User login
Recognize and treat iron deficiency anemia in pregnant women
All mammalian life is dependent on a continuous supply of molecular oxygen. Molecular oxygen is carried to cells by noncovalent binding to the iron moiety in the hemoglobin of red blood cells. It is utilized within cells by noncovalent binding to the iron moiety in various microsomal and mitochondrial proteins, including myoglobin and cytochromes. Consequently, to efficiently utilize molecular oxygen all mammalian life is dependent on an adequate supply of iron. Surprisingly, in an era of high technology precision medicine, many pregnant women are iron deficient, anemic, and not receiving adequate iron supplementation.
Iron deficiency is prevalent in women and pregnant women
Women often become iron deficient because of pregnancy or heavy menstrual bleeding. During pregnancy, maternal iron is provided to supply the needs of the fetus and placenta. Additional iron is needed to expand maternal red blood cell volume and replace iron lost due to bleeding at delivery. In the National Health and Nutrition Examination Survey (NHANES) of 1988–1994, 11% of women aged 16 to 49 years were iron deficient. By contrast, less than 1% of men aged 16 to 49 years were iron deficient.1
In a NHANES study from 1999–2006, risk factors for iron deficiency included multiparity, current pregnancy, and regular menstrual cycles. Use of hormonal contraception reduced the rate of iron deficiency.2 Using the same data, the prevalences of iron deficiency during the first, second, and third trimesters of pregnancy were reported to be 7%, 14%, and 30%, respectively.3 In addition to pregnancy and menstrual bleeding there are many other medical problems that may contribute to iron deficiency, including Helicobacter pylori (H pylori) infection, gastritis, celiac disease, and bariatric surgery.
Iron deficiency anemia may be associated with adverse pregnancy outcomes
In a retrospective study of 75,660 singleton pregnancies, 7,977 women were diagnosed with iron deficiency anemia when they were admitted for delivery. Compared with pregnant women without iron deficiency, the presence of iron deficiency increased the risk of:
- blood transfusion (odds ratio [OR], 5.48; 95% confidence interval [CI], 4.57–6.58)
- preterm delivery (OR, 1.54; 95% CI, 1.36–1.76)
- cesarean delivery (OR, 1.30; 95% CI, 1.13–1.49)
- 5-minute Apgar score <7 (OR, 2.21; 95% CI, 1.84–2.64)
- intensive care unit (ICU) admission (OR, 1.28; 95% CI, 1.20–1.39).4
In a systematic review and meta-analysis of 26 studies, maternal anemia (mostly iron deficiency anemia) was associated with a higher risk of low birth weight (relative risk [RR], 1.31; 95% CI, 1.13–1.51), preterm birth (RR, 1.63; 95% CI, 1.33–2.01), perinatal mortality (RR, 1.51; 95% CI, 1.30–1.76), and neonatal mortality (RR, 2.72; 95% CI, 1.19–6.25).5
In a clinical trial, pregnant women were randomly assigned to receive folic acid alone; folic acid plus iron supplements; or 15 vitamins and minerals, including folic acid and iron. At delivery, women in the iron-folic acid and the 15 vitamin and minerals groups had higher hemoglobin concentrations than the folic acid monotherapy group. Among 4,697 live births, women in the iron-folic acid group had significantly fewer preterm births (<34 weeks’ gestation) than the folic acid group (RR, 0.50; 95% CI, 0.27–0.94; P = .031).6 Data from additional randomized trials are needed to further clarify the effect of iron supplementation on obstetric outcomes.
Related article:
Treating polycystic ovary syndrome: Start using dual medical therapy
The diagnosis of iron deficiency is optimized by measuring serum ferritin
Serum ferritin measurement is an excellent test of iron deficiency. We recommend that all pregnant women have serum ferritin measured at the first prenatal visit and at the beginning of the third trimester to assess maternal iron stores. In pregnancy, the Centers for Disease Control and Prevention and the World Health Organization define anemia as a hemoglobin level of less than 11 g/dL or hematocrit less than 33% in the first and third trimesters. If a pregnant woman is not anemic, a serum ferritin level less than 15 ng/mL indicates iron deficiency.7 Some experts believe that in pregnant women who are not anemic, a serum ferritin level between 15 and 30 ng/mL may also indicate iron deficiency.8 If the pregnant woman is anemic and does not have another cause of the anemia, a serum ferritin level less than 40 ng/mL is indicative of iron deficiency.7
Ferritin is an acute phase reactant and levels may be falsely elevated due to chronic or acute inflammation, liver disease, renal failure, metabolic syndrome, or malignancy. Some women with iron deficiency due to bariatric surgery or malabsorption also have vitamin B12 and, less commonly, folate deficiency, which can contribute to the development of anemia (see “Diagnosis of anemia, iron deficiency, and iron deficiency anemia in pregnancy.”) Clinicians are often advised that a mean corpuscular volume demonstrating microcytosis is the “best test” to assess a patient for iron deficiency. However, reduced iron availability and low ferritin precede microcytosis. Hence microcytosis is a lagging measure and iron deficiency is diagnosed at an earlier stage by ferritin.
Requirements for a diagnosis of anemia in pregnancy
The American College of Obstetricians and Gynecologists recommends obtaining a hemoglobin and hematocrit test at the first prenatal visit and at the beginning of the third trimester of pregnancy.1
If the hemoglobin concentration is less than 11 g/dL, or hematocrit is less than 33%, anemia is present.2,3
If anemia is diagnosed, additional testing to investigate potential causes of anemia includes hemoglobin electrophoresis and measurement of vitamin B12 and folate levels. Many obstetricians perform hemoglobin electrophoresis on all their pregnant patients as part of the routine prenatal screen.
Requirements for a diagnosis of iron deficiency in pregnancy
We recommend obtaining a ferritin measurement at the first prenatal visit and at the beginning of the third trimester.
In pregnant women with anemia, iron deficiency is present if the ferritin is less than 40 ng/mL.
If a pregnant woman is not anemic, iron deficiency is present if the ferritin is less than 15 ng/mL.4
Requirements for a diagnosis of iron deficiency anemia
Hemoglobin concentration less than 11 g/dL, or hematocrit less than 33% (diagnosis of anemia).
PLUS
Ferritin less than 40 ng/mL (diagnosis of iron deficiency in an anemic woman)
PLUS
Evaluation for other known major causes of anemia, including blood loss, hemolysis, bone marrow disease, medications that suppress bone marrow function, kidney disease, malignancy, hemoglobinopathy, and vitamin B12 or folate deficiency.
References
- Guidelines for Perinatal Care. 8th ed. Washington DC: American Academy of Pediatrics, American College of Obstetricians and Gynecologists;2017.
- Centers for Disease Control and Prevention. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep. 1989;38(22):400-404.
- World Health Organization. Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. World Health Organization: Geneva, Switzerland; 2001. http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. Accessed November 8, 2017.
- Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency: an overview. J Gen Intern Med. 1992;7(2):145-153.
Dietary iron
Iron in food is present in heme (meat, poultry, fish) and non-heme forms (grains, plant food, supplements). Heme iron is better absorbed than non-heme iron. Foods rich in non-heme iron include spinach, lentils, prune juice, dried prunes, and fortified cereals. Absorption of non-heme iron can be increased by vitamin C or vitamin C–rich foods (broccoli, bell peppers, cantaloupe, grapefruit, oranges, strawberries, and tomatoes). Absorption of non-heme iron is reduced by consumption of dairy products, coffee, tea, and chocolate.
Oral iron treatment
Oral iron is an effective treatment for iron deficiency9,10 and is inexpensive, safe, and widely available. The CDC recommends that all pregnant women take a 30 mg/day iron supplement, unless they have hemochromatosis.11 For women with a low ferritin level and anemia, iron supplementation should be increased to 30 to 120 mg daily.11 Not all prenatal vitamins contain iron; those that do typically contain 17 to 28 mg of elemental iron per dose.
Many pregnant women taking oral iron, especially at doses greater than 30 mg daily, have gastrointestinal side effects, which cause them to discontinue the iron therapy.12 Taking iron supplementation on an intermittent basis may help to reduce gastrointestinal side effects and improve iron stores.13
In the past, a standard approach to the treatment of iron deficiency anemia was oral ferrous sulfate 325 mg (65 mg elemental iron) spaced in 3 doses each day for a total daily dose of 195 mg elemental iron. However, recent absorption studies concluded that maximal absorption of iron occurs with a dose in the range of 40 to 80 mg of elemental iron daily. Greater doses do not result in more iron absorption and are associated with more side effects.14,15 (See “Start using alternate-day oral iron dosing, and stop using daily iron dosing.”)
Recent research reports alternate-day oral iron dosing compared with daily oral iron dosing results in higher absorption of iron.
Details of the study
A total of 40 iron deficient women (mean serum ferritin level, 14 ng/mL) were randomly assigned to receive a daily dose of 60 mg of elemental iron (325 mg of ferrous sulfate) for 14 days or an alternate-day dose of 60 mg for 28 days. A small amount of radioactive iron was added to the oral medication to assess iron absorption. The primary outcome was fractional and total iron absorption, calculated by measuring radioactive iron in circulating red blood cells 14 days after the final oral iron dose.
Alternate-day iron dosing, compared with daily dosing, resulted in a higher fraction of the iron dose being absorbed (22% vs 16%; P = .0013). In addition, alternate-day iron dosing resulted in greater cumulative total iron absorption (175 mg vs 131 mg; P = .001). Nausea was reported less frequently by women in the alternate-day dosing group (11%) than in the daily iron dose group (29%).
The investigators concluded that prescribing iron as a single alternate-day
dose may be a superior dosing regimen compared with daily dosing.
Reference
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4(11):e524–e533.
Oral iron should not be taken in close approximation to the consumption of milk, cereals, tea, coffee, eggs, or calcium supplements. The absorption of oral iron is enhanced by the consumption of orange juice or 250 mg of vitamin C. Gastrointestinal side effects include nausea, flatulence, constipation, diarrhea, epigastric distress, and vomiting. If gastrointestinal side effects occur, interventions that might improve tolerability include: reduce the dose of iron or administer intermittently or use a low dose of oral iron, where dosing can be more easily titrated.
We re-check ferritin and hemoglobin levels 2 to 4 weeks after initiation of oral iron therapy and expect to see a hemoglobin rise of 1 g/dL if the therapy is effective.
Intravenous iron treatment
For women with iron deficiency anemia who cannot tolerate oral iron or in whom oral iron treatment has not resolved their anemia, intravenous (IV) iron treatment may be an optimal approach. Women in the third trimester of pregnancy with iron deficiency anemia have very little time to consume sufficient quantities of oral iron in food and supplements to restore their deficiency and reverse their anemia. Consequently, treatment with IV iron may be especially appropriate for women with iron deficiency anemia in the third trimester of pregnancy. Prior gastric surgery, including gastric bypass, results in reduced gastric acid production and causes severe impairment of intestinal absorption of iron. Patients with malabsorption syndromes, including celiac disease, also may have limited absorption of oral iron. These populations of pregnant women may particularly benefit from the use of IV iron. In pregnant women IV iron has fewer gastrointestinal side effects than oral iron.16
Many severely iron deficient patients need 1,000 mg of iron to resolve their deficit. In order to avoid giving multiple standard doses (200 mg per infusion, with 5 infusions over many days), some centers have explored the use of 1 large dose of IV iron (1,000 mg of low molecular weight iron dextran administered over 1 hour) (INFeD, Watson Pharma).17–19 This is not a regimen that is specifically approved by the US Food and Drug Administration. An alternative regimen is to administer 750 mg of ferrous carboxymaltose (Injectafer, Luitpold Pharmaceuticals) over 15 minutes, which is an FDA-approved regimen.18 Many hematologists prefer to administer multiple smaller doses of iron. For example, in our practice, pregnant women are commonly treated with IV iron sucrose (300 mg) every 2 weeks for 3 doses. To increase access of pregnant women to IV iron treatment, obstetricians need to work with hematologists and infusion centers to create collaborative protocols to expeditiously treat women in the third trimester.
There is an epidemic of iron deficiency in pregnant women in the United States. In an era of high technology medicine, it is surprising that iron deficiency remains an unsolved obstetric problem in our country.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277(12):973–976.
- Miller EM. Iron status and reproduction in US women: National Health and Nutrition Examination Survey 1999–2006. PLoS One. 2014;9(11):e112216.
- Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93(6):1312–1320.
- Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55(12):2799–2806.
- Rahmann MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495–504.
- Zeng L, Dibley MJ, Cheng Y, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:a2001.
- Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency: an overview. J Gen Intern Med. 1992;7(2):145–153.
- van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol. 1998;103(3):817–824.
- Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015(7);CD004736.
- Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2015;162(8):566–576.
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1–29.
- Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117383.
- Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015(10);CD009997.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981–1989.
- Schrier SL. So you know how to treat iron deficiency anemia. Blood. 2015;126(17):1971.
- Breymann C, Milman N, Mezzacasa A, Bernard R, Dudenhausen J; FER-ASAP investigators. Ferric carboxymaltose vs oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinatal Med. 2017;45(4):443–453.
- Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol. 2011;86(10):860–862.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91(1):31–38.
- Wong L, Smith S, Gilstrop M, et al. Safety and efficacy of rapid (1,000 mg in 1 hr) intravenous iron dextran for treatment of maternal iron deficient anemia of pregnancy. Am J Hematol. 2016;91(6):590–593.
All mammalian life is dependent on a continuous supply of molecular oxygen. Molecular oxygen is carried to cells by noncovalent binding to the iron moiety in the hemoglobin of red blood cells. It is utilized within cells by noncovalent binding to the iron moiety in various microsomal and mitochondrial proteins, including myoglobin and cytochromes. Consequently, to efficiently utilize molecular oxygen all mammalian life is dependent on an adequate supply of iron. Surprisingly, in an era of high technology precision medicine, many pregnant women are iron deficient, anemic, and not receiving adequate iron supplementation.
Iron deficiency is prevalent in women and pregnant women
Women often become iron deficient because of pregnancy or heavy menstrual bleeding. During pregnancy, maternal iron is provided to supply the needs of the fetus and placenta. Additional iron is needed to expand maternal red blood cell volume and replace iron lost due to bleeding at delivery. In the National Health and Nutrition Examination Survey (NHANES) of 1988–1994, 11% of women aged 16 to 49 years were iron deficient. By contrast, less than 1% of men aged 16 to 49 years were iron deficient.1
In a NHANES study from 1999–2006, risk factors for iron deficiency included multiparity, current pregnancy, and regular menstrual cycles. Use of hormonal contraception reduced the rate of iron deficiency.2 Using the same data, the prevalences of iron deficiency during the first, second, and third trimesters of pregnancy were reported to be 7%, 14%, and 30%, respectively.3 In addition to pregnancy and menstrual bleeding there are many other medical problems that may contribute to iron deficiency, including Helicobacter pylori (H pylori) infection, gastritis, celiac disease, and bariatric surgery.
Iron deficiency anemia may be associated with adverse pregnancy outcomes
In a retrospective study of 75,660 singleton pregnancies, 7,977 women were diagnosed with iron deficiency anemia when they were admitted for delivery. Compared with pregnant women without iron deficiency, the presence of iron deficiency increased the risk of:
- blood transfusion (odds ratio [OR], 5.48; 95% confidence interval [CI], 4.57–6.58)
- preterm delivery (OR, 1.54; 95% CI, 1.36–1.76)
- cesarean delivery (OR, 1.30; 95% CI, 1.13–1.49)
- 5-minute Apgar score <7 (OR, 2.21; 95% CI, 1.84–2.64)
- intensive care unit (ICU) admission (OR, 1.28; 95% CI, 1.20–1.39).4
In a systematic review and meta-analysis of 26 studies, maternal anemia (mostly iron deficiency anemia) was associated with a higher risk of low birth weight (relative risk [RR], 1.31; 95% CI, 1.13–1.51), preterm birth (RR, 1.63; 95% CI, 1.33–2.01), perinatal mortality (RR, 1.51; 95% CI, 1.30–1.76), and neonatal mortality (RR, 2.72; 95% CI, 1.19–6.25).5
In a clinical trial, pregnant women were randomly assigned to receive folic acid alone; folic acid plus iron supplements; or 15 vitamins and minerals, including folic acid and iron. At delivery, women in the iron-folic acid and the 15 vitamin and minerals groups had higher hemoglobin concentrations than the folic acid monotherapy group. Among 4,697 live births, women in the iron-folic acid group had significantly fewer preterm births (<34 weeks’ gestation) than the folic acid group (RR, 0.50; 95% CI, 0.27–0.94; P = .031).6 Data from additional randomized trials are needed to further clarify the effect of iron supplementation on obstetric outcomes.
Related article:
Treating polycystic ovary syndrome: Start using dual medical therapy
The diagnosis of iron deficiency is optimized by measuring serum ferritin
Serum ferritin measurement is an excellent test of iron deficiency. We recommend that all pregnant women have serum ferritin measured at the first prenatal visit and at the beginning of the third trimester to assess maternal iron stores. In pregnancy, the Centers for Disease Control and Prevention and the World Health Organization define anemia as a hemoglobin level of less than 11 g/dL or hematocrit less than 33% in the first and third trimesters. If a pregnant woman is not anemic, a serum ferritin level less than 15 ng/mL indicates iron deficiency.7 Some experts believe that in pregnant women who are not anemic, a serum ferritin level between 15 and 30 ng/mL may also indicate iron deficiency.8 If the pregnant woman is anemic and does not have another cause of the anemia, a serum ferritin level less than 40 ng/mL is indicative of iron deficiency.7
Ferritin is an acute phase reactant and levels may be falsely elevated due to chronic or acute inflammation, liver disease, renal failure, metabolic syndrome, or malignancy. Some women with iron deficiency due to bariatric surgery or malabsorption also have vitamin B12 and, less commonly, folate deficiency, which can contribute to the development of anemia (see “Diagnosis of anemia, iron deficiency, and iron deficiency anemia in pregnancy.”) Clinicians are often advised that a mean corpuscular volume demonstrating microcytosis is the “best test” to assess a patient for iron deficiency. However, reduced iron availability and low ferritin precede microcytosis. Hence microcytosis is a lagging measure and iron deficiency is diagnosed at an earlier stage by ferritin.
Requirements for a diagnosis of anemia in pregnancy
The American College of Obstetricians and Gynecologists recommends obtaining a hemoglobin and hematocrit test at the first prenatal visit and at the beginning of the third trimester of pregnancy.1
If the hemoglobin concentration is less than 11 g/dL, or hematocrit is less than 33%, anemia is present.2,3
If anemia is diagnosed, additional testing to investigate potential causes of anemia includes hemoglobin electrophoresis and measurement of vitamin B12 and folate levels. Many obstetricians perform hemoglobin electrophoresis on all their pregnant patients as part of the routine prenatal screen.
Requirements for a diagnosis of iron deficiency in pregnancy
We recommend obtaining a ferritin measurement at the first prenatal visit and at the beginning of the third trimester.
In pregnant women with anemia, iron deficiency is present if the ferritin is less than 40 ng/mL.
If a pregnant woman is not anemic, iron deficiency is present if the ferritin is less than 15 ng/mL.4
Requirements for a diagnosis of iron deficiency anemia
Hemoglobin concentration less than 11 g/dL, or hematocrit less than 33% (diagnosis of anemia).
PLUS
Ferritin less than 40 ng/mL (diagnosis of iron deficiency in an anemic woman)
PLUS
Evaluation for other known major causes of anemia, including blood loss, hemolysis, bone marrow disease, medications that suppress bone marrow function, kidney disease, malignancy, hemoglobinopathy, and vitamin B12 or folate deficiency.
References
- Guidelines for Perinatal Care. 8th ed. Washington DC: American Academy of Pediatrics, American College of Obstetricians and Gynecologists;2017.
- Centers for Disease Control and Prevention. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep. 1989;38(22):400-404.
- World Health Organization. Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. World Health Organization: Geneva, Switzerland; 2001. http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. Accessed November 8, 2017.
- Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency: an overview. J Gen Intern Med. 1992;7(2):145-153.
Dietary iron
Iron in food is present in heme (meat, poultry, fish) and non-heme forms (grains, plant food, supplements). Heme iron is better absorbed than non-heme iron. Foods rich in non-heme iron include spinach, lentils, prune juice, dried prunes, and fortified cereals. Absorption of non-heme iron can be increased by vitamin C or vitamin C–rich foods (broccoli, bell peppers, cantaloupe, grapefruit, oranges, strawberries, and tomatoes). Absorption of non-heme iron is reduced by consumption of dairy products, coffee, tea, and chocolate.
Oral iron treatment
Oral iron is an effective treatment for iron deficiency9,10 and is inexpensive, safe, and widely available. The CDC recommends that all pregnant women take a 30 mg/day iron supplement, unless they have hemochromatosis.11 For women with a low ferritin level and anemia, iron supplementation should be increased to 30 to 120 mg daily.11 Not all prenatal vitamins contain iron; those that do typically contain 17 to 28 mg of elemental iron per dose.
Many pregnant women taking oral iron, especially at doses greater than 30 mg daily, have gastrointestinal side effects, which cause them to discontinue the iron therapy.12 Taking iron supplementation on an intermittent basis may help to reduce gastrointestinal side effects and improve iron stores.13
In the past, a standard approach to the treatment of iron deficiency anemia was oral ferrous sulfate 325 mg (65 mg elemental iron) spaced in 3 doses each day for a total daily dose of 195 mg elemental iron. However, recent absorption studies concluded that maximal absorption of iron occurs with a dose in the range of 40 to 80 mg of elemental iron daily. Greater doses do not result in more iron absorption and are associated with more side effects.14,15 (See “Start using alternate-day oral iron dosing, and stop using daily iron dosing.”)
Recent research reports alternate-day oral iron dosing compared with daily oral iron dosing results in higher absorption of iron.
Details of the study
A total of 40 iron deficient women (mean serum ferritin level, 14 ng/mL) were randomly assigned to receive a daily dose of 60 mg of elemental iron (325 mg of ferrous sulfate) for 14 days or an alternate-day dose of 60 mg for 28 days. A small amount of radioactive iron was added to the oral medication to assess iron absorption. The primary outcome was fractional and total iron absorption, calculated by measuring radioactive iron in circulating red blood cells 14 days after the final oral iron dose.
Alternate-day iron dosing, compared with daily dosing, resulted in a higher fraction of the iron dose being absorbed (22% vs 16%; P = .0013). In addition, alternate-day iron dosing resulted in greater cumulative total iron absorption (175 mg vs 131 mg; P = .001). Nausea was reported less frequently by women in the alternate-day dosing group (11%) than in the daily iron dose group (29%).
The investigators concluded that prescribing iron as a single alternate-day
dose may be a superior dosing regimen compared with daily dosing.
Reference
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4(11):e524–e533.
Oral iron should not be taken in close approximation to the consumption of milk, cereals, tea, coffee, eggs, or calcium supplements. The absorption of oral iron is enhanced by the consumption of orange juice or 250 mg of vitamin C. Gastrointestinal side effects include nausea, flatulence, constipation, diarrhea, epigastric distress, and vomiting. If gastrointestinal side effects occur, interventions that might improve tolerability include: reduce the dose of iron or administer intermittently or use a low dose of oral iron, where dosing can be more easily titrated.
We re-check ferritin and hemoglobin levels 2 to 4 weeks after initiation of oral iron therapy and expect to see a hemoglobin rise of 1 g/dL if the therapy is effective.
Intravenous iron treatment
For women with iron deficiency anemia who cannot tolerate oral iron or in whom oral iron treatment has not resolved their anemia, intravenous (IV) iron treatment may be an optimal approach. Women in the third trimester of pregnancy with iron deficiency anemia have very little time to consume sufficient quantities of oral iron in food and supplements to restore their deficiency and reverse their anemia. Consequently, treatment with IV iron may be especially appropriate for women with iron deficiency anemia in the third trimester of pregnancy. Prior gastric surgery, including gastric bypass, results in reduced gastric acid production and causes severe impairment of intestinal absorption of iron. Patients with malabsorption syndromes, including celiac disease, also may have limited absorption of oral iron. These populations of pregnant women may particularly benefit from the use of IV iron. In pregnant women IV iron has fewer gastrointestinal side effects than oral iron.16
Many severely iron deficient patients need 1,000 mg of iron to resolve their deficit. In order to avoid giving multiple standard doses (200 mg per infusion, with 5 infusions over many days), some centers have explored the use of 1 large dose of IV iron (1,000 mg of low molecular weight iron dextran administered over 1 hour) (INFeD, Watson Pharma).17–19 This is not a regimen that is specifically approved by the US Food and Drug Administration. An alternative regimen is to administer 750 mg of ferrous carboxymaltose (Injectafer, Luitpold Pharmaceuticals) over 15 minutes, which is an FDA-approved regimen.18 Many hematologists prefer to administer multiple smaller doses of iron. For example, in our practice, pregnant women are commonly treated with IV iron sucrose (300 mg) every 2 weeks for 3 doses. To increase access of pregnant women to IV iron treatment, obstetricians need to work with hematologists and infusion centers to create collaborative protocols to expeditiously treat women in the third trimester.
There is an epidemic of iron deficiency in pregnant women in the United States. In an era of high technology medicine, it is surprising that iron deficiency remains an unsolved obstetric problem in our country.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
All mammalian life is dependent on a continuous supply of molecular oxygen. Molecular oxygen is carried to cells by noncovalent binding to the iron moiety in the hemoglobin of red blood cells. It is utilized within cells by noncovalent binding to the iron moiety in various microsomal and mitochondrial proteins, including myoglobin and cytochromes. Consequently, to efficiently utilize molecular oxygen all mammalian life is dependent on an adequate supply of iron. Surprisingly, in an era of high technology precision medicine, many pregnant women are iron deficient, anemic, and not receiving adequate iron supplementation.
Iron deficiency is prevalent in women and pregnant women
Women often become iron deficient because of pregnancy or heavy menstrual bleeding. During pregnancy, maternal iron is provided to supply the needs of the fetus and placenta. Additional iron is needed to expand maternal red blood cell volume and replace iron lost due to bleeding at delivery. In the National Health and Nutrition Examination Survey (NHANES) of 1988–1994, 11% of women aged 16 to 49 years were iron deficient. By contrast, less than 1% of men aged 16 to 49 years were iron deficient.1
In a NHANES study from 1999–2006, risk factors for iron deficiency included multiparity, current pregnancy, and regular menstrual cycles. Use of hormonal contraception reduced the rate of iron deficiency.2 Using the same data, the prevalences of iron deficiency during the first, second, and third trimesters of pregnancy were reported to be 7%, 14%, and 30%, respectively.3 In addition to pregnancy and menstrual bleeding there are many other medical problems that may contribute to iron deficiency, including Helicobacter pylori (H pylori) infection, gastritis, celiac disease, and bariatric surgery.
Iron deficiency anemia may be associated with adverse pregnancy outcomes
In a retrospective study of 75,660 singleton pregnancies, 7,977 women were diagnosed with iron deficiency anemia when they were admitted for delivery. Compared with pregnant women without iron deficiency, the presence of iron deficiency increased the risk of:
- blood transfusion (odds ratio [OR], 5.48; 95% confidence interval [CI], 4.57–6.58)
- preterm delivery (OR, 1.54; 95% CI, 1.36–1.76)
- cesarean delivery (OR, 1.30; 95% CI, 1.13–1.49)
- 5-minute Apgar score <7 (OR, 2.21; 95% CI, 1.84–2.64)
- intensive care unit (ICU) admission (OR, 1.28; 95% CI, 1.20–1.39).4
In a systematic review and meta-analysis of 26 studies, maternal anemia (mostly iron deficiency anemia) was associated with a higher risk of low birth weight (relative risk [RR], 1.31; 95% CI, 1.13–1.51), preterm birth (RR, 1.63; 95% CI, 1.33–2.01), perinatal mortality (RR, 1.51; 95% CI, 1.30–1.76), and neonatal mortality (RR, 2.72; 95% CI, 1.19–6.25).5
In a clinical trial, pregnant women were randomly assigned to receive folic acid alone; folic acid plus iron supplements; or 15 vitamins and minerals, including folic acid and iron. At delivery, women in the iron-folic acid and the 15 vitamin and minerals groups had higher hemoglobin concentrations than the folic acid monotherapy group. Among 4,697 live births, women in the iron-folic acid group had significantly fewer preterm births (<34 weeks’ gestation) than the folic acid group (RR, 0.50; 95% CI, 0.27–0.94; P = .031).6 Data from additional randomized trials are needed to further clarify the effect of iron supplementation on obstetric outcomes.
Related article:
Treating polycystic ovary syndrome: Start using dual medical therapy
The diagnosis of iron deficiency is optimized by measuring serum ferritin
Serum ferritin measurement is an excellent test of iron deficiency. We recommend that all pregnant women have serum ferritin measured at the first prenatal visit and at the beginning of the third trimester to assess maternal iron stores. In pregnancy, the Centers for Disease Control and Prevention and the World Health Organization define anemia as a hemoglobin level of less than 11 g/dL or hematocrit less than 33% in the first and third trimesters. If a pregnant woman is not anemic, a serum ferritin level less than 15 ng/mL indicates iron deficiency.7 Some experts believe that in pregnant women who are not anemic, a serum ferritin level between 15 and 30 ng/mL may also indicate iron deficiency.8 If the pregnant woman is anemic and does not have another cause of the anemia, a serum ferritin level less than 40 ng/mL is indicative of iron deficiency.7
Ferritin is an acute phase reactant and levels may be falsely elevated due to chronic or acute inflammation, liver disease, renal failure, metabolic syndrome, or malignancy. Some women with iron deficiency due to bariatric surgery or malabsorption also have vitamin B12 and, less commonly, folate deficiency, which can contribute to the development of anemia (see “Diagnosis of anemia, iron deficiency, and iron deficiency anemia in pregnancy.”) Clinicians are often advised that a mean corpuscular volume demonstrating microcytosis is the “best test” to assess a patient for iron deficiency. However, reduced iron availability and low ferritin precede microcytosis. Hence microcytosis is a lagging measure and iron deficiency is diagnosed at an earlier stage by ferritin.
Requirements for a diagnosis of anemia in pregnancy
The American College of Obstetricians and Gynecologists recommends obtaining a hemoglobin and hematocrit test at the first prenatal visit and at the beginning of the third trimester of pregnancy.1
If the hemoglobin concentration is less than 11 g/dL, or hematocrit is less than 33%, anemia is present.2,3
If anemia is diagnosed, additional testing to investigate potential causes of anemia includes hemoglobin electrophoresis and measurement of vitamin B12 and folate levels. Many obstetricians perform hemoglobin electrophoresis on all their pregnant patients as part of the routine prenatal screen.
Requirements for a diagnosis of iron deficiency in pregnancy
We recommend obtaining a ferritin measurement at the first prenatal visit and at the beginning of the third trimester.
In pregnant women with anemia, iron deficiency is present if the ferritin is less than 40 ng/mL.
If a pregnant woman is not anemic, iron deficiency is present if the ferritin is less than 15 ng/mL.4
Requirements for a diagnosis of iron deficiency anemia
Hemoglobin concentration less than 11 g/dL, or hematocrit less than 33% (diagnosis of anemia).
PLUS
Ferritin less than 40 ng/mL (diagnosis of iron deficiency in an anemic woman)
PLUS
Evaluation for other known major causes of anemia, including blood loss, hemolysis, bone marrow disease, medications that suppress bone marrow function, kidney disease, malignancy, hemoglobinopathy, and vitamin B12 or folate deficiency.
References
- Guidelines for Perinatal Care. 8th ed. Washington DC: American Academy of Pediatrics, American College of Obstetricians and Gynecologists;2017.
- Centers for Disease Control and Prevention. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep. 1989;38(22):400-404.
- World Health Organization. Iron deficiency anaemia: assessment, prevention and control. A guide for programme managers. World Health Organization: Geneva, Switzerland; 2001. http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. Accessed November 8, 2017.
- Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency: an overview. J Gen Intern Med. 1992;7(2):145-153.
Dietary iron
Iron in food is present in heme (meat, poultry, fish) and non-heme forms (grains, plant food, supplements). Heme iron is better absorbed than non-heme iron. Foods rich in non-heme iron include spinach, lentils, prune juice, dried prunes, and fortified cereals. Absorption of non-heme iron can be increased by vitamin C or vitamin C–rich foods (broccoli, bell peppers, cantaloupe, grapefruit, oranges, strawberries, and tomatoes). Absorption of non-heme iron is reduced by consumption of dairy products, coffee, tea, and chocolate.
Oral iron treatment
Oral iron is an effective treatment for iron deficiency9,10 and is inexpensive, safe, and widely available. The CDC recommends that all pregnant women take a 30 mg/day iron supplement, unless they have hemochromatosis.11 For women with a low ferritin level and anemia, iron supplementation should be increased to 30 to 120 mg daily.11 Not all prenatal vitamins contain iron; those that do typically contain 17 to 28 mg of elemental iron per dose.
Many pregnant women taking oral iron, especially at doses greater than 30 mg daily, have gastrointestinal side effects, which cause them to discontinue the iron therapy.12 Taking iron supplementation on an intermittent basis may help to reduce gastrointestinal side effects and improve iron stores.13
In the past, a standard approach to the treatment of iron deficiency anemia was oral ferrous sulfate 325 mg (65 mg elemental iron) spaced in 3 doses each day for a total daily dose of 195 mg elemental iron. However, recent absorption studies concluded that maximal absorption of iron occurs with a dose in the range of 40 to 80 mg of elemental iron daily. Greater doses do not result in more iron absorption and are associated with more side effects.14,15 (See “Start using alternate-day oral iron dosing, and stop using daily iron dosing.”)
Recent research reports alternate-day oral iron dosing compared with daily oral iron dosing results in higher absorption of iron.
Details of the study
A total of 40 iron deficient women (mean serum ferritin level, 14 ng/mL) were randomly assigned to receive a daily dose of 60 mg of elemental iron (325 mg of ferrous sulfate) for 14 days or an alternate-day dose of 60 mg for 28 days. A small amount of radioactive iron was added to the oral medication to assess iron absorption. The primary outcome was fractional and total iron absorption, calculated by measuring radioactive iron in circulating red blood cells 14 days after the final oral iron dose.
Alternate-day iron dosing, compared with daily dosing, resulted in a higher fraction of the iron dose being absorbed (22% vs 16%; P = .0013). In addition, alternate-day iron dosing resulted in greater cumulative total iron absorption (175 mg vs 131 mg; P = .001). Nausea was reported less frequently by women in the alternate-day dosing group (11%) than in the daily iron dose group (29%).
The investigators concluded that prescribing iron as a single alternate-day
dose may be a superior dosing regimen compared with daily dosing.
Reference
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4(11):e524–e533.
Oral iron should not be taken in close approximation to the consumption of milk, cereals, tea, coffee, eggs, or calcium supplements. The absorption of oral iron is enhanced by the consumption of orange juice or 250 mg of vitamin C. Gastrointestinal side effects include nausea, flatulence, constipation, diarrhea, epigastric distress, and vomiting. If gastrointestinal side effects occur, interventions that might improve tolerability include: reduce the dose of iron or administer intermittently or use a low dose of oral iron, where dosing can be more easily titrated.
We re-check ferritin and hemoglobin levels 2 to 4 weeks after initiation of oral iron therapy and expect to see a hemoglobin rise of 1 g/dL if the therapy is effective.
Intravenous iron treatment
For women with iron deficiency anemia who cannot tolerate oral iron or in whom oral iron treatment has not resolved their anemia, intravenous (IV) iron treatment may be an optimal approach. Women in the third trimester of pregnancy with iron deficiency anemia have very little time to consume sufficient quantities of oral iron in food and supplements to restore their deficiency and reverse their anemia. Consequently, treatment with IV iron may be especially appropriate for women with iron deficiency anemia in the third trimester of pregnancy. Prior gastric surgery, including gastric bypass, results in reduced gastric acid production and causes severe impairment of intestinal absorption of iron. Patients with malabsorption syndromes, including celiac disease, also may have limited absorption of oral iron. These populations of pregnant women may particularly benefit from the use of IV iron. In pregnant women IV iron has fewer gastrointestinal side effects than oral iron.16
Many severely iron deficient patients need 1,000 mg of iron to resolve their deficit. In order to avoid giving multiple standard doses (200 mg per infusion, with 5 infusions over many days), some centers have explored the use of 1 large dose of IV iron (1,000 mg of low molecular weight iron dextran administered over 1 hour) (INFeD, Watson Pharma).17–19 This is not a regimen that is specifically approved by the US Food and Drug Administration. An alternative regimen is to administer 750 mg of ferrous carboxymaltose (Injectafer, Luitpold Pharmaceuticals) over 15 minutes, which is an FDA-approved regimen.18 Many hematologists prefer to administer multiple smaller doses of iron. For example, in our practice, pregnant women are commonly treated with IV iron sucrose (300 mg) every 2 weeks for 3 doses. To increase access of pregnant women to IV iron treatment, obstetricians need to work with hematologists and infusion centers to create collaborative protocols to expeditiously treat women in the third trimester.
There is an epidemic of iron deficiency in pregnant women in the United States. In an era of high technology medicine, it is surprising that iron deficiency remains an unsolved obstetric problem in our country.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277(12):973–976.
- Miller EM. Iron status and reproduction in US women: National Health and Nutrition Examination Survey 1999–2006. PLoS One. 2014;9(11):e112216.
- Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93(6):1312–1320.
- Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55(12):2799–2806.
- Rahmann MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495–504.
- Zeng L, Dibley MJ, Cheng Y, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:a2001.
- Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency: an overview. J Gen Intern Med. 1992;7(2):145–153.
- van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol. 1998;103(3):817–824.
- Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015(7);CD004736.
- Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2015;162(8):566–576.
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1–29.
- Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117383.
- Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015(10);CD009997.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981–1989.
- Schrier SL. So you know how to treat iron deficiency anemia. Blood. 2015;126(17):1971.
- Breymann C, Milman N, Mezzacasa A, Bernard R, Dudenhausen J; FER-ASAP investigators. Ferric carboxymaltose vs oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinatal Med. 2017;45(4):443–453.
- Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol. 2011;86(10):860–862.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91(1):31–38.
- Wong L, Smith S, Gilstrop M, et al. Safety and efficacy of rapid (1,000 mg in 1 hr) intravenous iron dextran for treatment of maternal iron deficient anemia of pregnancy. Am J Hematol. 2016;91(6):590–593.
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277(12):973–976.
- Miller EM. Iron status and reproduction in US women: National Health and Nutrition Examination Survey 1999–2006. PLoS One. 2014;9(11):e112216.
- Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93(6):1312–1320.
- Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55(12):2799–2806.
- Rahmann MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495–504.
- Zeng L, Dibley MJ, Cheng Y, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:a2001.
- Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency: an overview. J Gen Intern Med. 1992;7(2):145–153.
- van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol. 1998;103(3):817–824.
- Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015(7);CD004736.
- Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2015;162(8):566–576.
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1–29.
- Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117383.
- Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015(10);CD009997.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981–1989.
- Schrier SL. So you know how to treat iron deficiency anemia. Blood. 2015;126(17):1971.
- Breymann C, Milman N, Mezzacasa A, Bernard R, Dudenhausen J; FER-ASAP investigators. Ferric carboxymaltose vs oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinatal Med. 2017;45(4):443–453.
- Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol. 2011;86(10):860–862.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91(1):31–38.
- Wong L, Smith S, Gilstrop M, et al. Safety and efficacy of rapid (1,000 mg in 1 hr) intravenous iron dextran for treatment of maternal iron deficient anemia of pregnancy. Am J Hematol. 2016;91(6):590–593.
Reduce maternal morbidity by the expeditious and decisive treatment of severe hypertension in pregnancy
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Obstetrician-gynecologists are deeply committed to reducing maternal mortality and severe morbidity. Hypertensive diseases of pregnancy, including preeclampsia and eclampsia, are important contributors to both maternal mortality and severe morbidity. Among US live births from 2011–2013 there were 1,078 pregnancy-related maternal deaths, and 10% were attributed to preeclampsia or eclampsia.1 Hypertensive disease of pregnancy is also a major cause of severe maternal morbidity, with an increased risk of acute renal failure, respiratory failure, and cerebrovascular events.2 Preeclampsia is associated with a 4-fold increased risk of thrombocytopenia and coagulopathy and a 2-fold increased risk of postpartum hemorrhage.3
Severe hypertension is defined as a systolic blood pressure (BP) ≥160 mm Hg or a diastolic BP ≥110 mm Hg on 2 measurements within 15 minutes.4,5 Severe hypertensive disease of pregnancy is a common clinical problem in obstetrics, requiring clinicians to respond expeditiously and decisively to minimize adverse maternal outcomes. Following the identification of severe hypertension, a diagnosis and management plan should be initiated within 30 to 60 minutes.4 Some experts recommend that treatment be initiated within 15 minutes of identifying severe hypertension in a pregnant woman.6
The American College of Obstetricians and Gynecologists recommends that obstetric programs adopt standardized guidelines for the management of women with preeclampsia or eclampsia.4 The National Partnership for Maternal Safety recommends that all obstetric programs develop care bundles to respond to severe hypertension.5 Key points in managing severe hypertension are summarized below.
Related article:
2017 Update on obstetrics: Preeclampsia prevention
1. Expeditiously initiate treatment of severe hypertension…
…with intravenous (IV) labetalol (administered as 20 mg/40 mg/80 mg sequential doses as needed) or hydralazine (administered as 10 mg/10 mg/20 mg/40 mg sequential doses as needed). Our preferred agent is labetalol, administered as a 20-mg IV infusion over 2 minutes. If the patient’s BP remains elevated 10 min after the initial dose, administer labetalol 40 mg as an IV infusion over 2 min. If her BP remains elevated 10 min after this dose, administer 80 mg of labetalol. If the BP continues to be elevated, hydralazine treatment can be initiated as described below.
Occasionally there are national shortages of labetalol or a patient has a low heart rate or contraindication such as heart disease or asthma prohibiting its use. If labetalol is not available, we use hydralazine administered as a 10-mg IV bolus over 2 min. If the BP remains elevated, every 20 min, an escalating dose of hydralazine is administered, first by repeating the 10-mg dose, then administering 20 mg, and finally 40 mg.
For women without IV access, we use oral nifedipine 10 mg to control hypertension only while awaiting the placement of an IV. If BP remains elevated after 30 min, a second dose of oral nifedipine 20 mg can be given with a plan to transition to IV agents as soon as possible. The risks of maternal tachycardia or overshoot hypotension with immediate release oral nifedipine limit its use in our clinical practice to this circumstance.
Once the BP is controlled, start maintenance oral hypertension therapy. Our first-line agent is labetalol 200 mg twice per day with a maximum dose of 800 mg 3 times daily (2,400 mg maximal daily dose).
2. Initiate treatment with magnesium sulfate
If the patient’s BP is ≥160/110 mm Hg or if her BP is ≥140/90 mm Hg with coexisting symptoms of severe preeclampsia (for example a severe headache), initiate magnesium sulfate treatment. A standard regimen is magnesium sulfate 4 to 6 g administered as an IV bolus over 20 min followed by the IV infusion of 2 g per hour. In our clinical opinion, if you plan on initiating IV antihypertensive treatment for severe hypertension you also should strongly consider starting magnesium sulfate to reduce the risk of an eclamptic seizure.
We also start magnesium sulfate therapy for women with severe hypertension and clinical symptoms or laboratory signs of preeclampsia even in the absence of proteinuria. Approximately 2% of women with preeclampsia will develop an eclamptic seizure and magnesium sulfate treatment significantly reduces the risk of seizure and may also reduce maternal mortality.7,8
Magnesium sulfate is contra-indicated in women with myasthenia gravis. In women with renal dysfunction, the loading dose can be given, but the continuous magnesium sulfate infusion should not be initiated until serum magnesium levels are assessed.
3. Consider administering maternal betamethasone
Treatment with betamethasone advances fetal maturation if the pregnancy is preterm (for example, <34 weeks of gestation). A major cause of neonatal morbidity and mortality for pregnancy complicated by severe hypertensive disease is premature delivery. Maternal glucocorticoid treatment reduces the risk of neonatal morbidity and mortality if preterm delivery is anticipated. However, do not delay delivery for antenatal corticosteroids for women with severe and persistent hypertension or symptoms of preeclampsia that do not resolve following treatment.
We also consider women with eclampsia, placental abruption, pulmonary edema, or severe laboratory derangements too unstable to delay delivery for 48 hours to achieve the maximum benefit of steroid treatment. If antenatal corticosteroids are administered in the late preterm period between 34 0/7 weeks and 36 6/7 weeks of gestation, obstetric management should not be altered and delivery should not be delayed.9
Related article:
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
4. Preeclampsia plus a severe headache is a toxic combination
For patients with this constellation either have a plan for delivery or keep them under close surveillance. Occasionally a woman >20 weeks pregnant with new onset hypertension and a headache is seen in an emergency department and is not assessed for proteinuria or other preeclampsia laboratory abnormalities. If the woman is diagnosed as having a migraine or tension headache and discharged home with a headache medicine they are at high risk for serious morbidity, including stroke.
Read about preeclampsia and thrombocytopenia, HELLP syndrome, more.
5. Preeclampsia plus thrombocytopenia complicates anesthesia options
If the platelet count falls too low (for instance, <70,000 platelets per µL), many anesthesiologists will not provide a regional anesthetic for delivery because of the risk of peridural bleeding. In addition, a low platelet count (<50,000 platelets per µL) significantly increases the risk of obstetric hemorrhage. Transfer of the patient to an obstetrics unit with a full-service blood bank capable of supporting multiple platelet transfusions may be warranted.
6. Preeclampsia plus dyspnea or chest pain increases the risk of severe maternal morbidity
Authors of a prospective study of 2,023 women with preeclampsia reported an increase in adverse maternal outcomes when the following factors were present: early gestational age, dyspnea, chest pain, oxygen saturation of SpO2 <93%, thrombocytopenia, elevated creatinine, or elevated aspartate transaminase concentration.10 If dyspnea is present, the patient may have pulmonary edema, pulmonary embolism, heart failure, acute asthma, or pneumonia. If the patient has chest pain the differential diagnosis includes pulmonary embolism, cardiac ischemia, cardiomyopathy, or another cardiac disease.
Consider obtaining a chest radiograph for pregnant women with dyspnea and a computed tomography pulmonary angiogram or lung scintigraphy (ventilation perfusion scan) if the chest radiograph is normal for women with chest pain.6,11 We obtain a transthoracic echocardiogram in cases of pulmonary edema to evaluate for the possibility of peripartum cardiomyopathy.
7. HELLP syndrome
The triad of hemolysis, elevated liver enzymes, and low platelet count (HELLP) is associated with an increased risk of maternal mortality and severe morbidity.12 In a study of 171 women with HELLP, factors that increased the risk for adverse maternal outcomes included12:
- aspartate aminotransferase (AST) levels >316 U/L
- alanine aminotransferase (ALT) levels >217 U/L
- total bilirubin levels >2.0 mg/dL
- lactate dehydrogenase (LDH) levels >1,290 U/L
- blood urea nitrogen test results >44 mg/dL
- platelet count <50,000 platelets per µL.
The clinical course of HELLP syndrome is characterized by progression and the potential for sudden and catastrophic deterioration. For example, some women with HELLP will suddenly develop a ruptured liver, pulmonary edema, or a stroke. The Society for Maternal-Fetal Medicine recommends against expectant management of women with HELLP syndrome.13
Related article:
Optimal obstetric care for women aged 40 and older
8. Delivery or expectant management?
Currently the only cure for preeclampsia is delivery. The Society for Maternal-Fetal Medicine recommends against expectant management of severe preeclampsia if certain problems occur (BOX).13 For women with preeclampsia who are less than 34 weeks’ gestation and do not have a contraindication to expectant management, consider transferring the patient to a tertiary maternal care center. In our practice, pregnant women with a hypertensive disorder are scheduled for an induction of labor and delivery at 37 weeks’ gestation.
The Society for Maternal-Fetal Medicine recommends delivery (not expectant management) in the presence of severe preeclampsia if any of the following are present13:
- eclampsia
- pulmonary edema
- disseminated intravascular coagulation
- renal insufficiency
- abruptio placentae
- abnormal fetal testing
- HELLP syndrome or persistent symptoms of severe preeclampsia.
In the United States, major obstetric causes of pregnancy-related death include sepsis, venous thromboembolism-pulmonary embolism, hemorrhage, and hypertensive disease of pregnancy. Other important causes of pregnancy-related death include cardiac disease, stroke, and pre-existing major medical disease including advanced cancer. In the United States there are approximately 17 pregnancy-related maternal deaths per 100,000 live births.1 Obstetricians are dedicated to reducing this excessively high rate of maternal death.
Given the US maternal death rate of 1 maternity death per 5,880 live births, over the course of a 40-year career, most obstetrician-gynecologists will have 1 or 2 of their pregnant patients die. From the perspective of an individual clinician, maternal death is an extremely rare event, with 1 death during every 20 years of practice. However, from a population perspective, maternal death in the United States is all too common compared to other developed countries. We can only reduce the rate of maternal death by working in interdisciplinary teams to ensure our obstetrics units are prepared to expeditiously diagnose and treat the most common obstetric causes of death and severe morbidity.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- Kuklina EV, Ayala C, Callaghan WM. Hyper-tensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306.
- Stevens S, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237–248.e16.
- Committee on Obstetric Practice. Committee Opinion No. 692: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;129(4):e90–e95.
- Bernstein PS, Martin JN Jr, Barton JR, et al. National Partnership for Maternal Safety: Consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017;130(2):347–357.
- Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119(2 pt 1):360–364.
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000-2008. Am J Obstet Gynecol. 2013;208(6):476.e1–e5.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320.
- von Dadelszen P, Payne B, Li J, et al; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377(9761):219–227.
- Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195(3):W214–W220.
- Erkilinç S, Eyi EG. Factors contributing to adverse maternal outcomes in patients with HELLP syndrome. J Matern Fetal Neonatal Med. 2017:1–7. doi:10.1080/14767058.2017.1359528.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198
Stop using codeine, oxycodone, hydrocodone, tramadol, and aspirin in women who are breastfeeding
In 2015 more than 30,000 deaths from opioid overdose were reported (FIGURE).1 More than 50% of the deaths were due to prescription opioids. The opioid crisis is a public health emergency and clinicians are diligently working to reduce both the number of opioid prescriptions and the doses prescribed per prescription.

In obstetrics, there is growing concern that narcotics used for the treatment of pain in women who are breastfeeding may increase the risk of adverse effects in newborns, including excessive sedation and respiratory depression. The American Academy of Pediatrics (AAP), the US Food and Drug Administration (FDA) and the American College of Obstetricians and Gynecologists (ACOG) recommend against the use of codeine and tramadol in women who are breastfeeding because their newborns may have adverse reactions, including excessive sleepiness, difficulty breathing, and potentially fatal breathing problems.2–4 In addition, there is growing concern that the use of oxycodone and hydrocodone should also be limited in women who are breastfeeding. In this article, I discuss the rationale for these recommendations.
Related article:
Landmark women’s health care remains law of the land
Codeine
Codeine is metabolized to morphine by CYP2D6 and CYP2D7. Both codeine and morphine are excreted into breast milk. Some women are ultrarapid metabolizers of codeine because of high levels of CYP2D6, resulting in higher concentrations of morphine in their breast milk and their breast fed newborn.2,5 In many women who are ultra-rapid metabolizers of codeine, CYP2D6 gene duplication or multiplication is the cause of the increased enzyme activity.6 Genotyping can identify some women who are ultrarapid metabolizers, but it is not currently utilized widely in clinical practice.
In the United States approximately 5% of women express high levels of CYP2D6 and are ultra-rapid metabolizers of codeine.4 In Ethiopia as many as 29% of women are ultrarapid metabolizers.7 Newborn central nervous system (CNS) depression is the most common adverse effect of fetal ingestion of excessive codeine and mor-phine from breast milk and may present as sedation, apnea, bradycardia, or cyanosis.8 Multiple newborn fatalities have been re-ported in the literature when lactating mothers who were ultrarapid metabolizers took co-deine. The FDA and ACOG recommend against the use of codeine in lactating women.
Hydrocodone
Hydrocodone, a hydrogenated ketone derivative of codeine, is metabolized by CYP2D6 to hydromorphone. Both hydrocodone and hydromorphone are present in breast milk. In lactating mothers taking hydrocodone, up to 9% of the dose may be ingested by the breastfeeding newborn.9 There is concern that hydrocodone use by women who are breastfeeding and are ultrarapid metabolizers may cause increased fetal consumption of hydromorphone resulting in adverse outcomes in the newborn. The AAP cautions against the use of hydrocodone.2
Oxycodone
Oxycodone is metabolized by CYP2D6 to oxymorphone and is concentrated into breast milk.10 Oxymorphone is more than 10 times more potent than oxycodone. In one study of lactating women taking oxycodone, codeine, or acetaminophen, the rates of neonate CNS depression were 20%, 17%, and 0.5%, respectively.11 The authors concluded that for mothers who are breastfeeding oxycodone was no safer than codeine because both medications were associated with a high rate of depression in the neonate. Newborns who develop CNS depression from exposure to oxycodone in breast milk will respond to naloxone treatment.12 The AAP recommends against prescribing oxycodone for women who are breastfeeding their infants.2
In a recent communication, the Society for Obstetric Anesthesia and Perinatology (SOAP) observed that in the United States, following cesarean delivery the majority of women receive oxycodone or hydrocodone.13 SOAP disagreed with the AAP recommendation against the use of oxycodone or hydrocodone in breastfeeding women. SOAP noted that all narcotics can produce adverse effects in newborns of breastfeeding women and that there are no good data that the prescription of oxycodone or hydrocodone is more risky than morphine or hydromorphone. However, based on their assessment of risk and benefit, pediatricians prioritize the use of acetaminophen and morphine and seldom use oxycodone or hydrocodone to treat moderate to severe pain in babies and children.
Tramadol
Tramadol is metabolized by CYP2D6 to O-desmethyltramadol. Both tramadol and O-desmethyltramadol are excreted into breast milk. In ultrarapid metabolizers, a greater concentration of O-desmethyltramadol is excreted into breast milk. The FDA reported that they identified no serious neonatal adverse events in the literature due to the use of tramadol by women who are breastfeeding. However, given that tramadol and its CYP2D6 metabolite enter breast milk and the potential for life-threatening respiratory de-pression in the infant, the FDA included tramadol in its warning about codeine.3
Codeine, hydrocodone, oxycodone, and tramadol are all metabolized to more potent metabolites by the CYP2D6 enzyme. Individuals with low CYP2D6 activity, representing about 5% of the US population, cannot fully activate these narcotics. Hence they may not get adequate pain relief when treated with codeine, oxycodone, hydrocodone, or tramadol. Given their resistance to these medications they may first be placed on a higher dose of the narcotic and then switched from a high ineffective dose of one of the agents activated by CYP2D6 to a high dose of morphine or hydromorphone. This can be dangerous because they may then receive an excessive dose of narcotic and develop respiratory depression.14
Read about how other pain medications affect breast milk.
Aspirin
There are very little high quality data about the use of aspirin in women breastfeeding and the effect on the neonate. If a mother takes aspirin, the drug will enter breast milk. It is estimated that the nursing baby receives about 4% to 8% of the mother’s dose. The World Health Organization recommends that aspirin is compatible with breastfeeding in occasional small doses, but repeated administration of aspirin in normal doses should be avoided in women who are breastfeeding. If chronic or high-dose aspirin therapy is recommended, the infant should be monitored for side effects including metabolic acidosis15 and coagulation disorders.16 The National Reye’s Syndrome Foundation recommends against the use of aspirin in women who are breastfeeding because of the theoretical risk of triggering Reye syndrome.17 Acetaminophen and ibuprofen are recommended by the WHO for chronic treatment of pain during breastfeeding.16
Acetaminophen and ibuprofen
For the medication treatment of pain in women who are breastfeeding, the WHO recommends the use of acetaminophen and ibuprofen.16 Acetaminophen is transferred from the maternal circulation into breast milk, but it is estimated that the dose to the nursing neonate is <0.3% of the maternal dose.18 In mothers taking ibuprofen 1600 mg daily, the concentration of ibuprofen in breast milk was below the level of laboratory detection (<1 mg/L).19 Ibuprofen treatment is thought to be safe for women who are breastfeeding because of its short half-life (2 hours), low excretion into milk, and few reported adverse effects in infants.
Morphine
Morphine is not metabolized by CYP2D6 and is excreted into breast milk. Many experts believe that women who are breastfeeding may take standard doses of oral morphine with few adverse effects in the newborn.20,21 For the treatment of moderate to severe pain in opioid-naive adults, morphine doses in the range of 10 mg orally every 4 hours up to 30 mg orally every 4 hours are prescribed. When using a solution of morphine, standard doses are 10 mg to 20 mg every 4 hours, as needed to treat pain. When using morphine tablets, standard doses are 15 mg to 30 mg every 4 hours. The WHO states that occasional doses of morphine are usually safe for women breastfeeding their newborn.16 The AAP recommends the use of morphine and hydromorphone when narcotic agents are needed to treat pain in breastfeeding women.2
Hydromorphone
Hydromorphone, a hydrogenated ketone derivative of morphine, is not metabolized by CYP2D6 and is excreted into breast milk. There are limited data on the safety of hydromorphone during breastfeeding. Breast milk concentrations of hydromorphone are low, and an occasional dose is likely associated with few adverse effects in the breastfeeding newborn.22 For the treatment of moderate to severe pain in opioid-naive adults, hydromorphone doses in the range of 2 mg orally every 4 hours up to 4 mg orally every 4 hours are prescribed. Like all narcotics, hydromorphone can result in central nervous system depression. If a mother ingests sufficient quantities of hydromorphone, respiratory depression in the breastfeeding newborn can occur. In one case report, a nursing mother was taking hydromorphone 4 mg every 4 hours for pain following a cesarean delivery. On day 6 following birth, her newborn was lethargic and she brought the infant to an emergency room. In the emergency room the infant became apneic and was successfully treated with naloxone, suggesting anarcotic overdose due to the presence of hydromorphone in breast milk.23 Hydromorphone should only be used at the lowest effective dose and for the shortest time possible.
Related article:
Should coffee consumption be added as an adjunct to the postoperative care of gynecologic oncology patients?
The bottom line
Pediatricians seldom prescribe codeine, oxycodone, hydrocodone, or tramadol for the treatment of pain in newborns or children. Pediatricians generally use acetaminophen and morphine for the treatment of pain in newborns. Although data from large, high quality clinical trials are not available, expert opinion recommends that acetaminophen and ibuprofen should be prescribed as first-line medications for the treatment of pain in women who are breastfeeding. Use of narcotics that are metabolized by CYP2D6 should be minimized or avoided in women who are breastfeeding. If narcotic medication is necessary, the lowest effective dose of morphine or hy-dromorphone should be prescribed for the shortest time possible. If morphine is prescribed to wo-men who are breastfeeding, they should be advised to observe their baby for signs of narcotic excess, including drowsiness, poor nursing, slow breathing, or low heart rate.
The goal of reducing morbidity and mortality from opioid use is a top public health priority. Obstetrician-gynecologists can contribute through the optimal use of opioid analgesics. Reducing the number of opioid prescriptions and the quantity of medication prescribed per prescription is an important first step in our effort to reduce opioid-related deaths.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Overdose Deaths—Number of Deaths from Opioid Drugs. National Institute on Drug Abuse website. . Update January 2017. Accessed September 14, 2017.
- Sachs HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3):e796–e809.
- US Food and Drug Administration. FDA Drug Safety Communication. FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/Drugs/DrugSafety/ucm118113.htm. Published April 2017. Accessed September 12, 2017.
- Practice advisory on codeine and tramadol for breast feeding women. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-on-Codeine-and-Tramadol-for-Breastfeeding-Women. Published April 27, 2017. Accessed September 12, 2017.
- Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatr Drugs. 2008;10(6):399–404.
- Langaee T, Hamadeh I, Chapman AB, Gums JG, Johnson JA. A novel simple method for determining CYP2D6 gene copy number and identifying allele(s) with duplication/multiplication. PLoS One. 2015;10(1):e0113808.
- Cascorbi I. Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17–22.
- Naumburg EG, Meny RG. Breast milk opioids and neonatal apnea. Am J Dis Child. 1988;142(1):11–12.
- Sauberan JB, Anderson PO, Lane JR, et al. Breast milk hydrocodone and hydromorphone levels in mothers using hydrocodone for postpartum pain. Obstet Gynecol. 2011;117(3):611–617.
- Seaton S, Reeves M, McLean S. Oxycodone as a component of multimodal analgesia for lactating mothers after Cesarean section: relationships between maternal plasma, breast milk and neonatal plasma levels. Aust N Z J Obstet Gynaecol. 2007;47(3):181–185.
- Lam J, Kelly L, Ciszkowski C, et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr. 2012;160(1):33–37.e2.
- Timm NL. Maternal use of oxycodone resulting in opioid intoxication in her breastfed neonate. J Pediatr. 2013;162(2):421–422.
- The Society for Obstetric Anesthesia and Perinatology. Comments in response to the ACOG/SMFM Practice Advisory on Codeine and Tramadol for Breastfeeding Women. The Society for Obstetric Anesthesia and Perinatology website. https://soap.org/soap-response-acog-smfm-advisory.pdf. Published June 10, 2017. Accessed August 28, 2017.
- Banning AM. Respiratory depression following medication change from tramadol to morphine [article in Danish]. Ugeskr Laeger. 1999;161(47):6500–6501.
- Clark JH, Wilson WG. A 16-day old breast-fed infant with metabolic acidosis caused by salicylate. Clin Pediatr (Phila). 1981;20(1):53–54.
- World Health Organization. Breastfeeding and maternal medication. Recommendations for drugs in the 11th WHO model list of essential drugs. http://apps.who.int/iris/bitstream/10665/62435/1/55732.pdf. Published 2002. Accessed September 12, 2017.
- Reye’s syndrome. National Reye’s Syndrome Foundation website. http://www.reyessyndrome.org. Accessed September 12, 2017.
- Berline CM Jr, Yaffe SJ, Ragni M. Disposition of acetaminophen in milk, saliva, and plasma of lactating women. Pediatr Pharmacol (New York). 1980;1(2):135–141.
- Townsend RJ, Benedetti TJ, Erickson SH, et al. Excretion of ibuprofen into breast milk. Am J Obstet Gynecol. 1984;149(2):184–186.
- Spigset O, Hägg S. Analgesics and breast-feeding: safety considerations. Paediatr Drugs. 2000;2(3):223–238.
- Bar-OZ B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Saf. 2003;26(13):925–935.
- Edwards JE, Rudy AC, Wermeling DP, Desai N, McNamara PJ. Hydromorphone transfer into breast milk after intranasal administration. Pharmacotherapy. 2003;23(2):153–158.
- Schultz ML, Kostic M, Kharasch S. A case of toxic breast-feeding [published online ahead of print January 6, 2017]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001009.
In 2015 more than 30,000 deaths from opioid overdose were reported (FIGURE).1 More than 50% of the deaths were due to prescription opioids. The opioid crisis is a public health emergency and clinicians are diligently working to reduce both the number of opioid prescriptions and the doses prescribed per prescription.

In obstetrics, there is growing concern that narcotics used for the treatment of pain in women who are breastfeeding may increase the risk of adverse effects in newborns, including excessive sedation and respiratory depression. The American Academy of Pediatrics (AAP), the US Food and Drug Administration (FDA) and the American College of Obstetricians and Gynecologists (ACOG) recommend against the use of codeine and tramadol in women who are breastfeeding because their newborns may have adverse reactions, including excessive sleepiness, difficulty breathing, and potentially fatal breathing problems.2–4 In addition, there is growing concern that the use of oxycodone and hydrocodone should also be limited in women who are breastfeeding. In this article, I discuss the rationale for these recommendations.
Related article:
Landmark women’s health care remains law of the land
Codeine
Codeine is metabolized to morphine by CYP2D6 and CYP2D7. Both codeine and morphine are excreted into breast milk. Some women are ultrarapid metabolizers of codeine because of high levels of CYP2D6, resulting in higher concentrations of morphine in their breast milk and their breast fed newborn.2,5 In many women who are ultra-rapid metabolizers of codeine, CYP2D6 gene duplication or multiplication is the cause of the increased enzyme activity.6 Genotyping can identify some women who are ultrarapid metabolizers, but it is not currently utilized widely in clinical practice.
In the United States approximately 5% of women express high levels of CYP2D6 and are ultra-rapid metabolizers of codeine.4 In Ethiopia as many as 29% of women are ultrarapid metabolizers.7 Newborn central nervous system (CNS) depression is the most common adverse effect of fetal ingestion of excessive codeine and mor-phine from breast milk and may present as sedation, apnea, bradycardia, or cyanosis.8 Multiple newborn fatalities have been re-ported in the literature when lactating mothers who were ultrarapid metabolizers took co-deine. The FDA and ACOG recommend against the use of codeine in lactating women.
Hydrocodone
Hydrocodone, a hydrogenated ketone derivative of codeine, is metabolized by CYP2D6 to hydromorphone. Both hydrocodone and hydromorphone are present in breast milk. In lactating mothers taking hydrocodone, up to 9% of the dose may be ingested by the breastfeeding newborn.9 There is concern that hydrocodone use by women who are breastfeeding and are ultrarapid metabolizers may cause increased fetal consumption of hydromorphone resulting in adverse outcomes in the newborn. The AAP cautions against the use of hydrocodone.2
Oxycodone
Oxycodone is metabolized by CYP2D6 to oxymorphone and is concentrated into breast milk.10 Oxymorphone is more than 10 times more potent than oxycodone. In one study of lactating women taking oxycodone, codeine, or acetaminophen, the rates of neonate CNS depression were 20%, 17%, and 0.5%, respectively.11 The authors concluded that for mothers who are breastfeeding oxycodone was no safer than codeine because both medications were associated with a high rate of depression in the neonate. Newborns who develop CNS depression from exposure to oxycodone in breast milk will respond to naloxone treatment.12 The AAP recommends against prescribing oxycodone for women who are breastfeeding their infants.2
In a recent communication, the Society for Obstetric Anesthesia and Perinatology (SOAP) observed that in the United States, following cesarean delivery the majority of women receive oxycodone or hydrocodone.13 SOAP disagreed with the AAP recommendation against the use of oxycodone or hydrocodone in breastfeeding women. SOAP noted that all narcotics can produce adverse effects in newborns of breastfeeding women and that there are no good data that the prescription of oxycodone or hydrocodone is more risky than morphine or hydromorphone. However, based on their assessment of risk and benefit, pediatricians prioritize the use of acetaminophen and morphine and seldom use oxycodone or hydrocodone to treat moderate to severe pain in babies and children.
Tramadol
Tramadol is metabolized by CYP2D6 to O-desmethyltramadol. Both tramadol and O-desmethyltramadol are excreted into breast milk. In ultrarapid metabolizers, a greater concentration of O-desmethyltramadol is excreted into breast milk. The FDA reported that they identified no serious neonatal adverse events in the literature due to the use of tramadol by women who are breastfeeding. However, given that tramadol and its CYP2D6 metabolite enter breast milk and the potential for life-threatening respiratory de-pression in the infant, the FDA included tramadol in its warning about codeine.3
Codeine, hydrocodone, oxycodone, and tramadol are all metabolized to more potent metabolites by the CYP2D6 enzyme. Individuals with low CYP2D6 activity, representing about 5% of the US population, cannot fully activate these narcotics. Hence they may not get adequate pain relief when treated with codeine, oxycodone, hydrocodone, or tramadol. Given their resistance to these medications they may first be placed on a higher dose of the narcotic and then switched from a high ineffective dose of one of the agents activated by CYP2D6 to a high dose of morphine or hydromorphone. This can be dangerous because they may then receive an excessive dose of narcotic and develop respiratory depression.14
Read about how other pain medications affect breast milk.
Aspirin
There are very little high quality data about the use of aspirin in women breastfeeding and the effect on the neonate. If a mother takes aspirin, the drug will enter breast milk. It is estimated that the nursing baby receives about 4% to 8% of the mother’s dose. The World Health Organization recommends that aspirin is compatible with breastfeeding in occasional small doses, but repeated administration of aspirin in normal doses should be avoided in women who are breastfeeding. If chronic or high-dose aspirin therapy is recommended, the infant should be monitored for side effects including metabolic acidosis15 and coagulation disorders.16 The National Reye’s Syndrome Foundation recommends against the use of aspirin in women who are breastfeeding because of the theoretical risk of triggering Reye syndrome.17 Acetaminophen and ibuprofen are recommended by the WHO for chronic treatment of pain during breastfeeding.16
Acetaminophen and ibuprofen
For the medication treatment of pain in women who are breastfeeding, the WHO recommends the use of acetaminophen and ibuprofen.16 Acetaminophen is transferred from the maternal circulation into breast milk, but it is estimated that the dose to the nursing neonate is <0.3% of the maternal dose.18 In mothers taking ibuprofen 1600 mg daily, the concentration of ibuprofen in breast milk was below the level of laboratory detection (<1 mg/L).19 Ibuprofen treatment is thought to be safe for women who are breastfeeding because of its short half-life (2 hours), low excretion into milk, and few reported adverse effects in infants.
Morphine
Morphine is not metabolized by CYP2D6 and is excreted into breast milk. Many experts believe that women who are breastfeeding may take standard doses of oral morphine with few adverse effects in the newborn.20,21 For the treatment of moderate to severe pain in opioid-naive adults, morphine doses in the range of 10 mg orally every 4 hours up to 30 mg orally every 4 hours are prescribed. When using a solution of morphine, standard doses are 10 mg to 20 mg every 4 hours, as needed to treat pain. When using morphine tablets, standard doses are 15 mg to 30 mg every 4 hours. The WHO states that occasional doses of morphine are usually safe for women breastfeeding their newborn.16 The AAP recommends the use of morphine and hydromorphone when narcotic agents are needed to treat pain in breastfeeding women.2
Hydromorphone
Hydromorphone, a hydrogenated ketone derivative of morphine, is not metabolized by CYP2D6 and is excreted into breast milk. There are limited data on the safety of hydromorphone during breastfeeding. Breast milk concentrations of hydromorphone are low, and an occasional dose is likely associated with few adverse effects in the breastfeeding newborn.22 For the treatment of moderate to severe pain in opioid-naive adults, hydromorphone doses in the range of 2 mg orally every 4 hours up to 4 mg orally every 4 hours are prescribed. Like all narcotics, hydromorphone can result in central nervous system depression. If a mother ingests sufficient quantities of hydromorphone, respiratory depression in the breastfeeding newborn can occur. In one case report, a nursing mother was taking hydromorphone 4 mg every 4 hours for pain following a cesarean delivery. On day 6 following birth, her newborn was lethargic and she brought the infant to an emergency room. In the emergency room the infant became apneic and was successfully treated with naloxone, suggesting anarcotic overdose due to the presence of hydromorphone in breast milk.23 Hydromorphone should only be used at the lowest effective dose and for the shortest time possible.
Related article:
Should coffee consumption be added as an adjunct to the postoperative care of gynecologic oncology patients?
The bottom line
Pediatricians seldom prescribe codeine, oxycodone, hydrocodone, or tramadol for the treatment of pain in newborns or children. Pediatricians generally use acetaminophen and morphine for the treatment of pain in newborns. Although data from large, high quality clinical trials are not available, expert opinion recommends that acetaminophen and ibuprofen should be prescribed as first-line medications for the treatment of pain in women who are breastfeeding. Use of narcotics that are metabolized by CYP2D6 should be minimized or avoided in women who are breastfeeding. If narcotic medication is necessary, the lowest effective dose of morphine or hy-dromorphone should be prescribed for the shortest time possible. If morphine is prescribed to wo-men who are breastfeeding, they should be advised to observe their baby for signs of narcotic excess, including drowsiness, poor nursing, slow breathing, or low heart rate.
The goal of reducing morbidity and mortality from opioid use is a top public health priority. Obstetrician-gynecologists can contribute through the optimal use of opioid analgesics. Reducing the number of opioid prescriptions and the quantity of medication prescribed per prescription is an important first step in our effort to reduce opioid-related deaths.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In 2015 more than 30,000 deaths from opioid overdose were reported (FIGURE).1 More than 50% of the deaths were due to prescription opioids. The opioid crisis is a public health emergency and clinicians are diligently working to reduce both the number of opioid prescriptions and the doses prescribed per prescription.

In obstetrics, there is growing concern that narcotics used for the treatment of pain in women who are breastfeeding may increase the risk of adverse effects in newborns, including excessive sedation and respiratory depression. The American Academy of Pediatrics (AAP), the US Food and Drug Administration (FDA) and the American College of Obstetricians and Gynecologists (ACOG) recommend against the use of codeine and tramadol in women who are breastfeeding because their newborns may have adverse reactions, including excessive sleepiness, difficulty breathing, and potentially fatal breathing problems.2–4 In addition, there is growing concern that the use of oxycodone and hydrocodone should also be limited in women who are breastfeeding. In this article, I discuss the rationale for these recommendations.
Related article:
Landmark women’s health care remains law of the land
Codeine
Codeine is metabolized to morphine by CYP2D6 and CYP2D7. Both codeine and morphine are excreted into breast milk. Some women are ultrarapid metabolizers of codeine because of high levels of CYP2D6, resulting in higher concentrations of morphine in their breast milk and their breast fed newborn.2,5 In many women who are ultra-rapid metabolizers of codeine, CYP2D6 gene duplication or multiplication is the cause of the increased enzyme activity.6 Genotyping can identify some women who are ultrarapid metabolizers, but it is not currently utilized widely in clinical practice.
In the United States approximately 5% of women express high levels of CYP2D6 and are ultra-rapid metabolizers of codeine.4 In Ethiopia as many as 29% of women are ultrarapid metabolizers.7 Newborn central nervous system (CNS) depression is the most common adverse effect of fetal ingestion of excessive codeine and mor-phine from breast milk and may present as sedation, apnea, bradycardia, or cyanosis.8 Multiple newborn fatalities have been re-ported in the literature when lactating mothers who were ultrarapid metabolizers took co-deine. The FDA and ACOG recommend against the use of codeine in lactating women.
Hydrocodone
Hydrocodone, a hydrogenated ketone derivative of codeine, is metabolized by CYP2D6 to hydromorphone. Both hydrocodone and hydromorphone are present in breast milk. In lactating mothers taking hydrocodone, up to 9% of the dose may be ingested by the breastfeeding newborn.9 There is concern that hydrocodone use by women who are breastfeeding and are ultrarapid metabolizers may cause increased fetal consumption of hydromorphone resulting in adverse outcomes in the newborn. The AAP cautions against the use of hydrocodone.2
Oxycodone
Oxycodone is metabolized by CYP2D6 to oxymorphone and is concentrated into breast milk.10 Oxymorphone is more than 10 times more potent than oxycodone. In one study of lactating women taking oxycodone, codeine, or acetaminophen, the rates of neonate CNS depression were 20%, 17%, and 0.5%, respectively.11 The authors concluded that for mothers who are breastfeeding oxycodone was no safer than codeine because both medications were associated with a high rate of depression in the neonate. Newborns who develop CNS depression from exposure to oxycodone in breast milk will respond to naloxone treatment.12 The AAP recommends against prescribing oxycodone for women who are breastfeeding their infants.2
In a recent communication, the Society for Obstetric Anesthesia and Perinatology (SOAP) observed that in the United States, following cesarean delivery the majority of women receive oxycodone or hydrocodone.13 SOAP disagreed with the AAP recommendation against the use of oxycodone or hydrocodone in breastfeeding women. SOAP noted that all narcotics can produce adverse effects in newborns of breastfeeding women and that there are no good data that the prescription of oxycodone or hydrocodone is more risky than morphine or hydromorphone. However, based on their assessment of risk and benefit, pediatricians prioritize the use of acetaminophen and morphine and seldom use oxycodone or hydrocodone to treat moderate to severe pain in babies and children.
Tramadol
Tramadol is metabolized by CYP2D6 to O-desmethyltramadol. Both tramadol and O-desmethyltramadol are excreted into breast milk. In ultrarapid metabolizers, a greater concentration of O-desmethyltramadol is excreted into breast milk. The FDA reported that they identified no serious neonatal adverse events in the literature due to the use of tramadol by women who are breastfeeding. However, given that tramadol and its CYP2D6 metabolite enter breast milk and the potential for life-threatening respiratory de-pression in the infant, the FDA included tramadol in its warning about codeine.3
Codeine, hydrocodone, oxycodone, and tramadol are all metabolized to more potent metabolites by the CYP2D6 enzyme. Individuals with low CYP2D6 activity, representing about 5% of the US population, cannot fully activate these narcotics. Hence they may not get adequate pain relief when treated with codeine, oxycodone, hydrocodone, or tramadol. Given their resistance to these medications they may first be placed on a higher dose of the narcotic and then switched from a high ineffective dose of one of the agents activated by CYP2D6 to a high dose of morphine or hydromorphone. This can be dangerous because they may then receive an excessive dose of narcotic and develop respiratory depression.14
Read about how other pain medications affect breast milk.
Aspirin
There are very little high quality data about the use of aspirin in women breastfeeding and the effect on the neonate. If a mother takes aspirin, the drug will enter breast milk. It is estimated that the nursing baby receives about 4% to 8% of the mother’s dose. The World Health Organization recommends that aspirin is compatible with breastfeeding in occasional small doses, but repeated administration of aspirin in normal doses should be avoided in women who are breastfeeding. If chronic or high-dose aspirin therapy is recommended, the infant should be monitored for side effects including metabolic acidosis15 and coagulation disorders.16 The National Reye’s Syndrome Foundation recommends against the use of aspirin in women who are breastfeeding because of the theoretical risk of triggering Reye syndrome.17 Acetaminophen and ibuprofen are recommended by the WHO for chronic treatment of pain during breastfeeding.16
Acetaminophen and ibuprofen
For the medication treatment of pain in women who are breastfeeding, the WHO recommends the use of acetaminophen and ibuprofen.16 Acetaminophen is transferred from the maternal circulation into breast milk, but it is estimated that the dose to the nursing neonate is <0.3% of the maternal dose.18 In mothers taking ibuprofen 1600 mg daily, the concentration of ibuprofen in breast milk was below the level of laboratory detection (<1 mg/L).19 Ibuprofen treatment is thought to be safe for women who are breastfeeding because of its short half-life (2 hours), low excretion into milk, and few reported adverse effects in infants.
Morphine
Morphine is not metabolized by CYP2D6 and is excreted into breast milk. Many experts believe that women who are breastfeeding may take standard doses of oral morphine with few adverse effects in the newborn.20,21 For the treatment of moderate to severe pain in opioid-naive adults, morphine doses in the range of 10 mg orally every 4 hours up to 30 mg orally every 4 hours are prescribed. When using a solution of morphine, standard doses are 10 mg to 20 mg every 4 hours, as needed to treat pain. When using morphine tablets, standard doses are 15 mg to 30 mg every 4 hours. The WHO states that occasional doses of morphine are usually safe for women breastfeeding their newborn.16 The AAP recommends the use of morphine and hydromorphone when narcotic agents are needed to treat pain in breastfeeding women.2
Hydromorphone
Hydromorphone, a hydrogenated ketone derivative of morphine, is not metabolized by CYP2D6 and is excreted into breast milk. There are limited data on the safety of hydromorphone during breastfeeding. Breast milk concentrations of hydromorphone are low, and an occasional dose is likely associated with few adverse effects in the breastfeeding newborn.22 For the treatment of moderate to severe pain in opioid-naive adults, hydromorphone doses in the range of 2 mg orally every 4 hours up to 4 mg orally every 4 hours are prescribed. Like all narcotics, hydromorphone can result in central nervous system depression. If a mother ingests sufficient quantities of hydromorphone, respiratory depression in the breastfeeding newborn can occur. In one case report, a nursing mother was taking hydromorphone 4 mg every 4 hours for pain following a cesarean delivery. On day 6 following birth, her newborn was lethargic and she brought the infant to an emergency room. In the emergency room the infant became apneic and was successfully treated with naloxone, suggesting anarcotic overdose due to the presence of hydromorphone in breast milk.23 Hydromorphone should only be used at the lowest effective dose and for the shortest time possible.
Related article:
Should coffee consumption be added as an adjunct to the postoperative care of gynecologic oncology patients?
The bottom line
Pediatricians seldom prescribe codeine, oxycodone, hydrocodone, or tramadol for the treatment of pain in newborns or children. Pediatricians generally use acetaminophen and morphine for the treatment of pain in newborns. Although data from large, high quality clinical trials are not available, expert opinion recommends that acetaminophen and ibuprofen should be prescribed as first-line medications for the treatment of pain in women who are breastfeeding. Use of narcotics that are metabolized by CYP2D6 should be minimized or avoided in women who are breastfeeding. If narcotic medication is necessary, the lowest effective dose of morphine or hy-dromorphone should be prescribed for the shortest time possible. If morphine is prescribed to wo-men who are breastfeeding, they should be advised to observe their baby for signs of narcotic excess, including drowsiness, poor nursing, slow breathing, or low heart rate.
The goal of reducing morbidity and mortality from opioid use is a top public health priority. Obstetrician-gynecologists can contribute through the optimal use of opioid analgesics. Reducing the number of opioid prescriptions and the quantity of medication prescribed per prescription is an important first step in our effort to reduce opioid-related deaths.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- National Overdose Deaths—Number of Deaths from Opioid Drugs. National Institute on Drug Abuse website. . Update January 2017. Accessed September 14, 2017.
- Sachs HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3):e796–e809.
- US Food and Drug Administration. FDA Drug Safety Communication. FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/Drugs/DrugSafety/ucm118113.htm. Published April 2017. Accessed September 12, 2017.
- Practice advisory on codeine and tramadol for breast feeding women. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-on-Codeine-and-Tramadol-for-Breastfeeding-Women. Published April 27, 2017. Accessed September 12, 2017.
- Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatr Drugs. 2008;10(6):399–404.
- Langaee T, Hamadeh I, Chapman AB, Gums JG, Johnson JA. A novel simple method for determining CYP2D6 gene copy number and identifying allele(s) with duplication/multiplication. PLoS One. 2015;10(1):e0113808.
- Cascorbi I. Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17–22.
- Naumburg EG, Meny RG. Breast milk opioids and neonatal apnea. Am J Dis Child. 1988;142(1):11–12.
- Sauberan JB, Anderson PO, Lane JR, et al. Breast milk hydrocodone and hydromorphone levels in mothers using hydrocodone for postpartum pain. Obstet Gynecol. 2011;117(3):611–617.
- Seaton S, Reeves M, McLean S. Oxycodone as a component of multimodal analgesia for lactating mothers after Cesarean section: relationships between maternal plasma, breast milk and neonatal plasma levels. Aust N Z J Obstet Gynaecol. 2007;47(3):181–185.
- Lam J, Kelly L, Ciszkowski C, et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr. 2012;160(1):33–37.e2.
- Timm NL. Maternal use of oxycodone resulting in opioid intoxication in her breastfed neonate. J Pediatr. 2013;162(2):421–422.
- The Society for Obstetric Anesthesia and Perinatology. Comments in response to the ACOG/SMFM Practice Advisory on Codeine and Tramadol for Breastfeeding Women. The Society for Obstetric Anesthesia and Perinatology website. https://soap.org/soap-response-acog-smfm-advisory.pdf. Published June 10, 2017. Accessed August 28, 2017.
- Banning AM. Respiratory depression following medication change from tramadol to morphine [article in Danish]. Ugeskr Laeger. 1999;161(47):6500–6501.
- Clark JH, Wilson WG. A 16-day old breast-fed infant with metabolic acidosis caused by salicylate. Clin Pediatr (Phila). 1981;20(1):53–54.
- World Health Organization. Breastfeeding and maternal medication. Recommendations for drugs in the 11th WHO model list of essential drugs. http://apps.who.int/iris/bitstream/10665/62435/1/55732.pdf. Published 2002. Accessed September 12, 2017.
- Reye’s syndrome. National Reye’s Syndrome Foundation website. http://www.reyessyndrome.org. Accessed September 12, 2017.
- Berline CM Jr, Yaffe SJ, Ragni M. Disposition of acetaminophen in milk, saliva, and plasma of lactating women. Pediatr Pharmacol (New York). 1980;1(2):135–141.
- Townsend RJ, Benedetti TJ, Erickson SH, et al. Excretion of ibuprofen into breast milk. Am J Obstet Gynecol. 1984;149(2):184–186.
- Spigset O, Hägg S. Analgesics and breast-feeding: safety considerations. Paediatr Drugs. 2000;2(3):223–238.
- Bar-OZ B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Saf. 2003;26(13):925–935.
- Edwards JE, Rudy AC, Wermeling DP, Desai N, McNamara PJ. Hydromorphone transfer into breast milk after intranasal administration. Pharmacotherapy. 2003;23(2):153–158.
- Schultz ML, Kostic M, Kharasch S. A case of toxic breast-feeding [published online ahead of print January 6, 2017]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001009.
- National Overdose Deaths—Number of Deaths from Opioid Drugs. National Institute on Drug Abuse website. . Update January 2017. Accessed September 14, 2017.
- Sachs HC; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132(3):e796–e809.
- US Food and Drug Administration. FDA Drug Safety Communication. FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/Drugs/DrugSafety/ucm118113.htm. Published April 2017. Accessed September 12, 2017.
- Practice advisory on codeine and tramadol for breast feeding women. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-Advisory-on-Codeine-and-Tramadol-for-Breastfeeding-Women. Published April 27, 2017. Accessed September 12, 2017.
- Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatr Drugs. 2008;10(6):399–404.
- Langaee T, Hamadeh I, Chapman AB, Gums JG, Johnson JA. A novel simple method for determining CYP2D6 gene copy number and identifying allele(s) with duplication/multiplication. PLoS One. 2015;10(1):e0113808.
- Cascorbi I. Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(suppl 2):17–22.
- Naumburg EG, Meny RG. Breast milk opioids and neonatal apnea. Am J Dis Child. 1988;142(1):11–12.
- Sauberan JB, Anderson PO, Lane JR, et al. Breast milk hydrocodone and hydromorphone levels in mothers using hydrocodone for postpartum pain. Obstet Gynecol. 2011;117(3):611–617.
- Seaton S, Reeves M, McLean S. Oxycodone as a component of multimodal analgesia for lactating mothers after Cesarean section: relationships between maternal plasma, breast milk and neonatal plasma levels. Aust N Z J Obstet Gynaecol. 2007;47(3):181–185.
- Lam J, Kelly L, Ciszkowski C, et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr. 2012;160(1):33–37.e2.
- Timm NL. Maternal use of oxycodone resulting in opioid intoxication in her breastfed neonate. J Pediatr. 2013;162(2):421–422.
- The Society for Obstetric Anesthesia and Perinatology. Comments in response to the ACOG/SMFM Practice Advisory on Codeine and Tramadol for Breastfeeding Women. The Society for Obstetric Anesthesia and Perinatology website. https://soap.org/soap-response-acog-smfm-advisory.pdf. Published June 10, 2017. Accessed August 28, 2017.
- Banning AM. Respiratory depression following medication change from tramadol to morphine [article in Danish]. Ugeskr Laeger. 1999;161(47):6500–6501.
- Clark JH, Wilson WG. A 16-day old breast-fed infant with metabolic acidosis caused by salicylate. Clin Pediatr (Phila). 1981;20(1):53–54.
- World Health Organization. Breastfeeding and maternal medication. Recommendations for drugs in the 11th WHO model list of essential drugs. http://apps.who.int/iris/bitstream/10665/62435/1/55732.pdf. Published 2002. Accessed September 12, 2017.
- Reye’s syndrome. National Reye’s Syndrome Foundation website. http://www.reyessyndrome.org. Accessed September 12, 2017.
- Berline CM Jr, Yaffe SJ, Ragni M. Disposition of acetaminophen in milk, saliva, and plasma of lactating women. Pediatr Pharmacol (New York). 1980;1(2):135–141.
- Townsend RJ, Benedetti TJ, Erickson SH, et al. Excretion of ibuprofen into breast milk. Am J Obstet Gynecol. 1984;149(2):184–186.
- Spigset O, Hägg S. Analgesics and breast-feeding: safety considerations. Paediatr Drugs. 2000;2(3):223–238.
- Bar-OZ B, Bulkowstein M, Benyamini L, et al. Use of antibiotic and analgesic drugs during lactation. Drug Saf. 2003;26(13):925–935.
- Edwards JE, Rudy AC, Wermeling DP, Desai N, McNamara PJ. Hydromorphone transfer into breast milk after intranasal administration. Pharmacotherapy. 2003;23(2):153–158.
- Schultz ML, Kostic M, Kharasch S. A case of toxic breast-feeding [published online ahead of print January 6, 2017]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001009.
Are combination estrogen-progestin oral contraceptives associated with an increased risk of cancer?
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
There are no large randomized clinical trials exploring the relationship between COCs and the risk of developing cancer. Many epidemiological studies, however, have investigated the possible association between COC use and the risk of cancer. Such prospective and retrospective studies consistently report that the use of COCs significantly decreases the risk of ovarian and endometrial cancer. The epidemiological data are less consistent concerning the possible association between COC use and the risk of breast cancer. Meta-analyses conclude that current use of COCs may be associated with a small increase in breast cancer risk. In addition, prolonged use of COCs may be associated with an increased risk of cervical cancer.
Ovarian cancer
- 0.78 (0.73–0.83) for 2.4 years
- 0.64 (0.59–0.69) for 6.8 years
- 0.56 (0.50–0.62) for 11.6 years
- 0.42 (0.36–0.49) for 18.3 years.
In the Royal College of General Practitioners Oral Contraceptive (RCGPOC) study, about 23,000 womenwho did not use COCs and 23,000 current users of COCs were recruited around 1968 and followed for a median of 41 years. In this study, current and recent use of COCs was associated with a decreased RR for ovarian cancer (0.49) and the risk reduction persisted for at least 35 years following COC discontinuation (RR, 0.50; 99% CI, 0.29–0.84).2
In the prospective Nurses’ Health Study (NHS) I, 121,700 nurses were recruited in 1976 and followed for more than 30 years.3 For nurses who reported using COCs for more than 5 years, the rate ratio for ovarian cancer at 20 years or less and greater than 20 years since last use was 0.58 (95% CI, 0.61–0.87) and 0.92 (95% CI, 0.61–1.39), respectively. These studies show that the association between COC use and a decreased risk of ovarian cancer persists for many years after discontinuing COCs.
Endometrial cancer
In the RCGPOC study of 46,000 women, the RR of endometrial cancer among current and recent users of COCs was 0.61, and the reduced risk (0.83) persisted for more than 35 years after discontinuing the COC.2
Related article:
2016 Update on cancer: Endometrial cancer
It is thought that the progestin in the COC provides most of the beneficial effect. Progestin-only contraceptives, such as depotmedroxyprogesterone acetate, progestin implants, and levonorgestrel-releasingintrauterine devices (LNG-IUDs) are also thought to reduce endometrial cancer risk. For instance, in a study of 93,842 Finnish women who used the LNG-IUD, the standardized incidence ratio for endometrial cancer was 0.50 among LNG-IUD users compared with the general population.5
Read about the effects of COC use in breast and cervical cancer.
Breast cancer
In the prospective NHS study of 116,608 nurses with 1,246,967 years of follow-up, the multivariate relative risk (mRR) of breast cancer with current COC use was 1.33 (95% CI, 1.03–1.73). Past use of COCs was not associated with a significantly increased risk of breast cancer (mRR, 1.12; 95% CI, 0.95–1.33; NS).7
In the RCGPOC study (approximately 46,000 women), current use of COCs was associated with an increased risk of breast cancer (incidence rate ratio [IRR], 1.48; 95% CI,1.10–1.97). Five to 15 years after stopping COCs, there was no significant association between prior COC use and breast cancer (IRR, 1.12; 99% CI, 0.91–1.39; NS).2
Related article:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
It is important to note that it is not possible to conclude from these data whether the reported association between current use of COCs and breast cancer is due to early and accelerated diagnosis of breast cancer, the biological effects of hormones contained in COCs on breast tissue and nascent tumors, or both. In addition, formulations of COCs prescribed in the 1960s and 1970s contained higher doses of estrogen, raising the possibility that the association between COCs and breast cancer is due to COC formulations that are no longer prescribed. However, in animal models and postmenopausal women certain combinations of estrogen plus progestin clearly influence breast cancer biology and cancer risk.8,9
Women carrying BRCA1 and BRCA2 mutations, which increase the risk of ovarian and breast cancer, are often counseled to consider bilateral salpingectomy between age 35 and 40 years to reduce the risk of developing ovarian cancer. An important clinical question is what is the impact of combination estrogen-progestin oral contraceptives (COC) use on ovarian and breast cancer risk among these women?
Meta-analyses of the association between COC use and ovarian cancer consistently report that COC use reduces the risk of ovarian cancer in women with clinically important BRCA1 and BRCA2 mutations.1,2 For example, a meta-analysis of 6 studies reported that women with BRCA1 and BRCA2 mutations who used COCs had a significantly decreased risk of ovarian cancer (odds ratio [OR], 0.58; 95% CI, 0.46–0.73).1
The association between COC use and breast cancer risk is not clear. One meta-analysis reported no significant association between COC use and breast cancer risk among BRCA mutation carriers (OR, 1.21; 95% CI, 0.93–1.58).1 Another meta-analysis reported a significant association between COC use before 1975 and breast cancer risk (RR, 1.47; 95% CI, 1.06–2.04) but not with recent low-estrogen formulations of COC (RR, 1.17; 95% CI, 0.74–1.86).2
Based on the available data, the Society of Gynecologic Oncologists recommends that women with clinically significant BRCA1 and BRCA2 mutations be offered chemoprevention with COCs because the benefit of ovarian cancer risk reduction outweighs the possible impact on breast cancer risk.3 A contrarian view-point espoused by some oncologists is that since women with BRCA mutations should have their ovaries removed prior to getting ovarian cancer, the clinical utility of recommending COC chemoprevention of ovarian cancer is largely irrelevant.
References
- Moorman PG, Havrilesky LJ, Gierisch JM, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31(33):4188–4198.
- Iodice S, Barile M, Rotmensz N, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Canc. 2010;46(12):2275–2284.
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120.
Cervical cancer
- less than 5 years, 0.73 (95% CI, 0.52–1.03)
- 5 to 9 years, 2.82 (95% CI, 1.46–5.42)
- ≥10 years, 4.03 (95% CI, 2.09–8.02).
It is not possible to conclude from these data whether the association between COC use and cervical cancer is due to the biological effects of hormones on the initiation and progression of HPV disease or confounding factors that have yet to be identified. It is known that estrogens and progestins influence the immune defense system of the lower genital tract, and this may be a pathway that influences the acquisition and progression of viral disease.12 From a clinical perspective, cervical cancer is largely preventable with HPV vaccination and screening. Therefore, the risk between COC use and cervical cancer is likely limited to women who have not been vaccinated and who are not actively participating in cervical cancer screening.
The bottom line
COC use markedly reduces the risk of ovarian and endometrial cancers, and slightly increases the risk of breast cancer. Prolonged COC use may be associated with an increased risk of cervical cancer. Using available epidemiological data, investigators attempted to project the impact of these competing risks on the approximate 12,300,000 females who live in Australia. Based on the pattern of COC use and the cancer incidence in Australia in 2010, the investigators calculated that COC use would cause about 105 breast and 52 cervical cancers and prevent 1,032 endometrial and 308 ovarian cancers.13 This analysis indicates that the balance of risks and benefits related to COC use and cancer generally favors COC use.
Prevention of unintended pregnancy is a major public health goal. Many women choose COCs as their preferred approach to preventing unintended pregnancy. Evaluated from a whole-life perspective the health benefits of COCs are substantial and represent a great advance in women’s health.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.
- Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314.
- Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–e9.
- Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. 2007;166(8):894–901.
- Collaborative Group on Epidemiological Studies on Endometrial Cancer. Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27,276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061–1070.
- Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 pt 1):292–299.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727.
- Hunter DJ, Colditz GA, Hankinson SE, et al. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2496–2502.
- Simões BM, Alferez DG, Howell SJ, Clarke RB. The role of steroid hormones in breast cancer stem cells. Endocr Relat Cancer. 2015;22(6):T177–T186.
- Chlebowski RT, Manson JE, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
- Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Fichorova RN, Chen PL, Morrison CS, et al. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio. 2015;6(5):e00221–e002215.
- Jordan SJ, Wilson LF, Nagle CM, et al. Cancers in Australia in 2010 attributable to and prevented by the use of combined oral contraceptives. Aust N Z J Public Health. 2015;39(5):441–445.
For the management of labor, patience is a virtue
During the past 45 years, the cesarean delivery (CD) rate in the United States has increased from 5.5% in 1970 to 33% from 2009 to 2013, followed by a small decrease to 32% in 2014 and 2015.1 Many clinical problems cause clinicians and patients to decide that CD is an optimal birth route, including: abnormal labor progress, abnormal or indeterminate fetal heart rate pattern, breech presentation, multiple gestation, macrosomia, placental and cord abnormalities, preeclampsia, prior uterine surgery, and prior CD.2 Recent secular trends that contribute to the current rate of CD include an adversarial liability environment,3,4 increasing rates of maternal obesity,5 and widespread use of continuous fetal-heart monitoring during labor.6
Wide variation in CD rate has been reported among countries, states, and hospitals. The variation is due, in part, to different perspectives about balancing the harms and benefits of vaginal delivery versus CD. In Europe, in 2010 the CD rates in Sweden and Italy were 17.1% and 38%, respectively.7 In 2010, among the states, Alaska had the lowest rate of CD at 22% and Kentucky had the highest rate at 40%.8 In 2015, the highest rate was 38%, in Mississippi (FIGURE).9 In 2014, among Massachusetts hospitals with more than 2,500 births, the CD rate ranged from a low of 22% to a high of 37%.10
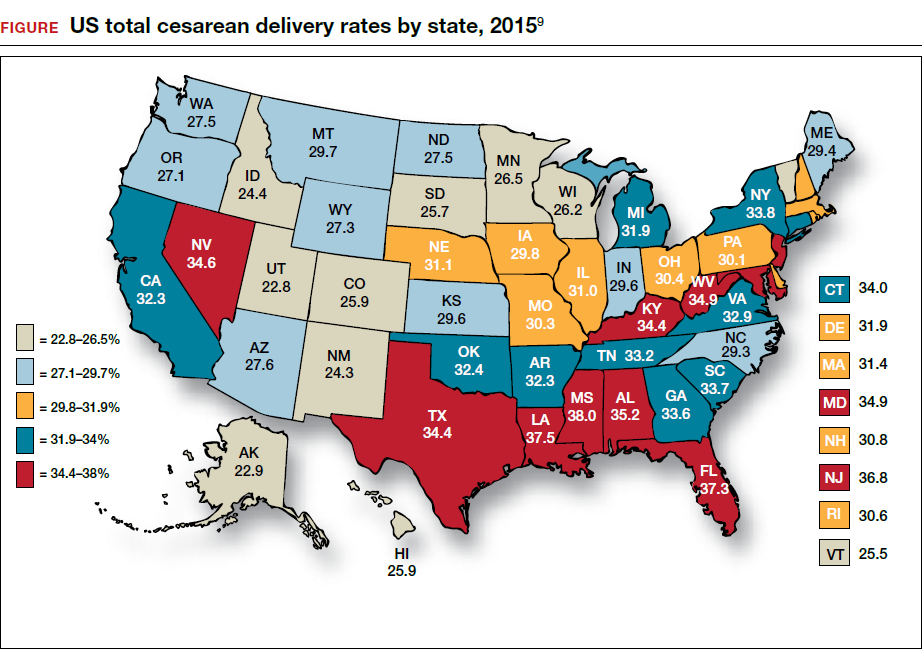
Clinicians, patients, policy experts, and the media are perplexed and troubled by the “high” US CD rate and the major variation in rate among countries, states, and hospitals. Labor management practices likely influence the rate of CD and diverse approaches to labor management likely account for the wide variation in CD rates.
A nationwide effort to standardize and continuously improve labor management might result in a decrease in the CD rate. Building on this opportunity, the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) have jointly recommended new labor management guidelines that may reduce the primary CD rate.8
The ACOG/SMFM guidelines encourage obstetricians to extend the time for labor progress in both the 1st and 2nd stages prior to recommending a CD.8 These new guidelines emphasize that for a modern obstetrician, patience is a virtue. There are 2 important caveats to this statement: to safely extend the length of time of labor requires both (1) a reassuring fetal heart rate tracing and (2) stable maternal health. If the fetus demonstrates a persistent worrisome Category II or a Category IIIheart-rate tracing, decisive intervention is necessary and permitting an extended labor would not be optimal. Similarly, if the mother has rapidly worsening preeclampsia it may not be wise to extend an induction of labor (IOL) over many days.
There are risks with extending the length of labor. An extended duration of the 1st stage of labor is associated with an increased rate of maternal chorioamnionitis and shoulder dystocia at birth.11 An extended duration of the 2nd stage of labor is associated with an increase in the rate of maternal chorioamnionitis, anal sphincter injury, uterine atony, and neonatal admission to an intensive care unit.12 Clinicians who adopt practices that permit an extended length of labor must weigh the benefits of avoiding a CD against these maternal and fetal complications.
Active phase redefined
Central to the ACOG/SMFM guidelines is a new definition of the active phase of labor. The research of Dr. Emmanuel Friedman indicated that at approximately 4 cm of cervical dilation many women in labor transition from the latent phase, a time of slow change in cervical dilation, to the active phase, a time of more rapid change in cervical dilation.13,14 However, more recent research indicates that the transition between the latent and active phase is difficult to precisely define, but more often occurs at about 6 cm of cervical dilation and not 4 cm of dilation.15 Adopting these new norms means that laboring women will spend much more time in the latent phase, a phase of labor in which patience is a virtue.
The ACOG/SMFM guidelines
Main takeaways from the ACOG/SMFM guidelines are summarized below. Interventions that address common obstetric issues and labor abnormalities are outlined below.
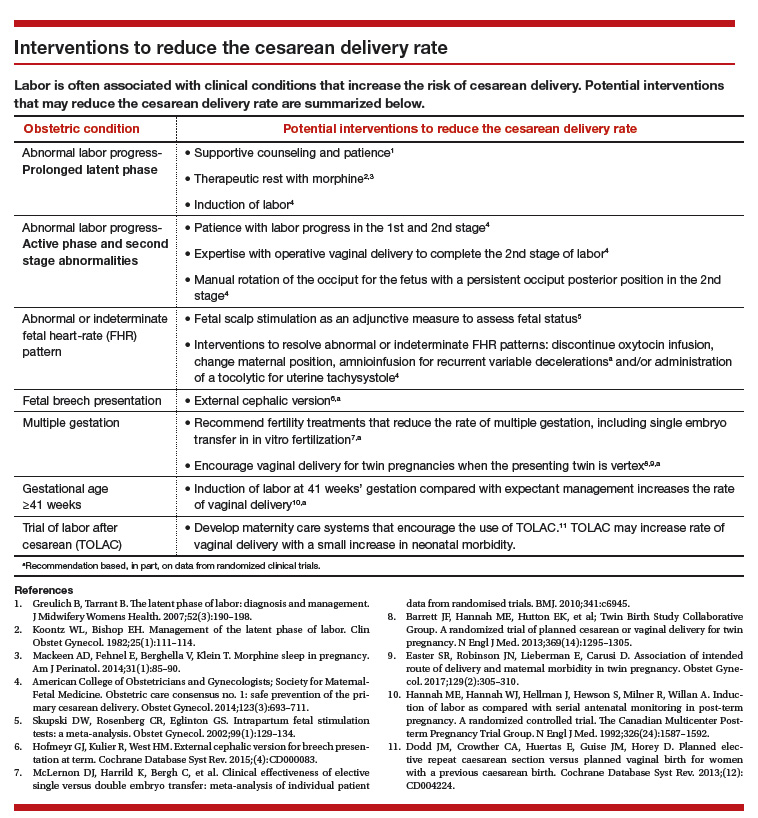
Do not perform CD for a prolonged latent phase of labor, defined as regular contractions of >20 hours duration in nulliparous women and >14 hours duration in multiparous women. Patience with a prolonged latent phase will be rewarded by the majority of women entering the active phase of labor. Alternatively, if appropriate, cervical ripening followed by oxytocin IOL and amniotomy will help the patient with a prolonged latent phase to enter the active phase of labor.16
For women with an unfavorable cervix as assessed by the Bishop score, cervical ripening should be performed prior to IOL. Use of cervical ripening prior to IOL increases the chance of achieving vaginal delivery within 24 hours and may result in a modest decrease in the rate of CD.17,18
Related article:
Should oxytocin and a Foley catheter be used concurrently for cervical ripening in induction of labor?
Failed IOL in the latent phase should only be diagnosed following 12 to 18 hours of both ruptured membranes and adequate contractions stimulated with oxytocin. The key ingredients for the successful management of the latent phase of labor are patience, oxytocin, and amniotomy.16
CD for the indication of active phase arrest requires cervical dilation ≥6 cm with ruptured membranes and no change in cervical dilation for ≥4 hours of adequate uterine activity. In the past, most obstetricians defined active phase arrest, a potential indication for CD, as the absence of cervical change for 2 or more hours in the presence of adequate uterine contractions and cervical dilation of at least 4 cm. Given the new definition of active phase arrest, slow but progressive progress in the 1st stage of labor is not an indication for CD.11,19
“A specific absolute maximum length of time spent in the 2nd stage beyond which all women should be offered an operative delivery has not been identified.”8 Diagnosis of arrest of labor in the 2nd stage may be considered after at least 2 hours of pushing in multiparous women and 3 hours of pushing in nulliparous women, especially if no fetal descent is occurring. The guidelines also state “longer durations may be appropriate on an individualized basis (eg, with use of epidural analgesia or with fetal malposition)” as long as fetal descent is observed.
Patience is a virtue, especially in the management of the 2nd stage of labor. Extending the 2nd stage up to 4 hours appears to be reasonably safe if the fetal status is reassuring and the mother is physiologically stable. In a study from San Francisco of 42,268 births with normal newborn outcomes, the 95th percentile for the length of the 2nd stage of labor for nulliparous women was 3.3 hours without an epidural and 5.6 hours with an epidural.20
In a study of 53,285 births, longer duration of pushing was associated with a small increase in the rate of neonatal adverse outcomes. In nulliparous women the rate of adverse neonatal outcomes increased from 1.3% with less than 60 minutes of pushing to 2.4% with greater than 240 minutes of pushing. Remarkably, even after 4 hours of pushing, 78% of nulliparous women who continued to push had a vaginal delivery.21 In this study, among nulliparous women the rate of anal sphincter injury increased from 5% with less than 60 minutes of pushing to 16% with greater than 240 minutes of pushing, and the rate of postpartum hemorrhage increased from 1% with less than 60 minutes of pushing to 3.3% with greater than 240 minutes of pushing.
I am not enthusiastic about patiently watching a labor extend into the 5th hour of the 2nd stage, especially if the fetus is at +2 station or lower. In a nulliparous woman, after 4 hours of managing the 2nd stage of labor, my patience is exhausted and I am inclined to identify a clear plan for delivery, either by enhanced labor coaching, operative vaginal delivery, or CD.
Operative vaginal delivery in the 2nd stage of labor is an acceptable alternative to CD. The rate of operative vaginal delivery in the United States has declined over the past 2 decades (TABLE). In Sweden in 2010 the operative vaginal delivery rate was 7.6% with a CD rate of 17.1%.7 In the United States in 2010 the operative delivery rate was 3.6%, and the CD rate was 33%.1 A renewed focus on operative vaginal delivery with ongoing training and team simulation for the procedure would increase our use of operative delivery and decrease the overall rate of CD.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
Encourage the detection of persistent fetal occiput posterior position by physical examination and/or ultrasound and consider manual rotation of the fetal occiput from the posterior to anterior position in the 2nd stage. Persistent occiput posterior is the most common fetal malposition.22 This malposition is associated with an increased rate of CD.23 There are few randomized trials of manual rotation of the fetal occiput from posterior to anterior position in the 2nd stage of labor, and the evidence is insufficient to determine the efficacy of manual rotation.24 Small nonrandomized studies report that manual rotation of the occiput from posterior to anterior position may reduce the CD rate.25–27
For persistent 2nd stage fetal occiput posterior position in a woman with an adequate pelvis, where manual rotation was not successful and the fetus is at +2 station or below, operative vaginal delivery is an option. “Vacuum or forceps?” and “If forceps, to rotate or not to rotate?” those are the clinical questions. Forceps delivery is more likely to be successfulthan vacuum delivery.28 Direct forceps delivery of the occiput posterior fetus is associated with more anal sphincter injuries than forceps delivery after successful rotation, but few clinicians regularly perform rotational forceps.29 In a study of 2,351 women in the 2nd stage of labor with the fetus at +2 station or below, compared with either forceps or vacuum delivery, CD was associated with more maternal infections and fewer perineal lacerations. Neonatal composite morbidity was not significantly different among the 3 routes of operative delivery.30
Amnioinfusion for repetitive variable decelerations of the fetal heart rate may reduce the risk of CD for an indeterminate fetal heart-rate pattern.31
IOL in a well-dated pregnancy at 41 weeks will reduce the risk of CD. In a large clinical trial, 3,407 women at 41 weeks of gestation were randomly assigned to IOL or expectant management. The rate of CD was significantly lower in the women assigned to IOL compared with expectant management (21% vs 25%, respectively; P = .03).32 The rate of neonatal morbidity was similar in the 2 groups.
Women with twin gestations and the first twin in a cephalic presentation may elect vaginal delivery. In a large clinical trial, 1,398 women with a twin gestation and the first twin in a cephalic presentation were randomly assigned to planned vaginal delivery (with cesarean only if necessary) or planned CD.33 The rate of CD was 44% and 91% for the women in the planned-vaginal and planned-cesarean groups, respectively. There was no significant difference in composite fetal or neonatal death or serious morbidity. The authors concluded that, for twin pregnancy with the presenting twin in the cephalic presentation, there were no demonstrated benefits of planned CD.
Develop maternity care systems that encourage the use of trial of labor after cesarean (TOLAC). The ACOG/SMFM guidelines focus on interventions to reduce the rate of primary CD and do not address the role of TOLAC in reducing CD rates. There are little data from clinical trials to assess the benefits and harms from TOLAC versus scheduled repeat CD.34 However, our experience with TOLAC in the 1990s strongly suggests that encouraging TOLAC will decrease the rate of CD. In 1996 the US rate of vaginal birth after cesarean (VBAC) peaked at 28%, and the rate of CD achieved a recent historic nadir of 21%. Growing concerns that TOLAC occasionally results in fetal harm was followed by a decrease in the VBAC rate to 12% in 2015.1 A recent study of obstetric practices in countries with high and low VBAC rates concluded that patient and clinician commitment and comfort with prioritizing TOLAC over scheduled repeat CD greatly influenced the VBAC rate.35
Related article:
Should lower uterine segment thickness measurement be included in the TOLAC decision-making process?
Labor management is an art
During labor obstetricians must balance the unique needs of mother and fetus, which requires great clinical skill and patience. Evolving concepts of normal labor progress necessitate that we change our expectations concerning the acceptable rate of progress in the 1st and 2nd stage of labor. Consistent application of these new labor guidelines may help to reduce the rate of CD.
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Matthews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1–70. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf. Accessed July 5, 2017.
- Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29–38.
- Localio AR, Lawthers AG, Bengtson JM, et al. Relationship between malpractice claims and cesarean delivery. JAMA. 1993;269(3):366–373.
- Cheng YW, Snowden JM, Handler SJ, Tager IB, Hubbard AE, Caughey AB. Litigation in obstetrics: does defensive medicine contribute to increases in cesarean delivery? J Matern Fetal Neonatal Med. 2014;27(16):1668–1675.
- Graham LE, Brunner Huber LR, Thompson ME, Ersek JL. Does amount of weight gain during pregnancy modify the association between obesity and cesarean section delivery? Birth. 2014;41(1):93–99.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;(5):CD006066.
- European Perinatal Health Report. Euro-Peristat website. http://www.europeristat.com/. Published 2012. Accessed July 5, 2017.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
- Cesarean delivery rate by state, 2015. Centers for Disease Control and Prevention website. https://www.cdc.gov/nchs/pressroom/sosmap/cesarean_births/cesareans.htm. Updated January 9, 2017. Accessed July 18, 2017.
- Baker CD, Land T; Massachusetts Department of Public Health. Massachusetts Births 2014. Massachusetts Executive Office of Health and Human Services website. http://www.mass.gov/eohhs/gov/departments/dph/programs/admin/dmoa/repi/birth-data.html. Published September 2015. Accessed July 5, 2017.
- Henry DE, Cheng YW, Shaffer BL, Kaimal AJ, Bianco K, Caughey AB. Perinatal outcomes in the setting of active phase arrest of labor. Obstet Gynecol. 2008;112(5):1109–1115.
- Rouse DJ, Weiner SJ, Bloom SL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1–e7.
- Friedman EZ. Labour: Clinical evaluation and management. Appleton-Century-Crofts: New York, NY; 1967.
- Friedman E. The graphic analysis of labor. Am J Obstet Gynecol. 1954;68(6):1568–1575.
- Zhang J, Landy HJ, Branch DW, et al; Consortium on Safe Labor. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
- Wei S, Wo BL, Qi HP, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2013;(8):CD006794.
- Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014;(6):CD003101.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Rouse DJ, Owen J, Savage KG, Hauth JC. Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98(4):550–554.
- Cheng YW, Shaffer BL, Nicholson JM, Caughey AB. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol. 2014;123(3):527–535.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Shriver National Institute of Child and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127(4):667–673.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125(3):695–709.
- Carseldine WJ, Phipps H, Zawada SF, et al. Does occiput posterior position in the second stage of labour increase the operative delivery rate? Aust N Z J Obstet Gynaecol. 2013;53(3):265–270.
- Phipps H, de Vries B, Hyett J, Osborn DA. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;(12):CD009298.
- Shaffer BL, Cheng YW, Vargas JE, Caughey AB. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24(1):65–72.
- Le Ray C, Serres P, Schmitz T, Cabrol D, Goffinet F. Manual rotation in occiput posterior or transverse positions: risk factors and consequences on the cesarean delivery rate. Obstet Gynecol. 2007;110(4):873–879.
- Reichman O, Gdansky E, Latinsky B, Labi S, Samueloff A. Digital rotation from occipito-posterior to occipito-anterior decreases the need for cesarean section. Eur J Obstet Gynecol Repro Biol. 2008;136:25–28.
- O’Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database Syst Rev. 2010;(11):CD005455.
- Hirsch E, Elue R, Wagner A Jr, et al. Severe perineal laceration during operative vaginal delivery: the impact of occiput posterior position. J Perinatol. 2014;34(12):898–900.
- Bailit JL, Grobman WA, Rice MM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Evaluation of delivery options for second-stage events. Am J Obstet Gynecol. 2016;214(5):638.e1–e10.
- Hofmeyr GJ, Lawrie TA. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database Syst Rev. 2012;1:CD000013.
- Hannah ME, Hannah WJ, Hellmann J, Hewson S, Milner R, Willan A. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326(24): 1587–1592.
- Barrett JF, Hannah ME, Hutton EK, et al; Twin Birth Study Collaborative Group. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med. 2013;369(14):1295–1305.
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat cesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev. 2013;(12):CD004224.
- Lundgren I, van Limbeek E, Vehvilainen-Julkunen K, Nilsson C. Clinicians’ views of factors of importance for improving the rate of VBAC (vaginal birth after caesarean section): a qualitative study from countries with high VBAC rates. BMC Pregnancy Childbirth. 2015;15:196.
During the past 45 years, the cesarean delivery (CD) rate in the United States has increased from 5.5% in 1970 to 33% from 2009 to 2013, followed by a small decrease to 32% in 2014 and 2015.1 Many clinical problems cause clinicians and patients to decide that CD is an optimal birth route, including: abnormal labor progress, abnormal or indeterminate fetal heart rate pattern, breech presentation, multiple gestation, macrosomia, placental and cord abnormalities, preeclampsia, prior uterine surgery, and prior CD.2 Recent secular trends that contribute to the current rate of CD include an adversarial liability environment,3,4 increasing rates of maternal obesity,5 and widespread use of continuous fetal-heart monitoring during labor.6
Wide variation in CD rate has been reported among countries, states, and hospitals. The variation is due, in part, to different perspectives about balancing the harms and benefits of vaginal delivery versus CD. In Europe, in 2010 the CD rates in Sweden and Italy were 17.1% and 38%, respectively.7 In 2010, among the states, Alaska had the lowest rate of CD at 22% and Kentucky had the highest rate at 40%.8 In 2015, the highest rate was 38%, in Mississippi (FIGURE).9 In 2014, among Massachusetts hospitals with more than 2,500 births, the CD rate ranged from a low of 22% to a high of 37%.10

Clinicians, patients, policy experts, and the media are perplexed and troubled by the “high” US CD rate and the major variation in rate among countries, states, and hospitals. Labor management practices likely influence the rate of CD and diverse approaches to labor management likely account for the wide variation in CD rates.
A nationwide effort to standardize and continuously improve labor management might result in a decrease in the CD rate. Building on this opportunity, the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) have jointly recommended new labor management guidelines that may reduce the primary CD rate.8
The ACOG/SMFM guidelines encourage obstetricians to extend the time for labor progress in both the 1st and 2nd stages prior to recommending a CD.8 These new guidelines emphasize that for a modern obstetrician, patience is a virtue. There are 2 important caveats to this statement: to safely extend the length of time of labor requires both (1) a reassuring fetal heart rate tracing and (2) stable maternal health. If the fetus demonstrates a persistent worrisome Category II or a Category IIIheart-rate tracing, decisive intervention is necessary and permitting an extended labor would not be optimal. Similarly, if the mother has rapidly worsening preeclampsia it may not be wise to extend an induction of labor (IOL) over many days.
There are risks with extending the length of labor. An extended duration of the 1st stage of labor is associated with an increased rate of maternal chorioamnionitis and shoulder dystocia at birth.11 An extended duration of the 2nd stage of labor is associated with an increase in the rate of maternal chorioamnionitis, anal sphincter injury, uterine atony, and neonatal admission to an intensive care unit.12 Clinicians who adopt practices that permit an extended length of labor must weigh the benefits of avoiding a CD against these maternal and fetal complications.
Active phase redefined
Central to the ACOG/SMFM guidelines is a new definition of the active phase of labor. The research of Dr. Emmanuel Friedman indicated that at approximately 4 cm of cervical dilation many women in labor transition from the latent phase, a time of slow change in cervical dilation, to the active phase, a time of more rapid change in cervical dilation.13,14 However, more recent research indicates that the transition between the latent and active phase is difficult to precisely define, but more often occurs at about 6 cm of cervical dilation and not 4 cm of dilation.15 Adopting these new norms means that laboring women will spend much more time in the latent phase, a phase of labor in which patience is a virtue.
The ACOG/SMFM guidelines
Main takeaways from the ACOG/SMFM guidelines are summarized below. Interventions that address common obstetric issues and labor abnormalities are outlined below.

Do not perform CD for a prolonged latent phase of labor, defined as regular contractions of >20 hours duration in nulliparous women and >14 hours duration in multiparous women. Patience with a prolonged latent phase will be rewarded by the majority of women entering the active phase of labor. Alternatively, if appropriate, cervical ripening followed by oxytocin IOL and amniotomy will help the patient with a prolonged latent phase to enter the active phase of labor.16
For women with an unfavorable cervix as assessed by the Bishop score, cervical ripening should be performed prior to IOL. Use of cervical ripening prior to IOL increases the chance of achieving vaginal delivery within 24 hours and may result in a modest decrease in the rate of CD.17,18
Related article:
Should oxytocin and a Foley catheter be used concurrently for cervical ripening in induction of labor?
Failed IOL in the latent phase should only be diagnosed following 12 to 18 hours of both ruptured membranes and adequate contractions stimulated with oxytocin. The key ingredients for the successful management of the latent phase of labor are patience, oxytocin, and amniotomy.16
CD for the indication of active phase arrest requires cervical dilation ≥6 cm with ruptured membranes and no change in cervical dilation for ≥4 hours of adequate uterine activity. In the past, most obstetricians defined active phase arrest, a potential indication for CD, as the absence of cervical change for 2 or more hours in the presence of adequate uterine contractions and cervical dilation of at least 4 cm. Given the new definition of active phase arrest, slow but progressive progress in the 1st stage of labor is not an indication for CD.11,19
“A specific absolute maximum length of time spent in the 2nd stage beyond which all women should be offered an operative delivery has not been identified.”8 Diagnosis of arrest of labor in the 2nd stage may be considered after at least 2 hours of pushing in multiparous women and 3 hours of pushing in nulliparous women, especially if no fetal descent is occurring. The guidelines also state “longer durations may be appropriate on an individualized basis (eg, with use of epidural analgesia or with fetal malposition)” as long as fetal descent is observed.
Patience is a virtue, especially in the management of the 2nd stage of labor. Extending the 2nd stage up to 4 hours appears to be reasonably safe if the fetal status is reassuring and the mother is physiologically stable. In a study from San Francisco of 42,268 births with normal newborn outcomes, the 95th percentile for the length of the 2nd stage of labor for nulliparous women was 3.3 hours without an epidural and 5.6 hours with an epidural.20
In a study of 53,285 births, longer duration of pushing was associated with a small increase in the rate of neonatal adverse outcomes. In nulliparous women the rate of adverse neonatal outcomes increased from 1.3% with less than 60 minutes of pushing to 2.4% with greater than 240 minutes of pushing. Remarkably, even after 4 hours of pushing, 78% of nulliparous women who continued to push had a vaginal delivery.21 In this study, among nulliparous women the rate of anal sphincter injury increased from 5% with less than 60 minutes of pushing to 16% with greater than 240 minutes of pushing, and the rate of postpartum hemorrhage increased from 1% with less than 60 minutes of pushing to 3.3% with greater than 240 minutes of pushing.
I am not enthusiastic about patiently watching a labor extend into the 5th hour of the 2nd stage, especially if the fetus is at +2 station or lower. In a nulliparous woman, after 4 hours of managing the 2nd stage of labor, my patience is exhausted and I am inclined to identify a clear plan for delivery, either by enhanced labor coaching, operative vaginal delivery, or CD.
Operative vaginal delivery in the 2nd stage of labor is an acceptable alternative to CD. The rate of operative vaginal delivery in the United States has declined over the past 2 decades (TABLE). In Sweden in 2010 the operative vaginal delivery rate was 7.6% with a CD rate of 17.1%.7 In the United States in 2010 the operative delivery rate was 3.6%, and the CD rate was 33%.1 A renewed focus on operative vaginal delivery with ongoing training and team simulation for the procedure would increase our use of operative delivery and decrease the overall rate of CD.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
Encourage the detection of persistent fetal occiput posterior position by physical examination and/or ultrasound and consider manual rotation of the fetal occiput from the posterior to anterior position in the 2nd stage. Persistent occiput posterior is the most common fetal malposition.22 This malposition is associated with an increased rate of CD.23 There are few randomized trials of manual rotation of the fetal occiput from posterior to anterior position in the 2nd stage of labor, and the evidence is insufficient to determine the efficacy of manual rotation.24 Small nonrandomized studies report that manual rotation of the occiput from posterior to anterior position may reduce the CD rate.25–27
For persistent 2nd stage fetal occiput posterior position in a woman with an adequate pelvis, where manual rotation was not successful and the fetus is at +2 station or below, operative vaginal delivery is an option. “Vacuum or forceps?” and “If forceps, to rotate or not to rotate?” those are the clinical questions. Forceps delivery is more likely to be successfulthan vacuum delivery.28 Direct forceps delivery of the occiput posterior fetus is associated with more anal sphincter injuries than forceps delivery after successful rotation, but few clinicians regularly perform rotational forceps.29 In a study of 2,351 women in the 2nd stage of labor with the fetus at +2 station or below, compared with either forceps or vacuum delivery, CD was associated with more maternal infections and fewer perineal lacerations. Neonatal composite morbidity was not significantly different among the 3 routes of operative delivery.30
Amnioinfusion for repetitive variable decelerations of the fetal heart rate may reduce the risk of CD for an indeterminate fetal heart-rate pattern.31
IOL in a well-dated pregnancy at 41 weeks will reduce the risk of CD. In a large clinical trial, 3,407 women at 41 weeks of gestation were randomly assigned to IOL or expectant management. The rate of CD was significantly lower in the women assigned to IOL compared with expectant management (21% vs 25%, respectively; P = .03).32 The rate of neonatal morbidity was similar in the 2 groups.
Women with twin gestations and the first twin in a cephalic presentation may elect vaginal delivery. In a large clinical trial, 1,398 women with a twin gestation and the first twin in a cephalic presentation were randomly assigned to planned vaginal delivery (with cesarean only if necessary) or planned CD.33 The rate of CD was 44% and 91% for the women in the planned-vaginal and planned-cesarean groups, respectively. There was no significant difference in composite fetal or neonatal death or serious morbidity. The authors concluded that, for twin pregnancy with the presenting twin in the cephalic presentation, there were no demonstrated benefits of planned CD.
Develop maternity care systems that encourage the use of trial of labor after cesarean (TOLAC). The ACOG/SMFM guidelines focus on interventions to reduce the rate of primary CD and do not address the role of TOLAC in reducing CD rates. There are little data from clinical trials to assess the benefits and harms from TOLAC versus scheduled repeat CD.34 However, our experience with TOLAC in the 1990s strongly suggests that encouraging TOLAC will decrease the rate of CD. In 1996 the US rate of vaginal birth after cesarean (VBAC) peaked at 28%, and the rate of CD achieved a recent historic nadir of 21%. Growing concerns that TOLAC occasionally results in fetal harm was followed by a decrease in the VBAC rate to 12% in 2015.1 A recent study of obstetric practices in countries with high and low VBAC rates concluded that patient and clinician commitment and comfort with prioritizing TOLAC over scheduled repeat CD greatly influenced the VBAC rate.35
Related article:
Should lower uterine segment thickness measurement be included in the TOLAC decision-making process?
Labor management is an art
During labor obstetricians must balance the unique needs of mother and fetus, which requires great clinical skill and patience. Evolving concepts of normal labor progress necessitate that we change our expectations concerning the acceptable rate of progress in the 1st and 2nd stage of labor. Consistent application of these new labor guidelines may help to reduce the rate of CD.
During the past 45 years, the cesarean delivery (CD) rate in the United States has increased from 5.5% in 1970 to 33% from 2009 to 2013, followed by a small decrease to 32% in 2014 and 2015.1 Many clinical problems cause clinicians and patients to decide that CD is an optimal birth route, including: abnormal labor progress, abnormal or indeterminate fetal heart rate pattern, breech presentation, multiple gestation, macrosomia, placental and cord abnormalities, preeclampsia, prior uterine surgery, and prior CD.2 Recent secular trends that contribute to the current rate of CD include an adversarial liability environment,3,4 increasing rates of maternal obesity,5 and widespread use of continuous fetal-heart monitoring during labor.6
Wide variation in CD rate has been reported among countries, states, and hospitals. The variation is due, in part, to different perspectives about balancing the harms and benefits of vaginal delivery versus CD. In Europe, in 2010 the CD rates in Sweden and Italy were 17.1% and 38%, respectively.7 In 2010, among the states, Alaska had the lowest rate of CD at 22% and Kentucky had the highest rate at 40%.8 In 2015, the highest rate was 38%, in Mississippi (FIGURE).9 In 2014, among Massachusetts hospitals with more than 2,500 births, the CD rate ranged from a low of 22% to a high of 37%.10

Clinicians, patients, policy experts, and the media are perplexed and troubled by the “high” US CD rate and the major variation in rate among countries, states, and hospitals. Labor management practices likely influence the rate of CD and diverse approaches to labor management likely account for the wide variation in CD rates.
A nationwide effort to standardize and continuously improve labor management might result in a decrease in the CD rate. Building on this opportunity, the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) have jointly recommended new labor management guidelines that may reduce the primary CD rate.8
The ACOG/SMFM guidelines encourage obstetricians to extend the time for labor progress in both the 1st and 2nd stages prior to recommending a CD.8 These new guidelines emphasize that for a modern obstetrician, patience is a virtue. There are 2 important caveats to this statement: to safely extend the length of time of labor requires both (1) a reassuring fetal heart rate tracing and (2) stable maternal health. If the fetus demonstrates a persistent worrisome Category II or a Category IIIheart-rate tracing, decisive intervention is necessary and permitting an extended labor would not be optimal. Similarly, if the mother has rapidly worsening preeclampsia it may not be wise to extend an induction of labor (IOL) over many days.
There are risks with extending the length of labor. An extended duration of the 1st stage of labor is associated with an increased rate of maternal chorioamnionitis and shoulder dystocia at birth.11 An extended duration of the 2nd stage of labor is associated with an increase in the rate of maternal chorioamnionitis, anal sphincter injury, uterine atony, and neonatal admission to an intensive care unit.12 Clinicians who adopt practices that permit an extended length of labor must weigh the benefits of avoiding a CD against these maternal and fetal complications.
Active phase redefined
Central to the ACOG/SMFM guidelines is a new definition of the active phase of labor. The research of Dr. Emmanuel Friedman indicated that at approximately 4 cm of cervical dilation many women in labor transition from the latent phase, a time of slow change in cervical dilation, to the active phase, a time of more rapid change in cervical dilation.13,14 However, more recent research indicates that the transition between the latent and active phase is difficult to precisely define, but more often occurs at about 6 cm of cervical dilation and not 4 cm of dilation.15 Adopting these new norms means that laboring women will spend much more time in the latent phase, a phase of labor in which patience is a virtue.
The ACOG/SMFM guidelines
Main takeaways from the ACOG/SMFM guidelines are summarized below. Interventions that address common obstetric issues and labor abnormalities are outlined below.

Do not perform CD for a prolonged latent phase of labor, defined as regular contractions of >20 hours duration in nulliparous women and >14 hours duration in multiparous women. Patience with a prolonged latent phase will be rewarded by the majority of women entering the active phase of labor. Alternatively, if appropriate, cervical ripening followed by oxytocin IOL and amniotomy will help the patient with a prolonged latent phase to enter the active phase of labor.16
For women with an unfavorable cervix as assessed by the Bishop score, cervical ripening should be performed prior to IOL. Use of cervical ripening prior to IOL increases the chance of achieving vaginal delivery within 24 hours and may result in a modest decrease in the rate of CD.17,18
Related article:
Should oxytocin and a Foley catheter be used concurrently for cervical ripening in induction of labor?
Failed IOL in the latent phase should only be diagnosed following 12 to 18 hours of both ruptured membranes and adequate contractions stimulated with oxytocin. The key ingredients for the successful management of the latent phase of labor are patience, oxytocin, and amniotomy.16
CD for the indication of active phase arrest requires cervical dilation ≥6 cm with ruptured membranes and no change in cervical dilation for ≥4 hours of adequate uterine activity. In the past, most obstetricians defined active phase arrest, a potential indication for CD, as the absence of cervical change for 2 or more hours in the presence of adequate uterine contractions and cervical dilation of at least 4 cm. Given the new definition of active phase arrest, slow but progressive progress in the 1st stage of labor is not an indication for CD.11,19
“A specific absolute maximum length of time spent in the 2nd stage beyond which all women should be offered an operative delivery has not been identified.”8 Diagnosis of arrest of labor in the 2nd stage may be considered after at least 2 hours of pushing in multiparous women and 3 hours of pushing in nulliparous women, especially if no fetal descent is occurring. The guidelines also state “longer durations may be appropriate on an individualized basis (eg, with use of epidural analgesia or with fetal malposition)” as long as fetal descent is observed.
Patience is a virtue, especially in the management of the 2nd stage of labor. Extending the 2nd stage up to 4 hours appears to be reasonably safe if the fetal status is reassuring and the mother is physiologically stable. In a study from San Francisco of 42,268 births with normal newborn outcomes, the 95th percentile for the length of the 2nd stage of labor for nulliparous women was 3.3 hours without an epidural and 5.6 hours with an epidural.20
In a study of 53,285 births, longer duration of pushing was associated with a small increase in the rate of neonatal adverse outcomes. In nulliparous women the rate of adverse neonatal outcomes increased from 1.3% with less than 60 minutes of pushing to 2.4% with greater than 240 minutes of pushing. Remarkably, even after 4 hours of pushing, 78% of nulliparous women who continued to push had a vaginal delivery.21 In this study, among nulliparous women the rate of anal sphincter injury increased from 5% with less than 60 minutes of pushing to 16% with greater than 240 minutes of pushing, and the rate of postpartum hemorrhage increased from 1% with less than 60 minutes of pushing to 3.3% with greater than 240 minutes of pushing.
I am not enthusiastic about patiently watching a labor extend into the 5th hour of the 2nd stage, especially if the fetus is at +2 station or lower. In a nulliparous woman, after 4 hours of managing the 2nd stage of labor, my patience is exhausted and I am inclined to identify a clear plan for delivery, either by enhanced labor coaching, operative vaginal delivery, or CD.
Operative vaginal delivery in the 2nd stage of labor is an acceptable alternative to CD. The rate of operative vaginal delivery in the United States has declined over the past 2 decades (TABLE). In Sweden in 2010 the operative vaginal delivery rate was 7.6% with a CD rate of 17.1%.7 In the United States in 2010 the operative delivery rate was 3.6%, and the CD rate was 33%.1 A renewed focus on operative vaginal delivery with ongoing training and team simulation for the procedure would increase our use of operative delivery and decrease the overall rate of CD.
Related article:
STOP using instruments to assist with delivery of the head at cesarean
Encourage the detection of persistent fetal occiput posterior position by physical examination and/or ultrasound and consider manual rotation of the fetal occiput from the posterior to anterior position in the 2nd stage. Persistent occiput posterior is the most common fetal malposition.22 This malposition is associated with an increased rate of CD.23 There are few randomized trials of manual rotation of the fetal occiput from posterior to anterior position in the 2nd stage of labor, and the evidence is insufficient to determine the efficacy of manual rotation.24 Small nonrandomized studies report that manual rotation of the occiput from posterior to anterior position may reduce the CD rate.25–27
For persistent 2nd stage fetal occiput posterior position in a woman with an adequate pelvis, where manual rotation was not successful and the fetus is at +2 station or below, operative vaginal delivery is an option. “Vacuum or forceps?” and “If forceps, to rotate or not to rotate?” those are the clinical questions. Forceps delivery is more likely to be successfulthan vacuum delivery.28 Direct forceps delivery of the occiput posterior fetus is associated with more anal sphincter injuries than forceps delivery after successful rotation, but few clinicians regularly perform rotational forceps.29 In a study of 2,351 women in the 2nd stage of labor with the fetus at +2 station or below, compared with either forceps or vacuum delivery, CD was associated with more maternal infections and fewer perineal lacerations. Neonatal composite morbidity was not significantly different among the 3 routes of operative delivery.30
Amnioinfusion for repetitive variable decelerations of the fetal heart rate may reduce the risk of CD for an indeterminate fetal heart-rate pattern.31
IOL in a well-dated pregnancy at 41 weeks will reduce the risk of CD. In a large clinical trial, 3,407 women at 41 weeks of gestation were randomly assigned to IOL or expectant management. The rate of CD was significantly lower in the women assigned to IOL compared with expectant management (21% vs 25%, respectively; P = .03).32 The rate of neonatal morbidity was similar in the 2 groups.
Women with twin gestations and the first twin in a cephalic presentation may elect vaginal delivery. In a large clinical trial, 1,398 women with a twin gestation and the first twin in a cephalic presentation were randomly assigned to planned vaginal delivery (with cesarean only if necessary) or planned CD.33 The rate of CD was 44% and 91% for the women in the planned-vaginal and planned-cesarean groups, respectively. There was no significant difference in composite fetal or neonatal death or serious morbidity. The authors concluded that, for twin pregnancy with the presenting twin in the cephalic presentation, there were no demonstrated benefits of planned CD.
Develop maternity care systems that encourage the use of trial of labor after cesarean (TOLAC). The ACOG/SMFM guidelines focus on interventions to reduce the rate of primary CD and do not address the role of TOLAC in reducing CD rates. There are little data from clinical trials to assess the benefits and harms from TOLAC versus scheduled repeat CD.34 However, our experience with TOLAC in the 1990s strongly suggests that encouraging TOLAC will decrease the rate of CD. In 1996 the US rate of vaginal birth after cesarean (VBAC) peaked at 28%, and the rate of CD achieved a recent historic nadir of 21%. Growing concerns that TOLAC occasionally results in fetal harm was followed by a decrease in the VBAC rate to 12% in 2015.1 A recent study of obstetric practices in countries with high and low VBAC rates concluded that patient and clinician commitment and comfort with prioritizing TOLAC over scheduled repeat CD greatly influenced the VBAC rate.35
Related article:
Should lower uterine segment thickness measurement be included in the TOLAC decision-making process?
Labor management is an art
During labor obstetricians must balance the unique needs of mother and fetus, which requires great clinical skill and patience. Evolving concepts of normal labor progress necessitate that we change our expectations concerning the acceptable rate of progress in the 1st and 2nd stage of labor. Consistent application of these new labor guidelines may help to reduce the rate of CD.
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Matthews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1–70. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf. Accessed July 5, 2017.
- Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29–38.
- Localio AR, Lawthers AG, Bengtson JM, et al. Relationship between malpractice claims and cesarean delivery. JAMA. 1993;269(3):366–373.
- Cheng YW, Snowden JM, Handler SJ, Tager IB, Hubbard AE, Caughey AB. Litigation in obstetrics: does defensive medicine contribute to increases in cesarean delivery? J Matern Fetal Neonatal Med. 2014;27(16):1668–1675.
- Graham LE, Brunner Huber LR, Thompson ME, Ersek JL. Does amount of weight gain during pregnancy modify the association between obesity and cesarean section delivery? Birth. 2014;41(1):93–99.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;(5):CD006066.
- European Perinatal Health Report. Euro-Peristat website. http://www.europeristat.com/. Published 2012. Accessed July 5, 2017.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
- Cesarean delivery rate by state, 2015. Centers for Disease Control and Prevention website. https://www.cdc.gov/nchs/pressroom/sosmap/cesarean_births/cesareans.htm. Updated January 9, 2017. Accessed July 18, 2017.
- Baker CD, Land T; Massachusetts Department of Public Health. Massachusetts Births 2014. Massachusetts Executive Office of Health and Human Services website. http://www.mass.gov/eohhs/gov/departments/dph/programs/admin/dmoa/repi/birth-data.html. Published September 2015. Accessed July 5, 2017.
- Henry DE, Cheng YW, Shaffer BL, Kaimal AJ, Bianco K, Caughey AB. Perinatal outcomes in the setting of active phase arrest of labor. Obstet Gynecol. 2008;112(5):1109–1115.
- Rouse DJ, Weiner SJ, Bloom SL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1–e7.
- Friedman EZ. Labour: Clinical evaluation and management. Appleton-Century-Crofts: New York, NY; 1967.
- Friedman E. The graphic analysis of labor. Am J Obstet Gynecol. 1954;68(6):1568–1575.
- Zhang J, Landy HJ, Branch DW, et al; Consortium on Safe Labor. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
- Wei S, Wo BL, Qi HP, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2013;(8):CD006794.
- Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014;(6):CD003101.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Rouse DJ, Owen J, Savage KG, Hauth JC. Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98(4):550–554.
- Cheng YW, Shaffer BL, Nicholson JM, Caughey AB. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol. 2014;123(3):527–535.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Shriver National Institute of Child and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127(4):667–673.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125(3):695–709.
- Carseldine WJ, Phipps H, Zawada SF, et al. Does occiput posterior position in the second stage of labour increase the operative delivery rate? Aust N Z J Obstet Gynaecol. 2013;53(3):265–270.
- Phipps H, de Vries B, Hyett J, Osborn DA. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;(12):CD009298.
- Shaffer BL, Cheng YW, Vargas JE, Caughey AB. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24(1):65–72.
- Le Ray C, Serres P, Schmitz T, Cabrol D, Goffinet F. Manual rotation in occiput posterior or transverse positions: risk factors and consequences on the cesarean delivery rate. Obstet Gynecol. 2007;110(4):873–879.
- Reichman O, Gdansky E, Latinsky B, Labi S, Samueloff A. Digital rotation from occipito-posterior to occipito-anterior decreases the need for cesarean section. Eur J Obstet Gynecol Repro Biol. 2008;136:25–28.
- O’Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database Syst Rev. 2010;(11):CD005455.
- Hirsch E, Elue R, Wagner A Jr, et al. Severe perineal laceration during operative vaginal delivery: the impact of occiput posterior position. J Perinatol. 2014;34(12):898–900.
- Bailit JL, Grobman WA, Rice MM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Evaluation of delivery options for second-stage events. Am J Obstet Gynecol. 2016;214(5):638.e1–e10.
- Hofmeyr GJ, Lawrie TA. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database Syst Rev. 2012;1:CD000013.
- Hannah ME, Hannah WJ, Hellmann J, Hewson S, Milner R, Willan A. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326(24): 1587–1592.
- Barrett JF, Hannah ME, Hutton EK, et al; Twin Birth Study Collaborative Group. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med. 2013;369(14):1295–1305.
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat cesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev. 2013;(12):CD004224.
- Lundgren I, van Limbeek E, Vehvilainen-Julkunen K, Nilsson C. Clinicians’ views of factors of importance for improving the rate of VBAC (vaginal birth after caesarean section): a qualitative study from countries with high VBAC rates. BMC Pregnancy Childbirth. 2015;15:196.
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Matthews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1–70. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf. Accessed July 5, 2017.
- Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29–38.
- Localio AR, Lawthers AG, Bengtson JM, et al. Relationship between malpractice claims and cesarean delivery. JAMA. 1993;269(3):366–373.
- Cheng YW, Snowden JM, Handler SJ, Tager IB, Hubbard AE, Caughey AB. Litigation in obstetrics: does defensive medicine contribute to increases in cesarean delivery? J Matern Fetal Neonatal Med. 2014;27(16):1668–1675.
- Graham LE, Brunner Huber LR, Thompson ME, Ersek JL. Does amount of weight gain during pregnancy modify the association between obesity and cesarean section delivery? Birth. 2014;41(1):93–99.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;(5):CD006066.
- European Perinatal Health Report. Euro-Peristat website. http://www.europeristat.com/. Published 2012. Accessed July 5, 2017.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711.
- Cesarean delivery rate by state, 2015. Centers for Disease Control and Prevention website. https://www.cdc.gov/nchs/pressroom/sosmap/cesarean_births/cesareans.htm. Updated January 9, 2017. Accessed July 18, 2017.
- Baker CD, Land T; Massachusetts Department of Public Health. Massachusetts Births 2014. Massachusetts Executive Office of Health and Human Services website. http://www.mass.gov/eohhs/gov/departments/dph/programs/admin/dmoa/repi/birth-data.html. Published September 2015. Accessed July 5, 2017.
- Henry DE, Cheng YW, Shaffer BL, Kaimal AJ, Bianco K, Caughey AB. Perinatal outcomes in the setting of active phase arrest of labor. Obstet Gynecol. 2008;112(5):1109–1115.
- Rouse DJ, Weiner SJ, Bloom SL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1–e7.
- Friedman EZ. Labour: Clinical evaluation and management. Appleton-Century-Crofts: New York, NY; 1967.
- Friedman E. The graphic analysis of labor. Am J Obstet Gynecol. 1954;68(6):1568–1575.
- Zhang J, Landy HJ, Branch DW, et al; Consortium on Safe Labor. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287.
- Wei S, Wo BL, Qi HP, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2013;(8):CD006794.
- Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014;(6):CD003101.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Rouse DJ, Owen J, Savage KG, Hauth JC. Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98(4):550–554.
- Cheng YW, Shaffer BL, Nicholson JM, Caughey AB. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol. 2014;123(3):527–535.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Shriver National Institute of Child and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127(4):667–673.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125(3):695–709.
- Carseldine WJ, Phipps H, Zawada SF, et al. Does occiput posterior position in the second stage of labour increase the operative delivery rate? Aust N Z J Obstet Gynaecol. 2013;53(3):265–270.
- Phipps H, de Vries B, Hyett J, Osborn DA. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;(12):CD009298.
- Shaffer BL, Cheng YW, Vargas JE, Caughey AB. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24(1):65–72.
- Le Ray C, Serres P, Schmitz T, Cabrol D, Goffinet F. Manual rotation in occiput posterior or transverse positions: risk factors and consequences on the cesarean delivery rate. Obstet Gynecol. 2007;110(4):873–879.
- Reichman O, Gdansky E, Latinsky B, Labi S, Samueloff A. Digital rotation from occipito-posterior to occipito-anterior decreases the need for cesarean section. Eur J Obstet Gynecol Repro Biol. 2008;136:25–28.
- O’Mahony F, Hofmeyr GJ, Menon V. Choice of instruments for assisted vaginal delivery. Cochrane Database Syst Rev. 2010;(11):CD005455.
- Hirsch E, Elue R, Wagner A Jr, et al. Severe perineal laceration during operative vaginal delivery: the impact of occiput posterior position. J Perinatol. 2014;34(12):898–900.
- Bailit JL, Grobman WA, Rice MM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Evaluation of delivery options for second-stage events. Am J Obstet Gynecol. 2016;214(5):638.e1–e10.
- Hofmeyr GJ, Lawrie TA. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database Syst Rev. 2012;1:CD000013.
- Hannah ME, Hannah WJ, Hellmann J, Hewson S, Milner R, Willan A. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian Multicenter Post-term Pregnancy Trial Group. N Engl J Med. 1992;326(24): 1587–1592.
- Barrett JF, Hannah ME, Hutton EK, et al; Twin Birth Study Collaborative Group. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med. 2013;369(14):1295–1305.
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat cesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev. 2013;(12):CD004224.
- Lundgren I, van Limbeek E, Vehvilainen-Julkunen K, Nilsson C. Clinicians’ views of factors of importance for improving the rate of VBAC (vaginal birth after caesarean section): a qualitative study from countries with high VBAC rates. BMC Pregnancy Childbirth. 2015;15:196.
Effective treatment of recurrent bacterial vaginosis
Bacterial vaginosis (BV) is caused by a complex change in vaginal bacterial flora, with a reduction in lactobacilli (which help maintain an acidic environment) and an increase in anaerobic gram-negative organisms including Gardnerella vaginalis species and Bacteroides, Prevotella, and Mobiluncus genera. Infection with G vaginalis is thought to trigger a cascade of changes in vaginal flora that leads to BV.1
BV is present in 30% to 50% of sexually active women, and of these women 50% to 75% have an abnormal vaginal discharge, which is gray, thin, and homogeneous and may have a fishy odor.2 In addition to causing an abnormal vaginal discharge, BV is a cause of postpartum fever, posthysterectomy vaginal cuff cellulitis, and postabortion infection, and it increases the risk of acquiring HIV, herpes simplex type 2, gonorrhea, chlamydia, and trichomoniasis infection.3
When using microscopy and the Amsel criteria, the diagnosis of BV is made when at least 3 of the following 4 criteria are present:
- homogeneous, thin, gray discharge
- vaginal pH >4.5
- positive whiff-amine test when applying a drop of 10% KOH to a sample of the vaginal discharge
- clue cells detected with microscopy on a saline wet mount.
If microscopy is not available, the Affirm VPIII test (BD Diagnostic Systems, Franklin Lakes, New Jersey) for DNA sequences of G vaginalis has high sensitivity and specificity.4 The OSOM BVBlue test (Sekisui Diagnostics, Lexington, Massachusetts), a Clinical Laboratory Improvement Amendments-waived point of service test, measures vaginal sialidase, which is produced by Gardnerella and other pathogens associated with BV.5 BV may be detected in routine cervical cytology testing and, if the patient is symptomatic, treatment is recommended.
Initial treatment of BV. The Centers for Disease Control and Prevention (CDC) has recommended 3 treatment regimens for BV and 4 alternative treatment options (TABLE).6 In addition to antimicrobial treatment, the CDC recommends that women with BV use condoms with sexual intercourse. The CDC also advises that clinicians should con-sider testing women with BV for HIV and other sexually transmitted infections.
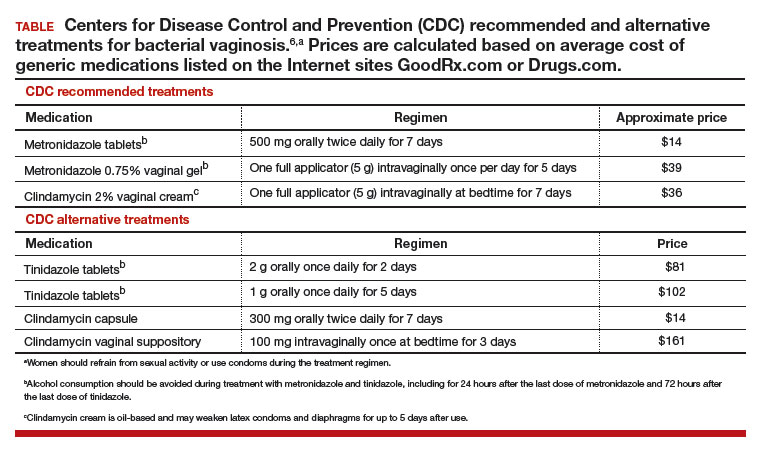
Related article:
Successful treatment of chronic vaginitis
Treatment of recurrent BV
A major problem with BV is that, although initial treatment is successful in about 80% of cases, up to 50% of women will have a recurrence of BV within 12 months of initial treatment.2 Preliminary studies suggest that for women with 3 or more episodes of BV, the regimens below may be effective.
Regimen 1
Following the completion of a CDC-recommended treatment regimen (see TABLE), prescribe metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 6 months.7
In a prospective randomized trial examining this regimen, following initial treatment with a 10-day metronidazole vaginal gel regimen 112 women were randomly assigned to chronic suppressive therapy with metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 16 weeks or a placebo. During the treatment period, recurrent BV was diagnosed in 26% of the women taking metronidazole gel and 59% of the women taking placebo.7 This regimen may be complicated by secondary vaginal candidiasis, which may be treated with a vaginal or oral antifungal agent.
Regimen 2
Initiate a 21-day course of vaginal boric acid capsules 600 mg once daily at bedtime and simultaneously prescribe a standard CDC treatment regimen (see TABLE). At the completion of the vaginal boric acid treatment initiate metronidazole vaginal gel 0.75% twice weekly for 6 months.8
NOTE: Boric acid can cause death if consumed orally.9 Boric acid capsules should be stored securely to ensure that they are not accidentally taken orally. Boric acid poisoning may present with vomiting, fever, skin rash, neutropenia, thrombocytopenia, metabolic acidosis, and renal failure.10 Boric acid should not be used by pregnant women because it is a teratogen.11
The bacterial organisms responsible for BV reside in a self-produced matrix, referred to as a biofilm, that protect the organisms from antimicrobial agents.12 Boric acid may prevent the formation of a biofilm and increase the effectiveness of anti-microbial treatment.
Regimen 3
Following the completion of a standard treatment regimen (see TABLE), prescribe oral metronidazole 2 g and fluconazole 150 mg administered once every month.13
In a randomized clinical trial, 310 female sex workers were randomly assigned to monthly treatment with oral metronidazole 2 g plus fluconazole 150 mg or placebo for up to 12 months.13 In the treatment and placebo groups episodes of BV were 199 and 326 per 100 person-years, respectively (hazard ratio, 0.55; 95% confidence interval, 0.49-0.63; P<.001). In Canada, a vaginal ovule containing both a high dose of metronidazole (500 mg) and nystatin (10,000 IU) is available and could be used intermittently to prevent recurrence.14
Treatment of partners
The CDC does not recommend treatment of the partners of women with BV because there are no definitive data to support such a recommendation. However, the 6 published clinical trials testing the utility of treating sex partners of women with BV have significant methodologic flaws, including underpowered studies and suboptimal antibiotic treatment regimens.15 Hence, whether partners should be treated remains an open question. Many experts believe that, in most cases, BV is a sexually transmitted disease.16,17 For women who have sex with women, the rate of BV concordance among partners is high. If one woman has diagnosed BV and symptoms are present in her partner, treatment of the partner is reasonable. For women with BV who have sex with men, sexual intercourse influences disease activity, and consistent use of condoms may reduce the rate of recurrence.18 Male circumcision may reduce the risk of BV in female partners.19
Related article:
Bacterial vaginosis: Meet patients' needs with effective diagnosis and treatment
Over-the-counter treatments
In women with BV it is thought that the vaginal administration of lactic acid can help restore the normal acidic pH of the vagina, encourage the growth of lactobacilli, and suppress the growth of the bacteria that cause BV.20 Many products containing lactic acid in a formulation for vaginal use are available (among them Luvena and Gynofit gel).
Lactobacilli play an important role in maintaining vaginal health. Lactobacillus rhamnosus and Lactobacillus reuteri are available for purchase as supplements for oral administration. It is thought that oral administration of lactobacilli can help improve the vaginal microbiome. In one clinical trial, 125 women with BV were randomly assigned to receive the combination of 1 week of metronidazole plus oral Lactobacillus twice daily for 30 days or metronidazole plus placebo.21 Resolution of symptoms was reported as 88% and 40% in the metronidazole-lactobacilli and metronidazole-placebo groups, respectively.21 By contrast, one systematic review of probiotic treatment of BV concluded that there is insufficient evidence to recommend for or against probiotic treatment of BV.22 Patients with recurrent BV commonly report that they believe a probiotic was helpful in resolving their symptoms.
On the horizon
In one trial, a single 2-g oral dose of secnidazole was as effective as a 7-day course of oral metronidazole 500 mg twice daily.23 In a small dose-finding study, a single dose of either secnidazole 1 g or 2 g was equally effective in treating BV.24 An effective single-dose treatment of BV would likely improve patient adherence with therapy. Symbiomix is preparing for FDA review of this medication (secnidazole, Solosec) for use in the United States.
BV is a prevalent problem and often adversely impacts a woman's quality of life and love relationships. BV recurrence is very common. Many women report that their BV was resistant to intermittet treatment and recurred, repetitively over many years. The 3 treatment options presented in this editorial may help to suppress the recurrence rate and improve symptoms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210(3):338-343.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478-1486.
- Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(suppl 1):S29-S35.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis. 2014;18(4):344-346.
- Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. Evaluation of a point-of-care test, BVBLue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
- 2015 Sexually transmitted disease treatment guidelines: Bacterial vaginosis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/tg2015/bv.htm. Updated June 4,2015. Accessed June 9, 2017.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
- Wong LC, Heimbach MD, Truscott DR, Duncan BD. Boric acid poisoning: report of 11 cases. Can Med Assoc J. 1964;90:1018-1023.
- Teshima D, Morishita K, Ueda Y, et al. Clinical management of boric acid ingestion: pharmacokinetic assessment of efficacy of hemodialysis for treatment of acute boric acid poisoning. J Pharmacobiodyn. 1992;15(6):287-294.
- Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E. Boric acid inhibits embryonic histone deacetylases: a suggested mechanism to explain boric acid-related teratogenicity. Toxicol Appl Pharmacol. 2007;220(2):178-185.
- Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis. 2015;61(4):601-606.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197(10):1361-1368.
- Sanchez S, Garcia PJ, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol. 2004;191(6):1898-1906.
- Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
- Muzny CA, Schwebke JR. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis. 2016;214(suppl 1):S1-S5.
- Vodstrcil LA, Walker SM, Hocking JS, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2015;60(7):1042-1053.
- Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
- Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200(1):42.e1-e7.
- O'Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200.
- Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8(6):1450-1454.
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
- Bohbot JM, Vicaut E, Fagnen D, Brauman M. Treatment of bacterial vaginosis: a multicenter, double-blind, double-dummy, randomised phase III study comparing secnidazole and metronidazole. Infect Dis Obstet Gynecol. 2010;2010. doi:10.1155/2010/705692.
- Núñez JT, Gómez G. Low-dose secnidazole in the treatment of bacterial vaginosis. Int J Gynaecol Obstet. 2005;88(3):281-285.
Bacterial vaginosis (BV) is caused by a complex change in vaginal bacterial flora, with a reduction in lactobacilli (which help maintain an acidic environment) and an increase in anaerobic gram-negative organisms including Gardnerella vaginalis species and Bacteroides, Prevotella, and Mobiluncus genera. Infection with G vaginalis is thought to trigger a cascade of changes in vaginal flora that leads to BV.1

BV is present in 30% to 50% of sexually active women, and of these women 50% to 75% have an abnormal vaginal discharge, which is gray, thin, and homogeneous and may have a fishy odor.2 In addition to causing an abnormal vaginal discharge, BV is a cause of postpartum fever, posthysterectomy vaginal cuff cellulitis, and postabortion infection, and it increases the risk of acquiring HIV, herpes simplex type 2, gonorrhea, chlamydia, and trichomoniasis infection.3
When using microscopy and the Amsel criteria, the diagnosis of BV is made when at least 3 of the following 4 criteria are present:
- homogeneous, thin, gray discharge
- vaginal pH >4.5
- positive whiff-amine test when applying a drop of 10% KOH to a sample of the vaginal discharge
- clue cells detected with microscopy on a saline wet mount.
If microscopy is not available, the Affirm VPIII test (BD Diagnostic Systems, Franklin Lakes, New Jersey) for DNA sequences of G vaginalis has high sensitivity and specificity.4 The OSOM BVBlue test (Sekisui Diagnostics, Lexington, Massachusetts), a Clinical Laboratory Improvement Amendments-waived point of service test, measures vaginal sialidase, which is produced by Gardnerella and other pathogens associated with BV.5 BV may be detected in routine cervical cytology testing and, if the patient is symptomatic, treatment is recommended.
Initial treatment of BV. The Centers for Disease Control and Prevention (CDC) has recommended 3 treatment regimens for BV and 4 alternative treatment options (TABLE).6 In addition to antimicrobial treatment, the CDC recommends that women with BV use condoms with sexual intercourse. The CDC also advises that clinicians should con-sider testing women with BV for HIV and other sexually transmitted infections.

Related article:
Successful treatment of chronic vaginitis
Treatment of recurrent BV
A major problem with BV is that, although initial treatment is successful in about 80% of cases, up to 50% of women will have a recurrence of BV within 12 months of initial treatment.2 Preliminary studies suggest that for women with 3 or more episodes of BV, the regimens below may be effective.
Regimen 1
Following the completion of a CDC-recommended treatment regimen (see TABLE), prescribe metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 6 months.7
In a prospective randomized trial examining this regimen, following initial treatment with a 10-day metronidazole vaginal gel regimen 112 women were randomly assigned to chronic suppressive therapy with metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 16 weeks or a placebo. During the treatment period, recurrent BV was diagnosed in 26% of the women taking metronidazole gel and 59% of the women taking placebo.7 This regimen may be complicated by secondary vaginal candidiasis, which may be treated with a vaginal or oral antifungal agent.
Regimen 2
Initiate a 21-day course of vaginal boric acid capsules 600 mg once daily at bedtime and simultaneously prescribe a standard CDC treatment regimen (see TABLE). At the completion of the vaginal boric acid treatment initiate metronidazole vaginal gel 0.75% twice weekly for 6 months.8
NOTE: Boric acid can cause death if consumed orally.9 Boric acid capsules should be stored securely to ensure that they are not accidentally taken orally. Boric acid poisoning may present with vomiting, fever, skin rash, neutropenia, thrombocytopenia, metabolic acidosis, and renal failure.10 Boric acid should not be used by pregnant women because it is a teratogen.11
The bacterial organisms responsible for BV reside in a self-produced matrix, referred to as a biofilm, that protect the organisms from antimicrobial agents.12 Boric acid may prevent the formation of a biofilm and increase the effectiveness of anti-microbial treatment.
Regimen 3
Following the completion of a standard treatment regimen (see TABLE), prescribe oral metronidazole 2 g and fluconazole 150 mg administered once every month.13
In a randomized clinical trial, 310 female sex workers were randomly assigned to monthly treatment with oral metronidazole 2 g plus fluconazole 150 mg or placebo for up to 12 months.13 In the treatment and placebo groups episodes of BV were 199 and 326 per 100 person-years, respectively (hazard ratio, 0.55; 95% confidence interval, 0.49-0.63; P<.001). In Canada, a vaginal ovule containing both a high dose of metronidazole (500 mg) and nystatin (10,000 IU) is available and could be used intermittently to prevent recurrence.14
Treatment of partners
The CDC does not recommend treatment of the partners of women with BV because there are no definitive data to support such a recommendation. However, the 6 published clinical trials testing the utility of treating sex partners of women with BV have significant methodologic flaws, including underpowered studies and suboptimal antibiotic treatment regimens.15 Hence, whether partners should be treated remains an open question. Many experts believe that, in most cases, BV is a sexually transmitted disease.16,17 For women who have sex with women, the rate of BV concordance among partners is high. If one woman has diagnosed BV and symptoms are present in her partner, treatment of the partner is reasonable. For women with BV who have sex with men, sexual intercourse influences disease activity, and consistent use of condoms may reduce the rate of recurrence.18 Male circumcision may reduce the risk of BV in female partners.19
Related article:
Bacterial vaginosis: Meet patients' needs with effective diagnosis and treatment
Over-the-counter treatments
In women with BV it is thought that the vaginal administration of lactic acid can help restore the normal acidic pH of the vagina, encourage the growth of lactobacilli, and suppress the growth of the bacteria that cause BV.20 Many products containing lactic acid in a formulation for vaginal use are available (among them Luvena and Gynofit gel).
Lactobacilli play an important role in maintaining vaginal health. Lactobacillus rhamnosus and Lactobacillus reuteri are available for purchase as supplements for oral administration. It is thought that oral administration of lactobacilli can help improve the vaginal microbiome. In one clinical trial, 125 women with BV were randomly assigned to receive the combination of 1 week of metronidazole plus oral Lactobacillus twice daily for 30 days or metronidazole plus placebo.21 Resolution of symptoms was reported as 88% and 40% in the metronidazole-lactobacilli and metronidazole-placebo groups, respectively.21 By contrast, one systematic review of probiotic treatment of BV concluded that there is insufficient evidence to recommend for or against probiotic treatment of BV.22 Patients with recurrent BV commonly report that they believe a probiotic was helpful in resolving their symptoms.
On the horizon
In one trial, a single 2-g oral dose of secnidazole was as effective as a 7-day course of oral metronidazole 500 mg twice daily.23 In a small dose-finding study, a single dose of either secnidazole 1 g or 2 g was equally effective in treating BV.24 An effective single-dose treatment of BV would likely improve patient adherence with therapy. Symbiomix is preparing for FDA review of this medication (secnidazole, Solosec) for use in the United States.
BV is a prevalent problem and often adversely impacts a woman's quality of life and love relationships. BV recurrence is very common. Many women report that their BV was resistant to intermittet treatment and recurred, repetitively over many years. The 3 treatment options presented in this editorial may help to suppress the recurrence rate and improve symptoms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Bacterial vaginosis (BV) is caused by a complex change in vaginal bacterial flora, with a reduction in lactobacilli (which help maintain an acidic environment) and an increase in anaerobic gram-negative organisms including Gardnerella vaginalis species and Bacteroides, Prevotella, and Mobiluncus genera. Infection with G vaginalis is thought to trigger a cascade of changes in vaginal flora that leads to BV.1

BV is present in 30% to 50% of sexually active women, and of these women 50% to 75% have an abnormal vaginal discharge, which is gray, thin, and homogeneous and may have a fishy odor.2 In addition to causing an abnormal vaginal discharge, BV is a cause of postpartum fever, posthysterectomy vaginal cuff cellulitis, and postabortion infection, and it increases the risk of acquiring HIV, herpes simplex type 2, gonorrhea, chlamydia, and trichomoniasis infection.3
When using microscopy and the Amsel criteria, the diagnosis of BV is made when at least 3 of the following 4 criteria are present:
- homogeneous, thin, gray discharge
- vaginal pH >4.5
- positive whiff-amine test when applying a drop of 10% KOH to a sample of the vaginal discharge
- clue cells detected with microscopy on a saline wet mount.
If microscopy is not available, the Affirm VPIII test (BD Diagnostic Systems, Franklin Lakes, New Jersey) for DNA sequences of G vaginalis has high sensitivity and specificity.4 The OSOM BVBlue test (Sekisui Diagnostics, Lexington, Massachusetts), a Clinical Laboratory Improvement Amendments-waived point of service test, measures vaginal sialidase, which is produced by Gardnerella and other pathogens associated with BV.5 BV may be detected in routine cervical cytology testing and, if the patient is symptomatic, treatment is recommended.
Initial treatment of BV. The Centers for Disease Control and Prevention (CDC) has recommended 3 treatment regimens for BV and 4 alternative treatment options (TABLE).6 In addition to antimicrobial treatment, the CDC recommends that women with BV use condoms with sexual intercourse. The CDC also advises that clinicians should con-sider testing women with BV for HIV and other sexually transmitted infections.

Related article:
Successful treatment of chronic vaginitis
Treatment of recurrent BV
A major problem with BV is that, although initial treatment is successful in about 80% of cases, up to 50% of women will have a recurrence of BV within 12 months of initial treatment.2 Preliminary studies suggest that for women with 3 or more episodes of BV, the regimens below may be effective.
Regimen 1
Following the completion of a CDC-recommended treatment regimen (see TABLE), prescribe metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 6 months.7
In a prospective randomized trial examining this regimen, following initial treatment with a 10-day metronidazole vaginal gel regimen 112 women were randomly assigned to chronic suppressive therapy with metronidazole vaginal gel 0.75%, one full applicator, twice weekly for 16 weeks or a placebo. During the treatment period, recurrent BV was diagnosed in 26% of the women taking metronidazole gel and 59% of the women taking placebo.7 This regimen may be complicated by secondary vaginal candidiasis, which may be treated with a vaginal or oral antifungal agent.
Regimen 2
Initiate a 21-day course of vaginal boric acid capsules 600 mg once daily at bedtime and simultaneously prescribe a standard CDC treatment regimen (see TABLE). At the completion of the vaginal boric acid treatment initiate metronidazole vaginal gel 0.75% twice weekly for 6 months.8
NOTE: Boric acid can cause death if consumed orally.9 Boric acid capsules should be stored securely to ensure that they are not accidentally taken orally. Boric acid poisoning may present with vomiting, fever, skin rash, neutropenia, thrombocytopenia, metabolic acidosis, and renal failure.10 Boric acid should not be used by pregnant women because it is a teratogen.11
The bacterial organisms responsible for BV reside in a self-produced matrix, referred to as a biofilm, that protect the organisms from antimicrobial agents.12 Boric acid may prevent the formation of a biofilm and increase the effectiveness of anti-microbial treatment.
Regimen 3
Following the completion of a standard treatment regimen (see TABLE), prescribe oral metronidazole 2 g and fluconazole 150 mg administered once every month.13
In a randomized clinical trial, 310 female sex workers were randomly assigned to monthly treatment with oral metronidazole 2 g plus fluconazole 150 mg or placebo for up to 12 months.13 In the treatment and placebo groups episodes of BV were 199 and 326 per 100 person-years, respectively (hazard ratio, 0.55; 95% confidence interval, 0.49-0.63; P<.001). In Canada, a vaginal ovule containing both a high dose of metronidazole (500 mg) and nystatin (10,000 IU) is available and could be used intermittently to prevent recurrence.14
Treatment of partners
The CDC does not recommend treatment of the partners of women with BV because there are no definitive data to support such a recommendation. However, the 6 published clinical trials testing the utility of treating sex partners of women with BV have significant methodologic flaws, including underpowered studies and suboptimal antibiotic treatment regimens.15 Hence, whether partners should be treated remains an open question. Many experts believe that, in most cases, BV is a sexually transmitted disease.16,17 For women who have sex with women, the rate of BV concordance among partners is high. If one woman has diagnosed BV and symptoms are present in her partner, treatment of the partner is reasonable. For women with BV who have sex with men, sexual intercourse influences disease activity, and consistent use of condoms may reduce the rate of recurrence.18 Male circumcision may reduce the risk of BV in female partners.19
Related article:
Bacterial vaginosis: Meet patients' needs with effective diagnosis and treatment
Over-the-counter treatments
In women with BV it is thought that the vaginal administration of lactic acid can help restore the normal acidic pH of the vagina, encourage the growth of lactobacilli, and suppress the growth of the bacteria that cause BV.20 Many products containing lactic acid in a formulation for vaginal use are available (among them Luvena and Gynofit gel).
Lactobacilli play an important role in maintaining vaginal health. Lactobacillus rhamnosus and Lactobacillus reuteri are available for purchase as supplements for oral administration. It is thought that oral administration of lactobacilli can help improve the vaginal microbiome. In one clinical trial, 125 women with BV were randomly assigned to receive the combination of 1 week of metronidazole plus oral Lactobacillus twice daily for 30 days or metronidazole plus placebo.21 Resolution of symptoms was reported as 88% and 40% in the metronidazole-lactobacilli and metronidazole-placebo groups, respectively.21 By contrast, one systematic review of probiotic treatment of BV concluded that there is insufficient evidence to recommend for or against probiotic treatment of BV.22 Patients with recurrent BV commonly report that they believe a probiotic was helpful in resolving their symptoms.
On the horizon
In one trial, a single 2-g oral dose of secnidazole was as effective as a 7-day course of oral metronidazole 500 mg twice daily.23 In a small dose-finding study, a single dose of either secnidazole 1 g or 2 g was equally effective in treating BV.24 An effective single-dose treatment of BV would likely improve patient adherence with therapy. Symbiomix is preparing for FDA review of this medication (secnidazole, Solosec) for use in the United States.
BV is a prevalent problem and often adversely impacts a woman's quality of life and love relationships. BV recurrence is very common. Many women report that their BV was resistant to intermittet treatment and recurred, repetitively over many years. The 3 treatment options presented in this editorial may help to suppress the recurrence rate and improve symptoms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210(3):338-343.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478-1486.
- Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(suppl 1):S29-S35.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis. 2014;18(4):344-346.
- Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. Evaluation of a point-of-care test, BVBLue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
- 2015 Sexually transmitted disease treatment guidelines: Bacterial vaginosis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/tg2015/bv.htm. Updated June 4,2015. Accessed June 9, 2017.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
- Wong LC, Heimbach MD, Truscott DR, Duncan BD. Boric acid poisoning: report of 11 cases. Can Med Assoc J. 1964;90:1018-1023.
- Teshima D, Morishita K, Ueda Y, et al. Clinical management of boric acid ingestion: pharmacokinetic assessment of efficacy of hemodialysis for treatment of acute boric acid poisoning. J Pharmacobiodyn. 1992;15(6):287-294.
- Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E. Boric acid inhibits embryonic histone deacetylases: a suggested mechanism to explain boric acid-related teratogenicity. Toxicol Appl Pharmacol. 2007;220(2):178-185.
- Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis. 2015;61(4):601-606.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197(10):1361-1368.
- Sanchez S, Garcia PJ, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol. 2004;191(6):1898-1906.
- Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
- Muzny CA, Schwebke JR. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis. 2016;214(suppl 1):S1-S5.
- Vodstrcil LA, Walker SM, Hocking JS, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2015;60(7):1042-1053.
- Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
- Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200(1):42.e1-e7.
- O'Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200.
- Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8(6):1450-1454.
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
- Bohbot JM, Vicaut E, Fagnen D, Brauman M. Treatment of bacterial vaginosis: a multicenter, double-blind, double-dummy, randomised phase III study comparing secnidazole and metronidazole. Infect Dis Obstet Gynecol. 2010;2010. doi:10.1155/2010/705692.
- Núñez JT, Gómez G. Low-dose secnidazole in the treatment of bacterial vaginosis. Int J Gynaecol Obstet. 2005;88(3):281-285.
- Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210(3):338-343.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478-1486.
- Murphy K, Mitchell CM. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J Infect Dis. 2016;214(suppl 1):S29-S35.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis. 2014;18(4):344-346.
- Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. Evaluation of a point-of-care test, BVBLue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
- 2015 Sexually transmitted disease treatment guidelines: Bacterial vaginosis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/tg2015/bv.htm. Updated June 4,2015. Accessed June 9, 2017.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
- Wong LC, Heimbach MD, Truscott DR, Duncan BD. Boric acid poisoning: report of 11 cases. Can Med Assoc J. 1964;90:1018-1023.
- Teshima D, Morishita K, Ueda Y, et al. Clinical management of boric acid ingestion: pharmacokinetic assessment of efficacy of hemodialysis for treatment of acute boric acid poisoning. J Pharmacobiodyn. 1992;15(6):287-294.
- Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E. Boric acid inhibits embryonic histone deacetylases: a suggested mechanism to explain boric acid-related teratogenicity. Toxicol Appl Pharmacol. 2007;220(2):178-185.
- Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis. 2015;61(4):601-606.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197(10):1361-1368.
- Sanchez S, Garcia PJ, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol. 2004;191(6):1898-1906.
- Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
- Muzny CA, Schwebke JR. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis. 2016;214(suppl 1):S1-S5.
- Vodstrcil LA, Walker SM, Hocking JS, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2015;60(7):1042-1053.
- Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
- Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners' genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200(1):42.e1-e7.
- O'Hanlon DE, Moench TR, Cone RA. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis. 2011;11:200.
- Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8(6):1450-1454.
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
- Bohbot JM, Vicaut E, Fagnen D, Brauman M. Treatment of bacterial vaginosis: a multicenter, double-blind, double-dummy, randomised phase III study comparing secnidazole and metronidazole. Infect Dis Obstet Gynecol. 2010;2010. doi:10.1155/2010/705692.
- Núñez JT, Gómez G. Low-dose secnidazole in the treatment of bacterial vaginosis. Int J Gynaecol Obstet. 2005;88(3):281-285.
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3

A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3

A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3

A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.
Treating polycystic ovary syndrome: Start using dual medical therapy
Using the Rotterdam criteria, the diagnosis of polycystic ovary syndrome (PCOS) is made in the presence of 2 of the following 3 criteria1:
- oligo-ovulation or anovulation
- hyperandrogenism manifested by the presence of either hirsutism or elevated hormone levels (including serum testosterone androstenedione and/or dehydroepiandrosterone sulfate)
- ultrasonography evidence of multifollicular ovaries (≥12 follicles with a diameter of 2 mm to 9 mm in one or both ovaries; FIGURE) or ovarian stromal volume of 10 mL or more.
Among reproductive-age women, the prevalence of PCOS has been reported to range from 8% to 13% for different populations.2 Most clinicians initiate treatment for PCOS with oral estrogen−progestin (OEP) monotherapy. OEP treatment has many beneficial hormonal effects, including:
- a resulting decrease in pituitary luteinizing hormone (LH) secretion, which decreases ovarian androgen production
- an increase in liver production of sex hormone−binding globulin (SHBG), which decreases free testosterone levels
- protection against the development of endometrial hyperplasia
- induction of regular uterine withdrawal bleeding.
However, OEP therapy neither improves metabolic indices (insulin sensitivity and visceral fat secretion of adipokines) nor blocks androgen action in the skin.
Dual medical treatment for PCOS can address the issues that monotherapy cannot and, along with providing guidance on improving diet and exercise, many experts support the initial therapy of PCOS with dual medical therapy (OEP plus metformin or spironolactone).
Advantages of OEP plus metformin
For many women with PCOS, the syndrome is characterized by abnormalities in both the reproductive (increase in LH secretion) and metabolic (insulin resistance and increased adipokines) systems. OEP monotherapy does not improve the metabolic abnormalities of PCOS. Combination treatment with both OEP plus metformin, along with diet and exercise, can best treat these combined abnormalities.
Data support dual therapy with metformin. In one small, randomized trial in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater reduction in serum androstenedione and a greater increase in SHBG than OEP monotherapy.3 In addition, weight loss and a reduction in waist-to-hip ratio only occurred in the OEP plus metformin group.3 In another small randomized study in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater decrease in free androgen index than OEP monotherapy.4
In my clinical opinion, women who may best benefit from OEP plus metformin therapy have one of the following factors indicating the presence of insulin resistance5:
- body mass index >30 kg/m2
- waist-to-hip ratio ≥0.85
- waist circumference >35 in (89 cm)
- acanthosis nigricans
- personal history of gestational diabetes
- family history of type 2 diabetes mellitus (T2DM) in a first-degree relative
- diagnosis of the metabolic syndrome.
My preferred treatment approach
Metformin is a low cost and safe treatment for metabolic dysfunction due to insulin resistance and excess adipokines. I often start PCOS treatment for my patients with an OEP plus metformin extended release (XR) 750 mg with dinner. If the patient tolerates this dose, I increase the dose to metformin XR 1,500 mg with dinner.
Adverse effects. The most common side effects of metformin are gastrointestinal, including abdominal discomfort, flatulence, borborygmi, diarrhea, and nausea. Metformin reduces serum vitamin B12 levels by 5% to 10%; therefore, ensuring adequate vitamin B12 intake (2.6 µg daily) is helpful.6 Although metformin does reduce vitamin B12 levels, there is no strong relationship between metformin and anemia or peripheral neuropathy.7 Lactic acidosis is a rare complication of metformin.
Beneficial effects. In the treatment of PCOS, metformin may have many beneficial effects, including8:
- decrease in insulin resistance
- decrease in harmful adipokines
- reduction in visceral fat
- reduction in the incidence of T2DM.
- oral norethindrone acetate 5 mg daily (which can lower luteinizing hormone levels and block ovulation) plus metformin
- norethindrone acetate 5 mg plus spironolactone
- levonorgestrel-intrauterine device plus metformin or spironolactone.
OEP plus spironolactone
Many women with PCOS have increased LH secretion and increased androgen activity in the skin due to increased 5-alpha reductase enzyme activity, which catalyzes the conversion of testosterone to the powerful intracellular androgen dihydrotestosterone.9 Women with PCOS may present with a chief problem report of hirsutism, acne, or female androgenetic alopecia. OEP plus spironolactone may be an optimal initial treatment for women with a dominant dermatologic manifestation of PCOS. OEP treatment results in a decrease in pituitary LH secretion and ovarian androgen production. Spironolactone adds to this therapeutic effect by blocking androgen action in the skin.
The data on dual therapy with spironolactone. Many dermatologists recommend spironolactone in combination with cosmetic measures for the treatment of acne, but there are only a few randomized trials that demonstrate its efficacy.10 In one trial spironolactone was demonstrated to be superior to placebo for the treatment of inflammatory acne.10 Authors of multiple randomized trials report that the antiandrogens, spironolactone, or finasteride are superior to metformin to treat hirsutism.11 In addition, a few small trials report that spironolactone plus OEP is superior to either OEP or metformin monotherapy for hirsutism.11 Clinical trials of spironolactone for hirsutism have been rated as “low quality” and additional controlled trials of OEP monotherapy versus OEP plus spironolactone are warranted.12
My preferred treatment approach
Spironolactone is effective in the treatment of hirsutism at doses ranging from 50 mg to 200 mg daily. I routinely use a dose of spironolactone 100 mg daily because this dose is near of the top of the dose-response curve and has few adverse effects (such as intermittent uterine bleeding or spotting). With spironolactone monotherapy at a dose of 200 mg, irregular uterine bleeding or spotting is common, but concomitant treatment with an OEP tends to minimize this side effect. In my practice I rarely have patients report irregular uterine bleeding or spotting with the combination treatment of an OEP and spironolactone 100 mg daily.
Contraindications. Spironolactone should not be given to women with renal insufficiency because it can cause hyperkalemia. However, it is not necessary to check potassium levels in young women taking spironolactone with normal creatinine levels.13
Triple therapy: OEP plus metformin plus spironolactone
Some experts strongly recommend the initial treatment of PCOS in adolescents and young women with triple therapy: OEP plus an insulin sensitizer plus an antiandrogen.14 This recommendation is based in part on the observation that OEP monotherapy may be associated with an increase in circulating adipokines and visceral fat mass as determined by dual-energy x-ray absorptiometry.15 By contrast, triple treatment with an OEP plus metformin plus an antiandrogen is associated with a decrease in circulating adipokines and visceral fat mass.
What is the best progestin for PCOS?
Any OEP is better than no OEP, regardless of the progestin used to treat the PCOS because ethinyl estradiol plus any synthetic progestin suppresses pituitary secretion of LH and decreases ovarian androgen production. However, for the treatment of acne, using a progestin that is less androgenic may be beneficial.16
In one study, 2,147 consecutive women who were taking a contraceptive and presented for treatment of acne were asked if their contraceptive had a positive impact on their acne. The percentage of women reporting that their contraceptive significantly improved their acne ranged from 26% for those taking drospirenone-ethinyl estradiol (EE) to 1% for those taking the etonogestrel subdermal implant (FIGURE).16 The US Food and Drug Administration has approved 4 OEP contraceptives for the treatment of acne (TABLE). The OEPs with drospirenone, norgestimate, desogestrel, or norethindrone acetate may be optimal choices for the treatment of acne caused by PCOS.
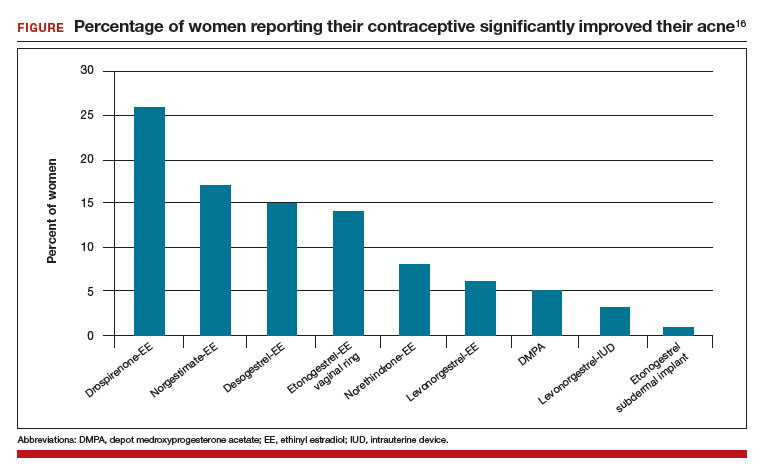
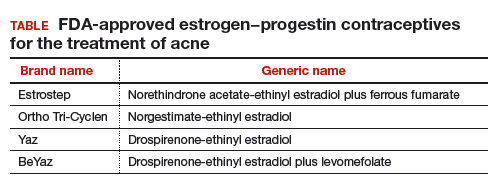
The bottom line
PCOS is a common endocrine disorder treated primarily by obstetricians-gynecologists. Among adolescents and young women with PCOS chief problem reports include irregular menses, hirsutism, obesity, acne, and infertility. Among mid-life women the presentation of PCOS often evolves into chronic medical problems, including obesity, metabolic syndrome, hyperlipidemia, hypertension, T2DM, cardiovascular disease, and endometrial cancer.17–19 To optimally treat the multiple pathophysiologic disorders manifested in PCOS, I recommend initial dual medical therapy with an OEP plus metformin or an OEP plus spironolactone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855.
- Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17(7):1729-1737.
- Cibula D, Fanta M, Vrbikova J, et al. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenism, SHBG and lipids in PCOS patients. Hum Reprod. 2005;20(1):180-184.
- Grundy SM, Brewer HB, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
- Niafar M, Hai F, Porhomayon J, Nader ND. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med. 2015;10(1):93-102.
- de Groot-Kamphuis DM, van Dijk PR, Groenier KH, Houweling St, Bilo HJ, Kleefstra N. Vitamin B12 deficiency and the lack of its consequences in type 2 diabetes patients using metformin. Neth J Med. 2013;71(7):386-390.
- Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162(2):193-212.
- Skalba P, Dabkowska-Huc A, Kazimierczak W, Samojedny A, Samojedny MP, Chelmicki Z. Content of 5-alph-reductase (type 1 and type 2) mRNA in dermal papillae from the lower abdominal region in women with hirsutism. Clin Exp Dermatol. 2006;31(4):564-570.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1153-1160.
- van Zuuren EJ, Fedorowicz Z. Interventions for hirsutism excluding laser and photoepilation therapy alone: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2016;175(1):45-61.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151(9):941-944.
- Ibanez L, de Zegher F. Low-dose combination of flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18(1):57-60.
- Ibanez L, de Zegher F. Ethinyl estradiol-drospirenone, flutamide-metformin or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89(4):1592-1597.
- Lortscher D, Admani S, Stur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Wang ET, Calderon-Margalit R, Cedars MI, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6-13.
- Joham AE, Raniasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(3):e447-e452.
- Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136(1):99-103.
Using the Rotterdam criteria, the diagnosis of polycystic ovary syndrome (PCOS) is made in the presence of 2 of the following 3 criteria1:
- oligo-ovulation or anovulation
- hyperandrogenism manifested by the presence of either hirsutism or elevated hormone levels (including serum testosterone androstenedione and/or dehydroepiandrosterone sulfate)
- ultrasonography evidence of multifollicular ovaries (≥12 follicles with a diameter of 2 mm to 9 mm in one or both ovaries; FIGURE) or ovarian stromal volume of 10 mL or more.

Among reproductive-age women, the prevalence of PCOS has been reported to range from 8% to 13% for different populations.2 Most clinicians initiate treatment for PCOS with oral estrogen−progestin (OEP) monotherapy. OEP treatment has many beneficial hormonal effects, including:
- a resulting decrease in pituitary luteinizing hormone (LH) secretion, which decreases ovarian androgen production
- an increase in liver production of sex hormone−binding globulin (SHBG), which decreases free testosterone levels
- protection against the development of endometrial hyperplasia
- induction of regular uterine withdrawal bleeding.
However, OEP therapy neither improves metabolic indices (insulin sensitivity and visceral fat secretion of adipokines) nor blocks androgen action in the skin.
Dual medical treatment for PCOS can address the issues that monotherapy cannot and, along with providing guidance on improving diet and exercise, many experts support the initial therapy of PCOS with dual medical therapy (OEP plus metformin or spironolactone).
Advantages of OEP plus metformin
For many women with PCOS, the syndrome is characterized by abnormalities in both the reproductive (increase in LH secretion) and metabolic (insulin resistance and increased adipokines) systems. OEP monotherapy does not improve the metabolic abnormalities of PCOS. Combination treatment with both OEP plus metformin, along with diet and exercise, can best treat these combined abnormalities.
Data support dual therapy with metformin. In one small, randomized trial in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater reduction in serum androstenedione and a greater increase in SHBG than OEP monotherapy.3 In addition, weight loss and a reduction in waist-to-hip ratio only occurred in the OEP plus metformin group.3 In another small randomized study in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater decrease in free androgen index than OEP monotherapy.4
In my clinical opinion, women who may best benefit from OEP plus metformin therapy have one of the following factors indicating the presence of insulin resistance5:
- body mass index >30 kg/m2
- waist-to-hip ratio ≥0.85
- waist circumference >35 in (89 cm)
- acanthosis nigricans
- personal history of gestational diabetes
- family history of type 2 diabetes mellitus (T2DM) in a first-degree relative
- diagnosis of the metabolic syndrome.
My preferred treatment approach
Metformin is a low cost and safe treatment for metabolic dysfunction due to insulin resistance and excess adipokines. I often start PCOS treatment for my patients with an OEP plus metformin extended release (XR) 750 mg with dinner. If the patient tolerates this dose, I increase the dose to metformin XR 1,500 mg with dinner.
Adverse effects. The most common side effects of metformin are gastrointestinal, including abdominal discomfort, flatulence, borborygmi, diarrhea, and nausea. Metformin reduces serum vitamin B12 levels by 5% to 10%; therefore, ensuring adequate vitamin B12 intake (2.6 µg daily) is helpful.6 Although metformin does reduce vitamin B12 levels, there is no strong relationship between metformin and anemia or peripheral neuropathy.7 Lactic acidosis is a rare complication of metformin.
Beneficial effects. In the treatment of PCOS, metformin may have many beneficial effects, including8:
- decrease in insulin resistance
- decrease in harmful adipokines
- reduction in visceral fat
- reduction in the incidence of T2DM.
- oral norethindrone acetate 5 mg daily (which can lower luteinizing hormone levels and block ovulation) plus metformin
- norethindrone acetate 5 mg plus spironolactone
- levonorgestrel-intrauterine device plus metformin or spironolactone.
OEP plus spironolactone
Many women with PCOS have increased LH secretion and increased androgen activity in the skin due to increased 5-alpha reductase enzyme activity, which catalyzes the conversion of testosterone to the powerful intracellular androgen dihydrotestosterone.9 Women with PCOS may present with a chief problem report of hirsutism, acne, or female androgenetic alopecia. OEP plus spironolactone may be an optimal initial treatment for women with a dominant dermatologic manifestation of PCOS. OEP treatment results in a decrease in pituitary LH secretion and ovarian androgen production. Spironolactone adds to this therapeutic effect by blocking androgen action in the skin.
The data on dual therapy with spironolactone. Many dermatologists recommend spironolactone in combination with cosmetic measures for the treatment of acne, but there are only a few randomized trials that demonstrate its efficacy.10 In one trial spironolactone was demonstrated to be superior to placebo for the treatment of inflammatory acne.10 Authors of multiple randomized trials report that the antiandrogens, spironolactone, or finasteride are superior to metformin to treat hirsutism.11 In addition, a few small trials report that spironolactone plus OEP is superior to either OEP or metformin monotherapy for hirsutism.11 Clinical trials of spironolactone for hirsutism have been rated as “low quality” and additional controlled trials of OEP monotherapy versus OEP plus spironolactone are warranted.12
My preferred treatment approach
Spironolactone is effective in the treatment of hirsutism at doses ranging from 50 mg to 200 mg daily. I routinely use a dose of spironolactone 100 mg daily because this dose is near of the top of the dose-response curve and has few adverse effects (such as intermittent uterine bleeding or spotting). With spironolactone monotherapy at a dose of 200 mg, irregular uterine bleeding or spotting is common, but concomitant treatment with an OEP tends to minimize this side effect. In my practice I rarely have patients report irregular uterine bleeding or spotting with the combination treatment of an OEP and spironolactone 100 mg daily.
Contraindications. Spironolactone should not be given to women with renal insufficiency because it can cause hyperkalemia. However, it is not necessary to check potassium levels in young women taking spironolactone with normal creatinine levels.13
Triple therapy: OEP plus metformin plus spironolactone
Some experts strongly recommend the initial treatment of PCOS in adolescents and young women with triple therapy: OEP plus an insulin sensitizer plus an antiandrogen.14 This recommendation is based in part on the observation that OEP monotherapy may be associated with an increase in circulating adipokines and visceral fat mass as determined by dual-energy x-ray absorptiometry.15 By contrast, triple treatment with an OEP plus metformin plus an antiandrogen is associated with a decrease in circulating adipokines and visceral fat mass.
What is the best progestin for PCOS?
Any OEP is better than no OEP, regardless of the progestin used to treat the PCOS because ethinyl estradiol plus any synthetic progestin suppresses pituitary secretion of LH and decreases ovarian androgen production. However, for the treatment of acne, using a progestin that is less androgenic may be beneficial.16
In one study, 2,147 consecutive women who were taking a contraceptive and presented for treatment of acne were asked if their contraceptive had a positive impact on their acne. The percentage of women reporting that their contraceptive significantly improved their acne ranged from 26% for those taking drospirenone-ethinyl estradiol (EE) to 1% for those taking the etonogestrel subdermal implant (FIGURE).16 The US Food and Drug Administration has approved 4 OEP contraceptives for the treatment of acne (TABLE). The OEPs with drospirenone, norgestimate, desogestrel, or norethindrone acetate may be optimal choices for the treatment of acne caused by PCOS.


The bottom line
PCOS is a common endocrine disorder treated primarily by obstetricians-gynecologists. Among adolescents and young women with PCOS chief problem reports include irregular menses, hirsutism, obesity, acne, and infertility. Among mid-life women the presentation of PCOS often evolves into chronic medical problems, including obesity, metabolic syndrome, hyperlipidemia, hypertension, T2DM, cardiovascular disease, and endometrial cancer.17–19 To optimally treat the multiple pathophysiologic disorders manifested in PCOS, I recommend initial dual medical therapy with an OEP plus metformin or an OEP plus spironolactone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Using the Rotterdam criteria, the diagnosis of polycystic ovary syndrome (PCOS) is made in the presence of 2 of the following 3 criteria1:
- oligo-ovulation or anovulation
- hyperandrogenism manifested by the presence of either hirsutism or elevated hormone levels (including serum testosterone androstenedione and/or dehydroepiandrosterone sulfate)
- ultrasonography evidence of multifollicular ovaries (≥12 follicles with a diameter of 2 mm to 9 mm in one or both ovaries; FIGURE) or ovarian stromal volume of 10 mL or more.

Among reproductive-age women, the prevalence of PCOS has been reported to range from 8% to 13% for different populations.2 Most clinicians initiate treatment for PCOS with oral estrogen−progestin (OEP) monotherapy. OEP treatment has many beneficial hormonal effects, including:
- a resulting decrease in pituitary luteinizing hormone (LH) secretion, which decreases ovarian androgen production
- an increase in liver production of sex hormone−binding globulin (SHBG), which decreases free testosterone levels
- protection against the development of endometrial hyperplasia
- induction of regular uterine withdrawal bleeding.
However, OEP therapy neither improves metabolic indices (insulin sensitivity and visceral fat secretion of adipokines) nor blocks androgen action in the skin.
Dual medical treatment for PCOS can address the issues that monotherapy cannot and, along with providing guidance on improving diet and exercise, many experts support the initial therapy of PCOS with dual medical therapy (OEP plus metformin or spironolactone).
Advantages of OEP plus metformin
For many women with PCOS, the syndrome is characterized by abnormalities in both the reproductive (increase in LH secretion) and metabolic (insulin resistance and increased adipokines) systems. OEP monotherapy does not improve the metabolic abnormalities of PCOS. Combination treatment with both OEP plus metformin, along with diet and exercise, can best treat these combined abnormalities.
Data support dual therapy with metformin. In one small, randomized trial in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater reduction in serum androstenedione and a greater increase in SHBG than OEP monotherapy.3 In addition, weight loss and a reduction in waist-to-hip ratio only occurred in the OEP plus metformin group.3 In another small randomized study in women with PCOS, OEP plus metformin (1,500 mg daily) resulted in a greater decrease in free androgen index than OEP monotherapy.4
In my clinical opinion, women who may best benefit from OEP plus metformin therapy have one of the following factors indicating the presence of insulin resistance5:
- body mass index >30 kg/m2
- waist-to-hip ratio ≥0.85
- waist circumference >35 in (89 cm)
- acanthosis nigricans
- personal history of gestational diabetes
- family history of type 2 diabetes mellitus (T2DM) in a first-degree relative
- diagnosis of the metabolic syndrome.
My preferred treatment approach
Metformin is a low cost and safe treatment for metabolic dysfunction due to insulin resistance and excess adipokines. I often start PCOS treatment for my patients with an OEP plus metformin extended release (XR) 750 mg with dinner. If the patient tolerates this dose, I increase the dose to metformin XR 1,500 mg with dinner.
Adverse effects. The most common side effects of metformin are gastrointestinal, including abdominal discomfort, flatulence, borborygmi, diarrhea, and nausea. Metformin reduces serum vitamin B12 levels by 5% to 10%; therefore, ensuring adequate vitamin B12 intake (2.6 µg daily) is helpful.6 Although metformin does reduce vitamin B12 levels, there is no strong relationship between metformin and anemia or peripheral neuropathy.7 Lactic acidosis is a rare complication of metformin.
Beneficial effects. In the treatment of PCOS, metformin may have many beneficial effects, including8:
- decrease in insulin resistance
- decrease in harmful adipokines
- reduction in visceral fat
- reduction in the incidence of T2DM.
- oral norethindrone acetate 5 mg daily (which can lower luteinizing hormone levels and block ovulation) plus metformin
- norethindrone acetate 5 mg plus spironolactone
- levonorgestrel-intrauterine device plus metformin or spironolactone.
OEP plus spironolactone
Many women with PCOS have increased LH secretion and increased androgen activity in the skin due to increased 5-alpha reductase enzyme activity, which catalyzes the conversion of testosterone to the powerful intracellular androgen dihydrotestosterone.9 Women with PCOS may present with a chief problem report of hirsutism, acne, or female androgenetic alopecia. OEP plus spironolactone may be an optimal initial treatment for women with a dominant dermatologic manifestation of PCOS. OEP treatment results in a decrease in pituitary LH secretion and ovarian androgen production. Spironolactone adds to this therapeutic effect by blocking androgen action in the skin.
The data on dual therapy with spironolactone. Many dermatologists recommend spironolactone in combination with cosmetic measures for the treatment of acne, but there are only a few randomized trials that demonstrate its efficacy.10 In one trial spironolactone was demonstrated to be superior to placebo for the treatment of inflammatory acne.10 Authors of multiple randomized trials report that the antiandrogens, spironolactone, or finasteride are superior to metformin to treat hirsutism.11 In addition, a few small trials report that spironolactone plus OEP is superior to either OEP or metformin monotherapy for hirsutism.11 Clinical trials of spironolactone for hirsutism have been rated as “low quality” and additional controlled trials of OEP monotherapy versus OEP plus spironolactone are warranted.12
My preferred treatment approach
Spironolactone is effective in the treatment of hirsutism at doses ranging from 50 mg to 200 mg daily. I routinely use a dose of spironolactone 100 mg daily because this dose is near of the top of the dose-response curve and has few adverse effects (such as intermittent uterine bleeding or spotting). With spironolactone monotherapy at a dose of 200 mg, irregular uterine bleeding or spotting is common, but concomitant treatment with an OEP tends to minimize this side effect. In my practice I rarely have patients report irregular uterine bleeding or spotting with the combination treatment of an OEP and spironolactone 100 mg daily.
Contraindications. Spironolactone should not be given to women with renal insufficiency because it can cause hyperkalemia. However, it is not necessary to check potassium levels in young women taking spironolactone with normal creatinine levels.13
Triple therapy: OEP plus metformin plus spironolactone
Some experts strongly recommend the initial treatment of PCOS in adolescents and young women with triple therapy: OEP plus an insulin sensitizer plus an antiandrogen.14 This recommendation is based in part on the observation that OEP monotherapy may be associated with an increase in circulating adipokines and visceral fat mass as determined by dual-energy x-ray absorptiometry.15 By contrast, triple treatment with an OEP plus metformin plus an antiandrogen is associated with a decrease in circulating adipokines and visceral fat mass.
What is the best progestin for PCOS?
Any OEP is better than no OEP, regardless of the progestin used to treat the PCOS because ethinyl estradiol plus any synthetic progestin suppresses pituitary secretion of LH and decreases ovarian androgen production. However, for the treatment of acne, using a progestin that is less androgenic may be beneficial.16
In one study, 2,147 consecutive women who were taking a contraceptive and presented for treatment of acne were asked if their contraceptive had a positive impact on their acne. The percentage of women reporting that their contraceptive significantly improved their acne ranged from 26% for those taking drospirenone-ethinyl estradiol (EE) to 1% for those taking the etonogestrel subdermal implant (FIGURE).16 The US Food and Drug Administration has approved 4 OEP contraceptives for the treatment of acne (TABLE). The OEPs with drospirenone, norgestimate, desogestrel, or norethindrone acetate may be optimal choices for the treatment of acne caused by PCOS.


The bottom line
PCOS is a common endocrine disorder treated primarily by obstetricians-gynecologists. Among adolescents and young women with PCOS chief problem reports include irregular menses, hirsutism, obesity, acne, and infertility. Among mid-life women the presentation of PCOS often evolves into chronic medical problems, including obesity, metabolic syndrome, hyperlipidemia, hypertension, T2DM, cardiovascular disease, and endometrial cancer.17–19 To optimally treat the multiple pathophysiologic disorders manifested in PCOS, I recommend initial dual medical therapy with an OEP plus metformin or an OEP plus spironolactone.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855.
- Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17(7):1729-1737.
- Cibula D, Fanta M, Vrbikova J, et al. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenism, SHBG and lipids in PCOS patients. Hum Reprod. 2005;20(1):180-184.
- Grundy SM, Brewer HB, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
- Niafar M, Hai F, Porhomayon J, Nader ND. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med. 2015;10(1):93-102.
- de Groot-Kamphuis DM, van Dijk PR, Groenier KH, Houweling St, Bilo HJ, Kleefstra N. Vitamin B12 deficiency and the lack of its consequences in type 2 diabetes patients using metformin. Neth J Med. 2013;71(7):386-390.
- Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162(2):193-212.
- Skalba P, Dabkowska-Huc A, Kazimierczak W, Samojedny A, Samojedny MP, Chelmicki Z. Content of 5-alph-reductase (type 1 and type 2) mRNA in dermal papillae from the lower abdominal region in women with hirsutism. Clin Exp Dermatol. 2006;31(4):564-570.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1153-1160.
- van Zuuren EJ, Fedorowicz Z. Interventions for hirsutism excluding laser and photoepilation therapy alone: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2016;175(1):45-61.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151(9):941-944.
- Ibanez L, de Zegher F. Low-dose combination of flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18(1):57-60.
- Ibanez L, de Zegher F. Ethinyl estradiol-drospirenone, flutamide-metformin or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89(4):1592-1597.
- Lortscher D, Admani S, Stur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Wang ET, Calderon-Margalit R, Cedars MI, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6-13.
- Joham AE, Raniasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(3):e447-e452.
- Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136(1):99-103.
- Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855.
- Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17(7):1729-1737.
- Cibula D, Fanta M, Vrbikova J, et al. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenism, SHBG and lipids in PCOS patients. Hum Reprod. 2005;20(1):180-184.
- Grundy SM, Brewer HB, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
- Niafar M, Hai F, Porhomayon J, Nader ND. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med. 2015;10(1):93-102.
- de Groot-Kamphuis DM, van Dijk PR, Groenier KH, Houweling St, Bilo HJ, Kleefstra N. Vitamin B12 deficiency and the lack of its consequences in type 2 diabetes patients using metformin. Neth J Med. 2013;71(7):386-390.
- Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010;162(2):193-212.
- Skalba P, Dabkowska-Huc A, Kazimierczak W, Samojedny A, Samojedny MP, Chelmicki Z. Content of 5-alph-reductase (type 1 and type 2) mRNA in dermal papillae from the lower abdominal region in women with hirsutism. Clin Exp Dermatol. 2006;31(4):564-570.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191.
- Swiglo BA, Cosma M, Flynn DN, et al. Clinical review: antiandrogens for the treatment of hirsutism: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1153-1160.
- van Zuuren EJ, Fedorowicz Z. Interventions for hirsutism excluding laser and photoepilation therapy alone: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol. 2016;175(1):45-61.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151(9):941-944.
- Ibanez L, de Zegher F. Low-dose combination of flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18(1):57-60.
- Ibanez L, de Zegher F. Ethinyl estradiol-drospirenone, flutamide-metformin or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab. 2004;89(4):1592-1597.
- Lortscher D, Admani S, Stur N, Eichenfield LF. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15(6):670-674.
- Wang ET, Calderon-Margalit R, Cedars MI, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6-13.
- Joham AE, Raniasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(3):e447-e452.
- Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136(1):99-103.
Why are there delays in the diagnosis of endometriosis?
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.
In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.

In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.

In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.
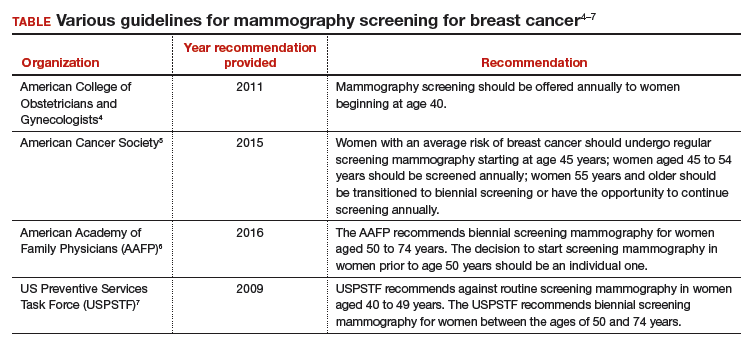
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.