User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Delta variant infects six vaccinated guests at outdoor wedding
In April, 92 people gathered in Texas for a wedding. To lower the chances of COVID-19 infection, the festivities were held outside under a large, open-air tent. All 92 guests were required to be fully vaccinated.
Despite those precautions, six people tested positive for the coronavirus and one of them died, Forbes magazine reported, citing a preprint published in medRxiv.
Researchers from Baylor College of Medicine said viral sequencing suggests “the strain containing the Delta variant was transmitted to wedding guests from two patients traveling from India. With no history of vaccine failure in these patients, our observations suggest these are true cases of vaccine breakthrough, mediated by the Delta variant.”
Three females and three males aged 53-69 tested positive for COVID-19. Three were overweight, but none had significant comorbidities or a history of failed vaccination.
The first people to get sick were a man and woman who traveled from India, Forbes reported. The man had no health problems, but the woman had diabetes. Both had gotten two doses of the Covaxin BBV152 vaccine before leaving India.
They tested positive for COVID-19 4 days after the wedding, and the man became so ill he was hospitalized. Six days after the wedding, he died, according to Forbes.
Two people who’d gotten the Pfizer/BioNTech vaccine and two people who received the Moderna vaccine interacted with the first two people, and they also tested positive. One of them, a man in his 60s, had to be hospitalized.
Forbes summed it up this way: “While the available COVID-19 vaccines can offer good protection against COVID-19, the protection is not perfect. As long as the pandemic is continuing, it is better to maintain multiple layers of COVID-19 precautions when you can.”
A version of this article first appeared on WebMD.com.
In April, 92 people gathered in Texas for a wedding. To lower the chances of COVID-19 infection, the festivities were held outside under a large, open-air tent. All 92 guests were required to be fully vaccinated.
Despite those precautions, six people tested positive for the coronavirus and one of them died, Forbes magazine reported, citing a preprint published in medRxiv.
Researchers from Baylor College of Medicine said viral sequencing suggests “the strain containing the Delta variant was transmitted to wedding guests from two patients traveling from India. With no history of vaccine failure in these patients, our observations suggest these are true cases of vaccine breakthrough, mediated by the Delta variant.”
Three females and three males aged 53-69 tested positive for COVID-19. Three were overweight, but none had significant comorbidities or a history of failed vaccination.
The first people to get sick were a man and woman who traveled from India, Forbes reported. The man had no health problems, but the woman had diabetes. Both had gotten two doses of the Covaxin BBV152 vaccine before leaving India.
They tested positive for COVID-19 4 days after the wedding, and the man became so ill he was hospitalized. Six days after the wedding, he died, according to Forbes.
Two people who’d gotten the Pfizer/BioNTech vaccine and two people who received the Moderna vaccine interacted with the first two people, and they also tested positive. One of them, a man in his 60s, had to be hospitalized.
Forbes summed it up this way: “While the available COVID-19 vaccines can offer good protection against COVID-19, the protection is not perfect. As long as the pandemic is continuing, it is better to maintain multiple layers of COVID-19 precautions when you can.”
A version of this article first appeared on WebMD.com.
In April, 92 people gathered in Texas for a wedding. To lower the chances of COVID-19 infection, the festivities were held outside under a large, open-air tent. All 92 guests were required to be fully vaccinated.
Despite those precautions, six people tested positive for the coronavirus and one of them died, Forbes magazine reported, citing a preprint published in medRxiv.
Researchers from Baylor College of Medicine said viral sequencing suggests “the strain containing the Delta variant was transmitted to wedding guests from two patients traveling from India. With no history of vaccine failure in these patients, our observations suggest these are true cases of vaccine breakthrough, mediated by the Delta variant.”
Three females and three males aged 53-69 tested positive for COVID-19. Three were overweight, but none had significant comorbidities or a history of failed vaccination.
The first people to get sick were a man and woman who traveled from India, Forbes reported. The man had no health problems, but the woman had diabetes. Both had gotten two doses of the Covaxin BBV152 vaccine before leaving India.
They tested positive for COVID-19 4 days after the wedding, and the man became so ill he was hospitalized. Six days after the wedding, he died, according to Forbes.
Two people who’d gotten the Pfizer/BioNTech vaccine and two people who received the Moderna vaccine interacted with the first two people, and they also tested positive. One of them, a man in his 60s, had to be hospitalized.
Forbes summed it up this way: “While the available COVID-19 vaccines can offer good protection against COVID-19, the protection is not perfect. As long as the pandemic is continuing, it is better to maintain multiple layers of COVID-19 precautions when you can.”
A version of this article first appeared on WebMD.com.
Medicare proposes direct payments to PAs, telehealth expansion
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
Do patients with cancer need a third shot of COVID vaccine?
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Long COVID symptoms reported by 6% of pediatric patients
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: New vaccinations drop as the case count rises
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.
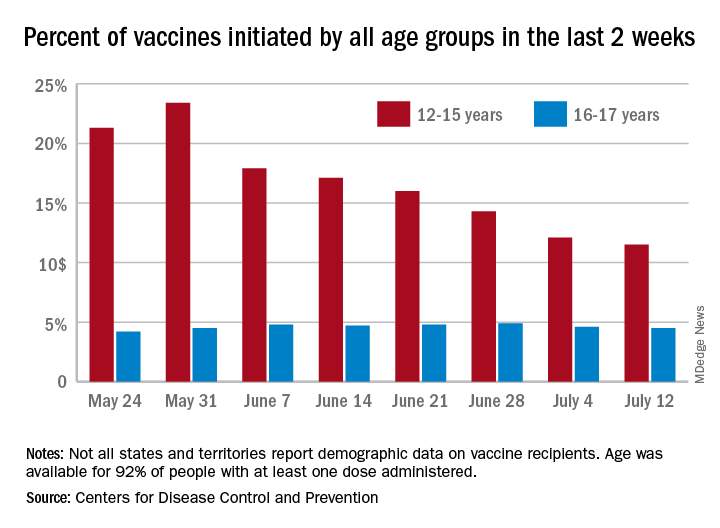
Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
Gender pay gap most pronounced in procedural specialties
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA to warn J&J that vaccine can increase Guillain-Barré risk: Media
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
Updates on COVID-19 guidance for sleep medicine
Background
Well into its second year, the worldwide COVID-19 pandemic continues to pose substantial challenges for health care access and delivery. Regulatory agencies such as the Centers for Disease Control (CDC) do not currently have guidance related to COVID-19 specific to sleep centers and laboratories. In March 2020, within days of the World Health Organization pandemic declaration, the American Academy of Sleep Medicine (AASM) posted detailed guidance on mitigation strategies for sleep medicine practices (COVID-19 Resources).
This initial guidance has been previously reported in this publication (Sullivan S, Gurubhagavatula I. CHEST Physician 2020 May 8), and the guidance has been periodically updated during the pandemic. It was restructured in mid-2020 to include sections summarizing CDC recommendations germane for sleep practices; additional sleep medicine-specific guidance from the AASM COVID-19 Task Force (TF); and a frequently asked questions (FAQ) section. The last major update from the task force occurred on Jan. 18, 2021, though subsequent posts – especially related to recent CDC changes in masking guidelines – were made in May 2021. The purpose of this article is to summarize these updates and to call attention to areas of ongoing interest to sleep medicine. Notably, the AASM Task Force guidance is nonbinding and offered as a framework for considering best practices in this evolving situation, acknowledging the importance of weighing local factors, conditions, and regulations, as well as the interests of and risks to the patient, staff, and providers.
Key updates
Data on exposure and transmission risks specific to sleep medicine
Measures for reducing viral transmission have been central to managing the spread of the virus in clinical settings. In its last major update, the AASM TF noted that no known outbreaks of COVID-19 related to sleep center exposure have been reported. A perspective and data published in the Journal of the American Medical Association concluded that hospital transmission of the virus “in the setting of universal masking is likely rare, even during periods of high community prevalence.” It also concluded that hospital-based outbreaks are more likely to occur in small workrooms and during mealtime when staff are less adherent to masking and physical distancing (Richterman A, et al. JAMA. 2020;324[21]:2155-6). The TF elaborated on considerations to reduce transmission, which include not just telework and foundational infection control practices, but also broader workplace considerations such as optimizing ventilation, taking advantage of outdoor spaces (e.g., for breaks and eating), scheduling to reduce interactions between personnel from different teams, minimizing contact in meeting/break rooms, removing tables and chairs from lounge areas, and following CDC guidance for effective facility operations.
Vaccination
In the January update, the AASM COVID-19 TF stated that, “sleep facility leaders should encourage staff and patients to be vaccinated in accordance with CDC guidance.” The role of the sleep medicine community in encouraging healthy sleep habits before and after vaccination was emphasized, pointing to evidence linking sleep and immunity, specifically between sleep duration and vaccination response (Healthy sleep and immune response to COVID-19 vaccination. 2021 Jan.).
In an FAQ update from March 26, 2021, considering whether continued COVID-19 testing was needed following full vaccination, the AASM advised testing prior to potential aerosol-generating procedures should be made on the basis of a risk-benefit assessment by the sleep clinician. Several considerations were highlighted, including recent COVID-19 infection, vaccination status of contacts, local prevalence of newer variants, and whether individuals are receiving positive airway pressure therapy. The TF focused on the vigilance for residents and staff in long-term care facilities, which have been associated with a number of outbreaks.
Masking in the context of the COVID-19 vaccine
The most significant change in recommendations is the recent relaxation of masking guidance by the CDC in the setting of the approval and distribution of COVID-19 vaccinations. In May, the CDC stated that fully vaccinated individuals can resume activities without masking or physically distancing except in scenarios of travel and where required by laws, regulations, and local businesses, due to the efficacy of the vaccines, increasing evidence of reduced asymptomatic carriage and transmission after vaccination, and anticipated increased uptake of vaccination. However, the CDC also noted that these updates did not apply to health care facilities, where the recommendation remains that patients and visitors should continue to mask throughout their stay. Additionally, fully vaccinated health care workers should continue to practice infection control measures while working with patients. On May 14, the AASM TF provided a detailed FAQ acknowledging the CDC’s new guidance, emphasizing that masking guidance in health care facilities remains unchanged, and encouraging individuals to follow CDC guidance regarding vaccination, noting that emergence of newer variants continues to be monitored, and existing vaccines still appear to induce neutralizing antibodies even if to a somewhat lower degree. The situation for pediatric sleep centers has been highlighted in particular because the potential risk posed by newer variants to children remains under investigation, and children under age 12 are not approved for vaccination.
Important caveats to discussions around vaccination status are the lack of a centralized method to identify vaccinated individuals, the unknown duration of immunity, and reports of the use of fake vaccine cards. At this time, in health care settings, vaccination status should not exempt mask usage for any individual.
Sleep medicine care for those with COVID-19
Regarding the duration of isolation and precautions for adults with COVID-19, the TF highlighted the CDC’s symptom-based strategy, rather than test-based strategy, for ending isolation of these patients, availing them of sleep medicine services in person.
In line with the CDC guidance, this approach indicates that scheduling in-person care such as polysomnography for a COVID-19–positive patient may be appropriate at least 10 days after symptom onset (or after a positive test if the patient never developed symptoms); or at least 20 days after symptom onset if the illness was severe; or if at least 90 days have elapsed since symptom onset, consider preappointment COVID-19 screening. In the context of immunocompromised individuals, involvement from infectious disease specialists may be needed to help guide decisions.
Patient communications
For many, a repercussion of the pandemic has been delaying care or avoiding addressing medical issues, including sleep disorders. The AASM encouraged practices to consider communicating with patients that delaying needed care can increase health risks; COVID-19 transmission to patients in health care settings has been low; effective safety procedures are in place; and whether remote/telehealth services are available.
Disparities in care
In addition to the specific guidance above, there are ongoing concerns regarding disparities in care resulting from a variety of sources and becoming more evident during the pandemic. Complex factors, ranging from economic, geographic, contextual, occupational, and others contribute to disparities that health care systems – and sleep medicine - have not been able to adequately address (Jackson CL and Johnson DA. J Clin Sleep Med. 16[8]:1401-2). More specific differences may include Internet access, reduced access due to socioeconomic barriers, transportation limitations, medical mistrust, and membership in a medically vulnerable group such as children, the elderly, and those with high acuity needs. For example, in pediatric patients there exist few evidence-based alternatives and guidelines to in-lab testing and care, which may have negatively impacted access to needed sleep medicine services (Sullivan S et al. J Clin Sleep Med. 2021 Mar 1;17[3]:361-2).
Economics in the COVID-19 pandemic
The economic effects of COVID-19 on medical institutions and in sleep medicine is a story that continues to unfold. Reductions in patient visits and elective procedures, infection control measures limiting capacity, increased costs to maintain such measures, and variability of responses by payer and region are just a few of the issues. The Centers for Medicare & Medicaid Services has employed waivers to increased flexibility and promote safe and effective care including the use of telemedicine during the public health emergency, but the future of these waivers remains uncertain. Alarmingly, a sizeable portion of sleep practices reported financial solvency concerns related to the pandemic (Ramar K. J Clin Sleep Med. 2020;16[11]:1939-42).
Conclusion
As the COVID-19 pandemic and related public health guidance continues to evolve, sleep medicine practices continue to adapt. Vaccination, new variants, changes in mask guidance, new outbreaks around the globe, financial and staffing uncertainties, as well as addressing disparities in care and outcomes that may be augmented by the pandemic remain salient areas of ongoing development.
Dr. Lee is a Postdoctoral and Pediatric Pulmonary Fellow, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, Stanford University School of Medicine; Dr. Sullivan is Clinical Professor, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, and by courtesy, Division of Sleep Medicine, Department of Psychiatry, Stanford University School of Medicine, Palo Alto, CA.
Background
Well into its second year, the worldwide COVID-19 pandemic continues to pose substantial challenges for health care access and delivery. Regulatory agencies such as the Centers for Disease Control (CDC) do not currently have guidance related to COVID-19 specific to sleep centers and laboratories. In March 2020, within days of the World Health Organization pandemic declaration, the American Academy of Sleep Medicine (AASM) posted detailed guidance on mitigation strategies for sleep medicine practices (COVID-19 Resources).
This initial guidance has been previously reported in this publication (Sullivan S, Gurubhagavatula I. CHEST Physician 2020 May 8), and the guidance has been periodically updated during the pandemic. It was restructured in mid-2020 to include sections summarizing CDC recommendations germane for sleep practices; additional sleep medicine-specific guidance from the AASM COVID-19 Task Force (TF); and a frequently asked questions (FAQ) section. The last major update from the task force occurred on Jan. 18, 2021, though subsequent posts – especially related to recent CDC changes in masking guidelines – were made in May 2021. The purpose of this article is to summarize these updates and to call attention to areas of ongoing interest to sleep medicine. Notably, the AASM Task Force guidance is nonbinding and offered as a framework for considering best practices in this evolving situation, acknowledging the importance of weighing local factors, conditions, and regulations, as well as the interests of and risks to the patient, staff, and providers.
Key updates
Data on exposure and transmission risks specific to sleep medicine
Measures for reducing viral transmission have been central to managing the spread of the virus in clinical settings. In its last major update, the AASM TF noted that no known outbreaks of COVID-19 related to sleep center exposure have been reported. A perspective and data published in the Journal of the American Medical Association concluded that hospital transmission of the virus “in the setting of universal masking is likely rare, even during periods of high community prevalence.” It also concluded that hospital-based outbreaks are more likely to occur in small workrooms and during mealtime when staff are less adherent to masking and physical distancing (Richterman A, et al. JAMA. 2020;324[21]:2155-6). The TF elaborated on considerations to reduce transmission, which include not just telework and foundational infection control practices, but also broader workplace considerations such as optimizing ventilation, taking advantage of outdoor spaces (e.g., for breaks and eating), scheduling to reduce interactions between personnel from different teams, minimizing contact in meeting/break rooms, removing tables and chairs from lounge areas, and following CDC guidance for effective facility operations.
Vaccination
In the January update, the AASM COVID-19 TF stated that, “sleep facility leaders should encourage staff and patients to be vaccinated in accordance with CDC guidance.” The role of the sleep medicine community in encouraging healthy sleep habits before and after vaccination was emphasized, pointing to evidence linking sleep and immunity, specifically between sleep duration and vaccination response (Healthy sleep and immune response to COVID-19 vaccination. 2021 Jan.).
In an FAQ update from March 26, 2021, considering whether continued COVID-19 testing was needed following full vaccination, the AASM advised testing prior to potential aerosol-generating procedures should be made on the basis of a risk-benefit assessment by the sleep clinician. Several considerations were highlighted, including recent COVID-19 infection, vaccination status of contacts, local prevalence of newer variants, and whether individuals are receiving positive airway pressure therapy. The TF focused on the vigilance for residents and staff in long-term care facilities, which have been associated with a number of outbreaks.
Masking in the context of the COVID-19 vaccine
The most significant change in recommendations is the recent relaxation of masking guidance by the CDC in the setting of the approval and distribution of COVID-19 vaccinations. In May, the CDC stated that fully vaccinated individuals can resume activities without masking or physically distancing except in scenarios of travel and where required by laws, regulations, and local businesses, due to the efficacy of the vaccines, increasing evidence of reduced asymptomatic carriage and transmission after vaccination, and anticipated increased uptake of vaccination. However, the CDC also noted that these updates did not apply to health care facilities, where the recommendation remains that patients and visitors should continue to mask throughout their stay. Additionally, fully vaccinated health care workers should continue to practice infection control measures while working with patients. On May 14, the AASM TF provided a detailed FAQ acknowledging the CDC’s new guidance, emphasizing that masking guidance in health care facilities remains unchanged, and encouraging individuals to follow CDC guidance regarding vaccination, noting that emergence of newer variants continues to be monitored, and existing vaccines still appear to induce neutralizing antibodies even if to a somewhat lower degree. The situation for pediatric sleep centers has been highlighted in particular because the potential risk posed by newer variants to children remains under investigation, and children under age 12 are not approved for vaccination.
Important caveats to discussions around vaccination status are the lack of a centralized method to identify vaccinated individuals, the unknown duration of immunity, and reports of the use of fake vaccine cards. At this time, in health care settings, vaccination status should not exempt mask usage for any individual.
Sleep medicine care for those with COVID-19
Regarding the duration of isolation and precautions for adults with COVID-19, the TF highlighted the CDC’s symptom-based strategy, rather than test-based strategy, for ending isolation of these patients, availing them of sleep medicine services in person.
In line with the CDC guidance, this approach indicates that scheduling in-person care such as polysomnography for a COVID-19–positive patient may be appropriate at least 10 days after symptom onset (or after a positive test if the patient never developed symptoms); or at least 20 days after symptom onset if the illness was severe; or if at least 90 days have elapsed since symptom onset, consider preappointment COVID-19 screening. In the context of immunocompromised individuals, involvement from infectious disease specialists may be needed to help guide decisions.
Patient communications
For many, a repercussion of the pandemic has been delaying care or avoiding addressing medical issues, including sleep disorders. The AASM encouraged practices to consider communicating with patients that delaying needed care can increase health risks; COVID-19 transmission to patients in health care settings has been low; effective safety procedures are in place; and whether remote/telehealth services are available.
Disparities in care
In addition to the specific guidance above, there are ongoing concerns regarding disparities in care resulting from a variety of sources and becoming more evident during the pandemic. Complex factors, ranging from economic, geographic, contextual, occupational, and others contribute to disparities that health care systems – and sleep medicine - have not been able to adequately address (Jackson CL and Johnson DA. J Clin Sleep Med. 16[8]:1401-2). More specific differences may include Internet access, reduced access due to socioeconomic barriers, transportation limitations, medical mistrust, and membership in a medically vulnerable group such as children, the elderly, and those with high acuity needs. For example, in pediatric patients there exist few evidence-based alternatives and guidelines to in-lab testing and care, which may have negatively impacted access to needed sleep medicine services (Sullivan S et al. J Clin Sleep Med. 2021 Mar 1;17[3]:361-2).
Economics in the COVID-19 pandemic
The economic effects of COVID-19 on medical institutions and in sleep medicine is a story that continues to unfold. Reductions in patient visits and elective procedures, infection control measures limiting capacity, increased costs to maintain such measures, and variability of responses by payer and region are just a few of the issues. The Centers for Medicare & Medicaid Services has employed waivers to increased flexibility and promote safe and effective care including the use of telemedicine during the public health emergency, but the future of these waivers remains uncertain. Alarmingly, a sizeable portion of sleep practices reported financial solvency concerns related to the pandemic (Ramar K. J Clin Sleep Med. 2020;16[11]:1939-42).
Conclusion
As the COVID-19 pandemic and related public health guidance continues to evolve, sleep medicine practices continue to adapt. Vaccination, new variants, changes in mask guidance, new outbreaks around the globe, financial and staffing uncertainties, as well as addressing disparities in care and outcomes that may be augmented by the pandemic remain salient areas of ongoing development.
Dr. Lee is a Postdoctoral and Pediatric Pulmonary Fellow, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, Stanford University School of Medicine; Dr. Sullivan is Clinical Professor, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, and by courtesy, Division of Sleep Medicine, Department of Psychiatry, Stanford University School of Medicine, Palo Alto, CA.
Background
Well into its second year, the worldwide COVID-19 pandemic continues to pose substantial challenges for health care access and delivery. Regulatory agencies such as the Centers for Disease Control (CDC) do not currently have guidance related to COVID-19 specific to sleep centers and laboratories. In March 2020, within days of the World Health Organization pandemic declaration, the American Academy of Sleep Medicine (AASM) posted detailed guidance on mitigation strategies for sleep medicine practices (COVID-19 Resources).
This initial guidance has been previously reported in this publication (Sullivan S, Gurubhagavatula I. CHEST Physician 2020 May 8), and the guidance has been periodically updated during the pandemic. It was restructured in mid-2020 to include sections summarizing CDC recommendations germane for sleep practices; additional sleep medicine-specific guidance from the AASM COVID-19 Task Force (TF); and a frequently asked questions (FAQ) section. The last major update from the task force occurred on Jan. 18, 2021, though subsequent posts – especially related to recent CDC changes in masking guidelines – were made in May 2021. The purpose of this article is to summarize these updates and to call attention to areas of ongoing interest to sleep medicine. Notably, the AASM Task Force guidance is nonbinding and offered as a framework for considering best practices in this evolving situation, acknowledging the importance of weighing local factors, conditions, and regulations, as well as the interests of and risks to the patient, staff, and providers.
Key updates
Data on exposure and transmission risks specific to sleep medicine
Measures for reducing viral transmission have been central to managing the spread of the virus in clinical settings. In its last major update, the AASM TF noted that no known outbreaks of COVID-19 related to sleep center exposure have been reported. A perspective and data published in the Journal of the American Medical Association concluded that hospital transmission of the virus “in the setting of universal masking is likely rare, even during periods of high community prevalence.” It also concluded that hospital-based outbreaks are more likely to occur in small workrooms and during mealtime when staff are less adherent to masking and physical distancing (Richterman A, et al. JAMA. 2020;324[21]:2155-6). The TF elaborated on considerations to reduce transmission, which include not just telework and foundational infection control practices, but also broader workplace considerations such as optimizing ventilation, taking advantage of outdoor spaces (e.g., for breaks and eating), scheduling to reduce interactions between personnel from different teams, minimizing contact in meeting/break rooms, removing tables and chairs from lounge areas, and following CDC guidance for effective facility operations.
Vaccination
In the January update, the AASM COVID-19 TF stated that, “sleep facility leaders should encourage staff and patients to be vaccinated in accordance with CDC guidance.” The role of the sleep medicine community in encouraging healthy sleep habits before and after vaccination was emphasized, pointing to evidence linking sleep and immunity, specifically between sleep duration and vaccination response (Healthy sleep and immune response to COVID-19 vaccination. 2021 Jan.).
In an FAQ update from March 26, 2021, considering whether continued COVID-19 testing was needed following full vaccination, the AASM advised testing prior to potential aerosol-generating procedures should be made on the basis of a risk-benefit assessment by the sleep clinician. Several considerations were highlighted, including recent COVID-19 infection, vaccination status of contacts, local prevalence of newer variants, and whether individuals are receiving positive airway pressure therapy. The TF focused on the vigilance for residents and staff in long-term care facilities, which have been associated with a number of outbreaks.
Masking in the context of the COVID-19 vaccine
The most significant change in recommendations is the recent relaxation of masking guidance by the CDC in the setting of the approval and distribution of COVID-19 vaccinations. In May, the CDC stated that fully vaccinated individuals can resume activities without masking or physically distancing except in scenarios of travel and where required by laws, regulations, and local businesses, due to the efficacy of the vaccines, increasing evidence of reduced asymptomatic carriage and transmission after vaccination, and anticipated increased uptake of vaccination. However, the CDC also noted that these updates did not apply to health care facilities, where the recommendation remains that patients and visitors should continue to mask throughout their stay. Additionally, fully vaccinated health care workers should continue to practice infection control measures while working with patients. On May 14, the AASM TF provided a detailed FAQ acknowledging the CDC’s new guidance, emphasizing that masking guidance in health care facilities remains unchanged, and encouraging individuals to follow CDC guidance regarding vaccination, noting that emergence of newer variants continues to be monitored, and existing vaccines still appear to induce neutralizing antibodies even if to a somewhat lower degree. The situation for pediatric sleep centers has been highlighted in particular because the potential risk posed by newer variants to children remains under investigation, and children under age 12 are not approved for vaccination.
Important caveats to discussions around vaccination status are the lack of a centralized method to identify vaccinated individuals, the unknown duration of immunity, and reports of the use of fake vaccine cards. At this time, in health care settings, vaccination status should not exempt mask usage for any individual.
Sleep medicine care for those with COVID-19
Regarding the duration of isolation and precautions for adults with COVID-19, the TF highlighted the CDC’s symptom-based strategy, rather than test-based strategy, for ending isolation of these patients, availing them of sleep medicine services in person.
In line with the CDC guidance, this approach indicates that scheduling in-person care such as polysomnography for a COVID-19–positive patient may be appropriate at least 10 days after symptom onset (or after a positive test if the patient never developed symptoms); or at least 20 days after symptom onset if the illness was severe; or if at least 90 days have elapsed since symptom onset, consider preappointment COVID-19 screening. In the context of immunocompromised individuals, involvement from infectious disease specialists may be needed to help guide decisions.
Patient communications
For many, a repercussion of the pandemic has been delaying care or avoiding addressing medical issues, including sleep disorders. The AASM encouraged practices to consider communicating with patients that delaying needed care can increase health risks; COVID-19 transmission to patients in health care settings has been low; effective safety procedures are in place; and whether remote/telehealth services are available.
Disparities in care
In addition to the specific guidance above, there are ongoing concerns regarding disparities in care resulting from a variety of sources and becoming more evident during the pandemic. Complex factors, ranging from economic, geographic, contextual, occupational, and others contribute to disparities that health care systems – and sleep medicine - have not been able to adequately address (Jackson CL and Johnson DA. J Clin Sleep Med. 16[8]:1401-2). More specific differences may include Internet access, reduced access due to socioeconomic barriers, transportation limitations, medical mistrust, and membership in a medically vulnerable group such as children, the elderly, and those with high acuity needs. For example, in pediatric patients there exist few evidence-based alternatives and guidelines to in-lab testing and care, which may have negatively impacted access to needed sleep medicine services (Sullivan S et al. J Clin Sleep Med. 2021 Mar 1;17[3]:361-2).
Economics in the COVID-19 pandemic
The economic effects of COVID-19 on medical institutions and in sleep medicine is a story that continues to unfold. Reductions in patient visits and elective procedures, infection control measures limiting capacity, increased costs to maintain such measures, and variability of responses by payer and region are just a few of the issues. The Centers for Medicare & Medicaid Services has employed waivers to increased flexibility and promote safe and effective care including the use of telemedicine during the public health emergency, but the future of these waivers remains uncertain. Alarmingly, a sizeable portion of sleep practices reported financial solvency concerns related to the pandemic (Ramar K. J Clin Sleep Med. 2020;16[11]:1939-42).
Conclusion
As the COVID-19 pandemic and related public health guidance continues to evolve, sleep medicine practices continue to adapt. Vaccination, new variants, changes in mask guidance, new outbreaks around the globe, financial and staffing uncertainties, as well as addressing disparities in care and outcomes that may be augmented by the pandemic remain salient areas of ongoing development.
Dr. Lee is a Postdoctoral and Pediatric Pulmonary Fellow, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, Stanford University School of Medicine; Dr. Sullivan is Clinical Professor, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, and by courtesy, Division of Sleep Medicine, Department of Psychiatry, Stanford University School of Medicine, Palo Alto, CA.
This month in the journal CHEST®
Editor’s picks
Hormone replacement therapy and development of new asthma. By Dr. E. Hansen et al.
Sex and gender omic biomarkers in men and women with COPD: Considerations for precision medicine. By Dr. D. Demeo.
Pulmonary function and radiological features in survivors of critical covid-19: A 3-month prospective cohort. By Dr. F. Barbe et al.
Characteristics and prevalence of domestic and occupational inhalational exposures across interstitial lung diseases. By Dr. C. Lee et al.
Identification and remediation of environmental exposures in patients with interstitial lung disease: Evidence review and practical considerations. By Dr. M. Salisbury et al.
How we do it: Creating an organizational culture for the chest physician. By Dr. J. Stoller et al..
Proposed quality metrics for lung cancer screening programs: A national lung cancer roundtable project. By Dr. P. Mazzone et al.
Editor’s picks
Editor’s picks
Hormone replacement therapy and development of new asthma. By Dr. E. Hansen et al.
Sex and gender omic biomarkers in men and women with COPD: Considerations for precision medicine. By Dr. D. Demeo.
Pulmonary function and radiological features in survivors of critical covid-19: A 3-month prospective cohort. By Dr. F. Barbe et al.
Characteristics and prevalence of domestic and occupational inhalational exposures across interstitial lung diseases. By Dr. C. Lee et al.
Identification and remediation of environmental exposures in patients with interstitial lung disease: Evidence review and practical considerations. By Dr. M. Salisbury et al.
How we do it: Creating an organizational culture for the chest physician. By Dr. J. Stoller et al..
Proposed quality metrics for lung cancer screening programs: A national lung cancer roundtable project. By Dr. P. Mazzone et al.
Hormone replacement therapy and development of new asthma. By Dr. E. Hansen et al.
Sex and gender omic biomarkers in men and women with COPD: Considerations for precision medicine. By Dr. D. Demeo.
Pulmonary function and radiological features in survivors of critical covid-19: A 3-month prospective cohort. By Dr. F. Barbe et al.
Characteristics and prevalence of domestic and occupational inhalational exposures across interstitial lung diseases. By Dr. C. Lee et al.
Identification and remediation of environmental exposures in patients with interstitial lung disease: Evidence review and practical considerations. By Dr. M. Salisbury et al.
How we do it: Creating an organizational culture for the chest physician. By Dr. J. Stoller et al..
Proposed quality metrics for lung cancer screening programs: A national lung cancer roundtable project. By Dr. P. Mazzone et al.
Get active while funding CHEST Foundation microgrants
The NetWorks Challenge 2021 is kicking off in July with a 25k to celebrate the Foundation’s 25th anniversary. This year, we’re asking each NetWork to participate in a physical challenge, virtually. Make your way to 25k by walking, running, biking – or any activity that suits you.
Through the challenge, you can engage in friendly competition while supporting the goals of the Foundation. This year, money raised will directly help us in addressing health disparities through our microgrants program and will support travel grants for doctors-in-training looking to attend CHEST 2021.
With your help, by participating in the NetWorks Challenge, we can fund grants that aim to lend a hand to those who need it the most. Expanding research capabilities, improving patient care, and giving access to medical equipment are just a few ways microgrants from the CHEST Foundation have been used in the past.
Salim Surani, MD, MSc, FCCP, is a long-time supporter of the NetWorks Challenge and the Foundation’s grants program. “Whatever the Foundation pays in terms of grants and awards not only impacts the recipient but also the community as a whole ... For me, it was a no-brainer to get involved in an organization that actually raises funding to support community, education, and research,” Dr. Surani said.
With your support, during the NetWorks Challenge, we can provide grants to more clinicians looking to make a difference in chest medicine.
Encourage your NetWork members to join you this summer in the race to 25k.
“When you work within the NetWorks and join together, and work along with the CHEST Foundation, the impact is much more powerful. I always believed that it is a privilege for us that we have the outlet at the CHEST Foundation to provide grants,” Dr. Surani said.
To learn more about this initiative and this year’s NetWorks Challenge, visit the CHEST Foundation’s website at https://foundation.chestnet.org/.
The NetWorks Challenge 2021 is kicking off in July with a 25k to celebrate the Foundation’s 25th anniversary. This year, we’re asking each NetWork to participate in a physical challenge, virtually. Make your way to 25k by walking, running, biking – or any activity that suits you.
Through the challenge, you can engage in friendly competition while supporting the goals of the Foundation. This year, money raised will directly help us in addressing health disparities through our microgrants program and will support travel grants for doctors-in-training looking to attend CHEST 2021.
With your help, by participating in the NetWorks Challenge, we can fund grants that aim to lend a hand to those who need it the most. Expanding research capabilities, improving patient care, and giving access to medical equipment are just a few ways microgrants from the CHEST Foundation have been used in the past.
Salim Surani, MD, MSc, FCCP, is a long-time supporter of the NetWorks Challenge and the Foundation’s grants program. “Whatever the Foundation pays in terms of grants and awards not only impacts the recipient but also the community as a whole ... For me, it was a no-brainer to get involved in an organization that actually raises funding to support community, education, and research,” Dr. Surani said.
With your support, during the NetWorks Challenge, we can provide grants to more clinicians looking to make a difference in chest medicine.
Encourage your NetWork members to join you this summer in the race to 25k.
“When you work within the NetWorks and join together, and work along with the CHEST Foundation, the impact is much more powerful. I always believed that it is a privilege for us that we have the outlet at the CHEST Foundation to provide grants,” Dr. Surani said.
To learn more about this initiative and this year’s NetWorks Challenge, visit the CHEST Foundation’s website at https://foundation.chestnet.org/.
The NetWorks Challenge 2021 is kicking off in July with a 25k to celebrate the Foundation’s 25th anniversary. This year, we’re asking each NetWork to participate in a physical challenge, virtually. Make your way to 25k by walking, running, biking – or any activity that suits you.
Through the challenge, you can engage in friendly competition while supporting the goals of the Foundation. This year, money raised will directly help us in addressing health disparities through our microgrants program and will support travel grants for doctors-in-training looking to attend CHEST 2021.
With your help, by participating in the NetWorks Challenge, we can fund grants that aim to lend a hand to those who need it the most. Expanding research capabilities, improving patient care, and giving access to medical equipment are just a few ways microgrants from the CHEST Foundation have been used in the past.
Salim Surani, MD, MSc, FCCP, is a long-time supporter of the NetWorks Challenge and the Foundation’s grants program. “Whatever the Foundation pays in terms of grants and awards not only impacts the recipient but also the community as a whole ... For me, it was a no-brainer to get involved in an organization that actually raises funding to support community, education, and research,” Dr. Surani said.
With your support, during the NetWorks Challenge, we can provide grants to more clinicians looking to make a difference in chest medicine.
Encourage your NetWork members to join you this summer in the race to 25k.
“When you work within the NetWorks and join together, and work along with the CHEST Foundation, the impact is much more powerful. I always believed that it is a privilege for us that we have the outlet at the CHEST Foundation to provide grants,” Dr. Surani said.
To learn more about this initiative and this year’s NetWorks Challenge, visit the CHEST Foundation’s website at https://foundation.chestnet.org/.