User login
High volume of noninvasive ventilation in hospitals doesn’t ensure good outcomes in acute COPD
Hospitals that frequently treat acute COPD exacerbations using noninvasive ventilation – a practice known to reduce mortality, length of stay, and the need for more invasive treatment – did not have better patient outcomes than did hospitals that used noninvasive ventilation less frequently, according to a report published in Annals of the American Thoracic Society.
Acute COPD exacerbations are “one of the few conditions with high-level evidence demonstrating the benefits of noninvasive ventilation in patients with respiratory distress,” and the treatment has been widely adopted for this patient population. However, for noninvasive ventilation to succeed, patients must be carefully selected and closely monitored, and a multidisciplinary team of nurses, respiratory therapists, and physicians must coordinate the treatment, often across multiple hospital settings, said Anuj B. Mehta, MD, of The Pulmonary Center, Boston University, and his associates.
Until now, it was not known whether hospitals with a high volume of noninvasive ventilation develop specialized expertise and thus deliver superior patient outcomes, or whether a high volume results from suboptimal patient selection or otherwise puts a strain on a hospital’s staff and thus produces poor outcomes. To examine this question, Dr. Mehta and his associates analyzed information in a database enrolling adults treated at 252 California hospitals for acute COPD exacerbation. They focused on 37,516 hospitalizations that occurred during a single year.
Overall, 9.3% of these patients received noninvasive ventilation. The median annual case volume of noninvasive ventilation for any indication was 64 per hospital. But rates of noninvasive ventilation varied widely across hospitals, with 40% of facilities significantly deviating from this median rate.
“Contrary to our hypothesis, we did not observe significantly lower COPD mortality” in hospitals with high volumes of noninvasive ventilation. For individual patients, admission to a hospital with a high volume of noninvasive ventilation was associated with significantly higher odds of treatment failure (adjusted OR, 1.95), and such failure was associated with significantly higher odds of death (adjusted OR, 1.81). In addition, at the hospital level, a high volume of noninvasive ventilation was associated with a significantly higher risk of treatment failure, which in turn was associated with higher patient mortality.
“Hospitals with higher total noninvasive ventilation case volume tended to use [it] in patients with more comorbidities and acute organ failures, suggesting potential overuse among patients at higher risk of treatment failure. ... [This] may partially explain why hospitals with high rates of using an evidence-based intervention did not achieve significant mortality benefits,” Dr. Mehta and his associates said (Ann Am Thorac Soc. 2016;13[10]:1752-9).
They added that the wide variation between hospitals in failure rates for noninvasive ventilation were likely attributable to unmeasured hospital factors, speculating that the site of treatment (regular ward vs. ICU); staffing ratios for nurses, respiratory therapists, and physicians; and the intensity of patient monitoring, such as the frequency of blood-gas measurement, may contribute.
“High rates of treatment failure at some hospitals suggest that further work is needed to maximize the real-world effectiveness of noninvasive ventilation, even for an indication [backed by] strong evidence,” the investigators said.
The National Institutes of Health; the National Heart, Lung, and Blood Institute; and Boston University supported the study. The investigators’ financial disclosures are available at www.atsjournals.org.
Eric Gartman, MD, FCCP, comments: It is unclear what conclusions can be drawn from this study given the likely heterogeneity between the included hospitals.
Eric Gartman, MD, FCCP, comments: It is unclear what conclusions can be drawn from this study given the likely heterogeneity between the included hospitals.
Eric Gartman, MD, FCCP, comments: It is unclear what conclusions can be drawn from this study given the likely heterogeneity between the included hospitals.
Hospitals that frequently treat acute COPD exacerbations using noninvasive ventilation – a practice known to reduce mortality, length of stay, and the need for more invasive treatment – did not have better patient outcomes than did hospitals that used noninvasive ventilation less frequently, according to a report published in Annals of the American Thoracic Society.
Acute COPD exacerbations are “one of the few conditions with high-level evidence demonstrating the benefits of noninvasive ventilation in patients with respiratory distress,” and the treatment has been widely adopted for this patient population. However, for noninvasive ventilation to succeed, patients must be carefully selected and closely monitored, and a multidisciplinary team of nurses, respiratory therapists, and physicians must coordinate the treatment, often across multiple hospital settings, said Anuj B. Mehta, MD, of The Pulmonary Center, Boston University, and his associates.
Until now, it was not known whether hospitals with a high volume of noninvasive ventilation develop specialized expertise and thus deliver superior patient outcomes, or whether a high volume results from suboptimal patient selection or otherwise puts a strain on a hospital’s staff and thus produces poor outcomes. To examine this question, Dr. Mehta and his associates analyzed information in a database enrolling adults treated at 252 California hospitals for acute COPD exacerbation. They focused on 37,516 hospitalizations that occurred during a single year.
Overall, 9.3% of these patients received noninvasive ventilation. The median annual case volume of noninvasive ventilation for any indication was 64 per hospital. But rates of noninvasive ventilation varied widely across hospitals, with 40% of facilities significantly deviating from this median rate.
“Contrary to our hypothesis, we did not observe significantly lower COPD mortality” in hospitals with high volumes of noninvasive ventilation. For individual patients, admission to a hospital with a high volume of noninvasive ventilation was associated with significantly higher odds of treatment failure (adjusted OR, 1.95), and such failure was associated with significantly higher odds of death (adjusted OR, 1.81). In addition, at the hospital level, a high volume of noninvasive ventilation was associated with a significantly higher risk of treatment failure, which in turn was associated with higher patient mortality.
“Hospitals with higher total noninvasive ventilation case volume tended to use [it] in patients with more comorbidities and acute organ failures, suggesting potential overuse among patients at higher risk of treatment failure. ... [This] may partially explain why hospitals with high rates of using an evidence-based intervention did not achieve significant mortality benefits,” Dr. Mehta and his associates said (Ann Am Thorac Soc. 2016;13[10]:1752-9).
They added that the wide variation between hospitals in failure rates for noninvasive ventilation were likely attributable to unmeasured hospital factors, speculating that the site of treatment (regular ward vs. ICU); staffing ratios for nurses, respiratory therapists, and physicians; and the intensity of patient monitoring, such as the frequency of blood-gas measurement, may contribute.
“High rates of treatment failure at some hospitals suggest that further work is needed to maximize the real-world effectiveness of noninvasive ventilation, even for an indication [backed by] strong evidence,” the investigators said.
The National Institutes of Health; the National Heart, Lung, and Blood Institute; and Boston University supported the study. The investigators’ financial disclosures are available at www.atsjournals.org.
Hospitals that frequently treat acute COPD exacerbations using noninvasive ventilation – a practice known to reduce mortality, length of stay, and the need for more invasive treatment – did not have better patient outcomes than did hospitals that used noninvasive ventilation less frequently, according to a report published in Annals of the American Thoracic Society.
Acute COPD exacerbations are “one of the few conditions with high-level evidence demonstrating the benefits of noninvasive ventilation in patients with respiratory distress,” and the treatment has been widely adopted for this patient population. However, for noninvasive ventilation to succeed, patients must be carefully selected and closely monitored, and a multidisciplinary team of nurses, respiratory therapists, and physicians must coordinate the treatment, often across multiple hospital settings, said Anuj B. Mehta, MD, of The Pulmonary Center, Boston University, and his associates.
Until now, it was not known whether hospitals with a high volume of noninvasive ventilation develop specialized expertise and thus deliver superior patient outcomes, or whether a high volume results from suboptimal patient selection or otherwise puts a strain on a hospital’s staff and thus produces poor outcomes. To examine this question, Dr. Mehta and his associates analyzed information in a database enrolling adults treated at 252 California hospitals for acute COPD exacerbation. They focused on 37,516 hospitalizations that occurred during a single year.
Overall, 9.3% of these patients received noninvasive ventilation. The median annual case volume of noninvasive ventilation for any indication was 64 per hospital. But rates of noninvasive ventilation varied widely across hospitals, with 40% of facilities significantly deviating from this median rate.
“Contrary to our hypothesis, we did not observe significantly lower COPD mortality” in hospitals with high volumes of noninvasive ventilation. For individual patients, admission to a hospital with a high volume of noninvasive ventilation was associated with significantly higher odds of treatment failure (adjusted OR, 1.95), and such failure was associated with significantly higher odds of death (adjusted OR, 1.81). In addition, at the hospital level, a high volume of noninvasive ventilation was associated with a significantly higher risk of treatment failure, which in turn was associated with higher patient mortality.
“Hospitals with higher total noninvasive ventilation case volume tended to use [it] in patients with more comorbidities and acute organ failures, suggesting potential overuse among patients at higher risk of treatment failure. ... [This] may partially explain why hospitals with high rates of using an evidence-based intervention did not achieve significant mortality benefits,” Dr. Mehta and his associates said (Ann Am Thorac Soc. 2016;13[10]:1752-9).
They added that the wide variation between hospitals in failure rates for noninvasive ventilation were likely attributable to unmeasured hospital factors, speculating that the site of treatment (regular ward vs. ICU); staffing ratios for nurses, respiratory therapists, and physicians; and the intensity of patient monitoring, such as the frequency of blood-gas measurement, may contribute.
“High rates of treatment failure at some hospitals suggest that further work is needed to maximize the real-world effectiveness of noninvasive ventilation, even for an indication [backed by] strong evidence,” the investigators said.
The National Institutes of Health; the National Heart, Lung, and Blood Institute; and Boston University supported the study. The investigators’ financial disclosures are available at www.atsjournals.org.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Key clinical point: Outcomes for patients with acute COPD exacerbations were no better at hospitals where noninvasive ventilation was frequently used.
Major finding: For individual patients, admission to a hospital with a high volume of noninvasive ventilation was associated with significantly higher odds of treatment failure (adjusted OR, 1.95), and such failure was associated with significantly higher odds of death (adjusted OR, 1.81).
Data source: A multicenter observational study involving 37,516 hospitalizations for COPD exacerbation at 252 California medical centers during a 1-year period.
Disclosures: The National Institutes of Health; the National Heart, Lung, and Blood Institute; and Boston University supported the study. The investigators’ financial disclosures are available at www.atsjournals.org.
Delayed cord clamping cuts anemia for 1 year
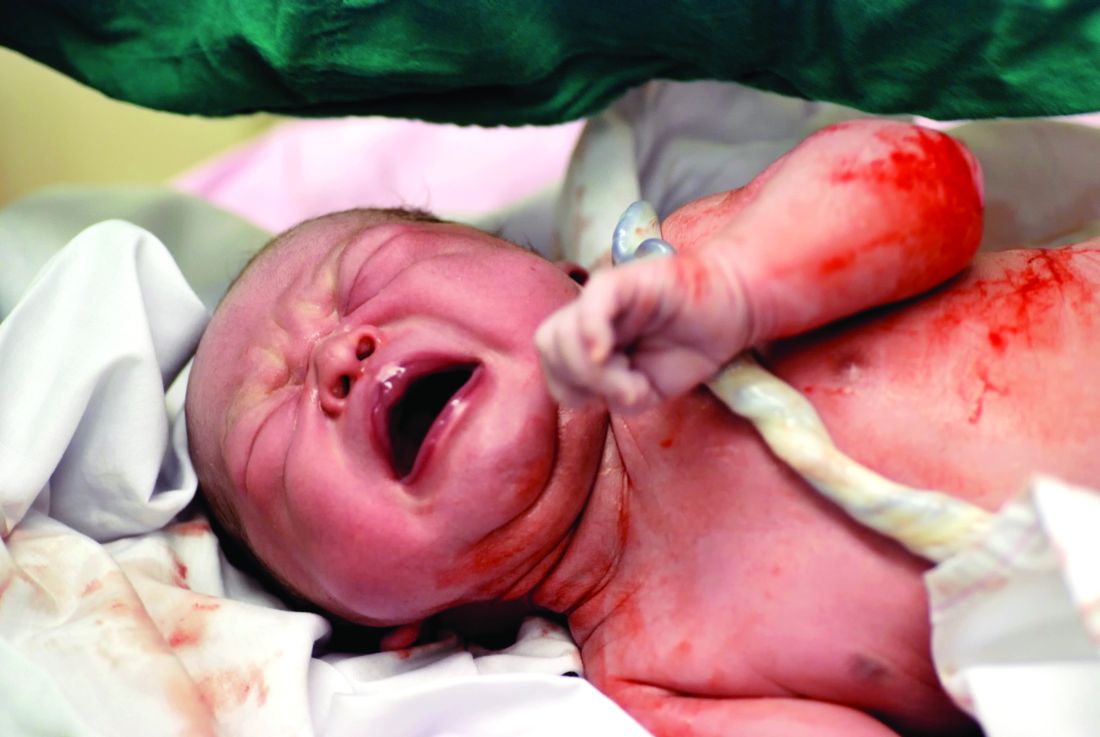
Delaying the clamping of the umbilical cord for 3 minutes after delivery decreased anemia for as long as 8-12 months in a population at high risk for the disorder, according to a report published online Jan. 17 in JAMA Pediatrics.
If early (within 1 minute of birth) cord clamping is avoided after delivery, fetoplacental blood moves into the newborn, augmenting his or her blood volume by 30%-40%. This is known to increase iron stores and prevent iron deficiency for at least 6 months, but until now there has been little evidence of how long this beneficial effect persists, said Ashish KC, MD, PhD, of International Maternal and Child Health, Uppsala (Sweden) University and the United Nations Children’s Fund, Lalitpur, Nepal, and associates.
The primary outcome measure – the hemoglobin level at 8 months of age – was a significant 0.2 g/dL higher after delayed clamping. Also, anemia was significantly less prevalent with delayed cord clamping (73.0% vs. 82.2%). This represents an 11% reduction in the risk of anemia and a 42% reduction in the risk of iron deficiency. “The relative risk for having iron deficiency anemia was 0.58, with a number needed to treat of 7,” Dr. KC and associates said (JAMA Ped. 2017 Jan 17. doi: 10.1001/jamapediatrics.2016.3971).
The benefits of delayed cord clamping persisted at 12 months of age; the mean hemoglobin was 0.3 g/dL higher in the delayed group. Anemia was less prevalent in the delayed clamping group; the relative risk was 0.91, the investigators said.
If this intervention were implemented worldwide, “this could translate to 5 million fewer infants with anemia at 8 months of age, with particular public health significance in South Asia and sub-Saharan Africa, where the prevalence of anemia is highest,” the investigators said. This study was supported by the Midwifery Society of Nepal, the Swedish Society of Medicine, the Little Child’s Foundation, the Swedish Society of Medical Research, and the United Nations Children’s Fund. Dr. KC and associates reported having no relevant financial disclosures.
Delaying the clamping of the umbilical cord for 3 minutes after delivery decreased anemia for as long as 8-12 months in a population at high risk for the disorder, according to a report published online Jan. 17 in JAMA Pediatrics.
If early (within 1 minute of birth) cord clamping is avoided after delivery, fetoplacental blood moves into the newborn, augmenting his or her blood volume by 30%-40%. This is known to increase iron stores and prevent iron deficiency for at least 6 months, but until now there has been little evidence of how long this beneficial effect persists, said Ashish KC, MD, PhD, of International Maternal and Child Health, Uppsala (Sweden) University and the United Nations Children’s Fund, Lalitpur, Nepal, and associates.
The primary outcome measure – the hemoglobin level at 8 months of age – was a significant 0.2 g/dL higher after delayed clamping. Also, anemia was significantly less prevalent with delayed cord clamping (73.0% vs. 82.2%). This represents an 11% reduction in the risk of anemia and a 42% reduction in the risk of iron deficiency. “The relative risk for having iron deficiency anemia was 0.58, with a number needed to treat of 7,” Dr. KC and associates said (JAMA Ped. 2017 Jan 17. doi: 10.1001/jamapediatrics.2016.3971).
The benefits of delayed cord clamping persisted at 12 months of age; the mean hemoglobin was 0.3 g/dL higher in the delayed group. Anemia was less prevalent in the delayed clamping group; the relative risk was 0.91, the investigators said.
If this intervention were implemented worldwide, “this could translate to 5 million fewer infants with anemia at 8 months of age, with particular public health significance in South Asia and sub-Saharan Africa, where the prevalence of anemia is highest,” the investigators said. This study was supported by the Midwifery Society of Nepal, the Swedish Society of Medicine, the Little Child’s Foundation, the Swedish Society of Medical Research, and the United Nations Children’s Fund. Dr. KC and associates reported having no relevant financial disclosures.
Delaying the clamping of the umbilical cord for 3 minutes after delivery decreased anemia for as long as 8-12 months in a population at high risk for the disorder, according to a report published online Jan. 17 in JAMA Pediatrics.
If early (within 1 minute of birth) cord clamping is avoided after delivery, fetoplacental blood moves into the newborn, augmenting his or her blood volume by 30%-40%. This is known to increase iron stores and prevent iron deficiency for at least 6 months, but until now there has been little evidence of how long this beneficial effect persists, said Ashish KC, MD, PhD, of International Maternal and Child Health, Uppsala (Sweden) University and the United Nations Children’s Fund, Lalitpur, Nepal, and associates.
The primary outcome measure – the hemoglobin level at 8 months of age – was a significant 0.2 g/dL higher after delayed clamping. Also, anemia was significantly less prevalent with delayed cord clamping (73.0% vs. 82.2%). This represents an 11% reduction in the risk of anemia and a 42% reduction in the risk of iron deficiency. “The relative risk for having iron deficiency anemia was 0.58, with a number needed to treat of 7,” Dr. KC and associates said (JAMA Ped. 2017 Jan 17. doi: 10.1001/jamapediatrics.2016.3971).
The benefits of delayed cord clamping persisted at 12 months of age; the mean hemoglobin was 0.3 g/dL higher in the delayed group. Anemia was less prevalent in the delayed clamping group; the relative risk was 0.91, the investigators said.
If this intervention were implemented worldwide, “this could translate to 5 million fewer infants with anemia at 8 months of age, with particular public health significance in South Asia and sub-Saharan Africa, where the prevalence of anemia is highest,” the investigators said. This study was supported by the Midwifery Society of Nepal, the Swedish Society of Medicine, the Little Child’s Foundation, the Swedish Society of Medical Research, and the United Nations Children’s Fund. Dr. KC and associates reported having no relevant financial disclosures.
FROM JAMA PEDIATRICS
Key clinical point: Delaying the clamping of the umbilical cord for 3 minutes decreased anemia for as long as 8-12 months in a population at high risk for the disorder.
Major finding: Delayed cord clamping yielded an 11% reduction in the risk of anemia and a 42% reduction in the risk of iron deficiency, compared with early cord clamping.
Data source: A prospective randomized trial involving 540 term and late preterm infants born in Nepal during a 7-week period
Disclosures: This study was supported by the Midwifery Society of Nepal, the Swedish Society of Medicine, the Little Child’s Foundation, the Swedish Society of Medical Research, and the United Nations Children’s Fund. Dr. KC and associates reported having no relevant financial disclosures.
Asthma ruled out in 33% of diagnosed adults
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
“The study by Aaron [et al.] is an important reminder that in addition to reviewing asthma symptoms and treatment, trying to understand if the diagnosis is still appropriate is an important part of clinical care.”
The study gives clinicians two insights: First, adults diagnosed as having asthma may not continue to have the disease years later, or at least may not require treatment indefinitely. And second, physiological testing is an essential component of diagnosis and will help avoid unnecessary treatment and missed alternative causes for signs and symptoms.
Helen M. Hollingsworth, MD, and George T. O’Connor, MD, are at the Pulmonary Center at Boston University. Dr. O’Connor is an associate editor of JAMA. He reported serving as a consultant for AstraZeneca and receiving grants from Janssen Pharmaceuticals. Dr. Hollingsworth and Dr. O’Connor made these remarks in an editorial accompanying Dr. Aaron’s report (JAMA 2017;317[3]:262-3).
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician during the preceding 5 years, according to a report published online Jan. 17 in JAMA.
In a prospective multicenter cohort study involving 613 asthma patients, 203 had no evidence of current asthma when they underwent serial assessments of respiratory symptoms, lung function, and bronchial provocation testing while not taking asthma medications. More than 90% of these 203 participants safely refrained from using the medications for an additional 1-year follow-up period, said Shawn D. Aaron, MD, of Ottawa (Ont.) Hospital Research Institute, and his associates in the Canadian Respiratory Research Network.
Some of these patients were likely misdiagnosed initially and some likely experienced remission since their initial diagnosis. Either way, reassessing asthma diagnoses may be warranted in many patients, the investigators said (JAMA 2017;317[3]:269-79).
To assess whether some patients could safely discontinue asthma medications because they no longer had the disease, the researchers performed a random sampling of the general adult population (approximately 17,000 people) living in urban, suburban, or rural areas in and around the 10 largest cities in Canada during a 3-year period. Those who reported that a member of the household had been diagnosed as having asthma within the previous 5 years were invited to participate in the study.
A total of 613 men and women (mean age, 51 years) completed the study, undergoing spirometry to assess airflow obstruction, methacholine challenges to assess airway hyperresponsiveness, clinical examination by a pulmonologist, and, if indicated, tapering and discontinuation of asthma medications. Those in whom asthma was ruled out were closely followed for 1 year, undergoing repeat bronchial challenge testing and reporting any worsening of asthma signs and symptoms.
At baseline, 87% of the participants said that they had recently used asthma medications and 45% said they used such medications daily. The remainder had already stopped using asthma medications, an indication that many patients can tell when their asthma has remitted (or was never present) and may adjust their medication use with or without a physician’s guidance, Dr. Aaron and his associates said.
Current asthma was confirmed in 62.3% of the study participants. The primary study outcome – the proportion of patients in whom a current asthma diagnosis was ruled out – was 33.1%, or 203 patients. Only 44% of these participants who did not have current asthma had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed. This indicates that “whenever possible, physicians should order objective tests, such as prebronchodilator and postbronchodilator spirometry, serial peak flow measurements, or bronchial challenge tests, to confirm asthma at the time of initial diagnosis,” the investigators said.
A total of 35% of the participants in whom asthma was ruled out had been using daily asthma medications. “Use of asthma medications in these patients presumably provided only risks for medication adverse effects and cost, with little opportunity for therapeutic benefit,” the researchers noted. Twelve patients – 2% of the study population – were found to have serious cardiorespiratory conditions that had been misdiagnosed as asthma: four people with ischemic heart disease (two requiring percutaneous coronary intervention), two with subglottic stenosis (both requiring airway dilation procedures), two with bronchiectasis, and one each with interstitial lung disease, pulmonary hypertension, sarcoidosis, and tracheobronchomalacia.
During the additional year of follow-up, 22 of the 203 patients in whom asthma had been ruled out had a positive bronchial challenge test result at 6 or 12 months. Six resumed using asthma medications, one was treated with a brief course of oral corticosteroid, and the others did not require asthma medications.
The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Asthma was ruled out in 33% of adults in the general Canadian population who had been diagnosed by a physician.
Major finding: Only 44% of the participants in whom asthma was ruled out had undergone objective testing before their initial diagnosis, compared with 56% of patients in whom asthma was confirmed.
Data source: A prospective multicenter cohort study involving 613 adults who had been diagnosed as having asthma during the preceding 5 years.
Disclosures: The Canadian Institutes of Health Research supported the study. Methapharm provided provocholine and Trudell Medical International provided the peak flow meters used in the study. Dr. Aaron reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
All systemic steroid guidelines for RA offer little guidance
Every current guideline and consensus statement regarding systemic glucocorticoid therapy in rheumatoid arthritis falls short of offering clinicians practical, evidence-based guidance, according to a systematic review of all 15 such documents published in 2011-2015 in English, French, German, and Spanish.
Despite the abundant and convincing evidence that systemic glucocorticoids are very beneficial and despite their widespread use for rheumatoid arthritis (RA), fewer than half of the existing guidelines explicitly recommend these agents. Even fewer specify the optimal treatment duration, and about one-third don’t even provide dosage recommendations.
Their report, published in Arthritis Care & Research, does not address intra-articular administration of glucocorticoids. The 15 sets of guidelines were developed by 13 different rheumatology associations and networks across five continents (Arthritis Care Res. 2016 Dec 28. doi: 10.1002/acr.23185).
In this comprehensive review of 3,742 relevant publications, there was general agreement that initial treatment should center on disease-modifying antirheumatic drugs (DMARDs) plus systemic glucocorticoids. If remission, or at least reduced disease activity with preserved function, doesn’t occur within 3-6 months, another synthetic or a biologic DMARD should be tried, with or without concomitant glucocorticoids. But none of the guidelines adequately address the timing or frequency of use, either in early RA or in established disease.
All the guidelines refer to using “low-dose” or the “lowest possible dose” to avoid adverse effects, but what they mean by that is unclear. Some define “low-dose” as less than or equal to 7.5 mg per day, but others define it at higher levels, up to 15 mg per day. “Moderate” and “high” doses are not defined at all, and some guidelines refer to unspecified “low to moderate doses” or “moderately high” doses. This causes “considerable confusion for the practicing clinician and investigators,” Mr. Palmowski and his associates noted.
Similarly, the guidelines are unclear as to which routes of administration are preferred, or are even acceptable. Most specifically mention oral systemic glucocorticoids; four mention the intramuscular route, with one guideline calling this preferable “in certain situations” because it facilitates better dosing control. One guideline recommends intravenous glucocorticoids, but only for severe extra-articular manifestations. And another advocates parenteral methylprednisolone in patients who have “weaning difficulties,” but gives no further details about using this route.
The guidelines all are similarly vague regarding the duration of glucocorticoid therapy. Two do not address treatment duration at all; the other 13 recommend “short-term” or “the shortest possible duration,” but do not adequately define these terms. Six guidelines discuss maximum treatment duration, but they define that as anywhere from 3 to 24 months. Only 4 of the 15 guidelines discuss treatment periods of longer than 6 months, and all of them are vague and sometimes contradict each other regarding such prolonged use.
The daily timing of glucocorticoid therapy is virtually ignored in all the guidelines, even though researchers have known for more than 50 years that the time of day when these agents are taken has a considerable impact on their efficacy and safety. Similarly, the use of delayed-release prednisone and the question of single versus multiple doses per day “remain to be tackled,” Mr. Palmowski and his associates said.
Tapering of glucocorticoid therapy to avoid triggering a recurrence is another topic given short shrift. Five of the guidelines don’t address it at all, and another five merely state that glucocorticoids should be tapered “as rapidly as possible.” The three guidelines that do stress the importance of gradual dose reductions offer conflicting advice about accomplishing that.
Existing guidelines also neglect to address patient-specific factors that may require special attention. Two explicitly allow the use of these agents during pregnancy, but the other 13 do not even consider the question. Three guidelines address possible interactions with other medications, but the other 12 do not. Only one guideline discusses the use of glucocorticoids in relation to common RA-associated cormorbidities such as cardiovascular disease and diabetes. And none of the guidelines make any age- or sex-specific recommendations.
Finally, many of the current guidelines fail to fully identify their funding sources. Five that never mentioned funding at all “were published (and probably funded) by rheumatology associations.” And at least two of the guidelines that ignored funding sources did receive support from pharmaceutical companies, Mr. Palmowski and his associates said.
This study was sponsored by the GLORIA (Glucocorticoid Low-dose Outcome in RA) Project of the European Commission’s Horizon 2020 Initiative. Mr. Palmowski reported having no relevant financial disclosures; his associates reported ties to Merck, Roche, Mundipharma, Pfizer, SUN, Novartis, Bristol-Myers Squibb, Takeda, UCB, Serono, and Horizon Pharma.
Some may read the report by Mr. Palmowski and his colleagues and fault the people who wrote the guidelines for these inadequacies. But the true culprit is the lack of good data on which to base guidelines.
And the data are lacking mainly because there is no funding for studies of dosing, treatment duration, timing of therapy, and patient-specific factors. Glucocorticoids are old drugs, so no industry sources would consider laying out the multiple millions of dollars necessary to conduct adequate research. Moreover, as many as 60% of all patients who could participate in such studies are already taking these agents, and this makes it difficult to determine their unique risk profiles.
Tina D. Mahajan, MD, is in the division of rheumatology and immunology at the University of Nebraska, Omaha. James R. O’Dell, MD, is chief of that division. The authors’ financial disclosures were not provided. Dr. Mahajan and Dr. O’Dell made these remarks in an editorial accompanying the report by Mr. Palmowski and his associates (Arthritis Care Res. 2016 Dec 28. doi: 10.1002/acr.23184).
Some may read the report by Mr. Palmowski and his colleagues and fault the people who wrote the guidelines for these inadequacies. But the true culprit is the lack of good data on which to base guidelines.
And the data are lacking mainly because there is no funding for studies of dosing, treatment duration, timing of therapy, and patient-specific factors. Glucocorticoids are old drugs, so no industry sources would consider laying out the multiple millions of dollars necessary to conduct adequate research. Moreover, as many as 60% of all patients who could participate in such studies are already taking these agents, and this makes it difficult to determine their unique risk profiles.
Tina D. Mahajan, MD, is in the division of rheumatology and immunology at the University of Nebraska, Omaha. James R. O’Dell, MD, is chief of that division. The authors’ financial disclosures were not provided. Dr. Mahajan and Dr. O’Dell made these remarks in an editorial accompanying the report by Mr. Palmowski and his associates (Arthritis Care Res. 2016 Dec 28. doi: 10.1002/acr.23184).
Some may read the report by Mr. Palmowski and his colleagues and fault the people who wrote the guidelines for these inadequacies. But the true culprit is the lack of good data on which to base guidelines.
And the data are lacking mainly because there is no funding for studies of dosing, treatment duration, timing of therapy, and patient-specific factors. Glucocorticoids are old drugs, so no industry sources would consider laying out the multiple millions of dollars necessary to conduct adequate research. Moreover, as many as 60% of all patients who could participate in such studies are already taking these agents, and this makes it difficult to determine their unique risk profiles.
Tina D. Mahajan, MD, is in the division of rheumatology and immunology at the University of Nebraska, Omaha. James R. O’Dell, MD, is chief of that division. The authors’ financial disclosures were not provided. Dr. Mahajan and Dr. O’Dell made these remarks in an editorial accompanying the report by Mr. Palmowski and his associates (Arthritis Care Res. 2016 Dec 28. doi: 10.1002/acr.23184).
Every current guideline and consensus statement regarding systemic glucocorticoid therapy in rheumatoid arthritis falls short of offering clinicians practical, evidence-based guidance, according to a systematic review of all 15 such documents published in 2011-2015 in English, French, German, and Spanish.
Despite the abundant and convincing evidence that systemic glucocorticoids are very beneficial and despite their widespread use for rheumatoid arthritis (RA), fewer than half of the existing guidelines explicitly recommend these agents. Even fewer specify the optimal treatment duration, and about one-third don’t even provide dosage recommendations.
Their report, published in Arthritis Care & Research, does not address intra-articular administration of glucocorticoids. The 15 sets of guidelines were developed by 13 different rheumatology associations and networks across five continents (Arthritis Care Res. 2016 Dec 28. doi: 10.1002/acr.23185).
In this comprehensive review of 3,742 relevant publications, there was general agreement that initial treatment should center on disease-modifying antirheumatic drugs (DMARDs) plus systemic glucocorticoids. If remission, or at least reduced disease activity with preserved function, doesn’t occur within 3-6 months, another synthetic or a biologic DMARD should be tried, with or without concomitant glucocorticoids. But none of the guidelines adequately address the timing or frequency of use, either in early RA or in established disease.
All the guidelines refer to using “low-dose” or the “lowest possible dose” to avoid adverse effects, but what they mean by that is unclear. Some define “low-dose” as less than or equal to 7.5 mg per day, but others define it at higher levels, up to 15 mg per day. “Moderate” and “high” doses are not defined at all, and some guidelines refer to unspecified “low to moderate doses” or “moderately high” doses. This causes “considerable confusion for the practicing clinician and investigators,” Mr. Palmowski and his associates noted.
Similarly, the guidelines are unclear as to which routes of administration are preferred, or are even acceptable. Most specifically mention oral systemic glucocorticoids; four mention the intramuscular route, with one guideline calling this preferable “in certain situations” because it facilitates better dosing control. One guideline recommends intravenous glucocorticoids, but only for severe extra-articular manifestations. And another advocates parenteral methylprednisolone in patients who have “weaning difficulties,” but gives no further details about using this route.
The guidelines all are similarly vague regarding the duration of glucocorticoid therapy. Two do not address treatment duration at all; the other 13 recommend “short-term” or “the shortest possible duration,” but do not adequately define these terms. Six guidelines discuss maximum treatment duration, but they define that as anywhere from 3 to 24 months. Only 4 of the 15 guidelines discuss treatment periods of longer than 6 months, and all of them are vague and sometimes contradict each other regarding such prolonged use.
The daily timing of glucocorticoid therapy is virtually ignored in all the guidelines, even though researchers have known for more than 50 years that the time of day when these agents are taken has a considerable impact on their efficacy and safety. Similarly, the use of delayed-release prednisone and the question of single versus multiple doses per day “remain to be tackled,” Mr. Palmowski and his associates said.
Tapering of glucocorticoid therapy to avoid triggering a recurrence is another topic given short shrift. Five of the guidelines don’t address it at all, and another five merely state that glucocorticoids should be tapered “as rapidly as possible.” The three guidelines that do stress the importance of gradual dose reductions offer conflicting advice about accomplishing that.
Existing guidelines also neglect to address patient-specific factors that may require special attention. Two explicitly allow the use of these agents during pregnancy, but the other 13 do not even consider the question. Three guidelines address possible interactions with other medications, but the other 12 do not. Only one guideline discusses the use of glucocorticoids in relation to common RA-associated cormorbidities such as cardiovascular disease and diabetes. And none of the guidelines make any age- or sex-specific recommendations.
Finally, many of the current guidelines fail to fully identify their funding sources. Five that never mentioned funding at all “were published (and probably funded) by rheumatology associations.” And at least two of the guidelines that ignored funding sources did receive support from pharmaceutical companies, Mr. Palmowski and his associates said.
This study was sponsored by the GLORIA (Glucocorticoid Low-dose Outcome in RA) Project of the European Commission’s Horizon 2020 Initiative. Mr. Palmowski reported having no relevant financial disclosures; his associates reported ties to Merck, Roche, Mundipharma, Pfizer, SUN, Novartis, Bristol-Myers Squibb, Takeda, UCB, Serono, and Horizon Pharma.
Every current guideline and consensus statement regarding systemic glucocorticoid therapy in rheumatoid arthritis falls short of offering clinicians practical, evidence-based guidance, according to a systematic review of all 15 such documents published in 2011-2015 in English, French, German, and Spanish.
Despite the abundant and convincing evidence that systemic glucocorticoids are very beneficial and despite their widespread use for rheumatoid arthritis (RA), fewer than half of the existing guidelines explicitly recommend these agents. Even fewer specify the optimal treatment duration, and about one-third don’t even provide dosage recommendations.
Their report, published in Arthritis Care & Research, does not address intra-articular administration of glucocorticoids. The 15 sets of guidelines were developed by 13 different rheumatology associations and networks across five continents (Arthritis Care Res. 2016 Dec 28. doi: 10.1002/acr.23185).
In this comprehensive review of 3,742 relevant publications, there was general agreement that initial treatment should center on disease-modifying antirheumatic drugs (DMARDs) plus systemic glucocorticoids. If remission, or at least reduced disease activity with preserved function, doesn’t occur within 3-6 months, another synthetic or a biologic DMARD should be tried, with or without concomitant glucocorticoids. But none of the guidelines adequately address the timing or frequency of use, either in early RA or in established disease.
All the guidelines refer to using “low-dose” or the “lowest possible dose” to avoid adverse effects, but what they mean by that is unclear. Some define “low-dose” as less than or equal to 7.5 mg per day, but others define it at higher levels, up to 15 mg per day. “Moderate” and “high” doses are not defined at all, and some guidelines refer to unspecified “low to moderate doses” or “moderately high” doses. This causes “considerable confusion for the practicing clinician and investigators,” Mr. Palmowski and his associates noted.
Similarly, the guidelines are unclear as to which routes of administration are preferred, or are even acceptable. Most specifically mention oral systemic glucocorticoids; four mention the intramuscular route, with one guideline calling this preferable “in certain situations” because it facilitates better dosing control. One guideline recommends intravenous glucocorticoids, but only for severe extra-articular manifestations. And another advocates parenteral methylprednisolone in patients who have “weaning difficulties,” but gives no further details about using this route.
The guidelines all are similarly vague regarding the duration of glucocorticoid therapy. Two do not address treatment duration at all; the other 13 recommend “short-term” or “the shortest possible duration,” but do not adequately define these terms. Six guidelines discuss maximum treatment duration, but they define that as anywhere from 3 to 24 months. Only 4 of the 15 guidelines discuss treatment periods of longer than 6 months, and all of them are vague and sometimes contradict each other regarding such prolonged use.
The daily timing of glucocorticoid therapy is virtually ignored in all the guidelines, even though researchers have known for more than 50 years that the time of day when these agents are taken has a considerable impact on their efficacy and safety. Similarly, the use of delayed-release prednisone and the question of single versus multiple doses per day “remain to be tackled,” Mr. Palmowski and his associates said.
Tapering of glucocorticoid therapy to avoid triggering a recurrence is another topic given short shrift. Five of the guidelines don’t address it at all, and another five merely state that glucocorticoids should be tapered “as rapidly as possible.” The three guidelines that do stress the importance of gradual dose reductions offer conflicting advice about accomplishing that.
Existing guidelines also neglect to address patient-specific factors that may require special attention. Two explicitly allow the use of these agents during pregnancy, but the other 13 do not even consider the question. Three guidelines address possible interactions with other medications, but the other 12 do not. Only one guideline discusses the use of glucocorticoids in relation to common RA-associated cormorbidities such as cardiovascular disease and diabetes. And none of the guidelines make any age- or sex-specific recommendations.
Finally, many of the current guidelines fail to fully identify their funding sources. Five that never mentioned funding at all “were published (and probably funded) by rheumatology associations.” And at least two of the guidelines that ignored funding sources did receive support from pharmaceutical companies, Mr. Palmowski and his associates said.
This study was sponsored by the GLORIA (Glucocorticoid Low-dose Outcome in RA) Project of the European Commission’s Horizon 2020 Initiative. Mr. Palmowski reported having no relevant financial disclosures; his associates reported ties to Merck, Roche, Mundipharma, Pfizer, SUN, Novartis, Bristol-Myers Squibb, Takeda, UCB, Serono, and Horizon Pharma.
Key clinical point:
Major finding: All 15 guidelines refer to using “low-dose” or the “lowest possible dose” of glucocorticoids to avoid adverse effects, but some define that as 7.5 mg per day while others define it at higher levels, all the way up to 15 mg per day.
Data source: A comprehensive, systematic review of all 15 guidelines and consensus statements regarding glucocorticoid therapy in RA that were published in 2011-2015 in English, French, German, and Spanish.
Disclosures: This study was sponsored by the GLORIA (Glucocorticoid Low-dose Outcome in RA) Project of the European Commission’s Horizon 2020 Initiative. Mr. Palmowski reported having no relevant financial disclosures; his associates reported ties to Merck, Roche, Mundipharma, Pfizer, SUN, Novartis, Bristol-Myers Squibb, Takeda, UCB, Serono, and Horizon Pharma.
177Lu-Dotatate for advanced midgut neuroendocrine tumors
177Lu-Dotatate, a radionuclide related to octreotide, reduced the risk of disease progression or death by 79% in a phase III trial involving 229 adults with advanced, progressive midgut neuroendocrine tumors, investigators reported in the New England Journal of Medicine.
“The response rate of 18% in the 177Lu-Dotatate group (as compared with 3% in the control group) is also notable, given that response rates above 5% have not been observed in large randomized clinical trials of other systemic therapies in this patient population,” said Jonathan R. Strosberg, MD, of the Moffitt Cancer Center, Tampa, and his associates.
All the study participants had metastatic disease that progressed despite first-line treatment using octreotide LAR; 80% had also undergone surgical resection, and nearly half had received some other form of systemic therapy before entering this study. The primary tumor site was the ileum in most patients, and most also had metastases in the liver, the lymph nodes, or both, “typically in the mesentery or retroperitoneum.”
The primary efficacy endpoint – the estimated rate of progression-free survival at month 20 – was 65.2% in the intervention group, compared with 10.8% in the control group. This represents a 79% lower risk of disease progression or death with 177Lu-Dotatate. An interim analysis of overall survival (an endpoint that cannot be determined definitively until more time has passed) showed a 60% lower risk of death in the intervention group than in the control group. Treatment response rates were 18% and 3%, respectively, Dr. Strosberg and his associates reported (New Engl J Med. 2017 Jan 12. doi:10.1056/NEJMoa1607427).
177Lu-Dotatate was administered together with a renal-protection agent, and there has been no evidence of renal toxic effects to date. Rates of low-grade hematologic toxicities were low. The most common adverse effects were nausea and vomiting, which were largely attributed to the renal-protection agent, as well as fatigue, asthenia, abdominal pain, and diarrhea. Adverse events leading to premature withdrawal from the trial developed in more of the control group (9%) than of the intervention group (6%).
177Lu-Dotatate, a radionuclide related to octreotide, reduced the risk of disease progression or death by 79% in a phase III trial involving 229 adults with advanced, progressive midgut neuroendocrine tumors, investigators reported in the New England Journal of Medicine.
“The response rate of 18% in the 177Lu-Dotatate group (as compared with 3% in the control group) is also notable, given that response rates above 5% have not been observed in large randomized clinical trials of other systemic therapies in this patient population,” said Jonathan R. Strosberg, MD, of the Moffitt Cancer Center, Tampa, and his associates.
All the study participants had metastatic disease that progressed despite first-line treatment using octreotide LAR; 80% had also undergone surgical resection, and nearly half had received some other form of systemic therapy before entering this study. The primary tumor site was the ileum in most patients, and most also had metastases in the liver, the lymph nodes, or both, “typically in the mesentery or retroperitoneum.”
The primary efficacy endpoint – the estimated rate of progression-free survival at month 20 – was 65.2% in the intervention group, compared with 10.8% in the control group. This represents a 79% lower risk of disease progression or death with 177Lu-Dotatate. An interim analysis of overall survival (an endpoint that cannot be determined definitively until more time has passed) showed a 60% lower risk of death in the intervention group than in the control group. Treatment response rates were 18% and 3%, respectively, Dr. Strosberg and his associates reported (New Engl J Med. 2017 Jan 12. doi:10.1056/NEJMoa1607427).
177Lu-Dotatate was administered together with a renal-protection agent, and there has been no evidence of renal toxic effects to date. Rates of low-grade hematologic toxicities were low. The most common adverse effects were nausea and vomiting, which were largely attributed to the renal-protection agent, as well as fatigue, asthenia, abdominal pain, and diarrhea. Adverse events leading to premature withdrawal from the trial developed in more of the control group (9%) than of the intervention group (6%).
177Lu-Dotatate, a radionuclide related to octreotide, reduced the risk of disease progression or death by 79% in a phase III trial involving 229 adults with advanced, progressive midgut neuroendocrine tumors, investigators reported in the New England Journal of Medicine.
“The response rate of 18% in the 177Lu-Dotatate group (as compared with 3% in the control group) is also notable, given that response rates above 5% have not been observed in large randomized clinical trials of other systemic therapies in this patient population,” said Jonathan R. Strosberg, MD, of the Moffitt Cancer Center, Tampa, and his associates.
All the study participants had metastatic disease that progressed despite first-line treatment using octreotide LAR; 80% had also undergone surgical resection, and nearly half had received some other form of systemic therapy before entering this study. The primary tumor site was the ileum in most patients, and most also had metastases in the liver, the lymph nodes, or both, “typically in the mesentery or retroperitoneum.”
The primary efficacy endpoint – the estimated rate of progression-free survival at month 20 – was 65.2% in the intervention group, compared with 10.8% in the control group. This represents a 79% lower risk of disease progression or death with 177Lu-Dotatate. An interim analysis of overall survival (an endpoint that cannot be determined definitively until more time has passed) showed a 60% lower risk of death in the intervention group than in the control group. Treatment response rates were 18% and 3%, respectively, Dr. Strosberg and his associates reported (New Engl J Med. 2017 Jan 12. doi:10.1056/NEJMoa1607427).
177Lu-Dotatate was administered together with a renal-protection agent, and there has been no evidence of renal toxic effects to date. Rates of low-grade hematologic toxicities were low. The most common adverse effects were nausea and vomiting, which were largely attributed to the renal-protection agent, as well as fatigue, asthenia, abdominal pain, and diarrhea. Adverse events leading to premature withdrawal from the trial developed in more of the control group (9%) than of the intervention group (6%).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: 177Lu-Dotatate cuts the risk of disease progression or death by 79% in patients with advanced progressive midgut neuroendocrine tumors.
Major finding: The primary efficacy endpoint – the estimated rate of progression-free survival at month 20 – was 65.2% in the intervention group, compared with 10.8% in the control group.
Data source: An international, open-label phase III randomized clinical trial involving 229 adults followed for a median of 14 months.
Disclosures: This study was sponsored by Advanced Accelerator Applications. Dr. Strosberg reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
USPSTF reaffirms need for folic acid supplements in pregnancy
The U.S. Preventive Services Task Force continues to recommend that all women planning or capable of pregnancy should take a daily supplement of 0.4-0.8 mg of folic acid to prevent neural tube defects in their offspring.
The task force “concludes with high certainty” that the benefits of such supplementation are substantial and the harms are minimal, according to the recommendation statement published online Jan. 10 in JAMA (2017;317[2]:183-9). The group based its updated recommendation on a systematic review of 24 studies performed since 2009 and involving 58,860 women. Although some newer studies have suggested that supplementation is no longer needed in this era of folic acid fortification of foods, “the USPSTF found no new substantial evidence ... that would lead to a change in its recommendation from 2009,” the researchers wrote.
The most recent data estimate that folic acid supplementation prevents neural tube defects in approximately 1,300 births each year.
This updated USPSTF recommendation is in accord with recommendations from the Health and Medicine Division of the National Academies (formerly the Institute of Medicine), the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the U.S. Public Health Service, the Centers for Disease Control and Prevention, the American Academy of Pediatrics, the American Academy of Neurology, and the American College of Medical Genetics and Genomics.
This work was supported solely by the USPSTF, an independent voluntary group mandated by Congress to assess preventive care services and funded by the Agency for Healthcare Research and Quality.
The USPSTF recommendation that all women of childbearing age take folic acid supplements is a prudent one. Ideally, it will educate all women who are planning or capable of pregnancy to follow this recommendation and thereby reduce the risk of these severe birth defects in their infants.
Should the USPSTF recommendation be rejected because fortified food is already providing sufficient folic acid to prevent neural tube defects? No. Too little is known about how folic acid prevents neural tube defects. For example, it is not known whether the tissue stores of folate in the developing embryo or the availability of folate in the serum during the all-important few days of neural tube closure is most important. Habitual use of folic acid supplements is a more reliable method of ensuring adequate levels than is diet. In theory, a woman might not consume sufficient enriched cereal grains during the critical period of approximately 1 week when the neural tube is closing. Exactly when folate must be available also is not known. In addition, some popular diets, such as low carbohydrate or gluten free, may reduce exposure to grains, limiting folic acid intake.
James L. Mills, MD, is in the Division of Intramural Population Health Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md. He reported having no relevant financial disclosures. These comments are adapted from an editorial accompanying the USPSTF report (JAMA. 2017;317 [2]:144-5).
The USPSTF recommendation that all women of childbearing age take folic acid supplements is a prudent one. Ideally, it will educate all women who are planning or capable of pregnancy to follow this recommendation and thereby reduce the risk of these severe birth defects in their infants.
Should the USPSTF recommendation be rejected because fortified food is already providing sufficient folic acid to prevent neural tube defects? No. Too little is known about how folic acid prevents neural tube defects. For example, it is not known whether the tissue stores of folate in the developing embryo or the availability of folate in the serum during the all-important few days of neural tube closure is most important. Habitual use of folic acid supplements is a more reliable method of ensuring adequate levels than is diet. In theory, a woman might not consume sufficient enriched cereal grains during the critical period of approximately 1 week when the neural tube is closing. Exactly when folate must be available also is not known. In addition, some popular diets, such as low carbohydrate or gluten free, may reduce exposure to grains, limiting folic acid intake.
James L. Mills, MD, is in the Division of Intramural Population Health Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md. He reported having no relevant financial disclosures. These comments are adapted from an editorial accompanying the USPSTF report (JAMA. 2017;317 [2]:144-5).
The USPSTF recommendation that all women of childbearing age take folic acid supplements is a prudent one. Ideally, it will educate all women who are planning or capable of pregnancy to follow this recommendation and thereby reduce the risk of these severe birth defects in their infants.
Should the USPSTF recommendation be rejected because fortified food is already providing sufficient folic acid to prevent neural tube defects? No. Too little is known about how folic acid prevents neural tube defects. For example, it is not known whether the tissue stores of folate in the developing embryo or the availability of folate in the serum during the all-important few days of neural tube closure is most important. Habitual use of folic acid supplements is a more reliable method of ensuring adequate levels than is diet. In theory, a woman might not consume sufficient enriched cereal grains during the critical period of approximately 1 week when the neural tube is closing. Exactly when folate must be available also is not known. In addition, some popular diets, such as low carbohydrate or gluten free, may reduce exposure to grains, limiting folic acid intake.
James L. Mills, MD, is in the Division of Intramural Population Health Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Md. He reported having no relevant financial disclosures. These comments are adapted from an editorial accompanying the USPSTF report (JAMA. 2017;317 [2]:144-5).
The U.S. Preventive Services Task Force continues to recommend that all women planning or capable of pregnancy should take a daily supplement of 0.4-0.8 mg of folic acid to prevent neural tube defects in their offspring.
The task force “concludes with high certainty” that the benefits of such supplementation are substantial and the harms are minimal, according to the recommendation statement published online Jan. 10 in JAMA (2017;317[2]:183-9). The group based its updated recommendation on a systematic review of 24 studies performed since 2009 and involving 58,860 women. Although some newer studies have suggested that supplementation is no longer needed in this era of folic acid fortification of foods, “the USPSTF found no new substantial evidence ... that would lead to a change in its recommendation from 2009,” the researchers wrote.
The most recent data estimate that folic acid supplementation prevents neural tube defects in approximately 1,300 births each year.
This updated USPSTF recommendation is in accord with recommendations from the Health and Medicine Division of the National Academies (formerly the Institute of Medicine), the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the U.S. Public Health Service, the Centers for Disease Control and Prevention, the American Academy of Pediatrics, the American Academy of Neurology, and the American College of Medical Genetics and Genomics.
This work was supported solely by the USPSTF, an independent voluntary group mandated by Congress to assess preventive care services and funded by the Agency for Healthcare Research and Quality.
The U.S. Preventive Services Task Force continues to recommend that all women planning or capable of pregnancy should take a daily supplement of 0.4-0.8 mg of folic acid to prevent neural tube defects in their offspring.
The task force “concludes with high certainty” that the benefits of such supplementation are substantial and the harms are minimal, according to the recommendation statement published online Jan. 10 in JAMA (2017;317[2]:183-9). The group based its updated recommendation on a systematic review of 24 studies performed since 2009 and involving 58,860 women. Although some newer studies have suggested that supplementation is no longer needed in this era of folic acid fortification of foods, “the USPSTF found no new substantial evidence ... that would lead to a change in its recommendation from 2009,” the researchers wrote.
The most recent data estimate that folic acid supplementation prevents neural tube defects in approximately 1,300 births each year.
This updated USPSTF recommendation is in accord with recommendations from the Health and Medicine Division of the National Academies (formerly the Institute of Medicine), the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the U.S. Public Health Service, the Centers for Disease Control and Prevention, the American Academy of Pediatrics, the American Academy of Neurology, and the American College of Medical Genetics and Genomics.
This work was supported solely by the USPSTF, an independent voluntary group mandated by Congress to assess preventive care services and funded by the Agency for Healthcare Research and Quality.
FROM JAMA
Key clinical point:
Major finding: Folic acid supplementation prevents neural tube defects in an estimated 1,300 births each year in the United States.
Data source: A systematic review of 24 studies (involving 58,860 women) that were performed since 2009 regarding the benefits and harms of folic acid supplementation.
Disclosures: This work was supported solely by the USPSTF, an independent voluntary group mandated by Congress to assess preventive care services and funded by the Agency for Healthcare Research and Quality.
Longer follow-up needed to track mesh explantation trends
Explantation of mesh used in ventral hernia repair occurs in approximately 1 of every 1,000 surgeries, but it’s a “hidden” morbidity because it almost always happens well after the 30- to 90-day window in which postoperative complications are typically reported in most registries and surveillance systems, according to a report in the Journal of the American College of Surgeons.
Mesh explantation usually occurs 1-3 years after implantation and triples the operative costs of the original ventral hernia repair. The rate of 1 per 1,000 surgeries and the massive increase in cost are comparable with those of occult injury of the common bile duct during cholecystectomy that later requires biliary reconstruction. But mesh explantation doesn’t generate the “profound attention” accorded to bile duct injury, perhaps because it develops much later in the postoperative course.
“It is surprising that mesh complications have not yet prompted similar concern,” said Kristy Kummerow Broman, MD, of the department of surgery, Vanderbilt University Medical Center, Nashville, Tenn., and her associates.
Until now, the frequency and cost of mesh explantation after ventral hernia repair in the general population have not been known. To make a reasonable estimate, the investigators constructed a cohort of 619,751 patients using information from inpatient and surgery databases for New York, California, and Florida between 2005 and 2011. Most of these were open procedures (91%), while 9% were laparoscopic.
During a mean follow-up of 3 years, 438 patients (0.7 per 1,000) underwent mesh explantation. This is a clinically significant incidence, and is likely an underestimate because ICD-9 and CPT coding for mesh removal is highly variable, Dr. Broman and her associates said.
This rate, for just three states during 3 years of follow-up, is nearly twice as high as the rate of mesh-related complications voluntarily reported to the FDA in post-marketing surveillance for the entire country during a 7-year period, they noted (J Am Coll Surg. 2017 Jan;224:35-42).
“It is paramount” that surgeons, manufacturers, and regulatory groups advocate mandatory reporting and “extend the surveillance for at least 1-3 years after implantation of a mesh device,” Dr. Broman and her associates said.
In this study, the median time to explantation was approximately 1 year (range, 2 days to 6 years), and 80% of explantations occurred within 2 years.
The median cumulative operative cost – excluding physician fees, nonsurgical medical costs, and the costs of patient disability and lost productivity – were $21,889 for patients requiring mesh explantation, compared with only $6,579 for those who did not. This finding highlights “the profound long-term implications of implantable devices in abdominal wall reconstruction,” they noted.
To put their findings in context, the investigators reviewed the literature regarding major bile duct injury during cholecystectomy. One large study on cases from 2001 to 2011 found that the rate of biliary reconstruction was comparable with that of explantation, at 0.8 to 1.1 per 1,000. Similarly, reoperation for bile duct injury approximately tripled the operative costs ($9,061 for patients who required biliary reconstruction vs $2,689 for those who didn’t). However, the $21,000 for mesh reoperation far exceeds the $9,000 for biliary reoperation.
This study was supported by the Department of Veterans Affairs, the VA Tennessee Valley Healthcare System, and the Americas Hernia Society Quality Collaborative. Dr. Broman reported having no relevant financial disclosures; her associates reported ties to Intuitive Surgical Solutions, Bard Davol, Ariste Medical, and Pfizer.
Explantation of mesh used in ventral hernia repair occurs in approximately 1 of every 1,000 surgeries, but it’s a “hidden” morbidity because it almost always happens well after the 30- to 90-day window in which postoperative complications are typically reported in most registries and surveillance systems, according to a report in the Journal of the American College of Surgeons.
Mesh explantation usually occurs 1-3 years after implantation and triples the operative costs of the original ventral hernia repair. The rate of 1 per 1,000 surgeries and the massive increase in cost are comparable with those of occult injury of the common bile duct during cholecystectomy that later requires biliary reconstruction. But mesh explantation doesn’t generate the “profound attention” accorded to bile duct injury, perhaps because it develops much later in the postoperative course.
“It is surprising that mesh complications have not yet prompted similar concern,” said Kristy Kummerow Broman, MD, of the department of surgery, Vanderbilt University Medical Center, Nashville, Tenn., and her associates.
Until now, the frequency and cost of mesh explantation after ventral hernia repair in the general population have not been known. To make a reasonable estimate, the investigators constructed a cohort of 619,751 patients using information from inpatient and surgery databases for New York, California, and Florida between 2005 and 2011. Most of these were open procedures (91%), while 9% were laparoscopic.
During a mean follow-up of 3 years, 438 patients (0.7 per 1,000) underwent mesh explantation. This is a clinically significant incidence, and is likely an underestimate because ICD-9 and CPT coding for mesh removal is highly variable, Dr. Broman and her associates said.
This rate, for just three states during 3 years of follow-up, is nearly twice as high as the rate of mesh-related complications voluntarily reported to the FDA in post-marketing surveillance for the entire country during a 7-year period, they noted (J Am Coll Surg. 2017 Jan;224:35-42).
“It is paramount” that surgeons, manufacturers, and regulatory groups advocate mandatory reporting and “extend the surveillance for at least 1-3 years after implantation of a mesh device,” Dr. Broman and her associates said.
In this study, the median time to explantation was approximately 1 year (range, 2 days to 6 years), and 80% of explantations occurred within 2 years.
The median cumulative operative cost – excluding physician fees, nonsurgical medical costs, and the costs of patient disability and lost productivity – were $21,889 for patients requiring mesh explantation, compared with only $6,579 for those who did not. This finding highlights “the profound long-term implications of implantable devices in abdominal wall reconstruction,” they noted.
To put their findings in context, the investigators reviewed the literature regarding major bile duct injury during cholecystectomy. One large study on cases from 2001 to 2011 found that the rate of biliary reconstruction was comparable with that of explantation, at 0.8 to 1.1 per 1,000. Similarly, reoperation for bile duct injury approximately tripled the operative costs ($9,061 for patients who required biliary reconstruction vs $2,689 for those who didn’t). However, the $21,000 for mesh reoperation far exceeds the $9,000 for biliary reoperation.
This study was supported by the Department of Veterans Affairs, the VA Tennessee Valley Healthcare System, and the Americas Hernia Society Quality Collaborative. Dr. Broman reported having no relevant financial disclosures; her associates reported ties to Intuitive Surgical Solutions, Bard Davol, Ariste Medical, and Pfizer.
Explantation of mesh used in ventral hernia repair occurs in approximately 1 of every 1,000 surgeries, but it’s a “hidden” morbidity because it almost always happens well after the 30- to 90-day window in which postoperative complications are typically reported in most registries and surveillance systems, according to a report in the Journal of the American College of Surgeons.
Mesh explantation usually occurs 1-3 years after implantation and triples the operative costs of the original ventral hernia repair. The rate of 1 per 1,000 surgeries and the massive increase in cost are comparable with those of occult injury of the common bile duct during cholecystectomy that later requires biliary reconstruction. But mesh explantation doesn’t generate the “profound attention” accorded to bile duct injury, perhaps because it develops much later in the postoperative course.
“It is surprising that mesh complications have not yet prompted similar concern,” said Kristy Kummerow Broman, MD, of the department of surgery, Vanderbilt University Medical Center, Nashville, Tenn., and her associates.
Until now, the frequency and cost of mesh explantation after ventral hernia repair in the general population have not been known. To make a reasonable estimate, the investigators constructed a cohort of 619,751 patients using information from inpatient and surgery databases for New York, California, and Florida between 2005 and 2011. Most of these were open procedures (91%), while 9% were laparoscopic.
During a mean follow-up of 3 years, 438 patients (0.7 per 1,000) underwent mesh explantation. This is a clinically significant incidence, and is likely an underestimate because ICD-9 and CPT coding for mesh removal is highly variable, Dr. Broman and her associates said.
This rate, for just three states during 3 years of follow-up, is nearly twice as high as the rate of mesh-related complications voluntarily reported to the FDA in post-marketing surveillance for the entire country during a 7-year period, they noted (J Am Coll Surg. 2017 Jan;224:35-42).
“It is paramount” that surgeons, manufacturers, and regulatory groups advocate mandatory reporting and “extend the surveillance for at least 1-3 years after implantation of a mesh device,” Dr. Broman and her associates said.
In this study, the median time to explantation was approximately 1 year (range, 2 days to 6 years), and 80% of explantations occurred within 2 years.
The median cumulative operative cost – excluding physician fees, nonsurgical medical costs, and the costs of patient disability and lost productivity – were $21,889 for patients requiring mesh explantation, compared with only $6,579 for those who did not. This finding highlights “the profound long-term implications of implantable devices in abdominal wall reconstruction,” they noted.
To put their findings in context, the investigators reviewed the literature regarding major bile duct injury during cholecystectomy. One large study on cases from 2001 to 2011 found that the rate of biliary reconstruction was comparable with that of explantation, at 0.8 to 1.1 per 1,000. Similarly, reoperation for bile duct injury approximately tripled the operative costs ($9,061 for patients who required biliary reconstruction vs $2,689 for those who didn’t). However, the $21,000 for mesh reoperation far exceeds the $9,000 for biliary reoperation.
This study was supported by the Department of Veterans Affairs, the VA Tennessee Valley Healthcare System, and the Americas Hernia Society Quality Collaborative. Dr. Broman reported having no relevant financial disclosures; her associates reported ties to Intuitive Surgical Solutions, Bard Davol, Ariste Medical, and Pfizer.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Explantation of mesh used in ventral hernia repair occurs in approximately 1 of every 1,000 surgeries, but it’s a “hidden” morbidity because it almost always happens well after the 30- to 90-day window in which postoperative complications are typically reported in most registries and surveillance systems.
Major finding: The 1/1,000 rate of mesh explantation, for three states during 3 years of follow-up, is nearly twice as high as the rate voluntarily reported to the Food and Drug Administration in post-marketing surveillance for the entire country during a 7-year period.
Data source: A longitudinal cohort study involving 619,751 adults undergoing ventral hernia repair in New York, California, and Florida who were followed for up to 6 years for mesh explantation.
Disclosures: This study was supported by the Department of Veterans Affairs, the VA Tennessee Valley Healthcare System, and the Americas Hernia Society Quality Collaborative. Dr. Broman reported having no relevant financial disclosures; her associates reported ties to Intuitive Surgical Solutions, Bard Davol, Ariste Medical, and Pfizer.
Lightweight mesh linked to longer LOS, worse QOL
Compared with midweight mesh, lightweight mesh was associated with more surgical site infection and longer hospital stay following open ventral hernia repair, according to a report published in the American Journal of Surgery.
In addition, lightweight mesh was associated with greater pain, more limitation of movement, and poorer quality of life for up to 2 years after the procedure, compared with midweight mesh.
Approximately 250,000 open ventral hernia repairs are performed in the Unites States each year, and mesh is used in 85% or more. Since heavyweight mesh was found to reduce abdominal wall mobility, which led to chronic discomfort in about 20% of cases, manufacturers turned to mesh that was more flexible, had reduced mass to decrease foreign-body reactions, but was strong enough to withstand the physiological stress that the abdominal wall is subjected to, the investigators noted.
They compared outcomes after hernia repairs using lightweight and midweight mesh by analyzing information in the International Hernia Mesh Registry database, which covers more than 30 medical centers in 10 countries. For this study, the researchers focused on 549 patients for whom surgeons had selected lightweight (34.2%) or midweight (47.7%) mesh. (The remaining 18.1% of cases used heavyweight mesh.)
Across the study groups, patients were similar for gender distribution; body mass index; race; and the presence of chronic obstructive pulmonary disease, asthma, and immunosuppression – factors that can heavily influence wound repair.
In an initial analysis of the data, midweight mesh was associated with significantly fewer superficial surgical site infections (1.2%) than lightweight mesh (4.8%), as well as a significantly shorter length of stay (3.6 days vs 5.3 days). However, rates of postoperative abdominal wall complications, abscesses, urinary tract infection, pneumonia, hematoma formation, seroma formation, ileus, deep vein thrombosis, and unplanned returns to the operating room were similar.
At 6-month follow-up, lightweight mesh was associated with significantly greater mesh sensation, abdominal discomfort, and movement limitation, as well as significantly worse overall quality of life (QOL), than midweight mesh. At 12 months, lightweight mesh was associated with significantly greater pain and limitation of movement and significantly worse QOL. At 24 months, lightweight mesh continued to be associated with movement limitation, but scores on other measures were similar to those with midweight mesh.
In a multivariate analysis that controlled for many potentially confounding variables, including smoking status, separation of the components of the mesh, the number of sutures anchoring the mesh, and the mesh location within the abdomen, midweight mesh was not associated with worse QOL scores at any time point. In contrast, lightweight mesh was associated with significantly worse QOL scores at 6 months, with an odds ratio of 2.64, and with significantly more pain at 12 months, with an OR of 2.58, Dr. Groene and his associates said (Am J Surg. 2016 Dec;212[6]:1054-62).
The investigators also noted that among their own hernia repair patients, lightweight mesh tends to fracture more easily than midweight mesh. Recent studies also have reported that over time, lightweight mesh is more likely to fail due to fracturing than midweight mesh, they added.
This study had no relevant financial relationships or sources of support. Dr. Groene and his associates reported having no financial conflicts of interest.
Compared with midweight mesh, lightweight mesh was associated with more surgical site infection and longer hospital stay following open ventral hernia repair, according to a report published in the American Journal of Surgery.
In addition, lightweight mesh was associated with greater pain, more limitation of movement, and poorer quality of life for up to 2 years after the procedure, compared with midweight mesh.
Approximately 250,000 open ventral hernia repairs are performed in the Unites States each year, and mesh is used in 85% or more. Since heavyweight mesh was found to reduce abdominal wall mobility, which led to chronic discomfort in about 20% of cases, manufacturers turned to mesh that was more flexible, had reduced mass to decrease foreign-body reactions, but was strong enough to withstand the physiological stress that the abdominal wall is subjected to, the investigators noted.
They compared outcomes after hernia repairs using lightweight and midweight mesh by analyzing information in the International Hernia Mesh Registry database, which covers more than 30 medical centers in 10 countries. For this study, the researchers focused on 549 patients for whom surgeons had selected lightweight (34.2%) or midweight (47.7%) mesh. (The remaining 18.1% of cases used heavyweight mesh.)
Across the study groups, patients were similar for gender distribution; body mass index; race; and the presence of chronic obstructive pulmonary disease, asthma, and immunosuppression – factors that can heavily influence wound repair.
In an initial analysis of the data, midweight mesh was associated with significantly fewer superficial surgical site infections (1.2%) than lightweight mesh (4.8%), as well as a significantly shorter length of stay (3.6 days vs 5.3 days). However, rates of postoperative abdominal wall complications, abscesses, urinary tract infection, pneumonia, hematoma formation, seroma formation, ileus, deep vein thrombosis, and unplanned returns to the operating room were similar.
At 6-month follow-up, lightweight mesh was associated with significantly greater mesh sensation, abdominal discomfort, and movement limitation, as well as significantly worse overall quality of life (QOL), than midweight mesh. At 12 months, lightweight mesh was associated with significantly greater pain and limitation of movement and significantly worse QOL. At 24 months, lightweight mesh continued to be associated with movement limitation, but scores on other measures were similar to those with midweight mesh.
In a multivariate analysis that controlled for many potentially confounding variables, including smoking status, separation of the components of the mesh, the number of sutures anchoring the mesh, and the mesh location within the abdomen, midweight mesh was not associated with worse QOL scores at any time point. In contrast, lightweight mesh was associated with significantly worse QOL scores at 6 months, with an odds ratio of 2.64, and with significantly more pain at 12 months, with an OR of 2.58, Dr. Groene and his associates said (Am J Surg. 2016 Dec;212[6]:1054-62).
The investigators also noted that among their own hernia repair patients, lightweight mesh tends to fracture more easily than midweight mesh. Recent studies also have reported that over time, lightweight mesh is more likely to fail due to fracturing than midweight mesh, they added.
This study had no relevant financial relationships or sources of support. Dr. Groene and his associates reported having no financial conflicts of interest.
Compared with midweight mesh, lightweight mesh was associated with more surgical site infection and longer hospital stay following open ventral hernia repair, according to a report published in the American Journal of Surgery.
In addition, lightweight mesh was associated with greater pain, more limitation of movement, and poorer quality of life for up to 2 years after the procedure, compared with midweight mesh.
Approximately 250,000 open ventral hernia repairs are performed in the Unites States each year, and mesh is used in 85% or more. Since heavyweight mesh was found to reduce abdominal wall mobility, which led to chronic discomfort in about 20% of cases, manufacturers turned to mesh that was more flexible, had reduced mass to decrease foreign-body reactions, but was strong enough to withstand the physiological stress that the abdominal wall is subjected to, the investigators noted.
They compared outcomes after hernia repairs using lightweight and midweight mesh by analyzing information in the International Hernia Mesh Registry database, which covers more than 30 medical centers in 10 countries. For this study, the researchers focused on 549 patients for whom surgeons had selected lightweight (34.2%) or midweight (47.7%) mesh. (The remaining 18.1% of cases used heavyweight mesh.)
Across the study groups, patients were similar for gender distribution; body mass index; race; and the presence of chronic obstructive pulmonary disease, asthma, and immunosuppression – factors that can heavily influence wound repair.
In an initial analysis of the data, midweight mesh was associated with significantly fewer superficial surgical site infections (1.2%) than lightweight mesh (4.8%), as well as a significantly shorter length of stay (3.6 days vs 5.3 days). However, rates of postoperative abdominal wall complications, abscesses, urinary tract infection, pneumonia, hematoma formation, seroma formation, ileus, deep vein thrombosis, and unplanned returns to the operating room were similar.
At 6-month follow-up, lightweight mesh was associated with significantly greater mesh sensation, abdominal discomfort, and movement limitation, as well as significantly worse overall quality of life (QOL), than midweight mesh. At 12 months, lightweight mesh was associated with significantly greater pain and limitation of movement and significantly worse QOL. At 24 months, lightweight mesh continued to be associated with movement limitation, but scores on other measures were similar to those with midweight mesh.
In a multivariate analysis that controlled for many potentially confounding variables, including smoking status, separation of the components of the mesh, the number of sutures anchoring the mesh, and the mesh location within the abdomen, midweight mesh was not associated with worse QOL scores at any time point. In contrast, lightweight mesh was associated with significantly worse QOL scores at 6 months, with an odds ratio of 2.64, and with significantly more pain at 12 months, with an OR of 2.58, Dr. Groene and his associates said (Am J Surg. 2016 Dec;212[6]:1054-62).
The investigators also noted that among their own hernia repair patients, lightweight mesh tends to fracture more easily than midweight mesh. Recent studies also have reported that over time, lightweight mesh is more likely to fail due to fracturing than midweight mesh, they added.
This study had no relevant financial relationships or sources of support. Dr. Groene and his associates reported having no financial conflicts of interest.
FROM THE AMERICAN JOURNAL OF SURGERY
Key clinical point: Compared with midweight mesh, lightweight mesh was associated with more surgical site infections and longer hospital stay in the short term and greater pain, more limitation of movement, and poorer quality of life for up to 2 years after open ventral hernia repair.
Major finding: In the short term, midweight mesh was associated with significantly fewer superficial surgical site infections (1.2%) than lightweight mesh (4.8%), as well as a significantly shorter length of stay (3.6 days vs 5.3 days).
Data source: An analysis of information in an international prospective registry of hernia mesh surgeries, which involved 549 adults.
Disclosures: This study had no relevant financial relationships or sources of support. Dr. Groene and his associates reported having no financial conflicts of interest.
‘Weekend warrior’ exercise pattern sufficient to cut mortality
The “weekend warrior” exercise pattern – having one or two rather than five to seven leisure-time activity sessions per week – may be sufficient to reduce all-cause, cardiovascular disease, and cancer mortality risks, according to a report published online Jan. 9 in JAMA Internal Medicine.
The World Health Organization and U.S. Department of Health & Human Services recommend that adults perform at least 150 minutes per week of moderate-intensity aerobic activity, at least 75 minutes per week of vigorous-intensity aerobic activity, or equivalent combinations, spread out over the week.
They performed a pooled analysis of data in eight household surveillance studies across England and Scotland, focusing on the self-reported physical activity patterns of 63,591 adults older than 40 years from 1994 through 2012. The mean age of the survey respondents was 58.6 years. A total of 62.8% were classified as inactive, 22.4% as insufficiently active (performing less than 150 minutes per week of moderate-intensity activity), 3.7% as weekend warriors, and 11.1% as regularly active.
There were 8,802 deaths because of all causes, including 2,780 deaths due to cardiovascular disease (CVD) and 2,526 deaths due to cancer, during 561,159 person-years of follow-up.
Compared with inactive participants, the hazard ratio (HR) for all-cause mortality was 0.69 for insufficiently active participants (a 31% reduction), 0.70 for weekend warriors (a 30% reduction), and 0.65 for regularly active participants (a 35% reduction).
The findings remained consistent for men and women alike and regardless of the presence or absence of obesity. However, because 95% of the study population was white, it is not known whether the findings apply to other racial or ethnic groups.
The study results suggest that some leisure-time physical activity is better than none, and that even as few as one to two sessions per week offer considerable health benefits to both men and women, even among obese adults, Dr. O’Donovan and his associates said.
The investigators did not elaborate on the finding that an “insufficient” activity level reduced mortality risks to nearly the same degree as a “weekend warrior” activity level.
The study was supported by the National Institute for Health Research; the Leicester Clinical Trials Unit; the Leicester-Loughborough Diet, Lifestyle, and Physical Activity Biomedical Research Unit; and the National Health and Medical Research Council. Dr. O’Donovan and his associates reported having no relevant financial disclosures.
In response to the question of whether exercise can wait for the weekend, the short answer is “perhaps.”
Meeting current guidelines for physical activity in only one or two sessions per week does yield substantial mortality benefit, but exercising more frequently yields even more.
In addition to studying the timing, frequency, and intensity of physical activity, we hope researchers also examine ways to promote its popularity in the general public.
Hannah Arem, PhD, is in the department of epidemiology and biostatistics at the Milken Institute School of Public Health, George Washington University, Washington. Loretta DiPietro, PhD, is in the department of exercise and nutrition sciences at the Milken Institute. Dr. Arem and Dr. DiPietro reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. O’Donovan’s report (JAMA Intern Med. 2017 Jan 9 [doi:10.1001/jamainternmend.2016.8050]).
In response to the question of whether exercise can wait for the weekend, the short answer is “perhaps.”
Meeting current guidelines for physical activity in only one or two sessions per week does yield substantial mortality benefit, but exercising more frequently yields even more.
In addition to studying the timing, frequency, and intensity of physical activity, we hope researchers also examine ways to promote its popularity in the general public.
Hannah Arem, PhD, is in the department of epidemiology and biostatistics at the Milken Institute School of Public Health, George Washington University, Washington. Loretta DiPietro, PhD, is in the department of exercise and nutrition sciences at the Milken Institute. Dr. Arem and Dr. DiPietro reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. O’Donovan’s report (JAMA Intern Med. 2017 Jan 9 [doi:10.1001/jamainternmend.2016.8050]).
In response to the question of whether exercise can wait for the weekend, the short answer is “perhaps.”
Meeting current guidelines for physical activity in only one or two sessions per week does yield substantial mortality benefit, but exercising more frequently yields even more.
In addition to studying the timing, frequency, and intensity of physical activity, we hope researchers also examine ways to promote its popularity in the general public.
Hannah Arem, PhD, is in the department of epidemiology and biostatistics at the Milken Institute School of Public Health, George Washington University, Washington. Loretta DiPietro, PhD, is in the department of exercise and nutrition sciences at the Milken Institute. Dr. Arem and Dr. DiPietro reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. O’Donovan’s report (JAMA Intern Med. 2017 Jan 9 [doi:10.1001/jamainternmend.2016.8050]).
The “weekend warrior” exercise pattern – having one or two rather than five to seven leisure-time activity sessions per week – may be sufficient to reduce all-cause, cardiovascular disease, and cancer mortality risks, according to a report published online Jan. 9 in JAMA Internal Medicine.
The World Health Organization and U.S. Department of Health & Human Services recommend that adults perform at least 150 minutes per week of moderate-intensity aerobic activity, at least 75 minutes per week of vigorous-intensity aerobic activity, or equivalent combinations, spread out over the week.
They performed a pooled analysis of data in eight household surveillance studies across England and Scotland, focusing on the self-reported physical activity patterns of 63,591 adults older than 40 years from 1994 through 2012. The mean age of the survey respondents was 58.6 years. A total of 62.8% were classified as inactive, 22.4% as insufficiently active (performing less than 150 minutes per week of moderate-intensity activity), 3.7% as weekend warriors, and 11.1% as regularly active.
There were 8,802 deaths because of all causes, including 2,780 deaths due to cardiovascular disease (CVD) and 2,526 deaths due to cancer, during 561,159 person-years of follow-up.
Compared with inactive participants, the hazard ratio (HR) for all-cause mortality was 0.69 for insufficiently active participants (a 31% reduction), 0.70 for weekend warriors (a 30% reduction), and 0.65 for regularly active participants (a 35% reduction).
The findings remained consistent for men and women alike and regardless of the presence or absence of obesity. However, because 95% of the study population was white, it is not known whether the findings apply to other racial or ethnic groups.
The study results suggest that some leisure-time physical activity is better than none, and that even as few as one to two sessions per week offer considerable health benefits to both men and women, even among obese adults, Dr. O’Donovan and his associates said.
The investigators did not elaborate on the finding that an “insufficient” activity level reduced mortality risks to nearly the same degree as a “weekend warrior” activity level.
The study was supported by the National Institute for Health Research; the Leicester Clinical Trials Unit; the Leicester-Loughborough Diet, Lifestyle, and Physical Activity Biomedical Research Unit; and the National Health and Medical Research Council. Dr. O’Donovan and his associates reported having no relevant financial disclosures.
The “weekend warrior” exercise pattern – having one or two rather than five to seven leisure-time activity sessions per week – may be sufficient to reduce all-cause, cardiovascular disease, and cancer mortality risks, according to a report published online Jan. 9 in JAMA Internal Medicine.
The World Health Organization and U.S. Department of Health & Human Services recommend that adults perform at least 150 minutes per week of moderate-intensity aerobic activity, at least 75 minutes per week of vigorous-intensity aerobic activity, or equivalent combinations, spread out over the week.
They performed a pooled analysis of data in eight household surveillance studies across England and Scotland, focusing on the self-reported physical activity patterns of 63,591 adults older than 40 years from 1994 through 2012. The mean age of the survey respondents was 58.6 years. A total of 62.8% were classified as inactive, 22.4% as insufficiently active (performing less than 150 minutes per week of moderate-intensity activity), 3.7% as weekend warriors, and 11.1% as regularly active.
There were 8,802 deaths because of all causes, including 2,780 deaths due to cardiovascular disease (CVD) and 2,526 deaths due to cancer, during 561,159 person-years of follow-up.
Compared with inactive participants, the hazard ratio (HR) for all-cause mortality was 0.69 for insufficiently active participants (a 31% reduction), 0.70 for weekend warriors (a 30% reduction), and 0.65 for regularly active participants (a 35% reduction).
The findings remained consistent for men and women alike and regardless of the presence or absence of obesity. However, because 95% of the study population was white, it is not known whether the findings apply to other racial or ethnic groups.
The study results suggest that some leisure-time physical activity is better than none, and that even as few as one to two sessions per week offer considerable health benefits to both men and women, even among obese adults, Dr. O’Donovan and his associates said.
The investigators did not elaborate on the finding that an “insufficient” activity level reduced mortality risks to nearly the same degree as a “weekend warrior” activity level.
The study was supported by the National Institute for Health Research; the Leicester Clinical Trials Unit; the Leicester-Loughborough Diet, Lifestyle, and Physical Activity Biomedical Research Unit; and the National Health and Medical Research Council. Dr. O’Donovan and his associates reported having no relevant financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The “weekend warrior” exercise pattern – having one or two rather than five to seven leisure-time activity sessions per week – may be sufficient to reduce mortality risks.
Major finding: Compared with inactive participants, the hazard ratio for all-cause mortality was 0.69 for insufficiently active participants (a 31% reduction), 0.70 for weekend warriors (a 30% reduction), and 0.65 for regularly active participants (a 35% reduction).
Data source: A pooled analysis of eight household surveillance studies in England and Scotland during 1994-2012, involving 63,591 adults older than 40 years.
Disclosures: The study was supported by the National Institute for Health Research; the Leicester Clinical Trials Unit; the Leicester-Loughborough Diet, Lifestyle, and Physical Activity Biomedical Research Unit; and the National Health and Medical Research Council. Dr. O’Donovan and his associates reported having no relevant financial disclosures.
Guidelines for diagnosing TB in adults, children
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus … there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted (Clin Infect Dis. 2016 Dec 8;64[2]:e1-33. doi: 10.1093/cid/ciw694).
Among the guidelines’ strongest recommendations:
• Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5-10 mL would be better.
• Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
• A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
• Rapid molecular drug susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
• Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
• For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
• For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
This work was supported by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America, with input from the American Academy of Pediatrics. Dr. Lewinsohn reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus … there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted (Clin Infect Dis. 2016 Dec 8;64[2]:e1-33. doi: 10.1093/cid/ciw694).
Among the guidelines’ strongest recommendations:
• Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5-10 mL would be better.
• Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
• A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
• Rapid molecular drug susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
• Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
• For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
• For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
This work was supported by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America, with input from the American Academy of Pediatrics. Dr. Lewinsohn reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus … there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted (Clin Infect Dis. 2016 Dec 8;64[2]:e1-33. doi: 10.1093/cid/ciw694).
Among the guidelines’ strongest recommendations:
• Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5-10 mL would be better.
• Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
• A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
• Rapid molecular drug susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
• Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
• For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
• For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
This work was supported by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America, with input from the American Academy of Pediatrics. Dr. Lewinsohn reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
Major finding: The clinical practice guideline includes 23 evidence-based recommendations concerning diagnostic testing for latent, pulmonary, or extrapulmonary tuberculosis in adults and children.
Data source: A compilation of 23 evidence-based recommendations about diagnostic testing for tuberculosis.
Disclosures: This work was supported by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America, with input from the American Academy of Pediatrics. Dr. Lewinsohn reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.