User login
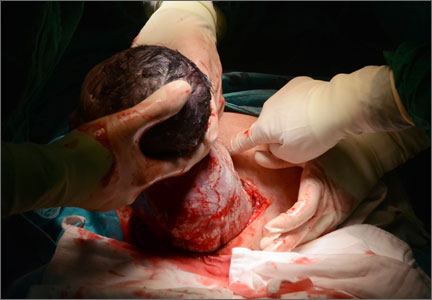
Hysterotomy incision and repair: Many options, many personal preferences
CASE: Your colleague’s hysterotomy practices vary from yours
You are in the hospital on a weekend inducing labor in your patient with hypertension. A colleague asks you to assist at a primary cesarean delivery for failure to progress in the second stage. You are glad to help. During the cesarean delivery, your colleague does not create a bladder flap, makes a superficial incision in the uterus and enters the uterine cavity bluntly with her index finger, uses blunt cephalad-caudad expansion of the uterine incision, and closes the uterine incision in a single-layer of continuous suture.
In your practice your general preference is to routinely dissect a bladder flap, enter the uterus using Allis clamps and sharp dissection; use blunt transverse expansion of the uterine incision; and close the uterine incision in two layers, locking the first layer. You wonder, is there any evidence that there is one best approach to managing the hysterotomy incision?
For many obstetrician-gynecologists, cesarean delivery is the major operation we perform most frequently. In planning and performing a cesarean delivery there are many technical surgical decision points, each with many options. A recent Cochrane review concluded that for most surgical options for uterine incision and closure, short-term maternal outcomes were similar among the options and that surgeons should use the techniques that they prefer and are comfortable performing.1 However, other authorities believe that the available evidence indicates that certain surgical techniques are associated with better maternal outcomes.2,3
In this editorial I focus on the varying surgical options available when performing a low transverse hysterotomy during cesarean delivery and the impact of these choices on maternal outcomes.
The bladder flap—surgeon’s choice
Theoretically, dissecting a bladder flap moves the dome of the bladder away from the anterior surface of the lower uterine segment, thereby protecting it from injury during the hysterotomy incision and repair. Three randomized trials have evaluated maternal outcomes following a hysterotomy with or without a bladder flap. All three trials reported that maternal outcomes were similar whether or not a bladder flap was created.4–6 In one trial, the creation of a bladder flap during a primary cesarean delivery was associated with increased adhesions between the parietal and visceral peritoneum and between the bladder and uterus at a repeat cesarean delivery.5
Some authorities have concluded that in most cesarean deliveries it is not necessary to create a bladder flap because the evidence does not indicate that it improves surgical outcomes.3 However, there may be clinical situations where a bladder flap is warranted. For example, during a repeat cesarean delivery, if the bladder is observed to be advanced high on the anterior uterine wall because of previous uterine surgery, a bladder flap may be helpful to ensure that the hysterotomy incision is performed in the lower uterine segment and not in the thickest, most muscular part of the uterine wall.
A second example is a case of arrested labor in the second stage with a deep transverse arrest of a macrosomic fetus. Lower segment lacerations may occur in this scenario, and some clinicians elect to dissect a bladder flap in anticipation of the risk of multiple extensions and a difficult hysterotomy repair. Since bladder injury occurs in less than 1% of cesarean deliveries, it would be difficult to perform a study with sufficient statistical power to determine whether creating a bladder flap influences the rate of bladder injury.7
Entering the uterine cavity—Try blunt entry
There are few clinical trial data to guide the technique for entering the uterine cavity. A major goal is to minimize the risk of a fetal laceration. One technique to reduce this risk is to superficially incise the uterus with a scalpel and then enter the uterus bluntly with a finger. Both the Misgav Ladach and modified Joel-Cohen techniques for cesarean delivery advocate the use of a superficial incision of the lower uterine segment with blunt entry into the uterine cavity.8,9 Other surgical options for entering the uterine cavity with minimal risk to the fetus include:
- Superficially incise the uterus with a scalpel and then apply Allis clamps to the upper and lower incision. Pull the tissue away from the underlying fetus before incising the final layer of uterine tissue and entering the cavity.10
- Apply the tip of the suction tubing with suction on and gently elevate the tissue trapped in the suction tip, incising the tissue to enter the uterus.
- Use a surgical device designed to reduce fetal lacerations (such as C-SAFE, CooperSurgical) to enter the uterus and extend the hysterotomy incision.11
Expanding the uterine incision—Use blunt expansion
Authors of a recent Cochrane meta-analysis analyzed five randomized controlled trials, involving
2,141 women, that evaluated blunt versus sharp expansion of a low transverse uterine incision.1 There was no difference in maternal febrile morbidity or major morbidity between the two techniques. However, blunt expansion of the uterine incision was associated with slightly less maternal blood loss and a lower risk of maternal blood transfusion than sharp incision (0.7% vs 3.1%).1 In another meta-analysis blunt expansion of the uterine incision with the surgeon’s fingers resulted in a smaller decrease in hematocrit and hemoglobin levels and fewer unintended extensions, but no difference in the rate of blood transfusion.12 Based on these findings some authorities recommend using blunt expansion of the uterine incision when a lower uterine segment incision is performed.3
One study, involving 811 women, compared cephalad-caudad blunt expansion versus transverse blunt expansion of the uterine incision.13 Cephalad-caudad blunt expansion compared with transverse blunt expansion resulted in a trend to less blood loss (398 mL versus 440 mL; P = .09), a significantly lower rate of unintended extension of the uterine incision (3.7% vs 7.4%, P = .03) and fewer cases with blood loss greater than 1,500 mL (0.2% vs 2.0%, P = .04). However, there was no difference in the rate of transfusion (0.7% vs 0.7%, P = 1.0) between cephalad-caudad versus transverse blunt expansion. Based on the results from this one trial, some authorities recommend that cephalad-caudad blunt extension be utilized rather than transverse blunt extension.3
Closing the uterine incision—One or two layers?
In the recent Cochrane meta-analysis, researchers compared outcomes of single-layer and two-layer closure of the uterine incision in 14 studies involving 13,890 women.1 There was no difference in rates of febrile morbidity (5.0% vs 5.1%), wound infection (9.4% vs 9.5%), or blood transfusion (2.1% vs 2.4%) between the two techniques. Authors of another systematic review of 20 trials of single- versus double-layer closure of the uterine incision concluded that, based on the available evidence from randomized trials, single- and double-layer closure appeared to produce similar outcomes.14 These authors cautioned, however, that based on nonrandomized studies, single layer closure might be associated with an increased risk of uterine rupture in a subsequent pregnancy.15,16
A uterine incision that was closed with a locked single-layer closure may be at an especially high risk of rupture during a subsequent trial of labor. In one analysis of relevant reports with heterogeneous study designs, the risk of uterine rupture during a trial of labor after a prior cesarean was 1.8% with a double-layer closure, 3.5% with an unlocked single-layer closure, and 6.2% with a locked single-layer closure.17 My perspective is that a double-layer closure generally is preferred because in a future pregnancy with a planned vaginal delivery, the double-layer closure may be associated with a lower rate of uterine rupture.
Some authorities recommend single-layer uterine closure if the patient is sure that she has no future plans to conceive. For example, a woman who is undergoing a tubal ligation at the time of cesarean delivery may be an optimal candidate for single-layer closure.3
Individualization and innovation in surgical care
Surgeons advance their skills by continually using the best evidence and advice from colleagues to guide changes in their practice. Many clinical situations present unique combinations of medical and anatomic problems, and surgeons need to use both creativity and expert judgment to solve these unique problems. Surgical choices that are guided by both the best evidence and hard-won clinical experience will result in optimal patient outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Dodd JM, Anderson ER, Gates S, Grivell RM. Surgical techniques for uterine incision and uterine closure at the time of cesarean section. Cochrane Database Sys Rev. 2014;7(3):CD004732.
2. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607–1617.
3. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: an updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
4. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98(6):1089–1092.
5. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in caesarean section on repeat caesarean delivery Eur J Obstet Gynecol. 2011;159(2):300–304.
6. Tuuli MG, Obido AO, Fogertey P, Roehl K, Stamilio D, Macones GA. Utility of the bladder flap at cesarean delivery. A randomized controlled trial. Obstet Gynecol. 2012;119(4):815–821.
7. Cahill AG, Stout MJ, Stamillo DM, Odibo AO, Peipert JF, Macones GA. Risk factors for bladder injury in patients with a prior hysterotomy. Obstet Gynecol. 2008;112(1):116–120.
8. Holmgren G, Sjoholm L, Stark M. The Misgav-Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78(7):615–621.
9. Wallin G, Fall O. Modified Joel-Cohen technique for cesarean delivery. Br J Obstet Gynaecol. 1999;106(3):221–226.
10. Gilstrap LC, Cunningham FG, Van Dorsten JP, eds. Operative Obstetrics, 2nd ed. New York, NY: McGraw Hill; 2002.
11. C SAFE. http://www.csafe.us/. Trumbull, CT: CooperSurgical, Inc.
12. Saad AF, Rahman M, Costantine MM, Saade GR. Blunt versus sharp uterine incision expansion during low transverse cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2014;211(6):684.e1–e11.
13. Cromi A, Ghezzi F, Di Naro E, Siesto G, Loverro G, Bolis P. Blunt expansion of the low transverse uterine incision at cesarean delivery: a randomized comparison of 2 techniques. Am J Obstet Gynecol. 2008;199(3):292.e1–e6.
14. Roberge S, Demers S, Berghella V, Chaillet N, Moore L, Bujold E. Impact of single- and double-layer closure on adverse outcomes and uterine scar defect: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014; 211(5):453–460.
15. Yasmin S, Sadaf J, Fatima N. Impact of methods for uterine incision closure on repeat cesarean section scar of lower uterine segment. J Coll Physicians Surg Pak. 2011;21(9): 522–526.
16. Bujold E, Mehta SH, Bujold C, Gauthier RJ. Interdelivery interval and uterine rupture. Am J Obstet Gynecol. 2002;187(5): 1199–1202.
17. Roberge S, Chaillet N, Boutin A, et al. Single- versus double-layer closure of the hysterotomy incision during cesarean delivery and risk of uterine rupture. Int J Gynaecol Obstet. 2011;115(1): 5–10.
CASE: Your colleague’s hysterotomy practices vary from yours
You are in the hospital on a weekend inducing labor in your patient with hypertension. A colleague asks you to assist at a primary cesarean delivery for failure to progress in the second stage. You are glad to help. During the cesarean delivery, your colleague does not create a bladder flap, makes a superficial incision in the uterus and enters the uterine cavity bluntly with her index finger, uses blunt cephalad-caudad expansion of the uterine incision, and closes the uterine incision in a single-layer of continuous suture.
In your practice your general preference is to routinely dissect a bladder flap, enter the uterus using Allis clamps and sharp dissection; use blunt transverse expansion of the uterine incision; and close the uterine incision in two layers, locking the first layer. You wonder, is there any evidence that there is one best approach to managing the hysterotomy incision?
For many obstetrician-gynecologists, cesarean delivery is the major operation we perform most frequently. In planning and performing a cesarean delivery there are many technical surgical decision points, each with many options. A recent Cochrane review concluded that for most surgical options for uterine incision and closure, short-term maternal outcomes were similar among the options and that surgeons should use the techniques that they prefer and are comfortable performing.1 However, other authorities believe that the available evidence indicates that certain surgical techniques are associated with better maternal outcomes.2,3
In this editorial I focus on the varying surgical options available when performing a low transverse hysterotomy during cesarean delivery and the impact of these choices on maternal outcomes.
The bladder flap—surgeon’s choice
Theoretically, dissecting a bladder flap moves the dome of the bladder away from the anterior surface of the lower uterine segment, thereby protecting it from injury during the hysterotomy incision and repair. Three randomized trials have evaluated maternal outcomes following a hysterotomy with or without a bladder flap. All three trials reported that maternal outcomes were similar whether or not a bladder flap was created.4–6 In one trial, the creation of a bladder flap during a primary cesarean delivery was associated with increased adhesions between the parietal and visceral peritoneum and between the bladder and uterus at a repeat cesarean delivery.5
Some authorities have concluded that in most cesarean deliveries it is not necessary to create a bladder flap because the evidence does not indicate that it improves surgical outcomes.3 However, there may be clinical situations where a bladder flap is warranted. For example, during a repeat cesarean delivery, if the bladder is observed to be advanced high on the anterior uterine wall because of previous uterine surgery, a bladder flap may be helpful to ensure that the hysterotomy incision is performed in the lower uterine segment and not in the thickest, most muscular part of the uterine wall.
A second example is a case of arrested labor in the second stage with a deep transverse arrest of a macrosomic fetus. Lower segment lacerations may occur in this scenario, and some clinicians elect to dissect a bladder flap in anticipation of the risk of multiple extensions and a difficult hysterotomy repair. Since bladder injury occurs in less than 1% of cesarean deliveries, it would be difficult to perform a study with sufficient statistical power to determine whether creating a bladder flap influences the rate of bladder injury.7
Entering the uterine cavity—Try blunt entry
There are few clinical trial data to guide the technique for entering the uterine cavity. A major goal is to minimize the risk of a fetal laceration. One technique to reduce this risk is to superficially incise the uterus with a scalpel and then enter the uterus bluntly with a finger. Both the Misgav Ladach and modified Joel-Cohen techniques for cesarean delivery advocate the use of a superficial incision of the lower uterine segment with blunt entry into the uterine cavity.8,9 Other surgical options for entering the uterine cavity with minimal risk to the fetus include:
- Superficially incise the uterus with a scalpel and then apply Allis clamps to the upper and lower incision. Pull the tissue away from the underlying fetus before incising the final layer of uterine tissue and entering the cavity.10
- Apply the tip of the suction tubing with suction on and gently elevate the tissue trapped in the suction tip, incising the tissue to enter the uterus.
- Use a surgical device designed to reduce fetal lacerations (such as C-SAFE, CooperSurgical) to enter the uterus and extend the hysterotomy incision.11
Expanding the uterine incision—Use blunt expansion
Authors of a recent Cochrane meta-analysis analyzed five randomized controlled trials, involving
2,141 women, that evaluated blunt versus sharp expansion of a low transverse uterine incision.1 There was no difference in maternal febrile morbidity or major morbidity between the two techniques. However, blunt expansion of the uterine incision was associated with slightly less maternal blood loss and a lower risk of maternal blood transfusion than sharp incision (0.7% vs 3.1%).1 In another meta-analysis blunt expansion of the uterine incision with the surgeon’s fingers resulted in a smaller decrease in hematocrit and hemoglobin levels and fewer unintended extensions, but no difference in the rate of blood transfusion.12 Based on these findings some authorities recommend using blunt expansion of the uterine incision when a lower uterine segment incision is performed.3
One study, involving 811 women, compared cephalad-caudad blunt expansion versus transverse blunt expansion of the uterine incision.13 Cephalad-caudad blunt expansion compared with transverse blunt expansion resulted in a trend to less blood loss (398 mL versus 440 mL; P = .09), a significantly lower rate of unintended extension of the uterine incision (3.7% vs 7.4%, P = .03) and fewer cases with blood loss greater than 1,500 mL (0.2% vs 2.0%, P = .04). However, there was no difference in the rate of transfusion (0.7% vs 0.7%, P = 1.0) between cephalad-caudad versus transverse blunt expansion. Based on the results from this one trial, some authorities recommend that cephalad-caudad blunt extension be utilized rather than transverse blunt extension.3
Closing the uterine incision—One or two layers?
In the recent Cochrane meta-analysis, researchers compared outcomes of single-layer and two-layer closure of the uterine incision in 14 studies involving 13,890 women.1 There was no difference in rates of febrile morbidity (5.0% vs 5.1%), wound infection (9.4% vs 9.5%), or blood transfusion (2.1% vs 2.4%) between the two techniques. Authors of another systematic review of 20 trials of single- versus double-layer closure of the uterine incision concluded that, based on the available evidence from randomized trials, single- and double-layer closure appeared to produce similar outcomes.14 These authors cautioned, however, that based on nonrandomized studies, single layer closure might be associated with an increased risk of uterine rupture in a subsequent pregnancy.15,16
A uterine incision that was closed with a locked single-layer closure may be at an especially high risk of rupture during a subsequent trial of labor. In one analysis of relevant reports with heterogeneous study designs, the risk of uterine rupture during a trial of labor after a prior cesarean was 1.8% with a double-layer closure, 3.5% with an unlocked single-layer closure, and 6.2% with a locked single-layer closure.17 My perspective is that a double-layer closure generally is preferred because in a future pregnancy with a planned vaginal delivery, the double-layer closure may be associated with a lower rate of uterine rupture.
Some authorities recommend single-layer uterine closure if the patient is sure that she has no future plans to conceive. For example, a woman who is undergoing a tubal ligation at the time of cesarean delivery may be an optimal candidate for single-layer closure.3
Individualization and innovation in surgical care
Surgeons advance their skills by continually using the best evidence and advice from colleagues to guide changes in their practice. Many clinical situations present unique combinations of medical and anatomic problems, and surgeons need to use both creativity and expert judgment to solve these unique problems. Surgical choices that are guided by both the best evidence and hard-won clinical experience will result in optimal patient outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Your colleague’s hysterotomy practices vary from yours
You are in the hospital on a weekend inducing labor in your patient with hypertension. A colleague asks you to assist at a primary cesarean delivery for failure to progress in the second stage. You are glad to help. During the cesarean delivery, your colleague does not create a bladder flap, makes a superficial incision in the uterus and enters the uterine cavity bluntly with her index finger, uses blunt cephalad-caudad expansion of the uterine incision, and closes the uterine incision in a single-layer of continuous suture.
In your practice your general preference is to routinely dissect a bladder flap, enter the uterus using Allis clamps and sharp dissection; use blunt transverse expansion of the uterine incision; and close the uterine incision in two layers, locking the first layer. You wonder, is there any evidence that there is one best approach to managing the hysterotomy incision?
For many obstetrician-gynecologists, cesarean delivery is the major operation we perform most frequently. In planning and performing a cesarean delivery there are many technical surgical decision points, each with many options. A recent Cochrane review concluded that for most surgical options for uterine incision and closure, short-term maternal outcomes were similar among the options and that surgeons should use the techniques that they prefer and are comfortable performing.1 However, other authorities believe that the available evidence indicates that certain surgical techniques are associated with better maternal outcomes.2,3
In this editorial I focus on the varying surgical options available when performing a low transverse hysterotomy during cesarean delivery and the impact of these choices on maternal outcomes.
The bladder flap—surgeon’s choice
Theoretically, dissecting a bladder flap moves the dome of the bladder away from the anterior surface of the lower uterine segment, thereby protecting it from injury during the hysterotomy incision and repair. Three randomized trials have evaluated maternal outcomes following a hysterotomy with or without a bladder flap. All three trials reported that maternal outcomes were similar whether or not a bladder flap was created.4–6 In one trial, the creation of a bladder flap during a primary cesarean delivery was associated with increased adhesions between the parietal and visceral peritoneum and between the bladder and uterus at a repeat cesarean delivery.5
Some authorities have concluded that in most cesarean deliveries it is not necessary to create a bladder flap because the evidence does not indicate that it improves surgical outcomes.3 However, there may be clinical situations where a bladder flap is warranted. For example, during a repeat cesarean delivery, if the bladder is observed to be advanced high on the anterior uterine wall because of previous uterine surgery, a bladder flap may be helpful to ensure that the hysterotomy incision is performed in the lower uterine segment and not in the thickest, most muscular part of the uterine wall.
A second example is a case of arrested labor in the second stage with a deep transverse arrest of a macrosomic fetus. Lower segment lacerations may occur in this scenario, and some clinicians elect to dissect a bladder flap in anticipation of the risk of multiple extensions and a difficult hysterotomy repair. Since bladder injury occurs in less than 1% of cesarean deliveries, it would be difficult to perform a study with sufficient statistical power to determine whether creating a bladder flap influences the rate of bladder injury.7
Entering the uterine cavity—Try blunt entry
There are few clinical trial data to guide the technique for entering the uterine cavity. A major goal is to minimize the risk of a fetal laceration. One technique to reduce this risk is to superficially incise the uterus with a scalpel and then enter the uterus bluntly with a finger. Both the Misgav Ladach and modified Joel-Cohen techniques for cesarean delivery advocate the use of a superficial incision of the lower uterine segment with blunt entry into the uterine cavity.8,9 Other surgical options for entering the uterine cavity with minimal risk to the fetus include:
- Superficially incise the uterus with a scalpel and then apply Allis clamps to the upper and lower incision. Pull the tissue away from the underlying fetus before incising the final layer of uterine tissue and entering the cavity.10
- Apply the tip of the suction tubing with suction on and gently elevate the tissue trapped in the suction tip, incising the tissue to enter the uterus.
- Use a surgical device designed to reduce fetal lacerations (such as C-SAFE, CooperSurgical) to enter the uterus and extend the hysterotomy incision.11
Expanding the uterine incision—Use blunt expansion
Authors of a recent Cochrane meta-analysis analyzed five randomized controlled trials, involving
2,141 women, that evaluated blunt versus sharp expansion of a low transverse uterine incision.1 There was no difference in maternal febrile morbidity or major morbidity between the two techniques. However, blunt expansion of the uterine incision was associated with slightly less maternal blood loss and a lower risk of maternal blood transfusion than sharp incision (0.7% vs 3.1%).1 In another meta-analysis blunt expansion of the uterine incision with the surgeon’s fingers resulted in a smaller decrease in hematocrit and hemoglobin levels and fewer unintended extensions, but no difference in the rate of blood transfusion.12 Based on these findings some authorities recommend using blunt expansion of the uterine incision when a lower uterine segment incision is performed.3
One study, involving 811 women, compared cephalad-caudad blunt expansion versus transverse blunt expansion of the uterine incision.13 Cephalad-caudad blunt expansion compared with transverse blunt expansion resulted in a trend to less blood loss (398 mL versus 440 mL; P = .09), a significantly lower rate of unintended extension of the uterine incision (3.7% vs 7.4%, P = .03) and fewer cases with blood loss greater than 1,500 mL (0.2% vs 2.0%, P = .04). However, there was no difference in the rate of transfusion (0.7% vs 0.7%, P = 1.0) between cephalad-caudad versus transverse blunt expansion. Based on the results from this one trial, some authorities recommend that cephalad-caudad blunt extension be utilized rather than transverse blunt extension.3
Closing the uterine incision—One or two layers?
In the recent Cochrane meta-analysis, researchers compared outcomes of single-layer and two-layer closure of the uterine incision in 14 studies involving 13,890 women.1 There was no difference in rates of febrile morbidity (5.0% vs 5.1%), wound infection (9.4% vs 9.5%), or blood transfusion (2.1% vs 2.4%) between the two techniques. Authors of another systematic review of 20 trials of single- versus double-layer closure of the uterine incision concluded that, based on the available evidence from randomized trials, single- and double-layer closure appeared to produce similar outcomes.14 These authors cautioned, however, that based on nonrandomized studies, single layer closure might be associated with an increased risk of uterine rupture in a subsequent pregnancy.15,16
A uterine incision that was closed with a locked single-layer closure may be at an especially high risk of rupture during a subsequent trial of labor. In one analysis of relevant reports with heterogeneous study designs, the risk of uterine rupture during a trial of labor after a prior cesarean was 1.8% with a double-layer closure, 3.5% with an unlocked single-layer closure, and 6.2% with a locked single-layer closure.17 My perspective is that a double-layer closure generally is preferred because in a future pregnancy with a planned vaginal delivery, the double-layer closure may be associated with a lower rate of uterine rupture.
Some authorities recommend single-layer uterine closure if the patient is sure that she has no future plans to conceive. For example, a woman who is undergoing a tubal ligation at the time of cesarean delivery may be an optimal candidate for single-layer closure.3
Individualization and innovation in surgical care
Surgeons advance their skills by continually using the best evidence and advice from colleagues to guide changes in their practice. Many clinical situations present unique combinations of medical and anatomic problems, and surgeons need to use both creativity and expert judgment to solve these unique problems. Surgical choices that are guided by both the best evidence and hard-won clinical experience will result in optimal patient outcomes.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Dodd JM, Anderson ER, Gates S, Grivell RM. Surgical techniques for uterine incision and uterine closure at the time of cesarean section. Cochrane Database Sys Rev. 2014;7(3):CD004732.
2. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607–1617.
3. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: an updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
4. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98(6):1089–1092.
5. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in caesarean section on repeat caesarean delivery Eur J Obstet Gynecol. 2011;159(2):300–304.
6. Tuuli MG, Obido AO, Fogertey P, Roehl K, Stamilio D, Macones GA. Utility of the bladder flap at cesarean delivery. A randomized controlled trial. Obstet Gynecol. 2012;119(4):815–821.
7. Cahill AG, Stout MJ, Stamillo DM, Odibo AO, Peipert JF, Macones GA. Risk factors for bladder injury in patients with a prior hysterotomy. Obstet Gynecol. 2008;112(1):116–120.
8. Holmgren G, Sjoholm L, Stark M. The Misgav-Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78(7):615–621.
9. Wallin G, Fall O. Modified Joel-Cohen technique for cesarean delivery. Br J Obstet Gynaecol. 1999;106(3):221–226.
10. Gilstrap LC, Cunningham FG, Van Dorsten JP, eds. Operative Obstetrics, 2nd ed. New York, NY: McGraw Hill; 2002.
11. C SAFE. http://www.csafe.us/. Trumbull, CT: CooperSurgical, Inc.
12. Saad AF, Rahman M, Costantine MM, Saade GR. Blunt versus sharp uterine incision expansion during low transverse cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2014;211(6):684.e1–e11.
13. Cromi A, Ghezzi F, Di Naro E, Siesto G, Loverro G, Bolis P. Blunt expansion of the low transverse uterine incision at cesarean delivery: a randomized comparison of 2 techniques. Am J Obstet Gynecol. 2008;199(3):292.e1–e6.
14. Roberge S, Demers S, Berghella V, Chaillet N, Moore L, Bujold E. Impact of single- and double-layer closure on adverse outcomes and uterine scar defect: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014; 211(5):453–460.
15. Yasmin S, Sadaf J, Fatima N. Impact of methods for uterine incision closure on repeat cesarean section scar of lower uterine segment. J Coll Physicians Surg Pak. 2011;21(9): 522–526.
16. Bujold E, Mehta SH, Bujold C, Gauthier RJ. Interdelivery interval and uterine rupture. Am J Obstet Gynecol. 2002;187(5): 1199–1202.
17. Roberge S, Chaillet N, Boutin A, et al. Single- versus double-layer closure of the hysterotomy incision during cesarean delivery and risk of uterine rupture. Int J Gynaecol Obstet. 2011;115(1): 5–10.
1. Dodd JM, Anderson ER, Gates S, Grivell RM. Surgical techniques for uterine incision and uterine closure at the time of cesarean section. Cochrane Database Sys Rev. 2014;7(3):CD004732.
2. Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607–1617.
3. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: an updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
4. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2001;98(6):1089–1092.
5. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in caesarean section on repeat caesarean delivery Eur J Obstet Gynecol. 2011;159(2):300–304.
6. Tuuli MG, Obido AO, Fogertey P, Roehl K, Stamilio D, Macones GA. Utility of the bladder flap at cesarean delivery. A randomized controlled trial. Obstet Gynecol. 2012;119(4):815–821.
7. Cahill AG, Stout MJ, Stamillo DM, Odibo AO, Peipert JF, Macones GA. Risk factors for bladder injury in patients with a prior hysterotomy. Obstet Gynecol. 2008;112(1):116–120.
8. Holmgren G, Sjoholm L, Stark M. The Misgav-Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78(7):615–621.
9. Wallin G, Fall O. Modified Joel-Cohen technique for cesarean delivery. Br J Obstet Gynaecol. 1999;106(3):221–226.
10. Gilstrap LC, Cunningham FG, Van Dorsten JP, eds. Operative Obstetrics, 2nd ed. New York, NY: McGraw Hill; 2002.
11. C SAFE. http://www.csafe.us/. Trumbull, CT: CooperSurgical, Inc.
12. Saad AF, Rahman M, Costantine MM, Saade GR. Blunt versus sharp uterine incision expansion during low transverse cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2014;211(6):684.e1–e11.
13. Cromi A, Ghezzi F, Di Naro E, Siesto G, Loverro G, Bolis P. Blunt expansion of the low transverse uterine incision at cesarean delivery: a randomized comparison of 2 techniques. Am J Obstet Gynecol. 2008;199(3):292.e1–e6.
14. Roberge S, Demers S, Berghella V, Chaillet N, Moore L, Bujold E. Impact of single- and double-layer closure on adverse outcomes and uterine scar defect: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014; 211(5):453–460.
15. Yasmin S, Sadaf J, Fatima N. Impact of methods for uterine incision closure on repeat cesarean section scar of lower uterine segment. J Coll Physicians Surg Pak. 2011;21(9): 522–526.
16. Bujold E, Mehta SH, Bujold C, Gauthier RJ. Interdelivery interval and uterine rupture. Am J Obstet Gynecol. 2002;187(5): 1199–1202.
17. Roberge S, Chaillet N, Boutin A, et al. Single- versus double-layer closure of the hysterotomy incision during cesarean delivery and risk of uterine rupture. Int J Gynaecol Obstet. 2011;115(1): 5–10.
Optimal pharmacologic treatment of nausea and vomiting of pregnancy
CASE: Pregnant patient seeks medication for her NVP
A 23-year-old G1P0 woman at 9 weeks’ gestation presents to your office with nausea and vomiting that is interfering with work. She has tried many changes in her daily habits. She has tried eating small, frequent meals; snacking on nuts and crackers; using lemon-scented products; and avoiding coffee and strong odors. Following an evaluation you diagnose nausea and vomiting of pregnancy (NVP). She asks, “Is there a medication for my nausea that is safe for my baby?”
Nausea with or without vomiting is a common problem for pregnant women between 6 and 14 weeks of gestation. In one study, nausea with or without vomiting was reported by 69% of patients and resulted in pharmacologic treatment in 15%.1 In a Cochrane review of NVP, investigators analyzed 37 trials involving treatments such as acupressure, acustimulation, acupuncture, ginger, chamomile, lemon oil, vitamin B6, and antiemetic medications. The authors concluded, “There is a lack of high-quality evidence to support any particular intervention.”2 Clinicians are challenged to effectively treat the symptoms of NVP and simultaneously to minimize the risk that the fetus will be exposed to a teratogen during the first trimester, a vulnerable period in organ development.
In this editorial, I briefly review nonpharmacologic options for NVP, but focus on current pharmacologic treatments. Of those available to ObGyns, what is the best first-choice treatment given recent and accumulated data regarding associated congenital anomalies?
Nonpharmacologic treatment
Although the authors of the Cochrane review did not identify high-quality evidence to support nonpharmacologic interventions, results of multiple randomized trials have demonstrated that ginger is effective in reducing pregnancy-associated nausea and vomiting.3 Ginger treatment is recommended at doses of 250 mg in capsules or syrup four times daily.
First-line pharmacologic treatment: Doxylamine plus pyridoxine
The US Food and Drug Administration (FDA) has approved the combination of doxylamine plus pyridoxine (vitamin B6) in a delayed-release formulation for treatment of NVP (Diclegis). Doxylamine is an antihistamine that blocks H1-receptor sites in the chemoreceptor trigger zone. It also diminishes vestibular stimulation and depresses labyrinthine activity through central anticholinergic activity. Its elimination half-life is 10 to 12 hours (Lexicomp). Each tablet contains doxylamine 10 mg and pyridoxine 10 mg. The starting dose is 2 tablets at bedtime.
If the woman has persistent symptoms, a third tablet is added, to be taken in the morning. If symptoms continue, a fourth tablet is recommended to be taken in the afternoon. In a large, randomized clinical trial, doxylamine-pyridoxine treatment reduced nausea, vomiting, and retching and improved perceived quality of life compared with placebo.4 The FDA assigned doxylamine-pyridoxine pregnancy category A because of the extensive evidence that it does not cause an increase in fetal malformations.5,6
If the delayed-release doxylamine-pyridoxine formulation (Diclegis) is not available to the patient, alternative formulations of doxylamine and pyridoxine can be prescribed. Pyridoxine is widely available over the counter as 25-mg tablets, and one tablet can be prescribed two or three times daily. Doxylamine is available as a chewable prescription medicine in 5-mg tablets (Aldex AN) and two tablets can be prescribed two or three times daily. Doxylamine is also available as a 25-mg over-the-counter tablet in Unisom SleepTabs. One-half tablet can be prescribed two or three times daily. The patient should be alerted that Unisom SleepGels contain diphenhydramine, not doxylamine.
Second-line pharmacologic treatment
Metoclopramide Metoclopramide is a dopamine antagonist. It enhances upper gastrointestinal motility, accelerates gastric emptying, and increases lower esophageal sphincter tone. At higher doses it blocks serotonin receptors in the chemoreceptor trigger zone. Its elimination half-life is 5 to 6 hours (Lexicomp). There are no large, randomized, placebo-controlled trials of oral metoclopramide for the treatment of nausea and vomiting of early pregnancy.
I am recommending metoclopramide as a second-line treatment for NVP because it appears to be effective and is not known to be associated with an increased risk of congenital malformations. Metoclopramide is widely used to prevent and treat intraoperative and postoperative nausea associated with cesarean delivery.7 In addition, intravenous (IV) metoclopramide is commonly used to treat women hospitalized with hyperemesis gravidarum. Results of randomized clinical trials demonstrate that when used to treat hyperemesis gravidarum, IV metoclopramide (10 mg every 8 hours) has similar efficacy to IV ondansetron (4 mg every 8 hours)8 and IV promethazine (25 mg every 8 hours).9 When using metoclopramide as an oral treatment for NVP, 10 mg every 8 hours is a commonly recommended regimen.
The FDA has assigned metoclopramide to pregnancy category B, which indicates that there is no evidence of fetal risk. Studies from Israel and Denmark show that metoclopramide is not associated with an increased risk of congenital malformations. In the study from Israel, among 3,458 infants born to women who had filled a prescription for metoclopramide during the first trimester of pregnancy, there was no increase in major congenital malformations, low birth weight, preterm delivery, or perinatal death.10 In the study from Denmark, among 28,486 infants born to mothers who had filled a prescription for metoclopramide in the first trimester there was no increase in congenital malformations or any of 20 individual categories of malformations, including neural tube defects, transposition of the great vessels, ventricular septal defect, atrial septal defect, tetralogy of Fallot, coarctation of the aorta, cleft lip or palate, anorectal atresia/stenosis, or limb reduction.11 The results of these two large studies are reassuring that metoclopramide is not associated with an increased risk of congenital malformations.
Metoclopramide can cause tardive dyskinesia, a serious movement disorder that may be irreversible with discontinuation of the drug. This risk increases with dose and length of treatment. The FDA recommends that clinicians avoid the use of metoclopramide for more than 12 weeks.
Third-line pharmacologic treatment: Ondansetron
In the United States ondansetron is commonly used to treat NVP.12 The drug is a selective 5-HT3 antagonist that blocks serotonin action in the central nervous system chemoreceptor trigger zone. The elimination half-life of ondansetron is 3 to 6 hours (Lexicomp).
The frequent use of ondansetron may be due, in part, to the perception that it is a very effective antiemetic. For example, in one small clinical trial, ondansetron 4 mg every 8 hours was reported to be superior to a combination of pyridoxine 25 mg every 8 hours plus doxylamine 12.5 mg every 8 hours.13 (Note that the pyridoxine and doxylamine tablets used in this trial were not in a combination delayed-release formulation.) I am recommending ondansetron as a third-line treatment for NVP because, although it is effective, it may be associated with an increased risk of fetal cardiac anomalies.
Is ondansetron associated with cardiac malformations?
The FDA has assigned ondansetron to pregnancy category B; however, there is concern that it may be associated with congenital heart defects. In a recent study of 1,349 infants born to Swedish women who had filled a prescription for ondansetron in early pregnancy, a significantly increased risk of cardiovascular defect (odds ratio [OR], 1.62; 95% confidence interval [CI], 1.04−2.14) and cardiac septum defect (OR, 2.05; 95% CI, 1.19−3.28) was reported.14 The cardiac anomalies were mostly atrial septal or ventricular septal defects.
In a second study, reported as an abstract, authors analyzed congenital malformations in 1,248 infants born to Danish women who filled a prescription for ondansetron in early pregnancy. These authors also found an increased risk of a congenital heart malformation (OR, 2.0; 95% CI, 1.3−3.1).15
A US case-control study showed an association between ondansetron use and cleft palate.1 The Swedish14 and Danish15 studies reported above did not find an association between ondansetron use and cleft palate.
The FDA issued a warning in June 2012 that at a dose of 32 mg, administered intravenously, ondansetron may prolong the QT interval and result in a potentially fatal heart arrhythmia, torsades de pointes.16 In the announcement the FDA did not alter the recommendations for oral dosing because there is no strong evidence that oral dosing is associated with clinically significant arrhythmias. Authors of a recent systematic review concluded that IV administration of large doses of ondansetron may cause cardiac arrhythmias, especially in patients with cardiac disease and those taking other drugs that prolong the QT interval, but that a single oral dose of ondansetron does not have a significant risk of causing an arrhythmia.17
Health Canada18 has advised that many commonly prescribed medications increase serotonin activity. When multiple drugs that each increase serotonin activity are prescribed in combination, the risk of serotonin syndrome is increased. Serotonin syndrome results in hyperthermia, agitation, tachycardia, and muscle twitching and can be fatal. Ondansetron was specifically mentioned in the Health Canada warning, but a search of the literature revealed very few reported cases of ondansetron being implicated in the serotonin syndrome.19
My bottom-line recommendations
NVP is a common obstetric problem. When oral pharmacologic therapy is indicated, first-line treatment should be with the FDA-approved combination of doxylamine-pyridoxine because it is both effective and associated with no known increased risk of congenital malformations. An effective second-line agent is metoclopramide. Based on very limited data, metoclopramide appears effective and is not associated with an increased risk of congenital malformations. However, it is not FDA approved for treatment of NVP. Ondansetron appears to be effective but its use in early pregnancy may be associated with congenital anomalies. Consequently, ondansetron should not be used to treat NVP unless first- and second-line treatments have been ineffective to treat the patient’s symptoms.
INSTANT POLL
Which of the following pharmacologic treatments of nausea with or without vomiting during pregnancy is your first-line medication choice?
• Ondansetron
• Metoclopramide
• Doxylamine-pyridoxine
• Meclizine Promethazine
• Trimethobenzamide
Visit the Quick Poll on the homepage, give your answer, and then see how other ObGyns have answered.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Anderka M, Mitchell AA, Louik C, Werler MMA, Hernandez-Diaz S, Rasmussen SA; National Birth Defects Prevention Study. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012;94(1):22–30.
2. Matthews A, Haas Dm, O’Mathuna DP, Dowswell T, Doyle M. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2014;(3):CD007575.
3. Borrelli F, Capasso R, Aviello G, Pittler MH, Izzo AA. Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstet Gynecol. 2005;105(4):849–856.
4. Koren G, Clark S, Hankins GD, et al. Effectiveness of delayed-release doxylamine and pyridoxine for nausea and vomiting of pregnancy: a randomized placebo controlled trial. Am J Obstet Gynecol. 2010;203(6):571.e1–7.
5. Einarson TR, Leeder JS, Koren G. A method for meta-analysis of epidemiologic studies. Drug Intell Clin Pharm. 1988;22(10):813–824.
6. McKeigue PM, Lamm SH, Linn S, Kutcher JS. Bendectin and birth defects. I. A meta-analysis of the epidemiologic studies. Teratology. 1994;50(1):27–37.
7. Mishriky BM, Habib AS. Metoclopramide for nausea and vomiting prophylaxis during and after cesarean delivery: a systematic review and meta-analysis. Br J Anaesth. 2012;108(3):374–383.
8. Abas MN, Tan PC, Azmi N, Omar SZ. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123(6):1272–1279.
9. Tan PC, Khine PP, Vallikkannu N, Omar SZ. Promethazine compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2010;115(5):975–981.
10. Matok I, Gorodischer R, Koren G, Sheiner E, Wiznitzer A, Levy A. The safety of metoclopramide use in the first trimester of pregnancy. N Engl J Med. 2009;360(24):2528–2535.
11. Pasternak B, Svanstrom H, Molgaard-Nielsen D, Melbye M, Hviid A. Metoclopramide in pregnancy and risk of major congenital malformations and fetal death. JAMA. 2013;310(15):1601–1611.
12. Koren G. Treating morning sickness in the United States—changes in prescribing are needed. Am J Obstet Gynecol. 2014;211(6):602–606.
13. Oliveira LG, Capp SM, You WB, Riffenburgh RH, Carstairs SH. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy : a randomized controlled trial. Obstet Gynecol. 2014;124(4):735–742.
14. Danielsson B, Wikner BN, Kallen B. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014;50:134–137.
15. Andersen JT, Jimenez-Solem E, Andersen NL, Poulsen HE. Ondansetron use in early pregnancy and the risk of congenital malformations—a registry based nationwide cohort study. Abstract presented at: 29th International Conference on Pharmacoepidemiology & Therapeutic Risk Management; August 25–28, 2013; Montreal, Canada. Abstract 25, Pregnancy Session 1. Pharmacoepidemiol Drug Saf. 2013;22(suppl 1):13–14.
16. US Food and Drug Administration. Ondansetron (Zofran) IV: drug safety communication - QT prolongation. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm310219.htm. Published June 29, 2012. Accessed December 26, 2014.
17. Freedman SB, Uleryk E, Rumantir M, Finkelstein Y. Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Ann Emerg Med. 2014;64(1):19–25.
18. Health Canada. Canadian Adverse Reaction Newsletter. 2003;13(3). http://www.hc-sc.gc.ca/dhp-mps/medeff/bulletin/carn-bcei_v13n3-eng.php. Published June 24, 2003. Accessed December 26, 2014.
19. Turkel SB, Nadala JG, Wincor MZ. Possible serotonin syndrome in association with 5-HT3 antagonist agents. Psychosomatics. 2001;42(3):258–260.
CASE: Pregnant patient seeks medication for her NVP
A 23-year-old G1P0 woman at 9 weeks’ gestation presents to your office with nausea and vomiting that is interfering with work. She has tried many changes in her daily habits. She has tried eating small, frequent meals; snacking on nuts and crackers; using lemon-scented products; and avoiding coffee and strong odors. Following an evaluation you diagnose nausea and vomiting of pregnancy (NVP). She asks, “Is there a medication for my nausea that is safe for my baby?”
Nausea with or without vomiting is a common problem for pregnant women between 6 and 14 weeks of gestation. In one study, nausea with or without vomiting was reported by 69% of patients and resulted in pharmacologic treatment in 15%.1 In a Cochrane review of NVP, investigators analyzed 37 trials involving treatments such as acupressure, acustimulation, acupuncture, ginger, chamomile, lemon oil, vitamin B6, and antiemetic medications. The authors concluded, “There is a lack of high-quality evidence to support any particular intervention.”2 Clinicians are challenged to effectively treat the symptoms of NVP and simultaneously to minimize the risk that the fetus will be exposed to a teratogen during the first trimester, a vulnerable period in organ development.
In this editorial, I briefly review nonpharmacologic options for NVP, but focus on current pharmacologic treatments. Of those available to ObGyns, what is the best first-choice treatment given recent and accumulated data regarding associated congenital anomalies?
Nonpharmacologic treatment
Although the authors of the Cochrane review did not identify high-quality evidence to support nonpharmacologic interventions, results of multiple randomized trials have demonstrated that ginger is effective in reducing pregnancy-associated nausea and vomiting.3 Ginger treatment is recommended at doses of 250 mg in capsules or syrup four times daily.
First-line pharmacologic treatment: Doxylamine plus pyridoxine
The US Food and Drug Administration (FDA) has approved the combination of doxylamine plus pyridoxine (vitamin B6) in a delayed-release formulation for treatment of NVP (Diclegis). Doxylamine is an antihistamine that blocks H1-receptor sites in the chemoreceptor trigger zone. It also diminishes vestibular stimulation and depresses labyrinthine activity through central anticholinergic activity. Its elimination half-life is 10 to 12 hours (Lexicomp). Each tablet contains doxylamine 10 mg and pyridoxine 10 mg. The starting dose is 2 tablets at bedtime.
If the woman has persistent symptoms, a third tablet is added, to be taken in the morning. If symptoms continue, a fourth tablet is recommended to be taken in the afternoon. In a large, randomized clinical trial, doxylamine-pyridoxine treatment reduced nausea, vomiting, and retching and improved perceived quality of life compared with placebo.4 The FDA assigned doxylamine-pyridoxine pregnancy category A because of the extensive evidence that it does not cause an increase in fetal malformations.5,6
If the delayed-release doxylamine-pyridoxine formulation (Diclegis) is not available to the patient, alternative formulations of doxylamine and pyridoxine can be prescribed. Pyridoxine is widely available over the counter as 25-mg tablets, and one tablet can be prescribed two or three times daily. Doxylamine is available as a chewable prescription medicine in 5-mg tablets (Aldex AN) and two tablets can be prescribed two or three times daily. Doxylamine is also available as a 25-mg over-the-counter tablet in Unisom SleepTabs. One-half tablet can be prescribed two or three times daily. The patient should be alerted that Unisom SleepGels contain diphenhydramine, not doxylamine.
Second-line pharmacologic treatment
Metoclopramide Metoclopramide is a dopamine antagonist. It enhances upper gastrointestinal motility, accelerates gastric emptying, and increases lower esophageal sphincter tone. At higher doses it blocks serotonin receptors in the chemoreceptor trigger zone. Its elimination half-life is 5 to 6 hours (Lexicomp). There are no large, randomized, placebo-controlled trials of oral metoclopramide for the treatment of nausea and vomiting of early pregnancy.
I am recommending metoclopramide as a second-line treatment for NVP because it appears to be effective and is not known to be associated with an increased risk of congenital malformations. Metoclopramide is widely used to prevent and treat intraoperative and postoperative nausea associated with cesarean delivery.7 In addition, intravenous (IV) metoclopramide is commonly used to treat women hospitalized with hyperemesis gravidarum. Results of randomized clinical trials demonstrate that when used to treat hyperemesis gravidarum, IV metoclopramide (10 mg every 8 hours) has similar efficacy to IV ondansetron (4 mg every 8 hours)8 and IV promethazine (25 mg every 8 hours).9 When using metoclopramide as an oral treatment for NVP, 10 mg every 8 hours is a commonly recommended regimen.
The FDA has assigned metoclopramide to pregnancy category B, which indicates that there is no evidence of fetal risk. Studies from Israel and Denmark show that metoclopramide is not associated with an increased risk of congenital malformations. In the study from Israel, among 3,458 infants born to women who had filled a prescription for metoclopramide during the first trimester of pregnancy, there was no increase in major congenital malformations, low birth weight, preterm delivery, or perinatal death.10 In the study from Denmark, among 28,486 infants born to mothers who had filled a prescription for metoclopramide in the first trimester there was no increase in congenital malformations or any of 20 individual categories of malformations, including neural tube defects, transposition of the great vessels, ventricular septal defect, atrial septal defect, tetralogy of Fallot, coarctation of the aorta, cleft lip or palate, anorectal atresia/stenosis, or limb reduction.11 The results of these two large studies are reassuring that metoclopramide is not associated with an increased risk of congenital malformations.
Metoclopramide can cause tardive dyskinesia, a serious movement disorder that may be irreversible with discontinuation of the drug. This risk increases with dose and length of treatment. The FDA recommends that clinicians avoid the use of metoclopramide for more than 12 weeks.
Third-line pharmacologic treatment: Ondansetron
In the United States ondansetron is commonly used to treat NVP.12 The drug is a selective 5-HT3 antagonist that blocks serotonin action in the central nervous system chemoreceptor trigger zone. The elimination half-life of ondansetron is 3 to 6 hours (Lexicomp).
The frequent use of ondansetron may be due, in part, to the perception that it is a very effective antiemetic. For example, in one small clinical trial, ondansetron 4 mg every 8 hours was reported to be superior to a combination of pyridoxine 25 mg every 8 hours plus doxylamine 12.5 mg every 8 hours.13 (Note that the pyridoxine and doxylamine tablets used in this trial were not in a combination delayed-release formulation.) I am recommending ondansetron as a third-line treatment for NVP because, although it is effective, it may be associated with an increased risk of fetal cardiac anomalies.
Is ondansetron associated with cardiac malformations?
The FDA has assigned ondansetron to pregnancy category B; however, there is concern that it may be associated with congenital heart defects. In a recent study of 1,349 infants born to Swedish women who had filled a prescription for ondansetron in early pregnancy, a significantly increased risk of cardiovascular defect (odds ratio [OR], 1.62; 95% confidence interval [CI], 1.04−2.14) and cardiac septum defect (OR, 2.05; 95% CI, 1.19−3.28) was reported.14 The cardiac anomalies were mostly atrial septal or ventricular septal defects.
In a second study, reported as an abstract, authors analyzed congenital malformations in 1,248 infants born to Danish women who filled a prescription for ondansetron in early pregnancy. These authors also found an increased risk of a congenital heart malformation (OR, 2.0; 95% CI, 1.3−3.1).15
A US case-control study showed an association between ondansetron use and cleft palate.1 The Swedish14 and Danish15 studies reported above did not find an association between ondansetron use and cleft palate.
The FDA issued a warning in June 2012 that at a dose of 32 mg, administered intravenously, ondansetron may prolong the QT interval and result in a potentially fatal heart arrhythmia, torsades de pointes.16 In the announcement the FDA did not alter the recommendations for oral dosing because there is no strong evidence that oral dosing is associated with clinically significant arrhythmias. Authors of a recent systematic review concluded that IV administration of large doses of ondansetron may cause cardiac arrhythmias, especially in patients with cardiac disease and those taking other drugs that prolong the QT interval, but that a single oral dose of ondansetron does not have a significant risk of causing an arrhythmia.17
Health Canada18 has advised that many commonly prescribed medications increase serotonin activity. When multiple drugs that each increase serotonin activity are prescribed in combination, the risk of serotonin syndrome is increased. Serotonin syndrome results in hyperthermia, agitation, tachycardia, and muscle twitching and can be fatal. Ondansetron was specifically mentioned in the Health Canada warning, but a search of the literature revealed very few reported cases of ondansetron being implicated in the serotonin syndrome.19
My bottom-line recommendations
NVP is a common obstetric problem. When oral pharmacologic therapy is indicated, first-line treatment should be with the FDA-approved combination of doxylamine-pyridoxine because it is both effective and associated with no known increased risk of congenital malformations. An effective second-line agent is metoclopramide. Based on very limited data, metoclopramide appears effective and is not associated with an increased risk of congenital malformations. However, it is not FDA approved for treatment of NVP. Ondansetron appears to be effective but its use in early pregnancy may be associated with congenital anomalies. Consequently, ondansetron should not be used to treat NVP unless first- and second-line treatments have been ineffective to treat the patient’s symptoms.
INSTANT POLL
Which of the following pharmacologic treatments of nausea with or without vomiting during pregnancy is your first-line medication choice?
• Ondansetron
• Metoclopramide
• Doxylamine-pyridoxine
• Meclizine Promethazine
• Trimethobenzamide
Visit the Quick Poll on the homepage, give your answer, and then see how other ObGyns have answered.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Pregnant patient seeks medication for her NVP
A 23-year-old G1P0 woman at 9 weeks’ gestation presents to your office with nausea and vomiting that is interfering with work. She has tried many changes in her daily habits. She has tried eating small, frequent meals; snacking on nuts and crackers; using lemon-scented products; and avoiding coffee and strong odors. Following an evaluation you diagnose nausea and vomiting of pregnancy (NVP). She asks, “Is there a medication for my nausea that is safe for my baby?”
Nausea with or without vomiting is a common problem for pregnant women between 6 and 14 weeks of gestation. In one study, nausea with or without vomiting was reported by 69% of patients and resulted in pharmacologic treatment in 15%.1 In a Cochrane review of NVP, investigators analyzed 37 trials involving treatments such as acupressure, acustimulation, acupuncture, ginger, chamomile, lemon oil, vitamin B6, and antiemetic medications. The authors concluded, “There is a lack of high-quality evidence to support any particular intervention.”2 Clinicians are challenged to effectively treat the symptoms of NVP and simultaneously to minimize the risk that the fetus will be exposed to a teratogen during the first trimester, a vulnerable period in organ development.
In this editorial, I briefly review nonpharmacologic options for NVP, but focus on current pharmacologic treatments. Of those available to ObGyns, what is the best first-choice treatment given recent and accumulated data regarding associated congenital anomalies?
Nonpharmacologic treatment
Although the authors of the Cochrane review did not identify high-quality evidence to support nonpharmacologic interventions, results of multiple randomized trials have demonstrated that ginger is effective in reducing pregnancy-associated nausea and vomiting.3 Ginger treatment is recommended at doses of 250 mg in capsules or syrup four times daily.
First-line pharmacologic treatment: Doxylamine plus pyridoxine
The US Food and Drug Administration (FDA) has approved the combination of doxylamine plus pyridoxine (vitamin B6) in a delayed-release formulation for treatment of NVP (Diclegis). Doxylamine is an antihistamine that blocks H1-receptor sites in the chemoreceptor trigger zone. It also diminishes vestibular stimulation and depresses labyrinthine activity through central anticholinergic activity. Its elimination half-life is 10 to 12 hours (Lexicomp). Each tablet contains doxylamine 10 mg and pyridoxine 10 mg. The starting dose is 2 tablets at bedtime.
If the woman has persistent symptoms, a third tablet is added, to be taken in the morning. If symptoms continue, a fourth tablet is recommended to be taken in the afternoon. In a large, randomized clinical trial, doxylamine-pyridoxine treatment reduced nausea, vomiting, and retching and improved perceived quality of life compared with placebo.4 The FDA assigned doxylamine-pyridoxine pregnancy category A because of the extensive evidence that it does not cause an increase in fetal malformations.5,6
If the delayed-release doxylamine-pyridoxine formulation (Diclegis) is not available to the patient, alternative formulations of doxylamine and pyridoxine can be prescribed. Pyridoxine is widely available over the counter as 25-mg tablets, and one tablet can be prescribed two or three times daily. Doxylamine is available as a chewable prescription medicine in 5-mg tablets (Aldex AN) and two tablets can be prescribed two or three times daily. Doxylamine is also available as a 25-mg over-the-counter tablet in Unisom SleepTabs. One-half tablet can be prescribed two or three times daily. The patient should be alerted that Unisom SleepGels contain diphenhydramine, not doxylamine.
Second-line pharmacologic treatment
Metoclopramide Metoclopramide is a dopamine antagonist. It enhances upper gastrointestinal motility, accelerates gastric emptying, and increases lower esophageal sphincter tone. At higher doses it blocks serotonin receptors in the chemoreceptor trigger zone. Its elimination half-life is 5 to 6 hours (Lexicomp). There are no large, randomized, placebo-controlled trials of oral metoclopramide for the treatment of nausea and vomiting of early pregnancy.
I am recommending metoclopramide as a second-line treatment for NVP because it appears to be effective and is not known to be associated with an increased risk of congenital malformations. Metoclopramide is widely used to prevent and treat intraoperative and postoperative nausea associated with cesarean delivery.7 In addition, intravenous (IV) metoclopramide is commonly used to treat women hospitalized with hyperemesis gravidarum. Results of randomized clinical trials demonstrate that when used to treat hyperemesis gravidarum, IV metoclopramide (10 mg every 8 hours) has similar efficacy to IV ondansetron (4 mg every 8 hours)8 and IV promethazine (25 mg every 8 hours).9 When using metoclopramide as an oral treatment for NVP, 10 mg every 8 hours is a commonly recommended regimen.
The FDA has assigned metoclopramide to pregnancy category B, which indicates that there is no evidence of fetal risk. Studies from Israel and Denmark show that metoclopramide is not associated with an increased risk of congenital malformations. In the study from Israel, among 3,458 infants born to women who had filled a prescription for metoclopramide during the first trimester of pregnancy, there was no increase in major congenital malformations, low birth weight, preterm delivery, or perinatal death.10 In the study from Denmark, among 28,486 infants born to mothers who had filled a prescription for metoclopramide in the first trimester there was no increase in congenital malformations or any of 20 individual categories of malformations, including neural tube defects, transposition of the great vessels, ventricular septal defect, atrial septal defect, tetralogy of Fallot, coarctation of the aorta, cleft lip or palate, anorectal atresia/stenosis, or limb reduction.11 The results of these two large studies are reassuring that metoclopramide is not associated with an increased risk of congenital malformations.
Metoclopramide can cause tardive dyskinesia, a serious movement disorder that may be irreversible with discontinuation of the drug. This risk increases with dose and length of treatment. The FDA recommends that clinicians avoid the use of metoclopramide for more than 12 weeks.
Third-line pharmacologic treatment: Ondansetron
In the United States ondansetron is commonly used to treat NVP.12 The drug is a selective 5-HT3 antagonist that blocks serotonin action in the central nervous system chemoreceptor trigger zone. The elimination half-life of ondansetron is 3 to 6 hours (Lexicomp).
The frequent use of ondansetron may be due, in part, to the perception that it is a very effective antiemetic. For example, in one small clinical trial, ondansetron 4 mg every 8 hours was reported to be superior to a combination of pyridoxine 25 mg every 8 hours plus doxylamine 12.5 mg every 8 hours.13 (Note that the pyridoxine and doxylamine tablets used in this trial were not in a combination delayed-release formulation.) I am recommending ondansetron as a third-line treatment for NVP because, although it is effective, it may be associated with an increased risk of fetal cardiac anomalies.
Is ondansetron associated with cardiac malformations?
The FDA has assigned ondansetron to pregnancy category B; however, there is concern that it may be associated with congenital heart defects. In a recent study of 1,349 infants born to Swedish women who had filled a prescription for ondansetron in early pregnancy, a significantly increased risk of cardiovascular defect (odds ratio [OR], 1.62; 95% confidence interval [CI], 1.04−2.14) and cardiac septum defect (OR, 2.05; 95% CI, 1.19−3.28) was reported.14 The cardiac anomalies were mostly atrial septal or ventricular septal defects.
In a second study, reported as an abstract, authors analyzed congenital malformations in 1,248 infants born to Danish women who filled a prescription for ondansetron in early pregnancy. These authors also found an increased risk of a congenital heart malformation (OR, 2.0; 95% CI, 1.3−3.1).15
A US case-control study showed an association between ondansetron use and cleft palate.1 The Swedish14 and Danish15 studies reported above did not find an association between ondansetron use and cleft palate.
The FDA issued a warning in June 2012 that at a dose of 32 mg, administered intravenously, ondansetron may prolong the QT interval and result in a potentially fatal heart arrhythmia, torsades de pointes.16 In the announcement the FDA did not alter the recommendations for oral dosing because there is no strong evidence that oral dosing is associated with clinically significant arrhythmias. Authors of a recent systematic review concluded that IV administration of large doses of ondansetron may cause cardiac arrhythmias, especially in patients with cardiac disease and those taking other drugs that prolong the QT interval, but that a single oral dose of ondansetron does not have a significant risk of causing an arrhythmia.17
Health Canada18 has advised that many commonly prescribed medications increase serotonin activity. When multiple drugs that each increase serotonin activity are prescribed in combination, the risk of serotonin syndrome is increased. Serotonin syndrome results in hyperthermia, agitation, tachycardia, and muscle twitching and can be fatal. Ondansetron was specifically mentioned in the Health Canada warning, but a search of the literature revealed very few reported cases of ondansetron being implicated in the serotonin syndrome.19
My bottom-line recommendations
NVP is a common obstetric problem. When oral pharmacologic therapy is indicated, first-line treatment should be with the FDA-approved combination of doxylamine-pyridoxine because it is both effective and associated with no known increased risk of congenital malformations. An effective second-line agent is metoclopramide. Based on very limited data, metoclopramide appears effective and is not associated with an increased risk of congenital malformations. However, it is not FDA approved for treatment of NVP. Ondansetron appears to be effective but its use in early pregnancy may be associated with congenital anomalies. Consequently, ondansetron should not be used to treat NVP unless first- and second-line treatments have been ineffective to treat the patient’s symptoms.
INSTANT POLL
Which of the following pharmacologic treatments of nausea with or without vomiting during pregnancy is your first-line medication choice?
• Ondansetron
• Metoclopramide
• Doxylamine-pyridoxine
• Meclizine Promethazine
• Trimethobenzamide
Visit the Quick Poll on the homepage, give your answer, and then see how other ObGyns have answered.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Anderka M, Mitchell AA, Louik C, Werler MMA, Hernandez-Diaz S, Rasmussen SA; National Birth Defects Prevention Study. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012;94(1):22–30.
2. Matthews A, Haas Dm, O’Mathuna DP, Dowswell T, Doyle M. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2014;(3):CD007575.
3. Borrelli F, Capasso R, Aviello G, Pittler MH, Izzo AA. Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstet Gynecol. 2005;105(4):849–856.
4. Koren G, Clark S, Hankins GD, et al. Effectiveness of delayed-release doxylamine and pyridoxine for nausea and vomiting of pregnancy: a randomized placebo controlled trial. Am J Obstet Gynecol. 2010;203(6):571.e1–7.
5. Einarson TR, Leeder JS, Koren G. A method for meta-analysis of epidemiologic studies. Drug Intell Clin Pharm. 1988;22(10):813–824.
6. McKeigue PM, Lamm SH, Linn S, Kutcher JS. Bendectin and birth defects. I. A meta-analysis of the epidemiologic studies. Teratology. 1994;50(1):27–37.
7. Mishriky BM, Habib AS. Metoclopramide for nausea and vomiting prophylaxis during and after cesarean delivery: a systematic review and meta-analysis. Br J Anaesth. 2012;108(3):374–383.
8. Abas MN, Tan PC, Azmi N, Omar SZ. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123(6):1272–1279.
9. Tan PC, Khine PP, Vallikkannu N, Omar SZ. Promethazine compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2010;115(5):975–981.
10. Matok I, Gorodischer R, Koren G, Sheiner E, Wiznitzer A, Levy A. The safety of metoclopramide use in the first trimester of pregnancy. N Engl J Med. 2009;360(24):2528–2535.
11. Pasternak B, Svanstrom H, Molgaard-Nielsen D, Melbye M, Hviid A. Metoclopramide in pregnancy and risk of major congenital malformations and fetal death. JAMA. 2013;310(15):1601–1611.
12. Koren G. Treating morning sickness in the United States—changes in prescribing are needed. Am J Obstet Gynecol. 2014;211(6):602–606.
13. Oliveira LG, Capp SM, You WB, Riffenburgh RH, Carstairs SH. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy : a randomized controlled trial. Obstet Gynecol. 2014;124(4):735–742.
14. Danielsson B, Wikner BN, Kallen B. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014;50:134–137.
15. Andersen JT, Jimenez-Solem E, Andersen NL, Poulsen HE. Ondansetron use in early pregnancy and the risk of congenital malformations—a registry based nationwide cohort study. Abstract presented at: 29th International Conference on Pharmacoepidemiology & Therapeutic Risk Management; August 25–28, 2013; Montreal, Canada. Abstract 25, Pregnancy Session 1. Pharmacoepidemiol Drug Saf. 2013;22(suppl 1):13–14.
16. US Food and Drug Administration. Ondansetron (Zofran) IV: drug safety communication - QT prolongation. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm310219.htm. Published June 29, 2012. Accessed December 26, 2014.
17. Freedman SB, Uleryk E, Rumantir M, Finkelstein Y. Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Ann Emerg Med. 2014;64(1):19–25.
18. Health Canada. Canadian Adverse Reaction Newsletter. 2003;13(3). http://www.hc-sc.gc.ca/dhp-mps/medeff/bulletin/carn-bcei_v13n3-eng.php. Published June 24, 2003. Accessed December 26, 2014.
19. Turkel SB, Nadala JG, Wincor MZ. Possible serotonin syndrome in association with 5-HT3 antagonist agents. Psychosomatics. 2001;42(3):258–260.
1. Anderka M, Mitchell AA, Louik C, Werler MMA, Hernandez-Diaz S, Rasmussen SA; National Birth Defects Prevention Study. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012;94(1):22–30.
2. Matthews A, Haas Dm, O’Mathuna DP, Dowswell T, Doyle M. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2014;(3):CD007575.
3. Borrelli F, Capasso R, Aviello G, Pittler MH, Izzo AA. Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstet Gynecol. 2005;105(4):849–856.
4. Koren G, Clark S, Hankins GD, et al. Effectiveness of delayed-release doxylamine and pyridoxine for nausea and vomiting of pregnancy: a randomized placebo controlled trial. Am J Obstet Gynecol. 2010;203(6):571.e1–7.
5. Einarson TR, Leeder JS, Koren G. A method for meta-analysis of epidemiologic studies. Drug Intell Clin Pharm. 1988;22(10):813–824.
6. McKeigue PM, Lamm SH, Linn S, Kutcher JS. Bendectin and birth defects. I. A meta-analysis of the epidemiologic studies. Teratology. 1994;50(1):27–37.
7. Mishriky BM, Habib AS. Metoclopramide for nausea and vomiting prophylaxis during and after cesarean delivery: a systematic review and meta-analysis. Br J Anaesth. 2012;108(3):374–383.
8. Abas MN, Tan PC, Azmi N, Omar SZ. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123(6):1272–1279.
9. Tan PC, Khine PP, Vallikkannu N, Omar SZ. Promethazine compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2010;115(5):975–981.
10. Matok I, Gorodischer R, Koren G, Sheiner E, Wiznitzer A, Levy A. The safety of metoclopramide use in the first trimester of pregnancy. N Engl J Med. 2009;360(24):2528–2535.
11. Pasternak B, Svanstrom H, Molgaard-Nielsen D, Melbye M, Hviid A. Metoclopramide in pregnancy and risk of major congenital malformations and fetal death. JAMA. 2013;310(15):1601–1611.
12. Koren G. Treating morning sickness in the United States—changes in prescribing are needed. Am J Obstet Gynecol. 2014;211(6):602–606.
13. Oliveira LG, Capp SM, You WB, Riffenburgh RH, Carstairs SH. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy : a randomized controlled trial. Obstet Gynecol. 2014;124(4):735–742.
14. Danielsson B, Wikner BN, Kallen B. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014;50:134–137.
15. Andersen JT, Jimenez-Solem E, Andersen NL, Poulsen HE. Ondansetron use in early pregnancy and the risk of congenital malformations—a registry based nationwide cohort study. Abstract presented at: 29th International Conference on Pharmacoepidemiology & Therapeutic Risk Management; August 25–28, 2013; Montreal, Canada. Abstract 25, Pregnancy Session 1. Pharmacoepidemiol Drug Saf. 2013;22(suppl 1):13–14.
16. US Food and Drug Administration. Ondansetron (Zofran) IV: drug safety communication - QT prolongation. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm310219.htm. Published June 29, 2012. Accessed December 26, 2014.
17. Freedman SB, Uleryk E, Rumantir M, Finkelstein Y. Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Ann Emerg Med. 2014;64(1):19–25.
18. Health Canada. Canadian Adverse Reaction Newsletter. 2003;13(3). http://www.hc-sc.gc.ca/dhp-mps/medeff/bulletin/carn-bcei_v13n3-eng.php. Published June 24, 2003. Accessed December 26, 2014.
19. Turkel SB, Nadala JG, Wincor MZ. Possible serotonin syndrome in association with 5-HT3 antagonist agents. Psychosomatics. 2001;42(3):258–260.
Dr. Robert L. Barbieri's Editors Picks for January 2015
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Click here for the rest of January 2015 issue.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Click here for the rest of January 2015 issue.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Click here for the rest of January 2015 issue.
Stop using the hCG discriminatory zone of 1,500 to 2,000 mIU/mL to guide intervention during early pregnancy
Approximately 15% of early pregnancies are complicated by pelvic or abdominal pain and uterine bleeding or spotting. In these situations, you must determine whether your patient has a viable intrauterine pregnancy, a pregnancy that will end in a miscarriage (spontaneous abortion), or an ectopic pregnancy.
To guide you to the correct diagnosis, a medical history and physical examination can be helpful. For example, a woman with a prior ectopic pregnancy who now has an early pregnancy complicated by pelvic pain and uterine bleeding is at high risk for an ectopic pregnancy. Physical examination also is important; if the pelvic examination reveals a dilated cervix with pregnancy tissue in the cervical os it is likely that a miscarriage is in progress. For most cases of early pregnancy complicated by pelvic pain and/or uterine bleeding, however, a pelvic sonogram and serial quantitative measurement of human chorionic gonadotropin (hCG) are needed to achieve the correct diagnosis.
Here I outline the clinical markers for transvaginal ultrasonography that indicate a viable or failed intrauterine pregnancy as well as an ectopic pregnancy. I also present data on single vs serial hCG measurement and discuss serial hCG levels that indicate viable or nonviable intrauterine or ectopic pregnancy.
Is an early gestation viable? Clinical evaluationTransvaginal pelvic ultrasoundTransvaginal and transabdominal ultrasonography play a critical role in evaluating early pregnancy problems. In a normal pregnancy, key developmental milestones that can be observed reliably on ultrasound are1,2:
- intrauterine gestational sac at 5 weeks
- yolk sac at 5.5 weeks
- embryonic pole and fetal heart beat at 6 to 6.5 weeks’ gestation.
A pelvic ultrasound also may provide evidence that an intrauterine pregnancy will fail and result in a miscarriage. Findings diagnostic of a failed intrauterine pregnancy include3:
- crown-rump length ≥7 mm and no fetal heartbeat
- mean sac diameter ≥25 mm and no embryo
- absence of an embryo with a heartbeat more than 2 weeks after an ultrasound scan that showed a gestational sac without a yolk sac
- absence of an embryo with a heartbeat more than 11 days after a scan that showed a gestational sac with a yolk sac.
Findings suspicious for a failing intrauterine pregnancy include3:
- crown-rump length <7 mm and no fetal heartbeat
- mean sac diameter of 16 to 24 mm and no embryo
- no heartbeat 7 to 13 days after an ultrasound scan that showed a gestational sac without a yolk sac
- no heartbeat 7 to 10 days after an ultrasound scan that showed a gestational sac with a yolk sac.
When it’s an ectopic pregnancy. Definitive ultrasonographic evidence of an ectopic pregnancy is identification of a fetal heartbeat outside the uterus or a gestational sac and yolk sac outside of the uterus. An adnexal mass can be identified on ultrasonography in most cases of ectopic pregnancy. In one study of 291 ectopic pregnancies, an adnexal mass was identified in 94% of cases, and a moderate to large amount of free pelvic fluid was found in 36% of cases.4 The adnexal masses included nonspecific (54% of all ectopic cases), a tubal ring without a yolk sac or embryo (25%), a yolk sac but no embryonic heartbeat (8%), and an embryo with cardiac activity (7%).
In clinical units with high-quality gynecologic ultrasonography available, most ectopic pregnancies will be detected on initial scan and only 10% to 15% of ectopic pregnancies will have an ultrasound finding of no intrauterine pregnancy and no evidence for an extrauterine pregnancy.5
Almost all (in the range of 99%) viable intrauterine pregnancies demonstrate an increase in hCG level of 53% or more over 48 hours, whereas only 21% of ectopic pregnancies demonstrate a rise of 53% or more.7
Most pregnancies that will end in a miscarriage demonstrate a decrease in hCG level over 48 hours. If the initial hCG value is 2,000 mIU/mL, 90% of pregnancies that will end in miscarriage will have an approximate 30% decrease in hCG over 48 hours. If the initial hCG is 1,000 mIU/mL, 95% of spontaneous abortions will have a 28% decline in 48 hours.7 About 10% of ectopic pregnancies also will demonstrate a 30% decrease in hCG over 48 hours.
A minor disadvantage of serial hCG measurements is that patients may become anxious and fearful as they await the result of life-altering test results.
When a gestation is found to be nonviable A viable intrauterine pregnancy is highly unlikely in a woman with no ultrasound evidence of an intrauterine pregnancy or an adnexal mass and an hCG level that rises very little, plateaus, or decreases over 48 hours. In this situation, a Karman cannula aspiration of uterine contents with rush pathology analysis can help clarify the likely diagnosis and guide therapy.
Women with documented villi on pathology likely are experiencing a miscarriage and can have their hCG level followed to resolution. Women with no documented villi and no decrease in hCG after the Karman
cannula aspiration can be presumed to have an ectopic pregnancy. If stable, these women may be candidates for treatment with methotrexate.8,9
Many experts have counseled against the use of a single hCG measurement in the discriminatory zone of 1,500 to 2,000 mIU/mL to trigger methotrexate treatment. Here is a sampling of their advice:
“An hCG level of 2,000 mIU/mL, without ultrasound findings of intrauterine pregnancy, while suggestive of abnormal pregnancy, is not diagnostic. Per the results of recent studies, it is reasonable to closely follow up rather than treat many of these early, stable cases of ectopic pregnancy.”
—Mehta et al.1
“Our data demonstrate that using a single value of serum hCG in a pregnancy of unknown location (PUL) population is of limited value.... A significant proportion of failing PULs and early intrauterine pregnancies in a PUL population have high serum hCG levels at presentation.”
—Condus et al.2
“The hCG discriminatory level should not be used to determine the management of a hemodynamically stable patient with suspected ectopic pregnancy, if sonography demonstrates no findings of intrauterine or ectopic pregnancy.”
—Doubilet et al.3
“There is almost no reason to give methotrexate on first encounter with a patient. If a patient is symptomatic with severe pain or signs of rupture, a surgical approach is indicated and methotrexate is contraindicated.”
—Barnhart et al.4
[When using the discriminatory zone]... “there is a chance of harming a viable intrauterine pregnancy, especially if the hCG level is 2000 to 3000 mIU/mL.... There is limited risk in taking a few extra days to make a definitive diagnosis in a woman with a pregnancy of unknown location who has no signs or symptoms of rupture and no ultrasonographic evidence of ectopic pregnancy.”
—Doubliet et al.3
“Viable intrauterine pregnancy is possible in patients with pregnancy of unknown location and hCG levels above the generally accept discriminatory zone, strict adherence to which can potentially disrupt a normal pregnancy. We support the need for judicious use of the hCG discriminatory level in hemodynamically stable patients with pregnancy of unknown location, and the decision to intervene should not be based solely on a single hCG level.”
—Ko and Cheung.5
References
- Mehta TS, Levine D, Beckwith B. Treatment of ectopic pregnancy: is a human chorionic gonadotropin level of 2,000 mIU/mL a reasonable threshold? Radiology. 1997;205(2):569–573.
- Condous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26(7):770–775.
- Doubilet PM, Benson CB, Bourne T, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
- Barnhart KT. Early pregnancy failure: beware of the pitfalls of modern management. Fertil Steril. 2012;98(5):1061–1065.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33(3):465–471.
Stop using the discriminatory zone and a single hCG measurement to trigger clinical intervention As noted above, a single hCG measurement is of very little value in determining whether an early pregnancy is a viable or nonviable intrauterine pregnancy or is ectopic. Many experts have reported that if a single hCG measurement shows a value of more than 1,500 mIU/mL and a pelvic ultrasound shows no intrauterine pregnancy, an ectopic or nonviable intrauterine pregnancy is likely. Some experts have used the presence of an hCG value of more than 1,500 mIU/mL plus an ultrasound scan without evidence of an intrauterine pregnancy to clinically diagnose the absence of a viable intrauterine pregnancy and administer methotrexate to treat a presumptive ectopic pregnancy. Many experts believe, however, that this approach will necessarily result in the treatment of viable intrauterine pregnancies with methotrexate.5,10
Based on one analysis, for 100 women with an initial hCG value between 2,000 and 3,000 mIU/mL and no intrauterine pregnancy or adnexal mass seen on ultrasound, follow-up will reveal that 65.5% had a failed intrauterine pregnancy, 33% had an ectopic pregnancy, and 1.5% had a viable intrauterine pregnancy.3,10,11 If all of these 100 women had been treated with methotrexate for a presumed ectopic pregnancy, approximately two women with a viable intrauterine pregnancy would have been exposed to methotrexate. This exposure would likely result in either a pregnancy loss or, if the pregnancy continues, an increased risk of fetal anomalies.
If the patient is obese, has fibroids, or has adenomyosis, she has an increased risk of an ultrasound failing to detect an early intrauterine pregnancy when the hCG value ranges from 1,500 to 3,000 mIU/mL.12 If the discriminatory zone is raised to 4,000 mIU/mL, the likelihood of mistakenly diagnosing a viable intrauterine pregnancy as a failed or ectopic pregnancy is much less (but not zero).
There is almost no clinical situation in which methotrexate should be given to a patient suspected of having an ectopic pregnancy on the first visit, unless ultrasound demonstrates an adnexal mass indicative of ectopic pregnancy.13,14 If the patient has severe pain or bleeding, or has signs consistent with a ruptured ectopic pregnancy, surgical intervention likely is warranted. If the patient is clinically stable, a safe option is to repeat the hCG measurement in 48 hours, with an ultrasound if indicated.
The discriminatory zone is an interesting and elegant idea. But in practice it is fraught with serious dangers, the greatest of which is methotrexate administration to a patient with a viable intrauterine gestation. My advice is that gynecologists should stop relying on a discriminatory zone of 1,500 to 2,000 mIU/mL to trigger clinical intervention.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Bree RL, Edwards M, Böhm-Vélez M, et al. Transvaginal sonography in the evaluation of normal early pregnancy: correlation with hCG level. AJR Am J Roentgenol. 1989;153(1):75–79.
2. Goldstein I, Zimmer EA, Tamir A, Peretz BA, Paldi E. Evaluation of normal gestational sac growth: appearance of embryonic heartbeat and embryo body movements using the transvaginal technique. Obstet Gynecol. 1991;77(6):885–888.
3. Doubilet PM, Benson CB, Bourne T, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
4. Frates MC, Doubilet PM, Peters HE, Benson CR. Adnexal sonographic findings in ectopic pregnancy and their correlation with tubal rupture and human chorionic gonadotropin levels. J Ultrasound Med. 2014;33(4):697–703.
5. Condous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26(7):770–775.
6. Barnhart KT. Clinical practice. Ectopic pregnancy. N Engl J Med. 2009;361(4):379–387.
7. Silva C, Sammel MD, Zhou L, et al. Human chorionic gonadotropin profile for women with ectopic pregnancy. Obstet Gynecol. 2006;107(3):605–610.
8. Shaunik A, Kulp J, Appleby DH, Sammel MD, Barnhart KT. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204(2):130.e1–6.
9. Brady P, Imudia AN, Awonuga AO, Wright DL, Syter AK, Toth TL. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101(2):420–426.
10. Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic discriminatory level. J Ultrasound Med. 2011;30(12):1637–1642.
11. Benson CB, Doubilet PM, Peters HE, Frates MC. Intrauterine fluid with ectopic pregnancy: a reappraisal. J Ultrasound Med. 2013;32(3):389–393.
12. Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33(3):465–471.
13. Barnhart KT. Early pregnancy failure: beware of the pitfalls of modern management. Fertil Steril. 2012;98(5):1061–1065.
14. Mehta TS, Levine D, Beckwith B. Treatment of ectopic pregnancy: is a human chorionic gonadotropin level of 2,000 mIU/mL a reasonable threshold? Radiology. 1997;205(2):569–573.
Approximately 15% of early pregnancies are complicated by pelvic or abdominal pain and uterine bleeding or spotting. In these situations, you must determine whether your patient has a viable intrauterine pregnancy, a pregnancy that will end in a miscarriage (spontaneous abortion), or an ectopic pregnancy.
To guide you to the correct diagnosis, a medical history and physical examination can be helpful. For example, a woman with a prior ectopic pregnancy who now has an early pregnancy complicated by pelvic pain and uterine bleeding is at high risk for an ectopic pregnancy. Physical examination also is important; if the pelvic examination reveals a dilated cervix with pregnancy tissue in the cervical os it is likely that a miscarriage is in progress. For most cases of early pregnancy complicated by pelvic pain and/or uterine bleeding, however, a pelvic sonogram and serial quantitative measurement of human chorionic gonadotropin (hCG) are needed to achieve the correct diagnosis.
Here I outline the clinical markers for transvaginal ultrasonography that indicate a viable or failed intrauterine pregnancy as well as an ectopic pregnancy. I also present data on single vs serial hCG measurement and discuss serial hCG levels that indicate viable or nonviable intrauterine or ectopic pregnancy.
Is an early gestation viable? Clinical evaluationTransvaginal pelvic ultrasoundTransvaginal and transabdominal ultrasonography play a critical role in evaluating early pregnancy problems. In a normal pregnancy, key developmental milestones that can be observed reliably on ultrasound are1,2:
- intrauterine gestational sac at 5 weeks
- yolk sac at 5.5 weeks
- embryonic pole and fetal heart beat at 6 to 6.5 weeks’ gestation.
A pelvic ultrasound also may provide evidence that an intrauterine pregnancy will fail and result in a miscarriage. Findings diagnostic of a failed intrauterine pregnancy include3:
- crown-rump length ≥7 mm and no fetal heartbeat
- mean sac diameter ≥25 mm and no embryo
- absence of an embryo with a heartbeat more than 2 weeks after an ultrasound scan that showed a gestational sac without a yolk sac
- absence of an embryo with a heartbeat more than 11 days after a scan that showed a gestational sac with a yolk sac.
Findings suspicious for a failing intrauterine pregnancy include3:
- crown-rump length <7 mm and no fetal heartbeat
- mean sac diameter of 16 to 24 mm and no embryo
- no heartbeat 7 to 13 days after an ultrasound scan that showed a gestational sac without a yolk sac
- no heartbeat 7 to 10 days after an ultrasound scan that showed a gestational sac with a yolk sac.
When it’s an ectopic pregnancy. Definitive ultrasonographic evidence of an ectopic pregnancy is identification of a fetal heartbeat outside the uterus or a gestational sac and yolk sac outside of the uterus. An adnexal mass can be identified on ultrasonography in most cases of ectopic pregnancy. In one study of 291 ectopic pregnancies, an adnexal mass was identified in 94% of cases, and a moderate to large amount of free pelvic fluid was found in 36% of cases.4 The adnexal masses included nonspecific (54% of all ectopic cases), a tubal ring without a yolk sac or embryo (25%), a yolk sac but no embryonic heartbeat (8%), and an embryo with cardiac activity (7%).
In clinical units with high-quality gynecologic ultrasonography available, most ectopic pregnancies will be detected on initial scan and only 10% to 15% of ectopic pregnancies will have an ultrasound finding of no intrauterine pregnancy and no evidence for an extrauterine pregnancy.5

Almost all (in the range of 99%) viable intrauterine pregnancies demonstrate an increase in hCG level of 53% or more over 48 hours, whereas only 21% of ectopic pregnancies demonstrate a rise of 53% or more.7
Most pregnancies that will end in a miscarriage demonstrate a decrease in hCG level over 48 hours. If the initial hCG value is 2,000 mIU/mL, 90% of pregnancies that will end in miscarriage will have an approximate 30% decrease in hCG over 48 hours. If the initial hCG is 1,000 mIU/mL, 95% of spontaneous abortions will have a 28% decline in 48 hours.7 About 10% of ectopic pregnancies also will demonstrate a 30% decrease in hCG over 48 hours.
A minor disadvantage of serial hCG measurements is that patients may become anxious and fearful as they await the result of life-altering test results.
When a gestation is found to be nonviable A viable intrauterine pregnancy is highly unlikely in a woman with no ultrasound evidence of an intrauterine pregnancy or an adnexal mass and an hCG level that rises very little, plateaus, or decreases over 48 hours. In this situation, a Karman cannula aspiration of uterine contents with rush pathology analysis can help clarify the likely diagnosis and guide therapy.
Women with documented villi on pathology likely are experiencing a miscarriage and can have their hCG level followed to resolution. Women with no documented villi and no decrease in hCG after the Karman
cannula aspiration can be presumed to have an ectopic pregnancy. If stable, these women may be candidates for treatment with methotrexate.8,9
Many experts have counseled against the use of a single hCG measurement in the discriminatory zone of 1,500 to 2,000 mIU/mL to trigger methotrexate treatment. Here is a sampling of their advice:
“An hCG level of 2,000 mIU/mL, without ultrasound findings of intrauterine pregnancy, while suggestive of abnormal pregnancy, is not diagnostic. Per the results of recent studies, it is reasonable to closely follow up rather than treat many of these early, stable cases of ectopic pregnancy.”
—Mehta et al.1
“Our data demonstrate that using a single value of serum hCG in a pregnancy of unknown location (PUL) population is of limited value.... A significant proportion of failing PULs and early intrauterine pregnancies in a PUL population have high serum hCG levels at presentation.”
—Condus et al.2
“The hCG discriminatory level should not be used to determine the management of a hemodynamically stable patient with suspected ectopic pregnancy, if sonography demonstrates no findings of intrauterine or ectopic pregnancy.”
—Doubilet et al.3
“There is almost no reason to give methotrexate on first encounter with a patient. If a patient is symptomatic with severe pain or signs of rupture, a surgical approach is indicated and methotrexate is contraindicated.”
—Barnhart et al.4
[When using the discriminatory zone]... “there is a chance of harming a viable intrauterine pregnancy, especially if the hCG level is 2000 to 3000 mIU/mL.... There is limited risk in taking a few extra days to make a definitive diagnosis in a woman with a pregnancy of unknown location who has no signs or symptoms of rupture and no ultrasonographic evidence of ectopic pregnancy.”
—Doubliet et al.3
“Viable intrauterine pregnancy is possible in patients with pregnancy of unknown location and hCG levels above the generally accept discriminatory zone, strict adherence to which can potentially disrupt a normal pregnancy. We support the need for judicious use of the hCG discriminatory level in hemodynamically stable patients with pregnancy of unknown location, and the decision to intervene should not be based solely on a single hCG level.”
—Ko and Cheung.5
References
- Mehta TS, Levine D, Beckwith B. Treatment of ectopic pregnancy: is a human chorionic gonadotropin level of 2,000 mIU/mL a reasonable threshold? Radiology. 1997;205(2):569–573.
- Condous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26(7):770–775.
- Doubilet PM, Benson CB, Bourne T, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
- Barnhart KT. Early pregnancy failure: beware of the pitfalls of modern management. Fertil Steril. 2012;98(5):1061–1065.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33(3):465–471.
Stop using the discriminatory zone and a single hCG measurement to trigger clinical intervention As noted above, a single hCG measurement is of very little value in determining whether an early pregnancy is a viable or nonviable intrauterine pregnancy or is ectopic. Many experts have reported that if a single hCG measurement shows a value of more than 1,500 mIU/mL and a pelvic ultrasound shows no intrauterine pregnancy, an ectopic or nonviable intrauterine pregnancy is likely. Some experts have used the presence of an hCG value of more than 1,500 mIU/mL plus an ultrasound scan without evidence of an intrauterine pregnancy to clinically diagnose the absence of a viable intrauterine pregnancy and administer methotrexate to treat a presumptive ectopic pregnancy. Many experts believe, however, that this approach will necessarily result in the treatment of viable intrauterine pregnancies with methotrexate.5,10
Based on one analysis, for 100 women with an initial hCG value between 2,000 and 3,000 mIU/mL and no intrauterine pregnancy or adnexal mass seen on ultrasound, follow-up will reveal that 65.5% had a failed intrauterine pregnancy, 33% had an ectopic pregnancy, and 1.5% had a viable intrauterine pregnancy.3,10,11 If all of these 100 women had been treated with methotrexate for a presumed ectopic pregnancy, approximately two women with a viable intrauterine pregnancy would have been exposed to methotrexate. This exposure would likely result in either a pregnancy loss or, if the pregnancy continues, an increased risk of fetal anomalies.
If the patient is obese, has fibroids, or has adenomyosis, she has an increased risk of an ultrasound failing to detect an early intrauterine pregnancy when the hCG value ranges from 1,500 to 3,000 mIU/mL.12 If the discriminatory zone is raised to 4,000 mIU/mL, the likelihood of mistakenly diagnosing a viable intrauterine pregnancy as a failed or ectopic pregnancy is much less (but not zero).
There is almost no clinical situation in which methotrexate should be given to a patient suspected of having an ectopic pregnancy on the first visit, unless ultrasound demonstrates an adnexal mass indicative of ectopic pregnancy.13,14 If the patient has severe pain or bleeding, or has signs consistent with a ruptured ectopic pregnancy, surgical intervention likely is warranted. If the patient is clinically stable, a safe option is to repeat the hCG measurement in 48 hours, with an ultrasound if indicated.
The discriminatory zone is an interesting and elegant idea. But in practice it is fraught with serious dangers, the greatest of which is methotrexate administration to a patient with a viable intrauterine gestation. My advice is that gynecologists should stop relying on a discriminatory zone of 1,500 to 2,000 mIU/mL to trigger clinical intervention.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Approximately 15% of early pregnancies are complicated by pelvic or abdominal pain and uterine bleeding or spotting. In these situations, you must determine whether your patient has a viable intrauterine pregnancy, a pregnancy that will end in a miscarriage (spontaneous abortion), or an ectopic pregnancy.
To guide you to the correct diagnosis, a medical history and physical examination can be helpful. For example, a woman with a prior ectopic pregnancy who now has an early pregnancy complicated by pelvic pain and uterine bleeding is at high risk for an ectopic pregnancy. Physical examination also is important; if the pelvic examination reveals a dilated cervix with pregnancy tissue in the cervical os it is likely that a miscarriage is in progress. For most cases of early pregnancy complicated by pelvic pain and/or uterine bleeding, however, a pelvic sonogram and serial quantitative measurement of human chorionic gonadotropin (hCG) are needed to achieve the correct diagnosis.
Here I outline the clinical markers for transvaginal ultrasonography that indicate a viable or failed intrauterine pregnancy as well as an ectopic pregnancy. I also present data on single vs serial hCG measurement and discuss serial hCG levels that indicate viable or nonviable intrauterine or ectopic pregnancy.
Is an early gestation viable? Clinical evaluationTransvaginal pelvic ultrasoundTransvaginal and transabdominal ultrasonography play a critical role in evaluating early pregnancy problems. In a normal pregnancy, key developmental milestones that can be observed reliably on ultrasound are1,2:
- intrauterine gestational sac at 5 weeks
- yolk sac at 5.5 weeks
- embryonic pole and fetal heart beat at 6 to 6.5 weeks’ gestation.
A pelvic ultrasound also may provide evidence that an intrauterine pregnancy will fail and result in a miscarriage. Findings diagnostic of a failed intrauterine pregnancy include3:
- crown-rump length ≥7 mm and no fetal heartbeat
- mean sac diameter ≥25 mm and no embryo
- absence of an embryo with a heartbeat more than 2 weeks after an ultrasound scan that showed a gestational sac without a yolk sac
- absence of an embryo with a heartbeat more than 11 days after a scan that showed a gestational sac with a yolk sac.
Findings suspicious for a failing intrauterine pregnancy include3:
- crown-rump length <7 mm and no fetal heartbeat
- mean sac diameter of 16 to 24 mm and no embryo
- no heartbeat 7 to 13 days after an ultrasound scan that showed a gestational sac without a yolk sac
- no heartbeat 7 to 10 days after an ultrasound scan that showed a gestational sac with a yolk sac.
When it’s an ectopic pregnancy. Definitive ultrasonographic evidence of an ectopic pregnancy is identification of a fetal heartbeat outside the uterus or a gestational sac and yolk sac outside of the uterus. An adnexal mass can be identified on ultrasonography in most cases of ectopic pregnancy. In one study of 291 ectopic pregnancies, an adnexal mass was identified in 94% of cases, and a moderate to large amount of free pelvic fluid was found in 36% of cases.4 The adnexal masses included nonspecific (54% of all ectopic cases), a tubal ring without a yolk sac or embryo (25%), a yolk sac but no embryonic heartbeat (8%), and an embryo with cardiac activity (7%).
In clinical units with high-quality gynecologic ultrasonography available, most ectopic pregnancies will be detected on initial scan and only 10% to 15% of ectopic pregnancies will have an ultrasound finding of no intrauterine pregnancy and no evidence for an extrauterine pregnancy.5

Almost all (in the range of 99%) viable intrauterine pregnancies demonstrate an increase in hCG level of 53% or more over 48 hours, whereas only 21% of ectopic pregnancies demonstrate a rise of 53% or more.7
Most pregnancies that will end in a miscarriage demonstrate a decrease in hCG level over 48 hours. If the initial hCG value is 2,000 mIU/mL, 90% of pregnancies that will end in miscarriage will have an approximate 30% decrease in hCG over 48 hours. If the initial hCG is 1,000 mIU/mL, 95% of spontaneous abortions will have a 28% decline in 48 hours.7 About 10% of ectopic pregnancies also will demonstrate a 30% decrease in hCG over 48 hours.
A minor disadvantage of serial hCG measurements is that patients may become anxious and fearful as they await the result of life-altering test results.
When a gestation is found to be nonviable A viable intrauterine pregnancy is highly unlikely in a woman with no ultrasound evidence of an intrauterine pregnancy or an adnexal mass and an hCG level that rises very little, plateaus, or decreases over 48 hours. In this situation, a Karman cannula aspiration of uterine contents with rush pathology analysis can help clarify the likely diagnosis and guide therapy.
Women with documented villi on pathology likely are experiencing a miscarriage and can have their hCG level followed to resolution. Women with no documented villi and no decrease in hCG after the Karman
cannula aspiration can be presumed to have an ectopic pregnancy. If stable, these women may be candidates for treatment with methotrexate.8,9
Many experts have counseled against the use of a single hCG measurement in the discriminatory zone of 1,500 to 2,000 mIU/mL to trigger methotrexate treatment. Here is a sampling of their advice:
“An hCG level of 2,000 mIU/mL, without ultrasound findings of intrauterine pregnancy, while suggestive of abnormal pregnancy, is not diagnostic. Per the results of recent studies, it is reasonable to closely follow up rather than treat many of these early, stable cases of ectopic pregnancy.”
—Mehta et al.1
“Our data demonstrate that using a single value of serum hCG in a pregnancy of unknown location (PUL) population is of limited value.... A significant proportion of failing PULs and early intrauterine pregnancies in a PUL population have high serum hCG levels at presentation.”
—Condus et al.2
“The hCG discriminatory level should not be used to determine the management of a hemodynamically stable patient with suspected ectopic pregnancy, if sonography demonstrates no findings of intrauterine or ectopic pregnancy.”
—Doubilet et al.3
“There is almost no reason to give methotrexate on first encounter with a patient. If a patient is symptomatic with severe pain or signs of rupture, a surgical approach is indicated and methotrexate is contraindicated.”
—Barnhart et al.4
[When using the discriminatory zone]... “there is a chance of harming a viable intrauterine pregnancy, especially if the hCG level is 2000 to 3000 mIU/mL.... There is limited risk in taking a few extra days to make a definitive diagnosis in a woman with a pregnancy of unknown location who has no signs or symptoms of rupture and no ultrasonographic evidence of ectopic pregnancy.”
—Doubliet et al.3
“Viable intrauterine pregnancy is possible in patients with pregnancy of unknown location and hCG levels above the generally accept discriminatory zone, strict adherence to which can potentially disrupt a normal pregnancy. We support the need for judicious use of the hCG discriminatory level in hemodynamically stable patients with pregnancy of unknown location, and the decision to intervene should not be based solely on a single hCG level.”
—Ko and Cheung.5
References
- Mehta TS, Levine D, Beckwith B. Treatment of ectopic pregnancy: is a human chorionic gonadotropin level of 2,000 mIU/mL a reasonable threshold? Radiology. 1997;205(2):569–573.
- Condous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26(7):770–775.
- Doubilet PM, Benson CB, Bourne T, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
- Barnhart KT. Early pregnancy failure: beware of the pitfalls of modern management. Fertil Steril. 2012;98(5):1061–1065.
- Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33(3):465–471.
Stop using the discriminatory zone and a single hCG measurement to trigger clinical intervention As noted above, a single hCG measurement is of very little value in determining whether an early pregnancy is a viable or nonviable intrauterine pregnancy or is ectopic. Many experts have reported that if a single hCG measurement shows a value of more than 1,500 mIU/mL and a pelvic ultrasound shows no intrauterine pregnancy, an ectopic or nonviable intrauterine pregnancy is likely. Some experts have used the presence of an hCG value of more than 1,500 mIU/mL plus an ultrasound scan without evidence of an intrauterine pregnancy to clinically diagnose the absence of a viable intrauterine pregnancy and administer methotrexate to treat a presumptive ectopic pregnancy. Many experts believe, however, that this approach will necessarily result in the treatment of viable intrauterine pregnancies with methotrexate.5,10
Based on one analysis, for 100 women with an initial hCG value between 2,000 and 3,000 mIU/mL and no intrauterine pregnancy or adnexal mass seen on ultrasound, follow-up will reveal that 65.5% had a failed intrauterine pregnancy, 33% had an ectopic pregnancy, and 1.5% had a viable intrauterine pregnancy.3,10,11 If all of these 100 women had been treated with methotrexate for a presumed ectopic pregnancy, approximately two women with a viable intrauterine pregnancy would have been exposed to methotrexate. This exposure would likely result in either a pregnancy loss or, if the pregnancy continues, an increased risk of fetal anomalies.
If the patient is obese, has fibroids, or has adenomyosis, she has an increased risk of an ultrasound failing to detect an early intrauterine pregnancy when the hCG value ranges from 1,500 to 3,000 mIU/mL.12 If the discriminatory zone is raised to 4,000 mIU/mL, the likelihood of mistakenly diagnosing a viable intrauterine pregnancy as a failed or ectopic pregnancy is much less (but not zero).
There is almost no clinical situation in which methotrexate should be given to a patient suspected of having an ectopic pregnancy on the first visit, unless ultrasound demonstrates an adnexal mass indicative of ectopic pregnancy.13,14 If the patient has severe pain or bleeding, or has signs consistent with a ruptured ectopic pregnancy, surgical intervention likely is warranted. If the patient is clinically stable, a safe option is to repeat the hCG measurement in 48 hours, with an ultrasound if indicated.
The discriminatory zone is an interesting and elegant idea. But in practice it is fraught with serious dangers, the greatest of which is methotrexate administration to a patient with a viable intrauterine gestation. My advice is that gynecologists should stop relying on a discriminatory zone of 1,500 to 2,000 mIU/mL to trigger clinical intervention.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Bree RL, Edwards M, Böhm-Vélez M, et al. Transvaginal sonography in the evaluation of normal early pregnancy: correlation with hCG level. AJR Am J Roentgenol. 1989;153(1):75–79.
2. Goldstein I, Zimmer EA, Tamir A, Peretz BA, Paldi E. Evaluation of normal gestational sac growth: appearance of embryonic heartbeat and embryo body movements using the transvaginal technique. Obstet Gynecol. 1991;77(6):885–888.
3. Doubilet PM, Benson CB, Bourne T, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
4. Frates MC, Doubilet PM, Peters HE, Benson CR. Adnexal sonographic findings in ectopic pregnancy and their correlation with tubal rupture and human chorionic gonadotropin levels. J Ultrasound Med. 2014;33(4):697–703.
5. Condous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26(7):770–775.
6. Barnhart KT. Clinical practice. Ectopic pregnancy. N Engl J Med. 2009;361(4):379–387.
7. Silva C, Sammel MD, Zhou L, et al. Human chorionic gonadotropin profile for women with ectopic pregnancy. Obstet Gynecol. 2006;107(3):605–610.
8. Shaunik A, Kulp J, Appleby DH, Sammel MD, Barnhart KT. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204(2):130.e1–6.
9. Brady P, Imudia AN, Awonuga AO, Wright DL, Syter AK, Toth TL. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101(2):420–426.
10. Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic discriminatory level. J Ultrasound Med. 2011;30(12):1637–1642.
11. Benson CB, Doubilet PM, Peters HE, Frates MC. Intrauterine fluid with ectopic pregnancy: a reappraisal. J Ultrasound Med. 2013;32(3):389–393.
12. Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33(3):465–471.
13. Barnhart KT. Early pregnancy failure: beware of the pitfalls of modern management. Fertil Steril. 2012;98(5):1061–1065.
14. Mehta TS, Levine D, Beckwith B. Treatment of ectopic pregnancy: is a human chorionic gonadotropin level of 2,000 mIU/mL a reasonable threshold? Radiology. 1997;205(2):569–573.
1. Bree RL, Edwards M, Böhm-Vélez M, et al. Transvaginal sonography in the evaluation of normal early pregnancy: correlation with hCG level. AJR Am J Roentgenol. 1989;153(1):75–79.
2. Goldstein I, Zimmer EA, Tamir A, Peretz BA, Paldi E. Evaluation of normal gestational sac growth: appearance of embryonic heartbeat and embryo body movements using the transvaginal technique. Obstet Gynecol. 1991;77(6):885–888.
3. Doubilet PM, Benson CB, Bourne T, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–1451.
4. Frates MC, Doubilet PM, Peters HE, Benson CR. Adnexal sonographic findings in ectopic pregnancy and their correlation with tubal rupture and human chorionic gonadotropin levels. J Ultrasound Med. 2014;33(4):697–703.
5. Condous G, Kirk E, Lu C, et al. Diagnostic accuracy of varying discriminatory zones for the prediction of ectopic pregnancy in women with pregnancy of unknown location. Ultrasound Obstet Gynecol. 2005;26(7):770–775.
6. Barnhart KT. Clinical practice. Ectopic pregnancy. N Engl J Med. 2009;361(4):379–387.
7. Silva C, Sammel MD, Zhou L, et al. Human chorionic gonadotropin profile for women with ectopic pregnancy. Obstet Gynecol. 2006;107(3):605–610.
8. Shaunik A, Kulp J, Appleby DH, Sammel MD, Barnhart KT. Utility of dilation and curettage in the diagnosis of pregnancy of unknown location. Am J Obstet Gynecol. 2011;204(2):130.e1–6.
9. Brady P, Imudia AN, Awonuga AO, Wright DL, Syter AK, Toth TL. Pregnancies of unknown location after in vitro fertilization: minimally invasive management with Karman cannula aspiration. Fertil Steril. 2014;101(2):420–426.
10. Doubilet PM, Benson CB. Further evidence against the reliability of the human chorionic discriminatory level. J Ultrasound Med. 2011;30(12):1637–1642.
11. Benson CB, Doubilet PM, Peters HE, Frates MC. Intrauterine fluid with ectopic pregnancy: a reappraisal. J Ultrasound Med. 2013;32(3):389–393.
12. Ko JK, Cheung VY. Time to revisit the human chorionic gonadotropin discriminatory level in the management of pregnancy of unknown location. J Ultrasound Med. 2014;33(3):465–471.
13. Barnhart KT. Early pregnancy failure: beware of the pitfalls of modern management. Fertil Steril. 2012;98(5):1061–1065.
14. Mehta TS, Levine D, Beckwith B. Treatment of ectopic pregnancy: is a human chorionic gonadotropin level of 2,000 mIU/mL a reasonable threshold? Radiology. 1997;205(2):569–573.
Nitrous oxide for labor pain
Neuraxial anesthesia, including epidural and combined spinal-epidural anesthetics, are the “gold standard” interventions for pain relief during labor because they provide a superb combination of reliable pain relief and safety for the mother and child.1 Many US birthing centers also offer additional options for managing labor pain, including continuous labor support,2 hydrotherapy,3 and parenteral opioids.4 In 2012, the US Food and Drug Administration (FDA) approved equipment to deliver a mixture of 50% nitrous oxide and 50% oxygen, which has offered a new option for laboring mothers.
Nitrous oxide is widely used for labor pain in the United Kingdom, Finland, Sweden, Canada, Australia, and New Zealand.5 In the United States, nitrous oxide has been a long-standing and common adjunct to general anesthetics, although it recently has fallen out of favor in place of better, more rapidly acting inhalation and intravenous general anesthetics. With these agents not suitable for labor analgesic use, however, nitrous oxide is undergoing a resurgence in popularity for obstetric analgesia in the United States, and we believe that it will evolve to have a prominent place among our interventions for labor pain.6 In this editorial, we detail the mechanism of action and the equipment’s use, as well as benefits for patients and cautions for clinicians.
How does nitrous oxide work?
Pharmacology. Nitrous oxide (N2O) was first synthesized by Joseph Priestley in 1772 and was used as an anesthetic for dental surgery in the mid-1800s. In the late 19th Century, nitrous oxide was tested as an agent for labor analgesia.7 It was introduced into clinical practice in the United Kingdom in the 1930s.8
The mechanism of action of nitrous oxide is not fully characterized. It is thought that the gas may produce analgesia by activating the endogenous opioid and noradrenergic systems, which in turn, modulate spinal cord transmission of pain signals.5
Administration to the laboring mother. For labor analgesia, nitrous oxide is typically administered as a mix of 50% N2O and 50% O2 using a portable unit with a gas mixer that is fed by small tanks of N2O and O2 or with a valve fed by a single tank containing a mixture of both N2O and O2. The portable units approved by the FDA contain an oxygen fail-safe system that ensures delivery of an appropriate oxygen concentration. The portable unit also contains a gas scavenging system that is attached to wall suction. The breathing circuit has a mask or a mouthpiece (according to patient preference) and demand valve. The patient places the mask over her nose and mouth, or uses just her mouth for the mouthpiece. With inhalation, the demand valve opens, releasing the gas mixture. On exhalation, the valve shunts the exhaled gases to the scavenging system.
Proper and safe use requires adherence to the principles of a true “patient-controlled” protocol. Only the patient is permitted to place the mask or mouthpiece over her nose and/or mouth. If the patient becomes drowsy, such that she cannot hold the mask to her face, then the internal demand valve will not deliver nitrous oxide and she will return to breathing room air. No one should hold the mask over the patient’s nose or mouth, and the mask should not be fixed in place with elastic bands because these actions may result in the inhalation of too much nitrous oxide.
Nitrous oxide has a rapid onset of action after inhalation and its action quickly dissipates after discontinuing inhalation. There is likely a dose-response relationship, with greater use of the nitrous oxide producing more drowsiness. With the intermittent inhalation method, the laboring patient using nitrous oxide is advised to initiate inhalation of nitrous oxide about 30 seconds before the onset of a contraction and discontinue inhalation at the peak of the contraction.
There is no time limit to the use of nitrous oxide. It can be used for hours during labor or only briefly for a particularly painful part of labor, such as during rapid cervical dilation or during the later portions of the second stage.
Patients report that nitrous oxide does not completely relieve pain but creates a diminished perception of the pain.9 As many as one-third of women are nonresponders and report no significant pain improvement with nitrous oxide use.10
The main side effects of inhalation of the gas are nausea, vomiting, dizziness, and drowsiness. Nausea has been reported in 5% to 40% of women, and vomiting has been reported in up to 15% of women using nitrous oxide.11
Cautions
Contraindications to nitrous oxide include a baseline arterial oxygenation saturation less than 95% on room air, acute asthma, emphysema, or pneumothorax, or any other air-filled compartment within the body, such as bowel obstruction or pneumocephalus. (Nitrous oxide can displace nitrogen from closed body spaces, which may lead to an increase in the volume of the closed space.12)
Nitrous oxide inactivates vitamin B12 by oxidation; therefore, vitamin B12 deficiency or related disorders may be considered a relative contraindication. However, compared with more extensive continuous use, such as during prolonged general anesthesia, intermittent use for a limited time during labor is associated with minimal to no hematologic effects.
If a laboring woman is using N2O, parenteral opioids should be administered only with great caution by an experienced clinician.
What do the data indicate?
The Agency for Healthcare Research and Quality (AHRQ) recently invited the Vanderbilt Evidence-based Practice Center to review the world literature on nitrous oxide for labor pain and to provide a summary of the research. Fifty-eight publications were identified, with 46 rated as poor quality.11,13 Given this overall poor quality of available research, many of the recommendations concerning the use of nitrous oxide for labor pain are based on clinical experience and expert opinion.
The experts concluded that, for the relief of labor pain, neuraxial anesthesia was more effective than nitrous oxide inhalation. In one randomized trial included in their systematic review, nulliparous laboring women were randomly assigned to neuraxial anesthesia or nitrous oxide plus meperidine.14 About 94% of nulliparous laboring women reported satisfaction with neuraxial anesthesia, compared with 54% treated with nitrous oxide and meperidine.14
Nitrous oxide is believed to be generally safe for mother and fetus. Its use does not impact the newborn Apgar score15 or alter uterine
contractility.16
Considering a nitrous oxide program for your birthing unit? Helpful hints to get started.
Catherine McGovern, RN, MSN, CNM
- Do your research to determine which type of equipment is right for the size and volume of your organization.
You need to consider ease of access and use for staff to bring this option to the bedside in a prompt and safe manner. Initial research includes visiting or speaking with practitioners on units currently using nitrous oxide. Use of nitrous oxide is growing, and networking is helpful in terms of planning your program. Making sure you have the correct gas line connectors for oxygen as well as for suction when using a scavenger system is a preliminary necessity. - Determine storage ability.
Your environmental safety officer is a good resource to determine location and regulations regarding safe storage as well as tank capacity. He or she also can help you determine where else in your organization nitrous oxide is used so you may be able to develop your unit-specific protocol from hospital-wide policy that is already in place. - Collaborate on a protocol.
After determining which type of equipment is best for you, propose the idea to committees that can contribute to the development of pain and sedation management protocols. The anesthesia department, pain committee, and postoperative pain management teams are knowledgeable resources and can help you write a safe protocol. Keep as the main focus the safe application and use of nitrous oxide for various patient populations. Potential medication interactions and contraindications for use should be discussed and included in a protocol.
One more department you want to include in your planning is infection control. For our unit, reviewing various types of equipment to determine the best infection control revealed some interesting design benefits to reduce infection risk. Because the nitrous oxide equipment would be mobile, the types of filter options, disposal options, and cleaning ability are important components for final equipment choice. - Include all parties in training and final roll out.
Once you develop your policy with input from all stakeholders, make sure you share it early and often before you go live. Include midwives, physicians, nurses, technicians, and administrative staff in training, which will help to dispel myths and increase awareness of availability within your unit. Provide background information to all trainees to ensure safe use and appropriate patient selection.
The most important determinant of success is the formation of an interprofessional team that works well together to develop a safe clinician- and patient-friendly program for the use of nitrous oxide.
Nitrous oxide, a bridge to an epidural or a natural childbirth
Many women start labor unsure about whether they want to use an epidural. For these women, nitrous oxide may be an option for reducing labor pain, thereby giving the woman more time to make a decision about whether to have an epidural anesthetic. In our practice, a significant percentage of women who use nitrous oxide early in labor subsequently request a neuraxial anesthetic. However, many women planning natural childbirth use nitrous oxide to reduce labor pain and successfully achieve their goal.
Postpartum pain reliever
Some women deliver without the use of any pain medicine. Sometimes birth is complicated by perineal lacerations requiring significant surgical repair. If a woman does not have adequate analgesia after injection of a local anesthetic, nitrous oxide may help reduce her pain during the perineal repair and facilitate quick completion of the procedure by allowing her to remain still. N2O also has been used to facilitate analgesia during manual removal of the placenta.
We predict an expanding role
There are many pharmacologic and nonpharmacologic options for managing labor pain, including a supportive birth environment, touch and massage, maternal positioning, relaxation and breathing techniques, continuous labor support, hydrotherapy, opioids, and neuraxial anesthesia. Midwives, labor nurses, and physicians have championed increasing the availability of nitrous oxide to laboring women in US birthing centers.17–20 With the FDA approval of inexpensive portable nitrous oxide units, it is likely that we will witness a resurgence of its use and gain important clinical experience in the role of nitrous oxide for managing labor pain.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Amin-Somuah M, Smyth R, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011;(12):CD000331.
2. Hodnett ED, Gates S, Hofmeyr JG, Sakala C. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;(7):CD003766.
3. Cluett ER, Burns E. Immersion in water in labour and birth. Cochrane Database Syst Rev. 2009;(2):CD000111.
4. Ullman R, Smith LA, Burns E, Mori R, Dowswell T. Parenteral opioids for maternal pain management in labor. Cochrane Database Syst Rev. 2010;(9):CD007396.
5. Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl Nature):S110–S126.
6. Klomp T, van Poppel M, Jones L, Lazet J, Di Nisio M, Lagro-Janssen A. Inhaled analgesia for pain management in labor. Cochrane Database Syst Rev. 2012;(9):CD009351.
7. Richards W, Parbrook G, Wilson J. Stanislav Klikovitch (1853-1910). Pioneer of nitrous oxide and oxygen analgesia. Anaesthesia. 1976;31(7):933–940.
8. Minnitt R. Self-administered anesthesia in childbirth. Br Med J. 1934;1:501–503.
9. Camann W, Alexander K. Easy labor: Every Woman’s Guide to Choosing Less Pain and More Joy during Childbirth. New York: Ballantine Books; 2007.
10. Rosen M, Mushin WW, Jones PL, Jones EV. Field trial of methoxyflurane, nitrous oxide, and trichloroethylene as obstetric analgesics. Br Med J. 1969;3(5665):263–267.
11. Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153–167.
12. Eger EI 2nd, Saidman LJ. Hazards of nitrous oxide anesthesia in bowel obstruction and pneumothorax. Anesthesiology. 1965;26:61–66.
13. Agency for Healthcare Research and Quality. Nitrous oxide for the management of labor pain. Comparative Effectiveness Review Number 67. August 2012. http://www.effectivehealthcare.ahrq.gov/ehc/products/260/1175/CER67_NitrousOxideLaborPain_FinalReport_20120817.pdf. Accessed November 7, 2014.
14. Leong EW, Sivanesaratnam V, Oh LL, Chan YK. Epidural analgesia in primigravidae in spontaneous labor at term: a prospective study. J Obstet Gynaecol Res. 2000;26(4):271–275.
15. Clinical trials of different concentrations of oxygen and nitrous oxide for obstetric analgesia. Report to the Medical Research Council of the Committee on Nitrous Oxide and Oxygen Analgesia in Midwifery. Br Med J. 1970;1(5698):709–713.
16. Vasicka A, Kretchmer H. Effect of conduction and inhalation anesthesia on uterine contractions. Am J Obstet Gynecol. 1961;82:600–611.
17. Rooks JP. Labor pain management other than neuraxial: what do we know and where do we go next? Birth. 2012;39(4):318–322.
18. American College of Nurse-Midwives. From the American College of Nurse-Midwives. Nitrous oxide for labor analgesia. J Midwifery Womens Health. 2010;55(3):292–296.
19. Bishop JT. Administration of nitrous oxide in labor: expanding the options for women. J Midwifery Womens Health. 2007;52(3):308–309.
20. Rooks JP. Nitrous oxide for pain in labor—why not in the United States? Birth. 2007;34(1):3–5.
Neuraxial anesthesia, including epidural and combined spinal-epidural anesthetics, are the “gold standard” interventions for pain relief during labor because they provide a superb combination of reliable pain relief and safety for the mother and child.1 Many US birthing centers also offer additional options for managing labor pain, including continuous labor support,2 hydrotherapy,3 and parenteral opioids.4 In 2012, the US Food and Drug Administration (FDA) approved equipment to deliver a mixture of 50% nitrous oxide and 50% oxygen, which has offered a new option for laboring mothers.
Nitrous oxide is widely used for labor pain in the United Kingdom, Finland, Sweden, Canada, Australia, and New Zealand.5 In the United States, nitrous oxide has been a long-standing and common adjunct to general anesthetics, although it recently has fallen out of favor in place of better, more rapidly acting inhalation and intravenous general anesthetics. With these agents not suitable for labor analgesic use, however, nitrous oxide is undergoing a resurgence in popularity for obstetric analgesia in the United States, and we believe that it will evolve to have a prominent place among our interventions for labor pain.6 In this editorial, we detail the mechanism of action and the equipment’s use, as well as benefits for patients and cautions for clinicians.
How does nitrous oxide work?
Pharmacology. Nitrous oxide (N2O) was first synthesized by Joseph Priestley in 1772 and was used as an anesthetic for dental surgery in the mid-1800s. In the late 19th Century, nitrous oxide was tested as an agent for labor analgesia.7 It was introduced into clinical practice in the United Kingdom in the 1930s.8
The mechanism of action of nitrous oxide is not fully characterized. It is thought that the gas may produce analgesia by activating the endogenous opioid and noradrenergic systems, which in turn, modulate spinal cord transmission of pain signals.5
Administration to the laboring mother. For labor analgesia, nitrous oxide is typically administered as a mix of 50% N2O and 50% O2 using a portable unit with a gas mixer that is fed by small tanks of N2O and O2 or with a valve fed by a single tank containing a mixture of both N2O and O2. The portable units approved by the FDA contain an oxygen fail-safe system that ensures delivery of an appropriate oxygen concentration. The portable unit also contains a gas scavenging system that is attached to wall suction. The breathing circuit has a mask or a mouthpiece (according to patient preference) and demand valve. The patient places the mask over her nose and mouth, or uses just her mouth for the mouthpiece. With inhalation, the demand valve opens, releasing the gas mixture. On exhalation, the valve shunts the exhaled gases to the scavenging system.
Proper and safe use requires adherence to the principles of a true “patient-controlled” protocol. Only the patient is permitted to place the mask or mouthpiece over her nose and/or mouth. If the patient becomes drowsy, such that she cannot hold the mask to her face, then the internal demand valve will not deliver nitrous oxide and she will return to breathing room air. No one should hold the mask over the patient’s nose or mouth, and the mask should not be fixed in place with elastic bands because these actions may result in the inhalation of too much nitrous oxide.
Nitrous oxide has a rapid onset of action after inhalation and its action quickly dissipates after discontinuing inhalation. There is likely a dose-response relationship, with greater use of the nitrous oxide producing more drowsiness. With the intermittent inhalation method, the laboring patient using nitrous oxide is advised to initiate inhalation of nitrous oxide about 30 seconds before the onset of a contraction and discontinue inhalation at the peak of the contraction.
There is no time limit to the use of nitrous oxide. It can be used for hours during labor or only briefly for a particularly painful part of labor, such as during rapid cervical dilation or during the later portions of the second stage.
Patients report that nitrous oxide does not completely relieve pain but creates a diminished perception of the pain.9 As many as one-third of women are nonresponders and report no significant pain improvement with nitrous oxide use.10
The main side effects of inhalation of the gas are nausea, vomiting, dizziness, and drowsiness. Nausea has been reported in 5% to 40% of women, and vomiting has been reported in up to 15% of women using nitrous oxide.11
Cautions
Contraindications to nitrous oxide include a baseline arterial oxygenation saturation less than 95% on room air, acute asthma, emphysema, or pneumothorax, or any other air-filled compartment within the body, such as bowel obstruction or pneumocephalus. (Nitrous oxide can displace nitrogen from closed body spaces, which may lead to an increase in the volume of the closed space.12)
Nitrous oxide inactivates vitamin B12 by oxidation; therefore, vitamin B12 deficiency or related disorders may be considered a relative contraindication. However, compared with more extensive continuous use, such as during prolonged general anesthesia, intermittent use for a limited time during labor is associated with minimal to no hematologic effects.
If a laboring woman is using N2O, parenteral opioids should be administered only with great caution by an experienced clinician.
What do the data indicate?
The Agency for Healthcare Research and Quality (AHRQ) recently invited the Vanderbilt Evidence-based Practice Center to review the world literature on nitrous oxide for labor pain and to provide a summary of the research. Fifty-eight publications were identified, with 46 rated as poor quality.11,13 Given this overall poor quality of available research, many of the recommendations concerning the use of nitrous oxide for labor pain are based on clinical experience and expert opinion.
The experts concluded that, for the relief of labor pain, neuraxial anesthesia was more effective than nitrous oxide inhalation. In one randomized trial included in their systematic review, nulliparous laboring women were randomly assigned to neuraxial anesthesia or nitrous oxide plus meperidine.14 About 94% of nulliparous laboring women reported satisfaction with neuraxial anesthesia, compared with 54% treated with nitrous oxide and meperidine.14
Nitrous oxide is believed to be generally safe for mother and fetus. Its use does not impact the newborn Apgar score15 or alter uterine
contractility.16
Considering a nitrous oxide program for your birthing unit? Helpful hints to get started.
Catherine McGovern, RN, MSN, CNM
- Do your research to determine which type of equipment is right for the size and volume of your organization.
You need to consider ease of access and use for staff to bring this option to the bedside in a prompt and safe manner. Initial research includes visiting or speaking with practitioners on units currently using nitrous oxide. Use of nitrous oxide is growing, and networking is helpful in terms of planning your program. Making sure you have the correct gas line connectors for oxygen as well as for suction when using a scavenger system is a preliminary necessity. - Determine storage ability.
Your environmental safety officer is a good resource to determine location and regulations regarding safe storage as well as tank capacity. He or she also can help you determine where else in your organization nitrous oxide is used so you may be able to develop your unit-specific protocol from hospital-wide policy that is already in place. - Collaborate on a protocol.
After determining which type of equipment is best for you, propose the idea to committees that can contribute to the development of pain and sedation management protocols. The anesthesia department, pain committee, and postoperative pain management teams are knowledgeable resources and can help you write a safe protocol. Keep as the main focus the safe application and use of nitrous oxide for various patient populations. Potential medication interactions and contraindications for use should be discussed and included in a protocol.
One more department you want to include in your planning is infection control. For our unit, reviewing various types of equipment to determine the best infection control revealed some interesting design benefits to reduce infection risk. Because the nitrous oxide equipment would be mobile, the types of filter options, disposal options, and cleaning ability are important components for final equipment choice. - Include all parties in training and final roll out.
Once you develop your policy with input from all stakeholders, make sure you share it early and often before you go live. Include midwives, physicians, nurses, technicians, and administrative staff in training, which will help to dispel myths and increase awareness of availability within your unit. Provide background information to all trainees to ensure safe use and appropriate patient selection.
The most important determinant of success is the formation of an interprofessional team that works well together to develop a safe clinician- and patient-friendly program for the use of nitrous oxide.
Nitrous oxide, a bridge to an epidural or a natural childbirth
Many women start labor unsure about whether they want to use an epidural. For these women, nitrous oxide may be an option for reducing labor pain, thereby giving the woman more time to make a decision about whether to have an epidural anesthetic. In our practice, a significant percentage of women who use nitrous oxide early in labor subsequently request a neuraxial anesthetic. However, many women planning natural childbirth use nitrous oxide to reduce labor pain and successfully achieve their goal.
Postpartum pain reliever
Some women deliver without the use of any pain medicine. Sometimes birth is complicated by perineal lacerations requiring significant surgical repair. If a woman does not have adequate analgesia after injection of a local anesthetic, nitrous oxide may help reduce her pain during the perineal repair and facilitate quick completion of the procedure by allowing her to remain still. N2O also has been used to facilitate analgesia during manual removal of the placenta.
We predict an expanding role
There are many pharmacologic and nonpharmacologic options for managing labor pain, including a supportive birth environment, touch and massage, maternal positioning, relaxation and breathing techniques, continuous labor support, hydrotherapy, opioids, and neuraxial anesthesia. Midwives, labor nurses, and physicians have championed increasing the availability of nitrous oxide to laboring women in US birthing centers.17–20 With the FDA approval of inexpensive portable nitrous oxide units, it is likely that we will witness a resurgence of its use and gain important clinical experience in the role of nitrous oxide for managing labor pain.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Neuraxial anesthesia, including epidural and combined spinal-epidural anesthetics, are the “gold standard” interventions for pain relief during labor because they provide a superb combination of reliable pain relief and safety for the mother and child.1 Many US birthing centers also offer additional options for managing labor pain, including continuous labor support,2 hydrotherapy,3 and parenteral opioids.4 In 2012, the US Food and Drug Administration (FDA) approved equipment to deliver a mixture of 50% nitrous oxide and 50% oxygen, which has offered a new option for laboring mothers.
Nitrous oxide is widely used for labor pain in the United Kingdom, Finland, Sweden, Canada, Australia, and New Zealand.5 In the United States, nitrous oxide has been a long-standing and common adjunct to general anesthetics, although it recently has fallen out of favor in place of better, more rapidly acting inhalation and intravenous general anesthetics. With these agents not suitable for labor analgesic use, however, nitrous oxide is undergoing a resurgence in popularity for obstetric analgesia in the United States, and we believe that it will evolve to have a prominent place among our interventions for labor pain.6 In this editorial, we detail the mechanism of action and the equipment’s use, as well as benefits for patients and cautions for clinicians.
How does nitrous oxide work?
Pharmacology. Nitrous oxide (N2O) was first synthesized by Joseph Priestley in 1772 and was used as an anesthetic for dental surgery in the mid-1800s. In the late 19th Century, nitrous oxide was tested as an agent for labor analgesia.7 It was introduced into clinical practice in the United Kingdom in the 1930s.8
The mechanism of action of nitrous oxide is not fully characterized. It is thought that the gas may produce analgesia by activating the endogenous opioid and noradrenergic systems, which in turn, modulate spinal cord transmission of pain signals.5
Administration to the laboring mother. For labor analgesia, nitrous oxide is typically administered as a mix of 50% N2O and 50% O2 using a portable unit with a gas mixer that is fed by small tanks of N2O and O2 or with a valve fed by a single tank containing a mixture of both N2O and O2. The portable units approved by the FDA contain an oxygen fail-safe system that ensures delivery of an appropriate oxygen concentration. The portable unit also contains a gas scavenging system that is attached to wall suction. The breathing circuit has a mask or a mouthpiece (according to patient preference) and demand valve. The patient places the mask over her nose and mouth, or uses just her mouth for the mouthpiece. With inhalation, the demand valve opens, releasing the gas mixture. On exhalation, the valve shunts the exhaled gases to the scavenging system.
Proper and safe use requires adherence to the principles of a true “patient-controlled” protocol. Only the patient is permitted to place the mask or mouthpiece over her nose and/or mouth. If the patient becomes drowsy, such that she cannot hold the mask to her face, then the internal demand valve will not deliver nitrous oxide and she will return to breathing room air. No one should hold the mask over the patient’s nose or mouth, and the mask should not be fixed in place with elastic bands because these actions may result in the inhalation of too much nitrous oxide.
Nitrous oxide has a rapid onset of action after inhalation and its action quickly dissipates after discontinuing inhalation. There is likely a dose-response relationship, with greater use of the nitrous oxide producing more drowsiness. With the intermittent inhalation method, the laboring patient using nitrous oxide is advised to initiate inhalation of nitrous oxide about 30 seconds before the onset of a contraction and discontinue inhalation at the peak of the contraction.
There is no time limit to the use of nitrous oxide. It can be used for hours during labor or only briefly for a particularly painful part of labor, such as during rapid cervical dilation or during the later portions of the second stage.
Patients report that nitrous oxide does not completely relieve pain but creates a diminished perception of the pain.9 As many as one-third of women are nonresponders and report no significant pain improvement with nitrous oxide use.10
The main side effects of inhalation of the gas are nausea, vomiting, dizziness, and drowsiness. Nausea has been reported in 5% to 40% of women, and vomiting has been reported in up to 15% of women using nitrous oxide.11
Cautions
Contraindications to nitrous oxide include a baseline arterial oxygenation saturation less than 95% on room air, acute asthma, emphysema, or pneumothorax, or any other air-filled compartment within the body, such as bowel obstruction or pneumocephalus. (Nitrous oxide can displace nitrogen from closed body spaces, which may lead to an increase in the volume of the closed space.12)
Nitrous oxide inactivates vitamin B12 by oxidation; therefore, vitamin B12 deficiency or related disorders may be considered a relative contraindication. However, compared with more extensive continuous use, such as during prolonged general anesthesia, intermittent use for a limited time during labor is associated with minimal to no hematologic effects.
If a laboring woman is using N2O, parenteral opioids should be administered only with great caution by an experienced clinician.
What do the data indicate?
The Agency for Healthcare Research and Quality (AHRQ) recently invited the Vanderbilt Evidence-based Practice Center to review the world literature on nitrous oxide for labor pain and to provide a summary of the research. Fifty-eight publications were identified, with 46 rated as poor quality.11,13 Given this overall poor quality of available research, many of the recommendations concerning the use of nitrous oxide for labor pain are based on clinical experience and expert opinion.
The experts concluded that, for the relief of labor pain, neuraxial anesthesia was more effective than nitrous oxide inhalation. In one randomized trial included in their systematic review, nulliparous laboring women were randomly assigned to neuraxial anesthesia or nitrous oxide plus meperidine.14 About 94% of nulliparous laboring women reported satisfaction with neuraxial anesthesia, compared with 54% treated with nitrous oxide and meperidine.14
Nitrous oxide is believed to be generally safe for mother and fetus. Its use does not impact the newborn Apgar score15 or alter uterine
contractility.16
Considering a nitrous oxide program for your birthing unit? Helpful hints to get started.
Catherine McGovern, RN, MSN, CNM
- Do your research to determine which type of equipment is right for the size and volume of your organization.
You need to consider ease of access and use for staff to bring this option to the bedside in a prompt and safe manner. Initial research includes visiting or speaking with practitioners on units currently using nitrous oxide. Use of nitrous oxide is growing, and networking is helpful in terms of planning your program. Making sure you have the correct gas line connectors for oxygen as well as for suction when using a scavenger system is a preliminary necessity. - Determine storage ability.
Your environmental safety officer is a good resource to determine location and regulations regarding safe storage as well as tank capacity. He or she also can help you determine where else in your organization nitrous oxide is used so you may be able to develop your unit-specific protocol from hospital-wide policy that is already in place. - Collaborate on a protocol.
After determining which type of equipment is best for you, propose the idea to committees that can contribute to the development of pain and sedation management protocols. The anesthesia department, pain committee, and postoperative pain management teams are knowledgeable resources and can help you write a safe protocol. Keep as the main focus the safe application and use of nitrous oxide for various patient populations. Potential medication interactions and contraindications for use should be discussed and included in a protocol.
One more department you want to include in your planning is infection control. For our unit, reviewing various types of equipment to determine the best infection control revealed some interesting design benefits to reduce infection risk. Because the nitrous oxide equipment would be mobile, the types of filter options, disposal options, and cleaning ability are important components for final equipment choice. - Include all parties in training and final roll out.
Once you develop your policy with input from all stakeholders, make sure you share it early and often before you go live. Include midwives, physicians, nurses, technicians, and administrative staff in training, which will help to dispel myths and increase awareness of availability within your unit. Provide background information to all trainees to ensure safe use and appropriate patient selection.
The most important determinant of success is the formation of an interprofessional team that works well together to develop a safe clinician- and patient-friendly program for the use of nitrous oxide.
Nitrous oxide, a bridge to an epidural or a natural childbirth
Many women start labor unsure about whether they want to use an epidural. For these women, nitrous oxide may be an option for reducing labor pain, thereby giving the woman more time to make a decision about whether to have an epidural anesthetic. In our practice, a significant percentage of women who use nitrous oxide early in labor subsequently request a neuraxial anesthetic. However, many women planning natural childbirth use nitrous oxide to reduce labor pain and successfully achieve their goal.
Postpartum pain reliever
Some women deliver without the use of any pain medicine. Sometimes birth is complicated by perineal lacerations requiring significant surgical repair. If a woman does not have adequate analgesia after injection of a local anesthetic, nitrous oxide may help reduce her pain during the perineal repair and facilitate quick completion of the procedure by allowing her to remain still. N2O also has been used to facilitate analgesia during manual removal of the placenta.
We predict an expanding role
There are many pharmacologic and nonpharmacologic options for managing labor pain, including a supportive birth environment, touch and massage, maternal positioning, relaxation and breathing techniques, continuous labor support, hydrotherapy, opioids, and neuraxial anesthesia. Midwives, labor nurses, and physicians have championed increasing the availability of nitrous oxide to laboring women in US birthing centers.17–20 With the FDA approval of inexpensive portable nitrous oxide units, it is likely that we will witness a resurgence of its use and gain important clinical experience in the role of nitrous oxide for managing labor pain.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Amin-Somuah M, Smyth R, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011;(12):CD000331.
2. Hodnett ED, Gates S, Hofmeyr JG, Sakala C. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;(7):CD003766.
3. Cluett ER, Burns E. Immersion in water in labour and birth. Cochrane Database Syst Rev. 2009;(2):CD000111.
4. Ullman R, Smith LA, Burns E, Mori R, Dowswell T. Parenteral opioids for maternal pain management in labor. Cochrane Database Syst Rev. 2010;(9):CD007396.
5. Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl Nature):S110–S126.
6. Klomp T, van Poppel M, Jones L, Lazet J, Di Nisio M, Lagro-Janssen A. Inhaled analgesia for pain management in labor. Cochrane Database Syst Rev. 2012;(9):CD009351.
7. Richards W, Parbrook G, Wilson J. Stanislav Klikovitch (1853-1910). Pioneer of nitrous oxide and oxygen analgesia. Anaesthesia. 1976;31(7):933–940.
8. Minnitt R. Self-administered anesthesia in childbirth. Br Med J. 1934;1:501–503.
9. Camann W, Alexander K. Easy labor: Every Woman’s Guide to Choosing Less Pain and More Joy during Childbirth. New York: Ballantine Books; 2007.
10. Rosen M, Mushin WW, Jones PL, Jones EV. Field trial of methoxyflurane, nitrous oxide, and trichloroethylene as obstetric analgesics. Br Med J. 1969;3(5665):263–267.
11. Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153–167.
12. Eger EI 2nd, Saidman LJ. Hazards of nitrous oxide anesthesia in bowel obstruction and pneumothorax. Anesthesiology. 1965;26:61–66.
13. Agency for Healthcare Research and Quality. Nitrous oxide for the management of labor pain. Comparative Effectiveness Review Number 67. August 2012. http://www.effectivehealthcare.ahrq.gov/ehc/products/260/1175/CER67_NitrousOxideLaborPain_FinalReport_20120817.pdf. Accessed November 7, 2014.
14. Leong EW, Sivanesaratnam V, Oh LL, Chan YK. Epidural analgesia in primigravidae in spontaneous labor at term: a prospective study. J Obstet Gynaecol Res. 2000;26(4):271–275.
15. Clinical trials of different concentrations of oxygen and nitrous oxide for obstetric analgesia. Report to the Medical Research Council of the Committee on Nitrous Oxide and Oxygen Analgesia in Midwifery. Br Med J. 1970;1(5698):709–713.
16. Vasicka A, Kretchmer H. Effect of conduction and inhalation anesthesia on uterine contractions. Am J Obstet Gynecol. 1961;82:600–611.
17. Rooks JP. Labor pain management other than neuraxial: what do we know and where do we go next? Birth. 2012;39(4):318–322.
18. American College of Nurse-Midwives. From the American College of Nurse-Midwives. Nitrous oxide for labor analgesia. J Midwifery Womens Health. 2010;55(3):292–296.
19. Bishop JT. Administration of nitrous oxide in labor: expanding the options for women. J Midwifery Womens Health. 2007;52(3):308–309.
20. Rooks JP. Nitrous oxide for pain in labor—why not in the United States? Birth. 2007;34(1):3–5.
1. Amin-Somuah M, Smyth R, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011;(12):CD000331.
2. Hodnett ED, Gates S, Hofmeyr JG, Sakala C. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;(7):CD003766.
3. Cluett ER, Burns E. Immersion in water in labour and birth. Cochrane Database Syst Rev. 2009;(2):CD000111.
4. Ullman R, Smith LA, Burns E, Mori R, Dowswell T. Parenteral opioids for maternal pain management in labor. Cochrane Database Syst Rev. 2010;(9):CD007396.
5. Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl Nature):S110–S126.
6. Klomp T, van Poppel M, Jones L, Lazet J, Di Nisio M, Lagro-Janssen A. Inhaled analgesia for pain management in labor. Cochrane Database Syst Rev. 2012;(9):CD009351.
7. Richards W, Parbrook G, Wilson J. Stanislav Klikovitch (1853-1910). Pioneer of nitrous oxide and oxygen analgesia. Anaesthesia. 1976;31(7):933–940.
8. Minnitt R. Self-administered anesthesia in childbirth. Br Med J. 1934;1:501–503.
9. Camann W, Alexander K. Easy labor: Every Woman’s Guide to Choosing Less Pain and More Joy during Childbirth. New York: Ballantine Books; 2007.
10. Rosen M, Mushin WW, Jones PL, Jones EV. Field trial of methoxyflurane, nitrous oxide, and trichloroethylene as obstetric analgesics. Br Med J. 1969;3(5665):263–267.
11. Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153–167.
12. Eger EI 2nd, Saidman LJ. Hazards of nitrous oxide anesthesia in bowel obstruction and pneumothorax. Anesthesiology. 1965;26:61–66.
13. Agency for Healthcare Research and Quality. Nitrous oxide for the management of labor pain. Comparative Effectiveness Review Number 67. August 2012. http://www.effectivehealthcare.ahrq.gov/ehc/products/260/1175/CER67_NitrousOxideLaborPain_FinalReport_20120817.pdf. Accessed November 7, 2014.
14. Leong EW, Sivanesaratnam V, Oh LL, Chan YK. Epidural analgesia in primigravidae in spontaneous labor at term: a prospective study. J Obstet Gynaecol Res. 2000;26(4):271–275.
15. Clinical trials of different concentrations of oxygen and nitrous oxide for obstetric analgesia. Report to the Medical Research Council of the Committee on Nitrous Oxide and Oxygen Analgesia in Midwifery. Br Med J. 1970;1(5698):709–713.
16. Vasicka A, Kretchmer H. Effect of conduction and inhalation anesthesia on uterine contractions. Am J Obstet Gynecol. 1961;82:600–611.
17. Rooks JP. Labor pain management other than neuraxial: what do we know and where do we go next? Birth. 2012;39(4):318–322.
18. American College of Nurse-Midwives. From the American College of Nurse-Midwives. Nitrous oxide for labor analgesia. J Midwifery Womens Health. 2010;55(3):292–296.
19. Bishop JT. Administration of nitrous oxide in labor: expanding the options for women. J Midwifery Womens Health. 2007;52(3):308–309.
20. Rooks JP. Nitrous oxide for pain in labor—why not in the United States? Birth. 2007;34(1):3–5.
Dr. Robert L. Barbieri’s Editor’s Picks November 2014
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s November 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the November 2014 issue here.
Letrozole versus clomiphene for ovulation induction
The three most common causes of infertility are anovulation, tubal occlusion, and abnormal semen parameters. The most common cause of anovulatory infertility is polycystic ovary syndrome (PCOS). Options for initial treatment of anovulatory infertility caused by PCOS include optimizing body mass index (BMI), clomiphene, clomiphene plus dexamethasone, and metformin (TABLE 1). If these low-cost interventions are not successful, high-cost interventions are often very effective treatments, and include follicle-stimulating hormone (FSH) injections, laparoscopic ovarian drilling, and in vitro fertilization.
For many couples, the high-cost interventions are prohibitively expensive. Recently, results of a high-quality randomized clinical trial published by Legro and colleagues in the New England Journal of Medicine indicate that letrozole is more effective than clomiphene for the treatment of anovulatory infertility in women with PCOS.1 Of great importance, letrozole was documented to be especially effective in women with a BMI greater than 30.3 kg/m2.
Letrozole is another low-cost option for couples with anovulatory infertility (TABLE 2), and you should consider it among your initial treatment choices. In this article, I outline when letrozole is your best first option for treatment.
Letrozole is more effective than clomiphene for ovulation induction in women with PCOS and BMI >30.3 kg/m2
Legro and colleagues1 randomly assigned 750 women with anovulatory infertility and PCOS to receive ovulation induction with either clomiphene or letrozole. The medications were prescribed using an escalating dose if ovulation did not occur. For clomiphene, the doses prescribed were 50 mg, 100 mg, and 150 mg. For letrozole, the doses were 2.5 mg, 5 mg, and 7.5 mg. The medications were given daily for 5 days on cycle days 3 to 7, following a spontaneous menses or a medroxyprogesterone acetate withdrawal bleed. Up to 5 cycles of ovulation induction were prescribed.
The ovulation rates for letrozole versus clomiphene were 61.7% and 48.3%, respectively (P<.001). The live birth rates for letrozole versus clomiphene were 27.5% and 19.1%, respectively (P = .007). Among women with a BMI of 30.3 kg/m2 or less, both letrozole and clomiphene treatment resulted in a similar live birth rate of approximately 30% to 35%. Among women with a BMI greater than 30.3 kg/m2, however, the live birth rates with letrozole versus clomiphene were approximately 20% and 10%, respectively.
Consequently, in my practice, I prioritize the use of letrozole for women with a BMI of 30 kg/m2 or greater.
Do not use anastrozole for ovulation induction
In a randomized trial of letrozole versus anastrozole for ovulation induction, 40 women with PCOS were randomly assigned to receive ovulation induction with letrozole (2.5 mg daily for 5 days) or anastrozole (1 mg daily for 5 days).2 The resulting ovulation rate was 84% for letrozole, compared with 60% for anastrozole (P<.05). The pregnancy rate also was significantly higher for letrozole (19% vs 10% for anastrozole, P<.05).
Investigators of two large randomized trials of anastrozole versus clomiphene reported that clomiphene was superior to anastrozole for induction of ovulation in the first cycle of treatment.3,4 Anastrozole, at doses of 1 mg, 5 mg, 10 mg, 20 mg, and 30 mg daily for 5 days, was less effective for ovulation induction in the first cycle of treatment than clomiphene at a dose of 50 mg.3,4
If an aromatase inhibitor is going to be prescribed for ovulation induction, I recommend the use of letrozole and recommend against the use of anastrozole.
Congenital malformations and ovulation induction
The administration of clomiphene or letrozole to pregnant rats has adverse fetal effects.5,6 For example, in pregnant rats a low dose of letrozole (0.003 mg/kg) has been reported to increase intrauterine mortality, fetal resorption, and postimplantation loss; decrease live births; and result in fetal anomalies, including dilation of the ureter and shortening of renal papillae.6
However, in the setting of ovulation induction, letrozole is not administered while the patient is pregnant and is discontinued many days before ovulation and conception. Consequently, the results observed in animal studies (with the medications administered to pregnant animals) may not be particularly relevant to the clinical situation where the fertility medication is discontinued before ovulation and conception.
It is important to exclude pregnancy prior to initiating treatment with letrozole or clomiphene.
Birth defects affect approximately 5% of newborns in the United States.7 The relative impact of maternal age, obesity, ovulation induction medicines, and a history of infertility on the rate of birth defects is not fully characterized and is a subject of intense research. To date, there is no strong and consistent evidence that ovulation induction agents, per se, significantly increase the rate of birth defects.
Tulandi and colleagues reported on 911 newborns conceived following ovulation induction with clomiphene or letrozole.8 Overall, the congenital malformation plus chromosomal abnormality rates associated with letrozole and clomiphene ovulation induction were 2.4% and 4.8%, respectively. The major congenital malformation rate for letrozole was 1.2%, and 3.0% for clomiphene.
Many women with anovulatory infertility and PCOS have a BMI of 30 kg/m2 or greater, and some are of advanced maternal age. It is known that women with such a BMI level have an increased risk of congenital malformations, including neural tube defects, spina bifida, septal anomalies, cleft palate, cleft lip, anorectal atresia, hydrocephaly, and limb reduction anomalies.9 The risk of gastroschisis is significantly reduced among obese pregnant women.9 Women aged 40 or older have an increased risk of having a fetus with cardiac defects, esophageal atresia, hypospadias, and craniosynostosis.10
Caution women of advanced maternal age with PCOS and a BMI of 30 kg/m2 or greater about the increased rate of congenital malformations associated with their age and elevated BMI.
Prioritize letrozole when BMI ≥30 kg/m2
I recommend that clomiphene should remain the first-line ovulation induction agent for women with PCOS and a BMI less than 30 kg/m2. This is because, among women with such a BMI level, both clomiphene and letrozole have similar efficacy, and clomiphene is approved by the US Food and Drug Administration for ovulation induction while letrozole is not.
However, for women with PCOS and a BMI of 30 kg/m2 or greater—a clinical situation where letrozole is about twice as effective as clomiphene—letrozole may be the preferred agent.
When prescribing letrozole, start with a dose of 2.5 mg daily for cycle days 3 to 7, following a spontaneous menses or progestin-induced bleed. If ovulation occurs, continue with the dose. If ovulation does not occur, increase the dose to 5 mg daily for cycle days 3 to 7. The maximal dose is 7.5 mg daily for cycle days 3 to 7. When prescribing letrozole, counsel your patient about the increased rate of congenital anomalies among women with an elevated BMI and the possible teratogenic effects of fertility medications.
The aromatase inhibitor letrozole is an important addition to our options for ovulation induction in women with PCOS. Will you start using letrozole for ovulation induction in your practice?
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129.
2. Al-Omari WR, Sulaiman WR, Al-Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2004;85(3):289–291.
3. Tredway D, Schertz JC, Bock D, Hemsey G, Diamond MP. Anastrozole vs. clomiphene citrate in infertile women with ovulatory dysfunction: a phase II, randomized, dose-finding study. Fertil Steril. 2011;95(5):1720–1724.
4. Tredway D, Schertz JC, Bock D, Hemsey G, Diamond MP. Anastrozole single-dose protocol in women with oligo- or anovulatory infertility: results of a randomized phase II dose-response study. Fertil Steril. 2011;95(5):1725–1729.
5. Clomid (clomiphene citrate tablets USP) [package insert]. Bridgewater, NJ: sanofi-aventis. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016131s026lbl.pdf. Revised October 2012. Accessed October 20, 2014.
6. Femara (letrozole tablets) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. https://www.pharma.us.novartis.com/product/pi/pdf/Femara.pdf. Revised January 2014. Accessed October 20, 2014.
7. Christianson A, Howson CP, Modell B. March of Dimes Global Report on Birth Defects: Executive Summary. White Plains NY: March of Dimes Birth Defects Foundation; 2006:2–9. http://www.marchofdimes.com/materials/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-execu tive-summary.pdf. Accessed October 20, 2014.
8. Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility with letrozole or clomiphene citrate. Fertil Steril. 2006;85(6):1761–1765.
9. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systemic review and meta-analysis. JAMA. 2009;301(6):636–650.
10. Gill SK, Broussard C, Devine O, Green RF, Rasmussen SA, Reefhuis J; National Birth Defects Prevention Study. Association between maternal age and birth defects of unknown etiology: United States, 1997–2007. Birth Defects Res A Clin Mol Teratol. 2012;94(12):1010–1018
The three most common causes of infertility are anovulation, tubal occlusion, and abnormal semen parameters. The most common cause of anovulatory infertility is polycystic ovary syndrome (PCOS). Options for initial treatment of anovulatory infertility caused by PCOS include optimizing body mass index (BMI), clomiphene, clomiphene plus dexamethasone, and metformin (TABLE 1). If these low-cost interventions are not successful, high-cost interventions are often very effective treatments, and include follicle-stimulating hormone (FSH) injections, laparoscopic ovarian drilling, and in vitro fertilization.
For many couples, the high-cost interventions are prohibitively expensive. Recently, results of a high-quality randomized clinical trial published by Legro and colleagues in the New England Journal of Medicine indicate that letrozole is more effective than clomiphene for the treatment of anovulatory infertility in women with PCOS.1 Of great importance, letrozole was documented to be especially effective in women with a BMI greater than 30.3 kg/m2.
Letrozole is another low-cost option for couples with anovulatory infertility (TABLE 2), and you should consider it among your initial treatment choices. In this article, I outline when letrozole is your best first option for treatment.
Letrozole is more effective than clomiphene for ovulation induction in women with PCOS and BMI >30.3 kg/m2
Legro and colleagues1 randomly assigned 750 women with anovulatory infertility and PCOS to receive ovulation induction with either clomiphene or letrozole. The medications were prescribed using an escalating dose if ovulation did not occur. For clomiphene, the doses prescribed were 50 mg, 100 mg, and 150 mg. For letrozole, the doses were 2.5 mg, 5 mg, and 7.5 mg. The medications were given daily for 5 days on cycle days 3 to 7, following a spontaneous menses or a medroxyprogesterone acetate withdrawal bleed. Up to 5 cycles of ovulation induction were prescribed.
The ovulation rates for letrozole versus clomiphene were 61.7% and 48.3%, respectively (P<.001). The live birth rates for letrozole versus clomiphene were 27.5% and 19.1%, respectively (P = .007). Among women with a BMI of 30.3 kg/m2 or less, both letrozole and clomiphene treatment resulted in a similar live birth rate of approximately 30% to 35%. Among women with a BMI greater than 30.3 kg/m2, however, the live birth rates with letrozole versus clomiphene were approximately 20% and 10%, respectively.
Consequently, in my practice, I prioritize the use of letrozole for women with a BMI of 30 kg/m2 or greater.
Do not use anastrozole for ovulation induction
In a randomized trial of letrozole versus anastrozole for ovulation induction, 40 women with PCOS were randomly assigned to receive ovulation induction with letrozole (2.5 mg daily for 5 days) or anastrozole (1 mg daily for 5 days).2 The resulting ovulation rate was 84% for letrozole, compared with 60% for anastrozole (P<.05). The pregnancy rate also was significantly higher for letrozole (19% vs 10% for anastrozole, P<.05).
Investigators of two large randomized trials of anastrozole versus clomiphene reported that clomiphene was superior to anastrozole for induction of ovulation in the first cycle of treatment.3,4 Anastrozole, at doses of 1 mg, 5 mg, 10 mg, 20 mg, and 30 mg daily for 5 days, was less effective for ovulation induction in the first cycle of treatment than clomiphene at a dose of 50 mg.3,4
If an aromatase inhibitor is going to be prescribed for ovulation induction, I recommend the use of letrozole and recommend against the use of anastrozole.
Congenital malformations and ovulation induction
The administration of clomiphene or letrozole to pregnant rats has adverse fetal effects.5,6 For example, in pregnant rats a low dose of letrozole (0.003 mg/kg) has been reported to increase intrauterine mortality, fetal resorption, and postimplantation loss; decrease live births; and result in fetal anomalies, including dilation of the ureter and shortening of renal papillae.6
However, in the setting of ovulation induction, letrozole is not administered while the patient is pregnant and is discontinued many days before ovulation and conception. Consequently, the results observed in animal studies (with the medications administered to pregnant animals) may not be particularly relevant to the clinical situation where the fertility medication is discontinued before ovulation and conception.
It is important to exclude pregnancy prior to initiating treatment with letrozole or clomiphene.
Birth defects affect approximately 5% of newborns in the United States.7 The relative impact of maternal age, obesity, ovulation induction medicines, and a history of infertility on the rate of birth defects is not fully characterized and is a subject of intense research. To date, there is no strong and consistent evidence that ovulation induction agents, per se, significantly increase the rate of birth defects.
Tulandi and colleagues reported on 911 newborns conceived following ovulation induction with clomiphene or letrozole.8 Overall, the congenital malformation plus chromosomal abnormality rates associated with letrozole and clomiphene ovulation induction were 2.4% and 4.8%, respectively. The major congenital malformation rate for letrozole was 1.2%, and 3.0% for clomiphene.
Many women with anovulatory infertility and PCOS have a BMI of 30 kg/m2 or greater, and some are of advanced maternal age. It is known that women with such a BMI level have an increased risk of congenital malformations, including neural tube defects, spina bifida, septal anomalies, cleft palate, cleft lip, anorectal atresia, hydrocephaly, and limb reduction anomalies.9 The risk of gastroschisis is significantly reduced among obese pregnant women.9 Women aged 40 or older have an increased risk of having a fetus with cardiac defects, esophageal atresia, hypospadias, and craniosynostosis.10
Caution women of advanced maternal age with PCOS and a BMI of 30 kg/m2 or greater about the increased rate of congenital malformations associated with their age and elevated BMI.
Prioritize letrozole when BMI ≥30 kg/m2
I recommend that clomiphene should remain the first-line ovulation induction agent for women with PCOS and a BMI less than 30 kg/m2. This is because, among women with such a BMI level, both clomiphene and letrozole have similar efficacy, and clomiphene is approved by the US Food and Drug Administration for ovulation induction while letrozole is not.
However, for women with PCOS and a BMI of 30 kg/m2 or greater—a clinical situation where letrozole is about twice as effective as clomiphene—letrozole may be the preferred agent.
When prescribing letrozole, start with a dose of 2.5 mg daily for cycle days 3 to 7, following a spontaneous menses or progestin-induced bleed. If ovulation occurs, continue with the dose. If ovulation does not occur, increase the dose to 5 mg daily for cycle days 3 to 7. The maximal dose is 7.5 mg daily for cycle days 3 to 7. When prescribing letrozole, counsel your patient about the increased rate of congenital anomalies among women with an elevated BMI and the possible teratogenic effects of fertility medications.
The aromatase inhibitor letrozole is an important addition to our options for ovulation induction in women with PCOS. Will you start using letrozole for ovulation induction in your practice?
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The three most common causes of infertility are anovulation, tubal occlusion, and abnormal semen parameters. The most common cause of anovulatory infertility is polycystic ovary syndrome (PCOS). Options for initial treatment of anovulatory infertility caused by PCOS include optimizing body mass index (BMI), clomiphene, clomiphene plus dexamethasone, and metformin (TABLE 1). If these low-cost interventions are not successful, high-cost interventions are often very effective treatments, and include follicle-stimulating hormone (FSH) injections, laparoscopic ovarian drilling, and in vitro fertilization.
For many couples, the high-cost interventions are prohibitively expensive. Recently, results of a high-quality randomized clinical trial published by Legro and colleagues in the New England Journal of Medicine indicate that letrozole is more effective than clomiphene for the treatment of anovulatory infertility in women with PCOS.1 Of great importance, letrozole was documented to be especially effective in women with a BMI greater than 30.3 kg/m2.
Letrozole is another low-cost option for couples with anovulatory infertility (TABLE 2), and you should consider it among your initial treatment choices. In this article, I outline when letrozole is your best first option for treatment.
Letrozole is more effective than clomiphene for ovulation induction in women with PCOS and BMI >30.3 kg/m2
Legro and colleagues1 randomly assigned 750 women with anovulatory infertility and PCOS to receive ovulation induction with either clomiphene or letrozole. The medications were prescribed using an escalating dose if ovulation did not occur. For clomiphene, the doses prescribed were 50 mg, 100 mg, and 150 mg. For letrozole, the doses were 2.5 mg, 5 mg, and 7.5 mg. The medications were given daily for 5 days on cycle days 3 to 7, following a spontaneous menses or a medroxyprogesterone acetate withdrawal bleed. Up to 5 cycles of ovulation induction were prescribed.
The ovulation rates for letrozole versus clomiphene were 61.7% and 48.3%, respectively (P<.001). The live birth rates for letrozole versus clomiphene were 27.5% and 19.1%, respectively (P = .007). Among women with a BMI of 30.3 kg/m2 or less, both letrozole and clomiphene treatment resulted in a similar live birth rate of approximately 30% to 35%. Among women with a BMI greater than 30.3 kg/m2, however, the live birth rates with letrozole versus clomiphene were approximately 20% and 10%, respectively.
Consequently, in my practice, I prioritize the use of letrozole for women with a BMI of 30 kg/m2 or greater.
Do not use anastrozole for ovulation induction
In a randomized trial of letrozole versus anastrozole for ovulation induction, 40 women with PCOS were randomly assigned to receive ovulation induction with letrozole (2.5 mg daily for 5 days) or anastrozole (1 mg daily for 5 days).2 The resulting ovulation rate was 84% for letrozole, compared with 60% for anastrozole (P<.05). The pregnancy rate also was significantly higher for letrozole (19% vs 10% for anastrozole, P<.05).
Investigators of two large randomized trials of anastrozole versus clomiphene reported that clomiphene was superior to anastrozole for induction of ovulation in the first cycle of treatment.3,4 Anastrozole, at doses of 1 mg, 5 mg, 10 mg, 20 mg, and 30 mg daily for 5 days, was less effective for ovulation induction in the first cycle of treatment than clomiphene at a dose of 50 mg.3,4
If an aromatase inhibitor is going to be prescribed for ovulation induction, I recommend the use of letrozole and recommend against the use of anastrozole.
Congenital malformations and ovulation induction
The administration of clomiphene or letrozole to pregnant rats has adverse fetal effects.5,6 For example, in pregnant rats a low dose of letrozole (0.003 mg/kg) has been reported to increase intrauterine mortality, fetal resorption, and postimplantation loss; decrease live births; and result in fetal anomalies, including dilation of the ureter and shortening of renal papillae.6
However, in the setting of ovulation induction, letrozole is not administered while the patient is pregnant and is discontinued many days before ovulation and conception. Consequently, the results observed in animal studies (with the medications administered to pregnant animals) may not be particularly relevant to the clinical situation where the fertility medication is discontinued before ovulation and conception.
It is important to exclude pregnancy prior to initiating treatment with letrozole or clomiphene.
Birth defects affect approximately 5% of newborns in the United States.7 The relative impact of maternal age, obesity, ovulation induction medicines, and a history of infertility on the rate of birth defects is not fully characterized and is a subject of intense research. To date, there is no strong and consistent evidence that ovulation induction agents, per se, significantly increase the rate of birth defects.
Tulandi and colleagues reported on 911 newborns conceived following ovulation induction with clomiphene or letrozole.8 Overall, the congenital malformation plus chromosomal abnormality rates associated with letrozole and clomiphene ovulation induction were 2.4% and 4.8%, respectively. The major congenital malformation rate for letrozole was 1.2%, and 3.0% for clomiphene.
Many women with anovulatory infertility and PCOS have a BMI of 30 kg/m2 or greater, and some are of advanced maternal age. It is known that women with such a BMI level have an increased risk of congenital malformations, including neural tube defects, spina bifida, septal anomalies, cleft palate, cleft lip, anorectal atresia, hydrocephaly, and limb reduction anomalies.9 The risk of gastroschisis is significantly reduced among obese pregnant women.9 Women aged 40 or older have an increased risk of having a fetus with cardiac defects, esophageal atresia, hypospadias, and craniosynostosis.10
Caution women of advanced maternal age with PCOS and a BMI of 30 kg/m2 or greater about the increased rate of congenital malformations associated with their age and elevated BMI.
Prioritize letrozole when BMI ≥30 kg/m2
I recommend that clomiphene should remain the first-line ovulation induction agent for women with PCOS and a BMI less than 30 kg/m2. This is because, among women with such a BMI level, both clomiphene and letrozole have similar efficacy, and clomiphene is approved by the US Food and Drug Administration for ovulation induction while letrozole is not.
However, for women with PCOS and a BMI of 30 kg/m2 or greater—a clinical situation where letrozole is about twice as effective as clomiphene—letrozole may be the preferred agent.
When prescribing letrozole, start with a dose of 2.5 mg daily for cycle days 3 to 7, following a spontaneous menses or progestin-induced bleed. If ovulation occurs, continue with the dose. If ovulation does not occur, increase the dose to 5 mg daily for cycle days 3 to 7. The maximal dose is 7.5 mg daily for cycle days 3 to 7. When prescribing letrozole, counsel your patient about the increased rate of congenital anomalies among women with an elevated BMI and the possible teratogenic effects of fertility medications.
The aromatase inhibitor letrozole is an important addition to our options for ovulation induction in women with PCOS. Will you start using letrozole for ovulation induction in your practice?
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129.
2. Al-Omari WR, Sulaiman WR, Al-Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2004;85(3):289–291.
3. Tredway D, Schertz JC, Bock D, Hemsey G, Diamond MP. Anastrozole vs. clomiphene citrate in infertile women with ovulatory dysfunction: a phase II, randomized, dose-finding study. Fertil Steril. 2011;95(5):1720–1724.
4. Tredway D, Schertz JC, Bock D, Hemsey G, Diamond MP. Anastrozole single-dose protocol in women with oligo- or anovulatory infertility: results of a randomized phase II dose-response study. Fertil Steril. 2011;95(5):1725–1729.
5. Clomid (clomiphene citrate tablets USP) [package insert]. Bridgewater, NJ: sanofi-aventis. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016131s026lbl.pdf. Revised October 2012. Accessed October 20, 2014.
6. Femara (letrozole tablets) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. https://www.pharma.us.novartis.com/product/pi/pdf/Femara.pdf. Revised January 2014. Accessed October 20, 2014.
7. Christianson A, Howson CP, Modell B. March of Dimes Global Report on Birth Defects: Executive Summary. White Plains NY: March of Dimes Birth Defects Foundation; 2006:2–9. http://www.marchofdimes.com/materials/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-execu tive-summary.pdf. Accessed October 20, 2014.
8. Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility with letrozole or clomiphene citrate. Fertil Steril. 2006;85(6):1761–1765.
9. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systemic review and meta-analysis. JAMA. 2009;301(6):636–650.
10. Gill SK, Broussard C, Devine O, Green RF, Rasmussen SA, Reefhuis J; National Birth Defects Prevention Study. Association between maternal age and birth defects of unknown etiology: United States, 1997–2007. Birth Defects Res A Clin Mol Teratol. 2012;94(12):1010–1018
1. Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129.
2. Al-Omari WR, Sulaiman WR, Al-Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2004;85(3):289–291.
3. Tredway D, Schertz JC, Bock D, Hemsey G, Diamond MP. Anastrozole vs. clomiphene citrate in infertile women with ovulatory dysfunction: a phase II, randomized, dose-finding study. Fertil Steril. 2011;95(5):1720–1724.
4. Tredway D, Schertz JC, Bock D, Hemsey G, Diamond MP. Anastrozole single-dose protocol in women with oligo- or anovulatory infertility: results of a randomized phase II dose-response study. Fertil Steril. 2011;95(5):1725–1729.
5. Clomid (clomiphene citrate tablets USP) [package insert]. Bridgewater, NJ: sanofi-aventis. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016131s026lbl.pdf. Revised October 2012. Accessed October 20, 2014.
6. Femara (letrozole tablets) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. https://www.pharma.us.novartis.com/product/pi/pdf/Femara.pdf. Revised January 2014. Accessed October 20, 2014.
7. Christianson A, Howson CP, Modell B. March of Dimes Global Report on Birth Defects: Executive Summary. White Plains NY: March of Dimes Birth Defects Foundation; 2006:2–9. http://www.marchofdimes.com/materials/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-execu tive-summary.pdf. Accessed October 20, 2014.
8. Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility with letrozole or clomiphene citrate. Fertil Steril. 2006;85(6):1761–1765.
9. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systemic review and meta-analysis. JAMA. 2009;301(6):636–650.
10. Gill SK, Broussard C, Devine O, Green RF, Rasmussen SA, Reefhuis J; National Birth Defects Prevention Study. Association between maternal age and birth defects of unknown etiology: United States, 1997–2007. Birth Defects Res A Clin Mol Teratol. 2012;94(12):1010–1018
Tackle the challenging shoulder dystocia emergency by practicing delivery of the posterior arm
CASE: McRobert’s maneuver fails
You are attempting an early term vaginal delivery of a 31-year-old G2P1 woman with type 2 diabetes mellitus and an estimated fetal weight of 4,100 g. The fetal head has delivered but retracted against the perineum, producing the “turtle sign.”
You call a shoulder dystocia emergency and request help. In sequence, you tell the mother to stop pushing, check for a nuchal cord, and cut a mediolateral episiotomy. Working seamlessly with your nurse, you place the patient at the edge of the bed, perform the McRobert’s maneuver, provide suprapubic pressure and apply gentle downward guidance to the fetal head. Unfortunately, with these maneuvers the baby does not deliver.
What is your next obstetric maneuver?
With alacrity, move on to an advanced maneuver. In this article, I outline your options for this advanced maneuver and describe the technique for execution. First, however, I discuss the amount of time you have to work with.
How long do you have to perform advanced maneuvers?
In managing a difficult shoulder dystocia, critical goals are to avoid permanent injury to the newborn, including brachial plexus injury, fetal asphyxia, central nervous system injury, and death. Many experts believe that the accoucheur has approximately 4 or 5 minutes to deliver the impacted fetus before the risk of these adverse outcomes rises substantially.1-3 In one study, a head-to-body delivery interval of less than 5 minutes and 5 minutes or longer were associated with rates of hypoxic ischemic encephalopathy of 0.5% and 24%, respectively.2
Stay calm, move on. Given the time pressure for management, it is important to initiate an advanced maneuver, such as rotation of the fetal body or delivery of the posterior arm, when the initial sequence of McRobert’s maneuver, suprapubic pressure, and gentle downward guidance on the fetal head do not result in delivery. Repetitively repeating these initial maneuvers will increase the risk of an adverse fetal outcome. Stay calm and quickly move on to an advanced maneuver.
Advanced maneuvers
The two advanced shoulder dystocia maneuvers that often result in a successful birth are:
- rotation of the fetal shoulders
- delivery of the posterior arm.4,5
In a prior editorial, I described in detail the Woods and Rubin rotational maneuvers.6 In this editorial, I focus on the technique for delivery of the posterior arm.
Delivery of the posterior arm
This maneuver to resolve difficult shoulder dystocia deliveries has been in the armamentarium of obstetricians since at least the mid-18th Century.7 The delivery of the posterior arm reduces the presenting fetal diameter from the larger bisacromial diameter to the smaller axilloacromial diameter. Experts estimate that this change results in a 2-cm decrease in the presenting fetal diameter, thereby facilitating delivery.8,9
In describing posterior arm delivery, it is important to clearly define the anatomy of the upper extremity. The arm is the portion of the upper extremity from the shoulder to the elbow joint. The long bone of the arm is the humerus. The forearm is the portion of the upper extremity from the elbow to the wrist. The long bones of the forearm are the radius and ulna.
Descriptions of how to deliver the posterior arm range from concise to detailed. A concise description recommends “inserting a hand in the vagina, grasping the fetal arm, and sweeping it across the chest.”9
These detailed instructions are provided by Dr. John Rodis, Chief of Obstetrics and Gynecology at St. Francis Hospital in Hartford Connecticut, in UpToDate:
Additional technical guidance. After grasping the fetal wrist and hand, pull the upper extremity against the fetal chest. Approaching the vaginal introitus, pull the wrist and hand toward the fetal ear nearest the maternal symphysis pubis.11 These maneuvers may result in a fracture to the humerus, but this complication is acceptable given the risk of fetal asphyxia and death.
Newborn injuries associated with shoulder dystocia | ||
| In a large retrospective study of 132,098 vaginal cephalic singleton births there were 2,018 cases of shoulder dystocia, representing a 1.5% rate of shoulder dystocia during vaginal birth.5A total of 101 neonatal injuries were reported in association with a shoulder dystocia, the most common being Erb’s palsy, clavicular fracture, and hypoxic ischemic encephalopathy. Some newborns incurred multiple injuries. | ||
| Type of injury | No. of newborns with injury | Rate of injury per 100 shoulder dystocias |
| Erb’s palsy | 60 | 3 |
| Clavicular fracture | 39 | 1.9 |
| Hypoxic ischemic encephalopathy | 6 | 0.3 |
| Klumpke’s palsy | 4 | 0.2 |
| Humerus fracture | 2 | 0.1 |
| Neonatal death | 0 | 0 |
Source: Hoffman, et al. Obstet Gynecol. 2011;117(6):1272–1278. | ||
Approaches to grasping the posterior arm
The posterior arm may be in one of three positions, and your approach to each position will be different.
Fetal hand near the chin. Delivery of the posterior arm is relatively easy when the fetal hand is in this position. Grasp the wrist gently and guide it out of the vagina. The fetal wrist should be pulled toward the fetal ear closest to the maternal symphysis.
Fetal hand on the abdomen. In this position, the operator can exert pressure on the antecubital fossa with the index and middle fingers, resulting in flexion of the forearm at the elbow. This will bring the fetal hand and wrist to the upper chest. The wrist then can be grasped and pronated over the fetal chest. The wrist and forearm are then pulled upward along the chest toward the fetal ear closest to the maternal symphysis.
Fetal upper extremity is extended with the hand next to the thigh. The most challenging situation is when the upper extremity of the fetus is extended along the trunk or behind the buttocks. In this situation the hand and wrist may be near the fetal thigh and very difficult to reach. In addition, the upper extremity may be tightly pinned between fetal trunk and maternal tissues, making it impossible to flex the forearm by gentle pressure on the antecubital fossa.
In this situation the operator’s hand must reach the fetal wrist and distal forearm, grasp these structures, and pull hard across the trunk to free the pinned upper extremity. The fetal wrist and distal forearm can be securely grasped using techniques pictured in the Figure. It can take 30 to 90 seconds for the operator to place a hand in the vagina, identify the posterior shoulder, follow the extended arm to the hand, and secure the wrist. Given the amount of time that it may take to accomplish the first steps of the maneuver, the nurse in the room should call out the time elapsed since the birth of the head at regular intervals to assist the obstetrician in pacing the speed of the intervention.
___________________________________________________________________________________________________
| 
| |
| Figure. When the fetal upper extremity is extended and the hand is near the fetal thigh the fetal upper extremity may be tightly pinned between maternal and fetal tissues. Gentle pressure in the antecubital fossa may not cause the forearm to flex toward the vaginal introitus. In this situation it may be very difficult to grasp the fetal wrist or forearm. The operator should be prepared to place their entire hand and forearm into the vagina to reach the fetal wrist (Top left). Two options for grasping the fetal wrist are with the index finger and middle finger (Top right), or by encircling the wrist with the thumb and index finger (Bottom left). For many obstetricians, the index and middle fingers extend much further from their wrist than the thumb. Consequently, when the fetal wrist and hand are against the fetal thigh it may be easier to reach the fetal wrist with the operator’s index and middle finger. However, many obstetricians find that the thumb and index finger provide a more secure grip of the fetal wrist. |
______________________________________________________________________________________________________
When the posterior arm is fully extended and pinned between fetal trunk and maternal tissues it can be very difficult to reach the fetal wrist. To help successfully complete the maneuver, the obstetrician should visualize placing his or her hand and entire forearm up to the elbow in the vagina to reach the fetal wrist. It may not be necessary to insert the entire forearm in the vagina, but the operator should visualize this step so he or she is prepared for the possibility.Surprisingly, the hollow of the sacrum often provides sufficient space for inserting the hand and entire forearm of the operator. In this process the operator’s hand and forearm may be strongly compressed by maternal and fetal tissues, cutting off circulation to the upper extremity. The operator’s upper extremity may quickly become numb, resulting in a reduction in tactile sensation and strength.
If the posterior arm is positioned behind the back of the fetus, maneuvers similar to those described above can be used to grasp the wrist and pull the arm to the anterior side of the fetal trunk, followed by delivery of the posterior arm.
Practice, practice, and practice some more
Obstetric emergencies create a rush of adrenaline and great stress for the obstetrician. This may adversely impact motor performance, decision-making, and communication skills.12 Low- and high-fidelity simulation exercises permit the obstetrics team to practice the sequence of maneuvers necessary to successfully resolve a shoulder dystocia, thereby reducing stress and improving performance when the emergency actually occurs.13 Simulating obstetric emergencies and visualizing the steps necessary to resolve an emergency are good approaches to prepare obstetricians for the most challenging emergencies. For the difficult to resolve shoulder dystocia, my recommendation is: “Deliver the posterior arm.”
Use this checklist to document a shoulder dystocia event
-Safety Checklists
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839–842.
2. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body delivery interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474–479.
3. Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318–322.
4. Leung TY, Stuart O, Suen SS, Sahota DS, Lau TK, Lao TT. Comparison of perinatal outcomes of shoulder dystocia alleviated by different type and sequence of manoeuvres: a retrospective review. BJOG. 2011;118(8):985–990.
5. Hoffman MK, Bailit KL, Branch DW, et al. A comparison of obstetric maneuvers for the acute management of should dystocia. Obstet Gynecol. 2011;117(6):1272–1278.
6. Barbieri RL. You are the second responder to a shoulder dystocia emergency. What do you do first? OBG Manag. 2013;25(????):10, 12, 15.
7. Beer E. History of extraction of the posterior arm to resolve shoulder dystocia. Obstet Gynecol Surv. 2006;61(3):149–151.
8. Kung J, Swan AV, Arulkumaran S. Delivery of the posterior arm reduces shoulder dimensions in shoulder dystocia. Int J Gynaecol Obstet. 2006;93(3):233–237.
9. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068–1072.
10. Rodis JF. Shoulder dystocia, intrapartum diagnosis, management and outcome. UpToDate, Waltham MA.
11. Mazzanti GA. Delivery of the anterior shoulder; a neglected art. Obstet Gynecol. 1959;13(5):603–607.
12. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5–10.
13. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513–517.
CASE: McRobert’s maneuver fails
You are attempting an early term vaginal delivery of a 31-year-old G2P1 woman with type 2 diabetes mellitus and an estimated fetal weight of 4,100 g. The fetal head has delivered but retracted against the perineum, producing the “turtle sign.”
You call a shoulder dystocia emergency and request help. In sequence, you tell the mother to stop pushing, check for a nuchal cord, and cut a mediolateral episiotomy. Working seamlessly with your nurse, you place the patient at the edge of the bed, perform the McRobert’s maneuver, provide suprapubic pressure and apply gentle downward guidance to the fetal head. Unfortunately, with these maneuvers the baby does not deliver.
What is your next obstetric maneuver?
With alacrity, move on to an advanced maneuver. In this article, I outline your options for this advanced maneuver and describe the technique for execution. First, however, I discuss the amount of time you have to work with.
How long do you have to perform advanced maneuvers?
In managing a difficult shoulder dystocia, critical goals are to avoid permanent injury to the newborn, including brachial plexus injury, fetal asphyxia, central nervous system injury, and death. Many experts believe that the accoucheur has approximately 4 or 5 minutes to deliver the impacted fetus before the risk of these adverse outcomes rises substantially.1-3 In one study, a head-to-body delivery interval of less than 5 minutes and 5 minutes or longer were associated with rates of hypoxic ischemic encephalopathy of 0.5% and 24%, respectively.2
Stay calm, move on. Given the time pressure for management, it is important to initiate an advanced maneuver, such as rotation of the fetal body or delivery of the posterior arm, when the initial sequence of McRobert’s maneuver, suprapubic pressure, and gentle downward guidance on the fetal head do not result in delivery. Repetitively repeating these initial maneuvers will increase the risk of an adverse fetal outcome. Stay calm and quickly move on to an advanced maneuver.
Advanced maneuvers
The two advanced shoulder dystocia maneuvers that often result in a successful birth are:
- rotation of the fetal shoulders
- delivery of the posterior arm.4,5
In a prior editorial, I described in detail the Woods and Rubin rotational maneuvers.6 In this editorial, I focus on the technique for delivery of the posterior arm.
Delivery of the posterior arm
This maneuver to resolve difficult shoulder dystocia deliveries has been in the armamentarium of obstetricians since at least the mid-18th Century.7 The delivery of the posterior arm reduces the presenting fetal diameter from the larger bisacromial diameter to the smaller axilloacromial diameter. Experts estimate that this change results in a 2-cm decrease in the presenting fetal diameter, thereby facilitating delivery.8,9
In describing posterior arm delivery, it is important to clearly define the anatomy of the upper extremity. The arm is the portion of the upper extremity from the shoulder to the elbow joint. The long bone of the arm is the humerus. The forearm is the portion of the upper extremity from the elbow to the wrist. The long bones of the forearm are the radius and ulna.
Descriptions of how to deliver the posterior arm range from concise to detailed. A concise description recommends “inserting a hand in the vagina, grasping the fetal arm, and sweeping it across the chest.”9
These detailed instructions are provided by Dr. John Rodis, Chief of Obstetrics and Gynecology at St. Francis Hospital in Hartford Connecticut, in UpToDate:
Additional technical guidance. After grasping the fetal wrist and hand, pull the upper extremity against the fetal chest. Approaching the vaginal introitus, pull the wrist and hand toward the fetal ear nearest the maternal symphysis pubis.11 These maneuvers may result in a fracture to the humerus, but this complication is acceptable given the risk of fetal asphyxia and death.
Newborn injuries associated with shoulder dystocia | ||
| In a large retrospective study of 132,098 vaginal cephalic singleton births there were 2,018 cases of shoulder dystocia, representing a 1.5% rate of shoulder dystocia during vaginal birth.5A total of 101 neonatal injuries were reported in association with a shoulder dystocia, the most common being Erb’s palsy, clavicular fracture, and hypoxic ischemic encephalopathy. Some newborns incurred multiple injuries. | ||
| Type of injury | No. of newborns with injury | Rate of injury per 100 shoulder dystocias |
| Erb’s palsy | 60 | 3 |
| Clavicular fracture | 39 | 1.9 |
| Hypoxic ischemic encephalopathy | 6 | 0.3 |
| Klumpke’s palsy | 4 | 0.2 |
| Humerus fracture | 2 | 0.1 |
| Neonatal death | 0 | 0 |
Source: Hoffman, et al. Obstet Gynecol. 2011;117(6):1272–1278. | ||
Approaches to grasping the posterior arm
The posterior arm may be in one of three positions, and your approach to each position will be different.
Fetal hand near the chin. Delivery of the posterior arm is relatively easy when the fetal hand is in this position. Grasp the wrist gently and guide it out of the vagina. The fetal wrist should be pulled toward the fetal ear closest to the maternal symphysis.
Fetal hand on the abdomen. In this position, the operator can exert pressure on the antecubital fossa with the index and middle fingers, resulting in flexion of the forearm at the elbow. This will bring the fetal hand and wrist to the upper chest. The wrist then can be grasped and pronated over the fetal chest. The wrist and forearm are then pulled upward along the chest toward the fetal ear closest to the maternal symphysis.
Fetal upper extremity is extended with the hand next to the thigh. The most challenging situation is when the upper extremity of the fetus is extended along the trunk or behind the buttocks. In this situation the hand and wrist may be near the fetal thigh and very difficult to reach. In addition, the upper extremity may be tightly pinned between fetal trunk and maternal tissues, making it impossible to flex the forearm by gentle pressure on the antecubital fossa.
In this situation the operator’s hand must reach the fetal wrist and distal forearm, grasp these structures, and pull hard across the trunk to free the pinned upper extremity. The fetal wrist and distal forearm can be securely grasped using techniques pictured in the Figure. It can take 30 to 90 seconds for the operator to place a hand in the vagina, identify the posterior shoulder, follow the extended arm to the hand, and secure the wrist. Given the amount of time that it may take to accomplish the first steps of the maneuver, the nurse in the room should call out the time elapsed since the birth of the head at regular intervals to assist the obstetrician in pacing the speed of the intervention.
___________________________________________________________________________________________________

| 
| |

| Figure. When the fetal upper extremity is extended and the hand is near the fetal thigh the fetal upper extremity may be tightly pinned between maternal and fetal tissues. Gentle pressure in the antecubital fossa may not cause the forearm to flex toward the vaginal introitus. In this situation it may be very difficult to grasp the fetal wrist or forearm. The operator should be prepared to place their entire hand and forearm into the vagina to reach the fetal wrist (Top left). Two options for grasping the fetal wrist are with the index finger and middle finger (Top right), or by encircling the wrist with the thumb and index finger (Bottom left). For many obstetricians, the index and middle fingers extend much further from their wrist than the thumb. Consequently, when the fetal wrist and hand are against the fetal thigh it may be easier to reach the fetal wrist with the operator’s index and middle finger. However, many obstetricians find that the thumb and index finger provide a more secure grip of the fetal wrist. |
______________________________________________________________________________________________________
When the posterior arm is fully extended and pinned between fetal trunk and maternal tissues it can be very difficult to reach the fetal wrist. To help successfully complete the maneuver, the obstetrician should visualize placing his or her hand and entire forearm up to the elbow in the vagina to reach the fetal wrist. It may not be necessary to insert the entire forearm in the vagina, but the operator should visualize this step so he or she is prepared for the possibility.Surprisingly, the hollow of the sacrum often provides sufficient space for inserting the hand and entire forearm of the operator. In this process the operator’s hand and forearm may be strongly compressed by maternal and fetal tissues, cutting off circulation to the upper extremity. The operator’s upper extremity may quickly become numb, resulting in a reduction in tactile sensation and strength.
If the posterior arm is positioned behind the back of the fetus, maneuvers similar to those described above can be used to grasp the wrist and pull the arm to the anterior side of the fetal trunk, followed by delivery of the posterior arm.
Practice, practice, and practice some more
Obstetric emergencies create a rush of adrenaline and great stress for the obstetrician. This may adversely impact motor performance, decision-making, and communication skills.12 Low- and high-fidelity simulation exercises permit the obstetrics team to practice the sequence of maneuvers necessary to successfully resolve a shoulder dystocia, thereby reducing stress and improving performance when the emergency actually occurs.13 Simulating obstetric emergencies and visualizing the steps necessary to resolve an emergency are good approaches to prepare obstetricians for the most challenging emergencies. For the difficult to resolve shoulder dystocia, my recommendation is: “Deliver the posterior arm.”
Use this checklist to document a shoulder dystocia event
-Safety Checklists
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: McRobert’s maneuver fails
You are attempting an early term vaginal delivery of a 31-year-old G2P1 woman with type 2 diabetes mellitus and an estimated fetal weight of 4,100 g. The fetal head has delivered but retracted against the perineum, producing the “turtle sign.”
You call a shoulder dystocia emergency and request help. In sequence, you tell the mother to stop pushing, check for a nuchal cord, and cut a mediolateral episiotomy. Working seamlessly with your nurse, you place the patient at the edge of the bed, perform the McRobert’s maneuver, provide suprapubic pressure and apply gentle downward guidance to the fetal head. Unfortunately, with these maneuvers the baby does not deliver.
What is your next obstetric maneuver?
With alacrity, move on to an advanced maneuver. In this article, I outline your options for this advanced maneuver and describe the technique for execution. First, however, I discuss the amount of time you have to work with.
How long do you have to perform advanced maneuvers?
In managing a difficult shoulder dystocia, critical goals are to avoid permanent injury to the newborn, including brachial plexus injury, fetal asphyxia, central nervous system injury, and death. Many experts believe that the accoucheur has approximately 4 or 5 minutes to deliver the impacted fetus before the risk of these adverse outcomes rises substantially.1-3 In one study, a head-to-body delivery interval of less than 5 minutes and 5 minutes or longer were associated with rates of hypoxic ischemic encephalopathy of 0.5% and 24%, respectively.2
Stay calm, move on. Given the time pressure for management, it is important to initiate an advanced maneuver, such as rotation of the fetal body or delivery of the posterior arm, when the initial sequence of McRobert’s maneuver, suprapubic pressure, and gentle downward guidance on the fetal head do not result in delivery. Repetitively repeating these initial maneuvers will increase the risk of an adverse fetal outcome. Stay calm and quickly move on to an advanced maneuver.
Advanced maneuvers
The two advanced shoulder dystocia maneuvers that often result in a successful birth are:
- rotation of the fetal shoulders
- delivery of the posterior arm.4,5
In a prior editorial, I described in detail the Woods and Rubin rotational maneuvers.6 In this editorial, I focus on the technique for delivery of the posterior arm.
Delivery of the posterior arm
This maneuver to resolve difficult shoulder dystocia deliveries has been in the armamentarium of obstetricians since at least the mid-18th Century.7 The delivery of the posterior arm reduces the presenting fetal diameter from the larger bisacromial diameter to the smaller axilloacromial diameter. Experts estimate that this change results in a 2-cm decrease in the presenting fetal diameter, thereby facilitating delivery.8,9
In describing posterior arm delivery, it is important to clearly define the anatomy of the upper extremity. The arm is the portion of the upper extremity from the shoulder to the elbow joint. The long bone of the arm is the humerus. The forearm is the portion of the upper extremity from the elbow to the wrist. The long bones of the forearm are the radius and ulna.
Descriptions of how to deliver the posterior arm range from concise to detailed. A concise description recommends “inserting a hand in the vagina, grasping the fetal arm, and sweeping it across the chest.”9
These detailed instructions are provided by Dr. John Rodis, Chief of Obstetrics and Gynecology at St. Francis Hospital in Hartford Connecticut, in UpToDate:
Additional technical guidance. After grasping the fetal wrist and hand, pull the upper extremity against the fetal chest. Approaching the vaginal introitus, pull the wrist and hand toward the fetal ear nearest the maternal symphysis pubis.11 These maneuvers may result in a fracture to the humerus, but this complication is acceptable given the risk of fetal asphyxia and death.
Newborn injuries associated with shoulder dystocia | ||
| In a large retrospective study of 132,098 vaginal cephalic singleton births there were 2,018 cases of shoulder dystocia, representing a 1.5% rate of shoulder dystocia during vaginal birth.5A total of 101 neonatal injuries were reported in association with a shoulder dystocia, the most common being Erb’s palsy, clavicular fracture, and hypoxic ischemic encephalopathy. Some newborns incurred multiple injuries. | ||
| Type of injury | No. of newborns with injury | Rate of injury per 100 shoulder dystocias |
| Erb’s palsy | 60 | 3 |
| Clavicular fracture | 39 | 1.9 |
| Hypoxic ischemic encephalopathy | 6 | 0.3 |
| Klumpke’s palsy | 4 | 0.2 |
| Humerus fracture | 2 | 0.1 |
| Neonatal death | 0 | 0 |
Source: Hoffman, et al. Obstet Gynecol. 2011;117(6):1272–1278. | ||
Approaches to grasping the posterior arm
The posterior arm may be in one of three positions, and your approach to each position will be different.
Fetal hand near the chin. Delivery of the posterior arm is relatively easy when the fetal hand is in this position. Grasp the wrist gently and guide it out of the vagina. The fetal wrist should be pulled toward the fetal ear closest to the maternal symphysis.
Fetal hand on the abdomen. In this position, the operator can exert pressure on the antecubital fossa with the index and middle fingers, resulting in flexion of the forearm at the elbow. This will bring the fetal hand and wrist to the upper chest. The wrist then can be grasped and pronated over the fetal chest. The wrist and forearm are then pulled upward along the chest toward the fetal ear closest to the maternal symphysis.
Fetal upper extremity is extended with the hand next to the thigh. The most challenging situation is when the upper extremity of the fetus is extended along the trunk or behind the buttocks. In this situation the hand and wrist may be near the fetal thigh and very difficult to reach. In addition, the upper extremity may be tightly pinned between fetal trunk and maternal tissues, making it impossible to flex the forearm by gentle pressure on the antecubital fossa.
In this situation the operator’s hand must reach the fetal wrist and distal forearm, grasp these structures, and pull hard across the trunk to free the pinned upper extremity. The fetal wrist and distal forearm can be securely grasped using techniques pictured in the Figure. It can take 30 to 90 seconds for the operator to place a hand in the vagina, identify the posterior shoulder, follow the extended arm to the hand, and secure the wrist. Given the amount of time that it may take to accomplish the first steps of the maneuver, the nurse in the room should call out the time elapsed since the birth of the head at regular intervals to assist the obstetrician in pacing the speed of the intervention.
___________________________________________________________________________________________________

| 
| |

| Figure. When the fetal upper extremity is extended and the hand is near the fetal thigh the fetal upper extremity may be tightly pinned between maternal and fetal tissues. Gentle pressure in the antecubital fossa may not cause the forearm to flex toward the vaginal introitus. In this situation it may be very difficult to grasp the fetal wrist or forearm. The operator should be prepared to place their entire hand and forearm into the vagina to reach the fetal wrist (Top left). Two options for grasping the fetal wrist are with the index finger and middle finger (Top right), or by encircling the wrist with the thumb and index finger (Bottom left). For many obstetricians, the index and middle fingers extend much further from their wrist than the thumb. Consequently, when the fetal wrist and hand are against the fetal thigh it may be easier to reach the fetal wrist with the operator’s index and middle finger. However, many obstetricians find that the thumb and index finger provide a more secure grip of the fetal wrist. |
______________________________________________________________________________________________________
When the posterior arm is fully extended and pinned between fetal trunk and maternal tissues it can be very difficult to reach the fetal wrist. To help successfully complete the maneuver, the obstetrician should visualize placing his or her hand and entire forearm up to the elbow in the vagina to reach the fetal wrist. It may not be necessary to insert the entire forearm in the vagina, but the operator should visualize this step so he or she is prepared for the possibility.Surprisingly, the hollow of the sacrum often provides sufficient space for inserting the hand and entire forearm of the operator. In this process the operator’s hand and forearm may be strongly compressed by maternal and fetal tissues, cutting off circulation to the upper extremity. The operator’s upper extremity may quickly become numb, resulting in a reduction in tactile sensation and strength.
If the posterior arm is positioned behind the back of the fetus, maneuvers similar to those described above can be used to grasp the wrist and pull the arm to the anterior side of the fetal trunk, followed by delivery of the posterior arm.
Practice, practice, and practice some more
Obstetric emergencies create a rush of adrenaline and great stress for the obstetrician. This may adversely impact motor performance, decision-making, and communication skills.12 Low- and high-fidelity simulation exercises permit the obstetrics team to practice the sequence of maneuvers necessary to successfully resolve a shoulder dystocia, thereby reducing stress and improving performance when the emergency actually occurs.13 Simulating obstetric emergencies and visualizing the steps necessary to resolve an emergency are good approaches to prepare obstetricians for the most challenging emergencies. For the difficult to resolve shoulder dystocia, my recommendation is: “Deliver the posterior arm.”
Use this checklist to document a shoulder dystocia event
-Safety Checklists
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839–842.
2. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body delivery interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474–479.
3. Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318–322.
4. Leung TY, Stuart O, Suen SS, Sahota DS, Lau TK, Lao TT. Comparison of perinatal outcomes of shoulder dystocia alleviated by different type and sequence of manoeuvres: a retrospective review. BJOG. 2011;118(8):985–990.
5. Hoffman MK, Bailit KL, Branch DW, et al. A comparison of obstetric maneuvers for the acute management of should dystocia. Obstet Gynecol. 2011;117(6):1272–1278.
6. Barbieri RL. You are the second responder to a shoulder dystocia emergency. What do you do first? OBG Manag. 2013;25(????):10, 12, 15.
7. Beer E. History of extraction of the posterior arm to resolve shoulder dystocia. Obstet Gynecol Surv. 2006;61(3):149–151.
8. Kung J, Swan AV, Arulkumaran S. Delivery of the posterior arm reduces shoulder dimensions in shoulder dystocia. Int J Gynaecol Obstet. 2006;93(3):233–237.
9. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068–1072.
10. Rodis JF. Shoulder dystocia, intrapartum diagnosis, management and outcome. UpToDate, Waltham MA.
11. Mazzanti GA. Delivery of the anterior shoulder; a neglected art. Obstet Gynecol. 1959;13(5):603–607.
12. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5–10.
13. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513–517.
1. Allen RH, Rosenbaum TC, Ghidini A, Poggi SH, Spong CY. Correlating head-to-body delivery intervals with neonatal depression in vaginal births that result in permanent brachial plexus injury. Am J Obstet Gynecol. 2002;187(4):839–842.
2. Leung TY, Stuart O, Sahota DS, Suen SS, Lau TK, Lao TT. Head-to-body delivery interval and risk of fetal acidosis and hypoxic ischaemic encephalopathy in shoulder dystocia: a retrospective review. BJOG. 2011;118(4):474–479.
3. Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318–322.
4. Leung TY, Stuart O, Suen SS, Sahota DS, Lau TK, Lao TT. Comparison of perinatal outcomes of shoulder dystocia alleviated by different type and sequence of manoeuvres: a retrospective review. BJOG. 2011;118(8):985–990.
5. Hoffman MK, Bailit KL, Branch DW, et al. A comparison of obstetric maneuvers for the acute management of should dystocia. Obstet Gynecol. 2011;117(6):1272–1278.
6. Barbieri RL. You are the second responder to a shoulder dystocia emergency. What do you do first? OBG Manag. 2013;25(????):10, 12, 15.
7. Beer E. History of extraction of the posterior arm to resolve shoulder dystocia. Obstet Gynecol Surv. 2006;61(3):149–151.
8. Kung J, Swan AV, Arulkumaran S. Delivery of the posterior arm reduces shoulder dimensions in shoulder dystocia. Int J Gynaecol Obstet. 2006;93(3):233–237.
9. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068–1072.
10. Rodis JF. Shoulder dystocia, intrapartum diagnosis, management and outcome. UpToDate, Waltham MA.
11. Mazzanti GA. Delivery of the anterior shoulder; a neglected art. Obstet Gynecol. 1959;13(5):603–607.
12. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5–10.
13. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513–517.
Farewell to indigo carmine
Suddenly, indigo carmine is in short supply throughout the United States. One manufacturer has stopped production because of a raw materials shortage; another is experiencing manufacturing delays. Neither company can estimate a resupply or product return date.1
Indigo carmine is approved by the US Food and Drug Administration (FDA) to localize ureteral orifices during cystoscopy and is commonly used in obstetrics and gynecology as a marker dye in the following additional situations:
- administered in a dilute solution via a catheter to back fill the bladder and test for bladder injury
- administered via a cannula in the uterine cavity to test the patency of the fallopian tubes
- injected into the amniotic fluid compartment to test for premature rupture of the membranes (PROM)
- injected into the amniotic fluid of a twin gestation to mark the amniotic fluid of one twin.
With this agent in short supply, we need to identify alternative marker dyes to use in our clinical practice. In this editorial, I provide a list of possible options to replace indigo carmine. Evidence supporting the use and safety of marker dyes in obstetrics and gynecology is based on small cohorts or case reports. The available evidence is of modest to low quality, and it is especially challenging to identify uncommon adverse effects. Consequently, expert opinion guides most practice recommendations.
Options to test the function of the ureters at cystoscopy
Given the lack of availability of indigo carmine, I recommend one of the following three options to test the function of the ureters at cystoscopy.
Partially fill the bladder with a solution of either sterile water or a 10% dextrose solution. (Experienced surgeons may prefer to use saline.) The turbulence of the interaction between the ureteral urine jet and instilled fluid in the bladder may permit visualization of the urine stream exiting the ureteral orifices as it swirls through the sterile water or dextrose solution. Both sterile water and a 10% dextrose solution offer a contrast in viscosity between the urine and the cystoscopy fluid, which may enhance the ability to detect the urine jet leaving the ureter.2
Administer IV methylene blue. Methylene blue is FDA approved for methemoglobinemia treatment. For this indication, it is administered intravenously at a dose of 1 to 2 mg/kgover 5 to 10 minutes. Paradoxically, when administered at a dose of >7 mg/kg, methylene blue can cause methemoglobinemia.3
Methylene blue often is provided as a 1% solution of 10 mg/mL in 10-mL vials. To use intravenous (IV) methylene blue to test ureteral function at cystoscopy, administer IV methylene blue 50 mg over 5 minutes. Use the cystoscope to view the colored urine exiting the ureteral orifices.4,5 Administering a small dose of IV furosemide may accelerate the appearance of methylene blue in the urine.
When to use caution. Methylene blue blocks serotonin metabolism by inhibiting monoamine oxidase and may precipitate a serotonin syndrome in patients taking selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or monoamine oxidase inhibitors (MAOIs).
Common findings in the serotonin syndrome include: temperature above 38° C (100.4° F), anxiety, agitation, delirium, clonus, tremor, and hypertonia.6 I recommend that you DO NOT use IV methylene blue in patients taking these medications.7,8
Great caution should be used before administering IV methylene blue in the presence of the following clinical situations:
- renal impairment
- G6PD deficiency
- pediatric patients.
Methylene blue never should be given by a subcutaneous or intrathecal route. In pregnant women it should never be injected into the amniotic fluid compartment.
Use preoperative oral phenazo-pyridine. If the preoperative plan includes a cystoscopy procedure to test ureteral function, administering oral phenazopyridine in the preoperative holding area will result in colorization of the urine within 30 minutes, and it will persist for approximately 4 to 5 hours. To use this approach, administer one dose of phenazopyridine (Pyridium, Azo-Gesic), 100 mg or 200 mg orally, 30 minutes to 1 hour before the planned surgical start time, in the preoperative holding area.9 During cystoscopy, the urine from the ureteral jet will be colored orange.
When to use caution. Phenazopyridine should not be administered to patients with G6PD deficiency.
Option to test for bladder injury
Methylene blue. If methylene blue is used to test the integrity of the bladder, I recommend diluting 10 mg methylene blue in 1 L normal saline and then instilling the dilute solution through a catheter into the bladder.10
It is unlikely that this technique will be associated with sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs, and MAOIs.
Option to test patency of the fallopian tubes (chromopertubation)
Methylene blue. If methylene blue is used via a cannula in the uterine cavity to test the patency of the fallopian tubes, I recommend diluting 10 mg methylene blue in 150 mL normal saline and using the dilute solution to test tubal patency.
It is unlikely that this process will lead to sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs and MAOIs. However, if intrauterine injection of the dilute dye solution results in extravasation of the dye into the pelvic veins, a significant amount of dye can enter the circulation.11 There are case reports of anaphylaxis following intrauterine injection of methylene blue to test tubal patency.12,13
Options to diagnose PROM and to use for twin amniocentesis
None. Most experts recommend against the intra-amniotic injection of methylene blue to diagnose PROM or in twin amniocentesis procedures. Methylene blue injected into the intra-amniotic fluid during amniocentesis in multiple gestations has been reported to cause fetal bowel obstruction or atresia.14,15 Fetal death also has been reported.16 Decades ago, indocyanine green, which is FDA approved to determine cardiac output, liver blood flow, and hepatic function, was reported to be useful to mark one sac of a twin gestation during amniocentesis.17 With modern ultrasonography technology, the need to rely on a dye to mark a sac of a twin has decreased significantly.
Our only option is to cope—effectively as possible Over decades, the medical community develops patterns of patient care that are critically dependent on the availability of key pharmaceuticals and devices. When a pharmaceutical or device suddenly becomes unavailable, it can disrupt important patterns of patient care. Imagine the impact on obstetrics practice if oxytocin became unavailable due to manufacturing shortages. Likely, both market forces and government regulation are the root cause of the shortfalls. Preventing the adverse consequences of the sudden loss of key pharmaceuticals and devices is an important priority to ensure optimal care of our patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Indigo carmine injection. American Society of Health-System Pharmacists (ASHP) Web site. http://www.ashp.org/menu/DrugShortages/CurrentShortages/Bulletin.aspx?id=861. Updated July 29, 2014. Accessed August 21, 2014.
2. Lin BL, Iwata Y. Modified cystoscopy to evaluate unilateral traumatic injury of the ureter during pelvic surgery. Am J Obstet Gynecol. 1990;162(5):1343–1344.
3. Lee M, Sharifi R. Methylene blue versus indigo carmine. Urology. 1996;47(5):783–784.
4. Thompson JD. Operative injuries to the ureter: prevention, recognition and management. In Rock JA, Thompson JD, eds. TeLinde’s Operative Gynecology. Philadelphia, PA: Lippincott Williams & Wilkins; 1997:1155.
5. Wang AC. The techniques of trocar insertion and intraoperative urethroscopy in tension-free vaginal taping: an experience of 600 cases. Acta Obstet Gynecol Scand. 2004;83(3):293–298.
6. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120.
7. Shah-Khan MG, Lovely J, Degnim AC. Safety of methylene blue dye for lymphatic mapping in patients taking selective serotonin reuptake inhibitors. Am J Surg. 2012;204(5):798–799.
8. Ng BK, Cameron AJ. The role of methylene blue in serotonin syndrome: a systematic review. Psychosomatics. 2010;51(3):194–200.
9. Hui JY, Harvey MA, Johnston SL. Confirmation of ureteric patency during cystoscopy using phenazopyridine HCl: a low-cost approach. J Obstet Gynaecol Can. 2009;31(9):845–849.
10. Moore CR, Shirodkar SP, Avallone MA, et al. Intravesical methylene blue facilitates precise identification of the diverticular neck during robot-assisted laparoscopic bladder diverticulectomy. J Laparoendosc Adv Surg Tech A. 2012;22(5):492–495.
11. Mhaskar R, Mhaskar AM. Methemoglobinemia following chromopertubation in treated pelvic tuberculosis. Int J Gynaecol Obstet. 2002;77(1):41–42.
12. Rzymski P, Wozniak J, Opala T, Wilczak M, Sajdak S. Anaphylactic reaction to methylene blue dye after laparoscopic chromopertubation. Int J Gynaecol Obstet. 2003;81(1):71–72.
13. Dewachter P, Mouton-Faivre C, Trechot P, Lieu JC, Mertes PM. Severe anaphylactic shock with methylene blue instillation. Anesth Analg. 2005;101(1):149–150.
14. McFadyen I. The dangers of intra-amniotic methylene blue. Br J Obstet Gynaecol. 1992;99(2):89–90.
15. Van der Pol JG, Wolf H, Boer K, et al. Jejunal atresia related to the use of methylene blue in genetic amniocentesis in twins. Br J Obstet Gynaecol. 1992;99(2):141–143.
16. Kidd SA, Lancaster PA, Anderson JC, et al. Fetal death after exposure to methylene blue dye during mid-trimester amniocentesis in twin pregnancy. Prenat Diagn. 1996;16(1):39–47.
17. Hobbins JC, Winsberg F, Blanchett M, et al. Section 5: fetal imaging. Prenat Diagn. 1981;1(5):35–38.
Suddenly, indigo carmine is in short supply throughout the United States. One manufacturer has stopped production because of a raw materials shortage; another is experiencing manufacturing delays. Neither company can estimate a resupply or product return date.1
Indigo carmine is approved by the US Food and Drug Administration (FDA) to localize ureteral orifices during cystoscopy and is commonly used in obstetrics and gynecology as a marker dye in the following additional situations:
- administered in a dilute solution via a catheter to back fill the bladder and test for bladder injury
- administered via a cannula in the uterine cavity to test the patency of the fallopian tubes
- injected into the amniotic fluid compartment to test for premature rupture of the membranes (PROM)
- injected into the amniotic fluid of a twin gestation to mark the amniotic fluid of one twin.
With this agent in short supply, we need to identify alternative marker dyes to use in our clinical practice. In this editorial, I provide a list of possible options to replace indigo carmine. Evidence supporting the use and safety of marker dyes in obstetrics and gynecology is based on small cohorts or case reports. The available evidence is of modest to low quality, and it is especially challenging to identify uncommon adverse effects. Consequently, expert opinion guides most practice recommendations.
Options to test the function of the ureters at cystoscopy
Given the lack of availability of indigo carmine, I recommend one of the following three options to test the function of the ureters at cystoscopy.
Partially fill the bladder with a solution of either sterile water or a 10% dextrose solution. (Experienced surgeons may prefer to use saline.) The turbulence of the interaction between the ureteral urine jet and instilled fluid in the bladder may permit visualization of the urine stream exiting the ureteral orifices as it swirls through the sterile water or dextrose solution. Both sterile water and a 10% dextrose solution offer a contrast in viscosity between the urine and the cystoscopy fluid, which may enhance the ability to detect the urine jet leaving the ureter.2
Administer IV methylene blue. Methylene blue is FDA approved for methemoglobinemia treatment. For this indication, it is administered intravenously at a dose of 1 to 2 mg/kgover 5 to 10 minutes. Paradoxically, when administered at a dose of >7 mg/kg, methylene blue can cause methemoglobinemia.3
Methylene blue often is provided as a 1% solution of 10 mg/mL in 10-mL vials. To use intravenous (IV) methylene blue to test ureteral function at cystoscopy, administer IV methylene blue 50 mg over 5 minutes. Use the cystoscope to view the colored urine exiting the ureteral orifices.4,5 Administering a small dose of IV furosemide may accelerate the appearance of methylene blue in the urine.
When to use caution. Methylene blue blocks serotonin metabolism by inhibiting monoamine oxidase and may precipitate a serotonin syndrome in patients taking selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or monoamine oxidase inhibitors (MAOIs).
Common findings in the serotonin syndrome include: temperature above 38° C (100.4° F), anxiety, agitation, delirium, clonus, tremor, and hypertonia.6 I recommend that you DO NOT use IV methylene blue in patients taking these medications.7,8
Great caution should be used before administering IV methylene blue in the presence of the following clinical situations:
- renal impairment
- G6PD deficiency
- pediatric patients.
Methylene blue never should be given by a subcutaneous or intrathecal route. In pregnant women it should never be injected into the amniotic fluid compartment.
Use preoperative oral phenazo-pyridine. If the preoperative plan includes a cystoscopy procedure to test ureteral function, administering oral phenazopyridine in the preoperative holding area will result in colorization of the urine within 30 minutes, and it will persist for approximately 4 to 5 hours. To use this approach, administer one dose of phenazopyridine (Pyridium, Azo-Gesic), 100 mg or 200 mg orally, 30 minutes to 1 hour before the planned surgical start time, in the preoperative holding area.9 During cystoscopy, the urine from the ureteral jet will be colored orange.
When to use caution. Phenazopyridine should not be administered to patients with G6PD deficiency.
Option to test for bladder injury
Methylene blue. If methylene blue is used to test the integrity of the bladder, I recommend diluting 10 mg methylene blue in 1 L normal saline and then instilling the dilute solution through a catheter into the bladder.10
It is unlikely that this technique will be associated with sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs, and MAOIs.
Option to test patency of the fallopian tubes (chromopertubation)
Methylene blue. If methylene blue is used via a cannula in the uterine cavity to test the patency of the fallopian tubes, I recommend diluting 10 mg methylene blue in 150 mL normal saline and using the dilute solution to test tubal patency.
It is unlikely that this process will lead to sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs and MAOIs. However, if intrauterine injection of the dilute dye solution results in extravasation of the dye into the pelvic veins, a significant amount of dye can enter the circulation.11 There are case reports of anaphylaxis following intrauterine injection of methylene blue to test tubal patency.12,13
Options to diagnose PROM and to use for twin amniocentesis
None. Most experts recommend against the intra-amniotic injection of methylene blue to diagnose PROM or in twin amniocentesis procedures. Methylene blue injected into the intra-amniotic fluid during amniocentesis in multiple gestations has been reported to cause fetal bowel obstruction or atresia.14,15 Fetal death also has been reported.16 Decades ago, indocyanine green, which is FDA approved to determine cardiac output, liver blood flow, and hepatic function, was reported to be useful to mark one sac of a twin gestation during amniocentesis.17 With modern ultrasonography technology, the need to rely on a dye to mark a sac of a twin has decreased significantly.
Our only option is to cope—effectively as possible Over decades, the medical community develops patterns of patient care that are critically dependent on the availability of key pharmaceuticals and devices. When a pharmaceutical or device suddenly becomes unavailable, it can disrupt important patterns of patient care. Imagine the impact on obstetrics practice if oxytocin became unavailable due to manufacturing shortages. Likely, both market forces and government regulation are the root cause of the shortfalls. Preventing the adverse consequences of the sudden loss of key pharmaceuticals and devices is an important priority to ensure optimal care of our patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Suddenly, indigo carmine is in short supply throughout the United States. One manufacturer has stopped production because of a raw materials shortage; another is experiencing manufacturing delays. Neither company can estimate a resupply or product return date.1
Indigo carmine is approved by the US Food and Drug Administration (FDA) to localize ureteral orifices during cystoscopy and is commonly used in obstetrics and gynecology as a marker dye in the following additional situations:
- administered in a dilute solution via a catheter to back fill the bladder and test for bladder injury
- administered via a cannula in the uterine cavity to test the patency of the fallopian tubes
- injected into the amniotic fluid compartment to test for premature rupture of the membranes (PROM)
- injected into the amniotic fluid of a twin gestation to mark the amniotic fluid of one twin.
With this agent in short supply, we need to identify alternative marker dyes to use in our clinical practice. In this editorial, I provide a list of possible options to replace indigo carmine. Evidence supporting the use and safety of marker dyes in obstetrics and gynecology is based on small cohorts or case reports. The available evidence is of modest to low quality, and it is especially challenging to identify uncommon adverse effects. Consequently, expert opinion guides most practice recommendations.
Options to test the function of the ureters at cystoscopy
Given the lack of availability of indigo carmine, I recommend one of the following three options to test the function of the ureters at cystoscopy.
Partially fill the bladder with a solution of either sterile water or a 10% dextrose solution. (Experienced surgeons may prefer to use saline.) The turbulence of the interaction between the ureteral urine jet and instilled fluid in the bladder may permit visualization of the urine stream exiting the ureteral orifices as it swirls through the sterile water or dextrose solution. Both sterile water and a 10% dextrose solution offer a contrast in viscosity between the urine and the cystoscopy fluid, which may enhance the ability to detect the urine jet leaving the ureter.2
Administer IV methylene blue. Methylene blue is FDA approved for methemoglobinemia treatment. For this indication, it is administered intravenously at a dose of 1 to 2 mg/kgover 5 to 10 minutes. Paradoxically, when administered at a dose of >7 mg/kg, methylene blue can cause methemoglobinemia.3
Methylene blue often is provided as a 1% solution of 10 mg/mL in 10-mL vials. To use intravenous (IV) methylene blue to test ureteral function at cystoscopy, administer IV methylene blue 50 mg over 5 minutes. Use the cystoscope to view the colored urine exiting the ureteral orifices.4,5 Administering a small dose of IV furosemide may accelerate the appearance of methylene blue in the urine.
When to use caution. Methylene blue blocks serotonin metabolism by inhibiting monoamine oxidase and may precipitate a serotonin syndrome in patients taking selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), or monoamine oxidase inhibitors (MAOIs).
Common findings in the serotonin syndrome include: temperature above 38° C (100.4° F), anxiety, agitation, delirium, clonus, tremor, and hypertonia.6 I recommend that you DO NOT use IV methylene blue in patients taking these medications.7,8
Great caution should be used before administering IV methylene blue in the presence of the following clinical situations:
- renal impairment
- G6PD deficiency
- pediatric patients.
Methylene blue never should be given by a subcutaneous or intrathecal route. In pregnant women it should never be injected into the amniotic fluid compartment.
Use preoperative oral phenazo-pyridine. If the preoperative plan includes a cystoscopy procedure to test ureteral function, administering oral phenazopyridine in the preoperative holding area will result in colorization of the urine within 30 minutes, and it will persist for approximately 4 to 5 hours. To use this approach, administer one dose of phenazopyridine (Pyridium, Azo-Gesic), 100 mg or 200 mg orally, 30 minutes to 1 hour before the planned surgical start time, in the preoperative holding area.9 During cystoscopy, the urine from the ureteral jet will be colored orange.
When to use caution. Phenazopyridine should not be administered to patients with G6PD deficiency.
Option to test for bladder injury
Methylene blue. If methylene blue is used to test the integrity of the bladder, I recommend diluting 10 mg methylene blue in 1 L normal saline and then instilling the dilute solution through a catheter into the bladder.10
It is unlikely that this technique will be associated with sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs, and MAOIs.
Option to test patency of the fallopian tubes (chromopertubation)
Methylene blue. If methylene blue is used via a cannula in the uterine cavity to test the patency of the fallopian tubes, I recommend diluting 10 mg methylene blue in 150 mL normal saline and using the dilute solution to test tubal patency.
It is unlikely that this process will lead to sufficient methylene blue absorption to cause a serotonin syndrome. Therefore, this technique can be used in patients taking SSRIs, SNRIs and MAOIs. However, if intrauterine injection of the dilute dye solution results in extravasation of the dye into the pelvic veins, a significant amount of dye can enter the circulation.11 There are case reports of anaphylaxis following intrauterine injection of methylene blue to test tubal patency.12,13
Options to diagnose PROM and to use for twin amniocentesis
None. Most experts recommend against the intra-amniotic injection of methylene blue to diagnose PROM or in twin amniocentesis procedures. Methylene blue injected into the intra-amniotic fluid during amniocentesis in multiple gestations has been reported to cause fetal bowel obstruction or atresia.14,15 Fetal death also has been reported.16 Decades ago, indocyanine green, which is FDA approved to determine cardiac output, liver blood flow, and hepatic function, was reported to be useful to mark one sac of a twin gestation during amniocentesis.17 With modern ultrasonography technology, the need to rely on a dye to mark a sac of a twin has decreased significantly.
Our only option is to cope—effectively as possible Over decades, the medical community develops patterns of patient care that are critically dependent on the availability of key pharmaceuticals and devices. When a pharmaceutical or device suddenly becomes unavailable, it can disrupt important patterns of patient care. Imagine the impact on obstetrics practice if oxytocin became unavailable due to manufacturing shortages. Likely, both market forces and government regulation are the root cause of the shortfalls. Preventing the adverse consequences of the sudden loss of key pharmaceuticals and devices is an important priority to ensure optimal care of our patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Indigo carmine injection. American Society of Health-System Pharmacists (ASHP) Web site. http://www.ashp.org/menu/DrugShortages/CurrentShortages/Bulletin.aspx?id=861. Updated July 29, 2014. Accessed August 21, 2014.
2. Lin BL, Iwata Y. Modified cystoscopy to evaluate unilateral traumatic injury of the ureter during pelvic surgery. Am J Obstet Gynecol. 1990;162(5):1343–1344.
3. Lee M, Sharifi R. Methylene blue versus indigo carmine. Urology. 1996;47(5):783–784.
4. Thompson JD. Operative injuries to the ureter: prevention, recognition and management. In Rock JA, Thompson JD, eds. TeLinde’s Operative Gynecology. Philadelphia, PA: Lippincott Williams & Wilkins; 1997:1155.
5. Wang AC. The techniques of trocar insertion and intraoperative urethroscopy in tension-free vaginal taping: an experience of 600 cases. Acta Obstet Gynecol Scand. 2004;83(3):293–298.
6. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120.
7. Shah-Khan MG, Lovely J, Degnim AC. Safety of methylene blue dye for lymphatic mapping in patients taking selective serotonin reuptake inhibitors. Am J Surg. 2012;204(5):798–799.
8. Ng BK, Cameron AJ. The role of methylene blue in serotonin syndrome: a systematic review. Psychosomatics. 2010;51(3):194–200.
9. Hui JY, Harvey MA, Johnston SL. Confirmation of ureteric patency during cystoscopy using phenazopyridine HCl: a low-cost approach. J Obstet Gynaecol Can. 2009;31(9):845–849.
10. Moore CR, Shirodkar SP, Avallone MA, et al. Intravesical methylene blue facilitates precise identification of the diverticular neck during robot-assisted laparoscopic bladder diverticulectomy. J Laparoendosc Adv Surg Tech A. 2012;22(5):492–495.
11. Mhaskar R, Mhaskar AM. Methemoglobinemia following chromopertubation in treated pelvic tuberculosis. Int J Gynaecol Obstet. 2002;77(1):41–42.
12. Rzymski P, Wozniak J, Opala T, Wilczak M, Sajdak S. Anaphylactic reaction to methylene blue dye after laparoscopic chromopertubation. Int J Gynaecol Obstet. 2003;81(1):71–72.
13. Dewachter P, Mouton-Faivre C, Trechot P, Lieu JC, Mertes PM. Severe anaphylactic shock with methylene blue instillation. Anesth Analg. 2005;101(1):149–150.
14. McFadyen I. The dangers of intra-amniotic methylene blue. Br J Obstet Gynaecol. 1992;99(2):89–90.
15. Van der Pol JG, Wolf H, Boer K, et al. Jejunal atresia related to the use of methylene blue in genetic amniocentesis in twins. Br J Obstet Gynaecol. 1992;99(2):141–143.
16. Kidd SA, Lancaster PA, Anderson JC, et al. Fetal death after exposure to methylene blue dye during mid-trimester amniocentesis in twin pregnancy. Prenat Diagn. 1996;16(1):39–47.
17. Hobbins JC, Winsberg F, Blanchett M, et al. Section 5: fetal imaging. Prenat Diagn. 1981;1(5):35–38.
1. Indigo carmine injection. American Society of Health-System Pharmacists (ASHP) Web site. http://www.ashp.org/menu/DrugShortages/CurrentShortages/Bulletin.aspx?id=861. Updated July 29, 2014. Accessed August 21, 2014.
2. Lin BL, Iwata Y. Modified cystoscopy to evaluate unilateral traumatic injury of the ureter during pelvic surgery. Am J Obstet Gynecol. 1990;162(5):1343–1344.
3. Lee M, Sharifi R. Methylene blue versus indigo carmine. Urology. 1996;47(5):783–784.
4. Thompson JD. Operative injuries to the ureter: prevention, recognition and management. In Rock JA, Thompson JD, eds. TeLinde’s Operative Gynecology. Philadelphia, PA: Lippincott Williams & Wilkins; 1997:1155.
5. Wang AC. The techniques of trocar insertion and intraoperative urethroscopy in tension-free vaginal taping: an experience of 600 cases. Acta Obstet Gynecol Scand. 2004;83(3):293–298.
6. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120.
7. Shah-Khan MG, Lovely J, Degnim AC. Safety of methylene blue dye for lymphatic mapping in patients taking selective serotonin reuptake inhibitors. Am J Surg. 2012;204(5):798–799.
8. Ng BK, Cameron AJ. The role of methylene blue in serotonin syndrome: a systematic review. Psychosomatics. 2010;51(3):194–200.
9. Hui JY, Harvey MA, Johnston SL. Confirmation of ureteric patency during cystoscopy using phenazopyridine HCl: a low-cost approach. J Obstet Gynaecol Can. 2009;31(9):845–849.
10. Moore CR, Shirodkar SP, Avallone MA, et al. Intravesical methylene blue facilitates precise identification of the diverticular neck during robot-assisted laparoscopic bladder diverticulectomy. J Laparoendosc Adv Surg Tech A. 2012;22(5):492–495.
11. Mhaskar R, Mhaskar AM. Methemoglobinemia following chromopertubation in treated pelvic tuberculosis. Int J Gynaecol Obstet. 2002;77(1):41–42.
12. Rzymski P, Wozniak J, Opala T, Wilczak M, Sajdak S. Anaphylactic reaction to methylene blue dye after laparoscopic chromopertubation. Int J Gynaecol Obstet. 2003;81(1):71–72.
13. Dewachter P, Mouton-Faivre C, Trechot P, Lieu JC, Mertes PM. Severe anaphylactic shock with methylene blue instillation. Anesth Analg. 2005;101(1):149–150.
14. McFadyen I. The dangers of intra-amniotic methylene blue. Br J Obstet Gynaecol. 1992;99(2):89–90.
15. Van der Pol JG, Wolf H, Boer K, et al. Jejunal atresia related to the use of methylene blue in genetic amniocentesis in twins. Br J Obstet Gynaecol. 1992;99(2):141–143.
16. Kidd SA, Lancaster PA, Anderson JC, et al. Fetal death after exposure to methylene blue dye during mid-trimester amniocentesis in twin pregnancy. Prenat Diagn. 1996;16(1):39–47.
17. Hobbins JC, Winsberg F, Blanchett M, et al. Section 5: fetal imaging. Prenat Diagn. 1981;1(5):35–38.
Transfer to the hospital for women planning a home birth: A high-risk obstetrics problem
CASE: Febrile laboring mother transferred to hospital
You are in the hospital managing the induction of labor for one of your nulliparous patients who is postterm. You are hoping for a quiet and uneventful shift.
At midnight the nursing administrator pages you and asks if you would please provide care to a pregnant woman attempting a home birth who is in labor and is being transferred to your hospital.
The woman is a 41-year-old G2P1 with one prior cesarean delivery who has attempted a trial of labor at home. According to the nursing administrator the patient has a temperature of 100.4°F and the most recent cervical examination shows her to be fully dilated at +3/5 station in an occiput posterior position. She has been fully dilated for 5 hours. The fetal heart rate, assessed by Doppler monitor, is reported to be reassuring.
What is your clinical plan?
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics recommend that pregnant women should deliver at certified birth centers or hospital-based obstetric units to optimize clinical outcomes for newborns and mothers.1,2 Both organizations also recognize a woman’s right to exercise her autonomy and choose a planned home birth.
In 2012, approximately 0.8% of pregnant women in the United States planned a home birth (31,500 home births and 3,999,386 total births).3 In 2009, three states had home birth rates above 1.9%, including Montana (2.6%), Oregon (1.96%), and Vermont (1.91%). Five states had home birth rates above 1.5%, including Idaho, Pennsylvania, Utah, Washington, and Wisconsin.4 Because planned home births may require transport to the hospital to complete the birth, all obstetric units should develop written plans for dealing with these high-risk patients.
Hospital transfer is common for women attempting a home birth
Many home birth experts regard the Netherlands as the country with the best organized and most successful home birth system that is fully integrated with hospital-based obstetric care. Approximately 23% of births in Holland occur at home supervised by a midwife. A key feature of the highly regulated Dutch system is that all pregnant women with a high-risk condition are required to give birth in a hospital and cannot have a home delivery. Consequently, only women with a low-risk pregnancy are permitted to attempt a home birth.
By contrast, in the United States, with a less well-regulated home birth system, women with high-risk conditions, such as one or more prior cesarean deliveries, may try to birth at home. In a Dutch study of 168,618 low-risk women attempting a home birth, 32% (N=53,809) were transferred to the hospital. Most of the transfers occurred during labor.5
In England about 2.8% of births occur at home. In a study of 16,840 planned home births in England, 21% (N=3,530) of the women were transferred to the hospital.6 Of the 3,530 transfers to the hospital, 70% were transferred before delivery and 30% after birth. In this study among 4,568 nulliparous women attempting home birth, 45% were transferred to the hospital. Among 12,272 multiparous women attempting home birth, 12% were transferred to the hospital.
Among the nulliparous women, but not among the multiparous women, there was a significantly increased risk of adverse newborn outcomes. Adverse newborn outcome was a composite measure that included perinatal death, stillbirth after the onset of labor, neonatal encephalopathy, meconium aspiration syndrome, brachial plexus injury, or fractured humerus or clavicle. The risk of an adverse newborn outcome among nulliparous women was 0.9% for those delivering at home and 0.5% for those delivering at the hospital.6
Your patient asks you, “Are home births safe for the baby?”
No large-scale randomized trials have compared home birth versus hospital birth.1 Consequently, the best evidence evaluating the risks and benefits of home birth is based on observational studies of large cohorts. Recent studies from the United States have reported that neonatal complications, including the risk of a low Apgar score and neonatal seizures, is significantly increased with planned home birth compared with birth at a hospital.2,3 In one study, the risk of a 5-minute Apgar score of zero was 1 in 615 home births and 1 in 6,493 hospital births.3 Studies from the Netherlands also have reported that planned home birth is associated with increased perinatal mortality and morbidity.4,5
Because planned home birth is associated with an increased risk of neonatal morbidity and mortality, some experts conclude that obstetricians have an ethical obligation to recommend against home birth and to respond to refusal of that recommendation with respectful persuasion.6
References
1. Olsen O, Clausen JA. Planned hospital birth versus planned home birth. Cochrane Database Syst Rev. 2012; (9):CD000352.
2. Grunebaum A, McCullough LB, Sapra KJ, et al. Apgar score of 0 at 5 minutes and neonatal seizures or serious neurologic dysfunction in relation to birth setting. Am J Obstet Gynecol. 2013;209(4):323.e1–e6.
3. Cheng YW, Snowden JM, King TL, Caughey AB. Selected perinatal outcomes associated with planned home births in the United States. Am J Obstet Gynecol. 2013;209(4).325.e1–e8.
4. Evers AC, Brouwers HA, Hukkelhoven CW, et al. Perinatal mortality and severe morbidity in low and high risk term pregnancies in the Netherlands: Prospective cohort study. BMJ. 2010;341:c5639.
5. Arabin B, Visser GHA. Comparison of obstetric care in Germany and in the Netherlands. J Health Med Informat. 2013;S11:014.
6. Chervenak FA, McCullough LB, Arabin B. Obstetric ethics: An essential dimension of planned home birth. Obstet Gynecol. 2011;117(5):1183–1187.
Interprofessional team care
For the woman planning a home birth, transfer from home to the hospital is a jarring experience. The woman may feel that she has not achieved a highly desired and important life goal. In a survey of women birthing in the Netherlands, transfer from home to the hospital was associated with a high rate of patient dissatisfaction with their birthing experience. Compared with women who were satisfied with their birth experience, women who were dissatisfied more often reported that the care providers at the hospital were rushed, insensitive, rude, inconsiderate, condescending, and unhelpful.7
Creating a positive birthing experience
Given that transfer to the hospital is associated with an increased rate of being dissatisfied with the birth experience, and that dissatisfied women may perceive their care providers negatively, it is important for the interprofessional hospital team to devote adequate time to listen the patient’s concerns, demonstrate a high degree of sensitivity, and be especially polite and helpful. It is probably best to avoid referring to the transfer as a “failed home birth.” Trust may be enhanced by asking open-ended questions about the patient’s expectations and expressing empathy for her situation. The hospital professional team might prioritize acknowledging the right of the woman to make informed choices and provide an overview of the standard procedures used at the hospital. The clinicians should explicitly state that the health of the mother and newborn are their top priority. The hospital team should also express confidence in the benefit of the standard practices they use to ensure a safe birth experience.
Successful negotiation: An art best achieved as a small group
When a laboring woman is transferred from home to the hospital, a negotiation begins with the hospital professionals about the best clinical path to a successful birth. The patient often arrives with a support team that includes her partner, a support person, and a midwife or trained birth attendant. These individuals often demonstrate strong group cohesion and may be skeptical of the benefits of hospital birthing practices including intravenous access, oxytocin administration, epidural anesthesia, and operative delivery. The goal for the patient and her support team and the hospital professionals is to achieve a safe birth for the baby and mother. Because the goal is aligned among all parties, the negotiation is focused on the clinical path that will best achieve the goal with minimal risks.
To enhance the likelihood of a successful negotiation, it is best if the team of hospital professionals, including an obstetrician, a senior nurse, and an obstetric anesthesiologist, jointly discuss hospital birthing practices with the patient and her support team. An obstetrician, negotiating independently, is in the difficult position of one professional trying to redirect the choices of a cohesive team of four individuals. Most experienced negotiators would not voluntarily enter a situation in which acting alone they needed to simultaneously negotiate with four people. A joint discussion between the interprofessional team and the patient reduces the opportunity for the patient and her team to generate disagreements among the hospital professionals.
An important issue is that the home midwife or trained birth attendant is not permitted to participate in the practice of medicine at the hospital. Only credentialed and licensed nurses, obstetricians, anesthesiologists, and pediatricians are permitted to participate in the practice of medicine at the hospital. It may be prudent to provide the home midwife a written statement from the hospital indicating that home midwives are not permitted to practice medicine at the institution.
Related article: Lay midwives and the ObGyn: Is collaboration risky? Lucia DiVenere, MA (Practice Management; May 2012)
Occasionally, negotiations between the hospital professional team and the patient and her support team are unsuccessful and the patient refuses the best advice of the hospital team. In these situations there should be a written plan of how the patient–clinician conflict will be communicated to other members of the hospital staff and hospital leadership. For example, another senior clinician may be asked to join in the planning process.
A high-risk patient population
In some cases of planned home birth, the patient and midwife have made management decisions that are inconsistent with standard obstetric protocols. Commonly encountered situations include 1) conservative home management of spontaneous rupture of the membranes at term, 2) prolonged conservative management of the arrest of the active phase of the first stage of labor, 3) prolonged second stage of labor, up to 24 hours in length, and 4) attempted home birth after multiple previous cesarean deliveries. I am also aware of multiple reports of attempted home birth of a fetus in the breech presentation.
On arrival to the hospital these patients and their newborns are at exceptionally high risk for adverse birth outcomes. If an adverse outcome were to occur, it would be unjust to assign sole or primary responsibility to the obstetrician for the adverse outcome. Hence, the hospital should have a written plan for helping to minimize the risk that the obstetrician, playing the role of Good Samaritan, will bear primary responsibility for an adverse outcome.
Related article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
CASE: Resolved
In the case presented above, the obstetrician, nurse, and obstetric anesthesiologist successfully negotiated with the patient. Intravenous access and an epidural anesthetic were established. Antibiotics were administered. Using ultrasound, the obstetrician confirmed that the fetus was in the occiput posterior position. The mother was exhausted from many hours of pushing and agreed to an operative delivery. Forceps were used to deliver a healthy baby and a perineal laceration was repaired.
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
2. American Academy of Pediatrics. Policy statement: Planned home birth Pediatrics. 2013;131(5):1016–1002.
3. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Matthews TH. Births: Final data for 2010. Natl Vital Stat Rep. 2012;61(1):1–72.
4. MacDorman MF, Mathews TJ, Declercq E. Home births in the United States, 1990-2009. NCHS Data Brief. 2012;(84):1–8.
5. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: A descriptive study. BJOG. 2008;115(5):570–578.
6. Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for health women with low risk pregnancies: The Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400.
7. Rijnders M, Baston H, Schonbeck Y, et al. Perinatal factors related to negative or positive recall of birth experience in women 3 years postpartum in the Netherlands. Birth. 2008;35:107–116.
CASE: Febrile laboring mother transferred to hospital
You are in the hospital managing the induction of labor for one of your nulliparous patients who is postterm. You are hoping for a quiet and uneventful shift.
At midnight the nursing administrator pages you and asks if you would please provide care to a pregnant woman attempting a home birth who is in labor and is being transferred to your hospital.
The woman is a 41-year-old G2P1 with one prior cesarean delivery who has attempted a trial of labor at home. According to the nursing administrator the patient has a temperature of 100.4°F and the most recent cervical examination shows her to be fully dilated at +3/5 station in an occiput posterior position. She has been fully dilated for 5 hours. The fetal heart rate, assessed by Doppler monitor, is reported to be reassuring.
What is your clinical plan?
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics recommend that pregnant women should deliver at certified birth centers or hospital-based obstetric units to optimize clinical outcomes for newborns and mothers.1,2 Both organizations also recognize a woman’s right to exercise her autonomy and choose a planned home birth.
In 2012, approximately 0.8% of pregnant women in the United States planned a home birth (31,500 home births and 3,999,386 total births).3 In 2009, three states had home birth rates above 1.9%, including Montana (2.6%), Oregon (1.96%), and Vermont (1.91%). Five states had home birth rates above 1.5%, including Idaho, Pennsylvania, Utah, Washington, and Wisconsin.4 Because planned home births may require transport to the hospital to complete the birth, all obstetric units should develop written plans for dealing with these high-risk patients.
Hospital transfer is common for women attempting a home birth
Many home birth experts regard the Netherlands as the country with the best organized and most successful home birth system that is fully integrated with hospital-based obstetric care. Approximately 23% of births in Holland occur at home supervised by a midwife. A key feature of the highly regulated Dutch system is that all pregnant women with a high-risk condition are required to give birth in a hospital and cannot have a home delivery. Consequently, only women with a low-risk pregnancy are permitted to attempt a home birth.
By contrast, in the United States, with a less well-regulated home birth system, women with high-risk conditions, such as one or more prior cesarean deliveries, may try to birth at home. In a Dutch study of 168,618 low-risk women attempting a home birth, 32% (N=53,809) were transferred to the hospital. Most of the transfers occurred during labor.5
In England about 2.8% of births occur at home. In a study of 16,840 planned home births in England, 21% (N=3,530) of the women were transferred to the hospital.6 Of the 3,530 transfers to the hospital, 70% were transferred before delivery and 30% after birth. In this study among 4,568 nulliparous women attempting home birth, 45% were transferred to the hospital. Among 12,272 multiparous women attempting home birth, 12% were transferred to the hospital.
Among the nulliparous women, but not among the multiparous women, there was a significantly increased risk of adverse newborn outcomes. Adverse newborn outcome was a composite measure that included perinatal death, stillbirth after the onset of labor, neonatal encephalopathy, meconium aspiration syndrome, brachial plexus injury, or fractured humerus or clavicle. The risk of an adverse newborn outcome among nulliparous women was 0.9% for those delivering at home and 0.5% for those delivering at the hospital.6
Your patient asks you, “Are home births safe for the baby?”
No large-scale randomized trials have compared home birth versus hospital birth.1 Consequently, the best evidence evaluating the risks and benefits of home birth is based on observational studies of large cohorts. Recent studies from the United States have reported that neonatal complications, including the risk of a low Apgar score and neonatal seizures, is significantly increased with planned home birth compared with birth at a hospital.2,3 In one study, the risk of a 5-minute Apgar score of zero was 1 in 615 home births and 1 in 6,493 hospital births.3 Studies from the Netherlands also have reported that planned home birth is associated with increased perinatal mortality and morbidity.4,5
Because planned home birth is associated with an increased risk of neonatal morbidity and mortality, some experts conclude that obstetricians have an ethical obligation to recommend against home birth and to respond to refusal of that recommendation with respectful persuasion.6
References
1. Olsen O, Clausen JA. Planned hospital birth versus planned home birth. Cochrane Database Syst Rev. 2012; (9):CD000352.
2. Grunebaum A, McCullough LB, Sapra KJ, et al. Apgar score of 0 at 5 minutes and neonatal seizures or serious neurologic dysfunction in relation to birth setting. Am J Obstet Gynecol. 2013;209(4):323.e1–e6.
3. Cheng YW, Snowden JM, King TL, Caughey AB. Selected perinatal outcomes associated with planned home births in the United States. Am J Obstet Gynecol. 2013;209(4).325.e1–e8.
4. Evers AC, Brouwers HA, Hukkelhoven CW, et al. Perinatal mortality and severe morbidity in low and high risk term pregnancies in the Netherlands: Prospective cohort study. BMJ. 2010;341:c5639.
5. Arabin B, Visser GHA. Comparison of obstetric care in Germany and in the Netherlands. J Health Med Informat. 2013;S11:014.
6. Chervenak FA, McCullough LB, Arabin B. Obstetric ethics: An essential dimension of planned home birth. Obstet Gynecol. 2011;117(5):1183–1187.
Interprofessional team care
For the woman planning a home birth, transfer from home to the hospital is a jarring experience. The woman may feel that she has not achieved a highly desired and important life goal. In a survey of women birthing in the Netherlands, transfer from home to the hospital was associated with a high rate of patient dissatisfaction with their birthing experience. Compared with women who were satisfied with their birth experience, women who were dissatisfied more often reported that the care providers at the hospital were rushed, insensitive, rude, inconsiderate, condescending, and unhelpful.7
Creating a positive birthing experience
Given that transfer to the hospital is associated with an increased rate of being dissatisfied with the birth experience, and that dissatisfied women may perceive their care providers negatively, it is important for the interprofessional hospital team to devote adequate time to listen the patient’s concerns, demonstrate a high degree of sensitivity, and be especially polite and helpful. It is probably best to avoid referring to the transfer as a “failed home birth.” Trust may be enhanced by asking open-ended questions about the patient’s expectations and expressing empathy for her situation. The hospital professional team might prioritize acknowledging the right of the woman to make informed choices and provide an overview of the standard procedures used at the hospital. The clinicians should explicitly state that the health of the mother and newborn are their top priority. The hospital team should also express confidence in the benefit of the standard practices they use to ensure a safe birth experience.
Successful negotiation: An art best achieved as a small group
When a laboring woman is transferred from home to the hospital, a negotiation begins with the hospital professionals about the best clinical path to a successful birth. The patient often arrives with a support team that includes her partner, a support person, and a midwife or trained birth attendant. These individuals often demonstrate strong group cohesion and may be skeptical of the benefits of hospital birthing practices including intravenous access, oxytocin administration, epidural anesthesia, and operative delivery. The goal for the patient and her support team and the hospital professionals is to achieve a safe birth for the baby and mother. Because the goal is aligned among all parties, the negotiation is focused on the clinical path that will best achieve the goal with minimal risks.
To enhance the likelihood of a successful negotiation, it is best if the team of hospital professionals, including an obstetrician, a senior nurse, and an obstetric anesthesiologist, jointly discuss hospital birthing practices with the patient and her support team. An obstetrician, negotiating independently, is in the difficult position of one professional trying to redirect the choices of a cohesive team of four individuals. Most experienced negotiators would not voluntarily enter a situation in which acting alone they needed to simultaneously negotiate with four people. A joint discussion between the interprofessional team and the patient reduces the opportunity for the patient and her team to generate disagreements among the hospital professionals.
An important issue is that the home midwife or trained birth attendant is not permitted to participate in the practice of medicine at the hospital. Only credentialed and licensed nurses, obstetricians, anesthesiologists, and pediatricians are permitted to participate in the practice of medicine at the hospital. It may be prudent to provide the home midwife a written statement from the hospital indicating that home midwives are not permitted to practice medicine at the institution.
Related article: Lay midwives and the ObGyn: Is collaboration risky? Lucia DiVenere, MA (Practice Management; May 2012)
Occasionally, negotiations between the hospital professional team and the patient and her support team are unsuccessful and the patient refuses the best advice of the hospital team. In these situations there should be a written plan of how the patient–clinician conflict will be communicated to other members of the hospital staff and hospital leadership. For example, another senior clinician may be asked to join in the planning process.
A high-risk patient population
In some cases of planned home birth, the patient and midwife have made management decisions that are inconsistent with standard obstetric protocols. Commonly encountered situations include 1) conservative home management of spontaneous rupture of the membranes at term, 2) prolonged conservative management of the arrest of the active phase of the first stage of labor, 3) prolonged second stage of labor, up to 24 hours in length, and 4) attempted home birth after multiple previous cesarean deliveries. I am also aware of multiple reports of attempted home birth of a fetus in the breech presentation.
On arrival to the hospital these patients and their newborns are at exceptionally high risk for adverse birth outcomes. If an adverse outcome were to occur, it would be unjust to assign sole or primary responsibility to the obstetrician for the adverse outcome. Hence, the hospital should have a written plan for helping to minimize the risk that the obstetrician, playing the role of Good Samaritan, will bear primary responsibility for an adverse outcome.
Related article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
CASE: Resolved
In the case presented above, the obstetrician, nurse, and obstetric anesthesiologist successfully negotiated with the patient. Intravenous access and an epidural anesthetic were established. Antibiotics were administered. Using ultrasound, the obstetrician confirmed that the fetus was in the occiput posterior position. The mother was exhausted from many hours of pushing and agreed to an operative delivery. Forceps were used to deliver a healthy baby and a perineal laceration was repaired.
CASE: Febrile laboring mother transferred to hospital
You are in the hospital managing the induction of labor for one of your nulliparous patients who is postterm. You are hoping for a quiet and uneventful shift.
At midnight the nursing administrator pages you and asks if you would please provide care to a pregnant woman attempting a home birth who is in labor and is being transferred to your hospital.
The woman is a 41-year-old G2P1 with one prior cesarean delivery who has attempted a trial of labor at home. According to the nursing administrator the patient has a temperature of 100.4°F and the most recent cervical examination shows her to be fully dilated at +3/5 station in an occiput posterior position. She has been fully dilated for 5 hours. The fetal heart rate, assessed by Doppler monitor, is reported to be reassuring.
What is your clinical plan?
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics recommend that pregnant women should deliver at certified birth centers or hospital-based obstetric units to optimize clinical outcomes for newborns and mothers.1,2 Both organizations also recognize a woman’s right to exercise her autonomy and choose a planned home birth.
In 2012, approximately 0.8% of pregnant women in the United States planned a home birth (31,500 home births and 3,999,386 total births).3 In 2009, three states had home birth rates above 1.9%, including Montana (2.6%), Oregon (1.96%), and Vermont (1.91%). Five states had home birth rates above 1.5%, including Idaho, Pennsylvania, Utah, Washington, and Wisconsin.4 Because planned home births may require transport to the hospital to complete the birth, all obstetric units should develop written plans for dealing with these high-risk patients.
Hospital transfer is common for women attempting a home birth
Many home birth experts regard the Netherlands as the country with the best organized and most successful home birth system that is fully integrated with hospital-based obstetric care. Approximately 23% of births in Holland occur at home supervised by a midwife. A key feature of the highly regulated Dutch system is that all pregnant women with a high-risk condition are required to give birth in a hospital and cannot have a home delivery. Consequently, only women with a low-risk pregnancy are permitted to attempt a home birth.
By contrast, in the United States, with a less well-regulated home birth system, women with high-risk conditions, such as one or more prior cesarean deliveries, may try to birth at home. In a Dutch study of 168,618 low-risk women attempting a home birth, 32% (N=53,809) were transferred to the hospital. Most of the transfers occurred during labor.5
In England about 2.8% of births occur at home. In a study of 16,840 planned home births in England, 21% (N=3,530) of the women were transferred to the hospital.6 Of the 3,530 transfers to the hospital, 70% were transferred before delivery and 30% after birth. In this study among 4,568 nulliparous women attempting home birth, 45% were transferred to the hospital. Among 12,272 multiparous women attempting home birth, 12% were transferred to the hospital.
Among the nulliparous women, but not among the multiparous women, there was a significantly increased risk of adverse newborn outcomes. Adverse newborn outcome was a composite measure that included perinatal death, stillbirth after the onset of labor, neonatal encephalopathy, meconium aspiration syndrome, brachial plexus injury, or fractured humerus or clavicle. The risk of an adverse newborn outcome among nulliparous women was 0.9% for those delivering at home and 0.5% for those delivering at the hospital.6
Your patient asks you, “Are home births safe for the baby?”
No large-scale randomized trials have compared home birth versus hospital birth.1 Consequently, the best evidence evaluating the risks and benefits of home birth is based on observational studies of large cohorts. Recent studies from the United States have reported that neonatal complications, including the risk of a low Apgar score and neonatal seizures, is significantly increased with planned home birth compared with birth at a hospital.2,3 In one study, the risk of a 5-minute Apgar score of zero was 1 in 615 home births and 1 in 6,493 hospital births.3 Studies from the Netherlands also have reported that planned home birth is associated with increased perinatal mortality and morbidity.4,5
Because planned home birth is associated with an increased risk of neonatal morbidity and mortality, some experts conclude that obstetricians have an ethical obligation to recommend against home birth and to respond to refusal of that recommendation with respectful persuasion.6
References
1. Olsen O, Clausen JA. Planned hospital birth versus planned home birth. Cochrane Database Syst Rev. 2012; (9):CD000352.
2. Grunebaum A, McCullough LB, Sapra KJ, et al. Apgar score of 0 at 5 minutes and neonatal seizures or serious neurologic dysfunction in relation to birth setting. Am J Obstet Gynecol. 2013;209(4):323.e1–e6.
3. Cheng YW, Snowden JM, King TL, Caughey AB. Selected perinatal outcomes associated with planned home births in the United States. Am J Obstet Gynecol. 2013;209(4).325.e1–e8.
4. Evers AC, Brouwers HA, Hukkelhoven CW, et al. Perinatal mortality and severe morbidity in low and high risk term pregnancies in the Netherlands: Prospective cohort study. BMJ. 2010;341:c5639.
5. Arabin B, Visser GHA. Comparison of obstetric care in Germany and in the Netherlands. J Health Med Informat. 2013;S11:014.
6. Chervenak FA, McCullough LB, Arabin B. Obstetric ethics: An essential dimension of planned home birth. Obstet Gynecol. 2011;117(5):1183–1187.
Interprofessional team care
For the woman planning a home birth, transfer from home to the hospital is a jarring experience. The woman may feel that she has not achieved a highly desired and important life goal. In a survey of women birthing in the Netherlands, transfer from home to the hospital was associated with a high rate of patient dissatisfaction with their birthing experience. Compared with women who were satisfied with their birth experience, women who were dissatisfied more often reported that the care providers at the hospital were rushed, insensitive, rude, inconsiderate, condescending, and unhelpful.7
Creating a positive birthing experience
Given that transfer to the hospital is associated with an increased rate of being dissatisfied with the birth experience, and that dissatisfied women may perceive their care providers negatively, it is important for the interprofessional hospital team to devote adequate time to listen the patient’s concerns, demonstrate a high degree of sensitivity, and be especially polite and helpful. It is probably best to avoid referring to the transfer as a “failed home birth.” Trust may be enhanced by asking open-ended questions about the patient’s expectations and expressing empathy for her situation. The hospital professional team might prioritize acknowledging the right of the woman to make informed choices and provide an overview of the standard procedures used at the hospital. The clinicians should explicitly state that the health of the mother and newborn are their top priority. The hospital team should also express confidence in the benefit of the standard practices they use to ensure a safe birth experience.
Successful negotiation: An art best achieved as a small group
When a laboring woman is transferred from home to the hospital, a negotiation begins with the hospital professionals about the best clinical path to a successful birth. The patient often arrives with a support team that includes her partner, a support person, and a midwife or trained birth attendant. These individuals often demonstrate strong group cohesion and may be skeptical of the benefits of hospital birthing practices including intravenous access, oxytocin administration, epidural anesthesia, and operative delivery. The goal for the patient and her support team and the hospital professionals is to achieve a safe birth for the baby and mother. Because the goal is aligned among all parties, the negotiation is focused on the clinical path that will best achieve the goal with minimal risks.
To enhance the likelihood of a successful negotiation, it is best if the team of hospital professionals, including an obstetrician, a senior nurse, and an obstetric anesthesiologist, jointly discuss hospital birthing practices with the patient and her support team. An obstetrician, negotiating independently, is in the difficult position of one professional trying to redirect the choices of a cohesive team of four individuals. Most experienced negotiators would not voluntarily enter a situation in which acting alone they needed to simultaneously negotiate with four people. A joint discussion between the interprofessional team and the patient reduces the opportunity for the patient and her team to generate disagreements among the hospital professionals.
An important issue is that the home midwife or trained birth attendant is not permitted to participate in the practice of medicine at the hospital. Only credentialed and licensed nurses, obstetricians, anesthesiologists, and pediatricians are permitted to participate in the practice of medicine at the hospital. It may be prudent to provide the home midwife a written statement from the hospital indicating that home midwives are not permitted to practice medicine at the institution.
Related article: Lay midwives and the ObGyn: Is collaboration risky? Lucia DiVenere, MA (Practice Management; May 2012)
Occasionally, negotiations between the hospital professional team and the patient and her support team are unsuccessful and the patient refuses the best advice of the hospital team. In these situations there should be a written plan of how the patient–clinician conflict will be communicated to other members of the hospital staff and hospital leadership. For example, another senior clinician may be asked to join in the planning process.
A high-risk patient population
In some cases of planned home birth, the patient and midwife have made management decisions that are inconsistent with standard obstetric protocols. Commonly encountered situations include 1) conservative home management of spontaneous rupture of the membranes at term, 2) prolonged conservative management of the arrest of the active phase of the first stage of labor, 3) prolonged second stage of labor, up to 24 hours in length, and 4) attempted home birth after multiple previous cesarean deliveries. I am also aware of multiple reports of attempted home birth of a fetus in the breech presentation.
On arrival to the hospital these patients and their newborns are at exceptionally high risk for adverse birth outcomes. If an adverse outcome were to occur, it would be unjust to assign sole or primary responsibility to the obstetrician for the adverse outcome. Hence, the hospital should have a written plan for helping to minimize the risk that the obstetrician, playing the role of Good Samaritan, will bear primary responsibility for an adverse outcome.
Related article: Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
CASE: Resolved
In the case presented above, the obstetrician, nurse, and obstetric anesthesiologist successfully negotiated with the patient. Intravenous access and an epidural anesthetic were established. Antibiotics were administered. Using ultrasound, the obstetrician confirmed that the fetus was in the occiput posterior position. The mother was exhausted from many hours of pushing and agreed to an operative delivery. Forceps were used to deliver a healthy baby and a perineal laceration was repaired.
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
2. American Academy of Pediatrics. Policy statement: Planned home birth Pediatrics. 2013;131(5):1016–1002.
3. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Matthews TH. Births: Final data for 2010. Natl Vital Stat Rep. 2012;61(1):1–72.
4. MacDorman MF, Mathews TJ, Declercq E. Home births in the United States, 1990-2009. NCHS Data Brief. 2012;(84):1–8.
5. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: A descriptive study. BJOG. 2008;115(5):570–578.
6. Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for health women with low risk pregnancies: The Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400.
7. Rijnders M, Baston H, Schonbeck Y, et al. Perinatal factors related to negative or positive recall of birth experience in women 3 years postpartum in the Netherlands. Birth. 2008;35:107–116.
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
2. American Academy of Pediatrics. Policy statement: Planned home birth Pediatrics. 2013;131(5):1016–1002.
3. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Matthews TH. Births: Final data for 2010. Natl Vital Stat Rep. 2012;61(1):1–72.
4. MacDorman MF, Mathews TJ, Declercq E. Home births in the United States, 1990-2009. NCHS Data Brief. 2012;(84):1–8.
5. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: A descriptive study. BJOG. 2008;115(5):570–578.
6. Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for health women with low risk pregnancies: The Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400.
7. Rijnders M, Baston H, Schonbeck Y, et al. Perinatal factors related to negative or positive recall of birth experience in women 3 years postpartum in the Netherlands. Birth. 2008;35:107–116.