User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Lung volume reduction methods show similar results for emphysema
BARCELONA – For patients with emphysema who are suitable candidates for lung volume reduction surgery, in a randomized trial.
Among patients with emphysema amenable to surgery, there were similar improvements between the treatment groups at 12-month follow-up as assessed by the iBODE score, a composite disease severity measure incorporating body mass index, airflow obstruction, dyspnea, and exercise capacity (incremental shuttle walk test), reported Sara Buttery, BSc, a research physiotherapist and PhD candidate at the National Heart and Lung Institute at Imperial College London.
“Until now there had been no direct comparison of the two to inform decision-making when a person seems to be suitable for either. Bronchoscopic lung volume reduction is a less invasive option and is thought to be ‘less risky’ but, until now, there has not been substantial research to support this,” she said at the annual congress of the European Respiratory Society.
Ms. Buttery and colleagues conducted a randomized, controlled, single-blinded superiority trial to see whether LVRS could be superior to BLVR with valves. They enrolled 88 patients (52% male) with a mean age of 64, and randomly assigned them to receive either LVRS (41 patients) or the less-invasive BLVR (47 patients).
As noted before, there were no significant differences in outcomes at 1 year, with similar degrees of improvement between the surgical techniques for both the composite iBODE score (–1.10 for LVRS vs. –0.82 for BLVR, nonsignificant), and for the individual components of the score.
In addition, the treatments were associated with similar reductions in gas trapping, with residual volume percentage predicted –36.1 with LVRS versus –30.5 with BLVR (nonsignificant).
One patient in each group died during the 12 months of follow-up. The death of the patient in the BLVR group was deemed to be treatment related; the death of the patient in the LVRS group was related to a noninfective exacerbation of chronic obstructive pulmonary disease.
Invited discussant Isabelle Opitz, MD, from University Hospital Zürich told Ms. Buttery: “I have to congratulate you for this very first randomized controlled trial comparing both procedures in a superiority design.”
She pointed out, however, that the number of patients lost to follow-up and crossover of some patients randomized to bronchoscopy raised questions about the powering of the study.
“We did a sensitivity analysis to have a look to see if there was any difference between the patients who did return and the ones who didn’t, and there was no difference at baseline between those patients.” Ms. Buttery said.
She noted that follow-up visits were hampered by the COVID-19 pandemic and the inability of many patients to come into the clinic.
Dr. Opitz also asked about COPD Assessment Test (CAT) scores that were included in the trial design but not reported in the presentation. Ms. Buttery said that the CAT results favored the LVRS group, and that the results would be included in a future economic analysis.
“The results from this first randomized controlled trial suggest that BLVR may be a good therapeutic option for those patients for whom either procedure is suitable,” said Alexander Mathioudakis, MD, PhD, from the University of Manchester (England), who was not involved with this study but commented on it in a press statement. “Lung volume reduction surgery is an invasive operation as it requires a small incision to be made in the chest, which is stitched up after the procedure. As such, it has risks associated with surgery and it takes longer to recover from than bronchoscopic lung volume reduction. On the other hand, endobronchial valves placement is also associated with side effects, such as pneumonia, or valve displacement. Therefore, both the safety and effectiveness of the two procedures need to be investigated further, in larger groups of patients, but the results from this trial are very encouraging.”
The study is supported by the U.K. National Institute of Health Research. Ms. Buttery, Dr. Opitz, and Dr. Mathioudakis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – For patients with emphysema who are suitable candidates for lung volume reduction surgery, in a randomized trial.
Among patients with emphysema amenable to surgery, there were similar improvements between the treatment groups at 12-month follow-up as assessed by the iBODE score, a composite disease severity measure incorporating body mass index, airflow obstruction, dyspnea, and exercise capacity (incremental shuttle walk test), reported Sara Buttery, BSc, a research physiotherapist and PhD candidate at the National Heart and Lung Institute at Imperial College London.
“Until now there had been no direct comparison of the two to inform decision-making when a person seems to be suitable for either. Bronchoscopic lung volume reduction is a less invasive option and is thought to be ‘less risky’ but, until now, there has not been substantial research to support this,” she said at the annual congress of the European Respiratory Society.
Ms. Buttery and colleagues conducted a randomized, controlled, single-blinded superiority trial to see whether LVRS could be superior to BLVR with valves. They enrolled 88 patients (52% male) with a mean age of 64, and randomly assigned them to receive either LVRS (41 patients) or the less-invasive BLVR (47 patients).
As noted before, there were no significant differences in outcomes at 1 year, with similar degrees of improvement between the surgical techniques for both the composite iBODE score (–1.10 for LVRS vs. –0.82 for BLVR, nonsignificant), and for the individual components of the score.
In addition, the treatments were associated with similar reductions in gas trapping, with residual volume percentage predicted –36.1 with LVRS versus –30.5 with BLVR (nonsignificant).
One patient in each group died during the 12 months of follow-up. The death of the patient in the BLVR group was deemed to be treatment related; the death of the patient in the LVRS group was related to a noninfective exacerbation of chronic obstructive pulmonary disease.
Invited discussant Isabelle Opitz, MD, from University Hospital Zürich told Ms. Buttery: “I have to congratulate you for this very first randomized controlled trial comparing both procedures in a superiority design.”
She pointed out, however, that the number of patients lost to follow-up and crossover of some patients randomized to bronchoscopy raised questions about the powering of the study.
“We did a sensitivity analysis to have a look to see if there was any difference between the patients who did return and the ones who didn’t, and there was no difference at baseline between those patients.” Ms. Buttery said.
She noted that follow-up visits were hampered by the COVID-19 pandemic and the inability of many patients to come into the clinic.
Dr. Opitz also asked about COPD Assessment Test (CAT) scores that were included in the trial design but not reported in the presentation. Ms. Buttery said that the CAT results favored the LVRS group, and that the results would be included in a future economic analysis.
“The results from this first randomized controlled trial suggest that BLVR may be a good therapeutic option for those patients for whom either procedure is suitable,” said Alexander Mathioudakis, MD, PhD, from the University of Manchester (England), who was not involved with this study but commented on it in a press statement. “Lung volume reduction surgery is an invasive operation as it requires a small incision to be made in the chest, which is stitched up after the procedure. As such, it has risks associated with surgery and it takes longer to recover from than bronchoscopic lung volume reduction. On the other hand, endobronchial valves placement is also associated with side effects, such as pneumonia, or valve displacement. Therefore, both the safety and effectiveness of the two procedures need to be investigated further, in larger groups of patients, but the results from this trial are very encouraging.”
The study is supported by the U.K. National Institute of Health Research. Ms. Buttery, Dr. Opitz, and Dr. Mathioudakis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – For patients with emphysema who are suitable candidates for lung volume reduction surgery, in a randomized trial.
Among patients with emphysema amenable to surgery, there were similar improvements between the treatment groups at 12-month follow-up as assessed by the iBODE score, a composite disease severity measure incorporating body mass index, airflow obstruction, dyspnea, and exercise capacity (incremental shuttle walk test), reported Sara Buttery, BSc, a research physiotherapist and PhD candidate at the National Heart and Lung Institute at Imperial College London.
“Until now there had been no direct comparison of the two to inform decision-making when a person seems to be suitable for either. Bronchoscopic lung volume reduction is a less invasive option and is thought to be ‘less risky’ but, until now, there has not been substantial research to support this,” she said at the annual congress of the European Respiratory Society.
Ms. Buttery and colleagues conducted a randomized, controlled, single-blinded superiority trial to see whether LVRS could be superior to BLVR with valves. They enrolled 88 patients (52% male) with a mean age of 64, and randomly assigned them to receive either LVRS (41 patients) or the less-invasive BLVR (47 patients).
As noted before, there were no significant differences in outcomes at 1 year, with similar degrees of improvement between the surgical techniques for both the composite iBODE score (–1.10 for LVRS vs. –0.82 for BLVR, nonsignificant), and for the individual components of the score.
In addition, the treatments were associated with similar reductions in gas trapping, with residual volume percentage predicted –36.1 with LVRS versus –30.5 with BLVR (nonsignificant).
One patient in each group died during the 12 months of follow-up. The death of the patient in the BLVR group was deemed to be treatment related; the death of the patient in the LVRS group was related to a noninfective exacerbation of chronic obstructive pulmonary disease.
Invited discussant Isabelle Opitz, MD, from University Hospital Zürich told Ms. Buttery: “I have to congratulate you for this very first randomized controlled trial comparing both procedures in a superiority design.”
She pointed out, however, that the number of patients lost to follow-up and crossover of some patients randomized to bronchoscopy raised questions about the powering of the study.
“We did a sensitivity analysis to have a look to see if there was any difference between the patients who did return and the ones who didn’t, and there was no difference at baseline between those patients.” Ms. Buttery said.
She noted that follow-up visits were hampered by the COVID-19 pandemic and the inability of many patients to come into the clinic.
Dr. Opitz also asked about COPD Assessment Test (CAT) scores that were included in the trial design but not reported in the presentation. Ms. Buttery said that the CAT results favored the LVRS group, and that the results would be included in a future economic analysis.
“The results from this first randomized controlled trial suggest that BLVR may be a good therapeutic option for those patients for whom either procedure is suitable,” said Alexander Mathioudakis, MD, PhD, from the University of Manchester (England), who was not involved with this study but commented on it in a press statement. “Lung volume reduction surgery is an invasive operation as it requires a small incision to be made in the chest, which is stitched up after the procedure. As such, it has risks associated with surgery and it takes longer to recover from than bronchoscopic lung volume reduction. On the other hand, endobronchial valves placement is also associated with side effects, such as pneumonia, or valve displacement. Therefore, both the safety and effectiveness of the two procedures need to be investigated further, in larger groups of patients, but the results from this trial are very encouraging.”
The study is supported by the U.K. National Institute of Health Research. Ms. Buttery, Dr. Opitz, and Dr. Mathioudakis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ERS 2022 CONGRESS
‘Dr. Caveman’ had a leg up on amputation
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
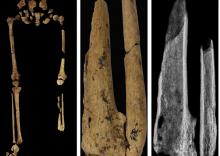
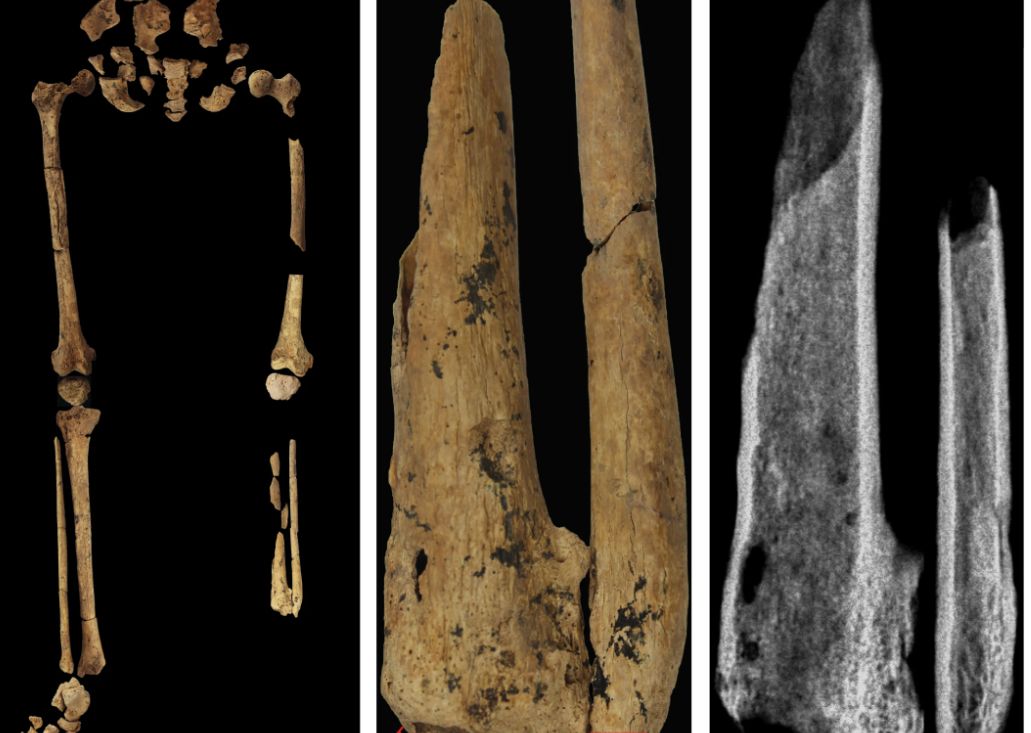
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
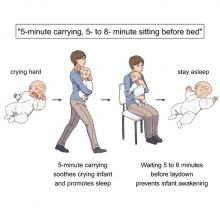
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
In NSCLC, not all EGFR mutations are the same
In non–small cell lung cancer (NSCLC), . However, there is a range of different EGFR mutations, and different mutation combinations can lead to different tumor characteristics that might in turn affect response to therapy.
A new real-world analysis of 159 NSCLC patients found that a combination of a mutation of the TP53 tumor suppressor gene and the EGFR Ex20 mutation is associated with worse disease outcomes, compared to patients with the EGFR Ex20 mutation alone. But the news wasn’t all bad. The same group of patients also responded better to ICB (immune checkpoint blockade) therapy than did the broader population of EGFR Ex20 patients.
The EGFR Ex20 mutation occurs in about 4% of NSCLC cases, while TP53 is quite common: The new study found a frequency of 43.9%. “We first have to mention that the findings regarding TP53 do not reach statistical significance; however, the trend is very strong, and results might be hampered due to small sample sizes. We think it is [appropriate] to exhaust more treatment options for these patients, especially targeted approaches with newer drugs that specifically target exon 20 insertions, as these drugs were not applied in our cohort,” Anna Kron, Dr. rer. medic., said in an email exchange. Dr. Kron presented the results at a poster session in Paris at the ESMO Congress. She is a researcher at University Hospital of Cologne, Germany.
The ImmunoTarget study, published in 2019, examined over 500 NSCLC patients with a range of driver mutations including EGFR and found that they responded poorly to ICIs in comparison to KRAS mutations.
But Dr. Kron’s group was not convinced. “Ex20 mutations differ clinically from other tyrosine kinase mutations in EGFR. We set out this study to rechallenge the paradigm of impaired benefit from ICI in EGFR-mutated patients, as we consider these mutations not interchangeable with other EGFR mutations,” Dr. Kron said.
“We would postulate that in EGFR Exon 20 mutations, ICI and specific inhibitors should be part of the therapeutic course. In patients with co-occurring TP53 mutations, treatment escalation could be considered,” Dr. Kron said.
The study included 159 patients with advanced NSCLC with the EGFR exon 20 insertion, who were treated between 2014 and 2020 at German hospitals. Among the patients, 37.7% were female; mean age at diagnosis was 65.87 years; 50.3% had a smoking history and 38.4% did not (data were unavailable for the rest); and 9.4% of tumors were stage I, 4.4% stage II, 8.2% stage IIIA, 3.8% stage IIIB, and 74.2% stage IV.
Over a follow-up of 4.1 years, there was a trend toward longer survival among patients with TP53 wild type (OS, 20 versus 12 months; P = .092). Sixty-six patients who received ICI therapy had better OS compared with those who did not (22 versus 10 months; P = .018). Among patients with co-occurring TP53 mutations, receipt of ICI therapy was associated with longer OS (16 versus 8 months; P = .048). There was a trend toward patients with TP53 wild type treated with ICI faring better than those who didn’t receive ICI (27.0 months versus 11.0 months; P = .109).
The researchers are continuing to study patients with EGFR Ex20 to better understand the role of TP53 and ICI therapy in these patients.
The study received no funding. Dr. Kron has no relevant financial disclosures.
In non–small cell lung cancer (NSCLC), . However, there is a range of different EGFR mutations, and different mutation combinations can lead to different tumor characteristics that might in turn affect response to therapy.
A new real-world analysis of 159 NSCLC patients found that a combination of a mutation of the TP53 tumor suppressor gene and the EGFR Ex20 mutation is associated with worse disease outcomes, compared to patients with the EGFR Ex20 mutation alone. But the news wasn’t all bad. The same group of patients also responded better to ICB (immune checkpoint blockade) therapy than did the broader population of EGFR Ex20 patients.
The EGFR Ex20 mutation occurs in about 4% of NSCLC cases, while TP53 is quite common: The new study found a frequency of 43.9%. “We first have to mention that the findings regarding TP53 do not reach statistical significance; however, the trend is very strong, and results might be hampered due to small sample sizes. We think it is [appropriate] to exhaust more treatment options for these patients, especially targeted approaches with newer drugs that specifically target exon 20 insertions, as these drugs were not applied in our cohort,” Anna Kron, Dr. rer. medic., said in an email exchange. Dr. Kron presented the results at a poster session in Paris at the ESMO Congress. She is a researcher at University Hospital of Cologne, Germany.
The ImmunoTarget study, published in 2019, examined over 500 NSCLC patients with a range of driver mutations including EGFR and found that they responded poorly to ICIs in comparison to KRAS mutations.
But Dr. Kron’s group was not convinced. “Ex20 mutations differ clinically from other tyrosine kinase mutations in EGFR. We set out this study to rechallenge the paradigm of impaired benefit from ICI in EGFR-mutated patients, as we consider these mutations not interchangeable with other EGFR mutations,” Dr. Kron said.
“We would postulate that in EGFR Exon 20 mutations, ICI and specific inhibitors should be part of the therapeutic course. In patients with co-occurring TP53 mutations, treatment escalation could be considered,” Dr. Kron said.
The study included 159 patients with advanced NSCLC with the EGFR exon 20 insertion, who were treated between 2014 and 2020 at German hospitals. Among the patients, 37.7% were female; mean age at diagnosis was 65.87 years; 50.3% had a smoking history and 38.4% did not (data were unavailable for the rest); and 9.4% of tumors were stage I, 4.4% stage II, 8.2% stage IIIA, 3.8% stage IIIB, and 74.2% stage IV.
Over a follow-up of 4.1 years, there was a trend toward longer survival among patients with TP53 wild type (OS, 20 versus 12 months; P = .092). Sixty-six patients who received ICI therapy had better OS compared with those who did not (22 versus 10 months; P = .018). Among patients with co-occurring TP53 mutations, receipt of ICI therapy was associated with longer OS (16 versus 8 months; P = .048). There was a trend toward patients with TP53 wild type treated with ICI faring better than those who didn’t receive ICI (27.0 months versus 11.0 months; P = .109).
The researchers are continuing to study patients with EGFR Ex20 to better understand the role of TP53 and ICI therapy in these patients.
The study received no funding. Dr. Kron has no relevant financial disclosures.
In non–small cell lung cancer (NSCLC), . However, there is a range of different EGFR mutations, and different mutation combinations can lead to different tumor characteristics that might in turn affect response to therapy.
A new real-world analysis of 159 NSCLC patients found that a combination of a mutation of the TP53 tumor suppressor gene and the EGFR Ex20 mutation is associated with worse disease outcomes, compared to patients with the EGFR Ex20 mutation alone. But the news wasn’t all bad. The same group of patients also responded better to ICB (immune checkpoint blockade) therapy than did the broader population of EGFR Ex20 patients.
The EGFR Ex20 mutation occurs in about 4% of NSCLC cases, while TP53 is quite common: The new study found a frequency of 43.9%. “We first have to mention that the findings regarding TP53 do not reach statistical significance; however, the trend is very strong, and results might be hampered due to small sample sizes. We think it is [appropriate] to exhaust more treatment options for these patients, especially targeted approaches with newer drugs that specifically target exon 20 insertions, as these drugs were not applied in our cohort,” Anna Kron, Dr. rer. medic., said in an email exchange. Dr. Kron presented the results at a poster session in Paris at the ESMO Congress. She is a researcher at University Hospital of Cologne, Germany.
The ImmunoTarget study, published in 2019, examined over 500 NSCLC patients with a range of driver mutations including EGFR and found that they responded poorly to ICIs in comparison to KRAS mutations.
But Dr. Kron’s group was not convinced. “Ex20 mutations differ clinically from other tyrosine kinase mutations in EGFR. We set out this study to rechallenge the paradigm of impaired benefit from ICI in EGFR-mutated patients, as we consider these mutations not interchangeable with other EGFR mutations,” Dr. Kron said.
“We would postulate that in EGFR Exon 20 mutations, ICI and specific inhibitors should be part of the therapeutic course. In patients with co-occurring TP53 mutations, treatment escalation could be considered,” Dr. Kron said.
The study included 159 patients with advanced NSCLC with the EGFR exon 20 insertion, who were treated between 2014 and 2020 at German hospitals. Among the patients, 37.7% were female; mean age at diagnosis was 65.87 years; 50.3% had a smoking history and 38.4% did not (data were unavailable for the rest); and 9.4% of tumors were stage I, 4.4% stage II, 8.2% stage IIIA, 3.8% stage IIIB, and 74.2% stage IV.
Over a follow-up of 4.1 years, there was a trend toward longer survival among patients with TP53 wild type (OS, 20 versus 12 months; P = .092). Sixty-six patients who received ICI therapy had better OS compared with those who did not (22 versus 10 months; P = .018). Among patients with co-occurring TP53 mutations, receipt of ICI therapy was associated with longer OS (16 versus 8 months; P = .048). There was a trend toward patients with TP53 wild type treated with ICI faring better than those who didn’t receive ICI (27.0 months versus 11.0 months; P = .109).
The researchers are continuing to study patients with EGFR Ex20 to better understand the role of TP53 and ICI therapy in these patients.
The study received no funding. Dr. Kron has no relevant financial disclosures.
FROM ESMO CONGRESS 2022
In early NSCLC, comorbidities linked to survival
Cardiometabolic and respiratory comorbidities are associated with worse survival in patients with non–small cell lung cancer (NSCLC), and new research suggests a potential mechanism.
Prior studies had shown mixed results when it came to these comorbidities and survival, according to study coauthor author Geoffrey Liu, MD, who is an epidemiology researcher at the University of Toronto Princess Margaret Cancer Centre. The new work represents data from multiple continents, from various ethnicities and cultures.
“We found that comorbidities had much greater impact on earlier than later stages of lung cancer, consistent with this previous study,” said Dr. Liu in an email. The study was presented by Miguel Garcia-Pardo, who is a researcher at University of Toronto Princess Margaret Cancer Centre, during a poster session at the annual meeting of the European Society for Medical Oncology.
“Deaths from [cardiometabolic] comorbidities were mainly from non–lung cancer competing causes, whereas the deaths from respiratory comorbidities were primarily driven by lung cancer specific survival, i.e., deaths from lung cancer itself. We conclude that Dr. Liu said.
Dr. Liu noted that controlling cardiometabolic risk factors like diabetes and hypertension is typically de-emphasized after diagnosis with early-stage lung cancer. The rationale is often that the lung cancer is a more acute concern than longer-term cardiometabolic risks. “The data from our analyses suggest a rethinking of this strategy. We need to pay more attention to controlling cardiovascular risk factors in early-stage lung cancer,” Dr. Liu said.
The findings also suggest that respiratory comorbidities should be managed more aggressively. That would allow more patients to undergo treatments like surgery and stereotactic radiation.
The Clinical Outcome Studies of the International Lung Cancer Consortium drew from two dozen studies conducted across five continents. It examined clinical, epidemiologic, genetic, and genomic factors and their potential influence on NSCLC outcomes. Cardiometabolic comorbidities included coronary artery disease, diabetes, vascular related diseases, and other heart diseases. Respiratory comorbidities included chronic obstructive pulmonary disease and asthma.
The analysis included 16,354 patients. Among patients with stage I NSCLC, there was an association between reduced overall survival (OS) and cardiometabolic comorbidity (adjusted hazard ratio, 1.17; P = .01) and respiratory comorbidity (aHR, 1.36; P < .001). For stage II/III patients, there was no significant association between OS and cardiometabolic comorbidities, but respiratory comorbidity was associated with worse OS (aHR, 1.15; P < .001). In stage 4, worse OS was associated with both cardiometabolic health comorbidity (aHR, 1.11; P = .03), but not respiratory comorbidity.
Among patients with stage IV NSCLC, there were no associations between overall survival or lung cancer–specific survival (LCSS) and respiratory or cardiometabolic risk factors. However, an examination of cause of death found a different pattern in patients with stage IB-IIIA disease: LCSS was worse among patients with respiratory comorbidities (aHR, 1.21; 95% CI, 1.09-1.34). Among those with cardiovascular comorbidities, the risk of non-NSCLC mortality was higher (aHR, 1.36; 95% CI, 1.15-1.63). The presence of respiratory comorbidity was associated with a reduced probability of undergoing surgical resection for both stage I (adjusted odds ratio, 0.45; 95% CI, 0.35-0.59) and stage II/III patients (aOR, 0.66; 95% CI, 0.53-0.80).
There was an association between non-NSCLC mortality and cardiometabolic comorbidities in stage IA (aHR, 1.37; 95% CI, 1.06-1.77) and in stages IB-IIIA (aHR, 1.32; 95% CI, 1.03-1.71) NSCLC. There were also associations between NSCLC mortality and respiratory comorbidity among stage IA (aHR, 1.51; 95% CI, 1.17-1.95) and stages IB-IIIA (aHR, 1.20; 95% CI, 1.06-1.36) NSCLC. There were no associations between respiratory comorbidity and non-NSCLC mortality.
Respiratory comorbidity was associated with a lower chance of undergoing surgical resection in stage IA (aHR, 0.54; 95% CI, 0.35-0.83) and stage IB-IIIA (aHR, 0.57; 95% CI, 0.46-0.70) cancers. Cardiometabolic comorbidity was associated with a lower rate of surgical resection only in stage 1B-3A patients (aHR, 0.73; 95% CI, 0.56-0.96). Among those who underwent resection, stage IA patients were less likely to die of lung cancer (aHR, 0.38; 95% CI, 0.28-0.52) but more likely to die of other causes (aHR, 1.73; 95% CI, 1.07-1.78). Stage IB-IIIA patients who underwent resection were less likely to die of lung cancer (aHR, 0.37; 95%, 0.32-0.42), but there was no significant association with non–lung cancer mortality.
The study was funded by the Lusi Wong Family Fund and the Alan Brown Chair. Dr. Liu has no relevant financial disclosures.
Cardiometabolic and respiratory comorbidities are associated with worse survival in patients with non–small cell lung cancer (NSCLC), and new research suggests a potential mechanism.
Prior studies had shown mixed results when it came to these comorbidities and survival, according to study coauthor author Geoffrey Liu, MD, who is an epidemiology researcher at the University of Toronto Princess Margaret Cancer Centre. The new work represents data from multiple continents, from various ethnicities and cultures.
“We found that comorbidities had much greater impact on earlier than later stages of lung cancer, consistent with this previous study,” said Dr. Liu in an email. The study was presented by Miguel Garcia-Pardo, who is a researcher at University of Toronto Princess Margaret Cancer Centre, during a poster session at the annual meeting of the European Society for Medical Oncology.
“Deaths from [cardiometabolic] comorbidities were mainly from non–lung cancer competing causes, whereas the deaths from respiratory comorbidities were primarily driven by lung cancer specific survival, i.e., deaths from lung cancer itself. We conclude that Dr. Liu said.
Dr. Liu noted that controlling cardiometabolic risk factors like diabetes and hypertension is typically de-emphasized after diagnosis with early-stage lung cancer. The rationale is often that the lung cancer is a more acute concern than longer-term cardiometabolic risks. “The data from our analyses suggest a rethinking of this strategy. We need to pay more attention to controlling cardiovascular risk factors in early-stage lung cancer,” Dr. Liu said.
The findings also suggest that respiratory comorbidities should be managed more aggressively. That would allow more patients to undergo treatments like surgery and stereotactic radiation.
The Clinical Outcome Studies of the International Lung Cancer Consortium drew from two dozen studies conducted across five continents. It examined clinical, epidemiologic, genetic, and genomic factors and their potential influence on NSCLC outcomes. Cardiometabolic comorbidities included coronary artery disease, diabetes, vascular related diseases, and other heart diseases. Respiratory comorbidities included chronic obstructive pulmonary disease and asthma.
The analysis included 16,354 patients. Among patients with stage I NSCLC, there was an association between reduced overall survival (OS) and cardiometabolic comorbidity (adjusted hazard ratio, 1.17; P = .01) and respiratory comorbidity (aHR, 1.36; P < .001). For stage II/III patients, there was no significant association between OS and cardiometabolic comorbidities, but respiratory comorbidity was associated with worse OS (aHR, 1.15; P < .001). In stage 4, worse OS was associated with both cardiometabolic health comorbidity (aHR, 1.11; P = .03), but not respiratory comorbidity.
Among patients with stage IV NSCLC, there were no associations between overall survival or lung cancer–specific survival (LCSS) and respiratory or cardiometabolic risk factors. However, an examination of cause of death found a different pattern in patients with stage IB-IIIA disease: LCSS was worse among patients with respiratory comorbidities (aHR, 1.21; 95% CI, 1.09-1.34). Among those with cardiovascular comorbidities, the risk of non-NSCLC mortality was higher (aHR, 1.36; 95% CI, 1.15-1.63). The presence of respiratory comorbidity was associated with a reduced probability of undergoing surgical resection for both stage I (adjusted odds ratio, 0.45; 95% CI, 0.35-0.59) and stage II/III patients (aOR, 0.66; 95% CI, 0.53-0.80).
There was an association between non-NSCLC mortality and cardiometabolic comorbidities in stage IA (aHR, 1.37; 95% CI, 1.06-1.77) and in stages IB-IIIA (aHR, 1.32; 95% CI, 1.03-1.71) NSCLC. There were also associations between NSCLC mortality and respiratory comorbidity among stage IA (aHR, 1.51; 95% CI, 1.17-1.95) and stages IB-IIIA (aHR, 1.20; 95% CI, 1.06-1.36) NSCLC. There were no associations between respiratory comorbidity and non-NSCLC mortality.
Respiratory comorbidity was associated with a lower chance of undergoing surgical resection in stage IA (aHR, 0.54; 95% CI, 0.35-0.83) and stage IB-IIIA (aHR, 0.57; 95% CI, 0.46-0.70) cancers. Cardiometabolic comorbidity was associated with a lower rate of surgical resection only in stage 1B-3A patients (aHR, 0.73; 95% CI, 0.56-0.96). Among those who underwent resection, stage IA patients were less likely to die of lung cancer (aHR, 0.38; 95% CI, 0.28-0.52) but more likely to die of other causes (aHR, 1.73; 95% CI, 1.07-1.78). Stage IB-IIIA patients who underwent resection were less likely to die of lung cancer (aHR, 0.37; 95%, 0.32-0.42), but there was no significant association with non–lung cancer mortality.
The study was funded by the Lusi Wong Family Fund and the Alan Brown Chair. Dr. Liu has no relevant financial disclosures.
Cardiometabolic and respiratory comorbidities are associated with worse survival in patients with non–small cell lung cancer (NSCLC), and new research suggests a potential mechanism.
Prior studies had shown mixed results when it came to these comorbidities and survival, according to study coauthor author Geoffrey Liu, MD, who is an epidemiology researcher at the University of Toronto Princess Margaret Cancer Centre. The new work represents data from multiple continents, from various ethnicities and cultures.
“We found that comorbidities had much greater impact on earlier than later stages of lung cancer, consistent with this previous study,” said Dr. Liu in an email. The study was presented by Miguel Garcia-Pardo, who is a researcher at University of Toronto Princess Margaret Cancer Centre, during a poster session at the annual meeting of the European Society for Medical Oncology.
“Deaths from [cardiometabolic] comorbidities were mainly from non–lung cancer competing causes, whereas the deaths from respiratory comorbidities were primarily driven by lung cancer specific survival, i.e., deaths from lung cancer itself. We conclude that Dr. Liu said.
Dr. Liu noted that controlling cardiometabolic risk factors like diabetes and hypertension is typically de-emphasized after diagnosis with early-stage lung cancer. The rationale is often that the lung cancer is a more acute concern than longer-term cardiometabolic risks. “The data from our analyses suggest a rethinking of this strategy. We need to pay more attention to controlling cardiovascular risk factors in early-stage lung cancer,” Dr. Liu said.
The findings also suggest that respiratory comorbidities should be managed more aggressively. That would allow more patients to undergo treatments like surgery and stereotactic radiation.
The Clinical Outcome Studies of the International Lung Cancer Consortium drew from two dozen studies conducted across five continents. It examined clinical, epidemiologic, genetic, and genomic factors and their potential influence on NSCLC outcomes. Cardiometabolic comorbidities included coronary artery disease, diabetes, vascular related diseases, and other heart diseases. Respiratory comorbidities included chronic obstructive pulmonary disease and asthma.
The analysis included 16,354 patients. Among patients with stage I NSCLC, there was an association between reduced overall survival (OS) and cardiometabolic comorbidity (adjusted hazard ratio, 1.17; P = .01) and respiratory comorbidity (aHR, 1.36; P < .001). For stage II/III patients, there was no significant association between OS and cardiometabolic comorbidities, but respiratory comorbidity was associated with worse OS (aHR, 1.15; P < .001). In stage 4, worse OS was associated with both cardiometabolic health comorbidity (aHR, 1.11; P = .03), but not respiratory comorbidity.
Among patients with stage IV NSCLC, there were no associations between overall survival or lung cancer–specific survival (LCSS) and respiratory or cardiometabolic risk factors. However, an examination of cause of death found a different pattern in patients with stage IB-IIIA disease: LCSS was worse among patients with respiratory comorbidities (aHR, 1.21; 95% CI, 1.09-1.34). Among those with cardiovascular comorbidities, the risk of non-NSCLC mortality was higher (aHR, 1.36; 95% CI, 1.15-1.63). The presence of respiratory comorbidity was associated with a reduced probability of undergoing surgical resection for both stage I (adjusted odds ratio, 0.45; 95% CI, 0.35-0.59) and stage II/III patients (aOR, 0.66; 95% CI, 0.53-0.80).
There was an association between non-NSCLC mortality and cardiometabolic comorbidities in stage IA (aHR, 1.37; 95% CI, 1.06-1.77) and in stages IB-IIIA (aHR, 1.32; 95% CI, 1.03-1.71) NSCLC. There were also associations between NSCLC mortality and respiratory comorbidity among stage IA (aHR, 1.51; 95% CI, 1.17-1.95) and stages IB-IIIA (aHR, 1.20; 95% CI, 1.06-1.36) NSCLC. There were no associations between respiratory comorbidity and non-NSCLC mortality.
Respiratory comorbidity was associated with a lower chance of undergoing surgical resection in stage IA (aHR, 0.54; 95% CI, 0.35-0.83) and stage IB-IIIA (aHR, 0.57; 95% CI, 0.46-0.70) cancers. Cardiometabolic comorbidity was associated with a lower rate of surgical resection only in stage 1B-3A patients (aHR, 0.73; 95% CI, 0.56-0.96). Among those who underwent resection, stage IA patients were less likely to die of lung cancer (aHR, 0.38; 95% CI, 0.28-0.52) but more likely to die of other causes (aHR, 1.73; 95% CI, 1.07-1.78). Stage IB-IIIA patients who underwent resection were less likely to die of lung cancer (aHR, 0.37; 95%, 0.32-0.42), but there was no significant association with non–lung cancer mortality.
The study was funded by the Lusi Wong Family Fund and the Alan Brown Chair. Dr. Liu has no relevant financial disclosures.
FROM ESMO CONGRESS 2022
‘Smoking gun–level’ evidence found linking air pollution with lung cancer
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
AT ESMO CONGRESS 2022
Heparin pretreatment may safely open arteries before STEMI cath
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests a large registry study.
An open infarct-related artery (IRA) at angiography on cath-lab arrival presents STEMI patients an opportunity for earlier reperfusion and a chance, in theory at least, for smaller infarcts and maybe improved clinical outcomes.
In the new analysis, which covers more than 40,000 patients with STEMI in Sweden, the 38% who received heparin before cath-lab arrival were 11% less likely to show IRA occlusion at angiography prior to direct percutaneous coronary intervention (PCI). They also showed a 13% lower 30-day mortality compared with patients who were started on heparin in the cath lab. Importantly, their risk of major bleeding in the hospital did not increase.
The “early reperfusion” associated with IRA patency at angiography “could have long-term benefit due to smaller infarct size,” potentially explaining the observed 30-day survival gain in the pretreatment group, Oskar Love Emilsson, Lund (Sweden) University, said in an interview.
Mr. Emilsson, a third-year medical student, reported the analysis at the annual congress of the European Society of Cardiology, and is lead author on its same-day publication in the journal EuroIntervention.
He mentioned a few cautions in interpreting the study, which is based primarily on data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). It included several sensitivity analyses that continued to back pretreatment heparin as a significant predictor of an unoccluded IRA but didn’t consistently support the 30-day mortality benefit seen in the primary analysis.
And, although the pretreatment group overall didn’t have more major bleeds, the risk did go up significantly for those older than 75 or those who weighed less than 60 kg (132 pounds) or underwent catheterization with an access route other than the radial artery. Extra caution should be exercised in such patients who receive heparin before cath-lab arrival for PCI, Mr. Emilsson observed.
“Our results suggest that heparin pretreatment might be a good option to improve patency of infarct related arteries in STEMI,” and potentially clinical outcomes, he said. “However, a definite answer would require a randomized controlled trial.”
Meanwhile, the current study may be the largest yet to look at clinical outcomes after pretreatment with unfractionated heparin before PCI for acute STEMI, the report states. There have been some observational studies, subanalyses of STEMI trials, and even a few limited randomized trials – including the HEAP trial published in 2000 – to weigh in on the subject. Some have supported the strategy, others have not.
“With rapid door-to-balloon times in STEMI, it can be challenging to show a significant difference between a prehospital heparin approach and heparin given in the lab,” observed Sunil V. Rao, MD, NYU Langone Health System, New York, who is not connected with the current study.
Many EDs in the United States have “a STEMI protocol that calls for an IV bolus of heparin. It would be tougher in the U.S. to give it in the ambulance but again, it’s not clear how much advantage that would really provide,” he told this news organization.
Support from randomized trials would be needed before the practice could be formally recommended. “The SCAAR registries have set the standard for how registries should be conducted,” Dr. Rao said. “This is a very well done observational study, but it is observational.”
The priority for STEMI patients, he added, “really should be to get them to the lab as fast as possible. If the ED protocol includes heparin before the cath lab, that’s great, but I don’t think we should delay getting these patients to the lab to accommodate pre–cath-lab heparin.”
The current analysis covered 41,631 patients with STEMI from 2008 through to 2016, of whom 38% were pretreated with heparin in an ambulance or the ED. The remaining 62% initiated heparin in the cath lab.
About one-third of the group had an open IRA at angiography. The adjusted risk ratio (RR) for IRA occlusion at angiography for patients pretreated vs. not pretreated with heparin was 0.89 (95% confidence interval [CI], 0.87-0.90).
The corresponding RR for death within 30 days was 0.87 (95% CI, 0.77-0.99), and for major in-hospital bleeding it was 1.01 (95% CI, 0.86-1.18).
The analysis was adjusted for other medications received before cath-lab arrival, especially a long list of antiplatelets and non-heparin antithrombins. That strengthens the case for heparin pretreatment as an independent predictor of an open IRA at initial angiography, Mr. Emilsson said.
Comparisons of propensity-score–matched subgroups of the total cohort, conducted separately for the IRA-occlusion endpoint and the endpoints of 30-day mortality and major bleeding, produced similar results.
Some observational data suggest that antiplatelet pretreatment with a P2Y12 inhibitor may promote IRA patency on angiography after cath lab arrival, Dr. Rao observed. “This indicates that there probably is a role of earlier antithrombotic therapy in STEMI patients, but the randomized trials have not shown a consistent benefit,” he said, referring in particular to the ATLANTIC trial.
Mr. Emilsson and Dr. Rao disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Gene mutations may drive lung cancer in never-smokers
Small cell lung cancer has traditionally been attributed almost exclusively to tobacco exposure, but some recent studies have suggested a higher than expected prevalence among nonsmokers. indicating that the subgroups may have unique disease characteristics. Key differences included a lower frequency of TP53 gene mutations and a higher frequency of epidermal growth factor receptor (EGFR) alterations in never smokers.
About 6.9% of small cell lung cancer patients in the CASPIAN study were nonsmokers, as were 3.0% in the IMpower133 study.
“Given that the pathogenesis of small cell lung cancer is often tied to the damaging effects of tobacco, we hypothesized that small cell lung cancer in never-smokers would possess distinct molecular attributes. Our data does not provide any solid evidence for any treatment implications, though it does raise therapeutic questions which we believe deserve further exploration,” said Michael Oh, MD, during a presentation of the study results at the annual meeting of the European Society for Medical Oncology. Dr. Oh is a fellow at the University of California, Los Angeles.
The topic is important clinically, according to Antonio Passaro, MD, PhD, who served as a discussant during the session. He noted that small cell lung cancer in never-smokers is the seventh-most common cause of cancer-related mortality worldwide. In non–small cell lung cancer, rates of tobacco-associated disease have been decreasing, but there are increases in diagnoses among never smokers. Nonsmoking small cell lung cancer patients do not have better prognoses, and novel therapies and advances like immunotherapy and low-dose CT lung cancer screening disproportionately benefit current or former smokers.
Potential risk factors for never-smokers include environmental exposures like radon gas, cooking oil vapors, indoor and outdoor wood burning, and genetic and viral factors. “At the present time we do not have the knowledge to identify the most important factor in development of lung cancer in never-smoking [patients],” said Dr. Passaro, who is a medical oncologist at the European Institute of Oncology in Milan.
He added that the current study results are interesting but need much more follow-up, such as “longitudinal studies combining detailed clinical annotation with tissue and blood sampling. Here there is a need for collaborative efforts.” Key questions include the roles of the genomic landscape in normal lung tissue may play, the lung micro-environment, genetic factors, and environmental exposures.
One key possibility is air pollution. “We know that lung cancer in never-smokers is frequent in some countries, for example in Asian countries and it is more frequent in the United States than in Europe, but to find an explanation to this kind of data is difficult at the present time,” Dr. Passaro said.
The researchers retrospectively analyzed data from 608 current or former smokers and 54 never-smokers with small cell lung cancer, with the latter making up 8% of the total population. 70.4% of never-smokers and 55.1% of current or former smokers were female (P = .031). There was no significant between-group difference with respect to age at diagnosis or race.
Somatic mutations were similar to what has been found in previous studies for current or former smokers. 85.2% had changes in TP53, compared with just 59.3% of never-smokers (Q < .001). Changes to EGFR were more common in never-smokers, occurring in 25.9% versus 2.6% (Q < .001). PIK3CA alterations were also more common in never-smokers (14.8% vs. 3.6%; Q = 0.022). There was no significant difference between the two groups with respect to changes in RB1.
Never smokers had tumors with less immune cell infiltration (P = .008), including fewer CD4+ T cells, CD8+ T cells, and macrophages. Their tumor mutation burden was also lower (median, 2.59 vs. 4.99; P < .001).
Dr. Oh has no relevant financial disclosures. Dr. Passaro has consulted, advised, and received research funding from a wide range of pharmaceutical companies.
Small cell lung cancer has traditionally been attributed almost exclusively to tobacco exposure, but some recent studies have suggested a higher than expected prevalence among nonsmokers. indicating that the subgroups may have unique disease characteristics. Key differences included a lower frequency of TP53 gene mutations and a higher frequency of epidermal growth factor receptor (EGFR) alterations in never smokers.
About 6.9% of small cell lung cancer patients in the CASPIAN study were nonsmokers, as were 3.0% in the IMpower133 study.
“Given that the pathogenesis of small cell lung cancer is often tied to the damaging effects of tobacco, we hypothesized that small cell lung cancer in never-smokers would possess distinct molecular attributes. Our data does not provide any solid evidence for any treatment implications, though it does raise therapeutic questions which we believe deserve further exploration,” said Michael Oh, MD, during a presentation of the study results at the annual meeting of the European Society for Medical Oncology. Dr. Oh is a fellow at the University of California, Los Angeles.
The topic is important clinically, according to Antonio Passaro, MD, PhD, who served as a discussant during the session. He noted that small cell lung cancer in never-smokers is the seventh-most common cause of cancer-related mortality worldwide. In non–small cell lung cancer, rates of tobacco-associated disease have been decreasing, but there are increases in diagnoses among never smokers. Nonsmoking small cell lung cancer patients do not have better prognoses, and novel therapies and advances like immunotherapy and low-dose CT lung cancer screening disproportionately benefit current or former smokers.
Potential risk factors for never-smokers include environmental exposures like radon gas, cooking oil vapors, indoor and outdoor wood burning, and genetic and viral factors. “At the present time we do not have the knowledge to identify the most important factor in development of lung cancer in never-smoking [patients],” said Dr. Passaro, who is a medical oncologist at the European Institute of Oncology in Milan.
He added that the current study results are interesting but need much more follow-up, such as “longitudinal studies combining detailed clinical annotation with tissue and blood sampling. Here there is a need for collaborative efforts.” Key questions include the roles of the genomic landscape in normal lung tissue may play, the lung micro-environment, genetic factors, and environmental exposures.
One key possibility is air pollution. “We know that lung cancer in never-smokers is frequent in some countries, for example in Asian countries and it is more frequent in the United States than in Europe, but to find an explanation to this kind of data is difficult at the present time,” Dr. Passaro said.
The researchers retrospectively analyzed data from 608 current or former smokers and 54 never-smokers with small cell lung cancer, with the latter making up 8% of the total population. 70.4% of never-smokers and 55.1% of current or former smokers were female (P = .031). There was no significant between-group difference with respect to age at diagnosis or race.
Somatic mutations were similar to what has been found in previous studies for current or former smokers. 85.2% had changes in TP53, compared with just 59.3% of never-smokers (Q < .001). Changes to EGFR were more common in never-smokers, occurring in 25.9% versus 2.6% (Q < .001). PIK3CA alterations were also more common in never-smokers (14.8% vs. 3.6%; Q = 0.022). There was no significant difference between the two groups with respect to changes in RB1.
Never smokers had tumors with less immune cell infiltration (P = .008), including fewer CD4+ T cells, CD8+ T cells, and macrophages. Their tumor mutation burden was also lower (median, 2.59 vs. 4.99; P < .001).
Dr. Oh has no relevant financial disclosures. Dr. Passaro has consulted, advised, and received research funding from a wide range of pharmaceutical companies.
Small cell lung cancer has traditionally been attributed almost exclusively to tobacco exposure, but some recent studies have suggested a higher than expected prevalence among nonsmokers. indicating that the subgroups may have unique disease characteristics. Key differences included a lower frequency of TP53 gene mutations and a higher frequency of epidermal growth factor receptor (EGFR) alterations in never smokers.
About 6.9% of small cell lung cancer patients in the CASPIAN study were nonsmokers, as were 3.0% in the IMpower133 study.
“Given that the pathogenesis of small cell lung cancer is often tied to the damaging effects of tobacco, we hypothesized that small cell lung cancer in never-smokers would possess distinct molecular attributes. Our data does not provide any solid evidence for any treatment implications, though it does raise therapeutic questions which we believe deserve further exploration,” said Michael Oh, MD, during a presentation of the study results at the annual meeting of the European Society for Medical Oncology. Dr. Oh is a fellow at the University of California, Los Angeles.
The topic is important clinically, according to Antonio Passaro, MD, PhD, who served as a discussant during the session. He noted that small cell lung cancer in never-smokers is the seventh-most common cause of cancer-related mortality worldwide. In non–small cell lung cancer, rates of tobacco-associated disease have been decreasing, but there are increases in diagnoses among never smokers. Nonsmoking small cell lung cancer patients do not have better prognoses, and novel therapies and advances like immunotherapy and low-dose CT lung cancer screening disproportionately benefit current or former smokers.
Potential risk factors for never-smokers include environmental exposures like radon gas, cooking oil vapors, indoor and outdoor wood burning, and genetic and viral factors. “At the present time we do not have the knowledge to identify the most important factor in development of lung cancer in never-smoking [patients],” said Dr. Passaro, who is a medical oncologist at the European Institute of Oncology in Milan.
He added that the current study results are interesting but need much more follow-up, such as “longitudinal studies combining detailed clinical annotation with tissue and blood sampling. Here there is a need for collaborative efforts.” Key questions include the roles of the genomic landscape in normal lung tissue may play, the lung micro-environment, genetic factors, and environmental exposures.
One key possibility is air pollution. “We know that lung cancer in never-smokers is frequent in some countries, for example in Asian countries and it is more frequent in the United States than in Europe, but to find an explanation to this kind of data is difficult at the present time,” Dr. Passaro said.
The researchers retrospectively analyzed data from 608 current or former smokers and 54 never-smokers with small cell lung cancer, with the latter making up 8% of the total population. 70.4% of never-smokers and 55.1% of current or former smokers were female (P = .031). There was no significant between-group difference with respect to age at diagnosis or race.
Somatic mutations were similar to what has been found in previous studies for current or former smokers. 85.2% had changes in TP53, compared with just 59.3% of never-smokers (Q < .001). Changes to EGFR were more common in never-smokers, occurring in 25.9% versus 2.6% (Q < .001). PIK3CA alterations were also more common in never-smokers (14.8% vs. 3.6%; Q = 0.022). There was no significant difference between the two groups with respect to changes in RB1.
Never smokers had tumors with less immune cell infiltration (P = .008), including fewer CD4+ T cells, CD8+ T cells, and macrophages. Their tumor mutation burden was also lower (median, 2.59 vs. 4.99; P < .001).
Dr. Oh has no relevant financial disclosures. Dr. Passaro has consulted, advised, and received research funding from a wide range of pharmaceutical companies.
AT ESMO CONGRESS 2022
Myocardial infarction in women younger than 50: Lessons to learn
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
Young women (under 50) are increasingly having heart attacks without doctors really knowing why. This is where the Young Women Presenting Acute Myocardial Infarction in France (WAMIF) study comes in, the results of which were presented in an e-poster at the annual congress of the European Society of Cardiology by Stéphane Manzo-Silberman, MD, Institute of Cardiology, Pitié-Salpétrière, Paris. The results (yet to be published) fight several of the preconceived ideas on the topic, Dr. Manzo-Silberman commented in an interview.
Significantly higher hospital death rates in women
“Cardiovascular disease is the main cause of death in women, killing seven times more than breast cancer,” notes Dr. Manzo-Silberman. The hospital death rate is significantly higher in women and, despite going down, is significantly higher than in men (more than double), particularly in women under 50. What’s more, in addition to the typical risk factors, women present specific risk factors related to hormone changes, high-risk inflammatory profiles, and thrombophilia.”
The WAMIF study was designed to determine the clinical, biological, and morphological features linked to hospital mortality after 12 months in women under 50. The prospective, observational study included all women in this age range from 30 sites in France between May 2017 and June 2019.
90% with retrosternal chest pain
The age of the 314 women enrolled was 44.9 years on average. Nearly two-thirds (192) presented with ST-segment elevation myocardial infarction and the other 122 without. In terms of symptoms, 91.6% of these women presented with typical chest pain, and 59.7% had related symptoms.
“With more than 90% having retrosternal pain, the idea that myocardial infarction presents with atypical symptoms in women has been widely challenged, despite the fact that more than half present with related symptoms and it isn’t known in which order these symptoms occur, Dr. Manzo-Silberman said in an interview. But what we can say is that if at any point a young woman mentions chest pain, even when occurring as part of several other symptoms, MI must be deemed a possibility until it has been ruled out.”
The risk profile revealed that 75.5% were smokers, 35% had a family history of heart disease, 33% had pregnancy complications, and 55% had recently experienced a stressful situation. The analysis also showed that cannabis use and oral contraception were primary risk factors in women younger than 35.
“With regard to risk factors, when designing this study we expected that lots of these young women would have largely atypical autoimmune conditions, with high levels of inflammation. We looked for everything, but this was not actually the case. Instead, we found very many women to have classic risk factors; three-quarters were smokers, a modifiable risk factor, which can largely be prevented. The other aspect concerns contraception, and it’s why I insist that gynecologists must be involved insofar as they must inform their patients how to manage their risk factors and tweak their contraception.”
Coronary angiography findings showed that only 1% received a normal result, 29.3% had vessel damage, and 14.6% had aortic dissection. “We were surprised again here because we expected that with young women we would see lots of heart attacks without obstruction, [in other words] normal coronary arteries, atypical forms of MI,” commented Dr. Manzo-Silberman. “In fact, most presented with atheroma, often obstructive lesions, or even triple-vessel disease, in nearly a third of the cohort. So that’s another misconception dispelled – we can’t just think that because a woman is young, nothing will be found. Coronary catheterization should be considered, and the diagnostic process should be completed in full.”
After 1 year, there had been two cancer-related deaths and 25 patients had undergone several angioplasty procedures. Nevertheless, 90.4% had not experienced any type of CV event, and 72% had not even had any symptoms.
“The final surprise was prognosis,” he said. “Previous studies, especially some authored by Viola Vaccarino, MD, PhD, showed an excess hospital rate in women and we had expected this to be the case here, but no hospital deaths were recorded. However, not far off 10% of women attended (at least once) the emergency department in the year following for recurrent chest pain which was not ischemic – ECG normal, troponin normal – so something was missing in their education as a patient.”
“So, there are improvements to be made in terms of secondary prevention, follow-up, and in the education of these young female patients who have experienced the major event that is a myocardial infarction,” concluded Dr. Manzo-Silberman.
This content was originally published on Medscape French edition.
FROM ESC CONGRESS 2022
Children and COVID: New cases took a downturn in September
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
Sleep Medicine Network
Nonrespiratory Sleep Section
Sleep in cancer patients
Sleep disturbance is among the most common symptoms in patients with cancer with an estimated prevalence of up to two out of three patients experiencing sleep disruption during their cancer journey.1,23,4
Common sleep disorders in cancer patients:
Insomnia: Cancer patients have at least a two-fold higher incidence of insomnia compared with the general population.5,6 Predisposing factors may include age, the presence of hyper-arousability,a prior history of insomnia, or a preexisting psychiatric disorder. Cancer-related factors include surgery, hospitalization, chemotherapy, hormonal therapy, radiation therapy, and use of steroids.7 If sedative-hypnotics are considered, they should be used in conjunction with cognitive and behavioral therapy for insomnia (CBT-I). Recent meta-analyses provide data to support a strong recommendation to utilize CBT-I to treat insomnia in cancer patients.6,8,9
Hypersomnolence: Hypersomnolence or excessive daytime sleepiness is a common symptom noted among cancer patients.10 Hypersomnia related to cancer can be often classified as either hypersomnia due to a medical condition or hypersomnia due to a drug or substance, especially for those patients taking opioid or other sedative medications.
Movement Disorders: Sleep movement disorders occur in patients with cancer and may be primary or attributable to chemotherapy-related neuropathy from therapy regimens, including platinum compounds, taxanes, vinca alkaloids, proteasome inhibitors, or thalidomide-based agents.11,12
Obstructive sleep apnea (OSA): OSA occurs in patients with cancer and may be increased in patients with specific cancers such as head and neck tumors.13 Patients with sleep apnea have a five-fold increased risk of cancer-related mortality, and several studies show an increased incidence of cancer in those with sleep apnea.14-16There is an increasing realization that not only sleep apnea, but sleep disturbance, in general, may be oncogenic based on increased autonomic tone, chronic stress, variation in the pituitary-hypothalamic axis, as well as circadian mechanisms.17
Early recognition/treatment of sleep issues is essential to improve quality of life in cancer patients.
Diwakar Balachandran, MD, FCCP
Member-at-Large
References
1. Balachandran DD, Miller MA, Faiz SA, Yennurajalingam S, Innominato PF. Evaluation and management of sleep and circadian rhythm disturbance in cancer. Curr Treat Options Oncol. 2021;22(9):81.
2. Yennurajalingam S, Balachandran D, Pedraza Cardozo SL, et al. Patient-reported sleep disturbance in advanced cancer: frequency, predictors and screening performance of the Edmonton Symptom Assessment System sleep item. BMJ Support Palliat Care. 2017;7(3):274-80.
3. Harris B, Ross J, Sanchez-Reilly S. Sleeping in the arms of cancer: A review of sleeping disorders among patients with cancer. Cancer J. 2014;20(5):299-305.
4. Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27(7):2747-53.
5. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292-8.
6. Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583-90.
7. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895-908.
8. Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113-24.
9. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20-8.
10. Jaumally BA, Das A, Cassell NC, et al. Excessive daytime sleepiness in cancer patients. Sleep Breath. 2021;25(2):1063-7.
11. Gewandter JS, Kleckner AS, Marshall JH, et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: an NIH Collaboratory study of claims data. Support Care Cancer. 2020;28(6):2553-62.
12. St Germain DC, O’Mara AM, Robinson JL, Torres AD, Minasian LM. Chemotherapy-induced peripheral neuropathy: Identifying the research gaps and associated changes to clinical trial design. Cancer. 2020;126(20):4602-13.
13. Faiz SA, Balachandran D, Hessel AC, et al. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist. 2014;19(11):1200-6.
14. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99-105.
15. Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742-8.
16. Martinez-Garcia MA, Campos-Rodriguez F, Barbe F. Cancer and OSA: Current evidence from human studies. Chest. 2016;150(2):451-63.
17. Gozal D, Farre R, Nieto FJ. Putative links between sleep apnea and cancer: From hypotheses to evolving evidence. Chest. 2015;148(5):1140-7.
Nonrespiratory Sleep Section
Sleep in cancer patients
Sleep disturbance is among the most common symptoms in patients with cancer with an estimated prevalence of up to two out of three patients experiencing sleep disruption during their cancer journey.1,23,4
Common sleep disorders in cancer patients:
Insomnia: Cancer patients have at least a two-fold higher incidence of insomnia compared with the general population.5,6 Predisposing factors may include age, the presence of hyper-arousability,a prior history of insomnia, or a preexisting psychiatric disorder. Cancer-related factors include surgery, hospitalization, chemotherapy, hormonal therapy, radiation therapy, and use of steroids.7 If sedative-hypnotics are considered, they should be used in conjunction with cognitive and behavioral therapy for insomnia (CBT-I). Recent meta-analyses provide data to support a strong recommendation to utilize CBT-I to treat insomnia in cancer patients.6,8,9
Hypersomnolence: Hypersomnolence or excessive daytime sleepiness is a common symptom noted among cancer patients.10 Hypersomnia related to cancer can be often classified as either hypersomnia due to a medical condition or hypersomnia due to a drug or substance, especially for those patients taking opioid or other sedative medications.
Movement Disorders: Sleep movement disorders occur in patients with cancer and may be primary or attributable to chemotherapy-related neuropathy from therapy regimens, including platinum compounds, taxanes, vinca alkaloids, proteasome inhibitors, or thalidomide-based agents.11,12
Obstructive sleep apnea (OSA): OSA occurs in patients with cancer and may be increased in patients with specific cancers such as head and neck tumors.13 Patients with sleep apnea have a five-fold increased risk of cancer-related mortality, and several studies show an increased incidence of cancer in those with sleep apnea.14-16There is an increasing realization that not only sleep apnea, but sleep disturbance, in general, may be oncogenic based on increased autonomic tone, chronic stress, variation in the pituitary-hypothalamic axis, as well as circadian mechanisms.17
Early recognition/treatment of sleep issues is essential to improve quality of life in cancer patients.
Diwakar Balachandran, MD, FCCP
Member-at-Large
References
1. Balachandran DD, Miller MA, Faiz SA, Yennurajalingam S, Innominato PF. Evaluation and management of sleep and circadian rhythm disturbance in cancer. Curr Treat Options Oncol. 2021;22(9):81.
2. Yennurajalingam S, Balachandran D, Pedraza Cardozo SL, et al. Patient-reported sleep disturbance in advanced cancer: frequency, predictors and screening performance of the Edmonton Symptom Assessment System sleep item. BMJ Support Palliat Care. 2017;7(3):274-80.
3. Harris B, Ross J, Sanchez-Reilly S. Sleeping in the arms of cancer: A review of sleeping disorders among patients with cancer. Cancer J. 2014;20(5):299-305.
4. Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27(7):2747-53.
5. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292-8.
6. Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583-90.
7. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895-908.
8. Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113-24.
9. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20-8.
10. Jaumally BA, Das A, Cassell NC, et al. Excessive daytime sleepiness in cancer patients. Sleep Breath. 2021;25(2):1063-7.
11. Gewandter JS, Kleckner AS, Marshall JH, et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: an NIH Collaboratory study of claims data. Support Care Cancer. 2020;28(6):2553-62.
12. St Germain DC, O’Mara AM, Robinson JL, Torres AD, Minasian LM. Chemotherapy-induced peripheral neuropathy: Identifying the research gaps and associated changes to clinical trial design. Cancer. 2020;126(20):4602-13.
13. Faiz SA, Balachandran D, Hessel AC, et al. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist. 2014;19(11):1200-6.
14. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99-105.
15. Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742-8.
16. Martinez-Garcia MA, Campos-Rodriguez F, Barbe F. Cancer and OSA: Current evidence from human studies. Chest. 2016;150(2):451-63.
17. Gozal D, Farre R, Nieto FJ. Putative links between sleep apnea and cancer: From hypotheses to evolving evidence. Chest. 2015;148(5):1140-7.
Nonrespiratory Sleep Section
Sleep in cancer patients
Sleep disturbance is among the most common symptoms in patients with cancer with an estimated prevalence of up to two out of three patients experiencing sleep disruption during their cancer journey.1,23,4
Common sleep disorders in cancer patients:
Insomnia: Cancer patients have at least a two-fold higher incidence of insomnia compared with the general population.5,6 Predisposing factors may include age, the presence of hyper-arousability,a prior history of insomnia, or a preexisting psychiatric disorder. Cancer-related factors include surgery, hospitalization, chemotherapy, hormonal therapy, radiation therapy, and use of steroids.7 If sedative-hypnotics are considered, they should be used in conjunction with cognitive and behavioral therapy for insomnia (CBT-I). Recent meta-analyses provide data to support a strong recommendation to utilize CBT-I to treat insomnia in cancer patients.6,8,9
Hypersomnolence: Hypersomnolence or excessive daytime sleepiness is a common symptom noted among cancer patients.10 Hypersomnia related to cancer can be often classified as either hypersomnia due to a medical condition or hypersomnia due to a drug or substance, especially for those patients taking opioid or other sedative medications.
Movement Disorders: Sleep movement disorders occur in patients with cancer and may be primary or attributable to chemotherapy-related neuropathy from therapy regimens, including platinum compounds, taxanes, vinca alkaloids, proteasome inhibitors, or thalidomide-based agents.11,12
Obstructive sleep apnea (OSA): OSA occurs in patients with cancer and may be increased in patients with specific cancers such as head and neck tumors.13 Patients with sleep apnea have a five-fold increased risk of cancer-related mortality, and several studies show an increased incidence of cancer in those with sleep apnea.14-16There is an increasing realization that not only sleep apnea, but sleep disturbance, in general, may be oncogenic based on increased autonomic tone, chronic stress, variation in the pituitary-hypothalamic axis, as well as circadian mechanisms.17
Early recognition/treatment of sleep issues is essential to improve quality of life in cancer patients.
Diwakar Balachandran, MD, FCCP
Member-at-Large
References
1. Balachandran DD, Miller MA, Faiz SA, Yennurajalingam S, Innominato PF. Evaluation and management of sleep and circadian rhythm disturbance in cancer. Curr Treat Options Oncol. 2021;22(9):81.
2. Yennurajalingam S, Balachandran D, Pedraza Cardozo SL, et al. Patient-reported sleep disturbance in advanced cancer: frequency, predictors and screening performance of the Edmonton Symptom Assessment System sleep item. BMJ Support Palliat Care. 2017;7(3):274-80.
3. Harris B, Ross J, Sanchez-Reilly S. Sleeping in the arms of cancer: A review of sleeping disorders among patients with cancer. Cancer J. 2014;20(5):299-305.
4. Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27(7):2747-53.
5. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292-8.
6. Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583-90.
7. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895-908.
8. Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113-24.
9. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20-8.
10. Jaumally BA, Das A, Cassell NC, et al. Excessive daytime sleepiness in cancer patients. Sleep Breath. 2021;25(2):1063-7.
11. Gewandter JS, Kleckner AS, Marshall JH, et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: an NIH Collaboratory study of claims data. Support Care Cancer. 2020;28(6):2553-62.
12. St Germain DC, O’Mara AM, Robinson JL, Torres AD, Minasian LM. Chemotherapy-induced peripheral neuropathy: Identifying the research gaps and associated changes to clinical trial design. Cancer. 2020;126(20):4602-13.
13. Faiz SA, Balachandran D, Hessel AC, et al. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist. 2014;19(11):1200-6.
14. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99-105.
15. Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742-8.
16. Martinez-Garcia MA, Campos-Rodriguez F, Barbe F. Cancer and OSA: Current evidence from human studies. Chest. 2016;150(2):451-63.
17. Gozal D, Farre R, Nieto FJ. Putative links between sleep apnea and cancer: From hypotheses to evolving evidence. Chest. 2015;148(5):1140-7.