User login
Breast cancer screening complexities
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
An oncologist’s view on screening mammography
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.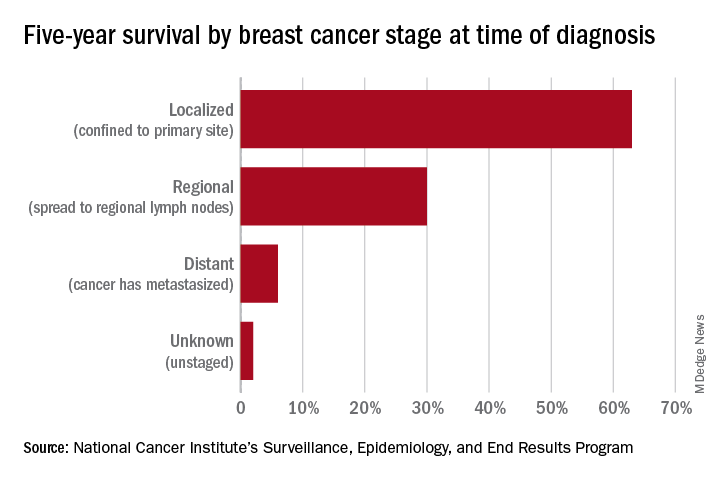
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.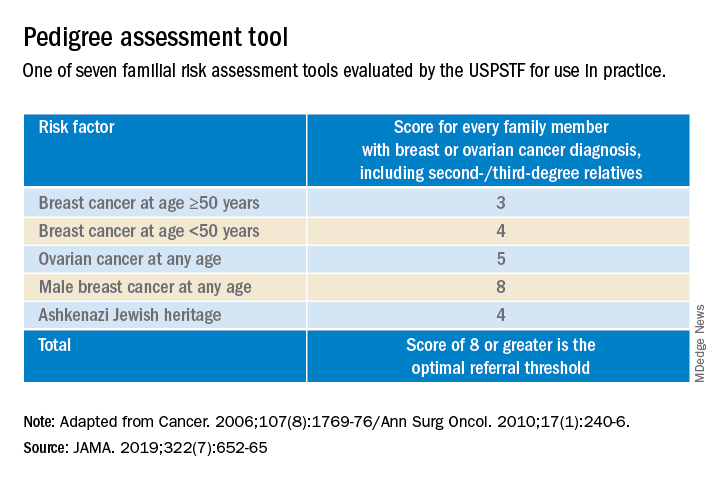
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.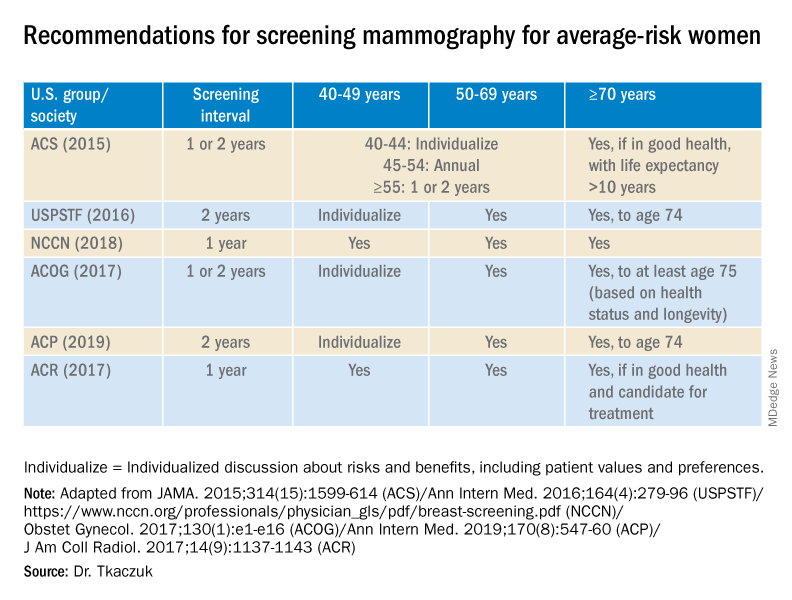
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
The scope of under- and overtreatment in older adults with cancer
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Because of physiological changes with aging and differences in cancer biology, caring for older adults (OAs) with cancer requires careful assessment and planning.
Clark Dumontier, MD, of Brigham and Women’s Hospital in Boston, and colleagues sought to define the meaning of the terms “undertreatment” and “overtreatment” for OAs with cancer in a scoping literature review published in the Journal of Clinical Oncology.
Though OAs are typically defined as adults aged 65 years and older, in this review, the authors defined OAs as patients aged 60 years and older.
The authors theorized that a scoping review of papers about this patient population could provide clues about limitations in the oncology literature and guidance about patient management and future research. Despite comprising the majority of cancer patients, OAs are underrepresented in clinical trials.
About scoping reviews
Scoping reviews are used to identify existing evidence in a field, clarify concepts or definitions in the literature, survey how research on a topic is conducted, and identify knowledge gaps. In addition, scoping reviews summarize available evidence without answering a discrete research question.
Industry standards for scoping reviews have been established by the Johanna Briggs Institute and Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews. According to these standards, scoping reviews should:
- Establish eligibility criteria with a rationale for each criterion clearly explained
- Search multiple databases in multiple languages
- Include “gray literature,” defined as studies that are unpublished or difficult to locate
- Have several independent reviewers screen titles and abstracts
- Ask multiple independent reviewers to review full text articles
- Present results with charts or diagrams that align with the review’s objective
- Graphically depict the decision process for including/excluding sources
- Identify implications for further research.
In their review, Dr. DuMontier and colleagues fulfilled many of the aforementioned criteria. The team searched three English-language databases for titles and abstracts that included the terms undertreatment and/or overtreatment, and were related to OAs with cancer, inclusive of all types of articles, cancer types, and treatments.
Definitions of undertreatment and overtreatment were extracted, and categories underlying these definitions were derived. Within a random subset of articles, two coauthors independently determined final categories of definitions and independently assigned those categories.
Findings and implications
To define OA, Dr. DuMontier and colleagues used a cutoff of 60 years or older. Articles mentioning undertreatment (n = 236), overtreatment (n = 71), or both (n = 51) met criteria for inclusion (n = 256), but only 14 articles (5.5%) explicitly provided formal definitions.
For most of the reviewed articles, the authors judged definitions from the surrounding context. In a random subset of 50 articles, there was a high level of agreement (87.1%; κ = 0.81) between two coauthors in independently assigning categories of definitions.
Undertreatment was applied to therapy that was less than recommended (148 articles; 62.7%) or less than recommended with worse outcomes (88 articles; 37.3%).
Overtreatment most commonly denoted intensive treatment of an OA in whom harms outweighed the benefits of treatment (38 articles; 53.5%) or intensive treatment of a cancer not expected to affect the OA during the patient’s remaining life (33 articles; 46.5%).
Overall, the authors found that undertreatment and overtreatment of OAs with cancer are imprecisely defined concepts. Formal geriatric assessment was recommended in just over half of articles, and only 26.2% recommended formal assessments of age-related vulnerabilities for management. The authors proposed definitions that accounted for both oncologic factors and geriatric domains.
Care of individual patients and clinical research
National Comprehensive Cancer Network (NCCN) guidelines for OAs with cancer recommend initial consideration of overall life expectancy. If a patient is a candidate for cancer treatment on that basis, the next recommended assessment is that of the patient’s capacity to understand the relevant information, appreciate the underlying values and overall medical situation, reason through decisions, and communicate a choice that is consistent with the patient’s articulated goals.
In the pretreatment evaluation of OAs in whom there are no concerns about tolerance to antineoplastic therapy, NCCN guidelines suggest geriatric screening with standardized tools and, if abnormal, comprehensive geriatric screening. The guidelines recommend considering alternative treatment options if nonmodifiable abnormalities are identified.
Referral to a geriatric clinical specialist, use of the Cancer and Aging Research Group’s Chemo Toxicity Calculator, and calculation of Chemotherapy Risk Assessment Scale for High-Age Patients score are specifically suggested if high-risk procedures (such as chemotherapy, radiation, or complex surgery, which most oncologists would consider to be “another day in the office”) are contemplated.
The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology are similarly detailed and endorse similar evaluations and management.
Employing disease-centric and geriatric domains
Dr. DuMontier and colleagues noted that, for OAs with comorbidity or psychosocial challenges, surrogate survival endpoints are unrelated to quality of life (QOL) outcomes. Nonetheless, QOL is valued by OAs at least as much as survival improvement.
Through no fault of their own, the authors’ conclusion that undertreatment and overtreatment are imperfectly defined concepts has a certain neutrality to it. However, the terms undertreatment and overtreatment are commonly used to signify that inappropriate treatment decisions were made. Therefore, the terms are inherently negative and pejorative.
As with most emotionally charged issues in oncology, it is ideal for professionals in our field to take charge when deficiencies exist. ASCO, NCCN, and the authors of this scoping review have provided a conceptual basis for doing so.
An integrated oncologist-geriatrician approach was shown to be effective in the randomized INTEGERATE trial, showing improved QOL, reduced hospital admissions, and reduced early treatment discontinuation from adverse events (ASCO 2020, Abstract 12011).
Therefore, those clinicians who have not formally, systematically, and routinely supplemented the traditional disease-centric endpoints with patient-centered criteria need to do so.
Similarly, a retrospective study published in JAMA Network Open demonstrated that geriatric and surgical comanagement of OAs with cancer was associated with significantly lower 90-day postoperative mortality and receipt of more supportive care services (physical therapy, occupational therapy, speech and swallow rehabilitation, and nutrition services), in comparison with management from the surgical service only.
These clinical and administrative changes will not only enhance patient management but also facilitate the clinical trials required to clarify optimal treatment intensity. As that occurs, we will be able to apply as much precision to the care of OAs with cancer as we do in other areas of cancer treatment.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Dumontier C et al. J Clin Oncol. 2020 Aug 1;38(22):2558-2569.
Cancer disparities: One of the most pressing public health issues
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
“The burden of cancer is not shouldered equally by all segments of the U.S. population,” the AACR adds. “The adverse differences in cancer burden that exist among certain population groups are one of the most pressing public health challenges that we face in the United States.”
AACR president Antoni Ribas, MD, PhD, gave some examples of these disparities at a September 16 Congressional briefing that focused on the inaugural AACR Cancer Disparities Progress Report 2020.
He noted that:
- Black men have more than double the rate of death from prostate cancer compared with men of other racial and ethnic groups.
- Hispanic children are 24% more likely to develop leukemia than non-Hispanic children.
- Non-Hispanic Black children and adolescents with cancer are more than 50% more likely to die from the cancer than non-Hispanic white children and adolescents with cancer.
- Women of low socioeconomic status with early stage ovarian cancer are 50% less likely to receive recommended care than are women of high socioeconomic status.
- In addition to racial and ethnic minority groups, other populations that bear a disproportionate burden when it comes to cancer include individuals lacking adequate health insurance coverage, immigrants, those with disabilities, residents in rural areas, and members of the lesbian, gay, bisexual, and transgender communities.
“It is absolutely unacceptable that advances in cancer care and treatment are not benefiting everyone equally,” Ribas commented.
Making progress against cancer
Progress being made against cancer was highlighted in another publication, the annual AACR Cancer Progress Report 2020.
U.S. cancer deaths declined by 29% between 1991 and 2017, translating to nearly 3 million cancer deaths avoided, the report notes. In addition, 5-year survival rates for all cancers combined increased from 49% in the mid-1970s to 70% for patients diagnosed from 2010-2016.
Between August 2019 and July 31 of this year, the U.S. Food and Drug Administration approved 20 new anticancer drugs for various cancer types and 15 new indications for previously approved cancer drugs, marking the highest number of approvals in one 12-month period since AACR started producing these reports 10 years ago.
A continuing reduction in the cigarette smoking rate among US adults, which is now below 14%, is contributing greatly to declines in lung cancer rates, which have largely driven the improvements in cancer survival, the AACR noted.
This report also notes that progress has been made toward reducing cancer disparities. Overall disparities in cancer death rates among racial and ethnic groups are less pronounced now than they have been in the past two decades. For example, the overall cancer death rate for African American patients was 33% higher than for White patients in 1990 but just 14% higher in 2016.
However, both reports agree that more must be done to reduce cancer disparities even further.
They highlight initiatives that are underway, including:
- The draft guidance issued by the FDA to promote diversification of clinical trial populations.
- The National Institutes of Health’s (NIH’s) Continuing Umbrella of Research Experiences (CURE) program supporting underrepresented students and scientists along their academic and research career pathway.
- The Centers for Disease Control and Prevention’s Racial and Ethnic Approaches to Community Health (REACH) program, a grant-making program focused on encouraging preventive behaviors in underserved communities.
- The NIH’s All of Us program, which is gathering information from the genomes of 1 million healthy individuals with a focus on recruitment from historically underrepresented populations.
Ribas also announced that AACR has established a task force to focus on racial inequalities in cancer research.
Eliminating disparities would save money, argued John D. Carpten, PhD, from the University of Southern California, Los Angeles, who chaired the steering committee that developed the AACR Cancer Disparities Progress Report.
Carpten noted research showing that eliminating disparities for racial and ethnic minorities between 2003 and 2006 would have reduced health care costs by more than $1 trillion in the United States. This underscores the potentially far-reaching impact of efforts to eliminate disparities, he said.
“Without a doubt, socioeconomics and inequities in access to quality care represent major factors influencing cancer health disparities, and these disparities will persist until we address these issues” he said.
Both progress reports culminate in a call to action, largely focused on the need for “unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for the NIH, NCI [National Cancer Institute], and FDA,” which is vital for accelerating the pace of progress.
The challenge is now compounded by the ongoing COVID-19 pandemic: Both progress reports note that racial and ethnic minorities, including African Americans, are not only affected disproportionately by cancer, but also by COVID-19, further highlighting the “stark inequities in health care.”
Ribas further called for action from national leadership and the scientific community.
“During this unprecedented time in our nation’s history, there is also a need for our nation’s leaders to take on a much bigger role in confronting and combating the structural and systemic racism that contributes to health disparities,” he said. The “pervasive racism and social injustices” that have contributed to disparities in both COVID-19 and cancer underscore the need for “the scientific community to step up and partner with Congress to assess and address this issue within the research community.”
This article first appeared on Medscape.com.
OTC ‘brain boosters’ may pose serious risks, experts say
, new research shows.
“Americans spend more than $600 million on over-the-counter smart pills every year, but we know very little about what is actually in these products,” said Pieter A. Cohen, MD, of the department of medicine at Harvard Medical School, Boston.
“Finding new combinations of drugs [that have] never been tested in humans in over-the-counter brain-boosting supplements is alarming,” said Dr. Cohen.
The study was published online Sept. 23 in Neurology Clinical Practice, a journal of the American Academy of Neurology.
Buyer beware
In a search of the National Institutes of Health Dietary Supplement Label Database and the Natural Medicines Database, Dr. Cohen and colleagues identified 10 supplements labeled as containing omberacetam, aniracetam, phenylpiracetam, or oxiracetam – four analogues of piracetam that are not approved for human use in the United States. Piracetam is also not approved in the United States.
In these 10 products, five unapproved drugs were discovered – omberacetam and aniracetam along with three others (phenibut, vinpocetine and picamilon).
By consuming the recommended serving size of these products, consumers could be exposed to pharmaceutical-level dosages of drugs including a maximum of 40.6 mg omberacetam (typical pharmacologic dose 10 mg), 502 mg of aniracetam (typical pharmacologic dose 200-750 mg), 15.4 mg of phenibut (typical dose 250-500 mg), 4.3 mg of vinpocetine (typical dose 5-40 mg), and 90.1 mg of picamilon (typical dose 50-200 mg), the study team reported.
Several drugs detected in these “smart” pills were not declared on the label, and several declared drugs were not detected in the products. For those products with drug quantities provided on the labels, three-quarters of declared quantities were inaccurate.
Consumers who use these cognitive enhancers could be exposed to amounts of these unapproved drugs that are fourfold greater than pharmaceutical dosages and combinations never tested in humans, the study team says. One product combined three different unapproved drugs and another product contained four different drugs.
“We have previously shown that these products may contain individual foreign drugs, but in our new study we found complex combinations of foreign drugs, up to four different drugs in a single product,” Dr. Cohen said.
The presence of these unapproved drugs in supplements, including at supratherapeutic dosages, suggests “serious risks to consumers and weaknesses in the regulatory framework under which supplements are permitted to be introduced in the U.S.,” Dr. Cohen and colleagues wrote.
“We should counsel our patients to avoid over-the-counter ‘smart pills’ until we can be assured as to the safety and efficacy of these products,” said Dr. Cohen.
Concerning findings
Glen R. Finney, MD, director of the Geisinger Memory and Cognition Program at the Neuroscience Institute, Geisinger Health System, Wilkes-Barre, Penn., said in an interview that two findings are very concerning: the lack of listed ingredients and especially the presence of unlisted drugs at active levels. “What if a person has a sensitivity or allergy to one of the unlisted drugs? This is a safety issue and a consumer issue,” Dr. Finney said.
Despite being widely promoted on television, “over-the-counter supplements are not regulated, so there is no guarantee that they contain what they claim, and there is very little evidence that they help memory and thinking even when they do have the ingredients they claim in the supplement,” said Dr. Finney,
“The best way to stay safe and help memory and thinking is to speak with your health providers about proven treatments that have good safety regulation, so you know what you’re getting, and what you’re getting from it,” Dr. Finney advised.
The study had no targeted funding. Dr. Cohen has collaborated in research with NSF International, received compensation from UptoDate, and received research support from Consumers Union and PEW Charitable Trusts. Dr. Finney has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
, new research shows.
“Americans spend more than $600 million on over-the-counter smart pills every year, but we know very little about what is actually in these products,” said Pieter A. Cohen, MD, of the department of medicine at Harvard Medical School, Boston.
“Finding new combinations of drugs [that have] never been tested in humans in over-the-counter brain-boosting supplements is alarming,” said Dr. Cohen.
The study was published online Sept. 23 in Neurology Clinical Practice, a journal of the American Academy of Neurology.
Buyer beware
In a search of the National Institutes of Health Dietary Supplement Label Database and the Natural Medicines Database, Dr. Cohen and colleagues identified 10 supplements labeled as containing omberacetam, aniracetam, phenylpiracetam, or oxiracetam – four analogues of piracetam that are not approved for human use in the United States. Piracetam is also not approved in the United States.
In these 10 products, five unapproved drugs were discovered – omberacetam and aniracetam along with three others (phenibut, vinpocetine and picamilon).
By consuming the recommended serving size of these products, consumers could be exposed to pharmaceutical-level dosages of drugs including a maximum of 40.6 mg omberacetam (typical pharmacologic dose 10 mg), 502 mg of aniracetam (typical pharmacologic dose 200-750 mg), 15.4 mg of phenibut (typical dose 250-500 mg), 4.3 mg of vinpocetine (typical dose 5-40 mg), and 90.1 mg of picamilon (typical dose 50-200 mg), the study team reported.
Several drugs detected in these “smart” pills were not declared on the label, and several declared drugs were not detected in the products. For those products with drug quantities provided on the labels, three-quarters of declared quantities were inaccurate.
Consumers who use these cognitive enhancers could be exposed to amounts of these unapproved drugs that are fourfold greater than pharmaceutical dosages and combinations never tested in humans, the study team says. One product combined three different unapproved drugs and another product contained four different drugs.
“We have previously shown that these products may contain individual foreign drugs, but in our new study we found complex combinations of foreign drugs, up to four different drugs in a single product,” Dr. Cohen said.
The presence of these unapproved drugs in supplements, including at supratherapeutic dosages, suggests “serious risks to consumers and weaknesses in the regulatory framework under which supplements are permitted to be introduced in the U.S.,” Dr. Cohen and colleagues wrote.
“We should counsel our patients to avoid over-the-counter ‘smart pills’ until we can be assured as to the safety and efficacy of these products,” said Dr. Cohen.
Concerning findings
Glen R. Finney, MD, director of the Geisinger Memory and Cognition Program at the Neuroscience Institute, Geisinger Health System, Wilkes-Barre, Penn., said in an interview that two findings are very concerning: the lack of listed ingredients and especially the presence of unlisted drugs at active levels. “What if a person has a sensitivity or allergy to one of the unlisted drugs? This is a safety issue and a consumer issue,” Dr. Finney said.
Despite being widely promoted on television, “over-the-counter supplements are not regulated, so there is no guarantee that they contain what they claim, and there is very little evidence that they help memory and thinking even when they do have the ingredients they claim in the supplement,” said Dr. Finney,
“The best way to stay safe and help memory and thinking is to speak with your health providers about proven treatments that have good safety regulation, so you know what you’re getting, and what you’re getting from it,” Dr. Finney advised.
The study had no targeted funding. Dr. Cohen has collaborated in research with NSF International, received compensation from UptoDate, and received research support from Consumers Union and PEW Charitable Trusts. Dr. Finney has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
, new research shows.
“Americans spend more than $600 million on over-the-counter smart pills every year, but we know very little about what is actually in these products,” said Pieter A. Cohen, MD, of the department of medicine at Harvard Medical School, Boston.
“Finding new combinations of drugs [that have] never been tested in humans in over-the-counter brain-boosting supplements is alarming,” said Dr. Cohen.
The study was published online Sept. 23 in Neurology Clinical Practice, a journal of the American Academy of Neurology.
Buyer beware
In a search of the National Institutes of Health Dietary Supplement Label Database and the Natural Medicines Database, Dr. Cohen and colleagues identified 10 supplements labeled as containing omberacetam, aniracetam, phenylpiracetam, or oxiracetam – four analogues of piracetam that are not approved for human use in the United States. Piracetam is also not approved in the United States.
In these 10 products, five unapproved drugs were discovered – omberacetam and aniracetam along with three others (phenibut, vinpocetine and picamilon).
By consuming the recommended serving size of these products, consumers could be exposed to pharmaceutical-level dosages of drugs including a maximum of 40.6 mg omberacetam (typical pharmacologic dose 10 mg), 502 mg of aniracetam (typical pharmacologic dose 200-750 mg), 15.4 mg of phenibut (typical dose 250-500 mg), 4.3 mg of vinpocetine (typical dose 5-40 mg), and 90.1 mg of picamilon (typical dose 50-200 mg), the study team reported.
Several drugs detected in these “smart” pills were not declared on the label, and several declared drugs were not detected in the products. For those products with drug quantities provided on the labels, three-quarters of declared quantities were inaccurate.
Consumers who use these cognitive enhancers could be exposed to amounts of these unapproved drugs that are fourfold greater than pharmaceutical dosages and combinations never tested in humans, the study team says. One product combined three different unapproved drugs and another product contained four different drugs.
“We have previously shown that these products may contain individual foreign drugs, but in our new study we found complex combinations of foreign drugs, up to four different drugs in a single product,” Dr. Cohen said.
The presence of these unapproved drugs in supplements, including at supratherapeutic dosages, suggests “serious risks to consumers and weaknesses in the regulatory framework under which supplements are permitted to be introduced in the U.S.,” Dr. Cohen and colleagues wrote.
“We should counsel our patients to avoid over-the-counter ‘smart pills’ until we can be assured as to the safety and efficacy of these products,” said Dr. Cohen.
Concerning findings
Glen R. Finney, MD, director of the Geisinger Memory and Cognition Program at the Neuroscience Institute, Geisinger Health System, Wilkes-Barre, Penn., said in an interview that two findings are very concerning: the lack of listed ingredients and especially the presence of unlisted drugs at active levels. “What if a person has a sensitivity or allergy to one of the unlisted drugs? This is a safety issue and a consumer issue,” Dr. Finney said.
Despite being widely promoted on television, “over-the-counter supplements are not regulated, so there is no guarantee that they contain what they claim, and there is very little evidence that they help memory and thinking even when they do have the ingredients they claim in the supplement,” said Dr. Finney,
“The best way to stay safe and help memory and thinking is to speak with your health providers about proven treatments that have good safety regulation, so you know what you’re getting, and what you’re getting from it,” Dr. Finney advised.
The study had no targeted funding. Dr. Cohen has collaborated in research with NSF International, received compensation from UptoDate, and received research support from Consumers Union and PEW Charitable Trusts. Dr. Finney has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY CLINICAL PRACTICE
Divergent findings with paclitaxel and nab-paclitaxel in TNBC
The trials, IMpassion130 and IMpassion131, both enrolled patients with metastatic or unresectable, locally advanced TNBC.
In IMpassion131, adding atezolizumab to paclitaxel did not improve progression-free survival (PFS) or overall survival (OS), regardless of programmed death–ligand 1 (PD-L1) expression.
In IMpassion130, adding atezolizumab to nab-paclitaxel did not improve OS in the intention-to-treat (ITT) population but did provide a “clinically meaningful” improvement in OS among PD-L1-positive patients, according to investigators.
IMpassion130 and IMpassion131 were presented during the same session at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.
Potential reasons for the different outcomes in the two studies require further exploration, according to David Miles, MD, of Mount Vernon Cancer Centre in Northwood, England, who presented the findings from IMpassion131.
ESMO discussant Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill, posited three possible explanations for the divergent findings. The steroids necessary with paclitaxel dosing may have had a negative effect on immune checkpoint inhibitor activity, differences in study populations may have played a role, or the divergent findings could be caused by chance.
Steroid use in IMpassion131 could have played a negative role because of its lympholytic activity, but other indications with steroid use have not demonstrated attenuated benefits, said Leisha A. Emens, MD, PhD, of the University of Pittsburgh Medical Center, who presented the findings from IMpassion130 at ESMO 2020.
“If I were a patient, based on the data to date, I would want nab-paclitaxel with atezolizumab,” Dr. Emens said.
Trial details
Both trials are phase 3, double-blind, placebo-controlled studies of women with metastatic or unresectable locally advanced TNBC who had received no prior therapy for advanced TNBC.
IMpassion130 included 451 patients randomized to atezolizumab plus nab-paclitaxel and 451 randomized to placebo plus nab-paclitaxel. Patients received nab-paclitaxel at a starting dose of 100 mg/m2 via IV infusion on days 1, 8, and 15 of each 28-day cycle for at least six cycles.
In both studies, patients received atezolizumab at 840 mg on days 1 and 15 of a 28-day cycle in their active treatment arms.
IMpassion131 included 651 patients randomized 2:1 to atezolizumab plus paclitaxel (n = 431) or placebo plus paclitaxel (n = 220). Patients received paclitaxel at 90 mg/m2 on days 1, 8, and 15 every 28 days until disease progression or unacceptable toxicity.
Baseline characteristics were well balanced between the treatment arms in both studies. Less than half of patients – 45% in IMpassion131 and 41% in IMpassion130 – were PD-L1 positive.
Results of IMpassion131
The primary endpoint in IMpassion131 was PFS, and there was no significant difference in PFS between the treatment arms.
“The primary objective of IMpassion131 was not met,” Dr. Miles said. “[The] addition of atezolizumab to paclitaxel did not significantly improve PFS in patients with PD-L1-positive metastatic triple-negative breast cancer.”
In the PD-L1-positive population, the median PFS was 5.7 months in the placebo arm and 6.0 months in the atezolizumab arm (stratified hazard ratio, 0.82, P = .20).
In the ITT population, the median PFS was 5.6 months in the control arm and 5.7 months in the atezolizumab arm (HR, 0.86).
In subgroup analyses, Dr. Miles noted, “There was no clue about adverse or beneficial effects in any subgroup.”
The updated OS analysis demonstrated no benefit with atezolizumab in the ITT population or the PD-L1-positive population. In fact, there was a trend toward better OS for the control group in the latter analysis.
In the PD-L1-positive population, the median OS was 28.3 months in the control arm and 22.1 months in the atezolizumab arm (HR, 1.12). The 2-year OS rates were 51% and 49%, respectively.
In the ITT population, the median OS was 22.8 months in the control arm and 19.2 months in the atezolizumab arm (HR, 1.11). The 2-year OS rates were 45% and 42%, respectively.
The safety profile of the atezolizumab-paclitaxel combination was consistent with known side effects of the individual drugs, Dr. Miles said. There were four fatal treatment-related adverse events in the atezolizumab arm.
Results of IMpassion130
Presenting the final OS analysis from IMpassion130, Dr. Emens noted that the study’s findings have led to recommendations for atezolizumab plus nab-paclitaxel as first-line treatment of PD-L1-positive TNBC in international guidelines.
The median OS in the ITT population was 18.7 months in the placebo arm and 21.0 months in the atezolizumab arm (stratified HR, 0.87, P = .077). The 3-year OS rates were 25% and 28%, respectively.
The median OS in the PD-L1-positive population was 17.9 months in the placebo arm and 25.4 months in the atezolizumab arm (HR, 0.67). The 3-year OS rates were 22% and 36%, respectively.
A P value is not available for the between-arm OS comparison in the PD-L1-positive population. OS was not formally tested in this group because the OS boundary for statistical significance was not crossed in the ITT population. However, Dr. Emens said there was a “clinically meaningful” OS benefit observed with atezolizumab in the PD-L1-positive patients.
Treatment withdrawals caused by adverse events were more common in the atezolizumab arm (19% vs. 8%). The most common of these was neuropathy, Dr. Emens said. However, she noted that atezolizumab-related adverse events were generally low grade and easily managed.
“These results support a positive benefit-risk profile for atezolizumab plus nab-paclitaxel as first-line therapy in patients with PD-L1-positive metastatic triple-negative breast cancer,” Dr. Emens concluded.
Both studies were funded by F. Hoffman–La Roche. Dr. Miles, Dr. Emens, and Dr. Carey disclosed financial relationships with Roche and other companies.
SOURCES: Miles D et al. ESMO 2020, Abstract LBA15; Emens LA et al. ESMO 2020, Abstract LBA16.
The trials, IMpassion130 and IMpassion131, both enrolled patients with metastatic or unresectable, locally advanced TNBC.
In IMpassion131, adding atezolizumab to paclitaxel did not improve progression-free survival (PFS) or overall survival (OS), regardless of programmed death–ligand 1 (PD-L1) expression.
In IMpassion130, adding atezolizumab to nab-paclitaxel did not improve OS in the intention-to-treat (ITT) population but did provide a “clinically meaningful” improvement in OS among PD-L1-positive patients, according to investigators.
IMpassion130 and IMpassion131 were presented during the same session at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.
Potential reasons for the different outcomes in the two studies require further exploration, according to David Miles, MD, of Mount Vernon Cancer Centre in Northwood, England, who presented the findings from IMpassion131.
ESMO discussant Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill, posited three possible explanations for the divergent findings. The steroids necessary with paclitaxel dosing may have had a negative effect on immune checkpoint inhibitor activity, differences in study populations may have played a role, or the divergent findings could be caused by chance.
Steroid use in IMpassion131 could have played a negative role because of its lympholytic activity, but other indications with steroid use have not demonstrated attenuated benefits, said Leisha A. Emens, MD, PhD, of the University of Pittsburgh Medical Center, who presented the findings from IMpassion130 at ESMO 2020.
“If I were a patient, based on the data to date, I would want nab-paclitaxel with atezolizumab,” Dr. Emens said.
Trial details
Both trials are phase 3, double-blind, placebo-controlled studies of women with metastatic or unresectable locally advanced TNBC who had received no prior therapy for advanced TNBC.
IMpassion130 included 451 patients randomized to atezolizumab plus nab-paclitaxel and 451 randomized to placebo plus nab-paclitaxel. Patients received nab-paclitaxel at a starting dose of 100 mg/m2 via IV infusion on days 1, 8, and 15 of each 28-day cycle for at least six cycles.
In both studies, patients received atezolizumab at 840 mg on days 1 and 15 of a 28-day cycle in their active treatment arms.
IMpassion131 included 651 patients randomized 2:1 to atezolizumab plus paclitaxel (n = 431) or placebo plus paclitaxel (n = 220). Patients received paclitaxel at 90 mg/m2 on days 1, 8, and 15 every 28 days until disease progression or unacceptable toxicity.
Baseline characteristics were well balanced between the treatment arms in both studies. Less than half of patients – 45% in IMpassion131 and 41% in IMpassion130 – were PD-L1 positive.
Results of IMpassion131
The primary endpoint in IMpassion131 was PFS, and there was no significant difference in PFS between the treatment arms.
“The primary objective of IMpassion131 was not met,” Dr. Miles said. “[The] addition of atezolizumab to paclitaxel did not significantly improve PFS in patients with PD-L1-positive metastatic triple-negative breast cancer.”
In the PD-L1-positive population, the median PFS was 5.7 months in the placebo arm and 6.0 months in the atezolizumab arm (stratified hazard ratio, 0.82, P = .20).
In the ITT population, the median PFS was 5.6 months in the control arm and 5.7 months in the atezolizumab arm (HR, 0.86).
In subgroup analyses, Dr. Miles noted, “There was no clue about adverse or beneficial effects in any subgroup.”
The updated OS analysis demonstrated no benefit with atezolizumab in the ITT population or the PD-L1-positive population. In fact, there was a trend toward better OS for the control group in the latter analysis.
In the PD-L1-positive population, the median OS was 28.3 months in the control arm and 22.1 months in the atezolizumab arm (HR, 1.12). The 2-year OS rates were 51% and 49%, respectively.
In the ITT population, the median OS was 22.8 months in the control arm and 19.2 months in the atezolizumab arm (HR, 1.11). The 2-year OS rates were 45% and 42%, respectively.
The safety profile of the atezolizumab-paclitaxel combination was consistent with known side effects of the individual drugs, Dr. Miles said. There were four fatal treatment-related adverse events in the atezolizumab arm.
Results of IMpassion130
Presenting the final OS analysis from IMpassion130, Dr. Emens noted that the study’s findings have led to recommendations for atezolizumab plus nab-paclitaxel as first-line treatment of PD-L1-positive TNBC in international guidelines.
The median OS in the ITT population was 18.7 months in the placebo arm and 21.0 months in the atezolizumab arm (stratified HR, 0.87, P = .077). The 3-year OS rates were 25% and 28%, respectively.
The median OS in the PD-L1-positive population was 17.9 months in the placebo arm and 25.4 months in the atezolizumab arm (HR, 0.67). The 3-year OS rates were 22% and 36%, respectively.
A P value is not available for the between-arm OS comparison in the PD-L1-positive population. OS was not formally tested in this group because the OS boundary for statistical significance was not crossed in the ITT population. However, Dr. Emens said there was a “clinically meaningful” OS benefit observed with atezolizumab in the PD-L1-positive patients.
Treatment withdrawals caused by adverse events were more common in the atezolizumab arm (19% vs. 8%). The most common of these was neuropathy, Dr. Emens said. However, she noted that atezolizumab-related adverse events were generally low grade and easily managed.
“These results support a positive benefit-risk profile for atezolizumab plus nab-paclitaxel as first-line therapy in patients with PD-L1-positive metastatic triple-negative breast cancer,” Dr. Emens concluded.
Both studies were funded by F. Hoffman–La Roche. Dr. Miles, Dr. Emens, and Dr. Carey disclosed financial relationships with Roche and other companies.
SOURCES: Miles D et al. ESMO 2020, Abstract LBA15; Emens LA et al. ESMO 2020, Abstract LBA16.
The trials, IMpassion130 and IMpassion131, both enrolled patients with metastatic or unresectable, locally advanced TNBC.
In IMpassion131, adding atezolizumab to paclitaxel did not improve progression-free survival (PFS) or overall survival (OS), regardless of programmed death–ligand 1 (PD-L1) expression.
In IMpassion130, adding atezolizumab to nab-paclitaxel did not improve OS in the intention-to-treat (ITT) population but did provide a “clinically meaningful” improvement in OS among PD-L1-positive patients, according to investigators.
IMpassion130 and IMpassion131 were presented during the same session at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.
Potential reasons for the different outcomes in the two studies require further exploration, according to David Miles, MD, of Mount Vernon Cancer Centre in Northwood, England, who presented the findings from IMpassion131.
ESMO discussant Lisa A. Carey, MD, of the University of North Carolina at Chapel Hill, posited three possible explanations for the divergent findings. The steroids necessary with paclitaxel dosing may have had a negative effect on immune checkpoint inhibitor activity, differences in study populations may have played a role, or the divergent findings could be caused by chance.
Steroid use in IMpassion131 could have played a negative role because of its lympholytic activity, but other indications with steroid use have not demonstrated attenuated benefits, said Leisha A. Emens, MD, PhD, of the University of Pittsburgh Medical Center, who presented the findings from IMpassion130 at ESMO 2020.
“If I were a patient, based on the data to date, I would want nab-paclitaxel with atezolizumab,” Dr. Emens said.
Trial details
Both trials are phase 3, double-blind, placebo-controlled studies of women with metastatic or unresectable locally advanced TNBC who had received no prior therapy for advanced TNBC.
IMpassion130 included 451 patients randomized to atezolizumab plus nab-paclitaxel and 451 randomized to placebo plus nab-paclitaxel. Patients received nab-paclitaxel at a starting dose of 100 mg/m2 via IV infusion on days 1, 8, and 15 of each 28-day cycle for at least six cycles.
In both studies, patients received atezolizumab at 840 mg on days 1 and 15 of a 28-day cycle in their active treatment arms.
IMpassion131 included 651 patients randomized 2:1 to atezolizumab plus paclitaxel (n = 431) or placebo plus paclitaxel (n = 220). Patients received paclitaxel at 90 mg/m2 on days 1, 8, and 15 every 28 days until disease progression or unacceptable toxicity.
Baseline characteristics were well balanced between the treatment arms in both studies. Less than half of patients – 45% in IMpassion131 and 41% in IMpassion130 – were PD-L1 positive.
Results of IMpassion131
The primary endpoint in IMpassion131 was PFS, and there was no significant difference in PFS between the treatment arms.
“The primary objective of IMpassion131 was not met,” Dr. Miles said. “[The] addition of atezolizumab to paclitaxel did not significantly improve PFS in patients with PD-L1-positive metastatic triple-negative breast cancer.”
In the PD-L1-positive population, the median PFS was 5.7 months in the placebo arm and 6.0 months in the atezolizumab arm (stratified hazard ratio, 0.82, P = .20).
In the ITT population, the median PFS was 5.6 months in the control arm and 5.7 months in the atezolizumab arm (HR, 0.86).
In subgroup analyses, Dr. Miles noted, “There was no clue about adverse or beneficial effects in any subgroup.”
The updated OS analysis demonstrated no benefit with atezolizumab in the ITT population or the PD-L1-positive population. In fact, there was a trend toward better OS for the control group in the latter analysis.
In the PD-L1-positive population, the median OS was 28.3 months in the control arm and 22.1 months in the atezolizumab arm (HR, 1.12). The 2-year OS rates were 51% and 49%, respectively.
In the ITT population, the median OS was 22.8 months in the control arm and 19.2 months in the atezolizumab arm (HR, 1.11). The 2-year OS rates were 45% and 42%, respectively.
The safety profile of the atezolizumab-paclitaxel combination was consistent with known side effects of the individual drugs, Dr. Miles said. There were four fatal treatment-related adverse events in the atezolizumab arm.
Results of IMpassion130
Presenting the final OS analysis from IMpassion130, Dr. Emens noted that the study’s findings have led to recommendations for atezolizumab plus nab-paclitaxel as first-line treatment of PD-L1-positive TNBC in international guidelines.
The median OS in the ITT population was 18.7 months in the placebo arm and 21.0 months in the atezolizumab arm (stratified HR, 0.87, P = .077). The 3-year OS rates were 25% and 28%, respectively.
The median OS in the PD-L1-positive population was 17.9 months in the placebo arm and 25.4 months in the atezolizumab arm (HR, 0.67). The 3-year OS rates were 22% and 36%, respectively.
A P value is not available for the between-arm OS comparison in the PD-L1-positive population. OS was not formally tested in this group because the OS boundary for statistical significance was not crossed in the ITT population. However, Dr. Emens said there was a “clinically meaningful” OS benefit observed with atezolizumab in the PD-L1-positive patients.
Treatment withdrawals caused by adverse events were more common in the atezolizumab arm (19% vs. 8%). The most common of these was neuropathy, Dr. Emens said. However, she noted that atezolizumab-related adverse events were generally low grade and easily managed.
“These results support a positive benefit-risk profile for atezolizumab plus nab-paclitaxel as first-line therapy in patients with PD-L1-positive metastatic triple-negative breast cancer,” Dr. Emens concluded.
Both studies were funded by F. Hoffman–La Roche. Dr. Miles, Dr. Emens, and Dr. Carey disclosed financial relationships with Roche and other companies.
SOURCES: Miles D et al. ESMO 2020, Abstract LBA15; Emens LA et al. ESMO 2020, Abstract LBA16.
FROM ESMO 2020
Global stomach cancer deaths decline as colorectal cancer deaths stagnate, rise
The data suggest fewer people are dying from stomach cancer, but in some countries, the risk of colorectal cancer death is increasing or declining much more slowly than other causes of premature death.
As for other cancers, in more than half of the countries analyzed, the risk of liver and prostate cancer death is on the rise in men, and the risk of lung cancer death is on the rise in women.
The global decrease in the risk of stomach cancer death may be explained by the fact that stomach cancer’s main cause is Helicobacter pylori infection, which correlates with general food hygiene, the study’s corresponding author Majid Ezzati, PhD, professor of global environmental health at Imperial College London, said in an interview.
“Factors such as more widespread electrification and refrigeration tend to drive the rates down,” he explained.
Dr. Ezzati and colleagues detailed their findings in the second edition of the NCD Countdown 2030 report, recently published in The Lancet.
The report revolves around the Sustainable Development Goal (SDG) target 3.4, which is to reduce premature deaths from NCDs by one-third between 2015 and 2030. The causes of death include cancer, cardiovascular disease, chronic respiratory disease, and diabetes, which are collectively known as NCD4. “Premature” deaths are defined as deaths in people aged 30-70 years.
SDG target 3.4 is still attainable, according to Dr. Ezzati and colleagues. However, their report showed that many countries are falling short of this goal.
The findings come from an analysis of 2016 World Health Organization global estimate data on age-, sex-, and cause-specific mortality for 176 countries and territories with at least 200,000 inhabitants. Mathematical modeling was used to assess the number of approaches countries used to accelerate declines in mortality.
Results of the analysis
“Trends in the risk of death from 2010 to 2016 varied considerably among NCD4 causes of death,” Dr. Ezzati and colleagues wrote.
Stomach cancer, ischemic and hemorrhagic stroke, ischemic heart disease, and chronic respiratory diseases had the fastest rates of decline among risks of premature death.
In fact, stomach cancer was the fastest declining cause of death in 45 countries (25.6%) among men and in 40 countries (22.7%) among women.
On the other hand, the risk of premature death from colorectal, liver, breast, prostate, and other cancers declined more slowly than the risk of premature death from other NCDs.
The risk of death from colorectal, liver, and prostate cancers in men and lung cancer in women rose in more than 50% of the countries surveyed.
“The median annual rate of change in the probability of dying prematurely from various causes ranged from +0.2% per year for lung cancer to –2.5% per year for hemorrhagic stroke in women, and from +0.5% per year for colorectal cancer to –1.8% per year for hemorrhagic stroke in men,” the investigators summarized.
Explaining the GI cancer results
“There are dramatic differences between the upper and lower GI tract, both in terms of anatomy/embryologic origin but also in terms of exposures,” observed Mark Lewis, MD, medical director of the gastrointestinal oncology program at Intermountain Healthcare in Salt Lake City, in an interview.
H. pylori infection, family history, and diet factor into stomach cancer risk, Dr. Lewis said.
While family history isn’t modifiable, “we are much better now at identifying and eradicating the potentially carcinogenic H. pylori bacterium. In terms of diet, the advent of modern refrigeration has made the prevalence of heavily salted/preserved foods decline,” he added.
A 14-day course of treatment (with a proton pump inhibitor and antibiotics) can eliminate H. pylori, Dr. Lewis continued. “The prophylactic effect against gastric cancer is massive, cutting risk by roughly half,” he said.
At least in the United States, colorectal cancer rates have declined in people 50 years and older, but rates have risen sharply in younger age groups, increasing by 2% annually in the last decade, according to statistics in CA: A Cancer Journal for Clinicians.
“One prevailing theory is prior antibiotic prescriptions [even in childhood] might perturb the microbiome of the lower GI tract and predispose to cancer,” Dr. Lewis said, pointing to a recent study in the British Journal of Cancer that identified an association between repeated antibiotic use and colorectal cancer.
Reducing NCD deaths
Dr. Ezzati and colleagues said six high-income countries – Denmark, Luxembourg, New Zealand, Norway, Singapore, and South Korea – are likely to meet SDG target 3.4 if they maintain or exceed average rates of decline seen during 2010-2016. Seventeen countries are on track to reach the target for women, and 15 countries are on track for men.
High-income countries in Asia-Pacific, western Europe, Australasia, and Canada have seen the lowest NCD4 mortality risk, whereas low- and middle-income countries in sub-Saharan Africa and men in central Asia and eastern Europe have seen the highest risk.
“To move forward, we must learn from those countries that are doing well and replicate their strategies to NCD prevention and healthcare,” Dr. Ezzati said in a statement. “Our analysis shows that every country still has options to achieve SDG target 3.4, but they need to address multiple diseases and have strong health systems.”
Increasing access to effective cancer screening and diagnosing and treating cancers earlier could help reduce long-term health consequences and premature deaths from cancer, according to Dr. Ezzati and colleagues. Screening would help even the playing field on cancer diagnosis and survival rates between higher-income countries and low- and middle-income countries.
“This approach will allow earlier diagnosis during precancerous or early stages of disease, followed by treatment of those cancers with effective treatment,” the authors stated.
Tobacco and alcohol interventions and increasing access to quality primary care would also help tamp down on NCD-related deaths.
The authors acknowledged that low-income countries, which may be struggling with other health crises such as COVID-19 and Ebola, may find it a challenge to stage such interventions.
“COVID-19 has exposed how a failure to invest in effective public health to prevent NCDs and provide health care for people living with NCDs can come back to bite us,” said Katie Dain, CEO of the NCD Alliance.
“The good news is that all countries can still meet the 2030 targets, with sound policies and smart investments. NCD prevention and treatment can no longer be seen a ‘nice to have.’ It must be considered as part of pandemic preparedness,” she added.
COVID-19 should serve as an impetus for governments to invest in healthier lifestyle and diet habits and curb alcohol and tobacco use, according to an editorial in The Lancet related to the analysis.
The current report updates 2018’s first NCD Countdown Report, which linked NCD4 conditions to approximately 32 million or 80% of NCD deaths. Unlike the recent report, 2018’s data didn’t focus on specific diseases.
The current report was funded by Research England. Dr. Ezzati received a charitable grant from the AstraZeneca Young Health Programme and personal fees from Prudential and Scor, outside of this report. None of the other authors reported competing interests. Dr. Lewis has no relevant disclosures except that he is a commentator for Medscape, which is owned by the same parent company as MDedge.
SOURCE: Bennett JE et al. Lancet. 2020 Sep 3. doi: 10.1016/S0140-6736(20)31761-X.
The data suggest fewer people are dying from stomach cancer, but in some countries, the risk of colorectal cancer death is increasing or declining much more slowly than other causes of premature death.
As for other cancers, in more than half of the countries analyzed, the risk of liver and prostate cancer death is on the rise in men, and the risk of lung cancer death is on the rise in women.
The global decrease in the risk of stomach cancer death may be explained by the fact that stomach cancer’s main cause is Helicobacter pylori infection, which correlates with general food hygiene, the study’s corresponding author Majid Ezzati, PhD, professor of global environmental health at Imperial College London, said in an interview.
“Factors such as more widespread electrification and refrigeration tend to drive the rates down,” he explained.
Dr. Ezzati and colleagues detailed their findings in the second edition of the NCD Countdown 2030 report, recently published in The Lancet.
The report revolves around the Sustainable Development Goal (SDG) target 3.4, which is to reduce premature deaths from NCDs by one-third between 2015 and 2030. The causes of death include cancer, cardiovascular disease, chronic respiratory disease, and diabetes, which are collectively known as NCD4. “Premature” deaths are defined as deaths in people aged 30-70 years.
SDG target 3.4 is still attainable, according to Dr. Ezzati and colleagues. However, their report showed that many countries are falling short of this goal.
The findings come from an analysis of 2016 World Health Organization global estimate data on age-, sex-, and cause-specific mortality for 176 countries and territories with at least 200,000 inhabitants. Mathematical modeling was used to assess the number of approaches countries used to accelerate declines in mortality.
Results of the analysis
“Trends in the risk of death from 2010 to 2016 varied considerably among NCD4 causes of death,” Dr. Ezzati and colleagues wrote.
Stomach cancer, ischemic and hemorrhagic stroke, ischemic heart disease, and chronic respiratory diseases had the fastest rates of decline among risks of premature death.
In fact, stomach cancer was the fastest declining cause of death in 45 countries (25.6%) among men and in 40 countries (22.7%) among women.
On the other hand, the risk of premature death from colorectal, liver, breast, prostate, and other cancers declined more slowly than the risk of premature death from other NCDs.
The risk of death from colorectal, liver, and prostate cancers in men and lung cancer in women rose in more than 50% of the countries surveyed.
“The median annual rate of change in the probability of dying prematurely from various causes ranged from +0.2% per year for lung cancer to –2.5% per year for hemorrhagic stroke in women, and from +0.5% per year for colorectal cancer to –1.8% per year for hemorrhagic stroke in men,” the investigators summarized.
Explaining the GI cancer results
“There are dramatic differences between the upper and lower GI tract, both in terms of anatomy/embryologic origin but also in terms of exposures,” observed Mark Lewis, MD, medical director of the gastrointestinal oncology program at Intermountain Healthcare in Salt Lake City, in an interview.
H. pylori infection, family history, and diet factor into stomach cancer risk, Dr. Lewis said.
While family history isn’t modifiable, “we are much better now at identifying and eradicating the potentially carcinogenic H. pylori bacterium. In terms of diet, the advent of modern refrigeration has made the prevalence of heavily salted/preserved foods decline,” he added.
A 14-day course of treatment (with a proton pump inhibitor and antibiotics) can eliminate H. pylori, Dr. Lewis continued. “The prophylactic effect against gastric cancer is massive, cutting risk by roughly half,” he said.
At least in the United States, colorectal cancer rates have declined in people 50 years and older, but rates have risen sharply in younger age groups, increasing by 2% annually in the last decade, according to statistics in CA: A Cancer Journal for Clinicians.
“One prevailing theory is prior antibiotic prescriptions [even in childhood] might perturb the microbiome of the lower GI tract and predispose to cancer,” Dr. Lewis said, pointing to a recent study in the British Journal of Cancer that identified an association between repeated antibiotic use and colorectal cancer.
Reducing NCD deaths
Dr. Ezzati and colleagues said six high-income countries – Denmark, Luxembourg, New Zealand, Norway, Singapore, and South Korea – are likely to meet SDG target 3.4 if they maintain or exceed average rates of decline seen during 2010-2016. Seventeen countries are on track to reach the target for women, and 15 countries are on track for men.
High-income countries in Asia-Pacific, western Europe, Australasia, and Canada have seen the lowest NCD4 mortality risk, whereas low- and middle-income countries in sub-Saharan Africa and men in central Asia and eastern Europe have seen the highest risk.
“To move forward, we must learn from those countries that are doing well and replicate their strategies to NCD prevention and healthcare,” Dr. Ezzati said in a statement. “Our analysis shows that every country still has options to achieve SDG target 3.4, but they need to address multiple diseases and have strong health systems.”
Increasing access to effective cancer screening and diagnosing and treating cancers earlier could help reduce long-term health consequences and premature deaths from cancer, according to Dr. Ezzati and colleagues. Screening would help even the playing field on cancer diagnosis and survival rates between higher-income countries and low- and middle-income countries.
“This approach will allow earlier diagnosis during precancerous or early stages of disease, followed by treatment of those cancers with effective treatment,” the authors stated.
Tobacco and alcohol interventions and increasing access to quality primary care would also help tamp down on NCD-related deaths.
The authors acknowledged that low-income countries, which may be struggling with other health crises such as COVID-19 and Ebola, may find it a challenge to stage such interventions.
“COVID-19 has exposed how a failure to invest in effective public health to prevent NCDs and provide health care for people living with NCDs can come back to bite us,” said Katie Dain, CEO of the NCD Alliance.
“The good news is that all countries can still meet the 2030 targets, with sound policies and smart investments. NCD prevention and treatment can no longer be seen a ‘nice to have.’ It must be considered as part of pandemic preparedness,” she added.
COVID-19 should serve as an impetus for governments to invest in healthier lifestyle and diet habits and curb alcohol and tobacco use, according to an editorial in The Lancet related to the analysis.
The current report updates 2018’s first NCD Countdown Report, which linked NCD4 conditions to approximately 32 million or 80% of NCD deaths. Unlike the recent report, 2018’s data didn’t focus on specific diseases.
The current report was funded by Research England. Dr. Ezzati received a charitable grant from the AstraZeneca Young Health Programme and personal fees from Prudential and Scor, outside of this report. None of the other authors reported competing interests. Dr. Lewis has no relevant disclosures except that he is a commentator for Medscape, which is owned by the same parent company as MDedge.
SOURCE: Bennett JE et al. Lancet. 2020 Sep 3. doi: 10.1016/S0140-6736(20)31761-X.
The data suggest fewer people are dying from stomach cancer, but in some countries, the risk of colorectal cancer death is increasing or declining much more slowly than other causes of premature death.
As for other cancers, in more than half of the countries analyzed, the risk of liver and prostate cancer death is on the rise in men, and the risk of lung cancer death is on the rise in women.
The global decrease in the risk of stomach cancer death may be explained by the fact that stomach cancer’s main cause is Helicobacter pylori infection, which correlates with general food hygiene, the study’s corresponding author Majid Ezzati, PhD, professor of global environmental health at Imperial College London, said in an interview.
“Factors such as more widespread electrification and refrigeration tend to drive the rates down,” he explained.
Dr. Ezzati and colleagues detailed their findings in the second edition of the NCD Countdown 2030 report, recently published in The Lancet.
The report revolves around the Sustainable Development Goal (SDG) target 3.4, which is to reduce premature deaths from NCDs by one-third between 2015 and 2030. The causes of death include cancer, cardiovascular disease, chronic respiratory disease, and diabetes, which are collectively known as NCD4. “Premature” deaths are defined as deaths in people aged 30-70 years.
SDG target 3.4 is still attainable, according to Dr. Ezzati and colleagues. However, their report showed that many countries are falling short of this goal.
The findings come from an analysis of 2016 World Health Organization global estimate data on age-, sex-, and cause-specific mortality for 176 countries and territories with at least 200,000 inhabitants. Mathematical modeling was used to assess the number of approaches countries used to accelerate declines in mortality.
Results of the analysis
“Trends in the risk of death from 2010 to 2016 varied considerably among NCD4 causes of death,” Dr. Ezzati and colleagues wrote.
Stomach cancer, ischemic and hemorrhagic stroke, ischemic heart disease, and chronic respiratory diseases had the fastest rates of decline among risks of premature death.
In fact, stomach cancer was the fastest declining cause of death in 45 countries (25.6%) among men and in 40 countries (22.7%) among women.
On the other hand, the risk of premature death from colorectal, liver, breast, prostate, and other cancers declined more slowly than the risk of premature death from other NCDs.
The risk of death from colorectal, liver, and prostate cancers in men and lung cancer in women rose in more than 50% of the countries surveyed.
“The median annual rate of change in the probability of dying prematurely from various causes ranged from +0.2% per year for lung cancer to –2.5% per year for hemorrhagic stroke in women, and from +0.5% per year for colorectal cancer to –1.8% per year for hemorrhagic stroke in men,” the investigators summarized.
Explaining the GI cancer results
“There are dramatic differences between the upper and lower GI tract, both in terms of anatomy/embryologic origin but also in terms of exposures,” observed Mark Lewis, MD, medical director of the gastrointestinal oncology program at Intermountain Healthcare in Salt Lake City, in an interview.
H. pylori infection, family history, and diet factor into stomach cancer risk, Dr. Lewis said.
While family history isn’t modifiable, “we are much better now at identifying and eradicating the potentially carcinogenic H. pylori bacterium. In terms of diet, the advent of modern refrigeration has made the prevalence of heavily salted/preserved foods decline,” he added.
A 14-day course of treatment (with a proton pump inhibitor and antibiotics) can eliminate H. pylori, Dr. Lewis continued. “The prophylactic effect against gastric cancer is massive, cutting risk by roughly half,” he said.
At least in the United States, colorectal cancer rates have declined in people 50 years and older, but rates have risen sharply in younger age groups, increasing by 2% annually in the last decade, according to statistics in CA: A Cancer Journal for Clinicians.
“One prevailing theory is prior antibiotic prescriptions [even in childhood] might perturb the microbiome of the lower GI tract and predispose to cancer,” Dr. Lewis said, pointing to a recent study in the British Journal of Cancer that identified an association between repeated antibiotic use and colorectal cancer.
Reducing NCD deaths
Dr. Ezzati and colleagues said six high-income countries – Denmark, Luxembourg, New Zealand, Norway, Singapore, and South Korea – are likely to meet SDG target 3.4 if they maintain or exceed average rates of decline seen during 2010-2016. Seventeen countries are on track to reach the target for women, and 15 countries are on track for men.
High-income countries in Asia-Pacific, western Europe, Australasia, and Canada have seen the lowest NCD4 mortality risk, whereas low- and middle-income countries in sub-Saharan Africa and men in central Asia and eastern Europe have seen the highest risk.
“To move forward, we must learn from those countries that are doing well and replicate their strategies to NCD prevention and healthcare,” Dr. Ezzati said in a statement. “Our analysis shows that every country still has options to achieve SDG target 3.4, but they need to address multiple diseases and have strong health systems.”
Increasing access to effective cancer screening and diagnosing and treating cancers earlier could help reduce long-term health consequences and premature deaths from cancer, according to Dr. Ezzati and colleagues. Screening would help even the playing field on cancer diagnosis and survival rates between higher-income countries and low- and middle-income countries.
“This approach will allow earlier diagnosis during precancerous or early stages of disease, followed by treatment of those cancers with effective treatment,” the authors stated.
Tobacco and alcohol interventions and increasing access to quality primary care would also help tamp down on NCD-related deaths.
The authors acknowledged that low-income countries, which may be struggling with other health crises such as COVID-19 and Ebola, may find it a challenge to stage such interventions.
“COVID-19 has exposed how a failure to invest in effective public health to prevent NCDs and provide health care for people living with NCDs can come back to bite us,” said Katie Dain, CEO of the NCD Alliance.
“The good news is that all countries can still meet the 2030 targets, with sound policies and smart investments. NCD prevention and treatment can no longer be seen a ‘nice to have.’ It must be considered as part of pandemic preparedness,” she added.
COVID-19 should serve as an impetus for governments to invest in healthier lifestyle and diet habits and curb alcohol and tobacco use, according to an editorial in The Lancet related to the analysis.
The current report updates 2018’s first NCD Countdown Report, which linked NCD4 conditions to approximately 32 million or 80% of NCD deaths. Unlike the recent report, 2018’s data didn’t focus on specific diseases.
The current report was funded by Research England. Dr. Ezzati received a charitable grant from the AstraZeneca Young Health Programme and personal fees from Prudential and Scor, outside of this report. None of the other authors reported competing interests. Dr. Lewis has no relevant disclosures except that he is a commentator for Medscape, which is owned by the same parent company as MDedge.
SOURCE: Bennett JE et al. Lancet. 2020 Sep 3. doi: 10.1016/S0140-6736(20)31761-X.
FROM THE LANCET
NCI may ‘kill’ major mammography trial, says adviser
Reevaluation comes after Medscape report
In September, an advisory board to the National Cancer Institute overwhelmingly voted to form a working group to review the continued funding and running of its largest cancer screening trial. But the lone abstaining board member suggested that the reevaluation was a smoke screen for ending the $100 million Tomosynthesis Mammography Imaging Screening Trial (TMIST).
“We’re going to have a working group to kill the trial,” Peter Adamson, MD, of Sanofi, commented in an interpretation of the NCI’s intent. The randomized trial, which began enrolling women in 2017, is comparing tomosynthesis, or three-dimensional (3-D) mammography, with older, less expensive 2-D technology.
The NCI’s move to reevaluate TMIST comes 6 months after Medscape Medical News reported that the projected 165,000-women study was lagging in enrollment of both patients and participating sites/physicians.
Enlisting sites is difficult, in part because radiologists – informed by their experience and previous research results – already believe that the 3-D technology is superior, commented two TMIST study investigators at that time.
Furthermore, experts who were quoted in the Medscape story said that radiology practice in the United States has substantially transitioned to 3-D and will continue to do so. This point was supported by a review of imaging market data.
At the September virtual meeting of the National Cancer Advisory Board, which was first reported by the Cancer Letter, Philip Castle, PhD, MPH, director of the NCI’s division of cancer prevention, said it was “time to reevaluate TMIST’s feasibility and relevance.”
There is also a need to “examine the utility of TMIST, given the unanticipated challenges due to the pandemic,” he said, referring to low accrual of patients in 2020.
TMIST is “a huge budget item,” and accrual has been a problem “for years – essentially since the trial began,” added Ned Sharpless, MD, director of the NCI.
Fourteen advisory board members voted in favor of creating the working group to review the trial. The lone abstention, as noted above, was from Dr. Adamson, who is global head of oncology development and pediatric innovation at Sanofi and is also professor emeritus, University of Pennsylvania, Philadelphia.
Dr. Adamson said that he was “not denying the impact” of COVID-19 but speculated that other clinical trials have had the same problem.
He also wondered whether COVID-19 was an “excuse” for bringing TMIST to a “public forum” and objected to the fact that TMIST investigators were not present and had not been invited to offer “an action plan” to remedy their trial’s woes.
Dr. Castle explained that COVID-19 had reduced TMIST’s monthly patient accrual by 50% from March to August, compared with recent preceding months. The underaccrual “will likely result in delayed outcomes and escalating costs.”
However, TMIST was staggeringly behind in accrual even before COVID-19. The trial has averaged less than 1,500 women a month over the past 2 years, yet it needed to average 5,500 a month to meet accrual goals, Dr. Castle noted.
Further, Dr. Castle suggested that TMIST’s contribution to mammography practice may be negligible, citing three factors: changes in the imaging market, earlier research results, and other ongoing major trials of 3-D mammography.
“[The] relevance of the [TMIST] data by the completion of the trial is uncertain because of 3-D market ... penetrance by the conclusion of the trial,” Dr. Castle said. Even with optimistic accrual projections, study completion and reporting would occur somewhere between 2029 and 2032 – not in 2025, as originally planned.
Currently, 68% of certified mammography facilities in the United States have one or more 3-D units, and 40% of all units are 3-D, he noted. (However, as previously reported, this latter statistic on total units is likely an undercount because all 3-D units have a built-in 2-D function, but the two components in a single machine are equally counted by the Food and Drug Administration – even when 3-D is exclusively used.)
Dr. Castle also summarized earlier observational and randomized trial data on tomosynthesis mammography: “Evidence is already available suggesting that 3-D is no worse and probably better than 2-D.”
Additionally, he reviewed three other European mammography trials involving 3-D technology (in the United Kingdom, Germany, and Norway) and said that they will contribute “additional informative data.”
Notably, Dr. Adamson did not object to these critical points but said the “approach” taken by the NCI advisory board at this meeting was “incredibly disturbing.”
Dr. Castle defended himself, saying “nowhere” in his presentation was there any mention that the trial would be “shut down.”
Dr. Sharpless then interjected: “There is no easy way to do this. TMIST is the most troubled of our trials [in prevention and screening] from an accrual point of view.”
TMIST investigators respond
A pair of TMIST investigators commented about the NCI’s planned reevaluation – and what it means.
Etta D. Pisano, MD, the study’s principal investigator, accented the positive: “We have heard nothing of suspending TMIST and are ready to work with NCI to reach TMIST endpoints more efficiently,” she said in an interview.
She also offered a positive spin on TMIST enrollment and trial site enlistment. “Enrollment quadrupled and trial sites doubled in the 14 months prior to the COVID-19 pandemic. An unheard of 19% of the 30,000 women enrolled at 99 sites are African American.”
The trial has “momentum,” said Dr. Pisano, who is also chief research officer at the American College of Radiology.
Dr. Castle, however, noted that enrollment is far behind schedule; the 30,000 women who have been enrolled so far represent less than a quarter of the planned enrollment of 165,000 women, due by the end of 2020.
TMIST is designed to learn whether 3-D mammography is better at finding – and reducing the rate of – potentially lethal cancers than older 2-D technology.
Most 3-D systems are used in conjunction with 2-D. First, two static images of the breast are taken (2-D), and then the unit moves in an arc, taking multiple images of the breast (3-D). This allows radiologists to flip through the images like pages in a book and is considered to offer a superior read of the breast.
TMIST uses “advanced” breast cancers as a novel surrogate outcome. The trial period is 4.5 years, during which women are screened annually to determine which imaging technology results in fewer advanced cancers. These include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease.
These malignancies correlate with breast cancer mortality, Dr. Pisano said.
“We need this trial to help us learn how to improve the process for identifying high-risk patients and how to utilize both of these technologies better,” said Mitchell Schnall, MD, PhD, from the University of Pennsylvania. He is cochair of the ECOG-ACRIN trial group, which is corunning TMIST.
“Look, we all know that 3-D sees more lesions, but consequently, more women are having additional procedures, including biopsies and excisions. The question that TMIST asks is whether that huge expenditure of time, money, and psychological stress is worth it in terms of better outcomes for each person who has the experience,” Dr. Schnall said in an interview.
“One size should not fit all in breast cancer screening, and the TMIST trial can show the way to a precision approach to screening,” he said.
But, earlier this year, Daniel Kopans, MD, professor of radiology, Harvard Medical School, Boston, said in an interview that TMIST is a “huge waste of money.”
He believes that “radiologists who are experienced in using [3-D] for screening will not go back.
“It would be malpractice since they know there are cancers that they will miss on [2-D] that they can find on tomosynthesis,” said Dr. Kopans. He was involved in the development of the first 3-D unit but no longer profits from its sales because his group’s patent has expired.
TMIST was conceived before 3-D mammogram took off clinically, which may explain the trial’s enrollment and site participation woes. In the lag time between the initial trial design (2012) and study start date (2017), clinical practice in the United States and Canada shifted quickly to embrace the newer technology.
This article first appeared on Medscape.com.
Reevaluation comes after Medscape report
Reevaluation comes after Medscape report
In September, an advisory board to the National Cancer Institute overwhelmingly voted to form a working group to review the continued funding and running of its largest cancer screening trial. But the lone abstaining board member suggested that the reevaluation was a smoke screen for ending the $100 million Tomosynthesis Mammography Imaging Screening Trial (TMIST).
“We’re going to have a working group to kill the trial,” Peter Adamson, MD, of Sanofi, commented in an interpretation of the NCI’s intent. The randomized trial, which began enrolling women in 2017, is comparing tomosynthesis, or three-dimensional (3-D) mammography, with older, less expensive 2-D technology.
The NCI’s move to reevaluate TMIST comes 6 months after Medscape Medical News reported that the projected 165,000-women study was lagging in enrollment of both patients and participating sites/physicians.
Enlisting sites is difficult, in part because radiologists – informed by their experience and previous research results – already believe that the 3-D technology is superior, commented two TMIST study investigators at that time.
Furthermore, experts who were quoted in the Medscape story said that radiology practice in the United States has substantially transitioned to 3-D and will continue to do so. This point was supported by a review of imaging market data.
At the September virtual meeting of the National Cancer Advisory Board, which was first reported by the Cancer Letter, Philip Castle, PhD, MPH, director of the NCI’s division of cancer prevention, said it was “time to reevaluate TMIST’s feasibility and relevance.”
There is also a need to “examine the utility of TMIST, given the unanticipated challenges due to the pandemic,” he said, referring to low accrual of patients in 2020.
TMIST is “a huge budget item,” and accrual has been a problem “for years – essentially since the trial began,” added Ned Sharpless, MD, director of the NCI.
Fourteen advisory board members voted in favor of creating the working group to review the trial. The lone abstention, as noted above, was from Dr. Adamson, who is global head of oncology development and pediatric innovation at Sanofi and is also professor emeritus, University of Pennsylvania, Philadelphia.
Dr. Adamson said that he was “not denying the impact” of COVID-19 but speculated that other clinical trials have had the same problem.
He also wondered whether COVID-19 was an “excuse” for bringing TMIST to a “public forum” and objected to the fact that TMIST investigators were not present and had not been invited to offer “an action plan” to remedy their trial’s woes.
Dr. Castle explained that COVID-19 had reduced TMIST’s monthly patient accrual by 50% from March to August, compared with recent preceding months. The underaccrual “will likely result in delayed outcomes and escalating costs.”
However, TMIST was staggeringly behind in accrual even before COVID-19. The trial has averaged less than 1,500 women a month over the past 2 years, yet it needed to average 5,500 a month to meet accrual goals, Dr. Castle noted.
Further, Dr. Castle suggested that TMIST’s contribution to mammography practice may be negligible, citing three factors: changes in the imaging market, earlier research results, and other ongoing major trials of 3-D mammography.
“[The] relevance of the [TMIST] data by the completion of the trial is uncertain because of 3-D market ... penetrance by the conclusion of the trial,” Dr. Castle said. Even with optimistic accrual projections, study completion and reporting would occur somewhere between 2029 and 2032 – not in 2025, as originally planned.
Currently, 68% of certified mammography facilities in the United States have one or more 3-D units, and 40% of all units are 3-D, he noted. (However, as previously reported, this latter statistic on total units is likely an undercount because all 3-D units have a built-in 2-D function, but the two components in a single machine are equally counted by the Food and Drug Administration – even when 3-D is exclusively used.)
Dr. Castle also summarized earlier observational and randomized trial data on tomosynthesis mammography: “Evidence is already available suggesting that 3-D is no worse and probably better than 2-D.”
Additionally, he reviewed three other European mammography trials involving 3-D technology (in the United Kingdom, Germany, and Norway) and said that they will contribute “additional informative data.”
Notably, Dr. Adamson did not object to these critical points but said the “approach” taken by the NCI advisory board at this meeting was “incredibly disturbing.”
Dr. Castle defended himself, saying “nowhere” in his presentation was there any mention that the trial would be “shut down.”
Dr. Sharpless then interjected: “There is no easy way to do this. TMIST is the most troubled of our trials [in prevention and screening] from an accrual point of view.”
TMIST investigators respond
A pair of TMIST investigators commented about the NCI’s planned reevaluation – and what it means.
Etta D. Pisano, MD, the study’s principal investigator, accented the positive: “We have heard nothing of suspending TMIST and are ready to work with NCI to reach TMIST endpoints more efficiently,” she said in an interview.
She also offered a positive spin on TMIST enrollment and trial site enlistment. “Enrollment quadrupled and trial sites doubled in the 14 months prior to the COVID-19 pandemic. An unheard of 19% of the 30,000 women enrolled at 99 sites are African American.”
The trial has “momentum,” said Dr. Pisano, who is also chief research officer at the American College of Radiology.
Dr. Castle, however, noted that enrollment is far behind schedule; the 30,000 women who have been enrolled so far represent less than a quarter of the planned enrollment of 165,000 women, due by the end of 2020.
TMIST is designed to learn whether 3-D mammography is better at finding – and reducing the rate of – potentially lethal cancers than older 2-D technology.
Most 3-D systems are used in conjunction with 2-D. First, two static images of the breast are taken (2-D), and then the unit moves in an arc, taking multiple images of the breast (3-D). This allows radiologists to flip through the images like pages in a book and is considered to offer a superior read of the breast.
TMIST uses “advanced” breast cancers as a novel surrogate outcome. The trial period is 4.5 years, during which women are screened annually to determine which imaging technology results in fewer advanced cancers. These include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease.
These malignancies correlate with breast cancer mortality, Dr. Pisano said.
“We need this trial to help us learn how to improve the process for identifying high-risk patients and how to utilize both of these technologies better,” said Mitchell Schnall, MD, PhD, from the University of Pennsylvania. He is cochair of the ECOG-ACRIN trial group, which is corunning TMIST.
“Look, we all know that 3-D sees more lesions, but consequently, more women are having additional procedures, including biopsies and excisions. The question that TMIST asks is whether that huge expenditure of time, money, and psychological stress is worth it in terms of better outcomes for each person who has the experience,” Dr. Schnall said in an interview.
“One size should not fit all in breast cancer screening, and the TMIST trial can show the way to a precision approach to screening,” he said.
But, earlier this year, Daniel Kopans, MD, professor of radiology, Harvard Medical School, Boston, said in an interview that TMIST is a “huge waste of money.”
He believes that “radiologists who are experienced in using [3-D] for screening will not go back.
“It would be malpractice since they know there are cancers that they will miss on [2-D] that they can find on tomosynthesis,” said Dr. Kopans. He was involved in the development of the first 3-D unit but no longer profits from its sales because his group’s patent has expired.
TMIST was conceived before 3-D mammogram took off clinically, which may explain the trial’s enrollment and site participation woes. In the lag time between the initial trial design (2012) and study start date (2017), clinical practice in the United States and Canada shifted quickly to embrace the newer technology.
This article first appeared on Medscape.com.
In September, an advisory board to the National Cancer Institute overwhelmingly voted to form a working group to review the continued funding and running of its largest cancer screening trial. But the lone abstaining board member suggested that the reevaluation was a smoke screen for ending the $100 million Tomosynthesis Mammography Imaging Screening Trial (TMIST).
“We’re going to have a working group to kill the trial,” Peter Adamson, MD, of Sanofi, commented in an interpretation of the NCI’s intent. The randomized trial, which began enrolling women in 2017, is comparing tomosynthesis, or three-dimensional (3-D) mammography, with older, less expensive 2-D technology.
The NCI’s move to reevaluate TMIST comes 6 months after Medscape Medical News reported that the projected 165,000-women study was lagging in enrollment of both patients and participating sites/physicians.
Enlisting sites is difficult, in part because radiologists – informed by their experience and previous research results – already believe that the 3-D technology is superior, commented two TMIST study investigators at that time.
Furthermore, experts who were quoted in the Medscape story said that radiology practice in the United States has substantially transitioned to 3-D and will continue to do so. This point was supported by a review of imaging market data.
At the September virtual meeting of the National Cancer Advisory Board, which was first reported by the Cancer Letter, Philip Castle, PhD, MPH, director of the NCI’s division of cancer prevention, said it was “time to reevaluate TMIST’s feasibility and relevance.”
There is also a need to “examine the utility of TMIST, given the unanticipated challenges due to the pandemic,” he said, referring to low accrual of patients in 2020.
TMIST is “a huge budget item,” and accrual has been a problem “for years – essentially since the trial began,” added Ned Sharpless, MD, director of the NCI.
Fourteen advisory board members voted in favor of creating the working group to review the trial. The lone abstention, as noted above, was from Dr. Adamson, who is global head of oncology development and pediatric innovation at Sanofi and is also professor emeritus, University of Pennsylvania, Philadelphia.
Dr. Adamson said that he was “not denying the impact” of COVID-19 but speculated that other clinical trials have had the same problem.
He also wondered whether COVID-19 was an “excuse” for bringing TMIST to a “public forum” and objected to the fact that TMIST investigators were not present and had not been invited to offer “an action plan” to remedy their trial’s woes.
Dr. Castle explained that COVID-19 had reduced TMIST’s monthly patient accrual by 50% from March to August, compared with recent preceding months. The underaccrual “will likely result in delayed outcomes and escalating costs.”
However, TMIST was staggeringly behind in accrual even before COVID-19. The trial has averaged less than 1,500 women a month over the past 2 years, yet it needed to average 5,500 a month to meet accrual goals, Dr. Castle noted.
Further, Dr. Castle suggested that TMIST’s contribution to mammography practice may be negligible, citing three factors: changes in the imaging market, earlier research results, and other ongoing major trials of 3-D mammography.
“[The] relevance of the [TMIST] data by the completion of the trial is uncertain because of 3-D market ... penetrance by the conclusion of the trial,” Dr. Castle said. Even with optimistic accrual projections, study completion and reporting would occur somewhere between 2029 and 2032 – not in 2025, as originally planned.
Currently, 68% of certified mammography facilities in the United States have one or more 3-D units, and 40% of all units are 3-D, he noted. (However, as previously reported, this latter statistic on total units is likely an undercount because all 3-D units have a built-in 2-D function, but the two components in a single machine are equally counted by the Food and Drug Administration – even when 3-D is exclusively used.)
Dr. Castle also summarized earlier observational and randomized trial data on tomosynthesis mammography: “Evidence is already available suggesting that 3-D is no worse and probably better than 2-D.”
Additionally, he reviewed three other European mammography trials involving 3-D technology (in the United Kingdom, Germany, and Norway) and said that they will contribute “additional informative data.”
Notably, Dr. Adamson did not object to these critical points but said the “approach” taken by the NCI advisory board at this meeting was “incredibly disturbing.”
Dr. Castle defended himself, saying “nowhere” in his presentation was there any mention that the trial would be “shut down.”
Dr. Sharpless then interjected: “There is no easy way to do this. TMIST is the most troubled of our trials [in prevention and screening] from an accrual point of view.”
TMIST investigators respond
A pair of TMIST investigators commented about the NCI’s planned reevaluation – and what it means.
Etta D. Pisano, MD, the study’s principal investigator, accented the positive: “We have heard nothing of suspending TMIST and are ready to work with NCI to reach TMIST endpoints more efficiently,” she said in an interview.
She also offered a positive spin on TMIST enrollment and trial site enlistment. “Enrollment quadrupled and trial sites doubled in the 14 months prior to the COVID-19 pandemic. An unheard of 19% of the 30,000 women enrolled at 99 sites are African American.”
The trial has “momentum,” said Dr. Pisano, who is also chief research officer at the American College of Radiology.
Dr. Castle, however, noted that enrollment is far behind schedule; the 30,000 women who have been enrolled so far represent less than a quarter of the planned enrollment of 165,000 women, due by the end of 2020.
TMIST is designed to learn whether 3-D mammography is better at finding – and reducing the rate of – potentially lethal cancers than older 2-D technology.
Most 3-D systems are used in conjunction with 2-D. First, two static images of the breast are taken (2-D), and then the unit moves in an arc, taking multiple images of the breast (3-D). This allows radiologists to flip through the images like pages in a book and is considered to offer a superior read of the breast.
TMIST uses “advanced” breast cancers as a novel surrogate outcome. The trial period is 4.5 years, during which women are screened annually to determine which imaging technology results in fewer advanced cancers. These include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease.
These malignancies correlate with breast cancer mortality, Dr. Pisano said.
“We need this trial to help us learn how to improve the process for identifying high-risk patients and how to utilize both of these technologies better,” said Mitchell Schnall, MD, PhD, from the University of Pennsylvania. He is cochair of the ECOG-ACRIN trial group, which is corunning TMIST.
“Look, we all know that 3-D sees more lesions, but consequently, more women are having additional procedures, including biopsies and excisions. The question that TMIST asks is whether that huge expenditure of time, money, and psychological stress is worth it in terms of better outcomes for each person who has the experience,” Dr. Schnall said in an interview.
“One size should not fit all in breast cancer screening, and the TMIST trial can show the way to a precision approach to screening,” he said.
But, earlier this year, Daniel Kopans, MD, professor of radiology, Harvard Medical School, Boston, said in an interview that TMIST is a “huge waste of money.”
He believes that “radiologists who are experienced in using [3-D] for screening will not go back.
“It would be malpractice since they know there are cancers that they will miss on [2-D] that they can find on tomosynthesis,” said Dr. Kopans. He was involved in the development of the first 3-D unit but no longer profits from its sales because his group’s patent has expired.
TMIST was conceived before 3-D mammogram took off clinically, which may explain the trial’s enrollment and site participation woes. In the lag time between the initial trial design (2012) and study start date (2017), clinical practice in the United States and Canada shifted quickly to embrace the newer technology.
This article first appeared on Medscape.com.
Abemaciclib cuts early recurrence in high-risk breast cancer
First advance in 20 years
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
First advance in 20 years
First advance in 20 years
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
FROM ESMO 2020
First-in-class ADC ups survival in mTNBC
The first-in-class drug is directed at trophoblast cell surface antigen 2 (Trop-2), which is highly expressed in breast cancer. Research on the drug was presented at the European Society for Medical Oncology Virtual Congress 2020.
ASCENT randomly assigned more than 500 patients who had metastatic TNBC and who had experienced disease progression after a median of four lines of therapy to receive either SG or physician’s choice of chemotherapy. SG significantly improved median progression-free survival (5.6 vs 1.7 months; hazard ratio [HR], 0.41; P < .0001) and median overall survival (12.1 vs 6.7 months; HR, 0.48; P < .0001).
The response rate was 35% for SG vs 5% for chemotherapy (P < .0001).
The study was presented by Aditya Bardia, MD, MPH, a medical oncologist at Massachusetts General Hospital, Boston, Massachusetts.
He said that because the safety profile of SG is consistent with previous reports and the treatment discontinuation rate was low, the clinical benefit “confirms the use of sacituzumab govitecan” in metastatic TNBC. This was a reference to the fact that the drug previously received accelerated approval on the basis of early data.
Bardia added that ongoing studies are evaluating the use of SG in earlier lines of therapy, including neoadjuvant settings in combination with other targeted agents, as well as in hormone receptor–positive metastatic breast cancer. One such study is the phase 3 TROPiCS-02 study, which is actively accruing patients.
Invited discussant Fatima Cardoso, MD, director of the Breast Unit at Champalimaud Clinical Center, Lisbon, Portugal, said the treatment algorithm for the management of TNBC will need to be updated in light of these results.
“In my opinion, we should now add sacituzumab govitecan as a new treatment option for patients treated with two or more lines of therapy,” she said.
She noted that the study design raised some questions over the way such trials should be conducted and the future sequencing of treatments.
Objections to study design and execution
Discussant Cardoso said the choice of progression-free survival as the primary endpoint for the trial was not ideal.
“In the metastatic setting, and particularly for triple-negative breast cancer, where the median survival is quite low and where each line of treatment, particularly after the second line, has a short duration, the primary endpoint should have been overall survival,” she commented.
“Luckily, we saw some results, but we could have missed it,” Cardoso said. She made “a plea to make sure that overall survival is at least the co–primary endpoint” in the future.
Cardoso also said it was not clear to her why the trial had to be stopped. “For the current patients, if there is no crossover, there is no benefit in stopping the trial,” she said.
She went on: “For future patients, it’s better to have the final results sufficiently powered.” She noted that the benefit seen in ASCENT was “moderate” and “so not a substantial breakthrough.” She added that it was “important not to stop trials too early.”
On the positive side, Cardoso said the median number of previous lines of therapy was “quite remarkable for this subtype, and it is important then for us to discuss sequencing,” particularly given that so many patients received checkpoint inhibitors before entering the trial.
Safety and a focus on diarrhea
Investigator Bardia explained that SG is an antibody-drug conjugate directed at Trop-2, which is highly expressed in breast cancer.
The antibody is linked to SN-38, the active metabolite of irinotecan, via a hydrolyzable linker that makes internalization and enzymatic cleavage by tumor cells unnecessary.
Hydrolysis of the linker releases SN-38 both within the tumor cell and extracellularly to induce a so-called bystander effect, in which neighboring tumors cells may be killed even if they do not express Trop-2.
On the basis of positive results from phase 1/2 trial data, SG was granted accelerated approval by the US Food and Drug Administration for patients with metastatic TNBC who experience disease progression after at least two prior therapies.
ASCENT was therefore a phase 3 confirmatory study. Patients with metastatic TNBC who had received at least two prior chemotherapy regimens were randomly assigned in a 1:1 ratio to receive intravenous SG on days 1 and 8 of a 21-day cycle or to receive physician’s choice of treatment.
Physicians could choose eribulin, vinorelbine, gemcitabine, or capecitabine.
The patients continued receiving treatment until disease progression or unacceptable toxicity occurred. On the unanimous recommendation of the data safety monitoring committee, the trial was ended early because of “compelling evidence of efficacy.”
In all, 267 patients were randomly assigned to receive SG. Of those patients, 15 remain on treatment. Treatment was discontinued for 199 patients who experienced disease progression. In the control arm, 262 patients were included, none of whom are still on treatment; 166 discontinued because of disease progression.
The current analysis is limited to 235 patients in the SG group and, in the control arm, 233 patients who did not have brain metastases. Patients with brain metastases will be the subject of a later analysis.
All but one patient in both treatment groups were women. The median age was approximately 54 years. The median number of prior treatment regimens was four. All patients had previously received chemotherapy, and between 26% and 29% have taken checkpoint inhibitors.
By the data cutoff of March 11, 2020, patients had received a median of seven treatment cycles with SG. Progression-free survival was adjudicated by blind, independent central review. The median duration of response was of borderline significance, at 6.3 months vs 3.6 months (P = .057).
Bardia showed that the results were consistent among all subgroups, including subgroups determined on the basis of age, number of prior therapies, whether patients had received prior immune checkpoint therapy, and the presence of liver metastases.
With respect to safety, the important grade 3 or higher treatment-related adverse events were neutropenia (seen in 51% of SG patients vs 33% of patients in the control arm), diarrhea (10% vs <1%), leukopenia (10% vs 5%), anemia (8% vs 5%), and febrile neutropenia (6% vs 2%).
Despite the fact that the adverse event rate was higher with SG than with physician’s choice of chemotherapy, the percentage of such events that led to treatment discontinuation was numerically lower, at 4.7% vs 5.4%.
Cardoso highlighted the “substantial percentage” of patients with diarrhea and nausea in the trial and noted that “all grades” of these adverse events “affect quality of life.”
The focus therefore should be on patient education, prophylaxis, and “the early management of side effects,” she said.
This point was taken up in the postpresentation debate. Bardia said the high rate of diarrhea “likely relates to the toxic payload, which is SN-38, which is known to cause diarrhea.
“Loperamide or immodium prophylaxis can be used in patients who receive this drug, and in general, our experience with the use of sacituzumab govitecan is you can control the diarrhea,” Bardia said.
He added: “There is also a different side effect that occurs during the infusion of SG, which is abdominal cramping and diarrhea, and that’s more of a cholinergic reaction. For that, atropine is the best medication to use.”
The study was funded by Immunomedics Inc. Bardia has disclosed financial ties with Immunomedics and multiple other pharmaceutical companies. Cardoso has disclosed financial ties to multiple drug companies.
This article first appeared on Medscape.com.
The first-in-class drug is directed at trophoblast cell surface antigen 2 (Trop-2), which is highly expressed in breast cancer. Research on the drug was presented at the European Society for Medical Oncology Virtual Congress 2020.
ASCENT randomly assigned more than 500 patients who had metastatic TNBC and who had experienced disease progression after a median of four lines of therapy to receive either SG or physician’s choice of chemotherapy. SG significantly improved median progression-free survival (5.6 vs 1.7 months; hazard ratio [HR], 0.41; P < .0001) and median overall survival (12.1 vs 6.7 months; HR, 0.48; P < .0001).
The response rate was 35% for SG vs 5% for chemotherapy (P < .0001).
The study was presented by Aditya Bardia, MD, MPH, a medical oncologist at Massachusetts General Hospital, Boston, Massachusetts.
He said that because the safety profile of SG is consistent with previous reports and the treatment discontinuation rate was low, the clinical benefit “confirms the use of sacituzumab govitecan” in metastatic TNBC. This was a reference to the fact that the drug previously received accelerated approval on the basis of early data.
Bardia added that ongoing studies are evaluating the use of SG in earlier lines of therapy, including neoadjuvant settings in combination with other targeted agents, as well as in hormone receptor–positive metastatic breast cancer. One such study is the phase 3 TROPiCS-02 study, which is actively accruing patients.
Invited discussant Fatima Cardoso, MD, director of the Breast Unit at Champalimaud Clinical Center, Lisbon, Portugal, said the treatment algorithm for the management of TNBC will need to be updated in light of these results.
“In my opinion, we should now add sacituzumab govitecan as a new treatment option for patients treated with two or more lines of therapy,” she said.
She noted that the study design raised some questions over the way such trials should be conducted and the future sequencing of treatments.
Objections to study design and execution
Discussant Cardoso said the choice of progression-free survival as the primary endpoint for the trial was not ideal.
“In the metastatic setting, and particularly for triple-negative breast cancer, where the median survival is quite low and where each line of treatment, particularly after the second line, has a short duration, the primary endpoint should have been overall survival,” she commented.
“Luckily, we saw some results, but we could have missed it,” Cardoso said. She made “a plea to make sure that overall survival is at least the co–primary endpoint” in the future.
Cardoso also said it was not clear to her why the trial had to be stopped. “For the current patients, if there is no crossover, there is no benefit in stopping the trial,” she said.
She went on: “For future patients, it’s better to have the final results sufficiently powered.” She noted that the benefit seen in ASCENT was “moderate” and “so not a substantial breakthrough.” She added that it was “important not to stop trials too early.”
On the positive side, Cardoso said the median number of previous lines of therapy was “quite remarkable for this subtype, and it is important then for us to discuss sequencing,” particularly given that so many patients received checkpoint inhibitors before entering the trial.
Safety and a focus on diarrhea
Investigator Bardia explained that SG is an antibody-drug conjugate directed at Trop-2, which is highly expressed in breast cancer.
The antibody is linked to SN-38, the active metabolite of irinotecan, via a hydrolyzable linker that makes internalization and enzymatic cleavage by tumor cells unnecessary.
Hydrolysis of the linker releases SN-38 both within the tumor cell and extracellularly to induce a so-called bystander effect, in which neighboring tumors cells may be killed even if they do not express Trop-2.
On the basis of positive results from phase 1/2 trial data, SG was granted accelerated approval by the US Food and Drug Administration for patients with metastatic TNBC who experience disease progression after at least two prior therapies.
ASCENT was therefore a phase 3 confirmatory study. Patients with metastatic TNBC who had received at least two prior chemotherapy regimens were randomly assigned in a 1:1 ratio to receive intravenous SG on days 1 and 8 of a 21-day cycle or to receive physician’s choice of treatment.
Physicians could choose eribulin, vinorelbine, gemcitabine, or capecitabine.
The patients continued receiving treatment until disease progression or unacceptable toxicity occurred. On the unanimous recommendation of the data safety monitoring committee, the trial was ended early because of “compelling evidence of efficacy.”
In all, 267 patients were randomly assigned to receive SG. Of those patients, 15 remain on treatment. Treatment was discontinued for 199 patients who experienced disease progression. In the control arm, 262 patients were included, none of whom are still on treatment; 166 discontinued because of disease progression.
The current analysis is limited to 235 patients in the SG group and, in the control arm, 233 patients who did not have brain metastases. Patients with brain metastases will be the subject of a later analysis.
All but one patient in both treatment groups were women. The median age was approximately 54 years. The median number of prior treatment regimens was four. All patients had previously received chemotherapy, and between 26% and 29% have taken checkpoint inhibitors.
By the data cutoff of March 11, 2020, patients had received a median of seven treatment cycles with SG. Progression-free survival was adjudicated by blind, independent central review. The median duration of response was of borderline significance, at 6.3 months vs 3.6 months (P = .057).
Bardia showed that the results were consistent among all subgroups, including subgroups determined on the basis of age, number of prior therapies, whether patients had received prior immune checkpoint therapy, and the presence of liver metastases.
With respect to safety, the important grade 3 or higher treatment-related adverse events were neutropenia (seen in 51% of SG patients vs 33% of patients in the control arm), diarrhea (10% vs <1%), leukopenia (10% vs 5%), anemia (8% vs 5%), and febrile neutropenia (6% vs 2%).
Despite the fact that the adverse event rate was higher with SG than with physician’s choice of chemotherapy, the percentage of such events that led to treatment discontinuation was numerically lower, at 4.7% vs 5.4%.
Cardoso highlighted the “substantial percentage” of patients with diarrhea and nausea in the trial and noted that “all grades” of these adverse events “affect quality of life.”
The focus therefore should be on patient education, prophylaxis, and “the early management of side effects,” she said.
This point was taken up in the postpresentation debate. Bardia said the high rate of diarrhea “likely relates to the toxic payload, which is SN-38, which is known to cause diarrhea.
“Loperamide or immodium prophylaxis can be used in patients who receive this drug, and in general, our experience with the use of sacituzumab govitecan is you can control the diarrhea,” Bardia said.
He added: “There is also a different side effect that occurs during the infusion of SG, which is abdominal cramping and diarrhea, and that’s more of a cholinergic reaction. For that, atropine is the best medication to use.”
The study was funded by Immunomedics Inc. Bardia has disclosed financial ties with Immunomedics and multiple other pharmaceutical companies. Cardoso has disclosed financial ties to multiple drug companies.
This article first appeared on Medscape.com.
The first-in-class drug is directed at trophoblast cell surface antigen 2 (Trop-2), which is highly expressed in breast cancer. Research on the drug was presented at the European Society for Medical Oncology Virtual Congress 2020.
ASCENT randomly assigned more than 500 patients who had metastatic TNBC and who had experienced disease progression after a median of four lines of therapy to receive either SG or physician’s choice of chemotherapy. SG significantly improved median progression-free survival (5.6 vs 1.7 months; hazard ratio [HR], 0.41; P < .0001) and median overall survival (12.1 vs 6.7 months; HR, 0.48; P < .0001).
The response rate was 35% for SG vs 5% for chemotherapy (P < .0001).
The study was presented by Aditya Bardia, MD, MPH, a medical oncologist at Massachusetts General Hospital, Boston, Massachusetts.
He said that because the safety profile of SG is consistent with previous reports and the treatment discontinuation rate was low, the clinical benefit “confirms the use of sacituzumab govitecan” in metastatic TNBC. This was a reference to the fact that the drug previously received accelerated approval on the basis of early data.
Bardia added that ongoing studies are evaluating the use of SG in earlier lines of therapy, including neoadjuvant settings in combination with other targeted agents, as well as in hormone receptor–positive metastatic breast cancer. One such study is the phase 3 TROPiCS-02 study, which is actively accruing patients.
Invited discussant Fatima Cardoso, MD, director of the Breast Unit at Champalimaud Clinical Center, Lisbon, Portugal, said the treatment algorithm for the management of TNBC will need to be updated in light of these results.
“In my opinion, we should now add sacituzumab govitecan as a new treatment option for patients treated with two or more lines of therapy,” she said.
She noted that the study design raised some questions over the way such trials should be conducted and the future sequencing of treatments.
Objections to study design and execution
Discussant Cardoso said the choice of progression-free survival as the primary endpoint for the trial was not ideal.
“In the metastatic setting, and particularly for triple-negative breast cancer, where the median survival is quite low and where each line of treatment, particularly after the second line, has a short duration, the primary endpoint should have been overall survival,” she commented.
“Luckily, we saw some results, but we could have missed it,” Cardoso said. She made “a plea to make sure that overall survival is at least the co–primary endpoint” in the future.
Cardoso also said it was not clear to her why the trial had to be stopped. “For the current patients, if there is no crossover, there is no benefit in stopping the trial,” she said.
She went on: “For future patients, it’s better to have the final results sufficiently powered.” She noted that the benefit seen in ASCENT was “moderate” and “so not a substantial breakthrough.” She added that it was “important not to stop trials too early.”
On the positive side, Cardoso said the median number of previous lines of therapy was “quite remarkable for this subtype, and it is important then for us to discuss sequencing,” particularly given that so many patients received checkpoint inhibitors before entering the trial.
Safety and a focus on diarrhea
Investigator Bardia explained that SG is an antibody-drug conjugate directed at Trop-2, which is highly expressed in breast cancer.
The antibody is linked to SN-38, the active metabolite of irinotecan, via a hydrolyzable linker that makes internalization and enzymatic cleavage by tumor cells unnecessary.
Hydrolysis of the linker releases SN-38 both within the tumor cell and extracellularly to induce a so-called bystander effect, in which neighboring tumors cells may be killed even if they do not express Trop-2.
On the basis of positive results from phase 1/2 trial data, SG was granted accelerated approval by the US Food and Drug Administration for patients with metastatic TNBC who experience disease progression after at least two prior therapies.
ASCENT was therefore a phase 3 confirmatory study. Patients with metastatic TNBC who had received at least two prior chemotherapy regimens were randomly assigned in a 1:1 ratio to receive intravenous SG on days 1 and 8 of a 21-day cycle or to receive physician’s choice of treatment.
Physicians could choose eribulin, vinorelbine, gemcitabine, or capecitabine.
The patients continued receiving treatment until disease progression or unacceptable toxicity occurred. On the unanimous recommendation of the data safety monitoring committee, the trial was ended early because of “compelling evidence of efficacy.”
In all, 267 patients were randomly assigned to receive SG. Of those patients, 15 remain on treatment. Treatment was discontinued for 199 patients who experienced disease progression. In the control arm, 262 patients were included, none of whom are still on treatment; 166 discontinued because of disease progression.
The current analysis is limited to 235 patients in the SG group and, in the control arm, 233 patients who did not have brain metastases. Patients with brain metastases will be the subject of a later analysis.
All but one patient in both treatment groups were women. The median age was approximately 54 years. The median number of prior treatment regimens was four. All patients had previously received chemotherapy, and between 26% and 29% have taken checkpoint inhibitors.
By the data cutoff of March 11, 2020, patients had received a median of seven treatment cycles with SG. Progression-free survival was adjudicated by blind, independent central review. The median duration of response was of borderline significance, at 6.3 months vs 3.6 months (P = .057).
Bardia showed that the results were consistent among all subgroups, including subgroups determined on the basis of age, number of prior therapies, whether patients had received prior immune checkpoint therapy, and the presence of liver metastases.
With respect to safety, the important grade 3 or higher treatment-related adverse events were neutropenia (seen in 51% of SG patients vs 33% of patients in the control arm), diarrhea (10% vs <1%), leukopenia (10% vs 5%), anemia (8% vs 5%), and febrile neutropenia (6% vs 2%).
Despite the fact that the adverse event rate was higher with SG than with physician’s choice of chemotherapy, the percentage of such events that led to treatment discontinuation was numerically lower, at 4.7% vs 5.4%.
Cardoso highlighted the “substantial percentage” of patients with diarrhea and nausea in the trial and noted that “all grades” of these adverse events “affect quality of life.”
The focus therefore should be on patient education, prophylaxis, and “the early management of side effects,” she said.
This point was taken up in the postpresentation debate. Bardia said the high rate of diarrhea “likely relates to the toxic payload, which is SN-38, which is known to cause diarrhea.
“Loperamide or immodium prophylaxis can be used in patients who receive this drug, and in general, our experience with the use of sacituzumab govitecan is you can control the diarrhea,” Bardia said.
He added: “There is also a different side effect that occurs during the infusion of SG, which is abdominal cramping and diarrhea, and that’s more of a cholinergic reaction. For that, atropine is the best medication to use.”
The study was funded by Immunomedics Inc. Bardia has disclosed financial ties with Immunomedics and multiple other pharmaceutical companies. Cardoso has disclosed financial ties to multiple drug companies.
This article first appeared on Medscape.com.
FROM ESMO 2020



