User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Universal CVD Risk Prediction Model Shows Good Performance
TOPLINE:
A universal cardiovascular disease (CVD) prediction tool performs well in patients with and without atherosclerotic CVD (ASCVD), a new study showed, suggesting this model could facilitate transition from primary to secondary prevention by streamlining risk classification.
METHODOLOGY:
- Researchers used different models to evaluate whether established CVD predictors, including age, sex, race, diabetes, systolic blood pressure, or smoking, are associated with major adverse cardiovascular events (MACEs), including myocardial infarction (MI), stroke, and heart failure (HF), among 9138 patients, mean age 63.8 years, in the Atherosclerosis Risk in Communities (ARIC) study.
- Of these, 609 had ASCVD (history of MI, ischemic stroke, or symptomatic peripheral artery disease) and 8529 did not.
- They extended their exploration to other predictors available in clinical practice, including family history of premature ASCVD, high-sensitivity C-reactive protein, lipoprotein(a), triglycerides, and apolipoprotein B, as well as predictors of HF such as body mass index and heart rate and blood-based cardiac biomarkers.
- An external validation analysis included 5322 participants in the Multi-Ethnic Study of Atherosclerosis (MESA).
- Over a median follow-up of 18.9 years, 3209 ARIC participants (35%) developed MACE for an incidence rate per 1000 person-years of 21.3 for MACE, 12.6 for MI/stroke, and 13.8 for HF.
TAKEAWAY:
- Of all candidate predictors, 10 variables (including established predictors and cardiac biomarkers) were included in the universal prediction model, which demonstrated good calibration in both those with ASCVD (hazard ratio [HR] C-statistic, 0.692; 95% CI, 0.650-0.735) and without ASCVD (HR C-statistic, 0.748; 95% CI, 0.726-0.770).
- As anticipated, the risk for MACE was generally lower in those with no prior ASCVD, but the 5-year risk in the highest quintile of predicted risk in those without ASCVD was higher than that in the lowest two quintiles of the ASCVD group.
- The universal risk prediction model was validated in the MESA community–based cohort; over a median follow-up of 13.7 years, 12% of participants with and without prior ASCVD developed MACE for an incidence rate per 1000 person-years of 10.2 for MACE, 7.4 for MI/stroke, and 4.3 for HF.
- The results were generally similar when examining individual outcomes (MI/stroke and HF) and for both no ASCVD and ASCVD groups across demographic subgroups by age, sex, and race.
IN PRACTICE:
The findings “support the importance of established predictors for classifying long-term CVD risk in both primary and secondary prevention settings,” the authors wrote, adding an advantage to this risk prediction approach could be to help providers and patients “further personalize secondary prevention.”
In an accompanying editorial, Pier Sergio Saba, MD, PhD, Clinical and Interventional Cardiology, Sassari University Hospital, Sassari, Italy, and others said the universal risk assessment approach “is conceptually promising” but noted patients with ASCVD represented only 7% of the study population, and this population was relatively young, potentially limiting the applicability of this risk model in older individuals. Before the risk model can be used in clinical settings, results need to be validated and given incorporation of cardiac biomarkers, “careful cost-benefit analyses may also be needed,” the editorial writers added.
SOURCE:
The study was conducted by Yejin Mok, PHD, MPH, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues. It was published online on January 29, 2024, in the Journal of the American College of Cardiology (JACC).
LIMITATIONS:
The somewhat limited number of study participants with prior ASCVD precluded researchers from quantifying the prognostic impact of ASCVD subtypes (eg, history of MI vs stroke vs peripheral artery disease). The study didn’t have data on some predictors recognized in guidelines (eg, coronary artery calcium and left ventricular ejection fraction). The ARIC analysis included only Black and White participants, and although models were validated in MESA, which included Chinese and Hispanic adults, extrapolation of results to more racially/ethnically diverse populations should be done with care.
DISCLOSURES:
The ARIC study received funding from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, and Department of Health and Human Services. The MESA study was supported by the NHLBI and National Center for Advancing Translational Sciences. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A universal cardiovascular disease (CVD) prediction tool performs well in patients with and without atherosclerotic CVD (ASCVD), a new study showed, suggesting this model could facilitate transition from primary to secondary prevention by streamlining risk classification.
METHODOLOGY:
- Researchers used different models to evaluate whether established CVD predictors, including age, sex, race, diabetes, systolic blood pressure, or smoking, are associated with major adverse cardiovascular events (MACEs), including myocardial infarction (MI), stroke, and heart failure (HF), among 9138 patients, mean age 63.8 years, in the Atherosclerosis Risk in Communities (ARIC) study.
- Of these, 609 had ASCVD (history of MI, ischemic stroke, or symptomatic peripheral artery disease) and 8529 did not.
- They extended their exploration to other predictors available in clinical practice, including family history of premature ASCVD, high-sensitivity C-reactive protein, lipoprotein(a), triglycerides, and apolipoprotein B, as well as predictors of HF such as body mass index and heart rate and blood-based cardiac biomarkers.
- An external validation analysis included 5322 participants in the Multi-Ethnic Study of Atherosclerosis (MESA).
- Over a median follow-up of 18.9 years, 3209 ARIC participants (35%) developed MACE for an incidence rate per 1000 person-years of 21.3 for MACE, 12.6 for MI/stroke, and 13.8 for HF.
TAKEAWAY:
- Of all candidate predictors, 10 variables (including established predictors and cardiac biomarkers) were included in the universal prediction model, which demonstrated good calibration in both those with ASCVD (hazard ratio [HR] C-statistic, 0.692; 95% CI, 0.650-0.735) and without ASCVD (HR C-statistic, 0.748; 95% CI, 0.726-0.770).
- As anticipated, the risk for MACE was generally lower in those with no prior ASCVD, but the 5-year risk in the highest quintile of predicted risk in those without ASCVD was higher than that in the lowest two quintiles of the ASCVD group.
- The universal risk prediction model was validated in the MESA community–based cohort; over a median follow-up of 13.7 years, 12% of participants with and without prior ASCVD developed MACE for an incidence rate per 1000 person-years of 10.2 for MACE, 7.4 for MI/stroke, and 4.3 for HF.
- The results were generally similar when examining individual outcomes (MI/stroke and HF) and for both no ASCVD and ASCVD groups across demographic subgroups by age, sex, and race.
IN PRACTICE:
The findings “support the importance of established predictors for classifying long-term CVD risk in both primary and secondary prevention settings,” the authors wrote, adding an advantage to this risk prediction approach could be to help providers and patients “further personalize secondary prevention.”
In an accompanying editorial, Pier Sergio Saba, MD, PhD, Clinical and Interventional Cardiology, Sassari University Hospital, Sassari, Italy, and others said the universal risk assessment approach “is conceptually promising” but noted patients with ASCVD represented only 7% of the study population, and this population was relatively young, potentially limiting the applicability of this risk model in older individuals. Before the risk model can be used in clinical settings, results need to be validated and given incorporation of cardiac biomarkers, “careful cost-benefit analyses may also be needed,” the editorial writers added.
SOURCE:
The study was conducted by Yejin Mok, PHD, MPH, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues. It was published online on January 29, 2024, in the Journal of the American College of Cardiology (JACC).
LIMITATIONS:
The somewhat limited number of study participants with prior ASCVD precluded researchers from quantifying the prognostic impact of ASCVD subtypes (eg, history of MI vs stroke vs peripheral artery disease). The study didn’t have data on some predictors recognized in guidelines (eg, coronary artery calcium and left ventricular ejection fraction). The ARIC analysis included only Black and White participants, and although models were validated in MESA, which included Chinese and Hispanic adults, extrapolation of results to more racially/ethnically diverse populations should be done with care.
DISCLOSURES:
The ARIC study received funding from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, and Department of Health and Human Services. The MESA study was supported by the NHLBI and National Center for Advancing Translational Sciences. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A universal cardiovascular disease (CVD) prediction tool performs well in patients with and without atherosclerotic CVD (ASCVD), a new study showed, suggesting this model could facilitate transition from primary to secondary prevention by streamlining risk classification.
METHODOLOGY:
- Researchers used different models to evaluate whether established CVD predictors, including age, sex, race, diabetes, systolic blood pressure, or smoking, are associated with major adverse cardiovascular events (MACEs), including myocardial infarction (MI), stroke, and heart failure (HF), among 9138 patients, mean age 63.8 years, in the Atherosclerosis Risk in Communities (ARIC) study.
- Of these, 609 had ASCVD (history of MI, ischemic stroke, or symptomatic peripheral artery disease) and 8529 did not.
- They extended their exploration to other predictors available in clinical practice, including family history of premature ASCVD, high-sensitivity C-reactive protein, lipoprotein(a), triglycerides, and apolipoprotein B, as well as predictors of HF such as body mass index and heart rate and blood-based cardiac biomarkers.
- An external validation analysis included 5322 participants in the Multi-Ethnic Study of Atherosclerosis (MESA).
- Over a median follow-up of 18.9 years, 3209 ARIC participants (35%) developed MACE for an incidence rate per 1000 person-years of 21.3 for MACE, 12.6 for MI/stroke, and 13.8 for HF.
TAKEAWAY:
- Of all candidate predictors, 10 variables (including established predictors and cardiac biomarkers) were included in the universal prediction model, which demonstrated good calibration in both those with ASCVD (hazard ratio [HR] C-statistic, 0.692; 95% CI, 0.650-0.735) and without ASCVD (HR C-statistic, 0.748; 95% CI, 0.726-0.770).
- As anticipated, the risk for MACE was generally lower in those with no prior ASCVD, but the 5-year risk in the highest quintile of predicted risk in those without ASCVD was higher than that in the lowest two quintiles of the ASCVD group.
- The universal risk prediction model was validated in the MESA community–based cohort; over a median follow-up of 13.7 years, 12% of participants with and without prior ASCVD developed MACE for an incidence rate per 1000 person-years of 10.2 for MACE, 7.4 for MI/stroke, and 4.3 for HF.
- The results were generally similar when examining individual outcomes (MI/stroke and HF) and for both no ASCVD and ASCVD groups across demographic subgroups by age, sex, and race.
IN PRACTICE:
The findings “support the importance of established predictors for classifying long-term CVD risk in both primary and secondary prevention settings,” the authors wrote, adding an advantage to this risk prediction approach could be to help providers and patients “further personalize secondary prevention.”
In an accompanying editorial, Pier Sergio Saba, MD, PhD, Clinical and Interventional Cardiology, Sassari University Hospital, Sassari, Italy, and others said the universal risk assessment approach “is conceptually promising” but noted patients with ASCVD represented only 7% of the study population, and this population was relatively young, potentially limiting the applicability of this risk model in older individuals. Before the risk model can be used in clinical settings, results need to be validated and given incorporation of cardiac biomarkers, “careful cost-benefit analyses may also be needed,” the editorial writers added.
SOURCE:
The study was conducted by Yejin Mok, PHD, MPH, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues. It was published online on January 29, 2024, in the Journal of the American College of Cardiology (JACC).
LIMITATIONS:
The somewhat limited number of study participants with prior ASCVD precluded researchers from quantifying the prognostic impact of ASCVD subtypes (eg, history of MI vs stroke vs peripheral artery disease). The study didn’t have data on some predictors recognized in guidelines (eg, coronary artery calcium and left ventricular ejection fraction). The ARIC analysis included only Black and White participants, and although models were validated in MESA, which included Chinese and Hispanic adults, extrapolation of results to more racially/ethnically diverse populations should be done with care.
DISCLOSURES:
The ARIC study received funding from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, and Department of Health and Human Services. The MESA study was supported by the NHLBI and National Center for Advancing Translational Sciences. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Switching From IV to Oral Antibiotics Safe for Patients, Study Shows
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to a recent observational study published in JAMA Network Open.
METHODOLOGY:
- Patients receiving antibiotics through an IV line risk developing a secondary infection; antibiotics received orally are considered safer.
- Researchers analyzed observational data from 914 adults with uncomplicated gram-negative bacteremia who received care in four hospitals in Denmark between 2018 and 2021.
- The outcomes of patients who were switched to oral antibiotics within 4 days after a positive blood culture were compared with those who continued to receive IV antibiotics for at least 5 days after the blood culture; participants in both groups received antibiotics for 7-14 days.
- Researchers assessed mortality rates over a 90-day window and used a target trial emulation method to conduct the study.
TAKEAWAY:
- Overall, 14.3% of patients who received prolonged IV treatment died, compared with 6.9% in the oral antibiotics group.
- In an intention-to-treat analysis, patients who were switched to oral antibiotics had a 22% lower risk for death within 90 days of initiation of treatment (relative risk [RR], 0.78; 95% CI, 0.60-1.10).
- In a per-protocol analysis, patients who switched to the oral route had a 1% lower odds of dying within 90 days (RR, 0.99; 95% CI, 0.70-1.40).
- Individuals who were switched to oral antibiotic treatment were younger than those who continued to receive antibiotics via the IV route (median age, 73 vs 76 years, respectively), had fewer comorbidities (four vs five), and were more likely to have community-acquired gram-negative bacteremia (89.4% vs 80.9%).
IN PRACTICE:
“These findings suggest that the mortality associated with early antibiotic stepdown treatment is comparable to that associated with receiving prolonged IV antibiotic treatment for individuals with uncomplicated gram-negative bacteremia,” the authors of the study wrote.
SOURCE:
The study was led by Sandra Tingsgård, MD, of the Center of Research & Department of Infectious Diseases at Copenhagen University Hospital–Amager and Hvidovre in Denmark.
LIMITATIONS:
The study was based on data from electronic health records, so some factors may not have been recorded or considered. The researchers identified few cases of multidrug-resistant infections, and the findings may not apply to those cases. Complicated cases and people who were not stabilized by day 4 were excluded from the analysis.
DISCLOSURES:
The authors report no disclosures or sources of funding.
A version of this article appeared on Medscape.com.
New Guidelines: Brain Death Is Equal to Heart Death, Says Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the New York University Grossman School of Medicine in New York City.
I think we had a breakthrough on a very controversial subject over the past month. Over and over again, debates have been breaking out, cases have been going to court, and fights have been coming to ethics committees about brain death. How do we know what brain death is, how do we diagnose it, and what rights do families have with respect to the diagnosis?
The American Academy of Neurology decided to form a task force, and they just issued guidelines on the definition, tests to use it, and the rights of families. They did a wonderful job, in my view. They›ve achieved clarity.
First, they tried to handle both adults and children. Children are, if you will, more difficult — and that’s been known — to test for brain death. Their brains are smaller. You get more interference and false signals coming from muscle or nerve activity that might be going on elsewhere in their bodies.
The guidelines say we’re going to try to see whether a person can breathe without support. If it’s an adult, one test over a 24-hour period would be sufficient. If you had them off the ventilator and they can’t breathe and show no signs of being able to do that, that’s a very fundamental test for brain death. For children, you’re going to have to do it twice. The guidelines are saying to be cautious.
Second, they say it’s very important to know the cause of the suspected brain death condition. If someone has a massive head injury, that’s different from a situation in which someone overdoses from drugs or drowns. Those conditions can be a little deceptive. In the case of drowning, sometimes the brain has protective mechanisms to protect circulation to the brain naturally for a little bit of time. I’m talking about minutes, not hours.
You want to be careful to make sure that you know the cause of the massive brain injury or insult that makes someone believe that the patient is brain-dead, whether it’s a stroke, an embolism, a bleed, a gunshot wound, or trauma to the head. Those factors really drive the certainty with which brain death should be pronounced. I think that’s very, very important.
They also said that brain death means the permanent loss of brain function. You may get a few cells still firing or you may be in a situation, because the life support is still there, where the body looks pink and perhaps might appear to still be alive to someone. When you know that the damage to the brain is so severe that there’s nothing that can be done to bring back the support of heart function, breathing, and most likely any ability to sense or feel anything, that is death.
I believe it’s very important, when talking to families, to say there are two ways that we pronounce people dead, and they’re equal: One is to say their heart has stopped, their breathing has stopped, and there’s nothing we can do to resuscitate them, which is cardiac death. The other is to say their brain has permanently ceased to function in any kind of integrated way. That means no heartbeat, no breathing, and no mental sensations. That is death.
In approaching families, it is critical that doctors and nurses don’t say, “Your relative is brain-dead.” That gives the family a sense that maybe they’re only “partially dead” or maybe there’s one key organ that has stopped working but maybe you can bring it back. Death is death. The law recognizes both cardiac death and brain death as death.
When you approach a family, if you believe that death has occurred, you say, “I’m very sorry. With regret, I have to tell you, your loved one is dead.” If they ask how you know, you can say, “We’ve determined it through brain death or through cardiac death.” You don’t give them a sense that people could be kind of dead, sort of dead, or nearly dead. Those states are comas or permanent vegetative states; they’re not the same as death.
What if the family says, “I don’t want you to do any testing. I don’t want to find out whether my relative is dead”? The American Academy of Neurology looked at this carefully and said that any test for death can be done without the permission or consent of the family. They said that because doctors need to know what steps to take to treat someone.
If a person is dead, then treatment is going to stop. It may not stop immediately. There may be issues about organ donation. There may be issues about gathering the family to come to the bedside to say goodbye, because many people think that’s more humane than saying goodbye at the morgue or in another setting.
This is all well and good, but patients cannot protect against bad news when it comes to death. We don’t want to be doing things to the dead that cost money or are futile because of death and using resources that might go to others.
We’ve got much more clarity than we have ever had with respect to the issue of brain death and how it works in any hospital. We have certain tests, including being off the ventilator and some other tests, that the guidelines supply. We know we have to be more careful with children. We want to know the etiology of the cause of the brain trauma, the devastating brain injury, to be sure that this is something that really is permanent cessation of integrated brain function.
We know that if you believe the person has died, you don’t need the consent of the family in order to do a brain-death test. You have to do it because there is no point in continuing treatment in expensive ICU settings and denying resources to others who might want to use those resources. The family can’t hold the medical team hostage.
We do know that when we approach someone with the determination, whatever it is, we should lead by saying that the person has died and then explain how that was determined, whether it be by cardiac death pronouncement — where you tried to resuscitate and the heart’s not beating — or brain-death analysis.
I’m Art Caplan at the Division of Medical Ethics at the NYU Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for: Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); serves as a contributing author and adviser for this news organization.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the New York University Grossman School of Medicine in New York City.
I think we had a breakthrough on a very controversial subject over the past month. Over and over again, debates have been breaking out, cases have been going to court, and fights have been coming to ethics committees about brain death. How do we know what brain death is, how do we diagnose it, and what rights do families have with respect to the diagnosis?
The American Academy of Neurology decided to form a task force, and they just issued guidelines on the definition, tests to use it, and the rights of families. They did a wonderful job, in my view. They›ve achieved clarity.
First, they tried to handle both adults and children. Children are, if you will, more difficult — and that’s been known — to test for brain death. Their brains are smaller. You get more interference and false signals coming from muscle or nerve activity that might be going on elsewhere in their bodies.
The guidelines say we’re going to try to see whether a person can breathe without support. If it’s an adult, one test over a 24-hour period would be sufficient. If you had them off the ventilator and they can’t breathe and show no signs of being able to do that, that’s a very fundamental test for brain death. For children, you’re going to have to do it twice. The guidelines are saying to be cautious.
Second, they say it’s very important to know the cause of the suspected brain death condition. If someone has a massive head injury, that’s different from a situation in which someone overdoses from drugs or drowns. Those conditions can be a little deceptive. In the case of drowning, sometimes the brain has protective mechanisms to protect circulation to the brain naturally for a little bit of time. I’m talking about minutes, not hours.
You want to be careful to make sure that you know the cause of the massive brain injury or insult that makes someone believe that the patient is brain-dead, whether it’s a stroke, an embolism, a bleed, a gunshot wound, or trauma to the head. Those factors really drive the certainty with which brain death should be pronounced. I think that’s very, very important.
They also said that brain death means the permanent loss of brain function. You may get a few cells still firing or you may be in a situation, because the life support is still there, where the body looks pink and perhaps might appear to still be alive to someone. When you know that the damage to the brain is so severe that there’s nothing that can be done to bring back the support of heart function, breathing, and most likely any ability to sense or feel anything, that is death.
I believe it’s very important, when talking to families, to say there are two ways that we pronounce people dead, and they’re equal: One is to say their heart has stopped, their breathing has stopped, and there’s nothing we can do to resuscitate them, which is cardiac death. The other is to say their brain has permanently ceased to function in any kind of integrated way. That means no heartbeat, no breathing, and no mental sensations. That is death.
In approaching families, it is critical that doctors and nurses don’t say, “Your relative is brain-dead.” That gives the family a sense that maybe they’re only “partially dead” or maybe there’s one key organ that has stopped working but maybe you can bring it back. Death is death. The law recognizes both cardiac death and brain death as death.
When you approach a family, if you believe that death has occurred, you say, “I’m very sorry. With regret, I have to tell you, your loved one is dead.” If they ask how you know, you can say, “We’ve determined it through brain death or through cardiac death.” You don’t give them a sense that people could be kind of dead, sort of dead, or nearly dead. Those states are comas or permanent vegetative states; they’re not the same as death.
What if the family says, “I don’t want you to do any testing. I don’t want to find out whether my relative is dead”? The American Academy of Neurology looked at this carefully and said that any test for death can be done without the permission or consent of the family. They said that because doctors need to know what steps to take to treat someone.
If a person is dead, then treatment is going to stop. It may not stop immediately. There may be issues about organ donation. There may be issues about gathering the family to come to the bedside to say goodbye, because many people think that’s more humane than saying goodbye at the morgue or in another setting.
This is all well and good, but patients cannot protect against bad news when it comes to death. We don’t want to be doing things to the dead that cost money or are futile because of death and using resources that might go to others.
We’ve got much more clarity than we have ever had with respect to the issue of brain death and how it works in any hospital. We have certain tests, including being off the ventilator and some other tests, that the guidelines supply. We know we have to be more careful with children. We want to know the etiology of the cause of the brain trauma, the devastating brain injury, to be sure that this is something that really is permanent cessation of integrated brain function.
We know that if you believe the person has died, you don’t need the consent of the family in order to do a brain-death test. You have to do it because there is no point in continuing treatment in expensive ICU settings and denying resources to others who might want to use those resources. The family can’t hold the medical team hostage.
We do know that when we approach someone with the determination, whatever it is, we should lead by saying that the person has died and then explain how that was determined, whether it be by cardiac death pronouncement — where you tried to resuscitate and the heart’s not beating — or brain-death analysis.
I’m Art Caplan at the Division of Medical Ethics at the NYU Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for: Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); serves as a contributing author and adviser for this news organization.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the New York University Grossman School of Medicine in New York City.
I think we had a breakthrough on a very controversial subject over the past month. Over and over again, debates have been breaking out, cases have been going to court, and fights have been coming to ethics committees about brain death. How do we know what brain death is, how do we diagnose it, and what rights do families have with respect to the diagnosis?
The American Academy of Neurology decided to form a task force, and they just issued guidelines on the definition, tests to use it, and the rights of families. They did a wonderful job, in my view. They›ve achieved clarity.
First, they tried to handle both adults and children. Children are, if you will, more difficult — and that’s been known — to test for brain death. Their brains are smaller. You get more interference and false signals coming from muscle or nerve activity that might be going on elsewhere in their bodies.
The guidelines say we’re going to try to see whether a person can breathe without support. If it’s an adult, one test over a 24-hour period would be sufficient. If you had them off the ventilator and they can’t breathe and show no signs of being able to do that, that’s a very fundamental test for brain death. For children, you’re going to have to do it twice. The guidelines are saying to be cautious.
Second, they say it’s very important to know the cause of the suspected brain death condition. If someone has a massive head injury, that’s different from a situation in which someone overdoses from drugs or drowns. Those conditions can be a little deceptive. In the case of drowning, sometimes the brain has protective mechanisms to protect circulation to the brain naturally for a little bit of time. I’m talking about minutes, not hours.
You want to be careful to make sure that you know the cause of the massive brain injury or insult that makes someone believe that the patient is brain-dead, whether it’s a stroke, an embolism, a bleed, a gunshot wound, or trauma to the head. Those factors really drive the certainty with which brain death should be pronounced. I think that’s very, very important.
They also said that brain death means the permanent loss of brain function. You may get a few cells still firing or you may be in a situation, because the life support is still there, where the body looks pink and perhaps might appear to still be alive to someone. When you know that the damage to the brain is so severe that there’s nothing that can be done to bring back the support of heart function, breathing, and most likely any ability to sense or feel anything, that is death.
I believe it’s very important, when talking to families, to say there are two ways that we pronounce people dead, and they’re equal: One is to say their heart has stopped, their breathing has stopped, and there’s nothing we can do to resuscitate them, which is cardiac death. The other is to say their brain has permanently ceased to function in any kind of integrated way. That means no heartbeat, no breathing, and no mental sensations. That is death.
In approaching families, it is critical that doctors and nurses don’t say, “Your relative is brain-dead.” That gives the family a sense that maybe they’re only “partially dead” or maybe there’s one key organ that has stopped working but maybe you can bring it back. Death is death. The law recognizes both cardiac death and brain death as death.
When you approach a family, if you believe that death has occurred, you say, “I’m very sorry. With regret, I have to tell you, your loved one is dead.” If they ask how you know, you can say, “We’ve determined it through brain death or through cardiac death.” You don’t give them a sense that people could be kind of dead, sort of dead, or nearly dead. Those states are comas or permanent vegetative states; they’re not the same as death.
What if the family says, “I don’t want you to do any testing. I don’t want to find out whether my relative is dead”? The American Academy of Neurology looked at this carefully and said that any test for death can be done without the permission or consent of the family. They said that because doctors need to know what steps to take to treat someone.
If a person is dead, then treatment is going to stop. It may not stop immediately. There may be issues about organ donation. There may be issues about gathering the family to come to the bedside to say goodbye, because many people think that’s more humane than saying goodbye at the morgue or in another setting.
This is all well and good, but patients cannot protect against bad news when it comes to death. We don’t want to be doing things to the dead that cost money or are futile because of death and using resources that might go to others.
We’ve got much more clarity than we have ever had with respect to the issue of brain death and how it works in any hospital. We have certain tests, including being off the ventilator and some other tests, that the guidelines supply. We know we have to be more careful with children. We want to know the etiology of the cause of the brain trauma, the devastating brain injury, to be sure that this is something that really is permanent cessation of integrated brain function.
We know that if you believe the person has died, you don’t need the consent of the family in order to do a brain-death test. You have to do it because there is no point in continuing treatment in expensive ICU settings and denying resources to others who might want to use those resources. The family can’t hold the medical team hostage.
We do know that when we approach someone with the determination, whatever it is, we should lead by saying that the person has died and then explain how that was determined, whether it be by cardiac death pronouncement — where you tried to resuscitate and the heart’s not beating — or brain-death analysis.
I’m Art Caplan at the Division of Medical Ethics at the NYU Grossman School of Medicine. Thanks for watching.
Dr. Caplan has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for: Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); serves as a contributing author and adviser for this news organization.
A version of this article appeared on Medscape.com.
Long COVID: Another Great Pretender
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
Circulating Tumor Cells Can Predict Progression in Stage 3 NSCLC
Circulating tumor cells (CTCs), the cells shed from a solid tumor into the bloodstream, may help doctors avoid having to do repeat needle biopsies on patients with unresectable non–small cell lung cancer.
Challenges to using CTCs clinically are that they are not abundant in the blood and have been difficult to isolate in patients with this type of cancer with commercially available assays.
In their paper, the authors show that an experimental nanotechnology can effectively isolate and measure CTCs in patients with stage 3 NSCLC. They also found that a precipitous drop in CTCs during chemoradiation treatment predicted significantly longer progression-free survival in those patients.
Study Results and Methods
For their research, study coauthors Shruti Jolly, MD, and Sunitha Nagrath, PhD, used a novel graphene oxide technology called the GO chip, developed more than a decade ago by Dr. Nagrath and her colleagues, to isolate CTCs from patients with stage 3 NSCLC. While a different technology, which is approved by the US Food and Drug Administration (FDA), uses a single antibody to pick up CTCs, the GO chip uses a cocktail of three antibodies to CTC proteins, making it more sensitive.
The 26 patients in the study (mean age 67, 27% female) all received radiation treatment for 6 weeks, plus weekly carboplatin and paclitaxel chemotherapy. Sixteen of the patients afterward went on to have immunotherapy with durvalumab. Blood was drawn at six fixed time points: before treatment, and at weeks 1, 4, 10, 18, and 30. CTCs were measured and analyzed with every draw.
Previous studies showed that absolute number of CTCs did not correlate with either tumor volume or progression-free survival in NSCLC.
Dr. Jolly and Dr. Nagrath sought to measure change in CTCs from baseline for each patient, having the patient serve as his or her own control. They found that patients whose individual CTC counts dropped by 75% or more between pretreatment and week 4 of chemoradiation saw a mean 21 months of progression-free survival compared with 7 months for patients whose CTCs dropped by less than 75% in the same period (P = .0076).
Dr. Jolly and Dr. Nagrath also aimed to determine, as an exploratory outcome of their study, whether other information collected from the CTCs could predict response to treatment with durvalumab immunotherapy. They found that having more than 50% of CTCs positive for the protein PD-L1 correlated to shorter progression-free survival among the 16 patients receiving durvalumab (P = .04).
“Every person’s tumor is unique in terms of its response to treatment,” said Dr. Jolly, a radiation oncologist and professor and associate chair of community practices in the Department of Radiation Oncology at the University of Michigan, Ann Arbor.
“Two people with a three-centimeter lung tumor will not necessarily shed the same amount of tumor cells into circulation. CTCs are reflective of disease burden; however, this is not related to the absolute numbers. That’s why we decided to use individualized baselines and look at the percentage of decrease,” she said.
Dr. Nagrath, professor of chemical and biomedical engineering at the University of Michigan, noted, in the same interview, that the findings argue for CTCs as a biomarker in stage 3 NSCLC.
“A lot of researchers who do lung cancer studies struggle with isolating lung cancer CTCs,” Dr. Nagrath said. “We showed, with repeated blood draws during treatment, what is changing at a molecular level and that you can see it with a simple blood draw. It also gives the proof of concept that if these cells are present, this is a good way to monitor and see if a treatment is working, even early in the treatment.” Moreover, she added, “many studies in lung cancer are in stage 4.”
Our study is unique as it followed patients with locally advanced tumors from their being treatment naive to all the way through immunotherapy,” she continued.
The University of Michigan has a patent on the GO chip technology, but thus far no company has made efforts to license it and submit it for approval. While “liquid biopsy” is an important emerging concept in lung cancer, there is little consensus yet as to which blood biomarkers — whether CTCs, circulating tumor DNA (ctDNA), or extracellular vesicles (EVs) — are most clinically relevant, Dr. Nagrath said.
The study’s small size is one of its weaknesses, according to the authors.
Findings are ‘Particularly Intriguing’
Majid Ebrahimi Warkiani, PhD, who was not involved in the study, described the new findings as “particularly intriguing [and] highlighting the efficacy of liquid biopsy using CTCs for predicting treatment outcomes.”
A challenge within the realm of CTCs lies in the community’s ongoing struggle to define and classify these cells accurately, Dr. Warkiani said in an interview.
“While surface protein markers offer valuable insights, emerging layers of analysis, such as metabolomics, are increasingly entering the scene to bolster the identification of putative cancer cells, alongside molecular tests like fluorescence in situ hybridization (FISH),” said Dr. Warkiani of the University of Technology Sydney in Australia. “The amalgamation of these approaches simultaneously presents a significant challenge, particularly in terms of standardization for patient care, unlike ctDNA, which faces fewer bottlenecks.
“The robustness of the research in this study is commendable. However, further clinical testing and randomized trials are imperative,” Dr. Warkiani continued. “Companies like Epic Sciences are actively engaged in advancing research and standardization in this field.”
The study by Dr. Jolly and Dr. Nagrath was funded by the National Institutes of Health. None of the study authors reported financial conflicts of interest. Dr. Warkiani reported no conflicts of interest related to his comment.
Circulating tumor cells (CTCs), the cells shed from a solid tumor into the bloodstream, may help doctors avoid having to do repeat needle biopsies on patients with unresectable non–small cell lung cancer.
Challenges to using CTCs clinically are that they are not abundant in the blood and have been difficult to isolate in patients with this type of cancer with commercially available assays.
In their paper, the authors show that an experimental nanotechnology can effectively isolate and measure CTCs in patients with stage 3 NSCLC. They also found that a precipitous drop in CTCs during chemoradiation treatment predicted significantly longer progression-free survival in those patients.
Study Results and Methods
For their research, study coauthors Shruti Jolly, MD, and Sunitha Nagrath, PhD, used a novel graphene oxide technology called the GO chip, developed more than a decade ago by Dr. Nagrath and her colleagues, to isolate CTCs from patients with stage 3 NSCLC. While a different technology, which is approved by the US Food and Drug Administration (FDA), uses a single antibody to pick up CTCs, the GO chip uses a cocktail of three antibodies to CTC proteins, making it more sensitive.
The 26 patients in the study (mean age 67, 27% female) all received radiation treatment for 6 weeks, plus weekly carboplatin and paclitaxel chemotherapy. Sixteen of the patients afterward went on to have immunotherapy with durvalumab. Blood was drawn at six fixed time points: before treatment, and at weeks 1, 4, 10, 18, and 30. CTCs were measured and analyzed with every draw.
Previous studies showed that absolute number of CTCs did not correlate with either tumor volume or progression-free survival in NSCLC.
Dr. Jolly and Dr. Nagrath sought to measure change in CTCs from baseline for each patient, having the patient serve as his or her own control. They found that patients whose individual CTC counts dropped by 75% or more between pretreatment and week 4 of chemoradiation saw a mean 21 months of progression-free survival compared with 7 months for patients whose CTCs dropped by less than 75% in the same period (P = .0076).
Dr. Jolly and Dr. Nagrath also aimed to determine, as an exploratory outcome of their study, whether other information collected from the CTCs could predict response to treatment with durvalumab immunotherapy. They found that having more than 50% of CTCs positive for the protein PD-L1 correlated to shorter progression-free survival among the 16 patients receiving durvalumab (P = .04).
“Every person’s tumor is unique in terms of its response to treatment,” said Dr. Jolly, a radiation oncologist and professor and associate chair of community practices in the Department of Radiation Oncology at the University of Michigan, Ann Arbor.
“Two people with a three-centimeter lung tumor will not necessarily shed the same amount of tumor cells into circulation. CTCs are reflective of disease burden; however, this is not related to the absolute numbers. That’s why we decided to use individualized baselines and look at the percentage of decrease,” she said.
Dr. Nagrath, professor of chemical and biomedical engineering at the University of Michigan, noted, in the same interview, that the findings argue for CTCs as a biomarker in stage 3 NSCLC.
“A lot of researchers who do lung cancer studies struggle with isolating lung cancer CTCs,” Dr. Nagrath said. “We showed, with repeated blood draws during treatment, what is changing at a molecular level and that you can see it with a simple blood draw. It also gives the proof of concept that if these cells are present, this is a good way to monitor and see if a treatment is working, even early in the treatment.” Moreover, she added, “many studies in lung cancer are in stage 4.”
Our study is unique as it followed patients with locally advanced tumors from their being treatment naive to all the way through immunotherapy,” she continued.
The University of Michigan has a patent on the GO chip technology, but thus far no company has made efforts to license it and submit it for approval. While “liquid biopsy” is an important emerging concept in lung cancer, there is little consensus yet as to which blood biomarkers — whether CTCs, circulating tumor DNA (ctDNA), or extracellular vesicles (EVs) — are most clinically relevant, Dr. Nagrath said.
The study’s small size is one of its weaknesses, according to the authors.
Findings are ‘Particularly Intriguing’
Majid Ebrahimi Warkiani, PhD, who was not involved in the study, described the new findings as “particularly intriguing [and] highlighting the efficacy of liquid biopsy using CTCs for predicting treatment outcomes.”
A challenge within the realm of CTCs lies in the community’s ongoing struggle to define and classify these cells accurately, Dr. Warkiani said in an interview.
“While surface protein markers offer valuable insights, emerging layers of analysis, such as metabolomics, are increasingly entering the scene to bolster the identification of putative cancer cells, alongside molecular tests like fluorescence in situ hybridization (FISH),” said Dr. Warkiani of the University of Technology Sydney in Australia. “The amalgamation of these approaches simultaneously presents a significant challenge, particularly in terms of standardization for patient care, unlike ctDNA, which faces fewer bottlenecks.
“The robustness of the research in this study is commendable. However, further clinical testing and randomized trials are imperative,” Dr. Warkiani continued. “Companies like Epic Sciences are actively engaged in advancing research and standardization in this field.”
The study by Dr. Jolly and Dr. Nagrath was funded by the National Institutes of Health. None of the study authors reported financial conflicts of interest. Dr. Warkiani reported no conflicts of interest related to his comment.
Circulating tumor cells (CTCs), the cells shed from a solid tumor into the bloodstream, may help doctors avoid having to do repeat needle biopsies on patients with unresectable non–small cell lung cancer.
Challenges to using CTCs clinically are that they are not abundant in the blood and have been difficult to isolate in patients with this type of cancer with commercially available assays.
In their paper, the authors show that an experimental nanotechnology can effectively isolate and measure CTCs in patients with stage 3 NSCLC. They also found that a precipitous drop in CTCs during chemoradiation treatment predicted significantly longer progression-free survival in those patients.
Study Results and Methods
For their research, study coauthors Shruti Jolly, MD, and Sunitha Nagrath, PhD, used a novel graphene oxide technology called the GO chip, developed more than a decade ago by Dr. Nagrath and her colleagues, to isolate CTCs from patients with stage 3 NSCLC. While a different technology, which is approved by the US Food and Drug Administration (FDA), uses a single antibody to pick up CTCs, the GO chip uses a cocktail of three antibodies to CTC proteins, making it more sensitive.
The 26 patients in the study (mean age 67, 27% female) all received radiation treatment for 6 weeks, plus weekly carboplatin and paclitaxel chemotherapy. Sixteen of the patients afterward went on to have immunotherapy with durvalumab. Blood was drawn at six fixed time points: before treatment, and at weeks 1, 4, 10, 18, and 30. CTCs were measured and analyzed with every draw.
Previous studies showed that absolute number of CTCs did not correlate with either tumor volume or progression-free survival in NSCLC.
Dr. Jolly and Dr. Nagrath sought to measure change in CTCs from baseline for each patient, having the patient serve as his or her own control. They found that patients whose individual CTC counts dropped by 75% or more between pretreatment and week 4 of chemoradiation saw a mean 21 months of progression-free survival compared with 7 months for patients whose CTCs dropped by less than 75% in the same period (P = .0076).
Dr. Jolly and Dr. Nagrath also aimed to determine, as an exploratory outcome of their study, whether other information collected from the CTCs could predict response to treatment with durvalumab immunotherapy. They found that having more than 50% of CTCs positive for the protein PD-L1 correlated to shorter progression-free survival among the 16 patients receiving durvalumab (P = .04).
“Every person’s tumor is unique in terms of its response to treatment,” said Dr. Jolly, a radiation oncologist and professor and associate chair of community practices in the Department of Radiation Oncology at the University of Michigan, Ann Arbor.
“Two people with a three-centimeter lung tumor will not necessarily shed the same amount of tumor cells into circulation. CTCs are reflective of disease burden; however, this is not related to the absolute numbers. That’s why we decided to use individualized baselines and look at the percentage of decrease,” she said.
Dr. Nagrath, professor of chemical and biomedical engineering at the University of Michigan, noted, in the same interview, that the findings argue for CTCs as a biomarker in stage 3 NSCLC.
“A lot of researchers who do lung cancer studies struggle with isolating lung cancer CTCs,” Dr. Nagrath said. “We showed, with repeated blood draws during treatment, what is changing at a molecular level and that you can see it with a simple blood draw. It also gives the proof of concept that if these cells are present, this is a good way to monitor and see if a treatment is working, even early in the treatment.” Moreover, she added, “many studies in lung cancer are in stage 4.”
Our study is unique as it followed patients with locally advanced tumors from their being treatment naive to all the way through immunotherapy,” she continued.
The University of Michigan has a patent on the GO chip technology, but thus far no company has made efforts to license it and submit it for approval. While “liquid biopsy” is an important emerging concept in lung cancer, there is little consensus yet as to which blood biomarkers — whether CTCs, circulating tumor DNA (ctDNA), or extracellular vesicles (EVs) — are most clinically relevant, Dr. Nagrath said.
The study’s small size is one of its weaknesses, according to the authors.
Findings are ‘Particularly Intriguing’
Majid Ebrahimi Warkiani, PhD, who was not involved in the study, described the new findings as “particularly intriguing [and] highlighting the efficacy of liquid biopsy using CTCs for predicting treatment outcomes.”
A challenge within the realm of CTCs lies in the community’s ongoing struggle to define and classify these cells accurately, Dr. Warkiani said in an interview.
“While surface protein markers offer valuable insights, emerging layers of analysis, such as metabolomics, are increasingly entering the scene to bolster the identification of putative cancer cells, alongside molecular tests like fluorescence in situ hybridization (FISH),” said Dr. Warkiani of the University of Technology Sydney in Australia. “The amalgamation of these approaches simultaneously presents a significant challenge, particularly in terms of standardization for patient care, unlike ctDNA, which faces fewer bottlenecks.
“The robustness of the research in this study is commendable. However, further clinical testing and randomized trials are imperative,” Dr. Warkiani continued. “Companies like Epic Sciences are actively engaged in advancing research and standardization in this field.”
The study by Dr. Jolly and Dr. Nagrath was funded by the National Institutes of Health. None of the study authors reported financial conflicts of interest. Dr. Warkiani reported no conflicts of interest related to his comment.
FROM CELL REPORTS
New Evidence Suggests Long COVID Could Be a Brain Injury
Brain fog is one of the most common, persistent complaints in patients with long COVID. It affects as many as 46% of patients who also deal with other cognitive concerns like memory loss and difficulty concentrating.
Now, researchers believe they know why. A new study has found that these symptoms may be the result of a viral-borne brain injury that may cause cognitive and mental health issues that persist for years.
The findings were based on a series of cognitive tests, self-reported symptoms, brain scans, and biomarkers.
Brain Deficits Equal to 20 Years of Brain Aging
As part of the preprint study, participants took a cognition test with their scores age-matched to those who had not suffered a serious bout of COVID-19. Then a blood sample was taken to look for specific biomarkers, showing that elevated levels of certain biomarkers were consistent with a brain injury. Using brain scans, researchers also found that certain regions of the brain associated with attention were reduced in volume.
Patients who participated in the study were “less accurate and slower” in their cognition, and suffered from at least one mental health condition, such as depression, anxiety, or posttraumatic stress disorder, according to researchers.
The brain deficits found in COVID-19 patients were equivalent to 20 years of brain aging and provided proof of what doctors have feared: that this virus can damage the brain and result in ongoing mental health issues.
“We found global deficits across cognition,” said lead study author Benedict Michael, PhD, director of the Infection Neuroscience Lab at the University of Liverpool in Liverpool, England. “The cognitive and memory problems that patients complained of were associated with neuroanatomical changes to the brain.”
Proof That Symptoms Aren’t ‘Figment’ of Patients’ Imaginations
Cognitive deficits were common among all patients, but the researchers said they don’t yet know whether the brain damage causes permanent cognitive decline. But the research provides patients who have been overlooked by some clinicians with proof that their conditions aren’t a figment of their imaginations, said Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina School of Medicine’s COVID Recovery Clinic.
“Even though we’re several years into this pandemic, there are still a lot of providers who don’t believe that their patients are experiencing these residual symptoms,” said Dr. Thompson, “That’s why the use of biomarkers is important, because it provides an objective indication that the brain has been compromised in some way.”
Some patients with long COVID have said that getting their doctors to believe they have a physical ailment has been a persistent problem throughout the pandemic and especially as it relates to the sometimes-vague collection of symptoms associated with brain fog. One study found that as many as 79% of study respondents reported negative interactions with their healthcare providers when they sought treatment for their long-COVID symptoms.
How Do COVID-Related Brain Injuries Happen?
Researchers are unsure what’s causing these brain injuries, though they have identified some clues. Previous research has suggested that such injuries might be the result of a lack of oxygen to the brain, especially in patients who were hospitalized, like those in this study, and were put on ventilators.
Brain scans have previously shown atrophy to the brain›s gray matter in COVID-19 patients, likely caused by inflammation from a heightened immune response rather than the virus itself. This inflammatory response seems to affect the central nervous system. As part of the new study, researchers found some neuroprotective effects of using steroids during hospitalization to reduce brain inflammation.
The results suggest that clinicians should overcome their skepticism and consider the possibility that their patients have suffered a brain injury and should be treated appropriately, said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University School of Medicine. “The old saying is that if it walks like a duck and talks like a duck, it’s a duck,” said Dr. Jackson.
He contends that treatments used for patients who have brain injuries have also been shown to be effective in treating long COVID–related brain fog symptoms. These may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for the treatment of related mental health concerns.
A New Path Forward
Treating long-COVID brain fog like a brain injury can help patients get back to some semblance of normalcy, researchers said. “What we’re seeing in terms of brain injury biomarkers and differences in brain scans correlates to real-life problems that these patients are dealing with on a daily basis,” said Dr. Jackson. These include problems at work and in life with multitasking, remembering details, meeting deadlines, synthesizing large amounts of information, and maintaining focus on the task at hand, he said.
There’s also a fear that even with treatment, the aging of the brain caused by the virus might have long-term repercussions and that this enduring injury may cause the early onset of dementia and Alzheimer’s disease in those who were already vulnerable to it. One study, from the National Institute of Neurological Disorders and Stroke (NINDS), found that in those infected with COVID-19 who already had dementia, the virus “rapidly accelerated structural and functional brain deterioration.”
“We already know the role that neuroinflammation plays in the brains of patients with Alzheimer’s disease,” said Dr. Thompson. “If long COVID is involved in prolonged inflammation of the brain, it goes a long way in explaining the mechanism underlying [the study’s reported] brain aging.”
Still More to Learn
In some ways, this study raises nearly as many questions as it does answers. While it provides concrete evidence around the damage the virus is doing to the brains of patients who contracted severe COVID-19, researchers don’t know about the impact on those who had less serious cases of the virus.
For Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System, the concern is that some long-COVID patients may be suffering from cognitive deficits that are more subtle but still impacting their daily lives, and that they’re not getting the help they need.
What’s more, said Dr. Al-Aly, it’s unclear whether the impacts of the brain damage are permanent or how to stop them from worsening. Researchers and clinicians need a better understanding of the mechanism that allows this virus to enter the brain and do structural damage. If it’s inflammation, will anti-inflammatory or antiviral medications work at preventing it? Will steroids help to offset the damage? “It’s critical we find some answers,” he said.
“SARS-CoV-2 isn’t going anywhere. It will continue to infect the population, so if this is indeed a virus that damages the brain in the long term or permanently, we need to figure out what can be done to stop it,” said Dr. Al-Aly.
A version of this article appeared on Medscape.com.
Brain fog is one of the most common, persistent complaints in patients with long COVID. It affects as many as 46% of patients who also deal with other cognitive concerns like memory loss and difficulty concentrating.
Now, researchers believe they know why. A new study has found that these symptoms may be the result of a viral-borne brain injury that may cause cognitive and mental health issues that persist for years.
The findings were based on a series of cognitive tests, self-reported symptoms, brain scans, and biomarkers.
Brain Deficits Equal to 20 Years of Brain Aging
As part of the preprint study, participants took a cognition test with their scores age-matched to those who had not suffered a serious bout of COVID-19. Then a blood sample was taken to look for specific biomarkers, showing that elevated levels of certain biomarkers were consistent with a brain injury. Using brain scans, researchers also found that certain regions of the brain associated with attention were reduced in volume.
Patients who participated in the study were “less accurate and slower” in their cognition, and suffered from at least one mental health condition, such as depression, anxiety, or posttraumatic stress disorder, according to researchers.
The brain deficits found in COVID-19 patients were equivalent to 20 years of brain aging and provided proof of what doctors have feared: that this virus can damage the brain and result in ongoing mental health issues.
“We found global deficits across cognition,” said lead study author Benedict Michael, PhD, director of the Infection Neuroscience Lab at the University of Liverpool in Liverpool, England. “The cognitive and memory problems that patients complained of were associated with neuroanatomical changes to the brain.”
Proof That Symptoms Aren’t ‘Figment’ of Patients’ Imaginations
Cognitive deficits were common among all patients, but the researchers said they don’t yet know whether the brain damage causes permanent cognitive decline. But the research provides patients who have been overlooked by some clinicians with proof that their conditions aren’t a figment of their imaginations, said Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina School of Medicine’s COVID Recovery Clinic.
“Even though we’re several years into this pandemic, there are still a lot of providers who don’t believe that their patients are experiencing these residual symptoms,” said Dr. Thompson, “That’s why the use of biomarkers is important, because it provides an objective indication that the brain has been compromised in some way.”
Some patients with long COVID have said that getting their doctors to believe they have a physical ailment has been a persistent problem throughout the pandemic and especially as it relates to the sometimes-vague collection of symptoms associated with brain fog. One study found that as many as 79% of study respondents reported negative interactions with their healthcare providers when they sought treatment for their long-COVID symptoms.
How Do COVID-Related Brain Injuries Happen?
Researchers are unsure what’s causing these brain injuries, though they have identified some clues. Previous research has suggested that such injuries might be the result of a lack of oxygen to the brain, especially in patients who were hospitalized, like those in this study, and were put on ventilators.
Brain scans have previously shown atrophy to the brain›s gray matter in COVID-19 patients, likely caused by inflammation from a heightened immune response rather than the virus itself. This inflammatory response seems to affect the central nervous system. As part of the new study, researchers found some neuroprotective effects of using steroids during hospitalization to reduce brain inflammation.
The results suggest that clinicians should overcome their skepticism and consider the possibility that their patients have suffered a brain injury and should be treated appropriately, said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University School of Medicine. “The old saying is that if it walks like a duck and talks like a duck, it’s a duck,” said Dr. Jackson.
He contends that treatments used for patients who have brain injuries have also been shown to be effective in treating long COVID–related brain fog symptoms. These may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for the treatment of related mental health concerns.
A New Path Forward
Treating long-COVID brain fog like a brain injury can help patients get back to some semblance of normalcy, researchers said. “What we’re seeing in terms of brain injury biomarkers and differences in brain scans correlates to real-life problems that these patients are dealing with on a daily basis,” said Dr. Jackson. These include problems at work and in life with multitasking, remembering details, meeting deadlines, synthesizing large amounts of information, and maintaining focus on the task at hand, he said.
There’s also a fear that even with treatment, the aging of the brain caused by the virus might have long-term repercussions and that this enduring injury may cause the early onset of dementia and Alzheimer’s disease in those who were already vulnerable to it. One study, from the National Institute of Neurological Disorders and Stroke (NINDS), found that in those infected with COVID-19 who already had dementia, the virus “rapidly accelerated structural and functional brain deterioration.”
“We already know the role that neuroinflammation plays in the brains of patients with Alzheimer’s disease,” said Dr. Thompson. “If long COVID is involved in prolonged inflammation of the brain, it goes a long way in explaining the mechanism underlying [the study’s reported] brain aging.”
Still More to Learn
In some ways, this study raises nearly as many questions as it does answers. While it provides concrete evidence around the damage the virus is doing to the brains of patients who contracted severe COVID-19, researchers don’t know about the impact on those who had less serious cases of the virus.
For Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System, the concern is that some long-COVID patients may be suffering from cognitive deficits that are more subtle but still impacting their daily lives, and that they’re not getting the help they need.
What’s more, said Dr. Al-Aly, it’s unclear whether the impacts of the brain damage are permanent or how to stop them from worsening. Researchers and clinicians need a better understanding of the mechanism that allows this virus to enter the brain and do structural damage. If it’s inflammation, will anti-inflammatory or antiviral medications work at preventing it? Will steroids help to offset the damage? “It’s critical we find some answers,” he said.
“SARS-CoV-2 isn’t going anywhere. It will continue to infect the population, so if this is indeed a virus that damages the brain in the long term or permanently, we need to figure out what can be done to stop it,” said Dr. Al-Aly.
A version of this article appeared on Medscape.com.
Brain fog is one of the most common, persistent complaints in patients with long COVID. It affects as many as 46% of patients who also deal with other cognitive concerns like memory loss and difficulty concentrating.
Now, researchers believe they know why. A new study has found that these symptoms may be the result of a viral-borne brain injury that may cause cognitive and mental health issues that persist for years.
The findings were based on a series of cognitive tests, self-reported symptoms, brain scans, and biomarkers.
Brain Deficits Equal to 20 Years of Brain Aging
As part of the preprint study, participants took a cognition test with their scores age-matched to those who had not suffered a serious bout of COVID-19. Then a blood sample was taken to look for specific biomarkers, showing that elevated levels of certain biomarkers were consistent with a brain injury. Using brain scans, researchers also found that certain regions of the brain associated with attention were reduced in volume.
Patients who participated in the study were “less accurate and slower” in their cognition, and suffered from at least one mental health condition, such as depression, anxiety, or posttraumatic stress disorder, according to researchers.
The brain deficits found in COVID-19 patients were equivalent to 20 years of brain aging and provided proof of what doctors have feared: that this virus can damage the brain and result in ongoing mental health issues.
“We found global deficits across cognition,” said lead study author Benedict Michael, PhD, director of the Infection Neuroscience Lab at the University of Liverpool in Liverpool, England. “The cognitive and memory problems that patients complained of were associated with neuroanatomical changes to the brain.”
Proof That Symptoms Aren’t ‘Figment’ of Patients’ Imaginations
Cognitive deficits were common among all patients, but the researchers said they don’t yet know whether the brain damage causes permanent cognitive decline. But the research provides patients who have been overlooked by some clinicians with proof that their conditions aren’t a figment of their imaginations, said Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina School of Medicine’s COVID Recovery Clinic.
“Even though we’re several years into this pandemic, there are still a lot of providers who don’t believe that their patients are experiencing these residual symptoms,” said Dr. Thompson, “That’s why the use of biomarkers is important, because it provides an objective indication that the brain has been compromised in some way.”
Some patients with long COVID have said that getting their doctors to believe they have a physical ailment has been a persistent problem throughout the pandemic and especially as it relates to the sometimes-vague collection of symptoms associated with brain fog. One study found that as many as 79% of study respondents reported negative interactions with their healthcare providers when they sought treatment for their long-COVID symptoms.
How Do COVID-Related Brain Injuries Happen?
Researchers are unsure what’s causing these brain injuries, though they have identified some clues. Previous research has suggested that such injuries might be the result of a lack of oxygen to the brain, especially in patients who were hospitalized, like those in this study, and were put on ventilators.
Brain scans have previously shown atrophy to the brain›s gray matter in COVID-19 patients, likely caused by inflammation from a heightened immune response rather than the virus itself. This inflammatory response seems to affect the central nervous system. As part of the new study, researchers found some neuroprotective effects of using steroids during hospitalization to reduce brain inflammation.
The results suggest that clinicians should overcome their skepticism and consider the possibility that their patients have suffered a brain injury and should be treated appropriately, said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University School of Medicine. “The old saying is that if it walks like a duck and talks like a duck, it’s a duck,” said Dr. Jackson.
He contends that treatments used for patients who have brain injuries have also been shown to be effective in treating long COVID–related brain fog symptoms. These may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for the treatment of related mental health concerns.
A New Path Forward
Treating long-COVID brain fog like a brain injury can help patients get back to some semblance of normalcy, researchers said. “What we’re seeing in terms of brain injury biomarkers and differences in brain scans correlates to real-life problems that these patients are dealing with on a daily basis,” said Dr. Jackson. These include problems at work and in life with multitasking, remembering details, meeting deadlines, synthesizing large amounts of information, and maintaining focus on the task at hand, he said.
There’s also a fear that even with treatment, the aging of the brain caused by the virus might have long-term repercussions and that this enduring injury may cause the early onset of dementia and Alzheimer’s disease in those who were already vulnerable to it. One study, from the National Institute of Neurological Disorders and Stroke (NINDS), found that in those infected with COVID-19 who already had dementia, the virus “rapidly accelerated structural and functional brain deterioration.”
“We already know the role that neuroinflammation plays in the brains of patients with Alzheimer’s disease,” said Dr. Thompson. “If long COVID is involved in prolonged inflammation of the brain, it goes a long way in explaining the mechanism underlying [the study’s reported] brain aging.”
Still More to Learn
In some ways, this study raises nearly as many questions as it does answers. While it provides concrete evidence around the damage the virus is doing to the brains of patients who contracted severe COVID-19, researchers don’t know about the impact on those who had less serious cases of the virus.
For Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System, the concern is that some long-COVID patients may be suffering from cognitive deficits that are more subtle but still impacting their daily lives, and that they’re not getting the help they need.
What’s more, said Dr. Al-Aly, it’s unclear whether the impacts of the brain damage are permanent or how to stop them from worsening. Researchers and clinicians need a better understanding of the mechanism that allows this virus to enter the brain and do structural damage. If it’s inflammation, will anti-inflammatory or antiviral medications work at preventing it? Will steroids help to offset the damage? “It’s critical we find some answers,” he said.
“SARS-CoV-2 isn’t going anywhere. It will continue to infect the population, so if this is indeed a virus that damages the brain in the long term or permanently, we need to figure out what can be done to stop it,” said Dr. Al-Aly.
A version of this article appeared on Medscape.com.
Healthcare Workers Face Increased Risks During the Pandemic
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
Healthcare workers have been at an increased risk for SARS-CoV-2 infection and mental distress such as anxiety and depression during the pandemic, according to new research.
The risk for infection was higher among healthcare workers in the first two waves of the pandemic and again during the fifth wave.
“Previous publications, including ours, suggested that the main problem was in the early weeks and months of the pandemic, but this paper shows that it continued until the later stages,” senior author Nicola Cherry, MD, an occupational epidemiologist at the University of Alberta in Edmonton, Canada, told this news organization.
The findings were published in the Canadian Journal of Public Health.
Wave Upon Wave
In the current study, the investigators sought to compare the risk for SARS-CoV-2 infection and mental distress among healthcare workers and among community referents (CRs). They examined the following waves of the COVID-19 pandemic:
- Wave 1: From March to June 2020 (4 months).
- Wave 2: From July 2020 to February 2021 (8 months).
- Wave 3: From March to June 2021 (4 months).
- Wave 4: From July to October 2021 (4 months).
- Wave 5 (Omicron): From November 2021 to March 2022 (5 months).
Healthcare workers in Alberta were asked at recruitment for consent to match their individual records to the Alberta Administrative Health Database. As the pandemic progressed, participants were also asked for consent to be linked to COVID-19 immunization records maintained by the provinces, as well as for the results of all polymerase chain reaction (PCR) testing for the SARS-CoV-2 virus.
The investigators matched 2959 healthcare workers to 14,546 CRs according to their age, sex, geographic location in Alberta, and number of physician claims from April 1, 2019, to March 31, 2020.
Incident SARS-CoV-2 infection was examined using PCR testing and the first date of a physician consultation at which the code for SARS-CoV-2 infection had been recorded. Mental health disorders were identified from physician records. They included anxiety disorders, stress and adjustment reactions, and depressive disorders.
Most (79.5%) of the healthcare workers were registered nurses, followed by physicians (16.1%), healthcare aides (2.4%), and licensed practical nurses (2.0%). Most participants (87.5%) were female. The median age at recruitment was 44 years.
Healthcare workers were at a greater risk for COVID-19 overall, with the first SARS-CoV-2 infection defined from either PCR tests (odds ratio [OR], 1.96) or from physician records (OR, 1.33). They were also at an increased risk for anxiety (adjusted OR, 1.25; P < .001), stress/adjustment reaction (adjusted OR, 1.52; P < .001), and depressive condition (adjusted OR, 1.39; P < .001). Moreover, the excess risks for stress/adjustment reactions and depressive conditions increased with successive waves during the pandemic, peaking in the fourth wave and continuing in the fifth wave.
“Although the increase was less in the middle of the phases of the pandemic, it came back with a vengeance during the last phase, which was the Omicron phase,” said Dr. Cherry.
“Employers of healthcare workers can’t assume that everything is now under control, that they know what they’re doing, and that there is no risk. We are now having some increases in COVID. It’s going to go on. The pandemic is not over in that sense, and infection control continues to be major,” she added.
The finding that mental health worsened among healthcare workers was not surprising, Dr. Cherry said. Even before the pandemic, studies had shown that healthcare workers were at a greater risk for depression than the population overall.
“There is a lot of need for care in mental health support of healthcare workers, whether during a pandemic or not,” said Dr. Cherry.
Nurses Are Suffering
Commenting on the research for this news organization, Farinaz Havaei, PhD, RN, assistant professor of nursing at the University of British Columbia in Vancouver, Canada, said, “This is a very important and timely study that draws on objective clinical and administrative data, as opposed to healthcare workers’ subjective reports.” Dr. Havaei did not participate in the research.
Overall, the findings are consistent with previous research that drew upon healthcare workers’ reports. They speak to the chronic and cumulative impact of COVID-19 and its associated stressors on the mental health and well-being of healthcare workers, said Dr. Havaei.
“The likelihood of stress/adjustment reaction and depression showed a relatively steady increase with increasing COVID-19 waves. This increase can likely be explained by healthcare workers’ depleting emotional reserves for coping with chronic workplace stressors such as concerns about exposure to COVID-19, inadequate staffing, and work overload,” she said. Witnessing the suffering and trauma of patients and their families likely added to this risk.
Dr. Havaei also pointed out that most of the study participants were nurses. The findings are consistent with prepandemic research that showed that the suboptimal conditions that nurses increasingly faced resulted in high levels of exhaustion and burnout.
“While I agree with the authors’ call for more mental health support for healthcare workers, I think prevention efforts that address the root cause of the problem should be prioritized,” she said.
From Heroes to Zeros
The same phenomena have been observed in the United States, said John Q. Young, MD, MPP, PhD, professor and chair of psychiatry at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. In various studies, Dr. Young and his colleagues have reported a strong association between exposure to the stressors of the pandemic and subsequent development of depression, anxiety, and posttraumatic stress disorder (PTSD) among healthcare workers.
“The findings from Alberta are remarkably consistent. In the beginning of the pandemic, there was a lot of acknowledgment of the work healthcare workers were doing. The fire department clapping as you leave work at night, being called heroes, even though a lot of healthcare workers feel uncomfortable with the hero language because they don’t feel like heroes. Yes, they’re afraid, but they are going to do what they need to do and help,” he said.
But as the pandemic continued, public sentiment changed, Dr. Young said. “They’ve gone from heroes to zeros. Now we are seeing the accumulated, chronic effects over months and years, and these are significant. Our healthcare workforce is vulnerable now. The reserves are low. There are serious shortages in nursing, with more retirements and more people leaving the field,” he said.
As part of a campaign to help healthcare workers cope, psychiatrists at Northwell Health have started a program called Stress First Aid at their Center for Traumatic Stress Response Resilience, where they train nurses, physicians, and other healthcare staff to use basic tools to recognize and respond to stress and distress in themselves and in their colleagues, said Dr. Young.
“For those healthcare workers who find that they are struggling and need more support, there is resilience coaching, which is one-on-one support. For those who need more clinical attention, there is a clinical program where our healthcare workers can meet with a psychologist, psychiatrist, or a therapist, to work through depression, PTSD, and anxiety. We didn’t have this before the pandemic, but it is now a big focus for our workforce,” he said. “We are trying to build resilience. The trauma is real.”
The study was supported by the College of Physicians and Surgeons of Alberta, the Canadian Institutes of Health Research, and the Canadian Immunology Task Force. Dr. Cherry and Dr. Havaei reported no relevant financial relationships. Dr. Young reported that he is senior vice president of behavioral health at Northwell.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF PUBLIC HEALTH
Near-Death Experiences During CPR: An Impetus for Better Care
If someone has been in cardiac arrest for 10 minutes, the brain is permanently damaged and there’s nothing to do, right?
Not so according to emerging evidence that suggests that the brain shows signs of electrical recovery for as long as an hour into ongoing cardiopulmonary resuscitation (CPR). This time between cardiac arrest and awakening can be a period of vivid experiences for the dying patient before they return to life — a phenomenon known as “recalled death.”
This should be an impetus to increase the use of devices that measure the quality of CPR and to find new treatments to restart the heart or prevent brain injury, experts advised. Cardiologists and critical care clinicians are among those who will need to manage patients in the aftermath.
said Jasmeet Soar, MD, consultant in Anesthetics & Intensive Care Medicine, North Bristol NHS Trust, Bristol, England, and an editor of the journal Resuscitation.
“We know that because if chest compressions are stopped, the person becomes unconscious again,” he said. “This CPR-induced consciousness has become more common when professionals do the CPR because resuscitation guidelines now place a much bigger focus on high-quality CPR — ‘push hard, push fast.’ ”
“People are giving up too soon on trying to revive individuals, and they should be trying more modern strategies, such as extracorporeal membrane oxygenation,” said Sam Parnia, MD, PhD, associate professor in the Department of Medicine at NYU Langone Health and director of critical care and resuscitation research at NYU Langone, New York City.
Brain Activity, Heightened Experiences
Two types of brain activity may occur when CPR works. The first, called CPR-induced consciousness, is when an individual recovers consciousness while in cardiac arrest. Signs of consciousness include combativeness, groaning, and eye-opening, Soar explained.
The second type is a perception of lucidity with recall of events, he said. “Patients who experience this may form memories that they can recall. We’re not sure whether that happens during CPR or while the patient is waking up during intensive care, or how the brain creates these memories, or if they’re real memories or coincidental, but it’s clear the brain does form them during the dying and recovery process.”
This latter phenomenon was explored in detail in a recent study led by Dr. Parnia.
In that study of 567 in-hospital patients with cardiac arrest from 25 centers in the United States and United Kingdom, 53 survived, 28 of those survivors were interviewed, and 11 reported memories or perceptions suggestive of consciousness.
Four types of experiences occurred:
- Recalled experiences of death: “I thought I heard my grandma [who had passed] saying ‘you need to go back.’”
- Emergence from coma during CPR/CPR-induced consciousness: “I remember when I came back and they were putting those two electrodes to my chest, and I remember the shock.”
- Emergence from coma in the post-resuscitation period: “I heard my partner saying [patient’s name] and my son saying ‘mom.’”
- Dreams and dream-like experiences: “[I] felt as though someone was holding my hand. It was very black; I couldn’t see anything.”
In a complementary cross-sectional study, 126 community cardiac arrest survivors reported similar experiences plus a fifth type, “delusions,” or “misattribution of medical events,” for example, “I heard my name, over and over again. All around me were things like demons and monsters. It felt like they were trying to tear off my body parts.”
“Many people label recalled experiences of death as ‘near-death’ experiences, but they’re not,” Dr. Parnia said. “Medically speaking, being near to death means your heart is about to stop. But the whole point is that these people are not near death. They actually died and came back from it.”
One of the big implications of the study, he said, is that “a lot of physicians are taught that somehow after, say, 3-5 minutes of oxygen deprivation, the brain dies. Our study showed this is not true. It showed that the brain may not be functioning, which is why they flatline. But if you’re able to resuscitate them appropriately, you can restore activity up to an hour later.”
Because some clinicians questioned or dismissed previous work in this area by Dr. Parnia and others, the latest study used EEG monitoring in a subset of 53 patients. Among those with evaluable EEG data, brain activity returned to normal or near-normal after flatlining in about 40% of images; spikes were seen in the delta (22%), theta (12%), alpha (6%), and beta (1%) waves associated with higher mental function.
“The team recorded what was happening in the brain during real-time CPR using various tests of consciousness, including EEG measurements and tests of visual and auditory awareness using a tablet with a special app and a Bluetooth headphone.”
“Incredibly, we found that even though the brain flatlines, which is what we expect when the heart stops, with professionally given CPR even up to about an hour after this, the brainwaves changed into normal to near-normal patterns,” Dr. Parnia said. “We were able to identify these brain waves in patients while they were being resuscitated, which confirms the fact that people can have lucid consciousness even though they appear to be unconscious.”
Asked what implications, if any, his work has for current definitions of brain death and cardiac death, Dr. Parnia said that the problem is that these are based on the concept of “a permanent irreversible loss of function,” but “that’s only relative to what medical treatments are developed at a given time.”
Potential Mechanism
Dr. Parnia and his team proposed a potential mechanism for recalled experiences of death. Essentially, when the brain flatlines, the dying brain removes natural inhibitory (braking) systems that are needed to support daily functioning. This disinhibition may open access to “new dimensions of reality, including lucid recall of stored memories from early childhood to death,” he said.
From a clinical perspective, he noted, “although the brain stops working when it flatlines, it does not die within 5 or 10 minutes of oxygen deprivation.”
This is contrary to what many doctors believe, and because of that, he said, “nobody has tried to find treatments or new ways to restart the heart or prevent brain injury. They think it’s futile. So, with this work, we’ve opened up the window to developing cocktails of drugs that could be given to patients who have technically gone through death to bring them back to life again.”
Probe Patients or Leave Well Enough Alone?
The findings have ramifications for clinicians who may be caring for patients who survive cardiac arrest, said Lance B. Becker, MD, professor and chair, Department of Emergency Medicine, Donald & Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, and chair, Department of Emergency Medicine at North Shore University Hospital, Manhasset, and Long Island Jewish Medical Center, Queens, New York.
“I’ve talked with a lot of patients who have had some kind of recalled experience around cardiac arrest and some who have had zero recall, as well, like in the paper,” he told this news organization. “The ones who do have an experience are sometimes mystified by it and have questions. And very often, clinicians don’t want to listen, don’t think it’s important, and downplay it.”
“I think it is important, and when people have important things happen to them, it’s really imperative that doctors listen, learn, and respond,” he said. “When I started in this field a long time ago, there were so few survivors that there wasn’t even a concept of survivorship,” he said.
Dr. Becker noted that it’s not uncommon for cardiac arrest survivors to have depression, problems with executive function, or a small brain injury they need to recover from. “Now survivorship organizations are springing up that these people can turn to, but clinicians still need to become more aware and sensitive to this.”
Not all are. “I had a number of patients who said I was the only doctor who ever asked them about what they experienced,” he recalled. “I was a young doctor at the time and didn’t exactly know what to say to them, but they were just happy to have a doctor who would listen to them and not be afraid to hear what they had to say.”
Recognizing that support is an issue, the American Heart Association released a scientific statement in 2020 on sudden cardiac arrest survivorship, which “expands the cardiac arrest resuscitation system of care to include patients, caregivers, and rehabilitative healthcare partnerships, which are central to cardiac survivorship.”
Soar has a more nuanced view of survivorship support, however. “I suspect some people are very glad to be alive, and that trying to dig deep and bring things out may actually be harmful,” he said. “It’s not as clear cut as everybody thinks.”
He noted that follow-up and rehabilitation should be an option for people who specifically need it who would need to be identified. “But human beings are resilient, and while some people will require help, not everybody will,” he said.
Better CPR, New Treatments
Experts in emergency and intensive care medicine studying survival after cardiac arrest hope to find ways to save patients before too much damage is done to the brain and other organs from loss of oxygen, Dr. Parnia said. He is the lead author in a recent multidisciplinary consensus statement on guidelines and standards for the study of death and recalled experiences of death.
“One of my bugbears is that our survival outcomes from cardiac arrest resuscitation have not changed very much for 60 years because we haven’t developed new treatments and innovative methods,” he said. “Unlike the rest of medicine, we’re living in the past.”
Currently, his team is developing cocktails of treatments. These include hypothermic circulatory arrest — cooling the body to stop blood circulation and brain function for up to 40 minutes — and giving magnesium, a brain-protective treatment, to people whose hearts stop.
Dr. Becker would like to see optimal care of patients with cardiac arrest. “The first step is to increase blood flow with good CPR and then measure whether CPR is working,” he said. Adding that despite the availability of devices that provide feedback on the quality of CPR, they’re rarely used. He cited ultrasound devices that measure the blood flow generated during CPR, compression meter devices that go between the patient’s chest and the rescuer’s hands that gauge the rate and depth of compression, and invasive devices that measure blood pressure during CPR.
His group is trying to design even better devices, he said. “An example would be a little probe that you could pop on the neck that would study blood flow to the brain with ultrasound, so that while you were pumping on the person, you could see if you’re making them better or not.”
“We also have some preliminary data showing that the American Heart Association recommended position on the chest for doing CPR is not the perfect place for everybody,” he said. The 2020 AHA guidelines recommended the center of the lower half of the sternum. At the 2023 American College of Emergency Physicians meeting, Dr. Becker›s team at Hofstra/Northwell presented data on 175 video-recorded adult cardiac arrests in their emergency department over more than 2 years, 22 of which involved at least one change of compression location (for a total of 29 location changes). They found that 41% of compression location changes were associated with return of spontaneous circulation.
For about a third of people, the hands need to be repositioned slightly. “This is not anything that is taught to the public because you can only figure it out if you have some kind of sensor that will let you know how you’re doing. That’s very achievable. We could have that in the future on every ambulance and even in people’s homes.”
When the person arrives at the hospital, he said, “we can make it easier and more likely that they can be put on extracorporeal membrane oxygenation (ECMO). We do that on selected patients in our hospital, even though it’s very difficult to do, because we know that when it’s done properly, it can change survival rates dramatically, from maybe 10%-50%.”
Dr. Dr. Becker, like Dr. Parnia, also favors the development of drug cocktails, and his team has been experimenting with various combinations in animal models. “We think those two things together — ECMO and a drug cocktail — would be a very powerful one to two knock out for cardiac arrest,” he said. “We have a long way to go — 10 or 20 years. But most people around the world working in this area believe that will be the future.”
Dr. Parnia’s study on recalled death was supported by The John Templeton Foundation, Resuscitation Council (UK), and New York University Grossman School of Medicine, with research support staff provided by the UK’s National Institutes for Health Research. Soar is the editor of the journal Resuscitation and receives payment from the publisher Elsevier. Dr. Becker’s institute has received grants from Philips Medical Systems, NIH, Zoll Medical Corp, Nihon Kohden, PCORI, BrainCool, and United Therapeutics. He has received advisory/consultancy honoraria from NIH, Nihon Kohden, HP, and Philips, and he holds several patents in hypothermia induction and reperfusion therapies and several pending patents involving the use of medical slurries as human coolant devices to create reperfusion cocktails and measurement of respiratory quotient.
A version of this article appeared on Medscape.com.
If someone has been in cardiac arrest for 10 minutes, the brain is permanently damaged and there’s nothing to do, right?
Not so according to emerging evidence that suggests that the brain shows signs of electrical recovery for as long as an hour into ongoing cardiopulmonary resuscitation (CPR). This time between cardiac arrest and awakening can be a period of vivid experiences for the dying patient before they return to life — a phenomenon known as “recalled death.”
This should be an impetus to increase the use of devices that measure the quality of CPR and to find new treatments to restart the heart or prevent brain injury, experts advised. Cardiologists and critical care clinicians are among those who will need to manage patients in the aftermath.
said Jasmeet Soar, MD, consultant in Anesthetics & Intensive Care Medicine, North Bristol NHS Trust, Bristol, England, and an editor of the journal Resuscitation.
“We know that because if chest compressions are stopped, the person becomes unconscious again,” he said. “This CPR-induced consciousness has become more common when professionals do the CPR because resuscitation guidelines now place a much bigger focus on high-quality CPR — ‘push hard, push fast.’ ”
“People are giving up too soon on trying to revive individuals, and they should be trying more modern strategies, such as extracorporeal membrane oxygenation,” said Sam Parnia, MD, PhD, associate professor in the Department of Medicine at NYU Langone Health and director of critical care and resuscitation research at NYU Langone, New York City.
Brain Activity, Heightened Experiences
Two types of brain activity may occur when CPR works. The first, called CPR-induced consciousness, is when an individual recovers consciousness while in cardiac arrest. Signs of consciousness include combativeness, groaning, and eye-opening, Soar explained.
The second type is a perception of lucidity with recall of events, he said. “Patients who experience this may form memories that they can recall. We’re not sure whether that happens during CPR or while the patient is waking up during intensive care, or how the brain creates these memories, or if they’re real memories or coincidental, but it’s clear the brain does form them during the dying and recovery process.”
This latter phenomenon was explored in detail in a recent study led by Dr. Parnia.
In that study of 567 in-hospital patients with cardiac arrest from 25 centers in the United States and United Kingdom, 53 survived, 28 of those survivors were interviewed, and 11 reported memories or perceptions suggestive of consciousness.
Four types of experiences occurred:
- Recalled experiences of death: “I thought I heard my grandma [who had passed] saying ‘you need to go back.’”
- Emergence from coma during CPR/CPR-induced consciousness: “I remember when I came back and they were putting those two electrodes to my chest, and I remember the shock.”
- Emergence from coma in the post-resuscitation period: “I heard my partner saying [patient’s name] and my son saying ‘mom.’”
- Dreams and dream-like experiences: “[I] felt as though someone was holding my hand. It was very black; I couldn’t see anything.”
In a complementary cross-sectional study, 126 community cardiac arrest survivors reported similar experiences plus a fifth type, “delusions,” or “misattribution of medical events,” for example, “I heard my name, over and over again. All around me were things like demons and monsters. It felt like they were trying to tear off my body parts.”
“Many people label recalled experiences of death as ‘near-death’ experiences, but they’re not,” Dr. Parnia said. “Medically speaking, being near to death means your heart is about to stop. But the whole point is that these people are not near death. They actually died and came back from it.”
One of the big implications of the study, he said, is that “a lot of physicians are taught that somehow after, say, 3-5 minutes of oxygen deprivation, the brain dies. Our study showed this is not true. It showed that the brain may not be functioning, which is why they flatline. But if you’re able to resuscitate them appropriately, you can restore activity up to an hour later.”
Because some clinicians questioned or dismissed previous work in this area by Dr. Parnia and others, the latest study used EEG monitoring in a subset of 53 patients. Among those with evaluable EEG data, brain activity returned to normal or near-normal after flatlining in about 40% of images; spikes were seen in the delta (22%), theta (12%), alpha (6%), and beta (1%) waves associated with higher mental function.
“The team recorded what was happening in the brain during real-time CPR using various tests of consciousness, including EEG measurements and tests of visual and auditory awareness using a tablet with a special app and a Bluetooth headphone.”
“Incredibly, we found that even though the brain flatlines, which is what we expect when the heart stops, with professionally given CPR even up to about an hour after this, the brainwaves changed into normal to near-normal patterns,” Dr. Parnia said. “We were able to identify these brain waves in patients while they were being resuscitated, which confirms the fact that people can have lucid consciousness even though they appear to be unconscious.”
Asked what implications, if any, his work has for current definitions of brain death and cardiac death, Dr. Parnia said that the problem is that these are based on the concept of “a permanent irreversible loss of function,” but “that’s only relative to what medical treatments are developed at a given time.”
Potential Mechanism
Dr. Parnia and his team proposed a potential mechanism for recalled experiences of death. Essentially, when the brain flatlines, the dying brain removes natural inhibitory (braking) systems that are needed to support daily functioning. This disinhibition may open access to “new dimensions of reality, including lucid recall of stored memories from early childhood to death,” he said.
From a clinical perspective, he noted, “although the brain stops working when it flatlines, it does not die within 5 or 10 minutes of oxygen deprivation.”
This is contrary to what many doctors believe, and because of that, he said, “nobody has tried to find treatments or new ways to restart the heart or prevent brain injury. They think it’s futile. So, with this work, we’ve opened up the window to developing cocktails of drugs that could be given to patients who have technically gone through death to bring them back to life again.”
Probe Patients or Leave Well Enough Alone?
The findings have ramifications for clinicians who may be caring for patients who survive cardiac arrest, said Lance B. Becker, MD, professor and chair, Department of Emergency Medicine, Donald & Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, and chair, Department of Emergency Medicine at North Shore University Hospital, Manhasset, and Long Island Jewish Medical Center, Queens, New York.
“I’ve talked with a lot of patients who have had some kind of recalled experience around cardiac arrest and some who have had zero recall, as well, like in the paper,” he told this news organization. “The ones who do have an experience are sometimes mystified by it and have questions. And very often, clinicians don’t want to listen, don’t think it’s important, and downplay it.”
“I think it is important, and when people have important things happen to them, it’s really imperative that doctors listen, learn, and respond,” he said. “When I started in this field a long time ago, there were so few survivors that there wasn’t even a concept of survivorship,” he said.
Dr. Becker noted that it’s not uncommon for cardiac arrest survivors to have depression, problems with executive function, or a small brain injury they need to recover from. “Now survivorship organizations are springing up that these people can turn to, but clinicians still need to become more aware and sensitive to this.”
Not all are. “I had a number of patients who said I was the only doctor who ever asked them about what they experienced,” he recalled. “I was a young doctor at the time and didn’t exactly know what to say to them, but they were just happy to have a doctor who would listen to them and not be afraid to hear what they had to say.”
Recognizing that support is an issue, the American Heart Association released a scientific statement in 2020 on sudden cardiac arrest survivorship, which “expands the cardiac arrest resuscitation system of care to include patients, caregivers, and rehabilitative healthcare partnerships, which are central to cardiac survivorship.”
Soar has a more nuanced view of survivorship support, however. “I suspect some people are very glad to be alive, and that trying to dig deep and bring things out may actually be harmful,” he said. “It’s not as clear cut as everybody thinks.”
He noted that follow-up and rehabilitation should be an option for people who specifically need it who would need to be identified. “But human beings are resilient, and while some people will require help, not everybody will,” he said.
Better CPR, New Treatments
Experts in emergency and intensive care medicine studying survival after cardiac arrest hope to find ways to save patients before too much damage is done to the brain and other organs from loss of oxygen, Dr. Parnia said. He is the lead author in a recent multidisciplinary consensus statement on guidelines and standards for the study of death and recalled experiences of death.
“One of my bugbears is that our survival outcomes from cardiac arrest resuscitation have not changed very much for 60 years because we haven’t developed new treatments and innovative methods,” he said. “Unlike the rest of medicine, we’re living in the past.”
Currently, his team is developing cocktails of treatments. These include hypothermic circulatory arrest — cooling the body to stop blood circulation and brain function for up to 40 minutes — and giving magnesium, a brain-protective treatment, to people whose hearts stop.
Dr. Becker would like to see optimal care of patients with cardiac arrest. “The first step is to increase blood flow with good CPR and then measure whether CPR is working,” he said. Adding that despite the availability of devices that provide feedback on the quality of CPR, they’re rarely used. He cited ultrasound devices that measure the blood flow generated during CPR, compression meter devices that go between the patient’s chest and the rescuer’s hands that gauge the rate and depth of compression, and invasive devices that measure blood pressure during CPR.
His group is trying to design even better devices, he said. “An example would be a little probe that you could pop on the neck that would study blood flow to the brain with ultrasound, so that while you were pumping on the person, you could see if you’re making them better or not.”
“We also have some preliminary data showing that the American Heart Association recommended position on the chest for doing CPR is not the perfect place for everybody,” he said. The 2020 AHA guidelines recommended the center of the lower half of the sternum. At the 2023 American College of Emergency Physicians meeting, Dr. Becker›s team at Hofstra/Northwell presented data on 175 video-recorded adult cardiac arrests in their emergency department over more than 2 years, 22 of which involved at least one change of compression location (for a total of 29 location changes). They found that 41% of compression location changes were associated with return of spontaneous circulation.
For about a third of people, the hands need to be repositioned slightly. “This is not anything that is taught to the public because you can only figure it out if you have some kind of sensor that will let you know how you’re doing. That’s very achievable. We could have that in the future on every ambulance and even in people’s homes.”
When the person arrives at the hospital, he said, “we can make it easier and more likely that they can be put on extracorporeal membrane oxygenation (ECMO). We do that on selected patients in our hospital, even though it’s very difficult to do, because we know that when it’s done properly, it can change survival rates dramatically, from maybe 10%-50%.”
Dr. Dr. Becker, like Dr. Parnia, also favors the development of drug cocktails, and his team has been experimenting with various combinations in animal models. “We think those two things together — ECMO and a drug cocktail — would be a very powerful one to two knock out for cardiac arrest,” he said. “We have a long way to go — 10 or 20 years. But most people around the world working in this area believe that will be the future.”
Dr. Parnia’s study on recalled death was supported by The John Templeton Foundation, Resuscitation Council (UK), and New York University Grossman School of Medicine, with research support staff provided by the UK’s National Institutes for Health Research. Soar is the editor of the journal Resuscitation and receives payment from the publisher Elsevier. Dr. Becker’s institute has received grants from Philips Medical Systems, NIH, Zoll Medical Corp, Nihon Kohden, PCORI, BrainCool, and United Therapeutics. He has received advisory/consultancy honoraria from NIH, Nihon Kohden, HP, and Philips, and he holds several patents in hypothermia induction and reperfusion therapies and several pending patents involving the use of medical slurries as human coolant devices to create reperfusion cocktails and measurement of respiratory quotient.
A version of this article appeared on Medscape.com.
If someone has been in cardiac arrest for 10 minutes, the brain is permanently damaged and there’s nothing to do, right?
Not so according to emerging evidence that suggests that the brain shows signs of electrical recovery for as long as an hour into ongoing cardiopulmonary resuscitation (CPR). This time between cardiac arrest and awakening can be a period of vivid experiences for the dying patient before they return to life — a phenomenon known as “recalled death.”
This should be an impetus to increase the use of devices that measure the quality of CPR and to find new treatments to restart the heart or prevent brain injury, experts advised. Cardiologists and critical care clinicians are among those who will need to manage patients in the aftermath.
said Jasmeet Soar, MD, consultant in Anesthetics & Intensive Care Medicine, North Bristol NHS Trust, Bristol, England, and an editor of the journal Resuscitation.
“We know that because if chest compressions are stopped, the person becomes unconscious again,” he said. “This CPR-induced consciousness has become more common when professionals do the CPR because resuscitation guidelines now place a much bigger focus on high-quality CPR — ‘push hard, push fast.’ ”
“People are giving up too soon on trying to revive individuals, and they should be trying more modern strategies, such as extracorporeal membrane oxygenation,” said Sam Parnia, MD, PhD, associate professor in the Department of Medicine at NYU Langone Health and director of critical care and resuscitation research at NYU Langone, New York City.
Brain Activity, Heightened Experiences
Two types of brain activity may occur when CPR works. The first, called CPR-induced consciousness, is when an individual recovers consciousness while in cardiac arrest. Signs of consciousness include combativeness, groaning, and eye-opening, Soar explained.
The second type is a perception of lucidity with recall of events, he said. “Patients who experience this may form memories that they can recall. We’re not sure whether that happens during CPR or while the patient is waking up during intensive care, or how the brain creates these memories, or if they’re real memories or coincidental, but it’s clear the brain does form them during the dying and recovery process.”
This latter phenomenon was explored in detail in a recent study led by Dr. Parnia.
In that study of 567 in-hospital patients with cardiac arrest from 25 centers in the United States and United Kingdom, 53 survived, 28 of those survivors were interviewed, and 11 reported memories or perceptions suggestive of consciousness.
Four types of experiences occurred:
- Recalled experiences of death: “I thought I heard my grandma [who had passed] saying ‘you need to go back.’”
- Emergence from coma during CPR/CPR-induced consciousness: “I remember when I came back and they were putting those two electrodes to my chest, and I remember the shock.”
- Emergence from coma in the post-resuscitation period: “I heard my partner saying [patient’s name] and my son saying ‘mom.’”
- Dreams and dream-like experiences: “[I] felt as though someone was holding my hand. It was very black; I couldn’t see anything.”
In a complementary cross-sectional study, 126 community cardiac arrest survivors reported similar experiences plus a fifth type, “delusions,” or “misattribution of medical events,” for example, “I heard my name, over and over again. All around me were things like demons and monsters. It felt like they were trying to tear off my body parts.”
“Many people label recalled experiences of death as ‘near-death’ experiences, but they’re not,” Dr. Parnia said. “Medically speaking, being near to death means your heart is about to stop. But the whole point is that these people are not near death. They actually died and came back from it.”
One of the big implications of the study, he said, is that “a lot of physicians are taught that somehow after, say, 3-5 minutes of oxygen deprivation, the brain dies. Our study showed this is not true. It showed that the brain may not be functioning, which is why they flatline. But if you’re able to resuscitate them appropriately, you can restore activity up to an hour later.”
Because some clinicians questioned or dismissed previous work in this area by Dr. Parnia and others, the latest study used EEG monitoring in a subset of 53 patients. Among those with evaluable EEG data, brain activity returned to normal or near-normal after flatlining in about 40% of images; spikes were seen in the delta (22%), theta (12%), alpha (6%), and beta (1%) waves associated with higher mental function.
“The team recorded what was happening in the brain during real-time CPR using various tests of consciousness, including EEG measurements and tests of visual and auditory awareness using a tablet with a special app and a Bluetooth headphone.”
“Incredibly, we found that even though the brain flatlines, which is what we expect when the heart stops, with professionally given CPR even up to about an hour after this, the brainwaves changed into normal to near-normal patterns,” Dr. Parnia said. “We were able to identify these brain waves in patients while they were being resuscitated, which confirms the fact that people can have lucid consciousness even though they appear to be unconscious.”
Asked what implications, if any, his work has for current definitions of brain death and cardiac death, Dr. Parnia said that the problem is that these are based on the concept of “a permanent irreversible loss of function,” but “that’s only relative to what medical treatments are developed at a given time.”
Potential Mechanism
Dr. Parnia and his team proposed a potential mechanism for recalled experiences of death. Essentially, when the brain flatlines, the dying brain removes natural inhibitory (braking) systems that are needed to support daily functioning. This disinhibition may open access to “new dimensions of reality, including lucid recall of stored memories from early childhood to death,” he said.
From a clinical perspective, he noted, “although the brain stops working when it flatlines, it does not die within 5 or 10 minutes of oxygen deprivation.”
This is contrary to what many doctors believe, and because of that, he said, “nobody has tried to find treatments or new ways to restart the heart or prevent brain injury. They think it’s futile. So, with this work, we’ve opened up the window to developing cocktails of drugs that could be given to patients who have technically gone through death to bring them back to life again.”
Probe Patients or Leave Well Enough Alone?
The findings have ramifications for clinicians who may be caring for patients who survive cardiac arrest, said Lance B. Becker, MD, professor and chair, Department of Emergency Medicine, Donald & Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, New York, and chair, Department of Emergency Medicine at North Shore University Hospital, Manhasset, and Long Island Jewish Medical Center, Queens, New York.
“I’ve talked with a lot of patients who have had some kind of recalled experience around cardiac arrest and some who have had zero recall, as well, like in the paper,” he told this news organization. “The ones who do have an experience are sometimes mystified by it and have questions. And very often, clinicians don’t want to listen, don’t think it’s important, and downplay it.”
“I think it is important, and when people have important things happen to them, it’s really imperative that doctors listen, learn, and respond,” he said. “When I started in this field a long time ago, there were so few survivors that there wasn’t even a concept of survivorship,” he said.
Dr. Becker noted that it’s not uncommon for cardiac arrest survivors to have depression, problems with executive function, or a small brain injury they need to recover from. “Now survivorship organizations are springing up that these people can turn to, but clinicians still need to become more aware and sensitive to this.”
Not all are. “I had a number of patients who said I was the only doctor who ever asked them about what they experienced,” he recalled. “I was a young doctor at the time and didn’t exactly know what to say to them, but they were just happy to have a doctor who would listen to them and not be afraid to hear what they had to say.”
Recognizing that support is an issue, the American Heart Association released a scientific statement in 2020 on sudden cardiac arrest survivorship, which “expands the cardiac arrest resuscitation system of care to include patients, caregivers, and rehabilitative healthcare partnerships, which are central to cardiac survivorship.”
Soar has a more nuanced view of survivorship support, however. “I suspect some people are very glad to be alive, and that trying to dig deep and bring things out may actually be harmful,” he said. “It’s not as clear cut as everybody thinks.”
He noted that follow-up and rehabilitation should be an option for people who specifically need it who would need to be identified. “But human beings are resilient, and while some people will require help, not everybody will,” he said.
Better CPR, New Treatments
Experts in emergency and intensive care medicine studying survival after cardiac arrest hope to find ways to save patients before too much damage is done to the brain and other organs from loss of oxygen, Dr. Parnia said. He is the lead author in a recent multidisciplinary consensus statement on guidelines and standards for the study of death and recalled experiences of death.
“One of my bugbears is that our survival outcomes from cardiac arrest resuscitation have not changed very much for 60 years because we haven’t developed new treatments and innovative methods,” he said. “Unlike the rest of medicine, we’re living in the past.”
Currently, his team is developing cocktails of treatments. These include hypothermic circulatory arrest — cooling the body to stop blood circulation and brain function for up to 40 minutes — and giving magnesium, a brain-protective treatment, to people whose hearts stop.
Dr. Becker would like to see optimal care of patients with cardiac arrest. “The first step is to increase blood flow with good CPR and then measure whether CPR is working,” he said. Adding that despite the availability of devices that provide feedback on the quality of CPR, they’re rarely used. He cited ultrasound devices that measure the blood flow generated during CPR, compression meter devices that go between the patient’s chest and the rescuer’s hands that gauge the rate and depth of compression, and invasive devices that measure blood pressure during CPR.
His group is trying to design even better devices, he said. “An example would be a little probe that you could pop on the neck that would study blood flow to the brain with ultrasound, so that while you were pumping on the person, you could see if you’re making them better or not.”
“We also have some preliminary data showing that the American Heart Association recommended position on the chest for doing CPR is not the perfect place for everybody,” he said. The 2020 AHA guidelines recommended the center of the lower half of the sternum. At the 2023 American College of Emergency Physicians meeting, Dr. Becker›s team at Hofstra/Northwell presented data on 175 video-recorded adult cardiac arrests in their emergency department over more than 2 years, 22 of which involved at least one change of compression location (for a total of 29 location changes). They found that 41% of compression location changes were associated with return of spontaneous circulation.
For about a third of people, the hands need to be repositioned slightly. “This is not anything that is taught to the public because you can only figure it out if you have some kind of sensor that will let you know how you’re doing. That’s very achievable. We could have that in the future on every ambulance and even in people’s homes.”
When the person arrives at the hospital, he said, “we can make it easier and more likely that they can be put on extracorporeal membrane oxygenation (ECMO). We do that on selected patients in our hospital, even though it’s very difficult to do, because we know that when it’s done properly, it can change survival rates dramatically, from maybe 10%-50%.”
Dr. Dr. Becker, like Dr. Parnia, also favors the development of drug cocktails, and his team has been experimenting with various combinations in animal models. “We think those two things together — ECMO and a drug cocktail — would be a very powerful one to two knock out for cardiac arrest,” he said. “We have a long way to go — 10 or 20 years. But most people around the world working in this area believe that will be the future.”
Dr. Parnia’s study on recalled death was supported by The John Templeton Foundation, Resuscitation Council (UK), and New York University Grossman School of Medicine, with research support staff provided by the UK’s National Institutes for Health Research. Soar is the editor of the journal Resuscitation and receives payment from the publisher Elsevier. Dr. Becker’s institute has received grants from Philips Medical Systems, NIH, Zoll Medical Corp, Nihon Kohden, PCORI, BrainCool, and United Therapeutics. He has received advisory/consultancy honoraria from NIH, Nihon Kohden, HP, and Philips, and he holds several patents in hypothermia induction and reperfusion therapies and several pending patents involving the use of medical slurries as human coolant devices to create reperfusion cocktails and measurement of respiratory quotient.
A version of this article appeared on Medscape.com.
How to Avoid the $400,000 Med School Debt Mistakes I Made
It’s not always great to be tops among your peers.
, according to Medscape Medical News’ 2023 Residents Salary and Debt Report.
I’m smack in that upper percentile. I amassed nearly a half million dollars in student debt and currently stand at roughly $400,000. Yay me.
As a naive twentysomething making a major life decision, I never thought my loans would amount to this inconceivable figure, the proverbial “mortgage without a roof” you hear student debt experts talk about.
This isn’t a story about how the student loan industry needs to be reformed or how education has become increasingly expensive or regrets about going to medical school.
It’s also not a story about how you should be handling basics like consolidating and refinancing and paying extra toward your principal.
It’s about my experience as a physician 13 years after signing that first promissory note. In short: I completely miscalculated the impact loans would have on my life.
I bought money to go to school. I can’t undo that. But over the past decade, I have learned a lot, particularly how those with their own mountain of debt — or who will inevitably wind up with one — can manage things better than I have.
Mistake #1: Loan Forgiveness Is More Complicated Than it Seems
My parents and I were aware of the Public Service Loan Forgiveness (PSLF) program which began in 2007 shortly before I started exploring medical school options. I wanted to help people, so working in the nonprofit sector sounded like a no-brainer. Making 120 payments while practicing at a qualifying institution didn’t sound hard.
Newsflash: Not all healthcare organizations are 501(c)3 programs that qualify as nonprofit for the PSLF program. You can’t just snap your fingers and land at one. I graduated from fellowship just as the COVID-19 pandemic began, which meant I was launching my medical career in the midst of hiring freezes and an overnight disappearance of job opportunities.
I had to take a 2-year hiatus from the nonprofit sector and found a part-time position with a local private practice group. It still stings. Had I been working for a qualified employer, I could have benefited from the student loan payment pause and been closer to applying for loan forgiveness.
Avoid it: Be brutally honest with yourself about what kind of medicine you want to practice — especially within the opportunities you have on hand. Private practice is very different from working for the nonprofit sector. I didn›t know that. When weighing career choices, immediately ask, “How will this impact how I pay my loans?” You may not like the answer, but you›ll always know where you stand financially.
Mistake #2: I Forgot to Factor in Life Goals
To be fair, some things were out of my control: Not getting into a state school with cheaper tuition rates, graduating at the start of a once-in-a-lifetime global pandemic. I wasn’t prepared for a changing job landscape. But there were also “expected” life events like getting married, developing a geographical preference, and having a child. I didn’t consider those either.
How about the “expected” goal of buying a home? For years I didn’t feel financially comfortable enough to take on a mortgage. For so long, my attitude has been don’t take on any more debt. (A special shout-out to my 6.8% interest rate which has contributed over a third of my total loan amount.)
This even affected how my husband and I would talk about what a future home might look like. There’s always a giant unwelcome guest casting a shadow over my thoughts.
Avoid it: Don’t compartmentalize your personal and professional lives. Your student loans will hang over both, and you need to be honest with yourself about what “upward mobility” really means to you while in debt. There’s a reason people say “live like a resident” until your loans are paid off. My husband and I finally worked our numbers to where we bought our first home this past year — a moment years in the making. I still drive around in my beloved Honda CR-V like it’s a Mercedes G-Wagon.
Mistake #3: I Didn’t Ask Questions
I regret not talking to a practicing physician about their experience with student loans. I didn’t know any. There weren’t any physicians in my extended family or my community network. I was a first-generation Pakistani American kid trying to figure it out.
It’s difficult because even today, many physicians aren’t comfortable discussing their financial circumstances. The lack of financial transparency and even financial literacy is astounding among young medical professionals. We live in a medical culture where no one talks about the money. I was too diffident and nervous to even try.
Avoid it: Don’t be afraid to have uncomfortable conversations about money. Don’t allow yourself to make even one passive decision. It’s your life.
If you can’t find someone in medicine to talk to about their financial journey, there are plenty of credible resources. Medscape Medical News has a Physician Business Academy with hot topics like personal finance. The White Coat Investor is literally bookmarked on all my electronic devices. KevinMD.com has a ton of resources and articles answering common financial questions about retirement, savings, and house buying. And Travis Hornsby with www.studentloanplanner.com has wonderful advice on all kinds of different loans.
There are no stupid questions. Just ask. You might be surprised by what people are willing to share.
Mistake #4: Playing it Casual With My Lenders
If $400,000 in debt doesn’t sound bad enough, imagine lots more. It turns out my loan carrier had me at a much higher loan balance because they’d inadvertently duplicated one of my loans in the total. I didn’t know that until I transferred my loans to another handler and it came to light.
Imagine my relief at having a lower total. Imagine my anger at myself for not checking sooner.
Avoid it: Do a thorough self-audit on all your loans more than once a year. Pretend they’re a patient with odd symptoms you can’t pin down and you have the luxury of doing every diagnostic test available. It’s not fun studying your own debt, but it’s the only way to really know how much you have.
Mistake #5: Not Leaving Room to Change My Mind
I underestimated how I would evolve and how my goals would change after having the letters “MD” after my name. I never dreamed that a nonprofit salary might not be enough.
A lot of us assume that the bedside is where we will find professional satisfaction. But you might be surprised. In a climate where we’re constantly being pushed to do more in a broken healthcare system, a landscape where misinformation and technology are forcing medicine to change, there might be little joy in working clinically full time. Then what do you do?
Because I elected to go the PSLF route, I’m tied to this decision. And while it still makes the most economic sense for me personally, it now limits my professional exploration and freedom.
Avoid it: Consider how much time you really want to spend in clinical medicine. Be mindful that you have to work at least 0.8 full time equivalent to qualify for the PSLF program. It’s very hard to predict the future, let alone your future, but just know you›ll have moments where you ask, “Do I really want to stay on this career track?” Will you be able to pivot? Can you live with it if the answer is no?
Looking Ahead
Let me be clear about one thing. Despite all the negativity I feel toward my student loans — guilt about the burden I brought to my marriage and my adult life, disappointment about the cost of becoming a successful physician, and frustration that this has turned out to be the most influential factor shaping my professional and personal choices — the one thing I don’t feel is shame.
I worked hard to get to this point in my life. I am proud of being a physician.
My student loan burden will follow me to the grave. But progress is also possible. I have friends that have paid their loans down by hustling, working hard, and dropping every penny toward them.
I also have friends that have had their loans forgiven. There are options. Everyone’s experience looks a little different. But don’t be naive: Student loans will color every financial decision you make.
I’m finding solace now in recently moving and finding work at a nonprofit institution. I’m back at it; 77 payments made, and 43 to go.
Well, technically I’ve made 93 payments. I’m still waiting for my loan servicer to get around to updating my account.
You really have to stay on top of those folks.
A version of this article appeared on Medscape.com.
It’s not always great to be tops among your peers.
, according to Medscape Medical News’ 2023 Residents Salary and Debt Report.
I’m smack in that upper percentile. I amassed nearly a half million dollars in student debt and currently stand at roughly $400,000. Yay me.
As a naive twentysomething making a major life decision, I never thought my loans would amount to this inconceivable figure, the proverbial “mortgage without a roof” you hear student debt experts talk about.
This isn’t a story about how the student loan industry needs to be reformed or how education has become increasingly expensive or regrets about going to medical school.
It’s also not a story about how you should be handling basics like consolidating and refinancing and paying extra toward your principal.
It’s about my experience as a physician 13 years after signing that first promissory note. In short: I completely miscalculated the impact loans would have on my life.
I bought money to go to school. I can’t undo that. But over the past decade, I have learned a lot, particularly how those with their own mountain of debt — or who will inevitably wind up with one — can manage things better than I have.
Mistake #1: Loan Forgiveness Is More Complicated Than it Seems
My parents and I were aware of the Public Service Loan Forgiveness (PSLF) program which began in 2007 shortly before I started exploring medical school options. I wanted to help people, so working in the nonprofit sector sounded like a no-brainer. Making 120 payments while practicing at a qualifying institution didn’t sound hard.
Newsflash: Not all healthcare organizations are 501(c)3 programs that qualify as nonprofit for the PSLF program. You can’t just snap your fingers and land at one. I graduated from fellowship just as the COVID-19 pandemic began, which meant I was launching my medical career in the midst of hiring freezes and an overnight disappearance of job opportunities.
I had to take a 2-year hiatus from the nonprofit sector and found a part-time position with a local private practice group. It still stings. Had I been working for a qualified employer, I could have benefited from the student loan payment pause and been closer to applying for loan forgiveness.
Avoid it: Be brutally honest with yourself about what kind of medicine you want to practice — especially within the opportunities you have on hand. Private practice is very different from working for the nonprofit sector. I didn›t know that. When weighing career choices, immediately ask, “How will this impact how I pay my loans?” You may not like the answer, but you›ll always know where you stand financially.
Mistake #2: I Forgot to Factor in Life Goals
To be fair, some things were out of my control: Not getting into a state school with cheaper tuition rates, graduating at the start of a once-in-a-lifetime global pandemic. I wasn’t prepared for a changing job landscape. But there were also “expected” life events like getting married, developing a geographical preference, and having a child. I didn’t consider those either.
How about the “expected” goal of buying a home? For years I didn’t feel financially comfortable enough to take on a mortgage. For so long, my attitude has been don’t take on any more debt. (A special shout-out to my 6.8% interest rate which has contributed over a third of my total loan amount.)
This even affected how my husband and I would talk about what a future home might look like. There’s always a giant unwelcome guest casting a shadow over my thoughts.
Avoid it: Don’t compartmentalize your personal and professional lives. Your student loans will hang over both, and you need to be honest with yourself about what “upward mobility” really means to you while in debt. There’s a reason people say “live like a resident” until your loans are paid off. My husband and I finally worked our numbers to where we bought our first home this past year — a moment years in the making. I still drive around in my beloved Honda CR-V like it’s a Mercedes G-Wagon.
Mistake #3: I Didn’t Ask Questions
I regret not talking to a practicing physician about their experience with student loans. I didn’t know any. There weren’t any physicians in my extended family or my community network. I was a first-generation Pakistani American kid trying to figure it out.
It’s difficult because even today, many physicians aren’t comfortable discussing their financial circumstances. The lack of financial transparency and even financial literacy is astounding among young medical professionals. We live in a medical culture where no one talks about the money. I was too diffident and nervous to even try.
Avoid it: Don’t be afraid to have uncomfortable conversations about money. Don’t allow yourself to make even one passive decision. It’s your life.
If you can’t find someone in medicine to talk to about their financial journey, there are plenty of credible resources. Medscape Medical News has a Physician Business Academy with hot topics like personal finance. The White Coat Investor is literally bookmarked on all my electronic devices. KevinMD.com has a ton of resources and articles answering common financial questions about retirement, savings, and house buying. And Travis Hornsby with www.studentloanplanner.com has wonderful advice on all kinds of different loans.
There are no stupid questions. Just ask. You might be surprised by what people are willing to share.
Mistake #4: Playing it Casual With My Lenders
If $400,000 in debt doesn’t sound bad enough, imagine lots more. It turns out my loan carrier had me at a much higher loan balance because they’d inadvertently duplicated one of my loans in the total. I didn’t know that until I transferred my loans to another handler and it came to light.
Imagine my relief at having a lower total. Imagine my anger at myself for not checking sooner.
Avoid it: Do a thorough self-audit on all your loans more than once a year. Pretend they’re a patient with odd symptoms you can’t pin down and you have the luxury of doing every diagnostic test available. It’s not fun studying your own debt, but it’s the only way to really know how much you have.
Mistake #5: Not Leaving Room to Change My Mind
I underestimated how I would evolve and how my goals would change after having the letters “MD” after my name. I never dreamed that a nonprofit salary might not be enough.
A lot of us assume that the bedside is where we will find professional satisfaction. But you might be surprised. In a climate where we’re constantly being pushed to do more in a broken healthcare system, a landscape where misinformation and technology are forcing medicine to change, there might be little joy in working clinically full time. Then what do you do?
Because I elected to go the PSLF route, I’m tied to this decision. And while it still makes the most economic sense for me personally, it now limits my professional exploration and freedom.
Avoid it: Consider how much time you really want to spend in clinical medicine. Be mindful that you have to work at least 0.8 full time equivalent to qualify for the PSLF program. It’s very hard to predict the future, let alone your future, but just know you›ll have moments where you ask, “Do I really want to stay on this career track?” Will you be able to pivot? Can you live with it if the answer is no?
Looking Ahead
Let me be clear about one thing. Despite all the negativity I feel toward my student loans — guilt about the burden I brought to my marriage and my adult life, disappointment about the cost of becoming a successful physician, and frustration that this has turned out to be the most influential factor shaping my professional and personal choices — the one thing I don’t feel is shame.
I worked hard to get to this point in my life. I am proud of being a physician.
My student loan burden will follow me to the grave. But progress is also possible. I have friends that have paid their loans down by hustling, working hard, and dropping every penny toward them.
I also have friends that have had their loans forgiven. There are options. Everyone’s experience looks a little different. But don’t be naive: Student loans will color every financial decision you make.
I’m finding solace now in recently moving and finding work at a nonprofit institution. I’m back at it; 77 payments made, and 43 to go.
Well, technically I’ve made 93 payments. I’m still waiting for my loan servicer to get around to updating my account.
You really have to stay on top of those folks.
A version of this article appeared on Medscape.com.
It’s not always great to be tops among your peers.
, according to Medscape Medical News’ 2023 Residents Salary and Debt Report.
I’m smack in that upper percentile. I amassed nearly a half million dollars in student debt and currently stand at roughly $400,000. Yay me.
As a naive twentysomething making a major life decision, I never thought my loans would amount to this inconceivable figure, the proverbial “mortgage without a roof” you hear student debt experts talk about.
This isn’t a story about how the student loan industry needs to be reformed or how education has become increasingly expensive or regrets about going to medical school.
It’s also not a story about how you should be handling basics like consolidating and refinancing and paying extra toward your principal.
It’s about my experience as a physician 13 years after signing that first promissory note. In short: I completely miscalculated the impact loans would have on my life.
I bought money to go to school. I can’t undo that. But over the past decade, I have learned a lot, particularly how those with their own mountain of debt — or who will inevitably wind up with one — can manage things better than I have.
Mistake #1: Loan Forgiveness Is More Complicated Than it Seems
My parents and I were aware of the Public Service Loan Forgiveness (PSLF) program which began in 2007 shortly before I started exploring medical school options. I wanted to help people, so working in the nonprofit sector sounded like a no-brainer. Making 120 payments while practicing at a qualifying institution didn’t sound hard.
Newsflash: Not all healthcare organizations are 501(c)3 programs that qualify as nonprofit for the PSLF program. You can’t just snap your fingers and land at one. I graduated from fellowship just as the COVID-19 pandemic began, which meant I was launching my medical career in the midst of hiring freezes and an overnight disappearance of job opportunities.
I had to take a 2-year hiatus from the nonprofit sector and found a part-time position with a local private practice group. It still stings. Had I been working for a qualified employer, I could have benefited from the student loan payment pause and been closer to applying for loan forgiveness.
Avoid it: Be brutally honest with yourself about what kind of medicine you want to practice — especially within the opportunities you have on hand. Private practice is very different from working for the nonprofit sector. I didn›t know that. When weighing career choices, immediately ask, “How will this impact how I pay my loans?” You may not like the answer, but you›ll always know where you stand financially.
Mistake #2: I Forgot to Factor in Life Goals
To be fair, some things were out of my control: Not getting into a state school with cheaper tuition rates, graduating at the start of a once-in-a-lifetime global pandemic. I wasn’t prepared for a changing job landscape. But there were also “expected” life events like getting married, developing a geographical preference, and having a child. I didn’t consider those either.
How about the “expected” goal of buying a home? For years I didn’t feel financially comfortable enough to take on a mortgage. For so long, my attitude has been don’t take on any more debt. (A special shout-out to my 6.8% interest rate which has contributed over a third of my total loan amount.)
This even affected how my husband and I would talk about what a future home might look like. There’s always a giant unwelcome guest casting a shadow over my thoughts.
Avoid it: Don’t compartmentalize your personal and professional lives. Your student loans will hang over both, and you need to be honest with yourself about what “upward mobility” really means to you while in debt. There’s a reason people say “live like a resident” until your loans are paid off. My husband and I finally worked our numbers to where we bought our first home this past year — a moment years in the making. I still drive around in my beloved Honda CR-V like it’s a Mercedes G-Wagon.
Mistake #3: I Didn’t Ask Questions
I regret not talking to a practicing physician about their experience with student loans. I didn’t know any. There weren’t any physicians in my extended family or my community network. I was a first-generation Pakistani American kid trying to figure it out.
It’s difficult because even today, many physicians aren’t comfortable discussing their financial circumstances. The lack of financial transparency and even financial literacy is astounding among young medical professionals. We live in a medical culture where no one talks about the money. I was too diffident and nervous to even try.
Avoid it: Don’t be afraid to have uncomfortable conversations about money. Don’t allow yourself to make even one passive decision. It’s your life.
If you can’t find someone in medicine to talk to about their financial journey, there are plenty of credible resources. Medscape Medical News has a Physician Business Academy with hot topics like personal finance. The White Coat Investor is literally bookmarked on all my electronic devices. KevinMD.com has a ton of resources and articles answering common financial questions about retirement, savings, and house buying. And Travis Hornsby with www.studentloanplanner.com has wonderful advice on all kinds of different loans.
There are no stupid questions. Just ask. You might be surprised by what people are willing to share.
Mistake #4: Playing it Casual With My Lenders
If $400,000 in debt doesn’t sound bad enough, imagine lots more. It turns out my loan carrier had me at a much higher loan balance because they’d inadvertently duplicated one of my loans in the total. I didn’t know that until I transferred my loans to another handler and it came to light.
Imagine my relief at having a lower total. Imagine my anger at myself for not checking sooner.
Avoid it: Do a thorough self-audit on all your loans more than once a year. Pretend they’re a patient with odd symptoms you can’t pin down and you have the luxury of doing every diagnostic test available. It’s not fun studying your own debt, but it’s the only way to really know how much you have.
Mistake #5: Not Leaving Room to Change My Mind
I underestimated how I would evolve and how my goals would change after having the letters “MD” after my name. I never dreamed that a nonprofit salary might not be enough.
A lot of us assume that the bedside is where we will find professional satisfaction. But you might be surprised. In a climate where we’re constantly being pushed to do more in a broken healthcare system, a landscape where misinformation and technology are forcing medicine to change, there might be little joy in working clinically full time. Then what do you do?
Because I elected to go the PSLF route, I’m tied to this decision. And while it still makes the most economic sense for me personally, it now limits my professional exploration and freedom.
Avoid it: Consider how much time you really want to spend in clinical medicine. Be mindful that you have to work at least 0.8 full time equivalent to qualify for the PSLF program. It’s very hard to predict the future, let alone your future, but just know you›ll have moments where you ask, “Do I really want to stay on this career track?” Will you be able to pivot? Can you live with it if the answer is no?
Looking Ahead
Let me be clear about one thing. Despite all the negativity I feel toward my student loans — guilt about the burden I brought to my marriage and my adult life, disappointment about the cost of becoming a successful physician, and frustration that this has turned out to be the most influential factor shaping my professional and personal choices — the one thing I don’t feel is shame.
I worked hard to get to this point in my life. I am proud of being a physician.
My student loan burden will follow me to the grave. But progress is also possible. I have friends that have paid their loans down by hustling, working hard, and dropping every penny toward them.
I also have friends that have had their loans forgiven. There are options. Everyone’s experience looks a little different. But don’t be naive: Student loans will color every financial decision you make.
I’m finding solace now in recently moving and finding work at a nonprofit institution. I’m back at it; 77 payments made, and 43 to go.
Well, technically I’ve made 93 payments. I’m still waiting for my loan servicer to get around to updating my account.
You really have to stay on top of those folks.
A version of this article appeared on Medscape.com.
Obesity and lung disease in the era of GLP-1 agonists
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).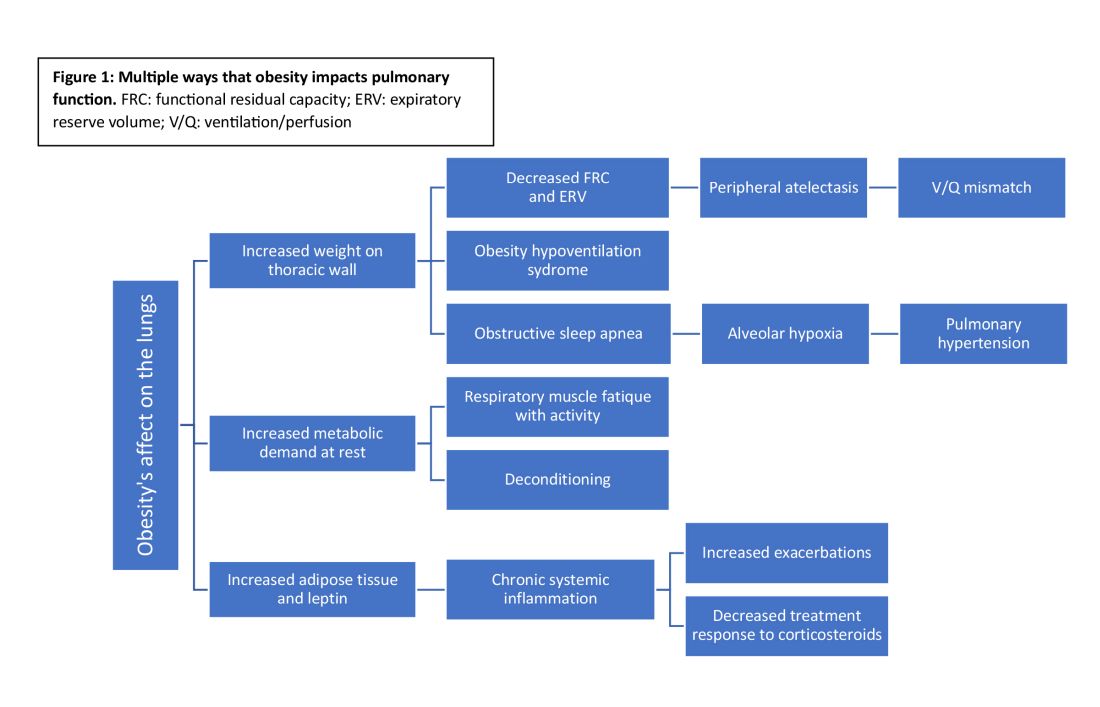
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?

