User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Janssen/J&J COVID-19 vaccine cuts transmission, new data show
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves cemiplimab-rwlc for NSCLC with PD-L1 expression
Specifically, the indication is for first-line treatment as monotherapy for patients with locally advanced or metastatic disease who are not candidates for surgical resection or definitive chemoradiotherapy and whose tumors have a high expression of programmed death–ligand 1 (PD-L1) (Tumor Proportion Score >50%), as determined by an FDA-approved test, with no EGFR, ALK, or ROS1 aberrations.
This is the third indication for cemiplimab-rlwc, a monoclonal antibody and PD-1 inhibitor.
In February, it was approved as the first immunotherapy to treat patients with locally advanced or metastatic basal cell carcinoma that was previously treated with a hedgehog pathway inhibitor or for whom a hedgehog inhibitor is inappropriate.
Cemiplimab-rlwc previously received FDA approval in 2018 for locally advanced or metastatic cutaneous squamous cell carcinoma for patients who were not eligible for curative surgery or radiotherapy. At the time, Karl Lewis, MD, a professor at the University of Colorado at Denver, Aurora, and a trial investigator, predicted that the drug “will change the treatment paradigm for patients with advanced basal cell carcinoma.”
Outperforms chemotherapy
The approval for use in NSCLC is based on results from the phase 3, open-label EMPOWER-Lung 1 trial, which randomly assigned 710 patients in a 1:1 ratio to receive either cemiplimab-rwlc or platinum-doublet chemotherapy. Patients had either locally advanced NSCLC and were not candidates for surgical resection or definitive chemoradiotherapy, or they had metastatic NSCLC.
Patients in the experimental arm received cemiplimab-rwlc 350 mg intravenously every 3 weeks. The primary efficacy outcome measures were overall survival (OS) and progression-free survival (PFS), determined on the basis of blinded independent central review.
Results showed statistically significant improvements in both outcomes. Median OS was 22.1 months with cemiplimab-rwlc versus 14.3 months with chemotherapy (hazard ratio, 0.68; P = .0022). Median PFS was 6.2 months versus 5.6 months (HR, 0.59; P < .0001).
The confirmed overall response rate was 37% for the cemiplimab arm versus 21% for the chemotherapy arm.
The most common adverse reactions (>10%) with cemiplimab-rlwc were musculoskeletal pain, rash, anemia, fatigue, decreased appetite, pneumonia, and cough.
This approval “means physicians and patients have a potent new treatment option against this deadly disease,” said Naiyer Rizvi, MD, Price Family Professor of Medicine, director of thoracic oncology, and codirector of cancer immunotherapy at Columbia University Irving Medical Center, New York, in a statement. He was a steering committee member on the EMPOWER-Lung-1 Trial.
“Notably, Libtayo was approved based on a pivotal trial where most chemotherapy patients crossed over to Libtayo following disease progression, and that allowed for frequently underrepresented patients who had pretreated and clinically stable brain metastases or who had locally advanced disease and were not candidates for definitive chemoradiation,” said Dr. Rizvi. “This gives doctors important new data when considering Libtayo for the varied patients and situations they treat in daily clinical practice.”
A version of this article first appeared on Medscape.com.
Specifically, the indication is for first-line treatment as monotherapy for patients with locally advanced or metastatic disease who are not candidates for surgical resection or definitive chemoradiotherapy and whose tumors have a high expression of programmed death–ligand 1 (PD-L1) (Tumor Proportion Score >50%), as determined by an FDA-approved test, with no EGFR, ALK, or ROS1 aberrations.
This is the third indication for cemiplimab-rlwc, a monoclonal antibody and PD-1 inhibitor.
In February, it was approved as the first immunotherapy to treat patients with locally advanced or metastatic basal cell carcinoma that was previously treated with a hedgehog pathway inhibitor or for whom a hedgehog inhibitor is inappropriate.
Cemiplimab-rlwc previously received FDA approval in 2018 for locally advanced or metastatic cutaneous squamous cell carcinoma for patients who were not eligible for curative surgery or radiotherapy. At the time, Karl Lewis, MD, a professor at the University of Colorado at Denver, Aurora, and a trial investigator, predicted that the drug “will change the treatment paradigm for patients with advanced basal cell carcinoma.”
Outperforms chemotherapy
The approval for use in NSCLC is based on results from the phase 3, open-label EMPOWER-Lung 1 trial, which randomly assigned 710 patients in a 1:1 ratio to receive either cemiplimab-rwlc or platinum-doublet chemotherapy. Patients had either locally advanced NSCLC and were not candidates for surgical resection or definitive chemoradiotherapy, or they had metastatic NSCLC.
Patients in the experimental arm received cemiplimab-rwlc 350 mg intravenously every 3 weeks. The primary efficacy outcome measures were overall survival (OS) and progression-free survival (PFS), determined on the basis of blinded independent central review.
Results showed statistically significant improvements in both outcomes. Median OS was 22.1 months with cemiplimab-rwlc versus 14.3 months with chemotherapy (hazard ratio, 0.68; P = .0022). Median PFS was 6.2 months versus 5.6 months (HR, 0.59; P < .0001).
The confirmed overall response rate was 37% for the cemiplimab arm versus 21% for the chemotherapy arm.
The most common adverse reactions (>10%) with cemiplimab-rlwc were musculoskeletal pain, rash, anemia, fatigue, decreased appetite, pneumonia, and cough.
This approval “means physicians and patients have a potent new treatment option against this deadly disease,” said Naiyer Rizvi, MD, Price Family Professor of Medicine, director of thoracic oncology, and codirector of cancer immunotherapy at Columbia University Irving Medical Center, New York, in a statement. He was a steering committee member on the EMPOWER-Lung-1 Trial.
“Notably, Libtayo was approved based on a pivotal trial where most chemotherapy patients crossed over to Libtayo following disease progression, and that allowed for frequently underrepresented patients who had pretreated and clinically stable brain metastases or who had locally advanced disease and were not candidates for definitive chemoradiation,” said Dr. Rizvi. “This gives doctors important new data when considering Libtayo for the varied patients and situations they treat in daily clinical practice.”
A version of this article first appeared on Medscape.com.
Specifically, the indication is for first-line treatment as monotherapy for patients with locally advanced or metastatic disease who are not candidates for surgical resection or definitive chemoradiotherapy and whose tumors have a high expression of programmed death–ligand 1 (PD-L1) (Tumor Proportion Score >50%), as determined by an FDA-approved test, with no EGFR, ALK, or ROS1 aberrations.
This is the third indication for cemiplimab-rlwc, a monoclonal antibody and PD-1 inhibitor.
In February, it was approved as the first immunotherapy to treat patients with locally advanced or metastatic basal cell carcinoma that was previously treated with a hedgehog pathway inhibitor or for whom a hedgehog inhibitor is inappropriate.
Cemiplimab-rlwc previously received FDA approval in 2018 for locally advanced or metastatic cutaneous squamous cell carcinoma for patients who were not eligible for curative surgery or radiotherapy. At the time, Karl Lewis, MD, a professor at the University of Colorado at Denver, Aurora, and a trial investigator, predicted that the drug “will change the treatment paradigm for patients with advanced basal cell carcinoma.”
Outperforms chemotherapy
The approval for use in NSCLC is based on results from the phase 3, open-label EMPOWER-Lung 1 trial, which randomly assigned 710 patients in a 1:1 ratio to receive either cemiplimab-rwlc or platinum-doublet chemotherapy. Patients had either locally advanced NSCLC and were not candidates for surgical resection or definitive chemoradiotherapy, or they had metastatic NSCLC.
Patients in the experimental arm received cemiplimab-rwlc 350 mg intravenously every 3 weeks. The primary efficacy outcome measures were overall survival (OS) and progression-free survival (PFS), determined on the basis of blinded independent central review.
Results showed statistically significant improvements in both outcomes. Median OS was 22.1 months with cemiplimab-rwlc versus 14.3 months with chemotherapy (hazard ratio, 0.68; P = .0022). Median PFS was 6.2 months versus 5.6 months (HR, 0.59; P < .0001).
The confirmed overall response rate was 37% for the cemiplimab arm versus 21% for the chemotherapy arm.
The most common adverse reactions (>10%) with cemiplimab-rlwc were musculoskeletal pain, rash, anemia, fatigue, decreased appetite, pneumonia, and cough.
This approval “means physicians and patients have a potent new treatment option against this deadly disease,” said Naiyer Rizvi, MD, Price Family Professor of Medicine, director of thoracic oncology, and codirector of cancer immunotherapy at Columbia University Irving Medical Center, New York, in a statement. He was a steering committee member on the EMPOWER-Lung-1 Trial.
“Notably, Libtayo was approved based on a pivotal trial where most chemotherapy patients crossed over to Libtayo following disease progression, and that allowed for frequently underrepresented patients who had pretreated and clinically stable brain metastases or who had locally advanced disease and were not candidates for definitive chemoradiation,” said Dr. Rizvi. “This gives doctors important new data when considering Libtayo for the varied patients and situations they treat in daily clinical practice.”
A version of this article first appeared on Medscape.com.
Loss of smell lingers post COVID-19
The findings illustrate that olfactory problems are common not only during the acute COVID-19 phase but also “in the long run” and that these problems should be “taken into consideration” when following up these patients, study investigator Johannes Frasnelli, MD, professor, department of anatomy, Université du Québec à Trois-Rivières, said in an interview.
Loss of the sense of smell can affect quality of life because it affects eating and drinking, and may even be dangerous, said Dr. Frasnelli. “If your sense of smell is impaired, you may unknowingly eat spoiled food, or you may not smell smoke or gas in your home,” he said. In addition, Dr. Frasnelli noted that an impaired sense of smell is associated with higher rates of depression. The findings will be presented at the annual meeting of the American Academy of Neurology in April.
‘Striking’ finding
Research shows that about 60% of patients with COVID-19 lose their sense of smell to some degree during the acute phase of the disease. “But we wanted to go further and look at the longer-term effects of loss of smell and taste,” said Dr. Frasnelli.
The analysis included 813 health care workers in the province of Quebec. For all the patients, SARS-CoV-2 infection was confirmed through testing with a nasopharyngeal viral swab.
Participants completed a 64-item online questionnaire that asked about three senses: olfactory; gustatory, which includes tastes such as sweet, sour, bitter, salty, savory and umami; and trigeminal, which includes sensations such as spiciness of hot peppers and “coolness” of mint.
They were asked to rate these on a scale of 0 (no perception) to 10 (very strong perception) before the infection, during the infection, and currently. They were also asked about other symptoms, including fatigue.
Most respondents had been infected in the first wave of the virus in March and April of 2020 and responded to the questionnaire an average of 5 months later.
The vast majority of respondents (84.1%) were women, which Dr. Frasnelli said was not surprising because women predominate in the health care field.
The analysis showed that average smell ratings were 8.98 before infection, 2.85 during the acute phase, and 7.41 when respondents answered the questionnaire. The sense of taste was less affected and recovered faster than did the sense of smell. Results for taste were 9.20 before infection, 3.59 during the acute phase, and 8.05 after COVID-19.
Among 580 respondents who indicated a compromised sense of smell during the acute phase, the average smell rating when answering the questionnaire was 6.89, compared to 9.03 before the infection. More than half (51.2%) reported not regaining full olfactory function.
The fact that the sense of smell had not returned to normal for half the participants so long after being infected is “novel and quite striking,” said Dr. Frasnelli.
However, he noted, this doesn’t necessarily mean all those with a compromised sense of smell “have huge problems.” In some cases, he said, the problem “is more subtle.”
Not a CNS problem?
Respondents also completed a chemosensory dysfunction home test (CD-HT). They were asked to prepare common household food items, such as peanut butter, sugar, salt, and vinegar, in a particular way – for example, to add sugar or salt to water – and provide feedback on how they smell and taste.
For this CD-HT analysis, 18.4% of respondents reported having persistent loss of smell. This, Dr. Frasnelli said, adds to evidence from self-reported responses and suggests that in some cases, the problem is more than senses not returning to normal.
“From the questionnaires, roughly 50% said their sense of smell is still not back to normal, and when we look at the CD home test, we see that almost 20% of subjects indeed have pretty strong impairment of their sense of smell,” he said.
The results showed no sex differences, although Dr. Frasnelli noted that most of the sample were women. “It’s tricky to look at the data with regard to sex because it’s a bit skewed,” he said.
Male respondents were older than female participants, but there was no difference in impairment between age groups. Dr. Frasnelli said this was “quite interesting,” inasmuch as older people usually lose some sense of smell.
The researchers have not yet examined whether the results differ by type of health care worker.
They also have not examined in detail whether infection severity affects the risk for extended olfactory impairment. Although some research suggests that the problem with smell is more common in less severe cases, Dr. Frasnelli noted this could be because loss of smell is not a huge problem for patients battling grave health problems.
As for other symptoms, many respondents reported lingering fatigue; some reported debilitating fatigue, said Dr. Frasnelli. However, he cautioned that this is difficult to interpret, because the participants were health care workers, many of whom returned to work during the pandemic and perhaps had not fully rested.
He also noted that he and his colleagues have not “made the link” between impaired smell and the degree of fatigue.
The COVID-19 virus appears to attack supporting sustentacular cells in the olfactory epithelium, not nerve cells.
“Right now, it seems that the smell problem is not a central nervous system problem but a peripheral problem,” said Dr. Frasnelli. “But we don’t know for sure; it may be that the virus somehow gets into the brain and some symptoms are caused by the effects of the infection on the brain.”
The researchers will extend their research with another questionnaire to assess senses 10-12 months after COVID-19.
Limitations of the study include the subjective nature of the smell and taste ratings and the single time point at which data were collected.
Confirmatory findings
Commenting on the research in an interview, Thomas Hummel, MD, professor, smell and taste clinic, department of otorhinolaryngology, Technische Universität Dresden (Germany), said the new results regarding loss of smell after COVID-19 are “very congruent” with what he and his colleagues have observed.
Research shows that up to one in five of those infected with SARS-CoV-2 experience olfactory loss. “While the numbers may vary a bit from study to study or lab to lab, I think 5% to 20% of post–COVID-19 patients exhibit long-term olfactory loss,” Dr. Hummel said.
His group has observed that “many more are not back to normal,” which conforms with what Dr. Frasnelli’s study reveals, said Dr. Hummel.
Also commenting on the research, Kenneth L. Tyler, MD, professor of neurology, University of Colorado at Denver, Aurora, and a fellow of the American Academy of Neurology, said the study was relatively large and the results “interesting.”
Although it “provides more evidence there’s a subset of patients with symptoms even well past the acute phase” of COVID-19, the results are “mostly confirmatory” and include “nothing super surprising,” Dr. Tyler said in an interview.
However, the investigators did attempt to make the study “a little more quantitative” and “to confirm the self-reporting with their validated CD home test,” he said.
Dr. Tyler wondered how representative the sample was and whether the study drew more participants with impaired senses. “If I had a loss of smell or taste, maybe I would be more likely to respond to such a survey,” he said.
He also noted the difficulty of separating loss of smell from loss of taste.
“If you lose your sense of smell, things don’t taste right, so it can be confounding as to how to separate out those two,” he noted.
The study was supported by the Foundation of the Université du Québec à Trois-Rivières and the Province of Quebec. Dr. Frasnelli has received royalties from Styriabooks in Austria for a book on olfaction published in 2019 and has received honoraria for speaking engagements. Dr. Hummel and Dr. Tyler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings illustrate that olfactory problems are common not only during the acute COVID-19 phase but also “in the long run” and that these problems should be “taken into consideration” when following up these patients, study investigator Johannes Frasnelli, MD, professor, department of anatomy, Université du Québec à Trois-Rivières, said in an interview.
Loss of the sense of smell can affect quality of life because it affects eating and drinking, and may even be dangerous, said Dr. Frasnelli. “If your sense of smell is impaired, you may unknowingly eat spoiled food, or you may not smell smoke or gas in your home,” he said. In addition, Dr. Frasnelli noted that an impaired sense of smell is associated with higher rates of depression. The findings will be presented at the annual meeting of the American Academy of Neurology in April.
‘Striking’ finding
Research shows that about 60% of patients with COVID-19 lose their sense of smell to some degree during the acute phase of the disease. “But we wanted to go further and look at the longer-term effects of loss of smell and taste,” said Dr. Frasnelli.
The analysis included 813 health care workers in the province of Quebec. For all the patients, SARS-CoV-2 infection was confirmed through testing with a nasopharyngeal viral swab.
Participants completed a 64-item online questionnaire that asked about three senses: olfactory; gustatory, which includes tastes such as sweet, sour, bitter, salty, savory and umami; and trigeminal, which includes sensations such as spiciness of hot peppers and “coolness” of mint.
They were asked to rate these on a scale of 0 (no perception) to 10 (very strong perception) before the infection, during the infection, and currently. They were also asked about other symptoms, including fatigue.
Most respondents had been infected in the first wave of the virus in March and April of 2020 and responded to the questionnaire an average of 5 months later.
The vast majority of respondents (84.1%) were women, which Dr. Frasnelli said was not surprising because women predominate in the health care field.
The analysis showed that average smell ratings were 8.98 before infection, 2.85 during the acute phase, and 7.41 when respondents answered the questionnaire. The sense of taste was less affected and recovered faster than did the sense of smell. Results for taste were 9.20 before infection, 3.59 during the acute phase, and 8.05 after COVID-19.
Among 580 respondents who indicated a compromised sense of smell during the acute phase, the average smell rating when answering the questionnaire was 6.89, compared to 9.03 before the infection. More than half (51.2%) reported not regaining full olfactory function.
The fact that the sense of smell had not returned to normal for half the participants so long after being infected is “novel and quite striking,” said Dr. Frasnelli.
However, he noted, this doesn’t necessarily mean all those with a compromised sense of smell “have huge problems.” In some cases, he said, the problem “is more subtle.”
Not a CNS problem?
Respondents also completed a chemosensory dysfunction home test (CD-HT). They were asked to prepare common household food items, such as peanut butter, sugar, salt, and vinegar, in a particular way – for example, to add sugar or salt to water – and provide feedback on how they smell and taste.
For this CD-HT analysis, 18.4% of respondents reported having persistent loss of smell. This, Dr. Frasnelli said, adds to evidence from self-reported responses and suggests that in some cases, the problem is more than senses not returning to normal.
“From the questionnaires, roughly 50% said their sense of smell is still not back to normal, and when we look at the CD home test, we see that almost 20% of subjects indeed have pretty strong impairment of their sense of smell,” he said.
The results showed no sex differences, although Dr. Frasnelli noted that most of the sample were women. “It’s tricky to look at the data with regard to sex because it’s a bit skewed,” he said.
Male respondents were older than female participants, but there was no difference in impairment between age groups. Dr. Frasnelli said this was “quite interesting,” inasmuch as older people usually lose some sense of smell.
The researchers have not yet examined whether the results differ by type of health care worker.
They also have not examined in detail whether infection severity affects the risk for extended olfactory impairment. Although some research suggests that the problem with smell is more common in less severe cases, Dr. Frasnelli noted this could be because loss of smell is not a huge problem for patients battling grave health problems.
As for other symptoms, many respondents reported lingering fatigue; some reported debilitating fatigue, said Dr. Frasnelli. However, he cautioned that this is difficult to interpret, because the participants were health care workers, many of whom returned to work during the pandemic and perhaps had not fully rested.
He also noted that he and his colleagues have not “made the link” between impaired smell and the degree of fatigue.
The COVID-19 virus appears to attack supporting sustentacular cells in the olfactory epithelium, not nerve cells.
“Right now, it seems that the smell problem is not a central nervous system problem but a peripheral problem,” said Dr. Frasnelli. “But we don’t know for sure; it may be that the virus somehow gets into the brain and some symptoms are caused by the effects of the infection on the brain.”
The researchers will extend their research with another questionnaire to assess senses 10-12 months after COVID-19.
Limitations of the study include the subjective nature of the smell and taste ratings and the single time point at which data were collected.
Confirmatory findings
Commenting on the research in an interview, Thomas Hummel, MD, professor, smell and taste clinic, department of otorhinolaryngology, Technische Universität Dresden (Germany), said the new results regarding loss of smell after COVID-19 are “very congruent” with what he and his colleagues have observed.
Research shows that up to one in five of those infected with SARS-CoV-2 experience olfactory loss. “While the numbers may vary a bit from study to study or lab to lab, I think 5% to 20% of post–COVID-19 patients exhibit long-term olfactory loss,” Dr. Hummel said.
His group has observed that “many more are not back to normal,” which conforms with what Dr. Frasnelli’s study reveals, said Dr. Hummel.
Also commenting on the research, Kenneth L. Tyler, MD, professor of neurology, University of Colorado at Denver, Aurora, and a fellow of the American Academy of Neurology, said the study was relatively large and the results “interesting.”
Although it “provides more evidence there’s a subset of patients with symptoms even well past the acute phase” of COVID-19, the results are “mostly confirmatory” and include “nothing super surprising,” Dr. Tyler said in an interview.
However, the investigators did attempt to make the study “a little more quantitative” and “to confirm the self-reporting with their validated CD home test,” he said.
Dr. Tyler wondered how representative the sample was and whether the study drew more participants with impaired senses. “If I had a loss of smell or taste, maybe I would be more likely to respond to such a survey,” he said.
He also noted the difficulty of separating loss of smell from loss of taste.
“If you lose your sense of smell, things don’t taste right, so it can be confounding as to how to separate out those two,” he noted.
The study was supported by the Foundation of the Université du Québec à Trois-Rivières and the Province of Quebec. Dr. Frasnelli has received royalties from Styriabooks in Austria for a book on olfaction published in 2019 and has received honoraria for speaking engagements. Dr. Hummel and Dr. Tyler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings illustrate that olfactory problems are common not only during the acute COVID-19 phase but also “in the long run” and that these problems should be “taken into consideration” when following up these patients, study investigator Johannes Frasnelli, MD, professor, department of anatomy, Université du Québec à Trois-Rivières, said in an interview.
Loss of the sense of smell can affect quality of life because it affects eating and drinking, and may even be dangerous, said Dr. Frasnelli. “If your sense of smell is impaired, you may unknowingly eat spoiled food, or you may not smell smoke or gas in your home,” he said. In addition, Dr. Frasnelli noted that an impaired sense of smell is associated with higher rates of depression. The findings will be presented at the annual meeting of the American Academy of Neurology in April.
‘Striking’ finding
Research shows that about 60% of patients with COVID-19 lose their sense of smell to some degree during the acute phase of the disease. “But we wanted to go further and look at the longer-term effects of loss of smell and taste,” said Dr. Frasnelli.
The analysis included 813 health care workers in the province of Quebec. For all the patients, SARS-CoV-2 infection was confirmed through testing with a nasopharyngeal viral swab.
Participants completed a 64-item online questionnaire that asked about three senses: olfactory; gustatory, which includes tastes such as sweet, sour, bitter, salty, savory and umami; and trigeminal, which includes sensations such as spiciness of hot peppers and “coolness” of mint.
They were asked to rate these on a scale of 0 (no perception) to 10 (very strong perception) before the infection, during the infection, and currently. They were also asked about other symptoms, including fatigue.
Most respondents had been infected in the first wave of the virus in March and April of 2020 and responded to the questionnaire an average of 5 months later.
The vast majority of respondents (84.1%) were women, which Dr. Frasnelli said was not surprising because women predominate in the health care field.
The analysis showed that average smell ratings were 8.98 before infection, 2.85 during the acute phase, and 7.41 when respondents answered the questionnaire. The sense of taste was less affected and recovered faster than did the sense of smell. Results for taste were 9.20 before infection, 3.59 during the acute phase, and 8.05 after COVID-19.
Among 580 respondents who indicated a compromised sense of smell during the acute phase, the average smell rating when answering the questionnaire was 6.89, compared to 9.03 before the infection. More than half (51.2%) reported not regaining full olfactory function.
The fact that the sense of smell had not returned to normal for half the participants so long after being infected is “novel and quite striking,” said Dr. Frasnelli.
However, he noted, this doesn’t necessarily mean all those with a compromised sense of smell “have huge problems.” In some cases, he said, the problem “is more subtle.”
Not a CNS problem?
Respondents also completed a chemosensory dysfunction home test (CD-HT). They were asked to prepare common household food items, such as peanut butter, sugar, salt, and vinegar, in a particular way – for example, to add sugar or salt to water – and provide feedback on how they smell and taste.
For this CD-HT analysis, 18.4% of respondents reported having persistent loss of smell. This, Dr. Frasnelli said, adds to evidence from self-reported responses and suggests that in some cases, the problem is more than senses not returning to normal.
“From the questionnaires, roughly 50% said their sense of smell is still not back to normal, and when we look at the CD home test, we see that almost 20% of subjects indeed have pretty strong impairment of their sense of smell,” he said.
The results showed no sex differences, although Dr. Frasnelli noted that most of the sample were women. “It’s tricky to look at the data with regard to sex because it’s a bit skewed,” he said.
Male respondents were older than female participants, but there was no difference in impairment between age groups. Dr. Frasnelli said this was “quite interesting,” inasmuch as older people usually lose some sense of smell.
The researchers have not yet examined whether the results differ by type of health care worker.
They also have not examined in detail whether infection severity affects the risk for extended olfactory impairment. Although some research suggests that the problem with smell is more common in less severe cases, Dr. Frasnelli noted this could be because loss of smell is not a huge problem for patients battling grave health problems.
As for other symptoms, many respondents reported lingering fatigue; some reported debilitating fatigue, said Dr. Frasnelli. However, he cautioned that this is difficult to interpret, because the participants were health care workers, many of whom returned to work during the pandemic and perhaps had not fully rested.
He also noted that he and his colleagues have not “made the link” between impaired smell and the degree of fatigue.
The COVID-19 virus appears to attack supporting sustentacular cells in the olfactory epithelium, not nerve cells.
“Right now, it seems that the smell problem is not a central nervous system problem but a peripheral problem,” said Dr. Frasnelli. “But we don’t know for sure; it may be that the virus somehow gets into the brain and some symptoms are caused by the effects of the infection on the brain.”
The researchers will extend their research with another questionnaire to assess senses 10-12 months after COVID-19.
Limitations of the study include the subjective nature of the smell and taste ratings and the single time point at which data were collected.
Confirmatory findings
Commenting on the research in an interview, Thomas Hummel, MD, professor, smell and taste clinic, department of otorhinolaryngology, Technische Universität Dresden (Germany), said the new results regarding loss of smell after COVID-19 are “very congruent” with what he and his colleagues have observed.
Research shows that up to one in five of those infected with SARS-CoV-2 experience olfactory loss. “While the numbers may vary a bit from study to study or lab to lab, I think 5% to 20% of post–COVID-19 patients exhibit long-term olfactory loss,” Dr. Hummel said.
His group has observed that “many more are not back to normal,” which conforms with what Dr. Frasnelli’s study reveals, said Dr. Hummel.
Also commenting on the research, Kenneth L. Tyler, MD, professor of neurology, University of Colorado at Denver, Aurora, and a fellow of the American Academy of Neurology, said the study was relatively large and the results “interesting.”
Although it “provides more evidence there’s a subset of patients with symptoms even well past the acute phase” of COVID-19, the results are “mostly confirmatory” and include “nothing super surprising,” Dr. Tyler said in an interview.
However, the investigators did attempt to make the study “a little more quantitative” and “to confirm the self-reporting with their validated CD home test,” he said.
Dr. Tyler wondered how representative the sample was and whether the study drew more participants with impaired senses. “If I had a loss of smell or taste, maybe I would be more likely to respond to such a survey,” he said.
He also noted the difficulty of separating loss of smell from loss of taste.
“If you lose your sense of smell, things don’t taste right, so it can be confounding as to how to separate out those two,” he noted.
The study was supported by the Foundation of the Université du Québec à Trois-Rivières and the Province of Quebec. Dr. Frasnelli has received royalties from Styriabooks in Austria for a book on olfaction published in 2019 and has received honoraria for speaking engagements. Dr. Hummel and Dr. Tyler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New cases of child COVID-19 drop for fifth straight week
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
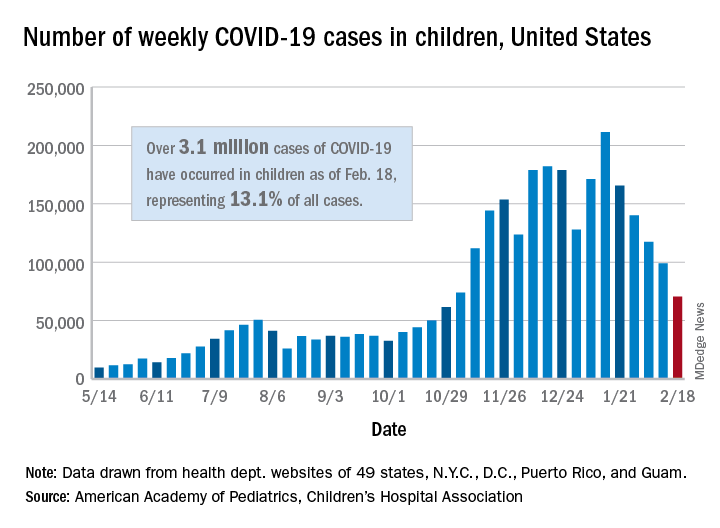
, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.
The fifth consecutive week with a decline has the number of new COVID-19 cases in children at its lowest level since late October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

, when 61,000 cases were reported, the AAP and CHA said in their weekly COVID-19 report.
The cumulative number of COVID-19 cases in children is now just over 3.1 million, which represents 13.1% of cases among all ages in the United States, based on data gathered from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
More children in California (439,000) have been infected than in any other state, while Illinois (176,000), Florida (145,000), Tennessee (137,000), Arizona (127,000), Ohio (121,000), and Pennsylvania (111,000) are the only other states with more than 100,000 cases, the AAP/CHA report shows.
Proportionally, the children of Wyoming have been hardest hit: Pediatric cases represent 19.4% of all cases in the state. The other four states with proportions of 18% or more are Alaska, Vermont, South Carolina, and Tennessee. Cumulative rates, however, tell a somewhat different story, as North Dakota leads with just over 8,500 cases per 100,000 children, followed by Tennessee (7,700 per 100,000) and Rhode Island (7,000 per 100,000), the AAP and CHA said.
Deaths in children, which had not been following the trend of fewer new cases over the last few weeks, dropped below double digits for the first time in a month. The six deaths that occurred during the week of Feb. 12-18 bring the total to 247 since the start of the pandemic in the 43 states, along with New York City and Guam, that are reporting such data, according to the report.
Variants spur new FDA guidance on COVID vaccines, tests, drugs
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
New light cast on type 2 MI aims to sharpen diagnosis, therapy
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.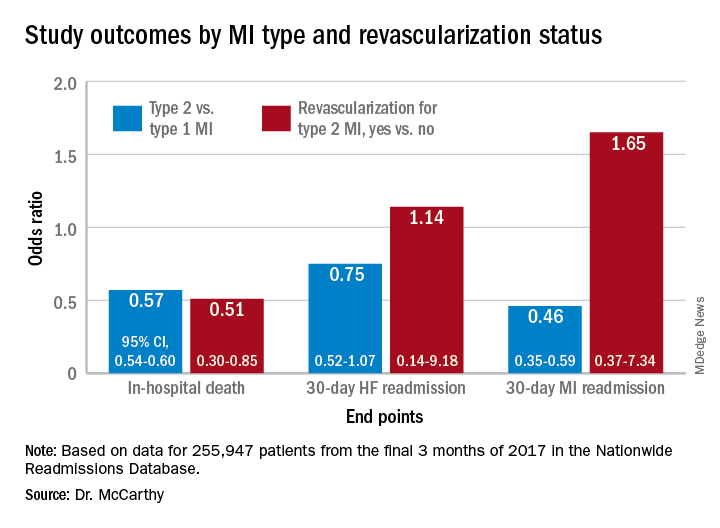
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.
Six-month follow-up shows continuing morbidity for COVID-19 survivors
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
In December 2019, a cluster of cases of what was first identified as a “mysterious pneumonia” was reported in the central Chinese city of Wuhan. Within a few short months, the disease had spread all over the world.
Wuhan was essentially “ground zero” for the novel coronavirus, or COVID-19, and now researchers report that many of the early survivors continue to experience a variety of lingering health issues.
At 6 months, for example, pulmonary and immune function have still not returned to normal in many of the patients who had been critically ill, said Zhiyong Peng, MD, PhD, an intensivist and medical researcher, in the department of critical care medicine, Zhonnan Hospital, Wuhan.
In addition, many are still experiencing varying degrees of psychiatric disability and physical morbidity.
The results of the report were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In 2020, Dr. Peng and colleagues conducted a single-center case series involving 138 patients with coronavirus pneumonia in order to describe the clinical characteristics of this new disease. Within this group, 26% of patients required admission to the intensive care unit and 4.3% died. As of Feb. 3, 2020, 26% required ICU care, 34.1% were discharged, 4.3% died, and 61.6% remained hospitalized. (JAMA. 2020 Mar 17;323[11]:1061-69) Not surprisingly, those requiring critical care experienced a higher rate of severe complications, including shock, arrythmias, acute cardiac injury and acute respiratory distress syndrome, compared with non-ICU patients.
“However, the long-term outcomes of survivors were unknown,” said Dr. Peng. Thus, the goal of the current study was to analyze the outcomes based on pulmonary function, physical morbidity, immunological status, health-related quality of life, cognitive impairment, and psychiatric disability.
The cohort included patients from four hospitals in Wuhan, who had been treated in the adult ICU and required mechanical ventilation (invasive or noninvasive), or had a high FiO2 concentration, or needed an intravenous infusion of vasopressors.
In all, 171 critically ill patients were admitted to the four designated hospitals, and of this group, 110 were discharged from ICU and 106 survived. At the 3-month follow-up, 92 patients were evaluated and at 6 months, 72 were evaluated.
Pulmonary function tests were performed, and all patients received a chest CT scan, and did the “6-minute walk test.” For immune function, lymphocyte counts and function assays were performed. The SF-36 questionnaire was used to evaluate health related quality of life, and cognitive and psychological assessments were conducted with a variety of tools including the Mini-Mental State Examination and Montreal Cognitive Assessment. Depression and anxiety were measured with Zung’s Self-Rating Anxiety Scale and the Hamilton Rating Scale.
At 3 months, 5 patients (5.4%) were seropositive for IgM and 9 (9.8%) were seronegative, while at 6 months, 9 patients (12.9%) tested seropositive for IgM and 12 (16.67%) tested seronegative.
A high proportion of patients also reported tachypnea after exercising (54%), heart palpitations (51.8%), fatigue (44.6%), and joint pain (20.5%).
In terms of lung function, survivors who had been intubated scored worse on pulmonary function tests and had a significant decrease in diffusing capacity for carbon monoxide (DLCO), compared with those who had not been intubated.
At 6 months, the DLCO remained at 76% of the predicted level, but the walking test and chest CT scan improved over time. “In multivariate analysis tracheostomy was a risk factor associated with distance walked in 6 minutes,” said Dr. Peng.
Other results showed that B cells were lower in survivors who had been intubated, compared with those who weren’t, and they were still low at 3 and 6 months, compared with normal values. T-cell subsets were also persistently low.
“Hyperfunction of T lymphocytes and hypofunction of NK cells were detected, which had not improved at 6 months,” said Dr. Peng.
Cognitive dysfunction and depression were reported in some survivors. Cognitive dysfunction at 3 months affected 12.8% of survivors, but it improved by 6 months, affecting on only 2.9% of the cohort (P = .029). However, rates of depression more than doubled from 3 to 6 months (20% vs. 47.8%, P < .001), and anxiety showed a slight increase (15.6% vs. 17.6%, P = .726).
“Further follow-up will be performed to confirm these findings,” Dr. Peng concluded.
Rahul Kashyap, MBBS, MBA, a research scientist and assistant professor of anesthesiology at the Mayo Clinic, Rochester, Minn., noted that currently the research from Wuhan is showing the follow-up for 6 months, but it takes time to gather and analyze the data. “I suspect we will be seeing results from the 1-year follow-up by June,” he said.
Dr. Kashyap, who was approached for an independent comment, also pointed out that in follow-up of SARS patients, some of them recovered but went on to develop chronic fatigue syndrome which is characterized by extreme fatigue that doesn’t improve with rest. “So the scientific community is contemplating if this will be true for patients with COVID-19 infection as well,” he said. “We have already seen that some of the ‘long haulers’ continue to have symptoms such as shortness of breath, joint pain, fatigue, loss of smell and taste, and even hearing loss in extreme cases.”
Some research is also confirming what has been reported from Wuhan. “Data from Ireland, that looked at 150 survivors, showed that almost 60% said they did not feel they were back to full health, regardless of the severity of the disease,” Dr. Kashyap said. “So, aside from Wuhan, we are now getting data from other sources that is similar. But what is interesting about the data from Ireland is that not all of the patients had severe illness or were in ICU.”
He added that data continue to come in from the United States and other countries, looking at long-term effects. “More and more patients are surviving as the care is getting better,” he said. “But beyond a year, we just don’t know yet.”
There was no outside sponsor listed. Dr. Peng and Dr. Kashyap have no disclosures.
FROM CCC50
Long-term CPAP use linked with more physical activity
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
FROM JOURNAL OF CLINICAL SLEEP MEDICINE
Organ transplant patient dies after receiving COVID-19–infected lungs
Doctors say a woman in Michigan contracted COVID-19 and died last fall 2 months after receiving a tainted double-lung transplant from a donor who turned out to harbor the virus that causes the disease – despite showing no signs of illness and initially testing negative.
Officials at the University of Michigan Medical School suggested it may be the first proven case of COVID-19 in the U.S. in which the virus was transmitted via an organ transplant. A surgeon who handled the donor lungs was also infected with the virus and fell ill but later recovered.
The incident appears to be isolated – the only confirmed case among nearly 40,000 transplants in 2020. But it has led to calls for more thorough testing of lung transplant donors, with samples taken from deep within the donor lungs as well as the nose and throat, said Dr. Daniel Kaul, director of Michigan Medicine’s transplant infectious disease service.
“We would absolutely not have used the lungs if we’d had a positive COVID-19 test,” said Dr. Kaul, who coauthored a report about the case in the American Journal of Transplantation.
The virus was transmitted when lungs from a woman from the Upper Midwest, who died after suffering a severe brain injury in a car accident, were transplanted into a woman with chronic obstructive lung disease at University Hospital in Ann Arbor. The nose and throat samples routinely collected from both organ donors and recipients tested negative for SARS-CoV-2, the virus that causes covid.
“All the screening that we normally do and are able to do, we did,” Dr. Kaul said.
Three days after the operation, however, the recipient spiked a fever; her blood pressure fell and her breathing became labored. Imaging showed signs of lung infection.
As her condition worsened, the patient developed septic shock and heart function problems. Doctors decided to test for SARS-CoV-2, Dr. Kaul said. Samples from her new lungs came back positive.
Suspicious about the origin of the infection, doctors returned to samples from the transplant donor. A molecular test of a swab from the donor’s nose and throat, taken 48 hours after her lungs were procured, had been negative for SARS-Cov-2. The donor’s family told doctors she had no history of recent travel or COVID-19 symptoms and no known exposure to anyone with the disease.
But doctors had kept a sample of fluid washed from deep within the donor lungs. When they tested that fluid, it was positive for the virus. Four days after the transplant, the surgeon who handled the donor lungs and performed the surgery tested positive, too. Genetic screening revealed that the transplant recipient and the surgeon had been infected by the donor. Ten other members of the transplant team tested negative for the virus.
The transplant recipient deteriorated rapidly, developing multisystem organ failure. Doctors tried known treatments for COVID-19, including remdesivir, a newly approved drug, and convalescent blood plasma from people previously infected with the disease. Eventually, she was placed on the last-resort option of ECMO, or extracorporeal membrane oxygenation, to no avail. Life support was withdrawn, and she died 61 days after the transplant.
Dr. Kaul called the incident “a tragic case.”
While the Michigan case marks the first confirmed incident in the U.S. of transmission through a transplant, others have been suspected. A recent Centers for Disease Control and Prevention report reviewed eight possible cases of what’s known as donor-derived infection that occurred last spring, but concluded the most likely source of transmission of the COVID-19 virus in those cases was in a community or health care setting.
Before this incident, it was not clear whether the COVID-19 virus could be transmitted through solid organ transplants, though it’s well documented with other respiratory viruses. Donor transmission of H1N1 2009 pandemic influenza has been detected almost exclusively in lung transplant recipients, Dr. Kaul noted.
While it’s not surprising that SARS-CoV-2 can be transmitted through infected lungs, it remains uncertain whether other organs affected by COVID-19 – hearts, livers and kidneys, for instance – can transmit the virus, too.
“It seems for non-lung donors that it may be very difficult to transmit COVID-19, even if the donor has COVID-19,” Dr. Kaul said.
Organ donors have been tested routinely for SARS-CoV-2 during the pandemic, though it’s not required by the Organ Procurement and Transplantation Network, or OPTN, which oversees transplants in the U.S. But the Michigan case underscores the need for more extensive sampling before transplant, especially in areas with high rates of covid transmission, Dr. Kaul said.
When it comes to lungs, that means making sure to test samples from the donor’s lower respiratory tract, as well as from the nose and throat. Obtaining and testing such samples from donors can be difficult to carry out in a timely fashion. There’s also the risk of introducing infection into the donated lungs, Dr. Kaul said.
Because no organs other than lungs were used, the Michigan case doesn’t provide insight into testing protocols for other organs.
Overall, viral transmissions from organ donors to recipients remain rare, occurring in fewer than 1% of transplant recipients, research shows. The medical risks facing ailing patients who reject a donor organ are generally far higher, said Dr. David Klassen, chief medical officer with the United Network for Organ Sharing, the federal contractor that runs the OPTN.
“The risks of turning down transplants are catastrophic,” he said. “I don’t think patients should be afraid of the transplant process.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Doctors say a woman in Michigan contracted COVID-19 and died last fall 2 months after receiving a tainted double-lung transplant from a donor who turned out to harbor the virus that causes the disease – despite showing no signs of illness and initially testing negative.
Officials at the University of Michigan Medical School suggested it may be the first proven case of COVID-19 in the U.S. in which the virus was transmitted via an organ transplant. A surgeon who handled the donor lungs was also infected with the virus and fell ill but later recovered.
The incident appears to be isolated – the only confirmed case among nearly 40,000 transplants in 2020. But it has led to calls for more thorough testing of lung transplant donors, with samples taken from deep within the donor lungs as well as the nose and throat, said Dr. Daniel Kaul, director of Michigan Medicine’s transplant infectious disease service.
“We would absolutely not have used the lungs if we’d had a positive COVID-19 test,” said Dr. Kaul, who coauthored a report about the case in the American Journal of Transplantation.
The virus was transmitted when lungs from a woman from the Upper Midwest, who died after suffering a severe brain injury in a car accident, were transplanted into a woman with chronic obstructive lung disease at University Hospital in Ann Arbor. The nose and throat samples routinely collected from both organ donors and recipients tested negative for SARS-CoV-2, the virus that causes covid.
“All the screening that we normally do and are able to do, we did,” Dr. Kaul said.
Three days after the operation, however, the recipient spiked a fever; her blood pressure fell and her breathing became labored. Imaging showed signs of lung infection.
As her condition worsened, the patient developed septic shock and heart function problems. Doctors decided to test for SARS-CoV-2, Dr. Kaul said. Samples from her new lungs came back positive.
Suspicious about the origin of the infection, doctors returned to samples from the transplant donor. A molecular test of a swab from the donor’s nose and throat, taken 48 hours after her lungs were procured, had been negative for SARS-Cov-2. The donor’s family told doctors she had no history of recent travel or COVID-19 symptoms and no known exposure to anyone with the disease.
But doctors had kept a sample of fluid washed from deep within the donor lungs. When they tested that fluid, it was positive for the virus. Four days after the transplant, the surgeon who handled the donor lungs and performed the surgery tested positive, too. Genetic screening revealed that the transplant recipient and the surgeon had been infected by the donor. Ten other members of the transplant team tested negative for the virus.
The transplant recipient deteriorated rapidly, developing multisystem organ failure. Doctors tried known treatments for COVID-19, including remdesivir, a newly approved drug, and convalescent blood plasma from people previously infected with the disease. Eventually, she was placed on the last-resort option of ECMO, or extracorporeal membrane oxygenation, to no avail. Life support was withdrawn, and she died 61 days after the transplant.
Dr. Kaul called the incident “a tragic case.”
While the Michigan case marks the first confirmed incident in the U.S. of transmission through a transplant, others have been suspected. A recent Centers for Disease Control and Prevention report reviewed eight possible cases of what’s known as donor-derived infection that occurred last spring, but concluded the most likely source of transmission of the COVID-19 virus in those cases was in a community or health care setting.
Before this incident, it was not clear whether the COVID-19 virus could be transmitted through solid organ transplants, though it’s well documented with other respiratory viruses. Donor transmission of H1N1 2009 pandemic influenza has been detected almost exclusively in lung transplant recipients, Dr. Kaul noted.
While it’s not surprising that SARS-CoV-2 can be transmitted through infected lungs, it remains uncertain whether other organs affected by COVID-19 – hearts, livers and kidneys, for instance – can transmit the virus, too.
“It seems for non-lung donors that it may be very difficult to transmit COVID-19, even if the donor has COVID-19,” Dr. Kaul said.
Organ donors have been tested routinely for SARS-CoV-2 during the pandemic, though it’s not required by the Organ Procurement and Transplantation Network, or OPTN, which oversees transplants in the U.S. But the Michigan case underscores the need for more extensive sampling before transplant, especially in areas with high rates of covid transmission, Dr. Kaul said.
When it comes to lungs, that means making sure to test samples from the donor’s lower respiratory tract, as well as from the nose and throat. Obtaining and testing such samples from donors can be difficult to carry out in a timely fashion. There’s also the risk of introducing infection into the donated lungs, Dr. Kaul said.
Because no organs other than lungs were used, the Michigan case doesn’t provide insight into testing protocols for other organs.
Overall, viral transmissions from organ donors to recipients remain rare, occurring in fewer than 1% of transplant recipients, research shows. The medical risks facing ailing patients who reject a donor organ are generally far higher, said Dr. David Klassen, chief medical officer with the United Network for Organ Sharing, the federal contractor that runs the OPTN.
“The risks of turning down transplants are catastrophic,” he said. “I don’t think patients should be afraid of the transplant process.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Doctors say a woman in Michigan contracted COVID-19 and died last fall 2 months after receiving a tainted double-lung transplant from a donor who turned out to harbor the virus that causes the disease – despite showing no signs of illness and initially testing negative.
Officials at the University of Michigan Medical School suggested it may be the first proven case of COVID-19 in the U.S. in which the virus was transmitted via an organ transplant. A surgeon who handled the donor lungs was also infected with the virus and fell ill but later recovered.
The incident appears to be isolated – the only confirmed case among nearly 40,000 transplants in 2020. But it has led to calls for more thorough testing of lung transplant donors, with samples taken from deep within the donor lungs as well as the nose and throat, said Dr. Daniel Kaul, director of Michigan Medicine’s transplant infectious disease service.
“We would absolutely not have used the lungs if we’d had a positive COVID-19 test,” said Dr. Kaul, who coauthored a report about the case in the American Journal of Transplantation.
The virus was transmitted when lungs from a woman from the Upper Midwest, who died after suffering a severe brain injury in a car accident, were transplanted into a woman with chronic obstructive lung disease at University Hospital in Ann Arbor. The nose and throat samples routinely collected from both organ donors and recipients tested negative for SARS-CoV-2, the virus that causes covid.
“All the screening that we normally do and are able to do, we did,” Dr. Kaul said.
Three days after the operation, however, the recipient spiked a fever; her blood pressure fell and her breathing became labored. Imaging showed signs of lung infection.
As her condition worsened, the patient developed septic shock and heart function problems. Doctors decided to test for SARS-CoV-2, Dr. Kaul said. Samples from her new lungs came back positive.
Suspicious about the origin of the infection, doctors returned to samples from the transplant donor. A molecular test of a swab from the donor’s nose and throat, taken 48 hours after her lungs were procured, had been negative for SARS-Cov-2. The donor’s family told doctors she had no history of recent travel or COVID-19 symptoms and no known exposure to anyone with the disease.
But doctors had kept a sample of fluid washed from deep within the donor lungs. When they tested that fluid, it was positive for the virus. Four days after the transplant, the surgeon who handled the donor lungs and performed the surgery tested positive, too. Genetic screening revealed that the transplant recipient and the surgeon had been infected by the donor. Ten other members of the transplant team tested negative for the virus.
The transplant recipient deteriorated rapidly, developing multisystem organ failure. Doctors tried known treatments for COVID-19, including remdesivir, a newly approved drug, and convalescent blood plasma from people previously infected with the disease. Eventually, she was placed on the last-resort option of ECMO, or extracorporeal membrane oxygenation, to no avail. Life support was withdrawn, and she died 61 days after the transplant.
Dr. Kaul called the incident “a tragic case.”
While the Michigan case marks the first confirmed incident in the U.S. of transmission through a transplant, others have been suspected. A recent Centers for Disease Control and Prevention report reviewed eight possible cases of what’s known as donor-derived infection that occurred last spring, but concluded the most likely source of transmission of the COVID-19 virus in those cases was in a community or health care setting.
Before this incident, it was not clear whether the COVID-19 virus could be transmitted through solid organ transplants, though it’s well documented with other respiratory viruses. Donor transmission of H1N1 2009 pandemic influenza has been detected almost exclusively in lung transplant recipients, Dr. Kaul noted.
While it’s not surprising that SARS-CoV-2 can be transmitted through infected lungs, it remains uncertain whether other organs affected by COVID-19 – hearts, livers and kidneys, for instance – can transmit the virus, too.
“It seems for non-lung donors that it may be very difficult to transmit COVID-19, even if the donor has COVID-19,” Dr. Kaul said.
Organ donors have been tested routinely for SARS-CoV-2 during the pandemic, though it’s not required by the Organ Procurement and Transplantation Network, or OPTN, which oversees transplants in the U.S. But the Michigan case underscores the need for more extensive sampling before transplant, especially in areas with high rates of covid transmission, Dr. Kaul said.
When it comes to lungs, that means making sure to test samples from the donor’s lower respiratory tract, as well as from the nose and throat. Obtaining and testing such samples from donors can be difficult to carry out in a timely fashion. There’s also the risk of introducing infection into the donated lungs, Dr. Kaul said.
Because no organs other than lungs were used, the Michigan case doesn’t provide insight into testing protocols for other organs.
Overall, viral transmissions from organ donors to recipients remain rare, occurring in fewer than 1% of transplant recipients, research shows. The medical risks facing ailing patients who reject a donor organ are generally far higher, said Dr. David Klassen, chief medical officer with the United Network for Organ Sharing, the federal contractor that runs the OPTN.
“The risks of turning down transplants are catastrophic,” he said. “I don’t think patients should be afraid of the transplant process.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Hospitalizations for food anaphylaxis triple, but deaths down in United Kingdom
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com
The rate of hospital admissions in the United Kingdom for food-induced anaphylaxis more than tripled over the 20 years from 1998 to 2018, but the case fatality rate fell by more than half, researchers report in BMJ.
“Cow’s milk is increasingly identified as the culprit allergen for fatal food reactions and is now the commonest cause of fatal anaphylaxis in children,” write Alessia Baseggio Conrado, PhD, a biochemist with the National Heart and Lung Institute at Imperial College London, and colleagues. “More education is needed to highlight the specific risks posed by cow’s milk to people who are allergic to increase awareness among food businesses.”
Whereas recognition of the risks posed by nut allergies has increased, people think milk allergy is mild, says senior author Paul. J. Turner, BMBCh, PhD, an allergist/immunologist at Imperial College. “This is often true in very young children, but school-aged children who still have milk allergy tend to have a more allergic profile, often with other allergies, including asthma,” Dr. Turner told this news organization. “Also, milk is very common in our diet, and you don’t need much milk to achieve a decent dose of allergen.”
During the study period, 101,891 people were hospitalized for anaphylaxis; 30,700 cases (30%) were coded as having been triggered by food.
These food-related admissions represent an increase from 1.23 to 4.04 per 100,000 population per year, for an annual increase of 5.7% (95% confidence interval, 5.5-5.9; P < .001), the authors write.
The largest jump occurred among children younger than 15 years, for whom admissions rose from 2.1 to 9.2 per 100,000 population per year, an annual increase of 6.6% (95% CI, 6.3-7.0). The annual increases were 5.9% (95% CI, 5.6-6.2) among persons aged 15 to 59 years and 2.1% (95% CI, 1.8-3.1) among those aged 60 years and older.
The investigators used data from England, Scotland, Wales, and Northern Ireland to track temporal trends and age and sex distributions for hospital admissions for which the primary diagnosis was anaphylaxis attributable to both food and nonfood triggers. These data were compared with nationally reported fatalities.
Over the 20-year period, 152 deaths were attributed to likely food-induced anaphylaxis. During that time, the case fatality rate for confirmed fatal food anaphylaxis fell from 0.7% to 0.19% (rate ratio, 0.931; 95% CI, 0.904-0.959; P < .001) and declined to 0.30% for suspected fatal food anaphylaxis (rate ratio, 0.970; 95% CI, 0.945-0.996; P = .024).
Between 1992 and 2018, at least 46% of all anaphylactic fatalities were deemed to be triggered by peanut or tree nut. Among school-aged children, 26% of anaphylactic fatalities were attributed to cow’s milk.
Not surprisingly, during the study period, there was an increase of 336% in prescriptions for adrenaline autoinjectors. Such prescriptions increased 11% per year.
Global trend
The data extend findings Dr. Turner and colleagues reported for England and Wales in 2014 regarding the entire United Kingdom population and align with epidemiologic trends in hospital admissions for anaphylaxis in the United States and Australia.
The researchers say better recognition and management of anaphylaxis could partly explain the decrease in fatalities, but the rise in hospitalizations remains puzzling. “Whether a true increase in the prevalence of anaphylaxis has occurred (rather than a reduction in the threshold to admit patients presenting with anaphylaxis) is unclear because evidence is lacking for an increase in prevalence of food allergy in the [United Kingdom] (and elsewhere) over the same time period,” they write.
Ronna L. Campbell, MD, PhD, an emergency physician at the Mayo Clinic in Rochester, Minn., has noted similar trends in the United States. “It may be that anaphylaxis recognition and diagnosis have improved, resulting in earlier administration of epinephrine,” Dr. Campbell said in an interview. “So while cases are increasing, earlier recognition and treatment result in decreased fatalities.” She is unaware of any new guidelines recommending increased hospitalization that would explain the puzzling rise in admissions.
According to the study authors, the clinical criteria used to diagnose anaphylaxis in the United Kingdom did not change during the study period. Although national guidance recommending the hospitalization of children younger than 16 who are suspected of having anaphylaxis was introduced in 2011 and may have boosted admissions, the year-on-year rate of increase has persisted since 2014. “Therefore the increase over the past 5 years cannot be attributed to the impact of the guidance,” they write.
The study was funded by grants from the U.K. Medical Research Council and U.K. Food Standards Agency. Two coauthors have disclosed financial relationships with industry outside of the submitted work. Dr. Conrado has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com









