User login
Simultaneous Cases of Carfilzomib-Induced Thrombotic Microangiopathy in 2 Patients With Multiple Myeloma
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
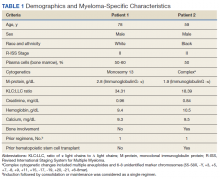
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
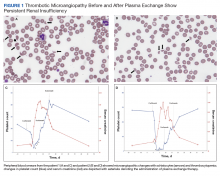
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
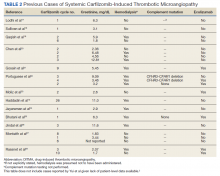
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
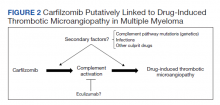
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
As a class of drugs, proteasome inhibitors are known to rarely cause drug-induced thrombotic microangiopathy (DITMA). In particular, carfilzomib is a second-generation, irreversible proteasome inhibitor approved for the treatment of relapsed, refractory multiple myeloma (MM) in combination with other therapeutic agents.1 Although generally well tolerated, carfilzomib has been associated with serious adverse events such as cardiovascular toxicity and DITMA.2-4 Thrombotic microangiopathy (TMA) is a life-threatening disorder characterized by thrombocytopenia, microangiopathic hemolytic anemia, and end-organ damage.5 Its occurrence secondary to carfilzomib has been reported only rarely in clinical trials of MM, and the most effective management of the disorder as well as the concurrent risk factors that contribute to its development remain incompletely understood.6,7 As a result, given both the expanding use of carfilzomib in practice and the morbidity of TMA, descriptions of carfilzomib-induced TMA from the real-world setting continue to provide important contributions to our understanding of the disorder.
At our US Department of Veterans Affairs (VA) medical center, 2 patients developed severe carfilzomib-induced TMA within days of one another. The presentation of simultaneous cases was highly unexpected and offered the unique opportunity to compare clinical features in real time. Here, we describe our 2 cases in detail, review their presentations and management in the context of the prior literature, and discuss potential insights gained into the disease.
Case Presentation
Case 1
A 78-year-old male patient was diagnosed with monoclonal gammopathy of undetermined significance in 2012 that progressed to Revised International Staging System stage II IgG-κ MM in 2016 due to worsening anemia with a hemoglobin level < 10 g/dL (Table 1). He was treated initially with 8 cycles of first-line bortezomib, lenalidomide, and dexamethasone, to which he achieved a partial response with > 50% reduction in serum M-protein. He then received 3 cycles of maintenance bortezomib until relapse, at which time he was switched to second-line therapy consisting of carfilzomib 20 mg/m2 on days 1 and 2 and 56 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 for subsequent cycles plus dexamethasone 20 mg twice weekly every 28 days.
After the patient received cycle 3, day 1 of carfilzomib, he developed subjective fevers, chills, and diarrhea. He missed his day 2 infusion and instead presented to the VA emergency department, where his vital signs were stable and laboratory tests were notable for the following levels: leukocytosis of20.3 K/µL (91.7% neutrophils), hemoglobin 12.4 g/dL (prior 13.5 g/dL), platelet count 171 K/µL, and creatinine 1.39 mg/dL (prior 1.13 g/dL). A chest X-ray demonstrated diffuse bilateral opacities concerning for edema vs infection, and he was started empirically on vancomycin, piperacillin-tazobactam, and azithromycin. His outpatient medications, which included acyclovir, aspirin, finasteride, oxybutynin, ranitidine, omega-3 fatty acids, fish oil, vitamin D, and senna, were continued as indicated.
On hospital day 2, the patient’s platelet count dropped to 81 K/µL and creatinine level rose to 1.78 mg/dL. He developed dark urine (urinalysis [UA] 3+ blood, 6-11 red blood cells per high power field [RBC/HPF]) and had laboratory tests suggestive of hemolysis, including lactic dehydrogenase (LDH) > 1,200 IU/L (reference range, 60-250 IU/L), haptoglobin < 30 mg/dL (reference range, 44-215 mg/dL), total bilirubin 3.2 mg/dL (reference range, 0.2-1.3 mg/dL; indirect bilirubin, 2.6 mg/dL), and a peripheral blood smear demonstrating moderate microangiopathy (Figure 1).
Workup for alternative causes of thrombocytopenia included a negative heparin-induced thrombocytopenia panel and a disseminated intravascular coagulation (DIC) panel showing elevated fibrinogen (515 mg/dL; reference range, 200-400 mg/dL) and mildly elevated international normalized ratio (INR) (1.3). Blood cultures were negative, and a 22-pathogen gastrointestinal polymerase chain reaction (PCR) panel failed to identify viral or bacterial pathogens, including Escherichia coli O157:H7. C3 (81 mg/dL; reference range, 90-180 mg/dL) and C4 (16 mg/dL; reference range, 16-47 mg/dL) complement levels were borderline to mildly reduced.
Based on this constellation of findings, a diagnosis of TMA was made, and the patient was started empirically on plasma exchange and pulse-dosed steroids. After 4 cycles of plasma exchange, the platelet count had normalized from its nadir of 29 K/µL. ADAMTS13 activity (98% enzyme activity) ruled out thrombotic thrombocytopenic purpura (TTP), and the patient continued to have anuric renal failure (creatinine, 8.62 mg/dL) necessitating the initiation of hemodialysis. Given persistent renal insufficiency, a diagnosis of atypical hemolytic uremic syndrome (HUS) was considered, and eculizumab 900 mg was administered on days 8 and 15 with stabilization of renal function. By the time of discharge on day 18, the patient’s creatinine level had decreased to 3.89 mg/dL, and platelet count was 403 K/µL. Creatinine normalized to 1.07 mg/dL by day 46.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for mutations in the following genes associated with atypical HUS: CFH, CFI, MCP (CD46), THBD, CFB, C3, DGKE, ADAMTS13, C4BPA, C4BPB, LMNA, CFHR1, CFHR3, CFHR4, and CFHR5. The patient subsequently remained off all antimyeloma therapy for > 1 year until eventually starting third-line pomalidomide plus dexamethasone without reinitiation of proteasome inhibitor therapy.
Case 2
A 59-year-old male patient, diagnosed in 2013 with ISS stage I IgG-κ MM after presenting with compression fractures, completed 8 cycles of cyclophosphamide, bortezomib, and dexamethasone before undergoing autologous hematopoietic stem cell transplantation with complete response (Table 1). He subsequently received single-agent maintenance bortezomib until relapse nearly 2 years later, at which time he started second-line carfilzomib 20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for cycle 1, followed by 27 mg/m2 on days 8, 9, 15, and 16 for cycles 2 to 8, lenalidomide 25 mg on days 1 to 21, and dexamethasone 40 mg weekly every 28 days. Serum free light chain levels normalized after 9 cycles, and he subsequently began maintenance carfilzomib 70 mg/m2 on days 1 and 15 plus lenalidomide 10 mg on days 1 to 21 every 28 days.
On the morning before admission, the patient received C6D17 of maintenance carfilzomib, which had been delayed from day 15 because of the holiday. Later that evening, he developed nausea, vomiting, and fever of 101.3 °F. He presented to the VA emergency department and was tachycardic (108 beats per minute) and hypotensive (86/55 mm Hg). Laboratory tests were notable for hemoglobin level 9.9 g/dL (prior 11.6 g/dL), platelet count 270 K/µL, and creatinine level 1.86 mg/dL (prior 1.12 mg/dL). A respiratory viral panel was positive for influenza A, and antimicrobial agents were eventually broadened to piperacillin-tazobactam, azithromycin, and oseltamivir. His outpatient medications, which included acyclovir, zoledronic acid, sulfamethoxazole/trimethoprim, aspirin, amlodipine, atorvastatin, omeprazole, zolpidem, calcium, vitamin D, loratadine, ascorbic acid, and prochlorperazine, were continued as indicated.
On hospital day 2, the patient’s platelet count declined from 211 to 57 K/µL. He developed tea-colored urine (UA 2+ blood, 0-2 RBC/HPF) and had laboratory tests suggestive of hemolysis, including LDH 910 IU/L (reference range, 60-250 IU/L), total bilirubin 3.3 mg/dL (reference range, 0.2-1.3 mg/dL; no direct or indirect available), and a peripheral blood smear demonstrating moderate microangiopathy. Although haptoglobin level was normal at this time (206 mg/dL; reference range, 44-215 mg/dL), it decreased to 42 mg/dL by the following day. Additional workup included a negative direct Coombs and a DIC panel showing elevated fibrinogen (596 mg/dL; reference range, 200-400 mg/dL) and mildly elevated INR (1.16). Blood cultures remained negative, and a 22-pathogen GI PCR panel identified no viral or bacterial pathogens, including E coli O157:H7. C3 (114 mg/dL; reference range, 90-180 mg/dL) and C4 (40 mg/dL; reference range, 16-47 mg/dL) complement levels were both normal.
Based on these findings, empiric treatment was started with plasma exchange and pulse-dosed steroids. The patient received 3 cycles of plasma exchange until the results of the ADAMTS13 activity ruled out TTP (63% enzyme activity). Over the next 6 days, his platelet count reached a nadir of 6 K/µL and creatinine level peaked at 10.36 mg/dL, necessitating the initiation of hemodialysis. Given severe renal insufficiency, a diagnosis of atypical HUS was again considered, and eculizumab 900 mg was administered on days 9 and 16 with stabilization of renal function. By the time of discharge on day 17, the patient’s creatinine level had decreased to 4.17 mg/dL and platelet count was 164 K/µL. Creatinine level normalized to 1.02 mg/dL by day 72.
Outpatient genetic testing through the BloodCenter of Wisconsin Diagnostic Laboratories was negative for gene mutations associated with atypical HUS. Approximately 1 month after discharge, the patient resumed maintenance lenalidomide alone without reinitiation of proteasome inhibitor therapy.
Discussion
In this case series, we describe the uncommon drug-related adverse event of TMA occurring in 2 patients with MM after receiving carfilzomib. Although the incidence of TMA disorders is low, reaching up to 2.8% in patients receiving carfilzomib plus cyclophosphamide and dexamethasone in the phase 2 CARDAMON trial, our experience suggests that a high index of suspicion for carfilzomib-induced TMA is warranted in the real-world setting.8 TMA syndromes, including TTP, HUS, and DITMA, are characterized by microvascular endothelial injury and thrombosis leading to thrombocytopenia and microangiopathic hemolytic anemia.5,9 Several drug culprits of DITMA are recognized, including quinine, gemcitabine, tacrolimus, and proteasome inhibitors (bortezomib, carfilzomib, ixazomib).10-12 In a real-world series of patients receiving proteasome inhibitor therapy, either carfilzomib (n=8) or bortezomib (n=3), common clinical features of DITMA included thrombocytopenia, microangiopathic hemolytic anemia, gastrointestinal symptoms, and renal insufficiency with or without a need for hemodialysis.2 Although DITMA has been described primarily as an early event, its occurrence after 12 months of proteasome inhibitor therapy has also been reported, both in this series and elsewhere, thereby suggesting an ongoing risk for DITMA throughout the duration of carfilzomib treatment.2,13
The diagnosis of DITMA can be challenging given its nonspecific symptoms that overlap with other TMA syndromes. Previous studies have proposed that for a drug to be associated with DITMA, there should be: (1) evidence of clinical and/or pathologic findings of TMA; (2) exclusion of alternative causes of TMA; (3) no other new drug exposures other than the suspected culprit medication; and (4) a lack of recurrence of TMA in absence of the drug.10 In the case of patients with MM, other causes of TMA have also been described, including the underlying plasma cell disorder itself and stem cell transplantation.14 In the 2 cases we have described, these alternative causes were considered unlikely given that only 1 patient underwent transplantation remotely and neither had a previous history of TMA secondary to their disease. With respect to other TMA syndromes, ADAMTS13 levels > 10% and negative stool studies for E coli O157:H7 suggested against TTP or typical HUS, respectively. No other drug culprits were identified, and the close timing between the receipt of carfilzomib and symptom onset supported a causal relationship.
Because specific therapies are lacking, management of DITMA has traditionally included drug discontinuation and supportive care for end-organ injury.5 The terminal complement inhibitor, eculizumab, improves hematologic abnormalities and renal function in patients with atypical HUS but its use for treating patients with DITMA is not standard.15 Therefore, the decision to administer eculizumab to our 2 patients was driven by their severe renal insufficiency without improvement after plasma exchange, which suggested a phenotype similar to atypical HUS. After administration of eculizumab, renal function stabilized and then gradually improved over weeks to months, a time course similar to that described in cases of patients with DITMA secondary to other anticancer therapies treated with eculizumab.16 Although these results suggest a potential role for eculizumab in proteasome inhibitor–induced TMA, distinguishing the benefit of eculizumab over drug discontinuation alone remains challenging, and well-designed prospective investigations are needed.
The clustered occurrence of our 2 cases is unique from previous reports that describe carfilzomib-induced TMA as a sporadic event (Table 2).13,17-28 Both immune-mediated and direct toxic effects have been proposed as mechanisms of DITMA, and while our cases do not differentiate between these mechanisms, we considered whether a combined model of initiation, whereby patient or environmental risk factors modulate occurrence of the disease in conjunction with the inciting drug, could explain the clustered occurrence of cases. In this series, drug manufacturing was not a shared risk factor as each patient received carfilzomib from different lot numbers. Furthermore, other patients at our center received carfilzomib from the same batches without developing DITMA. We also considered the role of infection given that 1 patient was diagnosed with influenza A and both presented with nonspecific, viral-like symptoms during the winter season. Interestingly, concurrent viral infections have been reported in other cases of carfilzomib-induced DITMA as well and have also been discussed as a trigger of atypical HUS.20,29 Finally, genetic testing was negative for complement pathway mutations that might predispose to complement dysregulation.
The absence of complement mutations in our 2 patients differs from a recent series describing heterozygous CFHR3-CHFR1 deletions in association with carfilzomib-induced TMA.22 In that report, the authors hypothesized that carfilzomib decreases expression of complement factor H (CFH), a negative regulator of complement activation, thereby leading to complement dysregulation in patients who are genetically predisposed. In a second series, plasma from patients with DITMA secondary to carfilzomib induced the deposition of the complement complex, C5b-9, on endothelial cells in culture, suggesting activation of the complement pathway.30 The effective use of eculizumab would also point to a role for complement activation, and ongoing investigations should aim to identify the triggers and mechanisms of complement dysregulation in this setting, especially for patients like ours in whom genetic testing for complement pathway mutations is negative (Figure 2).
Conclusions
DITMA is a known risk of proteasome inhibitors and is listed as a safety warning in the prescribing information for bortezomib, carfilzomib, and ixazomib.12 Given the overall rarity of this adverse event, the simultaneous presentation of our 2 cases was unexpected and underscores the need for heightened awareness in clinical practice. In addition, while no underlying complement mutations were identified, eculizumab was used in both cases to successfully stabilize renal function. Further research investigating the efficacy of eculizumab and the role of complement activation in proteasome inhibitor–induced TMA will be valuable.
Acknowledgments
The authors would like to thank the patients whose histories are reported in this manuscript as well as the physicians and staff who provided care during the hospitalizations and beyond. We also thank Oscar Silva, MD, PhD, for his assistance in reviewing and formatting the peripheral blood smear images.
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
1. McBride A, Klaus JO, Stockeri-Goldstein K. Carfilzomib: a second-generation proteasome inhibitor for the treatment of multiple myeloma. Am J Health Syst Pharm. 2015;72(5):353-360. doi:10.2146/ajhp130281
2. Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. doi:10.1002/ajh.24447
3. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. doi:10.1182/bloodadvances.2016003269
4. Chari A, Stewart AK, Russell SD, et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018;2(13):1633-1644. doi:10.1182/bloodadvances.2017015545
5. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. doi:10.1056/NEJMra1312353
6. Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. doi:10.1016/S1470-2045(15)00464-7
7. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186-197. doi:10.1016/S0140-6736(20)30734-0
8. Camilleri M, Cuadrado M, Phillips E, et al. Thrombotic microangiopathy in untreated myeloma patients receiving carfilzomib, cyclophosphamide and dexamethasone on the CARDAMON study. Br J Haematol. 2021;193(4):750-760. doi:10.1111/bjh.17377
9. Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. doi:10.1182/blood-2016-11-743104
10. Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systemic review of published reports. Blood. 2015;125(4):616-618. doi:10.1182/blood-2014-11-611335
11. Saleem R, Reese JA, George JN. Drug-induced thrombotic-microangiopathy: an updated systematic review, 2014-2018. Am J Hematol. 2018;93(9):E241-E243. doi:10.1002/ajh.25208
12 Nguyen MN, Nayernama A, Jones SC, Kanapuru B, Gormley N, Waldron PE. Proteasome inhibitor-associated thrombotic microangiopathy: a review of cases reported to the FDA adverse event reporting system and published in the literature. Am J Hematol. 2020;95(9):E218-E222. doi:10.1002/ajh.25832
13. Haddadin M, Al-Sadawi M, Madanat S, et al. Late presentation of carfilzomib associated thrombotic microangiopathy. Am J Med Case Rep. 2019;7(10):240-243. doi:10.12691/ajmcr-7-10-5
14 Portuguese AJ, Gleber C, Passero Jr FC, Lipe B. A review of thrombotic microangiopathies in multiple myeloma. Leuk Res. 2019;85:106195. doi:10.1016/j.leukres.2019.106195
15. Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. doi:10.1056/NEJMoa1208981
16. Olson SR, Lu E, Sulpizio E, Shatzel JJ, Rueda JF, DeLoughery TG. When to stop eculizumab in complement-mediated thrombotic microangiopathies. Am J Nephrol. 2018;48(2):96-107. doi:10.1159/000492033
17. Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. doi:10.1093/ckj/sfv059
18. Sullivan MR, Danilov AV, Lansigan F, Dunbar NM. Carfilzomib associated thrombotic microangiopathy initially treated with therapeutic plasma exchange. J Clin Apher., 2015;30(5):308-310. doi:10.1002/jca.21371
19. Qaqish I, Schlam IM, Chakkera HA, Fonseca R, Adamski J. Carfilzomib: a cause of drug associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54(3):401-404. doi:10.1016/j.transci.2016.03.002
20. Chen Y, Ooi M, Lim SF, et al. Thrombotic microangiopathy during carfilzomib use: case series in Singapore. Blood Cancer J. 2016;6(7):e450. doi:10.1038/bcj.2016.62
21. Gosain R, Gill A, Fuqua J, et al. Gemcitabine and carfilzomib induced thrombotic microangiopathy: eculizumab as a life-saving treatment. Clin Case Rep. 2017;5(12):1926-1930. doi:10.1002/ccr3.1214
22. Portuguese AJ, Lipe B. Carfilzomib-induced aHUS responds to early eculizumab and may be associated with heterozygrous CFHR3-CFHR1 deletion. Blood Adv. 2018;2(23):3443-3446. doi:10.1182/bloodadvances.2018027532
23. Moliz C, Gutiérrez E, Cavero T, Redondo B, Praga M. Eculizumab as a treatment for atypical hemolytic syndrome secondary to carfilzomib. Nefrologia (Engl Ed). 2019;39(1):86-88. doi:10.1016/j.nefro.2018.02.005
24. Jeyaraman P, Borah P, Singh A, et al., Thrombotic microangiopathy after carfilzomib in a very young myeloma patient. Blood Cells Mol Dis. 2020;81:102400. doi:10.1016/j.bcmd.2019.102400
25. Bhutani D, Assal A, Mapara MY, Prinzing S, Lentzsch S. Case report: carfilzomib-induced thrombotic microangiopathy with complement activation treated successfully with eculizumab. Clin Lymphoma Myeloma Leuk. 2020;20(4):e155-e157. doi:10.1016/j.clml.2020.01.016
26. Jindal N, Jandial A, Jain A, et al. Carfilzomib-induced thrombotic microangiopathy: a case based review. Hematol Oncol Stem Cell Ther. 2020;S1658-3876(20)30118-7. doi:10.1016/j.hemonc.2020.07.001
27. Monteith BE, Venner CP, Reece DE, et al. Drug-induced thrombotic microangiopathy with concurrent proteasome inhibitor use in the treatment of multiple myeloma: a case series and review of the literature. Clin Lymphoma Myeloma Leuk. 2020;20(11):e791-e780. doi:10.1016/j.clml.2020.04.014
28. Rassner M, Baur R, Wäsch R, et al. Two cases of carfilzomib-induced thrombotic microangiopathy successfully treated with eculizumab in multiple myeloma. BMC Nephrol. 2021;22(1):32. doi:10.1186/s12882-020-02226-5
29. Kavanagh D, Goodship THJ. Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology Am Soc Hematol Educ Program. 2011;2011:15-20. doi:10.1182/asheducation-2011.1.15
30. Blasco M, Martínez-Roca A, Rodríguez-Lobato LG, et al. Complement as the enabler of carfilzomib-induced thrombotic microangiopathy. Br J Haematol. 2021;193(1):181-187. doi:10.1111/bjh.16796
‘Unexpected’: Breast cancer spreads most during sleep
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a discovery the investigators called “striking and unexpected.”
“This has not been shown before [and] we were surprised, indeed,” Nicola Aceto, PhD, professor of molecular oncology, Swiss Federal Institute of Technology Zürich, said in an interview.
The findings carry potential implications for the timing of biopsy and treatment of metastasis-prone cancers, the authors said.
The study was published online in Nature.
Circulating tumor cells (CTCs) are generally believed to shed constantly or following particular events such as surgery or physical activity; however, the processes that regulate tumor cell metastasis and how circadian rhythms may play into tumorigenesis remain unclear.
To better understand these processes, Dr. Aceto and colleagues collected blood samples from 30 women with breast cancer at 4:00 a.m. and 10:00 a.m. – times representing the body’s resting and active phases, respectively.
The researchers observed that more than 78% of all CTCs obtained were from samples taken during the resting phase.
This finding is astounding, Harrison Ball, a PhD candidate, and Sunitha Nagrath, PhD, with the University of Michigan, Ann Arbor, wrote in Nature News & Views .
Dr. Aceto and colleagues also found that CTCs generated at night divide more quickly and therefore have a higher potential to metastasize, compared with those generated during the day, which “are devoid of metastatic ability,” according to the authors, who obtained similar results in a series of mouse models.
The team also observed that key circadian rhythm hormones (such as melatonin, testosterone, and glucocorticoids) regulate CTC generation, and insulin promotes tumor cell proliferation in a time-dependent manner, suggesting a “need for time-controlled approaches for the characterization and treatment of breast cancer,” the authors wrote.
Practice changing?
Dr. Ball and Dr. Nagrath said the time-dependent nature of CTC dynamics could very well transform how cancer patients are assessed and treated.
“The data pointing to CTC proliferation and release during the rest phase suggest that doctors might need to become more conscious of when to administer specific treatments,” they wrote.
Both cautioned, however, that large clinical trials would be needed before any consideration of circadian rhythms is incorporated into standard practice. It’s also unclear whether these results in breast cancer hold true for other tumor types.
Mariana G. Figueiro, PhD, who was not involved in the research, agreed that, if studies confirm more metastatic spread at night, “there is an opportunity to treat patients at strategic times.”
Dr. Figueiro, of the Icahn School of Medicine at Mount Sinai, New York, also saw a potential impact on the timing of blood draws. “I think tightening up on how people do biopsies and bloodwork based on circadian time is important.”
Marleen Meyers, MD, agreed that these findings could have many clinical implications.
“The most obvious is that the time of day [that] treatment is administered may influence efficacy,” said Dr. Meyers, clinical professor of medicine at New York University Langone’s Perlmutter Cancer Center.
But, Dr. Meyers noted, the benefits of treating someone at night would need to be weighed against the downsides of interrupting a person’s normal sleep-wake cycle. “If this finding is clinically important it will be a challenge incorporating this into clinical care,” she said.
The study had no funding reported. Dr. Aceto is a cofounder and member of the board of PAGE Therapeutics, listed as an inventor in patent applications related to circulating tumor cells, a paid consultant for several companies, and a Novartis shareholder. One coauthor is a cofounder of PAGE Therapeutics. All other authors declare no competing interests. Dr. Meyers and Dr. Figueiro reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE
Good chemo vs. bad chemo: When too much is a bad thing
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
FROM ASCO 2022
Racial/ethnic disparities exacerbated maternal death rise during 2020 pandemic.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
FROM JAMA NETWORK OPEN
My picks for best of ASCO 2022
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
AT ASCO 2022
Acupuncture deep needling technique points to greater tension headache relief
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Artificial intelligence colonoscopy system shows promise
A new artificial intelligence (AI) system can help expert endoscopists improve their colonoscopies, a new study indicates.
Endoscopists using the computer program SKOUT (Iterative Scopes) achieved a 27% better detection rate of adenomas per colonoscopy, compared with endoscopists working without computer assistance, said lead author Aasma Shaukat, MD, MPH, director of outcomes research in the division of gastroenterology and hepatology at New York University.
The study showed that AI colonoscopy systems can work in a routine population of U.S. patients, Dr. Shaukat said in an interview.
“As gastroenterologists, we are very excited,” she said.
The study was published online in Gastroenterology and was presented at the annual Digestive Disease® Week.
Previous research has shown that experienced endoscopists miss many polyps. To improve their detection rate, multiple companies have used machine learning to develop algorithms to identify suspicious areas.
“Once the computer sees the polyp, it puts a bounding box around it,” said Dr. Shaukat. “It draws the attention of the endoscopist to it. It assists the endoscopist but doesn’t replace the endoscopist.”
The Food and Drug Administration has approved two such systems: EndoScreener (Wision AI) and GI Genius (Cosmo Pharmaceuticals).
The SKOUT algorithm was trained on 3,616 full-length colonoscopy procedure videos from multiple centers. In bench testing, it achieved a 93.5% polyp-level true positive rate and a 2.3% false positive rate.
Randomized trial pits AI against standard procedure
To see how well the system works in the clinic, Dr. Shaukat and colleagues recruited 22 U.S. board-certified gastroenterologists from five academic and community centers. The gastroenterologists all had a minimum adenoma detection rate of 25%, defined as the number of colonoscopies in which at least one adenoma is found, divided by the number of colonoscopies performed. All the gastroenterologists had performed a minimum of 1,000 colonoscopy procedures.
The researchers randomly assigned 682 patients to undergo colonoscopy with the SKOUT and 677 to undergo colonoscopy using the standard procedure. The patients were aged 40 years or older and were scheduled for either screening or surveillance.
The endoscopists who received computer assistance detected 1.05 adenomas per colonoscopy versus 0.83 for those who did not have computer assistance, a statistically significant difference.
The proportion of resections with clinically significant histology was 71.7% with standard colonoscopies versus 67.4% with computer-assisted colonoscopies. This fell within the 14% margin that the researchers had set to show noninferiority for the computer system.
“The important thing is not just detecting all polyps but the polyps we care about, which are adenomas, and doing so without increasing the false positive rate,” said Dr. Shaukat.
The adenoma detection rate was 43.9% for the standard procedure and 47.8% for the computer-assisted procedure. This difference was not statistically significant, but Dr. Shaukat argued that the adenoma detection rate is not the best measure of success, because endoscopists sometimes stop looking for polyps once they find one.
The overall sessile serrated lesion detection rate for the standard colonoscopies was 16.0% versus 12.6% for the computer-assisted colonoscopies, which also was not statistically significant.
Next steps
This study is important because it was a large, multicenter trial in the United States, said Omer Ahmad, BSc, MBBS, MRCP, a gastroenterologist and clinical researcher at University College London, who was not involved in the study. Most of the trials of AI have been in China or Europe. “It was very important just to see this replicated in the U.S. population.”
The average procedure time was 15.41 minutes for the standard colonoscopies versus 15.82 minutes for the computer-assisted colonoscopies, which was not statistically different.
“It is important to note that the studies so far suggest that false positives do not have a significant impact on workflow,” said Dr. Ahmad.
The next crucial step in evaluating AI colonoscopy will be to track the effects over the long term, said Dr. Shaukat.
“As these technologies get approved and we see them in practice, we need to see that it’s leading to some outcome, like reduced colon cancer,” she said.
That also may be necessary before payers in the United States are willing to pay the additional cost for this technology, she added.
In the meantime, Dr. Ahmad said computer assistance is improving his own colonoscopies.
“I have found the systems have spotted some polyps that I may have otherwise missed,” he said. “There is a false positive rate, but for me, it doesn’t distract from my workflow.”
He believes the systems will be particularly helpful in improving the performance of less-skilled endoscopists.
He is also looking forward to systems that can help complete the reports needed at the end of each colonoscopy. “Most of us dislike having to write a laborious report and having to code everything at the end of the procedure,” he said.
The study was funded by Iterative Scopes. Dr. Shaukat reported having received research funding to her institution for the current study from Iterative Scopes and consulting fees from Freenome and Medtronic. Dr. Ahmad reports receiving speaker fees from the Canadian Association of Gastroenterology/Medtronic.
A version of this article first appeared on Medscape.com.
A new artificial intelligence (AI) system can help expert endoscopists improve their colonoscopies, a new study indicates.
Endoscopists using the computer program SKOUT (Iterative Scopes) achieved a 27% better detection rate of adenomas per colonoscopy, compared with endoscopists working without computer assistance, said lead author Aasma Shaukat, MD, MPH, director of outcomes research in the division of gastroenterology and hepatology at New York University.
The study showed that AI colonoscopy systems can work in a routine population of U.S. patients, Dr. Shaukat said in an interview.
“As gastroenterologists, we are very excited,” she said.
The study was published online in Gastroenterology and was presented at the annual Digestive Disease® Week.
Previous research has shown that experienced endoscopists miss many polyps. To improve their detection rate, multiple companies have used machine learning to develop algorithms to identify suspicious areas.
“Once the computer sees the polyp, it puts a bounding box around it,” said Dr. Shaukat. “It draws the attention of the endoscopist to it. It assists the endoscopist but doesn’t replace the endoscopist.”
The Food and Drug Administration has approved two such systems: EndoScreener (Wision AI) and GI Genius (Cosmo Pharmaceuticals).
The SKOUT algorithm was trained on 3,616 full-length colonoscopy procedure videos from multiple centers. In bench testing, it achieved a 93.5% polyp-level true positive rate and a 2.3% false positive rate.
Randomized trial pits AI against standard procedure
To see how well the system works in the clinic, Dr. Shaukat and colleagues recruited 22 U.S. board-certified gastroenterologists from five academic and community centers. The gastroenterologists all had a minimum adenoma detection rate of 25%, defined as the number of colonoscopies in which at least one adenoma is found, divided by the number of colonoscopies performed. All the gastroenterologists had performed a minimum of 1,000 colonoscopy procedures.
The researchers randomly assigned 682 patients to undergo colonoscopy with the SKOUT and 677 to undergo colonoscopy using the standard procedure. The patients were aged 40 years or older and were scheduled for either screening or surveillance.
The endoscopists who received computer assistance detected 1.05 adenomas per colonoscopy versus 0.83 for those who did not have computer assistance, a statistically significant difference.
The proportion of resections with clinically significant histology was 71.7% with standard colonoscopies versus 67.4% with computer-assisted colonoscopies. This fell within the 14% margin that the researchers had set to show noninferiority for the computer system.
“The important thing is not just detecting all polyps but the polyps we care about, which are adenomas, and doing so without increasing the false positive rate,” said Dr. Shaukat.
The adenoma detection rate was 43.9% for the standard procedure and 47.8% for the computer-assisted procedure. This difference was not statistically significant, but Dr. Shaukat argued that the adenoma detection rate is not the best measure of success, because endoscopists sometimes stop looking for polyps once they find one.
The overall sessile serrated lesion detection rate for the standard colonoscopies was 16.0% versus 12.6% for the computer-assisted colonoscopies, which also was not statistically significant.
Next steps
This study is important because it was a large, multicenter trial in the United States, said Omer Ahmad, BSc, MBBS, MRCP, a gastroenterologist and clinical researcher at University College London, who was not involved in the study. Most of the trials of AI have been in China or Europe. “It was very important just to see this replicated in the U.S. population.”
The average procedure time was 15.41 minutes for the standard colonoscopies versus 15.82 minutes for the computer-assisted colonoscopies, which was not statistically different.
“It is important to note that the studies so far suggest that false positives do not have a significant impact on workflow,” said Dr. Ahmad.
The next crucial step in evaluating AI colonoscopy will be to track the effects over the long term, said Dr. Shaukat.
“As these technologies get approved and we see them in practice, we need to see that it’s leading to some outcome, like reduced colon cancer,” she said.
That also may be necessary before payers in the United States are willing to pay the additional cost for this technology, she added.
In the meantime, Dr. Ahmad said computer assistance is improving his own colonoscopies.
“I have found the systems have spotted some polyps that I may have otherwise missed,” he said. “There is a false positive rate, but for me, it doesn’t distract from my workflow.”
He believes the systems will be particularly helpful in improving the performance of less-skilled endoscopists.
He is also looking forward to systems that can help complete the reports needed at the end of each colonoscopy. “Most of us dislike having to write a laborious report and having to code everything at the end of the procedure,” he said.
The study was funded by Iterative Scopes. Dr. Shaukat reported having received research funding to her institution for the current study from Iterative Scopes and consulting fees from Freenome and Medtronic. Dr. Ahmad reports receiving speaker fees from the Canadian Association of Gastroenterology/Medtronic.
A version of this article first appeared on Medscape.com.
A new artificial intelligence (AI) system can help expert endoscopists improve their colonoscopies, a new study indicates.
Endoscopists using the computer program SKOUT (Iterative Scopes) achieved a 27% better detection rate of adenomas per colonoscopy, compared with endoscopists working without computer assistance, said lead author Aasma Shaukat, MD, MPH, director of outcomes research in the division of gastroenterology and hepatology at New York University.
The study showed that AI colonoscopy systems can work in a routine population of U.S. patients, Dr. Shaukat said in an interview.
“As gastroenterologists, we are very excited,” she said.
The study was published online in Gastroenterology and was presented at the annual Digestive Disease® Week.
Previous research has shown that experienced endoscopists miss many polyps. To improve their detection rate, multiple companies have used machine learning to develop algorithms to identify suspicious areas.
“Once the computer sees the polyp, it puts a bounding box around it,” said Dr. Shaukat. “It draws the attention of the endoscopist to it. It assists the endoscopist but doesn’t replace the endoscopist.”
The Food and Drug Administration has approved two such systems: EndoScreener (Wision AI) and GI Genius (Cosmo Pharmaceuticals).
The SKOUT algorithm was trained on 3,616 full-length colonoscopy procedure videos from multiple centers. In bench testing, it achieved a 93.5% polyp-level true positive rate and a 2.3% false positive rate.
Randomized trial pits AI against standard procedure
To see how well the system works in the clinic, Dr. Shaukat and colleagues recruited 22 U.S. board-certified gastroenterologists from five academic and community centers. The gastroenterologists all had a minimum adenoma detection rate of 25%, defined as the number of colonoscopies in which at least one adenoma is found, divided by the number of colonoscopies performed. All the gastroenterologists had performed a minimum of 1,000 colonoscopy procedures.
The researchers randomly assigned 682 patients to undergo colonoscopy with the SKOUT and 677 to undergo colonoscopy using the standard procedure. The patients were aged 40 years or older and were scheduled for either screening or surveillance.
The endoscopists who received computer assistance detected 1.05 adenomas per colonoscopy versus 0.83 for those who did not have computer assistance, a statistically significant difference.
The proportion of resections with clinically significant histology was 71.7% with standard colonoscopies versus 67.4% with computer-assisted colonoscopies. This fell within the 14% margin that the researchers had set to show noninferiority for the computer system.
“The important thing is not just detecting all polyps but the polyps we care about, which are adenomas, and doing so without increasing the false positive rate,” said Dr. Shaukat.
The adenoma detection rate was 43.9% for the standard procedure and 47.8% for the computer-assisted procedure. This difference was not statistically significant, but Dr. Shaukat argued that the adenoma detection rate is not the best measure of success, because endoscopists sometimes stop looking for polyps once they find one.
The overall sessile serrated lesion detection rate for the standard colonoscopies was 16.0% versus 12.6% for the computer-assisted colonoscopies, which also was not statistically significant.
Next steps
This study is important because it was a large, multicenter trial in the United States, said Omer Ahmad, BSc, MBBS, MRCP, a gastroenterologist and clinical researcher at University College London, who was not involved in the study. Most of the trials of AI have been in China or Europe. “It was very important just to see this replicated in the U.S. population.”
The average procedure time was 15.41 minutes for the standard colonoscopies versus 15.82 minutes for the computer-assisted colonoscopies, which was not statistically different.
“It is important to note that the studies so far suggest that false positives do not have a significant impact on workflow,” said Dr. Ahmad.
The next crucial step in evaluating AI colonoscopy will be to track the effects over the long term, said Dr. Shaukat.
“As these technologies get approved and we see them in practice, we need to see that it’s leading to some outcome, like reduced colon cancer,” she said.
That also may be necessary before payers in the United States are willing to pay the additional cost for this technology, she added.
In the meantime, Dr. Ahmad said computer assistance is improving his own colonoscopies.
“I have found the systems have spotted some polyps that I may have otherwise missed,” he said. “There is a false positive rate, but for me, it doesn’t distract from my workflow.”
He believes the systems will be particularly helpful in improving the performance of less-skilled endoscopists.
He is also looking forward to systems that can help complete the reports needed at the end of each colonoscopy. “Most of us dislike having to write a laborious report and having to code everything at the end of the procedure,” he said.
The study was funded by Iterative Scopes. Dr. Shaukat reported having received research funding to her institution for the current study from Iterative Scopes and consulting fees from Freenome and Medtronic. Dr. Ahmad reports receiving speaker fees from the Canadian Association of Gastroenterology/Medtronic.
A version of this article first appeared on Medscape.com.
$150K: Average industry payment to top 1% of oncologists
A small number of U.S. medical oncologists make more than $100,000 a year in general payments from drug companies, a new study shows.
These high-payment physicians represent just 1% of all U.S. medical oncologists, yet they account for 37% of industry payments. These oncologists often hold important leadership positions, draft treatment guidelines, and sit on journal editorial boards.
The findings highlight a risk for “perceived and real conflict of interest,” corresponding author Christopher Booth, MD, of Queen’s University Cancer Research Center, Kingston, Ont., said in an interview. “Because of the leadership positions they hold, the potential impact of this small group of physicians on oncology practice and policy may be substantial.”
The study was published online in JCO Oncology Practice.
‘We have a problem’
It’s no secret that many oncologists have financial relationships with pharmaceutical companies. They receive payments for research initiatives, but they also receive more general, personal payments in the form of honoraria, consultant fees, gifts, and reimbursement for travel and meals.
Prior studies have shown that these payments are typically modest, but a small subset of medical oncologists receive more than $100,000 annually. Dr. Booth and colleagues wanted to know more about the characteristics of these “high-payment” oncologists.
Using the national Open Payments database, the researchers identified a total of 139 medical oncologists who practice in the United States and who received $100,000 or more in general payments linked to cancer medications in 2018.
In U.S. dollars, the median payment was $154,613, and the total was $24.2 million.
The majority (95%) of high-payment oncologists were active in clinical work, 56% worked in an academic setting, 31% worked at National Cancer Institute–designated cancer centers, and 23% worked at National Comprehensive Cancer Network (NCCN) centers.
Many were based in California (17%), Texas (12%), Florida (10%), and New York (8%).
Most currently hold or have held hospital leadership positions (60%) or faculty appointments (72%) and 21% have held leadership positions in specialty associations in the past 5 years. Nearly one-quarter (24%) have served on journal editorial boards, and 10% have authored clinical practice guidelines in the past 5 years.
More specifically, three physicians authored NCCN guidelines, and two authored American Society of Clinical Oncology guidelines during 2016-2021; one guideline was published in 2018 when payments were made.
“Oncology specialty associations, guideline panels, and journal editorial boards should reconsider if it is appropriate for physicians with such large payments to hold these high-profile positions,” Dr. Booth said.
Following publication of the study, some oncologists took to Twitter with reactions, including Manni Mohyuddin, MD (@ManniMD1), from the Huntsman Cancer Institute, University of Utah, Salt Lake City, who wrote: “I recognize that some conflict of interest ‘may’ be unavoidable in order to run trials. But when greater than TWICE the average American household annual salary is taken in payments from industry by those in leadership/editorial roles, we have a problem.”
Weighing in on the results, ASCO CEO Clifford A. Hudis, MD, told this news organization that the “limitations of the study make it difficult to draw conclusions about the scope or potential impact of these payments on care.”
For example, he explained, some payments attributed to individuals may have been made directly to the physicians’ institutions or employers for sponsored research expenses.
Dr. Hudis also noted that the payments examined in the study were made in 2018, whereas the potentially relevant leadership positions could have been attained at a different time.
Furthermore, in 2020, an editorial appeared in Cancer, showing that errors in Open Payments are “fairly common,” Dr. Hudis said. It’s also unclear whether the reported financial relationships were appropriately disclosed and were managed at the time under relevant conflict of interest policies, he said.
“The question left unanswered by this study is whether or not these relationships influence patient care,” said Dr. Hudis. He noted that decisions about care should come from physicians and patients who are informed of the best available, unbiased, peer-reviewed, scientific evidence.
“The potential impact of financial conflicts of interest on this effort is an issue of concern, even if this study does not directly address it,” Dr. Hudis said.
The study had no specific funding. Dr. Booth has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article. Dr. Hudis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A small number of U.S. medical oncologists make more than $100,000 a year in general payments from drug companies, a new study shows.
These high-payment physicians represent just 1% of all U.S. medical oncologists, yet they account for 37% of industry payments. These oncologists often hold important leadership positions, draft treatment guidelines, and sit on journal editorial boards.
The findings highlight a risk for “perceived and real conflict of interest,” corresponding author Christopher Booth, MD, of Queen’s University Cancer Research Center, Kingston, Ont., said in an interview. “Because of the leadership positions they hold, the potential impact of this small group of physicians on oncology practice and policy may be substantial.”
The study was published online in JCO Oncology Practice.
‘We have a problem’
It’s no secret that many oncologists have financial relationships with pharmaceutical companies. They receive payments for research initiatives, but they also receive more general, personal payments in the form of honoraria, consultant fees, gifts, and reimbursement for travel and meals.
Prior studies have shown that these payments are typically modest, but a small subset of medical oncologists receive more than $100,000 annually. Dr. Booth and colleagues wanted to know more about the characteristics of these “high-payment” oncologists.
Using the national Open Payments database, the researchers identified a total of 139 medical oncologists who practice in the United States and who received $100,000 or more in general payments linked to cancer medications in 2018.
In U.S. dollars, the median payment was $154,613, and the total was $24.2 million.
The majority (95%) of high-payment oncologists were active in clinical work, 56% worked in an academic setting, 31% worked at National Cancer Institute–designated cancer centers, and 23% worked at National Comprehensive Cancer Network (NCCN) centers.
Many were based in California (17%), Texas (12%), Florida (10%), and New York (8%).
Most currently hold or have held hospital leadership positions (60%) or faculty appointments (72%) and 21% have held leadership positions in specialty associations in the past 5 years. Nearly one-quarter (24%) have served on journal editorial boards, and 10% have authored clinical practice guidelines in the past 5 years.
More specifically, three physicians authored NCCN guidelines, and two authored American Society of Clinical Oncology guidelines during 2016-2021; one guideline was published in 2018 when payments were made.
“Oncology specialty associations, guideline panels, and journal editorial boards should reconsider if it is appropriate for physicians with such large payments to hold these high-profile positions,” Dr. Booth said.
Following publication of the study, some oncologists took to Twitter with reactions, including Manni Mohyuddin, MD (@ManniMD1), from the Huntsman Cancer Institute, University of Utah, Salt Lake City, who wrote: “I recognize that some conflict of interest ‘may’ be unavoidable in order to run trials. But when greater than TWICE the average American household annual salary is taken in payments from industry by those in leadership/editorial roles, we have a problem.”
Weighing in on the results, ASCO CEO Clifford A. Hudis, MD, told this news organization that the “limitations of the study make it difficult to draw conclusions about the scope or potential impact of these payments on care.”
For example, he explained, some payments attributed to individuals may have been made directly to the physicians’ institutions or employers for sponsored research expenses.
Dr. Hudis also noted that the payments examined in the study were made in 2018, whereas the potentially relevant leadership positions could have been attained at a different time.
Furthermore, in 2020, an editorial appeared in Cancer, showing that errors in Open Payments are “fairly common,” Dr. Hudis said. It’s also unclear whether the reported financial relationships were appropriately disclosed and were managed at the time under relevant conflict of interest policies, he said.
“The question left unanswered by this study is whether or not these relationships influence patient care,” said Dr. Hudis. He noted that decisions about care should come from physicians and patients who are informed of the best available, unbiased, peer-reviewed, scientific evidence.
“The potential impact of financial conflicts of interest on this effort is an issue of concern, even if this study does not directly address it,” Dr. Hudis said.
The study had no specific funding. Dr. Booth has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article. Dr. Hudis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A small number of U.S. medical oncologists make more than $100,000 a year in general payments from drug companies, a new study shows.
These high-payment physicians represent just 1% of all U.S. medical oncologists, yet they account for 37% of industry payments. These oncologists often hold important leadership positions, draft treatment guidelines, and sit on journal editorial boards.
The findings highlight a risk for “perceived and real conflict of interest,” corresponding author Christopher Booth, MD, of Queen’s University Cancer Research Center, Kingston, Ont., said in an interview. “Because of the leadership positions they hold, the potential impact of this small group of physicians on oncology practice and policy may be substantial.”
The study was published online in JCO Oncology Practice.
‘We have a problem’
It’s no secret that many oncologists have financial relationships with pharmaceutical companies. They receive payments for research initiatives, but they also receive more general, personal payments in the form of honoraria, consultant fees, gifts, and reimbursement for travel and meals.
Prior studies have shown that these payments are typically modest, but a small subset of medical oncologists receive more than $100,000 annually. Dr. Booth and colleagues wanted to know more about the characteristics of these “high-payment” oncologists.
Using the national Open Payments database, the researchers identified a total of 139 medical oncologists who practice in the United States and who received $100,000 or more in general payments linked to cancer medications in 2018.
In U.S. dollars, the median payment was $154,613, and the total was $24.2 million.
The majority (95%) of high-payment oncologists were active in clinical work, 56% worked in an academic setting, 31% worked at National Cancer Institute–designated cancer centers, and 23% worked at National Comprehensive Cancer Network (NCCN) centers.
Many were based in California (17%), Texas (12%), Florida (10%), and New York (8%).
Most currently hold or have held hospital leadership positions (60%) or faculty appointments (72%) and 21% have held leadership positions in specialty associations in the past 5 years. Nearly one-quarter (24%) have served on journal editorial boards, and 10% have authored clinical practice guidelines in the past 5 years.
More specifically, three physicians authored NCCN guidelines, and two authored American Society of Clinical Oncology guidelines during 2016-2021; one guideline was published in 2018 when payments were made.
“Oncology specialty associations, guideline panels, and journal editorial boards should reconsider if it is appropriate for physicians with such large payments to hold these high-profile positions,” Dr. Booth said.
Following publication of the study, some oncologists took to Twitter with reactions, including Manni Mohyuddin, MD (@ManniMD1), from the Huntsman Cancer Institute, University of Utah, Salt Lake City, who wrote: “I recognize that some conflict of interest ‘may’ be unavoidable in order to run trials. But when greater than TWICE the average American household annual salary is taken in payments from industry by those in leadership/editorial roles, we have a problem.”
Weighing in on the results, ASCO CEO Clifford A. Hudis, MD, told this news organization that the “limitations of the study make it difficult to draw conclusions about the scope or potential impact of these payments on care.”
For example, he explained, some payments attributed to individuals may have been made directly to the physicians’ institutions or employers for sponsored research expenses.
Dr. Hudis also noted that the payments examined in the study were made in 2018, whereas the potentially relevant leadership positions could have been attained at a different time.
Furthermore, in 2020, an editorial appeared in Cancer, showing that errors in Open Payments are “fairly common,” Dr. Hudis said. It’s also unclear whether the reported financial relationships were appropriately disclosed and were managed at the time under relevant conflict of interest policies, he said.
“The question left unanswered by this study is whether or not these relationships influence patient care,” said Dr. Hudis. He noted that decisions about care should come from physicians and patients who are informed of the best available, unbiased, peer-reviewed, scientific evidence.
“The potential impact of financial conflicts of interest on this effort is an issue of concern, even if this study does not directly address it,” Dr. Hudis said.
The study had no specific funding. Dr. Booth has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article. Dr. Hudis has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JCO ONCOLOGY PRACTICE
Evidence still lacking that vitamins prevent CVD, cancer: USPSTF
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
FROM JAMA
ER+/HER2− BC: Age and Ki-67 index predict nodal response to NAC
Key clinical point: In estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) neoadjuvant chemotherapy (NAC) should be considered to enable axillary conservation in patients aged <50 years with Ki67 ≥20%.
Major finding: Both Ki67 ≥20% (adjusted odds ratio [aOR] 2.60; P = .04) and age <50 years (aOR 2.44; P = .01) were significant independent predictors of nodal pathological complete response (pCR). Younger patients (<50 years) had a higher nodal pCR when Ki67 index was ≥20% vs. <20% (35.8% vs. 14.3%; P = .02), whereas older patients (≥50 years) had an extremely low nodal pCR when Ki67 was <20% vs. ≥20% (2.6% vs. 21%; P = .008).
Study details: This study included 315 patients with node-positive, stage I-III, ER+/HER2− BC who were treated with NAC followed by surgery.
Disclosures: This work was partly supported by the National Institutes of Health Mayo Clinic Breast SPORE grant. Dr. Goetz and Dr. Boughey declared having research collaboration and receiving grants, funding, personal fees, or consulting fees from several sources.
Source: Boughey JC et al. Neoadjuvant chemotherapy and nodal response rates in luminal breast cancer: effects of age and tumor Ki67. Ann Surg Oncol. 2022 (May 15). Doi: 10.1245/s10434-022-11871-z
Key clinical point: In estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) neoadjuvant chemotherapy (NAC) should be considered to enable axillary conservation in patients aged <50 years with Ki67 ≥20%.
Major finding: Both Ki67 ≥20% (adjusted odds ratio [aOR] 2.60; P = .04) and age <50 years (aOR 2.44; P = .01) were significant independent predictors of nodal pathological complete response (pCR). Younger patients (<50 years) had a higher nodal pCR when Ki67 index was ≥20% vs. <20% (35.8% vs. 14.3%; P = .02), whereas older patients (≥50 years) had an extremely low nodal pCR when Ki67 was <20% vs. ≥20% (2.6% vs. 21%; P = .008).
Study details: This study included 315 patients with node-positive, stage I-III, ER+/HER2− BC who were treated with NAC followed by surgery.
Disclosures: This work was partly supported by the National Institutes of Health Mayo Clinic Breast SPORE grant. Dr. Goetz and Dr. Boughey declared having research collaboration and receiving grants, funding, personal fees, or consulting fees from several sources.
Source: Boughey JC et al. Neoadjuvant chemotherapy and nodal response rates in luminal breast cancer: effects of age and tumor Ki67. Ann Surg Oncol. 2022 (May 15). Doi: 10.1245/s10434-022-11871-z
Key clinical point: In estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer (BC) neoadjuvant chemotherapy (NAC) should be considered to enable axillary conservation in patients aged <50 years with Ki67 ≥20%.
Major finding: Both Ki67 ≥20% (adjusted odds ratio [aOR] 2.60; P = .04) and age <50 years (aOR 2.44; P = .01) were significant independent predictors of nodal pathological complete response (pCR). Younger patients (<50 years) had a higher nodal pCR when Ki67 index was ≥20% vs. <20% (35.8% vs. 14.3%; P = .02), whereas older patients (≥50 years) had an extremely low nodal pCR when Ki67 was <20% vs. ≥20% (2.6% vs. 21%; P = .008).
Study details: This study included 315 patients with node-positive, stage I-III, ER+/HER2− BC who were treated with NAC followed by surgery.
Disclosures: This work was partly supported by the National Institutes of Health Mayo Clinic Breast SPORE grant. Dr. Goetz and Dr. Boughey declared having research collaboration and receiving grants, funding, personal fees, or consulting fees from several sources.
Source: Boughey JC et al. Neoadjuvant chemotherapy and nodal response rates in luminal breast cancer: effects of age and tumor Ki67. Ann Surg Oncol. 2022 (May 15). Doi: 10.1245/s10434-022-11871-z