User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


COVID-19 in children: New cases on the rise again
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
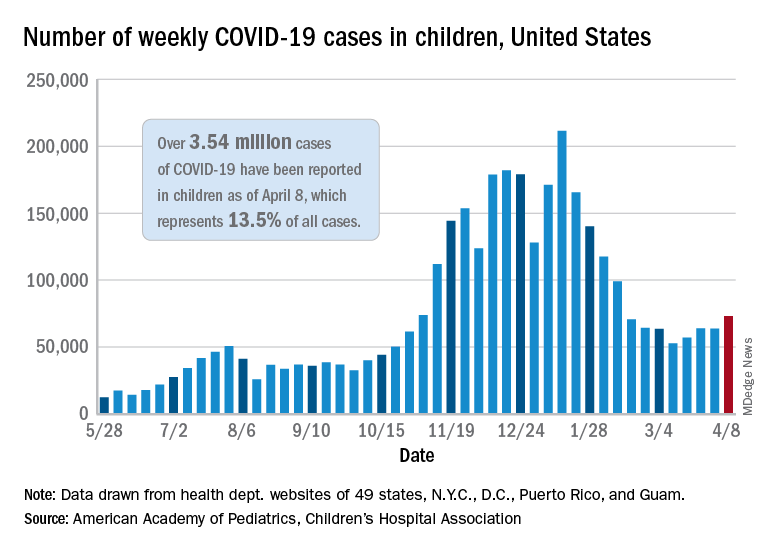
Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
Arthritis drug may curb myocardial damage in acute STEMI
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Medtronic recall of almost 240,000 ICDs is class I, FDA says
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
The Food and Drug Administration has declared Medtronic’s recall of seven models of defibrillating cardiac rhythm devices, caused by a risk for premature battery depletion, as class I, which implies a potential risk for serious injury or death. A total of 444 complaints, but no deaths, have been reported in association with the 239,171 affected devices, the agency said in a statement on April 12, 2021.
Physicians were notified of the company’s recall in early February. It covered implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy–defibrillator (CRT-D) models Evera, Viva, Brava, Claria, Amplia, Compia, and Visia distributed from Aug. 31, 2012 to May 9, 2018.
The devices could be subject to “an unexpected and rapid decrease in battery life” because of a possible short circuit that could lead to a device-replacement alert “earlier than expected.” Some devices may experience full battery depletion “within as little as 1 day” after such an alert.
“If the user does not respond to the first warning, the device may stop functioning. The likelihood that this issue will occur is constant after approximately 3 years after device use,” the announcement said.
Medtronic recommends device replacement no more than 1 week after such an early warning for patients who are not pacing dependent or who have them for primary prevention, but right away for pacing-dependent patients.
A version of this article first appeared on Medscape.com
Next winter may be rough: Models predict ‘considerable surge’ of COVID
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
It’s likely the United States will see another surge of COVID-19 this winter, warned Christopher Murray, MD, director of the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
Speaking at the national conference of State of Reform on April 8, Dr. Murray cited the seasonality of the SARS-CoV-2 virus, which wanes in the summer and waxes in the winter. The “optimistic forecast” of IHME, which has modeled the course of the pandemic for the past 13 months, is that daily deaths will rise a bit in the next month, then decline from May through August, he said.
“Summer should be fairly quiet in terms of COVID, if vaccinations rise and people don’t stop wearing masks,” Dr. Murray said.
But he added that “a considerable surge will occur over next winter,” because the new variants are more transmissible, and people will likely relax social distancing and mask wearing. The IHME predicts that the percentage of Americans who usually don masks will decline from 73% today to 21% by Aug. 1.
With a rapid decline in mask use and a rise in mobility, there will still be more than 1,000 deaths each day by July 1, Dr. Murray said. In a forecast released the day after Dr. Murray spoke, the IHME predicted that by Aug. 1, there will be a total of 618,523 U.S. deaths from COVID-19. Deaths could be as high as 696,651 if mobility among the vaccinated returns to prepandemic levels, the institute forecasts.
Based on cell phone data, Dr. Murray said, the amount of mobility in the United States has already risen to the level of March 2020, when the pandemic was just getting underway.
Decreased infections
If there’s one piece of good news in the latest IHME report, it’s that the estimated number of people infected (including those not tested) will drop from 111,581 today to a projected 17,502 on Aug. 1. But in a worst-case scenario, with sharply higher mobility among vaccinated people, the case count on that date would only fall to 73,842.
The SARS-CoV-2 variants are another factor of concern. Dr. Murray distinguished between variants like the one first identified in the U.K. (B.1.1.7) and other “escape variants.”
B.1.1.7, which is now the dominant strain in the United States, increases transmission but doesn’t necessarily escape the immune system or vaccines, he explained.
In contrast, if someone is infected with a variant such as the South African or the Brazilian mutations, he said, a previous COVID-19 infection might not protect the person, and vaccines are less effective against those variants.
Cross-variant immunity may range from 0% to 60% for escape variants, based on the slim amount of data now available, Dr. Murray said. In his view, these variants will be the long-term driver of the pandemic in the United States, while the United Kingdom variant is the short-term driver.
The latest data, he said, show that the Pfizer/BioNTech and Moderna vaccines are 75% effective against the escape variants, with lower efficacy for other vaccines. But booster shots may still be required to protect people against some variants.
Human factors
Human behavior will also help determine the course of the pandemic, he noted. Vaccine hesitancy, for example, is still high in the United States.
By the end of May, he predicted, about 180 million people will have received about two doses of vaccine. After that, he said, “vaccination will flatline due to lack of demand.” The two unknowns are how much campaigns to promote vaccination will increase vaccine confidence, and when children will be vaccinated.
In the United States, he said, 69% of adults have been vaccinated or want to get a shot. But that percentage has dropped 5 points since February, and vaccine confidence varies by state.
Dr. Murray emphasized that the winter surge he predicts can be blocked if people change their behaviors. These include a rise in vaccine confidence to 80% and continued mask wearing by most people.
However, if vaccine confidence and mask wearing decline, state governments continue to drop social distancing rules, and the uptake of boosters is low, the winter surge could be more serious, he said.
Double surge
Murray also raised the possibility of a double surge of COVID-19 and influenza this winter. Widely expected last winter, this double surge never materialized here or elsewhere, partly because of mask wearing. But Dr. Murray said it could happen this year: History shows that the flu tends to be stronger in years after weak outbreaks.
He advised hospitals to prepare now for whatever might come later this year. Public health authorities, he said, should speed up vaccination, monitor variants closely with additional sequencing, and try to modify behavior in high-risk groups.
Asked to explain the recent surge of COVID-19 cases in Michigan, Dr. Murray attributed it partly to the spread of the B.1.1.7 (U.K.) variant. But he noted that the U.K. variant has expanded even more widely in some other states that haven’t had an explosive surge like Michigan’s.
Moreover, he noted, Michigan doesn’t have low mask use or high mobility. So the upward spiral of COVID-19 infections there is very concerning, he said.
In regard to the role of children as reservoirs of the virus, Dr. Murray pointed out that views on this have changed around the world. For a while, people thought kids didn’t spread COVID-19 very much. That view shifted when U.K. data showed that child transmission of the B.1.1.7 variant increased by half to 9% of contacts in comparison with the original virus strain.
Dutch data, similarly, showed schools contributing to the latest outbreaks, and some European nations have closed schools. In the United States, the trend is to open them.
A version of this article first appeared on Medscape.com.
Remote cardio visits expand access for underserved during COVID
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA, CDC urge pause of J&J COVID vaccine
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
OCS heart system earns hard-won backing of FDA panel
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
Presurgical nivo/chemo boosts pCR rates in NSCLC
Patients with resectable non–small cell lung cancer (NSCLC) often receive treatment before they undergo surgery. For such patients who achieve a pathologic complete response (pCR), the chances of survival are improved.
However, only a small percentage of patients achieve a pCR with neoadjuvant chemotherapy alone.
Adding the immune checkpoint inhibitor nivolumab to platinum-doublet chemotherapy in the neoadjuvant setting boosts the success rate.
Results from the CheckMate 816 trial show that pCR rates improved from 2.2% with chemotherapy alone to 24% when nivolumab was added.
This difference translated into an odds ratio for achieving a pCR with nivolumab plus chemotherapy of 13.94 (P < .0001), reported Patrick M. Forde, MBBCh, from the Johns Hopkins Kimmel Cancer Center, Baltimore.
This primary endpoint, pCR, was defined as complete regression in both the primary tumor and lymph nodes.
“The magnitude of pCR with nivo plus chemo was similar in stage 1B, II, and stage IIIA disease, as well as in both squamous and nonsquamous histologies,” he added.
Dr. Forde presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT003).
The higher pCR rates were seen regardless of PD-L1 expression or tumor mutational burden, Dr. Forde said.
The benefit was also seen when the researchers considered only those patients who subsequently underwent resection (pCR rate of 30.5% with the combination versus 3.2% with chemotherapy alone) and when only the primary tumor was considered (pCR rate of 25.7% vs. 2.8%, respectively).
Change in trial design
Invited discussant Jhanelle Gray, MD, from the Moffitt Cancer Center, Tampa, pointed out that the CheckMate 816 trial originally included an experimental arm with double immunotherapy – ipilimumab plus nivolumab – added onto chemotherapy.
However, this third arm was closed after other trials reported promising results from adding a single immunotherapy onto chemotherapy. For example, results of the single-arm NADIM phase 2 study showed a 77.1% progression-free survival rate at 24 months with the combination of nivolumab, paclitaxel, and carboplatin, and the phase 2 KEYNOTE-021 trial showed that adding pembrolizumab to a standard platinum-doublet chemotherapy regimen nearly doubled response rates among patients with previously untreated advanced nonsquamous NSCLC (although there was no advantage in overall survival).
“Even with the change in trial design, patient characteristics were well balanced between the two arms, and the study met its primary endpoint in the intent-to-treat population,” she said.
Dr. Gray also commented that the choice of pCR as a primary endpoint is “intriguing, and the question remains if it represents a valid surrogate endpoint for survival.”
She noted that a meta-analysis of 32 neoadjuvant chemotherapy-based studies in NSCLC, presented at the 2020 European Society of Medical Oncology annual meeting, showed clear associations of pCR and major pathologic response to both overall survival and event-free survival.
“As these findings were established in a backdrop of chemotherapy, work is needed to confirm these findings in the setting of immunotherapy in particular, as at times, radiographic findings do not correlate with histological findings,” Dr. Gray said.
CheckMate 816 particulars
CheckMate 816 was conducted in 358 patients with newly diagnosed NSCLC with resectable stage IB tumors of at least 4 cm up to stage IIIA tumors, good performance status, and no known EGFR mutations or ALK alterations.
Patients were randomly assigned on an equal basis to receive either nivolumab at 360 mg plus chemotherapy every 3 weeks for three cycles or chemotherapy alone.
Surgery was planned within 6 week after neoadjuvant therapy. Patients could receive (at the investigator’s discretion) adjuvant chemotherapy with or without radiotherapy but no further immunotherapy during follow-up.
In this analysis, patients who did not undergo surgery or for whom evaluable tissue samples were not available were counted among those whose conditions did not respond to therapy.
Major pathologic response rate (≤10% residual viable tumor cells in the primary lung tumor and sampled lymph nodes), which was a secondary endpoint, was also significantly better, at 36.9% versus 8.9%, translating into an OR of 5.70 (95% confidence interval, 3.16-10.26).
In a subset of patients, the investigators assessed clearance of circulating tumor DNA (ctDNA) from day 1 of the first cycle to day 1 of the third cycle using a highly sensitive tumor-informed approach. They found that ctDNA was notably higher with the combination than with chemotherapy alone and that ctDNA clearance correlated with pCR.
Safety similar
“Remarkably, safety was quite similar across the two treatment arms,” Dr. Forde said.
The addition of nivolumab to chemotherapy did not appear to increase either treatment-related adverse events or adverse events of any cause. Grade 3-4 adverse events occurred in 41% of patients in the combination arm versus 44% in the chemotherapy-alone arm.
Treatment-related adverse events leading to discontinuation occurred in 10% of patients in each arm.
Two patients in the nivolumab-chemotherapy arm died from surgically related adverse events (one pulmonary embolism and one aortic rupture). These events were deemed to be unrelated to the study drug.
The investigators are continuing to assess event-free survival and overall survival.
CheckMate 816 is funded by Bristol-Myers Squibb and Ono Pharmaceutical. Dr. Forde has received grants/research support and advisory fees from Bristol-Myers Squibb and others. Dr. Gray has consulted for and has received grant/research support from Bristol-Myers Squibb and others.
A version of this article first appeared on Medscape.com.
Patients with resectable non–small cell lung cancer (NSCLC) often receive treatment before they undergo surgery. For such patients who achieve a pathologic complete response (pCR), the chances of survival are improved.
However, only a small percentage of patients achieve a pCR with neoadjuvant chemotherapy alone.
Adding the immune checkpoint inhibitor nivolumab to platinum-doublet chemotherapy in the neoadjuvant setting boosts the success rate.
Results from the CheckMate 816 trial show that pCR rates improved from 2.2% with chemotherapy alone to 24% when nivolumab was added.
This difference translated into an odds ratio for achieving a pCR with nivolumab plus chemotherapy of 13.94 (P < .0001), reported Patrick M. Forde, MBBCh, from the Johns Hopkins Kimmel Cancer Center, Baltimore.
This primary endpoint, pCR, was defined as complete regression in both the primary tumor and lymph nodes.
“The magnitude of pCR with nivo plus chemo was similar in stage 1B, II, and stage IIIA disease, as well as in both squamous and nonsquamous histologies,” he added.
Dr. Forde presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT003).
The higher pCR rates were seen regardless of PD-L1 expression or tumor mutational burden, Dr. Forde said.
The benefit was also seen when the researchers considered only those patients who subsequently underwent resection (pCR rate of 30.5% with the combination versus 3.2% with chemotherapy alone) and when only the primary tumor was considered (pCR rate of 25.7% vs. 2.8%, respectively).
Change in trial design
Invited discussant Jhanelle Gray, MD, from the Moffitt Cancer Center, Tampa, pointed out that the CheckMate 816 trial originally included an experimental arm with double immunotherapy – ipilimumab plus nivolumab – added onto chemotherapy.
However, this third arm was closed after other trials reported promising results from adding a single immunotherapy onto chemotherapy. For example, results of the single-arm NADIM phase 2 study showed a 77.1% progression-free survival rate at 24 months with the combination of nivolumab, paclitaxel, and carboplatin, and the phase 2 KEYNOTE-021 trial showed that adding pembrolizumab to a standard platinum-doublet chemotherapy regimen nearly doubled response rates among patients with previously untreated advanced nonsquamous NSCLC (although there was no advantage in overall survival).
“Even with the change in trial design, patient characteristics were well balanced between the two arms, and the study met its primary endpoint in the intent-to-treat population,” she said.
Dr. Gray also commented that the choice of pCR as a primary endpoint is “intriguing, and the question remains if it represents a valid surrogate endpoint for survival.”
She noted that a meta-analysis of 32 neoadjuvant chemotherapy-based studies in NSCLC, presented at the 2020 European Society of Medical Oncology annual meeting, showed clear associations of pCR and major pathologic response to both overall survival and event-free survival.
“As these findings were established in a backdrop of chemotherapy, work is needed to confirm these findings in the setting of immunotherapy in particular, as at times, radiographic findings do not correlate with histological findings,” Dr. Gray said.
CheckMate 816 particulars
CheckMate 816 was conducted in 358 patients with newly diagnosed NSCLC with resectable stage IB tumors of at least 4 cm up to stage IIIA tumors, good performance status, and no known EGFR mutations or ALK alterations.
Patients were randomly assigned on an equal basis to receive either nivolumab at 360 mg plus chemotherapy every 3 weeks for three cycles or chemotherapy alone.
Surgery was planned within 6 week after neoadjuvant therapy. Patients could receive (at the investigator’s discretion) adjuvant chemotherapy with or without radiotherapy but no further immunotherapy during follow-up.
In this analysis, patients who did not undergo surgery or for whom evaluable tissue samples were not available were counted among those whose conditions did not respond to therapy.
Major pathologic response rate (≤10% residual viable tumor cells in the primary lung tumor and sampled lymph nodes), which was a secondary endpoint, was also significantly better, at 36.9% versus 8.9%, translating into an OR of 5.70 (95% confidence interval, 3.16-10.26).
In a subset of patients, the investigators assessed clearance of circulating tumor DNA (ctDNA) from day 1 of the first cycle to day 1 of the third cycle using a highly sensitive tumor-informed approach. They found that ctDNA was notably higher with the combination than with chemotherapy alone and that ctDNA clearance correlated with pCR.
Safety similar
“Remarkably, safety was quite similar across the two treatment arms,” Dr. Forde said.
The addition of nivolumab to chemotherapy did not appear to increase either treatment-related adverse events or adverse events of any cause. Grade 3-4 adverse events occurred in 41% of patients in the combination arm versus 44% in the chemotherapy-alone arm.
Treatment-related adverse events leading to discontinuation occurred in 10% of patients in each arm.
Two patients in the nivolumab-chemotherapy arm died from surgically related adverse events (one pulmonary embolism and one aortic rupture). These events were deemed to be unrelated to the study drug.
The investigators are continuing to assess event-free survival and overall survival.
CheckMate 816 is funded by Bristol-Myers Squibb and Ono Pharmaceutical. Dr. Forde has received grants/research support and advisory fees from Bristol-Myers Squibb and others. Dr. Gray has consulted for and has received grant/research support from Bristol-Myers Squibb and others.
A version of this article first appeared on Medscape.com.
Patients with resectable non–small cell lung cancer (NSCLC) often receive treatment before they undergo surgery. For such patients who achieve a pathologic complete response (pCR), the chances of survival are improved.
However, only a small percentage of patients achieve a pCR with neoadjuvant chemotherapy alone.
Adding the immune checkpoint inhibitor nivolumab to platinum-doublet chemotherapy in the neoadjuvant setting boosts the success rate.
Results from the CheckMate 816 trial show that pCR rates improved from 2.2% with chemotherapy alone to 24% when nivolumab was added.
This difference translated into an odds ratio for achieving a pCR with nivolumab plus chemotherapy of 13.94 (P < .0001), reported Patrick M. Forde, MBBCh, from the Johns Hopkins Kimmel Cancer Center, Baltimore.
This primary endpoint, pCR, was defined as complete regression in both the primary tumor and lymph nodes.
“The magnitude of pCR with nivo plus chemo was similar in stage 1B, II, and stage IIIA disease, as well as in both squamous and nonsquamous histologies,” he added.
Dr. Forde presented the new data at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT003).
The higher pCR rates were seen regardless of PD-L1 expression or tumor mutational burden, Dr. Forde said.
The benefit was also seen when the researchers considered only those patients who subsequently underwent resection (pCR rate of 30.5% with the combination versus 3.2% with chemotherapy alone) and when only the primary tumor was considered (pCR rate of 25.7% vs. 2.8%, respectively).
Change in trial design
Invited discussant Jhanelle Gray, MD, from the Moffitt Cancer Center, Tampa, pointed out that the CheckMate 816 trial originally included an experimental arm with double immunotherapy – ipilimumab plus nivolumab – added onto chemotherapy.
However, this third arm was closed after other trials reported promising results from adding a single immunotherapy onto chemotherapy. For example, results of the single-arm NADIM phase 2 study showed a 77.1% progression-free survival rate at 24 months with the combination of nivolumab, paclitaxel, and carboplatin, and the phase 2 KEYNOTE-021 trial showed that adding pembrolizumab to a standard platinum-doublet chemotherapy regimen nearly doubled response rates among patients with previously untreated advanced nonsquamous NSCLC (although there was no advantage in overall survival).
“Even with the change in trial design, patient characteristics were well balanced between the two arms, and the study met its primary endpoint in the intent-to-treat population,” she said.
Dr. Gray also commented that the choice of pCR as a primary endpoint is “intriguing, and the question remains if it represents a valid surrogate endpoint for survival.”
She noted that a meta-analysis of 32 neoadjuvant chemotherapy-based studies in NSCLC, presented at the 2020 European Society of Medical Oncology annual meeting, showed clear associations of pCR and major pathologic response to both overall survival and event-free survival.
“As these findings were established in a backdrop of chemotherapy, work is needed to confirm these findings in the setting of immunotherapy in particular, as at times, radiographic findings do not correlate with histological findings,” Dr. Gray said.
CheckMate 816 particulars
CheckMate 816 was conducted in 358 patients with newly diagnosed NSCLC with resectable stage IB tumors of at least 4 cm up to stage IIIA tumors, good performance status, and no known EGFR mutations or ALK alterations.
Patients were randomly assigned on an equal basis to receive either nivolumab at 360 mg plus chemotherapy every 3 weeks for three cycles or chemotherapy alone.
Surgery was planned within 6 week after neoadjuvant therapy. Patients could receive (at the investigator’s discretion) adjuvant chemotherapy with or without radiotherapy but no further immunotherapy during follow-up.
In this analysis, patients who did not undergo surgery or for whom evaluable tissue samples were not available were counted among those whose conditions did not respond to therapy.
Major pathologic response rate (≤10% residual viable tumor cells in the primary lung tumor and sampled lymph nodes), which was a secondary endpoint, was also significantly better, at 36.9% versus 8.9%, translating into an OR of 5.70 (95% confidence interval, 3.16-10.26).
In a subset of patients, the investigators assessed clearance of circulating tumor DNA (ctDNA) from day 1 of the first cycle to day 1 of the third cycle using a highly sensitive tumor-informed approach. They found that ctDNA was notably higher with the combination than with chemotherapy alone and that ctDNA clearance correlated with pCR.
Safety similar
“Remarkably, safety was quite similar across the two treatment arms,” Dr. Forde said.
The addition of nivolumab to chemotherapy did not appear to increase either treatment-related adverse events or adverse events of any cause. Grade 3-4 adverse events occurred in 41% of patients in the combination arm versus 44% in the chemotherapy-alone arm.
Treatment-related adverse events leading to discontinuation occurred in 10% of patients in each arm.
Two patients in the nivolumab-chemotherapy arm died from surgically related adverse events (one pulmonary embolism and one aortic rupture). These events were deemed to be unrelated to the study drug.
The investigators are continuing to assess event-free survival and overall survival.
CheckMate 816 is funded by Bristol-Myers Squibb and Ono Pharmaceutical. Dr. Forde has received grants/research support and advisory fees from Bristol-Myers Squibb and others. Dr. Gray has consulted for and has received grant/research support from Bristol-Myers Squibb and others.
A version of this article first appeared on Medscape.com.
FROM AACR 2021
Study IDs most common lingering symptoms 8 months after mild COVID
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Communicating serious news over video. Bringing protocols to the forefront. Sleep and burnout in health care workers. Lung cancer screening.
Palliative and end-of-life care
Communicating serious news over video
Critical care consultation using telemedicine is increasingly prevalent. Having serious conversations regarding end-of life care over video can be extremely challenging. Here are some suggestions and sample phrases to make palliative-focused conversations more successful
Prior to initiating the conversation, communicate with the bedside team. Ensure they want you to discuss palliative options and make an outline of discussion topics together. Identify and include all important decision-makers. Family may need to be connected over a digital meeting platform such as Zoom© or WEBex© and arrange for interpreter services if needed.
Prepare the virtual meeting place ahead of time. Test the connection, and make sure the audiovisual quality is clear. Have the camera centrally positioned, and ensure adequate lighting to easily see facial expressions. Remove distracting background furniture, and clear your space of confidential material. Have a quiet area planned to avoid interruptions (J Gen Intern Med. 2020 Oct 27:1-4. doi: 10.1007/s11606-020-06278-z. Online ahead of print).
Open the conversation with introductions, and explore perceptions with open-ended questions: “So I know where to begin, tell me about your understanding of what has been happening?” Get a sense of the patient’s previous function, quality of life, and their values as an individual. Maintain good eye contact throughout. When ready to give an update, use simple language and avoid details: “Unfortunately, your condition is worse. You have not been responding to treatments as hoped. Your lungs are needing much more support, and I’m worried they are not going to get better.”Anticipate emotions, and provide empathetic responses: “I wish we had better news. This must be overwhelming for you” (Back, et al. Ann Int Med. 2020;172[11]:759). Finally, offer a recommendation. Most patients and families are interested in your advice and want guidance. Use the patient’s previously stated values to support your recommendation.
Andrew Badke, MD
Steering Committee Member
Respiratory care
COVID-19 pandemic bringing protocols to the forefront
COVID-19 has health care organizations threatened like never before. Staffing requirements, equipment necessities, and personnel training happen in a whirlwind.1 Information could change daily/hourly, and the need to protocolize guidelines became more evident each day.
While protocols have existed long before COVID-19, many institutions and organizations responded to the ever-changing pandemic by creating clinical practice guidelines (CPGs) to help not only their experienced staff members but also the non-traditional ICU caregivers thrust onto the front lines.3 Organizations worked on PPE protocols, respiratory care management, and ECMO guidelines to name a few.2,3 Protocols with algorithms and CPGs have been shown to reduce patient harm and improve standardization and communication.
A CPG is a general principle that guides the management of care, in which specific questions are posed, a literature review is completed, and the quality of the research evaluated. The questions are answered using the strength of the available research. CPG decision points are then based on the evidence or on the consensus of experts, resulting in a protocol that are descriptions of detailed behaviors to be followed in specific situations. These behaviors are provided in a list format or a flow diagram. Using a universal language for protocols with algorithms has aided many hospitals ensure effective care for patients and has even helped develop multidisciplinary relationships not present prior to the pandemic (onepagericu.com).
DeDe Gardner, RRT, DrPh, FCCP
Donna Tanner, RRT-ACCS, MBA, FCCP
Steering Committee Members
1. epub JAMA 2/2021.
2. WHO, Guidelines 1/2021.
3. CHEST, Clinical Resources.
4. Curr Treat Options Ped 2015,1:347
Sleep medicine
Time to move the dial: Sleep and burnout in health care workers during the COVID-19 pandemic
Although the interaction between sleep, mood disorders, and burnout is well established, many of us are still sleep-deprived. A cross-sectional study of over 800 health care workers during the pandemic stay-at-home orders in March 2020 reported that those working in-person had shorter sleep times and worse mood, while those with longer sleep times had improved mood (Conroy DA, et al. J Clin Sleep Med. 2021;17[2]:185). Even prior to COVID-19, many trainees were facing issues with sleep deprivation and burnout (Sharp M, et al. Chest. 2021;159[2]:733).
One year into the pandemic, we continue to face a unique set of hardships, exacerbating underlying sleep disorders such as insomnia, feelings of burnout, and mental health problems. An international team led by Dr. Joel Goh calculated the cost of burnout and its economic impact on the nation’s health care system and estimated this at $4.6 billion per year (Han S, et al. Ann Intern Med. 2019;170[11]:784). National medical organizations, including the National Academy of Medicine and the American Medical Association, have also placed greater emphasis on clinician well-being and resilience. Practical frameworks for creating wellness during the pandemic exist; however. senior-level executive champions are critical for implementation (Adibe B, et al. N Engl J Med Catalyst. Jun 2020). While the long-term impact remains unknown, the current state of sleep and mental health problems and the cost of burnout should be a warning to health systems and institutions to implement remedial interventions now.(“Taking action against burnout: A systems approach to professional well-being,” National Academies of Sciences, Engineering, and Medicine, October 2019.
Nancy H. Stewart, DO, MS, Steering Committee Member
Thoracic oncology
Impact of COVID-19 on lung cancer screening
Lung cancer is the leading cause of cancer-related death worldwide and COVID-19 is making this worse. Prior to the COVID-19 pandemic, despite evidence of improved mortality, the uptake of lung cancer screening (LCS) was quite low with only 4% of those eligible having undergone screening in 2015 (Jemal A, et al. JAMA Oncol. 2017;3[9]:1278).
As the COVID-19 pandemic unfolded, health care resources were re-allocated to critically ill patients and areas, and nonurgent care was postponed. Therefore, LCS programs were halted (Mazzone PJ, et al. Chest. 2020;158[1]:406). This led to concerns that fewer patients would undergo screening and more patients would experience delays in cancer diagnosis.
Using population-based modeling, researchers in England estimated the COVID-19 pandemic will result in decreased lung cancer survival and a subsequent increase in avoidable cancer deaths (Maringe C, et al. Lancet Oncol. 2020;21[8]:1023). And in fact, investigators in Spain found fewer new lung cancer diagnoses during the COVID-19 pandemic compared with the same time-period pre-pandemic, and those that were diagnosed were later stage disease (Reyes R, et al. IASCL World Conference. 2020. A3700).
As we learn more about COVID-19 and communities become vaccinated, it becomes critical to both resume LCS programs and improve participation. While the pandemic has hampered efforts to screening patients, it has also facilitated the uptake of new technologies such as telemedicine. In March 2020, due to the COVID-19 pandemic, the Centers for Medicare and Medicaid Services relaxed the rules for telehealth, and now covers shared decisions making (SDM) virtual visits for LCS (Centers for Medicare & Medicaid Services, “Telehealth Services.” ICN MLN901705, March 2020). This new tool, amongst others, could increase access to LCS, facilitate more widespread adoption of screening, and ultimately improve lung cancer outcomes.
Max Wayne, MD, and Jose Cardenas-Garcia, MD
Steering Committee Members
Palliative and end-of-life care
Communicating serious news over video
Critical care consultation using telemedicine is increasingly prevalent. Having serious conversations regarding end-of life care over video can be extremely challenging. Here are some suggestions and sample phrases to make palliative-focused conversations more successful
Prior to initiating the conversation, communicate with the bedside team. Ensure they want you to discuss palliative options and make an outline of discussion topics together. Identify and include all important decision-makers. Family may need to be connected over a digital meeting platform such as Zoom© or WEBex© and arrange for interpreter services if needed.
Prepare the virtual meeting place ahead of time. Test the connection, and make sure the audiovisual quality is clear. Have the camera centrally positioned, and ensure adequate lighting to easily see facial expressions. Remove distracting background furniture, and clear your space of confidential material. Have a quiet area planned to avoid interruptions (J Gen Intern Med. 2020 Oct 27:1-4. doi: 10.1007/s11606-020-06278-z. Online ahead of print).
Open the conversation with introductions, and explore perceptions with open-ended questions: “So I know where to begin, tell me about your understanding of what has been happening?” Get a sense of the patient’s previous function, quality of life, and their values as an individual. Maintain good eye contact throughout. When ready to give an update, use simple language and avoid details: “Unfortunately, your condition is worse. You have not been responding to treatments as hoped. Your lungs are needing much more support, and I’m worried they are not going to get better.”Anticipate emotions, and provide empathetic responses: “I wish we had better news. This must be overwhelming for you” (Back, et al. Ann Int Med. 2020;172[11]:759). Finally, offer a recommendation. Most patients and families are interested in your advice and want guidance. Use the patient’s previously stated values to support your recommendation.
Andrew Badke, MD
Steering Committee Member
Respiratory care
COVID-19 pandemic bringing protocols to the forefront
COVID-19 has health care organizations threatened like never before. Staffing requirements, equipment necessities, and personnel training happen in a whirlwind.1 Information could change daily/hourly, and the need to protocolize guidelines became more evident each day.
While protocols have existed long before COVID-19, many institutions and organizations responded to the ever-changing pandemic by creating clinical practice guidelines (CPGs) to help not only their experienced staff members but also the non-traditional ICU caregivers thrust onto the front lines.3 Organizations worked on PPE protocols, respiratory care management, and ECMO guidelines to name a few.2,3 Protocols with algorithms and CPGs have been shown to reduce patient harm and improve standardization and communication.
A CPG is a general principle that guides the management of care, in which specific questions are posed, a literature review is completed, and the quality of the research evaluated. The questions are answered using the strength of the available research. CPG decision points are then based on the evidence or on the consensus of experts, resulting in a protocol that are descriptions of detailed behaviors to be followed in specific situations. These behaviors are provided in a list format or a flow diagram. Using a universal language for protocols with algorithms has aided many hospitals ensure effective care for patients and has even helped develop multidisciplinary relationships not present prior to the pandemic (onepagericu.com).
DeDe Gardner, RRT, DrPh, FCCP
Donna Tanner, RRT-ACCS, MBA, FCCP
Steering Committee Members
1. epub JAMA 2/2021.
2. WHO, Guidelines 1/2021.
3. CHEST, Clinical Resources.
4. Curr Treat Options Ped 2015,1:347
Sleep medicine
Time to move the dial: Sleep and burnout in health care workers during the COVID-19 pandemic
Although the interaction between sleep, mood disorders, and burnout is well established, many of us are still sleep-deprived. A cross-sectional study of over 800 health care workers during the pandemic stay-at-home orders in March 2020 reported that those working in-person had shorter sleep times and worse mood, while those with longer sleep times had improved mood (Conroy DA, et al. J Clin Sleep Med. 2021;17[2]:185). Even prior to COVID-19, many trainees were facing issues with sleep deprivation and burnout (Sharp M, et al. Chest. 2021;159[2]:733).
One year into the pandemic, we continue to face a unique set of hardships, exacerbating underlying sleep disorders such as insomnia, feelings of burnout, and mental health problems. An international team led by Dr. Joel Goh calculated the cost of burnout and its economic impact on the nation’s health care system and estimated this at $4.6 billion per year (Han S, et al. Ann Intern Med. 2019;170[11]:784). National medical organizations, including the National Academy of Medicine and the American Medical Association, have also placed greater emphasis on clinician well-being and resilience. Practical frameworks for creating wellness during the pandemic exist; however. senior-level executive champions are critical for implementation (Adibe B, et al. N Engl J Med Catalyst. Jun 2020). While the long-term impact remains unknown, the current state of sleep and mental health problems and the cost of burnout should be a warning to health systems and institutions to implement remedial interventions now.(“Taking action against burnout: A systems approach to professional well-being,” National Academies of Sciences, Engineering, and Medicine, October 2019.
Nancy H. Stewart, DO, MS, Steering Committee Member
Thoracic oncology
Impact of COVID-19 on lung cancer screening
Lung cancer is the leading cause of cancer-related death worldwide and COVID-19 is making this worse. Prior to the COVID-19 pandemic, despite evidence of improved mortality, the uptake of lung cancer screening (LCS) was quite low with only 4% of those eligible having undergone screening in 2015 (Jemal A, et al. JAMA Oncol. 2017;3[9]:1278).
As the COVID-19 pandemic unfolded, health care resources were re-allocated to critically ill patients and areas, and nonurgent care was postponed. Therefore, LCS programs were halted (Mazzone PJ, et al. Chest. 2020;158[1]:406). This led to concerns that fewer patients would undergo screening and more patients would experience delays in cancer diagnosis.
Using population-based modeling, researchers in England estimated the COVID-19 pandemic will result in decreased lung cancer survival and a subsequent increase in avoidable cancer deaths (Maringe C, et al. Lancet Oncol. 2020;21[8]:1023). And in fact, investigators in Spain found fewer new lung cancer diagnoses during the COVID-19 pandemic compared with the same time-period pre-pandemic, and those that were diagnosed were later stage disease (Reyes R, et al. IASCL World Conference. 2020. A3700).
As we learn more about COVID-19 and communities become vaccinated, it becomes critical to both resume LCS programs and improve participation. While the pandemic has hampered efforts to screening patients, it has also facilitated the uptake of new technologies such as telemedicine. In March 2020, due to the COVID-19 pandemic, the Centers for Medicare and Medicaid Services relaxed the rules for telehealth, and now covers shared decisions making (SDM) virtual visits for LCS (Centers for Medicare & Medicaid Services, “Telehealth Services.” ICN MLN901705, March 2020). This new tool, amongst others, could increase access to LCS, facilitate more widespread adoption of screening, and ultimately improve lung cancer outcomes.
Max Wayne, MD, and Jose Cardenas-Garcia, MD
Steering Committee Members
Palliative and end-of-life care
Communicating serious news over video
Critical care consultation using telemedicine is increasingly prevalent. Having serious conversations regarding end-of life care over video can be extremely challenging. Here are some suggestions and sample phrases to make palliative-focused conversations more successful
Prior to initiating the conversation, communicate with the bedside team. Ensure they want you to discuss palliative options and make an outline of discussion topics together. Identify and include all important decision-makers. Family may need to be connected over a digital meeting platform such as Zoom© or WEBex© and arrange for interpreter services if needed.
Prepare the virtual meeting place ahead of time. Test the connection, and make sure the audiovisual quality is clear. Have the camera centrally positioned, and ensure adequate lighting to easily see facial expressions. Remove distracting background furniture, and clear your space of confidential material. Have a quiet area planned to avoid interruptions (J Gen Intern Med. 2020 Oct 27:1-4. doi: 10.1007/s11606-020-06278-z. Online ahead of print).
Open the conversation with introductions, and explore perceptions with open-ended questions: “So I know where to begin, tell me about your understanding of what has been happening?” Get a sense of the patient’s previous function, quality of life, and their values as an individual. Maintain good eye contact throughout. When ready to give an update, use simple language and avoid details: “Unfortunately, your condition is worse. You have not been responding to treatments as hoped. Your lungs are needing much more support, and I’m worried they are not going to get better.”Anticipate emotions, and provide empathetic responses: “I wish we had better news. This must be overwhelming for you” (Back, et al. Ann Int Med. 2020;172[11]:759). Finally, offer a recommendation. Most patients and families are interested in your advice and want guidance. Use the patient’s previously stated values to support your recommendation.
Andrew Badke, MD
Steering Committee Member
Respiratory care
COVID-19 pandemic bringing protocols to the forefront
COVID-19 has health care organizations threatened like never before. Staffing requirements, equipment necessities, and personnel training happen in a whirlwind.1 Information could change daily/hourly, and the need to protocolize guidelines became more evident each day.
While protocols have existed long before COVID-19, many institutions and organizations responded to the ever-changing pandemic by creating clinical practice guidelines (CPGs) to help not only their experienced staff members but also the non-traditional ICU caregivers thrust onto the front lines.3 Organizations worked on PPE protocols, respiratory care management, and ECMO guidelines to name a few.2,3 Protocols with algorithms and CPGs have been shown to reduce patient harm and improve standardization and communication.
A CPG is a general principle that guides the management of care, in which specific questions are posed, a literature review is completed, and the quality of the research evaluated. The questions are answered using the strength of the available research. CPG decision points are then based on the evidence or on the consensus of experts, resulting in a protocol that are descriptions of detailed behaviors to be followed in specific situations. These behaviors are provided in a list format or a flow diagram. Using a universal language for protocols with algorithms has aided many hospitals ensure effective care for patients and has even helped develop multidisciplinary relationships not present prior to the pandemic (onepagericu.com).
DeDe Gardner, RRT, DrPh, FCCP
Donna Tanner, RRT-ACCS, MBA, FCCP
Steering Committee Members
1. epub JAMA 2/2021.
2. WHO, Guidelines 1/2021.
3. CHEST, Clinical Resources.
4. Curr Treat Options Ped 2015,1:347
Sleep medicine
Time to move the dial: Sleep and burnout in health care workers during the COVID-19 pandemic
Although the interaction between sleep, mood disorders, and burnout is well established, many of us are still sleep-deprived. A cross-sectional study of over 800 health care workers during the pandemic stay-at-home orders in March 2020 reported that those working in-person had shorter sleep times and worse mood, while those with longer sleep times had improved mood (Conroy DA, et al. J Clin Sleep Med. 2021;17[2]:185). Even prior to COVID-19, many trainees were facing issues with sleep deprivation and burnout (Sharp M, et al. Chest. 2021;159[2]:733).
One year into the pandemic, we continue to face a unique set of hardships, exacerbating underlying sleep disorders such as insomnia, feelings of burnout, and mental health problems. An international team led by Dr. Joel Goh calculated the cost of burnout and its economic impact on the nation’s health care system and estimated this at $4.6 billion per year (Han S, et al. Ann Intern Med. 2019;170[11]:784). National medical organizations, including the National Academy of Medicine and the American Medical Association, have also placed greater emphasis on clinician well-being and resilience. Practical frameworks for creating wellness during the pandemic exist; however. senior-level executive champions are critical for implementation (Adibe B, et al. N Engl J Med Catalyst. Jun 2020). While the long-term impact remains unknown, the current state of sleep and mental health problems and the cost of burnout should be a warning to health systems and institutions to implement remedial interventions now.(“Taking action against burnout: A systems approach to professional well-being,” National Academies of Sciences, Engineering, and Medicine, October 2019.
Nancy H. Stewart, DO, MS, Steering Committee Member
Thoracic oncology
Impact of COVID-19 on lung cancer screening
Lung cancer is the leading cause of cancer-related death worldwide and COVID-19 is making this worse. Prior to the COVID-19 pandemic, despite evidence of improved mortality, the uptake of lung cancer screening (LCS) was quite low with only 4% of those eligible having undergone screening in 2015 (Jemal A, et al. JAMA Oncol. 2017;3[9]:1278).
As the COVID-19 pandemic unfolded, health care resources were re-allocated to critically ill patients and areas, and nonurgent care was postponed. Therefore, LCS programs were halted (Mazzone PJ, et al. Chest. 2020;158[1]:406). This led to concerns that fewer patients would undergo screening and more patients would experience delays in cancer diagnosis.
Using population-based modeling, researchers in England estimated the COVID-19 pandemic will result in decreased lung cancer survival and a subsequent increase in avoidable cancer deaths (Maringe C, et al. Lancet Oncol. 2020;21[8]:1023). And in fact, investigators in Spain found fewer new lung cancer diagnoses during the COVID-19 pandemic compared with the same time-period pre-pandemic, and those that were diagnosed were later stage disease (Reyes R, et al. IASCL World Conference. 2020. A3700).
As we learn more about COVID-19 and communities become vaccinated, it becomes critical to both resume LCS programs and improve participation. While the pandemic has hampered efforts to screening patients, it has also facilitated the uptake of new technologies such as telemedicine. In March 2020, due to the COVID-19 pandemic, the Centers for Medicare and Medicaid Services relaxed the rules for telehealth, and now covers shared decisions making (SDM) virtual visits for LCS (Centers for Medicare & Medicaid Services, “Telehealth Services.” ICN MLN901705, March 2020). This new tool, amongst others, could increase access to LCS, facilitate more widespread adoption of screening, and ultimately improve lung cancer outcomes.
Max Wayne, MD, and Jose Cardenas-Garcia, MD
Steering Committee Members














