User login
Immuno-oncology combos show promise in renal cell cancer
a new review finds. Based on initial data, all appear to show advantages over the standard first-line treatment with the older targeted-therapy drug sunitinib.
However, the review, published in the International Journal of Urology, cautions that uncertainty remains because of the “absence of long-term prognostic as well as safety data regarding these combination therapies.”
The review, led by Ken-ichi Harada MD, PhD, of Kobe (Japan) University, notes that the introduction of targeted therapies and immuno-oncology drugs over the last 2 decades has revolutionized the treatment of advanced renal cell carcinoma. Multiple combination therapies based on immuno-oncology drugs are now recommended by treatment guidelines.
However, the lack of head-to-head data means that “it is still challenging for physicians to make the best decision on first-line therapy,” the authors wrote.
In the review, the authors recapped the evidence regarding several combination therapies:
- Ipilimumab plus nivolumab, a combination of two monoclonal antibodies, has shown higher overall survival than sunitinib in multiple studies. Treatment-related adverse events are common, however, with one trial reporting that they led 69% of patients to discontinue treatment. Even so, “ipilimumab plus nivolumab therapy continues to demonstrate durable efficacy benefits over sunitinib in advanced renal cell carcinoma patients classified into intermediate or poor risk group after long-term follow-up.”
- Avelumab, a monoclonal antibody, plus the tyrosine kinase inhibitor (TKI) axitinib has not shown better overall survival rates versus sunitinib in a single trial, although there are signs of better progression-free survival. “Accordingly, avelumab plus axitinib is either not or discreetly recommended as a standard first-line therapy for advanced renal cell carcinoma patients by major clinical guidelines.”
- Pembrolizumab, a monoclonal antibody, plus axitinib has shown better progression-free survival and overall survival than sunitinib in a single trial. “Accordingly, pembrolizumab plus axitinib could be expected to have a powerful impact on favorable long-term cancer control with less frequent occurrence of severe adverse events, considering almost equivalent landmark overall survival to ipilimumab plus nivolumab.”
- Nivolumab plus cabozantinib, a TKI, beat sunitinib in a single trial in terms of progression-free survival and overall survival. “Nivolumab plus cabozantinib could be regarded as an efficacious therapeutic option for untreated advanced renal cell carcinoma patients with manageable safety.”
- Pembrolizumab plus lenvatinib, a TKI, showed better overall survival versus sunitinib in a single trial.
“These findings suggest that pembrolizumab plus lenvatinib could provide marked benefits with regard to cancer control in treatment-naive advanced renal cell carcinoma patients, and that caution should be exercised regarding the safety profile, considering the initial introduction of lenvatinib in the field of urological malignancies,” the authors wrote.
When compared against each other, most of these treatments appear to perform similarly, the authors wrote. With the exception of avelumab plus axitinib, all “showed almost similar advantages for the improvement of overall survival compared with sunitinib, judging from hazard ratios, and all five immuno-oncology drug-based combination therapies, particularly pembrolizumab plus lenvatinib, significantly prolonged progression-free survival, compared with sunitinib.”
No study funding was reported. The authors report various disclosures including relationships to Novartis, Pfizer, Ono, Takeda, MSD, Merck, and Bristol-Myers Squibb.
a new review finds. Based on initial data, all appear to show advantages over the standard first-line treatment with the older targeted-therapy drug sunitinib.
However, the review, published in the International Journal of Urology, cautions that uncertainty remains because of the “absence of long-term prognostic as well as safety data regarding these combination therapies.”
The review, led by Ken-ichi Harada MD, PhD, of Kobe (Japan) University, notes that the introduction of targeted therapies and immuno-oncology drugs over the last 2 decades has revolutionized the treatment of advanced renal cell carcinoma. Multiple combination therapies based on immuno-oncology drugs are now recommended by treatment guidelines.
However, the lack of head-to-head data means that “it is still challenging for physicians to make the best decision on first-line therapy,” the authors wrote.
In the review, the authors recapped the evidence regarding several combination therapies:
- Ipilimumab plus nivolumab, a combination of two monoclonal antibodies, has shown higher overall survival than sunitinib in multiple studies. Treatment-related adverse events are common, however, with one trial reporting that they led 69% of patients to discontinue treatment. Even so, “ipilimumab plus nivolumab therapy continues to demonstrate durable efficacy benefits over sunitinib in advanced renal cell carcinoma patients classified into intermediate or poor risk group after long-term follow-up.”
- Avelumab, a monoclonal antibody, plus the tyrosine kinase inhibitor (TKI) axitinib has not shown better overall survival rates versus sunitinib in a single trial, although there are signs of better progression-free survival. “Accordingly, avelumab plus axitinib is either not or discreetly recommended as a standard first-line therapy for advanced renal cell carcinoma patients by major clinical guidelines.”
- Pembrolizumab, a monoclonal antibody, plus axitinib has shown better progression-free survival and overall survival than sunitinib in a single trial. “Accordingly, pembrolizumab plus axitinib could be expected to have a powerful impact on favorable long-term cancer control with less frequent occurrence of severe adverse events, considering almost equivalent landmark overall survival to ipilimumab plus nivolumab.”
- Nivolumab plus cabozantinib, a TKI, beat sunitinib in a single trial in terms of progression-free survival and overall survival. “Nivolumab plus cabozantinib could be regarded as an efficacious therapeutic option for untreated advanced renal cell carcinoma patients with manageable safety.”
- Pembrolizumab plus lenvatinib, a TKI, showed better overall survival versus sunitinib in a single trial.
“These findings suggest that pembrolizumab plus lenvatinib could provide marked benefits with regard to cancer control in treatment-naive advanced renal cell carcinoma patients, and that caution should be exercised regarding the safety profile, considering the initial introduction of lenvatinib in the field of urological malignancies,” the authors wrote.
When compared against each other, most of these treatments appear to perform similarly, the authors wrote. With the exception of avelumab plus axitinib, all “showed almost similar advantages for the improvement of overall survival compared with sunitinib, judging from hazard ratios, and all five immuno-oncology drug-based combination therapies, particularly pembrolizumab plus lenvatinib, significantly prolonged progression-free survival, compared with sunitinib.”
No study funding was reported. The authors report various disclosures including relationships to Novartis, Pfizer, Ono, Takeda, MSD, Merck, and Bristol-Myers Squibb.
a new review finds. Based on initial data, all appear to show advantages over the standard first-line treatment with the older targeted-therapy drug sunitinib.
However, the review, published in the International Journal of Urology, cautions that uncertainty remains because of the “absence of long-term prognostic as well as safety data regarding these combination therapies.”
The review, led by Ken-ichi Harada MD, PhD, of Kobe (Japan) University, notes that the introduction of targeted therapies and immuno-oncology drugs over the last 2 decades has revolutionized the treatment of advanced renal cell carcinoma. Multiple combination therapies based on immuno-oncology drugs are now recommended by treatment guidelines.
However, the lack of head-to-head data means that “it is still challenging for physicians to make the best decision on first-line therapy,” the authors wrote.
In the review, the authors recapped the evidence regarding several combination therapies:
- Ipilimumab plus nivolumab, a combination of two monoclonal antibodies, has shown higher overall survival than sunitinib in multiple studies. Treatment-related adverse events are common, however, with one trial reporting that they led 69% of patients to discontinue treatment. Even so, “ipilimumab plus nivolumab therapy continues to demonstrate durable efficacy benefits over sunitinib in advanced renal cell carcinoma patients classified into intermediate or poor risk group after long-term follow-up.”
- Avelumab, a monoclonal antibody, plus the tyrosine kinase inhibitor (TKI) axitinib has not shown better overall survival rates versus sunitinib in a single trial, although there are signs of better progression-free survival. “Accordingly, avelumab plus axitinib is either not or discreetly recommended as a standard first-line therapy for advanced renal cell carcinoma patients by major clinical guidelines.”
- Pembrolizumab, a monoclonal antibody, plus axitinib has shown better progression-free survival and overall survival than sunitinib in a single trial. “Accordingly, pembrolizumab plus axitinib could be expected to have a powerful impact on favorable long-term cancer control with less frequent occurrence of severe adverse events, considering almost equivalent landmark overall survival to ipilimumab plus nivolumab.”
- Nivolumab plus cabozantinib, a TKI, beat sunitinib in a single trial in terms of progression-free survival and overall survival. “Nivolumab plus cabozantinib could be regarded as an efficacious therapeutic option for untreated advanced renal cell carcinoma patients with manageable safety.”
- Pembrolizumab plus lenvatinib, a TKI, showed better overall survival versus sunitinib in a single trial.
“These findings suggest that pembrolizumab plus lenvatinib could provide marked benefits with regard to cancer control in treatment-naive advanced renal cell carcinoma patients, and that caution should be exercised regarding the safety profile, considering the initial introduction of lenvatinib in the field of urological malignancies,” the authors wrote.
When compared against each other, most of these treatments appear to perform similarly, the authors wrote. With the exception of avelumab plus axitinib, all “showed almost similar advantages for the improvement of overall survival compared with sunitinib, judging from hazard ratios, and all five immuno-oncology drug-based combination therapies, particularly pembrolizumab plus lenvatinib, significantly prolonged progression-free survival, compared with sunitinib.”
No study funding was reported. The authors report various disclosures including relationships to Novartis, Pfizer, Ono, Takeda, MSD, Merck, and Bristol-Myers Squibb.
FROM THE INTERNATIONAL JOURNAL OF UROLOGY
Targeted therapy for renal cell cancer linked to higher cardiac risk
Patients on targeted therapy were more likely to develop conditions such as heart attacks and stroke than were those who took cytokine therapy (adjusted hazard ratio, 1.80; 95% confidence interval [CI] 1.19-2.74), according to a retrospective Taiwanese study reports.
“These findings may inform the evaluation of cardiovascular risk when considering targeted cancer therapies for patients with advanced renal cell carcinoma in real-world clinical practice,” wrote the authors of the report, which appeared in JACC: CardioOncology.
The study notes that one kind of targeted therapy – tyrosine kinase inhibitors with anti–vascular endothelial growth factor (VEGFR-TKI) have been linked to higher rates of major adverse cardiovascular events (1.38-22.7). There have also been reports linking another kind of targeted therapy, mechanistic target of rapamycin inhibitors (mTOR), to major adverse cardiovascular events.
In the new study, Dong-Yi Chen, MD, of Chang Gung University, Taiwan, and colleagues, tracked patients with renal cell carcinoma who underwent treatment with targeted therapy (sunitinib, sorafenib, pazopanib, everolimus, or temsirolimus, (n = 2,257, 81%) or cytokine therapy (interleukin-2 or interferon gamma, n = 528, 19%) from 2007 to 2018.
The two groups had similar gender, age and socioeconomic levels. Combined, the groups were 74% male, the median age was 63, and 68% had hypertension.
After stabilized inverse probability of treatment weighting, the adjusted incidence rates of major cardiovascular events were 6.65 and 3.36 per 100 person-years in the targeted and cytokine therapy groups, respectively. “The higher cardiovascular risk of the targeted group was driven primarily by the VEGFR TKI–treated patients,” the authors wrote.
Two drugs were linked to statistically significant higher rates of major cardiovascular adverse events compared with the reference drug sunitinib: the VEGFR TKI sorafenib (univariable HR, 1.94, 95% CI, 1.11-3.39), P = .021) and the mTOR temsirolimus (univariable HR, 2.11, 95% CI, 1.24-3.59, P = .006). Sunitinib was by far the most commonly used targeted therapy drug.
Among patients on targeted therapy, several factors were linked to higher rates of major cardiovascular events, such as baseline history of heart failure (HR, 3.88, 95% CI, 2.25-6.71), atrial fibrillation (HR, 3.60, 95% CI, 2.16-5.99), venous thromboembolism (HR, 2.50, 95% CI, 1.27-4.92), ischemic stroke (HR, 1.88, 95% CI, 1.14-3.11), and age at least 65 years (HR, 1.81, 95% CI, 1.27-2.58).
According to the authors, there are several theories about why targeted therapy may boost the risk of major adverse cardiovascular risk. “VEGF signaling inhibitors have been associated with hypertension,” which is a risk factor for cardiac death, they noted. Also, “multi-receptor TKIs, including VEGFR and platelet-derived growth factor receptor inhibitors, could destabilize the coronary microvascular endothelial network and reduce coronary flow reserve, leading to an increased risk for thrombosis and arterial ischemic events, including myocardial infarction and ischemic stroke.”
The study was funded by Chang Gung Memorial Hospital.
Patients on targeted therapy were more likely to develop conditions such as heart attacks and stroke than were those who took cytokine therapy (adjusted hazard ratio, 1.80; 95% confidence interval [CI] 1.19-2.74), according to a retrospective Taiwanese study reports.
“These findings may inform the evaluation of cardiovascular risk when considering targeted cancer therapies for patients with advanced renal cell carcinoma in real-world clinical practice,” wrote the authors of the report, which appeared in JACC: CardioOncology.
The study notes that one kind of targeted therapy – tyrosine kinase inhibitors with anti–vascular endothelial growth factor (VEGFR-TKI) have been linked to higher rates of major adverse cardiovascular events (1.38-22.7). There have also been reports linking another kind of targeted therapy, mechanistic target of rapamycin inhibitors (mTOR), to major adverse cardiovascular events.
In the new study, Dong-Yi Chen, MD, of Chang Gung University, Taiwan, and colleagues, tracked patients with renal cell carcinoma who underwent treatment with targeted therapy (sunitinib, sorafenib, pazopanib, everolimus, or temsirolimus, (n = 2,257, 81%) or cytokine therapy (interleukin-2 or interferon gamma, n = 528, 19%) from 2007 to 2018.
The two groups had similar gender, age and socioeconomic levels. Combined, the groups were 74% male, the median age was 63, and 68% had hypertension.
After stabilized inverse probability of treatment weighting, the adjusted incidence rates of major cardiovascular events were 6.65 and 3.36 per 100 person-years in the targeted and cytokine therapy groups, respectively. “The higher cardiovascular risk of the targeted group was driven primarily by the VEGFR TKI–treated patients,” the authors wrote.
Two drugs were linked to statistically significant higher rates of major cardiovascular adverse events compared with the reference drug sunitinib: the VEGFR TKI sorafenib (univariable HR, 1.94, 95% CI, 1.11-3.39), P = .021) and the mTOR temsirolimus (univariable HR, 2.11, 95% CI, 1.24-3.59, P = .006). Sunitinib was by far the most commonly used targeted therapy drug.
Among patients on targeted therapy, several factors were linked to higher rates of major cardiovascular events, such as baseline history of heart failure (HR, 3.88, 95% CI, 2.25-6.71), atrial fibrillation (HR, 3.60, 95% CI, 2.16-5.99), venous thromboembolism (HR, 2.50, 95% CI, 1.27-4.92), ischemic stroke (HR, 1.88, 95% CI, 1.14-3.11), and age at least 65 years (HR, 1.81, 95% CI, 1.27-2.58).
According to the authors, there are several theories about why targeted therapy may boost the risk of major adverse cardiovascular risk. “VEGF signaling inhibitors have been associated with hypertension,” which is a risk factor for cardiac death, they noted. Also, “multi-receptor TKIs, including VEGFR and platelet-derived growth factor receptor inhibitors, could destabilize the coronary microvascular endothelial network and reduce coronary flow reserve, leading to an increased risk for thrombosis and arterial ischemic events, including myocardial infarction and ischemic stroke.”
The study was funded by Chang Gung Memorial Hospital.
Patients on targeted therapy were more likely to develop conditions such as heart attacks and stroke than were those who took cytokine therapy (adjusted hazard ratio, 1.80; 95% confidence interval [CI] 1.19-2.74), according to a retrospective Taiwanese study reports.
“These findings may inform the evaluation of cardiovascular risk when considering targeted cancer therapies for patients with advanced renal cell carcinoma in real-world clinical practice,” wrote the authors of the report, which appeared in JACC: CardioOncology.
The study notes that one kind of targeted therapy – tyrosine kinase inhibitors with anti–vascular endothelial growth factor (VEGFR-TKI) have been linked to higher rates of major adverse cardiovascular events (1.38-22.7). There have also been reports linking another kind of targeted therapy, mechanistic target of rapamycin inhibitors (mTOR), to major adverse cardiovascular events.
In the new study, Dong-Yi Chen, MD, of Chang Gung University, Taiwan, and colleagues, tracked patients with renal cell carcinoma who underwent treatment with targeted therapy (sunitinib, sorafenib, pazopanib, everolimus, or temsirolimus, (n = 2,257, 81%) or cytokine therapy (interleukin-2 or interferon gamma, n = 528, 19%) from 2007 to 2018.
The two groups had similar gender, age and socioeconomic levels. Combined, the groups were 74% male, the median age was 63, and 68% had hypertension.
After stabilized inverse probability of treatment weighting, the adjusted incidence rates of major cardiovascular events were 6.65 and 3.36 per 100 person-years in the targeted and cytokine therapy groups, respectively. “The higher cardiovascular risk of the targeted group was driven primarily by the VEGFR TKI–treated patients,” the authors wrote.
Two drugs were linked to statistically significant higher rates of major cardiovascular adverse events compared with the reference drug sunitinib: the VEGFR TKI sorafenib (univariable HR, 1.94, 95% CI, 1.11-3.39), P = .021) and the mTOR temsirolimus (univariable HR, 2.11, 95% CI, 1.24-3.59, P = .006). Sunitinib was by far the most commonly used targeted therapy drug.
Among patients on targeted therapy, several factors were linked to higher rates of major cardiovascular events, such as baseline history of heart failure (HR, 3.88, 95% CI, 2.25-6.71), atrial fibrillation (HR, 3.60, 95% CI, 2.16-5.99), venous thromboembolism (HR, 2.50, 95% CI, 1.27-4.92), ischemic stroke (HR, 1.88, 95% CI, 1.14-3.11), and age at least 65 years (HR, 1.81, 95% CI, 1.27-2.58).
According to the authors, there are several theories about why targeted therapy may boost the risk of major adverse cardiovascular risk. “VEGF signaling inhibitors have been associated with hypertension,” which is a risk factor for cardiac death, they noted. Also, “multi-receptor TKIs, including VEGFR and platelet-derived growth factor receptor inhibitors, could destabilize the coronary microvascular endothelial network and reduce coronary flow reserve, leading to an increased risk for thrombosis and arterial ischemic events, including myocardial infarction and ischemic stroke.”
The study was funded by Chang Gung Memorial Hospital.
FROM JACC: CARDIOONCOLOGY
Heed cardiac risk of BTKis for CLL
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
FROM BLOOD ADVANCES
Drug shortages plague hematology, but preparedness helps
Just before he took a call from a reporter asking about the impact of drug shortages in hematology, Bill Greene, PharmD, chief pharmaceutical officer at St. Jude Children’s Research Hospital, had spent an hour on the phone overseeing his institution’s response to a hematology drug shortage. The chemotherapy drug fludarabine, used to treat chronic lymphocytic leukemia, was in short supply.
“There are 5 different manufacturers, but none of them have had drug available over the past 2 weeks,” Dr. Greene said. “We’re trying to chase some emergency supplies to be able to continue treatment for patients who’ve had their treatments initiated and planned.”
Over the past several years, this predicament has become common at hematology clinics across the country. In fact, management of scarce medication resources has become a significant part of Dr. Greene’s workload these days, as critical drugs fail to show up on time or manufacturer supplies run low at his hospital in Memphis.
This shortage of hematology drugs got a new dose of national attention, thanks to a recent episode of CBS News’ “60 Minutes.” Through interviews with physicians and parents of children who suddenly could not get vital medications, the report highlighted the recent shortage of another leukemia drug, vincristine.
“As a cancer mom, we shouldn’t be fighting for our children to get a drug that is needed,” Cyndi Valenta was quoted as saying. She recalled that when the shortage began in 2019, her 13-year-old son, a leukemia patient at Loma Linda (Calif.) University Hospital, felt frightened. Ms. Valenta said she felt a “gut-wrenching feeling of just fear and anger.” They were finally able to get doses of the drug after launching a social media campaign.
Such drug shortages are especially widespread in oncology and hematology, according to a survey of oncology pharmacists at 68 organizations nationwide. Published in the May 2022 issue of Oncology Practice, the study showed that 63% of institutions reported one or more drug shortages every month, with a 34% increase in 2019, compared with 2018. Treatment delays, reduced doses, or alternative regimens were reported by 75% of respondents, the authors wrote.
The pharmacists surveyed between May 2019 and July 2020 were asked about the three most hard-to-get chemotherapy and supportive care agents. Vincristine topped the list, followed by vinblastine, IVIG, leucovorin, and BCG, as well as difficult-to-obtain ropine, erwinia asparaginase, etoposide, and leuprolide. Several of these drugs are used to treat conditions such as lymphoma and leukemia.
Eighty-two percent of respondents reported shortages of decitabine (IV), often used as part of a cocktail with vinblastine and other drugs to treat Hodgkin lymphoma.
The reasons for drug shortages are varied. The CBS News report declared that “pharmaceutical companies have stopped producing many life-saving generic drugs because they make too little profit,” and it suggested that the federal government isn’t doing enough.
But government action actually might be making a difference. According to the FDA, the number of new drug shortages has fallen dramatically from 250 in 2011 to 41 in 2021, and the number of prevented drug shortages rose from nearly 200 to more than 300 over that same period. Still, the number of ongoing drug shortages has risen from around 40 in 2017 to about 80 in 2021.
Reasons for the paucity of certain drugs are often unclear. In a June 12, 2022 post, for example, the American Society of Health-System Pharmacists’ drug shortage database noted that the chemotherapy drug fludarabine was in short supply and provided details about when some of the 5 manufacturers expected to have it available. (This is the shortage that Dr. Greene was trying to manage.) But 4 of the 5 manufacturers “did not provide a reason,” and the fifth blamed manufacturing delays.
“There’s a lot of closely held trade secrets that hinder the ability to share good information,” said Dr. Greene. To make things more complicated, shipping times are often unreliable. “The product doesn’t show up today, we place another order. Sometimes it will show up tomorrow, sometimes it doesn’t,” he said. “If you’re not tracking it carefully, you deplete your own supply.”
Patients’ families have grown used to dealing with drug shortages, and “they’re less quick to blame personnel at our institution.”
How can hematologists cope with this issue? “The best thing in the immediate term is to advocate for their hospital to have a pharmacist dedicated to shortage monitoring and taking proactive steps to obviate shortages,” hematologist/oncologist Andrew Hantel, MD, an instructor at Dana-Farber Cancer Institute, Harvard Medical School, Boston, said in an interview.
“We have ongoing communications with other large cancer centers and the FDA to recognize shortages early and develop plans to make sure we stay ahead of them,” Dr. Hantel said. “Most often this involves assessing supply, use rates, alternative manufacturers, and additional measures the Food and Drug Administration can take (for example, importation), and occasionally working with clinical teams to see if other medications are feasible alternatives.”
If a drug is unavailable, it can also be helpful to discuss alternative approaches. “We did not have any frank shortages of vincristine,” Dr. Hantel said, “but we did focus on conservation measures and considered different ethically appropriate ways to distribute vincristine if there was a point at which we did not have enough for everyone who needed it.”
If a drug is in short supply, options can include delaying treatment, giving an alternative, or providing the rest of the regimen without the scarce drug, he said. In a 2021 report in The Lancet Hematology, Dr. Hantel and his colleagues offered “model solutions for ethical allocation during cancer medicine shortages.”
The authors of the May 2022 drug-shortage report highlighted an alternative regimen in hematology. They noted that manufacturing delays have limited the supply of dacarbazine, used for Hodgkin lymphoma. Due to the current shortages, they wrote, clinicians are considering the use of escalated bleomycin, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone, replacing dacarbazine with procarbazine and using the doxorubicin, bleomycin, vinblastine, procarbazine, and prednisone regimen, or replacing dacarbazine with cyclophosphamide.
Dr. Greene emphasized the importance of tracking the news and the drug shortage websites run by the FDA and the American Society of Health-System Pharmacists.
It’s also crucial to have a good relationship with your wholesaler, he added, and to communicate about these problems within your facility. At his hospital, the pharmaceutical staff holds a multi-disciplinary meeting at least weekly to discuss the supply of medications. As he put it, “it’s a challenging environment.”
Dr. Greene and Dr. Hantel reported no relevant disclosures.
Just before he took a call from a reporter asking about the impact of drug shortages in hematology, Bill Greene, PharmD, chief pharmaceutical officer at St. Jude Children’s Research Hospital, had spent an hour on the phone overseeing his institution’s response to a hematology drug shortage. The chemotherapy drug fludarabine, used to treat chronic lymphocytic leukemia, was in short supply.
“There are 5 different manufacturers, but none of them have had drug available over the past 2 weeks,” Dr. Greene said. “We’re trying to chase some emergency supplies to be able to continue treatment for patients who’ve had their treatments initiated and planned.”
Over the past several years, this predicament has become common at hematology clinics across the country. In fact, management of scarce medication resources has become a significant part of Dr. Greene’s workload these days, as critical drugs fail to show up on time or manufacturer supplies run low at his hospital in Memphis.
This shortage of hematology drugs got a new dose of national attention, thanks to a recent episode of CBS News’ “60 Minutes.” Through interviews with physicians and parents of children who suddenly could not get vital medications, the report highlighted the recent shortage of another leukemia drug, vincristine.
“As a cancer mom, we shouldn’t be fighting for our children to get a drug that is needed,” Cyndi Valenta was quoted as saying. She recalled that when the shortage began in 2019, her 13-year-old son, a leukemia patient at Loma Linda (Calif.) University Hospital, felt frightened. Ms. Valenta said she felt a “gut-wrenching feeling of just fear and anger.” They were finally able to get doses of the drug after launching a social media campaign.
Such drug shortages are especially widespread in oncology and hematology, according to a survey of oncology pharmacists at 68 organizations nationwide. Published in the May 2022 issue of Oncology Practice, the study showed that 63% of institutions reported one or more drug shortages every month, with a 34% increase in 2019, compared with 2018. Treatment delays, reduced doses, or alternative regimens were reported by 75% of respondents, the authors wrote.
The pharmacists surveyed between May 2019 and July 2020 were asked about the three most hard-to-get chemotherapy and supportive care agents. Vincristine topped the list, followed by vinblastine, IVIG, leucovorin, and BCG, as well as difficult-to-obtain ropine, erwinia asparaginase, etoposide, and leuprolide. Several of these drugs are used to treat conditions such as lymphoma and leukemia.
Eighty-two percent of respondents reported shortages of decitabine (IV), often used as part of a cocktail with vinblastine and other drugs to treat Hodgkin lymphoma.
The reasons for drug shortages are varied. The CBS News report declared that “pharmaceutical companies have stopped producing many life-saving generic drugs because they make too little profit,” and it suggested that the federal government isn’t doing enough.
But government action actually might be making a difference. According to the FDA, the number of new drug shortages has fallen dramatically from 250 in 2011 to 41 in 2021, and the number of prevented drug shortages rose from nearly 200 to more than 300 over that same period. Still, the number of ongoing drug shortages has risen from around 40 in 2017 to about 80 in 2021.
Reasons for the paucity of certain drugs are often unclear. In a June 12, 2022 post, for example, the American Society of Health-System Pharmacists’ drug shortage database noted that the chemotherapy drug fludarabine was in short supply and provided details about when some of the 5 manufacturers expected to have it available. (This is the shortage that Dr. Greene was trying to manage.) But 4 of the 5 manufacturers “did not provide a reason,” and the fifth blamed manufacturing delays.
“There’s a lot of closely held trade secrets that hinder the ability to share good information,” said Dr. Greene. To make things more complicated, shipping times are often unreliable. “The product doesn’t show up today, we place another order. Sometimes it will show up tomorrow, sometimes it doesn’t,” he said. “If you’re not tracking it carefully, you deplete your own supply.”
Patients’ families have grown used to dealing with drug shortages, and “they’re less quick to blame personnel at our institution.”
How can hematologists cope with this issue? “The best thing in the immediate term is to advocate for their hospital to have a pharmacist dedicated to shortage monitoring and taking proactive steps to obviate shortages,” hematologist/oncologist Andrew Hantel, MD, an instructor at Dana-Farber Cancer Institute, Harvard Medical School, Boston, said in an interview.
“We have ongoing communications with other large cancer centers and the FDA to recognize shortages early and develop plans to make sure we stay ahead of them,” Dr. Hantel said. “Most often this involves assessing supply, use rates, alternative manufacturers, and additional measures the Food and Drug Administration can take (for example, importation), and occasionally working with clinical teams to see if other medications are feasible alternatives.”
If a drug is unavailable, it can also be helpful to discuss alternative approaches. “We did not have any frank shortages of vincristine,” Dr. Hantel said, “but we did focus on conservation measures and considered different ethically appropriate ways to distribute vincristine if there was a point at which we did not have enough for everyone who needed it.”
If a drug is in short supply, options can include delaying treatment, giving an alternative, or providing the rest of the regimen without the scarce drug, he said. In a 2021 report in The Lancet Hematology, Dr. Hantel and his colleagues offered “model solutions for ethical allocation during cancer medicine shortages.”
The authors of the May 2022 drug-shortage report highlighted an alternative regimen in hematology. They noted that manufacturing delays have limited the supply of dacarbazine, used for Hodgkin lymphoma. Due to the current shortages, they wrote, clinicians are considering the use of escalated bleomycin, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone, replacing dacarbazine with procarbazine and using the doxorubicin, bleomycin, vinblastine, procarbazine, and prednisone regimen, or replacing dacarbazine with cyclophosphamide.
Dr. Greene emphasized the importance of tracking the news and the drug shortage websites run by the FDA and the American Society of Health-System Pharmacists.
It’s also crucial to have a good relationship with your wholesaler, he added, and to communicate about these problems within your facility. At his hospital, the pharmaceutical staff holds a multi-disciplinary meeting at least weekly to discuss the supply of medications. As he put it, “it’s a challenging environment.”
Dr. Greene and Dr. Hantel reported no relevant disclosures.
Just before he took a call from a reporter asking about the impact of drug shortages in hematology, Bill Greene, PharmD, chief pharmaceutical officer at St. Jude Children’s Research Hospital, had spent an hour on the phone overseeing his institution’s response to a hematology drug shortage. The chemotherapy drug fludarabine, used to treat chronic lymphocytic leukemia, was in short supply.
“There are 5 different manufacturers, but none of them have had drug available over the past 2 weeks,” Dr. Greene said. “We’re trying to chase some emergency supplies to be able to continue treatment for patients who’ve had their treatments initiated and planned.”
Over the past several years, this predicament has become common at hematology clinics across the country. In fact, management of scarce medication resources has become a significant part of Dr. Greene’s workload these days, as critical drugs fail to show up on time or manufacturer supplies run low at his hospital in Memphis.
This shortage of hematology drugs got a new dose of national attention, thanks to a recent episode of CBS News’ “60 Minutes.” Through interviews with physicians and parents of children who suddenly could not get vital medications, the report highlighted the recent shortage of another leukemia drug, vincristine.
“As a cancer mom, we shouldn’t be fighting for our children to get a drug that is needed,” Cyndi Valenta was quoted as saying. She recalled that when the shortage began in 2019, her 13-year-old son, a leukemia patient at Loma Linda (Calif.) University Hospital, felt frightened. Ms. Valenta said she felt a “gut-wrenching feeling of just fear and anger.” They were finally able to get doses of the drug after launching a social media campaign.
Such drug shortages are especially widespread in oncology and hematology, according to a survey of oncology pharmacists at 68 organizations nationwide. Published in the May 2022 issue of Oncology Practice, the study showed that 63% of institutions reported one or more drug shortages every month, with a 34% increase in 2019, compared with 2018. Treatment delays, reduced doses, or alternative regimens were reported by 75% of respondents, the authors wrote.
The pharmacists surveyed between May 2019 and July 2020 were asked about the three most hard-to-get chemotherapy and supportive care agents. Vincristine topped the list, followed by vinblastine, IVIG, leucovorin, and BCG, as well as difficult-to-obtain ropine, erwinia asparaginase, etoposide, and leuprolide. Several of these drugs are used to treat conditions such as lymphoma and leukemia.
Eighty-two percent of respondents reported shortages of decitabine (IV), often used as part of a cocktail with vinblastine and other drugs to treat Hodgkin lymphoma.
The reasons for drug shortages are varied. The CBS News report declared that “pharmaceutical companies have stopped producing many life-saving generic drugs because they make too little profit,” and it suggested that the federal government isn’t doing enough.
But government action actually might be making a difference. According to the FDA, the number of new drug shortages has fallen dramatically from 250 in 2011 to 41 in 2021, and the number of prevented drug shortages rose from nearly 200 to more than 300 over that same period. Still, the number of ongoing drug shortages has risen from around 40 in 2017 to about 80 in 2021.
Reasons for the paucity of certain drugs are often unclear. In a June 12, 2022 post, for example, the American Society of Health-System Pharmacists’ drug shortage database noted that the chemotherapy drug fludarabine was in short supply and provided details about when some of the 5 manufacturers expected to have it available. (This is the shortage that Dr. Greene was trying to manage.) But 4 of the 5 manufacturers “did not provide a reason,” and the fifth blamed manufacturing delays.
“There’s a lot of closely held trade secrets that hinder the ability to share good information,” said Dr. Greene. To make things more complicated, shipping times are often unreliable. “The product doesn’t show up today, we place another order. Sometimes it will show up tomorrow, sometimes it doesn’t,” he said. “If you’re not tracking it carefully, you deplete your own supply.”
Patients’ families have grown used to dealing with drug shortages, and “they’re less quick to blame personnel at our institution.”
How can hematologists cope with this issue? “The best thing in the immediate term is to advocate for their hospital to have a pharmacist dedicated to shortage monitoring and taking proactive steps to obviate shortages,” hematologist/oncologist Andrew Hantel, MD, an instructor at Dana-Farber Cancer Institute, Harvard Medical School, Boston, said in an interview.
“We have ongoing communications with other large cancer centers and the FDA to recognize shortages early and develop plans to make sure we stay ahead of them,” Dr. Hantel said. “Most often this involves assessing supply, use rates, alternative manufacturers, and additional measures the Food and Drug Administration can take (for example, importation), and occasionally working with clinical teams to see if other medications are feasible alternatives.”
If a drug is unavailable, it can also be helpful to discuss alternative approaches. “We did not have any frank shortages of vincristine,” Dr. Hantel said, “but we did focus on conservation measures and considered different ethically appropriate ways to distribute vincristine if there was a point at which we did not have enough for everyone who needed it.”
If a drug is in short supply, options can include delaying treatment, giving an alternative, or providing the rest of the regimen without the scarce drug, he said. In a 2021 report in The Lancet Hematology, Dr. Hantel and his colleagues offered “model solutions for ethical allocation during cancer medicine shortages.”
The authors of the May 2022 drug-shortage report highlighted an alternative regimen in hematology. They noted that manufacturing delays have limited the supply of dacarbazine, used for Hodgkin lymphoma. Due to the current shortages, they wrote, clinicians are considering the use of escalated bleomycin, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone, replacing dacarbazine with procarbazine and using the doxorubicin, bleomycin, vinblastine, procarbazine, and prednisone regimen, or replacing dacarbazine with cyclophosphamide.
Dr. Greene emphasized the importance of tracking the news and the drug shortage websites run by the FDA and the American Society of Health-System Pharmacists.
It’s also crucial to have a good relationship with your wholesaler, he added, and to communicate about these problems within your facility. At his hospital, the pharmaceutical staff holds a multi-disciplinary meeting at least weekly to discuss the supply of medications. As he put it, “it’s a challenging environment.”
Dr. Greene and Dr. Hantel reported no relevant disclosures.
If nuclear disaster strikes, U.S. hematologists stand ready
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.
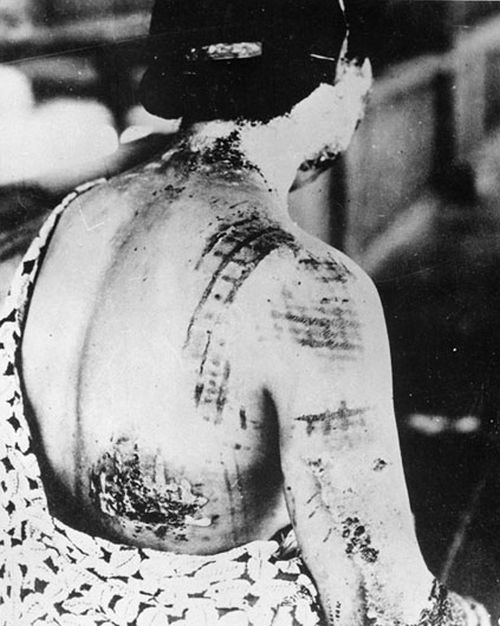
“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”
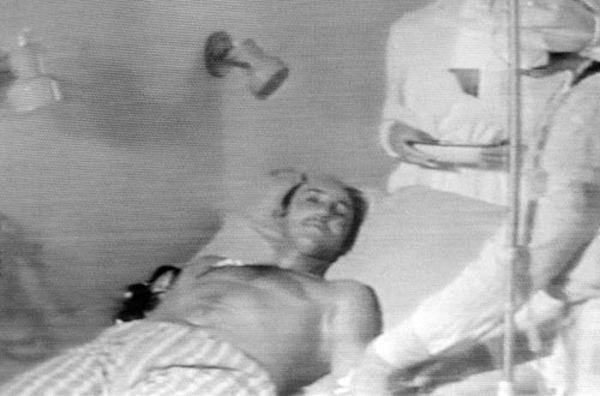
And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
Are headache clinical trials representative of the general patient population?
DENVER – In a debate over whether headache trials are representative of patients, one neurologist declared that they tend to leave out a variety of subjects with many types of headaches – the young, the old, the pregnant, and those without migraines, among others. But her counterpart defended migraine trials in particular, arguing that they’re evolving to become more valuable as researchers address their limitations.
At the core of the debate at the annual meeting of the American Headache Society were sharp divisions over how much the limitations of headache clinical trials matter. Both neurologists – Jan Brandes, MD, of Nashville (Tenn.) Neuroscience Group, and Amy Gelfand, MD, of the University of California at San Francisco, agree that they exist. But they diverged on how much they matter.
Exclusion/inclusion criteria are good
Dr. Brandes argued that randomized controlled trials “remain the single best study design,” and she said migraine headache trials have improved over the past couple of decades.
Eligibility criteria, for example, have expanded to allow patients with more subtypes of migraines to participate, she said. “Another change has been the establishment of guidelines or inclusion criteria that allow patients who have stable and treated hypertension, stable depression, and stable anxiety disorders that are controlled and treated and not interfering with the disease you’re studying.”
In essence, she said, “the exclusion/inclusion criteria are good.”
It’s also a positive change that longer patient-reported outcomes are included in trials, she said.
Exclusion/inclusion criteria are too restrictive
But Dr. Gelfand criticized the inclusion criteria in migraine trials, noting it includes “a lot of amazing complexity.” Trials often will limit participation to subjects aged 18-65, even though people have high rates of headaches, she said, and they frequently overrepresent men. Pregnant and lactating women are often omitted, too, even if a trial is examining a behavioral intervention. In some cases, lactating women may be breastfeeding for a year or two, she noted.
“The vast majority of births in the United States, 92%, are to females who are between the ages of 20 and 39. That is also the age range where migraine is most prevalent,” she said. Yes, certain new agents shouldn’t be tested for the first time in pregnant women because of the risk, she said, “but we need to grapple with the fact that migraine is affecting people who are also going to be pregnant and lactating.”
Many other criteria limit the subjects in headache trials, she said. The studies are “almost exclusively” of drugs for migraines, leaving out many people with other types such as adolescents with new persistent headaches. “Where are the trials for them?” she asked.
Other groups that are left out include those whose headaches that are due to a head injury, a viral infection such as COVID-19, or even vaccination against COVID-19, she said. “There are an infinite number of questions here that we are currently not even attempting to answer.”
Non-Whites are also poorly represented in trials, she said, and studies often don’t include data about non-Whites. “Race data exists. Where do we get off not even reporting it?”
Room for improvement
For her part, Dr. Brandes said less-common headache disorders are best studied in pragmatic trials until they can be better understood. “We need to understand pathophysiology better for some of these other disorders, particularly things like continuous headache and posttraumatic headache. Then we can begin to expand that.”
She added that randomized clinical trials are now underway regarding secondary headache related to COVID-19.
Dr. Brandes did not report disclosures. Dr. Gelfand had no disclosures.
DENVER – In a debate over whether headache trials are representative of patients, one neurologist declared that they tend to leave out a variety of subjects with many types of headaches – the young, the old, the pregnant, and those without migraines, among others. But her counterpart defended migraine trials in particular, arguing that they’re evolving to become more valuable as researchers address their limitations.
At the core of the debate at the annual meeting of the American Headache Society were sharp divisions over how much the limitations of headache clinical trials matter. Both neurologists – Jan Brandes, MD, of Nashville (Tenn.) Neuroscience Group, and Amy Gelfand, MD, of the University of California at San Francisco, agree that they exist. But they diverged on how much they matter.
Exclusion/inclusion criteria are good
Dr. Brandes argued that randomized controlled trials “remain the single best study design,” and she said migraine headache trials have improved over the past couple of decades.
Eligibility criteria, for example, have expanded to allow patients with more subtypes of migraines to participate, she said. “Another change has been the establishment of guidelines or inclusion criteria that allow patients who have stable and treated hypertension, stable depression, and stable anxiety disorders that are controlled and treated and not interfering with the disease you’re studying.”
In essence, she said, “the exclusion/inclusion criteria are good.”
It’s also a positive change that longer patient-reported outcomes are included in trials, she said.
Exclusion/inclusion criteria are too restrictive
But Dr. Gelfand criticized the inclusion criteria in migraine trials, noting it includes “a lot of amazing complexity.” Trials often will limit participation to subjects aged 18-65, even though people have high rates of headaches, she said, and they frequently overrepresent men. Pregnant and lactating women are often omitted, too, even if a trial is examining a behavioral intervention. In some cases, lactating women may be breastfeeding for a year or two, she noted.
“The vast majority of births in the United States, 92%, are to females who are between the ages of 20 and 39. That is also the age range where migraine is most prevalent,” she said. Yes, certain new agents shouldn’t be tested for the first time in pregnant women because of the risk, she said, “but we need to grapple with the fact that migraine is affecting people who are also going to be pregnant and lactating.”
Many other criteria limit the subjects in headache trials, she said. The studies are “almost exclusively” of drugs for migraines, leaving out many people with other types such as adolescents with new persistent headaches. “Where are the trials for them?” she asked.
Other groups that are left out include those whose headaches that are due to a head injury, a viral infection such as COVID-19, or even vaccination against COVID-19, she said. “There are an infinite number of questions here that we are currently not even attempting to answer.”
Non-Whites are also poorly represented in trials, she said, and studies often don’t include data about non-Whites. “Race data exists. Where do we get off not even reporting it?”
Room for improvement
For her part, Dr. Brandes said less-common headache disorders are best studied in pragmatic trials until they can be better understood. “We need to understand pathophysiology better for some of these other disorders, particularly things like continuous headache and posttraumatic headache. Then we can begin to expand that.”
She added that randomized clinical trials are now underway regarding secondary headache related to COVID-19.
Dr. Brandes did not report disclosures. Dr. Gelfand had no disclosures.
DENVER – In a debate over whether headache trials are representative of patients, one neurologist declared that they tend to leave out a variety of subjects with many types of headaches – the young, the old, the pregnant, and those without migraines, among others. But her counterpart defended migraine trials in particular, arguing that they’re evolving to become more valuable as researchers address their limitations.
At the core of the debate at the annual meeting of the American Headache Society were sharp divisions over how much the limitations of headache clinical trials matter. Both neurologists – Jan Brandes, MD, of Nashville (Tenn.) Neuroscience Group, and Amy Gelfand, MD, of the University of California at San Francisco, agree that they exist. But they diverged on how much they matter.
Exclusion/inclusion criteria are good
Dr. Brandes argued that randomized controlled trials “remain the single best study design,” and she said migraine headache trials have improved over the past couple of decades.
Eligibility criteria, for example, have expanded to allow patients with more subtypes of migraines to participate, she said. “Another change has been the establishment of guidelines or inclusion criteria that allow patients who have stable and treated hypertension, stable depression, and stable anxiety disorders that are controlled and treated and not interfering with the disease you’re studying.”
In essence, she said, “the exclusion/inclusion criteria are good.”
It’s also a positive change that longer patient-reported outcomes are included in trials, she said.
Exclusion/inclusion criteria are too restrictive
But Dr. Gelfand criticized the inclusion criteria in migraine trials, noting it includes “a lot of amazing complexity.” Trials often will limit participation to subjects aged 18-65, even though people have high rates of headaches, she said, and they frequently overrepresent men. Pregnant and lactating women are often omitted, too, even if a trial is examining a behavioral intervention. In some cases, lactating women may be breastfeeding for a year or two, she noted.
“The vast majority of births in the United States, 92%, are to females who are between the ages of 20 and 39. That is also the age range where migraine is most prevalent,” she said. Yes, certain new agents shouldn’t be tested for the first time in pregnant women because of the risk, she said, “but we need to grapple with the fact that migraine is affecting people who are also going to be pregnant and lactating.”
Many other criteria limit the subjects in headache trials, she said. The studies are “almost exclusively” of drugs for migraines, leaving out many people with other types such as adolescents with new persistent headaches. “Where are the trials for them?” she asked.
Other groups that are left out include those whose headaches that are due to a head injury, a viral infection such as COVID-19, or even vaccination against COVID-19, she said. “There are an infinite number of questions here that we are currently not even attempting to answer.”
Non-Whites are also poorly represented in trials, she said, and studies often don’t include data about non-Whites. “Race data exists. Where do we get off not even reporting it?”
Room for improvement
For her part, Dr. Brandes said less-common headache disorders are best studied in pragmatic trials until they can be better understood. “We need to understand pathophysiology better for some of these other disorders, particularly things like continuous headache and posttraumatic headache. Then we can begin to expand that.”
She added that randomized clinical trials are now underway regarding secondary headache related to COVID-19.
Dr. Brandes did not report disclosures. Dr. Gelfand had no disclosures.
AT AHS 2022
No more ‘escape hatch’: Post Roe, new worries about meds linked to birth defects
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Good chemo vs. bad chemo: When too much is a bad thing
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
A new study finds that mortality is significantly higher among patients with advanced solid tumors who are admitted to the hospital for chemotherapy treatment.
The findings – released in a poster session at the annual meeting of the American Society of Clinical Oncology – found that patients with solid tumors were more likely to be treated for nonurgent indications, not be referred to palliative care, and die within 60 days, compared with patients with hematologic malignancies.
Decisions about inpatient chemotherapy should not be uniform and instead should be based on a case-by-case basis, said Natalie Berger, MD, a hematologist-oncologist at Mount Sinai Hospital,, New York, and the study’s lead author.
Inpatient chemotherapy can be appropriate in certain situations, such as when chemotherapy must be given in the hospital and when it must be administered quickly after a patient presents with cancer symptoms and needs relief, she said.
However, “sometimes patients are admitted due to infection, side effects of chemotherapy or cancer, or for reasons unrelated to their cancer, and chemotherapy may be administered when it is not appropriate. It is also overutilized at the end of life which can lead to more aggressive end-of-life care rather than focusing on quality of life and supportive care,” Dr. Berger said.
The study is based on a retrospective chart review of 880 patients admitted to Mount Sinai Hospital between January 2016 and December 2017 to receive chemotherapy.
They found that the type of tumor was used to determine the urgency of an in-hospital stay for chemotherapy (odds ratio, 0.42; 95% CI, 0.25-0.72; P = .001). Patients with solid tumors or older patients or patients with a functional impairment score (Karnofsky Performance Scale) of 50% were less likely to respond to chemotherapy. There was also a decrease in quality of life among these patients, but only 46% of patients with solid tumors and 15% of patients with hematologic malignancies met with a palliative care professional.
One-third (34%) of patients with solid tumors didn’t have urgent indications, 43% of patients had no response to inpatient chemotherapy, and 20% died within 60 days, compared with patients with hematologic malignancies (19%, 19%, and 9%, respectively).
“There are many reasons why this [high mortality rate in patients with solid tumors] may be happening. Solid tumor patients are more often admitted at a later stage of their cancer when they are sicker, and they were also less likely to have a response to inpatient chemotherapy. Older patients and patients with a poor performance status were also less likely to respond to chemotherapy. This indicates that these patients were sicker, and chemotherapy use may not have been appropriate and palliative care may be underutilized,” she said.
Dr. Berger and colleagues have created a standardized protocol to assess “the appropriateness” of inpatient chemotherapy, improve quality of life, and reduce chemotherapy and health care utilization at the end of life. The protocol has been implemented as a pilot program at Mount Sinai Hospital, Dr. Berger said.
“Any inpatient chemotherapy case that meets standard accepted criteria for required inpatient administration are auto-approved through the electronic survey. For cases outside of standard criteria, further information must be inputted to determine appropriateness of inpatient treatment and are then scored electronically and reviewed by committee physicians and pharmacists,” she said.
Gabriel A. Brooks, MD, MPH, an oncologist with Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not affiliated with the study, said that inpatient chemotherapy treatment is under scrutiny elsewhere as well.
“There has been recognition that patients who are otherwise sick enough to require hospital admission are often too sick to benefit from chemotherapy,” although there are exceptions. “There is certainly a movement to limit inpatient chemotherapy to situations where it is most likely to be beneficial. Some of this is driven by cost pressures. For instance, Medicare pays for inpatient hospitalizations using the DRG [diagnosis-related group] system. Hospitals cannot charge a la carte for treatments given in the hospital. Instead, they are reimbursed at a fixed rate based on the hospital diagnoses. This will often lead to poor reimbursement of high-cost cancer treatments.”
Dr. Brooks said the study offers insight into who’s getting inpatient chemotherapy. However, “what I can’t tell from this poster is how often the solid tumor patients are getting first-line chemotherapy [as] these patients may be presenting late or may have a potentially treatable cancer with a narrow closing window for treatment versus later-line chemotherapy.”
He also noted that patient and family wishes are missing from the research. “This is critical. Patients and families should be informed that inpatient chemotherapy may not provide the benefit they are hoping for, especially for patients with solid tumors starting later lines of therapy. Patients should be informed that there are alternatives to inpatient chemotherapy, such as hospice referral or waiting for possible outpatient treatment – if their condition improves. But when a patient wants to try inpatient chemotherapy and their doctor wants to offer it, then it is likely a reasonable thing to try.”
Going forward, he said, “qualitative study is needed to better understand when and why inpatient chemotherapy is used. There are likely some clear good uses and some clear bad uses of inpatient chemotherapy. Can outpatient regimens be substituted for the regimens where patients are directly admitted? Or, can outpatient protocols be devised for these regimens? Are there specific situations where inpatient chemotherapy is the right thing (leukemia, esophageal cancer with worsening dysphagia, etc.)?”
No study funding was received.
FROM ASCO 2022
Twin study offers new insight into genetics of migraine
DENVER – , even though testosterone is thought to be protective. The findings, presented at the annual meeting of the American Headache Society, also hint at a possible role played by the prenatal environment.
The study marks the first time a large-scale twin dataset has been used to assess sex differences in underlying genetic factors of migraine, lead author Morgan Fitzgerald, a senior research associate at the University of California at San Diego, said in a presentation at the conference. The findings were previously published in Frontiers in Pain Research.
More than genetics
The researchers analyzed data regarding 51,872 participants in the Swedish Twin Registry. According to Dr. Fitzgerald, the database is ideal because it is large and includes both genders.
Per the database, female twins were more likely to have migraines without aura than were male twins (17.6% vs. 5.5%, respectively), reflecting global numbers that suggest 18% of females and 6% of males have migraines each year.
To better understand heritability, the researchers compared identical twins with fraternal twins, and looked for gender-related correlations, Dr. Fitzgerald said.
One analysis suggests that migraine is equally heritable in men and women with a broad sense heritability of 0.45 (95% confidence interval [CI], 0.40-0.50). However, another analysis model provides evidence “that there are differences in the underlying genetic factors contributing to migraine across males and females,” she said.
Unexpectedly, the researchers also found that females with male twins were more likely to have migraines than were those with female twins (odds ratio, 1.51, 95% CI, 1.26-1.81) even though males are less affected by the headaches.
“These results suggest that the prominent sex difference in migraine prevalence is not entirely accounted for by genetic factors, while demonstrating that masculinization of the prenatal environment may increase migraine risk for females,” the authors wrote in the published study. “This effect points to a potential prenatal neuroendocrine factor in the development of migraine.”
Probing the migraine gender gap
Commenting on the research, University of Texas at Dallas neuroscientist and headache researcher Gregory Dussor, PhD, said the new study is “a very unique approach to address the question of nature versus nurture in migraine. It was well designed and used robust statistical modeling.”
As for the findings, “the conclusion that genetics do not explain sex differences in migraine risk by themselves is not surprising given how big of a role hormones in later life are likely to play in the disease and how many factors there are that can influence hormone levels,” he said.
“On the other hand, the surprising part of the findings was that the presence of a male co-twin increases risk of migraine in females. I would have expected to see the opposite, given the lower prevalence of migraine in males and the seemingly protective role that male hormones can play in migraine.”
Overall, the study adds to data implicating environment and hormones in the migraine gender gap, he said. “One thing I wonder from this study is what influence a female co-twin growing up with a male co-twin can have on migraine susceptibility. That female co-twin may end up with a very different set of childhood experiences than if she was with another female co-twin. Twins generally spend an enormous amount of time together and the same sex versus opposite sex experiences are likely to be quite different. This may have an influence on migraine later in life.”
As for the value of the study in terms of diagnosis, treatment, or prevention of migraine, Dr. Dussor said, “it’s possible it could help to identify risk factors for higher migraine susceptibility but it’s far too early to know how this could be used.”
The authors have no disclosures. Dr. Dussor disclosed an NIH-funded grant to study the role of the hormone prolactin in preclinical migraine models.
DENVER – , even though testosterone is thought to be protective. The findings, presented at the annual meeting of the American Headache Society, also hint at a possible role played by the prenatal environment.
The study marks the first time a large-scale twin dataset has been used to assess sex differences in underlying genetic factors of migraine, lead author Morgan Fitzgerald, a senior research associate at the University of California at San Diego, said in a presentation at the conference. The findings were previously published in Frontiers in Pain Research.
More than genetics
The researchers analyzed data regarding 51,872 participants in the Swedish Twin Registry. According to Dr. Fitzgerald, the database is ideal because it is large and includes both genders.
Per the database, female twins were more likely to have migraines without aura than were male twins (17.6% vs. 5.5%, respectively), reflecting global numbers that suggest 18% of females and 6% of males have migraines each year.
To better understand heritability, the researchers compared identical twins with fraternal twins, and looked for gender-related correlations, Dr. Fitzgerald said.
One analysis suggests that migraine is equally heritable in men and women with a broad sense heritability of 0.45 (95% confidence interval [CI], 0.40-0.50). However, another analysis model provides evidence “that there are differences in the underlying genetic factors contributing to migraine across males and females,” she said.
Unexpectedly, the researchers also found that females with male twins were more likely to have migraines than were those with female twins (odds ratio, 1.51, 95% CI, 1.26-1.81) even though males are less affected by the headaches.
“These results suggest that the prominent sex difference in migraine prevalence is not entirely accounted for by genetic factors, while demonstrating that masculinization of the prenatal environment may increase migraine risk for females,” the authors wrote in the published study. “This effect points to a potential prenatal neuroendocrine factor in the development of migraine.”
Probing the migraine gender gap
Commenting on the research, University of Texas at Dallas neuroscientist and headache researcher Gregory Dussor, PhD, said the new study is “a very unique approach to address the question of nature versus nurture in migraine. It was well designed and used robust statistical modeling.”
As for the findings, “the conclusion that genetics do not explain sex differences in migraine risk by themselves is not surprising given how big of a role hormones in later life are likely to play in the disease and how many factors there are that can influence hormone levels,” he said.
“On the other hand, the surprising part of the findings was that the presence of a male co-twin increases risk of migraine in females. I would have expected to see the opposite, given the lower prevalence of migraine in males and the seemingly protective role that male hormones can play in migraine.”
Overall, the study adds to data implicating environment and hormones in the migraine gender gap, he said. “One thing I wonder from this study is what influence a female co-twin growing up with a male co-twin can have on migraine susceptibility. That female co-twin may end up with a very different set of childhood experiences than if she was with another female co-twin. Twins generally spend an enormous amount of time together and the same sex versus opposite sex experiences are likely to be quite different. This may have an influence on migraine later in life.”
As for the value of the study in terms of diagnosis, treatment, or prevention of migraine, Dr. Dussor said, “it’s possible it could help to identify risk factors for higher migraine susceptibility but it’s far too early to know how this could be used.”
The authors have no disclosures. Dr. Dussor disclosed an NIH-funded grant to study the role of the hormone prolactin in preclinical migraine models.
DENVER – , even though testosterone is thought to be protective. The findings, presented at the annual meeting of the American Headache Society, also hint at a possible role played by the prenatal environment.
The study marks the first time a large-scale twin dataset has been used to assess sex differences in underlying genetic factors of migraine, lead author Morgan Fitzgerald, a senior research associate at the University of California at San Diego, said in a presentation at the conference. The findings were previously published in Frontiers in Pain Research.
More than genetics
The researchers analyzed data regarding 51,872 participants in the Swedish Twin Registry. According to Dr. Fitzgerald, the database is ideal because it is large and includes both genders.
Per the database, female twins were more likely to have migraines without aura than were male twins (17.6% vs. 5.5%, respectively), reflecting global numbers that suggest 18% of females and 6% of males have migraines each year.
To better understand heritability, the researchers compared identical twins with fraternal twins, and looked for gender-related correlations, Dr. Fitzgerald said.
One analysis suggests that migraine is equally heritable in men and women with a broad sense heritability of 0.45 (95% confidence interval [CI], 0.40-0.50). However, another analysis model provides evidence “that there are differences in the underlying genetic factors contributing to migraine across males and females,” she said.
Unexpectedly, the researchers also found that females with male twins were more likely to have migraines than were those with female twins (odds ratio, 1.51, 95% CI, 1.26-1.81) even though males are less affected by the headaches.
“These results suggest that the prominent sex difference in migraine prevalence is not entirely accounted for by genetic factors, while demonstrating that masculinization of the prenatal environment may increase migraine risk for females,” the authors wrote in the published study. “This effect points to a potential prenatal neuroendocrine factor in the development of migraine.”
Probing the migraine gender gap
Commenting on the research, University of Texas at Dallas neuroscientist and headache researcher Gregory Dussor, PhD, said the new study is “a very unique approach to address the question of nature versus nurture in migraine. It was well designed and used robust statistical modeling.”
As for the findings, “the conclusion that genetics do not explain sex differences in migraine risk by themselves is not surprising given how big of a role hormones in later life are likely to play in the disease and how many factors there are that can influence hormone levels,” he said.
“On the other hand, the surprising part of the findings was that the presence of a male co-twin increases risk of migraine in females. I would have expected to see the opposite, given the lower prevalence of migraine in males and the seemingly protective role that male hormones can play in migraine.”
Overall, the study adds to data implicating environment and hormones in the migraine gender gap, he said. “One thing I wonder from this study is what influence a female co-twin growing up with a male co-twin can have on migraine susceptibility. That female co-twin may end up with a very different set of childhood experiences than if she was with another female co-twin. Twins generally spend an enormous amount of time together and the same sex versus opposite sex experiences are likely to be quite different. This may have an influence on migraine later in life.”
As for the value of the study in terms of diagnosis, treatment, or prevention of migraine, Dr. Dussor said, “it’s possible it could help to identify risk factors for higher migraine susceptibility but it’s far too early to know how this could be used.”
The authors have no disclosures. Dr. Dussor disclosed an NIH-funded grant to study the role of the hormone prolactin in preclinical migraine models.
AT AHS 2022
Autoimmune disorder drugs top list of meds linked to headache
DENVER – in a federal side effect database that anyone can contribute to, according to a new study presented at the annual meeting of the American Headache Society.
“Surprising findings included the significant number of immunosuppressants and immunomodulators present in the data,” study lead author Brett Musialowicz, a medical student at Robert Wood Johnson Medical School, New Brunswich, N.J., said in an interview. “Additionally, our data provides evidence that suggests that several medications belonging to these drug classes were less likely to be associated with medication-induced headaches,” raising questions about the mechanism.
Drugs most frequently linked to headaches
The researchers launched their study to better understand headache as a side effect of medication use, Mr. Musialowicz said. They analyzed entries from the Food and Drug Administration’s Adverse Event Reporting System for the period from July 2018 to March 2020 and listed the top 30 most commonly reported medications linked to headaches and their reported odds ratio. According to a website devoted to pharmacovigilance training, ROR refers to “the odds of a certain event occurring with your medicinal product, compared with the odds of the same event occurring with all other medicinal products in the database.”
After generic and brand name data was consolidated, the drug most frequently linked to headaches was apremilast with 8,672 reports, followed by adalimumab (5,357), tofacitinib (4,276), fingolimod (4,123), and etanercept (4,111). These drugs treat autoimmune disorders such as psoriasis, multiple sclerosis, and Crohn’s disease.
The other drugs in the top 15 ranked by frequency are treatments for hepatitis C (4 drugs), pulmonary arterial hypertension (4 drugs), arthritis (1 drug), and asthma (1 drug).
Of the top 30 drugs most frequently linked to headaches, the pulmonary hypertension drug epoprostenol – ranked 23rd – had the highest ROR at 12.8. The next highest were the hepatitis C drugs glecaprevir and pibrentasvir, tied at 10th in the frequency analysis and both with an ROR of 9.4.
“Pulmonary arterial dilators and vasodilators are believed to cause headaches by sensitizing extracranial arteries. Clinical evidence suggests there a vascular component to some types of headache,” Mr. Musialowicz said. “Monoclonal antibodies are suggested to cause headache by means of an immune response. Several monoclonal antibodies are in trials targeting [the calcitonin gene-related peptide] receptor, which is believed to be involved in migraine headache. These trials will help further elucidate the mechanisms of headache and potential drugs to treat these conditions.”
Is the data useful?
Stewart Tepper, MD, a neurologist at Geisel School of Medicine at Dartmouth, Hanover, N.H., who’s familiar with the study findings, discounted the new research in an interview. He noted that any member of the public can contribute to the federal database of adverse effects (drug manufacturers are required to contribute to it), and the data says nothing about denominators.
“It’s not a reasonable way to evaluate adverse effects, to just have everyone and their uncle saying ‘This particular drug did this to me.’ It’s not in any way useful,” he said. However, he added that the database sometimes “gives you a bit of a signal so you can go back and try to get scientifically collected data.”
When asked to respond, study coauthor and neurologist Pengfei (Phil) Zhang, MD, of Robert Wood Johnson Medical School, noted that the FDA created the database “for a reason.” He also noted that the researchers used a statistical analysis technique – ROR – that was invented to adjust for weaknesses in databases.
No study funding is reported. Mr. Musialowicz reported no disclosures. Dr. Zhang has received honorarium from Alder Biopharmaceuticals, Board Vitals, and Fieve Clinical Research. He collaborates with Headache Science Incorporated without receiving financial support, and he has ownership interest in Cymbeline. Another author reports research grant support from the American Epilepsy Society and the New Jersey Health Foundation. Dr. Tepper reported multiple disclosures.
DENVER – in a federal side effect database that anyone can contribute to, according to a new study presented at the annual meeting of the American Headache Society.
“Surprising findings included the significant number of immunosuppressants and immunomodulators present in the data,” study lead author Brett Musialowicz, a medical student at Robert Wood Johnson Medical School, New Brunswich, N.J., said in an interview. “Additionally, our data provides evidence that suggests that several medications belonging to these drug classes were less likely to be associated with medication-induced headaches,” raising questions about the mechanism.
Drugs most frequently linked to headaches
The researchers launched their study to better understand headache as a side effect of medication use, Mr. Musialowicz said. They analyzed entries from the Food and Drug Administration’s Adverse Event Reporting System for the period from July 2018 to March 2020 and listed the top 30 most commonly reported medications linked to headaches and their reported odds ratio. According to a website devoted to pharmacovigilance training, ROR refers to “the odds of a certain event occurring with your medicinal product, compared with the odds of the same event occurring with all other medicinal products in the database.”
After generic and brand name data was consolidated, the drug most frequently linked to headaches was apremilast with 8,672 reports, followed by adalimumab (5,357), tofacitinib (4,276), fingolimod (4,123), and etanercept (4,111). These drugs treat autoimmune disorders such as psoriasis, multiple sclerosis, and Crohn’s disease.
The other drugs in the top 15 ranked by frequency are treatments for hepatitis C (4 drugs), pulmonary arterial hypertension (4 drugs), arthritis (1 drug), and asthma (1 drug).
Of the top 30 drugs most frequently linked to headaches, the pulmonary hypertension drug epoprostenol – ranked 23rd – had the highest ROR at 12.8. The next highest were the hepatitis C drugs glecaprevir and pibrentasvir, tied at 10th in the frequency analysis and both with an ROR of 9.4.
“Pulmonary arterial dilators and vasodilators are believed to cause headaches by sensitizing extracranial arteries. Clinical evidence suggests there a vascular component to some types of headache,” Mr. Musialowicz said. “Monoclonal antibodies are suggested to cause headache by means of an immune response. Several monoclonal antibodies are in trials targeting [the calcitonin gene-related peptide] receptor, which is believed to be involved in migraine headache. These trials will help further elucidate the mechanisms of headache and potential drugs to treat these conditions.”
Is the data useful?
Stewart Tepper, MD, a neurologist at Geisel School of Medicine at Dartmouth, Hanover, N.H., who’s familiar with the study findings, discounted the new research in an interview. He noted that any member of the public can contribute to the federal database of adverse effects (drug manufacturers are required to contribute to it), and the data says nothing about denominators.
“It’s not a reasonable way to evaluate adverse effects, to just have everyone and their uncle saying ‘This particular drug did this to me.’ It’s not in any way useful,” he said. However, he added that the database sometimes “gives you a bit of a signal so you can go back and try to get scientifically collected data.”
When asked to respond, study coauthor and neurologist Pengfei (Phil) Zhang, MD, of Robert Wood Johnson Medical School, noted that the FDA created the database “for a reason.” He also noted that the researchers used a statistical analysis technique – ROR – that was invented to adjust for weaknesses in databases.
No study funding is reported. Mr. Musialowicz reported no disclosures. Dr. Zhang has received honorarium from Alder Biopharmaceuticals, Board Vitals, and Fieve Clinical Research. He collaborates with Headache Science Incorporated without receiving financial support, and he has ownership interest in Cymbeline. Another author reports research grant support from the American Epilepsy Society and the New Jersey Health Foundation. Dr. Tepper reported multiple disclosures.
DENVER – in a federal side effect database that anyone can contribute to, according to a new study presented at the annual meeting of the American Headache Society.
“Surprising findings included the significant number of immunosuppressants and immunomodulators present in the data,” study lead author Brett Musialowicz, a medical student at Robert Wood Johnson Medical School, New Brunswich, N.J., said in an interview. “Additionally, our data provides evidence that suggests that several medications belonging to these drug classes were less likely to be associated with medication-induced headaches,” raising questions about the mechanism.
Drugs most frequently linked to headaches
The researchers launched their study to better understand headache as a side effect of medication use, Mr. Musialowicz said. They analyzed entries from the Food and Drug Administration’s Adverse Event Reporting System for the period from July 2018 to March 2020 and listed the top 30 most commonly reported medications linked to headaches and their reported odds ratio. According to a website devoted to pharmacovigilance training, ROR refers to “the odds of a certain event occurring with your medicinal product, compared with the odds of the same event occurring with all other medicinal products in the database.”
After generic and brand name data was consolidated, the drug most frequently linked to headaches was apremilast with 8,672 reports, followed by adalimumab (5,357), tofacitinib (4,276), fingolimod (4,123), and etanercept (4,111). These drugs treat autoimmune disorders such as psoriasis, multiple sclerosis, and Crohn’s disease.
The other drugs in the top 15 ranked by frequency are treatments for hepatitis C (4 drugs), pulmonary arterial hypertension (4 drugs), arthritis (1 drug), and asthma (1 drug).
Of the top 30 drugs most frequently linked to headaches, the pulmonary hypertension drug epoprostenol – ranked 23rd – had the highest ROR at 12.8. The next highest were the hepatitis C drugs glecaprevir and pibrentasvir, tied at 10th in the frequency analysis and both with an ROR of 9.4.
“Pulmonary arterial dilators and vasodilators are believed to cause headaches by sensitizing extracranial arteries. Clinical evidence suggests there a vascular component to some types of headache,” Mr. Musialowicz said. “Monoclonal antibodies are suggested to cause headache by means of an immune response. Several monoclonal antibodies are in trials targeting [the calcitonin gene-related peptide] receptor, which is believed to be involved in migraine headache. These trials will help further elucidate the mechanisms of headache and potential drugs to treat these conditions.”
Is the data useful?
Stewart Tepper, MD, a neurologist at Geisel School of Medicine at Dartmouth, Hanover, N.H., who’s familiar with the study findings, discounted the new research in an interview. He noted that any member of the public can contribute to the federal database of adverse effects (drug manufacturers are required to contribute to it), and the data says nothing about denominators.
“It’s not a reasonable way to evaluate adverse effects, to just have everyone and their uncle saying ‘This particular drug did this to me.’ It’s not in any way useful,” he said. However, he added that the database sometimes “gives you a bit of a signal so you can go back and try to get scientifically collected data.”
When asked to respond, study coauthor and neurologist Pengfei (Phil) Zhang, MD, of Robert Wood Johnson Medical School, noted that the FDA created the database “for a reason.” He also noted that the researchers used a statistical analysis technique – ROR – that was invented to adjust for weaknesses in databases.
No study funding is reported. Mr. Musialowicz reported no disclosures. Dr. Zhang has received honorarium from Alder Biopharmaceuticals, Board Vitals, and Fieve Clinical Research. He collaborates with Headache Science Incorporated without receiving financial support, and he has ownership interest in Cymbeline. Another author reports research grant support from the American Epilepsy Society and the New Jersey Health Foundation. Dr. Tepper reported multiple disclosures.
AT AHS 2022





