User login
Posttraumatic epilepsy is common, even after ‘mild’ TBI
, new research suggests.
Results from a multicenter, prospective cohort study showed 2.7% of nearly 1,500 participants with TBI reported also having posttraumatic epilepsy, and these patients had significantly worse outcomes than those without posttraumatic epilepsy.
“Posttraumatic epilepsy is common even in so-called mild TBI, and we should be on the lookout for patients reporting these kinds of spells,” said coinvestigator Ramon Diaz-Arrastia, MD, PhD, professor of neurology and director of the TBI Clinical Research Center, University of Pennsylvania, Philadelphia.
Dr. Diaz-Arrastia said he dislikes the term “mild TBI” because many of these injuries have “pretty substantial consequences.”
The findings were published online Dec. 29 in JAMA Network Open.
Novel study
Seizures can occur after TBI, most commonly after a severe brain injury, such as those leading to coma or bleeding in the brain or requiring surgical intervention. However, there have been “hints” that some patients with milder brain injuries are also at increased risk for epilepsy, said Dr. Diaz-Arrastia.
To investigate, the researchers assessed data from the large, multicenter Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) database. Participants with TBI, defined as a Glasgow Coma Scale (GCS) score of 3-15, had presented to a level I trauma center within 24 hours of a head trauma needing evaluation with a CT scan.
The study included patients with relatively mild TBI (GCS score, 13-15), which is a “novel feature” of the study, the authors noted. Most prior studies of posttraumatic epilepsy focused on moderate to severe TBI.
The researchers included two sex- and age-matched control groups. The orthopedic trauma control (OTC) group consisted of patients with isolated trauma to the limbs, pelvis, and/or ribs. The “friend” or peer control group had backgrounds and lifestyles similar to those with TBI but had no history of TBI, concussion, or traumatic injury in the previous year.
The analysis included 1,885 participants (mean age, 41.3 years; 65.8% men). Of these, 1,493 had TBI, 182 were in the OTC group, and 210 were in the friends group. At 6- and 12-month follow-ups, investigators administered the Epilepsy Screening Questionnaire (ESQ), developed by the National Institute of Neurological Disorders and Stroke (NINDS).
Confirmatory data
Participants were asked about experiencing uncontrolled movements, unexplained changes in mental state, and repeated unusual attacks or convulsions, and whether they had been told they had epilepsy or seizures. If they answered yes to any of these questions, they received second-level screening, which asked about seizures.
Patients were deemed to have posttraumatic epilepsy if they answered affirmatively to any first-level screening item, experienced seizures 7 days after injury, and were diagnosed with epilepsy.
The primary outcome was rate of positive posttraumatic epilepsy diagnoses. At 12 months, 2.7% of those with TBI reported a posttraumatic epilepsy diagnosis compared with none of either of the control groups (P < .001).
This rate is consistent with prior literature and is “pretty close to what we expected,” said Dr. Diaz-Arrastia.
Among those with TBI and posttraumatic epilepsy, 12.2% had GCS scores of 3-8 (severe), 5.3% had scores of 9-12 (moderate), and 0.9% had scores of 13-15 (mild). That figure for mild TBI is not insignificant, said Dr. Diaz-Arrastia.
“Probably 90% of all those coming to the emergency room with a brain injury are diagnosed with mild TBI not requiring admission,” he noted.
The risk for posttraumatic epilepsy was higher the more severe the head injury, and among those with hemorrhage on head CT imaging. In patients with mild TBI, hemorrhage was associated with a two- to threefold risk of developing posttraumatic epilepsy.
“This prospective observational study confirms the epidemiologic data that even after mild brain injury, there is an increased risk for epilepsy,” said Dr. Diaz-Arrastia.
Universal screening?
The researchers also looked at whether seizures worsen other outcomes. Compared with those who had TBI but not posttraumatic epilepsy, those with posttraumatic epilepsy had significantly lower Glasgow Outcome Scale Extended (GOSE) scores (mean, 4.7 vs. 6.1; P < .001), higher Brief Symptom Inventory (BSI) scores (58.6 vs. 50.2; P = .02), and higher Rivermead Cognitive Metric (RCM) scores (5.3 vs. 3.1; P = .002) at 12 months after adjustment for age, initial GCS score, and imaging findings.
Higher GOSE and RCM scores reflect better outcomes, but a higher score on the BSI, which assesses overall mood, reflects a worse outcome, the investigators noted.
Previous evidence suggests prophylactic use of antiepileptic drugs in patients with TBI does not reduce risks. These drugs “are neither 100% safe nor 100% effective,” said Dr. Diaz-Arrastia. Some studies showed that certain agents actually worsen outcomes, he added.
What the field needs instead are antiepileptogenic drugs – those that interfere with the maladaptive synaptic plasticity that ends up in an epileptic circuit, he noted.
The new results suggest screening for posttraumatic epilepsy using the NINDS-ESQ “should be done pretty much routinely as a follow-up for all brain injuries,” Dr. Diaz-Arrastia said.
The investigators plan to have study participants assessed by an epileptologist later. A significant number of people with TBI, he noted, won’t develop posttraumatic epilepsy until 1-5 years after their injury – and even later in some cases.
A limitation of the study was that some patients reporting posttraumatic epilepsy may have had psychogenic nonepileptiform seizures, which are common in TBI patients, the investigators noted.
The study was supported by grants from One Mind, National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS, and Department of Defence. Dr. Diaz-Arrastia reported receiving grants from the NIH, NINDS, and DOD during the conduct of the study.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a multicenter, prospective cohort study showed 2.7% of nearly 1,500 participants with TBI reported also having posttraumatic epilepsy, and these patients had significantly worse outcomes than those without posttraumatic epilepsy.
“Posttraumatic epilepsy is common even in so-called mild TBI, and we should be on the lookout for patients reporting these kinds of spells,” said coinvestigator Ramon Diaz-Arrastia, MD, PhD, professor of neurology and director of the TBI Clinical Research Center, University of Pennsylvania, Philadelphia.
Dr. Diaz-Arrastia said he dislikes the term “mild TBI” because many of these injuries have “pretty substantial consequences.”
The findings were published online Dec. 29 in JAMA Network Open.
Novel study
Seizures can occur after TBI, most commonly after a severe brain injury, such as those leading to coma or bleeding in the brain or requiring surgical intervention. However, there have been “hints” that some patients with milder brain injuries are also at increased risk for epilepsy, said Dr. Diaz-Arrastia.
To investigate, the researchers assessed data from the large, multicenter Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) database. Participants with TBI, defined as a Glasgow Coma Scale (GCS) score of 3-15, had presented to a level I trauma center within 24 hours of a head trauma needing evaluation with a CT scan.
The study included patients with relatively mild TBI (GCS score, 13-15), which is a “novel feature” of the study, the authors noted. Most prior studies of posttraumatic epilepsy focused on moderate to severe TBI.
The researchers included two sex- and age-matched control groups. The orthopedic trauma control (OTC) group consisted of patients with isolated trauma to the limbs, pelvis, and/or ribs. The “friend” or peer control group had backgrounds and lifestyles similar to those with TBI but had no history of TBI, concussion, or traumatic injury in the previous year.
The analysis included 1,885 participants (mean age, 41.3 years; 65.8% men). Of these, 1,493 had TBI, 182 were in the OTC group, and 210 were in the friends group. At 6- and 12-month follow-ups, investigators administered the Epilepsy Screening Questionnaire (ESQ), developed by the National Institute of Neurological Disorders and Stroke (NINDS).
Confirmatory data
Participants were asked about experiencing uncontrolled movements, unexplained changes in mental state, and repeated unusual attacks or convulsions, and whether they had been told they had epilepsy or seizures. If they answered yes to any of these questions, they received second-level screening, which asked about seizures.
Patients were deemed to have posttraumatic epilepsy if they answered affirmatively to any first-level screening item, experienced seizures 7 days after injury, and were diagnosed with epilepsy.
The primary outcome was rate of positive posttraumatic epilepsy diagnoses. At 12 months, 2.7% of those with TBI reported a posttraumatic epilepsy diagnosis compared with none of either of the control groups (P < .001).
This rate is consistent with prior literature and is “pretty close to what we expected,” said Dr. Diaz-Arrastia.
Among those with TBI and posttraumatic epilepsy, 12.2% had GCS scores of 3-8 (severe), 5.3% had scores of 9-12 (moderate), and 0.9% had scores of 13-15 (mild). That figure for mild TBI is not insignificant, said Dr. Diaz-Arrastia.
“Probably 90% of all those coming to the emergency room with a brain injury are diagnosed with mild TBI not requiring admission,” he noted.
The risk for posttraumatic epilepsy was higher the more severe the head injury, and among those with hemorrhage on head CT imaging. In patients with mild TBI, hemorrhage was associated with a two- to threefold risk of developing posttraumatic epilepsy.
“This prospective observational study confirms the epidemiologic data that even after mild brain injury, there is an increased risk for epilepsy,” said Dr. Diaz-Arrastia.
Universal screening?
The researchers also looked at whether seizures worsen other outcomes. Compared with those who had TBI but not posttraumatic epilepsy, those with posttraumatic epilepsy had significantly lower Glasgow Outcome Scale Extended (GOSE) scores (mean, 4.7 vs. 6.1; P < .001), higher Brief Symptom Inventory (BSI) scores (58.6 vs. 50.2; P = .02), and higher Rivermead Cognitive Metric (RCM) scores (5.3 vs. 3.1; P = .002) at 12 months after adjustment for age, initial GCS score, and imaging findings.
Higher GOSE and RCM scores reflect better outcomes, but a higher score on the BSI, which assesses overall mood, reflects a worse outcome, the investigators noted.
Previous evidence suggests prophylactic use of antiepileptic drugs in patients with TBI does not reduce risks. These drugs “are neither 100% safe nor 100% effective,” said Dr. Diaz-Arrastia. Some studies showed that certain agents actually worsen outcomes, he added.
What the field needs instead are antiepileptogenic drugs – those that interfere with the maladaptive synaptic plasticity that ends up in an epileptic circuit, he noted.
The new results suggest screening for posttraumatic epilepsy using the NINDS-ESQ “should be done pretty much routinely as a follow-up for all brain injuries,” Dr. Diaz-Arrastia said.
The investigators plan to have study participants assessed by an epileptologist later. A significant number of people with TBI, he noted, won’t develop posttraumatic epilepsy until 1-5 years after their injury – and even later in some cases.
A limitation of the study was that some patients reporting posttraumatic epilepsy may have had psychogenic nonepileptiform seizures, which are common in TBI patients, the investigators noted.
The study was supported by grants from One Mind, National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS, and Department of Defence. Dr. Diaz-Arrastia reported receiving grants from the NIH, NINDS, and DOD during the conduct of the study.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a multicenter, prospective cohort study showed 2.7% of nearly 1,500 participants with TBI reported also having posttraumatic epilepsy, and these patients had significantly worse outcomes than those without posttraumatic epilepsy.
“Posttraumatic epilepsy is common even in so-called mild TBI, and we should be on the lookout for patients reporting these kinds of spells,” said coinvestigator Ramon Diaz-Arrastia, MD, PhD, professor of neurology and director of the TBI Clinical Research Center, University of Pennsylvania, Philadelphia.
Dr. Diaz-Arrastia said he dislikes the term “mild TBI” because many of these injuries have “pretty substantial consequences.”
The findings were published online Dec. 29 in JAMA Network Open.
Novel study
Seizures can occur after TBI, most commonly after a severe brain injury, such as those leading to coma or bleeding in the brain or requiring surgical intervention. However, there have been “hints” that some patients with milder brain injuries are also at increased risk for epilepsy, said Dr. Diaz-Arrastia.
To investigate, the researchers assessed data from the large, multicenter Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) database. Participants with TBI, defined as a Glasgow Coma Scale (GCS) score of 3-15, had presented to a level I trauma center within 24 hours of a head trauma needing evaluation with a CT scan.
The study included patients with relatively mild TBI (GCS score, 13-15), which is a “novel feature” of the study, the authors noted. Most prior studies of posttraumatic epilepsy focused on moderate to severe TBI.
The researchers included two sex- and age-matched control groups. The orthopedic trauma control (OTC) group consisted of patients with isolated trauma to the limbs, pelvis, and/or ribs. The “friend” or peer control group had backgrounds and lifestyles similar to those with TBI but had no history of TBI, concussion, or traumatic injury in the previous year.
The analysis included 1,885 participants (mean age, 41.3 years; 65.8% men). Of these, 1,493 had TBI, 182 were in the OTC group, and 210 were in the friends group. At 6- and 12-month follow-ups, investigators administered the Epilepsy Screening Questionnaire (ESQ), developed by the National Institute of Neurological Disorders and Stroke (NINDS).
Confirmatory data
Participants were asked about experiencing uncontrolled movements, unexplained changes in mental state, and repeated unusual attacks or convulsions, and whether they had been told they had epilepsy or seizures. If they answered yes to any of these questions, they received second-level screening, which asked about seizures.
Patients were deemed to have posttraumatic epilepsy if they answered affirmatively to any first-level screening item, experienced seizures 7 days after injury, and were diagnosed with epilepsy.
The primary outcome was rate of positive posttraumatic epilepsy diagnoses. At 12 months, 2.7% of those with TBI reported a posttraumatic epilepsy diagnosis compared with none of either of the control groups (P < .001).
This rate is consistent with prior literature and is “pretty close to what we expected,” said Dr. Diaz-Arrastia.
Among those with TBI and posttraumatic epilepsy, 12.2% had GCS scores of 3-8 (severe), 5.3% had scores of 9-12 (moderate), and 0.9% had scores of 13-15 (mild). That figure for mild TBI is not insignificant, said Dr. Diaz-Arrastia.
“Probably 90% of all those coming to the emergency room with a brain injury are diagnosed with mild TBI not requiring admission,” he noted.
The risk for posttraumatic epilepsy was higher the more severe the head injury, and among those with hemorrhage on head CT imaging. In patients with mild TBI, hemorrhage was associated with a two- to threefold risk of developing posttraumatic epilepsy.
“This prospective observational study confirms the epidemiologic data that even after mild brain injury, there is an increased risk for epilepsy,” said Dr. Diaz-Arrastia.
Universal screening?
The researchers also looked at whether seizures worsen other outcomes. Compared with those who had TBI but not posttraumatic epilepsy, those with posttraumatic epilepsy had significantly lower Glasgow Outcome Scale Extended (GOSE) scores (mean, 4.7 vs. 6.1; P < .001), higher Brief Symptom Inventory (BSI) scores (58.6 vs. 50.2; P = .02), and higher Rivermead Cognitive Metric (RCM) scores (5.3 vs. 3.1; P = .002) at 12 months after adjustment for age, initial GCS score, and imaging findings.
Higher GOSE and RCM scores reflect better outcomes, but a higher score on the BSI, which assesses overall mood, reflects a worse outcome, the investigators noted.
Previous evidence suggests prophylactic use of antiepileptic drugs in patients with TBI does not reduce risks. These drugs “are neither 100% safe nor 100% effective,” said Dr. Diaz-Arrastia. Some studies showed that certain agents actually worsen outcomes, he added.
What the field needs instead are antiepileptogenic drugs – those that interfere with the maladaptive synaptic plasticity that ends up in an epileptic circuit, he noted.
The new results suggest screening for posttraumatic epilepsy using the NINDS-ESQ “should be done pretty much routinely as a follow-up for all brain injuries,” Dr. Diaz-Arrastia said.
The investigators plan to have study participants assessed by an epileptologist later. A significant number of people with TBI, he noted, won’t develop posttraumatic epilepsy until 1-5 years after their injury – and even later in some cases.
A limitation of the study was that some patients reporting posttraumatic epilepsy may have had psychogenic nonepileptiform seizures, which are common in TBI patients, the investigators noted.
The study was supported by grants from One Mind, National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS, and Department of Defence. Dr. Diaz-Arrastia reported receiving grants from the NIH, NINDS, and DOD during the conduct of the study.
A version of this article first appeared on Medscape.com.
Herpes Zoster Following a Nucleoside-Modified Messenger RNA COVID-19 Vaccine
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
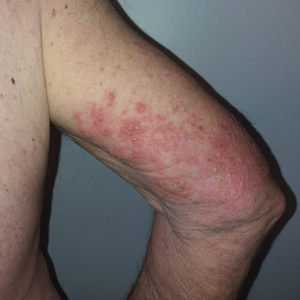
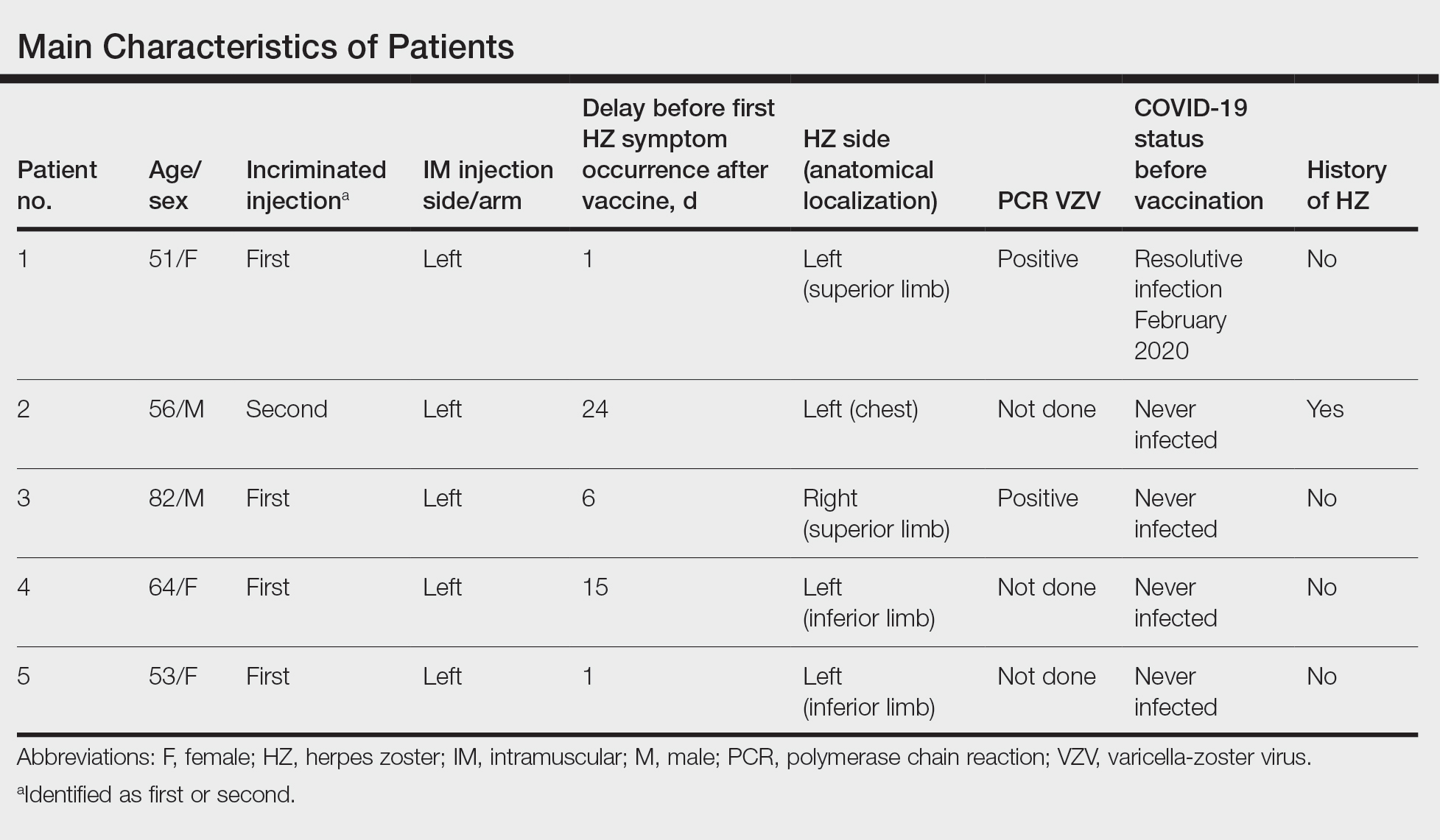
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.
Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5
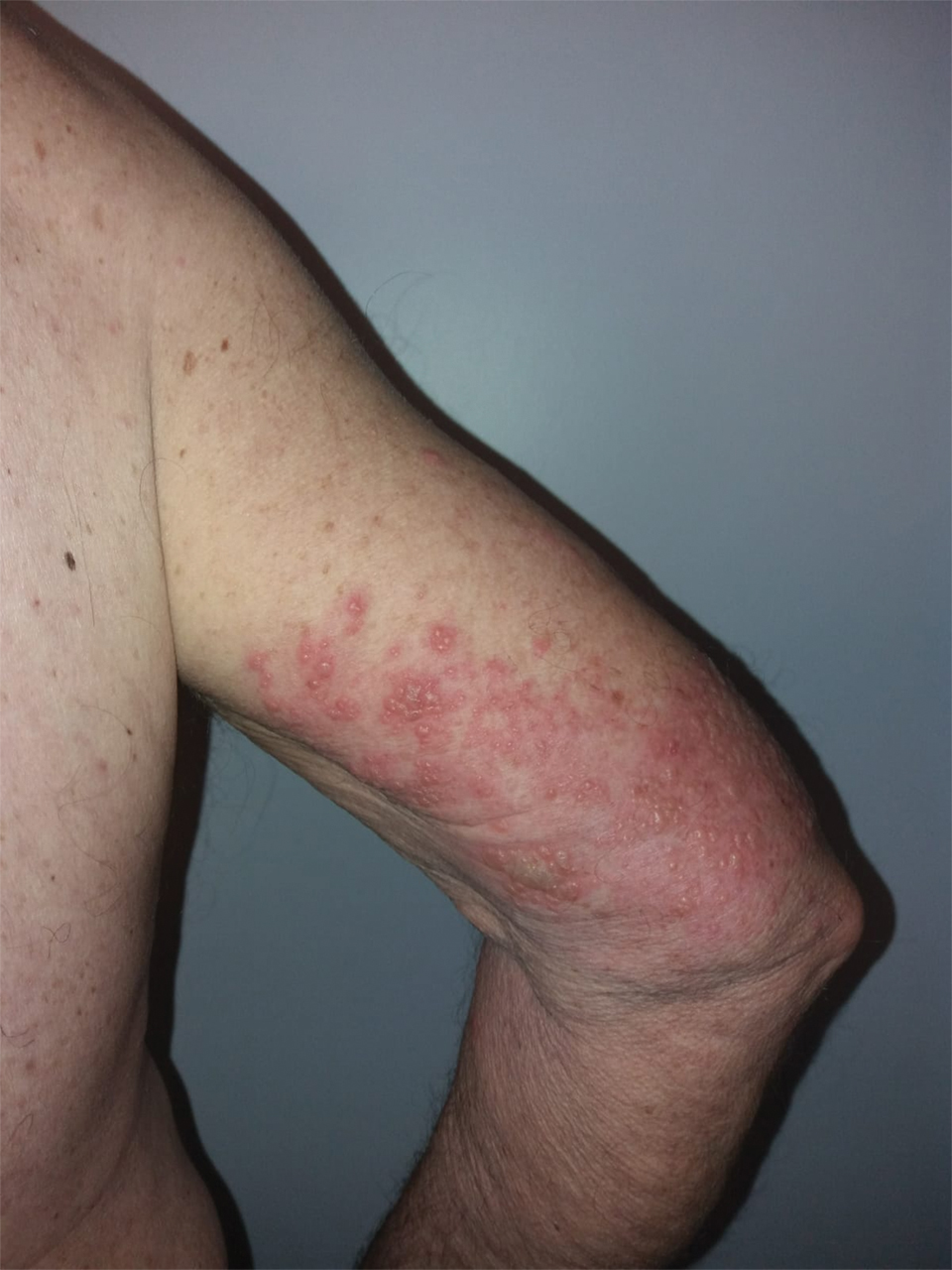
Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9
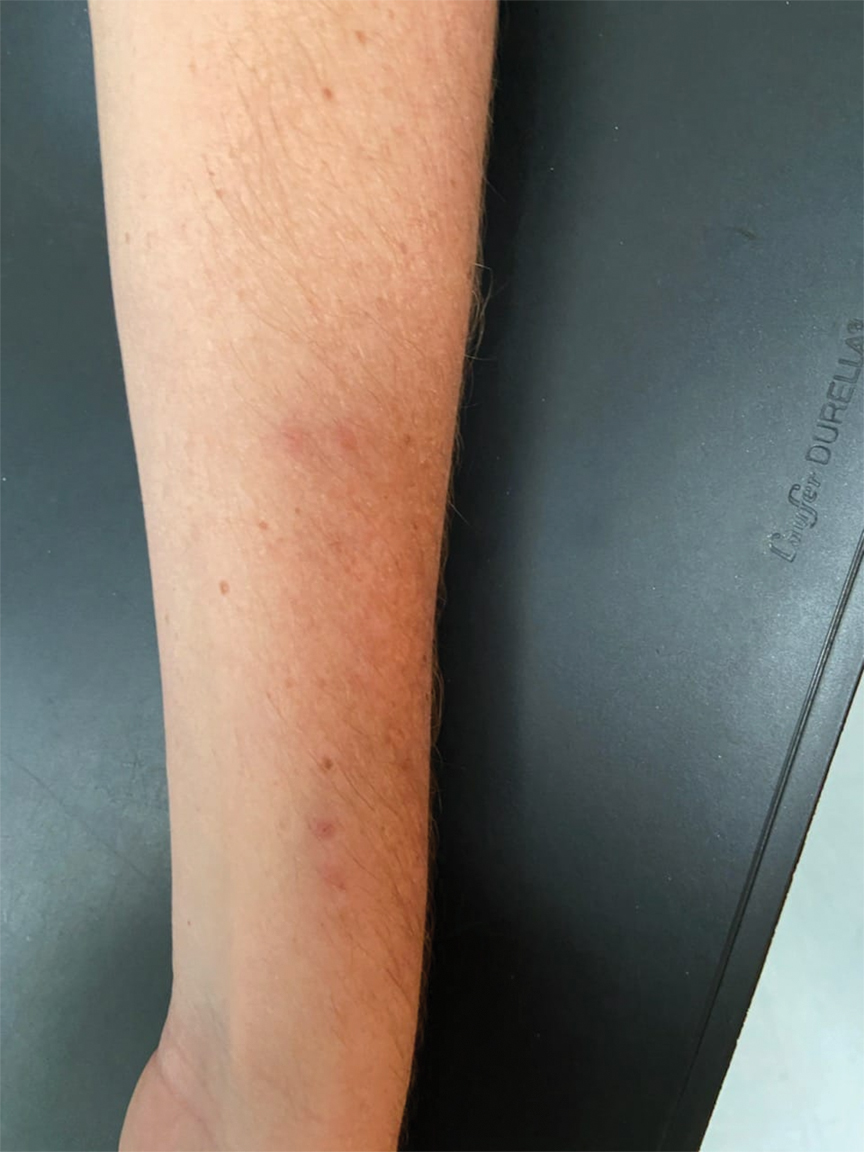
In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.

Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5

Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9

In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.

Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5

Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9

In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Practice Points
- Herpes zoster (HZ) has been reported following COVID-19 vaccination.
- Postinjection pain is common with COVID-19 vaccination, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay in onset between the injection and the symptoms.
- When indicated, the second vaccine dose should not be avoided in patients who are diagnosed with HZ.
Serious problems rare in ages 5-11 from COVID vaccine
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
Pandemic poses short- and long-term risks to babies, especially boys
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
DLBCL: PFS but no OS benefit with polatuzumab-vedotin add-on
Two-year progression free survival (PFS) was 76.7% for the 440 patients randomized to polatuzumab-vedotin (PV) add-on, versus 70.2% for the 439 randomized to R-CHOP, which translated to a 27% reduction in the risk of progression, relapse, or death (P = .02). However, overall survival (OS) at 2 years was just under 89% in both arms of the trial, dubbed POLARIX. Toxicity was comparable between the two arms.
The investigators swapped out the vincristine in R-CHOP for PV to avoid overlapping neurotoxic side effects and called their modified regimen “pola-R-CHP.”
“We believe these results support use of pola-R-CHP in the initial management of patients with DLBCL,” senior investigator Gilles Salles, MD, PhD, a hematologic oncologist at Memorial Sloan Cancer Center in New York, said at the American Society of Hematology annual meeting.
The study (ASH 2021 abstract LBA-1), was published simultaneously in the New England Journal of Medicine.
Worth the cost?
The investigators reported that the median follow up of 28.2 months may simply have been too short to see if the PFS benefit translates into better overall survival. Also, newer treatments for relapsed/refractory disease might have masked any OS benefit.
With the PFS benefit, however, “what we think we are seeing is a deeper, more profound complete remission that hopefully will translate into [better] overall survival, but it may be a while until that can be demonstrated,” said Jane N. Winter, MD, a hematologic oncologist at Northwestern University, Chicago, who moderated Dr. Salles’ presentation.
“If the improvement in PFS at 2 years represents a true higher cure rate and plateau rather than a simple delay in relapse,” the “results from the POLARIX trial are likely to be practice-changing,” blood cancer specialist Ajay K. Gopal, MD, professor of medicine at the University of Washington, Seattle, told this news organization when asked for comment.
With additional OS results pending, an audience member at ASH wondered if “the cost of this highly expensive monoclonal antibody drug conjugate is worth the small improvement in PFS.”
“We have to further study this point, but at this moment what is important is to have a treatment with better efficacy and no more toxicity” than R-CHOP, lead investigator Herve Tilly, MD, a hematologic oncologist at the University of Rouen, France, said at the meeting.
Dr. Gopal said the cost concerns are legitimate, but also pointed out that they “may be somewhat offset by the potential reduction in downstream use of expensive cellular therapies.”
The findings support his assertion. With reduced PFS, R-CHOP subjects were more likely than were pola-R-CHP subjects to go on to subsequent lines of therapy (30.3% versus 22.5%).
PV is already approved in the United States for relapsed or refractory DLBCL in combination with bendamustine and rituximab after failure of at least two previous regimens.
Defining a target population
R-CHOP – rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – has been the first-line standard of care for DLBCL for 2 decades, but it cures only about 60%-70% of patients. Researchers have tried for years to improve the cure rate by adding novel agents and other means, but outcomes haven’t been clinically meaningful, the investigators explained.
Polatuzumab, the antibody component of PV, zeroes in on a ubiquitous target on mature B-cell lymphomas, delivering vedotin, a potent microtubule inhibitor, directly to tumor cells.
Study subjects were treatment naive and a median of 65 years old with intermediate-risk or high-risk DLBCL. About a third had activated B-cell–like DLBCL, and almost two-thirds had baseline International Prognostic Index (IPI) scores between 3 and 5.
Each arm of the trial underwent six treatment cycles, plus two cycles of rituximab monotherapy.
On subgroup analysis, PFS benefit clustered among higher risk patients, namely patients older than 60 years, those with IPI scores between 3 and 5, and patients with the activated B-cell–like subtype.
Younger patients, subjects with lower IPI scores, patients with bulky disease, and those who had germinal-center B-cell–like DLBCL “did not show a clear [PFS] benefit,” the study team said.
Ongoing trial in the elderly
Adverse events in POLARIX were in line with the component drugs’ known toxicity profiles, with no new safety signals identified.
The most common grade 3/4 adverse events were neutropenia (28.3% in the pola-R-CHP group and 30.8% in the R-CHOP group), febrile neutropenia (13.8% and 8.0%, respectively), and anemia (12.0% and 8.4%). A bit over 6% of subjects in both arms discontinued because of adverse events.
The higher incidence of febrile neutropenia with pola-R-CHP “did not translate into a higher overall incidence of infection, treatment discontinuation, or dose reductions,” the investigators said.
They noted that patients with lymphoma arising from previously diagnosed indolent lymphoma, those with a primary mediastinal lymphoma, and people older than 80 years were not included in the study. A phase 3 trial in patients 75 years and up is recruiting.
The work was funded by PV maker Genentech/Roche. Many of the investigators disclosed ties to the companies, including Dr. Tilly, an adviser and speaker for Roche, and Dr. Salles, an adviser for Genentech. Three investigators were Genentech employees. Dr. Gopal is a consultant for Genentech/Roche. Dr. Winter did not have any ties to the companies.
Two-year progression free survival (PFS) was 76.7% for the 440 patients randomized to polatuzumab-vedotin (PV) add-on, versus 70.2% for the 439 randomized to R-CHOP, which translated to a 27% reduction in the risk of progression, relapse, or death (P = .02). However, overall survival (OS) at 2 years was just under 89% in both arms of the trial, dubbed POLARIX. Toxicity was comparable between the two arms.
The investigators swapped out the vincristine in R-CHOP for PV to avoid overlapping neurotoxic side effects and called their modified regimen “pola-R-CHP.”
“We believe these results support use of pola-R-CHP in the initial management of patients with DLBCL,” senior investigator Gilles Salles, MD, PhD, a hematologic oncologist at Memorial Sloan Cancer Center in New York, said at the American Society of Hematology annual meeting.
The study (ASH 2021 abstract LBA-1), was published simultaneously in the New England Journal of Medicine.
Worth the cost?
The investigators reported that the median follow up of 28.2 months may simply have been too short to see if the PFS benefit translates into better overall survival. Also, newer treatments for relapsed/refractory disease might have masked any OS benefit.
With the PFS benefit, however, “what we think we are seeing is a deeper, more profound complete remission that hopefully will translate into [better] overall survival, but it may be a while until that can be demonstrated,” said Jane N. Winter, MD, a hematologic oncologist at Northwestern University, Chicago, who moderated Dr. Salles’ presentation.
“If the improvement in PFS at 2 years represents a true higher cure rate and plateau rather than a simple delay in relapse,” the “results from the POLARIX trial are likely to be practice-changing,” blood cancer specialist Ajay K. Gopal, MD, professor of medicine at the University of Washington, Seattle, told this news organization when asked for comment.
With additional OS results pending, an audience member at ASH wondered if “the cost of this highly expensive monoclonal antibody drug conjugate is worth the small improvement in PFS.”
“We have to further study this point, but at this moment what is important is to have a treatment with better efficacy and no more toxicity” than R-CHOP, lead investigator Herve Tilly, MD, a hematologic oncologist at the University of Rouen, France, said at the meeting.
Dr. Gopal said the cost concerns are legitimate, but also pointed out that they “may be somewhat offset by the potential reduction in downstream use of expensive cellular therapies.”
The findings support his assertion. With reduced PFS, R-CHOP subjects were more likely than were pola-R-CHP subjects to go on to subsequent lines of therapy (30.3% versus 22.5%).
PV is already approved in the United States for relapsed or refractory DLBCL in combination with bendamustine and rituximab after failure of at least two previous regimens.
Defining a target population
R-CHOP – rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – has been the first-line standard of care for DLBCL for 2 decades, but it cures only about 60%-70% of patients. Researchers have tried for years to improve the cure rate by adding novel agents and other means, but outcomes haven’t been clinically meaningful, the investigators explained.
Polatuzumab, the antibody component of PV, zeroes in on a ubiquitous target on mature B-cell lymphomas, delivering vedotin, a potent microtubule inhibitor, directly to tumor cells.
Study subjects were treatment naive and a median of 65 years old with intermediate-risk or high-risk DLBCL. About a third had activated B-cell–like DLBCL, and almost two-thirds had baseline International Prognostic Index (IPI) scores between 3 and 5.
Each arm of the trial underwent six treatment cycles, plus two cycles of rituximab monotherapy.
On subgroup analysis, PFS benefit clustered among higher risk patients, namely patients older than 60 years, those with IPI scores between 3 and 5, and patients with the activated B-cell–like subtype.
Younger patients, subjects with lower IPI scores, patients with bulky disease, and those who had germinal-center B-cell–like DLBCL “did not show a clear [PFS] benefit,” the study team said.
Ongoing trial in the elderly
Adverse events in POLARIX were in line with the component drugs’ known toxicity profiles, with no new safety signals identified.
The most common grade 3/4 adverse events were neutropenia (28.3% in the pola-R-CHP group and 30.8% in the R-CHOP group), febrile neutropenia (13.8% and 8.0%, respectively), and anemia (12.0% and 8.4%). A bit over 6% of subjects in both arms discontinued because of adverse events.
The higher incidence of febrile neutropenia with pola-R-CHP “did not translate into a higher overall incidence of infection, treatment discontinuation, or dose reductions,” the investigators said.
They noted that patients with lymphoma arising from previously diagnosed indolent lymphoma, those with a primary mediastinal lymphoma, and people older than 80 years were not included in the study. A phase 3 trial in patients 75 years and up is recruiting.
The work was funded by PV maker Genentech/Roche. Many of the investigators disclosed ties to the companies, including Dr. Tilly, an adviser and speaker for Roche, and Dr. Salles, an adviser for Genentech. Three investigators were Genentech employees. Dr. Gopal is a consultant for Genentech/Roche. Dr. Winter did not have any ties to the companies.
Two-year progression free survival (PFS) was 76.7% for the 440 patients randomized to polatuzumab-vedotin (PV) add-on, versus 70.2% for the 439 randomized to R-CHOP, which translated to a 27% reduction in the risk of progression, relapse, or death (P = .02). However, overall survival (OS) at 2 years was just under 89% in both arms of the trial, dubbed POLARIX. Toxicity was comparable between the two arms.
The investigators swapped out the vincristine in R-CHOP for PV to avoid overlapping neurotoxic side effects and called their modified regimen “pola-R-CHP.”
“We believe these results support use of pola-R-CHP in the initial management of patients with DLBCL,” senior investigator Gilles Salles, MD, PhD, a hematologic oncologist at Memorial Sloan Cancer Center in New York, said at the American Society of Hematology annual meeting.
The study (ASH 2021 abstract LBA-1), was published simultaneously in the New England Journal of Medicine.
Worth the cost?
The investigators reported that the median follow up of 28.2 months may simply have been too short to see if the PFS benefit translates into better overall survival. Also, newer treatments for relapsed/refractory disease might have masked any OS benefit.
With the PFS benefit, however, “what we think we are seeing is a deeper, more profound complete remission that hopefully will translate into [better] overall survival, but it may be a while until that can be demonstrated,” said Jane N. Winter, MD, a hematologic oncologist at Northwestern University, Chicago, who moderated Dr. Salles’ presentation.
“If the improvement in PFS at 2 years represents a true higher cure rate and plateau rather than a simple delay in relapse,” the “results from the POLARIX trial are likely to be practice-changing,” blood cancer specialist Ajay K. Gopal, MD, professor of medicine at the University of Washington, Seattle, told this news organization when asked for comment.
With additional OS results pending, an audience member at ASH wondered if “the cost of this highly expensive monoclonal antibody drug conjugate is worth the small improvement in PFS.”
“We have to further study this point, but at this moment what is important is to have a treatment with better efficacy and no more toxicity” than R-CHOP, lead investigator Herve Tilly, MD, a hematologic oncologist at the University of Rouen, France, said at the meeting.
Dr. Gopal said the cost concerns are legitimate, but also pointed out that they “may be somewhat offset by the potential reduction in downstream use of expensive cellular therapies.”
The findings support his assertion. With reduced PFS, R-CHOP subjects were more likely than were pola-R-CHP subjects to go on to subsequent lines of therapy (30.3% versus 22.5%).
PV is already approved in the United States for relapsed or refractory DLBCL in combination with bendamustine and rituximab after failure of at least two previous regimens.
Defining a target population
R-CHOP – rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – has been the first-line standard of care for DLBCL for 2 decades, but it cures only about 60%-70% of patients. Researchers have tried for years to improve the cure rate by adding novel agents and other means, but outcomes haven’t been clinically meaningful, the investigators explained.
Polatuzumab, the antibody component of PV, zeroes in on a ubiquitous target on mature B-cell lymphomas, delivering vedotin, a potent microtubule inhibitor, directly to tumor cells.
Study subjects were treatment naive and a median of 65 years old with intermediate-risk or high-risk DLBCL. About a third had activated B-cell–like DLBCL, and almost two-thirds had baseline International Prognostic Index (IPI) scores between 3 and 5.
Each arm of the trial underwent six treatment cycles, plus two cycles of rituximab monotherapy.
On subgroup analysis, PFS benefit clustered among higher risk patients, namely patients older than 60 years, those with IPI scores between 3 and 5, and patients with the activated B-cell–like subtype.
Younger patients, subjects with lower IPI scores, patients with bulky disease, and those who had germinal-center B-cell–like DLBCL “did not show a clear [PFS] benefit,” the study team said.
Ongoing trial in the elderly
Adverse events in POLARIX were in line with the component drugs’ known toxicity profiles, with no new safety signals identified.
The most common grade 3/4 adverse events were neutropenia (28.3% in the pola-R-CHP group and 30.8% in the R-CHOP group), febrile neutropenia (13.8% and 8.0%, respectively), and anemia (12.0% and 8.4%). A bit over 6% of subjects in both arms discontinued because of adverse events.
The higher incidence of febrile neutropenia with pola-R-CHP “did not translate into a higher overall incidence of infection, treatment discontinuation, or dose reductions,” the investigators said.
They noted that patients with lymphoma arising from previously diagnosed indolent lymphoma, those with a primary mediastinal lymphoma, and people older than 80 years were not included in the study. A phase 3 trial in patients 75 years and up is recruiting.
The work was funded by PV maker Genentech/Roche. Many of the investigators disclosed ties to the companies, including Dr. Tilly, an adviser and speaker for Roche, and Dr. Salles, an adviser for Genentech. Three investigators were Genentech employees. Dr. Gopal is a consultant for Genentech/Roche. Dr. Winter did not have any ties to the companies.
REPORTING FROM ASH 2021
NHL: As a second-line treatment in phase 3 trial, tisa-cel disappoints
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
according to results of a randomized, phase 3 trial.
The chimeric antigen receptor (CAR) T-cell therapy did not improve event-free survival (EFS) in this phase 3 BELINDA study, potentially because of study design decisions or imbalances in relevant patient characteristics, according to the study investigators.
Despite the negative result, insights from this study will inform the development of future clinical trials of CAR T-cell therapy, said BELINDA investigator Michael R. Bishop, MD, of the David and Etta Jonas Center for Cellular Therapy, University of Chicago.
Findings of BELINDA, presented at the annual meeting of the American Society of Hematology, stand in contrast to two other high-profile CAR T-cell therapy studies also presented at the meeting. Those other studies demonstrated significant improvements in EFS in the second-line treatment of large B-cell lymphomas.
“All of us are excited to see that the other two trials were positive, and we were hoping that ours would be as well, but there are significant differences in the trial design,” Dr. Bishop said in a press conference held at the ASH meeting.
Tisagenlecleucel (tisa-cel), an anti-CD19 CAR T-cell therapy, is already approved by the Food and Drug Administration for the treatment of patients with relapsed or refractory large B-cell lymphomas after at least two other lines of systemic therapy.
The aim of the pivotal phase 3, randomized, multicenter BELINDA study was to evaluate tisa-cel earlier in the course of treatment for patients with more aggressive disease, according to Dr. Bishop.
About two-thirds of non-Hodgkin lymphoma patients will be cured with first-line treatment. However, very poor outcomes are seen among patients with disease that does not respond to the initial treatment or that reoccurs shortly afterward, Dr. Bishop said.
The standard of care approach for those patients is second-line therapy, he noted, usually with combination chemoimmunotherapy, followed by autologous stem cell transplant if the disease responds to chemotherapy.
“Unfortunately, only a minority of those patients will be found to have chemotherapy-sensitive disease and be able to go on to autologous stem cell transplantation,” Dr. Bishop said. “And even in that subgroup of patients, the outcomes are relatively poor.”
Accordingly, the phase 3 BELINDA study enrolled patients with aggressive non-Hodgkin lymphomas that either did not respond to first-line treatment or that reoccurred within 12 months.
The primary endpoint of the study was EFS, defined as the time from randomization to either stable or progressive disease at or after a week 12 assessment or to any-cause death at any time.
While that primary endpoint was not met for tisa-cel versus standard of care therapy, two other randomized, phase 3 studies presented at the ASH meeting did demonstrate that CAR T-cell therapy extended EFS when given as a second-line lymphoma treatment.
In the randomized, phase 3 ZUMA-7 trial, axicabtagene ciloleucel (axi-cel) significantly improved EFS versus standard of care in the treatment of patients with large B-cell lymphoma refractory to or relapsed within 12 months of adequate first-line therapy, according to investigators.
Similarly, the investigators said that treatment with lisocabtagene maraleucel (liso-cel) led to a significant improvement in EFS in TRANSFORM, a randomized, phase 3 clinical trial that enrolled patients with large B-cell lymphoma that was refractory to first-line therapy or else relapsed within 12 months of that treatment.
“It’s very possible that either or both the patient characteristics and the study design is what led to the difference in the top-line study results,” lymphoma specialist Andrew M. Evens, DO, said in an interview.
There were substantial differences between the studies in terms of what was allowed as optional bridging therapy and salvage therapy, according to Dr. Evens, associate director for clinical services and director of the lymphoma program at Rutgers Cancer Institute in New Brunswick, N.J.
“In ZUMA-7, they only allowed steroids as bridging therapy,” said Dr. Evens, who was not an investigator on any of the three second-line CAR T-cell studies.
In the BELINDA study, optional platinum-based chemotherapy bridging treatment allowed in one arm of the study could have potentially delayed tisa-cel infusion until after the week 6 assessment, study investigators reported in their ASH meeting abstract.
Differences in lymphodepleting therapy prior to CAR T-cell therapy could have also played a role. According to Dr. Bishop, the total doses of cyclophosphamide and fludarabine in BELINDA were 900 mg/m2 and 75 mg/m2, respectively, while in the other two trials, doses were 1,500 mg/m2 and 90 mg/m2, respectively.
Lymphodepleting chemotherapy is “extremely important” in the success of CAR T-cell therapeutic approaches, he noted at the press conference.
Dr. Bishop reported receiving consultancy fees from Arcellx, Autolus Therapeutics, Bristol-Myers Squibb, CRISPR, Kite/Gilead, and Novartis. He also reported research funding from Bristol-Myers Squibb and Kite/Gilead.
FROM ASH 2021
Are newer migraine therapies better? It depends
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
The findings, published in JAMA Network Open, “may imply that triptans will remain the current mainstay of specific acute migraine treatment,” suggested senior author Shuu-Jiun Wang, MD, from the National Yang Ming Chiao Tung University, and the Taipei Veterans General Hospital, both in Taipei, Taiwan, and his coauthors. However, lasmiditan (a 5-hydroxytryptamine1F receptor agonist) and rimegepant and ubrogepant (both calcitonin gene-related peptide [CGRP] antagonists) might still have unique advantages, since triptans are contraindicated for patients with cardiovascular risks, they said.
The systemic review and meta-analysis showed that, for the outcome of pain freedom and pain relief at 2 hours after the dose, the three newer agents worked better than placebo, but were inferior to most triptans. However, ubrogepant and rimegepant, which received U.S. Food and Drug Administration approval for the treatment of acute migraine in adults in December 2019 and February 2020, respectively, might be associated with fewer risks of adverse events (AEs), compared with triptans. “These new effective therapeutic options enrich the therapeutic categories of specific acute migraine treatments and may provide an opportunity to decrease the risks of barbiturate or opioid overuse or addiction,” they wrote.
The meta-analysis included 64 randomized, controlled trials involving 46,442 participants (74%-87% female across studies; age range, 36-43 years). All studies examined clinically relevant outcomes in patients with International Headache Society criteria for migraine, and compared currently available migraine-specific acute treatments with each other or placebo. The drugs were examined at doses with widespread clinical use and included: ergotamine, dihydroergotamine, sumatriptan, zolmitriptan, naratriptan, rizatriptan, almotriptan, eletriptan, frovatriptan, lasmiditan, rimegepant, and ubrogepant.
The findings showed that all drug treatments were associated with a higher odds ratio for pain freedom, compared with placebo, except for sumatriptan, 10-mg nasal spray. The most effective drug was eletriptan 40 mg (OR, 5.59), and the least effective was lasmiditan 50 mg (OR, 1.65). Most triptans were associated with higher ORs for both pain freedom and pain relief at 2 hours, compared with lasmiditan, rimegepant, or ubrogepant, while comparisons between lasmiditan, rimegepant, and ubrogepant for these outcomes showed no statistically significant difference, they reported.
Lasmiditan was associated with the highest risk of any AEs, “however, the AEs were tolerable and were not considered serious. … Therefore, we suggest that the benefits should be weighed against the risk of its AEs when considering the clinical application of lasmiditan,” they wrote. Certain triptans (rizatriptan, sumatriptan, and zolmitriptan) were also associated with a higher risk of any AEs, compared with the CGRP antagonists. “Nevertheless, most of the AEs were mild to moderate, and the percentages of serious AEs were low (0.0%-2.1%).”
Finally, the authors noted that their observations of successful treatment with 5-hydroxytriptamine1F receptor agonists and CGRP antagonists “reveals that vasoconstriction is not essential for antimigraine therapy.” which could have implications for future pharmaceutical development.
Older and newer medications each have advantages
“Triptans will be around for a long time, but the newer medications are here to stay,” said Alan M. Rapoport, MD, in reaction to the study. “Before this publication, we knew that the 2-hour efficacy results of the newer medications were not quite as good as the faster-acting triptans; and after this network meta-analysis we are more sure of that,” said Dr. Rapoport, of the department of neurology at University of California, Los Angeles. “But the fact that the three newer medications do not constrict blood vessels and can easily be given even to patients with contraindications to triptans, or patients that simply are at greater risk due to obesity, smoking history, family history, diabetes, lack of exercise, or higher lipid levels, puts them into a desirable category.”
Calling it a “very carefully done” systematic review, Dr. Rapoport had a few caveats about the strength of the research. The trials that were included were not identically designed and were performed in different areas, by different investigators, on different patients, he noted. They were also not head-to-head trials “which ensures that the resultant data are more pure.” The studies also looked only at rapid results at 2 hours after dosing. “In my experience, patients are often satisfied with the response times from these newer agents; and doctors and patients both are happy that they are not vasoconstrictive,” he said. “The researchers also omitted studies looking at zolmitriptan nasal spray, which I have found to be rapid in onset and efficacious with few adverse events.”
Finally, Dr. Rapoport noted that one condition not examined in the review was medication overuse headache (MOH), which is “a major problem with patients that have high-frequency episodic migraine and chronic migraine. To our knowledge thus far, the two gepants (ubrogepant and rimegepant) do not appear to cause MOH when taken frequently, and these agents may end up being a treatment for this condition.”
Dr Wang reported receiving personal fees from Eli Lilly, Daiichi-Sankyo, Norvatis Taiwan, Biogen, Pfizer, and Bayer; and grants from AbbVie, Norvatis, Eli Lilly, Taiwan Ministry of Technology and Science, Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. No other disclosures were reported. Dr. Rapoport serves as an advisor for AbbVie, Amgen, Biohaven, Cala Health, Satsuma, Teva Pharmaceutical Industries, Theranica, Xoc and Zosano; he is on the Speakers Bureau of AbbVie, Amgen, Biohaven, Lundbeck and Teva Pharmaceutical Industries. He is Editor-in-Chief of Neurology Reviews.
The study was funded by the Ministry of Science and Technology, Taiwan, Ministry of Education, Taiwan, and the Brain Research Center, National Yang Ming Chiao Tung University.
FROM JAMA NETWORK OPEN
TKI/BiTE combo extends survival of older patients with Ph+ALL
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Older patients with acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) are often not fit enough to withstand intensive chemotherapy and stem cell transplants, but remissions with alternative therapies are usually short lived.
Now, results from an ongoing study suggest that the combination of the
The new results were reported by investigators in the SWOG Cancer Research Network and come from a cohort of 25 patients with a median age of 73 years with newly diagnosed Ph+ALL or ALL with dasatinib-sensitive fusions of mutations (Ph-like ALL).
Nearly all (23 of 25 patients, 92%) had complete remissions, and 5 of 16 patients for whom minimal residual disease (MRD) data were available were MRD negative at day 28, said Anjali Advani, MD, from the Cleveland Clinic.
At a median follow-up of 1.7 years, the estimated 3-year disease-free survival rate was 80%, and the estimated overall survival rate was 85%, the investigators reported in a poster presentation at the annual meeting of the American Society of Hematology.
“I think the biggest question will be longer-term follow-up. We clearly see high remission rates in this population, but the issue is whether in these elderly patients who are not candidates for chemo we can prolong remission by the addition of other treatments, such as blinatumomab,” she said in an interview with this news organization.
“The follow-up is reasonable at this point, and as we get longer follow-up, if the current 3-year survival estimates hold up, that would be very encouraging,” she said.
Early promise
A leukemia specialist who was not involved in the study told this news organization that the results are promising, but added that it’s too early to make definitive judgments about the efficacy of the combination.
“People have used just a tyrosine kinase inhibitor and prednisone in these patients and gotten remissions, but they just don’t last,” said Peter Emanuel, MD, from CHI St. Vincent Infirmary in Little Rock, Ark.
“The promise with this approach is that you’re getting a longer-lasting remission – maybe not a cure, but a longer-lasting remission – without having to use intensive chemotherapy,” he said.
“It’s still a pretty small study, so I think this is going to require a bigger trial, looking at more patients, but it’s certainly very encouraging and very promising,” he added.
Hanno Hock, MD, PhD, a leukemia researcher at the Mass General Cancer Center in Boston, said in an interview that “the whole idea here is to add this newer agent, blinatumomab, to make those good initial responses more durable, and it looks like it is able to do that with very impressive initial data,” he said.
“The caveat is that this is still early, and one needs to wait and see how it all pans out, but it’s very well tolerated, and definitely the next logical step in trying to offer something to people who cannot tolerate more aggressive therapy such as transplant,” Dr. Hock added.
Study results
The new results come from a feasibility cohort of patients enrolled in the SWOG S1318 trial, which studied blinatumomab plus chemotherapy and prednisone in older patients with Ph-ALL, as well as blinatumomab, dasatinib, and prednisone in older adults with Ph+ ALL.
Patients 65 and older with newly diagnosed or relapsed/refractory Ph+ALL or Ph-like ALL and no central nervous system disease were eligible for the arm of the trial described here. All patients with data reported in this analysis had newly diagnosed ALL.
Patients first received a single induction cycle of dasatinib and prednisone and were then evaluated for response. Patients with a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) would then undergo prednisone tapering while continuing dasatinib until day 84. Patients without a CR or CRi at day 28 who had remissions by day 56 then also continued dasatinib until day 84.
Those patients still in remission at day 84 went on to three cycles of blinatumomab and dasatinib, followed by dasatinib and prednisone maintenance until unacceptable toxicity or disease progression. Patients may remain on maintenance for up to 10 years after registration.
Patients who do not have a CR or CRi by day 84 can receive reinduction with up to two total cycles of blinatumomab, with those who get a remission moving on to the blinatumomab/ dasatinib combination and those who do not going off protocol.
Of the 25 patients, 23 had a CR following dasatinib/prednisone induction. As noted, 5 of 16 patients evaluable for MRD were MRD negative.
Four patients did not receive postremission therapy, two because of adverse events, one who went on to transplant, and one because of insurance issues.
In a safety review early in the study, 4 of 12 evaluable patients were found to have dose-limiting toxicities, including one case each of grade 3 dyspnea and gastrointestinal pain (in a single patient), hypertension, dyspnea, and hyperglycemia.
These adverse events were deemed acceptable by both U.S. Food and Drug Administration and National Cancer Institute reviewers, and this arm of the study was allowed to continue, Dr. Advani noted.
The study was funded by grants from the National Institutes of Health. Dr. Advani disclosed financial relationships with several companies. Dr. Emanuel and Dr. Hock have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASH 2021
‘Outstanding data’: Mosunetuzumab in r/r follicular lymphoma
An experimental bi-specific monoclonal antibody known as mosunetuzumab has induced high response rates and long-duration responses as monotherapy for patients with heavily pretreated, relapsed or refractory follicular lymphoma in a phase 2 expansion study.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab, reported L. Elizabeth Budde, MD, PhD, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
In contrast, the complete response rate for historical controls was just 14% (P < .0001), Dr. Budde noted.
“We have seen deep and durable responses in heavily pretreated, high-risk relapsed/refractory follicular lymphoma patients with fixed-duration treatment. We also observed a very favorable tolerability profile, with most cytokine release syndrome confined to cycle 1 and low grade, and treatment administration is without mandatory hospitalization,” she commented.
Budde was speaking at a press briefing prior to her presentation of the data at the annual meeting of the American Society of Hematology (ASH), held in a hybrid live/virtual format.
The manufacturer, Genentech, said in a statement that based on these “highly positive results,” it plans to submit the new data to the U.S. Food and Drug Administration (FDA) in the near future for approval consideration.
If approved, mosunetuzumab has the potential to be a first-in-class CD20xCD3 T-cell engaging bispecific antibody in non-Hodgkin lymphoma, the company added.
“Outstanding” data
A lymphoma specialist who was not involved in the study told this news organization that he was favorably impressed by the findings.
“To me, the single-agent data looks really outstanding, with a response rate of 80%, a complete response rate of 60%, and a median duration of response of 23 months, and really very acceptable rates of cytokine release syndrome,” commented Brad S. Kahl, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis.
“I think as a single agent — if it does get approval — it will be a really valuable addition to the armamentarium in follicular lymphoma,” he said.
Dr. Kahl pointed to a separate phase 1b study, also presented at the meeting, suggesting that the combination of mosunetuzumab and lenalidomide (Revlimid) was safe and showed promising antitumor activity in patients with follicular lymphoma that has relapsed after at least 1 line of therapy.
“I’m very interested to see how mosunetuzumab plus lenalidomide pans out in the long run,” he said.
Study details
Mosunetuzumab engages T cells and redirects them to eliminate malignant B cells. It has the potential to be used as an off-the-shelf product, Dr. Budde said.
In the single-arm phase 2 expansion trial, Dr. Budde and colleagues enrolled 90 patients with grades 1 to 3a follicular lymphoma whose disease relapsed or was refractory to at least two prior lines of therapy, including at least one anti-CD20 monoclonal antibody, and at least one alkylating agent.
Patients were treated with step-up dosing for the first 21-day cycle to mitigate the cytokine release syndrome. They then received eight cycles if they had a complete response, and 17 cycles if they had a partial response or stable disease after eight cycles.
The primary endpoint was complete response rate by independent review, which was 60%, and the overall response rate (ORR), a secondary efficacy endpoint, was 80%.
There were no significant differences in CR or ORR rates among subgroups according to patient age, number of prior lines of therapy, relapsed or refractory disease to last prior line of therapy, double-refractory disease, or disease progression within 24 months of primary therapy.
The median duration of response among all responders was 22.8 months, with a median time to first response of 1.4 months. The 12- and 18-months event-free rates were 62% and 57%, respectively.
The safety profile was manageable, Dr. Budde said, with grade 3 or 4 drug-related adverse events occurring in about half of patients, and serious adverse events occurring in a third.
There were two deaths during the study, but neither was judged to be related to mosunetuzumab, and there were only two events leading to drug discontinuation.
Cytokine release syndrome (CRS) of any grade occurred in 40 patients (44.4%), but only 1 patient each had a grade 3 or 4 CR. The median time to CRS onset was 5.2 hours in cycle 1, and 26.6 hours in subsequent cycles. The median duration of CRS was 3 days. Ten patients had CRS managed with corticosteroids, and seven had it managed with tocilizumab.
Immune effector cell-associated neurotoxicity syndrome (ICANS) events were infrequent, and all were grade 1 or 2 in severity.
The study was supported by Genentech. Dr. Budde disclosed consulting for the company and others. Dr. Kahl has previously disclosed financial considerations with AbbVie.
A version of this article first appeared on Medscape.com.
This article was updated 12/12/21.
An experimental bi-specific monoclonal antibody known as mosunetuzumab has induced high response rates and long-duration responses as monotherapy for patients with heavily pretreated, relapsed or refractory follicular lymphoma in a phase 2 expansion study.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab, reported L. Elizabeth Budde, MD, PhD, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
In contrast, the complete response rate for historical controls was just 14% (P < .0001), Dr. Budde noted.
“We have seen deep and durable responses in heavily pretreated, high-risk relapsed/refractory follicular lymphoma patients with fixed-duration treatment. We also observed a very favorable tolerability profile, with most cytokine release syndrome confined to cycle 1 and low grade, and treatment administration is without mandatory hospitalization,” she commented.
Budde was speaking at a press briefing prior to her presentation of the data at the annual meeting of the American Society of Hematology (ASH), held in a hybrid live/virtual format.
The manufacturer, Genentech, said in a statement that based on these “highly positive results,” it plans to submit the new data to the U.S. Food and Drug Administration (FDA) in the near future for approval consideration.
If approved, mosunetuzumab has the potential to be a first-in-class CD20xCD3 T-cell engaging bispecific antibody in non-Hodgkin lymphoma, the company added.
“Outstanding” data
A lymphoma specialist who was not involved in the study told this news organization that he was favorably impressed by the findings.
“To me, the single-agent data looks really outstanding, with a response rate of 80%, a complete response rate of 60%, and a median duration of response of 23 months, and really very acceptable rates of cytokine release syndrome,” commented Brad S. Kahl, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis.
“I think as a single agent — if it does get approval — it will be a really valuable addition to the armamentarium in follicular lymphoma,” he said.
Dr. Kahl pointed to a separate phase 1b study, also presented at the meeting, suggesting that the combination of mosunetuzumab and lenalidomide (Revlimid) was safe and showed promising antitumor activity in patients with follicular lymphoma that has relapsed after at least 1 line of therapy.
“I’m very interested to see how mosunetuzumab plus lenalidomide pans out in the long run,” he said.
Study details
Mosunetuzumab engages T cells and redirects them to eliminate malignant B cells. It has the potential to be used as an off-the-shelf product, Dr. Budde said.
In the single-arm phase 2 expansion trial, Dr. Budde and colleagues enrolled 90 patients with grades 1 to 3a follicular lymphoma whose disease relapsed or was refractory to at least two prior lines of therapy, including at least one anti-CD20 monoclonal antibody, and at least one alkylating agent.
Patients were treated with step-up dosing for the first 21-day cycle to mitigate the cytokine release syndrome. They then received eight cycles if they had a complete response, and 17 cycles if they had a partial response or stable disease after eight cycles.
The primary endpoint was complete response rate by independent review, which was 60%, and the overall response rate (ORR), a secondary efficacy endpoint, was 80%.
There were no significant differences in CR or ORR rates among subgroups according to patient age, number of prior lines of therapy, relapsed or refractory disease to last prior line of therapy, double-refractory disease, or disease progression within 24 months of primary therapy.
The median duration of response among all responders was 22.8 months, with a median time to first response of 1.4 months. The 12- and 18-months event-free rates were 62% and 57%, respectively.
The safety profile was manageable, Dr. Budde said, with grade 3 or 4 drug-related adverse events occurring in about half of patients, and serious adverse events occurring in a third.
There were two deaths during the study, but neither was judged to be related to mosunetuzumab, and there were only two events leading to drug discontinuation.
Cytokine release syndrome (CRS) of any grade occurred in 40 patients (44.4%), but only 1 patient each had a grade 3 or 4 CR. The median time to CRS onset was 5.2 hours in cycle 1, and 26.6 hours in subsequent cycles. The median duration of CRS was 3 days. Ten patients had CRS managed with corticosteroids, and seven had it managed with tocilizumab.
Immune effector cell-associated neurotoxicity syndrome (ICANS) events were infrequent, and all were grade 1 or 2 in severity.
The study was supported by Genentech. Dr. Budde disclosed consulting for the company and others. Dr. Kahl has previously disclosed financial considerations with AbbVie.
A version of this article first appeared on Medscape.com.
This article was updated 12/12/21.
An experimental bi-specific monoclonal antibody known as mosunetuzumab has induced high response rates and long-duration responses as monotherapy for patients with heavily pretreated, relapsed or refractory follicular lymphoma in a phase 2 expansion study.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab, reported L. Elizabeth Budde, MD, PhD, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
In contrast, the complete response rate for historical controls was just 14% (P < .0001), Dr. Budde noted.
“We have seen deep and durable responses in heavily pretreated, high-risk relapsed/refractory follicular lymphoma patients with fixed-duration treatment. We also observed a very favorable tolerability profile, with most cytokine release syndrome confined to cycle 1 and low grade, and treatment administration is without mandatory hospitalization,” she commented.
Budde was speaking at a press briefing prior to her presentation of the data at the annual meeting of the American Society of Hematology (ASH), held in a hybrid live/virtual format.
The manufacturer, Genentech, said in a statement that based on these “highly positive results,” it plans to submit the new data to the U.S. Food and Drug Administration (FDA) in the near future for approval consideration.
If approved, mosunetuzumab has the potential to be a first-in-class CD20xCD3 T-cell engaging bispecific antibody in non-Hodgkin lymphoma, the company added.
“Outstanding” data
A lymphoma specialist who was not involved in the study told this news organization that he was favorably impressed by the findings.
“To me, the single-agent data looks really outstanding, with a response rate of 80%, a complete response rate of 60%, and a median duration of response of 23 months, and really very acceptable rates of cytokine release syndrome,” commented Brad S. Kahl, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis.
“I think as a single agent — if it does get approval — it will be a really valuable addition to the armamentarium in follicular lymphoma,” he said.
Dr. Kahl pointed to a separate phase 1b study, also presented at the meeting, suggesting that the combination of mosunetuzumab and lenalidomide (Revlimid) was safe and showed promising antitumor activity in patients with follicular lymphoma that has relapsed after at least 1 line of therapy.
“I’m very interested to see how mosunetuzumab plus lenalidomide pans out in the long run,” he said.
Study details
Mosunetuzumab engages T cells and redirects them to eliminate malignant B cells. It has the potential to be used as an off-the-shelf product, Dr. Budde said.
In the single-arm phase 2 expansion trial, Dr. Budde and colleagues enrolled 90 patients with grades 1 to 3a follicular lymphoma whose disease relapsed or was refractory to at least two prior lines of therapy, including at least one anti-CD20 monoclonal antibody, and at least one alkylating agent.
Patients were treated with step-up dosing for the first 21-day cycle to mitigate the cytokine release syndrome. They then received eight cycles if they had a complete response, and 17 cycles if they had a partial response or stable disease after eight cycles.
The primary endpoint was complete response rate by independent review, which was 60%, and the overall response rate (ORR), a secondary efficacy endpoint, was 80%.
There were no significant differences in CR or ORR rates among subgroups according to patient age, number of prior lines of therapy, relapsed or refractory disease to last prior line of therapy, double-refractory disease, or disease progression within 24 months of primary therapy.
The median duration of response among all responders was 22.8 months, with a median time to first response of 1.4 months. The 12- and 18-months event-free rates were 62% and 57%, respectively.
The safety profile was manageable, Dr. Budde said, with grade 3 or 4 drug-related adverse events occurring in about half of patients, and serious adverse events occurring in a third.
There were two deaths during the study, but neither was judged to be related to mosunetuzumab, and there were only two events leading to drug discontinuation.
Cytokine release syndrome (CRS) of any grade occurred in 40 patients (44.4%), but only 1 patient each had a grade 3 or 4 CR. The median time to CRS onset was 5.2 hours in cycle 1, and 26.6 hours in subsequent cycles. The median duration of CRS was 3 days. Ten patients had CRS managed with corticosteroids, and seven had it managed with tocilizumab.
Immune effector cell-associated neurotoxicity syndrome (ICANS) events were infrequent, and all were grade 1 or 2 in severity.
The study was supported by Genentech. Dr. Budde disclosed consulting for the company and others. Dr. Kahl has previously disclosed financial considerations with AbbVie.
A version of this article first appeared on Medscape.com.
This article was updated 12/12/21.
AT ASH 2021
‘Remarkable’ results with CAR T cells could make chemo obsolete
ATLANTA — Chimeric antigen receptor, results of the phase 3 ZUMA-7 and TRANSFORM trials suggest.
In the ZUMA-7 trial, at a median follow-up of 24.9 months, patients randomly assigned to receive CAR T-cell therapy with axicabtagene ciloleucel, or axi-cell (Yescarta) had a median event-free survival (EFS) of 8.3 months, compared with 2 months for patients randomly assigned to standard-of-care chemoimmunotherapy, reported Frederick L. Locke, MD, from the Moffitt Cancer Center in Tampa, Fla.
In TRANSFORM, comparing the CAR T construct lisocabtagene maraleucel, or liso-cel (Breyanzi) with standard-of-care second-line chemotherapy, median EFS was 10.1 months with liso-cel, compared with 2.3 months with standard of care, reported Manali Kamdar, MD, from the University of Colorado Cancer Center in Aurora.
The trials differed slightly in eligibility criteria and other details, but their overall results show great promise for improving second-line therapy for patients with relapsed or refractory LBCL, commented Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer in Vancouver, Canada.
“It’s really remarkable that the results are so far in favor of the CAR T-cell therapy that I think it’s inevitable that this will become the standard of care,” Dr. Sehn commented. She was not an investigator in either of the two trials.
Dr. Sehn was speaking at a press briefing here during the annual meeting of the American Society of Hematology. The new data from the two studies were presented at oral sessions, and the results from ZUMA-7 were also simultaneously published in the New England Journal of Medicine.
“For somebody who treats patients with large B-cell lymphoma like I do, it’s incredibly frustrating when patients fail frontline therapy,” Dr. Sehn said. “We come into the second line with more chemotherapy and at higher doses to try and slam things down hard. Particularly for the patients who were enrolled in these studies, which were the worst of the worst — the patients who are either refractory to chemotherapy or relapsed relatively early, within 1 year — it’s not surprising that coming in with a novel approach and a cellular therapy that has a proven curative capacity may have outperformed coming in with more chemotherapy.”
In an interview with this news organization, Dr. Locke said that, based on the findings of the ZUMA-7 trial that he presented, it’s likely that chemotherapy in the second-line setting for relapsed/refractory LBCL will largely fall by the wayside.
The first question is to identify the patients who can tolerate CAR T-cell therapy. “We need to refer these patients to a CAR T-cell center to make that decision. That decision really can’t be made in the local oncologist’s office,” he said. “That being said, there are patients who need urgent therapy, and they may need to get second-line chemotherapy right away.”
“What we know with CAR T cells is that older patients and patients with comorbidities can get these therapies safely, so to me there is no obvious patient who can’t get CAR T-cell therapy,” he added.
Also at the briefing, Dr. Kamdar, who presented the TRANSFORM trial results, remarked that “in my opinion, this is a breakthrough therapy, which has shown superiority over standard of care, in terms of not just efficacy but also an extremely favorable safety profile,” she said at a briefing.
For patients with LBCL for whom first-line therapy has failed, chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) has been the standard of care, but only about 25% of patients who are candidates for ASCT achieve durable remissions, Dr. Kamdar noted.
Both ZUMA-7 and TRANSFORM were designed to test whether moving CAR T-cell therapy forward into the second line could improve outcomes.
ZUMA-7 results
THE ZUMA-7 trial randomly assigned 180 patients to receive CAR T-cell therapy with axi-cell and 179 patients to standard of care. This consisted of two or three cycles of investigator-selected, protocol-defined chemoimmunotherapy, with patients who had a complete or partial response going onto ASCT.
As noted, the primary endpoint of EFS according to blinded central review favored axi-cel, with 24-month event-free survival rates of 41% vs. 16% for standard of care. The difference translated into a hazard ratio (HR) for progression or death of 0.40 (P < .001).
In all, 65% of patients had a complete response (CR) to axi-cel, compared with 32% with standard of care. The respective overall response rates were 83% and 50% (P < .001).
Dr. Locke pointed out that 94% of the patients assigned to axi-cel received definitive therapy, compared with the 36% of patients in the standard-of-care arm who went on to ASCT.
In an interim analysis, 2-year estimated overall survival was 61% with axi-cel vs. 52% with standard of care, although this difference was not statistically significant.
Median overall survival was not reached with axi-cel, compared with 35.1 months with standard-of-care.
Grade 3 or higher adverse events occurred in 91% of patients with CAR T, and 83% with the standard of care. In the axi-cel arm, 6% of patients had grade 3 or higher cytokine release syndrome (CRS), and 21% had grade 3 or higher neurologic events, although there were no deaths related to CRS or neurologic events.
TRANSFORM results
The TRANSFORM trial had broader eligibility criteria than ZUMA-7, including patients who had diffuse LBCL not otherwise specified (de novo or transformed from indolent NHL), high-grade BCL (double- or triple-hit) with DLBCL histology, follicular lymphoma grade 3B, primary mediastinal LBCL, or T-cell/histocyte-rich LBCL.
A total of 184 patients were randomly assigned, 92 in each group, to receive either liso-cel or standard-of-care. Patients assigned to liso-cel were allowed to have bridging therapy, and crossover to liso-cel was allowed for patients assigned to standard of care who either did not have a response by week 9 after randomization, had disease progression at any time, or started a new antineoplastic therapy after ASCT.
As noted before, the primary endpoint of EFS significantly favored CAR T-cell therapy, with a hazard ratio of 0.349 (P < .0001).
The EFS rates at 6 months were 63.3% with liso-cel vs 33.4% with standard of care, and the EFS rates at 12 months were 44.5% vs. 23.7%, respectively.
“Overall survival data were still immature at the time of this analysis, but show a trend favoring liso-cel, despite crossover,” Dr. Kamdar said.
Grade 3 or higher adverse events (AEs) occurred in 92% of patients on liso-cell and 87% of patients on standard of care. There was one treatment-related death in the liso-cel arm, and two in the standard of care arm, both from grade 3 or higher AEs. Neutropenia, anemia, and thrombocytopenia were the most common treatment-emergent AEs in each group.
ZUMA-7 is supported by Kite. Dr. Locke disclosed serving as a scientific advisor to Kite and relationships with other companies. TRANSFORM is supported by Celgene (BMS). Dr. Kamdar disclosed consultancy fees from BMS and others.
A version of this article first appeared on Medscape.com.
ATLANTA — Chimeric antigen receptor, results of the phase 3 ZUMA-7 and TRANSFORM trials suggest.
In the ZUMA-7 trial, at a median follow-up of 24.9 months, patients randomly assigned to receive CAR T-cell therapy with axicabtagene ciloleucel, or axi-cell (Yescarta) had a median event-free survival (EFS) of 8.3 months, compared with 2 months for patients randomly assigned to standard-of-care chemoimmunotherapy, reported Frederick L. Locke, MD, from the Moffitt Cancer Center in Tampa, Fla.
In TRANSFORM, comparing the CAR T construct lisocabtagene maraleucel, or liso-cel (Breyanzi) with standard-of-care second-line chemotherapy, median EFS was 10.1 months with liso-cel, compared with 2.3 months with standard of care, reported Manali Kamdar, MD, from the University of Colorado Cancer Center in Aurora.
The trials differed slightly in eligibility criteria and other details, but their overall results show great promise for improving second-line therapy for patients with relapsed or refractory LBCL, commented Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer in Vancouver, Canada.
“It’s really remarkable that the results are so far in favor of the CAR T-cell therapy that I think it’s inevitable that this will become the standard of care,” Dr. Sehn commented. She was not an investigator in either of the two trials.
Dr. Sehn was speaking at a press briefing here during the annual meeting of the American Society of Hematology. The new data from the two studies were presented at oral sessions, and the results from ZUMA-7 were also simultaneously published in the New England Journal of Medicine.
“For somebody who treats patients with large B-cell lymphoma like I do, it’s incredibly frustrating when patients fail frontline therapy,” Dr. Sehn said. “We come into the second line with more chemotherapy and at higher doses to try and slam things down hard. Particularly for the patients who were enrolled in these studies, which were the worst of the worst — the patients who are either refractory to chemotherapy or relapsed relatively early, within 1 year — it’s not surprising that coming in with a novel approach and a cellular therapy that has a proven curative capacity may have outperformed coming in with more chemotherapy.”
In an interview with this news organization, Dr. Locke said that, based on the findings of the ZUMA-7 trial that he presented, it’s likely that chemotherapy in the second-line setting for relapsed/refractory LBCL will largely fall by the wayside.
The first question is to identify the patients who can tolerate CAR T-cell therapy. “We need to refer these patients to a CAR T-cell center to make that decision. That decision really can’t be made in the local oncologist’s office,” he said. “That being said, there are patients who need urgent therapy, and they may need to get second-line chemotherapy right away.”
“What we know with CAR T cells is that older patients and patients with comorbidities can get these therapies safely, so to me there is no obvious patient who can’t get CAR T-cell therapy,” he added.
Also at the briefing, Dr. Kamdar, who presented the TRANSFORM trial results, remarked that “in my opinion, this is a breakthrough therapy, which has shown superiority over standard of care, in terms of not just efficacy but also an extremely favorable safety profile,” she said at a briefing.
For patients with LBCL for whom first-line therapy has failed, chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) has been the standard of care, but only about 25% of patients who are candidates for ASCT achieve durable remissions, Dr. Kamdar noted.
Both ZUMA-7 and TRANSFORM were designed to test whether moving CAR T-cell therapy forward into the second line could improve outcomes.
ZUMA-7 results
THE ZUMA-7 trial randomly assigned 180 patients to receive CAR T-cell therapy with axi-cell and 179 patients to standard of care. This consisted of two or three cycles of investigator-selected, protocol-defined chemoimmunotherapy, with patients who had a complete or partial response going onto ASCT.
As noted, the primary endpoint of EFS according to blinded central review favored axi-cel, with 24-month event-free survival rates of 41% vs. 16% for standard of care. The difference translated into a hazard ratio (HR) for progression or death of 0.40 (P < .001).
In all, 65% of patients had a complete response (CR) to axi-cel, compared with 32% with standard of care. The respective overall response rates were 83% and 50% (P < .001).
Dr. Locke pointed out that 94% of the patients assigned to axi-cel received definitive therapy, compared with the 36% of patients in the standard-of-care arm who went on to ASCT.
In an interim analysis, 2-year estimated overall survival was 61% with axi-cel vs. 52% with standard of care, although this difference was not statistically significant.
Median overall survival was not reached with axi-cel, compared with 35.1 months with standard-of-care.
Grade 3 or higher adverse events occurred in 91% of patients with CAR T, and 83% with the standard of care. In the axi-cel arm, 6% of patients had grade 3 or higher cytokine release syndrome (CRS), and 21% had grade 3 or higher neurologic events, although there were no deaths related to CRS or neurologic events.
TRANSFORM results
The TRANSFORM trial had broader eligibility criteria than ZUMA-7, including patients who had diffuse LBCL not otherwise specified (de novo or transformed from indolent NHL), high-grade BCL (double- or triple-hit) with DLBCL histology, follicular lymphoma grade 3B, primary mediastinal LBCL, or T-cell/histocyte-rich LBCL.
A total of 184 patients were randomly assigned, 92 in each group, to receive either liso-cel or standard-of-care. Patients assigned to liso-cel were allowed to have bridging therapy, and crossover to liso-cel was allowed for patients assigned to standard of care who either did not have a response by week 9 after randomization, had disease progression at any time, or started a new antineoplastic therapy after ASCT.
As noted before, the primary endpoint of EFS significantly favored CAR T-cell therapy, with a hazard ratio of 0.349 (P < .0001).
The EFS rates at 6 months were 63.3% with liso-cel vs 33.4% with standard of care, and the EFS rates at 12 months were 44.5% vs. 23.7%, respectively.
“Overall survival data were still immature at the time of this analysis, but show a trend favoring liso-cel, despite crossover,” Dr. Kamdar said.
Grade 3 or higher adverse events (AEs) occurred in 92% of patients on liso-cell and 87% of patients on standard of care. There was one treatment-related death in the liso-cel arm, and two in the standard of care arm, both from grade 3 or higher AEs. Neutropenia, anemia, and thrombocytopenia were the most common treatment-emergent AEs in each group.
ZUMA-7 is supported by Kite. Dr. Locke disclosed serving as a scientific advisor to Kite and relationships with other companies. TRANSFORM is supported by Celgene (BMS). Dr. Kamdar disclosed consultancy fees from BMS and others.
A version of this article first appeared on Medscape.com.
ATLANTA — Chimeric antigen receptor, results of the phase 3 ZUMA-7 and TRANSFORM trials suggest.
In the ZUMA-7 trial, at a median follow-up of 24.9 months, patients randomly assigned to receive CAR T-cell therapy with axicabtagene ciloleucel, or axi-cell (Yescarta) had a median event-free survival (EFS) of 8.3 months, compared with 2 months for patients randomly assigned to standard-of-care chemoimmunotherapy, reported Frederick L. Locke, MD, from the Moffitt Cancer Center in Tampa, Fla.
In TRANSFORM, comparing the CAR T construct lisocabtagene maraleucel, or liso-cel (Breyanzi) with standard-of-care second-line chemotherapy, median EFS was 10.1 months with liso-cel, compared with 2.3 months with standard of care, reported Manali Kamdar, MD, from the University of Colorado Cancer Center in Aurora.
The trials differed slightly in eligibility criteria and other details, but their overall results show great promise for improving second-line therapy for patients with relapsed or refractory LBCL, commented Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer in Vancouver, Canada.
“It’s really remarkable that the results are so far in favor of the CAR T-cell therapy that I think it’s inevitable that this will become the standard of care,” Dr. Sehn commented. She was not an investigator in either of the two trials.
Dr. Sehn was speaking at a press briefing here during the annual meeting of the American Society of Hematology. The new data from the two studies were presented at oral sessions, and the results from ZUMA-7 were also simultaneously published in the New England Journal of Medicine.
“For somebody who treats patients with large B-cell lymphoma like I do, it’s incredibly frustrating when patients fail frontline therapy,” Dr. Sehn said. “We come into the second line with more chemotherapy and at higher doses to try and slam things down hard. Particularly for the patients who were enrolled in these studies, which were the worst of the worst — the patients who are either refractory to chemotherapy or relapsed relatively early, within 1 year — it’s not surprising that coming in with a novel approach and a cellular therapy that has a proven curative capacity may have outperformed coming in with more chemotherapy.”
In an interview with this news organization, Dr. Locke said that, based on the findings of the ZUMA-7 trial that he presented, it’s likely that chemotherapy in the second-line setting for relapsed/refractory LBCL will largely fall by the wayside.
The first question is to identify the patients who can tolerate CAR T-cell therapy. “We need to refer these patients to a CAR T-cell center to make that decision. That decision really can’t be made in the local oncologist’s office,” he said. “That being said, there are patients who need urgent therapy, and they may need to get second-line chemotherapy right away.”
“What we know with CAR T cells is that older patients and patients with comorbidities can get these therapies safely, so to me there is no obvious patient who can’t get CAR T-cell therapy,” he added.
Also at the briefing, Dr. Kamdar, who presented the TRANSFORM trial results, remarked that “in my opinion, this is a breakthrough therapy, which has shown superiority over standard of care, in terms of not just efficacy but also an extremely favorable safety profile,” she said at a briefing.
For patients with LBCL for whom first-line therapy has failed, chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) has been the standard of care, but only about 25% of patients who are candidates for ASCT achieve durable remissions, Dr. Kamdar noted.
Both ZUMA-7 and TRANSFORM were designed to test whether moving CAR T-cell therapy forward into the second line could improve outcomes.
ZUMA-7 results
THE ZUMA-7 trial randomly assigned 180 patients to receive CAR T-cell therapy with axi-cell and 179 patients to standard of care. This consisted of two or three cycles of investigator-selected, protocol-defined chemoimmunotherapy, with patients who had a complete or partial response going onto ASCT.
As noted, the primary endpoint of EFS according to blinded central review favored axi-cel, with 24-month event-free survival rates of 41% vs. 16% for standard of care. The difference translated into a hazard ratio (HR) for progression or death of 0.40 (P < .001).
In all, 65% of patients had a complete response (CR) to axi-cel, compared with 32% with standard of care. The respective overall response rates were 83% and 50% (P < .001).
Dr. Locke pointed out that 94% of the patients assigned to axi-cel received definitive therapy, compared with the 36% of patients in the standard-of-care arm who went on to ASCT.
In an interim analysis, 2-year estimated overall survival was 61% with axi-cel vs. 52% with standard of care, although this difference was not statistically significant.
Median overall survival was not reached with axi-cel, compared with 35.1 months with standard-of-care.
Grade 3 or higher adverse events occurred in 91% of patients with CAR T, and 83% with the standard of care. In the axi-cel arm, 6% of patients had grade 3 or higher cytokine release syndrome (CRS), and 21% had grade 3 or higher neurologic events, although there were no deaths related to CRS or neurologic events.
TRANSFORM results
The TRANSFORM trial had broader eligibility criteria than ZUMA-7, including patients who had diffuse LBCL not otherwise specified (de novo or transformed from indolent NHL), high-grade BCL (double- or triple-hit) with DLBCL histology, follicular lymphoma grade 3B, primary mediastinal LBCL, or T-cell/histocyte-rich LBCL.
A total of 184 patients were randomly assigned, 92 in each group, to receive either liso-cel or standard-of-care. Patients assigned to liso-cel were allowed to have bridging therapy, and crossover to liso-cel was allowed for patients assigned to standard of care who either did not have a response by week 9 after randomization, had disease progression at any time, or started a new antineoplastic therapy after ASCT.
As noted before, the primary endpoint of EFS significantly favored CAR T-cell therapy, with a hazard ratio of 0.349 (P < .0001).
The EFS rates at 6 months were 63.3% with liso-cel vs 33.4% with standard of care, and the EFS rates at 12 months were 44.5% vs. 23.7%, respectively.
“Overall survival data were still immature at the time of this analysis, but show a trend favoring liso-cel, despite crossover,” Dr. Kamdar said.
Grade 3 or higher adverse events (AEs) occurred in 92% of patients on liso-cell and 87% of patients on standard of care. There was one treatment-related death in the liso-cel arm, and two in the standard of care arm, both from grade 3 or higher AEs. Neutropenia, anemia, and thrombocytopenia were the most common treatment-emergent AEs in each group.
ZUMA-7 is supported by Kite. Dr. Locke disclosed serving as a scientific advisor to Kite and relationships with other companies. TRANSFORM is supported by Celgene (BMS). Dr. Kamdar disclosed consultancy fees from BMS and others.
A version of this article first appeared on Medscape.com.
AT ASH 2021