User login
Ductal carcinoma in situ increases risk of dying from breast cancer by threefold
Key clinical point: Women with ductal carcinoma in situ (DCIS) had a 3-fold increased risk of dying from breast cancer than women without DCIS.
Major finding: Among the cohort, 1,540 women with DCIS died of breast cancer. The expected number of deaths from breast cancer in the cancer-free cohort was 458.
Study details: Cohort study of 144,524 women diagnosed with DCIS from 1995 to 2014.
Disclosures: The authors report no conflicts of interest.
Source: Giannakeas, V, et al. JAMA Netw Open. 2020;3(9):e2017124. doi:10.1001/jamanetworkopen.2020.17124
Key clinical point: Women with ductal carcinoma in situ (DCIS) had a 3-fold increased risk of dying from breast cancer than women without DCIS.
Major finding: Among the cohort, 1,540 women with DCIS died of breast cancer. The expected number of deaths from breast cancer in the cancer-free cohort was 458.
Study details: Cohort study of 144,524 women diagnosed with DCIS from 1995 to 2014.
Disclosures: The authors report no conflicts of interest.
Source: Giannakeas, V, et al. JAMA Netw Open. 2020;3(9):e2017124. doi:10.1001/jamanetworkopen.2020.17124
Key clinical point: Women with ductal carcinoma in situ (DCIS) had a 3-fold increased risk of dying from breast cancer than women without DCIS.
Major finding: Among the cohort, 1,540 women with DCIS died of breast cancer. The expected number of deaths from breast cancer in the cancer-free cohort was 458.
Study details: Cohort study of 144,524 women diagnosed with DCIS from 1995 to 2014.
Disclosures: The authors report no conflicts of interest.
Source: Giannakeas, V, et al. JAMA Netw Open. 2020;3(9):e2017124. doi:10.1001/jamanetworkopen.2020.17124
Women 70 and older are divided on age-based guidelines for breast cancer treatment
Key clinical point: In women aged 70 or older, there are skeptical views on age-based guidelines for breast cancer treatment and difficulty in interpretating the rationale for treatment de-escalation in low-risk, early-stage hormone receptor–positive breast cancer.
Major finding: Approximately 40% of participants stated they would proceed with sentinel lymph node biopsy (SLNB) despite evidence that omission is safe. Conversely, 73% stated they would omit postlumpectomy radiotherapy.
Study details: A qualitative study with 30 female participants, with a median age of 72 years and without a previous diagnosis of breast cancer.
Disclosures: Dr Jagsi reported receiving grants from the National Institutes of Health (NIH), Komen Foundation, Doris Duke Foundation, Blue Cross Blue Shield of Michigan for the Michigan Radiation Oncology Quality Consortium, and Genentech; grants and personal fees from Greenwall Foundation; personal fees from Amgen, Vizient, Sherinian & Hassostock, and Dressman, Benziger, and Lavelle; and options as compensation for her advisory board role from Equity Quotient; she also reported being an uncompensated founding member of TIME’S UP Healthcare and a member of the American Society of Clinical Oncology Board of Directors. No other disclosures were reported.
Source: Wang, T, et al. JAMA Netw Open. 2020;3(9):e2017129. doi:10.1001/jamanetworkopen.2020.17129
Key clinical point: In women aged 70 or older, there are skeptical views on age-based guidelines for breast cancer treatment and difficulty in interpretating the rationale for treatment de-escalation in low-risk, early-stage hormone receptor–positive breast cancer.
Major finding: Approximately 40% of participants stated they would proceed with sentinel lymph node biopsy (SLNB) despite evidence that omission is safe. Conversely, 73% stated they would omit postlumpectomy radiotherapy.
Study details: A qualitative study with 30 female participants, with a median age of 72 years and without a previous diagnosis of breast cancer.
Disclosures: Dr Jagsi reported receiving grants from the National Institutes of Health (NIH), Komen Foundation, Doris Duke Foundation, Blue Cross Blue Shield of Michigan for the Michigan Radiation Oncology Quality Consortium, and Genentech; grants and personal fees from Greenwall Foundation; personal fees from Amgen, Vizient, Sherinian & Hassostock, and Dressman, Benziger, and Lavelle; and options as compensation for her advisory board role from Equity Quotient; she also reported being an uncompensated founding member of TIME’S UP Healthcare and a member of the American Society of Clinical Oncology Board of Directors. No other disclosures were reported.
Source: Wang, T, et al. JAMA Netw Open. 2020;3(9):e2017129. doi:10.1001/jamanetworkopen.2020.17129
Key clinical point: In women aged 70 or older, there are skeptical views on age-based guidelines for breast cancer treatment and difficulty in interpretating the rationale for treatment de-escalation in low-risk, early-stage hormone receptor–positive breast cancer.
Major finding: Approximately 40% of participants stated they would proceed with sentinel lymph node biopsy (SLNB) despite evidence that omission is safe. Conversely, 73% stated they would omit postlumpectomy radiotherapy.
Study details: A qualitative study with 30 female participants, with a median age of 72 years and without a previous diagnosis of breast cancer.
Disclosures: Dr Jagsi reported receiving grants from the National Institutes of Health (NIH), Komen Foundation, Doris Duke Foundation, Blue Cross Blue Shield of Michigan for the Michigan Radiation Oncology Quality Consortium, and Genentech; grants and personal fees from Greenwall Foundation; personal fees from Amgen, Vizient, Sherinian & Hassostock, and Dressman, Benziger, and Lavelle; and options as compensation for her advisory board role from Equity Quotient; she also reported being an uncompensated founding member of TIME’S UP Healthcare and a member of the American Society of Clinical Oncology Board of Directors. No other disclosures were reported.
Source: Wang, T, et al. JAMA Netw Open. 2020;3(9):e2017129. doi:10.1001/jamanetworkopen.2020.17129
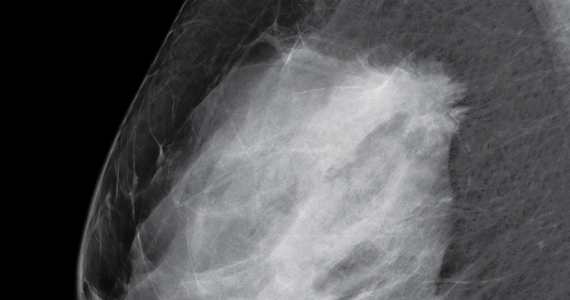
Beyond baseline, DBT no better than mammography for dense breasts
Key clinical point: In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the initial exam.
Major finding: For baseline screening in women aged 50-59 years, recall rates per 1,000 exams dropped from 241 with DM to 204 with DBT. Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT. On follow-up exams, recall and cancer detection rates varied by patients’ age and breast density.
Study details: Review of 1,584,079 screenings in women aged 40-79 years.
Disclosures: The research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. The study lead reported grants from GE Healthcare.
Source: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
Key clinical point: In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the initial exam.
Major finding: For baseline screening in women aged 50-59 years, recall rates per 1,000 exams dropped from 241 with DM to 204 with DBT. Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT. On follow-up exams, recall and cancer detection rates varied by patients’ age and breast density.
Study details: Review of 1,584,079 screenings in women aged 40-79 years.
Disclosures: The research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. The study lead reported grants from GE Healthcare.
Source: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
Key clinical point: In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the initial exam.
Major finding: For baseline screening in women aged 50-59 years, recall rates per 1,000 exams dropped from 241 with DM to 204 with DBT. Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT. On follow-up exams, recall and cancer detection rates varied by patients’ age and breast density.
Study details: Review of 1,584,079 screenings in women aged 40-79 years.
Disclosures: The research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. The study lead reported grants from GE Healthcare.
Source: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
Study supports multigene panel testing for all breast cancer patients with second primary cancers
Key clinical point: All patients with breast cancer who develop a second primary cancer should undergo multigene panel testing, according to researchers.
Major finding: Mutation rates in BRCA1/2-negative breast cancer patients with multiple primary cancers were approximately 7% to 9%, compared with about 4% to 5% in BRCA1/2-negative patients with a single breast cancer.
Study details: A comparison of mutation rates in 1,000 high-risk breast cancer patients (551 with multiple primary cancers and 449 with a single breast cancer) and 1,804 familial breast cancer patients (340 with multiple primaries and 1,464 with a single breast cancer).
Disclosures: This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Some authors disclosed relationships with a range of companies.
Source: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
Key clinical point: All patients with breast cancer who develop a second primary cancer should undergo multigene panel testing, according to researchers.
Major finding: Mutation rates in BRCA1/2-negative breast cancer patients with multiple primary cancers were approximately 7% to 9%, compared with about 4% to 5% in BRCA1/2-negative patients with a single breast cancer.
Study details: A comparison of mutation rates in 1,000 high-risk breast cancer patients (551 with multiple primary cancers and 449 with a single breast cancer) and 1,804 familial breast cancer patients (340 with multiple primaries and 1,464 with a single breast cancer).
Disclosures: This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Some authors disclosed relationships with a range of companies.
Source: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
Key clinical point: All patients with breast cancer who develop a second primary cancer should undergo multigene panel testing, according to researchers.
Major finding: Mutation rates in BRCA1/2-negative breast cancer patients with multiple primary cancers were approximately 7% to 9%, compared with about 4% to 5% in BRCA1/2-negative patients with a single breast cancer.
Study details: A comparison of mutation rates in 1,000 high-risk breast cancer patients (551 with multiple primary cancers and 449 with a single breast cancer) and 1,804 familial breast cancer patients (340 with multiple primaries and 1,464 with a single breast cancer).
Disclosures: This research was supported by grants from government agencies and foundations as well as the University of Pennsylvania. Some authors disclosed relationships with a range of companies.
Source: Maxwell KN et al. JCO Precis Oncol. 2020. doi: 10.1200/PO.19.00301.
AI algorithm on par with radiologists as mammogram reader
Key clinical point: An artificial intelligence computer algorithm performed on par with, and in some cases exceeded, radiologists in reading mammograms from women undergoing routine screening.
Major finding: When operating at a specificity of 96.6%, the sensitivity was 81.9% for the algorithm, 77.4% for first-reader radiologists, and 80.1% for second-reader radiologists.
Study details: A comparison of algorithm and radiologist assessments of mammograms in 8,805 women, 739 of whom were diagnosed with breast cancer.
Disclosures: The research was funded by the Stockholm County Council. The investigators disclosed financial relationships with the Swedish Research Council, the Swedish Cancer Society, Stockholm City Council, Collective Minds Radiology, and Pfizer.
Source: Salim M et al. JAMA Oncol. 2020 Aug 27. doi: 10.1001/jamaoncol.2020.3321.
Key clinical point: An artificial intelligence computer algorithm performed on par with, and in some cases exceeded, radiologists in reading mammograms from women undergoing routine screening.
Major finding: When operating at a specificity of 96.6%, the sensitivity was 81.9% for the algorithm, 77.4% for first-reader radiologists, and 80.1% for second-reader radiologists.
Study details: A comparison of algorithm and radiologist assessments of mammograms in 8,805 women, 739 of whom were diagnosed with breast cancer.
Disclosures: The research was funded by the Stockholm County Council. The investigators disclosed financial relationships with the Swedish Research Council, the Swedish Cancer Society, Stockholm City Council, Collective Minds Radiology, and Pfizer.
Source: Salim M et al. JAMA Oncol. 2020 Aug 27. doi: 10.1001/jamaoncol.2020.3321.
Key clinical point: An artificial intelligence computer algorithm performed on par with, and in some cases exceeded, radiologists in reading mammograms from women undergoing routine screening.
Major finding: When operating at a specificity of 96.6%, the sensitivity was 81.9% for the algorithm, 77.4% for first-reader radiologists, and 80.1% for second-reader radiologists.
Study details: A comparison of algorithm and radiologist assessments of mammograms in 8,805 women, 739 of whom were diagnosed with breast cancer.
Disclosures: The research was funded by the Stockholm County Council. The investigators disclosed financial relationships with the Swedish Research Council, the Swedish Cancer Society, Stockholm City Council, Collective Minds Radiology, and Pfizer.
Source: Salim M et al. JAMA Oncol. 2020 Aug 27. doi: 10.1001/jamaoncol.2020.3321.
How ObGyns can best work with radiologists to optimize screening for patients with dense breasts
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
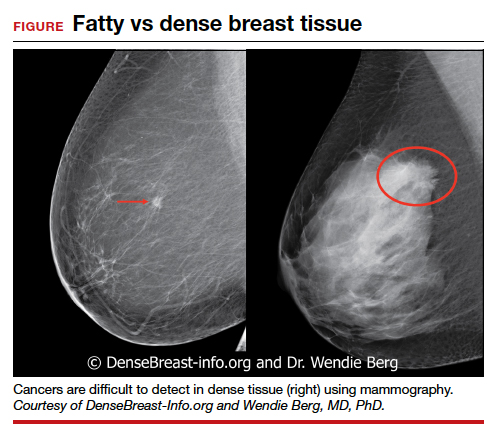
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.
MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
If your ObGyn practices are anything like ours, every time there is news coverage of a study regarding mammography or about efforts to pass a breast density inform law, your phone rings with patient calls. In fact, every density inform law enacted in the United States, except for in Illinois, directs patients to their referring provider—generally their ObGyn—to discuss the screening and risk implications of dense breast tissue.
The steady increased awareness of breast density means that we, as ObGyns and other primary care providers (PCPs), have additional responsibilities in managing the breast health of our patients. This includes guiding discussions with patients about what breast density means and whether supplemental screening beyond mammography might be beneficial.
As members of the Medical Advisory Board for DenseBreast-info.org (an online educational resource dedicated to providing breast density information to patients and health care professionals), we are aware of the growing body of evidence demonstrating improved detection of early breast cancer using supplemental screening in dense breasts. However, we know that there is confusion among clinicians about how and when to facilitate tailored screening for women with dense breasts or other breast cancer risk factors. Here we answer 6 questions focusing on how to navigate patient discussions around the topic and the best way to collaborate with radiologists to improve breast care for patients.
Play an active role
1. What role should ObGyns and PCPs play in women’s breast health?
Elizabeth Etkin-Kramer, MD: I am a firm believer that ObGyns and all women’s health providers should be able to assess their patients’ risk of breast cancer and explain the process for managing this risk with their patients. This explanation includes the clinical implications of breast density and when supplemental screening should be employed. It is also important for providers to know when to offer genetic testing and when a patient’s personal or family history indicates supplemental screening with breast magnetic resonance imaging (MRI).
DaCarla M. Albright, MD: I absolutely agree that PCPs, ObGyns, and family practitioners should spend the time to be educated about breast density and supplemental screening options. While the exact role providers play in managing patients’ breast health may vary depending on the practice type or location, the need for knowledge and comfort when talking with patients to help them make informed decisions is critical. Breast health and screening, including the importance of breast density, happen to be a particular interest of mine. I have participated in educational webinars, invited lectures, and breast cancer awareness media events on this topic in the past.
Continue to: Join forces with imaging centers...
Join forces with imaging centers
2. How can ObGyns and radiologists collaborate most effectively to use screening results to personalize breast care for patients?
Dr. Etkin-Kramer: It is important to have a close relationship with the radiologists that read our patients’ mammograms. We need to be able to easily contact the radiologist and quickly get clarification on a patient’s report or discuss next steps. Imaging centers should consider running outreach programs to educate their referring providers on how to risk assess, with this assessment inclusive of breast density. Dinner lectures or grand round meetings are effective to facilitate communication between the radiology community and the ObGyn community. Finally, as we all know, supplemental screening is often subject to copays and deductibles per insurance coverage. If advocacy groups, who are working to eliminate these types of costs, cannot get insurers to waive these payments, we need a less expensive self-pay option.
Dr. Albright: I definitely have and encourage an open line of communication between my practice and breast radiology, as well as our breast surgeons and cancer center to set up consultations as needed. We also invite our radiologists as guests to monthly practice meetings or grand rounds within our department to further improve access and open communication, as this environment is one in which greater provider education on density and adjunctive screening can be achieved.
Know when to refer a high-risk patient
3. Most ObGyns routinely collect family history and perform formal risk assessment. What do you need to know about referring patients to a high-risk program?
Dr. Etkin-Kramer: It is important as ObGyns to be knowledgeable about breast and ovarian cancer risk assessment and genetic testing for cancer susceptibility genes. Our patients expect that of us. I am comfortable doing risk assessment in my office, but I sometimes refer to other specialists in the community if the patient needs additional counseling. For risk assessment, I look at family and personal history, breast density, and other factors that might lead me to believe the patient might carry a hereditary cancer susceptibility gene, including Ashkenazi Jewish ancestry.1 When indicated, I check lifetime as well as short-term (5- to 10-year) risk, usually using Breast Cancer Surveillance Consortium (BCSC) or Tyrer-Cuzick/International Breast Cancer Intervention Study (IBIS) models, as these include breast density.
I discuss risk-reducing medications. The US Preventive Services Task Force recommends these agents if my patient’s 5-year risk of breast cancer is 1.67% or greater, and I strongly recommend chemoprevention when the patient’s 5-year BCSC risk exceeds 3%, provided likely benefits exceed risks.2,3 I discuss adding screening breast MRI if lifetime risk by Tyrer-Cuzick exceeds 20%. (Note that Gail and BCSC models are not recommended to be used to determine risk for purposes of supplemental screening with MRI as they do not consider paternal family history nor age of relatives at diagnosis.)
Dr. Albright: ObGyns should be able to ascertain a pertinent history and identify patients at risk for breast cancer based on their personal history, family history, and breast imaging/biopsy history, if relevant. We also need to improve our discussions of supplemental screening for patients who have heterogeneously dense or extremely dense breast tissue. I sense that some ObGyns may rely heavily on the radiologist to suggest supplemental screening, but patients actually look to ObGyns as their providers to have this knowledge and give them direction.
Since I practice at a large academic medical center, I have the opportunity to refer patients to our Breast Cancer Genetics Program because I may be limited on time for counseling in the office and do not want to miss salient details. With all of the information I have ascertained about the patient, I am able to determine and encourage appropriate screening and assure insurance coverage for adjunctive breast MRI when appropriate.
Continue to: Consider how you order patients’ screening to reduce barriers and cost...
Consider how you order patients’ screening to reduce barriers and cost
4. How would you suggest reducing barriers when referring patients for supplemental screening, such as MRI for high-risk women or ultrasound for those with dense breasts? Would you prefer it if such screening could be performed without additional script/referral? How does insurance coverage factor in?
Dr. Etkin-Kramer: I would love for a screening mammogram with possible ultrasound, on one script, to be the norm. One of the centers that I work with accepts a script written this way. Further, when a patient receives screening at a freestanding facility as opposed to a hospital, the fee for the supplemental screening may be lower because they do not add on a facility fee.
Dr. Albright: We have an order in our electronic health record that allows for screening mammography but adds on diagnostic mammography/bilateral ultrasonography, if indicated by imaging. I am mostly ordering that option now for all of my screening patients; rarely have I had issues with insurance accepting that script. As for when ordering an MRI, I always try to ensure that I have done the patient’s personal risk assessment and included that lifetime breast cancer risk on the order. If the risk is 20% or higher, I typically do not have any insurance coverage issues. If I am ordering MRI as supplemental screening, I typically order the “Fast MRI” protocol that our center offers. This order incurs a $299 out-of-pocket cost for the patient. Any patient with heterogeneously or extremely dense breasts on mammography should have this option, but it requires patient education, discussion with the provider, and an additional cost. I definitely think that insurers need to consider covering supplemental screening, since breast density is reportable in a majority of the US states and will soon be the national standard.
Pearls for guiding patients
5. How do you discuss breast density and the need for supplemental screening with your patients?
Dr. Etkin-Kramer: I strongly feel that my patients need to know when a screening test has limited ability to do its job. This is the case with dense breasts. Visuals help; when discussing breast density, I like the images supplied by DenseBreast-info.org (FIGURE). I explain the two implications of dense tissue:
- First, dense tissue makes it harder to visualize cancers in the breast—the denser the breasts, the less likely the radiologist can pick up a cancer, so mammographic sensitivity for extremely dense breasts can be as low as 25% to 50%.
- Second, high breast density adds to the risk of developing breast cancer. I explain that supplemental screening will pick up additional cancers in women with dense breasts. For example, breast ultrasound will pick up about 2-3/1000 additional breast cancers per year and MRI or molecular breast imaging (MBI) will pick up much more, perhaps 10/1000.

MRI is more invasive than an ultrasound and uses gadolinium, and MBI has more radiation. Supplemental screening is not endorsed by ACOG’s most recent Committee Opinion from 2017; 4 however, patients may choose to have it done. This is where shared-decision making is important.
I strongly recommend that all women’s health care providers complete the CME course on the DenseBreast-info.org website. “ Breast Density: Why It Matters ” is a certified educational program for referring physicians that helps health care professionals learn about breast density, its associated risks, and how best to guide patients regarding breast cancer screening.
Continue to: Dr. Albright...
Dr. Albright: When I discuss breast density, I make sure that patients understand that their mammogram determines the density of their breast tissue. I review that in the higher density categories (heterogeneously dense or extremely dense), there is a higher risk of missing cancer, and that these categories are also associated with a higher risk of breast cancer. I also discuss the potential need for supplemental screening, for which my institution primarily offers Fast MRI. However, we can offer breast ultrasonography instead as an option, especially for those concerned about gadolinium exposure. Our center offers either of these supplemental screenings at a cost of $299. I also review the lack of coverage for supplemental screening by some insurance carriers, as both providers and patients may need to advocate for insurer coverage of adjunct studies.
Educational resources
6. What reference materials, illustrations, or other tools do you use to educate your patients?
Dr. Etkin-Kramer: I frequently use handouts printed from the DenseBreast-info.org website, and there is now a brand new patient fact sheet that I have just started using. I also have an example of breast density categories from fatty replaced to extremely dense on my computer, and I am putting it on a new smart board.
Dr. Albright: The extensive resources available at DenseBreast-info.org can improve both patient and provider knowledge of these important issues, so I suggest patients visit that website, and I use many of the images and visuals to help explain breast density. I even use the materials from the website for educating my resident trainees on breast health and screening. ●
Nearly 16,000 children (up to age 19 years) face cancer-related treatment every year.1 For girls and young women, undergoing chest radiotherapy puts them at higher risk for secondary breast cancer. In fact, they have a 30% chance of developing such cancer by age 50—a risk that is similar to women with a BRCA1 mutation.2 Therefore, current recommendations for breast cancer screening among those who have undergone childhood chest radiation (≥20 Gy) are to begin annual mammography, with adjunct magnetic resonance imaging (MRI), at age 25 years (or 8 years after chest radiotherapy).3
To determine the benefits and risks of these recommendations, as well as of similar strategies, Yeh and colleagues performed simulation modeling using data from the Childhood Cancer Survivor Study and two CISNET (Cancer Intervention and Surveillance Modeling Network) models.4 For their study they targeted a cohort of female childhood cancer survivors having undergone chest radiotherapy and evaluated breast cancer screening with the following strategies:
- mammography plus MRI, starting at ages 25, 30, or 35 years and continuing to age 74
- MRI alone, starting at ages 25, 30, or 35 years and continuing to age 74.
They found that both strategies reduced the risk of breast cancer in the targeted cohort but that screening beginning at the earliest ages prevented most deaths. No screening at all was associated with a 10% to 11% lifetime risk of breast cancer, but mammography plus MRI beginning at age 25 reduced that risk by 56% to 71% depending on the model. Screening with MRI alone reduced mortality risk by 56% to 62%. When considering cost per quality adjusted life-year gained, the researchers found that screening beginning at age 30 to be the most cost-effective.4
Yeh and colleagues addressed concerns with mammography and radiation. Although they said the associated amount of radiation exposure is small, the use of mammography in women younger than age 30 is controversial—and not recommended by the American Cancer Society or the National Comprehensive Cancer Network.5,6
Bottom line. Yeh and colleagues conclude that MRI screening, with or without mammography, beginning between the ages of 25 and 30 should be emphasized in screening guidelines. They note the importance of insurance coverage for MRI in those at risk for breast cancer due to childhood radiation exposure.4
References
- National Cancer Institute. How common is cancer in children? https://www.cancer.gov/types/childhood-cancers/child-adolescentcancers-fact-sheet#how-common-is-cancer-in-children. Accessed September 25, 2020.
- Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217- 2223.
- Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http:// www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf. Accessed September 25, 2020.
- Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation. Ann Intern Med. 2020;173:331-341.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast cancer screening and diagnosis version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed September 25, 2020.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
- Bharucha PP, Chiu KE, Francois FM, et al. Genetic testing and screening recommendations for patients with hereditary breast cancer. RadioGraphics. 2020;40:913-936.
- Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327-2333.
- Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22:3230-3235.
- American College of Obstetricians and Gynecologists. Committee opinion no. 625: management of women with dense breasts diagnosed by mammography [published correction appears in Obstet Gynecol. 2016;127:166]. Obstet Gynecol. 2015;125(3):750-751.
Restarting breast cancer screening after disruption not so simple
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
FROM EBCC-12 VIRTUAL CONFERENCE
Radiotherapy planning scans reveal breast cancer patients’ CVD risk
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
Radiotherapy planning scans may be a rich untapped source of information for estimating the risk of cardiovascular disease (CVD) in breast cancer patients, a large study suggests.
Researchers found that breast cancer patients with a coronary artery calcifications (CAC) score exceeding 400 had nearly four times the adjusted risk of fatal and nonfatal CVD events when compared with patients who had a CAC score of 0.
Patients with scores exceeding 400 also had more than eight times the risk of coronary heart disease events. The associations were especially strong in the subset of patients who received anthracycline-containing chemotherapy.
Helena Verkooijen, MD, PhD, of University Medical Center Utrecht (the Netherlands) presented these findings at the 12th European Breast Cancer Conference.
Dr. Verkooijen noted that, over the past 50 years, breast cancer has dramatically declined as a cause of death among breast cancer survivors, while CVD has continued to account for about 20% of the total deaths in this population.
CACs are sometimes incidentally seen in radiotherapy planning CT scans. “Right now, this information is not often used for patient stratification or informing patients about their cardiovascular risk, and this is a pity, because we know that it is an independent risk factor, and, often, the presence of calcifications can occur in the absence of other cardiovascular risk factors,” Dr. Verkooijen said.
Study details
Dr. Verkooijen and and colleagues from the Bragataston Study Group retrospectively studied 15,919 breast cancer patients who had radiotherapy planning CT scans during 2004-2016 at three Dutch institutions.
The researchers used an automated deep-learning algorithm (described in Radiology) to detect and quantify coronary calcium in planning CT scans and calculate CAC scores, classifying them into five categories.
The median follow-up was 51.6 months. Most women (70%) did not have any calcium detected in their coronary arteries (CAC score of 0), while 3% fell into the highest category (CAC score of >400).
The incidence of nonfatal and fatal CVD events increased with CAC score:
- 5.1% with a score of 0.
- 8.5% with a score of 1-10.
- 13.5% with a score of 11-100.
- 17.6% with a score of 101-400.
- 28.0% with a score greater than 400.
In analyses adjusted for age, laterality of radiation, and receipt of cardiotoxic agents – anthracyclines and trastuzumab – women with a score exceeding 400 had sharply elevated adjusted risks of CVD events (hazard ratio, 3.7), of coronary heart disease events specifically (HR, 8.2), and of death from any cause (HR, 2.8), when compared with peers who had a CAC score of 0.
On further scrutiny of CVD events, the pattern was similar regardless of whether radiation was left- or right-sided. However, the association was stronger among women who received anthracyclines as compared with counterparts who did not, with a nearly six-fold higher risk for those with highest versus lowest CAC scores.
When the women were surveyed, nearly 90% said they wanted to be informed about their CAC score and associated CVD risk, even in the absence of evidence-based risk reduction strategies.
Applying the results
“We believe that this is the first time that anyone has conducted a study on this topic on a scale like this, and we show that it is possible to relatively easily identify women at a very high risk of CVD,” Dr. Verkooijen said. “But what do we do with this information, because these scans are not made to answer this question. … This is information that we get that we haven’t really requested. I think we should only use this information when we have really shown that we can help patients reduce their risk of cardiovascular disease.”
To that end, Dr. Verkooijen and colleagues are planning additional research that will look at the potential benefit of referring high-risk patients for cardioprevention strategies and at the role of using the CAC score to personalize treatment strategies.
“This is an interesting and novel approach to predicting cardiac events for patients undergoing breast cancer treatment,” Meena S. Moran, MD, of Yale University in New Haven, Conn., commented in an interview.
The approach would likely be feasible in typical practice with widespread availability of the automated algorithm and might even alter treatment planning in real time, she said. “From the standpoint of radiation oncology, it would mean running the software to generate a CAC score, which would allow for modifications in decision-making during treatment planning, such as whether or not to include the internal mammary nodal chain in a patient who may be in the ‘gray zone’ for regional nodal radiation. For example, if a patient has a high CAC score, plus if they have received (or are receiving) cardiotoxic drugs, radiation oncologists can use that information as an additional factor to consider in the decision-making of whether or not to include the internal mammary chain, which inevitably can increase the dose delivered to the heart,” Dr. Moran elaborated.
Dr. Verkooijen’s study was supported by the Dutch Cancer Society, the European Commission, the Dutch Digestive Foundation, the Netherlands Organisation for Scientific Research, and Elekta. Dr. Verkooijen and Dr. Moran disclosed no conflicts of interest.
SOURCE: Gal R et al. EBCC-12 Virtual Congress, Abstract 7.
FROM EBCC-12 VIRTUAL CONFERENCE
Stroke may be the first symptom of COVID-19 in younger patients
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Neurology
Nerve damage linked to prone positioning in COVID-19
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.
“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF ANAESTHESIOLOGY