User login
Germline testing in advanced cancer can lead to targeted treatment
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
FROM ASCO 2020
Oncologists’ income and satisfaction are up
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.
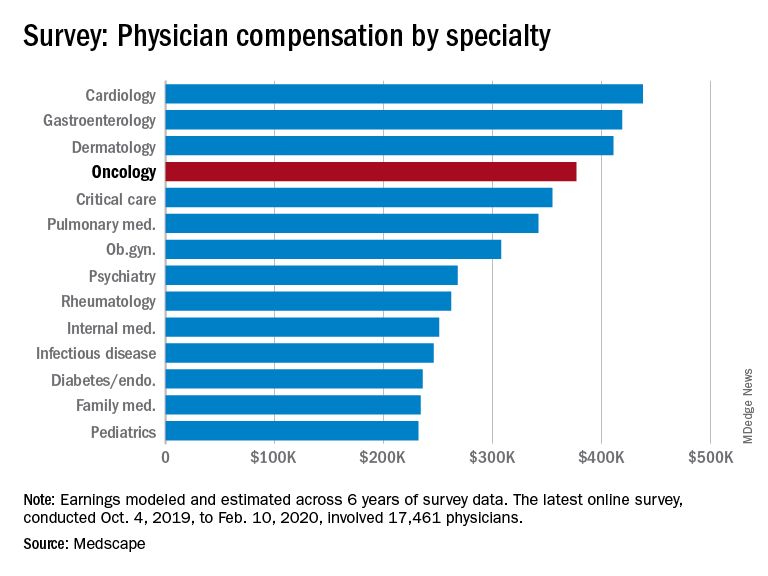
At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Tumor molecular profiling may help identify ‘exceptional responders’
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
First PARP inhibitor approved for metastatic prostate cancer
A completely new approach to the treatment of prostate cancer is now available to clinicians through the approval of the first PARP inhibitor for use in certain patients with this disease.
Rucaparib (Rubraca, Clovis Oncology) is the first PARP inhibitor approved for use in patients with metastatic castration-resistant prostate cancer (mCRPC) that harbors deleterious BRCA mutations (germline and/or somatic). The drug is indicated for use in patients who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy.
The drug is already marketed for use in ovarian cancer.
The new prostate cancer indication was granted an accelerated approval by the US Food and Drug Administration (FDA) on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial. A confirmatory phase 3 trial, TRITON3, is currently underway.
“Standard treatment options for men with mCRPC have been limited to androgen receptor–targeting therapies, taxane chemotherapy, radium-223, and sipuleucel-T,” said Wassim Abida, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, in a statement.
“Rucaparib is the first in a class of drugs to become newly available to patients with mCRPC who harbor a deleterious BRCA mutation,” said Abida, who is also the principal investigator of the TRITON2 study. “Given the level and duration of responses observed with rucaparib in men with mCRPC and these mutations, it represents an important and timely new treatment option for this patient population.”
Other indications, another PARP inhibitor
Rucaparib is already approved for the treatment of women with advanced BRCA mutation–positive ovarian cancer who have received two or more prior chemotherapies. It is also approved as maintenance treatment for patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who demonstrate a complete or partial response to platinum-based chemotherapy, regardless of BRCA status.
Another PARP inhibitor, olaparib (Lynparza, AstraZeneca), is awaiting approval for use in prostate cancer in men with BRCA mutations. That pending approval is based on results from the phase 3 PROfound trial, which was hailed as a “landmark trial” when it was presented last year. The results showed a significant improved in disease-free progression. The company recently announced that there was also a significant improvement in overall survival.
Olaparib is already approved for the maintenance treatment of platinum-sensitive relapsed ovarian cancer regardless of BRCA status and as first-line maintenance treatment in BRCA-mutated advanced ovarian cancer following response to platinum-based chemotherapy. It is also approved for germline BRCA-mutated HER2-negative metastatic breast cancer previously treated with chemotherapy and for the maintenance treatment of germline BRCA-mutated advanced pancreatic cancer following first-line platinum-based chemotherapy.
Details of the TRITON2 study
The accelerated approval for use of rucaparib in BRCA prostate cancer was based on efficacy data from the multicenter, single-arm TRITON2 clinical trial. The cohort included 62 patients with a BRCA (germline and/or somatic) mutation and measurable disease; 115 patients with a BRCA (germline and/or somatic) mutation and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency (HRD)–positive mCRPC.
The major efficacy outcomes were objective response rate (ORR) and duration of response. Confirmed PSA response rate was also a prespecified endpoint. Data were assessed by independent radiologic review.
For the patients with measurable disease and a BRCA mutation, the ORR was 44%. The ORR was similar for patients with a germline BRCA mutation.
Median duration of response was not evaluable at data cutoff but ranged from 1.7 to 24+ months. Of the 27 patients with a confirmed objective response, 15 (56%) patients showed a response that lasted 6 months or longer.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%.
The safety evaluation was based on an analysis of the 209 patients with HRD-positive mCRPC and included 115 with deleterious BRCA mutations. The most common adverse events (≥20%; grade 1-4) in the patients with BRCA mutations were fatigue/asthenia (62%), nausea (52%), anemia (43%), AST/ALT elevation (33%), decreased appetite (28%), rash (27%), constipation (27%), thrombocytopenia (25%), vomiting (22%), and diarrhea (20%).
Rucaparib has been associated with hematologic toxicity, including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). However, MDS/AML was not observed in the TRITON2 study, regardless of HRD mutation.
Confirmation with TRITON3
A phase 3, randomized, open-label study, TRITON3, is currently underway and is expected to serve as the confirmatory study for the accelerated approval in mCRPC. TRITON3 is comparing rucaparib with physician’s choice of therapy in patients with mCRPC who have specific gene alterations, including BRCA and ATM alterations, and who have experienced disease progression after androgen receptor–directed therapy but have not yet received chemotherapy. The primary endpoint for TRITON3 is radiographic progression-free survival.
This article first appeared on Medscape.com.
A completely new approach to the treatment of prostate cancer is now available to clinicians through the approval of the first PARP inhibitor for use in certain patients with this disease.
Rucaparib (Rubraca, Clovis Oncology) is the first PARP inhibitor approved for use in patients with metastatic castration-resistant prostate cancer (mCRPC) that harbors deleterious BRCA mutations (germline and/or somatic). The drug is indicated for use in patients who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy.
The drug is already marketed for use in ovarian cancer.
The new prostate cancer indication was granted an accelerated approval by the US Food and Drug Administration (FDA) on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial. A confirmatory phase 3 trial, TRITON3, is currently underway.
“Standard treatment options for men with mCRPC have been limited to androgen receptor–targeting therapies, taxane chemotherapy, radium-223, and sipuleucel-T,” said Wassim Abida, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, in a statement.
“Rucaparib is the first in a class of drugs to become newly available to patients with mCRPC who harbor a deleterious BRCA mutation,” said Abida, who is also the principal investigator of the TRITON2 study. “Given the level and duration of responses observed with rucaparib in men with mCRPC and these mutations, it represents an important and timely new treatment option for this patient population.”
Other indications, another PARP inhibitor
Rucaparib is already approved for the treatment of women with advanced BRCA mutation–positive ovarian cancer who have received two or more prior chemotherapies. It is also approved as maintenance treatment for patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who demonstrate a complete or partial response to platinum-based chemotherapy, regardless of BRCA status.
Another PARP inhibitor, olaparib (Lynparza, AstraZeneca), is awaiting approval for use in prostate cancer in men with BRCA mutations. That pending approval is based on results from the phase 3 PROfound trial, which was hailed as a “landmark trial” when it was presented last year. The results showed a significant improved in disease-free progression. The company recently announced that there was also a significant improvement in overall survival.
Olaparib is already approved for the maintenance treatment of platinum-sensitive relapsed ovarian cancer regardless of BRCA status and as first-line maintenance treatment in BRCA-mutated advanced ovarian cancer following response to platinum-based chemotherapy. It is also approved for germline BRCA-mutated HER2-negative metastatic breast cancer previously treated with chemotherapy and for the maintenance treatment of germline BRCA-mutated advanced pancreatic cancer following first-line platinum-based chemotherapy.
Details of the TRITON2 study
The accelerated approval for use of rucaparib in BRCA prostate cancer was based on efficacy data from the multicenter, single-arm TRITON2 clinical trial. The cohort included 62 patients with a BRCA (germline and/or somatic) mutation and measurable disease; 115 patients with a BRCA (germline and/or somatic) mutation and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency (HRD)–positive mCRPC.
The major efficacy outcomes were objective response rate (ORR) and duration of response. Confirmed PSA response rate was also a prespecified endpoint. Data were assessed by independent radiologic review.
For the patients with measurable disease and a BRCA mutation, the ORR was 44%. The ORR was similar for patients with a germline BRCA mutation.
Median duration of response was not evaluable at data cutoff but ranged from 1.7 to 24+ months. Of the 27 patients with a confirmed objective response, 15 (56%) patients showed a response that lasted 6 months or longer.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%.
The safety evaluation was based on an analysis of the 209 patients with HRD-positive mCRPC and included 115 with deleterious BRCA mutations. The most common adverse events (≥20%; grade 1-4) in the patients with BRCA mutations were fatigue/asthenia (62%), nausea (52%), anemia (43%), AST/ALT elevation (33%), decreased appetite (28%), rash (27%), constipation (27%), thrombocytopenia (25%), vomiting (22%), and diarrhea (20%).
Rucaparib has been associated with hematologic toxicity, including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). However, MDS/AML was not observed in the TRITON2 study, regardless of HRD mutation.
Confirmation with TRITON3
A phase 3, randomized, open-label study, TRITON3, is currently underway and is expected to serve as the confirmatory study for the accelerated approval in mCRPC. TRITON3 is comparing rucaparib with physician’s choice of therapy in patients with mCRPC who have specific gene alterations, including BRCA and ATM alterations, and who have experienced disease progression after androgen receptor–directed therapy but have not yet received chemotherapy. The primary endpoint for TRITON3 is radiographic progression-free survival.
This article first appeared on Medscape.com.
A completely new approach to the treatment of prostate cancer is now available to clinicians through the approval of the first PARP inhibitor for use in certain patients with this disease.
Rucaparib (Rubraca, Clovis Oncology) is the first PARP inhibitor approved for use in patients with metastatic castration-resistant prostate cancer (mCRPC) that harbors deleterious BRCA mutations (germline and/or somatic). The drug is indicated for use in patients who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy.
The drug is already marketed for use in ovarian cancer.
The new prostate cancer indication was granted an accelerated approval by the US Food and Drug Administration (FDA) on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial. A confirmatory phase 3 trial, TRITON3, is currently underway.
“Standard treatment options for men with mCRPC have been limited to androgen receptor–targeting therapies, taxane chemotherapy, radium-223, and sipuleucel-T,” said Wassim Abida, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, in a statement.
“Rucaparib is the first in a class of drugs to become newly available to patients with mCRPC who harbor a deleterious BRCA mutation,” said Abida, who is also the principal investigator of the TRITON2 study. “Given the level and duration of responses observed with rucaparib in men with mCRPC and these mutations, it represents an important and timely new treatment option for this patient population.”
Other indications, another PARP inhibitor
Rucaparib is already approved for the treatment of women with advanced BRCA mutation–positive ovarian cancer who have received two or more prior chemotherapies. It is also approved as maintenance treatment for patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who demonstrate a complete or partial response to platinum-based chemotherapy, regardless of BRCA status.
Another PARP inhibitor, olaparib (Lynparza, AstraZeneca), is awaiting approval for use in prostate cancer in men with BRCA mutations. That pending approval is based on results from the phase 3 PROfound trial, which was hailed as a “landmark trial” when it was presented last year. The results showed a significant improved in disease-free progression. The company recently announced that there was also a significant improvement in overall survival.
Olaparib is already approved for the maintenance treatment of platinum-sensitive relapsed ovarian cancer regardless of BRCA status and as first-line maintenance treatment in BRCA-mutated advanced ovarian cancer following response to platinum-based chemotherapy. It is also approved for germline BRCA-mutated HER2-negative metastatic breast cancer previously treated with chemotherapy and for the maintenance treatment of germline BRCA-mutated advanced pancreatic cancer following first-line platinum-based chemotherapy.
Details of the TRITON2 study
The accelerated approval for use of rucaparib in BRCA prostate cancer was based on efficacy data from the multicenter, single-arm TRITON2 clinical trial. The cohort included 62 patients with a BRCA (germline and/or somatic) mutation and measurable disease; 115 patients with a BRCA (germline and/or somatic) mutation and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency (HRD)–positive mCRPC.
The major efficacy outcomes were objective response rate (ORR) and duration of response. Confirmed PSA response rate was also a prespecified endpoint. Data were assessed by independent radiologic review.
For the patients with measurable disease and a BRCA mutation, the ORR was 44%. The ORR was similar for patients with a germline BRCA mutation.
Median duration of response was not evaluable at data cutoff but ranged from 1.7 to 24+ months. Of the 27 patients with a confirmed objective response, 15 (56%) patients showed a response that lasted 6 months or longer.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%.
The safety evaluation was based on an analysis of the 209 patients with HRD-positive mCRPC and included 115 with deleterious BRCA mutations. The most common adverse events (≥20%; grade 1-4) in the patients with BRCA mutations were fatigue/asthenia (62%), nausea (52%), anemia (43%), AST/ALT elevation (33%), decreased appetite (28%), rash (27%), constipation (27%), thrombocytopenia (25%), vomiting (22%), and diarrhea (20%).
Rucaparib has been associated with hematologic toxicity, including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). However, MDS/AML was not observed in the TRITON2 study, regardless of HRD mutation.
Confirmation with TRITON3
A phase 3, randomized, open-label study, TRITON3, is currently underway and is expected to serve as the confirmatory study for the accelerated approval in mCRPC. TRITON3 is comparing rucaparib with physician’s choice of therapy in patients with mCRPC who have specific gene alterations, including BRCA and ATM alterations, and who have experienced disease progression after androgen receptor–directed therapy but have not yet received chemotherapy. The primary endpoint for TRITON3 is radiographic progression-free survival.
This article first appeared on Medscape.com.
Mammography cuts risk for fatal breast cancers: New data
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Neoadjuvant nivolumab ‘promising’ in Merkel cell carcinoma
Neoadjuvant nivolumab (Opdivo, Bristol-Myers Squibb) could improve outcomes for patients with high-risk resectable Merkel cell carcinoma (MCC), say researchers reporting the first trial in this patient population.
The results come from a cohort of 39 patients with stage IIA-IV disease, all of whom received nivolumab 240 mg intravenously on days 1 and 15, with surgery planned for day 29. About half achieved a pathologic complete response (pCR) and/or a radiographic response. Recurrence-free survival was also prolonged among responders, and no relapses were observed at a median follow-up of 19.3 months.
“Additional investigation of these promising findings is warranted,” say the investigators.
“To our knowledge, this is the first study to examine neoadjuvant anti-PD-1 therapy in MCC,” said lead author Suzanne Topalian, MD, professor of surgery at the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins Medicine, Baltimore, Maryland. “While the results seem encouraging, I think that clinical trial experience with a greater number of patients and follow-up for a longer time period will be needed to assess whether this treatment approach should become standard for high-risk resectable MCC.”
The study was published online April 23 in the Journal of Clinical Oncology.
Rare and Aggressive Skin Cancer
MCC is a rare and aggressive neuroendocrine skin cancer that is diagnosed in approximately 1600 people each year in the United States, note the authors.
The annual incidence of new MCC cases rose by 95% between 2000 and 2013, as previously reported by Medscape Medical News.
Although nearly two thirds of patients present with localized disease, regional and distant metastases often occur. Until recently, cytotoxic chemotherapy was the primary systemic therapy for advanced MCC. Although patients frequently respond to chemotherapy, the duration tends to be very limited, and 95% of patients experience disease progression within 1 year, the authors comment.
Growing evidence supports the hypothesis that MCC is an immunogenic cancer. Clinical trials of PD-L1 inhibitors have demonstrated increased progression-free and overall survival compared with chemotherapy when used either in the first-line or second-line setting, as reported by Medscape Medical News.
“Neoadjuvant anti-PD-1 cancer therapy is still early in development, and there are many aspects deserving careful consideration,” Topalian told Medscape Medical News. “They are centered on balancing patient risk and benefit, finding an optimal presurgical treatment interval, exploring treatment combinations, determining if anti-PD-1 therapy should be continued after surgery, and defining biomarkers to guide treatment strategies for individual patients.”
Study Details
Topian and colleagues investigated neoadjuvant use of nivolumab in the CheckMate 358 study, which involved 39 patients with stage IIA-IV MCC (of whom 36 went on to surgery).
The median age of the patients was 68 years, and at enrollment, most patients (66.7%) had stage III disease. For 35 patients, tumors were evaluable for MCPyV status; 22 (62.9%) were positive. Quantifiable tumor cell PD-L1 expression occurred in 27 patients; expression was 1% or higher for seven of those patients (25.9%).
The primary endpoint for the neoadjuvant cohort was safety and tolerability of nivolumab, as measured by treatment-related adverse events (TRAEs) and surgical delays, defined as the proportion of patients who experienced TRAE-related delays of more than 4 weeks from the planned surgery date.
Exploratory endpoints included pCR, radiographic response, recurrence-free survival, overall survival, association of tumor MCPyV status, and PD-L1 expression with efficacy.
The median follow-up was 20.3 months. TRAEs of any grade were reported in 18 patients (46.2%). Three (7.7%) experienced a grade 3/4 event. TRAEs with potential immunologic causes occurred in six (15.4%) patients (two TRAEs were of grade 3/4).
Among those who underwent surgery, 17 (47.2%) achieved a pCR. Among patients who were radiographically evaluable (n = 33), 29 (87.9%) achieved some degree of radiographic tumor reduction. Within this group, 18 (54.5%) experienced tumor reductions of 30% or greater (the median change in tumor burden from baseline was −32.8%). Responses were observed regardless of tumor MCPyV, PD-L1, or TMB status.
Survival Outcomes
The median relapse-free survival was not reached for any of the patients who underwent surgery. At 12 and 24 months postoperatively, rates were 77.5% and 68.5%, respectively.
Among patients with pCR, relapse-free survival at 12 months was 100.0%, compared with 59.6% among those without pCR. At 24 months, it was 88.9% for those with pCR, vs 52.2% for those without pCR (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.01 – 0.93]).
Relapse-free survival was also higher for patients with radiographic tumor reduction of ≥30% in comparison with those with reduction of <30% or progression. At 12 months, it was 100.0%, vs 56.5%; at 24 months, it was 90.9%, vs 48.5% (HR, 0.11; 95% CI, 0.01 – 0.87). No substantial difference was noted between patient subgroups with respect to tumor MCPyV status or PD-L1 expression.
Median overall survival was not reached in the entire cohort. At 12 and 24 months following the first dose of nivolumab, rates were 93.2% and 79.4%, respectively. For the patients who underwent surgery and were evaluable for pathologic response or radiographic response, 100.0% and 88.9% of patients with pCR and all patients with radiographic tumor reduction of ≥30% were alive at 12 and 24 months, respectively.
Overall survival rates among patients who underwent surgery were comparable in subgroups with regard to tumor MCPyV status and PD-L1 expression.
The study was supported by Bristol-Myers Squibb and Ono, the Mark Foundation for Cancer Research, and the National Cancer Institute. Topalian reports relationships with Aduro, Potenza, Five Prime, Tizona, DNAtrix, FLX Bio, WindMIL, Dragonfly, ERVAXX, Trieza, Amgen, MedImmune, Merck, Compugen, DNAtrix, FLX Bio, Dynavax, Immunomic, Janssen Oncology, Immunocore, Bristol-Myers Squibb, Compugen Arbor, and NexImmune. Several coauthors have also disclosed relationships with industry.
This article first appeared on Medscape.com.
Neoadjuvant nivolumab (Opdivo, Bristol-Myers Squibb) could improve outcomes for patients with high-risk resectable Merkel cell carcinoma (MCC), say researchers reporting the first trial in this patient population.
The results come from a cohort of 39 patients with stage IIA-IV disease, all of whom received nivolumab 240 mg intravenously on days 1 and 15, with surgery planned for day 29. About half achieved a pathologic complete response (pCR) and/or a radiographic response. Recurrence-free survival was also prolonged among responders, and no relapses were observed at a median follow-up of 19.3 months.
“Additional investigation of these promising findings is warranted,” say the investigators.
“To our knowledge, this is the first study to examine neoadjuvant anti-PD-1 therapy in MCC,” said lead author Suzanne Topalian, MD, professor of surgery at the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins Medicine, Baltimore, Maryland. “While the results seem encouraging, I think that clinical trial experience with a greater number of patients and follow-up for a longer time period will be needed to assess whether this treatment approach should become standard for high-risk resectable MCC.”
The study was published online April 23 in the Journal of Clinical Oncology.
Rare and Aggressive Skin Cancer
MCC is a rare and aggressive neuroendocrine skin cancer that is diagnosed in approximately 1600 people each year in the United States, note the authors.
The annual incidence of new MCC cases rose by 95% between 2000 and 2013, as previously reported by Medscape Medical News.
Although nearly two thirds of patients present with localized disease, regional and distant metastases often occur. Until recently, cytotoxic chemotherapy was the primary systemic therapy for advanced MCC. Although patients frequently respond to chemotherapy, the duration tends to be very limited, and 95% of patients experience disease progression within 1 year, the authors comment.
Growing evidence supports the hypothesis that MCC is an immunogenic cancer. Clinical trials of PD-L1 inhibitors have demonstrated increased progression-free and overall survival compared with chemotherapy when used either in the first-line or second-line setting, as reported by Medscape Medical News.
“Neoadjuvant anti-PD-1 cancer therapy is still early in development, and there are many aspects deserving careful consideration,” Topalian told Medscape Medical News. “They are centered on balancing patient risk and benefit, finding an optimal presurgical treatment interval, exploring treatment combinations, determining if anti-PD-1 therapy should be continued after surgery, and defining biomarkers to guide treatment strategies for individual patients.”
Study Details
Topian and colleagues investigated neoadjuvant use of nivolumab in the CheckMate 358 study, which involved 39 patients with stage IIA-IV MCC (of whom 36 went on to surgery).
The median age of the patients was 68 years, and at enrollment, most patients (66.7%) had stage III disease. For 35 patients, tumors were evaluable for MCPyV status; 22 (62.9%) were positive. Quantifiable tumor cell PD-L1 expression occurred in 27 patients; expression was 1% or higher for seven of those patients (25.9%).
The primary endpoint for the neoadjuvant cohort was safety and tolerability of nivolumab, as measured by treatment-related adverse events (TRAEs) and surgical delays, defined as the proportion of patients who experienced TRAE-related delays of more than 4 weeks from the planned surgery date.
Exploratory endpoints included pCR, radiographic response, recurrence-free survival, overall survival, association of tumor MCPyV status, and PD-L1 expression with efficacy.
The median follow-up was 20.3 months. TRAEs of any grade were reported in 18 patients (46.2%). Three (7.7%) experienced a grade 3/4 event. TRAEs with potential immunologic causes occurred in six (15.4%) patients (two TRAEs were of grade 3/4).
Among those who underwent surgery, 17 (47.2%) achieved a pCR. Among patients who were radiographically evaluable (n = 33), 29 (87.9%) achieved some degree of radiographic tumor reduction. Within this group, 18 (54.5%) experienced tumor reductions of 30% or greater (the median change in tumor burden from baseline was −32.8%). Responses were observed regardless of tumor MCPyV, PD-L1, or TMB status.
Survival Outcomes
The median relapse-free survival was not reached for any of the patients who underwent surgery. At 12 and 24 months postoperatively, rates were 77.5% and 68.5%, respectively.
Among patients with pCR, relapse-free survival at 12 months was 100.0%, compared with 59.6% among those without pCR. At 24 months, it was 88.9% for those with pCR, vs 52.2% for those without pCR (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.01 – 0.93]).
Relapse-free survival was also higher for patients with radiographic tumor reduction of ≥30% in comparison with those with reduction of <30% or progression. At 12 months, it was 100.0%, vs 56.5%; at 24 months, it was 90.9%, vs 48.5% (HR, 0.11; 95% CI, 0.01 – 0.87). No substantial difference was noted between patient subgroups with respect to tumor MCPyV status or PD-L1 expression.
Median overall survival was not reached in the entire cohort. At 12 and 24 months following the first dose of nivolumab, rates were 93.2% and 79.4%, respectively. For the patients who underwent surgery and were evaluable for pathologic response or radiographic response, 100.0% and 88.9% of patients with pCR and all patients with radiographic tumor reduction of ≥30% were alive at 12 and 24 months, respectively.
Overall survival rates among patients who underwent surgery were comparable in subgroups with regard to tumor MCPyV status and PD-L1 expression.
The study was supported by Bristol-Myers Squibb and Ono, the Mark Foundation for Cancer Research, and the National Cancer Institute. Topalian reports relationships with Aduro, Potenza, Five Prime, Tizona, DNAtrix, FLX Bio, WindMIL, Dragonfly, ERVAXX, Trieza, Amgen, MedImmune, Merck, Compugen, DNAtrix, FLX Bio, Dynavax, Immunomic, Janssen Oncology, Immunocore, Bristol-Myers Squibb, Compugen Arbor, and NexImmune. Several coauthors have also disclosed relationships with industry.
This article first appeared on Medscape.com.
Neoadjuvant nivolumab (Opdivo, Bristol-Myers Squibb) could improve outcomes for patients with high-risk resectable Merkel cell carcinoma (MCC), say researchers reporting the first trial in this patient population.
The results come from a cohort of 39 patients with stage IIA-IV disease, all of whom received nivolumab 240 mg intravenously on days 1 and 15, with surgery planned for day 29. About half achieved a pathologic complete response (pCR) and/or a radiographic response. Recurrence-free survival was also prolonged among responders, and no relapses were observed at a median follow-up of 19.3 months.
“Additional investigation of these promising findings is warranted,” say the investigators.
“To our knowledge, this is the first study to examine neoadjuvant anti-PD-1 therapy in MCC,” said lead author Suzanne Topalian, MD, professor of surgery at the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins Medicine, Baltimore, Maryland. “While the results seem encouraging, I think that clinical trial experience with a greater number of patients and follow-up for a longer time period will be needed to assess whether this treatment approach should become standard for high-risk resectable MCC.”
The study was published online April 23 in the Journal of Clinical Oncology.
Rare and Aggressive Skin Cancer
MCC is a rare and aggressive neuroendocrine skin cancer that is diagnosed in approximately 1600 people each year in the United States, note the authors.
The annual incidence of new MCC cases rose by 95% between 2000 and 2013, as previously reported by Medscape Medical News.
Although nearly two thirds of patients present with localized disease, regional and distant metastases often occur. Until recently, cytotoxic chemotherapy was the primary systemic therapy for advanced MCC. Although patients frequently respond to chemotherapy, the duration tends to be very limited, and 95% of patients experience disease progression within 1 year, the authors comment.
Growing evidence supports the hypothesis that MCC is an immunogenic cancer. Clinical trials of PD-L1 inhibitors have demonstrated increased progression-free and overall survival compared with chemotherapy when used either in the first-line or second-line setting, as reported by Medscape Medical News.
“Neoadjuvant anti-PD-1 cancer therapy is still early in development, and there are many aspects deserving careful consideration,” Topalian told Medscape Medical News. “They are centered on balancing patient risk and benefit, finding an optimal presurgical treatment interval, exploring treatment combinations, determining if anti-PD-1 therapy should be continued after surgery, and defining biomarkers to guide treatment strategies for individual patients.”
Study Details
Topian and colleagues investigated neoadjuvant use of nivolumab in the CheckMate 358 study, which involved 39 patients with stage IIA-IV MCC (of whom 36 went on to surgery).
The median age of the patients was 68 years, and at enrollment, most patients (66.7%) had stage III disease. For 35 patients, tumors were evaluable for MCPyV status; 22 (62.9%) were positive. Quantifiable tumor cell PD-L1 expression occurred in 27 patients; expression was 1% or higher for seven of those patients (25.9%).
The primary endpoint for the neoadjuvant cohort was safety and tolerability of nivolumab, as measured by treatment-related adverse events (TRAEs) and surgical delays, defined as the proportion of patients who experienced TRAE-related delays of more than 4 weeks from the planned surgery date.
Exploratory endpoints included pCR, radiographic response, recurrence-free survival, overall survival, association of tumor MCPyV status, and PD-L1 expression with efficacy.
The median follow-up was 20.3 months. TRAEs of any grade were reported in 18 patients (46.2%). Three (7.7%) experienced a grade 3/4 event. TRAEs with potential immunologic causes occurred in six (15.4%) patients (two TRAEs were of grade 3/4).
Among those who underwent surgery, 17 (47.2%) achieved a pCR. Among patients who were radiographically evaluable (n = 33), 29 (87.9%) achieved some degree of radiographic tumor reduction. Within this group, 18 (54.5%) experienced tumor reductions of 30% or greater (the median change in tumor burden from baseline was −32.8%). Responses were observed regardless of tumor MCPyV, PD-L1, or TMB status.
Survival Outcomes
The median relapse-free survival was not reached for any of the patients who underwent surgery. At 12 and 24 months postoperatively, rates were 77.5% and 68.5%, respectively.
Among patients with pCR, relapse-free survival at 12 months was 100.0%, compared with 59.6% among those without pCR. At 24 months, it was 88.9% for those with pCR, vs 52.2% for those without pCR (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.01 – 0.93]).
Relapse-free survival was also higher for patients with radiographic tumor reduction of ≥30% in comparison with those with reduction of <30% or progression. At 12 months, it was 100.0%, vs 56.5%; at 24 months, it was 90.9%, vs 48.5% (HR, 0.11; 95% CI, 0.01 – 0.87). No substantial difference was noted between patient subgroups with respect to tumor MCPyV status or PD-L1 expression.
Median overall survival was not reached in the entire cohort. At 12 and 24 months following the first dose of nivolumab, rates were 93.2% and 79.4%, respectively. For the patients who underwent surgery and were evaluable for pathologic response or radiographic response, 100.0% and 88.9% of patients with pCR and all patients with radiographic tumor reduction of ≥30% were alive at 12 and 24 months, respectively.
Overall survival rates among patients who underwent surgery were comparable in subgroups with regard to tumor MCPyV status and PD-L1 expression.
The study was supported by Bristol-Myers Squibb and Ono, the Mark Foundation for Cancer Research, and the National Cancer Institute. Topalian reports relationships with Aduro, Potenza, Five Prime, Tizona, DNAtrix, FLX Bio, WindMIL, Dragonfly, ERVAXX, Trieza, Amgen, MedImmune, Merck, Compugen, DNAtrix, FLX Bio, Dynavax, Immunomic, Janssen Oncology, Immunocore, Bristol-Myers Squibb, Compugen Arbor, and NexImmune. Several coauthors have also disclosed relationships with industry.
This article first appeared on Medscape.com.
Guidelines on delaying cancer surgery during COVID-19
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer care and COVID-19 in Seattle, the first U.S. epicenter
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
Disruptions in cancer care in the era of COVID-19
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
Should routine colon cancer screening start at 45, not 50?
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
SAN FRANCISCO – For years, 50 years old has been the age at which screening for colorectal cancer (CRC) began in the United States, but recently, one group lowered the starting age to 45 years.
This move by the American Cancer Society in 2018 was made in reaction to reports of an increase in the incidence of CRC in younger adults.
However, other groups have stayed with the benchmark 50 years. This includes the U.S. Preventive Services Task Force and the National Comprehensive Cancer Network.
Should the age be lowered in view of the mounting reports of an increase in CRC in younger adults? Experts argued both for and against the move here at the 2020 Gastrointestinal Cancers Symposium.
“We’re having this debate because the health of more than 20 million Americans is in the balance,” commented David Weinberg, MD, MSc, chairman of the department of medicine at Fox Chase Cancer Center in Philadelphia. “This is not just an academic discussion.” If the screening age shifts to 5 years earlier, the impact nationally would be about 30,000 colorectal cancers and 11,000 deaths averted.
“It will take about 11 million additional colonoscopies ... and the overall bill would be $10 billion. That’s not a small number, but if the country has the resources and we want to do this, I would say we can,” argued Uri Ladabaum, MD, director of the gastrointestinal cancer prevention program and the clinical chief of the division of gastroenterology and hepatology at Stanford (Calif.) University.
Lower the age
Dr. Ladabaum argued in favor of lowering the age to 45 years to start screening. “In life, 60 may be the new 40, but for colorectal cancer screening, 45 is definitely the new 50,” he said. Anticipating arguments against such a move, he focused on several points.
First, the magnitude of the problem is certainly not small, he noted, pointing to a 2017 study showing that colorectal cancer rates have increased by 1%-2.4% annually since the mid-1980s in persons aged 20-39 years and by 0.5%-1.3% since the mid-1990s in adults aged 40-54 years (J Natl Cancer Inst. 2017;109:djw322). Rectal cancer incidence has been increasing even more rapidly, at a rate of about 3.2% annually during 1974-2013 in adults aged 20-29 years.
Overall, people who were born around 1990 and later have double the risk of colon cancer (incidence rate ratio, 2.40) and quadruple the risk of rectal cancer (IRR, 4.32) as compared with those born circa 1950.
“Thus, 45- to 49-year-olds are beginning to look like yesterday’s 50- to 54-year-olds used to be,” said Dr. Ladabaum.
One issue that has been raised is lead-time bias, with the burning question: Are the cancers found in adults in their 40s simply the same ones that would have eventually been detected in their 50s? Dr. Ladabaum argued that they are not, referencing a 2019 study showing that among persons aged 40 through 49 years, the disease was diagnosed at later stages (JAMA. 2019;321:1933-4).
For those aged 40- 49 years, there was a significant increase in incidence during 1995-2015. The proportion of distant cancers increased significantly (from 21.7% to 26.6%; P less than .001), and the authors of the study had noted that this increase of 4.9% could not be explained by a decrease in unstaged cases. “In the early ’90s and mid-’90s, we began to see an increase in all stages,” Dr. Ladabaum noted. “And the most important thing here is the distant cancers over time. They’ve gone up.” If the only explanation was lead time bias in people aged 40- 49 years, then a person screened and diagnosed with cancer at age 48 would have earlier-stage disease than if it had been found at age 51. “So is this all lead-time bias?” he said. “I think the answer is no.”
Next, Dr. Ladabaum tackled the issue of whether benefit/risk ratio of CRC screening is different among younger vs. older adults. This is difficult to tease out, he suggested, as the data are sparse and there were no controlled studies to date to address that. One study from Taiwan, which looked at the outcomes of fecal immunochemical testing (FIT), showed that in different age groups, the hazard ratio for detecting cancer in those with positive results is higher in younger people vs. older ones (J Clin Gastroenterol. 2016 Oct;50[9]:761-8).
“Indirect evidence shows that if we do a FIT test and it’s positive, it probably means something,” he said. “But is there something magical that at age 50 and older – that it becomes a screenable disease, and through age 49 it’s not screenable? I would say no. Biology is not like this.”
Finally, is it cost effective to start earlier? In a modeling projection published last summer by Dr. Ladabaum and colleagues, starting at 45 years would avert about four colorectal cancers and two colorectal cancer deaths per 1,000 people, and the cohort would gain approximately 14 quality-adjusted life years (Gastroenterology. 2019;157:137-48).
“The incremental cost per quality-adjusted life year gained is highly acceptable,” said Dr. Ladabaum. “This is well within the range of what’s considered cost-effective in the United States – under $35,000 for colonoscopy, and under $8,000 for fecal immunochemical tests.”
Therefore, the answer is yes, it is cost effective, he concluded.
Not so fast ...
Arguing the case against a lowering of the starting age, Dr. Weinberg agreed with Dr. Ladabaum that colon cancer risk for younger people is rising.
That risk has increased from 5.9/100,000 to 7.2/100,000, which is a relative increase of 22%, he noted.
“That is what newspapers will want to put on their headlines to sensationalize it – that the risk of colon cancer for young people is up by over 20%,” said Dr. Weinberg. “But that represents an absolute risk of just 1.3 more people/100,000. Put in some context, 99.9% of people in their 40s will not develop colon cancer.”
This observation was not to “make light of the remainder,” he emphasized, but “the overwhelming majority are not going to get this disease at this age,” he noted.
“It’s also not entirely clear that starting screening at 45 is the right answer,” he added.
“Taken to a somewhat ridiculous extreme,” he continued, “why not start at 40? You’ll catch more people that way, but no one is advocating that.”
A better understanding of the factors contributing to the increased CRC incidence seen in younger adults is very important, he argued, and he suggested that some of it may occur because of screening, along with factors such as obesity, diabetes, and childhood exposures.
Modeling has been done to calculate the risk and benefit of early screening, but while useful for decision making, models are “usually wrong and sit near the bottom of the evidence hierarchy.”
“Models help inform decisions, but they don’t define the standard of care,” Dr. Weinberg said. “No one would vaccinate a population based on a model.”
Dr. Weinberg also emphasized that the new recommendation from the American Cancer Society to start screening at age 45 years is qualified. This means that while “clear evidence of benefit exists,” so does uncertainty about whether “the benefits really outweigh the harms.”
The current evidence for reducing the screening age is not yet clear, he believes, and he questioned the premise that age should be the only criteria for cancer stratification.
Dr. Weinberg cited one study that looked at early-onset colon cancer (ages 18-49 years) and compared it with two groups: patients diagnosed at 50 years and older and matched controls (Clin Gastroenterol Hepatol. 2019;S1542-3565[19]31108-5). Besides age, the study authors identified several nonmodifiable risk factors that were associated with early-onset disease, including sex, race, history of inflammatory bowel disease (IBD), and family history of colorectal cancer.
“Being male was a risk factor and having a family history increased your risk by three times,” said Dr. Weinberg. “And I would note that this study had removed people, at least as best one can, who were known to have syndromic risks of early-onset colon cancer (such as familial adenomatous polyposis.”
Earlier screening (by age 40 years) should already be taking place in people with such syndromes, he commented, as well as those with a known family history and IBD. “Those recommendations were there before the ACS, and we don’t necessarily need another one,” he added.
To make things a little more confusing, a second recent study, using National Cancer Data Base data from 2004 to 2015, identified another set of factors associated with colon cancer in younger adults (Cancer. 2019 Nov 1;125:3828-35). This study showed diagnosis younger than 50 years rose only in non-Hispanic white men, in Hispanic and non-Hispanic white women, and in those living in urban vs. rural areas.
“But it gets more interesting,” Dr. Weinberg pointed out. “Risk increased over time for people in the highest zip code income quartile and those with private insurance, and risk was lower for people with Medicaid and no insurance at all,” he noted. “Well, that smacks of access to me,” he commented.
Another issue is the possibility of lead-time bias. During 1975-2015, incidence rose over time, according to Surveillance, Epidemiology, and End Results data (J Natl Cancer Inst. 2017 Aug;109:djw322). Screening of persons younger than age 49 years also more than doubled, from a low level in 2000 of about 6% to more than 15% by 2010. As screening increases, the incidence increases, Dr. Weinberg pointed out. “But mortality doesn’t change. And despite what Dr. Ladabaum said a moment ago about lead-time bias, that is textbook lead-time bias in any epidemiology study.”
Finally, it is essential to carefully weigh the benefit against the risk, Dr. Weinberg said.
A core principle of population screening is to create more future health benefits than harms, and if the screening age is lowered, several million additional colonoscopies will be performed.
Colonoscopy reduces the colorectal cancer mortality risk by about 75%, and the incidence of the disease is 7.2/100,000 in the younger age group. But colonoscopy-specific mortality – just having the test – is associated with a death rate of 7/100,000,” Dr. Weinberg pointed out. “Let’s not forget that there is a risk associated with this procedure.” (Gastrointest Endosc. 2011 Oct;74:745-52).
Dr. Weinberg emphasized that everyone wants to reduce the burden of cancer, and models are helpful for that purpose. “They’re obviously thought provoking, but they’re not adequate to drive change without additional evidence of clinical and cost-effectiveness,” he said. “These are important questions that need better data.”
He added that without changing the current screening protocol, “we could certainly emphasize more than ever the impact of family history and IBD on colon cancer risk and colon cancer prevention.”
“And certainly, there’s plenty of evidence that patients with a known family history of colon cancer are not getting screened at the right age or with the right frequency,” Dr. Weinberg concluded. “We can do better. All of us can do better.”
Dr. Weinberg has disclosed relationships with Fujifilm and Exact Sciences. Dr. Ladabaum has disclosed relationships with Lean Medical, Universal Dx, Clinical Genomics, Medtronic, Modus GI, and Quorum Consulting.
This article first appeared on Medscape.com.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM