User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Some physicians still lack access to COVID-19 vaccines
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
Should all women be routinely screened for lung cancer?
especially those with a history of breast cancer, according to a new study published in BJS Open.
The 2021 screening guidelines include adults aged between 50 and 80 years who have a 20–pack-year smoking history and currently smoke or have quit within the past 15 years, but the guidelines do not include nonsmokers or patients with a history of previous malignancies, such as breast cancer.
Led by Daniela Molena, MD, a thoracic surgeon and director of esophageal surgery at Memorial Sloan Kettering Cancer Center, New York, researchers conducted an analysis of 2,192 women with first-time lung cancer who underwent lung resections at Memorial Sloan Kettering between January 2000 and December 2017. The study’s objective was to determine stage at diagnosis, survival, and eligibility for lung cancer screening among patients with lung cancer who had a previous breast cancer diagnosis and those who did not have a history of breast cancer.
Only 331 (15.1%) patients were previously diagnosed with breast cancer, which was not statistically significant. “Overall, there were no statistically significant differences in genomic or oncogenic pathway alterations between the two groups, which suggests that lung cancer in patients who previously had breast cancer may not be affected at the genomic level by the previous breast cancer,” the authors wrote.
However, at 58.4%, more than half of patients in the study (1,281 patients) were prior smokers and only 33.3% met the USPSTF criteria for lung cancer screening, which the authors said was concerning.
“The most important finding of the study was that a high percentage of women with lung cancer, regardless of breast cancer history, did not meet the current USPSTF criteria for lung cancer screening. This is very important given the observation that nearly half of the women included in the study did not have a history of smoking. As such, the role of imaging for other causes, such as cancer surveillance, becomes especially important for early cancer diagnosis,” Dr. Molena and colleagues wrote. “To reduce late-stage cancer diagnoses, further assessment of guidelines for lung cancer screening for all women may be needed.”
Instead, for almost half of women in the study group with a history of breast cancer, the lung cancer was detected on a routine follow-up imaging scan.
USPSTF guidelines for lung cancer screening do not include previous malignancy as a high-risk feature requiring evaluation, which may explain why so few women in this study were screened for lung cancer, even though lung cancer is more common in breast cancer survivors than the general population. Approximately 10% of women who have had breast cancer will develop a second malignancy within 10 years and in most cases, it will be lung cancer. Plus, according to the National Cancer Institute, breast, lung, and colorectal cancers are the three most common cancers in women and account for approximately 50% of all new cancer diagnoses in women in 2020.
A 2018 analysis published in Frontiers in Oncology found that, of more than 6,000 women with secondary primary lung cancer after having had breast cancer, 42% had distant-stage disease at the time of diagnosis which, Dr. Molena and colleagues said, suggests an ongoing need to update screening recommendations.
“Given that lung cancer has a 5-year overall survival rate of less than 20% (highlighting the benefits of early-stage diagnosis), a better understanding of lung cancer in women with a history of breast cancer could have important implications for screening and surveillance,” the authors wrote.
Estrogen is known to play a role in the development of lung cancer by activating the epidermal growth factor receptor (EGFR). Previous research has shown an increased risk of lung cancer in patients with estrogen receptor–negative, progesterone receptor–negative, HER2-negative, or triple-negative breast cancer.
“Antiestrogen treatment has been demonstrated to decrease the incidence of lung cancer and has been associated with improved long-term survival in patients with lung cancer after breast cancer. Future studies should seek to identify high-risk populations on the basis of hormone receptor status and antiestrogen therapy use,” the authors wrote.
The authors noted a number of limitations to the study, including the single hospital as the sole source of data, plus, the analysis did not account for the length of time since patients quit smoking and a lung cancer diagnosis. Nor did it consider other risk factors, such as radiation, chemotherapy, or antiestrogen therapies.
The authors did not disclose any study-related conflicts of interests.
This article was updated 3/2/22.
especially those with a history of breast cancer, according to a new study published in BJS Open.
The 2021 screening guidelines include adults aged between 50 and 80 years who have a 20–pack-year smoking history and currently smoke or have quit within the past 15 years, but the guidelines do not include nonsmokers or patients with a history of previous malignancies, such as breast cancer.
Led by Daniela Molena, MD, a thoracic surgeon and director of esophageal surgery at Memorial Sloan Kettering Cancer Center, New York, researchers conducted an analysis of 2,192 women with first-time lung cancer who underwent lung resections at Memorial Sloan Kettering between January 2000 and December 2017. The study’s objective was to determine stage at diagnosis, survival, and eligibility for lung cancer screening among patients with lung cancer who had a previous breast cancer diagnosis and those who did not have a history of breast cancer.
Only 331 (15.1%) patients were previously diagnosed with breast cancer, which was not statistically significant. “Overall, there were no statistically significant differences in genomic or oncogenic pathway alterations between the two groups, which suggests that lung cancer in patients who previously had breast cancer may not be affected at the genomic level by the previous breast cancer,” the authors wrote.
However, at 58.4%, more than half of patients in the study (1,281 patients) were prior smokers and only 33.3% met the USPSTF criteria for lung cancer screening, which the authors said was concerning.
“The most important finding of the study was that a high percentage of women with lung cancer, regardless of breast cancer history, did not meet the current USPSTF criteria for lung cancer screening. This is very important given the observation that nearly half of the women included in the study did not have a history of smoking. As such, the role of imaging for other causes, such as cancer surveillance, becomes especially important for early cancer diagnosis,” Dr. Molena and colleagues wrote. “To reduce late-stage cancer diagnoses, further assessment of guidelines for lung cancer screening for all women may be needed.”
Instead, for almost half of women in the study group with a history of breast cancer, the lung cancer was detected on a routine follow-up imaging scan.
USPSTF guidelines for lung cancer screening do not include previous malignancy as a high-risk feature requiring evaluation, which may explain why so few women in this study were screened for lung cancer, even though lung cancer is more common in breast cancer survivors than the general population. Approximately 10% of women who have had breast cancer will develop a second malignancy within 10 years and in most cases, it will be lung cancer. Plus, according to the National Cancer Institute, breast, lung, and colorectal cancers are the three most common cancers in women and account for approximately 50% of all new cancer diagnoses in women in 2020.
A 2018 analysis published in Frontiers in Oncology found that, of more than 6,000 women with secondary primary lung cancer after having had breast cancer, 42% had distant-stage disease at the time of diagnosis which, Dr. Molena and colleagues said, suggests an ongoing need to update screening recommendations.
“Given that lung cancer has a 5-year overall survival rate of less than 20% (highlighting the benefits of early-stage diagnosis), a better understanding of lung cancer in women with a history of breast cancer could have important implications for screening and surveillance,” the authors wrote.
Estrogen is known to play a role in the development of lung cancer by activating the epidermal growth factor receptor (EGFR). Previous research has shown an increased risk of lung cancer in patients with estrogen receptor–negative, progesterone receptor–negative, HER2-negative, or triple-negative breast cancer.
“Antiestrogen treatment has been demonstrated to decrease the incidence of lung cancer and has been associated with improved long-term survival in patients with lung cancer after breast cancer. Future studies should seek to identify high-risk populations on the basis of hormone receptor status and antiestrogen therapy use,” the authors wrote.
The authors noted a number of limitations to the study, including the single hospital as the sole source of data, plus, the analysis did not account for the length of time since patients quit smoking and a lung cancer diagnosis. Nor did it consider other risk factors, such as radiation, chemotherapy, or antiestrogen therapies.
The authors did not disclose any study-related conflicts of interests.
This article was updated 3/2/22.
especially those with a history of breast cancer, according to a new study published in BJS Open.
The 2021 screening guidelines include adults aged between 50 and 80 years who have a 20–pack-year smoking history and currently smoke or have quit within the past 15 years, but the guidelines do not include nonsmokers or patients with a history of previous malignancies, such as breast cancer.
Led by Daniela Molena, MD, a thoracic surgeon and director of esophageal surgery at Memorial Sloan Kettering Cancer Center, New York, researchers conducted an analysis of 2,192 women with first-time lung cancer who underwent lung resections at Memorial Sloan Kettering between January 2000 and December 2017. The study’s objective was to determine stage at diagnosis, survival, and eligibility for lung cancer screening among patients with lung cancer who had a previous breast cancer diagnosis and those who did not have a history of breast cancer.
Only 331 (15.1%) patients were previously diagnosed with breast cancer, which was not statistically significant. “Overall, there were no statistically significant differences in genomic or oncogenic pathway alterations between the two groups, which suggests that lung cancer in patients who previously had breast cancer may not be affected at the genomic level by the previous breast cancer,” the authors wrote.
However, at 58.4%, more than half of patients in the study (1,281 patients) were prior smokers and only 33.3% met the USPSTF criteria for lung cancer screening, which the authors said was concerning.
“The most important finding of the study was that a high percentage of women with lung cancer, regardless of breast cancer history, did not meet the current USPSTF criteria for lung cancer screening. This is very important given the observation that nearly half of the women included in the study did not have a history of smoking. As such, the role of imaging for other causes, such as cancer surveillance, becomes especially important for early cancer diagnosis,” Dr. Molena and colleagues wrote. “To reduce late-stage cancer diagnoses, further assessment of guidelines for lung cancer screening for all women may be needed.”
Instead, for almost half of women in the study group with a history of breast cancer, the lung cancer was detected on a routine follow-up imaging scan.
USPSTF guidelines for lung cancer screening do not include previous malignancy as a high-risk feature requiring evaluation, which may explain why so few women in this study were screened for lung cancer, even though lung cancer is more common in breast cancer survivors than the general population. Approximately 10% of women who have had breast cancer will develop a second malignancy within 10 years and in most cases, it will be lung cancer. Plus, according to the National Cancer Institute, breast, lung, and colorectal cancers are the three most common cancers in women and account for approximately 50% of all new cancer diagnoses in women in 2020.
A 2018 analysis published in Frontiers in Oncology found that, of more than 6,000 women with secondary primary lung cancer after having had breast cancer, 42% had distant-stage disease at the time of diagnosis which, Dr. Molena and colleagues said, suggests an ongoing need to update screening recommendations.
“Given that lung cancer has a 5-year overall survival rate of less than 20% (highlighting the benefits of early-stage diagnosis), a better understanding of lung cancer in women with a history of breast cancer could have important implications for screening and surveillance,” the authors wrote.
Estrogen is known to play a role in the development of lung cancer by activating the epidermal growth factor receptor (EGFR). Previous research has shown an increased risk of lung cancer in patients with estrogen receptor–negative, progesterone receptor–negative, HER2-negative, or triple-negative breast cancer.
“Antiestrogen treatment has been demonstrated to decrease the incidence of lung cancer and has been associated with improved long-term survival in patients with lung cancer after breast cancer. Future studies should seek to identify high-risk populations on the basis of hormone receptor status and antiestrogen therapy use,” the authors wrote.
The authors noted a number of limitations to the study, including the single hospital as the sole source of data, plus, the analysis did not account for the length of time since patients quit smoking and a lung cancer diagnosis. Nor did it consider other risk factors, such as radiation, chemotherapy, or antiestrogen therapies.
The authors did not disclose any study-related conflicts of interests.
This article was updated 3/2/22.
FROM BJS OPEN
Children and COVID: New cases down to pre-Omicron level
New cases of COVID-19 in U.S. children dropped for the fifth consecutive week, but the rate of decline slowed considerably, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The national count of new cases has now fallen for five straight weeks since peaking Jan. 14-20, and this week’s figure is the lowest since the pre-Omicron days of mid-November, based on data collected by the AAP and CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.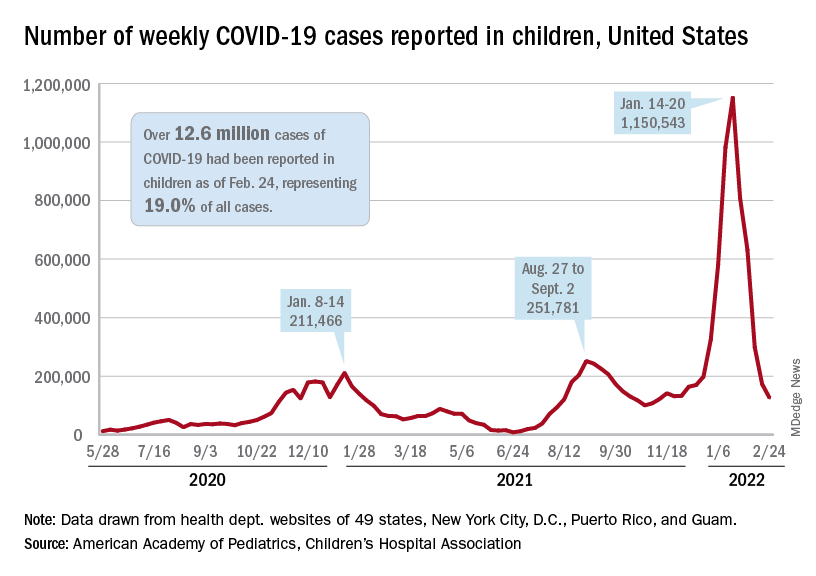
Over 12.6 million pediatric cases have been reported by those jurisdictions since the start of the pandemic, representing 19.0% of all cases in the United States, the AAP and CHA said in their weekly COVID report.
The highest cumulative rate among the states, 27.5%, can be found in Vermont, followed by New Hampshire (26.7%) and Alaska (26.6%). Alabama’s 12.1% is lower than any other jurisdiction, but the state stopped reporting during the summer of 2021, just as the Delta surge was beginning. The next two lowest states, Florida (12.8%) and Utah (13.9%), both define children as those aged 0-14 years, so the state with the lowest rate and no qualifiers is Idaho at 14.3%, the AAP/CHA data show.
The downward trend in new cases is reflected in other national measures. The daily rate of new hospital admissions for children aged 0-17 years was 0.32 per 100,000 population on Feb. 26, which is a drop of 75% since admissions peaked at 1.25 per 100,000 on Jan. 15, according to the Centers for Disease Control and Prevention.
The most recent 7-day average (Feb. 20-26) for child admissions with confirmed COVID-19 was 237 per day, compared with 914 per day during the peak week of Jan. 10-16. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits by age group, are down even more. The 7-day average was 1.2% on Feb. 25 for children aged 0-11 years, compared with a peak of 13.9% in mid-January, the CDC said on its COVID Data Tracker. The current rates for older children are even lower.
The decline of the Omicron surge over the last few weeks is allowing states to end mask mandates in schools around the country. The governors of California, Oregon, and Washington just announced that their states will be lifting their mask requirements on March 11, and New York State will end its mandate on March 2, while New York City is scheduled to go mask-free as of March 7, according to District Administration.
Those types of government moves, however, do not seem to be entirely supported by the public. In a survey conducted Feb. 9-21 by the Kaiser Family Foundation, 43% of the 1,502 respondents said that all students and staff should be required to wear masks in schools, while 40% said that there should be no mask requirements at all.
New cases of COVID-19 in U.S. children dropped for the fifth consecutive week, but the rate of decline slowed considerably, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The national count of new cases has now fallen for five straight weeks since peaking Jan. 14-20, and this week’s figure is the lowest since the pre-Omicron days of mid-November, based on data collected by the AAP and CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Over 12.6 million pediatric cases have been reported by those jurisdictions since the start of the pandemic, representing 19.0% of all cases in the United States, the AAP and CHA said in their weekly COVID report.
The highest cumulative rate among the states, 27.5%, can be found in Vermont, followed by New Hampshire (26.7%) and Alaska (26.6%). Alabama’s 12.1% is lower than any other jurisdiction, but the state stopped reporting during the summer of 2021, just as the Delta surge was beginning. The next two lowest states, Florida (12.8%) and Utah (13.9%), both define children as those aged 0-14 years, so the state with the lowest rate and no qualifiers is Idaho at 14.3%, the AAP/CHA data show.
The downward trend in new cases is reflected in other national measures. The daily rate of new hospital admissions for children aged 0-17 years was 0.32 per 100,000 population on Feb. 26, which is a drop of 75% since admissions peaked at 1.25 per 100,000 on Jan. 15, according to the Centers for Disease Control and Prevention.
The most recent 7-day average (Feb. 20-26) for child admissions with confirmed COVID-19 was 237 per day, compared with 914 per day during the peak week of Jan. 10-16. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits by age group, are down even more. The 7-day average was 1.2% on Feb. 25 for children aged 0-11 years, compared with a peak of 13.9% in mid-January, the CDC said on its COVID Data Tracker. The current rates for older children are even lower.
The decline of the Omicron surge over the last few weeks is allowing states to end mask mandates in schools around the country. The governors of California, Oregon, and Washington just announced that their states will be lifting their mask requirements on March 11, and New York State will end its mandate on March 2, while New York City is scheduled to go mask-free as of March 7, according to District Administration.
Those types of government moves, however, do not seem to be entirely supported by the public. In a survey conducted Feb. 9-21 by the Kaiser Family Foundation, 43% of the 1,502 respondents said that all students and staff should be required to wear masks in schools, while 40% said that there should be no mask requirements at all.
New cases of COVID-19 in U.S. children dropped for the fifth consecutive week, but the rate of decline slowed considerably, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The national count of new cases has now fallen for five straight weeks since peaking Jan. 14-20, and this week’s figure is the lowest since the pre-Omicron days of mid-November, based on data collected by the AAP and CHA from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Over 12.6 million pediatric cases have been reported by those jurisdictions since the start of the pandemic, representing 19.0% of all cases in the United States, the AAP and CHA said in their weekly COVID report.
The highest cumulative rate among the states, 27.5%, can be found in Vermont, followed by New Hampshire (26.7%) and Alaska (26.6%). Alabama’s 12.1% is lower than any other jurisdiction, but the state stopped reporting during the summer of 2021, just as the Delta surge was beginning. The next two lowest states, Florida (12.8%) and Utah (13.9%), both define children as those aged 0-14 years, so the state with the lowest rate and no qualifiers is Idaho at 14.3%, the AAP/CHA data show.
The downward trend in new cases is reflected in other national measures. The daily rate of new hospital admissions for children aged 0-17 years was 0.32 per 100,000 population on Feb. 26, which is a drop of 75% since admissions peaked at 1.25 per 100,000 on Jan. 15, according to the Centers for Disease Control and Prevention.
The most recent 7-day average (Feb. 20-26) for child admissions with confirmed COVID-19 was 237 per day, compared with 914 per day during the peak week of Jan. 10-16. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits by age group, are down even more. The 7-day average was 1.2% on Feb. 25 for children aged 0-11 years, compared with a peak of 13.9% in mid-January, the CDC said on its COVID Data Tracker. The current rates for older children are even lower.
The decline of the Omicron surge over the last few weeks is allowing states to end mask mandates in schools around the country. The governors of California, Oregon, and Washington just announced that their states will be lifting their mask requirements on March 11, and New York State will end its mandate on March 2, while New York City is scheduled to go mask-free as of March 7, according to District Administration.
Those types of government moves, however, do not seem to be entirely supported by the public. In a survey conducted Feb. 9-21 by the Kaiser Family Foundation, 43% of the 1,502 respondents said that all students and staff should be required to wear masks in schools, while 40% said that there should be no mask requirements at all.
Elective surgery should be delayed 7 weeks after COVID-19 infection for unvaccinated patients, statement recommends
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
Lung cancer drug price trends cause alarm, highlight need for reform
The findings underscore the need for price reform, according to the investigators, who analyzed prices for 17 brand-name medications used for treating metastatic non–small cell lung cancer (NSCLC).
Prices increased during the study period and correlated within each drug class, Aakash Desai, MBBS, and colleagues from the Mayo Clinic, Rochester, Minn., found.
“Because numerous new drugs have been approved for the treatment of NSCLC in recent years, we sought to specifically study the price competition among drugs used to treat this cancer subtype,” they explained, noting that for most drug classes price increases outpaced changes in the consumer price index for prescription medications and the inflation rate.
The findings were published Jan. 25, 2022, in JAMA Network Open.
Multiple brand-name medications across several drug classes, including four immune checkpoint inhibitors (pembrolizumab, nivolumab, atezolizumab, and durvalumab), five epidermal growth factor receptor inhibitors (gefitinib, afatinib, erlotinib, osimertinib, and dacomitinib), five anaplastic lymphoma kinase inhibitors (crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib), two BRAF inhibitors (dabrafenib, vemurafenib), and one MEK inhibitor (trametinib) were included in the analysis.
Median Pearson correlation coefficients approached 1.0 for all drug classes, indicating that prices increased despite within-class drug competition. Median values ranged from 0.898 for epidermal growth factor inhibitors to 0.999 for anaplastic lymphoma kinase inhibitors and BRAF and MEK inhibitors, the investigators found.
Median compounded annual growth rates (CAGRs) were 1.81% for immune checkpoint inhibitors, 2.56% for epidermal growth factor receptor inhibitors, 2.46% for anaplastic lymphoma kinase and ROS1 inhibitors, and 3.06% for BRAF and MEK inhibitors.
“With the exception of the immunotherapy class, the median cost CAGR outpaced the annual growth rate of the consumer price index for prescription drugs at 2.10% and, for all classes, the average yearly inflation rate of 1.75% during the same period,” they wrote.
Also of note, only one price decrease occurred among all therapeutic classes studied.
“This was observed for erlotinib between 2019 and 2020, and it corresponded with the introduction of a generic competitor to the market,” the authors said.
The findings are reminiscent of an earlier study that showed a 25% increase in the price of 24 patented injectable anticancer agents in the United States over a period of 8 years after launch.
“These increases in cost were not offset by supplemental U.S. Food and Drug Administration approvals, new competitors, or new off-label indications. Thus, price increases over time were not substantially reduced by market competition and increased at similar rates among drugs within the same class,” they wrote, adding that “although one might expect oncology drug prices to decrease over time after market entry, the list price of most anticancer agents increases paradoxically.”
The “lock-step price increases” observed without evidence of price competition in this analysis raise concerns about the affordability of promising oncology drugs, they said, concluding that “academic, industry, and government partnerships should be developed to address the high costs of prescription oncology drugs, which may soon be unaffordable for most patients if the trends discovered in the present study continue.”
Dr. Desai reported having no disclosures.
The findings underscore the need for price reform, according to the investigators, who analyzed prices for 17 brand-name medications used for treating metastatic non–small cell lung cancer (NSCLC).
Prices increased during the study period and correlated within each drug class, Aakash Desai, MBBS, and colleagues from the Mayo Clinic, Rochester, Minn., found.
“Because numerous new drugs have been approved for the treatment of NSCLC in recent years, we sought to specifically study the price competition among drugs used to treat this cancer subtype,” they explained, noting that for most drug classes price increases outpaced changes in the consumer price index for prescription medications and the inflation rate.
The findings were published Jan. 25, 2022, in JAMA Network Open.
Multiple brand-name medications across several drug classes, including four immune checkpoint inhibitors (pembrolizumab, nivolumab, atezolizumab, and durvalumab), five epidermal growth factor receptor inhibitors (gefitinib, afatinib, erlotinib, osimertinib, and dacomitinib), five anaplastic lymphoma kinase inhibitors (crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib), two BRAF inhibitors (dabrafenib, vemurafenib), and one MEK inhibitor (trametinib) were included in the analysis.
Median Pearson correlation coefficients approached 1.0 for all drug classes, indicating that prices increased despite within-class drug competition. Median values ranged from 0.898 for epidermal growth factor inhibitors to 0.999 for anaplastic lymphoma kinase inhibitors and BRAF and MEK inhibitors, the investigators found.
Median compounded annual growth rates (CAGRs) were 1.81% for immune checkpoint inhibitors, 2.56% for epidermal growth factor receptor inhibitors, 2.46% for anaplastic lymphoma kinase and ROS1 inhibitors, and 3.06% for BRAF and MEK inhibitors.
“With the exception of the immunotherapy class, the median cost CAGR outpaced the annual growth rate of the consumer price index for prescription drugs at 2.10% and, for all classes, the average yearly inflation rate of 1.75% during the same period,” they wrote.
Also of note, only one price decrease occurred among all therapeutic classes studied.
“This was observed for erlotinib between 2019 and 2020, and it corresponded with the introduction of a generic competitor to the market,” the authors said.
The findings are reminiscent of an earlier study that showed a 25% increase in the price of 24 patented injectable anticancer agents in the United States over a period of 8 years after launch.
“These increases in cost were not offset by supplemental U.S. Food and Drug Administration approvals, new competitors, or new off-label indications. Thus, price increases over time were not substantially reduced by market competition and increased at similar rates among drugs within the same class,” they wrote, adding that “although one might expect oncology drug prices to decrease over time after market entry, the list price of most anticancer agents increases paradoxically.”
The “lock-step price increases” observed without evidence of price competition in this analysis raise concerns about the affordability of promising oncology drugs, they said, concluding that “academic, industry, and government partnerships should be developed to address the high costs of prescription oncology drugs, which may soon be unaffordable for most patients if the trends discovered in the present study continue.”
Dr. Desai reported having no disclosures.
The findings underscore the need for price reform, according to the investigators, who analyzed prices for 17 brand-name medications used for treating metastatic non–small cell lung cancer (NSCLC).
Prices increased during the study period and correlated within each drug class, Aakash Desai, MBBS, and colleagues from the Mayo Clinic, Rochester, Minn., found.
“Because numerous new drugs have been approved for the treatment of NSCLC in recent years, we sought to specifically study the price competition among drugs used to treat this cancer subtype,” they explained, noting that for most drug classes price increases outpaced changes in the consumer price index for prescription medications and the inflation rate.
The findings were published Jan. 25, 2022, in JAMA Network Open.
Multiple brand-name medications across several drug classes, including four immune checkpoint inhibitors (pembrolizumab, nivolumab, atezolizumab, and durvalumab), five epidermal growth factor receptor inhibitors (gefitinib, afatinib, erlotinib, osimertinib, and dacomitinib), five anaplastic lymphoma kinase inhibitors (crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib), two BRAF inhibitors (dabrafenib, vemurafenib), and one MEK inhibitor (trametinib) were included in the analysis.
Median Pearson correlation coefficients approached 1.0 for all drug classes, indicating that prices increased despite within-class drug competition. Median values ranged from 0.898 for epidermal growth factor inhibitors to 0.999 for anaplastic lymphoma kinase inhibitors and BRAF and MEK inhibitors, the investigators found.
Median compounded annual growth rates (CAGRs) were 1.81% for immune checkpoint inhibitors, 2.56% for epidermal growth factor receptor inhibitors, 2.46% for anaplastic lymphoma kinase and ROS1 inhibitors, and 3.06% for BRAF and MEK inhibitors.
“With the exception of the immunotherapy class, the median cost CAGR outpaced the annual growth rate of the consumer price index for prescription drugs at 2.10% and, for all classes, the average yearly inflation rate of 1.75% during the same period,” they wrote.
Also of note, only one price decrease occurred among all therapeutic classes studied.
“This was observed for erlotinib between 2019 and 2020, and it corresponded with the introduction of a generic competitor to the market,” the authors said.
The findings are reminiscent of an earlier study that showed a 25% increase in the price of 24 patented injectable anticancer agents in the United States over a period of 8 years after launch.
“These increases in cost were not offset by supplemental U.S. Food and Drug Administration approvals, new competitors, or new off-label indications. Thus, price increases over time were not substantially reduced by market competition and increased at similar rates among drugs within the same class,” they wrote, adding that “although one might expect oncology drug prices to decrease over time after market entry, the list price of most anticancer agents increases paradoxically.”
The “lock-step price increases” observed without evidence of price competition in this analysis raise concerns about the affordability of promising oncology drugs, they said, concluding that “academic, industry, and government partnerships should be developed to address the high costs of prescription oncology drugs, which may soon be unaffordable for most patients if the trends discovered in the present study continue.”
Dr. Desai reported having no disclosures.
FROM JAMA NETWORK OPEN
Nasal microbiota show promise as polyp predictor
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease shows steady increase in U.S. over 15+ years
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
COVID-19 vaccines do not trigger sudden hearing loss: Study
Anecdotal reports have linked the vaccines against COVID-19 to the sudden loss of hearing in some people. But a new study has found no evidence for such a connection with any of the three approved shots.
The analysis of data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System (VAERS) found that
“We’re not finding a signal,” said Eric J. Formeister, MD, a neurotology fellow at the Johns Hopkins University, Baltimore, and the first author of the U.S. study, which appeared Feb. 24 in JAMA Otolaryngology – Head and Neck Surgery.
Dr. Formeister and colleagues undertook the study in response to reports of hearing problems, including hearing loss and tinnitus, that occurred soon after COVID-19 vaccination.
They analyzed reports of sudden hearing loss, experienced within 21 days of vaccination, logged in VAERS. Anyone can report a potential event to the database, which does not require medical documentation in support of the adverse event. To minimize potential misdiagnoses, Dr. Formeister and colleagues reviewed only those reports that indicated that a doctor had diagnosed sudden hearing loss, leaving 555 cases (305 in women; mean age 54 years) between December 2020 and July 2021.
Dividing these reports by the total doses of vaccines administered in the United States during that period yielded an incidence rate of 0.6 cases of sudden hearing loss for every 100,000 people, Dr. Formeister and colleagues reported.
When the researchers divided all cases of hearing loss in the VAERS database (2,170) by the number of people who had received two doses of vaccine, the incidence rate increased to 28 per 100,000 people. For comparison, the authors reported, the incidence of sudden hearing loss within the United States population is between 11 and 77 per 100,000 people, depending on age.
“There was not an increase in cases of sudden [sensorineural] hearing loss associated with COVID-19 vaccination compared to previously published reports before the COVID-19 vaccination era,” study coauthor Elliott D. Kozin, MD, assistant professor of otolaryngology–head and neck surgery at Harvard Medical School, Boston, said in an interview.
Another reassuring sign: If hearing loss were linked to the vaccines, the researchers said, they would expect to see an increase in the number of complaints in lockstep with an increase in the number of doses administered. However, the opposite was true. “[T]he rate of reports per 100,000 doses decreased across the vaccination period, despite large concomitant increases in the absolute number of vaccine doses administered per week,” the researchers reported.
They also looked at case reports of 21 men and women who had experienced sudden hearing loss after having received COVID-19 vaccines, to see if they could discern any clinically relevant signs of people most likely to experience the adverse event. However, the group had a range of preexisting conditions and varying times after receiving a vaccine when their hearing loss occurred, leading Dr. Formeister’s team to conclude that they could find no clear markers of risk.
“When we examined patients across several institutions, there was no obvious pattern. The patient demographics and clinical findings were variable,” Dr. Kozin said. A provisional interpretation of this data, he added, is that no link exists between COVID-19 vaccination and predictable hearing deficits, although the analysis covered a small number of patients.
“Association does not necessarily imply a causal relationship,” said Michael Brenner, MD, FACS, associate professor of otolaryngology–head and neck surgery at the University of Michigan, Ann Arbor. Dr. Brenner, who was not involved in the study, said any hearing loss attributed to the COVID-19 vaccines could have had other causes besides the injections.
But a second study, also published in JAMA Otolaryngology – Head and Neck Surgery on Feb. 24, leaves open the possibility of a link. Researchers in Israel looked for increases in steroid prescriptions used to treat sudden hearing loss as vaccination with the Pfizer version of the shot became widespread in that country. Their conclusion: The vaccine might be associated with a slightly increased risk of sudden hearing loss, although if so, that risk is likely “very small” and the benefits of vaccination “outweigh its potential association” with the side effect.
Dr. Brenner agreed. “The evidence supports [the] clear public health benefit of COVID-19 vaccination, and the scale of those benefits dwarfs associations with hearing, which are of uncertain significance,” he said.
A version of this article first appeared on Medscape.com.
Anecdotal reports have linked the vaccines against COVID-19 to the sudden loss of hearing in some people. But a new study has found no evidence for such a connection with any of the three approved shots.
The analysis of data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System (VAERS) found that
“We’re not finding a signal,” said Eric J. Formeister, MD, a neurotology fellow at the Johns Hopkins University, Baltimore, and the first author of the U.S. study, which appeared Feb. 24 in JAMA Otolaryngology – Head and Neck Surgery.
Dr. Formeister and colleagues undertook the study in response to reports of hearing problems, including hearing loss and tinnitus, that occurred soon after COVID-19 vaccination.
They analyzed reports of sudden hearing loss, experienced within 21 days of vaccination, logged in VAERS. Anyone can report a potential event to the database, which does not require medical documentation in support of the adverse event. To minimize potential misdiagnoses, Dr. Formeister and colleagues reviewed only those reports that indicated that a doctor had diagnosed sudden hearing loss, leaving 555 cases (305 in women; mean age 54 years) between December 2020 and July 2021.
Dividing these reports by the total doses of vaccines administered in the United States during that period yielded an incidence rate of 0.6 cases of sudden hearing loss for every 100,000 people, Dr. Formeister and colleagues reported.
When the researchers divided all cases of hearing loss in the VAERS database (2,170) by the number of people who had received two doses of vaccine, the incidence rate increased to 28 per 100,000 people. For comparison, the authors reported, the incidence of sudden hearing loss within the United States population is between 11 and 77 per 100,000 people, depending on age.
“There was not an increase in cases of sudden [sensorineural] hearing loss associated with COVID-19 vaccination compared to previously published reports before the COVID-19 vaccination era,” study coauthor Elliott D. Kozin, MD, assistant professor of otolaryngology–head and neck surgery at Harvard Medical School, Boston, said in an interview.
Another reassuring sign: If hearing loss were linked to the vaccines, the researchers said, they would expect to see an increase in the number of complaints in lockstep with an increase in the number of doses administered. However, the opposite was true. “[T]he rate of reports per 100,000 doses decreased across the vaccination period, despite large concomitant increases in the absolute number of vaccine doses administered per week,” the researchers reported.
They also looked at case reports of 21 men and women who had experienced sudden hearing loss after having received COVID-19 vaccines, to see if they could discern any clinically relevant signs of people most likely to experience the adverse event. However, the group had a range of preexisting conditions and varying times after receiving a vaccine when their hearing loss occurred, leading Dr. Formeister’s team to conclude that they could find no clear markers of risk.
“When we examined patients across several institutions, there was no obvious pattern. The patient demographics and clinical findings were variable,” Dr. Kozin said. A provisional interpretation of this data, he added, is that no link exists between COVID-19 vaccination and predictable hearing deficits, although the analysis covered a small number of patients.
“Association does not necessarily imply a causal relationship,” said Michael Brenner, MD, FACS, associate professor of otolaryngology–head and neck surgery at the University of Michigan, Ann Arbor. Dr. Brenner, who was not involved in the study, said any hearing loss attributed to the COVID-19 vaccines could have had other causes besides the injections.
But a second study, also published in JAMA Otolaryngology – Head and Neck Surgery on Feb. 24, leaves open the possibility of a link. Researchers in Israel looked for increases in steroid prescriptions used to treat sudden hearing loss as vaccination with the Pfizer version of the shot became widespread in that country. Their conclusion: The vaccine might be associated with a slightly increased risk of sudden hearing loss, although if so, that risk is likely “very small” and the benefits of vaccination “outweigh its potential association” with the side effect.
Dr. Brenner agreed. “The evidence supports [the] clear public health benefit of COVID-19 vaccination, and the scale of those benefits dwarfs associations with hearing, which are of uncertain significance,” he said.
A version of this article first appeared on Medscape.com.
Anecdotal reports have linked the vaccines against COVID-19 to the sudden loss of hearing in some people. But a new study has found no evidence for such a connection with any of the three approved shots.
The analysis of data from the Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System (VAERS) found that
“We’re not finding a signal,” said Eric J. Formeister, MD, a neurotology fellow at the Johns Hopkins University, Baltimore, and the first author of the U.S. study, which appeared Feb. 24 in JAMA Otolaryngology – Head and Neck Surgery.
Dr. Formeister and colleagues undertook the study in response to reports of hearing problems, including hearing loss and tinnitus, that occurred soon after COVID-19 vaccination.
They analyzed reports of sudden hearing loss, experienced within 21 days of vaccination, logged in VAERS. Anyone can report a potential event to the database, which does not require medical documentation in support of the adverse event. To minimize potential misdiagnoses, Dr. Formeister and colleagues reviewed only those reports that indicated that a doctor had diagnosed sudden hearing loss, leaving 555 cases (305 in women; mean age 54 years) between December 2020 and July 2021.
Dividing these reports by the total doses of vaccines administered in the United States during that period yielded an incidence rate of 0.6 cases of sudden hearing loss for every 100,000 people, Dr. Formeister and colleagues reported.
When the researchers divided all cases of hearing loss in the VAERS database (2,170) by the number of people who had received two doses of vaccine, the incidence rate increased to 28 per 100,000 people. For comparison, the authors reported, the incidence of sudden hearing loss within the United States population is between 11 and 77 per 100,000 people, depending on age.
“There was not an increase in cases of sudden [sensorineural] hearing loss associated with COVID-19 vaccination compared to previously published reports before the COVID-19 vaccination era,” study coauthor Elliott D. Kozin, MD, assistant professor of otolaryngology–head and neck surgery at Harvard Medical School, Boston, said in an interview.
Another reassuring sign: If hearing loss were linked to the vaccines, the researchers said, they would expect to see an increase in the number of complaints in lockstep with an increase in the number of doses administered. However, the opposite was true. “[T]he rate of reports per 100,000 doses decreased across the vaccination period, despite large concomitant increases in the absolute number of vaccine doses administered per week,” the researchers reported.
They also looked at case reports of 21 men and women who had experienced sudden hearing loss after having received COVID-19 vaccines, to see if they could discern any clinically relevant signs of people most likely to experience the adverse event. However, the group had a range of preexisting conditions and varying times after receiving a vaccine when their hearing loss occurred, leading Dr. Formeister’s team to conclude that they could find no clear markers of risk.
“When we examined patients across several institutions, there was no obvious pattern. The patient demographics and clinical findings were variable,” Dr. Kozin said. A provisional interpretation of this data, he added, is that no link exists between COVID-19 vaccination and predictable hearing deficits, although the analysis covered a small number of patients.
“Association does not necessarily imply a causal relationship,” said Michael Brenner, MD, FACS, associate professor of otolaryngology–head and neck surgery at the University of Michigan, Ann Arbor. Dr. Brenner, who was not involved in the study, said any hearing loss attributed to the COVID-19 vaccines could have had other causes besides the injections.
But a second study, also published in JAMA Otolaryngology – Head and Neck Surgery on Feb. 24, leaves open the possibility of a link. Researchers in Israel looked for increases in steroid prescriptions used to treat sudden hearing loss as vaccination with the Pfizer version of the shot became widespread in that country. Their conclusion: The vaccine might be associated with a slightly increased risk of sudden hearing loss, although if so, that risk is likely “very small” and the benefits of vaccination “outweigh its potential association” with the side effect.
Dr. Brenner agreed. “The evidence supports [the] clear public health benefit of COVID-19 vaccination, and the scale of those benefits dwarfs associations with hearing, which are of uncertain significance,” he said.
A version of this article first appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY – HEAD AND NECK SURGERY
Ukrainian physicians ‘ready to die for their freedom’
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Nasogastric tubes. Foley catheter kits. Hydrogel anti-burn bandages and transfusion bags. Heparin, atropine, tramadol.
These items are just a few of some two dozen critical medical supplies that physicians in Ukraine desperately need, according to Leo Wolansky, MD, a Ukrainian-American radiologist and president of the Ukrainian Medical Association of North America (UMANA).
Dr. Wolansky founded a teaching program with an organization called Friends of Radiology in Ukraine in 1996 and has been running courses for specialists there ever since. He last visited the country in 2019, before the COVID-19 pandemic, but has remained in contact with his medical colleagues by phone and email. Over the weekend of Feb. 26-27, UMANA held a fundraiser for Ukraine, raising more than $17,000.
Question: Where is your family from, and do you have relatives in the country now?
Dr. Wolansky: My family is from two different parts of Ukraine. My mother was from central Ukraine. Her father, Ivan Sharyj, was part of the students’ militia that fought at the famous battle of Kruty in 1918. Four hundred Ukrainian militia fought against 5,000 professional Russian soldiers and were massacred. He later wrote the first eye-witness account. Afterwards, he had the opportunity to flee Ukraine but chose to stay under a pseudonym. Eventually, during Stalin’s purges [1929-1933], the regime found him, arrested him, tortured him, and executed him. My mother was seven when she saw her father arrested, never to return home. My father was from Western Ukraine, which did not have a long history of Russian occupation. His mother’s family was very patriotic; her first cousin, Stepan Vytvytskyi, eventually became the president of Ukraine in exile from 1955-1964.
I have second and more distant cousins in Kyiv. My wife has first cousins in Western Ukraine. They and my doctor colleagues are suffering greatly but are ready to die for their freedom.
Question: The Russian invasion of Ukraine has put tremendous stress on the Ukrainian people, including the country’s medical professionals. How do doctors in these kinds of situations handle casualties they can’t prevent? How do they work around that sense that everything is out of their control?
Dr. Wolansky: A lot of infrastructural things are being disrupted; there are limitations that you wouldn’t normally encounter. Ukraine has been developing a lot of sophisticated medical technology, but it still has room to grow. Under these circumstances, when there are bombs going off and transportation is being disrupted, it creates very new and significant obstacles to surmount. It still has not risen to massive casualties, and we can just pray that it does not, but in times of war, a very different kind of medicine is practiced.
But remember, Ukraine has been at war since 2014, when Russia took Crimea and invaded the Eastern provinces. The doctors there are not unfamiliar with war injuries. At our conferences in Ukraine, I have seen radiological presentations of injuries sustained in war – gunshots, fractures, and amputations – as well as other kinds of traumatic injuries. You’re going for a kind of more emergent treatment: to transfuse, to maintain peoples’ blood pressure, put bandages on, sterilize and sanitize wounds to prevent infections. I imagine there will be many field hospitals set up between now and the next few weeks to deal with the acute injuries.
Question: Ukraine has struggled with high rates of HIV and multidrug-resistant tuberculosis, as well as a lack of resources for treating patients with mental illness. Meanwhile, the country has had more that 5 million cases of COVID-19 and an estimated 112,000 deaths from the disease. Are you concerned about an exacerbation of infection rates, including of COVID, particularly among refugees and those who become homeless?
Dr. Wolansky: Because COVID ran pretty rampant in Ukraine, I think that – at a high cost – there is a level of natural immunity in the population. And the weather is going to be getting warmer soon, and respiratory viruses are cyclic in nature, so I don’t know if that’s going to be a big complicating factor. However, people get sick all the time, and the prognosis for them is going to be much worse than it otherwise might be. If you have a heart attack, your chances were way better when the roads were clear and people weren’t shooting at you.
Right now, it’s very regional where the infrastructure is being destroyed. The West, where I used to go, is in much better shape than the East because it has not been the focus of Russian attacks. But Kyiv could turn into a very big humanitarian crisis very quickly if there’s no electricity, no water. All sorts of medical conditions could be greatly exacerbated, and some new health crises could arise from water contamination, bombs causing buildings to collapse, and other problems. Whatever the illness is, it’s going to be harder to take care of it.
Questions: Doctors Without Borders announced that it was suspending its operations in Ukraine because of the invasion – missions that included HIV care in Severodonetsk, tuberculosis care in Zhytomyr, and improving health care access in Donetsk in eastern Ukraine, according to the aid group. What do doctors in Ukraine need most acutely now, other than peace?
Dr. Wolansky: Obviously, money is valuable, and military protection, which would prevent additional damage to their infrastructure. One thing that bears mentioning. There’s been a fair amount of coverage of this, but I’ve witnessed it first-hand: The Ukrainian people are fiercely patriotic, and there’s really no way their spirit can be conquered. The USSR invaded Afghanistan, and after years of thinking they were in command, they left because they could no longer take the guerilla warfare and the constant sniper attacks. Ukraine’s population is many times larger than Afghanistan’s; there’s no way they can be subdued. And remember, the Ukrainian people have been free for 30 years – generations of young people have known no other way of life. They are not going to give that up.
A version of this article first appeared on Medscape.com.
Older age for menopause raises risk for lung cancer
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.

