User login
CCC19, other registries help define COVID/cancer landscape
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
FROM AACR: COVID-19 and CANCER
Genetic differences by ancestry shouldn’t impact efficacy of prostate cancer therapies
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
FROM CLINICAL CANCER RESEARCH
Ticagrelor/aspirin combo: Fewer repeat strokes and deaths, but more bleeds
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, new data show. However, severe bleeding was more common in the ticagrelor/aspirin group than in the aspirin-only group.
“We found that ticagrelor plus aspirin reduced the risk of stroke or death, compared to aspirin alone in patients presenting acutely with stroke or TIA,” reported lead author S. Claiborne Johnston, MD, PhD, dean and vice president for medical affairs, Dell Medical School, the University of Texas, Austin.
Although the combination also increased the risk for major hemorrhage, that increase was small and would not overwhelm the benefit, he said.
The study was published online July 16 in The New England Journal of Medicine.
Attractive properties
“Lots of patients have stroke in the days to weeks after first presenting with a stroke or TIA,” said Dr. Johnston, who is also the Frank and Charmaine Denius Distinguished Dean’s Chair at Dell Medical School. “Aspirin has been the standard of care but is only partially effective. Clopidogrel plus aspirin is another option that has recently been proven, [but] ticagrelor has attractive properties as an antiplatelet agent and works synergistically with aspirin,” he added.
Ticagrelor is a direct-acting antiplatelet agent that does not depend on metabolic activation and that “reversibly binds” and inhibits the P2Y12 receptor on platelets. Previous research has evaluated clopidogrel and aspirin for the secondary prevention of ischemic stroke or TIA. In an earlier trial, ticagrelor was no better than aspirin in preventing these subsequent events. However, the investigators noted that the combination of the two drugs has not been well studied.
The randomized, placebo-controlled, double-blind trial involved 11,016 patients at 414 sites in 28 countries. Patients who had experienced mild to moderate acute noncardioembolic ischemic stroke (mean age, 65 years; 39% women; roughly 54% White) were randomly assigned to receive either ticagrelor plus aspirin (n = 5,523) or aspirin alone (n = 5,493) for 30 days. Of these patients, 91% had sustained a stroke, and 9% had sustained a TIA.
Thirty days was chosen as the treatment period because the risk for subsequent stroke tends to occur mainly in the first month after an acute ischemic stroke or TIA. The primary outcome was “a composite of stroke or death in a time-to-first-event analysis from randomization to 30 days of follow-up.” For the study, “stroke” encompassed ischemic, hemorrhagic, or stroke of undetermined type, and “death” included deaths of all causes. Secondary outcomes included first subsequent ischemic stroke and disability (defined as a score of >1 on the Rankin Scale).
Almost all patients (99.5%) were taking aspirin during the treatment period, and most were also taking an antihypertensive and a statin (74% and 83%, respectively).
Patients in the ticagrelor/aspirin group had fewer primary-outcome events in comparison with those in the aspirin-only group (303 patients [5.5%] vs. 362 patients [6.6%]; hazard ratio, 0.83; 95% confidence interval, 0.71-0.96; P = 0.02). Incidence of subsequent ischemic stroke were similarly lower in the ticagrelor/aspirin group in comparison with the aspirin-only group (276 patients [5.0%] vs. 345 patients [6.3%]; HR, 0.79; 95% CI, 0.68-0.93; P = .004).
On the other hand, there was no significant difference between the groups in the incidence of overall disability (23.8% of the patients in the ticagrelor/aspirin group and in 24.1% of the patients in the aspirin group; odds ratio, 0.98; 95% CI, 0.89-1.07; P = .61).
There were differences between the groups in severe bleeding, which occurred in 28 patients (0.5%) in the ticagrelor/aspirin group and in seven patients (0.15) in the ticagrelor group (HR, 3.99; 95% CI, 1.74-9.14; P = .001). Moreover, more patients in the ticagrelor/aspirin group experienced a composite of intracranial hemorrhage or fatal bleeding compared with the aspirin-only group (0.4% vs 0.1%). Fatal bleeding occurred in 0.2% of patients in the ticagrelor/aspirin group versus 0.1% of patients in the aspirin group. More patients in the ticagrelor-aspirin group permanently discontinued the treatment because of bleeding than in the aspirin-only group (2.8% vs. 0.6%).
“The benefit from treatment with ticagrelor/aspirin, as compared with aspirin alone, would be expected to result in a number needed to treat of 92 to prevent one primary outcome event, and a number needed to harm of 263 for severe bleeding,” the authors noted.
Risks versus benefits
Commenting on the study, Konark Malhotra, MD, a vascular neurologist at Allegheny Health Network, Pittsburgh, noted that ticagrelor is an antiplatelet medication “that adds to the armamentarium of stroke neurologists for the treatment of mild acute ischemic or high-risk TIA patients.” Dr. Malhotra, who was not involved with the study, added that the “combined use of ticagrelor and aspirin is effective in the reduction of ischemic events, however, at the expense of increased risk of bleeding events.”
In an accompanying editorial, Peter Rothwell, MD, PhD, of the Wolfson Center for Prevention of Stroke and Dementia, Nuffield Department of Clinical Neurosciences at the University of Oxford (England) who was not involved with the study, suggested that the “bleeding risk associated with ticagrelor and aspirin might exceed the benefit among lower-risk patients who make up the majority in practice, and so the results should not be overgeneralized.” Moreover, “regardless of which combination of antiplatelet therapy is favored for the high-risk minority, all patients should receive aspirin immediately after TIA, unless aspirin is contraindicated.”
He noted that “too many patients are sent home from emergency departments without this simple treatment that substantially reduces the risk and severity of early recurrent stroke.”
The study was supported by AstraZeneca. Dr. Johnston has received a grant from AstraZeneca and nonfinancial support from SANOFI. Dr. Rothwell has received personal fees from Bayer and BMS. Dr. Malhotra has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From New England Journal of Medicine
In remission for 10 years: Long-term toxicity data on CAR T cells
When a patient with cancer hears there isn’t much left that doctors can do, it always stays fresh in the mind.
Doug Olson was first diagnosed with chronic lymphocytic leukemia (CLL) over 20 years ago, in 1996. For several years, his doctors used the watch-and-wait approach. But then his cancer progressed and needed treatment. By 2010, it had mutated so much that it no longer responded to standard therapy.
He was rapidly running out of options. Back then, the only treatment left was a bone marrow transplant. Without one, his doctors said, he would have 1 or 2 years left to live.
“I was really trying to avoid a bone marrow transplant. You’re playing your last card if that doesn’t work. It’s a pretty rough procedure,” Olson told Medscape Medical News.
Looking back, Olson counts himself as lucky – for being in the right place, at the right time, with the right doctor. His oncologist was David Porter, MD, the principal investigator on a trial at the University of Pennsylvania that was investigating a brand new approach to treating cancer: chimeric antigen receptor (CAR) T-cell therapy.
CAR T-cell therapy uses a patient’s own T cells engineered to express a receptor that targets proteins on cancer cells. CAR T cells are considered “living drugs” because they expand inside the body and stick around for years – maybe for a lifetime – to fight the cancer if it tries to come back.
“I was certainly intrigued by the approach. It had worked in mice, and it was the sort of thing that looked like it would work,” Olson recalled.
Science is not a foreign language to Olson. He holds a PhD in medicinal chemistry, spent most of his career in the in vitro diagnostics industry, and currently acts as chief executive officer of Buhlmann Diagnostics Corp.
So he read the clinical protocol for the first in-human trial of CAR T cells and agreed to become patient number two.
Olson’s T cells were harvested, engineered to attack the CD19 antigen found on malignant and normal B lymphocytes, and then were expanded into millions in the lab. After undergoing preconditioning with chemotherapy to minimize rejection and boost the CAR T cells’ expansion inside the body, he received several infusions of the new therapy over the course of 3 days.
Nothing really happened for 2 weeks. Then he developed severe flu-like symptoms – so bad that he was hospitalized.
Ironically, getting sick was a sign that the CAR T cells were working. Olson was experiencing one of the main short-term effects of CAR T-cell therapy: cytokine release syndrome. Symptoms include extremely high fevers and dangerous drops in blood pressure that can potentially cause end-organ damage.
In the early trials of these products, some patients experienced such a severe reaction that they needed intensive care, and some died. With increasing clinical experience, doctors have learned to control the reaction with the use of steroids and interleukein-6 inhibitors such as tocilizumab (Actemra).
Fortunately for Olson, the reaction passed, and he was eventually discharged.
Then the “aha moment” happened. Four weeks after receiving the CAR T cells, Olson found out that he was cancer free.
“It still gives me shivers,” he said. “Dr Porter said, ‘Your bone marrow’s completely free. We just can’t find a cancer cell anywhere.’ “
The remission has lasted, and it is now 10 years later.
Balancing long-term risks vs benefits
Long-term data have been accumulating for these novel therapies since Olson’s treatment in 2010. This is particularly important for CAR T-cell therapy, because of its longevity. Because these are living cells and are expected to persist in the body for years, there is great interest in longer-term data, especially the risks for toxicity.
The FDA requires clinical follow-up for at least 15 years for patients treated with CAR T-cell therapy or any other genetically modified cells.
So far, most of the experience with CAR T cells comes from anti-CD19-directed therapy, which has shown “remarkable” remission rates in the 50% to 85% range, said Nirali Shah, MD, head of the hematologic malignancies section of the Pediatric Oncology Branch at the National Cancer Institute (NCI).
The most recent results presented at this year’s annual meeting of the American Society of Clinical Oncology support earlier efficacy data, she noted. In the longest follow-up to date, researchers reported remissions lasting over 9 years in patients with relapsed/refractory B-cell lymphoma or CLL treated with Kite›s axicaptagene cilleucel (Yescarta), one of two anti-CD19-directed CAR T-cell therapies approved by the FDA in 2017 (the other is Novartis’ tisagenlecleucel [Kymriah]).
This study included 43 patients and showed an overall remission rate of 76%. Complete remission was achieved in 54% of patients, and 22% had partial remission.
The other focus is long-term safety. Although some of the long-term adverse effects are known and are manageable, others fall into the theoretical realm. In early May 2020, the NCI held a multidisciplinary virtual conference on CAR T-cell therapy «to encourage collaborative research about the subacute and potentially long-term toxicity profile of these treatments.»
“We know just a little at this point about late- and long-term effects of CAR-T therapy, because we are relatively early in the era of CAR T cells,” said Merav Bar, MD, from the Fred Hutchinson Cancer Research Center in Seattle, Washington.
B-cell aplasia and risk for new infections
What is known is that B-cell aplasia represents the most common long-term adverse effect of CAR T-cell therapy. B-cell aplasia results when anti-CD19 CAR-T therapy wipes out healthy B cells as well as the malignant ones responsible for leukemia/lymphoma.
As major players in the immune system, B cells are a key defense against viruses. So B-cell aplasia represents a very specific type of immunosuppression. It is generally less severe than immunosuppression that occurs after organ transplant, which hits the immune system pretty much across the board and carries a much higher risk for infection.
The main concern is what happens when someone with B-cell aplasia encounters a new pathogen, such as SARS-CoV-2.
After infection, B cells generate memory cells, which are not killed off by anti-CD19 therapy and that stick around for life. So a patient such as Olson would still make antibodies that fight infections they experienced before receiving CAR-T therapy, such as childhood chickenpox. But now they are unable to make new memory cells, so these patients receive monthly immunoglobulin infusions to protect against pathogens they have not previously encountered.
Olson takes this in stride and says he isn’t overly worried about COVID-19. He follows the recommended precautions for a man his age. He wears a mask, washes his hands frequently, and tries to maintain social distancing. But he doesn’t stay locked up in his New Hampshire home.
“I took the attitude when I was diagnosed with cancer that I’m going to live my life,” he said. “Quality of life to me is more important than quantity.”
Neuropsychiatric toxicity
Another problem is the possibility of neuropsychiatric toxicity. Past studies have reported a wide range of such toxicities associated with CAR T-cell therapy, including seizures and hallucinations. Most have occurred early in the course of treatment and appear to be short-lived and reversible. However, there remain questions about long-term neuropsychiatric problems.
In a long-term study of 40 patients with relapsed/refractory CLL, non-Hodgkin lymphoma, and ALL, nearly half of patients (47.5%, 19/40) self-reported at least one clinically meaningful negative neuropsychiatric outcome (anxiety, depression, or cognitive difficulty) 1 to 5 years after anti-CD19 CAR T-cell therapy. In addition, 37.5% (15/40) self-reported cognitive difficulties.
“Patients with more severe neurotoxicity showed a trend for more cognitive difficulties afterwards,» said Bar, senior author of the study.
However, teasing out the role that CAR T-cell therapy plays in these problems poses a challenge. All of these patients had been heavily pretreated with previous cancer therapy, which has also been associated with neuropsychiatric problems.
“So far, we don’t know what caused it,” Bar said. “Nevertheless, people need to pay attention to neuropsychiatric symptoms in CAR T-cell therapy. It is important to continue to monitor these patients for these issues.”
Graft-vs-host disease
Another potential problem is graft-vs-host disease (GVHD). This is not uncommon after hematopoietic stem cell transplants. It develops when the donor T cells view antigens on healthy recipient cells as foreign and attack them.
For patients who are treated with CAR T cells, GVHD is mostly a concern among individuals who have previously had a transplant and who are already at increased risk for it.
In a study of late effects among 86 adults treated with anti-CD19 CAR T cells for relapsed/refractory non-Hodgkin lymphoma, Bar and colleagues found that GVHD occurred only among patients who had received a previous donor stem cell transplant. Of these, 20% (3/15) developed GVHD about 28 months after CAR-T therapy.
“The data for CAR T cells causing GVHD really hasn’t shown that it’s a huge problem, although we have seen it and are continuing to monitor for it,” the NCI’s Shah commented to Medscape Medical News.
Other Long-term Adverse Effects
A range of other long-term adverse effects have been reported with CAR-T therapy, including prolonged cytopenias (reduced mature blood cells), myelodysplasia (bone marrow failure), and second malignancies.
In the study with the longest follow-up to date, 16% (7/43) of patients developed second malignancies, which is comparable to data from Bar’s study in Seattle (15%, 13/86). The researchers in this study consider this rate to be no higher than expected: these patients had already received extensive chemotherapy, which increases the risk for other cancers, they point out.
However, this brings up theoretical concerns about the long-term effects of gene modification. CAR T cells are engineered using retroviruses (mainly lentiviruses), which randomly insert the CAR genes into the host genome. Doing so may cause mutations that could promote cancer. These lentiviruses also carry the theoretical risk of becoming capable of viral replication once inside the body.
To address these concerns, viruses used to engineer CAR T cells go through comprehensive safety testing. After therapy, patients are checked every few months during the first year and annually after that.
So far, there have been no reports of cancers associated with CAR T-cell therapy.
“Any type of cancer is a very theoretical risk,” Bar told Medscape Medical News. «Most likely the malignancies in our study were related to prior treatment that the patients received. None of them had any evidence of replication-competent lentivirus, or any other evidence that the malignancies were related to the CAR T cells.»
Another theoretical concern is the possibility of new-onset autoimmune disease, although, once again, no cases have been reported so far.
“We think of it as a theoretic possibility. Whenever you jack up the immune system, autoimmune disease is a potential risk,” said Carl June, MD, director of the Center for Cellular Immunotherapies at the University of Pennsylvania.
June was the co–principal investigator of the trial in which Olson participated. He is also the inventor on patents for CAR T cells licensed by the University of Pennsylvania to Novartis and Tmunity and is a scientific founder with equity in Tmunity.
Still, autoimmunity could occur, and scientists are looking out for it.
“We are continuing to be vigilant in our monitoring for autoimmune disease,” Shah added. “We’ve been doing CAR T-cell therapy since 2012, and I think we have yet to see true autoimmunity beyond GVHD.”
Future directions
In the 10 years since Olson received CAR T-cell therapy, an entire industry has sprung up. Over 100 companies worldwide are now developing CAR T-cell therapies targeting various antigens. These therapies are directed at about 60 different tumor types, including solid tumors. Nearly 200 clinical trials are underway, though most are still in early stages: as of September 2019, only 5% had reached phase 3.
Clinical data show promising results for CAR T-cell therapy directed against CD22 (overexpressed on ALL cells), and BCMA (found on almost all multiple myeloma cells). Yet questions remain as to whether CAR T cells will be as effective if they target antigens other than CD19 or cells other than B lymphocytes. One of the biggest research questions is whether they will be effective against solid tumors.
One research avenue being watched with great interest is the development of universal CAR T cells. So far, such products are at very early stages of development (phase 1 trials), but they are attractive because of the potential advantages they offer over bespoke CAR T cells. Automating the process holds the promise of immediate availability, standardizing production, expanding access, and lowering costs. And because the T cells for this universal product come from healthy donors, they may function better than T cells that have been battered and bruised by past cancer treatments, or even the cancer itself.
However, precisely because they are developed from healthy donor T cells, universal CAR T cells may pose increased risk for GVHD. Scientists are trying to get around this problem by engineering universal CAR T cells that lack the T-cell receptor involved in GVHD.
There are also other concerns. Nature has a penchant for mutation. Engineering CAR T cells without T-cell receptors means the body may no longer detect or reject a universal CAR T cell if it goes rogue. Also, gene insertion in universal CAR-T therapy is targeted rather than random (as in bespoke CAR T cells), which could create off-target effects. Both issues create a theoretical risk of such products inducing an untreatable CAR T-cell therapy–associated cancer.
“The theoretic risk with universal cells is that their safety profile may not be as good for long term,” June commented.
Hope for the future
From that first trial in which June and Porter used CAR T cells, two of three patients they treated are still alive 10 years later.
Olson is one of these two, and he still undergoes monitoring every 3 months to check for relapse. So far, none of his tests have shown signs of his cancer returning.
After going into remission, Doug spent the next 6 to 9 months regaining his health and strength.
“I figured if I had this amazing treatment that saved my life, I had an obligation to stay alive,” he said. “I’d better not die of something like a heart attack!”
He took up long distance running and has completed six half marathons. He became involved in the Leukemia and Lymphoma Society, participating in fund-raising and helping newly diagnosed patients. Over the years, he has also given talks for researchers, people with cancer, and healthcare providers.
Doug is now 73. Today, he marvels at how rapidly the CAR-T field has progressed.
“Twenty years ago, if you had cancer, your prospects weren’t nearly as good as these days. In 2010, people still didn’t believe in CAR T-cell therapy,” he said. “My goal always in telling my story is a message of hope.”
This article first appeared on Medscape.com.
When a patient with cancer hears there isn’t much left that doctors can do, it always stays fresh in the mind.
Doug Olson was first diagnosed with chronic lymphocytic leukemia (CLL) over 20 years ago, in 1996. For several years, his doctors used the watch-and-wait approach. But then his cancer progressed and needed treatment. By 2010, it had mutated so much that it no longer responded to standard therapy.
He was rapidly running out of options. Back then, the only treatment left was a bone marrow transplant. Without one, his doctors said, he would have 1 or 2 years left to live.
“I was really trying to avoid a bone marrow transplant. You’re playing your last card if that doesn’t work. It’s a pretty rough procedure,” Olson told Medscape Medical News.
Looking back, Olson counts himself as lucky – for being in the right place, at the right time, with the right doctor. His oncologist was David Porter, MD, the principal investigator on a trial at the University of Pennsylvania that was investigating a brand new approach to treating cancer: chimeric antigen receptor (CAR) T-cell therapy.
CAR T-cell therapy uses a patient’s own T cells engineered to express a receptor that targets proteins on cancer cells. CAR T cells are considered “living drugs” because they expand inside the body and stick around for years – maybe for a lifetime – to fight the cancer if it tries to come back.
“I was certainly intrigued by the approach. It had worked in mice, and it was the sort of thing that looked like it would work,” Olson recalled.
Science is not a foreign language to Olson. He holds a PhD in medicinal chemistry, spent most of his career in the in vitro diagnostics industry, and currently acts as chief executive officer of Buhlmann Diagnostics Corp.
So he read the clinical protocol for the first in-human trial of CAR T cells and agreed to become patient number two.
Olson’s T cells were harvested, engineered to attack the CD19 antigen found on malignant and normal B lymphocytes, and then were expanded into millions in the lab. After undergoing preconditioning with chemotherapy to minimize rejection and boost the CAR T cells’ expansion inside the body, he received several infusions of the new therapy over the course of 3 days.
Nothing really happened for 2 weeks. Then he developed severe flu-like symptoms – so bad that he was hospitalized.
Ironically, getting sick was a sign that the CAR T cells were working. Olson was experiencing one of the main short-term effects of CAR T-cell therapy: cytokine release syndrome. Symptoms include extremely high fevers and dangerous drops in blood pressure that can potentially cause end-organ damage.
In the early trials of these products, some patients experienced such a severe reaction that they needed intensive care, and some died. With increasing clinical experience, doctors have learned to control the reaction with the use of steroids and interleukein-6 inhibitors such as tocilizumab (Actemra).
Fortunately for Olson, the reaction passed, and he was eventually discharged.
Then the “aha moment” happened. Four weeks after receiving the CAR T cells, Olson found out that he was cancer free.
“It still gives me shivers,” he said. “Dr Porter said, ‘Your bone marrow’s completely free. We just can’t find a cancer cell anywhere.’ “
The remission has lasted, and it is now 10 years later.
Balancing long-term risks vs benefits
Long-term data have been accumulating for these novel therapies since Olson’s treatment in 2010. This is particularly important for CAR T-cell therapy, because of its longevity. Because these are living cells and are expected to persist in the body for years, there is great interest in longer-term data, especially the risks for toxicity.
The FDA requires clinical follow-up for at least 15 years for patients treated with CAR T-cell therapy or any other genetically modified cells.
So far, most of the experience with CAR T cells comes from anti-CD19-directed therapy, which has shown “remarkable” remission rates in the 50% to 85% range, said Nirali Shah, MD, head of the hematologic malignancies section of the Pediatric Oncology Branch at the National Cancer Institute (NCI).
The most recent results presented at this year’s annual meeting of the American Society of Clinical Oncology support earlier efficacy data, she noted. In the longest follow-up to date, researchers reported remissions lasting over 9 years in patients with relapsed/refractory B-cell lymphoma or CLL treated with Kite›s axicaptagene cilleucel (Yescarta), one of two anti-CD19-directed CAR T-cell therapies approved by the FDA in 2017 (the other is Novartis’ tisagenlecleucel [Kymriah]).
This study included 43 patients and showed an overall remission rate of 76%. Complete remission was achieved in 54% of patients, and 22% had partial remission.
The other focus is long-term safety. Although some of the long-term adverse effects are known and are manageable, others fall into the theoretical realm. In early May 2020, the NCI held a multidisciplinary virtual conference on CAR T-cell therapy «to encourage collaborative research about the subacute and potentially long-term toxicity profile of these treatments.»
“We know just a little at this point about late- and long-term effects of CAR-T therapy, because we are relatively early in the era of CAR T cells,” said Merav Bar, MD, from the Fred Hutchinson Cancer Research Center in Seattle, Washington.
B-cell aplasia and risk for new infections
What is known is that B-cell aplasia represents the most common long-term adverse effect of CAR T-cell therapy. B-cell aplasia results when anti-CD19 CAR-T therapy wipes out healthy B cells as well as the malignant ones responsible for leukemia/lymphoma.
As major players in the immune system, B cells are a key defense against viruses. So B-cell aplasia represents a very specific type of immunosuppression. It is generally less severe than immunosuppression that occurs after organ transplant, which hits the immune system pretty much across the board and carries a much higher risk for infection.
The main concern is what happens when someone with B-cell aplasia encounters a new pathogen, such as SARS-CoV-2.
After infection, B cells generate memory cells, which are not killed off by anti-CD19 therapy and that stick around for life. So a patient such as Olson would still make antibodies that fight infections they experienced before receiving CAR-T therapy, such as childhood chickenpox. But now they are unable to make new memory cells, so these patients receive monthly immunoglobulin infusions to protect against pathogens they have not previously encountered.
Olson takes this in stride and says he isn’t overly worried about COVID-19. He follows the recommended precautions for a man his age. He wears a mask, washes his hands frequently, and tries to maintain social distancing. But he doesn’t stay locked up in his New Hampshire home.
“I took the attitude when I was diagnosed with cancer that I’m going to live my life,” he said. “Quality of life to me is more important than quantity.”
Neuropsychiatric toxicity
Another problem is the possibility of neuropsychiatric toxicity. Past studies have reported a wide range of such toxicities associated with CAR T-cell therapy, including seizures and hallucinations. Most have occurred early in the course of treatment and appear to be short-lived and reversible. However, there remain questions about long-term neuropsychiatric problems.
In a long-term study of 40 patients with relapsed/refractory CLL, non-Hodgkin lymphoma, and ALL, nearly half of patients (47.5%, 19/40) self-reported at least one clinically meaningful negative neuropsychiatric outcome (anxiety, depression, or cognitive difficulty) 1 to 5 years after anti-CD19 CAR T-cell therapy. In addition, 37.5% (15/40) self-reported cognitive difficulties.
“Patients with more severe neurotoxicity showed a trend for more cognitive difficulties afterwards,» said Bar, senior author of the study.
However, teasing out the role that CAR T-cell therapy plays in these problems poses a challenge. All of these patients had been heavily pretreated with previous cancer therapy, which has also been associated with neuropsychiatric problems.
“So far, we don’t know what caused it,” Bar said. “Nevertheless, people need to pay attention to neuropsychiatric symptoms in CAR T-cell therapy. It is important to continue to monitor these patients for these issues.”
Graft-vs-host disease
Another potential problem is graft-vs-host disease (GVHD). This is not uncommon after hematopoietic stem cell transplants. It develops when the donor T cells view antigens on healthy recipient cells as foreign and attack them.
For patients who are treated with CAR T cells, GVHD is mostly a concern among individuals who have previously had a transplant and who are already at increased risk for it.
In a study of late effects among 86 adults treated with anti-CD19 CAR T cells for relapsed/refractory non-Hodgkin lymphoma, Bar and colleagues found that GVHD occurred only among patients who had received a previous donor stem cell transplant. Of these, 20% (3/15) developed GVHD about 28 months after CAR-T therapy.
“The data for CAR T cells causing GVHD really hasn’t shown that it’s a huge problem, although we have seen it and are continuing to monitor for it,” the NCI’s Shah commented to Medscape Medical News.
Other Long-term Adverse Effects
A range of other long-term adverse effects have been reported with CAR-T therapy, including prolonged cytopenias (reduced mature blood cells), myelodysplasia (bone marrow failure), and second malignancies.
In the study with the longest follow-up to date, 16% (7/43) of patients developed second malignancies, which is comparable to data from Bar’s study in Seattle (15%, 13/86). The researchers in this study consider this rate to be no higher than expected: these patients had already received extensive chemotherapy, which increases the risk for other cancers, they point out.
However, this brings up theoretical concerns about the long-term effects of gene modification. CAR T cells are engineered using retroviruses (mainly lentiviruses), which randomly insert the CAR genes into the host genome. Doing so may cause mutations that could promote cancer. These lentiviruses also carry the theoretical risk of becoming capable of viral replication once inside the body.
To address these concerns, viruses used to engineer CAR T cells go through comprehensive safety testing. After therapy, patients are checked every few months during the first year and annually after that.
So far, there have been no reports of cancers associated with CAR T-cell therapy.
“Any type of cancer is a very theoretical risk,” Bar told Medscape Medical News. «Most likely the malignancies in our study were related to prior treatment that the patients received. None of them had any evidence of replication-competent lentivirus, or any other evidence that the malignancies were related to the CAR T cells.»
Another theoretical concern is the possibility of new-onset autoimmune disease, although, once again, no cases have been reported so far.
“We think of it as a theoretic possibility. Whenever you jack up the immune system, autoimmune disease is a potential risk,” said Carl June, MD, director of the Center for Cellular Immunotherapies at the University of Pennsylvania.
June was the co–principal investigator of the trial in which Olson participated. He is also the inventor on patents for CAR T cells licensed by the University of Pennsylvania to Novartis and Tmunity and is a scientific founder with equity in Tmunity.
Still, autoimmunity could occur, and scientists are looking out for it.
“We are continuing to be vigilant in our monitoring for autoimmune disease,” Shah added. “We’ve been doing CAR T-cell therapy since 2012, and I think we have yet to see true autoimmunity beyond GVHD.”
Future directions
In the 10 years since Olson received CAR T-cell therapy, an entire industry has sprung up. Over 100 companies worldwide are now developing CAR T-cell therapies targeting various antigens. These therapies are directed at about 60 different tumor types, including solid tumors. Nearly 200 clinical trials are underway, though most are still in early stages: as of September 2019, only 5% had reached phase 3.
Clinical data show promising results for CAR T-cell therapy directed against CD22 (overexpressed on ALL cells), and BCMA (found on almost all multiple myeloma cells). Yet questions remain as to whether CAR T cells will be as effective if they target antigens other than CD19 or cells other than B lymphocytes. One of the biggest research questions is whether they will be effective against solid tumors.
One research avenue being watched with great interest is the development of universal CAR T cells. So far, such products are at very early stages of development (phase 1 trials), but they are attractive because of the potential advantages they offer over bespoke CAR T cells. Automating the process holds the promise of immediate availability, standardizing production, expanding access, and lowering costs. And because the T cells for this universal product come from healthy donors, they may function better than T cells that have been battered and bruised by past cancer treatments, or even the cancer itself.
However, precisely because they are developed from healthy donor T cells, universal CAR T cells may pose increased risk for GVHD. Scientists are trying to get around this problem by engineering universal CAR T cells that lack the T-cell receptor involved in GVHD.
There are also other concerns. Nature has a penchant for mutation. Engineering CAR T cells without T-cell receptors means the body may no longer detect or reject a universal CAR T cell if it goes rogue. Also, gene insertion in universal CAR-T therapy is targeted rather than random (as in bespoke CAR T cells), which could create off-target effects. Both issues create a theoretical risk of such products inducing an untreatable CAR T-cell therapy–associated cancer.
“The theoretic risk with universal cells is that their safety profile may not be as good for long term,” June commented.
Hope for the future
From that first trial in which June and Porter used CAR T cells, two of three patients they treated are still alive 10 years later.
Olson is one of these two, and he still undergoes monitoring every 3 months to check for relapse. So far, none of his tests have shown signs of his cancer returning.
After going into remission, Doug spent the next 6 to 9 months regaining his health and strength.
“I figured if I had this amazing treatment that saved my life, I had an obligation to stay alive,” he said. “I’d better not die of something like a heart attack!”
He took up long distance running and has completed six half marathons. He became involved in the Leukemia and Lymphoma Society, participating in fund-raising and helping newly diagnosed patients. Over the years, he has also given talks for researchers, people with cancer, and healthcare providers.
Doug is now 73. Today, he marvels at how rapidly the CAR-T field has progressed.
“Twenty years ago, if you had cancer, your prospects weren’t nearly as good as these days. In 2010, people still didn’t believe in CAR T-cell therapy,” he said. “My goal always in telling my story is a message of hope.”
This article first appeared on Medscape.com.
When a patient with cancer hears there isn’t much left that doctors can do, it always stays fresh in the mind.
Doug Olson was first diagnosed with chronic lymphocytic leukemia (CLL) over 20 years ago, in 1996. For several years, his doctors used the watch-and-wait approach. But then his cancer progressed and needed treatment. By 2010, it had mutated so much that it no longer responded to standard therapy.
He was rapidly running out of options. Back then, the only treatment left was a bone marrow transplant. Without one, his doctors said, he would have 1 or 2 years left to live.
“I was really trying to avoid a bone marrow transplant. You’re playing your last card if that doesn’t work. It’s a pretty rough procedure,” Olson told Medscape Medical News.
Looking back, Olson counts himself as lucky – for being in the right place, at the right time, with the right doctor. His oncologist was David Porter, MD, the principal investigator on a trial at the University of Pennsylvania that was investigating a brand new approach to treating cancer: chimeric antigen receptor (CAR) T-cell therapy.
CAR T-cell therapy uses a patient’s own T cells engineered to express a receptor that targets proteins on cancer cells. CAR T cells are considered “living drugs” because they expand inside the body and stick around for years – maybe for a lifetime – to fight the cancer if it tries to come back.
“I was certainly intrigued by the approach. It had worked in mice, and it was the sort of thing that looked like it would work,” Olson recalled.
Science is not a foreign language to Olson. He holds a PhD in medicinal chemistry, spent most of his career in the in vitro diagnostics industry, and currently acts as chief executive officer of Buhlmann Diagnostics Corp.
So he read the clinical protocol for the first in-human trial of CAR T cells and agreed to become patient number two.
Olson’s T cells were harvested, engineered to attack the CD19 antigen found on malignant and normal B lymphocytes, and then were expanded into millions in the lab. After undergoing preconditioning with chemotherapy to minimize rejection and boost the CAR T cells’ expansion inside the body, he received several infusions of the new therapy over the course of 3 days.
Nothing really happened for 2 weeks. Then he developed severe flu-like symptoms – so bad that he was hospitalized.
Ironically, getting sick was a sign that the CAR T cells were working. Olson was experiencing one of the main short-term effects of CAR T-cell therapy: cytokine release syndrome. Symptoms include extremely high fevers and dangerous drops in blood pressure that can potentially cause end-organ damage.
In the early trials of these products, some patients experienced such a severe reaction that they needed intensive care, and some died. With increasing clinical experience, doctors have learned to control the reaction with the use of steroids and interleukein-6 inhibitors such as tocilizumab (Actemra).
Fortunately for Olson, the reaction passed, and he was eventually discharged.
Then the “aha moment” happened. Four weeks after receiving the CAR T cells, Olson found out that he was cancer free.
“It still gives me shivers,” he said. “Dr Porter said, ‘Your bone marrow’s completely free. We just can’t find a cancer cell anywhere.’ “
The remission has lasted, and it is now 10 years later.
Balancing long-term risks vs benefits
Long-term data have been accumulating for these novel therapies since Olson’s treatment in 2010. This is particularly important for CAR T-cell therapy, because of its longevity. Because these are living cells and are expected to persist in the body for years, there is great interest in longer-term data, especially the risks for toxicity.
The FDA requires clinical follow-up for at least 15 years for patients treated with CAR T-cell therapy or any other genetically modified cells.
So far, most of the experience with CAR T cells comes from anti-CD19-directed therapy, which has shown “remarkable” remission rates in the 50% to 85% range, said Nirali Shah, MD, head of the hematologic malignancies section of the Pediatric Oncology Branch at the National Cancer Institute (NCI).
The most recent results presented at this year’s annual meeting of the American Society of Clinical Oncology support earlier efficacy data, she noted. In the longest follow-up to date, researchers reported remissions lasting over 9 years in patients with relapsed/refractory B-cell lymphoma or CLL treated with Kite›s axicaptagene cilleucel (Yescarta), one of two anti-CD19-directed CAR T-cell therapies approved by the FDA in 2017 (the other is Novartis’ tisagenlecleucel [Kymriah]).
This study included 43 patients and showed an overall remission rate of 76%. Complete remission was achieved in 54% of patients, and 22% had partial remission.
The other focus is long-term safety. Although some of the long-term adverse effects are known and are manageable, others fall into the theoretical realm. In early May 2020, the NCI held a multidisciplinary virtual conference on CAR T-cell therapy «to encourage collaborative research about the subacute and potentially long-term toxicity profile of these treatments.»
“We know just a little at this point about late- and long-term effects of CAR-T therapy, because we are relatively early in the era of CAR T cells,” said Merav Bar, MD, from the Fred Hutchinson Cancer Research Center in Seattle, Washington.
B-cell aplasia and risk for new infections
What is known is that B-cell aplasia represents the most common long-term adverse effect of CAR T-cell therapy. B-cell aplasia results when anti-CD19 CAR-T therapy wipes out healthy B cells as well as the malignant ones responsible for leukemia/lymphoma.
As major players in the immune system, B cells are a key defense against viruses. So B-cell aplasia represents a very specific type of immunosuppression. It is generally less severe than immunosuppression that occurs after organ transplant, which hits the immune system pretty much across the board and carries a much higher risk for infection.
The main concern is what happens when someone with B-cell aplasia encounters a new pathogen, such as SARS-CoV-2.
After infection, B cells generate memory cells, which are not killed off by anti-CD19 therapy and that stick around for life. So a patient such as Olson would still make antibodies that fight infections they experienced before receiving CAR-T therapy, such as childhood chickenpox. But now they are unable to make new memory cells, so these patients receive monthly immunoglobulin infusions to protect against pathogens they have not previously encountered.
Olson takes this in stride and says he isn’t overly worried about COVID-19. He follows the recommended precautions for a man his age. He wears a mask, washes his hands frequently, and tries to maintain social distancing. But he doesn’t stay locked up in his New Hampshire home.
“I took the attitude when I was diagnosed with cancer that I’m going to live my life,” he said. “Quality of life to me is more important than quantity.”
Neuropsychiatric toxicity
Another problem is the possibility of neuropsychiatric toxicity. Past studies have reported a wide range of such toxicities associated with CAR T-cell therapy, including seizures and hallucinations. Most have occurred early in the course of treatment and appear to be short-lived and reversible. However, there remain questions about long-term neuropsychiatric problems.
In a long-term study of 40 patients with relapsed/refractory CLL, non-Hodgkin lymphoma, and ALL, nearly half of patients (47.5%, 19/40) self-reported at least one clinically meaningful negative neuropsychiatric outcome (anxiety, depression, or cognitive difficulty) 1 to 5 years after anti-CD19 CAR T-cell therapy. In addition, 37.5% (15/40) self-reported cognitive difficulties.
“Patients with more severe neurotoxicity showed a trend for more cognitive difficulties afterwards,» said Bar, senior author of the study.
However, teasing out the role that CAR T-cell therapy plays in these problems poses a challenge. All of these patients had been heavily pretreated with previous cancer therapy, which has also been associated with neuropsychiatric problems.
“So far, we don’t know what caused it,” Bar said. “Nevertheless, people need to pay attention to neuropsychiatric symptoms in CAR T-cell therapy. It is important to continue to monitor these patients for these issues.”
Graft-vs-host disease
Another potential problem is graft-vs-host disease (GVHD). This is not uncommon after hematopoietic stem cell transplants. It develops when the donor T cells view antigens on healthy recipient cells as foreign and attack them.
For patients who are treated with CAR T cells, GVHD is mostly a concern among individuals who have previously had a transplant and who are already at increased risk for it.
In a study of late effects among 86 adults treated with anti-CD19 CAR T cells for relapsed/refractory non-Hodgkin lymphoma, Bar and colleagues found that GVHD occurred only among patients who had received a previous donor stem cell transplant. Of these, 20% (3/15) developed GVHD about 28 months after CAR-T therapy.
“The data for CAR T cells causing GVHD really hasn’t shown that it’s a huge problem, although we have seen it and are continuing to monitor for it,” the NCI’s Shah commented to Medscape Medical News.
Other Long-term Adverse Effects
A range of other long-term adverse effects have been reported with CAR-T therapy, including prolonged cytopenias (reduced mature blood cells), myelodysplasia (bone marrow failure), and second malignancies.
In the study with the longest follow-up to date, 16% (7/43) of patients developed second malignancies, which is comparable to data from Bar’s study in Seattle (15%, 13/86). The researchers in this study consider this rate to be no higher than expected: these patients had already received extensive chemotherapy, which increases the risk for other cancers, they point out.
However, this brings up theoretical concerns about the long-term effects of gene modification. CAR T cells are engineered using retroviruses (mainly lentiviruses), which randomly insert the CAR genes into the host genome. Doing so may cause mutations that could promote cancer. These lentiviruses also carry the theoretical risk of becoming capable of viral replication once inside the body.
To address these concerns, viruses used to engineer CAR T cells go through comprehensive safety testing. After therapy, patients are checked every few months during the first year and annually after that.
So far, there have been no reports of cancers associated with CAR T-cell therapy.
“Any type of cancer is a very theoretical risk,” Bar told Medscape Medical News. «Most likely the malignancies in our study were related to prior treatment that the patients received. None of them had any evidence of replication-competent lentivirus, or any other evidence that the malignancies were related to the CAR T cells.»
Another theoretical concern is the possibility of new-onset autoimmune disease, although, once again, no cases have been reported so far.
“We think of it as a theoretic possibility. Whenever you jack up the immune system, autoimmune disease is a potential risk,” said Carl June, MD, director of the Center for Cellular Immunotherapies at the University of Pennsylvania.
June was the co–principal investigator of the trial in which Olson participated. He is also the inventor on patents for CAR T cells licensed by the University of Pennsylvania to Novartis and Tmunity and is a scientific founder with equity in Tmunity.
Still, autoimmunity could occur, and scientists are looking out for it.
“We are continuing to be vigilant in our monitoring for autoimmune disease,” Shah added. “We’ve been doing CAR T-cell therapy since 2012, and I think we have yet to see true autoimmunity beyond GVHD.”
Future directions
In the 10 years since Olson received CAR T-cell therapy, an entire industry has sprung up. Over 100 companies worldwide are now developing CAR T-cell therapies targeting various antigens. These therapies are directed at about 60 different tumor types, including solid tumors. Nearly 200 clinical trials are underway, though most are still in early stages: as of September 2019, only 5% had reached phase 3.
Clinical data show promising results for CAR T-cell therapy directed against CD22 (overexpressed on ALL cells), and BCMA (found on almost all multiple myeloma cells). Yet questions remain as to whether CAR T cells will be as effective if they target antigens other than CD19 or cells other than B lymphocytes. One of the biggest research questions is whether they will be effective against solid tumors.
One research avenue being watched with great interest is the development of universal CAR T cells. So far, such products are at very early stages of development (phase 1 trials), but they are attractive because of the potential advantages they offer over bespoke CAR T cells. Automating the process holds the promise of immediate availability, standardizing production, expanding access, and lowering costs. And because the T cells for this universal product come from healthy donors, they may function better than T cells that have been battered and bruised by past cancer treatments, or even the cancer itself.
However, precisely because they are developed from healthy donor T cells, universal CAR T cells may pose increased risk for GVHD. Scientists are trying to get around this problem by engineering universal CAR T cells that lack the T-cell receptor involved in GVHD.
There are also other concerns. Nature has a penchant for mutation. Engineering CAR T cells without T-cell receptors means the body may no longer detect or reject a universal CAR T cell if it goes rogue. Also, gene insertion in universal CAR-T therapy is targeted rather than random (as in bespoke CAR T cells), which could create off-target effects. Both issues create a theoretical risk of such products inducing an untreatable CAR T-cell therapy–associated cancer.
“The theoretic risk with universal cells is that their safety profile may not be as good for long term,” June commented.
Hope for the future
From that first trial in which June and Porter used CAR T cells, two of three patients they treated are still alive 10 years later.
Olson is one of these two, and he still undergoes monitoring every 3 months to check for relapse. So far, none of his tests have shown signs of his cancer returning.
After going into remission, Doug spent the next 6 to 9 months regaining his health and strength.
“I figured if I had this amazing treatment that saved my life, I had an obligation to stay alive,” he said. “I’d better not die of something like a heart attack!”
He took up long distance running and has completed six half marathons. He became involved in the Leukemia and Lymphoma Society, participating in fund-raising and helping newly diagnosed patients. Over the years, he has also given talks for researchers, people with cancer, and healthcare providers.
Doug is now 73. Today, he marvels at how rapidly the CAR-T field has progressed.
“Twenty years ago, if you had cancer, your prospects weren’t nearly as good as these days. In 2010, people still didn’t believe in CAR T-cell therapy,” he said. “My goal always in telling my story is a message of hope.”
This article first appeared on Medscape.com.
Cardiovascular risk factors tied to midlife cognitive decline
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research shows. The findings suggest that the relationship between CVRFs and cognition becomes evident much earlier than previously realized. Investigators found that individuals who smoked were 65% more likely to have accelerated cognitive decline, those with hypertension were 87% more likely, and individuals with diabetes had nearly a 200% increased risk.
“What is new here is that almost no one has looked at cardiovascular risk factors in such a young age [mean, 50 years] and cognitive change in middle age from 50 to 55 or so. Almost all other studies have looked at mid- or late-life cardiovascular risk factors and late-life cognition or dementia,” said study investigator Kristine Yaffe, MD.
The research was published online July 15 in Neurology.
New insight
Previous research has shown a strong association between CVRFs and a greater risk for cognitive decline and dementia in late life, but the investigators note that data about the influence of CVRFs on cognition in midlife are “sparse.” Longitudinal studies have also shown that several cognitive domains – particularly processing speed and executive function – may start to decline in midlife, but whether CVRFs, many of which also emerge in midlife, contribute to these changes is unclear.
To assess the effect of CVRFs on cognitive changes in midlife, the investigators analyzed data from the ongoing Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a multicenter longitudinal study designed to measure risk factors for coronary artery disease in a large cohort of Black and White men and women.
The analysis was based on data from 2,675 participants who underwent CVRF assessment and cognitive testing at baseline and 5 years later. At baseline, participants’ mean age was 50.2 years. Approximately 57% of participants were women, 55% were White, and the mean number of years of education was 15. At study outset, 43% (n = 1,133) of participants were considered obese, 31% (n = 826) had hypertension, 15% (n = 701) were current smokers, 11% (n = 290) had diabetes, and 9% (n = 248) had high cholesterol.
Cognition was assessed using the Digit Symbol Substitution Test, which measures processing speed and executive function; the Stroop Test, which measures executive function; and the Rey Auditory Verbal Learning Test, which measures verbal memory.
Dose-dependent effect
Overall results showed that, for 5% of participants, cognitive decline was accelerated at 5 years. In unadjusted models, the odds of developing accelerated cognitive decline over 5 years was associated with hypertension (7.5% vs. 4.3%; odds ratio, 1.79, 95% confidence interval, 1.27-2.52), diabetes (10.3% vs. 4.7%; OR, 2.33; 95% CI, 1.53-3.56), and smoking (7.7% current smokers vs. 4.3% never smokers; OR, 1.87; 95% CI, 1.21-2.90). After adjusting for age, sex, and race, the associations remained significant.
The researchers found no significant effect of high cholesterol (6.9% vs. 5.2%; OR, 1.35; 95% CI, 0.80-2.28) or obesity (6.1% vs. 4.8%; OR, 1.29; 95% CI, 0.92-1.82) on accelerated cognitive decline.
Compared with participants with no CVRFs, the likelihood of accelerated cognitive decline was higher for individuals with one or two risk factors (OR, 1.94; 95% CI, 1.16-3.25) and was higher still for those with three or more risk factors (OR, 3.51; 95% CI, 2.05-6.00).
The fact that there was no association between midlife cognitive decline and obesity or high cholesterol did not come as a surprise, said Dr. Yaffe. “Most studies have not shown a consistent finding with high cholesterol and later-life cognition, so it is not surprising we did not see one in midlife, when there is not as much cognitive change.”
The study’s results, said Dr. Yaffe, provide physicians with another good reason to help patients address CVRFs and to work with them to lower blood pressure, stop smoking, reduce diabetes incidence, or control diabetes.
Dr. Yaffe said she and her colleagues plan further research into CVRFs and accelerated cognitive decline. “We want to know if this earlier cognitive decline [in midlife] is connected to greater decline later in life. We also want to know if improving these risk factors in midlife might prevent or slow dementia later.”
More to explore
Commenting on the findings, Michelle M. Mielke, PhD, professor of epidemiology and neurology at Mayo Clinic, Rochester, Minn., said one of the study’s main implications “is that the prevention and treatment of midlife hypertension and diabetes and smoking cessation directly impacts shorter-term changes in cognition.”
She added that the study also provides a foundation for answering further questions about the effects of CVRFs on cognition in midlife. For example, questions about sex differences remain unanswered. Men develop CVRFs earlier than women, but the investigators did not provide the prevalence of cardiovascular risk factors by sex.
“It was also not reported whether a specific midlife cardiovascular risk factor was more strongly associated with accelerated cognitive decline for women or for men,” she said. In addition, the mean age of the population at baseline is the approximate age of the onset of menopause, after which cardiovascular risk factors increase among women.
“Additional research is needed to understand the emergence of cardiovascular risk factors pre- versus post menopause on subsequent cognition and also consider the use of menopausal hormone therapy,” said Dr. Mielke.
“Another future research avenue is to further understand the impact of antihypertensive and diabetes medications,” she continued. “For example, in the current study, it was not clear how many [participants] with hypertension were treated versus untreated and whether this impacted subsequent cognition. Similarly, it is not known whether specific antihypertensives are more beneficial for cognition in midlife.”
CARDIA is supported by the National Heart, Lung, and Blood Institute; the University of Alabama at Birmingham; Northwestern University, Chicago; the University of Minnesota; and the Kaiser Foundation Research Institute. Dr. Yaffe serves on data safety monitoring boards for Eli Lilly and studies sponsored by the National Institute on Aging. She is a board member of Alector and is a member of the Beeson Scientific Advisory Board and the Global Council on Brain Health. Dr. Mielke has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Early data support further study of ivosidenib in mIDH1 glioma
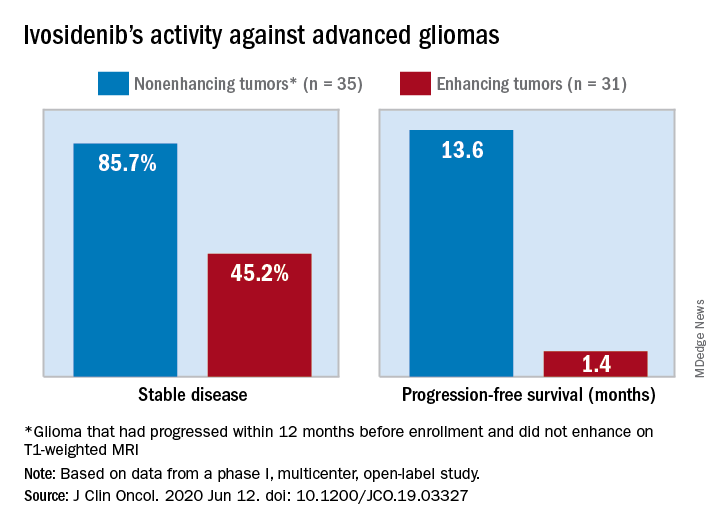
The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327

The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327

The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Still no clear answer on intranasal insulin for MCI and Alzheimer’s disease
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
The randomized trial of nearly 300 patients showed that, although one insulin administration device produced marked benefit in terms of change in mean score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12) over 12 months, reliability was inconsistent. A second device, used on the majority of patients in the study’s intention-to-treat population, showed no difference in these measures between patients who did and those who did not receive intranasal insulin.
“The primary analysis of the study showed no benefit of intranasal insulin on any measures of cognition or cerebrospinal fluid Alzheimer’s disease biomarkers when using the new device,” said principal investigator Suzanne Craft, PhD.
“But when we looked at our planned secondary analysis with the original device – which has been successful in previous studies – we saw quite a different picture,” added Dr. Craft, director of the Alzheimer’s Disease Research Center at Wake Forest University, Winston-Salem, N.C.
“We found a pronounced benefit with that device, such that after 18 months of administration, participants who had been receiving insulin from the beginning of the study had a large and clinically significant advantage in the primary outcome measure.”
Dr. Craft described the findings as complex. “The primary results were negative,” she added. “But the secondary results replicated those of several earlier studies when we used the same device that was used in those.”
The study was published online June 22 in JAMA Neurology.
Important for brain function
Insulin has been shown to play several important roles in brain function. The hormone is associated with a variety of cognitive functions, including memory. Through its association with vasoreactivity, lipid metabolism, and inflammation, insulin also plays an important role in vascular function.
“In the normal brain in healthy individuals, insulin is very important for synaptic function and viability. Insulin also promotes dendritic growth and facilitates synaptic health. Through this role, it plays an important part in memory,” said Dr. Craft. Given these connections, it is not surprising that reduced insulin levels or activity in brain and cerebrospinal fluid have been documented in some, but not all, studies of Alzheimer’s disease. Markers of insulin resistance also have been detected in both neuronally derived exosomes and brain tissue from adults with Alzheimer’s disease.
In light of the several important roles that insulin plays in the brain – coupled with the evidence connecting dysregulation of brain insulin and AD pathology – restoring brain insulin function may offer therapeutic benefit for adults suffering either Alzheimer’s disease or MCI. “There are a number of ways to do this,” said Dr. Craft. “But one of the approaches that we’ve focused on is providing insulin directly to the brain through intranasal administration. “By doing this, you circumvent potential issues if you administered insulin systemically.”
Previous research has shown that through this mode of administration, insulin can bypass the blood-brain barrier and reach the brain through olfactory and trigeminal perivascular channels, with little effect on peripheral insulin or blood glucose levels.
As previously reported, an earlier pilot study, also conducted by Dr. Craft and her team, showed that 4 months of daily intranasal administration of 20 IU or 40 IU of insulin preserved cognitive performance in individuals with Alzheimer’s disease or MCI.
Deeper dive
In the current investigation, the researchers wanted to broaden these findings in a larger, longer, randomized double-blinded clinical trial. The investigators assessed the efficacy of intranasal insulin on cognition, function, and biomarkers of Alzheimer’s disease, as well as the safety and feasibility of the delivery method. The multicenter trial was conducted from 2014 to 2018 and included 27 sites.
Study participants were between the ages of 55 and 85 years and had been diagnosed with amnestic MCI or Alzheimer’s disease on the basis of National Institute on Aging–Alzheimer Association criteria, a score of 20 or higher on the Mini–Mental State Examination, a clinical dementia rating of 0.5 or 1.0, or a delayed logical memory score within a specified range.
In total, 289 participants were randomly assigned to receive 40 IU of insulin or placebo for 12 months, followed by a 6-month open-label extension phase. The first 49 participants (32 men; mean age, 71.9 years) underwent insulin administration with the same device the investigators used in previous trials.
Of these, 45 completed the blinded phase, and 42 completed the open-label extension. When this device, which uses an electronic nebulizer-like delivery system, proved unreliable, the researchers switched to a second device, which uses a liquid hydrofluoroalkane propellant to deliver a metered dose of insulin through a nose tip without electronic assistance. Device 2 was used for the remaining 240 participants (123 men; mean age, 70.8 years). These patients became the study’s primary intention-to-treat population.
The study’s primary outcome was the mean change in score on the Alzheimer Disease Assessment Scale–Cognitive Subscale 12 (ADAS-cog-12), which was evaluated at 3-month intervals.
Secondary clinical outcomes were assessed at 6-month intervals. These included the mean change in scores for the Alzheimer Disease Cooperative Study Activities of Daily Living Scale for Mild Cognitive Impairment and the Clinical Dementia Rating Scale Sum of Boxes.
Safety and adherence were also assessed during each study visit. Physical and neurologic examinations were performed at baseline and at months 6, 12, and 18.
Of the primary intention-to-treat population of 240 patients, 121 were randomly assigned to receive intranasal insulin. The remaining 119 received placebo and served as controls. The two groups were demographically comparable.
Better cognitive performance
A total of 215 participants completed the blinded phase; 198 participants completed the open-label extension. Discontinuation rates were comparable in both arms. The researchers found no differences between groups with respect to mean change in ADAS-cog-12 score from baseline to month 12 (0.0258 points; 95% confidence interval, –1.771 to 1.822 points; P = .98). The two groups also proved comparable in terms of performance on all other cognitive tests.
The open-label portion yielded similar results. Participants originally assigned to the insulin arm and their counterparts in the placebo arm did not differ with respect to mean score change on the ADAS-cog-12 test (or any other outcome) at either month 15 or 18.
Cerebrospinal fluid insulin levels were unchanged between groups, as were blood glucose and hemoglobin A1c values. Indeed, levels of A-beta42, A-beta40, total tau protein, and tau p-181 were comparable for the patients who received intranasal insulin and those who received placebo.
The most common adverse events were infections, injuries, respiratory disorders, and nervous system disorders, though these did not differ between groups. In addition, there were no differences between groups with respect to severity of adverse events; most were rated as mild.
In contrast with the intention-to-treat population, the study’s secondary analysis – using data from the original administration device – yielded markedly different results. In the blinded phase, patients who received insulin had better ADAS-cog-12 performance at 12 months (−2.81 points; 95% CI, −6.09 to 0.45 points; P = .09) and nominally significant effects at 6 months (−3.78 points; 95% CI, −6.79 to −0.78 points; P = .01).
Device type critical
These effects persisted in the open-label analyses. Patients who received intranasal insulin had superior ADAS-cog-12 scores at month 15 (−5.70 points; 95% CI, −9.62 to −1.79 points; P = .004) and month 18 (−5.78 points; 95% CI, −10.55 to −1.01 points; P = .02), compared with their counterparts who received insulin via the second device. This part of the study also showed that, although individual biomarkers did not differ significantly between the two arms, the ratios of A-beta42 to A-beta40 (P = .01) and A-beta42 to total tau (P = .03) increased with use of the first device. The number, type, and severity of adverse events were comparable between the insulin and placebo groups in this arm of the study.
The mixed results revealed by the trial demonstrate that the device used for intranasal insulin administration is paramount in determining the therapy’s potential efficacy. “Our take-home message is that the device is a very important factor for these studies and that one needs to validate their ability to effectively deliver insulin to the CNS,” said Dr. Craft.
“We were quite confident that the first device was able to do that. On the other hand, the second device has never been tested in that way, and we still don’t know whether or not that device was able to successfully deliver insulin,” she said.
The investigators recognize the need for more research in the field. Such studies, Dr. Craft noted, will utilize administration devices that have been previously verified to have the ability to deliver insulin to the central nervous system. “We’re currently testing several devices,” she noted. “We’re using a protocol where we administer insulin with the devices and then conduct a lumbar puncture about 30 minutes later to verify that it is actually raising insulin levels in the cerebrospinal fluid.”
Not a failure
Commenting on the findings, Samuel E. Gandy, MD, PhD, who was not involved in the study, said the research illustrates the challenge when a new therapy, a new delivery device, and a cohort of cognitively impaired patients collide. “The result is not quite a slam dunk but is also by no means a failure,” commented Dr. Gandy, Mount Sinai Chair in Alzheimer’s Research at Mount Sinai Medical Center, New York.
“One looks forward to future iterations of the Craft et al. approach, wherein the trialists tweak the ligand and/or the delivery schedule and/or the device and/or the disease and/or the disease stage,” Dr. Gandy added. “Another ligand, VGF, also holds promise for intranasal delivery, based on work from Steve Salton, Michelle Ehrlich, and Eric Schadt, all from Mount Sinai. Perhaps the nose knows!”
For Dr. Craft, the potential upside of intranasal insulin for these patients is significant and warrants further investigation. “I understand why people who are not familiar with prior research in this area might be skeptical of our enthusiasm, given the results in the intention-to-treat population,” she said. “But those of us who have been working along with this for a while now, we feel like we’ve got to do the next study. But we need to have a device that we know works,” Dr. Craft added.
“If this is real, then there may be a very large clinical benefit in symptomatic patients, and there’s nothing so far that has really improved symptomatic disease.”
The study was supported by the National Institute on Aging. Eli Lilly provided diluent placebo for the blinded phase and insulin for the open-label phase of the clinical trial at no cost. Dr. Craft received grants from the National Institute on Aging and nonfinancial support from Eli Lilly during the conduct of the study and personal fees from T3D Therapeutics and vTv Therapeutics outside the submitted work.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY
HSCT or systemic treatment should be offered to HIV+ patients with lymphoma
Systemic or hematopoietic stem cell transplantation (HSCT) treatment of HIV-positive lymphoma patients resulted in improved outcomes, compared with nonsystemic treatment, according to the results of a large database study.
Researchers Thejus T. Jayakrishnan, MD, and colleagues examined patients with lymphoma diagnosed between 2004 and 2015 from the National Cancer Database. Patients were categorized as HIV positive and HIV negative. First-line lymphoma treatment was categorized as no systemic therapy reported, systemic therapy, or HSCT. Multivariate analysis was used to predict treatment and survival, according to Dr. Jayakrishnan, a resident at the department of internal medicine, Allegheny Health Network, Pittsburgh.
A total of 11,160 HIV-positive vs. 349,607 HIV-negative patients were analyzed, including mostly men, with a comorbidity index of 0. The most common lymphoma among HIV-positive patients was diffuse large B-cell lymphoma, according to the report in Clinical Lymphoma, Myeloma & Leukemia.
Among HIV-positive patients, 792 had no systemic treatment, 10,328 underwent systemic treatment, and 40 received HSCT treatment. The results showed that treatment of HIV-positive lymphoma patients resulted in improved outcomes: 3-year overall survival was 43.6% for nonsystemic treatment versus 58.1% for systemic (hazard ratio, 0.56; 95% confidence interval, 0.52-0.61; P < .005) versus 62.2% for HSCT therapy (HR, 0.42; 95% CI, 0.14-1.3; P = .08), the lack of significance in the latter could be caused in part by the small number of patients treated. Outcomes for both treatment regimens were lower, however, compared with non-HIV patients.
“The present study demonstrates improvement in survival outcomes for HIV-positive patients with lymphoma with treatments when feasible, but these outcomes are poor when compared to HIV-negative patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Jayakrishnan TT et al. Clin Lymph Myeloma Leuk. 2020 Feb 20. doi: 10.1016/j.clml.2020.06.003.
Systemic or hematopoietic stem cell transplantation (HSCT) treatment of HIV-positive lymphoma patients resulted in improved outcomes, compared with nonsystemic treatment, according to the results of a large database study.
Researchers Thejus T. Jayakrishnan, MD, and colleagues examined patients with lymphoma diagnosed between 2004 and 2015 from the National Cancer Database. Patients were categorized as HIV positive and HIV negative. First-line lymphoma treatment was categorized as no systemic therapy reported, systemic therapy, or HSCT. Multivariate analysis was used to predict treatment and survival, according to Dr. Jayakrishnan, a resident at the department of internal medicine, Allegheny Health Network, Pittsburgh.
A total of 11,160 HIV-positive vs. 349,607 HIV-negative patients were analyzed, including mostly men, with a comorbidity index of 0. The most common lymphoma among HIV-positive patients was diffuse large B-cell lymphoma, according to the report in Clinical Lymphoma, Myeloma & Leukemia.
Among HIV-positive patients, 792 had no systemic treatment, 10,328 underwent systemic treatment, and 40 received HSCT treatment. The results showed that treatment of HIV-positive lymphoma patients resulted in improved outcomes: 3-year overall survival was 43.6% for nonsystemic treatment versus 58.1% for systemic (hazard ratio, 0.56; 95% confidence interval, 0.52-0.61; P < .005) versus 62.2% for HSCT therapy (HR, 0.42; 95% CI, 0.14-1.3; P = .08), the lack of significance in the latter could be caused in part by the small number of patients treated. Outcomes for both treatment regimens were lower, however, compared with non-HIV patients.
“The present study demonstrates improvement in survival outcomes for HIV-positive patients with lymphoma with treatments when feasible, but these outcomes are poor when compared to HIV-negative patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Jayakrishnan TT et al. Clin Lymph Myeloma Leuk. 2020 Feb 20. doi: 10.1016/j.clml.2020.06.003.
Systemic or hematopoietic stem cell transplantation (HSCT) treatment of HIV-positive lymphoma patients resulted in improved outcomes, compared with nonsystemic treatment, according to the results of a large database study.
Researchers Thejus T. Jayakrishnan, MD, and colleagues examined patients with lymphoma diagnosed between 2004 and 2015 from the National Cancer Database. Patients were categorized as HIV positive and HIV negative. First-line lymphoma treatment was categorized as no systemic therapy reported, systemic therapy, or HSCT. Multivariate analysis was used to predict treatment and survival, according to Dr. Jayakrishnan, a resident at the department of internal medicine, Allegheny Health Network, Pittsburgh.
A total of 11,160 HIV-positive vs. 349,607 HIV-negative patients were analyzed, including mostly men, with a comorbidity index of 0. The most common lymphoma among HIV-positive patients was diffuse large B-cell lymphoma, according to the report in Clinical Lymphoma, Myeloma & Leukemia.
Among HIV-positive patients, 792 had no systemic treatment, 10,328 underwent systemic treatment, and 40 received HSCT treatment. The results showed that treatment of HIV-positive lymphoma patients resulted in improved outcomes: 3-year overall survival was 43.6% for nonsystemic treatment versus 58.1% for systemic (hazard ratio, 0.56; 95% confidence interval, 0.52-0.61; P < .005) versus 62.2% for HSCT therapy (HR, 0.42; 95% CI, 0.14-1.3; P = .08), the lack of significance in the latter could be caused in part by the small number of patients treated. Outcomes for both treatment regimens were lower, however, compared with non-HIV patients.
“The present study demonstrates improvement in survival outcomes for HIV-positive patients with lymphoma with treatments when feasible, but these outcomes are poor when compared to HIV-negative patients,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Jayakrishnan TT et al. Clin Lymph Myeloma Leuk. 2020 Feb 20. doi: 10.1016/j.clml.2020.06.003.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Blood biomarker detects concussion, shows severity, predicts recovery
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
New hope for ALS
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE

