User login
Should you adopt the practice of vaginal cleansing with povidone-iodine prior to cesarean delivery?
There are approximately 4,000,000 births annually in the United States, and about 32% of them occur by cesarean delivery. Compared with vaginal birth, cesarean delivery is associated with an increased risk of endometritis (defined as fever plus uterine or abdominal tenderness). Although surgical complications cannot be eliminated entirely, surgeons are deeply dedicated to the continuous improvement of surgical practice in order to reduce the risk of complications.
With cesarean delivery, many surgical practices have been adopted universally to reduce postoperative complications, including administration of intravenous (IV) antibiotics before skin incision to minimize postoperative infection and the use of postoperative mechanical or pharmacologic interventions to help prevent venous thromboembolism and pulmonary embolism. Preoperative vaginal cleansing with povidone-iodine may reduce the risk of postoperative endometritis, but the practice is not currently common in the United States.
Should you adopt a policy of preoperative vaginal cleansing prior to cesarean delivery? The data suggest perhaps you should.
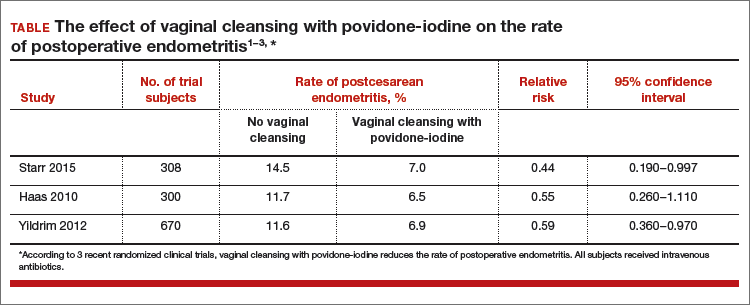
Data-driven support for povidone-iodine precesareanThree large randomized trials published within the past 10 years concluded that preoperative vaginal cleansing with povidone-iodine reduced the risk of postcesarean endometritis in women who also received prophylactic IV antibiotics (TABLE).1−3 Vaginal cleansing did not reduce the rate of postpartum fever or wound infection in these studies.
Clinical factors that increased the risk of postpartum endometritis independent of vaginal cleansing included:
- extended duration of cesarean surgery
- being in labor prior to cesarean delivery
- ruptured membranes
- advanced cervical examination
- maternal anemia
- use of intrapartum internal monitors
- prior history of genitourinary infection.
Authors of two recent, large nonrandomized studies also have reported that vaginal cleansing reduced the risk of postcesarean endometritis.4,5 By contrast, investigators from one large trial from 2001 did not observe a decrease in endometritis with vaginal cleansing.6
To test the impact of metronidazole vaginal gel on post‑cesarean endometritis, 224 women undergoing cesarean delivery for various indications were randomly assigned to placebo vaginal gel or metronidazole vaginal gel 5 g prior to surgery initiation.1 Most women also received intravenous antibiotics. The rates of endometritis were 17% and 7% in the placebo and metronidazole groups, respectively (relative risk, 0.42; 95% confidence interval, 0.19−0.92).
Vaginal antibiotic administration shows promise as an alternative to povidone-iodine cleansing in the prevention of postcesarean endometritis. Additional randomized clinical trials are necessary to fully evaluate the benefits and risks of this practice.
Reference
1. Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745−750.
Cochrane review of precesarean vaginal cleansingAuthors of a Cochrane review, in which they synthesized 7 studies involving 2,635 women, reported that vaginal cleansing with povidone-iodine immediately before cesarean delivery was associated with a reduced risk of postcesarean endometritis: 8.3% vs 4.3% in the control and vaginal cleansing groups, respectively, (risk ratio [RR], 0.45; 95% confidence interval [CI], 0.25−0.81).7
The positive effect of vaginal cleansing was particularly noteworthy in the subgroup of women with ruptured membranes (3 trials involving 272 women). The rates of endometritis in the control versus vaginal cleansing groups were 17.9% and 4.3%, respectively (RR, 0.24; 95% CI, 0.10−0.55).
Women who went into labor prior to cesarean delivery (523 women from 3 trials) also benefitted from vaginal cleansing, with endometritis rates of 13.0% and 7.4% in the control and vaginal cleansing groups, respectively (RR, 0.56; 95% CI, 0.34−0.95).
In this review, again, vaginal cleansing did not significantly reduce the rate of postoperative fever or wound infection.
The American College of Obstetricians and Gynecologists has noted that chlorohexidine gluconate solutions with high concentrations of alcohol are contraindicated for vaginal cleansing.1 However, although not approved for vaginal cleansing, solutions of chlorohexidine gluconate with low alcohol content (4% alcohol concentration) are safe and may be effective for off-label use as vaginal cleansings.
Reference
1. American College of Obstetricians and Gynecologists Women’s Health Care Physicians; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgical cleansing of the vagina. Obstet Gynecol. 2013;122(3):718−720.
Is vaginal cleansing prior to cesarean delivery best practice?In the United States, precesarean vaginal cleansing is not a common practice. To close the gap between current practice and what is potentially a best practice, two approaches to using vaginal cleansing could be instituted in delivery units.
Approach #1: A liberal clinical protocol. In this scenario, all women (who are not allergic to iodine or povidone-iodine) undergoing cesarean delivery should undergo vaginal cleansing. The World Health Organization conditionally recommends vaginal cleansing for all women undergoing a cesarean delivery.8
Approach #2: A focused clinical protocol. For this protocol, only women (again, who are not allergic to iodine or povidone-iodine) who have ruptured membranes or are in labor upon advanced cervical examination should receive vaginal cleansing.
The advantage of a liberal protocol is that vaginal preparation becomes embedded within the standard practice of cesarean delivery and, hence, is seldom overlooked. The upside of the focused protocol is that only those women most likely to benefit receive the intervention.
Tell me what you thinkWill you consider using vaginal cleansing in your practice? Please let me know your views on vaginal cleansing for cesarean delivery, as well as your clinical pearls on cesarean delivery surgery, at obgmanagement.com. In addition, weigh in on the Quick Poll posted to OBG Management’s homepage. Send your letter to the editor to [email protected].
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2015;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to cesarean delivery reduce the risk of endometritis?A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-cesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Memon S, Qazi RA, Bibi S, Parveen N. Effect of preoperative vaginal cleansing with an antiseptic solution to reduce post caesarean infectious morbidity. J Pak Med Assoc. 2011;61(12):1179–1183.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone-iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to
There are approximately 4,000,000 births annually in the United States, and about 32% of them occur by cesarean delivery. Compared with vaginal birth, cesarean delivery is associated with an increased risk of endometritis (defined as fever plus uterine or abdominal tenderness). Although surgical complications cannot be eliminated entirely, surgeons are deeply dedicated to the continuous improvement of surgical practice in order to reduce the risk of complications.
With cesarean delivery, many surgical practices have been adopted universally to reduce postoperative complications, including administration of intravenous (IV) antibiotics before skin incision to minimize postoperative infection and the use of postoperative mechanical or pharmacologic interventions to help prevent venous thromboembolism and pulmonary embolism. Preoperative vaginal cleansing with povidone-iodine may reduce the risk of postoperative endometritis, but the practice is not currently common in the United States.
Should you adopt a policy of preoperative vaginal cleansing prior to cesarean delivery? The data suggest perhaps you should.
Data-driven support for povidone-iodine precesareanThree large randomized trials published within the past 10 years concluded that preoperative vaginal cleansing with povidone-iodine reduced the risk of postcesarean endometritis in women who also received prophylactic IV antibiotics (TABLE).1−3 Vaginal cleansing did not reduce the rate of postpartum fever or wound infection in these studies.

Clinical factors that increased the risk of postpartum endometritis independent of vaginal cleansing included:
- extended duration of cesarean surgery
- being in labor prior to cesarean delivery
- ruptured membranes
- advanced cervical examination
- maternal anemia
- use of intrapartum internal monitors
- prior history of genitourinary infection.
Authors of two recent, large nonrandomized studies also have reported that vaginal cleansing reduced the risk of postcesarean endometritis.4,5 By contrast, investigators from one large trial from 2001 did not observe a decrease in endometritis with vaginal cleansing.6
To test the impact of metronidazole vaginal gel on post‑cesarean endometritis, 224 women undergoing cesarean delivery for various indications were randomly assigned to placebo vaginal gel or metronidazole vaginal gel 5 g prior to surgery initiation.1 Most women also received intravenous antibiotics. The rates of endometritis were 17% and 7% in the placebo and metronidazole groups, respectively (relative risk, 0.42; 95% confidence interval, 0.19−0.92).
Vaginal antibiotic administration shows promise as an alternative to povidone-iodine cleansing in the prevention of postcesarean endometritis. Additional randomized clinical trials are necessary to fully evaluate the benefits and risks of this practice.
Reference
1. Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745−750.
Cochrane review of precesarean vaginal cleansingAuthors of a Cochrane review, in which they synthesized 7 studies involving 2,635 women, reported that vaginal cleansing with povidone-iodine immediately before cesarean delivery was associated with a reduced risk of postcesarean endometritis: 8.3% vs 4.3% in the control and vaginal cleansing groups, respectively, (risk ratio [RR], 0.45; 95% confidence interval [CI], 0.25−0.81).7
The positive effect of vaginal cleansing was particularly noteworthy in the subgroup of women with ruptured membranes (3 trials involving 272 women). The rates of endometritis in the control versus vaginal cleansing groups were 17.9% and 4.3%, respectively (RR, 0.24; 95% CI, 0.10−0.55).
Women who went into labor prior to cesarean delivery (523 women from 3 trials) also benefitted from vaginal cleansing, with endometritis rates of 13.0% and 7.4% in the control and vaginal cleansing groups, respectively (RR, 0.56; 95% CI, 0.34−0.95).
In this review, again, vaginal cleansing did not significantly reduce the rate of postoperative fever or wound infection.
The American College of Obstetricians and Gynecologists has noted that chlorohexidine gluconate solutions with high concentrations of alcohol are contraindicated for vaginal cleansing.1 However, although not approved for vaginal cleansing, solutions of chlorohexidine gluconate with low alcohol content (4% alcohol concentration) are safe and may be effective for off-label use as vaginal cleansings.
Reference
1. American College of Obstetricians and Gynecologists Women’s Health Care Physicians; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgical cleansing of the vagina. Obstet Gynecol. 2013;122(3):718−720.
Is vaginal cleansing prior to cesarean delivery best practice?In the United States, precesarean vaginal cleansing is not a common practice. To close the gap between current practice and what is potentially a best practice, two approaches to using vaginal cleansing could be instituted in delivery units.
Approach #1: A liberal clinical protocol. In this scenario, all women (who are not allergic to iodine or povidone-iodine) undergoing cesarean delivery should undergo vaginal cleansing. The World Health Organization conditionally recommends vaginal cleansing for all women undergoing a cesarean delivery.8
Approach #2: A focused clinical protocol. For this protocol, only women (again, who are not allergic to iodine or povidone-iodine) who have ruptured membranes or are in labor upon advanced cervical examination should receive vaginal cleansing.
The advantage of a liberal protocol is that vaginal preparation becomes embedded within the standard practice of cesarean delivery and, hence, is seldom overlooked. The upside of the focused protocol is that only those women most likely to benefit receive the intervention.
Tell me what you thinkWill you consider using vaginal cleansing in your practice? Please let me know your views on vaginal cleansing for cesarean delivery, as well as your clinical pearls on cesarean delivery surgery, at obgmanagement.com. In addition, weigh in on the Quick Poll posted to OBG Management’s homepage. Send your letter to the editor to [email protected].
There are approximately 4,000,000 births annually in the United States, and about 32% of them occur by cesarean delivery. Compared with vaginal birth, cesarean delivery is associated with an increased risk of endometritis (defined as fever plus uterine or abdominal tenderness). Although surgical complications cannot be eliminated entirely, surgeons are deeply dedicated to the continuous improvement of surgical practice in order to reduce the risk of complications.
With cesarean delivery, many surgical practices have been adopted universally to reduce postoperative complications, including administration of intravenous (IV) antibiotics before skin incision to minimize postoperative infection and the use of postoperative mechanical or pharmacologic interventions to help prevent venous thromboembolism and pulmonary embolism. Preoperative vaginal cleansing with povidone-iodine may reduce the risk of postoperative endometritis, but the practice is not currently common in the United States.
Should you adopt a policy of preoperative vaginal cleansing prior to cesarean delivery? The data suggest perhaps you should.
Data-driven support for povidone-iodine precesareanThree large randomized trials published within the past 10 years concluded that preoperative vaginal cleansing with povidone-iodine reduced the risk of postcesarean endometritis in women who also received prophylactic IV antibiotics (TABLE).1−3 Vaginal cleansing did not reduce the rate of postpartum fever or wound infection in these studies.

Clinical factors that increased the risk of postpartum endometritis independent of vaginal cleansing included:
- extended duration of cesarean surgery
- being in labor prior to cesarean delivery
- ruptured membranes
- advanced cervical examination
- maternal anemia
- use of intrapartum internal monitors
- prior history of genitourinary infection.
Authors of two recent, large nonrandomized studies also have reported that vaginal cleansing reduced the risk of postcesarean endometritis.4,5 By contrast, investigators from one large trial from 2001 did not observe a decrease in endometritis with vaginal cleansing.6
To test the impact of metronidazole vaginal gel on post‑cesarean endometritis, 224 women undergoing cesarean delivery for various indications were randomly assigned to placebo vaginal gel or metronidazole vaginal gel 5 g prior to surgery initiation.1 Most women also received intravenous antibiotics. The rates of endometritis were 17% and 7% in the placebo and metronidazole groups, respectively (relative risk, 0.42; 95% confidence interval, 0.19−0.92).
Vaginal antibiotic administration shows promise as an alternative to povidone-iodine cleansing in the prevention of postcesarean endometritis. Additional randomized clinical trials are necessary to fully evaluate the benefits and risks of this practice.
Reference
1. Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745−750.
Cochrane review of precesarean vaginal cleansingAuthors of a Cochrane review, in which they synthesized 7 studies involving 2,635 women, reported that vaginal cleansing with povidone-iodine immediately before cesarean delivery was associated with a reduced risk of postcesarean endometritis: 8.3% vs 4.3% in the control and vaginal cleansing groups, respectively, (risk ratio [RR], 0.45; 95% confidence interval [CI], 0.25−0.81).7
The positive effect of vaginal cleansing was particularly noteworthy in the subgroup of women with ruptured membranes (3 trials involving 272 women). The rates of endometritis in the control versus vaginal cleansing groups were 17.9% and 4.3%, respectively (RR, 0.24; 95% CI, 0.10−0.55).
Women who went into labor prior to cesarean delivery (523 women from 3 trials) also benefitted from vaginal cleansing, with endometritis rates of 13.0% and 7.4% in the control and vaginal cleansing groups, respectively (RR, 0.56; 95% CI, 0.34−0.95).
In this review, again, vaginal cleansing did not significantly reduce the rate of postoperative fever or wound infection.
The American College of Obstetricians and Gynecologists has noted that chlorohexidine gluconate solutions with high concentrations of alcohol are contraindicated for vaginal cleansing.1 However, although not approved for vaginal cleansing, solutions of chlorohexidine gluconate with low alcohol content (4% alcohol concentration) are safe and may be effective for off-label use as vaginal cleansings.
Reference
1. American College of Obstetricians and Gynecologists Women’s Health Care Physicians; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgical cleansing of the vagina. Obstet Gynecol. 2013;122(3):718−720.
Is vaginal cleansing prior to cesarean delivery best practice?In the United States, precesarean vaginal cleansing is not a common practice. To close the gap between current practice and what is potentially a best practice, two approaches to using vaginal cleansing could be instituted in delivery units.
Approach #1: A liberal clinical protocol. In this scenario, all women (who are not allergic to iodine or povidone-iodine) undergoing cesarean delivery should undergo vaginal cleansing. The World Health Organization conditionally recommends vaginal cleansing for all women undergoing a cesarean delivery.8
Approach #2: A focused clinical protocol. For this protocol, only women (again, who are not allergic to iodine or povidone-iodine) who have ruptured membranes or are in labor upon advanced cervical examination should receive vaginal cleansing.
The advantage of a liberal protocol is that vaginal preparation becomes embedded within the standard practice of cesarean delivery and, hence, is seldom overlooked. The upside of the focused protocol is that only those women most likely to benefit receive the intervention.
Tell me what you thinkWill you consider using vaginal cleansing in your practice? Please let me know your views on vaginal cleansing for cesarean delivery, as well as your clinical pearls on cesarean delivery surgery, at obgmanagement.com. In addition, weigh in on the Quick Poll posted to OBG Management’s homepage. Send your letter to the editor to [email protected].
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2015;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to cesarean delivery reduce the risk of endometritis?A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-cesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Memon S, Qazi RA, Bibi S, Parveen N. Effect of preoperative vaginal cleansing with an antiseptic solution to reduce post caesarean infectious morbidity. J Pak Med Assoc. 2011;61(12):1179–1183.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone-iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to
- Starr RV, Zurawski J, Ismail M. Preoperative vaginal preparation with povidone-iodine and the risk of postcesarean endometritis. Obstet Gynecol. 2015;105(5 pt 1):1024–1029.
- Haas DM, Pazouki F, Smith RR, et al. Vaginal cleansing before cesarean delivery to reduce postoperative infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2010;202(3):310.e1–e6.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to cesarean delivery reduce the risk of endometritis?A randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(11):2316–2321.
- Asghania M, Mirblouk F, Shakiba M, Faraji R. Preoperative vaginal preparation with povidone-iodine on post-cesarean infectious morbidity. J Obstet Gynaecol. 2011;31(5):400–403.
- Memon S, Qazi RA, Bibi S, Parveen N. Effect of preoperative vaginal cleansing with an antiseptic solution to reduce post caesarean infectious morbidity. J Pak Med Assoc. 2011;61(12):1179–1183.
- Reid VC, Hartmann KE, McMahon M, Fry EP. Vaginal preparation with povidone-iodine and postcesarean infectious morbidity: a randomized controlled trial. Obstet Gynecol. 2001;97(1):147–152.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Yildrim G, Gungorduk K, Asicioglu O, et al. Does vaginal preparation with povidone-iodine prior to
Should newborns at 22 or 23 weeks’ gestational age be aggressively resuscitated?
For many decades the limit of viability was believed to be approximately 24 weeks of gestation. In many medical centers, newborns delivered at less than 25 weeks are evaluated in the delivery room and the decision to resuscitate is based on the infant’s clinical response. In the past, aggressive and extended resuscitation of newborns at 22 and 23 weeks was not common because the prognosis was bleak and clinicians did not want to inflict unnecessary pain when the chances for survival were limited. Recent advances in obstetric and pediatric care, however, have resulted in the survival of some infants born at 22 weeks’ gestation, calling into question long-held beliefs about the limits of viability.
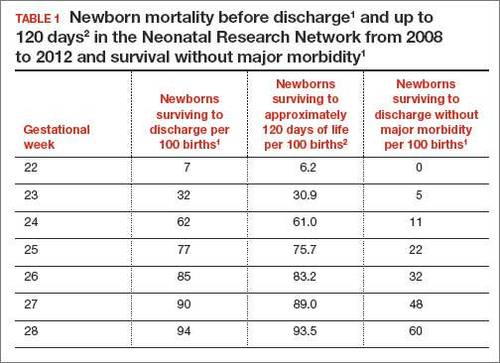
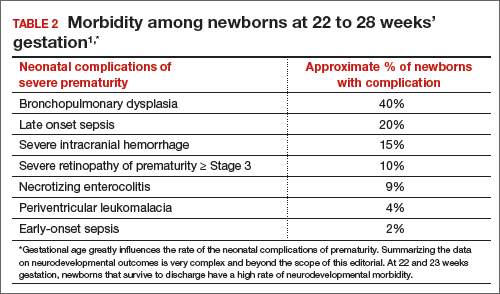
In 2 recent reports, investigators used data from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network to acquire detailed information about newborn survival and morbidity at 22 through 28 weeks’ gestation (TABLES 1 and 2).1,2 These data show that the survival of newborns at 23 through 27 weeks’ gestation is increasing, albeit slowly. Survival, without major morbidity, is gradually improving for newborns at 25 through 28 weeks.1,2 But what is the prognosis for a fetus born at 22 or 23 weeks?
There are several aspects of this issue to consider, including accurate dating of the gestational age and current viability outcomes data.
Determining the limit of viability: Accurate dating is essentialThe limit of viability is the milestone in gestation when there is a high probability of extrauterine survival. A major challenge in studies of the limit of viability for newborns is that accurate gestational dating is not always available. For example, in recent reports from the NICHD Neonatal Research Network the gestational age was determined by the best obstetric estimate, or the Ballard or Dubowitz examination, of the newborn.1,2
It is well known that ultrasound dating early in gestation is a better estimate of gestational age than last menstrual period, uterine sizing, or pediatric examination of the newborn. Hence, the available data are limited by the absence of precise gestational dating with early ultrasound. Data on the limit of viability with large numbers of births between 22 and 24 weeks with early ultrasound dating would help to refine our understanding of the limit of viability.
At 23 weeks, each day of in utero development is criticalThe importance of each additional day spent in utero during the 23rd week of gestation was demonstrated in a small cohort in 2001.4 Overall, during the 23rd week of gestation the survival of newborns to discharge was 33%.4 This finding is similar to the survival rate reported by the NICHD Neonatal Research Network in 2012.1 However, survival was vastly different early, compared with later, in the 23rd week4:
- from 23 weeks 0 days to 23 weeks 2 days: no newborn survived
- at 23 weeks 3 days and 23 weeks 4 days: 40% of newborns survived
- at 23 weeks 5 days and 23 weeks 6 days: 63% of newborns survived (a similar survival rate of 24-week gestations was reported by the NICHD Neonatal Research Network1).
The development of the fetus across the 23rd week of gestation appears to be critical to newborn survival. Hence, every day of in utero development during the 23rd week is critically important. A great challenge for obstetricians is how to approach the woman with threatened preterm birth at 22 weeks 0 days’ gestation. If the woman delivers within a few days, the likelihood of survival is minimal. However, if the pregnancy can be extended to 23 weeks and 5 days, survival rates increase significantly.
Aligning the actions of birth team, mother, and familyFactors that influence the limit of viability include:
- gestational age
- gender of the fetus (Females are more likely than males to survive.)
- treatment of the mother with glucocorticoids prior to birth
- newborn weight.
To increase the likelihood of newborn survival, obstetricians need to treat women at risk for preterm birth with antenatal glucocorticoids and antibiotics for rupture of membranes and to limit fetal stress during the birth process. Guidelines have evolved to encourage clinicians to treat women at preterm birth risk with glucocorticoids either at:
- 23 weeks’ gestation or
- 22 weeks’ gestation, if birth is anticipated to occur at 23 weeks or later.5
At birth, pediatricians are then faced with the very difficult decision of whether or not to aggressively resuscitate the severely preterm infant. Complex medical, social, and ethical issues ultimately guide pediatricians’ actions in this challenging situation. It is important for their actions to be in consensus with the obstetrician, the mother, and the mother’s family and for a consensus to be reached. Dissonant plans may increase adverse outcomes for the newborn. In one study when pediatricians and obstetricians were not aligned in their actions, the risk of death of an extremely preterm newborn significantly increased.6
Prior to birth, team meetings that include the obstetricians, pediatricians, mother, and family will help to set expectations about the course of care and, in turn, improve perceived outcomes.5 If feasible, obstetricians and pediatricians should develop joint institutional guidelines about the general approach to pregnant women when birth may occur at 22 or 23 weeks’ gestation.5
A neonatal outcomes predictor
The National Institute of Child Health and Human Development provides a Web-based tool for estimating newborn outcomes based on gestational age (22 to 25 weeks), birth weight, gender, singleton or multiple gestation, and exposure to antenatal glucocorticoid treatment. The outcomes tool provides estimates for survival and survival with severe morbidity. It uses data collected by the Neonatal Research Network to predict outcomes. To access the outcomes data assessment, visit https://www.nichd.nih.gov/about/org/der/branches/ppb/programs/epbo/Pages/epbo_case.aspx.
Is aggressive management of preterm birth and neonatal resuscitation a self-fulfilling prophecy?The beliefs and training of clinicians may influence the outcome of extremely preterm newborns. For example, if obstetricians and pediatricians focus on the fact that birth at 23 weeks is not likely to result in survival without severe morbidity, they may withhold key interventions such as antenatal glucocorticoids, antibiotics for rupture of the membranes, and aggressive newborn resuscitation.7 Consequently the likelihood of survival may be reduced.
If clinicians believe in maximal interventions for all newborns at 22 and 23 weeks’ gestation, their actions may result in a small increase in newborn survival—but at the cost of painful and unnecessary interventions in many newborns who are destined to die. Finding the right balance along the broad spectrum from expectant management to aggressive and extended resuscitation is challenging. Clearly there is no “right answer” with these extremely difficult decisions.
Future trends in the limit of viabilityIn 1963, Jacqueline Bouvier Kennedy, at 34 weeks’ gestation, went into preterm labor and delivered her son Patrick at a community hospital. Patrick developed respiratory distress syndrome and was transferred to the Boston Children’s Hospital. He died shortly thereafter.8 Would Patrick have survived if he had been delivered at an institution capable of providing high-risk obstetric and newborn services? Would such modern interventions as antenatal glucocorticoids, antibiotics for ruptured membranes, liberal use of cesarean delivery, and aggressive neonatal resuscitation have improved his chances for survival?
From our current perspective, it is surprising that a 34-week newborn died shortly after birth. With modern obstetric and pediatric care that scenario is unusual. It is possible that future advances in medical care will push the limit of viability to 22 weeks’ gestation. Future generations of clinicians may be surprised that the medicine we practice today is so limited.
However, given our current resources, it is unlikely that newborns at 22 weeks’ gestation will survive, or survive without severe morbidity. Consequently, routine aggressive resuscitation of newborns at 22 weeks should be approached with caution. At 23 weeks and later, many newborns will survive and a few will survive without severe morbidity. Given the complexity of the issues, the approach to resuscitation of infants at 22 and 23 weeks must account for the perspectives of the birth mother and her family, obstetricians, and pediatricians. Managing threatened preterm birth at 22 and 23 weeks is one of our greatest challenges as obstetricians, and we need to meet this challenge with grace and skill.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–1051.
- Patel RM, Kandefer S, Walsh MC, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340.
- Donovan EF, Tyson JE, Ehrenkranz RA, et al. Inaccuracy of Ballard scores before 28 weeks’ gestation. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1999;135(2 pt 1):147–152.
- McElrath TF, Robinson JN, Ecker JL, Ringer SA, Norwitz ER. Neonatal outcome of infants born at 23 weeks’ gestation. Obstet Gynecol. 2001;97(1):49–52.
- Raju TN, Mercer BM, Burchfield DJ, Joseph GF Jr. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(5):1083–1096.
- Guinsburg R, Branco de Almeida MF, dos Santos Rodrigues Sadeck L, et al; Brazilian Network on Neonatal Research. Proactive management of extreme prematurity: disagreement between obstetricians and neonatologists. J Perinatol. 2012;32(12):913-919.
- Tucker Emonds B, McKenzie F, Farrow V, Raglan G, Schulkin J. A national survey of obstetricians’ attitudes toward and practice of periviable interventions. J Perinatol. 2015;35(5):338–343.
- Altman LK. A Kennedy baby’s life and death. New York Times. http://www.nytimes.com/2013/07/30/health/a-kennedy-babys-life-and-death.html?_r=0. Published July 29, 2013. Accessed November 19, 2015.
For many decades the limit of viability was believed to be approximately 24 weeks of gestation. In many medical centers, newborns delivered at less than 25 weeks are evaluated in the delivery room and the decision to resuscitate is based on the infant’s clinical response. In the past, aggressive and extended resuscitation of newborns at 22 and 23 weeks was not common because the prognosis was bleak and clinicians did not want to inflict unnecessary pain when the chances for survival were limited. Recent advances in obstetric and pediatric care, however, have resulted in the survival of some infants born at 22 weeks’ gestation, calling into question long-held beliefs about the limits of viability.
In 2 recent reports, investigators used data from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network to acquire detailed information about newborn survival and morbidity at 22 through 28 weeks’ gestation (TABLES 1 and 2).1,2 These data show that the survival of newborns at 23 through 27 weeks’ gestation is increasing, albeit slowly. Survival, without major morbidity, is gradually improving for newborns at 25 through 28 weeks.1,2 But what is the prognosis for a fetus born at 22 or 23 weeks?


There are several aspects of this issue to consider, including accurate dating of the gestational age and current viability outcomes data.
Determining the limit of viability: Accurate dating is essentialThe limit of viability is the milestone in gestation when there is a high probability of extrauterine survival. A major challenge in studies of the limit of viability for newborns is that accurate gestational dating is not always available. For example, in recent reports from the NICHD Neonatal Research Network the gestational age was determined by the best obstetric estimate, or the Ballard or Dubowitz examination, of the newborn.1,2
It is well known that ultrasound dating early in gestation is a better estimate of gestational age than last menstrual period, uterine sizing, or pediatric examination of the newborn. Hence, the available data are limited by the absence of precise gestational dating with early ultrasound. Data on the limit of viability with large numbers of births between 22 and 24 weeks with early ultrasound dating would help to refine our understanding of the limit of viability.
At 23 weeks, each day of in utero development is criticalThe importance of each additional day spent in utero during the 23rd week of gestation was demonstrated in a small cohort in 2001.4 Overall, during the 23rd week of gestation the survival of newborns to discharge was 33%.4 This finding is similar to the survival rate reported by the NICHD Neonatal Research Network in 2012.1 However, survival was vastly different early, compared with later, in the 23rd week4:
- from 23 weeks 0 days to 23 weeks 2 days: no newborn survived
- at 23 weeks 3 days and 23 weeks 4 days: 40% of newborns survived
- at 23 weeks 5 days and 23 weeks 6 days: 63% of newborns survived (a similar survival rate of 24-week gestations was reported by the NICHD Neonatal Research Network1).
The development of the fetus across the 23rd week of gestation appears to be critical to newborn survival. Hence, every day of in utero development during the 23rd week is critically important. A great challenge for obstetricians is how to approach the woman with threatened preterm birth at 22 weeks 0 days’ gestation. If the woman delivers within a few days, the likelihood of survival is minimal. However, if the pregnancy can be extended to 23 weeks and 5 days, survival rates increase significantly.
Aligning the actions of birth team, mother, and familyFactors that influence the limit of viability include:
- gestational age
- gender of the fetus (Females are more likely than males to survive.)
- treatment of the mother with glucocorticoids prior to birth
- newborn weight.
To increase the likelihood of newborn survival, obstetricians need to treat women at risk for preterm birth with antenatal glucocorticoids and antibiotics for rupture of membranes and to limit fetal stress during the birth process. Guidelines have evolved to encourage clinicians to treat women at preterm birth risk with glucocorticoids either at:
- 23 weeks’ gestation or
- 22 weeks’ gestation, if birth is anticipated to occur at 23 weeks or later.5
At birth, pediatricians are then faced with the very difficult decision of whether or not to aggressively resuscitate the severely preterm infant. Complex medical, social, and ethical issues ultimately guide pediatricians’ actions in this challenging situation. It is important for their actions to be in consensus with the obstetrician, the mother, and the mother’s family and for a consensus to be reached. Dissonant plans may increase adverse outcomes for the newborn. In one study when pediatricians and obstetricians were not aligned in their actions, the risk of death of an extremely preterm newborn significantly increased.6
Prior to birth, team meetings that include the obstetricians, pediatricians, mother, and family will help to set expectations about the course of care and, in turn, improve perceived outcomes.5 If feasible, obstetricians and pediatricians should develop joint institutional guidelines about the general approach to pregnant women when birth may occur at 22 or 23 weeks’ gestation.5
A neonatal outcomes predictor
The National Institute of Child Health and Human Development provides a Web-based tool for estimating newborn outcomes based on gestational age (22 to 25 weeks), birth weight, gender, singleton or multiple gestation, and exposure to antenatal glucocorticoid treatment. The outcomes tool provides estimates for survival and survival with severe morbidity. It uses data collected by the Neonatal Research Network to predict outcomes. To access the outcomes data assessment, visit https://www.nichd.nih.gov/about/org/der/branches/ppb/programs/epbo/Pages/epbo_case.aspx.
Is aggressive management of preterm birth and neonatal resuscitation a self-fulfilling prophecy?The beliefs and training of clinicians may influence the outcome of extremely preterm newborns. For example, if obstetricians and pediatricians focus on the fact that birth at 23 weeks is not likely to result in survival without severe morbidity, they may withhold key interventions such as antenatal glucocorticoids, antibiotics for rupture of the membranes, and aggressive newborn resuscitation.7 Consequently the likelihood of survival may be reduced.
If clinicians believe in maximal interventions for all newborns at 22 and 23 weeks’ gestation, their actions may result in a small increase in newborn survival—but at the cost of painful and unnecessary interventions in many newborns who are destined to die. Finding the right balance along the broad spectrum from expectant management to aggressive and extended resuscitation is challenging. Clearly there is no “right answer” with these extremely difficult decisions.
Future trends in the limit of viabilityIn 1963, Jacqueline Bouvier Kennedy, at 34 weeks’ gestation, went into preterm labor and delivered her son Patrick at a community hospital. Patrick developed respiratory distress syndrome and was transferred to the Boston Children’s Hospital. He died shortly thereafter.8 Would Patrick have survived if he had been delivered at an institution capable of providing high-risk obstetric and newborn services? Would such modern interventions as antenatal glucocorticoids, antibiotics for ruptured membranes, liberal use of cesarean delivery, and aggressive neonatal resuscitation have improved his chances for survival?
From our current perspective, it is surprising that a 34-week newborn died shortly after birth. With modern obstetric and pediatric care that scenario is unusual. It is possible that future advances in medical care will push the limit of viability to 22 weeks’ gestation. Future generations of clinicians may be surprised that the medicine we practice today is so limited.
However, given our current resources, it is unlikely that newborns at 22 weeks’ gestation will survive, or survive without severe morbidity. Consequently, routine aggressive resuscitation of newborns at 22 weeks should be approached with caution. At 23 weeks and later, many newborns will survive and a few will survive without severe morbidity. Given the complexity of the issues, the approach to resuscitation of infants at 22 and 23 weeks must account for the perspectives of the birth mother and her family, obstetricians, and pediatricians. Managing threatened preterm birth at 22 and 23 weeks is one of our greatest challenges as obstetricians, and we need to meet this challenge with grace and skill.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
For many decades the limit of viability was believed to be approximately 24 weeks of gestation. In many medical centers, newborns delivered at less than 25 weeks are evaluated in the delivery room and the decision to resuscitate is based on the infant’s clinical response. In the past, aggressive and extended resuscitation of newborns at 22 and 23 weeks was not common because the prognosis was bleak and clinicians did not want to inflict unnecessary pain when the chances for survival were limited. Recent advances in obstetric and pediatric care, however, have resulted in the survival of some infants born at 22 weeks’ gestation, calling into question long-held beliefs about the limits of viability.
In 2 recent reports, investigators used data from the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network to acquire detailed information about newborn survival and morbidity at 22 through 28 weeks’ gestation (TABLES 1 and 2).1,2 These data show that the survival of newborns at 23 through 27 weeks’ gestation is increasing, albeit slowly. Survival, without major morbidity, is gradually improving for newborns at 25 through 28 weeks.1,2 But what is the prognosis for a fetus born at 22 or 23 weeks?


There are several aspects of this issue to consider, including accurate dating of the gestational age and current viability outcomes data.
Determining the limit of viability: Accurate dating is essentialThe limit of viability is the milestone in gestation when there is a high probability of extrauterine survival. A major challenge in studies of the limit of viability for newborns is that accurate gestational dating is not always available. For example, in recent reports from the NICHD Neonatal Research Network the gestational age was determined by the best obstetric estimate, or the Ballard or Dubowitz examination, of the newborn.1,2
It is well known that ultrasound dating early in gestation is a better estimate of gestational age than last menstrual period, uterine sizing, or pediatric examination of the newborn. Hence, the available data are limited by the absence of precise gestational dating with early ultrasound. Data on the limit of viability with large numbers of births between 22 and 24 weeks with early ultrasound dating would help to refine our understanding of the limit of viability.
At 23 weeks, each day of in utero development is criticalThe importance of each additional day spent in utero during the 23rd week of gestation was demonstrated in a small cohort in 2001.4 Overall, during the 23rd week of gestation the survival of newborns to discharge was 33%.4 This finding is similar to the survival rate reported by the NICHD Neonatal Research Network in 2012.1 However, survival was vastly different early, compared with later, in the 23rd week4:
- from 23 weeks 0 days to 23 weeks 2 days: no newborn survived
- at 23 weeks 3 days and 23 weeks 4 days: 40% of newborns survived
- at 23 weeks 5 days and 23 weeks 6 days: 63% of newborns survived (a similar survival rate of 24-week gestations was reported by the NICHD Neonatal Research Network1).
The development of the fetus across the 23rd week of gestation appears to be critical to newborn survival. Hence, every day of in utero development during the 23rd week is critically important. A great challenge for obstetricians is how to approach the woman with threatened preterm birth at 22 weeks 0 days’ gestation. If the woman delivers within a few days, the likelihood of survival is minimal. However, if the pregnancy can be extended to 23 weeks and 5 days, survival rates increase significantly.
Aligning the actions of birth team, mother, and familyFactors that influence the limit of viability include:
- gestational age
- gender of the fetus (Females are more likely than males to survive.)
- treatment of the mother with glucocorticoids prior to birth
- newborn weight.
To increase the likelihood of newborn survival, obstetricians need to treat women at risk for preterm birth with antenatal glucocorticoids and antibiotics for rupture of membranes and to limit fetal stress during the birth process. Guidelines have evolved to encourage clinicians to treat women at preterm birth risk with glucocorticoids either at:
- 23 weeks’ gestation or
- 22 weeks’ gestation, if birth is anticipated to occur at 23 weeks or later.5
At birth, pediatricians are then faced with the very difficult decision of whether or not to aggressively resuscitate the severely preterm infant. Complex medical, social, and ethical issues ultimately guide pediatricians’ actions in this challenging situation. It is important for their actions to be in consensus with the obstetrician, the mother, and the mother’s family and for a consensus to be reached. Dissonant plans may increase adverse outcomes for the newborn. In one study when pediatricians and obstetricians were not aligned in their actions, the risk of death of an extremely preterm newborn significantly increased.6
Prior to birth, team meetings that include the obstetricians, pediatricians, mother, and family will help to set expectations about the course of care and, in turn, improve perceived outcomes.5 If feasible, obstetricians and pediatricians should develop joint institutional guidelines about the general approach to pregnant women when birth may occur at 22 or 23 weeks’ gestation.5
A neonatal outcomes predictor
The National Institute of Child Health and Human Development provides a Web-based tool for estimating newborn outcomes based on gestational age (22 to 25 weeks), birth weight, gender, singleton or multiple gestation, and exposure to antenatal glucocorticoid treatment. The outcomes tool provides estimates for survival and survival with severe morbidity. It uses data collected by the Neonatal Research Network to predict outcomes. To access the outcomes data assessment, visit https://www.nichd.nih.gov/about/org/der/branches/ppb/programs/epbo/Pages/epbo_case.aspx.
Is aggressive management of preterm birth and neonatal resuscitation a self-fulfilling prophecy?The beliefs and training of clinicians may influence the outcome of extremely preterm newborns. For example, if obstetricians and pediatricians focus on the fact that birth at 23 weeks is not likely to result in survival without severe morbidity, they may withhold key interventions such as antenatal glucocorticoids, antibiotics for rupture of the membranes, and aggressive newborn resuscitation.7 Consequently the likelihood of survival may be reduced.
If clinicians believe in maximal interventions for all newborns at 22 and 23 weeks’ gestation, their actions may result in a small increase in newborn survival—but at the cost of painful and unnecessary interventions in many newborns who are destined to die. Finding the right balance along the broad spectrum from expectant management to aggressive and extended resuscitation is challenging. Clearly there is no “right answer” with these extremely difficult decisions.
Future trends in the limit of viabilityIn 1963, Jacqueline Bouvier Kennedy, at 34 weeks’ gestation, went into preterm labor and delivered her son Patrick at a community hospital. Patrick developed respiratory distress syndrome and was transferred to the Boston Children’s Hospital. He died shortly thereafter.8 Would Patrick have survived if he had been delivered at an institution capable of providing high-risk obstetric and newborn services? Would such modern interventions as antenatal glucocorticoids, antibiotics for ruptured membranes, liberal use of cesarean delivery, and aggressive neonatal resuscitation have improved his chances for survival?
From our current perspective, it is surprising that a 34-week newborn died shortly after birth. With modern obstetric and pediatric care that scenario is unusual. It is possible that future advances in medical care will push the limit of viability to 22 weeks’ gestation. Future generations of clinicians may be surprised that the medicine we practice today is so limited.
However, given our current resources, it is unlikely that newborns at 22 weeks’ gestation will survive, or survive without severe morbidity. Consequently, routine aggressive resuscitation of newborns at 22 weeks should be approached with caution. At 23 weeks and later, many newborns will survive and a few will survive without severe morbidity. Given the complexity of the issues, the approach to resuscitation of infants at 22 and 23 weeks must account for the perspectives of the birth mother and her family, obstetricians, and pediatricians. Managing threatened preterm birth at 22 and 23 weeks is one of our greatest challenges as obstetricians, and we need to meet this challenge with grace and skill.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–1051.
- Patel RM, Kandefer S, Walsh MC, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340.
- Donovan EF, Tyson JE, Ehrenkranz RA, et al. Inaccuracy of Ballard scores before 28 weeks’ gestation. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1999;135(2 pt 1):147–152.
- McElrath TF, Robinson JN, Ecker JL, Ringer SA, Norwitz ER. Neonatal outcome of infants born at 23 weeks’ gestation. Obstet Gynecol. 2001;97(1):49–52.
- Raju TN, Mercer BM, Burchfield DJ, Joseph GF Jr. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(5):1083–1096.
- Guinsburg R, Branco de Almeida MF, dos Santos Rodrigues Sadeck L, et al; Brazilian Network on Neonatal Research. Proactive management of extreme prematurity: disagreement between obstetricians and neonatologists. J Perinatol. 2012;32(12):913-919.
- Tucker Emonds B, McKenzie F, Farrow V, Raglan G, Schulkin J. A national survey of obstetricians’ attitudes toward and practice of periviable interventions. J Perinatol. 2015;35(5):338–343.
- Altman LK. A Kennedy baby’s life and death. New York Times. http://www.nytimes.com/2013/07/30/health/a-kennedy-babys-life-and-death.html?_r=0. Published July 29, 2013. Accessed November 19, 2015.
- Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–1051.
- Patel RM, Kandefer S, Walsh MC, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340.
- Donovan EF, Tyson JE, Ehrenkranz RA, et al. Inaccuracy of Ballard scores before 28 weeks’ gestation. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1999;135(2 pt 1):147–152.
- McElrath TF, Robinson JN, Ecker JL, Ringer SA, Norwitz ER. Neonatal outcome of infants born at 23 weeks’ gestation. Obstet Gynecol. 2001;97(1):49–52.
- Raju TN, Mercer BM, Burchfield DJ, Joseph GF Jr. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123(5):1083–1096.
- Guinsburg R, Branco de Almeida MF, dos Santos Rodrigues Sadeck L, et al; Brazilian Network on Neonatal Research. Proactive management of extreme prematurity: disagreement between obstetricians and neonatologists. J Perinatol. 2012;32(12):913-919.
- Tucker Emonds B, McKenzie F, Farrow V, Raglan G, Schulkin J. A national survey of obstetricians’ attitudes toward and practice of periviable interventions. J Perinatol. 2015;35(5):338–343.
- Altman LK. A Kennedy baby’s life and death. New York Times. http://www.nytimes.com/2013/07/30/health/a-kennedy-babys-life-and-death.html?_r=0. Published July 29, 2013. Accessed November 19, 2015.
Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?
Hysterectomy for benign disease is a very effective operation to treat moderate to severe uterine bleeding or pain caused by uterine problems. There are 3 main surgical approaches to performing a hysterectomy: vaginal, laparoscopic, and abdominal.
“Abdominal hysterectomy” is a term that indicates the procedure was performed using a relatively large incision in the abdominal wall. It also is possible for 2 surgical routes to be combined into one operation, such as a laparoscopically assisted vaginal hysterectomy or a laparoscopic hysterectomy with a mini-laparotomy incision to remove the uterus.
Substantial evidence indicates that vaginal and laparoscopic approaches to hysterectomy result in superior outcomes when compared with abdominal hysterectomy. In this editorial I highlight that data, as well as offer concrete ways in which we can increase the use of vaginal and laparoscopic hysterectomy while reducing the current reliance on an abdominal approach.
Vaginal and laparoscopic hysterectomy are associated with more rapid recoveryAuthors of a meta-analysis of 47 randomized trials involving 5,102 women concluded that women who underwent vaginal and laparoscopic hysterectomy had faster return to full activity, compared with women who had an abdominal hysterectomy. Compared with vaginal hysterectomy, the abdominal approach required an additional 12 days of postoperative recovery before return to normal activities and 1 additional day of postoperative hospitalization.1
In the same meta-analysis, when compared with the laparoscopic approach, abdominal hysterectomy required 15 additional days to return to normal activity and 2.6 more days of postoperative hospitalization.1 The evidence indicates that to maximize rapid return of the patient to full activity, we should reduce the use of abdominal hysterectomy for benign disease.
Abdominal hysterectomy is the most frequent US surgical approach In the United States in 2010, the rates of hysterectomy by route were 56% abdominal, 25% laparoscopic, and 19% vaginal.2 In contrast to US practice, French, German, and Australian gynecologists prioritize the vaginal route. In France in 2004, the rates of hysterectomy by route were 48% vaginal, 27% laparoscopic, and 25% abdominal.3 In Australia and Germany, vaginal hysterectomy is performed in 39% and 55% of all hysterectomy cases, respectively—a greater rate of vaginal hysterectomy than observed in the United States (TABLE 1).4,5
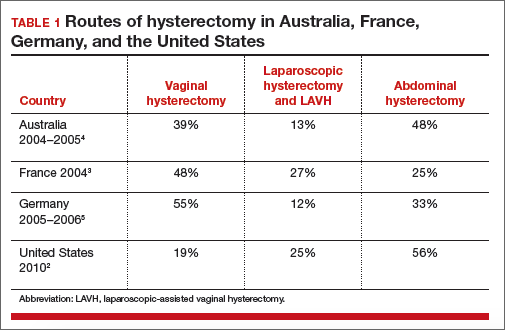
Our goal should be 40% or less for abdominal hysterectomy. Based on the experience of French,3 Australian,4 and German5 surgeons, a realistic goal is to reduce the use of abdominal hysterectomy in the United States to a rate of 40% or less and to increase the use of vaginal and laparoscopic hysterectomy to a combined rate of 60% or more.
Perceived contraindications for vaginal hysterectomy may not be valid
Surgeons may avoid selecting a vaginal route for hysterectomy for benign uterine disease when the patient has a markedly enlarged uterus (for example, >16 weeks’ size) or a markedly enlarged cervix or lower uterine segment. The large uterus may be difficult to remove through the vagina and an enlarged cervix or lower uterine segment may make it difficult to enter the peritoneal cavity.
However, large uteri can be removed through the vagina using uterine reduction techniques, including uterine bisection and intramyometrial coring. In one randomized clinical trial,1 women with enlarged uteri were randomly assigned to vaginal or abdominal hysterectomy. Both approaches were successful in removing large uteri. When compared with abdominal hysterectomy, the vaginal approach was associated with shorter operative time, less postoperative fever, less postoperative pain, and fewer hospital days following surgery.
Reference
How will we increase vaginal and laparoscopic hysterectomy for benign disease?In order to reduce the use of abdominal hysterectomy, a multipronged effort is needed:
- Leaders in gynecology need to champion the use of vaginal and laparoscopic hysterectomy.
- Educators in gynecology need to refocus and intensify surgical training to ensure that trainees are confident in their ability to perform both vaginal and laparoscopic hysterectomy.
- Hospital departments need to provide the continuing education and senior surgical mentoring that will facilitate reducing the use of abdominal hysterectomy.
- Quality review committees need to review the indication for abdominal hysterectomy procedures and question whether they could be better performed by a vaginal or laparoscopic route.
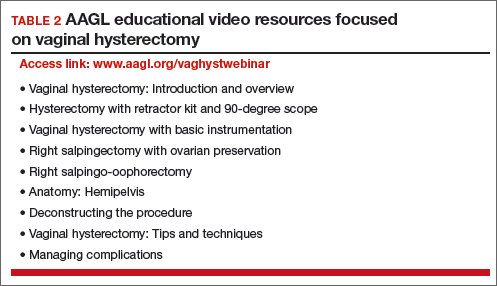
AAGL launches comprehensive video cooperative. A major new educational video offering on vaginal hysterectomy recently was released by the AAGL (TABLE 2). Produced by the AAGL and cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons, this resource includes detailed videos focused on basic instrumentation and technique, techniques for adnexal surgery at vaginal hysterectomy, and managing complications. I believe this resource will be of great value as momentum builds to increase the use of vaginal hysterectomy. In addition, OBG Management will continue to publish major articles by leading surgeons focused on vaginal hysterectomy.
Are you a champion of vaginal and laparoscopic hysterectomy? Every hospital should identify champions of these approaches. These master surgeons could help advance the capability of the hospital staff to confidently and safely prioritize the use of vaginal and laparoscopic hysterectomy for benign disease by mentoring other surgeons. If we reduce the use of abdominal hysterectomy we will improve outcomes and significantly advance women’s health.
Select OBG Management publications on vaginal surgery and minimally invasive gynecology
Transforming vaginal hysterectomy: 7 solutions to the most
daunting challenges
Rosanne M. Kho, MD (July 2014)
The Extracorporeal C-Incision Tissue Extraction ExCITE technique
Mireille D. Truong, MD, and Arnold P. Advincula, MD (November 2014)
Update on vaginal hysterectomy
Barbara S. Levy, MD (September 2015)
The ExCITE technique, Part 2: Simulation made simple
Mireille D. Truong, MD, and Arnold P. Advincula, MD (Coming soon)
The following articles are based on the master class in vaginal hysterectomy produced by AAGL and cosponsored by ACOG and SGS.
Vaginal hysterectomy with basic instrumentation
Barbara S. Levy, MD (October 2015)
Technique for salpingectomy and salpingo-oophorectomy
John B. Gebhart, MD, MS (In this issue, page 26)
Managing complications in vaginal hysterectomy
John B. Gebhart, MD, MS (Coming soon)
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;(8):CD003677. doi:10.1002/14651858.CD003677.pub5.
- Cohen SL, Vitonis AF, Einarsson JI. Updated hysterectomy surveillance and factors associated with minimally invasive hysterectomy. JSLS. 2014;18(3):e2014.00096.
- David-Montefiore E, Rouzier R, Chapron C, Darai E; Collegiale d’Obstétrique et Gynécologie de Paris-Ile de France. Surgical routes and complications of hysterectomy for benign disorders: a prospective observational study in French university hospitals. Hum Reprod. 2007;22(1):260–265.
- Hill E, Graham M, Shelley J. Hysterectomy trends in Australia—between 2000/01 and 2004/05. Aust N Z J Obstet Gynaecol. 2010;50(2):153–158.
- Stang A, Merrill RM, Kuss O. Nationwide rates of conversion from laparoscopic or vaginal hysterectomy to open abdominal hysterectomy in Germany. Eur J Epidemiol. 2011;26(2):125–133.
Hysterectomy for benign disease is a very effective operation to treat moderate to severe uterine bleeding or pain caused by uterine problems. There are 3 main surgical approaches to performing a hysterectomy: vaginal, laparoscopic, and abdominal.
“Abdominal hysterectomy” is a term that indicates the procedure was performed using a relatively large incision in the abdominal wall. It also is possible for 2 surgical routes to be combined into one operation, such as a laparoscopically assisted vaginal hysterectomy or a laparoscopic hysterectomy with a mini-laparotomy incision to remove the uterus.
Substantial evidence indicates that vaginal and laparoscopic approaches to hysterectomy result in superior outcomes when compared with abdominal hysterectomy. In this editorial I highlight that data, as well as offer concrete ways in which we can increase the use of vaginal and laparoscopic hysterectomy while reducing the current reliance on an abdominal approach.
Vaginal and laparoscopic hysterectomy are associated with more rapid recoveryAuthors of a meta-analysis of 47 randomized trials involving 5,102 women concluded that women who underwent vaginal and laparoscopic hysterectomy had faster return to full activity, compared with women who had an abdominal hysterectomy. Compared with vaginal hysterectomy, the abdominal approach required an additional 12 days of postoperative recovery before return to normal activities and 1 additional day of postoperative hospitalization.1
In the same meta-analysis, when compared with the laparoscopic approach, abdominal hysterectomy required 15 additional days to return to normal activity and 2.6 more days of postoperative hospitalization.1 The evidence indicates that to maximize rapid return of the patient to full activity, we should reduce the use of abdominal hysterectomy for benign disease.
Abdominal hysterectomy is the most frequent US surgical approach In the United States in 2010, the rates of hysterectomy by route were 56% abdominal, 25% laparoscopic, and 19% vaginal.2 In contrast to US practice, French, German, and Australian gynecologists prioritize the vaginal route. In France in 2004, the rates of hysterectomy by route were 48% vaginal, 27% laparoscopic, and 25% abdominal.3 In Australia and Germany, vaginal hysterectomy is performed in 39% and 55% of all hysterectomy cases, respectively—a greater rate of vaginal hysterectomy than observed in the United States (TABLE 1).4,5

Our goal should be 40% or less for abdominal hysterectomy. Based on the experience of French,3 Australian,4 and German5 surgeons, a realistic goal is to reduce the use of abdominal hysterectomy in the United States to a rate of 40% or less and to increase the use of vaginal and laparoscopic hysterectomy to a combined rate of 60% or more.
Perceived contraindications for vaginal hysterectomy may not be valid
Surgeons may avoid selecting a vaginal route for hysterectomy for benign uterine disease when the patient has a markedly enlarged uterus (for example, >16 weeks’ size) or a markedly enlarged cervix or lower uterine segment. The large uterus may be difficult to remove through the vagina and an enlarged cervix or lower uterine segment may make it difficult to enter the peritoneal cavity.
However, large uteri can be removed through the vagina using uterine reduction techniques, including uterine bisection and intramyometrial coring. In one randomized clinical trial,1 women with enlarged uteri were randomly assigned to vaginal or abdominal hysterectomy. Both approaches were successful in removing large uteri. When compared with abdominal hysterectomy, the vaginal approach was associated with shorter operative time, less postoperative fever, less postoperative pain, and fewer hospital days following surgery.
Reference
How will we increase vaginal and laparoscopic hysterectomy for benign disease?In order to reduce the use of abdominal hysterectomy, a multipronged effort is needed:
- Leaders in gynecology need to champion the use of vaginal and laparoscopic hysterectomy.
- Educators in gynecology need to refocus and intensify surgical training to ensure that trainees are confident in their ability to perform both vaginal and laparoscopic hysterectomy.
- Hospital departments need to provide the continuing education and senior surgical mentoring that will facilitate reducing the use of abdominal hysterectomy.
- Quality review committees need to review the indication for abdominal hysterectomy procedures and question whether they could be better performed by a vaginal or laparoscopic route.
AAGL launches comprehensive video cooperative. A major new educational video offering on vaginal hysterectomy recently was released by the AAGL (TABLE 2). Produced by the AAGL and cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons, this resource includes detailed videos focused on basic instrumentation and technique, techniques for adnexal surgery at vaginal hysterectomy, and managing complications. I believe this resource will be of great value as momentum builds to increase the use of vaginal hysterectomy. In addition, OBG Management will continue to publish major articles by leading surgeons focused on vaginal hysterectomy.

Are you a champion of vaginal and laparoscopic hysterectomy? Every hospital should identify champions of these approaches. These master surgeons could help advance the capability of the hospital staff to confidently and safely prioritize the use of vaginal and laparoscopic hysterectomy for benign disease by mentoring other surgeons. If we reduce the use of abdominal hysterectomy we will improve outcomes and significantly advance women’s health.
Select OBG Management publications on vaginal surgery and minimally invasive gynecology
Transforming vaginal hysterectomy: 7 solutions to the most
daunting challenges
Rosanne M. Kho, MD (July 2014)
The Extracorporeal C-Incision Tissue Extraction ExCITE technique
Mireille D. Truong, MD, and Arnold P. Advincula, MD (November 2014)
Update on vaginal hysterectomy
Barbara S. Levy, MD (September 2015)
The ExCITE technique, Part 2: Simulation made simple
Mireille D. Truong, MD, and Arnold P. Advincula, MD (Coming soon)
The following articles are based on the master class in vaginal hysterectomy produced by AAGL and cosponsored by ACOG and SGS.
Vaginal hysterectomy with basic instrumentation
Barbara S. Levy, MD (October 2015)
Technique for salpingectomy and salpingo-oophorectomy
John B. Gebhart, MD, MS (In this issue, page 26)
Managing complications in vaginal hysterectomy
John B. Gebhart, MD, MS (Coming soon)
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Hysterectomy for benign disease is a very effective operation to treat moderate to severe uterine bleeding or pain caused by uterine problems. There are 3 main surgical approaches to performing a hysterectomy: vaginal, laparoscopic, and abdominal.
“Abdominal hysterectomy” is a term that indicates the procedure was performed using a relatively large incision in the abdominal wall. It also is possible for 2 surgical routes to be combined into one operation, such as a laparoscopically assisted vaginal hysterectomy or a laparoscopic hysterectomy with a mini-laparotomy incision to remove the uterus.
Substantial evidence indicates that vaginal and laparoscopic approaches to hysterectomy result in superior outcomes when compared with abdominal hysterectomy. In this editorial I highlight that data, as well as offer concrete ways in which we can increase the use of vaginal and laparoscopic hysterectomy while reducing the current reliance on an abdominal approach.
Vaginal and laparoscopic hysterectomy are associated with more rapid recoveryAuthors of a meta-analysis of 47 randomized trials involving 5,102 women concluded that women who underwent vaginal and laparoscopic hysterectomy had faster return to full activity, compared with women who had an abdominal hysterectomy. Compared with vaginal hysterectomy, the abdominal approach required an additional 12 days of postoperative recovery before return to normal activities and 1 additional day of postoperative hospitalization.1
In the same meta-analysis, when compared with the laparoscopic approach, abdominal hysterectomy required 15 additional days to return to normal activity and 2.6 more days of postoperative hospitalization.1 The evidence indicates that to maximize rapid return of the patient to full activity, we should reduce the use of abdominal hysterectomy for benign disease.
Abdominal hysterectomy is the most frequent US surgical approach In the United States in 2010, the rates of hysterectomy by route were 56% abdominal, 25% laparoscopic, and 19% vaginal.2 In contrast to US practice, French, German, and Australian gynecologists prioritize the vaginal route. In France in 2004, the rates of hysterectomy by route were 48% vaginal, 27% laparoscopic, and 25% abdominal.3 In Australia and Germany, vaginal hysterectomy is performed in 39% and 55% of all hysterectomy cases, respectively—a greater rate of vaginal hysterectomy than observed in the United States (TABLE 1).4,5

Our goal should be 40% or less for abdominal hysterectomy. Based on the experience of French,3 Australian,4 and German5 surgeons, a realistic goal is to reduce the use of abdominal hysterectomy in the United States to a rate of 40% or less and to increase the use of vaginal and laparoscopic hysterectomy to a combined rate of 60% or more.
Perceived contraindications for vaginal hysterectomy may not be valid
Surgeons may avoid selecting a vaginal route for hysterectomy for benign uterine disease when the patient has a markedly enlarged uterus (for example, >16 weeks’ size) or a markedly enlarged cervix or lower uterine segment. The large uterus may be difficult to remove through the vagina and an enlarged cervix or lower uterine segment may make it difficult to enter the peritoneal cavity.
However, large uteri can be removed through the vagina using uterine reduction techniques, including uterine bisection and intramyometrial coring. In one randomized clinical trial,1 women with enlarged uteri were randomly assigned to vaginal or abdominal hysterectomy. Both approaches were successful in removing large uteri. When compared with abdominal hysterectomy, the vaginal approach was associated with shorter operative time, less postoperative fever, less postoperative pain, and fewer hospital days following surgery.
Reference
How will we increase vaginal and laparoscopic hysterectomy for benign disease?In order to reduce the use of abdominal hysterectomy, a multipronged effort is needed:
- Leaders in gynecology need to champion the use of vaginal and laparoscopic hysterectomy.
- Educators in gynecology need to refocus and intensify surgical training to ensure that trainees are confident in their ability to perform both vaginal and laparoscopic hysterectomy.
- Hospital departments need to provide the continuing education and senior surgical mentoring that will facilitate reducing the use of abdominal hysterectomy.
- Quality review committees need to review the indication for abdominal hysterectomy procedures and question whether they could be better performed by a vaginal or laparoscopic route.
AAGL launches comprehensive video cooperative. A major new educational video offering on vaginal hysterectomy recently was released by the AAGL (TABLE 2). Produced by the AAGL and cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons, this resource includes detailed videos focused on basic instrumentation and technique, techniques for adnexal surgery at vaginal hysterectomy, and managing complications. I believe this resource will be of great value as momentum builds to increase the use of vaginal hysterectomy. In addition, OBG Management will continue to publish major articles by leading surgeons focused on vaginal hysterectomy.

Are you a champion of vaginal and laparoscopic hysterectomy? Every hospital should identify champions of these approaches. These master surgeons could help advance the capability of the hospital staff to confidently and safely prioritize the use of vaginal and laparoscopic hysterectomy for benign disease by mentoring other surgeons. If we reduce the use of abdominal hysterectomy we will improve outcomes and significantly advance women’s health.
Select OBG Management publications on vaginal surgery and minimally invasive gynecology
Transforming vaginal hysterectomy: 7 solutions to the most
daunting challenges
Rosanne M. Kho, MD (July 2014)
The Extracorporeal C-Incision Tissue Extraction ExCITE technique
Mireille D. Truong, MD, and Arnold P. Advincula, MD (November 2014)
Update on vaginal hysterectomy
Barbara S. Levy, MD (September 2015)
The ExCITE technique, Part 2: Simulation made simple
Mireille D. Truong, MD, and Arnold P. Advincula, MD (Coming soon)
The following articles are based on the master class in vaginal hysterectomy produced by AAGL and cosponsored by ACOG and SGS.
Vaginal hysterectomy with basic instrumentation
Barbara S. Levy, MD (October 2015)
Technique for salpingectomy and salpingo-oophorectomy
John B. Gebhart, MD, MS (In this issue, page 26)
Managing complications in vaginal hysterectomy
John B. Gebhart, MD, MS (Coming soon)
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;(8):CD003677. doi:10.1002/14651858.CD003677.pub5.
- Cohen SL, Vitonis AF, Einarsson JI. Updated hysterectomy surveillance and factors associated with minimally invasive hysterectomy. JSLS. 2014;18(3):e2014.00096.
- David-Montefiore E, Rouzier R, Chapron C, Darai E; Collegiale d’Obstétrique et Gynécologie de Paris-Ile de France. Surgical routes and complications of hysterectomy for benign disorders: a prospective observational study in French university hospitals. Hum Reprod. 2007;22(1):260–265.
- Hill E, Graham M, Shelley J. Hysterectomy trends in Australia—between 2000/01 and 2004/05. Aust N Z J Obstet Gynaecol. 2010;50(2):153–158.
- Stang A, Merrill RM, Kuss O. Nationwide rates of conversion from laparoscopic or vaginal hysterectomy to open abdominal hysterectomy in Germany. Eur J Epidemiol. 2011;26(2):125–133.
- Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;(8):CD003677. doi:10.1002/14651858.CD003677.pub5.
- Cohen SL, Vitonis AF, Einarsson JI. Updated hysterectomy surveillance and factors associated with minimally invasive hysterectomy. JSLS. 2014;18(3):e2014.00096.
- David-Montefiore E, Rouzier R, Chapron C, Darai E; Collegiale d’Obstétrique et Gynécologie de Paris-Ile de France. Surgical routes and complications of hysterectomy for benign disorders: a prospective observational study in French university hospitals. Hum Reprod. 2007;22(1):260–265.
- Hill E, Graham M, Shelley J. Hysterectomy trends in Australia—between 2000/01 and 2004/05. Aust N Z J Obstet Gynaecol. 2010;50(2):153–158.
- Stang A, Merrill RM, Kuss O. Nationwide rates of conversion from laparoscopic or vaginal hysterectomy to open abdominal hysterectomy in Germany. Eur J Epidemiol. 2011;26(2):125–133.
Does hormone therapy reduce mortality in recently menopausal women?
Clinicians work to maximize the quality of life and longevity of every patient. For women with moderate to severe menopausal symptoms, oral estrogen therapy can improve quality of life, but at the cost of significant adverse effects. The Women’s Health Initiative (WHI) reported that for postmenopausal women with a uterus, conjugated estrogen plus medroxyprogesterone acetate (CEE+MPA) hormone therapy (HT) versus placebo significantly increased the risk of cardiovascular events (relative risk [RR], 1.13), breast cancer (RR, 1.24), stroke (RR, 1.37), deep vein thrombosis (RR, 1.87), and pulmonary embolism (RR, 1.98).1 In postmeno pausal women without a uterus, CEE HT did not increase the risk of breast cancer (RR, 0.79), compared with placebo, but it did significantly in crease the risk of cardiovascular events (RR, 1.11), stroke (RR, 1.35), deep vein thrombosis (RR, 1.48), and pulmonary embolism (RR, 1.35).1
Clinicians prescribing estrogen must individualize therapy according to its benefits and risks. An important issue that has received insufficient at tention is, “What is the effect of HT on mortality in recently menopausal women?” Here, I examine this issue.
HT reduces mortality in recently menopausal women
Pooling the results of the WHI CEE+MPA and CEE-only trials reveals that there were 70 deaths in the HT-treated groups and 98 deaths in the placebo groups among women aged 50 to 59 years.1 With 4,706 and 4,259 women alive at the conclusion of the study in the HT and placebo groups, respectively, the women in the placebo group had significantly more deaths than the women in the HT-treated groups (Fisher exact test, P = .0194, χ2 test with Yates correction, P = .0226).
Using pooled data from the WHI, the RR of death in the HT versus placebo group was estimated at 0.70 (95% confidence interval [CI], 0.51−0.96), representing approximately 5 fewer deaths per 1,000 women per 5 years of therapy.2 In women aged 60 to 69 years and 70 to 79 years there were no significant differences in death rates between the HT- and placebo-treated women.
My interpretation of these results is that HT likely is associated with a reduced risk of death in recently menopausal women, but not in women distant from menopause onset.
Cochrane review of HT and mortality
Consistent with the WHI findings, authors of a recent Cochrane meta-analysis of 19 randomized trials including 40,410 menopausal women reported that HT significantly increased the risk of stroke (RR, 1.24; 95% CI, 1.10−1.41), venous thromboembolism (RR, 1.92; 95% CI, 1.36−2.69), and pulmonary emboli (RR, 1.81; 95% CI, 1.32−2.48).3 However, among women treated with oral HT within 10 years after the start of menopause, there was a reduced risk of coronary heart disease (RR, 0.52; 95% CI, 0.29−0.96). Using data from 5 clinical trials, the Cochrane meta-analysis researchers reported that, compared with placebo, HT reduced mortality (RR, 0.70; 95% CI, 0.52−0.95).3
Results of the Cochrane meta-analysis are consistent with those of a previous meta-analysis of 19 randomized trials involving 16,000 women. In this analysis, investigators found a reduced risk of death in recently menopausal women treated with hormone therapy (RR, 0.73; 95% CI, 0.52−0.96).4
Early menopause, HT, and mortality
Authors of multiple large epidemiologic studies have reported that early menopause is associated with an increased risk of death if HT is not initiated.5−7 For example, results of a study of women in Olmsted County, Minnesota, conducted from 1950 to 1987, indicated that, for women younger than age 45 years who underwent bilateral oophorectomy, the risk of death was increased among those who did not initiate HT, compared with women who did not undergo oophorectomy (hazard ratio [HR], 1.84; 95% CI, 1.27−2.68; P = .001).7
By contrast, women younger than 45 years who underwent bilateral oophorectomy and initiated estrogen therapy did not have an increased risk of death compared with women who did not undergo oophorectomy (HR, 0.65; 95% CI, 0.30−1.41; P = .28).7 An excess number of cardiovascular events appeared to account for the increased mortality among women with early surgical menopause who did not initiate HT.
The “timing hypothesis” proposes that the initiation of HT soon after the onset of menopause is associated with beneficial cardiovascular effects, but initiation more than 10 years after the onset of menopause is not associated with beneficial cardiovascular effects. The timing hypothesis is supported by the finding that, in recently menopausal women, HT is associated with reduced carotid intima-media thickness (CIMT), compared with placebo.8 Greater CIMT thickness is associated with an increased risk of cardiovascular events.
In my experience, few primary care clinicians are aware of these data. Often, these clinicians over-emphasize the risks and withhold HT in this vulnerable group of women.
HT: Minimizing the risks of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer
Results of multiple studies have shown that certain HT regimens increase the risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Is it possible to prescribe HT in a way that reduces these risks?
Results of observational studies indicate that, compared with oral estrogen therapy, transdermal HT is associated with a lower risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer (TABLE).9−15
Reducing the risk of stroke caused by HT is an important goal. In a study of 15,710 women who had stroke and 59,958 control women aged 50 to 79 years, transdermal estradiol at a dose of 50 µg or less daily was not associated with an increased risk of stroke, compared with HT nonuse (rate ratio, 0.81; 95% CI, 0.62−1.05).9 Compared with HT nonuse, the use of oral estrogen (rate ratio, 1.28; 95% CI, 1.15−1.42) or transdermal estradiol 50 µg or greater daily (rate ratio, 1.89; 95% CI, 1.15−3.11) was associated with an increased risk of stroke.9
Reducing the risks of deep venous thromboembolism (VTE) and pulmonary embolism caused by HT is an important goal. In a meta-analysis of the risk of VTE with HT, compared with nonusers, oral estrogen therapy was associated with a significantly increased risk of VTE (odds ratio [OR], 2.5; 95% CI, 1.9−3.4). Compared with nonuse, transdermal estrogen therapy was not associated with an increased risk of VTE (OR, 1.2; 95% CI, 0.9−1.7).11 In a study comparing oral versus transdermal estradiol, transdermal estradiol was associated with a reduced risk of pulmonary embolism (0.46 [95% CI, 0.22−0.97]).13
Reducing the risk of breast cancer caused by HT is an important goal. Results of one study showed that the combination of oral estrogen plus synthetic progestin was associated with an increased risk of breast cancer, compared with nonuse (RR, 1.5; 95% CI, 1.1−1.9). By contrast, the combination of transdermal estradiol plus micronized progesterone was not associated with an increased risk of breast cancer, compared with nonuse (RR, 0.9; 95% CI, 0.7−1.2).15
The bottom line
In recently menopausal women with moderate to severe hot flashes, HT improves quality of life and appears to decrease mortality. However, HT with oral estrogen plus synthetic progestin is associated with an increased risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Compared with oral estrogen, transdermal estradiol treatment is associated with a lower risk of stroke, deep vein thrombosis, and pulmonary embolism. Compared with oral estrogen plus a synthetic progestin, transdermal estradiol plus micronized progesterone is associated with a lower risk of breast cancer. The benefits of HT are likely maximized by initiating therapy in the perimenopause transition or early in the postmenopause, and the risks are minimized by using transdermal estradiol.16−18
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353−1368.
- Santen RJ, Allred DC, Ardoin SP, et al. J Clin Endocrinol Metab. 2010;95(suppl 1):S1−S66.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in postmenopausal women. Cochrane Database Syst Rev. 2015;3:CD002229.
- Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger post-menopausal women. Am J Med. 2009;122(11):1016−1022.
- Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease: The Framingham Study. Ann Intern Med. 1978;89(2):157−161.
- Stampfer MJ, Colditz GA, Willet WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses Health Study. N Engl J Med. 1991;325(11):756−762.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15−23.
- Hodis HN, Mack WJ, Shoupe D, et al. Testing the menopausal hormone therapy timing hypothesis: the early versus late intervention trial with estradiol [abstract 13283]. American Heart Association Meeting 2014. Circulation. 2014;130:A13283.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010;8(5):979−986.
- Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227−1231.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340−345.
- Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052−1059.
- Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277−2286.
- Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448−454.
- L’Hermite M. HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric. 2013;16(suppl 1):44−53.
- Simon JA. What’s new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1):3−10.
- Mueck AO. Postmenopausal hormone replacement therapy and cardiovascular disease: the value of transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1): 11−17.
Clinicians work to maximize the quality of life and longevity of every patient. For women with moderate to severe menopausal symptoms, oral estrogen therapy can improve quality of life, but at the cost of significant adverse effects. The Women’s Health Initiative (WHI) reported that for postmenopausal women with a uterus, conjugated estrogen plus medroxyprogesterone acetate (CEE+MPA) hormone therapy (HT) versus placebo significantly increased the risk of cardiovascular events (relative risk [RR], 1.13), breast cancer (RR, 1.24), stroke (RR, 1.37), deep vein thrombosis (RR, 1.87), and pulmonary embolism (RR, 1.98).1 In postmeno pausal women without a uterus, CEE HT did not increase the risk of breast cancer (RR, 0.79), compared with placebo, but it did significantly in crease the risk of cardiovascular events (RR, 1.11), stroke (RR, 1.35), deep vein thrombosis (RR, 1.48), and pulmonary embolism (RR, 1.35).1
Clinicians prescribing estrogen must individualize therapy according to its benefits and risks. An important issue that has received insufficient at tention is, “What is the effect of HT on mortality in recently menopausal women?” Here, I examine this issue.
HT reduces mortality in recently menopausal women
Pooling the results of the WHI CEE+MPA and CEE-only trials reveals that there were 70 deaths in the HT-treated groups and 98 deaths in the placebo groups among women aged 50 to 59 years.1 With 4,706 and 4,259 women alive at the conclusion of the study in the HT and placebo groups, respectively, the women in the placebo group had significantly more deaths than the women in the HT-treated groups (Fisher exact test, P = .0194, χ2 test with Yates correction, P = .0226).
Using pooled data from the WHI, the RR of death in the HT versus placebo group was estimated at 0.70 (95% confidence interval [CI], 0.51−0.96), representing approximately 5 fewer deaths per 1,000 women per 5 years of therapy.2 In women aged 60 to 69 years and 70 to 79 years there were no significant differences in death rates between the HT- and placebo-treated women.
My interpretation of these results is that HT likely is associated with a reduced risk of death in recently menopausal women, but not in women distant from menopause onset.
Cochrane review of HT and mortality
Consistent with the WHI findings, authors of a recent Cochrane meta-analysis of 19 randomized trials including 40,410 menopausal women reported that HT significantly increased the risk of stroke (RR, 1.24; 95% CI, 1.10−1.41), venous thromboembolism (RR, 1.92; 95% CI, 1.36−2.69), and pulmonary emboli (RR, 1.81; 95% CI, 1.32−2.48).3 However, among women treated with oral HT within 10 years after the start of menopause, there was a reduced risk of coronary heart disease (RR, 0.52; 95% CI, 0.29−0.96). Using data from 5 clinical trials, the Cochrane meta-analysis researchers reported that, compared with placebo, HT reduced mortality (RR, 0.70; 95% CI, 0.52−0.95).3
Results of the Cochrane meta-analysis are consistent with those of a previous meta-analysis of 19 randomized trials involving 16,000 women. In this analysis, investigators found a reduced risk of death in recently menopausal women treated with hormone therapy (RR, 0.73; 95% CI, 0.52−0.96).4
Early menopause, HT, and mortality
Authors of multiple large epidemiologic studies have reported that early menopause is associated with an increased risk of death if HT is not initiated.5−7 For example, results of a study of women in Olmsted County, Minnesota, conducted from 1950 to 1987, indicated that, for women younger than age 45 years who underwent bilateral oophorectomy, the risk of death was increased among those who did not initiate HT, compared with women who did not undergo oophorectomy (hazard ratio [HR], 1.84; 95% CI, 1.27−2.68; P = .001).7
By contrast, women younger than 45 years who underwent bilateral oophorectomy and initiated estrogen therapy did not have an increased risk of death compared with women who did not undergo oophorectomy (HR, 0.65; 95% CI, 0.30−1.41; P = .28).7 An excess number of cardiovascular events appeared to account for the increased mortality among women with early surgical menopause who did not initiate HT.
The “timing hypothesis” proposes that the initiation of HT soon after the onset of menopause is associated with beneficial cardiovascular effects, but initiation more than 10 years after the onset of menopause is not associated with beneficial cardiovascular effects. The timing hypothesis is supported by the finding that, in recently menopausal women, HT is associated with reduced carotid intima-media thickness (CIMT), compared with placebo.8 Greater CIMT thickness is associated with an increased risk of cardiovascular events.
In my experience, few primary care clinicians are aware of these data. Often, these clinicians over-emphasize the risks and withhold HT in this vulnerable group of women.
HT: Minimizing the risks of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer
Results of multiple studies have shown that certain HT regimens increase the risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Is it possible to prescribe HT in a way that reduces these risks?
Results of observational studies indicate that, compared with oral estrogen therapy, transdermal HT is associated with a lower risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer (TABLE).9−15
Reducing the risk of stroke caused by HT is an important goal. In a study of 15,710 women who had stroke and 59,958 control women aged 50 to 79 years, transdermal estradiol at a dose of 50 µg or less daily was not associated with an increased risk of stroke, compared with HT nonuse (rate ratio, 0.81; 95% CI, 0.62−1.05).9 Compared with HT nonuse, the use of oral estrogen (rate ratio, 1.28; 95% CI, 1.15−1.42) or transdermal estradiol 50 µg or greater daily (rate ratio, 1.89; 95% CI, 1.15−3.11) was associated with an increased risk of stroke.9
Reducing the risks of deep venous thromboembolism (VTE) and pulmonary embolism caused by HT is an important goal. In a meta-analysis of the risk of VTE with HT, compared with nonusers, oral estrogen therapy was associated with a significantly increased risk of VTE (odds ratio [OR], 2.5; 95% CI, 1.9−3.4). Compared with nonuse, transdermal estrogen therapy was not associated with an increased risk of VTE (OR, 1.2; 95% CI, 0.9−1.7).11 In a study comparing oral versus transdermal estradiol, transdermal estradiol was associated with a reduced risk of pulmonary embolism (0.46 [95% CI, 0.22−0.97]).13
Reducing the risk of breast cancer caused by HT is an important goal. Results of one study showed that the combination of oral estrogen plus synthetic progestin was associated with an increased risk of breast cancer, compared with nonuse (RR, 1.5; 95% CI, 1.1−1.9). By contrast, the combination of transdermal estradiol plus micronized progesterone was not associated with an increased risk of breast cancer, compared with nonuse (RR, 0.9; 95% CI, 0.7−1.2).15
The bottom line
In recently menopausal women with moderate to severe hot flashes, HT improves quality of life and appears to decrease mortality. However, HT with oral estrogen plus synthetic progestin is associated with an increased risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Compared with oral estrogen, transdermal estradiol treatment is associated with a lower risk of stroke, deep vein thrombosis, and pulmonary embolism. Compared with oral estrogen plus a synthetic progestin, transdermal estradiol plus micronized progesterone is associated with a lower risk of breast cancer. The benefits of HT are likely maximized by initiating therapy in the perimenopause transition or early in the postmenopause, and the risks are minimized by using transdermal estradiol.16−18
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Clinicians work to maximize the quality of life and longevity of every patient. For women with moderate to severe menopausal symptoms, oral estrogen therapy can improve quality of life, but at the cost of significant adverse effects. The Women’s Health Initiative (WHI) reported that for postmenopausal women with a uterus, conjugated estrogen plus medroxyprogesterone acetate (CEE+MPA) hormone therapy (HT) versus placebo significantly increased the risk of cardiovascular events (relative risk [RR], 1.13), breast cancer (RR, 1.24), stroke (RR, 1.37), deep vein thrombosis (RR, 1.87), and pulmonary embolism (RR, 1.98).1 In postmeno pausal women without a uterus, CEE HT did not increase the risk of breast cancer (RR, 0.79), compared with placebo, but it did significantly in crease the risk of cardiovascular events (RR, 1.11), stroke (RR, 1.35), deep vein thrombosis (RR, 1.48), and pulmonary embolism (RR, 1.35).1
Clinicians prescribing estrogen must individualize therapy according to its benefits and risks. An important issue that has received insufficient at tention is, “What is the effect of HT on mortality in recently menopausal women?” Here, I examine this issue.
HT reduces mortality in recently menopausal women
Pooling the results of the WHI CEE+MPA and CEE-only trials reveals that there were 70 deaths in the HT-treated groups and 98 deaths in the placebo groups among women aged 50 to 59 years.1 With 4,706 and 4,259 women alive at the conclusion of the study in the HT and placebo groups, respectively, the women in the placebo group had significantly more deaths than the women in the HT-treated groups (Fisher exact test, P = .0194, χ2 test with Yates correction, P = .0226).
Using pooled data from the WHI, the RR of death in the HT versus placebo group was estimated at 0.70 (95% confidence interval [CI], 0.51−0.96), representing approximately 5 fewer deaths per 1,000 women per 5 years of therapy.2 In women aged 60 to 69 years and 70 to 79 years there were no significant differences in death rates between the HT- and placebo-treated women.
My interpretation of these results is that HT likely is associated with a reduced risk of death in recently menopausal women, but not in women distant from menopause onset.
Cochrane review of HT and mortality
Consistent with the WHI findings, authors of a recent Cochrane meta-analysis of 19 randomized trials including 40,410 menopausal women reported that HT significantly increased the risk of stroke (RR, 1.24; 95% CI, 1.10−1.41), venous thromboembolism (RR, 1.92; 95% CI, 1.36−2.69), and pulmonary emboli (RR, 1.81; 95% CI, 1.32−2.48).3 However, among women treated with oral HT within 10 years after the start of menopause, there was a reduced risk of coronary heart disease (RR, 0.52; 95% CI, 0.29−0.96). Using data from 5 clinical trials, the Cochrane meta-analysis researchers reported that, compared with placebo, HT reduced mortality (RR, 0.70; 95% CI, 0.52−0.95).3
Results of the Cochrane meta-analysis are consistent with those of a previous meta-analysis of 19 randomized trials involving 16,000 women. In this analysis, investigators found a reduced risk of death in recently menopausal women treated with hormone therapy (RR, 0.73; 95% CI, 0.52−0.96).4
Early menopause, HT, and mortality
Authors of multiple large epidemiologic studies have reported that early menopause is associated with an increased risk of death if HT is not initiated.5−7 For example, results of a study of women in Olmsted County, Minnesota, conducted from 1950 to 1987, indicated that, for women younger than age 45 years who underwent bilateral oophorectomy, the risk of death was increased among those who did not initiate HT, compared with women who did not undergo oophorectomy (hazard ratio [HR], 1.84; 95% CI, 1.27−2.68; P = .001).7
By contrast, women younger than 45 years who underwent bilateral oophorectomy and initiated estrogen therapy did not have an increased risk of death compared with women who did not undergo oophorectomy (HR, 0.65; 95% CI, 0.30−1.41; P = .28).7 An excess number of cardiovascular events appeared to account for the increased mortality among women with early surgical menopause who did not initiate HT.
The “timing hypothesis” proposes that the initiation of HT soon after the onset of menopause is associated with beneficial cardiovascular effects, but initiation more than 10 years after the onset of menopause is not associated with beneficial cardiovascular effects. The timing hypothesis is supported by the finding that, in recently menopausal women, HT is associated with reduced carotid intima-media thickness (CIMT), compared with placebo.8 Greater CIMT thickness is associated with an increased risk of cardiovascular events.
In my experience, few primary care clinicians are aware of these data. Often, these clinicians over-emphasize the risks and withhold HT in this vulnerable group of women.
HT: Minimizing the risks of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer
Results of multiple studies have shown that certain HT regimens increase the risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Is it possible to prescribe HT in a way that reduces these risks?
Results of observational studies indicate that, compared with oral estrogen therapy, transdermal HT is associated with a lower risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer (TABLE).9−15
Reducing the risk of stroke caused by HT is an important goal. In a study of 15,710 women who had stroke and 59,958 control women aged 50 to 79 years, transdermal estradiol at a dose of 50 µg or less daily was not associated with an increased risk of stroke, compared with HT nonuse (rate ratio, 0.81; 95% CI, 0.62−1.05).9 Compared with HT nonuse, the use of oral estrogen (rate ratio, 1.28; 95% CI, 1.15−1.42) or transdermal estradiol 50 µg or greater daily (rate ratio, 1.89; 95% CI, 1.15−3.11) was associated with an increased risk of stroke.9
Reducing the risks of deep venous thromboembolism (VTE) and pulmonary embolism caused by HT is an important goal. In a meta-analysis of the risk of VTE with HT, compared with nonusers, oral estrogen therapy was associated with a significantly increased risk of VTE (odds ratio [OR], 2.5; 95% CI, 1.9−3.4). Compared with nonuse, transdermal estrogen therapy was not associated with an increased risk of VTE (OR, 1.2; 95% CI, 0.9−1.7).11 In a study comparing oral versus transdermal estradiol, transdermal estradiol was associated with a reduced risk of pulmonary embolism (0.46 [95% CI, 0.22−0.97]).13
Reducing the risk of breast cancer caused by HT is an important goal. Results of one study showed that the combination of oral estrogen plus synthetic progestin was associated with an increased risk of breast cancer, compared with nonuse (RR, 1.5; 95% CI, 1.1−1.9). By contrast, the combination of transdermal estradiol plus micronized progesterone was not associated with an increased risk of breast cancer, compared with nonuse (RR, 0.9; 95% CI, 0.7−1.2).15
The bottom line
In recently menopausal women with moderate to severe hot flashes, HT improves quality of life and appears to decrease mortality. However, HT with oral estrogen plus synthetic progestin is associated with an increased risk of stroke, deep vein thrombosis, pulmonary embolism, and breast cancer. Compared with oral estrogen, transdermal estradiol treatment is associated with a lower risk of stroke, deep vein thrombosis, and pulmonary embolism. Compared with oral estrogen plus a synthetic progestin, transdermal estradiol plus micronized progesterone is associated with a lower risk of breast cancer. The benefits of HT are likely maximized by initiating therapy in the perimenopause transition or early in the postmenopause, and the risks are minimized by using transdermal estradiol.16−18
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353−1368.
- Santen RJ, Allred DC, Ardoin SP, et al. J Clin Endocrinol Metab. 2010;95(suppl 1):S1−S66.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in postmenopausal women. Cochrane Database Syst Rev. 2015;3:CD002229.
- Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger post-menopausal women. Am J Med. 2009;122(11):1016−1022.
- Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease: The Framingham Study. Ann Intern Med. 1978;89(2):157−161.
- Stampfer MJ, Colditz GA, Willet WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses Health Study. N Engl J Med. 1991;325(11):756−762.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15−23.
- Hodis HN, Mack WJ, Shoupe D, et al. Testing the menopausal hormone therapy timing hypothesis: the early versus late intervention trial with estradiol [abstract 13283]. American Heart Association Meeting 2014. Circulation. 2014;130:A13283.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010;8(5):979−986.
- Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227−1231.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340−345.
- Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052−1059.
- Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277−2286.
- Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448−454.
- L’Hermite M. HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric. 2013;16(suppl 1):44−53.
- Simon JA. What’s new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1):3−10.
- Mueck AO. Postmenopausal hormone replacement therapy and cardiovascular disease: the value of transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1): 11−17.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353−1368.
- Santen RJ, Allred DC, Ardoin SP, et al. J Clin Endocrinol Metab. 2010;95(suppl 1):S1−S66.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in postmenopausal women. Cochrane Database Syst Rev. 2015;3:CD002229.
- Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian meta-analysis of hormone therapy and mortality in younger post-menopausal women. Am J Med. 2009;122(11):1016−1022.
- Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease: The Framingham Study. Ann Intern Med. 1978;89(2):157−161.
- Stampfer MJ, Colditz GA, Willet WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses Health Study. N Engl J Med. 1991;325(11):756−762.
- Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15−23.
- Hodis HN, Mack WJ, Shoupe D, et al. Testing the menopausal hormone therapy timing hypothesis: the early versus late intervention trial with estradiol [abstract 13283]. American Heart Association Meeting 2014. Circulation. 2014;130:A13283.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519
- Renoux C, Dell’Aniello S, Suissa S. Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost. 2010;8(5):979−986.
- Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227−1231.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340−345.
- Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18(10):1052−1059.
- Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277−2286.
- Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448−454.
- L’Hermite M. HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric. 2013;16(suppl 1):44−53.
- Simon JA. What’s new in hormone replacement therapy: focus on transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1):3−10.
- Mueck AO. Postmenopausal hormone replacement therapy and cardiovascular disease: the value of transdermal estradiol and micronized progesterone. Climacteric. 2012;15(suppl 1): 11−17.
Reducing maternal mortality in the United States—Let’s get organized!
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
Why is breast density a weighty matter?
Case: Patient seeks clarification and next steps on her breast density classification
Your patient, a 51-year-old postmenopausal woman (G0P0) in good health, had an annual screening mammogram that showed no evidence of malignancy. She is white and has a mother with a history of breast cancer. She has never had a breast biopsy. Following the mammogram, she received a letter from the imaging center, stating:
She calls your office and asks, “What should I do next?”
Breasts are composed of fibrous, glandular, and adipose tissue. If the breasts contain a lot of fibrous and glandular tissue, and little adipose tissue, they are considered to be “dense.” Using mammography, the current standard is to report the density of breast tissue using 4 categories:
- almost entirely fatty
- scattered fibroglandular densities
- heterogeneously dense
- extremely dense.
Dense breast tissue is defined to include the 2 categories heterogeneously dense and extremely dense.
Observational studies have reported that dense breast tissue is associated with an increased risk of breast cancer, and dense breast tissue makes it more difficult to detect breast cancer on mammography. According to data from the Breast Cancer Surveillance Consortium, among women aged 50 or older, the relative risk of breast cancer stratified by the 4 categories of breast density is 0.59, 1.00, 1.46, and 1.77, for almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, and extremely dense, respectively.1 In one study, the sensitivity of mammography to detect breast cancer was 82% to 88% for women with nondense breasts and 62% to 69% in women with dense breasts.2 These data have catalyzed investigators to explore the use of supplemental imaging to enhance cancer detection in women with dense breasts.
The link between breast density and breast cancer risk and reduced sensitivity of mammography also has catalyzed activists and legislators to champion breast density notification laws, which have passed in more than 20 states. These laws require facilities that perform mammography to notify women with dense breasts that this finding is associated with an increased risk of breast cancer and that dense breasts reduce the ability of mammography to detect cancer. In some states, the law mandates that women with dense breasts be offered supplemental ultrasound imaging and that insurers must cover the cost of the ultrasound studies. Many of the laws recommend that the patient discuss the situation with the clinician who ordered the mammogram.
When I first saw the recommendation for patients to contact me about how to manage dense breasts, my initial response was, “Who? Me?” I felt ill equipped to provide any useful advice and suspected that many of my patients knew more than I about this issue.
Based on a review of the evidence, my current clinical recommendation is outlined in the 2 options below, including a low-resource utilization option and a high-resource utilization option. For patients, physicians, and health systems that are concerned that excessive breast cancer screening tests might cause more harm than benefit, the identification of dense breasts on mammogram is unlikely to be a trigger to perform any additional testing. In this situation, the pragmatic low-resource option is most relevant.
Alternatively, for patients and physicians who strongly believe in the value of screening mammography (see “Utilize tomosynthesis digital mammography technology for your patients” below), a reasonable strategy is to recommend that women with dense breasts and an increased risk for breast cancer be offered supplemental imaging.
In this editorial I elaborate these 2 approaches to breast cancer screening in women with dense breasts.
Utilize tomosynthesis digital mammography technology for your patients
Mammograms are the primary modality used for breast cancer screening because screening mammography has been shown to reduce breast cancer deaths by 15% to 30%.1,2 Annual or biennial mammograms are recommended for women aged 40 years or older by many professional organizations, including the American College of Obstetricians and Gynecologists and the American College of Radiology. However, mammography screening programs have been criticized because of false-positive tests resulting in unnecessary biopsies, limited sensitivity, and the theoretical risk of over-diagnosing clinically insignificant cancers.3,4
Mammography technology continues to evolve. Film-based mammography has been replaced by digital mammography. Tomosynthesis digital mammography, also known as 3-D mammography, is now replacing standard digital mammography.5
With tomosynthesis, digital mammography image acquisition is performed using an x-ray source that moves through an arc across the breast with the capture of a series of images from different angles and reconstruction of the data into thin slices approximately 1 mm in width. The presentation of breast images in thin slices permits superior detection of lesions. In addition, the collected images can be reconstructed to present a virtual 2-D image for analysis.
Tomosynthesis has been demonstrated to increase the sensitivity of mammography to detect cancer and reduce false-positive examinations. In a study of 454,850 mammography examinations, investigators found that the invasive cancer detection rate per 1,000 studies increased from 2.9 with standard digital mammography to 4.1 with tomosynthesis.6
Tomosynthesis also reduces the patient recall rate to perform additional views or subsequent ultrasound. In one large study, the recall rate was 12% for standard digital mammography and 8.4% for tomosynthesis.7
The limitations of tomosynthesis include higher costs and higher radiation doses.
If the technology is available, I recommend that women have their mammograms using the best technology, tomosynthesis digital mammography.8
References
1. Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806.
2. Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786.
3. US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendations statement. Ann Intern Med. 2009;151(10):716–726, W-236.
4. Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammograms. JAMA Intern Med. 2014;174(3):448–454.
5. Destounis SV, Morgan R, Areino A. Screening for dense breasts: digital tomosynthesis. AJR Am J Roentgenol. 2015;204(2):261–264.
6. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
7. Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269(3):694–700.
8. Pisano ED, Yaffe MJ. Breast cancer screening: should tomosynthesis replace digital mammography? JAMA. 2014;311(24):2488–2489.
A pragmatic, low-resource utilization screening approach for women with dense breasts
There are no published randomized clinical trials that provide high-quality evidence on what to do if dense breasts are identified on mammography.3 Authors of observational studies have evaluated the potential role of supplemental imaging, including ultrasound and magnetic resonance imaging (MRI), in the management of dense breast tissue (see “Supplemental breast cancer screening modalities” below). Supplemental imaging involves complex trade-offs, balancing the potential benefit of identifying occult early breast cancer lesions not identified by mammography with the risk of subjecting many women without cancer to additional testing and unnecessary biopsies.
A pragmatic, low-resource utilization plan for women with dense breasts involves emphasizing that mammography is the best available screening tool and that annual or biennial mammography is the foundation of all current approaches to breast cancer screening. Supplemental imaging is unnecessary with this approach because there is no evidence that it reduces breast cancer mortality. There is, however, substantial evidence that using supplemental imaging for all women with dense breasts will result in little benefit and great costs, including many unnecessary biopsies.1,4 Women with dense breasts also could consider annual clinical breast examination.
Supplemental breast cancer screening modalities
Ultrasound and magnetic resonance imaging (MRI) are available as supplemental imaging, although ultrasound is the only supplemental imaging test that is specifically approved for women with dense breasts. Among the clinically available imaging modalities, MRI can detect the greatest number of cancers.
Ultrasound
In women with dense breasts, ultrasound can detect another 3 to 4 cancers that were not detected by mammography. However, ultrasound imaging generates many false positive results that lead to additional biopsies. According to one analysis, compared with mammography alone, mammography plus ultrasound would prevent 0.36 breast cancer deaths and cause 354 additional biopsies per 1,000 women with dense breasts screened biennially for 25 years.1
Ultrasound commonly is used to follow up an abnormal mammogram to further evaluate masses and differentiate cysts from solid tumors. Ultrasound is also a useful breast-imaging tool for women who are pregnant. In 2012, the US Food and Drug Administration approved an automated breast ultrasound device to be used for supplemental imaging of asymptomatic women with dense breasts and a mammogram negative for cancer. This device may facilitate the use of ultrasound for supplemental imaging of women with dense breasts on mammography.
Magnetic resonance imaging
MRI can detect the greatest number of cancers of any clinically available modality.
It is almost never covered by insurance for women whose only breast cancer risk factor is the identification of dense breasts on mammography. The cost of MRI testing is, however, typically covered for women at very high risk for breast cancer.
Women who are known to be at very high risk for breast cancer should begin annual clinical breast examinations at age 25 years and alternate between screening mammography and screening MRI every 6 months or annually. These women include:
- carriers of clinically significant BRCA1 or BRCA2 mutations
- carriers of other high-risk genetic mutations such as Cowden syndrome (PTEN mutation), Lai-Fraumeni syndrome (TP53 mutation), and Peutz-Jeghers syndrome
- genetically untested women with a first-degree relative with a BRCA mutation.
Women who had thoracic radiation before age 30 also should be considered for this screening protocol beginning 8 to 10 years after the radiation exposure or at age 25 years.2
References
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. CRICO Breast Care Management Algorithm. CRICO; Cambridge, Massachusetts; 2014. https://www.rmf.harvard.edu/~/media/Files/_Global/KC/PDFs/Guidelines/cricormfbca2014_locked.pdf. Accessed July 19, 2015.
A high-resource utilization screening approach
There are no randomized trials to help guide recommendations about how to respond to a finding of dense breasts on mammography. In addition to breast density, many factors influence breast cancer risk, including a patient’s:
- age
- family history
- history of previous breast biopsies
- many reproductive factors, including early age of menarche and late childbearing.
Women with both dense breasts and an increased risk of breast cancer may reap the greatest benefit from supplemental imaging, such as ultrasonography. Therefore, a two-step approach can help.
Step 1: Assess breast cancer risk. This can be accomplished using one of many calculators. Three that are commonly used are the:
- National Cancer Institute (NCI) Breast Cancer Surveillance Consortium (BCSC) calculator5
- NCI Breast Cancer Risk Assessment Tool, Gail model (BRCAT)6
- IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model).7
The BCSC calculator uses age, race/ethnicity, first-degree relatives with breast cancer, a history of a breast biopsy, and breast density to calculate a 5-year risk of developing breast cancer.
The BCRAT tool uses current age, race/ethnicity, age at menarche, age at first live-birth of a child, number of first-degree relatives with breast cancer, a history of breast biopsies, and the identification of atypical hyperplasia to calculate a 5-year risk of breast cancer.
The IBIS model uses many more variables, including a detailed family history to calculate a 10-year and lifetime risk of breast cancer. If a patient has ductal carcinoma in situ, lobular carcinoma in situ, chest irradiation before age 30 years, or known BRCA1 or BRCA2 mutations, she is instructed not to use the risk calculators because they are at very high risk for breast cancer, and they need an individualized intensive plan for monitoring and prevention (see MRI section in “Supplemental breast cancer screening modalities” above).
Step 2: Use breast density and breast cancer risk to develop a screening plan. The NIH Breast Cancer Surveillance Consortium has published data estimating the risk that a woman with a mammogram negative for cancer will develop breast cancer within the next 12 months (based on her age, breast density, and breast cancer risk—calculated with the BCSC tool).8
It reported an increased risk of breast cancer diagnosed within 12 months following a mammogram that was negative for cancer in women with extremely dense breasts and a BCSC 5-year risk of breast cancer of 1.67% or greater and in women with heterogeneously dense breasts and a BCSC 5-year risk of breast cancer of 2.5% or greater.8
Using these cutoffs it is estimated that 24% of all women with heterogeneously or extremely dense breasts would be offered supplemental screening with a modality such as ultrasound, and 76% would be guided not to have supplemental screening because their risk of developing breast cancer in the 12 months following their negative mammogram is low.
If this guidance is followed, it would require 694 supplemental ultrasound studies and many biopsies to detect 1 additional breast cancer, significantly increasing overall health care costs.8 In many states insurers do not cover supplemental ultrasound imaging of the breasts. In most states insurers require preauthorization for supplemental MRI of the breasts. You need to know the insurance practices in the state to help guide decision making about supplemental imaging. The approach described above is consistent with the American College of Obstetricians and Gynecologists recommendation that women with dense breasts, who are asymptomaticand have no additional risk factors for breast cancer, do not need to be offered supplemental imaging.9
Case: Next steps
The BCSC calculator reveals that the 51-year-old woman with a family history of breast cancer and a mammogram showing extremely dense breasts has a 5-year risk of breast cancer of 2.68%. Given that this risk is elevated, this patient could be offered supplemental ultrasound screening and annual breast clinical examination. In addition, she could be further counseled about breast cancer chemoprevention options.10
Women with a strong family history of breast and/or ovarian cancer also could be referred for genetic counseling and BRCA testing.11 The risk of having a BRCA mutation can be calculated using the BRCAPRO tool.12
Most women with dense breast tissue on mammography will never develop breast cancer. Yet the presence of dense breast tissue both increases the risk of breast cancer and decreases the sensitivity of mammography to detect cancer. There are no high-quality data from randomized trials to help guide our recommendations concerning the management of dense breasts identified on mammography. Yet many states have laws that suggest patients ask you to provide advice about breast density.
Patients, clinicians, and health systems vary in their confidence in the clinical value of breast cancer screening programs. Consequently, there is no “right answer” to this vexing problem. The standard of care is to support a range of options tailored to the specific clinical characteristics and needs of each patient.
Many states mandate that patients receive letters from their mammography center that report on breast density. In many states the law requires that the letter contain a statement that dense breasts increase the risk of breast cancer and reduce the ability of mammography to detect breast cancer. Do you believe these letters:
b) are beneficial because they provide the patient important information
c) both a and b
To weigh in and send your Letter to the Editor, visit obgmanagement.com and look for the “Quick Poll” on the right side of the home page.
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175.
3. Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;4:CD009632.
4. Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs. mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163.
5. Breast Cancer Surveillance Consortium risk calculator. BCSC Web site. https://tools.bcsc-scc.org/BC5yearRisk/intro.htm. Updated February 13, 2015. Accessed July 17, 2015.
6. NCI Breast Cancer Risk Assessment Tool (Gail model). National Cancer Institute Web site. http://www.cancer.gov/BCRISKTOOL/. Accessed July 17, 2015.
7. IBIS Breast Cancer Risk Evaluation Tool. http://www.ems-trials.org/riskevaluator/. Updated January 9, 2015. Accessed July 17, 2015.
8. Kerlikowske K, Zhu W, Tosteson AN, et al; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer. Ann Intern Med. 2015;162(10):673–681.
9. Committee on Gynecologic Practice. Committee Opinion No. 625: Management of women with dense breasts diagnosed by mammography. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;125(3): 750–751.
10. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(34):2942–2962.
11. Profato JL, Arun BK. Genetic risk assessment for breast and gynecological malignancies. Curr Opin Obstet Gynecol. 2015;27(1):1–5.
12. BRCAPRO. BayesMendel Lab. Harvard University Web site. http://bcb.dfci.harvard.edu/bayesmendel/brcapro.php. Accessed July 19, 2015.
Case: Patient seeks clarification and next steps on her breast density classification
Your patient, a 51-year-old postmenopausal woman (G0P0) in good health, had an annual screening mammogram that showed no evidence of malignancy. She is white and has a mother with a history of breast cancer. She has never had a breast biopsy. Following the mammogram, she received a letter from the imaging center, stating:
She calls your office and asks, “What should I do next?”
Breasts are composed of fibrous, glandular, and adipose tissue. If the breasts contain a lot of fibrous and glandular tissue, and little adipose tissue, they are considered to be “dense.” Using mammography, the current standard is to report the density of breast tissue using 4 categories:
- almost entirely fatty
- scattered fibroglandular densities
- heterogeneously dense
- extremely dense.
Dense breast tissue is defined to include the 2 categories heterogeneously dense and extremely dense.
Observational studies have reported that dense breast tissue is associated with an increased risk of breast cancer, and dense breast tissue makes it more difficult to detect breast cancer on mammography. According to data from the Breast Cancer Surveillance Consortium, among women aged 50 or older, the relative risk of breast cancer stratified by the 4 categories of breast density is 0.59, 1.00, 1.46, and 1.77, for almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, and extremely dense, respectively.1 In one study, the sensitivity of mammography to detect breast cancer was 82% to 88% for women with nondense breasts and 62% to 69% in women with dense breasts.2 These data have catalyzed investigators to explore the use of supplemental imaging to enhance cancer detection in women with dense breasts.
The link between breast density and breast cancer risk and reduced sensitivity of mammography also has catalyzed activists and legislators to champion breast density notification laws, which have passed in more than 20 states. These laws require facilities that perform mammography to notify women with dense breasts that this finding is associated with an increased risk of breast cancer and that dense breasts reduce the ability of mammography to detect cancer. In some states, the law mandates that women with dense breasts be offered supplemental ultrasound imaging and that insurers must cover the cost of the ultrasound studies. Many of the laws recommend that the patient discuss the situation with the clinician who ordered the mammogram.
When I first saw the recommendation for patients to contact me about how to manage dense breasts, my initial response was, “Who? Me?” I felt ill equipped to provide any useful advice and suspected that many of my patients knew more than I about this issue.
Based on a review of the evidence, my current clinical recommendation is outlined in the 2 options below, including a low-resource utilization option and a high-resource utilization option. For patients, physicians, and health systems that are concerned that excessive breast cancer screening tests might cause more harm than benefit, the identification of dense breasts on mammogram is unlikely to be a trigger to perform any additional testing. In this situation, the pragmatic low-resource option is most relevant.
Alternatively, for patients and physicians who strongly believe in the value of screening mammography (see “Utilize tomosynthesis digital mammography technology for your patients” below), a reasonable strategy is to recommend that women with dense breasts and an increased risk for breast cancer be offered supplemental imaging.
In this editorial I elaborate these 2 approaches to breast cancer screening in women with dense breasts.
Utilize tomosynthesis digital mammography technology for your patients
Mammograms are the primary modality used for breast cancer screening because screening mammography has been shown to reduce breast cancer deaths by 15% to 30%.1,2 Annual or biennial mammograms are recommended for women aged 40 years or older by many professional organizations, including the American College of Obstetricians and Gynecologists and the American College of Radiology. However, mammography screening programs have been criticized because of false-positive tests resulting in unnecessary biopsies, limited sensitivity, and the theoretical risk of over-diagnosing clinically insignificant cancers.3,4
Mammography technology continues to evolve. Film-based mammography has been replaced by digital mammography. Tomosynthesis digital mammography, also known as 3-D mammography, is now replacing standard digital mammography.5
With tomosynthesis, digital mammography image acquisition is performed using an x-ray source that moves through an arc across the breast with the capture of a series of images from different angles and reconstruction of the data into thin slices approximately 1 mm in width. The presentation of breast images in thin slices permits superior detection of lesions. In addition, the collected images can be reconstructed to present a virtual 2-D image for analysis.
Tomosynthesis has been demonstrated to increase the sensitivity of mammography to detect cancer and reduce false-positive examinations. In a study of 454,850 mammography examinations, investigators found that the invasive cancer detection rate per 1,000 studies increased from 2.9 with standard digital mammography to 4.1 with tomosynthesis.6
Tomosynthesis also reduces the patient recall rate to perform additional views or subsequent ultrasound. In one large study, the recall rate was 12% for standard digital mammography and 8.4% for tomosynthesis.7
The limitations of tomosynthesis include higher costs and higher radiation doses.
If the technology is available, I recommend that women have their mammograms using the best technology, tomosynthesis digital mammography.8
References
1. Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806.
2. Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786.
3. US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendations statement. Ann Intern Med. 2009;151(10):716–726, W-236.
4. Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammograms. JAMA Intern Med. 2014;174(3):448–454.
5. Destounis SV, Morgan R, Areino A. Screening for dense breasts: digital tomosynthesis. AJR Am J Roentgenol. 2015;204(2):261–264.
6. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
7. Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269(3):694–700.
8. Pisano ED, Yaffe MJ. Breast cancer screening: should tomosynthesis replace digital mammography? JAMA. 2014;311(24):2488–2489.
A pragmatic, low-resource utilization screening approach for women with dense breasts
There are no published randomized clinical trials that provide high-quality evidence on what to do if dense breasts are identified on mammography.3 Authors of observational studies have evaluated the potential role of supplemental imaging, including ultrasound and magnetic resonance imaging (MRI), in the management of dense breast tissue (see “Supplemental breast cancer screening modalities” below). Supplemental imaging involves complex trade-offs, balancing the potential benefit of identifying occult early breast cancer lesions not identified by mammography with the risk of subjecting many women without cancer to additional testing and unnecessary biopsies.
A pragmatic, low-resource utilization plan for women with dense breasts involves emphasizing that mammography is the best available screening tool and that annual or biennial mammography is the foundation of all current approaches to breast cancer screening. Supplemental imaging is unnecessary with this approach because there is no evidence that it reduces breast cancer mortality. There is, however, substantial evidence that using supplemental imaging for all women with dense breasts will result in little benefit and great costs, including many unnecessary biopsies.1,4 Women with dense breasts also could consider annual clinical breast examination.
Supplemental breast cancer screening modalities
Ultrasound and magnetic resonance imaging (MRI) are available as supplemental imaging, although ultrasound is the only supplemental imaging test that is specifically approved for women with dense breasts. Among the clinically available imaging modalities, MRI can detect the greatest number of cancers.
Ultrasound
In women with dense breasts, ultrasound can detect another 3 to 4 cancers that were not detected by mammography. However, ultrasound imaging generates many false positive results that lead to additional biopsies. According to one analysis, compared with mammography alone, mammography plus ultrasound would prevent 0.36 breast cancer deaths and cause 354 additional biopsies per 1,000 women with dense breasts screened biennially for 25 years.1
Ultrasound commonly is used to follow up an abnormal mammogram to further evaluate masses and differentiate cysts from solid tumors. Ultrasound is also a useful breast-imaging tool for women who are pregnant. In 2012, the US Food and Drug Administration approved an automated breast ultrasound device to be used for supplemental imaging of asymptomatic women with dense breasts and a mammogram negative for cancer. This device may facilitate the use of ultrasound for supplemental imaging of women with dense breasts on mammography.
Magnetic resonance imaging
MRI can detect the greatest number of cancers of any clinically available modality.
It is almost never covered by insurance for women whose only breast cancer risk factor is the identification of dense breasts on mammography. The cost of MRI testing is, however, typically covered for women at very high risk for breast cancer.
Women who are known to be at very high risk for breast cancer should begin annual clinical breast examinations at age 25 years and alternate between screening mammography and screening MRI every 6 months or annually. These women include:
- carriers of clinically significant BRCA1 or BRCA2 mutations
- carriers of other high-risk genetic mutations such as Cowden syndrome (PTEN mutation), Lai-Fraumeni syndrome (TP53 mutation), and Peutz-Jeghers syndrome
- genetically untested women with a first-degree relative with a BRCA mutation.
Women who had thoracic radiation before age 30 also should be considered for this screening protocol beginning 8 to 10 years after the radiation exposure or at age 25 years.2
References
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. CRICO Breast Care Management Algorithm. CRICO; Cambridge, Massachusetts; 2014. https://www.rmf.harvard.edu/~/media/Files/_Global/KC/PDFs/Guidelines/cricormfbca2014_locked.pdf. Accessed July 19, 2015.
A high-resource utilization screening approach
There are no randomized trials to help guide recommendations about how to respond to a finding of dense breasts on mammography. In addition to breast density, many factors influence breast cancer risk, including a patient’s:
- age
- family history
- history of previous breast biopsies
- many reproductive factors, including early age of menarche and late childbearing.
Women with both dense breasts and an increased risk of breast cancer may reap the greatest benefit from supplemental imaging, such as ultrasonography. Therefore, a two-step approach can help.
Step 1: Assess breast cancer risk. This can be accomplished using one of many calculators. Three that are commonly used are the:
- National Cancer Institute (NCI) Breast Cancer Surveillance Consortium (BCSC) calculator5
- NCI Breast Cancer Risk Assessment Tool, Gail model (BRCAT)6
- IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model).7
The BCSC calculator uses age, race/ethnicity, first-degree relatives with breast cancer, a history of a breast biopsy, and breast density to calculate a 5-year risk of developing breast cancer.
The BCRAT tool uses current age, race/ethnicity, age at menarche, age at first live-birth of a child, number of first-degree relatives with breast cancer, a history of breast biopsies, and the identification of atypical hyperplasia to calculate a 5-year risk of breast cancer.
The IBIS model uses many more variables, including a detailed family history to calculate a 10-year and lifetime risk of breast cancer. If a patient has ductal carcinoma in situ, lobular carcinoma in situ, chest irradiation before age 30 years, or known BRCA1 or BRCA2 mutations, she is instructed not to use the risk calculators because they are at very high risk for breast cancer, and they need an individualized intensive plan for monitoring and prevention (see MRI section in “Supplemental breast cancer screening modalities” above).
Step 2: Use breast density and breast cancer risk to develop a screening plan. The NIH Breast Cancer Surveillance Consortium has published data estimating the risk that a woman with a mammogram negative for cancer will develop breast cancer within the next 12 months (based on her age, breast density, and breast cancer risk—calculated with the BCSC tool).8
It reported an increased risk of breast cancer diagnosed within 12 months following a mammogram that was negative for cancer in women with extremely dense breasts and a BCSC 5-year risk of breast cancer of 1.67% or greater and in women with heterogeneously dense breasts and a BCSC 5-year risk of breast cancer of 2.5% or greater.8
Using these cutoffs it is estimated that 24% of all women with heterogeneously or extremely dense breasts would be offered supplemental screening with a modality such as ultrasound, and 76% would be guided not to have supplemental screening because their risk of developing breast cancer in the 12 months following their negative mammogram is low.
If this guidance is followed, it would require 694 supplemental ultrasound studies and many biopsies to detect 1 additional breast cancer, significantly increasing overall health care costs.8 In many states insurers do not cover supplemental ultrasound imaging of the breasts. In most states insurers require preauthorization for supplemental MRI of the breasts. You need to know the insurance practices in the state to help guide decision making about supplemental imaging. The approach described above is consistent with the American College of Obstetricians and Gynecologists recommendation that women with dense breasts, who are asymptomaticand have no additional risk factors for breast cancer, do not need to be offered supplemental imaging.9
Case: Next steps
The BCSC calculator reveals that the 51-year-old woman with a family history of breast cancer and a mammogram showing extremely dense breasts has a 5-year risk of breast cancer of 2.68%. Given that this risk is elevated, this patient could be offered supplemental ultrasound screening and annual breast clinical examination. In addition, she could be further counseled about breast cancer chemoprevention options.10
Women with a strong family history of breast and/or ovarian cancer also could be referred for genetic counseling and BRCA testing.11 The risk of having a BRCA mutation can be calculated using the BRCAPRO tool.12
Most women with dense breast tissue on mammography will never develop breast cancer. Yet the presence of dense breast tissue both increases the risk of breast cancer and decreases the sensitivity of mammography to detect cancer. There are no high-quality data from randomized trials to help guide our recommendations concerning the management of dense breasts identified on mammography. Yet many states have laws that suggest patients ask you to provide advice about breast density.
Patients, clinicians, and health systems vary in their confidence in the clinical value of breast cancer screening programs. Consequently, there is no “right answer” to this vexing problem. The standard of care is to support a range of options tailored to the specific clinical characteristics and needs of each patient.
Many states mandate that patients receive letters from their mammography center that report on breast density. In many states the law requires that the letter contain a statement that dense breasts increase the risk of breast cancer and reduce the ability of mammography to detect breast cancer. Do you believe these letters:
b) are beneficial because they provide the patient important information
c) both a and b
To weigh in and send your Letter to the Editor, visit obgmanagement.com and look for the “Quick Poll” on the right side of the home page.
Case: Patient seeks clarification and next steps on her breast density classification
Your patient, a 51-year-old postmenopausal woman (G0P0) in good health, had an annual screening mammogram that showed no evidence of malignancy. She is white and has a mother with a history of breast cancer. She has never had a breast biopsy. Following the mammogram, she received a letter from the imaging center, stating:
She calls your office and asks, “What should I do next?”
Breasts are composed of fibrous, glandular, and adipose tissue. If the breasts contain a lot of fibrous and glandular tissue, and little adipose tissue, they are considered to be “dense.” Using mammography, the current standard is to report the density of breast tissue using 4 categories:
- almost entirely fatty
- scattered fibroglandular densities
- heterogeneously dense
- extremely dense.
Dense breast tissue is defined to include the 2 categories heterogeneously dense and extremely dense.
Observational studies have reported that dense breast tissue is associated with an increased risk of breast cancer, and dense breast tissue makes it more difficult to detect breast cancer on mammography. According to data from the Breast Cancer Surveillance Consortium, among women aged 50 or older, the relative risk of breast cancer stratified by the 4 categories of breast density is 0.59, 1.00, 1.46, and 1.77, for almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, and extremely dense, respectively.1 In one study, the sensitivity of mammography to detect breast cancer was 82% to 88% for women with nondense breasts and 62% to 69% in women with dense breasts.2 These data have catalyzed investigators to explore the use of supplemental imaging to enhance cancer detection in women with dense breasts.
The link between breast density and breast cancer risk and reduced sensitivity of mammography also has catalyzed activists and legislators to champion breast density notification laws, which have passed in more than 20 states. These laws require facilities that perform mammography to notify women with dense breasts that this finding is associated with an increased risk of breast cancer and that dense breasts reduce the ability of mammography to detect cancer. In some states, the law mandates that women with dense breasts be offered supplemental ultrasound imaging and that insurers must cover the cost of the ultrasound studies. Many of the laws recommend that the patient discuss the situation with the clinician who ordered the mammogram.
When I first saw the recommendation for patients to contact me about how to manage dense breasts, my initial response was, “Who? Me?” I felt ill equipped to provide any useful advice and suspected that many of my patients knew more than I about this issue.
Based on a review of the evidence, my current clinical recommendation is outlined in the 2 options below, including a low-resource utilization option and a high-resource utilization option. For patients, physicians, and health systems that are concerned that excessive breast cancer screening tests might cause more harm than benefit, the identification of dense breasts on mammogram is unlikely to be a trigger to perform any additional testing. In this situation, the pragmatic low-resource option is most relevant.
Alternatively, for patients and physicians who strongly believe in the value of screening mammography (see “Utilize tomosynthesis digital mammography technology for your patients” below), a reasonable strategy is to recommend that women with dense breasts and an increased risk for breast cancer be offered supplemental imaging.
In this editorial I elaborate these 2 approaches to breast cancer screening in women with dense breasts.
Utilize tomosynthesis digital mammography technology for your patients
Mammograms are the primary modality used for breast cancer screening because screening mammography has been shown to reduce breast cancer deaths by 15% to 30%.1,2 Annual or biennial mammograms are recommended for women aged 40 years or older by many professional organizations, including the American College of Obstetricians and Gynecologists and the American College of Radiology. However, mammography screening programs have been criticized because of false-positive tests resulting in unnecessary biopsies, limited sensitivity, and the theoretical risk of over-diagnosing clinically insignificant cancers.3,4
Mammography technology continues to evolve. Film-based mammography has been replaced by digital mammography. Tomosynthesis digital mammography, also known as 3-D mammography, is now replacing standard digital mammography.5
With tomosynthesis, digital mammography image acquisition is performed using an x-ray source that moves through an arc across the breast with the capture of a series of images from different angles and reconstruction of the data into thin slices approximately 1 mm in width. The presentation of breast images in thin slices permits superior detection of lesions. In addition, the collected images can be reconstructed to present a virtual 2-D image for analysis.
Tomosynthesis has been demonstrated to increase the sensitivity of mammography to detect cancer and reduce false-positive examinations. In a study of 454,850 mammography examinations, investigators found that the invasive cancer detection rate per 1,000 studies increased from 2.9 with standard digital mammography to 4.1 with tomosynthesis.6
Tomosynthesis also reduces the patient recall rate to perform additional views or subsequent ultrasound. In one large study, the recall rate was 12% for standard digital mammography and 8.4% for tomosynthesis.7
The limitations of tomosynthesis include higher costs and higher radiation doses.
If the technology is available, I recommend that women have their mammograms using the best technology, tomosynthesis digital mammography.8
References
1. Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42(5):793–806.
2. Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786.
3. US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendations statement. Ann Intern Med. 2009;151(10):716–726, W-236.
4. Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammograms. JAMA Intern Med. 2014;174(3):448–454.
5. Destounis SV, Morgan R, Areino A. Screening for dense breasts: digital tomosynthesis. AJR Am J Roentgenol. 2015;204(2):261–264.
6. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
7. Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269(3):694–700.
8. Pisano ED, Yaffe MJ. Breast cancer screening: should tomosynthesis replace digital mammography? JAMA. 2014;311(24):2488–2489.
A pragmatic, low-resource utilization screening approach for women with dense breasts
There are no published randomized clinical trials that provide high-quality evidence on what to do if dense breasts are identified on mammography.3 Authors of observational studies have evaluated the potential role of supplemental imaging, including ultrasound and magnetic resonance imaging (MRI), in the management of dense breast tissue (see “Supplemental breast cancer screening modalities” below). Supplemental imaging involves complex trade-offs, balancing the potential benefit of identifying occult early breast cancer lesions not identified by mammography with the risk of subjecting many women without cancer to additional testing and unnecessary biopsies.
A pragmatic, low-resource utilization plan for women with dense breasts involves emphasizing that mammography is the best available screening tool and that annual or biennial mammography is the foundation of all current approaches to breast cancer screening. Supplemental imaging is unnecessary with this approach because there is no evidence that it reduces breast cancer mortality. There is, however, substantial evidence that using supplemental imaging for all women with dense breasts will result in little benefit and great costs, including many unnecessary biopsies.1,4 Women with dense breasts also could consider annual clinical breast examination.
Supplemental breast cancer screening modalities
Ultrasound and magnetic resonance imaging (MRI) are available as supplemental imaging, although ultrasound is the only supplemental imaging test that is specifically approved for women with dense breasts. Among the clinically available imaging modalities, MRI can detect the greatest number of cancers.
Ultrasound
In women with dense breasts, ultrasound can detect another 3 to 4 cancers that were not detected by mammography. However, ultrasound imaging generates many false positive results that lead to additional biopsies. According to one analysis, compared with mammography alone, mammography plus ultrasound would prevent 0.36 breast cancer deaths and cause 354 additional biopsies per 1,000 women with dense breasts screened biennially for 25 years.1
Ultrasound commonly is used to follow up an abnormal mammogram to further evaluate masses and differentiate cysts from solid tumors. Ultrasound is also a useful breast-imaging tool for women who are pregnant. In 2012, the US Food and Drug Administration approved an automated breast ultrasound device to be used for supplemental imaging of asymptomatic women with dense breasts and a mammogram negative for cancer. This device may facilitate the use of ultrasound for supplemental imaging of women with dense breasts on mammography.
Magnetic resonance imaging
MRI can detect the greatest number of cancers of any clinically available modality.
It is almost never covered by insurance for women whose only breast cancer risk factor is the identification of dense breasts on mammography. The cost of MRI testing is, however, typically covered for women at very high risk for breast cancer.
Women who are known to be at very high risk for breast cancer should begin annual clinical breast examinations at age 25 years and alternate between screening mammography and screening MRI every 6 months or annually. These women include:
- carriers of clinically significant BRCA1 or BRCA2 mutations
- carriers of other high-risk genetic mutations such as Cowden syndrome (PTEN mutation), Lai-Fraumeni syndrome (TP53 mutation), and Peutz-Jeghers syndrome
- genetically untested women with a first-degree relative with a BRCA mutation.
Women who had thoracic radiation before age 30 also should be considered for this screening protocol beginning 8 to 10 years after the radiation exposure or at age 25 years.2
References
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. CRICO Breast Care Management Algorithm. CRICO; Cambridge, Massachusetts; 2014. https://www.rmf.harvard.edu/~/media/Files/_Global/KC/PDFs/Guidelines/cricormfbca2014_locked.pdf. Accessed July 19, 2015.
A high-resource utilization screening approach
There are no randomized trials to help guide recommendations about how to respond to a finding of dense breasts on mammography. In addition to breast density, many factors influence breast cancer risk, including a patient’s:
- age
- family history
- history of previous breast biopsies
- many reproductive factors, including early age of menarche and late childbearing.
Women with both dense breasts and an increased risk of breast cancer may reap the greatest benefit from supplemental imaging, such as ultrasonography. Therefore, a two-step approach can help.
Step 1: Assess breast cancer risk. This can be accomplished using one of many calculators. Three that are commonly used are the:
- National Cancer Institute (NCI) Breast Cancer Surveillance Consortium (BCSC) calculator5
- NCI Breast Cancer Risk Assessment Tool, Gail model (BRCAT)6
- IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model).7
The BCSC calculator uses age, race/ethnicity, first-degree relatives with breast cancer, a history of a breast biopsy, and breast density to calculate a 5-year risk of developing breast cancer.
The BCRAT tool uses current age, race/ethnicity, age at menarche, age at first live-birth of a child, number of first-degree relatives with breast cancer, a history of breast biopsies, and the identification of atypical hyperplasia to calculate a 5-year risk of breast cancer.
The IBIS model uses many more variables, including a detailed family history to calculate a 10-year and lifetime risk of breast cancer. If a patient has ductal carcinoma in situ, lobular carcinoma in situ, chest irradiation before age 30 years, or known BRCA1 or BRCA2 mutations, she is instructed not to use the risk calculators because they are at very high risk for breast cancer, and they need an individualized intensive plan for monitoring and prevention (see MRI section in “Supplemental breast cancer screening modalities” above).
Step 2: Use breast density and breast cancer risk to develop a screening plan. The NIH Breast Cancer Surveillance Consortium has published data estimating the risk that a woman with a mammogram negative for cancer will develop breast cancer within the next 12 months (based on her age, breast density, and breast cancer risk—calculated with the BCSC tool).8
It reported an increased risk of breast cancer diagnosed within 12 months following a mammogram that was negative for cancer in women with extremely dense breasts and a BCSC 5-year risk of breast cancer of 1.67% or greater and in women with heterogeneously dense breasts and a BCSC 5-year risk of breast cancer of 2.5% or greater.8
Using these cutoffs it is estimated that 24% of all women with heterogeneously or extremely dense breasts would be offered supplemental screening with a modality such as ultrasound, and 76% would be guided not to have supplemental screening because their risk of developing breast cancer in the 12 months following their negative mammogram is low.
If this guidance is followed, it would require 694 supplemental ultrasound studies and many biopsies to detect 1 additional breast cancer, significantly increasing overall health care costs.8 In many states insurers do not cover supplemental ultrasound imaging of the breasts. In most states insurers require preauthorization for supplemental MRI of the breasts. You need to know the insurance practices in the state to help guide decision making about supplemental imaging. The approach described above is consistent with the American College of Obstetricians and Gynecologists recommendation that women with dense breasts, who are asymptomaticand have no additional risk factors for breast cancer, do not need to be offered supplemental imaging.9
Case: Next steps
The BCSC calculator reveals that the 51-year-old woman with a family history of breast cancer and a mammogram showing extremely dense breasts has a 5-year risk of breast cancer of 2.68%. Given that this risk is elevated, this patient could be offered supplemental ultrasound screening and annual breast clinical examination. In addition, she could be further counseled about breast cancer chemoprevention options.10
Women with a strong family history of breast and/or ovarian cancer also could be referred for genetic counseling and BRCA testing.11 The risk of having a BRCA mutation can be calculated using the BRCAPRO tool.12
Most women with dense breast tissue on mammography will never develop breast cancer. Yet the presence of dense breast tissue both increases the risk of breast cancer and decreases the sensitivity of mammography to detect cancer. There are no high-quality data from randomized trials to help guide our recommendations concerning the management of dense breasts identified on mammography. Yet many states have laws that suggest patients ask you to provide advice about breast density.
Patients, clinicians, and health systems vary in their confidence in the clinical value of breast cancer screening programs. Consequently, there is no “right answer” to this vexing problem. The standard of care is to support a range of options tailored to the specific clinical characteristics and needs of each patient.
Many states mandate that patients receive letters from their mammography center that report on breast density. In many states the law requires that the letter contain a statement that dense breasts increase the risk of breast cancer and reduce the ability of mammography to detect breast cancer. Do you believe these letters:
b) are beneficial because they provide the patient important information
c) both a and b
To weigh in and send your Letter to the Editor, visit obgmanagement.com and look for the “Quick Poll” on the right side of the home page.
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175.
3. Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;4:CD009632.
4. Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs. mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163.
5. Breast Cancer Surveillance Consortium risk calculator. BCSC Web site. https://tools.bcsc-scc.org/BC5yearRisk/intro.htm. Updated February 13, 2015. Accessed July 17, 2015.
6. NCI Breast Cancer Risk Assessment Tool (Gail model). National Cancer Institute Web site. http://www.cancer.gov/BCRISKTOOL/. Accessed July 17, 2015.
7. IBIS Breast Cancer Risk Evaluation Tool. http://www.ems-trials.org/riskevaluator/. Updated January 9, 2015. Accessed July 17, 2015.
8. Kerlikowske K, Zhu W, Tosteson AN, et al; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer. Ann Intern Med. 2015;162(10):673–681.
9. Committee on Gynecologic Practice. Committee Opinion No. 625: Management of women with dense breasts diagnosed by mammography. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;125(3): 750–751.
10. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(34):2942–2962.
11. Profato JL, Arun BK. Genetic risk assessment for breast and gynecological malignancies. Curr Opin Obstet Gynecol. 2015;27(1):1–5.
12. BRCAPRO. BayesMendel Lab. Harvard University Web site. http://bcb.dfci.harvard.edu/bayesmendel/brcapro.php. Accessed July 19, 2015.
1. Sprague BL, Stout KN, Schechter MD, et al. Benefits, harms and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–166.
2. Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175.
3. Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;4:CD009632.
4. Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs. mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299(18):2151–2163.
5. Breast Cancer Surveillance Consortium risk calculator. BCSC Web site. https://tools.bcsc-scc.org/BC5yearRisk/intro.htm. Updated February 13, 2015. Accessed July 17, 2015.
6. NCI Breast Cancer Risk Assessment Tool (Gail model). National Cancer Institute Web site. http://www.cancer.gov/BCRISKTOOL/. Accessed July 17, 2015.
7. IBIS Breast Cancer Risk Evaluation Tool. http://www.ems-trials.org/riskevaluator/. Updated January 9, 2015. Accessed July 17, 2015.
8. Kerlikowske K, Zhu W, Tosteson AN, et al; Breast Cancer Surveillance Consortium. Identifying women with dense breasts at high risk for interval cancer. Ann Intern Med. 2015;162(10):673–681.
9. Committee on Gynecologic Practice. Committee Opinion No. 625: Management of women with dense breasts diagnosed by mammography. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;125(3): 750–751.
10. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(34):2942–2962.
11. Profato JL, Arun BK. Genetic risk assessment for breast and gynecological malignancies. Curr Opin Obstet Gynecol. 2015;27(1):1–5.
12. BRCAPRO. BayesMendel Lab. Harvard University Web site. http://bcb.dfci.harvard.edu/bayesmendel/brcapro.php. Accessed July 19, 2015.
Advances in protection against oncogenic human papillomaviruses: The 9-valent vaccine
When Dr. Harald zur Hausen received the 2008 Nobel Prize in Physiology or Medicine for his discovery of the link between human papillomavirus (HPV) infections and genital cancers, he completed a 40-year odyssey to prove that viruses caused human cancer. Initially, zur Hausen, working in the University of Pennsylvania laboratory of the noted virologists Drs. Werner and Gertrude Henle, discovered that the Epstein-Barr virus was involved in the development of Burkitt lymphoma.1 On return to his native Germany, he sought a link between HPV and genital tumors.2
First he isolated HPV 6 and HPV 11 directly from genital warts.3 Then zur Hausen utilized the nucleic acid sequences from HPV6 and the technique of low stringency hybridization to discover HPV 16 and HPV 18 in cervical cancer specimens.4,5 Oncogenic HPV DNA contains 2 genes that produce the oncoproteins E6 and E7. E6 increases the degradation of p53 and E7 inactivates the retino-blastoma protein.6 The double-hit inactivation of 2 tumor suppressor genes, p53 and retinoblastoma protein, increases the mitotic activity of the infected cells, eventually leading to cancer.
zur Hausen tried to persuade companies to develop anti-HPV vaccines but was rebuffed for years. Today, building on his research, we have HPV vaccines that are 2-valent (against HPV types 16 and 18), 4-valent (against HPV types 6, 11, 16, and 18), and 9-valent (against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58). zur Hausen richly deserved the Nobel Prize for his life-saving discoveries.
Cervical, vulvar, and vaginal cancers
HPV types 16 and 18 cause about 70% of cervical cancers. HPV types 31, 33, 45, 52, and 58 cause about 20% of cervical cancers.7 The 2-, 4-, and 9-valent HPV vaccines have been demonstrated to prevent premalignant cervical disease, including cervical intraepithelial neoplasia (CIN) 2 and CIN 3 and adenocarcinoma in situ.8–11 The development of a 9-valent HPV vaccine is an important advance because it provides more complete immunization against cancer causing viruses.
Approximately 70% of vaginal cancers are caused by HPV infections.12 Among squamous cell vulvar cancers, HPV is detected in approximately 70% of cancers with warty or basaloid histology and 12% of cancers with keratinizing histology.13 In vulvar cancer, HPV 16, 33, and 18 are the most common types detected, representing 73%, 7%, and 5% of cases, respectively. The HPV 4- and 9-valent vaccines have been reported to reduce precancerous lesions of the vagina and vulva.9,11 In most trials, vaccinations that occur before exposure to HPV through sexual encounters appear to provide greater protection than vaccinations that occur after HPV infection.
Anal cancer
Approximately 90% of anal cancers are caused by HPV infection, and HPV types 16 and 18 are detected in 81% and 4% of anal cancers, respectively.14 Among men who have sex with men, the HPV 4-valent vaccine reduced the rate of anal intraepithelial neoplasia, a precursor to anal cancer, by 50%.15 Women receiving the HPV 2-valent vaccine had an 84% reduction in the detection of anal cancer involving HPV types 16 and 18.16
Penile cancer
Approximately 48% of penile cancers harbor oncogenic HPV types.17 Among penile cancers the prevalence of HPV varies from 22% in verrucous cancer to 66% in basaloid and warty cancer. The most prevalent HPV types were 16, 6, and 18, which were observed in 31%, 7%, and 7% of the cancers, respectively.17 Penile cancer is not common and there are no studies directly demonstrating that HPV vaccination prevents penile cancer.
Oropharyngeal cancer
The rate of oropharyngeal cancer caused by HPV is rising rapidly and increasing more rapidly among men than among women.18 Remarkably, HPV-induced oropharyngeal cancer is projected to become more common than cervical cancer in 2020.18
In one report, 72% of oropharyngeal cancers harbored HPV 16, and antibodies against the HPV 16 oncoproteins E6 and E7 were detected in the blood of 64% of the cancer cases.19 In a case control study, having 6 or more lifetime oral-sex partners was associated with a 3.4-fold (95% confidence interval, 1.5 to 6.5) increased risk of developing oropharyngeal cancer.19
According to a population survey, 10% of men and 3.6% of women harbor HPV viruses in their oropharynx.20 In this study approximately 50% of the HPV viruses detected were high-risk types, with the following rank-order prevalence from highest to lowest: 16, 66, 51, 39, 56, 52, 59, 18, 53, 45, 35, 33, and 31.20 Theoretically, the 9-valent vaccine, with protection against HPV types 16, 18, 31, 33, 45, and 52, may be an optimal choice to prevent HPV-induced oropharyngeal cancer because of its broad coverage.
No study has yet proven that HPV vaccination reduces the risk of developing oropharyngeal cancer, but one study demonstrated that vaccination of girls against HPV types 16 and 18 reduced oral carriage of HPV 16 and HPV 18 by 93%.21 Vaccinating boys against HPV has been reported to be cost effective because it could reduce the high health care expenditures associated with treating oropharyngeal cancer.22
Will you be an immunization champion?
Although HPV vaccination reduces the disease burden of cervical, vulvar, vaginal, and anal neoplasia, the CDC reported that, as of 2013, only 38% of girls and 14% of boys in the United States had received 3 doses of HPV vaccine.23 The realization that oropharyngeal cancer caused by HPV is rapidly increasing may provide another catalyst to redouble our efforts to increase the vaccination rates for both boys and girls.
zur Hausen and many other experts have passionately advocated for vaccinating all boys and girls in order to maximize the beneficial effects of HPV vaccination.24 Every clinician can become an immunization champion by advocating that all boys and girls be vaccinated against HPV.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. zur Hausen H, Schulte-Holthausen H, Klein G, et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228(5276):1056–1058.
2. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-specific DNA sequences in human tumors: I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650–656.
3. Gissmann L, deVilliers EM, zur Hausen H. Analysis of human genital warts (condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int J Cancer. 1982;29(2):143–146.
4. Dürst M, Gissman L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA. 1983;80(12):3812–3815.
5. Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheulen W, zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3(5):1151–1157.
6. Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of human papillomavirus type 16 are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–4423.
7. Serrano B, Alemany L. Tous S, et al. Potential impact of a 9-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7(1):38.
8. Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomized study in young women. Lancet. 2009;374(9686):301–314.
9. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943.
10. FUTURE II Study Group. Quadrivalent vaccine against human papilloma virus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927.
11. Joura EA, Giuliano AR, Iversen OE, et al; Broad Spectrum HPV Vaccine Study. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8): 711–723.
12. Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012; 30(suppl 5):F12–F23.
13. de Sanjose S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49(16):3450–3461.
14. Alemany L, Saunier M, Alvarado-Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107.
15. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–1585.
16. Kreimer AR, González P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12(9):862–870.
17. Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20(4):449–457.
18. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301.
19. D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956.
20. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012; 307(7):693–703.
21. Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomised clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329.
22. Graham DM, Isaranuwatchai W, Habbous S, et al. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer [published online ahead of print April 13, 2015]. Cancer. doi: 10.1002/cncr.29111.
23. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624.
24. Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
When Dr. Harald zur Hausen received the 2008 Nobel Prize in Physiology or Medicine for his discovery of the link between human papillomavirus (HPV) infections and genital cancers, he completed a 40-year odyssey to prove that viruses caused human cancer. Initially, zur Hausen, working in the University of Pennsylvania laboratory of the noted virologists Drs. Werner and Gertrude Henle, discovered that the Epstein-Barr virus was involved in the development of Burkitt lymphoma.1 On return to his native Germany, he sought a link between HPV and genital tumors.2
First he isolated HPV 6 and HPV 11 directly from genital warts.3 Then zur Hausen utilized the nucleic acid sequences from HPV6 and the technique of low stringency hybridization to discover HPV 16 and HPV 18 in cervical cancer specimens.4,5 Oncogenic HPV DNA contains 2 genes that produce the oncoproteins E6 and E7. E6 increases the degradation of p53 and E7 inactivates the retino-blastoma protein.6 The double-hit inactivation of 2 tumor suppressor genes, p53 and retinoblastoma protein, increases the mitotic activity of the infected cells, eventually leading to cancer.
zur Hausen tried to persuade companies to develop anti-HPV vaccines but was rebuffed for years. Today, building on his research, we have HPV vaccines that are 2-valent (against HPV types 16 and 18), 4-valent (against HPV types 6, 11, 16, and 18), and 9-valent (against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58). zur Hausen richly deserved the Nobel Prize for his life-saving discoveries.
Cervical, vulvar, and vaginal cancers
HPV types 16 and 18 cause about 70% of cervical cancers. HPV types 31, 33, 45, 52, and 58 cause about 20% of cervical cancers.7 The 2-, 4-, and 9-valent HPV vaccines have been demonstrated to prevent premalignant cervical disease, including cervical intraepithelial neoplasia (CIN) 2 and CIN 3 and adenocarcinoma in situ.8–11 The development of a 9-valent HPV vaccine is an important advance because it provides more complete immunization against cancer causing viruses.
Approximately 70% of vaginal cancers are caused by HPV infections.12 Among squamous cell vulvar cancers, HPV is detected in approximately 70% of cancers with warty or basaloid histology and 12% of cancers with keratinizing histology.13 In vulvar cancer, HPV 16, 33, and 18 are the most common types detected, representing 73%, 7%, and 5% of cases, respectively. The HPV 4- and 9-valent vaccines have been reported to reduce precancerous lesions of the vagina and vulva.9,11 In most trials, vaccinations that occur before exposure to HPV through sexual encounters appear to provide greater protection than vaccinations that occur after HPV infection.
Anal cancer
Approximately 90% of anal cancers are caused by HPV infection, and HPV types 16 and 18 are detected in 81% and 4% of anal cancers, respectively.14 Among men who have sex with men, the HPV 4-valent vaccine reduced the rate of anal intraepithelial neoplasia, a precursor to anal cancer, by 50%.15 Women receiving the HPV 2-valent vaccine had an 84% reduction in the detection of anal cancer involving HPV types 16 and 18.16
Penile cancer
Approximately 48% of penile cancers harbor oncogenic HPV types.17 Among penile cancers the prevalence of HPV varies from 22% in verrucous cancer to 66% in basaloid and warty cancer. The most prevalent HPV types were 16, 6, and 18, which were observed in 31%, 7%, and 7% of the cancers, respectively.17 Penile cancer is not common and there are no studies directly demonstrating that HPV vaccination prevents penile cancer.
Oropharyngeal cancer
The rate of oropharyngeal cancer caused by HPV is rising rapidly and increasing more rapidly among men than among women.18 Remarkably, HPV-induced oropharyngeal cancer is projected to become more common than cervical cancer in 2020.18
In one report, 72% of oropharyngeal cancers harbored HPV 16, and antibodies against the HPV 16 oncoproteins E6 and E7 were detected in the blood of 64% of the cancer cases.19 In a case control study, having 6 or more lifetime oral-sex partners was associated with a 3.4-fold (95% confidence interval, 1.5 to 6.5) increased risk of developing oropharyngeal cancer.19
According to a population survey, 10% of men and 3.6% of women harbor HPV viruses in their oropharynx.20 In this study approximately 50% of the HPV viruses detected were high-risk types, with the following rank-order prevalence from highest to lowest: 16, 66, 51, 39, 56, 52, 59, 18, 53, 45, 35, 33, and 31.20 Theoretically, the 9-valent vaccine, with protection against HPV types 16, 18, 31, 33, 45, and 52, may be an optimal choice to prevent HPV-induced oropharyngeal cancer because of its broad coverage.
No study has yet proven that HPV vaccination reduces the risk of developing oropharyngeal cancer, but one study demonstrated that vaccination of girls against HPV types 16 and 18 reduced oral carriage of HPV 16 and HPV 18 by 93%.21 Vaccinating boys against HPV has been reported to be cost effective because it could reduce the high health care expenditures associated with treating oropharyngeal cancer.22
Will you be an immunization champion?
Although HPV vaccination reduces the disease burden of cervical, vulvar, vaginal, and anal neoplasia, the CDC reported that, as of 2013, only 38% of girls and 14% of boys in the United States had received 3 doses of HPV vaccine.23 The realization that oropharyngeal cancer caused by HPV is rapidly increasing may provide another catalyst to redouble our efforts to increase the vaccination rates for both boys and girls.
zur Hausen and many other experts have passionately advocated for vaccinating all boys and girls in order to maximize the beneficial effects of HPV vaccination.24 Every clinician can become an immunization champion by advocating that all boys and girls be vaccinated against HPV.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
When Dr. Harald zur Hausen received the 2008 Nobel Prize in Physiology or Medicine for his discovery of the link between human papillomavirus (HPV) infections and genital cancers, he completed a 40-year odyssey to prove that viruses caused human cancer. Initially, zur Hausen, working in the University of Pennsylvania laboratory of the noted virologists Drs. Werner and Gertrude Henle, discovered that the Epstein-Barr virus was involved in the development of Burkitt lymphoma.1 On return to his native Germany, he sought a link between HPV and genital tumors.2
First he isolated HPV 6 and HPV 11 directly from genital warts.3 Then zur Hausen utilized the nucleic acid sequences from HPV6 and the technique of low stringency hybridization to discover HPV 16 and HPV 18 in cervical cancer specimens.4,5 Oncogenic HPV DNA contains 2 genes that produce the oncoproteins E6 and E7. E6 increases the degradation of p53 and E7 inactivates the retino-blastoma protein.6 The double-hit inactivation of 2 tumor suppressor genes, p53 and retinoblastoma protein, increases the mitotic activity of the infected cells, eventually leading to cancer.
zur Hausen tried to persuade companies to develop anti-HPV vaccines but was rebuffed for years. Today, building on his research, we have HPV vaccines that are 2-valent (against HPV types 16 and 18), 4-valent (against HPV types 6, 11, 16, and 18), and 9-valent (against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58). zur Hausen richly deserved the Nobel Prize for his life-saving discoveries.
Cervical, vulvar, and vaginal cancers
HPV types 16 and 18 cause about 70% of cervical cancers. HPV types 31, 33, 45, 52, and 58 cause about 20% of cervical cancers.7 The 2-, 4-, and 9-valent HPV vaccines have been demonstrated to prevent premalignant cervical disease, including cervical intraepithelial neoplasia (CIN) 2 and CIN 3 and adenocarcinoma in situ.8–11 The development of a 9-valent HPV vaccine is an important advance because it provides more complete immunization against cancer causing viruses.
Approximately 70% of vaginal cancers are caused by HPV infections.12 Among squamous cell vulvar cancers, HPV is detected in approximately 70% of cancers with warty or basaloid histology and 12% of cancers with keratinizing histology.13 In vulvar cancer, HPV 16, 33, and 18 are the most common types detected, representing 73%, 7%, and 5% of cases, respectively. The HPV 4- and 9-valent vaccines have been reported to reduce precancerous lesions of the vagina and vulva.9,11 In most trials, vaccinations that occur before exposure to HPV through sexual encounters appear to provide greater protection than vaccinations that occur after HPV infection.
Anal cancer
Approximately 90% of anal cancers are caused by HPV infection, and HPV types 16 and 18 are detected in 81% and 4% of anal cancers, respectively.14 Among men who have sex with men, the HPV 4-valent vaccine reduced the rate of anal intraepithelial neoplasia, a precursor to anal cancer, by 50%.15 Women receiving the HPV 2-valent vaccine had an 84% reduction in the detection of anal cancer involving HPV types 16 and 18.16
Penile cancer
Approximately 48% of penile cancers harbor oncogenic HPV types.17 Among penile cancers the prevalence of HPV varies from 22% in verrucous cancer to 66% in basaloid and warty cancer. The most prevalent HPV types were 16, 6, and 18, which were observed in 31%, 7%, and 7% of the cancers, respectively.17 Penile cancer is not common and there are no studies directly demonstrating that HPV vaccination prevents penile cancer.
Oropharyngeal cancer
The rate of oropharyngeal cancer caused by HPV is rising rapidly and increasing more rapidly among men than among women.18 Remarkably, HPV-induced oropharyngeal cancer is projected to become more common than cervical cancer in 2020.18
In one report, 72% of oropharyngeal cancers harbored HPV 16, and antibodies against the HPV 16 oncoproteins E6 and E7 were detected in the blood of 64% of the cancer cases.19 In a case control study, having 6 or more lifetime oral-sex partners was associated with a 3.4-fold (95% confidence interval, 1.5 to 6.5) increased risk of developing oropharyngeal cancer.19
According to a population survey, 10% of men and 3.6% of women harbor HPV viruses in their oropharynx.20 In this study approximately 50% of the HPV viruses detected were high-risk types, with the following rank-order prevalence from highest to lowest: 16, 66, 51, 39, 56, 52, 59, 18, 53, 45, 35, 33, and 31.20 Theoretically, the 9-valent vaccine, with protection against HPV types 16, 18, 31, 33, 45, and 52, may be an optimal choice to prevent HPV-induced oropharyngeal cancer because of its broad coverage.
No study has yet proven that HPV vaccination reduces the risk of developing oropharyngeal cancer, but one study demonstrated that vaccination of girls against HPV types 16 and 18 reduced oral carriage of HPV 16 and HPV 18 by 93%.21 Vaccinating boys against HPV has been reported to be cost effective because it could reduce the high health care expenditures associated with treating oropharyngeal cancer.22
Will you be an immunization champion?
Although HPV vaccination reduces the disease burden of cervical, vulvar, vaginal, and anal neoplasia, the CDC reported that, as of 2013, only 38% of girls and 14% of boys in the United States had received 3 doses of HPV vaccine.23 The realization that oropharyngeal cancer caused by HPV is rapidly increasing may provide another catalyst to redouble our efforts to increase the vaccination rates for both boys and girls.
zur Hausen and many other experts have passionately advocated for vaccinating all boys and girls in order to maximize the beneficial effects of HPV vaccination.24 Every clinician can become an immunization champion by advocating that all boys and girls be vaccinated against HPV.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. zur Hausen H, Schulte-Holthausen H, Klein G, et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228(5276):1056–1058.
2. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-specific DNA sequences in human tumors: I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650–656.
3. Gissmann L, deVilliers EM, zur Hausen H. Analysis of human genital warts (condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int J Cancer. 1982;29(2):143–146.
4. Dürst M, Gissman L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA. 1983;80(12):3812–3815.
5. Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheulen W, zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3(5):1151–1157.
6. Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of human papillomavirus type 16 are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–4423.
7. Serrano B, Alemany L. Tous S, et al. Potential impact of a 9-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7(1):38.
8. Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomized study in young women. Lancet. 2009;374(9686):301–314.
9. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943.
10. FUTURE II Study Group. Quadrivalent vaccine against human papilloma virus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927.
11. Joura EA, Giuliano AR, Iversen OE, et al; Broad Spectrum HPV Vaccine Study. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8): 711–723.
12. Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012; 30(suppl 5):F12–F23.
13. de Sanjose S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49(16):3450–3461.
14. Alemany L, Saunier M, Alvarado-Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107.
15. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–1585.
16. Kreimer AR, González P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12(9):862–870.
17. Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20(4):449–457.
18. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301.
19. D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956.
20. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012; 307(7):693–703.
21. Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomised clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329.
22. Graham DM, Isaranuwatchai W, Habbous S, et al. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer [published online ahead of print April 13, 2015]. Cancer. doi: 10.1002/cncr.29111.
23. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624.
24. Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
1. zur Hausen H, Schulte-Holthausen H, Klein G, et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228(5276):1056–1058.
2. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-specific DNA sequences in human tumors: I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650–656.
3. Gissmann L, deVilliers EM, zur Hausen H. Analysis of human genital warts (condylomata acuminata) and other genital tumors for human papillomavirus type 6 DNA. Int J Cancer. 1982;29(2):143–146.
4. Dürst M, Gissman L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci USA. 1983;80(12):3812–3815.
5. Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheulen W, zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3(5):1151–1157.
6. Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of human papillomavirus type 16 are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–4423.
7. Serrano B, Alemany L. Tous S, et al. Potential impact of a 9-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7(1):38.
8. Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomized study in young women. Lancet. 2009;374(9686):301–314.
9. Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943.
10. FUTURE II Study Group. Quadrivalent vaccine against human papilloma virus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927.
11. Joura EA, Giuliano AR, Iversen OE, et al; Broad Spectrum HPV Vaccine Study. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8): 711–723.
12. Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012; 30(suppl 5):F12–F23.
13. de Sanjose S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49(16):3450–3461.
14. Alemany L, Saunier M, Alvarado-Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107.
15. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–1585.
16. Kreimer AR, González P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12(9):862–870.
17. Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20(4):449–457.
18. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301.
19. D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956.
20. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012; 307(7):693–703.
21. Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomised clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329.
22. Graham DM, Isaranuwatchai W, Habbous S, et al. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer [published online ahead of print April 13, 2015]. Cancer. doi: 10.1002/cncr.29111.
23. Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624.
24. Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
Why is obstetrics and gynecology a popular career choice for medical students?
Every year graduating medical students participate in the exciting, challenging, and anxiety-provoking process of applying to residency programs. After thousands of miles of travel, dozens of hotel overnight stays, and many interviews, the students are matched to their residency training site and begin specialty training. The first residency “Match” (conducted by the National Resident Matching Program) occurred in 1952 with 6,000 applicants and 10,400 available PGY-1 positions. In the 2014 Match, 34,270 applicants vied for 26,678 PGY-1 positions.1
Of great interest to medical students and educators are the relative balance of applicants and residency positions in each specialty, and the magnitude of risk that a US medical student will not match to his or her chosen specialty. For students who have devoted years preparing for residency training in a chosen specialty the day they learn that they have not matched is heartbreaking, painful, and a test of their resilience. The Match does sponsor a supplemental offer and acceptance program that helps unmatched applicants to identify unfilled positions. This process helps unmatched applicants to continue their professional development without a delay.
When researching for this editorial, I consulted the National Resident Matching Program’s Results and Data 2014 Main Residency Match.1 The TABLE shows the percentage of US medical school seniors who ranked a specialty as their only choice and did not match. The fields of neurosurgery, otolaryngology, plastic surgery, and orthopedic surgery had the greatest number of US medical school seniors (more than 17%) who ranked a specialty as their only choice and did not match in 2014. The fields of physical medicine and rehabilitation, dermatology, general surgery-categorical, obstetrics and gynecology, and radiation oncology had 6% to 11% of US seniors who ranked a specialty as their only choice not match in 2014. By contrast, almost all US applicants successfully matched in the fields of medicine-pediatrics, diagnostic radiology, anesthesiology, pathology, internal medicine-categorical, neurology, pediatrics-categorical, family medicine, emergency medicine, and psychiatry.
Clearly, obstetrics and gynecology is a popular career choice among medical students. Why might that be so?
Deeply meaningful relationships, continuity of care, plus surgical challenges
Students select a career in obstetrics and gynecology for many reasons. During their clinical experience in obstetrics and gynecology students often experience deeply meaningful relationships with patients at poignant life milestones, including conception, birth, and major surgery. In addition, students recognize that the field offers the opportunity to develop continuity relationships with patients and perform surgical procedures. Primary care specialists often develop deeply rewarding relationships with patients and their families that extend over decades, but they do not perform many surgical procedures. Procedure specialists, including general and orthopedic surgeons, perform hundreds of operations each year, but seldom have the opportunity to develop relationships with patients that last decades. Obstetrics and gynecology offers the combination of long-term continuity relationships with patients and training in surgical procedures.
Many other aspects of the field are attractive to students. Students report that their passion for the field was catalyzed by many factors and experiences, including:
- experiences with obstetricians and gynecologists who were superb role models
- the opportunity to support women and advocate for their needs over an entire lifetime
- the challenge of integrating unique cultural and religious perspectives with the medicine of family planning, sexuality, fertility, and birth
- the scientific and technical complexity of rapidly evolving diagnostic, medical, and surgical treatments, including comprehensive genetic testing and minimally invasive surgery techniques
- the opportunity to care for underserved women both domestically and globally
- delivering babies!
Renew your enthusiasm for our field—mentor!
For practicing obstetricians and gynecologists the combined challenges of complex cases with unfortunate clinical outcomes, ever growing administrative burdens, and the difficulty of balancing work and personal-life may cause them to doubt the wisdom of choosing to train in the field. One of the best ways to erase these doubts is to mentor one of the about
1,250 newly minted physicians who will start their training in obstetrics and gynecology in the summer of 2015 or one of the approximately 1,300 US medical students who will apply to enter the field in 2016.
Medical students have a world of opportunity in front of them, with dozens of exciting career options. The fact that so many students select a career in our field is heartening. These students will become excellent obstetrician-gynecologistsand dedicate themselves to advancingthe health of the 150 million women in the United States.
Would you select a career in obstetrics and gynecology again? Answer the Quick Poll on the home page and see how others have voted.
Why did you select a career in obstetrics and gynecology? Tell us at [email protected] Please include your name and city and state.
Reference
1. National Resident Matching Program. Results and Data: 2014 Main Residency Match. National Resident Matching Program; Washington DC. April 2014.
Every year graduating medical students participate in the exciting, challenging, and anxiety-provoking process of applying to residency programs. After thousands of miles of travel, dozens of hotel overnight stays, and many interviews, the students are matched to their residency training site and begin specialty training. The first residency “Match” (conducted by the National Resident Matching Program) occurred in 1952 with 6,000 applicants and 10,400 available PGY-1 positions. In the 2014 Match, 34,270 applicants vied for 26,678 PGY-1 positions.1
Of great interest to medical students and educators are the relative balance of applicants and residency positions in each specialty, and the magnitude of risk that a US medical student will not match to his or her chosen specialty. For students who have devoted years preparing for residency training in a chosen specialty the day they learn that they have not matched is heartbreaking, painful, and a test of their resilience. The Match does sponsor a supplemental offer and acceptance program that helps unmatched applicants to identify unfilled positions. This process helps unmatched applicants to continue their professional development without a delay.
When researching for this editorial, I consulted the National Resident Matching Program’s Results and Data 2014 Main Residency Match.1 The TABLE shows the percentage of US medical school seniors who ranked a specialty as their only choice and did not match. The fields of neurosurgery, otolaryngology, plastic surgery, and orthopedic surgery had the greatest number of US medical school seniors (more than 17%) who ranked a specialty as their only choice and did not match in 2014. The fields of physical medicine and rehabilitation, dermatology, general surgery-categorical, obstetrics and gynecology, and radiation oncology had 6% to 11% of US seniors who ranked a specialty as their only choice not match in 2014. By contrast, almost all US applicants successfully matched in the fields of medicine-pediatrics, diagnostic radiology, anesthesiology, pathology, internal medicine-categorical, neurology, pediatrics-categorical, family medicine, emergency medicine, and psychiatry.
Clearly, obstetrics and gynecology is a popular career choice among medical students. Why might that be so?
Deeply meaningful relationships, continuity of care, plus surgical challenges
Students select a career in obstetrics and gynecology for many reasons. During their clinical experience in obstetrics and gynecology students often experience deeply meaningful relationships with patients at poignant life milestones, including conception, birth, and major surgery. In addition, students recognize that the field offers the opportunity to develop continuity relationships with patients and perform surgical procedures. Primary care specialists often develop deeply rewarding relationships with patients and their families that extend over decades, but they do not perform many surgical procedures. Procedure specialists, including general and orthopedic surgeons, perform hundreds of operations each year, but seldom have the opportunity to develop relationships with patients that last decades. Obstetrics and gynecology offers the combination of long-term continuity relationships with patients and training in surgical procedures.
Many other aspects of the field are attractive to students. Students report that their passion for the field was catalyzed by many factors and experiences, including:
- experiences with obstetricians and gynecologists who were superb role models
- the opportunity to support women and advocate for their needs over an entire lifetime
- the challenge of integrating unique cultural and religious perspectives with the medicine of family planning, sexuality, fertility, and birth
- the scientific and technical complexity of rapidly evolving diagnostic, medical, and surgical treatments, including comprehensive genetic testing and minimally invasive surgery techniques
- the opportunity to care for underserved women both domestically and globally
- delivering babies!
Renew your enthusiasm for our field—mentor!
For practicing obstetricians and gynecologists the combined challenges of complex cases with unfortunate clinical outcomes, ever growing administrative burdens, and the difficulty of balancing work and personal-life may cause them to doubt the wisdom of choosing to train in the field. One of the best ways to erase these doubts is to mentor one of the about
1,250 newly minted physicians who will start their training in obstetrics and gynecology in the summer of 2015 or one of the approximately 1,300 US medical students who will apply to enter the field in 2016.
Medical students have a world of opportunity in front of them, with dozens of exciting career options. The fact that so many students select a career in our field is heartening. These students will become excellent obstetrician-gynecologistsand dedicate themselves to advancingthe health of the 150 million women in the United States.
Would you select a career in obstetrics and gynecology again? Answer the Quick Poll on the home page and see how others have voted.
Why did you select a career in obstetrics and gynecology? Tell us at [email protected] Please include your name and city and state.
Every year graduating medical students participate in the exciting, challenging, and anxiety-provoking process of applying to residency programs. After thousands of miles of travel, dozens of hotel overnight stays, and many interviews, the students are matched to their residency training site and begin specialty training. The first residency “Match” (conducted by the National Resident Matching Program) occurred in 1952 with 6,000 applicants and 10,400 available PGY-1 positions. In the 2014 Match, 34,270 applicants vied for 26,678 PGY-1 positions.1
Of great interest to medical students and educators are the relative balance of applicants and residency positions in each specialty, and the magnitude of risk that a US medical student will not match to his or her chosen specialty. For students who have devoted years preparing for residency training in a chosen specialty the day they learn that they have not matched is heartbreaking, painful, and a test of their resilience. The Match does sponsor a supplemental offer and acceptance program that helps unmatched applicants to identify unfilled positions. This process helps unmatched applicants to continue their professional development without a delay.
When researching for this editorial, I consulted the National Resident Matching Program’s Results and Data 2014 Main Residency Match.1 The TABLE shows the percentage of US medical school seniors who ranked a specialty as their only choice and did not match. The fields of neurosurgery, otolaryngology, plastic surgery, and orthopedic surgery had the greatest number of US medical school seniors (more than 17%) who ranked a specialty as their only choice and did not match in 2014. The fields of physical medicine and rehabilitation, dermatology, general surgery-categorical, obstetrics and gynecology, and radiation oncology had 6% to 11% of US seniors who ranked a specialty as their only choice not match in 2014. By contrast, almost all US applicants successfully matched in the fields of medicine-pediatrics, diagnostic radiology, anesthesiology, pathology, internal medicine-categorical, neurology, pediatrics-categorical, family medicine, emergency medicine, and psychiatry.
Clearly, obstetrics and gynecology is a popular career choice among medical students. Why might that be so?
Deeply meaningful relationships, continuity of care, plus surgical challenges
Students select a career in obstetrics and gynecology for many reasons. During their clinical experience in obstetrics and gynecology students often experience deeply meaningful relationships with patients at poignant life milestones, including conception, birth, and major surgery. In addition, students recognize that the field offers the opportunity to develop continuity relationships with patients and perform surgical procedures. Primary care specialists often develop deeply rewarding relationships with patients and their families that extend over decades, but they do not perform many surgical procedures. Procedure specialists, including general and orthopedic surgeons, perform hundreds of operations each year, but seldom have the opportunity to develop relationships with patients that last decades. Obstetrics and gynecology offers the combination of long-term continuity relationships with patients and training in surgical procedures.
Many other aspects of the field are attractive to students. Students report that their passion for the field was catalyzed by many factors and experiences, including:
- experiences with obstetricians and gynecologists who were superb role models
- the opportunity to support women and advocate for their needs over an entire lifetime
- the challenge of integrating unique cultural and religious perspectives with the medicine of family planning, sexuality, fertility, and birth
- the scientific and technical complexity of rapidly evolving diagnostic, medical, and surgical treatments, including comprehensive genetic testing and minimally invasive surgery techniques
- the opportunity to care for underserved women both domestically and globally
- delivering babies!
Renew your enthusiasm for our field—mentor!
For practicing obstetricians and gynecologists the combined challenges of complex cases with unfortunate clinical outcomes, ever growing administrative burdens, and the difficulty of balancing work and personal-life may cause them to doubt the wisdom of choosing to train in the field. One of the best ways to erase these doubts is to mentor one of the about
1,250 newly minted physicians who will start their training in obstetrics and gynecology in the summer of 2015 or one of the approximately 1,300 US medical students who will apply to enter the field in 2016.
Medical students have a world of opportunity in front of them, with dozens of exciting career options. The fact that so many students select a career in our field is heartening. These students will become excellent obstetrician-gynecologistsand dedicate themselves to advancingthe health of the 150 million women in the United States.
Would you select a career in obstetrics and gynecology again? Answer the Quick Poll on the home page and see how others have voted.
Why did you select a career in obstetrics and gynecology? Tell us at [email protected] Please include your name and city and state.
Reference
1. National Resident Matching Program. Results and Data: 2014 Main Residency Match. National Resident Matching Program; Washington DC. April 2014.
Reference
1. National Resident Matching Program. Results and Data: 2014 Main Residency Match. National Resident Matching Program; Washington DC. April 2014.
Uterus transplantation: Medical breakthrough or surgical folly?
Case: Patient asks for transplantation referral
During an annual ObGyn visit, a 28-year-old G0 with congenital absence of the uterus excitedly tells you about the news report of the first birth following uterus transplantation. She always has dreamed of becoming pregnant, and this medical breakthrough has spurred her imagination of what might be. You ask if she would consider adoption or a gestational carrier. Responding that she prefers to carry her own pregnancy, she asks you to refer her to a uterus transplantation program. You promise to look into this option for her. As she opens the door to leave your office, she mentions that her mother has volunteered to be the uterus donor.
Later, you have misgivings about making a referral for uterus transplantation. You wonder: Is this procedure an appropriate use of health care resources? Do its risks outweigh the benefits?
In September 2014, a 36-year-old Swedish woman gave birth following uterus transplantation. A 61-year-old family friend donated the uterus for the procedure.1 Prior to this breakthrough, women without a uterus had 3 reproductive alternatives: remain childless, adopt a child, or use a gestational carrier to give birth to their child. In many countries and some religions there are prohibitions against the use of a gestational carrier, leaving adoption as the only option to parenthood.
The first successful uterus transplantation did not occur by serendipity; a decade of careful work led to this breakthrough.2–4 Remarkably, it is now proven that this type of transplantation can result in the successful birth of a baby—but at what cost?
The Brännström Uterus Transplantation Program: A medical breakthrough
Dr. Mats Brännström at the University of Gothenburg, Sweden, is the leader of the courageous and innovative team that developed the world’s first successful uterus transplantation program. The team required a broad range of expertise and skills and included physicians, scientists, and support staff from Sahlgrenska University Hospital and Stockholm IVF in Sweden; University of Valencia, Spain; Griffith University, Australia; and the Cleveland Clinic, Florida. Two recent publications report on the outcomes of the first 9 uterus transplants.5,6
The successful protocol. The first step in the program is an exhaustive medical and psychosocial evaluation of the prospective uterus donor and recipient. Among the first 9 uterus recipients, 8 women had congenital absence of the uterus and 1 woman had a hysterectomy for cervical cancer. The uterus donors were mothers (in 5 cases), a mother-in-law, a sister, an aunt, and a friend.
After the recipient is approved for uterus transplantation, she undergoes in vitro fertilization (IVF) with cryopreservation of all embryos. IVF is recommended because it may not be possible to include the fallopian tubes in the uterus transplant or the tubes may not function properly following transplantation. The donor organ is harvested, using a modified radical hysterectomy with extended vascular pedicles, and transplanted into the pelvis of the recipient.
Following transplantation, immunosuppressive medications are prescribed daily to reduce the risk of organ rejection. The recipient is followed on a regular basis with physical examination and cervical biopsy to identify histologic markers of organ rejection. Episodes of rejection are treated with glucocorticoids and adjustment in the dose of immunosuppression medications. Fertility treatment with the recipient’s previously cryopreserved embryo begins 1 year following transplantation.
A unique feature of uterus transplantation is that the organ can be removed after childbearing is complete, thereby limiting lifetime exposure to immunosuppressive medications.
Uterus transplantation: Surgical folly?
Transplantation of a uterus involves major surgery. The inescapable reality is that the procedure will cause complications in some donors and recipients.
Specific complications faced. In the Brännström series, 1 uterus donor developed a postoperative ureterovaginal fistula, likely caused by extensive dissection of her ureters. This donor needed an additional operation to repair the fistula. Two of the 9 uterus transplants failed. One uterus was removed from the recipient 3 days after transplantation due to vascular occlusion and 1 uterus was removed 105 days after transplantation due to chronic infection resistant to antibiotic treatment. Seven of the transplants were successful and functioning in situ 12 months after transplantation as evidenced by regular menstrual bleeding. Five of the 7 recipients had rejection episodes, as demonstrated by the histology of cervix biopsies. Two of the recipients had 3 episodes of rejection. The rejection episodes were treated successfully with glucocorticoids and adjustment of immunosuppression medications.
Pregnancy in women with uterus transplantation is high risk because of the complications caused by immunosuppressive drugs and the high blood flow through the vascular grafts.7–9 In the Brännström series, the agents utilized for immunosuppression included mycophenolate mofetil, azathioprine, tacrolimus, and glucocorticoids. Mycophenolate mofetil is a potent teratogen and routinely is discontinued prior to initiating attempts at pregnancy. Azathioprine is associated with an increased rate of congenital anomalies, but the benefits of this immunosuppressive are believed to outweigh the risks for most pregnant women with an organ transplant. Tacrolimus increases the risk of developing hypertension, preeclampsia, and intrauterine growth restriction during pregnancy.
In the Brännström case report, the woman who became pregnant following uterus transplantation took tacrolimus and azathioprine to prevent organ rejection both before and during her pregnancy. Not unexpectedly, she developed preeclampsia with severe features at 31 weeks and 5 days. After admission to the hospital, a worrisome fetal heart rate pattern developed and a cesarean delivery was performed. The newborn male weighed 1,775 g, and no congenital anomalies were observed. During pregnancy, blood flow to the uterus is in the range of 500 mL/min, the equivalent of 1 unit of whole blood per minute.10 This torrential pulsating flow may increase the risk of a vascular catastrophe such as the rupture of a major artery at one of the graft anastomoses, potentially causing the death of the fetus or mother. Much more experience will be needed to fully characterize the pattern of pregnancy complications that occurs following uterus transplantation.
The cost issue. Uterus transplantation is an extremely expensive medical procedure. In the United States each transplantation is likely to cost hundreds of thousands of dollars. Health care resources used to support uterus transplantation are not available for other pressing medical needs. Given that it is an experimental procedure, it is unlikely that health insurance will reimburse the costs of the medical care. Transplantation programs will need to seek major donors to support the costs, as was done in the Brännström program, or identify patients capable of paying for the transplant. If programs plan to have most patients pay for the procedure, bioethical concerns of equitable access and fair selection of recipients will need to be addressed.
Ethics. Uterus transplantation raises many bioethical concerns and programs need to engage biomedical ethicists to guide their activities.11–13 Careful attention to thorough informed consent, risk-benefit analysis, equitable access, and fair selection of participants will be critical to running an ethical program. To reduce the risks of the procedure, programs likely will explore the use of uteri obtained from women who are brain dead or cadavers to spare altruistic living donors from undergoing hysterectomy surgery.
“Group of fools” or Nobel Prize in wait?
On December 23, 1954, the first successful kidney transplant was performed by Dr. Joseph E. Murray and his team at the Peter Bent Brigham Hospital, a predecessor to the Brigham and Women’s Hospital.14 His small group of physicians worked for years to perfect the kidney transplantation technique in the laboratory prior to attempting the case. A key to their success was the decision to perform the transplant with identical twins as the donor and recipient.
In the 1950s there was great controversy about whether kidney transplantation was a medical breakthrough or surgical folly. The lead surgical team was referred to as the “group of fools” by some colleagues. But Dr. Murray and his team succeeded in their efforts and opened the field of solid organ transplant. Recognizing the importance of his accomplishment, the Nobel Prize Committee awarded Dr. Murray the 1990 Nobel Prize in Physiology or Medicine. Dr. E. Donnell Thomas, a co-recipient of the award, was simultaneously recognized for developing bone marrow transplantation as a treatment for leukemia.
A medical breakthrough…
Organ transplantation medicine initially focused on the treatment of life-threatening diseases, including kidney, heart, lung, and liver failure. With recent innovations in composite tissue transplants, including face and limb, transplantation medicine is evolving to expand its focus to the repair of functional deficits that are not life threatening but do significantly impact quality of life. Uterus transplantation is an example of the new era of using transplants to repair functional deficits. The clinicians and patients involved in these innovative programs are courageous pioneers opening new vistas and helping to realize previously impossible dreams. In our time, many stakeholders are likely to conclude that uterus transplantation is a surgical folly. However, I predict that our children will conclude that uterus transplantation represents a medical breakthrough.
Share your thoughts on this article! Send your Letter to the Editor to Please include your name and the city and state in which you practice.
Weigh in at the Quick Poll on the homepage. Send your answers to these cases and any comments to [email protected]. Please include your name and the city and state in which you practice.
1. Brännström M, Johannesson L, Bokstrom H, et al. Livebirth after uterus transplantation. Lancet. 2015;385(9968):607–616.
2. Johannesson L, Enskog A, Mölne J, et al. Preclinical report on allogeneic uterus transplantation in nonhuman primates. Hum Reprod. 2013;28(1):189–198.
3. Brännström M, Diaz-Garcia C, Hanafy A, Olausson M, Tzakis A. Uterus transplantation: animal research and human possibilities. Fertil Steril. 2012;97(6):1269–1276.
4. Brännström M, Wranning CA, Altchek A. Experimental uterus transplantation. Hum Reprod Update. 2010;16(3):329–345.
5. Brännström M, Johannesson L, Dahm-Kähler P, et al. First clinical uterus transplant trial: a six-month report. Fertil Steril. 2014;101(5):1228–1236.
6. Johannesson L, Kvarnstrom N, Mölne J, et al. Uterus transplantation trial: 1-year outcome. Fertil Steril. 2015;103(1):199–204.
7. Concepcion BP, Schaefer HM. Caring for the pregnant kidney transplant recipient. Clin Transplant. 2011;25(6):821–829.
8. Rupley DM, Janda AM, Kapeles SR, Wilson TM, Berman D, Mathur AK. Preconception counseling, fertility and pregnancy complications after abdominal organ transplantation: a survey and cohort study of 532 recipients. Clin Transplant. 2014;28(9):937–945.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354(12):1281–1293.
10. Metcalfe J, Romney SL, Ramsey LH, Reid DH, Burwell CS. Estimation of uterine blood flow in normal human pregnancy at term. J Clin Invest. 1955;34(11):1632–1638.
11. Olausson M, Johannesson L, Brattgård D, et al. Ethics of uterus transplantation with live donors. Fertil Steril. 2014;102(1):40–43.
12. Del Priore G, Saso S, Meslin EM, et al. Uterine transplantation—a real possibility? The Indianapolis consensus. Hum Reprod. 2013;28(2):288–291.
13. Brosens I, Ghaem-Maghami S, Pijnenborg R. Uterus transplantation in the human: a complex surgical, medical and ethical challenge. Human Reprod. 2013;28(2):292–293.
14. Desai SP, Desai MS, Wood DN, Maddi R, Leeson S, Tilney NL. A semi-centennial report on the participants depicted in Joel Babb’s portrait, “The First Successful Kidney Transplantation”. Am J Transplant. 2007;7(7):1683–1688.
Case: Patient asks for transplantation referral
During an annual ObGyn visit, a 28-year-old G0 with congenital absence of the uterus excitedly tells you about the news report of the first birth following uterus transplantation. She always has dreamed of becoming pregnant, and this medical breakthrough has spurred her imagination of what might be. You ask if she would consider adoption or a gestational carrier. Responding that she prefers to carry her own pregnancy, she asks you to refer her to a uterus transplantation program. You promise to look into this option for her. As she opens the door to leave your office, she mentions that her mother has volunteered to be the uterus donor.
Later, you have misgivings about making a referral for uterus transplantation. You wonder: Is this procedure an appropriate use of health care resources? Do its risks outweigh the benefits?
In September 2014, a 36-year-old Swedish woman gave birth following uterus transplantation. A 61-year-old family friend donated the uterus for the procedure.1 Prior to this breakthrough, women without a uterus had 3 reproductive alternatives: remain childless, adopt a child, or use a gestational carrier to give birth to their child. In many countries and some religions there are prohibitions against the use of a gestational carrier, leaving adoption as the only option to parenthood.
The first successful uterus transplantation did not occur by serendipity; a decade of careful work led to this breakthrough.2–4 Remarkably, it is now proven that this type of transplantation can result in the successful birth of a baby—but at what cost?
The Brännström Uterus Transplantation Program: A medical breakthrough
Dr. Mats Brännström at the University of Gothenburg, Sweden, is the leader of the courageous and innovative team that developed the world’s first successful uterus transplantation program. The team required a broad range of expertise and skills and included physicians, scientists, and support staff from Sahlgrenska University Hospital and Stockholm IVF in Sweden; University of Valencia, Spain; Griffith University, Australia; and the Cleveland Clinic, Florida. Two recent publications report on the outcomes of the first 9 uterus transplants.5,6
The successful protocol. The first step in the program is an exhaustive medical and psychosocial evaluation of the prospective uterus donor and recipient. Among the first 9 uterus recipients, 8 women had congenital absence of the uterus and 1 woman had a hysterectomy for cervical cancer. The uterus donors were mothers (in 5 cases), a mother-in-law, a sister, an aunt, and a friend.
After the recipient is approved for uterus transplantation, she undergoes in vitro fertilization (IVF) with cryopreservation of all embryos. IVF is recommended because it may not be possible to include the fallopian tubes in the uterus transplant or the tubes may not function properly following transplantation. The donor organ is harvested, using a modified radical hysterectomy with extended vascular pedicles, and transplanted into the pelvis of the recipient.
Following transplantation, immunosuppressive medications are prescribed daily to reduce the risk of organ rejection. The recipient is followed on a regular basis with physical examination and cervical biopsy to identify histologic markers of organ rejection. Episodes of rejection are treated with glucocorticoids and adjustment in the dose of immunosuppression medications. Fertility treatment with the recipient’s previously cryopreserved embryo begins 1 year following transplantation.
A unique feature of uterus transplantation is that the organ can be removed after childbearing is complete, thereby limiting lifetime exposure to immunosuppressive medications.
Uterus transplantation: Surgical folly?
Transplantation of a uterus involves major surgery. The inescapable reality is that the procedure will cause complications in some donors and recipients.
Specific complications faced. In the Brännström series, 1 uterus donor developed a postoperative ureterovaginal fistula, likely caused by extensive dissection of her ureters. This donor needed an additional operation to repair the fistula. Two of the 9 uterus transplants failed. One uterus was removed from the recipient 3 days after transplantation due to vascular occlusion and 1 uterus was removed 105 days after transplantation due to chronic infection resistant to antibiotic treatment. Seven of the transplants were successful and functioning in situ 12 months after transplantation as evidenced by regular menstrual bleeding. Five of the 7 recipients had rejection episodes, as demonstrated by the histology of cervix biopsies. Two of the recipients had 3 episodes of rejection. The rejection episodes were treated successfully with glucocorticoids and adjustment of immunosuppression medications.
Pregnancy in women with uterus transplantation is high risk because of the complications caused by immunosuppressive drugs and the high blood flow through the vascular grafts.7–9 In the Brännström series, the agents utilized for immunosuppression included mycophenolate mofetil, azathioprine, tacrolimus, and glucocorticoids. Mycophenolate mofetil is a potent teratogen and routinely is discontinued prior to initiating attempts at pregnancy. Azathioprine is associated with an increased rate of congenital anomalies, but the benefits of this immunosuppressive are believed to outweigh the risks for most pregnant women with an organ transplant. Tacrolimus increases the risk of developing hypertension, preeclampsia, and intrauterine growth restriction during pregnancy.
In the Brännström case report, the woman who became pregnant following uterus transplantation took tacrolimus and azathioprine to prevent organ rejection both before and during her pregnancy. Not unexpectedly, she developed preeclampsia with severe features at 31 weeks and 5 days. After admission to the hospital, a worrisome fetal heart rate pattern developed and a cesarean delivery was performed. The newborn male weighed 1,775 g, and no congenital anomalies were observed. During pregnancy, blood flow to the uterus is in the range of 500 mL/min, the equivalent of 1 unit of whole blood per minute.10 This torrential pulsating flow may increase the risk of a vascular catastrophe such as the rupture of a major artery at one of the graft anastomoses, potentially causing the death of the fetus or mother. Much more experience will be needed to fully characterize the pattern of pregnancy complications that occurs following uterus transplantation.
The cost issue. Uterus transplantation is an extremely expensive medical procedure. In the United States each transplantation is likely to cost hundreds of thousands of dollars. Health care resources used to support uterus transplantation are not available for other pressing medical needs. Given that it is an experimental procedure, it is unlikely that health insurance will reimburse the costs of the medical care. Transplantation programs will need to seek major donors to support the costs, as was done in the Brännström program, or identify patients capable of paying for the transplant. If programs plan to have most patients pay for the procedure, bioethical concerns of equitable access and fair selection of recipients will need to be addressed.
Ethics. Uterus transplantation raises many bioethical concerns and programs need to engage biomedical ethicists to guide their activities.11–13 Careful attention to thorough informed consent, risk-benefit analysis, equitable access, and fair selection of participants will be critical to running an ethical program. To reduce the risks of the procedure, programs likely will explore the use of uteri obtained from women who are brain dead or cadavers to spare altruistic living donors from undergoing hysterectomy surgery.
“Group of fools” or Nobel Prize in wait?
On December 23, 1954, the first successful kidney transplant was performed by Dr. Joseph E. Murray and his team at the Peter Bent Brigham Hospital, a predecessor to the Brigham and Women’s Hospital.14 His small group of physicians worked for years to perfect the kidney transplantation technique in the laboratory prior to attempting the case. A key to their success was the decision to perform the transplant with identical twins as the donor and recipient.
In the 1950s there was great controversy about whether kidney transplantation was a medical breakthrough or surgical folly. The lead surgical team was referred to as the “group of fools” by some colleagues. But Dr. Murray and his team succeeded in their efforts and opened the field of solid organ transplant. Recognizing the importance of his accomplishment, the Nobel Prize Committee awarded Dr. Murray the 1990 Nobel Prize in Physiology or Medicine. Dr. E. Donnell Thomas, a co-recipient of the award, was simultaneously recognized for developing bone marrow transplantation as a treatment for leukemia.
A medical breakthrough…
Organ transplantation medicine initially focused on the treatment of life-threatening diseases, including kidney, heart, lung, and liver failure. With recent innovations in composite tissue transplants, including face and limb, transplantation medicine is evolving to expand its focus to the repair of functional deficits that are not life threatening but do significantly impact quality of life. Uterus transplantation is an example of the new era of using transplants to repair functional deficits. The clinicians and patients involved in these innovative programs are courageous pioneers opening new vistas and helping to realize previously impossible dreams. In our time, many stakeholders are likely to conclude that uterus transplantation is a surgical folly. However, I predict that our children will conclude that uterus transplantation represents a medical breakthrough.
Share your thoughts on this article! Send your Letter to the Editor to Please include your name and the city and state in which you practice.
Weigh in at the Quick Poll on the homepage. Send your answers to these cases and any comments to [email protected]. Please include your name and the city and state in which you practice.
Case: Patient asks for transplantation referral
During an annual ObGyn visit, a 28-year-old G0 with congenital absence of the uterus excitedly tells you about the news report of the first birth following uterus transplantation. She always has dreamed of becoming pregnant, and this medical breakthrough has spurred her imagination of what might be. You ask if she would consider adoption or a gestational carrier. Responding that she prefers to carry her own pregnancy, she asks you to refer her to a uterus transplantation program. You promise to look into this option for her. As she opens the door to leave your office, she mentions that her mother has volunteered to be the uterus donor.
Later, you have misgivings about making a referral for uterus transplantation. You wonder: Is this procedure an appropriate use of health care resources? Do its risks outweigh the benefits?
In September 2014, a 36-year-old Swedish woman gave birth following uterus transplantation. A 61-year-old family friend donated the uterus for the procedure.1 Prior to this breakthrough, women without a uterus had 3 reproductive alternatives: remain childless, adopt a child, or use a gestational carrier to give birth to their child. In many countries and some religions there are prohibitions against the use of a gestational carrier, leaving adoption as the only option to parenthood.
The first successful uterus transplantation did not occur by serendipity; a decade of careful work led to this breakthrough.2–4 Remarkably, it is now proven that this type of transplantation can result in the successful birth of a baby—but at what cost?
The Brännström Uterus Transplantation Program: A medical breakthrough
Dr. Mats Brännström at the University of Gothenburg, Sweden, is the leader of the courageous and innovative team that developed the world’s first successful uterus transplantation program. The team required a broad range of expertise and skills and included physicians, scientists, and support staff from Sahlgrenska University Hospital and Stockholm IVF in Sweden; University of Valencia, Spain; Griffith University, Australia; and the Cleveland Clinic, Florida. Two recent publications report on the outcomes of the first 9 uterus transplants.5,6
The successful protocol. The first step in the program is an exhaustive medical and psychosocial evaluation of the prospective uterus donor and recipient. Among the first 9 uterus recipients, 8 women had congenital absence of the uterus and 1 woman had a hysterectomy for cervical cancer. The uterus donors were mothers (in 5 cases), a mother-in-law, a sister, an aunt, and a friend.
After the recipient is approved for uterus transplantation, she undergoes in vitro fertilization (IVF) with cryopreservation of all embryos. IVF is recommended because it may not be possible to include the fallopian tubes in the uterus transplant or the tubes may not function properly following transplantation. The donor organ is harvested, using a modified radical hysterectomy with extended vascular pedicles, and transplanted into the pelvis of the recipient.
Following transplantation, immunosuppressive medications are prescribed daily to reduce the risk of organ rejection. The recipient is followed on a regular basis with physical examination and cervical biopsy to identify histologic markers of organ rejection. Episodes of rejection are treated with glucocorticoids and adjustment in the dose of immunosuppression medications. Fertility treatment with the recipient’s previously cryopreserved embryo begins 1 year following transplantation.
A unique feature of uterus transplantation is that the organ can be removed after childbearing is complete, thereby limiting lifetime exposure to immunosuppressive medications.
Uterus transplantation: Surgical folly?
Transplantation of a uterus involves major surgery. The inescapable reality is that the procedure will cause complications in some donors and recipients.
Specific complications faced. In the Brännström series, 1 uterus donor developed a postoperative ureterovaginal fistula, likely caused by extensive dissection of her ureters. This donor needed an additional operation to repair the fistula. Two of the 9 uterus transplants failed. One uterus was removed from the recipient 3 days after transplantation due to vascular occlusion and 1 uterus was removed 105 days after transplantation due to chronic infection resistant to antibiotic treatment. Seven of the transplants were successful and functioning in situ 12 months after transplantation as evidenced by regular menstrual bleeding. Five of the 7 recipients had rejection episodes, as demonstrated by the histology of cervix biopsies. Two of the recipients had 3 episodes of rejection. The rejection episodes were treated successfully with glucocorticoids and adjustment of immunosuppression medications.
Pregnancy in women with uterus transplantation is high risk because of the complications caused by immunosuppressive drugs and the high blood flow through the vascular grafts.7–9 In the Brännström series, the agents utilized for immunosuppression included mycophenolate mofetil, azathioprine, tacrolimus, and glucocorticoids. Mycophenolate mofetil is a potent teratogen and routinely is discontinued prior to initiating attempts at pregnancy. Azathioprine is associated with an increased rate of congenital anomalies, but the benefits of this immunosuppressive are believed to outweigh the risks for most pregnant women with an organ transplant. Tacrolimus increases the risk of developing hypertension, preeclampsia, and intrauterine growth restriction during pregnancy.
In the Brännström case report, the woman who became pregnant following uterus transplantation took tacrolimus and azathioprine to prevent organ rejection both before and during her pregnancy. Not unexpectedly, she developed preeclampsia with severe features at 31 weeks and 5 days. After admission to the hospital, a worrisome fetal heart rate pattern developed and a cesarean delivery was performed. The newborn male weighed 1,775 g, and no congenital anomalies were observed. During pregnancy, blood flow to the uterus is in the range of 500 mL/min, the equivalent of 1 unit of whole blood per minute.10 This torrential pulsating flow may increase the risk of a vascular catastrophe such as the rupture of a major artery at one of the graft anastomoses, potentially causing the death of the fetus or mother. Much more experience will be needed to fully characterize the pattern of pregnancy complications that occurs following uterus transplantation.
The cost issue. Uterus transplantation is an extremely expensive medical procedure. In the United States each transplantation is likely to cost hundreds of thousands of dollars. Health care resources used to support uterus transplantation are not available for other pressing medical needs. Given that it is an experimental procedure, it is unlikely that health insurance will reimburse the costs of the medical care. Transplantation programs will need to seek major donors to support the costs, as was done in the Brännström program, or identify patients capable of paying for the transplant. If programs plan to have most patients pay for the procedure, bioethical concerns of equitable access and fair selection of recipients will need to be addressed.
Ethics. Uterus transplantation raises many bioethical concerns and programs need to engage biomedical ethicists to guide their activities.11–13 Careful attention to thorough informed consent, risk-benefit analysis, equitable access, and fair selection of participants will be critical to running an ethical program. To reduce the risks of the procedure, programs likely will explore the use of uteri obtained from women who are brain dead or cadavers to spare altruistic living donors from undergoing hysterectomy surgery.
“Group of fools” or Nobel Prize in wait?
On December 23, 1954, the first successful kidney transplant was performed by Dr. Joseph E. Murray and his team at the Peter Bent Brigham Hospital, a predecessor to the Brigham and Women’s Hospital.14 His small group of physicians worked for years to perfect the kidney transplantation technique in the laboratory prior to attempting the case. A key to their success was the decision to perform the transplant with identical twins as the donor and recipient.
In the 1950s there was great controversy about whether kidney transplantation was a medical breakthrough or surgical folly. The lead surgical team was referred to as the “group of fools” by some colleagues. But Dr. Murray and his team succeeded in their efforts and opened the field of solid organ transplant. Recognizing the importance of his accomplishment, the Nobel Prize Committee awarded Dr. Murray the 1990 Nobel Prize in Physiology or Medicine. Dr. E. Donnell Thomas, a co-recipient of the award, was simultaneously recognized for developing bone marrow transplantation as a treatment for leukemia.
A medical breakthrough…
Organ transplantation medicine initially focused on the treatment of life-threatening diseases, including kidney, heart, lung, and liver failure. With recent innovations in composite tissue transplants, including face and limb, transplantation medicine is evolving to expand its focus to the repair of functional deficits that are not life threatening but do significantly impact quality of life. Uterus transplantation is an example of the new era of using transplants to repair functional deficits. The clinicians and patients involved in these innovative programs are courageous pioneers opening new vistas and helping to realize previously impossible dreams. In our time, many stakeholders are likely to conclude that uterus transplantation is a surgical folly. However, I predict that our children will conclude that uterus transplantation represents a medical breakthrough.
Share your thoughts on this article! Send your Letter to the Editor to Please include your name and the city and state in which you practice.
Weigh in at the Quick Poll on the homepage. Send your answers to these cases and any comments to [email protected]. Please include your name and the city and state in which you practice.
1. Brännström M, Johannesson L, Bokstrom H, et al. Livebirth after uterus transplantation. Lancet. 2015;385(9968):607–616.
2. Johannesson L, Enskog A, Mölne J, et al. Preclinical report on allogeneic uterus transplantation in nonhuman primates. Hum Reprod. 2013;28(1):189–198.
3. Brännström M, Diaz-Garcia C, Hanafy A, Olausson M, Tzakis A. Uterus transplantation: animal research and human possibilities. Fertil Steril. 2012;97(6):1269–1276.
4. Brännström M, Wranning CA, Altchek A. Experimental uterus transplantation. Hum Reprod Update. 2010;16(3):329–345.
5. Brännström M, Johannesson L, Dahm-Kähler P, et al. First clinical uterus transplant trial: a six-month report. Fertil Steril. 2014;101(5):1228–1236.
6. Johannesson L, Kvarnstrom N, Mölne J, et al. Uterus transplantation trial: 1-year outcome. Fertil Steril. 2015;103(1):199–204.
7. Concepcion BP, Schaefer HM. Caring for the pregnant kidney transplant recipient. Clin Transplant. 2011;25(6):821–829.
8. Rupley DM, Janda AM, Kapeles SR, Wilson TM, Berman D, Mathur AK. Preconception counseling, fertility and pregnancy complications after abdominal organ transplantation: a survey and cohort study of 532 recipients. Clin Transplant. 2014;28(9):937–945.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354(12):1281–1293.
10. Metcalfe J, Romney SL, Ramsey LH, Reid DH, Burwell CS. Estimation of uterine blood flow in normal human pregnancy at term. J Clin Invest. 1955;34(11):1632–1638.
11. Olausson M, Johannesson L, Brattgård D, et al. Ethics of uterus transplantation with live donors. Fertil Steril. 2014;102(1):40–43.
12. Del Priore G, Saso S, Meslin EM, et al. Uterine transplantation—a real possibility? The Indianapolis consensus. Hum Reprod. 2013;28(2):288–291.
13. Brosens I, Ghaem-Maghami S, Pijnenborg R. Uterus transplantation in the human: a complex surgical, medical and ethical challenge. Human Reprod. 2013;28(2):292–293.
14. Desai SP, Desai MS, Wood DN, Maddi R, Leeson S, Tilney NL. A semi-centennial report on the participants depicted in Joel Babb’s portrait, “The First Successful Kidney Transplantation”. Am J Transplant. 2007;7(7):1683–1688.
1. Brännström M, Johannesson L, Bokstrom H, et al. Livebirth after uterus transplantation. Lancet. 2015;385(9968):607–616.
2. Johannesson L, Enskog A, Mölne J, et al. Preclinical report on allogeneic uterus transplantation in nonhuman primates. Hum Reprod. 2013;28(1):189–198.
3. Brännström M, Diaz-Garcia C, Hanafy A, Olausson M, Tzakis A. Uterus transplantation: animal research and human possibilities. Fertil Steril. 2012;97(6):1269–1276.
4. Brännström M, Wranning CA, Altchek A. Experimental uterus transplantation. Hum Reprod Update. 2010;16(3):329–345.
5. Brännström M, Johannesson L, Dahm-Kähler P, et al. First clinical uterus transplant trial: a six-month report. Fertil Steril. 2014;101(5):1228–1236.
6. Johannesson L, Kvarnstrom N, Mölne J, et al. Uterus transplantation trial: 1-year outcome. Fertil Steril. 2015;103(1):199–204.
7. Concepcion BP, Schaefer HM. Caring for the pregnant kidney transplant recipient. Clin Transplant. 2011;25(6):821–829.
8. Rupley DM, Janda AM, Kapeles SR, Wilson TM, Berman D, Mathur AK. Preconception counseling, fertility and pregnancy complications after abdominal organ transplantation: a survey and cohort study of 532 recipients. Clin Transplant. 2014;28(9):937–945.
9. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—effects on mother and child. N Engl J Med. 2006;354(12):1281–1293.
10. Metcalfe J, Romney SL, Ramsey LH, Reid DH, Burwell CS. Estimation of uterine blood flow in normal human pregnancy at term. J Clin Invest. 1955;34(11):1632–1638.
11. Olausson M, Johannesson L, Brattgård D, et al. Ethics of uterus transplantation with live donors. Fertil Steril. 2014;102(1):40–43.
12. Del Priore G, Saso S, Meslin EM, et al. Uterine transplantation—a real possibility? The Indianapolis consensus. Hum Reprod. 2013;28(2):288–291.
13. Brosens I, Ghaem-Maghami S, Pijnenborg R. Uterus transplantation in the human: a complex surgical, medical and ethical challenge. Human Reprod. 2013;28(2):292–293.
14. Desai SP, Desai MS, Wood DN, Maddi R, Leeson S, Tilney NL. A semi-centennial report on the participants depicted in Joel Babb’s portrait, “The First Successful Kidney Transplantation”. Am J Transplant. 2007;7(7):1683–1688.
What is the risk that a patient will have an occult uterine cancer at myomectomy?
Wright and colleagues analyzed the risk of diagnosing an occult uterine cancer at the time of myomectomy using an administrative database, which contained information on 41,777 myomectomy surgeries performed at 496 hospitals from 2006 to 2012. They reported that 76 uterine corpus cancers (ICD-9 codes 179.x and 182.x) were detected, for a rate of 1 occult cancer identified per 550 myomectomy cases. The risk of diagnosing an occult uterine cancer increased with age.
Study limitations
A major weakness of the study is that the administrative database did not provide information about the histologic type of uterine corpus cancer. Uterine leiomyosarcoma is a highly aggressive cancer, while endometrial stromal sarcoma is a more indolent cancer. Additionally, the authors were not able to perform a histologic reassessment of the slides of the 76 cases reported as having uterine corpus cancer in order to confirm the diagnosis. Although the investigators provide age-specific information about the risk of uterine cancer, they did not have information on the menopausal status of the women.
Strengths of the study
Prior to these study results there were few data about the risk of diagnosing an occult cancer at the time of myomectomy. This very large study of more than 41,000 myomectomy cases will help clinicians fully counsel women about the risk of detecting an occult uterine cancer at the time of myomectomy.
What this evidence means for practice
Women aged 50 years or older should be advised against having a myomectomy given the 1 in 154 and 1 in 31 risk of identifying an occult uterine corpus cancer at the time of surgery in women aged 50 to 59 years and 60 years or older, respectively. Given an average age of menopause of 51 years, these data support the guidance of the US Food and Drug Administration that open electric power morcellation should not be used in surgery on uterine tumors in women who are perimenopausal or postmenopausal.
–Robert L. Barbieri, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Wright and colleagues analyzed the risk of diagnosing an occult uterine cancer at the time of myomectomy using an administrative database, which contained information on 41,777 myomectomy surgeries performed at 496 hospitals from 2006 to 2012. They reported that 76 uterine corpus cancers (ICD-9 codes 179.x and 182.x) were detected, for a rate of 1 occult cancer identified per 550 myomectomy cases. The risk of diagnosing an occult uterine cancer increased with age.
Study limitations
A major weakness of the study is that the administrative database did not provide information about the histologic type of uterine corpus cancer. Uterine leiomyosarcoma is a highly aggressive cancer, while endometrial stromal sarcoma is a more indolent cancer. Additionally, the authors were not able to perform a histologic reassessment of the slides of the 76 cases reported as having uterine corpus cancer in order to confirm the diagnosis. Although the investigators provide age-specific information about the risk of uterine cancer, they did not have information on the menopausal status of the women.
Strengths of the study
Prior to these study results there were few data about the risk of diagnosing an occult cancer at the time of myomectomy. This very large study of more than 41,000 myomectomy cases will help clinicians fully counsel women about the risk of detecting an occult uterine cancer at the time of myomectomy.
What this evidence means for practice
Women aged 50 years or older should be advised against having a myomectomy given the 1 in 154 and 1 in 31 risk of identifying an occult uterine corpus cancer at the time of surgery in women aged 50 to 59 years and 60 years or older, respectively. Given an average age of menopause of 51 years, these data support the guidance of the US Food and Drug Administration that open electric power morcellation should not be used in surgery on uterine tumors in women who are perimenopausal or postmenopausal.
–Robert L. Barbieri, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Wright and colleagues analyzed the risk of diagnosing an occult uterine cancer at the time of myomectomy using an administrative database, which contained information on 41,777 myomectomy surgeries performed at 496 hospitals from 2006 to 2012. They reported that 76 uterine corpus cancers (ICD-9 codes 179.x and 182.x) were detected, for a rate of 1 occult cancer identified per 550 myomectomy cases. The risk of diagnosing an occult uterine cancer increased with age.
Study limitations
A major weakness of the study is that the administrative database did not provide information about the histologic type of uterine corpus cancer. Uterine leiomyosarcoma is a highly aggressive cancer, while endometrial stromal sarcoma is a more indolent cancer. Additionally, the authors were not able to perform a histologic reassessment of the slides of the 76 cases reported as having uterine corpus cancer in order to confirm the diagnosis. Although the investigators provide age-specific information about the risk of uterine cancer, they did not have information on the menopausal status of the women.
Strengths of the study
Prior to these study results there were few data about the risk of diagnosing an occult cancer at the time of myomectomy. This very large study of more than 41,000 myomectomy cases will help clinicians fully counsel women about the risk of detecting an occult uterine cancer at the time of myomectomy.
What this evidence means for practice
Women aged 50 years or older should be advised against having a myomectomy given the 1 in 154 and 1 in 31 risk of identifying an occult uterine corpus cancer at the time of surgery in women aged 50 to 59 years and 60 years or older, respectively. Given an average age of menopause of 51 years, these data support the guidance of the US Food and Drug Administration that open electric power morcellation should not be used in surgery on uterine tumors in women who are perimenopausal or postmenopausal.
–Robert L. Barbieri, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.