User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Liberalized European sports cardiology guidelines break new ground
New guidelines on sports cardiology from the European Society of Cardiology break fresh ground by green-lighting participation in vigorous competitive sports by selected patients with stable coronary artery disease, heart failure, or mild arrhythmias.
These liberalized guidelines, released at the virtual annual congress of the European Society of Cardiology, thus move well beyond the standard exercise advice to engage in about 150 minutes per week of moderate physical activity, typically defined as brisk walking or its equivalent.
The guidelines reflect a conviction that exercise is powerful medicine for patients with cardiovascular disease and also affords a means to help curb the epidemics of diabetes and obesity that drive cardiovascular risk, according to Antonio Pelliccia, MD, who cochaired the 24-member task force of European and American experts that developed the guidelines.
In a session highlighting the new sports cardiology guidelines, Mats Borjesson, MD, head of the Center for Health and Performance at Gothenburg (Sweden) University, summarized the section devoted to patients with stable coronary artery disease: “If you have established CAD and a low risk of adverse events during exercise, you are eligible for high-intensity exercise and competitive sports. But if you have persistent ischemia despite medical treatment, or symptoms, then you’re only eligible for leisure-time subthreshold activity.”
Dr. Pelliccia put this new recommendation into context.
“We are not talking anymore in this particular disease just about cardiac rehabilitation or leisure-time activity, but we are also opening the border and talking about competitive sports activity in selected patients where you have the evidence for low risk of exercise-induced adverse events. This is a major achievement now for what is the major disease in our adult population,” said Dr. Pelliccia, chief of cardiology at the Institute of Sports Medicine and Science at the Italian National Olympic Committee and professor of sports cardiology at La Sapienza University of Rome.
The recommendation for individualized consideration of all types of exercise, even including vigorous competitive sports, in low-risk patients with CAD gets a class IIa, level of evidence (LOE) C recommendation in the new guidelines. That’s a big step down from a ringing class Ia endorsement, but since sports cardiology is a relatively young field with little evidence that’s based on randomized trials, the guidelines are rife with many other class IIa, LOE C recommendations as well.
“The level of evidence is rather low, so these guidelines are very much the personal perspective of the expert panel,” explained Martin Halle, MD, professor and head of the department of prevention, rehabilitation, and sports cardiology at Technical University of Munich.
The high-risk features for exercise-induced cardiac adverse events in patients with longstanding stable CAD, as cited in the guidelines, include a critical coronary stenosis, defined as a more than 70% lesion in a major coronary artery or a greater than 50% stenosis in the left main, and/or a fractional flow reserve score of less than 0.8; a left ventricular ejection fraction of 50% or less with wall-motion abnormalities; inducible myocardial ischemia on maximal exercise testing; nonsustained ventricular tachycardia; polymorphic or very frequent ventricular premature beats at rest and during maximum stress; and a recent acute coronary syndrome (ACS). These features call for an exercise prescription tailored to remain below the patient’s angina and ischemia thresholds.
“It’s important for cardiologists out there to understand that we definitely need a maximal exercise test. In somebody who is running and has an ACS and then wants to start running again, 200 watts on an ergometer is too low. We have to push them up to the end, and then if everything is okay – left ventricular function is okay, no ischemia, no arrhythmias under exercise testing – then it’s fine,” Dr. Halle said.
Dr. Pelliccia added that close follow-up is needed, because this is an evolving disease.”
Exercise and heart failure
Massimo F. Piepoli, MD, PhD, noted that the guidelines give a class IIb, LOE C recommendation for consideration of high-intensity recreational endurance and power sports in patients with heart failure with either midrange or preserved ejection fraction, provided they are stable, asymptomatic, on optimal guideline-directed medical therapy, and without abnormalities on a maximal exercise stress test.
However, such intense physical activity is not recommended in patients with heart failure with reduced ejection fraction, regardless of their symptom status, added Dr. Piepoli of Guglielmo da Saliceto Hospital in Placenza, Italy.
“We’re talking here, I think for the first time, about possible competitive sports participation in individuals with heart failure, depending on their clinical condition. We are really opening the barriers to sports participation, even in these patients in whom we never thought of it before,” Dr. Pelliccia observed.
Valvular heart disease and exercise
Guidelines panelist Sabiha Gati, MRCP, PhD, said asymptomatic individuals with mild valvular abnormalities can participate in all recreational and competitive sports; that’s a class I, LOE C recommendation.
“Moderate regurgitant lesions are better tolerated than stenotic lesions, and those with preserved systolic function, good functional capacity, without any exercise-induced arrhythmias or ischemia or abnormal hemodynamic response are considered to be low risk and can participate in all sports,” added Dr. Gati, a cardiologist at Royal Brompton Hospital, London.
The two most common valvular abnormalities encountered in clinical practice are bicuspid aortic valve and mitral valve prolapse. Dr. Gati noted that, while mitral valve prolapse has a benign prognosis in the great majority of affected individuals, the presence of specific features indicative of increased risk for sudden cardiac death precludes participation in strenuous exercise. These include T-wave inversion in the inferior leads on a 12-lead ECG, long QT, bileaflet mitral valve prolapse, basal inferolateral wall fibrosis, severe mitral regurgitation, or a family history of sudden cardiac death.
Bicuspid aortic valve has a prevalence of 1%-2% in the general population. It can be associated with aortic stenosis, aortic regurgitation, and increased risk of ascending aortic aneurysm and dissection. Since it remains unclear whether intensive exercise accelerates aortic dilatation, a cautious approach to sports participation is recommended in patients with an ascending aorta above the normal limit of 40 mm, she said.
The 80-page ESC sports cardiology guidelines, published online simultaneously with their presentation, cover a broad range of additional topics, including exercise recommendations for the general public, for the elderly, as well as for patients with cardiomyopathies, adult congenital heart disease, arrhythmias, and channelopathies. Gaps in evidence are also highlighted.
SOURCE: Pelliccia A. ESC 2020 and Eur Heart J. 2020 Aug 29. doi: 10.1093/eurheartj/ehaa605.
New guidelines on sports cardiology from the European Society of Cardiology break fresh ground by green-lighting participation in vigorous competitive sports by selected patients with stable coronary artery disease, heart failure, or mild arrhythmias.
These liberalized guidelines, released at the virtual annual congress of the European Society of Cardiology, thus move well beyond the standard exercise advice to engage in about 150 minutes per week of moderate physical activity, typically defined as brisk walking or its equivalent.
The guidelines reflect a conviction that exercise is powerful medicine for patients with cardiovascular disease and also affords a means to help curb the epidemics of diabetes and obesity that drive cardiovascular risk, according to Antonio Pelliccia, MD, who cochaired the 24-member task force of European and American experts that developed the guidelines.
In a session highlighting the new sports cardiology guidelines, Mats Borjesson, MD, head of the Center for Health and Performance at Gothenburg (Sweden) University, summarized the section devoted to patients with stable coronary artery disease: “If you have established CAD and a low risk of adverse events during exercise, you are eligible for high-intensity exercise and competitive sports. But if you have persistent ischemia despite medical treatment, or symptoms, then you’re only eligible for leisure-time subthreshold activity.”
Dr. Pelliccia put this new recommendation into context.
“We are not talking anymore in this particular disease just about cardiac rehabilitation or leisure-time activity, but we are also opening the border and talking about competitive sports activity in selected patients where you have the evidence for low risk of exercise-induced adverse events. This is a major achievement now for what is the major disease in our adult population,” said Dr. Pelliccia, chief of cardiology at the Institute of Sports Medicine and Science at the Italian National Olympic Committee and professor of sports cardiology at La Sapienza University of Rome.
The recommendation for individualized consideration of all types of exercise, even including vigorous competitive sports, in low-risk patients with CAD gets a class IIa, level of evidence (LOE) C recommendation in the new guidelines. That’s a big step down from a ringing class Ia endorsement, but since sports cardiology is a relatively young field with little evidence that’s based on randomized trials, the guidelines are rife with many other class IIa, LOE C recommendations as well.
“The level of evidence is rather low, so these guidelines are very much the personal perspective of the expert panel,” explained Martin Halle, MD, professor and head of the department of prevention, rehabilitation, and sports cardiology at Technical University of Munich.
The high-risk features for exercise-induced cardiac adverse events in patients with longstanding stable CAD, as cited in the guidelines, include a critical coronary stenosis, defined as a more than 70% lesion in a major coronary artery or a greater than 50% stenosis in the left main, and/or a fractional flow reserve score of less than 0.8; a left ventricular ejection fraction of 50% or less with wall-motion abnormalities; inducible myocardial ischemia on maximal exercise testing; nonsustained ventricular tachycardia; polymorphic or very frequent ventricular premature beats at rest and during maximum stress; and a recent acute coronary syndrome (ACS). These features call for an exercise prescription tailored to remain below the patient’s angina and ischemia thresholds.
“It’s important for cardiologists out there to understand that we definitely need a maximal exercise test. In somebody who is running and has an ACS and then wants to start running again, 200 watts on an ergometer is too low. We have to push them up to the end, and then if everything is okay – left ventricular function is okay, no ischemia, no arrhythmias under exercise testing – then it’s fine,” Dr. Halle said.
Dr. Pelliccia added that close follow-up is needed, because this is an evolving disease.”
Exercise and heart failure
Massimo F. Piepoli, MD, PhD, noted that the guidelines give a class IIb, LOE C recommendation for consideration of high-intensity recreational endurance and power sports in patients with heart failure with either midrange or preserved ejection fraction, provided they are stable, asymptomatic, on optimal guideline-directed medical therapy, and without abnormalities on a maximal exercise stress test.
However, such intense physical activity is not recommended in patients with heart failure with reduced ejection fraction, regardless of their symptom status, added Dr. Piepoli of Guglielmo da Saliceto Hospital in Placenza, Italy.
“We’re talking here, I think for the first time, about possible competitive sports participation in individuals with heart failure, depending on their clinical condition. We are really opening the barriers to sports participation, even in these patients in whom we never thought of it before,” Dr. Pelliccia observed.
Valvular heart disease and exercise
Guidelines panelist Sabiha Gati, MRCP, PhD, said asymptomatic individuals with mild valvular abnormalities can participate in all recreational and competitive sports; that’s a class I, LOE C recommendation.
“Moderate regurgitant lesions are better tolerated than stenotic lesions, and those with preserved systolic function, good functional capacity, without any exercise-induced arrhythmias or ischemia or abnormal hemodynamic response are considered to be low risk and can participate in all sports,” added Dr. Gati, a cardiologist at Royal Brompton Hospital, London.
The two most common valvular abnormalities encountered in clinical practice are bicuspid aortic valve and mitral valve prolapse. Dr. Gati noted that, while mitral valve prolapse has a benign prognosis in the great majority of affected individuals, the presence of specific features indicative of increased risk for sudden cardiac death precludes participation in strenuous exercise. These include T-wave inversion in the inferior leads on a 12-lead ECG, long QT, bileaflet mitral valve prolapse, basal inferolateral wall fibrosis, severe mitral regurgitation, or a family history of sudden cardiac death.
Bicuspid aortic valve has a prevalence of 1%-2% in the general population. It can be associated with aortic stenosis, aortic regurgitation, and increased risk of ascending aortic aneurysm and dissection. Since it remains unclear whether intensive exercise accelerates aortic dilatation, a cautious approach to sports participation is recommended in patients with an ascending aorta above the normal limit of 40 mm, she said.
The 80-page ESC sports cardiology guidelines, published online simultaneously with their presentation, cover a broad range of additional topics, including exercise recommendations for the general public, for the elderly, as well as for patients with cardiomyopathies, adult congenital heart disease, arrhythmias, and channelopathies. Gaps in evidence are also highlighted.
SOURCE: Pelliccia A. ESC 2020 and Eur Heart J. 2020 Aug 29. doi: 10.1093/eurheartj/ehaa605.
New guidelines on sports cardiology from the European Society of Cardiology break fresh ground by green-lighting participation in vigorous competitive sports by selected patients with stable coronary artery disease, heart failure, or mild arrhythmias.
These liberalized guidelines, released at the virtual annual congress of the European Society of Cardiology, thus move well beyond the standard exercise advice to engage in about 150 minutes per week of moderate physical activity, typically defined as brisk walking or its equivalent.
The guidelines reflect a conviction that exercise is powerful medicine for patients with cardiovascular disease and also affords a means to help curb the epidemics of diabetes and obesity that drive cardiovascular risk, according to Antonio Pelliccia, MD, who cochaired the 24-member task force of European and American experts that developed the guidelines.
In a session highlighting the new sports cardiology guidelines, Mats Borjesson, MD, head of the Center for Health and Performance at Gothenburg (Sweden) University, summarized the section devoted to patients with stable coronary artery disease: “If you have established CAD and a low risk of adverse events during exercise, you are eligible for high-intensity exercise and competitive sports. But if you have persistent ischemia despite medical treatment, or symptoms, then you’re only eligible for leisure-time subthreshold activity.”
Dr. Pelliccia put this new recommendation into context.
“We are not talking anymore in this particular disease just about cardiac rehabilitation or leisure-time activity, but we are also opening the border and talking about competitive sports activity in selected patients where you have the evidence for low risk of exercise-induced adverse events. This is a major achievement now for what is the major disease in our adult population,” said Dr. Pelliccia, chief of cardiology at the Institute of Sports Medicine and Science at the Italian National Olympic Committee and professor of sports cardiology at La Sapienza University of Rome.
The recommendation for individualized consideration of all types of exercise, even including vigorous competitive sports, in low-risk patients with CAD gets a class IIa, level of evidence (LOE) C recommendation in the new guidelines. That’s a big step down from a ringing class Ia endorsement, but since sports cardiology is a relatively young field with little evidence that’s based on randomized trials, the guidelines are rife with many other class IIa, LOE C recommendations as well.
“The level of evidence is rather low, so these guidelines are very much the personal perspective of the expert panel,” explained Martin Halle, MD, professor and head of the department of prevention, rehabilitation, and sports cardiology at Technical University of Munich.
The high-risk features for exercise-induced cardiac adverse events in patients with longstanding stable CAD, as cited in the guidelines, include a critical coronary stenosis, defined as a more than 70% lesion in a major coronary artery or a greater than 50% stenosis in the left main, and/or a fractional flow reserve score of less than 0.8; a left ventricular ejection fraction of 50% or less with wall-motion abnormalities; inducible myocardial ischemia on maximal exercise testing; nonsustained ventricular tachycardia; polymorphic or very frequent ventricular premature beats at rest and during maximum stress; and a recent acute coronary syndrome (ACS). These features call for an exercise prescription tailored to remain below the patient’s angina and ischemia thresholds.
“It’s important for cardiologists out there to understand that we definitely need a maximal exercise test. In somebody who is running and has an ACS and then wants to start running again, 200 watts on an ergometer is too low. We have to push them up to the end, and then if everything is okay – left ventricular function is okay, no ischemia, no arrhythmias under exercise testing – then it’s fine,” Dr. Halle said.
Dr. Pelliccia added that close follow-up is needed, because this is an evolving disease.”
Exercise and heart failure
Massimo F. Piepoli, MD, PhD, noted that the guidelines give a class IIb, LOE C recommendation for consideration of high-intensity recreational endurance and power sports in patients with heart failure with either midrange or preserved ejection fraction, provided they are stable, asymptomatic, on optimal guideline-directed medical therapy, and without abnormalities on a maximal exercise stress test.
However, such intense physical activity is not recommended in patients with heart failure with reduced ejection fraction, regardless of their symptom status, added Dr. Piepoli of Guglielmo da Saliceto Hospital in Placenza, Italy.
“We’re talking here, I think for the first time, about possible competitive sports participation in individuals with heart failure, depending on their clinical condition. We are really opening the barriers to sports participation, even in these patients in whom we never thought of it before,” Dr. Pelliccia observed.
Valvular heart disease and exercise
Guidelines panelist Sabiha Gati, MRCP, PhD, said asymptomatic individuals with mild valvular abnormalities can participate in all recreational and competitive sports; that’s a class I, LOE C recommendation.
“Moderate regurgitant lesions are better tolerated than stenotic lesions, and those with preserved systolic function, good functional capacity, without any exercise-induced arrhythmias or ischemia or abnormal hemodynamic response are considered to be low risk and can participate in all sports,” added Dr. Gati, a cardiologist at Royal Brompton Hospital, London.
The two most common valvular abnormalities encountered in clinical practice are bicuspid aortic valve and mitral valve prolapse. Dr. Gati noted that, while mitral valve prolapse has a benign prognosis in the great majority of affected individuals, the presence of specific features indicative of increased risk for sudden cardiac death precludes participation in strenuous exercise. These include T-wave inversion in the inferior leads on a 12-lead ECG, long QT, bileaflet mitral valve prolapse, basal inferolateral wall fibrosis, severe mitral regurgitation, or a family history of sudden cardiac death.
Bicuspid aortic valve has a prevalence of 1%-2% in the general population. It can be associated with aortic stenosis, aortic regurgitation, and increased risk of ascending aortic aneurysm and dissection. Since it remains unclear whether intensive exercise accelerates aortic dilatation, a cautious approach to sports participation is recommended in patients with an ascending aorta above the normal limit of 40 mm, she said.
The 80-page ESC sports cardiology guidelines, published online simultaneously with their presentation, cover a broad range of additional topics, including exercise recommendations for the general public, for the elderly, as well as for patients with cardiomyopathies, adult congenital heart disease, arrhythmias, and channelopathies. Gaps in evidence are also highlighted.
SOURCE: Pelliccia A. ESC 2020 and Eur Heart J. 2020 Aug 29. doi: 10.1093/eurheartj/ehaa605.
FROM ESC CONGRESS 2020
Many providers don’t follow hypertension guidelines
Many health care professionals are not following current, evidence-based guidelines to screen for and diagnose hypertension, and appear to have substantial gaps in knowledge, beliefs, and use of recommended practices, results from a large survey suggest.
“One surprising finding was that there was so much trust in the stethoscope, because the automated monitors are a better way to take blood pressure,” lead author Beverly Green, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The results of the survey were presented Sept. 10 at the virtual joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The U.S. Preventive Services Task Force (USPSTF) and the American Heart Association/American College of Cardiology recommend out-of-office blood pressure measurements – via ambulatory blood pressure monitoring (ABPM) or home BP monitoring – before making a new diagnosis of hypertension.
To gauge provider knowledge, beliefs, and practices related to BP diagnostic tests, the researchers surveyed 282 providers: 102 medical assistants (MA), 28 licensed practical nurses (LPNs), 33 registered nurses (RNs), 86 primary care physicians, and 33 advanced practitioners (APs).
More than three-quarters of providers (79%) felt that BP measured manually with a stethoscope and ABPM were “very or highly” accurate ways to measure BP when making a new diagnosis of hypertension.
Most did not think that automated clinic BPs, home BP, or kiosk BP measurements were very or highly accurate.
Nearly all providers surveyed (96%) reported that they “always or almost always” rely on clinic BP measurements when diagnosing hypertension, but the majority of physicians/APs would prefer using ABPM (61%) if available.
The problem with ABPM, said Dr. Green, is “it’s just not very available or convenient for patients, and a lot of providers think that patients won’t tolerate it.” Yet, without it, there is a risk for misclassification, she said.
Karen A. Griffin, MD, who chairs the AHA Council on Hypertension, said it became “customary to use clinic BP since ABPM was not previously reimbursed for the routine diagnosis of hypertension.
“Now that the payment for ABPM has been expanded, the number of machines at most institutions is not adequate for the need. Consequently, it will take some time to catch up with the current guidelines for diagnosing hypertension,” she said in an interview.
The provider survey by Dr. Green and colleagues also shows slow uptake of updated thresholds for high blood pressure.
Eighty-four percent of physicians/APs and 68% of MA/LPN/RNs said they used a clinic BP threshold of at least 140/90 mm Hg for making a new diagnosis of hypertension.
Only 3.5% and 9.0%, respectively, reported using the updated threshold of at least 130/80 mm Hg put forth in 2017.
Dr. Griffin said part of this stems from the fact that the survey began before the updated guidelines were released in 2017, “not to mention the fact that some societies have opposed the new threshold of 130/80 mm Hg.”
“I think, with time, the data on morbidity and mortality associated with the goal of 130/80 mm Hg will hopefully convince those who have not yet implemented these new guidelines that it is a safe and effective BP goal,” Dr. Griffin said.
This research had no specific funding. Dr. Green and Dr. Griffin have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Many health care professionals are not following current, evidence-based guidelines to screen for and diagnose hypertension, and appear to have substantial gaps in knowledge, beliefs, and use of recommended practices, results from a large survey suggest.
“One surprising finding was that there was so much trust in the stethoscope, because the automated monitors are a better way to take blood pressure,” lead author Beverly Green, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The results of the survey were presented Sept. 10 at the virtual joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The U.S. Preventive Services Task Force (USPSTF) and the American Heart Association/American College of Cardiology recommend out-of-office blood pressure measurements – via ambulatory blood pressure monitoring (ABPM) or home BP monitoring – before making a new diagnosis of hypertension.
To gauge provider knowledge, beliefs, and practices related to BP diagnostic tests, the researchers surveyed 282 providers: 102 medical assistants (MA), 28 licensed practical nurses (LPNs), 33 registered nurses (RNs), 86 primary care physicians, and 33 advanced practitioners (APs).
More than three-quarters of providers (79%) felt that BP measured manually with a stethoscope and ABPM were “very or highly” accurate ways to measure BP when making a new diagnosis of hypertension.
Most did not think that automated clinic BPs, home BP, or kiosk BP measurements were very or highly accurate.
Nearly all providers surveyed (96%) reported that they “always or almost always” rely on clinic BP measurements when diagnosing hypertension, but the majority of physicians/APs would prefer using ABPM (61%) if available.
The problem with ABPM, said Dr. Green, is “it’s just not very available or convenient for patients, and a lot of providers think that patients won’t tolerate it.” Yet, without it, there is a risk for misclassification, she said.
Karen A. Griffin, MD, who chairs the AHA Council on Hypertension, said it became “customary to use clinic BP since ABPM was not previously reimbursed for the routine diagnosis of hypertension.
“Now that the payment for ABPM has been expanded, the number of machines at most institutions is not adequate for the need. Consequently, it will take some time to catch up with the current guidelines for diagnosing hypertension,” she said in an interview.
The provider survey by Dr. Green and colleagues also shows slow uptake of updated thresholds for high blood pressure.
Eighty-four percent of physicians/APs and 68% of MA/LPN/RNs said they used a clinic BP threshold of at least 140/90 mm Hg for making a new diagnosis of hypertension.
Only 3.5% and 9.0%, respectively, reported using the updated threshold of at least 130/80 mm Hg put forth in 2017.
Dr. Griffin said part of this stems from the fact that the survey began before the updated guidelines were released in 2017, “not to mention the fact that some societies have opposed the new threshold of 130/80 mm Hg.”
“I think, with time, the data on morbidity and mortality associated with the goal of 130/80 mm Hg will hopefully convince those who have not yet implemented these new guidelines that it is a safe and effective BP goal,” Dr. Griffin said.
This research had no specific funding. Dr. Green and Dr. Griffin have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Many health care professionals are not following current, evidence-based guidelines to screen for and diagnose hypertension, and appear to have substantial gaps in knowledge, beliefs, and use of recommended practices, results from a large survey suggest.
“One surprising finding was that there was so much trust in the stethoscope, because the automated monitors are a better way to take blood pressure,” lead author Beverly Green, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The results of the survey were presented Sept. 10 at the virtual joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The U.S. Preventive Services Task Force (USPSTF) and the American Heart Association/American College of Cardiology recommend out-of-office blood pressure measurements – via ambulatory blood pressure monitoring (ABPM) or home BP monitoring – before making a new diagnosis of hypertension.
To gauge provider knowledge, beliefs, and practices related to BP diagnostic tests, the researchers surveyed 282 providers: 102 medical assistants (MA), 28 licensed practical nurses (LPNs), 33 registered nurses (RNs), 86 primary care physicians, and 33 advanced practitioners (APs).
More than three-quarters of providers (79%) felt that BP measured manually with a stethoscope and ABPM were “very or highly” accurate ways to measure BP when making a new diagnosis of hypertension.
Most did not think that automated clinic BPs, home BP, or kiosk BP measurements were very or highly accurate.
Nearly all providers surveyed (96%) reported that they “always or almost always” rely on clinic BP measurements when diagnosing hypertension, but the majority of physicians/APs would prefer using ABPM (61%) if available.
The problem with ABPM, said Dr. Green, is “it’s just not very available or convenient for patients, and a lot of providers think that patients won’t tolerate it.” Yet, without it, there is a risk for misclassification, she said.
Karen A. Griffin, MD, who chairs the AHA Council on Hypertension, said it became “customary to use clinic BP since ABPM was not previously reimbursed for the routine diagnosis of hypertension.
“Now that the payment for ABPM has been expanded, the number of machines at most institutions is not adequate for the need. Consequently, it will take some time to catch up with the current guidelines for diagnosing hypertension,” she said in an interview.
The provider survey by Dr. Green and colleagues also shows slow uptake of updated thresholds for high blood pressure.
Eighty-four percent of physicians/APs and 68% of MA/LPN/RNs said they used a clinic BP threshold of at least 140/90 mm Hg for making a new diagnosis of hypertension.
Only 3.5% and 9.0%, respectively, reported using the updated threshold of at least 130/80 mm Hg put forth in 2017.
Dr. Griffin said part of this stems from the fact that the survey began before the updated guidelines were released in 2017, “not to mention the fact that some societies have opposed the new threshold of 130/80 mm Hg.”
“I think, with time, the data on morbidity and mortality associated with the goal of 130/80 mm Hg will hopefully convince those who have not yet implemented these new guidelines that it is a safe and effective BP goal,” Dr. Griffin said.
This research had no specific funding. Dr. Green and Dr. Griffin have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
COVID-19 and the psychological side effects of PPE
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at [email protected].
Children and COVID-19: New cases may be leveling off
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
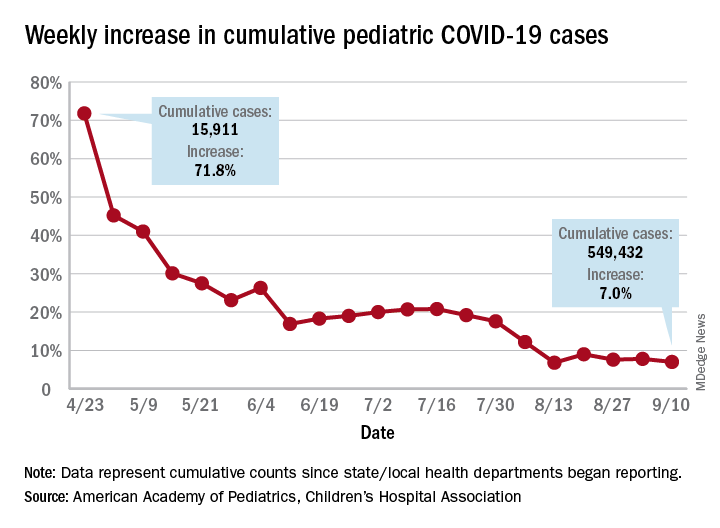
the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
Conspiracy theories
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Tough to tell COVID from smoke inhalation symptoms — And flu season’s coming
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
COVID-19 outcomes no worse in patients on TNF inhibitors or methotrexate
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Study confirms link between PAP apnea treatment and dementia onset
Obstructive sleep apnea (OSA) treatment with positive airway pressure (PAP) therapy was associated with a lower odds of incident Alzheimer’s disease and other dementia in a large retrospective cohort study of Medicare patients with the sleep disorder.
The study builds on research linking OSA to poor cognitive outcomes and dementia syndromes. With use of a 5% random sample of Medicare beneficiaries (more than 2.7 million) and their claims data, investigators identified approximately 53,000 who had an OSA diagnosis prior to 2011.
Of these Medicare beneficiaries, 78% with OSA were identified as “PAP-treated” based on having at least one durable medical equipment claim for PAP equipment. And of those treated, 74% were identified as “PAP adherent” based on having more than two PAP equipment claims separated by at least a month, said Galit Levi Dunietz, PhD, MPH, at the virtual annual meeting of the Associated Professional Sleep Societies.
Dr. Dunietz and her coinvestigators used logistic regression to examine the associations between PAP treatment and PAP treatment adherence, and incident ICD-9 diagnoses of Alzheimer’s disease (AD), mild cognitive impairment (MCI), and dementia not otherwise specified (DNOS) over the period 2011-2013.
After adjustments for potential confounders (age, sex, race, stroke, hypertension, cardiovascular disease, and depression), OSA treatment was associated with a significantly lower odds of a diagnosis of AD (odds ratio, 0.78; 95% confidence interval 0.69-0.89) or DNOS (OR, 0.69; 95% CI, 0.55-0.85), as well as nonsignificantly lower odds of MCI diagnosis (OR, 0.82; 95% CI, 0.66-1.02).
“People who are treated for OSA have a 22% reduced odds of being diagnosed with AD and a 31% reduced odds of getting DNOS,” said Dr. Dunietz, from the University of Michigan in Ann Arbor, in an interview after the meeting. “The 18% reduced odds of mild cognitive disorder is not really significant because the upper bound is 1.02, but we consider it approaching significance.”
Adherence to treatment was significantly associated with lower odds of AD, but not with significantly lower odds of DNOS or MCI, she said. OSA was confirmed by ICD-9 diagnosis codes plus the presence of relevant polysomnography current procedural terminology code.
All told, the findings “suggest that PAP therapy for OSA may lower short-term risk for dementia in older persons,” Dr. Dunietz and her co-nvestigators said in their poster presentation. “If a causal pathway exists between OSA and dementia, treatment of OSA may offer new opportunities to improve cognitive outcomes in older adults with OSA.”
Andrew W. Varga, MD, of the division of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai and the Mount Sinai Integrative Sleep Center, both in New York, said that cognitive impairment is now a recognized clinical consequence of OSA and that OSA treatment could be a target for the prevention of cognitive impairment and Alzheimer’s disease in particular.
“I absolutely bring it up with patients in their 60s and 70s. I’m honest – I say, there seems to be more and more evidence for links between apnea and Alzheimer’s in particular. I tell them we don’t know 100% whether PAP reverses any of this, but it stands to reason that it does,” said Dr. Varga, who was asked to comment on the study and related research.
An analysis published several years ago in Neurology from the Alzheimer’s Disease Neuroimaging Initiative cohort found that patients with self-reported sleep apnea had a younger age of MCI or AD onset (about 10 years) and that patients who used continuous positive airway pressure had a delayed age of onset. “Those who had a subjective diagnosis of sleep apnea and who also reported using CPAP as treatment seemed to go in the opposite direction,” said Dr. Varga, a coauthor of that study. “They had an onset of AD that looked just like people who had no sleep apnea.”
While this study was limited by sleep apnea being self-reported – and by the lack of severity data – the newly reported study may be limited by the use of ICD codes and the fact that OSA is often entered into patient’s chart before diagnosis is confirmed through a sleep study, Dr. Varga said.
“The field is mature enough that we should be thinking of doing honest and rigorous clinical trials for sleep apnea with cognitive outcomes being a main measure of interest,” he said. “The issue we’re struggling with in the field is that such a trial would not be short.”
There are several theories for the link between OSA and cognitive impairment, he said, including disruptions in sleep architecture leading to increased production of amyloid and tau and/or decreased “clearance” of extracellular amyloid, neuronal sensitivity to hypoxia, and cardiovascular comorbidities.
Dr. Dunietz’s study was supported by The American Academy of Sleep Medicine Foundation. She reported having no disclosures. Dr. Varga said he has no relevant disclosures.
Obstructive sleep apnea (OSA) treatment with positive airway pressure (PAP) therapy was associated with a lower odds of incident Alzheimer’s disease and other dementia in a large retrospective cohort study of Medicare patients with the sleep disorder.
The study builds on research linking OSA to poor cognitive outcomes and dementia syndromes. With use of a 5% random sample of Medicare beneficiaries (more than 2.7 million) and their claims data, investigators identified approximately 53,000 who had an OSA diagnosis prior to 2011.
Of these Medicare beneficiaries, 78% with OSA were identified as “PAP-treated” based on having at least one durable medical equipment claim for PAP equipment. And of those treated, 74% were identified as “PAP adherent” based on having more than two PAP equipment claims separated by at least a month, said Galit Levi Dunietz, PhD, MPH, at the virtual annual meeting of the Associated Professional Sleep Societies.
Dr. Dunietz and her coinvestigators used logistic regression to examine the associations between PAP treatment and PAP treatment adherence, and incident ICD-9 diagnoses of Alzheimer’s disease (AD), mild cognitive impairment (MCI), and dementia not otherwise specified (DNOS) over the period 2011-2013.
After adjustments for potential confounders (age, sex, race, stroke, hypertension, cardiovascular disease, and depression), OSA treatment was associated with a significantly lower odds of a diagnosis of AD (odds ratio, 0.78; 95% confidence interval 0.69-0.89) or DNOS (OR, 0.69; 95% CI, 0.55-0.85), as well as nonsignificantly lower odds of MCI diagnosis (OR, 0.82; 95% CI, 0.66-1.02).
“People who are treated for OSA have a 22% reduced odds of being diagnosed with AD and a 31% reduced odds of getting DNOS,” said Dr. Dunietz, from the University of Michigan in Ann Arbor, in an interview after the meeting. “The 18% reduced odds of mild cognitive disorder is not really significant because the upper bound is 1.02, but we consider it approaching significance.”
Adherence to treatment was significantly associated with lower odds of AD, but not with significantly lower odds of DNOS or MCI, she said. OSA was confirmed by ICD-9 diagnosis codes plus the presence of relevant polysomnography current procedural terminology code.
All told, the findings “suggest that PAP therapy for OSA may lower short-term risk for dementia in older persons,” Dr. Dunietz and her co-nvestigators said in their poster presentation. “If a causal pathway exists between OSA and dementia, treatment of OSA may offer new opportunities to improve cognitive outcomes in older adults with OSA.”
Andrew W. Varga, MD, of the division of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai and the Mount Sinai Integrative Sleep Center, both in New York, said that cognitive impairment is now a recognized clinical consequence of OSA and that OSA treatment could be a target for the prevention of cognitive impairment and Alzheimer’s disease in particular.
“I absolutely bring it up with patients in their 60s and 70s. I’m honest – I say, there seems to be more and more evidence for links between apnea and Alzheimer’s in particular. I tell them we don’t know 100% whether PAP reverses any of this, but it stands to reason that it does,” said Dr. Varga, who was asked to comment on the study and related research.
An analysis published several years ago in Neurology from the Alzheimer’s Disease Neuroimaging Initiative cohort found that patients with self-reported sleep apnea had a younger age of MCI or AD onset (about 10 years) and that patients who used continuous positive airway pressure had a delayed age of onset. “Those who had a subjective diagnosis of sleep apnea and who also reported using CPAP as treatment seemed to go in the opposite direction,” said Dr. Varga, a coauthor of that study. “They had an onset of AD that looked just like people who had no sleep apnea.”
While this study was limited by sleep apnea being self-reported – and by the lack of severity data – the newly reported study may be limited by the use of ICD codes and the fact that OSA is often entered into patient’s chart before diagnosis is confirmed through a sleep study, Dr. Varga said.
“The field is mature enough that we should be thinking of doing honest and rigorous clinical trials for sleep apnea with cognitive outcomes being a main measure of interest,” he said. “The issue we’re struggling with in the field is that such a trial would not be short.”
There are several theories for the link between OSA and cognitive impairment, he said, including disruptions in sleep architecture leading to increased production of amyloid and tau and/or decreased “clearance” of extracellular amyloid, neuronal sensitivity to hypoxia, and cardiovascular comorbidities.
Dr. Dunietz’s study was supported by The American Academy of Sleep Medicine Foundation. She reported having no disclosures. Dr. Varga said he has no relevant disclosures.
Obstructive sleep apnea (OSA) treatment with positive airway pressure (PAP) therapy was associated with a lower odds of incident Alzheimer’s disease and other dementia in a large retrospective cohort study of Medicare patients with the sleep disorder.
The study builds on research linking OSA to poor cognitive outcomes and dementia syndromes. With use of a 5% random sample of Medicare beneficiaries (more than 2.7 million) and their claims data, investigators identified approximately 53,000 who had an OSA diagnosis prior to 2011.
Of these Medicare beneficiaries, 78% with OSA were identified as “PAP-treated” based on having at least one durable medical equipment claim for PAP equipment. And of those treated, 74% were identified as “PAP adherent” based on having more than two PAP equipment claims separated by at least a month, said Galit Levi Dunietz, PhD, MPH, at the virtual annual meeting of the Associated Professional Sleep Societies.
Dr. Dunietz and her coinvestigators used logistic regression to examine the associations between PAP treatment and PAP treatment adherence, and incident ICD-9 diagnoses of Alzheimer’s disease (AD), mild cognitive impairment (MCI), and dementia not otherwise specified (DNOS) over the period 2011-2013.
After adjustments for potential confounders (age, sex, race, stroke, hypertension, cardiovascular disease, and depression), OSA treatment was associated with a significantly lower odds of a diagnosis of AD (odds ratio, 0.78; 95% confidence interval 0.69-0.89) or DNOS (OR, 0.69; 95% CI, 0.55-0.85), as well as nonsignificantly lower odds of MCI diagnosis (OR, 0.82; 95% CI, 0.66-1.02).
“People who are treated for OSA have a 22% reduced odds of being diagnosed with AD and a 31% reduced odds of getting DNOS,” said Dr. Dunietz, from the University of Michigan in Ann Arbor, in an interview after the meeting. “The 18% reduced odds of mild cognitive disorder is not really significant because the upper bound is 1.02, but we consider it approaching significance.”
Adherence to treatment was significantly associated with lower odds of AD, but not with significantly lower odds of DNOS or MCI, she said. OSA was confirmed by ICD-9 diagnosis codes plus the presence of relevant polysomnography current procedural terminology code.
All told, the findings “suggest that PAP therapy for OSA may lower short-term risk for dementia in older persons,” Dr. Dunietz and her co-nvestigators said in their poster presentation. “If a causal pathway exists between OSA and dementia, treatment of OSA may offer new opportunities to improve cognitive outcomes in older adults with OSA.”
Andrew W. Varga, MD, of the division of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai and the Mount Sinai Integrative Sleep Center, both in New York, said that cognitive impairment is now a recognized clinical consequence of OSA and that OSA treatment could be a target for the prevention of cognitive impairment and Alzheimer’s disease in particular.
“I absolutely bring it up with patients in their 60s and 70s. I’m honest – I say, there seems to be more and more evidence for links between apnea and Alzheimer’s in particular. I tell them we don’t know 100% whether PAP reverses any of this, but it stands to reason that it does,” said Dr. Varga, who was asked to comment on the study and related research.
An analysis published several years ago in Neurology from the Alzheimer’s Disease Neuroimaging Initiative cohort found that patients with self-reported sleep apnea had a younger age of MCI or AD onset (about 10 years) and that patients who used continuous positive airway pressure had a delayed age of onset. “Those who had a subjective diagnosis of sleep apnea and who also reported using CPAP as treatment seemed to go in the opposite direction,” said Dr. Varga, a coauthor of that study. “They had an onset of AD that looked just like people who had no sleep apnea.”
While this study was limited by sleep apnea being self-reported – and by the lack of severity data – the newly reported study may be limited by the use of ICD codes and the fact that OSA is often entered into patient’s chart before diagnosis is confirmed through a sleep study, Dr. Varga said.
“The field is mature enough that we should be thinking of doing honest and rigorous clinical trials for sleep apnea with cognitive outcomes being a main measure of interest,” he said. “The issue we’re struggling with in the field is that such a trial would not be short.”
There are several theories for the link between OSA and cognitive impairment, he said, including disruptions in sleep architecture leading to increased production of amyloid and tau and/or decreased “clearance” of extracellular amyloid, neuronal sensitivity to hypoxia, and cardiovascular comorbidities.
Dr. Dunietz’s study was supported by The American Academy of Sleep Medicine Foundation. She reported having no disclosures. Dr. Varga said he has no relevant disclosures.
FROM SLEEP 2020
Novel calculator predicts cancer risk in patients with CVD
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
FROM ESC CONGRESS 2020
Physician income drops, burnout spikes globally in pandemic
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.