User login
EHR Report: Don’t let the electronic health record do the driving
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.
The secret to the care of the patient ... is in caring for the patient.
-Francis W. Peabody, MD1
Last month I received a call from a man who was upset about the way he was treated in our office. He had presented with depression and felt insulted by one of our resident physicians in the way he had interacted with him during his visit. I offered to see him the next day.
When I walked into the exam room, I noticed that his eyes were bloodshot and he was fidgeting in his chair. He explained that it was difficult for him to address this issue, but he had been taken aback at his previous visit to our office when the doctor who saw him, after introducing himself, proceeded to sit down, open his computer, and start typing. The patient went on to describe that the physician – while staring at his computer screen – first acknowledged that he was being seen for depression and then immediately asked him if he had any plans to commit suicide. He did not have any suicidal plans, but he felt strongly that being asked about suicide as the first question in the doctor’s interview missed the point of his visit. He was having trouble concentrating, he felt down, and he was having difficulty sleeping at night, all contributing to trouble both at work and in his personal life. Suicide was not a concern of his. He shook his head. He said he understood that we, as doctors, had to put information into the computer, but he also felt that the doctor’s main goal during that visit appeared to be to get through the forms on the computer rather than taking care of him. He admonished that physicians also need to remember that there is a patient in the room and that we should pay attention to the patient first. The computer should be second. I couldn’t have said it better myself. I told him that I would look into what happened, and then we continued with his visit.
You can already see where this discussion is going. The odd thing about the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Medicare’s quality payment program, is that, unless we are careful, the result of the program may be the opposite of what it’s intended to accomplish. By leading to an over-focus on documentation of the quality of care, we are at risk of diminishing the quality of care itself. In essence, many of the requirements appear to simply be more advanced versions of the meaningful (meaningless?) use provisions with which we have previously grappled. It is clear that we should assess the quality of care that is given and that physician payment should be influenced by that care. It is also clear that the only reasonable way to measure the care provided is by collecting data from the EHR. The problem is that the sophistication of the EHR has not caught up to the sophistication of our goals.
Our challenge as physicians who care for patients therefore occurs at an individual level for each of us. How do we provide the necessary documentation scattered throughout our digital charts to satisfy reporting requirements, yet still meet the very real needs of patients to have their voices heard and their emotions acknowledged? The Physician Charter by the American Board of Internal Medicine discusses “the primacy of patient welfare” as a core tenant of medical practice. It goes on to state that “administrative exigencies must not compromise this principle.”2 Given competing demands, how do we continue to accomplish these goals which are often in conflict with one another?
We cannot provide an answer to this question because unfortunately – or perhaps fortunately – the answer does not come in the form of a clear algorithm of behaviors or a form that we can click on. However that does not mean that it cannot be done. Simply being mindful of how important personal interaction is to our patients will help us stay focused on patient needs. In fact, one of the most exciting aspects of our digital age (and our use of EHRs) is that the need to actually connect with people is more important than ever, and prioritizing this stands to reward those individuals who continue to pay attention to patients. In a future column, we will discuss suggestions and strategies for integrating the EHR into truly patient-centered care. In the early 1920s, Dr. Francis W. Peabody said, “The treatment of a disease may be entirely impersonal: the care of the patient must be completely personal.”1 Medical competency is essential and documentation is required, but neither alone is sufficient for the care of patients.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington Memorial Hospital. He is also a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. Peabody FW. The care of the patient. JAMA. 1927;88:877-82.
2. The Physician Charter. American Board of Internal Medicine Foundation at http://abimfoundation.org/what-we-do/physician-charter.
GOLD guidelines for the management of COPD – 2017 update
Chronic obstructive lung disease (COPD) is the third leading cause of death in the United States1 and a major cause of mortality and morbidity around the world. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) released a new “2017 Report”2 with modified recommendations for the diagnosis, management, and prevention of COPD. The report contains several changes that are relevant to the primary care provider that will be outlined below.
Redefining COPD
GOLD’s definition of COPD was changed in its 2017 Report: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitations that are due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.” The report emphasizes that “COPD may be punctuated by periods of acute worsening of respiratory symptoms, called exacerbations.” Note that the terms “emphysema” and “chronic bronchitis” have been removed in favor of a more comprehensive description of the pathophysiology of COPD. Importantly, the report states that cough and sputum production for at least 3 months in each of 2 consecutive years, previously accepted as diagnostic criteria, are present in only a minority of patients. It is noted that chronic respiratory symptoms may exist without spirometric changes and many patients (usually smokers) have structural evidence of COPD without airflow limitation.
Changes to COPD initial assessment
The primary criterion for diagnosis is unchanged: post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.70. Spirometry remains important to confirm the diagnosis in those with classic symptoms of dyspnea, chronic cough, and/or sputum production with a history of exposure to noxious particles or gases.
The GOLD assessment system previously incorporated spirometry and included an “ABCD” system such that patients in group A are least severe. Spirometry has been progressively deemphasized in favor of symptom-based classification and the 2017 Report, for the first time, dissociates spirometric findings from severity classification.
The new system uses symptom severity and exacerbation risk to classify COPD. Two specific standardized COPD symptom measurement tools, The Modified British Medical Research Council (mMRC) questionnaire and COPD Assessment Test (CAT), are reported by GOLD as the most widely used. Low symptom severity is considered an mMRC less than or equal to 1 or CAT less than or equal to 9, high symptom severity is considered an mMRC greater than or equal to 2 or CAT greater than or equal to 10. Low risk of exacerbation is defined as no more than one exacerbation not resulting in hospital admission in the last 12 months; high risk of exacerbation is defined as at least two exacerbations or any exacerbations resulting in hospital admission in the last 12 months. Symptom severity and exacerbation risk is divided into four quadrants:
• GOLD group A: Low symptom severity, low exacerbation risk.
• GOLD group B: High symptom severity, low exacerbation risk.
• GOLD group C: Low symptom severity, high exacerbation risk.
• GOLD group D: High symptom severity, high exacerbation risk.
Changes to prevention and management of stable COPD
Smoking cessation remains important in the prevention of COPD. The 2017 Report reflects the U.S. Preventive Services Task Force’s guidelines for smoking cessation: Offer nicotine replacement, cessation counseling, and pharmacotherapy (varenicline, bupropion or nortriptyline). There is insufficient evidence to support the use of e-cigarettes. Influenza and pneumococcal vaccinations are recommended. Pulmonary rehabilitation remains important.
The 2017 Report includes an expanded discussion of COPD medications. The role of short-acting bronchodilators (SABD) in COPD remains prominent. Changes include a stronger recommendation to use combination short-acting beta-agonists and short-acting muscarinic antagonists (SABA/SAMA) as these seem to be superior to SABD monotherapy in improving symptoms and FEV1.
There were several changes to the pharmacologic treatment algorithm. For the first time, GOLD proposes escalation strategies. Preference is given to LABA/LAMA (long-acting beta-agonist/long-acting muscarinic antagonists) combinations over LABA/ICS (long-acting beta-agonist/inhaled corticosteroid) combinations as a mainstay of treatment. The rationale for this change is that LABA/LAMAs give greater bronchodilation compared with LABA/ICS, and one study showed a decreased rate of exacerbations compared to LABA/ICS in patients with a history of exacerbations. In addition, patients with COPD who receive ICS appear to have a higher risk of developing pneumonia. GOLD recommendations are:
• Group A: Start with single bronchodilator (short- or long-acting), escalate to alternative class of bronchodilator if necessary.
• Group B: Start with LABA or LAMA, escalate to LABA/LAMA if symptoms persist.
• Group C: Start with LAMA, escalate to LABA/LAMA (preferred) or LABA/ICS if exacerbations continue.
• Group D: Start with LABA/LAMA (preferred) or LAMA monotherapy, escalate to LABA/LAMA/ICS (preferred) or try LABA/ICS before escalating to LAMA/LABA/ICS if symptoms persist or exacerbations continue; roflumilast and/or a macrolide may be considered if further exacerbations occur with LABA/LAMA/ICS.
Bottom line
1. GOLD classification of COPD severity is now based on clinical criteria alone: symptom assessment and risk for exacerbation.
2. SABA/SAMA combination therapy seems to be superior to either SABA or SAMA alone.
3. Patients in group A (milder symptoms, low exacerbation risk) may be initiated on either short- or long-acting bronchodilator therapy.
4. Patients in group B (milder symptoms, increased exacerbation risk) should be initiated on LAMA monotherapy.
5. LABA/LAMA combination therapy seems to be superior to LABA/ICS combination therapy and should be used when long-acting bronchodilator monotherapy fails to control symptoms or reduce exacerbations.
References
1. CDC MMWR 11/23/12
2. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 at http://goldcopd.org (accessed 3/10/2017)
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Lent is chief resident in the program.
Chronic obstructive lung disease (COPD) is the third leading cause of death in the United States1 and a major cause of mortality and morbidity around the world. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) released a new “2017 Report”2 with modified recommendations for the diagnosis, management, and prevention of COPD. The report contains several changes that are relevant to the primary care provider that will be outlined below.
Redefining COPD
GOLD’s definition of COPD was changed in its 2017 Report: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitations that are due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.” The report emphasizes that “COPD may be punctuated by periods of acute worsening of respiratory symptoms, called exacerbations.” Note that the terms “emphysema” and “chronic bronchitis” have been removed in favor of a more comprehensive description of the pathophysiology of COPD. Importantly, the report states that cough and sputum production for at least 3 months in each of 2 consecutive years, previously accepted as diagnostic criteria, are present in only a minority of patients. It is noted that chronic respiratory symptoms may exist without spirometric changes and many patients (usually smokers) have structural evidence of COPD without airflow limitation.
Changes to COPD initial assessment
The primary criterion for diagnosis is unchanged: post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.70. Spirometry remains important to confirm the diagnosis in those with classic symptoms of dyspnea, chronic cough, and/or sputum production with a history of exposure to noxious particles or gases.
The GOLD assessment system previously incorporated spirometry and included an “ABCD” system such that patients in group A are least severe. Spirometry has been progressively deemphasized in favor of symptom-based classification and the 2017 Report, for the first time, dissociates spirometric findings from severity classification.
The new system uses symptom severity and exacerbation risk to classify COPD. Two specific standardized COPD symptom measurement tools, The Modified British Medical Research Council (mMRC) questionnaire and COPD Assessment Test (CAT), are reported by GOLD as the most widely used. Low symptom severity is considered an mMRC less than or equal to 1 or CAT less than or equal to 9, high symptom severity is considered an mMRC greater than or equal to 2 or CAT greater than or equal to 10. Low risk of exacerbation is defined as no more than one exacerbation not resulting in hospital admission in the last 12 months; high risk of exacerbation is defined as at least two exacerbations or any exacerbations resulting in hospital admission in the last 12 months. Symptom severity and exacerbation risk is divided into four quadrants:
• GOLD group A: Low symptom severity, low exacerbation risk.
• GOLD group B: High symptom severity, low exacerbation risk.
• GOLD group C: Low symptom severity, high exacerbation risk.
• GOLD group D: High symptom severity, high exacerbation risk.
Changes to prevention and management of stable COPD
Smoking cessation remains important in the prevention of COPD. The 2017 Report reflects the U.S. Preventive Services Task Force’s guidelines for smoking cessation: Offer nicotine replacement, cessation counseling, and pharmacotherapy (varenicline, bupropion or nortriptyline). There is insufficient evidence to support the use of e-cigarettes. Influenza and pneumococcal vaccinations are recommended. Pulmonary rehabilitation remains important.
The 2017 Report includes an expanded discussion of COPD medications. The role of short-acting bronchodilators (SABD) in COPD remains prominent. Changes include a stronger recommendation to use combination short-acting beta-agonists and short-acting muscarinic antagonists (SABA/SAMA) as these seem to be superior to SABD monotherapy in improving symptoms and FEV1.
There were several changes to the pharmacologic treatment algorithm. For the first time, GOLD proposes escalation strategies. Preference is given to LABA/LAMA (long-acting beta-agonist/long-acting muscarinic antagonists) combinations over LABA/ICS (long-acting beta-agonist/inhaled corticosteroid) combinations as a mainstay of treatment. The rationale for this change is that LABA/LAMAs give greater bronchodilation compared with LABA/ICS, and one study showed a decreased rate of exacerbations compared to LABA/ICS in patients with a history of exacerbations. In addition, patients with COPD who receive ICS appear to have a higher risk of developing pneumonia. GOLD recommendations are:
• Group A: Start with single bronchodilator (short- or long-acting), escalate to alternative class of bronchodilator if necessary.
• Group B: Start with LABA or LAMA, escalate to LABA/LAMA if symptoms persist.
• Group C: Start with LAMA, escalate to LABA/LAMA (preferred) or LABA/ICS if exacerbations continue.
• Group D: Start with LABA/LAMA (preferred) or LAMA monotherapy, escalate to LABA/LAMA/ICS (preferred) or try LABA/ICS before escalating to LAMA/LABA/ICS if symptoms persist or exacerbations continue; roflumilast and/or a macrolide may be considered if further exacerbations occur with LABA/LAMA/ICS.
Bottom line
1. GOLD classification of COPD severity is now based on clinical criteria alone: symptom assessment and risk for exacerbation.
2. SABA/SAMA combination therapy seems to be superior to either SABA or SAMA alone.
3. Patients in group A (milder symptoms, low exacerbation risk) may be initiated on either short- or long-acting bronchodilator therapy.
4. Patients in group B (milder symptoms, increased exacerbation risk) should be initiated on LAMA monotherapy.
5. LABA/LAMA combination therapy seems to be superior to LABA/ICS combination therapy and should be used when long-acting bronchodilator monotherapy fails to control symptoms or reduce exacerbations.
References
1. CDC MMWR 11/23/12
2. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 at http://goldcopd.org (accessed 3/10/2017)
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Lent is chief resident in the program.
Chronic obstructive lung disease (COPD) is the third leading cause of death in the United States1 and a major cause of mortality and morbidity around the world. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) released a new “2017 Report”2 with modified recommendations for the diagnosis, management, and prevention of COPD. The report contains several changes that are relevant to the primary care provider that will be outlined below.
Redefining COPD
GOLD’s definition of COPD was changed in its 2017 Report: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitations that are due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.” The report emphasizes that “COPD may be punctuated by periods of acute worsening of respiratory symptoms, called exacerbations.” Note that the terms “emphysema” and “chronic bronchitis” have been removed in favor of a more comprehensive description of the pathophysiology of COPD. Importantly, the report states that cough and sputum production for at least 3 months in each of 2 consecutive years, previously accepted as diagnostic criteria, are present in only a minority of patients. It is noted that chronic respiratory symptoms may exist without spirometric changes and many patients (usually smokers) have structural evidence of COPD without airflow limitation.
Changes to COPD initial assessment
The primary criterion for diagnosis is unchanged: post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.70. Spirometry remains important to confirm the diagnosis in those with classic symptoms of dyspnea, chronic cough, and/or sputum production with a history of exposure to noxious particles or gases.
The GOLD assessment system previously incorporated spirometry and included an “ABCD” system such that patients in group A are least severe. Spirometry has been progressively deemphasized in favor of symptom-based classification and the 2017 Report, for the first time, dissociates spirometric findings from severity classification.
The new system uses symptom severity and exacerbation risk to classify COPD. Two specific standardized COPD symptom measurement tools, The Modified British Medical Research Council (mMRC) questionnaire and COPD Assessment Test (CAT), are reported by GOLD as the most widely used. Low symptom severity is considered an mMRC less than or equal to 1 or CAT less than or equal to 9, high symptom severity is considered an mMRC greater than or equal to 2 or CAT greater than or equal to 10. Low risk of exacerbation is defined as no more than one exacerbation not resulting in hospital admission in the last 12 months; high risk of exacerbation is defined as at least two exacerbations or any exacerbations resulting in hospital admission in the last 12 months. Symptom severity and exacerbation risk is divided into four quadrants:
• GOLD group A: Low symptom severity, low exacerbation risk.
• GOLD group B: High symptom severity, low exacerbation risk.
• GOLD group C: Low symptom severity, high exacerbation risk.
• GOLD group D: High symptom severity, high exacerbation risk.
Changes to prevention and management of stable COPD
Smoking cessation remains important in the prevention of COPD. The 2017 Report reflects the U.S. Preventive Services Task Force’s guidelines for smoking cessation: Offer nicotine replacement, cessation counseling, and pharmacotherapy (varenicline, bupropion or nortriptyline). There is insufficient evidence to support the use of e-cigarettes. Influenza and pneumococcal vaccinations are recommended. Pulmonary rehabilitation remains important.
The 2017 Report includes an expanded discussion of COPD medications. The role of short-acting bronchodilators (SABD) in COPD remains prominent. Changes include a stronger recommendation to use combination short-acting beta-agonists and short-acting muscarinic antagonists (SABA/SAMA) as these seem to be superior to SABD monotherapy in improving symptoms and FEV1.
There were several changes to the pharmacologic treatment algorithm. For the first time, GOLD proposes escalation strategies. Preference is given to LABA/LAMA (long-acting beta-agonist/long-acting muscarinic antagonists) combinations over LABA/ICS (long-acting beta-agonist/inhaled corticosteroid) combinations as a mainstay of treatment. The rationale for this change is that LABA/LAMAs give greater bronchodilation compared with LABA/ICS, and one study showed a decreased rate of exacerbations compared to LABA/ICS in patients with a history of exacerbations. In addition, patients with COPD who receive ICS appear to have a higher risk of developing pneumonia. GOLD recommendations are:
• Group A: Start with single bronchodilator (short- or long-acting), escalate to alternative class of bronchodilator if necessary.
• Group B: Start with LABA or LAMA, escalate to LABA/LAMA if symptoms persist.
• Group C: Start with LAMA, escalate to LABA/LAMA (preferred) or LABA/ICS if exacerbations continue.
• Group D: Start with LABA/LAMA (preferred) or LAMA monotherapy, escalate to LABA/LAMA/ICS (preferred) or try LABA/ICS before escalating to LAMA/LABA/ICS if symptoms persist or exacerbations continue; roflumilast and/or a macrolide may be considered if further exacerbations occur with LABA/LAMA/ICS.
Bottom line
1. GOLD classification of COPD severity is now based on clinical criteria alone: symptom assessment and risk for exacerbation.
2. SABA/SAMA combination therapy seems to be superior to either SABA or SAMA alone.
3. Patients in group A (milder symptoms, low exacerbation risk) may be initiated on either short- or long-acting bronchodilator therapy.
4. Patients in group B (milder symptoms, increased exacerbation risk) should be initiated on LAMA monotherapy.
5. LABA/LAMA combination therapy seems to be superior to LABA/ICS combination therapy and should be used when long-acting bronchodilator monotherapy fails to control symptoms or reduce exacerbations.
References
1. CDC MMWR 11/23/12
2. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 at http://goldcopd.org (accessed 3/10/2017)
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital. Dr. Lent is chief resident in the program.
Restoring the promise of (really) meaningful use
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reducing CV risk in diabetes: An ADA update
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.

Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
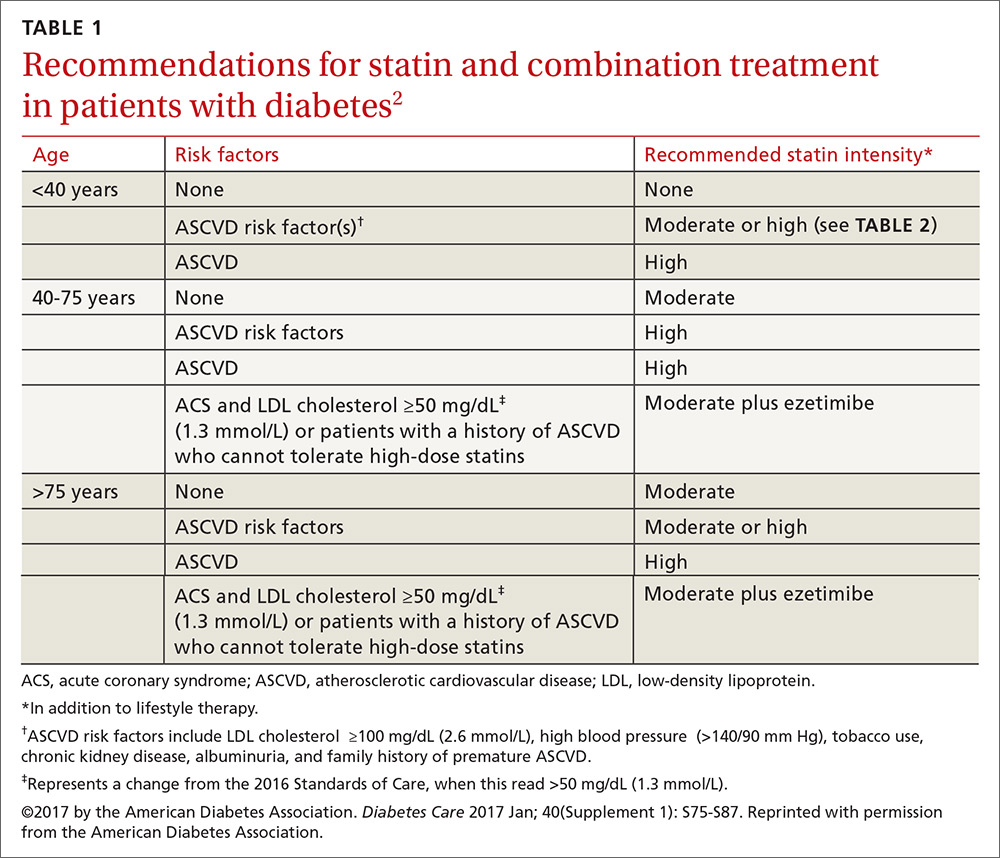
Who should get a statin, and how do I choose the optimum dosage?
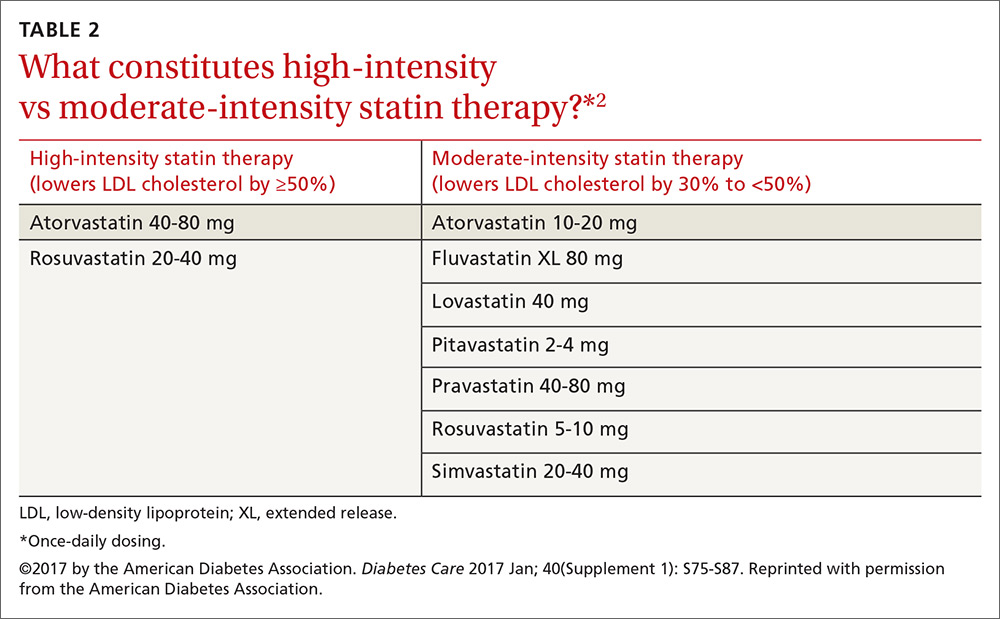
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.

Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.

Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37

For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.

Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.

Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37

For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
The EHR Report: Communication, social media, and legal vulnerability
Social media is now a part of everyday life. From Twitter, with its 140 character limit, to Facebook to Linkedin, there is a world of possibilities for communicating with friends, family, colleagues, and others online. Communication is good, but electronic media is a minefield for medical professionals who do not think carefully before they post.
The stories in the news about health care professionals who have posted obviously inflammatory material online, perhaps in a fit of rage, and have had their careers impacted or ended are just the tip of the iceberg. HIPAA violations have received a good deal of attention, with a well-known example being the doctor who was accused of posting a selfie with Joan Rivers, who was unconscious on the operating table. These examples, however, represent obvious violations of HIPAA and are infractions that most physicians would readily identify. Other examples may not be as obvious.
We know of one case where a nurse on the staff of a physicians’ office posted on Facebook that work was grueling that day because he felt under the weather with suspected flu. This may seem, at first, to be an innocuous communication. And that’s all it was, until, the son of an immunosuppressed man who had an appointment at that doctor’s office was flabbergasted to hear from a mutual friend that one of the nurses in the office was at work despite having the flu. He demanded to speak with the office manager and made sure that his father was not seen by that nurse. It may seem like an unlikely coincidence, but, in medical-legal circles, unlikely events occur all the time.
Many people who use social media will check in or post when they are out with friends or colleagues blowing off steam. For example, you might post something on social media about a holiday party you are attending. But, consider what happens if, at work the next day, something goes wrong, your care is called into question, and a lawsuit ensues. Your post may be innocent, but it now falls into the hands of the patient’s attorney. When you are having your deposition taken, the lawyer pulls your social media post out of a stack of papers and grills you about where you were, what you were doing, how late you stayed out, whether you were drinking, how much, and so on. Maybe you explain to him that you were only at the holiday party for an hour and did not have a single drink. That attorney, however, is not required to take your word for it and can ask you who you were with. All of a sudden, your friends and colleagues are being served with subpoenas for their depositions and being examined about what you did that night. Possibly, the lawyer is sending a subpoena for your credit card receipt and the restaurant’s billing records to determine what you ordered that night.
You should never rely on the false assumption that even “private” messages sent directly to other people will truly remain private. One of us was recently involved in a case where this worked to our advantage. A 30-year-old woman claimed that her family doctor never recommended that she see a gastroenterologist. A friend of the patient testified in a deposition that the two of them had discussed her medical care in private messages on Facebook. After the court ordered that the patient turn over her private Facebook messages, we learned that she told her friend that the doctor had indeed made the recommendation for her to see that specialist.
This cautionary tale doesn’t just apply to social media. Keep in mind that, if you are involved in litigation, attorneys can subpoena the records from your cellular phone provider. All cell phone text message are archived by the cellular provider and can be retrieved under subpoena. You may innocently be blowing off steam to a spouse or friend about a difficult patient or bad outcome but later have those text messages used against you in litigation.
The various social media platforms can be great tools for all kinds of professionals to share interesting information and further their professional development. However, everybody, especially the medical professional, needs to think before they post or send a message. We must also remember that, once information is out in cyberspace, it remains there and can never be truly erased. In other words, you can never unring the proverbial bell. It is important to think about the potential impact of that communication before posting and electronically communicating. Only communicate something that you would be comfortable defending in court.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Mr. Shear is an associate attorney in the health care department at Marshall Dennehey Warner Coleman & Goggin in Pittsburgh. He represents physicians, medical professionals, and hospitals in medical malpractice actions.
Social media is now a part of everyday life. From Twitter, with its 140 character limit, to Facebook to Linkedin, there is a world of possibilities for communicating with friends, family, colleagues, and others online. Communication is good, but electronic media is a minefield for medical professionals who do not think carefully before they post.
The stories in the news about health care professionals who have posted obviously inflammatory material online, perhaps in a fit of rage, and have had their careers impacted or ended are just the tip of the iceberg. HIPAA violations have received a good deal of attention, with a well-known example being the doctor who was accused of posting a selfie with Joan Rivers, who was unconscious on the operating table. These examples, however, represent obvious violations of HIPAA and are infractions that most physicians would readily identify. Other examples may not be as obvious.
We know of one case where a nurse on the staff of a physicians’ office posted on Facebook that work was grueling that day because he felt under the weather with suspected flu. This may seem, at first, to be an innocuous communication. And that’s all it was, until, the son of an immunosuppressed man who had an appointment at that doctor’s office was flabbergasted to hear from a mutual friend that one of the nurses in the office was at work despite having the flu. He demanded to speak with the office manager and made sure that his father was not seen by that nurse. It may seem like an unlikely coincidence, but, in medical-legal circles, unlikely events occur all the time.
Many people who use social media will check in or post when they are out with friends or colleagues blowing off steam. For example, you might post something on social media about a holiday party you are attending. But, consider what happens if, at work the next day, something goes wrong, your care is called into question, and a lawsuit ensues. Your post may be innocent, but it now falls into the hands of the patient’s attorney. When you are having your deposition taken, the lawyer pulls your social media post out of a stack of papers and grills you about where you were, what you were doing, how late you stayed out, whether you were drinking, how much, and so on. Maybe you explain to him that you were only at the holiday party for an hour and did not have a single drink. That attorney, however, is not required to take your word for it and can ask you who you were with. All of a sudden, your friends and colleagues are being served with subpoenas for their depositions and being examined about what you did that night. Possibly, the lawyer is sending a subpoena for your credit card receipt and the restaurant’s billing records to determine what you ordered that night.
You should never rely on the false assumption that even “private” messages sent directly to other people will truly remain private. One of us was recently involved in a case where this worked to our advantage. A 30-year-old woman claimed that her family doctor never recommended that she see a gastroenterologist. A friend of the patient testified in a deposition that the two of them had discussed her medical care in private messages on Facebook. After the court ordered that the patient turn over her private Facebook messages, we learned that she told her friend that the doctor had indeed made the recommendation for her to see that specialist.
This cautionary tale doesn’t just apply to social media. Keep in mind that, if you are involved in litigation, attorneys can subpoena the records from your cellular phone provider. All cell phone text message are archived by the cellular provider and can be retrieved under subpoena. You may innocently be blowing off steam to a spouse or friend about a difficult patient or bad outcome but later have those text messages used against you in litigation.
The various social media platforms can be great tools for all kinds of professionals to share interesting information and further their professional development. However, everybody, especially the medical professional, needs to think before they post or send a message. We must also remember that, once information is out in cyberspace, it remains there and can never be truly erased. In other words, you can never unring the proverbial bell. It is important to think about the potential impact of that communication before posting and electronically communicating. Only communicate something that you would be comfortable defending in court.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Mr. Shear is an associate attorney in the health care department at Marshall Dennehey Warner Coleman & Goggin in Pittsburgh. He represents physicians, medical professionals, and hospitals in medical malpractice actions.
Social media is now a part of everyday life. From Twitter, with its 140 character limit, to Facebook to Linkedin, there is a world of possibilities for communicating with friends, family, colleagues, and others online. Communication is good, but electronic media is a minefield for medical professionals who do not think carefully before they post.
The stories in the news about health care professionals who have posted obviously inflammatory material online, perhaps in a fit of rage, and have had their careers impacted or ended are just the tip of the iceberg. HIPAA violations have received a good deal of attention, with a well-known example being the doctor who was accused of posting a selfie with Joan Rivers, who was unconscious on the operating table. These examples, however, represent obvious violations of HIPAA and are infractions that most physicians would readily identify. Other examples may not be as obvious.
We know of one case where a nurse on the staff of a physicians’ office posted on Facebook that work was grueling that day because he felt under the weather with suspected flu. This may seem, at first, to be an innocuous communication. And that’s all it was, until, the son of an immunosuppressed man who had an appointment at that doctor’s office was flabbergasted to hear from a mutual friend that one of the nurses in the office was at work despite having the flu. He demanded to speak with the office manager and made sure that his father was not seen by that nurse. It may seem like an unlikely coincidence, but, in medical-legal circles, unlikely events occur all the time.
Many people who use social media will check in or post when they are out with friends or colleagues blowing off steam. For example, you might post something on social media about a holiday party you are attending. But, consider what happens if, at work the next day, something goes wrong, your care is called into question, and a lawsuit ensues. Your post may be innocent, but it now falls into the hands of the patient’s attorney. When you are having your deposition taken, the lawyer pulls your social media post out of a stack of papers and grills you about where you were, what you were doing, how late you stayed out, whether you were drinking, how much, and so on. Maybe you explain to him that you were only at the holiday party for an hour and did not have a single drink. That attorney, however, is not required to take your word for it and can ask you who you were with. All of a sudden, your friends and colleagues are being served with subpoenas for their depositions and being examined about what you did that night. Possibly, the lawyer is sending a subpoena for your credit card receipt and the restaurant’s billing records to determine what you ordered that night.
You should never rely on the false assumption that even “private” messages sent directly to other people will truly remain private. One of us was recently involved in a case where this worked to our advantage. A 30-year-old woman claimed that her family doctor never recommended that she see a gastroenterologist. A friend of the patient testified in a deposition that the two of them had discussed her medical care in private messages on Facebook. After the court ordered that the patient turn over her private Facebook messages, we learned that she told her friend that the doctor had indeed made the recommendation for her to see that specialist.
This cautionary tale doesn’t just apply to social media. Keep in mind that, if you are involved in litigation, attorneys can subpoena the records from your cellular phone provider. All cell phone text message are archived by the cellular provider and can be retrieved under subpoena. You may innocently be blowing off steam to a spouse or friend about a difficult patient or bad outcome but later have those text messages used against you in litigation.
The various social media platforms can be great tools for all kinds of professionals to share interesting information and further their professional development. However, everybody, especially the medical professional, needs to think before they post or send a message. We must also remember that, once information is out in cyberspace, it remains there and can never be truly erased. In other words, you can never unring the proverbial bell. It is important to think about the potential impact of that communication before posting and electronically communicating. Only communicate something that you would be comfortable defending in court.
Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Mr. Shear is an associate attorney in the health care department at Marshall Dennehey Warner Coleman & Goggin in Pittsburgh. He represents physicians, medical professionals, and hospitals in medical malpractice actions.
Management of chronic insomnia in adults
Most adults experience problems with sleep from time to time, and 6%-10% meet diagnostic criteria for chronic insomnia. Many of these patients present to their primary care clinicians looking for help. This clinical practice guideline from the American College of Physicians provides recommendations based on a review of studies published during the previous decade, which were assessed in terms of the strength of the recommendation and the quality of evidence. The guideline resulted in two recommendations:
1: All adult patients should receive cognitive behavioral therapy for insomnia (CBT-I) as the initial treatment for chronic insomnia. (strong recommendation)
2: Clinicians should use a shared decision-making approach discussing the benefits, harms, and costs of short-term use of medications, to decide whether to add pharmacological therapy in adults with chronic insomnia in whom cognitive behavioral therapy for insomnia (CBT-I) alone was unsuccessful. (weak recommendation)
Cognitive behavioral therapy for insomnia (CBT-I)
Cognitive behavioral therapy for insomnia encompasses a variety of measures that aim to change patients’ habits and beliefs associated with sleep. These measures include general “sleep hygiene” interventions, as well as stimulus control, sleep restriction, relaxation training, and cognitive reframing. With sleep hygiene, patients are educated about environmental factors that affect sleep, such as avoiding caffeine late in the day, limiting alcohol intake, having a regular sleep schedule, avoiding napping, the importance of exercise, and the importance of a quiet dark room in which to sleep. Examples of stimulus control include going to bed only when sleepy, and avoiding reading and watching TV in the bedroom. Sleep restriction limits the time in bed with strict sleep and wake-up times, gradually increasing time in bed as sleep efficiency improves.
Clinicians may find it surprising that this guideline makes such a strong, clear case for the primacy of behavioral measures in the treatment of chronic insomnia. The authors make a number of points in support of this position.
First, the effects of behavioral interventions appear to be robust – at least comparable to the short-term effects of medications – and often significantly better. For example, various studies of CBT-I show a decrease in sleep onset latency (how long it takes to fall asleep after going to bed) of between 12 and 31 minutes and an increase in total sleep time of 40 minutes. This compares favorably to the short-term effects of commonly used sleep medications.
Second, the effects of behavioral interventions are long-lasting compared with medications, which are usually approved for only short-term use, lose effectiveness over time, and have no benefit at all once they’re no longer being taken. Finally, there appear to be no harms associated with CBT-I, compared with significant adverse effects of medications.
One challenge is that access to effective behavioral interventions for insomnia can be an issue. On the other hand, a number of behavioral delivery methods were examined, and found to be effective, including in-person individual or group therapy, telephone- or Web-based modules or apps, and self-help books. An editorial accompanying the guidelines calls for efforts to increase the availability of behavioral modalities for insomnia.
Pharmacologic therapy
The recommendation to use pharmacologic therapy for insomnia is much more qualified than that for CBT-I, with language about shared decision-making, discussion of risks and benefits, emphasis on short-term use, and a provision that it be used only after an unsatisfactory trial of CBT-I alone. In addition, this recommendation is classified as “weak,” and the associated evidence “low-quality.” Medications reviewed included eszopiclone, zaleplon, zolpidem, orexin receptor antagonist, melatonin, ramelteon, and benzodiazepines.
There are several reasons why pharmacologic therapy is deemphasized. First, as noted above, the effects of commonly used medications are modest. As an example, typical patients with chronic insomnia will have sleep-onset latency of 60-70 minutes. Medications reviewed for this guideline decreased this time by approximately 10-20 minutes in short-term studies, so patients still took 40-60 minutes to fall asleep. Similar modest short-term effects were seen in terms of increasing total sleep time.
A second issue with pharmacologic therapy is that while many patients with chronic insomnia seek to use medications long term, the available studies have tended to look only at short-term use, and those studies with longer duration show a diminution of medication effect over time.
Finally, there are significant adverse effects associated with sedative-hypnotic medications, including somnolence, anxiety, confusion, and disturbance in attention. This is problematic, considering that these are precisely the symptoms that patients may be hoping to avoid when they take medications to help them sleep. Even patients who may not feel impaired often show demonstrable deficits in attention and performance following use of sleep medications; this issue is reflected in the boxed warnings that accompany several commonly prescribed agents.
It is noted in the evidence reviews that there are differences among the available medications. The nonbenzodiazepine hypnotics eszopiclone and zolpidem as well as the orexin receptor antagonist suvorexant improved short-term sleep quality, though the effect was small and there was significant evidence of harm as described above. Benzodiazepine hypnotics, melatonin agonists, and antidepressants studied had little or low-quality evidence to support efficacy on improving sleep. For melatonin and ramelteon, the evidence review notes that adverse effects did not differ between the medication and the placebo groups, though two open-label longer-term studies showed evidence of adverse effects with ramelteon. It is also important to note that patients studied in medication trials were mostly healthy middle-aged individuals; it is possible that the side effects of sleep medications may be greater in those who are older or more infirm.
Bottom line
This guideline from the American College of Physicians strongly endorses the use of tailored cognitive behavioral therapy modalities for the initial treatment of patients with chronic insomnia. Medications are given a weak recommendation for a limited back-up role.
Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
Qaseem A, et al. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016;165:125-33.
Brasure M. Psychological and Behavioral Interventions for Managing Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern Med. 2016;165:113-24.
Wilt TJ, et al. Pharmacologic Treatment of Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-12.
Most adults experience problems with sleep from time to time, and 6%-10% meet diagnostic criteria for chronic insomnia. Many of these patients present to their primary care clinicians looking for help. This clinical practice guideline from the American College of Physicians provides recommendations based on a review of studies published during the previous decade, which were assessed in terms of the strength of the recommendation and the quality of evidence. The guideline resulted in two recommendations:
1: All adult patients should receive cognitive behavioral therapy for insomnia (CBT-I) as the initial treatment for chronic insomnia. (strong recommendation)
2: Clinicians should use a shared decision-making approach discussing the benefits, harms, and costs of short-term use of medications, to decide whether to add pharmacological therapy in adults with chronic insomnia in whom cognitive behavioral therapy for insomnia (CBT-I) alone was unsuccessful. (weak recommendation)
Cognitive behavioral therapy for insomnia (CBT-I)
Cognitive behavioral therapy for insomnia encompasses a variety of measures that aim to change patients’ habits and beliefs associated with sleep. These measures include general “sleep hygiene” interventions, as well as stimulus control, sleep restriction, relaxation training, and cognitive reframing. With sleep hygiene, patients are educated about environmental factors that affect sleep, such as avoiding caffeine late in the day, limiting alcohol intake, having a regular sleep schedule, avoiding napping, the importance of exercise, and the importance of a quiet dark room in which to sleep. Examples of stimulus control include going to bed only when sleepy, and avoiding reading and watching TV in the bedroom. Sleep restriction limits the time in bed with strict sleep and wake-up times, gradually increasing time in bed as sleep efficiency improves.
Clinicians may find it surprising that this guideline makes such a strong, clear case for the primacy of behavioral measures in the treatment of chronic insomnia. The authors make a number of points in support of this position.
First, the effects of behavioral interventions appear to be robust – at least comparable to the short-term effects of medications – and often significantly better. For example, various studies of CBT-I show a decrease in sleep onset latency (how long it takes to fall asleep after going to bed) of between 12 and 31 minutes and an increase in total sleep time of 40 minutes. This compares favorably to the short-term effects of commonly used sleep medications.
Second, the effects of behavioral interventions are long-lasting compared with medications, which are usually approved for only short-term use, lose effectiveness over time, and have no benefit at all once they’re no longer being taken. Finally, there appear to be no harms associated with CBT-I, compared with significant adverse effects of medications.
One challenge is that access to effective behavioral interventions for insomnia can be an issue. On the other hand, a number of behavioral delivery methods were examined, and found to be effective, including in-person individual or group therapy, telephone- or Web-based modules or apps, and self-help books. An editorial accompanying the guidelines calls for efforts to increase the availability of behavioral modalities for insomnia.
Pharmacologic therapy
The recommendation to use pharmacologic therapy for insomnia is much more qualified than that for CBT-I, with language about shared decision-making, discussion of risks and benefits, emphasis on short-term use, and a provision that it be used only after an unsatisfactory trial of CBT-I alone. In addition, this recommendation is classified as “weak,” and the associated evidence “low-quality.” Medications reviewed included eszopiclone, zaleplon, zolpidem, orexin receptor antagonist, melatonin, ramelteon, and benzodiazepines.
There are several reasons why pharmacologic therapy is deemphasized. First, as noted above, the effects of commonly used medications are modest. As an example, typical patients with chronic insomnia will have sleep-onset latency of 60-70 minutes. Medications reviewed for this guideline decreased this time by approximately 10-20 minutes in short-term studies, so patients still took 40-60 minutes to fall asleep. Similar modest short-term effects were seen in terms of increasing total sleep time.
A second issue with pharmacologic therapy is that while many patients with chronic insomnia seek to use medications long term, the available studies have tended to look only at short-term use, and those studies with longer duration show a diminution of medication effect over time.
Finally, there are significant adverse effects associated with sedative-hypnotic medications, including somnolence, anxiety, confusion, and disturbance in attention. This is problematic, considering that these are precisely the symptoms that patients may be hoping to avoid when they take medications to help them sleep. Even patients who may not feel impaired often show demonstrable deficits in attention and performance following use of sleep medications; this issue is reflected in the boxed warnings that accompany several commonly prescribed agents.
It is noted in the evidence reviews that there are differences among the available medications. The nonbenzodiazepine hypnotics eszopiclone and zolpidem as well as the orexin receptor antagonist suvorexant improved short-term sleep quality, though the effect was small and there was significant evidence of harm as described above. Benzodiazepine hypnotics, melatonin agonists, and antidepressants studied had little or low-quality evidence to support efficacy on improving sleep. For melatonin and ramelteon, the evidence review notes that adverse effects did not differ between the medication and the placebo groups, though two open-label longer-term studies showed evidence of adverse effects with ramelteon. It is also important to note that patients studied in medication trials were mostly healthy middle-aged individuals; it is possible that the side effects of sleep medications may be greater in those who are older or more infirm.
Bottom line
This guideline from the American College of Physicians strongly endorses the use of tailored cognitive behavioral therapy modalities for the initial treatment of patients with chronic insomnia. Medications are given a weak recommendation for a limited back-up role.
Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
Qaseem A, et al. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016;165:125-33.
Brasure M. Psychological and Behavioral Interventions for Managing Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern Med. 2016;165:113-24.
Wilt TJ, et al. Pharmacologic Treatment of Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-12.
Most adults experience problems with sleep from time to time, and 6%-10% meet diagnostic criteria for chronic insomnia. Many of these patients present to their primary care clinicians looking for help. This clinical practice guideline from the American College of Physicians provides recommendations based on a review of studies published during the previous decade, which were assessed in terms of the strength of the recommendation and the quality of evidence. The guideline resulted in two recommendations:
1: All adult patients should receive cognitive behavioral therapy for insomnia (CBT-I) as the initial treatment for chronic insomnia. (strong recommendation)
2: Clinicians should use a shared decision-making approach discussing the benefits, harms, and costs of short-term use of medications, to decide whether to add pharmacological therapy in adults with chronic insomnia in whom cognitive behavioral therapy for insomnia (CBT-I) alone was unsuccessful. (weak recommendation)
Cognitive behavioral therapy for insomnia (CBT-I)
Cognitive behavioral therapy for insomnia encompasses a variety of measures that aim to change patients’ habits and beliefs associated with sleep. These measures include general “sleep hygiene” interventions, as well as stimulus control, sleep restriction, relaxation training, and cognitive reframing. With sleep hygiene, patients are educated about environmental factors that affect sleep, such as avoiding caffeine late in the day, limiting alcohol intake, having a regular sleep schedule, avoiding napping, the importance of exercise, and the importance of a quiet dark room in which to sleep. Examples of stimulus control include going to bed only when sleepy, and avoiding reading and watching TV in the bedroom. Sleep restriction limits the time in bed with strict sleep and wake-up times, gradually increasing time in bed as sleep efficiency improves.
Clinicians may find it surprising that this guideline makes such a strong, clear case for the primacy of behavioral measures in the treatment of chronic insomnia. The authors make a number of points in support of this position.
First, the effects of behavioral interventions appear to be robust – at least comparable to the short-term effects of medications – and often significantly better. For example, various studies of CBT-I show a decrease in sleep onset latency (how long it takes to fall asleep after going to bed) of between 12 and 31 minutes and an increase in total sleep time of 40 minutes. This compares favorably to the short-term effects of commonly used sleep medications.
Second, the effects of behavioral interventions are long-lasting compared with medications, which are usually approved for only short-term use, lose effectiveness over time, and have no benefit at all once they’re no longer being taken. Finally, there appear to be no harms associated with CBT-I, compared with significant adverse effects of medications.
One challenge is that access to effective behavioral interventions for insomnia can be an issue. On the other hand, a number of behavioral delivery methods were examined, and found to be effective, including in-person individual or group therapy, telephone- or Web-based modules or apps, and self-help books. An editorial accompanying the guidelines calls for efforts to increase the availability of behavioral modalities for insomnia.
Pharmacologic therapy
The recommendation to use pharmacologic therapy for insomnia is much more qualified than that for CBT-I, with language about shared decision-making, discussion of risks and benefits, emphasis on short-term use, and a provision that it be used only after an unsatisfactory trial of CBT-I alone. In addition, this recommendation is classified as “weak,” and the associated evidence “low-quality.” Medications reviewed included eszopiclone, zaleplon, zolpidem, orexin receptor antagonist, melatonin, ramelteon, and benzodiazepines.
There are several reasons why pharmacologic therapy is deemphasized. First, as noted above, the effects of commonly used medications are modest. As an example, typical patients with chronic insomnia will have sleep-onset latency of 60-70 minutes. Medications reviewed for this guideline decreased this time by approximately 10-20 minutes in short-term studies, so patients still took 40-60 minutes to fall asleep. Similar modest short-term effects were seen in terms of increasing total sleep time.
A second issue with pharmacologic therapy is that while many patients with chronic insomnia seek to use medications long term, the available studies have tended to look only at short-term use, and those studies with longer duration show a diminution of medication effect over time.
Finally, there are significant adverse effects associated with sedative-hypnotic medications, including somnolence, anxiety, confusion, and disturbance in attention. This is problematic, considering that these are precisely the symptoms that patients may be hoping to avoid when they take medications to help them sleep. Even patients who may not feel impaired often show demonstrable deficits in attention and performance following use of sleep medications; this issue is reflected in the boxed warnings that accompany several commonly prescribed agents.
It is noted in the evidence reviews that there are differences among the available medications. The nonbenzodiazepine hypnotics eszopiclone and zolpidem as well as the orexin receptor antagonist suvorexant improved short-term sleep quality, though the effect was small and there was significant evidence of harm as described above. Benzodiazepine hypnotics, melatonin agonists, and antidepressants studied had little or low-quality evidence to support efficacy on improving sleep. For melatonin and ramelteon, the evidence review notes that adverse effects did not differ between the medication and the placebo groups, though two open-label longer-term studies showed evidence of adverse effects with ramelteon. It is also important to note that patients studied in medication trials were mostly healthy middle-aged individuals; it is possible that the side effects of sleep medications may be greater in those who are older or more infirm.
Bottom line
This guideline from the American College of Physicians strongly endorses the use of tailored cognitive behavioral therapy modalities for the initial treatment of patients with chronic insomnia. Medications are given a weak recommendation for a limited back-up role.
Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
Qaseem A, et al. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016;165:125-33.
Brasure M. Psychological and Behavioral Interventions for Managing Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern Med. 2016;165:113-24.
Wilt TJ, et al. Pharmacologic Treatment of Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-12.
Two boys, a dog, and our electronic health records
“Speak clearly, if you speak at all; carve every word before you let it fall.” – Oliver Wendell Holmes Sr.
One of our favorite stories is that of two boys talking to one another with a dog sitting nearby. One boy says to the other, “I taught my dog how to whistle.” Skeptically, the other boy responds, “Really? I don’t hear him whistling.” The first boys then replies, “I said I taught him. I didn’t say he learned!”
We spend a lot of time as physicians going over information with our patients, yet, according to the best data available, they retain only a small portion of what we tell them. Medication adherence rates for chronic disease range from 30% to 70%, showing that many doses of important medications are missed. Patients often don’t even remember the last instructions we give them as they are walking out of the office. This raises questions about both the way we explain information and how we can use the tools at our disposal to enhance the communication so vital to patient outcomes.
Obviously, we need to consider our words carefully and focus on teaching, not just speaking. What sets teaching apart from speaking is consideration of the learner. The better we understand our patients’ perspectives, the better the knowledge transfer will be. A simple way to address this may be better eye contact.
We have all heard the expression “the eyes are a window to the soul.” Yet, we now have computers that acts as a virtual shades, covering that window and drawing our gaze away from our patients. These shades can blind us to important clues, impeding communication and leading to misunderstanding, missed opportunity, and even patient harm. This is why some practices have chosen to use scribes to handle documentation, freeing up physicians’ eyes and addressing another obstacle to communication: time.
One of the most cited complaints from physicians is lack of time. There is an ever-growing demand on us to see more patients, manage more data, and “check off more boxes” to meet bureaucratic requirements. It should come as no surprise that these impede good patient care. We are thankful that attempts to modernize payment models are recognizing this problem. For example, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) helps to blaze the trail by focusing on care quality, practice improvement, and patient satisfaction for incentive payments. While these are early steps, they certainly point to a future more concerned with value than with volume.
As we move toward that future, we need to acknowledge that information technology can be both the problem and the answer. The current state of health IT is far from perfect. The tools we use have been designed, seemingly, around financial performance or developed to meet government requirements. It appears that neither physicians nor patients were consulted to ensure their usability or utility. Step No. 1 was getting EHRs out there. Steps 2-10 will be making them useful to clinicians, patients, and health care systems. Part of that utility will come in their ability to enhance communication.
Take patient portals, for example. The “meaningful use program” set as a requirement the ability for patients to “view, download, or transmit” their health information through electronic means. EHR vendors complied with this request but seem to have missed the intent of the measure. Patients accessing the information often are confronted with a morass of technical jargon and unfamiliar medical terms, which may even be offensive. For example, we recently spoke to a parent of a teenager with moderate intellectual disabilities. A hold-out ICD-9 code on the teen’s chart translated to her portal as “318.0 – Imbecile.” Her mother was appropriately upset, and she decided to leave the practice.
As we begin to understand technology’s advantages – and learn its pitfalls – we believe EHR vendors must enhance their offerings while engaging both providers and patients in the process of improvement. We also believe physicians need to leverage the entire care team to realize the software’s full potential. This approach may present new challenges in communication, but it also presents new opportunities. We hope that this collaborative approach will allow physicians to have more time to spend connecting with patients, leading to enhanced understanding and satisfaction.
Our knowledge of human health and disease is growing more sophisticated and so is the challenge of imparting that knowledge to patients. It is critical to find ways to do so that are relevant and understandable and give patients the tools they need to reinforce and remember what we say. This is one of the promises that we are just beginning to see fulfilled by modern EHR technology. Unlike the boy who was trying to teach his dog to whistle, our words have deep impact, and our roles as educators have never been more important.
This article was updated 3/24/17.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
“Speak clearly, if you speak at all; carve every word before you let it fall.” – Oliver Wendell Holmes Sr.
One of our favorite stories is that of two boys talking to one another with a dog sitting nearby. One boy says to the other, “I taught my dog how to whistle.” Skeptically, the other boy responds, “Really? I don’t hear him whistling.” The first boys then replies, “I said I taught him. I didn’t say he learned!”
We spend a lot of time as physicians going over information with our patients, yet, according to the best data available, they retain only a small portion of what we tell them. Medication adherence rates for chronic disease range from 30% to 70%, showing that many doses of important medications are missed. Patients often don’t even remember the last instructions we give them as they are walking out of the office. This raises questions about both the way we explain information and how we can use the tools at our disposal to enhance the communication so vital to patient outcomes.
Obviously, we need to consider our words carefully and focus on teaching, not just speaking. What sets teaching apart from speaking is consideration of the learner. The better we understand our patients’ perspectives, the better the knowledge transfer will be. A simple way to address this may be better eye contact.
We have all heard the expression “the eyes are a window to the soul.” Yet, we now have computers that acts as a virtual shades, covering that window and drawing our gaze away from our patients. These shades can blind us to important clues, impeding communication and leading to misunderstanding, missed opportunity, and even patient harm. This is why some practices have chosen to use scribes to handle documentation, freeing up physicians’ eyes and addressing another obstacle to communication: time.
One of the most cited complaints from physicians is lack of time. There is an ever-growing demand on us to see more patients, manage more data, and “check off more boxes” to meet bureaucratic requirements. It should come as no surprise that these impede good patient care. We are thankful that attempts to modernize payment models are recognizing this problem. For example, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) helps to blaze the trail by focusing on care quality, practice improvement, and patient satisfaction for incentive payments. While these are early steps, they certainly point to a future more concerned with value than with volume.
As we move toward that future, we need to acknowledge that information technology can be both the problem and the answer. The current state of health IT is far from perfect. The tools we use have been designed, seemingly, around financial performance or developed to meet government requirements. It appears that neither physicians nor patients were consulted to ensure their usability or utility. Step No. 1 was getting EHRs out there. Steps 2-10 will be making them useful to clinicians, patients, and health care systems. Part of that utility will come in their ability to enhance communication.
Take patient portals, for example. The “meaningful use program” set as a requirement the ability for patients to “view, download, or transmit” their health information through electronic means. EHR vendors complied with this request but seem to have missed the intent of the measure. Patients accessing the information often are confronted with a morass of technical jargon and unfamiliar medical terms, which may even be offensive. For example, we recently spoke to a parent of a teenager with moderate intellectual disabilities. A hold-out ICD-9 code on the teen’s chart translated to her portal as “318.0 – Imbecile.” Her mother was appropriately upset, and she decided to leave the practice.
As we begin to understand technology’s advantages – and learn its pitfalls – we believe EHR vendors must enhance their offerings while engaging both providers and patients in the process of improvement. We also believe physicians need to leverage the entire care team to realize the software’s full potential. This approach may present new challenges in communication, but it also presents new opportunities. We hope that this collaborative approach will allow physicians to have more time to spend connecting with patients, leading to enhanced understanding and satisfaction.
Our knowledge of human health and disease is growing more sophisticated and so is the challenge of imparting that knowledge to patients. It is critical to find ways to do so that are relevant and understandable and give patients the tools they need to reinforce and remember what we say. This is one of the promises that we are just beginning to see fulfilled by modern EHR technology. Unlike the boy who was trying to teach his dog to whistle, our words have deep impact, and our roles as educators have never been more important.
This article was updated 3/24/17.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
“Speak clearly, if you speak at all; carve every word before you let it fall.” – Oliver Wendell Holmes Sr.
One of our favorite stories is that of two boys talking to one another with a dog sitting nearby. One boy says to the other, “I taught my dog how to whistle.” Skeptically, the other boy responds, “Really? I don’t hear him whistling.” The first boys then replies, “I said I taught him. I didn’t say he learned!”
We spend a lot of time as physicians going over information with our patients, yet, according to the best data available, they retain only a small portion of what we tell them. Medication adherence rates for chronic disease range from 30% to 70%, showing that many doses of important medications are missed. Patients often don’t even remember the last instructions we give them as they are walking out of the office. This raises questions about both the way we explain information and how we can use the tools at our disposal to enhance the communication so vital to patient outcomes.
Obviously, we need to consider our words carefully and focus on teaching, not just speaking. What sets teaching apart from speaking is consideration of the learner. The better we understand our patients’ perspectives, the better the knowledge transfer will be. A simple way to address this may be better eye contact.
We have all heard the expression “the eyes are a window to the soul.” Yet, we now have computers that acts as a virtual shades, covering that window and drawing our gaze away from our patients. These shades can blind us to important clues, impeding communication and leading to misunderstanding, missed opportunity, and even patient harm. This is why some practices have chosen to use scribes to handle documentation, freeing up physicians’ eyes and addressing another obstacle to communication: time.
One of the most cited complaints from physicians is lack of time. There is an ever-growing demand on us to see more patients, manage more data, and “check off more boxes” to meet bureaucratic requirements. It should come as no surprise that these impede good patient care. We are thankful that attempts to modernize payment models are recognizing this problem. For example, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) helps to blaze the trail by focusing on care quality, practice improvement, and patient satisfaction for incentive payments. While these are early steps, they certainly point to a future more concerned with value than with volume.
As we move toward that future, we need to acknowledge that information technology can be both the problem and the answer. The current state of health IT is far from perfect. The tools we use have been designed, seemingly, around financial performance or developed to meet government requirements. It appears that neither physicians nor patients were consulted to ensure their usability or utility. Step No. 1 was getting EHRs out there. Steps 2-10 will be making them useful to clinicians, patients, and health care systems. Part of that utility will come in their ability to enhance communication.
Take patient portals, for example. The “meaningful use program” set as a requirement the ability for patients to “view, download, or transmit” their health information through electronic means. EHR vendors complied with this request but seem to have missed the intent of the measure. Patients accessing the information often are confronted with a morass of technical jargon and unfamiliar medical terms, which may even be offensive. For example, we recently spoke to a parent of a teenager with moderate intellectual disabilities. A hold-out ICD-9 code on the teen’s chart translated to her portal as “318.0 – Imbecile.” Her mother was appropriately upset, and she decided to leave the practice.
As we begin to understand technology’s advantages – and learn its pitfalls – we believe EHR vendors must enhance their offerings while engaging both providers and patients in the process of improvement. We also believe physicians need to leverage the entire care team to realize the software’s full potential. This approach may present new challenges in communication, but it also presents new opportunities. We hope that this collaborative approach will allow physicians to have more time to spend connecting with patients, leading to enhanced understanding and satisfaction.
Our knowledge of human health and disease is growing more sophisticated and so is the challenge of imparting that knowledge to patients. It is critical to find ways to do so that are relevant and understandable and give patients the tools they need to reinforce and remember what we say. This is one of the promises that we are just beginning to see fulfilled by modern EHR technology. Unlike the boy who was trying to teach his dog to whistle, our words have deep impact, and our roles as educators have never been more important.
This article was updated 3/24/17.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Our new year’s resolutions
Be at war with your vices, at peace with your neighbors, and let every new year find you a better person.
– Benjamin Franklin
Traditionally, the new year is a time for reflection, looking back to review what could have been done better, and looking forward to the opportunity to rectify those inadequacies over the coming year. We thought we would take this opportunity to look at our use of the electronic health record and think about the things we might do over the next year to make our lives easier and our charting better.
Top of our list is a renewed commitment to finish our notes by the end of each session. Too many physicians we know rush through patient hours and then are left with 10-20 notes to finish at the end of the day. Realistically, this is when we least feel like completing notes. Such work encroaches on personal and family time, likely contributes to the burnout that has been increasing among physicians, and is much less likely to accurately represent the encounter than notes completed in real time.
As we have spoken with many of our colleagues, it has become clear to us that many clinicians have learned how to be “just proficient enough” in their use of their EHR; they are not pulling out their hair every 5 minutes in frustration, but they have not taken the extra time and effort that are needed to optimize their productivity. To efficiently use an EHR requires some time spent designing templates and macros to make it easy to repetitively carry out common tasks.
A lot of physicians – particularly physicians over 40 years of age – are still typing their notes with the ol’ two-finger hunt-and-peck technique. This is incredibly time consuming, inefficient, and frustrating.
While many solutions have been proposed, including having a scribe walk around with the doctor, the simplest and easiest to implement is voice transcription. Even though medical transcription software is expensive, the return on investment is large for those who do not type well. After a short period of training on the software, notes are generally of higher quality and are finished considerably faster than when typing. The technology also has the ability to learn the names of frequently used consultants, medications, and procedures, so users don’t even have to type uncommon names or words.
Another area in which we hope to advance over the next year is working more effectively as a team to share the documentation burden. Nurses and medical assistants – within the boundaries of their licensing – can be empowered to document in predefined areas of the chart as much as possible.
For example, given the fact that the prevalence of depression is about twice as high in patients with diabetes as it is in the general population, our medical assistants now screen our diabetes patients with a PHQ-2 depression screen and record the results in the chart. This has been good for our patients, satisfying for our medical assistants, and has offloaded this task from the doctors.
We need to think of more areas where we can facilitate team care and really make everyone – physicians, nurses, front staff, and patients – more satisfied with the care that is being given.
Most EHRs have a reminder function – the ability to prompt a user to follow up on an abnormal x-ray or lab results in case a patient does not come back into the office as recommended. Our sense is that most of us are not using this function. It is worth finding out how to use it and giving it a try.
Patient portals have gained a lot of traction over the past few years. For a little while, we were really making an effort to have patients register, so that currently many (but by far not most) of our patients have signed up. We want to make better use of this fantastic resource.
We say “fantastic” because when we talk to patients (or friends or family) who use the portal, they have shared that it really makes their lives easier. They are able to see their labs, ponder the meaning of their results (perhaps of a slightly high glucose or an LDL cholesterol level), and if they have questions, they can correspond electronically with their care providers. It enhances care and allows us to spend less time on the phone, while giving patients better access to information.
New year’s resolutions are an opportunity for reflection and optimism. As we look back on the past year, we should learn from our experience and approach the year in front of us with greater enthusiasm, in the hope that through that enthusiasm we can continue to grow, be better and healthier, and simply be more like the people we want to be.
The electronic health record affects all of our interactions with patients and colleagues, and, when not used optimally, encroaches into our personal and family lives. It is a perfect place to focus during the new year to enable us to have more productive, effective, and happier times both in the office and at home.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Be at war with your vices, at peace with your neighbors, and let every new year find you a better person.
– Benjamin Franklin
Traditionally, the new year is a time for reflection, looking back to review what could have been done better, and looking forward to the opportunity to rectify those inadequacies over the coming year. We thought we would take this opportunity to look at our use of the electronic health record and think about the things we might do over the next year to make our lives easier and our charting better.
Top of our list is a renewed commitment to finish our notes by the end of each session. Too many physicians we know rush through patient hours and then are left with 10-20 notes to finish at the end of the day. Realistically, this is when we least feel like completing notes. Such work encroaches on personal and family time, likely contributes to the burnout that has been increasing among physicians, and is much less likely to accurately represent the encounter than notes completed in real time.
As we have spoken with many of our colleagues, it has become clear to us that many clinicians have learned how to be “just proficient enough” in their use of their EHR; they are not pulling out their hair every 5 minutes in frustration, but they have not taken the extra time and effort that are needed to optimize their productivity. To efficiently use an EHR requires some time spent designing templates and macros to make it easy to repetitively carry out common tasks.
A lot of physicians – particularly physicians over 40 years of age – are still typing their notes with the ol’ two-finger hunt-and-peck technique. This is incredibly time consuming, inefficient, and frustrating.
While many solutions have been proposed, including having a scribe walk around with the doctor, the simplest and easiest to implement is voice transcription. Even though medical transcription software is expensive, the return on investment is large for those who do not type well. After a short period of training on the software, notes are generally of higher quality and are finished considerably faster than when typing. The technology also has the ability to learn the names of frequently used consultants, medications, and procedures, so users don’t even have to type uncommon names or words.
Another area in which we hope to advance over the next year is working more effectively as a team to share the documentation burden. Nurses and medical assistants – within the boundaries of their licensing – can be empowered to document in predefined areas of the chart as much as possible.
For example, given the fact that the prevalence of depression is about twice as high in patients with diabetes as it is in the general population, our medical assistants now screen our diabetes patients with a PHQ-2 depression screen and record the results in the chart. This has been good for our patients, satisfying for our medical assistants, and has offloaded this task from the doctors.
We need to think of more areas where we can facilitate team care and really make everyone – physicians, nurses, front staff, and patients – more satisfied with the care that is being given.
Most EHRs have a reminder function – the ability to prompt a user to follow up on an abnormal x-ray or lab results in case a patient does not come back into the office as recommended. Our sense is that most of us are not using this function. It is worth finding out how to use it and giving it a try.
Patient portals have gained a lot of traction over the past few years. For a little while, we were really making an effort to have patients register, so that currently many (but by far not most) of our patients have signed up. We want to make better use of this fantastic resource.
We say “fantastic” because when we talk to patients (or friends or family) who use the portal, they have shared that it really makes their lives easier. They are able to see their labs, ponder the meaning of their results (perhaps of a slightly high glucose or an LDL cholesterol level), and if they have questions, they can correspond electronically with their care providers. It enhances care and allows us to spend less time on the phone, while giving patients better access to information.
New year’s resolutions are an opportunity for reflection and optimism. As we look back on the past year, we should learn from our experience and approach the year in front of us with greater enthusiasm, in the hope that through that enthusiasm we can continue to grow, be better and healthier, and simply be more like the people we want to be.
The electronic health record affects all of our interactions with patients and colleagues, and, when not used optimally, encroaches into our personal and family lives. It is a perfect place to focus during the new year to enable us to have more productive, effective, and happier times both in the office and at home.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Be at war with your vices, at peace with your neighbors, and let every new year find you a better person.
– Benjamin Franklin
Traditionally, the new year is a time for reflection, looking back to review what could have been done better, and looking forward to the opportunity to rectify those inadequacies over the coming year. We thought we would take this opportunity to look at our use of the electronic health record and think about the things we might do over the next year to make our lives easier and our charting better.
Top of our list is a renewed commitment to finish our notes by the end of each session. Too many physicians we know rush through patient hours and then are left with 10-20 notes to finish at the end of the day. Realistically, this is when we least feel like completing notes. Such work encroaches on personal and family time, likely contributes to the burnout that has been increasing among physicians, and is much less likely to accurately represent the encounter than notes completed in real time.
As we have spoken with many of our colleagues, it has become clear to us that many clinicians have learned how to be “just proficient enough” in their use of their EHR; they are not pulling out their hair every 5 minutes in frustration, but they have not taken the extra time and effort that are needed to optimize their productivity. To efficiently use an EHR requires some time spent designing templates and macros to make it easy to repetitively carry out common tasks.
A lot of physicians – particularly physicians over 40 years of age – are still typing their notes with the ol’ two-finger hunt-and-peck technique. This is incredibly time consuming, inefficient, and frustrating.
While many solutions have been proposed, including having a scribe walk around with the doctor, the simplest and easiest to implement is voice transcription. Even though medical transcription software is expensive, the return on investment is large for those who do not type well. After a short period of training on the software, notes are generally of higher quality and are finished considerably faster than when typing. The technology also has the ability to learn the names of frequently used consultants, medications, and procedures, so users don’t even have to type uncommon names or words.
Another area in which we hope to advance over the next year is working more effectively as a team to share the documentation burden. Nurses and medical assistants – within the boundaries of their licensing – can be empowered to document in predefined areas of the chart as much as possible.
For example, given the fact that the prevalence of depression is about twice as high in patients with diabetes as it is in the general population, our medical assistants now screen our diabetes patients with a PHQ-2 depression screen and record the results in the chart. This has been good for our patients, satisfying for our medical assistants, and has offloaded this task from the doctors.
We need to think of more areas where we can facilitate team care and really make everyone – physicians, nurses, front staff, and patients – more satisfied with the care that is being given.
Most EHRs have a reminder function – the ability to prompt a user to follow up on an abnormal x-ray or lab results in case a patient does not come back into the office as recommended. Our sense is that most of us are not using this function. It is worth finding out how to use it and giving it a try.
Patient portals have gained a lot of traction over the past few years. For a little while, we were really making an effort to have patients register, so that currently many (but by far not most) of our patients have signed up. We want to make better use of this fantastic resource.
We say “fantastic” because when we talk to patients (or friends or family) who use the portal, they have shared that it really makes their lives easier. They are able to see their labs, ponder the meaning of their results (perhaps of a slightly high glucose or an LDL cholesterol level), and if they have questions, they can correspond electronically with their care providers. It enhances care and allows us to spend less time on the phone, while giving patients better access to information.
New year’s resolutions are an opportunity for reflection and optimism. As we look back on the past year, we should learn from our experience and approach the year in front of us with greater enthusiasm, in the hope that through that enthusiasm we can continue to grow, be better and healthier, and simply be more like the people we want to be.
The electronic health record affects all of our interactions with patients and colleagues, and, when not used optimally, encroaches into our personal and family lives. It is a perfect place to focus during the new year to enable us to have more productive, effective, and happier times both in the office and at home.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Clinical Guidelines: ADA 2017 Standards of Medical Care in Diabetes
In 2012, 29.1 million Americans, or 9.3% of the population, had diabetes. Of this number, 21 million were diagnosed, and 8.1 million were undiagnosed. Each year almost 1.5 million Americans receive a new diagnosis of diabetes. The management of diabetes relies upon excellent primary care. Each year the American Diabetes Association reviews new evidence and publishes an updated Standards of Care in the January issue of Diabetes Care. Here we give a short overview of the guidelines with emphasis on fundamentals and changes in the standards over the past year.
Self-management education and support, nutrition therapy, and physical activity
All patients should participate in ongoing diabetes self-management education (DSME) to facilitate the knowledge, skills, and abilities necessary to obtain optimal self-care and incorporate the needs, goals, and life experiences of the person with diabetes as they face new challenges throughout a lifetime of diabetes.
In addition, each patient should receive individualized medical nutrition therapy (MNT) provided by a registered dietitian with knowledge regarding diabetes-specific MNT. Most patients should increase aerobic physical activity to 150 min/week. Providers should encourage patients to reduce the amount of time spent sedentary by briefly standing, walking, or performing other light physical activities every 30 minutes.
Glycemic targets
A reasonable hemoglobin A1c goal for many diabetic nonpregnant adults is less than 7%. A less stringent goal under 8% may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular and macrovascular complications, and extensive comorbid conditions. HbA1c measurements should be done at diagnosis and routinely to monitor glycemic control. To aid in achieving glycemic targets, self-monitoring blood glucose (SMBG) allows patients to evaluate their individual response to therapy. Integrating SMBG data into diabetes management can help guide MNT, adjust medications, determine physical activity requirements, and prevent hypoglycemia. Individuals at risk for hypoglycemia should be asked about symptomatic and asymptomatic hypoglycemia at each encounter and counseled regarding treatment of hypoglycemic events.
Obesity management
There is strong and consistent evidence that obesity management may be beneficial in the treatment of type 2 diabetes. For overweight and obese patients with type 2 diabetes, interventions should be high intensity (more than 16 sessions in 6 months) and focus on diet, physical activity, and behavioral therapy designed to achieve a greater than 5% weight loss (energy deficit of 500-750 kcal/day).
For select patients, weight loss medications may be effective as adjuncts to lifestyle changes. When choosing additional pharmacologic interventions to improve glycemic control in overweight or obese patients, providers should use medications that promote weight loss or are weight neutral including metformin, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl peptidase-4 inhibitors (DPP-4) versus those that cause weight gain such as insulin secretagogues, thiazolidinediones, and insulin.
Metabolic surgery should be recommended to patients with type 2 diabetes and body mass index above 40 kg/m2 (BMI above 37.5 kg/m2 in Asian Americans), regardless of adequate glycemic control and for patients with BMI above 35 kg/m2 (more than 32.5 kg/m2 in Asian Americans) without adequate glycemic control despite lifestyle modifications and optimal medical therapy. Metabolic surgery should be considered for appropriate candidates with BMIs as low as 30 if hyperglycemia is inadequately controlled despite optical medical control by either oral or injectable medications.
CV disease and risk management: BP, lipids, antiplatelet therapy, and glycemic medication management
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality for individuals with diabetes. Screening for atherosclerotic cardiovascular disease is not recommended; rather, the emphasis is on careful risk factor management.
If systolic blood pressure (SBP) is confirmed to be above 140 mm Hg and/or the diastolic blood pressure (DBP) is confirmed to be above 90 mm Hg, pharmacologic therapy should be initiated. A meta-analysis of randomized trials of adults with type 2 diabetes comparing intensive blood pressure targets (upper limit of 130 mm Hg SBP and 80 mm Hg DBP) with standard targets (upper limit of 140-160 mm Hg SBP and 85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MI. There was a statistically significant, 35% relative risk (RR) reduction in stroke with intensive targets, but intensive targets were associated with an increased risk for adverse events such as hypotension and syncope. Recommendations suggest that antihypertension treatment in adults with diabetes without albuminuria should include any of the classes of medication demonstrated to reduce cardiovascular events in patients with diabetes, such as ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, or dihydropyridine calcium-channel blockers. ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and elevated urine albumin/creatinine ratios (above 30 mg/g creatinine). The standards also suggest consideration of administering one or more antihypertensive medications at bedtime, which may improve cardiovascular outcomes.
For patients aged 40-75 years who have diabetes without additional atherosclerotic CV disease risk factors, a moderate-intensity statin should be considered. If there are additional cardiovascular risk factors, then a high-intensity statin should be considered. For patients who are younger than 40 years of age and have diabetes with additional atherosclerotic CV disease risk factors, a less strong recommendation is to consider using moderate-intensity or high-intensity statins. For patients older than 75 years with diabetes without additional atherosclerotic CV disease risk factors, consider using moderate-intensity statin therapy; high-intensity statin therapy may be considered in older adults with risk factors for atherosclerotic cardiovascular disease.
Both women and men who are at least 50 years old and have diabetes with at least one additional cardiovascular risk factor should consider taking daily aspirin therapy (75-162 mg/day) if they do not have any risk for excessive bleeding.
In patients with long-standing suboptimally controlled type 2 diabetes and established atherosclerotic CV disease, empagliflozin or liraglutide should be considered as they have been shown to reduce cardiovascular and all-cause mortality when added to standard care.
Microvascular disease and foot care
Large prospective studies have demonstrated that optimized glucose control can reduce the onset and progression of diabetic microvascular complications. Diabetic kidney disease occurs in about 20%-40% of persons with diabetes. Annual screening includes estimated glomerular filtration rate and spot urine albumin-to-creatinine ratio. Treatment includes ACE inhibitors or ARBs in addition to a target blood pressure of under 140/90 mm Hg.
Diabetic retinopathy screening includes a dilated eye exam by an eye care professional. Treatment includes laser photocoagulation therapy for high risk nonproliferative retinopathy or proliferative retinopathy, or intravitreal injections of antivascular endothelial growth factor agents for central-involved diabetic macular edema.
Diabetic peripheral neuropathy screening includes annual 10-g monofilament and 128-HZ tuning fork vibration sensation. Medications for painful diabetic neuropathy may include gabapentin, pregabalin, duloxetine, and other agents.
Neuropathy and vascular disease are contributors to diabetic foot ulcers and amputation. A comprehensive foot examination along with appropriate risk factor oriented history to include neuropathic and vascular components (pulses, claudication) should be performed annually, while all patients with diabetes should have their feet checked at every visit.
Older adults
Prioritizing treatment goals in older adults is important in this heterogeneous population. Cardiovascular risk factor treatment is likely to be beneficial.
In setting HbA1c goals, functional status, and comorbid conditions should be considered. Metformin can still be a first-line agent for many older adults with type 2 diabetes, with consideration to renal status (creatinine clearance above 30 mL/min per 1.73 m2) and heart failure. DPP-4s have few side effects and low risk of hypoglycemia. GLP-1 receptor agonists have a low risk of hypoglycemia but may be associated with GI side effects and weight loss. SGLT-2 inhibitors have a low risk of hypoglycemia, and attention should be paid to renal thresholds for use. Thiazolidinediones should be used cautiously in those with heart failure or at elevated fracture risk. Sulfonylureas should be used cautiously because of their elevated risk of hypoglycemia. When used, a short-acting sulfonylurea – such as glipizide – is preferred, as long-acting sulfonylureas are contraindicated because of even greater hypoglycemic risk. Single-injection basal insulin may be appropriate for many with ease of use and efficacy.
The bottom line
Diabetes is a rapidly changing field and each year the American Diabetes Association updates the Standards of Medical Care document to be consistent with the latest evidence. Highlights of the standards include emphasis on diabetes self-management education, individualized glycemic goal setting, obesity management, setting blood pressure targets to less than 140/90 mm Hg, as well as statins and daily aspirin for most people with diabetes. In addition, ADA now recommends the use of specific antihyperglycemic medications to reduce cardiovascular and all-cause mortality in patients with diabetes and established cardiovascular disease.
Reference
American Diabetes Association Standards of Medical Care in Diabetes – 2017. Diabetes Care 2017; 40 (sup 1):S1-S138
Dr. Skolnik is professor of family and community medicine, Temple University School of Medicine, Philadelphia, and associate director, Family Medicine Residency Program, Abington-Jefferson Health, Abington, Pa. Dr. Johnson is associate professor at the University of North Dakota School of Medicine and Health Sciences, and practices at the Altru Diabetes Center, Grand Forks. Ms. Neuman practices at St. Mark’s Hospital, Salt Lake City.
In 2012, 29.1 million Americans, or 9.3% of the population, had diabetes. Of this number, 21 million were diagnosed, and 8.1 million were undiagnosed. Each year almost 1.5 million Americans receive a new diagnosis of diabetes. The management of diabetes relies upon excellent primary care. Each year the American Diabetes Association reviews new evidence and publishes an updated Standards of Care in the January issue of Diabetes Care. Here we give a short overview of the guidelines with emphasis on fundamentals and changes in the standards over the past year.
Self-management education and support, nutrition therapy, and physical activity
All patients should participate in ongoing diabetes self-management education (DSME) to facilitate the knowledge, skills, and abilities necessary to obtain optimal self-care and incorporate the needs, goals, and life experiences of the person with diabetes as they face new challenges throughout a lifetime of diabetes.
In addition, each patient should receive individualized medical nutrition therapy (MNT) provided by a registered dietitian with knowledge regarding diabetes-specific MNT. Most patients should increase aerobic physical activity to 150 min/week. Providers should encourage patients to reduce the amount of time spent sedentary by briefly standing, walking, or performing other light physical activities every 30 minutes.
Glycemic targets
A reasonable hemoglobin A1c goal for many diabetic nonpregnant adults is less than 7%. A less stringent goal under 8% may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular and macrovascular complications, and extensive comorbid conditions. HbA1c measurements should be done at diagnosis and routinely to monitor glycemic control. To aid in achieving glycemic targets, self-monitoring blood glucose (SMBG) allows patients to evaluate their individual response to therapy. Integrating SMBG data into diabetes management can help guide MNT, adjust medications, determine physical activity requirements, and prevent hypoglycemia. Individuals at risk for hypoglycemia should be asked about symptomatic and asymptomatic hypoglycemia at each encounter and counseled regarding treatment of hypoglycemic events.
Obesity management
There is strong and consistent evidence that obesity management may be beneficial in the treatment of type 2 diabetes. For overweight and obese patients with type 2 diabetes, interventions should be high intensity (more than 16 sessions in 6 months) and focus on diet, physical activity, and behavioral therapy designed to achieve a greater than 5% weight loss (energy deficit of 500-750 kcal/day).
For select patients, weight loss medications may be effective as adjuncts to lifestyle changes. When choosing additional pharmacologic interventions to improve glycemic control in overweight or obese patients, providers should use medications that promote weight loss or are weight neutral including metformin, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl peptidase-4 inhibitors (DPP-4) versus those that cause weight gain such as insulin secretagogues, thiazolidinediones, and insulin.
Metabolic surgery should be recommended to patients with type 2 diabetes and body mass index above 40 kg/m2 (BMI above 37.5 kg/m2 in Asian Americans), regardless of adequate glycemic control and for patients with BMI above 35 kg/m2 (more than 32.5 kg/m2 in Asian Americans) without adequate glycemic control despite lifestyle modifications and optimal medical therapy. Metabolic surgery should be considered for appropriate candidates with BMIs as low as 30 if hyperglycemia is inadequately controlled despite optical medical control by either oral or injectable medications.
CV disease and risk management: BP, lipids, antiplatelet therapy, and glycemic medication management
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality for individuals with diabetes. Screening for atherosclerotic cardiovascular disease is not recommended; rather, the emphasis is on careful risk factor management.
If systolic blood pressure (SBP) is confirmed to be above 140 mm Hg and/or the diastolic blood pressure (DBP) is confirmed to be above 90 mm Hg, pharmacologic therapy should be initiated. A meta-analysis of randomized trials of adults with type 2 diabetes comparing intensive blood pressure targets (upper limit of 130 mm Hg SBP and 80 mm Hg DBP) with standard targets (upper limit of 140-160 mm Hg SBP and 85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MI. There was a statistically significant, 35% relative risk (RR) reduction in stroke with intensive targets, but intensive targets were associated with an increased risk for adverse events such as hypotension and syncope. Recommendations suggest that antihypertension treatment in adults with diabetes without albuminuria should include any of the classes of medication demonstrated to reduce cardiovascular events in patients with diabetes, such as ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, or dihydropyridine calcium-channel blockers. ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and elevated urine albumin/creatinine ratios (above 30 mg/g creatinine). The standards also suggest consideration of administering one or more antihypertensive medications at bedtime, which may improve cardiovascular outcomes.
For patients aged 40-75 years who have diabetes without additional atherosclerotic CV disease risk factors, a moderate-intensity statin should be considered. If there are additional cardiovascular risk factors, then a high-intensity statin should be considered. For patients who are younger than 40 years of age and have diabetes with additional atherosclerotic CV disease risk factors, a less strong recommendation is to consider using moderate-intensity or high-intensity statins. For patients older than 75 years with diabetes without additional atherosclerotic CV disease risk factors, consider using moderate-intensity statin therapy; high-intensity statin therapy may be considered in older adults with risk factors for atherosclerotic cardiovascular disease.
Both women and men who are at least 50 years old and have diabetes with at least one additional cardiovascular risk factor should consider taking daily aspirin therapy (75-162 mg/day) if they do not have any risk for excessive bleeding.
In patients with long-standing suboptimally controlled type 2 diabetes and established atherosclerotic CV disease, empagliflozin or liraglutide should be considered as they have been shown to reduce cardiovascular and all-cause mortality when added to standard care.
Microvascular disease and foot care
Large prospective studies have demonstrated that optimized glucose control can reduce the onset and progression of diabetic microvascular complications. Diabetic kidney disease occurs in about 20%-40% of persons with diabetes. Annual screening includes estimated glomerular filtration rate and spot urine albumin-to-creatinine ratio. Treatment includes ACE inhibitors or ARBs in addition to a target blood pressure of under 140/90 mm Hg.
Diabetic retinopathy screening includes a dilated eye exam by an eye care professional. Treatment includes laser photocoagulation therapy for high risk nonproliferative retinopathy or proliferative retinopathy, or intravitreal injections of antivascular endothelial growth factor agents for central-involved diabetic macular edema.
Diabetic peripheral neuropathy screening includes annual 10-g monofilament and 128-HZ tuning fork vibration sensation. Medications for painful diabetic neuropathy may include gabapentin, pregabalin, duloxetine, and other agents.
Neuropathy and vascular disease are contributors to diabetic foot ulcers and amputation. A comprehensive foot examination along with appropriate risk factor oriented history to include neuropathic and vascular components (pulses, claudication) should be performed annually, while all patients with diabetes should have their feet checked at every visit.
Older adults
Prioritizing treatment goals in older adults is important in this heterogeneous population. Cardiovascular risk factor treatment is likely to be beneficial.
In setting HbA1c goals, functional status, and comorbid conditions should be considered. Metformin can still be a first-line agent for many older adults with type 2 diabetes, with consideration to renal status (creatinine clearance above 30 mL/min per 1.73 m2) and heart failure. DPP-4s have few side effects and low risk of hypoglycemia. GLP-1 receptor agonists have a low risk of hypoglycemia but may be associated with GI side effects and weight loss. SGLT-2 inhibitors have a low risk of hypoglycemia, and attention should be paid to renal thresholds for use. Thiazolidinediones should be used cautiously in those with heart failure or at elevated fracture risk. Sulfonylureas should be used cautiously because of their elevated risk of hypoglycemia. When used, a short-acting sulfonylurea – such as glipizide – is preferred, as long-acting sulfonylureas are contraindicated because of even greater hypoglycemic risk. Single-injection basal insulin may be appropriate for many with ease of use and efficacy.
The bottom line
Diabetes is a rapidly changing field and each year the American Diabetes Association updates the Standards of Medical Care document to be consistent with the latest evidence. Highlights of the standards include emphasis on diabetes self-management education, individualized glycemic goal setting, obesity management, setting blood pressure targets to less than 140/90 mm Hg, as well as statins and daily aspirin for most people with diabetes. In addition, ADA now recommends the use of specific antihyperglycemic medications to reduce cardiovascular and all-cause mortality in patients with diabetes and established cardiovascular disease.
Reference
American Diabetes Association Standards of Medical Care in Diabetes – 2017. Diabetes Care 2017; 40 (sup 1):S1-S138
Dr. Skolnik is professor of family and community medicine, Temple University School of Medicine, Philadelphia, and associate director, Family Medicine Residency Program, Abington-Jefferson Health, Abington, Pa. Dr. Johnson is associate professor at the University of North Dakota School of Medicine and Health Sciences, and practices at the Altru Diabetes Center, Grand Forks. Ms. Neuman practices at St. Mark’s Hospital, Salt Lake City.
In 2012, 29.1 million Americans, or 9.3% of the population, had diabetes. Of this number, 21 million were diagnosed, and 8.1 million were undiagnosed. Each year almost 1.5 million Americans receive a new diagnosis of diabetes. The management of diabetes relies upon excellent primary care. Each year the American Diabetes Association reviews new evidence and publishes an updated Standards of Care in the January issue of Diabetes Care. Here we give a short overview of the guidelines with emphasis on fundamentals and changes in the standards over the past year.
Self-management education and support, nutrition therapy, and physical activity
All patients should participate in ongoing diabetes self-management education (DSME) to facilitate the knowledge, skills, and abilities necessary to obtain optimal self-care and incorporate the needs, goals, and life experiences of the person with diabetes as they face new challenges throughout a lifetime of diabetes.
In addition, each patient should receive individualized medical nutrition therapy (MNT) provided by a registered dietitian with knowledge regarding diabetes-specific MNT. Most patients should increase aerobic physical activity to 150 min/week. Providers should encourage patients to reduce the amount of time spent sedentary by briefly standing, walking, or performing other light physical activities every 30 minutes.
Glycemic targets
A reasonable hemoglobin A1c goal for many diabetic nonpregnant adults is less than 7%. A less stringent goal under 8% may be appropriate for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular and macrovascular complications, and extensive comorbid conditions. HbA1c measurements should be done at diagnosis and routinely to monitor glycemic control. To aid in achieving glycemic targets, self-monitoring blood glucose (SMBG) allows patients to evaluate their individual response to therapy. Integrating SMBG data into diabetes management can help guide MNT, adjust medications, determine physical activity requirements, and prevent hypoglycemia. Individuals at risk for hypoglycemia should be asked about symptomatic and asymptomatic hypoglycemia at each encounter and counseled regarding treatment of hypoglycemic events.
Obesity management
There is strong and consistent evidence that obesity management may be beneficial in the treatment of type 2 diabetes. For overweight and obese patients with type 2 diabetes, interventions should be high intensity (more than 16 sessions in 6 months) and focus on diet, physical activity, and behavioral therapy designed to achieve a greater than 5% weight loss (energy deficit of 500-750 kcal/day).
For select patients, weight loss medications may be effective as adjuncts to lifestyle changes. When choosing additional pharmacologic interventions to improve glycemic control in overweight or obese patients, providers should use medications that promote weight loss or are weight neutral including metformin, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl peptidase-4 inhibitors (DPP-4) versus those that cause weight gain such as insulin secretagogues, thiazolidinediones, and insulin.
Metabolic surgery should be recommended to patients with type 2 diabetes and body mass index above 40 kg/m2 (BMI above 37.5 kg/m2 in Asian Americans), regardless of adequate glycemic control and for patients with BMI above 35 kg/m2 (more than 32.5 kg/m2 in Asian Americans) without adequate glycemic control despite lifestyle modifications and optimal medical therapy. Metabolic surgery should be considered for appropriate candidates with BMIs as low as 30 if hyperglycemia is inadequately controlled despite optical medical control by either oral or injectable medications.
CV disease and risk management: BP, lipids, antiplatelet therapy, and glycemic medication management
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality for individuals with diabetes. Screening for atherosclerotic cardiovascular disease is not recommended; rather, the emphasis is on careful risk factor management.
If systolic blood pressure (SBP) is confirmed to be above 140 mm Hg and/or the diastolic blood pressure (DBP) is confirmed to be above 90 mm Hg, pharmacologic therapy should be initiated. A meta-analysis of randomized trials of adults with type 2 diabetes comparing intensive blood pressure targets (upper limit of 130 mm Hg SBP and 80 mm Hg DBP) with standard targets (upper limit of 140-160 mm Hg SBP and 85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MI. There was a statistically significant, 35% relative risk (RR) reduction in stroke with intensive targets, but intensive targets were associated with an increased risk for adverse events such as hypotension and syncope. Recommendations suggest that antihypertension treatment in adults with diabetes without albuminuria should include any of the classes of medication demonstrated to reduce cardiovascular events in patients with diabetes, such as ACE inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, or dihydropyridine calcium-channel blockers. ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and elevated urine albumin/creatinine ratios (above 30 mg/g creatinine). The standards also suggest consideration of administering one or more antihypertensive medications at bedtime, which may improve cardiovascular outcomes.
For patients aged 40-75 years who have diabetes without additional atherosclerotic CV disease risk factors, a moderate-intensity statin should be considered. If there are additional cardiovascular risk factors, then a high-intensity statin should be considered. For patients who are younger than 40 years of age and have diabetes with additional atherosclerotic CV disease risk factors, a less strong recommendation is to consider using moderate-intensity or high-intensity statins. For patients older than 75 years with diabetes without additional atherosclerotic CV disease risk factors, consider using moderate-intensity statin therapy; high-intensity statin therapy may be considered in older adults with risk factors for atherosclerotic cardiovascular disease.
Both women and men who are at least 50 years old and have diabetes with at least one additional cardiovascular risk factor should consider taking daily aspirin therapy (75-162 mg/day) if they do not have any risk for excessive bleeding.
In patients with long-standing suboptimally controlled type 2 diabetes and established atherosclerotic CV disease, empagliflozin or liraglutide should be considered as they have been shown to reduce cardiovascular and all-cause mortality when added to standard care.
Microvascular disease and foot care
Large prospective studies have demonstrated that optimized glucose control can reduce the onset and progression of diabetic microvascular complications. Diabetic kidney disease occurs in about 20%-40% of persons with diabetes. Annual screening includes estimated glomerular filtration rate and spot urine albumin-to-creatinine ratio. Treatment includes ACE inhibitors or ARBs in addition to a target blood pressure of under 140/90 mm Hg.
Diabetic retinopathy screening includes a dilated eye exam by an eye care professional. Treatment includes laser photocoagulation therapy for high risk nonproliferative retinopathy or proliferative retinopathy, or intravitreal injections of antivascular endothelial growth factor agents for central-involved diabetic macular edema.
Diabetic peripheral neuropathy screening includes annual 10-g monofilament and 128-HZ tuning fork vibration sensation. Medications for painful diabetic neuropathy may include gabapentin, pregabalin, duloxetine, and other agents.
Neuropathy and vascular disease are contributors to diabetic foot ulcers and amputation. A comprehensive foot examination along with appropriate risk factor oriented history to include neuropathic and vascular components (pulses, claudication) should be performed annually, while all patients with diabetes should have their feet checked at every visit.
Older adults
Prioritizing treatment goals in older adults is important in this heterogeneous population. Cardiovascular risk factor treatment is likely to be beneficial.
In setting HbA1c goals, functional status, and comorbid conditions should be considered. Metformin can still be a first-line agent for many older adults with type 2 diabetes, with consideration to renal status (creatinine clearance above 30 mL/min per 1.73 m2) and heart failure. DPP-4s have few side effects and low risk of hypoglycemia. GLP-1 receptor agonists have a low risk of hypoglycemia but may be associated with GI side effects and weight loss. SGLT-2 inhibitors have a low risk of hypoglycemia, and attention should be paid to renal thresholds for use. Thiazolidinediones should be used cautiously in those with heart failure or at elevated fracture risk. Sulfonylureas should be used cautiously because of their elevated risk of hypoglycemia. When used, a short-acting sulfonylurea – such as glipizide – is preferred, as long-acting sulfonylureas are contraindicated because of even greater hypoglycemic risk. Single-injection basal insulin may be appropriate for many with ease of use and efficacy.
The bottom line
Diabetes is a rapidly changing field and each year the American Diabetes Association updates the Standards of Medical Care document to be consistent with the latest evidence. Highlights of the standards include emphasis on diabetes self-management education, individualized glycemic goal setting, obesity management, setting blood pressure targets to less than 140/90 mm Hg, as well as statins and daily aspirin for most people with diabetes. In addition, ADA now recommends the use of specific antihyperglycemic medications to reduce cardiovascular and all-cause mortality in patients with diabetes and established cardiovascular disease.
Reference
American Diabetes Association Standards of Medical Care in Diabetes – 2017. Diabetes Care 2017; 40 (sup 1):S1-S138
Dr. Skolnik is professor of family and community medicine, Temple University School of Medicine, Philadelphia, and associate director, Family Medicine Residency Program, Abington-Jefferson Health, Abington, Pa. Dr. Johnson is associate professor at the University of North Dakota School of Medicine and Health Sciences, and practices at the Altru Diabetes Center, Grand Forks. Ms. Neuman practices at St. Mark’s Hospital, Salt Lake City.
Treatment of depression – nonpharmacologic vs. pharmacologic
Major depressive disorder (MDD) affects 16% of adults in the United States at some point in their lives. It is one of the most important causes of disability, time off from work, and personal distress, accounting for more than 8 million office visits per year.
Recent information shows that while 8% of the population screens positive for depression, only a quarter of those with depression receive treatment. Most patients with depression are cared for by primary care physicians, not psychiatrists.1 It is important that primary care physicians are familiar with the range of evidence-based treatments for depression and their relative efficacy. Most patients with depression receive antidepressant medication and less than one-third of patients receive some form of psychotherapy.1 The American College of Physicians guideline reviews the evidence regarding the relative efficacy and safety of second-generation antidepressants and nonpharmacologic treatment of depression.2
Outcomes evaluated in this guideline include response, remission, functional capacity, quality of life, reduction of suicidality or hospitalizations, and harms.
The pharmacotherapy treatment of depression, as assessed in this guideline, are second-generation antidepressants (SGAs), which include selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and selective serotonin norepinephrine reuptake inhibitors. Previous reviews have shown that the SGAs have similar efficacy and safety with the side effects varying among the different medications; common side effects include constipation, diarrhea, nausea, decreased sexual ability, dizziness, headache, insomnia, and fatigue.
The strongest evidence, rated as moderate quality, comes from trials comparing SGAs to a form of psychotherapy called cognitive-behavioral therapy (CBT). CBT uses the technique of “collaborative empiricism” to question patients maladaptive beliefs, and by examining those beliefs, help patients to take on interpretations of reality that are less biased by their initial negative thoughts. Through these “cognitive” exercises, patients begin to take on healthier, more-adaptive approaches to the social, physical, and emotional challenges in their lives. These interpretations are then “tested” in the real world, the behavioral aspect of CBT. Studies that ranged in time from 8 to 52 weeks in patients with MDD showed SGAs and CBT to have equal efficacy with regard to response and remission of depression to therapy. Combining SGA and CBT, compared with SGA alone, did not show a difference in outcomes of response to therapy or remission of depression, though patients who received both therapies had some improved efficacy in work function.
When SGAs were compared with interpersonal therapy, psychodynamic therapy, St. John’s wort, acupuncture, and exercise, there was low-quality evidence that these interventions performed with equal efficacy to SGAs. Two trials of exercise, compared with sertraline, had moderate-quality evidence showing similar efficacy between the two treatments.
When patients have an incomplete response to initial treatment with an SGA, there was no difference in response or remission when using a strategy of switching from one SGA to another versus switching to cognitive therapy. Similarly, with regard to augmentation, CBT appears to work equally to augmenting initial SGA therapy with bupropion or buspirone.
The guidelines discuss that, with regard to adverse effects, while the discontinuation rates of SGAs and CBT are similar, CBT likely has fewer side effects. In addition, it is important to recognize that CBT has lower relapse rate associated with its use than do SGAs. This is presumably because once a skill set is developed when learning CBT, those skills can continue to be used long term.
The bottom line
Most patients who experience depression are cared for by their primary care physician. Treatments for depression include psychotherapy, complementary and alternative medicine (CAM), exercise, and pharmacotherapy. After discussion with the patient, the American College of Physicians recommends choosing either cognitive-behavioral therapy or second-generation antidepressants when treating depression.
References
1. Olfson M, Blanco C, Marcus SC. Treatment of Adult Depression in the United States. JAMA Intern Med. 2016 Oct;176(10):1482-91.
2. Qaseem A, et al. Nonpharmacologic Versus Pharmacologic Treatment of Adult Patients With Major Depressive Disorder: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016 Mar 1;164:350-59.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Aaron Sutton is a behavioral therapy consultant in the family medicine residency program at Abington Memorial Hospital.
Major depressive disorder (MDD) affects 16% of adults in the United States at some point in their lives. It is one of the most important causes of disability, time off from work, and personal distress, accounting for more than 8 million office visits per year.
Recent information shows that while 8% of the population screens positive for depression, only a quarter of those with depression receive treatment. Most patients with depression are cared for by primary care physicians, not psychiatrists.1 It is important that primary care physicians are familiar with the range of evidence-based treatments for depression and their relative efficacy. Most patients with depression receive antidepressant medication and less than one-third of patients receive some form of psychotherapy.1 The American College of Physicians guideline reviews the evidence regarding the relative efficacy and safety of second-generation antidepressants and nonpharmacologic treatment of depression.2
Outcomes evaluated in this guideline include response, remission, functional capacity, quality of life, reduction of suicidality or hospitalizations, and harms.
The pharmacotherapy treatment of depression, as assessed in this guideline, are second-generation antidepressants (SGAs), which include selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and selective serotonin norepinephrine reuptake inhibitors. Previous reviews have shown that the SGAs have similar efficacy and safety with the side effects varying among the different medications; common side effects include constipation, diarrhea, nausea, decreased sexual ability, dizziness, headache, insomnia, and fatigue.
The strongest evidence, rated as moderate quality, comes from trials comparing SGAs to a form of psychotherapy called cognitive-behavioral therapy (CBT). CBT uses the technique of “collaborative empiricism” to question patients maladaptive beliefs, and by examining those beliefs, help patients to take on interpretations of reality that are less biased by their initial negative thoughts. Through these “cognitive” exercises, patients begin to take on healthier, more-adaptive approaches to the social, physical, and emotional challenges in their lives. These interpretations are then “tested” in the real world, the behavioral aspect of CBT. Studies that ranged in time from 8 to 52 weeks in patients with MDD showed SGAs and CBT to have equal efficacy with regard to response and remission of depression to therapy. Combining SGA and CBT, compared with SGA alone, did not show a difference in outcomes of response to therapy or remission of depression, though patients who received both therapies had some improved efficacy in work function.
When SGAs were compared with interpersonal therapy, psychodynamic therapy, St. John’s wort, acupuncture, and exercise, there was low-quality evidence that these interventions performed with equal efficacy to SGAs. Two trials of exercise, compared with sertraline, had moderate-quality evidence showing similar efficacy between the two treatments.
When patients have an incomplete response to initial treatment with an SGA, there was no difference in response or remission when using a strategy of switching from one SGA to another versus switching to cognitive therapy. Similarly, with regard to augmentation, CBT appears to work equally to augmenting initial SGA therapy with bupropion or buspirone.
The guidelines discuss that, with regard to adverse effects, while the discontinuation rates of SGAs and CBT are similar, CBT likely has fewer side effects. In addition, it is important to recognize that CBT has lower relapse rate associated with its use than do SGAs. This is presumably because once a skill set is developed when learning CBT, those skills can continue to be used long term.
The bottom line
Most patients who experience depression are cared for by their primary care physician. Treatments for depression include psychotherapy, complementary and alternative medicine (CAM), exercise, and pharmacotherapy. After discussion with the patient, the American College of Physicians recommends choosing either cognitive-behavioral therapy or second-generation antidepressants when treating depression.
References
1. Olfson M, Blanco C, Marcus SC. Treatment of Adult Depression in the United States. JAMA Intern Med. 2016 Oct;176(10):1482-91.
2. Qaseem A, et al. Nonpharmacologic Versus Pharmacologic Treatment of Adult Patients With Major Depressive Disorder: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016 Mar 1;164:350-59.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Aaron Sutton is a behavioral therapy consultant in the family medicine residency program at Abington Memorial Hospital.
Major depressive disorder (MDD) affects 16% of adults in the United States at some point in their lives. It is one of the most important causes of disability, time off from work, and personal distress, accounting for more than 8 million office visits per year.
Recent information shows that while 8% of the population screens positive for depression, only a quarter of those with depression receive treatment. Most patients with depression are cared for by primary care physicians, not psychiatrists.1 It is important that primary care physicians are familiar with the range of evidence-based treatments for depression and their relative efficacy. Most patients with depression receive antidepressant medication and less than one-third of patients receive some form of psychotherapy.1 The American College of Physicians guideline reviews the evidence regarding the relative efficacy and safety of second-generation antidepressants and nonpharmacologic treatment of depression.2
Outcomes evaluated in this guideline include response, remission, functional capacity, quality of life, reduction of suicidality or hospitalizations, and harms.
The pharmacotherapy treatment of depression, as assessed in this guideline, are second-generation antidepressants (SGAs), which include selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and selective serotonin norepinephrine reuptake inhibitors. Previous reviews have shown that the SGAs have similar efficacy and safety with the side effects varying among the different medications; common side effects include constipation, diarrhea, nausea, decreased sexual ability, dizziness, headache, insomnia, and fatigue.
The strongest evidence, rated as moderate quality, comes from trials comparing SGAs to a form of psychotherapy called cognitive-behavioral therapy (CBT). CBT uses the technique of “collaborative empiricism” to question patients maladaptive beliefs, and by examining those beliefs, help patients to take on interpretations of reality that are less biased by their initial negative thoughts. Through these “cognitive” exercises, patients begin to take on healthier, more-adaptive approaches to the social, physical, and emotional challenges in their lives. These interpretations are then “tested” in the real world, the behavioral aspect of CBT. Studies that ranged in time from 8 to 52 weeks in patients with MDD showed SGAs and CBT to have equal efficacy with regard to response and remission of depression to therapy. Combining SGA and CBT, compared with SGA alone, did not show a difference in outcomes of response to therapy or remission of depression, though patients who received both therapies had some improved efficacy in work function.
When SGAs were compared with interpersonal therapy, psychodynamic therapy, St. John’s wort, acupuncture, and exercise, there was low-quality evidence that these interventions performed with equal efficacy to SGAs. Two trials of exercise, compared with sertraline, had moderate-quality evidence showing similar efficacy between the two treatments.
When patients have an incomplete response to initial treatment with an SGA, there was no difference in response or remission when using a strategy of switching from one SGA to another versus switching to cognitive therapy. Similarly, with regard to augmentation, CBT appears to work equally to augmenting initial SGA therapy with bupropion or buspirone.
The guidelines discuss that, with regard to adverse effects, while the discontinuation rates of SGAs and CBT are similar, CBT likely has fewer side effects. In addition, it is important to recognize that CBT has lower relapse rate associated with its use than do SGAs. This is presumably because once a skill set is developed when learning CBT, those skills can continue to be used long term.
The bottom line
Most patients who experience depression are cared for by their primary care physician. Treatments for depression include psychotherapy, complementary and alternative medicine (CAM), exercise, and pharmacotherapy. After discussion with the patient, the American College of Physicians recommends choosing either cognitive-behavioral therapy or second-generation antidepressants when treating depression.
References
1. Olfson M, Blanco C, Marcus SC. Treatment of Adult Depression in the United States. JAMA Intern Med. 2016 Oct;176(10):1482-91.
2. Qaseem A, et al. Nonpharmacologic Versus Pharmacologic Treatment of Adult Patients With Major Depressive Disorder: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016 Mar 1;164:350-59.
Dr. Skolnik is associate director of the family medicine residency program at Abington (Pa.) Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia. Aaron Sutton is a behavioral therapy consultant in the family medicine residency program at Abington Memorial Hospital.










