User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


How to make visits run more smoothly and be more productive
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
Most COVID-19 survivors return to work within 2 years
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
FROM THE LANCET RESPIRATORY MEDICINE
Myositis guidelines aim to standardize adult and pediatric care
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
FROM BSR 2022
Section reports
Pulmonary vascular & cardiovascular network: Cardiovascular medicine & surgery section
Targeted temperature management (TTM) after cardiac arrest: How cool?
Recent randomized control trials, TTM2 (Dankiewicz J. N Engl J Med. 2021;384:2283) and HYPERION (Lascarrou J-B. N Engl J Med. 2019;381:2327), of therapeutic hypothermia, as opposed to normothermia, in patients who remain comatose after return of spontaneous circulation (ROSC) after cardiac arrest have produced conflicting results regarding survival and neurologic benefit. TTM2 reported no benefit to cooling to 33°C, while HYPERION found improved neurologic outcome at 90 days in patients cooled to 33°C. The European Resuscitation Council (ERC) and European Society of Intensive Care Medicine (ESICM) recently released an evidence review and guideline for adults who remain comatose after cardiac arrest (Sandroni C. Intensive Care Med. 2022;48:261). These guidelines recommend continuous monitoring of core temperature in all patients who remain comatose after cardiac arrest, and preventing fever (>37.7°C) for 72 hours, but with no recommendation of target temperature of 32°C vs 36°C.
Differences in patient populations, presenting rhythm during arrest, duration of CPR, and time to target temperature likely each contribute to the disparate conclusions of previous trials. For example, HYPERION enrolled patients with out of hospital cardiac arrest with initial nonshockable rhythms and found benefit to cooling to 33°C. In comparison, TTM2 enrolled all patients with ROSC following arrest (regardless of rhythm), including patients with in-hospital cardiac arrest and found no benefit in therapeutic cooling. Differences in patient populations are underscored by the widely differing percentage of patients with good neurologic outcome in their respective control groups: approximately 30% in the TTM2 trial and 6% in HYPERION. The guidelines leave significant room for clinical judgment in employing therapeutic cooling but encourage the continuous monitoring of core temperature and active avoidance of fever.
Fiore Mastroianna, MD
Section Member-at-Large
Chest infections & disaster response network: Chest infections section
Update on LTBI treatment: Ensuring success by simplifying, shortening, and completing treatment
My patient has a positive IGRA test result – what’s next?
If TB disease is ruled out by clinical, radiographic, and microbiologic assessment (if indicated), then latent TB infection (LTBI) is established, and treatment should be offered, guided by shared-decision making between provider and patient.
What options are available?
While the former standard 9-month regimen of isoniazid-monotherapy can be shortened to 6 months, shorter rifamycin-based regimens are now preferred in most cases and include:
4 months rifampin daily, 3 months isoniazid plus rifampin daily, or 3 months isoniazid plus rifapentine weekly. In addition, 1 month of isoniazid plus rifapentine daily has recently been shown to be effective in people with HIV.
How to choose?
Rifamycin-based regimens have been shown to have less hepatotoxicity and higher completion rates. Drug-drug interactions are of potential concern, for example, in patients receiving anticoagulation or treatment for HIV. The clinician should be aware of rifamycins causing a flu-like illness that may be treatment-limiting. In patients with known contact to drug-resistant TB, regimens are individualized.
How to monitor?
Adherence and completion are the keys to success. Directly observed therapy may be indicated in certain scenarios. Baseline and monthly blood work is recommended for people with risk factors for hepatic or bone marrow toxicity. More importantly, patients should be instructed to discontinue LTBI medications and call the clinician with any new symptoms. HIV testing should be offered to all patients if status is unknown. Clinicians are encouraged to reach out to one of four regional TB Centers of Excellence for guidance.
Sebastian Kurz, MD, FCCP
Amee Patrawalla, MD, MPH, FCCP
Section Members-at-Large
References
Testing and Treatment of Latent Tuberculosis Infection in the United States: Clinical Recommendations. A Guide for Health Care Providers and Public Health Programs. Copyright © 2021 by the National Society of Tuberculosis Clinicians and National Tuberculosis Controllers Association
1. Shah, D. Latent tuberculosis infection. N Engl J Med. 2021;385:2271-80.
2. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):111-115.
3. Swindells et. al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380:1001-11.
Thoracic oncology & chest procedures network: Lung cancer section
Adjuvant and neoadjuvant therapies in early stage lung cancer
Since the discovery of the epidermal growth factor receptor (EGFR) mutation in 2004 and the development of checkpoint blockade in 2006, personalized treatment options for non–small cell lung cancer (NSCLC) have exploded, but targeted systemic therapy medications were only recommended among patients with metastatic or locally advanced disease (Rivera MP, Matthay RA. Clin Chest Med. 2020;41[1]:ix-xi). However, in November 2020, the National Comprehensive Cancer Network (NCCN) updated guidelines to recommend EGFR testing in surgically resected stage IB-IIIA adenocarcinoma, and to consider adjuvant osimerintib in those who were mutation-positive (NCCN. Nov 2020). Interim analysis of an ongoing phase-3 trial showed 89% of patients in the osimertinib group were alive and disease-free at 24 months compared with 52% in the placebo group (hazard ratio 0.20, P < .001) (Wu YL, et al. N Engl J Med. 2020;383[18]:1711-23).
The FDA has also recently approved the use of neoadjuvant and adjuvant immunotherapy in combination with platinum-based chemotherapy. Nivolumab is now approved as neoadjuvant therapy in patients with resectable IB-IIIA NSCLC regardless of PDL-1 status. The Checkmate-816 trial showed increased median event-free survival in the immunotherapy plus chemotherapy group of 31.6 months vs 20.8 months in the chemotherapy-only group (FDA.gov. 2022, Mar 4). Atezolizumab is also now approved for adjuvant treatment following resection and platinum-based chemotherapy in patients with stage II to IIIA NSCLC whose tumors have PD-L1 expression on ≥ 1% of tumor cells. Median disease-free survival was not reached in patients in the atezolizumab groups vs 35.3 months in the best supportive care group (FDA.gov. 2021, Oct 15). With so many advances in the personalized treatment among all stages of NSCLC, this is a hopeful new chapter in the care of patients with NSCLC.
More information: https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManager?fileManagerId=11259
Sohini Ghosh, MD
Section Member-at-Large
Diffuse lung disease and lung transplant network: Lung transplant section
Continuous distribution for lung transplant: Overhauling the wait list
Determining how to allocate the scarce resource of donor lungs to patients is a difficult task and evaluated continuously for potential improvement. Since 2005, in the United States, lung transplant recipients have been selected based primarily on location within a Donor Service Area and by lung allocation score (LAS), a composite score of urgency for transplant. This was updated in 2017 to an allocation by highest LAS within 250 nautical miles from the donor hospital. Factors such as blood type compatibility and height are also considered. Implementation of the LAS improved the sickest patients’ access to transplants while not worsening 1-year mortality (Egan TM. Semin Respir Crit Care Med. 2018;39[02]:126-37). Unfortunately, geographic hard boundaries mean a high proportion of low LAS (<50) patients receive local donors while high LAS patients receive national offers or die while on the waitlist (Iribarne A, et al. Clin Transplant. 2016:30:688-93).
A new model that employs continuous distribution has been developed based on concerns regarding equity and improving allocation. This model would prioritize patients based on factors including medical priority, efficient management of organ placement (distance), expected posttransplant outcomes, and patient access (equity). By creating a composite of these without a geographic boundary, patients would be considered more on urgency within realistic constraints of distance and outcomes.
The Organ Procurement and Transplantation Network has officially approved continuous distribution, with implementation planned for 2022; details regarding the new scoring system are to be published and further research will need to be undertaken to determine if it meets the goal of overall improvement in patient access, equity, and outcomes.
Grant A. Turner, MD, MHA
Laura Frye, MD
Section Members-at-Large
Critical care network: Non-respiratory critical care section
Update from the non-respiratory critical care section
As you’ve probably noticed, there have been some changes here at CHEST involving the Networks. Leadership here at CHEST has been hard at work restructuring the networks to make them more closely aligned with relevant clinical disciplines, and, ultimately, allow for greater participation. I am proud to have been given stewardship of the new Non-Respiratory Critical Care Section of the Critical Care Network.
So, what exactly is Non-Respiratory Critical Care? Well, that’s where I need your help. You see this network is meant to reflect the needs and wants of CHEST members like you. We need you, dear readers, to join in this venture and help us guide the content that this section will ultimately create for our members.
If you’re interested in critical care, but you don’t see your particular area of interest anywhere else in the current structure ... guess what? You’ve found the right place!
My Infectious Diseases and Nephro peeps? Welcome! Are you a surgical or anesthesia intensivist? Don’t be shy. ECMO people, let’s hear some chatter!Is therapeutic hypothermia your thing? Come on in. The water’s freezing. 33 degrees just like you folks like it. Or is it 36? Not sure. Anyway, see what I’m talking about? We really need your help!You can get involved by clicking on the Membership & Community tab at the CHEST website. Once you’re a member, you can even nominate yourself to run for the Steering Committee elections which are held periodically. Hope to see you soon!
Deep Ramachandran, MD, FCCP
Section Chair
Sleep medicine network: Non-respiratory sleep section
Unusual suspects? Breakthrough in the treatment of idiopathic hypersomnia
Idiopathic hypersomnia (IH) is a rare and debilitating disorder defined by its excessive daytime sleepiness, sleep inertia, prolonged nighttime sleep, and long, unrefreshing naps (AASM. ICSD 3rd ed. 2014). Gamma-aminobutyric acid (GABA) is one of the main inhibitory neurotransmitters in the nervous system. It is through the potentiation of GABA that substances such as alcohol and benzodiazepines yield their effects. It is also hypothesized that the “brain fog” experienced in IH may be a consequence of either higher levels of an endogenous benzodiazepines in the cerebral spinal fluid or the presence of a GABA-enhancing peptide (Rye DB. Science Transl Med. 2012;Med 4:161ra151).
Sodium oxybate (SXB), a compound that likely has its therapeutic effect through the potentiation of GABA receptors, is an effective treatment option for cataplexy and sleepiness in narcolepsy. Although there may be some overlap between narcolepsy and IH in both diagnosis and treatment (Bassetti C, et al. Brain. 1997;120:1423), it would perhaps be entirely counterintuitive (given SXB’s pharmacology) to imagine using SXB as a plausible treatment option in IH. It was, however, investigated in the treatment of refractory hypersomnia and IH. In the retrospective study looking at 46 subjects treated with SXB, 71% experienced improvement of their severe sleep inertia, 55% had a decrease in their excessive daytime sleepiness, and 52% reported a shortened nighttime sleep time (Leu-Semenescu S, et al. Sleep Med. 2016;17:38).
In a recent double-blind, randomized control trial, the lower-sodium oxybate (LXB) was trialed in 154 patients with IH. It demonstrated statistically significant and clinically meaningful improvements (compared with placebo) in the Epworth Sleepiness Scale score (P <.0001) and in the Idiopathic Hypersomnia Severity Scale (P <.0001). The effects were seen both during the up titration of LXB and the benefits were maintained during the stable phase of the intervention (Dauvilliers Y, et al. Lancet Neurol. 2022;21(1):53). In August 2021, LXB (initially launched in 2020 for the treatment of narcolepsy) is now the first FDA-approved medication to treat IH in adults. It is curious, however, that LXB’s understood therapeutic effects are secondary to the “potentiation” of the very GABA receptor we have believed to be the root cause of the debilitating symptoms in IH. Could this discovery lend to further insights into the origins of this condition?
Ruckshanda Majid, MD, FCCP
Pulmonary vascular & cardiovascular network: Cardiovascular medicine & surgery section
Targeted temperature management (TTM) after cardiac arrest: How cool?
Recent randomized control trials, TTM2 (Dankiewicz J. N Engl J Med. 2021;384:2283) and HYPERION (Lascarrou J-B. N Engl J Med. 2019;381:2327), of therapeutic hypothermia, as opposed to normothermia, in patients who remain comatose after return of spontaneous circulation (ROSC) after cardiac arrest have produced conflicting results regarding survival and neurologic benefit. TTM2 reported no benefit to cooling to 33°C, while HYPERION found improved neurologic outcome at 90 days in patients cooled to 33°C. The European Resuscitation Council (ERC) and European Society of Intensive Care Medicine (ESICM) recently released an evidence review and guideline for adults who remain comatose after cardiac arrest (Sandroni C. Intensive Care Med. 2022;48:261). These guidelines recommend continuous monitoring of core temperature in all patients who remain comatose after cardiac arrest, and preventing fever (>37.7°C) for 72 hours, but with no recommendation of target temperature of 32°C vs 36°C.
Differences in patient populations, presenting rhythm during arrest, duration of CPR, and time to target temperature likely each contribute to the disparate conclusions of previous trials. For example, HYPERION enrolled patients with out of hospital cardiac arrest with initial nonshockable rhythms and found benefit to cooling to 33°C. In comparison, TTM2 enrolled all patients with ROSC following arrest (regardless of rhythm), including patients with in-hospital cardiac arrest and found no benefit in therapeutic cooling. Differences in patient populations are underscored by the widely differing percentage of patients with good neurologic outcome in their respective control groups: approximately 30% in the TTM2 trial and 6% in HYPERION. The guidelines leave significant room for clinical judgment in employing therapeutic cooling but encourage the continuous monitoring of core temperature and active avoidance of fever.
Fiore Mastroianna, MD
Section Member-at-Large
Chest infections & disaster response network: Chest infections section
Update on LTBI treatment: Ensuring success by simplifying, shortening, and completing treatment
My patient has a positive IGRA test result – what’s next?
If TB disease is ruled out by clinical, radiographic, and microbiologic assessment (if indicated), then latent TB infection (LTBI) is established, and treatment should be offered, guided by shared-decision making between provider and patient.
What options are available?
While the former standard 9-month regimen of isoniazid-monotherapy can be shortened to 6 months, shorter rifamycin-based regimens are now preferred in most cases and include:
4 months rifampin daily, 3 months isoniazid plus rifampin daily, or 3 months isoniazid plus rifapentine weekly. In addition, 1 month of isoniazid plus rifapentine daily has recently been shown to be effective in people with HIV.
How to choose?
Rifamycin-based regimens have been shown to have less hepatotoxicity and higher completion rates. Drug-drug interactions are of potential concern, for example, in patients receiving anticoagulation or treatment for HIV. The clinician should be aware of rifamycins causing a flu-like illness that may be treatment-limiting. In patients with known contact to drug-resistant TB, regimens are individualized.
How to monitor?
Adherence and completion are the keys to success. Directly observed therapy may be indicated in certain scenarios. Baseline and monthly blood work is recommended for people with risk factors for hepatic or bone marrow toxicity. More importantly, patients should be instructed to discontinue LTBI medications and call the clinician with any new symptoms. HIV testing should be offered to all patients if status is unknown. Clinicians are encouraged to reach out to one of four regional TB Centers of Excellence for guidance.
Sebastian Kurz, MD, FCCP
Amee Patrawalla, MD, MPH, FCCP
Section Members-at-Large
References
Testing and Treatment of Latent Tuberculosis Infection in the United States: Clinical Recommendations. A Guide for Health Care Providers and Public Health Programs. Copyright © 2021 by the National Society of Tuberculosis Clinicians and National Tuberculosis Controllers Association
1. Shah, D. Latent tuberculosis infection. N Engl J Med. 2021;385:2271-80.
2. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):111-115.
3. Swindells et. al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380:1001-11.
Thoracic oncology & chest procedures network: Lung cancer section
Adjuvant and neoadjuvant therapies in early stage lung cancer
Since the discovery of the epidermal growth factor receptor (EGFR) mutation in 2004 and the development of checkpoint blockade in 2006, personalized treatment options for non–small cell lung cancer (NSCLC) have exploded, but targeted systemic therapy medications were only recommended among patients with metastatic or locally advanced disease (Rivera MP, Matthay RA. Clin Chest Med. 2020;41[1]:ix-xi). However, in November 2020, the National Comprehensive Cancer Network (NCCN) updated guidelines to recommend EGFR testing in surgically resected stage IB-IIIA adenocarcinoma, and to consider adjuvant osimerintib in those who were mutation-positive (NCCN. Nov 2020). Interim analysis of an ongoing phase-3 trial showed 89% of patients in the osimertinib group were alive and disease-free at 24 months compared with 52% in the placebo group (hazard ratio 0.20, P < .001) (Wu YL, et al. N Engl J Med. 2020;383[18]:1711-23).
The FDA has also recently approved the use of neoadjuvant and adjuvant immunotherapy in combination with platinum-based chemotherapy. Nivolumab is now approved as neoadjuvant therapy in patients with resectable IB-IIIA NSCLC regardless of PDL-1 status. The Checkmate-816 trial showed increased median event-free survival in the immunotherapy plus chemotherapy group of 31.6 months vs 20.8 months in the chemotherapy-only group (FDA.gov. 2022, Mar 4). Atezolizumab is also now approved for adjuvant treatment following resection and platinum-based chemotherapy in patients with stage II to IIIA NSCLC whose tumors have PD-L1 expression on ≥ 1% of tumor cells. Median disease-free survival was not reached in patients in the atezolizumab groups vs 35.3 months in the best supportive care group (FDA.gov. 2021, Oct 15). With so many advances in the personalized treatment among all stages of NSCLC, this is a hopeful new chapter in the care of patients with NSCLC.
More information: https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManager?fileManagerId=11259
Sohini Ghosh, MD
Section Member-at-Large
Diffuse lung disease and lung transplant network: Lung transplant section
Continuous distribution for lung transplant: Overhauling the wait list
Determining how to allocate the scarce resource of donor lungs to patients is a difficult task and evaluated continuously for potential improvement. Since 2005, in the United States, lung transplant recipients have been selected based primarily on location within a Donor Service Area and by lung allocation score (LAS), a composite score of urgency for transplant. This was updated in 2017 to an allocation by highest LAS within 250 nautical miles from the donor hospital. Factors such as blood type compatibility and height are also considered. Implementation of the LAS improved the sickest patients’ access to transplants while not worsening 1-year mortality (Egan TM. Semin Respir Crit Care Med. 2018;39[02]:126-37). Unfortunately, geographic hard boundaries mean a high proportion of low LAS (<50) patients receive local donors while high LAS patients receive national offers or die while on the waitlist (Iribarne A, et al. Clin Transplant. 2016:30:688-93).
A new model that employs continuous distribution has been developed based on concerns regarding equity and improving allocation. This model would prioritize patients based on factors including medical priority, efficient management of organ placement (distance), expected posttransplant outcomes, and patient access (equity). By creating a composite of these without a geographic boundary, patients would be considered more on urgency within realistic constraints of distance and outcomes.
The Organ Procurement and Transplantation Network has officially approved continuous distribution, with implementation planned for 2022; details regarding the new scoring system are to be published and further research will need to be undertaken to determine if it meets the goal of overall improvement in patient access, equity, and outcomes.
Grant A. Turner, MD, MHA
Laura Frye, MD
Section Members-at-Large
Critical care network: Non-respiratory critical care section
Update from the non-respiratory critical care section
As you’ve probably noticed, there have been some changes here at CHEST involving the Networks. Leadership here at CHEST has been hard at work restructuring the networks to make them more closely aligned with relevant clinical disciplines, and, ultimately, allow for greater participation. I am proud to have been given stewardship of the new Non-Respiratory Critical Care Section of the Critical Care Network.
So, what exactly is Non-Respiratory Critical Care? Well, that’s where I need your help. You see this network is meant to reflect the needs and wants of CHEST members like you. We need you, dear readers, to join in this venture and help us guide the content that this section will ultimately create for our members.
If you’re interested in critical care, but you don’t see your particular area of interest anywhere else in the current structure ... guess what? You’ve found the right place!
My Infectious Diseases and Nephro peeps? Welcome! Are you a surgical or anesthesia intensivist? Don’t be shy. ECMO people, let’s hear some chatter!Is therapeutic hypothermia your thing? Come on in. The water’s freezing. 33 degrees just like you folks like it. Or is it 36? Not sure. Anyway, see what I’m talking about? We really need your help!You can get involved by clicking on the Membership & Community tab at the CHEST website. Once you’re a member, you can even nominate yourself to run for the Steering Committee elections which are held periodically. Hope to see you soon!
Deep Ramachandran, MD, FCCP
Section Chair
Sleep medicine network: Non-respiratory sleep section
Unusual suspects? Breakthrough in the treatment of idiopathic hypersomnia
Idiopathic hypersomnia (IH) is a rare and debilitating disorder defined by its excessive daytime sleepiness, sleep inertia, prolonged nighttime sleep, and long, unrefreshing naps (AASM. ICSD 3rd ed. 2014). Gamma-aminobutyric acid (GABA) is one of the main inhibitory neurotransmitters in the nervous system. It is through the potentiation of GABA that substances such as alcohol and benzodiazepines yield their effects. It is also hypothesized that the “brain fog” experienced in IH may be a consequence of either higher levels of an endogenous benzodiazepines in the cerebral spinal fluid or the presence of a GABA-enhancing peptide (Rye DB. Science Transl Med. 2012;Med 4:161ra151).
Sodium oxybate (SXB), a compound that likely has its therapeutic effect through the potentiation of GABA receptors, is an effective treatment option for cataplexy and sleepiness in narcolepsy. Although there may be some overlap between narcolepsy and IH in both diagnosis and treatment (Bassetti C, et al. Brain. 1997;120:1423), it would perhaps be entirely counterintuitive (given SXB’s pharmacology) to imagine using SXB as a plausible treatment option in IH. It was, however, investigated in the treatment of refractory hypersomnia and IH. In the retrospective study looking at 46 subjects treated with SXB, 71% experienced improvement of their severe sleep inertia, 55% had a decrease in their excessive daytime sleepiness, and 52% reported a shortened nighttime sleep time (Leu-Semenescu S, et al. Sleep Med. 2016;17:38).
In a recent double-blind, randomized control trial, the lower-sodium oxybate (LXB) was trialed in 154 patients with IH. It demonstrated statistically significant and clinically meaningful improvements (compared with placebo) in the Epworth Sleepiness Scale score (P <.0001) and in the Idiopathic Hypersomnia Severity Scale (P <.0001). The effects were seen both during the up titration of LXB and the benefits were maintained during the stable phase of the intervention (Dauvilliers Y, et al. Lancet Neurol. 2022;21(1):53). In August 2021, LXB (initially launched in 2020 for the treatment of narcolepsy) is now the first FDA-approved medication to treat IH in adults. It is curious, however, that LXB’s understood therapeutic effects are secondary to the “potentiation” of the very GABA receptor we have believed to be the root cause of the debilitating symptoms in IH. Could this discovery lend to further insights into the origins of this condition?
Ruckshanda Majid, MD, FCCP
Pulmonary vascular & cardiovascular network: Cardiovascular medicine & surgery section
Targeted temperature management (TTM) after cardiac arrest: How cool?
Recent randomized control trials, TTM2 (Dankiewicz J. N Engl J Med. 2021;384:2283) and HYPERION (Lascarrou J-B. N Engl J Med. 2019;381:2327), of therapeutic hypothermia, as opposed to normothermia, in patients who remain comatose after return of spontaneous circulation (ROSC) after cardiac arrest have produced conflicting results regarding survival and neurologic benefit. TTM2 reported no benefit to cooling to 33°C, while HYPERION found improved neurologic outcome at 90 days in patients cooled to 33°C. The European Resuscitation Council (ERC) and European Society of Intensive Care Medicine (ESICM) recently released an evidence review and guideline for adults who remain comatose after cardiac arrest (Sandroni C. Intensive Care Med. 2022;48:261). These guidelines recommend continuous monitoring of core temperature in all patients who remain comatose after cardiac arrest, and preventing fever (>37.7°C) for 72 hours, but with no recommendation of target temperature of 32°C vs 36°C.
Differences in patient populations, presenting rhythm during arrest, duration of CPR, and time to target temperature likely each contribute to the disparate conclusions of previous trials. For example, HYPERION enrolled patients with out of hospital cardiac arrest with initial nonshockable rhythms and found benefit to cooling to 33°C. In comparison, TTM2 enrolled all patients with ROSC following arrest (regardless of rhythm), including patients with in-hospital cardiac arrest and found no benefit in therapeutic cooling. Differences in patient populations are underscored by the widely differing percentage of patients with good neurologic outcome in their respective control groups: approximately 30% in the TTM2 trial and 6% in HYPERION. The guidelines leave significant room for clinical judgment in employing therapeutic cooling but encourage the continuous monitoring of core temperature and active avoidance of fever.
Fiore Mastroianna, MD
Section Member-at-Large
Chest infections & disaster response network: Chest infections section
Update on LTBI treatment: Ensuring success by simplifying, shortening, and completing treatment
My patient has a positive IGRA test result – what’s next?
If TB disease is ruled out by clinical, radiographic, and microbiologic assessment (if indicated), then latent TB infection (LTBI) is established, and treatment should be offered, guided by shared-decision making between provider and patient.
What options are available?
While the former standard 9-month regimen of isoniazid-monotherapy can be shortened to 6 months, shorter rifamycin-based regimens are now preferred in most cases and include:
4 months rifampin daily, 3 months isoniazid plus rifampin daily, or 3 months isoniazid plus rifapentine weekly. In addition, 1 month of isoniazid plus rifapentine daily has recently been shown to be effective in people with HIV.
How to choose?
Rifamycin-based regimens have been shown to have less hepatotoxicity and higher completion rates. Drug-drug interactions are of potential concern, for example, in patients receiving anticoagulation or treatment for HIV. The clinician should be aware of rifamycins causing a flu-like illness that may be treatment-limiting. In patients with known contact to drug-resistant TB, regimens are individualized.
How to monitor?
Adherence and completion are the keys to success. Directly observed therapy may be indicated in certain scenarios. Baseline and monthly blood work is recommended for people with risk factors for hepatic or bone marrow toxicity. More importantly, patients should be instructed to discontinue LTBI medications and call the clinician with any new symptoms. HIV testing should be offered to all patients if status is unknown. Clinicians are encouraged to reach out to one of four regional TB Centers of Excellence for guidance.
Sebastian Kurz, MD, FCCP
Amee Patrawalla, MD, MPH, FCCP
Section Members-at-Large
References
Testing and Treatment of Latent Tuberculosis Infection in the United States: Clinical Recommendations. A Guide for Health Care Providers and Public Health Programs. Copyright © 2021 by the National Society of Tuberculosis Clinicians and National Tuberculosis Controllers Association
1. Shah, D. Latent tuberculosis infection. N Engl J Med. 2021;385:2271-80.
2. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017 Jan 15;64(2):111-115.
3. Swindells et. al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380:1001-11.
Thoracic oncology & chest procedures network: Lung cancer section
Adjuvant and neoadjuvant therapies in early stage lung cancer
Since the discovery of the epidermal growth factor receptor (EGFR) mutation in 2004 and the development of checkpoint blockade in 2006, personalized treatment options for non–small cell lung cancer (NSCLC) have exploded, but targeted systemic therapy medications were only recommended among patients with metastatic or locally advanced disease (Rivera MP, Matthay RA. Clin Chest Med. 2020;41[1]:ix-xi). However, in November 2020, the National Comprehensive Cancer Network (NCCN) updated guidelines to recommend EGFR testing in surgically resected stage IB-IIIA adenocarcinoma, and to consider adjuvant osimerintib in those who were mutation-positive (NCCN. Nov 2020). Interim analysis of an ongoing phase-3 trial showed 89% of patients in the osimertinib group were alive and disease-free at 24 months compared with 52% in the placebo group (hazard ratio 0.20, P < .001) (Wu YL, et al. N Engl J Med. 2020;383[18]:1711-23).
The FDA has also recently approved the use of neoadjuvant and adjuvant immunotherapy in combination with platinum-based chemotherapy. Nivolumab is now approved as neoadjuvant therapy in patients with resectable IB-IIIA NSCLC regardless of PDL-1 status. The Checkmate-816 trial showed increased median event-free survival in the immunotherapy plus chemotherapy group of 31.6 months vs 20.8 months in the chemotherapy-only group (FDA.gov. 2022, Mar 4). Atezolizumab is also now approved for adjuvant treatment following resection and platinum-based chemotherapy in patients with stage II to IIIA NSCLC whose tumors have PD-L1 expression on ≥ 1% of tumor cells. Median disease-free survival was not reached in patients in the atezolizumab groups vs 35.3 months in the best supportive care group (FDA.gov. 2021, Oct 15). With so many advances in the personalized treatment among all stages of NSCLC, this is a hopeful new chapter in the care of patients with NSCLC.
More information: https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManager?fileManagerId=11259
Sohini Ghosh, MD
Section Member-at-Large
Diffuse lung disease and lung transplant network: Lung transplant section
Continuous distribution for lung transplant: Overhauling the wait list
Determining how to allocate the scarce resource of donor lungs to patients is a difficult task and evaluated continuously for potential improvement. Since 2005, in the United States, lung transplant recipients have been selected based primarily on location within a Donor Service Area and by lung allocation score (LAS), a composite score of urgency for transplant. This was updated in 2017 to an allocation by highest LAS within 250 nautical miles from the donor hospital. Factors such as blood type compatibility and height are also considered. Implementation of the LAS improved the sickest patients’ access to transplants while not worsening 1-year mortality (Egan TM. Semin Respir Crit Care Med. 2018;39[02]:126-37). Unfortunately, geographic hard boundaries mean a high proportion of low LAS (<50) patients receive local donors while high LAS patients receive national offers or die while on the waitlist (Iribarne A, et al. Clin Transplant. 2016:30:688-93).
A new model that employs continuous distribution has been developed based on concerns regarding equity and improving allocation. This model would prioritize patients based on factors including medical priority, efficient management of organ placement (distance), expected posttransplant outcomes, and patient access (equity). By creating a composite of these without a geographic boundary, patients would be considered more on urgency within realistic constraints of distance and outcomes.
The Organ Procurement and Transplantation Network has officially approved continuous distribution, with implementation planned for 2022; details regarding the new scoring system are to be published and further research will need to be undertaken to determine if it meets the goal of overall improvement in patient access, equity, and outcomes.
Grant A. Turner, MD, MHA
Laura Frye, MD
Section Members-at-Large
Critical care network: Non-respiratory critical care section
Update from the non-respiratory critical care section
As you’ve probably noticed, there have been some changes here at CHEST involving the Networks. Leadership here at CHEST has been hard at work restructuring the networks to make them more closely aligned with relevant clinical disciplines, and, ultimately, allow for greater participation. I am proud to have been given stewardship of the new Non-Respiratory Critical Care Section of the Critical Care Network.
So, what exactly is Non-Respiratory Critical Care? Well, that’s where I need your help. You see this network is meant to reflect the needs and wants of CHEST members like you. We need you, dear readers, to join in this venture and help us guide the content that this section will ultimately create for our members.
If you’re interested in critical care, but you don’t see your particular area of interest anywhere else in the current structure ... guess what? You’ve found the right place!
My Infectious Diseases and Nephro peeps? Welcome! Are you a surgical or anesthesia intensivist? Don’t be shy. ECMO people, let’s hear some chatter!Is therapeutic hypothermia your thing? Come on in. The water’s freezing. 33 degrees just like you folks like it. Or is it 36? Not sure. Anyway, see what I’m talking about? We really need your help!You can get involved by clicking on the Membership & Community tab at the CHEST website. Once you’re a member, you can even nominate yourself to run for the Steering Committee elections which are held periodically. Hope to see you soon!
Deep Ramachandran, MD, FCCP
Section Chair
Sleep medicine network: Non-respiratory sleep section
Unusual suspects? Breakthrough in the treatment of idiopathic hypersomnia
Idiopathic hypersomnia (IH) is a rare and debilitating disorder defined by its excessive daytime sleepiness, sleep inertia, prolonged nighttime sleep, and long, unrefreshing naps (AASM. ICSD 3rd ed. 2014). Gamma-aminobutyric acid (GABA) is one of the main inhibitory neurotransmitters in the nervous system. It is through the potentiation of GABA that substances such as alcohol and benzodiazepines yield their effects. It is also hypothesized that the “brain fog” experienced in IH may be a consequence of either higher levels of an endogenous benzodiazepines in the cerebral spinal fluid or the presence of a GABA-enhancing peptide (Rye DB. Science Transl Med. 2012;Med 4:161ra151).
Sodium oxybate (SXB), a compound that likely has its therapeutic effect through the potentiation of GABA receptors, is an effective treatment option for cataplexy and sleepiness in narcolepsy. Although there may be some overlap between narcolepsy and IH in both diagnosis and treatment (Bassetti C, et al. Brain. 1997;120:1423), it would perhaps be entirely counterintuitive (given SXB’s pharmacology) to imagine using SXB as a plausible treatment option in IH. It was, however, investigated in the treatment of refractory hypersomnia and IH. In the retrospective study looking at 46 subjects treated with SXB, 71% experienced improvement of their severe sleep inertia, 55% had a decrease in their excessive daytime sleepiness, and 52% reported a shortened nighttime sleep time (Leu-Semenescu S, et al. Sleep Med. 2016;17:38).
In a recent double-blind, randomized control trial, the lower-sodium oxybate (LXB) was trialed in 154 patients with IH. It demonstrated statistically significant and clinically meaningful improvements (compared with placebo) in the Epworth Sleepiness Scale score (P <.0001) and in the Idiopathic Hypersomnia Severity Scale (P <.0001). The effects were seen both during the up titration of LXB and the benefits were maintained during the stable phase of the intervention (Dauvilliers Y, et al. Lancet Neurol. 2022;21(1):53). In August 2021, LXB (initially launched in 2020 for the treatment of narcolepsy) is now the first FDA-approved medication to treat IH in adults. It is curious, however, that LXB’s understood therapeutic effects are secondary to the “potentiation” of the very GABA receptor we have believed to be the root cause of the debilitating symptoms in IH. Could this discovery lend to further insights into the origins of this condition?
Ruckshanda Majid, MD, FCCP
The quest for a good night’s sleep: An update on pharmacologic therapy for insomnia
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
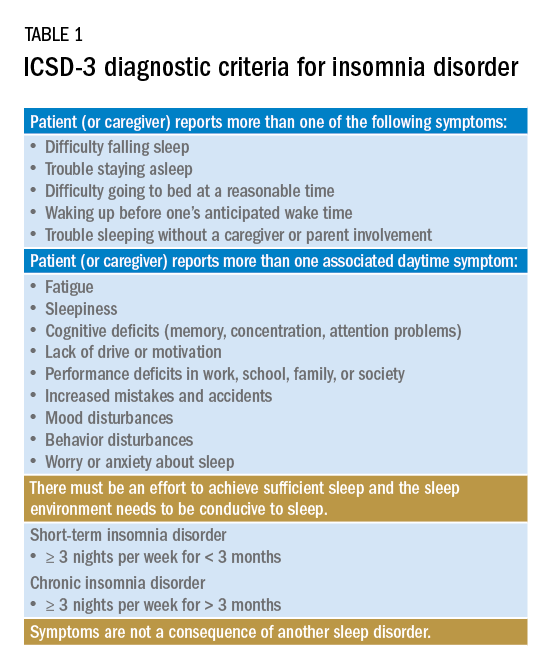
For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.
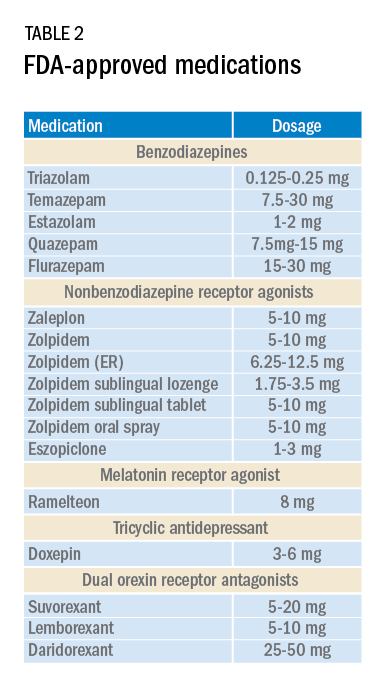
Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.
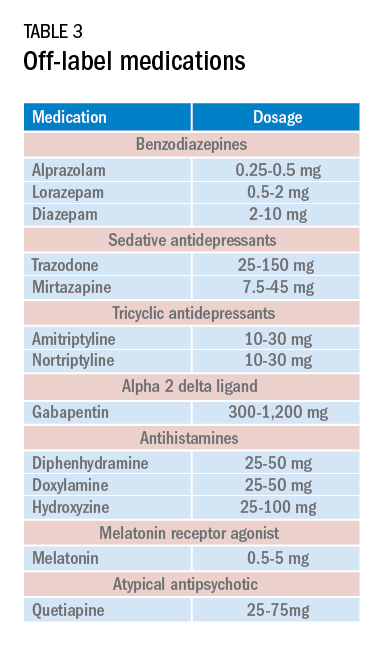
Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).
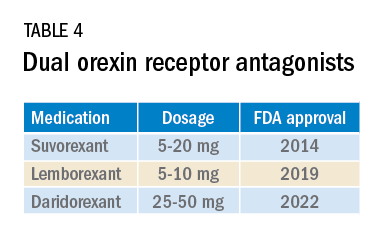
Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).
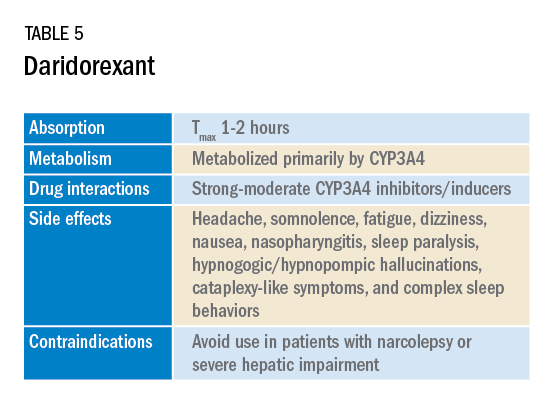
Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).

For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.

Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.

Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).

Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).

Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).

For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.

Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.

Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).

Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).

Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
President’s report
There is little I enjoy more than an opportunity to get together with old friends.
I write this missive on the return trip from a week of CHEST leadership meetings held last month, and I find myself filled with joy, awe, and great appreciation for the hard work our volunteers contribute to making the American College of Chest Physicians an extraordinarily productive and successful organization. This year’s meetings meant more than any I can ever recall from the past, in the context of a return to in-person gatherings that let our members share laughs, stories, and even a game or two of laser tag in the context of celebrating good times and friendship. And while some great works were accomplished by our committees, some of which I will enumerate below, the highlight of the week was definitely the esprit de corps that was on broad display.
Our Membership Committee meeting was led by Vice-Chair Marie Budev, DO, FCCP. While this committee is tasked with the critical duty of reviewing applications for the prestigious FCCP designation, they are just as importantly tasked with promoting membership, to our domestic and international colleagues. This is a challenging task, because different members prioritize the variety of benefits from CHEST differently; some focus on access to our educational offerings, both throughout the year and at our annual meeting, while others find greater value in the chance to network with colleagues from around the world and to participate in leadership in an international society. Making sure that we are helping our members realize these benefits, while also identifying (and potentially enhancing) those opportunities in which members are most interested is a challenging task, and I very much enjoyed watching these folks brainstorm ways that we could further increase the value of joining CHEST for current and potential future members.
The Guidelines Oversight Committee, chaired by Lisa Moores, MD, FCCP, is responsible for the oversight of CHEST’s evidence-based guidelines. As our clinical guidelines are among the most highly regarded of all of the things we publish, the members of this committee take special care to ensure that the subjects selected for review as part of the guideline process meet strict criteria. They receive dozens of proposals for new guidelines each year and carefully examine each one to identify the potential public health impact, to ensure the availability of literature in the space worthy of review, and to provide the opportunity to illuminate areas where there are significant clinical uncertainties, often due to new treatments or diagnostic tests. Watching committee members meticulously debate the merits of the many good ideas received to finalize a short list of topics for guideline development in the coming year was incredibly informative and validated my longstanding perception that our members are some of the best clinical minds in the pulmonary, critical care, and sleep fields in the world.
The Professional Standards Committee (PSC), chaired by Scott Manaker, MD, PhD, FCCP, has the important duty of developing CHEST’s conflict of interest (COI) policy, as well as reviewing all potential COI among CHEST leaders and members of our guideline panels. While this may sound a little dry, the fascinating part of this meeting was the ongoing discussion of what constitutes a meaningful COI. As one would expect, many of the best medical experts in the world have relationships with pharmaceutical and medical device companies, who often seek the counsel and participation of high-performing, high-volume clinicians for research trials. CHEST has extremely strict rules with regard to COI among its many levels of leadership, but the question of what constitutes a potentially problematic COI for the large number of folks who volunteer their time and energy to teach at one of our many courses is an interesting (albeit possibly philosophical) question. Since PSC members cannot observe every CHEST faculty interaction, we rely on our members to let us know if they perceive any potential bias in faculty teaching (and we so very much appreciate those of you who bring the extremely rare concerns to our attention!), but this is an area that we continue to watch very closely, as we continue to ensure that all CHEST education is accurate, unbiased, and the best available throughout the world.
The reformulated Council of Networks met under the leadership of Angel Coz Yataco, MD, FCCP, and Cassie Kennedy, MD, FCCP, to discuss how to best engage our members in the new structure, which comprises seven Networks and 22 component Sections. The Council’s primary charges are to develop educational content, to review project applications from Sections, and to serve as expert consultants to the President in their specific clinical domains. While the new configuration provides a significant increase in leadership positions for our members, as well as more formal opportunities to cultivate relationships across different Sections, I have received a few emails from colleagues who were concerned about elimination of certain former Networks, or the placement of a specific Section under a specific Network. Some of these concerns were discussed at the April meeting. While there will be some growing pains, listening to the thoughtful discussion that ensued validated my belief that Drs. Coz and Kennedy are the right folks to be leading the Council as it matures into this new and stronger structure.
While I also had the opportunity to hear reports from the Training and Transitions Committee, the Education Committee, and the Council of Global Governors, I wanted to briefly mention the Scientific Program Committee and its Innovations Group. While we are looking forward to seeing everyone in Nashville this October, I cannot tell you how excited I am about some of the new things we have in store for our first in-person annual meeting in 3 years. (Literally ... I am absolutely sworn to secrecy!) But under the leadership of Program Chair Subani Chandra, MD, FCCP, and my two other “Chief Fun Officers” Aneesa Das, MD, FCCP, and William Kelly, MD, FCCP, I can say that attendees are going to be in for a heck of a lot of fun. Oh, and there’s going to be some education there, also.
In closing, I want to reiterate how much of a pleasure and privilege it has been to sit in the President’s chair over the first few months of 2022. If any of the committees I’ve described above sound interesting to you, please strongly consider throwing your hat into the ring when nominations open up in the coming months. Getting involved at CHEST has been one of the best experiences of my career, and I expect you’ll feel the same way after you join in the fun. As always, I remain available to you, either by emailing me at [email protected] or messaging me on Twitter @ChestPrez. And, please come find me in Nashville in October, either to say hello, or to challenge me to a game of laser tag. ... I’m not very hard to beat.
Until next time,
David
There is little I enjoy more than an opportunity to get together with old friends.
I write this missive on the return trip from a week of CHEST leadership meetings held last month, and I find myself filled with joy, awe, and great appreciation for the hard work our volunteers contribute to making the American College of Chest Physicians an extraordinarily productive and successful organization. This year’s meetings meant more than any I can ever recall from the past, in the context of a return to in-person gatherings that let our members share laughs, stories, and even a game or two of laser tag in the context of celebrating good times and friendship. And while some great works were accomplished by our committees, some of which I will enumerate below, the highlight of the week was definitely the esprit de corps that was on broad display.
Our Membership Committee meeting was led by Vice-Chair Marie Budev, DO, FCCP. While this committee is tasked with the critical duty of reviewing applications for the prestigious FCCP designation, they are just as importantly tasked with promoting membership, to our domestic and international colleagues. This is a challenging task, because different members prioritize the variety of benefits from CHEST differently; some focus on access to our educational offerings, both throughout the year and at our annual meeting, while others find greater value in the chance to network with colleagues from around the world and to participate in leadership in an international society. Making sure that we are helping our members realize these benefits, while also identifying (and potentially enhancing) those opportunities in which members are most interested is a challenging task, and I very much enjoyed watching these folks brainstorm ways that we could further increase the value of joining CHEST for current and potential future members.
The Guidelines Oversight Committee, chaired by Lisa Moores, MD, FCCP, is responsible for the oversight of CHEST’s evidence-based guidelines. As our clinical guidelines are among the most highly regarded of all of the things we publish, the members of this committee take special care to ensure that the subjects selected for review as part of the guideline process meet strict criteria. They receive dozens of proposals for new guidelines each year and carefully examine each one to identify the potential public health impact, to ensure the availability of literature in the space worthy of review, and to provide the opportunity to illuminate areas where there are significant clinical uncertainties, often due to new treatments or diagnostic tests. Watching committee members meticulously debate the merits of the many good ideas received to finalize a short list of topics for guideline development in the coming year was incredibly informative and validated my longstanding perception that our members are some of the best clinical minds in the pulmonary, critical care, and sleep fields in the world.
The Professional Standards Committee (PSC), chaired by Scott Manaker, MD, PhD, FCCP, has the important duty of developing CHEST’s conflict of interest (COI) policy, as well as reviewing all potential COI among CHEST leaders and members of our guideline panels. While this may sound a little dry, the fascinating part of this meeting was the ongoing discussion of what constitutes a meaningful COI. As one would expect, many of the best medical experts in the world have relationships with pharmaceutical and medical device companies, who often seek the counsel and participation of high-performing, high-volume clinicians for research trials. CHEST has extremely strict rules with regard to COI among its many levels of leadership, but the question of what constitutes a potentially problematic COI for the large number of folks who volunteer their time and energy to teach at one of our many courses is an interesting (albeit possibly philosophical) question. Since PSC members cannot observe every CHEST faculty interaction, we rely on our members to let us know if they perceive any potential bias in faculty teaching (and we so very much appreciate those of you who bring the extremely rare concerns to our attention!), but this is an area that we continue to watch very closely, as we continue to ensure that all CHEST education is accurate, unbiased, and the best available throughout the world.
The reformulated Council of Networks met under the leadership of Angel Coz Yataco, MD, FCCP, and Cassie Kennedy, MD, FCCP, to discuss how to best engage our members in the new structure, which comprises seven Networks and 22 component Sections. The Council’s primary charges are to develop educational content, to review project applications from Sections, and to serve as expert consultants to the President in their specific clinical domains. While the new configuration provides a significant increase in leadership positions for our members, as well as more formal opportunities to cultivate relationships across different Sections, I have received a few emails from colleagues who were concerned about elimination of certain former Networks, or the placement of a specific Section under a specific Network. Some of these concerns were discussed at the April meeting. While there will be some growing pains, listening to the thoughtful discussion that ensued validated my belief that Drs. Coz and Kennedy are the right folks to be leading the Council as it matures into this new and stronger structure.
While I also had the opportunity to hear reports from the Training and Transitions Committee, the Education Committee, and the Council of Global Governors, I wanted to briefly mention the Scientific Program Committee and its Innovations Group. While we are looking forward to seeing everyone in Nashville this October, I cannot tell you how excited I am about some of the new things we have in store for our first in-person annual meeting in 3 years. (Literally ... I am absolutely sworn to secrecy!) But under the leadership of Program Chair Subani Chandra, MD, FCCP, and my two other “Chief Fun Officers” Aneesa Das, MD, FCCP, and William Kelly, MD, FCCP, I can say that attendees are going to be in for a heck of a lot of fun. Oh, and there’s going to be some education there, also.
In closing, I want to reiterate how much of a pleasure and privilege it has been to sit in the President’s chair over the first few months of 2022. If any of the committees I’ve described above sound interesting to you, please strongly consider throwing your hat into the ring when nominations open up in the coming months. Getting involved at CHEST has been one of the best experiences of my career, and I expect you’ll feel the same way after you join in the fun. As always, I remain available to you, either by emailing me at [email protected] or messaging me on Twitter @ChestPrez. And, please come find me in Nashville in October, either to say hello, or to challenge me to a game of laser tag. ... I’m not very hard to beat.
Until next time,
David
There is little I enjoy more than an opportunity to get together with old friends.
I write this missive on the return trip from a week of CHEST leadership meetings held last month, and I find myself filled with joy, awe, and great appreciation for the hard work our volunteers contribute to making the American College of Chest Physicians an extraordinarily productive and successful organization. This year’s meetings meant more than any I can ever recall from the past, in the context of a return to in-person gatherings that let our members share laughs, stories, and even a game or two of laser tag in the context of celebrating good times and friendship. And while some great works were accomplished by our committees, some of which I will enumerate below, the highlight of the week was definitely the esprit de corps that was on broad display.
Our Membership Committee meeting was led by Vice-Chair Marie Budev, DO, FCCP. While this committee is tasked with the critical duty of reviewing applications for the prestigious FCCP designation, they are just as importantly tasked with promoting membership, to our domestic and international colleagues. This is a challenging task, because different members prioritize the variety of benefits from CHEST differently; some focus on access to our educational offerings, both throughout the year and at our annual meeting, while others find greater value in the chance to network with colleagues from around the world and to participate in leadership in an international society. Making sure that we are helping our members realize these benefits, while also identifying (and potentially enhancing) those opportunities in which members are most interested is a challenging task, and I very much enjoyed watching these folks brainstorm ways that we could further increase the value of joining CHEST for current and potential future members.
The Guidelines Oversight Committee, chaired by Lisa Moores, MD, FCCP, is responsible for the oversight of CHEST’s evidence-based guidelines. As our clinical guidelines are among the most highly regarded of all of the things we publish, the members of this committee take special care to ensure that the subjects selected for review as part of the guideline process meet strict criteria. They receive dozens of proposals for new guidelines each year and carefully examine each one to identify the potential public health impact, to ensure the availability of literature in the space worthy of review, and to provide the opportunity to illuminate areas where there are significant clinical uncertainties, often due to new treatments or diagnostic tests. Watching committee members meticulously debate the merits of the many good ideas received to finalize a short list of topics for guideline development in the coming year was incredibly informative and validated my longstanding perception that our members are some of the best clinical minds in the pulmonary, critical care, and sleep fields in the world.
The Professional Standards Committee (PSC), chaired by Scott Manaker, MD, PhD, FCCP, has the important duty of developing CHEST’s conflict of interest (COI) policy, as well as reviewing all potential COI among CHEST leaders and members of our guideline panels. While this may sound a little dry, the fascinating part of this meeting was the ongoing discussion of what constitutes a meaningful COI. As one would expect, many of the best medical experts in the world have relationships with pharmaceutical and medical device companies, who often seek the counsel and participation of high-performing, high-volume clinicians for research trials. CHEST has extremely strict rules with regard to COI among its many levels of leadership, but the question of what constitutes a potentially problematic COI for the large number of folks who volunteer their time and energy to teach at one of our many courses is an interesting (albeit possibly philosophical) question. Since PSC members cannot observe every CHEST faculty interaction, we rely on our members to let us know if they perceive any potential bias in faculty teaching (and we so very much appreciate those of you who bring the extremely rare concerns to our attention!), but this is an area that we continue to watch very closely, as we continue to ensure that all CHEST education is accurate, unbiased, and the best available throughout the world.
The reformulated Council of Networks met under the leadership of Angel Coz Yataco, MD, FCCP, and Cassie Kennedy, MD, FCCP, to discuss how to best engage our members in the new structure, which comprises seven Networks and 22 component Sections. The Council’s primary charges are to develop educational content, to review project applications from Sections, and to serve as expert consultants to the President in their specific clinical domains. While the new configuration provides a significant increase in leadership positions for our members, as well as more formal opportunities to cultivate relationships across different Sections, I have received a few emails from colleagues who were concerned about elimination of certain former Networks, or the placement of a specific Section under a specific Network. Some of these concerns were discussed at the April meeting. While there will be some growing pains, listening to the thoughtful discussion that ensued validated my belief that Drs. Coz and Kennedy are the right folks to be leading the Council as it matures into this new and stronger structure.
While I also had the opportunity to hear reports from the Training and Transitions Committee, the Education Committee, and the Council of Global Governors, I wanted to briefly mention the Scientific Program Committee and its Innovations Group. While we are looking forward to seeing everyone in Nashville this October, I cannot tell you how excited I am about some of the new things we have in store for our first in-person annual meeting in 3 years. (Literally ... I am absolutely sworn to secrecy!) But under the leadership of Program Chair Subani Chandra, MD, FCCP, and my two other “Chief Fun Officers” Aneesa Das, MD, FCCP, and William Kelly, MD, FCCP, I can say that attendees are going to be in for a heck of a lot of fun. Oh, and there’s going to be some education there, also.
In closing, I want to reiterate how much of a pleasure and privilege it has been to sit in the President’s chair over the first few months of 2022. If any of the committees I’ve described above sound interesting to you, please strongly consider throwing your hat into the ring when nominations open up in the coming months. Getting involved at CHEST has been one of the best experiences of my career, and I expect you’ll feel the same way after you join in the fun. As always, I remain available to you, either by emailing me at [email protected] or messaging me on Twitter @ChestPrez. And, please come find me in Nashville in October, either to say hello, or to challenge me to a game of laser tag. ... I’m not very hard to beat.
Until next time,
David
Introducing new CHEST Physician editorial board member
Welcome to Corinne Young, MSN, FNP-C, FCCP, who recently joined the CHEST Physician editorial board to represent and advocate for the perspective of advanced practice providers on the interdisciplinary team.
Young is a nurse practitioner and director of APP and Clinical Services for Colorado Springs Pulmonary Consultants in Colorado. She also is the founder and president of the Association of Pulmonary Advanced Practice Providers, which she created with support from CHEST staff and leaders, who encouraged her to create a community around advocating for and developing credentialing opportunities for this population.
The idea began early in Young’s career, when, after joining CHEST and attending educational events, she was struck by the lack of standardization in practice she saw among APPs.
“Every time I would be at the CHEST meeting, if I happened to bump into another APP, I would assault them with questions because I didn’t know what the norm was—and come to find out, nobody did,” she said. “Our organization came out of that, and our goal is to eventually standardize the education and knowledge base of APPs.”
Because there is not an option for a national certification specifically for pulmonary medicine for APPs, Young instead attained the FCCP to demonstrate her clinical competency and knowledge. She also immersed herself in the education and community of CHEST, working on the former Clinical Research & Quality Improvement NetWork Committee and Interprofessional Team NetWork Committee, serving on the Scientific Program Committee, and developing patient education on asthma, among other projects.
Now, as a member of the CHEST Physician Editorial Board, Young hopes to build awareness among clinicians of the importance of APPs on the care team and to support another option for APPs to access high-quality education and content to help them build their knowledge and enhance the care they deliver.
“It’s important that CHEST Physician is interested in an APP perspective being included,” she said. “It’s validation that we’re part of the team, that we’re included in all aspects of care including areas outside of direct care: in education, in the literature. ... That they feel our contributions are important.”
When she isn’t working with CHEST or caring for patients, Young and her husband competitively team rope, a rodeo event in which two people work together to rope a steer. Although they were unable to attend, they qualified for the world series in the sport last year, and hope to qualify again this year.
Please join us in welcoming Corinne Young to the CHEST Physician editorial board.
Welcome to Corinne Young, MSN, FNP-C, FCCP, who recently joined the CHEST Physician editorial board to represent and advocate for the perspective of advanced practice providers on the interdisciplinary team.
Young is a nurse practitioner and director of APP and Clinical Services for Colorado Springs Pulmonary Consultants in Colorado. She also is the founder and president of the Association of Pulmonary Advanced Practice Providers, which she created with support from CHEST staff and leaders, who encouraged her to create a community around advocating for and developing credentialing opportunities for this population.
The idea began early in Young’s career, when, after joining CHEST and attending educational events, she was struck by the lack of standardization in practice she saw among APPs.
“Every time I would be at the CHEST meeting, if I happened to bump into another APP, I would assault them with questions because I didn’t know what the norm was—and come to find out, nobody did,” she said. “Our organization came out of that, and our goal is to eventually standardize the education and knowledge base of APPs.”
Because there is not an option for a national certification specifically for pulmonary medicine for APPs, Young instead attained the FCCP to demonstrate her clinical competency and knowledge. She also immersed herself in the education and community of CHEST, working on the former Clinical Research & Quality Improvement NetWork Committee and Interprofessional Team NetWork Committee, serving on the Scientific Program Committee, and developing patient education on asthma, among other projects.
Now, as a member of the CHEST Physician Editorial Board, Young hopes to build awareness among clinicians of the importance of APPs on the care team and to support another option for APPs to access high-quality education and content to help them build their knowledge and enhance the care they deliver.
“It’s important that CHEST Physician is interested in an APP perspective being included,” she said. “It’s validation that we’re part of the team, that we’re included in all aspects of care including areas outside of direct care: in education, in the literature. ... That they feel our contributions are important.”
When she isn’t working with CHEST or caring for patients, Young and her husband competitively team rope, a rodeo event in which two people work together to rope a steer. Although they were unable to attend, they qualified for the world series in the sport last year, and hope to qualify again this year.
Please join us in welcoming Corinne Young to the CHEST Physician editorial board.
Welcome to Corinne Young, MSN, FNP-C, FCCP, who recently joined the CHEST Physician editorial board to represent and advocate for the perspective of advanced practice providers on the interdisciplinary team.
Young is a nurse practitioner and director of APP and Clinical Services for Colorado Springs Pulmonary Consultants in Colorado. She also is the founder and president of the Association of Pulmonary Advanced Practice Providers, which she created with support from CHEST staff and leaders, who encouraged her to create a community around advocating for and developing credentialing opportunities for this population.
The idea began early in Young’s career, when, after joining CHEST and attending educational events, she was struck by the lack of standardization in practice she saw among APPs.
“Every time I would be at the CHEST meeting, if I happened to bump into another APP, I would assault them with questions because I didn’t know what the norm was—and come to find out, nobody did,” she said. “Our organization came out of that, and our goal is to eventually standardize the education and knowledge base of APPs.”
Because there is not an option for a national certification specifically for pulmonary medicine for APPs, Young instead attained the FCCP to demonstrate her clinical competency and knowledge. She also immersed herself in the education and community of CHEST, working on the former Clinical Research & Quality Improvement NetWork Committee and Interprofessional Team NetWork Committee, serving on the Scientific Program Committee, and developing patient education on asthma, among other projects.
Now, as a member of the CHEST Physician Editorial Board, Young hopes to build awareness among clinicians of the importance of APPs on the care team and to support another option for APPs to access high-quality education and content to help them build their knowledge and enhance the care they deliver.
“It’s important that CHEST Physician is interested in an APP perspective being included,” she said. “It’s validation that we’re part of the team, that we’re included in all aspects of care including areas outside of direct care: in education, in the literature. ... That they feel our contributions are important.”
When she isn’t working with CHEST or caring for patients, Young and her husband competitively team rope, a rodeo event in which two people work together to rope a steer. Although they were unable to attend, they qualified for the world series in the sport last year, and hope to qualify again this year.
Please join us in welcoming Corinne Young to the CHEST Physician editorial board.
This month in the journal CHEST®
Editor’s picks
Genetic Associations and Architecture of Asthma-COPD Overlap. By Catherine John, et al.
Emerging Nonpulmonary Complications for Adults With Cystic Fibrosis. By Dr. Melanie Chin, et al.
Aspirin as a Treatment for ARDS: A Randomized, Placebo-Controlled Clinical Trial. By Dr. Philip Toner, et al.
PICU in the MICU: How Adult ICUs Can Support Pediatric Care in Public Health Emergencies. By Dr. Mary A. King, et al.
Association of BMI and Change in Weight With Mortality in Patients With Fibrotic Interstitial Lung Disease. By Dr. Alessia Comes, et al.
Off-Label Use and Inappropriate Dosing of Direct Oral Anticoagulants in Cardiopulmonary Disease. By Dr. Ayman A. Hussein, et al.
Editor’s picks
Editor’s picks
Genetic Associations and Architecture of Asthma-COPD Overlap. By Catherine John, et al.
Emerging Nonpulmonary Complications for Adults With Cystic Fibrosis. By Dr. Melanie Chin, et al.
Aspirin as a Treatment for ARDS: A Randomized, Placebo-Controlled Clinical Trial. By Dr. Philip Toner, et al.
PICU in the MICU: How Adult ICUs Can Support Pediatric Care in Public Health Emergencies. By Dr. Mary A. King, et al.
Association of BMI and Change in Weight With Mortality in Patients With Fibrotic Interstitial Lung Disease. By Dr. Alessia Comes, et al.
Off-Label Use and Inappropriate Dosing of Direct Oral Anticoagulants in Cardiopulmonary Disease. By Dr. Ayman A. Hussein, et al.
Genetic Associations and Architecture of Asthma-COPD Overlap. By Catherine John, et al.
Emerging Nonpulmonary Complications for Adults With Cystic Fibrosis. By Dr. Melanie Chin, et al.
Aspirin as a Treatment for ARDS: A Randomized, Placebo-Controlled Clinical Trial. By Dr. Philip Toner, et al.
PICU in the MICU: How Adult ICUs Can Support Pediatric Care in Public Health Emergencies. By Dr. Mary A. King, et al.
Association of BMI and Change in Weight With Mortality in Patients With Fibrotic Interstitial Lung Disease. By Dr. Alessia Comes, et al.
Off-Label Use and Inappropriate Dosing of Direct Oral Anticoagulants in Cardiopulmonary Disease. By Dr. Ayman A. Hussein, et al.
Coming together for a night of philanthropy and fun
Although attendees will be watching “The Test of the Champion” with bated breath, the upcoming Belmont Stakes Dinner and Auction on June 11 in New York City is about much more than a famous horse race. It’s about community – the vibrant community of clinicians, patients, advocates, and more who support the mission to crush lung disease.
The event started small with a Sunday brunch at the home of CHEST President-Elect Doreen Addrizzo-Harris, MD, FCCP, where attendees gathered to learn more from their host about the CHEST Foundation’s many initiatives. However, over the years, Dr. Addrizzo-Harris leaned on her own community of colleagues, family, friends, and patients to build an event that now boasts hundreds of attendees. But despite all that has changed, the Belmont Stakes Dinner and Auction is still dedicated to raising awareness about the CHEST Foundation and fundraising for initiatives to develop patient education and improve care.
In addition to a plated dinner, silent auction, cocktail reception, and rooftop after-party, this year’s event will feature speeches from two long-time patient advocates living with chronic lung conditions, Fred Schick and Betsy Glaeser.
For Dr. Addrizzo-Harris, spotlighting that unique patient perspective is particularly meaningful because the core focus of CHEST and the CHEST Foundation is to improve care and, by extension, patients’ lives.
Visit foundation.chestnet.org to read a blog post with more information about Schick and Glaeser’s work advocating for others with lung disease, find more details about the Belmont Stakes Dinner and Auction, and reserve your seat for this night of philanthropy and fun.
Although attendees will be watching “The Test of the Champion” with bated breath, the upcoming Belmont Stakes Dinner and Auction on June 11 in New York City is about much more than a famous horse race. It’s about community – the vibrant community of clinicians, patients, advocates, and more who support the mission to crush lung disease.
The event started small with a Sunday brunch at the home of CHEST President-Elect Doreen Addrizzo-Harris, MD, FCCP, where attendees gathered to learn more from their host about the CHEST Foundation’s many initiatives. However, over the years, Dr. Addrizzo-Harris leaned on her own community of colleagues, family, friends, and patients to build an event that now boasts hundreds of attendees. But despite all that has changed, the Belmont Stakes Dinner and Auction is still dedicated to raising awareness about the CHEST Foundation and fundraising for initiatives to develop patient education and improve care.
In addition to a plated dinner, silent auction, cocktail reception, and rooftop after-party, this year’s event will feature speeches from two long-time patient advocates living with chronic lung conditions, Fred Schick and Betsy Glaeser.
For Dr. Addrizzo-Harris, spotlighting that unique patient perspective is particularly meaningful because the core focus of CHEST and the CHEST Foundation is to improve care and, by extension, patients’ lives.
Visit foundation.chestnet.org to read a blog post with more information about Schick and Glaeser’s work advocating for others with lung disease, find more details about the Belmont Stakes Dinner and Auction, and reserve your seat for this night of philanthropy and fun.
Although attendees will be watching “The Test of the Champion” with bated breath, the upcoming Belmont Stakes Dinner and Auction on June 11 in New York City is about much more than a famous horse race. It’s about community – the vibrant community of clinicians, patients, advocates, and more who support the mission to crush lung disease.
The event started small with a Sunday brunch at the home of CHEST President-Elect Doreen Addrizzo-Harris, MD, FCCP, where attendees gathered to learn more from their host about the CHEST Foundation’s many initiatives. However, over the years, Dr. Addrizzo-Harris leaned on her own community of colleagues, family, friends, and patients to build an event that now boasts hundreds of attendees. But despite all that has changed, the Belmont Stakes Dinner and Auction is still dedicated to raising awareness about the CHEST Foundation and fundraising for initiatives to develop patient education and improve care.
In addition to a plated dinner, silent auction, cocktail reception, and rooftop after-party, this year’s event will feature speeches from two long-time patient advocates living with chronic lung conditions, Fred Schick and Betsy Glaeser.
For Dr. Addrizzo-Harris, spotlighting that unique patient perspective is particularly meaningful because the core focus of CHEST and the CHEST Foundation is to improve care and, by extension, patients’ lives.
Visit foundation.chestnet.org to read a blog post with more information about Schick and Glaeser’s work advocating for others with lung disease, find more details about the Belmont Stakes Dinner and Auction, and reserve your seat for this night of philanthropy and fun.
Supporting the Harold Amos Medical Faculty Development program
In 2020, the CHEST Foundation embarked on a bold new initiative to build trust, identify, and remove barriers, and promote health care access for all in order to help fight lung disease. As part of that, we recognize that racial and ethnic minorities have been underrepresented in medical professions, contributing to these barriers to patient care.
We recognize that advocating for these groups and increasing the number of medical professors who represent people of color, ethnic minority groups, or who come from an historically disadvantaged community also increases the number of role models in our communities and can help stimulate greater interest among minority students in the health care professions. This year, CHEST is joining ATS and ALA in funding the Harold Amos Medical Faculty Development program, and the CHEST Foundation will be raising funds to support these fellowship recipients.
Harold Amos, PhD, was the first African American to chair a department, now the Department of Microbiology and Medical Genetics, of the Harvard Medical School. Dr. Amos worked tirelessly to recruit and mentor minority and disadvantaged students to careers in academic medicine and science. He was a founding member of the National Advisory Committee of the Robert Wood Johnson Foundation’s Minority Medical Faculty Development Program in 1983 and served as the Program’s National Program Director between 1989 and 1993. Dr. Amos remained active with the program until his death in 2003.
This program exists to continue Dr. Amos’s legacy and to increase the number of faculty from historically disadvantaged backgrounds who can achieve senior rank in academic medicine, dentistry, or nursing and who will encourage and foster the development of succeeding classes of such physicians, dentists, and nurse-scientists. The impact of this program is clear.
Key results
- Over the past 30 years, 241 scholars had completed all 4 years of the program (as of 2012). More than three-quarters remained in academic medicine, including 57 professors, 76 associate professors, and 56 assistant professors.
- Many program alumni have earned professional honors and become influential leaders in the health care field. For example, three direct institutes at the National Institutes of Health, and 10 have been elected to the Institute of Medicine.
- Alumni have received hundreds of awards and honors, including a MacArthur Fellowship “genius” award.
- Alumni have reached positions of influence in academia that enable them to help correct the underrepresentation of minorities in the health professions and address health disparities.
Former scholars are:
- Members of admission, intern, and faculty selection committees
- On review boards for clinical protocols and research studies
- Officers of professional societies and on editorial boards of academic journals
CHEST is proud to join with ATS and ALA to support this incredible program. We recognize that the impact on the past is only the start. By supporting this initiative, we are also looking to address the challenges of the future as the health care landscape continues to evolve. Ensuring that this program reaches the right groups and continues to promote Dr. Amos’s legacy is integral not only to the success of the program but also to aid us in being able to care for our diverse and unique patient populations. The CHEST Foundation is raising funds to support future fellowship recipients. Join us at our next Viva la Vino wine tasting event on July 14 at 7:00 PM CT. All proceeds go to benefit this important initiative, and you can learn more about the work the Foundation does in a relaxed, social environment.
In 2020, the CHEST Foundation embarked on a bold new initiative to build trust, identify, and remove barriers, and promote health care access for all in order to help fight lung disease. As part of that, we recognize that racial and ethnic minorities have been underrepresented in medical professions, contributing to these barriers to patient care.
We recognize that advocating for these groups and increasing the number of medical professors who represent people of color, ethnic minority groups, or who come from an historically disadvantaged community also increases the number of role models in our communities and can help stimulate greater interest among minority students in the health care professions. This year, CHEST is joining ATS and ALA in funding the Harold Amos Medical Faculty Development program, and the CHEST Foundation will be raising funds to support these fellowship recipients.
Harold Amos, PhD, was the first African American to chair a department, now the Department of Microbiology and Medical Genetics, of the Harvard Medical School. Dr. Amos worked tirelessly to recruit and mentor minority and disadvantaged students to careers in academic medicine and science. He was a founding member of the National Advisory Committee of the Robert Wood Johnson Foundation’s Minority Medical Faculty Development Program in 1983 and served as the Program’s National Program Director between 1989 and 1993. Dr. Amos remained active with the program until his death in 2003.
This program exists to continue Dr. Amos’s legacy and to increase the number of faculty from historically disadvantaged backgrounds who can achieve senior rank in academic medicine, dentistry, or nursing and who will encourage and foster the development of succeeding classes of such physicians, dentists, and nurse-scientists. The impact of this program is clear.
Key results
- Over the past 30 years, 241 scholars had completed all 4 years of the program (as of 2012). More than three-quarters remained in academic medicine, including 57 professors, 76 associate professors, and 56 assistant professors.
- Many program alumni have earned professional honors and become influential leaders in the health care field. For example, three direct institutes at the National Institutes of Health, and 10 have been elected to the Institute of Medicine.
- Alumni have received hundreds of awards and honors, including a MacArthur Fellowship “genius” award.
- Alumni have reached positions of influence in academia that enable them to help correct the underrepresentation of minorities in the health professions and address health disparities.
Former scholars are:
- Members of admission, intern, and faculty selection committees
- On review boards for clinical protocols and research studies
- Officers of professional societies and on editorial boards of academic journals
CHEST is proud to join with ATS and ALA to support this incredible program. We recognize that the impact on the past is only the start. By supporting this initiative, we are also looking to address the challenges of the future as the health care landscape continues to evolve. Ensuring that this program reaches the right groups and continues to promote Dr. Amos’s legacy is integral not only to the success of the program but also to aid us in being able to care for our diverse and unique patient populations. The CHEST Foundation is raising funds to support future fellowship recipients. Join us at our next Viva la Vino wine tasting event on July 14 at 7:00 PM CT. All proceeds go to benefit this important initiative, and you can learn more about the work the Foundation does in a relaxed, social environment.
In 2020, the CHEST Foundation embarked on a bold new initiative to build trust, identify, and remove barriers, and promote health care access for all in order to help fight lung disease. As part of that, we recognize that racial and ethnic minorities have been underrepresented in medical professions, contributing to these barriers to patient care.
We recognize that advocating for these groups and increasing the number of medical professors who represent people of color, ethnic minority groups, or who come from an historically disadvantaged community also increases the number of role models in our communities and can help stimulate greater interest among minority students in the health care professions. This year, CHEST is joining ATS and ALA in funding the Harold Amos Medical Faculty Development program, and the CHEST Foundation will be raising funds to support these fellowship recipients.
Harold Amos, PhD, was the first African American to chair a department, now the Department of Microbiology and Medical Genetics, of the Harvard Medical School. Dr. Amos worked tirelessly to recruit and mentor minority and disadvantaged students to careers in academic medicine and science. He was a founding member of the National Advisory Committee of the Robert Wood Johnson Foundation’s Minority Medical Faculty Development Program in 1983 and served as the Program’s National Program Director between 1989 and 1993. Dr. Amos remained active with the program until his death in 2003.
This program exists to continue Dr. Amos’s legacy and to increase the number of faculty from historically disadvantaged backgrounds who can achieve senior rank in academic medicine, dentistry, or nursing and who will encourage and foster the development of succeeding classes of such physicians, dentists, and nurse-scientists. The impact of this program is clear.
Key results
- Over the past 30 years, 241 scholars had completed all 4 years of the program (as of 2012). More than three-quarters remained in academic medicine, including 57 professors, 76 associate professors, and 56 assistant professors.
- Many program alumni have earned professional honors and become influential leaders in the health care field. For example, three direct institutes at the National Institutes of Health, and 10 have been elected to the Institute of Medicine.
- Alumni have received hundreds of awards and honors, including a MacArthur Fellowship “genius” award.
- Alumni have reached positions of influence in academia that enable them to help correct the underrepresentation of minorities in the health professions and address health disparities.
Former scholars are:
- Members of admission, intern, and faculty selection committees
- On review boards for clinical protocols and research studies
- Officers of professional societies and on editorial boards of academic journals
CHEST is proud to join with ATS and ALA to support this incredible program. We recognize that the impact on the past is only the start. By supporting this initiative, we are also looking to address the challenges of the future as the health care landscape continues to evolve. Ensuring that this program reaches the right groups and continues to promote Dr. Amos’s legacy is integral not only to the success of the program but also to aid us in being able to care for our diverse and unique patient populations. The CHEST Foundation is raising funds to support future fellowship recipients. Join us at our next Viva la Vino wine tasting event on July 14 at 7:00 PM CT. All proceeds go to benefit this important initiative, and you can learn more about the work the Foundation does in a relaxed, social environment.













