User login
Primary Capsule-Deficient Cutaneous Cryptococcosis in a Sporotrichoid Pattern in an Immunocompetent Host
Cryptococcosis is an opportunistic yeast infection caused by Cryptococcus neoformans that remains the most common systemic fungal infection in immunosuppressed patients and often presents with signs of meningitis. Cutaneous cryptococcosis occurs in 10% to 20% of systemic Cryptococcus infections and usually is secondary to hematogenous dissemination in patients with an underlying disease, particularly human immunodeficiency virus. Primary cutaneous cryptococcosis (PCC) is a more rare clinical identity that is characterized by skin lesions confined to 1 body region, often presenting as a whitlow or phlegmon with positive culture for C neoformans and no evidence of simultaneous dissemination. We report a rare case of PCC in a 73-year-old man with intact cell-mediated immunity.
Case Report
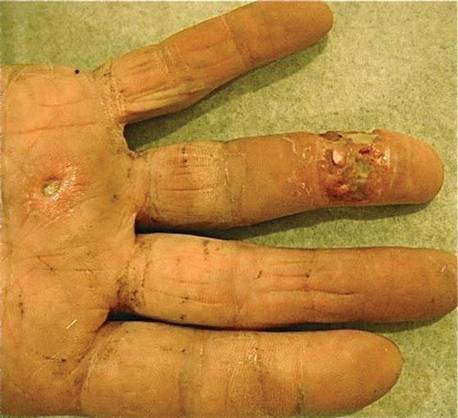
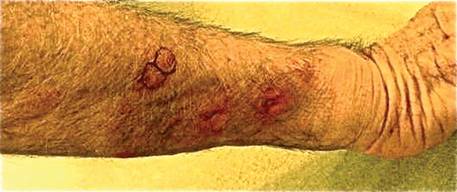
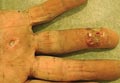
A 73-year-old man who was a beef farmer presented on primary care referral with multiple red nodules and ulcers on the right third and fourth digits and distal forearm following abrasion to the region. The patient reported that the lesions had started as painful nodules that would open and drain. He had been taking oral ciprofloxacin and oral ketoconazole for 3 days as prescribed by his primary care physician but had not begun to see results. He denied any travel or exposure to roses, fish tanks, or any sick contacts. A review of systems was negative for fever, night sweats, malaise, headache, or any other systemic symptoms. Physical examination revealed multiple 2- to 6-mm nodules and ulcers distributed in a sporotrichoid pattern on the right hand (Figure 1) and arm (Figure 2). Lymphadenopathy was absent and the rest of the examination revealed no abnormalities.
|
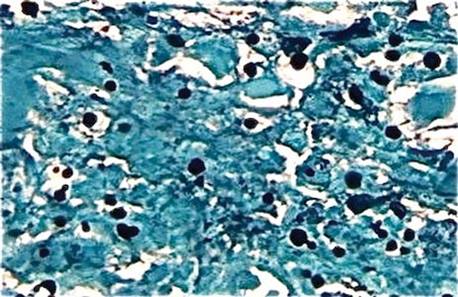
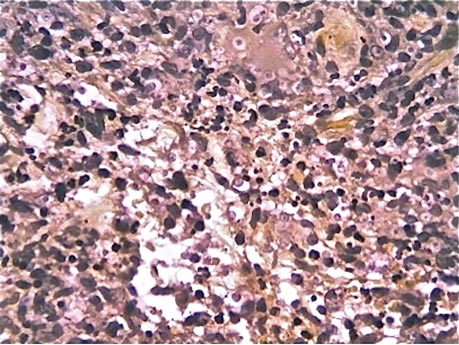
Initially, 4 punch biopsies of the right hand and arm were obtained and sent for Gram staining, tissue culture (bacterial and fungal), and histopathologic review. A presumptive diagnosis of sporotrichosis was made, with change of treatment pending culture. On routine hematoxylin and eosin staining, marked acute and chronic granulomatous inflammation with microabscesses was noted. Acid-fast bacilli staining was negative. Follow-up Gomori methenamine-silver (GMS) staining showed numerous fungal spores with narrow base budding (Figure 3). Subsequent mucicarmine staining did not reveal dark red capsules characteristic of Cryptococcus (Figure 4). The pathology report indicated that the findings may represent sporotrichosis in the appropriate clinical setting, but GMS staining could not definitively classify Sporothrix schenckii or rule out other fungal infections without tissue culture. Before culture results could be obtained, the patient returned 2 weeks later for suture removal at which point the prior medications were stopped and itraconazole 200 mg once daily was initiated.
Upon receiving the culture results, a diagnosis of primary capsule-deficient cutaneous cryptococcosis was made. The lesions showed clinical improvement at 1-month follow-up, and treatment with itraconazole was continued with monthly liver function tests. After 5 months of continued improvement, the itraconazole dose was decreased to 100 mg once daily for 1 month. The patient was free of lesions and any sequelae at 6-month and 1-year follow-up.
Comment
Cryptococcosis is caused by C neoformans, an opportunistic, basidiomycetous, yeastlike fungus1 that presents as a yeast in both the environment and tissue and normally is associated with immunocompromised host infection, especially in individuals with human immunodeficiency virus. The most common route of infection is through the lungs as respiratory droplets followed by hematogenous dissemination to the central nervous system and skin, with meningitis being the most common clinical manifestation and Cryptococcus being the most common cause of fungal meningitis worldwide.2 Cutaneous involvement after hematogenous spread (secondary cutaneous cryptococcosis) is reported in 10% to 20% of systemic Cryptococcus cases, while PCC is limited to rare cases in which trauma or abrasions to the affected site are notable risk factors.2,3
|
Cryptococcus can produce a myriad of skin manifestations including but not limited to nodules, ulcers, plaques, pustules, vesicobullous lesions, and draining sinuses. Neuville et al1 found that cellulitis, cutaneous ulcers, and whitlows were the most common presenting clinical features in PCC. Whitlows also have been reported as a rare presentation in secondary cutaneous cryptococcosis yielding to the much more prevalent presentation of umbilicated papules resembling molluscum contagiosum.1 This polymorphic identity can therefore mimic not only other dermatoses and neoplasms but other infections such as bacterial cellulitis, herpes simplex virus, and molluscum contagiosum, especially in disseminated cryptococcosis, making microscopic assessment crucial for the diagnostic confirmation of cutaneous cryptococcosis. The differential diagnosis includes sporotrichosis and Mycobacterium marinum due to the lymphatic distribution of the lesions as well as squamous cell carcinoma. Our initial diagnosis of sporotrichosis was assumptive until mycological data could be obtained.
The histopathology patterns characteristic of C neoformans infection fall into either a paucireactive pattern with myriads of densely packed organisms with mucoid gelatinous capsules that cause minimal tissue reaction or a mixed suppurative and granulomatous reaction with varying degrees of necrosis.4 The granulomatous form can affect histiocytes, giant cells, lymphocytes, and fibroblasts. These findings along with the characteristic carminophilic capsule of C neoformans allows for a prompt diagnosis. However, the C neoformans spore somewhat characteristically measures 3 to 20 mm in diameter and stains well with periodic acid–Schiff stain and GMS.4,5 Therefore, the lack of capsule broadened our early differential to include Histoplasma capsulatum, S schenckii, Paracoccidioides brasiliensis, and even Blastomyces dermatitidis.
Neuville et al1 proposed the following criteria for the diagnosis of PCC: the absence of dissemination and predominantly a solitary skin lesion on unclothed areas presenting as a whitlow or phlegmon, a history of skin injury or damage leading to direct inoculation, participation in outdoor activities, exposure to bird droppings, and isolation of C neoformans serotype D. Other factors that strongly support PCC diagnosis over squamous cell carcinoma (based on a review of the literature) are rural residential environment, older age, equal prevalence among men and women, and lack of underlying disease. Presence of these factors seem to favor PCC over squamous cell carcinoma, as some still consider the existence of PCC in general to be controversial because skin manifestations represent a sentinel finding indicative of disseminated disease.1,3
The fungus can be found worldwide as an ubiquitous saprophyte of soil, especially if the soil is enriched with pigeon droppings. A link between C neoformans and pigeons has been suggested, with dried avian excreta allowing the yeast to abundantly grow because of its high nitrogen content.5,6 Other possible sites include decaying wood, fruits, vegetables, and dust.1 There are 4 main serotypes and 3 varieties of C neoformans: C neoformans var grubii (serotype A; worldwide distribution), C neoformans var gattii (serotypes B and C; more circumscribed diffusion and distribution including subtropical regions of Australia, Central Africa, South Asia, and California), and C neoformans var neoformans (serotype D; worldwide distribution).3,7 A literature review indicated that known cases of serotype D (global incidence, 9%) tended to produce cutaneous lesions without systemic involvement.7 Microscopically, the most important characteristic feature found in all serotypes is the polysaccharide capsule, which normally acts as an important virulence factor.6 This capsule as well as detection of the budding yeast can be visualized with india ink (cerebrospinal fluid), methylene blue, or mucicarmine staining. The latex agglutination test for cryptococcal antigen has been used as a serologic test for cerebrospinal fluid, blood, and urine with a sensitivity of 86% to 95%.8
Treatment of cryptococcal disease depends on location and severity of lesions. Many cases of PCC spontaneously resolve, but it is a recommended practice to treat the lesions via incision, local irrigation and debridement, and anti-inflammatory and antifungal agents.9 Antifungal therapy with amphotericin B with or without flucytosine was the standard of therapy. The newer oral azole compounds (eg, ketoconazole, fluconazole, itraconazole) are effective against Cryptococcus, making them the probable treatment in immunocompetent patients because of fewer side effects. Nonetheless, these drugs should be maintained for several weeks or even months to achieve complete resolution of PCC.10
Our patient’s clinical presentation, physical findings, and treatment response seemed to fit well with a diagnosis of PCC, particularly the solitary skin lesions on unclothed areas of the skin; history of skin injury, participation in farming, or exposure to bird droppings (eg, contaminated soil, manure); isolation of C neoformans; and lack of evidence of disseminated disease. Once a diagnosis of PCC is made, however, evaluation of a patient’s immune system and other systemic involvement must be performed, as solitary skin lesions can be the only symptom and an early marker of disseminated disease. Inclusion of a lumbar puncture in the absence of localizing signs is not required in the workup of PCC, with the emergence of more cases of PCC being required before conclusive recommendations can be made. A strong history and physical examination, including pertinent details such as local trauma and exposure to bird droppings, along with the criteria provided by Neuville et al1 and laboratory information may be sufficient to diagnose PCC; close monitoring should be continued.1 Luckily, of the reported cases of PCC in immunocompetent individuals, oral antifungal therapy usually has been curative.2,3 The fact that our patient did not develop generalized disease could be explained by the presence of the possible serotype D, low virulence of the capsule-deficient strain, or perhaps some other immunologic mechanism of defense.
1. Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity [published online ahead of print January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
2. Werchniak AE, Baughman RD. Primary cutaneous cryptococcosis in an elderly man. Clin Exp Dermatol. 2004;29:159-160.
3. Pau M, Lallai C, Aste N, et al. Primary cutaneous cryptococcosis in an immunocompetent host [published online ahead of print March 14, 2009]. Mycoses. 2010;53:256-258.
4. Ramdial PK, Calonje E, Sing Y, et al. Molluscum-like cutaneous cryptococcosis: a histopathological and pathogenetic appraisal [published online ahead of print June 4, 2008]. J Cutan Pathol. 2008;35:1007-1013.
5. Vogelaers D, Petrovic M, Deroo M, et al. A case of primary cutaneous cryptococcosis. Eur J Clin Microbiol Infect Dis. 1997;16:150-152.
6. Naka W, Masuda M, Konohana A, et al. Primary cutaneous cryptococcosis and Cryptococcus neoformans serotype D. Clin Exp Dermatol. 1995;20:221-225.
7. Xiujiao X, Aie X. Two cases of cutaneous cryptococcosis. Mycoses. 2005;48:238-241.
8. Murray PR, Rosenthal KS, Pfaller MA, eds. Medical Microbiology. 6th ed. Philadelphia, PA: Mosby Elsevier; 2009.
9. Moreno Castillo JL, Del Negro G, Heins-Vaccari E, et al. Primary cutaneous cryptococcosis. Mycopathologia. 1986;96:25-28.
10. Joshi S, Wattal C, Duggal L, et al. Cutaneous cryptococcosis. J Assoc Physicians India. 2004;52:242-243.
Cryptococcosis is an opportunistic yeast infection caused by Cryptococcus neoformans that remains the most common systemic fungal infection in immunosuppressed patients and often presents with signs of meningitis. Cutaneous cryptococcosis occurs in 10% to 20% of systemic Cryptococcus infections and usually is secondary to hematogenous dissemination in patients with an underlying disease, particularly human immunodeficiency virus. Primary cutaneous cryptococcosis (PCC) is a more rare clinical identity that is characterized by skin lesions confined to 1 body region, often presenting as a whitlow or phlegmon with positive culture for C neoformans and no evidence of simultaneous dissemination. We report a rare case of PCC in a 73-year-old man with intact cell-mediated immunity.
Case Report
A 73-year-old man who was a beef farmer presented on primary care referral with multiple red nodules and ulcers on the right third and fourth digits and distal forearm following abrasion to the region. The patient reported that the lesions had started as painful nodules that would open and drain. He had been taking oral ciprofloxacin and oral ketoconazole for 3 days as prescribed by his primary care physician but had not begun to see results. He denied any travel or exposure to roses, fish tanks, or any sick contacts. A review of systems was negative for fever, night sweats, malaise, headache, or any other systemic symptoms. Physical examination revealed multiple 2- to 6-mm nodules and ulcers distributed in a sporotrichoid pattern on the right hand (Figure 1) and arm (Figure 2). Lymphadenopathy was absent and the rest of the examination revealed no abnormalities.
|
Initially, 4 punch biopsies of the right hand and arm were obtained and sent for Gram staining, tissue culture (bacterial and fungal), and histopathologic review. A presumptive diagnosis of sporotrichosis was made, with change of treatment pending culture. On routine hematoxylin and eosin staining, marked acute and chronic granulomatous inflammation with microabscesses was noted. Acid-fast bacilli staining was negative. Follow-up Gomori methenamine-silver (GMS) staining showed numerous fungal spores with narrow base budding (Figure 3). Subsequent mucicarmine staining did not reveal dark red capsules characteristic of Cryptococcus (Figure 4). The pathology report indicated that the findings may represent sporotrichosis in the appropriate clinical setting, but GMS staining could not definitively classify Sporothrix schenckii or rule out other fungal infections without tissue culture. Before culture results could be obtained, the patient returned 2 weeks later for suture removal at which point the prior medications were stopped and itraconazole 200 mg once daily was initiated.
Upon receiving the culture results, a diagnosis of primary capsule-deficient cutaneous cryptococcosis was made. The lesions showed clinical improvement at 1-month follow-up, and treatment with itraconazole was continued with monthly liver function tests. After 5 months of continued improvement, the itraconazole dose was decreased to 100 mg once daily for 1 month. The patient was free of lesions and any sequelae at 6-month and 1-year follow-up.
Comment
Cryptococcosis is caused by C neoformans, an opportunistic, basidiomycetous, yeastlike fungus1 that presents as a yeast in both the environment and tissue and normally is associated with immunocompromised host infection, especially in individuals with human immunodeficiency virus. The most common route of infection is through the lungs as respiratory droplets followed by hematogenous dissemination to the central nervous system and skin, with meningitis being the most common clinical manifestation and Cryptococcus being the most common cause of fungal meningitis worldwide.2 Cutaneous involvement after hematogenous spread (secondary cutaneous cryptococcosis) is reported in 10% to 20% of systemic Cryptococcus cases, while PCC is limited to rare cases in which trauma or abrasions to the affected site are notable risk factors.2,3
|
Cryptococcus can produce a myriad of skin manifestations including but not limited to nodules, ulcers, plaques, pustules, vesicobullous lesions, and draining sinuses. Neuville et al1 found that cellulitis, cutaneous ulcers, and whitlows were the most common presenting clinical features in PCC. Whitlows also have been reported as a rare presentation in secondary cutaneous cryptococcosis yielding to the much more prevalent presentation of umbilicated papules resembling molluscum contagiosum.1 This polymorphic identity can therefore mimic not only other dermatoses and neoplasms but other infections such as bacterial cellulitis, herpes simplex virus, and molluscum contagiosum, especially in disseminated cryptococcosis, making microscopic assessment crucial for the diagnostic confirmation of cutaneous cryptococcosis. The differential diagnosis includes sporotrichosis and Mycobacterium marinum due to the lymphatic distribution of the lesions as well as squamous cell carcinoma. Our initial diagnosis of sporotrichosis was assumptive until mycological data could be obtained.
The histopathology patterns characteristic of C neoformans infection fall into either a paucireactive pattern with myriads of densely packed organisms with mucoid gelatinous capsules that cause minimal tissue reaction or a mixed suppurative and granulomatous reaction with varying degrees of necrosis.4 The granulomatous form can affect histiocytes, giant cells, lymphocytes, and fibroblasts. These findings along with the characteristic carminophilic capsule of C neoformans allows for a prompt diagnosis. However, the C neoformans spore somewhat characteristically measures 3 to 20 mm in diameter and stains well with periodic acid–Schiff stain and GMS.4,5 Therefore, the lack of capsule broadened our early differential to include Histoplasma capsulatum, S schenckii, Paracoccidioides brasiliensis, and even Blastomyces dermatitidis.
Neuville et al1 proposed the following criteria for the diagnosis of PCC: the absence of dissemination and predominantly a solitary skin lesion on unclothed areas presenting as a whitlow or phlegmon, a history of skin injury or damage leading to direct inoculation, participation in outdoor activities, exposure to bird droppings, and isolation of C neoformans serotype D. Other factors that strongly support PCC diagnosis over squamous cell carcinoma (based on a review of the literature) are rural residential environment, older age, equal prevalence among men and women, and lack of underlying disease. Presence of these factors seem to favor PCC over squamous cell carcinoma, as some still consider the existence of PCC in general to be controversial because skin manifestations represent a sentinel finding indicative of disseminated disease.1,3
The fungus can be found worldwide as an ubiquitous saprophyte of soil, especially if the soil is enriched with pigeon droppings. A link between C neoformans and pigeons has been suggested, with dried avian excreta allowing the yeast to abundantly grow because of its high nitrogen content.5,6 Other possible sites include decaying wood, fruits, vegetables, and dust.1 There are 4 main serotypes and 3 varieties of C neoformans: C neoformans var grubii (serotype A; worldwide distribution), C neoformans var gattii (serotypes B and C; more circumscribed diffusion and distribution including subtropical regions of Australia, Central Africa, South Asia, and California), and C neoformans var neoformans (serotype D; worldwide distribution).3,7 A literature review indicated that known cases of serotype D (global incidence, 9%) tended to produce cutaneous lesions without systemic involvement.7 Microscopically, the most important characteristic feature found in all serotypes is the polysaccharide capsule, which normally acts as an important virulence factor.6 This capsule as well as detection of the budding yeast can be visualized with india ink (cerebrospinal fluid), methylene blue, or mucicarmine staining. The latex agglutination test for cryptococcal antigen has been used as a serologic test for cerebrospinal fluid, blood, and urine with a sensitivity of 86% to 95%.8
Treatment of cryptococcal disease depends on location and severity of lesions. Many cases of PCC spontaneously resolve, but it is a recommended practice to treat the lesions via incision, local irrigation and debridement, and anti-inflammatory and antifungal agents.9 Antifungal therapy with amphotericin B with or without flucytosine was the standard of therapy. The newer oral azole compounds (eg, ketoconazole, fluconazole, itraconazole) are effective against Cryptococcus, making them the probable treatment in immunocompetent patients because of fewer side effects. Nonetheless, these drugs should be maintained for several weeks or even months to achieve complete resolution of PCC.10
Our patient’s clinical presentation, physical findings, and treatment response seemed to fit well with a diagnosis of PCC, particularly the solitary skin lesions on unclothed areas of the skin; history of skin injury, participation in farming, or exposure to bird droppings (eg, contaminated soil, manure); isolation of C neoformans; and lack of evidence of disseminated disease. Once a diagnosis of PCC is made, however, evaluation of a patient’s immune system and other systemic involvement must be performed, as solitary skin lesions can be the only symptom and an early marker of disseminated disease. Inclusion of a lumbar puncture in the absence of localizing signs is not required in the workup of PCC, with the emergence of more cases of PCC being required before conclusive recommendations can be made. A strong history and physical examination, including pertinent details such as local trauma and exposure to bird droppings, along with the criteria provided by Neuville et al1 and laboratory information may be sufficient to diagnose PCC; close monitoring should be continued.1 Luckily, of the reported cases of PCC in immunocompetent individuals, oral antifungal therapy usually has been curative.2,3 The fact that our patient did not develop generalized disease could be explained by the presence of the possible serotype D, low virulence of the capsule-deficient strain, or perhaps some other immunologic mechanism of defense.
Cryptococcosis is an opportunistic yeast infection caused by Cryptococcus neoformans that remains the most common systemic fungal infection in immunosuppressed patients and often presents with signs of meningitis. Cutaneous cryptococcosis occurs in 10% to 20% of systemic Cryptococcus infections and usually is secondary to hematogenous dissemination in patients with an underlying disease, particularly human immunodeficiency virus. Primary cutaneous cryptococcosis (PCC) is a more rare clinical identity that is characterized by skin lesions confined to 1 body region, often presenting as a whitlow or phlegmon with positive culture for C neoformans and no evidence of simultaneous dissemination. We report a rare case of PCC in a 73-year-old man with intact cell-mediated immunity.
Case Report
A 73-year-old man who was a beef farmer presented on primary care referral with multiple red nodules and ulcers on the right third and fourth digits and distal forearm following abrasion to the region. The patient reported that the lesions had started as painful nodules that would open and drain. He had been taking oral ciprofloxacin and oral ketoconazole for 3 days as prescribed by his primary care physician but had not begun to see results. He denied any travel or exposure to roses, fish tanks, or any sick contacts. A review of systems was negative for fever, night sweats, malaise, headache, or any other systemic symptoms. Physical examination revealed multiple 2- to 6-mm nodules and ulcers distributed in a sporotrichoid pattern on the right hand (Figure 1) and arm (Figure 2). Lymphadenopathy was absent and the rest of the examination revealed no abnormalities.
|
Initially, 4 punch biopsies of the right hand and arm were obtained and sent for Gram staining, tissue culture (bacterial and fungal), and histopathologic review. A presumptive diagnosis of sporotrichosis was made, with change of treatment pending culture. On routine hematoxylin and eosin staining, marked acute and chronic granulomatous inflammation with microabscesses was noted. Acid-fast bacilli staining was negative. Follow-up Gomori methenamine-silver (GMS) staining showed numerous fungal spores with narrow base budding (Figure 3). Subsequent mucicarmine staining did not reveal dark red capsules characteristic of Cryptococcus (Figure 4). The pathology report indicated that the findings may represent sporotrichosis in the appropriate clinical setting, but GMS staining could not definitively classify Sporothrix schenckii or rule out other fungal infections without tissue culture. Before culture results could be obtained, the patient returned 2 weeks later for suture removal at which point the prior medications were stopped and itraconazole 200 mg once daily was initiated.
Upon receiving the culture results, a diagnosis of primary capsule-deficient cutaneous cryptococcosis was made. The lesions showed clinical improvement at 1-month follow-up, and treatment with itraconazole was continued with monthly liver function tests. After 5 months of continued improvement, the itraconazole dose was decreased to 100 mg once daily for 1 month. The patient was free of lesions and any sequelae at 6-month and 1-year follow-up.
Comment
Cryptococcosis is caused by C neoformans, an opportunistic, basidiomycetous, yeastlike fungus1 that presents as a yeast in both the environment and tissue and normally is associated with immunocompromised host infection, especially in individuals with human immunodeficiency virus. The most common route of infection is through the lungs as respiratory droplets followed by hematogenous dissemination to the central nervous system and skin, with meningitis being the most common clinical manifestation and Cryptococcus being the most common cause of fungal meningitis worldwide.2 Cutaneous involvement after hematogenous spread (secondary cutaneous cryptococcosis) is reported in 10% to 20% of systemic Cryptococcus cases, while PCC is limited to rare cases in which trauma or abrasions to the affected site are notable risk factors.2,3
|
Cryptococcus can produce a myriad of skin manifestations including but not limited to nodules, ulcers, plaques, pustules, vesicobullous lesions, and draining sinuses. Neuville et al1 found that cellulitis, cutaneous ulcers, and whitlows were the most common presenting clinical features in PCC. Whitlows also have been reported as a rare presentation in secondary cutaneous cryptococcosis yielding to the much more prevalent presentation of umbilicated papules resembling molluscum contagiosum.1 This polymorphic identity can therefore mimic not only other dermatoses and neoplasms but other infections such as bacterial cellulitis, herpes simplex virus, and molluscum contagiosum, especially in disseminated cryptococcosis, making microscopic assessment crucial for the diagnostic confirmation of cutaneous cryptococcosis. The differential diagnosis includes sporotrichosis and Mycobacterium marinum due to the lymphatic distribution of the lesions as well as squamous cell carcinoma. Our initial diagnosis of sporotrichosis was assumptive until mycological data could be obtained.
The histopathology patterns characteristic of C neoformans infection fall into either a paucireactive pattern with myriads of densely packed organisms with mucoid gelatinous capsules that cause minimal tissue reaction or a mixed suppurative and granulomatous reaction with varying degrees of necrosis.4 The granulomatous form can affect histiocytes, giant cells, lymphocytes, and fibroblasts. These findings along with the characteristic carminophilic capsule of C neoformans allows for a prompt diagnosis. However, the C neoformans spore somewhat characteristically measures 3 to 20 mm in diameter and stains well with periodic acid–Schiff stain and GMS.4,5 Therefore, the lack of capsule broadened our early differential to include Histoplasma capsulatum, S schenckii, Paracoccidioides brasiliensis, and even Blastomyces dermatitidis.
Neuville et al1 proposed the following criteria for the diagnosis of PCC: the absence of dissemination and predominantly a solitary skin lesion on unclothed areas presenting as a whitlow or phlegmon, a history of skin injury or damage leading to direct inoculation, participation in outdoor activities, exposure to bird droppings, and isolation of C neoformans serotype D. Other factors that strongly support PCC diagnosis over squamous cell carcinoma (based on a review of the literature) are rural residential environment, older age, equal prevalence among men and women, and lack of underlying disease. Presence of these factors seem to favor PCC over squamous cell carcinoma, as some still consider the existence of PCC in general to be controversial because skin manifestations represent a sentinel finding indicative of disseminated disease.1,3
The fungus can be found worldwide as an ubiquitous saprophyte of soil, especially if the soil is enriched with pigeon droppings. A link between C neoformans and pigeons has been suggested, with dried avian excreta allowing the yeast to abundantly grow because of its high nitrogen content.5,6 Other possible sites include decaying wood, fruits, vegetables, and dust.1 There are 4 main serotypes and 3 varieties of C neoformans: C neoformans var grubii (serotype A; worldwide distribution), C neoformans var gattii (serotypes B and C; more circumscribed diffusion and distribution including subtropical regions of Australia, Central Africa, South Asia, and California), and C neoformans var neoformans (serotype D; worldwide distribution).3,7 A literature review indicated that known cases of serotype D (global incidence, 9%) tended to produce cutaneous lesions without systemic involvement.7 Microscopically, the most important characteristic feature found in all serotypes is the polysaccharide capsule, which normally acts as an important virulence factor.6 This capsule as well as detection of the budding yeast can be visualized with india ink (cerebrospinal fluid), methylene blue, or mucicarmine staining. The latex agglutination test for cryptococcal antigen has been used as a serologic test for cerebrospinal fluid, blood, and urine with a sensitivity of 86% to 95%.8
Treatment of cryptococcal disease depends on location and severity of lesions. Many cases of PCC spontaneously resolve, but it is a recommended practice to treat the lesions via incision, local irrigation and debridement, and anti-inflammatory and antifungal agents.9 Antifungal therapy with amphotericin B with or without flucytosine was the standard of therapy. The newer oral azole compounds (eg, ketoconazole, fluconazole, itraconazole) are effective against Cryptococcus, making them the probable treatment in immunocompetent patients because of fewer side effects. Nonetheless, these drugs should be maintained for several weeks or even months to achieve complete resolution of PCC.10
Our patient’s clinical presentation, physical findings, and treatment response seemed to fit well with a diagnosis of PCC, particularly the solitary skin lesions on unclothed areas of the skin; history of skin injury, participation in farming, or exposure to bird droppings (eg, contaminated soil, manure); isolation of C neoformans; and lack of evidence of disseminated disease. Once a diagnosis of PCC is made, however, evaluation of a patient’s immune system and other systemic involvement must be performed, as solitary skin lesions can be the only symptom and an early marker of disseminated disease. Inclusion of a lumbar puncture in the absence of localizing signs is not required in the workup of PCC, with the emergence of more cases of PCC being required before conclusive recommendations can be made. A strong history and physical examination, including pertinent details such as local trauma and exposure to bird droppings, along with the criteria provided by Neuville et al1 and laboratory information may be sufficient to diagnose PCC; close monitoring should be continued.1 Luckily, of the reported cases of PCC in immunocompetent individuals, oral antifungal therapy usually has been curative.2,3 The fact that our patient did not develop generalized disease could be explained by the presence of the possible serotype D, low virulence of the capsule-deficient strain, or perhaps some other immunologic mechanism of defense.
1. Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity [published online ahead of print January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
2. Werchniak AE, Baughman RD. Primary cutaneous cryptococcosis in an elderly man. Clin Exp Dermatol. 2004;29:159-160.
3. Pau M, Lallai C, Aste N, et al. Primary cutaneous cryptococcosis in an immunocompetent host [published online ahead of print March 14, 2009]. Mycoses. 2010;53:256-258.
4. Ramdial PK, Calonje E, Sing Y, et al. Molluscum-like cutaneous cryptococcosis: a histopathological and pathogenetic appraisal [published online ahead of print June 4, 2008]. J Cutan Pathol. 2008;35:1007-1013.
5. Vogelaers D, Petrovic M, Deroo M, et al. A case of primary cutaneous cryptococcosis. Eur J Clin Microbiol Infect Dis. 1997;16:150-152.
6. Naka W, Masuda M, Konohana A, et al. Primary cutaneous cryptococcosis and Cryptococcus neoformans serotype D. Clin Exp Dermatol. 1995;20:221-225.
7. Xiujiao X, Aie X. Two cases of cutaneous cryptococcosis. Mycoses. 2005;48:238-241.
8. Murray PR, Rosenthal KS, Pfaller MA, eds. Medical Microbiology. 6th ed. Philadelphia, PA: Mosby Elsevier; 2009.
9. Moreno Castillo JL, Del Negro G, Heins-Vaccari E, et al. Primary cutaneous cryptococcosis. Mycopathologia. 1986;96:25-28.
10. Joshi S, Wattal C, Duggal L, et al. Cutaneous cryptococcosis. J Assoc Physicians India. 2004;52:242-243.
1. Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity [published online ahead of print January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
2. Werchniak AE, Baughman RD. Primary cutaneous cryptococcosis in an elderly man. Clin Exp Dermatol. 2004;29:159-160.
3. Pau M, Lallai C, Aste N, et al. Primary cutaneous cryptococcosis in an immunocompetent host [published online ahead of print March 14, 2009]. Mycoses. 2010;53:256-258.
4. Ramdial PK, Calonje E, Sing Y, et al. Molluscum-like cutaneous cryptococcosis: a histopathological and pathogenetic appraisal [published online ahead of print June 4, 2008]. J Cutan Pathol. 2008;35:1007-1013.
5. Vogelaers D, Petrovic M, Deroo M, et al. A case of primary cutaneous cryptococcosis. Eur J Clin Microbiol Infect Dis. 1997;16:150-152.
6. Naka W, Masuda M, Konohana A, et al. Primary cutaneous cryptococcosis and Cryptococcus neoformans serotype D. Clin Exp Dermatol. 1995;20:221-225.
7. Xiujiao X, Aie X. Two cases of cutaneous cryptococcosis. Mycoses. 2005;48:238-241.
8. Murray PR, Rosenthal KS, Pfaller MA, eds. Medical Microbiology. 6th ed. Philadelphia, PA: Mosby Elsevier; 2009.
9. Moreno Castillo JL, Del Negro G, Heins-Vaccari E, et al. Primary cutaneous cryptococcosis. Mycopathologia. 1986;96:25-28.
10. Joshi S, Wattal C, Duggal L, et al. Cutaneous cryptococcosis. J Assoc Physicians India. 2004;52:242-243.
Practice Points
- Cryptococcus neoformans is an encapsulated yeast that is ubiquitous in the environment and is especially abundant in soil enriched with pigeon droppings.
- Immunocompetent hosts often are asymptomatic or have only mild pulmonary disease, while disseminated disease affects the lungs, central nervous system, bones, and skin in immunocompromised hosts.
- Diagnostic tests include india ink or mucicarmine staining to highlight characteristic capsules or the latex agglutination test to measure circulating capsular antigen.