermittent exercise as well. Strength training, for example, requires relatively short periods of exertion, with plenty of rest in between.
The one choice you don’t want to make
While there are lots of good exercise options for someone with asthma, there’s one clearly bad choice, according to Dr. George: “Avoiding exercise.”
Being inactive puts one at higher risk for obesity and all the health problems that go with it. And allowing one’s fitness level to decline makes it much harder to move when one needs or wants to.
Any choice is better than that one.
A version of this article first appeared on WebMD.com.
Your patient bequeathed money to you: Can you accept it?
Michael Victoroff, MD, described the phone call he received from an attorney asking a thorny ethics question involving a patient’s gift to another physician. Dr. Victoroff, a past member of the ethics committee of the American Academy of Family Physicians, had definite thoughts about it.
“The attorney was representing the daughters of an elderly gentleman who had moved from the East Coast to Colorado to be closer to them,” said Dr. Victoroff, who teaches bioethics in the MBA program at the University of Denver and also practices at the University of Colorado School of Medicine.
“The father visited his new primary care physician frequently because he had multiple health issues.”
The patient was happy with the doctor’s medical care and over time that they developed a friendship. Dr. Victoroff emphasized that no sexual or romantic impropriety ever took place between the patient and his physician.
“But the social relationship went beyond the ordinary doctor-patient boundaries. The patient ultimately named the doctor as his health care proxy in the event that he became unable to make decisions regarding his care. He also mentioned he was going to leave her $100,000 in his will,” says Dr. Victoroff.
The physician did accept the role of proxy, “which raises a whole host of ethical issues,” says Dr. Victoroff. As it happened, she was never called upon to exercise that decision-making authority, since the patient died suddenly and was mentally competent at the time.
for her to accept such a substantial bequest from a patient, and they hired an attorney to contest the will.
No law against it
Dennis Hursh, attorney and managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, noted in an interview that, “the problem isn’t legal per se. Rather, the problem is an ethical one.”
Legally speaking, there’s no prohibition against receiving a bequest or other form of gift from a patient. “People are free to dispose of their estates in whatever way they see fit, and no law technically precludes a physician from accepting a bequest,” says Dr. Victoroff. “But this presupposes there is nothing improper going on, such as extortion, deception, coercion, or exercising undue influence.”
The issue of bequeathing money to their physician gained attention in a recent case that took place in Australia. Peter Alexakis, MD, received a whopping bequest of $24 million from a patient. The elderly patient had changed his will to name Dr. Alexakis as the sole beneficiary – after Dr. Alexakis had visited him at home 92 times during the preceding months. The original heirs filed a lawsuit in Australia’s Supreme Court against Dr. Alexakis, contesting the will.
The lawsuit was unsuccessful in court, but Dr. Alexakis was found guilty of malpractice by Australia’s Health Care Complaints Commission after being reported to the HCCC by the palliative care physicians who were treating the patient. They alleged that Dr. Alexakis had interfered with their care of the patient. The more serious allegation was that the doctor had engaged in a deliberate strategy to exploit the relationship for financial gain.
Dr. Alexakis was chastised by the HCCC for engaging in “obtuse” and “suspicious” behavior and for “blurring the boundaries of the doctor-patient relationship.”
There are three domains – legal, ethical, and practical – when it comes to accepting bequests or any gifts from patients, says Dr. Victoroff.
“[In] the legal domain, for example, if you receive a bequest from anyone, patient or otherwise, you have to know your local laws about estates and taxes and so forth and obey them,” he said.
Attorney Hursh pointed out that the Australian doctor wasn’t found guilty of wrongdoing in a court of law but rather of unethical conduct by the Australian medical licensing entity.
Patients giving gifts is often a part of a physician’s life
When Ian Schorr, MD, first started out in practice, he was surprised that patients began bringing him gifts of food to express gratitude for his care.
“I thought it was unethical to accept their gifts, so I turned them down and wouldn’t accept so much as a cookie,” Dr. Schorr, a now-retired ophthalmologist, told this news organization. “But that changed because my office staff told me that some patients were feeling disappointed and insulted. I realized that some people want to express appreciation in ways that go beyond a monetary payment.”
The next time he received a gift from a patient, he “accepted it gracefully.” And he wrote a thank you note, which he continued to do any time he received a gift from a patient.
Kenneth Prager, MD, professor of clinical medicine, director of clinical ethics and chairman of the Medical Ethics Committee at Columbia University Medical Center, New York, says, “I have literally received hundreds of gifts, the vast majority being tokens of patients’ appreciation,” he said. “I’ll get boxes of chocolate or cakes, or sometimes articles of clothing.”
Occasionally, Dr. Prager receives a “somewhat larger gift” – for example, two tickets to a baseball game. “To reject these gifts would be a slap in the face to the patient,” he says, but “where it gets more ethically cloudy is when a gift is very substantial.”
Dr. Prager has never been offered a “substantial” gift or bequest personally. “But a patient whose brother I cared for has indicated that she has left instructions in her will to endow an associate chair of ethics in my honor, and I didn’t decline that,” he said.
The AMA Code of Ethics confirms that accepting gifts offered “as an expression of gratitude or a reflection of the patient’s cultural tradition” can “enhance the patient-physician relationship.” But sometimes gifts “may signal psychological needs that require the physician’s attention.” Accepting such gifts is “likely to damage the patient-physician relationship.”
Potential damage to the therapeutic relationship applies to all physicians but especially for psychiatrists and mental health professionals. “There are more stringent ethical requirements when it comes to these disciplines, where gift-giving gets into the territory of transference or may have particular psychological meaning, and accepting the gift may muddy the therapeutic waters,” Dr. Victoroff said.
Impact on the patient’s family and on other patients
The AMA statement encourages physicians to be “sensitive to the gift’s value, relative to the patient’s or physician’s means.” Physicians should decline gifts that are “disproportionately or inappropriately large, or when the physician would be uncomfortable to have colleagues know the gift had been accepted.”
They should also decline a bequest from a patient if they have reason to believe that to accept it “would present an emotional or financial hardship to the patient’s family.”
“If Bill Gates were leaving $100,000 to his doctor, I imagine Melinda would be just fine,” Mr. Hursh said. “But under ordinary circumstances, if the patient’s family might feel the impact of the bequest, it would be unethical to accept it and could be grounds for revocation of the doctor’s license.”
The AMA statement also warns physicians that by offering a gift, some patients may be seeking to “secure or influence care or to secure preferential treatment,” which can “undermine physicians’ obligation to provide services fairly to all patients.”
For this reason, bequests are “sticky,” said Laurel Lyckholm, MD, professor of hematology and oncology at West Virginia University School of Medicine. In the case of institutions where patients or community members donate money, “we know whose names are on the plaques that hang on the hospital walls, so it’s a delicate balance. What if there’s only one bed or one ventilator? Will the wife of the donor get preferential treatment?”
Follow institutional policy
A “very small gift, such as a fruitcake, is fine,” says Dr. Lyckholm, author of an essay on accepting gifts from patients. She said there’s a dollar amount ($15) that her institution mandates, above which a gift – even food – is considered too expensive to accept. “I was a nurse before I became a physician, and people always tried to give us gifts because we were so close to the minute-by-minute care of the patients,” she said. “We were not allowed to accept money or anything lavish.”
But in the case of small gifts, “the risk-benefit analysis is that there’s much more risk not to take it and to hurt the patient’s feelings.”
Gifts above $15 are given to charity. “I explain to patients that I’m not allowed to take such a large gift, but I’d love to give it to the hospital’s Rosenbaum Family House that provides patients and their relatives with lodging, or to the homeless shelter in Morgantown.”
Dr. Lyckholm, who serves on the ethics committee at J.W. Ruby Memorial Hospital, once was offered expensive tickets and said to the patient, “This is so incredibly thoughtful and kind, but I can’t accept them. I would like to give the tickets to a charity that can auction them off.”
She advises physicians to find out their institution’s policies. Many institutions have policies about what gifts their staff – whether physicians, nurses, or other health care professionals – can accept.
Passing the ‘smell test’
Accepting a large gift from a patient could potentially make it look like you might have exercised undue influence.
“That concern brings us to the third domain, which is very practical and all about appearances and perceptions,” Dr. Victoroff said.
He noted that there is “an inherent power differential between a physician and a patient. The very nature of the relationship can create a risk of ‘undue influence’ on the doctor’s part, even if it’s not apparent to the doctor.” For this reason, it’s necessary to be utterly transparent about how the bequest came about.
He suggests that if a patient informs you that he or she would like to leave money to you, it might be wise to suggest a meeting with the patient’s family, thus establishing some transparency.
It may not be possible to meet with the patient’s family for logistical reasons or because the patient would prefer not to involve their family in their estate planning. But in any case, it’s advisable to document any conversation in the patient’s chart, Dr. Victoroff advised.
“You should make a contemporaneous note that the patient initiated the suggestion and that you counseled them about the implications, no differently than you would with an interaction of a clinical nature,” he suggests. That way, if money has been left to you and is disputed, there’s a clear record that you didn’t solicit it or use any undue influence to bring it about.
He also recommended getting advice from a trusted colleague or a member of your institution’s ethics committee. “Taking time to get a second opinion about an ethical question is a safeguard, like having a chaperone in the room during an examination.”
Ultimately, “there is no human relationship without potential conflicts of interest. Our job is to manage those as best as we can, and sunlight is the best antidote to bad appearances,” Dr. Victoroff said.
A version of this article appeared on Medscape.com.
Michael Victoroff, MD, described the phone call he received from an attorney asking a thorny ethics question involving a patient’s gift to another physician. Dr. Victoroff, a past member of the ethics committee of the American Academy of Family Physicians, had definite thoughts about it.
“The attorney was representing the daughters of an elderly gentleman who had moved from the East Coast to Colorado to be closer to them,” said Dr. Victoroff, who teaches bioethics in the MBA program at the University of Denver and also practices at the University of Colorado School of Medicine.
“The father visited his new primary care physician frequently because he had multiple health issues.”
The patient was happy with the doctor’s medical care and over time that they developed a friendship. Dr. Victoroff emphasized that no sexual or romantic impropriety ever took place between the patient and his physician.
“But the social relationship went beyond the ordinary doctor-patient boundaries. The patient ultimately named the doctor as his health care proxy in the event that he became unable to make decisions regarding his care. He also mentioned he was going to leave her $100,000 in his will,” says Dr. Victoroff.
The physician did accept the role of proxy, “which raises a whole host of ethical issues,” says Dr. Victoroff. As it happened, she was never called upon to exercise that decision-making authority, since the patient died suddenly and was mentally competent at the time.
for her to accept such a substantial bequest from a patient, and they hired an attorney to contest the will.
No law against it
Dennis Hursh, attorney and managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, noted in an interview that, “the problem isn’t legal per se. Rather, the problem is an ethical one.”
Legally speaking, there’s no prohibition against receiving a bequest or other form of gift from a patient. “People are free to dispose of their estates in whatever way they see fit, and no law technically precludes a physician from accepting a bequest,” says Dr. Victoroff. “But this presupposes there is nothing improper going on, such as extortion, deception, coercion, or exercising undue influence.”
The issue of bequeathing money to their physician gained attention in a recent case that took place in Australia. Peter Alexakis, MD, received a whopping bequest of $24 million from a patient. The elderly patient had changed his will to name Dr. Alexakis as the sole beneficiary – after Dr. Alexakis had visited him at home 92 times during the preceding months. The original heirs filed a lawsuit in Australia’s Supreme Court against Dr. Alexakis, contesting the will.
The lawsuit was unsuccessful in court, but Dr. Alexakis was found guilty of malpractice by Australia’s Health Care Complaints Commission after being reported to the HCCC by the palliative care physicians who were treating the patient. They alleged that Dr. Alexakis had interfered with their care of the patient. The more serious allegation was that the doctor had engaged in a deliberate strategy to exploit the relationship for financial gain.
Dr. Alexakis was chastised by the HCCC for engaging in “obtuse” and “suspicious” behavior and for “blurring the boundaries of the doctor-patient relationship.”
There are three domains – legal, ethical, and practical – when it comes to accepting bequests or any gifts from patients, says Dr. Victoroff.
“[In] the legal domain, for example, if you receive a bequest from anyone, patient or otherwise, you have to know your local laws about estates and taxes and so forth and obey them,” he said.
Attorney Hursh pointed out that the Australian doctor wasn’t found guilty of wrongdoing in a court of law but rather of unethical conduct by the Australian medical licensing entity.
Patients giving gifts is often a part of a physician’s life
When Ian Schorr, MD, first started out in practice, he was surprised that patients began bringing him gifts of food to express gratitude for his care.
“I thought it was unethical to accept their gifts, so I turned them down and wouldn’t accept so much as a cookie,” Dr. Schorr, a now-retired ophthalmologist, told this news organization. “But that changed because my office staff told me that some patients were feeling disappointed and insulted. I realized that some people want to express appreciation in ways that go beyond a monetary payment.”
The next time he received a gift from a patient, he “accepted it gracefully.” And he wrote a thank you note, which he continued to do any time he received a gift from a patient.
Kenneth Prager, MD, professor of clinical medicine, director of clinical ethics and chairman of the Medical Ethics Committee at Columbia University Medical Center, New York, says, “I have literally received hundreds of gifts, the vast majority being tokens of patients’ appreciation,” he said. “I’ll get boxes of chocolate or cakes, or sometimes articles of clothing.”
Occasionally, Dr. Prager receives a “somewhat larger gift” – for example, two tickets to a baseball game. “To reject these gifts would be a slap in the face to the patient,” he says, but “where it gets more ethically cloudy is when a gift is very substantial.”
Dr. Prager has never been offered a “substantial” gift or bequest personally. “But a patient whose brother I cared for has indicated that she has left instructions in her will to endow an associate chair of ethics in my honor, and I didn’t decline that,” he said.
The AMA Code of Ethics confirms that accepting gifts offered “as an expression of gratitude or a reflection of the patient’s cultural tradition” can “enhance the patient-physician relationship.” But sometimes gifts “may signal psychological needs that require the physician’s attention.” Accepting such gifts is “likely to damage the patient-physician relationship.”
Potential damage to the therapeutic relationship applies to all physicians but especially for psychiatrists and mental health professionals. “There are more stringent ethical requirements when it comes to these disciplines, where gift-giving gets into the territory of transference or may have particular psychological meaning, and accepting the gift may muddy the therapeutic waters,” Dr. Victoroff said.
Impact on the patient’s family and on other patients
The AMA statement encourages physicians to be “sensitive to the gift’s value, relative to the patient’s or physician’s means.” Physicians should decline gifts that are “disproportionately or inappropriately large, or when the physician would be uncomfortable to have colleagues know the gift had been accepted.”
They should also decline a bequest from a patient if they have reason to believe that to accept it “would present an emotional or financial hardship to the patient’s family.”
“If Bill Gates were leaving $100,000 to his doctor, I imagine Melinda would be just fine,” Mr. Hursh said. “But under ordinary circumstances, if the patient’s family might feel the impact of the bequest, it would be unethical to accept it and could be grounds for revocation of the doctor’s license.”
The AMA statement also warns physicians that by offering a gift, some patients may be seeking to “secure or influence care or to secure preferential treatment,” which can “undermine physicians’ obligation to provide services fairly to all patients.”
For this reason, bequests are “sticky,” said Laurel Lyckholm, MD, professor of hematology and oncology at West Virginia University School of Medicine. In the case of institutions where patients or community members donate money, “we know whose names are on the plaques that hang on the hospital walls, so it’s a delicate balance. What if there’s only one bed or one ventilator? Will the wife of the donor get preferential treatment?”
Follow institutional policy
A “very small gift, such as a fruitcake, is fine,” says Dr. Lyckholm, author of an essay on accepting gifts from patients. She said there’s a dollar amount ($15) that her institution mandates, above which a gift – even food – is considered too expensive to accept. “I was a nurse before I became a physician, and people always tried to give us gifts because we were so close to the minute-by-minute care of the patients,” she said. “We were not allowed to accept money or anything lavish.”
But in the case of small gifts, “the risk-benefit analysis is that there’s much more risk not to take it and to hurt the patient’s feelings.”
Gifts above $15 are given to charity. “I explain to patients that I’m not allowed to take such a large gift, but I’d love to give it to the hospital’s Rosenbaum Family House that provides patients and their relatives with lodging, or to the homeless shelter in Morgantown.”
Dr. Lyckholm, who serves on the ethics committee at J.W. Ruby Memorial Hospital, once was offered expensive tickets and said to the patient, “This is so incredibly thoughtful and kind, but I can’t accept them. I would like to give the tickets to a charity that can auction them off.”
She advises physicians to find out their institution’s policies. Many institutions have policies about what gifts their staff – whether physicians, nurses, or other health care professionals – can accept.
Passing the ‘smell test’
Accepting a large gift from a patient could potentially make it look like you might have exercised undue influence.
“That concern brings us to the third domain, which is very practical and all about appearances and perceptions,” Dr. Victoroff said.
He noted that there is “an inherent power differential between a physician and a patient. The very nature of the relationship can create a risk of ‘undue influence’ on the doctor’s part, even if it’s not apparent to the doctor.” For this reason, it’s necessary to be utterly transparent about how the bequest came about.
He suggests that if a patient informs you that he or she would like to leave money to you, it might be wise to suggest a meeting with the patient’s family, thus establishing some transparency.
It may not be possible to meet with the patient’s family for logistical reasons or because the patient would prefer not to involve their family in their estate planning. But in any case, it’s advisable to document any conversation in the patient’s chart, Dr. Victoroff advised.
“You should make a contemporaneous note that the patient initiated the suggestion and that you counseled them about the implications, no differently than you would with an interaction of a clinical nature,” he suggests. That way, if money has been left to you and is disputed, there’s a clear record that you didn’t solicit it or use any undue influence to bring it about.
He also recommended getting advice from a trusted colleague or a member of your institution’s ethics committee. “Taking time to get a second opinion about an ethical question is a safeguard, like having a chaperone in the room during an examination.”
Ultimately, “there is no human relationship without potential conflicts of interest. Our job is to manage those as best as we can, and sunlight is the best antidote to bad appearances,” Dr. Victoroff said.
A version of this article appeared on Medscape.com.
Michael Victoroff, MD, described the phone call he received from an attorney asking a thorny ethics question involving a patient’s gift to another physician. Dr. Victoroff, a past member of the ethics committee of the American Academy of Family Physicians, had definite thoughts about it.
“The attorney was representing the daughters of an elderly gentleman who had moved from the East Coast to Colorado to be closer to them,” said Dr. Victoroff, who teaches bioethics in the MBA program at the University of Denver and also practices at the University of Colorado School of Medicine.
“The father visited his new primary care physician frequently because he had multiple health issues.”
The patient was happy with the doctor’s medical care and over time that they developed a friendship. Dr. Victoroff emphasized that no sexual or romantic impropriety ever took place between the patient and his physician.
“But the social relationship went beyond the ordinary doctor-patient boundaries. The patient ultimately named the doctor as his health care proxy in the event that he became unable to make decisions regarding his care. He also mentioned he was going to leave her $100,000 in his will,” says Dr. Victoroff.
The physician did accept the role of proxy, “which raises a whole host of ethical issues,” says Dr. Victoroff. As it happened, she was never called upon to exercise that decision-making authority, since the patient died suddenly and was mentally competent at the time.
for her to accept such a substantial bequest from a patient, and they hired an attorney to contest the will.
No law against it
Dennis Hursh, attorney and managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, noted in an interview that, “the problem isn’t legal per se. Rather, the problem is an ethical one.”
Legally speaking, there’s no prohibition against receiving a bequest or other form of gift from a patient. “People are free to dispose of their estates in whatever way they see fit, and no law technically precludes a physician from accepting a bequest,” says Dr. Victoroff. “But this presupposes there is nothing improper going on, such as extortion, deception, coercion, or exercising undue influence.”
The issue of bequeathing money to their physician gained attention in a recent case that took place in Australia. Peter Alexakis, MD, received a whopping bequest of $24 million from a patient. The elderly patient had changed his will to name Dr. Alexakis as the sole beneficiary – after Dr. Alexakis had visited him at home 92 times during the preceding months. The original heirs filed a lawsuit in Australia’s Supreme Court against Dr. Alexakis, contesting the will.
The lawsuit was unsuccessful in court, but Dr. Alexakis was found guilty of malpractice by Australia’s Health Care Complaints Commission after being reported to the HCCC by the palliative care physicians who were treating the patient. They alleged that Dr. Alexakis had interfered with their care of the patient. The more serious allegation was that the doctor had engaged in a deliberate strategy to exploit the relationship for financial gain.
Dr. Alexakis was chastised by the HCCC for engaging in “obtuse” and “suspicious” behavior and for “blurring the boundaries of the doctor-patient relationship.”
There are three domains – legal, ethical, and practical – when it comes to accepting bequests or any gifts from patients, says Dr. Victoroff.
“[In] the legal domain, for example, if you receive a bequest from anyone, patient or otherwise, you have to know your local laws about estates and taxes and so forth and obey them,” he said.
Attorney Hursh pointed out that the Australian doctor wasn’t found guilty of wrongdoing in a court of law but rather of unethical conduct by the Australian medical licensing entity.
Patients giving gifts is often a part of a physician’s life
When Ian Schorr, MD, first started out in practice, he was surprised that patients began bringing him gifts of food to express gratitude for his care.
“I thought it was unethical to accept their gifts, so I turned them down and wouldn’t accept so much as a cookie,” Dr. Schorr, a now-retired ophthalmologist, told this news organization. “But that changed because my office staff told me that some patients were feeling disappointed and insulted. I realized that some people want to express appreciation in ways that go beyond a monetary payment.”
The next time he received a gift from a patient, he “accepted it gracefully.” And he wrote a thank you note, which he continued to do any time he received a gift from a patient.
Kenneth Prager, MD, professor of clinical medicine, director of clinical ethics and chairman of the Medical Ethics Committee at Columbia University Medical Center, New York, says, “I have literally received hundreds of gifts, the vast majority being tokens of patients’ appreciation,” he said. “I’ll get boxes of chocolate or cakes, or sometimes articles of clothing.”
Occasionally, Dr. Prager receives a “somewhat larger gift” – for example, two tickets to a baseball game. “To reject these gifts would be a slap in the face to the patient,” he says, but “where it gets more ethically cloudy is when a gift is very substantial.”
Dr. Prager has never been offered a “substantial” gift or bequest personally. “But a patient whose brother I cared for has indicated that she has left instructions in her will to endow an associate chair of ethics in my honor, and I didn’t decline that,” he said.
The AMA Code of Ethics confirms that accepting gifts offered “as an expression of gratitude or a reflection of the patient’s cultural tradition” can “enhance the patient-physician relationship.” But sometimes gifts “may signal psychological needs that require the physician’s attention.” Accepting such gifts is “likely to damage the patient-physician relationship.”
Potential damage to the therapeutic relationship applies to all physicians but especially for psychiatrists and mental health professionals. “There are more stringent ethical requirements when it comes to these disciplines, where gift-giving gets into the territory of transference or may have particular psychological meaning, and accepting the gift may muddy the therapeutic waters,” Dr. Victoroff said.
Impact on the patient’s family and on other patients
The AMA statement encourages physicians to be “sensitive to the gift’s value, relative to the patient’s or physician’s means.” Physicians should decline gifts that are “disproportionately or inappropriately large, or when the physician would be uncomfortable to have colleagues know the gift had been accepted.”
They should also decline a bequest from a patient if they have reason to believe that to accept it “would present an emotional or financial hardship to the patient’s family.”
“If Bill Gates were leaving $100,000 to his doctor, I imagine Melinda would be just fine,” Mr. Hursh said. “But under ordinary circumstances, if the patient’s family might feel the impact of the bequest, it would be unethical to accept it and could be grounds for revocation of the doctor’s license.”
The AMA statement also warns physicians that by offering a gift, some patients may be seeking to “secure or influence care or to secure preferential treatment,” which can “undermine physicians’ obligation to provide services fairly to all patients.”
For this reason, bequests are “sticky,” said Laurel Lyckholm, MD, professor of hematology and oncology at West Virginia University School of Medicine. In the case of institutions where patients or community members donate money, “we know whose names are on the plaques that hang on the hospital walls, so it’s a delicate balance. What if there’s only one bed or one ventilator? Will the wife of the donor get preferential treatment?”
Follow institutional policy
A “very small gift, such as a fruitcake, is fine,” says Dr. Lyckholm, author of an essay on accepting gifts from patients. She said there’s a dollar amount ($15) that her institution mandates, above which a gift – even food – is considered too expensive to accept. “I was a nurse before I became a physician, and people always tried to give us gifts because we were so close to the minute-by-minute care of the patients,” she said. “We were not allowed to accept money or anything lavish.”
But in the case of small gifts, “the risk-benefit analysis is that there’s much more risk not to take it and to hurt the patient’s feelings.”
Gifts above $15 are given to charity. “I explain to patients that I’m not allowed to take such a large gift, but I’d love to give it to the hospital’s Rosenbaum Family House that provides patients and their relatives with lodging, or to the homeless shelter in Morgantown.”
Dr. Lyckholm, who serves on the ethics committee at J.W. Ruby Memorial Hospital, once was offered expensive tickets and said to the patient, “This is so incredibly thoughtful and kind, but I can’t accept them. I would like to give the tickets to a charity that can auction them off.”
She advises physicians to find out their institution’s policies. Many institutions have policies about what gifts their staff – whether physicians, nurses, or other health care professionals – can accept.
Passing the ‘smell test’
Accepting a large gift from a patient could potentially make it look like you might have exercised undue influence.
“That concern brings us to the third domain, which is very practical and all about appearances and perceptions,” Dr. Victoroff said.
He noted that there is “an inherent power differential between a physician and a patient. The very nature of the relationship can create a risk of ‘undue influence’ on the doctor’s part, even if it’s not apparent to the doctor.” For this reason, it’s necessary to be utterly transparent about how the bequest came about.
He suggests that if a patient informs you that he or she would like to leave money to you, it might be wise to suggest a meeting with the patient’s family, thus establishing some transparency.
It may not be possible to meet with the patient’s family for logistical reasons or because the patient would prefer not to involve their family in their estate planning. But in any case, it’s advisable to document any conversation in the patient’s chart, Dr. Victoroff advised.
“You should make a contemporaneous note that the patient initiated the suggestion and that you counseled them about the implications, no differently than you would with an interaction of a clinical nature,” he suggests. That way, if money has been left to you and is disputed, there’s a clear record that you didn’t solicit it or use any undue influence to bring it about.
He also recommended getting advice from a trusted colleague or a member of your institution’s ethics committee. “Taking time to get a second opinion about an ethical question is a safeguard, like having a chaperone in the room during an examination.”
Ultimately, “there is no human relationship without potential conflicts of interest. Our job is to manage those as best as we can, and sunlight is the best antidote to bad appearances,” Dr. Victoroff said.
A version of this article appeared on Medscape.com.
Sepsis too often neglected in hospitals
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
Five ways to avert a malpractice lawsuit with better EHR techniques
Although most physicians have gotten used to working with EHRs, despite their irritations, the use of EHRs has contributed to a growing number of malpractice lawsuits. Defense attorneys say that
According to a study in the Journal of Patient Safety, more than 30% of all EHR-related malpractice cases are associated with medication errors; 28% with diagnosis; and 31% with a complication of treatment, such as entering wrong information, entering information in the wrong place, and overlooking EHR flags and warnings for interactions or contraindications.
The study gave these examples of EHR-related errors that led to patient harm and ultimately to malpractice lawsuits:
- A discharge order omitted a patient’s medication that prevented strokes; the patient had a stroke days later.
- An electronic order for morphine failed to state the upper dose limit; the patient died.
- A physician meant to click on “discontinue” for an anticoagulant but mistakenly clicked on “continue” for home use.
Catching potential issues such as drug interactions or critical medical history that should inform treatment is more important than ever. “We know from safety engineering principles that just relying on vigilance is not a long-term safety strategy,” says Aaron Zach Hettinger, MD, chief research information officer at MedStar Health Research Institute, Washington, D.C. “So, it’s critical that we design these safe systems and leverage the data that’s in them.”
Here are five smart EHR practices to help protect your patients’ health and your own liability.
1. Double-check dropdown boxes
When it comes to user error, it’s easy to click the wrong choice from a drop-down menu. Better to take the time to explain your answer in a box, even if it takes a few more minutes. Or if you are choosing from a menu, proofread any information it auto-fills in the chart.
Dr. Hettinger says you can strike a balance between these templated approaches to diagnosis and long-term care by working with third-party systems and your organization or vendor IT department to help with follow-up questions to keep populated data in check.
“Make sure you have a back-end system that can help monitor that structured data,” says Dr. Hettinger. Structured data are the patient’s demographic information, like name, address, age, height, weight, vital signs, and data elements like diagnosis, medications, and lab results. “Wherever you can leverage the underlying tools that are part of the electronic health record to make sure that we’re constantly checking the right results, that helps reduce the workload so that clinicians can focus on taking care of the patients and doing the right thing and not be as focused on entering data into the system.”
2. Supplement EHR notes with direct communication
The failure to diagnose cancer because one physician doesn’t know what another physician saw in an imaging report is one of the most common claims in the cases he tries, says Aaron Boeder, a plaintiff’s medical negligence lawyer in Chicago.
Physicians often assume that if they put a note in the electronic chart, others will look for it, but Mr. Boeder says it’s far more prudent to communicate directly.
“Let’s say a radiologist interprets a scan and sees what might be cancer,” he says. “If the ordering doctor is an orthopedist who’s ordered a CT scan for DVT, there’s going to be a report for that scan. It’s going to get auto-populated back into that physician’s note,” says Mr. Boeder.
The physician may or may not look at it, but it will be in their note, and they’re supposed to follow up on it because they ordered the scan. “But they may not follow up on it, and they may not get a call from the radiologist,” he says.
“Next thing you know, 2 or 3 years later, that patient is diagnosed with very advanced cancer.”
3. Tailor auto-fill information to your common practices
Suppose, as a physician, you find that you need to change a default setting time and time again. Dr. Hettinger says it’s worth your time to take an extra couple of minutes to work with your vendor or your health system to try and make changes to auto-population settings that align with your practices.
“Let’s say a default dose of 20 milligrams of a medication is what automatically pops up, but in reality, your practice is to use a smaller dose because it’s safer, even though they’re all within the acceptable realm of what you would order,” he says. “Rather than have the default to the higher dose, see if you can change the default to a lower dose. And that way, you don’t have to catch yourself every time.”
If your auto-fills are amounts that constantly need changing, an interruption could easily knock you off course before you make that correction.
“If there are ways to have the system defaults be safer or more in line with your clinical practice, and especially across a group, then you’re designing a safer system and not relying on vigilance or memory prone to interruptions,” says Dr. Hettinger.
4. Curb the copy and paste
It’s tempting to copy a note from a previous patient visit and make only minimal changes as needed, but you risk including outdated information if you do. Even if you’re repeating questions asked by the intake nurse, it is safer to not to rely on that information, says Beth Kanik, a defense medical malpractice attorney in Atlanta.
“If it later goes into litigation, the argument then becomes that it looks like you didn’t do your job,” says Ms. Kanik. “Instead, try to ask questions in a way that would elicit responses that may be a little different than what the nurse got, so that it’s clear you asked the questions and didn’t just simply rely upon someone else’s information.”
5. Separate typing from listening
While EHR may be an excellent tool for data collection and safety checking, it’s not a stand-in for doctor-patient interaction. As technology practices push medicine toward more and more efficiency, Mr. Boeder says it’s most often listening over all else that makes the difference in the quality of care. And good listening requires full attention.
“A real concern for physicians is the number of visits they’re expected to accomplish in a set amount of time,” says Mr. Boeder. “Often this translates into a doctor talking to a patient while typing notes or while reading a note from the last time the patient was in.”
Taking the time to pause after entering data and briefly reviewing your understanding of what your patient has told you can be invaluable and may save you – and your patient – problems later.
“In so many cases, it comes down to people not being heard,” says Mr. Boeder. “So listen to what your patients are saying.”
A version of this article first appeared on Medscape.com.
Although most physicians have gotten used to working with EHRs, despite their irritations, the use of EHRs has contributed to a growing number of malpractice lawsuits. Defense attorneys say that
According to a study in the Journal of Patient Safety, more than 30% of all EHR-related malpractice cases are associated with medication errors; 28% with diagnosis; and 31% with a complication of treatment, such as entering wrong information, entering information in the wrong place, and overlooking EHR flags and warnings for interactions or contraindications.
The study gave these examples of EHR-related errors that led to patient harm and ultimately to malpractice lawsuits:
- A discharge order omitted a patient’s medication that prevented strokes; the patient had a stroke days later.
- An electronic order for morphine failed to state the upper dose limit; the patient died.
- A physician meant to click on “discontinue” for an anticoagulant but mistakenly clicked on “continue” for home use.
Catching potential issues such as drug interactions or critical medical history that should inform treatment is more important than ever. “We know from safety engineering principles that just relying on vigilance is not a long-term safety strategy,” says Aaron Zach Hettinger, MD, chief research information officer at MedStar Health Research Institute, Washington, D.C. “So, it’s critical that we design these safe systems and leverage the data that’s in them.”
Here are five smart EHR practices to help protect your patients’ health and your own liability.
1. Double-check dropdown boxes
When it comes to user error, it’s easy to click the wrong choice from a drop-down menu. Better to take the time to explain your answer in a box, even if it takes a few more minutes. Or if you are choosing from a menu, proofread any information it auto-fills in the chart.
Dr. Hettinger says you can strike a balance between these templated approaches to diagnosis and long-term care by working with third-party systems and your organization or vendor IT department to help with follow-up questions to keep populated data in check.
“Make sure you have a back-end system that can help monitor that structured data,” says Dr. Hettinger. Structured data are the patient’s demographic information, like name, address, age, height, weight, vital signs, and data elements like diagnosis, medications, and lab results. “Wherever you can leverage the underlying tools that are part of the electronic health record to make sure that we’re constantly checking the right results, that helps reduce the workload so that clinicians can focus on taking care of the patients and doing the right thing and not be as focused on entering data into the system.”
2. Supplement EHR notes with direct communication
The failure to diagnose cancer because one physician doesn’t know what another physician saw in an imaging report is one of the most common claims in the cases he tries, says Aaron Boeder, a plaintiff’s medical negligence lawyer in Chicago.
Physicians often assume that if they put a note in the electronic chart, others will look for it, but Mr. Boeder says it’s far more prudent to communicate directly.
“Let’s say a radiologist interprets a scan and sees what might be cancer,” he says. “If the ordering doctor is an orthopedist who’s ordered a CT scan for DVT, there’s going to be a report for that scan. It’s going to get auto-populated back into that physician’s note,” says Mr. Boeder.
The physician may or may not look at it, but it will be in their note, and they’re supposed to follow up on it because they ordered the scan. “But they may not follow up on it, and they may not get a call from the radiologist,” he says.
“Next thing you know, 2 or 3 years later, that patient is diagnosed with very advanced cancer.”
3. Tailor auto-fill information to your common practices
Suppose, as a physician, you find that you need to change a default setting time and time again. Dr. Hettinger says it’s worth your time to take an extra couple of minutes to work with your vendor or your health system to try and make changes to auto-population settings that align with your practices.
“Let’s say a default dose of 20 milligrams of a medication is what automatically pops up, but in reality, your practice is to use a smaller dose because it’s safer, even though they’re all within the acceptable realm of what you would order,” he says. “Rather than have the default to the higher dose, see if you can change the default to a lower dose. And that way, you don’t have to catch yourself every time.”
If your auto-fills are amounts that constantly need changing, an interruption could easily knock you off course before you make that correction.
“If there are ways to have the system defaults be safer or more in line with your clinical practice, and especially across a group, then you’re designing a safer system and not relying on vigilance or memory prone to interruptions,” says Dr. Hettinger.
4. Curb the copy and paste
It’s tempting to copy a note from a previous patient visit and make only minimal changes as needed, but you risk including outdated information if you do. Even if you’re repeating questions asked by the intake nurse, it is safer to not to rely on that information, says Beth Kanik, a defense medical malpractice attorney in Atlanta.
“If it later goes into litigation, the argument then becomes that it looks like you didn’t do your job,” says Ms. Kanik. “Instead, try to ask questions in a way that would elicit responses that may be a little different than what the nurse got, so that it’s clear you asked the questions and didn’t just simply rely upon someone else’s information.”
5. Separate typing from listening
While EHR may be an excellent tool for data collection and safety checking, it’s not a stand-in for doctor-patient interaction. As technology practices push medicine toward more and more efficiency, Mr. Boeder says it’s most often listening over all else that makes the difference in the quality of care. And good listening requires full attention.
“A real concern for physicians is the number of visits they’re expected to accomplish in a set amount of time,” says Mr. Boeder. “Often this translates into a doctor talking to a patient while typing notes or while reading a note from the last time the patient was in.”
Taking the time to pause after entering data and briefly reviewing your understanding of what your patient has told you can be invaluable and may save you – and your patient – problems later.
“In so many cases, it comes down to people not being heard,” says Mr. Boeder. “So listen to what your patients are saying.”
A version of this article first appeared on Medscape.com.
Although most physicians have gotten used to working with EHRs, despite their irritations, the use of EHRs has contributed to a growing number of malpractice lawsuits. Defense attorneys say that
According to a study in the Journal of Patient Safety, more than 30% of all EHR-related malpractice cases are associated with medication errors; 28% with diagnosis; and 31% with a complication of treatment, such as entering wrong information, entering information in the wrong place, and overlooking EHR flags and warnings for interactions or contraindications.
The study gave these examples of EHR-related errors that led to patient harm and ultimately to malpractice lawsuits:
- A discharge order omitted a patient’s medication that prevented strokes; the patient had a stroke days later.
- An electronic order for morphine failed to state the upper dose limit; the patient died.
- A physician meant to click on “discontinue” for an anticoagulant but mistakenly clicked on “continue” for home use.
Catching potential issues such as drug interactions or critical medical history that should inform treatment is more important than ever. “We know from safety engineering principles that just relying on vigilance is not a long-term safety strategy,” says Aaron Zach Hettinger, MD, chief research information officer at MedStar Health Research Institute, Washington, D.C. “So, it’s critical that we design these safe systems and leverage the data that’s in them.”
Here are five smart EHR practices to help protect your patients’ health and your own liability.
1. Double-check dropdown boxes
When it comes to user error, it’s easy to click the wrong choice from a drop-down menu. Better to take the time to explain your answer in a box, even if it takes a few more minutes. Or if you are choosing from a menu, proofread any information it auto-fills in the chart.
Dr. Hettinger says you can strike a balance between these templated approaches to diagnosis and long-term care by working with third-party systems and your organization or vendor IT department to help with follow-up questions to keep populated data in check.
“Make sure you have a back-end system that can help monitor that structured data,” says Dr. Hettinger. Structured data are the patient’s demographic information, like name, address, age, height, weight, vital signs, and data elements like diagnosis, medications, and lab results. “Wherever you can leverage the underlying tools that are part of the electronic health record to make sure that we’re constantly checking the right results, that helps reduce the workload so that clinicians can focus on taking care of the patients and doing the right thing and not be as focused on entering data into the system.”
2. Supplement EHR notes with direct communication
The failure to diagnose cancer because one physician doesn’t know what another physician saw in an imaging report is one of the most common claims in the cases he tries, says Aaron Boeder, a plaintiff’s medical negligence lawyer in Chicago.
Physicians often assume that if they put a note in the electronic chart, others will look for it, but Mr. Boeder says it’s far more prudent to communicate directly.
“Let’s say a radiologist interprets a scan and sees what might be cancer,” he says. “If the ordering doctor is an orthopedist who’s ordered a CT scan for DVT, there’s going to be a report for that scan. It’s going to get auto-populated back into that physician’s note,” says Mr. Boeder.
The physician may or may not look at it, but it will be in their note, and they’re supposed to follow up on it because they ordered the scan. “But they may not follow up on it, and they may not get a call from the radiologist,” he says.
“Next thing you know, 2 or 3 years later, that patient is diagnosed with very advanced cancer.”
3. Tailor auto-fill information to your common practices
Suppose, as a physician, you find that you need to change a default setting time and time again. Dr. Hettinger says it’s worth your time to take an extra couple of minutes to work with your vendor or your health system to try and make changes to auto-population settings that align with your practices.
“Let’s say a default dose of 20 milligrams of a medication is what automatically pops up, but in reality, your practice is to use a smaller dose because it’s safer, even though they’re all within the acceptable realm of what you would order,” he says. “Rather than have the default to the higher dose, see if you can change the default to a lower dose. And that way, you don’t have to catch yourself every time.”
If your auto-fills are amounts that constantly need changing, an interruption could easily knock you off course before you make that correction.
“If there are ways to have the system defaults be safer or more in line with your clinical practice, and especially across a group, then you’re designing a safer system and not relying on vigilance or memory prone to interruptions,” says Dr. Hettinger.
4. Curb the copy and paste
It’s tempting to copy a note from a previous patient visit and make only minimal changes as needed, but you risk including outdated information if you do. Even if you’re repeating questions asked by the intake nurse, it is safer to not to rely on that information, says Beth Kanik, a defense medical malpractice attorney in Atlanta.
“If it later goes into litigation, the argument then becomes that it looks like you didn’t do your job,” says Ms. Kanik. “Instead, try to ask questions in a way that would elicit responses that may be a little different than what the nurse got, so that it’s clear you asked the questions and didn’t just simply rely upon someone else’s information.”
5. Separate typing from listening
While EHR may be an excellent tool for data collection and safety checking, it’s not a stand-in for doctor-patient interaction. As technology practices push medicine toward more and more efficiency, Mr. Boeder says it’s most often listening over all else that makes the difference in the quality of care. And good listening requires full attention.
“A real concern for physicians is the number of visits they’re expected to accomplish in a set amount of time,” says Mr. Boeder. “Often this translates into a doctor talking to a patient while typing notes or while reading a note from the last time the patient was in.”
Taking the time to pause after entering data and briefly reviewing your understanding of what your patient has told you can be invaluable and may save you – and your patient – problems later.
“In so many cases, it comes down to people not being heard,” says Mr. Boeder. “So listen to what your patients are saying.”
A version of this article first appeared on Medscape.com.
Domestic violence in health care is real and underreported
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.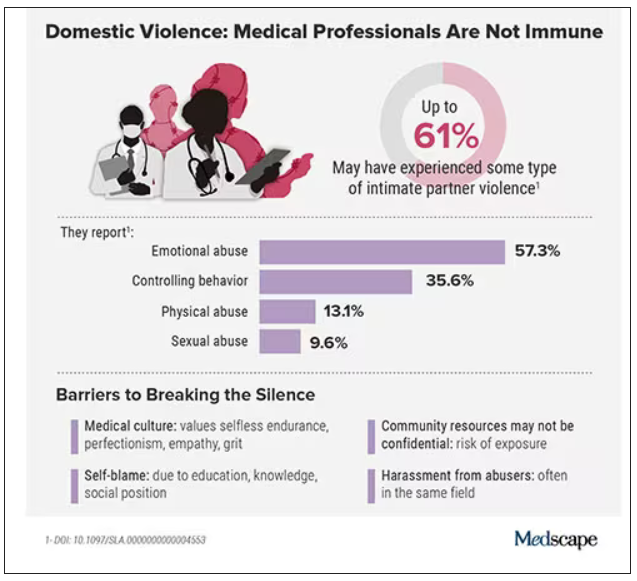
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
One in five doctors with long COVID can no longer work: Survey
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Resident creates AI alternative to U.S. News med school ranking
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Q&A: What to know about the new BA 2.86 COVID variant
The Centers for Disease Control and Prevention and the World Health Organization have dubbed the BA 2.86 variant of COVID-19 as a variant to watch.
So far, only 26 cases of “Pirola,” as the new variant is being called, have been identified: 10 in Denmark, four each in Sweden and the United States, three in South Africa, two in Portugal, and one each the United Kingdom, Israel, and Canada. BA 2.86 is a subvariant of Omicron, but according to reports from the CDC, the strain has many more mutations than the ones that came before it.
With so many facts still unknown about this new variant, this news organization asked experts what people need to be aware of as it continues to spread.
What is unique about the BA 2.86 variant?
“It is unique in that it has more than three mutations on the spike protein,” said Purvi S. Parikh, MD, an infectious disease expert at New York University’s Langone Health. The virus uses the spike proteins to enter our cells.
This “may mean it will be more transmissible, cause more severe disease, and/or our vaccines and treatments may not work as well, as compared to other variants,” she said.
What do we need to watch with BA 2.86 going forward?
“We don’t know if this variant will be associated with a change in the disease severity. We currently see increased numbers of cases in general, even though we don’t yet see the BA.2.86 in our system,” said Heba Mostafa, PhD, director of the molecular virology laboratory at Johns Hopkins Hospital in Baltimore.
“It is important to monitor BA.2.86 (and other variants) and understand how its evolution impacts the number of cases and disease outcomes,” she said. “We should all be aware of the current increase in cases, though, and try to get tested and be treated as soon as possible, as antivirals should be effective against the circulating variants.”
What should doctors know?
Dr. Parikh said doctors should generally expect more COVID cases in their clinics and make sure to screen patients even if their symptoms are mild.
“We have tools that can be used – antivirals like Paxlovid are still efficacious with current dominant strains such as EG.5,” she said. “And encourage your patients to get their boosters, mask, wash hands, and social distance.”
How well can our vaccines fight BA 2.86?
“Vaccine coverage for the BA.2.86 is an area of uncertainty right now,” said Dr. Mostafa.
In its report, the CDC said scientists are still figuring out how well the updated COVID vaccine works. It’s expected to be available in the fall, and for now, they believe the new shot will still make infections less severe, new variants and all.
Prior vaccinations and infections have created antibodies in many people, and that will likely provide some protection, Dr. Mostafa said. “When we experienced the Omicron wave in December 2021, even though the variant was distant from what circulated before its emergence and was associated with a very large increase in the number of cases, vaccinations were still protective against severe disease.”
What is the most important thing to keep track of when it comes to this variant?
According to Dr. Parikh, “it’s most important to monitor how transmissible [BA 2.86] is, how severe it is, and if our current treatments and vaccines work.”
Dr. Mostafa said how well the new variants escape existing antibody protection should also be studied and watched closely.
What does this stage of the virus mutation tell us about where we are in the pandemic?
The history of the coronavirus over the past few years shows that variants with many changes evolve and can spread very quickly, Dr. Mostafa said. “Now that the virus is endemic, it is essential to monitor, update vaccinations if necessary, diagnose, treat, and implement infection control measures when necessary.”
With the limited data we have so far, experts seem to agree that while the variant’s makeup raises some red flags, it is too soon to jump to any conclusions about how easy it is to catch it and the ways it may change how the virus impacts those who contract it.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention and the World Health Organization have dubbed the BA 2.86 variant of COVID-19 as a variant to watch.
So far, only 26 cases of “Pirola,” as the new variant is being called, have been identified: 10 in Denmark, four each in Sweden and the United States, three in South Africa, two in Portugal, and one each the United Kingdom, Israel, and Canada. BA 2.86 is a subvariant of Omicron, but according to reports from the CDC, the strain has many more mutations than the ones that came before it.
With so many facts still unknown about this new variant, this news organization asked experts what people need to be aware of as it continues to spread.
What is unique about the BA 2.86 variant?
“It is unique in that it has more than three mutations on the spike protein,” said Purvi S. Parikh, MD, an infectious disease expert at New York University’s Langone Health. The virus uses the spike proteins to enter our cells.
This “may mean it will be more transmissible, cause more severe disease, and/or our vaccines and treatments may not work as well, as compared to other variants,” she said.
What do we need to watch with BA 2.86 going forward?
“We don’t know if this variant will be associated with a change in the disease severity. We currently see increased numbers of cases in general, even though we don’t yet see the BA.2.86 in our system,” said Heba Mostafa, PhD, director of the molecular virology laboratory at Johns Hopkins Hospital in Baltimore.
“It is important to monitor BA.2.86 (and other variants) and understand how its evolution impacts the number of cases and disease outcomes,” she said. “We should all be aware of the current increase in cases, though, and try to get tested and be treated as soon as possible, as antivirals should be effective against the circulating variants.”
What should doctors know?
Dr. Parikh said doctors should generally expect more COVID cases in their clinics and make sure to screen patients even if their symptoms are mild.
“We have tools that can be used – antivirals like Paxlovid are still efficacious with current dominant strains such as EG.5,” she said. “And encourage your patients to get their boosters, mask, wash hands, and social distance.”
How well can our vaccines fight BA 2.86?
“Vaccine coverage for the BA.2.86 is an area of uncertainty right now,” said Dr. Mostafa.
In its report, the CDC said scientists are still figuring out how well the updated COVID vaccine works. It’s expected to be available in the fall, and for now, they believe the new shot will still make infections less severe, new variants and all.
Prior vaccinations and infections have created antibodies in many people, and that will likely provide some protection, Dr. Mostafa said. “When we experienced the Omicron wave in December 2021, even though the variant was distant from what circulated before its emergence and was associated with a very large increase in the number of cases, vaccinations were still protective against severe disease.”
What is the most important thing to keep track of when it comes to this variant?
According to Dr. Parikh, “it’s most important to monitor how transmissible [BA 2.86] is, how severe it is, and if our current treatments and vaccines work.”
Dr. Mostafa said how well the new variants escape existing antibody protection should also be studied and watched closely.
What does this stage of the virus mutation tell us about where we are in the pandemic?
The history of the coronavirus over the past few years shows that variants with many changes evolve and can spread very quickly, Dr. Mostafa said. “Now that the virus is endemic, it is essential to monitor, update vaccinations if necessary, diagnose, treat, and implement infection control measures when necessary.”
With the limited data we have so far, experts seem to agree that while the variant’s makeup raises some red flags, it is too soon to jump to any conclusions about how easy it is to catch it and the ways it may change how the virus impacts those who contract it.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention and the World Health Organization have dubbed the BA 2.86 variant of COVID-19 as a variant to watch.
So far, only 26 cases of “Pirola,” as the new variant is being called, have been identified: 10 in Denmark, four each in Sweden and the United States, three in South Africa, two in Portugal, and one each the United Kingdom, Israel, and Canada. BA 2.86 is a subvariant of Omicron, but according to reports from the CDC, the strain has many more mutations than the ones that came before it.
With so many facts still unknown about this new variant, this news organization asked experts what people need to be aware of as it continues to spread.
What is unique about the BA 2.86 variant?
“It is unique in that it has more than three mutations on the spike protein,” said Purvi S. Parikh, MD, an infectious disease expert at New York University’s Langone Health. The virus uses the spike proteins to enter our cells.
This “may mean it will be more transmissible, cause more severe disease, and/or our vaccines and treatments may not work as well, as compared to other variants,” she said.
What do we need to watch with BA 2.86 going forward?
“We don’t know if this variant will be associated with a change in the disease severity. We currently see increased numbers of cases in general, even though we don’t yet see the BA.2.86 in our system,” said Heba Mostafa, PhD, director of the molecular virology laboratory at Johns Hopkins Hospital in Baltimore.
“It is important to monitor BA.2.86 (and other variants) and understand how its evolution impacts the number of cases and disease outcomes,” she said. “We should all be aware of the current increase in cases, though, and try to get tested and be treated as soon as possible, as antivirals should be effective against the circulating variants.”
What should doctors know?
Dr. Parikh said doctors should generally expect more COVID cases in their clinics and make sure to screen patients even if their symptoms are mild.
“We have tools that can be used – antivirals like Paxlovid are still efficacious with current dominant strains such as EG.5,” she said. “And encourage your patients to get their boosters, mask, wash hands, and social distance.”
How well can our vaccines fight BA 2.86?
“Vaccine coverage for the BA.2.86 is an area of uncertainty right now,” said Dr. Mostafa.
In its report, the CDC said scientists are still figuring out how well the updated COVID vaccine works. It’s expected to be available in the fall, and for now, they believe the new shot will still make infections less severe, new variants and all.
Prior vaccinations and infections have created antibodies in many people, and that will likely provide some protection, Dr. Mostafa said. “When we experienced the Omicron wave in December 2021, even though the variant was distant from what circulated before its emergence and was associated with a very large increase in the number of cases, vaccinations were still protective against severe disease.”
What is the most important thing to keep track of when it comes to this variant?
According to Dr. Parikh, “it’s most important to monitor how transmissible [BA 2.86] is, how severe it is, and if our current treatments and vaccines work.”
Dr. Mostafa said how well the new variants escape existing antibody protection should also be studied and watched closely.
What does this stage of the virus mutation tell us about where we are in the pandemic?
The history of the coronavirus over the past few years shows that variants with many changes evolve and can spread very quickly, Dr. Mostafa said. “Now that the virus is endemic, it is essential to monitor, update vaccinations if necessary, diagnose, treat, and implement infection control measures when necessary.”
With the limited data we have so far, experts seem to agree that while the variant’s makeup raises some red flags, it is too soon to jump to any conclusions about how easy it is to catch it and the ways it may change how the virus impacts those who contract it.
A version of this article first appeared on WebMD.com.