User login
More New Therapeutics for Psoriasis
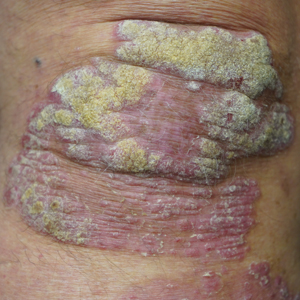
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
Commentary: A New Drug, and Pediatric Concerns, February 2023
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
Commentary: Evaluating Recent Drug Developments in Atopic Dermatitis, January 2023
When I hear about a new drug for inflammatory skin disease that has a novel target, the first thing I do is Google what happens when you have a deficiency in that pathway. For OX40, the first thing that comes up is "inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood."1 That doesn't make this target seem appealing to me. While I might use a new drug targeting this pathway if other options fail, drugs targeting this pathway would not be my first choice, even if clinical trial safety data looked good. Clinical trials are powered to assess efficacy and common safety issues but tend not to be large enough to fully characterize rare risks.
Black box warnings on topical calcineurin inhibitors seem dumb to me. I think black box warnings on topical calcineurin inhibitors would be truly ridiculous, even laughable, except that laughing is not appropriate because these misguided warnings may be hurting our patients. These black box warnings on topical calcineurin inhibitors may exemplify the limitations of governmental bureaucracies. There doesn't seem to be a strong rationale for why these black box warnings were placed on topical calcineurin inhibitors initially. Why regulators haven't removed these black box warnings since then is baffling, as topical calcineurin inhibitors are considerably safer for patients than the alternative, topical corticosteroids. We have good evidence that topical calcineurin inhibitors do not cause cancer in our patients. The continued presence of black box warnings on these products may undermine the credibility of FDA-mandated black box warnings on other products.
Hedderson and colleagues state, in a study of cardiovascular events and atopic dermatitis, "VTE [venous thromboembolism] and DVT [deep vein thrombosis] IRs [incidence rates] were markedly higher in this study than have been observed in the general US adult population (VTE: 2.0 [current study] vs. 1.1; DVT: 1.6 [current study] vs. 0.66 per 1000 person-years." I think that's misleading. The difference of only 1 in 1000 doesn't seem like a markedly higher rate to me and it's also unlikely to be clinically meaningful. Even if there is some increased relative risk of some type of cardiovascular event, even if the rate is doubled, that doesn't mean we need to screen or intervene. We need to be mindful of the absolute risks and not be moved by relative risks. We need to see cost-effectiveness studies showing that an intervention is valuable before we conclude that we should be doing some screening or intervention to chase down and increase the relative risk for some potential adverse event.
Additional Reference
- Byun M, Ma CS, Akçay A et al. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med. 2013;210(9):1743–1759. Doi: 10.1084/jem.20130592
When I hear about a new drug for inflammatory skin disease that has a novel target, the first thing I do is Google what happens when you have a deficiency in that pathway. For OX40, the first thing that comes up is "inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood."1 That doesn't make this target seem appealing to me. While I might use a new drug targeting this pathway if other options fail, drugs targeting this pathway would not be my first choice, even if clinical trial safety data looked good. Clinical trials are powered to assess efficacy and common safety issues but tend not to be large enough to fully characterize rare risks.
Black box warnings on topical calcineurin inhibitors seem dumb to me. I think black box warnings on topical calcineurin inhibitors would be truly ridiculous, even laughable, except that laughing is not appropriate because these misguided warnings may be hurting our patients. These black box warnings on topical calcineurin inhibitors may exemplify the limitations of governmental bureaucracies. There doesn't seem to be a strong rationale for why these black box warnings were placed on topical calcineurin inhibitors initially. Why regulators haven't removed these black box warnings since then is baffling, as topical calcineurin inhibitors are considerably safer for patients than the alternative, topical corticosteroids. We have good evidence that topical calcineurin inhibitors do not cause cancer in our patients. The continued presence of black box warnings on these products may undermine the credibility of FDA-mandated black box warnings on other products.
Hedderson and colleagues state, in a study of cardiovascular events and atopic dermatitis, "VTE [venous thromboembolism] and DVT [deep vein thrombosis] IRs [incidence rates] were markedly higher in this study than have been observed in the general US adult population (VTE: 2.0 [current study] vs. 1.1; DVT: 1.6 [current study] vs. 0.66 per 1000 person-years." I think that's misleading. The difference of only 1 in 1000 doesn't seem like a markedly higher rate to me and it's also unlikely to be clinically meaningful. Even if there is some increased relative risk of some type of cardiovascular event, even if the rate is doubled, that doesn't mean we need to screen or intervene. We need to be mindful of the absolute risks and not be moved by relative risks. We need to see cost-effectiveness studies showing that an intervention is valuable before we conclude that we should be doing some screening or intervention to chase down and increase the relative risk for some potential adverse event.
Additional Reference
- Byun M, Ma CS, Akçay A et al. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med. 2013;210(9):1743–1759. Doi: 10.1084/jem.20130592
When I hear about a new drug for inflammatory skin disease that has a novel target, the first thing I do is Google what happens when you have a deficiency in that pathway. For OX40, the first thing that comes up is "inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood."1 That doesn't make this target seem appealing to me. While I might use a new drug targeting this pathway if other options fail, drugs targeting this pathway would not be my first choice, even if clinical trial safety data looked good. Clinical trials are powered to assess efficacy and common safety issues but tend not to be large enough to fully characterize rare risks.
Black box warnings on topical calcineurin inhibitors seem dumb to me. I think black box warnings on topical calcineurin inhibitors would be truly ridiculous, even laughable, except that laughing is not appropriate because these misguided warnings may be hurting our patients. These black box warnings on topical calcineurin inhibitors may exemplify the limitations of governmental bureaucracies. There doesn't seem to be a strong rationale for why these black box warnings were placed on topical calcineurin inhibitors initially. Why regulators haven't removed these black box warnings since then is baffling, as topical calcineurin inhibitors are considerably safer for patients than the alternative, topical corticosteroids. We have good evidence that topical calcineurin inhibitors do not cause cancer in our patients. The continued presence of black box warnings on these products may undermine the credibility of FDA-mandated black box warnings on other products.
Hedderson and colleagues state, in a study of cardiovascular events and atopic dermatitis, "VTE [venous thromboembolism] and DVT [deep vein thrombosis] IRs [incidence rates] were markedly higher in this study than have been observed in the general US adult population (VTE: 2.0 [current study] vs. 1.1; DVT: 1.6 [current study] vs. 0.66 per 1000 person-years." I think that's misleading. The difference of only 1 in 1000 doesn't seem like a markedly higher rate to me and it's also unlikely to be clinically meaningful. Even if there is some increased relative risk of some type of cardiovascular event, even if the rate is doubled, that doesn't mean we need to screen or intervene. We need to be mindful of the absolute risks and not be moved by relative risks. We need to see cost-effectiveness studies showing that an intervention is valuable before we conclude that we should be doing some screening or intervention to chase down and increase the relative risk for some potential adverse event.
Additional Reference
- Byun M, Ma CS, Akçay A et al. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J Exp Med. 2013;210(9):1743–1759. Doi: 10.1084/jem.20130592
Factors Influencing Patient Preferences for Phototherapy: A Survey Study
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Practice Points
- Patients have different priorities when selecting phototherapy, including safety, costs, effectiveness, insurance issues, and convenience.
- By offering and educating patients on all forms of phototherapy, dermatologists may help guide patients to their optimal treatment plan according to patient priorities.
Views and Beliefs of Vitiligo Patients in Online Discussion Forums: A Qualitative Study
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25
Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.
An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25

Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.

An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
Vitiligo is a chronic dermatologic condition that negatively affects quality of life (QOL), with substantial burden on the psychosocial well-being of patients.1 There is no cure, and current treatment modalities are aimed at controlling the chronic relapsing condition.1-3 Despite topical and cosmetic treatments for stabilization and repigmentation, vitiligo remains unpredictable.3
All genders, races, ethnicities, and socioeconomic classes are equally affected.4 The underlying etiology of vitiligo remains unknown to a great extent and is more poorly understood by the general public compared with other skin diseases (eg, acne).5 Patients with vitiligo experience social withdrawal, decreased sense of self-esteem, anxiety, depression, and suicidal ideation.5,6 Stigmatization has the greatest impact on QOL, with strong correlations between avoidance behaviors and lesion concealment.6-8 Although the condition is especially disfiguring for darker skin types, lighter skin types also are substantially affected, with similar overall self-reported stress.6,7
Individuals with chronic illnesses such as vitiligo turn to online communities for health information and social support, commiserating with others who have the same condition.9,10 Online forums are platforms for asynchronous peer-to-peer exchange of disease-related information for better management of long-term disease.11 Moreover, of all available internet resources, online forum posts are the most commonly accessed source of information (91%) for patients following visits with their doctors.12
Qualitative research involving chronic skin conditions and the information exchanged in online forums has been conducted for patients with acne, psoriasis, and atopic dermatitis, but not for patients with vitiligo.13-16 Although online questionnaires have been administered to patients with vitiligo, the content within online forums is not well characterized.2,17
The purpose of this qualitative study was to evaluate the online content exchanged by individuals with vitiligo to better understand the general attitudes and help-seeking behaviors in online forums.
Methods
Study Design—This qualitative study sought to investigate health beliefs and messages about vitiligo posted by users in US-based online discussion forums. An interpretive research paradigm was utilized so that all content collected in online forums were the views expressed by individuals.18-20 An integrated approach was used in the development of the coding manual, with pre-established major themes and subthemes as a guiding framework.16,21,22 We adhered to an inductive grounded method by means of de novo line-by-line coding, such that we had flexibility for new subthemes to emerge throughout the duration of the entire coding process.23
Individual posts and subsequent replies embedded within public online forums were used as the collected data source. Google was utilized as the primary search engine to identify forums pertaining to vitiligo, as 80% of US adults with chronic disease report that their inquiries for health information start with Google, Bing, or Yahoo.24 The institutional review board at the Wake Forest School of Medicine (Winston-Salem, North Carolina) granted approval of the study (IRB00063073). Online forums were considered “property” of the public domain and were accessible to all, eliminating the need for written informed consent.24-26
Search Criteria—We conducted our forum search in February 2020 with a systematic approach using predetermined phrases—online forum vitiligo support, vitiligo online message board, and vitiligo forums—which yielded more than 358,171 total results (eTable 1). Threads were identified in chronological order (from newest to oldest) based on how they appeared during each internet search, and all Google results for the respective search phrases were reviewed. Dates of selected threads ranged from 2005 to 2020. Only sites with US domains were included. Posts that either included views and understandings of vitiligo or belonged to a thread that contained a vitiligo discussion were deemed relevant for inclusion. Forums were excluded if registration or means of payment was required to view posts, if there were fewer than 2 user replies to a thread, if threads contained patient photographs, or if no posts had been made in the last 2 years (rendering the thread inactive). No social media platforms, such as Facebook, or formal online platforms, such as MyVitiligoTeam, were included in the search. A no-fee-for-access was chosen for this study, as the majority of those with a chronic condition who encounter a required paywall find the information elsewhere.25

Data Analysis—A total of 39 online forums were deemed relevant to the topic of vitiligo; 9 of them met inclusion criteria (eTable 2). The messages within the forums were copied verbatim into a password-encrypted text document, and usernames in the threads were de-identified, ensuring user confidentiality.

An inductive thematic analysis was utilized to explore the views and beliefs of online forum users discussing vitiligo. One author (M.B.G.) read the extracted message threads, developed an initial codebook, and established a finalized version with the agreement of another author (A.M.B.)(eTable 3). The forums were independently coded (M.B.G. and A.M.B.) in a line-by-line manner according to the codebook. Discrepancies were documented and resolved. Data saturation was adequately achieved, such that no new themes emerged during the iterative coding process. NVivo was used for qualitative analysis.

Results
Nine forums met inclusion criteria, comprising 105 pages of text. There were 61 total discussion threads, with 382 anonymous contributing users. Most users initiated a thread by posting either a question, an advice statement, or a request for help. The psychosocial impact of the disease permeated multiple domains,including personal relationships and daily life. Several threads discussed treatment, including effective camouflage and makeup, as well as peer validation of physician-prescribed treatments, along with threads dedicated to “cures” or homeopathy regimens. In several instances, commercial product endorsement, testimonials, and marketing links were reposted by the same user multiple times.
Inductive thematic analysis highlighted diverse themes and subthemes related to the beliefs and perspectives of users with vitiligo or with relatives or friends with vitiligo: psychosocial impact, disease management and camouflage/concealment, alternative medicine/homeopathy/cures, interactions with the public and health care providers, and skin tone and race. Quotes from individuals were included to demonstrate themes and subthemes.
Psychosocial Impact: QOL, Sources of Support, and Coping—There was a broad range of comments on how patients cope with and view their vitiligo. Some individuals felt vitiligo made them special, and others were at peace with and accepted their condition. In contrast, others reported the disease had devastated them and interfered with relationships. Individuals shared their stories of grief and hardships through childhood and adulthood and their concerns, especially on affected visible areas or the potential for disease progression. Users were vocal about how vitiligo affected their daily routines and lives, sharing how they felt uncomfortable outside the home, no longer engaged in swimming or exposing their legs, and preferred to stay inside instead. Some users adopted a “tough love” approach to coping, sharing how they have learned to either embrace their vitiligo or “live with it.” Some examples include:
“My best advice is go with the flow, vitiligo is not the worst thing that can happen.”
“I hate my life with vitiligo yet really I feel so selfish that there is much worse suffering in the world than a few white patches.”
Other advice was very practical:
“I hope it isn’t vanity that is tearing you apart because that is only skin deep. Make a fashion statement with hats.”
Some users acknowledged and adopted the mantra that vitiligo is not a somatic condition or “physical ailment,” while others emphasized its pervasive psychological burden:
“I still deal with this psychologically . . . You must keep a positive attitude and frame of mind . . . Vitiligo will not kill you, but you do need to stay strong and keep your head up emotionally.”
“I am just really thankful that I have a disease that will not kill me or that has [not] affected me physically at all. I consider myself lucky.”
Disease Management: Treatment, Vitiligo Course, Advice-Seeking, Camouflage—The range of information discussed for treatment was highly variable. There were many accounts in which users advised others to seek professional help, namely that of a dermatologist, for a formal assessment. Many expressed frustrations with treatments and their ineffectiveness, to which the majority of users said to consult with a professional and to remain patient and hopeful/optimistic:
“The best thing to do would be to take an appointment with a dermatologist and have the discoloration checked out. That’s the only way to know whether it is vitiligo or not.”
“My way of dealing with it is to gain control by camouflage.”
“The calming effect of being in control of my vitiligo, whether with concealers, self-tan or anything else, has stopped my feelings of despair.”
Beliefs on Alternative Medicine: Homeopathy and Alternative Regimens—Although some threads started with a post asking for the best treatments, others initiated a discussion by posting “best herbal treatments for cure” or “how to cure my vitiligo,” emphasizing the beliefs and wishes for a cure for vitiligo. Alternative therapies that users endorsed included apple cider vinegar, toothpaste, vitamins, and Ayurvedic treatment, among others. Dietary plans were popular, with users claiming success with dietary alterations in stopping and preventing lesion progression. For example, individuals felt that avoidance of sugar, meat, dairy, and citrus fruits or drinks and consumption of only filtered water were crucial to preventing further lesion spread and resulted in their “cure”:
“Don’t eat chocolate, wine (made of grapes), coffee, or tea if you don’t want to have vitiligo or let it get worse. Take Vitamin B, biotin, and nuts for Vitamin E.”
Other dangerous messages pitted treatments by health professionals against beliefs in homeopathy:
“I feel that vitiligo treatment is all in your diet and vitamins. All that medicine and UV lights is a no-no . . .w ith every medicine there is a side effect. The doctors could be healing your vitiligo and severely damaging you inside and out, and you won’t know until years later.”
There was a minor presence of users advising against homeopathy and the associated misinformation and inaccurate claims on curing vitiligo, though this group was small in comparison to the number of users posting outlandish claims on cure:
“There is no cure . . . It’s where your immune system attacks your skin cells causing loss of pigmentation. The skin that has lost the pigmentation can’t be reversed.”
Interactions With the Public and Health Care Providers—Those with vitiligo encounter unique situations in public and in their daily lives. Many of the accounts shared anecdotal stories on how patients have handled the stigma and discrimination faced:
“I have had to face discrimination at school, public places, college, functions, and every new person I have met has asked me this: ‘how did this happen?’”
Those with vitiligo even stated how they wished others would deal with their condition out in public, hoping that others would directly ask what the lesions were instead of the more hurtful staring. There were many stories in which users said others feel vitiligo was contagious or “dirty” and stressed that the condition is not infectious:
“I refer to myself as ‘camo-man’ and reassure people I come into contact with that it is not contagious.”
“Once I was eating at a restaurant . . . and a little girl said to her mom, ‘Look, Mom, that lady doesn’t wash her arms, look how dirty they are.’ That just broke my heart.”
Skin Tone and Implications—The belief that vitiligo lesions are less dramatic or less anxiety provoking for individuals with lighter skin was noted by users themselves and by health care providers in certain cases. Skin tone and its impact on QOL was confusing and contentious. Some users with fair skin stated their vitiligo was “less of an annoyance” or “less obvious” compared with individuals with darker complexions. Conversely, other accounts of self-reported White users vehemently stressed the anxieties felt by depigmented lesions, despite being “already white at baseline.”
“Was told by my dermatologist (upon diagnosis) that ‘You’re lucky you’re not African American—it shows up on them much worse. You’re so fair, it doesn’t really matter.’ ”
“You didn’t say what race you are. I could imagine it has a bigger impact if you are anything other than White.”
Comment
Patients Looking for Cures—The general attitude within the forums was uplifting and encouraging, with users detailing how they respond to others in public and sharing their personal perspectives. We found a mix of information regarding disease management and treatment of vitiligo. Overall, there was uncertainty about treatments, with individuals expressing concern that their treatments were ineffective or had failed or that better alternatives would be more suitable for their condition. We found many anecdotal endorsements of homeopathic remedies for vitiligo, with users boasting that their disease had not only been cured but had never returned. Some users completely denounced these statements, while other threads seemed to revolve completely around “cure” discussions with no dissenting voices. The number of discussions related to homeopathy was concerning. Furthermore, there often were no moderators within threads to remove cure-related content, whether commercially endorsed or anecdotal. It is plausible that supplements and vitamins recommended by some physicians may be incorrectly interpreted as a “cure” in online discussions. Our findings are consistent with prior reports that forums are a platform to express dissatisfaction with treatment and the need for additional treatment options.15,22
Concern Expressed by Health Care Providers—Prior qualitative research has described how patients with chronic dermatologic conditions believe that health care providers minimize patients’ psychological distress.27,28 We found several accounts in which an individual had explicitly stated their provider had “belittled” the extent and impact of vitiligo when comparing skin phototypes. This suggests either that physicians underestimate the impact of vitiligo on their patients or that physicians are not expressing enough empathic concern about the impact the condition has on those affected.
Cosmetic Aspects of Vitiligo—Few clinical trials have investigated QOL and cosmetic acceptability of treatments as outcome measures.29 We found several instances in which users with vitiligo had reported being dismissed as having a “cosmetic disease,” consistent with other work demonstrating the negative impact on such dismissals.22 Moreover, concealment and camouflage techniques frequently were discussed, demonstrating the relevance of cosmetic management as an important research topic.
Trustworthy Sources of Health Information—Patients still view physicians as trustworthy and a key source of health care information and advice.30-32 Patients with vitiligo who have been directed to reliable information sources often express gratitude22 and want health professionals to remain an important source in their health information-seeking.31 Given the range in information discussed online, it may be valuable to invite patients to share what information they have encountered online.
Our study highlights the conflicting health information and advice shared by users in online forums, complicating an already psychologically burdensome condition. Guiding patients to credible, moderated sites and resources that are accurate, understandable, and easy to access may help dispel the conflicting messages and stories discussed in the online community.
Study Strengths and Limitations—Limitations included reporting bias and reliance on self-reported information on the diagnosis and extent of individuals’ vitiligo. Excluding social media websites and platforms from the data collection is a limitation to comprehensively assessing the topic of internet users with vitiligo. Many social media platforms direct patients and their family members to support groups and therefore may have excluded these particular individuals. Social media platforms were excluded from our research owing to the prerequisite of creating user accounts or registering as an online member. Our inclusion criteria were specific to forums that did not require registering or creating an account and were therefore freely accessible to all internet viewers. There is an inherent lack of context present in online forums, preventing data collection on individuals’ demographics and socioeconomic backgrounds. However, anonymity may have allowed individuals to express their thoughts more freely.
An integrated approach, along with our sampling method of online forums not requiring registration, allows for greater transferability and understanding of the health needs of the general public with vitiligo.
Conclusion
Individuals with vitiligo continue to seek peer psychosocial support for the physical and emotional management of their disease. Counseling those with vitiligo about cosmetic concealment options, homeopathy, and treatment scams remains paramount. Directing patients to evidence-based resources, along with providing structured sources of support, may help to improve the psychosocial burden and QOL experienced by patients with vitiligo. Connecting patients with local and national support groups moderated by physicians, such as the Global Vitiligo Foundation (https://globalvitiligofoundation.org/), may provide benefit to patients with vitiligo.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38:419-431.
- Ezzedine K, Sheth V, Rodrigues M, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73:883-885.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo—part 2—classification, histopathology and treatment. An Bras Dermatol. 2014;89:784-790.
- Alkhateeb A, Fain PR, Thody A, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208-214.
- Nguyen CM, Beroukhim K, Danesh MJ, et al. The psychosocial impact of acne, vitiligo, and psoriasis: a review. Clin Cosmet Investig Dermatol. 2016;9:383-392.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Grimes PE, Billips M. Childhood vitiligo: clinical spectrum and therapeutic approaches. In: Hann SK, Nordlund JJ, eds. Vitiligo: A Monograph on the Basic and Clinical Science. Blackwell Science; 2000.
- Sawant NS, Vanjari NA, Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019:6879412.
- Liu Y, Kornfield R, Shaw BR, et al. When support is needed: social support solicitation and provision in an online alcohol use disorder forum. Digit Health. 2017;3:2055207617704274.
- Health 2.0. The Economist. 2007;384:14.
- Fox S. Peer-to-peer health care. Pew Research Center. February 28, 2011. Accessed December 14, 2021. https://www.pewinternet.org/wp-content/uploads/sites/9/media/Files/Reports/2011/Pew_P2PHealthcare_2011.pdf
- Li N, Orrange S, Kravitz RL, et al. Reasons for and predictors of patients’ online health information seeking following a medical appointment. Fam Pract. 2014;31:550-556.
- Idriss SZ, Kvedar JC, Watson AJ. The role of online support communities: benefits of expanded social networks to patients with psoriasis. Arch Dermatol. 2009;145:46-51.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol 2017;176:1500-1507.
- Santer M, Chandler D, Lown M, et al. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol. 2017;177:751-757.
- Santer M, Burgess H, Yardley L, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract. 2012;62:e261-e267.
- Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the Vitiligo Society. Clin Exp Dermatol. 2010;35:736-739.
- Guba EG, Lincoln YS. Competing paradigms in qualitative research. In: Denzin NK, Lincoln YS, eds. Handbook of Qualitative Research. Sage Publications, Inc; 1994:105-117.
- Lincoln YS. Emerging criteria for quality in qualitative and interpretive research. Qualitative Inquiry. 2016;1:275-289.
- O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245-1251.
- Teasdale EJ, Muller I, Santer M. Carers’ views of topical corticosteroid use in childhood eczema: a qualitative study of online discussion forums. Br J Dermatol. 2017;176:1500-1507.
- Teasdale E, Muller I, Sani AA, et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open. 2018;8:e018652.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772.
- Hewson C, Buchanan T, Brown I, et al. Ethics Guidelines for Internet-mediated Research. The British Psychological Society; 2017.
- Coulson NS. Sharing, supporting and sobriety: a qualitative analysis of messages posted to alcohol-related online discussion forums in the United Kingdom. J Subst Use. 2014;19:176-180.
- Attard A, Coulson NS. A thematic analysis of patient communication in Parkinson’s disease online support group discussion forums. Comput Hum Behav. 2012;28:500-506.
- Nelson PA, Chew-Graham CA, Griffiths CE, et al. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol. 2013;168:354-361.
- Gore C, Johnson RJ, Caress AL, et al. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy. 2005;60:938-943.
- Eleftheriadou V, Thomas KS, Whitton ME, et al. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804-814.
- Tan SS, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
- Sillence E, Briggs P, Harris PR, et al. How do patients evaluate and make use of online health information? Soc Sci Med. 2007;64:1853-1862.
- Hay MC, Cadigan RJ, Khanna D, et al. Prepared patients: internet information seeking by new rheumatology patients. Arthritis Rheum. 2008;59:575-582.
Practice Points
- Online forums provide invaluable insight on vitiligo disease management, psychosocial impact, and burden on quality of life. Patient care can be improved by inquiring where patients seek information and whether online forums are utilized.
- Commonly discussed topics in online forums were cosmetic concealment of vitiligo lesions and homeopathy or “cure” discussions. Health care providers can engage in honest conversations about evidence-based medical treatments for vitiligo. The interest in cosmetic management highlights a relevant research area in this field.
- Health care providers can better serve patients with vitiligo by providing online resources that are reputable and can help guide patients to credible internet sources such as the Global Vitiligo Foundation.
Anecdote Increases Patient Willingness to Take a Biologic Medication for Psoriasis
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Practice Points
- Patients often are apprehensive to start biologic medications for their psoriasis.
- Clinical trial evidence of a biologic medication’s efficacy and safety as well as anecdotes of patient experiences appear to be important factors for patients when considering taking a medication.
- The use of an anecdote—alone or in combination with clinical trial evidence—to help patients overcome fears of starting a biologic medication for their psoriasis may be an effective way to improve patients’ willingness to take treatment.
Psoriasis Highlights From AADVMX 2021
Key studies on psoriasis presented at the American Academy of Dermatology Virtual Meeting Experience (AAD VMX) 2021included data on new topical treatments and biological therapies.
Dr Steven Feldman, of Wake Forest School of Medicine, reviews trial data demonstrating the efficacy of a topical formulation of roflumilast, a phosphodiesterase type 4 (PDE-4) inhibitor previously used in oral systemic form to treat psoriasis.
He also discusses a meta-analysis of the efficacy of biologics favoring newer treatments, such as drugs targeting IL-17 and IL-23.
Dr Feldman reviews the results of two pivotal phase 3 trials presented at the meeting. The POETYK study examined deucravacitinib, a TYK2 inhibitor. In a head-to-head comparison, deucravacitinib was found to be more effective and better tolerated than apremilast in treating psoriasis. BE RADIANT, another head-to-head study, compared the IL-17 blockers bimekizumab and secukinumab. The year-long study favored bimekizumab, though it was associated with a higher risk for candidiasis.
Finally, Dr Feldman discusses the significance of a study showing that psoriasis patients have an approximately 20% higher risk for COVID-19 infection compared with a control group.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Key studies on psoriasis presented at the American Academy of Dermatology Virtual Meeting Experience (AAD VMX) 2021included data on new topical treatments and biological therapies.
Dr Steven Feldman, of Wake Forest School of Medicine, reviews trial data demonstrating the efficacy of a topical formulation of roflumilast, a phosphodiesterase type 4 (PDE-4) inhibitor previously used in oral systemic form to treat psoriasis.
He also discusses a meta-analysis of the efficacy of biologics favoring newer treatments, such as drugs targeting IL-17 and IL-23.
Dr Feldman reviews the results of two pivotal phase 3 trials presented at the meeting. The POETYK study examined deucravacitinib, a TYK2 inhibitor. In a head-to-head comparison, deucravacitinib was found to be more effective and better tolerated than apremilast in treating psoriasis. BE RADIANT, another head-to-head study, compared the IL-17 blockers bimekizumab and secukinumab. The year-long study favored bimekizumab, though it was associated with a higher risk for candidiasis.
Finally, Dr Feldman discusses the significance of a study showing that psoriasis patients have an approximately 20% higher risk for COVID-19 infection compared with a control group.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Key studies on psoriasis presented at the American Academy of Dermatology Virtual Meeting Experience (AAD VMX) 2021included data on new topical treatments and biological therapies.
Dr Steven Feldman, of Wake Forest School of Medicine, reviews trial data demonstrating the efficacy of a topical formulation of roflumilast, a phosphodiesterase type 4 (PDE-4) inhibitor previously used in oral systemic form to treat psoriasis.
He also discusses a meta-analysis of the efficacy of biologics favoring newer treatments, such as drugs targeting IL-17 and IL-23.
Dr Feldman reviews the results of two pivotal phase 3 trials presented at the meeting. The POETYK study examined deucravacitinib, a TYK2 inhibitor. In a head-to-head comparison, deucravacitinib was found to be more effective and better tolerated than apremilast in treating psoriasis. BE RADIANT, another head-to-head study, compared the IL-17 blockers bimekizumab and secukinumab. The year-long study favored bimekizumab, though it was associated with a higher risk for candidiasis.
Finally, Dr Feldman discusses the significance of a study showing that psoriasis patients have an approximately 20% higher risk for COVID-19 infection compared with a control group.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies

Gaps in Treatment Guidelines for Atopic Dermatitis
Treatment options for atopic dermatitis have evolved significantly in the past several years, but the current guidelines have yet to catch up.
Drs Steven Feldman and Lindsay Strowd, from Wake Forest School of Medicine, discuss gaps in the American Academy of Dermatology treatment guidelines for atopic dermatitis.
The current guidelines have not been updated to include medications approved for atopic dermatitis, including crisaborole, a steroid-sparing ointment used to treat mild to moderate disease in patients 3 months of age and older.
Another drug that has been approved since the 2014 guidelines is the biologic dupilumab, which is a monoclonal antibody that acts on the IL-4 receptor. The agent inhibits the binding of IL-4 receptors to the principal cytokines responsible for mediating the disease. Dupilumab is administered by injection and is approved for patients 6 years and older with moderate to severe disease.
The doctors also discuss therapies for atopic dermatitis currently in development, including topical and oral JAK inhibitors. They agree that the long-term benefit of topical JAK inhibitors may be limited, but that oral JAK inhibitors have the potential to be more effective than dupilumab and more acceptable to patients who do not like injections.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Lindsay C. Strowd, MD, Associate Professor, Vice Chair, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Lindsay C. Strowd, MD, has disclosed no relevant financial relationships.
Treatment options for atopic dermatitis have evolved significantly in the past several years, but the current guidelines have yet to catch up.
Drs Steven Feldman and Lindsay Strowd, from Wake Forest School of Medicine, discuss gaps in the American Academy of Dermatology treatment guidelines for atopic dermatitis.
The current guidelines have not been updated to include medications approved for atopic dermatitis, including crisaborole, a steroid-sparing ointment used to treat mild to moderate disease in patients 3 months of age and older.
Another drug that has been approved since the 2014 guidelines is the biologic dupilumab, which is a monoclonal antibody that acts on the IL-4 receptor. The agent inhibits the binding of IL-4 receptors to the principal cytokines responsible for mediating the disease. Dupilumab is administered by injection and is approved for patients 6 years and older with moderate to severe disease.
The doctors also discuss therapies for atopic dermatitis currently in development, including topical and oral JAK inhibitors. They agree that the long-term benefit of topical JAK inhibitors may be limited, but that oral JAK inhibitors have the potential to be more effective than dupilumab and more acceptable to patients who do not like injections.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Lindsay C. Strowd, MD, Associate Professor, Vice Chair, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Lindsay C. Strowd, MD, has disclosed no relevant financial relationships.
Treatment options for atopic dermatitis have evolved significantly in the past several years, but the current guidelines have yet to catch up.
Drs Steven Feldman and Lindsay Strowd, from Wake Forest School of Medicine, discuss gaps in the American Academy of Dermatology treatment guidelines for atopic dermatitis.
The current guidelines have not been updated to include medications approved for atopic dermatitis, including crisaborole, a steroid-sparing ointment used to treat mild to moderate disease in patients 3 months of age and older.
Another drug that has been approved since the 2014 guidelines is the biologic dupilumab, which is a monoclonal antibody that acts on the IL-4 receptor. The agent inhibits the binding of IL-4 receptors to the principal cytokines responsible for mediating the disease. Dupilumab is administered by injection and is approved for patients 6 years and older with moderate to severe disease.
The doctors also discuss therapies for atopic dermatitis currently in development, including topical and oral JAK inhibitors. They agree that the long-term benefit of topical JAK inhibitors may be limited, but that oral JAK inhibitors have the potential to be more effective than dupilumab and more acceptable to patients who do not like injections.
--
Steven R. Feldman, MD, PhD, Professor, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Steven R. Feldman, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AbbVie; Alvotech; Advance Medical; Almirall; Arena; Bristol-Myers Squibb; Caremark; Amgen; Celgene; Galderma Laboratories; Gerson Lehrman Group; Guidepoint Global; Helsinn; Janssen; Kikaku; Leo Pharma; Eli Lilly and Company; Merck; Mylan; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sienna; Sun Pharma; Suncare Research; Xenoport
Serve(d) as a speaker for: AbbVie; Amgen; Celgene; Janssen; Leo Pharma; Eli Lilly and Company; Novartis; Ortho Dermatology; Pfizer; Regeneron; Sanofi; Sun Pharma
Receive(d) grant support from: AbbVie; Amgen; Celgene; Galderma Laboratories; Janssen; Eli Lilly and Company; Novartis; Pfizer; Regeneron; Sanofi
Receive(d) royalties from: Informa; UpToDate; Xlibris
Holds stock in: Causa Technologies; Medical Quality Enhancement Corporation
Serves as founder and chief technology officer for: Causa Technologies
Lindsay C. Strowd, MD, Associate Professor, Vice Chair, Department of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina
Lindsay C. Strowd, MD, has disclosed no relevant financial relationships.

Clinical Case-Viewing Sessions in Dermatology: The Patient Perspective
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
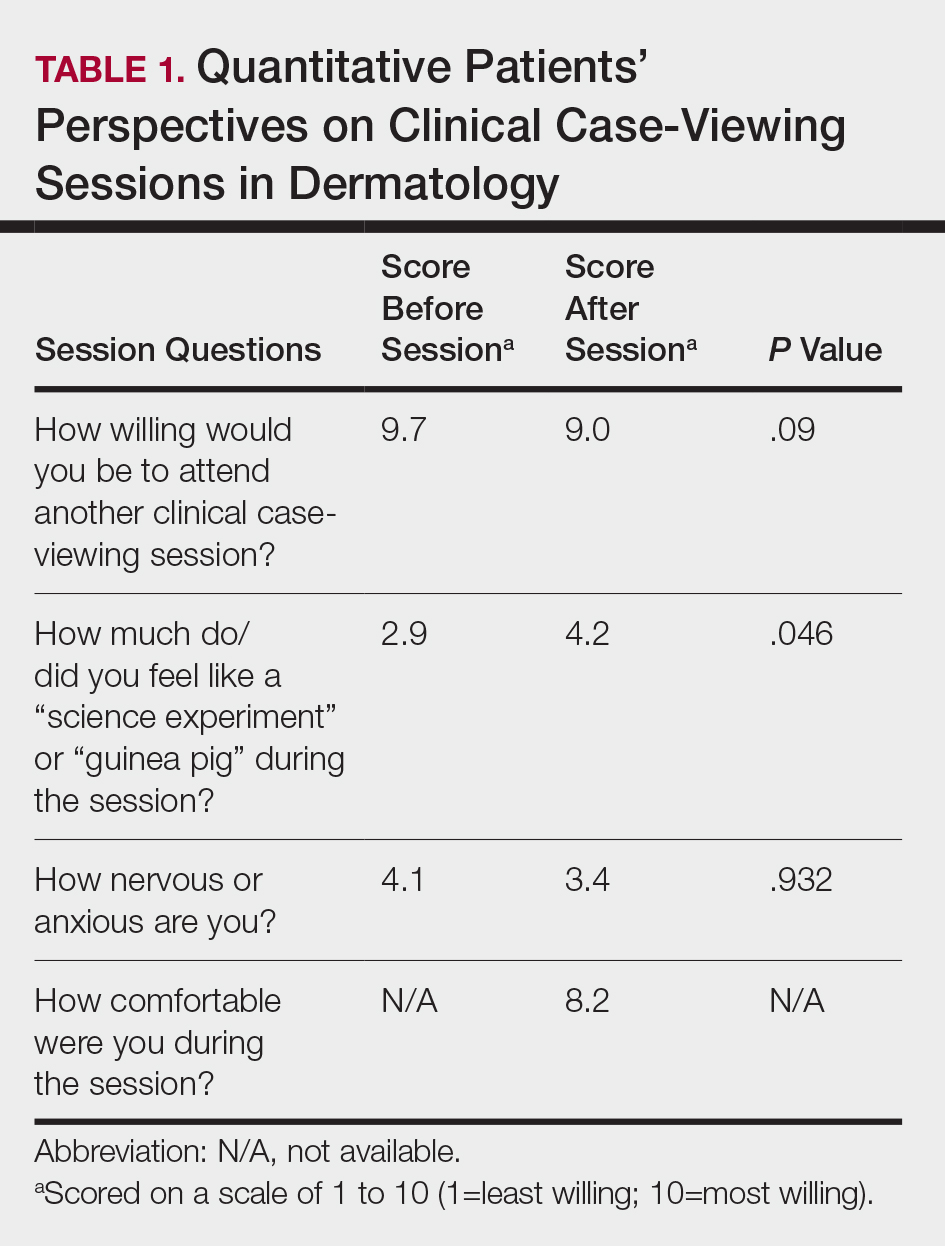
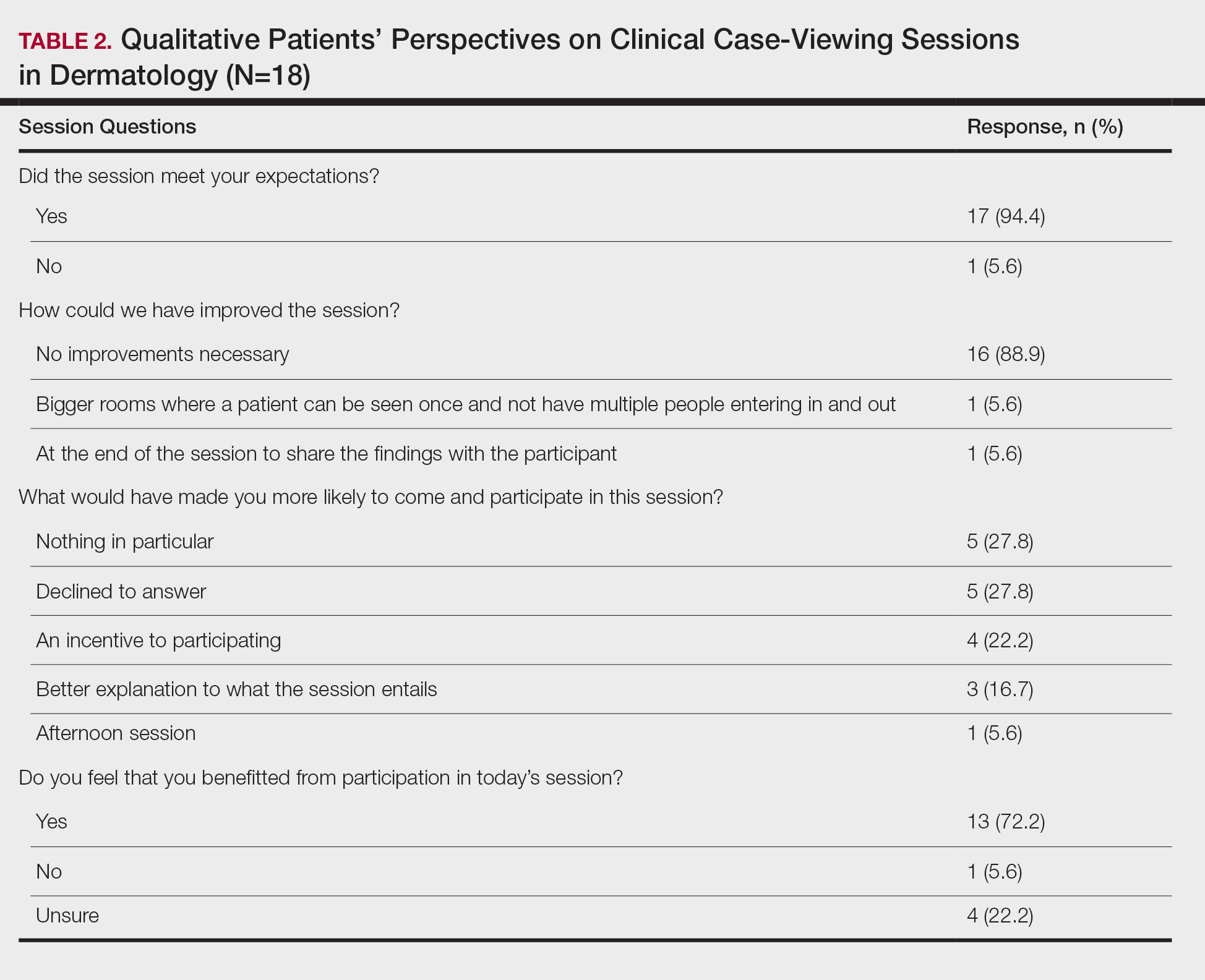
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.


The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.


The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
Practice Points
- Patient willingness to attend dermatology clinical case-viewing (CCV) sessions is relatively unchanged before and after the session.
- Participants generally consider CCV sessions to be a positive experience.
Adherence to Topical Treatment Can Improve Treatment-Resistant Moderate Psoriasis
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.
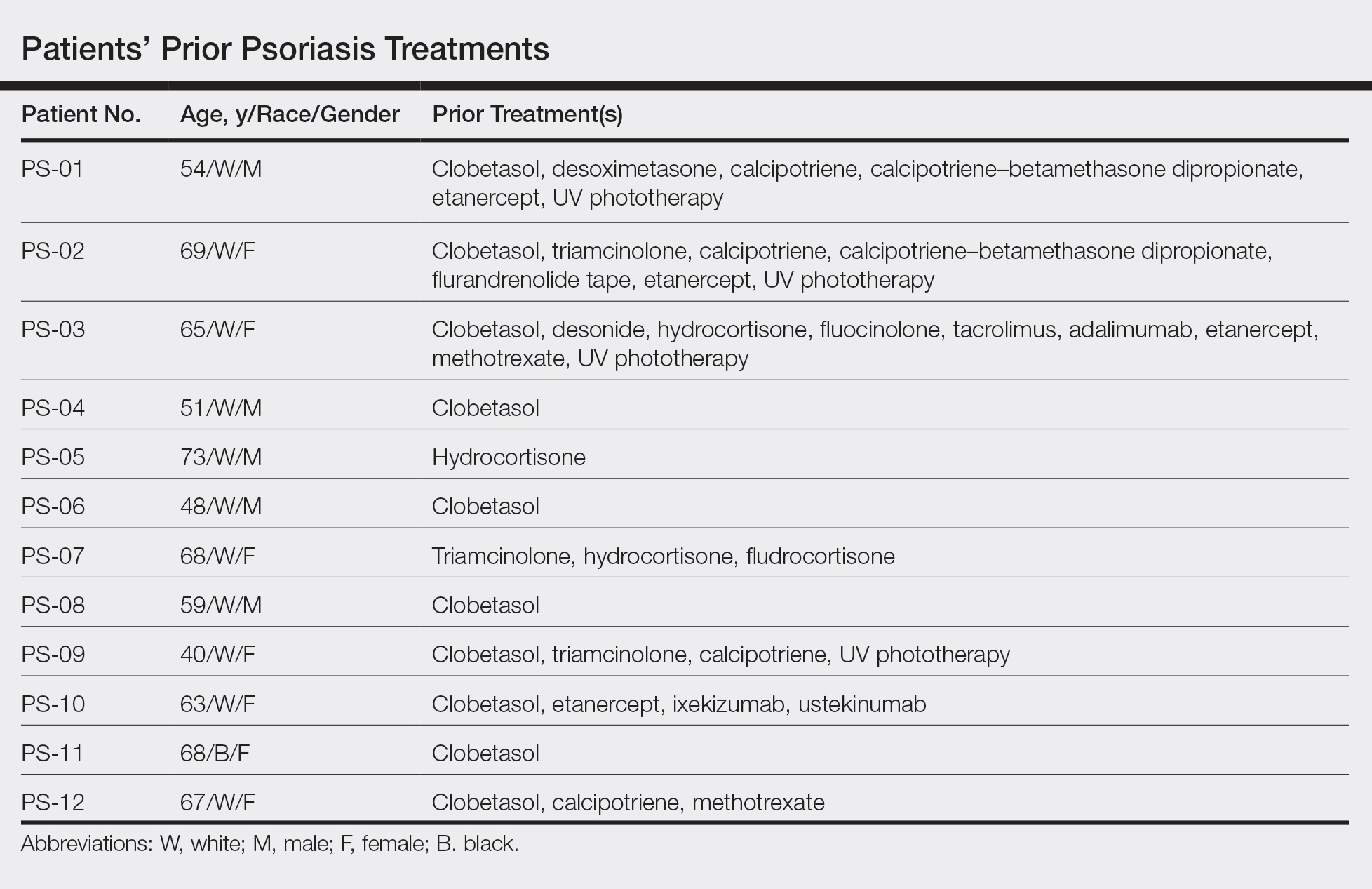
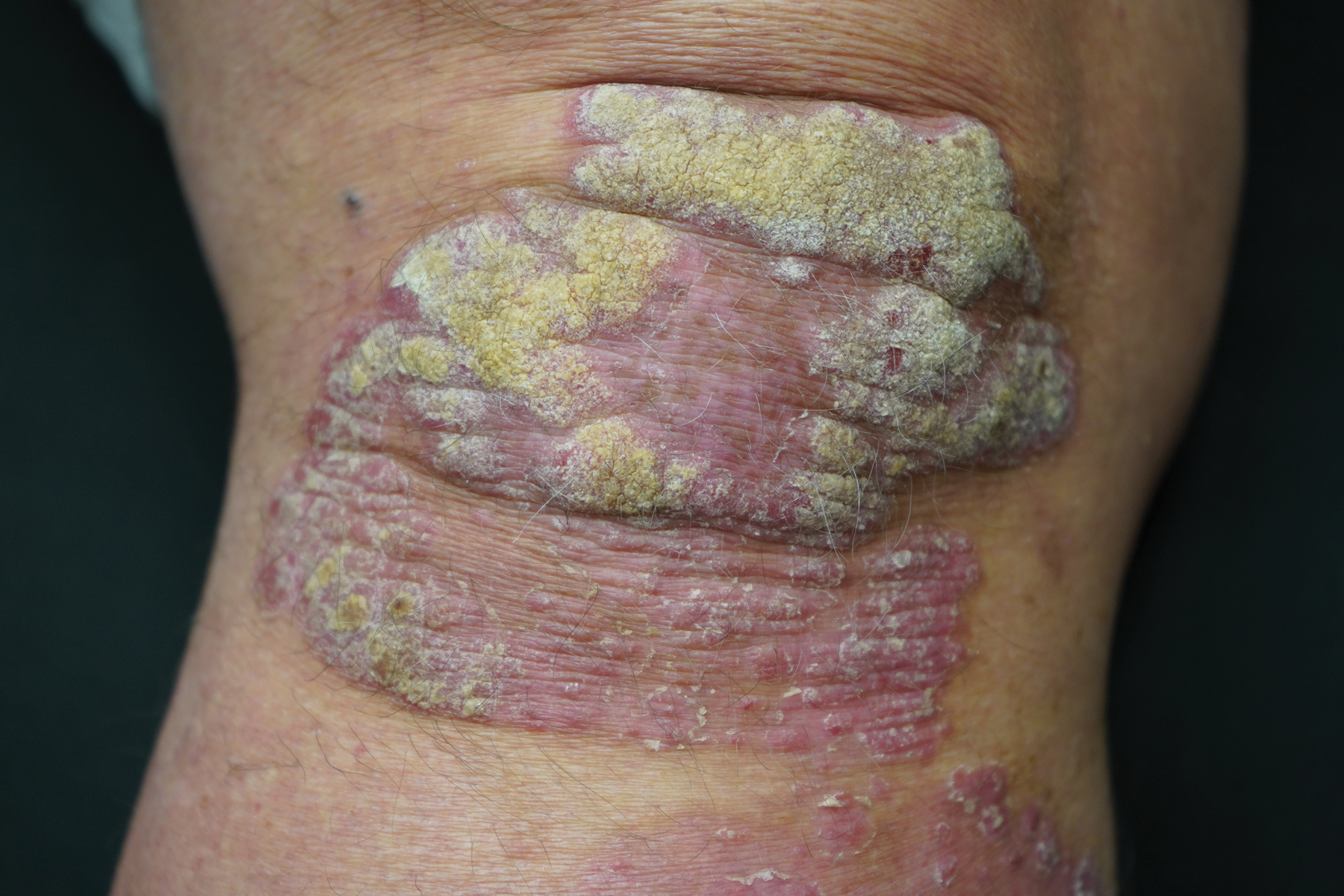
All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).
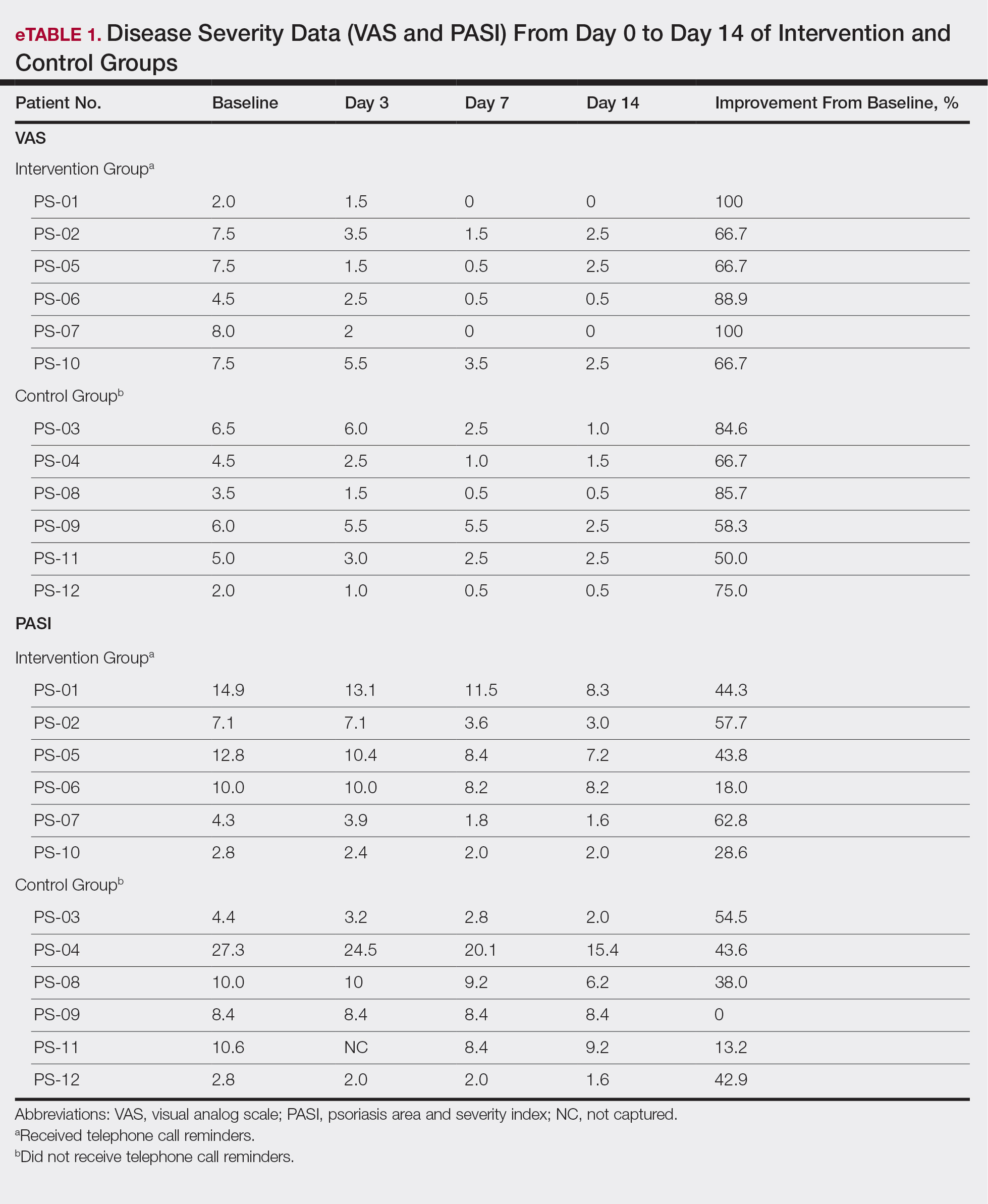
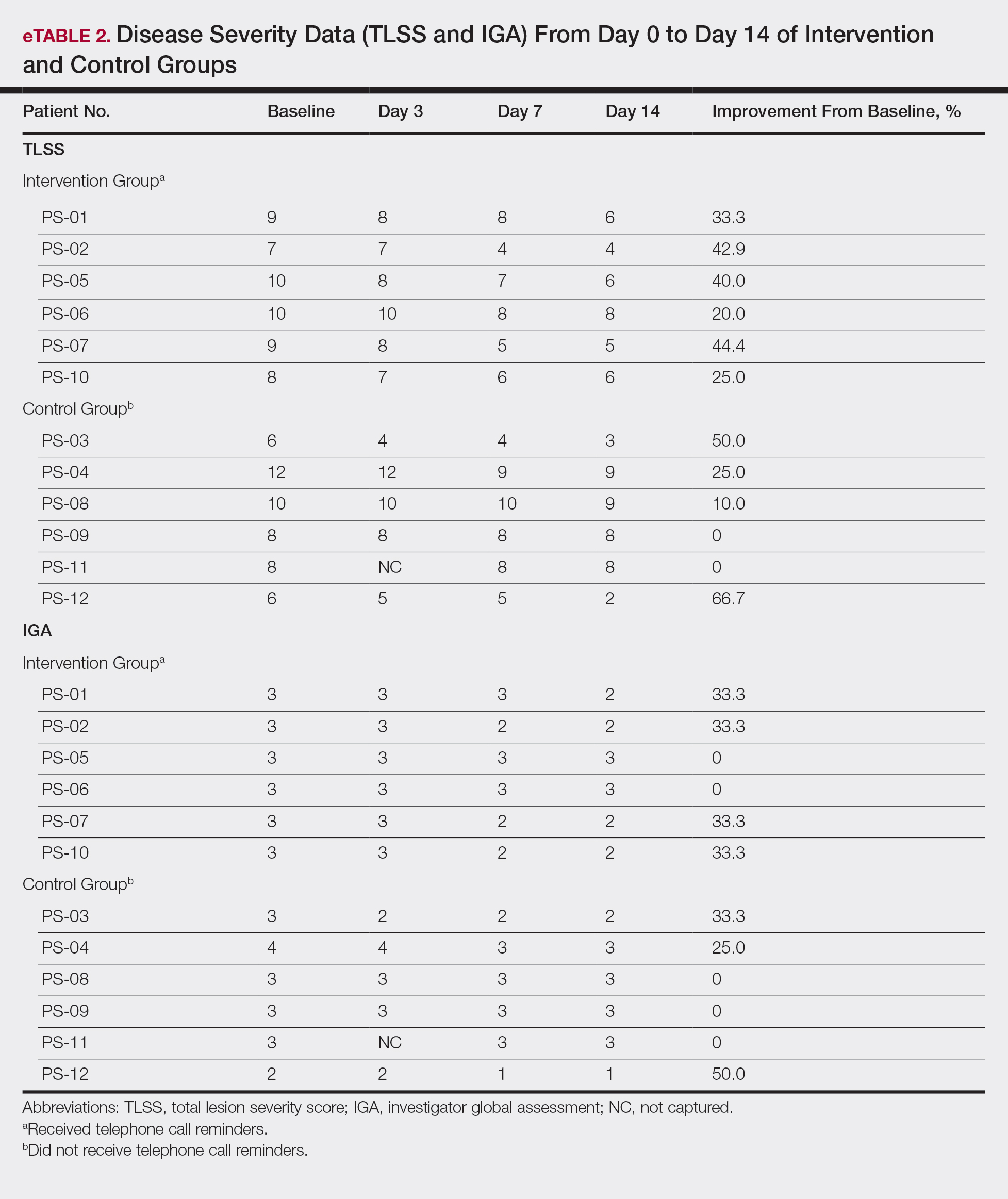
The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.


All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).


The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
High-potency topical corticosteroids are first-line treatments for psoriasis, but many patients report that they are ineffective or lose effectiveness over time.1-5 The mechanism underlying the lack or loss of activity is not well characterized but may be due to poor adherence to treatment. Adherence to topical treatment is poor in the short run and even worse in the long run.6,7 We evaluated 12 patients with psoriasis resistant to topical corticosteroids to determine if they would respond to topical corticosteroids under conditions designed to promote adherence to treatment.
Methods
This open-label, randomized, single-center clinical study recruited 12 patients with plaque psoriasis that previously failed treatment with topical corticosteroids and other therapies (Table). We stratified disease by body surface area: mild (<3%), moderate (3%–10%), and severe (>10%). Inclusion criteria included adult patients with plaque psoriasis amenable to topical corticosteroid therapy, ability to comply with requirements of the study, and a history of failed topical corticosteroid treatment (Figure). Patients were excluded if they were pregnant, breastfeeding, had conditions that would affect adherence or potentially bias results (eg, dementia, Alzheimer disease), had a history of allergy or sensitivity to corticosteroids, and had a history of drug hypersensitivity.


All patients received desoximetasone spray 0.25% twice daily for 14 days. At the baseline visit, 6 patients were randomly selected to also receive a twice-daily reminder telephone call. Study visits occurred frequently—at baseline and on days 3, 7, and 14—to further assure good adherence to the treatment regimen.
During visits, disease severity was scored using the visual analog scale for pruritus, psoriasis area and severity index (PASI), total lesion severity score (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
The study was designed to assess the number of topical treatment–resistant patients who would improve with topical treatment but was not designed or powered to test if the telephone call reminders increased adherence.
Results
All patients completed the study; 10 of 12 patients (83.3%) had previously used topical clobetasol and it failed (Table). At the 2-week end-of-study visit, most patients improved on all measures. Patients who received telephone call reminders improved more than patients who did not. All 12 patients (100%) reported relief of itching; 11 of 12 (91.7%) had an improved PASI; 10 of 12 (83.3%) had an improved TLSS; and 7 of 12 (58.3%) had an improved IGA (eTables 1 and 2).


The percentage reduction in pruritus ranged from 66.7% to 100% and 50.0% to 85.7% with and without telephone call reminders, respectively. Improvement in PASI ranged from 18.0% to 62.8% and 0% to 54.5% with and without telephone call reminders, respectively. Improvement in TLSS and IGA was of lower magnitude but showed a similar pattern, with numerically greater improvement in the telephone call reminders group compared to the group that was not called (eTable 2). No patients showed a worse score for pruritus on the visual analog scale, PASI, TLSS, or IGA.
Discussion
Topical corticosteroids are highly effective for psoriasis in clinical trials, with clearance in 2 to 4 weeks in 60% to 80% of patients, a rapidity of response not matched by even the most potent biologic treatments.8,9 However, topical corticosteroids are not always effective in clinical practice. There may be primary inefficacy (they do not work at first) or secondary inefficacy (a previously effective treatment loses efficacy over time).10 Poor adherence can explain both phenomena. Primary adherence occurs when patients fill their prescription; secondary adherence occurs when patients follow the medication recommendations.11 Primary nonadherence is common in patients with psoriasis; in one study, 50% of psoriasis prescriptions were not filled.12 Secondary adherence also is poor and declines over time; electronic monitoring revealed adherence to topical treatments in psoriasis patients decreased from 85% initially to 51% at the end of 8 weeks.7 Given the high efficacy of topical corticosteroids in clinical trials and the poor adherence to topical treatment in patients with psoriasis, we anticipated that psoriasis that is resistant to topical corticosteroids would improve rapidly under conditions designed to promote adherence.
As expected, disease improved in almost every patient in this small cohort when they were given a potent topical corticosteroid, even though they previously reported that their psoriasis was resistant to potent topical corticosteroids. Although this study enrolled only a small cohort, it appears that the majority of patients with limited psoriasis that was reported to be resistant to topical treatment can see a response to topical treatment under conditions designed to encourage good adherence.
We believe that the good outcomes seen in our study were a result of good adherence. Although the desoximetasone spray 0.25% used in this study is a superpotent topical corticosteroid,8 the response to treatment was unlikely due to changing corticosteroid potency because 10 of 12 patients had tried another superpotent topical corticosteroid (clobetasol) and it failed. We chose a spray product for this study rather than an ointment to promote adherence; however, this choice limited the ability to assess adherence directly, as adherence-monitoring devices for spray delivery systems are not readily available.
Our study was limited by the small sample size and brief duration of treatment. However, the effect size is so large (ie, the topical treatment was so effective) that only a small sample size and brief treatment duration were needed to show that a high percentage of patients with psoriasis that had previously failed treatment with topical corticosteroids can in fact respond to this treatment.
We used telephone calls as reminders in 50% of patients to further encourage adherence. The study was not designed or powered to assess the effect of the telephone call reminders, but patients receiving those calls appeared to have slightly greater reduction in disease severity. Nonetheless, twice-daily telephone call reminders are unlikely to be a wanted or practical intervention; other approaches to encourage adherence are needed.
Frequent follow-up visits were incorporated in our study design to maximize adherence. Although it might not be feasible for clinical practices to schedule follow-up visits as often as in our study, other approaches such as virtual visits and electronic interaction might provide a practical alternative. Multifaceted approaches to increasing adherence include encouraging patients to participate in the treatment plan, prescribing therapy consistent with a patient’s preferred vehicle, and extensive patient education.13 If patients do not respond as expected, poor adherence can be considered. Other potential causes of poor outcomes include error in diagnosis; resistance to the prescribed treatment; concomitant infection; irritant exposure; and, in the case of biologics, antidrug antibody formation.14,15
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
- Feldman SR, Fleischer AB Jr, Cooper JZ. New topical treatments change the pattern of treatment of psoriasis: dermatologists remain the primary providers of this care. Int J Dermatol. 2000;39:41-44.
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis. 2007;80(suppl 5):12-19.
- Saleem MD, Negus D, Feldman SR. Topical 0.25% desoximetasone spray efficacy for moderate to severe plaque psoriasis: a randomized clinical trial. J Dermatolog Treat. 2018;29:32-35.
- Mraz S, Leonardi C, Colón LE, et al. Different treatment outcomes with different formulations of clobetasol propionate 0.05% for the treatment of plaque psoriasis. J Dermatolog Treat. 2008;19:354-359.
- Chiricozzi A, Pimpinelli N, Ricceri F, et al. Treatment of psoriasis with topical agents: recommendations from a Tuscany Consensus. Dermatol Ther. 2017;30:e12549.
- Carroll CL, Feldman SR, Camacho FT, et al. Adherence to topical therapy decreases during the course of an 8-week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. J Am Acad Dermatol. 2004;51:212-216.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015;14:835-840.
- Beutner K, Chakrabarty A, Lemke S, et al. An intra-individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. J Drugs Dermatol. 2006;5:357-360.
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378.
- Blais L, Kettani FZ, Forget A, et al. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2016;73:91-97.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Zschocke I, Mrowietz U, Karakasili E, et al. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4-9.
- Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol. 2015;56:241-251.
- Varada S, Tintle SJ, Gottlieb AB. Apremilast for the treatment of psoriatic arthritis. Expert Rev Clin Pharmacol. 2014;7:239-250.
Practice Points
- Most patients with psoriasis are good candidates for topical treatment.
- Topical treatment of psoriasis often is ineffective.
- Topical treatment of psoriasis can be rapidly effective, even in patients who reported disease that was resistant to topical treatment.