User login
Maternal health benefits of breastfeeding
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
Elagolix: A new treatment for pelvic pain caused by endometriosis
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
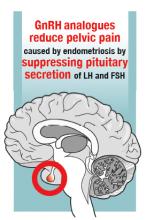
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
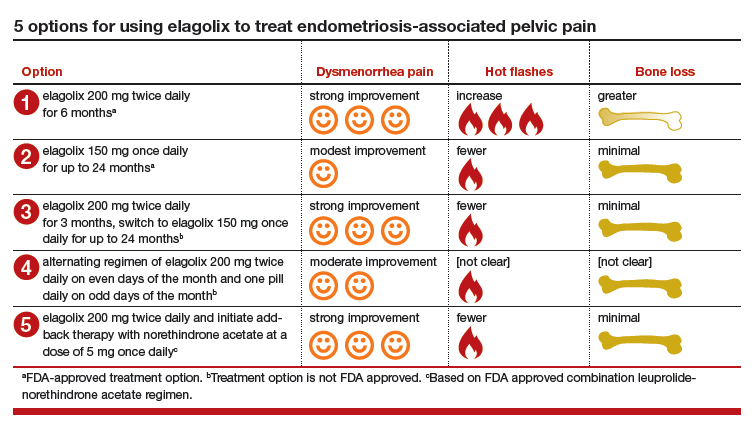
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3
Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is the presence of tissue resembling endometrial glands and stroma outside of the uterine cavity. Women with endometriosis often present for medical care with at least one of 3 problems: pelvic pain, infertility, and/or an adnexal mass due to endometriosis.1 Many clinical observations demonstrate that endometriosis lesions require estrogen to grow and maintain their viability, including that: (1) endometriosis is uncommon before puberty or after menopause, (2) surgical removal of both ovaries results in regression of endometriosis lesions, and (3) gonadotropin-releasing hormone (GnRH) analogues cause a hypo‑estrogenic hormonal environment, resulting in regression of endometriosis lesions and improvement in pelvic pain. Since endometriosis lesions require estrogen to maintain their viability, suppressing estradiol is a logical approach to hormonal treatment of the disease.
The estrogen threshold hypothesis
The estradiol concentration that causes endometriosis lesions to grow or regress varies among women, but a concentration less than 20 pg/mL usually causes lesions to regress, and a concentration greater than 60 pg/mL usually supports lesion growth and maintains lesion viability.2 Although an estradiol concentration below 20 pg/mL may cause lesions to regress, it also is associated with moderate to severe hot flashes and accelerated bone loss. These adverse effects limit the use of strong suppression of estrogen as a long-term treatment strategy. The estrogen threshold hypothesis posits that gently suppressing estradiol to a concentration between 20 and 45 pg/mL may simultaneously cause endometriosis lesions to regress, resulting in reduced pelvic pain, minimal bone loss, and few hot flashes.2
Building on the estrogen threshold hypothesis, clinicians have two options for treatment of pelvic pain caused by endometriosis:
- strong suppression of estradiol to a concentration below 20 pg/mL
- gentle suppression of estradiol to a concentration in the range of 20 to 45 pg/mL.
Strong suppression of estradiol to levels below 20 pg/mL will reliably induce amenorrhea and cause regression of endometriosis lesions, thereby reducing pelvic pain. Strong suppression of estradiol also will cause moderate to severe hot flashes and accelerated bone loss in many women. By contrast, gentle suppression of circulating estradiol to a concentration in the range of 20 to 45 pg/mL may result in amenorrhea or oligomenorrhea, suppression of the growth of endometriosis lesions, a modest reduction in pelvic pain, mild hot flashes, and minimal bone loss.
Recently, the US Food and Drug Administration (FDA) approved elagolix, an oral GnRH antagonist, for treatment of endometriosis.3 Elagolix blocks GnRH receptors in the pituitary gland, resulting in reduced production of luteinizing hormone and follicle stimulating hormone and a decrease in sex steroid secretion in the ovarian follicles, which leads to a reduction in the production and circulating concentration of estradiol. The FDA approved two doses of elagolix: 150 mg once daily for up to 24 months and 200 mg twice daily for up to 6 months. Importantly, elagolix at a dose of 150 mg once daily results in a mean circulating estradiol concentration of 41 pg/mL, indicating gentle suppression of ovarian estradiol production, and 200 mg twice daily results in a mean circulating ovarian estradiol concentration of 12 pg/mL, indicating strong suppression of ovarian estradiol production.3 For clinicians treating women with pelvic pain caused by endometriosis, these two elagolix regimens permit the individualization of hormonal therapy to the unique needs of each woman.
Continue to: Safety information for elagolix
- Contraindications: Elagolix should not be prescribed to women who are currently pregnant or have known osteoporosis or severe hepatic impairment. Elagolix should not be used in women taking cyclosporine or gemfibrozil (organic anion transporting polypeptide inhibitors).
- Elagolix may cause dose-dependent bone loss.
- Elagolix reduces menstrual bleeding, which may make it difficult to recognize the occurrence of pregnancy. Nonhormonal contraceptives should be utilized during elagolix treatment.
- Elagolix may be associated with an increase in reported depressive symptoms and mood changes.
- Elagolix may be associated with an increase in alanine aminotransferase more than 3 times the upper limit of the reference range. If elevated liver function tests are detected, the benefits and risks of continuing elagolix treatment should be evaluated.
Elagolix benefits and adverse effects
In one large clinical trial (Elaris Endometriosis I), 872 women were randomly assigned to treatment with one of two doses of elagolix (200 mg twice daily [high-dose group] or 150 mgonce daily [low-dose group]) or placebo.4 After 3 months of treatment, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of women in the high-dose, low-dose, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). In addition, at 3 months, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of women in the high-dose, low-dose, and placebo groups, respectively (low-dose vs placebo, P<.01; high-dose vs placebo, P<.001). Hot flashes that were severe enough to be classified as adverse events by study participants were reported by 42%, 24%, and 7% of the women in the high-dose, low-dose, and placebo groups, respectively. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47%, and hip/femoral/neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose, low-dose, and placebo groups, respectively.
Another large clinical trial of elagolix for treatment of pelvic pain caused by endometriosis (Elaris II) involving 817 women produced results that were similar to those reported in Elaris I.4 The elagolix continuation studies, Elaris III and IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.5
Depot leuprolide acetate and nafarelin acetate
Depot leuprolide acetate and nafarelin acetate are GnRH analogues approved by the FDA more than 25 years ago for treatment of pelvic pain caused by endometriosis. Over the past two decades, depot leuprolide acetate has been one of the most commonly used hormonal treatments for endometriosis in the United States. A 3-month formulation of depot leuprolide acetate with an 11.25-mg injection has resulted in mean circulating estradiol concentrations of 8 pg/mL, indicating very strong suppression of estradiol production.6 A twice-daily 200-µg dose of nafarelin acetate nasal spray has resulted in a circulating estradiol concentration of approximately 28 pg/mL, indicating gentle suppression of estradiol production.7
At current prices, elagolix treatment is substantially less expensive than treatment with leuprolide or nafarelin. In addition, many women in my practice prefer to use an oral medication over an intramuscular injection or a nasal spray medication. It is likely that clinicians and patients will evolve to prioritize and favor elagolix therapy over depot leuprolide or nafarelin treatment.
Continue to: 5 options for using elagolix
5 options for using elagolix
There are many potential options for using elagolix in the treatment of pelvic pain caused by endometriosis.
Option 1. Prescribe elagolix 200 mg twice daily for 6 months to achieve strong suppression of estradiol and marked improvement in dysmenorrhea, although at the cost of more hot flashes and greater bone loss.
Option 2. Prescribe elagolix 150 mg once daily for up to 24 months to achieve gentle suppression of estradiol and modest improvement in dysmenorrhea with fewer hot flashes and minimal bone loss.
Options 1 and 2 have been studied in high quality clinical trials involving more than 1,500 women and are approved by the FDA.
Option 3. Initiate treatment with elagolix 200 mg twice daily for 3 months, immediately accruing the benefits of strong suppression of estradiol, and then switch to elagolix 150 mg once daily for up to 24 months to achieve continuing pain control with fewer adverse effects. This regimen combines strong initial suppression of estradiol, which will result in marked improvement in dysmenorrhea, along with long-term gentle suppression of estradiol, which is likely to maintain decreased pain symptoms with minimal long-term bone loss and fewer hot flashes.
Option 4. Prescribe an alternating regimen of elagolix 200 mg twice daily on even days of the month (two pills daily is an even number of pills) and one pill daily on odd days of the month (1 pill daily is an odd number of pills). This regimen should produce a mean estradiol concentration between 12 and 41 pg/mL, resulting in moderate rather than strong or gentle suppression of estradiol.
Options 3 and 4 are based on extrapolation using our knowledge about the hormonal treatment of endometriosis and are not regimens approved by the FDA.
Option 5. Prescribe elagolix 200 mg twice daily and initiate add-back therapy with norethindrone acetate 5 mg once daily. Substantial evidence supports the combination of a GnRH analogue that strongly suppresses estradiol production with norethindrone acetate add-back, which helps mitigate the bone loss that occurs with strong suppression of estradiol and reduces the frequency of moderate to severe hot flashes.
Option 5 is based on extrapolation from high-quality studies of leuprolide acetate depot plus norethindrone acetate add-back.8 The combination regimen is approved by the FDA.3

Elagolix availability increases treatment choices for women
Pelvic pain caused by endometriosis is common, affecting approximately 8% of women of reproductive age.9 Endometriosis is a vexing disease because diagnosis is often delayed many years after the onset of symptoms, causing great frustration among patients.10 Some effective hormonal treatment options, including danazol and depot leuprolide, are poorly tolerated by patients because of adverse effects, including weight gain (danazol), hot flashes, and bone loss (depot leuprolide). Combination oral contraceptives used in a continuous or cyclic fashion often result in inadequate improvement in pelvic pain.11 The synthesis of an orally active, small-molecule GnRH antagonist is an innovative advance in endocrine pharmacology. The Elaris Endometriosis clinical trials have demonstrated that elagolix is effective in the treatment of pelvic pain caused by endometriosis.4,5 A great advantage of elagolix is that dosing can be tailored for each patient to achieve reduction in pain while minimizing unwanted adverse effects such as hot flashes and bone loss. In Elaris Endometriosis I, fewer than 10% of women discontinued elagolix due to adverse effects.4 Elagolix is also less expensive than depot leuprolide and nafarelin.
Millions of women in the United States have pelvic pain caused by endometriosis. Obstetrician-gynecologists are the clinicians best trained to care for these women, and patients trust that we will effectively treat their problem.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131:557-571.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Orlissa [package insert]. North Chicago, IL: AbbVie Inc; 2018.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017; 377: 28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Lupron Depot [package insert]. North Chicago, IL: Abbott Laboratories: 2012.
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. a multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318:485-489.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Missmer SA, Hankinson SE, Spiegelman D, et al. The incidence of laparoscopically-confirmed endometriosis by demographic, anthropomorphic and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8,10-11,16.
- Jensen JT, Schlaff W, Gordon K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: a systematic review of the evidence. Fertil Steril. 2018;110:137-152.
Postpartum hemorrhage: Aortic compression to reduce pelvic bleeding
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
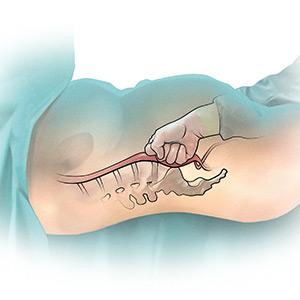
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11
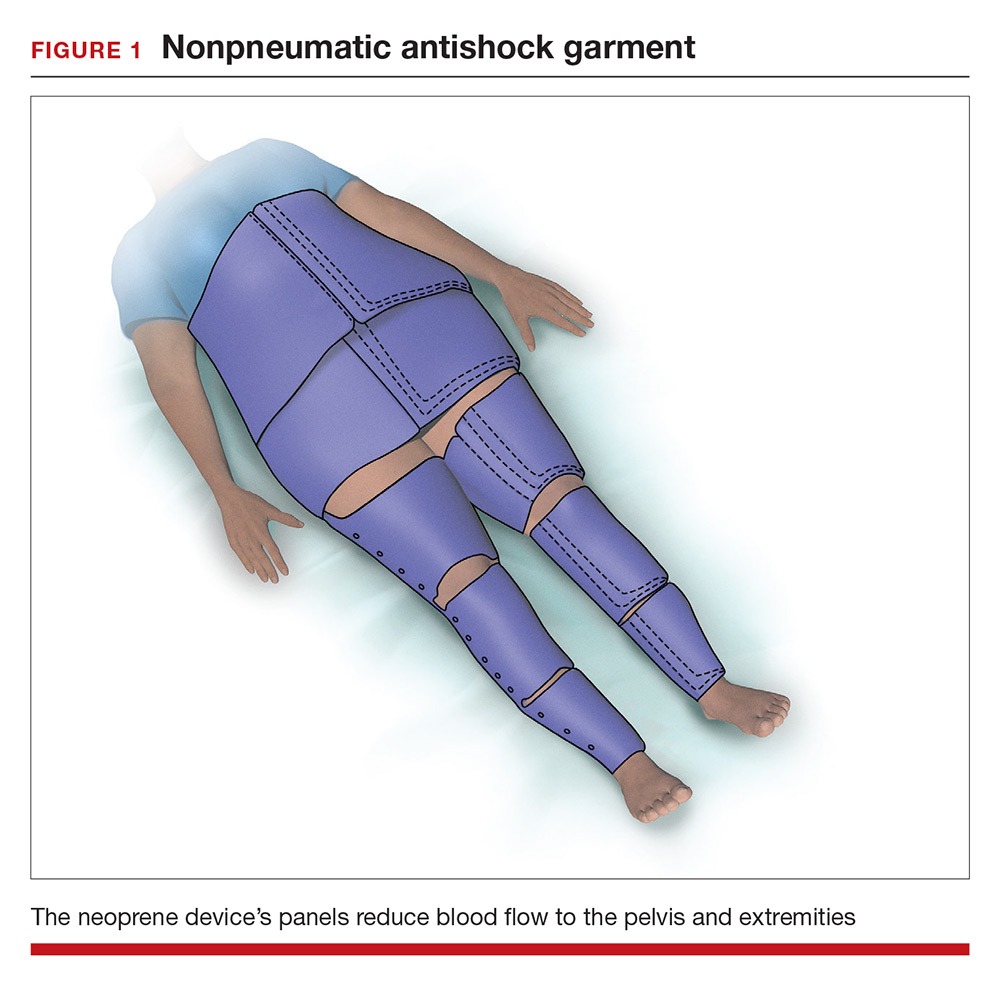
In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
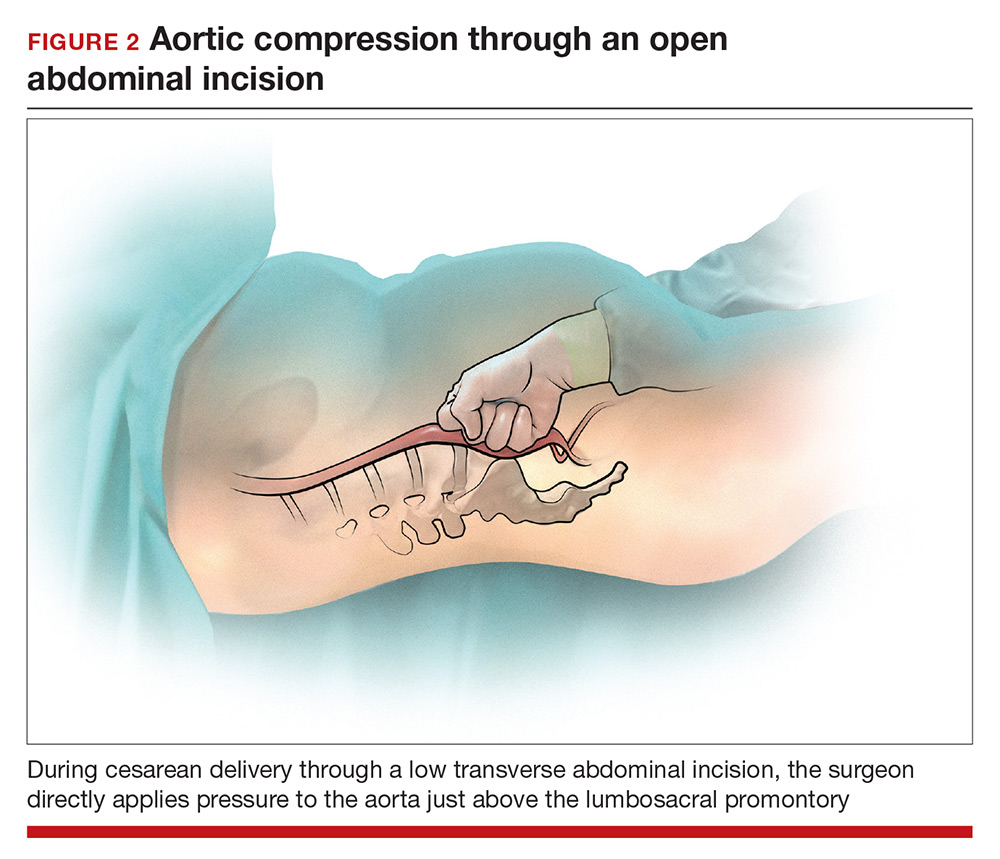
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.
Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11

In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.

Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11

In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.

Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
Optimize the medical treatment of endometriosis—Use all available medications
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).
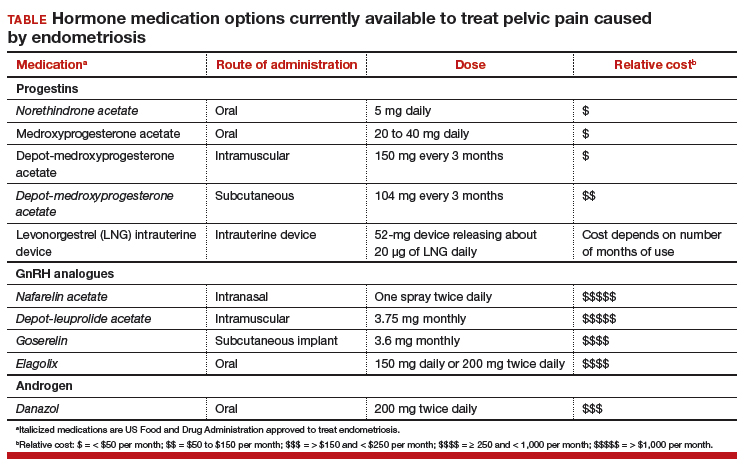
Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).

Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).

Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Are we ready for primary HPV testing for the prevention of cervical cancer?
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.

Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.

Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cervical cancer screening represents one of the great public health successes of the 20th Century. Two physician-scientists, George Papanicolaou, MD, PhD (1883–1962), and Harald zur Hausen, MD (1936–), made extraordinary contributions to the evolution of effective cervical cancer screening programs. Dr. Papanicolaou led development of the iconic Pap smear, creating techniques for collecting specimens and using cytologic techniques to identify cervical cancer and its precursors, and Dr. zur Hausen discovered the association of human papillomavirus (HPV) infection with cervical cancer.1,2
Although it is but a distant memory, in the 1930s cervical and uterine cancer caused more deaths among women than breast, lung, or ovarian cancer. The successful deployment of Pap smear screening resulted in a decrease in cervical cancer rates in developed countries. Cervical cancer deaths remain common in many parts of the world, however. Cervical cancer screening programs can reduce cervical cancer incidence by greater than 80%.3 In the United States between 1973 and 2006, the invasive cervical cancer age-adjusted incidence rates dropped from 10.28 to 3.97 per 100,000 women.4
HPV causes cervical cancer
Dr. zur Hausen dedicated his career to identifying viral causes of human cancer. In his Nobel Laureate autobiography, he reported that during his 2-year rotating residency, he loved his obstetrics and gynecology experience, but found it “physically highly demanding” and decided to focus his career in microbiology and immunology.5 After proving that herpes simplex virus did not cause cervical cancer he began to explore the role of HPV in the disease process. He first identified HPV types 6 and 11 and showed that these agents caused genital warts. He then used low-stringency hybridization techniques to identify HPV types 16 and 18 in specimens of cervical cancer. Later, he and his colleagues proved that two HPV proteins, E6 and E7, interfere with the function of cell cycle control proteins p53 and retinoblastoma protein, resulting in dysregulated cell growth and cancer.2 These findings permitted the development of both HPV vaccines and nucleic acid–based tests to identify high-risk oncogenic HPV (hrHPV) in cells and tissue specimens.
HPV vaccination
Dr. zur Hausen was an energetic and vocal advocate for the development and widescale deployment of HPV vaccines, including vaccination of males and females.6 Initially his ideas were rejected by the pharmaceutical industry, but eventually, with advances in virology and vaccine development, multiple companies pursued the development of HPV vaccines, the first cancer prevention vaccines. The best approach to cervical cancer prevention is intensive population-wide HPV vaccination of both boys and girls before exposure to the HPV virus. Beyond its beneficial effect on the incidence of cervical cancer, HPV vaccination also reduces the population incidence of anal, vulvar, and oropharyngeal cancer.7 Prevention of oropharyngeal cancer is especially important for men, supporting the recommendation for vaccination of all boys.8
Population-wide HPV vaccination will result in a lower prevalence of cervical cancer precursors and reduce the sensitivity of cytology, thereby making primary HPV screening more attractive.9 Based on one modelling study, universal HPV vaccination can reduce cervical cancer rates by greater than 50% over current levels, and introduction of primary HPV screening will reduce cervical cancer rates by an additional 20%.10 In an era of widespread vaccination for HPV, screening for cervical cancer should be intensified for nonvaccinated women.10
Read about Primary cervical cancer screening with cytology
Primary cervical cancer screening with cytology
Primary screening with cervical cytology alone remains an option supported by many authorities and professional society guidelines.11 Most studies report that HPV testing has greater sensitivity than cervical cytology alone, especially for the detection of adenocarcinoma of the cervix.12 In one Canadian study, 10,154 women were randomly assigned to HPV or cervical cytology testing. The sensitivity of HPV testing and cervical cytology for detecting cervical intraepithelial neoplasia grade 2 or 3 was 95% and 55%, respectively, with a specificity of 94% and 97%, respectively.13 When used together the sensitivity and specificity of cotesting was 100% and 93%, respectively, but resulted in an increased number of colposcopies, which may be costly and add stress for the patient. Many countries are beginning to move away from cervical cancer screening with cytology or cotesting to programs built upon a foundation of primary HPV testing.
Primary cervical cancer screening with HPV testing
The knowledge that hrHPV is a more sensitive test for cervical cancer and its precursors, as well as the relatively lower sensitivity of cytology, is the foundation for transitioning from primary screening with cervical cytology to primary screening with HPV testing. In the Netherlands14 and Australia15,16 HPV testing with reflex cytology is the nationwide approach to cervical cancer screening. The basic components of the Dutch primary HPV screening program, as explained by Dr. Lai van Zulyan Mandres, are14:
- Samples are collected by a general practitioner and sent to one of 5 central testing facilities for DNA testing for hrHPV.
- If all previous samples tested negative, the screening occurs at ages 30, 35, 40, 50, and 60 years, a minimum of 5 screens per woman.
- If there is a history of a previously positive hrHPV, the screening is intensified, with additional specimens collected at ages 45, 55, and 60 years.
- If the sample is hrHPV negative, the patient continues screening at the standard intervals. No cytology testing is performed.
- If the sample is hrHPV positive, reflex cytology is performed using the original collected sample. If the cytology shows no intraepithelial lesion or malignancy (NILM), another specimen is obtained for cytology within 6 months. If the second cytology specimen shows atypical squamous cells of undetermined significance (ASCUS) or a more worrisome cytology finding, the patient is sent for colposcopy. If two NILM cytology specimens have been obtained, the patient resumes primary hrHPV screening every 5 years.
- If the specimen is hrHPV positive and cytology is ASCUS or more worrisome the patient is referred for colposcopy (FIGURE).14 The Dutch estimate that primary hrHPV screening will reduce the number of cervical cytology specimens by 90% annually.

Australia also has implemented nationwide primary HPV testing for cervical cancer screening. This change was implemented following a 10-year program of universal school-based vaccination of girls and boys, and biennial cytology screening for all women. The Australian screening program initiates hrHPV testing at age 25 years and thereafter every 5 years until age 74. If the hrHPV test is positive, reflex testing for HPV types 16 and 18 are performed on the original specimen along with cervical cytology. Women who test positive for HPV 16 or 18 are immediately referred for colposcopy. If the hrHPV test is positive and reflex testing for HPV 16 and 18 is negative, cervical cytology demonstrating ASCUS, low- or high-grade squamous intraepithelial lesions, or more worrisome results trigger a referral for colposcopy. The Australian program supports testing of self-collected vaginal samples for women who are underscreened or have never been screened.15,16
Read about Pros and cons of switching approaches
Pros and cons of switching approaches
Deployment of new technology often yields benefits and challenges. A putative benefit of primary HPV screening is a reduction in health care costs without an increase in cervical cancer deaths. Another benefit of primary HPV screening is that it may enable self-collection of specimens for analysis, thereby increasing access to cervical cancer screening for underserved and marginalized populations of women who are not currently participating in cervical cancer screening programs.17 One challenge is that many women are unaware that hrHPV is the cause of most invasive cervical cancers. The detection of hrHPV in a woman in a long-term relationship who was previously negative for hrHPV may cause the emotions of surprise, fear, anxiety, and anger, thereby stressing the relationship.18
Another concern is that many women are worried about no longer receiving the familiar “Pap smear” cancer screening test in which they have tremendous faith. When Australia transitioned to primary HPV screening, more than 70,000 women signed a petition to “save women’s lives” by permitting continued access to the cervical cytology testing.19 Primary HPV testing may result in a transient increase in the number of women referred for colposcopy, potentially overwhelming the capacity of the health care system to deliver this vital service.20,21 The HPV types that most often cause cervical cancer may vary among countries. For example, in Thailand, HPV 52 and 58 are frequently detected in women with high-grade squamous lesions, and including these subtypes in reflex genotyping may be of regional benefit.22
Primary cervical cancer screening with HPV testing: When will it be used widely in the United States?
In contrast to the United States, the Netherlands is a small, densely populated country that has a highly integrated health system with centralized laboratory centers, a nationwide electronic health record, and clinicians organized to perform as an integrated team. These features ensure that all lifetime tests results are available in one record, that HPV testing is highly standardized, and that clinicians will follow a prescribed care pathway. The Netherlands’ health system is organized to support the successful transition, in a single step, to primary HPV testing. The United States is the third most populous country in the world, following China and India, with a diverse approach to health care, a highly mobile population, no single interoperable electronic health record, and minimal central control of clinical practice. The United States is not organized to make a “big bang” transition to primary HPV cervical cancer screening. It is likely that the introduction of primary HPV screening will occur first in highly integrated health systems that control the clinical, laboratory, and electronic records of a large population.
The results of the ATHENA study provide a clear clinical algorithm for implementing a primary HPV screening program for cervical cancer in the United States.23–25 Samples are collected for hrHPV testing at a specified interval, 3 or 5 years, beginning at age 25 years. Women younger than age 25 years should be screened with cytology alone. Detection of hrHPV results in reflex viral typing for HPV 16 and 18. Women with samples positive for HPV 16 and 18 are immediately referred for colposcopy. Samples positive for hrHPV and negative for HPV 16 and 18 have reflex cytology testing performed on the original HPV specimen. If cytology testing reports NILM, repeat cotesting is performed in one year. If cytology testing reports ASCUS or a more concerning result, the woman is referred for colposcopy.
Malcolm Gladwell, in his book The Tipping Point, identified 3 processes that help push an innovative new approach from obscurity into widespread use.26 First, authoritative voices that can catalyze change need to consistently communicate their shared vision for the future. Second, there must be a clear message that galvanizes the many to change their approach. Third, the historical context must be supportive of the change. Over the next decade we are likely to hit a tipping point and transition from cervical cancer screening that relies on cervical cytology to an approach that prioritizes hrHPV testing. When that change will occur in the United States is unclear. But our colleagues in other countries already have transitioned to primary hrHPV testing for cervical cancer screening.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
- Hinsey JC. George Nicholas Papanicolaou, May 13, 1883–February 19, 1962. Acta Cytol. 1962;6:483–486.
- zur Hausen H. Papillomaviruses in human cancers. Proc Assoc Am Physicians. 1999;111(6):581–587.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol 10: Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health (Larchmt). 2012;21(10):1031–1037.
- Harold zur Hausen-Biographical. Nobelprize.org website. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html. Accessed June 19, 2018.
- Michels KB, zur Hausen H. HPV vaccine for all. Lancet. 2009;374(9686):268–270.
- Hansen BT, Campbell S, Nygaard M. Long-term incidence of HPV-related cancers, and cases preventable by HPV vaccination: a registry-based study in Norway. BMJ Open. 2018;8(2):e019005.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27(5):6–8.
- Massad LS. Anticipating the impact of human papillomavirus vaccination on US cervical cancer prevention strategies. J Low Genit Tract Dis. 2018;22(2):123–125.
- Castanon A, Landy R, Pesola F, Windridge P, Sasieni P. Prediction of cervical cancer incidence in England, UK, up to 2040, under four scenarios: a modeling study. Lancet Public Health. 2018;3(1):e34–e43.
- Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891.
- Moukarzel LA, Angarita AM, VandenBussche C, et al. Preinvasive and invasive cervical adenocarcinoma: preceding low-risk or negative Pap result increases time to diagnosis. J Low Genital Tract Dis. 2017;21(2):91–96.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588.
- van Zuylen-Manders L. Primary HPV screening: The Dutch experience. http://www.britishcytology.org.uk/resources/Primary_HPV_screening_The_Dutch_experience.pdf. Accessed June 19, 2018.
- Hammond I, Canfell K, Saville M. A new era for cervical cancer screening in Australia: watch this space! Aust N Z J Obstet Gynaecol. 2017;57(5):499–501.
- Canfell K, Saville M, Caruana M, et al. Protocol for Compass: a randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8(1):e016700.
- Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: a qualitative study of stakeholder perspectives. Curr Oncol. 2018;25(1):e8–e16.
- Patel H, Moss EL, Sherman SM. HPV primary cervical cancer screening in England: women’s awareness and attitudes. Psychooncology. 2018;27(6):1559–1564.
- Obermair HM, Dodd RH, Bonner C, Jansen J, McCaffery K. “It has saved thousands of lives, so why change it?” Content analysis of objections to cervical cancer screening programme changes in Australia. BMJ Open. 2018;8(2):e019171.
- Hall MT, Simms KT, Lew JB, Smith MA, Saville M, Canfell K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017-2035: Example from Australia. PLoS One. 2018;13(2):e0185332.
- Rebolj M, Bonde J, Preisler S, Ejegod D, Rygaard C, Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11(1):e0147326.
- Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Srisomboon J, Intaraphet S, Siriaunkgul S. Genotyping for human papillomavirus (HPV) 16/18/52/58 has a higher performance than HPV16/18 genotyping in triaging women with positive high-risk HPV test in Northern Thailand. PLoS One. 2016;11(6):e0158184.
- Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Gladwell M. The Tipping Point: How Little Things Can Make a Big Difference. New York, New York: Little Brown; 2000.
Delivering bad news in obstetric practice
Obstetrics is a field filled with joyful experiences highlighted by pregnancy, childbirth, and the growth of healthy families. The field is also filled with many experiences that are sorrowful, including failure to conceive after infertility treatment, miscarriage, ultrasound-detected fetal anomalies, fetuses with genetic problems, fetal and neonatal demise, extremely premature birth, and birth injury. For decades oncologists have evolved their approach to discussing bad news with cancer patients. In the distant past, oncologists often kept a cancer diagnosis from the patient, preferring to spare them the stress of the news. In the modern era of transparency, however, oncologists now uniformly strive to keep patients informed of their situation and have adopted structured approaches to delivering bad news. An adverse pregnancy outcome such as a miscarriage or fetal loss may trigger emotional responses as intense as those experienced by a person hearing about a cancer diagnosis. Women who have recently experienced a miscarriage report emotional responses ranging from “a little disappointed” to “in shock” and “for it to be taken away was crushing.”1 As obstetricians, we can advance our practice by adopting a structured approach to delivering bad news, building on the lessons from cancer medicine. Improving the quality of our communication about adverse pregnancy events will reduce emotional distress and enable patients and families to more effectively cope with challenging situations.

Communicating bad news: The facts, the emotional response, and the impact on identity
Clinicians need to be cognizant that a conversation about bad news is 3 interwoven conversations that involve facts, emotional responses, and an altered self-identity. In addition to communicating the facts of the event in clear language, clinicians need to simultaneously monitor and manage the emotional responses to the adverse event and the impact on the participants’ sense of self.2 Clinicians are steeped in scientific tradition and method, and as experts we are naturally drawn to a discussion of the facts.
However, a discussion about bad news is highly likely to trigger an emotional response in the patient and the clinician. For example, when a clinician tells the patient about delivery events that resulted in an unexpected newborn injury, the patient may become angry and the clinician may be fearful, anxious, and defensive. Managing the emotions of all participants in the conversation is important for an optimal outcome.
An adverse event also may cause those involved to think about their self-identity. A key feature of bad news is that it alters patients’ expectations about their future, juxtaposing the reality of their outcome with the preferable outcome that may have been. Following a stillbirth during her first pregnancy the patient may be wondering, “Will I ever be a mother?”, “Did I cause the loss?”, and “Does all life end in death?” A traumatic event also may impact the self-identity of the clinician. Following a delivery where the newborn was injured, the clinician may be wondering, “Am I a good or bad clinician?”, “Did I do something wrong?”, “Is it time for me to retire from obstetrical practice?”
Following an adverse pregnancy outcome some patients are consumed with intense grief. This may require the patient and her family to move through a series of emotions (similar to those who receive a new diagnosis of cancer), including denial, anger, bargaining, depression, and acceptance.
Responses to grief
Kubler-Ross identified these 5 psychological coping mechanisms that are often used by people experiencing grief: denial, anger, bargaining, depression, and acceptance.3 The goal of the clinician is to help grieving patients move through these stages in an appropriate fashion and not get stuck in the stages of denial, anger, and/or depression. Following a difficult pregnancy some patients and their family members become stuck in a state dominated by anger, rage, and resentment. This is fertile ground for the growth of a professional liability case. Denial and anger are adaptive short-term defenses to protecting self-identity. In time, most people engage in more constructive responses, accept the adverse event, and plan for the future. Kubler-Ross observed that hope helps people survive through a time of great suffering and is present throughout the response to grief. Clinicians can play an important role in ensuring that a flame of hope is kept burning throughout the process of responding to and grieving bad outcomes.
A structured approach to delivering bad news: SPIKES
Dr. Robert Buckman, an oncologist, has proposed using a structured approach, SPIKES, to guide conversations focused on delivering bad news.4–6 SPIKES is focused on trying to deeply understand the patient’s level of knowledge, emotions, and perspective before providing medical information and support. SPIKES consists of 6 key steps.
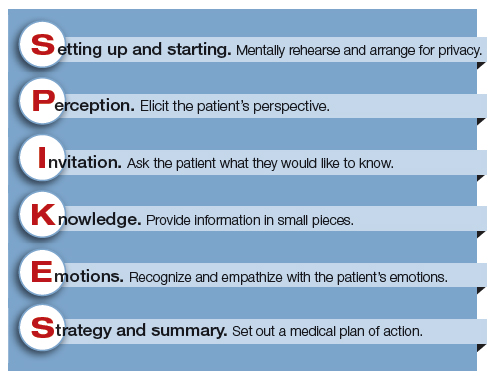
1. Setting up and starting. Mentally rehearse and arrange for privacy. Make sure the patient’s support people are present. Sit down, use open body language, eye contact, and/or touch to make a connection with the patient. Create room for a dialogue by using open-ended questions, silent pauses, listening first, and encouraging the patient to provide their perspective.
2. Perception. Elicit the patient’s perspective. Assess what the patient believes and feels. Assess vocabulary and comprehension.
3. Invitation. Ask the patient what they would like to know. Obtain permission to share knowledge.
4. Knowledge. Provide information in small pieces, always checking back on the patient’s understanding. Use plain language that aligns with the patient’s vocabulary and understanding.
5. Emotions. Explore, explicitly recognize, and empathize with the patient’s emotions.
6. Strategy and summary. Set out a medical plan of action. Express a commitment to be available for the patient as she embarks on the care plan. Arrange for a follow-up conversation.
Some studies have indicated that having a protocol such as SPIKES for delivering bad news helps clinicians to navigate this challenging process, which in turn improves patient satisfaction with disclosure.7 Simulation training focused on communicating bad news could be better utilized to help clinicians practice this skill, similar to the simulation exercises used to practice common clinical problems like hemorrhage and shoulder dystocia.8,9
Physician responses to bad outcomes
Over a career in clinical practice, physicians experience many bad outcomes that expose them to the contagion of sadness and grief. Despite this vicarious trauma, they must always present a professional persona, placing the patient’s needs above their own pain. Due to these experiences, clinicians may become isolated, depressed, and burned out. Drs. Michael and Enid Balint recognized the adverse effect of a lifetime of exposure to suffering and pain. They proposed that physicians could mitigate the trauma of these experiences by participating in small group meetings with a trained leader to discuss their most difficult clinical experiences in a confidential and supportive environment.10,11 By sharing clinical experiences, feelings, and stories with trusted colleagues, physicians can channel painful experiences into a greater understanding of the empathy and compassion needed to care for themselves, their colleagues, and patients. Clinical practice is invariably punctuated by occasional adverse outcomes necessitating that we effectively manage the process of delivering bad news, simultaneously caring for ourselves and our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Flink-Bochacki R, Hamm ME, Borrero S, Chen BA, Achilles SL, Chang JC. Family planning and counseling desires of women who have experienced miscarriage. Obstet Gynecol. 2018;131(4):625-631.
- Stone D, Patton B, Heen S. Difficult conversations. How to discuss what matters most. Penguin Books: New York, NY; 1999:9-10.
- Kubler-Ross E. On death and dying. MacMillan: New York, NY; 1969.
- Buckman R. How to break bad news. A guide for health care professionals. The Johns Hopkins University Press: Baltimore, MD; 1992.
- Buckman R. Practical plans for difficult conversations in medicine. Strategies that work in breaking bad news. The Johns Hopkins University Press: Baltimore, MD; 2010.
- Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES-a six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302-311.
- Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet. 2004;363(9405):312-319.
- Colletti L, Gruppen L, Barclay M, Stern D. Teaching students to break bad news. Am J Surg. 2001;182(1):20-23.
- Rosenbaum ME, Ferguson KJ, Lobas JG. Teaching medical students and residents skills for delivering bad news: a review of strategies. Acad Med. 2004;79(2):107-117.
- Balint M. The doctor, his patient and the illness. Pitman: London, England; 1957.
- Salinksky J. Balint groups and the Balint method. The Balint Society website. https://balint.co.uk/about/the-balint-method/. Accessed May 17, 2018.
Obstetrics is a field filled with joyful experiences highlighted by pregnancy, childbirth, and the growth of healthy families. The field is also filled with many experiences that are sorrowful, including failure to conceive after infertility treatment, miscarriage, ultrasound-detected fetal anomalies, fetuses with genetic problems, fetal and neonatal demise, extremely premature birth, and birth injury. For decades oncologists have evolved their approach to discussing bad news with cancer patients. In the distant past, oncologists often kept a cancer diagnosis from the patient, preferring to spare them the stress of the news. In the modern era of transparency, however, oncologists now uniformly strive to keep patients informed of their situation and have adopted structured approaches to delivering bad news. An adverse pregnancy outcome such as a miscarriage or fetal loss may trigger emotional responses as intense as those experienced by a person hearing about a cancer diagnosis. Women who have recently experienced a miscarriage report emotional responses ranging from “a little disappointed” to “in shock” and “for it to be taken away was crushing.”1 As obstetricians, we can advance our practice by adopting a structured approach to delivering bad news, building on the lessons from cancer medicine. Improving the quality of our communication about adverse pregnancy events will reduce emotional distress and enable patients and families to more effectively cope with challenging situations.

Communicating bad news: The facts, the emotional response, and the impact on identity
Clinicians need to be cognizant that a conversation about bad news is 3 interwoven conversations that involve facts, emotional responses, and an altered self-identity. In addition to communicating the facts of the event in clear language, clinicians need to simultaneously monitor and manage the emotional responses to the adverse event and the impact on the participants’ sense of self.2 Clinicians are steeped in scientific tradition and method, and as experts we are naturally drawn to a discussion of the facts.
However, a discussion about bad news is highly likely to trigger an emotional response in the patient and the clinician. For example, when a clinician tells the patient about delivery events that resulted in an unexpected newborn injury, the patient may become angry and the clinician may be fearful, anxious, and defensive. Managing the emotions of all participants in the conversation is important for an optimal outcome.
An adverse event also may cause those involved to think about their self-identity. A key feature of bad news is that it alters patients’ expectations about their future, juxtaposing the reality of their outcome with the preferable outcome that may have been. Following a stillbirth during her first pregnancy the patient may be wondering, “Will I ever be a mother?”, “Did I cause the loss?”, and “Does all life end in death?” A traumatic event also may impact the self-identity of the clinician. Following a delivery where the newborn was injured, the clinician may be wondering, “Am I a good or bad clinician?”, “Did I do something wrong?”, “Is it time for me to retire from obstetrical practice?”
Following an adverse pregnancy outcome some patients are consumed with intense grief. This may require the patient and her family to move through a series of emotions (similar to those who receive a new diagnosis of cancer), including denial, anger, bargaining, depression, and acceptance.
Responses to grief
Kubler-Ross identified these 5 psychological coping mechanisms that are often used by people experiencing grief: denial, anger, bargaining, depression, and acceptance.3 The goal of the clinician is to help grieving patients move through these stages in an appropriate fashion and not get stuck in the stages of denial, anger, and/or depression. Following a difficult pregnancy some patients and their family members become stuck in a state dominated by anger, rage, and resentment. This is fertile ground for the growth of a professional liability case. Denial and anger are adaptive short-term defenses to protecting self-identity. In time, most people engage in more constructive responses, accept the adverse event, and plan for the future. Kubler-Ross observed that hope helps people survive through a time of great suffering and is present throughout the response to grief. Clinicians can play an important role in ensuring that a flame of hope is kept burning throughout the process of responding to and grieving bad outcomes.
A structured approach to delivering bad news: SPIKES
Dr. Robert Buckman, an oncologist, has proposed using a structured approach, SPIKES, to guide conversations focused on delivering bad news.4–6 SPIKES is focused on trying to deeply understand the patient’s level of knowledge, emotions, and perspective before providing medical information and support. SPIKES consists of 6 key steps.

1. Setting up and starting. Mentally rehearse and arrange for privacy. Make sure the patient’s support people are present. Sit down, use open body language, eye contact, and/or touch to make a connection with the patient. Create room for a dialogue by using open-ended questions, silent pauses, listening first, and encouraging the patient to provide their perspective.
2. Perception. Elicit the patient’s perspective. Assess what the patient believes and feels. Assess vocabulary and comprehension.
3. Invitation. Ask the patient what they would like to know. Obtain permission to share knowledge.
4. Knowledge. Provide information in small pieces, always checking back on the patient’s understanding. Use plain language that aligns with the patient’s vocabulary and understanding.
5. Emotions. Explore, explicitly recognize, and empathize with the patient’s emotions.
6. Strategy and summary. Set out a medical plan of action. Express a commitment to be available for the patient as she embarks on the care plan. Arrange for a follow-up conversation.
Some studies have indicated that having a protocol such as SPIKES for delivering bad news helps clinicians to navigate this challenging process, which in turn improves patient satisfaction with disclosure.7 Simulation training focused on communicating bad news could be better utilized to help clinicians practice this skill, similar to the simulation exercises used to practice common clinical problems like hemorrhage and shoulder dystocia.8,9
Physician responses to bad outcomes
Over a career in clinical practice, physicians experience many bad outcomes that expose them to the contagion of sadness and grief. Despite this vicarious trauma, they must always present a professional persona, placing the patient’s needs above their own pain. Due to these experiences, clinicians may become isolated, depressed, and burned out. Drs. Michael and Enid Balint recognized the adverse effect of a lifetime of exposure to suffering and pain. They proposed that physicians could mitigate the trauma of these experiences by participating in small group meetings with a trained leader to discuss their most difficult clinical experiences in a confidential and supportive environment.10,11 By sharing clinical experiences, feelings, and stories with trusted colleagues, physicians can channel painful experiences into a greater understanding of the empathy and compassion needed to care for themselves, their colleagues, and patients. Clinical practice is invariably punctuated by occasional adverse outcomes necessitating that we effectively manage the process of delivering bad news, simultaneously caring for ourselves and our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Obstetrics is a field filled with joyful experiences highlighted by pregnancy, childbirth, and the growth of healthy families. The field is also filled with many experiences that are sorrowful, including failure to conceive after infertility treatment, miscarriage, ultrasound-detected fetal anomalies, fetuses with genetic problems, fetal and neonatal demise, extremely premature birth, and birth injury. For decades oncologists have evolved their approach to discussing bad news with cancer patients. In the distant past, oncologists often kept a cancer diagnosis from the patient, preferring to spare them the stress of the news. In the modern era of transparency, however, oncologists now uniformly strive to keep patients informed of their situation and have adopted structured approaches to delivering bad news. An adverse pregnancy outcome such as a miscarriage or fetal loss may trigger emotional responses as intense as those experienced by a person hearing about a cancer diagnosis. Women who have recently experienced a miscarriage report emotional responses ranging from “a little disappointed” to “in shock” and “for it to be taken away was crushing.”1 As obstetricians, we can advance our practice by adopting a structured approach to delivering bad news, building on the lessons from cancer medicine. Improving the quality of our communication about adverse pregnancy events will reduce emotional distress and enable patients and families to more effectively cope with challenging situations.

Communicating bad news: The facts, the emotional response, and the impact on identity
Clinicians need to be cognizant that a conversation about bad news is 3 interwoven conversations that involve facts, emotional responses, and an altered self-identity. In addition to communicating the facts of the event in clear language, clinicians need to simultaneously monitor and manage the emotional responses to the adverse event and the impact on the participants’ sense of self.2 Clinicians are steeped in scientific tradition and method, and as experts we are naturally drawn to a discussion of the facts.
However, a discussion about bad news is highly likely to trigger an emotional response in the patient and the clinician. For example, when a clinician tells the patient about delivery events that resulted in an unexpected newborn injury, the patient may become angry and the clinician may be fearful, anxious, and defensive. Managing the emotions of all participants in the conversation is important for an optimal outcome.
An adverse event also may cause those involved to think about their self-identity. A key feature of bad news is that it alters patients’ expectations about their future, juxtaposing the reality of their outcome with the preferable outcome that may have been. Following a stillbirth during her first pregnancy the patient may be wondering, “Will I ever be a mother?”, “Did I cause the loss?”, and “Does all life end in death?” A traumatic event also may impact the self-identity of the clinician. Following a delivery where the newborn was injured, the clinician may be wondering, “Am I a good or bad clinician?”, “Did I do something wrong?”, “Is it time for me to retire from obstetrical practice?”
Following an adverse pregnancy outcome some patients are consumed with intense grief. This may require the patient and her family to move through a series of emotions (similar to those who receive a new diagnosis of cancer), including denial, anger, bargaining, depression, and acceptance.
Responses to grief
Kubler-Ross identified these 5 psychological coping mechanisms that are often used by people experiencing grief: denial, anger, bargaining, depression, and acceptance.3 The goal of the clinician is to help grieving patients move through these stages in an appropriate fashion and not get stuck in the stages of denial, anger, and/or depression. Following a difficult pregnancy some patients and their family members become stuck in a state dominated by anger, rage, and resentment. This is fertile ground for the growth of a professional liability case. Denial and anger are adaptive short-term defenses to protecting self-identity. In time, most people engage in more constructive responses, accept the adverse event, and plan for the future. Kubler-Ross observed that hope helps people survive through a time of great suffering and is present throughout the response to grief. Clinicians can play an important role in ensuring that a flame of hope is kept burning throughout the process of responding to and grieving bad outcomes.
A structured approach to delivering bad news: SPIKES
Dr. Robert Buckman, an oncologist, has proposed using a structured approach, SPIKES, to guide conversations focused on delivering bad news.4–6 SPIKES is focused on trying to deeply understand the patient’s level of knowledge, emotions, and perspective before providing medical information and support. SPIKES consists of 6 key steps.

1. Setting up and starting. Mentally rehearse and arrange for privacy. Make sure the patient’s support people are present. Sit down, use open body language, eye contact, and/or touch to make a connection with the patient. Create room for a dialogue by using open-ended questions, silent pauses, listening first, and encouraging the patient to provide their perspective.
2. Perception. Elicit the patient’s perspective. Assess what the patient believes and feels. Assess vocabulary and comprehension.
3. Invitation. Ask the patient what they would like to know. Obtain permission to share knowledge.
4. Knowledge. Provide information in small pieces, always checking back on the patient’s understanding. Use plain language that aligns with the patient’s vocabulary and understanding.
5. Emotions. Explore, explicitly recognize, and empathize with the patient’s emotions.
6. Strategy and summary. Set out a medical plan of action. Express a commitment to be available for the patient as she embarks on the care plan. Arrange for a follow-up conversation.
Some studies have indicated that having a protocol such as SPIKES for delivering bad news helps clinicians to navigate this challenging process, which in turn improves patient satisfaction with disclosure.7 Simulation training focused on communicating bad news could be better utilized to help clinicians practice this skill, similar to the simulation exercises used to practice common clinical problems like hemorrhage and shoulder dystocia.8,9
Physician responses to bad outcomes
Over a career in clinical practice, physicians experience many bad outcomes that expose them to the contagion of sadness and grief. Despite this vicarious trauma, they must always present a professional persona, placing the patient’s needs above their own pain. Due to these experiences, clinicians may become isolated, depressed, and burned out. Drs. Michael and Enid Balint recognized the adverse effect of a lifetime of exposure to suffering and pain. They proposed that physicians could mitigate the trauma of these experiences by participating in small group meetings with a trained leader to discuss their most difficult clinical experiences in a confidential and supportive environment.10,11 By sharing clinical experiences, feelings, and stories with trusted colleagues, physicians can channel painful experiences into a greater understanding of the empathy and compassion needed to care for themselves, their colleagues, and patients. Clinical practice is invariably punctuated by occasional adverse outcomes necessitating that we effectively manage the process of delivering bad news, simultaneously caring for ourselves and our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Flink-Bochacki R, Hamm ME, Borrero S, Chen BA, Achilles SL, Chang JC. Family planning and counseling desires of women who have experienced miscarriage. Obstet Gynecol. 2018;131(4):625-631.
- Stone D, Patton B, Heen S. Difficult conversations. How to discuss what matters most. Penguin Books: New York, NY; 1999:9-10.
- Kubler-Ross E. On death and dying. MacMillan: New York, NY; 1969.
- Buckman R. How to break bad news. A guide for health care professionals. The Johns Hopkins University Press: Baltimore, MD; 1992.
- Buckman R. Practical plans for difficult conversations in medicine. Strategies that work in breaking bad news. The Johns Hopkins University Press: Baltimore, MD; 2010.
- Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES-a six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302-311.
- Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet. 2004;363(9405):312-319.
- Colletti L, Gruppen L, Barclay M, Stern D. Teaching students to break bad news. Am J Surg. 2001;182(1):20-23.
- Rosenbaum ME, Ferguson KJ, Lobas JG. Teaching medical students and residents skills for delivering bad news: a review of strategies. Acad Med. 2004;79(2):107-117.
- Balint M. The doctor, his patient and the illness. Pitman: London, England; 1957.
- Salinksky J. Balint groups and the Balint method. The Balint Society website. https://balint.co.uk/about/the-balint-method/. Accessed May 17, 2018.
- Flink-Bochacki R, Hamm ME, Borrero S, Chen BA, Achilles SL, Chang JC. Family planning and counseling desires of women who have experienced miscarriage. Obstet Gynecol. 2018;131(4):625-631.
- Stone D, Patton B, Heen S. Difficult conversations. How to discuss what matters most. Penguin Books: New York, NY; 1999:9-10.
- Kubler-Ross E. On death and dying. MacMillan: New York, NY; 1969.
- Buckman R. How to break bad news. A guide for health care professionals. The Johns Hopkins University Press: Baltimore, MD; 1992.
- Buckman R. Practical plans for difficult conversations in medicine. Strategies that work in breaking bad news. The Johns Hopkins University Press: Baltimore, MD; 2010.
- Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP. SPIKES-a six-step protocol for delivering bad news: application to the patient with cancer. Oncologist. 2000;5(4):302-311.
- Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet. 2004;363(9405):312-319.
- Colletti L, Gruppen L, Barclay M, Stern D. Teaching students to break bad news. Am J Surg. 2001;182(1):20-23.
- Rosenbaum ME, Ferguson KJ, Lobas JG. Teaching medical students and residents skills for delivering bad news: a review of strategies. Acad Med. 2004;79(2):107-117.
- Balint M. The doctor, his patient and the illness. Pitman: London, England; 1957.
- Salinksky J. Balint groups and the Balint method. The Balint Society website. https://balint.co.uk/about/the-balint-method/. Accessed May 17, 2018.
Tactics for reducing the rate of surgical site infection following cesarean delivery
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
Hidradenitis suppurativa: An underdiagnosed skin problem of women
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9
HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9

HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9

HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The new normal in blood pressure diagnosis and management: Lower is better
For many years, the approach to the diagnosis of hypertension was straight-forward—multiple blood pressure (BP) measurements ≥140/90 mm Hg established the diagnosis of hypertension, a disease associated with an increased risk of adverse cardiovascular events, including myocardial infarction and stroke. For more than a decade, hypertension experts have argued that a BP ≥130/80 mm Hg should establish the diagnosis of hypertension. Many clinicians resisted the change because it would markedly increase the number of asymptomatic adults with the diagnosis, increasing the number needing treatment. However, the findings of the Systolic Blood Pressure Intervention Trial (SPRINT) and other observational studies have catalyzed the American College of Cardiology (ACC) and the American Heart Association (AHA) to redefine normal BP as <120/80 mm Hg.1 This change will expand the diagnosis of hypertension to include up to 50% of American adults.1 In addition, the new definition of normal BP will result in the greater use of lifestyle interventions and antihypertensive medications to achieve the new normal, a BP of <120/80 mm Hg.
The new definition of hypertension
The new definition of hypertension is of particular importance for people at risk for developing cardiovascular disease (CVD) 1,2 and is summarized here.
- Normal BP: systolic BP (SBP) <120 mm Hg and diastolic BP (DBP) <80 mm Hg
- Elevated BP: SBP 120–129 mm Hg and DBP <80 mm Hg
- Stage 1 hypertension: SBP 130–139 mm Hg or DBP 80–89 mm Hg.
- Stage 2 hypertension: SBP ≥140 mm Hg or DBP ≥90 mm Hg.
The new definition of hypertension will markedly increase the number of mid-life adults eligible to be treated for hypertension. I summarize the approach to treating hypertension in this article.
For mid-life adults, a SBP of <120 mm Hg is better for the heart
The heart is a pump, and not surprisingly, if a pump can achieve its job at a lower rather than a higher pressure, it is likely to last longer. The SPRINT study clearly demonstrated that in elderly hypertensive adults, an SBP target of <120 mm Hg is associated with fewer deaths than a SBP in the range of 130 to 140 mm Hg.3
In the SPRINT trial, 9,361 people with a mean age, body mass index, and BP of 68 years, 30 kg/m2 and 140/78 mm Hg, respectively, were randomly assigned to intensive treatment of SBP to a goal of <120 mm Hg or to a target of <140 mm Hg. After 1 year of treatment, the intensive treatment group had a mean SBP of 121 mm Hg and the standard treatment group had a mean SBP of 136 mm Hg. To achieve a SBP <120 mm Hg, most patients required 3 antihypertensive medications, in contrast to the 2 antihypertensive medications typically needed to achieve a SBP in the range of 130 to 140 mm Hg.
After a median of 3.3 years of follow-up, significantly fewer deaths occurred in the intensive treatment group than in the standard treatment group, including deaths from all causes (3.3% vs 4.5%, P = .003) and deaths from CVD (0.8% vs 1.4%; P = .005). In addition, the risk of developing heart failure was lower in the intensive treatment than in the standard treatment group (1.3% vs 2.1%, P = .002). There was no difference between the 2 groups in the risk of stroke (1.3% vs 1.5%, P = .50) or myocardial infarction (2.1% vs 2.5%, P = .19). The rate of syncope was higher in the intensive treatment group (2.3% vs 1.7% in the standard treatment group, P = .05).3 Self-reported mental and physical health and satisfaction with treatment was similar in both groups.4
The investigators concluded that among people at risk for CVD, targeting a SBP of <120 mm Hg as compared to <140 mm Hg resulted in lower rates of heart failure and death, two clinically meaningful endpoints.
Read about nonpharmacologic interventions and antihypertensive medications to treat hypertension.
Diet and exercise
Nonpharmacologic interventions, including diet and exercise, are recommended for all people with a BP >120/80 mm Hg. In most situations, antihypertensive medications are not necessary if the patient has elevated BP (SBP 120–129 mm Hg and DBP <80 mm Hg) or Stage 1 hypertension (SBP 130–139 mm Hg or DBP 80–89 mm Hg) and a 10-year CVD risk of less than 10% using the ACC/AHA cardiovascular risk calculator5 (see http://www.cvriskcalcula tor.com/). For people at low risk for CVD, nonpharmacologic interventions, including diet and exercise, are often sufficient treatment.
The Dietary Approaches to Stop Hypertension (DASH) diet emphasizes increasing consumption of fruits, vegetables, low-fat dairy, whole-grains, fish, poultry, and nuts and decreasing the consumption of red meats, sugary drinks, sweets, sodium, and saturated and trans-fats. In randomized trials, the DASH diet is associated with a reduction in BP of approximately 5 mm Hg systolic and 3 mm Hg diastolic.6 The DASH trial monitored weight changes and adjusted calorie intake to ensure a stabilized weight throughout the study. Hence, the positive effect of the DASH diet was observed in the absence of any weight loss. Weight loss also can decrease BP with every 1- to 2-lb weight loss, reducing SBP by approximately 1 mm Hg.7 Combining the DASH diet with a low-sodium diet is especially important in people with high sodium intake, and is reported to reduce SBP by 5 to 20 mm Hg.8 Reducing the consumption of alcohol can result in a reduction of SBP and DBP in the range of 3 and 2 mm Hg, respectively.9
Exercising for 40 minutes, 3 to 4 times per week is associated with a reduction of SBP and DBP of approximately 5 and 3 mm Hg, respectively.10 Although the studies are of low quality, meditation is reported to decrease SBP and DBP by 4 and 2 mm Hg, respectively.11
Antihypertensive medications
For all mid-life adults with Stage 2hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg) or with both clinical CVD and Stage 1 hypertension, antihypertensive medications are recommended.1 For people with Stage 1 hypertension and a 10-year CVD risk of ≥10%, antihypertensive medications are recommended. The target BP is <130/80 mm Hg for most people.
The recommended antihypertensive medications include thiazide diuretics, calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs). Many patients with Stage 2 hypertension will need treatment with 2 agents of different classes to achieve a BP <130/80 mm Hg. Some experts believe that an optimal 2-agent regimen includes an ACE or ARB plus a CCB based on the results of the ACCOMPLISH trial.12 In this trial, 11,506 adults with hypertension and at very high risk for CVD, were randomly assigned to treatment with an ACE inhibitor plus CCB or with an ACE inhibitor plus hydrochlorothiazide. The BP achieved in both groups was approximately 132/73 mm Hg. The study was stopped after 3 years because participants in the ACE/thiazide group had a higher rate of adverse cardiovascular events (myocardial infarction, stroke, or death) than those in the ACE/CCB group (11.8% vs 9.6%; hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.72–0.90; P<.001). Compared to the ACE/thiazide group, the ACE/CCB group had a significantly lower rate of fatal and nonfatal myocardial infarction (2.2% vs 2.8%; HR, 0.78; 95% CI, 0.62–0.99; P = .04) and a lower rate of death from cardiovascular causes (1.9% vs 2.3%; HR, 0.80; 95% CI, 0.62–1.03, P = .08).
Worldwide, approximately 1 billion adults have a SBP ≥140 mm Hg.13 In the United States, 32% of adult women have Stage 2 hypertension or are taking an antihypertensive medication (TABLE).1 There is a generally linear relationship between increasing SBP and DBP and an increased risk of a cardiovascular event, including heart failure, myocardial infarction, and stroke. An increase of SBP of 20 mm Hg or DBP of 10 mm Hg above a baseline BP of 115/75 mm Hg doubles the risk of death from CVD.14 For adults at risk for CVD, intensive treatment of hypertension clearly reduces the risk of a life-changing cardiovascular event.

It will probably take many years for the new SBP target of <120 mm Hg to be fully accepted by clinicians and patients because, although achieving a SBP of <120 mm Hg will decrease cardiovascular events, it is a very difficult target to achieve, requiring treatment with 3 antihypertensive medications for most patients. The early diagnosis and intensive treatment of hypertension is challenging because it requires clinicians to initiate a multi-decade course of treatment of asymptomatic people with the goal of preventing a life-altering cardiovascular event, including stroke and myocardial infarction.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published online ahead of print November 7, 2017]. J Am Coll Cardiol. doi:10.1016/j.jacc.2017.11.005.
- Cifu AS, Davis AM. Prevention, detection, evaluation and management of high blood pressure in adults. JAMA. 2017;318(21):2132–2134.
- Wright JT Jr, Williamson JD, Whelton PK; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116.
- Berlowitz DR, Foy CG, Kazis LE, et al; SPRINT Research Group. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med. 2017;377(8):733–744.
- American College of Cardiology and American Heart Association. Heart risk calculator. http://www.cvriskcalculator.com/. Accessed January 22, 2018.
- Moore TJ, Vollmer WM, Appel LJ, et al. Effect of dietary patterns of ambulatory blood pressure results from the Dietary Approaches to Stop Hypertension (DASH) Trial. DASH Collaborative Research Group. Hypertension. 1999;34(3):472–477.
- Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153(7):849–858.
- Juraschek SP, Miller ER, Weaver CM, Appel LJ. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017;70(23):2841–2848.
- Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38(5):1112–1117.
- Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens. 2013;31(4):639–648.
- Bai Z, Chang J, Chen C, Li P, Yang K, Chi I. Investigating the effect of transcendental meditation on blood pressure: a systematic review and meta-analysis. J Hum Hypertens. 2015;29(11):653–662.
- Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428.
- Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317(2):165–182.
- Swedish Council on Health Technology Assessment. Moderately elevated blood pressure: a systematic review. https://www.ncbi.nlm.nih.gov/books/NBK448011/. Published September 2008. Accessed January 22, 2018.
For many years, the approach to the diagnosis of hypertension was straight-forward—multiple blood pressure (BP) measurements ≥140/90 mm Hg established the diagnosis of hypertension, a disease associated with an increased risk of adverse cardiovascular events, including myocardial infarction and stroke. For more than a decade, hypertension experts have argued that a BP ≥130/80 mm Hg should establish the diagnosis of hypertension. Many clinicians resisted the change because it would markedly increase the number of asymptomatic adults with the diagnosis, increasing the number needing treatment. However, the findings of the Systolic Blood Pressure Intervention Trial (SPRINT) and other observational studies have catalyzed the American College of Cardiology (ACC) and the American Heart Association (AHA) to redefine normal BP as <120/80 mm Hg.1 This change will expand the diagnosis of hypertension to include up to 50% of American adults.1 In addition, the new definition of normal BP will result in the greater use of lifestyle interventions and antihypertensive medications to achieve the new normal, a BP of <120/80 mm Hg.
The new definition of hypertension
The new definition of hypertension is of particular importance for people at risk for developing cardiovascular disease (CVD) 1,2 and is summarized here.
- Normal BP: systolic BP (SBP) <120 mm Hg and diastolic BP (DBP) <80 mm Hg
- Elevated BP: SBP 120–129 mm Hg and DBP <80 mm Hg
- Stage 1 hypertension: SBP 130–139 mm Hg or DBP 80–89 mm Hg.
- Stage 2 hypertension: SBP ≥140 mm Hg or DBP ≥90 mm Hg.
The new definition of hypertension will markedly increase the number of mid-life adults eligible to be treated for hypertension. I summarize the approach to treating hypertension in this article.
For mid-life adults, a SBP of <120 mm Hg is better for the heart
The heart is a pump, and not surprisingly, if a pump can achieve its job at a lower rather than a higher pressure, it is likely to last longer. The SPRINT study clearly demonstrated that in elderly hypertensive adults, an SBP target of <120 mm Hg is associated with fewer deaths than a SBP in the range of 130 to 140 mm Hg.3
In the SPRINT trial, 9,361 people with a mean age, body mass index, and BP of 68 years, 30 kg/m2 and 140/78 mm Hg, respectively, were randomly assigned to intensive treatment of SBP to a goal of <120 mm Hg or to a target of <140 mm Hg. After 1 year of treatment, the intensive treatment group had a mean SBP of 121 mm Hg and the standard treatment group had a mean SBP of 136 mm Hg. To achieve a SBP <120 mm Hg, most patients required 3 antihypertensive medications, in contrast to the 2 antihypertensive medications typically needed to achieve a SBP in the range of 130 to 140 mm Hg.
After a median of 3.3 years of follow-up, significantly fewer deaths occurred in the intensive treatment group than in the standard treatment group, including deaths from all causes (3.3% vs 4.5%, P = .003) and deaths from CVD (0.8% vs 1.4%; P = .005). In addition, the risk of developing heart failure was lower in the intensive treatment than in the standard treatment group (1.3% vs 2.1%, P = .002). There was no difference between the 2 groups in the risk of stroke (1.3% vs 1.5%, P = .50) or myocardial infarction (2.1% vs 2.5%, P = .19). The rate of syncope was higher in the intensive treatment group (2.3% vs 1.7% in the standard treatment group, P = .05).3 Self-reported mental and physical health and satisfaction with treatment was similar in both groups.4
The investigators concluded that among people at risk for CVD, targeting a SBP of <120 mm Hg as compared to <140 mm Hg resulted in lower rates of heart failure and death, two clinically meaningful endpoints.
Read about nonpharmacologic interventions and antihypertensive medications to treat hypertension.
Diet and exercise
Nonpharmacologic interventions, including diet and exercise, are recommended for all people with a BP >120/80 mm Hg. In most situations, antihypertensive medications are not necessary if the patient has elevated BP (SBP 120–129 mm Hg and DBP <80 mm Hg) or Stage 1 hypertension (SBP 130–139 mm Hg or DBP 80–89 mm Hg) and a 10-year CVD risk of less than 10% using the ACC/AHA cardiovascular risk calculator5 (see http://www.cvriskcalcula tor.com/). For people at low risk for CVD, nonpharmacologic interventions, including diet and exercise, are often sufficient treatment.
The Dietary Approaches to Stop Hypertension (DASH) diet emphasizes increasing consumption of fruits, vegetables, low-fat dairy, whole-grains, fish, poultry, and nuts and decreasing the consumption of red meats, sugary drinks, sweets, sodium, and saturated and trans-fats. In randomized trials, the DASH diet is associated with a reduction in BP of approximately 5 mm Hg systolic and 3 mm Hg diastolic.6 The DASH trial monitored weight changes and adjusted calorie intake to ensure a stabilized weight throughout the study. Hence, the positive effect of the DASH diet was observed in the absence of any weight loss. Weight loss also can decrease BP with every 1- to 2-lb weight loss, reducing SBP by approximately 1 mm Hg.7 Combining the DASH diet with a low-sodium diet is especially important in people with high sodium intake, and is reported to reduce SBP by 5 to 20 mm Hg.8 Reducing the consumption of alcohol can result in a reduction of SBP and DBP in the range of 3 and 2 mm Hg, respectively.9
Exercising for 40 minutes, 3 to 4 times per week is associated with a reduction of SBP and DBP of approximately 5 and 3 mm Hg, respectively.10 Although the studies are of low quality, meditation is reported to decrease SBP and DBP by 4 and 2 mm Hg, respectively.11
Antihypertensive medications
For all mid-life adults with Stage 2hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg) or with both clinical CVD and Stage 1 hypertension, antihypertensive medications are recommended.1 For people with Stage 1 hypertension and a 10-year CVD risk of ≥10%, antihypertensive medications are recommended. The target BP is <130/80 mm Hg for most people.
The recommended antihypertensive medications include thiazide diuretics, calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs). Many patients with Stage 2 hypertension will need treatment with 2 agents of different classes to achieve a BP <130/80 mm Hg. Some experts believe that an optimal 2-agent regimen includes an ACE or ARB plus a CCB based on the results of the ACCOMPLISH trial.12 In this trial, 11,506 adults with hypertension and at very high risk for CVD, were randomly assigned to treatment with an ACE inhibitor plus CCB or with an ACE inhibitor plus hydrochlorothiazide. The BP achieved in both groups was approximately 132/73 mm Hg. The study was stopped after 3 years because participants in the ACE/thiazide group had a higher rate of adverse cardiovascular events (myocardial infarction, stroke, or death) than those in the ACE/CCB group (11.8% vs 9.6%; hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.72–0.90; P<.001). Compared to the ACE/thiazide group, the ACE/CCB group had a significantly lower rate of fatal and nonfatal myocardial infarction (2.2% vs 2.8%; HR, 0.78; 95% CI, 0.62–0.99; P = .04) and a lower rate of death from cardiovascular causes (1.9% vs 2.3%; HR, 0.80; 95% CI, 0.62–1.03, P = .08).
Worldwide, approximately 1 billion adults have a SBP ≥140 mm Hg.13 In the United States, 32% of adult women have Stage 2 hypertension or are taking an antihypertensive medication (TABLE).1 There is a generally linear relationship between increasing SBP and DBP and an increased risk of a cardiovascular event, including heart failure, myocardial infarction, and stroke. An increase of SBP of 20 mm Hg or DBP of 10 mm Hg above a baseline BP of 115/75 mm Hg doubles the risk of death from CVD.14 For adults at risk for CVD, intensive treatment of hypertension clearly reduces the risk of a life-changing cardiovascular event.

It will probably take many years for the new SBP target of <120 mm Hg to be fully accepted by clinicians and patients because, although achieving a SBP of <120 mm Hg will decrease cardiovascular events, it is a very difficult target to achieve, requiring treatment with 3 antihypertensive medications for most patients. The early diagnosis and intensive treatment of hypertension is challenging because it requires clinicians to initiate a multi-decade course of treatment of asymptomatic people with the goal of preventing a life-altering cardiovascular event, including stroke and myocardial infarction.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
For many years, the approach to the diagnosis of hypertension was straight-forward—multiple blood pressure (BP) measurements ≥140/90 mm Hg established the diagnosis of hypertension, a disease associated with an increased risk of adverse cardiovascular events, including myocardial infarction and stroke. For more than a decade, hypertension experts have argued that a BP ≥130/80 mm Hg should establish the diagnosis of hypertension. Many clinicians resisted the change because it would markedly increase the number of asymptomatic adults with the diagnosis, increasing the number needing treatment. However, the findings of the Systolic Blood Pressure Intervention Trial (SPRINT) and other observational studies have catalyzed the American College of Cardiology (ACC) and the American Heart Association (AHA) to redefine normal BP as <120/80 mm Hg.1 This change will expand the diagnosis of hypertension to include up to 50% of American adults.1 In addition, the new definition of normal BP will result in the greater use of lifestyle interventions and antihypertensive medications to achieve the new normal, a BP of <120/80 mm Hg.
The new definition of hypertension
The new definition of hypertension is of particular importance for people at risk for developing cardiovascular disease (CVD) 1,2 and is summarized here.
- Normal BP: systolic BP (SBP) <120 mm Hg and diastolic BP (DBP) <80 mm Hg
- Elevated BP: SBP 120–129 mm Hg and DBP <80 mm Hg
- Stage 1 hypertension: SBP 130–139 mm Hg or DBP 80–89 mm Hg.
- Stage 2 hypertension: SBP ≥140 mm Hg or DBP ≥90 mm Hg.
The new definition of hypertension will markedly increase the number of mid-life adults eligible to be treated for hypertension. I summarize the approach to treating hypertension in this article.
For mid-life adults, a SBP of <120 mm Hg is better for the heart
The heart is a pump, and not surprisingly, if a pump can achieve its job at a lower rather than a higher pressure, it is likely to last longer. The SPRINT study clearly demonstrated that in elderly hypertensive adults, an SBP target of <120 mm Hg is associated with fewer deaths than a SBP in the range of 130 to 140 mm Hg.3
In the SPRINT trial, 9,361 people with a mean age, body mass index, and BP of 68 years, 30 kg/m2 and 140/78 mm Hg, respectively, were randomly assigned to intensive treatment of SBP to a goal of <120 mm Hg or to a target of <140 mm Hg. After 1 year of treatment, the intensive treatment group had a mean SBP of 121 mm Hg and the standard treatment group had a mean SBP of 136 mm Hg. To achieve a SBP <120 mm Hg, most patients required 3 antihypertensive medications, in contrast to the 2 antihypertensive medications typically needed to achieve a SBP in the range of 130 to 140 mm Hg.
After a median of 3.3 years of follow-up, significantly fewer deaths occurred in the intensive treatment group than in the standard treatment group, including deaths from all causes (3.3% vs 4.5%, P = .003) and deaths from CVD (0.8% vs 1.4%; P = .005). In addition, the risk of developing heart failure was lower in the intensive treatment than in the standard treatment group (1.3% vs 2.1%, P = .002). There was no difference between the 2 groups in the risk of stroke (1.3% vs 1.5%, P = .50) or myocardial infarction (2.1% vs 2.5%, P = .19). The rate of syncope was higher in the intensive treatment group (2.3% vs 1.7% in the standard treatment group, P = .05).3 Self-reported mental and physical health and satisfaction with treatment was similar in both groups.4
The investigators concluded that among people at risk for CVD, targeting a SBP of <120 mm Hg as compared to <140 mm Hg resulted in lower rates of heart failure and death, two clinically meaningful endpoints.
Read about nonpharmacologic interventions and antihypertensive medications to treat hypertension.
Diet and exercise
Nonpharmacologic interventions, including diet and exercise, are recommended for all people with a BP >120/80 mm Hg. In most situations, antihypertensive medications are not necessary if the patient has elevated BP (SBP 120–129 mm Hg and DBP <80 mm Hg) or Stage 1 hypertension (SBP 130–139 mm Hg or DBP 80–89 mm Hg) and a 10-year CVD risk of less than 10% using the ACC/AHA cardiovascular risk calculator5 (see http://www.cvriskcalcula tor.com/). For people at low risk for CVD, nonpharmacologic interventions, including diet and exercise, are often sufficient treatment.
The Dietary Approaches to Stop Hypertension (DASH) diet emphasizes increasing consumption of fruits, vegetables, low-fat dairy, whole-grains, fish, poultry, and nuts and decreasing the consumption of red meats, sugary drinks, sweets, sodium, and saturated and trans-fats. In randomized trials, the DASH diet is associated with a reduction in BP of approximately 5 mm Hg systolic and 3 mm Hg diastolic.6 The DASH trial monitored weight changes and adjusted calorie intake to ensure a stabilized weight throughout the study. Hence, the positive effect of the DASH diet was observed in the absence of any weight loss. Weight loss also can decrease BP with every 1- to 2-lb weight loss, reducing SBP by approximately 1 mm Hg.7 Combining the DASH diet with a low-sodium diet is especially important in people with high sodium intake, and is reported to reduce SBP by 5 to 20 mm Hg.8 Reducing the consumption of alcohol can result in a reduction of SBP and DBP in the range of 3 and 2 mm Hg, respectively.9
Exercising for 40 minutes, 3 to 4 times per week is associated with a reduction of SBP and DBP of approximately 5 and 3 mm Hg, respectively.10 Although the studies are of low quality, meditation is reported to decrease SBP and DBP by 4 and 2 mm Hg, respectively.11
Antihypertensive medications
For all mid-life adults with Stage 2hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg) or with both clinical CVD and Stage 1 hypertension, antihypertensive medications are recommended.1 For people with Stage 1 hypertension and a 10-year CVD risk of ≥10%, antihypertensive medications are recommended. The target BP is <130/80 mm Hg for most people.
The recommended antihypertensive medications include thiazide diuretics, calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs). Many patients with Stage 2 hypertension will need treatment with 2 agents of different classes to achieve a BP <130/80 mm Hg. Some experts believe that an optimal 2-agent regimen includes an ACE or ARB plus a CCB based on the results of the ACCOMPLISH trial.12 In this trial, 11,506 adults with hypertension and at very high risk for CVD, were randomly assigned to treatment with an ACE inhibitor plus CCB or with an ACE inhibitor plus hydrochlorothiazide. The BP achieved in both groups was approximately 132/73 mm Hg. The study was stopped after 3 years because participants in the ACE/thiazide group had a higher rate of adverse cardiovascular events (myocardial infarction, stroke, or death) than those in the ACE/CCB group (11.8% vs 9.6%; hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.72–0.90; P<.001). Compared to the ACE/thiazide group, the ACE/CCB group had a significantly lower rate of fatal and nonfatal myocardial infarction (2.2% vs 2.8%; HR, 0.78; 95% CI, 0.62–0.99; P = .04) and a lower rate of death from cardiovascular causes (1.9% vs 2.3%; HR, 0.80; 95% CI, 0.62–1.03, P = .08).
Worldwide, approximately 1 billion adults have a SBP ≥140 mm Hg.13 In the United States, 32% of adult women have Stage 2 hypertension or are taking an antihypertensive medication (TABLE).1 There is a generally linear relationship between increasing SBP and DBP and an increased risk of a cardiovascular event, including heart failure, myocardial infarction, and stroke. An increase of SBP of 20 mm Hg or DBP of 10 mm Hg above a baseline BP of 115/75 mm Hg doubles the risk of death from CVD.14 For adults at risk for CVD, intensive treatment of hypertension clearly reduces the risk of a life-changing cardiovascular event.

It will probably take many years for the new SBP target of <120 mm Hg to be fully accepted by clinicians and patients because, although achieving a SBP of <120 mm Hg will decrease cardiovascular events, it is a very difficult target to achieve, requiring treatment with 3 antihypertensive medications for most patients. The early diagnosis and intensive treatment of hypertension is challenging because it requires clinicians to initiate a multi-decade course of treatment of asymptomatic people with the goal of preventing a life-altering cardiovascular event, including stroke and myocardial infarction.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published online ahead of print November 7, 2017]. J Am Coll Cardiol. doi:10.1016/j.jacc.2017.11.005.
- Cifu AS, Davis AM. Prevention, detection, evaluation and management of high blood pressure in adults. JAMA. 2017;318(21):2132–2134.
- Wright JT Jr, Williamson JD, Whelton PK; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116.
- Berlowitz DR, Foy CG, Kazis LE, et al; SPRINT Research Group. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med. 2017;377(8):733–744.
- American College of Cardiology and American Heart Association. Heart risk calculator. http://www.cvriskcalculator.com/. Accessed January 22, 2018.
- Moore TJ, Vollmer WM, Appel LJ, et al. Effect of dietary patterns of ambulatory blood pressure results from the Dietary Approaches to Stop Hypertension (DASH) Trial. DASH Collaborative Research Group. Hypertension. 1999;34(3):472–477.
- Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153(7):849–858.
- Juraschek SP, Miller ER, Weaver CM, Appel LJ. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017;70(23):2841–2848.
- Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38(5):1112–1117.
- Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens. 2013;31(4):639–648.
- Bai Z, Chang J, Chen C, Li P, Yang K, Chi I. Investigating the effect of transcendental meditation on blood pressure: a systematic review and meta-analysis. J Hum Hypertens. 2015;29(11):653–662.
- Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428.
- Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317(2):165–182.
- Swedish Council on Health Technology Assessment. Moderately elevated blood pressure: a systematic review. https://www.ncbi.nlm.nih.gov/books/NBK448011/. Published September 2008. Accessed January 22, 2018.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published online ahead of print November 7, 2017]. J Am Coll Cardiol. doi:10.1016/j.jacc.2017.11.005.
- Cifu AS, Davis AM. Prevention, detection, evaluation and management of high blood pressure in adults. JAMA. 2017;318(21):2132–2134.
- Wright JT Jr, Williamson JD, Whelton PK; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116.
- Berlowitz DR, Foy CG, Kazis LE, et al; SPRINT Research Group. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med. 2017;377(8):733–744.
- American College of Cardiology and American Heart Association. Heart risk calculator. http://www.cvriskcalculator.com/. Accessed January 22, 2018.
- Moore TJ, Vollmer WM, Appel LJ, et al. Effect of dietary patterns of ambulatory blood pressure results from the Dietary Approaches to Stop Hypertension (DASH) Trial. DASH Collaborative Research Group. Hypertension. 1999;34(3):472–477.
- Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153(7):849–858.
- Juraschek SP, Miller ER, Weaver CM, Appel LJ. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017;70(23):2841–2848.
- Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38(5):1112–1117.
- Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens. 2013;31(4):639–648.
- Bai Z, Chang J, Chen C, Li P, Yang K, Chi I. Investigating the effect of transcendental meditation on blood pressure: a systematic review and meta-analysis. J Hum Hypertens. 2015;29(11):653–662.
- Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428.
- Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317(2):165–182.
- Swedish Council on Health Technology Assessment. Moderately elevated blood pressure: a systematic review. https://www.ncbi.nlm.nih.gov/books/NBK448011/. Published September 2008. Accessed January 22, 2018.
30 years in service to you, our community of women’s health clinicians
The mission of

OBG Management : Print and electronic portals for knowledge acquisition
Experienced clinicians acquire new knowledge and refresh established concepts through discussions with trusted colleagues and by reading journals and books that contain information relevant to their practice. A continuing trend in professional development is the accelerating transition from a reliance on print media (print journals and books) to electronic information delivery. Many clinicians continue to enjoy reading medical journals and magazines. ObGyns are no different; 96% report reading the print edition of medical journals.1 At
However, in the time-pressured setting of office- and hospital-based patient care, critical information is now frequently accessed through an electronic portal that is web based and focused on immediately answering a high priority question necessary for optimal patient care.
The information base needed to practice medicine is massive and continues to grow rapidly. No single print textbook or journal can cover this vast information base. Libraries of print material are cumbersome to use and ordinarily not accessible at the site of patient care. Electronic portals are the only means of providing immediate access to all medical knowledge. Electronic technology enables the aggregation of vast amounts of information in a database that is rapidly accessible from anywhere, and new search technology is making it easier to quickly locate the information you need.
The next frontier in medical information exchange is the application of artificial intelligence to cull “answers” from the vast aggregation of data. By combining all available medical information and artificial intelligence processes, in the near future, clinicians will be able to instantaneously get an answer to a question they have about how to care for a specific patient. A decade ago, when a question was entered into an Internet search engine, the response was typically a list of potential websites where the answer might be located. In the past few years, with the integration of huge databases and artificial intelligence, some advanced search engines now provide a specific answer to a question, followed by a list of relevant websites. For example, if you enter this question: “What countries have the greatest number of people?” into the Google search tool, in less than 1 second a direct answer is provided: “China has the world’s largest population (1.4 billion), followed by India (1.3 billion). The next most populous nations—the United States, Indonesia, Brazil and Pakistan—combined have less than 1 billion people.” The next step in medical information communication will be the deployment of artificial intelligence systems that can directly answer a query from a clinician about a specific patient.
Our distinguished Editorial Board and authors—the heart and mind of OBG Management
The editorial team at

Improving clinician wellness and resilience and reducing burnout
Clinicians throughout the world are reporting decreased levels of professional fulfillment and increased levels of burnout.2–4 This epidemic is likely caused by many factors, including the deployment of poorly designed electronic health systems, the administrative guidance for clinicians to work faster with fewer support staff, increasing administrative and secretarial burden on clinicians, and institutional constraints on clinician autonomy. Many of these problems only can be addressed at the level of the health system, but some are in the control of individual clinicians.
In the upcoming years,
The gratitude exercise
Showing more gratitude to those who have been most meaningful in your life may increase your wellness. Try the gratitude exercise outlined below.
To prepare for the exercise you will need about 15 minutes of uninterrupted time, a quiet room, and a method for recording your thoughts (pen/paper, electronic word processor, voice recorder).
Sit quietly and close your eyes. Spend 5 minutes thinking about the people in your life whose contributions have had the greatest positive impact on your development. Think deeply about the importance of their role in your life. Select one of those important people.
Open your eyes and spend 10 minutes expressing in writing your thoughts and feelings about that person. Once you have completed expressing yourself in writing, commit to reading your words, verbatim, to the person within the following 48 hours. This could be done by voice communication, video conferencing, or in-person.
The future of obstetrics and gynecology is bright
Medical students are electing to pursue a career in obstetrics and gynecology in record numbers. The students entering the field and the residents currently in training are superbly prepared and have demonstrated their commitment to advancing reproductive health by experiences in advocacy, research, and community service. We need to ensure that these super-star young physicians are able to have a 40-year career that is productive and fulfilling.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kantar Media. Sources and Interactions. Medical/Surgical Edition. Kantar Media; New York, New York; 2017.
- Dyrbye LN, West CP, Satele D, et al. Burnout among U.S. medical students, residents and early career physicians relative to the general U.S. population. Acad Med. 2014;89(3):443-451.
- Vandenbroeck S, Van Gerven E, De Witte H, Vanhaecht K, Godderis L. Burnout in Belgian physicians and nurses. Occup Med (London). 2017;67(7):546-554.
- Siu C, Yuen SK, Cheung A. Burnout among public doctors in Hong Kong: cross-sectional survey. Hong Kong Med J. 2012;18(3):186-192.
- Cheng ST, Tsui PK, Lam JH. Improving mental health in health care practitioners: randomized controlled trial of a gratitude intervention. J Consult Clin Psychol. 2015;83(1):177-186.
The mission of

OBG Management : Print and electronic portals for knowledge acquisition
Experienced clinicians acquire new knowledge and refresh established concepts through discussions with trusted colleagues and by reading journals and books that contain information relevant to their practice. A continuing trend in professional development is the accelerating transition from a reliance on print media (print journals and books) to electronic information delivery. Many clinicians continue to enjoy reading medical journals and magazines. ObGyns are no different; 96% report reading the print edition of medical journals.1 At
However, in the time-pressured setting of office- and hospital-based patient care, critical information is now frequently accessed through an electronic portal that is web based and focused on immediately answering a high priority question necessary for optimal patient care.
The information base needed to practice medicine is massive and continues to grow rapidly. No single print textbook or journal can cover this vast information base. Libraries of print material are cumbersome to use and ordinarily not accessible at the site of patient care. Electronic portals are the only means of providing immediate access to all medical knowledge. Electronic technology enables the aggregation of vast amounts of information in a database that is rapidly accessible from anywhere, and new search technology is making it easier to quickly locate the information you need.
The next frontier in medical information exchange is the application of artificial intelligence to cull “answers” from the vast aggregation of data. By combining all available medical information and artificial intelligence processes, in the near future, clinicians will be able to instantaneously get an answer to a question they have about how to care for a specific patient. A decade ago, when a question was entered into an Internet search engine, the response was typically a list of potential websites where the answer might be located. In the past few years, with the integration of huge databases and artificial intelligence, some advanced search engines now provide a specific answer to a question, followed by a list of relevant websites. For example, if you enter this question: “What countries have the greatest number of people?” into the Google search tool, in less than 1 second a direct answer is provided: “China has the world’s largest population (1.4 billion), followed by India (1.3 billion). The next most populous nations—the United States, Indonesia, Brazil and Pakistan—combined have less than 1 billion people.” The next step in medical information communication will be the deployment of artificial intelligence systems that can directly answer a query from a clinician about a specific patient.
Our distinguished Editorial Board and authors—the heart and mind of OBG Management
The editorial team at

Improving clinician wellness and resilience and reducing burnout
Clinicians throughout the world are reporting decreased levels of professional fulfillment and increased levels of burnout.2–4 This epidemic is likely caused by many factors, including the deployment of poorly designed electronic health systems, the administrative guidance for clinicians to work faster with fewer support staff, increasing administrative and secretarial burden on clinicians, and institutional constraints on clinician autonomy. Many of these problems only can be addressed at the level of the health system, but some are in the control of individual clinicians.
In the upcoming years,
The gratitude exercise
Showing more gratitude to those who have been most meaningful in your life may increase your wellness. Try the gratitude exercise outlined below.
To prepare for the exercise you will need about 15 minutes of uninterrupted time, a quiet room, and a method for recording your thoughts (pen/paper, electronic word processor, voice recorder).
Sit quietly and close your eyes. Spend 5 minutes thinking about the people in your life whose contributions have had the greatest positive impact on your development. Think deeply about the importance of their role in your life. Select one of those important people.
Open your eyes and spend 10 minutes expressing in writing your thoughts and feelings about that person. Once you have completed expressing yourself in writing, commit to reading your words, verbatim, to the person within the following 48 hours. This could be done by voice communication, video conferencing, or in-person.
The future of obstetrics and gynecology is bright
Medical students are electing to pursue a career in obstetrics and gynecology in record numbers. The students entering the field and the residents currently in training are superbly prepared and have demonstrated their commitment to advancing reproductive health by experiences in advocacy, research, and community service. We need to ensure that these super-star young physicians are able to have a 40-year career that is productive and fulfilling.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The mission of

OBG Management : Print and electronic portals for knowledge acquisition
Experienced clinicians acquire new knowledge and refresh established concepts through discussions with trusted colleagues and by reading journals and books that contain information relevant to their practice. A continuing trend in professional development is the accelerating transition from a reliance on print media (print journals and books) to electronic information delivery. Many clinicians continue to enjoy reading medical journals and magazines. ObGyns are no different; 96% report reading the print edition of medical journals.1 At
However, in the time-pressured setting of office- and hospital-based patient care, critical information is now frequently accessed through an electronic portal that is web based and focused on immediately answering a high priority question necessary for optimal patient care.
The information base needed to practice medicine is massive and continues to grow rapidly. No single print textbook or journal can cover this vast information base. Libraries of print material are cumbersome to use and ordinarily not accessible at the site of patient care. Electronic portals are the only means of providing immediate access to all medical knowledge. Electronic technology enables the aggregation of vast amounts of information in a database that is rapidly accessible from anywhere, and new search technology is making it easier to quickly locate the information you need.
The next frontier in medical information exchange is the application of artificial intelligence to cull “answers” from the vast aggregation of data. By combining all available medical information and artificial intelligence processes, in the near future, clinicians will be able to instantaneously get an answer to a question they have about how to care for a specific patient. A decade ago, when a question was entered into an Internet search engine, the response was typically a list of potential websites where the answer might be located. In the past few years, with the integration of huge databases and artificial intelligence, some advanced search engines now provide a specific answer to a question, followed by a list of relevant websites. For example, if you enter this question: “What countries have the greatest number of people?” into the Google search tool, in less than 1 second a direct answer is provided: “China has the world’s largest population (1.4 billion), followed by India (1.3 billion). The next most populous nations—the United States, Indonesia, Brazil and Pakistan—combined have less than 1 billion people.” The next step in medical information communication will be the deployment of artificial intelligence systems that can directly answer a query from a clinician about a specific patient.
Our distinguished Editorial Board and authors—the heart and mind of OBG Management
The editorial team at

Improving clinician wellness and resilience and reducing burnout
Clinicians throughout the world are reporting decreased levels of professional fulfillment and increased levels of burnout.2–4 This epidemic is likely caused by many factors, including the deployment of poorly designed electronic health systems, the administrative guidance for clinicians to work faster with fewer support staff, increasing administrative and secretarial burden on clinicians, and institutional constraints on clinician autonomy. Many of these problems only can be addressed at the level of the health system, but some are in the control of individual clinicians.
In the upcoming years,
The gratitude exercise
Showing more gratitude to those who have been most meaningful in your life may increase your wellness. Try the gratitude exercise outlined below.
To prepare for the exercise you will need about 15 minutes of uninterrupted time, a quiet room, and a method for recording your thoughts (pen/paper, electronic word processor, voice recorder).
Sit quietly and close your eyes. Spend 5 minutes thinking about the people in your life whose contributions have had the greatest positive impact on your development. Think deeply about the importance of their role in your life. Select one of those important people.
Open your eyes and spend 10 minutes expressing in writing your thoughts and feelings about that person. Once you have completed expressing yourself in writing, commit to reading your words, verbatim, to the person within the following 48 hours. This could be done by voice communication, video conferencing, or in-person.
The future of obstetrics and gynecology is bright
Medical students are electing to pursue a career in obstetrics and gynecology in record numbers. The students entering the field and the residents currently in training are superbly prepared and have demonstrated their commitment to advancing reproductive health by experiences in advocacy, research, and community service. We need to ensure that these super-star young physicians are able to have a 40-year career that is productive and fulfilling.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kantar Media. Sources and Interactions. Medical/Surgical Edition. Kantar Media; New York, New York; 2017.
- Dyrbye LN, West CP, Satele D, et al. Burnout among U.S. medical students, residents and early career physicians relative to the general U.S. population. Acad Med. 2014;89(3):443-451.
- Vandenbroeck S, Van Gerven E, De Witte H, Vanhaecht K, Godderis L. Burnout in Belgian physicians and nurses. Occup Med (London). 2017;67(7):546-554.
- Siu C, Yuen SK, Cheung A. Burnout among public doctors in Hong Kong: cross-sectional survey. Hong Kong Med J. 2012;18(3):186-192.
- Cheng ST, Tsui PK, Lam JH. Improving mental health in health care practitioners: randomized controlled trial of a gratitude intervention. J Consult Clin Psychol. 2015;83(1):177-186.
- Kantar Media. Sources and Interactions. Medical/Surgical Edition. Kantar Media; New York, New York; 2017.
- Dyrbye LN, West CP, Satele D, et al. Burnout among U.S. medical students, residents and early career physicians relative to the general U.S. population. Acad Med. 2014;89(3):443-451.
- Vandenbroeck S, Van Gerven E, De Witte H, Vanhaecht K, Godderis L. Burnout in Belgian physicians and nurses. Occup Med (London). 2017;67(7):546-554.
- Siu C, Yuen SK, Cheung A. Burnout among public doctors in Hong Kong: cross-sectional survey. Hong Kong Med J. 2012;18(3):186-192.
- Cheng ST, Tsui PK, Lam JH. Improving mental health in health care practitioners: randomized controlled trial of a gratitude intervention. J Consult Clin Psychol. 2015;83(1):177-186.