User login
Long-acting reversible contraceptives and acne in adolescents
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
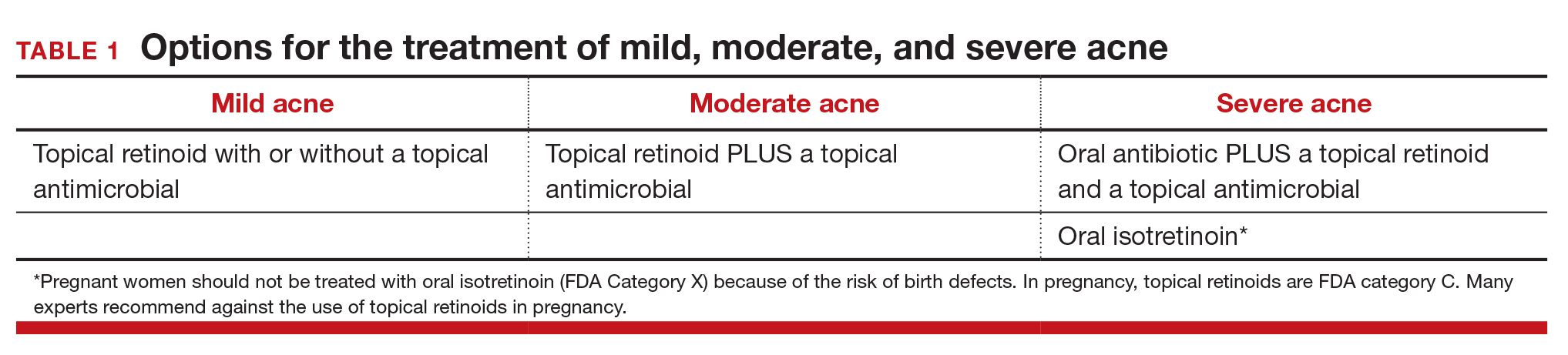
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.
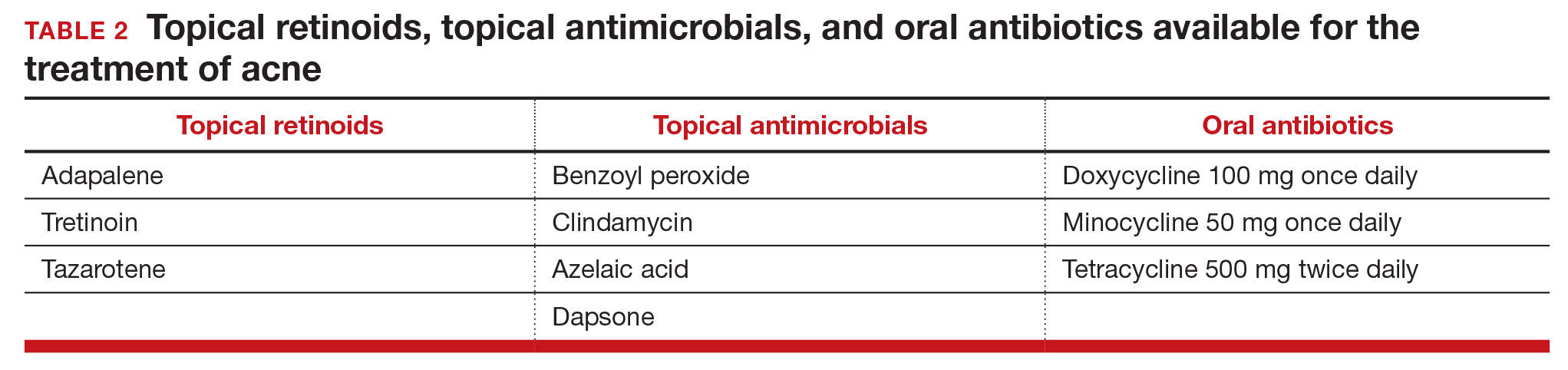
Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.


Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Examining the impact of contraception on acne in adolescents is clinically important because acne affects about 85% of adolescents, and contraceptives may influence the course of acne disease. Estrogen-progestin contraceptives cause a significant improvement in acne.1,2 By contrast, the levonorgestrel-releasing intrauterine device and the etonogestrel contraceptive implant may exacerbate acne. In this editorial we review the hormonal contraception−acne relationship, available acne treatments, and appropriate management.
Related article:
Your teenage patient and contraception: Think “long-acting” first
Combination oral contraception and acne
As noted, combination oral contraceptives generally result in acne improvement.1,2 Estrogen-progestin contraceptives improve the condition through two mechanisms. Primarily, estrogen-progestin contraceptives suppress pituitary luteinizing hormone secretion, thereby decreasing ovarian testosterone production. These contraceptives also increase liver production of sex hormone-binding globulin (SHBG), thereby increasing bound testosterone and decreasing free testosterone. The decrease in ovarian testosterone production and the increase in SHBG-bound testosterone reduce sebum production, resulting in acne improvement.
The US Food and Drug Administration has approved 4 estrogen-progestin contraceptives for acne treatment:
- Estrostep (norethindrone acetate-ethinyl estradiol plus ferrous fumarate)
- Ortho Tri-Cyclen (norgestimate-ethinyl estradiol)
- Yaz (drospirenone-ethinyl estradiol)
- BeYaz (drospirenone-ethinyl estradiol plus levomefolate).
LARC and acne
The levonorgestrel intrauterine devices (LNG-IUDs), including the levonorgestrel intrauterine systems Mirena, Liletta, Skyla, and Kyleena, and the etonogestrel implant (Nexplanon) are among the most effective contraceptives available for women. Over the last decade there has been a marked increase in the use of LARC. In 2002, 1.3% of women aged 15 to 24 years used an IUD or progestin implant, and this percentage increased to 10% by 2013.3
Progestin-containing LARC may cause acne to worsen. In a large 3-year prospective study of more than 2,900 women using the progestin implant or the copper IUD (ParaGard), use of the progestin implant was associated with a higher rate of reported acne than the copper IUD (18% vs 13%, respectively; relative risk, 1.4; 95% confidence interval, 1.20−1.56; P<.0001).4 In a retrospective review of 991 women who used the etonogestrel implant, 24% of the women requested that the implant be removed; the 3 most common reasons for removal were: bleeding disturbances (45%), worsening acne, (12%) and desire to conceive (12%).5
Similar differences in reported acne are seen between the LNG-IUD and the copper IUD. In a study of 320 women using the LNG-IUD and the copper IUD, an increase in acne was reported by 17% and 7%, respectively (P<.025).6 In a small prospective study of the LNG-IUD versus the copper IUD over the first 12 months of use, use of the LNG-IUD was associated with a statistically significant worsening of acne scores while use of the copper IUD had no impact on acne scores.7
Related article:
Overcoming LARC complications: 7 case challenges
In a study of 2,147 consecutive women using a hormonal contraceptive who presented to a dermatologist for the treatment of acne, patients were asked to assess how the contraceptive affected their acne. By type of contraceptive, the percent of women who reported that the contraceptive made their acne worse was: LNG-IUD, 36%; progestin implant, 33%; depot medroxyprogesterone acetate (MPA), 27%; levonorgestrel-ethinyl estradiol oral contraceptive, 10%; norgestimate-ethinyl estradiol (EE), 6%; etonogestrel-EE vaginal ring, 4%; drospirenone-EE, 3%; and desogestrel-EE, 2%. The percent of women who reported that the contraceptive significantly improved their acne was: drospirenone-EE, 26%; norgestimate-EE, 17%; desogestrel-EE, 15%; etonogestrel-EE vaginal ring, 14%; norethindrone-EE, 8%; levonorgestrel-EE, 6%; depot MPA, 5%; LNG-IUD, 3%; and progestin implant, 1%.8
In adolescents with acne, switching from an estrogen-progestin contraceptive to a LNG-IUD or an etonogestrel implant may cause the patient to report that her acne has worsened. As mentioned, combination estrogen-progestin contraceptives reduce free testosterone, thereby improving acne. When an estrogen-progestin contraceptive is discontinued, free testosterone levels will increase. If a LARC method is initiated and the patient’s acne worsens, the patient may attribute this change to the LARC. For clinicians planning on switching a patient from an estrogen-progestin contraceptive to a LNG-IUD or etonogestrel implant, evaluation of current acne symptoms and acne history may be particularly important.
Acne treatment
Acne is caused by follicular hyperproliferation and abnormal desquamation, excess sebum production, proliferation of Propionibacterium acnes, and inflammation.
First-line agents. An expert guideline developed under the auspices of the American Academy of Dermatology recommends that topical agents including retinoids and antimicrobials be first-line treatments for acne.9,10
Topical retinoids are the primary component of topical acne treatment and can be used as monotherapy or in combination with topical antimicrobials (TABLE 1). Three topical retinoids are approved for use in the United States: tretinoin, adapalene, and tazarotene. Adapalene is available by prescription, 0.1% and 0.3% gel, and over the counter, 0.1% gel (Differin Gel) (TABLE 2). The topical retinoids are applied once daily at bedtime and can cause local skin irritation and dryness. Pregnant women should not be treated with topical retinoids.


Topical antimicrobials for the treatment of acne include: benzoyl peroxide, clindamycin, azelaic acid, and dapsone. Clindamycin is only recommended for use in combination with benzoyl peroxide in order to reduce the development of bacterial resistance to the antibiotic.
Related article:
Does the risk of unplanned pregnancy outweigh the risk of VTE from hormonal contraception?
Approach to mild, moderate, and severe acne. In adolescents with mild acne a topical retinoid or benzoyl peroxide can be used as monotherapy or used together. Referral to a dermatologist is recommended for moderate to severe acne. Moderate acne is treated with combination topical therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). Severe acne is treated with 3 months of oral antibiotics plus topical combination therapy (benzoyl peroxide plus a topical retinoid, a topical antibiotic, or both). In cases of severe nodular acne or acne that produces scarring the patient may require oral isotretinoin treatment.
Acne management for adolescents seeking LARC
Given the data that the LNG-IUD and the etonogestrel implant may worsen acne, it may be wise to preemptively ensure that adolescents with acne who are initiating these contraceptives are also being adequately treated for their acne. Gynecologists should provide anticipatory guidance for adolescents with mild acne who initiate progestin-based LARC. Topical benzoyl peroxide is available over-the-counter and can be recommended to these patients. Follow-up in clinic a few months after initiation also may be helpful to assess side effects.
In moderate and severe cases, coordination with dermatology is recommended. For these patients, gynecologists could consider prescribing a topical retinoid or antibiotic medication in conjunction with a new progestin-based LARC method. Those with severe acne also may benefit from concurrent use of oral contraceptives. In adolescents who do not tolerate progestin-based LARC, the copper IUD is a highly effective alternative and can be paired with estrogen-progestin contraception for acne treatment.
Related article:
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Acne is but one consideration for contraceptive choice
With the above methods, acne can be managed in adolescents seeking a LNG-IUD or implant and should not be considered a contraindication or reason to avoid progestin-based LARC. Adolescents are more likely to continue LARC than estrogen-progestin contraceptives and LARC methods are associated with substantially lower pregnancy rates in this patient population.11 LARC is recommended as first-line contraception for adolescents by both the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.12,13
In choosing contraception with your adolescent patient, the risk of unintended pregnancy should be weighed against the risk of acne and other potential side effects. Do not select a contraceptive based on the presence or absence of acne disease. However, be aware that contraceptives can either improve or worsen acne. Patients with mild and moderate acne disease should be considered for treatment with topical retinoids and/or antimicrobial agents.

Dr. Barbieri reports no financial relationships relevant to this article.
Dr. Roe reports receiving grant or research support from the Society of Family Planning.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;(7):CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71(3):450-459.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15 to 44: United States 2011-2013. Natl Health Stat Report. 2015;(86):1-14.
- Bahamondes L, Brache V, Meirik O, Ali M, Habib N, Landoulsi S; WHO Study Group on Contraceptive Implants for Women. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527-2538.
- Bitzer J, Tschudin S, Adler J; Swiss Implanon Study Group. Acceptability and side-effects of Implanon in Switzerland: a retrospective study by the Implanon Swiss Study Group. Eur J Contracept Reprod Health Care. 2004;9(4):278-284.
- Nilsson CG, Luukkainen T, Diaz J, Allonen H. Clinical performance of a new levonorgestrel-releasing intrauterine device. A randomized comparison with a Nova-T-copper device. Contraception. 1982;25(4):345-356.
- Kelekci S, Kelecki KH, Yilmaz B. Effects of levonorgestrel-releasing intrauterine system and T380A intrauterine copper device on dysmenorrhea and days of bleeding in women with and without adenomyosis. Contraception. 2012;86(5):458-463.
- Lortscher D, Admani S, Satur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
- Roman CJ, Cifu AD, Stein SL. Management of acne vulgaris. JAMA. 2016;316(13):1402-1403.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998-2007.
- American Academy of Pediatrics Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- American College of Obstetricians and Gynecologists Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group. Committee Opinion No. 539. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120(4):983-988.
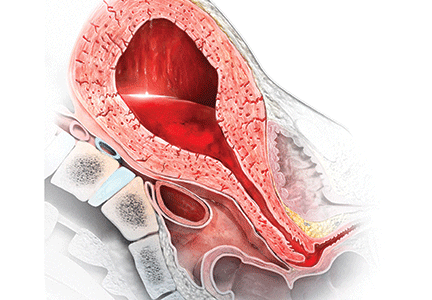
Management of wound complications following obstetric anal sphincter injury (OASIS)
During vaginal delivery spontaneous perineal trauma and extension of episiotomy incisions are common. A severe perineal laceration that extends into or through the anal sphincter complex is referred to as an obstetric anal sphincter injury (OASIS) and requires meticulous repair. Following the repair of an OASIS, serious wound complications, including dehiscence and infection, may occur. In Europe the reported rate of OASIS varies widely among countries, with a rate of 0.1% in Romania, possibly due to underreporting, and 4.9% in Iceland.1 In the United States the rates of 3rd- and 4th-degree lacerations were reported to be 3.3% and 1.1%, respectively.2
Risk factors for OASIS include forceps delivery (odds ratio [OR], 5.50), vacuum-assisted delivery (OR, 3.98), and midline episiotomy (OR, 3.82).3 Additional risk factors for severe perineal injury at vaginal delivery include nulliparity (adjusted odds ratio [aOR], 2.58), delivery from a persistent occiput posterior position (aOR, 2.24), and above-average newborn birth weight (aOR, 1.28).4
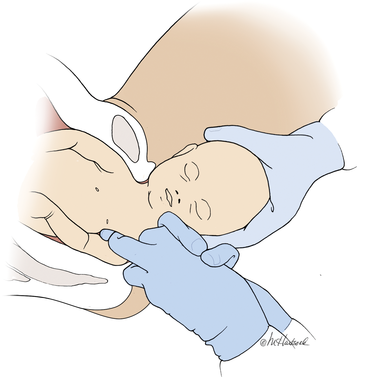
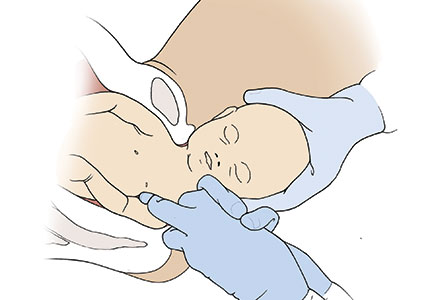
In a meta-analysis of randomized trials, the researchers reported that restrictive use of episiotomy reduced the risk of severe perineal trauma (relative risk [RR], 0.67) but increased the risk of anterior perineal trauma (RR, 1.84).5 The American College of Obstetricians and Gynecologists (ACOG) recommends that episiotomy should not be a routine practice and is best restricted to use in a limited number of cases where fetal and maternal benefit is likely.6 In addition, ACOG recommends that if episiotomy is indicated, a mediolateral incision is favored over a midline incision. In my practice I perform only mediolateral episiotomy incisions. However, mediolateral episiotomy may be associated with an increased risk of postpartum perineal pain and dyspareunia.7 Use of warm compresses applied to the perineum during the second stage of labor may reduce the risk of 3rd- and 4th-degree lacerations.8 Techniques to ensure that the fetal head and shoulders are birthed in a slow and controlled fashion may decrease the risk of OASIS.9 See the TABLE, “Four maneuvers to control and slow the birth of the fetal head.”10–14

Related article:
Stop performing median episiotomy!
Wound complications following the repair of a 3rd- or 4th-degree laceration are reported to occur in approximately 5% to 10% of cases.15 The most common wound complications are dehiscence, infection, abscess formation, pain, sexual dysfunction, and anal incontinence. Minor wound complications, including superficial epithelial separation, can be managed expectantly. However, major wound complications need intensive treatment.
In one study of 21 women who had a major wound complication following the repair of a 4th-degree laceration, 53% had dehiscence plus infection, 33% had dehiscence alone, and 14% had infection alone.16 Major wound complications present at a mean of 5 days after delivery, with a wide range from 1 to 17 days following delivery.17 In a study of 144 cases of wound breakdown following a perineal laceration repair, the major risk factors for wound breakdown were episiotomy (aOR, 11.1), smoking (aOR, 6.4), midwife repair of laceration (aOR, 4.7), use of chromic suture (aOR, 3.9), and operative vaginal delivery (aOR, 3.4).18 In one study of 66 women with a wound complication following the repair of a 3rd- or 4th-degree laceration, clinical risk factors for a wound complication were cigarette smoking (OR, 4.04), 4th-degree laceration (OR, 1.89), and operative vaginal delivery (OR, 1.76).19 The use of intrapartum antibiotics appears to be protective (OR, 0.29) against wound complications following a major perineal laceration.19
Approach to the patient with a dehisced and infected perineal wound
Historically, wound dehiscence following surgical repair of a perineal injury was managed by allowing the wound to slowly close. This approach adversely impacts the quality of life of the affected woman because it may take weeks for the wound to heal. One small randomized trial17 and multiple case series20–24 report that an active multistep management algorithm permits early closure of the majority of these wounds, thereby accelerating the patient’s full recovery. Delayed primary (within 72 hours postpartum) or early secondary reconstruction (within 14 days of delivery) has been demonstrated to be safe with acceptable long-term functional out-comes.25 The modern approach to the treatment of a patient with an infected wound dehiscence following a severe perineal injury involves 3 steps.
Related article:
It’s time to restrict the use of episiotomy
Step 1. Restore tissue to health
The dehisced wound is cultured and, if infection is present, treatment is initiated with intravenous antibiotics appropriate for an infection with colorectal flora. One antibiotic option includes a cephalosporin (cefotetan 2 g IV every 6 hours) plus metronidazole (500 mg IV every 8 hours).
In the operating room, the wound should be thoroughly assessed, cleansed, and debrided. This step includes irrigation of the wound with a warm fluid, mechanical debridement, and sharp dissection of necrotic tissue. If the wound is infected, removal of stitches that are visible in the open wound is recommended.
Often more than one session of debridement may be needed to obtain wound edges that are free from exudate and show granulation at the wound margins. Between debridement sessions, wet-to-dry dressings are utilized. Two to 10 days of wound care may be needed before an attempt is made to close the wound. The wound is suitable for repair when there is no infected tissue and granulation tissue is present. Some surgeons prefer a mechanical bowel preparation regimen just before surgically closing the open wound. This may prevent early bowel movements and provide for tissue healing after surgery.26 The same preparations recommended for colonoscopy can be considered prior to surgical repair.
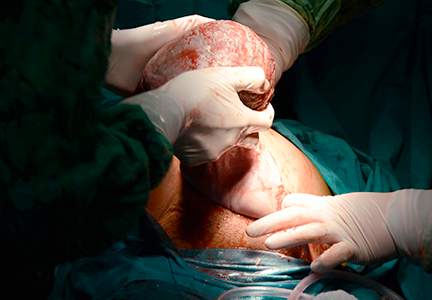
Step 2. Surgically close the wound
The wound is surgically closed in the operating room. If in Step 1 the assessment of the wound shows major trauma, assistance from a urogynecologist may be warranted. Surgical management of a perineal wound dehiscence requires a clear understanding of perineal anatomy and the structures contained between the vagina and the anorectum.
Six key structures may be involved in perineal injury: the anorectal mucosa, internal anal sphincter, external anal sphincter, vaginal wall and perineal skin, bulbocavernosus muscle, and transverse perineal muscles. It is important to definitively identify the individual structures that need to be repaired. Careful dissection is then carried out to mobilize these structures for repair. Additional debridement may be necessary to remove excess granulation tissue.
Anorectal mucosa repair. With repair of a 4th-degree perineal wound dehiscence, the apex of the defect in the anorectal mucosa is identified. The defect is repaired beginning at the apex using closely spaced interrupted sutures or a running suture of 3-0 or 4-0 polyglactin 910. Adequate tissue bites that will resist tearing should be taken. If interrupted sutures are used, tying the knots within the anorectal canal prevents them from being located within the healing wound.
Internal anal sphincter repair. After the anorectal mucosa is closed, attention is turned to reapproximation of the internal anal sphincter. The ends of a torn internal anal sphincter are often located lateral to the anorectal mucosa and appear as shiny gray-white fibrous tissue. The surgeon’s gloved index finger can be placed within the anorectal canal to aid in identification of the internal anal sphincter, as it tends to have a rubbery feel. Additionally, while the surgeon’s gloved index finger is in the anorectal canal, the surgeon’s gloved thumb can be used to retract the anorectal mucosa slightly medial and inferior so that adequate bites of the internal anal sphincter can be taken on each side.
Alternatively, Allis clamps canbe placed on the ends of the retracted internal anal sphincter to facilitate repair. Suture selection for repair of the internal anal sphincter can include 3-0 polyglactin 910 or 3-0 monofilament, delayed-absorbable suture such as polydioxanone sulfate (PDS). Some surgeons prefer delayed-absorbable suture (PDS) for this layer given the internal anal sphincter is constantly contracting and relaxing as it samples stool.26 This layer also can be closed with either interrupted sutures or a running suture.
External anal sphincter repair. After the anorectal mucosa and internal anal sphincter defects are reapproximated, attention is turned to the external anal sphincter. Like the internal anal sphincter, the ends of the external anal sphincter are often retracted laterally and must be definitively identified and mobilized in order to ensure an adequate tension-free repair. It is important to include the fascial sheath in the repair of the external anal sphincter.27 Allis clamps can be used to grasp the ends of the torn muscle after they are identified.
We recommend 0 or 2-0 PDS for repair of the external sphincter. Repair can be performed using either an end-to-end or overlapping technique. An end-to-end repair traditionally involves reapproximating the ends of the torn muscle and its overlying fascial sheath using interrupted sutures placed at four quadrants (12:00, 3:00, 6:00, 9:00).
In contrast, in an overlapping repair, the ends of the muscle are brought together with mattress sutures. Suture is passed top down through the medial aspect of the more superior muscle flap and top down through the inferior muscle flap more laterally. The same suture is then passed bottom up through the inferior muscle flap more laterally and finally bottom up through the medial aspect of the more superior muscle flap. Two to four mattress sutures are usually placed. After all sutures are placed, they are tied securely.
An overlapping repair results in a greater amount of tissue contact between the two torn muscle ends. However, adequate mobility of the external anal sphincter is necessary to perform this type of repair.
Vaginal wall and perineal body repair. After the anal sphincters have been repaired, the vaginal wall and remainder of the perineal body are reconstructed using the same techniques involved in a 2nd-degree laceration repair. Care must be taken to retrieve and reapproximate the torn ends of the bulbocavernosus muscles, which are also often retracted laterally and superiorly. After the bulbocavernosus and transverse perineal muscles are brought together in the midline, the posterior vaginal wall should be perpendicular to the perineum.
An alternative to surgical closure of a 2nd-degree dehiscence is the use of vacuum-assisted wound closure. Disadvantages of this approach include difficulty in maintaining a vacuum seal in the perineal region and the risk of wound contamination with feces. In one case report, 3 weeks of vacuum-assisted wound closure resulted in healing of a 10-cm wound dehiscence that occurred 5 days following a forceps-assisted vaginal delivery with a mediolateral episiotomy.28
Step 3. Ensure complete healing of the wound
Superb postoperative wound care helps to ensure a quick return to full recovery. Wound care should include regularly scheduled sitz baths (at least 3 times daily) followed by drying the perineum. It is preferable to provide a liquid diet that avoids frequent bowel movements in the initial 3 postoperative days. Stool softeners and fiber supplementation are recommended when a full diet is resumed. Some surgeons have found mineral oil (1 to 2 tablespoons daily) effective in producing soft stools that are easy to pass.26 Ensuring soft stool consistency is important to help prevent repair breakdown that may occur with passage of hard stools, fecal impaction, and/or straining during defecation.
We recommend follow-up 1 to 2 weeks after surgery to assess wound healing. No vaginal intercourseis permitted until complete healing is achieved.
Use a surgical checklist
All obstetricians and midwives strive to reduce the risk of OASIS at vaginal birth. When OASIS occurs, it is often useful to use a surgical checklist to ensure the execution of all steps in the management of the repair and recovery process.29 It is heartbreaking to see an OASIS repair breakdown in the week following a vaginal delivery. But by following the 3 steps outlined here, the secondary repair is likely to be successful and will quickly return most patients to full health.
Related article:
Develop and use a checklist for 3rd- and 4th-degree perineal lacerations

The authors report no financial relationships relevant to this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Blondel B, Alexander S, Bjarnadottir RI, et al; Euro-Peristat Scientific Committee. Variations in rates of severe perineal tears and episiotomies in 20 European countries: a study based on routine national data in Euro-Perstat Project. Acta Obstet Gynecol Scand. 2016;95(7):746–754.
- Friedman AM, Ananth CV, Predergast E, D’Alton ME, Wright JD. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125(4):927–937.
- Pergialiotis V, Vlachos D, Protopapas A, Pappa K, Vlachos G. Risk factors for severe perineal lacerations during childbirth. Int J Gynecol Obstet. 2014;125(1):6–14.
- Schmitz T, Alberti C, Andriss B, Moutafoff C, Oury JF, Sibony O. Identification of women at high risk for severe perineal lacerations. Eur J Obstet Gynecol Reprod Biol. 2014;182:11–15.
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.
- ACOG Committee on Practice Bulletins—Obstetrics. Practice bulletin no. 165: Prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2016;128(1):e1–e15.
- Sartore A, De Seta F, Maso G, Pregazzi R, Grimaldi E, Guaschino S. The effects of mediolateral episiotomy on pelvic floor function after vaginal delivery. Obstet Gynecol. 2004;103(4):669–673.
- Aasheim V, Nilsen AB, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2011;(12):CD006672.
- Harvey MA, Pierce M, Alter JE, et al; Society of Obstetricians and Gynaecologists of Canada. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynaecol Can. 2015;37(12):1131–1148.
- Jonsson ER, Elfaghi I, Rydhstrom H, Herbst A. Modified Ritgen’s maneuver for anal sphincter injury at delivery: a randomized controlled trial. Obstet Gynecol. 2008;112(2 pt 1):212–217.
- Williams JW. Obstetrics: A Text-book for the Use of Students and Practitioners. New York, NY: D Appleton and Co; 1903:288.
- Cunningham FG. The Ritgen maneuver: another sacred cow questioned. Obstet Gynecol. 2008;112(2 pt 1):210–211.
- Myrfield K, Brook C, Creedy D. Reducing perineal trauma: implications of flexion and extension of the fetal head during birth. Midwifery. 1997;13:197–201.
- Ostergaard Poulsen M, Lund Madsen M, Skriver-Moller AC, Overgaard C. Does the Finnish intervention prevent obstetrical anal sphincter injuries? A systematic review of the literature. BMJ Open. 2015;5:e008346.
- Kamel A, Khaled M. Episiotomy and obstetric perineal wound dehiscence: beyond soreness. J Obstet Gynaecol. 2014;34(3):215–217.
- Goldaber KG, Wendel PJ, McIntire DD, Wendel GD Jr. Postpartum perineal morbidity after fourth-degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489–493.
- Monberg J, Hammen S. Ruptured episiotomia resutured primarily. Acta Obstet Gynecol Scand. 1987;66(2):163–164.
- Jallad K, Steele SE, Barber MD. Breakdown of perineal laceration repair after vaginal delivery: a case-control study. Female Pelvic Med Reconstr Surg. 2016;22(4):276–279.
- Stock L, Basham E, Gossett DR, Lewicky-Gaupp C. Factors associated with wound complications in women with obstetric anal sphincter injuries (OASIS). Am J Obstet Gynecol. 2013;208(4):327.e1–e8.
- Hauth JC, Gilstrap LC 3rd, Ward SC, Hankins GD. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67(6):806–809.
- Hankins GD, Hauth JC, Gilstrap LC 3rd, Hammond TL, Yeomans ER, Snyder RR. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75(1):48–51.
- Ramin SM, Ramus RM, Little BB, Gilstrap LC 3rd. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167(4 pt 1):1104–1107.
- Arona AJ, Al-Marayati L, Grimes DA, Ballard CA. Early secondary repair of third- and fourth-degree perineal lacerations after outpatient wound preparation. Obstet Gynecol. 1995;86(2):294–296.
- Uygur D, Yesildaglar N, Kis S, Sipahi T. Early repair of episiotomy dehiscence. Aust N Z J Obstet Gynaecol. 2004;44(3):244–246.
- Soerensen MM, Bek KM, Buntzen S, Hojberg KE, Laurberg S. Long-term outcome of delayed primary or early secondary reconstruction of the anal sphincter after obstetrical injury. Dis Colon Rectum. 2008;51(3):312–317.
- Delancey JOL, Berger MB. Surgical approaches to postobstetrical perineal body defects (rectovaginal fistula and chronic third and fourth-degree lacerations). Clin Obstet Gynecol. 2010;53(1):134–144.
- Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician. 2003;68(8):1585–1590.
- Aviki EM, Batalden RP, del Carmen MG, Berkowitz LR. Vacuum-assisted closure for episiotomy dehiscence. Obstet Gynecol. 2015;126(3):530–533.
- Barbieri RL. Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. OBG Manag. 2013;25(8):8–12.
During vaginal delivery spontaneous perineal trauma and extension of episiotomy incisions are common. A severe perineal laceration that extends into or through the anal sphincter complex is referred to as an obstetric anal sphincter injury (OASIS) and requires meticulous repair. Following the repair of an OASIS, serious wound complications, including dehiscence and infection, may occur. In Europe the reported rate of OASIS varies widely among countries, with a rate of 0.1% in Romania, possibly due to underreporting, and 4.9% in Iceland.1 In the United States the rates of 3rd- and 4th-degree lacerations were reported to be 3.3% and 1.1%, respectively.2
Risk factors for OASIS include forceps delivery (odds ratio [OR], 5.50), vacuum-assisted delivery (OR, 3.98), and midline episiotomy (OR, 3.82).3 Additional risk factors for severe perineal injury at vaginal delivery include nulliparity (adjusted odds ratio [aOR], 2.58), delivery from a persistent occiput posterior position (aOR, 2.24), and above-average newborn birth weight (aOR, 1.28).4
In a meta-analysis of randomized trials, the researchers reported that restrictive use of episiotomy reduced the risk of severe perineal trauma (relative risk [RR], 0.67) but increased the risk of anterior perineal trauma (RR, 1.84).5 The American College of Obstetricians and Gynecologists (ACOG) recommends that episiotomy should not be a routine practice and is best restricted to use in a limited number of cases where fetal and maternal benefit is likely.6 In addition, ACOG recommends that if episiotomy is indicated, a mediolateral incision is favored over a midline incision. In my practice I perform only mediolateral episiotomy incisions. However, mediolateral episiotomy may be associated with an increased risk of postpartum perineal pain and dyspareunia.7 Use of warm compresses applied to the perineum during the second stage of labor may reduce the risk of 3rd- and 4th-degree lacerations.8 Techniques to ensure that the fetal head and shoulders are birthed in a slow and controlled fashion may decrease the risk of OASIS.9 See the TABLE, “Four maneuvers to control and slow the birth of the fetal head.”10–14

Related article:
Stop performing median episiotomy!
Wound complications following the repair of a 3rd- or 4th-degree laceration are reported to occur in approximately 5% to 10% of cases.15 The most common wound complications are dehiscence, infection, abscess formation, pain, sexual dysfunction, and anal incontinence. Minor wound complications, including superficial epithelial separation, can be managed expectantly. However, major wound complications need intensive treatment.
In one study of 21 women who had a major wound complication following the repair of a 4th-degree laceration, 53% had dehiscence plus infection, 33% had dehiscence alone, and 14% had infection alone.16 Major wound complications present at a mean of 5 days after delivery, with a wide range from 1 to 17 days following delivery.17 In a study of 144 cases of wound breakdown following a perineal laceration repair, the major risk factors for wound breakdown were episiotomy (aOR, 11.1), smoking (aOR, 6.4), midwife repair of laceration (aOR, 4.7), use of chromic suture (aOR, 3.9), and operative vaginal delivery (aOR, 3.4).18 In one study of 66 women with a wound complication following the repair of a 3rd- or 4th-degree laceration, clinical risk factors for a wound complication were cigarette smoking (OR, 4.04), 4th-degree laceration (OR, 1.89), and operative vaginal delivery (OR, 1.76).19 The use of intrapartum antibiotics appears to be protective (OR, 0.29) against wound complications following a major perineal laceration.19
Approach to the patient with a dehisced and infected perineal wound
Historically, wound dehiscence following surgical repair of a perineal injury was managed by allowing the wound to slowly close. This approach adversely impacts the quality of life of the affected woman because it may take weeks for the wound to heal. One small randomized trial17 and multiple case series20–24 report that an active multistep management algorithm permits early closure of the majority of these wounds, thereby accelerating the patient’s full recovery. Delayed primary (within 72 hours postpartum) or early secondary reconstruction (within 14 days of delivery) has been demonstrated to be safe with acceptable long-term functional out-comes.25 The modern approach to the treatment of a patient with an infected wound dehiscence following a severe perineal injury involves 3 steps.
Related article:
It’s time to restrict the use of episiotomy
Step 1. Restore tissue to health
The dehisced wound is cultured and, if infection is present, treatment is initiated with intravenous antibiotics appropriate for an infection with colorectal flora. One antibiotic option includes a cephalosporin (cefotetan 2 g IV every 6 hours) plus metronidazole (500 mg IV every 8 hours).
In the operating room, the wound should be thoroughly assessed, cleansed, and debrided. This step includes irrigation of the wound with a warm fluid, mechanical debridement, and sharp dissection of necrotic tissue. If the wound is infected, removal of stitches that are visible in the open wound is recommended.
Often more than one session of debridement may be needed to obtain wound edges that are free from exudate and show granulation at the wound margins. Between debridement sessions, wet-to-dry dressings are utilized. Two to 10 days of wound care may be needed before an attempt is made to close the wound. The wound is suitable for repair when there is no infected tissue and granulation tissue is present. Some surgeons prefer a mechanical bowel preparation regimen just before surgically closing the open wound. This may prevent early bowel movements and provide for tissue healing after surgery.26 The same preparations recommended for colonoscopy can be considered prior to surgical repair.
Step 2. Surgically close the wound
The wound is surgically closed in the operating room. If in Step 1 the assessment of the wound shows major trauma, assistance from a urogynecologist may be warranted. Surgical management of a perineal wound dehiscence requires a clear understanding of perineal anatomy and the structures contained between the vagina and the anorectum.
Six key structures may be involved in perineal injury: the anorectal mucosa, internal anal sphincter, external anal sphincter, vaginal wall and perineal skin, bulbocavernosus muscle, and transverse perineal muscles. It is important to definitively identify the individual structures that need to be repaired. Careful dissection is then carried out to mobilize these structures for repair. Additional debridement may be necessary to remove excess granulation tissue.
Anorectal mucosa repair. With repair of a 4th-degree perineal wound dehiscence, the apex of the defect in the anorectal mucosa is identified. The defect is repaired beginning at the apex using closely spaced interrupted sutures or a running suture of 3-0 or 4-0 polyglactin 910. Adequate tissue bites that will resist tearing should be taken. If interrupted sutures are used, tying the knots within the anorectal canal prevents them from being located within the healing wound.
Internal anal sphincter repair. After the anorectal mucosa is closed, attention is turned to reapproximation of the internal anal sphincter. The ends of a torn internal anal sphincter are often located lateral to the anorectal mucosa and appear as shiny gray-white fibrous tissue. The surgeon’s gloved index finger can be placed within the anorectal canal to aid in identification of the internal anal sphincter, as it tends to have a rubbery feel. Additionally, while the surgeon’s gloved index finger is in the anorectal canal, the surgeon’s gloved thumb can be used to retract the anorectal mucosa slightly medial and inferior so that adequate bites of the internal anal sphincter can be taken on each side.
Alternatively, Allis clamps canbe placed on the ends of the retracted internal anal sphincter to facilitate repair. Suture selection for repair of the internal anal sphincter can include 3-0 polyglactin 910 or 3-0 monofilament, delayed-absorbable suture such as polydioxanone sulfate (PDS). Some surgeons prefer delayed-absorbable suture (PDS) for this layer given the internal anal sphincter is constantly contracting and relaxing as it samples stool.26 This layer also can be closed with either interrupted sutures or a running suture.
External anal sphincter repair. After the anorectal mucosa and internal anal sphincter defects are reapproximated, attention is turned to the external anal sphincter. Like the internal anal sphincter, the ends of the external anal sphincter are often retracted laterally and must be definitively identified and mobilized in order to ensure an adequate tension-free repair. It is important to include the fascial sheath in the repair of the external anal sphincter.27 Allis clamps can be used to grasp the ends of the torn muscle after they are identified.
We recommend 0 or 2-0 PDS for repair of the external sphincter. Repair can be performed using either an end-to-end or overlapping technique. An end-to-end repair traditionally involves reapproximating the ends of the torn muscle and its overlying fascial sheath using interrupted sutures placed at four quadrants (12:00, 3:00, 6:00, 9:00).
In contrast, in an overlapping repair, the ends of the muscle are brought together with mattress sutures. Suture is passed top down through the medial aspect of the more superior muscle flap and top down through the inferior muscle flap more laterally. The same suture is then passed bottom up through the inferior muscle flap more laterally and finally bottom up through the medial aspect of the more superior muscle flap. Two to four mattress sutures are usually placed. After all sutures are placed, they are tied securely.
An overlapping repair results in a greater amount of tissue contact between the two torn muscle ends. However, adequate mobility of the external anal sphincter is necessary to perform this type of repair.
Vaginal wall and perineal body repair. After the anal sphincters have been repaired, the vaginal wall and remainder of the perineal body are reconstructed using the same techniques involved in a 2nd-degree laceration repair. Care must be taken to retrieve and reapproximate the torn ends of the bulbocavernosus muscles, which are also often retracted laterally and superiorly. After the bulbocavernosus and transverse perineal muscles are brought together in the midline, the posterior vaginal wall should be perpendicular to the perineum.
An alternative to surgical closure of a 2nd-degree dehiscence is the use of vacuum-assisted wound closure. Disadvantages of this approach include difficulty in maintaining a vacuum seal in the perineal region and the risk of wound contamination with feces. In one case report, 3 weeks of vacuum-assisted wound closure resulted in healing of a 10-cm wound dehiscence that occurred 5 days following a forceps-assisted vaginal delivery with a mediolateral episiotomy.28
Step 3. Ensure complete healing of the wound
Superb postoperative wound care helps to ensure a quick return to full recovery. Wound care should include regularly scheduled sitz baths (at least 3 times daily) followed by drying the perineum. It is preferable to provide a liquid diet that avoids frequent bowel movements in the initial 3 postoperative days. Stool softeners and fiber supplementation are recommended when a full diet is resumed. Some surgeons have found mineral oil (1 to 2 tablespoons daily) effective in producing soft stools that are easy to pass.26 Ensuring soft stool consistency is important to help prevent repair breakdown that may occur with passage of hard stools, fecal impaction, and/or straining during defecation.
We recommend follow-up 1 to 2 weeks after surgery to assess wound healing. No vaginal intercourseis permitted until complete healing is achieved.
Use a surgical checklist
All obstetricians and midwives strive to reduce the risk of OASIS at vaginal birth. When OASIS occurs, it is often useful to use a surgical checklist to ensure the execution of all steps in the management of the repair and recovery process.29 It is heartbreaking to see an OASIS repair breakdown in the week following a vaginal delivery. But by following the 3 steps outlined here, the secondary repair is likely to be successful and will quickly return most patients to full health.
Related article:
Develop and use a checklist for 3rd- and 4th-degree perineal lacerations

The authors report no financial relationships relevant to this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
During vaginal delivery spontaneous perineal trauma and extension of episiotomy incisions are common. A severe perineal laceration that extends into or through the anal sphincter complex is referred to as an obstetric anal sphincter injury (OASIS) and requires meticulous repair. Following the repair of an OASIS, serious wound complications, including dehiscence and infection, may occur. In Europe the reported rate of OASIS varies widely among countries, with a rate of 0.1% in Romania, possibly due to underreporting, and 4.9% in Iceland.1 In the United States the rates of 3rd- and 4th-degree lacerations were reported to be 3.3% and 1.1%, respectively.2
Risk factors for OASIS include forceps delivery (odds ratio [OR], 5.50), vacuum-assisted delivery (OR, 3.98), and midline episiotomy (OR, 3.82).3 Additional risk factors for severe perineal injury at vaginal delivery include nulliparity (adjusted odds ratio [aOR], 2.58), delivery from a persistent occiput posterior position (aOR, 2.24), and above-average newborn birth weight (aOR, 1.28).4
In a meta-analysis of randomized trials, the researchers reported that restrictive use of episiotomy reduced the risk of severe perineal trauma (relative risk [RR], 0.67) but increased the risk of anterior perineal trauma (RR, 1.84).5 The American College of Obstetricians and Gynecologists (ACOG) recommends that episiotomy should not be a routine practice and is best restricted to use in a limited number of cases where fetal and maternal benefit is likely.6 In addition, ACOG recommends that if episiotomy is indicated, a mediolateral incision is favored over a midline incision. In my practice I perform only mediolateral episiotomy incisions. However, mediolateral episiotomy may be associated with an increased risk of postpartum perineal pain and dyspareunia.7 Use of warm compresses applied to the perineum during the second stage of labor may reduce the risk of 3rd- and 4th-degree lacerations.8 Techniques to ensure that the fetal head and shoulders are birthed in a slow and controlled fashion may decrease the risk of OASIS.9 See the TABLE, “Four maneuvers to control and slow the birth of the fetal head.”10–14

Related article:
Stop performing median episiotomy!
Wound complications following the repair of a 3rd- or 4th-degree laceration are reported to occur in approximately 5% to 10% of cases.15 The most common wound complications are dehiscence, infection, abscess formation, pain, sexual dysfunction, and anal incontinence. Minor wound complications, including superficial epithelial separation, can be managed expectantly. However, major wound complications need intensive treatment.
In one study of 21 women who had a major wound complication following the repair of a 4th-degree laceration, 53% had dehiscence plus infection, 33% had dehiscence alone, and 14% had infection alone.16 Major wound complications present at a mean of 5 days after delivery, with a wide range from 1 to 17 days following delivery.17 In a study of 144 cases of wound breakdown following a perineal laceration repair, the major risk factors for wound breakdown were episiotomy (aOR, 11.1), smoking (aOR, 6.4), midwife repair of laceration (aOR, 4.7), use of chromic suture (aOR, 3.9), and operative vaginal delivery (aOR, 3.4).18 In one study of 66 women with a wound complication following the repair of a 3rd- or 4th-degree laceration, clinical risk factors for a wound complication were cigarette smoking (OR, 4.04), 4th-degree laceration (OR, 1.89), and operative vaginal delivery (OR, 1.76).19 The use of intrapartum antibiotics appears to be protective (OR, 0.29) against wound complications following a major perineal laceration.19
Approach to the patient with a dehisced and infected perineal wound
Historically, wound dehiscence following surgical repair of a perineal injury was managed by allowing the wound to slowly close. This approach adversely impacts the quality of life of the affected woman because it may take weeks for the wound to heal. One small randomized trial17 and multiple case series20–24 report that an active multistep management algorithm permits early closure of the majority of these wounds, thereby accelerating the patient’s full recovery. Delayed primary (within 72 hours postpartum) or early secondary reconstruction (within 14 days of delivery) has been demonstrated to be safe with acceptable long-term functional out-comes.25 The modern approach to the treatment of a patient with an infected wound dehiscence following a severe perineal injury involves 3 steps.
Related article:
It’s time to restrict the use of episiotomy
Step 1. Restore tissue to health
The dehisced wound is cultured and, if infection is present, treatment is initiated with intravenous antibiotics appropriate for an infection with colorectal flora. One antibiotic option includes a cephalosporin (cefotetan 2 g IV every 6 hours) plus metronidazole (500 mg IV every 8 hours).
In the operating room, the wound should be thoroughly assessed, cleansed, and debrided. This step includes irrigation of the wound with a warm fluid, mechanical debridement, and sharp dissection of necrotic tissue. If the wound is infected, removal of stitches that are visible in the open wound is recommended.
Often more than one session of debridement may be needed to obtain wound edges that are free from exudate and show granulation at the wound margins. Between debridement sessions, wet-to-dry dressings are utilized. Two to 10 days of wound care may be needed before an attempt is made to close the wound. The wound is suitable for repair when there is no infected tissue and granulation tissue is present. Some surgeons prefer a mechanical bowel preparation regimen just before surgically closing the open wound. This may prevent early bowel movements and provide for tissue healing after surgery.26 The same preparations recommended for colonoscopy can be considered prior to surgical repair.
Step 2. Surgically close the wound
The wound is surgically closed in the operating room. If in Step 1 the assessment of the wound shows major trauma, assistance from a urogynecologist may be warranted. Surgical management of a perineal wound dehiscence requires a clear understanding of perineal anatomy and the structures contained between the vagina and the anorectum.
Six key structures may be involved in perineal injury: the anorectal mucosa, internal anal sphincter, external anal sphincter, vaginal wall and perineal skin, bulbocavernosus muscle, and transverse perineal muscles. It is important to definitively identify the individual structures that need to be repaired. Careful dissection is then carried out to mobilize these structures for repair. Additional debridement may be necessary to remove excess granulation tissue.
Anorectal mucosa repair. With repair of a 4th-degree perineal wound dehiscence, the apex of the defect in the anorectal mucosa is identified. The defect is repaired beginning at the apex using closely spaced interrupted sutures or a running suture of 3-0 or 4-0 polyglactin 910. Adequate tissue bites that will resist tearing should be taken. If interrupted sutures are used, tying the knots within the anorectal canal prevents them from being located within the healing wound.
Internal anal sphincter repair. After the anorectal mucosa is closed, attention is turned to reapproximation of the internal anal sphincter. The ends of a torn internal anal sphincter are often located lateral to the anorectal mucosa and appear as shiny gray-white fibrous tissue. The surgeon’s gloved index finger can be placed within the anorectal canal to aid in identification of the internal anal sphincter, as it tends to have a rubbery feel. Additionally, while the surgeon’s gloved index finger is in the anorectal canal, the surgeon’s gloved thumb can be used to retract the anorectal mucosa slightly medial and inferior so that adequate bites of the internal anal sphincter can be taken on each side.
Alternatively, Allis clamps canbe placed on the ends of the retracted internal anal sphincter to facilitate repair. Suture selection for repair of the internal anal sphincter can include 3-0 polyglactin 910 or 3-0 monofilament, delayed-absorbable suture such as polydioxanone sulfate (PDS). Some surgeons prefer delayed-absorbable suture (PDS) for this layer given the internal anal sphincter is constantly contracting and relaxing as it samples stool.26 This layer also can be closed with either interrupted sutures or a running suture.
External anal sphincter repair. After the anorectal mucosa and internal anal sphincter defects are reapproximated, attention is turned to the external anal sphincter. Like the internal anal sphincter, the ends of the external anal sphincter are often retracted laterally and must be definitively identified and mobilized in order to ensure an adequate tension-free repair. It is important to include the fascial sheath in the repair of the external anal sphincter.27 Allis clamps can be used to grasp the ends of the torn muscle after they are identified.
We recommend 0 or 2-0 PDS for repair of the external sphincter. Repair can be performed using either an end-to-end or overlapping technique. An end-to-end repair traditionally involves reapproximating the ends of the torn muscle and its overlying fascial sheath using interrupted sutures placed at four quadrants (12:00, 3:00, 6:00, 9:00).
In contrast, in an overlapping repair, the ends of the muscle are brought together with mattress sutures. Suture is passed top down through the medial aspect of the more superior muscle flap and top down through the inferior muscle flap more laterally. The same suture is then passed bottom up through the inferior muscle flap more laterally and finally bottom up through the medial aspect of the more superior muscle flap. Two to four mattress sutures are usually placed. After all sutures are placed, they are tied securely.
An overlapping repair results in a greater amount of tissue contact between the two torn muscle ends. However, adequate mobility of the external anal sphincter is necessary to perform this type of repair.
Vaginal wall and perineal body repair. After the anal sphincters have been repaired, the vaginal wall and remainder of the perineal body are reconstructed using the same techniques involved in a 2nd-degree laceration repair. Care must be taken to retrieve and reapproximate the torn ends of the bulbocavernosus muscles, which are also often retracted laterally and superiorly. After the bulbocavernosus and transverse perineal muscles are brought together in the midline, the posterior vaginal wall should be perpendicular to the perineum.
An alternative to surgical closure of a 2nd-degree dehiscence is the use of vacuum-assisted wound closure. Disadvantages of this approach include difficulty in maintaining a vacuum seal in the perineal region and the risk of wound contamination with feces. In one case report, 3 weeks of vacuum-assisted wound closure resulted in healing of a 10-cm wound dehiscence that occurred 5 days following a forceps-assisted vaginal delivery with a mediolateral episiotomy.28
Step 3. Ensure complete healing of the wound
Superb postoperative wound care helps to ensure a quick return to full recovery. Wound care should include regularly scheduled sitz baths (at least 3 times daily) followed by drying the perineum. It is preferable to provide a liquid diet that avoids frequent bowel movements in the initial 3 postoperative days. Stool softeners and fiber supplementation are recommended when a full diet is resumed. Some surgeons have found mineral oil (1 to 2 tablespoons daily) effective in producing soft stools that are easy to pass.26 Ensuring soft stool consistency is important to help prevent repair breakdown that may occur with passage of hard stools, fecal impaction, and/or straining during defecation.
We recommend follow-up 1 to 2 weeks after surgery to assess wound healing. No vaginal intercourseis permitted until complete healing is achieved.
Use a surgical checklist
All obstetricians and midwives strive to reduce the risk of OASIS at vaginal birth. When OASIS occurs, it is often useful to use a surgical checklist to ensure the execution of all steps in the management of the repair and recovery process.29 It is heartbreaking to see an OASIS repair breakdown in the week following a vaginal delivery. But by following the 3 steps outlined here, the secondary repair is likely to be successful and will quickly return most patients to full health.
Related article:
Develop and use a checklist for 3rd- and 4th-degree perineal lacerations

The authors report no financial relationships relevant to this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Blondel B, Alexander S, Bjarnadottir RI, et al; Euro-Peristat Scientific Committee. Variations in rates of severe perineal tears and episiotomies in 20 European countries: a study based on routine national data in Euro-Perstat Project. Acta Obstet Gynecol Scand. 2016;95(7):746–754.
- Friedman AM, Ananth CV, Predergast E, D’Alton ME, Wright JD. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125(4):927–937.
- Pergialiotis V, Vlachos D, Protopapas A, Pappa K, Vlachos G. Risk factors for severe perineal lacerations during childbirth. Int J Gynecol Obstet. 2014;125(1):6–14.
- Schmitz T, Alberti C, Andriss B, Moutafoff C, Oury JF, Sibony O. Identification of women at high risk for severe perineal lacerations. Eur J Obstet Gynecol Reprod Biol. 2014;182:11–15.
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.
- ACOG Committee on Practice Bulletins—Obstetrics. Practice bulletin no. 165: Prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2016;128(1):e1–e15.
- Sartore A, De Seta F, Maso G, Pregazzi R, Grimaldi E, Guaschino S. The effects of mediolateral episiotomy on pelvic floor function after vaginal delivery. Obstet Gynecol. 2004;103(4):669–673.
- Aasheim V, Nilsen AB, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2011;(12):CD006672.
- Harvey MA, Pierce M, Alter JE, et al; Society of Obstetricians and Gynaecologists of Canada. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynaecol Can. 2015;37(12):1131–1148.
- Jonsson ER, Elfaghi I, Rydhstrom H, Herbst A. Modified Ritgen’s maneuver for anal sphincter injury at delivery: a randomized controlled trial. Obstet Gynecol. 2008;112(2 pt 1):212–217.
- Williams JW. Obstetrics: A Text-book for the Use of Students and Practitioners. New York, NY: D Appleton and Co; 1903:288.
- Cunningham FG. The Ritgen maneuver: another sacred cow questioned. Obstet Gynecol. 2008;112(2 pt 1):210–211.
- Myrfield K, Brook C, Creedy D. Reducing perineal trauma: implications of flexion and extension of the fetal head during birth. Midwifery. 1997;13:197–201.
- Ostergaard Poulsen M, Lund Madsen M, Skriver-Moller AC, Overgaard C. Does the Finnish intervention prevent obstetrical anal sphincter injuries? A systematic review of the literature. BMJ Open. 2015;5:e008346.
- Kamel A, Khaled M. Episiotomy and obstetric perineal wound dehiscence: beyond soreness. J Obstet Gynaecol. 2014;34(3):215–217.
- Goldaber KG, Wendel PJ, McIntire DD, Wendel GD Jr. Postpartum perineal morbidity after fourth-degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489–493.
- Monberg J, Hammen S. Ruptured episiotomia resutured primarily. Acta Obstet Gynecol Scand. 1987;66(2):163–164.
- Jallad K, Steele SE, Barber MD. Breakdown of perineal laceration repair after vaginal delivery: a case-control study. Female Pelvic Med Reconstr Surg. 2016;22(4):276–279.
- Stock L, Basham E, Gossett DR, Lewicky-Gaupp C. Factors associated with wound complications in women with obstetric anal sphincter injuries (OASIS). Am J Obstet Gynecol. 2013;208(4):327.e1–e8.
- Hauth JC, Gilstrap LC 3rd, Ward SC, Hankins GD. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67(6):806–809.
- Hankins GD, Hauth JC, Gilstrap LC 3rd, Hammond TL, Yeomans ER, Snyder RR. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75(1):48–51.
- Ramin SM, Ramus RM, Little BB, Gilstrap LC 3rd. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167(4 pt 1):1104–1107.
- Arona AJ, Al-Marayati L, Grimes DA, Ballard CA. Early secondary repair of third- and fourth-degree perineal lacerations after outpatient wound preparation. Obstet Gynecol. 1995;86(2):294–296.
- Uygur D, Yesildaglar N, Kis S, Sipahi T. Early repair of episiotomy dehiscence. Aust N Z J Obstet Gynaecol. 2004;44(3):244–246.
- Soerensen MM, Bek KM, Buntzen S, Hojberg KE, Laurberg S. Long-term outcome of delayed primary or early secondary reconstruction of the anal sphincter after obstetrical injury. Dis Colon Rectum. 2008;51(3):312–317.
- Delancey JOL, Berger MB. Surgical approaches to postobstetrical perineal body defects (rectovaginal fistula and chronic third and fourth-degree lacerations). Clin Obstet Gynecol. 2010;53(1):134–144.
- Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician. 2003;68(8):1585–1590.
- Aviki EM, Batalden RP, del Carmen MG, Berkowitz LR. Vacuum-assisted closure for episiotomy dehiscence. Obstet Gynecol. 2015;126(3):530–533.
- Barbieri RL. Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. OBG Manag. 2013;25(8):8–12.
- Blondel B, Alexander S, Bjarnadottir RI, et al; Euro-Peristat Scientific Committee. Variations in rates of severe perineal tears and episiotomies in 20 European countries: a study based on routine national data in Euro-Perstat Project. Acta Obstet Gynecol Scand. 2016;95(7):746–754.
- Friedman AM, Ananth CV, Predergast E, D’Alton ME, Wright JD. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125(4):927–937.
- Pergialiotis V, Vlachos D, Protopapas A, Pappa K, Vlachos G. Risk factors for severe perineal lacerations during childbirth. Int J Gynecol Obstet. 2014;125(1):6–14.
- Schmitz T, Alberti C, Andriss B, Moutafoff C, Oury JF, Sibony O. Identification of women at high risk for severe perineal lacerations. Eur J Obstet Gynecol Reprod Biol. 2014;182:11–15.
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;(1):CD000081.
- ACOG Committee on Practice Bulletins—Obstetrics. Practice bulletin no. 165: Prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2016;128(1):e1–e15.
- Sartore A, De Seta F, Maso G, Pregazzi R, Grimaldi E, Guaschino S. The effects of mediolateral episiotomy on pelvic floor function after vaginal delivery. Obstet Gynecol. 2004;103(4):669–673.
- Aasheim V, Nilsen AB, Lukasse M, Reinar LM. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2011;(12):CD006672.
- Harvey MA, Pierce M, Alter JE, et al; Society of Obstetricians and Gynaecologists of Canada. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynaecol Can. 2015;37(12):1131–1148.
- Jonsson ER, Elfaghi I, Rydhstrom H, Herbst A. Modified Ritgen’s maneuver for anal sphincter injury at delivery: a randomized controlled trial. Obstet Gynecol. 2008;112(2 pt 1):212–217.
- Williams JW. Obstetrics: A Text-book for the Use of Students and Practitioners. New York, NY: D Appleton and Co; 1903:288.
- Cunningham FG. The Ritgen maneuver: another sacred cow questioned. Obstet Gynecol. 2008;112(2 pt 1):210–211.
- Myrfield K, Brook C, Creedy D. Reducing perineal trauma: implications of flexion and extension of the fetal head during birth. Midwifery. 1997;13:197–201.
- Ostergaard Poulsen M, Lund Madsen M, Skriver-Moller AC, Overgaard C. Does the Finnish intervention prevent obstetrical anal sphincter injuries? A systematic review of the literature. BMJ Open. 2015;5:e008346.
- Kamel A, Khaled M. Episiotomy and obstetric perineal wound dehiscence: beyond soreness. J Obstet Gynaecol. 2014;34(3):215–217.
- Goldaber KG, Wendel PJ, McIntire DD, Wendel GD Jr. Postpartum perineal morbidity after fourth-degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489–493.
- Monberg J, Hammen S. Ruptured episiotomia resutured primarily. Acta Obstet Gynecol Scand. 1987;66(2):163–164.
- Jallad K, Steele SE, Barber MD. Breakdown of perineal laceration repair after vaginal delivery: a case-control study. Female Pelvic Med Reconstr Surg. 2016;22(4):276–279.
- Stock L, Basham E, Gossett DR, Lewicky-Gaupp C. Factors associated with wound complications in women with obstetric anal sphincter injuries (OASIS). Am J Obstet Gynecol. 2013;208(4):327.e1–e8.
- Hauth JC, Gilstrap LC 3rd, Ward SC, Hankins GD. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67(6):806–809.
- Hankins GD, Hauth JC, Gilstrap LC 3rd, Hammond TL, Yeomans ER, Snyder RR. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75(1):48–51.
- Ramin SM, Ramus RM, Little BB, Gilstrap LC 3rd. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167(4 pt 1):1104–1107.
- Arona AJ, Al-Marayati L, Grimes DA, Ballard CA. Early secondary repair of third- and fourth-degree perineal lacerations after outpatient wound preparation. Obstet Gynecol. 1995;86(2):294–296.
- Uygur D, Yesildaglar N, Kis S, Sipahi T. Early repair of episiotomy dehiscence. Aust N Z J Obstet Gynaecol. 2004;44(3):244–246.
- Soerensen MM, Bek KM, Buntzen S, Hojberg KE, Laurberg S. Long-term outcome of delayed primary or early secondary reconstruction of the anal sphincter after obstetrical injury. Dis Colon Rectum. 2008;51(3):312–317.
- Delancey JOL, Berger MB. Surgical approaches to postobstetrical perineal body defects (rectovaginal fistula and chronic third and fourth-degree lacerations). Clin Obstet Gynecol. 2010;53(1):134–144.
- Leeman L, Spearman M, Rogers R. Repair of obstetric perineal lacerations. Am Fam Physician. 2003;68(8):1585–1590.
- Aviki EM, Batalden RP, del Carmen MG, Berkowitz LR. Vacuum-assisted closure for episiotomy dehiscence. Obstet Gynecol. 2015;126(3):530–533.
- Barbieri RL. Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. OBG Manag. 2013;25(8):8–12.
Do you utilize vasopressin in your difficult cesarean delivery surgeries?
Vasopressin is often used to reduce blood loss in gynecologic surgery. Results of randomized clinical trials indicate that its use reduces blood loss in many gynecologic surgery procedures, including hysterectomy, myomectomy, cervical conization, and second trimester pregnancy termination.1−7 In contrast to the widespread use of dilute vasopressin injection in gynecology surgery, obstetricians in the United States seldom use vasopressin to reduce blood loss in difficult cesarean delivery surgery. Although there is very little direct evidence from clinical trials on the value of vasopressin in obstetric surgery, high-quality evidence from relevant gynecologic surgery and case reports from obstetricians support its use during difficult cesarean delivery surgery.
Biology of oxytocin and vasopressin
Oxytocin and vasopressin are fraternal twin nanopeptides that differ by only two amino acids and are secreted from the posterior pituitary. The human uterus contains both oxytocin and vasopressin receptors; stimulation of either receptor causes uterine contraction. Vasopressin receptor activation also causes vasoconstriction and platelet activation.
Given the similar biochemistry of oxytocin and vasopressin it is not surprising that each hormone is capable of binding to both oxytocin and vasopressin receptors. The affinity of oxytocin for the oxytocin and vasopressin receptors as expressed as an inhibition constant is 6.8 nM and 35 nM, respectively. Vasopressin’s affinity for the oxytocin and vasopressin V1a receptors is 48 nM and 1.4 nM, respectively.8
Administering vasopressin into the uterus will achieve a high concentration of the hormone, which stimulates both the oxytocin and vasopressin receptors, resulting in uterine contraction, vasoconstriction, and platelet activation. Of particular importance to obstetricians is that following a prolonged labor or administration of oxytocin, myometrial oxytocin receptors may be downregulated, but vasopressin receptors may remain functional.9,10
Vasopressin regulates plasma volume, blood pressure, osmolality, and uterine contractility. The vasopressin V1a receptor is present on vascular smooth muscle cells, platelets, and uterine myocytes. Activating this receptor causes vasoconstriction, platelet activation, and uterine contraction.
Vasopressin reduces surgical blood loss in two ways. The first major mechanism is through vasoconstriction.11 Second, in uterine surgery specifically, vasopressin stimulates uterine contraction. The hormone exerts its antidiuretic action through the V2 receptor in the kidney.
Optimal vasopressin dose
In gynecologic surgery, the vasopressin doses utilized to reduce blood loss range from 5 U to 20 U diluted in 20 mL to 200 mL of saline. Randomized trial results indicate that a vasopressin dose of 4 U is effective in reducing blood loss during second trimester pregnancy termination,7 and a dose of 3 U is effective in reducing blood loss during cervical conization.5,6 There is insufficient obstetric literature to determine the optimal dose of vasopressin to reduce blood loss in difficult cesarean delivery sur- gery, but doses similar to those used in gynecologic surgery should be considered.
Possible effects of vasopressin overdosing. In gynecologic surgery, injection of vasopressin has been reported to cause bradycardia, hypotension, myocardial infarction, and cardiovascular collapse.12 Given that multiple vasoactive medications may be given to a patient undergoing a complex cesarean delivery, including oxytocin, methergine, and ephedrine, it is important for the obstetrician to use the lowest effective dose of vasopressin necessary to facilitate control of blood loss. The obstetrician needs to communicate with the anesthesiologist and coordinate the use of dilute vasopressin with other vasoactive medications.
Avoid intravascular injection of vasopressin. I prefer to inject vasopressin in the subserosa of the uterus rather than to inject it in a highly vascular area such as the subendometrium or near the uterine artery and vein.
Vasopressin reduces blood loss during hysterectomy
One randomized trial has reported that the administration of 10 U of vasopressin diluted in saline into the lower uterine segment reduced blood loss at abdominal hysterectomy in nonpregnant women compared with an injection of saline alone (445 mL vs 748 mL of blood loss, respectively).1 There are no clinical trials of the use of vasopressin in cesarean hysterectomy. However, abdominal hysterectomy procedures and cesarean hysterectomy are similar, and vasopressin likely helps to reduce blood loss at cesarean hysterectomy.
Vasopressin reduces blood loss during myomectomy
Authors of 3 small, randomized clinical trials in nonpregnant women have reported that the intramyometrial injection of dilute vasopressin reduces blood loss during myomectomy surgery.2−4 The vasopressin doses in the 3 trials ranged from 5 U of vasopressin in 100 mL of saline to 20 U of vasopressin in 20 mL of saline. A Cochrane meta-analyis of the 3 studies concluded that, at myomectomy, the intramyometrial injection of dilute vasopressin was associated with a significant reduction in blood loss compared with placebo (246 mL vs 483 mL, respectively).13
There are great similarities between myomectomy in the nonpregnant and pregnant uterus. Given the clinical trials data that support the use of vasopressin to reduce blood loss during myomectomy in the nonpregnant uterus, it is likely that vasopressin also would reduce blood loss during myomectomy performed at the time of a cesarean delivery.
At cesarean delivery, elective myomectomy of intramural fibroids is generally not recommended because of the risk of massive blood loss. Clinicians often remove large pedunculated fibroids because this surgery does not usually cause massive bleeding. However, on occasion it may be necessary to perform a myomectomy on intramural myoma(s) in order to close a hysterotomy incision.
For myomectomy surgery performed at the time of cesarean delivery, many techniques have been utilized to reduce blood loss, including:
- intravenous oxytocin infusion14,15
- injection of oxytocin into the myoma pseudocapsule15
- electrosurgery16−18
- argon beam coagulator19
- uterine tourniquet20
- premyomectomy placement of a uterine U stitch21 or purse string suture22
- O’Leary sutures23,24
- temporary balloon occlusion of pelvic arteries25
- vasopressin injection.26
Given the widespread use of vasopressin injection in gynecologic surgery to reduce blood loss at myomectomy, obstetricians should consider using vasopressin in their cesarean myomectomy surgery.
Use of vasopressin during cesarean delivery for placenta previa may reduce blood loss
Women with a complete placenta previa require a cesarean delivery to safely birth their baby. Cesarean deliveries performed for this indication are associated with an increased risk of hemorrhage. In one case series of 59 patients with placenta previa undergoing cesarean delivery, 4 U of vasopressin diluted in 20 mL of saline was injected into the placental implantation site to reduce blood loss. Among the patients receiving vasopressin in- jection, the blood loss was 1,149 mL. Among 50 women with placenta previa who did not receive vasopressin injection, the blood loss was 1,634 mL.27
Obstetric surgery and vasopressin: The time has come
As obstetricians and gynecologists we constantly strive to improve the effectiveness of our surgical procedures and reduce adverse outcomes, including infection and blood loss. The use of vasopressin is widely accepted in gynecologic surgery as an adjuvant that reduces blood loss. The time has come to expand the use of vasopressin in difficult obstetric surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97:867–872.
- Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Brit J Obstet Gynaecol. 1994;101:435–437.
- Assaf A. Adhesions after laparoscopic myomectomy effect of the technique used. Gynaecol Endosc. 1999;8(4):225–229.
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertil Steril. 2011;95(2):762–766.
- Sabol ED, Gibson JL, Bowes WA Jr. Vasopressin injection in cervical conization. A double-blind study. Obstet Gynecol. 1971;37(4):596–601.
- Martin-Hirsch PP, Keep SL, Bryant A. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;(6):CD001421.
- Schulz KE, Grimes DA, Christensen DD. Vasopressin reduces blood loss from second trimester dilatation and evacuation abortion. Lancet. 1985;2(8451):353–356.
- Akerlund M, Bossmar T, Brouard R, et al. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects in isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999;106(10):1047–1053.
- Akerlund M. Involvement of oxytocin and vasopressin in the pathophysiology of preterm labor and primary dysmenorrhea. Prog Brain Res. 2002;139:359–365.
- Helmer H, Hacki T, Schneeberger C, et al. Oxytocinand vasopressin 1a receptor gene expression in the cycling or pregnant human uterus. Am J Obstet Gynecol. 1998;179(6 pt 1):1572–1578.
- Wing DA, Goharkhay N, Felix JC, Rostamkhani M, Naidu YM, Kovacs BW. Expression of the oxytocin and V1a vasopressin receptors in human myometrium during differing physiological states and following misoprostol administration. Gynecol Obstet Invest. 2006;62(4):181–185.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014; (8):CD005355.
- Tinelli A, Malvasi A, Mynbaev OA, et al. The surgical outcome of intracapsular cesarean myomectomy. A match control study. J Matern Fetal Neonatal Med. 2014;27(1):66–71.
- Brown D, Fletcher HM, Myrie MO, Reid M. Caesarean myomectomy—a safe procedure. A retrospective case controlled study. J Obstet Gynaecol. 1999;19(2):139–141.
- Kaymak O, Ustunyrt E, Okyay RE, Kalyoncu S, Mollamahmutoglu L. Myomectomy during cesarean section. Int J Gynaecol Obstet. 2005;89(2):90–93.
- Park BJ, Kim YW. Safety of cesarean myomectomy. J Obstet Gynaecol Res. 2009;35(5):906–911.
- Kim YS, Choi SD, Bae DH. Risk factors for complications in patients undergoing myomectomy at the time of cesarean section. J Obstet Gynaecol Res. 2010;36(3):550–554.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynecol Obstet. 1999;67(3):189–190.
- Incebiyik A, Hilali NG, Camuzcuoglu A, Vural M, Camuzcuoglu H. Myomectomy during caesarean: a retrospective evaluation of 6 cases. Arch Gynecol Obstet. 2014;289(3):569–573.
- Cobellis L, Pecori E, Cobellis G. Hemostatic technique for myomectomy during cesarean section. Int J Gynaecol Obstet. 2002;79(3):261–262.
- Lee JH, Cho DH. Myomectomy using purse-string suture during cesarean section. Arch Gynecol Obstet. 2011;283(suppl 1):S35–S37.
- Desai BR, Patted SS, Pujar YV, Sheriqar BY, Das SR, Ruge JC. A novel technique of selective uterine devascularization before myomectomy at the time of cesarean section: a pilot study. Fertil Steril. 2010;94(1):362–364.
- Sapmaz E, Celik H, Altungul A. Bilateral ascending uterine artery ligation vs. tourniquet use for hemostasis in cesarean myomectomy. A comparison. J Reprod Med. 2003;48(12):950–954.
- Sparic R, Malvasi A, Kadija S, Babovic I, Nejkovic L, Tinelli A. Cesarean myomectomy trends and controveries: an appraisal [published online ahead of print July 17, 2016]. J Matern Fetal Neonatal Med. doi:10.1080/14767058.2016.1205024.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing cesarean section. J Obstet Gynaecol Res. 2010;36(2):284–290.
- Kato S, Tanabe A, Kanki K, et al. Local injection of vasopressin reduces the blood loss during cesarean section in placenta previa. J Obstet Gynaecol Res. 2014;40(5):1249–1256.
Vasopressin is often used to reduce blood loss in gynecologic surgery. Results of randomized clinical trials indicate that its use reduces blood loss in many gynecologic surgery procedures, including hysterectomy, myomectomy, cervical conization, and second trimester pregnancy termination.1−7 In contrast to the widespread use of dilute vasopressin injection in gynecology surgery, obstetricians in the United States seldom use vasopressin to reduce blood loss in difficult cesarean delivery surgery. Although there is very little direct evidence from clinical trials on the value of vasopressin in obstetric surgery, high-quality evidence from relevant gynecologic surgery and case reports from obstetricians support its use during difficult cesarean delivery surgery.
Biology of oxytocin and vasopressin
Oxytocin and vasopressin are fraternal twin nanopeptides that differ by only two amino acids and are secreted from the posterior pituitary. The human uterus contains both oxytocin and vasopressin receptors; stimulation of either receptor causes uterine contraction. Vasopressin receptor activation also causes vasoconstriction and platelet activation.
Given the similar biochemistry of oxytocin and vasopressin it is not surprising that each hormone is capable of binding to both oxytocin and vasopressin receptors. The affinity of oxytocin for the oxytocin and vasopressin receptors as expressed as an inhibition constant is 6.8 nM and 35 nM, respectively. Vasopressin’s affinity for the oxytocin and vasopressin V1a receptors is 48 nM and 1.4 nM, respectively.8
Administering vasopressin into the uterus will achieve a high concentration of the hormone, which stimulates both the oxytocin and vasopressin receptors, resulting in uterine contraction, vasoconstriction, and platelet activation. Of particular importance to obstetricians is that following a prolonged labor or administration of oxytocin, myometrial oxytocin receptors may be downregulated, but vasopressin receptors may remain functional.9,10
Vasopressin regulates plasma volume, blood pressure, osmolality, and uterine contractility. The vasopressin V1a receptor is present on vascular smooth muscle cells, platelets, and uterine myocytes. Activating this receptor causes vasoconstriction, platelet activation, and uterine contraction.
Vasopressin reduces surgical blood loss in two ways. The first major mechanism is through vasoconstriction.11 Second, in uterine surgery specifically, vasopressin stimulates uterine contraction. The hormone exerts its antidiuretic action through the V2 receptor in the kidney.
Optimal vasopressin dose
In gynecologic surgery, the vasopressin doses utilized to reduce blood loss range from 5 U to 20 U diluted in 20 mL to 200 mL of saline. Randomized trial results indicate that a vasopressin dose of 4 U is effective in reducing blood loss during second trimester pregnancy termination,7 and a dose of 3 U is effective in reducing blood loss during cervical conization.5,6 There is insufficient obstetric literature to determine the optimal dose of vasopressin to reduce blood loss in difficult cesarean delivery sur- gery, but doses similar to those used in gynecologic surgery should be considered.
Possible effects of vasopressin overdosing. In gynecologic surgery, injection of vasopressin has been reported to cause bradycardia, hypotension, myocardial infarction, and cardiovascular collapse.12 Given that multiple vasoactive medications may be given to a patient undergoing a complex cesarean delivery, including oxytocin, methergine, and ephedrine, it is important for the obstetrician to use the lowest effective dose of vasopressin necessary to facilitate control of blood loss. The obstetrician needs to communicate with the anesthesiologist and coordinate the use of dilute vasopressin with other vasoactive medications.
Avoid intravascular injection of vasopressin. I prefer to inject vasopressin in the subserosa of the uterus rather than to inject it in a highly vascular area such as the subendometrium or near the uterine artery and vein.
Vasopressin reduces blood loss during hysterectomy
One randomized trial has reported that the administration of 10 U of vasopressin diluted in saline into the lower uterine segment reduced blood loss at abdominal hysterectomy in nonpregnant women compared with an injection of saline alone (445 mL vs 748 mL of blood loss, respectively).1 There are no clinical trials of the use of vasopressin in cesarean hysterectomy. However, abdominal hysterectomy procedures and cesarean hysterectomy are similar, and vasopressin likely helps to reduce blood loss at cesarean hysterectomy.
Vasopressin reduces blood loss during myomectomy
Authors of 3 small, randomized clinical trials in nonpregnant women have reported that the intramyometrial injection of dilute vasopressin reduces blood loss during myomectomy surgery.2−4 The vasopressin doses in the 3 trials ranged from 5 U of vasopressin in 100 mL of saline to 20 U of vasopressin in 20 mL of saline. A Cochrane meta-analyis of the 3 studies concluded that, at myomectomy, the intramyometrial injection of dilute vasopressin was associated with a significant reduction in blood loss compared with placebo (246 mL vs 483 mL, respectively).13
There are great similarities between myomectomy in the nonpregnant and pregnant uterus. Given the clinical trials data that support the use of vasopressin to reduce blood loss during myomectomy in the nonpregnant uterus, it is likely that vasopressin also would reduce blood loss during myomectomy performed at the time of a cesarean delivery.
At cesarean delivery, elective myomectomy of intramural fibroids is generally not recommended because of the risk of massive blood loss. Clinicians often remove large pedunculated fibroids because this surgery does not usually cause massive bleeding. However, on occasion it may be necessary to perform a myomectomy on intramural myoma(s) in order to close a hysterotomy incision.
For myomectomy surgery performed at the time of cesarean delivery, many techniques have been utilized to reduce blood loss, including:
- intravenous oxytocin infusion14,15
- injection of oxytocin into the myoma pseudocapsule15
- electrosurgery16−18
- argon beam coagulator19
- uterine tourniquet20
- premyomectomy placement of a uterine U stitch21 or purse string suture22
- O’Leary sutures23,24
- temporary balloon occlusion of pelvic arteries25
- vasopressin injection.26
Given the widespread use of vasopressin injection in gynecologic surgery to reduce blood loss at myomectomy, obstetricians should consider using vasopressin in their cesarean myomectomy surgery.
Use of vasopressin during cesarean delivery for placenta previa may reduce blood loss
Women with a complete placenta previa require a cesarean delivery to safely birth their baby. Cesarean deliveries performed for this indication are associated with an increased risk of hemorrhage. In one case series of 59 patients with placenta previa undergoing cesarean delivery, 4 U of vasopressin diluted in 20 mL of saline was injected into the placental implantation site to reduce blood loss. Among the patients receiving vasopressin in- jection, the blood loss was 1,149 mL. Among 50 women with placenta previa who did not receive vasopressin injection, the blood loss was 1,634 mL.27
Obstetric surgery and vasopressin: The time has come
As obstetricians and gynecologists we constantly strive to improve the effectiveness of our surgical procedures and reduce adverse outcomes, including infection and blood loss. The use of vasopressin is widely accepted in gynecologic surgery as an adjuvant that reduces blood loss. The time has come to expand the use of vasopressin in difficult obstetric surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Vasopressin is often used to reduce blood loss in gynecologic surgery. Results of randomized clinical trials indicate that its use reduces blood loss in many gynecologic surgery procedures, including hysterectomy, myomectomy, cervical conization, and second trimester pregnancy termination.1−7 In contrast to the widespread use of dilute vasopressin injection in gynecology surgery, obstetricians in the United States seldom use vasopressin to reduce blood loss in difficult cesarean delivery surgery. Although there is very little direct evidence from clinical trials on the value of vasopressin in obstetric surgery, high-quality evidence from relevant gynecologic surgery and case reports from obstetricians support its use during difficult cesarean delivery surgery.
Biology of oxytocin and vasopressin
Oxytocin and vasopressin are fraternal twin nanopeptides that differ by only two amino acids and are secreted from the posterior pituitary. The human uterus contains both oxytocin and vasopressin receptors; stimulation of either receptor causes uterine contraction. Vasopressin receptor activation also causes vasoconstriction and platelet activation.
Given the similar biochemistry of oxytocin and vasopressin it is not surprising that each hormone is capable of binding to both oxytocin and vasopressin receptors. The affinity of oxytocin for the oxytocin and vasopressin receptors as expressed as an inhibition constant is 6.8 nM and 35 nM, respectively. Vasopressin’s affinity for the oxytocin and vasopressin V1a receptors is 48 nM and 1.4 nM, respectively.8
Administering vasopressin into the uterus will achieve a high concentration of the hormone, which stimulates both the oxytocin and vasopressin receptors, resulting in uterine contraction, vasoconstriction, and platelet activation. Of particular importance to obstetricians is that following a prolonged labor or administration of oxytocin, myometrial oxytocin receptors may be downregulated, but vasopressin receptors may remain functional.9,10
Vasopressin regulates plasma volume, blood pressure, osmolality, and uterine contractility. The vasopressin V1a receptor is present on vascular smooth muscle cells, platelets, and uterine myocytes. Activating this receptor causes vasoconstriction, platelet activation, and uterine contraction.
Vasopressin reduces surgical blood loss in two ways. The first major mechanism is through vasoconstriction.11 Second, in uterine surgery specifically, vasopressin stimulates uterine contraction. The hormone exerts its antidiuretic action through the V2 receptor in the kidney.
Optimal vasopressin dose
In gynecologic surgery, the vasopressin doses utilized to reduce blood loss range from 5 U to 20 U diluted in 20 mL to 200 mL of saline. Randomized trial results indicate that a vasopressin dose of 4 U is effective in reducing blood loss during second trimester pregnancy termination,7 and a dose of 3 U is effective in reducing blood loss during cervical conization.5,6 There is insufficient obstetric literature to determine the optimal dose of vasopressin to reduce blood loss in difficult cesarean delivery sur- gery, but doses similar to those used in gynecologic surgery should be considered.
Possible effects of vasopressin overdosing. In gynecologic surgery, injection of vasopressin has been reported to cause bradycardia, hypotension, myocardial infarction, and cardiovascular collapse.12 Given that multiple vasoactive medications may be given to a patient undergoing a complex cesarean delivery, including oxytocin, methergine, and ephedrine, it is important for the obstetrician to use the lowest effective dose of vasopressin necessary to facilitate control of blood loss. The obstetrician needs to communicate with the anesthesiologist and coordinate the use of dilute vasopressin with other vasoactive medications.
Avoid intravascular injection of vasopressin. I prefer to inject vasopressin in the subserosa of the uterus rather than to inject it in a highly vascular area such as the subendometrium or near the uterine artery and vein.
Vasopressin reduces blood loss during hysterectomy
One randomized trial has reported that the administration of 10 U of vasopressin diluted in saline into the lower uterine segment reduced blood loss at abdominal hysterectomy in nonpregnant women compared with an injection of saline alone (445 mL vs 748 mL of blood loss, respectively).1 There are no clinical trials of the use of vasopressin in cesarean hysterectomy. However, abdominal hysterectomy procedures and cesarean hysterectomy are similar, and vasopressin likely helps to reduce blood loss at cesarean hysterectomy.
Vasopressin reduces blood loss during myomectomy
Authors of 3 small, randomized clinical trials in nonpregnant women have reported that the intramyometrial injection of dilute vasopressin reduces blood loss during myomectomy surgery.2−4 The vasopressin doses in the 3 trials ranged from 5 U of vasopressin in 100 mL of saline to 20 U of vasopressin in 20 mL of saline. A Cochrane meta-analyis of the 3 studies concluded that, at myomectomy, the intramyometrial injection of dilute vasopressin was associated with a significant reduction in blood loss compared with placebo (246 mL vs 483 mL, respectively).13
There are great similarities between myomectomy in the nonpregnant and pregnant uterus. Given the clinical trials data that support the use of vasopressin to reduce blood loss during myomectomy in the nonpregnant uterus, it is likely that vasopressin also would reduce blood loss during myomectomy performed at the time of a cesarean delivery.
At cesarean delivery, elective myomectomy of intramural fibroids is generally not recommended because of the risk of massive blood loss. Clinicians often remove large pedunculated fibroids because this surgery does not usually cause massive bleeding. However, on occasion it may be necessary to perform a myomectomy on intramural myoma(s) in order to close a hysterotomy incision.
For myomectomy surgery performed at the time of cesarean delivery, many techniques have been utilized to reduce blood loss, including:
- intravenous oxytocin infusion14,15
- injection of oxytocin into the myoma pseudocapsule15
- electrosurgery16−18
- argon beam coagulator19
- uterine tourniquet20
- premyomectomy placement of a uterine U stitch21 or purse string suture22
- O’Leary sutures23,24
- temporary balloon occlusion of pelvic arteries25
- vasopressin injection.26
Given the widespread use of vasopressin injection in gynecologic surgery to reduce blood loss at myomectomy, obstetricians should consider using vasopressin in their cesarean myomectomy surgery.
Use of vasopressin during cesarean delivery for placenta previa may reduce blood loss
Women with a complete placenta previa require a cesarean delivery to safely birth their baby. Cesarean deliveries performed for this indication are associated with an increased risk of hemorrhage. In one case series of 59 patients with placenta previa undergoing cesarean delivery, 4 U of vasopressin diluted in 20 mL of saline was injected into the placental implantation site to reduce blood loss. Among the patients receiving vasopressin in- jection, the blood loss was 1,149 mL. Among 50 women with placenta previa who did not receive vasopressin injection, the blood loss was 1,634 mL.27
Obstetric surgery and vasopressin: The time has come
As obstetricians and gynecologists we constantly strive to improve the effectiveness of our surgical procedures and reduce adverse outcomes, including infection and blood loss. The use of vasopressin is widely accepted in gynecologic surgery as an adjuvant that reduces blood loss. The time has come to expand the use of vasopressin in difficult obstetric surgery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97:867–872.
- Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Brit J Obstet Gynaecol. 1994;101:435–437.
- Assaf A. Adhesions after laparoscopic myomectomy effect of the technique used. Gynaecol Endosc. 1999;8(4):225–229.
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertil Steril. 2011;95(2):762–766.
- Sabol ED, Gibson JL, Bowes WA Jr. Vasopressin injection in cervical conization. A double-blind study. Obstet Gynecol. 1971;37(4):596–601.
- Martin-Hirsch PP, Keep SL, Bryant A. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;(6):CD001421.
- Schulz KE, Grimes DA, Christensen DD. Vasopressin reduces blood loss from second trimester dilatation and evacuation abortion. Lancet. 1985;2(8451):353–356.
- Akerlund M, Bossmar T, Brouard R, et al. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects in isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999;106(10):1047–1053.
- Akerlund M. Involvement of oxytocin and vasopressin in the pathophysiology of preterm labor and primary dysmenorrhea. Prog Brain Res. 2002;139:359–365.
- Helmer H, Hacki T, Schneeberger C, et al. Oxytocinand vasopressin 1a receptor gene expression in the cycling or pregnant human uterus. Am J Obstet Gynecol. 1998;179(6 pt 1):1572–1578.
- Wing DA, Goharkhay N, Felix JC, Rostamkhani M, Naidu YM, Kovacs BW. Expression of the oxytocin and V1a vasopressin receptors in human myometrium during differing physiological states and following misoprostol administration. Gynecol Obstet Invest. 2006;62(4):181–185.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014; (8):CD005355.
- Tinelli A, Malvasi A, Mynbaev OA, et al. The surgical outcome of intracapsular cesarean myomectomy. A match control study. J Matern Fetal Neonatal Med. 2014;27(1):66–71.
- Brown D, Fletcher HM, Myrie MO, Reid M. Caesarean myomectomy—a safe procedure. A retrospective case controlled study. J Obstet Gynaecol. 1999;19(2):139–141.
- Kaymak O, Ustunyrt E, Okyay RE, Kalyoncu S, Mollamahmutoglu L. Myomectomy during cesarean section. Int J Gynaecol Obstet. 2005;89(2):90–93.
- Park BJ, Kim YW. Safety of cesarean myomectomy. J Obstet Gynaecol Res. 2009;35(5):906–911.
- Kim YS, Choi SD, Bae DH. Risk factors for complications in patients undergoing myomectomy at the time of cesarean section. J Obstet Gynaecol Res. 2010;36(3):550–554.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynecol Obstet. 1999;67(3):189–190.
- Incebiyik A, Hilali NG, Camuzcuoglu A, Vural M, Camuzcuoglu H. Myomectomy during caesarean: a retrospective evaluation of 6 cases. Arch Gynecol Obstet. 2014;289(3):569–573.
- Cobellis L, Pecori E, Cobellis G. Hemostatic technique for myomectomy during cesarean section. Int J Gynaecol Obstet. 2002;79(3):261–262.
- Lee JH, Cho DH. Myomectomy using purse-string suture during cesarean section. Arch Gynecol Obstet. 2011;283(suppl 1):S35–S37.
- Desai BR, Patted SS, Pujar YV, Sheriqar BY, Das SR, Ruge JC. A novel technique of selective uterine devascularization before myomectomy at the time of cesarean section: a pilot study. Fertil Steril. 2010;94(1):362–364.
- Sapmaz E, Celik H, Altungul A. Bilateral ascending uterine artery ligation vs. tourniquet use for hemostasis in cesarean myomectomy. A comparison. J Reprod Med. 2003;48(12):950–954.
- Sparic R, Malvasi A, Kadija S, Babovic I, Nejkovic L, Tinelli A. Cesarean myomectomy trends and controveries: an appraisal [published online ahead of print July 17, 2016]. J Matern Fetal Neonatal Med. doi:10.1080/14767058.2016.1205024.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing cesarean section. J Obstet Gynaecol Res. 2010;36(2):284–290.
- Kato S, Tanabe A, Kanki K, et al. Local injection of vasopressin reduces the blood loss during cesarean section in placenta previa. J Obstet Gynaecol Res. 2014;40(5):1249–1256.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97:867–872.
- Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Brit J Obstet Gynaecol. 1994;101:435–437.
- Assaf A. Adhesions after laparoscopic myomectomy effect of the technique used. Gynaecol Endosc. 1999;8(4):225–229.
- Zhao F, Jiao Y, Guo Z, Hou R, Wang M. Evaluation of loop ligation of larger myoma pseudocapsule combined with vasopressin on laparoscopic myomectomy. Fertil Steril. 2011;95(2):762–766.
- Sabol ED, Gibson JL, Bowes WA Jr. Vasopressin injection in cervical conization. A double-blind study. Obstet Gynecol. 1971;37(4):596–601.
- Martin-Hirsch PP, Keep SL, Bryant A. Interventions for preventing blood loss during the treatment of cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;(6):CD001421.
- Schulz KE, Grimes DA, Christensen DD. Vasopressin reduces blood loss from second trimester dilatation and evacuation abortion. Lancet. 1985;2(8451):353–356.
- Akerlund M, Bossmar T, Brouard R, et al. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects in isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999;106(10):1047–1053.
- Akerlund M. Involvement of oxytocin and vasopressin in the pathophysiology of preterm labor and primary dysmenorrhea. Prog Brain Res. 2002;139:359–365.
- Helmer H, Hacki T, Schneeberger C, et al. Oxytocinand vasopressin 1a receptor gene expression in the cycling or pregnant human uterus. Am J Obstet Gynecol. 1998;179(6 pt 1):1572–1578.
- Wing DA, Goharkhay N, Felix JC, Rostamkhani M, Naidu YM, Kovacs BW. Expression of the oxytocin and V1a vasopressin receptors in human myometrium during differing physiological states and following misoprostol administration. Gynecol Obstet Invest. 2006;62(4):181–185.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014; (8):CD005355.
- Tinelli A, Malvasi A, Mynbaev OA, et al. The surgical outcome of intracapsular cesarean myomectomy. A match control study. J Matern Fetal Neonatal Med. 2014;27(1):66–71.
- Brown D, Fletcher HM, Myrie MO, Reid M. Caesarean myomectomy—a safe procedure. A retrospective case controlled study. J Obstet Gynaecol. 1999;19(2):139–141.
- Kaymak O, Ustunyrt E, Okyay RE, Kalyoncu S, Mollamahmutoglu L. Myomectomy during cesarean section. Int J Gynaecol Obstet. 2005;89(2):90–93.
- Park BJ, Kim YW. Safety of cesarean myomectomy. J Obstet Gynaecol Res. 2009;35(5):906–911.
- Kim YS, Choi SD, Bae DH. Risk factors for complications in patients undergoing myomectomy at the time of cesarean section. J Obstet Gynaecol Res. 2010;36(3):550–554.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynecol Obstet. 1999;67(3):189–190.
- Incebiyik A, Hilali NG, Camuzcuoglu A, Vural M, Camuzcuoglu H. Myomectomy during caesarean: a retrospective evaluation of 6 cases. Arch Gynecol Obstet. 2014;289(3):569–573.
- Cobellis L, Pecori E, Cobellis G. Hemostatic technique for myomectomy during cesarean section. Int J Gynaecol Obstet. 2002;79(3):261–262.
- Lee JH, Cho DH. Myomectomy using purse-string suture during cesarean section. Arch Gynecol Obstet. 2011;283(suppl 1):S35–S37.
- Desai BR, Patted SS, Pujar YV, Sheriqar BY, Das SR, Ruge JC. A novel technique of selective uterine devascularization before myomectomy at the time of cesarean section: a pilot study. Fertil Steril. 2010;94(1):362–364.
- Sapmaz E, Celik H, Altungul A. Bilateral ascending uterine artery ligation vs. tourniquet use for hemostasis in cesarean myomectomy. A comparison. J Reprod Med. 2003;48(12):950–954.
- Sparic R, Malvasi A, Kadija S, Babovic I, Nejkovic L, Tinelli A. Cesarean myomectomy trends and controveries: an appraisal [published online ahead of print July 17, 2016]. J Matern Fetal Neonatal Med. doi:10.1080/14767058.2016.1205024.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing cesarean section. J Obstet Gynaecol Res. 2010;36(2):284–290.
- Kato S, Tanabe A, Kanki K, et al. Local injection of vasopressin reduces the blood loss during cesarean section in placenta previa. J Obstet Gynaecol Res. 2014;40(5):1249–1256.
Have you measured lactate in your sick obstetrics and gynecology patients during the past year?
Lactate measurement is widely used in emergency departments (EDs) and intensive care units (ICUs) to facilitate the early diagnosis and management of sepsis, severe trauma, ischemic bowel, and necrotizing fasciitis. Measuring lactate levels is much less commonly utilized in the practice of obstetrics and gynecology; increasing measurement in our practices may improve our early recognition and treatment of women with severe sepsis and other serious diseases.
Lactate physiology
The metabolism of glucose in the Embden-Meyerhof pathway results in the production of pyruvate and the high-energy compounds ATP and NADH. Pyruvate can enter 3 alternative metabolic pathways: 1) the mitochondrial Krebs cycle, 2) conversion to lactate in the cell cytosol, or 3) conversion back to glucose in the process of gluconeogenesis.
Under aerobic conditions, most pyruvate enters the Krebs cycle and little is converted to lactate. Molecular oxygen is an absolute requirement for Krebs cycle activity. Under anaerobic conditions, pyruvate cannot enter the Krebs cycle and is preferentially converted to lactate.1
An elevated lactate level is a sensitive marker for tissue hypoxia caused by a variety of diseases, including sepsis, trauma, ischemic bowel, and necrotizing fasciitis. With sepsis, additional mechanisms also contribute to the increase in lactate, including increased glycolysis, impaired lactate clearance, and activation of inflammatory cells that shift cellular metabolism toward lactate production.2,3
The normal range for venous plasma lactate in adults is 0.5 to 2.2 mM, although the normal range may vary because of differences in local laboratory methods. Arterial, capillary, and venous lactate are all highly positively correlated.4 Venous lactate concentrations between 2.3 and 3.9 mM are suggestive of mild physiologic dysfunction, and values ≥4.0 mM are consistent with severe physiologic dysfunction. In hospitalized patients, sepsis is one of the most common causes of a lactate level ≥4 mM.5
In many patients with an elevated lactate concentration the anion gap is also increased—but this is not always the case. In fact, in one large observational study, among patients with sepsis and a lactate concentration ≥4 mM, approximately 25% had a normal bicarbonate level and normal anion gap.6
Elevated lactate levels applied in obstetric and gynecologic practice
CASE 1. Obstetric practice: Hernia identified during labor
A 30-year-old woman (G1P0) presents in early labor at 37 weeks’ gestation. Two years prior to the pregnancy she had a Roux-en-Y gastric bypass and lost more than 100 lb. In addition to reporting lower abdominal pain occurring during contractions, she reports the new onset of mid-epigastric pain. A surgical consult is requested. The initial white blood cell count is 6,290 per uL, and the lactate level is 1.0 mM.
The surgeon consulted orders a computed tomography (CT) scan with oral contrast, but the patient has difficulty retaining the oral contrast due to her nausea, delaying the performance of the CT scan. Three hours following admission a follow-up lactate measurement is 3.3 mM, and an emergency CT scan is performed.
The CT scan shows an internal hernia with swirling of the mesenteric vessels and twisting of the small bowel mesentery. An urgent cesarean delivery and repair of the internal hernia is performed.
The patient and her newborn do well postoperatively. The postoperative lactate level is 0.8 mM.
In pregnant women with a past history of a Roux-en-Y gastric bypass and abdominal discomfort who are in labor it is challenging to rapidly diagnose internal hernias and other bowel problems.7−9 In this case, the increased lactate level from 1.0 mM to 3.3 mM raised concern for ischemic bowel and triggered the emergency CT and urgent exploratory laparotomy and cesarean delivery.
Up to 14% of maternal deaths in the United States are due to infection.10 In many of these cases, there is a delay in sepsis recognition because previously healthy pregnant women with sepsis may not manifest classic signs such as fever, hypotension, or mental status changes until late in the disease course. Measurement of lactate can facilitate the early recognition of severe sepsis in pregnant women, thereby accelerating and focusing their treatment.11
To reduce mortality due to sepsis, aggressive intervention needs to occur within the first 6 hours following the onset of the infection.
CASE 2. Gynecologic practice: Bacterial infection identified in the presence of abdominal pain and vomiting
A 40-year-old woman presents to the ED 5 days following a myomectomy, with nausea, vomiting, and abdominal pain. Her vital signs reveal: temperature, 98.4°F (36.9°C); heart rate, 122 bpm; blood pressure, 115/70 mm Hg; and white blood cell count, 6,270 per uL. Her lactate level is 4.0 mM. She is admitted to the ICU with a presumptive diagnosis of severe sepsis and treatment with broad-spectrum antibiotics is initiated. Twenty-four hours following admission, gram-negative rods are identified in blood cultures that are later identified to be Bacteroides fragilis.
For the past 2 decades there has been a concerted national effort to reduce mortality caused by sepsis through early diagnosis and aggressive treatment of sepsis in an ICU setting. Observational studies have reported that an elevated lactate level is an excellent early biomarker for sepsis and may be observed prior to the onset of fever, elevated white blood cell count, or hypotension.6 For example, in one large study of patients with sepsis and a lactate measurement ≥4 mM, only 50% of patients had a systolic blood pressure <90 mm Hg.
Elevated lactate levels also are associated with an increased risk of death. Among 13,932 consecutive patients admitted to an ICU in Alberta, Canada, the mortality rate among patients with a venous or arterial lactate >2 mM was 20%, compared with a mortality rate of 5% for patients with a lactate level ≤2 mM.12 In a study of 1,278 patients with infection admitted to the hospital from the ED, mortality increased as baseline lactate concentration rose. For lactate concentrations of 0 to 2.4, 2.5 to 3.9, and ≥4.0 mM, mortality rates were 5%, 9%, and 28%, respectively.13
In patients with sepsis, serial measurement of lactate can help to guide treatment. In a randomized trial, 348 patients admitted to an ICU with a lactate ≥3 mM were randomly assigned to standard treatment, in which the clinicians had no knowledge of patients’ lactate levels, or to an experimental group, in which the clinicians were provided lactate measurement results every 2 hours. Compared with clinicians in the control group, the clinicians with access to frequent lactate measurements administered more fluids and vasodilators to their patients. Compared with patients in the control group, the hospital mortality rate was lower when the clinicians had access to frequent lactate measurements (34% vs 44%, respectively; adjusted hazard ratio, 0.61; 95% confidence interval, 0.43−0.87; P = .0006).14
Elevated lactate levels in the fetus and newborn
The physiologic status of the newborn is routinely assessed with the Apgar score. Umbilical artery and venous blood gases, including measurement of pH, are often used as a corroborating biomarker. Most studies report that umbilical artery or vein lactate measurement is as useful as a pH measurement in assessing newborn physiologic status. The normal range of lactate in fetuses and newborns is not precisely defined, with values between 3.5 and 7 mM being cited as the upper limit of normal.15−18
In many countries (but not the United States), in utero fetal status during labor is assessed by fetal scalp sampling of blood and measurement of either pH or lactate. Fetal scalp sampling is difficult and often very little blood is obtained, making it difficult to measure pH. A Cochrane review reported that in 2 randomized trials, fetal scalp sampling produced a successful measurement of lactate in 99% of attempts, while a pH result could only be obtained in 79% of cases due to an inadequate volume of blood or clotted blood.19
Increased lactate measurement can help our patients
Measuring lactate in order to rapidly identify patients with major physiologic derangements is practiced widely in EDs and ICUs. There is significant opportunity to increase the use of lactate measurement in obstetrics and gynecology. Increasing this use will help to rapidly identify women with severe sepsis and other diseases, leading to more rapid intervention and improved outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clinic Proc. 2013;88(10):1127−1140.
- Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care. 2015;3:39.
- Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149(1):252−261.
- Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74.
- Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567−573.
- Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368−1377.
- Caranta DG, Lee AM, Pennington D, Zelig CM. Complications from Roux-en-Y gastric bypass mistaken for medical complications in gravid patients. Obstet Gynecol. 2014;124(2 part 2 suppl 1):464−466.
- Moore KA, Ouyang DW, Whang EE. Maternal and fetal deaths after gastric bypass surgery for morbid obesity. N Engl J Med. 2004;351(7):721−722.
- Loar PV 3rd, Sanchez-Ramos L, Kaunitz AM, Kerwin AJ, Diaz J. Maternal death caused by midgut volvulus after bariatric surgery. Am J Obstet Gynecol. 2005;193(5):1748−1749.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
- Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol. 2015;32(5):481−486.
- Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperprolactinemia in the critically ill. Crit Care. 2009;13(3):R90.
- Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524−528.
- Jansen TC, van Bommel J, Schoonderbeek FJ, et al; LACTATE Study Group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752−761.
- Suidan JS, Young BK. Outcome of fetuses with lactic acidemia. Am J Obstet Gynecol. 1984;150(1):33−37.
- Tuuli MG, Stout MJ, Macones GA, Cahill AG. Umbilical cord venous lactate for predicting arterial lactic acidemia and neonatal morbidity at term. Obstet Gynecol. 2016;127(4):674−680.
- Shirey T, St. Pierre J, Winkelman J. Cord lactate, pH, and blood gases from healthy neonates. Gynecol Obstet Invest. 1996;41(1):15−19.
- Heinis AM, Spaanderman ME, Gunnewiek JM, Lotgering FK. Scalp blood lactate for intra-partum assessment of fetal metabolic acidosis. Acta Obstet Gynecol Scand. 2011;90(10):1107−1114.
- East CE, Leader LR, Sheehan P, Henshall NE, Colditz PB, Lau R. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev. 2015;(5):CD006174.
Lactate measurement is widely used in emergency departments (EDs) and intensive care units (ICUs) to facilitate the early diagnosis and management of sepsis, severe trauma, ischemic bowel, and necrotizing fasciitis. Measuring lactate levels is much less commonly utilized in the practice of obstetrics and gynecology; increasing measurement in our practices may improve our early recognition and treatment of women with severe sepsis and other serious diseases.
Lactate physiology
The metabolism of glucose in the Embden-Meyerhof pathway results in the production of pyruvate and the high-energy compounds ATP and NADH. Pyruvate can enter 3 alternative metabolic pathways: 1) the mitochondrial Krebs cycle, 2) conversion to lactate in the cell cytosol, or 3) conversion back to glucose in the process of gluconeogenesis.
Under aerobic conditions, most pyruvate enters the Krebs cycle and little is converted to lactate. Molecular oxygen is an absolute requirement for Krebs cycle activity. Under anaerobic conditions, pyruvate cannot enter the Krebs cycle and is preferentially converted to lactate.1
An elevated lactate level is a sensitive marker for tissue hypoxia caused by a variety of diseases, including sepsis, trauma, ischemic bowel, and necrotizing fasciitis. With sepsis, additional mechanisms also contribute to the increase in lactate, including increased glycolysis, impaired lactate clearance, and activation of inflammatory cells that shift cellular metabolism toward lactate production.2,3
The normal range for venous plasma lactate in adults is 0.5 to 2.2 mM, although the normal range may vary because of differences in local laboratory methods. Arterial, capillary, and venous lactate are all highly positively correlated.4 Venous lactate concentrations between 2.3 and 3.9 mM are suggestive of mild physiologic dysfunction, and values ≥4.0 mM are consistent with severe physiologic dysfunction. In hospitalized patients, sepsis is one of the most common causes of a lactate level ≥4 mM.5
In many patients with an elevated lactate concentration the anion gap is also increased—but this is not always the case. In fact, in one large observational study, among patients with sepsis and a lactate concentration ≥4 mM, approximately 25% had a normal bicarbonate level and normal anion gap.6
Elevated lactate levels applied in obstetric and gynecologic practice
CASE 1. Obstetric practice: Hernia identified during labor
A 30-year-old woman (G1P0) presents in early labor at 37 weeks’ gestation. Two years prior to the pregnancy she had a Roux-en-Y gastric bypass and lost more than 100 lb. In addition to reporting lower abdominal pain occurring during contractions, she reports the new onset of mid-epigastric pain. A surgical consult is requested. The initial white blood cell count is 6,290 per uL, and the lactate level is 1.0 mM.
The surgeon consulted orders a computed tomography (CT) scan with oral contrast, but the patient has difficulty retaining the oral contrast due to her nausea, delaying the performance of the CT scan. Three hours following admission a follow-up lactate measurement is 3.3 mM, and an emergency CT scan is performed.
The CT scan shows an internal hernia with swirling of the mesenteric vessels and twisting of the small bowel mesentery. An urgent cesarean delivery and repair of the internal hernia is performed.
The patient and her newborn do well postoperatively. The postoperative lactate level is 0.8 mM.
In pregnant women with a past history of a Roux-en-Y gastric bypass and abdominal discomfort who are in labor it is challenging to rapidly diagnose internal hernias and other bowel problems.7−9 In this case, the increased lactate level from 1.0 mM to 3.3 mM raised concern for ischemic bowel and triggered the emergency CT and urgent exploratory laparotomy and cesarean delivery.
Up to 14% of maternal deaths in the United States are due to infection.10 In many of these cases, there is a delay in sepsis recognition because previously healthy pregnant women with sepsis may not manifest classic signs such as fever, hypotension, or mental status changes until late in the disease course. Measurement of lactate can facilitate the early recognition of severe sepsis in pregnant women, thereby accelerating and focusing their treatment.11
To reduce mortality due to sepsis, aggressive intervention needs to occur within the first 6 hours following the onset of the infection.
CASE 2. Gynecologic practice: Bacterial infection identified in the presence of abdominal pain and vomiting
A 40-year-old woman presents to the ED 5 days following a myomectomy, with nausea, vomiting, and abdominal pain. Her vital signs reveal: temperature, 98.4°F (36.9°C); heart rate, 122 bpm; blood pressure, 115/70 mm Hg; and white blood cell count, 6,270 per uL. Her lactate level is 4.0 mM. She is admitted to the ICU with a presumptive diagnosis of severe sepsis and treatment with broad-spectrum antibiotics is initiated. Twenty-four hours following admission, gram-negative rods are identified in blood cultures that are later identified to be Bacteroides fragilis.
For the past 2 decades there has been a concerted national effort to reduce mortality caused by sepsis through early diagnosis and aggressive treatment of sepsis in an ICU setting. Observational studies have reported that an elevated lactate level is an excellent early biomarker for sepsis and may be observed prior to the onset of fever, elevated white blood cell count, or hypotension.6 For example, in one large study of patients with sepsis and a lactate measurement ≥4 mM, only 50% of patients had a systolic blood pressure <90 mm Hg.
Elevated lactate levels also are associated with an increased risk of death. Among 13,932 consecutive patients admitted to an ICU in Alberta, Canada, the mortality rate among patients with a venous or arterial lactate >2 mM was 20%, compared with a mortality rate of 5% for patients with a lactate level ≤2 mM.12 In a study of 1,278 patients with infection admitted to the hospital from the ED, mortality increased as baseline lactate concentration rose. For lactate concentrations of 0 to 2.4, 2.5 to 3.9, and ≥4.0 mM, mortality rates were 5%, 9%, and 28%, respectively.13
In patients with sepsis, serial measurement of lactate can help to guide treatment. In a randomized trial, 348 patients admitted to an ICU with a lactate ≥3 mM were randomly assigned to standard treatment, in which the clinicians had no knowledge of patients’ lactate levels, or to an experimental group, in which the clinicians were provided lactate measurement results every 2 hours. Compared with clinicians in the control group, the clinicians with access to frequent lactate measurements administered more fluids and vasodilators to their patients. Compared with patients in the control group, the hospital mortality rate was lower when the clinicians had access to frequent lactate measurements (34% vs 44%, respectively; adjusted hazard ratio, 0.61; 95% confidence interval, 0.43−0.87; P = .0006).14
Elevated lactate levels in the fetus and newborn
The physiologic status of the newborn is routinely assessed with the Apgar score. Umbilical artery and venous blood gases, including measurement of pH, are often used as a corroborating biomarker. Most studies report that umbilical artery or vein lactate measurement is as useful as a pH measurement in assessing newborn physiologic status. The normal range of lactate in fetuses and newborns is not precisely defined, with values between 3.5 and 7 mM being cited as the upper limit of normal.15−18
In many countries (but not the United States), in utero fetal status during labor is assessed by fetal scalp sampling of blood and measurement of either pH or lactate. Fetal scalp sampling is difficult and often very little blood is obtained, making it difficult to measure pH. A Cochrane review reported that in 2 randomized trials, fetal scalp sampling produced a successful measurement of lactate in 99% of attempts, while a pH result could only be obtained in 79% of cases due to an inadequate volume of blood or clotted blood.19
Increased lactate measurement can help our patients
Measuring lactate in order to rapidly identify patients with major physiologic derangements is practiced widely in EDs and ICUs. There is significant opportunity to increase the use of lactate measurement in obstetrics and gynecology. Increasing this use will help to rapidly identify women with severe sepsis and other diseases, leading to more rapid intervention and improved outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Lactate measurement is widely used in emergency departments (EDs) and intensive care units (ICUs) to facilitate the early diagnosis and management of sepsis, severe trauma, ischemic bowel, and necrotizing fasciitis. Measuring lactate levels is much less commonly utilized in the practice of obstetrics and gynecology; increasing measurement in our practices may improve our early recognition and treatment of women with severe sepsis and other serious diseases.
Lactate physiology
The metabolism of glucose in the Embden-Meyerhof pathway results in the production of pyruvate and the high-energy compounds ATP and NADH. Pyruvate can enter 3 alternative metabolic pathways: 1) the mitochondrial Krebs cycle, 2) conversion to lactate in the cell cytosol, or 3) conversion back to glucose in the process of gluconeogenesis.
Under aerobic conditions, most pyruvate enters the Krebs cycle and little is converted to lactate. Molecular oxygen is an absolute requirement for Krebs cycle activity. Under anaerobic conditions, pyruvate cannot enter the Krebs cycle and is preferentially converted to lactate.1
An elevated lactate level is a sensitive marker for tissue hypoxia caused by a variety of diseases, including sepsis, trauma, ischemic bowel, and necrotizing fasciitis. With sepsis, additional mechanisms also contribute to the increase in lactate, including increased glycolysis, impaired lactate clearance, and activation of inflammatory cells that shift cellular metabolism toward lactate production.2,3
The normal range for venous plasma lactate in adults is 0.5 to 2.2 mM, although the normal range may vary because of differences in local laboratory methods. Arterial, capillary, and venous lactate are all highly positively correlated.4 Venous lactate concentrations between 2.3 and 3.9 mM are suggestive of mild physiologic dysfunction, and values ≥4.0 mM are consistent with severe physiologic dysfunction. In hospitalized patients, sepsis is one of the most common causes of a lactate level ≥4 mM.5
In many patients with an elevated lactate concentration the anion gap is also increased—but this is not always the case. In fact, in one large observational study, among patients with sepsis and a lactate concentration ≥4 mM, approximately 25% had a normal bicarbonate level and normal anion gap.6
Elevated lactate levels applied in obstetric and gynecologic practice
CASE 1. Obstetric practice: Hernia identified during labor
A 30-year-old woman (G1P0) presents in early labor at 37 weeks’ gestation. Two years prior to the pregnancy she had a Roux-en-Y gastric bypass and lost more than 100 lb. In addition to reporting lower abdominal pain occurring during contractions, she reports the new onset of mid-epigastric pain. A surgical consult is requested. The initial white blood cell count is 6,290 per uL, and the lactate level is 1.0 mM.
The surgeon consulted orders a computed tomography (CT) scan with oral contrast, but the patient has difficulty retaining the oral contrast due to her nausea, delaying the performance of the CT scan. Three hours following admission a follow-up lactate measurement is 3.3 mM, and an emergency CT scan is performed.
The CT scan shows an internal hernia with swirling of the mesenteric vessels and twisting of the small bowel mesentery. An urgent cesarean delivery and repair of the internal hernia is performed.
The patient and her newborn do well postoperatively. The postoperative lactate level is 0.8 mM.
In pregnant women with a past history of a Roux-en-Y gastric bypass and abdominal discomfort who are in labor it is challenging to rapidly diagnose internal hernias and other bowel problems.7−9 In this case, the increased lactate level from 1.0 mM to 3.3 mM raised concern for ischemic bowel and triggered the emergency CT and urgent exploratory laparotomy and cesarean delivery.
Up to 14% of maternal deaths in the United States are due to infection.10 In many of these cases, there is a delay in sepsis recognition because previously healthy pregnant women with sepsis may not manifest classic signs such as fever, hypotension, or mental status changes until late in the disease course. Measurement of lactate can facilitate the early recognition of severe sepsis in pregnant women, thereby accelerating and focusing their treatment.11
To reduce mortality due to sepsis, aggressive intervention needs to occur within the first 6 hours following the onset of the infection.
CASE 2. Gynecologic practice: Bacterial infection identified in the presence of abdominal pain and vomiting
A 40-year-old woman presents to the ED 5 days following a myomectomy, with nausea, vomiting, and abdominal pain. Her vital signs reveal: temperature, 98.4°F (36.9°C); heart rate, 122 bpm; blood pressure, 115/70 mm Hg; and white blood cell count, 6,270 per uL. Her lactate level is 4.0 mM. She is admitted to the ICU with a presumptive diagnosis of severe sepsis and treatment with broad-spectrum antibiotics is initiated. Twenty-four hours following admission, gram-negative rods are identified in blood cultures that are later identified to be Bacteroides fragilis.
For the past 2 decades there has been a concerted national effort to reduce mortality caused by sepsis through early diagnosis and aggressive treatment of sepsis in an ICU setting. Observational studies have reported that an elevated lactate level is an excellent early biomarker for sepsis and may be observed prior to the onset of fever, elevated white blood cell count, or hypotension.6 For example, in one large study of patients with sepsis and a lactate measurement ≥4 mM, only 50% of patients had a systolic blood pressure <90 mm Hg.
Elevated lactate levels also are associated with an increased risk of death. Among 13,932 consecutive patients admitted to an ICU in Alberta, Canada, the mortality rate among patients with a venous or arterial lactate >2 mM was 20%, compared with a mortality rate of 5% for patients with a lactate level ≤2 mM.12 In a study of 1,278 patients with infection admitted to the hospital from the ED, mortality increased as baseline lactate concentration rose. For lactate concentrations of 0 to 2.4, 2.5 to 3.9, and ≥4.0 mM, mortality rates were 5%, 9%, and 28%, respectively.13
In patients with sepsis, serial measurement of lactate can help to guide treatment. In a randomized trial, 348 patients admitted to an ICU with a lactate ≥3 mM were randomly assigned to standard treatment, in which the clinicians had no knowledge of patients’ lactate levels, or to an experimental group, in which the clinicians were provided lactate measurement results every 2 hours. Compared with clinicians in the control group, the clinicians with access to frequent lactate measurements administered more fluids and vasodilators to their patients. Compared with patients in the control group, the hospital mortality rate was lower when the clinicians had access to frequent lactate measurements (34% vs 44%, respectively; adjusted hazard ratio, 0.61; 95% confidence interval, 0.43−0.87; P = .0006).14
Elevated lactate levels in the fetus and newborn
The physiologic status of the newborn is routinely assessed with the Apgar score. Umbilical artery and venous blood gases, including measurement of pH, are often used as a corroborating biomarker. Most studies report that umbilical artery or vein lactate measurement is as useful as a pH measurement in assessing newborn physiologic status. The normal range of lactate in fetuses and newborns is not precisely defined, with values between 3.5 and 7 mM being cited as the upper limit of normal.15−18
In many countries (but not the United States), in utero fetal status during labor is assessed by fetal scalp sampling of blood and measurement of either pH or lactate. Fetal scalp sampling is difficult and often very little blood is obtained, making it difficult to measure pH. A Cochrane review reported that in 2 randomized trials, fetal scalp sampling produced a successful measurement of lactate in 99% of attempts, while a pH result could only be obtained in 79% of cases due to an inadequate volume of blood or clotted blood.19
Increased lactate measurement can help our patients
Measuring lactate in order to rapidly identify patients with major physiologic derangements is practiced widely in EDs and ICUs. There is significant opportunity to increase the use of lactate measurement in obstetrics and gynecology. Increasing this use will help to rapidly identify women with severe sepsis and other diseases, leading to more rapid intervention and improved outcomes.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clinic Proc. 2013;88(10):1127−1140.
- Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care. 2015;3:39.
- Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149(1):252−261.
- Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74.
- Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567−573.
- Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368−1377.
- Caranta DG, Lee AM, Pennington D, Zelig CM. Complications from Roux-en-Y gastric bypass mistaken for medical complications in gravid patients. Obstet Gynecol. 2014;124(2 part 2 suppl 1):464−466.
- Moore KA, Ouyang DW, Whang EE. Maternal and fetal deaths after gastric bypass surgery for morbid obesity. N Engl J Med. 2004;351(7):721−722.
- Loar PV 3rd, Sanchez-Ramos L, Kaunitz AM, Kerwin AJ, Diaz J. Maternal death caused by midgut volvulus after bariatric surgery. Am J Obstet Gynecol. 2005;193(5):1748−1749.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
- Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol. 2015;32(5):481−486.
- Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperprolactinemia in the critically ill. Crit Care. 2009;13(3):R90.
- Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524−528.
- Jansen TC, van Bommel J, Schoonderbeek FJ, et al; LACTATE Study Group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752−761.
- Suidan JS, Young BK. Outcome of fetuses with lactic acidemia. Am J Obstet Gynecol. 1984;150(1):33−37.
- Tuuli MG, Stout MJ, Macones GA, Cahill AG. Umbilical cord venous lactate for predicting arterial lactic acidemia and neonatal morbidity at term. Obstet Gynecol. 2016;127(4):674−680.
- Shirey T, St. Pierre J, Winkelman J. Cord lactate, pH, and blood gases from healthy neonates. Gynecol Obstet Invest. 1996;41(1):15−19.
- Heinis AM, Spaanderman ME, Gunnewiek JM, Lotgering FK. Scalp blood lactate for intra-partum assessment of fetal metabolic acidosis. Acta Obstet Gynecol Scand. 2011;90(10):1107−1114.
- East CE, Leader LR, Sheehan P, Henshall NE, Colditz PB, Lau R. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev. 2015;(5):CD006174.
- Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clinic Proc. 2013;88(10):1127−1140.
- Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care. 2015;3:39.
- Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149(1):252−261.
- Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74.
- Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567−573.
- Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368−1377.
- Caranta DG, Lee AM, Pennington D, Zelig CM. Complications from Roux-en-Y gastric bypass mistaken for medical complications in gravid patients. Obstet Gynecol. 2014;124(2 part 2 suppl 1):464−466.
- Moore KA, Ouyang DW, Whang EE. Maternal and fetal deaths after gastric bypass surgery for morbid obesity. N Engl J Med. 2004;351(7):721−722.
- Loar PV 3rd, Sanchez-Ramos L, Kaunitz AM, Kerwin AJ, Diaz J. Maternal death caused by midgut volvulus after bariatric surgery. Am J Obstet Gynecol. 2005;193(5):1748−1749.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
- Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol. 2015;32(5):481−486.
- Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperprolactinemia in the critically ill. Crit Care. 2009;13(3):R90.
- Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524−528.
- Jansen TC, van Bommel J, Schoonderbeek FJ, et al; LACTATE Study Group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752−761.
- Suidan JS, Young BK. Outcome of fetuses with lactic acidemia. Am J Obstet Gynecol. 1984;150(1):33−37.
- Tuuli MG, Stout MJ, Macones GA, Cahill AG. Umbilical cord venous lactate for predicting arterial lactic acidemia and neonatal morbidity at term. Obstet Gynecol. 2016;127(4):674−680.
- Shirey T, St. Pierre J, Winkelman J. Cord lactate, pH, and blood gases from healthy neonates. Gynecol Obstet Invest. 1996;41(1):15−19.
- Heinis AM, Spaanderman ME, Gunnewiek JM, Lotgering FK. Scalp blood lactate for intra-partum assessment of fetal metabolic acidosis. Acta Obstet Gynecol Scand. 2011;90(10):1107−1114.
- East CE, Leader LR, Sheehan P, Henshall NE, Colditz PB, Lau R. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev. 2015;(5):CD006174.
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.
The TCu380A copper-IUD is effective up to 12 years
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13
Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.

The TCu380A copper-IUD is effective up to 12 years
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13

Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
One of the most important medical interventions to improve maternal-child health is providing effective contraception to men and women of reproductive age. The 52-mg levonorgestrel-intrauterine device (LNG-IUD; Mirena) is one of the most effective forms of reversible contraception available to women, with a failure rate of 1.1% over 5 years of use.1 The TCu380A copper-IUD (ParaGard), another highly effective reversible contraceptive, is reported to have failure rates of approximately 1.4% and 2.2%, over 5 and 10 years of use.2
An interesting question is whether—in certain clinical situations—a single IUD can be used for longer than the currently recommended 5 and 10 years for a Mirena IUD and a ParaGard IUD, respectively.
The LNG-IUD containing 52 mg LNG may be effective up to 7 years
The US Food and Drug Administration (FDA) package insert for the Mirena 52-mg LNG-IUD states that the device is “indicated for contraception for up to 5 years. Thereafter if continued contraception is desired, the system should be replaced.”1 The FDA package insert for the levonorgestrel-releasing intrauterine system, Liletta 52-mg LNG-IUD, states that it is “indicated for prevention of pregnancy up to 3 years.”3 The FDA guidance is based on data submitted to the agency by the manufacturers to support the approval process. Completing large-scale clinical trials that extend past 5 years or more is challenging, because of the cost and the loss of study participants to follow-up. Hence, few clinical trials of contraceptive IUDs continue for more than 5 to 10 years.
Although the FDA-approved indication for Mirena and Liletta is 5 and 3 years, respectively, evidence suggests that the 52-mg LNG-IUD is an effective contraceptive beyond 5 years. In fact, multiple studies report that this IUD is an effective contraceptive for at least 6 or 7 years (TABLE 1).4–9 Among 895 women using the 52-mg LNG-IUD for 6 to 7 years, only 1 pregnancy was reported in the last year of use. In that case, the IUD was in the cervix and partially expelled from the uterus.8 These data indicate that the 52-mg LNG-IUD is likely an effective contraceptive for up to 7 years, with pregnancy rates below 1% in the last year of use.

The TCu380A copper-IUD is effective up to 12 years
The currently available TCu380A copper-IUD (ParaGard) is FDA approved for 10 years.2 Studies evaluating the efficacy of this copper-IUD are limited, but those that have been published reported that it is effective for at least 12 years and possibly up to 20 years (TABLE 2).10−13

Recently I saw a patient who had a copper-IUD (ParaGard, TCu380A) inserted as a teen after a birth, and had successfully used the same device for 17 years. She presented for removal of the IUD so that she could attempt conception. After removal of the IUD, copper wire was visible on the device. Long-term studies of the TCu220 copper-IUD, which contains less copper than the ParaGard, report pregnancies with the use of the device beyond 10 years.12 These devices, which are not available in the United States, should not be used past their recommended interval.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.
- Mirena [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; July 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. Accessed July 28, 2016.
- ParaGard [package insert]. N. Tonawanda, NY: FEI Women’s Health LLC; revised September 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf. Accessed July 28, 2016.
- Liletta [package insert]. Parsippany, NJ: Actavis Pharma, Inc; February 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206229s000lbl.pdf. Accessed July 28, 2016.
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the copper T380 Ag IUDS. Contraception. 1991;44(5):473–480.
- Díaz J, Faúndes A, Díaz M, Marchi N. Evaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, Brazil. Contraception. 1993;47(2):169–175.
- Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78(8):716–721.
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, Bahamondes L. Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception. 2009;80(1):84–89.
- McNicholas C, Maddipati R, Zhao Q, Swor E, Peipert JF. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599–604.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498–506.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495–503.
- Bahamondes L, Faundes A, Sobreira-Lima B, Liu-Filho JF, Pecci P, Matera S. TCu 380A IUD: a reversible permanent contraceptive method in women over 35 years of age. Contraception. 2005;72(5):337–341.
- United Nations Development Programme. Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Sivin I. Utility and drawbacks of continuous use of a copper T IUD for 20 years. Contraception. 2007;75(6 suppl):S70–S75.
Protecting the newborn brain—the final frontier in obstetric and neonatal care
During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.
The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.
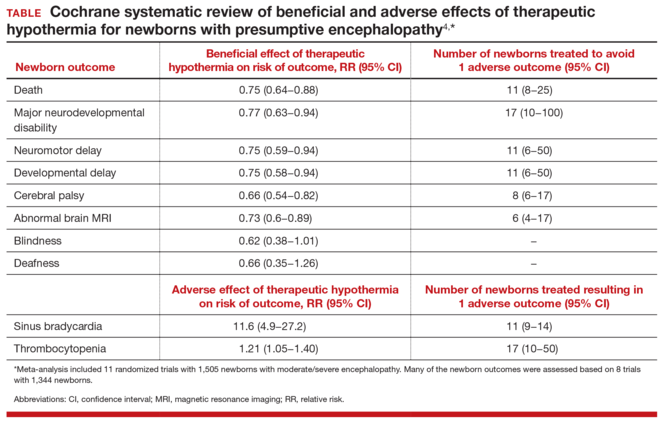
Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.
During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.

The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.

Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.

The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.

Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.
- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.
Stop using rectal misoprostol for the treatment of postpartum hemorrhage caused by uterine atony
Most authorities recommend that, following delivery, all women should receive a uterotonic medication to reduce the risk of postpartum hemorrhage (PPH).1 In the United States, the preferred uterotonic for this preventive effort is oxytocin—a low-cost, highly effective agent that typically is administered as an intravenous (IV) infusion or intramuscular (IM) injection. Unfortunately, even with the universal administration of oxytocin in the third stage of labor, PPH occurs in about 3% of vaginal deliveries.
A key decision in treating a PPH due to uterine atony is treatment with an optimal uterotonic. The options include:
- additional oxytocin
- carboprost tromethamine(Hemabate)
- methylergonovine (Methergine)
- misoprostol.
Many obstetricians choose rectal misoprostol alone or in combination with oxytocin as the preferred treatment of PPH. However, evidence from clinical trials and pharmacokinetic studies suggest that rectal misoprostol is not an optimal choice if parenteral uterotonics are available. Here I pre-sent this evidence and urge you to stop the practice of using rectal misoprostol in efforts to manage PPH.
RCTs do not support the use of rectal misoprostolRandomized clinical trials (RCTs) have not demonstrated that misoprostol is superior to oxytocin for the treatment of PPH caused by uterine atony.2 For example, Blum and colleagues studied 31,055 women who received oxytocin (by IV or IM route) at vaginal delivery and observed that 809 (3%) developed a PPH.3 The women who developed PPH were randomly assigned to treatment with misoprostol 800 µg sublingual or oxytocin 40 U in 1,000 mL as an IV infusion over 15 minutes.
Both oxytocin and misoprostol had similar efficacy for controlling bleeding within 20 minutes (90% and 89%, respectively). Fewer women had blood loss of 1,000 mL or greater when treated with oxytocin compared with misoprostol (1% vs 3%, respectively; P = .062). In addition, oxytocin was associated with fewer temperature elevations of 38°C (100.4°F) or above (15% vs 22% for misoprostol, P = .007) and fewer temperature elevations of 40°C (104°F) or above (0.2% vs 1.2% for misoprostol, P = .11).
In another trial, women with a vaginal delivery who were not treated with a uterotonic in the third stage were monitored for the development of a PPH.4 PPH did develop in 1,422 women, who were then randomly assigned to receive oxytocin (10 U IV or IM) plus a placebo tablet or oxytocin plus misoprostol (600 µg sublingual).
Comparing oxytocin alone versus oxytocin plus misoprostol, there was no difference in blood loss of 500 mL or greater after treatment initiation (14% vs 14%). However, 90 minutes following treatment, temperature elevations occurred much more often in the women who received oxytocin plus misoprostol compared with the women who received oxytocin alone (temperature ≥38°C: 58% vs 19%; temperature ≥40°C: 6.8% vs 0.4%).
Bottom line: If you have access to oxytocin, there is no advantage to using misoprostol to treat a PPH due to uterine atony.5
Rectal misoprostol does not achieve optimal circulating concentrations of the drugMisoprostol tablets are formulated for oral administration, not rectal administration. The studies in the TABLE show that rectal administration of misoprostol results in lower circulating concentration of the medication compared to oral, buccal, or vaginal administration.6−8 After rectal administration it takes about 60 minutes to reach the peak circulating concentration of misoprostol.6,7 By contrast, parenteral oxytocin, methylergonovine, and carboprost tromethamine reach peak serum concentration much more quickly after administration.

In a study of misoprostol stimulation of uterine contractility as measured by an intrauterine pressure catheter, buccal administration resulted in higher peak uterine tone than rectal administration (49 vs 31 mm Hg).8 In addition, time to onset of uterine contractility was 41 minutes and 103 minutes, respectively, for buccal and rectal administration.
These studies show that rectal misoprostol is associated with lower serum concentrations, longer time to onset of uterine contraction, and less contractility than buccal administration. The one advantage of rectal administration is that it has a longer duration of action than the oral, buccal, or sublingual routes. In pharmacokinetic comparisons of buccal versus sublingual administration of misoprostol, the sublingual route results in greater peak concentration, which may cause more adverse effects.9,10
Misoprostol is a useful uterotonic if parenteral agents are not available
Worldwide, approximately one maternal death occurs every 7 minutes. Postpartum hemorrhage (PPH) is a common cause of maternal death. Oxytocin, methylergonovine, and carboprost tromethamine should be stored in a refrigerated environment to ensure the stability and bioavailability of the drug. In settings in which reliable refrigeration is not available, misoprostol, a medication that is heat-stable, is often used to prevent and treat PPH.
One approach to preventing PPH is to provide 600 µg of misoprostol to women delivering at home without a skilled birth attendant that they can self- administer after the delivery.1,2 Another approach is to recommend that skilled birth attendants administer misoprostol following the delivery.3
Although I am recommending that we not use rectal misoprostol to treat PPH in the United States, it is clear that misoprostol plays an important role in preventing PPH in countries where parenteral uterotonics are not available. If a clinician in the United States was involved in a home birth complicated by PPH due to uterine atony, and if misoprostol was the only available uterotonic, it would be wise to administer it promptly.
References
- Rajbhandari S, Hodgins S, Sanghvi H, McPherson R, Pradhan YV, Baqui AH; Misoprostol Study Group. Expanding uterotonic protection following childbirth through community-based distribution of misoprostol: operations research study in Nepal. Int J Gynaecol Obstet. 2010;108(3):282–288.
- Sanghvi H, Ansari N, Prata NJ, Gibson H, Ehsan AT, Smith JM. Prevention of postpartum hemorrhage at home birth in Afghanistan. Int J Gynaecol Obstet. 2010;108(3):276–281.
- Prata N, Mbaruku G, Campbell M, Potts M, Vahidnia F. Controlling postpartum hemorrhage after home births in Tanzania. Int J Gynaecol Obstet. 2005;90(1):51–55.
Prioritize oxytocin, methergine, and carboprost tromethamineWhen treating PPH, administration of oxytocin, methylergonovine, or carboprost tromethamine rapidly provides therapeutic concentration of medication. For oxytocin, 40 U in 1 L, administered at a rate sufficient to control atony, or 10 U IM injection are often effective in controlling bleeding due to atony. Carboprost tromethamine 0.25 mg administered intramuscularly every 15 minutes up to 8 doses provides an excellent second-line agent. Carboprost tromethamine is contraindicated for women with asthma.
Methylergonovine 0.2 mg administered intramuscularly only can be given every 2 to 4 hours. Consequently, because time is of the essence in managing a severe PPH, it is unusual to be able to administer more than one dose of the agent during the course of treatment. Methylergonovine is contraindicated for women with hypertension.
There is scant evidence that misoprostol is more effective than oxytocin, and misoprostol clearly causes a higher rate of elevated temperature than any of the parenteral uterotonic agents. In your practice stop using rectal misoprostol for the treatment of PPH caused by uterine atony, and prioritize the use of parenteral uterotonics.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Gibbons KJ, Albright CM, Rouse DJ. Postpartum hemorrhage in the developed world: whither misoprostol? Am J Obstet Gynecol. 2013;208(3):181−183.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375(9710):217−223.
- Widmer M, Blum J, Hofmeyr GJ, et al. Misoprostolas an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010;375(9728):1808−1813.
- Weeks A. The prevention and treatment of postpartum hemorrhage: what do we know and where do we go to next? BJOG. 2015;122(2):202−210.
- Khan RU, El-Refaey H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynecol. 2003;101(5 pt 1):968−974.
- Khan RU, El-Refaey H, Sharma S, Sooranna D, Stafford M. Oral, rectal and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103(5 pt 1):866−870.
- Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 pt 1):582−590.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71(1):22−25.
- Frye LJ, Byrne ME, Winikoff B. A crossover pharmacokinetic study of misoprostol by the oral, sublingual and buccal routes [published online ahead of print April 22, 2016]. Eur J Contracept Reprod Health Care. doi:10.3109/13625187.2016.1168799.

Most authorities recommend that, following delivery, all women should receive a uterotonic medication to reduce the risk of postpartum hemorrhage (PPH).1 In the United States, the preferred uterotonic for this preventive effort is oxytocin—a low-cost, highly effective agent that typically is administered as an intravenous (IV) infusion or intramuscular (IM) injection. Unfortunately, even with the universal administration of oxytocin in the third stage of labor, PPH occurs in about 3% of vaginal deliveries.
A key decision in treating a PPH due to uterine atony is treatment with an optimal uterotonic. The options include:
- additional oxytocin
- carboprost tromethamine(Hemabate)
- methylergonovine (Methergine)
- misoprostol.
Many obstetricians choose rectal misoprostol alone or in combination with oxytocin as the preferred treatment of PPH. However, evidence from clinical trials and pharmacokinetic studies suggest that rectal misoprostol is not an optimal choice if parenteral uterotonics are available. Here I pre-sent this evidence and urge you to stop the practice of using rectal misoprostol in efforts to manage PPH.
RCTs do not support the use of rectal misoprostolRandomized clinical trials (RCTs) have not demonstrated that misoprostol is superior to oxytocin for the treatment of PPH caused by uterine atony.2 For example, Blum and colleagues studied 31,055 women who received oxytocin (by IV or IM route) at vaginal delivery and observed that 809 (3%) developed a PPH.3 The women who developed PPH were randomly assigned to treatment with misoprostol 800 µg sublingual or oxytocin 40 U in 1,000 mL as an IV infusion over 15 minutes.
Both oxytocin and misoprostol had similar efficacy for controlling bleeding within 20 minutes (90% and 89%, respectively). Fewer women had blood loss of 1,000 mL or greater when treated with oxytocin compared with misoprostol (1% vs 3%, respectively; P = .062). In addition, oxytocin was associated with fewer temperature elevations of 38°C (100.4°F) or above (15% vs 22% for misoprostol, P = .007) and fewer temperature elevations of 40°C (104°F) or above (0.2% vs 1.2% for misoprostol, P = .11).
In another trial, women with a vaginal delivery who were not treated with a uterotonic in the third stage were monitored for the development of a PPH.4 PPH did develop in 1,422 women, who were then randomly assigned to receive oxytocin (10 U IV or IM) plus a placebo tablet or oxytocin plus misoprostol (600 µg sublingual).
Comparing oxytocin alone versus oxytocin plus misoprostol, there was no difference in blood loss of 500 mL or greater after treatment initiation (14% vs 14%). However, 90 minutes following treatment, temperature elevations occurred much more often in the women who received oxytocin plus misoprostol compared with the women who received oxytocin alone (temperature ≥38°C: 58% vs 19%; temperature ≥40°C: 6.8% vs 0.4%).
Bottom line: If you have access to oxytocin, there is no advantage to using misoprostol to treat a PPH due to uterine atony.5
Rectal misoprostol does not achieve optimal circulating concentrations of the drugMisoprostol tablets are formulated for oral administration, not rectal administration. The studies in the TABLE show that rectal administration of misoprostol results in lower circulating concentration of the medication compared to oral, buccal, or vaginal administration.6−8 After rectal administration it takes about 60 minutes to reach the peak circulating concentration of misoprostol.6,7 By contrast, parenteral oxytocin, methylergonovine, and carboprost tromethamine reach peak serum concentration much more quickly after administration.

In a study of misoprostol stimulation of uterine contractility as measured by an intrauterine pressure catheter, buccal administration resulted in higher peak uterine tone than rectal administration (49 vs 31 mm Hg).8 In addition, time to onset of uterine contractility was 41 minutes and 103 minutes, respectively, for buccal and rectal administration.
These studies show that rectal misoprostol is associated with lower serum concentrations, longer time to onset of uterine contraction, and less contractility than buccal administration. The one advantage of rectal administration is that it has a longer duration of action than the oral, buccal, or sublingual routes. In pharmacokinetic comparisons of buccal versus sublingual administration of misoprostol, the sublingual route results in greater peak concentration, which may cause more adverse effects.9,10
Misoprostol is a useful uterotonic if parenteral agents are not available
Worldwide, approximately one maternal death occurs every 7 minutes. Postpartum hemorrhage (PPH) is a common cause of maternal death. Oxytocin, methylergonovine, and carboprost tromethamine should be stored in a refrigerated environment to ensure the stability and bioavailability of the drug. In settings in which reliable refrigeration is not available, misoprostol, a medication that is heat-stable, is often used to prevent and treat PPH.
One approach to preventing PPH is to provide 600 µg of misoprostol to women delivering at home without a skilled birth attendant that they can self- administer after the delivery.1,2 Another approach is to recommend that skilled birth attendants administer misoprostol following the delivery.3
Although I am recommending that we not use rectal misoprostol to treat PPH in the United States, it is clear that misoprostol plays an important role in preventing PPH in countries where parenteral uterotonics are not available. If a clinician in the United States was involved in a home birth complicated by PPH due to uterine atony, and if misoprostol was the only available uterotonic, it would be wise to administer it promptly.
References
- Rajbhandari S, Hodgins S, Sanghvi H, McPherson R, Pradhan YV, Baqui AH; Misoprostol Study Group. Expanding uterotonic protection following childbirth through community-based distribution of misoprostol: operations research study in Nepal. Int J Gynaecol Obstet. 2010;108(3):282–288.
- Sanghvi H, Ansari N, Prata NJ, Gibson H, Ehsan AT, Smith JM. Prevention of postpartum hemorrhage at home birth in Afghanistan. Int J Gynaecol Obstet. 2010;108(3):276–281.
- Prata N, Mbaruku G, Campbell M, Potts M, Vahidnia F. Controlling postpartum hemorrhage after home births in Tanzania. Int J Gynaecol Obstet. 2005;90(1):51–55.
Prioritize oxytocin, methergine, and carboprost tromethamineWhen treating PPH, administration of oxytocin, methylergonovine, or carboprost tromethamine rapidly provides therapeutic concentration of medication. For oxytocin, 40 U in 1 L, administered at a rate sufficient to control atony, or 10 U IM injection are often effective in controlling bleeding due to atony. Carboprost tromethamine 0.25 mg administered intramuscularly every 15 minutes up to 8 doses provides an excellent second-line agent. Carboprost tromethamine is contraindicated for women with asthma.
Methylergonovine 0.2 mg administered intramuscularly only can be given every 2 to 4 hours. Consequently, because time is of the essence in managing a severe PPH, it is unusual to be able to administer more than one dose of the agent during the course of treatment. Methylergonovine is contraindicated for women with hypertension.
There is scant evidence that misoprostol is more effective than oxytocin, and misoprostol clearly causes a higher rate of elevated temperature than any of the parenteral uterotonic agents. In your practice stop using rectal misoprostol for the treatment of PPH caused by uterine atony, and prioritize the use of parenteral uterotonics.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Most authorities recommend that, following delivery, all women should receive a uterotonic medication to reduce the risk of postpartum hemorrhage (PPH).1 In the United States, the preferred uterotonic for this preventive effort is oxytocin—a low-cost, highly effective agent that typically is administered as an intravenous (IV) infusion or intramuscular (IM) injection. Unfortunately, even with the universal administration of oxytocin in the third stage of labor, PPH occurs in about 3% of vaginal deliveries.
A key decision in treating a PPH due to uterine atony is treatment with an optimal uterotonic. The options include:
- additional oxytocin
- carboprost tromethamine(Hemabate)
- methylergonovine (Methergine)
- misoprostol.
Many obstetricians choose rectal misoprostol alone or in combination with oxytocin as the preferred treatment of PPH. However, evidence from clinical trials and pharmacokinetic studies suggest that rectal misoprostol is not an optimal choice if parenteral uterotonics are available. Here I pre-sent this evidence and urge you to stop the practice of using rectal misoprostol in efforts to manage PPH.
RCTs do not support the use of rectal misoprostolRandomized clinical trials (RCTs) have not demonstrated that misoprostol is superior to oxytocin for the treatment of PPH caused by uterine atony.2 For example, Blum and colleagues studied 31,055 women who received oxytocin (by IV or IM route) at vaginal delivery and observed that 809 (3%) developed a PPH.3 The women who developed PPH were randomly assigned to treatment with misoprostol 800 µg sublingual or oxytocin 40 U in 1,000 mL as an IV infusion over 15 minutes.
Both oxytocin and misoprostol had similar efficacy for controlling bleeding within 20 minutes (90% and 89%, respectively). Fewer women had blood loss of 1,000 mL or greater when treated with oxytocin compared with misoprostol (1% vs 3%, respectively; P = .062). In addition, oxytocin was associated with fewer temperature elevations of 38°C (100.4°F) or above (15% vs 22% for misoprostol, P = .007) and fewer temperature elevations of 40°C (104°F) or above (0.2% vs 1.2% for misoprostol, P = .11).
In another trial, women with a vaginal delivery who were not treated with a uterotonic in the third stage were monitored for the development of a PPH.4 PPH did develop in 1,422 women, who were then randomly assigned to receive oxytocin (10 U IV or IM) plus a placebo tablet or oxytocin plus misoprostol (600 µg sublingual).
Comparing oxytocin alone versus oxytocin plus misoprostol, there was no difference in blood loss of 500 mL or greater after treatment initiation (14% vs 14%). However, 90 minutes following treatment, temperature elevations occurred much more often in the women who received oxytocin plus misoprostol compared with the women who received oxytocin alone (temperature ≥38°C: 58% vs 19%; temperature ≥40°C: 6.8% vs 0.4%).
Bottom line: If you have access to oxytocin, there is no advantage to using misoprostol to treat a PPH due to uterine atony.5
Rectal misoprostol does not achieve optimal circulating concentrations of the drugMisoprostol tablets are formulated for oral administration, not rectal administration. The studies in the TABLE show that rectal administration of misoprostol results in lower circulating concentration of the medication compared to oral, buccal, or vaginal administration.6−8 After rectal administration it takes about 60 minutes to reach the peak circulating concentration of misoprostol.6,7 By contrast, parenteral oxytocin, methylergonovine, and carboprost tromethamine reach peak serum concentration much more quickly after administration.

In a study of misoprostol stimulation of uterine contractility as measured by an intrauterine pressure catheter, buccal administration resulted in higher peak uterine tone than rectal administration (49 vs 31 mm Hg).8 In addition, time to onset of uterine contractility was 41 minutes and 103 minutes, respectively, for buccal and rectal administration.
These studies show that rectal misoprostol is associated with lower serum concentrations, longer time to onset of uterine contraction, and less contractility than buccal administration. The one advantage of rectal administration is that it has a longer duration of action than the oral, buccal, or sublingual routes. In pharmacokinetic comparisons of buccal versus sublingual administration of misoprostol, the sublingual route results in greater peak concentration, which may cause more adverse effects.9,10
Misoprostol is a useful uterotonic if parenteral agents are not available
Worldwide, approximately one maternal death occurs every 7 minutes. Postpartum hemorrhage (PPH) is a common cause of maternal death. Oxytocin, methylergonovine, and carboprost tromethamine should be stored in a refrigerated environment to ensure the stability and bioavailability of the drug. In settings in which reliable refrigeration is not available, misoprostol, a medication that is heat-stable, is often used to prevent and treat PPH.
One approach to preventing PPH is to provide 600 µg of misoprostol to women delivering at home without a skilled birth attendant that they can self- administer after the delivery.1,2 Another approach is to recommend that skilled birth attendants administer misoprostol following the delivery.3
Although I am recommending that we not use rectal misoprostol to treat PPH in the United States, it is clear that misoprostol plays an important role in preventing PPH in countries where parenteral uterotonics are not available. If a clinician in the United States was involved in a home birth complicated by PPH due to uterine atony, and if misoprostol was the only available uterotonic, it would be wise to administer it promptly.
References
- Rajbhandari S, Hodgins S, Sanghvi H, McPherson R, Pradhan YV, Baqui AH; Misoprostol Study Group. Expanding uterotonic protection following childbirth through community-based distribution of misoprostol: operations research study in Nepal. Int J Gynaecol Obstet. 2010;108(3):282–288.
- Sanghvi H, Ansari N, Prata NJ, Gibson H, Ehsan AT, Smith JM. Prevention of postpartum hemorrhage at home birth in Afghanistan. Int J Gynaecol Obstet. 2010;108(3):276–281.
- Prata N, Mbaruku G, Campbell M, Potts M, Vahidnia F. Controlling postpartum hemorrhage after home births in Tanzania. Int J Gynaecol Obstet. 2005;90(1):51–55.
Prioritize oxytocin, methergine, and carboprost tromethamineWhen treating PPH, administration of oxytocin, methylergonovine, or carboprost tromethamine rapidly provides therapeutic concentration of medication. For oxytocin, 40 U in 1 L, administered at a rate sufficient to control atony, or 10 U IM injection are often effective in controlling bleeding due to atony. Carboprost tromethamine 0.25 mg administered intramuscularly every 15 minutes up to 8 doses provides an excellent second-line agent. Carboprost tromethamine is contraindicated for women with asthma.
Methylergonovine 0.2 mg administered intramuscularly only can be given every 2 to 4 hours. Consequently, because time is of the essence in managing a severe PPH, it is unusual to be able to administer more than one dose of the agent during the course of treatment. Methylergonovine is contraindicated for women with hypertension.
There is scant evidence that misoprostol is more effective than oxytocin, and misoprostol clearly causes a higher rate of elevated temperature than any of the parenteral uterotonic agents. In your practice stop using rectal misoprostol for the treatment of PPH caused by uterine atony, and prioritize the use of parenteral uterotonics.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Gibbons KJ, Albright CM, Rouse DJ. Postpartum hemorrhage in the developed world: whither misoprostol? Am J Obstet Gynecol. 2013;208(3):181−183.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375(9710):217−223.
- Widmer M, Blum J, Hofmeyr GJ, et al. Misoprostolas an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010;375(9728):1808−1813.
- Weeks A. The prevention and treatment of postpartum hemorrhage: what do we know and where do we go to next? BJOG. 2015;122(2):202−210.
- Khan RU, El-Refaey H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynecol. 2003;101(5 pt 1):968−974.
- Khan RU, El-Refaey H, Sharma S, Sooranna D, Stafford M. Oral, rectal and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103(5 pt 1):866−870.
- Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 pt 1):582−590.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71(1):22−25.
- Frye LJ, Byrne ME, Winikoff B. A crossover pharmacokinetic study of misoprostol by the oral, sublingual and buccal routes [published online ahead of print April 22, 2016]. Eur J Contracept Reprod Health Care. doi:10.3109/13625187.2016.1168799.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Gibbons KJ, Albright CM, Rouse DJ. Postpartum hemorrhage in the developed world: whither misoprostol? Am J Obstet Gynecol. 2013;208(3):181−183.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375(9710):217−223.
- Widmer M, Blum J, Hofmeyr GJ, et al. Misoprostolas an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010;375(9728):1808−1813.
- Weeks A. The prevention and treatment of postpartum hemorrhage: what do we know and where do we go to next? BJOG. 2015;122(2):202−210.
- Khan RU, El-Refaey H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynecol. 2003;101(5 pt 1):968−974.
- Khan RU, El-Refaey H, Sharma S, Sooranna D, Stafford M. Oral, rectal and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103(5 pt 1):866−870.
- Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 pt 1):582−590.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71(1):22−25.
- Frye LJ, Byrne ME, Winikoff B. A crossover pharmacokinetic study of misoprostol by the oral, sublingual and buccal routes [published online ahead of print April 22, 2016]. Eur J Contracept Reprod Health Care. doi:10.3109/13625187.2016.1168799.
Start offering aspirin to pregnant women at high risk for preeclampsia
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7
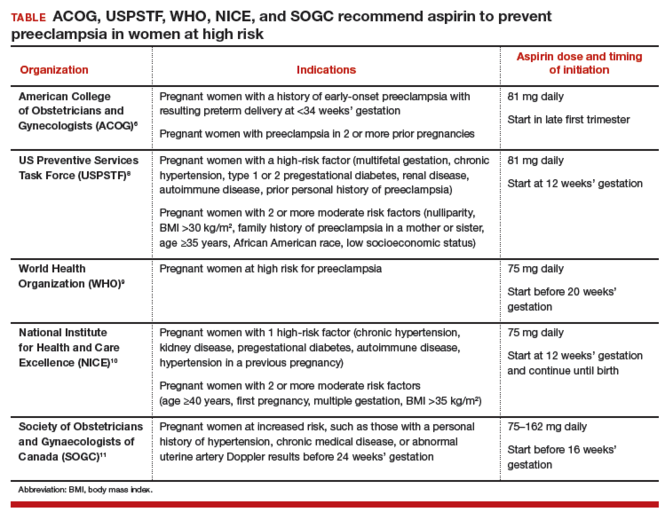
An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7

An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7

An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
Intractable shoulder dystocia: A posterior axilla maneuver may save the day
Shoulder dystocia is an unpredictable obstetric emergency that challenges all obstetricians and midwives. In response to a shoulder dystocia emergency, most clinicians implement a sequence of well-practiced steps that begin with early recognition of the problem, clear communication of the emergency with delivery room staff, and a call for help to available clinicians. Management steps may include:
- instructing the mother to stop pushing and moving the mother's buttocks to the edge of the bed
- ensuring there is not a tight nuchal cord
- committing to avoiding the use of excessive force on the fetal head and neck
- considering performing an episiotomy
- performing the McRoberts maneuver combined with suprapubic pressure
- using a rotational maneuver, such as the Woods maneuver or the Rubin maneuver
- delivering the posterior arm
- considering the Gaskin all-four maneuver.
When initial management steps are not enoughIf this sequence of steps does not result in successful vaginal delivery, additional options include: clavicle fracture, cephalic replacement followed by cesarean delivery (Zavanelli maneuver), symphysiotomy, or fundal pressure combined with a rotational maneuver. Another simple intervention that is not discussed widely in medical textbooks or taught during training is the posterior axilla maneuver.
Posterior axilla maneuversVarying posterior axilla maneuvers have been described by many expert obstetricians, including Willughby (17th Century),1 Holman (1963),2 Schramm (1983),3 Menticoglou (2006),4 and Hofmeyr and Cluver (2009, 2015).5−7
Willughby maneuverPercival Willughby’s (1596−1685) description of a posterior axilla maneuver was brief1:
After the head is born, if the child through the greatness of the shoulders, should stick at the neck, let the midwife put her fingers under the child's armpit and give it a nudge, thrusting it to the other side with her finger, drawing the child or she may quickly bring forth the shoulders, without offering to put it forth by her hands clasped about the neck, which might endanger the breaking of the neck.
Holman maneuverHolman described a maneuver with the following steps2:
- perform an episiotomy
- place a finger in the posterior axilla and draw the posterior shoulder down along the pelvic axis
- simultaneously have an assistant perform suprapubic pressure and
- if necessary, insert two supinated fingers under the pubic arch and press and rock the anterior shoulder, tilting the anterior shoulder toward the hollow of the sacrum while simultaneously gently pulling the posterior axilla along the pelvic axis.
Schramm maneuverSchramm, working with a population enriched with women with diabetes, frequently encountered shoulder dystocia and recommended3:
If the posterior axilla can be reached—in other words, if the posterior shoulder is engaged—in my experience it can always be delivered by rotating it to the anterior position while at the same time applying traction....I normally place 1 or 2 fingers of my right hand in the posterior axilla and “scruff” the neck with my left hand, applying both rotation and traction. Because this grip is somewhat insecure, the resultant tractive force is limited and I consider this manoeuvre to be the most effective and least traumatic method of relieving moderate to severe obstruction.
Practice your shoulder dystocia maneuvers using simulation
Obstetric emergencies trigger a rush of adrenaline and great stress for the obstetrician and delivery room team. This may adversely impact motor performance, decision making, and communication skills.1 Low- and high-fidelity simulation exercises create an environment in which the obstetrics team can practice the sequence of maneuvers and seamless teamwork needed to successfully resolve a shoulder dystocia.2,3 Implementing a shoulder dystocia protocol and practicing the protocol using team-based simulation may help to reduce the adverse outcomes of shoulder dystocia.3,4
Reference
1. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5−10.
2. Crofts JF, Fox R, Ellis D, Winter C, Hinshaw K, Draycott TJ. Observations from 450 shoulder dystocia simulations. Obstet Gynecol. 2008;112(4):906−912.
3. Draycott TJ, Crofts JF, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14−20.
4. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513−517.
Manipulation of the posterior axilla |
|
The right and left third fingers are locked into the posterior axilla, one finger from the front and one from the back of the fetus. Gentle downward guidance is provided by the fingers to draw the posterior shoulder down and out along the curve of the sacrum, thus releasing the anterior shoulder.4 In this drawing, an assistant gently holds the head up. |
Menticoglou maneuverMenticoglou noted that delivery of the posterior arm generally resolves almost all cases of shoulder dystocia. However, if the posterior arm is extended and trapped between the fetus and maternal pelvic side-wall, it may be difficult to deliver the posterior arm. In these cases he recommended having an assistant gently hold, not pull, the fetal head upward and, at the same time, having the obstetrician get on one knee, placing the middle fingers of both hands into the posterior axilla of the fetus.4
The right middle finger is placed into the axilla from the left side of the maternal pelvis, and the left middle finger is placed into the axilla from the right side of the maternal pelvis, resulting in the two middle fingers overlapping in the fetal axilla (FIGURE).4 Gentle force is then used to pull the posterior shoulder and arm downward and outward along the curve of the sacrum. Once the shoulder has emerged from the pelvis, the posterior arm is delivered. Alternatively, if the posterior shoulder is brought well down into the pelvis, another attempt can be made at delivering the posterior arm.4
My preferred approach. The Menticoglou maneuver is my preferred posterior axilla maneuver because it can be accomplished rapidly; requires no equipment, such as a sling catheter; and the obstetrician has good tactile feedback throughout the application of gentle force.
Hofmeyr-Cluver maneuverIn cases of difficult shoulder dystocia, Dr. William Smellie (1762)8 recommended placing one or two fingers in the anterior or posterior fetal axilla and gentling pulling on the axilla to deliver the body. If the axillae were too high to reach, he recommended using a blunt hook in the axilla to draw forth the impacted child. He advised caution when using a blunt hook because the fetus might be injured or lacerated.
Instead of using a hook, Hofmeyr and Cluver5−7 have recommended using a catheter sling to deliver the posterior shoulder. In this maneuver, a loop of a suction catheter or firm urinary catheter is placed over the obstetrician’s index finger and the loop is pushed through the posterior axilla, back to front, with guidance from the index finger. The index finger of the opposite hand is used to catch the loop and pull the catheter through, creating a single-stranded sling that is positioned in the axilla. Gentle force is then applied to the sling in the axis of the pelvis to deliver the posterior shoulder.
“If the posterior arm does not follow it is then swept out easily because room has been created by delivering the posterior shoulder. If the aforementioned procedure fails, the sling can be used to rotate the shoulder. To perform a rotational maneuver, sling traction is directed laterally towards the side of the baby’s back then anteriorly while digital pressure is applied behind the anterior shoulder to assist rotation.”7
Use ACOG’s checklist for documenting a shoulder dystocia
Following the resolution of a shoulder dystocia, it is important to gather all the necessary facts to complete a detailed medical record entry describing the situation and interventions used. The checklist from the American College of Obstetricians and Gynecologists (ACOG) helps you to prepare a standardized medical record entry that is comprehensive.
My experience is that “free form” medical record entries describing the events at a shoulder dystocia event are generally not optimally organized, creating future problems when the case is reviewed.
ACOG obstetric checklists are available for download at http://www.acog.org/Resources-And-Publications, or use your web browser to search for “ACOG Shoulder Dystocia checklist.”
With scant literature, know the benefits and risksThe world’s literature on posterior axilla maneuvers to resolve shoulder dystocia consists of case series and individual case reports.2−7 Hence, the quality of the data supporting this intervention is not optimal, and risks associated with the maneuver are not well characterized. Application of a controlled and gentle force to the posterior axilla may cause fracture of the fetal humerus5 or dislocation of the fetal shoulder. The posterior axilla maneuver also may increase the risk of a maternal third- or fourth-degree perineal laceration.
As a general rule, as the number of maneuvers used to resolve a difficult shoulder dystocia increase, the risk of neonatal injury increases.9 Since the posterior axilla maneuver typically is only attempted after multiple previous maneuvers have failed, the risk of fetal injury is increased. However, as time passes and a shoulder dystocia remains unresolved for 4 or 5 minutes, the risk of neurologic injury and fetal death increases.10
In resolving a shoulder dystocia, speed and skill are essential. A posterior axilla maneuver can be performed more rapidly than a Zavanelli maneuver or a symphysiotomy. Although manipulation of the posterior axilla and arm may cause a fracture of the humerus, this complication is a modest price to pay for preventing permanent fetal brain injury or fetal death.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Willughby P. Observations in midwifery. New York, NY: MW Books; 1972:312−313.
- Holman MS. A new manoeuvre for delivery of an impacted shoulder based on a mechanical analysis. S Afr Med J. 1963;37:247−249.
- Schramm M. Impacted shoulders—a personal experience. Aust N Z J Obstet Gynaecol. 1983;23(1):28−31.
- Menticoglou SM. A modified technique to deliver the posterior arm in severe shoulder dystocia. Obstet Gynecol. 2006;108(3 pt 2):755−757.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction: a technique for intractable shoulder dystocia. Obstet Gynecol. 2009;113(2 pt 2):486–488.
- Hofmeyr GJ, Cluver CA. Posterior axilla sling traction for intractable shoulder dystocia. BJOG. 2009;116(13):1818−1820.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction for shoulder dystocia: case review and a new method for shoulder rotation with the sling. Am J Obstet Gynecol. 2015;212(6):784.e1−e7.
- Smellie W. A treatise on the theory and practice of midwifery. 4th ed. London, England; 1762:226−227.
- Hoffman MK, Bailit JL, Branch DW, et al; Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272−1278.
- Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318−322.
Shoulder dystocia is an unpredictable obstetric emergency that challenges all obstetricians and midwives. In response to a shoulder dystocia emergency, most clinicians implement a sequence of well-practiced steps that begin with early recognition of the problem, clear communication of the emergency with delivery room staff, and a call for help to available clinicians. Management steps may include:
- instructing the mother to stop pushing and moving the mother's buttocks to the edge of the bed
- ensuring there is not a tight nuchal cord
- committing to avoiding the use of excessive force on the fetal head and neck
- considering performing an episiotomy
- performing the McRoberts maneuver combined with suprapubic pressure
- using a rotational maneuver, such as the Woods maneuver or the Rubin maneuver
- delivering the posterior arm
- considering the Gaskin all-four maneuver.
When initial management steps are not enoughIf this sequence of steps does not result in successful vaginal delivery, additional options include: clavicle fracture, cephalic replacement followed by cesarean delivery (Zavanelli maneuver), symphysiotomy, or fundal pressure combined with a rotational maneuver. Another simple intervention that is not discussed widely in medical textbooks or taught during training is the posterior axilla maneuver.
Posterior axilla maneuversVarying posterior axilla maneuvers have been described by many expert obstetricians, including Willughby (17th Century),1 Holman (1963),2 Schramm (1983),3 Menticoglou (2006),4 and Hofmeyr and Cluver (2009, 2015).5−7
Willughby maneuverPercival Willughby’s (1596−1685) description of a posterior axilla maneuver was brief1:
After the head is born, if the child through the greatness of the shoulders, should stick at the neck, let the midwife put her fingers under the child's armpit and give it a nudge, thrusting it to the other side with her finger, drawing the child or she may quickly bring forth the shoulders, without offering to put it forth by her hands clasped about the neck, which might endanger the breaking of the neck.
Holman maneuverHolman described a maneuver with the following steps2:
- perform an episiotomy
- place a finger in the posterior axilla and draw the posterior shoulder down along the pelvic axis
- simultaneously have an assistant perform suprapubic pressure and
- if necessary, insert two supinated fingers under the pubic arch and press and rock the anterior shoulder, tilting the anterior shoulder toward the hollow of the sacrum while simultaneously gently pulling the posterior axilla along the pelvic axis.
Schramm maneuverSchramm, working with a population enriched with women with diabetes, frequently encountered shoulder dystocia and recommended3:
If the posterior axilla can be reached—in other words, if the posterior shoulder is engaged—in my experience it can always be delivered by rotating it to the anterior position while at the same time applying traction....I normally place 1 or 2 fingers of my right hand in the posterior axilla and “scruff” the neck with my left hand, applying both rotation and traction. Because this grip is somewhat insecure, the resultant tractive force is limited and I consider this manoeuvre to be the most effective and least traumatic method of relieving moderate to severe obstruction.
Practice your shoulder dystocia maneuvers using simulation
Obstetric emergencies trigger a rush of adrenaline and great stress for the obstetrician and delivery room team. This may adversely impact motor performance, decision making, and communication skills.1 Low- and high-fidelity simulation exercises create an environment in which the obstetrics team can practice the sequence of maneuvers and seamless teamwork needed to successfully resolve a shoulder dystocia.2,3 Implementing a shoulder dystocia protocol and practicing the protocol using team-based simulation may help to reduce the adverse outcomes of shoulder dystocia.3,4
Reference
1. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5−10.
2. Crofts JF, Fox R, Ellis D, Winter C, Hinshaw K, Draycott TJ. Observations from 450 shoulder dystocia simulations. Obstet Gynecol. 2008;112(4):906−912.
3. Draycott TJ, Crofts JF, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14−20.
4. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513−517.
Manipulation of the posterior axilla |
|
The right and left third fingers are locked into the posterior axilla, one finger from the front and one from the back of the fetus. Gentle downward guidance is provided by the fingers to draw the posterior shoulder down and out along the curve of the sacrum, thus releasing the anterior shoulder.4 In this drawing, an assistant gently holds the head up. |
Menticoglou maneuverMenticoglou noted that delivery of the posterior arm generally resolves almost all cases of shoulder dystocia. However, if the posterior arm is extended and trapped between the fetus and maternal pelvic side-wall, it may be difficult to deliver the posterior arm. In these cases he recommended having an assistant gently hold, not pull, the fetal head upward and, at the same time, having the obstetrician get on one knee, placing the middle fingers of both hands into the posterior axilla of the fetus.4
The right middle finger is placed into the axilla from the left side of the maternal pelvis, and the left middle finger is placed into the axilla from the right side of the maternal pelvis, resulting in the two middle fingers overlapping in the fetal axilla (FIGURE).4 Gentle force is then used to pull the posterior shoulder and arm downward and outward along the curve of the sacrum. Once the shoulder has emerged from the pelvis, the posterior arm is delivered. Alternatively, if the posterior shoulder is brought well down into the pelvis, another attempt can be made at delivering the posterior arm.4
My preferred approach. The Menticoglou maneuver is my preferred posterior axilla maneuver because it can be accomplished rapidly; requires no equipment, such as a sling catheter; and the obstetrician has good tactile feedback throughout the application of gentle force.
Hofmeyr-Cluver maneuverIn cases of difficult shoulder dystocia, Dr. William Smellie (1762)8 recommended placing one or two fingers in the anterior or posterior fetal axilla and gentling pulling on the axilla to deliver the body. If the axillae were too high to reach, he recommended using a blunt hook in the axilla to draw forth the impacted child. He advised caution when using a blunt hook because the fetus might be injured or lacerated.
Instead of using a hook, Hofmeyr and Cluver5−7 have recommended using a catheter sling to deliver the posterior shoulder. In this maneuver, a loop of a suction catheter or firm urinary catheter is placed over the obstetrician’s index finger and the loop is pushed through the posterior axilla, back to front, with guidance from the index finger. The index finger of the opposite hand is used to catch the loop and pull the catheter through, creating a single-stranded sling that is positioned in the axilla. Gentle force is then applied to the sling in the axis of the pelvis to deliver the posterior shoulder.
“If the posterior arm does not follow it is then swept out easily because room has been created by delivering the posterior shoulder. If the aforementioned procedure fails, the sling can be used to rotate the shoulder. To perform a rotational maneuver, sling traction is directed laterally towards the side of the baby’s back then anteriorly while digital pressure is applied behind the anterior shoulder to assist rotation.”7
Use ACOG’s checklist for documenting a shoulder dystocia
Following the resolution of a shoulder dystocia, it is important to gather all the necessary facts to complete a detailed medical record entry describing the situation and interventions used. The checklist from the American College of Obstetricians and Gynecologists (ACOG) helps you to prepare a standardized medical record entry that is comprehensive.
My experience is that “free form” medical record entries describing the events at a shoulder dystocia event are generally not optimally organized, creating future problems when the case is reviewed.
ACOG obstetric checklists are available for download at http://www.acog.org/Resources-And-Publications, or use your web browser to search for “ACOG Shoulder Dystocia checklist.”
With scant literature, know the benefits and risksThe world’s literature on posterior axilla maneuvers to resolve shoulder dystocia consists of case series and individual case reports.2−7 Hence, the quality of the data supporting this intervention is not optimal, and risks associated with the maneuver are not well characterized. Application of a controlled and gentle force to the posterior axilla may cause fracture of the fetal humerus5 or dislocation of the fetal shoulder. The posterior axilla maneuver also may increase the risk of a maternal third- or fourth-degree perineal laceration.
As a general rule, as the number of maneuvers used to resolve a difficult shoulder dystocia increase, the risk of neonatal injury increases.9 Since the posterior axilla maneuver typically is only attempted after multiple previous maneuvers have failed, the risk of fetal injury is increased. However, as time passes and a shoulder dystocia remains unresolved for 4 or 5 minutes, the risk of neurologic injury and fetal death increases.10
In resolving a shoulder dystocia, speed and skill are essential. A posterior axilla maneuver can be performed more rapidly than a Zavanelli maneuver or a symphysiotomy. Although manipulation of the posterior axilla and arm may cause a fracture of the humerus, this complication is a modest price to pay for preventing permanent fetal brain injury or fetal death.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Shoulder dystocia is an unpredictable obstetric emergency that challenges all obstetricians and midwives. In response to a shoulder dystocia emergency, most clinicians implement a sequence of well-practiced steps that begin with early recognition of the problem, clear communication of the emergency with delivery room staff, and a call for help to available clinicians. Management steps may include:
- instructing the mother to stop pushing and moving the mother's buttocks to the edge of the bed
- ensuring there is not a tight nuchal cord
- committing to avoiding the use of excessive force on the fetal head and neck
- considering performing an episiotomy
- performing the McRoberts maneuver combined with suprapubic pressure
- using a rotational maneuver, such as the Woods maneuver or the Rubin maneuver
- delivering the posterior arm
- considering the Gaskin all-four maneuver.
When initial management steps are not enoughIf this sequence of steps does not result in successful vaginal delivery, additional options include: clavicle fracture, cephalic replacement followed by cesarean delivery (Zavanelli maneuver), symphysiotomy, or fundal pressure combined with a rotational maneuver. Another simple intervention that is not discussed widely in medical textbooks or taught during training is the posterior axilla maneuver.
Posterior axilla maneuversVarying posterior axilla maneuvers have been described by many expert obstetricians, including Willughby (17th Century),1 Holman (1963),2 Schramm (1983),3 Menticoglou (2006),4 and Hofmeyr and Cluver (2009, 2015).5−7
Willughby maneuverPercival Willughby’s (1596−1685) description of a posterior axilla maneuver was brief1:
After the head is born, if the child through the greatness of the shoulders, should stick at the neck, let the midwife put her fingers under the child's armpit and give it a nudge, thrusting it to the other side with her finger, drawing the child or she may quickly bring forth the shoulders, without offering to put it forth by her hands clasped about the neck, which might endanger the breaking of the neck.
Holman maneuverHolman described a maneuver with the following steps2:
- perform an episiotomy
- place a finger in the posterior axilla and draw the posterior shoulder down along the pelvic axis
- simultaneously have an assistant perform suprapubic pressure and
- if necessary, insert two supinated fingers under the pubic arch and press and rock the anterior shoulder, tilting the anterior shoulder toward the hollow of the sacrum while simultaneously gently pulling the posterior axilla along the pelvic axis.
Schramm maneuverSchramm, working with a population enriched with women with diabetes, frequently encountered shoulder dystocia and recommended3:
If the posterior axilla can be reached—in other words, if the posterior shoulder is engaged—in my experience it can always be delivered by rotating it to the anterior position while at the same time applying traction....I normally place 1 or 2 fingers of my right hand in the posterior axilla and “scruff” the neck with my left hand, applying both rotation and traction. Because this grip is somewhat insecure, the resultant tractive force is limited and I consider this manoeuvre to be the most effective and least traumatic method of relieving moderate to severe obstruction.
Practice your shoulder dystocia maneuvers using simulation
Obstetric emergencies trigger a rush of adrenaline and great stress for the obstetrician and delivery room team. This may adversely impact motor performance, decision making, and communication skills.1 Low- and high-fidelity simulation exercises create an environment in which the obstetrics team can practice the sequence of maneuvers and seamless teamwork needed to successfully resolve a shoulder dystocia.2,3 Implementing a shoulder dystocia protocol and practicing the protocol using team-based simulation may help to reduce the adverse outcomes of shoulder dystocia.3,4
Reference
1. Wetzel CM, Kneebone RL, Woloshynowych M, et al. The effects of stress on surgical performance. Am J Surg. 2006;191(1):5−10.
2. Crofts JF, Fox R, Ellis D, Winter C, Hinshaw K, Draycott TJ. Observations from 450 shoulder dystocia simulations. Obstet Gynecol. 2008;112(4):906−912.
3. Draycott TJ, Crofts JF, Ash JP, et al. Improving neonatal outcome through practical shoulder dystocia training. Obstet Gynecol. 2008;112(1):14−20.
4. Grobman WA, Miller D, Burke C, Hornbogen A, Tam K, Costello R. Outcomes associated with introduction of a shoulder dystocia protocol. Am J Obstet Gynecol. 2011;205(6):513−517.
Manipulation of the posterior axilla |
|
The right and left third fingers are locked into the posterior axilla, one finger from the front and one from the back of the fetus. Gentle downward guidance is provided by the fingers to draw the posterior shoulder down and out along the curve of the sacrum, thus releasing the anterior shoulder.4 In this drawing, an assistant gently holds the head up. |
Menticoglou maneuverMenticoglou noted that delivery of the posterior arm generally resolves almost all cases of shoulder dystocia. However, if the posterior arm is extended and trapped between the fetus and maternal pelvic side-wall, it may be difficult to deliver the posterior arm. In these cases he recommended having an assistant gently hold, not pull, the fetal head upward and, at the same time, having the obstetrician get on one knee, placing the middle fingers of both hands into the posterior axilla of the fetus.4
The right middle finger is placed into the axilla from the left side of the maternal pelvis, and the left middle finger is placed into the axilla from the right side of the maternal pelvis, resulting in the two middle fingers overlapping in the fetal axilla (FIGURE).4 Gentle force is then used to pull the posterior shoulder and arm downward and outward along the curve of the sacrum. Once the shoulder has emerged from the pelvis, the posterior arm is delivered. Alternatively, if the posterior shoulder is brought well down into the pelvis, another attempt can be made at delivering the posterior arm.4
My preferred approach. The Menticoglou maneuver is my preferred posterior axilla maneuver because it can be accomplished rapidly; requires no equipment, such as a sling catheter; and the obstetrician has good tactile feedback throughout the application of gentle force.
Hofmeyr-Cluver maneuverIn cases of difficult shoulder dystocia, Dr. William Smellie (1762)8 recommended placing one or two fingers in the anterior or posterior fetal axilla and gentling pulling on the axilla to deliver the body. If the axillae were too high to reach, he recommended using a blunt hook in the axilla to draw forth the impacted child. He advised caution when using a blunt hook because the fetus might be injured or lacerated.
Instead of using a hook, Hofmeyr and Cluver5−7 have recommended using a catheter sling to deliver the posterior shoulder. In this maneuver, a loop of a suction catheter or firm urinary catheter is placed over the obstetrician’s index finger and the loop is pushed through the posterior axilla, back to front, with guidance from the index finger. The index finger of the opposite hand is used to catch the loop and pull the catheter through, creating a single-stranded sling that is positioned in the axilla. Gentle force is then applied to the sling in the axis of the pelvis to deliver the posterior shoulder.
“If the posterior arm does not follow it is then swept out easily because room has been created by delivering the posterior shoulder. If the aforementioned procedure fails, the sling can be used to rotate the shoulder. To perform a rotational maneuver, sling traction is directed laterally towards the side of the baby’s back then anteriorly while digital pressure is applied behind the anterior shoulder to assist rotation.”7
Use ACOG’s checklist for documenting a shoulder dystocia
Following the resolution of a shoulder dystocia, it is important to gather all the necessary facts to complete a detailed medical record entry describing the situation and interventions used. The checklist from the American College of Obstetricians and Gynecologists (ACOG) helps you to prepare a standardized medical record entry that is comprehensive.
My experience is that “free form” medical record entries describing the events at a shoulder dystocia event are generally not optimally organized, creating future problems when the case is reviewed.
ACOG obstetric checklists are available for download at http://www.acog.org/Resources-And-Publications, or use your web browser to search for “ACOG Shoulder Dystocia checklist.”
With scant literature, know the benefits and risksThe world’s literature on posterior axilla maneuvers to resolve shoulder dystocia consists of case series and individual case reports.2−7 Hence, the quality of the data supporting this intervention is not optimal, and risks associated with the maneuver are not well characterized. Application of a controlled and gentle force to the posterior axilla may cause fracture of the fetal humerus5 or dislocation of the fetal shoulder. The posterior axilla maneuver also may increase the risk of a maternal third- or fourth-degree perineal laceration.
As a general rule, as the number of maneuvers used to resolve a difficult shoulder dystocia increase, the risk of neonatal injury increases.9 Since the posterior axilla maneuver typically is only attempted after multiple previous maneuvers have failed, the risk of fetal injury is increased. However, as time passes and a shoulder dystocia remains unresolved for 4 or 5 minutes, the risk of neurologic injury and fetal death increases.10
In resolving a shoulder dystocia, speed and skill are essential. A posterior axilla maneuver can be performed more rapidly than a Zavanelli maneuver or a symphysiotomy. Although manipulation of the posterior axilla and arm may cause a fracture of the humerus, this complication is a modest price to pay for preventing permanent fetal brain injury or fetal death.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Willughby P. Observations in midwifery. New York, NY: MW Books; 1972:312−313.
- Holman MS. A new manoeuvre for delivery of an impacted shoulder based on a mechanical analysis. S Afr Med J. 1963;37:247−249.
- Schramm M. Impacted shoulders—a personal experience. Aust N Z J Obstet Gynaecol. 1983;23(1):28−31.
- Menticoglou SM. A modified technique to deliver the posterior arm in severe shoulder dystocia. Obstet Gynecol. 2006;108(3 pt 2):755−757.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction: a technique for intractable shoulder dystocia. Obstet Gynecol. 2009;113(2 pt 2):486–488.
- Hofmeyr GJ, Cluver CA. Posterior axilla sling traction for intractable shoulder dystocia. BJOG. 2009;116(13):1818−1820.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction for shoulder dystocia: case review and a new method for shoulder rotation with the sling. Am J Obstet Gynecol. 2015;212(6):784.e1−e7.
- Smellie W. A treatise on the theory and practice of midwifery. 4th ed. London, England; 1762:226−227.
- Hoffman MK, Bailit JL, Branch DW, et al; Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272−1278.
- Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318−322.
- Willughby P. Observations in midwifery. New York, NY: MW Books; 1972:312−313.
- Holman MS. A new manoeuvre for delivery of an impacted shoulder based on a mechanical analysis. S Afr Med J. 1963;37:247−249.
- Schramm M. Impacted shoulders—a personal experience. Aust N Z J Obstet Gynaecol. 1983;23(1):28−31.
- Menticoglou SM. A modified technique to deliver the posterior arm in severe shoulder dystocia. Obstet Gynecol. 2006;108(3 pt 2):755−757.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction: a technique for intractable shoulder dystocia. Obstet Gynecol. 2009;113(2 pt 2):486–488.
- Hofmeyr GJ, Cluver CA. Posterior axilla sling traction for intractable shoulder dystocia. BJOG. 2009;116(13):1818−1820.
- Cluver CA, Hofmeyr GJ. Posterior axilla sling traction for shoulder dystocia: case review and a new method for shoulder rotation with the sling. Am J Obstet Gynecol. 2015;212(6):784.e1−e7.
- Smellie W. A treatise on the theory and practice of midwifery. 4th ed. London, England; 1762:226−227.
- Hoffman MK, Bailit JL, Branch DW, et al; Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272−1278.
- Lerner H, Durlacher K, Smith S, Hamilton E. Relationship between head-to-body delivery interval in shoulder dystocia and neonatal depression. Obstet Gynecol. 2011;118(2 pt 1):318−322.
In this article
- Menticoglou maneuver
- Importance of simulation
Can we solve the problem of inadequate contraception for women at high risk for adverse pregnancy outcomes?
In the United States contraception practices are slowly improving, with robust evidence for the increased use of long-acting reversible contraceptives and preliminary data that the unintended pregnancy rate may be decreasing for the first time in many years.1 There remains a major gap in contraception practice, however: US women with chronic disease who are at high risk for adverse pregnancy outcomes are not receiving adequate contraceptive counseling or adequate contraception.2,3 In one study, the majority of women with hypertension, diabetes, epilepsy, stroke, heart disease, lupus, or thrombophilia were not using a prescription contraceptive.3
Recently, I have seen women with major medical problems, who have had many visits with specialists and primary care clinicians, but who have not had their contraceptive needs prioritized. Here are but a few examples:
- We recently cared for a patient with heart disease and severe pulmonary hypertension, who had many procedures performed by cardiologists and cardiac surgeons, but contraception had not been prioritized as one of her foremost medical needs.
- A young woman who had a pulmonary embolism 1-month postpartum reported that her primary care clinician said that she could never use any hormonal contraceptive, including the progestin-only pill, progestin-implant, and the progestin-releasing intrauterine device (IUD). She was not taking a contraceptive and had an unplanned pregnancy.
- A middle-aged woman with diabetes and a glycosylated hemoglo‑ bin A1c (HbA1c) value greater than 10% was regularly seeing her primary care clinician, but was not using an effective contraceptive. She became pregnant with a fetus that had a major congenital anomaly.
Clearly, there is a major gap between current and optimal contraceptive services for women with chronic medical problems. Women with diabetes and heart disease are affected substantially, as the evidence I present in this editorial indicates. Studies also show that women taking teratogenic medications do not receive the vital counseling that they should regarding contraception. Given the potential detrimental adverse events to both mother and fetus, obstetrician-gynecologists are poised to offer solutions to this concerning inadequacy of care.
DiabetesWomen with diabetes and an abnormally elevated HbA1c level are at high risk for many adverse pregnancy outcomes, including major congenital malformations and intrauterine fetal demise.4 Unfortunately, results of many studies indicate that women with diabetes are not receiving adequate contraceptive services.5–7 In one review of records at Kaiser Permanente Northern California, investigators reported that 62% of 122,921 healthy women, but only 48% of 8,182 women with diabetes, received contraceptive counseling, a contraceptive prescription, or contraceptive services.5
Why is it that so many women with diabetes do not receive contraceptive services? One possibility is that clinicians are reluctant to prescribe oral hormonal contraceptives that contain estrogen to their patients with diabetes because of a perceived increased risk of cardiovascular events.8 In the Kaiser study, 31% of the healthy women, and only 13% of the women with diabetes, were using a pill, patch, or ring (most of which contain estrogen).5 In this same study, the rate of utilization of an IUD was similar in the healthy (6.5%) and diabetic (5.6%) women. The IUD is known to be safe for use in women with diabetes.9
The low rate of utilization of intrauterine contraception by women with diabetes is a gap that gynecologists are well positioned to help solve.
Heart diseaseIn developed countries, a major cause of maternal mortality is pregnancy among women with congenital or acquired heart disease.10 Misinformation is a common problem in contraceptive counseling. In a recent study of 83 sexually active women with congenital heart disease, 6 women were told that they could not use an IUD or progestin-implant because they were unsafe for those with repaired congenital heart disease.11 In this cohort of women, who were at high risk for adverse pregnancy outcomes, 45% of pregnancies were unplanned, similar to the rate among healthy women.
On a positive note, authors of a small study from Maryland found that, among women with heart disease, the self-reported use of a contraceptive increased from 60% prepregnancy to 93% following delivery.12
Clearly, patient interaction with qualified women’s health clinicians can increase contraceptive use in those with high-risk medical issues.
Teratogenic medicationsShould reproductive-age women taking long-term methotrexate for treatment of rheumatoid conditions receive contraceptive counseling? The answer is clearly, “yes.” Methotrexate can cause fetal death or major congenital malformations, such as absence of digits and oxycephaly (premature closure of the skull sutures). All women of reproductive age prescribed known teratogens should receive effective contraception. Unfortunately, data do not indicate this is occurring.
In one study of 1,694 adolescents and young women aged 14 to 25 years who were prescribed a teratogen, only 29% received documented contraception counseling, and only 11% received a contraceptive prescription or were documented to be actively using a contraceptive.13 The most commonly prescribed teratogens in this study were topiramate, methotrexate, and isoretinoin. Among the specialists who prescribed the medications, dermatologists documented contraceptive counseling in 47% of visits—likely because of the federally mandated risk mitigation system for prescribing isoretinoin. Neurologists and hematologists were least likely to document contraceptive counseling, at 16% and 28%, respectively.13
In a study of 488,175 women aged 15 to 44 years receiving care from clinicians at Kaiser Permanente Northern California, contraceptive counseling documentation was compared among women prescribed US Food and Drug Administration (FDA) category A or B medications (nonteratogenic) versus FDA category D or X medications (teratogenic).14 The rate at which women had no contraceptive counseling recorded was similar whether a teratogenic (47.6%) or nonteratogenic (46%) medication was prescribed. Clearly, there is a gap between current and optimal practice when teratogens are prescribed to women of reproductive age.
What could improve contraceptive services for women with serious medical problems?One promising approach is to include contraception status as a vital sign for all women and men of reproductive age. Most electronic medical records prioritize assessment of such health vital signs as allergies, smoking status, depression screening, falls prevention, blood pressure, temperature, heart rate, weight, and height. Contraception status is of equal importance to these vital signs in women and men of reproductive age and should be routinely documented.
Another intervention is to create a standard of care in which reproductive-age women with major medical problems are routinely referred to a clinician who has the time and skill to provide a comprehensive contraception visit. Health systems could take greater responsibility for managing the contraception practices of their members. For example, within a given accountable care organization the electronic health record could be used to identify adult women of reproductive age with diabetes and an HbA1c level greater than 7%. These women could be contacted to ascertain their contraception status and their need for a contraception health visit. Electronic health records could be utilized to identify all reproductive-age women taking a teratogenic medication. A computer-generated alert could be sent to the responsible clinician recommending referral to an obstetrician-gynecologist for a contraceptive services visit.15
Pharmacists could be more proactive in highlighting the importance of contraception for women prescribed teratogens and in recommending a contraceptive visit. In some states pharmacists can offer an oral hormonal contraceptive to women who are prescribed a teratogen and at risk for becoming pregnant.
How do you propose to address lack of counseling?As an experienced clinician, you likely have ideas about how to improve contraceptive counseling for women with significant medical problems. Please let me know what interventions you think would best improve the use of contraception in this group of high-risk women by emailing me at OBG Management: [email protected].
Tell us…What are your ideas to improve contraception counseling for women with significant medical problems that put them at high risk for adverse pregnancy outcomes?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- DeNoble AE, Hall KS, Xu X, Zochowski MK, Piehl K, Dalton VK. Receipt of prescription contraception by commercially insured women with chronic medical conditions. Obstet Gynecol. 2014;123(6):1213–1220.
- Champaloux SW, Tepper NK, Curtis KM, et al. Contraceptive use among women with medical conditions in a nationwide privately insured population. Obstet Gynecol. 2015;126(6):1151–1159.
- Klingensmith GJ, Pyle L, Nadeau KJ, et al; TODAY Study Group. Pregnancy outcomes in youth with type 2 diabetes: the TODAY study experience. Diabetes Care. 2016;39(1):122–129.
- Schwarz EB, Postlethwaite D, Hung YY, Lantzman E, Armstrong MA, Horberg MA. Provision of contraceptive services to women with diabetes mellitus. J Gen Int Med. 2011;27(2):196–201.
- Schwarz EB, Maselli J, Gonzales R. Contraceptive counseling of diabetic women of reproductive age. Obstet Gynecol. 2006;107(5):1070–1074.
- Chuang CH, Chase GA, Bensyl DM, Weisman CS. Contraceptive use by diabetic and obese women. Womens Health Issues. 2005;15(4):167–173.
- Lidegaard O. Hormonal contraception, thrombosis and age. Expert Opin Drug Safe. 2014;13(10):1353–1360.
- Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013:814062. doi.10.1155/2013/814062.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetric outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346–354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363–369.
- Perritt JB, Burke A, Jasmshidli R, Wang J, Fox M. Contraception counseling, pregnancy intention and contraception use in women with medical problems: an analysis of data from the Maryland Pregnancy Risk Assessment Monitoring System (PRAMS). Contraception. 2013;88(2):263–268.
- Stancil SL, Miller M, Briggs H, Lynch D, Goggin K, Kearns G. Contraceptive provision to adolescent females prescribed teratogenic medications. Pediatrics. 2016;137(1):1–8.
- Schwarz EB, Postlewaite DA, Hung YY, Armstrong MA. Documentation of contraception and pregnancy when prescribing potentially teratogenic medications for reproductive-age women. Ann Int Med. 2007;147(6):370–376.
- Mody SK, Wu J, Ornelas M, et al. Using the electronic medical record to refer women taking category D or X medications for teratogen and contraceptive counseling. Birth Defects Res A Clin Mol Teratol. 2015;103(7):644–647.
In the United States contraception practices are slowly improving, with robust evidence for the increased use of long-acting reversible contraceptives and preliminary data that the unintended pregnancy rate may be decreasing for the first time in many years.1 There remains a major gap in contraception practice, however: US women with chronic disease who are at high risk for adverse pregnancy outcomes are not receiving adequate contraceptive counseling or adequate contraception.2,3 In one study, the majority of women with hypertension, diabetes, epilepsy, stroke, heart disease, lupus, or thrombophilia were not using a prescription contraceptive.3
Recently, I have seen women with major medical problems, who have had many visits with specialists and primary care clinicians, but who have not had their contraceptive needs prioritized. Here are but a few examples:
- We recently cared for a patient with heart disease and severe pulmonary hypertension, who had many procedures performed by cardiologists and cardiac surgeons, but contraception had not been prioritized as one of her foremost medical needs.
- A young woman who had a pulmonary embolism 1-month postpartum reported that her primary care clinician said that she could never use any hormonal contraceptive, including the progestin-only pill, progestin-implant, and the progestin-releasing intrauterine device (IUD). She was not taking a contraceptive and had an unplanned pregnancy.
- A middle-aged woman with diabetes and a glycosylated hemoglo‑ bin A1c (HbA1c) value greater than 10% was regularly seeing her primary care clinician, but was not using an effective contraceptive. She became pregnant with a fetus that had a major congenital anomaly.
Clearly, there is a major gap between current and optimal contraceptive services for women with chronic medical problems. Women with diabetes and heart disease are affected substantially, as the evidence I present in this editorial indicates. Studies also show that women taking teratogenic medications do not receive the vital counseling that they should regarding contraception. Given the potential detrimental adverse events to both mother and fetus, obstetrician-gynecologists are poised to offer solutions to this concerning inadequacy of care.
DiabetesWomen with diabetes and an abnormally elevated HbA1c level are at high risk for many adverse pregnancy outcomes, including major congenital malformations and intrauterine fetal demise.4 Unfortunately, results of many studies indicate that women with diabetes are not receiving adequate contraceptive services.5–7 In one review of records at Kaiser Permanente Northern California, investigators reported that 62% of 122,921 healthy women, but only 48% of 8,182 women with diabetes, received contraceptive counseling, a contraceptive prescription, or contraceptive services.5
Why is it that so many women with diabetes do not receive contraceptive services? One possibility is that clinicians are reluctant to prescribe oral hormonal contraceptives that contain estrogen to their patients with diabetes because of a perceived increased risk of cardiovascular events.8 In the Kaiser study, 31% of the healthy women, and only 13% of the women with diabetes, were using a pill, patch, or ring (most of which contain estrogen).5 In this same study, the rate of utilization of an IUD was similar in the healthy (6.5%) and diabetic (5.6%) women. The IUD is known to be safe for use in women with diabetes.9
The low rate of utilization of intrauterine contraception by women with diabetes is a gap that gynecologists are well positioned to help solve.
Heart diseaseIn developed countries, a major cause of maternal mortality is pregnancy among women with congenital or acquired heart disease.10 Misinformation is a common problem in contraceptive counseling. In a recent study of 83 sexually active women with congenital heart disease, 6 women were told that they could not use an IUD or progestin-implant because they were unsafe for those with repaired congenital heart disease.11 In this cohort of women, who were at high risk for adverse pregnancy outcomes, 45% of pregnancies were unplanned, similar to the rate among healthy women.
On a positive note, authors of a small study from Maryland found that, among women with heart disease, the self-reported use of a contraceptive increased from 60% prepregnancy to 93% following delivery.12
Clearly, patient interaction with qualified women’s health clinicians can increase contraceptive use in those with high-risk medical issues.
Teratogenic medicationsShould reproductive-age women taking long-term methotrexate for treatment of rheumatoid conditions receive contraceptive counseling? The answer is clearly, “yes.” Methotrexate can cause fetal death or major congenital malformations, such as absence of digits and oxycephaly (premature closure of the skull sutures). All women of reproductive age prescribed known teratogens should receive effective contraception. Unfortunately, data do not indicate this is occurring.
In one study of 1,694 adolescents and young women aged 14 to 25 years who were prescribed a teratogen, only 29% received documented contraception counseling, and only 11% received a contraceptive prescription or were documented to be actively using a contraceptive.13 The most commonly prescribed teratogens in this study were topiramate, methotrexate, and isoretinoin. Among the specialists who prescribed the medications, dermatologists documented contraceptive counseling in 47% of visits—likely because of the federally mandated risk mitigation system for prescribing isoretinoin. Neurologists and hematologists were least likely to document contraceptive counseling, at 16% and 28%, respectively.13
In a study of 488,175 women aged 15 to 44 years receiving care from clinicians at Kaiser Permanente Northern California, contraceptive counseling documentation was compared among women prescribed US Food and Drug Administration (FDA) category A or B medications (nonteratogenic) versus FDA category D or X medications (teratogenic).14 The rate at which women had no contraceptive counseling recorded was similar whether a teratogenic (47.6%) or nonteratogenic (46%) medication was prescribed. Clearly, there is a gap between current and optimal practice when teratogens are prescribed to women of reproductive age.
What could improve contraceptive services for women with serious medical problems?One promising approach is to include contraception status as a vital sign for all women and men of reproductive age. Most electronic medical records prioritize assessment of such health vital signs as allergies, smoking status, depression screening, falls prevention, blood pressure, temperature, heart rate, weight, and height. Contraception status is of equal importance to these vital signs in women and men of reproductive age and should be routinely documented.
Another intervention is to create a standard of care in which reproductive-age women with major medical problems are routinely referred to a clinician who has the time and skill to provide a comprehensive contraception visit. Health systems could take greater responsibility for managing the contraception practices of their members. For example, within a given accountable care organization the electronic health record could be used to identify adult women of reproductive age with diabetes and an HbA1c level greater than 7%. These women could be contacted to ascertain their contraception status and their need for a contraception health visit. Electronic health records could be utilized to identify all reproductive-age women taking a teratogenic medication. A computer-generated alert could be sent to the responsible clinician recommending referral to an obstetrician-gynecologist for a contraceptive services visit.15
Pharmacists could be more proactive in highlighting the importance of contraception for women prescribed teratogens and in recommending a contraceptive visit. In some states pharmacists can offer an oral hormonal contraceptive to women who are prescribed a teratogen and at risk for becoming pregnant.
How do you propose to address lack of counseling?As an experienced clinician, you likely have ideas about how to improve contraceptive counseling for women with significant medical problems. Please let me know what interventions you think would best improve the use of contraception in this group of high-risk women by emailing me at OBG Management: [email protected].
Tell us…What are your ideas to improve contraception counseling for women with significant medical problems that put them at high risk for adverse pregnancy outcomes?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice
In the United States contraception practices are slowly improving, with robust evidence for the increased use of long-acting reversible contraceptives and preliminary data that the unintended pregnancy rate may be decreasing for the first time in many years.1 There remains a major gap in contraception practice, however: US women with chronic disease who are at high risk for adverse pregnancy outcomes are not receiving adequate contraceptive counseling or adequate contraception.2,3 In one study, the majority of women with hypertension, diabetes, epilepsy, stroke, heart disease, lupus, or thrombophilia were not using a prescription contraceptive.3
Recently, I have seen women with major medical problems, who have had many visits with specialists and primary care clinicians, but who have not had their contraceptive needs prioritized. Here are but a few examples:
- We recently cared for a patient with heart disease and severe pulmonary hypertension, who had many procedures performed by cardiologists and cardiac surgeons, but contraception had not been prioritized as one of her foremost medical needs.
- A young woman who had a pulmonary embolism 1-month postpartum reported that her primary care clinician said that she could never use any hormonal contraceptive, including the progestin-only pill, progestin-implant, and the progestin-releasing intrauterine device (IUD). She was not taking a contraceptive and had an unplanned pregnancy.
- A middle-aged woman with diabetes and a glycosylated hemoglo‑ bin A1c (HbA1c) value greater than 10% was regularly seeing her primary care clinician, but was not using an effective contraceptive. She became pregnant with a fetus that had a major congenital anomaly.
Clearly, there is a major gap between current and optimal contraceptive services for women with chronic medical problems. Women with diabetes and heart disease are affected substantially, as the evidence I present in this editorial indicates. Studies also show that women taking teratogenic medications do not receive the vital counseling that they should regarding contraception. Given the potential detrimental adverse events to both mother and fetus, obstetrician-gynecologists are poised to offer solutions to this concerning inadequacy of care.
DiabetesWomen with diabetes and an abnormally elevated HbA1c level are at high risk for many adverse pregnancy outcomes, including major congenital malformations and intrauterine fetal demise.4 Unfortunately, results of many studies indicate that women with diabetes are not receiving adequate contraceptive services.5–7 In one review of records at Kaiser Permanente Northern California, investigators reported that 62% of 122,921 healthy women, but only 48% of 8,182 women with diabetes, received contraceptive counseling, a contraceptive prescription, or contraceptive services.5
Why is it that so many women with diabetes do not receive contraceptive services? One possibility is that clinicians are reluctant to prescribe oral hormonal contraceptives that contain estrogen to their patients with diabetes because of a perceived increased risk of cardiovascular events.8 In the Kaiser study, 31% of the healthy women, and only 13% of the women with diabetes, were using a pill, patch, or ring (most of which contain estrogen).5 In this same study, the rate of utilization of an IUD was similar in the healthy (6.5%) and diabetic (5.6%) women. The IUD is known to be safe for use in women with diabetes.9
The low rate of utilization of intrauterine contraception by women with diabetes is a gap that gynecologists are well positioned to help solve.
Heart diseaseIn developed countries, a major cause of maternal mortality is pregnancy among women with congenital or acquired heart disease.10 Misinformation is a common problem in contraceptive counseling. In a recent study of 83 sexually active women with congenital heart disease, 6 women were told that they could not use an IUD or progestin-implant because they were unsafe for those with repaired congenital heart disease.11 In this cohort of women, who were at high risk for adverse pregnancy outcomes, 45% of pregnancies were unplanned, similar to the rate among healthy women.
On a positive note, authors of a small study from Maryland found that, among women with heart disease, the self-reported use of a contraceptive increased from 60% prepregnancy to 93% following delivery.12
Clearly, patient interaction with qualified women’s health clinicians can increase contraceptive use in those with high-risk medical issues.
Teratogenic medicationsShould reproductive-age women taking long-term methotrexate for treatment of rheumatoid conditions receive contraceptive counseling? The answer is clearly, “yes.” Methotrexate can cause fetal death or major congenital malformations, such as absence of digits and oxycephaly (premature closure of the skull sutures). All women of reproductive age prescribed known teratogens should receive effective contraception. Unfortunately, data do not indicate this is occurring.
In one study of 1,694 adolescents and young women aged 14 to 25 years who were prescribed a teratogen, only 29% received documented contraception counseling, and only 11% received a contraceptive prescription or were documented to be actively using a contraceptive.13 The most commonly prescribed teratogens in this study were topiramate, methotrexate, and isoretinoin. Among the specialists who prescribed the medications, dermatologists documented contraceptive counseling in 47% of visits—likely because of the federally mandated risk mitigation system for prescribing isoretinoin. Neurologists and hematologists were least likely to document contraceptive counseling, at 16% and 28%, respectively.13
In a study of 488,175 women aged 15 to 44 years receiving care from clinicians at Kaiser Permanente Northern California, contraceptive counseling documentation was compared among women prescribed US Food and Drug Administration (FDA) category A or B medications (nonteratogenic) versus FDA category D or X medications (teratogenic).14 The rate at which women had no contraceptive counseling recorded was similar whether a teratogenic (47.6%) or nonteratogenic (46%) medication was prescribed. Clearly, there is a gap between current and optimal practice when teratogens are prescribed to women of reproductive age.
What could improve contraceptive services for women with serious medical problems?One promising approach is to include contraception status as a vital sign for all women and men of reproductive age. Most electronic medical records prioritize assessment of such health vital signs as allergies, smoking status, depression screening, falls prevention, blood pressure, temperature, heart rate, weight, and height. Contraception status is of equal importance to these vital signs in women and men of reproductive age and should be routinely documented.
Another intervention is to create a standard of care in which reproductive-age women with major medical problems are routinely referred to a clinician who has the time and skill to provide a comprehensive contraception visit. Health systems could take greater responsibility for managing the contraception practices of their members. For example, within a given accountable care organization the electronic health record could be used to identify adult women of reproductive age with diabetes and an HbA1c level greater than 7%. These women could be contacted to ascertain their contraception status and their need for a contraception health visit. Electronic health records could be utilized to identify all reproductive-age women taking a teratogenic medication. A computer-generated alert could be sent to the responsible clinician recommending referral to an obstetrician-gynecologist for a contraceptive services visit.15
Pharmacists could be more proactive in highlighting the importance of contraception for women prescribed teratogens and in recommending a contraceptive visit. In some states pharmacists can offer an oral hormonal contraceptive to women who are prescribed a teratogen and at risk for becoming pregnant.
How do you propose to address lack of counseling?As an experienced clinician, you likely have ideas about how to improve contraceptive counseling for women with significant medical problems. Please let me know what interventions you think would best improve the use of contraception in this group of high-risk women by emailing me at OBG Management: [email protected].
Tell us…What are your ideas to improve contraception counseling for women with significant medical problems that put them at high risk for adverse pregnancy outcomes?
Send your letter to the editor to [email protected]. Please include the city and state in which you practice
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- DeNoble AE, Hall KS, Xu X, Zochowski MK, Piehl K, Dalton VK. Receipt of prescription contraception by commercially insured women with chronic medical conditions. Obstet Gynecol. 2014;123(6):1213–1220.
- Champaloux SW, Tepper NK, Curtis KM, et al. Contraceptive use among women with medical conditions in a nationwide privately insured population. Obstet Gynecol. 2015;126(6):1151–1159.
- Klingensmith GJ, Pyle L, Nadeau KJ, et al; TODAY Study Group. Pregnancy outcomes in youth with type 2 diabetes: the TODAY study experience. Diabetes Care. 2016;39(1):122–129.
- Schwarz EB, Postlethwaite D, Hung YY, Lantzman E, Armstrong MA, Horberg MA. Provision of contraceptive services to women with diabetes mellitus. J Gen Int Med. 2011;27(2):196–201.
- Schwarz EB, Maselli J, Gonzales R. Contraceptive counseling of diabetic women of reproductive age. Obstet Gynecol. 2006;107(5):1070–1074.
- Chuang CH, Chase GA, Bensyl DM, Weisman CS. Contraceptive use by diabetic and obese women. Womens Health Issues. 2005;15(4):167–173.
- Lidegaard O. Hormonal contraception, thrombosis and age. Expert Opin Drug Safe. 2014;13(10):1353–1360.
- Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013:814062. doi.10.1155/2013/814062.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetric outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346–354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363–369.
- Perritt JB, Burke A, Jasmshidli R, Wang J, Fox M. Contraception counseling, pregnancy intention and contraception use in women with medical problems: an analysis of data from the Maryland Pregnancy Risk Assessment Monitoring System (PRAMS). Contraception. 2013;88(2):263–268.
- Stancil SL, Miller M, Briggs H, Lynch D, Goggin K, Kearns G. Contraceptive provision to adolescent females prescribed teratogenic medications. Pediatrics. 2016;137(1):1–8.
- Schwarz EB, Postlewaite DA, Hung YY, Armstrong MA. Documentation of contraception and pregnancy when prescribing potentially teratogenic medications for reproductive-age women. Ann Int Med. 2007;147(6):370–376.
- Mody SK, Wu J, Ornelas M, et al. Using the electronic medical record to refer women taking category D or X medications for teratogen and contraceptive counseling. Birth Defects Res A Clin Mol Teratol. 2015;103(7):644–647.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- DeNoble AE, Hall KS, Xu X, Zochowski MK, Piehl K, Dalton VK. Receipt of prescription contraception by commercially insured women with chronic medical conditions. Obstet Gynecol. 2014;123(6):1213–1220.
- Champaloux SW, Tepper NK, Curtis KM, et al. Contraceptive use among women with medical conditions in a nationwide privately insured population. Obstet Gynecol. 2015;126(6):1151–1159.
- Klingensmith GJ, Pyle L, Nadeau KJ, et al; TODAY Study Group. Pregnancy outcomes in youth with type 2 diabetes: the TODAY study experience. Diabetes Care. 2016;39(1):122–129.
- Schwarz EB, Postlethwaite D, Hung YY, Lantzman E, Armstrong MA, Horberg MA. Provision of contraceptive services to women with diabetes mellitus. J Gen Int Med. 2011;27(2):196–201.
- Schwarz EB, Maselli J, Gonzales R. Contraceptive counseling of diabetic women of reproductive age. Obstet Gynecol. 2006;107(5):1070–1074.
- Chuang CH, Chase GA, Bensyl DM, Weisman CS. Contraceptive use by diabetic and obese women. Womens Health Issues. 2005;15(4):167–173.
- Lidegaard O. Hormonal contraception, thrombosis and age. Expert Opin Drug Safe. 2014;13(10):1353–1360.
- Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013:814062. doi.10.1155/2013/814062.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetric outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346–354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363–369.
- Perritt JB, Burke A, Jasmshidli R, Wang J, Fox M. Contraception counseling, pregnancy intention and contraception use in women with medical problems: an analysis of data from the Maryland Pregnancy Risk Assessment Monitoring System (PRAMS). Contraception. 2013;88(2):263–268.
- Stancil SL, Miller M, Briggs H, Lynch D, Goggin K, Kearns G. Contraceptive provision to adolescent females prescribed teratogenic medications. Pediatrics. 2016;137(1):1–8.
- Schwarz EB, Postlewaite DA, Hung YY, Armstrong MA. Documentation of contraception and pregnancy when prescribing potentially teratogenic medications for reproductive-age women. Ann Int Med. 2007;147(6):370–376.
- Mody SK, Wu J, Ornelas M, et al. Using the electronic medical record to refer women taking category D or X medications for teratogen and contraceptive counseling. Birth Defects Res A Clin Mol Teratol. 2015;103(7):644–647.