User login
Here on Earth
We live inundated with promises that technology will solve our most challenging problems, yet we are regularly disappointed when it does not. New technological solutions seem to appear daily, and we feel like we are falling behind if we do not jump to join the people who are implementing, selling, or imposing new solutions. Often these solutions are offered before the problem is even fully understood, and no assessment has been made to determine if the solution actually helps to solve the challenge identified. With 80% of us now having transitioned to EHRs, we know full well their benefits as well as their pitfalls. While we have mostly accommodated to electronic documentation, we are now at the point where we are beginning to explore some of the most exciting potential benefits of our EHRs – population health, enhanced data on medication adherence, and improved patient communication. As we look at this next stage of growth, we are reminded of a lesson from an old joke:
A rabbi dies and goes to heaven. When he gets there he is given an old robe and a wooden walking stick and is told to get in line to the entrance to heaven. While the rabbi waits in the long line, a taxi driver walks up and is greeted by a group of angels blowing their horns announcing his arrival. One of the angels walks over to the driver and gives him a flowing white satin robe and a golden walking stick. Another angel then escorts him to the front of the line.
The angel turned toward him, smiled, and shook his head. “Yes, yes,” the angel replied, “We know all that. But, here in heaven we care about results, not intent. While you gave your sermons, people slept. When the cab driver drove, people prayed.”
As we look ahead to the next generation of electronic health records, there are going to be many creative ideas of how to use them to help patients improve their health and take care of their diseases. One of the more notable new technologies over the last 5 years is the development of wearable health devices. Innovations like the Apple Watch, Fitbit, and other wearables allow us to track our activity and diet, and encourage us to behave better. They do this by providing constant feedback on how we are doing, and they offer the ability to use social groups to encourage sustained behavioral change. Some devices tell us regularly how far we have walked while others let us know when we have been sitting too long. As we input information about diet, the devices and their associated apps give us feedback on how we are adhering to our dietary goals. Some even allow data to be funneled into the EHR so that physicians can review the behavioral changes and track patient progress. The challenge that arises is that the technology itself is so fascinating and so filled with promise that it is easy to forget what is most important: ensuring it works not just to keep us engaged and busy but also to help us accomplish the real goals we have defined for its use.
Wearable technology is now the most recent and dramatic example of how the excitement over technology may be outpacing its utility. Most of us have tried (or have patients, friends and family who have tried) wearable technology solutions to track and encourage behavioral change. A recent article published in JAMA looked at more than 400 individuals randomized to a standard behavioral weight-loss intervention vs. a technology-enhanced weight loss intervention using a wearable device over 24 months. It was fairly obvious that the group with the wearable device would do better, and have improved fitness and more weight loss. It was obvious … except that is not what happened. Both groups improved equally in fitness, and the standard intervention group lost significantly more weight over 24 months than did the wearable technology group.
There are many reasons that this might have happened. It may be that the idea of this quick feedback loop is in itself flawed, or it may be that the devices and/or the dietary input is simply imprecise, causing people to think that they are doing better than they really are (and then modifying their behavior in the wrong direction). Whatever the explanation, seeing those results, I think again of the moral handed down though generations by that old joke – that here on earth we need to care less about intent and more about results.
Reference
Jakicic JM, et al. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss The IDEA Randomized Clinical Trial. JAMA. 2016;316[11]:1161-71. doi: 10.1001/jama.2016.12858
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
We live inundated with promises that technology will solve our most challenging problems, yet we are regularly disappointed when it does not. New technological solutions seem to appear daily, and we feel like we are falling behind if we do not jump to join the people who are implementing, selling, or imposing new solutions. Often these solutions are offered before the problem is even fully understood, and no assessment has been made to determine if the solution actually helps to solve the challenge identified. With 80% of us now having transitioned to EHRs, we know full well their benefits as well as their pitfalls. While we have mostly accommodated to electronic documentation, we are now at the point where we are beginning to explore some of the most exciting potential benefits of our EHRs – population health, enhanced data on medication adherence, and improved patient communication. As we look at this next stage of growth, we are reminded of a lesson from an old joke:
A rabbi dies and goes to heaven. When he gets there he is given an old robe and a wooden walking stick and is told to get in line to the entrance to heaven. While the rabbi waits in the long line, a taxi driver walks up and is greeted by a group of angels blowing their horns announcing his arrival. One of the angels walks over to the driver and gives him a flowing white satin robe and a golden walking stick. Another angel then escorts him to the front of the line.
The angel turned toward him, smiled, and shook his head. “Yes, yes,” the angel replied, “We know all that. But, here in heaven we care about results, not intent. While you gave your sermons, people slept. When the cab driver drove, people prayed.”
As we look ahead to the next generation of electronic health records, there are going to be many creative ideas of how to use them to help patients improve their health and take care of their diseases. One of the more notable new technologies over the last 5 years is the development of wearable health devices. Innovations like the Apple Watch, Fitbit, and other wearables allow us to track our activity and diet, and encourage us to behave better. They do this by providing constant feedback on how we are doing, and they offer the ability to use social groups to encourage sustained behavioral change. Some devices tell us regularly how far we have walked while others let us know when we have been sitting too long. As we input information about diet, the devices and their associated apps give us feedback on how we are adhering to our dietary goals. Some even allow data to be funneled into the EHR so that physicians can review the behavioral changes and track patient progress. The challenge that arises is that the technology itself is so fascinating and so filled with promise that it is easy to forget what is most important: ensuring it works not just to keep us engaged and busy but also to help us accomplish the real goals we have defined for its use.
Wearable technology is now the most recent and dramatic example of how the excitement over technology may be outpacing its utility. Most of us have tried (or have patients, friends and family who have tried) wearable technology solutions to track and encourage behavioral change. A recent article published in JAMA looked at more than 400 individuals randomized to a standard behavioral weight-loss intervention vs. a technology-enhanced weight loss intervention using a wearable device over 24 months. It was fairly obvious that the group with the wearable device would do better, and have improved fitness and more weight loss. It was obvious … except that is not what happened. Both groups improved equally in fitness, and the standard intervention group lost significantly more weight over 24 months than did the wearable technology group.
There are many reasons that this might have happened. It may be that the idea of this quick feedback loop is in itself flawed, or it may be that the devices and/or the dietary input is simply imprecise, causing people to think that they are doing better than they really are (and then modifying their behavior in the wrong direction). Whatever the explanation, seeing those results, I think again of the moral handed down though generations by that old joke – that here on earth we need to care less about intent and more about results.
Reference
Jakicic JM, et al. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss The IDEA Randomized Clinical Trial. JAMA. 2016;316[11]:1161-71. doi: 10.1001/jama.2016.12858
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
We live inundated with promises that technology will solve our most challenging problems, yet we are regularly disappointed when it does not. New technological solutions seem to appear daily, and we feel like we are falling behind if we do not jump to join the people who are implementing, selling, or imposing new solutions. Often these solutions are offered before the problem is even fully understood, and no assessment has been made to determine if the solution actually helps to solve the challenge identified. With 80% of us now having transitioned to EHRs, we know full well their benefits as well as their pitfalls. While we have mostly accommodated to electronic documentation, we are now at the point where we are beginning to explore some of the most exciting potential benefits of our EHRs – population health, enhanced data on medication adherence, and improved patient communication. As we look at this next stage of growth, we are reminded of a lesson from an old joke:
A rabbi dies and goes to heaven. When he gets there he is given an old robe and a wooden walking stick and is told to get in line to the entrance to heaven. While the rabbi waits in the long line, a taxi driver walks up and is greeted by a group of angels blowing their horns announcing his arrival. One of the angels walks over to the driver and gives him a flowing white satin robe and a golden walking stick. Another angel then escorts him to the front of the line.
The angel turned toward him, smiled, and shook his head. “Yes, yes,” the angel replied, “We know all that. But, here in heaven we care about results, not intent. While you gave your sermons, people slept. When the cab driver drove, people prayed.”
As we look ahead to the next generation of electronic health records, there are going to be many creative ideas of how to use them to help patients improve their health and take care of their diseases. One of the more notable new technologies over the last 5 years is the development of wearable health devices. Innovations like the Apple Watch, Fitbit, and other wearables allow us to track our activity and diet, and encourage us to behave better. They do this by providing constant feedback on how we are doing, and they offer the ability to use social groups to encourage sustained behavioral change. Some devices tell us regularly how far we have walked while others let us know when we have been sitting too long. As we input information about diet, the devices and their associated apps give us feedback on how we are adhering to our dietary goals. Some even allow data to be funneled into the EHR so that physicians can review the behavioral changes and track patient progress. The challenge that arises is that the technology itself is so fascinating and so filled with promise that it is easy to forget what is most important: ensuring it works not just to keep us engaged and busy but also to help us accomplish the real goals we have defined for its use.
Wearable technology is now the most recent and dramatic example of how the excitement over technology may be outpacing its utility. Most of us have tried (or have patients, friends and family who have tried) wearable technology solutions to track and encourage behavioral change. A recent article published in JAMA looked at more than 400 individuals randomized to a standard behavioral weight-loss intervention vs. a technology-enhanced weight loss intervention using a wearable device over 24 months. It was fairly obvious that the group with the wearable device would do better, and have improved fitness and more weight loss. It was obvious … except that is not what happened. Both groups improved equally in fitness, and the standard intervention group lost significantly more weight over 24 months than did the wearable technology group.
There are many reasons that this might have happened. It may be that the idea of this quick feedback loop is in itself flawed, or it may be that the devices and/or the dietary input is simply imprecise, causing people to think that they are doing better than they really are (and then modifying their behavior in the wrong direction). Whatever the explanation, seeing those results, I think again of the moral handed down though generations by that old joke – that here on earth we need to care less about intent and more about results.
Reference
Jakicic JM, et al. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss The IDEA Randomized Clinical Trial. JAMA. 2016;316[11]:1161-71. doi: 10.1001/jama.2016.12858
Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
The EHR Report: Seeing through a glass, darkly
Recently, we were invited to take part in an case presentation focused on a young female patient. The reason for our specific invitation was because a key component in the patient’s case was centered on her electronic health record. This kind of story was not new to us – in fact, stories like these are becoming almost common everywhere. But this particular conference promised to be very special, because the patient herself was asked to take an active role and present the story from a unique perspective – her own.
Seated on stage in a hospital gown and accompanied by her I.V. pole, the patient related a 4-month history of symptoms. She had obviously told the story dozens of times – through seemingly endless encounters – to her primary physician, hospital residents, medical students, emergency physicians, and just about anyone else who would lend an ear. Listening to her share her story with a large audience while still a patient in the hospital was incredibly powerful; it was difficult not to become emotional with her as she welled up with tears. She told a complex, though very coherent tale that included her past medical and family histories, her employment, her hobbies, and her unusual signs and symptoms as they developed over an extended period of time. When the patient was done, her case was presented from another perspective: the way it was recorded in her electronic record. For a brief moment, those seated in the audience were confused. Then the theme of the conference became evident – these were completely different stories.
This was no case of mistaken identity or registration error. The chart presented at the conference did belong to the patient, but the story told by the chart was wrong. Reading through the chart, it would be easy to come away with the same sense as her care team; this must simply be a common illness that wasn’t responding to conventional treatment. Encounter after encounter, a new plan was devised to address the presumed diagnosis. But the patient’s telling of the history barely mentioned any symptoms related to that diagnosis. Her version focused more on how her life was affected, how she could no longer take care of her daughter, how she could no longer exercise (which she did avidly), and how she was sinking deeper into despair and losing hope. Woven through all of this were the historical details and seemingly obvious physical manifestations that might easily disclose the real cause of her symptoms. A few basic questions about her family history would also reveal multiple immediate family members who suffered from the same disease! But even if these questions had been asked, and even if the story had been heard, the image in the mirror – her chart – did not reflect an accurate understanding of the patient.
We often solicit comments from readers, and the response is alway encouraging. It is clear that our colleagues in the medical community feel a strong sense of obligation to their patients and care deeply for maintaining the sanctity of the physician-patient relationship. However, many feel the electronic health record has become a barrier to developing and nurturing that relationship, standing in the way understanding their stories. One poignant letter from a cardiologist in Florida, Eugene H. Eisman, MD, does a beautiful job in crystallizing this sentiment. Dr. Eisman writes:
“Many of my patients end up hospitalized where I do not have privileges. Almost every attending is attentive enough to send me a discharge summary. These, however, are EHR-generated. The patient may have been hospitalized for 3 days, yet the summary is seven pages long. It is filled with total nonsense, such as whether the patient had traveled to North Africa (even though he was hospitalized with a fractured hip while skiing in Colorado). The attending has managed to cut and paste reports of every chest film and CBC, and I have pages of normal studies. The final diagnosis and discharge medications are difficult to find in this morass of words. I cannot force myself to read this document, and it is thrown into his chart after a cursory glance. Yet, I can’t sleep at night. Is there buried in this seven-page document a discovery of malignancy, etc.?”
Dr. Eisman’s words are powerful because they reveal an oft-overlooked truth about modern medical records. The patient’s chart, once considered a sacred text containing the key inflection points in a patient’s story, has become merely a filing cabinet in which to stuff every piece of data about the patient, no matter how mundane or trivial. No thought need be put into preserving the important details, because now absolutely every detail can be included. We have become so overloaded with the unimportant, that we may lose the truly critical in this sea of information. It has become, therefore, imperative that physicians rediscover the patient in their story, and not rely solely on the poor reflection we may find in their chart.
Thousands of years ago, the apostle Paul wrote that “we see now as through a glass, darkly.” Borrowing from his original meaning, these ancient words have been quoted throughout literature to describe an “incomplete understanding,” often mixed with a state of despair. Today, we might think of the electronic record as the glass – or mirror – reflecting the patient’s story. Ironically, in spite of having more information than ever, the image we see may be incomplete, and possibly even wrong altogether. While the amount of available data may at first glance appear enlightening, the reflection in the glass may be rather dark indeed.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. An avid programmer, he has published software for handheld devices in partnership with national organizations, and he is always looking for new ways to bring evidence-based medicine to the point of care. Neil Skolnik, MD, is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is also editor-in-chief of Redi-Reference Inc., a software company that creates mobile apps.
Recently, we were invited to take part in an case presentation focused on a young female patient. The reason for our specific invitation was because a key component in the patient’s case was centered on her electronic health record. This kind of story was not new to us – in fact, stories like these are becoming almost common everywhere. But this particular conference promised to be very special, because the patient herself was asked to take an active role and present the story from a unique perspective – her own.
Seated on stage in a hospital gown and accompanied by her I.V. pole, the patient related a 4-month history of symptoms. She had obviously told the story dozens of times – through seemingly endless encounters – to her primary physician, hospital residents, medical students, emergency physicians, and just about anyone else who would lend an ear. Listening to her share her story with a large audience while still a patient in the hospital was incredibly powerful; it was difficult not to become emotional with her as she welled up with tears. She told a complex, though very coherent tale that included her past medical and family histories, her employment, her hobbies, and her unusual signs and symptoms as they developed over an extended period of time. When the patient was done, her case was presented from another perspective: the way it was recorded in her electronic record. For a brief moment, those seated in the audience were confused. Then the theme of the conference became evident – these were completely different stories.
This was no case of mistaken identity or registration error. The chart presented at the conference did belong to the patient, but the story told by the chart was wrong. Reading through the chart, it would be easy to come away with the same sense as her care team; this must simply be a common illness that wasn’t responding to conventional treatment. Encounter after encounter, a new plan was devised to address the presumed diagnosis. But the patient’s telling of the history barely mentioned any symptoms related to that diagnosis. Her version focused more on how her life was affected, how she could no longer take care of her daughter, how she could no longer exercise (which she did avidly), and how she was sinking deeper into despair and losing hope. Woven through all of this were the historical details and seemingly obvious physical manifestations that might easily disclose the real cause of her symptoms. A few basic questions about her family history would also reveal multiple immediate family members who suffered from the same disease! But even if these questions had been asked, and even if the story had been heard, the image in the mirror – her chart – did not reflect an accurate understanding of the patient.
We often solicit comments from readers, and the response is alway encouraging. It is clear that our colleagues in the medical community feel a strong sense of obligation to their patients and care deeply for maintaining the sanctity of the physician-patient relationship. However, many feel the electronic health record has become a barrier to developing and nurturing that relationship, standing in the way understanding their stories. One poignant letter from a cardiologist in Florida, Eugene H. Eisman, MD, does a beautiful job in crystallizing this sentiment. Dr. Eisman writes:
“Many of my patients end up hospitalized where I do not have privileges. Almost every attending is attentive enough to send me a discharge summary. These, however, are EHR-generated. The patient may have been hospitalized for 3 days, yet the summary is seven pages long. It is filled with total nonsense, such as whether the patient had traveled to North Africa (even though he was hospitalized with a fractured hip while skiing in Colorado). The attending has managed to cut and paste reports of every chest film and CBC, and I have pages of normal studies. The final diagnosis and discharge medications are difficult to find in this morass of words. I cannot force myself to read this document, and it is thrown into his chart after a cursory glance. Yet, I can’t sleep at night. Is there buried in this seven-page document a discovery of malignancy, etc.?”
Dr. Eisman’s words are powerful because they reveal an oft-overlooked truth about modern medical records. The patient’s chart, once considered a sacred text containing the key inflection points in a patient’s story, has become merely a filing cabinet in which to stuff every piece of data about the patient, no matter how mundane or trivial. No thought need be put into preserving the important details, because now absolutely every detail can be included. We have become so overloaded with the unimportant, that we may lose the truly critical in this sea of information. It has become, therefore, imperative that physicians rediscover the patient in their story, and not rely solely on the poor reflection we may find in their chart.
Thousands of years ago, the apostle Paul wrote that “we see now as through a glass, darkly.” Borrowing from his original meaning, these ancient words have been quoted throughout literature to describe an “incomplete understanding,” often mixed with a state of despair. Today, we might think of the electronic record as the glass – or mirror – reflecting the patient’s story. Ironically, in spite of having more information than ever, the image we see may be incomplete, and possibly even wrong altogether. While the amount of available data may at first glance appear enlightening, the reflection in the glass may be rather dark indeed.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. An avid programmer, he has published software for handheld devices in partnership with national organizations, and he is always looking for new ways to bring evidence-based medicine to the point of care. Neil Skolnik, MD, is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is also editor-in-chief of Redi-Reference Inc., a software company that creates mobile apps.
Recently, we were invited to take part in an case presentation focused on a young female patient. The reason for our specific invitation was because a key component in the patient’s case was centered on her electronic health record. This kind of story was not new to us – in fact, stories like these are becoming almost common everywhere. But this particular conference promised to be very special, because the patient herself was asked to take an active role and present the story from a unique perspective – her own.
Seated on stage in a hospital gown and accompanied by her I.V. pole, the patient related a 4-month history of symptoms. She had obviously told the story dozens of times – through seemingly endless encounters – to her primary physician, hospital residents, medical students, emergency physicians, and just about anyone else who would lend an ear. Listening to her share her story with a large audience while still a patient in the hospital was incredibly powerful; it was difficult not to become emotional with her as she welled up with tears. She told a complex, though very coherent tale that included her past medical and family histories, her employment, her hobbies, and her unusual signs and symptoms as they developed over an extended period of time. When the patient was done, her case was presented from another perspective: the way it was recorded in her electronic record. For a brief moment, those seated in the audience were confused. Then the theme of the conference became evident – these were completely different stories.
This was no case of mistaken identity or registration error. The chart presented at the conference did belong to the patient, but the story told by the chart was wrong. Reading through the chart, it would be easy to come away with the same sense as her care team; this must simply be a common illness that wasn’t responding to conventional treatment. Encounter after encounter, a new plan was devised to address the presumed diagnosis. But the patient’s telling of the history barely mentioned any symptoms related to that diagnosis. Her version focused more on how her life was affected, how she could no longer take care of her daughter, how she could no longer exercise (which she did avidly), and how she was sinking deeper into despair and losing hope. Woven through all of this were the historical details and seemingly obvious physical manifestations that might easily disclose the real cause of her symptoms. A few basic questions about her family history would also reveal multiple immediate family members who suffered from the same disease! But even if these questions had been asked, and even if the story had been heard, the image in the mirror – her chart – did not reflect an accurate understanding of the patient.
We often solicit comments from readers, and the response is alway encouraging. It is clear that our colleagues in the medical community feel a strong sense of obligation to their patients and care deeply for maintaining the sanctity of the physician-patient relationship. However, many feel the electronic health record has become a barrier to developing and nurturing that relationship, standing in the way understanding their stories. One poignant letter from a cardiologist in Florida, Eugene H. Eisman, MD, does a beautiful job in crystallizing this sentiment. Dr. Eisman writes:
“Many of my patients end up hospitalized where I do not have privileges. Almost every attending is attentive enough to send me a discharge summary. These, however, are EHR-generated. The patient may have been hospitalized for 3 days, yet the summary is seven pages long. It is filled with total nonsense, such as whether the patient had traveled to North Africa (even though he was hospitalized with a fractured hip while skiing in Colorado). The attending has managed to cut and paste reports of every chest film and CBC, and I have pages of normal studies. The final diagnosis and discharge medications are difficult to find in this morass of words. I cannot force myself to read this document, and it is thrown into his chart after a cursory glance. Yet, I can’t sleep at night. Is there buried in this seven-page document a discovery of malignancy, etc.?”
Dr. Eisman’s words are powerful because they reveal an oft-overlooked truth about modern medical records. The patient’s chart, once considered a sacred text containing the key inflection points in a patient’s story, has become merely a filing cabinet in which to stuff every piece of data about the patient, no matter how mundane or trivial. No thought need be put into preserving the important details, because now absolutely every detail can be included. We have become so overloaded with the unimportant, that we may lose the truly critical in this sea of information. It has become, therefore, imperative that physicians rediscover the patient in their story, and not rely solely on the poor reflection we may find in their chart.
Thousands of years ago, the apostle Paul wrote that “we see now as through a glass, darkly.” Borrowing from his original meaning, these ancient words have been quoted throughout literature to describe an “incomplete understanding,” often mixed with a state of despair. Today, we might think of the electronic record as the glass – or mirror – reflecting the patient’s story. Ironically, in spite of having more information than ever, the image we see may be incomplete, and possibly even wrong altogether. While the amount of available data may at first glance appear enlightening, the reflection in the glass may be rather dark indeed.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. An avid programmer, he has published software for handheld devices in partnership with national organizations, and he is always looking for new ways to bring evidence-based medicine to the point of care. Neil Skolnik, MD, is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. He is also editor-in-chief of Redi-Reference Inc., a software company that creates mobile apps.
Brief resolved unexplained events (formerly apparent life-threatening events) and evaluation of lower-risk infants
In this new clinical practice guideline, the American Academy of Pediatrics has recommended that the term “apparent life-threatening events” (ALTEs) be replaced with a new term, “brief resolved unexplained events” (BRUEs). ALTE, proposed in 1986 to replace the term near-SIDS (sudden infant death syndrome), has been defined as an episode that is frightening to the observer and characterized by some combination of apnea, color change, marked change in muscle tone, and/or choking or gagging. Many of these children undergo a comprehensive work-up in addition to the initial history and physical. Children may be admitted for observation, and the admission often includes further evaluation with cardiorespiratory monitoring, labs, and occasionally specialized studies. These tests are usually normal, and patients are discharged home, leaving families to continue to worry that there is an undetected underlying problem.
The ALTE definition is often vague in determination and dependent on a subjective report from caregivers and their perception of the severity of the event. The new term BRUE is based on more stringent, objective criteria. BRUE is defined as occurring in children less than 1 year of age, where an observer reports a sudden, brief now-resolved episode with one or more of the following:
• Cyanosis or pallor.
• Absent, decreased, or irregular breathing.
• A marked change in tone (hypertonia or hypotonia).
• An altered level of responsiveness.
• No explanation for a qualifying event after an appropriate history and physical are conducted.
The BRUE definition differs from that of an ALTE. First, the “life-threatening” qualifier has been removed from both the title and diagnostic criteria. This allows providers to approach the patient with more objectivity, and allows clinical decision making to stem from the evaluation of the child rather than the perceived severity of the event.
“Color change” has been more strictly defined to be only cyanosis or pallor. In the ALTE definition, redness or rubor was an acceptable criterion for diagnosis; however, this is a common finding in healthy newborns.
“Change in muscle tone” has been more specifically defined and must be characterized as hypertonia or hypotonia. Characterizing the change in tone assists providers in investigating specific underlying causes. “Altered level of responsiveness” is a new criterion.
There is a notable absence of “choking or gagging” from the BRUE definition. These are often signs of reflux and upper respiratory infections in the infant. By the very nature of the definition, if a child is diagnosed with an underlying illness, this excludes the diagnosis of BRUE.
Identifying risk factors for repeat events
In addition to using new criteria for diagnosis, providers are also able to characterize infants as higher risk and lower risk. If a child truly has a BRUE, he/she may be diagnosed as higher risk or lower risk for a recurrent episode or SIDS.
A lower-risk infant has the following characteristics:
• Over 60 days old.
• Gestational age greater than 32 weeks; postconception age over 45 weeks.
• First BRUE.
• Duration of event under 1 minute.
• No CPR required by a trained medical provider (not parents).
• No concerning history and physical findings.
Children who are identified as being at higher risk would benefit from further work-up beyond a thorough history and physical. Additional testing may reveal the underlying cause of the episode (congenital cardiac disease, underlying metabolic disorder, abuse), thereby excluding the diagnosis of BRUE. By further characterizing the diagnosis, the new definition allows providers to avoid unnecessary studies in otherwise healthy children.
Key action statements and recommendations
Action statements are recommended for the evaluation of children who are classified as lower risk with BRUE. While not all the action statements can be covered in this review, for lower-risk individuals, clinicians:
• Do not need to admit infants solely for cardiorespiratory monitoring.
• Should not start home cardiorespiratory monitoring, obtain an overnight polysomnogram, a chest radiograph, or an echocardiogram.
• Assess for risk factors in order to detect any possible child abuse.
• Should not obtain neuroimaging to detect neurologic disorders or child abuse, and should not perform an EEG to detect a neurologic disorder.
• Are strongly recommended to refrain from doing a WBC, blood culture, or lumbar puncture with cerebrospinal fluid studies to rule out an occult bacterial infection.
• Should avoid doing an extensive work-up for underlying gastroesophageal reflux (e.g., upper gastrointestinal tract series, endoscopy, pH probe, ultrasound).
• Are encouraged to educate parents and families about BRUEs, and offer resources for CPR training for families and caregivers.
Limitations
While there are many benefits to these new guidelines, there are challenges. ALTE is ingrained in clinical practice, and it may take time for a uniform acceptance of the change in terminology and criteria. Additional limitations come with the lack of evidence of outcomes and impact, as all studies available are based on ALTE criteria, and data will lag in evaluating the utility of conceptualizing events as BRUEs.
The bottom line
BRUE has been proposed to replace the term ALTE for an unexplained witnessed event as defined above. BRUEs differ from ALTEs in that the criteria are more strictly defined, and they allow providers to stratify children as lower risk or higher risk for a recurrent episode or SIDS. By identifying a child’s risk, providers are able to appropriately utilize resources to refrain from doing an extensive medical work-up in a child who is otherwise healthy and low risk for a serious event.
Reference
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Cavanaugh is a second-year resident in the Abington-Jefferson Family Medicine Residency Program.
In this new clinical practice guideline, the American Academy of Pediatrics has recommended that the term “apparent life-threatening events” (ALTEs) be replaced with a new term, “brief resolved unexplained events” (BRUEs). ALTE, proposed in 1986 to replace the term near-SIDS (sudden infant death syndrome), has been defined as an episode that is frightening to the observer and characterized by some combination of apnea, color change, marked change in muscle tone, and/or choking or gagging. Many of these children undergo a comprehensive work-up in addition to the initial history and physical. Children may be admitted for observation, and the admission often includes further evaluation with cardiorespiratory monitoring, labs, and occasionally specialized studies. These tests are usually normal, and patients are discharged home, leaving families to continue to worry that there is an undetected underlying problem.
The ALTE definition is often vague in determination and dependent on a subjective report from caregivers and their perception of the severity of the event. The new term BRUE is based on more stringent, objective criteria. BRUE is defined as occurring in children less than 1 year of age, where an observer reports a sudden, brief now-resolved episode with one or more of the following:
• Cyanosis or pallor.
• Absent, decreased, or irregular breathing.
• A marked change in tone (hypertonia or hypotonia).
• An altered level of responsiveness.
• No explanation for a qualifying event after an appropriate history and physical are conducted.
The BRUE definition differs from that of an ALTE. First, the “life-threatening” qualifier has been removed from both the title and diagnostic criteria. This allows providers to approach the patient with more objectivity, and allows clinical decision making to stem from the evaluation of the child rather than the perceived severity of the event.
“Color change” has been more strictly defined to be only cyanosis or pallor. In the ALTE definition, redness or rubor was an acceptable criterion for diagnosis; however, this is a common finding in healthy newborns.
“Change in muscle tone” has been more specifically defined and must be characterized as hypertonia or hypotonia. Characterizing the change in tone assists providers in investigating specific underlying causes. “Altered level of responsiveness” is a new criterion.
There is a notable absence of “choking or gagging” from the BRUE definition. These are often signs of reflux and upper respiratory infections in the infant. By the very nature of the definition, if a child is diagnosed with an underlying illness, this excludes the diagnosis of BRUE.
Identifying risk factors for repeat events
In addition to using new criteria for diagnosis, providers are also able to characterize infants as higher risk and lower risk. If a child truly has a BRUE, he/she may be diagnosed as higher risk or lower risk for a recurrent episode or SIDS.
A lower-risk infant has the following characteristics:
• Over 60 days old.
• Gestational age greater than 32 weeks; postconception age over 45 weeks.
• First BRUE.
• Duration of event under 1 minute.
• No CPR required by a trained medical provider (not parents).
• No concerning history and physical findings.
Children who are identified as being at higher risk would benefit from further work-up beyond a thorough history and physical. Additional testing may reveal the underlying cause of the episode (congenital cardiac disease, underlying metabolic disorder, abuse), thereby excluding the diagnosis of BRUE. By further characterizing the diagnosis, the new definition allows providers to avoid unnecessary studies in otherwise healthy children.
Key action statements and recommendations
Action statements are recommended for the evaluation of children who are classified as lower risk with BRUE. While not all the action statements can be covered in this review, for lower-risk individuals, clinicians:
• Do not need to admit infants solely for cardiorespiratory monitoring.
• Should not start home cardiorespiratory monitoring, obtain an overnight polysomnogram, a chest radiograph, or an echocardiogram.
• Assess for risk factors in order to detect any possible child abuse.
• Should not obtain neuroimaging to detect neurologic disorders or child abuse, and should not perform an EEG to detect a neurologic disorder.
• Are strongly recommended to refrain from doing a WBC, blood culture, or lumbar puncture with cerebrospinal fluid studies to rule out an occult bacterial infection.
• Should avoid doing an extensive work-up for underlying gastroesophageal reflux (e.g., upper gastrointestinal tract series, endoscopy, pH probe, ultrasound).
• Are encouraged to educate parents and families about BRUEs, and offer resources for CPR training for families and caregivers.
Limitations
While there are many benefits to these new guidelines, there are challenges. ALTE is ingrained in clinical practice, and it may take time for a uniform acceptance of the change in terminology and criteria. Additional limitations come with the lack of evidence of outcomes and impact, as all studies available are based on ALTE criteria, and data will lag in evaluating the utility of conceptualizing events as BRUEs.
The bottom line
BRUE has been proposed to replace the term ALTE for an unexplained witnessed event as defined above. BRUEs differ from ALTEs in that the criteria are more strictly defined, and they allow providers to stratify children as lower risk or higher risk for a recurrent episode or SIDS. By identifying a child’s risk, providers are able to appropriately utilize resources to refrain from doing an extensive medical work-up in a child who is otherwise healthy and low risk for a serious event.
Reference
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Cavanaugh is a second-year resident in the Abington-Jefferson Family Medicine Residency Program.
In this new clinical practice guideline, the American Academy of Pediatrics has recommended that the term “apparent life-threatening events” (ALTEs) be replaced with a new term, “brief resolved unexplained events” (BRUEs). ALTE, proposed in 1986 to replace the term near-SIDS (sudden infant death syndrome), has been defined as an episode that is frightening to the observer and characterized by some combination of apnea, color change, marked change in muscle tone, and/or choking or gagging. Many of these children undergo a comprehensive work-up in addition to the initial history and physical. Children may be admitted for observation, and the admission often includes further evaluation with cardiorespiratory monitoring, labs, and occasionally specialized studies. These tests are usually normal, and patients are discharged home, leaving families to continue to worry that there is an undetected underlying problem.
The ALTE definition is often vague in determination and dependent on a subjective report from caregivers and their perception of the severity of the event. The new term BRUE is based on more stringent, objective criteria. BRUE is defined as occurring in children less than 1 year of age, where an observer reports a sudden, brief now-resolved episode with one or more of the following:
• Cyanosis or pallor.
• Absent, decreased, or irregular breathing.
• A marked change in tone (hypertonia or hypotonia).
• An altered level of responsiveness.
• No explanation for a qualifying event after an appropriate history and physical are conducted.
The BRUE definition differs from that of an ALTE. First, the “life-threatening” qualifier has been removed from both the title and diagnostic criteria. This allows providers to approach the patient with more objectivity, and allows clinical decision making to stem from the evaluation of the child rather than the perceived severity of the event.
“Color change” has been more strictly defined to be only cyanosis or pallor. In the ALTE definition, redness or rubor was an acceptable criterion for diagnosis; however, this is a common finding in healthy newborns.
“Change in muscle tone” has been more specifically defined and must be characterized as hypertonia or hypotonia. Characterizing the change in tone assists providers in investigating specific underlying causes. “Altered level of responsiveness” is a new criterion.
There is a notable absence of “choking or gagging” from the BRUE definition. These are often signs of reflux and upper respiratory infections in the infant. By the very nature of the definition, if a child is diagnosed with an underlying illness, this excludes the diagnosis of BRUE.
Identifying risk factors for repeat events
In addition to using new criteria for diagnosis, providers are also able to characterize infants as higher risk and lower risk. If a child truly has a BRUE, he/she may be diagnosed as higher risk or lower risk for a recurrent episode or SIDS.
A lower-risk infant has the following characteristics:
• Over 60 days old.
• Gestational age greater than 32 weeks; postconception age over 45 weeks.
• First BRUE.
• Duration of event under 1 minute.
• No CPR required by a trained medical provider (not parents).
• No concerning history and physical findings.
Children who are identified as being at higher risk would benefit from further work-up beyond a thorough history and physical. Additional testing may reveal the underlying cause of the episode (congenital cardiac disease, underlying metabolic disorder, abuse), thereby excluding the diagnosis of BRUE. By further characterizing the diagnosis, the new definition allows providers to avoid unnecessary studies in otherwise healthy children.
Key action statements and recommendations
Action statements are recommended for the evaluation of children who are classified as lower risk with BRUE. While not all the action statements can be covered in this review, for lower-risk individuals, clinicians:
• Do not need to admit infants solely for cardiorespiratory monitoring.
• Should not start home cardiorespiratory monitoring, obtain an overnight polysomnogram, a chest radiograph, or an echocardiogram.
• Assess for risk factors in order to detect any possible child abuse.
• Should not obtain neuroimaging to detect neurologic disorders or child abuse, and should not perform an EEG to detect a neurologic disorder.
• Are strongly recommended to refrain from doing a WBC, blood culture, or lumbar puncture with cerebrospinal fluid studies to rule out an occult bacterial infection.
• Should avoid doing an extensive work-up for underlying gastroesophageal reflux (e.g., upper gastrointestinal tract series, endoscopy, pH probe, ultrasound).
• Are encouraged to educate parents and families about BRUEs, and offer resources for CPR training for families and caregivers.
Limitations
While there are many benefits to these new guidelines, there are challenges. ALTE is ingrained in clinical practice, and it may take time for a uniform acceptance of the change in terminology and criteria. Additional limitations come with the lack of evidence of outcomes and impact, as all studies available are based on ALTE criteria, and data will lag in evaluating the utility of conceptualizing events as BRUEs.
The bottom line
BRUE has been proposed to replace the term ALTE for an unexplained witnessed event as defined above. BRUEs differ from ALTEs in that the criteria are more strictly defined, and they allow providers to stratify children as lower risk or higher risk for a recurrent episode or SIDS. By identifying a child’s risk, providers are able to appropriately utilize resources to refrain from doing an extensive medical work-up in a child who is otherwise healthy and low risk for a serious event.
Reference
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Cavanaugh is a second-year resident in the Abington-Jefferson Family Medicine Residency Program.
The EHR Report: The vortex that sucks you in
Recently, a colleague of ours described the office electronic health record as “the vortex that sucks you in.” This statement occurred during a departmental meeting focused on physician burnout. When members of the department were asked about what things they felt contributed to a feeling of dissatisfaction with work, the electronic health record quickly emerged as a common denominator of dissatisfaction. There were certainly other contributors – the changing and challenging medical environment, fighting with insurance companies, decreased autonomy over practice decisions – but far and away the most cited contributor to dissatisfaction among members of the department was the EHR.
The reasons that EHRs have led to dissatisfaction seem to have changed over the last few years. Initially, physicians found it difficult to suddenly adapt practice styles developed over many years to the new world of electronic documentation. Suddenly they needed to type (or in the case of many, hunt and peck) notes into the history of present illness and fit patient histories into templates seemingly developed by engineers rather than physicians. Now, while most of us have adapted to the logistics of the EHR, there is no escaping the increasing demands for more and more information. There is also ongoing frustration with the lack of control in deciding whether information is relevant for the patient, as well as disparity between the promise and expectation of what electronic records should deliver and what we experience each day in front of us.
Given the degree to which EHRs are contributing to physician dissatisfaction and burnout, it is incumbent upon us to figure out ways to make the EHR work better for clinicians. The literature describes burnout as “a syndrome characterized by a loss of enthusiasm for work (emotional exhaustion), feelings of cynicism (depersonalization), and a low sense of personal accomplishment.” In a recent study, almost half of all physicians described at least one symptom of burnout. Interestingly, physician burnout is greatest in primary care specialties. Surprisingly, compared with other working adults in the United States, physicians are more likely to have symptoms of burnout (38% vs 28%) as well as express dissatisfaction with their work-life balance (40% vs. 23%).1 This issue is important because burnout – in addition to its negative effects on physicians’ experience and quality of life – can erode the quality of the care they give, increase the risk of medical errors, and lead to early ending of lifelong careers.2 The literature suggests that the high prevalence of burnout among U.S. physicians means that “the problem lies more with the system and environment in which physicians work rather than being due to innate vulnerabilities in a few susceptible individuals.” Not surprisingly, we have received letters from readers of our column over many years discussing how the entry of EHRs into their practice was a critical influence in their decisions to retire early.
In our discussion after the department meeting, several physicians described the need to do charting at night from home in order to have their work accomplished for the next day. This is not surprising to any of us who work in primary care and use EHRs. The ability to have access to the EHR anytime and from anywhere is a classic double-edged sword. It is certainly convenient to be able to complete our charting from home without having to stay late in the office on nights and weekends. Unfortunately, bringing work home also erodes into time that could otherwise be spent with family and pursuing other interests.
This is just one of many frustrations. Another common issue is superfluous documentation on the part of specialists. Often, the information is entered by physician extenders or using canned macros to “pad” the note. Sifting through paragraphs of this irrelevant – and sometimes inaccurate – information in consultant notes devalues the integrity of the interaction. It also minimizes the time that was actually spent in the office doing the real hard work of medicine instead of the rudimentary work of documenting things that were either never said or mentioned briefly in passing.
The week after our department meeting was the first week of work for our new interns. Rounding in one of our nursing homes, I handed the intern a patient’s chart and began to explain how the chart was organized – where the orders, progress notes, and labs were located in the chart. The intern had an odd smile on her face. I asked her what was wrong. She replied, “I didn’t know anyone still had paper charts; how do you enter a note there?”
So we come full circle. You can’t miss what you never had. Younger physicians do not resent the EHR, nor can they perceive the EHR to be contributing to discontent. That is not to say that it does not contribute; it is just difficult to identify problems when the way things are is what you have always known. The issue of EHRs contributing to physician burnout is real, and we need to learn more about its causes. Please email us with your thoughts about the aspects of EHRs that you find most frustrating or challenging. Our goal in hearing from you is that it is only by knowing the challenges that we face that we can begin to formulate solutions to overcome those challenges and together make tomorrow’s practice better than today’s.
References
1. Shanafelt TD et al. Burnout and satisfaction with work-life balance among U.S. physicians relative to the general U.S. population. Arch Intern Med. 2012;172(18):1377-85.
2. Shanafelt TD, Balch CM, Bechamps G et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
Recently, a colleague of ours described the office electronic health record as “the vortex that sucks you in.” This statement occurred during a departmental meeting focused on physician burnout. When members of the department were asked about what things they felt contributed to a feeling of dissatisfaction with work, the electronic health record quickly emerged as a common denominator of dissatisfaction. There were certainly other contributors – the changing and challenging medical environment, fighting with insurance companies, decreased autonomy over practice decisions – but far and away the most cited contributor to dissatisfaction among members of the department was the EHR.
The reasons that EHRs have led to dissatisfaction seem to have changed over the last few years. Initially, physicians found it difficult to suddenly adapt practice styles developed over many years to the new world of electronic documentation. Suddenly they needed to type (or in the case of many, hunt and peck) notes into the history of present illness and fit patient histories into templates seemingly developed by engineers rather than physicians. Now, while most of us have adapted to the logistics of the EHR, there is no escaping the increasing demands for more and more information. There is also ongoing frustration with the lack of control in deciding whether information is relevant for the patient, as well as disparity between the promise and expectation of what electronic records should deliver and what we experience each day in front of us.
Given the degree to which EHRs are contributing to physician dissatisfaction and burnout, it is incumbent upon us to figure out ways to make the EHR work better for clinicians. The literature describes burnout as “a syndrome characterized by a loss of enthusiasm for work (emotional exhaustion), feelings of cynicism (depersonalization), and a low sense of personal accomplishment.” In a recent study, almost half of all physicians described at least one symptom of burnout. Interestingly, physician burnout is greatest in primary care specialties. Surprisingly, compared with other working adults in the United States, physicians are more likely to have symptoms of burnout (38% vs 28%) as well as express dissatisfaction with their work-life balance (40% vs. 23%).1 This issue is important because burnout – in addition to its negative effects on physicians’ experience and quality of life – can erode the quality of the care they give, increase the risk of medical errors, and lead to early ending of lifelong careers.2 The literature suggests that the high prevalence of burnout among U.S. physicians means that “the problem lies more with the system and environment in which physicians work rather than being due to innate vulnerabilities in a few susceptible individuals.” Not surprisingly, we have received letters from readers of our column over many years discussing how the entry of EHRs into their practice was a critical influence in their decisions to retire early.
In our discussion after the department meeting, several physicians described the need to do charting at night from home in order to have their work accomplished for the next day. This is not surprising to any of us who work in primary care and use EHRs. The ability to have access to the EHR anytime and from anywhere is a classic double-edged sword. It is certainly convenient to be able to complete our charting from home without having to stay late in the office on nights and weekends. Unfortunately, bringing work home also erodes into time that could otherwise be spent with family and pursuing other interests.
This is just one of many frustrations. Another common issue is superfluous documentation on the part of specialists. Often, the information is entered by physician extenders or using canned macros to “pad” the note. Sifting through paragraphs of this irrelevant – and sometimes inaccurate – information in consultant notes devalues the integrity of the interaction. It also minimizes the time that was actually spent in the office doing the real hard work of medicine instead of the rudimentary work of documenting things that were either never said or mentioned briefly in passing.
The week after our department meeting was the first week of work for our new interns. Rounding in one of our nursing homes, I handed the intern a patient’s chart and began to explain how the chart was organized – where the orders, progress notes, and labs were located in the chart. The intern had an odd smile on her face. I asked her what was wrong. She replied, “I didn’t know anyone still had paper charts; how do you enter a note there?”
So we come full circle. You can’t miss what you never had. Younger physicians do not resent the EHR, nor can they perceive the EHR to be contributing to discontent. That is not to say that it does not contribute; it is just difficult to identify problems when the way things are is what you have always known. The issue of EHRs contributing to physician burnout is real, and we need to learn more about its causes. Please email us with your thoughts about the aspects of EHRs that you find most frustrating or challenging. Our goal in hearing from you is that it is only by knowing the challenges that we face that we can begin to formulate solutions to overcome those challenges and together make tomorrow’s practice better than today’s.
References
1. Shanafelt TD et al. Burnout and satisfaction with work-life balance among U.S. physicians relative to the general U.S. population. Arch Intern Med. 2012;172(18):1377-85.
2. Shanafelt TD, Balch CM, Bechamps G et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
Recently, a colleague of ours described the office electronic health record as “the vortex that sucks you in.” This statement occurred during a departmental meeting focused on physician burnout. When members of the department were asked about what things they felt contributed to a feeling of dissatisfaction with work, the electronic health record quickly emerged as a common denominator of dissatisfaction. There were certainly other contributors – the changing and challenging medical environment, fighting with insurance companies, decreased autonomy over practice decisions – but far and away the most cited contributor to dissatisfaction among members of the department was the EHR.
The reasons that EHRs have led to dissatisfaction seem to have changed over the last few years. Initially, physicians found it difficult to suddenly adapt practice styles developed over many years to the new world of electronic documentation. Suddenly they needed to type (or in the case of many, hunt and peck) notes into the history of present illness and fit patient histories into templates seemingly developed by engineers rather than physicians. Now, while most of us have adapted to the logistics of the EHR, there is no escaping the increasing demands for more and more information. There is also ongoing frustration with the lack of control in deciding whether information is relevant for the patient, as well as disparity between the promise and expectation of what electronic records should deliver and what we experience each day in front of us.
Given the degree to which EHRs are contributing to physician dissatisfaction and burnout, it is incumbent upon us to figure out ways to make the EHR work better for clinicians. The literature describes burnout as “a syndrome characterized by a loss of enthusiasm for work (emotional exhaustion), feelings of cynicism (depersonalization), and a low sense of personal accomplishment.” In a recent study, almost half of all physicians described at least one symptom of burnout. Interestingly, physician burnout is greatest in primary care specialties. Surprisingly, compared with other working adults in the United States, physicians are more likely to have symptoms of burnout (38% vs 28%) as well as express dissatisfaction with their work-life balance (40% vs. 23%).1 This issue is important because burnout – in addition to its negative effects on physicians’ experience and quality of life – can erode the quality of the care they give, increase the risk of medical errors, and lead to early ending of lifelong careers.2 The literature suggests that the high prevalence of burnout among U.S. physicians means that “the problem lies more with the system and environment in which physicians work rather than being due to innate vulnerabilities in a few susceptible individuals.” Not surprisingly, we have received letters from readers of our column over many years discussing how the entry of EHRs into their practice was a critical influence in their decisions to retire early.
In our discussion after the department meeting, several physicians described the need to do charting at night from home in order to have their work accomplished for the next day. This is not surprising to any of us who work in primary care and use EHRs. The ability to have access to the EHR anytime and from anywhere is a classic double-edged sword. It is certainly convenient to be able to complete our charting from home without having to stay late in the office on nights and weekends. Unfortunately, bringing work home also erodes into time that could otherwise be spent with family and pursuing other interests.
This is just one of many frustrations. Another common issue is superfluous documentation on the part of specialists. Often, the information is entered by physician extenders or using canned macros to “pad” the note. Sifting through paragraphs of this irrelevant – and sometimes inaccurate – information in consultant notes devalues the integrity of the interaction. It also minimizes the time that was actually spent in the office doing the real hard work of medicine instead of the rudimentary work of documenting things that were either never said or mentioned briefly in passing.
The week after our department meeting was the first week of work for our new interns. Rounding in one of our nursing homes, I handed the intern a patient’s chart and began to explain how the chart was organized – where the orders, progress notes, and labs were located in the chart. The intern had an odd smile on her face. I asked her what was wrong. She replied, “I didn’t know anyone still had paper charts; how do you enter a note there?”
So we come full circle. You can’t miss what you never had. Younger physicians do not resent the EHR, nor can they perceive the EHR to be contributing to discontent. That is not to say that it does not contribute; it is just difficult to identify problems when the way things are is what you have always known. The issue of EHRs contributing to physician burnout is real, and we need to learn more about its causes. Please email us with your thoughts about the aspects of EHRs that you find most frustrating or challenging. Our goal in hearing from you is that it is only by knowing the challenges that we face that we can begin to formulate solutions to overcome those challenges and together make tomorrow’s practice better than today’s.
References
1. Shanafelt TD et al. Burnout and satisfaction with work-life balance among U.S. physicians relative to the general U.S. population. Arch Intern Med. 2012;172(18):1377-85.
2. Shanafelt TD, Balch CM, Bechamps G et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
Clinical Guidelines: Update in acne treatment
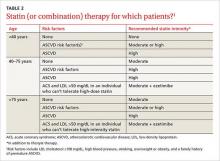
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
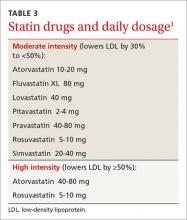
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Clinical Guidelines: Update in acne treatment
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
Acne affects 85% of teenagers but can frequently persist into adulthood. It causes significant physical and psychological effects for patients including facial scarring, depression, and decreased self-esteem. The initial approach to acne is determined according to presenting severity, emphasizing topical treatment for milder disease and the addition of oral therapy as disease becomes more severe.
Treatment of mild acne can begin with either benzoyl peroxide (BP) or a topical retinoid (TR). Another option for slightly more severe acne is to start with the initially suggested treatment for moderately severe acne, which is topical combination therapy with BP plus a topical antibiotic; TR plus BP; or TR plus BP in combination with a topical antibiotic. Combination therapy can be given either with separate application of the different medicines or by using fixed combination products that include the separate components in one formulation.
BP is an antibacterial agent, with mild comedolytic properties, and it often is added to topical antibiotic therapy to increase effectiveness and reduce the development of resistance. BP is available in strengths from 2.5% to 10% and in a variety of formulations, which can be used as leave-on or wash-off agents. Common side effects include dose dependent skin irritation and bleaching of fabric.
Topical antibiotics, including clindamycin and erythromycin, work through both their antimicrobial and anti-inflammatory affects. Monotherapy with topical antibiotics is no longer recommended; instead they should be used in combination with BP to prevent bacterial resistance. The preferred topical antibiotic is clindamycin 1% solution or gel. Clindamycin is available in a combination with BP, which may enhance compliance with the treatment regimen.
Topical retinoids are vitamin A derivatives that are the core of treatment. They are effective for all forms of acne and should always be used in the treatment of comedonal acne. There are currently three active agents available: tretinoin (0.025%-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% and 0.3% cream or 0.1% lotion), and tazarotene (0.05% and 0.1% cream, gel, or foam). Combination products are available containing clindamycin and BP. The main side effects of retinoids include dryness, peeling, erythema, and skin irritation. Reducing the frequency of application or potency used may be helpful for limiting these side effects. Topical retinoids increase the risk of photosensitivity, so patients should be counseled on daily sunscreen use, and their use is contraindicated in pregnancy.
Dapsone is an alternative topical treatment for mild acne. Topical dapsone is primarily effective in reducing inflammatory lesions, and seems to be more beneficial for female patients. Dapsone can be combined with topical retinoids if comedonal lesions are present.
Moderate acne can be treated with either topical combination therapy as described above, or systemic antibiotics plus a TR and BP, with or without the addition of a topical antibiotic as well. Female patients may also consider combined oral contraceptives or spironolactone for the treatment of moderate acne.
Systemic antibiotics have been used in the treatment of acne vulgaris for many years, and they are indicated for use in moderate to severe acne. They should always be used in combination with topical therapies, specifically a retinoid or BP. Generally, systemic antibiotics should be used for the shortest possible duration, often 3 months, to prevent the development of bacterial resistance. Tetracyclines and macrolides have the strongest evidence for efficacy. Doxycycline and minocycline are considered equally effective and are the preferred first-line oral antibiotics. Azithromycin has been studied in a variety of pulse dose regimens, and is a good alternative for patients who are not candidates for tetracyclines.
Combined oral contraceptive pills (COCs) are another option for the treatment of acne in female patients. COCs improve acne through their antiandrogenic effects. Spironolactone also has antiandrogen properties, and while it is not FDA approved for the treatment of acne, the AAD guidelines support selective use in women. Spironolactone has been studied at doses from 50 to 200 mg daily and has shown clinically significant improvement in acne. Side effects include diuresis, menstrual irregularities, breast tenderness, and rare hyperkalemia.
Severe acne is treated with an oral antibiotic plus topical combination therapy, or oral isotretinoin, with the addition where appropriate of COCs or oral spironolactone.
Oral isotretinoin, an isomer of retinoic acid, is approved by the FDA for the treatment of severe recalcitrant acne. It causes decreased sebum production, acne lesions, and scarring. It can also be considered in the treatment of moderate acne that is resistant to other treatments, relapses quickly, or produces significant scarring or psychosocial distress. Serum cholesterol, triglycerides, and transaminases can rise during treatment, and should be monitored. Because of the risk of teratogenic effects, the FDA has mandated that all patients receiving isotretinoin must participate in the iPLEDGE risk management program, which requires abstinence or two forms of birth control. While isotretinoin requires monitoring and carries the possibility of significant side effects, it is an effective treatment option for patients with severe recalcitrant acne.
The bottom line
Acne is commonly treated by primary care physicians. A clear approach of graded treatment based on severity of disease yields improvement in outcomes. Mild acne should be treated with benzoyl peroxide, retinoids or a combinations of topical treatments. Systemic antibiotics should be combined with topical therapies for moderate to severe acne. Female patients may also consider using combined oral contraceptives and spironolactone. Oral isotretinoin is an effective option for severe acne, but requires close monitoring.
References
Guidelines of care for the management of acne vulgaris (J Am Acad Dermatol. 2016;74[5]:945-73.e33. doi: 10.1016/j.jaad.2015.12.037. Epub 2016 Feb 17).
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Marriott is an attending family physician at Capital Health Primary Care in Hamilton, N.J.
CDC guidelines: Opioids for chronic pain
Opioids are commonly prescribed for chronic pain, and in fact approximately 20% of patients presenting with noncancer pain symptoms will receive an opioid prescription in a physician’s office. Opioid prescriptions increased 7% per capita between 2007 and 2012. Along with this increase in prescriptions has been a proportional increase in opioid-related deaths.
The Centers for Disease Control and Prevention guidelines provides recommendations aimed at primary care clinicians for prescribing opioid medications for chronic pain in the outpatient setting. Chronic pain is defined as pain that lasts longer than 3 months, which may be a result of underlying medical disease, injury, medical treatment, or unknown causes. The target population for the guidelines are patients over 18 years of age with chronic noncancer pain. The guidelines are not intended for the clinical care of patients at the end of life, palliative care, or active cancer patients. Special populations that are addressed in the guidelines include older adults, pregnant women, and patients with a history of substance use disorder.
After a detailed review of the evidence, the CDC summarized 12 recommendations for physicians when prescribing opioids for chronic pain, outlined below, and divided them into three sections. All of the recommendations are category A, meaning they apply to most patients. The exception is recommendation No. 10, as it pertains to urine drug testing, which is a category B recommendation, meaning that different choices may be appropriate for some patients.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic treatment and nonopioid pharmacologic treatments are preferred for chronic pain. Opioids are not a first-line option and should be combined with nonpharmacologic therapy and nonopioid medications if appropriate.
2. Treatment goals should be established with all patients when initiating therapy. Continuation of opioid treatment should occur only if improvement in pain and/or function continues to outweigh the risks of the treatment.
3. Discussion of the risks and realistic benefits of opioid therapy should occur with patients at initiation and during the treatment course.
Opioid selection, dosage, duration, and discontinuation
4. When starting opioid therapy for chronic pain immediate-release opioids should be used initially. Extended-release/long-acting opioids are associated with a higher risk of overdose when treatment is initiated with these; therefore, extended-release/long-acting opioids should be used only for patients with severe, continuous pain and only after a patient has received immediate-release opioids for at least 1 week.
5. Opioids should be prescribed at the lowest effective dose. Avoid dosages greater than 90-mg morphine equivalents per day, and exercise caution at doses greater than 50-mg morphine equivalents per day.
6. Because most chronic pain is initially treated as acute pain, when treating acute pain be sure to use the lowest dose, and prescribe only the amount anticipated to be required for the acute injury/complaint. Prescriptions of longer than 7 days for acute pain are usually not necessary.
7. Evaluate the benefits and harms of opioid prescription within 1-4 weeks of initiation or dose escalation. Always consider tapering or discontinuation if goals are not being met.
8. Evaluate risk factors for opioid-related harms both when initiating medications and periodically during treatment. Risk factors include a history of substance use disorder, high opioid doses, or benzodiazepine use.
Assessing risk and addressing harms of opioid use
9. Review patients’ prescription drug monitoring program to help determine the risk for overdose. Intervals of review may range from when each prescription is given to every 3 months.
10. Utilize urine drug screening when initiating medication and periodically (at least annually). Discuss all unexpected results with the lab, patient, and possibly toxicologist. Repeatedly negative urine drug screens indicate the patient is not taking a prescribed opioid, and therefore medication can be discontinued without a taper.
11. Avoid prescribing a benzodiazepine and opioids concurrently because of a higher risk of fatal overdose.
12. Arrange treatment for patients with opioid-use disorder such as referral to a medication-assisted treatment center for buprenorphine or methadone treatment.
Special populations
The CDC addressed several special populations for which special care when initiating or titrating opioid therapy should be taken:
• Patients with sleep-disordered breathing. Any patient with moderate-to-severe sleep-disordered breathing, including sleep apnea, should avoid opioids if possible; if opioids can’t be avoided, slow titration and lower starting doses should be used.
• Pregnant women and reproductive age women. Use in pregnancy can lead to additional risks for both mother and fetus; therefore, initiation of opioids for chronic pain in any reproductive age woman should include this information so a proper joint decision may be made between patient and clinician. The risks include preterm delivery, birth defects, stillbirth, poor fetal growth, and neonatal abstinence syndrome.
• Patients older than 65. Because of decreasing renal function, this population is at risk for the accumulation of opioids and may be unable to tolerate nonopioid pharmacologic therapy such as NSAIDs as a result of comorbidities. When opioids are necessary, the recommendations indicate a need for fall risk assessment, monitoring for cognitive impairment, and an appropriate bowel regimen.
• Patients with mental health conditions. These patients pose a high risk for overdose both because of polypharmacy, specifically benzodiazepine use, and mental instability. Make sure patients are being optimally treated for their mental health disorders, and when possible consider the use of tricyclic antidepressants or serotonin-norepinephrine reuptake inhibitors for additional pain relief.
• Patients with substance use disorders. Those who use illicit substances contribute to a significant proportion of deaths related to opioid use. However, the previously recommended risk assessment tools were found to be inaccurate and should not provide clinicians with a sense of security when prescribing to this patient population. In addition, consulting a substance use disorder specialist and/or pain specialist may be best for this population.
The bottom line
Opioid use has been increasing steadily in the United States with a proportional increase in opioid overdoses. The CDC guidelines present a strategy to prescribing opioids that emphasizes caution, careful decision making, and monitoring when prescribing opioids in order to best control pain while mitigating the risks of opioid use disorder and overdose.
Reference
CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-50.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Carcia is currently a third-year resident and chief resident in the family medicine program at Abington Memorial Hospital.
Opioids are commonly prescribed for chronic pain, and in fact approximately 20% of patients presenting with noncancer pain symptoms will receive an opioid prescription in a physician’s office. Opioid prescriptions increased 7% per capita between 2007 and 2012. Along with this increase in prescriptions has been a proportional increase in opioid-related deaths.
The Centers for Disease Control and Prevention guidelines provides recommendations aimed at primary care clinicians for prescribing opioid medications for chronic pain in the outpatient setting. Chronic pain is defined as pain that lasts longer than 3 months, which may be a result of underlying medical disease, injury, medical treatment, or unknown causes. The target population for the guidelines are patients over 18 years of age with chronic noncancer pain. The guidelines are not intended for the clinical care of patients at the end of life, palliative care, or active cancer patients. Special populations that are addressed in the guidelines include older adults, pregnant women, and patients with a history of substance use disorder.
After a detailed review of the evidence, the CDC summarized 12 recommendations for physicians when prescribing opioids for chronic pain, outlined below, and divided them into three sections. All of the recommendations are category A, meaning they apply to most patients. The exception is recommendation No. 10, as it pertains to urine drug testing, which is a category B recommendation, meaning that different choices may be appropriate for some patients.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic treatment and nonopioid pharmacologic treatments are preferred for chronic pain. Opioids are not a first-line option and should be combined with nonpharmacologic therapy and nonopioid medications if appropriate.
2. Treatment goals should be established with all patients when initiating therapy. Continuation of opioid treatment should occur only if improvement in pain and/or function continues to outweigh the risks of the treatment.
3. Discussion of the risks and realistic benefits of opioid therapy should occur with patients at initiation and during the treatment course.
Opioid selection, dosage, duration, and discontinuation
4. When starting opioid therapy for chronic pain immediate-release opioids should be used initially. Extended-release/long-acting opioids are associated with a higher risk of overdose when treatment is initiated with these; therefore, extended-release/long-acting opioids should be used only for patients with severe, continuous pain and only after a patient has received immediate-release opioids for at least 1 week.
5. Opioids should be prescribed at the lowest effective dose. Avoid dosages greater than 90-mg morphine equivalents per day, and exercise caution at doses greater than 50-mg morphine equivalents per day.
6. Because most chronic pain is initially treated as acute pain, when treating acute pain be sure to use the lowest dose, and prescribe only the amount anticipated to be required for the acute injury/complaint. Prescriptions of longer than 7 days for acute pain are usually not necessary.
7. Evaluate the benefits and harms of opioid prescription within 1-4 weeks of initiation or dose escalation. Always consider tapering or discontinuation if goals are not being met.
8. Evaluate risk factors for opioid-related harms both when initiating medications and periodically during treatment. Risk factors include a history of substance use disorder, high opioid doses, or benzodiazepine use.
Assessing risk and addressing harms of opioid use
9. Review patients’ prescription drug monitoring program to help determine the risk for overdose. Intervals of review may range from when each prescription is given to every 3 months.
10. Utilize urine drug screening when initiating medication and periodically (at least annually). Discuss all unexpected results with the lab, patient, and possibly toxicologist. Repeatedly negative urine drug screens indicate the patient is not taking a prescribed opioid, and therefore medication can be discontinued without a taper.
11. Avoid prescribing a benzodiazepine and opioids concurrently because of a higher risk of fatal overdose.
12. Arrange treatment for patients with opioid-use disorder such as referral to a medication-assisted treatment center for buprenorphine or methadone treatment.
Special populations
The CDC addressed several special populations for which special care when initiating or titrating opioid therapy should be taken:
• Patients with sleep-disordered breathing. Any patient with moderate-to-severe sleep-disordered breathing, including sleep apnea, should avoid opioids if possible; if opioids can’t be avoided, slow titration and lower starting doses should be used.
• Pregnant women and reproductive age women. Use in pregnancy can lead to additional risks for both mother and fetus; therefore, initiation of opioids for chronic pain in any reproductive age woman should include this information so a proper joint decision may be made between patient and clinician. The risks include preterm delivery, birth defects, stillbirth, poor fetal growth, and neonatal abstinence syndrome.
• Patients older than 65. Because of decreasing renal function, this population is at risk for the accumulation of opioids and may be unable to tolerate nonopioid pharmacologic therapy such as NSAIDs as a result of comorbidities. When opioids are necessary, the recommendations indicate a need for fall risk assessment, monitoring for cognitive impairment, and an appropriate bowel regimen.
• Patients with mental health conditions. These patients pose a high risk for overdose both because of polypharmacy, specifically benzodiazepine use, and mental instability. Make sure patients are being optimally treated for their mental health disorders, and when possible consider the use of tricyclic antidepressants or serotonin-norepinephrine reuptake inhibitors for additional pain relief.
• Patients with substance use disorders. Those who use illicit substances contribute to a significant proportion of deaths related to opioid use. However, the previously recommended risk assessment tools were found to be inaccurate and should not provide clinicians with a sense of security when prescribing to this patient population. In addition, consulting a substance use disorder specialist and/or pain specialist may be best for this population.
The bottom line
Opioid use has been increasing steadily in the United States with a proportional increase in opioid overdoses. The CDC guidelines present a strategy to prescribing opioids that emphasizes caution, careful decision making, and monitoring when prescribing opioids in order to best control pain while mitigating the risks of opioid use disorder and overdose.
Reference
CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-50.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Carcia is currently a third-year resident and chief resident in the family medicine program at Abington Memorial Hospital.
Opioids are commonly prescribed for chronic pain, and in fact approximately 20% of patients presenting with noncancer pain symptoms will receive an opioid prescription in a physician’s office. Opioid prescriptions increased 7% per capita between 2007 and 2012. Along with this increase in prescriptions has been a proportional increase in opioid-related deaths.
The Centers for Disease Control and Prevention guidelines provides recommendations aimed at primary care clinicians for prescribing opioid medications for chronic pain in the outpatient setting. Chronic pain is defined as pain that lasts longer than 3 months, which may be a result of underlying medical disease, injury, medical treatment, or unknown causes. The target population for the guidelines are patients over 18 years of age with chronic noncancer pain. The guidelines are not intended for the clinical care of patients at the end of life, palliative care, or active cancer patients. Special populations that are addressed in the guidelines include older adults, pregnant women, and patients with a history of substance use disorder.
After a detailed review of the evidence, the CDC summarized 12 recommendations for physicians when prescribing opioids for chronic pain, outlined below, and divided them into three sections. All of the recommendations are category A, meaning they apply to most patients. The exception is recommendation No. 10, as it pertains to urine drug testing, which is a category B recommendation, meaning that different choices may be appropriate for some patients.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic treatment and nonopioid pharmacologic treatments are preferred for chronic pain. Opioids are not a first-line option and should be combined with nonpharmacologic therapy and nonopioid medications if appropriate.
2. Treatment goals should be established with all patients when initiating therapy. Continuation of opioid treatment should occur only if improvement in pain and/or function continues to outweigh the risks of the treatment.
3. Discussion of the risks and realistic benefits of opioid therapy should occur with patients at initiation and during the treatment course.
Opioid selection, dosage, duration, and discontinuation
4. When starting opioid therapy for chronic pain immediate-release opioids should be used initially. Extended-release/long-acting opioids are associated with a higher risk of overdose when treatment is initiated with these; therefore, extended-release/long-acting opioids should be used only for patients with severe, continuous pain and only after a patient has received immediate-release opioids for at least 1 week.
5. Opioids should be prescribed at the lowest effective dose. Avoid dosages greater than 90-mg morphine equivalents per day, and exercise caution at doses greater than 50-mg morphine equivalents per day.
6. Because most chronic pain is initially treated as acute pain, when treating acute pain be sure to use the lowest dose, and prescribe only the amount anticipated to be required for the acute injury/complaint. Prescriptions of longer than 7 days for acute pain are usually not necessary.
7. Evaluate the benefits and harms of opioid prescription within 1-4 weeks of initiation or dose escalation. Always consider tapering or discontinuation if goals are not being met.
8. Evaluate risk factors for opioid-related harms both when initiating medications and periodically during treatment. Risk factors include a history of substance use disorder, high opioid doses, or benzodiazepine use.
Assessing risk and addressing harms of opioid use
9. Review patients’ prescription drug monitoring program to help determine the risk for overdose. Intervals of review may range from when each prescription is given to every 3 months.
10. Utilize urine drug screening when initiating medication and periodically (at least annually). Discuss all unexpected results with the lab, patient, and possibly toxicologist. Repeatedly negative urine drug screens indicate the patient is not taking a prescribed opioid, and therefore medication can be discontinued without a taper.
11. Avoid prescribing a benzodiazepine and opioids concurrently because of a higher risk of fatal overdose.
12. Arrange treatment for patients with opioid-use disorder such as referral to a medication-assisted treatment center for buprenorphine or methadone treatment.
Special populations
The CDC addressed several special populations for which special care when initiating or titrating opioid therapy should be taken:
• Patients with sleep-disordered breathing. Any patient with moderate-to-severe sleep-disordered breathing, including sleep apnea, should avoid opioids if possible; if opioids can’t be avoided, slow titration and lower starting doses should be used.
• Pregnant women and reproductive age women. Use in pregnancy can lead to additional risks for both mother and fetus; therefore, initiation of opioids for chronic pain in any reproductive age woman should include this information so a proper joint decision may be made between patient and clinician. The risks include preterm delivery, birth defects, stillbirth, poor fetal growth, and neonatal abstinence syndrome.
• Patients older than 65. Because of decreasing renal function, this population is at risk for the accumulation of opioids and may be unable to tolerate nonopioid pharmacologic therapy such as NSAIDs as a result of comorbidities. When opioids are necessary, the recommendations indicate a need for fall risk assessment, monitoring for cognitive impairment, and an appropriate bowel regimen.
• Patients with mental health conditions. These patients pose a high risk for overdose both because of polypharmacy, specifically benzodiazepine use, and mental instability. Make sure patients are being optimally treated for their mental health disorders, and when possible consider the use of tricyclic antidepressants or serotonin-norepinephrine reuptake inhibitors for additional pain relief.
• Patients with substance use disorders. Those who use illicit substances contribute to a significant proportion of deaths related to opioid use. However, the previously recommended risk assessment tools were found to be inaccurate and should not provide clinicians with a sense of security when prescribing to this patient population. In addition, consulting a substance use disorder specialist and/or pain specialist may be best for this population.
The bottom line
Opioid use has been increasing steadily in the United States with a proportional increase in opioid overdoses. The CDC guidelines present a strategy to prescribing opioids that emphasizes caution, careful decision making, and monitoring when prescribing opioids in order to best control pain while mitigating the risks of opioid use disorder and overdose.
Reference
CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-50.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia. Dr. Carcia is currently a third-year resident and chief resident in the family medicine program at Abington Memorial Hospital.
EHR Report: Smith vs. Smith: Errors in the era of EHRs
George Smith (DOB 2/12/51) is a 65-year-old male patient with a history of hypertension and hyperlipidemia who presents to his local emergency department complaining of worsening dyspnea. He has been suffering with a “chest cold” for the past week, and has also noticed a gradual increase in chest discomfort. The patient is unsure if this is related to exertion or due to his nonproductive cough, but describes the sensation as a “tightness that seems to be getting worse.” The emergency physician is appropriately concerned about a cardiac cause for his symptoms, but is reassured after a check of his electronic health record reveals a recent nuclear treadmill stress test showing normal myocardial perfusion and excellent exercise tolerance, with a low probability of coronary disease.
The only problem is that George Smith never had a stress test. In fact, it’s his twin brother James Smith – also with a birth date of 2/12/51 and a home in the same city – who just had the study done in preparation for surgery. The mix-up in the records began 3 weeks ago, when a tech in the cardiac testing department made an error registering James for his stress test, and now the results of his study have filed into the chart of his twin brother. Fortunately for George, the primary care physician who cares for both brothers happens to be in the emergency department seeing a different patient. He is “curbsided” by the ED doc and recognizes the identification error before the patient is to be discharged home.
This alarming situation – a fictionalized version of a story that happens regularly in hospitals all across the United States – highlights several serious problems with electronic health records. With all of their claimed advantages, EHRs have created a tremendous number of new complications. Some are obvious, such as increased documentation time, connectivity issues, hardware failures, and superfluous “overdocumentation.” But the more troubling issues with electronic records are the ones that are much subtler. Specifically, as the case above highlights, there is the tendency to “lose the forest in the trees” of the EHR, and actually make mistakes that can have devastating consequences. This month we want to cast a light on how electronic tools designed to improve quality and safety actually can compromise them, beginning with the unfortunate reality that …
Modern conveniences can make errors more convenient as well
One of the great advantages of a well-designed electronic record is the ease of locating information when you need it; by entering a few pieces of information such as a last name and date of birth, we can find the needed data in seconds. Unfortunately, this simple and elegant system has exposed a weakness in the people using it: confirmation bias – the idea that we all tend to see what we want to see. This is an adaptive behavior that we all develop to improve efficiency and successfully navigate all of the conscious and subconscious decisions we make throughout the day. Typically, confirmation bias serves to make our lives easier, but in the case above, it didn’t help Mr. Smith; on the contrary, it almost led to disastrous consequences. The error was fortunately recognized by his astute primary care physician, but this case could have ended much differently. The experience should serve as a reminder to us that …
We can easily lose the big picture
The days of hunting for missing patient charts are thankfully long gone, but there are a few critical aspects of paper records that have been lost in the translation to electronic form. One such missing piece was noted by a colleague when first transitioning to an EHR. After a day or two of struggling with the new software, he lamented “I’m missing the big picture!” He had lost the advantage of glancing at a paper chart and instantly recalling the details about his patients that he had compiled over many years of care. For many physicians like him, this may mean reviewing handwritten notes or jottings in the margin of the chart, but sometimes just the appearance of the chart itself is enough to trigger an intellectual or emotional response.
This notion simply doesn’t exist in the world of electronic “charts,” which are all uniform by design. In the quest to simplify workflow and encourage muscle memory, EHR designers have eschewed the intangible experience of holding a yellowing, dog-eared, overflowing patient folder. Instead, physicians now find themselves holding the same PC or tablet as they walk into every patient encounter, left with only a name and date of birth to distinguish one patient from the next. Even worse, the mere definition of a patient chart has moved from a physical construct to a metaphysical one. Charts can be anywhere and everywhere, and can be edited by any end user at virtually any point of care. This opens up almost limitless opportunities for error, and unfortunately …
Errors can last a lifetime
With each episode of care, the charts of the two Mr. Smiths could become more enmeshed, and the histories harder to untangle. (In this case, a passing reference to the stress test results in the ED intern’s history and physical of George Smith may perpetuate the mistake, even though the error has been caught this time.) When mistakes like this are identified, hundreds of collective staff hours can be required to unweave comingled medical records, even when they don’t result in patient harm. It is therefore critical to develop safeguards to prevent them from occurring in the first place, with efforts that include training programs, workflow process improvement, and technology enhancement.
Ultimately, it may be impossible to prevent all documentation errors. However, by focusing on the big picture and considering patient safety first, we can raise awareness of these and other critical issues and develop the tools and training necessary to make mistakes possible to avoid.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
George Smith (DOB 2/12/51) is a 65-year-old male patient with a history of hypertension and hyperlipidemia who presents to his local emergency department complaining of worsening dyspnea. He has been suffering with a “chest cold” for the past week, and has also noticed a gradual increase in chest discomfort. The patient is unsure if this is related to exertion or due to his nonproductive cough, but describes the sensation as a “tightness that seems to be getting worse.” The emergency physician is appropriately concerned about a cardiac cause for his symptoms, but is reassured after a check of his electronic health record reveals a recent nuclear treadmill stress test showing normal myocardial perfusion and excellent exercise tolerance, with a low probability of coronary disease.
The only problem is that George Smith never had a stress test. In fact, it’s his twin brother James Smith – also with a birth date of 2/12/51 and a home in the same city – who just had the study done in preparation for surgery. The mix-up in the records began 3 weeks ago, when a tech in the cardiac testing department made an error registering James for his stress test, and now the results of his study have filed into the chart of his twin brother. Fortunately for George, the primary care physician who cares for both brothers happens to be in the emergency department seeing a different patient. He is “curbsided” by the ED doc and recognizes the identification error before the patient is to be discharged home.
This alarming situation – a fictionalized version of a story that happens regularly in hospitals all across the United States – highlights several serious problems with electronic health records. With all of their claimed advantages, EHRs have created a tremendous number of new complications. Some are obvious, such as increased documentation time, connectivity issues, hardware failures, and superfluous “overdocumentation.” But the more troubling issues with electronic records are the ones that are much subtler. Specifically, as the case above highlights, there is the tendency to “lose the forest in the trees” of the EHR, and actually make mistakes that can have devastating consequences. This month we want to cast a light on how electronic tools designed to improve quality and safety actually can compromise them, beginning with the unfortunate reality that …
Modern conveniences can make errors more convenient as well
One of the great advantages of a well-designed electronic record is the ease of locating information when you need it; by entering a few pieces of information such as a last name and date of birth, we can find the needed data in seconds. Unfortunately, this simple and elegant system has exposed a weakness in the people using it: confirmation bias – the idea that we all tend to see what we want to see. This is an adaptive behavior that we all develop to improve efficiency and successfully navigate all of the conscious and subconscious decisions we make throughout the day. Typically, confirmation bias serves to make our lives easier, but in the case above, it didn’t help Mr. Smith; on the contrary, it almost led to disastrous consequences. The error was fortunately recognized by his astute primary care physician, but this case could have ended much differently. The experience should serve as a reminder to us that …
We can easily lose the big picture
The days of hunting for missing patient charts are thankfully long gone, but there are a few critical aspects of paper records that have been lost in the translation to electronic form. One such missing piece was noted by a colleague when first transitioning to an EHR. After a day or two of struggling with the new software, he lamented “I’m missing the big picture!” He had lost the advantage of glancing at a paper chart and instantly recalling the details about his patients that he had compiled over many years of care. For many physicians like him, this may mean reviewing handwritten notes or jottings in the margin of the chart, but sometimes just the appearance of the chart itself is enough to trigger an intellectual or emotional response.
This notion simply doesn’t exist in the world of electronic “charts,” which are all uniform by design. In the quest to simplify workflow and encourage muscle memory, EHR designers have eschewed the intangible experience of holding a yellowing, dog-eared, overflowing patient folder. Instead, physicians now find themselves holding the same PC or tablet as they walk into every patient encounter, left with only a name and date of birth to distinguish one patient from the next. Even worse, the mere definition of a patient chart has moved from a physical construct to a metaphysical one. Charts can be anywhere and everywhere, and can be edited by any end user at virtually any point of care. This opens up almost limitless opportunities for error, and unfortunately …
Errors can last a lifetime
With each episode of care, the charts of the two Mr. Smiths could become more enmeshed, and the histories harder to untangle. (In this case, a passing reference to the stress test results in the ED intern’s history and physical of George Smith may perpetuate the mistake, even though the error has been caught this time.) When mistakes like this are identified, hundreds of collective staff hours can be required to unweave comingled medical records, even when they don’t result in patient harm. It is therefore critical to develop safeguards to prevent them from occurring in the first place, with efforts that include training programs, workflow process improvement, and technology enhancement.
Ultimately, it may be impossible to prevent all documentation errors. However, by focusing on the big picture and considering patient safety first, we can raise awareness of these and other critical issues and develop the tools and training necessary to make mistakes possible to avoid.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
George Smith (DOB 2/12/51) is a 65-year-old male patient with a history of hypertension and hyperlipidemia who presents to his local emergency department complaining of worsening dyspnea. He has been suffering with a “chest cold” for the past week, and has also noticed a gradual increase in chest discomfort. The patient is unsure if this is related to exertion or due to his nonproductive cough, but describes the sensation as a “tightness that seems to be getting worse.” The emergency physician is appropriately concerned about a cardiac cause for his symptoms, but is reassured after a check of his electronic health record reveals a recent nuclear treadmill stress test showing normal myocardial perfusion and excellent exercise tolerance, with a low probability of coronary disease.
The only problem is that George Smith never had a stress test. In fact, it’s his twin brother James Smith – also with a birth date of 2/12/51 and a home in the same city – who just had the study done in preparation for surgery. The mix-up in the records began 3 weeks ago, when a tech in the cardiac testing department made an error registering James for his stress test, and now the results of his study have filed into the chart of his twin brother. Fortunately for George, the primary care physician who cares for both brothers happens to be in the emergency department seeing a different patient. He is “curbsided” by the ED doc and recognizes the identification error before the patient is to be discharged home.
This alarming situation – a fictionalized version of a story that happens regularly in hospitals all across the United States – highlights several serious problems with electronic health records. With all of their claimed advantages, EHRs have created a tremendous number of new complications. Some are obvious, such as increased documentation time, connectivity issues, hardware failures, and superfluous “overdocumentation.” But the more troubling issues with electronic records are the ones that are much subtler. Specifically, as the case above highlights, there is the tendency to “lose the forest in the trees” of the EHR, and actually make mistakes that can have devastating consequences. This month we want to cast a light on how electronic tools designed to improve quality and safety actually can compromise them, beginning with the unfortunate reality that …
Modern conveniences can make errors more convenient as well
One of the great advantages of a well-designed electronic record is the ease of locating information when you need it; by entering a few pieces of information such as a last name and date of birth, we can find the needed data in seconds. Unfortunately, this simple and elegant system has exposed a weakness in the people using it: confirmation bias – the idea that we all tend to see what we want to see. This is an adaptive behavior that we all develop to improve efficiency and successfully navigate all of the conscious and subconscious decisions we make throughout the day. Typically, confirmation bias serves to make our lives easier, but in the case above, it didn’t help Mr. Smith; on the contrary, it almost led to disastrous consequences. The error was fortunately recognized by his astute primary care physician, but this case could have ended much differently. The experience should serve as a reminder to us that …
We can easily lose the big picture
The days of hunting for missing patient charts are thankfully long gone, but there are a few critical aspects of paper records that have been lost in the translation to electronic form. One such missing piece was noted by a colleague when first transitioning to an EHR. After a day or two of struggling with the new software, he lamented “I’m missing the big picture!” He had lost the advantage of glancing at a paper chart and instantly recalling the details about his patients that he had compiled over many years of care. For many physicians like him, this may mean reviewing handwritten notes or jottings in the margin of the chart, but sometimes just the appearance of the chart itself is enough to trigger an intellectual or emotional response.
This notion simply doesn’t exist in the world of electronic “charts,” which are all uniform by design. In the quest to simplify workflow and encourage muscle memory, EHR designers have eschewed the intangible experience of holding a yellowing, dog-eared, overflowing patient folder. Instead, physicians now find themselves holding the same PC or tablet as they walk into every patient encounter, left with only a name and date of birth to distinguish one patient from the next. Even worse, the mere definition of a patient chart has moved from a physical construct to a metaphysical one. Charts can be anywhere and everywhere, and can be edited by any end user at virtually any point of care. This opens up almost limitless opportunities for error, and unfortunately …
Errors can last a lifetime
With each episode of care, the charts of the two Mr. Smiths could become more enmeshed, and the histories harder to untangle. (In this case, a passing reference to the stress test results in the ED intern’s history and physical of George Smith may perpetuate the mistake, even though the error has been caught this time.) When mistakes like this are identified, hundreds of collective staff hours can be required to unweave comingled medical records, even when they don’t result in patient harm. It is therefore critical to develop safeguards to prevent them from occurring in the first place, with efforts that include training programs, workflow process improvement, and technology enhancement.
Ultimately, it may be impossible to prevent all documentation errors. However, by focusing on the big picture and considering patient safety first, we can raise awareness of these and other critical issues and develop the tools and training necessary to make mistakes possible to avoid.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University, Philadelphia.
Diabetes update: Your guide to the latest ADA standards
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
Prevention of diabetes, as well as early detection and treatment of both prediabetes and diabetes, is critical to the health of our country. Because evidence-based guidelines are key to our ability to effectively address the nation’s diabetes epidemic, the American Diabetes Association (ADA) updates its “Standards of Medical Care in Diabetes” annually to incorporate new evidence or clarifications.
The 2016 standards,1 available at professional.diabetes.org/jfp, are a valuable resource. Among the latest revisions: an expansion in screening recommendations, a change in the age at which aspirin therapy for women should be considered, and a change in A1C goals for pregnant women with diabetes.
As members of the ADA’s primary care advisory group, we use a question and answer format in the summary that follows to highlight recent revisions and review other recommendations that are of particular relevance to physicians in primary care. It is important to note, however, that ADA recommendations are not intended to preclude clinical judgment and should be applied in the context of excellent medical care.
Diagnosis and screening
Have the 2016 ADA standards changed the way diabetes is diagnosed?
No. The criteria for a diagnosis of diabetes did not change. Diabetes and prediabetes are still screened for and diagnosed with any of the following: a fasting plasma glucose (FPG); a 2-hour 75-g oral glucose tolerance test (OGTT); a random plasma glucose >200 mg/dL with symptoms of hyperglycemia; or A1C criteria (TABLE 1).1,2 The wording was changed, however, to make it clear that no one test is preferred over another for diagnosis.
Yes. In addition to screening asymptomatic adults of any age who are overweight or obese and have one or more additional risk factors for diabetes, the 2016 standards recommend screening all adults 45 years and older, regardless of weight.
Is an A1C <7% the recommended treatment goal for everyone with diabetes?
No. An A1C <7% is considered reasonable for most, but not all, nonpregnant adults. In the last few years, the ADA has focused more on individualized targets.
Tighter control (<6.5%)—which is associated with lower rates of eye disease, kidney disease, and nerve damage—may be appropriate for patients who have no significant hypoglycemia, no cardiovascular disease (CVD), a shorter duration of diabetes, or a longer expected lifespan.
Conversely, a higher target (<8%) may be appropriate for patients who are older, have longstanding diabetes, advanced macrovascular or microvascular disease, established complications, or a limited life expectancy.3,4
Pregnancy. The 2016 standards have a new target for pregnant women with diabetes: The ADA previously recommended an A1C <6% for this patient population, but now recommends a target A1C between 6% and 6.5%. This may be tightened or relaxed, however, depending on individual risk of hypoglycemia.
In focusing on individualized targets and hypoglycemia avoidance, the ADA notes that attention must be paid to fasting, pre-meal, and post-meal blood glucose levels to achieve treatment goals. The 2016 standards emphasize the importance of patient-centered diabetes care, aligned with a coordinated, team-based chronic care model.
Diabetes self-management education and support is indicated for those who are newly diagnosed, and should be provided periodically based on glucose control and progression of the disease. All patients should receive education on hypoglycemia risk and treatment.
Prediabetes and prevention
What is prediabetes and what can I do to prevent patients with prediabetes from developing diabetes?
Patients with impaired glucose tolerance, impaired fasting glucose, or an A1C between 5.7% and 6.4% are considered to have prediabetes and are at risk for developing type 2 diabetes.
Family physicians should refer patients with prediabetes to intensive diet, physical activity, and behavioral counseling programs like those based on the Diabetes Prevention Program study (www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx). Goals should include a minimum 7% weight loss and moderate-intensity physical activity, such as brisk walking, for at least 150 minutes per week.
Lifestyle modification programs have been shown to be very effective in preventing diabetes, with about a 58% reduction in the risk of developing type 2 diabetes after 3 years.5 The 2016 standards added a recommendation that physicians encourage the use of new technology, such as text messaging or smart phone apps, to support such efforts.
Should I consider initiating oral antiglycemics in patients with prediabetes?
Yes. Pharmacologic agents, including metformin, acarbose, and pioglitazone, have been shown to decrease progression from prediabetes to type 2 diabetes. Thus, antiglycemics should be considered for certain patients. Metformin is especially appropriate for women with a history of gestational diabetes, patients who are younger than 60 years, and those who have a body mass index (BMI) ≥35 kg/m2.6
How often should I screen patients with prediabetes?
Patients with prediabetes should be screened annually. Such individuals should also be screened and treated for modifiable cardiovascular risk factors. There is strong evidence that the treatment of obesity can be beneficial for those at any stage of the diabetes spectrum.
Obesity management
What do the 2016 ADA standards recommend for obese patients with diabetes?
With more than two-thirds of Americans either overweight or obese, the ADA added a new section on obesity management and calls on health care providers to:
- weigh patients and calculate and document their BMI at every visit, and
- counsel those who are overweight or obese on the benefits of even modest weight loss.
The ADA recommends a sustained weight loss of 5%, which can improve glycemic control and reduce the need for diabetes medications,7-9 although weight loss of ≥7% is optimal. Physicians are also called on to assess each patient’s readiness to engage in therapeutic lifestyle change to maintain a modest weight loss.
Treatment for obesity can include therapeutic lifestyle change (reduction in calories, increase in physical activity) and behavioral therapy. For refractory patients, pharmacologic therapy and bariatric surgery may be considered.
Interventions should be high-intensity (≥16 sessions in 6 months) and focus on diet, physical activity, and behavioral strategies to achieve a 500 to 750 calorie deficit per day.10 Long-term (≥1 year) comprehensive weight maintenance programs should be prescribed for those who achieve short-term weight loss.11,12 Such programs should provide at least monthly contact and encourage ongoing monitoring of body weight (weekly or more frequently), continued consumption of a reduced-calorie diet, and participation in high levels of physical activity (200 to 300 minutes per week).
Glycemic treatment
What are some of the key factors that distinguish the different type 2 diabetes medications from one another?
An increasing understanding of diabetes pathophysiology has led to a wider array of medications, making treatment more complex than ever. It is important for physicians to have a strong working knowledge of the various classes of antidiabetic agents and the subtleties between drugs in the same class to best individualize treatment.
Here are the highlights of each class of medication listed in the ADA/European Association for the Study of Diabetes algorithm for the management of type 2 diabetes,13 which is available at http://care.diabetesjournals.org/content/38/1/140/F2.large.jpg):
Metformin is the preferred initial medication for all patients who can tolerate it and have no contraindications. The drug is cost-effective, weight neutral, and has had positive cardiovascular and mortality outcomes in long-term studies. Adverse gastrointestinal (GI) effects, including nausea, diarrhea, and dyspepsia, are common but can be reduced with a slow titration of the drug. Metformin should be used with caution in those with renal disease. The dose should be reduced if the estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2 and the drug discontinued if eGFR <30 mL/min/1.73 m2.
Sulfonylureas/meglitinides stimulate insulin secretion in a glucose-independent manner. They are cost-effective and have high efficacy early in the disease and with initial use, but the effect wanes as the disease progresses. This class of drugs is associated with weight gain and hypoglycemia. Second-generation sulfonylureas (glipizide, glimepiride) are recommended; meglitinides are more expensive than sulfonylureas.
Thiazolidinediones work to improve insulin sensitivity in the periphery and have a low risk of hypoglycemia. They have been associated with fluid retention, weight gain, and worsening of pre-existing congestive heart failure, but previous cardiovascular concerns (with rosiglitazone)14 and bladder cancer risks (with pioglitazone)15-17 have been refuted. Thiazolidinediones are contraindicated in those with Class III and IV congestive heart failure, however, and patients taking them require careful monitoring for weight gain, fluid retention, and exacerbation of heart failure.
Dipeptidyl peptidase-4 inhibitors (DPP4Is) work to reduce the breakdown of endogenous incretin hormones. These oral agents increase insulin secretion in a glucose-dependent manner; more insulin is secreted when glucose is higher and less when glucose is closer to normal. This means that there is a much lower risk of hypoglycemia when a DPP4I is used as monotherapy.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), which are injectable, also work via incretin hormones and stimulate insulin in a glucose-dependent manner. They are associated with weight loss and low rates of hypoglycemia. Adverse GI effects are common with this class of drugs, but can be reduced by titrating the medication and avoiding overeating. GLP-1RAs can be taken twice daily to once weekly, depending on the specific agent.
Sodium glucose transporter 2 inhibitors (SGLT2Is) are oral agents and the newest class of antidiabetes drugs. The drugs help block the reabsorption of glucose, thereby lowering glucose levels, blood pressure, and weight in many patients. The most common adverse effects are urinary tract and genital yeast infections. SGLT2Is should not be given to patients with advanced renal disease (chronic kidney disease Stages 3B-5) because they will not be effectively absorbed.
The US Food and Drug Administration (FDA) recently issued a warning about the risk of ketoacidosis with these agents,18 and patients should be advised to stop taking them and to seek immediate medical attention if they develop symptoms of ketoacidosis, such as excessive thirst, frequent urination, nausea and vomiting, abdominal pain, weakness or fatigue, shortness of breath, fruity-scented breath, or confusion.
Insulin is eventually needed by most patients with type 2 diabetes who live long enough to see the disease progress. The most common adverse effects are weight gain and hypoglycemia. There are many types of insulin, but only one that is delivered via inhalation—human insulin inhaled powder. Inhaled insulin, however, has the potential for adverse pulmonary effects, including cough and reduction of peak expiratory flow. Therefore, pulmonary function testing is recommended prior to its use.
Treatment goal attainment should be evaluated every 3 months, and treatment titrated at 3-month intervals if goals are not achieved. The ADA/European Association for the Study of Diabetes’ algorithm indicates that patients are likely to need insulin a year after diagnosis if their A1C goal has not been achieved or maintained.13
The following medications are not included in the algorithm but are included in the 2016 standards, and may be helpful for certain patients:
Alpha-glucosidase inhibitors delay the absorption of glucose from the proximal to distal GI tract, thereby reducing postprandial hyperglycemia. Flatulence and leakage of stool—the most common adverse effects—have limited their use in the United States.
Bile acid sequestrants (colesevelam) treat both hyperlipidemia and diabetes. The medications work by reducing glucose absorption from the GI tract. They reduce postprandial hyperglycemia, with a low risk of hypoglycemia. Colesevelam’s use is limited, however, because of the number of pills needed (6 daily).
Bromocriptine affects satiety levels via the central nervous system, and is available in a specific formulation for the treatment of diabetes. “First-dose” hypotension, however, is an adverse effect of considerable concern.1
Pramlintide, an injectable amylin mimetic given to patients on prandial insulin, can reduce postprandial glucose levels. The most common adverse effects are upper GI symptoms and hypoglycemia. Due to the adverse effects and the need for an injection with each meal, pramlintide is used infrequently.
Cardiovascular risk reduction
Has the ADA revised its recommendations for cardiovascular disease risk management?
Yes. There have been several changes. The first is in terminology, with atherosclerotic cardiovascular disease (ASCVD) replacing CVD alone. While new recommendations for statin therapy for adults older than 40 years (TABLE 2)1 were also added, the emphasis remains on therapeutic lifestyle change as an effective treatment for hypertension. These modifications should include at least 150 minutes of moderate physical activity per week and, for most patients, a reduction in total calories, saturated fat, and sodium.
It is important to remind patients that to maximize the benefits in terms of treating hyperglycemia, hypertension, and dyslipidemia, such changes must be maintained over the long term.
Aspirin therapy. The ADA also revised its recommendation regarding aspirin therapy. Based on new evidence in the treatment of women with ASCVD risk, the standards now call for considering aspirin therapy (75-162 mg/d) in both women and men ≥50 years as a primary prevention strategy for those with type 1 or type 2 diabetes with a 10-year ASCVD risk of >10%. (The previous standards recommended this only for women older than 60 years.)
Antiplatelet therapy is now recommended for patients younger than 50 years with multiple risk factors, and as secondary prevention in those with a history of ASCVD.19-21
Hypertension. The ADA’s recommendations for treating hypertension in patients with diabetes have not changed; the goal remains <140/<90 mm Hg. Lower targets may be appropriate for younger patients, those with albuminuria, and individuals with additional CVD risk factors; however, systolic pressure <130 mm Hg has not been shown to reduce CVD outcomes, and diastolic pressure <70 mm Hg has been associated with higher mortality.22
Optimal medication and lifestyle therapy are important to achieve goals, with avoidance of undue treatment burden. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), but not both, should be included as part of treatment. Other agents, such as a thiazide diuretic, may be needed to achieve individual goals. Serum creatinine/eGFR and serum potassium levels should be monitored with the use of diuretics.
Lipids. The 2016 standards include notable changes in lipid management. The ADA sees a role for ezetimibe for select patients, based on studies such as the IMPROVE IT trial23 that included participants with diabetes. The ADA also added a table highlighting statin recommendations and delineating high and moderate-intensity statins (TABLE 3).1 Those younger than 40 years with no other risk factors may not need a statin, but patients ages 40 or older will need moderate- to high-intensity statin therapy to effectively lower ASCVD risk.24-28
These recommendations reflect a comprehensive plan to reduce ASCVD in this at-risk population, which should also include lifestyle modification, including smoking prevention and quit strategies, as needed.
Microvascular complications
DIABETIC KIDNEY DISEASE
How should I diagnose nephropathy?
The ADA changed the terminology, referring to “diabetic kidney disease” (DKD) rather than nephropathy to highlight the fact that the focus is on kidney disease directly linked to diabetes.
Other recommendations include an annual assessment of urinary albumin (eg, spot urine albumin-to-creatinine ratio and eGFR) for patients who have had type 1 diabetes for ≥5 years and all patients who have type 2 diabetes. Two out of 3 abnormal specimens collected within a 3- to 6-month period indicate the presence of albuminuria.
What can be done to prevent or slow the progression of DKD?
Optimal BP and glycemic control are key,29-35 along with diet and medication. For patients with DKD, dietary protein intake should be 0.8 g/kg body weight per day. ACE inhibitors and ARBs have been shown to slow the decline in eGFR in patients with elevated urinary albumin excretion (≥30 mg/day).
However, neither an ACE inhibitor nor an ARB is recommended for the primary prevention of DKD in patients who have normal BP, normal urine albumin-to-creatinine ratio (<30 mg/g), and normal eGFR. In addition, combined use of an ACE inhibitor and an ARB should be avoided, as it provides no additional benefit and increases the risk of adverse effects.29
RETINOPATHY
How should I manage retinopathy in patients with diabetes?
As with the management of DKD, it is important to optimize glycemic and BP control to reduce the risk, or slow the progression, of retinopathy. Intensive diabetes management, with the goal of achieving near-normal glycemic levels, has been shown in large prospective randomized studies to prevent or delay the onset and progression of diabetic retinopathy.33,36 The presence of retinopathy is not a contraindication to aspirin therapy for ASCVD prevention, as aspirin does not increase the risk of retinal hemorrhage.
When should patients with diabetes be screened for retinopathy?
Patients with type 1 diabetes should have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist within 5 years of the onset of diabetes. Those with type 2 diabetes should have such an exam shortly after diagnosis. The exam should be repeated annually; if there is no evidence of retinopathy, however, 2-year intervals may be considered.
PERIPHERAL NEUROPATHY
When and how should I screen patients with diabetes for neuropathy?
All patients should be screened for diabetic peripheral neuropathy (DPN) starting at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes, and continued at least annually thereafter. Assessment should include a detailed history and 10-g monofilament testing, as well as at least one of the following tests: pinprick, temperature, and vibration sensation.
It is important, too, to screen patients with more advanced diabetes for signs and symptoms of autonomic neuropathy. Signs and symptoms may include resting tachycardia, exercise intolerance, orthostatic hypotension, gastroparesis, constipation, impaired neurovascular function, and autonomic failure in response to hypoglycemia. In men, diabetic autonomic neuropathy may cause erectile dysfunction and/or retrograde ejaculation.
How should I manage patients who have DPN?
Tight glycemic control is the only measure that has been shown to prevent or delay the development of DPN or cardiac autonomic neuropathy in patients with type 1 diabetes,37,38 and to slow the progression of neuropathy in some patients with type 2 diabetes.39
The FDA has approved pregabalin, duloxetine, and tapentadol for the treatment of pain associated with DPN. Tricyclic antidepressants, gabapentin, venlafaxine, carbamazepine, tramadol, and topical capsaicin, although not approved for the treatment of painful DPN, may also be effective in treating neuropathic pain.
For those with autonomic neuropathy, dietary changes and prokinetic agents such as erythromycin may alleviate gastroparesis. Due to extrapyramidal adverse effects, metoclopramide is reserved for the most severe and unresponsive cases. Recurrent urinary tract infections, pyelonephritis, incontinence, or palpable bladder should prompt an evaluation for bladder dysfunction. Controlling lipids and BP, quitting smoking, and making other lifestyle changes can reduce both the development and the progression of autonomic neuropathy.
FOOT CARE/PERIPHERAL ARTERIAL DISEASE
What does the ADA recommend regarding foot care for patients with diabetes?
The ADA’s standards recommend an annual comprehensive foot examination to identify risk factors predictive of ulcers and potential amputations. The exam should start with inspection and assessment of foot pulses and should seek to identify loss of peripheral sensation. The examination should include inspection of the skin, assessment of foot deformities, neurologic assessment including 10-g monofilament testing and pinprick or vibration testing or assessment of ankle reflexes, and vascular assessment, including pulses in the legs and feet.40
It is also important to screen patients for peripheral arterial disease (PAD), with a comprehensive medical history and physical exam of pulses. Ankle-brachial index testing (ABI) should be performed in patients with signs or symptoms of PAD, including claudication or skin and hair changes in the lower extremities. ABI may be considered for all patients with diabetes starting at age 50 and in those younger than 50 years who have risk factors.41
Which patients with diabetes are at higher risk for foot complications?
The following are risk factors for foot complications: previous amputation, prior foot ulcer, peripheral neuropathy, foot deformity, peripheral vascular disease, visual impairment, peripheral neuropathy (especially if on dialysis), poor glycemic control, and smoking. Patients with high-risk foot conditions should be educated about their risk and appropriate management.
A well-fitted walking shoe that cushions the feet and redistributes pressure is one option to help patients. Patients with bony deformities may need extra wide or deep shoes and patients with more advanced disease may need custom-fitted shoes.
When should patients be referred to a foot specialist?
Refer patients to a foot care specialist for ongoing preventive care and lifelong surveillance if they smoke or have a history of lower-extremity complications, a loss of protective sensation, structural abnormalities, or PAD.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(Suppl 1). Available at: http://care.diabetesjournals.org/site/misc/2016-Standards-of-Care.pdf. Accessed March 28, 2016.
2. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
3. Lipska KJ, Ross JS, Miao Y, et al. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362.
4. Vijan S, Sussman JB, Yudkin JS, et al. Effect of patients’ risks and p on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234.
5. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
6. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737.
7. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905–912.
8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
9. Pastors JG, Warshaw H, Daly A, et al. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–613.
10. Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force Recommendation. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed March 28, 2016.
11. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and metaanalysis. Obesity (Silver Spring). 2006;14:1283–1293.
12. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;99:14–23.
13. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149.
14. Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195.
15. Balaji V, Seshiah V, Ashtalakshmi G, et al. Retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–427.
16. Kuo HW, Tiao MM, Ho SC, et al. Pioglitazone use and the risk of bladder cancer. Kaohsiung J Med Sci. 2014;30:94–97.
17. Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: a propensity score matched cohort study. Br J Clin Pharmacol. 2013;75:254-259.
18. US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Accessed December 11, 2015.
19. Huxley RR, Peters SAE, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206.
20. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551.
21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973-1980.
22. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
23. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
24. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485.
25. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
26. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
27. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316.
28. Nissen SE, Tuzcu EM, Schoenhagen P, et al; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080.
29. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713.
30. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an American Diabetes Association Consensus Conference. Diabetes Care. 2014;37:2864–2883.
31. The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720.
32. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
33. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853.
34. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
35. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430.
36. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
37. Chew EY, Ambrosius WT, Davis MD, et al; ACCORD Study Group; ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244.
38. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528.
39. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:31–38.
40. Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765.
41. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
42. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Available at: http://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Accessed April 8, 2016.
Clinical Guidelines: Evaluating suspected acute pulmonary embolism
When a patient comes to the office or emergency department complaining of shortness of breath, acute pulmonary embolism is a diagnosis that must be considered.
The signs and symptoms of pulmonary embolism (PE) – which include tachycardia, shortness of breath, and chest pain – are nonspecific. So, it is important to have a well-thought-out approach to make the diagnosis in patients who have PE and avoid unnecessary tests and risks in patients with a low likelihood of PE.
New guidelines from the American College of Physicians suggest a graded approach to diagnostic testing based on a patient’s estimated pre-test probability of having PE.
When deciding whether to test for PE and which test to order, it is essential to determine the likelihood that a patient’s symptoms are due to PE. Validated decision-support tests include the Well’s Criteria, Pulmonary Embolism Rule-Out Criteria (PERC), and the revised Geneva score. Although they have been recommended for years, these tests often are not used. The guidelines note that the accuracy of an experienced clinician’s gut sense appears to be similar to that of the validated decision tools.
The first decision is whether to do any testing at all. PERC was developed in response to the growing and inappropriate use of D-dimer testing to rule out PE in situations with very low clinical suspicion. The ACP guidelines discuss a meta-analysis of 12 studies, which determined that the overall proportion of missed PEs in patients who had a negative PERC score was 0.3%.
PERC criteria are age younger than 50 years, heart rate less than 100 beats per minute, oxygen saturation greater than 94% on room air, no unilateral leg swelling, no hemoptysis, no surgery or trauma, no history of venous thromboembolism, and no estrogen use. The PERC test is negative when an individual meets all of the criteria above.
It is recommended that, in patients felt to be at low risk and whose PERC test is negative, then no other workup should be done. In this circumstance, “the risk of PE is lower than the risk of testing.”
If the PERC test is positive, then low-risk patients should undergo a highly sensitive D-dimer test. When used this way, the PERC tool decreases the use of D-dimer testing in patients who otherwise would have been tested inappropriately.
For intermediate-risk patients, the first test to obtain is a highly sensitive D-dimer test. The ACP guidelines summarized two recent studies – one using the Well’s Criteria and the other a revised Geneva score – which examined D-dimer testing specifically in intermediate-risk groups.
The studies evaluated 1,679 and 330 patients, respectively, at intermediate risk and found that a normal D-dimer level in these patients was 99.5% and 100% sensitive, respectively, for excluding PE on CT. Therefore, patients at both low- and intermediate-level risk should be screened first with a D-dimer rather than going directly to imaging.
When evaluating D-dimer results, a threshold of greater than 500 ng/mL usually indicates a positive test. However, in patients older than 50 years, the guidelines note that it may be more accurate to use a D-dimer level equal to a patient’s age multiplied by 10 ng/mL. Because it is a very sensitive test, a negative D-dimer test indicates no further testing is needed, and there is no reason to obtain a CT scan.
In patients believed to be at high risk of having PE, either through the use of a validated clinical decision tool or by clinical gestalt, a negative D-dimer test is not sensitive enough to rule out PE. Patients who are deemed to be at high risk of having PE should go directly to evaluation by CT pulmonary angiography (CTPA). Pulmonary ventilation/perfusion scanning should be used when CTPA is unavailable or contraindicated.
The bottom line
• The first step when evaluating a patient is to determine his or her pretest probability of PE using either a clinical tool or clinical judgment. The Well’s Criteria and Geneva score have been validated and are considered equally accurate, and neither has been shown to be superior to risk stratification using clinical gestalt.
• Low pretest probability of PE: First, use the PERC criteria. In those patients who meet all eight rule-out criteria, DO NOT order a D-dimer; the risk of PE is lower than the risk of testing. Those who do not meet all eight criteria should undergo a D-dimer. A normal D-dimer level is sufficient to rule out PE, and imaging studies are not needed. An elevated plasma D-dimer, ideally adjusted for age, should prompt evaluation by CTPA.
• Intermediate pretest probability of PE: D-dimer testing is the first step. A negative D-dimer has sufficient negative predictive value to eliminate the need for further testing. An elevated D-dimer, ideally adjusted for age, should prompt evaluation by CTPA.
• High pretest probability of PE: In patients with a high pretest probability secondary to either clinical gestalt or a clinical prediction tool, evaluation by CTPA is warranted.
References
• “Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians.” Ann Intern Med. 2015 Nov 3;163(9):701-11.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Vandergrift is a first-year resident in the program.
When a patient comes to the office or emergency department complaining of shortness of breath, acute pulmonary embolism is a diagnosis that must be considered.
The signs and symptoms of pulmonary embolism (PE) – which include tachycardia, shortness of breath, and chest pain – are nonspecific. So, it is important to have a well-thought-out approach to make the diagnosis in patients who have PE and avoid unnecessary tests and risks in patients with a low likelihood of PE.
New guidelines from the American College of Physicians suggest a graded approach to diagnostic testing based on a patient’s estimated pre-test probability of having PE.
When deciding whether to test for PE and which test to order, it is essential to determine the likelihood that a patient’s symptoms are due to PE. Validated decision-support tests include the Well’s Criteria, Pulmonary Embolism Rule-Out Criteria (PERC), and the revised Geneva score. Although they have been recommended for years, these tests often are not used. The guidelines note that the accuracy of an experienced clinician’s gut sense appears to be similar to that of the validated decision tools.
The first decision is whether to do any testing at all. PERC was developed in response to the growing and inappropriate use of D-dimer testing to rule out PE in situations with very low clinical suspicion. The ACP guidelines discuss a meta-analysis of 12 studies, which determined that the overall proportion of missed PEs in patients who had a negative PERC score was 0.3%.
PERC criteria are age younger than 50 years, heart rate less than 100 beats per minute, oxygen saturation greater than 94% on room air, no unilateral leg swelling, no hemoptysis, no surgery or trauma, no history of venous thromboembolism, and no estrogen use. The PERC test is negative when an individual meets all of the criteria above.
It is recommended that, in patients felt to be at low risk and whose PERC test is negative, then no other workup should be done. In this circumstance, “the risk of PE is lower than the risk of testing.”
If the PERC test is positive, then low-risk patients should undergo a highly sensitive D-dimer test. When used this way, the PERC tool decreases the use of D-dimer testing in patients who otherwise would have been tested inappropriately.
For intermediate-risk patients, the first test to obtain is a highly sensitive D-dimer test. The ACP guidelines summarized two recent studies – one using the Well’s Criteria and the other a revised Geneva score – which examined D-dimer testing specifically in intermediate-risk groups.
The studies evaluated 1,679 and 330 patients, respectively, at intermediate risk and found that a normal D-dimer level in these patients was 99.5% and 100% sensitive, respectively, for excluding PE on CT. Therefore, patients at both low- and intermediate-level risk should be screened first with a D-dimer rather than going directly to imaging.
When evaluating D-dimer results, a threshold of greater than 500 ng/mL usually indicates a positive test. However, in patients older than 50 years, the guidelines note that it may be more accurate to use a D-dimer level equal to a patient’s age multiplied by 10 ng/mL. Because it is a very sensitive test, a negative D-dimer test indicates no further testing is needed, and there is no reason to obtain a CT scan.
In patients believed to be at high risk of having PE, either through the use of a validated clinical decision tool or by clinical gestalt, a negative D-dimer test is not sensitive enough to rule out PE. Patients who are deemed to be at high risk of having PE should go directly to evaluation by CT pulmonary angiography (CTPA). Pulmonary ventilation/perfusion scanning should be used when CTPA is unavailable or contraindicated.
The bottom line
• The first step when evaluating a patient is to determine his or her pretest probability of PE using either a clinical tool or clinical judgment. The Well’s Criteria and Geneva score have been validated and are considered equally accurate, and neither has been shown to be superior to risk stratification using clinical gestalt.
• Low pretest probability of PE: First, use the PERC criteria. In those patients who meet all eight rule-out criteria, DO NOT order a D-dimer; the risk of PE is lower than the risk of testing. Those who do not meet all eight criteria should undergo a D-dimer. A normal D-dimer level is sufficient to rule out PE, and imaging studies are not needed. An elevated plasma D-dimer, ideally adjusted for age, should prompt evaluation by CTPA.
• Intermediate pretest probability of PE: D-dimer testing is the first step. A negative D-dimer has sufficient negative predictive value to eliminate the need for further testing. An elevated D-dimer, ideally adjusted for age, should prompt evaluation by CTPA.
• High pretest probability of PE: In patients with a high pretest probability secondary to either clinical gestalt or a clinical prediction tool, evaluation by CTPA is warranted.
References
• “Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians.” Ann Intern Med. 2015 Nov 3;163(9):701-11.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Vandergrift is a first-year resident in the program.
When a patient comes to the office or emergency department complaining of shortness of breath, acute pulmonary embolism is a diagnosis that must be considered.
The signs and symptoms of pulmonary embolism (PE) – which include tachycardia, shortness of breath, and chest pain – are nonspecific. So, it is important to have a well-thought-out approach to make the diagnosis in patients who have PE and avoid unnecessary tests and risks in patients with a low likelihood of PE.
New guidelines from the American College of Physicians suggest a graded approach to diagnostic testing based on a patient’s estimated pre-test probability of having PE.
When deciding whether to test for PE and which test to order, it is essential to determine the likelihood that a patient’s symptoms are due to PE. Validated decision-support tests include the Well’s Criteria, Pulmonary Embolism Rule-Out Criteria (PERC), and the revised Geneva score. Although they have been recommended for years, these tests often are not used. The guidelines note that the accuracy of an experienced clinician’s gut sense appears to be similar to that of the validated decision tools.
The first decision is whether to do any testing at all. PERC was developed in response to the growing and inappropriate use of D-dimer testing to rule out PE in situations with very low clinical suspicion. The ACP guidelines discuss a meta-analysis of 12 studies, which determined that the overall proportion of missed PEs in patients who had a negative PERC score was 0.3%.
PERC criteria are age younger than 50 years, heart rate less than 100 beats per minute, oxygen saturation greater than 94% on room air, no unilateral leg swelling, no hemoptysis, no surgery or trauma, no history of venous thromboembolism, and no estrogen use. The PERC test is negative when an individual meets all of the criteria above.
It is recommended that, in patients felt to be at low risk and whose PERC test is negative, then no other workup should be done. In this circumstance, “the risk of PE is lower than the risk of testing.”
If the PERC test is positive, then low-risk patients should undergo a highly sensitive D-dimer test. When used this way, the PERC tool decreases the use of D-dimer testing in patients who otherwise would have been tested inappropriately.
For intermediate-risk patients, the first test to obtain is a highly sensitive D-dimer test. The ACP guidelines summarized two recent studies – one using the Well’s Criteria and the other a revised Geneva score – which examined D-dimer testing specifically in intermediate-risk groups.
The studies evaluated 1,679 and 330 patients, respectively, at intermediate risk and found that a normal D-dimer level in these patients was 99.5% and 100% sensitive, respectively, for excluding PE on CT. Therefore, patients at both low- and intermediate-level risk should be screened first with a D-dimer rather than going directly to imaging.
When evaluating D-dimer results, a threshold of greater than 500 ng/mL usually indicates a positive test. However, in patients older than 50 years, the guidelines note that it may be more accurate to use a D-dimer level equal to a patient’s age multiplied by 10 ng/mL. Because it is a very sensitive test, a negative D-dimer test indicates no further testing is needed, and there is no reason to obtain a CT scan.
In patients believed to be at high risk of having PE, either through the use of a validated clinical decision tool or by clinical gestalt, a negative D-dimer test is not sensitive enough to rule out PE. Patients who are deemed to be at high risk of having PE should go directly to evaluation by CT pulmonary angiography (CTPA). Pulmonary ventilation/perfusion scanning should be used when CTPA is unavailable or contraindicated.
The bottom line
• The first step when evaluating a patient is to determine his or her pretest probability of PE using either a clinical tool or clinical judgment. The Well’s Criteria and Geneva score have been validated and are considered equally accurate, and neither has been shown to be superior to risk stratification using clinical gestalt.
• Low pretest probability of PE: First, use the PERC criteria. In those patients who meet all eight rule-out criteria, DO NOT order a D-dimer; the risk of PE is lower than the risk of testing. Those who do not meet all eight criteria should undergo a D-dimer. A normal D-dimer level is sufficient to rule out PE, and imaging studies are not needed. An elevated plasma D-dimer, ideally adjusted for age, should prompt evaluation by CTPA.
• Intermediate pretest probability of PE: D-dimer testing is the first step. A negative D-dimer has sufficient negative predictive value to eliminate the need for further testing. An elevated D-dimer, ideally adjusted for age, should prompt evaluation by CTPA.
• High pretest probability of PE: In patients with a high pretest probability secondary to either clinical gestalt or a clinical prediction tool, evaluation by CTPA is warranted.
References
• “Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians.” Ann Intern Med. 2015 Nov 3;163(9):701-11.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Vandergrift is a first-year resident in the program.