User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


No increase seen in children’s cumulative COVID-19 burden
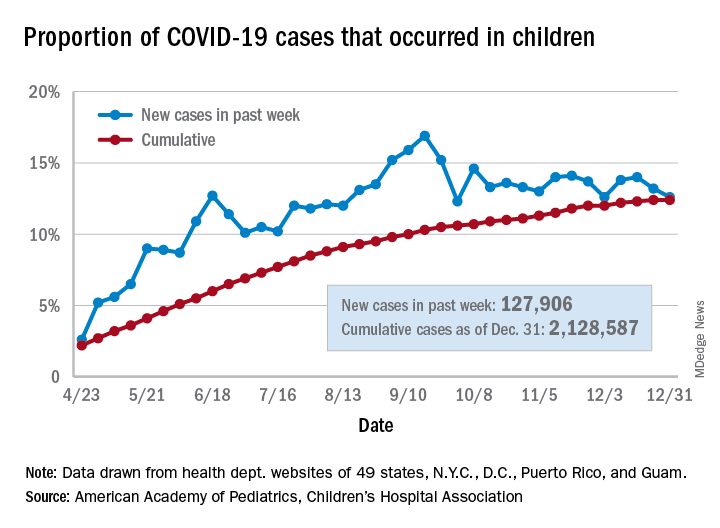
Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.

Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.

Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.
Annual WCC visits significantly limit asthma worsening
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
FROM PEDIATRICS
Microvascular injury of brain, olfactory bulb seen in COVID-19
new research suggests.
Postmortem MRI brain scans of 13 patients who died from COVID-19 showed abnormalities in 10 of the participants. Of these, nine showed punctate hyperintensities, “which represented areas of microvascular injury and fibrinogen leakage,” the investigators reported. Immunostaining also showed a thinning of the basal lamina in five of these patients.
Further analyses showed punctate hypointensities linked to congested blood vessels in 10 patients. These areas were “interpreted as microhemorrhages,” the researchers noted.
There was no evidence of viral infection, including SARS-CoV-2.
“These findings may inform the interpretation of changes observed on [MRI] of punctate hyperintensities and linear hypointensities in patients with COVID-19,” wrote Myoung-Hwa Lee, PhD, a research fellow at the National Institute of Neurological Disorders and Stroke, and colleagues. The findings were published online Dec. 30 in a “correspondence” piece in the New England Journal of Medicine.
Interpret with caution
The investigators examined brains from a convenience sample of 19 patients (mean age, 50 years), all of whom died from COVID-19 between March and July 2020.
An 11.7-tesla scanner was used to obtain magnetic resonance microscopy images for 13 of the patients. In order to scan the olfactory bulb, the scanner was set at a resolution of 25 mcm; for the brain, it was set at 100 mcm.
Chromogenic immunostaining was used to assess brain abnormalities found in 10 of the patients. Multiplex fluorescence imaging was also used for some of the patients.
For 18 study participants, a histopathological brain examination was performed. In the patients who also had medical histories available to the researchers, five had mild respiratory syndrome, four had acute respiratory distress syndrome, two had pulmonary embolism, one had delirium, and three had unknown symptoms.
The punctate hyperintensities found on magnetic resonance microscopy were also found on histopathological exam. Collagen IV immunostaining showed a thinning in the basal lamina of endothelial cells in these areas.
In addition to congested blood vessels, punctate hypointensities were linked to areas of fibrinogen leakage – but also to “relatively intact vasculature,” the investigators reported.
“There was minimal perivascular inflammation in the specimens examined, but there was no vascular occlusion,” they added.
SARS-CoV-2 was also not found in any of the participants. “It is possible that the virus was cleared by the time of death or that viral copy numbers were below the level of detection by our assays,” the researchers noted.
In 13 of the patients, hypertrophic astrocytes, macrophage infiltrates, and perivascular-activated microglia were found. Eight patients showed CD3+ and CD8+ T cells in spaces and lumens next to endothelial cells.
Finally, five patients showed activated microglia next to neurons. This is “suggestive of neuronophagia in the olfactory bulb, substantial nigra, dorsal motor nucleus of the vagal nerve, and the pre-Bötzinger complex in the medulla, which is involved in the generation of spontaneous rhythmic breathing,” wrote the investigators.
In summary, vascular pathology was found in 10 cases, perivascular infiltrates were present in 13 cases, acute ischemic hypoxic neurons were present in 6 cases, and changes suggestive of neuronophagia were present in 5 cases.
The researchers noted that, although the study findings may be helpful when interpreting brain changes on MRI scan in this patient population, availability of clinical information for the participants was limited.
Therefore, “no conclusions can be drawn in relation to neurologic features of COVID-19,” they wrote.
The study was funded by NINDS. Dr. Lee and all but one of the other investigators reported no relevant financial relationships; the remaining investigator reported having received grants from NINDS during the conduct of this study.
A version of this article first appeared on Medscape.com.
new research suggests.
Postmortem MRI brain scans of 13 patients who died from COVID-19 showed abnormalities in 10 of the participants. Of these, nine showed punctate hyperintensities, “which represented areas of microvascular injury and fibrinogen leakage,” the investigators reported. Immunostaining also showed a thinning of the basal lamina in five of these patients.
Further analyses showed punctate hypointensities linked to congested blood vessels in 10 patients. These areas were “interpreted as microhemorrhages,” the researchers noted.
There was no evidence of viral infection, including SARS-CoV-2.
“These findings may inform the interpretation of changes observed on [MRI] of punctate hyperintensities and linear hypointensities in patients with COVID-19,” wrote Myoung-Hwa Lee, PhD, a research fellow at the National Institute of Neurological Disorders and Stroke, and colleagues. The findings were published online Dec. 30 in a “correspondence” piece in the New England Journal of Medicine.
Interpret with caution
The investigators examined brains from a convenience sample of 19 patients (mean age, 50 years), all of whom died from COVID-19 between March and July 2020.
An 11.7-tesla scanner was used to obtain magnetic resonance microscopy images for 13 of the patients. In order to scan the olfactory bulb, the scanner was set at a resolution of 25 mcm; for the brain, it was set at 100 mcm.
Chromogenic immunostaining was used to assess brain abnormalities found in 10 of the patients. Multiplex fluorescence imaging was also used for some of the patients.
For 18 study participants, a histopathological brain examination was performed. In the patients who also had medical histories available to the researchers, five had mild respiratory syndrome, four had acute respiratory distress syndrome, two had pulmonary embolism, one had delirium, and three had unknown symptoms.
The punctate hyperintensities found on magnetic resonance microscopy were also found on histopathological exam. Collagen IV immunostaining showed a thinning in the basal lamina of endothelial cells in these areas.
In addition to congested blood vessels, punctate hypointensities were linked to areas of fibrinogen leakage – but also to “relatively intact vasculature,” the investigators reported.
“There was minimal perivascular inflammation in the specimens examined, but there was no vascular occlusion,” they added.
SARS-CoV-2 was also not found in any of the participants. “It is possible that the virus was cleared by the time of death or that viral copy numbers were below the level of detection by our assays,” the researchers noted.
In 13 of the patients, hypertrophic astrocytes, macrophage infiltrates, and perivascular-activated microglia were found. Eight patients showed CD3+ and CD8+ T cells in spaces and lumens next to endothelial cells.
Finally, five patients showed activated microglia next to neurons. This is “suggestive of neuronophagia in the olfactory bulb, substantial nigra, dorsal motor nucleus of the vagal nerve, and the pre-Bötzinger complex in the medulla, which is involved in the generation of spontaneous rhythmic breathing,” wrote the investigators.
In summary, vascular pathology was found in 10 cases, perivascular infiltrates were present in 13 cases, acute ischemic hypoxic neurons were present in 6 cases, and changes suggestive of neuronophagia were present in 5 cases.
The researchers noted that, although the study findings may be helpful when interpreting brain changes on MRI scan in this patient population, availability of clinical information for the participants was limited.
Therefore, “no conclusions can be drawn in relation to neurologic features of COVID-19,” they wrote.
The study was funded by NINDS. Dr. Lee and all but one of the other investigators reported no relevant financial relationships; the remaining investigator reported having received grants from NINDS during the conduct of this study.
A version of this article first appeared on Medscape.com.
new research suggests.
Postmortem MRI brain scans of 13 patients who died from COVID-19 showed abnormalities in 10 of the participants. Of these, nine showed punctate hyperintensities, “which represented areas of microvascular injury and fibrinogen leakage,” the investigators reported. Immunostaining also showed a thinning of the basal lamina in five of these patients.
Further analyses showed punctate hypointensities linked to congested blood vessels in 10 patients. These areas were “interpreted as microhemorrhages,” the researchers noted.
There was no evidence of viral infection, including SARS-CoV-2.
“These findings may inform the interpretation of changes observed on [MRI] of punctate hyperintensities and linear hypointensities in patients with COVID-19,” wrote Myoung-Hwa Lee, PhD, a research fellow at the National Institute of Neurological Disorders and Stroke, and colleagues. The findings were published online Dec. 30 in a “correspondence” piece in the New England Journal of Medicine.
Interpret with caution
The investigators examined brains from a convenience sample of 19 patients (mean age, 50 years), all of whom died from COVID-19 between March and July 2020.
An 11.7-tesla scanner was used to obtain magnetic resonance microscopy images for 13 of the patients. In order to scan the olfactory bulb, the scanner was set at a resolution of 25 mcm; for the brain, it was set at 100 mcm.
Chromogenic immunostaining was used to assess brain abnormalities found in 10 of the patients. Multiplex fluorescence imaging was also used for some of the patients.
For 18 study participants, a histopathological brain examination was performed. In the patients who also had medical histories available to the researchers, five had mild respiratory syndrome, four had acute respiratory distress syndrome, two had pulmonary embolism, one had delirium, and three had unknown symptoms.
The punctate hyperintensities found on magnetic resonance microscopy were also found on histopathological exam. Collagen IV immunostaining showed a thinning in the basal lamina of endothelial cells in these areas.
In addition to congested blood vessels, punctate hypointensities were linked to areas of fibrinogen leakage – but also to “relatively intact vasculature,” the investigators reported.
“There was minimal perivascular inflammation in the specimens examined, but there was no vascular occlusion,” they added.
SARS-CoV-2 was also not found in any of the participants. “It is possible that the virus was cleared by the time of death or that viral copy numbers were below the level of detection by our assays,” the researchers noted.
In 13 of the patients, hypertrophic astrocytes, macrophage infiltrates, and perivascular-activated microglia were found. Eight patients showed CD3+ and CD8+ T cells in spaces and lumens next to endothelial cells.
Finally, five patients showed activated microglia next to neurons. This is “suggestive of neuronophagia in the olfactory bulb, substantial nigra, dorsal motor nucleus of the vagal nerve, and the pre-Bötzinger complex in the medulla, which is involved in the generation of spontaneous rhythmic breathing,” wrote the investigators.
In summary, vascular pathology was found in 10 cases, perivascular infiltrates were present in 13 cases, acute ischemic hypoxic neurons were present in 6 cases, and changes suggestive of neuronophagia were present in 5 cases.
The researchers noted that, although the study findings may be helpful when interpreting brain changes on MRI scan in this patient population, availability of clinical information for the participants was limited.
Therefore, “no conclusions can be drawn in relation to neurologic features of COVID-19,” they wrote.
The study was funded by NINDS. Dr. Lee and all but one of the other investigators reported no relevant financial relationships; the remaining investigator reported having received grants from NINDS during the conduct of this study.
A version of this article first appeared on Medscape.com.
Experts debate wisdom of delaying second COVID-19 vaccine dose
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New evidence shows that COVID-19 invades the brain
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
U.S. hits 20 million cases as COVID variant spreads
The United States started 2021 they way it ended 2020: Setting new records amidst the coronavirus pandemic.
The country passed the 20 million mark for coronavirus cases on Friday, setting the mark sometime around noon, according to Johns Hopkins University’s COVID-19 tracker. The total is nearly twice as many as the next worst country – India, which has 10.28 million cases.
Along with the case count, more than 346,000 Americans have now died of COVID-19, the disease caused by the coronavirus. That is 77% more fatalities than Brazil, which ranks second globally with 194,949 deaths.
More than 125,370 coronavirus patients were hospitalized on Thursday, the fourth record-setting day in a row, according to the COVID Tracking Project.
Going by official tallies, it took 292 days for the United States to reach its first 10 million cases, and just 54 more days to double it, CNN reported.
Meanwhile, 12.41 million doses of COVID-19 vaccines have been distributed in the United States as of Wednesday, according to the Centers for Disease Control and Prevention. Yet only 2.8 million people have received the first of a two-shot regimen.
The slower-than-hoped-for rollout of the Pfizer and Moderna vaccines comes as a new variant of the coronavirus has emerged in a third state. Florida officials announced a confirmed case of the new variant – believed to have originated in the United Kingdom – in Martin County in southeast Florida.
The state health department said on Twitter that the patient is a man in his 20s with no history of travel. The department said it is working with the CDC to investigate.
The variant has also been confirmed in cases in Colorado and California. It is believed to be more contagious. The BBC reported that the new variant increases the reproduction, or “R number,” by 0.4 and 0.7. The UK’s most recent R number has been estimated at 1.1-1.3, meaning anyone who has the coronavirus could be assumed to spread it to up to 1.3 people.
The R number needs to be below 1.0 for the spread of the virus to fall.
“There is a huge difference in how easily the variant virus spreads,” Professor Axel Gandy of London’s Imperial College told BBC News. “This is the most serious change in the virus since the epidemic began.”
A version of this article first appeared on WebMD.com.
The United States started 2021 they way it ended 2020: Setting new records amidst the coronavirus pandemic.
The country passed the 20 million mark for coronavirus cases on Friday, setting the mark sometime around noon, according to Johns Hopkins University’s COVID-19 tracker. The total is nearly twice as many as the next worst country – India, which has 10.28 million cases.
Along with the case count, more than 346,000 Americans have now died of COVID-19, the disease caused by the coronavirus. That is 77% more fatalities than Brazil, which ranks second globally with 194,949 deaths.
More than 125,370 coronavirus patients were hospitalized on Thursday, the fourth record-setting day in a row, according to the COVID Tracking Project.
Going by official tallies, it took 292 days for the United States to reach its first 10 million cases, and just 54 more days to double it, CNN reported.
Meanwhile, 12.41 million doses of COVID-19 vaccines have been distributed in the United States as of Wednesday, according to the Centers for Disease Control and Prevention. Yet only 2.8 million people have received the first of a two-shot regimen.
The slower-than-hoped-for rollout of the Pfizer and Moderna vaccines comes as a new variant of the coronavirus has emerged in a third state. Florida officials announced a confirmed case of the new variant – believed to have originated in the United Kingdom – in Martin County in southeast Florida.
The state health department said on Twitter that the patient is a man in his 20s with no history of travel. The department said it is working with the CDC to investigate.
The variant has also been confirmed in cases in Colorado and California. It is believed to be more contagious. The BBC reported that the new variant increases the reproduction, or “R number,” by 0.4 and 0.7. The UK’s most recent R number has been estimated at 1.1-1.3, meaning anyone who has the coronavirus could be assumed to spread it to up to 1.3 people.
The R number needs to be below 1.0 for the spread of the virus to fall.
“There is a huge difference in how easily the variant virus spreads,” Professor Axel Gandy of London’s Imperial College told BBC News. “This is the most serious change in the virus since the epidemic began.”
A version of this article first appeared on WebMD.com.
The United States started 2021 they way it ended 2020: Setting new records amidst the coronavirus pandemic.
The country passed the 20 million mark for coronavirus cases on Friday, setting the mark sometime around noon, according to Johns Hopkins University’s COVID-19 tracker. The total is nearly twice as many as the next worst country – India, which has 10.28 million cases.
Along with the case count, more than 346,000 Americans have now died of COVID-19, the disease caused by the coronavirus. That is 77% more fatalities than Brazil, which ranks second globally with 194,949 deaths.
More than 125,370 coronavirus patients were hospitalized on Thursday, the fourth record-setting day in a row, according to the COVID Tracking Project.
Going by official tallies, it took 292 days for the United States to reach its first 10 million cases, and just 54 more days to double it, CNN reported.
Meanwhile, 12.41 million doses of COVID-19 vaccines have been distributed in the United States as of Wednesday, according to the Centers for Disease Control and Prevention. Yet only 2.8 million people have received the first of a two-shot regimen.
The slower-than-hoped-for rollout of the Pfizer and Moderna vaccines comes as a new variant of the coronavirus has emerged in a third state. Florida officials announced a confirmed case of the new variant – believed to have originated in the United Kingdom – in Martin County in southeast Florida.
The state health department said on Twitter that the patient is a man in his 20s with no history of travel. The department said it is working with the CDC to investigate.
The variant has also been confirmed in cases in Colorado and California. It is believed to be more contagious. The BBC reported that the new variant increases the reproduction, or “R number,” by 0.4 and 0.7. The UK’s most recent R number has been estimated at 1.1-1.3, meaning anyone who has the coronavirus could be assumed to spread it to up to 1.3 people.
The R number needs to be below 1.0 for the spread of the virus to fall.
“There is a huge difference in how easily the variant virus spreads,” Professor Axel Gandy of London’s Imperial College told BBC News. “This is the most serious change in the virus since the epidemic began.”
A version of this article first appeared on WebMD.com.
Medicaid to cover routine costs for patients in trials
A boost for patients with cancer and other serious illnesses.
Congress has ordered the holdouts among U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
A massive wrap-up spending/COVID-19 relief bill that was signed into law Dec. 27 carried with it a mandate on Medicaid. States are ordered to put in place Medicaid payment policies for routine items and services, such as the cost of physician visits or laboratory tests, that are provided in connection with participation in clinical trials for serious and life-threatening conditions. The law includes a January 2022 target date for this coverage through Medicaid.
Medicare and other large insurers already pick up the tab for these kinds of expenses, leaving Medicaid as an outlier, ASCO noted in a press statement. ASCO and other cancer groups have for years pressed Medicaid to cover routine expenses for people participating in clinical trials. Already, 15 states, including California, require their Medicaid programs to cover these expenses, according to ASCO.
“We believe that the trials can bring extra benefits to patients,” said Monica M. Bertagnolli, MD, of Dana-Farber Cancer Institute, Boston. Dr. Bertagnolli has worked for years to secure Medicaid coverage for expenses connected to clinical trials.
Although Medicaid covers costs of standard care for cancer patients, people enrolled in the program may have concerns about participating in clinical studies, said Dr. Bertagnolli, chair of the Association for Clinical Oncology, which was established by ASCO to promote wider access to cancer care. Having extra medical expenses may be more than these patients can tolerate.
“Many of them just say, ‘I can’t take that financial risk, so I’ll just stay with standard of care,’ “ Dr. Bertagnolli said in an interview.
Equity issues
Medicaid has expanded greatly, owing to financial aid provided to states through the Affordable Care Act of 2010.
To date, 38 of 50 U.S. states have accepted federal aid to lift income limits for Medicaid eligibility, according to a tally kept by the nonprofit Kaiser Family Foundation. This Medicaid expansion has given more of the nation’s working poor access to health.care, including cancer treatment. Between 2013 and January 2020, enrollment in Medicaid in expansion states increased by about 12.4 million, according to the Medicaid and CHIP Payment and Access Commission.
Medicaid is the nation’s dominant health insurer. Enrollment has been around 70 million in recent months.
That tops the 61 million enrolled in Medicare, the federal program for people aged 65 and older and those with disabilities. (There’s some overlap between Medicare and Medicaid. About 12.8 million persons were dually eligible for these programs in 2018.) UnitedHealth, a giant private insurer, has about 43 million domestic customers.
Medicaid also serves many of the groups of people for which researchers have been seeking to increase participation in clinical trials. ASCO’s Association for Clinical Oncology and dozens of its partners raised this point in a letter to congressional leaders on Feb. 15, 2020.
“Lack of participation in clinical trials from the Medicaid population means these patients are being excluded from potentially life-saving trials and are not reflected in the outcome of the clinical research,” the groups wrote. “Increased access to clinical trial participation for Medicaid enrollees helps ensure medical research results more accurately capture and reflect the populations of this country.”
The ACA’s Medicaid expansion is working to address some of the racial gaps in insurance coverage, according to a January 2020 report from the nonprofit Commonwealth Fund.
Black and Hispanic adults are almost twice as likely as are White adults to have incomes that are less than 200% of the federal poverty level, according to the Commonwealth Fund report. The report also said that people in these groups reported significantly higher rates of cost-related problems in receiving care before the Medicaid expansion began in 2014.
The uninsured rate for Black adults dropped from 24.4% in 2013 to 14.4% in 2018; the rate for Hispanic adults fell from 40.2% to 24.9%, according to the Commonwealth Fund report.
There are concerns, though, about attempts by some governors to impose onerous restrictions on adults enrolled in Medicaid, Dr. Bertagnolli said. She was president of ASCO in 2018 when the group called on the Centers for Medicare & Medicaid Services to reject state requests to create restrictions that could hinder people’s access to cancer screening or care.
The Trump administration encouraged governors to adopt work requirements. As a result, a dozen states approved these policies, according to a November report from the nonprofit Center on Budget and Policy Priorities. The efforts were blocked by courts.
Data from the limited period of implementation in Arkansas, Michigan, and New Hampshire provide evidence that these kinds of requirements don’t work as intended, according to the CBPP report.
“In all three states, evidence suggests that people who were working and people with serious health needs who should have been eligible for exemptions lost coverage or were at risk of losing coverage due to red tape,” CBPP analysts Jennifer Wagner and Jessica Schubel wrote in their report.
In 2019, The New England Journal of Medicine published an article about the early stages of the Arkansas experiment with Medicaid work rules. Almost 17,000 adults lost their health care coverage in the initial months of implementation, but there appeared to be no significant difference in employment, Benjamin Sommers, MD, PhD, of the Harvard School of Public Health, Boston, and colleagues wrote in their article.
For many people in Arkansas, coverage was lost because of difficulties in reporting compliance with the Medicaid work rule, not because of the employment mandate itself, according to the authors. More than 95% of persons who were targeted by Arkansas’ Medicaid work policy already met its requirements or should have been exempt, they wrote.
Democrats have tended to oppose efforts to attach work requirements, which can include volunteer activities or career training, to Medicaid. Dr. Bertagnolli said there is a need to guard against any future bid to add work requirements to the program.
Extra bureaucratic hurdles may pose an especially tough burden on working adults enrolled in Medicaid, she said.
People who qualify for the program may already be worried about their finances while juggling continued demands of child care and employment, she said. They don’t need to be put at risk of losing access to medical care over administrative rules while undergoing cancer treatment, she said.
“We have to take care of people who are sick. That’s just the way it is,” Dr. Bertagnolli said.
A version of this article first appeared on Medscape.com.
A boost for patients with cancer and other serious illnesses.
A boost for patients with cancer and other serious illnesses.
Congress has ordered the holdouts among U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
A massive wrap-up spending/COVID-19 relief bill that was signed into law Dec. 27 carried with it a mandate on Medicaid. States are ordered to put in place Medicaid payment policies for routine items and services, such as the cost of physician visits or laboratory tests, that are provided in connection with participation in clinical trials for serious and life-threatening conditions. The law includes a January 2022 target date for this coverage through Medicaid.
Medicare and other large insurers already pick up the tab for these kinds of expenses, leaving Medicaid as an outlier, ASCO noted in a press statement. ASCO and other cancer groups have for years pressed Medicaid to cover routine expenses for people participating in clinical trials. Already, 15 states, including California, require their Medicaid programs to cover these expenses, according to ASCO.
“We believe that the trials can bring extra benefits to patients,” said Monica M. Bertagnolli, MD, of Dana-Farber Cancer Institute, Boston. Dr. Bertagnolli has worked for years to secure Medicaid coverage for expenses connected to clinical trials.
Although Medicaid covers costs of standard care for cancer patients, people enrolled in the program may have concerns about participating in clinical studies, said Dr. Bertagnolli, chair of the Association for Clinical Oncology, which was established by ASCO to promote wider access to cancer care. Having extra medical expenses may be more than these patients can tolerate.
“Many of them just say, ‘I can’t take that financial risk, so I’ll just stay with standard of care,’ “ Dr. Bertagnolli said in an interview.
Equity issues
Medicaid has expanded greatly, owing to financial aid provided to states through the Affordable Care Act of 2010.
To date, 38 of 50 U.S. states have accepted federal aid to lift income limits for Medicaid eligibility, according to a tally kept by the nonprofit Kaiser Family Foundation. This Medicaid expansion has given more of the nation’s working poor access to health.care, including cancer treatment. Between 2013 and January 2020, enrollment in Medicaid in expansion states increased by about 12.4 million, according to the Medicaid and CHIP Payment and Access Commission.
Medicaid is the nation’s dominant health insurer. Enrollment has been around 70 million in recent months.
That tops the 61 million enrolled in Medicare, the federal program for people aged 65 and older and those with disabilities. (There’s some overlap between Medicare and Medicaid. About 12.8 million persons were dually eligible for these programs in 2018.) UnitedHealth, a giant private insurer, has about 43 million domestic customers.
Medicaid also serves many of the groups of people for which researchers have been seeking to increase participation in clinical trials. ASCO’s Association for Clinical Oncology and dozens of its partners raised this point in a letter to congressional leaders on Feb. 15, 2020.
“Lack of participation in clinical trials from the Medicaid population means these patients are being excluded from potentially life-saving trials and are not reflected in the outcome of the clinical research,” the groups wrote. “Increased access to clinical trial participation for Medicaid enrollees helps ensure medical research results more accurately capture and reflect the populations of this country.”
The ACA’s Medicaid expansion is working to address some of the racial gaps in insurance coverage, according to a January 2020 report from the nonprofit Commonwealth Fund.
Black and Hispanic adults are almost twice as likely as are White adults to have incomes that are less than 200% of the federal poverty level, according to the Commonwealth Fund report. The report also said that people in these groups reported significantly higher rates of cost-related problems in receiving care before the Medicaid expansion began in 2014.
The uninsured rate for Black adults dropped from 24.4% in 2013 to 14.4% in 2018; the rate for Hispanic adults fell from 40.2% to 24.9%, according to the Commonwealth Fund report.
There are concerns, though, about attempts by some governors to impose onerous restrictions on adults enrolled in Medicaid, Dr. Bertagnolli said. She was president of ASCO in 2018 when the group called on the Centers for Medicare & Medicaid Services to reject state requests to create restrictions that could hinder people’s access to cancer screening or care.
The Trump administration encouraged governors to adopt work requirements. As a result, a dozen states approved these policies, according to a November report from the nonprofit Center on Budget and Policy Priorities. The efforts were blocked by courts.
Data from the limited period of implementation in Arkansas, Michigan, and New Hampshire provide evidence that these kinds of requirements don’t work as intended, according to the CBPP report.
“In all three states, evidence suggests that people who were working and people with serious health needs who should have been eligible for exemptions lost coverage or were at risk of losing coverage due to red tape,” CBPP analysts Jennifer Wagner and Jessica Schubel wrote in their report.
In 2019, The New England Journal of Medicine published an article about the early stages of the Arkansas experiment with Medicaid work rules. Almost 17,000 adults lost their health care coverage in the initial months of implementation, but there appeared to be no significant difference in employment, Benjamin Sommers, MD, PhD, of the Harvard School of Public Health, Boston, and colleagues wrote in their article.
For many people in Arkansas, coverage was lost because of difficulties in reporting compliance with the Medicaid work rule, not because of the employment mandate itself, according to the authors. More than 95% of persons who were targeted by Arkansas’ Medicaid work policy already met its requirements or should have been exempt, they wrote.
Democrats have tended to oppose efforts to attach work requirements, which can include volunteer activities or career training, to Medicaid. Dr. Bertagnolli said there is a need to guard against any future bid to add work requirements to the program.
Extra bureaucratic hurdles may pose an especially tough burden on working adults enrolled in Medicaid, she said.
People who qualify for the program may already be worried about their finances while juggling continued demands of child care and employment, she said. They don’t need to be put at risk of losing access to medical care over administrative rules while undergoing cancer treatment, she said.
“We have to take care of people who are sick. That’s just the way it is,” Dr. Bertagnolli said.
A version of this article first appeared on Medscape.com.
Congress has ordered the holdouts among U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
A massive wrap-up spending/COVID-19 relief bill that was signed into law Dec. 27 carried with it a mandate on Medicaid. States are ordered to put in place Medicaid payment policies for routine items and services, such as the cost of physician visits or laboratory tests, that are provided in connection with participation in clinical trials for serious and life-threatening conditions. The law includes a January 2022 target date for this coverage through Medicaid.
Medicare and other large insurers already pick up the tab for these kinds of expenses, leaving Medicaid as an outlier, ASCO noted in a press statement. ASCO and other cancer groups have for years pressed Medicaid to cover routine expenses for people participating in clinical trials. Already, 15 states, including California, require their Medicaid programs to cover these expenses, according to ASCO.
“We believe that the trials can bring extra benefits to patients,” said Monica M. Bertagnolli, MD, of Dana-Farber Cancer Institute, Boston. Dr. Bertagnolli has worked for years to secure Medicaid coverage for expenses connected to clinical trials.
Although Medicaid covers costs of standard care for cancer patients, people enrolled in the program may have concerns about participating in clinical studies, said Dr. Bertagnolli, chair of the Association for Clinical Oncology, which was established by ASCO to promote wider access to cancer care. Having extra medical expenses may be more than these patients can tolerate.
“Many of them just say, ‘I can’t take that financial risk, so I’ll just stay with standard of care,’ “ Dr. Bertagnolli said in an interview.
Equity issues
Medicaid has expanded greatly, owing to financial aid provided to states through the Affordable Care Act of 2010.
To date, 38 of 50 U.S. states have accepted federal aid to lift income limits for Medicaid eligibility, according to a tally kept by the nonprofit Kaiser Family Foundation. This Medicaid expansion has given more of the nation’s working poor access to health.care, including cancer treatment. Between 2013 and January 2020, enrollment in Medicaid in expansion states increased by about 12.4 million, according to the Medicaid and CHIP Payment and Access Commission.
Medicaid is the nation’s dominant health insurer. Enrollment has been around 70 million in recent months.
That tops the 61 million enrolled in Medicare, the federal program for people aged 65 and older and those with disabilities. (There’s some overlap between Medicare and Medicaid. About 12.8 million persons were dually eligible for these programs in 2018.) UnitedHealth, a giant private insurer, has about 43 million domestic customers.
Medicaid also serves many of the groups of people for which researchers have been seeking to increase participation in clinical trials. ASCO’s Association for Clinical Oncology and dozens of its partners raised this point in a letter to congressional leaders on Feb. 15, 2020.
“Lack of participation in clinical trials from the Medicaid population means these patients are being excluded from potentially life-saving trials and are not reflected in the outcome of the clinical research,” the groups wrote. “Increased access to clinical trial participation for Medicaid enrollees helps ensure medical research results more accurately capture and reflect the populations of this country.”
The ACA’s Medicaid expansion is working to address some of the racial gaps in insurance coverage, according to a January 2020 report from the nonprofit Commonwealth Fund.
Black and Hispanic adults are almost twice as likely as are White adults to have incomes that are less than 200% of the federal poverty level, according to the Commonwealth Fund report. The report also said that people in these groups reported significantly higher rates of cost-related problems in receiving care before the Medicaid expansion began in 2014.
The uninsured rate for Black adults dropped from 24.4% in 2013 to 14.4% in 2018; the rate for Hispanic adults fell from 40.2% to 24.9%, according to the Commonwealth Fund report.
There are concerns, though, about attempts by some governors to impose onerous restrictions on adults enrolled in Medicaid, Dr. Bertagnolli said. She was president of ASCO in 2018 when the group called on the Centers for Medicare & Medicaid Services to reject state requests to create restrictions that could hinder people’s access to cancer screening or care.
The Trump administration encouraged governors to adopt work requirements. As a result, a dozen states approved these policies, according to a November report from the nonprofit Center on Budget and Policy Priorities. The efforts were blocked by courts.
Data from the limited period of implementation in Arkansas, Michigan, and New Hampshire provide evidence that these kinds of requirements don’t work as intended, according to the CBPP report.
“In all three states, evidence suggests that people who were working and people with serious health needs who should have been eligible for exemptions lost coverage or were at risk of losing coverage due to red tape,” CBPP analysts Jennifer Wagner and Jessica Schubel wrote in their report.
In 2019, The New England Journal of Medicine published an article about the early stages of the Arkansas experiment with Medicaid work rules. Almost 17,000 adults lost their health care coverage in the initial months of implementation, but there appeared to be no significant difference in employment, Benjamin Sommers, MD, PhD, of the Harvard School of Public Health, Boston, and colleagues wrote in their article.
For many people in Arkansas, coverage was lost because of difficulties in reporting compliance with the Medicaid work rule, not because of the employment mandate itself, according to the authors. More than 95% of persons who were targeted by Arkansas’ Medicaid work policy already met its requirements or should have been exempt, they wrote.
Democrats have tended to oppose efforts to attach work requirements, which can include volunteer activities or career training, to Medicaid. Dr. Bertagnolli said there is a need to guard against any future bid to add work requirements to the program.
Extra bureaucratic hurdles may pose an especially tough burden on working adults enrolled in Medicaid, she said.
People who qualify for the program may already be worried about their finances while juggling continued demands of child care and employment, she said. They don’t need to be put at risk of losing access to medical care over administrative rules while undergoing cancer treatment, she said.
“We have to take care of people who are sick. That’s just the way it is,” Dr. Bertagnolli said.
A version of this article first appeared on Medscape.com.
COVID-19 mortality in hospitalized HF patients: Nearly 1 in 4
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NETs a possible therapeutic target for COVID-19 thrombosis?
Researchers in Madrid may have found a clue to the pathogenesis of ST-segment elevation myocardial infarction (STEMI) in patients with COVID-19; it might also offer a therapeutic target to counter the hypercoagulability seen with COVID-19.
In a case series of five patients with COVID-19 who had an STEMI, neutrophil extracellular traps (NETs) were detected in coronary thrombi of all five patients. The median density was 66%, which is significantly higher than that seen in a historical series of patients with STEMI. In that series, NETs were found in only two-thirds of patients; in that series, the median density was 19%.
In the patients with COVID-19 and STEMI and in the patients reported in the prepandemic historical series from 2015, intracoronary aspirates were obtained during percutaneous coronary intervention using a thrombus aspiration device.
Histologically, findings in the patients from 2015 differed from those of patients with COVID-19. In the patients with COVID, thrombi were composed mostly of fibrin and polymorphonuclear cells. None showed fragments of atherosclerotic plaque or iron deposits indicative of previous episodes of plaque rupture. In contrast, 65% of thrombi from the 2015 series contained plaque fragments.
Ana Blasco, MD, PhD, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, and colleagues report their findings in an article published online Dec. 29 in JAMA Cardiology.
Commenting on the findings in an interview, Irene Lang, MD, from the Medical University of Vienna said, “This is really a very small series, purely observational, and suffering from the problem that acute STEMI is uncommon in COVID-19, but it does serve to demonstrate once more the abundance of NETs in acute myocardial infarction.”
“NETs are very much at the cutting edge of thrombosis research, and NET formation provides yet another link between inflammation and clot formation,” added Peter Libby, MD, from Harvard Medical School and Brigham and Women’s Hospital, Boston.
“Multiple observations have shown thrombosis of arteries large and small, microvessels, and veins in COVID-19. The observations of Blasco et al. add to the growing literature about NETs as contributors to the havoc wrought in multiple organs in advanced COVID-19,” he added in an email exchange with this news organization.
Neither Dr. Lang nor Dr. Libby were involved in this research; both have been actively studying NETs and their contribution to cardiothrombotic disease in recent years.
NETs are newly recognized contributors to venous and arterial thrombosis. These weblike DNA strands are extruded by activated or dying neutrophils and have protein mediators that ensnare pathogens while minimizing damage to the host cell.
First described in 2004, exaggerated NET formation has also been linked to the initiation and accretion of inflammation and thrombosis.
“NETs thus furnish a previously unsuspected link between inflammation, innate immunity, thrombosis, oxidative stress, and cardiovascular diseases,” Dr. Libby and his coauthors wrote in an article on the topic published in Circulation Research earlier this year.
Limiting NET formation or “dissolving” existing NETs could provide a therapeutic avenue not just for patients with COVID-19 but for all patients with thrombotic disease.
“The concept of NETs as a therapeutic target is appealing, in and out of COVID times,” said Dr. Lang.
“I personally believe that the work helps to raise awareness for the potential use of deoxyribonuclease (DNase), an enzyme that acts to clear NETs by dissolving the DNA strands, in the acute treatment of STEMI. Rapid injection of engineered recombinant DNases could potentially wipe away coronary obstructions, ideally before they may cause damage to the myocardium,” she added.
Dr. Blasco and colleagues and Dr. Lang have disclosed no relevant financial relationships. Dr. Libby is an unpaid consultant or member of the advisory board for a number of companies.
A version of this article first appeared on Medscape.com.
Researchers in Madrid may have found a clue to the pathogenesis of ST-segment elevation myocardial infarction (STEMI) in patients with COVID-19; it might also offer a therapeutic target to counter the hypercoagulability seen with COVID-19.
In a case series of five patients with COVID-19 who had an STEMI, neutrophil extracellular traps (NETs) were detected in coronary thrombi of all five patients. The median density was 66%, which is significantly higher than that seen in a historical series of patients with STEMI. In that series, NETs were found in only two-thirds of patients; in that series, the median density was 19%.
In the patients with COVID-19 and STEMI and in the patients reported in the prepandemic historical series from 2015, intracoronary aspirates were obtained during percutaneous coronary intervention using a thrombus aspiration device.
Histologically, findings in the patients from 2015 differed from those of patients with COVID-19. In the patients with COVID, thrombi were composed mostly of fibrin and polymorphonuclear cells. None showed fragments of atherosclerotic plaque or iron deposits indicative of previous episodes of plaque rupture. In contrast, 65% of thrombi from the 2015 series contained plaque fragments.
Ana Blasco, MD, PhD, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, and colleagues report their findings in an article published online Dec. 29 in JAMA Cardiology.
Commenting on the findings in an interview, Irene Lang, MD, from the Medical University of Vienna said, “This is really a very small series, purely observational, and suffering from the problem that acute STEMI is uncommon in COVID-19, but it does serve to demonstrate once more the abundance of NETs in acute myocardial infarction.”
“NETs are very much at the cutting edge of thrombosis research, and NET formation provides yet another link between inflammation and clot formation,” added Peter Libby, MD, from Harvard Medical School and Brigham and Women’s Hospital, Boston.
“Multiple observations have shown thrombosis of arteries large and small, microvessels, and veins in COVID-19. The observations of Blasco et al. add to the growing literature about NETs as contributors to the havoc wrought in multiple organs in advanced COVID-19,” he added in an email exchange with this news organization.
Neither Dr. Lang nor Dr. Libby were involved in this research; both have been actively studying NETs and their contribution to cardiothrombotic disease in recent years.
NETs are newly recognized contributors to venous and arterial thrombosis. These weblike DNA strands are extruded by activated or dying neutrophils and have protein mediators that ensnare pathogens while minimizing damage to the host cell.
First described in 2004, exaggerated NET formation has also been linked to the initiation and accretion of inflammation and thrombosis.
“NETs thus furnish a previously unsuspected link between inflammation, innate immunity, thrombosis, oxidative stress, and cardiovascular diseases,” Dr. Libby and his coauthors wrote in an article on the topic published in Circulation Research earlier this year.
Limiting NET formation or “dissolving” existing NETs could provide a therapeutic avenue not just for patients with COVID-19 but for all patients with thrombotic disease.
“The concept of NETs as a therapeutic target is appealing, in and out of COVID times,” said Dr. Lang.
“I personally believe that the work helps to raise awareness for the potential use of deoxyribonuclease (DNase), an enzyme that acts to clear NETs by dissolving the DNA strands, in the acute treatment of STEMI. Rapid injection of engineered recombinant DNases could potentially wipe away coronary obstructions, ideally before they may cause damage to the myocardium,” she added.
Dr. Blasco and colleagues and Dr. Lang have disclosed no relevant financial relationships. Dr. Libby is an unpaid consultant or member of the advisory board for a number of companies.
A version of this article first appeared on Medscape.com.
Researchers in Madrid may have found a clue to the pathogenesis of ST-segment elevation myocardial infarction (STEMI) in patients with COVID-19; it might also offer a therapeutic target to counter the hypercoagulability seen with COVID-19.
In a case series of five patients with COVID-19 who had an STEMI, neutrophil extracellular traps (NETs) were detected in coronary thrombi of all five patients. The median density was 66%, which is significantly higher than that seen in a historical series of patients with STEMI. In that series, NETs were found in only two-thirds of patients; in that series, the median density was 19%.
In the patients with COVID-19 and STEMI and in the patients reported in the prepandemic historical series from 2015, intracoronary aspirates were obtained during percutaneous coronary intervention using a thrombus aspiration device.
Histologically, findings in the patients from 2015 differed from those of patients with COVID-19. In the patients with COVID, thrombi were composed mostly of fibrin and polymorphonuclear cells. None showed fragments of atherosclerotic plaque or iron deposits indicative of previous episodes of plaque rupture. In contrast, 65% of thrombi from the 2015 series contained plaque fragments.
Ana Blasco, MD, PhD, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, and colleagues report their findings in an article published online Dec. 29 in JAMA Cardiology.
Commenting on the findings in an interview, Irene Lang, MD, from the Medical University of Vienna said, “This is really a very small series, purely observational, and suffering from the problem that acute STEMI is uncommon in COVID-19, but it does serve to demonstrate once more the abundance of NETs in acute myocardial infarction.”
“NETs are very much at the cutting edge of thrombosis research, and NET formation provides yet another link between inflammation and clot formation,” added Peter Libby, MD, from Harvard Medical School and Brigham and Women’s Hospital, Boston.
“Multiple observations have shown thrombosis of arteries large and small, microvessels, and veins in COVID-19. The observations of Blasco et al. add to the growing literature about NETs as contributors to the havoc wrought in multiple organs in advanced COVID-19,” he added in an email exchange with this news organization.
Neither Dr. Lang nor Dr. Libby were involved in this research; both have been actively studying NETs and their contribution to cardiothrombotic disease in recent years.
NETs are newly recognized contributors to venous and arterial thrombosis. These weblike DNA strands are extruded by activated or dying neutrophils and have protein mediators that ensnare pathogens while minimizing damage to the host cell.
First described in 2004, exaggerated NET formation has also been linked to the initiation and accretion of inflammation and thrombosis.
“NETs thus furnish a previously unsuspected link between inflammation, innate immunity, thrombosis, oxidative stress, and cardiovascular diseases,” Dr. Libby and his coauthors wrote in an article on the topic published in Circulation Research earlier this year.
Limiting NET formation or “dissolving” existing NETs could provide a therapeutic avenue not just for patients with COVID-19 but for all patients with thrombotic disease.
“The concept of NETs as a therapeutic target is appealing, in and out of COVID times,” said Dr. Lang.
“I personally believe that the work helps to raise awareness for the potential use of deoxyribonuclease (DNase), an enzyme that acts to clear NETs by dissolving the DNA strands, in the acute treatment of STEMI. Rapid injection of engineered recombinant DNases could potentially wipe away coronary obstructions, ideally before they may cause damage to the myocardium,” she added.
Dr. Blasco and colleagues and Dr. Lang have disclosed no relevant financial relationships. Dr. Libby is an unpaid consultant or member of the advisory board for a number of companies.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine rollout faces delays
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .