User login
iPad app puts cognitive screening in the hands of MS patients
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
REPORTING FROM THE CMSC ANNUAL MEETING
New agents may bring hope for SLE patients
SANDESTIN, FLA. – Several drugs approved for other conditions may also have good effect in patients with systemic lupus erythematosus, Michelle Petri, MD, said in an interview at the annual Congress of Clinical Rheumatology.
The molecules target several different disease pathways, said Dr. Petri, director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
Ustekinumab (Stelara) has accumulated the most data so far. A phase 2 study presented last fall at the annual meeting of the American College of Rheumatology found that it conferred significant benefits relative to placebo, including a 60% responder rate (29% better than placebo), a significantly lower flare rate, and improvements in musculoskeletal and mucocutaneous disease features. The rate of serious adverse events was acceptable (8.3% vs. 9.5% for placebo).
Baricitinib is also being investigated in SLE, Dr. Petri said. A phase 2 study conducted by Eli Lilly closed late last year and will be reported on June 13 at the European League Against Rheumatism’s opening plenary session (Wallace et al. EULAR 2018 abstract OP0019).
The three-armed, placebo-controlled study comprised 314 patients who were randomized to placebo or one of two baricitinib doses, given orally for 24 weeks. The primary outcome was remission of arthritis and/or rash as measured by the SLE Disease Activity Index 2000 (SLEDAI-2K). Secondary endpoints included responder rate, change from baseline in the SLEDAI-2K, change in the Global Assessment of Disease Activity score, and pharmacokinetic measures.
Dr. Petri disclosed relationships with Amgen, Boston Pharmaceuticals, Bristol-Myers Squibb, EMD Serono, Janssen, Novartis, and GlaxoSmithKline.
SANDESTIN, FLA. – Several drugs approved for other conditions may also have good effect in patients with systemic lupus erythematosus, Michelle Petri, MD, said in an interview at the annual Congress of Clinical Rheumatology.
The molecules target several different disease pathways, said Dr. Petri, director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
Ustekinumab (Stelara) has accumulated the most data so far. A phase 2 study presented last fall at the annual meeting of the American College of Rheumatology found that it conferred significant benefits relative to placebo, including a 60% responder rate (29% better than placebo), a significantly lower flare rate, and improvements in musculoskeletal and mucocutaneous disease features. The rate of serious adverse events was acceptable (8.3% vs. 9.5% for placebo).
Baricitinib is also being investigated in SLE, Dr. Petri said. A phase 2 study conducted by Eli Lilly closed late last year and will be reported on June 13 at the European League Against Rheumatism’s opening plenary session (Wallace et al. EULAR 2018 abstract OP0019).
The three-armed, placebo-controlled study comprised 314 patients who were randomized to placebo or one of two baricitinib doses, given orally for 24 weeks. The primary outcome was remission of arthritis and/or rash as measured by the SLE Disease Activity Index 2000 (SLEDAI-2K). Secondary endpoints included responder rate, change from baseline in the SLEDAI-2K, change in the Global Assessment of Disease Activity score, and pharmacokinetic measures.
Dr. Petri disclosed relationships with Amgen, Boston Pharmaceuticals, Bristol-Myers Squibb, EMD Serono, Janssen, Novartis, and GlaxoSmithKline.
SANDESTIN, FLA. – Several drugs approved for other conditions may also have good effect in patients with systemic lupus erythematosus, Michelle Petri, MD, said in an interview at the annual Congress of Clinical Rheumatology.
The molecules target several different disease pathways, said Dr. Petri, director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
Ustekinumab (Stelara) has accumulated the most data so far. A phase 2 study presented last fall at the annual meeting of the American College of Rheumatology found that it conferred significant benefits relative to placebo, including a 60% responder rate (29% better than placebo), a significantly lower flare rate, and improvements in musculoskeletal and mucocutaneous disease features. The rate of serious adverse events was acceptable (8.3% vs. 9.5% for placebo).
Baricitinib is also being investigated in SLE, Dr. Petri said. A phase 2 study conducted by Eli Lilly closed late last year and will be reported on June 13 at the European League Against Rheumatism’s opening plenary session (Wallace et al. EULAR 2018 abstract OP0019).
The three-armed, placebo-controlled study comprised 314 patients who were randomized to placebo or one of two baricitinib doses, given orally for 24 weeks. The primary outcome was remission of arthritis and/or rash as measured by the SLE Disease Activity Index 2000 (SLEDAI-2K). Secondary endpoints included responder rate, change from baseline in the SLEDAI-2K, change in the Global Assessment of Disease Activity score, and pharmacokinetic measures.
Dr. Petri disclosed relationships with Amgen, Boston Pharmaceuticals, Bristol-Myers Squibb, EMD Serono, Janssen, Novartis, and GlaxoSmithKline.
EXPERT ANALYSIS FROM CCR 18
MDR Candida auris is on the move
MADRID – The anticipated global emergence of multidrug resistant Candida auris is now an established fact, but a case study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress demonstrates just how devastating an outbreak can be to a medical facility and its surgical ICU patients.
The dangerous invasive infection is spreading through Asia, Europe, and the Americas, causing potentially fatal candidemias and proving devilishly difficult to eradicate in health care facilities once it becomes established.
Several multidrug resistant (MDR) C. auris outbreaks were reported at the ECCMID meeting. Most troubling: a continuing outbreak in a hospital in Valencia, Spain, in which 17 patients have died – a 41% fatality rate among those who developed a fulminant C. auris candidemia, Javier Pemán, MD, said at the meeting. The strain appeared to be a clonal population not previously identified in published reports.
“C. auris is hard to remove from the hospital environment,” once it becomes established, said Dr. Pemán of La Fe University and Polytechnic Hospital, Valencia. “When an outbreak lasts for months, as ours has, it is difficult, but necessary, to maintain control measures, identify it early in the lab, and isolate and treat patients early with combination therapy.”
He and his team have relied primarily on a combination of amphotericin B and echinocandin (AMB+ECN), although, he added, the optimal dosing and treatment time aren’t known, and many C. auris isolates are echinocandin resistant.
MDR C. auris first appearedin Tokyo in 2009. It then spread to South Korea around 2011, and then appeared across Asia and Western Europe. Its first appearance in Spain was the 2016 Le Fe outbreak.
According to the Centers for Disease Control and Prevention, single cases have appeared in Austria, Belgium, Malaysia, Norway, and the United Arab Emirates. Canada, Colombia, France, Germany, India, Israel, Japan, Kenya, Kuwait, Oman, Pakistan, Panama, South Korea, South Africa, Spain, the United Arab Emirates, the United Kingdom, and Venezuela have experienced multiple outbreaks.
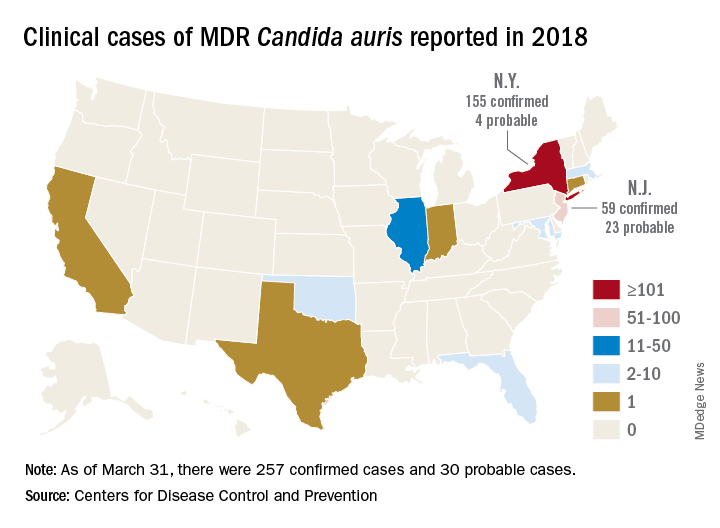
The CDC has recorded 257 confirmed and 30 probable cases of MDR C. auris in the United States as of March 31, 2018. Most of these occurred in New York City and New Jersey; a number of patients had recent stays in hospitals in India, Pakistan, South Africa, the UAE, and Venezuela.
Jacques Meis, MD, of the department of medical microbiology and infectious diseases at Canisius Wilhelmina Hospital, Nijmegen, the Netherlands, set the stage for an extended discussion of C. auris at the meeting.
“This is a multidrug resistant yeast that has emerged in the last decade. Some rare isolates are resistant to all three major antifungal classes. Unlike other Candida species, it seems to persist for prolonged periods in health care environments and to colonize patients’ skin. It behaves rather like resistant bacteria.”
Once established in a health care setting – often an intensive care ward – C. auris poses major infection controls challenges and can be very hard to identify and eradicate, said Dr. Meis.
The identification problem is well known. The 2016 CDC alert noted that “commercially available biochemical-based tests, including API strips and VITEK-2, used in many U.S. laboratories to identify fungi, cannot differentiate C. auris from related species. Because of these challenges, clinical laboratories have misidentified the organism as C. haemulonii and Saccharomyces cerevisiae.”
“It’s often misidentified as other Candida species or as Saccharomyces when we investigate with biochemical methods. C. auris is best identified using Matrix Assisted Laser Desorption/Ionization time of flight mass spectrometry (MALDI-TOF),” said Dr. Meis.
Among the presentations at ECCMID were a report of a U.K. outbreak that affected 70 patients in a neuroscience ICU. It was traced to axillary skin-surface temperature probes, and eradicated only after those probes were removed. More than 90% of the isolates were resistant to fluconazole, voriconazole, and posaconazole; 18% were amphotericin resistant.
A poster described the microbiological characteristics of 50 C. auris isolates taken from 11 hospitals in Korea.
Dr. Pemán described the outbreak in Valencia, which began in April 2016; the report was simultaneously published in the online journal Mycoses (2018 Apr 14. doi: 10.1111/myc.12781).
The index case was a 66-year-old man with hepatocellular carcinoma who underwent a liver resection at Hospital Le Fe in April 2016. During his stay in the surgical ICU (SICU), he developed a fungal infection from an unknown, highly fluconazole-resistant yeast. The pathogen was twice misidentified, first as C. haemulonii and then as S. cerevisiae.
Three weeks later, the patient in the adjacent bed developed a similar infection. Sequencing of the internal transcribed spacer confirmed both as a Candida isolate – an organism previously unknown in Spain.
The SICU setup was apparently very conducive to the C. auris life cycle, Dr. Pemán said. It’s a relatively open ward divided into three rooms with 12 beds in each. There are no isolation beds, and dozens of workers have access to the ward every day, including clinical and cleaning staff.
After identifying the second isolate, Dr. Pemán said, infection control staff went into action. They instituted contact precautions in the SICU, and took regular cultures from newly admitted patients and cultures of every SICU patient every 7 days.
“We also started an intense search for more cases throughout the hospital and in 101 SICU workers. Of 305 samples from hands and ears, we found nothing.” They reviewed all the prior fluconazole-resistant Candida isolates; C. auris was not present in the hospital before the index case.
Three weeks after case 2, six new SICU patients tested positive for C. auris (two blood cultures, one vascular line, one respiratory specimen, two rectal swabs, and one urinary tract sample).
“We reinforced contact precautions in colonized and infected patients and started a twice-daily environmental cleaning practice with quaternary ammonium around them,” said Dr. Pemán. They instituted a proactive hospital-wide hand hygiene campaign and spread the word about the outbreak.
By July, there were 11 new colonized patients, 3 of whom developed candidemia. These patients were grouped in the same SICU ward and underwent daily skin treatments with 4% aqueous chlorhexidine wipes.
The environmental inspection found C. auris on beds, tables, walls, and the floor all around infected patients. The pathogen also was living on IV pumps, computer keyboards, and bedside tables. Blood pressure cuffs were a favorite haunt: 19 of 36 samples in the adjacent ICU were positive. These data were separately reported at ECCMID.
Despite all of these efforts at eradication, infections continued to rise. By November, there were 24 newly colonized patients and nine new candidemia episodes in SICU and regular ICU patients. In December, a new infection control bundle began: A surveillance nurse in the C. auris SICU ward was in charge of compliance; any patient with any yeast growth in culture was isolated, and staff used 2% alcohol chlorhexidine wipes before and after IV catheter handling. Staff also washed down all surfaces three times daily with a disinfectant.
Patients could leave isolation after three consecutive C. auris–negative cultures. After discharge, an ultraviolet light decontamination procedure disinfected each patient room.
The pathogen was almost unbelievably resilient, Dr. Pemán noted in the Mycoses article. “In some cases, C. auris was recovered from walls after cleaning with cationic surface–active products ... it was not known until very recently that these products, as well as quaternary ammonium disinfectants, cannot effectively remove C. auris from surfaces.”
As a result of the previous measures, the outbreak slowed down during December 2016, with two new candidemia cases, but by February, the outbreak resumed with 50 new cases and 18 candidemias detected. Cases continued to emerge throughout 2017.
By September 2017, 250 patients had been colonized; 116 of these were included in the Mycoses report. There were 30 episodes of candidemia (26%); of these, 17 died by 30 days (41.4%). Spondylodiscitis and endocarditis each developed in two patients and one developed ventriculitis.
A separate poster by Dr. Pemán and his colleagues gave more details:
• A 52-year-old woman with C. auris–induced endocarditis died after 4 weeks of treatment with AMB+ECN and flucytosine. She had undergone a prosthetic heart valve placement for Ebstein’s anomaly.
• A 71-year-old man with hydrocephalus developed a C. auris–induced infection of his ventriculoperitoneal shunt; he also had undergone cardiovascular surgery and had an ischemic cardiomyopathy. He died despite shunt removal and 8 weeks of AMB+ECN.
• A 71-year-old man who underwent cardiovascular surgery and received a prosthetic heart valve developed endocarditis. He is alive and at last report, on week 26 of AMB+ECN, flucytosine, and isavuconazole.
• A 68-year-old man who underwent abdominal surgery for hepatocellular carcinoma developed spondylodiscitis and is alive after 24 weeks of AMB+ECN.
• A 48-year old female multiple trauma patient developed spondylodiscitis and is alive after 48 weeks of treatment with AMB+ECN.
A multivariate analysis determined that antibacterial treatment increased the risk of candidemia by almost 30 times (odds ratio, 29.59). The next highest risk was neutropenia (OR, 20.7) and then simply being a hospital and SICU patient. Dr. Pemán’s poster said, “In the 16 months before the index case, La Fe recorded 89 candidemias, none caused by C. auris. In the 16 months afterward, there were 154 candidemias, largely C. auris. Before April 2016, C. parapsilosis accounted for the largest portion of candidemias (46%) followed by C. albicans. After the index case, C. auris accounted for 42%, followed by C. parapsilosis (21%) and C. albicans (18%).”
Because of its fluconazole resistance, patients with C. auris received a combined antifungal treatment of liposomal amphotericin B 3 mg/kg per day for 5 days, and a standard dose of echinocandin for 3 weeks. Many C. auris strains are echinocandin resistant, Dr. Pemán noted. This particular strain was clonal, different from any other previously reported, he said.
“Our results confirm those previously reported by other authors, that C. auris is grouped in different independent clusters according to its geographical origin. Although all Spanish isolates were genotypically distinct from Indian, Omani, U.K., and Venezuelan isolates, there seems to be some connection with South African isolates.”
Hospital Le Fe continues to struggle with C. auris. As of March, 335 patients have tested positive for the pathogen, and 80 have developed candidemias.
“We feel we may be approaching the end of this episode, but it’s really not possible to be sure,” he said.
Dr. Pemán had no relevant financial disclosures.
SOURCE: ECCMID 2018 Peman et al. S0067.
MADRID – The anticipated global emergence of multidrug resistant Candida auris is now an established fact, but a case study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress demonstrates just how devastating an outbreak can be to a medical facility and its surgical ICU patients.
The dangerous invasive infection is spreading through Asia, Europe, and the Americas, causing potentially fatal candidemias and proving devilishly difficult to eradicate in health care facilities once it becomes established.
Several multidrug resistant (MDR) C. auris outbreaks were reported at the ECCMID meeting. Most troubling: a continuing outbreak in a hospital in Valencia, Spain, in which 17 patients have died – a 41% fatality rate among those who developed a fulminant C. auris candidemia, Javier Pemán, MD, said at the meeting. The strain appeared to be a clonal population not previously identified in published reports.
“C. auris is hard to remove from the hospital environment,” once it becomes established, said Dr. Pemán of La Fe University and Polytechnic Hospital, Valencia. “When an outbreak lasts for months, as ours has, it is difficult, but necessary, to maintain control measures, identify it early in the lab, and isolate and treat patients early with combination therapy.”
He and his team have relied primarily on a combination of amphotericin B and echinocandin (AMB+ECN), although, he added, the optimal dosing and treatment time aren’t known, and many C. auris isolates are echinocandin resistant.
MDR C. auris first appearedin Tokyo in 2009. It then spread to South Korea around 2011, and then appeared across Asia and Western Europe. Its first appearance in Spain was the 2016 Le Fe outbreak.
According to the Centers for Disease Control and Prevention, single cases have appeared in Austria, Belgium, Malaysia, Norway, and the United Arab Emirates. Canada, Colombia, France, Germany, India, Israel, Japan, Kenya, Kuwait, Oman, Pakistan, Panama, South Korea, South Africa, Spain, the United Arab Emirates, the United Kingdom, and Venezuela have experienced multiple outbreaks.
The CDC has recorded 257 confirmed and 30 probable cases of MDR C. auris in the United States as of March 31, 2018. Most of these occurred in New York City and New Jersey; a number of patients had recent stays in hospitals in India, Pakistan, South Africa, the UAE, and Venezuela.

Jacques Meis, MD, of the department of medical microbiology and infectious diseases at Canisius Wilhelmina Hospital, Nijmegen, the Netherlands, set the stage for an extended discussion of C. auris at the meeting.
“This is a multidrug resistant yeast that has emerged in the last decade. Some rare isolates are resistant to all three major antifungal classes. Unlike other Candida species, it seems to persist for prolonged periods in health care environments and to colonize patients’ skin. It behaves rather like resistant bacteria.”
Once established in a health care setting – often an intensive care ward – C. auris poses major infection controls challenges and can be very hard to identify and eradicate, said Dr. Meis.
The identification problem is well known. The 2016 CDC alert noted that “commercially available biochemical-based tests, including API strips and VITEK-2, used in many U.S. laboratories to identify fungi, cannot differentiate C. auris from related species. Because of these challenges, clinical laboratories have misidentified the organism as C. haemulonii and Saccharomyces cerevisiae.”
“It’s often misidentified as other Candida species or as Saccharomyces when we investigate with biochemical methods. C. auris is best identified using Matrix Assisted Laser Desorption/Ionization time of flight mass spectrometry (MALDI-TOF),” said Dr. Meis.
Among the presentations at ECCMID were a report of a U.K. outbreak that affected 70 patients in a neuroscience ICU. It was traced to axillary skin-surface temperature probes, and eradicated only after those probes were removed. More than 90% of the isolates were resistant to fluconazole, voriconazole, and posaconazole; 18% were amphotericin resistant.
A poster described the microbiological characteristics of 50 C. auris isolates taken from 11 hospitals in Korea.
Dr. Pemán described the outbreak in Valencia, which began in April 2016; the report was simultaneously published in the online journal Mycoses (2018 Apr 14. doi: 10.1111/myc.12781).
The index case was a 66-year-old man with hepatocellular carcinoma who underwent a liver resection at Hospital Le Fe in April 2016. During his stay in the surgical ICU (SICU), he developed a fungal infection from an unknown, highly fluconazole-resistant yeast. The pathogen was twice misidentified, first as C. haemulonii and then as S. cerevisiae.
Three weeks later, the patient in the adjacent bed developed a similar infection. Sequencing of the internal transcribed spacer confirmed both as a Candida isolate – an organism previously unknown in Spain.
The SICU setup was apparently very conducive to the C. auris life cycle, Dr. Pemán said. It’s a relatively open ward divided into three rooms with 12 beds in each. There are no isolation beds, and dozens of workers have access to the ward every day, including clinical and cleaning staff.
After identifying the second isolate, Dr. Pemán said, infection control staff went into action. They instituted contact precautions in the SICU, and took regular cultures from newly admitted patients and cultures of every SICU patient every 7 days.
“We also started an intense search for more cases throughout the hospital and in 101 SICU workers. Of 305 samples from hands and ears, we found nothing.” They reviewed all the prior fluconazole-resistant Candida isolates; C. auris was not present in the hospital before the index case.
Three weeks after case 2, six new SICU patients tested positive for C. auris (two blood cultures, one vascular line, one respiratory specimen, two rectal swabs, and one urinary tract sample).
“We reinforced contact precautions in colonized and infected patients and started a twice-daily environmental cleaning practice with quaternary ammonium around them,” said Dr. Pemán. They instituted a proactive hospital-wide hand hygiene campaign and spread the word about the outbreak.
By July, there were 11 new colonized patients, 3 of whom developed candidemia. These patients were grouped in the same SICU ward and underwent daily skin treatments with 4% aqueous chlorhexidine wipes.
The environmental inspection found C. auris on beds, tables, walls, and the floor all around infected patients. The pathogen also was living on IV pumps, computer keyboards, and bedside tables. Blood pressure cuffs were a favorite haunt: 19 of 36 samples in the adjacent ICU were positive. These data were separately reported at ECCMID.
Despite all of these efforts at eradication, infections continued to rise. By November, there were 24 newly colonized patients and nine new candidemia episodes in SICU and regular ICU patients. In December, a new infection control bundle began: A surveillance nurse in the C. auris SICU ward was in charge of compliance; any patient with any yeast growth in culture was isolated, and staff used 2% alcohol chlorhexidine wipes before and after IV catheter handling. Staff also washed down all surfaces three times daily with a disinfectant.
Patients could leave isolation after three consecutive C. auris–negative cultures. After discharge, an ultraviolet light decontamination procedure disinfected each patient room.
The pathogen was almost unbelievably resilient, Dr. Pemán noted in the Mycoses article. “In some cases, C. auris was recovered from walls after cleaning with cationic surface–active products ... it was not known until very recently that these products, as well as quaternary ammonium disinfectants, cannot effectively remove C. auris from surfaces.”
As a result of the previous measures, the outbreak slowed down during December 2016, with two new candidemia cases, but by February, the outbreak resumed with 50 new cases and 18 candidemias detected. Cases continued to emerge throughout 2017.
By September 2017, 250 patients had been colonized; 116 of these were included in the Mycoses report. There were 30 episodes of candidemia (26%); of these, 17 died by 30 days (41.4%). Spondylodiscitis and endocarditis each developed in two patients and one developed ventriculitis.
A separate poster by Dr. Pemán and his colleagues gave more details:
• A 52-year-old woman with C. auris–induced endocarditis died after 4 weeks of treatment with AMB+ECN and flucytosine. She had undergone a prosthetic heart valve placement for Ebstein’s anomaly.
• A 71-year-old man with hydrocephalus developed a C. auris–induced infection of his ventriculoperitoneal shunt; he also had undergone cardiovascular surgery and had an ischemic cardiomyopathy. He died despite shunt removal and 8 weeks of AMB+ECN.
• A 71-year-old man who underwent cardiovascular surgery and received a prosthetic heart valve developed endocarditis. He is alive and at last report, on week 26 of AMB+ECN, flucytosine, and isavuconazole.
• A 68-year-old man who underwent abdominal surgery for hepatocellular carcinoma developed spondylodiscitis and is alive after 24 weeks of AMB+ECN.
• A 48-year old female multiple trauma patient developed spondylodiscitis and is alive after 48 weeks of treatment with AMB+ECN.
A multivariate analysis determined that antibacterial treatment increased the risk of candidemia by almost 30 times (odds ratio, 29.59). The next highest risk was neutropenia (OR, 20.7) and then simply being a hospital and SICU patient. Dr. Pemán’s poster said, “In the 16 months before the index case, La Fe recorded 89 candidemias, none caused by C. auris. In the 16 months afterward, there were 154 candidemias, largely C. auris. Before April 2016, C. parapsilosis accounted for the largest portion of candidemias (46%) followed by C. albicans. After the index case, C. auris accounted for 42%, followed by C. parapsilosis (21%) and C. albicans (18%).”
Because of its fluconazole resistance, patients with C. auris received a combined antifungal treatment of liposomal amphotericin B 3 mg/kg per day for 5 days, and a standard dose of echinocandin for 3 weeks. Many C. auris strains are echinocandin resistant, Dr. Pemán noted. This particular strain was clonal, different from any other previously reported, he said.
“Our results confirm those previously reported by other authors, that C. auris is grouped in different independent clusters according to its geographical origin. Although all Spanish isolates were genotypically distinct from Indian, Omani, U.K., and Venezuelan isolates, there seems to be some connection with South African isolates.”
Hospital Le Fe continues to struggle with C. auris. As of March, 335 patients have tested positive for the pathogen, and 80 have developed candidemias.
“We feel we may be approaching the end of this episode, but it’s really not possible to be sure,” he said.
Dr. Pemán had no relevant financial disclosures.
SOURCE: ECCMID 2018 Peman et al. S0067.
MADRID – The anticipated global emergence of multidrug resistant Candida auris is now an established fact, but a case study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress demonstrates just how devastating an outbreak can be to a medical facility and its surgical ICU patients.
The dangerous invasive infection is spreading through Asia, Europe, and the Americas, causing potentially fatal candidemias and proving devilishly difficult to eradicate in health care facilities once it becomes established.
Several multidrug resistant (MDR) C. auris outbreaks were reported at the ECCMID meeting. Most troubling: a continuing outbreak in a hospital in Valencia, Spain, in which 17 patients have died – a 41% fatality rate among those who developed a fulminant C. auris candidemia, Javier Pemán, MD, said at the meeting. The strain appeared to be a clonal population not previously identified in published reports.
“C. auris is hard to remove from the hospital environment,” once it becomes established, said Dr. Pemán of La Fe University and Polytechnic Hospital, Valencia. “When an outbreak lasts for months, as ours has, it is difficult, but necessary, to maintain control measures, identify it early in the lab, and isolate and treat patients early with combination therapy.”
He and his team have relied primarily on a combination of amphotericin B and echinocandin (AMB+ECN), although, he added, the optimal dosing and treatment time aren’t known, and many C. auris isolates are echinocandin resistant.
MDR C. auris first appearedin Tokyo in 2009. It then spread to South Korea around 2011, and then appeared across Asia and Western Europe. Its first appearance in Spain was the 2016 Le Fe outbreak.
According to the Centers for Disease Control and Prevention, single cases have appeared in Austria, Belgium, Malaysia, Norway, and the United Arab Emirates. Canada, Colombia, France, Germany, India, Israel, Japan, Kenya, Kuwait, Oman, Pakistan, Panama, South Korea, South Africa, Spain, the United Arab Emirates, the United Kingdom, and Venezuela have experienced multiple outbreaks.
The CDC has recorded 257 confirmed and 30 probable cases of MDR C. auris in the United States as of March 31, 2018. Most of these occurred in New York City and New Jersey; a number of patients had recent stays in hospitals in India, Pakistan, South Africa, the UAE, and Venezuela.

Jacques Meis, MD, of the department of medical microbiology and infectious diseases at Canisius Wilhelmina Hospital, Nijmegen, the Netherlands, set the stage for an extended discussion of C. auris at the meeting.
“This is a multidrug resistant yeast that has emerged in the last decade. Some rare isolates are resistant to all three major antifungal classes. Unlike other Candida species, it seems to persist for prolonged periods in health care environments and to colonize patients’ skin. It behaves rather like resistant bacteria.”
Once established in a health care setting – often an intensive care ward – C. auris poses major infection controls challenges and can be very hard to identify and eradicate, said Dr. Meis.
The identification problem is well known. The 2016 CDC alert noted that “commercially available biochemical-based tests, including API strips and VITEK-2, used in many U.S. laboratories to identify fungi, cannot differentiate C. auris from related species. Because of these challenges, clinical laboratories have misidentified the organism as C. haemulonii and Saccharomyces cerevisiae.”
“It’s often misidentified as other Candida species or as Saccharomyces when we investigate with biochemical methods. C. auris is best identified using Matrix Assisted Laser Desorption/Ionization time of flight mass spectrometry (MALDI-TOF),” said Dr. Meis.
Among the presentations at ECCMID were a report of a U.K. outbreak that affected 70 patients in a neuroscience ICU. It was traced to axillary skin-surface temperature probes, and eradicated only after those probes were removed. More than 90% of the isolates were resistant to fluconazole, voriconazole, and posaconazole; 18% were amphotericin resistant.
A poster described the microbiological characteristics of 50 C. auris isolates taken from 11 hospitals in Korea.
Dr. Pemán described the outbreak in Valencia, which began in April 2016; the report was simultaneously published in the online journal Mycoses (2018 Apr 14. doi: 10.1111/myc.12781).
The index case was a 66-year-old man with hepatocellular carcinoma who underwent a liver resection at Hospital Le Fe in April 2016. During his stay in the surgical ICU (SICU), he developed a fungal infection from an unknown, highly fluconazole-resistant yeast. The pathogen was twice misidentified, first as C. haemulonii and then as S. cerevisiae.
Three weeks later, the patient in the adjacent bed developed a similar infection. Sequencing of the internal transcribed spacer confirmed both as a Candida isolate – an organism previously unknown in Spain.
The SICU setup was apparently very conducive to the C. auris life cycle, Dr. Pemán said. It’s a relatively open ward divided into three rooms with 12 beds in each. There are no isolation beds, and dozens of workers have access to the ward every day, including clinical and cleaning staff.
After identifying the second isolate, Dr. Pemán said, infection control staff went into action. They instituted contact precautions in the SICU, and took regular cultures from newly admitted patients and cultures of every SICU patient every 7 days.
“We also started an intense search for more cases throughout the hospital and in 101 SICU workers. Of 305 samples from hands and ears, we found nothing.” They reviewed all the prior fluconazole-resistant Candida isolates; C. auris was not present in the hospital before the index case.
Three weeks after case 2, six new SICU patients tested positive for C. auris (two blood cultures, one vascular line, one respiratory specimen, two rectal swabs, and one urinary tract sample).
“We reinforced contact precautions in colonized and infected patients and started a twice-daily environmental cleaning practice with quaternary ammonium around them,” said Dr. Pemán. They instituted a proactive hospital-wide hand hygiene campaign and spread the word about the outbreak.
By July, there were 11 new colonized patients, 3 of whom developed candidemia. These patients were grouped in the same SICU ward and underwent daily skin treatments with 4% aqueous chlorhexidine wipes.
The environmental inspection found C. auris on beds, tables, walls, and the floor all around infected patients. The pathogen also was living on IV pumps, computer keyboards, and bedside tables. Blood pressure cuffs were a favorite haunt: 19 of 36 samples in the adjacent ICU were positive. These data were separately reported at ECCMID.
Despite all of these efforts at eradication, infections continued to rise. By November, there were 24 newly colonized patients and nine new candidemia episodes in SICU and regular ICU patients. In December, a new infection control bundle began: A surveillance nurse in the C. auris SICU ward was in charge of compliance; any patient with any yeast growth in culture was isolated, and staff used 2% alcohol chlorhexidine wipes before and after IV catheter handling. Staff also washed down all surfaces three times daily with a disinfectant.
Patients could leave isolation after three consecutive C. auris–negative cultures. After discharge, an ultraviolet light decontamination procedure disinfected each patient room.
The pathogen was almost unbelievably resilient, Dr. Pemán noted in the Mycoses article. “In some cases, C. auris was recovered from walls after cleaning with cationic surface–active products ... it was not known until very recently that these products, as well as quaternary ammonium disinfectants, cannot effectively remove C. auris from surfaces.”
As a result of the previous measures, the outbreak slowed down during December 2016, with two new candidemia cases, but by February, the outbreak resumed with 50 new cases and 18 candidemias detected. Cases continued to emerge throughout 2017.
By September 2017, 250 patients had been colonized; 116 of these were included in the Mycoses report. There were 30 episodes of candidemia (26%); of these, 17 died by 30 days (41.4%). Spondylodiscitis and endocarditis each developed in two patients and one developed ventriculitis.
A separate poster by Dr. Pemán and his colleagues gave more details:
• A 52-year-old woman with C. auris–induced endocarditis died after 4 weeks of treatment with AMB+ECN and flucytosine. She had undergone a prosthetic heart valve placement for Ebstein’s anomaly.
• A 71-year-old man with hydrocephalus developed a C. auris–induced infection of his ventriculoperitoneal shunt; he also had undergone cardiovascular surgery and had an ischemic cardiomyopathy. He died despite shunt removal and 8 weeks of AMB+ECN.
• A 71-year-old man who underwent cardiovascular surgery and received a prosthetic heart valve developed endocarditis. He is alive and at last report, on week 26 of AMB+ECN, flucytosine, and isavuconazole.
• A 68-year-old man who underwent abdominal surgery for hepatocellular carcinoma developed spondylodiscitis and is alive after 24 weeks of AMB+ECN.
• A 48-year old female multiple trauma patient developed spondylodiscitis and is alive after 48 weeks of treatment with AMB+ECN.
A multivariate analysis determined that antibacterial treatment increased the risk of candidemia by almost 30 times (odds ratio, 29.59). The next highest risk was neutropenia (OR, 20.7) and then simply being a hospital and SICU patient. Dr. Pemán’s poster said, “In the 16 months before the index case, La Fe recorded 89 candidemias, none caused by C. auris. In the 16 months afterward, there were 154 candidemias, largely C. auris. Before April 2016, C. parapsilosis accounted for the largest portion of candidemias (46%) followed by C. albicans. After the index case, C. auris accounted for 42%, followed by C. parapsilosis (21%) and C. albicans (18%).”
Because of its fluconazole resistance, patients with C. auris received a combined antifungal treatment of liposomal amphotericin B 3 mg/kg per day for 5 days, and a standard dose of echinocandin for 3 weeks. Many C. auris strains are echinocandin resistant, Dr. Pemán noted. This particular strain was clonal, different from any other previously reported, he said.
“Our results confirm those previously reported by other authors, that C. auris is grouped in different independent clusters according to its geographical origin. Although all Spanish isolates were genotypically distinct from Indian, Omani, U.K., and Venezuelan isolates, there seems to be some connection with South African isolates.”
Hospital Le Fe continues to struggle with C. auris. As of March, 335 patients have tested positive for the pathogen, and 80 have developed candidemias.
“We feel we may be approaching the end of this episode, but it’s really not possible to be sure,” he said.
Dr. Pemán had no relevant financial disclosures.
SOURCE: ECCMID 2018 Peman et al. S0067.
REPORTING FROM ECCMID 2018
SAMHSA’s new general embarks on a new mission
Elinore F. McCance-Katz, MD, PhD, is by turns passionate and impatient, empathetic and brusquely no-nonsense. She is a person who knows what she wants to do and knows how to do it.
Accomplishing that on the idealogic battlefield of a presidential appointment is the focus of her considerable energy.
Dr. McCance-Katz carries the newly minted banner of assistant secretary for mental health and substance use. Her troops comprise more than 100 related federal agencies. Her charge is at once simple and mind-numbingly complex: Overhaul the nation’s mental health care system. for their illnesses before they can begin to heal.
It’s the precise opposite of the Substance Abuse and Mental Health Services Administration’s prior targeting, which focused heavily on providing healthy social support for patients with mental health and substance abuse disorders. To Dr. McCance-Katz’s way of thinking, dollars spent on such are largely wasted, because terribly ill people simply can’t function in healthy ways until they begin to get better. Why, she reasoned, should the government waste money on peer-support services while ignoring – or even openly rejecting – evidence-based psychiatric treatment paradigms that are proven to help heal?
It’s this philosophical dichotomy – combined with what she perceived as a palpable dismissal of psychiatric science – that drove her away from her first SAMHSA appointment as the agency’s first chief medical officer. She aired her frustration in an editorial, published 2 years ago in Psychiatric Times.
“There is a perceptible hostility toward psychiatric medicine: a resistance to addressing the treatment needs of those with serious mental illness and a questioning by some at SAMHSA as to whether mental disorders even exist – for example, is psychosis just a ‘different way of thinking for some experiencing stress?’ … Nowhere in SAMHSA’s strategic initiatives is psychiatric treatment of mental illness a priority. The occasional vague reference to treatment is no substitute for the urgent need for programs that address these issues.”
In the same letter, she outlined a new battle plan, one that includes funding for better outpatient treatments, more psychiatric hospital beds, clinician education and support, and money to beef up the dwindling supply of psychiatrists, advanced-practice psychiatric nurses, and psychologists.
In an exclusive video interview, Dr. McCance-Katz sat down with MDedge Psychiatry to discuss the path that brought her to this station and the long road ahead.
Elinore F. McCance-Katz, MD, PhD, is by turns passionate and impatient, empathetic and brusquely no-nonsense. She is a person who knows what she wants to do and knows how to do it.
Accomplishing that on the idealogic battlefield of a presidential appointment is the focus of her considerable energy.
Dr. McCance-Katz carries the newly minted banner of assistant secretary for mental health and substance use. Her troops comprise more than 100 related federal agencies. Her charge is at once simple and mind-numbingly complex: Overhaul the nation’s mental health care system. for their illnesses before they can begin to heal.
It’s the precise opposite of the Substance Abuse and Mental Health Services Administration’s prior targeting, which focused heavily on providing healthy social support for patients with mental health and substance abuse disorders. To Dr. McCance-Katz’s way of thinking, dollars spent on such are largely wasted, because terribly ill people simply can’t function in healthy ways until they begin to get better. Why, she reasoned, should the government waste money on peer-support services while ignoring – or even openly rejecting – evidence-based psychiatric treatment paradigms that are proven to help heal?
It’s this philosophical dichotomy – combined with what she perceived as a palpable dismissal of psychiatric science – that drove her away from her first SAMHSA appointment as the agency’s first chief medical officer. She aired her frustration in an editorial, published 2 years ago in Psychiatric Times.
“There is a perceptible hostility toward psychiatric medicine: a resistance to addressing the treatment needs of those with serious mental illness and a questioning by some at SAMHSA as to whether mental disorders even exist – for example, is psychosis just a ‘different way of thinking for some experiencing stress?’ … Nowhere in SAMHSA’s strategic initiatives is psychiatric treatment of mental illness a priority. The occasional vague reference to treatment is no substitute for the urgent need for programs that address these issues.”
In the same letter, she outlined a new battle plan, one that includes funding for better outpatient treatments, more psychiatric hospital beds, clinician education and support, and money to beef up the dwindling supply of psychiatrists, advanced-practice psychiatric nurses, and psychologists.
In an exclusive video interview, Dr. McCance-Katz sat down with MDedge Psychiatry to discuss the path that brought her to this station and the long road ahead.
Elinore F. McCance-Katz, MD, PhD, is by turns passionate and impatient, empathetic and brusquely no-nonsense. She is a person who knows what she wants to do and knows how to do it.
Accomplishing that on the idealogic battlefield of a presidential appointment is the focus of her considerable energy.
Dr. McCance-Katz carries the newly minted banner of assistant secretary for mental health and substance use. Her troops comprise more than 100 related federal agencies. Her charge is at once simple and mind-numbingly complex: Overhaul the nation’s mental health care system. for their illnesses before they can begin to heal.
It’s the precise opposite of the Substance Abuse and Mental Health Services Administration’s prior targeting, which focused heavily on providing healthy social support for patients with mental health and substance abuse disorders. To Dr. McCance-Katz’s way of thinking, dollars spent on such are largely wasted, because terribly ill people simply can’t function in healthy ways until they begin to get better. Why, she reasoned, should the government waste money on peer-support services while ignoring – or even openly rejecting – evidence-based psychiatric treatment paradigms that are proven to help heal?
It’s this philosophical dichotomy – combined with what she perceived as a palpable dismissal of psychiatric science – that drove her away from her first SAMHSA appointment as the agency’s first chief medical officer. She aired her frustration in an editorial, published 2 years ago in Psychiatric Times.
“There is a perceptible hostility toward psychiatric medicine: a resistance to addressing the treatment needs of those with serious mental illness and a questioning by some at SAMHSA as to whether mental disorders even exist – for example, is psychosis just a ‘different way of thinking for some experiencing stress?’ … Nowhere in SAMHSA’s strategic initiatives is psychiatric treatment of mental illness a priority. The occasional vague reference to treatment is no substitute for the urgent need for programs that address these issues.”
In the same letter, she outlined a new battle plan, one that includes funding for better outpatient treatments, more psychiatric hospital beds, clinician education and support, and money to beef up the dwindling supply of psychiatrists, advanced-practice psychiatric nurses, and psychologists.
In an exclusive video interview, Dr. McCance-Katz sat down with MDedge Psychiatry to discuss the path that brought her to this station and the long road ahead.
For Gram-negative bacteremias, 7 days of antibiotics is enough
MADRID – Seven days of antibiotic therapy was just as effective as 14 days for patients with Gram-negative bacteremias.
The shorter course was associated with similar cure rates and a faster return to normal activities, Dafna Yahav, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“In patients hospitalized with Gram-negative bacteremia and sepsis, a course of 7 antibiotic days was not inferior to 14 days, and resulted in a more rapid return to baseline activity, “ said Dr. Yahav of the Rabin Medical Center, Petah Tikva, Israel. “This could lead to a change in accepted management algorithms and shortened antibiotic therapy. Potentially, though we did not show this in our trial, it may lead to reduced cost, reduced development of resistance, and fewer adverse events.”
During the past few years, a new dogma has emerged in antibiotic treatment paradigms, she said: Shorter is better. Brad Spellberg, MD, described this concept in his 2016 editorial in JAMA Internal Medicine, “The new antibiotic mantra” (Sep 1;176[9]:1254-5).
In it, Dr. Spellberg, of the University of Southern California, Los Angeles, addressed the long-held view that a full 10- or 14-day course of antibiotics was necessary to decrease the risk of creating a resistant strain, even if clinical symptoms were long resolved.
However, he noted, there is little evidence supporting the idea that longer courses suppress the rise of resistance – and, in fact, some data support the opposite.
“To the contrary, specifically for pneumonia, studies have shown that longer courses of therapy result in more emergence of antibiotic resistance, which is consistent with everything we know about natural selection, the driver of antibiotic resistance,” he noted. “In only a few types of infections does resistance emerge at the site of infection; rather, resistance typically emerges off target, among colonizing flora away from the site of infection. Thus, all that is achieved by treating an infection with antibiotics for longer than the patient has symptoms is increased selective pressure driving antibiotic resistance among our colonizing microbial flora.”
The European Union and Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America have all recently updated their antibiotic stewardship guidelines to include a strong recommendation for the shortest effective duration of antimicrobial therapy.
However, most of the supporting data were drawn from randomized, controlled studies of patients with lung, skin, and kidney infections. Short-course treatments have not been adequately studied in bacteremia patients, Dr. Yahav said.
The aim of her study, which was investigator initiated and received no external funding, was to demonstrate the noninferiority of 7 days of antibiotic therapy, compared with 14 days, in patients with bacteremia arising from Gram-negative infections.
The randomized, open-label study comprised 604 patients in three hospitals: two in Israel and one in Italy. Patients were eligible if they had an aerobic Gram-negative bacteremia of any infection source that was either community- or hospital acquired. The medication choice was left up to the treating physician. Patients were assessed at discharge, and at days 30 and 90.
The primary outcome was a composite 90-day endpoint of all-cause mortality, clinical failure (relapse, new local complications, or distant complications), and readmission or hospital stay longer than 14 days. There were a number of secondary outcomes, including new infection, emergence of antibiotic resistance, total hospital and total antibiotic days, time to return to baseline activity, and adverse events.
The cohort was a mean of 71 years old. About 60% were functionally independent, and the mean Charlson comorbidity score was 2. Most of the infections (90%) were nosocomial. The urinary tract was the largest source of infection (69%). Other sources were abdominal, respiratory, central venous catheter, and skin or soft tissue.
Escherichia coli was the most common infective organism (62%), followed by Klebsiella species and Enterobacteriaceae. A small number of patients had Acinetobacter and Pseudomonas infections.
In the intent-to-treat analysis, the primary composite outcome of all-cause mortality or extended hospital stay occurred in 46% of the 7-day group and 50% of the 14-day group – not significantly different. The results were nearly identical in the per-protocol analysis (46% vs. 49.6%).
Likewise, none of the secondary outcomes posted a significant difference in favor of one treatment arm, including relapse (2.9% vs. 2.7%) and resistance development (10.8% vs. 9.7%).
Dr. Yahav pointed out that total antibiotic-use days were significantly less in the 7-day group, (5 days) than in the 14-day group (10 days). Patients in the short-duration group returned to their normal activities a day earlier than those in the longer-term group (2 days vs. 3 days), a difference that was statistically significant.
The total hospital stay from randomization to day 90 was only half a day shorter in the short-term group (mean, 3 days vs. 3.5 days). That was not a significant finding.
There were some differences in adverse events, although none was statistically significant. The short-duration arm had slightly more cases of kidney injury (0.5%), fewer cases of liver function abnormalities (–1.5%), and half as many rashes (two vs. four). There were two cases of Clostridium difficile in the short-use arm and one in the long-use arm, also not a significant difference.
A subgroup analysis looked at outcomes among the different sources of infection (urinary tract vs. other), whether empirical antibiotics were used, and whether the induced resistance was multdrug or non–multidrug. All of those differences hovered close to the null, but generally favored short antibiotic treatment, Dr. Yahav noted.
“I would conclude from these data that is generally safe to stop antibiotics after 7 days of covering antibiotics for Gram-negative bacteremia patients, if they are hemodynamically stable and nonneutropenic at 7 days, and have no uncontrolled source of infection,” she concluded.
The investigator-initiated study had no outside funding.
SOURCE: Yahav D et al. ECCMID 2018. Oral abstract O1120.
MADRID – Seven days of antibiotic therapy was just as effective as 14 days for patients with Gram-negative bacteremias.
The shorter course was associated with similar cure rates and a faster return to normal activities, Dafna Yahav, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“In patients hospitalized with Gram-negative bacteremia and sepsis, a course of 7 antibiotic days was not inferior to 14 days, and resulted in a more rapid return to baseline activity, “ said Dr. Yahav of the Rabin Medical Center, Petah Tikva, Israel. “This could lead to a change in accepted management algorithms and shortened antibiotic therapy. Potentially, though we did not show this in our trial, it may lead to reduced cost, reduced development of resistance, and fewer adverse events.”
During the past few years, a new dogma has emerged in antibiotic treatment paradigms, she said: Shorter is better. Brad Spellberg, MD, described this concept in his 2016 editorial in JAMA Internal Medicine, “The new antibiotic mantra” (Sep 1;176[9]:1254-5).
In it, Dr. Spellberg, of the University of Southern California, Los Angeles, addressed the long-held view that a full 10- or 14-day course of antibiotics was necessary to decrease the risk of creating a resistant strain, even if clinical symptoms were long resolved.
However, he noted, there is little evidence supporting the idea that longer courses suppress the rise of resistance – and, in fact, some data support the opposite.
“To the contrary, specifically for pneumonia, studies have shown that longer courses of therapy result in more emergence of antibiotic resistance, which is consistent with everything we know about natural selection, the driver of antibiotic resistance,” he noted. “In only a few types of infections does resistance emerge at the site of infection; rather, resistance typically emerges off target, among colonizing flora away from the site of infection. Thus, all that is achieved by treating an infection with antibiotics for longer than the patient has symptoms is increased selective pressure driving antibiotic resistance among our colonizing microbial flora.”
The European Union and Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America have all recently updated their antibiotic stewardship guidelines to include a strong recommendation for the shortest effective duration of antimicrobial therapy.
However, most of the supporting data were drawn from randomized, controlled studies of patients with lung, skin, and kidney infections. Short-course treatments have not been adequately studied in bacteremia patients, Dr. Yahav said.
The aim of her study, which was investigator initiated and received no external funding, was to demonstrate the noninferiority of 7 days of antibiotic therapy, compared with 14 days, in patients with bacteremia arising from Gram-negative infections.
The randomized, open-label study comprised 604 patients in three hospitals: two in Israel and one in Italy. Patients were eligible if they had an aerobic Gram-negative bacteremia of any infection source that was either community- or hospital acquired. The medication choice was left up to the treating physician. Patients were assessed at discharge, and at days 30 and 90.
The primary outcome was a composite 90-day endpoint of all-cause mortality, clinical failure (relapse, new local complications, or distant complications), and readmission or hospital stay longer than 14 days. There were a number of secondary outcomes, including new infection, emergence of antibiotic resistance, total hospital and total antibiotic days, time to return to baseline activity, and adverse events.
The cohort was a mean of 71 years old. About 60% were functionally independent, and the mean Charlson comorbidity score was 2. Most of the infections (90%) were nosocomial. The urinary tract was the largest source of infection (69%). Other sources were abdominal, respiratory, central venous catheter, and skin or soft tissue.
Escherichia coli was the most common infective organism (62%), followed by Klebsiella species and Enterobacteriaceae. A small number of patients had Acinetobacter and Pseudomonas infections.
In the intent-to-treat analysis, the primary composite outcome of all-cause mortality or extended hospital stay occurred in 46% of the 7-day group and 50% of the 14-day group – not significantly different. The results were nearly identical in the per-protocol analysis (46% vs. 49.6%).
Likewise, none of the secondary outcomes posted a significant difference in favor of one treatment arm, including relapse (2.9% vs. 2.7%) and resistance development (10.8% vs. 9.7%).
Dr. Yahav pointed out that total antibiotic-use days were significantly less in the 7-day group, (5 days) than in the 14-day group (10 days). Patients in the short-duration group returned to their normal activities a day earlier than those in the longer-term group (2 days vs. 3 days), a difference that was statistically significant.
The total hospital stay from randomization to day 90 was only half a day shorter in the short-term group (mean, 3 days vs. 3.5 days). That was not a significant finding.
There were some differences in adverse events, although none was statistically significant. The short-duration arm had slightly more cases of kidney injury (0.5%), fewer cases of liver function abnormalities (–1.5%), and half as many rashes (two vs. four). There were two cases of Clostridium difficile in the short-use arm and one in the long-use arm, also not a significant difference.
A subgroup analysis looked at outcomes among the different sources of infection (urinary tract vs. other), whether empirical antibiotics were used, and whether the induced resistance was multdrug or non–multidrug. All of those differences hovered close to the null, but generally favored short antibiotic treatment, Dr. Yahav noted.
“I would conclude from these data that is generally safe to stop antibiotics after 7 days of covering antibiotics for Gram-negative bacteremia patients, if they are hemodynamically stable and nonneutropenic at 7 days, and have no uncontrolled source of infection,” she concluded.
The investigator-initiated study had no outside funding.
SOURCE: Yahav D et al. ECCMID 2018. Oral abstract O1120.
MADRID – Seven days of antibiotic therapy was just as effective as 14 days for patients with Gram-negative bacteremias.
The shorter course was associated with similar cure rates and a faster return to normal activities, Dafna Yahav, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“In patients hospitalized with Gram-negative bacteremia and sepsis, a course of 7 antibiotic days was not inferior to 14 days, and resulted in a more rapid return to baseline activity, “ said Dr. Yahav of the Rabin Medical Center, Petah Tikva, Israel. “This could lead to a change in accepted management algorithms and shortened antibiotic therapy. Potentially, though we did not show this in our trial, it may lead to reduced cost, reduced development of resistance, and fewer adverse events.”
During the past few years, a new dogma has emerged in antibiotic treatment paradigms, she said: Shorter is better. Brad Spellberg, MD, described this concept in his 2016 editorial in JAMA Internal Medicine, “The new antibiotic mantra” (Sep 1;176[9]:1254-5).
In it, Dr. Spellberg, of the University of Southern California, Los Angeles, addressed the long-held view that a full 10- or 14-day course of antibiotics was necessary to decrease the risk of creating a resistant strain, even if clinical symptoms were long resolved.
However, he noted, there is little evidence supporting the idea that longer courses suppress the rise of resistance – and, in fact, some data support the opposite.
“To the contrary, specifically for pneumonia, studies have shown that longer courses of therapy result in more emergence of antibiotic resistance, which is consistent with everything we know about natural selection, the driver of antibiotic resistance,” he noted. “In only a few types of infections does resistance emerge at the site of infection; rather, resistance typically emerges off target, among colonizing flora away from the site of infection. Thus, all that is achieved by treating an infection with antibiotics for longer than the patient has symptoms is increased selective pressure driving antibiotic resistance among our colonizing microbial flora.”
The European Union and Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America have all recently updated their antibiotic stewardship guidelines to include a strong recommendation for the shortest effective duration of antimicrobial therapy.
However, most of the supporting data were drawn from randomized, controlled studies of patients with lung, skin, and kidney infections. Short-course treatments have not been adequately studied in bacteremia patients, Dr. Yahav said.
The aim of her study, which was investigator initiated and received no external funding, was to demonstrate the noninferiority of 7 days of antibiotic therapy, compared with 14 days, in patients with bacteremia arising from Gram-negative infections.
The randomized, open-label study comprised 604 patients in three hospitals: two in Israel and one in Italy. Patients were eligible if they had an aerobic Gram-negative bacteremia of any infection source that was either community- or hospital acquired. The medication choice was left up to the treating physician. Patients were assessed at discharge, and at days 30 and 90.
The primary outcome was a composite 90-day endpoint of all-cause mortality, clinical failure (relapse, new local complications, or distant complications), and readmission or hospital stay longer than 14 days. There were a number of secondary outcomes, including new infection, emergence of antibiotic resistance, total hospital and total antibiotic days, time to return to baseline activity, and adverse events.
The cohort was a mean of 71 years old. About 60% were functionally independent, and the mean Charlson comorbidity score was 2. Most of the infections (90%) were nosocomial. The urinary tract was the largest source of infection (69%). Other sources were abdominal, respiratory, central venous catheter, and skin or soft tissue.
Escherichia coli was the most common infective organism (62%), followed by Klebsiella species and Enterobacteriaceae. A small number of patients had Acinetobacter and Pseudomonas infections.
In the intent-to-treat analysis, the primary composite outcome of all-cause mortality or extended hospital stay occurred in 46% of the 7-day group and 50% of the 14-day group – not significantly different. The results were nearly identical in the per-protocol analysis (46% vs. 49.6%).
Likewise, none of the secondary outcomes posted a significant difference in favor of one treatment arm, including relapse (2.9% vs. 2.7%) and resistance development (10.8% vs. 9.7%).
Dr. Yahav pointed out that total antibiotic-use days were significantly less in the 7-day group, (5 days) than in the 14-day group (10 days). Patients in the short-duration group returned to their normal activities a day earlier than those in the longer-term group (2 days vs. 3 days), a difference that was statistically significant.
The total hospital stay from randomization to day 90 was only half a day shorter in the short-term group (mean, 3 days vs. 3.5 days). That was not a significant finding.
There were some differences in adverse events, although none was statistically significant. The short-duration arm had slightly more cases of kidney injury (0.5%), fewer cases of liver function abnormalities (–1.5%), and half as many rashes (two vs. four). There were two cases of Clostridium difficile in the short-use arm and one in the long-use arm, also not a significant difference.
A subgroup analysis looked at outcomes among the different sources of infection (urinary tract vs. other), whether empirical antibiotics were used, and whether the induced resistance was multdrug or non–multidrug. All of those differences hovered close to the null, but generally favored short antibiotic treatment, Dr. Yahav noted.
“I would conclude from these data that is generally safe to stop antibiotics after 7 days of covering antibiotics for Gram-negative bacteremia patients, if they are hemodynamically stable and nonneutropenic at 7 days, and have no uncontrolled source of infection,” she concluded.
The investigator-initiated study had no outside funding.
SOURCE: Yahav D et al. ECCMID 2018. Oral abstract O1120.
REPORTING FROM ECCMID 2018
Key clinical point: Two weeks of antibiotic treatment conferred no benefits over 7 days of treatment in patients with Gram-negative bacteremias.
Major finding: All-cause mortality and extended hospital stay occurred in 46% of the 7-day group and 50% of the 14-day group – not significantly different.
Study details: The randomized, open-label trial comprised 604 patients.
Disclosures: The investigator-initiated study had no external funding. Dr. Yahav had no financial disclosures.
Source: Yahav D et al. ECCMID 2018. Oral Abstract O1120.
VIDEO: Calming microglia might control fibromyalgia
SANDESTIN, FLA. – Activated microglia may be a root cause of fibromyalgia, and bringing them back to a resting state an effective path to symptom relief.
Jarred Younger, PhD, is particularly interested in dextronaltrexone, the right-handed isomer of the drug commonly employed in addiction medicine, for calming microglia in fibromyalgia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Unlike the commercially available levo-naltrexone, which binds at both the mu-opioid receptor and Toll-like receptor 4 (TLR4), dextronaltrexone blocks only TLR4. Blocking this receptor interferes with the cells’ ability to recruit peripheral immune cells, which may enter the brain, release cytokines, and induce a proinflammatory environment. By targeting only TLR4 and sparing opioid receptors, , Dr. Younger said in a video interview at the annual Congress of Clinical Rheumatology.
He already has investigated low-dose levo-naltrexone in a small positive crossover trial in 31 fibromyalgia patients. While taking the drug, patients reported significantly less pain and improved mood.
Dr. Younger also recently published a study suggesting that low-dose naltrexone actively improves peripheral proinflammatory cytokine levels.
The placebo-controlled crossover trial enrolled eight women with moderately severe fibromyalgia who took 4.5 mg naltrexone daily for 8 weeks. Compared with baseline, they had significantly reduced plasma levels of a variety of interleukin (IL) subtypes. Also reduced were interferon-alpha, transforming growth factor-alpha and -beta, TNF-alpha, and granulocyte-colony stimulating factor. Patients experienced a mean 15% reduction in fibromyalgia pain and an 18% reduction in overall symptoms.
But proving the drug’s method of action continues to be a challenge, he admitted. It’s not easy to observe microglial trafficking and cellular response to immune signaling in the brain.
Dr. Younger is preparing to launch an innovative PET study that should prove whether activated microglia are recruiting peripheral leukocytes into the brains of fibromyalgia patients. He intends to isolate T and B cells from blood, tag them with a PET radioligand, and reinject them into the subject.
“Since those cells are tagged, a few days later, we can scan the person and see if those cells made it into the brain,” Dr. Younger explained. “If we find T cells and B cells in the brain, that’s clear evidence that the peripheral immune system is attacking and infiltrating the brain, which would be very good in telling us what’s going on in fibromyalgia.”
Low-dose naltrexone is not approved for treating fibromyalgia, he noted. However, during the discussion period after Dr. Younger’s presentation, a number of physicians said they have been using the drug in fibromyalgia patients; some said it has been useful for patients with multiple sclerosis, as well.
Dr. Younger had no relevant financial disclosures.
SANDESTIN, FLA. – Activated microglia may be a root cause of fibromyalgia, and bringing them back to a resting state an effective path to symptom relief.
Jarred Younger, PhD, is particularly interested in dextronaltrexone, the right-handed isomer of the drug commonly employed in addiction medicine, for calming microglia in fibromyalgia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Unlike the commercially available levo-naltrexone, which binds at both the mu-opioid receptor and Toll-like receptor 4 (TLR4), dextronaltrexone blocks only TLR4. Blocking this receptor interferes with the cells’ ability to recruit peripheral immune cells, which may enter the brain, release cytokines, and induce a proinflammatory environment. By targeting only TLR4 and sparing opioid receptors, , Dr. Younger said in a video interview at the annual Congress of Clinical Rheumatology.
He already has investigated low-dose levo-naltrexone in a small positive crossover trial in 31 fibromyalgia patients. While taking the drug, patients reported significantly less pain and improved mood.
Dr. Younger also recently published a study suggesting that low-dose naltrexone actively improves peripheral proinflammatory cytokine levels.
The placebo-controlled crossover trial enrolled eight women with moderately severe fibromyalgia who took 4.5 mg naltrexone daily for 8 weeks. Compared with baseline, they had significantly reduced plasma levels of a variety of interleukin (IL) subtypes. Also reduced were interferon-alpha, transforming growth factor-alpha and -beta, TNF-alpha, and granulocyte-colony stimulating factor. Patients experienced a mean 15% reduction in fibromyalgia pain and an 18% reduction in overall symptoms.
But proving the drug’s method of action continues to be a challenge, he admitted. It’s not easy to observe microglial trafficking and cellular response to immune signaling in the brain.
Dr. Younger is preparing to launch an innovative PET study that should prove whether activated microglia are recruiting peripheral leukocytes into the brains of fibromyalgia patients. He intends to isolate T and B cells from blood, tag them with a PET radioligand, and reinject them into the subject.
“Since those cells are tagged, a few days later, we can scan the person and see if those cells made it into the brain,” Dr. Younger explained. “If we find T cells and B cells in the brain, that’s clear evidence that the peripheral immune system is attacking and infiltrating the brain, which would be very good in telling us what’s going on in fibromyalgia.”
Low-dose naltrexone is not approved for treating fibromyalgia, he noted. However, during the discussion period after Dr. Younger’s presentation, a number of physicians said they have been using the drug in fibromyalgia patients; some said it has been useful for patients with multiple sclerosis, as well.
Dr. Younger had no relevant financial disclosures.
SANDESTIN, FLA. – Activated microglia may be a root cause of fibromyalgia, and bringing them back to a resting state an effective path to symptom relief.
Jarred Younger, PhD, is particularly interested in dextronaltrexone, the right-handed isomer of the drug commonly employed in addiction medicine, for calming microglia in fibromyalgia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Unlike the commercially available levo-naltrexone, which binds at both the mu-opioid receptor and Toll-like receptor 4 (TLR4), dextronaltrexone blocks only TLR4. Blocking this receptor interferes with the cells’ ability to recruit peripheral immune cells, which may enter the brain, release cytokines, and induce a proinflammatory environment. By targeting only TLR4 and sparing opioid receptors, , Dr. Younger said in a video interview at the annual Congress of Clinical Rheumatology.
He already has investigated low-dose levo-naltrexone in a small positive crossover trial in 31 fibromyalgia patients. While taking the drug, patients reported significantly less pain and improved mood.
Dr. Younger also recently published a study suggesting that low-dose naltrexone actively improves peripheral proinflammatory cytokine levels.
The placebo-controlled crossover trial enrolled eight women with moderately severe fibromyalgia who took 4.5 mg naltrexone daily for 8 weeks. Compared with baseline, they had significantly reduced plasma levels of a variety of interleukin (IL) subtypes. Also reduced were interferon-alpha, transforming growth factor-alpha and -beta, TNF-alpha, and granulocyte-colony stimulating factor. Patients experienced a mean 15% reduction in fibromyalgia pain and an 18% reduction in overall symptoms.
But proving the drug’s method of action continues to be a challenge, he admitted. It’s not easy to observe microglial trafficking and cellular response to immune signaling in the brain.
Dr. Younger is preparing to launch an innovative PET study that should prove whether activated microglia are recruiting peripheral leukocytes into the brains of fibromyalgia patients. He intends to isolate T and B cells from blood, tag them with a PET radioligand, and reinject them into the subject.
“Since those cells are tagged, a few days later, we can scan the person and see if those cells made it into the brain,” Dr. Younger explained. “If we find T cells and B cells in the brain, that’s clear evidence that the peripheral immune system is attacking and infiltrating the brain, which would be very good in telling us what’s going on in fibromyalgia.”
Low-dose naltrexone is not approved for treating fibromyalgia, he noted. However, during the discussion period after Dr. Younger’s presentation, a number of physicians said they have been using the drug in fibromyalgia patients; some said it has been useful for patients with multiple sclerosis, as well.
Dr. Younger had no relevant financial disclosures.
REPORTING FROM CCR 2018
Three days of beta-lactam beat clinically stable CAP
MADRID – Three days of beta-lactam therapy was just as effective as 8 days for clinically stable patients presenting with community-acquired pneumonia.
In a randomized, placebo-controlled trial, 15-day cure rates were 69.9% in patients who took 3 days of antibiotics and 61.2% in those who took 8 days – a nonsignificant difference, Aurélien Dinh, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Reducing treatment time now appears to be manageable and effective in a number of infectious diseases,” Dr. Dinh explained. “Although there are some limits, surely, this change in practice might lead to reduced rates of multidrug-resistant bacteria, fewer adverse events, and surely lower costs.”
The French PTC Trial (Short Duration Treatment of Non-Severe Community-Acquired Pneumonia) randomized 310 patients (mean age, 73.5 years) to either short- or long-course treatment with a beta-lactam antibiotic. Patients were eligible for the study if they were admitted to the hospital for community-acquired pneumonia based on respiratory signs, fever of 38° C or higher, and evidence of new infiltrate on chest radiograph.
All patients were treated with 3 days of amoxicillin/clavulanic acid (Augmentin) or third-generation cephalosporin. Those who had responded clinically by day 3 entered the 5-day randomization period, receiving placebo or 5 more days of active therapy with the same agent.
Clinical requirements for randomization included being afebrile with stable heart and respiratory rate, a systolic blood pressure of at least 90 mm Hg, and oxygen saturation of at least 90%.
The primary endpoint was clinical cure at day 15: no fever, absence of or improvement in respiratory symptoms (dyspnea, cough, purulent sputum, and cackles), and no need for additional antibiotic treatment for any indication.
Secondary endpoints were cure at day 30, 30-day mortality, adverse events, length of stay, return to usual activities by day 30, and quality of life at day 30.
Many of the generally elderly patient cohort had comorbid illnesses, including diabetes (about 20%), chronic obstructive pulmonary disease (about 35%), and coronary insufficiency (about 14%). About 20% were active smokers. Less than 10% had gotten a pneumococcal vaccine in the past 5 years.
At admission, more than half of patients were dyspneic, 80% had cough, and 39% had purulent sputum. The median PSI/PORT Score was 82.
After 3 days of treatment, clinical cure was not significantly different between the 3- and 8-day groups, either in the intent-to-treat analysis (69.9% vs. 61.2%) or in the per-protocol analysis (75.7% vs. 68.7%).
Because the trial had closed days before the ECCMID meeting, only the primary endpoints were available for discussion, Dr. Dinh said. Investigators are analyzing the secondary endpoint data, which he said would be published at a later date.
Despite the positive results, Dr. Dinh cautioned against using the study as justification for a one-size-fits-all treatment for community-acquired pneumonia.
“Although I think we demonstrated that 3 days of treatment with beta-lactam is not inferior to 8 days, this cannot be imposed without regard to individual patient status,” he cautioned. Such a treatment paradigm would not be advisable for patients with moderately severe pneumonia, who were excluded from the study, or those with compromised immune systems.
Nor does Dr. Dinh expect wholesale clinical embracing of the encouraging results, which bolster the ever-accumulating data in favor of shorter courses of antibiotics for some infectious diseases.
“I think there is a chance that clinicians who normally treat for 9 or 10 days may now feel comfortable reducing to 7,” he said with a chuckle.
The French Ministry of Health sponsored the study. Dr. Dinh had no competing financial interests.
SOURCE: Dinh et al. ECCMID 2018, Oral Abstract O1126.
MADRID – Three days of beta-lactam therapy was just as effective as 8 days for clinically stable patients presenting with community-acquired pneumonia.
In a randomized, placebo-controlled trial, 15-day cure rates were 69.9% in patients who took 3 days of antibiotics and 61.2% in those who took 8 days – a nonsignificant difference, Aurélien Dinh, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Reducing treatment time now appears to be manageable and effective in a number of infectious diseases,” Dr. Dinh explained. “Although there are some limits, surely, this change in practice might lead to reduced rates of multidrug-resistant bacteria, fewer adverse events, and surely lower costs.”
The French PTC Trial (Short Duration Treatment of Non-Severe Community-Acquired Pneumonia) randomized 310 patients (mean age, 73.5 years) to either short- or long-course treatment with a beta-lactam antibiotic. Patients were eligible for the study if they were admitted to the hospital for community-acquired pneumonia based on respiratory signs, fever of 38° C or higher, and evidence of new infiltrate on chest radiograph.
All patients were treated with 3 days of amoxicillin/clavulanic acid (Augmentin) or third-generation cephalosporin. Those who had responded clinically by day 3 entered the 5-day randomization period, receiving placebo or 5 more days of active therapy with the same agent.
Clinical requirements for randomization included being afebrile with stable heart and respiratory rate, a systolic blood pressure of at least 90 mm Hg, and oxygen saturation of at least 90%.
The primary endpoint was clinical cure at day 15: no fever, absence of or improvement in respiratory symptoms (dyspnea, cough, purulent sputum, and cackles), and no need for additional antibiotic treatment for any indication.
Secondary endpoints were cure at day 30, 30-day mortality, adverse events, length of stay, return to usual activities by day 30, and quality of life at day 30.
Many of the generally elderly patient cohort had comorbid illnesses, including diabetes (about 20%), chronic obstructive pulmonary disease (about 35%), and coronary insufficiency (about 14%). About 20% were active smokers. Less than 10% had gotten a pneumococcal vaccine in the past 5 years.
At admission, more than half of patients were dyspneic, 80% had cough, and 39% had purulent sputum. The median PSI/PORT Score was 82.
After 3 days of treatment, clinical cure was not significantly different between the 3- and 8-day groups, either in the intent-to-treat analysis (69.9% vs. 61.2%) or in the per-protocol analysis (75.7% vs. 68.7%).
Because the trial had closed days before the ECCMID meeting, only the primary endpoints were available for discussion, Dr. Dinh said. Investigators are analyzing the secondary endpoint data, which he said would be published at a later date.
Despite the positive results, Dr. Dinh cautioned against using the study as justification for a one-size-fits-all treatment for community-acquired pneumonia.
“Although I think we demonstrated that 3 days of treatment with beta-lactam is not inferior to 8 days, this cannot be imposed without regard to individual patient status,” he cautioned. Such a treatment paradigm would not be advisable for patients with moderately severe pneumonia, who were excluded from the study, or those with compromised immune systems.
Nor does Dr. Dinh expect wholesale clinical embracing of the encouraging results, which bolster the ever-accumulating data in favor of shorter courses of antibiotics for some infectious diseases.
“I think there is a chance that clinicians who normally treat for 9 or 10 days may now feel comfortable reducing to 7,” he said with a chuckle.
The French Ministry of Health sponsored the study. Dr. Dinh had no competing financial interests.
SOURCE: Dinh et al. ECCMID 2018, Oral Abstract O1126.
MADRID – Three days of beta-lactam therapy was just as effective as 8 days for clinically stable patients presenting with community-acquired pneumonia.
In a randomized, placebo-controlled trial, 15-day cure rates were 69.9% in patients who took 3 days of antibiotics and 61.2% in those who took 8 days – a nonsignificant difference, Aurélien Dinh, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Reducing treatment time now appears to be manageable and effective in a number of infectious diseases,” Dr. Dinh explained. “Although there are some limits, surely, this change in practice might lead to reduced rates of multidrug-resistant bacteria, fewer adverse events, and surely lower costs.”
The French PTC Trial (Short Duration Treatment of Non-Severe Community-Acquired Pneumonia) randomized 310 patients (mean age, 73.5 years) to either short- or long-course treatment with a beta-lactam antibiotic. Patients were eligible for the study if they were admitted to the hospital for community-acquired pneumonia based on respiratory signs, fever of 38° C or higher, and evidence of new infiltrate on chest radiograph.
All patients were treated with 3 days of amoxicillin/clavulanic acid (Augmentin) or third-generation cephalosporin. Those who had responded clinically by day 3 entered the 5-day randomization period, receiving placebo or 5 more days of active therapy with the same agent.
Clinical requirements for randomization included being afebrile with stable heart and respiratory rate, a systolic blood pressure of at least 90 mm Hg, and oxygen saturation of at least 90%.
The primary endpoint was clinical cure at day 15: no fever, absence of or improvement in respiratory symptoms (dyspnea, cough, purulent sputum, and cackles), and no need for additional antibiotic treatment for any indication.
Secondary endpoints were cure at day 30, 30-day mortality, adverse events, length of stay, return to usual activities by day 30, and quality of life at day 30.
Many of the generally elderly patient cohort had comorbid illnesses, including diabetes (about 20%), chronic obstructive pulmonary disease (about 35%), and coronary insufficiency (about 14%). About 20% were active smokers. Less than 10% had gotten a pneumococcal vaccine in the past 5 years.
At admission, more than half of patients were dyspneic, 80% had cough, and 39% had purulent sputum. The median PSI/PORT Score was 82.
After 3 days of treatment, clinical cure was not significantly different between the 3- and 8-day groups, either in the intent-to-treat analysis (69.9% vs. 61.2%) or in the per-protocol analysis (75.7% vs. 68.7%).
Because the trial had closed days before the ECCMID meeting, only the primary endpoints were available for discussion, Dr. Dinh said. Investigators are analyzing the secondary endpoint data, which he said would be published at a later date.
Despite the positive results, Dr. Dinh cautioned against using the study as justification for a one-size-fits-all treatment for community-acquired pneumonia.
“Although I think we demonstrated that 3 days of treatment with beta-lactam is not inferior to 8 days, this cannot be imposed without regard to individual patient status,” he cautioned. Such a treatment paradigm would not be advisable for patients with moderately severe pneumonia, who were excluded from the study, or those with compromised immune systems.
Nor does Dr. Dinh expect wholesale clinical embracing of the encouraging results, which bolster the ever-accumulating data in favor of shorter courses of antibiotics for some infectious diseases.
“I think there is a chance that clinicians who normally treat for 9 or 10 days may now feel comfortable reducing to 7,” he said with a chuckle.
The French Ministry of Health sponsored the study. Dr. Dinh had no competing financial interests.
SOURCE: Dinh et al. ECCMID 2018, Oral Abstract O1126.
REPORTING FROM ECCMID 2018
Key clinical point: Three days of beta-lactam treatment were as effective as 8 days in curing clinically stable patients with community-acquired pneumonia.
Major finding: Cure rates at 15 days were 69.9% in the 3-day group, compared with 61.2% in the 8-day group, a nonsignificant difference.
Study details: The placebo-controlled study randomized 310 patients to treatment.
Disclosures: The French Ministry of Health sponsored the trial. Dr. Dinh had no financial disclosures.
Source: Dinh et al. ECCMID 2018, oral abstract O1126.
VIDEO: Characteristic flora define intestinal microbiome in scleroderma
SANDESTIN, FLA. – Scleroderma patients appear to have a characteristic microbiome composition, which is consistent in samples taken around the world.
These patients showed decreased populations of beneficial commensal flora and increased populations of proinflammatory species, Elizabeth Volkmann, MD, said at the annual Congress of Clinical Rheumatology.
Furthermore, specific species seem to correlate with specific gastrointestinal symptoms, said Dr. Volkmann of the University of California, Los Angeles. “Features also unexpectedly overlap with the consortium typical for Crohn’s disease, a disease with both inflammatory and fibrosing phenotype,” she said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her recent exploration of this topic included 17 patients with scleroderma and GI symptoms and 17 matched healthy controls (BMJ Open Gastro. 2017;3:e000134). Everyone underwent a bowel prep and colonoscopy, during which cecum and sigmoid mucosal lavage samples were obtained. Those samples underwent RNA sequencing.
In addition to quantifying the species present, Dr. Volkmann sought to associate populations with symptoms. The primary assessment tool was the GIT 2.0, which measures distention/bloating; diarrhea; fecal soilage; constipation; emotional well-being; and social functioning.
Similar to the findings in inflammatory disease states, scleroderma patients had decreased levels of commensal Clostridia, a class of Firmicutes that is established in early infancy and very important in the maintenance of gut homeostasis. They also showed a decreased proportion of Faecalibacterium, a genus with anti-inflammatory activity; this finding has been observed in patients with Crohn’s disease.
Patients also showed relative increases in pathobionts. These are potentially pathological organisms that, under normal circumstances, live symbiotically. Janet Chow, PhD, who coined the term in a 2011 paper, said these species are typically proinflammatory (Curr Opin Immunol. 2011 Aug; 23[4]:473-80).
“Organisms proposed as pathobionts are associated with chronic inflammatory conditions – unlike opportunistic pathogens, which often cause acute infections and are typically acquired from the environment or other parts of the body. In addition, pathobionts are innocuous to the host under normal conditions,” wrote Dr. Chow of the California Institute of Technology, Pasadena.
In Dr. Volkmann’s study, Bifidobacterium and Lactobacillus, which are usually reduced in proinflammatory disorders, were relatively abundant in patients, compared with controls.
She noted specific associations with both symptoms. Parabacteroides and Enterobacteriaceae were associated with increased constipation. Prevotella was associated with increased diarrhea and increased distention/bloating.
Her results are consistent with a Swedish study (Arthritis Res Ther. 2016 Nov 1;18[1]:278) and three Italian studies conducted in Rome, Milan, and Piacenza.
“It’s fascinating that we seem to be identifying a consistent microbiome profile for scleroderma patients,” Dr. Volkmann said.
Dr. Volkmann had no relevant financial disclosures.
SANDESTIN, FLA. – Scleroderma patients appear to have a characteristic microbiome composition, which is consistent in samples taken around the world.
These patients showed decreased populations of beneficial commensal flora and increased populations of proinflammatory species, Elizabeth Volkmann, MD, said at the annual Congress of Clinical Rheumatology.
Furthermore, specific species seem to correlate with specific gastrointestinal symptoms, said Dr. Volkmann of the University of California, Los Angeles. “Features also unexpectedly overlap with the consortium typical for Crohn’s disease, a disease with both inflammatory and fibrosing phenotype,” she said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her recent exploration of this topic included 17 patients with scleroderma and GI symptoms and 17 matched healthy controls (BMJ Open Gastro. 2017;3:e000134). Everyone underwent a bowel prep and colonoscopy, during which cecum and sigmoid mucosal lavage samples were obtained. Those samples underwent RNA sequencing.
In addition to quantifying the species present, Dr. Volkmann sought to associate populations with symptoms. The primary assessment tool was the GIT 2.0, which measures distention/bloating; diarrhea; fecal soilage; constipation; emotional well-being; and social functioning.
Similar to the findings in inflammatory disease states, scleroderma patients had decreased levels of commensal Clostridia, a class of Firmicutes that is established in early infancy and very important in the maintenance of gut homeostasis. They also showed a decreased proportion of Faecalibacterium, a genus with anti-inflammatory activity; this finding has been observed in patients with Crohn’s disease.
Patients also showed relative increases in pathobionts. These are potentially pathological organisms that, under normal circumstances, live symbiotically. Janet Chow, PhD, who coined the term in a 2011 paper, said these species are typically proinflammatory (Curr Opin Immunol. 2011 Aug; 23[4]:473-80).
“Organisms proposed as pathobionts are associated with chronic inflammatory conditions – unlike opportunistic pathogens, which often cause acute infections and are typically acquired from the environment or other parts of the body. In addition, pathobionts are innocuous to the host under normal conditions,” wrote Dr. Chow of the California Institute of Technology, Pasadena.
In Dr. Volkmann’s study, Bifidobacterium and Lactobacillus, which are usually reduced in proinflammatory disorders, were relatively abundant in patients, compared with controls.
She noted specific associations with both symptoms. Parabacteroides and Enterobacteriaceae were associated with increased constipation. Prevotella was associated with increased diarrhea and increased distention/bloating.
Her results are consistent with a Swedish study (Arthritis Res Ther. 2016 Nov 1;18[1]:278) and three Italian studies conducted in Rome, Milan, and Piacenza.
“It’s fascinating that we seem to be identifying a consistent microbiome profile for scleroderma patients,” Dr. Volkmann said.
Dr. Volkmann had no relevant financial disclosures.
SANDESTIN, FLA. – Scleroderma patients appear to have a characteristic microbiome composition, which is consistent in samples taken around the world.
These patients showed decreased populations of beneficial commensal flora and increased populations of proinflammatory species, Elizabeth Volkmann, MD, said at the annual Congress of Clinical Rheumatology.
Furthermore, specific species seem to correlate with specific gastrointestinal symptoms, said Dr. Volkmann of the University of California, Los Angeles. “Features also unexpectedly overlap with the consortium typical for Crohn’s disease, a disease with both inflammatory and fibrosing phenotype,” she said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Her recent exploration of this topic included 17 patients with scleroderma and GI symptoms and 17 matched healthy controls (BMJ Open Gastro. 2017;3:e000134). Everyone underwent a bowel prep and colonoscopy, during which cecum and sigmoid mucosal lavage samples were obtained. Those samples underwent RNA sequencing.
In addition to quantifying the species present, Dr. Volkmann sought to associate populations with symptoms. The primary assessment tool was the GIT 2.0, which measures distention/bloating; diarrhea; fecal soilage; constipation; emotional well-being; and social functioning.
Similar to the findings in inflammatory disease states, scleroderma patients had decreased levels of commensal Clostridia, a class of Firmicutes that is established in early infancy and very important in the maintenance of gut homeostasis. They also showed a decreased proportion of Faecalibacterium, a genus with anti-inflammatory activity; this finding has been observed in patients with Crohn’s disease.
Patients also showed relative increases in pathobionts. These are potentially pathological organisms that, under normal circumstances, live symbiotically. Janet Chow, PhD, who coined the term in a 2011 paper, said these species are typically proinflammatory (Curr Opin Immunol. 2011 Aug; 23[4]:473-80).
“Organisms proposed as pathobionts are associated with chronic inflammatory conditions – unlike opportunistic pathogens, which often cause acute infections and are typically acquired from the environment or other parts of the body. In addition, pathobionts are innocuous to the host under normal conditions,” wrote Dr. Chow of the California Institute of Technology, Pasadena.
In Dr. Volkmann’s study, Bifidobacterium and Lactobacillus, which are usually reduced in proinflammatory disorders, were relatively abundant in patients, compared with controls.
She noted specific associations with both symptoms. Parabacteroides and Enterobacteriaceae were associated with increased constipation. Prevotella was associated with increased diarrhea and increased distention/bloating.
Her results are consistent with a Swedish study (Arthritis Res Ther. 2016 Nov 1;18[1]:278) and three Italian studies conducted in Rome, Milan, and Piacenza.
“It’s fascinating that we seem to be identifying a consistent microbiome profile for scleroderma patients,” Dr. Volkmann said.
Dr. Volkmann had no relevant financial disclosures.
REPORTING FROM CCR 18
VIDEO: Let clinical scenario, not imaging, guide sarcoidosis treatment
SANDESTIN, FLA. – Don’t be a slave to imaging when evaluating the patient with sarcoidosis.
“Sometimes, the worst-looking patients [on imaging] have the best prognosis,” Daniel Culver, DO, said at the annual Congress of Clinical Rheumatology. Patients with Löfgren’s syndrome are a very good example of this tenet, he said in an interview. Scans can look alarming, with multiple widespread granulomas. But Löfgren’s is generally a benign condition, despite its threatening mien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Instead of imaging, “Let two things drive your decision to treat: danger to an organ, and quality of life,” said Dr. Culver, a pulmonologist and director of the Sarcoidosis Center of Excellence at the Cleveland Clinic in Ohio; he is also president of the World Association for Sarcoidosis.
He agrees with a decision schema published in 2015 (Clin Chest Med. 2015;36[4]:751-67).
Six factors weigh in favor of treatment:
- Symptomatic disease.
- Impaired organ function.
- Disease endangering an organ.
- Progressive disease.
- Clear-cut disease activity.
- Low likelihood of remission.
These must be balanced – with patient input as the fulcrum – against five factors that favor conservative management:
- Minimal symptoms.
- Good organ function.
- Low risk of danger to organs.
- Inactive disease.
- Higher likelihood of remission.
The decision to embark on a treatment program, usually starting with a steroid-based regimen, can’t be taken lightly, Dr. Culver said. A 2017 study showed that steroids pose a cumulative risk of toxicities for sarcoidosis patients (Respir Med. 2017 Nov;132:9-14). Patients who started steroids faced more than a doubling in the risk of a toxic side effect by 96 months when compared with those who didn’t. But even short-term steroid use increased the risk of a toxicity, Dr. Culver said. The study noted that problems can begin to occur in as little as 1 month, at a cumulative dose as low as 1 g.
For patients who fall onto the “treat” side of the risk teeter-totter, Dr. Culver recommended starting with an initial course of prednisone at 20-30 mg daily for no more than 4 weeks. Responders can taper to less than 10 mg/day. Those who continue to do well can maintain low-dose prednisone for up to 12 months and then complete the taper. Patients who relapse can add an immune modulator (methotrexate, azathioprine, leflunomide, or mycophenolate).
Those who have an inadequate response to the initial prednisone course should then get an immune modulator. If they do well, that can be maintained; a second modulator can be brought on board if necessary.
For those who don’t respond at all to the initial prednisone course, it’s necessary to proceed immediately to an immunosuppressive regimen to prevent irreversible fibrosis.
Dr. Culver noted associations with multiple pharmaceutical companies, but said none were relevant to his talk.
SOURCE: Culver D. CCR 2018.
SANDESTIN, FLA. – Don’t be a slave to imaging when evaluating the patient with sarcoidosis.
“Sometimes, the worst-looking patients [on imaging] have the best prognosis,” Daniel Culver, DO, said at the annual Congress of Clinical Rheumatology. Patients with Löfgren’s syndrome are a very good example of this tenet, he said in an interview. Scans can look alarming, with multiple widespread granulomas. But Löfgren’s is generally a benign condition, despite its threatening mien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Instead of imaging, “Let two things drive your decision to treat: danger to an organ, and quality of life,” said Dr. Culver, a pulmonologist and director of the Sarcoidosis Center of Excellence at the Cleveland Clinic in Ohio; he is also president of the World Association for Sarcoidosis.
He agrees with a decision schema published in 2015 (Clin Chest Med. 2015;36[4]:751-67).
Six factors weigh in favor of treatment:
- Symptomatic disease.
- Impaired organ function.
- Disease endangering an organ.
- Progressive disease.
- Clear-cut disease activity.
- Low likelihood of remission.
These must be balanced – with patient input as the fulcrum – against five factors that favor conservative management:
- Minimal symptoms.
- Good organ function.
- Low risk of danger to organs.
- Inactive disease.
- Higher likelihood of remission.
The decision to embark on a treatment program, usually starting with a steroid-based regimen, can’t be taken lightly, Dr. Culver said. A 2017 study showed that steroids pose a cumulative risk of toxicities for sarcoidosis patients (Respir Med. 2017 Nov;132:9-14). Patients who started steroids faced more than a doubling in the risk of a toxic side effect by 96 months when compared with those who didn’t. But even short-term steroid use increased the risk of a toxicity, Dr. Culver said. The study noted that problems can begin to occur in as little as 1 month, at a cumulative dose as low as 1 g.
For patients who fall onto the “treat” side of the risk teeter-totter, Dr. Culver recommended starting with an initial course of prednisone at 20-30 mg daily for no more than 4 weeks. Responders can taper to less than 10 mg/day. Those who continue to do well can maintain low-dose prednisone for up to 12 months and then complete the taper. Patients who relapse can add an immune modulator (methotrexate, azathioprine, leflunomide, or mycophenolate).
Those who have an inadequate response to the initial prednisone course should then get an immune modulator. If they do well, that can be maintained; a second modulator can be brought on board if necessary.
For those who don’t respond at all to the initial prednisone course, it’s necessary to proceed immediately to an immunosuppressive regimen to prevent irreversible fibrosis.
Dr. Culver noted associations with multiple pharmaceutical companies, but said none were relevant to his talk.
SOURCE: Culver D. CCR 2018.
SANDESTIN, FLA. – Don’t be a slave to imaging when evaluating the patient with sarcoidosis.
“Sometimes, the worst-looking patients [on imaging] have the best prognosis,” Daniel Culver, DO, said at the annual Congress of Clinical Rheumatology. Patients with Löfgren’s syndrome are a very good example of this tenet, he said in an interview. Scans can look alarming, with multiple widespread granulomas. But Löfgren’s is generally a benign condition, despite its threatening mien.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Instead of imaging, “Let two things drive your decision to treat: danger to an organ, and quality of life,” said Dr. Culver, a pulmonologist and director of the Sarcoidosis Center of Excellence at the Cleveland Clinic in Ohio; he is also president of the World Association for Sarcoidosis.
He agrees with a decision schema published in 2015 (Clin Chest Med. 2015;36[4]:751-67).
Six factors weigh in favor of treatment:
- Symptomatic disease.
- Impaired organ function.
- Disease endangering an organ.
- Progressive disease.
- Clear-cut disease activity.
- Low likelihood of remission.
These must be balanced – with patient input as the fulcrum – against five factors that favor conservative management:
- Minimal symptoms.
- Good organ function.
- Low risk of danger to organs.
- Inactive disease.
- Higher likelihood of remission.
The decision to embark on a treatment program, usually starting with a steroid-based regimen, can’t be taken lightly, Dr. Culver said. A 2017 study showed that steroids pose a cumulative risk of toxicities for sarcoidosis patients (Respir Med. 2017 Nov;132:9-14). Patients who started steroids faced more than a doubling in the risk of a toxic side effect by 96 months when compared with those who didn’t. But even short-term steroid use increased the risk of a toxicity, Dr. Culver said. The study noted that problems can begin to occur in as little as 1 month, at a cumulative dose as low as 1 g.
For patients who fall onto the “treat” side of the risk teeter-totter, Dr. Culver recommended starting with an initial course of prednisone at 20-30 mg daily for no more than 4 weeks. Responders can taper to less than 10 mg/day. Those who continue to do well can maintain low-dose prednisone for up to 12 months and then complete the taper. Patients who relapse can add an immune modulator (methotrexate, azathioprine, leflunomide, or mycophenolate).
Those who have an inadequate response to the initial prednisone course should then get an immune modulator. If they do well, that can be maintained; a second modulator can be brought on board if necessary.
For those who don’t respond at all to the initial prednisone course, it’s necessary to proceed immediately to an immunosuppressive regimen to prevent irreversible fibrosis.
Dr. Culver noted associations with multiple pharmaceutical companies, but said none were relevant to his talk.
SOURCE: Culver D. CCR 2018.
REPORTING FROM CCR 18
VIDEO: Dual studies seek answers in isolated skin vasculitis
SANDESTIN, FLA. – Patients with isolated skin vasculitis have always faced a frustrating clinical problem with no clear solution.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ARAMIS (A Randomized Multicenter Study for Isolated Skin Vasculitis) and its linked genetic investigation, CUTIS (Clinical Transcriptomics in Systemic Vasculitis), may finally identify not only optimal treatments but also insight into the root causes and predictors of treatment response, Christian Pagnoux, MD, said at the annual Congress of Clinical Rheumatology.
“Isolated skin vasculitis is a much-understudied disease, with only one clinical trial to guide our treatment,” said Dr. Pagnoux of the Mount Sinai Hospital, Toronto. In 1995, a 3-month trial randomized 41 patients to skin emollients or to colchicine 0.5 mg/day. Colchicine wasn’t significantly better, but some who had attained remission on it relapsed after discontinuing the drug, which suggested there might be some benefit (Arch Dermatol. 1995;131[12]:1399-1402).
That hint of efficacy in just three patients 23 years ago forms the sole basis of the typical treatment for this disorder: colchicine, Dr. Pagnoux said. “We know that it doesn’t work, yet we continue to prescribe it. Patients deserve better.”
ARAMIS and CUTIS are the first attempts since then at solving this puzzle. ARAMIS is now recruiting about 90 patients in 10 North American medical centers. The three-armed crossover trial will randomize patients to colchicine 0.6 mg twice a day, dapsone 150 mg/day, or azathioprine 2 mg/kg per day for 6 months. Nonresponders can then be rerandomized to one of the other two study drugs for another 6 months. The primary endpoint is clinical response. Secondary endpoints include changes in physician and patient global assessment of response, Skindex29 score, health-related quality of life, and the Patient-Reported Outcomes Measurement Information System.
ARAMIS patients may also participate in CUTIS, the linked histopathologic and genetic investigation. More broad-ranging than ARAMIS, CUTIS is seeking 50 patients with several forms of idiopathic vasculitis, including cryoglobulinemic vasculitis, drug-induced vasculitis, eosinophilic granulomatosis with polyangiitis, IgA vasculitis, isolated cutaneous vasculitis, granulomatosis with polyangiitis, microscopic polyangiitis, polyarteritis nodosa, and urticarial vasculitis.
The study will examine histopathologic and transcriptomic characteristics in punch biopsies of the lesions. “We very much hope that gene expression profiling on these lesions will help define novel pathways and help us to classify and target therapies,” Dr. Pagnoux said.
To learn more about these studies and refer patients into them, visit the Rare Disease Network pages for ARAMIS and CUTIS.
Dr. Pagnoux had no financial disclosures relevant to either study.
SOURCE: Pagnoux C. CCR 2018
SANDESTIN, FLA. – Patients with isolated skin vasculitis have always faced a frustrating clinical problem with no clear solution.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ARAMIS (A Randomized Multicenter Study for Isolated Skin Vasculitis) and its linked genetic investigation, CUTIS (Clinical Transcriptomics in Systemic Vasculitis), may finally identify not only optimal treatments but also insight into the root causes and predictors of treatment response, Christian Pagnoux, MD, said at the annual Congress of Clinical Rheumatology.
“Isolated skin vasculitis is a much-understudied disease, with only one clinical trial to guide our treatment,” said Dr. Pagnoux of the Mount Sinai Hospital, Toronto. In 1995, a 3-month trial randomized 41 patients to skin emollients or to colchicine 0.5 mg/day. Colchicine wasn’t significantly better, but some who had attained remission on it relapsed after discontinuing the drug, which suggested there might be some benefit (Arch Dermatol. 1995;131[12]:1399-1402).
That hint of efficacy in just three patients 23 years ago forms the sole basis of the typical treatment for this disorder: colchicine, Dr. Pagnoux said. “We know that it doesn’t work, yet we continue to prescribe it. Patients deserve better.”
ARAMIS and CUTIS are the first attempts since then at solving this puzzle. ARAMIS is now recruiting about 90 patients in 10 North American medical centers. The three-armed crossover trial will randomize patients to colchicine 0.6 mg twice a day, dapsone 150 mg/day, or azathioprine 2 mg/kg per day for 6 months. Nonresponders can then be rerandomized to one of the other two study drugs for another 6 months. The primary endpoint is clinical response. Secondary endpoints include changes in physician and patient global assessment of response, Skindex29 score, health-related quality of life, and the Patient-Reported Outcomes Measurement Information System.
ARAMIS patients may also participate in CUTIS, the linked histopathologic and genetic investigation. More broad-ranging than ARAMIS, CUTIS is seeking 50 patients with several forms of idiopathic vasculitis, including cryoglobulinemic vasculitis, drug-induced vasculitis, eosinophilic granulomatosis with polyangiitis, IgA vasculitis, isolated cutaneous vasculitis, granulomatosis with polyangiitis, microscopic polyangiitis, polyarteritis nodosa, and urticarial vasculitis.
The study will examine histopathologic and transcriptomic characteristics in punch biopsies of the lesions. “We very much hope that gene expression profiling on these lesions will help define novel pathways and help us to classify and target therapies,” Dr. Pagnoux said.
To learn more about these studies and refer patients into them, visit the Rare Disease Network pages for ARAMIS and CUTIS.
Dr. Pagnoux had no financial disclosures relevant to either study.
SOURCE: Pagnoux C. CCR 2018
SANDESTIN, FLA. – Patients with isolated skin vasculitis have always faced a frustrating clinical problem with no clear solution.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ARAMIS (A Randomized Multicenter Study for Isolated Skin Vasculitis) and its linked genetic investigation, CUTIS (Clinical Transcriptomics in Systemic Vasculitis), may finally identify not only optimal treatments but also insight into the root causes and predictors of treatment response, Christian Pagnoux, MD, said at the annual Congress of Clinical Rheumatology.
“Isolated skin vasculitis is a much-understudied disease, with only one clinical trial to guide our treatment,” said Dr. Pagnoux of the Mount Sinai Hospital, Toronto. In 1995, a 3-month trial randomized 41 patients to skin emollients or to colchicine 0.5 mg/day. Colchicine wasn’t significantly better, but some who had attained remission on it relapsed after discontinuing the drug, which suggested there might be some benefit (Arch Dermatol. 1995;131[12]:1399-1402).
That hint of efficacy in just three patients 23 years ago forms the sole basis of the typical treatment for this disorder: colchicine, Dr. Pagnoux said. “We know that it doesn’t work, yet we continue to prescribe it. Patients deserve better.”
ARAMIS and CUTIS are the first attempts since then at solving this puzzle. ARAMIS is now recruiting about 90 patients in 10 North American medical centers. The three-armed crossover trial will randomize patients to colchicine 0.6 mg twice a day, dapsone 150 mg/day, or azathioprine 2 mg/kg per day for 6 months. Nonresponders can then be rerandomized to one of the other two study drugs for another 6 months. The primary endpoint is clinical response. Secondary endpoints include changes in physician and patient global assessment of response, Skindex29 score, health-related quality of life, and the Patient-Reported Outcomes Measurement Information System.
ARAMIS patients may also participate in CUTIS, the linked histopathologic and genetic investigation. More broad-ranging than ARAMIS, CUTIS is seeking 50 patients with several forms of idiopathic vasculitis, including cryoglobulinemic vasculitis, drug-induced vasculitis, eosinophilic granulomatosis with polyangiitis, IgA vasculitis, isolated cutaneous vasculitis, granulomatosis with polyangiitis, microscopic polyangiitis, polyarteritis nodosa, and urticarial vasculitis.
The study will examine histopathologic and transcriptomic characteristics in punch biopsies of the lesions. “We very much hope that gene expression profiling on these lesions will help define novel pathways and help us to classify and target therapies,” Dr. Pagnoux said.
To learn more about these studies and refer patients into them, visit the Rare Disease Network pages for ARAMIS and CUTIS.
Dr. Pagnoux had no financial disclosures relevant to either study.
SOURCE: Pagnoux C. CCR 2018
REPORTING FROM CCR 18