User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


CDC panel recommends Pfizer COVID-19 boosters for ages 12-15
The CDC had already said 16- and 17-year-olds “may” receive a Pfizer booster but the new recommendation adds the 12- to 15-year-old group and strengthens the “may” to “should” for 16- and 17-year-olds.
The committee voted 13-1 to recommend the booster for ages 12-17. CDC Director Rochelle Walensky, MD, must still approve the recommendation for it to take effect.
The vote comes after the FDA on Jan. 3 authorized the Pfizer vaccine booster dose for 12- to 15-year-olds.
The FDA action updated the authorization for the Pfizer vaccine, and the agency also shortened the recommended time between a second dose and the booster to 5 months or more (from 6 months). A third primary series dose is also now authorized for certain immunocompromised children between 5 and 11 years old. Full details are available in an FDA news release.
The CDC on Jan. 4 also backed the shortened time frame and a third primary series dose for some immunocompromised children 5-11 years old. But the CDC delayed a decision on a booster for 12- to 15-year-olds until it heard from its Advisory Committee on Immunization Practices on Jan. 5.
The decision came as school districts nationwide are wrestling with decisions of whether to keep schools open or revert to a virtual format as cases surge, and as pediatric COVID-19 cases and hospitalizations reach new highs.
The only dissenting vote came from Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University in Nashville, Tenn.
She said after the vote, “I am just fine with kids getting a booster. This is not me against all boosters. I just really want the U.S. to move forward with all kids.”
Dr. Talbot said earlier in the comment period, “If we divert our public health from the unvaccinated to the vaccinated, we are not going to make a big impact. Boosters are incredibly important but they won’t solve this problem of the crowded hospitals.”
She said vaccinating the unvaccinated must be the priority.
“If you are a parent out there who has not yet vaccinated your child because you have questions, please, please talk to a health care provider,” she said.
Among the 13 supporters of the recommendation was Oliver Brooks, MD, chief medical officer of Watts HealthCare Corporation in Los Angeles.
Dr. Brooks said extending the population for boosters is another tool in the toolbox.
“If it’s a hammer, we should hit that nail hard,” he said.
Sara Oliver, MD, ACIP’s lead for the COVID-19 work group, presented the case behind the recommendation.
She noted the soaring Omicron cases.
“As of Jan. 3, the 7-day average had reached an all-time high of nearly 500,000 cases,” Dr. Oliver noted.
Since this summer, she said, adolescents have had a higher rate of incidence than that of adults.
“The majority of COVID cases continue to occur among the unvaccinated,” she said, “with unvaccinated 12- to 17-year-olds having a 7-times-higher risk of testing positive for SARS-CoV-2 compared to vaccinated 12- to 17-year-olds. Unvaccinated 12- to 17-year-olds have around 11 times higher risk of hospitalization than vaccinated 12- to 17-year-olds.
“Vaccine effectiveness in adolescents 12-15 years old remains high,” Dr. Oliver said, but evidence shows there may be “some waning over time.”
Discussion of risk centered on myocarditis.
Dr. Oliver said myocarditis rates reported after the Pfizer vaccine in Israel across all populations as of Dec. 15 show that “the rates of myocarditis after a third dose are lower than what is seen after the second dose.”
A version of this article first appeared on WebMD.com.
The CDC had already said 16- and 17-year-olds “may” receive a Pfizer booster but the new recommendation adds the 12- to 15-year-old group and strengthens the “may” to “should” for 16- and 17-year-olds.
The committee voted 13-1 to recommend the booster for ages 12-17. CDC Director Rochelle Walensky, MD, must still approve the recommendation for it to take effect.
The vote comes after the FDA on Jan. 3 authorized the Pfizer vaccine booster dose for 12- to 15-year-olds.
The FDA action updated the authorization for the Pfizer vaccine, and the agency also shortened the recommended time between a second dose and the booster to 5 months or more (from 6 months). A third primary series dose is also now authorized for certain immunocompromised children between 5 and 11 years old. Full details are available in an FDA news release.
The CDC on Jan. 4 also backed the shortened time frame and a third primary series dose for some immunocompromised children 5-11 years old. But the CDC delayed a decision on a booster for 12- to 15-year-olds until it heard from its Advisory Committee on Immunization Practices on Jan. 5.
The decision came as school districts nationwide are wrestling with decisions of whether to keep schools open or revert to a virtual format as cases surge, and as pediatric COVID-19 cases and hospitalizations reach new highs.
The only dissenting vote came from Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University in Nashville, Tenn.
She said after the vote, “I am just fine with kids getting a booster. This is not me against all boosters. I just really want the U.S. to move forward with all kids.”
Dr. Talbot said earlier in the comment period, “If we divert our public health from the unvaccinated to the vaccinated, we are not going to make a big impact. Boosters are incredibly important but they won’t solve this problem of the crowded hospitals.”
She said vaccinating the unvaccinated must be the priority.
“If you are a parent out there who has not yet vaccinated your child because you have questions, please, please talk to a health care provider,” she said.
Among the 13 supporters of the recommendation was Oliver Brooks, MD, chief medical officer of Watts HealthCare Corporation in Los Angeles.
Dr. Brooks said extending the population for boosters is another tool in the toolbox.
“If it’s a hammer, we should hit that nail hard,” he said.
Sara Oliver, MD, ACIP’s lead for the COVID-19 work group, presented the case behind the recommendation.
She noted the soaring Omicron cases.
“As of Jan. 3, the 7-day average had reached an all-time high of nearly 500,000 cases,” Dr. Oliver noted.
Since this summer, she said, adolescents have had a higher rate of incidence than that of adults.
“The majority of COVID cases continue to occur among the unvaccinated,” she said, “with unvaccinated 12- to 17-year-olds having a 7-times-higher risk of testing positive for SARS-CoV-2 compared to vaccinated 12- to 17-year-olds. Unvaccinated 12- to 17-year-olds have around 11 times higher risk of hospitalization than vaccinated 12- to 17-year-olds.
“Vaccine effectiveness in adolescents 12-15 years old remains high,” Dr. Oliver said, but evidence shows there may be “some waning over time.”
Discussion of risk centered on myocarditis.
Dr. Oliver said myocarditis rates reported after the Pfizer vaccine in Israel across all populations as of Dec. 15 show that “the rates of myocarditis after a third dose are lower than what is seen after the second dose.”
A version of this article first appeared on WebMD.com.
The CDC had already said 16- and 17-year-olds “may” receive a Pfizer booster but the new recommendation adds the 12- to 15-year-old group and strengthens the “may” to “should” for 16- and 17-year-olds.
The committee voted 13-1 to recommend the booster for ages 12-17. CDC Director Rochelle Walensky, MD, must still approve the recommendation for it to take effect.
The vote comes after the FDA on Jan. 3 authorized the Pfizer vaccine booster dose for 12- to 15-year-olds.
The FDA action updated the authorization for the Pfizer vaccine, and the agency also shortened the recommended time between a second dose and the booster to 5 months or more (from 6 months). A third primary series dose is also now authorized for certain immunocompromised children between 5 and 11 years old. Full details are available in an FDA news release.
The CDC on Jan. 4 also backed the shortened time frame and a third primary series dose for some immunocompromised children 5-11 years old. But the CDC delayed a decision on a booster for 12- to 15-year-olds until it heard from its Advisory Committee on Immunization Practices on Jan. 5.
The decision came as school districts nationwide are wrestling with decisions of whether to keep schools open or revert to a virtual format as cases surge, and as pediatric COVID-19 cases and hospitalizations reach new highs.
The only dissenting vote came from Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University in Nashville, Tenn.
She said after the vote, “I am just fine with kids getting a booster. This is not me against all boosters. I just really want the U.S. to move forward with all kids.”
Dr. Talbot said earlier in the comment period, “If we divert our public health from the unvaccinated to the vaccinated, we are not going to make a big impact. Boosters are incredibly important but they won’t solve this problem of the crowded hospitals.”
She said vaccinating the unvaccinated must be the priority.
“If you are a parent out there who has not yet vaccinated your child because you have questions, please, please talk to a health care provider,” she said.
Among the 13 supporters of the recommendation was Oliver Brooks, MD, chief medical officer of Watts HealthCare Corporation in Los Angeles.
Dr. Brooks said extending the population for boosters is another tool in the toolbox.
“If it’s a hammer, we should hit that nail hard,” he said.
Sara Oliver, MD, ACIP’s lead for the COVID-19 work group, presented the case behind the recommendation.
She noted the soaring Omicron cases.
“As of Jan. 3, the 7-day average had reached an all-time high of nearly 500,000 cases,” Dr. Oliver noted.
Since this summer, she said, adolescents have had a higher rate of incidence than that of adults.
“The majority of COVID cases continue to occur among the unvaccinated,” she said, “with unvaccinated 12- to 17-year-olds having a 7-times-higher risk of testing positive for SARS-CoV-2 compared to vaccinated 12- to 17-year-olds. Unvaccinated 12- to 17-year-olds have around 11 times higher risk of hospitalization than vaccinated 12- to 17-year-olds.
“Vaccine effectiveness in adolescents 12-15 years old remains high,” Dr. Oliver said, but evidence shows there may be “some waning over time.”
Discussion of risk centered on myocarditis.
Dr. Oliver said myocarditis rates reported after the Pfizer vaccine in Israel across all populations as of Dec. 15 show that “the rates of myocarditis after a third dose are lower than what is seen after the second dose.”
A version of this article first appeared on WebMD.com.
Who needs self-driving cars when we’ve got goldfish?
If a fish can drive …
Have you ever seen a sparrow swim? Have you ever seen an elephant fly? How about a goldfish driving a car? Well, one of these is not just something out of a children’s book.
In a recent study, investigators from Ben-Gurion University did the impossible and got a fish to drive a robotic car on land. How?
No, there wasn’t a tiny steering wheel inside the tank. The researchers created a tank with video recognition ability to sync with the fish. This video shows that the car, on which the tank sat, would navigate in the direction that the fish swam. The goal was to get the fish to “drive” toward a visual target, and with a little training the fish was successful regardless of start point, the researchers explained.
So what does that tell us about the brain and behavior? Shachar Givon, who was part of the research team, said the “study hints that navigational ability is universal rather than specific to the environment.”
The study’s domain transfer methodology (putting one species in the environment of another and have them cope with an unfamiliar task) shows that other animals also have the cognitive ability to transfer skills from one terrestrial environment to another.
That leads us to lesson two. Goldfish are much smarter than we think. So please don’t tap on the glass.
We prefer ‘It’s not writing a funny LOTME article’!
So many medical journals spend all their time grappling with such silly dilemmas as curing cancer or beating COVID-19. Boring! Fortunately, the BMJ dares to stand above the rest by dedicating its Christmas issue to answering the real issues in medicine. And what was the biggest question? Which is the more accurate idiom: “It’s not rocket science,” or “It’s not brain surgery”?
English researchers collected data from 329 aerospace engineers and 72 neurosurgeons who took the Great British Intelligence Test and compared the results against 18,000 people in the general public.
The engineers and neurosurgeons were basically identical in four of the six domains, but neurosurgeons had the advantage when it came to semantic problem solving and engineers had an edge at mental manipulation and attention. The aerospace engineers were identical to the public in all domains, but neurosurgeons held an advantage in problem-solving speed and a disadvantage in memory recall speed.
The researchers noted that exposure to Latin and Greek etymologies during their education gave neurosurgeons the advantage in semantic problem solving, while the aerospace engineers’ advantage in mental manipulation stems from skills taught during engineering training.
But is there a definitive answer to the question? If you’ve got an easy task in front of you, which is more accurate to say: “It’s not rocket science” or “It’s not brain surgery”? Can we get a drum roll?
It’s not brain surgery! At least, as long as the task doesn’t involve rapid problem solving. The investigators hedged further by saying that “It’s a walk in the park” is probably more accurate. Plus, “other specialties might deserve to be on that pedestal, and future work should aim to determine the most deserving profession,” they wrote. Well, at least we’ve got something to look forward to in BMJ’s next Christmas issue.
For COVID-19, a syringe is the sheep of things to come
The logical approach to fighting COVID-19 hasn’t really worked with a lot of people, so how about something more emotional?
People love animals, so they might be a good way to promote the use of vaccines and masks. Puppies are awfully cute, and so are koalas and pandas. And who can say no to a sea otter?
Well, forget it. Instead, we’ve got elephants … and sheep … and goats. Oh my.
First, elephant Santas. The Jirasartwitthaya school in Ayutthaya, Thailand, was recently visited by five elephants in Santa Claus costumes who handed out hand sanitizer and face masks to the students, Reuters said.
“I’m so glad that I got a balloon from the elephant. My heart is pounding very fast,” student Biuon Greham said. And balloons. The elephants handed out sanitizer and masks and balloons. There’s a sentence we never thought we’d write.
And those sheep and goats we mentioned? That was a different party.
Hanspeter Etzold, who “works with shepherds, companies, and animals to run team-building events in the northern German town of Schneverdingen,” according to Reuters, had an idea to promote the use of the COVID-19 vaccine. And yes, it involved sheep and goats.
Mr. Etzold worked with shepherd Wiebke Schmidt-Kochan, who arranged her 700 goats and sheep into the shape of a 100-meter-long syringe using bits of bread laying on the ground. “Sheep are such likable animals – maybe they can get the message over better,” Mr. Etzold told AP.
If those are the carrots in an animals-as-carrots-and-sticks approach, then maybe this golf-club-chomping crab could be the stick. We’re certainly not going to argue with it.
To be or not to be … seen
Increased Zoom meetings have been another side effect of the COVID-19 pandemic as more and more people have been working and learning from home.
A recent study from Washington State University looked at two groups of people who Zoomed on a regular basis: employees and students. Individuals who made the change to remote work/learning were surveyed in the summer and fall of 2020. They completed assessments with questions on their work/classes and their level of self-consciousness.
Those with low self-esteem did not enjoy having to see themselves on camera, and those with higher self-esteem actually enjoyed it more. “Most people believe that seeing yourself during virtual meetings contributes to making the overall experience worse, but that’s not what showed up in my data,” said Kristine Kuhn, PhD, the study’s author.
Dr. Kuhn found that having the choice of whether to have the camera on made a big difference in how the participants felt. Having that control made it a more positive experience. Most professors/bosses would probably like to see the faces of those in the Zoom meetings, but it might be better to let people choose for themselves. The unbrushed-hair club would certainly agree.
If a fish can drive …
Have you ever seen a sparrow swim? Have you ever seen an elephant fly? How about a goldfish driving a car? Well, one of these is not just something out of a children’s book.
In a recent study, investigators from Ben-Gurion University did the impossible and got a fish to drive a robotic car on land. How?
No, there wasn’t a tiny steering wheel inside the tank. The researchers created a tank with video recognition ability to sync with the fish. This video shows that the car, on which the tank sat, would navigate in the direction that the fish swam. The goal was to get the fish to “drive” toward a visual target, and with a little training the fish was successful regardless of start point, the researchers explained.
So what does that tell us about the brain and behavior? Shachar Givon, who was part of the research team, said the “study hints that navigational ability is universal rather than specific to the environment.”
The study’s domain transfer methodology (putting one species in the environment of another and have them cope with an unfamiliar task) shows that other animals also have the cognitive ability to transfer skills from one terrestrial environment to another.
That leads us to lesson two. Goldfish are much smarter than we think. So please don’t tap on the glass.
We prefer ‘It’s not writing a funny LOTME article’!
So many medical journals spend all their time grappling with such silly dilemmas as curing cancer or beating COVID-19. Boring! Fortunately, the BMJ dares to stand above the rest by dedicating its Christmas issue to answering the real issues in medicine. And what was the biggest question? Which is the more accurate idiom: “It’s not rocket science,” or “It’s not brain surgery”?
English researchers collected data from 329 aerospace engineers and 72 neurosurgeons who took the Great British Intelligence Test and compared the results against 18,000 people in the general public.
The engineers and neurosurgeons were basically identical in four of the six domains, but neurosurgeons had the advantage when it came to semantic problem solving and engineers had an edge at mental manipulation and attention. The aerospace engineers were identical to the public in all domains, but neurosurgeons held an advantage in problem-solving speed and a disadvantage in memory recall speed.
The researchers noted that exposure to Latin and Greek etymologies during their education gave neurosurgeons the advantage in semantic problem solving, while the aerospace engineers’ advantage in mental manipulation stems from skills taught during engineering training.
But is there a definitive answer to the question? If you’ve got an easy task in front of you, which is more accurate to say: “It’s not rocket science” or “It’s not brain surgery”? Can we get a drum roll?
It’s not brain surgery! At least, as long as the task doesn’t involve rapid problem solving. The investigators hedged further by saying that “It’s a walk in the park” is probably more accurate. Plus, “other specialties might deserve to be on that pedestal, and future work should aim to determine the most deserving profession,” they wrote. Well, at least we’ve got something to look forward to in BMJ’s next Christmas issue.
For COVID-19, a syringe is the sheep of things to come
The logical approach to fighting COVID-19 hasn’t really worked with a lot of people, so how about something more emotional?
People love animals, so they might be a good way to promote the use of vaccines and masks. Puppies are awfully cute, and so are koalas and pandas. And who can say no to a sea otter?
Well, forget it. Instead, we’ve got elephants … and sheep … and goats. Oh my.
First, elephant Santas. The Jirasartwitthaya school in Ayutthaya, Thailand, was recently visited by five elephants in Santa Claus costumes who handed out hand sanitizer and face masks to the students, Reuters said.
“I’m so glad that I got a balloon from the elephant. My heart is pounding very fast,” student Biuon Greham said. And balloons. The elephants handed out sanitizer and masks and balloons. There’s a sentence we never thought we’d write.
And those sheep and goats we mentioned? That was a different party.
Hanspeter Etzold, who “works with shepherds, companies, and animals to run team-building events in the northern German town of Schneverdingen,” according to Reuters, had an idea to promote the use of the COVID-19 vaccine. And yes, it involved sheep and goats.
Mr. Etzold worked with shepherd Wiebke Schmidt-Kochan, who arranged her 700 goats and sheep into the shape of a 100-meter-long syringe using bits of bread laying on the ground. “Sheep are such likable animals – maybe they can get the message over better,” Mr. Etzold told AP.
If those are the carrots in an animals-as-carrots-and-sticks approach, then maybe this golf-club-chomping crab could be the stick. We’re certainly not going to argue with it.
To be or not to be … seen
Increased Zoom meetings have been another side effect of the COVID-19 pandemic as more and more people have been working and learning from home.
A recent study from Washington State University looked at two groups of people who Zoomed on a regular basis: employees and students. Individuals who made the change to remote work/learning were surveyed in the summer and fall of 2020. They completed assessments with questions on their work/classes and their level of self-consciousness.
Those with low self-esteem did not enjoy having to see themselves on camera, and those with higher self-esteem actually enjoyed it more. “Most people believe that seeing yourself during virtual meetings contributes to making the overall experience worse, but that’s not what showed up in my data,” said Kristine Kuhn, PhD, the study’s author.
Dr. Kuhn found that having the choice of whether to have the camera on made a big difference in how the participants felt. Having that control made it a more positive experience. Most professors/bosses would probably like to see the faces of those in the Zoom meetings, but it might be better to let people choose for themselves. The unbrushed-hair club would certainly agree.
If a fish can drive …
Have you ever seen a sparrow swim? Have you ever seen an elephant fly? How about a goldfish driving a car? Well, one of these is not just something out of a children’s book.
In a recent study, investigators from Ben-Gurion University did the impossible and got a fish to drive a robotic car on land. How?
No, there wasn’t a tiny steering wheel inside the tank. The researchers created a tank with video recognition ability to sync with the fish. This video shows that the car, on which the tank sat, would navigate in the direction that the fish swam. The goal was to get the fish to “drive” toward a visual target, and with a little training the fish was successful regardless of start point, the researchers explained.
So what does that tell us about the brain and behavior? Shachar Givon, who was part of the research team, said the “study hints that navigational ability is universal rather than specific to the environment.”
The study’s domain transfer methodology (putting one species in the environment of another and have them cope with an unfamiliar task) shows that other animals also have the cognitive ability to transfer skills from one terrestrial environment to another.
That leads us to lesson two. Goldfish are much smarter than we think. So please don’t tap on the glass.
We prefer ‘It’s not writing a funny LOTME article’!
So many medical journals spend all their time grappling with such silly dilemmas as curing cancer or beating COVID-19. Boring! Fortunately, the BMJ dares to stand above the rest by dedicating its Christmas issue to answering the real issues in medicine. And what was the biggest question? Which is the more accurate idiom: “It’s not rocket science,” or “It’s not brain surgery”?
English researchers collected data from 329 aerospace engineers and 72 neurosurgeons who took the Great British Intelligence Test and compared the results against 18,000 people in the general public.
The engineers and neurosurgeons were basically identical in four of the six domains, but neurosurgeons had the advantage when it came to semantic problem solving and engineers had an edge at mental manipulation and attention. The aerospace engineers were identical to the public in all domains, but neurosurgeons held an advantage in problem-solving speed and a disadvantage in memory recall speed.
The researchers noted that exposure to Latin and Greek etymologies during their education gave neurosurgeons the advantage in semantic problem solving, while the aerospace engineers’ advantage in mental manipulation stems from skills taught during engineering training.
But is there a definitive answer to the question? If you’ve got an easy task in front of you, which is more accurate to say: “It’s not rocket science” or “It’s not brain surgery”? Can we get a drum roll?
It’s not brain surgery! At least, as long as the task doesn’t involve rapid problem solving. The investigators hedged further by saying that “It’s a walk in the park” is probably more accurate. Plus, “other specialties might deserve to be on that pedestal, and future work should aim to determine the most deserving profession,” they wrote. Well, at least we’ve got something to look forward to in BMJ’s next Christmas issue.
For COVID-19, a syringe is the sheep of things to come
The logical approach to fighting COVID-19 hasn’t really worked with a lot of people, so how about something more emotional?
People love animals, so they might be a good way to promote the use of vaccines and masks. Puppies are awfully cute, and so are koalas and pandas. And who can say no to a sea otter?
Well, forget it. Instead, we’ve got elephants … and sheep … and goats. Oh my.
First, elephant Santas. The Jirasartwitthaya school in Ayutthaya, Thailand, was recently visited by five elephants in Santa Claus costumes who handed out hand sanitizer and face masks to the students, Reuters said.
“I’m so glad that I got a balloon from the elephant. My heart is pounding very fast,” student Biuon Greham said. And balloons. The elephants handed out sanitizer and masks and balloons. There’s a sentence we never thought we’d write.
And those sheep and goats we mentioned? That was a different party.
Hanspeter Etzold, who “works with shepherds, companies, and animals to run team-building events in the northern German town of Schneverdingen,” according to Reuters, had an idea to promote the use of the COVID-19 vaccine. And yes, it involved sheep and goats.
Mr. Etzold worked with shepherd Wiebke Schmidt-Kochan, who arranged her 700 goats and sheep into the shape of a 100-meter-long syringe using bits of bread laying on the ground. “Sheep are such likable animals – maybe they can get the message over better,” Mr. Etzold told AP.
If those are the carrots in an animals-as-carrots-and-sticks approach, then maybe this golf-club-chomping crab could be the stick. We’re certainly not going to argue with it.
To be or not to be … seen
Increased Zoom meetings have been another side effect of the COVID-19 pandemic as more and more people have been working and learning from home.
A recent study from Washington State University looked at two groups of people who Zoomed on a regular basis: employees and students. Individuals who made the change to remote work/learning were surveyed in the summer and fall of 2020. They completed assessments with questions on their work/classes and their level of self-consciousness.
Those with low self-esteem did not enjoy having to see themselves on camera, and those with higher self-esteem actually enjoyed it more. “Most people believe that seeing yourself during virtual meetings contributes to making the overall experience worse, but that’s not what showed up in my data,” said Kristine Kuhn, PhD, the study’s author.
Dr. Kuhn found that having the choice of whether to have the camera on made a big difference in how the participants felt. Having that control made it a more positive experience. Most professors/bosses would probably like to see the faces of those in the Zoom meetings, but it might be better to let people choose for themselves. The unbrushed-hair club would certainly agree.
CDC defends new COVID guidance as doctors raise concerns
, Director Rochelle Walenksy, MD, said during a White House briefing Jan. 5.
Health officials recently shortened the recommended COVID-19 isolation and quarantine period from 10 days to 5, creating confusion amid an outbreak of the highly transmissible Omicron variant, which now accounts for 95% of cases in the United States.
Then, in slightly updated guidance, the CDC recommended using an at-home antigen test after 5 days of isolation if possible, even though these tests having aren’t as sensitive to the Omicron variant, according to the FDA.
“After we released our recs early last week, it became very clear people were interested in using the rapid test, though not authorized for this purpose after the end of their isolation period,” Dr. Walensky said. “We then provided guidance on how they should be used.”
“If that test is negative, people really do need to understand they must continue to wear their mask for those 5 days,” Dr. Walensky said.
But for many, the CDC guidelines are murky and seem to always change.
“Nearly 2 years into this pandemic, with Omicron cases surging across the country, the American people should be able to count on the Centers for Disease Control and Prevention for timely, accurate, clear guidance to protect themselves, their loved ones, and their communities,” American Medical Association president Gerald Harmon, MD, said in a statement. “Instead, the new recommendations on quarantine and isolation are not only confusing, but are risking further spread of the virus.”
About 31% of people remain infectious 5 days after a positive COVID-19 test, Dr. Harmon said, quoting the CDC’s own rationale for changing its guidance.
“With hundreds of thousands of new cases daily and more than a million positive reported cases on January 3, tens of thousands – potentially hundreds of thousands of people – could return to work and school infectious if they follow the CDC’s new guidance on ending isolation after 5 days without a negative test,” he said. “Physicians are concerned that these recommendations put our patients at risk and could further overwhelm our health care system.”
Instead, Dr. Harmon said a negative test should be required for ending isolation.
“Reemerging without knowing one’s status unnecessarily risks further transmission of the virus,” he said.
Meanwhile, also during the White House briefing, officials said that early data continue to show that Omicron infections are less severe than those from other variants, but skyrocketing cases will still put a strain on the health care system.
“The big caveat is we should not be complacent,” presidential Chief Medical Adviser Anthony Fauci, MD, said a White House briefing Jan. 5.
He added that Omicron “could still stress our hospital system because a certain proportion of a large volume of cases, no matter what, are going to be severe.”
Cases continue to increase greatly. This week’s 7-day daily average of infections is 491,700 -- an increase of 98% over last week, Dr. Walensky said. Hospitalizations, while lagging behind case numbers, are still rising significantly: The daily average is 14,800 admissions, up 63% from last week. Daily deaths this week are 1,200, an increase of only 5%.
Dr. Walensky continues to encourage vaccinations, boosters, and other precautions.
“Vaccines and boosters are protecting people from the severe and tragic outcomes that can occur from COVID-19 infection,” she said. “Get vaccinated and get boosted if eligible, wear a mask, stay home when you’re sick, and take a test if you have symptoms or are looking for greater reassurance before you gather with others.”
A version of this article first appeared on WebMD.com.
, Director Rochelle Walenksy, MD, said during a White House briefing Jan. 5.
Health officials recently shortened the recommended COVID-19 isolation and quarantine period from 10 days to 5, creating confusion amid an outbreak of the highly transmissible Omicron variant, which now accounts for 95% of cases in the United States.
Then, in slightly updated guidance, the CDC recommended using an at-home antigen test after 5 days of isolation if possible, even though these tests having aren’t as sensitive to the Omicron variant, according to the FDA.
“After we released our recs early last week, it became very clear people were interested in using the rapid test, though not authorized for this purpose after the end of their isolation period,” Dr. Walensky said. “We then provided guidance on how they should be used.”
“If that test is negative, people really do need to understand they must continue to wear their mask for those 5 days,” Dr. Walensky said.
But for many, the CDC guidelines are murky and seem to always change.
“Nearly 2 years into this pandemic, with Omicron cases surging across the country, the American people should be able to count on the Centers for Disease Control and Prevention for timely, accurate, clear guidance to protect themselves, their loved ones, and their communities,” American Medical Association president Gerald Harmon, MD, said in a statement. “Instead, the new recommendations on quarantine and isolation are not only confusing, but are risking further spread of the virus.”
About 31% of people remain infectious 5 days after a positive COVID-19 test, Dr. Harmon said, quoting the CDC’s own rationale for changing its guidance.
“With hundreds of thousands of new cases daily and more than a million positive reported cases on January 3, tens of thousands – potentially hundreds of thousands of people – could return to work and school infectious if they follow the CDC’s new guidance on ending isolation after 5 days without a negative test,” he said. “Physicians are concerned that these recommendations put our patients at risk and could further overwhelm our health care system.”
Instead, Dr. Harmon said a negative test should be required for ending isolation.
“Reemerging without knowing one’s status unnecessarily risks further transmission of the virus,” he said.
Meanwhile, also during the White House briefing, officials said that early data continue to show that Omicron infections are less severe than those from other variants, but skyrocketing cases will still put a strain on the health care system.
“The big caveat is we should not be complacent,” presidential Chief Medical Adviser Anthony Fauci, MD, said a White House briefing Jan. 5.
He added that Omicron “could still stress our hospital system because a certain proportion of a large volume of cases, no matter what, are going to be severe.”
Cases continue to increase greatly. This week’s 7-day daily average of infections is 491,700 -- an increase of 98% over last week, Dr. Walensky said. Hospitalizations, while lagging behind case numbers, are still rising significantly: The daily average is 14,800 admissions, up 63% from last week. Daily deaths this week are 1,200, an increase of only 5%.
Dr. Walensky continues to encourage vaccinations, boosters, and other precautions.
“Vaccines and boosters are protecting people from the severe and tragic outcomes that can occur from COVID-19 infection,” she said. “Get vaccinated and get boosted if eligible, wear a mask, stay home when you’re sick, and take a test if you have symptoms or are looking for greater reassurance before you gather with others.”
A version of this article first appeared on WebMD.com.
, Director Rochelle Walenksy, MD, said during a White House briefing Jan. 5.
Health officials recently shortened the recommended COVID-19 isolation and quarantine period from 10 days to 5, creating confusion amid an outbreak of the highly transmissible Omicron variant, which now accounts for 95% of cases in the United States.
Then, in slightly updated guidance, the CDC recommended using an at-home antigen test after 5 days of isolation if possible, even though these tests having aren’t as sensitive to the Omicron variant, according to the FDA.
“After we released our recs early last week, it became very clear people were interested in using the rapid test, though not authorized for this purpose after the end of their isolation period,” Dr. Walensky said. “We then provided guidance on how they should be used.”
“If that test is negative, people really do need to understand they must continue to wear their mask for those 5 days,” Dr. Walensky said.
But for many, the CDC guidelines are murky and seem to always change.
“Nearly 2 years into this pandemic, with Omicron cases surging across the country, the American people should be able to count on the Centers for Disease Control and Prevention for timely, accurate, clear guidance to protect themselves, their loved ones, and their communities,” American Medical Association president Gerald Harmon, MD, said in a statement. “Instead, the new recommendations on quarantine and isolation are not only confusing, but are risking further spread of the virus.”
About 31% of people remain infectious 5 days after a positive COVID-19 test, Dr. Harmon said, quoting the CDC’s own rationale for changing its guidance.
“With hundreds of thousands of new cases daily and more than a million positive reported cases on January 3, tens of thousands – potentially hundreds of thousands of people – could return to work and school infectious if they follow the CDC’s new guidance on ending isolation after 5 days without a negative test,” he said. “Physicians are concerned that these recommendations put our patients at risk and could further overwhelm our health care system.”
Instead, Dr. Harmon said a negative test should be required for ending isolation.
“Reemerging without knowing one’s status unnecessarily risks further transmission of the virus,” he said.
Meanwhile, also during the White House briefing, officials said that early data continue to show that Omicron infections are less severe than those from other variants, but skyrocketing cases will still put a strain on the health care system.
“The big caveat is we should not be complacent,” presidential Chief Medical Adviser Anthony Fauci, MD, said a White House briefing Jan. 5.
He added that Omicron “could still stress our hospital system because a certain proportion of a large volume of cases, no matter what, are going to be severe.”
Cases continue to increase greatly. This week’s 7-day daily average of infections is 491,700 -- an increase of 98% over last week, Dr. Walensky said. Hospitalizations, while lagging behind case numbers, are still rising significantly: The daily average is 14,800 admissions, up 63% from last week. Daily deaths this week are 1,200, an increase of only 5%.
Dr. Walensky continues to encourage vaccinations, boosters, and other precautions.
“Vaccines and boosters are protecting people from the severe and tragic outcomes that can occur from COVID-19 infection,” she said. “Get vaccinated and get boosted if eligible, wear a mask, stay home when you’re sick, and take a test if you have symptoms or are looking for greater reassurance before you gather with others.”
A version of this article first appeared on WebMD.com.
Frail COPD patients at high risk of disability and death
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
FROM CHEST
COVID affects executive functioning in young to middle-age adults: Study
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MEDRXIV
Lung cancer risk misperceptions impede lifesaving screenings
according to analysis of data from the SUMMIT study recently published in the Journal of Thoracic Oncology. Such an approach may be more effective than trying to change risk perceptions.
While 1-year survival among patients diagnosed with early-stage lung cancer is 88%, it is only 19% for those diagnosed with advanced disease. But only 27% of patients are diagnosed with early-stage disease. Screening high-risk asymptomatic adults using LDCT detects early-stage disease and significantly reduces lung cancer mortality, according to Samantha L. Quaife, PhD, of the Wolfson Institute of Population Health at Queen Mary University of London, and associates.
The effectiveness and equity of LDCT lung cancer screening as a population-level early detection strategy is compromised by low uptake among high-risk groups, the authors wrote.
In the United States, only 2% of eligible smokers have been screened since screening was first recommended in 2013. To provide a scientific evidence base for intervention, an understanding of factors making high-risk groups less likely to participate in LDCT screening is critical, Dr. Quaife and colleagues wrote.
Their longitudinal cohort study evaluating psychological correlates of lung cancer screening uptake included 44,000 ever-smokers (aged 55-77 years) who were invited to mail a self-regulatory questionnaire for lung cancer screening. Eligibility for LDCT lung cancer screening and inclusion in the SUMMIT study were further determined through telephone and in-person Lung Health Check (LHC) appointments. The primary outcome was uptake of the invitation to book an LHC appointment by telephone.
Of those invited, 7,966 (18.1%) returned the questionnaire with 7,730 (45% female; mean age, about 64 years) linked to screening uptake data. About 30% reported being current smokers with high tobacco dependence (60.3% smoking within 30 minutes of waking). The analysis from Dr. Quaife and colleagues looked at psychological correlates of lung cancer screening uptake using a psychometrically validated self-regulatory questionnaire for lung cancer screening (SRQ-LCS) to measure psychological constructs hypothesized to be associated with uptake which included consequences, emotional representation, coherence (lung cancer knowledge), treatment control, personal control, risk perception, perceived stigma, response efficacy of smoking cessation, early diagnosis behavioral response, survival from lung cancer, and treatment intention.
Among those who perceived early diagnosis to be more beneficial as a behavioral response, the positive association with uptake was strongest (adjusted odds ratio, 1.37; 95% confidence interval, 1.33-1.41). Those who perceived greater personal control (aOR, 1.09; 95% CI, 1.05-1.11) or believed their risk of lung cancer was high (aOR, 1.08; 95% CI, 1.05-1.10) were also more likely to respond. Other uptake increases were found for those who perceived smoking cessation as an effective means of reducing lung cancer risk or thought the chances of surviving early-stage lung cancer were good or fair (P < .01), and for those who perceived lung cancer as stigmatized (aOR, 1.26; 95% CI, 1.14-1.40). Most of these constructs were also perceived more negatively by current than former smokers.
Income, employment, education, social class, and housing conditions were significantly associated with many of the constructs. Greater affluence correlated with perceived personal control and benefit from early diagnosis, but more negative perceptions of the consequences of lung cancer. Also, those from more affluent areas were more likely to perceive lung cancer to be stigmatized and perceive smoking cessation to be less effective in reducing risk. Current daily smokers were less willing to be treated for early-stage disease, more pessimistic about survival, but had the highest-risk perception scores, at odds with their lower participation in lung screening trials. This contradiction, Dr. Quaife and colleagues suggested, may be explained by current smokers also holding more negative perceptions associated with lower uptake, including negative perceptions of lung cancer controllability, early diagnosis and survival, lower willingness to be treated, and belief that smoking cessation is less effective in reducing risk. All of these undermine positive responses to their high perceived risk.
“These findings pinpoint specific psychological targets for intervention,” the authors wrote. Experimental studies investigating the methods and mechanisms through which these perceptions could be changed are needed.
The study was funded by Cancer Research UK Population Research Fellowship (C50664/A24460) awarded to Dr. Quaife. The study investigators declared no support from financial organizations that might have an interest in the submitted work in the previous 3 years.
according to analysis of data from the SUMMIT study recently published in the Journal of Thoracic Oncology. Such an approach may be more effective than trying to change risk perceptions.
While 1-year survival among patients diagnosed with early-stage lung cancer is 88%, it is only 19% for those diagnosed with advanced disease. But only 27% of patients are diagnosed with early-stage disease. Screening high-risk asymptomatic adults using LDCT detects early-stage disease and significantly reduces lung cancer mortality, according to Samantha L. Quaife, PhD, of the Wolfson Institute of Population Health at Queen Mary University of London, and associates.
The effectiveness and equity of LDCT lung cancer screening as a population-level early detection strategy is compromised by low uptake among high-risk groups, the authors wrote.
In the United States, only 2% of eligible smokers have been screened since screening was first recommended in 2013. To provide a scientific evidence base for intervention, an understanding of factors making high-risk groups less likely to participate in LDCT screening is critical, Dr. Quaife and colleagues wrote.
Their longitudinal cohort study evaluating psychological correlates of lung cancer screening uptake included 44,000 ever-smokers (aged 55-77 years) who were invited to mail a self-regulatory questionnaire for lung cancer screening. Eligibility for LDCT lung cancer screening and inclusion in the SUMMIT study were further determined through telephone and in-person Lung Health Check (LHC) appointments. The primary outcome was uptake of the invitation to book an LHC appointment by telephone.
Of those invited, 7,966 (18.1%) returned the questionnaire with 7,730 (45% female; mean age, about 64 years) linked to screening uptake data. About 30% reported being current smokers with high tobacco dependence (60.3% smoking within 30 minutes of waking). The analysis from Dr. Quaife and colleagues looked at psychological correlates of lung cancer screening uptake using a psychometrically validated self-regulatory questionnaire for lung cancer screening (SRQ-LCS) to measure psychological constructs hypothesized to be associated with uptake which included consequences, emotional representation, coherence (lung cancer knowledge), treatment control, personal control, risk perception, perceived stigma, response efficacy of smoking cessation, early diagnosis behavioral response, survival from lung cancer, and treatment intention.
Among those who perceived early diagnosis to be more beneficial as a behavioral response, the positive association with uptake was strongest (adjusted odds ratio, 1.37; 95% confidence interval, 1.33-1.41). Those who perceived greater personal control (aOR, 1.09; 95% CI, 1.05-1.11) or believed their risk of lung cancer was high (aOR, 1.08; 95% CI, 1.05-1.10) were also more likely to respond. Other uptake increases were found for those who perceived smoking cessation as an effective means of reducing lung cancer risk or thought the chances of surviving early-stage lung cancer were good or fair (P < .01), and for those who perceived lung cancer as stigmatized (aOR, 1.26; 95% CI, 1.14-1.40). Most of these constructs were also perceived more negatively by current than former smokers.
Income, employment, education, social class, and housing conditions were significantly associated with many of the constructs. Greater affluence correlated with perceived personal control and benefit from early diagnosis, but more negative perceptions of the consequences of lung cancer. Also, those from more affluent areas were more likely to perceive lung cancer to be stigmatized and perceive smoking cessation to be less effective in reducing risk. Current daily smokers were less willing to be treated for early-stage disease, more pessimistic about survival, but had the highest-risk perception scores, at odds with their lower participation in lung screening trials. This contradiction, Dr. Quaife and colleagues suggested, may be explained by current smokers also holding more negative perceptions associated with lower uptake, including negative perceptions of lung cancer controllability, early diagnosis and survival, lower willingness to be treated, and belief that smoking cessation is less effective in reducing risk. All of these undermine positive responses to their high perceived risk.
“These findings pinpoint specific psychological targets for intervention,” the authors wrote. Experimental studies investigating the methods and mechanisms through which these perceptions could be changed are needed.
The study was funded by Cancer Research UK Population Research Fellowship (C50664/A24460) awarded to Dr. Quaife. The study investigators declared no support from financial organizations that might have an interest in the submitted work in the previous 3 years.
according to analysis of data from the SUMMIT study recently published in the Journal of Thoracic Oncology. Such an approach may be more effective than trying to change risk perceptions.
While 1-year survival among patients diagnosed with early-stage lung cancer is 88%, it is only 19% for those diagnosed with advanced disease. But only 27% of patients are diagnosed with early-stage disease. Screening high-risk asymptomatic adults using LDCT detects early-stage disease and significantly reduces lung cancer mortality, according to Samantha L. Quaife, PhD, of the Wolfson Institute of Population Health at Queen Mary University of London, and associates.
The effectiveness and equity of LDCT lung cancer screening as a population-level early detection strategy is compromised by low uptake among high-risk groups, the authors wrote.
In the United States, only 2% of eligible smokers have been screened since screening was first recommended in 2013. To provide a scientific evidence base for intervention, an understanding of factors making high-risk groups less likely to participate in LDCT screening is critical, Dr. Quaife and colleagues wrote.
Their longitudinal cohort study evaluating psychological correlates of lung cancer screening uptake included 44,000 ever-smokers (aged 55-77 years) who were invited to mail a self-regulatory questionnaire for lung cancer screening. Eligibility for LDCT lung cancer screening and inclusion in the SUMMIT study were further determined through telephone and in-person Lung Health Check (LHC) appointments. The primary outcome was uptake of the invitation to book an LHC appointment by telephone.
Of those invited, 7,966 (18.1%) returned the questionnaire with 7,730 (45% female; mean age, about 64 years) linked to screening uptake data. About 30% reported being current smokers with high tobacco dependence (60.3% smoking within 30 minutes of waking). The analysis from Dr. Quaife and colleagues looked at psychological correlates of lung cancer screening uptake using a psychometrically validated self-regulatory questionnaire for lung cancer screening (SRQ-LCS) to measure psychological constructs hypothesized to be associated with uptake which included consequences, emotional representation, coherence (lung cancer knowledge), treatment control, personal control, risk perception, perceived stigma, response efficacy of smoking cessation, early diagnosis behavioral response, survival from lung cancer, and treatment intention.
Among those who perceived early diagnosis to be more beneficial as a behavioral response, the positive association with uptake was strongest (adjusted odds ratio, 1.37; 95% confidence interval, 1.33-1.41). Those who perceived greater personal control (aOR, 1.09; 95% CI, 1.05-1.11) or believed their risk of lung cancer was high (aOR, 1.08; 95% CI, 1.05-1.10) were also more likely to respond. Other uptake increases were found for those who perceived smoking cessation as an effective means of reducing lung cancer risk or thought the chances of surviving early-stage lung cancer were good or fair (P < .01), and for those who perceived lung cancer as stigmatized (aOR, 1.26; 95% CI, 1.14-1.40). Most of these constructs were also perceived more negatively by current than former smokers.
Income, employment, education, social class, and housing conditions were significantly associated with many of the constructs. Greater affluence correlated with perceived personal control and benefit from early diagnosis, but more negative perceptions of the consequences of lung cancer. Also, those from more affluent areas were more likely to perceive lung cancer to be stigmatized and perceive smoking cessation to be less effective in reducing risk. Current daily smokers were less willing to be treated for early-stage disease, more pessimistic about survival, but had the highest-risk perception scores, at odds with their lower participation in lung screening trials. This contradiction, Dr. Quaife and colleagues suggested, may be explained by current smokers also holding more negative perceptions associated with lower uptake, including negative perceptions of lung cancer controllability, early diagnosis and survival, lower willingness to be treated, and belief that smoking cessation is less effective in reducing risk. All of these undermine positive responses to their high perceived risk.
“These findings pinpoint specific psychological targets for intervention,” the authors wrote. Experimental studies investigating the methods and mechanisms through which these perceptions could be changed are needed.
The study was funded by Cancer Research UK Population Research Fellowship (C50664/A24460) awarded to Dr. Quaife. The study investigators declared no support from financial organizations that might have an interest in the submitted work in the previous 3 years.
FROM THE JOURNAL OF THORACIC ONCOLOGY
Children and COVID: New cases, admissions are higher than ever
Weekly COVID-19 cases in children passed 300,000 for the first time since the pandemic started, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The rate of new COVID-related hospital admissions also reached a new high of 0.74 per 100,000 children as of Dec. 31. The highest rate seen before the current Omicron-fueled surge was 0.47 per 100,000 in early September, data from the Centers for Disease Control and Prevention show.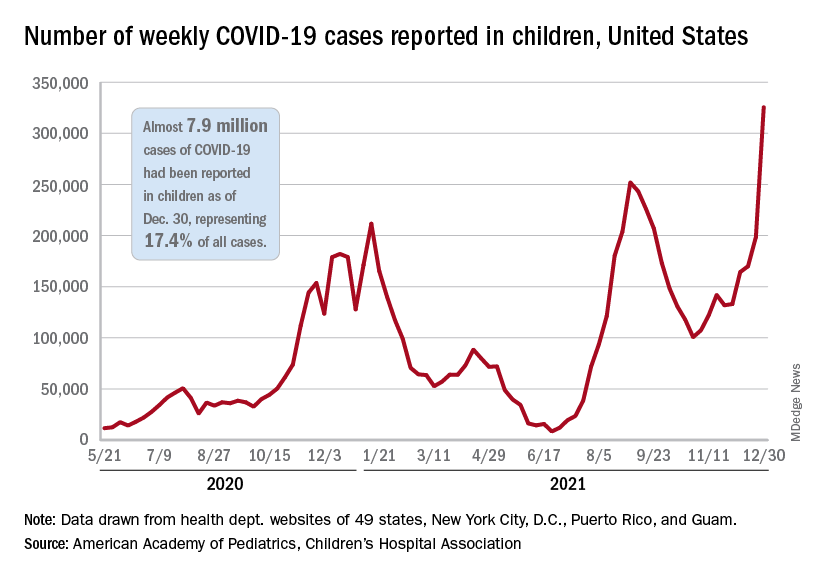
and exceeding the previous week’s count by almost 64%, the AAP and CHA said in their weekly COVID report.
New cases were up in all four regions of the United States, with the Northeast adding the most newly infected children while setting a new high for the fifth consecutive week. The South was just behind for the week but still well off the record it reached in September, the Midwest was third but recorded its busiest week ever, and the West was fourth and nowhere near its previous high, the AAP/CHA report indicated.
The total number of child cases since the pandemic began is almost 7.9 million, they said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. That figure represents 17.4% of all cases reported in the United States, and the cumulative rate of COVID infection is up to almost 10,500 per 100,000 children, meaning that 1 in 10 children have been infected.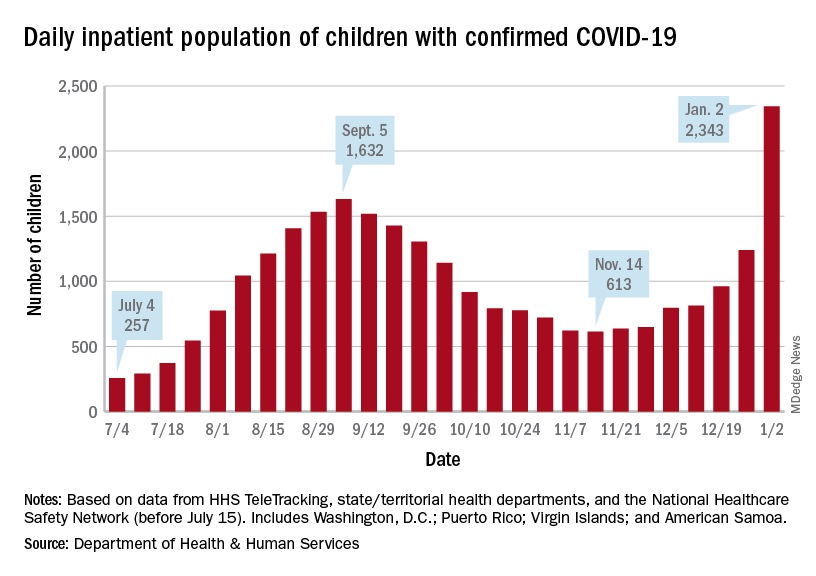
Children are still less likely to be hospitalized than adults, but the gap appears to be closing. On Jan. 2 there were 2,343 children and 87,690 adults in the hospital with confirmed COVID, a ratio of 37 adults for each child, but on Sept. 5, at the height of the previous surge, the ratio of hospitalized adults (93,647) to children (1,632) was 57:1, according to data from the Department of Health & Human Services.
New admissions show a similar pattern: The 0.74 admissions per 100,000 children recorded on Dec. 31 was lower than, for example, adults aged 30-39 years (2.7 per 100,000) or 50-59 years (4.25 per 100,000), but on Sept. 5 the corresponding figures were 0.46 (children), 2.74 (ages 30-39), and 5.03 (aged 50-59), based on the HHS data.
A look at vaccinations
The vaccination response to Omicron, however, has been more subdued and somewhat inconsistent. Vaccine initiation, not surprisingly, was down among eligible children for the week of Dec. 23-29. Before that, both the 5- to 11-year-olds and 12- to 15-year-olds were down for the second week of December and then up a bit (5.6% and 14.3%, respectively) during the third week, while the 16- to 17-year-olds, increased initiation by 63.2%, CDC’s COVID Data Tracker shows.
Less than a quarter (23.5%) of children aged 5-11 received at least one dose of the vaccine in the first 2 months of their eligibility, and only 14.7% are fully vaccinated. Among the older children, coverage looks like this: at least one dose for 61.2% of 12- to 15-year-olds and 67.4% of 16- to 17-year-olds and full vaccination for 51.3% and 57.6%, respectively, the CDC said.
At the state level, Massachusetts and Hawaii have the highest rates for children aged 12-17 years, with 86% having received a least one dose, and Vermont is highest for children aged 5-11 at 56%. The lowest rates can be found in Wyoming (38%) for 12- to 17-year-olds and in Mississippi (6%) for 5- to 11-year-olds, the AAP said in a separate report.
Weekly COVID-19 cases in children passed 300,000 for the first time since the pandemic started, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The rate of new COVID-related hospital admissions also reached a new high of 0.74 per 100,000 children as of Dec. 31. The highest rate seen before the current Omicron-fueled surge was 0.47 per 100,000 in early September, data from the Centers for Disease Control and Prevention show.
and exceeding the previous week’s count by almost 64%, the AAP and CHA said in their weekly COVID report.
New cases were up in all four regions of the United States, with the Northeast adding the most newly infected children while setting a new high for the fifth consecutive week. The South was just behind for the week but still well off the record it reached in September, the Midwest was third but recorded its busiest week ever, and the West was fourth and nowhere near its previous high, the AAP/CHA report indicated.
The total number of child cases since the pandemic began is almost 7.9 million, they said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. That figure represents 17.4% of all cases reported in the United States, and the cumulative rate of COVID infection is up to almost 10,500 per 100,000 children, meaning that 1 in 10 children have been infected.
Children are still less likely to be hospitalized than adults, but the gap appears to be closing. On Jan. 2 there were 2,343 children and 87,690 adults in the hospital with confirmed COVID, a ratio of 37 adults for each child, but on Sept. 5, at the height of the previous surge, the ratio of hospitalized adults (93,647) to children (1,632) was 57:1, according to data from the Department of Health & Human Services.
New admissions show a similar pattern: The 0.74 admissions per 100,000 children recorded on Dec. 31 was lower than, for example, adults aged 30-39 years (2.7 per 100,000) or 50-59 years (4.25 per 100,000), but on Sept. 5 the corresponding figures were 0.46 (children), 2.74 (ages 30-39), and 5.03 (aged 50-59), based on the HHS data.
A look at vaccinations
The vaccination response to Omicron, however, has been more subdued and somewhat inconsistent. Vaccine initiation, not surprisingly, was down among eligible children for the week of Dec. 23-29. Before that, both the 5- to 11-year-olds and 12- to 15-year-olds were down for the second week of December and then up a bit (5.6% and 14.3%, respectively) during the third week, while the 16- to 17-year-olds, increased initiation by 63.2%, CDC’s COVID Data Tracker shows.
Less than a quarter (23.5%) of children aged 5-11 received at least one dose of the vaccine in the first 2 months of their eligibility, and only 14.7% are fully vaccinated. Among the older children, coverage looks like this: at least one dose for 61.2% of 12- to 15-year-olds and 67.4% of 16- to 17-year-olds and full vaccination for 51.3% and 57.6%, respectively, the CDC said.
At the state level, Massachusetts and Hawaii have the highest rates for children aged 12-17 years, with 86% having received a least one dose, and Vermont is highest for children aged 5-11 at 56%. The lowest rates can be found in Wyoming (38%) for 12- to 17-year-olds and in Mississippi (6%) for 5- to 11-year-olds, the AAP said in a separate report.
Weekly COVID-19 cases in children passed 300,000 for the first time since the pandemic started, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The rate of new COVID-related hospital admissions also reached a new high of 0.74 per 100,000 children as of Dec. 31. The highest rate seen before the current Omicron-fueled surge was 0.47 per 100,000 in early September, data from the Centers for Disease Control and Prevention show.
and exceeding the previous week’s count by almost 64%, the AAP and CHA said in their weekly COVID report.
New cases were up in all four regions of the United States, with the Northeast adding the most newly infected children while setting a new high for the fifth consecutive week. The South was just behind for the week but still well off the record it reached in September, the Midwest was third but recorded its busiest week ever, and the West was fourth and nowhere near its previous high, the AAP/CHA report indicated.
The total number of child cases since the pandemic began is almost 7.9 million, they said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. That figure represents 17.4% of all cases reported in the United States, and the cumulative rate of COVID infection is up to almost 10,500 per 100,000 children, meaning that 1 in 10 children have been infected.
Children are still less likely to be hospitalized than adults, but the gap appears to be closing. On Jan. 2 there were 2,343 children and 87,690 adults in the hospital with confirmed COVID, a ratio of 37 adults for each child, but on Sept. 5, at the height of the previous surge, the ratio of hospitalized adults (93,647) to children (1,632) was 57:1, according to data from the Department of Health & Human Services.
New admissions show a similar pattern: The 0.74 admissions per 100,000 children recorded on Dec. 31 was lower than, for example, adults aged 30-39 years (2.7 per 100,000) or 50-59 years (4.25 per 100,000), but on Sept. 5 the corresponding figures were 0.46 (children), 2.74 (ages 30-39), and 5.03 (aged 50-59), based on the HHS data.
A look at vaccinations
The vaccination response to Omicron, however, has been more subdued and somewhat inconsistent. Vaccine initiation, not surprisingly, was down among eligible children for the week of Dec. 23-29. Before that, both the 5- to 11-year-olds and 12- to 15-year-olds were down for the second week of December and then up a bit (5.6% and 14.3%, respectively) during the third week, while the 16- to 17-year-olds, increased initiation by 63.2%, CDC’s COVID Data Tracker shows.
Less than a quarter (23.5%) of children aged 5-11 received at least one dose of the vaccine in the first 2 months of their eligibility, and only 14.7% are fully vaccinated. Among the older children, coverage looks like this: at least one dose for 61.2% of 12- to 15-year-olds and 67.4% of 16- to 17-year-olds and full vaccination for 51.3% and 57.6%, respectively, the CDC said.
At the state level, Massachusetts and Hawaii have the highest rates for children aged 12-17 years, with 86% having received a least one dose, and Vermont is highest for children aged 5-11 at 56%. The lowest rates can be found in Wyoming (38%) for 12- to 17-year-olds and in Mississippi (6%) for 5- to 11-year-olds, the AAP said in a separate report.
Asthma treatment does not appear to raise risk of neuropsychiatric disease
Use of a leukotriene receptor antagonist (LTRA) for asthma management did not increase the risk of neuropsychiatric disease, based on data from more than 60,000 asthma patients.
Although LTRAs are established as an effective drug for asthma, the U.S. Food and Drug Administration warnings of the risk for neuropsychiatric (NP) drug reactions – including a boxed warning for montelukast (Singulair) – has raised concerns, writes Ji-Su Shim, MD, of Ewha Womans University, Seoul, South Korea, and colleagues.
However, evidence for such an association is limited, and previous studies have focused only on children and adolescents, and on a single LTRA (montelukast), the researchers say.
In a study published Dec. 1 in the Journal of Allergy and Clinical Immunology: In Practice, the researchers used a Korean national health insurance database to identify 61,571 adult patients with asthma aged 40 years and older between Jan. 2002 and Dec. 2015 with no history of LTRA use.
The patients underwent screening examinations between Jan. 2009 and Dec. 2010, which marked the start of a follow-up period ending on Dec. 31, 2015. The median age of the study population was 61 years, and the mean follow-up period for NPs or other outcomes was approximately 47.6 months for LTRA users and 46.5 months for nonusers. Overall, 11.1% of the study population used pranlukast (Onon), 11% used montelukast, and 0.24% used zafirlukast (Accolate).
A total of 12,168 patients took an LTRA during the follow-up period. The hazard ratio for newly diagnosed neuropsychiatric diseases was not significantly different between LTRA users and nonusers (hazard ratio, 1.01; P = .952) in an adjusted model that included age, sex, pack-years of smoking, alcohol use, physical activity, body mass index, comorbid conditions, other respiratory diseases, and use of other asthma medications.
(75.4% vs. 76.1% for dementia, 12.7% vs. 12.8% for mood disorders, and 5.6% vs. 3.5% for panic disorders).
A subgroup analysis for associations between the duration of LTRA use and NP disease risk also showed no significant difference between LTRA users and nonusers.
“The mechanism of the development of NP symptoms by LTRAs has not been identified,” the researchers write in their discussion of the study findings. “Because most of NP side effects due to montelukast occur in few patients within 2 weeks of drug administration, it also may have relation with the presence of some genetic polymorphisms involving modification of the normal action or metabolism of LTRAs,” they explained.
The FDA’s boxed warning for montelukast noting the risk of serious mental health side effects has renewed interest in the relationship between NPs and LTRAs, the researchers noted. However, the current study findings support previous randomized controlled trials and larger studies, and the current warnings are based mainly on pharmacovigilance studies, case series, and case reports, they said.
The study findings were limited by several factors, including the retrospective design, the potential for misclassification of asthma diagnosis, the exclusion of temporary NP symptoms that might prompt LTRA discontinuation, and the inability to detect possible differences in ethnicities other than Korean, the researchers note.
However, the results suggest that adverse NP symptoms should not prevent physicians from prescribing LTRAs to selected patients with asthma. Instead, the physician should accompany the prescription with “a word of caution in case any mood changes might occur,” the investigators wrote.
“Further studies, such as randomized controlled trials, are needed to reveal the association between the use of LTRAs and the risk of NP events and/or diseases,” they concluded.
Potential genetic predisposition may drive cases
The relatively rare occurrence of NP symptoms in asthma patients using LTRAs has prompted questions from the medical community on whether the relationship really exists, writes Désirée Larenas-Linnemann, MD, of Médica Sur Clinical Foundation and Hospital, Mexico City, in an accompanying editorial ).
The current study provides information about medications and possible adverse drug reactions, but “great care should be taken in the interpretation of the results from such a study,” she notes. Limitations include not only the possible misclassification of asthma and the homogeneous study population, but also the fact that some NPs, such as dementia, are already common in older adults..
Dr. Larenas-Linnemann shared a story of one of her patients, a 2½-year-old boy who began exhibiting hyperactivity and other strange behaviors while on an LRTA. The toddler’s father had previously reported “horrible nightmares, strange thoughts, and to feel upset, unsecure until he suspended the medication.” Cases such as this support a potential genetic predisposition, with drug metabolism playing a role, and clinicians should take genetic backgrounds into account, she said.
“Even though the current study did not show an association between LTRA use or duration of exposure and the occurrence of NP diseases in Korean adults with asthma, this does not imply such a relationship might be present in other age groups (children-adolescents-adults up to 50 years) or in patients with a different genetic background,” she emphasized.
However, “In the meantime, although LTRA should continue to be prescribed if indicated, an index of suspicion for possible NP effects should be maintained,” Dr. Larenas-Linnemann concluded.
“This study is timely, since the boxed warning for montelukast was issued approximately 1 year ago by the FDA,” Thomas B. Casale, MD, of the University of South Florida, Tampa, said in an interview.
Dr. Casale said he was not surprised by the findings, “since most of the data implicating a potential link between the use of montelukast and neuropsychiatric disorders have not been particularly compelling,” and much of the current information comes from case reports and retrospective studies.
“Furthermore, the data appeared to be somewhat stronger in the pediatric population,” Dr. Casale noted. “This study focused on elderly patients (mean age 61) and included two other leukotriene modifiers. The number of patients receiving montelukast was small (56), which may have also confounded the results,” he noted.
As for clinical implications, “I don’t think this study will change practice,” Dr. Casale said. “As indicated, it is in an elderly population, included only a limited number of patients receiving montelukast, and was in a Korean cohort. All of these factors could have influenced the results,” and the data may not be generalizable to patients elsewhere, including the United States, he said. “Also, the study only included patients with asthma and in the United States; the approval for rhinitis is another important indication to study,” he noted.
Additional research is needed in the form of better prospective studies examining the potential link between montelukast and neuropsychiatric disorders in both the pediatric and adult populations having either asthma or rhinitis, Dr. Casale concluded.
The study received no outside funding. The researchers and Dr. Casale have disclosed no relevant financial relationships. Dr. Larenas-Linnemann disclosed personal fees from Allakos, Armstrong, AstraZeneca, Chiesi, DBV Technologies, Grünenthal, GSK, Mylan/Viatris, Menarini, MSD, Novartis, Pfizer, Sanofi, Siegfried, UCB, Alakos, Gossamer, and Carnot, and grants from Sanofi, AstraZeneca, Novartis, Circassia, UCB, GSK, and the Purina Institute.
A version of this article first appeared on Medscape.com.
Use of a leukotriene receptor antagonist (LTRA) for asthma management did not increase the risk of neuropsychiatric disease, based on data from more than 60,000 asthma patients.
Although LTRAs are established as an effective drug for asthma, the U.S. Food and Drug Administration warnings of the risk for neuropsychiatric (NP) drug reactions – including a boxed warning for montelukast (Singulair) – has raised concerns, writes Ji-Su Shim, MD, of Ewha Womans University, Seoul, South Korea, and colleagues.
However, evidence for such an association is limited, and previous studies have focused only on children and adolescents, and on a single LTRA (montelukast), the researchers say.
In a study published Dec. 1 in the Journal of Allergy and Clinical Immunology: In Practice, the researchers used a Korean national health insurance database to identify 61,571 adult patients with asthma aged 40 years and older between Jan. 2002 and Dec. 2015 with no history of LTRA use.
The patients underwent screening examinations between Jan. 2009 and Dec. 2010, which marked the start of a follow-up period ending on Dec. 31, 2015. The median age of the study population was 61 years, and the mean follow-up period for NPs or other outcomes was approximately 47.6 months for LTRA users and 46.5 months for nonusers. Overall, 11.1% of the study population used pranlukast (Onon), 11% used montelukast, and 0.24% used zafirlukast (Accolate).
A total of 12,168 patients took an LTRA during the follow-up period. The hazard ratio for newly diagnosed neuropsychiatric diseases was not significantly different between LTRA users and nonusers (hazard ratio, 1.01; P = .952) in an adjusted model that included age, sex, pack-years of smoking, alcohol use, physical activity, body mass index, comorbid conditions, other respiratory diseases, and use of other asthma medications.
(75.4% vs. 76.1% for dementia, 12.7% vs. 12.8% for mood disorders, and 5.6% vs. 3.5% for panic disorders).
A subgroup analysis for associations between the duration of LTRA use and NP disease risk also showed no significant difference between LTRA users and nonusers.
“The mechanism of the development of NP symptoms by LTRAs has not been identified,” the researchers write in their discussion of the study findings. “Because most of NP side effects due to montelukast occur in few patients within 2 weeks of drug administration, it also may have relation with the presence of some genetic polymorphisms involving modification of the normal action or metabolism of LTRAs,” they explained.
The FDA’s boxed warning for montelukast noting the risk of serious mental health side effects has renewed interest in the relationship between NPs and LTRAs, the researchers noted. However, the current study findings support previous randomized controlled trials and larger studies, and the current warnings are based mainly on pharmacovigilance studies, case series, and case reports, they said.
The study findings were limited by several factors, including the retrospective design, the potential for misclassification of asthma diagnosis, the exclusion of temporary NP symptoms that might prompt LTRA discontinuation, and the inability to detect possible differences in ethnicities other than Korean, the researchers note.
However, the results suggest that adverse NP symptoms should not prevent physicians from prescribing LTRAs to selected patients with asthma. Instead, the physician should accompany the prescription with “a word of caution in case any mood changes might occur,” the investigators wrote.
“Further studies, such as randomized controlled trials, are needed to reveal the association between the use of LTRAs and the risk of NP events and/or diseases,” they concluded.
Potential genetic predisposition may drive cases
The relatively rare occurrence of NP symptoms in asthma patients using LTRAs has prompted questions from the medical community on whether the relationship really exists, writes Désirée Larenas-Linnemann, MD, of Médica Sur Clinical Foundation and Hospital, Mexico City, in an accompanying editorial ).
The current study provides information about medications and possible adverse drug reactions, but “great care should be taken in the interpretation of the results from such a study,” she notes. Limitations include not only the possible misclassification of asthma and the homogeneous study population, but also the fact that some NPs, such as dementia, are already common in older adults..
Dr. Larenas-Linnemann shared a story of one of her patients, a 2½-year-old boy who began exhibiting hyperactivity and other strange behaviors while on an LRTA. The toddler’s father had previously reported “horrible nightmares, strange thoughts, and to feel upset, unsecure until he suspended the medication.” Cases such as this support a potential genetic predisposition, with drug metabolism playing a role, and clinicians should take genetic backgrounds into account, she said.
“Even though the current study did not show an association between LTRA use or duration of exposure and the occurrence of NP diseases in Korean adults with asthma, this does not imply such a relationship might be present in other age groups (children-adolescents-adults up to 50 years) or in patients with a different genetic background,” she emphasized.
However, “In the meantime, although LTRA should continue to be prescribed if indicated, an index of suspicion for possible NP effects should be maintained,” Dr. Larenas-Linnemann concluded.
“This study is timely, since the boxed warning for montelukast was issued approximately 1 year ago by the FDA,” Thomas B. Casale, MD, of the University of South Florida, Tampa, said in an interview.
Dr. Casale said he was not surprised by the findings, “since most of the data implicating a potential link between the use of montelukast and neuropsychiatric disorders have not been particularly compelling,” and much of the current information comes from case reports and retrospective studies.
“Furthermore, the data appeared to be somewhat stronger in the pediatric population,” Dr. Casale noted. “This study focused on elderly patients (mean age 61) and included two other leukotriene modifiers. The number of patients receiving montelukast was small (56), which may have also confounded the results,” he noted.
As for clinical implications, “I don’t think this study will change practice,” Dr. Casale said. “As indicated, it is in an elderly population, included only a limited number of patients receiving montelukast, and was in a Korean cohort. All of these factors could have influenced the results,” and the data may not be generalizable to patients elsewhere, including the United States, he said. “Also, the study only included patients with asthma and in the United States; the approval for rhinitis is another important indication to study,” he noted.
Additional research is needed in the form of better prospective studies examining the potential link between montelukast and neuropsychiatric disorders in both the pediatric and adult populations having either asthma or rhinitis, Dr. Casale concluded.
The study received no outside funding. The researchers and Dr. Casale have disclosed no relevant financial relationships. Dr. Larenas-Linnemann disclosed personal fees from Allakos, Armstrong, AstraZeneca, Chiesi, DBV Technologies, Grünenthal, GSK, Mylan/Viatris, Menarini, MSD, Novartis, Pfizer, Sanofi, Siegfried, UCB, Alakos, Gossamer, and Carnot, and grants from Sanofi, AstraZeneca, Novartis, Circassia, UCB, GSK, and the Purina Institute.
A version of this article first appeared on Medscape.com.
Use of a leukotriene receptor antagonist (LTRA) for asthma management did not increase the risk of neuropsychiatric disease, based on data from more than 60,000 asthma patients.
Although LTRAs are established as an effective drug for asthma, the U.S. Food and Drug Administration warnings of the risk for neuropsychiatric (NP) drug reactions – including a boxed warning for montelukast (Singulair) – has raised concerns, writes Ji-Su Shim, MD, of Ewha Womans University, Seoul, South Korea, and colleagues.
However, evidence for such an association is limited, and previous studies have focused only on children and adolescents, and on a single LTRA (montelukast), the researchers say.
In a study published Dec. 1 in the Journal of Allergy and Clinical Immunology: In Practice, the researchers used a Korean national health insurance database to identify 61,571 adult patients with asthma aged 40 years and older between Jan. 2002 and Dec. 2015 with no history of LTRA use.
The patients underwent screening examinations between Jan. 2009 and Dec. 2010, which marked the start of a follow-up period ending on Dec. 31, 2015. The median age of the study population was 61 years, and the mean follow-up period for NPs or other outcomes was approximately 47.6 months for LTRA users and 46.5 months for nonusers. Overall, 11.1% of the study population used pranlukast (Onon), 11% used montelukast, and 0.24% used zafirlukast (Accolate).
A total of 12,168 patients took an LTRA during the follow-up period. The hazard ratio for newly diagnosed neuropsychiatric diseases was not significantly different between LTRA users and nonusers (hazard ratio, 1.01; P = .952) in an adjusted model that included age, sex, pack-years of smoking, alcohol use, physical activity, body mass index, comorbid conditions, other respiratory diseases, and use of other asthma medications.
(75.4% vs. 76.1% for dementia, 12.7% vs. 12.8% for mood disorders, and 5.6% vs. 3.5% for panic disorders).
A subgroup analysis for associations between the duration of LTRA use and NP disease risk also showed no significant difference between LTRA users and nonusers.
“The mechanism of the development of NP symptoms by LTRAs has not been identified,” the researchers write in their discussion of the study findings. “Because most of NP side effects due to montelukast occur in few patients within 2 weeks of drug administration, it also may have relation with the presence of some genetic polymorphisms involving modification of the normal action or metabolism of LTRAs,” they explained.
The FDA’s boxed warning for montelukast noting the risk of serious mental health side effects has renewed interest in the relationship between NPs and LTRAs, the researchers noted. However, the current study findings support previous randomized controlled trials and larger studies, and the current warnings are based mainly on pharmacovigilance studies, case series, and case reports, they said.
The study findings were limited by several factors, including the retrospective design, the potential for misclassification of asthma diagnosis, the exclusion of temporary NP symptoms that might prompt LTRA discontinuation, and the inability to detect possible differences in ethnicities other than Korean, the researchers note.
However, the results suggest that adverse NP symptoms should not prevent physicians from prescribing LTRAs to selected patients with asthma. Instead, the physician should accompany the prescription with “a word of caution in case any mood changes might occur,” the investigators wrote.
“Further studies, such as randomized controlled trials, are needed to reveal the association between the use of LTRAs and the risk of NP events and/or diseases,” they concluded.
Potential genetic predisposition may drive cases
The relatively rare occurrence of NP symptoms in asthma patients using LTRAs has prompted questions from the medical community on whether the relationship really exists, writes Désirée Larenas-Linnemann, MD, of Médica Sur Clinical Foundation and Hospital, Mexico City, in an accompanying editorial ).
The current study provides information about medications and possible adverse drug reactions, but “great care should be taken in the interpretation of the results from such a study,” she notes. Limitations include not only the possible misclassification of asthma and the homogeneous study population, but also the fact that some NPs, such as dementia, are already common in older adults..
Dr. Larenas-Linnemann shared a story of one of her patients, a 2½-year-old boy who began exhibiting hyperactivity and other strange behaviors while on an LRTA. The toddler’s father had previously reported “horrible nightmares, strange thoughts, and to feel upset, unsecure until he suspended the medication.” Cases such as this support a potential genetic predisposition, with drug metabolism playing a role, and clinicians should take genetic backgrounds into account, she said.
“Even though the current study did not show an association between LTRA use or duration of exposure and the occurrence of NP diseases in Korean adults with asthma, this does not imply such a relationship might be present in other age groups (children-adolescents-adults up to 50 years) or in patients with a different genetic background,” she emphasized.
However, “In the meantime, although LTRA should continue to be prescribed if indicated, an index of suspicion for possible NP effects should be maintained,” Dr. Larenas-Linnemann concluded.
“This study is timely, since the boxed warning for montelukast was issued approximately 1 year ago by the FDA,” Thomas B. Casale, MD, of the University of South Florida, Tampa, said in an interview.
Dr. Casale said he was not surprised by the findings, “since most of the data implicating a potential link between the use of montelukast and neuropsychiatric disorders have not been particularly compelling,” and much of the current information comes from case reports and retrospective studies.
“Furthermore, the data appeared to be somewhat stronger in the pediatric population,” Dr. Casale noted. “This study focused on elderly patients (mean age 61) and included two other leukotriene modifiers. The number of patients receiving montelukast was small (56), which may have also confounded the results,” he noted.
As for clinical implications, “I don’t think this study will change practice,” Dr. Casale said. “As indicated, it is in an elderly population, included only a limited number of patients receiving montelukast, and was in a Korean cohort. All of these factors could have influenced the results,” and the data may not be generalizable to patients elsewhere, including the United States, he said. “Also, the study only included patients with asthma and in the United States; the approval for rhinitis is another important indication to study,” he noted.
Additional research is needed in the form of better prospective studies examining the potential link between montelukast and neuropsychiatric disorders in both the pediatric and adult populations having either asthma or rhinitis, Dr. Casale concluded.
The study received no outside funding. The researchers and Dr. Casale have disclosed no relevant financial relationships. Dr. Larenas-Linnemann disclosed personal fees from Allakos, Armstrong, AstraZeneca, Chiesi, DBV Technologies, Grünenthal, GSK, Mylan/Viatris, Menarini, MSD, Novartis, Pfizer, Sanofi, Siegfried, UCB, Alakos, Gossamer, and Carnot, and grants from Sanofi, AstraZeneca, Novartis, Circassia, UCB, GSK, and the Purina Institute.
A version of this article first appeared on Medscape.com.
Peanut desensitization plummets 1 month after avoiding exposure
Children with peanut allergies treated with peanut oral immunotherapy for 3 years can tolerate increasingly higher exposures to peanuts. But avoidance of peanut-protein exposure for just a single month after the treatment leads to rapid and substantial decreases in tolerance, findings from a small study show.
The findings “underscore the fact that the desensitization achieved with peanut oral immunotherapy is a transient immune state,” report the authors of the study, published in December in The Journal of Allergy and Clinical Immunology: In Practice.
Therefore, “adherence to dosing [in peanut immunotherapy] is very important, and clinicians should expect a decline in tolerance with lapse in dosing,” first author Carla M. Davis, MD, director of the Texas Children’s Hospital Food Allergy Program at Baylor College of Medicine, Houston, told this news organization.
Oral immunotherapy, involving small exposures to peanut protein to build up desensitization, has been shown to mitigate allergic reactions, and, as reported by this news organization, the first peanut oral immunotherapy drug recently received approval from the U.S. Food and Drug Administration.
However, current approaches involve very low daily exposure of about 300 mg of peanut protein, equivalent to only about one to two peanuts, and research is lacking regarding the maximum tolerated doses, as well as on how long the tolerance is sustained if maintenance therapy is discontinued. “For the peanut-allergic population that would like to eat more than 1-2 peanuts, an achievable dose is currently unknown,” the study authors write. “The critical question, of the maximum tolerated dose achieved after POIT, has not been answered.”
To evaluate those issues in their phase 2 study, Dr. Davis and her colleagues enrolled 28 subjects between the ages of 5 and 13 with a diagnosis of eosinophilic esophagitis and peanut allergy.
The treatment protocol included a 1-year buildup phase of oral immunotherapy, followed by a 2-year daily maintenance phase with a dose of 3,900 mg of peanut protein.
After consenting, 11 patients dropped out of the study due to a lack of interest, and two more withdrew after failing to tolerate their first dose, leaving 15 who started treatment in the study, with a mean age of 8.7 years (range, 5.2-12.5 years), and 47% female.
Twelve patients reached the maintenance dose of 3,900 mg over a median of 13 months, and double-blind, placebo-controlled peanut challenges showed that, on average, their mean maximum cumulative tolerated dose after 12 months increased by 12,063 mg (P < .001), and the mean dose triggering a reaction increased by 15,667 mg.
Of the 12 patients, 11 (91.7%) were able to successfully tolerate at least 10,725 mg after 12 months of treatment, and six patients (50.0%) successfully tolerated at least 15,225 mg.
Two patients were able to tolerate up to the maximum cumulative target dose of 26,225 mg, equivalent to more than 105 peanuts.
“The ability to tolerate [greater than] 100 peanuts following peanut oral immunotherapy has never before been demonstrated and gives insight into the potential for food oral immunotherapy to be utilized in a subset of patients who have an immunologic phenotype accepting of this therapy,” the authors write.
“Understanding the risk of ingestion of peanut protein higher than the prescribed peanut oral immunotherapy maintenance dose will improve the safe, practical use of [the therapy],” they add.
Tolerance plummets with avoidance
In the protocol’s third phase, after the 3-year buildup and maintenance therapy, daily peanut exposure was avoided for 30 days, and among the six patients who participated, the mean maximum cumulative tolerated dose declined to just 2,783 mg, and the reaction dose dropped to 4,614 mg (P = .03).
“This was a disappointing finding, because we thought the desensitization would last longer after such a long period of treatment,” Dr. Davis said.
While the avoidance period was only a month, Dr. Davis said she expects the rebound in sensitivity would continue if avoidance was prolonged. “Other studies indicate the decline in tolerance would continue over time, [and] we believe it would continue to decline,” she said.
Further analysis of peanut allergy biomarkers showed significant decreases in skin prick test wheal size and cytokine expression within the first 6 weeks of initiation of the peanut oral immunotherapy. The patterns were reversed during the 1-month avoidance, with both measures increasing.
Of note, the changes in biomarkers varied significantly among the participants.
In terms of adverse events, eight patients (53%) required one or two doses of epinephrine during the study, with all but two patients receiving the epinephrine during the 12-month buildup phase, consistent with previous studies.
In commenting on the study, Richard L. Wasserman, MD, PhD, medical director of pediatric allergy and immunology at Medical City Children’s Hospital, Dallas, noted that the findings pertain to the subset of peanut oral immunotherapy patients (about 30%) who want to be able to eat peanuts.
“Most families just want protection against accidental ingestion, and these observations don’t relate to those patients,” he said in an interview.
Dr. Wasserman noted that his approach with patients is to wait until 3 years of daily maintenance after buildup (as opposed to 2 years in the study) before considering an avoidance challenge.
“When our patients pass a sustained unresponsiveness challenge, we recommend continued exposure of 2,000 mg at least weekly,” he explained.
Dr. Wasserman added that the study’s findings on biomarker changes were notable.
“The eventual reduction in peanut serum IgE in all of their patients is very interesting,” he said. “Many of our patients’ peanut serum IgE plateaus after 2 or 3 years.”
And he added, “This report suggests that we should be making patients aware that they may further decrease their peanut serum IgE by increasing their maintenance dose.”
The study was funded by the Scurlock Foundation/Waring Family Foundation and the Texas Children’s Hospital food allergy program. Dr. Davis is a consultant for Aimmune, DBV, and Moonlight Therapeutics. Dr. Wasserman is a consultant for Aimmune and DBV.
A version of this article first appeared on Medscape.com.
Children with peanut allergies treated with peanut oral immunotherapy for 3 years can tolerate increasingly higher exposures to peanuts. But avoidance of peanut-protein exposure for just a single month after the treatment leads to rapid and substantial decreases in tolerance, findings from a small study show.
The findings “underscore the fact that the desensitization achieved with peanut oral immunotherapy is a transient immune state,” report the authors of the study, published in December in The Journal of Allergy and Clinical Immunology: In Practice.
Therefore, “adherence to dosing [in peanut immunotherapy] is very important, and clinicians should expect a decline in tolerance with lapse in dosing,” first author Carla M. Davis, MD, director of the Texas Children’s Hospital Food Allergy Program at Baylor College of Medicine, Houston, told this news organization.
Oral immunotherapy, involving small exposures to peanut protein to build up desensitization, has been shown to mitigate allergic reactions, and, as reported by this news organization, the first peanut oral immunotherapy drug recently received approval from the U.S. Food and Drug Administration.
However, current approaches involve very low daily exposure of about 300 mg of peanut protein, equivalent to only about one to two peanuts, and research is lacking regarding the maximum tolerated doses, as well as on how long the tolerance is sustained if maintenance therapy is discontinued. “For the peanut-allergic population that would like to eat more than 1-2 peanuts, an achievable dose is currently unknown,” the study authors write. “The critical question, of the maximum tolerated dose achieved after POIT, has not been answered.”
To evaluate those issues in their phase 2 study, Dr. Davis and her colleagues enrolled 28 subjects between the ages of 5 and 13 with a diagnosis of eosinophilic esophagitis and peanut allergy.
The treatment protocol included a 1-year buildup phase of oral immunotherapy, followed by a 2-year daily maintenance phase with a dose of 3,900 mg of peanut protein.
After consenting, 11 patients dropped out of the study due to a lack of interest, and two more withdrew after failing to tolerate their first dose, leaving 15 who started treatment in the study, with a mean age of 8.7 years (range, 5.2-12.5 years), and 47% female.
Twelve patients reached the maintenance dose of 3,900 mg over a median of 13 months, and double-blind, placebo-controlled peanut challenges showed that, on average, their mean maximum cumulative tolerated dose after 12 months increased by 12,063 mg (P < .001), and the mean dose triggering a reaction increased by 15,667 mg.
Of the 12 patients, 11 (91.7%) were able to successfully tolerate at least 10,725 mg after 12 months of treatment, and six patients (50.0%) successfully tolerated at least 15,225 mg.
Two patients were able to tolerate up to the maximum cumulative target dose of 26,225 mg, equivalent to more than 105 peanuts.
“The ability to tolerate [greater than] 100 peanuts following peanut oral immunotherapy has never before been demonstrated and gives insight into the potential for food oral immunotherapy to be utilized in a subset of patients who have an immunologic phenotype accepting of this therapy,” the authors write.
“Understanding the risk of ingestion of peanut protein higher than the prescribed peanut oral immunotherapy maintenance dose will improve the safe, practical use of [the therapy],” they add.
Tolerance plummets with avoidance
In the protocol’s third phase, after the 3-year buildup and maintenance therapy, daily peanut exposure was avoided for 30 days, and among the six patients who participated, the mean maximum cumulative tolerated dose declined to just 2,783 mg, and the reaction dose dropped to 4,614 mg (P = .03).
“This was a disappointing finding, because we thought the desensitization would last longer after such a long period of treatment,” Dr. Davis said.
While the avoidance period was only a month, Dr. Davis said she expects the rebound in sensitivity would continue if avoidance was prolonged. “Other studies indicate the decline in tolerance would continue over time, [and] we believe it would continue to decline,” she said.
Further analysis of peanut allergy biomarkers showed significant decreases in skin prick test wheal size and cytokine expression within the first 6 weeks of initiation of the peanut oral immunotherapy. The patterns were reversed during the 1-month avoidance, with both measures increasing.
Of note, the changes in biomarkers varied significantly among the participants.
In terms of adverse events, eight patients (53%) required one or two doses of epinephrine during the study, with all but two patients receiving the epinephrine during the 12-month buildup phase, consistent with previous studies.
In commenting on the study, Richard L. Wasserman, MD, PhD, medical director of pediatric allergy and immunology at Medical City Children’s Hospital, Dallas, noted that the findings pertain to the subset of peanut oral immunotherapy patients (about 30%) who want to be able to eat peanuts.
“Most families just want protection against accidental ingestion, and these observations don’t relate to those patients,” he said in an interview.
Dr. Wasserman noted that his approach with patients is to wait until 3 years of daily maintenance after buildup (as opposed to 2 years in the study) before considering an avoidance challenge.
“When our patients pass a sustained unresponsiveness challenge, we recommend continued exposure of 2,000 mg at least weekly,” he explained.
Dr. Wasserman added that the study’s findings on biomarker changes were notable.
“The eventual reduction in peanut serum IgE in all of their patients is very interesting,” he said. “Many of our patients’ peanut serum IgE plateaus after 2 or 3 years.”
And he added, “This report suggests that we should be making patients aware that they may further decrease their peanut serum IgE by increasing their maintenance dose.”
The study was funded by the Scurlock Foundation/Waring Family Foundation and the Texas Children’s Hospital food allergy program. Dr. Davis is a consultant for Aimmune, DBV, and Moonlight Therapeutics. Dr. Wasserman is a consultant for Aimmune and DBV.
A version of this article first appeared on Medscape.com.
Children with peanut allergies treated with peanut oral immunotherapy for 3 years can tolerate increasingly higher exposures to peanuts. But avoidance of peanut-protein exposure for just a single month after the treatment leads to rapid and substantial decreases in tolerance, findings from a small study show.
The findings “underscore the fact that the desensitization achieved with peanut oral immunotherapy is a transient immune state,” report the authors of the study, published in December in The Journal of Allergy and Clinical Immunology: In Practice.
Therefore, “adherence to dosing [in peanut immunotherapy] is very important, and clinicians should expect a decline in tolerance with lapse in dosing,” first author Carla M. Davis, MD, director of the Texas Children’s Hospital Food Allergy Program at Baylor College of Medicine, Houston, told this news organization.
Oral immunotherapy, involving small exposures to peanut protein to build up desensitization, has been shown to mitigate allergic reactions, and, as reported by this news organization, the first peanut oral immunotherapy drug recently received approval from the U.S. Food and Drug Administration.
However, current approaches involve very low daily exposure of about 300 mg of peanut protein, equivalent to only about one to two peanuts, and research is lacking regarding the maximum tolerated doses, as well as on how long the tolerance is sustained if maintenance therapy is discontinued. “For the peanut-allergic population that would like to eat more than 1-2 peanuts, an achievable dose is currently unknown,” the study authors write. “The critical question, of the maximum tolerated dose achieved after POIT, has not been answered.”
To evaluate those issues in their phase 2 study, Dr. Davis and her colleagues enrolled 28 subjects between the ages of 5 and 13 with a diagnosis of eosinophilic esophagitis and peanut allergy.
The treatment protocol included a 1-year buildup phase of oral immunotherapy, followed by a 2-year daily maintenance phase with a dose of 3,900 mg of peanut protein.
After consenting, 11 patients dropped out of the study due to a lack of interest, and two more withdrew after failing to tolerate their first dose, leaving 15 who started treatment in the study, with a mean age of 8.7 years (range, 5.2-12.5 years), and 47% female.
Twelve patients reached the maintenance dose of 3,900 mg over a median of 13 months, and double-blind, placebo-controlled peanut challenges showed that, on average, their mean maximum cumulative tolerated dose after 12 months increased by 12,063 mg (P < .001), and the mean dose triggering a reaction increased by 15,667 mg.
Of the 12 patients, 11 (91.7%) were able to successfully tolerate at least 10,725 mg after 12 months of treatment, and six patients (50.0%) successfully tolerated at least 15,225 mg.
Two patients were able to tolerate up to the maximum cumulative target dose of 26,225 mg, equivalent to more than 105 peanuts.
“The ability to tolerate [greater than] 100 peanuts following peanut oral immunotherapy has never before been demonstrated and gives insight into the potential for food oral immunotherapy to be utilized in a subset of patients who have an immunologic phenotype accepting of this therapy,” the authors write.
“Understanding the risk of ingestion of peanut protein higher than the prescribed peanut oral immunotherapy maintenance dose will improve the safe, practical use of [the therapy],” they add.
Tolerance plummets with avoidance
In the protocol’s third phase, after the 3-year buildup and maintenance therapy, daily peanut exposure was avoided for 30 days, and among the six patients who participated, the mean maximum cumulative tolerated dose declined to just 2,783 mg, and the reaction dose dropped to 4,614 mg (P = .03).
“This was a disappointing finding, because we thought the desensitization would last longer after such a long period of treatment,” Dr. Davis said.
While the avoidance period was only a month, Dr. Davis said she expects the rebound in sensitivity would continue if avoidance was prolonged. “Other studies indicate the decline in tolerance would continue over time, [and] we believe it would continue to decline,” she said.
Further analysis of peanut allergy biomarkers showed significant decreases in skin prick test wheal size and cytokine expression within the first 6 weeks of initiation of the peanut oral immunotherapy. The patterns were reversed during the 1-month avoidance, with both measures increasing.
Of note, the changes in biomarkers varied significantly among the participants.
In terms of adverse events, eight patients (53%) required one or two doses of epinephrine during the study, with all but two patients receiving the epinephrine during the 12-month buildup phase, consistent with previous studies.
In commenting on the study, Richard L. Wasserman, MD, PhD, medical director of pediatric allergy and immunology at Medical City Children’s Hospital, Dallas, noted that the findings pertain to the subset of peanut oral immunotherapy patients (about 30%) who want to be able to eat peanuts.
“Most families just want protection against accidental ingestion, and these observations don’t relate to those patients,” he said in an interview.
Dr. Wasserman noted that his approach with patients is to wait until 3 years of daily maintenance after buildup (as opposed to 2 years in the study) before considering an avoidance challenge.
“When our patients pass a sustained unresponsiveness challenge, we recommend continued exposure of 2,000 mg at least weekly,” he explained.
Dr. Wasserman added that the study’s findings on biomarker changes were notable.
“The eventual reduction in peanut serum IgE in all of their patients is very interesting,” he said. “Many of our patients’ peanut serum IgE plateaus after 2 or 3 years.”
And he added, “This report suggests that we should be making patients aware that they may further decrease their peanut serum IgE by increasing their maintenance dose.”
The study was funded by the Scurlock Foundation/Waring Family Foundation and the Texas Children’s Hospital food allergy program. Dr. Davis is a consultant for Aimmune, DBV, and Moonlight Therapeutics. Dr. Wasserman is a consultant for Aimmune and DBV.
A version of this article first appeared on Medscape.com.
New CDC COVID-19 isolation guidelines still up for debate among experts
It’s a true Goldilocks debate: , with some calling them suitable, some saying they’re “reckless,” and at least one expert saying they’re “right in the middle.”
The controversy may lead to more updates. On Jan. 2, Anthony S. Fauci, MD, President Joe Biden’s chief medical adviser, said on CNN’s State of the Union that he anticipates further clarification of the guidelines soon.
Sparking the most debate: Infected people are not told to test before leaving isolation, the vaccinated and unvaccinated who are exposed are given some of the same advice, and the mask advice is not specific enough.
As issued on Dec. 27, the guidelines for the general public recommend:
- Anyone who tests positive should stay home and isolate for 5 days (instead of 10) and if the person has no symptoms or the symptoms resolve after 5 days, leaving the house is okay. A mask should be worn around others for 5 more days. In the event of a fever, the person must stay home until it resolves.
- If people are exposed to someone infected with COVID-19 and they have been boosted, finished the primary series of either the Pfizer or Moderna vaccine within the past 6 months, or finished the primary series of the Johnson & Johnson vaccine within the past 2 months, they should wear a mask around others for 10 days and, if possible, test on day 5. However, if symptoms develop, they should get a test and stay home.
- If people are exposed to someone infected with COVID-19 and they are unvaccinated or are more than 6 months out from their second dose of the Pfizer or Moderna vaccine (or more than 2 months after the J&J vaccine) and not boosted, they should quarantine for 5 days and then wear a mask for 5 more days. If quarantine is impossible, a mask should be worn for 10 days. A test on day 5 is suggested if possible. If symptoms occur, they should quarantine and test.
On social media and in interviews with this news organization, public health experts expressed an array of opinions.
A tweet from Eric Topol, MD, editor-in-chief of Medscape, posted the day after the new guidelines came out, had an empty box and this: “The data that support the new @CDCgov 5 day isolation period without a negative test.”
In a tweet on Jan. 2, Ashish K. Jha, MD, MPH, dean of the Brown University School of Public Health, said: “Hearing that CDC considering adding testing to isolation guidelines. That would be great. I’ve been arguing for a while that serial negative antigen tests provide a lot of confidence that someone is not contagious.”
Michael Mina, MD, PhD, chief science officer of eMed, a digital point-of-care platform enabling at-home diagnostic testing, tweeted: “CDC’s new guidance to drop isolation of positives to 5 days without a negative test is reckless. Some [people] stay infectious 3 days, some 12. I absolutely don’t want to sit next to someone who turned [positive] 5 days ago and hasn’t tested Neg. Test Neg to leave isolation early is just smart.”
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and an infectious disease specialist, disagrees. Typically, he said, an infected person sheds virus for 7 days.
“If you are asymptomatic, the chances that you are shedding a significant amount of virus is very, very small,” he said in an interview.
Under debate
Testing: While many public health experts say a recommendation to test before leaving isolation is needed, CDC Director Rochelle Walensky, MD, explained testing was not recommended before leaving isolation because PCR testing can stay positive up to 12 weeks after a person is first infected with COVID-19.
Asked why there was not a recommendation for a rapid antigen test before leaving isolation, Dr. Walensky told CNN that it is not known how these tests perform at the end of infection and that the tests are not Food and Drug Administration–authorized for that purpose.
And while the guidelines suggest that those exposed – whether they are boosted, vaccinated, or not – should test on day 5 if possible, that recommendation should be stronger, some said. “At the very least recommend a test in those who can get it done,” said Dr. Topol.
However, making that recommendation is difficult when experts know how difficult it is for people to obtain tests now, William Schaffner, MD, professor of preventive medicine and an infectious disease specialist at Vanderbilt University, Nashville, Tenn., said in an interview.
“I am sure this was intensely debated,” Dr. Schaffner said of the recommendation on testing.
Vaccination status categories: Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security, Baltimore, questioned the scientific basis behind treating the fully vaccinated (with two mRNA or one J&J vaccine) who are exposed ‘’as the equivalent of the unvaccinated when it comes to the quarantine requirement since the fully vaccinated are protected against what matters.”
Dr. Topol agreed: Guidelines “should be different for vaccinated versus unvaccinated.”
The recommendations for the exposed should definitely be simpler, Dr. Offit said. “I think it would be much simpler to just say, ‘If you are exposed, mask for 10 days,’ “ regardless of vaccination status.
Masks: The guidelines should also be more specific about the type of masks, Dr. Topol said. They should spell out that the masks need to be N95 or KN95, he said.
Science-driven or economy-driven? Was the guidance changed due more to concerns about the economy than to scientific information about infection and transmission? “It was,” Dr. Topol said.
Dr. Adalja sees it differently. “While it is true that this updated guidance will help the economy, it is based on a scientific foundation and should have been issued much earlier than it was.”
Tough decisions
The agency is walking a tightrope, Dr. Schaffner said, adding that he is in general agreement with what the CDC is trying to do. “The tightrope is between the public health ideal and trying to determine what will be acceptable,’’ he said.
The revised guidelines are more practical than before, others said. “The goal is harm reduction and many people just don’t do any isolation if they are faced with a 10-day period,” Dr. Adalja said.
Before issuing the new guidance, the CDC looked at the accumulating science and also took into account stresses on the health care system and other factors, Dr. Schaffner said. “Is it perfect?” Dr. Schaffner said of the new guideline. “No. Is it carefree? No. It’s right in the middle.”
Dr. Schaffner does think the messages about the new recommendations and how they were decided upon could have been communicated better, and in a more understandable manner. Some experts, for instance, led with the economy and the need for people to return to work and school when explaining the guidelines and then brought up the science behind the revisions.
That order should have been reversed, Dr. Schaffner said.
A version of this article first appeared on Medscape.com.
It’s a true Goldilocks debate: , with some calling them suitable, some saying they’re “reckless,” and at least one expert saying they’re “right in the middle.”
The controversy may lead to more updates. On Jan. 2, Anthony S. Fauci, MD, President Joe Biden’s chief medical adviser, said on CNN’s State of the Union that he anticipates further clarification of the guidelines soon.
Sparking the most debate: Infected people are not told to test before leaving isolation, the vaccinated and unvaccinated who are exposed are given some of the same advice, and the mask advice is not specific enough.
As issued on Dec. 27, the guidelines for the general public recommend:
- Anyone who tests positive should stay home and isolate for 5 days (instead of 10) and if the person has no symptoms or the symptoms resolve after 5 days, leaving the house is okay. A mask should be worn around others for 5 more days. In the event of a fever, the person must stay home until it resolves.
- If people are exposed to someone infected with COVID-19 and they have been boosted, finished the primary series of either the Pfizer or Moderna vaccine within the past 6 months, or finished the primary series of the Johnson & Johnson vaccine within the past 2 months, they should wear a mask around others for 10 days and, if possible, test on day 5. However, if symptoms develop, they should get a test and stay home.
- If people are exposed to someone infected with COVID-19 and they are unvaccinated or are more than 6 months out from their second dose of the Pfizer or Moderna vaccine (or more than 2 months after the J&J vaccine) and not boosted, they should quarantine for 5 days and then wear a mask for 5 more days. If quarantine is impossible, a mask should be worn for 10 days. A test on day 5 is suggested if possible. If symptoms occur, they should quarantine and test.
On social media and in interviews with this news organization, public health experts expressed an array of opinions.
A tweet from Eric Topol, MD, editor-in-chief of Medscape, posted the day after the new guidelines came out, had an empty box and this: “The data that support the new @CDCgov 5 day isolation period without a negative test.”
In a tweet on Jan. 2, Ashish K. Jha, MD, MPH, dean of the Brown University School of Public Health, said: “Hearing that CDC considering adding testing to isolation guidelines. That would be great. I’ve been arguing for a while that serial negative antigen tests provide a lot of confidence that someone is not contagious.”
Michael Mina, MD, PhD, chief science officer of eMed, a digital point-of-care platform enabling at-home diagnostic testing, tweeted: “CDC’s new guidance to drop isolation of positives to 5 days without a negative test is reckless. Some [people] stay infectious 3 days, some 12. I absolutely don’t want to sit next to someone who turned [positive] 5 days ago and hasn’t tested Neg. Test Neg to leave isolation early is just smart.”
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and an infectious disease specialist, disagrees. Typically, he said, an infected person sheds virus for 7 days.
“If you are asymptomatic, the chances that you are shedding a significant amount of virus is very, very small,” he said in an interview.
Under debate
Testing: While many public health experts say a recommendation to test before leaving isolation is needed, CDC Director Rochelle Walensky, MD, explained testing was not recommended before leaving isolation because PCR testing can stay positive up to 12 weeks after a person is first infected with COVID-19.
Asked why there was not a recommendation for a rapid antigen test before leaving isolation, Dr. Walensky told CNN that it is not known how these tests perform at the end of infection and that the tests are not Food and Drug Administration–authorized for that purpose.
And while the guidelines suggest that those exposed – whether they are boosted, vaccinated, or not – should test on day 5 if possible, that recommendation should be stronger, some said. “At the very least recommend a test in those who can get it done,” said Dr. Topol.
However, making that recommendation is difficult when experts know how difficult it is for people to obtain tests now, William Schaffner, MD, professor of preventive medicine and an infectious disease specialist at Vanderbilt University, Nashville, Tenn., said in an interview.
“I am sure this was intensely debated,” Dr. Schaffner said of the recommendation on testing.
Vaccination status categories: Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security, Baltimore, questioned the scientific basis behind treating the fully vaccinated (with two mRNA or one J&J vaccine) who are exposed ‘’as the equivalent of the unvaccinated when it comes to the quarantine requirement since the fully vaccinated are protected against what matters.”
Dr. Topol agreed: Guidelines “should be different for vaccinated versus unvaccinated.”
The recommendations for the exposed should definitely be simpler, Dr. Offit said. “I think it would be much simpler to just say, ‘If you are exposed, mask for 10 days,’ “ regardless of vaccination status.
Masks: The guidelines should also be more specific about the type of masks, Dr. Topol said. They should spell out that the masks need to be N95 or KN95, he said.
Science-driven or economy-driven? Was the guidance changed due more to concerns about the economy than to scientific information about infection and transmission? “It was,” Dr. Topol said.
Dr. Adalja sees it differently. “While it is true that this updated guidance will help the economy, it is based on a scientific foundation and should have been issued much earlier than it was.”
Tough decisions
The agency is walking a tightrope, Dr. Schaffner said, adding that he is in general agreement with what the CDC is trying to do. “The tightrope is between the public health ideal and trying to determine what will be acceptable,’’ he said.
The revised guidelines are more practical than before, others said. “The goal is harm reduction and many people just don’t do any isolation if they are faced with a 10-day period,” Dr. Adalja said.
Before issuing the new guidance, the CDC looked at the accumulating science and also took into account stresses on the health care system and other factors, Dr. Schaffner said. “Is it perfect?” Dr. Schaffner said of the new guideline. “No. Is it carefree? No. It’s right in the middle.”
Dr. Schaffner does think the messages about the new recommendations and how they were decided upon could have been communicated better, and in a more understandable manner. Some experts, for instance, led with the economy and the need for people to return to work and school when explaining the guidelines and then brought up the science behind the revisions.
That order should have been reversed, Dr. Schaffner said.
A version of this article first appeared on Medscape.com.
It’s a true Goldilocks debate: , with some calling them suitable, some saying they’re “reckless,” and at least one expert saying they’re “right in the middle.”
The controversy may lead to more updates. On Jan. 2, Anthony S. Fauci, MD, President Joe Biden’s chief medical adviser, said on CNN’s State of the Union that he anticipates further clarification of the guidelines soon.
Sparking the most debate: Infected people are not told to test before leaving isolation, the vaccinated and unvaccinated who are exposed are given some of the same advice, and the mask advice is not specific enough.
As issued on Dec. 27, the guidelines for the general public recommend:
- Anyone who tests positive should stay home and isolate for 5 days (instead of 10) and if the person has no symptoms or the symptoms resolve after 5 days, leaving the house is okay. A mask should be worn around others for 5 more days. In the event of a fever, the person must stay home until it resolves.
- If people are exposed to someone infected with COVID-19 and they have been boosted, finished the primary series of either the Pfizer or Moderna vaccine within the past 6 months, or finished the primary series of the Johnson & Johnson vaccine within the past 2 months, they should wear a mask around others for 10 days and, if possible, test on day 5. However, if symptoms develop, they should get a test and stay home.
- If people are exposed to someone infected with COVID-19 and they are unvaccinated or are more than 6 months out from their second dose of the Pfizer or Moderna vaccine (or more than 2 months after the J&J vaccine) and not boosted, they should quarantine for 5 days and then wear a mask for 5 more days. If quarantine is impossible, a mask should be worn for 10 days. A test on day 5 is suggested if possible. If symptoms occur, they should quarantine and test.
On social media and in interviews with this news organization, public health experts expressed an array of opinions.
A tweet from Eric Topol, MD, editor-in-chief of Medscape, posted the day after the new guidelines came out, had an empty box and this: “The data that support the new @CDCgov 5 day isolation period without a negative test.”
In a tweet on Jan. 2, Ashish K. Jha, MD, MPH, dean of the Brown University School of Public Health, said: “Hearing that CDC considering adding testing to isolation guidelines. That would be great. I’ve been arguing for a while that serial negative antigen tests provide a lot of confidence that someone is not contagious.”
Michael Mina, MD, PhD, chief science officer of eMed, a digital point-of-care platform enabling at-home diagnostic testing, tweeted: “CDC’s new guidance to drop isolation of positives to 5 days without a negative test is reckless. Some [people] stay infectious 3 days, some 12. I absolutely don’t want to sit next to someone who turned [positive] 5 days ago and hasn’t tested Neg. Test Neg to leave isolation early is just smart.”
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and an infectious disease specialist, disagrees. Typically, he said, an infected person sheds virus for 7 days.
“If you are asymptomatic, the chances that you are shedding a significant amount of virus is very, very small,” he said in an interview.
Under debate
Testing: While many public health experts say a recommendation to test before leaving isolation is needed, CDC Director Rochelle Walensky, MD, explained testing was not recommended before leaving isolation because PCR testing can stay positive up to 12 weeks after a person is first infected with COVID-19.
Asked why there was not a recommendation for a rapid antigen test before leaving isolation, Dr. Walensky told CNN that it is not known how these tests perform at the end of infection and that the tests are not Food and Drug Administration–authorized for that purpose.
And while the guidelines suggest that those exposed – whether they are boosted, vaccinated, or not – should test on day 5 if possible, that recommendation should be stronger, some said. “At the very least recommend a test in those who can get it done,” said Dr. Topol.
However, making that recommendation is difficult when experts know how difficult it is for people to obtain tests now, William Schaffner, MD, professor of preventive medicine and an infectious disease specialist at Vanderbilt University, Nashville, Tenn., said in an interview.
“I am sure this was intensely debated,” Dr. Schaffner said of the recommendation on testing.
Vaccination status categories: Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security, Baltimore, questioned the scientific basis behind treating the fully vaccinated (with two mRNA or one J&J vaccine) who are exposed ‘’as the equivalent of the unvaccinated when it comes to the quarantine requirement since the fully vaccinated are protected against what matters.”
Dr. Topol agreed: Guidelines “should be different for vaccinated versus unvaccinated.”
The recommendations for the exposed should definitely be simpler, Dr. Offit said. “I think it would be much simpler to just say, ‘If you are exposed, mask for 10 days,’ “ regardless of vaccination status.
Masks: The guidelines should also be more specific about the type of masks, Dr. Topol said. They should spell out that the masks need to be N95 or KN95, he said.
Science-driven or economy-driven? Was the guidance changed due more to concerns about the economy than to scientific information about infection and transmission? “It was,” Dr. Topol said.
Dr. Adalja sees it differently. “While it is true that this updated guidance will help the economy, it is based on a scientific foundation and should have been issued much earlier than it was.”
Tough decisions
The agency is walking a tightrope, Dr. Schaffner said, adding that he is in general agreement with what the CDC is trying to do. “The tightrope is between the public health ideal and trying to determine what will be acceptable,’’ he said.
The revised guidelines are more practical than before, others said. “The goal is harm reduction and many people just don’t do any isolation if they are faced with a 10-day period,” Dr. Adalja said.
Before issuing the new guidance, the CDC looked at the accumulating science and also took into account stresses on the health care system and other factors, Dr. Schaffner said. “Is it perfect?” Dr. Schaffner said of the new guideline. “No. Is it carefree? No. It’s right in the middle.”
Dr. Schaffner does think the messages about the new recommendations and how they were decided upon could have been communicated better, and in a more understandable manner. Some experts, for instance, led with the economy and the need for people to return to work and school when explaining the guidelines and then brought up the science behind the revisions.
That order should have been reversed, Dr. Schaffner said.
A version of this article first appeared on Medscape.com.




