User login
Excess weight over lifetime hikes risk for colorectal cancer
Excess weight over a lifetime may play a greater role in a person’s risk for colorectal cancer (CRC) than previously thought, according to new research.
In their paper published online March 17 in JAMA Oncology, the authors liken the cumulative effects of a lifetime with overweight or obesity to the increased risk of cancer the more people smoke over time.
This population-based, case-control study was led by Xiangwei Li, MSc, of the division of clinical epidemiology and aging research at the German Cancer Research Center in Heidelberg.
It looked at height and self-reported weight documented in 10-year increments starting at age 20 years up to the current age for 5,635 people with CRC compared with 4,515 people in a control group.
Odds for colorectal cancer increased substantially over the decades when people carried the excess weight long term compared with participants who remained within the normal weight range during the period.
Coauthor Hermann Brenner, MD, MPH, a colleague in Li’s division at the German Cancer Research Center, said in an interview that a key message in the research is that “overweight and obesity are likely to increase the risk of colorectal cancer more strongly than suggested by previous studies that typically had considered body weight only at a single point of time.”
The researchers used a measure of weighted number of years lived with overweight or obesity (WYOs) determined by multiplying excess body mass index by number of years the person carried the excess weight.
They found a link between WYOs and CRC risk, with adjusted odds ratios (ORs) increasing from 1.25 (95% confidence interval [CI], 1.09-1.44) to 2.54 (95% CI, 2.24-2.89) from the first to the fourth quartile of WYOs, compared with people who stayed within normal weight parameters.
The odds went up substantially the longer the time carrying the excess weight.
“Each SD increment in WYOs was associated with an increase of CRC risk by 55% (adjusted OR, 1.55; 95% CI, 1.46-1.64),” the authors wrote. “This OR was higher than the OR per SD increase of excess body mass index at any single point of time, which ranged from 1.04 (95% CI, 0.93-1.16) to 1.27 (95% CI 1.16-1.39).”
Dr. Brenner said that although this study focused on colorectal cancer, “the same is likely to apply for other cancers and other chronic diseases.”
Prevention of overweight and obesity to reduce burden of cancer and other chronic diseases “should become a public health priority,” he said.
Preventing overweight in childhood is important
Overweight and obesity increasingly are starting in childhood, he noted, and may be a lifelong burden.
Therefore, “efforts to prevent their development in childhood, adolescence, and young adulthood are particularly important,” Dr. Brenner said.
The average age of the patients was 68 years in both the CRC and control groups. There were more men than women in both groups: 59.7% were men in the CRC group and 61.1% were men in the control group.
“Our proposed concept of WYOs is comparable to the concept of pack-years in that WYOs can be considered a weighted measure of years lived with the exposure, with weights reflecting the intensity of exposure,” the authors wrote.
Study helps confirm what is becoming more clear to researchers
Kimmie Ng, MD, MPH, a professor at Harvard Medical School and oncologist at Dana-Farber Cancer Institute, both in Boston, said in an interview that the study helps confirm what is becoming more clear to researchers.
“We do think that exposures over the life course are the ones that will be most strongly contributing to a risk of colorectal cancer as an adult,” she said. “With obesity, what we think is happening is that it’s setting up this milieu of chronic inflammation and insulin resistance and we know those two factors can lead to higher rates of colorectal cancer development and increased tumor growth.”
She said the ideal, but impractical, way to do the study would be to follow healthy people from childhood and document their weight over a lifetime. In this case-control study, people were asked to recall their weight at different time periods, which is a limitation and could lead to recall bias.
But the study is important, Dr. Ng said, and it adds convincing evidence that addressing the link between excess weight and CRC and chronic diseases should be a public health priority. “With the recent rise in young-onset colorectal cancer since the 1990s there has been a lot of interest in looking at whether obesity is a major contributor to that rising trend,” Dr. Ng noted. “If obesity is truly linked to colorectal cancer, these rising rates of obesity are very worrisome for potentially leading to more colorectal cancers in young adulthood and beyond.“
The study authors and Dr. Ng report no relevant financial relationships.
The new research was funded by the German Research Council, the Interdisciplinary Research Program of the National Center for Tumor Diseases, Germany, and the German Federal Ministry of Education and Research.
Excess weight over a lifetime may play a greater role in a person’s risk for colorectal cancer (CRC) than previously thought, according to new research.
In their paper published online March 17 in JAMA Oncology, the authors liken the cumulative effects of a lifetime with overweight or obesity to the increased risk of cancer the more people smoke over time.
This population-based, case-control study was led by Xiangwei Li, MSc, of the division of clinical epidemiology and aging research at the German Cancer Research Center in Heidelberg.
It looked at height and self-reported weight documented in 10-year increments starting at age 20 years up to the current age for 5,635 people with CRC compared with 4,515 people in a control group.
Odds for colorectal cancer increased substantially over the decades when people carried the excess weight long term compared with participants who remained within the normal weight range during the period.
Coauthor Hermann Brenner, MD, MPH, a colleague in Li’s division at the German Cancer Research Center, said in an interview that a key message in the research is that “overweight and obesity are likely to increase the risk of colorectal cancer more strongly than suggested by previous studies that typically had considered body weight only at a single point of time.”
The researchers used a measure of weighted number of years lived with overweight or obesity (WYOs) determined by multiplying excess body mass index by number of years the person carried the excess weight.
They found a link between WYOs and CRC risk, with adjusted odds ratios (ORs) increasing from 1.25 (95% confidence interval [CI], 1.09-1.44) to 2.54 (95% CI, 2.24-2.89) from the first to the fourth quartile of WYOs, compared with people who stayed within normal weight parameters.
The odds went up substantially the longer the time carrying the excess weight.
“Each SD increment in WYOs was associated with an increase of CRC risk by 55% (adjusted OR, 1.55; 95% CI, 1.46-1.64),” the authors wrote. “This OR was higher than the OR per SD increase of excess body mass index at any single point of time, which ranged from 1.04 (95% CI, 0.93-1.16) to 1.27 (95% CI 1.16-1.39).”
Dr. Brenner said that although this study focused on colorectal cancer, “the same is likely to apply for other cancers and other chronic diseases.”
Prevention of overweight and obesity to reduce burden of cancer and other chronic diseases “should become a public health priority,” he said.
Preventing overweight in childhood is important
Overweight and obesity increasingly are starting in childhood, he noted, and may be a lifelong burden.
Therefore, “efforts to prevent their development in childhood, adolescence, and young adulthood are particularly important,” Dr. Brenner said.
The average age of the patients was 68 years in both the CRC and control groups. There were more men than women in both groups: 59.7% were men in the CRC group and 61.1% were men in the control group.
“Our proposed concept of WYOs is comparable to the concept of pack-years in that WYOs can be considered a weighted measure of years lived with the exposure, with weights reflecting the intensity of exposure,” the authors wrote.
Study helps confirm what is becoming more clear to researchers
Kimmie Ng, MD, MPH, a professor at Harvard Medical School and oncologist at Dana-Farber Cancer Institute, both in Boston, said in an interview that the study helps confirm what is becoming more clear to researchers.
“We do think that exposures over the life course are the ones that will be most strongly contributing to a risk of colorectal cancer as an adult,” she said. “With obesity, what we think is happening is that it’s setting up this milieu of chronic inflammation and insulin resistance and we know those two factors can lead to higher rates of colorectal cancer development and increased tumor growth.”
She said the ideal, but impractical, way to do the study would be to follow healthy people from childhood and document their weight over a lifetime. In this case-control study, people were asked to recall their weight at different time periods, which is a limitation and could lead to recall bias.
But the study is important, Dr. Ng said, and it adds convincing evidence that addressing the link between excess weight and CRC and chronic diseases should be a public health priority. “With the recent rise in young-onset colorectal cancer since the 1990s there has been a lot of interest in looking at whether obesity is a major contributor to that rising trend,” Dr. Ng noted. “If obesity is truly linked to colorectal cancer, these rising rates of obesity are very worrisome for potentially leading to more colorectal cancers in young adulthood and beyond.“
The study authors and Dr. Ng report no relevant financial relationships.
The new research was funded by the German Research Council, the Interdisciplinary Research Program of the National Center for Tumor Diseases, Germany, and the German Federal Ministry of Education and Research.
Excess weight over a lifetime may play a greater role in a person’s risk for colorectal cancer (CRC) than previously thought, according to new research.
In their paper published online March 17 in JAMA Oncology, the authors liken the cumulative effects of a lifetime with overweight or obesity to the increased risk of cancer the more people smoke over time.
This population-based, case-control study was led by Xiangwei Li, MSc, of the division of clinical epidemiology and aging research at the German Cancer Research Center in Heidelberg.
It looked at height and self-reported weight documented in 10-year increments starting at age 20 years up to the current age for 5,635 people with CRC compared with 4,515 people in a control group.
Odds for colorectal cancer increased substantially over the decades when people carried the excess weight long term compared with participants who remained within the normal weight range during the period.
Coauthor Hermann Brenner, MD, MPH, a colleague in Li’s division at the German Cancer Research Center, said in an interview that a key message in the research is that “overweight and obesity are likely to increase the risk of colorectal cancer more strongly than suggested by previous studies that typically had considered body weight only at a single point of time.”
The researchers used a measure of weighted number of years lived with overweight or obesity (WYOs) determined by multiplying excess body mass index by number of years the person carried the excess weight.
They found a link between WYOs and CRC risk, with adjusted odds ratios (ORs) increasing from 1.25 (95% confidence interval [CI], 1.09-1.44) to 2.54 (95% CI, 2.24-2.89) from the first to the fourth quartile of WYOs, compared with people who stayed within normal weight parameters.
The odds went up substantially the longer the time carrying the excess weight.
“Each SD increment in WYOs was associated with an increase of CRC risk by 55% (adjusted OR, 1.55; 95% CI, 1.46-1.64),” the authors wrote. “This OR was higher than the OR per SD increase of excess body mass index at any single point of time, which ranged from 1.04 (95% CI, 0.93-1.16) to 1.27 (95% CI 1.16-1.39).”
Dr. Brenner said that although this study focused on colorectal cancer, “the same is likely to apply for other cancers and other chronic diseases.”
Prevention of overweight and obesity to reduce burden of cancer and other chronic diseases “should become a public health priority,” he said.
Preventing overweight in childhood is important
Overweight and obesity increasingly are starting in childhood, he noted, and may be a lifelong burden.
Therefore, “efforts to prevent their development in childhood, adolescence, and young adulthood are particularly important,” Dr. Brenner said.
The average age of the patients was 68 years in both the CRC and control groups. There were more men than women in both groups: 59.7% were men in the CRC group and 61.1% were men in the control group.
“Our proposed concept of WYOs is comparable to the concept of pack-years in that WYOs can be considered a weighted measure of years lived with the exposure, with weights reflecting the intensity of exposure,” the authors wrote.
Study helps confirm what is becoming more clear to researchers
Kimmie Ng, MD, MPH, a professor at Harvard Medical School and oncologist at Dana-Farber Cancer Institute, both in Boston, said in an interview that the study helps confirm what is becoming more clear to researchers.
“We do think that exposures over the life course are the ones that will be most strongly contributing to a risk of colorectal cancer as an adult,” she said. “With obesity, what we think is happening is that it’s setting up this milieu of chronic inflammation and insulin resistance and we know those two factors can lead to higher rates of colorectal cancer development and increased tumor growth.”
She said the ideal, but impractical, way to do the study would be to follow healthy people from childhood and document their weight over a lifetime. In this case-control study, people were asked to recall their weight at different time periods, which is a limitation and could lead to recall bias.
But the study is important, Dr. Ng said, and it adds convincing evidence that addressing the link between excess weight and CRC and chronic diseases should be a public health priority. “With the recent rise in young-onset colorectal cancer since the 1990s there has been a lot of interest in looking at whether obesity is a major contributor to that rising trend,” Dr. Ng noted. “If obesity is truly linked to colorectal cancer, these rising rates of obesity are very worrisome for potentially leading to more colorectal cancers in young adulthood and beyond.“
The study authors and Dr. Ng report no relevant financial relationships.
The new research was funded by the German Research Council, the Interdisciplinary Research Program of the National Center for Tumor Diseases, Germany, and the German Federal Ministry of Education and Research.
FROM JAMA ONCOLOGY
Doctors treat osteoporosis with hormone therapy against guidelines
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
Ways to lessen toxic effects of chemo in older adults
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
Hematocrit, White Blood Cells, and Thrombotic Events in the Veteran Population With Polycythemia Vera
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
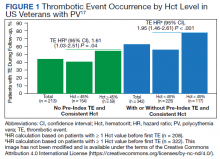
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
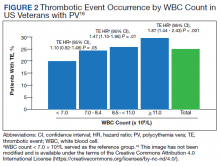
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
Polycythemia vera (PV) is a rare myeloproliferative neoplasm affecting 44 to 57 individuals per 100,000 in the United States.1,2 It is characterized by somatic mutations in the hematopoietic stem cell, resulting in hyperproliferation of mature myeloid lineage cells.2 Sustained erythrocytosis is a hallmark of PV, although many patients also have leukocytosis and thrombocytosis.2,3 These patients have increased inherent thrombotic risk with arterial events reported to occur at rates of 7 to 21/1000 person-years and venous thrombotic events at 5 to 20/1000 person-years.4-7 Thrombotic and cardiovascular events are leading causes of morbidity and mortality, resulting in a reduced overall survival of patients with PV compared with the general population.3,8-10
Blood Cell Counts and Thrombotic Events in PV
Treatment strategies for patients with PV mainly aim to prevent or manage thrombotic and bleeding complications through normalization of blood counts.11 Hematocrit (Hct) control has been reported to be associated with reduced thrombotic risk in patients with PV. This was shown and popularized by the prospective, randomized Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) trial in which participants were randomized 1:1 to maintaining either a low (< 45%) or high (45%-50%) Hct for 5 years to examine the long-term effects of more- or less-intensive cytoreductive therapy.12 Patients in the low-Hct group were found to have a lower rate of death from cardiovascular events or major thrombosis (1.1/100 person-years in the low-Hct group vs 4.4 in the high-Hct group; hazard ratio [HR], 3.91; 95% confidence interval [CI], 1.45-10.53; P = .007). Likewise, cardiovascular events occurred at a lower rate in patients in the low-Hct group compared with the high-Hct group (4.4% vs 10.9% of patients, respectively; HR, 2.69; 95% CI, 1.19-6.12; P = .02).12
Leukocytosis has also been linked to elevated risk for vascular events as shown in several studies, including the real-world European Collaboration on Low-Dose Aspirin in PV (ECLAP) observational study and a post hoc subanalysis of the CYTO-PV study.13,14 In a multivariate, time-dependent analysis in ECLAP, patients with white blood cell (WBC) counts > 15 × 109/L had a significant increase in the risk of thrombosis compared with those who had lower WBC counts, with higher WBC count more strongly associated with arterial than venous thromboembolism.13 In CYTO-PV, a significant correlation between elevated WBC count (≥ 11 × 109/L vs reference level of < 7 × 109/L) and time-dependent risk of major thrombosis was shown (HR, 3.9; 95% CI, 1.24-12.3; P = .02).14 Likewise, WBC count ≥ 11 × 109/L was found to be a predictor of subsequent venous events in a separate single-center multivariate analysis of patients with PV.8
Although CYTO-PV remains one of the largest prospective landmark studies in PV demonstrating the impact of Hct control on thrombosis, it is worthwhile to note that the patients in the high-Hct group who received less frequent myelosuppressive therapy with hydroxyurea than the low-Hct group also had higher WBC counts.12,15 Work is needed to determine the relative effects of high Hct and high WBC counts on PV independent of each other.
The Veteran Population with PV
Two recently published retrospective analyses from Parasuraman and colleagues used data from the Veterans Health Administration (VHA), the largest integrated health care system in the US, with an aim to replicate findings from CYTO-PV in a real-world population.16,17 The 2 analyses focused independently on the effects of Hct control and WBC count on the risk of a thrombotic event in patients with PV.
In the first retrospective analysis, 213 patients with PV and no prior thrombosis were placed into groups based on whether Hct levels were consistently either < 45% or ≥ 45% throughout the study period.17 The mean follow-up time was 2.3 years, during which 44.1% of patients experienced a thrombotic event (Figure 1). Patients with Hct levels < 45% had a lower rate of thrombotic events compared to those with levels ≥ 45% (40.3% vs 54.2%, respectively; HR, 1.61; 95% CI, 1.03-2.51; P = .04). In a sensitivity analysis that included patients with pre-index thrombotic events (N = 342), similar results were noted (55.6% vs 76.9% between the < 45% and ≥ 45% groups, respectively; HR, 1.95; 95% CI, 1.46-2.61; P < .001).
In the second analysis, the authors investigated the relationship between WBC counts and thrombotic events.16 Evaluable patients (N = 1565) were grouped into 1 of 4 cohorts based on the last WBC measurement taken during the study period before a thrombotic event or through the end of follow-up: (1) WBC < 7.0 × 109/L, (2) 7.0 to 8.4 × 109/L, (3) 8.5 to < 11.0 × 109/L, or (4) ≥ 11.0 × 109/L. Mean follow-up time ranged from 3.6 to 4.5 years among WBC count cohorts, during which 24.9% of patients experienced a thrombotic event. Compared with the reference cohort (WBC < 7.0 × 109/L), a significant positive association between WBC counts and thrombotic event occurrence was observed among patients with WBC counts of 8.5 to < 11.0 × 109/L (HR, 1.47; 95% CI, 1.10-1.96; P < .01) and ≥ 11 × 109/L (HR, 1.87; 95% CI, 1.44-2.43; P < .001) (Figure 2).16 When including all patients in a sensitivity analysis regardless of whether they experienced thrombotic events before the index date (N = 1876), similar results were obtained (7.0-8.4 × 109/L group: HR, 1.22; 95% CI, 0.97-1.55; P = .0959; 8.5 - 11.0 × 109/L group: HR, 1.41; 95% CI, 1.10-1.81; P = .0062; ≥ 11.0 × 109/L group: HR, 1.53; 95% CI, 1.23-1.91; P < .001; compared with < 7.0 × 109/L reference group). Rates of phlebotomy and cytoreductive treatments were similar across groups.16
Some limitations to these studies are attributable to their retrospective design, reliance on health records, and the VHA population characteristics, which differ from the general population. For example, in this analysis, patients with PV in the VHA population had significantly increased risk of thrombotic events, even at a lower WBC count threshold (≥ 8.5 × 109/L) compared with those reported in CYTO-PV (≥ 11 × 109/L). Furthermore, approximately one-third of patients had elevated WBC levels, compared with 25.5% in the CYTO-PV study.14,16 This is most likely due to the unique nature of the VHA patient population, who are predominantly older adult men and generally have a higher comorbidity burden. A notable pre-index comorbidity burden was reported in the VHA population in the Hct analysis, even when compared to patients with PV in the general US population (Charlson Comorbidity Index score, 1.3 vs 0.8).6,17 Comorbid conditions such as hypertension, diabetes, and tobacco use, which are most common among the VHA population, are independently associated with higher risk of cardiovascular and thrombotic events.18,19 However, whether these higher levels of comorbidities affected the type of treatments they received was not elucidated, and the effectiveness of treatments to maintain target Hct levels was not addressed in the study.
Current PV Management and Future Implications
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology in myeloproliferative neoplasms recommend maintaining Hct levels < 45% in patients with PV.11 Patients with high-risk disease (age ≥ 60 years and/or history of thrombosis) are monitored for new thrombosis or bleeding and are managed for their cardiovascular risk factors. In addition, they receive low-dose aspirin (81-100 mg/day), undergo phlebotomy to maintain an Hct < 45%, and are managed with pharmacologic cytoreductive therapy. Cytoreductive therapy primarily consists of hydroxyurea or peginterferon alfa-2a for younger patients. Ruxolitinib, a Janus kinase (JAK1)/JAK2 inhibitor, is now approved by the US Food and Drug Administration as second-line treatment for those with PV that is intolerant or unresponsive to hydroxyurea or peginterferon alfa-2a treatments.11,20 However, the role of cytoreductive therapy is not clear for patients with low-risk disease (age < 60 years and no history of thrombosis). These patients are managed for their cardiovascular risk factors, undergo phlebotomy to maintain an Hct < 45%, are maintained on low-dose aspirin (81-100 mg/day), and are monitored for indications for cytoreductive therapy, which include any new thrombosis or disease-related major bleeding, frequent or persistent need for phlebotomy with poor tolerance for the procedure, splenomegaly, thrombocytosis, leukocytosis, and disease-related symptoms (eg, aquagenic pruritus, night sweats, fatigue).
Even though the current guidelines recommend maintaining a target Hct of < 45% in patients with high-risk PV, the role of Hct as the main determinant of thrombotic risk in patients with PV is still debated.21 In JAK2V617F-positive essential thrombocythemia, Hct levels are usually normal but risk of thrombosis is nevertheless still significant.22 The risk of thrombosis is significantly lower in primary familial and congenital polycythemia and much lower in secondary erythrocytosis such as cyanotic heart disease, long-term native dwellers of high altitude, and those with high-oxygen–affinity hemoglobins.21,23 In secondary erythrocytosis from hypoxia or upregulated hypoxic pathway such as hypoxia inducible factor-2α (HIF-2α) mutation and Chuvash erythrocytosis, the risk of thrombosis is more associated with the upregulated HIF pathway and its downstream consequences, rather than the elevated Hct level.24
However, most current literature supports the association of increased risk of thrombosis with higher Hct and high WBC count in patients with PV. In addition, the underlying mechanism of thrombogenesis still remains elusive; it is likely a complex process that involves interactions among multiple components, including elevated blood counts arising from clonal hematopoiesis, JAK2V617F allele burden, and platelet and WBC activation and their interaction with endothelial cells and inflammatory cytokines.25
Nevertheless, Hct control and aspirin use are current standard of care for patients with PV to mitigate thrombotic risk, and the results from the 2 analyses by Parasuraman and colleagues, using real-world data from the VHA, support the current practice guidelines to maintain Hct < 45% in these patients. They also provide additional support for considering WBC counts when determining patient risk and treatment plans. Although treatment response criteria from the European LeukemiaNet include achieving normal WBC levels to decrease the risk of thrombosis, current NCCN guidelines do not include WBC counts as a component for establishing patient risk or provide a target WBC count to guide patient management.11,26,27 Updates to these practice guidelines may be warranted. In addition, further study is needed to understand the mechanism of thrombogenesis in PV and other myeloproliferative disorders in order to develop novel therapeutic targets and improve patient outcomes.
Acknowledgments
Writing assistance was provided by Tania Iqbal, PhD, an employee of ICON (North Wales, PA), and was funded by Incyte Corporation (Wilmington, DE).
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
1. Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595-600. doi:10.3109/10428194.2013.813500
2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi:10.1182/blood-2016-03-643544
3. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874-1881. doi:10.1038/leu.2013.163
4. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. doi:10.1200/JCO.2005.07.062
5. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110(3):840-846. doi:10.1182/blood-2006-12-064287
6. Goyal RK, Davis KL, Cote I, Mounedji N, Kaye JA. Increased incidence of thromboembolic event rates in patients diagnosed with polycythemia vera: results from an observational cohort study. Blood (ASH Annual Meeting Abstracts). 2014;124:4840. doi:10.1182/blood.V124.21.4840.4840
7. Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. doi:10.1182/blood-2014-07-591610 8. Cerquozzi S, Barraco D, Lasho T, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7(12):662. doi:10.1038/s41408-017-0035-6
9. Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965-1976. doi:10.1007/s00277-014-2205-y
10. Hultcrantz M, Kristinsson SY, Andersson TM-L, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995-3001. doi:10.1200/JCO.2012.42.1925
11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in myeloproliferative neoplasms (Version 1.2020). Accessed March 3, 2022. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
12. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. doi:10.1056/NEJMoa1208500
13. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446-2452. doi:10.1182/blood-2006-08-042515
14. Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126(4):560-561. doi:10.1182/blood-2015-04-638593
15. Prchal JT, Gordeuk VR. Treatment target in polycythemia vera. N Engl J Med. 2013;368(16):1555-1556. doi:10.1056/NEJMc1301262
16. Parasuraman S, Yu J, Paranagama D, et al. Elevated white blood cell levels and thrombotic events in patients with polycythemia vera: a real-world analysis of Veterans Health Administration data. Clin Lymphoma Myeloma Leuk. 2020;20(2):63-69. doi:10.1016/j.clml.2019.11.010
17. Parasuraman S, Yu J, Paranagama D, et al. Hematocrit levels and thrombotic events in patients with polycythemia vera: an analysis of Veterans Health Administration data. Ann Hematol. 2019;98(11):2533-2539. doi:10.1007/s00277-019-03793-w
18. WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345. doi:10.1016/S2214-109X(19)30318-3.
19. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. doi:10.1161/CIRCULATIONAHA.107.699579
20. Jakafi. Package insert. Incyte Corporation; 2020.
21. Gordeuk VR, Key NS, Prchal JT. Re-evaluation of hematocrit as a determinant of thrombotic risk in erythrocytosis. Haematologica. 2019;104(4):653-658. doi:10.3324/haematol.2018.210732
22. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857-5859. doi:10.1182/blood-2011-02-339002
23. Perloff JK, Marelli AJ, Miner PD. Risk of stroke in adults with cyanotic congenital heart disease. Circulation. 1993;87(6):1954-1959. doi:10.1161/01.cir.87.6.1954
24. Gordeuk VR, Miasnikova GY, Sergueeva AI, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90. doi:10.3324/haematol.2019.216267
25. Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015;29(4):215-221. doi:10.1016/j.blre.2014.12.002
26. Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. doi:10.1038/s41375-018-0077-1
27. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. doi:10.1182/blood-2013-01-478891
Restoring ‘sixth sense’ may reduce falls in Alzheimer’s
(AD), new research confirms.
Falls are twice as common in patients with AD versu older individuals without the disorder and significantly increase the likelihood of institutionalization.
However, researchers recorded fewer falls in patients with a better functioning vestibular system, which detects head movements and plays a critical role in spatial orientation, posture, gait, and balance.
The results suggest that improving vestibular function with currently available therapies may prevent falls, something the researchers will investigate in a new clinical trial launching next month.
“One of the most dangerous and impactful symptoms in terms of function in patients with Alzheimer’s disease is their increased predisposition to falls,” study investigator Yuri Agrawal, MD, department of otolaryngology–head and neck surgery, Johns Hopkins University School of Medicine, Baltimore, said in an interview. “Alzheimer’s is the sixth leading cause of death in the U.S., and some people actually say that that high mortality rate is because of their predisposition to falls and the injuries that occur.”
The study was published online Feb. 14 in the Journal of Alzheimer’s Disease.
The ‘sixth hidden sense’
The vestibular system consists of three semicircular canals, which detect rotational head movement, and two otolith organs called the utricle and the saccule, which sense linear head movements and the orientation of the head with respect to gravity.
“We call the vestibular system the sixth hidden sense because it’s not a conscious perception like taste or smell,” Dr. Agrawal said. “It’s constantly providing input to our brain about where we are in space.”
Dr. Agrawal and colleagues previously reported that vestibular loss is twice as common in Alzheimer’s patients as in cognitively unimpaired age-matched controls. Now, they wanted to know if this sensory loss was associated with an increased risk for falls in this population.
The study included 48 patients age greater than or equal to 60 years with mild-to-moderate AD between 2018 and 2020. They also included an age-matched control group of healthy controls with no cognitive impairment.
Researchers assessed vestibular function at baseline by measuring semicircular canal and saccular function. One test required participants to wear goggles and complete a series of tests with their eyes open and closed while researchers recorded their eye movement with video-oculography. They also measured participants’ balance using the Berg Balance Scale.
Relative to matched controls, AD patients exhibited increased lateral instability when their eyes were open (P = .033) and closed (P = .042). Studies suggest that lateral stability declines more quickly with age and that instability with eyes closed is the single biggest predictor of incident falls in community-dwelling adults.
To determine if poor vestibular function increased fall risk in patients with AD, researchers followed the cohort for up to 2 years.
“We found that patients with vestibular loss at baseline were 50% more likely to fall, adjusting for other factors that could contribute to that,” Dr. Agrawal said.
Specifically, better semicircular canal function was significantly associated with lower likelihood of falls, even after adjusting for confounders (adjusted hazard ratio, 0.65; P = .009).
Can therapy help?
Commenting on the findings, James Burke, MD, PhD, professor of neurology at Duke University Medical Center, Durham, N.C., said that the finding that impaired vestibular function is associated with increased falls “significantly advances our understanding of the topic” and suggests that treating vestibular dysfunction could reduce falls in Alzheimer’s patients.
“Screening patients with Alzheimer’s disease for impaired vestibular function could lead to identification of individuals at high risk of falls and target those who would benefit from vestibular therapy,” he said.
Vestibular rehabilitation therapy is often used to treat a number of disorders related to vestibular function loss. There are also studies underway to measure the efficacy of a vestibular implant that works much like a cochlear implant.
While evaluation of vestibular function is currently not routinely included in AD care, studies such as these suggest it may be time to consider adding it to the standard of care, Jennifer Coto, PhD, assistant professor of otolaryngology at the University of Miami Miller School of Medicine, said in an interview.
“Best practice guidelines for management of Alzheimer’s patients should be revised to include routine vestibular evaluation and support from a multidisciplinary team that may address other crucial areas of functioning, particularly psychological functioning, sleep, and independence,” she said.
“Future research also needs to evaluate the effectiveness of vestibular therapy in patients with Alzheimer’s and the benefits of early identification and intervention for preventing recurrent falls.”
Dr. Agrawal is leading a 5-year, $3.5 million National Institute on Aging study that seeks to do just that. Enrollment in the study begins next month. Patients will complete an initial in-person screening, but the remainder of the study will be conducted virtually.
Therapies will be noninvasive, nonpharmaceutical, and performed in participants’ homes. If the therapy is successful at reducing falls, Dr. Agrawal said the virtual design would significantly broaden its potential patient reach.
The study was funded by the National Institute on Aging. Study authors’ disclosures are reported in the original article. Dr. Coto and Dr. Burke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AD), new research confirms.
Falls are twice as common in patients with AD versu older individuals without the disorder and significantly increase the likelihood of institutionalization.
However, researchers recorded fewer falls in patients with a better functioning vestibular system, which detects head movements and plays a critical role in spatial orientation, posture, gait, and balance.
The results suggest that improving vestibular function with currently available therapies may prevent falls, something the researchers will investigate in a new clinical trial launching next month.
“One of the most dangerous and impactful symptoms in terms of function in patients with Alzheimer’s disease is their increased predisposition to falls,” study investigator Yuri Agrawal, MD, department of otolaryngology–head and neck surgery, Johns Hopkins University School of Medicine, Baltimore, said in an interview. “Alzheimer’s is the sixth leading cause of death in the U.S., and some people actually say that that high mortality rate is because of their predisposition to falls and the injuries that occur.”
The study was published online Feb. 14 in the Journal of Alzheimer’s Disease.
The ‘sixth hidden sense’
The vestibular system consists of three semicircular canals, which detect rotational head movement, and two otolith organs called the utricle and the saccule, which sense linear head movements and the orientation of the head with respect to gravity.
“We call the vestibular system the sixth hidden sense because it’s not a conscious perception like taste or smell,” Dr. Agrawal said. “It’s constantly providing input to our brain about where we are in space.”
Dr. Agrawal and colleagues previously reported that vestibular loss is twice as common in Alzheimer’s patients as in cognitively unimpaired age-matched controls. Now, they wanted to know if this sensory loss was associated with an increased risk for falls in this population.
The study included 48 patients age greater than or equal to 60 years with mild-to-moderate AD between 2018 and 2020. They also included an age-matched control group of healthy controls with no cognitive impairment.
Researchers assessed vestibular function at baseline by measuring semicircular canal and saccular function. One test required participants to wear goggles and complete a series of tests with their eyes open and closed while researchers recorded their eye movement with video-oculography. They also measured participants’ balance using the Berg Balance Scale.
Relative to matched controls, AD patients exhibited increased lateral instability when their eyes were open (P = .033) and closed (P = .042). Studies suggest that lateral stability declines more quickly with age and that instability with eyes closed is the single biggest predictor of incident falls in community-dwelling adults.
To determine if poor vestibular function increased fall risk in patients with AD, researchers followed the cohort for up to 2 years.
“We found that patients with vestibular loss at baseline were 50% more likely to fall, adjusting for other factors that could contribute to that,” Dr. Agrawal said.
Specifically, better semicircular canal function was significantly associated with lower likelihood of falls, even after adjusting for confounders (adjusted hazard ratio, 0.65; P = .009).
Can therapy help?
Commenting on the findings, James Burke, MD, PhD, professor of neurology at Duke University Medical Center, Durham, N.C., said that the finding that impaired vestibular function is associated with increased falls “significantly advances our understanding of the topic” and suggests that treating vestibular dysfunction could reduce falls in Alzheimer’s patients.
“Screening patients with Alzheimer’s disease for impaired vestibular function could lead to identification of individuals at high risk of falls and target those who would benefit from vestibular therapy,” he said.
Vestibular rehabilitation therapy is often used to treat a number of disorders related to vestibular function loss. There are also studies underway to measure the efficacy of a vestibular implant that works much like a cochlear implant.
While evaluation of vestibular function is currently not routinely included in AD care, studies such as these suggest it may be time to consider adding it to the standard of care, Jennifer Coto, PhD, assistant professor of otolaryngology at the University of Miami Miller School of Medicine, said in an interview.
“Best practice guidelines for management of Alzheimer’s patients should be revised to include routine vestibular evaluation and support from a multidisciplinary team that may address other crucial areas of functioning, particularly psychological functioning, sleep, and independence,” she said.
“Future research also needs to evaluate the effectiveness of vestibular therapy in patients with Alzheimer’s and the benefits of early identification and intervention for preventing recurrent falls.”
Dr. Agrawal is leading a 5-year, $3.5 million National Institute on Aging study that seeks to do just that. Enrollment in the study begins next month. Patients will complete an initial in-person screening, but the remainder of the study will be conducted virtually.
Therapies will be noninvasive, nonpharmaceutical, and performed in participants’ homes. If the therapy is successful at reducing falls, Dr. Agrawal said the virtual design would significantly broaden its potential patient reach.
The study was funded by the National Institute on Aging. Study authors’ disclosures are reported in the original article. Dr. Coto and Dr. Burke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AD), new research confirms.
Falls are twice as common in patients with AD versu older individuals without the disorder and significantly increase the likelihood of institutionalization.
However, researchers recorded fewer falls in patients with a better functioning vestibular system, which detects head movements and plays a critical role in spatial orientation, posture, gait, and balance.
The results suggest that improving vestibular function with currently available therapies may prevent falls, something the researchers will investigate in a new clinical trial launching next month.
“One of the most dangerous and impactful symptoms in terms of function in patients with Alzheimer’s disease is their increased predisposition to falls,” study investigator Yuri Agrawal, MD, department of otolaryngology–head and neck surgery, Johns Hopkins University School of Medicine, Baltimore, said in an interview. “Alzheimer’s is the sixth leading cause of death in the U.S., and some people actually say that that high mortality rate is because of their predisposition to falls and the injuries that occur.”
The study was published online Feb. 14 in the Journal of Alzheimer’s Disease.
The ‘sixth hidden sense’
The vestibular system consists of three semicircular canals, which detect rotational head movement, and two otolith organs called the utricle and the saccule, which sense linear head movements and the orientation of the head with respect to gravity.
“We call the vestibular system the sixth hidden sense because it’s not a conscious perception like taste or smell,” Dr. Agrawal said. “It’s constantly providing input to our brain about where we are in space.”
Dr. Agrawal and colleagues previously reported that vestibular loss is twice as common in Alzheimer’s patients as in cognitively unimpaired age-matched controls. Now, they wanted to know if this sensory loss was associated with an increased risk for falls in this population.
The study included 48 patients age greater than or equal to 60 years with mild-to-moderate AD between 2018 and 2020. They also included an age-matched control group of healthy controls with no cognitive impairment.
Researchers assessed vestibular function at baseline by measuring semicircular canal and saccular function. One test required participants to wear goggles and complete a series of tests with their eyes open and closed while researchers recorded their eye movement with video-oculography. They also measured participants’ balance using the Berg Balance Scale.
Relative to matched controls, AD patients exhibited increased lateral instability when their eyes were open (P = .033) and closed (P = .042). Studies suggest that lateral stability declines more quickly with age and that instability with eyes closed is the single biggest predictor of incident falls in community-dwelling adults.
To determine if poor vestibular function increased fall risk in patients with AD, researchers followed the cohort for up to 2 years.
“We found that patients with vestibular loss at baseline were 50% more likely to fall, adjusting for other factors that could contribute to that,” Dr. Agrawal said.
Specifically, better semicircular canal function was significantly associated with lower likelihood of falls, even after adjusting for confounders (adjusted hazard ratio, 0.65; P = .009).
Can therapy help?
Commenting on the findings, James Burke, MD, PhD, professor of neurology at Duke University Medical Center, Durham, N.C., said that the finding that impaired vestibular function is associated with increased falls “significantly advances our understanding of the topic” and suggests that treating vestibular dysfunction could reduce falls in Alzheimer’s patients.
“Screening patients with Alzheimer’s disease for impaired vestibular function could lead to identification of individuals at high risk of falls and target those who would benefit from vestibular therapy,” he said.
Vestibular rehabilitation therapy is often used to treat a number of disorders related to vestibular function loss. There are also studies underway to measure the efficacy of a vestibular implant that works much like a cochlear implant.
While evaluation of vestibular function is currently not routinely included in AD care, studies such as these suggest it may be time to consider adding it to the standard of care, Jennifer Coto, PhD, assistant professor of otolaryngology at the University of Miami Miller School of Medicine, said in an interview.
“Best practice guidelines for management of Alzheimer’s patients should be revised to include routine vestibular evaluation and support from a multidisciplinary team that may address other crucial areas of functioning, particularly psychological functioning, sleep, and independence,” she said.
“Future research also needs to evaluate the effectiveness of vestibular therapy in patients with Alzheimer’s and the benefits of early identification and intervention for preventing recurrent falls.”
Dr. Agrawal is leading a 5-year, $3.5 million National Institute on Aging study that seeks to do just that. Enrollment in the study begins next month. Patients will complete an initial in-person screening, but the remainder of the study will be conducted virtually.
Therapies will be noninvasive, nonpharmaceutical, and performed in participants’ homes. If the therapy is successful at reducing falls, Dr. Agrawal said the virtual design would significantly broaden its potential patient reach.
The study was funded by the National Institute on Aging. Study authors’ disclosures are reported in the original article. Dr. Coto and Dr. Burke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Just one extra drink a day may change the brain
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
FDA approves first PARP inhibitor for early BRCA+ breast cancer
BRCA+ breast cancer
Specifically, the new approval is for the adjuvant treatment of adult patients with high-risk early-stage HER2-negative, BRCA-mutated breast cancer who have completed chemotherapy and local treatment.
The FDA also approved BRACAnalysis CDx (Myriad Genetics), a companion diagnostic test to identify patients who may benefit from olaparib.
The latest approval was based on phase 3 OlympiA trial results, which showed a 42% improvement in invasive and distant disease-free survival with olaparib in comparison with placebo. Data from OlympiaA and other clinical studies also confirm BRACAnalysis CDx as “an effective test for patients deciding on their best treatment options,” Myriad Genetics noted in a press release.
The OlympiA results, as reported by this news organization, were presented during the plenary session of the American Society of Clinical Oncology 2021 annual meeting and were published in the New England Journal of Medicine.
Those findings prompted an ASCO “rapid recommendation” updating of ASCO’s 2020 guidelines for the management of hereditary breast cancer.
The latest results from OlympiA show that olaparib reduced the risk of death by 32% (hazard ratio, 0.68) in comparison with placebo, according to a company press release announcing the approval. Overall survival data are slated for presentation at a European Society for Medical Oncology Virtual Plenary session on March 16, 2022.
A version of this article first appeared on Medscape.com.
Specifically, the new approval is for the adjuvant treatment of adult patients with high-risk early-stage HER2-negative, BRCA-mutated breast cancer who have completed chemotherapy and local treatment.
The FDA also approved BRACAnalysis CDx (Myriad Genetics), a companion diagnostic test to identify patients who may benefit from olaparib.
The latest approval was based on phase 3 OlympiA trial results, which showed a 42% improvement in invasive and distant disease-free survival with olaparib in comparison with placebo. Data from OlympiaA and other clinical studies also confirm BRACAnalysis CDx as “an effective test for patients deciding on their best treatment options,” Myriad Genetics noted in a press release.
The OlympiA results, as reported by this news organization, were presented during the plenary session of the American Society of Clinical Oncology 2021 annual meeting and were published in the New England Journal of Medicine.
Those findings prompted an ASCO “rapid recommendation” updating of ASCO’s 2020 guidelines for the management of hereditary breast cancer.
The latest results from OlympiA show that olaparib reduced the risk of death by 32% (hazard ratio, 0.68) in comparison with placebo, according to a company press release announcing the approval. Overall survival data are slated for presentation at a European Society for Medical Oncology Virtual Plenary session on March 16, 2022.
A version of this article first appeared on Medscape.com.
Specifically, the new approval is for the adjuvant treatment of adult patients with high-risk early-stage HER2-negative, BRCA-mutated breast cancer who have completed chemotherapy and local treatment.
The FDA also approved BRACAnalysis CDx (Myriad Genetics), a companion diagnostic test to identify patients who may benefit from olaparib.
The latest approval was based on phase 3 OlympiA trial results, which showed a 42% improvement in invasive and distant disease-free survival with olaparib in comparison with placebo. Data from OlympiaA and other clinical studies also confirm BRACAnalysis CDx as “an effective test for patients deciding on their best treatment options,” Myriad Genetics noted in a press release.
The OlympiA results, as reported by this news organization, were presented during the plenary session of the American Society of Clinical Oncology 2021 annual meeting and were published in the New England Journal of Medicine.
Those findings prompted an ASCO “rapid recommendation” updating of ASCO’s 2020 guidelines for the management of hereditary breast cancer.
The latest results from OlympiA show that olaparib reduced the risk of death by 32% (hazard ratio, 0.68) in comparison with placebo, according to a company press release announcing the approval. Overall survival data are slated for presentation at a European Society for Medical Oncology Virtual Plenary session on March 16, 2022.
A version of this article first appeared on Medscape.com.
BRCA+ breast cancer
BRCA+ breast cancer
Appropriate cancer screening for women with dense breasts
We have been interested in the quiz series focused on breast cancer screening for women with dense breasts presented in
The concerns with breast cancer in particular
Breast cancer is not cervical cancer. It isn’t one disease. It is a multitude of diseases that happen to show up in the breast. Some are relatively slow-growing—the kinds of cancers that lend themselves to screening and to early intervention. But other cancers are rapidly-growing; they show up no matter how often or what modality we use for screening. Our goal should be to find an approach to screening that can diagnose breast cancer at a stage where we can intervene and positively impact breast cancer specific and overall mortality.
Screening guidelines vary
The variety of screening guidelines published by different professional organizations reflect differing assumptions and sets of values related to the early diagnosis and treatment of breast cancer. (For a comprehensive table of current screening guidelines, see https://www.cdc.gov/cancer/breast/pdf/breast-cancer-screening-guidelines-508.pdf.)
ACOG’s approach—to offer screening at age 40 but to begin by at least age 50 and, through shared decision making with the patient, screen every 1 or 2 years—is focused on capturing as many cases as we can identify, while minimizing the harms of false-positives.1 The perspective of the US Preventive Services Task Force (USPSTF) recommendations (to screen every 2 years beginning at age 50) is at the population level, a cost-effective approach that will have the greatest benefit while minimizing harms in the population at large.2 The American Society of Breast Surgeons recommends screening to begin by age 40.3 Like the breast surgeons, radiologists dedicated to breast imaging are focused on an individual rather than a population level. They strive to identify each and every instance of possible cancer, and therefore recommend annual screening beginning at age 40.4 However, with more aggressive screening in average-risk women many cases of ductal carcinoma in situ (DCIS) are identified—a lesion that, if not detected, may not impact the woman’s health during her lifetime—representing what some might call “overdiagnosis.” Yet there may be some instances in which the DCIS might affect an individual woman’s health. Unfortunately, we can’t prospectively distinguish between the first and the second types of cases.
We follow American College of Obstetricians and Gynecologists and US Preventive Services Task Force guidelines in discussing screening (both its hazards and benefits) with our average-risk patients beginning at age 40. We talk about risk factors for breast cancer, including breast density, but let patients know that no specific additional imaging is advised, and that density is more common in younger women (one consideration in earlier screening) and is quite common in general. Although we do not send follow-up letters to patients with dense breasts, we do educate our staff so that they can respond appropriately should patients call with questions.
Of course, we all bring to the table values that will impact the decisions that we make for ourselves and for our patients. What an ObGyn might suggest may differ from what a radiologist might suggest. Although we follow recommendations made by the radiologist at screening, an ObGyn wants to take care of the whole human being. We are concerned with bones, heart, everything about the patient, so we approach a patient in a different way. These priorities are reflected in the current varying breast cancer screening guidelines.
Continue to: Research on breast cancer screening varies by design...
Research on breast cancer screening varies by design
There has not been a randomized clinical trial conducted on screening mammography since the days of the analog mammogram. The research that has been conducted is difficult to compare due to variations in screening ages and intervals, technology sensitivity, and patient adherence with recommended screening. Treatments for breast cancer also have changed dramatically over time, so the findings of older studies may no longer be relevant to current breast cancer screening. The kind of analysis that needs to be done is an interrupted time series, where you can look at the trajectory of breast cancer survival and whether screening mammography shifts that survival in any way.
One specific study from Australia measured the impact of newer available breast cancer treatments, including tamoxifen for women with receptor-positive tumors and newer chemotherapy strategies.5 The authors analyzed screening mammography trends in one large province where women aged 50 to 69 were offered biennial screening. Trends from the 1990s showed that more women were being screened over time. Simultaneously, however, advances in therapy were entering clinical practice. The researchers pointed to a substantial decline in mortality from breast cancer from the early 1980s until 2013. But their conclusion was that none of the decline in mortality for breast cancer could be attributed to screening mammography when they looked at time trends; from their perspective all of the important decline in breast cancer mortality resulted from better treatment. They concluded that government programs should not support screening mammography.5
That is a recommendation that we do not support. However, we do recognize the conundrum that mammography is less sensitive among those who have dense breasts. In order to have congruent professional guidelines, we support research funding to determine which types, starting ages, and intervals of screening would be best in various patient populations. The USPSTF cites data from studies performed in the 1980s based on outdated technology; more recent (and relevant) randomized clinical trials have not been performed, and yet this information is critical to provide sufficient evidence to develop appropriate guidelines.
Our recommendations for gathering new data
The kind of data we would find most valuable would assess how different screening strategies impact overall mortality and breast cancer-specific mortality. It would require decades of follow-up—which of course means that screening technology will change over that time. A surrogate for evaluating overall survival is to look at interval cancers, which are all breast cancers diagnosed following negative mammograms and prior to the next screening. These cancers may or may not be biologically active, again focusing us on the need to look at overall survival of the patient. In addition, reducing breast cancer mortality may not reduce overall mortality, because the treatment for breast cancer may cause heart disease, or osteoporosis, or something else that impacts overall survival. These are important considerations for women and physicians who are making choices on treatment. What matters to a patient are 2 overlapping questions:
- Do I have a life-threatening condition or do I not?
- Has screening identified a condition that might lead to treatment that’s unnecessary?
The problem is that with breast cancer we can’t tell the difference. We do not understand the biological potential of a lesion when we evaluate an image on MRI, or computed tomography (CT), or mammography.
A re-look at presented data
A trial conducted by Bakker and colleagues6 was discussed by the authors of the DenseBreast-info.org quiz in which they recommended breast MRI for all women with extremely dense breasts (but no other risk factors for breast cancer) detected on screening mammograms.7 The Bakker study was large and conducted in the Netherlands. The primary outcome of the trial was to compare the incidence of interval breast cancers of women aged 50 to 75 randomly assigned to MRI versus those assigned to continued screening mammography every 2 years. Importantly, among the more than 8,000 women who were assigned to MRI, 59%, or fewer than two-thirds, chose to actually undergo MRI.
Among women randomized to MRI, 20 interval cancers were found—4 were diagnosed in those who actually had MRIs, and 16 were diagnosed among women who were randomized to MRI but didn’t undergo the study. Among women assigned to screening mammography only, 161 interval cancers were diagnosed among more than 32,000 women screened. The primary outcome findings were 2.5 interval cancers per 1,000 screenings among women randomly assigned to MRI, and 5 interval cancers per 1,000 screenings among those randomly assigned to mammography only.6
Because the trial included women aged 50 and older, we can’t apply these results to younger women, who often undergo screening mammography in the United States. In addition, the majority of the population in the Netherlands are of Western European ethnicity, a less-diverse population of women than in the United States. Furthermore, among the tumors that were detected in the MRI group, a larger proportion were DCIS, early-stage tumors, well differentiated, and hormone receptor-positive. This observation supports that many of the MRI-detected tumors were cases of overdiagnosis, or the detection of tumors destined not to cause clinical problems for the patient during her lifetime, or for which earlier diagnosis would impact survival.
We also know that treatment of these small ER-positive tumors carries risks for patients, as we may treat them by depriving a patient of estrogen for the rest of her life, with potential consequences of sexual dysfunction, osteoporosis, and perhaps cardiovascular disease depending on her age at the time of that diagnosis. Weighing the risks and benefits of not only treatment but also use of more sensitive screening techniques such as MRI is extremely important. Although Bakker and colleagues’ study results are interesting, we do not feel they support routinely recommending MRI for women found to have extremely dense breasts with mammography.
Overdiagnosis: A difficult concept
One reason overdiagnosis is so challenging to understand is that it can’t be directly measured, which makes comprehending it that much more problematic for clinicians and our patients.
One way to help grasp the overall issue is to compare screening mammography with cervical and colon cancer screening.
We are well aware that cervical cancer screening has reduced the incidence of mortality from invasive cervical cancer.8 We can argue very validly that the biggest success in any cancer screening program in history and globally has been cervical cancer screening. Our specialty, in particular, should feel proud about this. Screening colonoscopy also has repeatedly been found to reduce colon cancer mortality.9 For breast cancer, decades of media messaging have emphasized the benefits of screening mammograms; however, in contrast with cervical cancer screening and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic or advanced disease. Danish authors pointed out in 2017 that screening mammography has not achieved the hoped for or the promised reduction in breast cancer mortality.10
A report published in the March 2022, issue of Annals of Internal Medicine used modeling techniques to estimate the incidence of overdiagnosis and concluded that, among women aged 50-74 years receiving biennial screening mammograms (consistent with USPSTF recommendations), more than 15% of screen-detected breast cancers would represent cases of overdiagnosis. Of note, the study authors found that, among screen-detected cancers, the proportion representing overdiagnosis among women in their 60s (16.7%) and early 70s (23.6%) was higher than among women in their 50s-60s (11.5%-11.6%).11
The former Chief Medical and Scientific Officer for the American Cancer Society Otis Brawley, MD, has stated that, at the same time that breast cancer screening should not be abandoned, “We must acknowledge that overdiagnosis is common. The benefits of screening have been overstated, and some patients considered as ‘cured’ from breast cancer have, in fact, been harmed by unneeded treatment.”12
“Everybody loves early detection,” said Donald Berry, PhD, from MD Anderson Cancer Center, “but it comes with harms.” He points out that mortality rates have improved for breast cancer, but he attributes it to improved treatment. “The harms [of screening] we know, but the benefits of screening are very uncertain.”13
The importance of health equity is receiving more attention. When examining equity according to breast cancer mortality, ethnic minority populations have worse cancer survival outcomes than White women; the mortality rate is 40% higher among Black women than among White women.1 Lower survival rates are also noted among lower socioeconomic groups and among women who live in rural areas. Lower survival rates among ethnic minority women are also noted for cervical and colorectal cancers.2
In the past, these disparities in mortality were attributed to the historically lower breast cancer screening rates among Black women compared with White women. However, decades of efforts to increase mammography rates have effectively addressed much of the racial/ethnic gap in screening rates.1 In fact, a 2021 study showed Black and Hispanic women to have 6% to 10% higher rates of breast, cervical, and colorectal cancer screening than White women according to US Preventive Services Task Force guidelines.2 The study authors point out that other national data have demonstrated similar results and conclude that “higher cancer mortality among racial/ethnic minority groups will not be reduced solely by increasing rates of cancer screening. Although preventive screenings and timely diagnosis are important elements of prognosis, they are just 2 elements of many along the cancer care continuum that need to be addressed to eliminate disparities in cancer mortality.”
Unfortunately, the randomized trials that have been conducted on mammography have been conducted overwhelmingly in White populations. National registry studies from the Netherlands and Sweden are not representative patient populations for the United States. Recently, the US government proposed an ambitious plan to cut cancer mortality rates and has promised vast amounts of research funding to achieve that goal.3 Hopefully, this funding will support studies which enroll diverse patient populations. We hope to gain knowledge on what elements along the cancer care continuum can be addressed to better reduce or eliminate cancer mortality inequities.
References
1. National Cancer Institute. SEER Explorer. https://seer.cancer.gov/explorer/. Accessed February 9, 2022.
2. Benavidez GA, Zgodic A, Zahnd WE, Eberth JM. Disparities in Meeting USPSTF Breast, Cervical, and Colorectal Cancer Screening Guidelines Among Women in the United States. Prev Chronic Dis. 2021;18:200315. doi: http://dx.doi.org/10.5888/pcd18.200315.
3. Stohlberg SG, Kolata G. Biden presents ambitious plan to cut cancer death rate in half. The New York Times. February 2, 2022.
Continue to: Limitations of breast MRI...
Limitations of breast MRI
Overall, MRI is a diagnostic and monitoring test. It is costlier than mammography, and because it is not recommended in guidelines as a screening modality for most women, it is not typically covered by insurance. Abbreviated (rapid) MRI is a non-standardized imaging strategy being used at a few health centers. It has a shorter protocol overall than MRI, so it takes less time than current MRI and is less expensive, but there are few data on sensitivity and specificity. It is yet to be determined which populations could benefit from this newer technology.
As mentioned, 41% of women in the Bakker et al trial who were randomly assigned to breast MRI chose not to proceed with that exam even though it would have been at no cost to them.6 Anecdotally, some patients who have undergone MRI say they would forgo it a second time as a screening modality because it was a very unpleasant, stressful experience. It’s not a perfect test, although it is more sensitive than mammography.
Other options for following up dense-breast screening. Besides MRI and abbreviated MRI, the following modalities can be used to evaluate women found to have dense breasts with screening mammograms: CT mammography with contrast, molecular breast imaging, and ultrasonography.
Screening and treatment advances
3D mammography. In the US, the great majority of screening mammography now is performed with tomosynthesis, or what our patients sometimes call 3D mammography. In fact, it is approaching standard of care. Women whose screening mammography includes tomosynthesis are less likely to experience a so-called callback for additional imaging with diagnostic mammography or breast ultrasonography.14
Liquid biopsy. A potential major advancement for making decisions about when to treat cancers in general involves determining the biological behavior of a tumor, based on analysis of either circulating tumor DNA or proteins in the blood. As more experience with this new technology accumulates, the role of liquid biopsies for breast cancer will expand.15 Liquid biopsies for screening remain investigational for now, but they hold tremendous potential.
Noninvasive proteomics. With the development of noninvasive proteomic biomarkers obtained from blood, saliva, or nipple aspiration fluid, there exists the possibility of not just evaluating an image of a tumor seen on a mammogram, but actually studying the biological characteristics of that lesion.16 The cost of this technology is far less in terms of resources than MRI or molecular-based imaging, and actually reveals the flaws with using image-based screening. With proteomics, we can tell whether or not a lump is generating proteins that are going to make that disease biologically meaningful, and treatment decisions can be based on that information. This idea has the potential to disrupt our current breast cancer screening paradigm.
Advocacy’s role in mandating legislation
Many advocacy groups lobby on Capitol Hill for legislation related to health care, but we don’t feel that is the best way to make scientific decisions, and it’s not the way to do medicine. Passionate people, who truly believe that their outcome would have been different had something else been done, have every right to advocate, and should. However, without longer-term data focusing on breast cancer and overall mortality, rather than surrogate outcomes like interval cancers, it is not clear that routinely recommending supplemental MRI will improve survival for women with extremely dense breasts. Unfortunately, overall, earlier diagnosis of highly aggressive breast cancer tumors does not result in better outcomes for patients. ●
- American College of Obstetricians and Gynecologists. Practice Bulletin number 179: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130: e1-e16. doi: 10.1097/AOG.0000000000002158.
- Sui AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296. doi: 10.7326/M15-2886.
- The American Society of Breast Surgeons. Position statement on screening mammography. https://www.breastsurgeons.org/docs /statements/Position-Statement-on-ScreeningMammography.pdf. Accessed February 15, 2022.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average-risk: update from the ACR and Society of Breast Imaging. J Am College Radiol. 2021;18:1280-1288.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Seitzman R, Berg W. Average-risk women with dense breasts—what breast screening is appropriate? OBG Manag. 2021;33:18-19. doi: 10.12788/obgm.0155.
- Gopalani SV, Janitz AE, Campbell JE. Cervical cancer incidence and mortality among non-hispanic African American and White women, United States, 1999-2015. J Natl Med Assoc. 2020;112:632-638. doi: 10.1016 /j.jnma.2020.06.007.
- Niikura R, Hirata Y, Suzuki N, et al. Colonoscopy reduces colorectal cancer mortality: a multicenter, long-term, colonoscopy-based cohort study. PLoS One. 2017;12:e0185294.
- Jørgensen KJ, Gøtzsche PC, Kalager M, et al. Breast cancer screening in Denmark. Ann Intern Med. 2017;167:524. doi: 10.7326/L17-0270.
- Ryser MD, Lange J, Inoue IL, et al. Estimation of breast cancer overdiagnosis in a U.S. breast screening cohort. Ann Intern Med. 2022 March 1. doi: 10.7326/M21-3577.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis. Ann Intern Med. 2017;166:364-365. doi:10.7326/M16-2850.
- Stohlberg SG, Kolata G. Biden presents ambitious plan to cut cancer death rate in half. The New York Times. February 2, 2022.
- Conant EF, Barlow WE, Herschorn SD, et al. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol. 2019;5:635-642. doi: 10.1001 /jamaoncol.2018.7078.
- Tay TK, Tan PH. Liquid biopsy in breast cancer: a focused review. Arch Pathol Lab Med. 2021;145: 678-686. doi: 10.5858/arpa.2019-0559-RA.
- Debald M, Wolgarten M, Walgenbach-Brunagel G, et al. Non-invasive proteomics—thinking about personalized breast cancer screening and treatment. EPMA J. 2010;1:413-420. doi: 10.1007 /s13167-010-0039-9.
We have been interested in the quiz series focused on breast cancer screening for women with dense breasts presented in
The concerns with breast cancer in particular
Breast cancer is not cervical cancer. It isn’t one disease. It is a multitude of diseases that happen to show up in the breast. Some are relatively slow-growing—the kinds of cancers that lend themselves to screening and to early intervention. But other cancers are rapidly-growing; they show up no matter how often or what modality we use for screening. Our goal should be to find an approach to screening that can diagnose breast cancer at a stage where we can intervene and positively impact breast cancer specific and overall mortality.
Screening guidelines vary
The variety of screening guidelines published by different professional organizations reflect differing assumptions and sets of values related to the early diagnosis and treatment of breast cancer. (For a comprehensive table of current screening guidelines, see https://www.cdc.gov/cancer/breast/pdf/breast-cancer-screening-guidelines-508.pdf.)
ACOG’s approach—to offer screening at age 40 but to begin by at least age 50 and, through shared decision making with the patient, screen every 1 or 2 years—is focused on capturing as many cases as we can identify, while minimizing the harms of false-positives.1 The perspective of the US Preventive Services Task Force (USPSTF) recommendations (to screen every 2 years beginning at age 50) is at the population level, a cost-effective approach that will have the greatest benefit while minimizing harms in the population at large.2 The American Society of Breast Surgeons recommends screening to begin by age 40.3 Like the breast surgeons, radiologists dedicated to breast imaging are focused on an individual rather than a population level. They strive to identify each and every instance of possible cancer, and therefore recommend annual screening beginning at age 40.4 However, with more aggressive screening in average-risk women many cases of ductal carcinoma in situ (DCIS) are identified—a lesion that, if not detected, may not impact the woman’s health during her lifetime—representing what some might call “overdiagnosis.” Yet there may be some instances in which the DCIS might affect an individual woman’s health. Unfortunately, we can’t prospectively distinguish between the first and the second types of cases.
We follow American College of Obstetricians and Gynecologists and US Preventive Services Task Force guidelines in discussing screening (both its hazards and benefits) with our average-risk patients beginning at age 40. We talk about risk factors for breast cancer, including breast density, but let patients know that no specific additional imaging is advised, and that density is more common in younger women (one consideration in earlier screening) and is quite common in general. Although we do not send follow-up letters to patients with dense breasts, we do educate our staff so that they can respond appropriately should patients call with questions.
Of course, we all bring to the table values that will impact the decisions that we make for ourselves and for our patients. What an ObGyn might suggest may differ from what a radiologist might suggest. Although we follow recommendations made by the radiologist at screening, an ObGyn wants to take care of the whole human being. We are concerned with bones, heart, everything about the patient, so we approach a patient in a different way. These priorities are reflected in the current varying breast cancer screening guidelines.
Continue to: Research on breast cancer screening varies by design...
Research on breast cancer screening varies by design
There has not been a randomized clinical trial conducted on screening mammography since the days of the analog mammogram. The research that has been conducted is difficult to compare due to variations in screening ages and intervals, technology sensitivity, and patient adherence with recommended screening. Treatments for breast cancer also have changed dramatically over time, so the findings of older studies may no longer be relevant to current breast cancer screening. The kind of analysis that needs to be done is an interrupted time series, where you can look at the trajectory of breast cancer survival and whether screening mammography shifts that survival in any way.
One specific study from Australia measured the impact of newer available breast cancer treatments, including tamoxifen for women with receptor-positive tumors and newer chemotherapy strategies.5 The authors analyzed screening mammography trends in one large province where women aged 50 to 69 were offered biennial screening. Trends from the 1990s showed that more women were being screened over time. Simultaneously, however, advances in therapy were entering clinical practice. The researchers pointed to a substantial decline in mortality from breast cancer from the early 1980s until 2013. But their conclusion was that none of the decline in mortality for breast cancer could be attributed to screening mammography when they looked at time trends; from their perspective all of the important decline in breast cancer mortality resulted from better treatment. They concluded that government programs should not support screening mammography.5
That is a recommendation that we do not support. However, we do recognize the conundrum that mammography is less sensitive among those who have dense breasts. In order to have congruent professional guidelines, we support research funding to determine which types, starting ages, and intervals of screening would be best in various patient populations. The USPSTF cites data from studies performed in the 1980s based on outdated technology; more recent (and relevant) randomized clinical trials have not been performed, and yet this information is critical to provide sufficient evidence to develop appropriate guidelines.
Our recommendations for gathering new data
The kind of data we would find most valuable would assess how different screening strategies impact overall mortality and breast cancer-specific mortality. It would require decades of follow-up—which of course means that screening technology will change over that time. A surrogate for evaluating overall survival is to look at interval cancers, which are all breast cancers diagnosed following negative mammograms and prior to the next screening. These cancers may or may not be biologically active, again focusing us on the need to look at overall survival of the patient. In addition, reducing breast cancer mortality may not reduce overall mortality, because the treatment for breast cancer may cause heart disease, or osteoporosis, or something else that impacts overall survival. These are important considerations for women and physicians who are making choices on treatment. What matters to a patient are 2 overlapping questions:
- Do I have a life-threatening condition or do I not?
- Has screening identified a condition that might lead to treatment that’s unnecessary?
The problem is that with breast cancer we can’t tell the difference. We do not understand the biological potential of a lesion when we evaluate an image on MRI, or computed tomography (CT), or mammography.
A re-look at presented data
A trial conducted by Bakker and colleagues6 was discussed by the authors of the DenseBreast-info.org quiz in which they recommended breast MRI for all women with extremely dense breasts (but no other risk factors for breast cancer) detected on screening mammograms.7 The Bakker study was large and conducted in the Netherlands. The primary outcome of the trial was to compare the incidence of interval breast cancers of women aged 50 to 75 randomly assigned to MRI versus those assigned to continued screening mammography every 2 years. Importantly, among the more than 8,000 women who were assigned to MRI, 59%, or fewer than two-thirds, chose to actually undergo MRI.
Among women randomized to MRI, 20 interval cancers were found—4 were diagnosed in those who actually had MRIs, and 16 were diagnosed among women who were randomized to MRI but didn’t undergo the study. Among women assigned to screening mammography only, 161 interval cancers were diagnosed among more than 32,000 women screened. The primary outcome findings were 2.5 interval cancers per 1,000 screenings among women randomly assigned to MRI, and 5 interval cancers per 1,000 screenings among those randomly assigned to mammography only.6
Because the trial included women aged 50 and older, we can’t apply these results to younger women, who often undergo screening mammography in the United States. In addition, the majority of the population in the Netherlands are of Western European ethnicity, a less-diverse population of women than in the United States. Furthermore, among the tumors that were detected in the MRI group, a larger proportion were DCIS, early-stage tumors, well differentiated, and hormone receptor-positive. This observation supports that many of the MRI-detected tumors were cases of overdiagnosis, or the detection of tumors destined not to cause clinical problems for the patient during her lifetime, or for which earlier diagnosis would impact survival.
We also know that treatment of these small ER-positive tumors carries risks for patients, as we may treat them by depriving a patient of estrogen for the rest of her life, with potential consequences of sexual dysfunction, osteoporosis, and perhaps cardiovascular disease depending on her age at the time of that diagnosis. Weighing the risks and benefits of not only treatment but also use of more sensitive screening techniques such as MRI is extremely important. Although Bakker and colleagues’ study results are interesting, we do not feel they support routinely recommending MRI for women found to have extremely dense breasts with mammography.
Overdiagnosis: A difficult concept
One reason overdiagnosis is so challenging to understand is that it can’t be directly measured, which makes comprehending it that much more problematic for clinicians and our patients.
One way to help grasp the overall issue is to compare screening mammography with cervical and colon cancer screening.
We are well aware that cervical cancer screening has reduced the incidence of mortality from invasive cervical cancer.8 We can argue very validly that the biggest success in any cancer screening program in history and globally has been cervical cancer screening. Our specialty, in particular, should feel proud about this. Screening colonoscopy also has repeatedly been found to reduce colon cancer mortality.9 For breast cancer, decades of media messaging have emphasized the benefits of screening mammograms; however, in contrast with cervical cancer screening and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic or advanced disease. Danish authors pointed out in 2017 that screening mammography has not achieved the hoped for or the promised reduction in breast cancer mortality.10
A report published in the March 2022, issue of Annals of Internal Medicine used modeling techniques to estimate the incidence of overdiagnosis and concluded that, among women aged 50-74 years receiving biennial screening mammograms (consistent with USPSTF recommendations), more than 15% of screen-detected breast cancers would represent cases of overdiagnosis. Of note, the study authors found that, among screen-detected cancers, the proportion representing overdiagnosis among women in their 60s (16.7%) and early 70s (23.6%) was higher than among women in their 50s-60s (11.5%-11.6%).11
The former Chief Medical and Scientific Officer for the American Cancer Society Otis Brawley, MD, has stated that, at the same time that breast cancer screening should not be abandoned, “We must acknowledge that overdiagnosis is common. The benefits of screening have been overstated, and some patients considered as ‘cured’ from breast cancer have, in fact, been harmed by unneeded treatment.”12
“Everybody loves early detection,” said Donald Berry, PhD, from MD Anderson Cancer Center, “but it comes with harms.” He points out that mortality rates have improved for breast cancer, but he attributes it to improved treatment. “The harms [of screening] we know, but the benefits of screening are very uncertain.”13
The importance of health equity is receiving more attention. When examining equity according to breast cancer mortality, ethnic minority populations have worse cancer survival outcomes than White women; the mortality rate is 40% higher among Black women than among White women.1 Lower survival rates are also noted among lower socioeconomic groups and among women who live in rural areas. Lower survival rates among ethnic minority women are also noted for cervical and colorectal cancers.2
In the past, these disparities in mortality were attributed to the historically lower breast cancer screening rates among Black women compared with White women. However, decades of efforts to increase mammography rates have effectively addressed much of the racial/ethnic gap in screening rates.1 In fact, a 2021 study showed Black and Hispanic women to have 6% to 10% higher rates of breast, cervical, and colorectal cancer screening than White women according to US Preventive Services Task Force guidelines.2 The study authors point out that other national data have demonstrated similar results and conclude that “higher cancer mortality among racial/ethnic minority groups will not be reduced solely by increasing rates of cancer screening. Although preventive screenings and timely diagnosis are important elements of prognosis, they are just 2 elements of many along the cancer care continuum that need to be addressed to eliminate disparities in cancer mortality.”
Unfortunately, the randomized trials that have been conducted on mammography have been conducted overwhelmingly in White populations. National registry studies from the Netherlands and Sweden are not representative patient populations for the United States. Recently, the US government proposed an ambitious plan to cut cancer mortality rates and has promised vast amounts of research funding to achieve that goal.3 Hopefully, this funding will support studies which enroll diverse patient populations. We hope to gain knowledge on what elements along the cancer care continuum can be addressed to better reduce or eliminate cancer mortality inequities.
References
1. National Cancer Institute. SEER Explorer. https://seer.cancer.gov/explorer/. Accessed February 9, 2022.
2. Benavidez GA, Zgodic A, Zahnd WE, Eberth JM. Disparities in Meeting USPSTF Breast, Cervical, and Colorectal Cancer Screening Guidelines Among Women in the United States. Prev Chronic Dis. 2021;18:200315. doi: http://dx.doi.org/10.5888/pcd18.200315.
3. Stohlberg SG, Kolata G. Biden presents ambitious plan to cut cancer death rate in half. The New York Times. February 2, 2022.
Continue to: Limitations of breast MRI...
Limitations of breast MRI
Overall, MRI is a diagnostic and monitoring test. It is costlier than mammography, and because it is not recommended in guidelines as a screening modality for most women, it is not typically covered by insurance. Abbreviated (rapid) MRI is a non-standardized imaging strategy being used at a few health centers. It has a shorter protocol overall than MRI, so it takes less time than current MRI and is less expensive, but there are few data on sensitivity and specificity. It is yet to be determined which populations could benefit from this newer technology.
As mentioned, 41% of women in the Bakker et al trial who were randomly assigned to breast MRI chose not to proceed with that exam even though it would have been at no cost to them.6 Anecdotally, some patients who have undergone MRI say they would forgo it a second time as a screening modality because it was a very unpleasant, stressful experience. It’s not a perfect test, although it is more sensitive than mammography.
Other options for following up dense-breast screening. Besides MRI and abbreviated MRI, the following modalities can be used to evaluate women found to have dense breasts with screening mammograms: CT mammography with contrast, molecular breast imaging, and ultrasonography.
Screening and treatment advances
3D mammography. In the US, the great majority of screening mammography now is performed with tomosynthesis, or what our patients sometimes call 3D mammography. In fact, it is approaching standard of care. Women whose screening mammography includes tomosynthesis are less likely to experience a so-called callback for additional imaging with diagnostic mammography or breast ultrasonography.14
Liquid biopsy. A potential major advancement for making decisions about when to treat cancers in general involves determining the biological behavior of a tumor, based on analysis of either circulating tumor DNA or proteins in the blood. As more experience with this new technology accumulates, the role of liquid biopsies for breast cancer will expand.15 Liquid biopsies for screening remain investigational for now, but they hold tremendous potential.
Noninvasive proteomics. With the development of noninvasive proteomic biomarkers obtained from blood, saliva, or nipple aspiration fluid, there exists the possibility of not just evaluating an image of a tumor seen on a mammogram, but actually studying the biological characteristics of that lesion.16 The cost of this technology is far less in terms of resources than MRI or molecular-based imaging, and actually reveals the flaws with using image-based screening. With proteomics, we can tell whether or not a lump is generating proteins that are going to make that disease biologically meaningful, and treatment decisions can be based on that information. This idea has the potential to disrupt our current breast cancer screening paradigm.
Advocacy’s role in mandating legislation
Many advocacy groups lobby on Capitol Hill for legislation related to health care, but we don’t feel that is the best way to make scientific decisions, and it’s not the way to do medicine. Passionate people, who truly believe that their outcome would have been different had something else been done, have every right to advocate, and should. However, without longer-term data focusing on breast cancer and overall mortality, rather than surrogate outcomes like interval cancers, it is not clear that routinely recommending supplemental MRI will improve survival for women with extremely dense breasts. Unfortunately, overall, earlier diagnosis of highly aggressive breast cancer tumors does not result in better outcomes for patients. ●
We have been interested in the quiz series focused on breast cancer screening for women with dense breasts presented in
The concerns with breast cancer in particular
Breast cancer is not cervical cancer. It isn’t one disease. It is a multitude of diseases that happen to show up in the breast. Some are relatively slow-growing—the kinds of cancers that lend themselves to screening and to early intervention. But other cancers are rapidly-growing; they show up no matter how often or what modality we use for screening. Our goal should be to find an approach to screening that can diagnose breast cancer at a stage where we can intervene and positively impact breast cancer specific and overall mortality.
Screening guidelines vary
The variety of screening guidelines published by different professional organizations reflect differing assumptions and sets of values related to the early diagnosis and treatment of breast cancer. (For a comprehensive table of current screening guidelines, see https://www.cdc.gov/cancer/breast/pdf/breast-cancer-screening-guidelines-508.pdf.)
ACOG’s approach—to offer screening at age 40 but to begin by at least age 50 and, through shared decision making with the patient, screen every 1 or 2 years—is focused on capturing as many cases as we can identify, while minimizing the harms of false-positives.1 The perspective of the US Preventive Services Task Force (USPSTF) recommendations (to screen every 2 years beginning at age 50) is at the population level, a cost-effective approach that will have the greatest benefit while minimizing harms in the population at large.2 The American Society of Breast Surgeons recommends screening to begin by age 40.3 Like the breast surgeons, radiologists dedicated to breast imaging are focused on an individual rather than a population level. They strive to identify each and every instance of possible cancer, and therefore recommend annual screening beginning at age 40.4 However, with more aggressive screening in average-risk women many cases of ductal carcinoma in situ (DCIS) are identified—a lesion that, if not detected, may not impact the woman’s health during her lifetime—representing what some might call “overdiagnosis.” Yet there may be some instances in which the DCIS might affect an individual woman’s health. Unfortunately, we can’t prospectively distinguish between the first and the second types of cases.
We follow American College of Obstetricians and Gynecologists and US Preventive Services Task Force guidelines in discussing screening (both its hazards and benefits) with our average-risk patients beginning at age 40. We talk about risk factors for breast cancer, including breast density, but let patients know that no specific additional imaging is advised, and that density is more common in younger women (one consideration in earlier screening) and is quite common in general. Although we do not send follow-up letters to patients with dense breasts, we do educate our staff so that they can respond appropriately should patients call with questions.
Of course, we all bring to the table values that will impact the decisions that we make for ourselves and for our patients. What an ObGyn might suggest may differ from what a radiologist might suggest. Although we follow recommendations made by the radiologist at screening, an ObGyn wants to take care of the whole human being. We are concerned with bones, heart, everything about the patient, so we approach a patient in a different way. These priorities are reflected in the current varying breast cancer screening guidelines.
Continue to: Research on breast cancer screening varies by design...
Research on breast cancer screening varies by design
There has not been a randomized clinical trial conducted on screening mammography since the days of the analog mammogram. The research that has been conducted is difficult to compare due to variations in screening ages and intervals, technology sensitivity, and patient adherence with recommended screening. Treatments for breast cancer also have changed dramatically over time, so the findings of older studies may no longer be relevant to current breast cancer screening. The kind of analysis that needs to be done is an interrupted time series, where you can look at the trajectory of breast cancer survival and whether screening mammography shifts that survival in any way.
One specific study from Australia measured the impact of newer available breast cancer treatments, including tamoxifen for women with receptor-positive tumors and newer chemotherapy strategies.5 The authors analyzed screening mammography trends in one large province where women aged 50 to 69 were offered biennial screening. Trends from the 1990s showed that more women were being screened over time. Simultaneously, however, advances in therapy were entering clinical practice. The researchers pointed to a substantial decline in mortality from breast cancer from the early 1980s until 2013. But their conclusion was that none of the decline in mortality for breast cancer could be attributed to screening mammography when they looked at time trends; from their perspective all of the important decline in breast cancer mortality resulted from better treatment. They concluded that government programs should not support screening mammography.5
That is a recommendation that we do not support. However, we do recognize the conundrum that mammography is less sensitive among those who have dense breasts. In order to have congruent professional guidelines, we support research funding to determine which types, starting ages, and intervals of screening would be best in various patient populations. The USPSTF cites data from studies performed in the 1980s based on outdated technology; more recent (and relevant) randomized clinical trials have not been performed, and yet this information is critical to provide sufficient evidence to develop appropriate guidelines.
Our recommendations for gathering new data
The kind of data we would find most valuable would assess how different screening strategies impact overall mortality and breast cancer-specific mortality. It would require decades of follow-up—which of course means that screening technology will change over that time. A surrogate for evaluating overall survival is to look at interval cancers, which are all breast cancers diagnosed following negative mammograms and prior to the next screening. These cancers may or may not be biologically active, again focusing us on the need to look at overall survival of the patient. In addition, reducing breast cancer mortality may not reduce overall mortality, because the treatment for breast cancer may cause heart disease, or osteoporosis, or something else that impacts overall survival. These are important considerations for women and physicians who are making choices on treatment. What matters to a patient are 2 overlapping questions:
- Do I have a life-threatening condition or do I not?
- Has screening identified a condition that might lead to treatment that’s unnecessary?
The problem is that with breast cancer we can’t tell the difference. We do not understand the biological potential of a lesion when we evaluate an image on MRI, or computed tomography (CT), or mammography.
A re-look at presented data
A trial conducted by Bakker and colleagues6 was discussed by the authors of the DenseBreast-info.org quiz in which they recommended breast MRI for all women with extremely dense breasts (but no other risk factors for breast cancer) detected on screening mammograms.7 The Bakker study was large and conducted in the Netherlands. The primary outcome of the trial was to compare the incidence of interval breast cancers of women aged 50 to 75 randomly assigned to MRI versus those assigned to continued screening mammography every 2 years. Importantly, among the more than 8,000 women who were assigned to MRI, 59%, or fewer than two-thirds, chose to actually undergo MRI.
Among women randomized to MRI, 20 interval cancers were found—4 were diagnosed in those who actually had MRIs, and 16 were diagnosed among women who were randomized to MRI but didn’t undergo the study. Among women assigned to screening mammography only, 161 interval cancers were diagnosed among more than 32,000 women screened. The primary outcome findings were 2.5 interval cancers per 1,000 screenings among women randomly assigned to MRI, and 5 interval cancers per 1,000 screenings among those randomly assigned to mammography only.6
Because the trial included women aged 50 and older, we can’t apply these results to younger women, who often undergo screening mammography in the United States. In addition, the majority of the population in the Netherlands are of Western European ethnicity, a less-diverse population of women than in the United States. Furthermore, among the tumors that were detected in the MRI group, a larger proportion were DCIS, early-stage tumors, well differentiated, and hormone receptor-positive. This observation supports that many of the MRI-detected tumors were cases of overdiagnosis, or the detection of tumors destined not to cause clinical problems for the patient during her lifetime, or for which earlier diagnosis would impact survival.
We also know that treatment of these small ER-positive tumors carries risks for patients, as we may treat them by depriving a patient of estrogen for the rest of her life, with potential consequences of sexual dysfunction, osteoporosis, and perhaps cardiovascular disease depending on her age at the time of that diagnosis. Weighing the risks and benefits of not only treatment but also use of more sensitive screening techniques such as MRI is extremely important. Although Bakker and colleagues’ study results are interesting, we do not feel they support routinely recommending MRI for women found to have extremely dense breasts with mammography.
Overdiagnosis: A difficult concept
One reason overdiagnosis is so challenging to understand is that it can’t be directly measured, which makes comprehending it that much more problematic for clinicians and our patients.
One way to help grasp the overall issue is to compare screening mammography with cervical and colon cancer screening.
We are well aware that cervical cancer screening has reduced the incidence of mortality from invasive cervical cancer.8 We can argue very validly that the biggest success in any cancer screening program in history and globally has been cervical cancer screening. Our specialty, in particular, should feel proud about this. Screening colonoscopy also has repeatedly been found to reduce colon cancer mortality.9 For breast cancer, decades of media messaging have emphasized the benefits of screening mammograms; however, in contrast with cervical cancer screening and colonoscopy, screening mammography has not reduced the incidence of breast cancer presenting with metastatic or advanced disease. Danish authors pointed out in 2017 that screening mammography has not achieved the hoped for or the promised reduction in breast cancer mortality.10
A report published in the March 2022, issue of Annals of Internal Medicine used modeling techniques to estimate the incidence of overdiagnosis and concluded that, among women aged 50-74 years receiving biennial screening mammograms (consistent with USPSTF recommendations), more than 15% of screen-detected breast cancers would represent cases of overdiagnosis. Of note, the study authors found that, among screen-detected cancers, the proportion representing overdiagnosis among women in their 60s (16.7%) and early 70s (23.6%) was higher than among women in their 50s-60s (11.5%-11.6%).11
The former Chief Medical and Scientific Officer for the American Cancer Society Otis Brawley, MD, has stated that, at the same time that breast cancer screening should not be abandoned, “We must acknowledge that overdiagnosis is common. The benefits of screening have been overstated, and some patients considered as ‘cured’ from breast cancer have, in fact, been harmed by unneeded treatment.”12
“Everybody loves early detection,” said Donald Berry, PhD, from MD Anderson Cancer Center, “but it comes with harms.” He points out that mortality rates have improved for breast cancer, but he attributes it to improved treatment. “The harms [of screening] we know, but the benefits of screening are very uncertain.”13
The importance of health equity is receiving more attention. When examining equity according to breast cancer mortality, ethnic minority populations have worse cancer survival outcomes than White women; the mortality rate is 40% higher among Black women than among White women.1 Lower survival rates are also noted among lower socioeconomic groups and among women who live in rural areas. Lower survival rates among ethnic minority women are also noted for cervical and colorectal cancers.2
In the past, these disparities in mortality were attributed to the historically lower breast cancer screening rates among Black women compared with White women. However, decades of efforts to increase mammography rates have effectively addressed much of the racial/ethnic gap in screening rates.1 In fact, a 2021 study showed Black and Hispanic women to have 6% to 10% higher rates of breast, cervical, and colorectal cancer screening than White women according to US Preventive Services Task Force guidelines.2 The study authors point out that other national data have demonstrated similar results and conclude that “higher cancer mortality among racial/ethnic minority groups will not be reduced solely by increasing rates of cancer screening. Although preventive screenings and timely diagnosis are important elements of prognosis, they are just 2 elements of many along the cancer care continuum that need to be addressed to eliminate disparities in cancer mortality.”
Unfortunately, the randomized trials that have been conducted on mammography have been conducted overwhelmingly in White populations. National registry studies from the Netherlands and Sweden are not representative patient populations for the United States. Recently, the US government proposed an ambitious plan to cut cancer mortality rates and has promised vast amounts of research funding to achieve that goal.3 Hopefully, this funding will support studies which enroll diverse patient populations. We hope to gain knowledge on what elements along the cancer care continuum can be addressed to better reduce or eliminate cancer mortality inequities.
References
1. National Cancer Institute. SEER Explorer. https://seer.cancer.gov/explorer/. Accessed February 9, 2022.
2. Benavidez GA, Zgodic A, Zahnd WE, Eberth JM. Disparities in Meeting USPSTF Breast, Cervical, and Colorectal Cancer Screening Guidelines Among Women in the United States. Prev Chronic Dis. 2021;18:200315. doi: http://dx.doi.org/10.5888/pcd18.200315.
3. Stohlberg SG, Kolata G. Biden presents ambitious plan to cut cancer death rate in half. The New York Times. February 2, 2022.
Continue to: Limitations of breast MRI...
Limitations of breast MRI
Overall, MRI is a diagnostic and monitoring test. It is costlier than mammography, and because it is not recommended in guidelines as a screening modality for most women, it is not typically covered by insurance. Abbreviated (rapid) MRI is a non-standardized imaging strategy being used at a few health centers. It has a shorter protocol overall than MRI, so it takes less time than current MRI and is less expensive, but there are few data on sensitivity and specificity. It is yet to be determined which populations could benefit from this newer technology.
As mentioned, 41% of women in the Bakker et al trial who were randomly assigned to breast MRI chose not to proceed with that exam even though it would have been at no cost to them.6 Anecdotally, some patients who have undergone MRI say they would forgo it a second time as a screening modality because it was a very unpleasant, stressful experience. It’s not a perfect test, although it is more sensitive than mammography.
Other options for following up dense-breast screening. Besides MRI and abbreviated MRI, the following modalities can be used to evaluate women found to have dense breasts with screening mammograms: CT mammography with contrast, molecular breast imaging, and ultrasonography.
Screening and treatment advances
3D mammography. In the US, the great majority of screening mammography now is performed with tomosynthesis, or what our patients sometimes call 3D mammography. In fact, it is approaching standard of care. Women whose screening mammography includes tomosynthesis are less likely to experience a so-called callback for additional imaging with diagnostic mammography or breast ultrasonography.14
Liquid biopsy. A potential major advancement for making decisions about when to treat cancers in general involves determining the biological behavior of a tumor, based on analysis of either circulating tumor DNA or proteins in the blood. As more experience with this new technology accumulates, the role of liquid biopsies for breast cancer will expand.15 Liquid biopsies for screening remain investigational for now, but they hold tremendous potential.
Noninvasive proteomics. With the development of noninvasive proteomic biomarkers obtained from blood, saliva, or nipple aspiration fluid, there exists the possibility of not just evaluating an image of a tumor seen on a mammogram, but actually studying the biological characteristics of that lesion.16 The cost of this technology is far less in terms of resources than MRI or molecular-based imaging, and actually reveals the flaws with using image-based screening. With proteomics, we can tell whether or not a lump is generating proteins that are going to make that disease biologically meaningful, and treatment decisions can be based on that information. This idea has the potential to disrupt our current breast cancer screening paradigm.
Advocacy’s role in mandating legislation
Many advocacy groups lobby on Capitol Hill for legislation related to health care, but we don’t feel that is the best way to make scientific decisions, and it’s not the way to do medicine. Passionate people, who truly believe that their outcome would have been different had something else been done, have every right to advocate, and should. However, without longer-term data focusing on breast cancer and overall mortality, rather than surrogate outcomes like interval cancers, it is not clear that routinely recommending supplemental MRI will improve survival for women with extremely dense breasts. Unfortunately, overall, earlier diagnosis of highly aggressive breast cancer tumors does not result in better outcomes for patients. ●
- American College of Obstetricians and Gynecologists. Practice Bulletin number 179: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130: e1-e16. doi: 10.1097/AOG.0000000000002158.
- Sui AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296. doi: 10.7326/M15-2886.
- The American Society of Breast Surgeons. Position statement on screening mammography. https://www.breastsurgeons.org/docs /statements/Position-Statement-on-ScreeningMammography.pdf. Accessed February 15, 2022.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average-risk: update from the ACR and Society of Breast Imaging. J Am College Radiol. 2021;18:1280-1288.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Seitzman R, Berg W. Average-risk women with dense breasts—what breast screening is appropriate? OBG Manag. 2021;33:18-19. doi: 10.12788/obgm.0155.
- Gopalani SV, Janitz AE, Campbell JE. Cervical cancer incidence and mortality among non-hispanic African American and White women, United States, 1999-2015. J Natl Med Assoc. 2020;112:632-638. doi: 10.1016 /j.jnma.2020.06.007.
- Niikura R, Hirata Y, Suzuki N, et al. Colonoscopy reduces colorectal cancer mortality: a multicenter, long-term, colonoscopy-based cohort study. PLoS One. 2017;12:e0185294.
- Jørgensen KJ, Gøtzsche PC, Kalager M, et al. Breast cancer screening in Denmark. Ann Intern Med. 2017;167:524. doi: 10.7326/L17-0270.
- Ryser MD, Lange J, Inoue IL, et al. Estimation of breast cancer overdiagnosis in a U.S. breast screening cohort. Ann Intern Med. 2022 March 1. doi: 10.7326/M21-3577.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis. Ann Intern Med. 2017;166:364-365. doi:10.7326/M16-2850.
- Stohlberg SG, Kolata G. Biden presents ambitious plan to cut cancer death rate in half. The New York Times. February 2, 2022.
- Conant EF, Barlow WE, Herschorn SD, et al. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol. 2019;5:635-642. doi: 10.1001 /jamaoncol.2018.7078.
- Tay TK, Tan PH. Liquid biopsy in breast cancer: a focused review. Arch Pathol Lab Med. 2021;145: 678-686. doi: 10.5858/arpa.2019-0559-RA.
- Debald M, Wolgarten M, Walgenbach-Brunagel G, et al. Non-invasive proteomics—thinking about personalized breast cancer screening and treatment. EPMA J. 2010;1:413-420. doi: 10.1007 /s13167-010-0039-9.
- American College of Obstetricians and Gynecologists. Practice Bulletin number 179: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130: e1-e16. doi: 10.1097/AOG.0000000000002158.
- Sui AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296. doi: 10.7326/M15-2886.
- The American Society of Breast Surgeons. Position statement on screening mammography. https://www.breastsurgeons.org/docs /statements/Position-Statement-on-ScreeningMammography.pdf. Accessed February 15, 2022.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average-risk: update from the ACR and Society of Breast Imaging. J Am College Radiol. 2021;18:1280-1288.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Seitzman R, Berg W. Average-risk women with dense breasts—what breast screening is appropriate? OBG Manag. 2021;33:18-19. doi: 10.12788/obgm.0155.
- Gopalani SV, Janitz AE, Campbell JE. Cervical cancer incidence and mortality among non-hispanic African American and White women, United States, 1999-2015. J Natl Med Assoc. 2020;112:632-638. doi: 10.1016 /j.jnma.2020.06.007.
- Niikura R, Hirata Y, Suzuki N, et al. Colonoscopy reduces colorectal cancer mortality: a multicenter, long-term, colonoscopy-based cohort study. PLoS One. 2017;12:e0185294.
- Jørgensen KJ, Gøtzsche PC, Kalager M, et al. Breast cancer screening in Denmark. Ann Intern Med. 2017;167:524. doi: 10.7326/L17-0270.
- Ryser MD, Lange J, Inoue IL, et al. Estimation of breast cancer overdiagnosis in a U.S. breast screening cohort. Ann Intern Med. 2022 March 1. doi: 10.7326/M21-3577.
- Brawley OW. Accepting the existence of breast cancer overdiagnosis. Ann Intern Med. 2017;166:364-365. doi:10.7326/M16-2850.
- Stohlberg SG, Kolata G. Biden presents ambitious plan to cut cancer death rate in half. The New York Times. February 2, 2022.
- Conant EF, Barlow WE, Herschorn SD, et al. Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol. 2019;5:635-642. doi: 10.1001 /jamaoncol.2018.7078.
- Tay TK, Tan PH. Liquid biopsy in breast cancer: a focused review. Arch Pathol Lab Med. 2021;145: 678-686. doi: 10.5858/arpa.2019-0559-RA.
- Debald M, Wolgarten M, Walgenbach-Brunagel G, et al. Non-invasive proteomics—thinking about personalized breast cancer screening and treatment. EPMA J. 2010;1:413-420. doi: 10.1007 /s13167-010-0039-9.
Targeting the endocannabinoid system in migraine
, according to Italian researchers at the University of Pavia, and the C. Mondino National Institute of Neurology Foundation. “The complexity of the endocannabinoid system calls for accurate biochemical and pharmacological characterization of any new compounds undergoing testing and development,” noted Rosaria Greco, PhD. She and her colleagues authored a review on the topic that was published online Feb. 18, 2022, in Headache.
Although cannabis has been investigated for both the treatment and prevention of migraine, evidence for its benefit is weak because of lack of controlled studies, they explained. Archival data from a large database “showed greater improvements in men than in women and suggested that concentrated preparations were more effective than flower consumption.” In addition, a small single-center study linked nabilone, a synthetic cannabinoid, to reductions in pain duration, intensity, and daily intake of analgesics among patients with medication overuse headache. Finally, a pilot study reported a reduction in pain intensity among patients with chronic migraine treated with a combination of tested a combination of delta-9-tetrahydrocannabinol and cannabidiol. “Methodologically sound studies are now needed to investigate the possible effects of cannabis in migraine treatment and to define strains, formulations, and dosage,” they noted.
Not just cannabis
In addition to exogenous cannabis, there are now preclinical studies suggesting other compounds that interact with the endocannabinoid system “are also able to modulate the pathways involved in migraine-related pain,” the study authors wrote. “But the road ahead is still long. Multiple molecules linked to the endocannabinoid system have emerged as potential therapeutic targets.
The complexity of the system demands caution and precise biochemical and pharmacological characterization of the new compounds to be tested and developed.”
Among these compounds are endogenous ligands such as N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol that specifically target CB1 and CB2 receptors. Additionally, there are endocannabinoid-based drugs that also target the CB1/CB2 receptors, as well as other substances, such as lipids (palmitoylethanolamide [PEA]) and enzymes, that do not bind to the CB1/CB2 receptors but are responsible for endocannabinoid biosynthesis.
There is some evidence that the endocannabinoid system may be dysfunctional in patients with migraine, and the authors noted their work has shown that PEA plasma levels are increased during experimentally triggered migraine-like attacks. Thus, some preclinical and preliminary evidence suggests that administration of PEA or anandamide may have analgesic and anti-inflammatory effects in migraine.
Another approach is the inhibition of endocannabinoid catabolic enzymes, which could circumvent the adverse effects associated with direct activation of CB receptors. “Endocannabinoid tone enhancement has been proposed as an alternative modality of activation of CB receptors and is possibly devoid of the psychotropic effects reported with CB receptor agonists,” noted the authors, who have shown in animal and preclinical studies that inhibition of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase can modulate migraine pain.
Yet another way of indirectly impacting CB receptors is through their allosteric ligands, an approach that “deserves further investigation”, and “might provide interesting leads for clinical development, given that it may have a favorable side-effect profile with limited psychomimetic and depressant effects,” wrote the authors. And finally, inhibition of N-acylethanolamine acid amide hydrolase, the enzyme that preferentially hydrolyzes PEA, might be a promising approach.
“The multiplicity of options and the wealth of data already obtained in animal models underscore the importance of further advancing research in this area,” the authors concluded.
Patients are taking cannabinoids; physicians should learn about them
Commenting on the paper, Alan Rapaport, MD, clinical professor of neurology at the University of California, Los Angeles, said “this well-done paper points out the complexity of the endocannabinoid system and the multiple ways of getting it to work for certain patients. It details some of the studies that show beneficial results in migraine, medication overuse headache, chronic migraine, and pain. Patients with headache, other types of pain, anxiety, nausea, sleep issues, and other symptoms are already taking cannabinoids, usually derived from the marijuana plant, that are not well regulated. A few are prescribed drugs which target CB1 and CB2 receptors. Patients often get relief of some of their symptoms, sometimes getting high and many times not.
“The paper makes the point that previous studies are often small, not carefully controlled, or well documented. We do need to start doing larger, properly designed studies and getting them into the literature. Doctors need to learn more about these treatments. The next step will be to get [Food and Drug Administration]–approved treatments, so physicians and nurses will know exactly what we are giving, the beneficial effects to expect in a certain percentage of patients, and the adverse events to warn our patients about. Cannabinoids have been tried by a large percentage of patients with headache and pain. Now we need to standardize the various treatments that are sure to be suggested in the future.”
The study was funded by the Migraine Research Foundation, and the Italian Ministry of Health. The study authors declared no conflicts of interest.
, according to Italian researchers at the University of Pavia, and the C. Mondino National Institute of Neurology Foundation. “The complexity of the endocannabinoid system calls for accurate biochemical and pharmacological characterization of any new compounds undergoing testing and development,” noted Rosaria Greco, PhD. She and her colleagues authored a review on the topic that was published online Feb. 18, 2022, in Headache.
Although cannabis has been investigated for both the treatment and prevention of migraine, evidence for its benefit is weak because of lack of controlled studies, they explained. Archival data from a large database “showed greater improvements in men than in women and suggested that concentrated preparations were more effective than flower consumption.” In addition, a small single-center study linked nabilone, a synthetic cannabinoid, to reductions in pain duration, intensity, and daily intake of analgesics among patients with medication overuse headache. Finally, a pilot study reported a reduction in pain intensity among patients with chronic migraine treated with a combination of tested a combination of delta-9-tetrahydrocannabinol and cannabidiol. “Methodologically sound studies are now needed to investigate the possible effects of cannabis in migraine treatment and to define strains, formulations, and dosage,” they noted.
Not just cannabis
In addition to exogenous cannabis, there are now preclinical studies suggesting other compounds that interact with the endocannabinoid system “are also able to modulate the pathways involved in migraine-related pain,” the study authors wrote. “But the road ahead is still long. Multiple molecules linked to the endocannabinoid system have emerged as potential therapeutic targets.
The complexity of the system demands caution and precise biochemical and pharmacological characterization of the new compounds to be tested and developed.”
Among these compounds are endogenous ligands such as N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol that specifically target CB1 and CB2 receptors. Additionally, there are endocannabinoid-based drugs that also target the CB1/CB2 receptors, as well as other substances, such as lipids (palmitoylethanolamide [PEA]) and enzymes, that do not bind to the CB1/CB2 receptors but are responsible for endocannabinoid biosynthesis.
There is some evidence that the endocannabinoid system may be dysfunctional in patients with migraine, and the authors noted their work has shown that PEA plasma levels are increased during experimentally triggered migraine-like attacks. Thus, some preclinical and preliminary evidence suggests that administration of PEA or anandamide may have analgesic and anti-inflammatory effects in migraine.
Another approach is the inhibition of endocannabinoid catabolic enzymes, which could circumvent the adverse effects associated with direct activation of CB receptors. “Endocannabinoid tone enhancement has been proposed as an alternative modality of activation of CB receptors and is possibly devoid of the psychotropic effects reported with CB receptor agonists,” noted the authors, who have shown in animal and preclinical studies that inhibition of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase can modulate migraine pain.
Yet another way of indirectly impacting CB receptors is through their allosteric ligands, an approach that “deserves further investigation”, and “might provide interesting leads for clinical development, given that it may have a favorable side-effect profile with limited psychomimetic and depressant effects,” wrote the authors. And finally, inhibition of N-acylethanolamine acid amide hydrolase, the enzyme that preferentially hydrolyzes PEA, might be a promising approach.
“The multiplicity of options and the wealth of data already obtained in animal models underscore the importance of further advancing research in this area,” the authors concluded.
Patients are taking cannabinoids; physicians should learn about them
Commenting on the paper, Alan Rapaport, MD, clinical professor of neurology at the University of California, Los Angeles, said “this well-done paper points out the complexity of the endocannabinoid system and the multiple ways of getting it to work for certain patients. It details some of the studies that show beneficial results in migraine, medication overuse headache, chronic migraine, and pain. Patients with headache, other types of pain, anxiety, nausea, sleep issues, and other symptoms are already taking cannabinoids, usually derived from the marijuana plant, that are not well regulated. A few are prescribed drugs which target CB1 and CB2 receptors. Patients often get relief of some of their symptoms, sometimes getting high and many times not.
“The paper makes the point that previous studies are often small, not carefully controlled, or well documented. We do need to start doing larger, properly designed studies and getting them into the literature. Doctors need to learn more about these treatments. The next step will be to get [Food and Drug Administration]–approved treatments, so physicians and nurses will know exactly what we are giving, the beneficial effects to expect in a certain percentage of patients, and the adverse events to warn our patients about. Cannabinoids have been tried by a large percentage of patients with headache and pain. Now we need to standardize the various treatments that are sure to be suggested in the future.”
The study was funded by the Migraine Research Foundation, and the Italian Ministry of Health. The study authors declared no conflicts of interest.
, according to Italian researchers at the University of Pavia, and the C. Mondino National Institute of Neurology Foundation. “The complexity of the endocannabinoid system calls for accurate biochemical and pharmacological characterization of any new compounds undergoing testing and development,” noted Rosaria Greco, PhD. She and her colleagues authored a review on the topic that was published online Feb. 18, 2022, in Headache.
Although cannabis has been investigated for both the treatment and prevention of migraine, evidence for its benefit is weak because of lack of controlled studies, they explained. Archival data from a large database “showed greater improvements in men than in women and suggested that concentrated preparations were more effective than flower consumption.” In addition, a small single-center study linked nabilone, a synthetic cannabinoid, to reductions in pain duration, intensity, and daily intake of analgesics among patients with medication overuse headache. Finally, a pilot study reported a reduction in pain intensity among patients with chronic migraine treated with a combination of tested a combination of delta-9-tetrahydrocannabinol and cannabidiol. “Methodologically sound studies are now needed to investigate the possible effects of cannabis in migraine treatment and to define strains, formulations, and dosage,” they noted.
Not just cannabis
In addition to exogenous cannabis, there are now preclinical studies suggesting other compounds that interact with the endocannabinoid system “are also able to modulate the pathways involved in migraine-related pain,” the study authors wrote. “But the road ahead is still long. Multiple molecules linked to the endocannabinoid system have emerged as potential therapeutic targets.
The complexity of the system demands caution and precise biochemical and pharmacological characterization of the new compounds to be tested and developed.”
Among these compounds are endogenous ligands such as N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol that specifically target CB1 and CB2 receptors. Additionally, there are endocannabinoid-based drugs that also target the CB1/CB2 receptors, as well as other substances, such as lipids (palmitoylethanolamide [PEA]) and enzymes, that do not bind to the CB1/CB2 receptors but are responsible for endocannabinoid biosynthesis.
There is some evidence that the endocannabinoid system may be dysfunctional in patients with migraine, and the authors noted their work has shown that PEA plasma levels are increased during experimentally triggered migraine-like attacks. Thus, some preclinical and preliminary evidence suggests that administration of PEA or anandamide may have analgesic and anti-inflammatory effects in migraine.
Another approach is the inhibition of endocannabinoid catabolic enzymes, which could circumvent the adverse effects associated with direct activation of CB receptors. “Endocannabinoid tone enhancement has been proposed as an alternative modality of activation of CB receptors and is possibly devoid of the psychotropic effects reported with CB receptor agonists,” noted the authors, who have shown in animal and preclinical studies that inhibition of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase can modulate migraine pain.
Yet another way of indirectly impacting CB receptors is through their allosteric ligands, an approach that “deserves further investigation”, and “might provide interesting leads for clinical development, given that it may have a favorable side-effect profile with limited psychomimetic and depressant effects,” wrote the authors. And finally, inhibition of N-acylethanolamine acid amide hydrolase, the enzyme that preferentially hydrolyzes PEA, might be a promising approach.
“The multiplicity of options and the wealth of data already obtained in animal models underscore the importance of further advancing research in this area,” the authors concluded.
Patients are taking cannabinoids; physicians should learn about them
Commenting on the paper, Alan Rapaport, MD, clinical professor of neurology at the University of California, Los Angeles, said “this well-done paper points out the complexity of the endocannabinoid system and the multiple ways of getting it to work for certain patients. It details some of the studies that show beneficial results in migraine, medication overuse headache, chronic migraine, and pain. Patients with headache, other types of pain, anxiety, nausea, sleep issues, and other symptoms are already taking cannabinoids, usually derived from the marijuana plant, that are not well regulated. A few are prescribed drugs which target CB1 and CB2 receptors. Patients often get relief of some of their symptoms, sometimes getting high and many times not.
“The paper makes the point that previous studies are often small, not carefully controlled, or well documented. We do need to start doing larger, properly designed studies and getting them into the literature. Doctors need to learn more about these treatments. The next step will be to get [Food and Drug Administration]–approved treatments, so physicians and nurses will know exactly what we are giving, the beneficial effects to expect in a certain percentage of patients, and the adverse events to warn our patients about. Cannabinoids have been tried by a large percentage of patients with headache and pain. Now we need to standardize the various treatments that are sure to be suggested in the future.”
The study was funded by the Migraine Research Foundation, and the Italian Ministry of Health. The study authors declared no conflicts of interest.
FROM HEADACHE
Amazonian indigenous groups have world’s lowest rate of dementia
What to know
- Only about 1% of members of the Tsimane and Moseten peoples of the Bolivian Amazon suffer from dementia, compared with 11% of people aged 65 and older in the United States.
- Underscoring the profound relationship between lifestyle and cognitive health, something about the preindustrial subsistence lifestyle of the groups appears to protect older tribe members from dementia.
- The rate of generally accepted as typical in aging is comparable between the tribes and rates in developed countries such as the United States.
- The Tsimane and Moseten people remain very physically active throughout their lives by fishing, hunting, and farming and experience less brain atrophy than their American and European peers.
- Indigenous populations elsewhere in the world have been found to have high rates of dementia, which are attributed to more contact with their nonindigenous neighbors and adoption of their lifestyles.
--From staff reports
This is a summary of the article, “Study: Some of the world’s lowest rates of dementia found in Amazonian indigenous groups,” published by Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, on March 9, 2022. The full article can be found on news.ucsb.edu.
A version of this article first appeared on Medscape.com.
What to know
- Only about 1% of members of the Tsimane and Moseten peoples of the Bolivian Amazon suffer from dementia, compared with 11% of people aged 65 and older in the United States.
- Underscoring the profound relationship between lifestyle and cognitive health, something about the preindustrial subsistence lifestyle of the groups appears to protect older tribe members from dementia.
- The rate of generally accepted as typical in aging is comparable between the tribes and rates in developed countries such as the United States.
- The Tsimane and Moseten people remain very physically active throughout their lives by fishing, hunting, and farming and experience less brain atrophy than their American and European peers.
- Indigenous populations elsewhere in the world have been found to have high rates of dementia, which are attributed to more contact with their nonindigenous neighbors and adoption of their lifestyles.
--From staff reports
This is a summary of the article, “Study: Some of the world’s lowest rates of dementia found in Amazonian indigenous groups,” published by Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, on March 9, 2022. The full article can be found on news.ucsb.edu.
A version of this article first appeared on Medscape.com.
What to know
- Only about 1% of members of the Tsimane and Moseten peoples of the Bolivian Amazon suffer from dementia, compared with 11% of people aged 65 and older in the United States.
- Underscoring the profound relationship between lifestyle and cognitive health, something about the preindustrial subsistence lifestyle of the groups appears to protect older tribe members from dementia.
- The rate of generally accepted as typical in aging is comparable between the tribes and rates in developed countries such as the United States.
- The Tsimane and Moseten people remain very physically active throughout their lives by fishing, hunting, and farming and experience less brain atrophy than their American and European peers.
- Indigenous populations elsewhere in the world have been found to have high rates of dementia, which are attributed to more contact with their nonindigenous neighbors and adoption of their lifestyles.
--From staff reports
This is a summary of the article, “Study: Some of the world’s lowest rates of dementia found in Amazonian indigenous groups,” published by Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, on March 9, 2022. The full article can be found on news.ucsb.edu.
A version of this article first appeared on Medscape.com.