User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Fibrosis progression flies below the radar in subclinical ILD
Subclinical or preclinical interstitial lung disease in patients with connective tissue diseases is not a benign entity, and many patients may experience progression of lung fibrosis before a diagnosis of ILD is made, investigators caution.
Among patients with connective tissue disease assessed with baseline and follow-up high-resolution CT scans for ILD, nearly one-fourth had evidence of ILD progression over a median of 4.5 years, reported Anna-Maria Hoffmann-Vold, MD, PhD, from Oslo University Hospital.
“Subclinical ILD is frequently present across all connective tissue diseases. It progresses over time in a substantial subgroup of people comparable to patients with clinical ILD, and our findings really question the terms ‘subclinical/preclinical ILD,’ which may potentially lead to a suboptimal watchful waiting management,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Jesse Roman, MD, CEO at the Jane & Leonard Korman Respiratory Institute at Thomas Jefferson University, Philadelphia, who was not involved in the study, commented that the findings regarding subclinical disease come as no surprise.
“The connective tissue disorders are linked to interstitial lung disease, and we believe that they are the primary causes of interstitial lung diseases in most countries,” he said in an interview.
“Basically, what you’re detecting is that if you can identify these people early, then you can see that they behave like any other patients with interstitial lung disease with progression, so most experts recommend that patients with any kind of connective tissue disorder be followed with either CT scans or pulmonary function tests, or carefully interviewed every time they come to identify any kind of very early interstitial lung disease – particularly in patients with rheumatoid arthritis, in patients with systemic sclerosis, and in patients with dermatomyositis,” Dr. Roman said.
He noted that when patients present with an idiopathic or undiagnosed condition suggestive of ILD, clinicians at his center will order serology tests to detect potential cases of subclinical connective tissue disorders.
Observational study
Dr. Hoffmann-Vold and colleagues looked at 525 patients with connective tissue diseases assessed for ILD at their center, including 296 with systemic sclerosis, 94 with anti-synthetase syndrome, and 135 with mixed connective tissue disease.
They used semiquantitative assessment to determine the prevalence of ILD, defining subclinical disease as ILD extent of less than 5% on high-resolution CT, preserved lung function with forced vital capacity (FVC) greater than 80% of predicted, and no respiratory symptoms.
Clinical ILD was defined as either ILD extent greater than 5%, or ILD extent below 5% but with respiratory symptoms and FVC below 80% of predicted.
They found that 44% of the patients had ILD on high-resolution CT, 43% had no evidence of ILD, and 13% had subclinical ILD.
In a comparison of patients without ILD and those with either clinical or subclinical ILD, they found that, while the mean patient age was about 51 in all three groups, men were more likely than women to have clinical ILD. A higher proportion of patients with clinical ILD (39%) died during the total observation period of about 13 years, compared with 22% of patients without ILD, and 18% of those with subclinical ILD.
As noted before, of 395 patients with baseline and follow-up high-resolution CT, 95 (24%) had evidence of lung fibrosis progression, with 38% of patients with subclinical ILD and 51% of patients with clinical ILD having progression during follow-up.
“In our connective tissue disease patients with ILD, the symptoms-define-disease argument would clearly lead to [the idea] that ILD is not a disease until patients become symptomatic, which we all know is frequently appearing in advanced stages of ILD,” Dr. Hoffmann-Vold said.
The study was funded by Oslo University Hospital. Dr. Hoffmann-Vold and Dr. Roman reported no relevant conflicts of interest to disclose.
Subclinical or preclinical interstitial lung disease in patients with connective tissue diseases is not a benign entity, and many patients may experience progression of lung fibrosis before a diagnosis of ILD is made, investigators caution.
Among patients with connective tissue disease assessed with baseline and follow-up high-resolution CT scans for ILD, nearly one-fourth had evidence of ILD progression over a median of 4.5 years, reported Anna-Maria Hoffmann-Vold, MD, PhD, from Oslo University Hospital.
“Subclinical ILD is frequently present across all connective tissue diseases. It progresses over time in a substantial subgroup of people comparable to patients with clinical ILD, and our findings really question the terms ‘subclinical/preclinical ILD,’ which may potentially lead to a suboptimal watchful waiting management,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Jesse Roman, MD, CEO at the Jane & Leonard Korman Respiratory Institute at Thomas Jefferson University, Philadelphia, who was not involved in the study, commented that the findings regarding subclinical disease come as no surprise.
“The connective tissue disorders are linked to interstitial lung disease, and we believe that they are the primary causes of interstitial lung diseases in most countries,” he said in an interview.
“Basically, what you’re detecting is that if you can identify these people early, then you can see that they behave like any other patients with interstitial lung disease with progression, so most experts recommend that patients with any kind of connective tissue disorder be followed with either CT scans or pulmonary function tests, or carefully interviewed every time they come to identify any kind of very early interstitial lung disease – particularly in patients with rheumatoid arthritis, in patients with systemic sclerosis, and in patients with dermatomyositis,” Dr. Roman said.
He noted that when patients present with an idiopathic or undiagnosed condition suggestive of ILD, clinicians at his center will order serology tests to detect potential cases of subclinical connective tissue disorders.
Observational study
Dr. Hoffmann-Vold and colleagues looked at 525 patients with connective tissue diseases assessed for ILD at their center, including 296 with systemic sclerosis, 94 with anti-synthetase syndrome, and 135 with mixed connective tissue disease.
They used semiquantitative assessment to determine the prevalence of ILD, defining subclinical disease as ILD extent of less than 5% on high-resolution CT, preserved lung function with forced vital capacity (FVC) greater than 80% of predicted, and no respiratory symptoms.
Clinical ILD was defined as either ILD extent greater than 5%, or ILD extent below 5% but with respiratory symptoms and FVC below 80% of predicted.
They found that 44% of the patients had ILD on high-resolution CT, 43% had no evidence of ILD, and 13% had subclinical ILD.
In a comparison of patients without ILD and those with either clinical or subclinical ILD, they found that, while the mean patient age was about 51 in all three groups, men were more likely than women to have clinical ILD. A higher proportion of patients with clinical ILD (39%) died during the total observation period of about 13 years, compared with 22% of patients without ILD, and 18% of those with subclinical ILD.
As noted before, of 395 patients with baseline and follow-up high-resolution CT, 95 (24%) had evidence of lung fibrosis progression, with 38% of patients with subclinical ILD and 51% of patients with clinical ILD having progression during follow-up.
“In our connective tissue disease patients with ILD, the symptoms-define-disease argument would clearly lead to [the idea] that ILD is not a disease until patients become symptomatic, which we all know is frequently appearing in advanced stages of ILD,” Dr. Hoffmann-Vold said.
The study was funded by Oslo University Hospital. Dr. Hoffmann-Vold and Dr. Roman reported no relevant conflicts of interest to disclose.
Subclinical or preclinical interstitial lung disease in patients with connective tissue diseases is not a benign entity, and many patients may experience progression of lung fibrosis before a diagnosis of ILD is made, investigators caution.
Among patients with connective tissue disease assessed with baseline and follow-up high-resolution CT scans for ILD, nearly one-fourth had evidence of ILD progression over a median of 4.5 years, reported Anna-Maria Hoffmann-Vold, MD, PhD, from Oslo University Hospital.
“Subclinical ILD is frequently present across all connective tissue diseases. It progresses over time in a substantial subgroup of people comparable to patients with clinical ILD, and our findings really question the terms ‘subclinical/preclinical ILD,’ which may potentially lead to a suboptimal watchful waiting management,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Jesse Roman, MD, CEO at the Jane & Leonard Korman Respiratory Institute at Thomas Jefferson University, Philadelphia, who was not involved in the study, commented that the findings regarding subclinical disease come as no surprise.
“The connective tissue disorders are linked to interstitial lung disease, and we believe that they are the primary causes of interstitial lung diseases in most countries,” he said in an interview.
“Basically, what you’re detecting is that if you can identify these people early, then you can see that they behave like any other patients with interstitial lung disease with progression, so most experts recommend that patients with any kind of connective tissue disorder be followed with either CT scans or pulmonary function tests, or carefully interviewed every time they come to identify any kind of very early interstitial lung disease – particularly in patients with rheumatoid arthritis, in patients with systemic sclerosis, and in patients with dermatomyositis,” Dr. Roman said.
He noted that when patients present with an idiopathic or undiagnosed condition suggestive of ILD, clinicians at his center will order serology tests to detect potential cases of subclinical connective tissue disorders.
Observational study
Dr. Hoffmann-Vold and colleagues looked at 525 patients with connective tissue diseases assessed for ILD at their center, including 296 with systemic sclerosis, 94 with anti-synthetase syndrome, and 135 with mixed connective tissue disease.
They used semiquantitative assessment to determine the prevalence of ILD, defining subclinical disease as ILD extent of less than 5% on high-resolution CT, preserved lung function with forced vital capacity (FVC) greater than 80% of predicted, and no respiratory symptoms.
Clinical ILD was defined as either ILD extent greater than 5%, or ILD extent below 5% but with respiratory symptoms and FVC below 80% of predicted.
They found that 44% of the patients had ILD on high-resolution CT, 43% had no evidence of ILD, and 13% had subclinical ILD.
In a comparison of patients without ILD and those with either clinical or subclinical ILD, they found that, while the mean patient age was about 51 in all three groups, men were more likely than women to have clinical ILD. A higher proportion of patients with clinical ILD (39%) died during the total observation period of about 13 years, compared with 22% of patients without ILD, and 18% of those with subclinical ILD.
As noted before, of 395 patients with baseline and follow-up high-resolution CT, 95 (24%) had evidence of lung fibrosis progression, with 38% of patients with subclinical ILD and 51% of patients with clinical ILD having progression during follow-up.
“In our connective tissue disease patients with ILD, the symptoms-define-disease argument would clearly lead to [the idea] that ILD is not a disease until patients become symptomatic, which we all know is frequently appearing in advanced stages of ILD,” Dr. Hoffmann-Vold said.
The study was funded by Oslo University Hospital. Dr. Hoffmann-Vold and Dr. Roman reported no relevant conflicts of interest to disclose.
FROM ERS 2021
New Moderna vaccine data ‘support’ booster shot after 8 months
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Three ‘bad news’ payment changes coming soon for physicians
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
COVID wars, part nine: The rise of iodine
Onions and iodine and COVID, oh my!
As surely as the sun rises, anti-vaxxers will come up with some wacky and dangerous new idea to prevent COVID. While perhaps nothing will top horse medication, gargling iodine (or spraying it into the nose) is also not a great idea.
Multiple social media posts have extolled the virtues of gargling Betadine (povidone iodine), which is a TOPICAL disinfectant commonly used in EDs and operating rooms. One post cited a paper by a Bangladeshi plastic surgeon who hypothesized on the subject, and if that’s not a peer-reviewed, rigorously researched source, we don’t know what is.
Perhaps unsurprisingly, actual medical experts do not recommend using Betadine to prevent COVID. Ingesting it can cause iodine poisoning and plenty of nasty GI side effects; while Betadine does make a diluted product safe for gargling use (used for the treatment of sore throats), it has not shown any effectiveness against viruses or COVID in particular.
A New York ED doctor summed it up best in the Rolling Stone article when he was told anti-vaxxers were gargling iodine: He offered a choice four-letter expletive, then said, “Of course they are.”
But wait! We’ve got a two-for-one deal on dubious COVID cures this week. Health experts in Myanmar (Burma to all the “Seinfeld” fans) and Thailand have been combating social media posts claiming that onion fumes will cure COVID. All you need to do is slice an onion in half, sniff it for a while, then chew on a second onion, and your COVID will be cured!
In what is surely the most radical understatement of the year, a professor in the department of preventive and social medicine at Chulalongkorn University, Bangkok, said in the AFP article that there is “no solid evidence” to support onion sniffing from “any clinical research.”
We’re just going to assume the expletives that surely followed were kept off the record.
Pro-Trump state governor encourages vaccination
Clearly, the politics of COVID-19 have been working against the science of COVID-19. Politicians can’t, or won’t, agree on what to do about it, and many prominent Republicans have been actively resisting vaccine and mask mandates.
There is at least one Republican governor who has wholeheartedly encouraged vaccination in his pro-Trump state. We’re talking about Gov. Jim Justice of West Virginia, and not for the first time.
The Washington Post has detailed his efforts to promote the COVID vaccine, and we would like to share a couple of examples.
In June he suggested that people who didn’t get vaccinated were “entering the death drawing.” He followed that by saying, “If I knew for certain that there was going to be eight or nine people die by next Tuesday, and I could be one of them if I don’t take the vaccine ... What in the world do you think I would do? I mean, I would run over top of somebody.”
More recently, Gov. Justice took on vaccine conspiracy theories.
“For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the very same people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Nuff said.
Jet lag may be a gut feeling
After a week-long vacation halfway around the world, it’s time to go back to your usual routine and time zone. But don’t forget about that free souvenir, jet lag. A disrupted circadian rhythm can be a real bummer, but researchers may have found the fix in your belly.
In a study funded by the U.S. Navy, researchers at the University of Colorado, Boulder, looked into how the presence of a prebiotic in one’s diet can have on the disrupted biological clocks. They’re not the same as probiotics, which help you stay regular in another way. Prebiotics work as food to help the good gut bacteria you already have. An earlier study had suggested that prebiotics may have a positive effect on the brain.
To test the theory, the researchers gave one group of rats their regular food while another group received food with two different prebiotics. After manipulating the rats’ light-dark cycle for 8 weeks to give the illusion of traveling to a time zone 12 hours ahead every week, they found that the rats who ate the prebiotics were able to bounce back faster.
The possibility of ingesting something to keep your body clock regular sounds like a dream, but the researchers don’t really advise you to snatch all the supplements you can at your local pharmacy just yet.
“If you know you are going to come into a challenge, you could take a look at some of the prebiotics that are available. Just realize that they are not customized yet, so it might work for you but it won’t work for your neighbor,” said senior author Monika Fleshner.
Until there’s more conclusive research, just be good to your bacteria.
How to make stuff up and influence people
You’ve probably heard that we use only 10% of our brain. It’s right up there with “the Earth is flat” and “an apple a day keeps the doctor away.”
The idea that we use only 10% of our brains can probably be traced back to the early 1900s, suggests Discover magazine, when psychologist William James wrote, “Compared with what we ought to be, we are only half awake. Our fires are damped, our drafts are checked. We are making use of only a small part of our possible mental and physical resources.”
There are many different takes on it, but it is indeed a myth that we use only 10% of our brains. Dale Carnegie, the public speaking teacher, seems to be the one who put the specific number of 10% on James’ idea in his 1936 book, “How to Win Friends and Influence People.”
“We think that people are excited by this pseudo fact because it’s very optimistic,” neuroscientist Sandra Aamodt told Discover. “Wouldn’t we all love to think our brains had some giant pool of untapped potential that we’re not using?”
The reality is, we do use our whole brain. Functional MRI shows that different parts of the brain are used for different things such as language and memories. “Not all at the same time, of course. But every part of the brain has a job to do,” the Discover article explained.
There are many things we don’t know about how the brain works, but at least you know you use more than 10%. After all, a brain just told you so.
Onions and iodine and COVID, oh my!
As surely as the sun rises, anti-vaxxers will come up with some wacky and dangerous new idea to prevent COVID. While perhaps nothing will top horse medication, gargling iodine (or spraying it into the nose) is also not a great idea.
Multiple social media posts have extolled the virtues of gargling Betadine (povidone iodine), which is a TOPICAL disinfectant commonly used in EDs and operating rooms. One post cited a paper by a Bangladeshi plastic surgeon who hypothesized on the subject, and if that’s not a peer-reviewed, rigorously researched source, we don’t know what is.
Perhaps unsurprisingly, actual medical experts do not recommend using Betadine to prevent COVID. Ingesting it can cause iodine poisoning and plenty of nasty GI side effects; while Betadine does make a diluted product safe for gargling use (used for the treatment of sore throats), it has not shown any effectiveness against viruses or COVID in particular.
A New York ED doctor summed it up best in the Rolling Stone article when he was told anti-vaxxers were gargling iodine: He offered a choice four-letter expletive, then said, “Of course they are.”
But wait! We’ve got a two-for-one deal on dubious COVID cures this week. Health experts in Myanmar (Burma to all the “Seinfeld” fans) and Thailand have been combating social media posts claiming that onion fumes will cure COVID. All you need to do is slice an onion in half, sniff it for a while, then chew on a second onion, and your COVID will be cured!
In what is surely the most radical understatement of the year, a professor in the department of preventive and social medicine at Chulalongkorn University, Bangkok, said in the AFP article that there is “no solid evidence” to support onion sniffing from “any clinical research.”
We’re just going to assume the expletives that surely followed were kept off the record.
Pro-Trump state governor encourages vaccination
Clearly, the politics of COVID-19 have been working against the science of COVID-19. Politicians can’t, or won’t, agree on what to do about it, and many prominent Republicans have been actively resisting vaccine and mask mandates.
There is at least one Republican governor who has wholeheartedly encouraged vaccination in his pro-Trump state. We’re talking about Gov. Jim Justice of West Virginia, and not for the first time.
The Washington Post has detailed his efforts to promote the COVID vaccine, and we would like to share a couple of examples.
In June he suggested that people who didn’t get vaccinated were “entering the death drawing.” He followed that by saying, “If I knew for certain that there was going to be eight or nine people die by next Tuesday, and I could be one of them if I don’t take the vaccine ... What in the world do you think I would do? I mean, I would run over top of somebody.”
More recently, Gov. Justice took on vaccine conspiracy theories.
“For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the very same people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Nuff said.
Jet lag may be a gut feeling
After a week-long vacation halfway around the world, it’s time to go back to your usual routine and time zone. But don’t forget about that free souvenir, jet lag. A disrupted circadian rhythm can be a real bummer, but researchers may have found the fix in your belly.
In a study funded by the U.S. Navy, researchers at the University of Colorado, Boulder, looked into how the presence of a prebiotic in one’s diet can have on the disrupted biological clocks. They’re not the same as probiotics, which help you stay regular in another way. Prebiotics work as food to help the good gut bacteria you already have. An earlier study had suggested that prebiotics may have a positive effect on the brain.
To test the theory, the researchers gave one group of rats their regular food while another group received food with two different prebiotics. After manipulating the rats’ light-dark cycle for 8 weeks to give the illusion of traveling to a time zone 12 hours ahead every week, they found that the rats who ate the prebiotics were able to bounce back faster.
The possibility of ingesting something to keep your body clock regular sounds like a dream, but the researchers don’t really advise you to snatch all the supplements you can at your local pharmacy just yet.
“If you know you are going to come into a challenge, you could take a look at some of the prebiotics that are available. Just realize that they are not customized yet, so it might work for you but it won’t work for your neighbor,” said senior author Monika Fleshner.
Until there’s more conclusive research, just be good to your bacteria.
How to make stuff up and influence people
You’ve probably heard that we use only 10% of our brain. It’s right up there with “the Earth is flat” and “an apple a day keeps the doctor away.”
The idea that we use only 10% of our brains can probably be traced back to the early 1900s, suggests Discover magazine, when psychologist William James wrote, “Compared with what we ought to be, we are only half awake. Our fires are damped, our drafts are checked. We are making use of only a small part of our possible mental and physical resources.”
There are many different takes on it, but it is indeed a myth that we use only 10% of our brains. Dale Carnegie, the public speaking teacher, seems to be the one who put the specific number of 10% on James’ idea in his 1936 book, “How to Win Friends and Influence People.”
“We think that people are excited by this pseudo fact because it’s very optimistic,” neuroscientist Sandra Aamodt told Discover. “Wouldn’t we all love to think our brains had some giant pool of untapped potential that we’re not using?”
The reality is, we do use our whole brain. Functional MRI shows that different parts of the brain are used for different things such as language and memories. “Not all at the same time, of course. But every part of the brain has a job to do,” the Discover article explained.
There are many things we don’t know about how the brain works, but at least you know you use more than 10%. After all, a brain just told you so.
Onions and iodine and COVID, oh my!
As surely as the sun rises, anti-vaxxers will come up with some wacky and dangerous new idea to prevent COVID. While perhaps nothing will top horse medication, gargling iodine (or spraying it into the nose) is also not a great idea.
Multiple social media posts have extolled the virtues of gargling Betadine (povidone iodine), which is a TOPICAL disinfectant commonly used in EDs and operating rooms. One post cited a paper by a Bangladeshi plastic surgeon who hypothesized on the subject, and if that’s not a peer-reviewed, rigorously researched source, we don’t know what is.
Perhaps unsurprisingly, actual medical experts do not recommend using Betadine to prevent COVID. Ingesting it can cause iodine poisoning and plenty of nasty GI side effects; while Betadine does make a diluted product safe for gargling use (used for the treatment of sore throats), it has not shown any effectiveness against viruses or COVID in particular.
A New York ED doctor summed it up best in the Rolling Stone article when he was told anti-vaxxers were gargling iodine: He offered a choice four-letter expletive, then said, “Of course they are.”
But wait! We’ve got a two-for-one deal on dubious COVID cures this week. Health experts in Myanmar (Burma to all the “Seinfeld” fans) and Thailand have been combating social media posts claiming that onion fumes will cure COVID. All you need to do is slice an onion in half, sniff it for a while, then chew on a second onion, and your COVID will be cured!
In what is surely the most radical understatement of the year, a professor in the department of preventive and social medicine at Chulalongkorn University, Bangkok, said in the AFP article that there is “no solid evidence” to support onion sniffing from “any clinical research.”
We’re just going to assume the expletives that surely followed were kept off the record.
Pro-Trump state governor encourages vaccination
Clearly, the politics of COVID-19 have been working against the science of COVID-19. Politicians can’t, or won’t, agree on what to do about it, and many prominent Republicans have been actively resisting vaccine and mask mandates.
There is at least one Republican governor who has wholeheartedly encouraged vaccination in his pro-Trump state. We’re talking about Gov. Jim Justice of West Virginia, and not for the first time.
The Washington Post has detailed his efforts to promote the COVID vaccine, and we would like to share a couple of examples.
In June he suggested that people who didn’t get vaccinated were “entering the death drawing.” He followed that by saying, “If I knew for certain that there was going to be eight or nine people die by next Tuesday, and I could be one of them if I don’t take the vaccine ... What in the world do you think I would do? I mean, I would run over top of somebody.”
More recently, Gov. Justice took on vaccine conspiracy theories.
“For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the very same people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Nuff said.
Jet lag may be a gut feeling
After a week-long vacation halfway around the world, it’s time to go back to your usual routine and time zone. But don’t forget about that free souvenir, jet lag. A disrupted circadian rhythm can be a real bummer, but researchers may have found the fix in your belly.
In a study funded by the U.S. Navy, researchers at the University of Colorado, Boulder, looked into how the presence of a prebiotic in one’s diet can have on the disrupted biological clocks. They’re not the same as probiotics, which help you stay regular in another way. Prebiotics work as food to help the good gut bacteria you already have. An earlier study had suggested that prebiotics may have a positive effect on the brain.
To test the theory, the researchers gave one group of rats their regular food while another group received food with two different prebiotics. After manipulating the rats’ light-dark cycle for 8 weeks to give the illusion of traveling to a time zone 12 hours ahead every week, they found that the rats who ate the prebiotics were able to bounce back faster.
The possibility of ingesting something to keep your body clock regular sounds like a dream, but the researchers don’t really advise you to snatch all the supplements you can at your local pharmacy just yet.
“If you know you are going to come into a challenge, you could take a look at some of the prebiotics that are available. Just realize that they are not customized yet, so it might work for you but it won’t work for your neighbor,” said senior author Monika Fleshner.
Until there’s more conclusive research, just be good to your bacteria.
How to make stuff up and influence people
You’ve probably heard that we use only 10% of our brain. It’s right up there with “the Earth is flat” and “an apple a day keeps the doctor away.”
The idea that we use only 10% of our brains can probably be traced back to the early 1900s, suggests Discover magazine, when psychologist William James wrote, “Compared with what we ought to be, we are only half awake. Our fires are damped, our drafts are checked. We are making use of only a small part of our possible mental and physical resources.”
There are many different takes on it, but it is indeed a myth that we use only 10% of our brains. Dale Carnegie, the public speaking teacher, seems to be the one who put the specific number of 10% on James’ idea in his 1936 book, “How to Win Friends and Influence People.”
“We think that people are excited by this pseudo fact because it’s very optimistic,” neuroscientist Sandra Aamodt told Discover. “Wouldn’t we all love to think our brains had some giant pool of untapped potential that we’re not using?”
The reality is, we do use our whole brain. Functional MRI shows that different parts of the brain are used for different things such as language and memories. “Not all at the same time, of course. But every part of the brain has a job to do,” the Discover article explained.
There are many things we don’t know about how the brain works, but at least you know you use more than 10%. After all, a brain just told you so.
Want to see what COVID strain you have? The government says no
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
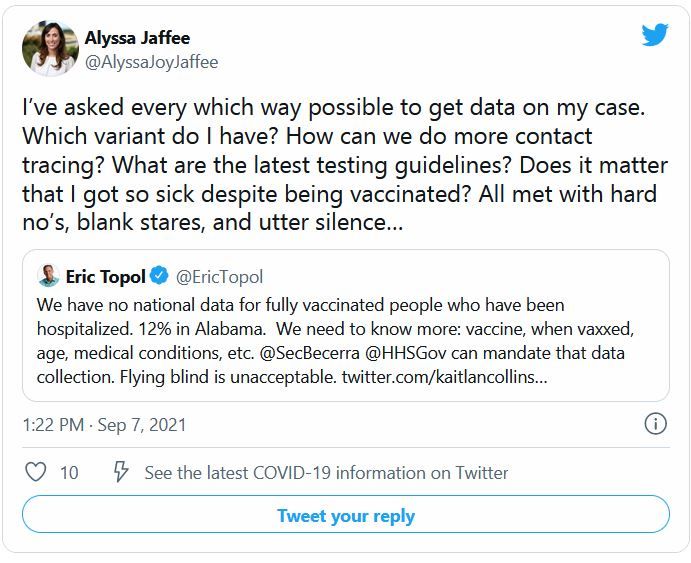
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.

The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.

The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Children and COVID: New cases down slightly from record high
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.
Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).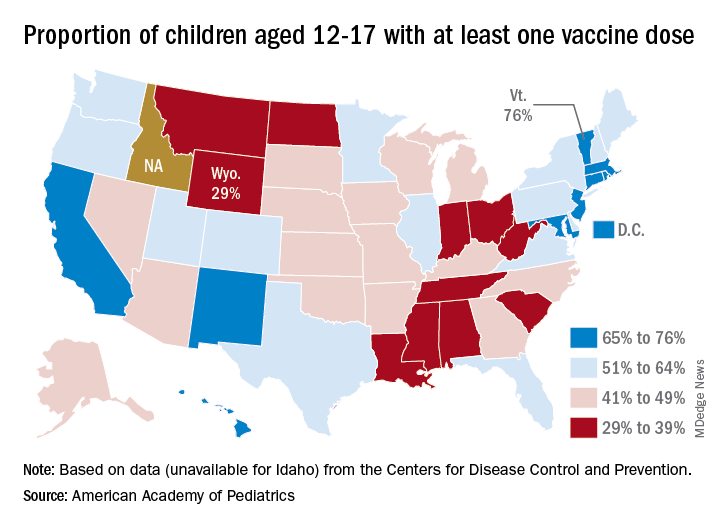
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Man dies after 43 full ICUs turn him away
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Obese children with asthma are resistant to ICS
Obese or overweight children with asthma could be using inhaled corticosteroids (ICS) to no avail, combined results from observational studies suggest.
Using Mendelian randomization, a method for reducing bias in observational studies, investigators from the University of Amsterdam Medical Center performed an analysis of data from four cross-sectional studies and one cohort study on a total of 1,511 children with asthma.
They showed that every 1-unit increase in the body mass index (BMI) z score was associated with a more than twofold higher odds ratio for exacerbation, reported Cristina Longo, PhD, a former postdoctoral fellow at AMC, and assistant professor of medicine at the University of Montreal.
“In this large, multicenter Mendelian randomization study, our findings support current evidence that children with higher BMI status respond inadequately to inhaled corticosteroids, and that this association is likely not explained by measured confounding or reverse causation,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Unmeasured confounding
The obese-asthma phenotype in children is characterized by reduced lung function, high symptom expression, poor response to ICS, and high health care utilization.
“While most observational studies suggest that weight status is associated with asthma exacerbations, despite using inhaled corticosteroids, it’s unclear whether these associations may be due to unmeasured confounding or reverse causation, which captures the idea that perhaps obesity is a consequence rather than a cause of uncontrolled severe asthma,” she said.
Traditional observational studies of the obesity-asthma link rely on comparing data on asthma in a target population and comparing nonobese patients with obese patients. The problem with this method, Dr. Longo contended, is that the exposure assignment – weight status – is not random, and could lead to bias from potential imbalance of confounders, leading to unintentionally biased results.
In contrast, Mendelian randomization uses genetic data to approximate random assignment of exposures, using a risk score for BMI based on genetic susceptibility. The score is based on the accumulation of genetic variants (single-nucleotide polymorphisms, or SNPs) that predispose individuals to obesity, with higher numbers of variants results in a higher risk score.
The scores are then used to determine the comparison groups for evaluating the obesity-asthma association.
Alphabet soup
Dr. Longo and colleagues analyzed data on a total 1,511 children enrolled in four observational studies (PACMAN, PAGES, HPR, CLARA) and one cohort study (ALSPAC).
They included children with an asthma diagnosis who used ICS and had available information on both BMI and genetics.
The Mendelian randomization analysis was based on a weighted allele score based on 97 SNPs predictive of BMI based on large-scale genomewide association studies. The exposure for the analysis was age- and sex-adjusted BMI z scores based on World Health Organization growth charts for children.
They found that using the Mendelian randomization approach, for each standard deviation increase in BMI, the OR for any parent-reported asthma exacerbations, including urgent care visits or use of oral corticosteroids, was 2.31 (95% confidence interval, 1.26-4.25).
In contrast, if the traditional observational model had been used, the OR would be a nonsignificant 1.10 (95% CI, 0.99-1.22).
“Treatment guidelines recommend steroids for children with asthma who have a higher-than-normal BMI,” Dr. Longo said in a statement. “Our research group felt that the one-size fits-all approach to treating children with asthma with inhaled steroids as their first-line treatment, particularly those with excess weight, warrants revision. At the very least, research identifying potential alternative treatments should be encouraged and prioritized, especially since 30% of children with asthma are also obese. With the childhood obesity epidemic rising, we expect this percentage to increase meaning this problem of poor control will be seen more frequently in routine clinical practice.”
Christopher E. Brightling, PhD, professor of respiratory medicine at the University of Leicester (England), commented that “this is very good and fascinating research with findings that are important and novel.
“It sheds light on the complex interplay between genes, weight, and response to inhaled corticosteroids, underscoring the need to combine drug treatments with lifestyle and diet modifications. Policy makers, health care providers and families need to do much more to tackle the growing obesity epidemic in young people,” he said.
Dr. Brightling was not involved in the study.
The study was supported by the ERS and the European Union’s H2020 research and innovation program. Dr. Longo was a Horizon 2020 Marie-Sklodowska Cure Respire-3 fellow. Dr. Brightling reported no relevant disclosures.
Obese or overweight children with asthma could be using inhaled corticosteroids (ICS) to no avail, combined results from observational studies suggest.
Using Mendelian randomization, a method for reducing bias in observational studies, investigators from the University of Amsterdam Medical Center performed an analysis of data from four cross-sectional studies and one cohort study on a total of 1,511 children with asthma.
They showed that every 1-unit increase in the body mass index (BMI) z score was associated with a more than twofold higher odds ratio for exacerbation, reported Cristina Longo, PhD, a former postdoctoral fellow at AMC, and assistant professor of medicine at the University of Montreal.
“In this large, multicenter Mendelian randomization study, our findings support current evidence that children with higher BMI status respond inadequately to inhaled corticosteroids, and that this association is likely not explained by measured confounding or reverse causation,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Unmeasured confounding
The obese-asthma phenotype in children is characterized by reduced lung function, high symptom expression, poor response to ICS, and high health care utilization.
“While most observational studies suggest that weight status is associated with asthma exacerbations, despite using inhaled corticosteroids, it’s unclear whether these associations may be due to unmeasured confounding or reverse causation, which captures the idea that perhaps obesity is a consequence rather than a cause of uncontrolled severe asthma,” she said.
Traditional observational studies of the obesity-asthma link rely on comparing data on asthma in a target population and comparing nonobese patients with obese patients. The problem with this method, Dr. Longo contended, is that the exposure assignment – weight status – is not random, and could lead to bias from potential imbalance of confounders, leading to unintentionally biased results.
In contrast, Mendelian randomization uses genetic data to approximate random assignment of exposures, using a risk score for BMI based on genetic susceptibility. The score is based on the accumulation of genetic variants (single-nucleotide polymorphisms, or SNPs) that predispose individuals to obesity, with higher numbers of variants results in a higher risk score.
The scores are then used to determine the comparison groups for evaluating the obesity-asthma association.
Alphabet soup
Dr. Longo and colleagues analyzed data on a total 1,511 children enrolled in four observational studies (PACMAN, PAGES, HPR, CLARA) and one cohort study (ALSPAC).
They included children with an asthma diagnosis who used ICS and had available information on both BMI and genetics.
The Mendelian randomization analysis was based on a weighted allele score based on 97 SNPs predictive of BMI based on large-scale genomewide association studies. The exposure for the analysis was age- and sex-adjusted BMI z scores based on World Health Organization growth charts for children.
They found that using the Mendelian randomization approach, for each standard deviation increase in BMI, the OR for any parent-reported asthma exacerbations, including urgent care visits or use of oral corticosteroids, was 2.31 (95% confidence interval, 1.26-4.25).
In contrast, if the traditional observational model had been used, the OR would be a nonsignificant 1.10 (95% CI, 0.99-1.22).
“Treatment guidelines recommend steroids for children with asthma who have a higher-than-normal BMI,” Dr. Longo said in a statement. “Our research group felt that the one-size fits-all approach to treating children with asthma with inhaled steroids as their first-line treatment, particularly those with excess weight, warrants revision. At the very least, research identifying potential alternative treatments should be encouraged and prioritized, especially since 30% of children with asthma are also obese. With the childhood obesity epidemic rising, we expect this percentage to increase meaning this problem of poor control will be seen more frequently in routine clinical practice.”
Christopher E. Brightling, PhD, professor of respiratory medicine at the University of Leicester (England), commented that “this is very good and fascinating research with findings that are important and novel.
“It sheds light on the complex interplay between genes, weight, and response to inhaled corticosteroids, underscoring the need to combine drug treatments with lifestyle and diet modifications. Policy makers, health care providers and families need to do much more to tackle the growing obesity epidemic in young people,” he said.
Dr. Brightling was not involved in the study.
The study was supported by the ERS and the European Union’s H2020 research and innovation program. Dr. Longo was a Horizon 2020 Marie-Sklodowska Cure Respire-3 fellow. Dr. Brightling reported no relevant disclosures.
Obese or overweight children with asthma could be using inhaled corticosteroids (ICS) to no avail, combined results from observational studies suggest.
Using Mendelian randomization, a method for reducing bias in observational studies, investigators from the University of Amsterdam Medical Center performed an analysis of data from four cross-sectional studies and one cohort study on a total of 1,511 children with asthma.
They showed that every 1-unit increase in the body mass index (BMI) z score was associated with a more than twofold higher odds ratio for exacerbation, reported Cristina Longo, PhD, a former postdoctoral fellow at AMC, and assistant professor of medicine at the University of Montreal.
“In this large, multicenter Mendelian randomization study, our findings support current evidence that children with higher BMI status respond inadequately to inhaled corticosteroids, and that this association is likely not explained by measured confounding or reverse causation,” she said in an oral abstract presentation during the European Respiratory Society International Congress.
Unmeasured confounding
The obese-asthma phenotype in children is characterized by reduced lung function, high symptom expression, poor response to ICS, and high health care utilization.
“While most observational studies suggest that weight status is associated with asthma exacerbations, despite using inhaled corticosteroids, it’s unclear whether these associations may be due to unmeasured confounding or reverse causation, which captures the idea that perhaps obesity is a consequence rather than a cause of uncontrolled severe asthma,” she said.
Traditional observational studies of the obesity-asthma link rely on comparing data on asthma in a target population and comparing nonobese patients with obese patients. The problem with this method, Dr. Longo contended, is that the exposure assignment – weight status – is not random, and could lead to bias from potential imbalance of confounders, leading to unintentionally biased results.
In contrast, Mendelian randomization uses genetic data to approximate random assignment of exposures, using a risk score for BMI based on genetic susceptibility. The score is based on the accumulation of genetic variants (single-nucleotide polymorphisms, or SNPs) that predispose individuals to obesity, with higher numbers of variants results in a higher risk score.
The scores are then used to determine the comparison groups for evaluating the obesity-asthma association.
Alphabet soup
Dr. Longo and colleagues analyzed data on a total 1,511 children enrolled in four observational studies (PACMAN, PAGES, HPR, CLARA) and one cohort study (ALSPAC).
They included children with an asthma diagnosis who used ICS and had available information on both BMI and genetics.
The Mendelian randomization analysis was based on a weighted allele score based on 97 SNPs predictive of BMI based on large-scale genomewide association studies. The exposure for the analysis was age- and sex-adjusted BMI z scores based on World Health Organization growth charts for children.
They found that using the Mendelian randomization approach, for each standard deviation increase in BMI, the OR for any parent-reported asthma exacerbations, including urgent care visits or use of oral corticosteroids, was 2.31 (95% confidence interval, 1.26-4.25).
In contrast, if the traditional observational model had been used, the OR would be a nonsignificant 1.10 (95% CI, 0.99-1.22).
“Treatment guidelines recommend steroids for children with asthma who have a higher-than-normal BMI,” Dr. Longo said in a statement. “Our research group felt that the one-size fits-all approach to treating children with asthma with inhaled steroids as their first-line treatment, particularly those with excess weight, warrants revision. At the very least, research identifying potential alternative treatments should be encouraged and prioritized, especially since 30% of children with asthma are also obese. With the childhood obesity epidemic rising, we expect this percentage to increase meaning this problem of poor control will be seen more frequently in routine clinical practice.”
Christopher E. Brightling, PhD, professor of respiratory medicine at the University of Leicester (England), commented that “this is very good and fascinating research with findings that are important and novel.
“It sheds light on the complex interplay between genes, weight, and response to inhaled corticosteroids, underscoring the need to combine drug treatments with lifestyle and diet modifications. Policy makers, health care providers and families need to do much more to tackle the growing obesity epidemic in young people,” he said.
Dr. Brightling was not involved in the study.
The study was supported by the ERS and the European Union’s H2020 research and innovation program. Dr. Longo was a Horizon 2020 Marie-Sklodowska Cure Respire-3 fellow. Dr. Brightling reported no relevant disclosures.
FROM ERS 2021
Feds slap UPMC, lead cardiothoracic surgeon with fraud lawsuit
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Pandemic strategies to boost trial enrollment should stay
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.