User login
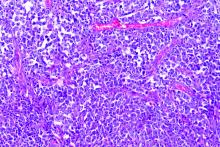
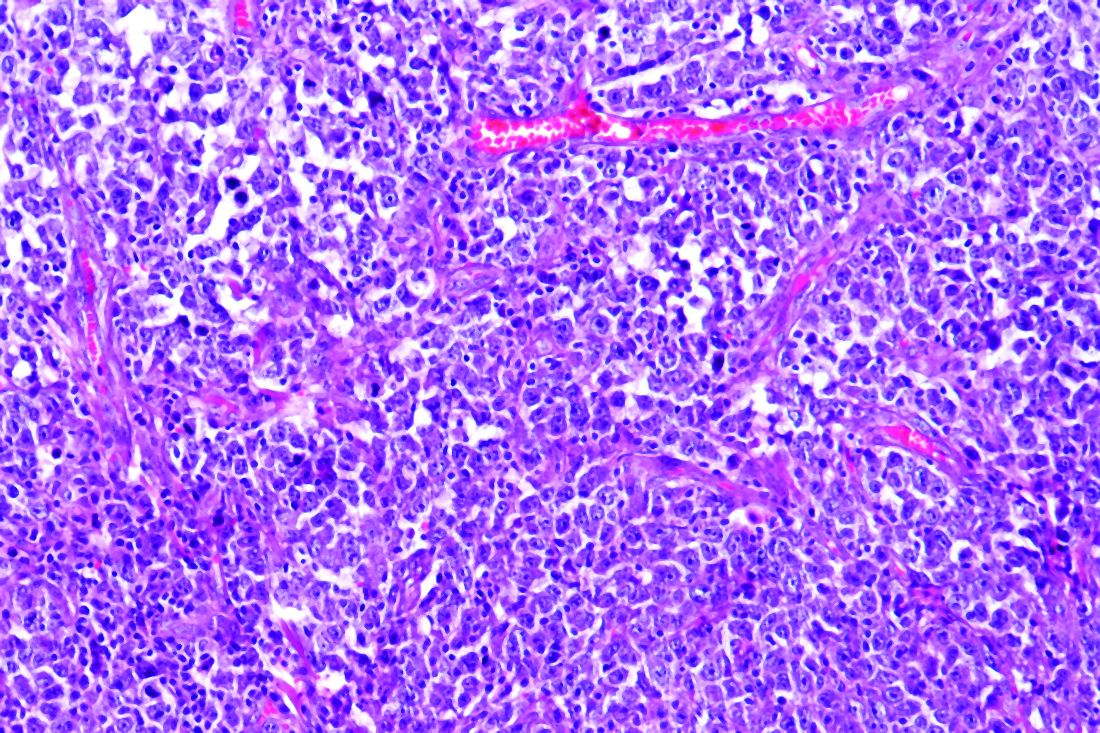
Pigment traits, sun sensitivity associated with risk of non-Hodgkin lymphomas and chronic lymphocytic leukemia
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
Risk factors for keratinocyte carcinomas, primarily pigment traits and sun sensitivity, were associated with the risk of developing non-Hodgkin lymphomas (NHL) and chronic lymphocytic leukemia (CLL) in an analysis of 92,097 women in France.
The presence of “many or very many nevi [moles]” was particularly associated with the risk of CLL among individuals in the E3N cohort, according to a report published online in Cancer Medicine. E3N is a prospective cohort of French women aged 40-65 years at inclusion in 1990. Researchers collected cancer data at baseline and every 2-3 years.
Hazard ratios and 95% confidence intervals for associations between patients pigmentary traits and sun exposure and their risk for CLL/NHL were estimated using Cox models, according to study author Louis-Marie Garcin, MD, of the Université Paris-Saclay, Villejuif, and colleagues.
Common etiology?
Among the 92,097 women included in the study, 622 incident cases of CLL/NHL were observed over a median of 24-years’ follow-up.
The presence of nevi was associated with CLL/NHL risk. The HR for “many or very many nevi” relative to “no nevi” was 1.56. The association with number of nevi was strongest for the risk of CLL, with an HR for “many or very many nevi” of 3.00 vs. 1.32 for NHL. In addition, the researchers found that women whose skin was highly sensitive to sunburn also had a higher risk of CLL (HR, 1.96), while no increased risk of NHL was observed. All HR values were within their respective 95% confidence intervals.
Relevant characteristics that were found to not be associated with added CLL/NHL risk were skin or hair color, number of freckles, and average daily UV dose during spring and summer in the location of residence at birth or at inclusion.
These observations suggest that CLL in particular may share some constitutional risk factors with keratinocyte cancers, according to the researchers.
“We report an association between nevi frequency and CLL/NHL risk, suggesting a partly common genetic etiology of these tumors. Future research should investigate common pathophysiological pathways that could promote the development of both skin carcinoma and CLL/NHL,” the researchers concluded.
The study was sponsored by the French government. The authors stated that they had no conflicts of interest.
SOURCE: Garcin L-M et al. Cancer Med. 2020. doi: 10.1002/cam4.3586.
FROM CANCER MEDICINE
Combo DAA treatments may benefit patients with resistant HCV genotype 3
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
FROM ANNALS OF HEPATOLOGY
Hemophilia, von Willebrand disease do not increase postop complications for ACL reconstruction
Patients with hemophilia A or von Willebrand disease undergoing anterior cruciate ligament (ACL) reconstruction had rates of postoperative complications and ACL reinjuries that were not significantly different from those control patients. However, the cost of health care utilization was significantly greater for the hemophilia A and von Willebrand disease patients, according to a large retrospective database study published online in The Knee.
All patients who underwent an ACL reconstruction from 2010 to 2014 in a large commercial database were assessed. Patients with hemophilia A, hemophilia B, and von Willebrand disease were identified. Patient demographics, cost of surgery, blood product use, concomitant injuries, repeat ACL injury, complications, and various operative variables were collected.
A total of 33 patients with hemophilia A, 3 with hemophilia B patients, 63 with von Willebrand disease and 103,478 control patients who had ACL reconstruction were compared, according to Connor Zale, MD, and colleagues at Penn State Hershey (Pa.) Medical Center.
Similar outcomes, higher costs
Complications – including length of hospital stay, postoperative hemorrhage within 14 days after surgery, infection rates within 90 days of surgery, lysis of adhesions or manipulation under anesthesia within 90 days of surgery, concomitant injuries to the knee, additional ACL injury within 1 year of surgery, deep-vein thrombosis, and pulmonary embolism – were not statistically different between the hemophilia/von Willebrand cohorts and the control group, according to the researchers.
However, surgery and postoperative care were costlier in the hemophilia A and von Willebrand cohorts. Total health care utilization within 30 days of ACL reconstruction was significantly more expensive for patients with hemophilia A ($25,982) and those with von Willebrand disease ($16,445), compared with those among controls ($12,887). In addition, the total health care utilization costs within 90 days of ACL reconstruction were significantly higher for patients with hemophilia A ($30,310) and those with von Willebrand disease ($20,355), compared with those among controls ($14,564), with all P values less than .001.
None of the patients with hemophilia A or those with von Willebrand received blood products perioperatively, had a known major hemarthrosis, or were readmitted within 30 or 90 days, the authors noted, adding that this finding differs from previous studies. The authors speculated that, since no blood products were administered and there was no significant difference in postoperative hemorrhage, the patients with hemophilia A were preoperatively optimized for an acceptable prothrombin time and international normalized ratio and/or were more effectively managed postoperatively.
“Many surgeons may be fearful of performing an ACL reconstruction on those with hemophilia A, hemophilia B, and von Willebrand disease due to concerns over risk of a major hemarthrosis and other complications postoperatively. This study observed that hemophilia A and von Willebrand disease patients who underwent an ACL reconstruction had rates of postoperative complications that were not statistically different than those who underwent ACL reconstructions and did not have a known hypocoagulable condition,” the researchers concluded.
The authors reported that they had no potential conflicts of interest to disclose.
SOURCE: Zale C et al. Knee. 2020;27(6):1729-34.
Patients with hemophilia A or von Willebrand disease undergoing anterior cruciate ligament (ACL) reconstruction had rates of postoperative complications and ACL reinjuries that were not significantly different from those control patients. However, the cost of health care utilization was significantly greater for the hemophilia A and von Willebrand disease patients, according to a large retrospective database study published online in The Knee.
All patients who underwent an ACL reconstruction from 2010 to 2014 in a large commercial database were assessed. Patients with hemophilia A, hemophilia B, and von Willebrand disease were identified. Patient demographics, cost of surgery, blood product use, concomitant injuries, repeat ACL injury, complications, and various operative variables were collected.
A total of 33 patients with hemophilia A, 3 with hemophilia B patients, 63 with von Willebrand disease and 103,478 control patients who had ACL reconstruction were compared, according to Connor Zale, MD, and colleagues at Penn State Hershey (Pa.) Medical Center.
Similar outcomes, higher costs
Complications – including length of hospital stay, postoperative hemorrhage within 14 days after surgery, infection rates within 90 days of surgery, lysis of adhesions or manipulation under anesthesia within 90 days of surgery, concomitant injuries to the knee, additional ACL injury within 1 year of surgery, deep-vein thrombosis, and pulmonary embolism – were not statistically different between the hemophilia/von Willebrand cohorts and the control group, according to the researchers.
However, surgery and postoperative care were costlier in the hemophilia A and von Willebrand cohorts. Total health care utilization within 30 days of ACL reconstruction was significantly more expensive for patients with hemophilia A ($25,982) and those with von Willebrand disease ($16,445), compared with those among controls ($12,887). In addition, the total health care utilization costs within 90 days of ACL reconstruction were significantly higher for patients with hemophilia A ($30,310) and those with von Willebrand disease ($20,355), compared with those among controls ($14,564), with all P values less than .001.
None of the patients with hemophilia A or those with von Willebrand received blood products perioperatively, had a known major hemarthrosis, or were readmitted within 30 or 90 days, the authors noted, adding that this finding differs from previous studies. The authors speculated that, since no blood products were administered and there was no significant difference in postoperative hemorrhage, the patients with hemophilia A were preoperatively optimized for an acceptable prothrombin time and international normalized ratio and/or were more effectively managed postoperatively.
“Many surgeons may be fearful of performing an ACL reconstruction on those with hemophilia A, hemophilia B, and von Willebrand disease due to concerns over risk of a major hemarthrosis and other complications postoperatively. This study observed that hemophilia A and von Willebrand disease patients who underwent an ACL reconstruction had rates of postoperative complications that were not statistically different than those who underwent ACL reconstructions and did not have a known hypocoagulable condition,” the researchers concluded.
The authors reported that they had no potential conflicts of interest to disclose.
SOURCE: Zale C et al. Knee. 2020;27(6):1729-34.
Patients with hemophilia A or von Willebrand disease undergoing anterior cruciate ligament (ACL) reconstruction had rates of postoperative complications and ACL reinjuries that were not significantly different from those control patients. However, the cost of health care utilization was significantly greater for the hemophilia A and von Willebrand disease patients, according to a large retrospective database study published online in The Knee.
All patients who underwent an ACL reconstruction from 2010 to 2014 in a large commercial database were assessed. Patients with hemophilia A, hemophilia B, and von Willebrand disease were identified. Patient demographics, cost of surgery, blood product use, concomitant injuries, repeat ACL injury, complications, and various operative variables were collected.
A total of 33 patients with hemophilia A, 3 with hemophilia B patients, 63 with von Willebrand disease and 103,478 control patients who had ACL reconstruction were compared, according to Connor Zale, MD, and colleagues at Penn State Hershey (Pa.) Medical Center.
Similar outcomes, higher costs
Complications – including length of hospital stay, postoperative hemorrhage within 14 days after surgery, infection rates within 90 days of surgery, lysis of adhesions or manipulation under anesthesia within 90 days of surgery, concomitant injuries to the knee, additional ACL injury within 1 year of surgery, deep-vein thrombosis, and pulmonary embolism – were not statistically different between the hemophilia/von Willebrand cohorts and the control group, according to the researchers.
However, surgery and postoperative care were costlier in the hemophilia A and von Willebrand cohorts. Total health care utilization within 30 days of ACL reconstruction was significantly more expensive for patients with hemophilia A ($25,982) and those with von Willebrand disease ($16,445), compared with those among controls ($12,887). In addition, the total health care utilization costs within 90 days of ACL reconstruction were significantly higher for patients with hemophilia A ($30,310) and those with von Willebrand disease ($20,355), compared with those among controls ($14,564), with all P values less than .001.
None of the patients with hemophilia A or those with von Willebrand received blood products perioperatively, had a known major hemarthrosis, or were readmitted within 30 or 90 days, the authors noted, adding that this finding differs from previous studies. The authors speculated that, since no blood products were administered and there was no significant difference in postoperative hemorrhage, the patients with hemophilia A were preoperatively optimized for an acceptable prothrombin time and international normalized ratio and/or were more effectively managed postoperatively.
“Many surgeons may be fearful of performing an ACL reconstruction on those with hemophilia A, hemophilia B, and von Willebrand disease due to concerns over risk of a major hemarthrosis and other complications postoperatively. This study observed that hemophilia A and von Willebrand disease patients who underwent an ACL reconstruction had rates of postoperative complications that were not statistically different than those who underwent ACL reconstructions and did not have a known hypocoagulable condition,” the researchers concluded.
The authors reported that they had no potential conflicts of interest to disclose.
SOURCE: Zale C et al. Knee. 2020;27(6):1729-34.
FROM THE KNEE
Key clinical point:
Major finding: Total health care utilization within 30 days of ACL reconstruction was significantly greater for hemophilia A ($25,982) and von Willebrand disease ($16,445) patients, compared with controls ($12,887).
Study details: A retrospective study of 33 patients with hemophilia A, 3 with hemophilia B, and 63 with von Willebrand factor, as well as 103,478 controls, who all underwent ACL reconstruction.
Disclosures: The authors reported that they had no potential conflicts of interest to disclose.
Source: Zale C et al. Knee. 2020;27(6):1729-34.
Opt-out policy at a syringe service program increased HIV/HCV testing
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
FROM INTERNATIONAL JOURNAL OF DRUG POLICY
Ibrutinib associated with decreased circulating malignant cells and restored T-cell function in CLL patients
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
Ibrutinib showed significant impact on circulating malignant and nonmalignant immune cells and was found to restore healthy T-cell function in patients with chronic lymphocytic leukemia (CLL), according to the results of a comparative study of CLL patients and healthy controls.
Researchers compared circulating counts of 21 immune blood cell subsets throughout the first year of treatment in 55 patients with relapsed/refractory (R/R) CLL from the RESONATE trial and 50 previously untreated CLL patients from the RESONATE-2 trial with 20 untreated age-matched healthy donors, according to a report published online in Leukemia Research.
In addition, T-cell function was assessed in response to T-cell–receptor stimulation in 21 patients with R/R CLL, compared with 18 age-matched healthy donors, according to Isabelle G. Solman, MS, an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. and colleagues.
Positive indicators
Ibrutinib significantly decreased pathologically high circulating B cells, regulatory T cells, effector/memory CD4+ and CD8+ T cells (including exhausted and chronically activated T cells), natural killer (NK) T cells, and myeloid-derived suppressor cells; preserved naive T cells and NK cells; and increased circulating classical monocytes, according to the researchers.
Ibrutinib also significantly restored T-cell proliferative ability, degranulation, and cytokine secretion. Over the same period, ofatumumab or chlorambucil did not confer the same spectrum of normalization as ibrutinib in multiple immune subsets that were examined, they added.
“These results establish that ibrutinib has a significant and likely positive impact on circulating malignant and nonmalignant immune cells and restores healthy T-cell function,” the researchers indicated.
“Ibrutinib has a significant, progressively positive impact on both malignant and nonmalignant immune cells in CLL. These positive effects on circulating nonmalignant immune cells may contribute to long-term CLL disease control, overall health status, and decreased susceptibility to infection,” they concluded.
The study was funded by Pharmacyclics, an AbbVie Company. Ms. Solman is an employee of Translational Medicine, Pharmacyclics, Sunnyvale, Calif. as were several other authors.
SOURCE: Solman IG et al. Leuk Res. 2020;97. doi: 10.1016/j.leukres.2020.106432.
FROM LEUKEMIA RESEARCH
Whales, seals, and dolphins: Will SARS-CoV-2–contaminated wastewater prove a killer?
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
FROM SCIENCE OF THE TOTAL ENVIRONMENT
Mouth splints decrease risk of post–dental extraction bleeding in hemophilia
Dental extractions can cause significant risk of bleeding in hemophilia patients being treated with factor replacements. However, mouth splints significantly decreased the risk of postextraction bleeding in these patients, according to Takahiro Yagyuu, DDS, of the department of oral and maxillofacial surgery, Nara Medical University, Kashihara, Japan, and colleagues.
The researchers performed a retrospective analysis of the medical records of hemophilia patients who underwent tooth extraction(s) between April 2006 and April 2019 at a single university hospital in Japan.
They conducted logistic regression analyses to identify risk/protective factors for postextraction bleeding in procedures involving patients receiving factor replacement therapy. Postextraction bleeding was defined as bleeding that could not be stopped by biting down on gauze and required medical treatment between 30 minutes and 14 days after the extraction, according to the report published online on in the British Journal of Oral & Maxillofacial Surgery.
A total of 130 extractions in 48 patients with hemophilia A and 21 extractions in 7 patients with hemophilia B were performed. Postextraction bleeding events were observed in 9 patients (16.3%) and 12 extractions (7.9%). On average, postextraction bleeding occurred 6 days after intervention and on the fifth postoperative day for extractions, according to the researchers.
Benefits of splints
The study found that the use of mouth splints significantly decreased the risk of postextraction bleeding (odds ratio, 0.13; P = .01) in hemophilia patients being treated with clotting factor replacements.
However, other factors in the study cohort, such as age, severity of hemophilia, duration of factor replacement therapy, gingival incision, bone removal, tooth separation, use of absorbable hemostats, wound closure, and the prescription of NSAIDs, were not significantly associated with postextraction bleeding, the researchers added.
“The use of mouth splints significantly decreased the risk of post-extraction bleeding. [In the future], we will conduct a prospective study to investigate the optimal type of splint and splint-wearing period to improve hemostatic management of tooth extraction in hemophilia patients,” the researchers concluded.
One author reported grants and personal fees from Bayer, Bioverativ, Chugai Pharmaceutical, Novo Nordisk, and Shire. A second author teaches a course endowed by Shire Japan. The other authors reported they had no conflicts.
SOURCE: Yagyuu T et al. Br J Oral Maxillofac Surg. 2020 Oct 11. doi: 10.1016/j.bjoms.2020.08.121.
Dental extractions can cause significant risk of bleeding in hemophilia patients being treated with factor replacements. However, mouth splints significantly decreased the risk of postextraction bleeding in these patients, according to Takahiro Yagyuu, DDS, of the department of oral and maxillofacial surgery, Nara Medical University, Kashihara, Japan, and colleagues.
The researchers performed a retrospective analysis of the medical records of hemophilia patients who underwent tooth extraction(s) between April 2006 and April 2019 at a single university hospital in Japan.
They conducted logistic regression analyses to identify risk/protective factors for postextraction bleeding in procedures involving patients receiving factor replacement therapy. Postextraction bleeding was defined as bleeding that could not be stopped by biting down on gauze and required medical treatment between 30 minutes and 14 days after the extraction, according to the report published online on in the British Journal of Oral & Maxillofacial Surgery.
A total of 130 extractions in 48 patients with hemophilia A and 21 extractions in 7 patients with hemophilia B were performed. Postextraction bleeding events were observed in 9 patients (16.3%) and 12 extractions (7.9%). On average, postextraction bleeding occurred 6 days after intervention and on the fifth postoperative day for extractions, according to the researchers.
Benefits of splints
The study found that the use of mouth splints significantly decreased the risk of postextraction bleeding (odds ratio, 0.13; P = .01) in hemophilia patients being treated with clotting factor replacements.
However, other factors in the study cohort, such as age, severity of hemophilia, duration of factor replacement therapy, gingival incision, bone removal, tooth separation, use of absorbable hemostats, wound closure, and the prescription of NSAIDs, were not significantly associated with postextraction bleeding, the researchers added.
“The use of mouth splints significantly decreased the risk of post-extraction bleeding. [In the future], we will conduct a prospective study to investigate the optimal type of splint and splint-wearing period to improve hemostatic management of tooth extraction in hemophilia patients,” the researchers concluded.
One author reported grants and personal fees from Bayer, Bioverativ, Chugai Pharmaceutical, Novo Nordisk, and Shire. A second author teaches a course endowed by Shire Japan. The other authors reported they had no conflicts.
SOURCE: Yagyuu T et al. Br J Oral Maxillofac Surg. 2020 Oct 11. doi: 10.1016/j.bjoms.2020.08.121.
Dental extractions can cause significant risk of bleeding in hemophilia patients being treated with factor replacements. However, mouth splints significantly decreased the risk of postextraction bleeding in these patients, according to Takahiro Yagyuu, DDS, of the department of oral and maxillofacial surgery, Nara Medical University, Kashihara, Japan, and colleagues.
The researchers performed a retrospective analysis of the medical records of hemophilia patients who underwent tooth extraction(s) between April 2006 and April 2019 at a single university hospital in Japan.
They conducted logistic regression analyses to identify risk/protective factors for postextraction bleeding in procedures involving patients receiving factor replacement therapy. Postextraction bleeding was defined as bleeding that could not be stopped by biting down on gauze and required medical treatment between 30 minutes and 14 days after the extraction, according to the report published online on in the British Journal of Oral & Maxillofacial Surgery.
A total of 130 extractions in 48 patients with hemophilia A and 21 extractions in 7 patients with hemophilia B were performed. Postextraction bleeding events were observed in 9 patients (16.3%) and 12 extractions (7.9%). On average, postextraction bleeding occurred 6 days after intervention and on the fifth postoperative day for extractions, according to the researchers.
Benefits of splints
The study found that the use of mouth splints significantly decreased the risk of postextraction bleeding (odds ratio, 0.13; P = .01) in hemophilia patients being treated with clotting factor replacements.
However, other factors in the study cohort, such as age, severity of hemophilia, duration of factor replacement therapy, gingival incision, bone removal, tooth separation, use of absorbable hemostats, wound closure, and the prescription of NSAIDs, were not significantly associated with postextraction bleeding, the researchers added.
“The use of mouth splints significantly decreased the risk of post-extraction bleeding. [In the future], we will conduct a prospective study to investigate the optimal type of splint and splint-wearing period to improve hemostatic management of tooth extraction in hemophilia patients,” the researchers concluded.
One author reported grants and personal fees from Bayer, Bioverativ, Chugai Pharmaceutical, Novo Nordisk, and Shire. A second author teaches a course endowed by Shire Japan. The other authors reported they had no conflicts.
SOURCE: Yagyuu T et al. Br J Oral Maxillofac Surg. 2020 Oct 11. doi: 10.1016/j.bjoms.2020.08.121.
FROM THE BRITISH JOURNAL OF ORAL AND MAXILLOFACIAL SURGERY
Socioeconomic factors affect survival of multiple myeloma patients
Disparities driven by socioeconomic factors have been shown to affect outcomes for patients with a variety of cancer types. Researchers found that this was also true for patients with multiple myeloma, according to a report published in Hematology/Oncology and Stem Cell Therapy.
In particular, survival was affected by a variety of socioeconomic factors.
Researchers conducting the study queried the National Cancer Database for patients diagnosed with multiple myeloma between 2004 and 2016. Only those 56,102 patients who received systemic therapy as the first-line treatment were included, according to Thejus T. Jayakrishnan, MD, of Allegheny Health Network, Pittsburgh, and colleagues.
Enrollment rates for therapy were calculated using receiving systemic therapy as the incident event of interest (numerator) over time to initiation of therapy (denominator). The incident rate ratios were analyzed using Poisson regression. A multivariate Cox proportional hazards model was used for survival analysis of 50,543 patients, and differences were determined as hazard ratios.
Significant differences
The study showed that therapy enrollment was significantly affected by race and sex (P < .005), with the enrollment rate for women and for non-Hispanic Blacks both being lower versus men and non-Hispanic Whites, respectively.
Advanced age, earlier year of diagnosis, lack of insurance or Medicaid, and higher comorbidity were found to be associated with poor survival (HR >1), whereas being a woman or a non-Hispanic Black (who were speculated to have more favorable cytogenetic profiles), having a higher income, and having treatment at an academic center were all associated with improved survival (each category at HR <1).
“Disparities in [multiple myeloma] exist and are caused by a complex interplay of multiple factors, with socioeconomic factors such as insurance and income playing a dominant role. The disparities not only exact high human cost but also negatively impact the economics of health care,” the researchers concluded.
The study was not funded and the authors reported that they had no relevant disclosures.
SOURCE: Jayakrishnan TT et al. Hematol Oncol Stem Cell Ther. 2020 Oct 10. doi: 10.1016/j.hemonc.2020.09.005.
Disparities driven by socioeconomic factors have been shown to affect outcomes for patients with a variety of cancer types. Researchers found that this was also true for patients with multiple myeloma, according to a report published in Hematology/Oncology and Stem Cell Therapy.
In particular, survival was affected by a variety of socioeconomic factors.
Researchers conducting the study queried the National Cancer Database for patients diagnosed with multiple myeloma between 2004 and 2016. Only those 56,102 patients who received systemic therapy as the first-line treatment were included, according to Thejus T. Jayakrishnan, MD, of Allegheny Health Network, Pittsburgh, and colleagues.
Enrollment rates for therapy were calculated using receiving systemic therapy as the incident event of interest (numerator) over time to initiation of therapy (denominator). The incident rate ratios were analyzed using Poisson regression. A multivariate Cox proportional hazards model was used for survival analysis of 50,543 patients, and differences were determined as hazard ratios.
Significant differences
The study showed that therapy enrollment was significantly affected by race and sex (P < .005), with the enrollment rate for women and for non-Hispanic Blacks both being lower versus men and non-Hispanic Whites, respectively.
Advanced age, earlier year of diagnosis, lack of insurance or Medicaid, and higher comorbidity were found to be associated with poor survival (HR >1), whereas being a woman or a non-Hispanic Black (who were speculated to have more favorable cytogenetic profiles), having a higher income, and having treatment at an academic center were all associated with improved survival (each category at HR <1).
“Disparities in [multiple myeloma] exist and are caused by a complex interplay of multiple factors, with socioeconomic factors such as insurance and income playing a dominant role. The disparities not only exact high human cost but also negatively impact the economics of health care,” the researchers concluded.
The study was not funded and the authors reported that they had no relevant disclosures.
SOURCE: Jayakrishnan TT et al. Hematol Oncol Stem Cell Ther. 2020 Oct 10. doi: 10.1016/j.hemonc.2020.09.005.
Disparities driven by socioeconomic factors have been shown to affect outcomes for patients with a variety of cancer types. Researchers found that this was also true for patients with multiple myeloma, according to a report published in Hematology/Oncology and Stem Cell Therapy.
In particular, survival was affected by a variety of socioeconomic factors.
Researchers conducting the study queried the National Cancer Database for patients diagnosed with multiple myeloma between 2004 and 2016. Only those 56,102 patients who received systemic therapy as the first-line treatment were included, according to Thejus T. Jayakrishnan, MD, of Allegheny Health Network, Pittsburgh, and colleagues.
Enrollment rates for therapy were calculated using receiving systemic therapy as the incident event of interest (numerator) over time to initiation of therapy (denominator). The incident rate ratios were analyzed using Poisson regression. A multivariate Cox proportional hazards model was used for survival analysis of 50,543 patients, and differences were determined as hazard ratios.
Significant differences
The study showed that therapy enrollment was significantly affected by race and sex (P < .005), with the enrollment rate for women and for non-Hispanic Blacks both being lower versus men and non-Hispanic Whites, respectively.
Advanced age, earlier year of diagnosis, lack of insurance or Medicaid, and higher comorbidity were found to be associated with poor survival (HR >1), whereas being a woman or a non-Hispanic Black (who were speculated to have more favorable cytogenetic profiles), having a higher income, and having treatment at an academic center were all associated with improved survival (each category at HR <1).
“Disparities in [multiple myeloma] exist and are caused by a complex interplay of multiple factors, with socioeconomic factors such as insurance and income playing a dominant role. The disparities not only exact high human cost but also negatively impact the economics of health care,” the researchers concluded.
The study was not funded and the authors reported that they had no relevant disclosures.
SOURCE: Jayakrishnan TT et al. Hematol Oncol Stem Cell Ther. 2020 Oct 10. doi: 10.1016/j.hemonc.2020.09.005.
FROM HEMATOLOGY/ONCOLOGY AND STEM CELL THERAPY
ESMO offers new clinical practice guideline for CLL
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
An updated European Society for Medical Oncology (ESMO) clinical practice guidelines were released to provide key recommendations on the management of chronic lymphocytic leukemia (CLL).
The guidelines were developed by a multidisciplinary group of experts from different institutions and countries in Europe and provide levels of evidence and grades of recommendation where applicable for issues regarding prognosis and treatment decisions in CLL. Such decisions depend on genetic and clinical factors such as age, stage, and comorbidities. The guidelines also focus on new therapies targeting B-cell-receptor pathways or defect mechanism of apoptosis, which have been found to induce long-lasting remissions. The guidelines were endorsed by the European Hematology Association (EHA) through the Scientific Working Group on CLL/European Research Initiative on CLL (ERIC), according to the report published online the Annals of Oncology.
These clinical practice guidelines were developed in accordance with the ESMO standard operating procedures for clinical practice guidelines development with use of relevant literature selected by the expert authors. Statements without grading were considered justified as standard clinical practice by the experts and the ESMO faculty.
Below are some highlights of the guidelines, which cover a wide array of topics regarding the diagnosis, staging, treatment, and progression of CLL disease.
Diagnosis
The guidelines indicate that CLL diagnosis is usually possible by immunophenotyping of peripheral blood only and that lymph node (LN) biopsy and/or bone marrow biopsy may be helpful if immunophenotyping is not conclusive for the diagnosis of CLL, according to Barbara Eichhorst, MD, of the University of Cologne (Germany) and colleagues on behalf of the ESMO guidelines committee.
Staging and risk assessment
Early asymptomatic-stage disease does not need further risk assessment, but after the first year, when all patients should be seen at 3-monthly intervals, patients can be followed every 3-12 months. The interval would depend on burden and dynamics of the disease obtained by the using history and physical examinations, including a careful palpation of all LN areas, spleen, and liver, as well as assessing complete blood cell count and differential count, according to the report.
Advanced- and symptomatic-stage disease requires a broader examination including imaging, history and status of relevant infections, and fluorescent in situ hybridization (FISH) assays for the detection of deletion of the chromosome 17 (del[17p]) affecting the tumor protein p53 expression and, in the absence of del(17p), TP53 sequencing for detection of TP53 gene mutation, according to the authors.
Prognostication
Two clinical staging systems are typically used in CLL. Both Binet and Rai staging systems separate three groups of patients with different prognosis, although “as a consequence of more effective therapy, the overall survival (OS) of patients with advanced stage has improved and the relevance of the staging systems for prognostication has decreased,” according to the report.
The recent addition of genetic markers has also proved highly relevant to identifying patients with different prognoses and to guide treatment.
Therapy
Although CLL is an incurable disease, choice and application of treatment are strongly tied to the length of survival, according to the authors. The guidelines recommend Binet and Rai staging with clinical symptoms as relevant for treatment indication. In addition, the identification of del(17p), TP53 mutations, and IGHV status are relevant for choice of therapy and should be assessed prior to treatment.
The guidelines discuss specific treatment modalities for various stages of the disease, from early stages to relapse.
For frontline therapy, different treatment strategies are available including continuous treatment with Bruton tyrosine kinase (BTK)–inhibitors, such as ibrutinib, until progression or time-limited therapy with ChT backbone and CD20 antibodies. In addition, the Food and Drug Administration and European Medicines Agency have recently approved the combination of venetoclax plus obinutuzumab for first-line therapy of CLL.
Treatment decisions should include an assessment of IGHV and TP53 status, as well as patient-related factors such as comedication, comorbidities, preferences, drug availability, and potential of treatment adherence, according to the guidelines.
In case of symptomatic relapse within 3 years after fixed-duration therapy or nonresponse to therapy, the guidelines recommend that the therapeutic regimen should be changed, regardless of the type of first-line either to venetoclax plus rituximab for 24 months or to ibrutinib, acalabrutinib, or other BTK inhibitors (if available) as continuous therapy.
The guidelines also discuss the possible roles for hematopoietic stem cell transplantation and cellular therapies, as well as the treatment of the various complications that can arise in patients with CLL, and dealing with various aspects of disease progression.
No external funds were provided for the production of the guidelines. The authors of the report and members of the ESMO Guidelines Committee reported numerous disclosures regarding pharmaceutical and biotechnology companies.
SOURCE: Eichhorst B et al. Ann Oncol. 2020 Oct 19. doi: 10.1016/j.annonc.2020.09.019.
FROM ANNALS OF ONCOLOGY
Older age, r/r disease in lymphoma patients tied to increased COVID-19 death rate
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
FROM ECLINICALMEDICINE