User login
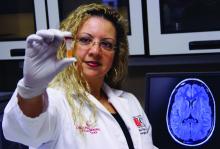
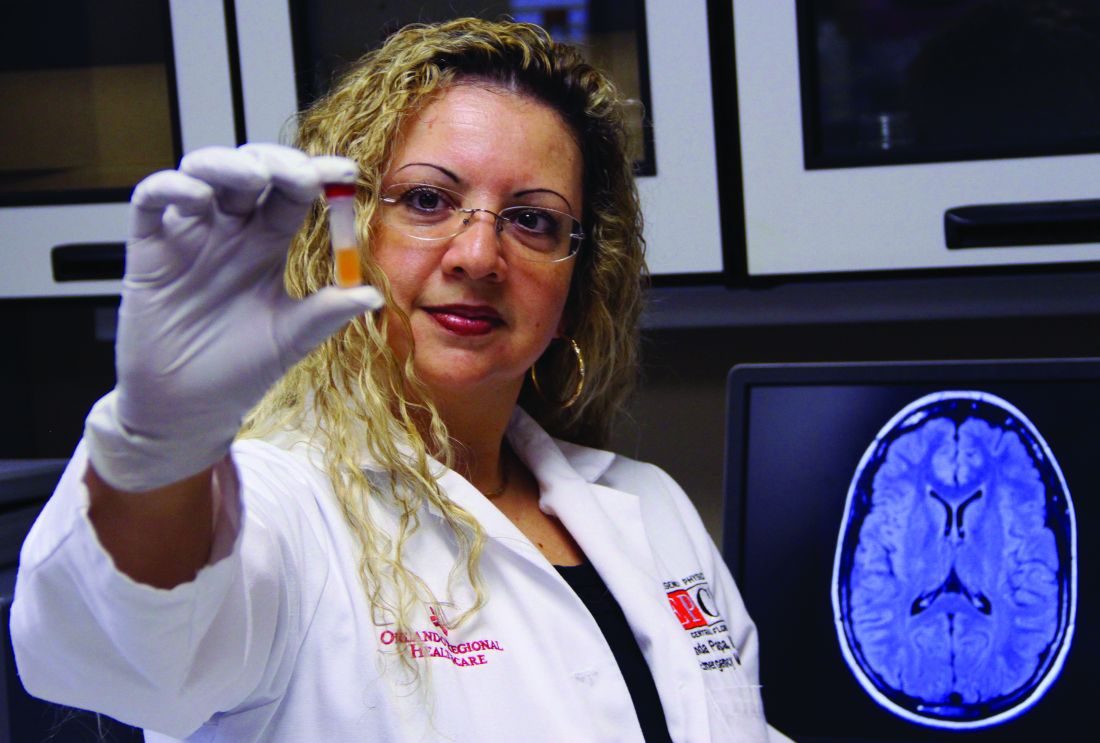
Blood test may reveal brain injury
researchers reported Aug. 26 in BMJ Paediatrics Open.
“GFAP outperformed UCH-L1 in detecting concussion in both children and adults within 4 hours of injury,” reported lead author Linda Papa, MD, and collaborators. Dr. Papa is an emergency medicine doctor at Orlando Health. “UCH-L1 was expressed at much higher levels than GFAP in those with nonconcussive trauma, particularly in children. Elevations of these biomarkers in nonconcussive head trauma suggest possible subconcussive brain injury. GFAP could be potentially useful to detect concussion for up to a week post injury.”
In 2018 the Food and Drug Administration approved the use of these biomarkers to guide CT scan ordering in adults with mild to moderate traumatic brain injury, but investigators have not established their ability to detect concussion in children or adults. Clinicians lack an objective measure to diagnose concussion acutely.
To assess the ability of GFAP and UCH-L1 to detect concussion, Dr. Papa and colleagues conducted a prospective cohort study. The researchers enrolled trauma patients of all ages at three level I trauma centers in the United States. They included patients with and without head trauma who had a Glasgow Coma Scale score of 15 and who presented within 4 hours of injury. Investigators screened for concussion symptoms, obtained biomarker data from 712 trauma patients, and conducted repeated blood sampling in adults.
They grouped patients by those with concussion (n = 371), those with head trauma without overt signs of concussion (n = 149), and those with peripheral trauma without head trauma or concussion (n = 192). The study included 175 children. Injury mechanisms included car crashes, falls, bicycle accidents, and sports injuries.
Patients with concussion had significantly higher GFAP concentrations, compared with patients with body trauma and patients with nonconcussive head trauma. UCH-L1 levels did not significantly differ between patients with concussion and head trauma controls, however.
“Based on these results, the potential utility of GFAP to distinguish concussion from body trauma controls over 7 days postinjury was fair to excellent,” with area under the receiver operating characteristics curves (AUCs) of 0.75-0.89, the researchers said. “UCH-L1’s ability was guarded and variable with AUCs from poor to good depending on timing of samples.” UCH-L1 demonstrated AUCs that ranged from 0.54 to 0.78; earlier samples performed better.
GFAP elevations in head trauma controls “may represent milder forms of concussion that do not elicit typical signs or symptoms associated with concussion,” the authors wrote. “These injuries may be irrelevant, or they may represent important trauma that is just below the level of clinical detection and referred to as subconcussive trauma. ... Biomarkers (such as GFAP and UCH-L1) could provide a more objective measure of injury and potentially identify those at risk for neurocognitive problems.”
The study was supported by the National Institute of Neurological Disorders and Stroke. Dr. Papa is an unpaid scientific consultant for Banyan Biomarkers, which developed kits to measure the biomarkers, and coauthors receive contract research funding from Banyan Biomarkers.
SOURCE: Papa L et al. BMJ Paediatr Open. 2019 Aug 26. doi: 10.1136/bmjpo-2019-000473.
researchers reported Aug. 26 in BMJ Paediatrics Open.
“GFAP outperformed UCH-L1 in detecting concussion in both children and adults within 4 hours of injury,” reported lead author Linda Papa, MD, and collaborators. Dr. Papa is an emergency medicine doctor at Orlando Health. “UCH-L1 was expressed at much higher levels than GFAP in those with nonconcussive trauma, particularly in children. Elevations of these biomarkers in nonconcussive head trauma suggest possible subconcussive brain injury. GFAP could be potentially useful to detect concussion for up to a week post injury.”
In 2018 the Food and Drug Administration approved the use of these biomarkers to guide CT scan ordering in adults with mild to moderate traumatic brain injury, but investigators have not established their ability to detect concussion in children or adults. Clinicians lack an objective measure to diagnose concussion acutely.
To assess the ability of GFAP and UCH-L1 to detect concussion, Dr. Papa and colleagues conducted a prospective cohort study. The researchers enrolled trauma patients of all ages at three level I trauma centers in the United States. They included patients with and without head trauma who had a Glasgow Coma Scale score of 15 and who presented within 4 hours of injury. Investigators screened for concussion symptoms, obtained biomarker data from 712 trauma patients, and conducted repeated blood sampling in adults.
They grouped patients by those with concussion (n = 371), those with head trauma without overt signs of concussion (n = 149), and those with peripheral trauma without head trauma or concussion (n = 192). The study included 175 children. Injury mechanisms included car crashes, falls, bicycle accidents, and sports injuries.
Patients with concussion had significantly higher GFAP concentrations, compared with patients with body trauma and patients with nonconcussive head trauma. UCH-L1 levels did not significantly differ between patients with concussion and head trauma controls, however.
“Based on these results, the potential utility of GFAP to distinguish concussion from body trauma controls over 7 days postinjury was fair to excellent,” with area under the receiver operating characteristics curves (AUCs) of 0.75-0.89, the researchers said. “UCH-L1’s ability was guarded and variable with AUCs from poor to good depending on timing of samples.” UCH-L1 demonstrated AUCs that ranged from 0.54 to 0.78; earlier samples performed better.
GFAP elevations in head trauma controls “may represent milder forms of concussion that do not elicit typical signs or symptoms associated with concussion,” the authors wrote. “These injuries may be irrelevant, or they may represent important trauma that is just below the level of clinical detection and referred to as subconcussive trauma. ... Biomarkers (such as GFAP and UCH-L1) could provide a more objective measure of injury and potentially identify those at risk for neurocognitive problems.”
The study was supported by the National Institute of Neurological Disorders and Stroke. Dr. Papa is an unpaid scientific consultant for Banyan Biomarkers, which developed kits to measure the biomarkers, and coauthors receive contract research funding from Banyan Biomarkers.
SOURCE: Papa L et al. BMJ Paediatr Open. 2019 Aug 26. doi: 10.1136/bmjpo-2019-000473.
researchers reported Aug. 26 in BMJ Paediatrics Open.
“GFAP outperformed UCH-L1 in detecting concussion in both children and adults within 4 hours of injury,” reported lead author Linda Papa, MD, and collaborators. Dr. Papa is an emergency medicine doctor at Orlando Health. “UCH-L1 was expressed at much higher levels than GFAP in those with nonconcussive trauma, particularly in children. Elevations of these biomarkers in nonconcussive head trauma suggest possible subconcussive brain injury. GFAP could be potentially useful to detect concussion for up to a week post injury.”
In 2018 the Food and Drug Administration approved the use of these biomarkers to guide CT scan ordering in adults with mild to moderate traumatic brain injury, but investigators have not established their ability to detect concussion in children or adults. Clinicians lack an objective measure to diagnose concussion acutely.
To assess the ability of GFAP and UCH-L1 to detect concussion, Dr. Papa and colleagues conducted a prospective cohort study. The researchers enrolled trauma patients of all ages at three level I trauma centers in the United States. They included patients with and without head trauma who had a Glasgow Coma Scale score of 15 and who presented within 4 hours of injury. Investigators screened for concussion symptoms, obtained biomarker data from 712 trauma patients, and conducted repeated blood sampling in adults.
They grouped patients by those with concussion (n = 371), those with head trauma without overt signs of concussion (n = 149), and those with peripheral trauma without head trauma or concussion (n = 192). The study included 175 children. Injury mechanisms included car crashes, falls, bicycle accidents, and sports injuries.
Patients with concussion had significantly higher GFAP concentrations, compared with patients with body trauma and patients with nonconcussive head trauma. UCH-L1 levels did not significantly differ between patients with concussion and head trauma controls, however.
“Based on these results, the potential utility of GFAP to distinguish concussion from body trauma controls over 7 days postinjury was fair to excellent,” with area under the receiver operating characteristics curves (AUCs) of 0.75-0.89, the researchers said. “UCH-L1’s ability was guarded and variable with AUCs from poor to good depending on timing of samples.” UCH-L1 demonstrated AUCs that ranged from 0.54 to 0.78; earlier samples performed better.
GFAP elevations in head trauma controls “may represent milder forms of concussion that do not elicit typical signs or symptoms associated with concussion,” the authors wrote. “These injuries may be irrelevant, or they may represent important trauma that is just below the level of clinical detection and referred to as subconcussive trauma. ... Biomarkers (such as GFAP and UCH-L1) could provide a more objective measure of injury and potentially identify those at risk for neurocognitive problems.”
The study was supported by the National Institute of Neurological Disorders and Stroke. Dr. Papa is an unpaid scientific consultant for Banyan Biomarkers, which developed kits to measure the biomarkers, and coauthors receive contract research funding from Banyan Biomarkers.
SOURCE: Papa L et al. BMJ Paediatr Open. 2019 Aug 26. doi: 10.1136/bmjpo-2019-000473.
FROM BMJ PAEDIATRICS OPEN
Key clinical point: Levels of glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase L1 (UCH-L1) are lowest in patients with nonconcussive body trauma, higher in patients with nonconcussive head trauma, and highest in patients with concussion.
Major finding: GFAP was fair to excellent at distinguishing concussion from body trauma, with area under the receiver operating characteristics curves of 0.75-0.89.
Study details: A prospective cohort study of 712 trauma patients of all ages at three level I trauma centers in the United States. The study included patients with and without head trauma who had a Glasgow Coma Scale score of 15 and presented within 4 hours of injury.
Disclosures: The study was supported by the National Institute of Neurological Disorders and Stroke. Dr. Papa is an unpaid scientific consultant for Banyan Biomarkers, which developed kits to measure the biomarkers. Coauthors receive contract research funding from Banyan Biomarkers.
Source: Papa L et al. BMJ Paediatr Open. 2019 Aug 26. doi: 10.1136/bmjpo-2019-000473.
Robert Cowan, MD, Discusses Patient Perspectives Study Outcomes
In a sampling of 300 individuals with a mean age of 41 years and nearly 16 self-reported headache days per month over the past 6 months, respondents agreed that they were willing to trade some degree of efficacy for less severe adverse events—namely weight gain and memory problems—and that they were even willing to pay more for these tradeoffs in some cases. On average, respondents were willing to pay:
- $84 more (95% confidence interval [CI], $64‐$103) per month to avoid a 10% weight gain
- $59 more (95% CI, $42‐$76) per month to avoid memory problems
- $35 more (95% CI, $20‐$51) per month to avoid a 5% weight gain, and
- $32 (95% CI, $18‐$46) per month to avoid thinking problems.
Within the pool, 81% of respondents confirmed that they had taken a prescription medicine to prevent migraine in the past 6 months.
I think the broad message of this study is important: migraine is not just about migraine or headache days per month. This should not come as news to anyone with more than a passing interest in this condition. As an epidemiological study, it is useful to understand how migraine patients, as a group, view the relative value of cost, side effects, etc. For clinicians, the value of this study is to remind us of the complexity we need to consider when prescribing a migraine treatment. Issues of co-morbidity, economic resources, type of work or daily activities, and most bothersome symptom all play into the decision process, and it is critical to have the patient expressly involved in this process. It is not just about migraine days. It never has been.
Implicit in this article is the reality that we now have a wide variety of pharmacologic and device options for treating migraine. In the past, this was not the case. There is no clear winner among the preventives in terms of headache or migraine days per month. Rather, as the article suggests, we now have the option of selecting our preventives based on a cost-benefit analysis. Because of the near parity in terms of efficacy, the choices are often based on accessibility, financial burden, delivery system, and side effect profile. Properly presented, this can be an empowering experience for the patient. Choices may involve the need for trialing one or more treatments before gaining access to a preferred treatment, or the willingness to risk the chance of an untoward side effect against the promise of a more convivial dosing regimen. This collaborative decision-making process helps center the locus of control with the patient and secure a healthy relationship between the provider and patient.
It should also be remembered that the promise of a given side effect profile is based on the observation in trials and does not reflect the probability of a given outcome in a single patient. Neither does the side effect profile generated in a controlled trial necessarily reflect the side effect profile in any given individual. Too often we fail to stress this to patients and a 1% risk becomes an unavoidable consequence or a promise of smooth sailing. Time spent educating patients (and providers) with regard to the interpretation of efficacy and risk data, is time well spent.
Dr. Cowan is a Higgins Professor of Neurology, Chief of the Division of Headache Medicine and the Department of Neurology and Neurosciences, and Director of the Center for Headache and Facial Pain, at Stanford University School of Medicine.
In a sampling of 300 individuals with a mean age of 41 years and nearly 16 self-reported headache days per month over the past 6 months, respondents agreed that they were willing to trade some degree of efficacy for less severe adverse events—namely weight gain and memory problems—and that they were even willing to pay more for these tradeoffs in some cases. On average, respondents were willing to pay:
- $84 more (95% confidence interval [CI], $64‐$103) per month to avoid a 10% weight gain
- $59 more (95% CI, $42‐$76) per month to avoid memory problems
- $35 more (95% CI, $20‐$51) per month to avoid a 5% weight gain, and
- $32 (95% CI, $18‐$46) per month to avoid thinking problems.
Within the pool, 81% of respondents confirmed that they had taken a prescription medicine to prevent migraine in the past 6 months.
I think the broad message of this study is important: migraine is not just about migraine or headache days per month. This should not come as news to anyone with more than a passing interest in this condition. As an epidemiological study, it is useful to understand how migraine patients, as a group, view the relative value of cost, side effects, etc. For clinicians, the value of this study is to remind us of the complexity we need to consider when prescribing a migraine treatment. Issues of co-morbidity, economic resources, type of work or daily activities, and most bothersome symptom all play into the decision process, and it is critical to have the patient expressly involved in this process. It is not just about migraine days. It never has been.
Implicit in this article is the reality that we now have a wide variety of pharmacologic and device options for treating migraine. In the past, this was not the case. There is no clear winner among the preventives in terms of headache or migraine days per month. Rather, as the article suggests, we now have the option of selecting our preventives based on a cost-benefit analysis. Because of the near parity in terms of efficacy, the choices are often based on accessibility, financial burden, delivery system, and side effect profile. Properly presented, this can be an empowering experience for the patient. Choices may involve the need for trialing one or more treatments before gaining access to a preferred treatment, or the willingness to risk the chance of an untoward side effect against the promise of a more convivial dosing regimen. This collaborative decision-making process helps center the locus of control with the patient and secure a healthy relationship between the provider and patient.
It should also be remembered that the promise of a given side effect profile is based on the observation in trials and does not reflect the probability of a given outcome in a single patient. Neither does the side effect profile generated in a controlled trial necessarily reflect the side effect profile in any given individual. Too often we fail to stress this to patients and a 1% risk becomes an unavoidable consequence or a promise of smooth sailing. Time spent educating patients (and providers) with regard to the interpretation of efficacy and risk data, is time well spent.
Dr. Cowan is a Higgins Professor of Neurology, Chief of the Division of Headache Medicine and the Department of Neurology and Neurosciences, and Director of the Center for Headache and Facial Pain, at Stanford University School of Medicine.
In a sampling of 300 individuals with a mean age of 41 years and nearly 16 self-reported headache days per month over the past 6 months, respondents agreed that they were willing to trade some degree of efficacy for less severe adverse events—namely weight gain and memory problems—and that they were even willing to pay more for these tradeoffs in some cases. On average, respondents were willing to pay:
- $84 more (95% confidence interval [CI], $64‐$103) per month to avoid a 10% weight gain
- $59 more (95% CI, $42‐$76) per month to avoid memory problems
- $35 more (95% CI, $20‐$51) per month to avoid a 5% weight gain, and
- $32 (95% CI, $18‐$46) per month to avoid thinking problems.
Within the pool, 81% of respondents confirmed that they had taken a prescription medicine to prevent migraine in the past 6 months.
I think the broad message of this study is important: migraine is not just about migraine or headache days per month. This should not come as news to anyone with more than a passing interest in this condition. As an epidemiological study, it is useful to understand how migraine patients, as a group, view the relative value of cost, side effects, etc. For clinicians, the value of this study is to remind us of the complexity we need to consider when prescribing a migraine treatment. Issues of co-morbidity, economic resources, type of work or daily activities, and most bothersome symptom all play into the decision process, and it is critical to have the patient expressly involved in this process. It is not just about migraine days. It never has been.
Implicit in this article is the reality that we now have a wide variety of pharmacologic and device options for treating migraine. In the past, this was not the case. There is no clear winner among the preventives in terms of headache or migraine days per month. Rather, as the article suggests, we now have the option of selecting our preventives based on a cost-benefit analysis. Because of the near parity in terms of efficacy, the choices are often based on accessibility, financial burden, delivery system, and side effect profile. Properly presented, this can be an empowering experience for the patient. Choices may involve the need for trialing one or more treatments before gaining access to a preferred treatment, or the willingness to risk the chance of an untoward side effect against the promise of a more convivial dosing regimen. This collaborative decision-making process helps center the locus of control with the patient and secure a healthy relationship between the provider and patient.
It should also be remembered that the promise of a given side effect profile is based on the observation in trials and does not reflect the probability of a given outcome in a single patient. Neither does the side effect profile generated in a controlled trial necessarily reflect the side effect profile in any given individual. Too often we fail to stress this to patients and a 1% risk becomes an unavoidable consequence or a promise of smooth sailing. Time spent educating patients (and providers) with regard to the interpretation of efficacy and risk data, is time well spent.
Dr. Cowan is a Higgins Professor of Neurology, Chief of the Division of Headache Medicine and the Department of Neurology and Neurosciences, and Director of the Center for Headache and Facial Pain, at Stanford University School of Medicine.
Coordination of Care Between Primary Care and Oncology for Patients With Prostate Cancer (FULL)
The following is a lightly edited transcript of a teleconference recorded in July 2018. The teleconference brought together health care providers from the Greater Los Angeles VA Health Care System (GLAVAHCS) to discuss the real-world processes for managing the treatment of patients with prostate cancer as they move between primary and specialist care.
William J. Aronson, MD. We are fortunate in having a superb medical record system at the Department of Veterans Affairs (VA) where we can all communicate with each other through a number of methods. Let’s start our discussion by reviewing an index patient that we see in our practice who has been treated with either radical prostatectomy or radiation therapy. One question to address is: Is there a point when the Urology or Radiation Oncology service can transition the patient’s entire care back to the primary care team? And if so, what would be the optimal way to accomplish this?
Nick, is there some point at which you discharge the patient from the radiation oncology service and give specific directions to primary care, or is it primarily just back to urology in your case?
Nicholas G. Nickols, MD, PhD. I have not discharged any patient from my clinic after definitive prostate cancer treatment. During treatment, patients are seen every week. Subsequently, I see them 6 weeks posttreatment, and then every 4 months for the first year, then every 6 months for the next 4 years, and then yearly after that. Although I never formally discharged a patient from my clinic, you can see based on the frequency of visits, that the patient will see more often than their primary care provider (PCP) toward the beginning. And then, after some years, the patient sees their primary more than they me. So it’s not an immediate hand off but rather a gradual transition. It’s important that the PCP is aware of what to look for especially for the late recurrences, late potential side effects, probably more significantly than the early side effects, how to manage them when appropriate, and when to ask the patient to see our team more frequently in follow-up.
William Aronson. We have a number of patients who travel tremendous distances to see us, and I tend to think that many of our follow-up patients, once things are stabilized with regards to management of their side effects, really could see their primary care doctors if we can give them specific instructions on, for example, when to get a prostate-specific antigen (PSA) test and when to refer back to us.
Alison, can you think of some specific cases where you feel like we’ve successfully done that?
Alison Neymark, MS. For the most part we haven’t discharged people, either. What we have done is transitioned them over to a phone clinic. In our department, we have 4 nurse practitioners (NPs) who each have a half-day of phone clinic where they call patients with their test results. Some of those patients are prostate cancer patients that we have been following for years. We schedule them for a phone call, whether it’s every 3 months, every 6 months or every year, to review the updated PSA level and to just check in with them by phone. It’s a win-win because it’s a really quick phone call to reassure the veteran that the PSA level is being followed, and it frees up an in-person appointment slot for another veteran.
We still have patients that prefer face-to-face visits, even though they know we’re not doing anything except discussing a PSA level with them—they just want that security of seeing our face. Some patients are very nervous, and they don’t necessarily want to be discharged, so to speak, back to primary care. Also, for those patients that travel a long distance to clinic, we offer an appointment in the video chat clinic, with the community-based outpatient clinics in Bakersfield and Santa Maria, California.
PSA Levels
William Aronson. I probably see a patient about every 4 to 6 weeks who has a low PSA after about 10 years and has a long distance to travel and mobility and other problems that make it difficult to come in.
The challenge that I have is, what is that specific guideline to give with regards to the rise in PSA? I think it all depends on the patients prostate cancer clinical features and comorbidities.
Nicholas Nickols. If a patient has been seen by me in follow-up a number of times and there’s really no active issues and there’s a low suspicion of recurrence, then I offer the patient the option of a phone follow-up as an alternative to face to face. Some of them accept that, but I ask that they agree to also see either urology or their PCP face to face. I will also remotely ensure that they’re getting the right laboratory tests, and if not, I’ll put those orders in.
With regard to when to refer a patient back for a suspected recurrence after definitive radiation therapy, there is an accepted definition of biochemical failure called the Phoenix definition, which is an absolute rise in 2 ng/mL of PSA over their posttreatment nadir. Often the posttreatment nadir, especially if they were on hormone therapy, will be close to 0. If the PSA gets to 2, that is a good trigger for a referral back to me and/or urology to discuss restaging and workup for a suspected recurrence.
For patients that are postsurgery and then subsequently get salvage radiation, it is not as clear when a restaging workup should be initiated. Currently, the imaging that is routine care is not very sensitive for detecting PSA in that setting until the PSA is around 0.8 ng/mL, and that’s with the most modern imaging available. Over time that may improve.
William Aronson. The other index patient to think about would be the patient who is on watchful waiting for their prostate cancer, which is to be distinguished from active surveillance. If someone’s on active surveillance, we’re regularly doing prostate biopsies and doing very close monitoring; but we also have patients who have multiple other medical problems, have a limited life expectancy, don’t have aggressive prostate cancer, and it’s extremely reasonable not to do a biopsy in those patients.
Again, those are patients where we do follow the PSA generally every 6 months. And I think there’s also scenarios there where it’s reasonable to refer back to primary care with specific instructions. These, again, are patients who had difficulty getting in to see us or have mobility issues, but it is also a way to limit patient visits if that’s their desire.
Peter Glassman, MBBS, MSc: I’m trained as both a general internist and board certified in hospice and palliative medicine. I currently provide primary care as well as palliative care. I view prostate cancer from the diagnosis through the treatment spectrum as a continuum. It starts with the PCP with an elevated PSA level or if the digital rectal exam has an abnormality, and then the role of the genitourinary (GU) practitioner becomes more significant during the active treatment and diagnostic phases.
Primary care doesn’t disappear, and I think there are 2 major issues that go along with that. First of all, we in primary care, because we take care of patients that often have other comorbidities, need to work with the patient on those comorbidities. Secondly, we need the information shared between the GU and primary care providers so that we can answer questions from our patients and have an understanding of what they’re going through and when.
As time goes on, we go through various phases: We may reach a cure, a quiescent period, active therapy, watchful waiting, or recurrence. Primary care gets involved as time goes on when the disease either becomes quiescent, is just being followed, or is considered cured. Clearly when you have watchful waiting, active treatment, or are in a recurrence, then GU takes the forefront.
I view it as a wave function. Primary care to GU with primary in smaller letters and then primary, if you will, in larger letters, GU becomes a lesser participant unless there is active therapy, watchful waiting or recurrence.
In doing a little bit of research, I found 2 very good and very helpful documents. One is the American Cancer Society (ACS) prostate cancer survivorship care guidelines (Box). And the other is a synopsis of the guidelines. What I liked was that the guidelines focused not only on what should be done for the initial period of prostate cancer, but also for many of the ancillary issues which we often don’t give voice to. The guidelines provide a structure, a foundation to work with our patients over time on their prostate cancer-related issues while, at the same time, being cognizant that we need to deal with their other comorbid conditions.
Modes of Communication
Alison Neymark. We find that including parameters for PSA monitoring in our Progress Notes in the electronic health record (EHR) the best way to communicate with other providers. We’ll say, “If PSA gets to this level, please refer back.” We try to make it clear because with the VA being a training facility, it could be a different resident/attending physician team that’s going to see the patient the next time he is in primary care.
Peter Glassman. Yes, we’re very lucky, as Bill talked about earlier and Alison just mentioned. We have the EHR, and Bill may remember this. Before the EHR, we were constantly fishing to find the most relevant notes. If a patient saw a GU practitioner the day before they saw me, I was often asking the patient what was said. Now we can just review the notes.
It’s a double-edged sword though because there are, of course, many notes in a medical record; and you have to look for the specific items. The EHR and documenting the medical record probably plays the primary role in getting information across. When you want to have an active handoff, or you need to communicate with each other, we have a variety of mechanisms, ranging from the phone to the Microsoft Skype Link (Redmond, WA) system that allows us to tap a message to a colleague.
And I’ve been here long enough that I’ve seen most permutations of how prostate cancer is diagnosed as well as shared among providers. Bill and I have shared patients. Alison and I have shared patients, not necessarily with prostate cancer, although that too. But we know how to communicate with each other. And of course, there’s paging if you need something more urgently.
William Aronson. We also use Microsoft Outlook e-mail, and encrypt the messages to keep them confidential and private. The other nice thing we have is there is a nationwide urology Outlook e-mail, so if any of us have any specific questions, through one e-mail we can send it around the country; and there’s usually multiple very useful responses. That’s another real strength of our system within the VA that helps patient care enormously.
Nicholas Nickols. Sometimes, if there’s a critical note that I absolutely want someone on the care team to read, I’ll add them as a cosigner; and that will pop up when they log in to the Computerized Patient Record System (CPRS) as something that they need to read.
If the patient lives particularly far or gets his care at another VA medical center and laboratory tests are needed, then I will reach out to their PCP via e-mail. If contact is not confirmed, I will reach out via phone or Skype.
Peter Glassman. The most helpful notes are those that are very specific as to what primary care is being asked to do and/or what urology is going to be doing. So, the more specific we get in the notes as to what is being addressed, I think that’s very helpful.
I have been here long enough that I’ve known both Alison and Bill; and if they have an issue, they will tap me a message. It wasn’t long ago that Bill sent a message to me, and we worked on a patient with prostate cancer who was going to be on long-term hormone therapy. We talked about osteoporosis management, and between us we worked out who was going to do what. Those are the kind of shared decision-making situations that are very, very helpful.
Alison Neymark. Also, GLAVAHCS has a home-based primary care team (HBPC), and a lot of the PCPs for that team are NPs. They know that they can contact me for their patients because a lot of those patients are on watchful waiting, and we do not necessarily need to see them face to face in clinic. Our urology team just needs to review updated lab results and how they are doing clinically. The HBPC NP who knows them best can contact me every 6 months or so, and we’ll discuss the case, which avoids making the patient come in, especially when they’re homebound. Those of us that have been working at the VA for many years have established good relationships. We feel very comfortable reaching out and talking to each other about these patients
Peter Glassman. Alison, I agree. When I can talk to my patients and say, “You know, we had that question about,” whatever the question might be, “and I contacted urology, and this is what they said.” It gives the patient confidence that we’re following up on the issues that they have and that we’re communicating with each other in a way that is to their benefit. And I think it’s very appreciated both by the provider as well as the patient.
William Aronson. Not infrequently I’ll have patients who have nonurologic issues, which I may first detect, or who have specific issues with their prostate cancer that can be comanaged. And I have found that when I send an encrypted e-mail to the PCP, it has been an extremely satisfying interaction; and we really get to the heart of the matter quickly for the sake of the veteran.
Veterans With Comorbidities
William Aronson. Posttraumatic stress disorder (PTSD) is a very significant and unique aspect of our patients, which is enormously important to recognize. For example, the side effects of prostate treatments can be very significant, whether radiation or surgery. Our patients understandably can be very fearful of the prostate cancer diagnosis and treatment side effects.
We know, for example, after a patient gets a diagnosis of prostate cancer, they’re at increased risk of cardiac death. That’s an especially important issue for our patients that there be an ongoing interaction between urology and primary care.
The ACS guidelines that Dr. Glassman referred to were enlightening. In many cases, primary care can look at the whole patient and their circumstances better than we can and may detect, for example, specific psychological issues that either they can manage or refer to other specialists.
Peter Glassman. One of the things that was highlighted in the ACS guideline is that in any population of men who have this disease, there’s going to be distress, anxiety, and full-fledged depression. Of course, there are psychosocial aspects of prostate cancer, such as sexual activity and intimacy with a partner that we often don’t explore but are probably playing an important role in the overall health of our patients. We need to be mindful of these psychosocial aspects and at least periodically ask them, “How are you doing with this? How are things at home?” And of course, we already use screeners for depression. As the article noted, distress and anxiety and other factors can make somebody’s life less optimal with poorer quality of life.
Dual Care Patients
Alison Neymark. Many patients whether they have Medicare, insurance through their spouse, or Kaiser Permanente through their job, choose to go to both places. The challenge is communicating with the non-VA providers because here at the VA we can communicate easily through Skype, Outlook e-mail, or CPRS, but for dual care patients who’s in charge? I encourage the veterans to choose whom they want to manage their care; we’re always here and happy to treat them, but they need to decide who’s in charge because I don’t want them to get into a situation where the differing opinions lead to a delay in care.
Nicholas Nickols. The communication when the patient is receiving care outside VA, either on a continuous basis or temporarily, is more of a challenge. We obviously can’t rely upon the messaging system, face-to-face contact is difficult, and they may not be able to use e-mail as well. So in those situations, usually a phone call is the best approach. I have found that the outside providers are happy to speak on the phone to coordinate care.
Peter Glassman. I agree, it does add a layer of complexity because we don’t readily have the notes, any information in front of us. That said, a lot of our patients can and do bring in information from outside specialists, and I’m hopeful that they share the information that we provide back to their outside doctors as well.
William Aronson. Some patient get nervous. They might decide they want care elsewhere, but they still want the VA available for them. I always let them know they should proceed in whatever way they prefer, but we’re always available and here for them. I try to empower them to make their own decisions and feel comfortable with them.
Nicholas Nickols. Notes from the outside, if they’re being referred for VA Choice or community care, do get uploaded into VistA Imaging and can be accessed, although it’s not instantaneous. Sometimes there’s a delay, but I have been able to access outside notes most of the time. If a patient goes through a clinic at the VA, the note is written in real time, and you can read it immediately.
Peter Glassman. That is true for patients that are within the VA system who receive contracted care either through Choice or through non-VA care that is contracted through VA. For somebody who is choosing to use 2 health care systems, that can provide more of a challenge because those notes don’t come to us. Over time, most of my patients have brought test results to me.
The thing with oncologic care, of course, is it’s a lot more complex. And it’s hard to know without reasonable documentation what’s been going on. At some level, you have to trust that the outside provider is doing whatever they need to do, or you have to take it upon yourself to do it within the system.
Alison Neymark. In my experience with the Choice Program, it really depends on the outside providers and how comfortable they are with the system that has been established to share records. Not all providers are going into that system and accessing it. I have had cases where I will see the non-VA provider’s note and it’ll say, “No documentation available for this consultation.” It just happens that they didn’t go into the system to review it. So it can be a challenge.
I’ve had good communication with the providers who use the system correctly. In some cases, just to make it easier, I will go ahead and communicate with them through encrypted e-mail, or I’ll talk to their care coordinators directly by phone.
Peter Glassman. Many, if not most, PCPs are going to take care of these patients, certainly within the VA, with their GU colleagues. And most of us feel comfortable using the current documentation system in a way that allows us to share information or at least to gather information about these patients.
One of the things that I think came out for me in looking at this was that there are guidelines or there are ideas out there on how to take better care of these patients. And I for one learned a fair bit just by going through these documents, which I’m very appreciative of. But it does highlight to me that we can give good care and provide good shared care for prostate cancer survivors. I think that is something that perhaps this discussion will highlight that not only are people doing that, but there are resources they can utilize that will help them get a more comprehensive picture of taking care of prostate cancer survivors in the primary care clinic.
The beauty of the VA system as a system is that as these issues come up that might affect the overall health of the veteran with prostate cancer, for example, psychosocial issues, we have many people that can address this that are experts in their area. And one of the great beauties of having an all-encompassing healthcare system is being able to use resources within the system, whether that be for other medical problems or other social or other psychological issues, that we ourselves are not expert in. We can reach out to our other colleagues and ask them for assistance. We have that available to help the patients. It’s really holistic.
We even have integrated medicine where we can help patients, hopefully, get back into a healthy lifestyle, for example, whereas we may not have that expertise or knowledge. We often think of this as sort of a shared decision between GU and primary care. But, in fact, it’s really the responsibility of many, many people of the system at large. We are very lucky to have that.
The following is a lightly edited transcript of a teleconference recorded in July 2018. The teleconference brought together health care providers from the Greater Los Angeles VA Health Care System (GLAVAHCS) to discuss the real-world processes for managing the treatment of patients with prostate cancer as they move between primary and specialist care.
William J. Aronson, MD. We are fortunate in having a superb medical record system at the Department of Veterans Affairs (VA) where we can all communicate with each other through a number of methods. Let’s start our discussion by reviewing an index patient that we see in our practice who has been treated with either radical prostatectomy or radiation therapy. One question to address is: Is there a point when the Urology or Radiation Oncology service can transition the patient’s entire care back to the primary care team? And if so, what would be the optimal way to accomplish this?
Nick, is there some point at which you discharge the patient from the radiation oncology service and give specific directions to primary care, or is it primarily just back to urology in your case?
Nicholas G. Nickols, MD, PhD. I have not discharged any patient from my clinic after definitive prostate cancer treatment. During treatment, patients are seen every week. Subsequently, I see them 6 weeks posttreatment, and then every 4 months for the first year, then every 6 months for the next 4 years, and then yearly after that. Although I never formally discharged a patient from my clinic, you can see based on the frequency of visits, that the patient will see more often than their primary care provider (PCP) toward the beginning. And then, after some years, the patient sees their primary more than they me. So it’s not an immediate hand off but rather a gradual transition. It’s important that the PCP is aware of what to look for especially for the late recurrences, late potential side effects, probably more significantly than the early side effects, how to manage them when appropriate, and when to ask the patient to see our team more frequently in follow-up.
William Aronson. We have a number of patients who travel tremendous distances to see us, and I tend to think that many of our follow-up patients, once things are stabilized with regards to management of their side effects, really could see their primary care doctors if we can give them specific instructions on, for example, when to get a prostate-specific antigen (PSA) test and when to refer back to us.
Alison, can you think of some specific cases where you feel like we’ve successfully done that?
Alison Neymark, MS. For the most part we haven’t discharged people, either. What we have done is transitioned them over to a phone clinic. In our department, we have 4 nurse practitioners (NPs) who each have a half-day of phone clinic where they call patients with their test results. Some of those patients are prostate cancer patients that we have been following for years. We schedule them for a phone call, whether it’s every 3 months, every 6 months or every year, to review the updated PSA level and to just check in with them by phone. It’s a win-win because it’s a really quick phone call to reassure the veteran that the PSA level is being followed, and it frees up an in-person appointment slot for another veteran.
We still have patients that prefer face-to-face visits, even though they know we’re not doing anything except discussing a PSA level with them—they just want that security of seeing our face. Some patients are very nervous, and they don’t necessarily want to be discharged, so to speak, back to primary care. Also, for those patients that travel a long distance to clinic, we offer an appointment in the video chat clinic, with the community-based outpatient clinics in Bakersfield and Santa Maria, California.
PSA Levels
William Aronson. I probably see a patient about every 4 to 6 weeks who has a low PSA after about 10 years and has a long distance to travel and mobility and other problems that make it difficult to come in.
The challenge that I have is, what is that specific guideline to give with regards to the rise in PSA? I think it all depends on the patients prostate cancer clinical features and comorbidities.
Nicholas Nickols. If a patient has been seen by me in follow-up a number of times and there’s really no active issues and there’s a low suspicion of recurrence, then I offer the patient the option of a phone follow-up as an alternative to face to face. Some of them accept that, but I ask that they agree to also see either urology or their PCP face to face. I will also remotely ensure that they’re getting the right laboratory tests, and if not, I’ll put those orders in.
With regard to when to refer a patient back for a suspected recurrence after definitive radiation therapy, there is an accepted definition of biochemical failure called the Phoenix definition, which is an absolute rise in 2 ng/mL of PSA over their posttreatment nadir. Often the posttreatment nadir, especially if they were on hormone therapy, will be close to 0. If the PSA gets to 2, that is a good trigger for a referral back to me and/or urology to discuss restaging and workup for a suspected recurrence.
For patients that are postsurgery and then subsequently get salvage radiation, it is not as clear when a restaging workup should be initiated. Currently, the imaging that is routine care is not very sensitive for detecting PSA in that setting until the PSA is around 0.8 ng/mL, and that’s with the most modern imaging available. Over time that may improve.
William Aronson. The other index patient to think about would be the patient who is on watchful waiting for their prostate cancer, which is to be distinguished from active surveillance. If someone’s on active surveillance, we’re regularly doing prostate biopsies and doing very close monitoring; but we also have patients who have multiple other medical problems, have a limited life expectancy, don’t have aggressive prostate cancer, and it’s extremely reasonable not to do a biopsy in those patients.
Again, those are patients where we do follow the PSA generally every 6 months. And I think there’s also scenarios there where it’s reasonable to refer back to primary care with specific instructions. These, again, are patients who had difficulty getting in to see us or have mobility issues, but it is also a way to limit patient visits if that’s their desire.
Peter Glassman, MBBS, MSc: I’m trained as both a general internist and board certified in hospice and palliative medicine. I currently provide primary care as well as palliative care. I view prostate cancer from the diagnosis through the treatment spectrum as a continuum. It starts with the PCP with an elevated PSA level or if the digital rectal exam has an abnormality, and then the role of the genitourinary (GU) practitioner becomes more significant during the active treatment and diagnostic phases.
Primary care doesn’t disappear, and I think there are 2 major issues that go along with that. First of all, we in primary care, because we take care of patients that often have other comorbidities, need to work with the patient on those comorbidities. Secondly, we need the information shared between the GU and primary care providers so that we can answer questions from our patients and have an understanding of what they’re going through and when.
As time goes on, we go through various phases: We may reach a cure, a quiescent period, active therapy, watchful waiting, or recurrence. Primary care gets involved as time goes on when the disease either becomes quiescent, is just being followed, or is considered cured. Clearly when you have watchful waiting, active treatment, or are in a recurrence, then GU takes the forefront.
I view it as a wave function. Primary care to GU with primary in smaller letters and then primary, if you will, in larger letters, GU becomes a lesser participant unless there is active therapy, watchful waiting or recurrence.
In doing a little bit of research, I found 2 very good and very helpful documents. One is the American Cancer Society (ACS) prostate cancer survivorship care guidelines (Box). And the other is a synopsis of the guidelines. What I liked was that the guidelines focused not only on what should be done for the initial period of prostate cancer, but also for many of the ancillary issues which we often don’t give voice to. The guidelines provide a structure, a foundation to work with our patients over time on their prostate cancer-related issues while, at the same time, being cognizant that we need to deal with their other comorbid conditions.
Modes of Communication
Alison Neymark. We find that including parameters for PSA monitoring in our Progress Notes in the electronic health record (EHR) the best way to communicate with other providers. We’ll say, “If PSA gets to this level, please refer back.” We try to make it clear because with the VA being a training facility, it could be a different resident/attending physician team that’s going to see the patient the next time he is in primary care.
Peter Glassman. Yes, we’re very lucky, as Bill talked about earlier and Alison just mentioned. We have the EHR, and Bill may remember this. Before the EHR, we were constantly fishing to find the most relevant notes. If a patient saw a GU practitioner the day before they saw me, I was often asking the patient what was said. Now we can just review the notes.
It’s a double-edged sword though because there are, of course, many notes in a medical record; and you have to look for the specific items. The EHR and documenting the medical record probably plays the primary role in getting information across. When you want to have an active handoff, or you need to communicate with each other, we have a variety of mechanisms, ranging from the phone to the Microsoft Skype Link (Redmond, WA) system that allows us to tap a message to a colleague.
And I’ve been here long enough that I’ve seen most permutations of how prostate cancer is diagnosed as well as shared among providers. Bill and I have shared patients. Alison and I have shared patients, not necessarily with prostate cancer, although that too. But we know how to communicate with each other. And of course, there’s paging if you need something more urgently.
William Aronson. We also use Microsoft Outlook e-mail, and encrypt the messages to keep them confidential and private. The other nice thing we have is there is a nationwide urology Outlook e-mail, so if any of us have any specific questions, through one e-mail we can send it around the country; and there’s usually multiple very useful responses. That’s another real strength of our system within the VA that helps patient care enormously.
Nicholas Nickols. Sometimes, if there’s a critical note that I absolutely want someone on the care team to read, I’ll add them as a cosigner; and that will pop up when they log in to the Computerized Patient Record System (CPRS) as something that they need to read.
If the patient lives particularly far or gets his care at another VA medical center and laboratory tests are needed, then I will reach out to their PCP via e-mail. If contact is not confirmed, I will reach out via phone or Skype.
Peter Glassman. The most helpful notes are those that are very specific as to what primary care is being asked to do and/or what urology is going to be doing. So, the more specific we get in the notes as to what is being addressed, I think that’s very helpful.
I have been here long enough that I’ve known both Alison and Bill; and if they have an issue, they will tap me a message. It wasn’t long ago that Bill sent a message to me, and we worked on a patient with prostate cancer who was going to be on long-term hormone therapy. We talked about osteoporosis management, and between us we worked out who was going to do what. Those are the kind of shared decision-making situations that are very, very helpful.
Alison Neymark. Also, GLAVAHCS has a home-based primary care team (HBPC), and a lot of the PCPs for that team are NPs. They know that they can contact me for their patients because a lot of those patients are on watchful waiting, and we do not necessarily need to see them face to face in clinic. Our urology team just needs to review updated lab results and how they are doing clinically. The HBPC NP who knows them best can contact me every 6 months or so, and we’ll discuss the case, which avoids making the patient come in, especially when they’re homebound. Those of us that have been working at the VA for many years have established good relationships. We feel very comfortable reaching out and talking to each other about these patients
Peter Glassman. Alison, I agree. When I can talk to my patients and say, “You know, we had that question about,” whatever the question might be, “and I contacted urology, and this is what they said.” It gives the patient confidence that we’re following up on the issues that they have and that we’re communicating with each other in a way that is to their benefit. And I think it’s very appreciated both by the provider as well as the patient.
William Aronson. Not infrequently I’ll have patients who have nonurologic issues, which I may first detect, or who have specific issues with their prostate cancer that can be comanaged. And I have found that when I send an encrypted e-mail to the PCP, it has been an extremely satisfying interaction; and we really get to the heart of the matter quickly for the sake of the veteran.
Veterans With Comorbidities
William Aronson. Posttraumatic stress disorder (PTSD) is a very significant and unique aspect of our patients, which is enormously important to recognize. For example, the side effects of prostate treatments can be very significant, whether radiation or surgery. Our patients understandably can be very fearful of the prostate cancer diagnosis and treatment side effects.
We know, for example, after a patient gets a diagnosis of prostate cancer, they’re at increased risk of cardiac death. That’s an especially important issue for our patients that there be an ongoing interaction between urology and primary care.
The ACS guidelines that Dr. Glassman referred to were enlightening. In many cases, primary care can look at the whole patient and their circumstances better than we can and may detect, for example, specific psychological issues that either they can manage or refer to other specialists.
Peter Glassman. One of the things that was highlighted in the ACS guideline is that in any population of men who have this disease, there’s going to be distress, anxiety, and full-fledged depression. Of course, there are psychosocial aspects of prostate cancer, such as sexual activity and intimacy with a partner that we often don’t explore but are probably playing an important role in the overall health of our patients. We need to be mindful of these psychosocial aspects and at least periodically ask them, “How are you doing with this? How are things at home?” And of course, we already use screeners for depression. As the article noted, distress and anxiety and other factors can make somebody’s life less optimal with poorer quality of life.
Dual Care Patients
Alison Neymark. Many patients whether they have Medicare, insurance through their spouse, or Kaiser Permanente through their job, choose to go to both places. The challenge is communicating with the non-VA providers because here at the VA we can communicate easily through Skype, Outlook e-mail, or CPRS, but for dual care patients who’s in charge? I encourage the veterans to choose whom they want to manage their care; we’re always here and happy to treat them, but they need to decide who’s in charge because I don’t want them to get into a situation where the differing opinions lead to a delay in care.
Nicholas Nickols. The communication when the patient is receiving care outside VA, either on a continuous basis or temporarily, is more of a challenge. We obviously can’t rely upon the messaging system, face-to-face contact is difficult, and they may not be able to use e-mail as well. So in those situations, usually a phone call is the best approach. I have found that the outside providers are happy to speak on the phone to coordinate care.
Peter Glassman. I agree, it does add a layer of complexity because we don’t readily have the notes, any information in front of us. That said, a lot of our patients can and do bring in information from outside specialists, and I’m hopeful that they share the information that we provide back to their outside doctors as well.
William Aronson. Some patient get nervous. They might decide they want care elsewhere, but they still want the VA available for them. I always let them know they should proceed in whatever way they prefer, but we’re always available and here for them. I try to empower them to make their own decisions and feel comfortable with them.
Nicholas Nickols. Notes from the outside, if they’re being referred for VA Choice or community care, do get uploaded into VistA Imaging and can be accessed, although it’s not instantaneous. Sometimes there’s a delay, but I have been able to access outside notes most of the time. If a patient goes through a clinic at the VA, the note is written in real time, and you can read it immediately.
Peter Glassman. That is true for patients that are within the VA system who receive contracted care either through Choice or through non-VA care that is contracted through VA. For somebody who is choosing to use 2 health care systems, that can provide more of a challenge because those notes don’t come to us. Over time, most of my patients have brought test results to me.
The thing with oncologic care, of course, is it’s a lot more complex. And it’s hard to know without reasonable documentation what’s been going on. At some level, you have to trust that the outside provider is doing whatever they need to do, or you have to take it upon yourself to do it within the system.
Alison Neymark. In my experience with the Choice Program, it really depends on the outside providers and how comfortable they are with the system that has been established to share records. Not all providers are going into that system and accessing it. I have had cases where I will see the non-VA provider’s note and it’ll say, “No documentation available for this consultation.” It just happens that they didn’t go into the system to review it. So it can be a challenge.
I’ve had good communication with the providers who use the system correctly. In some cases, just to make it easier, I will go ahead and communicate with them through encrypted e-mail, or I’ll talk to their care coordinators directly by phone.
Peter Glassman. Many, if not most, PCPs are going to take care of these patients, certainly within the VA, with their GU colleagues. And most of us feel comfortable using the current documentation system in a way that allows us to share information or at least to gather information about these patients.
One of the things that I think came out for me in looking at this was that there are guidelines or there are ideas out there on how to take better care of these patients. And I for one learned a fair bit just by going through these documents, which I’m very appreciative of. But it does highlight to me that we can give good care and provide good shared care for prostate cancer survivors. I think that is something that perhaps this discussion will highlight that not only are people doing that, but there are resources they can utilize that will help them get a more comprehensive picture of taking care of prostate cancer survivors in the primary care clinic.
The beauty of the VA system as a system is that as these issues come up that might affect the overall health of the veteran with prostate cancer, for example, psychosocial issues, we have many people that can address this that are experts in their area. And one of the great beauties of having an all-encompassing healthcare system is being able to use resources within the system, whether that be for other medical problems or other social or other psychological issues, that we ourselves are not expert in. We can reach out to our other colleagues and ask them for assistance. We have that available to help the patients. It’s really holistic.
We even have integrated medicine where we can help patients, hopefully, get back into a healthy lifestyle, for example, whereas we may not have that expertise or knowledge. We often think of this as sort of a shared decision between GU and primary care. But, in fact, it’s really the responsibility of many, many people of the system at large. We are very lucky to have that.
The following is a lightly edited transcript of a teleconference recorded in July 2018. The teleconference brought together health care providers from the Greater Los Angeles VA Health Care System (GLAVAHCS) to discuss the real-world processes for managing the treatment of patients with prostate cancer as they move between primary and specialist care.
William J. Aronson, MD. We are fortunate in having a superb medical record system at the Department of Veterans Affairs (VA) where we can all communicate with each other through a number of methods. Let’s start our discussion by reviewing an index patient that we see in our practice who has been treated with either radical prostatectomy or radiation therapy. One question to address is: Is there a point when the Urology or Radiation Oncology service can transition the patient’s entire care back to the primary care team? And if so, what would be the optimal way to accomplish this?
Nick, is there some point at which you discharge the patient from the radiation oncology service and give specific directions to primary care, or is it primarily just back to urology in your case?
Nicholas G. Nickols, MD, PhD. I have not discharged any patient from my clinic after definitive prostate cancer treatment. During treatment, patients are seen every week. Subsequently, I see them 6 weeks posttreatment, and then every 4 months for the first year, then every 6 months for the next 4 years, and then yearly after that. Although I never formally discharged a patient from my clinic, you can see based on the frequency of visits, that the patient will see more often than their primary care provider (PCP) toward the beginning. And then, after some years, the patient sees their primary more than they me. So it’s not an immediate hand off but rather a gradual transition. It’s important that the PCP is aware of what to look for especially for the late recurrences, late potential side effects, probably more significantly than the early side effects, how to manage them when appropriate, and when to ask the patient to see our team more frequently in follow-up.
William Aronson. We have a number of patients who travel tremendous distances to see us, and I tend to think that many of our follow-up patients, once things are stabilized with regards to management of their side effects, really could see their primary care doctors if we can give them specific instructions on, for example, when to get a prostate-specific antigen (PSA) test and when to refer back to us.
Alison, can you think of some specific cases where you feel like we’ve successfully done that?
Alison Neymark, MS. For the most part we haven’t discharged people, either. What we have done is transitioned them over to a phone clinic. In our department, we have 4 nurse practitioners (NPs) who each have a half-day of phone clinic where they call patients with their test results. Some of those patients are prostate cancer patients that we have been following for years. We schedule them for a phone call, whether it’s every 3 months, every 6 months or every year, to review the updated PSA level and to just check in with them by phone. It’s a win-win because it’s a really quick phone call to reassure the veteran that the PSA level is being followed, and it frees up an in-person appointment slot for another veteran.
We still have patients that prefer face-to-face visits, even though they know we’re not doing anything except discussing a PSA level with them—they just want that security of seeing our face. Some patients are very nervous, and they don’t necessarily want to be discharged, so to speak, back to primary care. Also, for those patients that travel a long distance to clinic, we offer an appointment in the video chat clinic, with the community-based outpatient clinics in Bakersfield and Santa Maria, California.
PSA Levels
William Aronson. I probably see a patient about every 4 to 6 weeks who has a low PSA after about 10 years and has a long distance to travel and mobility and other problems that make it difficult to come in.
The challenge that I have is, what is that specific guideline to give with regards to the rise in PSA? I think it all depends on the patients prostate cancer clinical features and comorbidities.
Nicholas Nickols. If a patient has been seen by me in follow-up a number of times and there’s really no active issues and there’s a low suspicion of recurrence, then I offer the patient the option of a phone follow-up as an alternative to face to face. Some of them accept that, but I ask that they agree to also see either urology or their PCP face to face. I will also remotely ensure that they’re getting the right laboratory tests, and if not, I’ll put those orders in.
With regard to when to refer a patient back for a suspected recurrence after definitive radiation therapy, there is an accepted definition of biochemical failure called the Phoenix definition, which is an absolute rise in 2 ng/mL of PSA over their posttreatment nadir. Often the posttreatment nadir, especially if they were on hormone therapy, will be close to 0. If the PSA gets to 2, that is a good trigger for a referral back to me and/or urology to discuss restaging and workup for a suspected recurrence.
For patients that are postsurgery and then subsequently get salvage radiation, it is not as clear when a restaging workup should be initiated. Currently, the imaging that is routine care is not very sensitive for detecting PSA in that setting until the PSA is around 0.8 ng/mL, and that’s with the most modern imaging available. Over time that may improve.
William Aronson. The other index patient to think about would be the patient who is on watchful waiting for their prostate cancer, which is to be distinguished from active surveillance. If someone’s on active surveillance, we’re regularly doing prostate biopsies and doing very close monitoring; but we also have patients who have multiple other medical problems, have a limited life expectancy, don’t have aggressive prostate cancer, and it’s extremely reasonable not to do a biopsy in those patients.
Again, those are patients where we do follow the PSA generally every 6 months. And I think there’s also scenarios there where it’s reasonable to refer back to primary care with specific instructions. These, again, are patients who had difficulty getting in to see us or have mobility issues, but it is also a way to limit patient visits if that’s their desire.
Peter Glassman, MBBS, MSc: I’m trained as both a general internist and board certified in hospice and palliative medicine. I currently provide primary care as well as palliative care. I view prostate cancer from the diagnosis through the treatment spectrum as a continuum. It starts with the PCP with an elevated PSA level or if the digital rectal exam has an abnormality, and then the role of the genitourinary (GU) practitioner becomes more significant during the active treatment and diagnostic phases.
Primary care doesn’t disappear, and I think there are 2 major issues that go along with that. First of all, we in primary care, because we take care of patients that often have other comorbidities, need to work with the patient on those comorbidities. Secondly, we need the information shared between the GU and primary care providers so that we can answer questions from our patients and have an understanding of what they’re going through and when.
As time goes on, we go through various phases: We may reach a cure, a quiescent period, active therapy, watchful waiting, or recurrence. Primary care gets involved as time goes on when the disease either becomes quiescent, is just being followed, or is considered cured. Clearly when you have watchful waiting, active treatment, or are in a recurrence, then GU takes the forefront.
I view it as a wave function. Primary care to GU with primary in smaller letters and then primary, if you will, in larger letters, GU becomes a lesser participant unless there is active therapy, watchful waiting or recurrence.
In doing a little bit of research, I found 2 very good and very helpful documents. One is the American Cancer Society (ACS) prostate cancer survivorship care guidelines (Box). And the other is a synopsis of the guidelines. What I liked was that the guidelines focused not only on what should be done for the initial period of prostate cancer, but also for many of the ancillary issues which we often don’t give voice to. The guidelines provide a structure, a foundation to work with our patients over time on their prostate cancer-related issues while, at the same time, being cognizant that we need to deal with their other comorbid conditions.
Modes of Communication
Alison Neymark. We find that including parameters for PSA monitoring in our Progress Notes in the electronic health record (EHR) the best way to communicate with other providers. We’ll say, “If PSA gets to this level, please refer back.” We try to make it clear because with the VA being a training facility, it could be a different resident/attending physician team that’s going to see the patient the next time he is in primary care.
Peter Glassman. Yes, we’re very lucky, as Bill talked about earlier and Alison just mentioned. We have the EHR, and Bill may remember this. Before the EHR, we were constantly fishing to find the most relevant notes. If a patient saw a GU practitioner the day before they saw me, I was often asking the patient what was said. Now we can just review the notes.
It’s a double-edged sword though because there are, of course, many notes in a medical record; and you have to look for the specific items. The EHR and documenting the medical record probably plays the primary role in getting information across. When you want to have an active handoff, or you need to communicate with each other, we have a variety of mechanisms, ranging from the phone to the Microsoft Skype Link (Redmond, WA) system that allows us to tap a message to a colleague.
And I’ve been here long enough that I’ve seen most permutations of how prostate cancer is diagnosed as well as shared among providers. Bill and I have shared patients. Alison and I have shared patients, not necessarily with prostate cancer, although that too. But we know how to communicate with each other. And of course, there’s paging if you need something more urgently.
William Aronson. We also use Microsoft Outlook e-mail, and encrypt the messages to keep them confidential and private. The other nice thing we have is there is a nationwide urology Outlook e-mail, so if any of us have any specific questions, through one e-mail we can send it around the country; and there’s usually multiple very useful responses. That’s another real strength of our system within the VA that helps patient care enormously.
Nicholas Nickols. Sometimes, if there’s a critical note that I absolutely want someone on the care team to read, I’ll add them as a cosigner; and that will pop up when they log in to the Computerized Patient Record System (CPRS) as something that they need to read.
If the patient lives particularly far or gets his care at another VA medical center and laboratory tests are needed, then I will reach out to their PCP via e-mail. If contact is not confirmed, I will reach out via phone or Skype.
Peter Glassman. The most helpful notes are those that are very specific as to what primary care is being asked to do and/or what urology is going to be doing. So, the more specific we get in the notes as to what is being addressed, I think that’s very helpful.
I have been here long enough that I’ve known both Alison and Bill; and if they have an issue, they will tap me a message. It wasn’t long ago that Bill sent a message to me, and we worked on a patient with prostate cancer who was going to be on long-term hormone therapy. We talked about osteoporosis management, and between us we worked out who was going to do what. Those are the kind of shared decision-making situations that are very, very helpful.
Alison Neymark. Also, GLAVAHCS has a home-based primary care team (HBPC), and a lot of the PCPs for that team are NPs. They know that they can contact me for their patients because a lot of those patients are on watchful waiting, and we do not necessarily need to see them face to face in clinic. Our urology team just needs to review updated lab results and how they are doing clinically. The HBPC NP who knows them best can contact me every 6 months or so, and we’ll discuss the case, which avoids making the patient come in, especially when they’re homebound. Those of us that have been working at the VA for many years have established good relationships. We feel very comfortable reaching out and talking to each other about these patients
Peter Glassman. Alison, I agree. When I can talk to my patients and say, “You know, we had that question about,” whatever the question might be, “and I contacted urology, and this is what they said.” It gives the patient confidence that we’re following up on the issues that they have and that we’re communicating with each other in a way that is to their benefit. And I think it’s very appreciated both by the provider as well as the patient.
William Aronson. Not infrequently I’ll have patients who have nonurologic issues, which I may first detect, or who have specific issues with their prostate cancer that can be comanaged. And I have found that when I send an encrypted e-mail to the PCP, it has been an extremely satisfying interaction; and we really get to the heart of the matter quickly for the sake of the veteran.
Veterans With Comorbidities
William Aronson. Posttraumatic stress disorder (PTSD) is a very significant and unique aspect of our patients, which is enormously important to recognize. For example, the side effects of prostate treatments can be very significant, whether radiation or surgery. Our patients understandably can be very fearful of the prostate cancer diagnosis and treatment side effects.
We know, for example, after a patient gets a diagnosis of prostate cancer, they’re at increased risk of cardiac death. That’s an especially important issue for our patients that there be an ongoing interaction between urology and primary care.
The ACS guidelines that Dr. Glassman referred to were enlightening. In many cases, primary care can look at the whole patient and their circumstances better than we can and may detect, for example, specific psychological issues that either they can manage or refer to other specialists.
Peter Glassman. One of the things that was highlighted in the ACS guideline is that in any population of men who have this disease, there’s going to be distress, anxiety, and full-fledged depression. Of course, there are psychosocial aspects of prostate cancer, such as sexual activity and intimacy with a partner that we often don’t explore but are probably playing an important role in the overall health of our patients. We need to be mindful of these psychosocial aspects and at least periodically ask them, “How are you doing with this? How are things at home?” And of course, we already use screeners for depression. As the article noted, distress and anxiety and other factors can make somebody’s life less optimal with poorer quality of life.
Dual Care Patients
Alison Neymark. Many patients whether they have Medicare, insurance through their spouse, or Kaiser Permanente through their job, choose to go to both places. The challenge is communicating with the non-VA providers because here at the VA we can communicate easily through Skype, Outlook e-mail, or CPRS, but for dual care patients who’s in charge? I encourage the veterans to choose whom they want to manage their care; we’re always here and happy to treat them, but they need to decide who’s in charge because I don’t want them to get into a situation where the differing opinions lead to a delay in care.
Nicholas Nickols. The communication when the patient is receiving care outside VA, either on a continuous basis or temporarily, is more of a challenge. We obviously can’t rely upon the messaging system, face-to-face contact is difficult, and they may not be able to use e-mail as well. So in those situations, usually a phone call is the best approach. I have found that the outside providers are happy to speak on the phone to coordinate care.
Peter Glassman. I agree, it does add a layer of complexity because we don’t readily have the notes, any information in front of us. That said, a lot of our patients can and do bring in information from outside specialists, and I’m hopeful that they share the information that we provide back to their outside doctors as well.
William Aronson. Some patient get nervous. They might decide they want care elsewhere, but they still want the VA available for them. I always let them know they should proceed in whatever way they prefer, but we’re always available and here for them. I try to empower them to make their own decisions and feel comfortable with them.
Nicholas Nickols. Notes from the outside, if they’re being referred for VA Choice or community care, do get uploaded into VistA Imaging and can be accessed, although it’s not instantaneous. Sometimes there’s a delay, but I have been able to access outside notes most of the time. If a patient goes through a clinic at the VA, the note is written in real time, and you can read it immediately.
Peter Glassman. That is true for patients that are within the VA system who receive contracted care either through Choice or through non-VA care that is contracted through VA. For somebody who is choosing to use 2 health care systems, that can provide more of a challenge because those notes don’t come to us. Over time, most of my patients have brought test results to me.
The thing with oncologic care, of course, is it’s a lot more complex. And it’s hard to know without reasonable documentation what’s been going on. At some level, you have to trust that the outside provider is doing whatever they need to do, or you have to take it upon yourself to do it within the system.
Alison Neymark. In my experience with the Choice Program, it really depends on the outside providers and how comfortable they are with the system that has been established to share records. Not all providers are going into that system and accessing it. I have had cases where I will see the non-VA provider’s note and it’ll say, “No documentation available for this consultation.” It just happens that they didn’t go into the system to review it. So it can be a challenge.
I’ve had good communication with the providers who use the system correctly. In some cases, just to make it easier, I will go ahead and communicate with them through encrypted e-mail, or I’ll talk to their care coordinators directly by phone.
Peter Glassman. Many, if not most, PCPs are going to take care of these patients, certainly within the VA, with their GU colleagues. And most of us feel comfortable using the current documentation system in a way that allows us to share information or at least to gather information about these patients.
One of the things that I think came out for me in looking at this was that there are guidelines or there are ideas out there on how to take better care of these patients. And I for one learned a fair bit just by going through these documents, which I’m very appreciative of. But it does highlight to me that we can give good care and provide good shared care for prostate cancer survivors. I think that is something that perhaps this discussion will highlight that not only are people doing that, but there are resources they can utilize that will help them get a more comprehensive picture of taking care of prostate cancer survivors in the primary care clinic.
The beauty of the VA system as a system is that as these issues come up that might affect the overall health of the veteran with prostate cancer, for example, psychosocial issues, we have many people that can address this that are experts in their area. And one of the great beauties of having an all-encompassing healthcare system is being able to use resources within the system, whether that be for other medical problems or other social or other psychological issues, that we ourselves are not expert in. We can reach out to our other colleagues and ask them for assistance. We have that available to help the patients. It’s really holistic.
We even have integrated medicine where we can help patients, hopefully, get back into a healthy lifestyle, for example, whereas we may not have that expertise or knowledge. We often think of this as sort of a shared decision between GU and primary care. But, in fact, it’s really the responsibility of many, many people of the system at large. We are very lucky to have that.
Oncologists agree with AI treatment decisions about half the time
When it comes to treatment recommendations for high-risk breast cancer, oncologists agree with a leading artificial intelligence platform about half the time, according to investigators.
In the first study of its kind, involving 10 Chinese oncologists, recommendation concordance with the Watson for Oncology treatment advisory tool (WfO) was generally lower for hormone receptor–positive and metastatic cancers than hormone receptor–negative and nonmetastatic cases, reported Fengrui Xu, MD, of the Academy of Military Medical Sciences in Beijing, and colleagues. Refinement could enable broad use of Watson, not to dictate treatment decisions, but instead to propose alternate treatment approaches and offer point-of-care access to relevant evidence.
“[WfO] is an example of a quantitative oncology clinical decision support that leverages the clinical expertise of oncologists at Memorial Sloan Kettering Cancer Center [MSKCC],” the investigators wrote in JCO Clinical Cancer Informatics. The platform uses machine-learning software to interpret patient scenarios in light from MSKCC training cases, MSKCC treatment guidelines, and more than 300 medical textbooks and journals.
To compare WfO with real-world decision makers, the investigators recruited three chief physicians, four attending physicians, and three fellows to provide treatment recommendations for 1,977 patients with complex breast cancer who were treated at 10 hospitals in China. Participating physicians shared the workload; each evaluated an average of 198 different cases.
On average, oncologists and WfO made the same treatment recommendations 56% of the time. Out of the different types of physicians, fellows were most likely to agree with WfO, based on a 68% concordance rate, compared with 54% for chief physicians and 49% for attending physicians. Including all physicians, concordance was lowest for hormone receptor–positive/HER2-positive disease (48%) and highest for triple-negative cases (71%). Adjuvant and metastatic therapies were also evaluated, with high concordance for adjuvant endocrine (78%) and targeted therapy (100%), compared with moderate concordance for first- (52%) and second-line metastatic therapy (50%). The investigators described concordance results as generally “modest;” however, they noted that such levels are promising.
“This degree of concordance is encouraging because therapeutic decisions in these cases are often difficult as a result of the current limits of medical knowledge for treating complex breast cancers and the presence of local contextual factors that affect physician treatment choices,” the investigators wrote. “It is important to note that nonconcordance does not imply that one treatment is correct for a given patient and another is not, nor does it necessarily diminish the potential value of a decision support system that provides access to supporting evidence and insight into its reasoning process.”
The study was funded by Zefei Jiang. The investigators reported affiliations with IBM Watson Health, Pharmaceutical Manufacturer Institution, Merck, and others.
SOURCE: Xu F et al. JCO Clin Cancer Inform. 2019 Aug 16. doi: 10.1200/CCI.18.00159.
When it comes to treatment recommendations for high-risk breast cancer, oncologists agree with a leading artificial intelligence platform about half the time, according to investigators.
In the first study of its kind, involving 10 Chinese oncologists, recommendation concordance with the Watson for Oncology treatment advisory tool (WfO) was generally lower for hormone receptor–positive and metastatic cancers than hormone receptor–negative and nonmetastatic cases, reported Fengrui Xu, MD, of the Academy of Military Medical Sciences in Beijing, and colleagues. Refinement could enable broad use of Watson, not to dictate treatment decisions, but instead to propose alternate treatment approaches and offer point-of-care access to relevant evidence.
“[WfO] is an example of a quantitative oncology clinical decision support that leverages the clinical expertise of oncologists at Memorial Sloan Kettering Cancer Center [MSKCC],” the investigators wrote in JCO Clinical Cancer Informatics. The platform uses machine-learning software to interpret patient scenarios in light from MSKCC training cases, MSKCC treatment guidelines, and more than 300 medical textbooks and journals.
To compare WfO with real-world decision makers, the investigators recruited three chief physicians, four attending physicians, and three fellows to provide treatment recommendations for 1,977 patients with complex breast cancer who were treated at 10 hospitals in China. Participating physicians shared the workload; each evaluated an average of 198 different cases.
On average, oncologists and WfO made the same treatment recommendations 56% of the time. Out of the different types of physicians, fellows were most likely to agree with WfO, based on a 68% concordance rate, compared with 54% for chief physicians and 49% for attending physicians. Including all physicians, concordance was lowest for hormone receptor–positive/HER2-positive disease (48%) and highest for triple-negative cases (71%). Adjuvant and metastatic therapies were also evaluated, with high concordance for adjuvant endocrine (78%) and targeted therapy (100%), compared with moderate concordance for first- (52%) and second-line metastatic therapy (50%). The investigators described concordance results as generally “modest;” however, they noted that such levels are promising.
“This degree of concordance is encouraging because therapeutic decisions in these cases are often difficult as a result of the current limits of medical knowledge for treating complex breast cancers and the presence of local contextual factors that affect physician treatment choices,” the investigators wrote. “It is important to note that nonconcordance does not imply that one treatment is correct for a given patient and another is not, nor does it necessarily diminish the potential value of a decision support system that provides access to supporting evidence and insight into its reasoning process.”
The study was funded by Zefei Jiang. The investigators reported affiliations with IBM Watson Health, Pharmaceutical Manufacturer Institution, Merck, and others.
SOURCE: Xu F et al. JCO Clin Cancer Inform. 2019 Aug 16. doi: 10.1200/CCI.18.00159.
When it comes to treatment recommendations for high-risk breast cancer, oncologists agree with a leading artificial intelligence platform about half the time, according to investigators.
In the first study of its kind, involving 10 Chinese oncologists, recommendation concordance with the Watson for Oncology treatment advisory tool (WfO) was generally lower for hormone receptor–positive and metastatic cancers than hormone receptor–negative and nonmetastatic cases, reported Fengrui Xu, MD, of the Academy of Military Medical Sciences in Beijing, and colleagues. Refinement could enable broad use of Watson, not to dictate treatment decisions, but instead to propose alternate treatment approaches and offer point-of-care access to relevant evidence.
“[WfO] is an example of a quantitative oncology clinical decision support that leverages the clinical expertise of oncologists at Memorial Sloan Kettering Cancer Center [MSKCC],” the investigators wrote in JCO Clinical Cancer Informatics. The platform uses machine-learning software to interpret patient scenarios in light from MSKCC training cases, MSKCC treatment guidelines, and more than 300 medical textbooks and journals.
To compare WfO with real-world decision makers, the investigators recruited three chief physicians, four attending physicians, and three fellows to provide treatment recommendations for 1,977 patients with complex breast cancer who were treated at 10 hospitals in China. Participating physicians shared the workload; each evaluated an average of 198 different cases.
On average, oncologists and WfO made the same treatment recommendations 56% of the time. Out of the different types of physicians, fellows were most likely to agree with WfO, based on a 68% concordance rate, compared with 54% for chief physicians and 49% for attending physicians. Including all physicians, concordance was lowest for hormone receptor–positive/HER2-positive disease (48%) and highest for triple-negative cases (71%). Adjuvant and metastatic therapies were also evaluated, with high concordance for adjuvant endocrine (78%) and targeted therapy (100%), compared with moderate concordance for first- (52%) and second-line metastatic therapy (50%). The investigators described concordance results as generally “modest;” however, they noted that such levels are promising.
“This degree of concordance is encouraging because therapeutic decisions in these cases are often difficult as a result of the current limits of medical knowledge for treating complex breast cancers and the presence of local contextual factors that affect physician treatment choices,” the investigators wrote. “It is important to note that nonconcordance does not imply that one treatment is correct for a given patient and another is not, nor does it necessarily diminish the potential value of a decision support system that provides access to supporting evidence and insight into its reasoning process.”
The study was funded by Zefei Jiang. The investigators reported affiliations with IBM Watson Health, Pharmaceutical Manufacturer Institution, Merck, and others.
SOURCE: Xu F et al. JCO Clin Cancer Inform. 2019 Aug 16. doi: 10.1200/CCI.18.00159.
FROM JCO CLINICAL CANCER INFORMATICS
Cancer survivors face more age-related deficits
Long-term survivors of cancer have more age-related functional deficits than do those who have not experienced cancer, and these deficits – as well as their cancer history – are both associated with a higher risk of all-cause mortality, a study has found.
A paper published in Cancer reported the outcomes of a population-based cohort study involving 1,723 female cancer survivors and 11,145 cancer-free women enrolled in the Iowa Women’s Health Study, who were followed for 10 years.
The analysis revealed that women with a history of cancer had significantly more deficits on a geriatric assessment compared with their age-matched controls without a history of cancer. While 66% of women without a cancer history had one or more deficits, 70% of those with a history had at least one age-related deficit, and they were significantly more likely to have two or more deficits.
Cancer survivors were significantly more likely to have two or more physical function limitations than were those without a history of cancer (42.4% vs. 36.9%, P less than .0001), to have two or more comorbidities (41.3% vs. 38.2%, P = .02) and to have poor general health (23.3% vs. 17.4%, P less than .0001). They were also significantly less likely to be underweight.
The study found that both cancer history and age-related functional deficits were predictors of mortality, even after adjustment for confounders such as chronological age, smoking, and physical activity levels. The highest mortality risk was seen in cancer survivors with two or more age-related health deficits, who had a twofold greater mortality risk compared with the noncancer controls with fewer than two health deficits.
Even individuals with a history of cancer but without any health deficits still had a 1.3-1.4-fold increased risk of mortality compared with individuals without a history of cancer and without health deficits.
“These results confirm the increased risk of mortality associated with GA domain deficits and extend the research by demonstrating that a cancer history is associated with an older functional age compared with aged-matched cancer-free individuals,” wrote Cindy K. Blair, PhD, of the department of internal medicine at the University of New Mexico, Albuquerque, and coauthors.
They noted that the study included very long-term cancer survivors who had survived for an average of 11 years before they underwent the geriatric assessment and were then followed for 10 years after that point.
“Further research is needed to identify older cancer survivors who are at risk of accelerated aging,” the authors wrote. “Interventions that target physical function, comorbidity, nutritional status, and general health are greatly needed to improve or maintain the quality of survivorship in older cancer survivors.”
The National Cancer Institute, the University of Minnesota Cancer Center, and the University of New Mexico Comprehensive Cancer Center supported the study. Two authors declared grants from the National Institutes of Health related to the study.
SOURCE: Blair C et al. Cancer 2019, Aug 16. doi: 10.1002/cncr.32449.
Long-term survivors of cancer have more age-related functional deficits than do those who have not experienced cancer, and these deficits – as well as their cancer history – are both associated with a higher risk of all-cause mortality, a study has found.
A paper published in Cancer reported the outcomes of a population-based cohort study involving 1,723 female cancer survivors and 11,145 cancer-free women enrolled in the Iowa Women’s Health Study, who were followed for 10 years.
The analysis revealed that women with a history of cancer had significantly more deficits on a geriatric assessment compared with their age-matched controls without a history of cancer. While 66% of women without a cancer history had one or more deficits, 70% of those with a history had at least one age-related deficit, and they were significantly more likely to have two or more deficits.
Cancer survivors were significantly more likely to have two or more physical function limitations than were those without a history of cancer (42.4% vs. 36.9%, P less than .0001), to have two or more comorbidities (41.3% vs. 38.2%, P = .02) and to have poor general health (23.3% vs. 17.4%, P less than .0001). They were also significantly less likely to be underweight.
The study found that both cancer history and age-related functional deficits were predictors of mortality, even after adjustment for confounders such as chronological age, smoking, and physical activity levels. The highest mortality risk was seen in cancer survivors with two or more age-related health deficits, who had a twofold greater mortality risk compared with the noncancer controls with fewer than two health deficits.
Even individuals with a history of cancer but without any health deficits still had a 1.3-1.4-fold increased risk of mortality compared with individuals without a history of cancer and without health deficits.
“These results confirm the increased risk of mortality associated with GA domain deficits and extend the research by demonstrating that a cancer history is associated with an older functional age compared with aged-matched cancer-free individuals,” wrote Cindy K. Blair, PhD, of the department of internal medicine at the University of New Mexico, Albuquerque, and coauthors.
They noted that the study included very long-term cancer survivors who had survived for an average of 11 years before they underwent the geriatric assessment and were then followed for 10 years after that point.
“Further research is needed to identify older cancer survivors who are at risk of accelerated aging,” the authors wrote. “Interventions that target physical function, comorbidity, nutritional status, and general health are greatly needed to improve or maintain the quality of survivorship in older cancer survivors.”
The National Cancer Institute, the University of Minnesota Cancer Center, and the University of New Mexico Comprehensive Cancer Center supported the study. Two authors declared grants from the National Institutes of Health related to the study.
SOURCE: Blair C et al. Cancer 2019, Aug 16. doi: 10.1002/cncr.32449.
Long-term survivors of cancer have more age-related functional deficits than do those who have not experienced cancer, and these deficits – as well as their cancer history – are both associated with a higher risk of all-cause mortality, a study has found.
A paper published in Cancer reported the outcomes of a population-based cohort study involving 1,723 female cancer survivors and 11,145 cancer-free women enrolled in the Iowa Women’s Health Study, who were followed for 10 years.
The analysis revealed that women with a history of cancer had significantly more deficits on a geriatric assessment compared with their age-matched controls without a history of cancer. While 66% of women without a cancer history had one or more deficits, 70% of those with a history had at least one age-related deficit, and they were significantly more likely to have two or more deficits.
Cancer survivors were significantly more likely to have two or more physical function limitations than were those without a history of cancer (42.4% vs. 36.9%, P less than .0001), to have two or more comorbidities (41.3% vs. 38.2%, P = .02) and to have poor general health (23.3% vs. 17.4%, P less than .0001). They were also significantly less likely to be underweight.
The study found that both cancer history and age-related functional deficits were predictors of mortality, even after adjustment for confounders such as chronological age, smoking, and physical activity levels. The highest mortality risk was seen in cancer survivors with two or more age-related health deficits, who had a twofold greater mortality risk compared with the noncancer controls with fewer than two health deficits.
Even individuals with a history of cancer but without any health deficits still had a 1.3-1.4-fold increased risk of mortality compared with individuals without a history of cancer and without health deficits.
“These results confirm the increased risk of mortality associated with GA domain deficits and extend the research by demonstrating that a cancer history is associated with an older functional age compared with aged-matched cancer-free individuals,” wrote Cindy K. Blair, PhD, of the department of internal medicine at the University of New Mexico, Albuquerque, and coauthors.
They noted that the study included very long-term cancer survivors who had survived for an average of 11 years before they underwent the geriatric assessment and were then followed for 10 years after that point.
“Further research is needed to identify older cancer survivors who are at risk of accelerated aging,” the authors wrote. “Interventions that target physical function, comorbidity, nutritional status, and general health are greatly needed to improve or maintain the quality of survivorship in older cancer survivors.”
The National Cancer Institute, the University of Minnesota Cancer Center, and the University of New Mexico Comprehensive Cancer Center supported the study. Two authors declared grants from the National Institutes of Health related to the study.
SOURCE: Blair C et al. Cancer 2019, Aug 16. doi: 10.1002/cncr.32449.
FROM CANCER
Diet, exercise don’t improve breast cancer-related lymphedema
Neither weight-loss nor home-based exercise programs improved outcomes for women with breast cancer–related lymphedema, investigators found.
Among 351 overweight breast cancer survivors with breast cancer–related lymphedema (BCRL), there were no significant differences at 1 year of follow-up in the percentage difference between limb volumes from baseline, regardless of whether patients had been randomly assigned to a home-based exercise program, weight-loss program, combined interventions, or to a facility-based lymphedema care–only program, reported Kathryn H. Schmitz, PhD, MPH, from Penn State University, Hershey, and colleagues.
“Our findings are contradictory to our own clinical experience, as we have received reports from patients with BCRL who have noted improvements in their lymphedema symptoms after weight loss. Possible explanations for this mismatch of clinical experience and empirical evidence include alterations in aspects of lymphedema, such as tissue composition, that remain challenging to measure with high reliability and validity,” they wrote in JAMA Oncology.
The findings suggest that breast cancer survivors with lymphedema may benefit more from facility-based exercise than home-based lymphedema care, the investigators wrote.
In the randomized Women in Steady Exercise Research (WISER) Survivor trial, the investigators enrolled 351 overweight breast cancer survivors with lymphedema and randomly assigned them to a 52-week home-based exercise program consisting of strength and resistance training twice per week and 180 minutes per week of walking (87 patients), a weight-loss program consisting of 20 weeks of meal replacement and 1 year of lifestyle-modification counseling (87 patients), a combination of the two programs (87 patients), or a facility-based lymphedema care program only (90 patients, control group).
The primary endpoint was originally intended to be lymphedema clinical events such as incident flare-ups or cellulitis, but was changed to the percentage of interlimb difference (that is, between the affected and unaffected limb) because of a reduction in funding that led to a reduction in the sample size.
There were no significant between-group differences at either baseline or 12 months in either the percentage of interlimb differences or in absolute differences, the investigators found.
Women assigned to the diet and exercise intervention lost significantly more weight than controls (P less than .001), but saw significant improvements in fitness only in the maximum amount of weight they could lift (P = .01).
“Multiple national organizations currently advise overweight women to achieve and maintain a healthy weight to improve the outcomes of previously diagnosed BCRL. The empirical evidence base, including data from the present study, does not support the assertion that weight loss as an intervention improves the hallmark measure of BCRL severity, percentage of interlimb difference,” Dr. Schmitz and colleagues wrote.
They acknowledged that BCRL is a long-term condition and that the 1-year follow-up period may have been too short to observe lymphedema exacerbation or related clinical events; therefore, the results may not apply to women with severe lymphedema, such as those with interlimb differences of greater than 30%.
The study was supported by various National Institutes of Health grants. Compression garments were supplied by BSN Medical. Dr. Schmitz reported receiving grants from the National Cancer Institute and nonfinancial support from BSN Medical during the conduct of the study, personal fees from Klose Training outside the submitted work, and a licensed patent for a Strength After Breast Cancer course.
SOURCE: Schmitz KH et al. JAMA Oncol. 2019 Aug 15. doi: 10.1001/jamaoncol.2019.2109..
Neither weight-loss nor home-based exercise programs improved outcomes for women with breast cancer–related lymphedema, investigators found.
Among 351 overweight breast cancer survivors with breast cancer–related lymphedema (BCRL), there were no significant differences at 1 year of follow-up in the percentage difference between limb volumes from baseline, regardless of whether patients had been randomly assigned to a home-based exercise program, weight-loss program, combined interventions, or to a facility-based lymphedema care–only program, reported Kathryn H. Schmitz, PhD, MPH, from Penn State University, Hershey, and colleagues.
“Our findings are contradictory to our own clinical experience, as we have received reports from patients with BCRL who have noted improvements in their lymphedema symptoms after weight loss. Possible explanations for this mismatch of clinical experience and empirical evidence include alterations in aspects of lymphedema, such as tissue composition, that remain challenging to measure with high reliability and validity,” they wrote in JAMA Oncology.
The findings suggest that breast cancer survivors with lymphedema may benefit more from facility-based exercise than home-based lymphedema care, the investigators wrote.
In the randomized Women in Steady Exercise Research (WISER) Survivor trial, the investigators enrolled 351 overweight breast cancer survivors with lymphedema and randomly assigned them to a 52-week home-based exercise program consisting of strength and resistance training twice per week and 180 minutes per week of walking (87 patients), a weight-loss program consisting of 20 weeks of meal replacement and 1 year of lifestyle-modification counseling (87 patients), a combination of the two programs (87 patients), or a facility-based lymphedema care program only (90 patients, control group).
The primary endpoint was originally intended to be lymphedema clinical events such as incident flare-ups or cellulitis, but was changed to the percentage of interlimb difference (that is, between the affected and unaffected limb) because of a reduction in funding that led to a reduction in the sample size.
There were no significant between-group differences at either baseline or 12 months in either the percentage of interlimb differences or in absolute differences, the investigators found.
Women assigned to the diet and exercise intervention lost significantly more weight than controls (P less than .001), but saw significant improvements in fitness only in the maximum amount of weight they could lift (P = .01).
“Multiple national organizations currently advise overweight women to achieve and maintain a healthy weight to improve the outcomes of previously diagnosed BCRL. The empirical evidence base, including data from the present study, does not support the assertion that weight loss as an intervention improves the hallmark measure of BCRL severity, percentage of interlimb difference,” Dr. Schmitz and colleagues wrote.
They acknowledged that BCRL is a long-term condition and that the 1-year follow-up period may have been too short to observe lymphedema exacerbation or related clinical events; therefore, the results may not apply to women with severe lymphedema, such as those with interlimb differences of greater than 30%.
The study was supported by various National Institutes of Health grants. Compression garments were supplied by BSN Medical. Dr. Schmitz reported receiving grants from the National Cancer Institute and nonfinancial support from BSN Medical during the conduct of the study, personal fees from Klose Training outside the submitted work, and a licensed patent for a Strength After Breast Cancer course.
SOURCE: Schmitz KH et al. JAMA Oncol. 2019 Aug 15. doi: 10.1001/jamaoncol.2019.2109..
Neither weight-loss nor home-based exercise programs improved outcomes for women with breast cancer–related lymphedema, investigators found.
Among 351 overweight breast cancer survivors with breast cancer–related lymphedema (BCRL), there were no significant differences at 1 year of follow-up in the percentage difference between limb volumes from baseline, regardless of whether patients had been randomly assigned to a home-based exercise program, weight-loss program, combined interventions, or to a facility-based lymphedema care–only program, reported Kathryn H. Schmitz, PhD, MPH, from Penn State University, Hershey, and colleagues.
“Our findings are contradictory to our own clinical experience, as we have received reports from patients with BCRL who have noted improvements in their lymphedema symptoms after weight loss. Possible explanations for this mismatch of clinical experience and empirical evidence include alterations in aspects of lymphedema, such as tissue composition, that remain challenging to measure with high reliability and validity,” they wrote in JAMA Oncology.
The findings suggest that breast cancer survivors with lymphedema may benefit more from facility-based exercise than home-based lymphedema care, the investigators wrote.
In the randomized Women in Steady Exercise Research (WISER) Survivor trial, the investigators enrolled 351 overweight breast cancer survivors with lymphedema and randomly assigned them to a 52-week home-based exercise program consisting of strength and resistance training twice per week and 180 minutes per week of walking (87 patients), a weight-loss program consisting of 20 weeks of meal replacement and 1 year of lifestyle-modification counseling (87 patients), a combination of the two programs (87 patients), or a facility-based lymphedema care program only (90 patients, control group).
The primary endpoint was originally intended to be lymphedema clinical events such as incident flare-ups or cellulitis, but was changed to the percentage of interlimb difference (that is, between the affected and unaffected limb) because of a reduction in funding that led to a reduction in the sample size.
There were no significant between-group differences at either baseline or 12 months in either the percentage of interlimb differences or in absolute differences, the investigators found.
Women assigned to the diet and exercise intervention lost significantly more weight than controls (P less than .001), but saw significant improvements in fitness only in the maximum amount of weight they could lift (P = .01).
“Multiple national organizations currently advise overweight women to achieve and maintain a healthy weight to improve the outcomes of previously diagnosed BCRL. The empirical evidence base, including data from the present study, does not support the assertion that weight loss as an intervention improves the hallmark measure of BCRL severity, percentage of interlimb difference,” Dr. Schmitz and colleagues wrote.
They acknowledged that BCRL is a long-term condition and that the 1-year follow-up period may have been too short to observe lymphedema exacerbation or related clinical events; therefore, the results may not apply to women with severe lymphedema, such as those with interlimb differences of greater than 30%.
The study was supported by various National Institutes of Health grants. Compression garments were supplied by BSN Medical. Dr. Schmitz reported receiving grants from the National Cancer Institute and nonfinancial support from BSN Medical during the conduct of the study, personal fees from Klose Training outside the submitted work, and a licensed patent for a Strength After Breast Cancer course.
SOURCE: Schmitz KH et al. JAMA Oncol. 2019 Aug 15. doi: 10.1001/jamaoncol.2019.2109..
FROM JAMA ONCOLOGY
Recurrence score may help predict chemotherapy benefit in grade 3 breast cancers
For patients with grade 3 breast cancer, recurrence score testing may have significant clinical value in determining which patients are likely to benefit from chemotherapy, according to investigators who recently reported results of a large, national cohort study.
Among patients with pN0/1 grade 3 invasive breast cancers, about one-third had a low recurrence score, which was associated with no early benefit from the addition of chemotherapy, wrote senior study author Jane E. Brock, MBBS, PhD, of Harvard Medical School, Boston, and coauthors.
Incorporating recurrence score testing into clinical decision making may help “tailor treatment recommendations” for patients with grade 3 invasive breast cancers, Dr. Brock and coauthors reported in JCO Precision Oncology.
“To our knowledge, these findings represent the largest analysis to date of the potential impact of recurrence score on the outcomes and management of grade 3 tumors, and suggest that the assumption that all pT1c/2 pN0/1, [estrogen receptor–positive] histopathologic grade 3 tumors are high risk and will consequently benefit from adjuvant chemotherapy may be unmerited,” the Dr. Brock and coauthors wrote in the report.
These findings “fill a gap” as the final results of the RxPONDER trial are awaited, according to investigators. Specifically, RxPONDER is designed to evaluate the potential benefit of adjuvant chemotherapy in pN0/1 patients with intermediate range recurrence scores.
Moreover, the findings complement the reported results of the TAILORx trial, which showed that chemotherapy does not provide a benefit in most low and intermediate recurrence score tumors, which suggests some patients may safely omit chemotherapy without affecting outcomes, the investigators added.
The present analysis included a total of 30,864 grade 3 breast cancers from the National Cancer Database, which represents more than 70% of new diagnoses in the United States, according to investigators.
Testing using the 21-gene Oncotype DX Breast Recurrence Score increased over time for pN0 cancers, from 53% in 2010 to 72% in 2015, investigators found; likewise, for pN1 cancers, testing increased from 16% to 36% over that time period. They also found that, overall, 30% of pN0 and 27.1% of pN1 cancers had a low recurrence score.
Adjuvant chemotherapy was not associated with any additional benefit in patients with low recurrence scores, according to the analysis.
For patients with intermediate recurrence scores, chemotherapy was linked to improved survival in univariable analyses, but following adjustment for clinical and pathologic characteristics, intermediate scores were not predictive of a significant overall survival benefit, investigators found.
By contrast, chemotherapy was associated with additional benefit in patients with high recurrence scores in both univariable and multivariable analyses.
For patients with pN0 grade 3 disease and high recurrence score, chemotherapy was linked to significantly improved overall survival, compared with that of patients who received no chemotherapy (hazard ratio, 0.63; 95% confidence interval, 0.43-0.90; P = .01), while a similar survival benefit was reported among patients with pN1 disease and high recurrence scores (HR, 0.24; 95% CI, 0.13-0.47; P less than .001).
These results suggest that recurrence score may aid in determining the anticipated benefit of chemotherapy in this heterogeneous cohort of patients, investigators wrote.
“Furthermore, our findings show significant variability in national patterns of recurrence testing and chemotherapy use for grade 3 disease, which suggests opportunities for more comprehensive national guidelines for recurrence score testing in high-grade tumors,” they concluded.
Dr. Brock reported no potential conflicts of interest. Coauthors provided disclosures related to AstraZeneca, Blade Therapeutics, Eisai, EMD Serono, Galena Biopharma, Genentech, Genomic Health, Novartis, Novartis Institutes for BioMedical Research, Peregrine, Puma Biotechnology, resTORbio, Roche, and others.
SOURCE: Brock JE et al. JCO Precis Oncol. 2019 Aug 14. doi: 10.1200/PO.19.00029.
For patients with grade 3 breast cancer, recurrence score testing may have significant clinical value in determining which patients are likely to benefit from chemotherapy, according to investigators who recently reported results of a large, national cohort study.
Among patients with pN0/1 grade 3 invasive breast cancers, about one-third had a low recurrence score, which was associated with no early benefit from the addition of chemotherapy, wrote senior study author Jane E. Brock, MBBS, PhD, of Harvard Medical School, Boston, and coauthors.
Incorporating recurrence score testing into clinical decision making may help “tailor treatment recommendations” for patients with grade 3 invasive breast cancers, Dr. Brock and coauthors reported in JCO Precision Oncology.
“To our knowledge, these findings represent the largest analysis to date of the potential impact of recurrence score on the outcomes and management of grade 3 tumors, and suggest that the assumption that all pT1c/2 pN0/1, [estrogen receptor–positive] histopathologic grade 3 tumors are high risk and will consequently benefit from adjuvant chemotherapy may be unmerited,” the Dr. Brock and coauthors wrote in the report.
These findings “fill a gap” as the final results of the RxPONDER trial are awaited, according to investigators. Specifically, RxPONDER is designed to evaluate the potential benefit of adjuvant chemotherapy in pN0/1 patients with intermediate range recurrence scores.
Moreover, the findings complement the reported results of the TAILORx trial, which showed that chemotherapy does not provide a benefit in most low and intermediate recurrence score tumors, which suggests some patients may safely omit chemotherapy without affecting outcomes, the investigators added.
The present analysis included a total of 30,864 grade 3 breast cancers from the National Cancer Database, which represents more than 70% of new diagnoses in the United States, according to investigators.
Testing using the 21-gene Oncotype DX Breast Recurrence Score increased over time for pN0 cancers, from 53% in 2010 to 72% in 2015, investigators found; likewise, for pN1 cancers, testing increased from 16% to 36% over that time period. They also found that, overall, 30% of pN0 and 27.1% of pN1 cancers had a low recurrence score.
Adjuvant chemotherapy was not associated with any additional benefit in patients with low recurrence scores, according to the analysis.
For patients with intermediate recurrence scores, chemotherapy was linked to improved survival in univariable analyses, but following adjustment for clinical and pathologic characteristics, intermediate scores were not predictive of a significant overall survival benefit, investigators found.
By contrast, chemotherapy was associated with additional benefit in patients with high recurrence scores in both univariable and multivariable analyses.
For patients with pN0 grade 3 disease and high recurrence score, chemotherapy was linked to significantly improved overall survival, compared with that of patients who received no chemotherapy (hazard ratio, 0.63; 95% confidence interval, 0.43-0.90; P = .01), while a similar survival benefit was reported among patients with pN1 disease and high recurrence scores (HR, 0.24; 95% CI, 0.13-0.47; P less than .001).
These results suggest that recurrence score may aid in determining the anticipated benefit of chemotherapy in this heterogeneous cohort of patients, investigators wrote.
“Furthermore, our findings show significant variability in national patterns of recurrence testing and chemotherapy use for grade 3 disease, which suggests opportunities for more comprehensive national guidelines for recurrence score testing in high-grade tumors,” they concluded.
Dr. Brock reported no potential conflicts of interest. Coauthors provided disclosures related to AstraZeneca, Blade Therapeutics, Eisai, EMD Serono, Galena Biopharma, Genentech, Genomic Health, Novartis, Novartis Institutes for BioMedical Research, Peregrine, Puma Biotechnology, resTORbio, Roche, and others.
SOURCE: Brock JE et al. JCO Precis Oncol. 2019 Aug 14. doi: 10.1200/PO.19.00029.
For patients with grade 3 breast cancer, recurrence score testing may have significant clinical value in determining which patients are likely to benefit from chemotherapy, according to investigators who recently reported results of a large, national cohort study.
Among patients with pN0/1 grade 3 invasive breast cancers, about one-third had a low recurrence score, which was associated with no early benefit from the addition of chemotherapy, wrote senior study author Jane E. Brock, MBBS, PhD, of Harvard Medical School, Boston, and coauthors.
Incorporating recurrence score testing into clinical decision making may help “tailor treatment recommendations” for patients with grade 3 invasive breast cancers, Dr. Brock and coauthors reported in JCO Precision Oncology.
“To our knowledge, these findings represent the largest analysis to date of the potential impact of recurrence score on the outcomes and management of grade 3 tumors, and suggest that the assumption that all pT1c/2 pN0/1, [estrogen receptor–positive] histopathologic grade 3 tumors are high risk and will consequently benefit from adjuvant chemotherapy may be unmerited,” the Dr. Brock and coauthors wrote in the report.
These findings “fill a gap” as the final results of the RxPONDER trial are awaited, according to investigators. Specifically, RxPONDER is designed to evaluate the potential benefit of adjuvant chemotherapy in pN0/1 patients with intermediate range recurrence scores.
Moreover, the findings complement the reported results of the TAILORx trial, which showed that chemotherapy does not provide a benefit in most low and intermediate recurrence score tumors, which suggests some patients may safely omit chemotherapy without affecting outcomes, the investigators added.
The present analysis included a total of 30,864 grade 3 breast cancers from the National Cancer Database, which represents more than 70% of new diagnoses in the United States, according to investigators.
Testing using the 21-gene Oncotype DX Breast Recurrence Score increased over time for pN0 cancers, from 53% in 2010 to 72% in 2015, investigators found; likewise, for pN1 cancers, testing increased from 16% to 36% over that time period. They also found that, overall, 30% of pN0 and 27.1% of pN1 cancers had a low recurrence score.
Adjuvant chemotherapy was not associated with any additional benefit in patients with low recurrence scores, according to the analysis.
For patients with intermediate recurrence scores, chemotherapy was linked to improved survival in univariable analyses, but following adjustment for clinical and pathologic characteristics, intermediate scores were not predictive of a significant overall survival benefit, investigators found.
By contrast, chemotherapy was associated with additional benefit in patients with high recurrence scores in both univariable and multivariable analyses.
For patients with pN0 grade 3 disease and high recurrence score, chemotherapy was linked to significantly improved overall survival, compared with that of patients who received no chemotherapy (hazard ratio, 0.63; 95% confidence interval, 0.43-0.90; P = .01), while a similar survival benefit was reported among patients with pN1 disease and high recurrence scores (HR, 0.24; 95% CI, 0.13-0.47; P less than .001).
These results suggest that recurrence score may aid in determining the anticipated benefit of chemotherapy in this heterogeneous cohort of patients, investigators wrote.
“Furthermore, our findings show significant variability in national patterns of recurrence testing and chemotherapy use for grade 3 disease, which suggests opportunities for more comprehensive national guidelines for recurrence score testing in high-grade tumors,” they concluded.
Dr. Brock reported no potential conflicts of interest. Coauthors provided disclosures related to AstraZeneca, Blade Therapeutics, Eisai, EMD Serono, Galena Biopharma, Genentech, Genomic Health, Novartis, Novartis Institutes for BioMedical Research, Peregrine, Puma Biotechnology, resTORbio, Roche, and others.
SOURCE: Brock JE et al. JCO Precis Oncol. 2019 Aug 14. doi: 10.1200/PO.19.00029.
FROM JCO PRECISION ONCOLOGY
USPSTF expands BRCA1/2 testing recommendations
The U.S. Preventive Services Task Force (USPSTF) has updated its recommendations on assessment of breast cancer susceptibility gene (BRCA)-related cancer, substantially expanding the pool of individuals for whom risk assessment, testing, and counseling would be warranted.
In its 2013 recommendation, the USPSTF said referral for genetic counseling and evaluation for BRCA1/2 testing was warranted for women who had a family history linked to increased risk of potentially harmful BRCA1/2 mutations.
The updated recommendations, just published in JAMA, expand the screening-eligible population to include those with personal cancer history, and more specifically call out ancestry linked to BRCA1/2 mutations as a risk factor (JAMA. 2019;322[7]:652-65. doi: 10.1001/jama.2019.10987).
“The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University, and coauthors of the task force report.
Positive results on the risk assessment tool should prompt genetic counseling, and genetic testing if indicated after counseling, the USPSTF added in its statement.
By contrast, the task force recommends against routine assessment, counseling, and testing in women with no family history, personal history, or ancestry linked to possibly harmful BRCA1/2 gene mutations, consistent with their previous recommendation.
Mutations of BRCA1/2 genes occur in an estimated 1 in 300-500 women in the general population, and account for 15% of ovarian cancer and up to 10% of breast cancer cases, according to the USPSTF.
Breast cancer risk is increased up to 65% by 70 years in those women with clinically significant BRCA1/2 mutations, while risk of ovarian, fallopian tube, or peritoneal cancer are increased by up to 39%, according to studies cited by the USPSTF.
Important step forward
Including women with prior breast and ovarian cancer in the screening-eligible population is an “important step forward,” Susan Domcheck, MD, and Mark Robson, MD, said in a related editorial.
“While further expansion of the USPSTF recommendation should be considered, the importance is clear: Identification of individuals at risk of carrying a BRCA1/2 mutation can be lifesaving and should be a part of routine medical care,” Dr. Domcheck and Dr. Robson said in their editorial, which appears in JAMA.
While the updated recommendations explicitly call out ancestry as a risk factor, they stop short of endorsing testing for unaffected Ashkenazi Jewish women with no family history, the authors said.
“However, the statement may be interpreted as a step toward supporting unselected testing in this group,” they added.
Among unselected individuals of Ashkenazi Jewish descent, 1 in 40 have 1 of 3 specific BRCA1 or BRCA2 founder mutations, according to one study cited by Dr. Domcheck and Dr. Robson.
More research needed
Current research is still “limited or lacking” to address many key questions about the benefits and harms of risk assessment, genetic counseling, and genetic testing in women without BRCA1/2-related cancer, according to authors of a literature review used by the USPSTF.
Notably, the ability of risk assessment, testing, and counseling to reduce cancer incidence and mortality among such women has not been directly evaluated by studies to date, said the review authors, led by Heidi D. Nelson, MD, MPH, of Oregon Health & Science University, Portland.
“Without effectiveness trials of intensive screening, practice standards have preceded supporting evidence,” said Dr. Nelson and coauthors noted in a report on the review findings.
In observational studies, mastectomy and oophorectomy have been associated with substantial reductions in subsequent cancer incidence and mortality; however, they are invasive procedures with potential complications, the authors noted.
“To determine the appropriateness of risk assessment and genetic testing for BRCA1/2 mutations as a preventive service in primary care, more information is needed about mutation prevalence and the effect of testing in the general population,” they added.
Researchers studying BRCA1/2 assessment as preventive service in primary care have generally looked at highly selected patient populations in referral centers, and have reported relatively short-term outcomes, they said.
Research is additionally needed on access to genetic testing and follow-up, effectiveness of risk stratification and multigene panels, and the impact of direct-to-consumer genetic testing, among other key questions, the authors of the review added.
Treatment implications
While the USPSTF recommendations do not mention systemic therapy, finding a BRCA mutation in a cancer patient today has important implications for treatment, said Rachel L. Yung, MD, and Larissa A. Korde, MD, MPH
Specifically, poly (ADP-ribose) polymerase (PARP) inhibitors have proved effective in certain BRCA-related cancers, Dr. Yung and Dr. Korde said in an editorial on the updated recommendations appearing in JAMA Oncology.
The Food and Drug Administration has already approved several PARP inhibitors for treatment of BRCA-linked metastatic breast or ovarian cancers, and studies are underway for other tumor types, including prostate and pancreatic cancers that harbor a BRCA mutation.
“Increasing awareness of BRCA mutation as a target for treatment will likely lead to an increase in the identification of patients with cancer harboring germline BRCA mutations, which in turn will increase the need for cascade testing for relatives of affected probands,” wrote Dr. Yung and Dr. Korde.
Addressing disparities in care
The USPSTF recommendations for BRCA risk assessment do not address disparities in testing referral and variation in breast cancer phenotypes among women of African ancestry, owing to lack of evidence, according to Lisa Newman, MD, MPH, of the Interdisciplinary Breast Program at New York–Presbyterian/Weill Cornell Medical Center, New York.
“Paradoxically, the data-driven basis for the USPSTF recommendation statement may magnify existing genetic testing disparities,” Dr. Newman wrote in an editorial that appears in JAMA Surgery.
Non-Hispanic black women in the United States have a twofold higher incidence of triple-negative breast cancer, which is a well documented risk factor for BRCA1 mutation carrier status, according to Dr. Newman.
Despite this, she added, genetic counseling and testing referrals remain “disproportionately low” among U.S. patients of African ancestry.
“It remains imperative for clinicians to exercise clinical judgment and to be mindful of patient subsets that do not necessarily fit into recommendations designed for the majority or general populations,” Dr. Newman concluded in her editorial.
The USPSTF is funded by the Agency for Healthcare Research and Quality. Members of the task force receive travel reimbursement and honoraria for participating in USPSTF meetings.
The U.S. Preventive Services Task Force (USPSTF) has updated its recommendations on assessment of breast cancer susceptibility gene (BRCA)-related cancer, substantially expanding the pool of individuals for whom risk assessment, testing, and counseling would be warranted.
In its 2013 recommendation, the USPSTF said referral for genetic counseling and evaluation for BRCA1/2 testing was warranted for women who had a family history linked to increased risk of potentially harmful BRCA1/2 mutations.
The updated recommendations, just published in JAMA, expand the screening-eligible population to include those with personal cancer history, and more specifically call out ancestry linked to BRCA1/2 mutations as a risk factor (JAMA. 2019;322[7]:652-65. doi: 10.1001/jama.2019.10987).
“The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University, and coauthors of the task force report.
Positive results on the risk assessment tool should prompt genetic counseling, and genetic testing if indicated after counseling, the USPSTF added in its statement.
By contrast, the task force recommends against routine assessment, counseling, and testing in women with no family history, personal history, or ancestry linked to possibly harmful BRCA1/2 gene mutations, consistent with their previous recommendation.
Mutations of BRCA1/2 genes occur in an estimated 1 in 300-500 women in the general population, and account for 15% of ovarian cancer and up to 10% of breast cancer cases, according to the USPSTF.
Breast cancer risk is increased up to 65% by 70 years in those women with clinically significant BRCA1/2 mutations, while risk of ovarian, fallopian tube, or peritoneal cancer are increased by up to 39%, according to studies cited by the USPSTF.
Important step forward
Including women with prior breast and ovarian cancer in the screening-eligible population is an “important step forward,” Susan Domcheck, MD, and Mark Robson, MD, said in a related editorial.
“While further expansion of the USPSTF recommendation should be considered, the importance is clear: Identification of individuals at risk of carrying a BRCA1/2 mutation can be lifesaving and should be a part of routine medical care,” Dr. Domcheck and Dr. Robson said in their editorial, which appears in JAMA.
While the updated recommendations explicitly call out ancestry as a risk factor, they stop short of endorsing testing for unaffected Ashkenazi Jewish women with no family history, the authors said.
“However, the statement may be interpreted as a step toward supporting unselected testing in this group,” they added.
Among unselected individuals of Ashkenazi Jewish descent, 1 in 40 have 1 of 3 specific BRCA1 or BRCA2 founder mutations, according to one study cited by Dr. Domcheck and Dr. Robson.
More research needed
Current research is still “limited or lacking” to address many key questions about the benefits and harms of risk assessment, genetic counseling, and genetic testing in women without BRCA1/2-related cancer, according to authors of a literature review used by the USPSTF.
Notably, the ability of risk assessment, testing, and counseling to reduce cancer incidence and mortality among such women has not been directly evaluated by studies to date, said the review authors, led by Heidi D. Nelson, MD, MPH, of Oregon Health & Science University, Portland.
“Without effectiveness trials of intensive screening, practice standards have preceded supporting evidence,” said Dr. Nelson and coauthors noted in a report on the review findings.
In observational studies, mastectomy and oophorectomy have been associated with substantial reductions in subsequent cancer incidence and mortality; however, they are invasive procedures with potential complications, the authors noted.
“To determine the appropriateness of risk assessment and genetic testing for BRCA1/2 mutations as a preventive service in primary care, more information is needed about mutation prevalence and the effect of testing in the general population,” they added.
Researchers studying BRCA1/2 assessment as preventive service in primary care have generally looked at highly selected patient populations in referral centers, and have reported relatively short-term outcomes, they said.
Research is additionally needed on access to genetic testing and follow-up, effectiveness of risk stratification and multigene panels, and the impact of direct-to-consumer genetic testing, among other key questions, the authors of the review added.
Treatment implications
While the USPSTF recommendations do not mention systemic therapy, finding a BRCA mutation in a cancer patient today has important implications for treatment, said Rachel L. Yung, MD, and Larissa A. Korde, MD, MPH
Specifically, poly (ADP-ribose) polymerase (PARP) inhibitors have proved effective in certain BRCA-related cancers, Dr. Yung and Dr. Korde said in an editorial on the updated recommendations appearing in JAMA Oncology.
The Food and Drug Administration has already approved several PARP inhibitors for treatment of BRCA-linked metastatic breast or ovarian cancers, and studies are underway for other tumor types, including prostate and pancreatic cancers that harbor a BRCA mutation.
“Increasing awareness of BRCA mutation as a target for treatment will likely lead to an increase in the identification of patients with cancer harboring germline BRCA mutations, which in turn will increase the need for cascade testing for relatives of affected probands,” wrote Dr. Yung and Dr. Korde.
Addressing disparities in care
The USPSTF recommendations for BRCA risk assessment do not address disparities in testing referral and variation in breast cancer phenotypes among women of African ancestry, owing to lack of evidence, according to Lisa Newman, MD, MPH, of the Interdisciplinary Breast Program at New York–Presbyterian/Weill Cornell Medical Center, New York.
“Paradoxically, the data-driven basis for the USPSTF recommendation statement may magnify existing genetic testing disparities,” Dr. Newman wrote in an editorial that appears in JAMA Surgery.
Non-Hispanic black women in the United States have a twofold higher incidence of triple-negative breast cancer, which is a well documented risk factor for BRCA1 mutation carrier status, according to Dr. Newman.
Despite this, she added, genetic counseling and testing referrals remain “disproportionately low” among U.S. patients of African ancestry.
“It remains imperative for clinicians to exercise clinical judgment and to be mindful of patient subsets that do not necessarily fit into recommendations designed for the majority or general populations,” Dr. Newman concluded in her editorial.
The USPSTF is funded by the Agency for Healthcare Research and Quality. Members of the task force receive travel reimbursement and honoraria for participating in USPSTF meetings.
The U.S. Preventive Services Task Force (USPSTF) has updated its recommendations on assessment of breast cancer susceptibility gene (BRCA)-related cancer, substantially expanding the pool of individuals for whom risk assessment, testing, and counseling would be warranted.
In its 2013 recommendation, the USPSTF said referral for genetic counseling and evaluation for BRCA1/2 testing was warranted for women who had a family history linked to increased risk of potentially harmful BRCA1/2 mutations.
The updated recommendations, just published in JAMA, expand the screening-eligible population to include those with personal cancer history, and more specifically call out ancestry linked to BRCA1/2 mutations as a risk factor (JAMA. 2019;322[7]:652-65. doi: 10.1001/jama.2019.10987).
“The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University, and coauthors of the task force report.
Positive results on the risk assessment tool should prompt genetic counseling, and genetic testing if indicated after counseling, the USPSTF added in its statement.
By contrast, the task force recommends against routine assessment, counseling, and testing in women with no family history, personal history, or ancestry linked to possibly harmful BRCA1/2 gene mutations, consistent with their previous recommendation.
Mutations of BRCA1/2 genes occur in an estimated 1 in 300-500 women in the general population, and account for 15% of ovarian cancer and up to 10% of breast cancer cases, according to the USPSTF.
Breast cancer risk is increased up to 65% by 70 years in those women with clinically significant BRCA1/2 mutations, while risk of ovarian, fallopian tube, or peritoneal cancer are increased by up to 39%, according to studies cited by the USPSTF.
Important step forward
Including women with prior breast and ovarian cancer in the screening-eligible population is an “important step forward,” Susan Domcheck, MD, and Mark Robson, MD, said in a related editorial.
“While further expansion of the USPSTF recommendation should be considered, the importance is clear: Identification of individuals at risk of carrying a BRCA1/2 mutation can be lifesaving and should be a part of routine medical care,” Dr. Domcheck and Dr. Robson said in their editorial, which appears in JAMA.
While the updated recommendations explicitly call out ancestry as a risk factor, they stop short of endorsing testing for unaffected Ashkenazi Jewish women with no family history, the authors said.
“However, the statement may be interpreted as a step toward supporting unselected testing in this group,” they added.
Among unselected individuals of Ashkenazi Jewish descent, 1 in 40 have 1 of 3 specific BRCA1 or BRCA2 founder mutations, according to one study cited by Dr. Domcheck and Dr. Robson.
More research needed
Current research is still “limited or lacking” to address many key questions about the benefits and harms of risk assessment, genetic counseling, and genetic testing in women without BRCA1/2-related cancer, according to authors of a literature review used by the USPSTF.
Notably, the ability of risk assessment, testing, and counseling to reduce cancer incidence and mortality among such women has not been directly evaluated by studies to date, said the review authors, led by Heidi D. Nelson, MD, MPH, of Oregon Health & Science University, Portland.
“Without effectiveness trials of intensive screening, practice standards have preceded supporting evidence,” said Dr. Nelson and coauthors noted in a report on the review findings.
In observational studies, mastectomy and oophorectomy have been associated with substantial reductions in subsequent cancer incidence and mortality; however, they are invasive procedures with potential complications, the authors noted.
“To determine the appropriateness of risk assessment and genetic testing for BRCA1/2 mutations as a preventive service in primary care, more information is needed about mutation prevalence and the effect of testing in the general population,” they added.
Researchers studying BRCA1/2 assessment as preventive service in primary care have generally looked at highly selected patient populations in referral centers, and have reported relatively short-term outcomes, they said.
Research is additionally needed on access to genetic testing and follow-up, effectiveness of risk stratification and multigene panels, and the impact of direct-to-consumer genetic testing, among other key questions, the authors of the review added.
Treatment implications
While the USPSTF recommendations do not mention systemic therapy, finding a BRCA mutation in a cancer patient today has important implications for treatment, said Rachel L. Yung, MD, and Larissa A. Korde, MD, MPH
Specifically, poly (ADP-ribose) polymerase (PARP) inhibitors have proved effective in certain BRCA-related cancers, Dr. Yung and Dr. Korde said in an editorial on the updated recommendations appearing in JAMA Oncology.
The Food and Drug Administration has already approved several PARP inhibitors for treatment of BRCA-linked metastatic breast or ovarian cancers, and studies are underway for other tumor types, including prostate and pancreatic cancers that harbor a BRCA mutation.
“Increasing awareness of BRCA mutation as a target for treatment will likely lead to an increase in the identification of patients with cancer harboring germline BRCA mutations, which in turn will increase the need for cascade testing for relatives of affected probands,” wrote Dr. Yung and Dr. Korde.
Addressing disparities in care
The USPSTF recommendations for BRCA risk assessment do not address disparities in testing referral and variation in breast cancer phenotypes among women of African ancestry, owing to lack of evidence, according to Lisa Newman, MD, MPH, of the Interdisciplinary Breast Program at New York–Presbyterian/Weill Cornell Medical Center, New York.
“Paradoxically, the data-driven basis for the USPSTF recommendation statement may magnify existing genetic testing disparities,” Dr. Newman wrote in an editorial that appears in JAMA Surgery.
Non-Hispanic black women in the United States have a twofold higher incidence of triple-negative breast cancer, which is a well documented risk factor for BRCA1 mutation carrier status, according to Dr. Newman.
Despite this, she added, genetic counseling and testing referrals remain “disproportionately low” among U.S. patients of African ancestry.
“It remains imperative for clinicians to exercise clinical judgment and to be mindful of patient subsets that do not necessarily fit into recommendations designed for the majority or general populations,” Dr. Newman concluded in her editorial.
The USPSTF is funded by the Agency for Healthcare Research and Quality. Members of the task force receive travel reimbursement and honoraria for participating in USPSTF meetings.
FROM JAMA
Immune Checkpoint Inhibitors for Urothelial Cancer: An Update on New Therapies (FULL)
An essential feature of cancer is its ability to evade the immune system. Multiple mechanisms are used for this purpose, including the disruption of antigen presentation and suppression of the immune response. The latter mechanism involves the activation of T-cell inhibition by recruiting regulatory T cells that weaken this response. Recent progress in understanding the ability of cancer to evade the immune system has paved the way to develop strategies to reverse this process and reactivate the immune system. Particularly, immune checkpoint signaling between T cells and tumor cells has been targeted with a new class of drug, immune checkpoint inhibitors. Immunotherapy has been an established and effective treatment in bladder cancer since 1976 when Morales and colleagues demonstrated that intravesical treatments with bacillus Calmette-Guérin can treat carcinoma in situ and prevent nonmuscle invasive urothelial cancer recurrence.1,2 This treatment elicits a cytotoxic response via antigenic presentation by bladder tumor cells.
Cytotoxic T-lymphocyte-associated protein (CTLA)-4, programmed death-1 (PD-1) and programmed death-ligand-1 (PD-L1) are molecules that downregulate the immune response and are targets of therapeutic antibodies that have demonstrated clinical efficacy across a wide range of malignancies. Five such agents—pembrolizumab, atezolizumab, nivolumab, avelumab and durvalumab—were recently approved by the US Food and Drug Administration (FDA) for clinical use in patients with advanced urothelial cancers.3 This class of agents also has been approved for several other malignancies, most notably in melanoma, non-small cell lung cancer, and renal cell carcinoma.3
Immune Biology
CTLA-4 is expressed on activated CD4 and CD8 T cells and competes with CD28 on T cells to interact with the costimulatory B7 proteins on antigen presenting cells. The CD28/B7 interaction promotes T-cell activation and effector functions, and the CTLA-4/B7 interaction inhibits them. In addition, PD-1 is a receptor expressed on CD4 and CD8 T cells, T regulatory (Treg) cells, B cells and natural killer (NK) cells that interacts with its ligand PD-L1 to suppress the immune response. Urothelial cancer possesses features that make it an adequate target for immunotherapeutic agents. Primarily, it is characterized by a high-mutation load, which lends itself to an increased expression of immunogenic antigens on tumor cells.4
Immunotherapy Treatments in Cisplatin-Ineligible Patients
Cisplatin-based chemotherapy is the first-line treatment and standard of care in unresectable or metastatic urothelial cancer. However, many patients are unable to receive cisplatin secondary to renal dysfunction, poor performance status, or other comorbidities. Alternative cytotoxic therapies in the first-line setting such as carboplatin-based regimens are associated with inferior outcomes and poor tolerability. There is, therefore, a need for effective and well-tolerated therapies in cisplatin-ineligible patients (Table).
In the phase 2 Keynote-052 trial, 370 cisplatin-ineligible patients were treated with the anti-PD-1 antibody pembrolizumab 200 mg every 3 weeks for up to 2 years.5At a median follow-up of 9.5 months, the objective response rate (+ORR) was 29% for the entire cohort, with a 7% complete response (CR) rate, and a 22% partial response (PR) rate.5 The median duration of response had not been reached at the time of analysis. Responses were seen regardless of PD-L1 expression, although high response rates were noted in patients whose tumors had PD-L1 expression > 10%. Pembrolizumab had an acceptable tolerability profile in this population. The most common grade 3 or 4 treatment-related adverse event (AE) was fatigue at 2%; 5% of patients discontinued therapy due to treatment related AEs, whereas 17% of patients had immune-mediated AEs.5
Similarly, in a single-arm phase 2 trial, atezolizumab, an anti-PD-L1 antibody, dosed at 1,200 mg every 3 weeks was used as first-line therapy in 119 patients with advanced urothelial cancer who were cisplatin ineligible. At a median follow-up of 17 months, the ORR was 23%, with a 9% CR rate. The median duration of response had not been reached. Median progression free survival (PFS) was 2.7 months, whereas overall survival (OS) was 16 months. Eight percent of patients had an AE leading to treatment discontinuation, and 17% had immune-mediated AEs.6 Both pembrolizumab and atezolizumab were granted FDA approval in 2017 for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-based chemotherapy.3
Immunotherapy Treatments After Progression With Cisplatin
Cytotoxic chemotherapy in the second-line setting with disease progression following platinum-based treatment has shown dismal responses, with a median OS of about 6 to 7 months.7 Immunotherapy provides an effective and a much-needed option in this scenario.
Five antibodies targeting the PD-1/PD-L1 pathway, pembrolizumab, nivolumab, atezolizumab, avelumab and durvalumab, have been granted FDA approval for patients who have progressed during or after platinum-based therapy (Table).3 In the phase 3 Keynote-045 trial, 542 patients were randomly assigned to receive either pembrolizumab 200 mg administered every 3 weeks or investigator’s choice chemotherapy (paclitaxel, docetaxel, or vinflunine).7 Median OS was 10.3 months in the pembrolizumab group and 7.4 months in the chemotherapy group (hazard ratio for death, 0.73; P = .002). Serious (grade 3 or above) treatment-related AEs were significantly less frequent with pembrolizumab (15% vs 49.4%).7 In a phase 2 trial, 270 patients were treated with nivolumab, a PD-1 inhibitor, at a dose of 3 mg/kg given every 2 weeks.8 The ORR was 19.6%, while the median OS for the entire cohort was 7 months. Responses were seen at all levels of PD-L1 expression, although in patients whose tumor expressed PD-L1 ≥1%, median OS was 11.3 months.8
It should be noted that in a large phase 3 trial comparing atezolizumab with chemotherapy in the second-line setting, ORR and OS were not statistically different between the 2 groups, although the duration of response was longer with atezolizumab.9 In early phase trials, avelumab and durvalumab, both PD-L1 inhibitors showed an ORR of about 17%, with higher ORR seen in patients with tumors positive for PD-L1 expression.10,11 The AE profile of immune checkpoint inhibitors is relatively favorable in clinical trials. The American Society of Clinical Oncology and National Comprehensive Cancer Network have jointly published evidence-based guidelines for the management of their immune related AEs.12
Future Directions
Several challenges have emerged with immunotherapy treatments. One issue is the relatively low ORRs for immune checkpoint inhibitors, ranging from 13.4% to 24% depending on the trial. Therefore, there is a need to identify reliable biomarkers and selection criteria to predict their efficacy and improve patient selection. Although tumor PD-L1 expression has shown some usefulness in this setting, responses have been noted in patients whose tumors have low or no expression of PD-L1. This low predictive accuracy is caused by several factors, including PD-L1 intratumor expression heterogeneity, primary vs metastatic site PD-L1 expression heterogeneity, lack of consensus on which PD-L1 assays and which value cutoffs to use, and the differences seen in marker expression depending on the freshness of the tissue specimen.
Other predictive biomarkers with potential include tumor gene expression profiles/tumor mutational load, T-cell and B-cell signatures. The optimal imaging modality and timing of this imaging for response assessment also is uncertain. So-called tumor pseudo-progression seen on imaging after treatment with these agents as a result of the immune/inflammatory response to the tumor is now a well-recognized phenomenon, but it can be challenging to differentiate from true disease progression. Other challenges include deciding on which immune checkpoint inhibitor to use given a lack of head-to-head comparisons of these immunotherapeutic agents, finding the proper drug doses to maximize efficacy, as well as determining the optimal duration of treatment in patients with continued response to immunotherapy. Many oncologists continue these treatments for up to 2 years in the setting of a significant or complete response.
Conclusion
Immune checkpoint inhibitors have emerged as pivotal treatments for patients with advanced urothelial cancer who are unfit to receive cisplatin in the first-line setting or who experience disease progression after cisplatin-based chemotherapy. This field continues to expand at a rapid pace due to multiple ongoing clinical trials assessing these agents, whether alone, in combination with cytotoxic, targeted, radiation therapies, or with other immune checkpoint inhibitors, both in the advanced as well as the neoadjuvant/adjuvant settings.
1. Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
2. Morales A. Treatment of carcinoma in situ of the bladder with BCG. Cancer Immunol Immunother. 1980;9 (1-2):69-72.
3. US Food and drug administration. FDA approved drug products. www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed July 5, 2018.
4. Farina MS, Lundgren KT, Bellmunt J. Immunotherapy in urothelial cancer: recent results and future perspectives. Drugs. 2017;77(10):1077-1089.
5. Balar AV, Castellano DE, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483-1492.
6. Balar AV, Galsky MD, Rosenberg JE, et al; IMvigor210 Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76.
7. Bellmunt J, de Wit R, Vaughn DJ, et al; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026.
8. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322.
9. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748-757.
10. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51-64.
11. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411.
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768.
An essential feature of cancer is its ability to evade the immune system. Multiple mechanisms are used for this purpose, including the disruption of antigen presentation and suppression of the immune response. The latter mechanism involves the activation of T-cell inhibition by recruiting regulatory T cells that weaken this response. Recent progress in understanding the ability of cancer to evade the immune system has paved the way to develop strategies to reverse this process and reactivate the immune system. Particularly, immune checkpoint signaling between T cells and tumor cells has been targeted with a new class of drug, immune checkpoint inhibitors. Immunotherapy has been an established and effective treatment in bladder cancer since 1976 when Morales and colleagues demonstrated that intravesical treatments with bacillus Calmette-Guérin can treat carcinoma in situ and prevent nonmuscle invasive urothelial cancer recurrence.1,2 This treatment elicits a cytotoxic response via antigenic presentation by bladder tumor cells.
Cytotoxic T-lymphocyte-associated protein (CTLA)-4, programmed death-1 (PD-1) and programmed death-ligand-1 (PD-L1) are molecules that downregulate the immune response and are targets of therapeutic antibodies that have demonstrated clinical efficacy across a wide range of malignancies. Five such agents—pembrolizumab, atezolizumab, nivolumab, avelumab and durvalumab—were recently approved by the US Food and Drug Administration (FDA) for clinical use in patients with advanced urothelial cancers.3 This class of agents also has been approved for several other malignancies, most notably in melanoma, non-small cell lung cancer, and renal cell carcinoma.3
Immune Biology
CTLA-4 is expressed on activated CD4 and CD8 T cells and competes with CD28 on T cells to interact with the costimulatory B7 proteins on antigen presenting cells. The CD28/B7 interaction promotes T-cell activation and effector functions, and the CTLA-4/B7 interaction inhibits them. In addition, PD-1 is a receptor expressed on CD4 and CD8 T cells, T regulatory (Treg) cells, B cells and natural killer (NK) cells that interacts with its ligand PD-L1 to suppress the immune response. Urothelial cancer possesses features that make it an adequate target for immunotherapeutic agents. Primarily, it is characterized by a high-mutation load, which lends itself to an increased expression of immunogenic antigens on tumor cells.4
Immunotherapy Treatments in Cisplatin-Ineligible Patients
Cisplatin-based chemotherapy is the first-line treatment and standard of care in unresectable or metastatic urothelial cancer. However, many patients are unable to receive cisplatin secondary to renal dysfunction, poor performance status, or other comorbidities. Alternative cytotoxic therapies in the first-line setting such as carboplatin-based regimens are associated with inferior outcomes and poor tolerability. There is, therefore, a need for effective and well-tolerated therapies in cisplatin-ineligible patients (Table).
In the phase 2 Keynote-052 trial, 370 cisplatin-ineligible patients were treated with the anti-PD-1 antibody pembrolizumab 200 mg every 3 weeks for up to 2 years.5At a median follow-up of 9.5 months, the objective response rate (+ORR) was 29% for the entire cohort, with a 7% complete response (CR) rate, and a 22% partial response (PR) rate.5 The median duration of response had not been reached at the time of analysis. Responses were seen regardless of PD-L1 expression, although high response rates were noted in patients whose tumors had PD-L1 expression > 10%. Pembrolizumab had an acceptable tolerability profile in this population. The most common grade 3 or 4 treatment-related adverse event (AE) was fatigue at 2%; 5% of patients discontinued therapy due to treatment related AEs, whereas 17% of patients had immune-mediated AEs.5
Similarly, in a single-arm phase 2 trial, atezolizumab, an anti-PD-L1 antibody, dosed at 1,200 mg every 3 weeks was used as first-line therapy in 119 patients with advanced urothelial cancer who were cisplatin ineligible. At a median follow-up of 17 months, the ORR was 23%, with a 9% CR rate. The median duration of response had not been reached. Median progression free survival (PFS) was 2.7 months, whereas overall survival (OS) was 16 months. Eight percent of patients had an AE leading to treatment discontinuation, and 17% had immune-mediated AEs.6 Both pembrolizumab and atezolizumab were granted FDA approval in 2017 for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-based chemotherapy.3
Immunotherapy Treatments After Progression With Cisplatin
Cytotoxic chemotherapy in the second-line setting with disease progression following platinum-based treatment has shown dismal responses, with a median OS of about 6 to 7 months.7 Immunotherapy provides an effective and a much-needed option in this scenario.
Five antibodies targeting the PD-1/PD-L1 pathway, pembrolizumab, nivolumab, atezolizumab, avelumab and durvalumab, have been granted FDA approval for patients who have progressed during or after platinum-based therapy (Table).3 In the phase 3 Keynote-045 trial, 542 patients were randomly assigned to receive either pembrolizumab 200 mg administered every 3 weeks or investigator’s choice chemotherapy (paclitaxel, docetaxel, or vinflunine).7 Median OS was 10.3 months in the pembrolizumab group and 7.4 months in the chemotherapy group (hazard ratio for death, 0.73; P = .002). Serious (grade 3 or above) treatment-related AEs were significantly less frequent with pembrolizumab (15% vs 49.4%).7 In a phase 2 trial, 270 patients were treated with nivolumab, a PD-1 inhibitor, at a dose of 3 mg/kg given every 2 weeks.8 The ORR was 19.6%, while the median OS for the entire cohort was 7 months. Responses were seen at all levels of PD-L1 expression, although in patients whose tumor expressed PD-L1 ≥1%, median OS was 11.3 months.8
It should be noted that in a large phase 3 trial comparing atezolizumab with chemotherapy in the second-line setting, ORR and OS were not statistically different between the 2 groups, although the duration of response was longer with atezolizumab.9 In early phase trials, avelumab and durvalumab, both PD-L1 inhibitors showed an ORR of about 17%, with higher ORR seen in patients with tumors positive for PD-L1 expression.10,11 The AE profile of immune checkpoint inhibitors is relatively favorable in clinical trials. The American Society of Clinical Oncology and National Comprehensive Cancer Network have jointly published evidence-based guidelines for the management of their immune related AEs.12
Future Directions
Several challenges have emerged with immunotherapy treatments. One issue is the relatively low ORRs for immune checkpoint inhibitors, ranging from 13.4% to 24% depending on the trial. Therefore, there is a need to identify reliable biomarkers and selection criteria to predict their efficacy and improve patient selection. Although tumor PD-L1 expression has shown some usefulness in this setting, responses have been noted in patients whose tumors have low or no expression of PD-L1. This low predictive accuracy is caused by several factors, including PD-L1 intratumor expression heterogeneity, primary vs metastatic site PD-L1 expression heterogeneity, lack of consensus on which PD-L1 assays and which value cutoffs to use, and the differences seen in marker expression depending on the freshness of the tissue specimen.
Other predictive biomarkers with potential include tumor gene expression profiles/tumor mutational load, T-cell and B-cell signatures. The optimal imaging modality and timing of this imaging for response assessment also is uncertain. So-called tumor pseudo-progression seen on imaging after treatment with these agents as a result of the immune/inflammatory response to the tumor is now a well-recognized phenomenon, but it can be challenging to differentiate from true disease progression. Other challenges include deciding on which immune checkpoint inhibitor to use given a lack of head-to-head comparisons of these immunotherapeutic agents, finding the proper drug doses to maximize efficacy, as well as determining the optimal duration of treatment in patients with continued response to immunotherapy. Many oncologists continue these treatments for up to 2 years in the setting of a significant or complete response.
Conclusion
Immune checkpoint inhibitors have emerged as pivotal treatments for patients with advanced urothelial cancer who are unfit to receive cisplatin in the first-line setting or who experience disease progression after cisplatin-based chemotherapy. This field continues to expand at a rapid pace due to multiple ongoing clinical trials assessing these agents, whether alone, in combination with cytotoxic, targeted, radiation therapies, or with other immune checkpoint inhibitors, both in the advanced as well as the neoadjuvant/adjuvant settings.
An essential feature of cancer is its ability to evade the immune system. Multiple mechanisms are used for this purpose, including the disruption of antigen presentation and suppression of the immune response. The latter mechanism involves the activation of T-cell inhibition by recruiting regulatory T cells that weaken this response. Recent progress in understanding the ability of cancer to evade the immune system has paved the way to develop strategies to reverse this process and reactivate the immune system. Particularly, immune checkpoint signaling between T cells and tumor cells has been targeted with a new class of drug, immune checkpoint inhibitors. Immunotherapy has been an established and effective treatment in bladder cancer since 1976 when Morales and colleagues demonstrated that intravesical treatments with bacillus Calmette-Guérin can treat carcinoma in situ and prevent nonmuscle invasive urothelial cancer recurrence.1,2 This treatment elicits a cytotoxic response via antigenic presentation by bladder tumor cells.
Cytotoxic T-lymphocyte-associated protein (CTLA)-4, programmed death-1 (PD-1) and programmed death-ligand-1 (PD-L1) are molecules that downregulate the immune response and are targets of therapeutic antibodies that have demonstrated clinical efficacy across a wide range of malignancies. Five such agents—pembrolizumab, atezolizumab, nivolumab, avelumab and durvalumab—were recently approved by the US Food and Drug Administration (FDA) for clinical use in patients with advanced urothelial cancers.3 This class of agents also has been approved for several other malignancies, most notably in melanoma, non-small cell lung cancer, and renal cell carcinoma.3
Immune Biology
CTLA-4 is expressed on activated CD4 and CD8 T cells and competes with CD28 on T cells to interact with the costimulatory B7 proteins on antigen presenting cells. The CD28/B7 interaction promotes T-cell activation and effector functions, and the CTLA-4/B7 interaction inhibits them. In addition, PD-1 is a receptor expressed on CD4 and CD8 T cells, T regulatory (Treg) cells, B cells and natural killer (NK) cells that interacts with its ligand PD-L1 to suppress the immune response. Urothelial cancer possesses features that make it an adequate target for immunotherapeutic agents. Primarily, it is characterized by a high-mutation load, which lends itself to an increased expression of immunogenic antigens on tumor cells.4
Immunotherapy Treatments in Cisplatin-Ineligible Patients
Cisplatin-based chemotherapy is the first-line treatment and standard of care in unresectable or metastatic urothelial cancer. However, many patients are unable to receive cisplatin secondary to renal dysfunction, poor performance status, or other comorbidities. Alternative cytotoxic therapies in the first-line setting such as carboplatin-based regimens are associated with inferior outcomes and poor tolerability. There is, therefore, a need for effective and well-tolerated therapies in cisplatin-ineligible patients (Table).
In the phase 2 Keynote-052 trial, 370 cisplatin-ineligible patients were treated with the anti-PD-1 antibody pembrolizumab 200 mg every 3 weeks for up to 2 years.5At a median follow-up of 9.5 months, the objective response rate (+ORR) was 29% for the entire cohort, with a 7% complete response (CR) rate, and a 22% partial response (PR) rate.5 The median duration of response had not been reached at the time of analysis. Responses were seen regardless of PD-L1 expression, although high response rates were noted in patients whose tumors had PD-L1 expression > 10%. Pembrolizumab had an acceptable tolerability profile in this population. The most common grade 3 or 4 treatment-related adverse event (AE) was fatigue at 2%; 5% of patients discontinued therapy due to treatment related AEs, whereas 17% of patients had immune-mediated AEs.5
Similarly, in a single-arm phase 2 trial, atezolizumab, an anti-PD-L1 antibody, dosed at 1,200 mg every 3 weeks was used as first-line therapy in 119 patients with advanced urothelial cancer who were cisplatin ineligible. At a median follow-up of 17 months, the ORR was 23%, with a 9% CR rate. The median duration of response had not been reached. Median progression free survival (PFS) was 2.7 months, whereas overall survival (OS) was 16 months. Eight percent of patients had an AE leading to treatment discontinuation, and 17% had immune-mediated AEs.6 Both pembrolizumab and atezolizumab were granted FDA approval in 2017 for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-based chemotherapy.3
Immunotherapy Treatments After Progression With Cisplatin
Cytotoxic chemotherapy in the second-line setting with disease progression following platinum-based treatment has shown dismal responses, with a median OS of about 6 to 7 months.7 Immunotherapy provides an effective and a much-needed option in this scenario.
Five antibodies targeting the PD-1/PD-L1 pathway, pembrolizumab, nivolumab, atezolizumab, avelumab and durvalumab, have been granted FDA approval for patients who have progressed during or after platinum-based therapy (Table).3 In the phase 3 Keynote-045 trial, 542 patients were randomly assigned to receive either pembrolizumab 200 mg administered every 3 weeks or investigator’s choice chemotherapy (paclitaxel, docetaxel, or vinflunine).7 Median OS was 10.3 months in the pembrolizumab group and 7.4 months in the chemotherapy group (hazard ratio for death, 0.73; P = .002). Serious (grade 3 or above) treatment-related AEs were significantly less frequent with pembrolizumab (15% vs 49.4%).7 In a phase 2 trial, 270 patients were treated with nivolumab, a PD-1 inhibitor, at a dose of 3 mg/kg given every 2 weeks.8 The ORR was 19.6%, while the median OS for the entire cohort was 7 months. Responses were seen at all levels of PD-L1 expression, although in patients whose tumor expressed PD-L1 ≥1%, median OS was 11.3 months.8
It should be noted that in a large phase 3 trial comparing atezolizumab with chemotherapy in the second-line setting, ORR and OS were not statistically different between the 2 groups, although the duration of response was longer with atezolizumab.9 In early phase trials, avelumab and durvalumab, both PD-L1 inhibitors showed an ORR of about 17%, with higher ORR seen in patients with tumors positive for PD-L1 expression.10,11 The AE profile of immune checkpoint inhibitors is relatively favorable in clinical trials. The American Society of Clinical Oncology and National Comprehensive Cancer Network have jointly published evidence-based guidelines for the management of their immune related AEs.12
Future Directions
Several challenges have emerged with immunotherapy treatments. One issue is the relatively low ORRs for immune checkpoint inhibitors, ranging from 13.4% to 24% depending on the trial. Therefore, there is a need to identify reliable biomarkers and selection criteria to predict their efficacy and improve patient selection. Although tumor PD-L1 expression has shown some usefulness in this setting, responses have been noted in patients whose tumors have low or no expression of PD-L1. This low predictive accuracy is caused by several factors, including PD-L1 intratumor expression heterogeneity, primary vs metastatic site PD-L1 expression heterogeneity, lack of consensus on which PD-L1 assays and which value cutoffs to use, and the differences seen in marker expression depending on the freshness of the tissue specimen.
Other predictive biomarkers with potential include tumor gene expression profiles/tumor mutational load, T-cell and B-cell signatures. The optimal imaging modality and timing of this imaging for response assessment also is uncertain. So-called tumor pseudo-progression seen on imaging after treatment with these agents as a result of the immune/inflammatory response to the tumor is now a well-recognized phenomenon, but it can be challenging to differentiate from true disease progression. Other challenges include deciding on which immune checkpoint inhibitor to use given a lack of head-to-head comparisons of these immunotherapeutic agents, finding the proper drug doses to maximize efficacy, as well as determining the optimal duration of treatment in patients with continued response to immunotherapy. Many oncologists continue these treatments for up to 2 years in the setting of a significant or complete response.
Conclusion
Immune checkpoint inhibitors have emerged as pivotal treatments for patients with advanced urothelial cancer who are unfit to receive cisplatin in the first-line setting or who experience disease progression after cisplatin-based chemotherapy. This field continues to expand at a rapid pace due to multiple ongoing clinical trials assessing these agents, whether alone, in combination with cytotoxic, targeted, radiation therapies, or with other immune checkpoint inhibitors, both in the advanced as well as the neoadjuvant/adjuvant settings.
1. Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
2. Morales A. Treatment of carcinoma in situ of the bladder with BCG. Cancer Immunol Immunother. 1980;9 (1-2):69-72.
3. US Food and drug administration. FDA approved drug products. www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed July 5, 2018.
4. Farina MS, Lundgren KT, Bellmunt J. Immunotherapy in urothelial cancer: recent results and future perspectives. Drugs. 2017;77(10):1077-1089.
5. Balar AV, Castellano DE, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483-1492.
6. Balar AV, Galsky MD, Rosenberg JE, et al; IMvigor210 Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76.
7. Bellmunt J, de Wit R, Vaughn DJ, et al; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026.
8. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322.
9. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748-757.
10. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51-64.
11. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411.
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768.
1. Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
2. Morales A. Treatment of carcinoma in situ of the bladder with BCG. Cancer Immunol Immunother. 1980;9 (1-2):69-72.
3. US Food and drug administration. FDA approved drug products. www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed July 5, 2018.
4. Farina MS, Lundgren KT, Bellmunt J. Immunotherapy in urothelial cancer: recent results and future perspectives. Drugs. 2017;77(10):1077-1089.
5. Balar AV, Castellano DE, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483-1492.
6. Balar AV, Galsky MD, Rosenberg JE, et al; IMvigor210 Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76.
7. Bellmunt J, de Wit R, Vaughn DJ, et al; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026.
8. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322.
9. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748-757.
10. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51-64.
11. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411.
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768.
Considerations for Psoriasis in Pregnancy
1. Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
2. Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
3. Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
4. Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
5. Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
6. Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
7. Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
8. Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
9. Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
10. Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
11. Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
12. Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
13. Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrow¬band UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
1. Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
2. Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
3. Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
4. Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
5. Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
6. Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
7. Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
8. Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
9. Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
10. Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
11. Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
12. Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
13. Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrow¬band UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
1. Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
2. Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
3. Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
4. Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
5. Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
6. Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
7. Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
8. Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
9. Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
10. Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
11. Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
12. Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
13. Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrow¬band UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.