User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Children and COVID: The Omicron surge has become a retreat
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 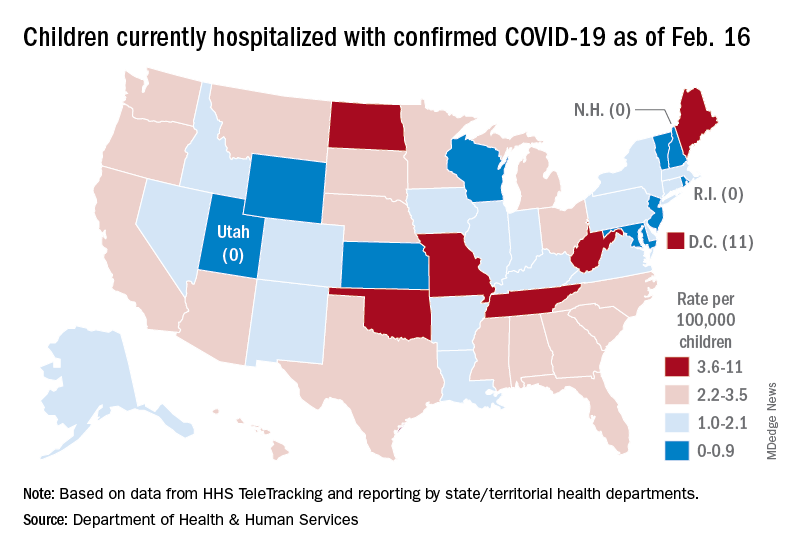
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
Subvariant may be more dangerous than original Omicron strain
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
Past President’s perspective
It’s January 1, 2022, as I write, and my CHEST presidency came to an end last night as the fireworks lit up the sky. With COVID-19 waxing and waning across the United States and around the world, I have been a wartime president. CHEST has not been able to do a number of the things that we would normally have done in person, including that there has not been an in-person CHEST annual meeting during my entire presidency. We have, nonetheless, achieved some important things that I will share with you.
If you’re a typical CHEST member, you probably don’t spend a lot of time wondering about CHEST’s finances, nor should you. Nevertheless, CHEST – your organization – does have to be fiscally responsible if we desire to continue our educational and research missions, and that is the job of your Board of Regents, your presidents, and your professional staff at the CHEST headquarters. I’m happy to tell you that your organization is in healthy financial condition, in spite of a challenging economic environment and, being forced into remote, online annual meetings and board reviews for 2 years. What that means to us and to you is that we get to maintain and improve our full array of educational activities, including our annual meeting, our journal, our board reviews, our hands-on courses at the CHEST headquarters, and our web content. And, we get to accelerate our advocacy activities for our patients and for the clinical folks who care for them (us!). CHEST is primed for emerging from this pandemic stronger, because we have had to make the most of every dollar we have, and more innovative, because that’s how we have done it. We are ready for new ways of interacting and for innovative new ways of delivering education, sponsoring research, fostering networking, and leading in the clinical arena of chest medicine.
During my time as CHEST President, many of us have become progressively more aware of the blatant inequities that continue in society – and, yes, even in medicine. Perhaps more than anything, it both saddens and angers me when anyone values or devalues someone else’s life because of the color of their skin, who they feel attracted to or love, the sex they were born with or their knowledge that nature gave them the wrong physical characteristics for their gender, what physical impairments they have, where they were born, where they were educated - or not, what language is their first language, or what opportunities they were presented with in their lives. Everyone deserves the opportunity to be who and what they are and to be respected for who they are, and everyone deserves the opportunity to excel. The strongest collaborations have diverse constituents with unified goals, and I want for CHEST to be among the strongest of professional collaborations. It has been deeply important to me during my presidency to champion these values, and we have worked hard to make CHEST an inclusive and diverse organization. Much remains to be done, but we did make some good progress this year.
We established a spirometry working group to look at the science around race-based adjustments for normal values, to call out if there are mistakes or omissions in that approach, and to propose the work that needs to be done to correct them. We invited the American Thoracic Society and the Canadian Thoracic Society to join us in this effort. Race is a social construct, not a physiologic principle, and some data suggest that apparent differences in physiology could actually reflect differences in socioeconomic status of study participants. In similar work, our nephrology colleagues demonstrated that apparent differences in normal glomerular filtration rate (GFR) are related to socio-economic and health care access issues; they called for labs to no longer report race-based norms for creatinine and GFR values. Our colleagues believe that race-based GFR norms have harmed patients by promoting delay in treatments aimed at preventing dialysis or by causing delays in the initiation of dialysis. In our world, asbestos companies have argued that African American and other populations of color should receive lower asbestosis settlements on the basis that they began with lower predicted lung function and, therefore, had been less damaged by exposure to asbestos. I am very interested to see our working group’s output. I think it could result in landmark changes in our evaluation and treatment of patients with lung diseases.
A very important undertaking for us this year was a top to bottom analysis of our own practices around diversity, equity, and inclusion. We started by taking lessons from the CHEST Foundation-sponsored listening tour across the nation. Many of our patients of color lack adequate access to the care they need, which informs our efforts in advocacy and health policy. We also learned that, as a profession, we have not earned the trust of our patients of color, and we must take steps to remedy that. CHEST began this effort by developing the First 5 Minutes program, which teaches all of us how to take the first moments of our interactions with patients to enhance our empathy and to establish trusting relationships with them. You will hear more about this program in the months to come.
CHEST is dedicated to ensuring that all of our members have equitable opportunities to take part in our learning activities, both as participants and as developers. Likewise, we want any member who desires to advance in our organization to have wide open opportunity to develop and use their skills. We hired a consulting firm who specializes in aiding nonprofits with their diversity, equity, and inclusion goals to help us find our weaknesses in that area. They spent several months interviewing members at all stages of their careers and in a variety of job types, with the goal of determining what it is like to be a CHEST member of color, a woman, a member of the LGBTQIA community, or a member of any group that has been made to feel “other.” We are currently working to turn their findings into concrete steps to make CHEST the most diverse and inclusive medical society possible. Finally, our consultants are helping us to ensure that the people we hire to work for our organization full time have equitable opportunities in their workplace, and that CHEST headquarters feels inclusive and is diverse for them.
COVID-19 rages on. In fact, daily case numbers at this writing are skyrocketing, higher than at any time during the pandemic, and hospitalization rates, while lower than with some of the previous waves, are following. Many of us are stressed, and in many of our ICUs, we have fewer nurses than we did at the outset of the pandemic. The CHEST COVID-19 task force continues on the job, though, with fresh content to match the current circumstances. These dedicated individuals, who I recognized with a Presidential Citation for 2021, have worked since the early days of the pandemic to scour the literature and the landscape to find the right data and the right experts to inform the topical infographics, reviews, webinars, and podcasts that are freely available to all and are posted on the CHEST website. I hope that you have availed yourself of the material there, and, if not, you have missed some valuable learning opportunities. Missed them in real time, that is; they are all on the site for you to use at will. We are optimistic that someday soon, there will be less of a need for the COVID-19 task force, but the members are all ready to continue their work until that time comes..
I’ve highlighted just a few of the higher profile things that CHEST achieved in 2021. It would be impossible for me to cover all that CHEST has accomplished this past year. My sources tell me that during my presidency, we generated, signed on, or declined to join nearly 100 advocacy statements on topics ranging from recall of home CPAP machines to access to appropriate supplemental oxygen for patients with interstitial lung disease, to the acquisition of a nebulizer company by a tobacco company. We held successful board review sessions and repeated our all online, yet interactive, version of the CHEST annual meeting, with more than 4,000 total attendees– not as large as an in-person meeting, but not terribly far off, either. I will add that our program chairs and their committee pivoted from a meeting in Vancouver to a meeting in Orlando to, with only 6 weeks’ notice, a meeting in the ether. We are fortunate to have worked with such talented and dedicated individuals, and all of us owe them a lot for their efforts.
If, as I say, I have been a wartime president, then the worldwide viral pandemic that directly affects those of us in chest medicine has been the war. In spite of the current tsunami of cases, I am optimistic that the war ends relatively soon. CHEST will not simply return to normalcy, though. Dr. David Schulman, a brilliant and innovative educator, has taken the leadership reins of the organization, and I foresee exhilarating times ahead.
We are making it through a challenging environment, and CHEST is stronger for it. I will look forward to seeing all of you in Nashville, when we, at long last, can look one another in the eye, shake one another’s hand, and enjoy the experience of the CHEST annual meeting together. And if you don’t mind me asking, when you see me in Nashville, will you please do exactly that?
It’s January 1, 2022, as I write, and my CHEST presidency came to an end last night as the fireworks lit up the sky. With COVID-19 waxing and waning across the United States and around the world, I have been a wartime president. CHEST has not been able to do a number of the things that we would normally have done in person, including that there has not been an in-person CHEST annual meeting during my entire presidency. We have, nonetheless, achieved some important things that I will share with you.
If you’re a typical CHEST member, you probably don’t spend a lot of time wondering about CHEST’s finances, nor should you. Nevertheless, CHEST – your organization – does have to be fiscally responsible if we desire to continue our educational and research missions, and that is the job of your Board of Regents, your presidents, and your professional staff at the CHEST headquarters. I’m happy to tell you that your organization is in healthy financial condition, in spite of a challenging economic environment and, being forced into remote, online annual meetings and board reviews for 2 years. What that means to us and to you is that we get to maintain and improve our full array of educational activities, including our annual meeting, our journal, our board reviews, our hands-on courses at the CHEST headquarters, and our web content. And, we get to accelerate our advocacy activities for our patients and for the clinical folks who care for them (us!). CHEST is primed for emerging from this pandemic stronger, because we have had to make the most of every dollar we have, and more innovative, because that’s how we have done it. We are ready for new ways of interacting and for innovative new ways of delivering education, sponsoring research, fostering networking, and leading in the clinical arena of chest medicine.
During my time as CHEST President, many of us have become progressively more aware of the blatant inequities that continue in society – and, yes, even in medicine. Perhaps more than anything, it both saddens and angers me when anyone values or devalues someone else’s life because of the color of their skin, who they feel attracted to or love, the sex they were born with or their knowledge that nature gave them the wrong physical characteristics for their gender, what physical impairments they have, where they were born, where they were educated - or not, what language is their first language, or what opportunities they were presented with in their lives. Everyone deserves the opportunity to be who and what they are and to be respected for who they are, and everyone deserves the opportunity to excel. The strongest collaborations have diverse constituents with unified goals, and I want for CHEST to be among the strongest of professional collaborations. It has been deeply important to me during my presidency to champion these values, and we have worked hard to make CHEST an inclusive and diverse organization. Much remains to be done, but we did make some good progress this year.
We established a spirometry working group to look at the science around race-based adjustments for normal values, to call out if there are mistakes or omissions in that approach, and to propose the work that needs to be done to correct them. We invited the American Thoracic Society and the Canadian Thoracic Society to join us in this effort. Race is a social construct, not a physiologic principle, and some data suggest that apparent differences in physiology could actually reflect differences in socioeconomic status of study participants. In similar work, our nephrology colleagues demonstrated that apparent differences in normal glomerular filtration rate (GFR) are related to socio-economic and health care access issues; they called for labs to no longer report race-based norms for creatinine and GFR values. Our colleagues believe that race-based GFR norms have harmed patients by promoting delay in treatments aimed at preventing dialysis or by causing delays in the initiation of dialysis. In our world, asbestos companies have argued that African American and other populations of color should receive lower asbestosis settlements on the basis that they began with lower predicted lung function and, therefore, had been less damaged by exposure to asbestos. I am very interested to see our working group’s output. I think it could result in landmark changes in our evaluation and treatment of patients with lung diseases.
A very important undertaking for us this year was a top to bottom analysis of our own practices around diversity, equity, and inclusion. We started by taking lessons from the CHEST Foundation-sponsored listening tour across the nation. Many of our patients of color lack adequate access to the care they need, which informs our efforts in advocacy and health policy. We also learned that, as a profession, we have not earned the trust of our patients of color, and we must take steps to remedy that. CHEST began this effort by developing the First 5 Minutes program, which teaches all of us how to take the first moments of our interactions with patients to enhance our empathy and to establish trusting relationships with them. You will hear more about this program in the months to come.
CHEST is dedicated to ensuring that all of our members have equitable opportunities to take part in our learning activities, both as participants and as developers. Likewise, we want any member who desires to advance in our organization to have wide open opportunity to develop and use their skills. We hired a consulting firm who specializes in aiding nonprofits with their diversity, equity, and inclusion goals to help us find our weaknesses in that area. They spent several months interviewing members at all stages of their careers and in a variety of job types, with the goal of determining what it is like to be a CHEST member of color, a woman, a member of the LGBTQIA community, or a member of any group that has been made to feel “other.” We are currently working to turn their findings into concrete steps to make CHEST the most diverse and inclusive medical society possible. Finally, our consultants are helping us to ensure that the people we hire to work for our organization full time have equitable opportunities in their workplace, and that CHEST headquarters feels inclusive and is diverse for them.
COVID-19 rages on. In fact, daily case numbers at this writing are skyrocketing, higher than at any time during the pandemic, and hospitalization rates, while lower than with some of the previous waves, are following. Many of us are stressed, and in many of our ICUs, we have fewer nurses than we did at the outset of the pandemic. The CHEST COVID-19 task force continues on the job, though, with fresh content to match the current circumstances. These dedicated individuals, who I recognized with a Presidential Citation for 2021, have worked since the early days of the pandemic to scour the literature and the landscape to find the right data and the right experts to inform the topical infographics, reviews, webinars, and podcasts that are freely available to all and are posted on the CHEST website. I hope that you have availed yourself of the material there, and, if not, you have missed some valuable learning opportunities. Missed them in real time, that is; they are all on the site for you to use at will. We are optimistic that someday soon, there will be less of a need for the COVID-19 task force, but the members are all ready to continue their work until that time comes..
I’ve highlighted just a few of the higher profile things that CHEST achieved in 2021. It would be impossible for me to cover all that CHEST has accomplished this past year. My sources tell me that during my presidency, we generated, signed on, or declined to join nearly 100 advocacy statements on topics ranging from recall of home CPAP machines to access to appropriate supplemental oxygen for patients with interstitial lung disease, to the acquisition of a nebulizer company by a tobacco company. We held successful board review sessions and repeated our all online, yet interactive, version of the CHEST annual meeting, with more than 4,000 total attendees– not as large as an in-person meeting, but not terribly far off, either. I will add that our program chairs and their committee pivoted from a meeting in Vancouver to a meeting in Orlando to, with only 6 weeks’ notice, a meeting in the ether. We are fortunate to have worked with such talented and dedicated individuals, and all of us owe them a lot for their efforts.
If, as I say, I have been a wartime president, then the worldwide viral pandemic that directly affects those of us in chest medicine has been the war. In spite of the current tsunami of cases, I am optimistic that the war ends relatively soon. CHEST will not simply return to normalcy, though. Dr. David Schulman, a brilliant and innovative educator, has taken the leadership reins of the organization, and I foresee exhilarating times ahead.
We are making it through a challenging environment, and CHEST is stronger for it. I will look forward to seeing all of you in Nashville, when we, at long last, can look one another in the eye, shake one another’s hand, and enjoy the experience of the CHEST annual meeting together. And if you don’t mind me asking, when you see me in Nashville, will you please do exactly that?
It’s January 1, 2022, as I write, and my CHEST presidency came to an end last night as the fireworks lit up the sky. With COVID-19 waxing and waning across the United States and around the world, I have been a wartime president. CHEST has not been able to do a number of the things that we would normally have done in person, including that there has not been an in-person CHEST annual meeting during my entire presidency. We have, nonetheless, achieved some important things that I will share with you.
If you’re a typical CHEST member, you probably don’t spend a lot of time wondering about CHEST’s finances, nor should you. Nevertheless, CHEST – your organization – does have to be fiscally responsible if we desire to continue our educational and research missions, and that is the job of your Board of Regents, your presidents, and your professional staff at the CHEST headquarters. I’m happy to tell you that your organization is in healthy financial condition, in spite of a challenging economic environment and, being forced into remote, online annual meetings and board reviews for 2 years. What that means to us and to you is that we get to maintain and improve our full array of educational activities, including our annual meeting, our journal, our board reviews, our hands-on courses at the CHEST headquarters, and our web content. And, we get to accelerate our advocacy activities for our patients and for the clinical folks who care for them (us!). CHEST is primed for emerging from this pandemic stronger, because we have had to make the most of every dollar we have, and more innovative, because that’s how we have done it. We are ready for new ways of interacting and for innovative new ways of delivering education, sponsoring research, fostering networking, and leading in the clinical arena of chest medicine.
During my time as CHEST President, many of us have become progressively more aware of the blatant inequities that continue in society – and, yes, even in medicine. Perhaps more than anything, it both saddens and angers me when anyone values or devalues someone else’s life because of the color of their skin, who they feel attracted to or love, the sex they were born with or their knowledge that nature gave them the wrong physical characteristics for their gender, what physical impairments they have, where they were born, where they were educated - or not, what language is their first language, or what opportunities they were presented with in their lives. Everyone deserves the opportunity to be who and what they are and to be respected for who they are, and everyone deserves the opportunity to excel. The strongest collaborations have diverse constituents with unified goals, and I want for CHEST to be among the strongest of professional collaborations. It has been deeply important to me during my presidency to champion these values, and we have worked hard to make CHEST an inclusive and diverse organization. Much remains to be done, but we did make some good progress this year.
We established a spirometry working group to look at the science around race-based adjustments for normal values, to call out if there are mistakes or omissions in that approach, and to propose the work that needs to be done to correct them. We invited the American Thoracic Society and the Canadian Thoracic Society to join us in this effort. Race is a social construct, not a physiologic principle, and some data suggest that apparent differences in physiology could actually reflect differences in socioeconomic status of study participants. In similar work, our nephrology colleagues demonstrated that apparent differences in normal glomerular filtration rate (GFR) are related to socio-economic and health care access issues; they called for labs to no longer report race-based norms for creatinine and GFR values. Our colleagues believe that race-based GFR norms have harmed patients by promoting delay in treatments aimed at preventing dialysis or by causing delays in the initiation of dialysis. In our world, asbestos companies have argued that African American and other populations of color should receive lower asbestosis settlements on the basis that they began with lower predicted lung function and, therefore, had been less damaged by exposure to asbestos. I am very interested to see our working group’s output. I think it could result in landmark changes in our evaluation and treatment of patients with lung diseases.
A very important undertaking for us this year was a top to bottom analysis of our own practices around diversity, equity, and inclusion. We started by taking lessons from the CHEST Foundation-sponsored listening tour across the nation. Many of our patients of color lack adequate access to the care they need, which informs our efforts in advocacy and health policy. We also learned that, as a profession, we have not earned the trust of our patients of color, and we must take steps to remedy that. CHEST began this effort by developing the First 5 Minutes program, which teaches all of us how to take the first moments of our interactions with patients to enhance our empathy and to establish trusting relationships with them. You will hear more about this program in the months to come.
CHEST is dedicated to ensuring that all of our members have equitable opportunities to take part in our learning activities, both as participants and as developers. Likewise, we want any member who desires to advance in our organization to have wide open opportunity to develop and use their skills. We hired a consulting firm who specializes in aiding nonprofits with their diversity, equity, and inclusion goals to help us find our weaknesses in that area. They spent several months interviewing members at all stages of their careers and in a variety of job types, with the goal of determining what it is like to be a CHEST member of color, a woman, a member of the LGBTQIA community, or a member of any group that has been made to feel “other.” We are currently working to turn their findings into concrete steps to make CHEST the most diverse and inclusive medical society possible. Finally, our consultants are helping us to ensure that the people we hire to work for our organization full time have equitable opportunities in their workplace, and that CHEST headquarters feels inclusive and is diverse for them.
COVID-19 rages on. In fact, daily case numbers at this writing are skyrocketing, higher than at any time during the pandemic, and hospitalization rates, while lower than with some of the previous waves, are following. Many of us are stressed, and in many of our ICUs, we have fewer nurses than we did at the outset of the pandemic. The CHEST COVID-19 task force continues on the job, though, with fresh content to match the current circumstances. These dedicated individuals, who I recognized with a Presidential Citation for 2021, have worked since the early days of the pandemic to scour the literature and the landscape to find the right data and the right experts to inform the topical infographics, reviews, webinars, and podcasts that are freely available to all and are posted on the CHEST website. I hope that you have availed yourself of the material there, and, if not, you have missed some valuable learning opportunities. Missed them in real time, that is; they are all on the site for you to use at will. We are optimistic that someday soon, there will be less of a need for the COVID-19 task force, but the members are all ready to continue their work until that time comes..
I’ve highlighted just a few of the higher profile things that CHEST achieved in 2021. It would be impossible for me to cover all that CHEST has accomplished this past year. My sources tell me that during my presidency, we generated, signed on, or declined to join nearly 100 advocacy statements on topics ranging from recall of home CPAP machines to access to appropriate supplemental oxygen for patients with interstitial lung disease, to the acquisition of a nebulizer company by a tobacco company. We held successful board review sessions and repeated our all online, yet interactive, version of the CHEST annual meeting, with more than 4,000 total attendees– not as large as an in-person meeting, but not terribly far off, either. I will add that our program chairs and their committee pivoted from a meeting in Vancouver to a meeting in Orlando to, with only 6 weeks’ notice, a meeting in the ether. We are fortunate to have worked with such talented and dedicated individuals, and all of us owe them a lot for their efforts.
If, as I say, I have been a wartime president, then the worldwide viral pandemic that directly affects those of us in chest medicine has been the war. In spite of the current tsunami of cases, I am optimistic that the war ends relatively soon. CHEST will not simply return to normalcy, though. Dr. David Schulman, a brilliant and innovative educator, has taken the leadership reins of the organization, and I foresee exhilarating times ahead.
We are making it through a challenging environment, and CHEST is stronger for it. I will look forward to seeing all of you in Nashville, when we, at long last, can look one another in the eye, shake one another’s hand, and enjoy the experience of the CHEST annual meeting together. And if you don’t mind me asking, when you see me in Nashville, will you please do exactly that?
Ivermectin does not stop progression to severe COVID: randomized trial
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICNE
Early in career, female academic docs earn less than males: study
Worse still, the earning potential of women in most specialties is $214,440 (or 10%) less than their male colleagues over the course of the first 10 years of their careers in academic medicine.
Among the vast majority of subspecialties, women’s starting salaries and their salaries 10 years into their careers were lower than their male colleagues in academic medicine, per the study in JAMA Network Open.
Eva Catenaccio, MD, an epilepsy fellow at Children’s Hospital of Philadelphia and the lead author of the study, told this news organization that the gender disparities in earning potential are “pervasive in academic medicine.” These earnings disparities, which occur in nearly all subspecialties and can reach hundreds of thousands of dollars in the first 10 years of an academic physician’s career, “are largely the result of gender differences in annual salary that start immediately after training,” she said.
Changing the timing of academic promotion and equalizing starting salary and salary growth can help close the salary gap, said Dr. Catenaccio.
The study also reveals that women could face a 1-year delay in promotion from assistant to associate professor, compared with men. This delay could reduce female physicians’ earning potential by a 10-year median of $26,042 (or 2%), whereas failure to be promoted at all could decrease the 10-year earning potential by a median of $218,724 (or 13%).
Across medicine more broadly, male physicians continue to earn 35% more than their female colleagues, according to the 2021 Medscape Physician Compensation Report. The biggest differences in take-home pay exist between male and female specialists, per the report. On average, male physicians earn $376,000, while women’s take-home pay is $283,000.
Medical schools and hospital leaders have a role to play
The earning potential during the first 10 years of post-training employment by gender was the most dramatic in neurosurgery, orthopedic surgery, and cardiology, per the study. Three subspecialties where women and men have similar earning potential include pediatric nephrology, pediatric neurology, and pediatric rheumatology.
The coauthors note that it’s commonly understood that women don’t negotiate as often or as successfully as their male colleagues. A 2019 study in JAMA Surgery of 606 male and female surgery residents revealed that while residents of both genders shared similar career goals, women had lower future salary expectations and a significantly more negative view of the salary negotiation process.
Dr. Catenaccio and her coauthors acknowledge that negotiation skills and financial literacy should be taught during medical school and postgraduate training. “However, the onus for ensuring salary equity should not fall on the individual candidate alone; rather, departmental and hospital leadership should take responsibility to ensure uniform starting salaries and prevent gender-based inequalities,” they wrote in the study.
“We hope that this study encourages academic medical institutions to increase transparency and equity around compensation, particularly for junior faculty,” Dr. Catenaccio said in an interview. “This will require both ensuring equal starting salaries and providing periodic adjustments throughout individuals’ careers to prevent divergence in earning potential by gender or any other individual characteristics.”
Harold Simon, MD, MBA, vice chair for faculty for the department of pediatrics and professor of pediatrics and emergency medicine at Emory University, Atlanta, told this news organization that “[i]ncreased transparency around compensation can enable women to advocate for equitable pay. However, the burden for ensuring equity should not fall on individuals but instead must be the primary responsibility of academic institutions.”
Specifically, Dr. Simon advocates for hospital leaders to “ensure equity among providers including compensation [as] a crucial part of maintaining a diverse workforce and, ultimately, providing balanced access to health care for patients.”
In addition, the authors call for periodic compensation evaluations and adjustments to help prevent gender-based salary differences among female and male physicians in academia. “This is absolutely necessary, both to develop future compensation plans and to address any pre-existing gender-based salary inequities for those women currently well into their careers,” they wrote in the study.
Data analysis was conducted from March to May 2021. Researchers used models to estimate the impacts of promotion timing and potential interventions, which include equalizing starting salaries and annual salary rates.
The study included compensation data for 24,593 female and 29,886 male academic physicians across 45 subspecialties. It relied on publicly available data from the Association of American Medical Colleges’ annual Medical School Faculty Salary Survey report.
A version of this article first appeared on Medscape.com.
Worse still, the earning potential of women in most specialties is $214,440 (or 10%) less than their male colleagues over the course of the first 10 years of their careers in academic medicine.
Among the vast majority of subspecialties, women’s starting salaries and their salaries 10 years into their careers were lower than their male colleagues in academic medicine, per the study in JAMA Network Open.
Eva Catenaccio, MD, an epilepsy fellow at Children’s Hospital of Philadelphia and the lead author of the study, told this news organization that the gender disparities in earning potential are “pervasive in academic medicine.” These earnings disparities, which occur in nearly all subspecialties and can reach hundreds of thousands of dollars in the first 10 years of an academic physician’s career, “are largely the result of gender differences in annual salary that start immediately after training,” she said.
Changing the timing of academic promotion and equalizing starting salary and salary growth can help close the salary gap, said Dr. Catenaccio.
The study also reveals that women could face a 1-year delay in promotion from assistant to associate professor, compared with men. This delay could reduce female physicians’ earning potential by a 10-year median of $26,042 (or 2%), whereas failure to be promoted at all could decrease the 10-year earning potential by a median of $218,724 (or 13%).
Across medicine more broadly, male physicians continue to earn 35% more than their female colleagues, according to the 2021 Medscape Physician Compensation Report. The biggest differences in take-home pay exist between male and female specialists, per the report. On average, male physicians earn $376,000, while women’s take-home pay is $283,000.
Medical schools and hospital leaders have a role to play
The earning potential during the first 10 years of post-training employment by gender was the most dramatic in neurosurgery, orthopedic surgery, and cardiology, per the study. Three subspecialties where women and men have similar earning potential include pediatric nephrology, pediatric neurology, and pediatric rheumatology.
The coauthors note that it’s commonly understood that women don’t negotiate as often or as successfully as their male colleagues. A 2019 study in JAMA Surgery of 606 male and female surgery residents revealed that while residents of both genders shared similar career goals, women had lower future salary expectations and a significantly more negative view of the salary negotiation process.
Dr. Catenaccio and her coauthors acknowledge that negotiation skills and financial literacy should be taught during medical school and postgraduate training. “However, the onus for ensuring salary equity should not fall on the individual candidate alone; rather, departmental and hospital leadership should take responsibility to ensure uniform starting salaries and prevent gender-based inequalities,” they wrote in the study.
“We hope that this study encourages academic medical institutions to increase transparency and equity around compensation, particularly for junior faculty,” Dr. Catenaccio said in an interview. “This will require both ensuring equal starting salaries and providing periodic adjustments throughout individuals’ careers to prevent divergence in earning potential by gender or any other individual characteristics.”
Harold Simon, MD, MBA, vice chair for faculty for the department of pediatrics and professor of pediatrics and emergency medicine at Emory University, Atlanta, told this news organization that “[i]ncreased transparency around compensation can enable women to advocate for equitable pay. However, the burden for ensuring equity should not fall on individuals but instead must be the primary responsibility of academic institutions.”
Specifically, Dr. Simon advocates for hospital leaders to “ensure equity among providers including compensation [as] a crucial part of maintaining a diverse workforce and, ultimately, providing balanced access to health care for patients.”
In addition, the authors call for periodic compensation evaluations and adjustments to help prevent gender-based salary differences among female and male physicians in academia. “This is absolutely necessary, both to develop future compensation plans and to address any pre-existing gender-based salary inequities for those women currently well into their careers,” they wrote in the study.
Data analysis was conducted from March to May 2021. Researchers used models to estimate the impacts of promotion timing and potential interventions, which include equalizing starting salaries and annual salary rates.
The study included compensation data for 24,593 female and 29,886 male academic physicians across 45 subspecialties. It relied on publicly available data from the Association of American Medical Colleges’ annual Medical School Faculty Salary Survey report.
A version of this article first appeared on Medscape.com.
Worse still, the earning potential of women in most specialties is $214,440 (or 10%) less than their male colleagues over the course of the first 10 years of their careers in academic medicine.
Among the vast majority of subspecialties, women’s starting salaries and their salaries 10 years into their careers were lower than their male colleagues in academic medicine, per the study in JAMA Network Open.
Eva Catenaccio, MD, an epilepsy fellow at Children’s Hospital of Philadelphia and the lead author of the study, told this news organization that the gender disparities in earning potential are “pervasive in academic medicine.” These earnings disparities, which occur in nearly all subspecialties and can reach hundreds of thousands of dollars in the first 10 years of an academic physician’s career, “are largely the result of gender differences in annual salary that start immediately after training,” she said.
Changing the timing of academic promotion and equalizing starting salary and salary growth can help close the salary gap, said Dr. Catenaccio.
The study also reveals that women could face a 1-year delay in promotion from assistant to associate professor, compared with men. This delay could reduce female physicians’ earning potential by a 10-year median of $26,042 (or 2%), whereas failure to be promoted at all could decrease the 10-year earning potential by a median of $218,724 (or 13%).
Across medicine more broadly, male physicians continue to earn 35% more than their female colleagues, according to the 2021 Medscape Physician Compensation Report. The biggest differences in take-home pay exist between male and female specialists, per the report. On average, male physicians earn $376,000, while women’s take-home pay is $283,000.
Medical schools and hospital leaders have a role to play
The earning potential during the first 10 years of post-training employment by gender was the most dramatic in neurosurgery, orthopedic surgery, and cardiology, per the study. Three subspecialties where women and men have similar earning potential include pediatric nephrology, pediatric neurology, and pediatric rheumatology.
The coauthors note that it’s commonly understood that women don’t negotiate as often or as successfully as their male colleagues. A 2019 study in JAMA Surgery of 606 male and female surgery residents revealed that while residents of both genders shared similar career goals, women had lower future salary expectations and a significantly more negative view of the salary negotiation process.
Dr. Catenaccio and her coauthors acknowledge that negotiation skills and financial literacy should be taught during medical school and postgraduate training. “However, the onus for ensuring salary equity should not fall on the individual candidate alone; rather, departmental and hospital leadership should take responsibility to ensure uniform starting salaries and prevent gender-based inequalities,” they wrote in the study.
“We hope that this study encourages academic medical institutions to increase transparency and equity around compensation, particularly for junior faculty,” Dr. Catenaccio said in an interview. “This will require both ensuring equal starting salaries and providing periodic adjustments throughout individuals’ careers to prevent divergence in earning potential by gender or any other individual characteristics.”
Harold Simon, MD, MBA, vice chair for faculty for the department of pediatrics and professor of pediatrics and emergency medicine at Emory University, Atlanta, told this news organization that “[i]ncreased transparency around compensation can enable women to advocate for equitable pay. However, the burden for ensuring equity should not fall on individuals but instead must be the primary responsibility of academic institutions.”
Specifically, Dr. Simon advocates for hospital leaders to “ensure equity among providers including compensation [as] a crucial part of maintaining a diverse workforce and, ultimately, providing balanced access to health care for patients.”
In addition, the authors call for periodic compensation evaluations and adjustments to help prevent gender-based salary differences among female and male physicians in academia. “This is absolutely necessary, both to develop future compensation plans and to address any pre-existing gender-based salary inequities for those women currently well into their careers,” they wrote in the study.
Data analysis was conducted from March to May 2021. Researchers used models to estimate the impacts of promotion timing and potential interventions, which include equalizing starting salaries and annual salary rates.
The study included compensation data for 24,593 female and 29,886 male academic physicians across 45 subspecialties. It relied on publicly available data from the Association of American Medical Colleges’ annual Medical School Faculty Salary Survey report.
A version of this article first appeared on Medscape.com.
Childhood-onset insomnia persists into adolescence and adulthood
Childhood-onset insomnia is a chronic problem in 43% of children, based on 15-year follow-up data from approximately 500 individuals.
Difficulty initiating or maintaining sleep (DIMS) is the most frequently reported insomnia symptom in children and teens, but longitudinal data on the trajectory of insomnia symptoms from childhood into adulthood are limited, Julio Fernandez-Mendoza, PhD, of Penn State University, Hershey, and colleagues wrote.
Previous studies have shown varying results, notably on the effect of objective short sleep duration (OSSD), they said. The extent to which the effect of OSSD on insomnia trajectories, and whether OSSD affects the development of insomnia in the transition to adulthood remains uncertain.
In a study published in Pediatrics, the researchers reviewed data from 502 children who enrolled at age 5-12 years between 2000 and 2005. The participants underwent laboratory polysomnography visits at baseline; 421 had a second laboratory visit between 2010 and 2013 (median age, 16 years), and 502 completed a structured self-reported survey between 2018 and 2021 at a median age of 24 years. At the first visit, 118 children met criteria for insomnia, defined as parent reports of often/moderate or very often/severe DIMS and/or use of over-the-counter or prescription sleep medications for DIMS. At the second visit, 120 children met the definition for insomnia.
Among children with insomnia symptoms at baseline, 53.7% had persistence of insomnia symptoms in adolescence and 61.9% had symptoms in young adulthood; 46.3% and 38.1% remitted at these times.
Among children with insomnia symptoms at adolescence, 57.5% and 42.5% had persistence and remittance, respectively, in young adulthood.
In children with insomnia at baseline, therefore, the most frequent developmental trajectory was persistence (43.3%) followed by remission (26.9% since childhood and 11.2% since adolescence) and a waxing and waning pattern (18.6%), the researchers said.
Among children with normal sleep at baseline, 69.7% retained normal sleep patterns in adolescence and 63.3% retained normal sleep in young adulthood; 30.3% and 36.7% developed insomnia in adolescence and young adulthood, respectively.
Overall, adult insomnia was reported by 22.0% and 20.8% of individuals with childhood and adolescent insomnia, respectively. In a multivariate analysis, the odds of adult insomnia were 2.6 times and 5.5 times higher among those with histories of short-sleeping in childhood and adolescence, respectively.
“The most common developmental trajectory for insomnia symptoms was that of persistence from childhood through young adulthood,” the researchers wrote in their discussion of the study.
“These 15-year longitudinal findings across three developmental stages indicate that insomnia symptoms should not be expected to developmentally remit in at least 40% of children and that adolescence is a critical developmental period for the adverse prognosis of the insomnia with short sleep duration phenotype,” they emphasized.
The study findings were limited by several factors including the collection of OSSD and other sleep data via a 1-night, 9-hour polysomnography, which might not be representative of habitual sleep at home, the researchers noted. Other limitations include the lack of polysomnography data to accompany the young adult survey and the inability to validate insomnia in young adults via strict diagnostic criteria.
However, the results reveal that the persistence of childhood insomnia is higher than suggested in previous studies, and that these children and adolescents, especially short sleepers, are at significantly increased risk of adult insomnia, the researchers concluded.
“Early sleep interventions are a priority, as clinicians should not expect insomnia symptoms to developmentally remit in a high proportion of children, although objective sleep measures may be indicated in adolescence to identify those with poorer long-term prognosis,” they said.
Pandemic prompts interest in sleep issues
The current study is important at this time because sleep disruptions in children and adolescents have increased over the course of the COVID-19 pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was especially surprised to see that adolescent insomnia will most likely not remit in young adulthood, as she had considered it a disorder of adolescence.
The study highlights the need for early intervention to manage insomnia in children. However, there are several barriers to such intervention. “Parents [of young children] are overwhelmed and just need sleep themselves, so they don’t always have the energy to work on good sleep habits in their children,” she said. Improving sleep habits in adolescents requires overcoming the barrier of the young patients’ attitudes. “For adolescents, they need to buy into the change.”
However, the take-home message for clinicians is that it is important to work to overcome these barriers and improve sleep in children and teens, because the longitudinal data suggest that the problem is “likely to persist and unlikely to remit,” for many, she said.
As for additional studies, “I would like to see more research done on neurologic and psychological causes of insomnia,” Dr. Kinsella said.
The study was supported in part by grants from the National Heart, Lung, and Blood Institute; National Institute of Mental Health; and the National Center for Advancing Translational Sciences of the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Childhood-onset insomnia is a chronic problem in 43% of children, based on 15-year follow-up data from approximately 500 individuals.
Difficulty initiating or maintaining sleep (DIMS) is the most frequently reported insomnia symptom in children and teens, but longitudinal data on the trajectory of insomnia symptoms from childhood into adulthood are limited, Julio Fernandez-Mendoza, PhD, of Penn State University, Hershey, and colleagues wrote.
Previous studies have shown varying results, notably on the effect of objective short sleep duration (OSSD), they said. The extent to which the effect of OSSD on insomnia trajectories, and whether OSSD affects the development of insomnia in the transition to adulthood remains uncertain.
In a study published in Pediatrics, the researchers reviewed data from 502 children who enrolled at age 5-12 years between 2000 and 2005. The participants underwent laboratory polysomnography visits at baseline; 421 had a second laboratory visit between 2010 and 2013 (median age, 16 years), and 502 completed a structured self-reported survey between 2018 and 2021 at a median age of 24 years. At the first visit, 118 children met criteria for insomnia, defined as parent reports of often/moderate or very often/severe DIMS and/or use of over-the-counter or prescription sleep medications for DIMS. At the second visit, 120 children met the definition for insomnia.
Among children with insomnia symptoms at baseline, 53.7% had persistence of insomnia symptoms in adolescence and 61.9% had symptoms in young adulthood; 46.3% and 38.1% remitted at these times.
Among children with insomnia symptoms at adolescence, 57.5% and 42.5% had persistence and remittance, respectively, in young adulthood.
In children with insomnia at baseline, therefore, the most frequent developmental trajectory was persistence (43.3%) followed by remission (26.9% since childhood and 11.2% since adolescence) and a waxing and waning pattern (18.6%), the researchers said.
Among children with normal sleep at baseline, 69.7% retained normal sleep patterns in adolescence and 63.3% retained normal sleep in young adulthood; 30.3% and 36.7% developed insomnia in adolescence and young adulthood, respectively.
Overall, adult insomnia was reported by 22.0% and 20.8% of individuals with childhood and adolescent insomnia, respectively. In a multivariate analysis, the odds of adult insomnia were 2.6 times and 5.5 times higher among those with histories of short-sleeping in childhood and adolescence, respectively.
“The most common developmental trajectory for insomnia symptoms was that of persistence from childhood through young adulthood,” the researchers wrote in their discussion of the study.
“These 15-year longitudinal findings across three developmental stages indicate that insomnia symptoms should not be expected to developmentally remit in at least 40% of children and that adolescence is a critical developmental period for the adverse prognosis of the insomnia with short sleep duration phenotype,” they emphasized.
The study findings were limited by several factors including the collection of OSSD and other sleep data via a 1-night, 9-hour polysomnography, which might not be representative of habitual sleep at home, the researchers noted. Other limitations include the lack of polysomnography data to accompany the young adult survey and the inability to validate insomnia in young adults via strict diagnostic criteria.
However, the results reveal that the persistence of childhood insomnia is higher than suggested in previous studies, and that these children and adolescents, especially short sleepers, are at significantly increased risk of adult insomnia, the researchers concluded.
“Early sleep interventions are a priority, as clinicians should not expect insomnia symptoms to developmentally remit in a high proportion of children, although objective sleep measures may be indicated in adolescence to identify those with poorer long-term prognosis,” they said.
Pandemic prompts interest in sleep issues
The current study is important at this time because sleep disruptions in children and adolescents have increased over the course of the COVID-19 pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was especially surprised to see that adolescent insomnia will most likely not remit in young adulthood, as she had considered it a disorder of adolescence.
The study highlights the need for early intervention to manage insomnia in children. However, there are several barriers to such intervention. “Parents [of young children] are overwhelmed and just need sleep themselves, so they don’t always have the energy to work on good sleep habits in their children,” she said. Improving sleep habits in adolescents requires overcoming the barrier of the young patients’ attitudes. “For adolescents, they need to buy into the change.”
However, the take-home message for clinicians is that it is important to work to overcome these barriers and improve sleep in children and teens, because the longitudinal data suggest that the problem is “likely to persist and unlikely to remit,” for many, she said.
As for additional studies, “I would like to see more research done on neurologic and psychological causes of insomnia,” Dr. Kinsella said.
The study was supported in part by grants from the National Heart, Lung, and Blood Institute; National Institute of Mental Health; and the National Center for Advancing Translational Sciences of the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Childhood-onset insomnia is a chronic problem in 43% of children, based on 15-year follow-up data from approximately 500 individuals.
Difficulty initiating or maintaining sleep (DIMS) is the most frequently reported insomnia symptom in children and teens, but longitudinal data on the trajectory of insomnia symptoms from childhood into adulthood are limited, Julio Fernandez-Mendoza, PhD, of Penn State University, Hershey, and colleagues wrote.
Previous studies have shown varying results, notably on the effect of objective short sleep duration (OSSD), they said. The extent to which the effect of OSSD on insomnia trajectories, and whether OSSD affects the development of insomnia in the transition to adulthood remains uncertain.
In a study published in Pediatrics, the researchers reviewed data from 502 children who enrolled at age 5-12 years between 2000 and 2005. The participants underwent laboratory polysomnography visits at baseline; 421 had a second laboratory visit between 2010 and 2013 (median age, 16 years), and 502 completed a structured self-reported survey between 2018 and 2021 at a median age of 24 years. At the first visit, 118 children met criteria for insomnia, defined as parent reports of often/moderate or very often/severe DIMS and/or use of over-the-counter or prescription sleep medications for DIMS. At the second visit, 120 children met the definition for insomnia.
Among children with insomnia symptoms at baseline, 53.7% had persistence of insomnia symptoms in adolescence and 61.9% had symptoms in young adulthood; 46.3% and 38.1% remitted at these times.
Among children with insomnia symptoms at adolescence, 57.5% and 42.5% had persistence and remittance, respectively, in young adulthood.
In children with insomnia at baseline, therefore, the most frequent developmental trajectory was persistence (43.3%) followed by remission (26.9% since childhood and 11.2% since adolescence) and a waxing and waning pattern (18.6%), the researchers said.
Among children with normal sleep at baseline, 69.7% retained normal sleep patterns in adolescence and 63.3% retained normal sleep in young adulthood; 30.3% and 36.7% developed insomnia in adolescence and young adulthood, respectively.
Overall, adult insomnia was reported by 22.0% and 20.8% of individuals with childhood and adolescent insomnia, respectively. In a multivariate analysis, the odds of adult insomnia were 2.6 times and 5.5 times higher among those with histories of short-sleeping in childhood and adolescence, respectively.
“The most common developmental trajectory for insomnia symptoms was that of persistence from childhood through young adulthood,” the researchers wrote in their discussion of the study.
“These 15-year longitudinal findings across three developmental stages indicate that insomnia symptoms should not be expected to developmentally remit in at least 40% of children and that adolescence is a critical developmental period for the adverse prognosis of the insomnia with short sleep duration phenotype,” they emphasized.
The study findings were limited by several factors including the collection of OSSD and other sleep data via a 1-night, 9-hour polysomnography, which might not be representative of habitual sleep at home, the researchers noted. Other limitations include the lack of polysomnography data to accompany the young adult survey and the inability to validate insomnia in young adults via strict diagnostic criteria.
However, the results reveal that the persistence of childhood insomnia is higher than suggested in previous studies, and that these children and adolescents, especially short sleepers, are at significantly increased risk of adult insomnia, the researchers concluded.
“Early sleep interventions are a priority, as clinicians should not expect insomnia symptoms to developmentally remit in a high proportion of children, although objective sleep measures may be indicated in adolescence to identify those with poorer long-term prognosis,” they said.
Pandemic prompts interest in sleep issues
The current study is important at this time because sleep disruptions in children and adolescents have increased over the course of the COVID-19 pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was especially surprised to see that adolescent insomnia will most likely not remit in young adulthood, as she had considered it a disorder of adolescence.
The study highlights the need for early intervention to manage insomnia in children. However, there are several barriers to such intervention. “Parents [of young children] are overwhelmed and just need sleep themselves, so they don’t always have the energy to work on good sleep habits in their children,” she said. Improving sleep habits in adolescents requires overcoming the barrier of the young patients’ attitudes. “For adolescents, they need to buy into the change.”
However, the take-home message for clinicians is that it is important to work to overcome these barriers and improve sleep in children and teens, because the longitudinal data suggest that the problem is “likely to persist and unlikely to remit,” for many, she said.
As for additional studies, “I would like to see more research done on neurologic and psychological causes of insomnia,” Dr. Kinsella said.
The study was supported in part by grants from the National Heart, Lung, and Blood Institute; National Institute of Mental Health; and the National Center for Advancing Translational Sciences of the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM PEDIATRICS
Long COVID is real and consists of these conditions – or does it?
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM CROI 2022
Early flu treatment of hospital CAP patients improves outcomes
Early initiation of the antiviral oseltamivir (Tamiflu) reduces the risk for death in patients hospitalized with community-acquired pneumonia (CAP) but patients have to be tested for influenza first and that is not happening often enough, a large observational cohort of adult patients indicates.
“Early testing allows for early treatment, and we found that early treatment was associated with reduced mortality so testing patients during the flu season is crucial,” senior author Michael Rothberg, MD, MPH, of the Cleveland Clinic said in an interview.
“Even during the flu season, most patients with CAP in our study went untested for influenza [even though] those who received early oseltamivir exhibited lower 14-day in-hospital case fatality ... suggesting more widespread testing might improve patient outcomes,” the authors added.
The study was published online Feb. 5, 2022, in the journal CHEST.
Premier database
Data from the Premier Database – a hospital discharge database with information from over 600 hospitals in the United States – were analyzed between July 2010 and June 2015. Microbiological laboratory data was provided by 179 hospitals. “For each year, we evaluated the total percentage of patients tested for influenza A/B within 3 days of hospitalization,” lead author Abhishek Deshpande, MD, PhD, Cleveland Clinic, and colleagues explained.
A total of 166,268 patients with CAP were included in the study, among which only about one-quarter were tested for influenza. Some 11.5% tested positive for the flu, the authors noted. Testing did increase from 15.4% in 2010 to 35.6% in 2015 and it was higher at close to 29% during the influenza season, compared with only about 8% during the summer months.
Patients who were tested for influenza were younger at age 66.6 years, compared with untested patients, who were 70 years of age (P < .001). Tested patients were also less likely to have been admitted from a nursing facility (P < .001), were less likely to have been hospitalized in the preceding 6 months (P < .001) and have fewer comorbidities than those who were not tested (P < .001).
“Both groups had similar illness severities on admission,” the authors observed, “but patients who were tested were less likely to die in the hospital within 14 days,” the authors reported – at 6.7% versus 10.9% for untested patients (P < .001).
More than 80% of patients who tested positive for influenza received an antibacterial on day 1 of their admission, compared with virtually all those who were either not tested or who tested negative, the investigators added (P < .001). The mean duration of antibacterial therapy among patients with a bacterial coinfection was not influenced by influenza test results.
However, among those who tested positive for influenza, almost 60% received oseltamivir on day 1 whereas roughly 30% received treatment on day 2 or later. In fact, almost all patients who received early oseltamivir were tested for influenza on day 1, the investigators pointed out. Patients who received early oseltamivir had a 25% lower risk of death within the first 14 days in hospital at an adjusted odds ratio of 0.75 (95% confidence interval, 0.59-0.96).
Early initiation of the antiviral also reduced the risk of requiring subsequent ICU care by 36% at an aOR of 0.64; invasive mechanical ventilation by 46% at an aOR of 0.54, and the need for vasopressor therapy by 47% at an aOR of 0.53. All results were within the 95% confidence levels.
Early use of antiviral therapy also reduced both the length of hospital stay and the cost of that stay by 12%.
ATS-IDSA guidelines
As Dr. Deshpande noted, the American Thoracic Society and the Infectious Diseases Society of America guidelines recommend testing and empiric treatment of influenza in patients hospitalized with CAP. “Testing more inpatients especially during the flu season can reduce other diagnostic testing and improve antimicrobial stewardship,” Deshpande noted.
Thus, while the rate of testing for influenza did increase over the 5-year study interval, “there is substantial room for improvement,” he added, as a positive test clearly does trigger the need for intervention. As Dr. Deshpande also noted, the past two influenza seasons have been mild, but influenza activity has again picked up lately again in many parts of the United States.
With the COVID-19 pandemic overwhelming influenza over the past few years, “differentiating between the two based on symptoms alone can be challenging,” he acknowledged, “and clinicians will need to test and treat accordingly.” This is particularly important given that this study clearly indicates that early treatment with an antiviral can lower the risk of short-term mortality in hospitalized CAP patients.
One limitation of the study was the lack of data on time of symptom onset, which may be an important confounder of the effect of oseltamivir on outcomes, the authors point out. Asked to comment on the findings, Barbara Jones, MD, University of Utah Health, Salt Lake City, noted that timely antivirals for patients with influenza are highly effective at mitigating severe disease and are thus strongly recommended by practice guidelines.
“However, it is hard for clinicians to keep influenza on the radar and change testing and treatment approaches according to the season and prevalence [of influenza infections],” she said in an interview. “This is an important study that highlights this challenge.
“We need a better understanding of the solutions that have been effective at improving influenza recognition and treatment, possibly by studying facilities that perform well at this process,” she said.
Dr. Deshpande reported receiving research funding to his institution from the Clorox Company and consultant fees from Merck.
A version of this article first appeared on Medscape.com.
Early initiation of the antiviral oseltamivir (Tamiflu) reduces the risk for death in patients hospitalized with community-acquired pneumonia (CAP) but patients have to be tested for influenza first and that is not happening often enough, a large observational cohort of adult patients indicates.
“Early testing allows for early treatment, and we found that early treatment was associated with reduced mortality so testing patients during the flu season is crucial,” senior author Michael Rothberg, MD, MPH, of the Cleveland Clinic said in an interview.
“Even during the flu season, most patients with CAP in our study went untested for influenza [even though] those who received early oseltamivir exhibited lower 14-day in-hospital case fatality ... suggesting more widespread testing might improve patient outcomes,” the authors added.
The study was published online Feb. 5, 2022, in the journal CHEST.
Premier database
Data from the Premier Database – a hospital discharge database with information from over 600 hospitals in the United States – were analyzed between July 2010 and June 2015. Microbiological laboratory data was provided by 179 hospitals. “For each year, we evaluated the total percentage of patients tested for influenza A/B within 3 days of hospitalization,” lead author Abhishek Deshpande, MD, PhD, Cleveland Clinic, and colleagues explained.
A total of 166,268 patients with CAP were included in the study, among which only about one-quarter were tested for influenza. Some 11.5% tested positive for the flu, the authors noted. Testing did increase from 15.4% in 2010 to 35.6% in 2015 and it was higher at close to 29% during the influenza season, compared with only about 8% during the summer months.
Patients who were tested for influenza were younger at age 66.6 years, compared with untested patients, who were 70 years of age (P < .001). Tested patients were also less likely to have been admitted from a nursing facility (P < .001), were less likely to have been hospitalized in the preceding 6 months (P < .001) and have fewer comorbidities than those who were not tested (P < .001).
“Both groups had similar illness severities on admission,” the authors observed, “but patients who were tested were less likely to die in the hospital within 14 days,” the authors reported – at 6.7% versus 10.9% for untested patients (P < .001).
More than 80% of patients who tested positive for influenza received an antibacterial on day 1 of their admission, compared with virtually all those who were either not tested or who tested negative, the investigators added (P < .001). The mean duration of antibacterial therapy among patients with a bacterial coinfection was not influenced by influenza test results.
However, among those who tested positive for influenza, almost 60% received oseltamivir on day 1 whereas roughly 30% received treatment on day 2 or later. In fact, almost all patients who received early oseltamivir were tested for influenza on day 1, the investigators pointed out. Patients who received early oseltamivir had a 25% lower risk of death within the first 14 days in hospital at an adjusted odds ratio of 0.75 (95% confidence interval, 0.59-0.96).
Early initiation of the antiviral also reduced the risk of requiring subsequent ICU care by 36% at an aOR of 0.64; invasive mechanical ventilation by 46% at an aOR of 0.54, and the need for vasopressor therapy by 47% at an aOR of 0.53. All results were within the 95% confidence levels.
Early use of antiviral therapy also reduced both the length of hospital stay and the cost of that stay by 12%.
ATS-IDSA guidelines
As Dr. Deshpande noted, the American Thoracic Society and the Infectious Diseases Society of America guidelines recommend testing and empiric treatment of influenza in patients hospitalized with CAP. “Testing more inpatients especially during the flu season can reduce other diagnostic testing and improve antimicrobial stewardship,” Deshpande noted.
Thus, while the rate of testing for influenza did increase over the 5-year study interval, “there is substantial room for improvement,” he added, as a positive test clearly does trigger the need for intervention. As Dr. Deshpande also noted, the past two influenza seasons have been mild, but influenza activity has again picked up lately again in many parts of the United States.
With the COVID-19 pandemic overwhelming influenza over the past few years, “differentiating between the two based on symptoms alone can be challenging,” he acknowledged, “and clinicians will need to test and treat accordingly.” This is particularly important given that this study clearly indicates that early treatment with an antiviral can lower the risk of short-term mortality in hospitalized CAP patients.
One limitation of the study was the lack of data on time of symptom onset, which may be an important confounder of the effect of oseltamivir on outcomes, the authors point out. Asked to comment on the findings, Barbara Jones, MD, University of Utah Health, Salt Lake City, noted that timely antivirals for patients with influenza are highly effective at mitigating severe disease and are thus strongly recommended by practice guidelines.
“However, it is hard for clinicians to keep influenza on the radar and change testing and treatment approaches according to the season and prevalence [of influenza infections],” she said in an interview. “This is an important study that highlights this challenge.
“We need a better understanding of the solutions that have been effective at improving influenza recognition and treatment, possibly by studying facilities that perform well at this process,” she said.
Dr. Deshpande reported receiving research funding to his institution from the Clorox Company and consultant fees from Merck.
A version of this article first appeared on Medscape.com.
Early initiation of the antiviral oseltamivir (Tamiflu) reduces the risk for death in patients hospitalized with community-acquired pneumonia (CAP) but patients have to be tested for influenza first and that is not happening often enough, a large observational cohort of adult patients indicates.
“Early testing allows for early treatment, and we found that early treatment was associated with reduced mortality so testing patients during the flu season is crucial,” senior author Michael Rothberg, MD, MPH, of the Cleveland Clinic said in an interview.
“Even during the flu season, most patients with CAP in our study went untested for influenza [even though] those who received early oseltamivir exhibited lower 14-day in-hospital case fatality ... suggesting more widespread testing might improve patient outcomes,” the authors added.
The study was published online Feb. 5, 2022, in the journal CHEST.
Premier database
Data from the Premier Database – a hospital discharge database with information from over 600 hospitals in the United States – were analyzed between July 2010 and June 2015. Microbiological laboratory data was provided by 179 hospitals. “For each year, we evaluated the total percentage of patients tested for influenza A/B within 3 days of hospitalization,” lead author Abhishek Deshpande, MD, PhD, Cleveland Clinic, and colleagues explained.
A total of 166,268 patients with CAP were included in the study, among which only about one-quarter were tested for influenza. Some 11.5% tested positive for the flu, the authors noted. Testing did increase from 15.4% in 2010 to 35.6% in 2015 and it was higher at close to 29% during the influenza season, compared with only about 8% during the summer months.
Patients who were tested for influenza were younger at age 66.6 years, compared with untested patients, who were 70 years of age (P < .001). Tested patients were also less likely to have been admitted from a nursing facility (P < .001), were less likely to have been hospitalized in the preceding 6 months (P < .001) and have fewer comorbidities than those who were not tested (P < .001).
“Both groups had similar illness severities on admission,” the authors observed, “but patients who were tested were less likely to die in the hospital within 14 days,” the authors reported – at 6.7% versus 10.9% for untested patients (P < .001).
More than 80% of patients who tested positive for influenza received an antibacterial on day 1 of their admission, compared with virtually all those who were either not tested or who tested negative, the investigators added (P < .001). The mean duration of antibacterial therapy among patients with a bacterial coinfection was not influenced by influenza test results.
However, among those who tested positive for influenza, almost 60% received oseltamivir on day 1 whereas roughly 30% received treatment on day 2 or later. In fact, almost all patients who received early oseltamivir were tested for influenza on day 1, the investigators pointed out. Patients who received early oseltamivir had a 25% lower risk of death within the first 14 days in hospital at an adjusted odds ratio of 0.75 (95% confidence interval, 0.59-0.96).
Early initiation of the antiviral also reduced the risk of requiring subsequent ICU care by 36% at an aOR of 0.64; invasive mechanical ventilation by 46% at an aOR of 0.54, and the need for vasopressor therapy by 47% at an aOR of 0.53. All results were within the 95% confidence levels.
Early use of antiviral therapy also reduced both the length of hospital stay and the cost of that stay by 12%.
ATS-IDSA guidelines
As Dr. Deshpande noted, the American Thoracic Society and the Infectious Diseases Society of America guidelines recommend testing and empiric treatment of influenza in patients hospitalized with CAP. “Testing more inpatients especially during the flu season can reduce other diagnostic testing and improve antimicrobial stewardship,” Deshpande noted.
Thus, while the rate of testing for influenza did increase over the 5-year study interval, “there is substantial room for improvement,” he added, as a positive test clearly does trigger the need for intervention. As Dr. Deshpande also noted, the past two influenza seasons have been mild, but influenza activity has again picked up lately again in many parts of the United States.
With the COVID-19 pandemic overwhelming influenza over the past few years, “differentiating between the two based on symptoms alone can be challenging,” he acknowledged, “and clinicians will need to test and treat accordingly.” This is particularly important given that this study clearly indicates that early treatment with an antiviral can lower the risk of short-term mortality in hospitalized CAP patients.
One limitation of the study was the lack of data on time of symptom onset, which may be an important confounder of the effect of oseltamivir on outcomes, the authors point out. Asked to comment on the findings, Barbara Jones, MD, University of Utah Health, Salt Lake City, noted that timely antivirals for patients with influenza are highly effective at mitigating severe disease and are thus strongly recommended by practice guidelines.
“However, it is hard for clinicians to keep influenza on the radar and change testing and treatment approaches according to the season and prevalence [of influenza infections],” she said in an interview. “This is an important study that highlights this challenge.
“We need a better understanding of the solutions that have been effective at improving influenza recognition and treatment, possibly by studying facilities that perform well at this process,” she said.
Dr. Deshpande reported receiving research funding to his institution from the Clorox Company and consultant fees from Merck.
A version of this article first appeared on Medscape.com.
FROM CHEST
About 73% of U.S. estimated to be immune to Omicron variant
, a university health institute says.
About half of eligible Americans have received booster shots, and about 80 million confirmed COVID-19 infections have been reported. Many more infections have occurred but haven’t been officially recorded, The Associated Press reported.
The high percentage of immunity from vaccination and previous infection tends to prevent or shorten new illnesses and reduce the amount of virus circulating overall. Health experts are now discussing whether the number is high enough to stop new waves or reduce the burden on hospitals.
“I am optimistic even if we have a surge in summer, cases will go up, but hospitalizations and deaths will not,” Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington in Seattle, told the AP.
Dr. Mokdad works on COVID-19 forecasting for the university’s Institute for Health Metrics and Evaluation, which has been a reliable model during the pandemic. Dr. Mokdad calculated the 73% number for the AP.
“We have changed,” he said. “We have been exposed to this virus and we know how to deal with it.”
The United States is now reporting about 125,000 new cases per day, according to the data tracker from the New York Times, marking a 68% decrease from the past 2 weeks. Hospitalizations are also down 39%, and about 2,300 new deaths are being reported daily, marking a 13% decline.
There will be more outbreaks as new variants emerge, immunity wanes, and some people remain unvaccinated, Dr. Mokdad said. But the coronavirus is no longer new, and the entire population is no longer “immunologically naive.” Scientists are now trying to understand how long booster protection will last against Omicron and how many people have been infected who had mild or no symptoms that were never reported.
By the end of the Omicron surge, about three out of four people in the United States will have been infected, Shaun Truelove, PhD, an epidemiologist and disease modeler at Johns Hopkins University, told the AP.
“We know it’s a huge proportion of the population,” he said. “This varies a lot by location, and in some areas, we expect the number infected to be closer to one in two.”
That means different regions and groups of people have different levels of protection and risk. In Virginia, for instance, disease modelers estimate that about 45% of residents have the highest level of immunity by being vaccinated and boosted or vaccinated with a recent Omicron infection. Another 47% have immunity that has waned somewhat.
“That’s going to be a nice shield of armor for our population as a whole,” Bryan Lewis, PhD, an epidemiologist who leads the University of Virginia’s COVID-19 modeling team, told the outlet. “If we do get to very low case rates, we certainly can ease back on some of these restrictions.”
About 7% of Virginians are considered the most vulnerable because they were never vaccinated or infected, he noted. Nationwide, about 80 million Americans are still vulnerable, the AP reported.
“The 26% who could still get Omicron right now have to be very careful,” Dr. Mokdad said.
The percentages will continue to change as immunity wanes and new variants circulate in the country. For now, the Institute for Health Metrics and Evaluation model estimates that about 63% to 81% of Americans are protected.
“We’ve reached a much better position for the coming months, but with waning immunity, we shouldn’t take it for granted,” Dr. Mokdad said.
A version of this article first appeared on WebMD.com.
, a university health institute says.
About half of eligible Americans have received booster shots, and about 80 million confirmed COVID-19 infections have been reported. Many more infections have occurred but haven’t been officially recorded, The Associated Press reported.
The high percentage of immunity from vaccination and previous infection tends to prevent or shorten new illnesses and reduce the amount of virus circulating overall. Health experts are now discussing whether the number is high enough to stop new waves or reduce the burden on hospitals.
“I am optimistic even if we have a surge in summer, cases will go up, but hospitalizations and deaths will not,” Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington in Seattle, told the AP.
Dr. Mokdad works on COVID-19 forecasting for the university’s Institute for Health Metrics and Evaluation, which has been a reliable model during the pandemic. Dr. Mokdad calculated the 73% number for the AP.
“We have changed,” he said. “We have been exposed to this virus and we know how to deal with it.”
The United States is now reporting about 125,000 new cases per day, according to the data tracker from the New York Times, marking a 68% decrease from the past 2 weeks. Hospitalizations are also down 39%, and about 2,300 new deaths are being reported daily, marking a 13% decline.
There will be more outbreaks as new variants emerge, immunity wanes, and some people remain unvaccinated, Dr. Mokdad said. But the coronavirus is no longer new, and the entire population is no longer “immunologically naive.” Scientists are now trying to understand how long booster protection will last against Omicron and how many people have been infected who had mild or no symptoms that were never reported.
By the end of the Omicron surge, about three out of four people in the United States will have been infected, Shaun Truelove, PhD, an epidemiologist and disease modeler at Johns Hopkins University, told the AP.
“We know it’s a huge proportion of the population,” he said. “This varies a lot by location, and in some areas, we expect the number infected to be closer to one in two.”
That means different regions and groups of people have different levels of protection and risk. In Virginia, for instance, disease modelers estimate that about 45% of residents have the highest level of immunity by being vaccinated and boosted or vaccinated with a recent Omicron infection. Another 47% have immunity that has waned somewhat.
“That’s going to be a nice shield of armor for our population as a whole,” Bryan Lewis, PhD, an epidemiologist who leads the University of Virginia’s COVID-19 modeling team, told the outlet. “If we do get to very low case rates, we certainly can ease back on some of these restrictions.”
About 7% of Virginians are considered the most vulnerable because they were never vaccinated or infected, he noted. Nationwide, about 80 million Americans are still vulnerable, the AP reported.
“The 26% who could still get Omicron right now have to be very careful,” Dr. Mokdad said.
The percentages will continue to change as immunity wanes and new variants circulate in the country. For now, the Institute for Health Metrics and Evaluation model estimates that about 63% to 81% of Americans are protected.
“We’ve reached a much better position for the coming months, but with waning immunity, we shouldn’t take it for granted,” Dr. Mokdad said.
A version of this article first appeared on WebMD.com.
, a university health institute says.
About half of eligible Americans have received booster shots, and about 80 million confirmed COVID-19 infections have been reported. Many more infections have occurred but haven’t been officially recorded, The Associated Press reported.
The high percentage of immunity from vaccination and previous infection tends to prevent or shorten new illnesses and reduce the amount of virus circulating overall. Health experts are now discussing whether the number is high enough to stop new waves or reduce the burden on hospitals.
“I am optimistic even if we have a surge in summer, cases will go up, but hospitalizations and deaths will not,” Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington in Seattle, told the AP.
Dr. Mokdad works on COVID-19 forecasting for the university’s Institute for Health Metrics and Evaluation, which has been a reliable model during the pandemic. Dr. Mokdad calculated the 73% number for the AP.
“We have changed,” he said. “We have been exposed to this virus and we know how to deal with it.”
The United States is now reporting about 125,000 new cases per day, according to the data tracker from the New York Times, marking a 68% decrease from the past 2 weeks. Hospitalizations are also down 39%, and about 2,300 new deaths are being reported daily, marking a 13% decline.
There will be more outbreaks as new variants emerge, immunity wanes, and some people remain unvaccinated, Dr. Mokdad said. But the coronavirus is no longer new, and the entire population is no longer “immunologically naive.” Scientists are now trying to understand how long booster protection will last against Omicron and how many people have been infected who had mild or no symptoms that were never reported.
By the end of the Omicron surge, about three out of four people in the United States will have been infected, Shaun Truelove, PhD, an epidemiologist and disease modeler at Johns Hopkins University, told the AP.
“We know it’s a huge proportion of the population,” he said. “This varies a lot by location, and in some areas, we expect the number infected to be closer to one in two.”
That means different regions and groups of people have different levels of protection and risk. In Virginia, for instance, disease modelers estimate that about 45% of residents have the highest level of immunity by being vaccinated and boosted or vaccinated with a recent Omicron infection. Another 47% have immunity that has waned somewhat.
“That’s going to be a nice shield of armor for our population as a whole,” Bryan Lewis, PhD, an epidemiologist who leads the University of Virginia’s COVID-19 modeling team, told the outlet. “If we do get to very low case rates, we certainly can ease back on some of these restrictions.”
About 7% of Virginians are considered the most vulnerable because they were never vaccinated or infected, he noted. Nationwide, about 80 million Americans are still vulnerable, the AP reported.
“The 26% who could still get Omicron right now have to be very careful,” Dr. Mokdad said.
The percentages will continue to change as immunity wanes and new variants circulate in the country. For now, the Institute for Health Metrics and Evaluation model estimates that about 63% to 81% of Americans are protected.
“We’ve reached a much better position for the coming months, but with waning immunity, we shouldn’t take it for granted,” Dr. Mokdad said.
A version of this article first appeared on WebMD.com.
When your medical error harmed a patient and you’re wracked with guilt
Peter Schwartz, MD, was chair of the department of obstetrics and gynecology at a hospital in Reading, Pa., in the mid-1990s when a young physician sought him out. The doctor, whom Dr. Schwartz regarded as talented and empathetic, was visibly shaken. The expectant mother they were caring for had just lost her unborn child.
“The doctor came into my office within an hour of the event and asked me to look at the case,” Dr. Schwartz recalled. “I could see that they had failed to recognize ominous changes in the fetal heart rate, and I faced the pain of having to tell them, ‘I think this could have been handled much better.’” Dr. Schwartz delivered the news as compassionately as he could, but a subsequent review confirmed his suspicion: The doctor had made a serious error.
“The doctor was devastated,” he said. “She got counseling and took time off, but in the end, she quit practicing medicine. She said, ‘If I keep practicing, something like that could happen again, and I don’t think I could handle it.’”
To err may be human, but in a health care setting, the harm can be catastrophic. that their feelings of guilt, shame, and self-doubt can lead to depression, anxiety, post-traumatic stress disorder, and even suicidal ideation. The trauma can be so profound that, in a now famous 2000 editorial in the British Medical Journal, Albert Wu, MD, gave the phenomenon a name: “second victim syndrome.”
Today, as quality improvement organizations and health systems work to address medical errors in a just and transparent way, they’re realizing that finding ways to help traumatized clinicians is integral to their efforts.
Are doctors really ‘second victims?’
Although the medical field is moving away from the term “second victim,” which patient advocates argue lacks a ring of accountability, the emotional trauma doctors and other clinicians endure is garnering increased attention. In the 2 decades since Dr. Wu wrote his editorial, research has shown that many types of adverse health care events can evoke traumatic responses. In fact, studies indicate that from 10.4% to 43.3% of health care workers may experience negative symptoms following an adverse event.
But for doctors – who have sworn an oath to do no harm – the emotional toll of having committed a serious medical error can be particularly burdensome and lingering. In a Dutch study involving more than 4,300 doctors and nurses, respondents who were involved in a patient safety incident that resulted in harm were nine times more likely to have negative symptoms lasting longer than 6 months than those who were involved in a near-miss experience.
“There’s a feeling of wanting to erase yourself,” says Danielle Ofri, MD, a New York internist and author of “When We Do Harm: A Doctor Confronts Medical Error.”
That emotional response can have a profound impact on the way medical errors are disclosed, investigated, and ultimately resolved, said Thomas Gallagher, MD, an internist and executive director of the Collaborative for Accountability and Improvement, a patient safety program at the University of Washington.
“When something goes wrong, as physicians, we don’t know what to do,” Dr. Gallagher says. “We feel awful, and often our human reflexes lead us astray. The doctor’s own emotions become barriers to addressing the situation.” For example, guilt and shame may lead doctors to try to hide or diminish their mistakes. Some doctors might try to shift blame, while others may feel so guilty they assume they were responsible for an outcome that was beyond their control.
Recognizing that clinicians’ responses to medical errors are inextricably tangled with how those events are addressed, a growing number of health systems are making clinician support a key element when dealing with medical errors.
Emotional first aid
Although it’s typical for physicians to feel isolated in the wake of errors, these experiences are far from unique. Research conducted by University of Missouri Health Care nurse scientist Susan Scott, RN, PhD, shows that just as most individuals experiencing grief pass through several distinct emotional stages, health care professionals who make errors go through emotional stages that may occur sequentially or concurrently.
An initial period of chaos is often followed by intrusive reflections, haunting re-enactments, and feelings of inadequacy. The doctor’s thinking moves from “How did that happen?” to “What did I miss?” to “What will people think about me?” As the error comes under scrutiny by quality improvement organizations, licensing boards, and/or lawyers, the doctor feels besieged. The doctor may want to reach out but is afraid to. According to Dr. Scott, only 15% of care providers ask for help.
Recognizing that physicians and other care providers rarely ask for support – or may not realize they need it – a growing number of health systems are implementing Communication and Resolution Programs (CRPs). Rather than respond to medical errors with a deny-and-defend mentality, CRPs emphasize transparency and accountability.
This approach, which the Agency for Healthcare Research and Quality has embraced and codified with its Communication and Optimal Resolution (CANDOR) toolkit, focuses on prompt incident reporting; communication with and support for patients, family members, and caregivers affected by the event; event analysis; quality improvement; and just resolution of the event, including apologies and financial compensation where appropriate.
The CANDOR toolkit, which includes a module entitled Care for the Caregiver, directs health systems to identify individuals and establish teams, led by representatives from patient safety and/or risk management, who can respond promptly to an event. After ensuring the patient is clinically stable and safe, the CANDOR process provides for immediate and ongoing emotional support to the patient, the family, and the caregiver.
“A lot of what CRPs are about is creating structures and processes that normalize an open and compassionate response to harm events in medicine,” says Dr. Gallagher, who estimates that between 400 and 500 health systems now have CRPs in place.
Wisdom through adversity
While clinicians experience many difficult and negative emotions in the wake of medical errors, how they move forward after the event varies markedly. Some, unable to come to terms with the trauma, may move to another institution or leave medicine entirely. Others, while occasionally reliving the trauma, learn to cope. For the most fortunate, enduring the trauma of a medical error can lead to growth, insight, and wisdom.
In an article published in the journal Academic Medicine, researchers asked 61 physicians who had made serious medical errors, “What helped you to cope positively?” Some of the most common responses – talking about their feelings with a peer, disclosing and apologizing for a mistake, and developing system changes to prevent additional errors – are baked into some health systems’ CRP programs. Other respondents said they dedicated themselves to learning from the mistake, becoming experts in a given field, or sharing what they learned from the experience through teaching.
Dr. Ofri said that after she made an error decades ago while managing a patient with diabetic ketoacidosis, her senior resident publicly berated her for it. The incident taught her a clinical lesson: Never remove an insulin drip without administering long-acting insulin. More importantly, the resident’s verbal thumping taught her about the corrosive effects of shame. Today, Dr. Ofri, who works in a teaching hospital, says that when meeting a new medical team, she begins by recounting her five biggest medical errors.
“I want them to come to me if they make a mistake,” she says. “I want to first make sure the patient is okay. But then I want to make sure the doctor is okay. I also want to know: What was it about the system that contributed to the error, and what can we do to prevent similar errors in the future?”
Acceptance and compassion
Time, experience, supportive peers, an understanding partner or spouse: all of these can help a doctor recover from the trauma of a mistake. “But they’re not an eraser,” Dr. Schwartz said.
Sometimes, doctors say, the path forward starts with acceptance.
Jan Bonhoeffer, MD, author of “Dare to Care: How to Survive and Thrive in Today’s Medical World,” tells a story about a mistake that transformed his life. In 2004, he was working in a busy London emergency department when an adolescent girl arrived complaining of breathing trouble. Dr. Bonhoeffer diagnosed her with asthma and discharged her with an inhaler. The next day, the girl was back in the hospital – this time in the ICU, intubated, and on a ventilator. Because he had failed to take an x-ray, Dr. Bonhoeffer missed the tumor growing in the girl’s chest.
Dr. Bonhoeffer was shattered by his error. “After that experience, I knew I wanted to make learning from my mistakes part of my daily practice,” he says. Now, at the end of each workday, Dr. Bonhoeffer takes an inventory of the day and reflects on all his actions, large and small, clinical and not. “I take a few minutes and think about everything I did and what I should have done differently,” he said. The daily practice can be humbling because it forces him to confront his errors, but it is also empowering, he said, “because the next day I get to make a different choice.”
Dr. Bonhoeffer added, “Doctors are fallible, and you have to be compassionate with yourself. Compassion isn’t sweet. It’s not motherhood and honey pies. It’s coming to terms with reality. It’s not a cure, but it’s healing.”
A version of this article first appeared on Medscape.com.
Peter Schwartz, MD, was chair of the department of obstetrics and gynecology at a hospital in Reading, Pa., in the mid-1990s when a young physician sought him out. The doctor, whom Dr. Schwartz regarded as talented and empathetic, was visibly shaken. The expectant mother they were caring for had just lost her unborn child.
“The doctor came into my office within an hour of the event and asked me to look at the case,” Dr. Schwartz recalled. “I could see that they had failed to recognize ominous changes in the fetal heart rate, and I faced the pain of having to tell them, ‘I think this could have been handled much better.’” Dr. Schwartz delivered the news as compassionately as he could, but a subsequent review confirmed his suspicion: The doctor had made a serious error.
“The doctor was devastated,” he said. “She got counseling and took time off, but in the end, she quit practicing medicine. She said, ‘If I keep practicing, something like that could happen again, and I don’t think I could handle it.’”
To err may be human, but in a health care setting, the harm can be catastrophic. that their feelings of guilt, shame, and self-doubt can lead to depression, anxiety, post-traumatic stress disorder, and even suicidal ideation. The trauma can be so profound that, in a now famous 2000 editorial in the British Medical Journal, Albert Wu, MD, gave the phenomenon a name: “second victim syndrome.”
Today, as quality improvement organizations and health systems work to address medical errors in a just and transparent way, they’re realizing that finding ways to help traumatized clinicians is integral to their efforts.
Are doctors really ‘second victims?’
Although the medical field is moving away from the term “second victim,” which patient advocates argue lacks a ring of accountability, the emotional trauma doctors and other clinicians endure is garnering increased attention. In the 2 decades since Dr. Wu wrote his editorial, research has shown that many types of adverse health care events can evoke traumatic responses. In fact, studies indicate that from 10.4% to 43.3% of health care workers may experience negative symptoms following an adverse event.
But for doctors – who have sworn an oath to do no harm – the emotional toll of having committed a serious medical error can be particularly burdensome and lingering. In a Dutch study involving more than 4,300 doctors and nurses, respondents who were involved in a patient safety incident that resulted in harm were nine times more likely to have negative symptoms lasting longer than 6 months than those who were involved in a near-miss experience.
“There’s a feeling of wanting to erase yourself,” says Danielle Ofri, MD, a New York internist and author of “When We Do Harm: A Doctor Confronts Medical Error.”
That emotional response can have a profound impact on the way medical errors are disclosed, investigated, and ultimately resolved, said Thomas Gallagher, MD, an internist and executive director of the Collaborative for Accountability and Improvement, a patient safety program at the University of Washington.
“When something goes wrong, as physicians, we don’t know what to do,” Dr. Gallagher says. “We feel awful, and often our human reflexes lead us astray. The doctor’s own emotions become barriers to addressing the situation.” For example, guilt and shame may lead doctors to try to hide or diminish their mistakes. Some doctors might try to shift blame, while others may feel so guilty they assume they were responsible for an outcome that was beyond their control.
Recognizing that clinicians’ responses to medical errors are inextricably tangled with how those events are addressed, a growing number of health systems are making clinician support a key element when dealing with medical errors.
Emotional first aid
Although it’s typical for physicians to feel isolated in the wake of errors, these experiences are far from unique. Research conducted by University of Missouri Health Care nurse scientist Susan Scott, RN, PhD, shows that just as most individuals experiencing grief pass through several distinct emotional stages, health care professionals who make errors go through emotional stages that may occur sequentially or concurrently.
An initial period of chaos is often followed by intrusive reflections, haunting re-enactments, and feelings of inadequacy. The doctor’s thinking moves from “How did that happen?” to “What did I miss?” to “What will people think about me?” As the error comes under scrutiny by quality improvement organizations, licensing boards, and/or lawyers, the doctor feels besieged. The doctor may want to reach out but is afraid to. According to Dr. Scott, only 15% of care providers ask for help.
Recognizing that physicians and other care providers rarely ask for support – or may not realize they need it – a growing number of health systems are implementing Communication and Resolution Programs (CRPs). Rather than respond to medical errors with a deny-and-defend mentality, CRPs emphasize transparency and accountability.
This approach, which the Agency for Healthcare Research and Quality has embraced and codified with its Communication and Optimal Resolution (CANDOR) toolkit, focuses on prompt incident reporting; communication with and support for patients, family members, and caregivers affected by the event; event analysis; quality improvement; and just resolution of the event, including apologies and financial compensation where appropriate.
The CANDOR toolkit, which includes a module entitled Care for the Caregiver, directs health systems to identify individuals and establish teams, led by representatives from patient safety and/or risk management, who can respond promptly to an event. After ensuring the patient is clinically stable and safe, the CANDOR process provides for immediate and ongoing emotional support to the patient, the family, and the caregiver.
“A lot of what CRPs are about is creating structures and processes that normalize an open and compassionate response to harm events in medicine,” says Dr. Gallagher, who estimates that between 400 and 500 health systems now have CRPs in place.
Wisdom through adversity
While clinicians experience many difficult and negative emotions in the wake of medical errors, how they move forward after the event varies markedly. Some, unable to come to terms with the trauma, may move to another institution or leave medicine entirely. Others, while occasionally reliving the trauma, learn to cope. For the most fortunate, enduring the trauma of a medical error can lead to growth, insight, and wisdom.
In an article published in the journal Academic Medicine, researchers asked 61 physicians who had made serious medical errors, “What helped you to cope positively?” Some of the most common responses – talking about their feelings with a peer, disclosing and apologizing for a mistake, and developing system changes to prevent additional errors – are baked into some health systems’ CRP programs. Other respondents said they dedicated themselves to learning from the mistake, becoming experts in a given field, or sharing what they learned from the experience through teaching.
Dr. Ofri said that after she made an error decades ago while managing a patient with diabetic ketoacidosis, her senior resident publicly berated her for it. The incident taught her a clinical lesson: Never remove an insulin drip without administering long-acting insulin. More importantly, the resident’s verbal thumping taught her about the corrosive effects of shame. Today, Dr. Ofri, who works in a teaching hospital, says that when meeting a new medical team, she begins by recounting her five biggest medical errors.
“I want them to come to me if they make a mistake,” she says. “I want to first make sure the patient is okay. But then I want to make sure the doctor is okay. I also want to know: What was it about the system that contributed to the error, and what can we do to prevent similar errors in the future?”
Acceptance and compassion
Time, experience, supportive peers, an understanding partner or spouse: all of these can help a doctor recover from the trauma of a mistake. “But they’re not an eraser,” Dr. Schwartz said.
Sometimes, doctors say, the path forward starts with acceptance.
Jan Bonhoeffer, MD, author of “Dare to Care: How to Survive and Thrive in Today’s Medical World,” tells a story about a mistake that transformed his life. In 2004, he was working in a busy London emergency department when an adolescent girl arrived complaining of breathing trouble. Dr. Bonhoeffer diagnosed her with asthma and discharged her with an inhaler. The next day, the girl was back in the hospital – this time in the ICU, intubated, and on a ventilator. Because he had failed to take an x-ray, Dr. Bonhoeffer missed the tumor growing in the girl’s chest.
Dr. Bonhoeffer was shattered by his error. “After that experience, I knew I wanted to make learning from my mistakes part of my daily practice,” he says. Now, at the end of each workday, Dr. Bonhoeffer takes an inventory of the day and reflects on all his actions, large and small, clinical and not. “I take a few minutes and think about everything I did and what I should have done differently,” he said. The daily practice can be humbling because it forces him to confront his errors, but it is also empowering, he said, “because the next day I get to make a different choice.”
Dr. Bonhoeffer added, “Doctors are fallible, and you have to be compassionate with yourself. Compassion isn’t sweet. It’s not motherhood and honey pies. It’s coming to terms with reality. It’s not a cure, but it’s healing.”
A version of this article first appeared on Medscape.com.
Peter Schwartz, MD, was chair of the department of obstetrics and gynecology at a hospital in Reading, Pa., in the mid-1990s when a young physician sought him out. The doctor, whom Dr. Schwartz regarded as talented and empathetic, was visibly shaken. The expectant mother they were caring for had just lost her unborn child.
“The doctor came into my office within an hour of the event and asked me to look at the case,” Dr. Schwartz recalled. “I could see that they had failed to recognize ominous changes in the fetal heart rate, and I faced the pain of having to tell them, ‘I think this could have been handled much better.’” Dr. Schwartz delivered the news as compassionately as he could, but a subsequent review confirmed his suspicion: The doctor had made a serious error.
“The doctor was devastated,” he said. “She got counseling and took time off, but in the end, she quit practicing medicine. She said, ‘If I keep practicing, something like that could happen again, and I don’t think I could handle it.’”
To err may be human, but in a health care setting, the harm can be catastrophic. that their feelings of guilt, shame, and self-doubt can lead to depression, anxiety, post-traumatic stress disorder, and even suicidal ideation. The trauma can be so profound that, in a now famous 2000 editorial in the British Medical Journal, Albert Wu, MD, gave the phenomenon a name: “second victim syndrome.”
Today, as quality improvement organizations and health systems work to address medical errors in a just and transparent way, they’re realizing that finding ways to help traumatized clinicians is integral to their efforts.
Are doctors really ‘second victims?’
Although the medical field is moving away from the term “second victim,” which patient advocates argue lacks a ring of accountability, the emotional trauma doctors and other clinicians endure is garnering increased attention. In the 2 decades since Dr. Wu wrote his editorial, research has shown that many types of adverse health care events can evoke traumatic responses. In fact, studies indicate that from 10.4% to 43.3% of health care workers may experience negative symptoms following an adverse event.
But for doctors – who have sworn an oath to do no harm – the emotional toll of having committed a serious medical error can be particularly burdensome and lingering. In a Dutch study involving more than 4,300 doctors and nurses, respondents who were involved in a patient safety incident that resulted in harm were nine times more likely to have negative symptoms lasting longer than 6 months than those who were involved in a near-miss experience.
“There’s a feeling of wanting to erase yourself,” says Danielle Ofri, MD, a New York internist and author of “When We Do Harm: A Doctor Confronts Medical Error.”
That emotional response can have a profound impact on the way medical errors are disclosed, investigated, and ultimately resolved, said Thomas Gallagher, MD, an internist and executive director of the Collaborative for Accountability and Improvement, a patient safety program at the University of Washington.
“When something goes wrong, as physicians, we don’t know what to do,” Dr. Gallagher says. “We feel awful, and often our human reflexes lead us astray. The doctor’s own emotions become barriers to addressing the situation.” For example, guilt and shame may lead doctors to try to hide or diminish their mistakes. Some doctors might try to shift blame, while others may feel so guilty they assume they were responsible for an outcome that was beyond their control.
Recognizing that clinicians’ responses to medical errors are inextricably tangled with how those events are addressed, a growing number of health systems are making clinician support a key element when dealing with medical errors.
Emotional first aid
Although it’s typical for physicians to feel isolated in the wake of errors, these experiences are far from unique. Research conducted by University of Missouri Health Care nurse scientist Susan Scott, RN, PhD, shows that just as most individuals experiencing grief pass through several distinct emotional stages, health care professionals who make errors go through emotional stages that may occur sequentially or concurrently.
An initial period of chaos is often followed by intrusive reflections, haunting re-enactments, and feelings of inadequacy. The doctor’s thinking moves from “How did that happen?” to “What did I miss?” to “What will people think about me?” As the error comes under scrutiny by quality improvement organizations, licensing boards, and/or lawyers, the doctor feels besieged. The doctor may want to reach out but is afraid to. According to Dr. Scott, only 15% of care providers ask for help.
Recognizing that physicians and other care providers rarely ask for support – or may not realize they need it – a growing number of health systems are implementing Communication and Resolution Programs (CRPs). Rather than respond to medical errors with a deny-and-defend mentality, CRPs emphasize transparency and accountability.
This approach, which the Agency for Healthcare Research and Quality has embraced and codified with its Communication and Optimal Resolution (CANDOR) toolkit, focuses on prompt incident reporting; communication with and support for patients, family members, and caregivers affected by the event; event analysis; quality improvement; and just resolution of the event, including apologies and financial compensation where appropriate.
The CANDOR toolkit, which includes a module entitled Care for the Caregiver, directs health systems to identify individuals and establish teams, led by representatives from patient safety and/or risk management, who can respond promptly to an event. After ensuring the patient is clinically stable and safe, the CANDOR process provides for immediate and ongoing emotional support to the patient, the family, and the caregiver.
“A lot of what CRPs are about is creating structures and processes that normalize an open and compassionate response to harm events in medicine,” says Dr. Gallagher, who estimates that between 400 and 500 health systems now have CRPs in place.
Wisdom through adversity
While clinicians experience many difficult and negative emotions in the wake of medical errors, how they move forward after the event varies markedly. Some, unable to come to terms with the trauma, may move to another institution or leave medicine entirely. Others, while occasionally reliving the trauma, learn to cope. For the most fortunate, enduring the trauma of a medical error can lead to growth, insight, and wisdom.
In an article published in the journal Academic Medicine, researchers asked 61 physicians who had made serious medical errors, “What helped you to cope positively?” Some of the most common responses – talking about their feelings with a peer, disclosing and apologizing for a mistake, and developing system changes to prevent additional errors – are baked into some health systems’ CRP programs. Other respondents said they dedicated themselves to learning from the mistake, becoming experts in a given field, or sharing what they learned from the experience through teaching.
Dr. Ofri said that after she made an error decades ago while managing a patient with diabetic ketoacidosis, her senior resident publicly berated her for it. The incident taught her a clinical lesson: Never remove an insulin drip without administering long-acting insulin. More importantly, the resident’s verbal thumping taught her about the corrosive effects of shame. Today, Dr. Ofri, who works in a teaching hospital, says that when meeting a new medical team, she begins by recounting her five biggest medical errors.
“I want them to come to me if they make a mistake,” she says. “I want to first make sure the patient is okay. But then I want to make sure the doctor is okay. I also want to know: What was it about the system that contributed to the error, and what can we do to prevent similar errors in the future?”
Acceptance and compassion
Time, experience, supportive peers, an understanding partner or spouse: all of these can help a doctor recover from the trauma of a mistake. “But they’re not an eraser,” Dr. Schwartz said.
Sometimes, doctors say, the path forward starts with acceptance.
Jan Bonhoeffer, MD, author of “Dare to Care: How to Survive and Thrive in Today’s Medical World,” tells a story about a mistake that transformed his life. In 2004, he was working in a busy London emergency department when an adolescent girl arrived complaining of breathing trouble. Dr. Bonhoeffer diagnosed her with asthma and discharged her with an inhaler. The next day, the girl was back in the hospital – this time in the ICU, intubated, and on a ventilator. Because he had failed to take an x-ray, Dr. Bonhoeffer missed the tumor growing in the girl’s chest.
Dr. Bonhoeffer was shattered by his error. “After that experience, I knew I wanted to make learning from my mistakes part of my daily practice,” he says. Now, at the end of each workday, Dr. Bonhoeffer takes an inventory of the day and reflects on all his actions, large and small, clinical and not. “I take a few minutes and think about everything I did and what I should have done differently,” he said. The daily practice can be humbling because it forces him to confront his errors, but it is also empowering, he said, “because the next day I get to make a different choice.”
Dr. Bonhoeffer added, “Doctors are fallible, and you have to be compassionate with yourself. Compassion isn’t sweet. It’s not motherhood and honey pies. It’s coming to terms with reality. It’s not a cure, but it’s healing.”
A version of this article first appeared on Medscape.com.