User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Patients fend for themselves to access highly touted COVID antibody treatments
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Biden’s COVID-19 challenge: 100 million vaccinations in the first 100 days. It won’t be easy.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
Many EM docs have treated COVID-19 patients without proper PPE: Survey
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
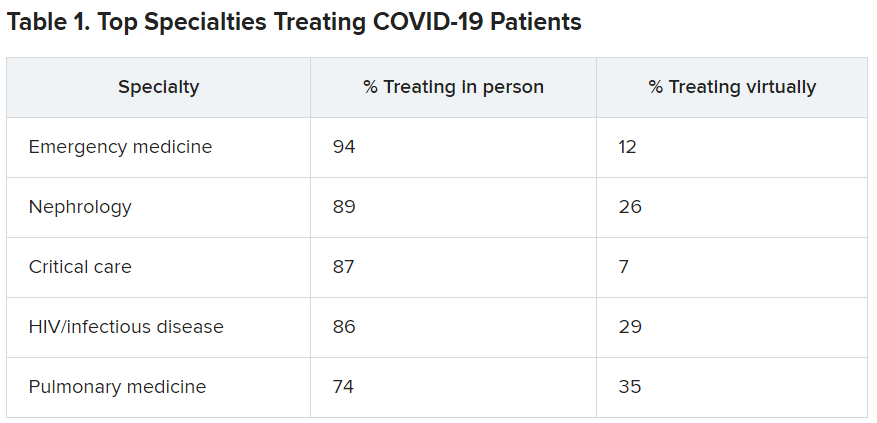
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
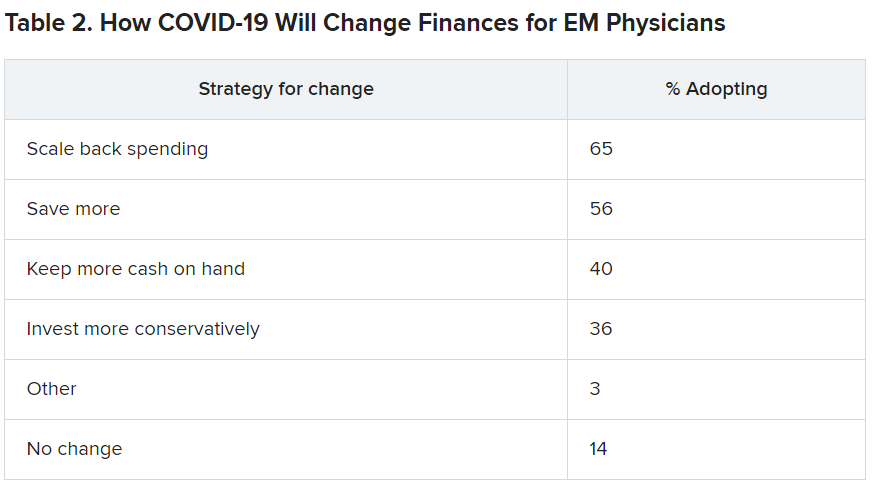
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Further warning on SGLT2 inhibitor use and DKA risk in COVID-19
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Latest weekly increase is largest yet
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
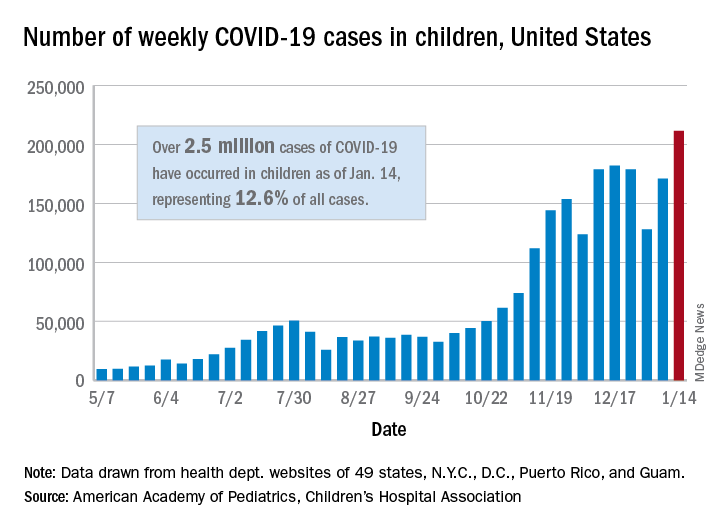
There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
Many ED visits may be preventable for NSCLC patients
The leading cause of ED visits in the study was the cancer itself, although many visits were unrelated to non–small cell lung cancer (NSCLC) or cancer therapy.
Less than 10% of ED visits were related to cancer therapy, but visits were much more common among patients receiving chemotherapy than among those receiving immunotherapy or tyrosine kinase inhibitors (TKIs).
Manan P. Shah, MD, and Joel W. Neal, MD, PhD, both of Stanford (Calif. ) University, reported these results in JCO Oncology Practice.
The authors noted that, in the United States, care of patients with cancer, among all diseases, leads to the highest per-person cost. Unplanned hospital visits are among the largest drivers of increased spending in advanced cancer care, accounting for 48% of spending. However, that spending may not be indicative of better quality care, but rather of inefficiency, according to the authors.
One registry spanning 2009-2012 and including more than 400,000 newly diagnosed cancer patients found lung cancer to have the third-highest rate of unplanned hospitalizations (after hepatobiliary and pancreatic cancer). Those findings were published in JCO Oncology Practice in 2018.
While the reason for presentation to the ED is often the cancer or its therapy in this population, there is a paucity of research on the relative contribution of factors leading to unplanned hospital visits.
Common precipitants of medical emergencies in advanced stages of lung cancer include pulmonary embolism, obstructive pneumonia, spinal cord compression caused by metastasis, and tumor progression or pleural effusion leading to respiratory failure.
Lung cancer therapies, such as TKIs, immunotherapy, and cytotoxic chemotherapy, can also cause various medical emergencies arising out of skin reactions, gastrointestinal dysfunction, organ inflammatory processes, and bone marrow suppression.
Identifying drivers of unplanned ED visits
The primary goals of Dr. Shah and Dr. Neal’s study were to understand the drivers of unplanned ED visits and identify potential strategies to prevent them.
Drawing from the Stanford Medicine Research Data Repository, the authors identified 97 NSCLC patients with 173 ED visits at Stanford.
Patients were sorted according to which of the three therapies they had been receiving in the 3 months prior to the unplanned visit – TKIs (n = 47), immunotherapy (n = 24), or chemotherapy (n = 26). Patients receiving a combination of chemotherapy and immunotherapy were classified under the immunotherapy category.
ED visits were divided into four categories: cancer related, therapy related, unrelated to cancer or therapy, and rule-out (when an outpatient provider sent the patient to rule out medico-oncologic emergencies such as pulmonary embolism or cord compression).
If the patient’s main complaint(s) began 2 or more days before presentation, the diagnostics or therapeutics could have been provided in an outpatient setting (e.g., in clinic or urgent care), and the patient was discharged directly from the ED, the encounter was classified as potentially preventable. Among these preventable encounters, those made during business hours were also labeled unnecessary because they could have been managed through the Stanford sick call system for same-day urgent visits.
Leading cause is cancer
Overall, the leading cause of ED visits was NSCLC itself (54%). The patient’s cancer contributed to 61% of ED visits in the TKI group, 49% in the immunotherapy group, and 42% in the chemotherapy group.
Many ED visits were deemed unrelated to cancer or its therapies – 30% in the TKI group, 26% in the immunotherapy group, and 32% in the chemotherapy group.
Rule-out cases contributed to 7% of ED visits in the TKI group, 14% in the immunotherapy group, and 5% in the chemotherapy group.
Overall, 9% of ED visits were therapy related. The smallest proportion of these was observed in the TKI group (2%), which was significantly smaller than in the immunotherapy group (12%), a rate also significantly smaller than in the chemotherapy group (21%, P < .001).
Most unplanned ED visits did not lead to admissions (55%), were for complaints that began 2 or more days prior to presentation (53%), led to diagnostics or therapeutics that could have been administered in an outpatient setting (48%), and were during business hours (52%).
As a result, 24% of visits were classified as preventable, and 10% were deemed unnecessary.
Preventive strategies
“Our study suggests that TKIs lead to fewer emergency room visits than immunotherapy and chemotherapy,” Dr. Shah said in an interview.
“Overall, this may not necessarily change which therapy we prescribe,” he added, “as TKI therapy is often first line for patients with targeted mutations. However, recognizing that those on chemotherapy or immunotherapy are at higher risk for emergency room visits, we may target preventative strategies, for example, nursing phone calls, telemonitoring of symptoms, and frequent video visits toward this high-risk population.”
Dr. Shah and Dr. Neal said it’s “reassuring” that TKIs and immunotherapy are small drivers of unplanned hospital care. However, they also said efforts aimed at reducing chemotherapy-related ED visits are warranted.
The authors speculated that early intervention, extension of ambulatory care, and patient education about outpatient avenues of care could eliminate a significant proportion (at least 20%) of unplanned ED visits by NSCLC patients.
There was no specific funding for this study. Dr. Shah disclosed no conflicts of interest. Dr. Neal disclosed relationships with many companies, including this news organization.
The leading cause of ED visits in the study was the cancer itself, although many visits were unrelated to non–small cell lung cancer (NSCLC) or cancer therapy.
Less than 10% of ED visits were related to cancer therapy, but visits were much more common among patients receiving chemotherapy than among those receiving immunotherapy or tyrosine kinase inhibitors (TKIs).
Manan P. Shah, MD, and Joel W. Neal, MD, PhD, both of Stanford (Calif. ) University, reported these results in JCO Oncology Practice.
The authors noted that, in the United States, care of patients with cancer, among all diseases, leads to the highest per-person cost. Unplanned hospital visits are among the largest drivers of increased spending in advanced cancer care, accounting for 48% of spending. However, that spending may not be indicative of better quality care, but rather of inefficiency, according to the authors.
One registry spanning 2009-2012 and including more than 400,000 newly diagnosed cancer patients found lung cancer to have the third-highest rate of unplanned hospitalizations (after hepatobiliary and pancreatic cancer). Those findings were published in JCO Oncology Practice in 2018.
While the reason for presentation to the ED is often the cancer or its therapy in this population, there is a paucity of research on the relative contribution of factors leading to unplanned hospital visits.
Common precipitants of medical emergencies in advanced stages of lung cancer include pulmonary embolism, obstructive pneumonia, spinal cord compression caused by metastasis, and tumor progression or pleural effusion leading to respiratory failure.
Lung cancer therapies, such as TKIs, immunotherapy, and cytotoxic chemotherapy, can also cause various medical emergencies arising out of skin reactions, gastrointestinal dysfunction, organ inflammatory processes, and bone marrow suppression.
Identifying drivers of unplanned ED visits
The primary goals of Dr. Shah and Dr. Neal’s study were to understand the drivers of unplanned ED visits and identify potential strategies to prevent them.
Drawing from the Stanford Medicine Research Data Repository, the authors identified 97 NSCLC patients with 173 ED visits at Stanford.
Patients were sorted according to which of the three therapies they had been receiving in the 3 months prior to the unplanned visit – TKIs (n = 47), immunotherapy (n = 24), or chemotherapy (n = 26). Patients receiving a combination of chemotherapy and immunotherapy were classified under the immunotherapy category.
ED visits were divided into four categories: cancer related, therapy related, unrelated to cancer or therapy, and rule-out (when an outpatient provider sent the patient to rule out medico-oncologic emergencies such as pulmonary embolism or cord compression).
If the patient’s main complaint(s) began 2 or more days before presentation, the diagnostics or therapeutics could have been provided in an outpatient setting (e.g., in clinic or urgent care), and the patient was discharged directly from the ED, the encounter was classified as potentially preventable. Among these preventable encounters, those made during business hours were also labeled unnecessary because they could have been managed through the Stanford sick call system for same-day urgent visits.
Leading cause is cancer
Overall, the leading cause of ED visits was NSCLC itself (54%). The patient’s cancer contributed to 61% of ED visits in the TKI group, 49% in the immunotherapy group, and 42% in the chemotherapy group.
Many ED visits were deemed unrelated to cancer or its therapies – 30% in the TKI group, 26% in the immunotherapy group, and 32% in the chemotherapy group.
Rule-out cases contributed to 7% of ED visits in the TKI group, 14% in the immunotherapy group, and 5% in the chemotherapy group.
Overall, 9% of ED visits were therapy related. The smallest proportion of these was observed in the TKI group (2%), which was significantly smaller than in the immunotherapy group (12%), a rate also significantly smaller than in the chemotherapy group (21%, P < .001).
Most unplanned ED visits did not lead to admissions (55%), were for complaints that began 2 or more days prior to presentation (53%), led to diagnostics or therapeutics that could have been administered in an outpatient setting (48%), and were during business hours (52%).
As a result, 24% of visits were classified as preventable, and 10% were deemed unnecessary.
Preventive strategies
“Our study suggests that TKIs lead to fewer emergency room visits than immunotherapy and chemotherapy,” Dr. Shah said in an interview.
“Overall, this may not necessarily change which therapy we prescribe,” he added, “as TKI therapy is often first line for patients with targeted mutations. However, recognizing that those on chemotherapy or immunotherapy are at higher risk for emergency room visits, we may target preventative strategies, for example, nursing phone calls, telemonitoring of symptoms, and frequent video visits toward this high-risk population.”
Dr. Shah and Dr. Neal said it’s “reassuring” that TKIs and immunotherapy are small drivers of unplanned hospital care. However, they also said efforts aimed at reducing chemotherapy-related ED visits are warranted.
The authors speculated that early intervention, extension of ambulatory care, and patient education about outpatient avenues of care could eliminate a significant proportion (at least 20%) of unplanned ED visits by NSCLC patients.
There was no specific funding for this study. Dr. Shah disclosed no conflicts of interest. Dr. Neal disclosed relationships with many companies, including this news organization.
The leading cause of ED visits in the study was the cancer itself, although many visits were unrelated to non–small cell lung cancer (NSCLC) or cancer therapy.
Less than 10% of ED visits were related to cancer therapy, but visits were much more common among patients receiving chemotherapy than among those receiving immunotherapy or tyrosine kinase inhibitors (TKIs).
Manan P. Shah, MD, and Joel W. Neal, MD, PhD, both of Stanford (Calif. ) University, reported these results in JCO Oncology Practice.
The authors noted that, in the United States, care of patients with cancer, among all diseases, leads to the highest per-person cost. Unplanned hospital visits are among the largest drivers of increased spending in advanced cancer care, accounting for 48% of spending. However, that spending may not be indicative of better quality care, but rather of inefficiency, according to the authors.
One registry spanning 2009-2012 and including more than 400,000 newly diagnosed cancer patients found lung cancer to have the third-highest rate of unplanned hospitalizations (after hepatobiliary and pancreatic cancer). Those findings were published in JCO Oncology Practice in 2018.
While the reason for presentation to the ED is often the cancer or its therapy in this population, there is a paucity of research on the relative contribution of factors leading to unplanned hospital visits.
Common precipitants of medical emergencies in advanced stages of lung cancer include pulmonary embolism, obstructive pneumonia, spinal cord compression caused by metastasis, and tumor progression or pleural effusion leading to respiratory failure.
Lung cancer therapies, such as TKIs, immunotherapy, and cytotoxic chemotherapy, can also cause various medical emergencies arising out of skin reactions, gastrointestinal dysfunction, organ inflammatory processes, and bone marrow suppression.
Identifying drivers of unplanned ED visits
The primary goals of Dr. Shah and Dr. Neal’s study were to understand the drivers of unplanned ED visits and identify potential strategies to prevent them.
Drawing from the Stanford Medicine Research Data Repository, the authors identified 97 NSCLC patients with 173 ED visits at Stanford.
Patients were sorted according to which of the three therapies they had been receiving in the 3 months prior to the unplanned visit – TKIs (n = 47), immunotherapy (n = 24), or chemotherapy (n = 26). Patients receiving a combination of chemotherapy and immunotherapy were classified under the immunotherapy category.
ED visits were divided into four categories: cancer related, therapy related, unrelated to cancer or therapy, and rule-out (when an outpatient provider sent the patient to rule out medico-oncologic emergencies such as pulmonary embolism or cord compression).
If the patient’s main complaint(s) began 2 or more days before presentation, the diagnostics or therapeutics could have been provided in an outpatient setting (e.g., in clinic or urgent care), and the patient was discharged directly from the ED, the encounter was classified as potentially preventable. Among these preventable encounters, those made during business hours were also labeled unnecessary because they could have been managed through the Stanford sick call system for same-day urgent visits.
Leading cause is cancer
Overall, the leading cause of ED visits was NSCLC itself (54%). The patient’s cancer contributed to 61% of ED visits in the TKI group, 49% in the immunotherapy group, and 42% in the chemotherapy group.
Many ED visits were deemed unrelated to cancer or its therapies – 30% in the TKI group, 26% in the immunotherapy group, and 32% in the chemotherapy group.
Rule-out cases contributed to 7% of ED visits in the TKI group, 14% in the immunotherapy group, and 5% in the chemotherapy group.
Overall, 9% of ED visits were therapy related. The smallest proportion of these was observed in the TKI group (2%), which was significantly smaller than in the immunotherapy group (12%), a rate also significantly smaller than in the chemotherapy group (21%, P < .001).
Most unplanned ED visits did not lead to admissions (55%), were for complaints that began 2 or more days prior to presentation (53%), led to diagnostics or therapeutics that could have been administered in an outpatient setting (48%), and were during business hours (52%).
As a result, 24% of visits were classified as preventable, and 10% were deemed unnecessary.
Preventive strategies
“Our study suggests that TKIs lead to fewer emergency room visits than immunotherapy and chemotherapy,” Dr. Shah said in an interview.
“Overall, this may not necessarily change which therapy we prescribe,” he added, “as TKI therapy is often first line for patients with targeted mutations. However, recognizing that those on chemotherapy or immunotherapy are at higher risk for emergency room visits, we may target preventative strategies, for example, nursing phone calls, telemonitoring of symptoms, and frequent video visits toward this high-risk population.”
Dr. Shah and Dr. Neal said it’s “reassuring” that TKIs and immunotherapy are small drivers of unplanned hospital care. However, they also said efforts aimed at reducing chemotherapy-related ED visits are warranted.
The authors speculated that early intervention, extension of ambulatory care, and patient education about outpatient avenues of care could eliminate a significant proportion (at least 20%) of unplanned ED visits by NSCLC patients.
There was no specific funding for this study. Dr. Shah disclosed no conflicts of interest. Dr. Neal disclosed relationships with many companies, including this news organization.
FROM JCO ONCOLOGY PRACTICE
American Academy of Sleep Medicine (AASM) advocates for year-round standard time
Although the United States has observed daylight saving time (DST) continuously, in some form, for the last 5 decades (Table), the twice a year switches have never been less popular. In 2019, an American Academy of Sleep Medicine (AASM) survey of more than 2,000 US adults found that 63% support the elimination of seasonal time changes in favor of a national, fixed, year-round time, and only 11% oppose it. Indeed, multiple states have pending legislations to adopt year-round daylight saving time or year-round standard time (Updated September 30, 2020, Congressional Research Service. https://crsreports.congress.gov. R45208 Daylight Saving Time. Accessed Dec 14, 2020). Adjacent states, to limit confusion to interstate travel and commerce, tend to lobby for similar changes together. Most importantly, because of the scientific evidence of detrimental health effects to the public and safety concerns, the American Academy of Sleep Medicine has issued a position statement for year-round standard time (Rishi MA, et al. Daylight saving time: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2020;16(10):1781).
Railroad industry successfully lobbied the US government for consistent time in the United States to keep transportation schedules uniform in 1883; standard time was implemented. When war efforts were over, DST was dropped. Some regions, such as New York and Chicago, maintained DST, but no national standard was applied. Retailers and the recreational activity industry advocated for DST to increase business after work in the afternoon and evenings. In 1966, Congress passed the Uniform Time Act of 1966 to implement 6 months of DST and 6 months of standard time (Waxman OB. The real reason why daylight saving time is a thing. https://time.com/4549397/daylight-saving-time-history-politics/; November 1, 2017. Accessed Dec 14, 2020). Local jurisdictions can opt out of DST, but it requires an act of congress to enforce perennial DST.
When the OPEC embargo occurred, the Emergency Daylight Saving Time Energy Conservation Act was enacted in 1973, but it was quickly ended in October 1974 due to its unpopularity. The dairy industry was opposed to earlier rise time that disrupted the animals’ feeding schedules and their farm operations (Feldman R. Five myths about daylight saving time. https://www.washingtonpost.com/opinions/five-myths-about-daylight-saving-time/2015/03/06/970092d4-c2c1-11e4-9271-610273846239_story.html. Accessed Dec 14, 2020.). Public safety was raised as a concern as early as 1975. The Department of Transportation found increased fatalities of school-aged children in the mornings from January to April of 1974 as compared with 1973. However, the National Bureau of Standards, that performed a review subsequently, stated that other factors might also be at play. Further extension of DST from 6 months of the year to the subsequent 7, and then 8 months per year were enacted in 1986 and 2005, respectively (The reasoning behind changing daylight saving. https://www.npr.org/templates/story/story.php?storyId=7779869. NPR. Accessed Nov 1, 2020.)
An exemption of a state from DST is allowable under existing law, but to establish permanent DST will require an act of Congress. Since then, Arizona and Hawaii, as well as US territories, such as Puerto Rico, Guam, American Samoa and Northern Mariana Islands, and US Virgin Islands, have all opted out of DST by state exemption. Because of Hawaii’s proximity to the equator, the timing of sunrise and sunset were fairly constant throughout the year that made DST unnecessary. The Navajo Nation in Arizona, because of its extension into adjacent New Mexico and Utah, participates in DST. Most of the countries along the tropics, parts of Australia, China, Japan, South Korea, India, and majority of African countries do not observe DST. The European Union has voted to abolish twice yearly change in time in 2021; and individual member states will be able to decide whether they wish to remain on permanent standard time or DST. Since 2015, more than 45 states have proposed legislation to change their observance of DST.
The human biological rhythm is most consistent with standard time (Antle M. Circadian rhythm expert argues against permanent daylight saving time. https://www.ucalgary.ca/news/circadian-rhythm-expert-argues-against-permanent-daylight-saving-time. Accessed Dec 14, 2020.). Since the biological clock for most individuals is not exactly 24 hours long, zeitgebers such as sunlight, exercise, and feeding behaviors are important time cues to foster a regular rhythm. Acutely, the adjustment to 1 hour’s sleep loss at the spring switch from standard time to DST generally requires several days to adapt (Kalidindi A. Daylight saving time is bad for your health. https://massivesci.com/articles/daylight-saving-savings-time-dst-november-standard-time. Accessed Dec 14, 2020.). During this adjustment period, the internal bodily functions are disrupted. The sense of sleepiness and fatigue are increased with earlier morning awakenings, and the inability to fall asleep earlier leads to symptoms of insomnia and poor sleep quality.
The health and economic costs due to accidents, injuries, and medical errors are now well known. Individual biological rhythm disruptions at the spring switch from standard time to DST with the loss of sleep likely contributes to higher risks of myocardial infarctions (Janszky I, et al. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008; 359(18):1966) that are not mostly seen during the fall switch from DST to standard time. An estimated 40 minutes of sleep loss occurs within the Sunday to Monday transition of DST in the spring. Medical errors, car crashes, suicide risks, and fatigue are all reportedly higher on the Monday after the spring switch. Some of these effects have been cited as remaining elevated through the first week and possibly chronically during the entire duration of DST. Some people have difficulty adapting to sleep loss from DST, creating social jetlag, and complaints of fatigue and increased prevalence of metabolic syndromes are more common in this population (Koopman ADM, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New Hoorn study. J Biol Rhythms. 2017 Aug:32(4):359; Roenneberg T, et al. Social jetlag and obesity. Curr Biol. 2012 May 22; 22(10):939). “Cyber-loafing,” describing those at work but who chose to peruse entertaining websites, reportedly occurred more during DST compared with the fall.
Delaying school start time has been associated with improved school attendance and performance. The American Academy of Pediatrics and AASM support delaying school start time; this measure has been adopted by California, and legislation is pending in other states (https://www.startschoollater.net/legislation.html). In spring, the loss of 1 hour’s sleep would negate any benefit of beginning the school day later. Students would suffer inattention, decrease ability to focus, and be less effective learners. Obesity and metabolic syndromes that have been found in adults, are also observed in children whose biological rhythms are delayed compared with their peers who have morning lark tendencies. Risks of mood disorder may be elevated at onset of DST due to earlier arise time or standard time when less sunlight is available in the evenings.
During the current pandemic with SARS-CoV-2, there are new reports of teens and college students able to obtain more sleep because of online education (How children’s sleep habits have changed in the pandemic. https://www.nytimes.com/2020/08/17/well/family/children-sleep-pandemic.html. Accessed Dec 14, 2020.) and they had more restful sleep and improved mood. This positive trend will be monitored closely with some schools returning to in-person instruction.
Societal costs of decreased productivity, on the job accidents and injuries, and increased risk of motor vehicle crashes (Robb D, et al. Accident rates and the impact of daylight saving transitions. Accid Anal Prev. 2018 Feb; 111:193), in addition to individual well-being, have also been reported. Energy savings that propelled arguments for DST did not translate into significant savings after all. Although less electricity was used with more abundance of sunlight in the afternoon, people drove more and used more gasoline to attend their after work activities.
Adaptation of a year-round time schedule will need to balance the impact and disruption to the health and well-being of its citizens, as well as the interests of its commercial sector. The argument for maintaining year-round standard time states that to prevent the loss of the 1 hour’s sleep that DST creates in the spring. Therefore, it preserves a more aligned biological rhythm, lowers the risks of preventable myocardial infarction, improves attention and focus, lessens daytime fatigue, and improves sense of well-being year round. Certainly, it will ensure that the teens who are likely to have later sleep schedules, will not lose more sleep and negate the benefit of starting school later.
Timeline for DST
1784 Benjamin Franklin advocated to rise earlier so as to burn fewer candles in the evenings.
1883 Railroads need standard time for operations.
1890 Merchants and retailers (clothing, cigars) advocated for longer shopping hours.
1916 Germany conserves energy.
1918 DST: fuel conservation during World War I.
1942 DST during World War II.
1963 “Chaos of clocks” needs uniform time for commerce.
1966 Uniform Time Act: DST 6 months per year.
1973 Emergency DST Energy Conservation Act: Arab oil embargo to extend DST to 8 months; ended prematurely in October 1974.
1986 Extended start date from last Sunday of April to first Sunday of November.
2005 Energy Act of 2005: 2nd week of March.
Dr. Yuen is Assistant Professor, UCSF Department of Internal Medicine-Pulmonary Department, and Adjunct Clinical Assistant Professor at Department of Psychiatry & Behavioral Sciences at Stanford (Calif.) University. Dr. Rishi is Consultant – Pulmonary, Critical Care and Sleep Medicine, Mayo Clinic Health System, Eau Claire, WI; and Assistant Professor of Medicine, Alix School of Medicine, Mayo Clinic, Rochester, MN.
Correction 3/16/21: A photo caption in an earlier version of this article misstated Dr. Kin Yuen's name.
Although the United States has observed daylight saving time (DST) continuously, in some form, for the last 5 decades (Table), the twice a year switches have never been less popular. In 2019, an American Academy of Sleep Medicine (AASM) survey of more than 2,000 US adults found that 63% support the elimination of seasonal time changes in favor of a national, fixed, year-round time, and only 11% oppose it. Indeed, multiple states have pending legislations to adopt year-round daylight saving time or year-round standard time (Updated September 30, 2020, Congressional Research Service. https://crsreports.congress.gov. R45208 Daylight Saving Time. Accessed Dec 14, 2020). Adjacent states, to limit confusion to interstate travel and commerce, tend to lobby for similar changes together. Most importantly, because of the scientific evidence of detrimental health effects to the public and safety concerns, the American Academy of Sleep Medicine has issued a position statement for year-round standard time (Rishi MA, et al. Daylight saving time: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2020;16(10):1781).
Railroad industry successfully lobbied the US government for consistent time in the United States to keep transportation schedules uniform in 1883; standard time was implemented. When war efforts were over, DST was dropped. Some regions, such as New York and Chicago, maintained DST, but no national standard was applied. Retailers and the recreational activity industry advocated for DST to increase business after work in the afternoon and evenings. In 1966, Congress passed the Uniform Time Act of 1966 to implement 6 months of DST and 6 months of standard time (Waxman OB. The real reason why daylight saving time is a thing. https://time.com/4549397/daylight-saving-time-history-politics/; November 1, 2017. Accessed Dec 14, 2020). Local jurisdictions can opt out of DST, but it requires an act of congress to enforce perennial DST.
When the OPEC embargo occurred, the Emergency Daylight Saving Time Energy Conservation Act was enacted in 1973, but it was quickly ended in October 1974 due to its unpopularity. The dairy industry was opposed to earlier rise time that disrupted the animals’ feeding schedules and their farm operations (Feldman R. Five myths about daylight saving time. https://www.washingtonpost.com/opinions/five-myths-about-daylight-saving-time/2015/03/06/970092d4-c2c1-11e4-9271-610273846239_story.html. Accessed Dec 14, 2020.). Public safety was raised as a concern as early as 1975. The Department of Transportation found increased fatalities of school-aged children in the mornings from January to April of 1974 as compared with 1973. However, the National Bureau of Standards, that performed a review subsequently, stated that other factors might also be at play. Further extension of DST from 6 months of the year to the subsequent 7, and then 8 months per year were enacted in 1986 and 2005, respectively (The reasoning behind changing daylight saving. https://www.npr.org/templates/story/story.php?storyId=7779869. NPR. Accessed Nov 1, 2020.)
An exemption of a state from DST is allowable under existing law, but to establish permanent DST will require an act of Congress. Since then, Arizona and Hawaii, as well as US territories, such as Puerto Rico, Guam, American Samoa and Northern Mariana Islands, and US Virgin Islands, have all opted out of DST by state exemption. Because of Hawaii’s proximity to the equator, the timing of sunrise and sunset were fairly constant throughout the year that made DST unnecessary. The Navajo Nation in Arizona, because of its extension into adjacent New Mexico and Utah, participates in DST. Most of the countries along the tropics, parts of Australia, China, Japan, South Korea, India, and majority of African countries do not observe DST. The European Union has voted to abolish twice yearly change in time in 2021; and individual member states will be able to decide whether they wish to remain on permanent standard time or DST. Since 2015, more than 45 states have proposed legislation to change their observance of DST.
The human biological rhythm is most consistent with standard time (Antle M. Circadian rhythm expert argues against permanent daylight saving time. https://www.ucalgary.ca/news/circadian-rhythm-expert-argues-against-permanent-daylight-saving-time. Accessed Dec 14, 2020.). Since the biological clock for most individuals is not exactly 24 hours long, zeitgebers such as sunlight, exercise, and feeding behaviors are important time cues to foster a regular rhythm. Acutely, the adjustment to 1 hour’s sleep loss at the spring switch from standard time to DST generally requires several days to adapt (Kalidindi A. Daylight saving time is bad for your health. https://massivesci.com/articles/daylight-saving-savings-time-dst-november-standard-time. Accessed Dec 14, 2020.). During this adjustment period, the internal bodily functions are disrupted. The sense of sleepiness and fatigue are increased with earlier morning awakenings, and the inability to fall asleep earlier leads to symptoms of insomnia and poor sleep quality.
The health and economic costs due to accidents, injuries, and medical errors are now well known. Individual biological rhythm disruptions at the spring switch from standard time to DST with the loss of sleep likely contributes to higher risks of myocardial infarctions (Janszky I, et al. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008; 359(18):1966) that are not mostly seen during the fall switch from DST to standard time. An estimated 40 minutes of sleep loss occurs within the Sunday to Monday transition of DST in the spring. Medical errors, car crashes, suicide risks, and fatigue are all reportedly higher on the Monday after the spring switch. Some of these effects have been cited as remaining elevated through the first week and possibly chronically during the entire duration of DST. Some people have difficulty adapting to sleep loss from DST, creating social jetlag, and complaints of fatigue and increased prevalence of metabolic syndromes are more common in this population (Koopman ADM, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New Hoorn study. J Biol Rhythms. 2017 Aug:32(4):359; Roenneberg T, et al. Social jetlag and obesity. Curr Biol. 2012 May 22; 22(10):939). “Cyber-loafing,” describing those at work but who chose to peruse entertaining websites, reportedly occurred more during DST compared with the fall.
Delaying school start time has been associated with improved school attendance and performance. The American Academy of Pediatrics and AASM support delaying school start time; this measure has been adopted by California, and legislation is pending in other states (https://www.startschoollater.net/legislation.html). In spring, the loss of 1 hour’s sleep would negate any benefit of beginning the school day later. Students would suffer inattention, decrease ability to focus, and be less effective learners. Obesity and metabolic syndromes that have been found in adults, are also observed in children whose biological rhythms are delayed compared with their peers who have morning lark tendencies. Risks of mood disorder may be elevated at onset of DST due to earlier arise time or standard time when less sunlight is available in the evenings.
During the current pandemic with SARS-CoV-2, there are new reports of teens and college students able to obtain more sleep because of online education (How children’s sleep habits have changed in the pandemic. https://www.nytimes.com/2020/08/17/well/family/children-sleep-pandemic.html. Accessed Dec 14, 2020.) and they had more restful sleep and improved mood. This positive trend will be monitored closely with some schools returning to in-person instruction.
Societal costs of decreased productivity, on the job accidents and injuries, and increased risk of motor vehicle crashes (Robb D, et al. Accident rates and the impact of daylight saving transitions. Accid Anal Prev. 2018 Feb; 111:193), in addition to individual well-being, have also been reported. Energy savings that propelled arguments for DST did not translate into significant savings after all. Although less electricity was used with more abundance of sunlight in the afternoon, people drove more and used more gasoline to attend their after work activities.
Adaptation of a year-round time schedule will need to balance the impact and disruption to the health and well-being of its citizens, as well as the interests of its commercial sector. The argument for maintaining year-round standard time states that to prevent the loss of the 1 hour’s sleep that DST creates in the spring. Therefore, it preserves a more aligned biological rhythm, lowers the risks of preventable myocardial infarction, improves attention and focus, lessens daytime fatigue, and improves sense of well-being year round. Certainly, it will ensure that the teens who are likely to have later sleep schedules, will not lose more sleep and negate the benefit of starting school later.
Timeline for DST
1784 Benjamin Franklin advocated to rise earlier so as to burn fewer candles in the evenings.
1883 Railroads need standard time for operations.
1890 Merchants and retailers (clothing, cigars) advocated for longer shopping hours.
1916 Germany conserves energy.
1918 DST: fuel conservation during World War I.
1942 DST during World War II.
1963 “Chaos of clocks” needs uniform time for commerce.
1966 Uniform Time Act: DST 6 months per year.
1973 Emergency DST Energy Conservation Act: Arab oil embargo to extend DST to 8 months; ended prematurely in October 1974.
1986 Extended start date from last Sunday of April to first Sunday of November.
2005 Energy Act of 2005: 2nd week of March.
Dr. Yuen is Assistant Professor, UCSF Department of Internal Medicine-Pulmonary Department, and Adjunct Clinical Assistant Professor at Department of Psychiatry & Behavioral Sciences at Stanford (Calif.) University. Dr. Rishi is Consultant – Pulmonary, Critical Care and Sleep Medicine, Mayo Clinic Health System, Eau Claire, WI; and Assistant Professor of Medicine, Alix School of Medicine, Mayo Clinic, Rochester, MN.
Correction 3/16/21: A photo caption in an earlier version of this article misstated Dr. Kin Yuen's name.
Although the United States has observed daylight saving time (DST) continuously, in some form, for the last 5 decades (Table), the twice a year switches have never been less popular. In 2019, an American Academy of Sleep Medicine (AASM) survey of more than 2,000 US adults found that 63% support the elimination of seasonal time changes in favor of a national, fixed, year-round time, and only 11% oppose it. Indeed, multiple states have pending legislations to adopt year-round daylight saving time or year-round standard time (Updated September 30, 2020, Congressional Research Service. https://crsreports.congress.gov. R45208 Daylight Saving Time. Accessed Dec 14, 2020). Adjacent states, to limit confusion to interstate travel and commerce, tend to lobby for similar changes together. Most importantly, because of the scientific evidence of detrimental health effects to the public and safety concerns, the American Academy of Sleep Medicine has issued a position statement for year-round standard time (Rishi MA, et al. Daylight saving time: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2020;16(10):1781).
Railroad industry successfully lobbied the US government for consistent time in the United States to keep transportation schedules uniform in 1883; standard time was implemented. When war efforts were over, DST was dropped. Some regions, such as New York and Chicago, maintained DST, but no national standard was applied. Retailers and the recreational activity industry advocated for DST to increase business after work in the afternoon and evenings. In 1966, Congress passed the Uniform Time Act of 1966 to implement 6 months of DST and 6 months of standard time (Waxman OB. The real reason why daylight saving time is a thing. https://time.com/4549397/daylight-saving-time-history-politics/; November 1, 2017. Accessed Dec 14, 2020). Local jurisdictions can opt out of DST, but it requires an act of congress to enforce perennial DST.
When the OPEC embargo occurred, the Emergency Daylight Saving Time Energy Conservation Act was enacted in 1973, but it was quickly ended in October 1974 due to its unpopularity. The dairy industry was opposed to earlier rise time that disrupted the animals’ feeding schedules and their farm operations (Feldman R. Five myths about daylight saving time. https://www.washingtonpost.com/opinions/five-myths-about-daylight-saving-time/2015/03/06/970092d4-c2c1-11e4-9271-610273846239_story.html. Accessed Dec 14, 2020.). Public safety was raised as a concern as early as 1975. The Department of Transportation found increased fatalities of school-aged children in the mornings from January to April of 1974 as compared with 1973. However, the National Bureau of Standards, that performed a review subsequently, stated that other factors might also be at play. Further extension of DST from 6 months of the year to the subsequent 7, and then 8 months per year were enacted in 1986 and 2005, respectively (The reasoning behind changing daylight saving. https://www.npr.org/templates/story/story.php?storyId=7779869. NPR. Accessed Nov 1, 2020.)
An exemption of a state from DST is allowable under existing law, but to establish permanent DST will require an act of Congress. Since then, Arizona and Hawaii, as well as US territories, such as Puerto Rico, Guam, American Samoa and Northern Mariana Islands, and US Virgin Islands, have all opted out of DST by state exemption. Because of Hawaii’s proximity to the equator, the timing of sunrise and sunset were fairly constant throughout the year that made DST unnecessary. The Navajo Nation in Arizona, because of its extension into adjacent New Mexico and Utah, participates in DST. Most of the countries along the tropics, parts of Australia, China, Japan, South Korea, India, and majority of African countries do not observe DST. The European Union has voted to abolish twice yearly change in time in 2021; and individual member states will be able to decide whether they wish to remain on permanent standard time or DST. Since 2015, more than 45 states have proposed legislation to change their observance of DST.
The human biological rhythm is most consistent with standard time (Antle M. Circadian rhythm expert argues against permanent daylight saving time. https://www.ucalgary.ca/news/circadian-rhythm-expert-argues-against-permanent-daylight-saving-time. Accessed Dec 14, 2020.). Since the biological clock for most individuals is not exactly 24 hours long, zeitgebers such as sunlight, exercise, and feeding behaviors are important time cues to foster a regular rhythm. Acutely, the adjustment to 1 hour’s sleep loss at the spring switch from standard time to DST generally requires several days to adapt (Kalidindi A. Daylight saving time is bad for your health. https://massivesci.com/articles/daylight-saving-savings-time-dst-november-standard-time. Accessed Dec 14, 2020.). During this adjustment period, the internal bodily functions are disrupted. The sense of sleepiness and fatigue are increased with earlier morning awakenings, and the inability to fall asleep earlier leads to symptoms of insomnia and poor sleep quality.
The health and economic costs due to accidents, injuries, and medical errors are now well known. Individual biological rhythm disruptions at the spring switch from standard time to DST with the loss of sleep likely contributes to higher risks of myocardial infarctions (Janszky I, et al. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008; 359(18):1966) that are not mostly seen during the fall switch from DST to standard time. An estimated 40 minutes of sleep loss occurs within the Sunday to Monday transition of DST in the spring. Medical errors, car crashes, suicide risks, and fatigue are all reportedly higher on the Monday after the spring switch. Some of these effects have been cited as remaining elevated through the first week and possibly chronically during the entire duration of DST. Some people have difficulty adapting to sleep loss from DST, creating social jetlag, and complaints of fatigue and increased prevalence of metabolic syndromes are more common in this population (Koopman ADM, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: The New Hoorn study. J Biol Rhythms. 2017 Aug:32(4):359; Roenneberg T, et al. Social jetlag and obesity. Curr Biol. 2012 May 22; 22(10):939). “Cyber-loafing,” describing those at work but who chose to peruse entertaining websites, reportedly occurred more during DST compared with the fall.
Delaying school start time has been associated with improved school attendance and performance. The American Academy of Pediatrics and AASM support delaying school start time; this measure has been adopted by California, and legislation is pending in other states (https://www.startschoollater.net/legislation.html). In spring, the loss of 1 hour’s sleep would negate any benefit of beginning the school day later. Students would suffer inattention, decrease ability to focus, and be less effective learners. Obesity and metabolic syndromes that have been found in adults, are also observed in children whose biological rhythms are delayed compared with their peers who have morning lark tendencies. Risks of mood disorder may be elevated at onset of DST due to earlier arise time or standard time when less sunlight is available in the evenings.
During the current pandemic with SARS-CoV-2, there are new reports of teens and college students able to obtain more sleep because of online education (How children’s sleep habits have changed in the pandemic. https://www.nytimes.com/2020/08/17/well/family/children-sleep-pandemic.html. Accessed Dec 14, 2020.) and they had more restful sleep and improved mood. This positive trend will be monitored closely with some schools returning to in-person instruction.
Societal costs of decreased productivity, on the job accidents and injuries, and increased risk of motor vehicle crashes (Robb D, et al. Accident rates and the impact of daylight saving transitions. Accid Anal Prev. 2018 Feb; 111:193), in addition to individual well-being, have also been reported. Energy savings that propelled arguments for DST did not translate into significant savings after all. Although less electricity was used with more abundance of sunlight in the afternoon, people drove more and used more gasoline to attend their after work activities.
Adaptation of a year-round time schedule will need to balance the impact and disruption to the health and well-being of its citizens, as well as the interests of its commercial sector. The argument for maintaining year-round standard time states that to prevent the loss of the 1 hour’s sleep that DST creates in the spring. Therefore, it preserves a more aligned biological rhythm, lowers the risks of preventable myocardial infarction, improves attention and focus, lessens daytime fatigue, and improves sense of well-being year round. Certainly, it will ensure that the teens who are likely to have later sleep schedules, will not lose more sleep and negate the benefit of starting school later.
Timeline for DST
1784 Benjamin Franklin advocated to rise earlier so as to burn fewer candles in the evenings.
1883 Railroads need standard time for operations.
1890 Merchants and retailers (clothing, cigars) advocated for longer shopping hours.
1916 Germany conserves energy.
1918 DST: fuel conservation during World War I.
1942 DST during World War II.
1963 “Chaos of clocks” needs uniform time for commerce.
1966 Uniform Time Act: DST 6 months per year.
1973 Emergency DST Energy Conservation Act: Arab oil embargo to extend DST to 8 months; ended prematurely in October 1974.
1986 Extended start date from last Sunday of April to first Sunday of November.
2005 Energy Act of 2005: 2nd week of March.
Dr. Yuen is Assistant Professor, UCSF Department of Internal Medicine-Pulmonary Department, and Adjunct Clinical Assistant Professor at Department of Psychiatry & Behavioral Sciences at Stanford (Calif.) University. Dr. Rishi is Consultant – Pulmonary, Critical Care and Sleep Medicine, Mayo Clinic Health System, Eau Claire, WI; and Assistant Professor of Medicine, Alix School of Medicine, Mayo Clinic, Rochester, MN.
Correction 3/16/21: A photo caption in an earlier version of this article misstated Dr. Kin Yuen's name.
Updates from the AMA House of Delegates: November 2020 special meeting
The American Medical Association (AMA) had its November 2020 AMA Special Meeting of the AMA House of Delegates (HOD) from November 13-17.
Delegates from over 170 societies (state societies, specialties, subspecialties, and uniformed services), including physicians, residents, and students, gathered virtually for the meeting(https://tinyurl.com/y7494mwa) to consider a wide array of proposals to help fulfill the AMA’s core mission of promoting medicine and improving public health. The AMA House of Delegates, also known as the “House” or the “HOD,” is the principal policy-making body of the AMA. This democratic forum represents the views and interests of a diverse group of member physicians from more than 170 societies. These delegates meet twice per year to establish policies on health; medical, professional, and governance matters; and the principles within which the AMA’s business activities are conducted.
During the COVID-19 pandemic, the AMA has been the leading physician and patient ally—voicing recommendations to key Congressional leaders and agency staff, state policymakers, and private sector stakeholders. Acting on both federal and state levels, examples of AMA’s recent efforts include actions in financial relief, telehealth, testing and vaccine development, health equity, and more.
CHEST is an active member, and through the HOD and Specialty and Service Society (SSS), CHEST can partner with AMA other societies to support each other on important regulatory issues. CHEST/Allergy Section Council (participants at this meeting were from the AAAAI, AAOA, AASM, ACAAI, ATS, CHEST, and SCCM) met before voting in the House to discuss pending business. The meeting was hosted by the current CHEST/Allergy council chair Dr. Wesley Vander Ark (AMA Delegate AAOA) and Jami Lucas, CEO AAOA.
Policy and resolutions
Overview of the process
Policies originate via resolutions submitted by individuals or societies. These resolutions then go to one of several Reference Committees for open discussion. These committees then report their recommendations back to the HOD, which then discusses and votes on the recommendations. In some instances, the question is referred for further studies by one of several Councils, which reports go to the Board of Trustees or back to the House. Details can be found in the April 2018 CHEST Physician® article on the process. (https://tinyurl.com/yacysxar).
This year, due to the virtual nature, prioritization matrix was utilized and based on urgency. Resolutions were divided into top priority, priority, medium priority, low priority, and not a priority.
The following reference committees convened at this Special Meeting Constitution & Bylaws, Medical Service, Legislation Medical Education, Public Health, Science and Technology, Finance and Medical Practice.
Some of the issues discussed at the House of Delegates are as follows:
Medical education
Continuing board certification (Adapted as a new policy)
The policy states that American Medical Association (AMA), through its Council on Medical Education, continue to work with the American Board of Medical Specialties (ABMS) and ABMS member boards to implement key recommendations outlined by the Continuing Board Certification: Vision for the Future Commission in its final report, including the development of new, integrated standards for continuing certification programs by 2020 that will address the Commission’s recommendations for flexibility in knowledge assessment and advancing practice, feedback to diplomates, and consistency.
Graduate medical education and the corporate practice of medicine (modified existing policy)
The existing policy was amended to urge AMA to continue to monitor issues, including waiver of due process requirements, created by corporate-owned graduate medical education sites.
Public health
Bullying in the Practice of Medicine
Health-care organizations, including academic medical centers, should establish policies to prevent and address bullying in their workplaces. An effective workplace policy should:
• Describe the management’s commitment to providing a safe and healthy workplace.
• Show the staff that their leaders are concerned about bullying and unprofessional behavior and that they take it seriously.
• Clearly define workplace violence, harassment, and bullying, specifically including intimidation, threats, and other forms of aggressive behavior.
• Specify to whom the policy applies (ie, medical staff, students, administration, patients, employees, contractors, vendors, etc).
• Define both expected and prohibited behaviors.
• Outline steps for individuals to take when they feel they are a victim of workplace bullying.
• Provide contact information for a confidential means for documenting and reporting incidents.
• Prohibit retaliation and ensure privacy and confidentiality.
• Document training requirements and establish clear expectations about the training objectives.
Availability of personal protective equipment (PPE)
That our American Medical Association actively support that physicians and health-care professionals are empowered to use workplace modifications to continue professional patient care when they determine such action to be appropriate and in the best interest of patient and physician wellbeing. Physicians and health-care professionals must be permitted to use their professional judgment and augment institution-provided PPE with additional, appropriately decontaminated, personally provided personal protective equipment (PPE) without penalty (Directive to Take Action); and be it further that AMA affirm that the medical staff of each healt-care institution should integrally be involved in disaster planning, strategy, and tactical management of ongoing crises (New HOD Policy).
AMA governance and finance
The establishment of private practice physicians’ section was approved.
Medical practice
Merit-based incentive payment system (MIPS)
That our American Medical Association (AMA) support legislation that ensures Medicare physician payment is sufficient to safeguard beneficiary access to care, replaces or supplements budget neutrality in MIPS with incentive payments, or implements positive annual physician payment updates. (Directive to Take Action).
Establishing professional services claims-based payment enhancement for activities associated with the COVID-19 pandemic
American Medical Association work with other interested parties to advocate for regulatory action on the part of the Centers for Medicare & Medicaid Services to implement a professional services claims-based payment enhancement to help recognize the enhanced, nonseparately reimbursable work performed by physicians during the COVID-19 Public Health Emergency. (Directive to Take Action).
This is just a small sampling of the activities and more information, including reports from the various Councils, are available on the AMA website, http://ama-assn.org.
CHEST members interested in the AMA policy-making process may observe any AMA-HOD meeting or participate in the AMA’s democratic processes. Attendees will also be able to increase their knowledge and skills at no cost. They will also be able to connect with more than 1,500 peers and other meeting attendees from across the country. CHEST members with the time (there are two 5-day meetings each year) and interest are invited to apply to be an official CHEST delegate to the AMA. Contact Jennifer Nemkovich at [email protected] for details.
Dr. Desai is with the Chicago Chest Center and AMITA Health Suburban Lung Associates; and the Division of Pulmonary, Critical Care, Sleep and Allergy, University of Illinois at Chicago. He is also the CHEST Delegate to the AMA House of Delegates.
The American Medical Association (AMA) had its November 2020 AMA Special Meeting of the AMA House of Delegates (HOD) from November 13-17.
Delegates from over 170 societies (state societies, specialties, subspecialties, and uniformed services), including physicians, residents, and students, gathered virtually for the meeting(https://tinyurl.com/y7494mwa) to consider a wide array of proposals to help fulfill the AMA’s core mission of promoting medicine and improving public health. The AMA House of Delegates, also known as the “House” or the “HOD,” is the principal policy-making body of the AMA. This democratic forum represents the views and interests of a diverse group of member physicians from more than 170 societies. These delegates meet twice per year to establish policies on health; medical, professional, and governance matters; and the principles within which the AMA’s business activities are conducted.
During the COVID-19 pandemic, the AMA has been the leading physician and patient ally—voicing recommendations to key Congressional leaders and agency staff, state policymakers, and private sector stakeholders. Acting on both federal and state levels, examples of AMA’s recent efforts include actions in financial relief, telehealth, testing and vaccine development, health equity, and more.
CHEST is an active member, and through the HOD and Specialty and Service Society (SSS), CHEST can partner with AMA other societies to support each other on important regulatory issues. CHEST/Allergy Section Council (participants at this meeting were from the AAAAI, AAOA, AASM, ACAAI, ATS, CHEST, and SCCM) met before voting in the House to discuss pending business. The meeting was hosted by the current CHEST/Allergy council chair Dr. Wesley Vander Ark (AMA Delegate AAOA) and Jami Lucas, CEO AAOA.
Policy and resolutions
Overview of the process
Policies originate via resolutions submitted by individuals or societies. These resolutions then go to one of several Reference Committees for open discussion. These committees then report their recommendations back to the HOD, which then discusses and votes on the recommendations. In some instances, the question is referred for further studies by one of several Councils, which reports go to the Board of Trustees or back to the House. Details can be found in the April 2018 CHEST Physician® article on the process. (https://tinyurl.com/yacysxar).
This year, due to the virtual nature, prioritization matrix was utilized and based on urgency. Resolutions were divided into top priority, priority, medium priority, low priority, and not a priority.
The following reference committees convened at this Special Meeting Constitution & Bylaws, Medical Service, Legislation Medical Education, Public Health, Science and Technology, Finance and Medical Practice.
Some of the issues discussed at the House of Delegates are as follows:
Medical education
Continuing board certification (Adapted as a new policy)
The policy states that American Medical Association (AMA), through its Council on Medical Education, continue to work with the American Board of Medical Specialties (ABMS) and ABMS member boards to implement key recommendations outlined by the Continuing Board Certification: Vision for the Future Commission in its final report, including the development of new, integrated standards for continuing certification programs by 2020 that will address the Commission’s recommendations for flexibility in knowledge assessment and advancing practice, feedback to diplomates, and consistency.
Graduate medical education and the corporate practice of medicine (modified existing policy)
The existing policy was amended to urge AMA to continue to monitor issues, including waiver of due process requirements, created by corporate-owned graduate medical education sites.
Public health
Bullying in the Practice of Medicine
Health-care organizations, including academic medical centers, should establish policies to prevent and address bullying in their workplaces. An effective workplace policy should:
• Describe the management’s commitment to providing a safe and healthy workplace.
• Show the staff that their leaders are concerned about bullying and unprofessional behavior and that they take it seriously.
• Clearly define workplace violence, harassment, and bullying, specifically including intimidation, threats, and other forms of aggressive behavior.
• Specify to whom the policy applies (ie, medical staff, students, administration, patients, employees, contractors, vendors, etc).
• Define both expected and prohibited behaviors.
• Outline steps for individuals to take when they feel they are a victim of workplace bullying.
• Provide contact information for a confidential means for documenting and reporting incidents.
• Prohibit retaliation and ensure privacy and confidentiality.
• Document training requirements and establish clear expectations about the training objectives.
Availability of personal protective equipment (PPE)
That our American Medical Association actively support that physicians and health-care professionals are empowered to use workplace modifications to continue professional patient care when they determine such action to be appropriate and in the best interest of patient and physician wellbeing. Physicians and health-care professionals must be permitted to use their professional judgment and augment institution-provided PPE with additional, appropriately decontaminated, personally provided personal protective equipment (PPE) without penalty (Directive to Take Action); and be it further that AMA affirm that the medical staff of each healt-care institution should integrally be involved in disaster planning, strategy, and tactical management of ongoing crises (New HOD Policy).
AMA governance and finance
The establishment of private practice physicians’ section was approved.
Medical practice
Merit-based incentive payment system (MIPS)
That our American Medical Association (AMA) support legislation that ensures Medicare physician payment is sufficient to safeguard beneficiary access to care, replaces or supplements budget neutrality in MIPS with incentive payments, or implements positive annual physician payment updates. (Directive to Take Action).
Establishing professional services claims-based payment enhancement for activities associated with the COVID-19 pandemic
American Medical Association work with other interested parties to advocate for regulatory action on the part of the Centers for Medicare & Medicaid Services to implement a professional services claims-based payment enhancement to help recognize the enhanced, nonseparately reimbursable work performed by physicians during the COVID-19 Public Health Emergency. (Directive to Take Action).
This is just a small sampling of the activities and more information, including reports from the various Councils, are available on the AMA website, http://ama-assn.org.
CHEST members interested in the AMA policy-making process may observe any AMA-HOD meeting or participate in the AMA’s democratic processes. Attendees will also be able to increase their knowledge and skills at no cost. They will also be able to connect with more than 1,500 peers and other meeting attendees from across the country. CHEST members with the time (there are two 5-day meetings each year) and interest are invited to apply to be an official CHEST delegate to the AMA. Contact Jennifer Nemkovich at [email protected] for details.
Dr. Desai is with the Chicago Chest Center and AMITA Health Suburban Lung Associates; and the Division of Pulmonary, Critical Care, Sleep and Allergy, University of Illinois at Chicago. He is also the CHEST Delegate to the AMA House of Delegates.
The American Medical Association (AMA) had its November 2020 AMA Special Meeting of the AMA House of Delegates (HOD) from November 13-17.
Delegates from over 170 societies (state societies, specialties, subspecialties, and uniformed services), including physicians, residents, and students, gathered virtually for the meeting(https://tinyurl.com/y7494mwa) to consider a wide array of proposals to help fulfill the AMA’s core mission of promoting medicine and improving public health. The AMA House of Delegates, also known as the “House” or the “HOD,” is the principal policy-making body of the AMA. This democratic forum represents the views and interests of a diverse group of member physicians from more than 170 societies. These delegates meet twice per year to establish policies on health; medical, professional, and governance matters; and the principles within which the AMA’s business activities are conducted.
During the COVID-19 pandemic, the AMA has been the leading physician and patient ally—voicing recommendations to key Congressional leaders and agency staff, state policymakers, and private sector stakeholders. Acting on both federal and state levels, examples of AMA’s recent efforts include actions in financial relief, telehealth, testing and vaccine development, health equity, and more.
CHEST is an active member, and through the HOD and Specialty and Service Society (SSS), CHEST can partner with AMA other societies to support each other on important regulatory issues. CHEST/Allergy Section Council (participants at this meeting were from the AAAAI, AAOA, AASM, ACAAI, ATS, CHEST, and SCCM) met before voting in the House to discuss pending business. The meeting was hosted by the current CHEST/Allergy council chair Dr. Wesley Vander Ark (AMA Delegate AAOA) and Jami Lucas, CEO AAOA.
Policy and resolutions
Overview of the process
Policies originate via resolutions submitted by individuals or societies. These resolutions then go to one of several Reference Committees for open discussion. These committees then report their recommendations back to the HOD, which then discusses and votes on the recommendations. In some instances, the question is referred for further studies by one of several Councils, which reports go to the Board of Trustees or back to the House. Details can be found in the April 2018 CHEST Physician® article on the process. (https://tinyurl.com/yacysxar).
This year, due to the virtual nature, prioritization matrix was utilized and based on urgency. Resolutions were divided into top priority, priority, medium priority, low priority, and not a priority.
The following reference committees convened at this Special Meeting Constitution & Bylaws, Medical Service, Legislation Medical Education, Public Health, Science and Technology, Finance and Medical Practice.
Some of the issues discussed at the House of Delegates are as follows:
Medical education
Continuing board certification (Adapted as a new policy)
The policy states that American Medical Association (AMA), through its Council on Medical Education, continue to work with the American Board of Medical Specialties (ABMS) and ABMS member boards to implement key recommendations outlined by the Continuing Board Certification: Vision for the Future Commission in its final report, including the development of new, integrated standards for continuing certification programs by 2020 that will address the Commission’s recommendations for flexibility in knowledge assessment and advancing practice, feedback to diplomates, and consistency.
Graduate medical education and the corporate practice of medicine (modified existing policy)
The existing policy was amended to urge AMA to continue to monitor issues, including waiver of due process requirements, created by corporate-owned graduate medical education sites.
Public health
Bullying in the Practice of Medicine
Health-care organizations, including academic medical centers, should establish policies to prevent and address bullying in their workplaces. An effective workplace policy should:
• Describe the management’s commitment to providing a safe and healthy workplace.
• Show the staff that their leaders are concerned about bullying and unprofessional behavior and that they take it seriously.
• Clearly define workplace violence, harassment, and bullying, specifically including intimidation, threats, and other forms of aggressive behavior.
• Specify to whom the policy applies (ie, medical staff, students, administration, patients, employees, contractors, vendors, etc).
• Define both expected and prohibited behaviors.
• Outline steps for individuals to take when they feel they are a victim of workplace bullying.
• Provide contact information for a confidential means for documenting and reporting incidents.
• Prohibit retaliation and ensure privacy and confidentiality.
• Document training requirements and establish clear expectations about the training objectives.
Availability of personal protective equipment (PPE)
That our American Medical Association actively support that physicians and health-care professionals are empowered to use workplace modifications to continue professional patient care when they determine such action to be appropriate and in the best interest of patient and physician wellbeing. Physicians and health-care professionals must be permitted to use their professional judgment and augment institution-provided PPE with additional, appropriately decontaminated, personally provided personal protective equipment (PPE) without penalty (Directive to Take Action); and be it further that AMA affirm that the medical staff of each healt-care institution should integrally be involved in disaster planning, strategy, and tactical management of ongoing crises (New HOD Policy).
AMA governance and finance
The establishment of private practice physicians’ section was approved.
Medical practice
Merit-based incentive payment system (MIPS)
That our American Medical Association (AMA) support legislation that ensures Medicare physician payment is sufficient to safeguard beneficiary access to care, replaces or supplements budget neutrality in MIPS with incentive payments, or implements positive annual physician payment updates. (Directive to Take Action).
Establishing professional services claims-based payment enhancement for activities associated with the COVID-19 pandemic
American Medical Association work with other interested parties to advocate for regulatory action on the part of the Centers for Medicare & Medicaid Services to implement a professional services claims-based payment enhancement to help recognize the enhanced, nonseparately reimbursable work performed by physicians during the COVID-19 Public Health Emergency. (Directive to Take Action).
This is just a small sampling of the activities and more information, including reports from the various Councils, are available on the AMA website, http://ama-assn.org.
CHEST members interested in the AMA policy-making process may observe any AMA-HOD meeting or participate in the AMA’s democratic processes. Attendees will also be able to increase their knowledge and skills at no cost. They will also be able to connect with more than 1,500 peers and other meeting attendees from across the country. CHEST members with the time (there are two 5-day meetings each year) and interest are invited to apply to be an official CHEST delegate to the AMA. Contact Jennifer Nemkovich at [email protected] for details.
Dr. Desai is with the Chicago Chest Center and AMITA Health Suburban Lung Associates; and the Division of Pulmonary, Critical Care, Sleep and Allergy, University of Illinois at Chicago. He is also the CHEST Delegate to the AMA House of Delegates.
Meet the new members of the CHEST Physician® Editorial Board
We’re happy to introduce these new board members whose primary responsibility is the active review each month of potential articles for publication that could have an impact on or be of interest to our health-care professional readership.
Carolyn M. D’Ambrosio, MD, FCCP, is the Program Director for the Harvard-Brigham and Women’s Hospital Fellowship in Pulmonary and Critical Care Medicine and is Associate Professor of Medicine at Harvard Medical School. Most recently, she was awarded the Pillar Award for Educational Program Leadership, the top award for program directors throughout the Mass General Brigham institutions. In addition to teaching and clinical work, Dr. D’Ambrosio has conducted research on sleep and menopause, sleep and breathing in infants, and participated as the sleep medicine expert in two systemic reviews on home sleep apnea testing and fixed vs auto-titrating CPAP. She continues her work in Medical Ethics as a Senior Ethics Consultant at Brigham and Women’s Hospital.
Jonathan (Jona) Ludmir, MD, FCCP
After completing internal medicine/pediatrics, cardiology, and critical care training, Dr. Ludmir joined the Massachusetts General Hospital staff as a cardiac intensivist and noninvasive cardiologist. His clinical focus is in the heart center ICU, the echocardiography lab, as well as in outpatient cardiology. Additionally, he is the lead physician for the Family-Centered Care Initiative, where he focuses on incorporating evidence-based guidelines and leads in the science of family-centered cardiovascular care delivery. Dr. Ludmir’s research focuses on identifying and addressing psychological symptoms in the ICU, optimizing ICU communication, and enhancing delivery of family-centered care.
Abbie Begnaud, MD, FCCP
Dr. Begnaud hails from south Louisiana and reveals that she attended her first CHEST Annual Meeting in 2011 in Hawaii, and she was “instantly hooked.” Clinically, she practices general pulmonology, critical care, and interventional pulmonology and focuses her research on lung cancer screening and health disparities. She has been on faculty at the University of Minnesota since 2013 and is passionate about lung cancer, health equity, and mentoring.
Shyam Subramanian, MD , FCCP
Dr. Subramanian is currently the Section Chief for Specialty Clinics and the Division Chief for Pulmonary/Critical Care and Sleep Medicine at Sutter Gould Medical Foundation, Tracy, California.
He previously was Systems Director at Baylor College of Medicine in Houston and Section Chief at Case Western Reserve University in Cleveland. Dr. Subramanian currently serves as Chair for the CHEST Clinical Pulmonary NetWork and has previously served as Chair of the Practice Operations NetWork. He is a member of the Executive Committee of the Council of NetWorks and the Scientific Program Committee for the CHEST Annual Meeting.
Mary Jo S. Farmer, MD, PhD, FCCP
Dr. Farmer is a pulmonary, critical care, and sleep medicine physician at Baystate Medical Center (Springfield, MA); Assistant Professor of Medicine University at Massachusetts Medical School – Baystate; and adjunct faculty Tufts University School of Medicine. Dr. Farmer serves as director of pulmonary hypertension services for the Pulmonary & Critical Care Division. Pulmonary vascular disease, interprofessional education, clinical trials research, endobronchial ultrasound, and medical student, resident, and fellow education are her major interests. She is a member of the CHEST Interprofessional NetWork and Clinical Pulmonary NetWork.
We’re happy to introduce these new board members whose primary responsibility is the active review each month of potential articles for publication that could have an impact on or be of interest to our health-care professional readership.
Carolyn M. D’Ambrosio, MD, FCCP, is the Program Director for the Harvard-Brigham and Women’s Hospital Fellowship in Pulmonary and Critical Care Medicine and is Associate Professor of Medicine at Harvard Medical School. Most recently, she was awarded the Pillar Award for Educational Program Leadership, the top award for program directors throughout the Mass General Brigham institutions. In addition to teaching and clinical work, Dr. D’Ambrosio has conducted research on sleep and menopause, sleep and breathing in infants, and participated as the sleep medicine expert in two systemic reviews on home sleep apnea testing and fixed vs auto-titrating CPAP. She continues her work in Medical Ethics as a Senior Ethics Consultant at Brigham and Women’s Hospital.
Jonathan (Jona) Ludmir, MD, FCCP
After completing internal medicine/pediatrics, cardiology, and critical care training, Dr. Ludmir joined the Massachusetts General Hospital staff as a cardiac intensivist and noninvasive cardiologist. His clinical focus is in the heart center ICU, the echocardiography lab, as well as in outpatient cardiology. Additionally, he is the lead physician for the Family-Centered Care Initiative, where he focuses on incorporating evidence-based guidelines and leads in the science of family-centered cardiovascular care delivery. Dr. Ludmir’s research focuses on identifying and addressing psychological symptoms in the ICU, optimizing ICU communication, and enhancing delivery of family-centered care.
Abbie Begnaud, MD, FCCP
Dr. Begnaud hails from south Louisiana and reveals that she attended her first CHEST Annual Meeting in 2011 in Hawaii, and she was “instantly hooked.” Clinically, she practices general pulmonology, critical care, and interventional pulmonology and focuses her research on lung cancer screening and health disparities. She has been on faculty at the University of Minnesota since 2013 and is passionate about lung cancer, health equity, and mentoring.
Shyam Subramanian, MD , FCCP
Dr. Subramanian is currently the Section Chief for Specialty Clinics and the Division Chief for Pulmonary/Critical Care and Sleep Medicine at Sutter Gould Medical Foundation, Tracy, California.
He previously was Systems Director at Baylor College of Medicine in Houston and Section Chief at Case Western Reserve University in Cleveland. Dr. Subramanian currently serves as Chair for the CHEST Clinical Pulmonary NetWork and has previously served as Chair of the Practice Operations NetWork. He is a member of the Executive Committee of the Council of NetWorks and the Scientific Program Committee for the CHEST Annual Meeting.
Mary Jo S. Farmer, MD, PhD, FCCP
Dr. Farmer is a pulmonary, critical care, and sleep medicine physician at Baystate Medical Center (Springfield, MA); Assistant Professor of Medicine University at Massachusetts Medical School – Baystate; and adjunct faculty Tufts University School of Medicine. Dr. Farmer serves as director of pulmonary hypertension services for the Pulmonary & Critical Care Division. Pulmonary vascular disease, interprofessional education, clinical trials research, endobronchial ultrasound, and medical student, resident, and fellow education are her major interests. She is a member of the CHEST Interprofessional NetWork and Clinical Pulmonary NetWork.
We’re happy to introduce these new board members whose primary responsibility is the active review each month of potential articles for publication that could have an impact on or be of interest to our health-care professional readership.
Carolyn M. D’Ambrosio, MD, FCCP, is the Program Director for the Harvard-Brigham and Women’s Hospital Fellowship in Pulmonary and Critical Care Medicine and is Associate Professor of Medicine at Harvard Medical School. Most recently, she was awarded the Pillar Award for Educational Program Leadership, the top award for program directors throughout the Mass General Brigham institutions. In addition to teaching and clinical work, Dr. D’Ambrosio has conducted research on sleep and menopause, sleep and breathing in infants, and participated as the sleep medicine expert in two systemic reviews on home sleep apnea testing and fixed vs auto-titrating CPAP. She continues her work in Medical Ethics as a Senior Ethics Consultant at Brigham and Women’s Hospital.
Jonathan (Jona) Ludmir, MD, FCCP
After completing internal medicine/pediatrics, cardiology, and critical care training, Dr. Ludmir joined the Massachusetts General Hospital staff as a cardiac intensivist and noninvasive cardiologist. His clinical focus is in the heart center ICU, the echocardiography lab, as well as in outpatient cardiology. Additionally, he is the lead physician for the Family-Centered Care Initiative, where he focuses on incorporating evidence-based guidelines and leads in the science of family-centered cardiovascular care delivery. Dr. Ludmir’s research focuses on identifying and addressing psychological symptoms in the ICU, optimizing ICU communication, and enhancing delivery of family-centered care.
Abbie Begnaud, MD, FCCP
Dr. Begnaud hails from south Louisiana and reveals that she attended her first CHEST Annual Meeting in 2011 in Hawaii, and she was “instantly hooked.” Clinically, she practices general pulmonology, critical care, and interventional pulmonology and focuses her research on lung cancer screening and health disparities. She has been on faculty at the University of Minnesota since 2013 and is passionate about lung cancer, health equity, and mentoring.
Shyam Subramanian, MD , FCCP
Dr. Subramanian is currently the Section Chief for Specialty Clinics and the Division Chief for Pulmonary/Critical Care and Sleep Medicine at Sutter Gould Medical Foundation, Tracy, California.
He previously was Systems Director at Baylor College of Medicine in Houston and Section Chief at Case Western Reserve University in Cleveland. Dr. Subramanian currently serves as Chair for the CHEST Clinical Pulmonary NetWork and has previously served as Chair of the Practice Operations NetWork. He is a member of the Executive Committee of the Council of NetWorks and the Scientific Program Committee for the CHEST Annual Meeting.
Mary Jo S. Farmer, MD, PhD, FCCP
Dr. Farmer is a pulmonary, critical care, and sleep medicine physician at Baystate Medical Center (Springfield, MA); Assistant Professor of Medicine University at Massachusetts Medical School – Baystate; and adjunct faculty Tufts University School of Medicine. Dr. Farmer serves as director of pulmonary hypertension services for the Pulmonary & Critical Care Division. Pulmonary vascular disease, interprofessional education, clinical trials research, endobronchial ultrasound, and medical student, resident, and fellow education are her major interests. She is a member of the CHEST Interprofessional NetWork and Clinical Pulmonary NetWork.
How the Foundation’s virtual listening tour aims to help patients like James
Constance Baker was juggling the dual stresses of mothering a newborn and raising a teenager when she noticed a skin patch on her father looked discolored. His breathing soon became labored, and the skin on his hands turned calloused. Then he passed out. Initially, doctors thought his problems were cardiovascular.
Since James didn’t have a primary doctor, Constance repeatedly took him to the emergency room to receive care. His frequent visits attracted the attention of a medical intern who ordered tests and asked James to see a specialist. More than half a year later, Constance and James met pulmonologist Dr. Demondes Haynes and learned the cause of James’ troubled breathing. James has a rare disease called scleroderma, which hardens patches of skin and created scarring of his lung tissue. He also had pulmonary hypertension. James needed rapid intervention with a complicated regimen of medication.
At first, James didn’t want to go along with the program, but Dr. Haynes’ attentive and gentle nature changed his mind. “Dr. Haynes always made us comfortable, taking the time to listen and show us his concern. He even explained that we wouldn’t have to worry about paying for anything, which was a huge relief.”
Before Dr. Haynes, James and Constance had never met a doctor who didn’t treat them like a case file. “He actually acknowledged our circumstances, which meant he acknowledged us.”
As a native Mississippian, Dr. Haynes knows the plight of many of his patients. “Not everyone with lung disease can access a pulmonologist, like me, and not everyone can afford appropriate treatment. You have to recognize these disparities in order to build a relationship of trust with your patients.”
James was ready to start treatment with Dr. Haynes’ guidance, but since he couldn’t read, he couldn’t understand how to put the medication together. That’s when Constance had to step up. They worked together to change and clean the tubing to the port by his heart and make his medication. “We leaned on each other a lot during that time, and you know what? We made it through.”
Even though James’ disease can be debilitating at times, and his care can seem completely overwhelming, Constance wouldn’t have it any other way. “It’s always been my father and I, just us two. He’s always taken care of me, and now it’s my turn to take care of him.”
Unfortunately, Constance and James’ story is not unique. So many patients don’t have access to doctors, specialists, and caregivers, and many aren’t empowered enough to take
their medications. These stories don’t get posted on Instagram and they don’t make the evening news. Underprivileged and underserved patients have been left behind – left without a voice.
That’s why the foundation launched its virtual listening tours across America in September. Our tours give patients, caregivers, and physicians the opportunity to raise issues that they believe are impacting health care in their communities.
How can physicians work to understand their patients better? How can patients learn to trust their providers? These are all the questions we aim to answer.
James is doing as well as he is because of his relationship with Dr. Haynes. What can we do with that information? We can listen, we can learn, and we can spread the word.
Read more about the work of the CHEST Foundation in its 2020 Impact Report at chestfoundation.org.
Constance Baker was juggling the dual stresses of mothering a newborn and raising a teenager when she noticed a skin patch on her father looked discolored. His breathing soon became labored, and the skin on his hands turned calloused. Then he passed out. Initially, doctors thought his problems were cardiovascular.
Since James didn’t have a primary doctor, Constance repeatedly took him to the emergency room to receive care. His frequent visits attracted the attention of a medical intern who ordered tests and asked James to see a specialist. More than half a year later, Constance and James met pulmonologist Dr. Demondes Haynes and learned the cause of James’ troubled breathing. James has a rare disease called scleroderma, which hardens patches of skin and created scarring of his lung tissue. He also had pulmonary hypertension. James needed rapid intervention with a complicated regimen of medication.
At first, James didn’t want to go along with the program, but Dr. Haynes’ attentive and gentle nature changed his mind. “Dr. Haynes always made us comfortable, taking the time to listen and show us his concern. He even explained that we wouldn’t have to worry about paying for anything, which was a huge relief.”
Before Dr. Haynes, James and Constance had never met a doctor who didn’t treat them like a case file. “He actually acknowledged our circumstances, which meant he acknowledged us.”
As a native Mississippian, Dr. Haynes knows the plight of many of his patients. “Not everyone with lung disease can access a pulmonologist, like me, and not everyone can afford appropriate treatment. You have to recognize these disparities in order to build a relationship of trust with your patients.”
James was ready to start treatment with Dr. Haynes’ guidance, but since he couldn’t read, he couldn’t understand how to put the medication together. That’s when Constance had to step up. They worked together to change and clean the tubing to the port by his heart and make his medication. “We leaned on each other a lot during that time, and you know what? We made it through.”
Even though James’ disease can be debilitating at times, and his care can seem completely overwhelming, Constance wouldn’t have it any other way. “It’s always been my father and I, just us two. He’s always taken care of me, and now it’s my turn to take care of him.”
Unfortunately, Constance and James’ story is not unique. So many patients don’t have access to doctors, specialists, and caregivers, and many aren’t empowered enough to take
their medications. These stories don’t get posted on Instagram and they don’t make the evening news. Underprivileged and underserved patients have been left behind – left without a voice.
That’s why the foundation launched its virtual listening tours across America in September. Our tours give patients, caregivers, and physicians the opportunity to raise issues that they believe are impacting health care in their communities.
How can physicians work to understand their patients better? How can patients learn to trust their providers? These are all the questions we aim to answer.
James is doing as well as he is because of his relationship with Dr. Haynes. What can we do with that information? We can listen, we can learn, and we can spread the word.
Read more about the work of the CHEST Foundation in its 2020 Impact Report at chestfoundation.org.
Constance Baker was juggling the dual stresses of mothering a newborn and raising a teenager when she noticed a skin patch on her father looked discolored. His breathing soon became labored, and the skin on his hands turned calloused. Then he passed out. Initially, doctors thought his problems were cardiovascular.
Since James didn’t have a primary doctor, Constance repeatedly took him to the emergency room to receive care. His frequent visits attracted the attention of a medical intern who ordered tests and asked James to see a specialist. More than half a year later, Constance and James met pulmonologist Dr. Demondes Haynes and learned the cause of James’ troubled breathing. James has a rare disease called scleroderma, which hardens patches of skin and created scarring of his lung tissue. He also had pulmonary hypertension. James needed rapid intervention with a complicated regimen of medication.
At first, James didn’t want to go along with the program, but Dr. Haynes’ attentive and gentle nature changed his mind. “Dr. Haynes always made us comfortable, taking the time to listen and show us his concern. He even explained that we wouldn’t have to worry about paying for anything, which was a huge relief.”
Before Dr. Haynes, James and Constance had never met a doctor who didn’t treat them like a case file. “He actually acknowledged our circumstances, which meant he acknowledged us.”
As a native Mississippian, Dr. Haynes knows the plight of many of his patients. “Not everyone with lung disease can access a pulmonologist, like me, and not everyone can afford appropriate treatment. You have to recognize these disparities in order to build a relationship of trust with your patients.”
James was ready to start treatment with Dr. Haynes’ guidance, but since he couldn’t read, he couldn’t understand how to put the medication together. That’s when Constance had to step up. They worked together to change and clean the tubing to the port by his heart and make his medication. “We leaned on each other a lot during that time, and you know what? We made it through.”
Even though James’ disease can be debilitating at times, and his care can seem completely overwhelming, Constance wouldn’t have it any other way. “It’s always been my father and I, just us two. He’s always taken care of me, and now it’s my turn to take care of him.”
Unfortunately, Constance and James’ story is not unique. So many patients don’t have access to doctors, specialists, and caregivers, and many aren’t empowered enough to take
their medications. These stories don’t get posted on Instagram and they don’t make the evening news. Underprivileged and underserved patients have been left behind – left without a voice.
That’s why the foundation launched its virtual listening tours across America in September. Our tours give patients, caregivers, and physicians the opportunity to raise issues that they believe are impacting health care in their communities.
How can physicians work to understand their patients better? How can patients learn to trust their providers? These are all the questions we aim to answer.
James is doing as well as he is because of his relationship with Dr. Haynes. What can we do with that information? We can listen, we can learn, and we can spread the word.
Read more about the work of the CHEST Foundation in its 2020 Impact Report at chestfoundation.org.








