User login
Aortic repair in Loeys-Dietz syndrome requires close follow-up
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005. Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths.
Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years.
“In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy.
Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
This report by Dr. Patel and his coauthors confirms the need for close surveillance of individuals with Loeys-Dietz syndrome who have had aortic operations, John S. Ikonomidis, MD, PhD, of the Medical University of South Carolina, Charleston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:413-4).
Dr. Ikonomidis noted this study is important because of its population size. “This is probably the largest single-center surgical report of this kind in the world,” he said.
The study highlighted a number of issues germane to LDS patients who have cardiovascular surgery, among them a critical need for genetic testing to help cardiac surgeons determine the disease genotype and what operation to perform, Dr. Ikonomidis said.
But Dr. Ikonomidis also pointed out the variation in aortic root size in the study patients. The smallest root in the series was 2 cm and 21 of 65 patients with a maximum root diameter smaller than 4 cm had root surgery. “This is a testament to the fact that surgical decision making in this population is dependent not just on the known genotype and aortic dimensions, but also on the rate of growth, aortic valve function, severity of noncardiac phenotype, and family history,” Dr. Ikonomidis said.
Dr. Ikonomidis had no financial relationships to disclose.
This report by Dr. Patel and his coauthors confirms the need for close surveillance of individuals with Loeys-Dietz syndrome who have had aortic operations, John S. Ikonomidis, MD, PhD, of the Medical University of South Carolina, Charleston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:413-4).
Dr. Ikonomidis noted this study is important because of its population size. “This is probably the largest single-center surgical report of this kind in the world,” he said.
The study highlighted a number of issues germane to LDS patients who have cardiovascular surgery, among them a critical need for genetic testing to help cardiac surgeons determine the disease genotype and what operation to perform, Dr. Ikonomidis said.
But Dr. Ikonomidis also pointed out the variation in aortic root size in the study patients. The smallest root in the series was 2 cm and 21 of 65 patients with a maximum root diameter smaller than 4 cm had root surgery. “This is a testament to the fact that surgical decision making in this population is dependent not just on the known genotype and aortic dimensions, but also on the rate of growth, aortic valve function, severity of noncardiac phenotype, and family history,” Dr. Ikonomidis said.
Dr. Ikonomidis had no financial relationships to disclose.
This report by Dr. Patel and his coauthors confirms the need for close surveillance of individuals with Loeys-Dietz syndrome who have had aortic operations, John S. Ikonomidis, MD, PhD, of the Medical University of South Carolina, Charleston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:413-4).
Dr. Ikonomidis noted this study is important because of its population size. “This is probably the largest single-center surgical report of this kind in the world,” he said.
The study highlighted a number of issues germane to LDS patients who have cardiovascular surgery, among them a critical need for genetic testing to help cardiac surgeons determine the disease genotype and what operation to perform, Dr. Ikonomidis said.
But Dr. Ikonomidis also pointed out the variation in aortic root size in the study patients. The smallest root in the series was 2 cm and 21 of 65 patients with a maximum root diameter smaller than 4 cm had root surgery. “This is a testament to the fact that surgical decision making in this population is dependent not just on the known genotype and aortic dimensions, but also on the rate of growth, aortic valve function, severity of noncardiac phenotype, and family history,” Dr. Ikonomidis said.
Dr. Ikonomidis had no financial relationships to disclose.
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005. Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths.
Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years.
“In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy.
Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005. Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths.
Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years.
“In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy.
Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
Key clinical point: Outcomes for aortic surgery in Loeys-Dietz syndrome are favorable, but reintervention rates are high.
Major finding: Patients require close postoperative follow-up with cardiovascular imaging.
Data source: Retrospective review of 79 patients who had cardiovascular surgery for LDS over 26 years at Johns Hopkins University.
Disclosure: Dr. Patel and his coauthors reported having no relevant financial disclosures.
Can a nomogram foretell invasive pulmonary adenocarcinoma?
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
EXPERT ANALYSIS FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Investigators developed a nomogram that may help predict the risk of invasive pulmonary adenocarcinoma for patients with a solitary peripheral subsolid nodule.
Major finding: This nomogram may help clinicians individualize each patient’s prognosis for invasive pulmonary adenocarcinoma and develop treatment plans accordingly.
Data source: Retrospective analysis of 273 consecutive patients who had surgery to remove a solitary peripheral subsolid nodule at a single center.
Disclosure: The investigators received support from the Zhejiang Province Science and Technology Plan. Dr. Jin and Dr. Cao reported having no relevant financial disclosures.
Will genome editing advance animal-to-human transplantation?
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
Key clinical point: CRISPR/Cas9 genome editing is advancing the creation of animal models for xenotransplantation into humans.
Major finding: Genome editing tools are moving xenotransplantation models quickly toward potential treatments for cardiopulmonary disease.
Data source: Expert opinion with literature review.
Disclosures: Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler reported having no relevant financial disclosures.
Pairing vascular reconstruction, pancreatic cancer resection
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: A more aggressive vascular resection and reconstruction in pancreatic cancer may improve outcomes and palliation in these patients.
Major finding: Mean survival with lateral venorrhaphy exceeded primary anastomosis and interposition graft (21 months vs. 13 months vs. 4 months).
Data source: Updated data of previously published single-center retrospective review of 183 patients who had Whipple procedure for pancreatic adenocarcinoma.
Disclosures: Dr. Fujitani reported having no financial disclosures.
VQI confirms improvements in vascular practice
CHICAGO – Five years after the Society for Vascular Surgery launched the Vascular Quality Initiative, participating centers are more likely to use chlorhexidine and have also cut their surgery times and reduced their transfusion rates, according to results presented at a symposium on vascular surgery sponsored by Northwestern University.
But more drastic have been the improvements once low-performing centers have made in these measures and others, Larry Kraiss, MD, of the University of Utah, Salt Lake City, said in reporting an update on VQI. “If you look at centers that had a big change in not using chlorhexidine to using chlorhexidine, the reduction of surgical site infections [SSI] in that subgroup was actually pretty significant,” said Dr. Kraiss, chair of the governing council of the SVS Patient Safety Organization, which oversees VQI.
These pivotal improvements came about after the VQI distributed what it calls COPI reports – for Center Opportunity Profile for Improvement – to participating centers. Currently, 379 centers in 46 states and Ontario participate in VQI, feeding data into 12 different vascular procedure registries ranging from peripheral vascular interventions to lower-extremity amputations. As of Nov. 1, 2016, 330,400 procedures had been submitted to VQI.
Dr. Kraiss called the COPI report the “workhorse” of the VQI. “It can give participating centers insight into what they can do to improve outcomes,” he said. It is one of three types of reports VQI provides. The others are benchmarking reports that show the masked ratings for all participating centers but confidentially highlight the rating of the individual center receiving the report; and reports for individual providers.
The most recent readout of the SSI COPI report compared measures in two periods: 2011-2012 and 2013-2014. In those periods, overall use of chlorhexidine rose from 66.6% to 81.2%; transfusion rates of more than 2 units fell from 14.4% to 11.5%; the share of procedures lasting 220 minutes or more fell from 50.2% to 47.7%; and SSI rate overall fell from 3.4% to 3.1%. While the change in SSI was not statistically significant, Dr. Kraiss said the 17 centers that had a large increase in chlorhexidine use did see statistically significant declines in SSI.
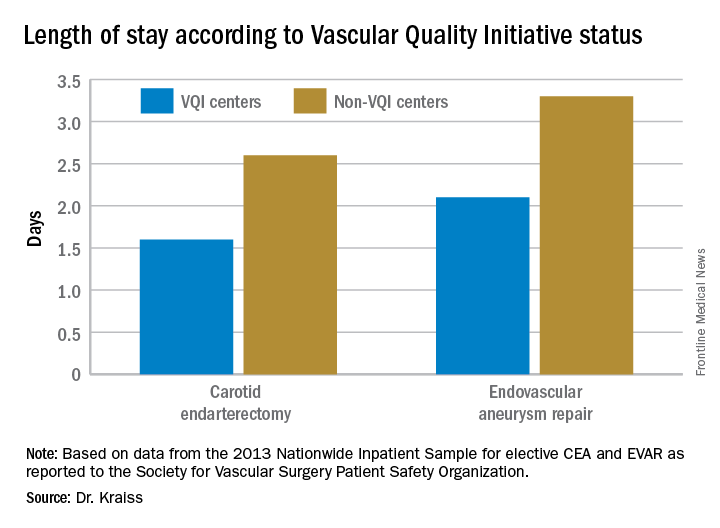
VQI also showed a 5-year survival rate of 79% of patients discharged with both statin and aspirin therapy vs. 61% for patients discharged without (J Vasc Surg. 2015;61[4]:1010-9). “This represents an opportunity to inform individual providers about how often they discharge patients on an aspirin and statin,” Dr. Kraiss said. Provider-targeted reports show how individual physicians rate in their region and nationwide.
VQI is more than a registry, Dr. Kraiss said; it’s also organized into 17 regional quality groups that provide surgeons a safe place to discuss VQI data and how to use that to encourage best practices. “There’s no risk of compromising or making the information identifiable,” he said. “It’s a matter of just getting together and trying to share best practices in a relatively informal environment, and hopefully through that drive quality improvement.
Other benefits of participating in VQI are that it can help surgeons comply with requirements for Medicare’s Merit-based Incentive Payment System (MIPS). VQI also offers opportunities to enroll in industry-sponsored clinical trials, which can help defray the cost of VQI participation, he said.
Dr. Kraiss had no relevant financial relationships to disclose.
CHICAGO – Five years after the Society for Vascular Surgery launched the Vascular Quality Initiative, participating centers are more likely to use chlorhexidine and have also cut their surgery times and reduced their transfusion rates, according to results presented at a symposium on vascular surgery sponsored by Northwestern University.
But more drastic have been the improvements once low-performing centers have made in these measures and others, Larry Kraiss, MD, of the University of Utah, Salt Lake City, said in reporting an update on VQI. “If you look at centers that had a big change in not using chlorhexidine to using chlorhexidine, the reduction of surgical site infections [SSI] in that subgroup was actually pretty significant,” said Dr. Kraiss, chair of the governing council of the SVS Patient Safety Organization, which oversees VQI.
These pivotal improvements came about after the VQI distributed what it calls COPI reports – for Center Opportunity Profile for Improvement – to participating centers. Currently, 379 centers in 46 states and Ontario participate in VQI, feeding data into 12 different vascular procedure registries ranging from peripheral vascular interventions to lower-extremity amputations. As of Nov. 1, 2016, 330,400 procedures had been submitted to VQI.
Dr. Kraiss called the COPI report the “workhorse” of the VQI. “It can give participating centers insight into what they can do to improve outcomes,” he said. It is one of three types of reports VQI provides. The others are benchmarking reports that show the masked ratings for all participating centers but confidentially highlight the rating of the individual center receiving the report; and reports for individual providers.
The most recent readout of the SSI COPI report compared measures in two periods: 2011-2012 and 2013-2014. In those periods, overall use of chlorhexidine rose from 66.6% to 81.2%; transfusion rates of more than 2 units fell from 14.4% to 11.5%; the share of procedures lasting 220 minutes or more fell from 50.2% to 47.7%; and SSI rate overall fell from 3.4% to 3.1%. While the change in SSI was not statistically significant, Dr. Kraiss said the 17 centers that had a large increase in chlorhexidine use did see statistically significant declines in SSI.

VQI also showed a 5-year survival rate of 79% of patients discharged with both statin and aspirin therapy vs. 61% for patients discharged without (J Vasc Surg. 2015;61[4]:1010-9). “This represents an opportunity to inform individual providers about how often they discharge patients on an aspirin and statin,” Dr. Kraiss said. Provider-targeted reports show how individual physicians rate in their region and nationwide.
VQI is more than a registry, Dr. Kraiss said; it’s also organized into 17 regional quality groups that provide surgeons a safe place to discuss VQI data and how to use that to encourage best practices. “There’s no risk of compromising or making the information identifiable,” he said. “It’s a matter of just getting together and trying to share best practices in a relatively informal environment, and hopefully through that drive quality improvement.
Other benefits of participating in VQI are that it can help surgeons comply with requirements for Medicare’s Merit-based Incentive Payment System (MIPS). VQI also offers opportunities to enroll in industry-sponsored clinical trials, which can help defray the cost of VQI participation, he said.
Dr. Kraiss had no relevant financial relationships to disclose.
CHICAGO – Five years after the Society for Vascular Surgery launched the Vascular Quality Initiative, participating centers are more likely to use chlorhexidine and have also cut their surgery times and reduced their transfusion rates, according to results presented at a symposium on vascular surgery sponsored by Northwestern University.
But more drastic have been the improvements once low-performing centers have made in these measures and others, Larry Kraiss, MD, of the University of Utah, Salt Lake City, said in reporting an update on VQI. “If you look at centers that had a big change in not using chlorhexidine to using chlorhexidine, the reduction of surgical site infections [SSI] in that subgroup was actually pretty significant,” said Dr. Kraiss, chair of the governing council of the SVS Patient Safety Organization, which oversees VQI.
These pivotal improvements came about after the VQI distributed what it calls COPI reports – for Center Opportunity Profile for Improvement – to participating centers. Currently, 379 centers in 46 states and Ontario participate in VQI, feeding data into 12 different vascular procedure registries ranging from peripheral vascular interventions to lower-extremity amputations. As of Nov. 1, 2016, 330,400 procedures had been submitted to VQI.
Dr. Kraiss called the COPI report the “workhorse” of the VQI. “It can give participating centers insight into what they can do to improve outcomes,” he said. It is one of three types of reports VQI provides. The others are benchmarking reports that show the masked ratings for all participating centers but confidentially highlight the rating of the individual center receiving the report; and reports for individual providers.
The most recent readout of the SSI COPI report compared measures in two periods: 2011-2012 and 2013-2014. In those periods, overall use of chlorhexidine rose from 66.6% to 81.2%; transfusion rates of more than 2 units fell from 14.4% to 11.5%; the share of procedures lasting 220 minutes or more fell from 50.2% to 47.7%; and SSI rate overall fell from 3.4% to 3.1%. While the change in SSI was not statistically significant, Dr. Kraiss said the 17 centers that had a large increase in chlorhexidine use did see statistically significant declines in SSI.

VQI also showed a 5-year survival rate of 79% of patients discharged with both statin and aspirin therapy vs. 61% for patients discharged without (J Vasc Surg. 2015;61[4]:1010-9). “This represents an opportunity to inform individual providers about how often they discharge patients on an aspirin and statin,” Dr. Kraiss said. Provider-targeted reports show how individual physicians rate in their region and nationwide.
VQI is more than a registry, Dr. Kraiss said; it’s also organized into 17 regional quality groups that provide surgeons a safe place to discuss VQI data and how to use that to encourage best practices. “There’s no risk of compromising or making the information identifiable,” he said. “It’s a matter of just getting together and trying to share best practices in a relatively informal environment, and hopefully through that drive quality improvement.
Other benefits of participating in VQI are that it can help surgeons comply with requirements for Medicare’s Merit-based Incentive Payment System (MIPS). VQI also offers opportunities to enroll in industry-sponsored clinical trials, which can help defray the cost of VQI participation, he said.
Dr. Kraiss had no relevant financial relationships to disclose.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: The Vascular Quality Initiative (VQI) provides comparative outcomes data that centers and surgeons can use to improve quality.
Major finding: Hospital length of stay for carotid endarterectomy averages 1.6 days for VQI centers vs. 2.6 days for nonparticipating centers.
Data source: VQI database.
Disclosures: Dr. Kraiss reported having no financial disclosures.
Semaglutide compares well with sitagliptin
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.
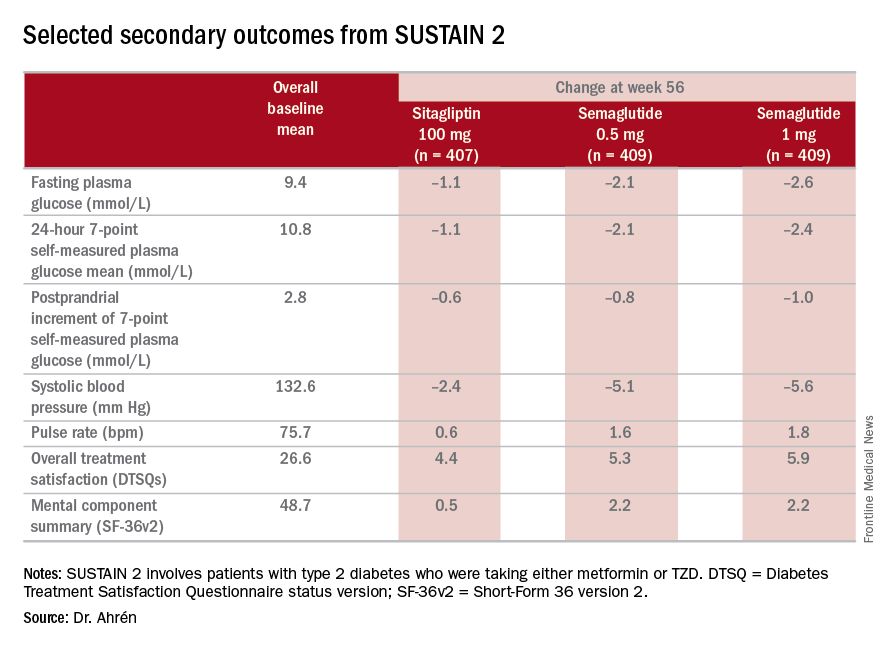
The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.

The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.

The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Investigators for a phase III trial have found weekly semaglutide superior to daily sitagliptin as add-on therapy for improving glycemic control and reducing body weight in type 2 diabetes.
Major finding: Semaglutide 0.5 and 1 mg showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c, compared with an average reduction of 0.5% for sitagliptin.
Data source: SUSTAIN 2 double-blind, randomized trial of 1,231 patients with type 2 diabetes taking either metformin or thiazolidinediones.
Disclosures: Dr. Ahrén disclosed relationships with Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, and Sanofi-Aventis Deutschland.
Vascular anomalies often misdiagnosed amidst confusion
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point:
Major finding: Use of imaging and a clearer understanding of the lack of neoplastic activity are key to more precisely diagnosing vascular malformations.
Data source: Review of literature and center experience.
Disclosure: Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
An alternative device for ESRD patients with central venous obstruction
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
Key clinical point: Combined graft-catheter device may preserve femoral access for hemodialysis for patients with central venous obstruction.
Major finding: One-year primary potency rate was 22% and secondary patency rate 60% for device recipients.
Data source: Literature review, including pooled results from eight studies involving 408 subjects.
Disclosures: Dr. Wong reported having no financial disclosures.
Now is time to embrace emerging PAD interventions
CHICAGO – Bioresorbable scaffolds, new drugs, adjuvant interventions, and stem and progenitor cell therapy will change how vascular surgeons treat peripheral artery disease in the next 5 years, so they must embrace these emerging treatments or run the risk of being displaced by other specialists, according to a presentation at a symposium on vascular surgery sponsored by Northwestern University.
“Vascular surgeons must position their practices to be the nexus for the evaluation and treatment of the patient and proactively engage in the critical trials of these new technologies,” said Patrick J. Geraghty, MD, of Washington University, St. Louis. “If our specialty fails to adapt to new treatment options, we risk getting sidelined as critical limb ischemia (CLI) treatment moves into a multimodality model.”
Dr. Geraghty focused on several future directions for PAD treatment: improved drug-eluting stents (DES) for superficial femoral artery disease; drug-coated balloons and modified DES for infrapopliteal disease; biologic modifiers for claudication and CLI; and bioresorbable, drug-eluting scaffolds for infrainguinal interventions.
“You’re not simply a plumber anymore; you’re a biological response modifier,” Dr. Geraghty said, explaining that biologic response modification technologies are the logical successor where standard surgical and endovascular techniques have either fallen short (as in early patency loss due to restenosis) or failed to offer effective alternatives (as in no-option advanced CLI patients). “And that takes many of us out of our comfort zone,” he said.
Dr. Geraghty noted the VIBRANT trial (J Vasc Surg. 2013;58[2]:386-95) and similar studies of non–drug eluting constructs identified early restenosis as the primary culprit in endovascular patency loss. “If you could reduce those early patency losses, you’d have an admirable primary patency rate for these complex lesions,” he said. “We’re able to reconstruct a vessel lumen. The question is, how to best maintain it?”
To answer that, Dr. Geraghty noted that the SIROCCO II trial (J Vasc Interv Radiol. 2005;16[3]:331-8) failed to show an advantage for a sirolimus-eluting stent over bare nitinol stent for superficial femoral artery (SFA) disease, but the subsequent Zilver PTX trial showed the benefits of paclitaxel-eluting stents over 5 years (Circulation. 2016;133[15]:1472-83).
He noted that drug-coated balloons (DCBs) trials have yielded mixed results in infrapopliteal intervention. Most notably, the multicenter In.Pact DEEP trial (Circulation. 2015;131[5]:495-502) failed to show treatment efficacy, Dr. Geraghty said. “The In.Pact DEEP results sharply contrasted with the positive data from trials of similar DCBs in the SFA” (N Engl J Med. 2015;373[2]:145-53).
With regard to DES for infrapopliteal disease, Dr. Geraghty noted the promise of positive results of the ACHILLES (J Am Coll Cardiol. 2012;60[22]:2290-5) and DESTINY (J Vasc Surg. 2012;55[2]:390-9) trials, along with the modest structural changes needed to convert from coronary to proximal tibial applications.
Bioresorbable vascular scaffolds (BVS) for CLI have also made recent advances. “It has been a slow road, but I’m happy that industry has pursued this aggressively,” Dr. Geraghty said. He pointed out that the ESPRIT I trial of bioresorbable everolimus-eluting vascular scaffolds in PAD involving the external iliac artery and SFA reported restenosis rates of 12.1% and 16.1% at 1 and 2 years, respectively (JACC Cardiovasc Interv. 2016;9[11]:1178-87). A trial of the Absorb BVS (Abbott) for short infrapopliteal lesions showed primary patency rates of 96% and 85% at 1 and 2 years, he said (JACC Cardiovasc Interv. 2016;9[7]:715-24).
“Vascular surgeons should be tracking BVS technology closely,” Dr. Geraghty said. “It achieves multiple desirable goals: immediate scaffolding for luminal restoration; mitigation of the restenotic stimulus via stent resorption; drug delivery for inhibition of restenosis; and the prospect of simpler re-interventions.”
Stem/progenitor cell therapies may also provide new solutions for no-option vasculature. One trial that showed “promising trends,” Dr. Geraghty said, is the RESTORE-CLI study of bone marrow aspiration (Mol Ther. 2012;20[6]:1280-6). “This trial reported a trend toward improved time to failure and reduced amputation-free survival, but did not meet its primary endpoint,” he said. “Likewise, the recently presented Biomet MOBILE data failed to meet its primary endpoint, but showed favorable trends in some treatment subgroups” (J Vasc Surg. 2011;54[6]:1650-8).
Dr. Geraghty noted that trial design in this field may need to change directions. “Look at the Delphi consensus matrices for the WIfI (Wound, Ischemia, foot Infection) Threatened limb Classification System (J Vasc Surg. 2014;59[1]:220-34). These show that complex wounds bear a significant risk of amputation, perhaps unmitigated by successful revascularization.” In addition, he called amputation-free survival “a rather blunt instrument” for evaluating how therapies impact limb outcomes and said it can confound the analysis of their effectiveness.
“Instead of confining the progenitor-cell therapies to no-option CLI trials, I’m eager to also see them investigated for treatment of claudication,” Dr. Geraghty said. “Can cell-based therapies possibly displace endovascular interventions as the first-line, least-harmful option for claudication?”
Dr. Geraghty also touched on intra/extravascular adjuvant therapies: antithrombin nanoparticles; inhibitory nanoparticles and polymeric wraps; and adventitial drug delivery techniques, among others.
“It’s critically important for vascular surgeons to position themselves for continued success in CLI treatment,” he said. “That involves aggressive practice branding, active trial participation, critical analysis of new technologies, and adoption of new, even disruptive, treatment modalities that show patient benefit.”
Dr. Geraghty disclosed stock ownership in Pulse Therapeutics; consultant fees from Bard Peripheral Vascular, Boston Scientific, Intact Vascular, Bard/Lutonix and Spectranetics; and serving as principal investigator for trials by Cook Medical, Bard/Lutonix, and Intact Vascular, with fees going to Washington University Medical School.
CHICAGO – Bioresorbable scaffolds, new drugs, adjuvant interventions, and stem and progenitor cell therapy will change how vascular surgeons treat peripheral artery disease in the next 5 years, so they must embrace these emerging treatments or run the risk of being displaced by other specialists, according to a presentation at a symposium on vascular surgery sponsored by Northwestern University.
“Vascular surgeons must position their practices to be the nexus for the evaluation and treatment of the patient and proactively engage in the critical trials of these new technologies,” said Patrick J. Geraghty, MD, of Washington University, St. Louis. “If our specialty fails to adapt to new treatment options, we risk getting sidelined as critical limb ischemia (CLI) treatment moves into a multimodality model.”
Dr. Geraghty focused on several future directions for PAD treatment: improved drug-eluting stents (DES) for superficial femoral artery disease; drug-coated balloons and modified DES for infrapopliteal disease; biologic modifiers for claudication and CLI; and bioresorbable, drug-eluting scaffolds for infrainguinal interventions.
“You’re not simply a plumber anymore; you’re a biological response modifier,” Dr. Geraghty said, explaining that biologic response modification technologies are the logical successor where standard surgical and endovascular techniques have either fallen short (as in early patency loss due to restenosis) or failed to offer effective alternatives (as in no-option advanced CLI patients). “And that takes many of us out of our comfort zone,” he said.
Dr. Geraghty noted the VIBRANT trial (J Vasc Surg. 2013;58[2]:386-95) and similar studies of non–drug eluting constructs identified early restenosis as the primary culprit in endovascular patency loss. “If you could reduce those early patency losses, you’d have an admirable primary patency rate for these complex lesions,” he said. “We’re able to reconstruct a vessel lumen. The question is, how to best maintain it?”
To answer that, Dr. Geraghty noted that the SIROCCO II trial (J Vasc Interv Radiol. 2005;16[3]:331-8) failed to show an advantage for a sirolimus-eluting stent over bare nitinol stent for superficial femoral artery (SFA) disease, but the subsequent Zilver PTX trial showed the benefits of paclitaxel-eluting stents over 5 years (Circulation. 2016;133[15]:1472-83).
He noted that drug-coated balloons (DCBs) trials have yielded mixed results in infrapopliteal intervention. Most notably, the multicenter In.Pact DEEP trial (Circulation. 2015;131[5]:495-502) failed to show treatment efficacy, Dr. Geraghty said. “The In.Pact DEEP results sharply contrasted with the positive data from trials of similar DCBs in the SFA” (N Engl J Med. 2015;373[2]:145-53).
With regard to DES for infrapopliteal disease, Dr. Geraghty noted the promise of positive results of the ACHILLES (J Am Coll Cardiol. 2012;60[22]:2290-5) and DESTINY (J Vasc Surg. 2012;55[2]:390-9) trials, along with the modest structural changes needed to convert from coronary to proximal tibial applications.
Bioresorbable vascular scaffolds (BVS) for CLI have also made recent advances. “It has been a slow road, but I’m happy that industry has pursued this aggressively,” Dr. Geraghty said. He pointed out that the ESPRIT I trial of bioresorbable everolimus-eluting vascular scaffolds in PAD involving the external iliac artery and SFA reported restenosis rates of 12.1% and 16.1% at 1 and 2 years, respectively (JACC Cardiovasc Interv. 2016;9[11]:1178-87). A trial of the Absorb BVS (Abbott) for short infrapopliteal lesions showed primary patency rates of 96% and 85% at 1 and 2 years, he said (JACC Cardiovasc Interv. 2016;9[7]:715-24).
“Vascular surgeons should be tracking BVS technology closely,” Dr. Geraghty said. “It achieves multiple desirable goals: immediate scaffolding for luminal restoration; mitigation of the restenotic stimulus via stent resorption; drug delivery for inhibition of restenosis; and the prospect of simpler re-interventions.”
Stem/progenitor cell therapies may also provide new solutions for no-option vasculature. One trial that showed “promising trends,” Dr. Geraghty said, is the RESTORE-CLI study of bone marrow aspiration (Mol Ther. 2012;20[6]:1280-6). “This trial reported a trend toward improved time to failure and reduced amputation-free survival, but did not meet its primary endpoint,” he said. “Likewise, the recently presented Biomet MOBILE data failed to meet its primary endpoint, but showed favorable trends in some treatment subgroups” (J Vasc Surg. 2011;54[6]:1650-8).
Dr. Geraghty noted that trial design in this field may need to change directions. “Look at the Delphi consensus matrices for the WIfI (Wound, Ischemia, foot Infection) Threatened limb Classification System (J Vasc Surg. 2014;59[1]:220-34). These show that complex wounds bear a significant risk of amputation, perhaps unmitigated by successful revascularization.” In addition, he called amputation-free survival “a rather blunt instrument” for evaluating how therapies impact limb outcomes and said it can confound the analysis of their effectiveness.
“Instead of confining the progenitor-cell therapies to no-option CLI trials, I’m eager to also see them investigated for treatment of claudication,” Dr. Geraghty said. “Can cell-based therapies possibly displace endovascular interventions as the first-line, least-harmful option for claudication?”
Dr. Geraghty also touched on intra/extravascular adjuvant therapies: antithrombin nanoparticles; inhibitory nanoparticles and polymeric wraps; and adventitial drug delivery techniques, among others.
“It’s critically important for vascular surgeons to position themselves for continued success in CLI treatment,” he said. “That involves aggressive practice branding, active trial participation, critical analysis of new technologies, and adoption of new, even disruptive, treatment modalities that show patient benefit.”
Dr. Geraghty disclosed stock ownership in Pulse Therapeutics; consultant fees from Bard Peripheral Vascular, Boston Scientific, Intact Vascular, Bard/Lutonix and Spectranetics; and serving as principal investigator for trials by Cook Medical, Bard/Lutonix, and Intact Vascular, with fees going to Washington University Medical School.
CHICAGO – Bioresorbable scaffolds, new drugs, adjuvant interventions, and stem and progenitor cell therapy will change how vascular surgeons treat peripheral artery disease in the next 5 years, so they must embrace these emerging treatments or run the risk of being displaced by other specialists, according to a presentation at a symposium on vascular surgery sponsored by Northwestern University.
“Vascular surgeons must position their practices to be the nexus for the evaluation and treatment of the patient and proactively engage in the critical trials of these new technologies,” said Patrick J. Geraghty, MD, of Washington University, St. Louis. “If our specialty fails to adapt to new treatment options, we risk getting sidelined as critical limb ischemia (CLI) treatment moves into a multimodality model.”
Dr. Geraghty focused on several future directions for PAD treatment: improved drug-eluting stents (DES) for superficial femoral artery disease; drug-coated balloons and modified DES for infrapopliteal disease; biologic modifiers for claudication and CLI; and bioresorbable, drug-eluting scaffolds for infrainguinal interventions.
“You’re not simply a plumber anymore; you’re a biological response modifier,” Dr. Geraghty said, explaining that biologic response modification technologies are the logical successor where standard surgical and endovascular techniques have either fallen short (as in early patency loss due to restenosis) or failed to offer effective alternatives (as in no-option advanced CLI patients). “And that takes many of us out of our comfort zone,” he said.
Dr. Geraghty noted the VIBRANT trial (J Vasc Surg. 2013;58[2]:386-95) and similar studies of non–drug eluting constructs identified early restenosis as the primary culprit in endovascular patency loss. “If you could reduce those early patency losses, you’d have an admirable primary patency rate for these complex lesions,” he said. “We’re able to reconstruct a vessel lumen. The question is, how to best maintain it?”
To answer that, Dr. Geraghty noted that the SIROCCO II trial (J Vasc Interv Radiol. 2005;16[3]:331-8) failed to show an advantage for a sirolimus-eluting stent over bare nitinol stent for superficial femoral artery (SFA) disease, but the subsequent Zilver PTX trial showed the benefits of paclitaxel-eluting stents over 5 years (Circulation. 2016;133[15]:1472-83).
He noted that drug-coated balloons (DCBs) trials have yielded mixed results in infrapopliteal intervention. Most notably, the multicenter In.Pact DEEP trial (Circulation. 2015;131[5]:495-502) failed to show treatment efficacy, Dr. Geraghty said. “The In.Pact DEEP results sharply contrasted with the positive data from trials of similar DCBs in the SFA” (N Engl J Med. 2015;373[2]:145-53).
With regard to DES for infrapopliteal disease, Dr. Geraghty noted the promise of positive results of the ACHILLES (J Am Coll Cardiol. 2012;60[22]:2290-5) and DESTINY (J Vasc Surg. 2012;55[2]:390-9) trials, along with the modest structural changes needed to convert from coronary to proximal tibial applications.
Bioresorbable vascular scaffolds (BVS) for CLI have also made recent advances. “It has been a slow road, but I’m happy that industry has pursued this aggressively,” Dr. Geraghty said. He pointed out that the ESPRIT I trial of bioresorbable everolimus-eluting vascular scaffolds in PAD involving the external iliac artery and SFA reported restenosis rates of 12.1% and 16.1% at 1 and 2 years, respectively (JACC Cardiovasc Interv. 2016;9[11]:1178-87). A trial of the Absorb BVS (Abbott) for short infrapopliteal lesions showed primary patency rates of 96% and 85% at 1 and 2 years, he said (JACC Cardiovasc Interv. 2016;9[7]:715-24).
“Vascular surgeons should be tracking BVS technology closely,” Dr. Geraghty said. “It achieves multiple desirable goals: immediate scaffolding for luminal restoration; mitigation of the restenotic stimulus via stent resorption; drug delivery for inhibition of restenosis; and the prospect of simpler re-interventions.”
Stem/progenitor cell therapies may also provide new solutions for no-option vasculature. One trial that showed “promising trends,” Dr. Geraghty said, is the RESTORE-CLI study of bone marrow aspiration (Mol Ther. 2012;20[6]:1280-6). “This trial reported a trend toward improved time to failure and reduced amputation-free survival, but did not meet its primary endpoint,” he said. “Likewise, the recently presented Biomet MOBILE data failed to meet its primary endpoint, but showed favorable trends in some treatment subgroups” (J Vasc Surg. 2011;54[6]:1650-8).
Dr. Geraghty noted that trial design in this field may need to change directions. “Look at the Delphi consensus matrices for the WIfI (Wound, Ischemia, foot Infection) Threatened limb Classification System (J Vasc Surg. 2014;59[1]:220-34). These show that complex wounds bear a significant risk of amputation, perhaps unmitigated by successful revascularization.” In addition, he called amputation-free survival “a rather blunt instrument” for evaluating how therapies impact limb outcomes and said it can confound the analysis of their effectiveness.
“Instead of confining the progenitor-cell therapies to no-option CLI trials, I’m eager to also see them investigated for treatment of claudication,” Dr. Geraghty said. “Can cell-based therapies possibly displace endovascular interventions as the first-line, least-harmful option for claudication?”
Dr. Geraghty also touched on intra/extravascular adjuvant therapies: antithrombin nanoparticles; inhibitory nanoparticles and polymeric wraps; and adventitial drug delivery techniques, among others.
“It’s critically important for vascular surgeons to position themselves for continued success in CLI treatment,” he said. “That involves aggressive practice branding, active trial participation, critical analysis of new technologies, and adoption of new, even disruptive, treatment modalities that show patient benefit.”
Dr. Geraghty disclosed stock ownership in Pulse Therapeutics; consultant fees from Bard Peripheral Vascular, Boston Scientific, Intact Vascular, Bard/Lutonix and Spectranetics; and serving as principal investigator for trials by Cook Medical, Bard/Lutonix, and Intact Vascular, with fees going to Washington University Medical School.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: Emerging treatments for lower-extremity interventions range from improved drug-eluting stents for the superficial femoral artery and infrapopliteal disease to bioresorbable, drug-eluting scaffolds for infrainguinal interventions.
Major finding: The future of minimally invasive revascularization hinges on reliably reopening stenosed or occluded arteries, maintaining vessel patency and using therapies to stimulate arteriogenesis or angiogenesis without reintervention.
Data source: Review of literature.
Disclosures: Dr. Geraghty disclosed stock ownership in Pulse Therapeutics; consultant fees from Bard Peripheral Vascular, Boston Scientific, Intact Vascular, Bard/Lutonix and Spectranetics; and serving as principal investigator for trials by Cook Medical, Bard/Lutonix, and Intact Vascular, with fees going to Washington University Medical School.
Echocardiography can benefit use of stented bovine graft for MVR in infants
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Preoperative echocardiography may help guide placement of modified stented jugular vein grafts in infants and small children with hypoplastic mitral and aortic valves.
Major finding: Echocardiography showed that a ratio of the narrowest subaortic region in systole to the actual mitral valve dimension of less than 0.5 was associated with postoperative left ventricular outflow tract obstruction.
Data source: Single-center, retrospective review of 24 patients who underwent mitral valve replacement with modified stented jugular vein grafts from March 2010 to March 2015.
Disclosures: Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.