User login
Tumor surroundings may hold key to TNBC treatment optimization
Tumor microenvironment (TME) profiles could help tailor treatment for triple-negative breast cancer (TNBC), with the TME profile of the immunomodulatory subtype of TNBC making it the most susceptible to the effects of the immune checkpoint inhibitors.
“This study allowed us to gain more insight into the complex interactions between tumor cells and their microenvironment, in particular immune cells,” wrote a team of Belgian and Canadian researchers. Their report is in the Journal of the National Cancer Institute.
At least five molecular subtypes of TNBC have been identified but little was previously known about the heterogeneity of their surroundings, noted Yacine Bareche, MSc, of Institut Jules Bordet, Université Libre de Bruxelles, Brussels, and associates. They looked at a series of 1,512 TNBC samples from four large and publicly available transcriptomic and genomic datasets and their TME, which is made up of many cell types – fibroblasts, adipose, and immune-inflammatory cells – and blood and lymphatic vascular networks.
The researchers explained that each of the four TNBC subtypes identified so far have “distinct mutational profiles, genomic alterations, and biological processes” which were matched by differences in their surrounding environments.
For instance, the immunomodulatory subtype of TNBC was associated with high expression of “adaptive immune-related gene signatures and a fully inflamed spatial pattern appearing to be the optimal candidate for immune check-point inhibitors.” By contrast, Mr. Bareche and coauthors said that “most mesenchymal stem-like and luminal androgen receptor TNBC tumors had an immunosuppressive phenotype with high expression levels of stromal gene signatures.”
The findings include “novel evidence” of how TNBC tumors may become resistant to the effects of immune checkpoint inhibitors.
The results demonstrate that each TNBC subtype is associated with specific TME profiles, “setting the ground for a rationale tailoring of immunotherapy in TNBC patients,” the researchers noted. Mr. Bareche and associates cautioned, however, that “prospective validation of our findings is warranted before their clinical implementation.”
The study was supported by a grant from the Breast Cancer Research Foundation. The researchers had no conflicts of interest.
SOURCE: Bareche Y et al. J Natl Cancer Inst. 2019 Oct 29. doi: 10.1093/jnci/djz208.
Of particular interest in the study by Bareche et al. is the different distribution of key immune targets. The observed distinctions between the immunomodulatory and basal-like TNBC subtypes, for example, might enable more rational trial design in which immunotherapy is preferentially evaluated in the more susceptible immunomodulatory tumors rather than basal-like tumors. Their findings also suggest that TNBC tumors other than the immunomodulatory subtype might need additional approaches to make them more susceptible to immune therapy or indeed require completely different treatment approaches. Immunologic differences between TNBC subtype microenvironments are highlighted but there are also higher-level domains identified – such as in the immune response, vascularization, stromal involvement and so on – that could make the research more generally applicable in the study and refinement of novel therapeutic strategies. Their work is just one of many steps forward in looking for predictive markers of a growing number of precision treatments for breast and other cancers.
Lior Z. Braunstein, MD, and Nadeem Riaz, MD, MSc are radiation oncologists at Memorial Sloan Kettering Cancer Center, New York. Dr. Riaz is associate director of genomics operations, Immunogenomics and Precision Oncology Platform. Their comments are summarized from the editorial accompanying the study by Bareche et al.; neither had conflicts of interest.
Of particular interest in the study by Bareche et al. is the different distribution of key immune targets. The observed distinctions between the immunomodulatory and basal-like TNBC subtypes, for example, might enable more rational trial design in which immunotherapy is preferentially evaluated in the more susceptible immunomodulatory tumors rather than basal-like tumors. Their findings also suggest that TNBC tumors other than the immunomodulatory subtype might need additional approaches to make them more susceptible to immune therapy or indeed require completely different treatment approaches. Immunologic differences between TNBC subtype microenvironments are highlighted but there are also higher-level domains identified – such as in the immune response, vascularization, stromal involvement and so on – that could make the research more generally applicable in the study and refinement of novel therapeutic strategies. Their work is just one of many steps forward in looking for predictive markers of a growing number of precision treatments for breast and other cancers.
Lior Z. Braunstein, MD, and Nadeem Riaz, MD, MSc are radiation oncologists at Memorial Sloan Kettering Cancer Center, New York. Dr. Riaz is associate director of genomics operations, Immunogenomics and Precision Oncology Platform. Their comments are summarized from the editorial accompanying the study by Bareche et al.; neither had conflicts of interest.
Of particular interest in the study by Bareche et al. is the different distribution of key immune targets. The observed distinctions between the immunomodulatory and basal-like TNBC subtypes, for example, might enable more rational trial design in which immunotherapy is preferentially evaluated in the more susceptible immunomodulatory tumors rather than basal-like tumors. Their findings also suggest that TNBC tumors other than the immunomodulatory subtype might need additional approaches to make them more susceptible to immune therapy or indeed require completely different treatment approaches. Immunologic differences between TNBC subtype microenvironments are highlighted but there are also higher-level domains identified – such as in the immune response, vascularization, stromal involvement and so on – that could make the research more generally applicable in the study and refinement of novel therapeutic strategies. Their work is just one of many steps forward in looking for predictive markers of a growing number of precision treatments for breast and other cancers.
Lior Z. Braunstein, MD, and Nadeem Riaz, MD, MSc are radiation oncologists at Memorial Sloan Kettering Cancer Center, New York. Dr. Riaz is associate director of genomics operations, Immunogenomics and Precision Oncology Platform. Their comments are summarized from the editorial accompanying the study by Bareche et al.; neither had conflicts of interest.
Tumor microenvironment (TME) profiles could help tailor treatment for triple-negative breast cancer (TNBC), with the TME profile of the immunomodulatory subtype of TNBC making it the most susceptible to the effects of the immune checkpoint inhibitors.
“This study allowed us to gain more insight into the complex interactions between tumor cells and their microenvironment, in particular immune cells,” wrote a team of Belgian and Canadian researchers. Their report is in the Journal of the National Cancer Institute.
At least five molecular subtypes of TNBC have been identified but little was previously known about the heterogeneity of their surroundings, noted Yacine Bareche, MSc, of Institut Jules Bordet, Université Libre de Bruxelles, Brussels, and associates. They looked at a series of 1,512 TNBC samples from four large and publicly available transcriptomic and genomic datasets and their TME, which is made up of many cell types – fibroblasts, adipose, and immune-inflammatory cells – and blood and lymphatic vascular networks.
The researchers explained that each of the four TNBC subtypes identified so far have “distinct mutational profiles, genomic alterations, and biological processes” which were matched by differences in their surrounding environments.
For instance, the immunomodulatory subtype of TNBC was associated with high expression of “adaptive immune-related gene signatures and a fully inflamed spatial pattern appearing to be the optimal candidate for immune check-point inhibitors.” By contrast, Mr. Bareche and coauthors said that “most mesenchymal stem-like and luminal androgen receptor TNBC tumors had an immunosuppressive phenotype with high expression levels of stromal gene signatures.”
The findings include “novel evidence” of how TNBC tumors may become resistant to the effects of immune checkpoint inhibitors.
The results demonstrate that each TNBC subtype is associated with specific TME profiles, “setting the ground for a rationale tailoring of immunotherapy in TNBC patients,” the researchers noted. Mr. Bareche and associates cautioned, however, that “prospective validation of our findings is warranted before their clinical implementation.”
The study was supported by a grant from the Breast Cancer Research Foundation. The researchers had no conflicts of interest.
SOURCE: Bareche Y et al. J Natl Cancer Inst. 2019 Oct 29. doi: 10.1093/jnci/djz208.
Tumor microenvironment (TME) profiles could help tailor treatment for triple-negative breast cancer (TNBC), with the TME profile of the immunomodulatory subtype of TNBC making it the most susceptible to the effects of the immune checkpoint inhibitors.
“This study allowed us to gain more insight into the complex interactions between tumor cells and their microenvironment, in particular immune cells,” wrote a team of Belgian and Canadian researchers. Their report is in the Journal of the National Cancer Institute.
At least five molecular subtypes of TNBC have been identified but little was previously known about the heterogeneity of their surroundings, noted Yacine Bareche, MSc, of Institut Jules Bordet, Université Libre de Bruxelles, Brussels, and associates. They looked at a series of 1,512 TNBC samples from four large and publicly available transcriptomic and genomic datasets and their TME, which is made up of many cell types – fibroblasts, adipose, and immune-inflammatory cells – and blood and lymphatic vascular networks.
The researchers explained that each of the four TNBC subtypes identified so far have “distinct mutational profiles, genomic alterations, and biological processes” which were matched by differences in their surrounding environments.
For instance, the immunomodulatory subtype of TNBC was associated with high expression of “adaptive immune-related gene signatures and a fully inflamed spatial pattern appearing to be the optimal candidate for immune check-point inhibitors.” By contrast, Mr. Bareche and coauthors said that “most mesenchymal stem-like and luminal androgen receptor TNBC tumors had an immunosuppressive phenotype with high expression levels of stromal gene signatures.”
The findings include “novel evidence” of how TNBC tumors may become resistant to the effects of immune checkpoint inhibitors.
The results demonstrate that each TNBC subtype is associated with specific TME profiles, “setting the ground for a rationale tailoring of immunotherapy in TNBC patients,” the researchers noted. Mr. Bareche and associates cautioned, however, that “prospective validation of our findings is warranted before their clinical implementation.”
The study was supported by a grant from the Breast Cancer Research Foundation. The researchers had no conflicts of interest.
SOURCE: Bareche Y et al. J Natl Cancer Inst. 2019 Oct 29. doi: 10.1093/jnci/djz208.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Though metastatic breast cancer survival is improving, rates vary by region
Though survival rates of patients with metastatic breast cancer (MBC) have increased over the last 2 decades, a new study has indicated disparities exist across regions and by variables like age and race.
“It appears from these results that we may be at a crossroads for MBC treatment and survival,” wrote Judith A. Malmgren, PhD, of the University of Washington and her coauthors. The study was published in Cancer. “Access to appropriate, timely, and up‐to‐date diagnosis, care, treatment, and surveillance could turn this fatal disease into a chronic and treatable phenomenon, depending on patient factors, molecular subtype, and insurance capacity to pay for treatment,” they said.
To determine how breast cancer outcomes might vary across regions, the researchers compared breast cancer–specific survival rates (BCSS) from Surveillance, Epidemiology, and End Results-9 (SEER-9) registry data minus a regional subset from the Seattle-Puget Sound (S-PS) region (n = 12,121) to patients from that S-PS region (n = 1,931) and to an individual cohort in that area (n = 261). Five-year BCSS rates were calculated for three time periods: 1990‐1998, 1999‐2004, and 2005‐2011.
All analyzed patients were diagnosed with a first primary, de novo, stage IV breast cancer between the ages of 25 and 84 years from 1990 to 2011. Patients in the SEER-9 group and the S-PS region had a mean age of 61 years, compared with the individual cohort’s mean age of 55 years. Patients in the individual cohort were more likely to reside in a major metropolitan area of over 1 million people, compared with the SEER group and the S-PS region (86% versus 61% and 58%, respectively).
Patients in the SEER-9 group had improved BCSS rates over the study period, from 19% in 1990-1998 (95% confidence interval, 18%-21%; P less than .001) to 26% in 2005-2011 (95% CI, 24%-27%; P less than .001). Patients in the S-PS region saw even greater improvements in BCSS rates, from 21% in 1990-1998 (95% CI, 18%-24%; P less than .001) to 35% in 2005-2011 (95% CI, 32%-39%; P less than .001). But the largest improvement in survival rates came from patients in the individual cohort, who went from 29% in 1990-1998 (95% CI, 18%-37%; P less than .001) to 56% in 2005-2011 (95% CI, 45%-65%; P = .004).
In a proportional hazards model for breast cancer–specific death, reduced hazard in the SEER-9 group was associated with surgery (hazard ratio, 0.58; 95% CI, 0.55-0.61; P less than .001), an age less than 70 (HR, 0.77; 95% CI, 0.73-0.82; P less than .001) and white race (HR, 0.84; 95% CI, 0.79-0.89; P less than .001). Similar associations were seen in the S-PS region with surgery (HR, 0.57; 95% CI, 0.50-0.66; P less than .001) and an age less than 70 (HR, 0.72; 95% CI, 0.62-0.84; P less than .001), but not white race.
The study results “indicate that the stage IV population that is living longer may be benefiting from many of the same therapies used to treat early breast cancer, especially for patients who are able to handle adjuvant chemotherapy treatment and are HR‐positive,” the researchers said. “However, the lag in survival improvement across different population‐based, geographic regions suggests that some groups and regions may benefit unequally from treatment advances as well as timely diagnosis.”
The study was funded by the Kaplan Cancer Research Fund, the Metastatic Breast Cancer Alliance, and the Surveillance, Epidemiology, and End Results Cancer Surveillance System program of the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Malmgren JA et al. Cancer. 2019 Oct 22. doi: 10.1002/cncr.32531.
Though survival rates of patients with metastatic breast cancer (MBC) have increased over the last 2 decades, a new study has indicated disparities exist across regions and by variables like age and race.
“It appears from these results that we may be at a crossroads for MBC treatment and survival,” wrote Judith A. Malmgren, PhD, of the University of Washington and her coauthors. The study was published in Cancer. “Access to appropriate, timely, and up‐to‐date diagnosis, care, treatment, and surveillance could turn this fatal disease into a chronic and treatable phenomenon, depending on patient factors, molecular subtype, and insurance capacity to pay for treatment,” they said.
To determine how breast cancer outcomes might vary across regions, the researchers compared breast cancer–specific survival rates (BCSS) from Surveillance, Epidemiology, and End Results-9 (SEER-9) registry data minus a regional subset from the Seattle-Puget Sound (S-PS) region (n = 12,121) to patients from that S-PS region (n = 1,931) and to an individual cohort in that area (n = 261). Five-year BCSS rates were calculated for three time periods: 1990‐1998, 1999‐2004, and 2005‐2011.
All analyzed patients were diagnosed with a first primary, de novo, stage IV breast cancer between the ages of 25 and 84 years from 1990 to 2011. Patients in the SEER-9 group and the S-PS region had a mean age of 61 years, compared with the individual cohort’s mean age of 55 years. Patients in the individual cohort were more likely to reside in a major metropolitan area of over 1 million people, compared with the SEER group and the S-PS region (86% versus 61% and 58%, respectively).
Patients in the SEER-9 group had improved BCSS rates over the study period, from 19% in 1990-1998 (95% confidence interval, 18%-21%; P less than .001) to 26% in 2005-2011 (95% CI, 24%-27%; P less than .001). Patients in the S-PS region saw even greater improvements in BCSS rates, from 21% in 1990-1998 (95% CI, 18%-24%; P less than .001) to 35% in 2005-2011 (95% CI, 32%-39%; P less than .001). But the largest improvement in survival rates came from patients in the individual cohort, who went from 29% in 1990-1998 (95% CI, 18%-37%; P less than .001) to 56% in 2005-2011 (95% CI, 45%-65%; P = .004).
In a proportional hazards model for breast cancer–specific death, reduced hazard in the SEER-9 group was associated with surgery (hazard ratio, 0.58; 95% CI, 0.55-0.61; P less than .001), an age less than 70 (HR, 0.77; 95% CI, 0.73-0.82; P less than .001) and white race (HR, 0.84; 95% CI, 0.79-0.89; P less than .001). Similar associations were seen in the S-PS region with surgery (HR, 0.57; 95% CI, 0.50-0.66; P less than .001) and an age less than 70 (HR, 0.72; 95% CI, 0.62-0.84; P less than .001), but not white race.
The study results “indicate that the stage IV population that is living longer may be benefiting from many of the same therapies used to treat early breast cancer, especially for patients who are able to handle adjuvant chemotherapy treatment and are HR‐positive,” the researchers said. “However, the lag in survival improvement across different population‐based, geographic regions suggests that some groups and regions may benefit unequally from treatment advances as well as timely diagnosis.”
The study was funded by the Kaplan Cancer Research Fund, the Metastatic Breast Cancer Alliance, and the Surveillance, Epidemiology, and End Results Cancer Surveillance System program of the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Malmgren JA et al. Cancer. 2019 Oct 22. doi: 10.1002/cncr.32531.
Though survival rates of patients with metastatic breast cancer (MBC) have increased over the last 2 decades, a new study has indicated disparities exist across regions and by variables like age and race.
“It appears from these results that we may be at a crossroads for MBC treatment and survival,” wrote Judith A. Malmgren, PhD, of the University of Washington and her coauthors. The study was published in Cancer. “Access to appropriate, timely, and up‐to‐date diagnosis, care, treatment, and surveillance could turn this fatal disease into a chronic and treatable phenomenon, depending on patient factors, molecular subtype, and insurance capacity to pay for treatment,” they said.
To determine how breast cancer outcomes might vary across regions, the researchers compared breast cancer–specific survival rates (BCSS) from Surveillance, Epidemiology, and End Results-9 (SEER-9) registry data minus a regional subset from the Seattle-Puget Sound (S-PS) region (n = 12,121) to patients from that S-PS region (n = 1,931) and to an individual cohort in that area (n = 261). Five-year BCSS rates were calculated for three time periods: 1990‐1998, 1999‐2004, and 2005‐2011.
All analyzed patients were diagnosed with a first primary, de novo, stage IV breast cancer between the ages of 25 and 84 years from 1990 to 2011. Patients in the SEER-9 group and the S-PS region had a mean age of 61 years, compared with the individual cohort’s mean age of 55 years. Patients in the individual cohort were more likely to reside in a major metropolitan area of over 1 million people, compared with the SEER group and the S-PS region (86% versus 61% and 58%, respectively).
Patients in the SEER-9 group had improved BCSS rates over the study period, from 19% in 1990-1998 (95% confidence interval, 18%-21%; P less than .001) to 26% in 2005-2011 (95% CI, 24%-27%; P less than .001). Patients in the S-PS region saw even greater improvements in BCSS rates, from 21% in 1990-1998 (95% CI, 18%-24%; P less than .001) to 35% in 2005-2011 (95% CI, 32%-39%; P less than .001). But the largest improvement in survival rates came from patients in the individual cohort, who went from 29% in 1990-1998 (95% CI, 18%-37%; P less than .001) to 56% in 2005-2011 (95% CI, 45%-65%; P = .004).
In a proportional hazards model for breast cancer–specific death, reduced hazard in the SEER-9 group was associated with surgery (hazard ratio, 0.58; 95% CI, 0.55-0.61; P less than .001), an age less than 70 (HR, 0.77; 95% CI, 0.73-0.82; P less than .001) and white race (HR, 0.84; 95% CI, 0.79-0.89; P less than .001). Similar associations were seen in the S-PS region with surgery (HR, 0.57; 95% CI, 0.50-0.66; P less than .001) and an age less than 70 (HR, 0.72; 95% CI, 0.62-0.84; P less than .001), but not white race.
The study results “indicate that the stage IV population that is living longer may be benefiting from many of the same therapies used to treat early breast cancer, especially for patients who are able to handle adjuvant chemotherapy treatment and are HR‐positive,” the researchers said. “However, the lag in survival improvement across different population‐based, geographic regions suggests that some groups and regions may benefit unequally from treatment advances as well as timely diagnosis.”
The study was funded by the Kaplan Cancer Research Fund, the Metastatic Breast Cancer Alliance, and the Surveillance, Epidemiology, and End Results Cancer Surveillance System program of the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Malmgren JA et al. Cancer. 2019 Oct 22. doi: 10.1002/cncr.32531.
FROM CANCER
Insomnia symptoms increase likelihood of stroke and heart disease
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
FROM NEUROLOGY
Key clinical point: The presence of insomnia symptoms increases the likelihood of cardiovascular or cerebrovascular disease during approximately 10 years of follow-up.
Major finding: After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13.
Study details: An analysis of data from 487,200 adults in China aged 30-79 years who completed a baseline survey during 2004-2008 and were followed through 2016.
Disclosures: This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
Source: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
New data further define role of PD-L1 status, immunotherapy in metastatic breast cancer
BARCELONA – Programmed death-ligand 1 (PD-L1) status in patients with advanced triple negative or HER2-positive breast cancer appears to identify distinct disease entities with varying likelihood of benefit from immune checkpoint inhibition, according to Giampaolo Bianchini, MD.
This observation, which contrasts with findings in other solid tumors and expands the road map to improved outcomes with immunotherapy for metastatic breast cancer, is based in part on new findings presented at the European Society for Medical Oncology Congress.
Among additional lessons from those findings: PD-L1 assays are not easily interchangeable, and studies with a “one size fits all” approach should be avoided, Dr. Bianchini, head of the Breast Cancer Group – Medical Oncology and clinical translational and immunotherapy research at Ospedale San Raffaele, Milan, said at the congress.
IMPassion130 and PD-L1 assays
In the phase 3 IMpassion130 trial assessing nanoparticle, albumin-bound (nab)-paclitaxel chemotherapy + either the anti-PD-L1 monoclonal antibody atezolizumab or placebo for the first-line treatment of metastatic triple-negative breast cancer (mTNBC), investigators used, and validated, the VENTANA PD-L1 SP142 assay to evaluate PD-L1 expression in immune cells (IC). PD-L1 positivity was defined using a 1% cutoff, meaning that PD-L1-stained IC encompassed at least 1% of the tumor area.
The trial demonstrated significantly improved progression-free survival (PFS) in the atezolizumab arm, both in the intention-to-treat (ITT) analysis (7.2 vs. 5.5 months in the placebo arm; hazard ratio, 0.80), and the PD-L1-positive subgroup (7.5 vs. 5.0 months; HR, 0.62), and the results were published in November 2018 (N Engl J Med. 2018; 379:2108-21).
“IMpassion130 is the first phase 3 trial demonstrating clinical benefit of cancer immunotherapy in patients with PD-L1-positive, metastatic triple-negative breast cancer,” Hope S. Rugo, MD, said at the congress. “The combination of atezolizumab and nab-paclitaxel is now approved in the United States and Europe for this indication.”
In addition, the SP142 antibody (which binds to PD-L1), at the 1% cutoff, predicted PFS and overall survival (OS) with atezolizumab + nab-paclitaxel, compared with nab-paclitaxel + placebo; the absolute improvement in OS in the PD-L1-positive population was 7 months (HR, 0.71), whereas no impact was seen in PFS or OS in patients who were PD-L1-negative using the SP142 assay, said Dr. Rugo, professor of hematology/oncology, and director of breast oncology and clinical trials education at the University of California, San Francisco.
Based on the IMPassion130 findings, the Food and Drug Administration approved the SP142 assay, using the 1% cutoff, as a “companion diagnostic device for selecting TNBC patients for atezolizumab.”
However, questions remain about how to best identify patients who could benefit from the atezolizumab + nab-paclitaxel combination, Dr. Rugo said.
Therefore, she and her colleagues performed a retrospective post hoc subgroup analysis of data from the trial to assess the performance and analytical concordance of the SP142 assay and two other commonly used PD-L1 immunohistochemistry (IHC) assays: the VENTANA SP263 IHC assay typically used as a companion diagnostic with durvalumab, and the Dako PD-L1 IHC 22C3 assay typically used with pembrolizumab.
In addition, the investigators assessed PD-L1 prevalence and clinical activity.
“We also included an evaluation of important factors related to PD-L1 testing and ... relationship to clinical outcome,” Dr. Rugo said.
In 614 biomarker-evaluable patients, representing 68% of the IMPassion130 ITT population, PD-L1-positive prevalence was 46% with the SP142 assay, 75% with the SP263 assay (also based on a 1% IC cutoff), and 81% with the 22C3 assay (with positivity defined as a combined positive score [CPS] of 1 or more based on an algorithm including both tumor and IC counts).
“Almost all SP142-positive cases are captured by either 22C3 or SP263. However, about a third of patients’ tumors were positive for PD-L1 using only one of the other two assays,” she noted, explaining that “this leads to suboptimal analytical concordance.”
The overall percentage agreement between SP142 and the other assays was only 64%-68%, she said.
Positive percentage agreement rates of 98% for both SP263 and 22C3 suggest that the patients identified as PD-L1 positive using the SP142 assay are captured by the other two assays. However, negative percentage agreement rates were less than 45%.
The HRs for PFS were 0.60 in SP142-positive patients, 0.64 in SP263-positive patients, and 0.68 in 22C3-positive patients, and the HRs for OS were 0.74, 0.75, and 0.78, respectively.
Subgroup analyses indicated that PFS and OS benefit with atezolizumab + nab-paclitaxel vs. nab-paclitaxel alone was greater in double-positive patients (those with SP142 positivity and either SP263 or 22C3 positivity) than in patients who were SP263-positive/SP142-negative or 22C3-positive/SP142-negative.
Dr. Rugo and her colleagues also found that the benefits with atezolizumab + nab-paclitaxel in PD-L1-positive patients were apparent regardless of the source of tissue for testing (breast or distant metastases).
They concluded that the findings of the assays are not equivalent; 22C3 and SP263 identified more patients as PD-L1 positive, and SP142-positivity was encompassed in positive tests for both.
“The clinical benefit in the 22C3-positive and the SP263-positive subgroups appear to be driven by the SP142-positive subgroup, and [SP142] identifies patients with the longest median progression-free and overall survival from the addition of atezolizumab to nab-paclitaxel,” she said “The SP142 assay with an IC cutoff of 1% or greater is the approved diagnostic test used to identify patients with metastatic triple-negative breast cancer who are most likely to benefit from the addition of the checkpoint inhibitor atezolizumab to nab-paclitaxel.”
As for whether the SP142 should be the assay of choice in other settings in which it hasn’t been validated, Dr. Rugo said it is advisable to use the assay that has been validated in a positive trial.
“That’s what we would generally do ... however, recognizing that some countries are not using SP142, and some sites may not have access, certainly you encompass that population in the patients whose tumors are positive by both other assays,” she said. “The risk is that you might overtreat, and the cost of treatment is greater.”
Excess toxicity is also a concern in that situation, she said, adding that “hopefully in the future we’ll be able to figure out ways to have even more patients benefit from the addition of immunotherapy so that won’t be an issue.”
“What this data shows is that you can feel secure that you are encompassing the patient population identified by the parent trial to benefit from the addition of atezolizumab by using either of the other two assays; you’re only missing 1% – so that’s very reasonable,” she said. “The risk is that you’re overtreating; it’s quite likely that there’s a population there that isn’t benefiting as much, but that’s a balance.”
The findings from IMPassion130 with regard to OS in the unselected population that included PD-L1-negative patients (18.7 vs. 21.0 months with vs. without atezolizumab; HR, 0.86) underscore the fact that “one size does not fit all” when it comes to immunotherapy benefit, Dr. Bianchini said.
This is further demonstrated by the post hoc analysis comparing IHC assays, he said, explaining that 63% of IMPassion130 patients who were considered PD-L1-negative based on the SP142 “actually scored as PD-L1-positive by the other tests.
“So the very clinically important question is if there is any evidence from the data that [the PD-L1-negative group] benefits in a significant way from the addition of atezolizumab,” he said. “I don’t see evidence for a clinical benefit, I see evidence to look for new biomarkers to identify a potential population who will benefit.”
The “absence of evidence is not evidence of absence,” he stressed, noting that it may be possible – with the right biomarkers – to identify PD-L1-negative patients who would benefit.
What the findings do show, however, is support for the FDA decision to approve the SP142 assay with an IC cutoff of 1% as a companion diagnostic tool, and that PD-L1 is ideally assessed using samples from both the primary and metastatic site, as the IMPassion130 data “do not inform whether PD-L1 assessment in primary and metastatic sites is equally informative,” he said.
In addition, Dr. Bianchini said the findings suggest that more information is needed about using different cutoffs for SP263 and 22C3, and he cautioned against “directly translating these finding to other disease settings or immune combinations.
“Defining new biomarkers to identify who within the PD-L1-negative group might benefit from this combination remains an unmet need,” he said. “For sure, I don’t see a space for the other tests to define this population,” he added.
KEYNOTE-119, KATE2, and future directions
Both the randomized, open-label, phase 3 KEYNOTE-119 study of the checkpoint inhibitor pembrolizumab vs. single-agent chemotherapy for mTNBC, and the phase 2 KATE2 trial of the antibody-drug conjugate trastuzumab emtansine (T-DM1) + either atezolizumab or placebo in previously treated HER2-positive breast cancer patients, failed to meet their respective primary study endpoints.
But the news isn’t all bad, Dr. Bianchini said.
For example, in KEYNOTE-119, second- or third-line pembrolizumab monotherapy did not significantly improve OS vs. chemotherapy for mTNBC, but the pembrolizumab treatment effect increased as PD-L1 enrichment increased, he explained.
Pembrolizumab showed promising antitumor activity and manageable safety in mTNBC in prior trials, and was therefore further assessed in the KEYNOTE-119 study of 601 patients with centrally confirmed TNBC, 1-2 prior systemic treatments for mTNBC, progression on the latest therapy, and a prior anthracycline or taxane, Javier Cortés, MD, PhD, of Instituto Oncológico, Madrid, reported at the congress.
Pembrolizumab was given at a dose of 200 mg every 3 weeks, and chemotherapy was physician’s choice of capecitabine, eribulin, gemcitabine, or vinorelbine.
At a median follow-up of 9.9 months in the pembrolizumab group and 10.9 months in the chemotherapy group, OS did not differ significantly between the groups; this was true overall, in patients with a CPS of 10 or greater, and in those with a CPS of 1 or greater.
In all-comers, the HR for OS was 0.97, compared with 0.78 in patients with CPS of 10 or greater, and 0.86 in those with CPS of 1 or greater, Dr. Cortés said.
“One of the most interesting exploratory analyses was OS in those patients with CPS of 20 or higher,” he said, noting that median OS in that group was 14.9 vs.12.5 months with pembrolizumab vs. with chemotherapy (HR, 0.58).
Pembrolizumab did not improve overall PFS, but again, the rates improved with higher CPS. Duration of response, however, was longer with pembrolizumab vs. chemotherapy (12.2 vs. 8.3 months overall; 12.2 vs. 6.5 months for CPS of 1 or greater; and not reached vs. 7.1 months for CPS of 10 or greater).
Grade 3-5 AEs occurred in 35% vs. 49% of patients in the pembrolizumab vs. chemotherapy groups, with nine deaths occurring in each, Dr. Cortés said, adding that treatment-related AEs occurred in 14% (with one death) and 36% (with two deaths), respectively, and grade 3-4 immune-mediated AEs and infusion reactions occurred in 3.2% vs. 1.0% (no deaths), respectively.
In the double-blind, signal-seeking KATE2 trial, as reported in 2018 at the San Antonio Breast Cancer Symposium, no overall PFS improvement was seen with atezolizumab + T-DM1 (median of 8.2 vs. 6.8 months; HR, 0.82; 12-month PFS 38% vs. 34%), but again, a possible benefit was seen in PD-L1-positive patients (8.5 vs. 4.1 months; HR, 0.60).
KATE2 included 202 patients with advanced HER2-positive breast cancer that progressed after treatment with T-DM1 and a taxane. They were randomized 2:1 to receive intravenous T-DM1 at a dose of 3.6 mg/kg plus atezolizumab (1,200 mg) or placebo every 3 weeks until loss of clinical benefit or intolerable toxicity.
The “overall survival and final safety results” show that at a median follow-up of 19.0 months in the atezolizumab arm and 18.2 months in the placebo arm, with 52 OS events reported, median OS was not reached in either arm and 1-year survival was similar in the two groups (89.1% and 89.0%), Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology and co-leader of the Hillman Cancer Immunology and Immunotherapy Program at Hillman Cancer Center, University of Pittsburgh Medical Center (UPMC) reported at the congress.
The 1-year OS rate in the PD-L1-positive subgroup, however, was numerically higher with vs. without atezolizumab (94.2% vs. 87.9%), said Dr. Emens, director of translational immunotherapy for the Women’s Cancer Research Center at UPMC.
Of note, all additional biomarkers of T-cell activation and quantity analyzed, including PD-L1 gene expression, CD8 gene expression, T effector signature gene expression, and stromal tumor infiltrating lymphocytes (TILs), were enriched in the PD-L1-positive subgroup vs. the PD-L1-negative patients.
Further, OS rates in other immune biomarker subgroups (those with PD-L1 RNA expression, CD8 RNA expression, and T effector signature at or below vs. above the median, and those with TILs less than 5% vs. 5% or greater) were consistent with those in the PD-L1 IC-positive subgroup, and the biggest difference between the atezolizumab and placebo arms related to stromal TILs, she said.
The safety profile in this final analysis was consistent with the known safety profile of each drug, she added, noting that grade 3 or greater AEs occurred in 52.6% vs. 44.8% of patients in the atezolizumab vs. placebo arms, and serious AEs – primarily pyrexia – occurred in 36.1% vs 20.9%, respectively.
The rate of grade 5 AEs was similar in the groups.
T-DM1 is indicated for the treatment of HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane, either separately or in combination, Dr. Emens said.
“In addition to its cytotoxic activity, T-DM1 may potentiate tumor immunity,” she explained, adding that KATE2 was designed to assess whether combining T-DM1 with atezolizumab, an anti-PD-L1 antibody that restores anti-tumor immunity, would result in greater clinical activity than either drug alone.
Although the number of OS events was small, the data suggest an OS benefit with the addition of atezolizumab to T-DM1, specifically in the PD-L1 IC-positive patients, but follow-up was short and the study lacked statistical power, therefore additional study of HER2-targeted agents with atezolizumab in previously treated HER2-positive, PD-L1 IC-positive advanced breast cancer is warranted, Dr. Emens concluded.
Indeed, the finding of improved OS in the PD-L1-positive subgroups of both KEYNOTE-119 and KATE2, is of interest, Dr. Bianchini said.
Both trials failed to meet their primary endpoints, but a closer look into KEYNOTE-119 shows that PD-L1 as a continuous biomarker (using CPS, 22C3) was associated with a “continuous and strong trend” toward improved ORR with the addition of pembrolizumab.
The ORR was 9.6% vs. 10.6% in unselected patients, compared with 26.3% vs. 11.5% in those with CPS of 20 or greater.
“And when you look at duration of response, you see an increase not just in the number ... but the quality of the response,” he said, noting that for PFS, as well, a trend toward superiority is seen “that is consistent with all the other endpoints.”
“So overall, the application of incrementally restrictive cut-off of CPS lends weight to the exploratory analysis showing better survival from pembrolizumab in tumors with CPS more than 20,” Dr. Bianchini said, noting that the “real question,” however, is whether the finding “is worth clinical implementation.
“We know a lot about the primary tumor and immune infiltration. We’ve learned ... that if you wait and look ahead at immune infiltration in the advanced stage, you find that the tumor becomes smart,” he said, explaining that tumor/immune co-evolution leads to increased immuno-editing and immune subversion and it becomes “much harder to just hit the tumor with PD-L1, because this is not the only mechanism of immune escape.”
A review of several studies shows that in similar populations defined by biomarkers, response rates in patients treated with checkpoint inhibitors decrease in the second- and third-line setting vs. the first-line setting, he said.
For example, pembrolizumab response rates in the first-line and second-line or greater setting in cohort B of the KEYNOTE-086 study were 21.4% and 5.7%, respectively, compared with 12.3% in the second- to third-line setting in KEYNOTE-119, he said.
Another consideration is whether monotherapy or combination therapy is preferable, and the data suggest that regardless of how PD-L1-positivity is defined (by CPS cutoff of 1 vs. 20, for example), most patients treated with monotherapy progress within the first 3 months, he said.
“I don’t see that this is a safe approach for the majority of these patients. So without better biomarkers, combinations should always be preferred, at least to avoid early progression,” Dr. Bianchini said, adding that the open question, then, is: “If we set the new standard in the first-line as the combination of nab-paclitaxel and atezolizumab for PD-L1-positive patients defined by the VENTANA [SP142 assay], should we continue with immune checkpoint [inhibition] using different combinations?”
“Of course, at the time the trial was designed, the results of IMpassion were not available, but it’s very important, because [the findings] add to the evidence that immunotherapy is extremely relevant for some patients,” he said.
KATE2 further demonstrated the importance of PD-L1 status, he said, adding that due to its limitations, including small sample size and short follow-up, longer follow-up is needed to better evaluate duration of response and PFS.
“Despite the trial limitations, the qualitative effect seen in all clinical endpoints – overall response rate, progression-free survival, overall survival – in PD-L1-positive tumors defined by SP142 ... provided strong and robust signals supporting the investigation of immune checkpoint inhibitors in HER-positive breast cancer,” he said, noting that “many trials are ongoing in the early setting and the advanced setting.”
In addition to the lessons of these trials with respect to the interchangeability of PD-L1 IHC assays and the value of PD-L1 assessment for identifying the likelihood of benefit from immune checkpoint inhibitors, the findings highlight the possibility that PD-L1-negative tumors require different immunotherapy approaches or alternative therapeutic strategies, and underscore that the benefit of immunotherapy in PD-L1-positive patients is still restricted to a minority.
“So new studies and approaches with immuno-oncology are needed, and we need more effective biomarkers, because we need to have precision oncology applied – we need to go in that direction,” he concluded.
Dr. Bianchini reported consultancy/honorarium and or advisory board activity associated with Roche, MSD, AstraZeneca, Pfizer, Chugai, EISAI, Lilly, Novartis, Amgen, Sanofi, Neopharm, and Genomic Health. The IMPassion30 trial was funded by F. Hoffmann-La Roche Ltd.; Dr. Rugo reported research grants, other funding, and or travel/accommodation/expenses from Pfizer, Novartis, Eli Lilly, Merck, OBI, EISAI, Plexxikon, Genentech/Roche, MacroGenics, PUMA, Mylan, Immunomedics, Daiichi Sankyo, and Celltrion. KEYNOTE-119 was funded by Merck Sharp & Dohme Corp.; Dr. Cortés and Dr. Emens reported numerous funding relationships but none with F. Hoffman-La Roche. KATE2 was funded by F. Hoffmann-La Roche.
Sources: IMPassion130; ESMO Abstract LBA20; KEYNOTE-119: ESMO Abstract LBA21; KATE2: ESMO Abstract 305O.
BARCELONA – Programmed death-ligand 1 (PD-L1) status in patients with advanced triple negative or HER2-positive breast cancer appears to identify distinct disease entities with varying likelihood of benefit from immune checkpoint inhibition, according to Giampaolo Bianchini, MD.
This observation, which contrasts with findings in other solid tumors and expands the road map to improved outcomes with immunotherapy for metastatic breast cancer, is based in part on new findings presented at the European Society for Medical Oncology Congress.
Among additional lessons from those findings: PD-L1 assays are not easily interchangeable, and studies with a “one size fits all” approach should be avoided, Dr. Bianchini, head of the Breast Cancer Group – Medical Oncology and clinical translational and immunotherapy research at Ospedale San Raffaele, Milan, said at the congress.
IMPassion130 and PD-L1 assays
In the phase 3 IMpassion130 trial assessing nanoparticle, albumin-bound (nab)-paclitaxel chemotherapy + either the anti-PD-L1 monoclonal antibody atezolizumab or placebo for the first-line treatment of metastatic triple-negative breast cancer (mTNBC), investigators used, and validated, the VENTANA PD-L1 SP142 assay to evaluate PD-L1 expression in immune cells (IC). PD-L1 positivity was defined using a 1% cutoff, meaning that PD-L1-stained IC encompassed at least 1% of the tumor area.
The trial demonstrated significantly improved progression-free survival (PFS) in the atezolizumab arm, both in the intention-to-treat (ITT) analysis (7.2 vs. 5.5 months in the placebo arm; hazard ratio, 0.80), and the PD-L1-positive subgroup (7.5 vs. 5.0 months; HR, 0.62), and the results were published in November 2018 (N Engl J Med. 2018; 379:2108-21).
“IMpassion130 is the first phase 3 trial demonstrating clinical benefit of cancer immunotherapy in patients with PD-L1-positive, metastatic triple-negative breast cancer,” Hope S. Rugo, MD, said at the congress. “The combination of atezolizumab and nab-paclitaxel is now approved in the United States and Europe for this indication.”
In addition, the SP142 antibody (which binds to PD-L1), at the 1% cutoff, predicted PFS and overall survival (OS) with atezolizumab + nab-paclitaxel, compared with nab-paclitaxel + placebo; the absolute improvement in OS in the PD-L1-positive population was 7 months (HR, 0.71), whereas no impact was seen in PFS or OS in patients who were PD-L1-negative using the SP142 assay, said Dr. Rugo, professor of hematology/oncology, and director of breast oncology and clinical trials education at the University of California, San Francisco.
Based on the IMPassion130 findings, the Food and Drug Administration approved the SP142 assay, using the 1% cutoff, as a “companion diagnostic device for selecting TNBC patients for atezolizumab.”
However, questions remain about how to best identify patients who could benefit from the atezolizumab + nab-paclitaxel combination, Dr. Rugo said.
Therefore, she and her colleagues performed a retrospective post hoc subgroup analysis of data from the trial to assess the performance and analytical concordance of the SP142 assay and two other commonly used PD-L1 immunohistochemistry (IHC) assays: the VENTANA SP263 IHC assay typically used as a companion diagnostic with durvalumab, and the Dako PD-L1 IHC 22C3 assay typically used with pembrolizumab.
In addition, the investigators assessed PD-L1 prevalence and clinical activity.
“We also included an evaluation of important factors related to PD-L1 testing and ... relationship to clinical outcome,” Dr. Rugo said.
In 614 biomarker-evaluable patients, representing 68% of the IMPassion130 ITT population, PD-L1-positive prevalence was 46% with the SP142 assay, 75% with the SP263 assay (also based on a 1% IC cutoff), and 81% with the 22C3 assay (with positivity defined as a combined positive score [CPS] of 1 or more based on an algorithm including both tumor and IC counts).
“Almost all SP142-positive cases are captured by either 22C3 or SP263. However, about a third of patients’ tumors were positive for PD-L1 using only one of the other two assays,” she noted, explaining that “this leads to suboptimal analytical concordance.”
The overall percentage agreement between SP142 and the other assays was only 64%-68%, she said.
Positive percentage agreement rates of 98% for both SP263 and 22C3 suggest that the patients identified as PD-L1 positive using the SP142 assay are captured by the other two assays. However, negative percentage agreement rates were less than 45%.
The HRs for PFS were 0.60 in SP142-positive patients, 0.64 in SP263-positive patients, and 0.68 in 22C3-positive patients, and the HRs for OS were 0.74, 0.75, and 0.78, respectively.
Subgroup analyses indicated that PFS and OS benefit with atezolizumab + nab-paclitaxel vs. nab-paclitaxel alone was greater in double-positive patients (those with SP142 positivity and either SP263 or 22C3 positivity) than in patients who were SP263-positive/SP142-negative or 22C3-positive/SP142-negative.
Dr. Rugo and her colleagues also found that the benefits with atezolizumab + nab-paclitaxel in PD-L1-positive patients were apparent regardless of the source of tissue for testing (breast or distant metastases).
They concluded that the findings of the assays are not equivalent; 22C3 and SP263 identified more patients as PD-L1 positive, and SP142-positivity was encompassed in positive tests for both.
“The clinical benefit in the 22C3-positive and the SP263-positive subgroups appear to be driven by the SP142-positive subgroup, and [SP142] identifies patients with the longest median progression-free and overall survival from the addition of atezolizumab to nab-paclitaxel,” she said “The SP142 assay with an IC cutoff of 1% or greater is the approved diagnostic test used to identify patients with metastatic triple-negative breast cancer who are most likely to benefit from the addition of the checkpoint inhibitor atezolizumab to nab-paclitaxel.”
As for whether the SP142 should be the assay of choice in other settings in which it hasn’t been validated, Dr. Rugo said it is advisable to use the assay that has been validated in a positive trial.
“That’s what we would generally do ... however, recognizing that some countries are not using SP142, and some sites may not have access, certainly you encompass that population in the patients whose tumors are positive by both other assays,” she said. “The risk is that you might overtreat, and the cost of treatment is greater.”
Excess toxicity is also a concern in that situation, she said, adding that “hopefully in the future we’ll be able to figure out ways to have even more patients benefit from the addition of immunotherapy so that won’t be an issue.”
“What this data shows is that you can feel secure that you are encompassing the patient population identified by the parent trial to benefit from the addition of atezolizumab by using either of the other two assays; you’re only missing 1% – so that’s very reasonable,” she said. “The risk is that you’re overtreating; it’s quite likely that there’s a population there that isn’t benefiting as much, but that’s a balance.”
The findings from IMPassion130 with regard to OS in the unselected population that included PD-L1-negative patients (18.7 vs. 21.0 months with vs. without atezolizumab; HR, 0.86) underscore the fact that “one size does not fit all” when it comes to immunotherapy benefit, Dr. Bianchini said.
This is further demonstrated by the post hoc analysis comparing IHC assays, he said, explaining that 63% of IMPassion130 patients who were considered PD-L1-negative based on the SP142 “actually scored as PD-L1-positive by the other tests.
“So the very clinically important question is if there is any evidence from the data that [the PD-L1-negative group] benefits in a significant way from the addition of atezolizumab,” he said. “I don’t see evidence for a clinical benefit, I see evidence to look for new biomarkers to identify a potential population who will benefit.”
The “absence of evidence is not evidence of absence,” he stressed, noting that it may be possible – with the right biomarkers – to identify PD-L1-negative patients who would benefit.
What the findings do show, however, is support for the FDA decision to approve the SP142 assay with an IC cutoff of 1% as a companion diagnostic tool, and that PD-L1 is ideally assessed using samples from both the primary and metastatic site, as the IMPassion130 data “do not inform whether PD-L1 assessment in primary and metastatic sites is equally informative,” he said.
In addition, Dr. Bianchini said the findings suggest that more information is needed about using different cutoffs for SP263 and 22C3, and he cautioned against “directly translating these finding to other disease settings or immune combinations.
“Defining new biomarkers to identify who within the PD-L1-negative group might benefit from this combination remains an unmet need,” he said. “For sure, I don’t see a space for the other tests to define this population,” he added.
KEYNOTE-119, KATE2, and future directions
Both the randomized, open-label, phase 3 KEYNOTE-119 study of the checkpoint inhibitor pembrolizumab vs. single-agent chemotherapy for mTNBC, and the phase 2 KATE2 trial of the antibody-drug conjugate trastuzumab emtansine (T-DM1) + either atezolizumab or placebo in previously treated HER2-positive breast cancer patients, failed to meet their respective primary study endpoints.
But the news isn’t all bad, Dr. Bianchini said.
For example, in KEYNOTE-119, second- or third-line pembrolizumab monotherapy did not significantly improve OS vs. chemotherapy for mTNBC, but the pembrolizumab treatment effect increased as PD-L1 enrichment increased, he explained.
Pembrolizumab showed promising antitumor activity and manageable safety in mTNBC in prior trials, and was therefore further assessed in the KEYNOTE-119 study of 601 patients with centrally confirmed TNBC, 1-2 prior systemic treatments for mTNBC, progression on the latest therapy, and a prior anthracycline or taxane, Javier Cortés, MD, PhD, of Instituto Oncológico, Madrid, reported at the congress.
Pembrolizumab was given at a dose of 200 mg every 3 weeks, and chemotherapy was physician’s choice of capecitabine, eribulin, gemcitabine, or vinorelbine.
At a median follow-up of 9.9 months in the pembrolizumab group and 10.9 months in the chemotherapy group, OS did not differ significantly between the groups; this was true overall, in patients with a CPS of 10 or greater, and in those with a CPS of 1 or greater.
In all-comers, the HR for OS was 0.97, compared with 0.78 in patients with CPS of 10 or greater, and 0.86 in those with CPS of 1 or greater, Dr. Cortés said.
“One of the most interesting exploratory analyses was OS in those patients with CPS of 20 or higher,” he said, noting that median OS in that group was 14.9 vs.12.5 months with pembrolizumab vs. with chemotherapy (HR, 0.58).
Pembrolizumab did not improve overall PFS, but again, the rates improved with higher CPS. Duration of response, however, was longer with pembrolizumab vs. chemotherapy (12.2 vs. 8.3 months overall; 12.2 vs. 6.5 months for CPS of 1 or greater; and not reached vs. 7.1 months for CPS of 10 or greater).
Grade 3-5 AEs occurred in 35% vs. 49% of patients in the pembrolizumab vs. chemotherapy groups, with nine deaths occurring in each, Dr. Cortés said, adding that treatment-related AEs occurred in 14% (with one death) and 36% (with two deaths), respectively, and grade 3-4 immune-mediated AEs and infusion reactions occurred in 3.2% vs. 1.0% (no deaths), respectively.
In the double-blind, signal-seeking KATE2 trial, as reported in 2018 at the San Antonio Breast Cancer Symposium, no overall PFS improvement was seen with atezolizumab + T-DM1 (median of 8.2 vs. 6.8 months; HR, 0.82; 12-month PFS 38% vs. 34%), but again, a possible benefit was seen in PD-L1-positive patients (8.5 vs. 4.1 months; HR, 0.60).
KATE2 included 202 patients with advanced HER2-positive breast cancer that progressed after treatment with T-DM1 and a taxane. They were randomized 2:1 to receive intravenous T-DM1 at a dose of 3.6 mg/kg plus atezolizumab (1,200 mg) or placebo every 3 weeks until loss of clinical benefit or intolerable toxicity.
The “overall survival and final safety results” show that at a median follow-up of 19.0 months in the atezolizumab arm and 18.2 months in the placebo arm, with 52 OS events reported, median OS was not reached in either arm and 1-year survival was similar in the two groups (89.1% and 89.0%), Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology and co-leader of the Hillman Cancer Immunology and Immunotherapy Program at Hillman Cancer Center, University of Pittsburgh Medical Center (UPMC) reported at the congress.
The 1-year OS rate in the PD-L1-positive subgroup, however, was numerically higher with vs. without atezolizumab (94.2% vs. 87.9%), said Dr. Emens, director of translational immunotherapy for the Women’s Cancer Research Center at UPMC.
Of note, all additional biomarkers of T-cell activation and quantity analyzed, including PD-L1 gene expression, CD8 gene expression, T effector signature gene expression, and stromal tumor infiltrating lymphocytes (TILs), were enriched in the PD-L1-positive subgroup vs. the PD-L1-negative patients.
Further, OS rates in other immune biomarker subgroups (those with PD-L1 RNA expression, CD8 RNA expression, and T effector signature at or below vs. above the median, and those with TILs less than 5% vs. 5% or greater) were consistent with those in the PD-L1 IC-positive subgroup, and the biggest difference between the atezolizumab and placebo arms related to stromal TILs, she said.
The safety profile in this final analysis was consistent with the known safety profile of each drug, she added, noting that grade 3 or greater AEs occurred in 52.6% vs. 44.8% of patients in the atezolizumab vs. placebo arms, and serious AEs – primarily pyrexia – occurred in 36.1% vs 20.9%, respectively.
The rate of grade 5 AEs was similar in the groups.
T-DM1 is indicated for the treatment of HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane, either separately or in combination, Dr. Emens said.
“In addition to its cytotoxic activity, T-DM1 may potentiate tumor immunity,” she explained, adding that KATE2 was designed to assess whether combining T-DM1 with atezolizumab, an anti-PD-L1 antibody that restores anti-tumor immunity, would result in greater clinical activity than either drug alone.
Although the number of OS events was small, the data suggest an OS benefit with the addition of atezolizumab to T-DM1, specifically in the PD-L1 IC-positive patients, but follow-up was short and the study lacked statistical power, therefore additional study of HER2-targeted agents with atezolizumab in previously treated HER2-positive, PD-L1 IC-positive advanced breast cancer is warranted, Dr. Emens concluded.
Indeed, the finding of improved OS in the PD-L1-positive subgroups of both KEYNOTE-119 and KATE2, is of interest, Dr. Bianchini said.
Both trials failed to meet their primary endpoints, but a closer look into KEYNOTE-119 shows that PD-L1 as a continuous biomarker (using CPS, 22C3) was associated with a “continuous and strong trend” toward improved ORR with the addition of pembrolizumab.
The ORR was 9.6% vs. 10.6% in unselected patients, compared with 26.3% vs. 11.5% in those with CPS of 20 or greater.
“And when you look at duration of response, you see an increase not just in the number ... but the quality of the response,” he said, noting that for PFS, as well, a trend toward superiority is seen “that is consistent with all the other endpoints.”
“So overall, the application of incrementally restrictive cut-off of CPS lends weight to the exploratory analysis showing better survival from pembrolizumab in tumors with CPS more than 20,” Dr. Bianchini said, noting that the “real question,” however, is whether the finding “is worth clinical implementation.
“We know a lot about the primary tumor and immune infiltration. We’ve learned ... that if you wait and look ahead at immune infiltration in the advanced stage, you find that the tumor becomes smart,” he said, explaining that tumor/immune co-evolution leads to increased immuno-editing and immune subversion and it becomes “much harder to just hit the tumor with PD-L1, because this is not the only mechanism of immune escape.”
A review of several studies shows that in similar populations defined by biomarkers, response rates in patients treated with checkpoint inhibitors decrease in the second- and third-line setting vs. the first-line setting, he said.
For example, pembrolizumab response rates in the first-line and second-line or greater setting in cohort B of the KEYNOTE-086 study were 21.4% and 5.7%, respectively, compared with 12.3% in the second- to third-line setting in KEYNOTE-119, he said.
Another consideration is whether monotherapy or combination therapy is preferable, and the data suggest that regardless of how PD-L1-positivity is defined (by CPS cutoff of 1 vs. 20, for example), most patients treated with monotherapy progress within the first 3 months, he said.
“I don’t see that this is a safe approach for the majority of these patients. So without better biomarkers, combinations should always be preferred, at least to avoid early progression,” Dr. Bianchini said, adding that the open question, then, is: “If we set the new standard in the first-line as the combination of nab-paclitaxel and atezolizumab for PD-L1-positive patients defined by the VENTANA [SP142 assay], should we continue with immune checkpoint [inhibition] using different combinations?”
“Of course, at the time the trial was designed, the results of IMpassion were not available, but it’s very important, because [the findings] add to the evidence that immunotherapy is extremely relevant for some patients,” he said.
KATE2 further demonstrated the importance of PD-L1 status, he said, adding that due to its limitations, including small sample size and short follow-up, longer follow-up is needed to better evaluate duration of response and PFS.
“Despite the trial limitations, the qualitative effect seen in all clinical endpoints – overall response rate, progression-free survival, overall survival – in PD-L1-positive tumors defined by SP142 ... provided strong and robust signals supporting the investigation of immune checkpoint inhibitors in HER-positive breast cancer,” he said, noting that “many trials are ongoing in the early setting and the advanced setting.”
In addition to the lessons of these trials with respect to the interchangeability of PD-L1 IHC assays and the value of PD-L1 assessment for identifying the likelihood of benefit from immune checkpoint inhibitors, the findings highlight the possibility that PD-L1-negative tumors require different immunotherapy approaches or alternative therapeutic strategies, and underscore that the benefit of immunotherapy in PD-L1-positive patients is still restricted to a minority.
“So new studies and approaches with immuno-oncology are needed, and we need more effective biomarkers, because we need to have precision oncology applied – we need to go in that direction,” he concluded.
Dr. Bianchini reported consultancy/honorarium and or advisory board activity associated with Roche, MSD, AstraZeneca, Pfizer, Chugai, EISAI, Lilly, Novartis, Amgen, Sanofi, Neopharm, and Genomic Health. The IMPassion30 trial was funded by F. Hoffmann-La Roche Ltd.; Dr. Rugo reported research grants, other funding, and or travel/accommodation/expenses from Pfizer, Novartis, Eli Lilly, Merck, OBI, EISAI, Plexxikon, Genentech/Roche, MacroGenics, PUMA, Mylan, Immunomedics, Daiichi Sankyo, and Celltrion. KEYNOTE-119 was funded by Merck Sharp & Dohme Corp.; Dr. Cortés and Dr. Emens reported numerous funding relationships but none with F. Hoffman-La Roche. KATE2 was funded by F. Hoffmann-La Roche.
Sources: IMPassion130; ESMO Abstract LBA20; KEYNOTE-119: ESMO Abstract LBA21; KATE2: ESMO Abstract 305O.
BARCELONA – Programmed death-ligand 1 (PD-L1) status in patients with advanced triple negative or HER2-positive breast cancer appears to identify distinct disease entities with varying likelihood of benefit from immune checkpoint inhibition, according to Giampaolo Bianchini, MD.
This observation, which contrasts with findings in other solid tumors and expands the road map to improved outcomes with immunotherapy for metastatic breast cancer, is based in part on new findings presented at the European Society for Medical Oncology Congress.
Among additional lessons from those findings: PD-L1 assays are not easily interchangeable, and studies with a “one size fits all” approach should be avoided, Dr. Bianchini, head of the Breast Cancer Group – Medical Oncology and clinical translational and immunotherapy research at Ospedale San Raffaele, Milan, said at the congress.
IMPassion130 and PD-L1 assays
In the phase 3 IMpassion130 trial assessing nanoparticle, albumin-bound (nab)-paclitaxel chemotherapy + either the anti-PD-L1 monoclonal antibody atezolizumab or placebo for the first-line treatment of metastatic triple-negative breast cancer (mTNBC), investigators used, and validated, the VENTANA PD-L1 SP142 assay to evaluate PD-L1 expression in immune cells (IC). PD-L1 positivity was defined using a 1% cutoff, meaning that PD-L1-stained IC encompassed at least 1% of the tumor area.
The trial demonstrated significantly improved progression-free survival (PFS) in the atezolizumab arm, both in the intention-to-treat (ITT) analysis (7.2 vs. 5.5 months in the placebo arm; hazard ratio, 0.80), and the PD-L1-positive subgroup (7.5 vs. 5.0 months; HR, 0.62), and the results were published in November 2018 (N Engl J Med. 2018; 379:2108-21).
“IMpassion130 is the first phase 3 trial demonstrating clinical benefit of cancer immunotherapy in patients with PD-L1-positive, metastatic triple-negative breast cancer,” Hope S. Rugo, MD, said at the congress. “The combination of atezolizumab and nab-paclitaxel is now approved in the United States and Europe for this indication.”
In addition, the SP142 antibody (which binds to PD-L1), at the 1% cutoff, predicted PFS and overall survival (OS) with atezolizumab + nab-paclitaxel, compared with nab-paclitaxel + placebo; the absolute improvement in OS in the PD-L1-positive population was 7 months (HR, 0.71), whereas no impact was seen in PFS or OS in patients who were PD-L1-negative using the SP142 assay, said Dr. Rugo, professor of hematology/oncology, and director of breast oncology and clinical trials education at the University of California, San Francisco.
Based on the IMPassion130 findings, the Food and Drug Administration approved the SP142 assay, using the 1% cutoff, as a “companion diagnostic device for selecting TNBC patients for atezolizumab.”
However, questions remain about how to best identify patients who could benefit from the atezolizumab + nab-paclitaxel combination, Dr. Rugo said.
Therefore, she and her colleagues performed a retrospective post hoc subgroup analysis of data from the trial to assess the performance and analytical concordance of the SP142 assay and two other commonly used PD-L1 immunohistochemistry (IHC) assays: the VENTANA SP263 IHC assay typically used as a companion diagnostic with durvalumab, and the Dako PD-L1 IHC 22C3 assay typically used with pembrolizumab.
In addition, the investigators assessed PD-L1 prevalence and clinical activity.
“We also included an evaluation of important factors related to PD-L1 testing and ... relationship to clinical outcome,” Dr. Rugo said.
In 614 biomarker-evaluable patients, representing 68% of the IMPassion130 ITT population, PD-L1-positive prevalence was 46% with the SP142 assay, 75% with the SP263 assay (also based on a 1% IC cutoff), and 81% with the 22C3 assay (with positivity defined as a combined positive score [CPS] of 1 or more based on an algorithm including both tumor and IC counts).
“Almost all SP142-positive cases are captured by either 22C3 or SP263. However, about a third of patients’ tumors were positive for PD-L1 using only one of the other two assays,” she noted, explaining that “this leads to suboptimal analytical concordance.”
The overall percentage agreement between SP142 and the other assays was only 64%-68%, she said.
Positive percentage agreement rates of 98% for both SP263 and 22C3 suggest that the patients identified as PD-L1 positive using the SP142 assay are captured by the other two assays. However, negative percentage agreement rates were less than 45%.
The HRs for PFS were 0.60 in SP142-positive patients, 0.64 in SP263-positive patients, and 0.68 in 22C3-positive patients, and the HRs for OS were 0.74, 0.75, and 0.78, respectively.
Subgroup analyses indicated that PFS and OS benefit with atezolizumab + nab-paclitaxel vs. nab-paclitaxel alone was greater in double-positive patients (those with SP142 positivity and either SP263 or 22C3 positivity) than in patients who were SP263-positive/SP142-negative or 22C3-positive/SP142-negative.
Dr. Rugo and her colleagues also found that the benefits with atezolizumab + nab-paclitaxel in PD-L1-positive patients were apparent regardless of the source of tissue for testing (breast or distant metastases).
They concluded that the findings of the assays are not equivalent; 22C3 and SP263 identified more patients as PD-L1 positive, and SP142-positivity was encompassed in positive tests for both.
“The clinical benefit in the 22C3-positive and the SP263-positive subgroups appear to be driven by the SP142-positive subgroup, and [SP142] identifies patients with the longest median progression-free and overall survival from the addition of atezolizumab to nab-paclitaxel,” she said “The SP142 assay with an IC cutoff of 1% or greater is the approved diagnostic test used to identify patients with metastatic triple-negative breast cancer who are most likely to benefit from the addition of the checkpoint inhibitor atezolizumab to nab-paclitaxel.”
As for whether the SP142 should be the assay of choice in other settings in which it hasn’t been validated, Dr. Rugo said it is advisable to use the assay that has been validated in a positive trial.
“That’s what we would generally do ... however, recognizing that some countries are not using SP142, and some sites may not have access, certainly you encompass that population in the patients whose tumors are positive by both other assays,” she said. “The risk is that you might overtreat, and the cost of treatment is greater.”
Excess toxicity is also a concern in that situation, she said, adding that “hopefully in the future we’ll be able to figure out ways to have even more patients benefit from the addition of immunotherapy so that won’t be an issue.”
“What this data shows is that you can feel secure that you are encompassing the patient population identified by the parent trial to benefit from the addition of atezolizumab by using either of the other two assays; you’re only missing 1% – so that’s very reasonable,” she said. “The risk is that you’re overtreating; it’s quite likely that there’s a population there that isn’t benefiting as much, but that’s a balance.”
The findings from IMPassion130 with regard to OS in the unselected population that included PD-L1-negative patients (18.7 vs. 21.0 months with vs. without atezolizumab; HR, 0.86) underscore the fact that “one size does not fit all” when it comes to immunotherapy benefit, Dr. Bianchini said.
This is further demonstrated by the post hoc analysis comparing IHC assays, he said, explaining that 63% of IMPassion130 patients who were considered PD-L1-negative based on the SP142 “actually scored as PD-L1-positive by the other tests.
“So the very clinically important question is if there is any evidence from the data that [the PD-L1-negative group] benefits in a significant way from the addition of atezolizumab,” he said. “I don’t see evidence for a clinical benefit, I see evidence to look for new biomarkers to identify a potential population who will benefit.”
The “absence of evidence is not evidence of absence,” he stressed, noting that it may be possible – with the right biomarkers – to identify PD-L1-negative patients who would benefit.
What the findings do show, however, is support for the FDA decision to approve the SP142 assay with an IC cutoff of 1% as a companion diagnostic tool, and that PD-L1 is ideally assessed using samples from both the primary and metastatic site, as the IMPassion130 data “do not inform whether PD-L1 assessment in primary and metastatic sites is equally informative,” he said.
In addition, Dr. Bianchini said the findings suggest that more information is needed about using different cutoffs for SP263 and 22C3, and he cautioned against “directly translating these finding to other disease settings or immune combinations.
“Defining new biomarkers to identify who within the PD-L1-negative group might benefit from this combination remains an unmet need,” he said. “For sure, I don’t see a space for the other tests to define this population,” he added.
KEYNOTE-119, KATE2, and future directions
Both the randomized, open-label, phase 3 KEYNOTE-119 study of the checkpoint inhibitor pembrolizumab vs. single-agent chemotherapy for mTNBC, and the phase 2 KATE2 trial of the antibody-drug conjugate trastuzumab emtansine (T-DM1) + either atezolizumab or placebo in previously treated HER2-positive breast cancer patients, failed to meet their respective primary study endpoints.
But the news isn’t all bad, Dr. Bianchini said.
For example, in KEYNOTE-119, second- or third-line pembrolizumab monotherapy did not significantly improve OS vs. chemotherapy for mTNBC, but the pembrolizumab treatment effect increased as PD-L1 enrichment increased, he explained.
Pembrolizumab showed promising antitumor activity and manageable safety in mTNBC in prior trials, and was therefore further assessed in the KEYNOTE-119 study of 601 patients with centrally confirmed TNBC, 1-2 prior systemic treatments for mTNBC, progression on the latest therapy, and a prior anthracycline or taxane, Javier Cortés, MD, PhD, of Instituto Oncológico, Madrid, reported at the congress.
Pembrolizumab was given at a dose of 200 mg every 3 weeks, and chemotherapy was physician’s choice of capecitabine, eribulin, gemcitabine, or vinorelbine.
At a median follow-up of 9.9 months in the pembrolizumab group and 10.9 months in the chemotherapy group, OS did not differ significantly between the groups; this was true overall, in patients with a CPS of 10 or greater, and in those with a CPS of 1 or greater.
In all-comers, the HR for OS was 0.97, compared with 0.78 in patients with CPS of 10 or greater, and 0.86 in those with CPS of 1 or greater, Dr. Cortés said.
“One of the most interesting exploratory analyses was OS in those patients with CPS of 20 or higher,” he said, noting that median OS in that group was 14.9 vs.12.5 months with pembrolizumab vs. with chemotherapy (HR, 0.58).
Pembrolizumab did not improve overall PFS, but again, the rates improved with higher CPS. Duration of response, however, was longer with pembrolizumab vs. chemotherapy (12.2 vs. 8.3 months overall; 12.2 vs. 6.5 months for CPS of 1 or greater; and not reached vs. 7.1 months for CPS of 10 or greater).
Grade 3-5 AEs occurred in 35% vs. 49% of patients in the pembrolizumab vs. chemotherapy groups, with nine deaths occurring in each, Dr. Cortés said, adding that treatment-related AEs occurred in 14% (with one death) and 36% (with two deaths), respectively, and grade 3-4 immune-mediated AEs and infusion reactions occurred in 3.2% vs. 1.0% (no deaths), respectively.
In the double-blind, signal-seeking KATE2 trial, as reported in 2018 at the San Antonio Breast Cancer Symposium, no overall PFS improvement was seen with atezolizumab + T-DM1 (median of 8.2 vs. 6.8 months; HR, 0.82; 12-month PFS 38% vs. 34%), but again, a possible benefit was seen in PD-L1-positive patients (8.5 vs. 4.1 months; HR, 0.60).
KATE2 included 202 patients with advanced HER2-positive breast cancer that progressed after treatment with T-DM1 and a taxane. They were randomized 2:1 to receive intravenous T-DM1 at a dose of 3.6 mg/kg plus atezolizumab (1,200 mg) or placebo every 3 weeks until loss of clinical benefit or intolerable toxicity.
The “overall survival and final safety results” show that at a median follow-up of 19.0 months in the atezolizumab arm and 18.2 months in the placebo arm, with 52 OS events reported, median OS was not reached in either arm and 1-year survival was similar in the two groups (89.1% and 89.0%), Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology and co-leader of the Hillman Cancer Immunology and Immunotherapy Program at Hillman Cancer Center, University of Pittsburgh Medical Center (UPMC) reported at the congress.
The 1-year OS rate in the PD-L1-positive subgroup, however, was numerically higher with vs. without atezolizumab (94.2% vs. 87.9%), said Dr. Emens, director of translational immunotherapy for the Women’s Cancer Research Center at UPMC.
Of note, all additional biomarkers of T-cell activation and quantity analyzed, including PD-L1 gene expression, CD8 gene expression, T effector signature gene expression, and stromal tumor infiltrating lymphocytes (TILs), were enriched in the PD-L1-positive subgroup vs. the PD-L1-negative patients.
Further, OS rates in other immune biomarker subgroups (those with PD-L1 RNA expression, CD8 RNA expression, and T effector signature at or below vs. above the median, and those with TILs less than 5% vs. 5% or greater) were consistent with those in the PD-L1 IC-positive subgroup, and the biggest difference between the atezolizumab and placebo arms related to stromal TILs, she said.
The safety profile in this final analysis was consistent with the known safety profile of each drug, she added, noting that grade 3 or greater AEs occurred in 52.6% vs. 44.8% of patients in the atezolizumab vs. placebo arms, and serious AEs – primarily pyrexia – occurred in 36.1% vs 20.9%, respectively.
The rate of grade 5 AEs was similar in the groups.
T-DM1 is indicated for the treatment of HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane, either separately or in combination, Dr. Emens said.
“In addition to its cytotoxic activity, T-DM1 may potentiate tumor immunity,” she explained, adding that KATE2 was designed to assess whether combining T-DM1 with atezolizumab, an anti-PD-L1 antibody that restores anti-tumor immunity, would result in greater clinical activity than either drug alone.
Although the number of OS events was small, the data suggest an OS benefit with the addition of atezolizumab to T-DM1, specifically in the PD-L1 IC-positive patients, but follow-up was short and the study lacked statistical power, therefore additional study of HER2-targeted agents with atezolizumab in previously treated HER2-positive, PD-L1 IC-positive advanced breast cancer is warranted, Dr. Emens concluded.
Indeed, the finding of improved OS in the PD-L1-positive subgroups of both KEYNOTE-119 and KATE2, is of interest, Dr. Bianchini said.
Both trials failed to meet their primary endpoints, but a closer look into KEYNOTE-119 shows that PD-L1 as a continuous biomarker (using CPS, 22C3) was associated with a “continuous and strong trend” toward improved ORR with the addition of pembrolizumab.
The ORR was 9.6% vs. 10.6% in unselected patients, compared with 26.3% vs. 11.5% in those with CPS of 20 or greater.
“And when you look at duration of response, you see an increase not just in the number ... but the quality of the response,” he said, noting that for PFS, as well, a trend toward superiority is seen “that is consistent with all the other endpoints.”
“So overall, the application of incrementally restrictive cut-off of CPS lends weight to the exploratory analysis showing better survival from pembrolizumab in tumors with CPS more than 20,” Dr. Bianchini said, noting that the “real question,” however, is whether the finding “is worth clinical implementation.
“We know a lot about the primary tumor and immune infiltration. We’ve learned ... that if you wait and look ahead at immune infiltration in the advanced stage, you find that the tumor becomes smart,” he said, explaining that tumor/immune co-evolution leads to increased immuno-editing and immune subversion and it becomes “much harder to just hit the tumor with PD-L1, because this is not the only mechanism of immune escape.”
A review of several studies shows that in similar populations defined by biomarkers, response rates in patients treated with checkpoint inhibitors decrease in the second- and third-line setting vs. the first-line setting, he said.
For example, pembrolizumab response rates in the first-line and second-line or greater setting in cohort B of the KEYNOTE-086 study were 21.4% and 5.7%, respectively, compared with 12.3% in the second- to third-line setting in KEYNOTE-119, he said.
Another consideration is whether monotherapy or combination therapy is preferable, and the data suggest that regardless of how PD-L1-positivity is defined (by CPS cutoff of 1 vs. 20, for example), most patients treated with monotherapy progress within the first 3 months, he said.
“I don’t see that this is a safe approach for the majority of these patients. So without better biomarkers, combinations should always be preferred, at least to avoid early progression,” Dr. Bianchini said, adding that the open question, then, is: “If we set the new standard in the first-line as the combination of nab-paclitaxel and atezolizumab for PD-L1-positive patients defined by the VENTANA [SP142 assay], should we continue with immune checkpoint [inhibition] using different combinations?”
“Of course, at the time the trial was designed, the results of IMpassion were not available, but it’s very important, because [the findings] add to the evidence that immunotherapy is extremely relevant for some patients,” he said.
KATE2 further demonstrated the importance of PD-L1 status, he said, adding that due to its limitations, including small sample size and short follow-up, longer follow-up is needed to better evaluate duration of response and PFS.
“Despite the trial limitations, the qualitative effect seen in all clinical endpoints – overall response rate, progression-free survival, overall survival – in PD-L1-positive tumors defined by SP142 ... provided strong and robust signals supporting the investigation of immune checkpoint inhibitors in HER-positive breast cancer,” he said, noting that “many trials are ongoing in the early setting and the advanced setting.”
In addition to the lessons of these trials with respect to the interchangeability of PD-L1 IHC assays and the value of PD-L1 assessment for identifying the likelihood of benefit from immune checkpoint inhibitors, the findings highlight the possibility that PD-L1-negative tumors require different immunotherapy approaches or alternative therapeutic strategies, and underscore that the benefit of immunotherapy in PD-L1-positive patients is still restricted to a minority.
“So new studies and approaches with immuno-oncology are needed, and we need more effective biomarkers, because we need to have precision oncology applied – we need to go in that direction,” he concluded.
Dr. Bianchini reported consultancy/honorarium and or advisory board activity associated with Roche, MSD, AstraZeneca, Pfizer, Chugai, EISAI, Lilly, Novartis, Amgen, Sanofi, Neopharm, and Genomic Health. The IMPassion30 trial was funded by F. Hoffmann-La Roche Ltd.; Dr. Rugo reported research grants, other funding, and or travel/accommodation/expenses from Pfizer, Novartis, Eli Lilly, Merck, OBI, EISAI, Plexxikon, Genentech/Roche, MacroGenics, PUMA, Mylan, Immunomedics, Daiichi Sankyo, and Celltrion. KEYNOTE-119 was funded by Merck Sharp & Dohme Corp.; Dr. Cortés and Dr. Emens reported numerous funding relationships but none with F. Hoffman-La Roche. KATE2 was funded by F. Hoffmann-La Roche.
Sources: IMPassion130; ESMO Abstract LBA20; KEYNOTE-119: ESMO Abstract LBA21; KATE2: ESMO Abstract 305O.
REPORTING FROM ESMO 2019
Adding pertuzumab shows benefit in ERBB2-positive breast cancer
Combination pertuzumab, trastuzumab, and docetaxel provided better responses than did placebo, trastuzumab, and docetaxel in Asian patients with ERBB2-positive early or locally advanced breast cancer, according to a phase 3 trial.
The safety profile of the combination regimen was similar between the treatment arms and in accordance with that of pertuzumab alone, reported Zhimin Shao, MD, of Fudan (Shanghai) University Cancer Center, and colleagues. The study was published in JAMA Oncology.
The randomized, placebo-controlled, phase 3 PEONY study included 329 women with ERBB2-positive early or locally advanced disease. The effects of adding pertuzumab to trastuzumab and docetaxel was studied in 23 centers throughout Asia.
Prior to surgery, study subjects in the treatment arm received intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg, trastuzumab at a loading dose of 8 mg/kg followed by 6 mg/kg, and 75 mg/m2 of docetaxel, while patients in the placebo arm received placebo, trastuzumab, and docetaxel. Both regimens were administered every 3 weeks for a total of four cycles.
Post surgery, study patients received intravenous fluorouracil, cyclophosphamide, and epirubicin for a total of 3 cycles, followed by pertuzumab plus trastuzumab or placebo plus trastuzumab for a total of 13 cycles.
The primary outcome was the total pathologic complete response rate assessed at the completion of surgery.
After analysis, the researchers found that total pathologic complete response rates were significantly higher for patients in the pertuzumab arm (39.3%) compared with the placebo arm (21.8%) (difference, 17.5%; P = .001).
With respect to safety, the rates of common adverse events were similar between the groups, with the exception of diarrhea (38.5% in the pertuzumab arm vs. 16.4% in the placebo arm). The incidences of serious toxicities were slightly higher in the pertuzumab arm (10.1%) compared with the placebo arm (8.2%).
“Of the most common grade 3 or higher adverse events, there was a higher incidence of neutropenia in the pertuzumab group (38.1% vs. 32.7%),” they reported.
The researchers acknowledged a key limitation of the study was the short duration of follow-up. As a result, some secondary outcome data were immature at the time of the analysis.
“The PEONY trial adds to the totality of the data showing the benefit of pertuzumab and trastuzumab with chemotherapy in ERBB2-positive early breast cancer,” they concluded.
The authors reported financial affiliations with F. Hoffmann-La Roche Ltd., which funded the study, and Genentech.
SOURCE: Shao Z et al. JAMA Oncol. 2019 Oct 24. doi: 10.1001/jamaoncol.2019.3692.
Combination pertuzumab, trastuzumab, and docetaxel provided better responses than did placebo, trastuzumab, and docetaxel in Asian patients with ERBB2-positive early or locally advanced breast cancer, according to a phase 3 trial.
The safety profile of the combination regimen was similar between the treatment arms and in accordance with that of pertuzumab alone, reported Zhimin Shao, MD, of Fudan (Shanghai) University Cancer Center, and colleagues. The study was published in JAMA Oncology.
The randomized, placebo-controlled, phase 3 PEONY study included 329 women with ERBB2-positive early or locally advanced disease. The effects of adding pertuzumab to trastuzumab and docetaxel was studied in 23 centers throughout Asia.
Prior to surgery, study subjects in the treatment arm received intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg, trastuzumab at a loading dose of 8 mg/kg followed by 6 mg/kg, and 75 mg/m2 of docetaxel, while patients in the placebo arm received placebo, trastuzumab, and docetaxel. Both regimens were administered every 3 weeks for a total of four cycles.
Post surgery, study patients received intravenous fluorouracil, cyclophosphamide, and epirubicin for a total of 3 cycles, followed by pertuzumab plus trastuzumab or placebo plus trastuzumab for a total of 13 cycles.
The primary outcome was the total pathologic complete response rate assessed at the completion of surgery.
After analysis, the researchers found that total pathologic complete response rates were significantly higher for patients in the pertuzumab arm (39.3%) compared with the placebo arm (21.8%) (difference, 17.5%; P = .001).
With respect to safety, the rates of common adverse events were similar between the groups, with the exception of diarrhea (38.5% in the pertuzumab arm vs. 16.4% in the placebo arm). The incidences of serious toxicities were slightly higher in the pertuzumab arm (10.1%) compared with the placebo arm (8.2%).
“Of the most common grade 3 or higher adverse events, there was a higher incidence of neutropenia in the pertuzumab group (38.1% vs. 32.7%),” they reported.
The researchers acknowledged a key limitation of the study was the short duration of follow-up. As a result, some secondary outcome data were immature at the time of the analysis.
“The PEONY trial adds to the totality of the data showing the benefit of pertuzumab and trastuzumab with chemotherapy in ERBB2-positive early breast cancer,” they concluded.
The authors reported financial affiliations with F. Hoffmann-La Roche Ltd., which funded the study, and Genentech.
SOURCE: Shao Z et al. JAMA Oncol. 2019 Oct 24. doi: 10.1001/jamaoncol.2019.3692.
Combination pertuzumab, trastuzumab, and docetaxel provided better responses than did placebo, trastuzumab, and docetaxel in Asian patients with ERBB2-positive early or locally advanced breast cancer, according to a phase 3 trial.
The safety profile of the combination regimen was similar between the treatment arms and in accordance with that of pertuzumab alone, reported Zhimin Shao, MD, of Fudan (Shanghai) University Cancer Center, and colleagues. The study was published in JAMA Oncology.
The randomized, placebo-controlled, phase 3 PEONY study included 329 women with ERBB2-positive early or locally advanced disease. The effects of adding pertuzumab to trastuzumab and docetaxel was studied in 23 centers throughout Asia.
Prior to surgery, study subjects in the treatment arm received intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg, trastuzumab at a loading dose of 8 mg/kg followed by 6 mg/kg, and 75 mg/m2 of docetaxel, while patients in the placebo arm received placebo, trastuzumab, and docetaxel. Both regimens were administered every 3 weeks for a total of four cycles.
Post surgery, study patients received intravenous fluorouracil, cyclophosphamide, and epirubicin for a total of 3 cycles, followed by pertuzumab plus trastuzumab or placebo plus trastuzumab for a total of 13 cycles.
The primary outcome was the total pathologic complete response rate assessed at the completion of surgery.
After analysis, the researchers found that total pathologic complete response rates were significantly higher for patients in the pertuzumab arm (39.3%) compared with the placebo arm (21.8%) (difference, 17.5%; P = .001).
With respect to safety, the rates of common adverse events were similar between the groups, with the exception of diarrhea (38.5% in the pertuzumab arm vs. 16.4% in the placebo arm). The incidences of serious toxicities were slightly higher in the pertuzumab arm (10.1%) compared with the placebo arm (8.2%).
“Of the most common grade 3 or higher adverse events, there was a higher incidence of neutropenia in the pertuzumab group (38.1% vs. 32.7%),” they reported.
The researchers acknowledged a key limitation of the study was the short duration of follow-up. As a result, some secondary outcome data were immature at the time of the analysis.
“The PEONY trial adds to the totality of the data showing the benefit of pertuzumab and trastuzumab with chemotherapy in ERBB2-positive early breast cancer,” they concluded.
The authors reported financial affiliations with F. Hoffmann-La Roche Ltd., which funded the study, and Genentech.
SOURCE: Shao Z et al. JAMA Oncol. 2019 Oct 24. doi: 10.1001/jamaoncol.2019.3692.
FROM JAMA ONCOLOGY
Interview with Clyde E. Markowitz, MD on switching therapies during MS treatment
Clyde E. Markowitz, MD, is the director of the Multiple Sclerosis Center at Penn Neuroscience Center and an Associate Professor of Neurology at the Perelman School of Medicine at the University of Pennsylvania. We sat down with Dr. Markowitz to talk about different multiple sclerosis (MS) therapies and how to determine when it might be time to switch a patient’s current regimen.
Why would an MS specialist switch a patient from one drug therapy to another?
The main reason we switch a patient from one treatment to another is usually related to an inadequate response to their current treatment. This can be seen when a patient is having new clinical symptoms suggestive of a relapse. Additional situations which would cause us to consider a switch in treatment include if the patient has had a new abnormalities seen on MRI scans, such as new T2 lesions or gadolinium- enhancing lesions. We might also switch a patient due to intolerance towards the medication they are on. For example, if they are experiencing flu-like symptoms or having Gastrointestinal issues.
In addition, the expectation that the treatment should slow the rate of progression may not be adequately demonstrating the desired effect. In that setting, we may consider a switch to a drug with a different mechanism of action to hopefully better control disease progression.
Laboratory abnormalities while on treatment might also be a consideration for a switch in therapy. Elevated LFTs, or low WBCs can occur on DMTs and may require a change in treatment. Patients on Natalizumab, require JC virus antibody testing. If the patient’s JCV Ab status changes from negative to positive or a rising index may require a change in therapy to avoid the development of PML.
What are some special considerations for patients during a switch in therapy?
We need to take into consideration the patient’s comorbidities. Does the patient have a history of diabetes, hypertension, cardiac concerns or a risk for infectious complications? What is the patient’s age? As individuals age the immune system becomes less robust at fighting infections or surveillance for malignancies. Some of the medications are immunosuppressive and might increase the risk of developing opportunistic infections or cancers.
Family planning should be taken into consideration during the discussion of which medications might be appropriate. Is the patient planning to have a pregnancy in the near future? Some medications might not be appropriate in that case.
Route of administration could be a factor to consider, since there are several medications that are administered as an infusion in a medical office or hospital setting. This could create issues for some patients who are employed and may have to miss work during these infusions. This could be as frequent as monthly or 2-3 times per year. Some patients just starting a new job, may feel uncomfortable taking time off or disclosing that they have MS leading to concerns for job security.
We also consider the side effects of the new treatment. What side effects and safety monitoring are required for a particular medication? Are there frequent blood tests, cardiac monitoring, dermatologic and ophthalmologic monitoring? How will this impact the patient’s quality of life?
In the end, it comes down to the level of monitoring required for a particular treatment, where the patient is in his or her life, and where he or she is in the disease course.
What are some potential complications when switching therapies?
When switching therapies, one of the bigger concerns is how quickly can we get the patient on the new therapy. Some medications when stopped can lead to return of disease activity or possibly lead to a rebound phenomenon with significant inflammatory activity. We focus on transitioning a patient quickly to a new drug that has a rapid mechanism of action thus limiting the amount of time that a patient is without a treatment. However, based on the mechanism of action of the drug you must consider if a wash out is necessary. The question is how quickly can the patient start the new drug thus preventing a rebound phenomenon. Ideally, no wash out would protect the patient best but might have safety concerns depending on the switch drug profile. If the switch was related to concerns for high JC virus antibody titer going off of natalizumab, there may be a need to make sure the patient does not have PML before making the switch. This may require MRIs and CSF analysis prior to switching.
Ultimately, we consider whether the drug we are switching the patient to is going to be more efficacious than the drug that the patient was previously on. We consider the safety and side effect profile of the new medication. We balance the risk of the disease with the risk of the medication. We must factor in the patient’s tolerance for risk as well and make the best decision with all the available factors considered.
Clyde E. Markowitz, MD, is the director of the Multiple Sclerosis Center at Penn Neuroscience Center and an Associate Professor of Neurology at the Perelman School of Medicine at the University of Pennsylvania. We sat down with Dr. Markowitz to talk about different multiple sclerosis (MS) therapies and how to determine when it might be time to switch a patient’s current regimen.
Why would an MS specialist switch a patient from one drug therapy to another?
The main reason we switch a patient from one treatment to another is usually related to an inadequate response to their current treatment. This can be seen when a patient is having new clinical symptoms suggestive of a relapse. Additional situations which would cause us to consider a switch in treatment include if the patient has had a new abnormalities seen on MRI scans, such as new T2 lesions or gadolinium- enhancing lesions. We might also switch a patient due to intolerance towards the medication they are on. For example, if they are experiencing flu-like symptoms or having Gastrointestinal issues.
In addition, the expectation that the treatment should slow the rate of progression may not be adequately demonstrating the desired effect. In that setting, we may consider a switch to a drug with a different mechanism of action to hopefully better control disease progression.
Laboratory abnormalities while on treatment might also be a consideration for a switch in therapy. Elevated LFTs, or low WBCs can occur on DMTs and may require a change in treatment. Patients on Natalizumab, require JC virus antibody testing. If the patient’s JCV Ab status changes from negative to positive or a rising index may require a change in therapy to avoid the development of PML.
What are some special considerations for patients during a switch in therapy?
We need to take into consideration the patient’s comorbidities. Does the patient have a history of diabetes, hypertension, cardiac concerns or a risk for infectious complications? What is the patient’s age? As individuals age the immune system becomes less robust at fighting infections or surveillance for malignancies. Some of the medications are immunosuppressive and might increase the risk of developing opportunistic infections or cancers.
Family planning should be taken into consideration during the discussion of which medications might be appropriate. Is the patient planning to have a pregnancy in the near future? Some medications might not be appropriate in that case.
Route of administration could be a factor to consider, since there are several medications that are administered as an infusion in a medical office or hospital setting. This could create issues for some patients who are employed and may have to miss work during these infusions. This could be as frequent as monthly or 2-3 times per year. Some patients just starting a new job, may feel uncomfortable taking time off or disclosing that they have MS leading to concerns for job security.
We also consider the side effects of the new treatment. What side effects and safety monitoring are required for a particular medication? Are there frequent blood tests, cardiac monitoring, dermatologic and ophthalmologic monitoring? How will this impact the patient’s quality of life?
In the end, it comes down to the level of monitoring required for a particular treatment, where the patient is in his or her life, and where he or she is in the disease course.
What are some potential complications when switching therapies?
When switching therapies, one of the bigger concerns is how quickly can we get the patient on the new therapy. Some medications when stopped can lead to return of disease activity or possibly lead to a rebound phenomenon with significant inflammatory activity. We focus on transitioning a patient quickly to a new drug that has a rapid mechanism of action thus limiting the amount of time that a patient is without a treatment. However, based on the mechanism of action of the drug you must consider if a wash out is necessary. The question is how quickly can the patient start the new drug thus preventing a rebound phenomenon. Ideally, no wash out would protect the patient best but might have safety concerns depending on the switch drug profile. If the switch was related to concerns for high JC virus antibody titer going off of natalizumab, there may be a need to make sure the patient does not have PML before making the switch. This may require MRIs and CSF analysis prior to switching.
Ultimately, we consider whether the drug we are switching the patient to is going to be more efficacious than the drug that the patient was previously on. We consider the safety and side effect profile of the new medication. We balance the risk of the disease with the risk of the medication. We must factor in the patient’s tolerance for risk as well and make the best decision with all the available factors considered.
Clyde E. Markowitz, MD, is the director of the Multiple Sclerosis Center at Penn Neuroscience Center and an Associate Professor of Neurology at the Perelman School of Medicine at the University of Pennsylvania. We sat down with Dr. Markowitz to talk about different multiple sclerosis (MS) therapies and how to determine when it might be time to switch a patient’s current regimen.
Why would an MS specialist switch a patient from one drug therapy to another?
The main reason we switch a patient from one treatment to another is usually related to an inadequate response to their current treatment. This can be seen when a patient is having new clinical symptoms suggestive of a relapse. Additional situations which would cause us to consider a switch in treatment include if the patient has had a new abnormalities seen on MRI scans, such as new T2 lesions or gadolinium- enhancing lesions. We might also switch a patient due to intolerance towards the medication they are on. For example, if they are experiencing flu-like symptoms or having Gastrointestinal issues.
In addition, the expectation that the treatment should slow the rate of progression may not be adequately demonstrating the desired effect. In that setting, we may consider a switch to a drug with a different mechanism of action to hopefully better control disease progression.
Laboratory abnormalities while on treatment might also be a consideration for a switch in therapy. Elevated LFTs, or low WBCs can occur on DMTs and may require a change in treatment. Patients on Natalizumab, require JC virus antibody testing. If the patient’s JCV Ab status changes from negative to positive or a rising index may require a change in therapy to avoid the development of PML.
What are some special considerations for patients during a switch in therapy?
We need to take into consideration the patient’s comorbidities. Does the patient have a history of diabetes, hypertension, cardiac concerns or a risk for infectious complications? What is the patient’s age? As individuals age the immune system becomes less robust at fighting infections or surveillance for malignancies. Some of the medications are immunosuppressive and might increase the risk of developing opportunistic infections or cancers.
Family planning should be taken into consideration during the discussion of which medications might be appropriate. Is the patient planning to have a pregnancy in the near future? Some medications might not be appropriate in that case.
Route of administration could be a factor to consider, since there are several medications that are administered as an infusion in a medical office or hospital setting. This could create issues for some patients who are employed and may have to miss work during these infusions. This could be as frequent as monthly or 2-3 times per year. Some patients just starting a new job, may feel uncomfortable taking time off or disclosing that they have MS leading to concerns for job security.
We also consider the side effects of the new treatment. What side effects and safety monitoring are required for a particular medication? Are there frequent blood tests, cardiac monitoring, dermatologic and ophthalmologic monitoring? How will this impact the patient’s quality of life?
In the end, it comes down to the level of monitoring required for a particular treatment, where the patient is in his or her life, and where he or she is in the disease course.
What are some potential complications when switching therapies?
When switching therapies, one of the bigger concerns is how quickly can we get the patient on the new therapy. Some medications when stopped can lead to return of disease activity or possibly lead to a rebound phenomenon with significant inflammatory activity. We focus on transitioning a patient quickly to a new drug that has a rapid mechanism of action thus limiting the amount of time that a patient is without a treatment. However, based on the mechanism of action of the drug you must consider if a wash out is necessary. The question is how quickly can the patient start the new drug thus preventing a rebound phenomenon. Ideally, no wash out would protect the patient best but might have safety concerns depending on the switch drug profile. If the switch was related to concerns for high JC virus antibody titer going off of natalizumab, there may be a need to make sure the patient does not have PML before making the switch. This may require MRIs and CSF analysis prior to switching.
Ultimately, we consider whether the drug we are switching the patient to is going to be more efficacious than the drug that the patient was previously on. We consider the safety and side effect profile of the new medication. We balance the risk of the disease with the risk of the medication. We must factor in the patient’s tolerance for risk as well and make the best decision with all the available factors considered.
FDA approves diroximel fumarate for relapsing MS
The Food and Drug Administration has approved diroximel fumarate (Vumerity) for the treatment of relapsing forms of multiple sclerosis (MS) in adults, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to an Oct. 30 announcement from its developers, Biogen and Alkermes.
The approval is based on pharmacokinetic studies that established the bioequivalence of diroximel fumarate and dimethyl fumarate (Tecfidera), and it relied in part on the safety and efficacy data for dimethyl fumarate, which was approved in 2013. Diroximel fumarate rapidly converts to monomethyl fumarate, the same active metabolite as dimethyl fumarate.
Diroximel fumarate may be better tolerated than dimethyl fumarate. A trial found that the newer drug has significantly better gastrointestinal tolerability, the developers of the drug announced in July. In addition, the drug application for diroximel fumarate included interim data from EVOLVE-MS-1, an ongoing, open-label, 2-year safety study evaluating diroximel fumarate in patients with relapsing-remitting MS. Researchers found a 6.3% rate of treatment discontinuation attributable to adverse events. Less than 1% of patients discontinued treatment because of gastrointestinal adverse events.
Serious side effects of diroximel fumarate may include allergic reaction, progressive multifocal leukoencephalopathy, decreases in white blood cell count, and liver problems. Flushing and stomach problems are the most common side effects, which may decrease over time.
Biogen plans to make diroximel fumarate available in the United States in the near future, the company said. Prescribing information is available online.
The Food and Drug Administration has approved diroximel fumarate (Vumerity) for the treatment of relapsing forms of multiple sclerosis (MS) in adults, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to an Oct. 30 announcement from its developers, Biogen and Alkermes.
The approval is based on pharmacokinetic studies that established the bioequivalence of diroximel fumarate and dimethyl fumarate (Tecfidera), and it relied in part on the safety and efficacy data for dimethyl fumarate, which was approved in 2013. Diroximel fumarate rapidly converts to monomethyl fumarate, the same active metabolite as dimethyl fumarate.
Diroximel fumarate may be better tolerated than dimethyl fumarate. A trial found that the newer drug has significantly better gastrointestinal tolerability, the developers of the drug announced in July. In addition, the drug application for diroximel fumarate included interim data from EVOLVE-MS-1, an ongoing, open-label, 2-year safety study evaluating diroximel fumarate in patients with relapsing-remitting MS. Researchers found a 6.3% rate of treatment discontinuation attributable to adverse events. Less than 1% of patients discontinued treatment because of gastrointestinal adverse events.
Serious side effects of diroximel fumarate may include allergic reaction, progressive multifocal leukoencephalopathy, decreases in white blood cell count, and liver problems. Flushing and stomach problems are the most common side effects, which may decrease over time.
Biogen plans to make diroximel fumarate available in the United States in the near future, the company said. Prescribing information is available online.
The Food and Drug Administration has approved diroximel fumarate (Vumerity) for the treatment of relapsing forms of multiple sclerosis (MS) in adults, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to an Oct. 30 announcement from its developers, Biogen and Alkermes.
The approval is based on pharmacokinetic studies that established the bioequivalence of diroximel fumarate and dimethyl fumarate (Tecfidera), and it relied in part on the safety and efficacy data for dimethyl fumarate, which was approved in 2013. Diroximel fumarate rapidly converts to monomethyl fumarate, the same active metabolite as dimethyl fumarate.
Diroximel fumarate may be better tolerated than dimethyl fumarate. A trial found that the newer drug has significantly better gastrointestinal tolerability, the developers of the drug announced in July. In addition, the drug application for diroximel fumarate included interim data from EVOLVE-MS-1, an ongoing, open-label, 2-year safety study evaluating diroximel fumarate in patients with relapsing-remitting MS. Researchers found a 6.3% rate of treatment discontinuation attributable to adverse events. Less than 1% of patients discontinued treatment because of gastrointestinal adverse events.
Serious side effects of diroximel fumarate may include allergic reaction, progressive multifocal leukoencephalopathy, decreases in white blood cell count, and liver problems. Flushing and stomach problems are the most common side effects, which may decrease over time.
Biogen plans to make diroximel fumarate available in the United States in the near future, the company said. Prescribing information is available online.
USPSTF recommendations on risk assessment, genetic counseling, and genetic testing for BRCA-related cancer
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.
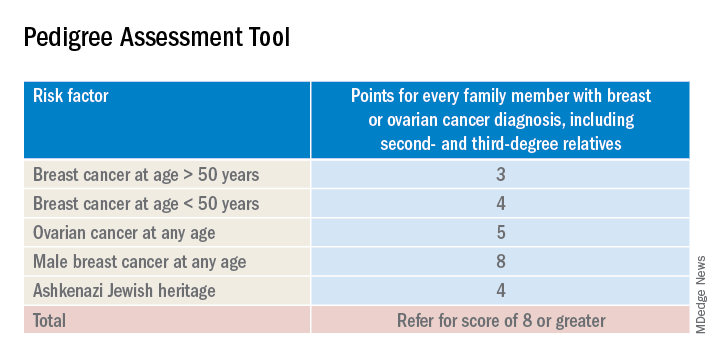
A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Breast cancer screening recommendations have evolved over the past decade. BRCA1/2 genes are tumor-suppressor genes. Mutations in these genes place women at an increased risk for developing breast, ovarian, fallopian tube, and peritoneal cancer. Detection of BRCA1/2 mutations through genetic screening can provide patients with more information about their cancer risk and can lead to discussion of prophylactic therapies. This includes increased screening frequency, medical therapy, and surgical interventions.
New USPSTF recommendations address who is at an increased risk for BRCA1/2 mutations. They recommend using screening tools focusing on family history that primary care physicians can utilize to determine who should be referred for genetic counseling to discuss the risks and benefits of genetic screening. The following are the task force’s two primary recommendations:
The USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 gene mutations with an appropriate brief familial risk assessment tool. Women with a positive result on the risk assessment tool should receive genetic counseling and, if indicated after counseling, genetic testing. (B recommendation)
The USPSTF recommends against routine risk assessment, genetic counseling, or genetic testing for women whose personal or family history or ancestry is not associated with potentially harmful BRCA1/2 gene mutations. (D recommendation)
Breast cancer is the second leading cause of cancer and cancer death for women in the United States. Ovarian cancer ranks fifth in cancer deaths for women in the U.S. By age 70, women with BRCA1/2 mutations have a 45%-65% cumulative lifetime risk of developing breast cancer.
Mutations in BRCA1, specifically, are associated with a 39% lifetime risk for ovarian, fallopian tube, and peritoneal cancer. In contrast, mutations in BRCA2 are associated with a 10%-17% lifetime risk.
The USPSTF also underscores the increased prevalence of BRCA1/2 mutations in the Ashkenazi Jewish population. Three out of seven familial risk assessment tools inquire about Jewish ancestry. This is because the Ashkenazi Jewish population have a higher prevalence of three founder mutations in the BRCA1/2 gene. A member of this population has a 1 in 40 chance of carrying one of these three mutations, whereas the general population has a 1 in 300 chance.
The USPSTF recommends a multistep process of screening. The first step is taking a family history of cancer. For women who have a family history of breast, ovarian, tubal, or peritoneal cancer or a personal history of these cancers, a brief familial risk assessment tool should be used to determine the need for referral for in-depth genetic counseling to determine the need for genetic testing.
It is important to recognize that the validated tools recommended by the USPSTF are specific for genetic risk assessment. General breast cancer risk assessment tools, including the National Cancer Institute Breast Cancer Risk Assessment Tool, which is based on the Gail model, are not recommended.
The sensitivity of the tools recommended by the USPSTF range between 77% and 100%. A number of tools are given as an option with no one tool being better than the other. Perhaps the easiest to implement of the validated tools recommended is the Pedigree Assessment Tool. For this tool, points are assigned for every family member with breast or ovarian cancer as indicated in the table below.

A positive result on a screening tool will lead primary care physicians to appropriately refer patients for genetic counseling. Genetic testing for BRCA1/2 mutations should be limited to those individuals whose personal and/or family history reflects an increased risk for gene mutations after detailed genetic assessment and counseling. The results of the genetic screening should assist a patient in their decision making.
Prophylactic treatment for BRCA1/2 mutation carriers are outside the scope of this recommendation. However the guidelines briefly review risk-reducing therapies including screening, medical, and surgical options. Medical therapy for patients who have BRCA1/2 mutations include the use of tamoxifen, raloxifene, and aromatase inhibitors. Surgical interventions include bilateral mastectomy and salpingo-oopherectomy.
Screening options include earlier and more frequent mammograms and breast MRIs. Screening is largely based on family history and the USPSTF acknowledges their uncertainty when screening women with an unknown family history. Male breast cancer, pancreatic cancer, prostate cancer, and melanoma are also associated with BRCA1/2 mutations. They are not included in this recommendation.
The bottom line
USPSTF recommended that primary care physicians should use familial risk assessment tools to screen women for BRCA1/2 mutations. This includes women with a personal and/or family history of breast, ovarian, tubal, or peritoneal cancer or women with a family history of BRCA1/2 gene mutations. Patients who test positive through one of the suggested screening tools should be referred for genetic counseling. This could lead to genetic testing and subsequent prophylactic therapies and/or increased screenings if the patient so desires. It is of importance to note the USPSTF recommends against routine screening of BRCA1/2 gene mutations for women who do not meet the above requirements.
Reference
USPSTF Recommendation: Assessment, counseling, and testing for BRCA-related cancer. JAMA. 2019;322(7):652-65. doi: 10.1001/jama.2019.10987.
Dr. Style is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Trastuzumab benefit lasts long-term in HER2+ breast cancer
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
Among patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer, adding trastuzumab to adjuvant chemotherapy reduces risk of recurrence for at least 10 years, according to investigators.
The benefit of trastuzumab was greater among patients with hormone receptor–positive (HR+) disease than those with HR– disease until the 5-year timepoint, after which HR status had no significant impact on recurrence rates, reported lead author Saranya Chumsri, MD, of the Mayo Clinic in Jacksonville, Fla., and colleagues. This finding echoes a pattern similar to that of HER2– breast cancer, in which patients with HR+ disease have relatively consistent risk of recurrence over time, whereas patients with HR– disease have an early risk of recurrence that decreases after 5 years.
“To the best of our knowledge, this analysis is the first to address the risk of late relapses in subsets of HER2+ breast cancer patients who were treated with adjuvant trastuzumab,” the investigators wrote. Their report is in Journal of Clinical Oncology.
They drew data from 3,177 patients with HER2+ breast cancer who were involved in two phase 3 studies: the North Central Cancer Treatment Group N9831 and National Surgical Adjuvant Breast and Bowel Project B-31 trials. Patients involved in the analysis received either standard adjuvant chemotherapy with cyclophosphamide and doxorubicin followed by weekly paclitaxel or the same chemotherapy regimen plus concurrent trastuzumab. The primary outcome was recurrence-free survival, which was defined as time from randomization until local, regional, or distant recurrence of breast cancer or breast cancer–related death. Kaplan-Meier estimates were performed to determine recurrence-free survival, while Cox proportional hazards regression models were used to determine factors that predicted relapse.
Including a median follow-up of 8 years across all patients, the analysis showed that those with HR+ breast cancer had a significantly higher estimated rate of recurrence-free survival than that of those with HR– disease after 5 years (81.49% vs. 74.65%) and 10 years (73.84% vs. 69.22%). Overall, a comparable level of benefit was derived from adding trastuzumab regardless of HR status (interaction P = .87). However, during the first 5 years, HR positivity predicted greater benefit from adding trastuzumab, as patients with HR+ disease had a 40% lower risk of relapse than that of those with HR– disease (hazard ratio, 0.60; P less than .001). Between years 5 and 10, the statistical significance of HR status faded (P = .12), suggesting that HR status is not a predictor of long-term recurrence.
“Given concerning adverse effects and potentially smaller benefit of extended adjuvant endocrine therapy, particularly in patients with N0 or N1 disease, our findings highlight the need to develop better risk prediction models and biomarkers to identify which patients have sufficient risk for late relapse to warrant the use of extended endocrine therapy in HER2+ breast cancer,” the investigators concluded.
The study was funded by the National Institutes of Health, the Breast Cancer Research Foundation, Bankhead-Coley Research Program, the DONNA Foundation, and Genentech. Dr. Chumsri disclosed a financial relationship with Merck. Coauthors disclosed ties with Merck, Novartis, Genentech, and NanoString Technologies.
SOURCE: Chumsri et al. J Clin Oncol. 2019 Oct 17. doi: 10.1200/JCO.19.00443.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Adding veliparib to chemotherapy improves PFS in BRCA-mutated breast cancer
BARCELONA – Adding veliparib to chemotherapy improved progression-free survival and provided a more durable benefit than did chemotherapy alone for HER2-negative advanced germline BRCA-associated breast cancer in the randomized, placebo-controlled, phase 3 BROCADE3 study.
Investigator-assessed median progression-free survival (PFS), the primary study endpoint, was 14.5 vs. 12.6 months in 337 patients randomized to receive the poly (ADP-ribose) polymerase inhibitor (PARPi) veliparib along with carboplatin/paclitaxel (CP) and 172 who received placebo and CP (hazard ratio, 0.71), Veronique C. Diéras, MD, reported at the European Society for Medical Oncology Congress.
“The benefit looks durable; more patients in the veliparib arm were still progression free at 2 years (34% vs. 20%), and at 3 years (26% vs. 11%),” said Dr. Diéras of Institut Curie, Paris, and Centre Eugene Marquis, Rennes, France.
The PFS benefit was confirmed by independent central review, and was apparent in all subgroups analyzed, except perhaps in patients with prior brain metastases, who comprised a very small group, she noted.
Among the secondary study endpoints were median overall survival (33.5 vs. 28.2 months at an interim analysis; HR, 0.95), clinical benefit rate (90.7% and 93.2% at 24 weeks), and objective response rate (75.8% and 74.1%).
“This is very important to note,” she said, referring to the high percentage of patients who benefited from CP alone. “But, again, if we look at the duration of response, in the veliparib arm the median duration of response was 14.7 months, whereas it was 11 months in the placebo arm.”
Participants in the double-blind trial were adults with a median age of 47 years who were randomized 2:1 to CP with veliparib or placebo for the treatment of germline BRCA1- or BRCA2-mutated metastatic breast cancer and had received no more than two prior lines of cytotoxic therapy; 48% were estrogen receptor– and/or progesterone receptor–negative, 8% had prior platinum therapy, 4% had a history of central nervous system metastases, and 19% had prior chemotherapy for metastatic disease.
They received placebo or veliparib at an oral dose of 120 mg twice daily on days −2 to 5 with carboplatin (area under the curve 6 on day 1) and weekly paclitaxel (80 mg/m2 on days 1, 8, and 15) in 21-day cycles until disease progression, and as allowed per study protocol, 44% crossed over from the placebo to veliparib group, Dr. Diéras said.
Common adverse events in the veliparib and placebo groups, respectively, included neutropenia (in 89% and 91% of patients), thrombocytopenia (81% and 71%), anemia (80% and 70%), and nausea (73% and 64%), she said, adding that the most common grade 3 adverse events were anemia (42% and 40%), neutropenia (81% and 84%), and thrombocytopenia (40% and 28%).
However, less than 10% of patients discontinued the study drug because of adverse events, she noted.
“In fact, the addition of veliparib to cytotoxic chemotherapy didn’t impair the administration of cytotoxic chemotherapy,” she said, adding that the mean number of CP cycles was 11 in both arms.
Select adverse events of special interest in the veliparib and placebo groups, respectively, included infection within 14 days of neutropenia (any grade, 37% and 36%; grade 3+, 5.4% and 1.8%), hemorrhage within 14 days of thrombocytopenia (any grade, 10% and 7%; grade 3+, 0.3% and 0%), and myelodysplastic syndromes (0.3% in both groups, with 0 grade 3+ events), she said.
“We know that germline BRCA-mutated breast cancers have increased sensitivity to platinum agents. Moreover, according to the concept of synthetic lethality, we do know also that these mutated tumors are very good candidates for PARP inhibition, so there is a strong rationale to combine a PARP inhibitor with cytotoxic chemotherapy with platinum,” she said.
Early studies of such combinations have been challenging because of exacerbation of myelosuppression, which may be the result of “the PARP trapping activity of some compounds,” but veliparib potently inhibits PARP with minimal PARP trapping, and thus may be better tolerated in combination with CP, she explained.
“In fact, in a phase 2 randomized trial – BROCADE2 – we did observe numerical increases in PFS and overall survival with [veliparib+CP],” she said.
In BROCADE3, the addition to veliparib to CP provided “a statistically significant and clinically meaningful benefit in patients with HER-negative advanced breast cancer and a germline BRCA mutation” without substantially altering the toxicity profile of C/P, she said.
“Considering these results, in my opinion, patients harboring BRCA mutations with advanced breast cancer [who are] candidates for chemotherapy should be offered this treatment option,” she concluded.
Invited discussant Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at Peter MacCallum Cancer Center, Victoria, Australia, said the investigators should be commended for conducting this phase 3 trial, and agreed that the approach is “reasonable to consider in a patient who does need chemotherapy.”
However, Dr. Loi said, it remains unclear if the PFS benefit seen in BROCADE3 is “due to the combination upfront and/or the monotherapy,” and suggested waiting for additional data before considering veliparib + CP as the new standard of care for germline BRCA1- and BRCA2-mutated advanced breast cancer.
“Current ESMO guidelines advise that single-agent chemotherapy be given sequentially in the absence of visceral crisis, therefore I think it’s important to await further for mature OS data and patient-reported outcomes,” she said, adding that it is also important to wait for the correlative analyses to “try to understand the rate of BRCA reversions and other resistance mechanisms in plasma.”
BROCADE3 was funded by AbbVie. Dr. Diéras reported advisory/consultancy roles for several pharmaceutical companies including AbbVie. Dr. Loi reported relationships with numerous pharmaceutical companies.
SOURCE: Diéras V et al. ESMO 2019, Abstract LBA9.
BARCELONA – Adding veliparib to chemotherapy improved progression-free survival and provided a more durable benefit than did chemotherapy alone for HER2-negative advanced germline BRCA-associated breast cancer in the randomized, placebo-controlled, phase 3 BROCADE3 study.
Investigator-assessed median progression-free survival (PFS), the primary study endpoint, was 14.5 vs. 12.6 months in 337 patients randomized to receive the poly (ADP-ribose) polymerase inhibitor (PARPi) veliparib along with carboplatin/paclitaxel (CP) and 172 who received placebo and CP (hazard ratio, 0.71), Veronique C. Diéras, MD, reported at the European Society for Medical Oncology Congress.
“The benefit looks durable; more patients in the veliparib arm were still progression free at 2 years (34% vs. 20%), and at 3 years (26% vs. 11%),” said Dr. Diéras of Institut Curie, Paris, and Centre Eugene Marquis, Rennes, France.
The PFS benefit was confirmed by independent central review, and was apparent in all subgroups analyzed, except perhaps in patients with prior brain metastases, who comprised a very small group, she noted.
Among the secondary study endpoints were median overall survival (33.5 vs. 28.2 months at an interim analysis; HR, 0.95), clinical benefit rate (90.7% and 93.2% at 24 weeks), and objective response rate (75.8% and 74.1%).
“This is very important to note,” she said, referring to the high percentage of patients who benefited from CP alone. “But, again, if we look at the duration of response, in the veliparib arm the median duration of response was 14.7 months, whereas it was 11 months in the placebo arm.”
Participants in the double-blind trial were adults with a median age of 47 years who were randomized 2:1 to CP with veliparib or placebo for the treatment of germline BRCA1- or BRCA2-mutated metastatic breast cancer and had received no more than two prior lines of cytotoxic therapy; 48% were estrogen receptor– and/or progesterone receptor–negative, 8% had prior platinum therapy, 4% had a history of central nervous system metastases, and 19% had prior chemotherapy for metastatic disease.
They received placebo or veliparib at an oral dose of 120 mg twice daily on days −2 to 5 with carboplatin (area under the curve 6 on day 1) and weekly paclitaxel (80 mg/m2 on days 1, 8, and 15) in 21-day cycles until disease progression, and as allowed per study protocol, 44% crossed over from the placebo to veliparib group, Dr. Diéras said.
Common adverse events in the veliparib and placebo groups, respectively, included neutropenia (in 89% and 91% of patients), thrombocytopenia (81% and 71%), anemia (80% and 70%), and nausea (73% and 64%), she said, adding that the most common grade 3 adverse events were anemia (42% and 40%), neutropenia (81% and 84%), and thrombocytopenia (40% and 28%).
However, less than 10% of patients discontinued the study drug because of adverse events, she noted.
“In fact, the addition of veliparib to cytotoxic chemotherapy didn’t impair the administration of cytotoxic chemotherapy,” she said, adding that the mean number of CP cycles was 11 in both arms.
Select adverse events of special interest in the veliparib and placebo groups, respectively, included infection within 14 days of neutropenia (any grade, 37% and 36%; grade 3+, 5.4% and 1.8%), hemorrhage within 14 days of thrombocytopenia (any grade, 10% and 7%; grade 3+, 0.3% and 0%), and myelodysplastic syndromes (0.3% in both groups, with 0 grade 3+ events), she said.
“We know that germline BRCA-mutated breast cancers have increased sensitivity to platinum agents. Moreover, according to the concept of synthetic lethality, we do know also that these mutated tumors are very good candidates for PARP inhibition, so there is a strong rationale to combine a PARP inhibitor with cytotoxic chemotherapy with platinum,” she said.
Early studies of such combinations have been challenging because of exacerbation of myelosuppression, which may be the result of “the PARP trapping activity of some compounds,” but veliparib potently inhibits PARP with minimal PARP trapping, and thus may be better tolerated in combination with CP, she explained.
“In fact, in a phase 2 randomized trial – BROCADE2 – we did observe numerical increases in PFS and overall survival with [veliparib+CP],” she said.
In BROCADE3, the addition to veliparib to CP provided “a statistically significant and clinically meaningful benefit in patients with HER-negative advanced breast cancer and a germline BRCA mutation” without substantially altering the toxicity profile of C/P, she said.
“Considering these results, in my opinion, patients harboring BRCA mutations with advanced breast cancer [who are] candidates for chemotherapy should be offered this treatment option,” she concluded.
Invited discussant Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at Peter MacCallum Cancer Center, Victoria, Australia, said the investigators should be commended for conducting this phase 3 trial, and agreed that the approach is “reasonable to consider in a patient who does need chemotherapy.”
However, Dr. Loi said, it remains unclear if the PFS benefit seen in BROCADE3 is “due to the combination upfront and/or the monotherapy,” and suggested waiting for additional data before considering veliparib + CP as the new standard of care for germline BRCA1- and BRCA2-mutated advanced breast cancer.
“Current ESMO guidelines advise that single-agent chemotherapy be given sequentially in the absence of visceral crisis, therefore I think it’s important to await further for mature OS data and patient-reported outcomes,” she said, adding that it is also important to wait for the correlative analyses to “try to understand the rate of BRCA reversions and other resistance mechanisms in plasma.”
BROCADE3 was funded by AbbVie. Dr. Diéras reported advisory/consultancy roles for several pharmaceutical companies including AbbVie. Dr. Loi reported relationships with numerous pharmaceutical companies.
SOURCE: Diéras V et al. ESMO 2019, Abstract LBA9.
BARCELONA – Adding veliparib to chemotherapy improved progression-free survival and provided a more durable benefit than did chemotherapy alone for HER2-negative advanced germline BRCA-associated breast cancer in the randomized, placebo-controlled, phase 3 BROCADE3 study.
Investigator-assessed median progression-free survival (PFS), the primary study endpoint, was 14.5 vs. 12.6 months in 337 patients randomized to receive the poly (ADP-ribose) polymerase inhibitor (PARPi) veliparib along with carboplatin/paclitaxel (CP) and 172 who received placebo and CP (hazard ratio, 0.71), Veronique C. Diéras, MD, reported at the European Society for Medical Oncology Congress.
“The benefit looks durable; more patients in the veliparib arm were still progression free at 2 years (34% vs. 20%), and at 3 years (26% vs. 11%),” said Dr. Diéras of Institut Curie, Paris, and Centre Eugene Marquis, Rennes, France.
The PFS benefit was confirmed by independent central review, and was apparent in all subgroups analyzed, except perhaps in patients with prior brain metastases, who comprised a very small group, she noted.
Among the secondary study endpoints were median overall survival (33.5 vs. 28.2 months at an interim analysis; HR, 0.95), clinical benefit rate (90.7% and 93.2% at 24 weeks), and objective response rate (75.8% and 74.1%).
“This is very important to note,” she said, referring to the high percentage of patients who benefited from CP alone. “But, again, if we look at the duration of response, in the veliparib arm the median duration of response was 14.7 months, whereas it was 11 months in the placebo arm.”
Participants in the double-blind trial were adults with a median age of 47 years who were randomized 2:1 to CP with veliparib or placebo for the treatment of germline BRCA1- or BRCA2-mutated metastatic breast cancer and had received no more than two prior lines of cytotoxic therapy; 48% were estrogen receptor– and/or progesterone receptor–negative, 8% had prior platinum therapy, 4% had a history of central nervous system metastases, and 19% had prior chemotherapy for metastatic disease.
They received placebo or veliparib at an oral dose of 120 mg twice daily on days −2 to 5 with carboplatin (area under the curve 6 on day 1) and weekly paclitaxel (80 mg/m2 on days 1, 8, and 15) in 21-day cycles until disease progression, and as allowed per study protocol, 44% crossed over from the placebo to veliparib group, Dr. Diéras said.
Common adverse events in the veliparib and placebo groups, respectively, included neutropenia (in 89% and 91% of patients), thrombocytopenia (81% and 71%), anemia (80% and 70%), and nausea (73% and 64%), she said, adding that the most common grade 3 adverse events were anemia (42% and 40%), neutropenia (81% and 84%), and thrombocytopenia (40% and 28%).
However, less than 10% of patients discontinued the study drug because of adverse events, she noted.
“In fact, the addition of veliparib to cytotoxic chemotherapy didn’t impair the administration of cytotoxic chemotherapy,” she said, adding that the mean number of CP cycles was 11 in both arms.
Select adverse events of special interest in the veliparib and placebo groups, respectively, included infection within 14 days of neutropenia (any grade, 37% and 36%; grade 3+, 5.4% and 1.8%), hemorrhage within 14 days of thrombocytopenia (any grade, 10% and 7%; grade 3+, 0.3% and 0%), and myelodysplastic syndromes (0.3% in both groups, with 0 grade 3+ events), she said.
“We know that germline BRCA-mutated breast cancers have increased sensitivity to platinum agents. Moreover, according to the concept of synthetic lethality, we do know also that these mutated tumors are very good candidates for PARP inhibition, so there is a strong rationale to combine a PARP inhibitor with cytotoxic chemotherapy with platinum,” she said.
Early studies of such combinations have been challenging because of exacerbation of myelosuppression, which may be the result of “the PARP trapping activity of some compounds,” but veliparib potently inhibits PARP with minimal PARP trapping, and thus may be better tolerated in combination with CP, she explained.
“In fact, in a phase 2 randomized trial – BROCADE2 – we did observe numerical increases in PFS and overall survival with [veliparib+CP],” she said.
In BROCADE3, the addition to veliparib to CP provided “a statistically significant and clinically meaningful benefit in patients with HER-negative advanced breast cancer and a germline BRCA mutation” without substantially altering the toxicity profile of C/P, she said.
“Considering these results, in my opinion, patients harboring BRCA mutations with advanced breast cancer [who are] candidates for chemotherapy should be offered this treatment option,” she concluded.
Invited discussant Sherene Loi, MBBS, PhD, head of the Translational Breast Cancer Genomics and Therapeutics Laboratory at Peter MacCallum Cancer Center, Victoria, Australia, said the investigators should be commended for conducting this phase 3 trial, and agreed that the approach is “reasonable to consider in a patient who does need chemotherapy.”
However, Dr. Loi said, it remains unclear if the PFS benefit seen in BROCADE3 is “due to the combination upfront and/or the monotherapy,” and suggested waiting for additional data before considering veliparib + CP as the new standard of care for germline BRCA1- and BRCA2-mutated advanced breast cancer.
“Current ESMO guidelines advise that single-agent chemotherapy be given sequentially in the absence of visceral crisis, therefore I think it’s important to await further for mature OS data and patient-reported outcomes,” she said, adding that it is also important to wait for the correlative analyses to “try to understand the rate of BRCA reversions and other resistance mechanisms in plasma.”
BROCADE3 was funded by AbbVie. Dr. Diéras reported advisory/consultancy roles for several pharmaceutical companies including AbbVie. Dr. Loi reported relationships with numerous pharmaceutical companies.
SOURCE: Diéras V et al. ESMO 2019, Abstract LBA9.
REPORTING FROM ESMO 2019










