User login
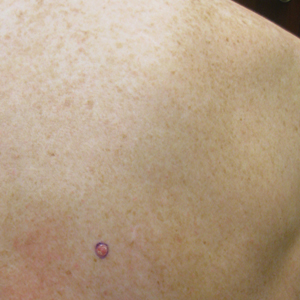
Role of Psoriasis in the Development of Merkel Cell Carcinoma
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
Neurologists publish consensus statement on stridor in MSA
The statement was published Oct. 1 in Neurology. In addition to reviewing the literature on the topic and providing recommendations, the authors described several areas for future research.
MSA is a rare neurodegenerative disorder that entails autonomic failure, cerebellar ataxia, and parkinsonism. Laryngeal stridor has a high positive predictive value in the diagnosis of MSA, but consensus about its definition and clinical implications had not been established previously. The Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) delle Scienze Neurologiche di Bologna (Italy) convened a consensus conference of experts in 2017 to determine diagnostic criteria for stridor in MSA, define its prognostic value, suggest treatment options, and indicate subjects for future research. The neurologists reviewed studies of any design that reported original data. They based their statements on 34 published articles, most of which were class III or IV.
The authors defined stridor in MSA as “a strained, high-pitched, harsh respiratory sound, mainly inspiratory, caused by laryngeal dysfunction leading to narrowing of the rima glottidis.” Stridor may occur exclusively during sleep or during sleep and wakefulness. It may be recognized during a clinical examination, through witness report, or through an audio recording. Neurologists may consider laryngoscopy to exclude mechanical lesions or functional vocal cord abnormalities related to other neurologic conditions, wrote the authors. Drug-induced sleep endoscopy and video polysomnography also may be considered.
Whether stridor, or certain features of stridor, affects survival in MSA is uncertain. “Stridor within 3 years of motor or autonomic symptom onset may shorten survival,” according to the statement. “However, identification of stridor onset may be difficult.” Moreover, stridor during wakefulness is considered to reflect a more advanced stage of disease, compared with stridor during sleep. Although stridor can be distressing for the patient and his or her caregivers, its influence on health-related quality of life has yet to be determined, according to the statement.
Continuous positive airway pressure (CPAP) during sleep can be a useful symptomatic treatment and should be considered a first-line therapy for stridor, wrote the authors. Tracheostomy, another effective symptomatic treatment, bypasses upper-airway obstruction at the larynx. “Persistent and severe stridor may require tracheostomy,” according to the statement. It is not certain whether CPAP improves survival in patients with MSA and stridor, and tracheostomy may improve survival. The literature contains insufficient evidence about whether minimally invasive procedures or botulinum toxin injections are effective symptomatic treatments for stridor, wrote the authors.
During their review of the literature, the authors identified what they considered to be several research gaps. The diagnosis of stridor remains challenging, and investigators should develop a questionnaire for detecting stridor, they wrote. A smartphone application also could be developed to recognize stridor automatically. “The relationship between stridor and other breathing disorders (i.e., central apneas and breathing rate abnormalities) and their respective contributions to disease prognosis and survival should be determined through a multicenter prospective study,” according to the statement. Finally, randomized controlled trials comparing CPAP and tracheostomy for various degrees of stridor could guide physicians’ choice of treatment.
The IRCCS funded the study. One of the authors is a section editor for Neurology, and other authors reported receiving honoraria from various companies such as Novartis, Sanofi, and UCB.
The statement was published Oct. 1 in Neurology. In addition to reviewing the literature on the topic and providing recommendations, the authors described several areas for future research.
MSA is a rare neurodegenerative disorder that entails autonomic failure, cerebellar ataxia, and parkinsonism. Laryngeal stridor has a high positive predictive value in the diagnosis of MSA, but consensus about its definition and clinical implications had not been established previously. The Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) delle Scienze Neurologiche di Bologna (Italy) convened a consensus conference of experts in 2017 to determine diagnostic criteria for stridor in MSA, define its prognostic value, suggest treatment options, and indicate subjects for future research. The neurologists reviewed studies of any design that reported original data. They based their statements on 34 published articles, most of which were class III or IV.
The authors defined stridor in MSA as “a strained, high-pitched, harsh respiratory sound, mainly inspiratory, caused by laryngeal dysfunction leading to narrowing of the rima glottidis.” Stridor may occur exclusively during sleep or during sleep and wakefulness. It may be recognized during a clinical examination, through witness report, or through an audio recording. Neurologists may consider laryngoscopy to exclude mechanical lesions or functional vocal cord abnormalities related to other neurologic conditions, wrote the authors. Drug-induced sleep endoscopy and video polysomnography also may be considered.
Whether stridor, or certain features of stridor, affects survival in MSA is uncertain. “Stridor within 3 years of motor or autonomic symptom onset may shorten survival,” according to the statement. “However, identification of stridor onset may be difficult.” Moreover, stridor during wakefulness is considered to reflect a more advanced stage of disease, compared with stridor during sleep. Although stridor can be distressing for the patient and his or her caregivers, its influence on health-related quality of life has yet to be determined, according to the statement.
Continuous positive airway pressure (CPAP) during sleep can be a useful symptomatic treatment and should be considered a first-line therapy for stridor, wrote the authors. Tracheostomy, another effective symptomatic treatment, bypasses upper-airway obstruction at the larynx. “Persistent and severe stridor may require tracheostomy,” according to the statement. It is not certain whether CPAP improves survival in patients with MSA and stridor, and tracheostomy may improve survival. The literature contains insufficient evidence about whether minimally invasive procedures or botulinum toxin injections are effective symptomatic treatments for stridor, wrote the authors.
During their review of the literature, the authors identified what they considered to be several research gaps. The diagnosis of stridor remains challenging, and investigators should develop a questionnaire for detecting stridor, they wrote. A smartphone application also could be developed to recognize stridor automatically. “The relationship between stridor and other breathing disorders (i.e., central apneas and breathing rate abnormalities) and their respective contributions to disease prognosis and survival should be determined through a multicenter prospective study,” according to the statement. Finally, randomized controlled trials comparing CPAP and tracheostomy for various degrees of stridor could guide physicians’ choice of treatment.
The IRCCS funded the study. One of the authors is a section editor for Neurology, and other authors reported receiving honoraria from various companies such as Novartis, Sanofi, and UCB.
The statement was published Oct. 1 in Neurology. In addition to reviewing the literature on the topic and providing recommendations, the authors described several areas for future research.
MSA is a rare neurodegenerative disorder that entails autonomic failure, cerebellar ataxia, and parkinsonism. Laryngeal stridor has a high positive predictive value in the diagnosis of MSA, but consensus about its definition and clinical implications had not been established previously. The Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) delle Scienze Neurologiche di Bologna (Italy) convened a consensus conference of experts in 2017 to determine diagnostic criteria for stridor in MSA, define its prognostic value, suggest treatment options, and indicate subjects for future research. The neurologists reviewed studies of any design that reported original data. They based their statements on 34 published articles, most of which were class III or IV.
The authors defined stridor in MSA as “a strained, high-pitched, harsh respiratory sound, mainly inspiratory, caused by laryngeal dysfunction leading to narrowing of the rima glottidis.” Stridor may occur exclusively during sleep or during sleep and wakefulness. It may be recognized during a clinical examination, through witness report, or through an audio recording. Neurologists may consider laryngoscopy to exclude mechanical lesions or functional vocal cord abnormalities related to other neurologic conditions, wrote the authors. Drug-induced sleep endoscopy and video polysomnography also may be considered.
Whether stridor, or certain features of stridor, affects survival in MSA is uncertain. “Stridor within 3 years of motor or autonomic symptom onset may shorten survival,” according to the statement. “However, identification of stridor onset may be difficult.” Moreover, stridor during wakefulness is considered to reflect a more advanced stage of disease, compared with stridor during sleep. Although stridor can be distressing for the patient and his or her caregivers, its influence on health-related quality of life has yet to be determined, according to the statement.
Continuous positive airway pressure (CPAP) during sleep can be a useful symptomatic treatment and should be considered a first-line therapy for stridor, wrote the authors. Tracheostomy, another effective symptomatic treatment, bypasses upper-airway obstruction at the larynx. “Persistent and severe stridor may require tracheostomy,” according to the statement. It is not certain whether CPAP improves survival in patients with MSA and stridor, and tracheostomy may improve survival. The literature contains insufficient evidence about whether minimally invasive procedures or botulinum toxin injections are effective symptomatic treatments for stridor, wrote the authors.
During their review of the literature, the authors identified what they considered to be several research gaps. The diagnosis of stridor remains challenging, and investigators should develop a questionnaire for detecting stridor, they wrote. A smartphone application also could be developed to recognize stridor automatically. “The relationship between stridor and other breathing disorders (i.e., central apneas and breathing rate abnormalities) and their respective contributions to disease prognosis and survival should be determined through a multicenter prospective study,” according to the statement. Finally, randomized controlled trials comparing CPAP and tracheostomy for various degrees of stridor could guide physicians’ choice of treatment.
The IRCCS funded the study. One of the authors is a section editor for Neurology, and other authors reported receiving honoraria from various companies such as Novartis, Sanofi, and UCB.
FROM NEUROLOGY
Increased Parkinson’s disease risk seen with bipolar disorder
Patients with bipolar disorder may be at increased risk of Parkinson’s disease in later life, according to a systematic review and meta-analysis published in JAMA Neurology.
Patrícia R. Faustino, MD, from the faculty of medicine at the University of Lisboa (Portgual), and coauthors reviewed and analyzed seven articles – four cohort studies and three cross-sectional studies – that reported data on idiopathic Parkinson’s disease in patients with bipolar disorder, compared with those without. The meta-analysis found that individuals with a previous diagnosis of bipolar disorder had a 235% higher risk of being later diagnosed with Parkinson’s disease. Even after removing studies with a high risk of bias, the risk was still 3.21 times higher in those with bipolar disorder, compared with those without.
“The pathophysiological rationale between bipolar disorder and Parkinson’s disease might be explained by the dopamine dysregulation hypothesis, which states that the cyclical process of bipolar disorder in manic states leads to a down-regulation of dopamine receptor sensitivity (depression phase), which is later compensated by up-regulation (manic state),” the authors wrote. “Over time, this phenomenon may lead to an overall reduction of dopaminergic activity, the prototypical Parkinson’s disease state.”
Subgroup analysis revealed that subgroups with shorter follow-up periods – less than 9 years – had a greater increase in the risk of a later Parkinson’s disease diagnosis. The authors noted that this could represent misdiagnosis of parkinsonism – possibly drug induced – as Parkinson’s disease. The researchers also raised the possibility that the increased risk of Parkinson’s disease in patients with bipolar disorder could relate to long-term lithium use, rather than being a causal relationship. “However, treatment with lithium is foundational in bipolar disorder, and so to separate the causal effect from a potential confounder would be particularly difficult,” they wrote.
One of the studies included did explore the use of lithium, and found that lithium monotherapy was associated with a significant increase in the risk of being diagnosed with Parkinson’s disease or taking antiparkinsonism medication, compared with antidepressant therapy. However the authors commented that the diagnostic code may not differentiate Parkinson’s disease from other causes of parkinsonism.
Given their findings, the authors suggested that, if patients with bipolar disorder present with parkinsonism features, it may not necessarily be drug induced. In these patients, they recommended an investigation for Parkinson’s disease, perhaps using functional neuroimaging “as Parkinson’s disease classically presents with nigrostriatal degeneration while drug-induced parkinsonism does not.”
Two authors declared grants and personal fees from the pharmaceutical sector. No other conflicts of interest were reported.
SOURCE: Faustino PR et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3446.
Patients with bipolar disorder may be at increased risk of Parkinson’s disease in later life, according to a systematic review and meta-analysis published in JAMA Neurology.
Patrícia R. Faustino, MD, from the faculty of medicine at the University of Lisboa (Portgual), and coauthors reviewed and analyzed seven articles – four cohort studies and three cross-sectional studies – that reported data on idiopathic Parkinson’s disease in patients with bipolar disorder, compared with those without. The meta-analysis found that individuals with a previous diagnosis of bipolar disorder had a 235% higher risk of being later diagnosed with Parkinson’s disease. Even after removing studies with a high risk of bias, the risk was still 3.21 times higher in those with bipolar disorder, compared with those without.
“The pathophysiological rationale between bipolar disorder and Parkinson’s disease might be explained by the dopamine dysregulation hypothesis, which states that the cyclical process of bipolar disorder in manic states leads to a down-regulation of dopamine receptor sensitivity (depression phase), which is later compensated by up-regulation (manic state),” the authors wrote. “Over time, this phenomenon may lead to an overall reduction of dopaminergic activity, the prototypical Parkinson’s disease state.”
Subgroup analysis revealed that subgroups with shorter follow-up periods – less than 9 years – had a greater increase in the risk of a later Parkinson’s disease diagnosis. The authors noted that this could represent misdiagnosis of parkinsonism – possibly drug induced – as Parkinson’s disease. The researchers also raised the possibility that the increased risk of Parkinson’s disease in patients with bipolar disorder could relate to long-term lithium use, rather than being a causal relationship. “However, treatment with lithium is foundational in bipolar disorder, and so to separate the causal effect from a potential confounder would be particularly difficult,” they wrote.
One of the studies included did explore the use of lithium, and found that lithium monotherapy was associated with a significant increase in the risk of being diagnosed with Parkinson’s disease or taking antiparkinsonism medication, compared with antidepressant therapy. However the authors commented that the diagnostic code may not differentiate Parkinson’s disease from other causes of parkinsonism.
Given their findings, the authors suggested that, if patients with bipolar disorder present with parkinsonism features, it may not necessarily be drug induced. In these patients, they recommended an investigation for Parkinson’s disease, perhaps using functional neuroimaging “as Parkinson’s disease classically presents with nigrostriatal degeneration while drug-induced parkinsonism does not.”
Two authors declared grants and personal fees from the pharmaceutical sector. No other conflicts of interest were reported.
SOURCE: Faustino PR et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3446.
Patients with bipolar disorder may be at increased risk of Parkinson’s disease in later life, according to a systematic review and meta-analysis published in JAMA Neurology.
Patrícia R. Faustino, MD, from the faculty of medicine at the University of Lisboa (Portgual), and coauthors reviewed and analyzed seven articles – four cohort studies and three cross-sectional studies – that reported data on idiopathic Parkinson’s disease in patients with bipolar disorder, compared with those without. The meta-analysis found that individuals with a previous diagnosis of bipolar disorder had a 235% higher risk of being later diagnosed with Parkinson’s disease. Even after removing studies with a high risk of bias, the risk was still 3.21 times higher in those with bipolar disorder, compared with those without.
“The pathophysiological rationale between bipolar disorder and Parkinson’s disease might be explained by the dopamine dysregulation hypothesis, which states that the cyclical process of bipolar disorder in manic states leads to a down-regulation of dopamine receptor sensitivity (depression phase), which is later compensated by up-regulation (manic state),” the authors wrote. “Over time, this phenomenon may lead to an overall reduction of dopaminergic activity, the prototypical Parkinson’s disease state.”
Subgroup analysis revealed that subgroups with shorter follow-up periods – less than 9 years – had a greater increase in the risk of a later Parkinson’s disease diagnosis. The authors noted that this could represent misdiagnosis of parkinsonism – possibly drug induced – as Parkinson’s disease. The researchers also raised the possibility that the increased risk of Parkinson’s disease in patients with bipolar disorder could relate to long-term lithium use, rather than being a causal relationship. “However, treatment with lithium is foundational in bipolar disorder, and so to separate the causal effect from a potential confounder would be particularly difficult,” they wrote.
One of the studies included did explore the use of lithium, and found that lithium monotherapy was associated with a significant increase in the risk of being diagnosed with Parkinson’s disease or taking antiparkinsonism medication, compared with antidepressant therapy. However the authors commented that the diagnostic code may not differentiate Parkinson’s disease from other causes of parkinsonism.
Given their findings, the authors suggested that, if patients with bipolar disorder present with parkinsonism features, it may not necessarily be drug induced. In these patients, they recommended an investigation for Parkinson’s disease, perhaps using functional neuroimaging “as Parkinson’s disease classically presents with nigrostriatal degeneration while drug-induced parkinsonism does not.”
Two authors declared grants and personal fees from the pharmaceutical sector. No other conflicts of interest were reported.
SOURCE: Faustino PR et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3446.
FROM JAMA NEUROLOGY
Accounting for sex may improve diagnosis of amnestic MCI
, according to an investigation published Oct. 9 in Neurology. Using sex-specific cut scores to define verbal memory impairment improves diagnostic accuracy and “may result in earlier detection of memory impairment in women and avoid false diagnoses in men,” wrote Erin E. Sundermann, PhD, assistant project scientist in psychiatry at the University of California, San Diego, and colleagues.
A diagnosis of aMCI generally requires a verbal memory deficit. Ample research demonstrates a female advantage on verbal memory tests, but normative data for these tests usually do not adjust for sex. Dr. Sundermann and colleagues previously showed that among men and women with aMCI and similar disease burden, women perform better on tests of verbal memory. Given these results, the investigators initiated a new study to test the hypothesis that using sex-specific norms and cut scores to identify memory impairment improves the accuracy of aMCI diagnosis, compared with non–sex-specific norms and cut scores.
An examination of ADNI data
Dr. Sundermann’s group extracted cross-sectional data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database in October 2016. They included participants without dementia for whom neuropsychologic and Alzheimer’s disease pathologic marker data were available at baseline. They excluded patients with a non-aMCI diagnosis based on typical and sex-specific criteria.
The researchers’ primary outcome was the Rey Auditory Verbal Learning Test (RAVLT). They also determined the presence or absence of the APOE e4 allele for each participant. Biomarker outcomes included the CSF ratio of hyperphosphorylated tau (p-tau) to beta-amyloid (A-beta), and cortical A-beta deposition.
Dr. Sundermann and colleagues applied the Jak/Bondi actuarial neuropsychologic diagnostic method to baseline data. This method relies on six neuropsychologic tests, including the RAVLT Learning and Delayed Recall. They subsequently derived two sets of normative data for the RAVLT outcomes in a normative sample of 1,620 patients enrolled in the Mayo Clinic Study of Aging (MCSA). The latter patients were considered normal controls at baseline and at least two follow-up visits at 15 months apart. One set of normative data was specific for age and education, and the other was specific for age, education, and sex. Dr. Sundermann’s group next applied the typical Jak/Bondi method and the sex-specific Jak/Bondi method to all ADNI participants’ data.
Biomarker analysis supported the hypothesis
The researchers included 985 participants (453 women) in their final sample. Approximately 94% of the population was white. Mean age was 72.9 years, and mean education duration was 16.3 years. Overall, women had a significantly lower mean age (71.9 years vs. 73.6 years), significantly fewer mean years of education (15.7 years vs. 16.7 years), and a significantly higher mean Mini-Mental State Examination score (28 vs. 28.1) compared with men. Compared with men’s scores, women’s scores on the RAVLT Learning (mean 42.3 vs. mean 35.6) and Delayed Recall (mean 6.2 vs. mean 4.5) were significantly higher.
When Dr. Sundermann and colleagues used typical cut scores, the frequency of aMCI diagnosis was significantly higher in men. Using sex-specific cut scores eliminated this sex difference, however. Among men, 184 (35%) were categorized as true positive, 293 (55%) as true negative, and 55 (10%) as false positive. No men were categorized as false negative. Among women, 120 (26%) were categorized as true positive, 288 (64%) as true negative, and 45 (10%) as false negative. No women were categorized as false positive.
The likelihood of cortical amyloid positivity in false negative women was 3.6 times greater than in true negative women but did not differ from that in true positive women. The likelihood of positivity for the CSF p-tau/A-beta ratio in false negative women was more than two times higher than in true negative women but did not differ from that in true positive women. The likelihood of having an APOE e4 allele in false negative women was almost fivefold higher than in true negative women but did not differ from that in true positive women.
The likelihood of cortical amyloid positivity in false positive men was less than that in true positive men (odds ratio [OR], 0.45) but did not differ from that in true negative men. The likelihood of positivity for CSF p-tau/A-beta ratio in false positive men was significantly less than in true positive men (OR, 0.47) but did not differ from that in true negative men. The likelihood of having the APOE e4 allele in false positive men was lower than that in true positive men (OR, 0.63) and higher than that in true negative men (OR, 1.50), but not significantly.
Results have implications for treatment
“If these results are confirmed, they have vital implications,” Dr. Sundermann said in a press release. “If women are inaccurately identified as having no problems with memory and thinking skills when they actually have MCI, then treatments are not being started, and they and their families are not planning ahead for their care or their financial or legal situations. And for men who are inaccurately diagnosed with MCI, they can be exposed to unneeded medications along with undue stress for them and their families.”
Among the limitations that the investigators acknowledged was the study’s cross-sectional, rather than longitudinal, design. In addition, the ADNI population that the researchers examined is a convenience sample of predominantly white and well-educated volunteers. The results therefore may not be generalizable to the broader U.S. population, wrote the authors.
Grants from the National Institutes of Health funded the study. Several of the investigators reported receiving honoraria from various pharmaceutical companies such as Mylan. One investigator sits on the editorial board for Neurology.
, according to an investigation published Oct. 9 in Neurology. Using sex-specific cut scores to define verbal memory impairment improves diagnostic accuracy and “may result in earlier detection of memory impairment in women and avoid false diagnoses in men,” wrote Erin E. Sundermann, PhD, assistant project scientist in psychiatry at the University of California, San Diego, and colleagues.
A diagnosis of aMCI generally requires a verbal memory deficit. Ample research demonstrates a female advantage on verbal memory tests, but normative data for these tests usually do not adjust for sex. Dr. Sundermann and colleagues previously showed that among men and women with aMCI and similar disease burden, women perform better on tests of verbal memory. Given these results, the investigators initiated a new study to test the hypothesis that using sex-specific norms and cut scores to identify memory impairment improves the accuracy of aMCI diagnosis, compared with non–sex-specific norms and cut scores.
An examination of ADNI data
Dr. Sundermann’s group extracted cross-sectional data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database in October 2016. They included participants without dementia for whom neuropsychologic and Alzheimer’s disease pathologic marker data were available at baseline. They excluded patients with a non-aMCI diagnosis based on typical and sex-specific criteria.
The researchers’ primary outcome was the Rey Auditory Verbal Learning Test (RAVLT). They also determined the presence or absence of the APOE e4 allele for each participant. Biomarker outcomes included the CSF ratio of hyperphosphorylated tau (p-tau) to beta-amyloid (A-beta), and cortical A-beta deposition.
Dr. Sundermann and colleagues applied the Jak/Bondi actuarial neuropsychologic diagnostic method to baseline data. This method relies on six neuropsychologic tests, including the RAVLT Learning and Delayed Recall. They subsequently derived two sets of normative data for the RAVLT outcomes in a normative sample of 1,620 patients enrolled in the Mayo Clinic Study of Aging (MCSA). The latter patients were considered normal controls at baseline and at least two follow-up visits at 15 months apart. One set of normative data was specific for age and education, and the other was specific for age, education, and sex. Dr. Sundermann’s group next applied the typical Jak/Bondi method and the sex-specific Jak/Bondi method to all ADNI participants’ data.
Biomarker analysis supported the hypothesis
The researchers included 985 participants (453 women) in their final sample. Approximately 94% of the population was white. Mean age was 72.9 years, and mean education duration was 16.3 years. Overall, women had a significantly lower mean age (71.9 years vs. 73.6 years), significantly fewer mean years of education (15.7 years vs. 16.7 years), and a significantly higher mean Mini-Mental State Examination score (28 vs. 28.1) compared with men. Compared with men’s scores, women’s scores on the RAVLT Learning (mean 42.3 vs. mean 35.6) and Delayed Recall (mean 6.2 vs. mean 4.5) were significantly higher.
When Dr. Sundermann and colleagues used typical cut scores, the frequency of aMCI diagnosis was significantly higher in men. Using sex-specific cut scores eliminated this sex difference, however. Among men, 184 (35%) were categorized as true positive, 293 (55%) as true negative, and 55 (10%) as false positive. No men were categorized as false negative. Among women, 120 (26%) were categorized as true positive, 288 (64%) as true negative, and 45 (10%) as false negative. No women were categorized as false positive.
The likelihood of cortical amyloid positivity in false negative women was 3.6 times greater than in true negative women but did not differ from that in true positive women. The likelihood of positivity for the CSF p-tau/A-beta ratio in false negative women was more than two times higher than in true negative women but did not differ from that in true positive women. The likelihood of having an APOE e4 allele in false negative women was almost fivefold higher than in true negative women but did not differ from that in true positive women.
The likelihood of cortical amyloid positivity in false positive men was less than that in true positive men (odds ratio [OR], 0.45) but did not differ from that in true negative men. The likelihood of positivity for CSF p-tau/A-beta ratio in false positive men was significantly less than in true positive men (OR, 0.47) but did not differ from that in true negative men. The likelihood of having the APOE e4 allele in false positive men was lower than that in true positive men (OR, 0.63) and higher than that in true negative men (OR, 1.50), but not significantly.
Results have implications for treatment
“If these results are confirmed, they have vital implications,” Dr. Sundermann said in a press release. “If women are inaccurately identified as having no problems with memory and thinking skills when they actually have MCI, then treatments are not being started, and they and their families are not planning ahead for their care or their financial or legal situations. And for men who are inaccurately diagnosed with MCI, they can be exposed to unneeded medications along with undue stress for them and their families.”
Among the limitations that the investigators acknowledged was the study’s cross-sectional, rather than longitudinal, design. In addition, the ADNI population that the researchers examined is a convenience sample of predominantly white and well-educated volunteers. The results therefore may not be generalizable to the broader U.S. population, wrote the authors.
Grants from the National Institutes of Health funded the study. Several of the investigators reported receiving honoraria from various pharmaceutical companies such as Mylan. One investigator sits on the editorial board for Neurology.
, according to an investigation published Oct. 9 in Neurology. Using sex-specific cut scores to define verbal memory impairment improves diagnostic accuracy and “may result in earlier detection of memory impairment in women and avoid false diagnoses in men,” wrote Erin E. Sundermann, PhD, assistant project scientist in psychiatry at the University of California, San Diego, and colleagues.
A diagnosis of aMCI generally requires a verbal memory deficit. Ample research demonstrates a female advantage on verbal memory tests, but normative data for these tests usually do not adjust for sex. Dr. Sundermann and colleagues previously showed that among men and women with aMCI and similar disease burden, women perform better on tests of verbal memory. Given these results, the investigators initiated a new study to test the hypothesis that using sex-specific norms and cut scores to identify memory impairment improves the accuracy of aMCI diagnosis, compared with non–sex-specific norms and cut scores.
An examination of ADNI data
Dr. Sundermann’s group extracted cross-sectional data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database in October 2016. They included participants without dementia for whom neuropsychologic and Alzheimer’s disease pathologic marker data were available at baseline. They excluded patients with a non-aMCI diagnosis based on typical and sex-specific criteria.
The researchers’ primary outcome was the Rey Auditory Verbal Learning Test (RAVLT). They also determined the presence or absence of the APOE e4 allele for each participant. Biomarker outcomes included the CSF ratio of hyperphosphorylated tau (p-tau) to beta-amyloid (A-beta), and cortical A-beta deposition.
Dr. Sundermann and colleagues applied the Jak/Bondi actuarial neuropsychologic diagnostic method to baseline data. This method relies on six neuropsychologic tests, including the RAVLT Learning and Delayed Recall. They subsequently derived two sets of normative data for the RAVLT outcomes in a normative sample of 1,620 patients enrolled in the Mayo Clinic Study of Aging (MCSA). The latter patients were considered normal controls at baseline and at least two follow-up visits at 15 months apart. One set of normative data was specific for age and education, and the other was specific for age, education, and sex. Dr. Sundermann’s group next applied the typical Jak/Bondi method and the sex-specific Jak/Bondi method to all ADNI participants’ data.
Biomarker analysis supported the hypothesis
The researchers included 985 participants (453 women) in their final sample. Approximately 94% of the population was white. Mean age was 72.9 years, and mean education duration was 16.3 years. Overall, women had a significantly lower mean age (71.9 years vs. 73.6 years), significantly fewer mean years of education (15.7 years vs. 16.7 years), and a significantly higher mean Mini-Mental State Examination score (28 vs. 28.1) compared with men. Compared with men’s scores, women’s scores on the RAVLT Learning (mean 42.3 vs. mean 35.6) and Delayed Recall (mean 6.2 vs. mean 4.5) were significantly higher.
When Dr. Sundermann and colleagues used typical cut scores, the frequency of aMCI diagnosis was significantly higher in men. Using sex-specific cut scores eliminated this sex difference, however. Among men, 184 (35%) were categorized as true positive, 293 (55%) as true negative, and 55 (10%) as false positive. No men were categorized as false negative. Among women, 120 (26%) were categorized as true positive, 288 (64%) as true negative, and 45 (10%) as false negative. No women were categorized as false positive.
The likelihood of cortical amyloid positivity in false negative women was 3.6 times greater than in true negative women but did not differ from that in true positive women. The likelihood of positivity for the CSF p-tau/A-beta ratio in false negative women was more than two times higher than in true negative women but did not differ from that in true positive women. The likelihood of having an APOE e4 allele in false negative women was almost fivefold higher than in true negative women but did not differ from that in true positive women.
The likelihood of cortical amyloid positivity in false positive men was less than that in true positive men (odds ratio [OR], 0.45) but did not differ from that in true negative men. The likelihood of positivity for CSF p-tau/A-beta ratio in false positive men was significantly less than in true positive men (OR, 0.47) but did not differ from that in true negative men. The likelihood of having the APOE e4 allele in false positive men was lower than that in true positive men (OR, 0.63) and higher than that in true negative men (OR, 1.50), but not significantly.
Results have implications for treatment
“If these results are confirmed, they have vital implications,” Dr. Sundermann said in a press release. “If women are inaccurately identified as having no problems with memory and thinking skills when they actually have MCI, then treatments are not being started, and they and their families are not planning ahead for their care or their financial or legal situations. And for men who are inaccurately diagnosed with MCI, they can be exposed to unneeded medications along with undue stress for them and their families.”
Among the limitations that the investigators acknowledged was the study’s cross-sectional, rather than longitudinal, design. In addition, the ADNI population that the researchers examined is a convenience sample of predominantly white and well-educated volunteers. The results therefore may not be generalizable to the broader U.S. population, wrote the authors.
Grants from the National Institutes of Health funded the study. Several of the investigators reported receiving honoraria from various pharmaceutical companies such as Mylan. One investigator sits on the editorial board for Neurology.
FROM NEUROLOGY
Risk of contralateral breast cancer highest for women under 40
Stray radiation exposure could be responsible for up to 28% of the contralateral breast cancer that occurs years after radiotherapy of the primary tumor.
The risk is highest among women initially treated when younger than 40 years, who had at lest 5 years’ lapse between the first and second occurrences, and had a higher genetic risk score, Gordon P. Watt, PhD, and colleagues reported in JAMA Network Open.
“These findings may support clinical decision making related to radiation treatment, particularly among women for whom other modalities may be considered,” wrote Dr. Watt of Memorial Sloan Kettering Cancer Center, New York, and coauthors. “For example, young women with a high [genetic risk] may consider partial-breast radiation therapy, rather than whole-breast radiation therapy when appropriate, opt for radiation therapy techniques that reduce integral dose (e.g., proton beam), or decide for non–radiation therapy–based locoregional management (e.g., mastectomy). These findings may be especially important in younger women with medially located breast cancers, where the scatter dose to the contralateral breast is likely to be higher.”
The investigators enrolled women who received a diagnosis for a first invasive local or regional breast cancer when they were younger than 55 years in a case-control study to investigate whether the NHEJ genetic risk score (GRS) could predict a woman’s risk of developing contralateral breast cancer after irradiation of a primary breast cancer. The risk score comprises 93 single nucleotide polymorphisms (SNPs) located in or near the seven genes in the NHEJ pathway, including DCLRE1C, LIG4, NHEJ1, PRKDC, XRCC4, XRCC5, and XRCC6. Dr. Watts’ team investigated risks associated with 69 of the SNPs.
The study comprised 3,732 women who were recruited from 2000 to 2012, with primary diagnosis occurring from 1985 to 2008. Of these, 1,521 had contralateral breast cancer; the remainder had unilateral disease and were used as controls.
At first diagnosis, they were aged a median of 46 years, although age ranged from 23 to 54 years. Most of the primary tumors (84%) were ductal. Among the recurrences, 9% were in situ, 63% local, 23% regional, and 2% distant. Stage was unknown on the remainder. Among these recurrent tumors, 54% were estrogen receptor positive and 24% were progesterone receptor positive, with unknown status on the remainder.
For women aged younger than 40 years at the time of the primary tumor, and at least 5 years out from treatment, radiation increased the risk of contralateral disease by 70%. But there was no significant risk to younger women with less than 5 years’ latency, or to women diagnosed at 40 years or older regardless of the time since treatment.
Age played a similar role when considering location-specific stray radiation dose. The risk was doubled in younger women who were exposed to at least 1 Gy and at least 5 years out from treatment, but there was no significantly increased risk to women older at first diagnosis.
Nor were the individual SNPs associated with increased risk. “The NHEJ GRS was approximately normally distributed and was dichotomized at the overall median for analysis; the median (range) GRS in the case group was 75 alleles and the median GRS in the control group was 74 alleles,” the investigators wrote.
But when examined by total GRS score, differences emerged.
“In the high NHEJ GRS group, among women who received the first diagnosis when they were younger than 40 years with a latency of 5 years or more, a stray radiation dose of 1.0 Gy or more was associated with threefold greater contralateral breast cancer risk, compared with no radiation exposure. In contrast, for women with an NHEJ GRS of 74 alleles or fewer in the same age and latency group, there was no association between radiation dose and contralateral breast cancer,” they wrote.
Again, there was no increased risk for women aged older than 40 years at first diagnosis.
“Based on these results, after a latency of 5 years or longer among women who received their first breast cancer diagnosis when they were younger than 40 years with a high NHEJ GRS, the population-attributable risk fraction of contralateral breast cancer attributable to stray radiation exposure to the contralateral breast was 28%. The corresponding population attributable risk fraction among women who received their first diagnosis when they were younger than 40 years after a latency of 5 years or more with a low NHEJ GRS was 18%,” the investigators wrote.
Five of the seven NHEJ pathway genes appeared to be driving these increased risks: LIG4, NHEJ1, XRCC4, XRCC5, and XRCC6. The expression quantitative trait loci (eQTLs) in each gene were always associated with a single direction of association; all risk alleles in XRCC4 were associated with decreased expression and all risk alleles in LIG4 were associated with increased expression.
“The consistent association of multiple NHEJ GRS risk alleles with eQTLs in a single direction suggests that the NHEJ GRS may be capturing the effect of SNP alleles on the transcription of one or more genes in the NHEJ pathway. This supports the hypothesis that the variation in this pathway may alter double-stranded DNA damage response, thereby increasing the risk of tumor development. However, the results from [the Genotype-Tissue Expression database] are drawn from multiple tissues that may not be appropriate proxies for breast tissue and the impact of genetic variation in the overall NHEJ pathway is likely to be complex,” the investigators wrote.
Dr. Watt had no financial disclosures. The research was funded by the National Cancer Institute.
SOURCE: Watt GP et al. JAMA Netw Open. 2019 Sep 27. doi: 10.1001/jamanetworkopen.2019.12259.
Stray radiation exposure could be responsible for up to 28% of the contralateral breast cancer that occurs years after radiotherapy of the primary tumor.
The risk is highest among women initially treated when younger than 40 years, who had at lest 5 years’ lapse between the first and second occurrences, and had a higher genetic risk score, Gordon P. Watt, PhD, and colleagues reported in JAMA Network Open.
“These findings may support clinical decision making related to radiation treatment, particularly among women for whom other modalities may be considered,” wrote Dr. Watt of Memorial Sloan Kettering Cancer Center, New York, and coauthors. “For example, young women with a high [genetic risk] may consider partial-breast radiation therapy, rather than whole-breast radiation therapy when appropriate, opt for radiation therapy techniques that reduce integral dose (e.g., proton beam), or decide for non–radiation therapy–based locoregional management (e.g., mastectomy). These findings may be especially important in younger women with medially located breast cancers, where the scatter dose to the contralateral breast is likely to be higher.”
The investigators enrolled women who received a diagnosis for a first invasive local or regional breast cancer when they were younger than 55 years in a case-control study to investigate whether the NHEJ genetic risk score (GRS) could predict a woman’s risk of developing contralateral breast cancer after irradiation of a primary breast cancer. The risk score comprises 93 single nucleotide polymorphisms (SNPs) located in or near the seven genes in the NHEJ pathway, including DCLRE1C, LIG4, NHEJ1, PRKDC, XRCC4, XRCC5, and XRCC6. Dr. Watts’ team investigated risks associated with 69 of the SNPs.
The study comprised 3,732 women who were recruited from 2000 to 2012, with primary diagnosis occurring from 1985 to 2008. Of these, 1,521 had contralateral breast cancer; the remainder had unilateral disease and were used as controls.
At first diagnosis, they were aged a median of 46 years, although age ranged from 23 to 54 years. Most of the primary tumors (84%) were ductal. Among the recurrences, 9% were in situ, 63% local, 23% regional, and 2% distant. Stage was unknown on the remainder. Among these recurrent tumors, 54% were estrogen receptor positive and 24% were progesterone receptor positive, with unknown status on the remainder.
For women aged younger than 40 years at the time of the primary tumor, and at least 5 years out from treatment, radiation increased the risk of contralateral disease by 70%. But there was no significant risk to younger women with less than 5 years’ latency, or to women diagnosed at 40 years or older regardless of the time since treatment.
Age played a similar role when considering location-specific stray radiation dose. The risk was doubled in younger women who were exposed to at least 1 Gy and at least 5 years out from treatment, but there was no significantly increased risk to women older at first diagnosis.
Nor were the individual SNPs associated with increased risk. “The NHEJ GRS was approximately normally distributed and was dichotomized at the overall median for analysis; the median (range) GRS in the case group was 75 alleles and the median GRS in the control group was 74 alleles,” the investigators wrote.
But when examined by total GRS score, differences emerged.
“In the high NHEJ GRS group, among women who received the first diagnosis when they were younger than 40 years with a latency of 5 years or more, a stray radiation dose of 1.0 Gy or more was associated with threefold greater contralateral breast cancer risk, compared with no radiation exposure. In contrast, for women with an NHEJ GRS of 74 alleles or fewer in the same age and latency group, there was no association between radiation dose and contralateral breast cancer,” they wrote.
Again, there was no increased risk for women aged older than 40 years at first diagnosis.
“Based on these results, after a latency of 5 years or longer among women who received their first breast cancer diagnosis when they were younger than 40 years with a high NHEJ GRS, the population-attributable risk fraction of contralateral breast cancer attributable to stray radiation exposure to the contralateral breast was 28%. The corresponding population attributable risk fraction among women who received their first diagnosis when they were younger than 40 years after a latency of 5 years or more with a low NHEJ GRS was 18%,” the investigators wrote.
Five of the seven NHEJ pathway genes appeared to be driving these increased risks: LIG4, NHEJ1, XRCC4, XRCC5, and XRCC6. The expression quantitative trait loci (eQTLs) in each gene were always associated with a single direction of association; all risk alleles in XRCC4 were associated with decreased expression and all risk alleles in LIG4 were associated with increased expression.
“The consistent association of multiple NHEJ GRS risk alleles with eQTLs in a single direction suggests that the NHEJ GRS may be capturing the effect of SNP alleles on the transcription of one or more genes in the NHEJ pathway. This supports the hypothesis that the variation in this pathway may alter double-stranded DNA damage response, thereby increasing the risk of tumor development. However, the results from [the Genotype-Tissue Expression database] are drawn from multiple tissues that may not be appropriate proxies for breast tissue and the impact of genetic variation in the overall NHEJ pathway is likely to be complex,” the investigators wrote.
Dr. Watt had no financial disclosures. The research was funded by the National Cancer Institute.
SOURCE: Watt GP et al. JAMA Netw Open. 2019 Sep 27. doi: 10.1001/jamanetworkopen.2019.12259.
Stray radiation exposure could be responsible for up to 28% of the contralateral breast cancer that occurs years after radiotherapy of the primary tumor.
The risk is highest among women initially treated when younger than 40 years, who had at lest 5 years’ lapse between the first and second occurrences, and had a higher genetic risk score, Gordon P. Watt, PhD, and colleagues reported in JAMA Network Open.
“These findings may support clinical decision making related to radiation treatment, particularly among women for whom other modalities may be considered,” wrote Dr. Watt of Memorial Sloan Kettering Cancer Center, New York, and coauthors. “For example, young women with a high [genetic risk] may consider partial-breast radiation therapy, rather than whole-breast radiation therapy when appropriate, opt for radiation therapy techniques that reduce integral dose (e.g., proton beam), or decide for non–radiation therapy–based locoregional management (e.g., mastectomy). These findings may be especially important in younger women with medially located breast cancers, where the scatter dose to the contralateral breast is likely to be higher.”
The investigators enrolled women who received a diagnosis for a first invasive local or regional breast cancer when they were younger than 55 years in a case-control study to investigate whether the NHEJ genetic risk score (GRS) could predict a woman’s risk of developing contralateral breast cancer after irradiation of a primary breast cancer. The risk score comprises 93 single nucleotide polymorphisms (SNPs) located in or near the seven genes in the NHEJ pathway, including DCLRE1C, LIG4, NHEJ1, PRKDC, XRCC4, XRCC5, and XRCC6. Dr. Watts’ team investigated risks associated with 69 of the SNPs.
The study comprised 3,732 women who were recruited from 2000 to 2012, with primary diagnosis occurring from 1985 to 2008. Of these, 1,521 had contralateral breast cancer; the remainder had unilateral disease and were used as controls.
At first diagnosis, they were aged a median of 46 years, although age ranged from 23 to 54 years. Most of the primary tumors (84%) were ductal. Among the recurrences, 9% were in situ, 63% local, 23% regional, and 2% distant. Stage was unknown on the remainder. Among these recurrent tumors, 54% were estrogen receptor positive and 24% were progesterone receptor positive, with unknown status on the remainder.
For women aged younger than 40 years at the time of the primary tumor, and at least 5 years out from treatment, radiation increased the risk of contralateral disease by 70%. But there was no significant risk to younger women with less than 5 years’ latency, or to women diagnosed at 40 years or older regardless of the time since treatment.
Age played a similar role when considering location-specific stray radiation dose. The risk was doubled in younger women who were exposed to at least 1 Gy and at least 5 years out from treatment, but there was no significantly increased risk to women older at first diagnosis.
Nor were the individual SNPs associated with increased risk. “The NHEJ GRS was approximately normally distributed and was dichotomized at the overall median for analysis; the median (range) GRS in the case group was 75 alleles and the median GRS in the control group was 74 alleles,” the investigators wrote.
But when examined by total GRS score, differences emerged.
“In the high NHEJ GRS group, among women who received the first diagnosis when they were younger than 40 years with a latency of 5 years or more, a stray radiation dose of 1.0 Gy or more was associated with threefold greater contralateral breast cancer risk, compared with no radiation exposure. In contrast, for women with an NHEJ GRS of 74 alleles or fewer in the same age and latency group, there was no association between radiation dose and contralateral breast cancer,” they wrote.
Again, there was no increased risk for women aged older than 40 years at first diagnosis.
“Based on these results, after a latency of 5 years or longer among women who received their first breast cancer diagnosis when they were younger than 40 years with a high NHEJ GRS, the population-attributable risk fraction of contralateral breast cancer attributable to stray radiation exposure to the contralateral breast was 28%. The corresponding population attributable risk fraction among women who received their first diagnosis when they were younger than 40 years after a latency of 5 years or more with a low NHEJ GRS was 18%,” the investigators wrote.
Five of the seven NHEJ pathway genes appeared to be driving these increased risks: LIG4, NHEJ1, XRCC4, XRCC5, and XRCC6. The expression quantitative trait loci (eQTLs) in each gene were always associated with a single direction of association; all risk alleles in XRCC4 were associated with decreased expression and all risk alleles in LIG4 were associated with increased expression.
“The consistent association of multiple NHEJ GRS risk alleles with eQTLs in a single direction suggests that the NHEJ GRS may be capturing the effect of SNP alleles on the transcription of one or more genes in the NHEJ pathway. This supports the hypothesis that the variation in this pathway may alter double-stranded DNA damage response, thereby increasing the risk of tumor development. However, the results from [the Genotype-Tissue Expression database] are drawn from multiple tissues that may not be appropriate proxies for breast tissue and the impact of genetic variation in the overall NHEJ pathway is likely to be complex,” the investigators wrote.
Dr. Watt had no financial disclosures. The research was funded by the National Cancer Institute.
SOURCE: Watt GP et al. JAMA Netw Open. 2019 Sep 27. doi: 10.1001/jamanetworkopen.2019.12259.
FROM JAMA NETWORK OPEN
Multigene testing for all patients with breast cancer may be cost-effective, life-saving
High-risk multigene testing for all patients with breast cancer may be a cost-effective way to reduce rates of breast cancer and ovarian cancer, according to investigators.
A microsimulation modeling study suggested that moving from standard BRCA testing based on clinical criteria or family history to widespread adoption of multigene testing could potentially save more than 2,400 lives per year in the United States, reported lead author Li Sun, MSc, of the London School of Hygiene & Tropical Medicine, and colleagues.
“Moving toward unselected BC testing may give an impetus for prevention in unaffected family members along with clinical implications for the patient with BC. Pathogenic variant carriers with newly diagnosed BC can opt for bilateral mastectomy rather than breast conservation at initial BC surgery,” the investigators wrote in JAMA Oncology. They noted that, presently, only 20%-30% of eligible patients are actually tested. “Testing all patients with breast cancer at diagnosis can increase testing access and uptake and identify many more pathogenic variant carriers for screening and prevention.”
To see if such a broad roll-out would be economically and medically feasible and beneficial, the investigators performed a modeling study involving 11,836 women with breast cancer, with data drawn from four large databases in the United Kingdom, the United States, and Australia.
The model compared annual costs, number of detected cases of breast cancer and ovarian cancer, and related rates of mortality between standard BRCA testing based on clinical criteria or family history versus high-risk multigene testing (BRCA1/BRCA2/PALB2) for all patients with breast cancer.
Resultant incremental cost-effectiveness ratios (ICERs) showed that in both the United Kingdom and the United States, multigene testing would cost, on average, significantly less than minimum willingness-to-pay thresholds of £30,000 per quality-adjusted life-year (QALY) and $100,000/QALY, respectively. In the United Kingdom, the estimated cost of multigene testing was £10,464/QALY from a payer’s perspective and £7,216/QALY from a societal perspective, the latter of which incorporates economic costs beyond the health care system. In the United States, these values rose to $65,661/QALY (payer perspective) and $61,618/QALY (societal perspective). Probabilistic sensitivity analysis suggested that multigene testing would be cost-effective for almost all health system simulations in the United Kingdom (98%-99%) and approximately two-thirds of those in the United States (64%-68%).
Epidemiologically, in the United Kingdom, multigene testing could potentially prevent 2,101 cases of breast cancer and ovarian cancer and 633 deaths per year, while in the United States, 9,733 cases might be prevented, with 2,406 lives saved.
“Our analysis suggests that an unselected testing strategy is extremely cost-effective for U.K. and U.S. health systems and provides a basis for change in current guidelines and policy to implement this strategy,” the investigators said.
The study was funded by the National Cancer Institute, the State of Washington, the Fred Hutchinson Cancer Research Center, and others. The investigators reported additional relationships with AstraZeneca, the Manchester National Institute for Health Research Biomedical Research Centre, Cancer Research UK, an others.
SOURCE: Sun et al. JAMA Oncol. 2019 Oct 3. doi: 10.1001/jamaoncol.2019.3323.
Although detection of more pathogenic variants among patients with breast cancer may initially appear beneficial, the hidden risks and costs associated with broader multigene testing would likely outweigh the benefits.
Present guidelines, such as those provided by the National Comprehensive Cancer Network, already include effective strategies for detecting BRCA1/2 variants, as only 0.6% of patients who do not meet screening guidelines are estimated to test positive
Beyond BRCA1/2, the clinical relevance of pathogenic variants becomes dubious, as many genetic aberrations are not actionable. Furthermore, misinterpretation of unfamiliar results may increase risks of treatment decisions that might actually harm patients. While some argue that broader testing could have benefits for family members, this is poorly supported with evidence.
Behind the allegedly falling costs of genetic sequencing, true costs are hidden with corporate policies that reduce patient financial liability. It is worth noting that two publicly held testing companies recently reported annual revenues of $144 million and $498 million, representing money drawn largely from third-party payers covering guideline-recommended testing. Instead of concrete science, the popularity of multigene testing is more likely driven by active marketing and technological convenience. More independent studies are needed.
Mark Robson, MD, is with the department of breast cancer medicine and clinical genetics at Memorial Sloan Kettering Cancer Center in New York. Susan Domchek, MD is with the Basser Center for BRCA at the University of Pennsylvania in Philadelphia. Dr. Robson disclosed financial relationships with AstraZeneca, McKesson, and Pfizer. Dr. Domchek reported personal fees from Clovis, AstraZeneca, and BMS. Their remarks are adapted from an editorial (JAMA Oncol. 2019 Oct 3. doi: 10.1001/jamaoncol.2019.4004).
Although detection of more pathogenic variants among patients with breast cancer may initially appear beneficial, the hidden risks and costs associated with broader multigene testing would likely outweigh the benefits.
Present guidelines, such as those provided by the National Comprehensive Cancer Network, already include effective strategies for detecting BRCA1/2 variants, as only 0.6% of patients who do not meet screening guidelines are estimated to test positive
Beyond BRCA1/2, the clinical relevance of pathogenic variants becomes dubious, as many genetic aberrations are not actionable. Furthermore, misinterpretation of unfamiliar results may increase risks of treatment decisions that might actually harm patients. While some argue that broader testing could have benefits for family members, this is poorly supported with evidence.
Behind the allegedly falling costs of genetic sequencing, true costs are hidden with corporate policies that reduce patient financial liability. It is worth noting that two publicly held testing companies recently reported annual revenues of $144 million and $498 million, representing money drawn largely from third-party payers covering guideline-recommended testing. Instead of concrete science, the popularity of multigene testing is more likely driven by active marketing and technological convenience. More independent studies are needed.
Mark Robson, MD, is with the department of breast cancer medicine and clinical genetics at Memorial Sloan Kettering Cancer Center in New York. Susan Domchek, MD is with the Basser Center for BRCA at the University of Pennsylvania in Philadelphia. Dr. Robson disclosed financial relationships with AstraZeneca, McKesson, and Pfizer. Dr. Domchek reported personal fees from Clovis, AstraZeneca, and BMS. Their remarks are adapted from an editorial (JAMA Oncol. 2019 Oct 3. doi: 10.1001/jamaoncol.2019.4004).
Although detection of more pathogenic variants among patients with breast cancer may initially appear beneficial, the hidden risks and costs associated with broader multigene testing would likely outweigh the benefits.
Present guidelines, such as those provided by the National Comprehensive Cancer Network, already include effective strategies for detecting BRCA1/2 variants, as only 0.6% of patients who do not meet screening guidelines are estimated to test positive
Beyond BRCA1/2, the clinical relevance of pathogenic variants becomes dubious, as many genetic aberrations are not actionable. Furthermore, misinterpretation of unfamiliar results may increase risks of treatment decisions that might actually harm patients. While some argue that broader testing could have benefits for family members, this is poorly supported with evidence.
Behind the allegedly falling costs of genetic sequencing, true costs are hidden with corporate policies that reduce patient financial liability. It is worth noting that two publicly held testing companies recently reported annual revenues of $144 million and $498 million, representing money drawn largely from third-party payers covering guideline-recommended testing. Instead of concrete science, the popularity of multigene testing is more likely driven by active marketing and technological convenience. More independent studies are needed.
Mark Robson, MD, is with the department of breast cancer medicine and clinical genetics at Memorial Sloan Kettering Cancer Center in New York. Susan Domchek, MD is with the Basser Center for BRCA at the University of Pennsylvania in Philadelphia. Dr. Robson disclosed financial relationships with AstraZeneca, McKesson, and Pfizer. Dr. Domchek reported personal fees from Clovis, AstraZeneca, and BMS. Their remarks are adapted from an editorial (JAMA Oncol. 2019 Oct 3. doi: 10.1001/jamaoncol.2019.4004).
High-risk multigene testing for all patients with breast cancer may be a cost-effective way to reduce rates of breast cancer and ovarian cancer, according to investigators.
A microsimulation modeling study suggested that moving from standard BRCA testing based on clinical criteria or family history to widespread adoption of multigene testing could potentially save more than 2,400 lives per year in the United States, reported lead author Li Sun, MSc, of the London School of Hygiene & Tropical Medicine, and colleagues.
“Moving toward unselected BC testing may give an impetus for prevention in unaffected family members along with clinical implications for the patient with BC. Pathogenic variant carriers with newly diagnosed BC can opt for bilateral mastectomy rather than breast conservation at initial BC surgery,” the investigators wrote in JAMA Oncology. They noted that, presently, only 20%-30% of eligible patients are actually tested. “Testing all patients with breast cancer at diagnosis can increase testing access and uptake and identify many more pathogenic variant carriers for screening and prevention.”
To see if such a broad roll-out would be economically and medically feasible and beneficial, the investigators performed a modeling study involving 11,836 women with breast cancer, with data drawn from four large databases in the United Kingdom, the United States, and Australia.
The model compared annual costs, number of detected cases of breast cancer and ovarian cancer, and related rates of mortality between standard BRCA testing based on clinical criteria or family history versus high-risk multigene testing (BRCA1/BRCA2/PALB2) for all patients with breast cancer.
Resultant incremental cost-effectiveness ratios (ICERs) showed that in both the United Kingdom and the United States, multigene testing would cost, on average, significantly less than minimum willingness-to-pay thresholds of £30,000 per quality-adjusted life-year (QALY) and $100,000/QALY, respectively. In the United Kingdom, the estimated cost of multigene testing was £10,464/QALY from a payer’s perspective and £7,216/QALY from a societal perspective, the latter of which incorporates economic costs beyond the health care system. In the United States, these values rose to $65,661/QALY (payer perspective) and $61,618/QALY (societal perspective). Probabilistic sensitivity analysis suggested that multigene testing would be cost-effective for almost all health system simulations in the United Kingdom (98%-99%) and approximately two-thirds of those in the United States (64%-68%).
Epidemiologically, in the United Kingdom, multigene testing could potentially prevent 2,101 cases of breast cancer and ovarian cancer and 633 deaths per year, while in the United States, 9,733 cases might be prevented, with 2,406 lives saved.
“Our analysis suggests that an unselected testing strategy is extremely cost-effective for U.K. and U.S. health systems and provides a basis for change in current guidelines and policy to implement this strategy,” the investigators said.
The study was funded by the National Cancer Institute, the State of Washington, the Fred Hutchinson Cancer Research Center, and others. The investigators reported additional relationships with AstraZeneca, the Manchester National Institute for Health Research Biomedical Research Centre, Cancer Research UK, an others.
SOURCE: Sun et al. JAMA Oncol. 2019 Oct 3. doi: 10.1001/jamaoncol.2019.3323.
High-risk multigene testing for all patients with breast cancer may be a cost-effective way to reduce rates of breast cancer and ovarian cancer, according to investigators.
A microsimulation modeling study suggested that moving from standard BRCA testing based on clinical criteria or family history to widespread adoption of multigene testing could potentially save more than 2,400 lives per year in the United States, reported lead author Li Sun, MSc, of the London School of Hygiene & Tropical Medicine, and colleagues.
“Moving toward unselected BC testing may give an impetus for prevention in unaffected family members along with clinical implications for the patient with BC. Pathogenic variant carriers with newly diagnosed BC can opt for bilateral mastectomy rather than breast conservation at initial BC surgery,” the investigators wrote in JAMA Oncology. They noted that, presently, only 20%-30% of eligible patients are actually tested. “Testing all patients with breast cancer at diagnosis can increase testing access and uptake and identify many more pathogenic variant carriers for screening and prevention.”
To see if such a broad roll-out would be economically and medically feasible and beneficial, the investigators performed a modeling study involving 11,836 women with breast cancer, with data drawn from four large databases in the United Kingdom, the United States, and Australia.
The model compared annual costs, number of detected cases of breast cancer and ovarian cancer, and related rates of mortality between standard BRCA testing based on clinical criteria or family history versus high-risk multigene testing (BRCA1/BRCA2/PALB2) for all patients with breast cancer.
Resultant incremental cost-effectiveness ratios (ICERs) showed that in both the United Kingdom and the United States, multigene testing would cost, on average, significantly less than minimum willingness-to-pay thresholds of £30,000 per quality-adjusted life-year (QALY) and $100,000/QALY, respectively. In the United Kingdom, the estimated cost of multigene testing was £10,464/QALY from a payer’s perspective and £7,216/QALY from a societal perspective, the latter of which incorporates economic costs beyond the health care system. In the United States, these values rose to $65,661/QALY (payer perspective) and $61,618/QALY (societal perspective). Probabilistic sensitivity analysis suggested that multigene testing would be cost-effective for almost all health system simulations in the United Kingdom (98%-99%) and approximately two-thirds of those in the United States (64%-68%).
Epidemiologically, in the United Kingdom, multigene testing could potentially prevent 2,101 cases of breast cancer and ovarian cancer and 633 deaths per year, while in the United States, 9,733 cases might be prevented, with 2,406 lives saved.
“Our analysis suggests that an unselected testing strategy is extremely cost-effective for U.K. and U.S. health systems and provides a basis for change in current guidelines and policy to implement this strategy,” the investigators said.
The study was funded by the National Cancer Institute, the State of Washington, the Fred Hutchinson Cancer Research Center, and others. The investigators reported additional relationships with AstraZeneca, the Manchester National Institute for Health Research Biomedical Research Centre, Cancer Research UK, an others.
SOURCE: Sun et al. JAMA Oncol. 2019 Oct 3. doi: 10.1001/jamaoncol.2019.3323.
FROM JAMA ONCOLOGY
Ribociclib/fulvestrant boosts survival in advanced breast cancer
BARCELONA – In postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer, the combination of ribociclib (Kisqali) and fulvestrant (Faslodex) was associated with a significant survival benefit, compared with fulvestrant alone, investigators reported.
Among 726 postmenopausal patients in MONALEESA-3 randomly assigned to receive either ribociclib or placebo plus fulvestrant who were followed for a median of 39.4 months, the median overall survival (OS) was not reached for patients assigned to ribociclib, compared with 40 months for patients in the control (fulvestrant-only) arm, reported Dennis J. Slamon, MD, PhD from the University of California, Los Angeles
“The combined data set of MONALEESA-3 and MONALEESA-7 represent some 1,400 patients, and the largest body of evidence for overall survival for any [cyclin-dependent kinases 4/6] inhibitor, and these data demonstrate a consistent, meaningful prolongation of survival irrespective of menopausal status, pre or post, and irrespective of whether the patient is treated in the first line or the second line,” he said at the European Society for Medical Oncology Congress.
Results of the MONALEESA-7 trial, which showed an overall survival advantage for adding ribociclib to endocrine therapy in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, were reported at the 2019 annual meeting of the American Society of Clinical Oncology.
MONALEESA-3 investigators enrolled 726 postmenopausal women with hormone receptor–positive/HER2-negative advanced breast cancer who had received not more than one prior line of endocrine therapy for advanced disease, stratified them by the presence of liver and/or lung metastases and prior endocrine therapy, and then randomly assigned them to receive intramuscular fulvestrant 500 mg every 28 days, with an additional dose on day 15 of cycle one, plus either ribociclib 600 mg per day, 3 weeks on and 1 week off for every cycle, or placebo.
Dr. Slamon presented results of the primary endpoint of progression-free survival at the 2018 ASCO annual meeting, which showed a median PFS with ribociclib plus fulvestrant of 20.5 months versus 12.8 months for fulvestrant alone (hazard ratio, 5.93; P less than .0001). The overall survival data presented by Dr. Slamon at ESMO 2019 were not mature at that time.
As noted, at a median follow-up of 39.4 months, median overall survival was not reached in the combination arm, compared with 40 months in the fulvestrant/placebo arm, translating into a HR for risk of death with ribociclib of 0.724 (P = .00455).
Estimated OS rates at 36 months were 67% in the ribociclib arm versus 58.2% in the placebo arm, and at 42 months were 57.8% and 45.9%, respectively.
The survival benefit appeared consistent both for patients treated in the first line (median OS not reached vs. 45.1 months; HR, 0.70) and for those treated after early relapse of therapy for early breast or in the second line (median OS, 40.2 vs. 32.5 months, respectively; HR, 0.73). In both comparisons, however, the 95% confidence interval crossed 1, indicating that the results were not statistically significant.
An analysis of OS by subgroup indicated significant benefit or trends for most categories except Asian race, and treatment in Asia or Latin America, although the numbers of patients in each of those subgroups was less than 20, making it difficult to draw significant inferences from the findings, Dr. Slamon cautioned.
A descriptive analysis of updated PFS results were very similar to those previously reported, with a median of 20.6 months with ribociclib versus 12.8 months with placebo (HR, 0.587). Median PFS by line of therapy was 33.6 versus 19.2 months, respectively, in the first line, and 14.5 versus 9.1 months in the second line (HR, 0.546 and 0.571; P values not shown).
“CDK4/6 inhibitors not only improve progression-free survival in first- and second-line metastatic breast cancer, it also translates into an overall survival improvement. What more do we really want?” said invited discussant Sybil Loibl, MD, from Goethe University in Frankfurt, Germany.
“Outcome improves irrespective of pretreatment, menopausal status, endocrine sensitivity and site of metastases, which is good knowledge for our patients,” she said.
To date there are no biomarkers identified that can select subgroups that could experience particular benefit from CDK4/6 inhibitors. It may require a meta-analysis of all available clinical trial data on these agents in both the first and second line therapy to reveal potential differences among subgroups, she said.
MONALEESA-3 is supported by Novartis. Dr. Slamon disclosed a consulting or advisory role, research funding, honoraria, and travel expenses from Novartis and others. Dr. Loibl disclosed honoraria and research grants from Novartis and others.
SOURCE: Slamon DJ et al. ESMO 2019, Abstract LBA7_PR.
BARCELONA – In postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer, the combination of ribociclib (Kisqali) and fulvestrant (Faslodex) was associated with a significant survival benefit, compared with fulvestrant alone, investigators reported.
Among 726 postmenopausal patients in MONALEESA-3 randomly assigned to receive either ribociclib or placebo plus fulvestrant who were followed for a median of 39.4 months, the median overall survival (OS) was not reached for patients assigned to ribociclib, compared with 40 months for patients in the control (fulvestrant-only) arm, reported Dennis J. Slamon, MD, PhD from the University of California, Los Angeles
“The combined data set of MONALEESA-3 and MONALEESA-7 represent some 1,400 patients, and the largest body of evidence for overall survival for any [cyclin-dependent kinases 4/6] inhibitor, and these data demonstrate a consistent, meaningful prolongation of survival irrespective of menopausal status, pre or post, and irrespective of whether the patient is treated in the first line or the second line,” he said at the European Society for Medical Oncology Congress.
Results of the MONALEESA-7 trial, which showed an overall survival advantage for adding ribociclib to endocrine therapy in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, were reported at the 2019 annual meeting of the American Society of Clinical Oncology.
MONALEESA-3 investigators enrolled 726 postmenopausal women with hormone receptor–positive/HER2-negative advanced breast cancer who had received not more than one prior line of endocrine therapy for advanced disease, stratified them by the presence of liver and/or lung metastases and prior endocrine therapy, and then randomly assigned them to receive intramuscular fulvestrant 500 mg every 28 days, with an additional dose on day 15 of cycle one, plus either ribociclib 600 mg per day, 3 weeks on and 1 week off for every cycle, or placebo.
Dr. Slamon presented results of the primary endpoint of progression-free survival at the 2018 ASCO annual meeting, which showed a median PFS with ribociclib plus fulvestrant of 20.5 months versus 12.8 months for fulvestrant alone (hazard ratio, 5.93; P less than .0001). The overall survival data presented by Dr. Slamon at ESMO 2019 were not mature at that time.
As noted, at a median follow-up of 39.4 months, median overall survival was not reached in the combination arm, compared with 40 months in the fulvestrant/placebo arm, translating into a HR for risk of death with ribociclib of 0.724 (P = .00455).
Estimated OS rates at 36 months were 67% in the ribociclib arm versus 58.2% in the placebo arm, and at 42 months were 57.8% and 45.9%, respectively.
The survival benefit appeared consistent both for patients treated in the first line (median OS not reached vs. 45.1 months; HR, 0.70) and for those treated after early relapse of therapy for early breast or in the second line (median OS, 40.2 vs. 32.5 months, respectively; HR, 0.73). In both comparisons, however, the 95% confidence interval crossed 1, indicating that the results were not statistically significant.
An analysis of OS by subgroup indicated significant benefit or trends for most categories except Asian race, and treatment in Asia or Latin America, although the numbers of patients in each of those subgroups was less than 20, making it difficult to draw significant inferences from the findings, Dr. Slamon cautioned.
A descriptive analysis of updated PFS results were very similar to those previously reported, with a median of 20.6 months with ribociclib versus 12.8 months with placebo (HR, 0.587). Median PFS by line of therapy was 33.6 versus 19.2 months, respectively, in the first line, and 14.5 versus 9.1 months in the second line (HR, 0.546 and 0.571; P values not shown).
“CDK4/6 inhibitors not only improve progression-free survival in first- and second-line metastatic breast cancer, it also translates into an overall survival improvement. What more do we really want?” said invited discussant Sybil Loibl, MD, from Goethe University in Frankfurt, Germany.
“Outcome improves irrespective of pretreatment, menopausal status, endocrine sensitivity and site of metastases, which is good knowledge for our patients,” she said.
To date there are no biomarkers identified that can select subgroups that could experience particular benefit from CDK4/6 inhibitors. It may require a meta-analysis of all available clinical trial data on these agents in both the first and second line therapy to reveal potential differences among subgroups, she said.
MONALEESA-3 is supported by Novartis. Dr. Slamon disclosed a consulting or advisory role, research funding, honoraria, and travel expenses from Novartis and others. Dr. Loibl disclosed honoraria and research grants from Novartis and others.
SOURCE: Slamon DJ et al. ESMO 2019, Abstract LBA7_PR.
BARCELONA – In postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer, the combination of ribociclib (Kisqali) and fulvestrant (Faslodex) was associated with a significant survival benefit, compared with fulvestrant alone, investigators reported.
Among 726 postmenopausal patients in MONALEESA-3 randomly assigned to receive either ribociclib or placebo plus fulvestrant who were followed for a median of 39.4 months, the median overall survival (OS) was not reached for patients assigned to ribociclib, compared with 40 months for patients in the control (fulvestrant-only) arm, reported Dennis J. Slamon, MD, PhD from the University of California, Los Angeles
“The combined data set of MONALEESA-3 and MONALEESA-7 represent some 1,400 patients, and the largest body of evidence for overall survival for any [cyclin-dependent kinases 4/6] inhibitor, and these data demonstrate a consistent, meaningful prolongation of survival irrespective of menopausal status, pre or post, and irrespective of whether the patient is treated in the first line or the second line,” he said at the European Society for Medical Oncology Congress.
Results of the MONALEESA-7 trial, which showed an overall survival advantage for adding ribociclib to endocrine therapy in premenopausal women with hormone receptor–positive, HER2-negative breast cancer, were reported at the 2019 annual meeting of the American Society of Clinical Oncology.
MONALEESA-3 investigators enrolled 726 postmenopausal women with hormone receptor–positive/HER2-negative advanced breast cancer who had received not more than one prior line of endocrine therapy for advanced disease, stratified them by the presence of liver and/or lung metastases and prior endocrine therapy, and then randomly assigned them to receive intramuscular fulvestrant 500 mg every 28 days, with an additional dose on day 15 of cycle one, plus either ribociclib 600 mg per day, 3 weeks on and 1 week off for every cycle, or placebo.
Dr. Slamon presented results of the primary endpoint of progression-free survival at the 2018 ASCO annual meeting, which showed a median PFS with ribociclib plus fulvestrant of 20.5 months versus 12.8 months for fulvestrant alone (hazard ratio, 5.93; P less than .0001). The overall survival data presented by Dr. Slamon at ESMO 2019 were not mature at that time.
As noted, at a median follow-up of 39.4 months, median overall survival was not reached in the combination arm, compared with 40 months in the fulvestrant/placebo arm, translating into a HR for risk of death with ribociclib of 0.724 (P = .00455).
Estimated OS rates at 36 months were 67% in the ribociclib arm versus 58.2% in the placebo arm, and at 42 months were 57.8% and 45.9%, respectively.
The survival benefit appeared consistent both for patients treated in the first line (median OS not reached vs. 45.1 months; HR, 0.70) and for those treated after early relapse of therapy for early breast or in the second line (median OS, 40.2 vs. 32.5 months, respectively; HR, 0.73). In both comparisons, however, the 95% confidence interval crossed 1, indicating that the results were not statistically significant.
An analysis of OS by subgroup indicated significant benefit or trends for most categories except Asian race, and treatment in Asia or Latin America, although the numbers of patients in each of those subgroups was less than 20, making it difficult to draw significant inferences from the findings, Dr. Slamon cautioned.
A descriptive analysis of updated PFS results were very similar to those previously reported, with a median of 20.6 months with ribociclib versus 12.8 months with placebo (HR, 0.587). Median PFS by line of therapy was 33.6 versus 19.2 months, respectively, in the first line, and 14.5 versus 9.1 months in the second line (HR, 0.546 and 0.571; P values not shown).
“CDK4/6 inhibitors not only improve progression-free survival in first- and second-line metastatic breast cancer, it also translates into an overall survival improvement. What more do we really want?” said invited discussant Sybil Loibl, MD, from Goethe University in Frankfurt, Germany.
“Outcome improves irrespective of pretreatment, menopausal status, endocrine sensitivity and site of metastases, which is good knowledge for our patients,” she said.
To date there are no biomarkers identified that can select subgroups that could experience particular benefit from CDK4/6 inhibitors. It may require a meta-analysis of all available clinical trial data on these agents in both the first and second line therapy to reveal potential differences among subgroups, she said.
MONALEESA-3 is supported by Novartis. Dr. Slamon disclosed a consulting or advisory role, research funding, honoraria, and travel expenses from Novartis and others. Dr. Loibl disclosed honoraria and research grants from Novartis and others.
SOURCE: Slamon DJ et al. ESMO 2019, Abstract LBA7_PR.
REPORTING FROM ESMO 2019
Cancer burden: Multiple metrics needed to clarify the big picture
A new analysis of 40 years of U.S. cancer data underscores the importance of looking at multiple metrics to discern the complex interplay of factors influencing cancer burden in the population. Findings showed that the epidemiologic signature – a composite of two or three key metrics – differed across cancer types and was favorable in some cases and unfavorable in others.
“Epidemiologic signatures that illustrate trends in population-based data on cancer burden provide insight into true cancer occurrence, overdiagnosis, and treatment advances,” explain the analysts, led by H. Gilbert Welch, MD, MPH, Center for Surgery and Public Health, Brigham and Women’s Hospital, Boston. “They are important indicators of the potential contribution of environmental exposures, primary preventive interventions, new treatments, and changing diagnostic and screening practices.”
Dr. Welch and colleagues analyzed data for the years 1975 through 2015, assessing juxtaposed trends in incidence, mortality, and, when available, metastatic incidence (cancer already metastatic at diagnosis) for 11 cancers individually and for all cancers combined. Incidence data combining invasive and in situ cancers were obtained from the original nine Surveillance, Epidemiology, and End Results (SEER) registries, and mortality data were obtained from the National Vital Statistics System.
The analysts then explored implications of the epidemiologic signatures as they pertain to true cancer occurrence (the underlying incidence of clinically meaningful cancer), overdiagnosis (detection of cancers that will not cause symptoms or death), and treatment advances.
Individual cancers
Findings of the analysis, published in a special report in the New England Journal of Medicine, revealed three broad categories of epidemiologic signatures having different implications for the public health and oncology fields.
Desirable signatures showed, for example, declining mortality against a backdrop of stable incidence over the 40-year period, signaling improved treatment, as seen for chronic myeloid leukemia following introduction of imatinib (Gleevec), according to the analysts. Lung cancer incidence and mortality rose and fell in tandem, reflecting an increase in smoking followed by a decrease in response to prevention efforts. Stomach, cervical, and colorectal cancers had both falling incidence – likely reflecting a true decline in occurrence related to prevention and/or screening detection and subsequent treatment of precancerous lesions – and falling mortality.
Undesirable signatures showed a rising incidence juxtaposed with stable mortality and stable or rising metastatic incidence, signaling likely overdiagnosis, Dr. Welch and colleagues proposed. Three cancers—thyroid cancer, kidney cancer, and melanoma—fell into this category; for thyroid cancer and melanoma, fairly recent upticks in metastatic incidence may reflect upstaging.
Finally, some signatures showed mixed signals, with rising incidence and falling mortality. Breast cancer incidence rose and stabilized, coinciding with introduction of screening mammography, and possibly reflecting an increase in true cancer occurrence or overdiagnosis (with stable metastatic incidence favoring the latter), the analysts speculate. Declining mortality since the 1990s may be due to improved treatment or screening, or both. Prostate cancer incidence rose sharply with introduction of prostate-specific antigen screening but then fell to initial levels, suggesting sensitivity of this cancer to diagnostic scrutiny. Falling metastatic incidence indicates screening leads to earlier diagnosis in some cases, while declining mortality starting in the 1990s may again reflect improved treatment or screening, or both.
All cancers
The epidemiologic signature for all cancers combined differed somewhat by sex. Women had a rising incidence during the 1980s that was mainly driven by lung and breast cancers, according to Dr. Welch and colleagues; a continued rise since the mid-1990s was largely driven by melanoma, kidney cancer, and thyroid cancer. Declining mortality since 1990 has been primarily due to reductions in deaths from breast and colorectal cancers, and, more recently, lung cancer.
Men had a “volatile pattern” in the incidence of all cancers combined that was attributable to prostate cancer trends; drops in lung and colorectal cancer incidences were offset by rises in melanoma and kidney cancer incidences, the analysts proposed. Declining mortality since 1990 was more marked than that among women and reflects a longer period of decline in lung cancer mortality, plus reductions in deaths from prostate cancer and colorectal cancer.
“Falling mortality means that there has been real progress against cancer in the past 40 years – largely reflecting improved treatment and the decline of a uniquely powerful causal factor: cigarette smoking,” Dr. Welch and colleagues noted. “The lack of an accompanying fall in incidence is an unfortunate side effect of early cancer-detection efforts.”
Dr. Welch reported that he had no relevant disclosures. The analysis did not receive any specific funding.
SOURCE: Welch HG et al. N Engl J Med. 2019;381:1378-86. doi: 10.1056/NEJMsr1905447.
A new analysis of 40 years of U.S. cancer data underscores the importance of looking at multiple metrics to discern the complex interplay of factors influencing cancer burden in the population. Findings showed that the epidemiologic signature – a composite of two or three key metrics – differed across cancer types and was favorable in some cases and unfavorable in others.
“Epidemiologic signatures that illustrate trends in population-based data on cancer burden provide insight into true cancer occurrence, overdiagnosis, and treatment advances,” explain the analysts, led by H. Gilbert Welch, MD, MPH, Center for Surgery and Public Health, Brigham and Women’s Hospital, Boston. “They are important indicators of the potential contribution of environmental exposures, primary preventive interventions, new treatments, and changing diagnostic and screening practices.”
Dr. Welch and colleagues analyzed data for the years 1975 through 2015, assessing juxtaposed trends in incidence, mortality, and, when available, metastatic incidence (cancer already metastatic at diagnosis) for 11 cancers individually and for all cancers combined. Incidence data combining invasive and in situ cancers were obtained from the original nine Surveillance, Epidemiology, and End Results (SEER) registries, and mortality data were obtained from the National Vital Statistics System.
The analysts then explored implications of the epidemiologic signatures as they pertain to true cancer occurrence (the underlying incidence of clinically meaningful cancer), overdiagnosis (detection of cancers that will not cause symptoms or death), and treatment advances.
Individual cancers
Findings of the analysis, published in a special report in the New England Journal of Medicine, revealed three broad categories of epidemiologic signatures having different implications for the public health and oncology fields.
Desirable signatures showed, for example, declining mortality against a backdrop of stable incidence over the 40-year period, signaling improved treatment, as seen for chronic myeloid leukemia following introduction of imatinib (Gleevec), according to the analysts. Lung cancer incidence and mortality rose and fell in tandem, reflecting an increase in smoking followed by a decrease in response to prevention efforts. Stomach, cervical, and colorectal cancers had both falling incidence – likely reflecting a true decline in occurrence related to prevention and/or screening detection and subsequent treatment of precancerous lesions – and falling mortality.
Undesirable signatures showed a rising incidence juxtaposed with stable mortality and stable or rising metastatic incidence, signaling likely overdiagnosis, Dr. Welch and colleagues proposed. Three cancers—thyroid cancer, kidney cancer, and melanoma—fell into this category; for thyroid cancer and melanoma, fairly recent upticks in metastatic incidence may reflect upstaging.
Finally, some signatures showed mixed signals, with rising incidence and falling mortality. Breast cancer incidence rose and stabilized, coinciding with introduction of screening mammography, and possibly reflecting an increase in true cancer occurrence or overdiagnosis (with stable metastatic incidence favoring the latter), the analysts speculate. Declining mortality since the 1990s may be due to improved treatment or screening, or both. Prostate cancer incidence rose sharply with introduction of prostate-specific antigen screening but then fell to initial levels, suggesting sensitivity of this cancer to diagnostic scrutiny. Falling metastatic incidence indicates screening leads to earlier diagnosis in some cases, while declining mortality starting in the 1990s may again reflect improved treatment or screening, or both.
All cancers
The epidemiologic signature for all cancers combined differed somewhat by sex. Women had a rising incidence during the 1980s that was mainly driven by lung and breast cancers, according to Dr. Welch and colleagues; a continued rise since the mid-1990s was largely driven by melanoma, kidney cancer, and thyroid cancer. Declining mortality since 1990 has been primarily due to reductions in deaths from breast and colorectal cancers, and, more recently, lung cancer.
Men had a “volatile pattern” in the incidence of all cancers combined that was attributable to prostate cancer trends; drops in lung and colorectal cancer incidences were offset by rises in melanoma and kidney cancer incidences, the analysts proposed. Declining mortality since 1990 was more marked than that among women and reflects a longer period of decline in lung cancer mortality, plus reductions in deaths from prostate cancer and colorectal cancer.
“Falling mortality means that there has been real progress against cancer in the past 40 years – largely reflecting improved treatment and the decline of a uniquely powerful causal factor: cigarette smoking,” Dr. Welch and colleagues noted. “The lack of an accompanying fall in incidence is an unfortunate side effect of early cancer-detection efforts.”
Dr. Welch reported that he had no relevant disclosures. The analysis did not receive any specific funding.
SOURCE: Welch HG et al. N Engl J Med. 2019;381:1378-86. doi: 10.1056/NEJMsr1905447.
A new analysis of 40 years of U.S. cancer data underscores the importance of looking at multiple metrics to discern the complex interplay of factors influencing cancer burden in the population. Findings showed that the epidemiologic signature – a composite of two or three key metrics – differed across cancer types and was favorable in some cases and unfavorable in others.
“Epidemiologic signatures that illustrate trends in population-based data on cancer burden provide insight into true cancer occurrence, overdiagnosis, and treatment advances,” explain the analysts, led by H. Gilbert Welch, MD, MPH, Center for Surgery and Public Health, Brigham and Women’s Hospital, Boston. “They are important indicators of the potential contribution of environmental exposures, primary preventive interventions, new treatments, and changing diagnostic and screening practices.”
Dr. Welch and colleagues analyzed data for the years 1975 through 2015, assessing juxtaposed trends in incidence, mortality, and, when available, metastatic incidence (cancer already metastatic at diagnosis) for 11 cancers individually and for all cancers combined. Incidence data combining invasive and in situ cancers were obtained from the original nine Surveillance, Epidemiology, and End Results (SEER) registries, and mortality data were obtained from the National Vital Statistics System.
The analysts then explored implications of the epidemiologic signatures as they pertain to true cancer occurrence (the underlying incidence of clinically meaningful cancer), overdiagnosis (detection of cancers that will not cause symptoms or death), and treatment advances.
Individual cancers
Findings of the analysis, published in a special report in the New England Journal of Medicine, revealed three broad categories of epidemiologic signatures having different implications for the public health and oncology fields.
Desirable signatures showed, for example, declining mortality against a backdrop of stable incidence over the 40-year period, signaling improved treatment, as seen for chronic myeloid leukemia following introduction of imatinib (Gleevec), according to the analysts. Lung cancer incidence and mortality rose and fell in tandem, reflecting an increase in smoking followed by a decrease in response to prevention efforts. Stomach, cervical, and colorectal cancers had both falling incidence – likely reflecting a true decline in occurrence related to prevention and/or screening detection and subsequent treatment of precancerous lesions – and falling mortality.
Undesirable signatures showed a rising incidence juxtaposed with stable mortality and stable or rising metastatic incidence, signaling likely overdiagnosis, Dr. Welch and colleagues proposed. Three cancers—thyroid cancer, kidney cancer, and melanoma—fell into this category; for thyroid cancer and melanoma, fairly recent upticks in metastatic incidence may reflect upstaging.
Finally, some signatures showed mixed signals, with rising incidence and falling mortality. Breast cancer incidence rose and stabilized, coinciding with introduction of screening mammography, and possibly reflecting an increase in true cancer occurrence or overdiagnosis (with stable metastatic incidence favoring the latter), the analysts speculate. Declining mortality since the 1990s may be due to improved treatment or screening, or both. Prostate cancer incidence rose sharply with introduction of prostate-specific antigen screening but then fell to initial levels, suggesting sensitivity of this cancer to diagnostic scrutiny. Falling metastatic incidence indicates screening leads to earlier diagnosis in some cases, while declining mortality starting in the 1990s may again reflect improved treatment or screening, or both.
All cancers
The epidemiologic signature for all cancers combined differed somewhat by sex. Women had a rising incidence during the 1980s that was mainly driven by lung and breast cancers, according to Dr. Welch and colleagues; a continued rise since the mid-1990s was largely driven by melanoma, kidney cancer, and thyroid cancer. Declining mortality since 1990 has been primarily due to reductions in deaths from breast and colorectal cancers, and, more recently, lung cancer.
Men had a “volatile pattern” in the incidence of all cancers combined that was attributable to prostate cancer trends; drops in lung and colorectal cancer incidences were offset by rises in melanoma and kidney cancer incidences, the analysts proposed. Declining mortality since 1990 was more marked than that among women and reflects a longer period of decline in lung cancer mortality, plus reductions in deaths from prostate cancer and colorectal cancer.
“Falling mortality means that there has been real progress against cancer in the past 40 years – largely reflecting improved treatment and the decline of a uniquely powerful causal factor: cigarette smoking,” Dr. Welch and colleagues noted. “The lack of an accompanying fall in incidence is an unfortunate side effect of early cancer-detection efforts.”
Dr. Welch reported that he had no relevant disclosures. The analysis did not receive any specific funding.
SOURCE: Welch HG et al. N Engl J Med. 2019;381:1378-86. doi: 10.1056/NEJMsr1905447.
FROM NEW ENGLAND JOURNAL OF MEDICINE
MONARCH 2: Abemaciclib plus fulvestrant improves overall survival
BARCELONA – Adding the CDK4/6 inhibitor abemaciclib to fulvestrant significantly improves overall survival in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer patients who progressed on prior endocrine therapy, according to findings from the phase 3 MONARCH 2 trial.
At a median follow-up of 47.7 months, overall survival – a secondary study endpoint – was 46.7 months in 446 patients randomized to receive abemaciclib and fulvestrant, compared with 37.3 months in 223 patients who received placebo and fulvestrant (hazard ratio, 0.757), George W. Sledge, MD, reported at the European Society for Medical Oncology Congress.
The overall survival benefit was consistent across stratification factors, which included site of metastasis (visceral, bone, or other) and resistance to prior endocrine therapy (primary versus secondary), but it was most pronounced in patients with visceral disease (HR, 0.675) and primary resistance to prior endocrine therapy (HR, 0.686), said Dr. Sledge, a professor of medicine at Stanford (Calif.) Medical Center.
Progression-free survival, the primary study endpoint, was 16.4 and 9.3 months at the previously reported 2-year follow-up in the treatment and placebo groups, respectively, and the current analysis showed progression-free survival to be “highly consistent” with those findings (16.9 vs. 9.3 months; HR, 0.563), he said.
“Of note, and of interest for further follow-up, a landmark analysis at 3 years shows that approximately three times as many patients on the abemaciclib arm remained progression free, compared to the control arm,” he added, also noting that time from randomization to postdiscontinuation chemotherapy was prolonged with abemaciclib, compared with placebo (50.2 vs. 22.1 months; HR, 0.625).
“A highly significant result,” he said.
At the 47.7 month follow-up, 17% and 4% of patients in the treatment and placebo groups, respectively, remained on treatment.
An additional exploratory analysis showed that 5.8% of patients receiving abemaciclib crossed over to another CDK4/6 inhibitor after discontinuation of therapy, compared with 17.0% of those in the placebo group.
“CDK4/6 inhibitors have emerged as standard-of-care treatment for patients with HR+, HER2– breast cancer,” he said, noting that abemaciclib is a selective CDK4/6 inhibitor with continuous, twice-daily oral administration. “It is approved for monotherapy after progression on endocrine therapy and prior chemotherapy in the metastatic setting, and ... in combination with endocrine therapy in the front-line setting and after progression.”
The global, randomized, double-blind MONARCH 2 trial assessed abemaciclib + fulvestrant in women with advanced endocrine therapy–resistant HR+, HER2– advanced breast cancer, including pre- or perimenopausal women with ovarian suppression and postmenopausal women.
Study participants were randomized 2:1 to receive 500 mg of fulvestrant per label instructions plus 150 mg of abemaciclib every 12 hours or placebo.
Treatment-emergent adverse events in MONARCH 2 were consistent with those previously reported in the primary analysis, Dr. Sledge said.
“Continued follow-up of MONARCH 2 is ongoing to further characterize the overall survival benefit and to look at exploratory efficacy and correlative endpoints,” he noted.
Speaking about the findings during a press conference at the meeting, Nadia Harbeck, MD, a professor at Ludwig Maximilians University, Munich, Germany, said they have important practice-changing implications.
“We were always struggling with whether to give these drugs first or second line, and I think now if we consider the first-line survival benefit ... [first-line use] should be standard of care,” she said, referring to CDK4/6 inhibitors and to findings from MONARCH 2 and prior studies of the inhibitors, including the MONALEESA-3 trial presented at the ESMO Congress.
MONALEESA-3 showed similar overall survival results with the CDK4/6 inhibitor ribociclib in postmenopausal women with HR+, HER2– advanced breast cancer.
“The data are highly clinically meaningful; I think they are going to make a huge impact on how we treat breast cancer,” Dr. Harbeck said.
Dr. Sledge has received trial support, research grants, and travel accommodations from Eli Lilly and Company; is a board member for Tessa Therapeutics; and is a consultant for Syndax, Symphogen, and Verseau Therapeutics. Dr. Harbeck disclosed financial relationships with Agendia, Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Genomic Health, Lilly, MSD, Nanostring, Novartis, Odonate, Pfizer, Roche, Sandoz/Hexal, and Seattle Genetics. She is also director of the West German Study Group and a member of the German AGO Breast Committee.
SOURCE: Sledge G et al., ESMO 2019, Abstract LBA6-PR.
BARCELONA – Adding the CDK4/6 inhibitor abemaciclib to fulvestrant significantly improves overall survival in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer patients who progressed on prior endocrine therapy, according to findings from the phase 3 MONARCH 2 trial.
At a median follow-up of 47.7 months, overall survival – a secondary study endpoint – was 46.7 months in 446 patients randomized to receive abemaciclib and fulvestrant, compared with 37.3 months in 223 patients who received placebo and fulvestrant (hazard ratio, 0.757), George W. Sledge, MD, reported at the European Society for Medical Oncology Congress.
The overall survival benefit was consistent across stratification factors, which included site of metastasis (visceral, bone, or other) and resistance to prior endocrine therapy (primary versus secondary), but it was most pronounced in patients with visceral disease (HR, 0.675) and primary resistance to prior endocrine therapy (HR, 0.686), said Dr. Sledge, a professor of medicine at Stanford (Calif.) Medical Center.
Progression-free survival, the primary study endpoint, was 16.4 and 9.3 months at the previously reported 2-year follow-up in the treatment and placebo groups, respectively, and the current analysis showed progression-free survival to be “highly consistent” with those findings (16.9 vs. 9.3 months; HR, 0.563), he said.
“Of note, and of interest for further follow-up, a landmark analysis at 3 years shows that approximately three times as many patients on the abemaciclib arm remained progression free, compared to the control arm,” he added, also noting that time from randomization to postdiscontinuation chemotherapy was prolonged with abemaciclib, compared with placebo (50.2 vs. 22.1 months; HR, 0.625).
“A highly significant result,” he said.
At the 47.7 month follow-up, 17% and 4% of patients in the treatment and placebo groups, respectively, remained on treatment.
An additional exploratory analysis showed that 5.8% of patients receiving abemaciclib crossed over to another CDK4/6 inhibitor after discontinuation of therapy, compared with 17.0% of those in the placebo group.
“CDK4/6 inhibitors have emerged as standard-of-care treatment for patients with HR+, HER2– breast cancer,” he said, noting that abemaciclib is a selective CDK4/6 inhibitor with continuous, twice-daily oral administration. “It is approved for monotherapy after progression on endocrine therapy and prior chemotherapy in the metastatic setting, and ... in combination with endocrine therapy in the front-line setting and after progression.”
The global, randomized, double-blind MONARCH 2 trial assessed abemaciclib + fulvestrant in women with advanced endocrine therapy–resistant HR+, HER2– advanced breast cancer, including pre- or perimenopausal women with ovarian suppression and postmenopausal women.
Study participants were randomized 2:1 to receive 500 mg of fulvestrant per label instructions plus 150 mg of abemaciclib every 12 hours or placebo.
Treatment-emergent adverse events in MONARCH 2 were consistent with those previously reported in the primary analysis, Dr. Sledge said.
“Continued follow-up of MONARCH 2 is ongoing to further characterize the overall survival benefit and to look at exploratory efficacy and correlative endpoints,” he noted.
Speaking about the findings during a press conference at the meeting, Nadia Harbeck, MD, a professor at Ludwig Maximilians University, Munich, Germany, said they have important practice-changing implications.
“We were always struggling with whether to give these drugs first or second line, and I think now if we consider the first-line survival benefit ... [first-line use] should be standard of care,” she said, referring to CDK4/6 inhibitors and to findings from MONARCH 2 and prior studies of the inhibitors, including the MONALEESA-3 trial presented at the ESMO Congress.
MONALEESA-3 showed similar overall survival results with the CDK4/6 inhibitor ribociclib in postmenopausal women with HR+, HER2– advanced breast cancer.
“The data are highly clinically meaningful; I think they are going to make a huge impact on how we treat breast cancer,” Dr. Harbeck said.
Dr. Sledge has received trial support, research grants, and travel accommodations from Eli Lilly and Company; is a board member for Tessa Therapeutics; and is a consultant for Syndax, Symphogen, and Verseau Therapeutics. Dr. Harbeck disclosed financial relationships with Agendia, Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Genomic Health, Lilly, MSD, Nanostring, Novartis, Odonate, Pfizer, Roche, Sandoz/Hexal, and Seattle Genetics. She is also director of the West German Study Group and a member of the German AGO Breast Committee.
SOURCE: Sledge G et al., ESMO 2019, Abstract LBA6-PR.
BARCELONA – Adding the CDK4/6 inhibitor abemaciclib to fulvestrant significantly improves overall survival in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer patients who progressed on prior endocrine therapy, according to findings from the phase 3 MONARCH 2 trial.
At a median follow-up of 47.7 months, overall survival – a secondary study endpoint – was 46.7 months in 446 patients randomized to receive abemaciclib and fulvestrant, compared with 37.3 months in 223 patients who received placebo and fulvestrant (hazard ratio, 0.757), George W. Sledge, MD, reported at the European Society for Medical Oncology Congress.
The overall survival benefit was consistent across stratification factors, which included site of metastasis (visceral, bone, or other) and resistance to prior endocrine therapy (primary versus secondary), but it was most pronounced in patients with visceral disease (HR, 0.675) and primary resistance to prior endocrine therapy (HR, 0.686), said Dr. Sledge, a professor of medicine at Stanford (Calif.) Medical Center.
Progression-free survival, the primary study endpoint, was 16.4 and 9.3 months at the previously reported 2-year follow-up in the treatment and placebo groups, respectively, and the current analysis showed progression-free survival to be “highly consistent” with those findings (16.9 vs. 9.3 months; HR, 0.563), he said.
“Of note, and of interest for further follow-up, a landmark analysis at 3 years shows that approximately three times as many patients on the abemaciclib arm remained progression free, compared to the control arm,” he added, also noting that time from randomization to postdiscontinuation chemotherapy was prolonged with abemaciclib, compared with placebo (50.2 vs. 22.1 months; HR, 0.625).
“A highly significant result,” he said.
At the 47.7 month follow-up, 17% and 4% of patients in the treatment and placebo groups, respectively, remained on treatment.
An additional exploratory analysis showed that 5.8% of patients receiving abemaciclib crossed over to another CDK4/6 inhibitor after discontinuation of therapy, compared with 17.0% of those in the placebo group.
“CDK4/6 inhibitors have emerged as standard-of-care treatment for patients with HR+, HER2– breast cancer,” he said, noting that abemaciclib is a selective CDK4/6 inhibitor with continuous, twice-daily oral administration. “It is approved for monotherapy after progression on endocrine therapy and prior chemotherapy in the metastatic setting, and ... in combination with endocrine therapy in the front-line setting and after progression.”
The global, randomized, double-blind MONARCH 2 trial assessed abemaciclib + fulvestrant in women with advanced endocrine therapy–resistant HR+, HER2– advanced breast cancer, including pre- or perimenopausal women with ovarian suppression and postmenopausal women.
Study participants were randomized 2:1 to receive 500 mg of fulvestrant per label instructions plus 150 mg of abemaciclib every 12 hours or placebo.
Treatment-emergent adverse events in MONARCH 2 were consistent with those previously reported in the primary analysis, Dr. Sledge said.
“Continued follow-up of MONARCH 2 is ongoing to further characterize the overall survival benefit and to look at exploratory efficacy and correlative endpoints,” he noted.
Speaking about the findings during a press conference at the meeting, Nadia Harbeck, MD, a professor at Ludwig Maximilians University, Munich, Germany, said they have important practice-changing implications.
“We were always struggling with whether to give these drugs first or second line, and I think now if we consider the first-line survival benefit ... [first-line use] should be standard of care,” she said, referring to CDK4/6 inhibitors and to findings from MONARCH 2 and prior studies of the inhibitors, including the MONALEESA-3 trial presented at the ESMO Congress.
MONALEESA-3 showed similar overall survival results with the CDK4/6 inhibitor ribociclib in postmenopausal women with HR+, HER2– advanced breast cancer.
“The data are highly clinically meaningful; I think they are going to make a huge impact on how we treat breast cancer,” Dr. Harbeck said.
Dr. Sledge has received trial support, research grants, and travel accommodations from Eli Lilly and Company; is a board member for Tessa Therapeutics; and is a consultant for Syndax, Symphogen, and Verseau Therapeutics. Dr. Harbeck disclosed financial relationships with Agendia, Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Genomic Health, Lilly, MSD, Nanostring, Novartis, Odonate, Pfizer, Roche, Sandoz/Hexal, and Seattle Genetics. She is also director of the West German Study Group and a member of the German AGO Breast Committee.
SOURCE: Sledge G et al., ESMO 2019, Abstract LBA6-PR.
REPORTING FROM ESMO 2019
Gene recurrence score helps predict successful combination therapy for early breast cancer
alone in a study of 1,389 women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer.
“The 21-gene recurrence score (RS) assay provides prognostic information for distant recurrence in hormone-receptor–positive, ERBB2-negative early breast cancer that is independent of clinicopathologic features and is also predictive of chemotherapy benefit when the RS is high,” wrote Joseph A. Sparano, MD, of Montefiore Medical Center, New York, and his colleagues. However, little is known about how this risk score applies to women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer, they said.
In the study published in JAMA Oncology, they identified 1,389 women with a recurrence score of 26-100 (the definition of a high recurrence risk). The average age of the patients was 56 years, and 71% were postmenopausal.
In addition to receiving endocrine therapy, the women were randomized to no chemotherapy (89 patients) or one of several chemotherapy regimens including docetaxel/cyclophosphamide (589 patients), anthracycline without a taxane (334 patients), an anthracycline and taxane (244 patients), cyclophosphamide/methotrexate/5-fluorouracil (52 patients), and other regimens (81 patients). Among those treated with chemotherapy, overall survival (OS) at 5 years was 96% and estimated rates of freedom from recurrence of breast cancer at a distant site, and from a distant and/or local regional site, at 5 years were 93% and 91%, respectively. At 5 years, the estimated rate of invasive disease–free survival (IDFS) was 88%.
When broken down by chemotherapy regimen, 5-year rates of freedom from recurrence of breast cancer at a distant site ranged from 92.3% to 95.5%, except for 88.5% for the cyclophosphamide/methotrexate/5-fluorouracil (CMF) group; the rate was 92.6% for patients in the no-chemotherapy group.
The 5-year rates of IDFS ranged from 84% to 91.3% in the chemotherapy groups, compared with 79.7% in the no-chemotherapy group.
The expected rates of distant recurrence in the overall patient population if treated with endocrine therapy alone was 78.8% at 5 years and 65.4% at 9 years, the researchers said. Rates among the patients with an RS of 26-30 if treated with endocrine therapy alone were 89.6% at 5 years and 80.6% at 9 years; rates for those with an RS of 31-100 were 70.7% and 54% for 5 and 9 years, respectively.
The study findings were limited by several factors including a lack of randomization to endocrine therapy alone and the relatively short follow-up period, the researchers noted. However, strengths include the large sample size and high rate of compliance with chemotherapy.
The results support data from previous studies and “add to the evidence base supporting the use of the 21-gene RS assay to guide the use of adjuvant chemotherapy in patients with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer,” they concluded.
The study was supported in part by the National Cancer Institute, the Canadian Cancer Society Research Institute, the Breast Cancer Research Foundation, the Komen Foundation, and the Breast Cancer Research Stamp issued by the United States Postal Service. Dr. Sparano disclosed grants from the National Cancer Institute. Of the remaining authors, several disclosed receiving personal or speaker fees from the assay manufacturer, Genomic Health; one author received funding from the company during the study.
SOURCE: Sparano J et al. JAMA Oncol. 2019 Sep 30. doi: 10.1001/jamaoncol.2019.4794.
alone in a study of 1,389 women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer.
“The 21-gene recurrence score (RS) assay provides prognostic information for distant recurrence in hormone-receptor–positive, ERBB2-negative early breast cancer that is independent of clinicopathologic features and is also predictive of chemotherapy benefit when the RS is high,” wrote Joseph A. Sparano, MD, of Montefiore Medical Center, New York, and his colleagues. However, little is known about how this risk score applies to women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer, they said.
In the study published in JAMA Oncology, they identified 1,389 women with a recurrence score of 26-100 (the definition of a high recurrence risk). The average age of the patients was 56 years, and 71% were postmenopausal.
In addition to receiving endocrine therapy, the women were randomized to no chemotherapy (89 patients) or one of several chemotherapy regimens including docetaxel/cyclophosphamide (589 patients), anthracycline without a taxane (334 patients), an anthracycline and taxane (244 patients), cyclophosphamide/methotrexate/5-fluorouracil (52 patients), and other regimens (81 patients). Among those treated with chemotherapy, overall survival (OS) at 5 years was 96% and estimated rates of freedom from recurrence of breast cancer at a distant site, and from a distant and/or local regional site, at 5 years were 93% and 91%, respectively. At 5 years, the estimated rate of invasive disease–free survival (IDFS) was 88%.
When broken down by chemotherapy regimen, 5-year rates of freedom from recurrence of breast cancer at a distant site ranged from 92.3% to 95.5%, except for 88.5% for the cyclophosphamide/methotrexate/5-fluorouracil (CMF) group; the rate was 92.6% for patients in the no-chemotherapy group.
The 5-year rates of IDFS ranged from 84% to 91.3% in the chemotherapy groups, compared with 79.7% in the no-chemotherapy group.
The expected rates of distant recurrence in the overall patient population if treated with endocrine therapy alone was 78.8% at 5 years and 65.4% at 9 years, the researchers said. Rates among the patients with an RS of 26-30 if treated with endocrine therapy alone were 89.6% at 5 years and 80.6% at 9 years; rates for those with an RS of 31-100 were 70.7% and 54% for 5 and 9 years, respectively.
The study findings were limited by several factors including a lack of randomization to endocrine therapy alone and the relatively short follow-up period, the researchers noted. However, strengths include the large sample size and high rate of compliance with chemotherapy.
The results support data from previous studies and “add to the evidence base supporting the use of the 21-gene RS assay to guide the use of adjuvant chemotherapy in patients with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer,” they concluded.
The study was supported in part by the National Cancer Institute, the Canadian Cancer Society Research Institute, the Breast Cancer Research Foundation, the Komen Foundation, and the Breast Cancer Research Stamp issued by the United States Postal Service. Dr. Sparano disclosed grants from the National Cancer Institute. Of the remaining authors, several disclosed receiving personal or speaker fees from the assay manufacturer, Genomic Health; one author received funding from the company during the study.
SOURCE: Sparano J et al. JAMA Oncol. 2019 Sep 30. doi: 10.1001/jamaoncol.2019.4794.
alone in a study of 1,389 women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer.
“The 21-gene recurrence score (RS) assay provides prognostic information for distant recurrence in hormone-receptor–positive, ERBB2-negative early breast cancer that is independent of clinicopathologic features and is also predictive of chemotherapy benefit when the RS is high,” wrote Joseph A. Sparano, MD, of Montefiore Medical Center, New York, and his colleagues. However, little is known about how this risk score applies to women with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer, they said.
In the study published in JAMA Oncology, they identified 1,389 women with a recurrence score of 26-100 (the definition of a high recurrence risk). The average age of the patients was 56 years, and 71% were postmenopausal.
In addition to receiving endocrine therapy, the women were randomized to no chemotherapy (89 patients) or one of several chemotherapy regimens including docetaxel/cyclophosphamide (589 patients), anthracycline without a taxane (334 patients), an anthracycline and taxane (244 patients), cyclophosphamide/methotrexate/5-fluorouracil (52 patients), and other regimens (81 patients). Among those treated with chemotherapy, overall survival (OS) at 5 years was 96% and estimated rates of freedom from recurrence of breast cancer at a distant site, and from a distant and/or local regional site, at 5 years were 93% and 91%, respectively. At 5 years, the estimated rate of invasive disease–free survival (IDFS) was 88%.
When broken down by chemotherapy regimen, 5-year rates of freedom from recurrence of breast cancer at a distant site ranged from 92.3% to 95.5%, except for 88.5% for the cyclophosphamide/methotrexate/5-fluorouracil (CMF) group; the rate was 92.6% for patients in the no-chemotherapy group.
The 5-year rates of IDFS ranged from 84% to 91.3% in the chemotherapy groups, compared with 79.7% in the no-chemotherapy group.
The expected rates of distant recurrence in the overall patient population if treated with endocrine therapy alone was 78.8% at 5 years and 65.4% at 9 years, the researchers said. Rates among the patients with an RS of 26-30 if treated with endocrine therapy alone were 89.6% at 5 years and 80.6% at 9 years; rates for those with an RS of 31-100 were 70.7% and 54% for 5 and 9 years, respectively.
The study findings were limited by several factors including a lack of randomization to endocrine therapy alone and the relatively short follow-up period, the researchers noted. However, strengths include the large sample size and high rate of compliance with chemotherapy.
The results support data from previous studies and “add to the evidence base supporting the use of the 21-gene RS assay to guide the use of adjuvant chemotherapy in patients with hormone receptor–positive, ERBB2-negative, axillary node–negative breast cancer,” they concluded.
The study was supported in part by the National Cancer Institute, the Canadian Cancer Society Research Institute, the Breast Cancer Research Foundation, the Komen Foundation, and the Breast Cancer Research Stamp issued by the United States Postal Service. Dr. Sparano disclosed grants from the National Cancer Institute. Of the remaining authors, several disclosed receiving personal or speaker fees from the assay manufacturer, Genomic Health; one author received funding from the company during the study.
SOURCE: Sparano J et al. JAMA Oncol. 2019 Sep 30. doi: 10.1001/jamaoncol.2019.4794.
FROM JAMA ONCOLOGY