User login
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6
One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.
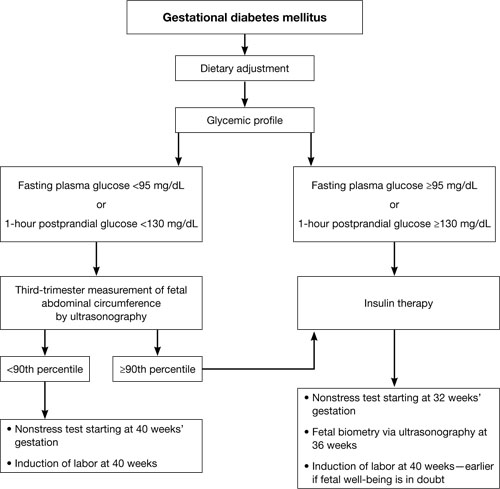
Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6

One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.

Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6

One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.

Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
