User login
Endocrinologist charged after bomb-making supplies found
An endocrinologist in Naples, Fla., faces multiple federal charges after police found homemade explosives and bomb-making supplies, as well as numerous illegal drugs, in his home.
Police were executing a search warrant at the home of Christy Daniel Cugini, MD, 63, on March 30 when they found the items, according to Collier County Sheriff’s Office (CCSO).
“An investigation continues and more charges could be brought,” the sheriff’s office said in a statement. As of April 1, Dr. Cugini was out on bond. His next court appearance is on April 26.
A search of his bedroom turned up marijuana, tramadol, oxycodone, and hydrocodone, the sheriff’s office said. According to nbcmiami.com, police also found 560 grams of marijuana and $20,000 in cash and jewelry in a safe.
“Some of the narcotics were in pill bottles with other people’s names on them. Many of the substances were of trafficking quantities. The search also turned up numerous items of narcotic paraphernalia, including heat seal bags, a vacuum sealer, and a scale,” the CCSO report said.
Charges against Dr. Cugini include narcotics trafficking; possession of marijuana with intent to sell/manufacture/deliver; possession of more than 20 grams of marijuana; possession of a controlled substance; and possession of narcotic paraphernalia, according to the report.
He was also charged with nine counts of making/possessing a destructive device.
The CCSO bomb squad was brought in to investigate the homemade explosive devices and supplies, including potassium nitrate and ammonium nitrate – which can be used as oxidizers – PVC pipe, and flash powders used in fireworks in Dr. Cugini’s house and garage.
Newsweek reported that the bomb squad found six red cylindrical devices about 4 inches long, according to information reported in an affidavit from Collier County Officer Jeffrey Tayar. They may have been intended to be a hand-tossed improvised explosive device, Mr. Tayar wrote.
An officer also found three other devices made up of PVC pipe attached to a small wood square. A rifle round was inserted into the PVC pipe, Mr. Tayar’s report said.
“The device could be placed on the ground in such a manner as to leave the rifle round facing up,” Mr. Tayar reportedly wrote. “If downward pressure were applied on the tip of the round ... the rifle round [would] discharge, launching the projectile portion of the round upward, presumably into the foot of the subject stepping on it.”
NBC News reported that deputies said Dr. Cugini appeared to live only with his young daughter.
He initially agreed to speak with deputies but then invoked his Miranda rights and stopped answering questions, NBC said.
Dr. Cugini’s profile has been removed from the Millennium Physician Group website.
His employer offered this statement via spokesperson Liza Fernandez: “We are shocked at the allegations regarding Dr. Christy Cugini. He has been placed on administrative leave until further notice. Millennium is committed to cooperating with law enforcement and is conducting an internal investigation.”
According to U.S. News & World Report, Dr. Cugini is affiliated with NCH Baker Hospital. He received his medical degree from Ross University School of Medicine, now located in Barbados, and has been practicing for more than 20 years.
Attempts to contact Dr. Cugini were unsuccessful.
A version of this article first appeared on Medscape.com.
An endocrinologist in Naples, Fla., faces multiple federal charges after police found homemade explosives and bomb-making supplies, as well as numerous illegal drugs, in his home.
Police were executing a search warrant at the home of Christy Daniel Cugini, MD, 63, on March 30 when they found the items, according to Collier County Sheriff’s Office (CCSO).
“An investigation continues and more charges could be brought,” the sheriff’s office said in a statement. As of April 1, Dr. Cugini was out on bond. His next court appearance is on April 26.
A search of his bedroom turned up marijuana, tramadol, oxycodone, and hydrocodone, the sheriff’s office said. According to nbcmiami.com, police also found 560 grams of marijuana and $20,000 in cash and jewelry in a safe.
“Some of the narcotics were in pill bottles with other people’s names on them. Many of the substances were of trafficking quantities. The search also turned up numerous items of narcotic paraphernalia, including heat seal bags, a vacuum sealer, and a scale,” the CCSO report said.
Charges against Dr. Cugini include narcotics trafficking; possession of marijuana with intent to sell/manufacture/deliver; possession of more than 20 grams of marijuana; possession of a controlled substance; and possession of narcotic paraphernalia, according to the report.
He was also charged with nine counts of making/possessing a destructive device.
The CCSO bomb squad was brought in to investigate the homemade explosive devices and supplies, including potassium nitrate and ammonium nitrate – which can be used as oxidizers – PVC pipe, and flash powders used in fireworks in Dr. Cugini’s house and garage.
Newsweek reported that the bomb squad found six red cylindrical devices about 4 inches long, according to information reported in an affidavit from Collier County Officer Jeffrey Tayar. They may have been intended to be a hand-tossed improvised explosive device, Mr. Tayar wrote.
An officer also found three other devices made up of PVC pipe attached to a small wood square. A rifle round was inserted into the PVC pipe, Mr. Tayar’s report said.
“The device could be placed on the ground in such a manner as to leave the rifle round facing up,” Mr. Tayar reportedly wrote. “If downward pressure were applied on the tip of the round ... the rifle round [would] discharge, launching the projectile portion of the round upward, presumably into the foot of the subject stepping on it.”
NBC News reported that deputies said Dr. Cugini appeared to live only with his young daughter.
He initially agreed to speak with deputies but then invoked his Miranda rights and stopped answering questions, NBC said.
Dr. Cugini’s profile has been removed from the Millennium Physician Group website.
His employer offered this statement via spokesperson Liza Fernandez: “We are shocked at the allegations regarding Dr. Christy Cugini. He has been placed on administrative leave until further notice. Millennium is committed to cooperating with law enforcement and is conducting an internal investigation.”
According to U.S. News & World Report, Dr. Cugini is affiliated with NCH Baker Hospital. He received his medical degree from Ross University School of Medicine, now located in Barbados, and has been practicing for more than 20 years.
Attempts to contact Dr. Cugini were unsuccessful.
A version of this article first appeared on Medscape.com.
An endocrinologist in Naples, Fla., faces multiple federal charges after police found homemade explosives and bomb-making supplies, as well as numerous illegal drugs, in his home.
Police were executing a search warrant at the home of Christy Daniel Cugini, MD, 63, on March 30 when they found the items, according to Collier County Sheriff’s Office (CCSO).
“An investigation continues and more charges could be brought,” the sheriff’s office said in a statement. As of April 1, Dr. Cugini was out on bond. His next court appearance is on April 26.
A search of his bedroom turned up marijuana, tramadol, oxycodone, and hydrocodone, the sheriff’s office said. According to nbcmiami.com, police also found 560 grams of marijuana and $20,000 in cash and jewelry in a safe.
“Some of the narcotics were in pill bottles with other people’s names on them. Many of the substances were of trafficking quantities. The search also turned up numerous items of narcotic paraphernalia, including heat seal bags, a vacuum sealer, and a scale,” the CCSO report said.
Charges against Dr. Cugini include narcotics trafficking; possession of marijuana with intent to sell/manufacture/deliver; possession of more than 20 grams of marijuana; possession of a controlled substance; and possession of narcotic paraphernalia, according to the report.
He was also charged with nine counts of making/possessing a destructive device.
The CCSO bomb squad was brought in to investigate the homemade explosive devices and supplies, including potassium nitrate and ammonium nitrate – which can be used as oxidizers – PVC pipe, and flash powders used in fireworks in Dr. Cugini’s house and garage.
Newsweek reported that the bomb squad found six red cylindrical devices about 4 inches long, according to information reported in an affidavit from Collier County Officer Jeffrey Tayar. They may have been intended to be a hand-tossed improvised explosive device, Mr. Tayar wrote.
An officer also found three other devices made up of PVC pipe attached to a small wood square. A rifle round was inserted into the PVC pipe, Mr. Tayar’s report said.
“The device could be placed on the ground in such a manner as to leave the rifle round facing up,” Mr. Tayar reportedly wrote. “If downward pressure were applied on the tip of the round ... the rifle round [would] discharge, launching the projectile portion of the round upward, presumably into the foot of the subject stepping on it.”
NBC News reported that deputies said Dr. Cugini appeared to live only with his young daughter.
He initially agreed to speak with deputies but then invoked his Miranda rights and stopped answering questions, NBC said.
Dr. Cugini’s profile has been removed from the Millennium Physician Group website.
His employer offered this statement via spokesperson Liza Fernandez: “We are shocked at the allegations regarding Dr. Christy Cugini. He has been placed on administrative leave until further notice. Millennium is committed to cooperating with law enforcement and is conducting an internal investigation.”
According to U.S. News & World Report, Dr. Cugini is affiliated with NCH Baker Hospital. He received his medical degree from Ross University School of Medicine, now located in Barbados, and has been practicing for more than 20 years.
Attempts to contact Dr. Cugini were unsuccessful.
A version of this article first appeared on Medscape.com.
Black inpatients at higher risk of poor safety outcomes
One expert says these findings should be a call to action for hospitals and physicians.
The Urban Institute, which is funded in part by the Robert Wood Johnson Foundation, looked at differences in Black and White patient safety measures among adults receiving inpatient care in 26 states.
Care quality was measured by the rate of preventable adverse hospital patient safety events per 1,000 at-risk discharges using data from the Agency for Healthcare Research and Quality (AHRQ).
Researchers compared experience by race on 11 patient safety indicators – four related to general patient safety, and seven linked to risk of adverse events with surgical procedures.
Surgical risk differences significant
The gaps were widest surrounding surgical care. Black patients were 7.9 percentage points more likely to be in a hospital considered low quality across all surgical safety measures. They were 4.9 percentage points more likely to be admitted to a hospital considered low quality across all general safety indicators.
“If you’re a Black patient getting surgery – relative to a White patient – in my study, you were 25% less likely to be in a hospital that prevented hemorrhage during surgery; you were 26% less likely to be in a hospital that prevented postoperative respiratory failure; and you were more than 30% less likely to be in a hospital that is effective in preventing postoperative sepsis,” Anuj Gangopadhyaya, PhD, senior research associate at the Urban Institute, said in an interview.
According to the report, Black patients were also 31.9% less likely than were White patients to be admitted into hospitals considered high quality in preventing pressure ulcers and 22.8% less likely to be in a hospital good at preventing iatrogenic pneumothorax.
Dr. Gangopadhyaya said this may be the first study to compare the numbers after the inception of the Affordable Care Act. These data were collected in 2017, 3 years after the core elements of the ACA kicked in.
He said that although the ACA has done much to narrow the racial gap in terms of insurance coverage, it has not been effective in reducing the heightened safety risk to Black patients in the hospital.
‘Shocking, though not surprising’
Uché Blackstock, MD, founder and CEO of Advancing Health Equity in New York City, called the findings “shocking, though not surprising.”
Though these data were collected before COVID-19, the pandemic has exposed profound racial inequities, she noted.
She cited the example of Susan Moore, MD, a Black physician in Carmel, Ind., who died from COVID-19 at age 52 in December after experiencing what she said was systemic racism in her care.
“We saw in the death of Dr. Susan Moore that even having a formal education and being a physician is not protective for Black patients. These findings only reaffirm what we already know – that Black patients receive worse and lower-quality care than White patients,” Dr. Blackstock said in an interview.
“These findings are not a result of Black patients’ individual choices as is often suggested, but rather the results of a health care system that has devalued the lives of Black patients and inherently provides poorer quality of care to them.”
Dr. Blackstock said this report represents a call to action.
Health care institutions must, she said, “look inward at the intentional and critical antiracism work that must be done on provider, organizational, and systems levels by allocating the necessary resources, continuing to track disaggregated health metrics, and committing to structural change within health care systems.”
Resources instead of penalties?
Dr. Gangopadhyaya said the second phase of the research will compare safety outcomes between Black and White patients in the same hospital. Those results will shed more light on what’s driving the differences in risk on safety measures.
He acknowledged that, particularly in an emergency, there is little choice involved with which hospital a patient enters. Patients typically go to a hospital in their neighborhood. And it’s well established that ZIP codes can determine health care outcomes.
But he suspects the differences cannot be explained simply by socioeconomic factors.
He pointed out that previous research has found disparities among Black and White patients in the same neighborhoods.
In one part of this study, researchers narrowed the comparison to Black and White adults with Medicare coverage, with similar provider networks and reimbursement structure, to test whether insurance was playing a significant role.
“Even among that group, you still see the persistent differences in the safety risks driven by the hospitals patients are admitted to,” Dr. Gangopadhyaya said.
He suggests two policy approaches to address the gaps: Either find ways for high-quality hospitals to reach more people of color, or find out what’s keeping the low-quality hospitals from implementing the practices that are effective in high-quality hospitals.
Currently, the ACA has penalties in place when hospitals score low for specific safety risks, he noted, saying that approach doesn’t appear to be working.
“Perhaps instead of penalizing hospitals, we might want to consider providing resources to hospitals that help them better adopt the successful protocols in their high-quality counterparts,” he said.
Dr. Gangopadhyaya has disclosed no relevant financial relationships. Dr. Blackstock has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 4/2/21.
One expert says these findings should be a call to action for hospitals and physicians.
The Urban Institute, which is funded in part by the Robert Wood Johnson Foundation, looked at differences in Black and White patient safety measures among adults receiving inpatient care in 26 states.
Care quality was measured by the rate of preventable adverse hospital patient safety events per 1,000 at-risk discharges using data from the Agency for Healthcare Research and Quality (AHRQ).
Researchers compared experience by race on 11 patient safety indicators – four related to general patient safety, and seven linked to risk of adverse events with surgical procedures.
Surgical risk differences significant
The gaps were widest surrounding surgical care. Black patients were 7.9 percentage points more likely to be in a hospital considered low quality across all surgical safety measures. They were 4.9 percentage points more likely to be admitted to a hospital considered low quality across all general safety indicators.
“If you’re a Black patient getting surgery – relative to a White patient – in my study, you were 25% less likely to be in a hospital that prevented hemorrhage during surgery; you were 26% less likely to be in a hospital that prevented postoperative respiratory failure; and you were more than 30% less likely to be in a hospital that is effective in preventing postoperative sepsis,” Anuj Gangopadhyaya, PhD, senior research associate at the Urban Institute, said in an interview.
According to the report, Black patients were also 31.9% less likely than were White patients to be admitted into hospitals considered high quality in preventing pressure ulcers and 22.8% less likely to be in a hospital good at preventing iatrogenic pneumothorax.
Dr. Gangopadhyaya said this may be the first study to compare the numbers after the inception of the Affordable Care Act. These data were collected in 2017, 3 years after the core elements of the ACA kicked in.
He said that although the ACA has done much to narrow the racial gap in terms of insurance coverage, it has not been effective in reducing the heightened safety risk to Black patients in the hospital.
‘Shocking, though not surprising’
Uché Blackstock, MD, founder and CEO of Advancing Health Equity in New York City, called the findings “shocking, though not surprising.”
Though these data were collected before COVID-19, the pandemic has exposed profound racial inequities, she noted.
She cited the example of Susan Moore, MD, a Black physician in Carmel, Ind., who died from COVID-19 at age 52 in December after experiencing what she said was systemic racism in her care.
“We saw in the death of Dr. Susan Moore that even having a formal education and being a physician is not protective for Black patients. These findings only reaffirm what we already know – that Black patients receive worse and lower-quality care than White patients,” Dr. Blackstock said in an interview.
“These findings are not a result of Black patients’ individual choices as is often suggested, but rather the results of a health care system that has devalued the lives of Black patients and inherently provides poorer quality of care to them.”
Dr. Blackstock said this report represents a call to action.
Health care institutions must, she said, “look inward at the intentional and critical antiracism work that must be done on provider, organizational, and systems levels by allocating the necessary resources, continuing to track disaggregated health metrics, and committing to structural change within health care systems.”
Resources instead of penalties?
Dr. Gangopadhyaya said the second phase of the research will compare safety outcomes between Black and White patients in the same hospital. Those results will shed more light on what’s driving the differences in risk on safety measures.
He acknowledged that, particularly in an emergency, there is little choice involved with which hospital a patient enters. Patients typically go to a hospital in their neighborhood. And it’s well established that ZIP codes can determine health care outcomes.
But he suspects the differences cannot be explained simply by socioeconomic factors.
He pointed out that previous research has found disparities among Black and White patients in the same neighborhoods.
In one part of this study, researchers narrowed the comparison to Black and White adults with Medicare coverage, with similar provider networks and reimbursement structure, to test whether insurance was playing a significant role.
“Even among that group, you still see the persistent differences in the safety risks driven by the hospitals patients are admitted to,” Dr. Gangopadhyaya said.
He suggests two policy approaches to address the gaps: Either find ways for high-quality hospitals to reach more people of color, or find out what’s keeping the low-quality hospitals from implementing the practices that are effective in high-quality hospitals.
Currently, the ACA has penalties in place when hospitals score low for specific safety risks, he noted, saying that approach doesn’t appear to be working.
“Perhaps instead of penalizing hospitals, we might want to consider providing resources to hospitals that help them better adopt the successful protocols in their high-quality counterparts,” he said.
Dr. Gangopadhyaya has disclosed no relevant financial relationships. Dr. Blackstock has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 4/2/21.
One expert says these findings should be a call to action for hospitals and physicians.
The Urban Institute, which is funded in part by the Robert Wood Johnson Foundation, looked at differences in Black and White patient safety measures among adults receiving inpatient care in 26 states.
Care quality was measured by the rate of preventable adverse hospital patient safety events per 1,000 at-risk discharges using data from the Agency for Healthcare Research and Quality (AHRQ).
Researchers compared experience by race on 11 patient safety indicators – four related to general patient safety, and seven linked to risk of adverse events with surgical procedures.
Surgical risk differences significant
The gaps were widest surrounding surgical care. Black patients were 7.9 percentage points more likely to be in a hospital considered low quality across all surgical safety measures. They were 4.9 percentage points more likely to be admitted to a hospital considered low quality across all general safety indicators.
“If you’re a Black patient getting surgery – relative to a White patient – in my study, you were 25% less likely to be in a hospital that prevented hemorrhage during surgery; you were 26% less likely to be in a hospital that prevented postoperative respiratory failure; and you were more than 30% less likely to be in a hospital that is effective in preventing postoperative sepsis,” Anuj Gangopadhyaya, PhD, senior research associate at the Urban Institute, said in an interview.
According to the report, Black patients were also 31.9% less likely than were White patients to be admitted into hospitals considered high quality in preventing pressure ulcers and 22.8% less likely to be in a hospital good at preventing iatrogenic pneumothorax.
Dr. Gangopadhyaya said this may be the first study to compare the numbers after the inception of the Affordable Care Act. These data were collected in 2017, 3 years after the core elements of the ACA kicked in.
He said that although the ACA has done much to narrow the racial gap in terms of insurance coverage, it has not been effective in reducing the heightened safety risk to Black patients in the hospital.
‘Shocking, though not surprising’
Uché Blackstock, MD, founder and CEO of Advancing Health Equity in New York City, called the findings “shocking, though not surprising.”
Though these data were collected before COVID-19, the pandemic has exposed profound racial inequities, she noted.
She cited the example of Susan Moore, MD, a Black physician in Carmel, Ind., who died from COVID-19 at age 52 in December after experiencing what she said was systemic racism in her care.
“We saw in the death of Dr. Susan Moore that even having a formal education and being a physician is not protective for Black patients. These findings only reaffirm what we already know – that Black patients receive worse and lower-quality care than White patients,” Dr. Blackstock said in an interview.
“These findings are not a result of Black patients’ individual choices as is often suggested, but rather the results of a health care system that has devalued the lives of Black patients and inherently provides poorer quality of care to them.”
Dr. Blackstock said this report represents a call to action.
Health care institutions must, she said, “look inward at the intentional and critical antiracism work that must be done on provider, organizational, and systems levels by allocating the necessary resources, continuing to track disaggregated health metrics, and committing to structural change within health care systems.”
Resources instead of penalties?
Dr. Gangopadhyaya said the second phase of the research will compare safety outcomes between Black and White patients in the same hospital. Those results will shed more light on what’s driving the differences in risk on safety measures.
He acknowledged that, particularly in an emergency, there is little choice involved with which hospital a patient enters. Patients typically go to a hospital in their neighborhood. And it’s well established that ZIP codes can determine health care outcomes.
But he suspects the differences cannot be explained simply by socioeconomic factors.
He pointed out that previous research has found disparities among Black and White patients in the same neighborhoods.
In one part of this study, researchers narrowed the comparison to Black and White adults with Medicare coverage, with similar provider networks and reimbursement structure, to test whether insurance was playing a significant role.
“Even among that group, you still see the persistent differences in the safety risks driven by the hospitals patients are admitted to,” Dr. Gangopadhyaya said.
He suggests two policy approaches to address the gaps: Either find ways for high-quality hospitals to reach more people of color, or find out what’s keeping the low-quality hospitals from implementing the practices that are effective in high-quality hospitals.
Currently, the ACA has penalties in place when hospitals score low for specific safety risks, he noted, saying that approach doesn’t appear to be working.
“Perhaps instead of penalizing hospitals, we might want to consider providing resources to hospitals that help them better adopt the successful protocols in their high-quality counterparts,” he said.
Dr. Gangopadhyaya has disclosed no relevant financial relationships. Dr. Blackstock has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 4/2/21.
Vaccine mismatch: What to do after dose 1 when plans change
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
Ideally, Americans receiving their Pfizer/BioNTech or Moderna COVID-19 vaccines will get both doses from the same manufacturer, said Gregory Poland, MD, a vaccinologist at the Mayo Clinic in Rochester, Minn.
After all, that’s how they were tested for efficacy and safety, and it was results from those studies that led to emergency use authorization (EUA) being granted by the Food and Drug Administration.
But states and countries have struggled to keep up with the demand for vaccine, and more flexible vaccination schedules could help.
So researchers are exploring whether it is safe and effective to get the first and second doses from different manufacturers. And they are even wondering whether mixing doses from different manufacturers could increase effectiveness, particularly in light of emerging variants.
It’s called the “interchangeability issue,” said Dr. Poland, who has gotten a steady stream of questions about it.
For example, a patient recently asked about options for his father, who had gotten his first dose of the AstraZeneca vaccine in Ecuador, but had since moved to the United States, where that product has not been approved for use.
Dr. Poland said in an interview that he prefaces each answer with: “I’ve got no science for what I’m about to tell you.”
In this particular case, he recommended that the man’s father talk with his doctor about his level of COVID-19 risk and consider whether he should gamble on the AstraZeneca vaccine getting approved in the United States soon, or whether he should ask for a second dose from one of the three vaccines currently approved.
On March 22, 2021, AstraZeneca released positive results from its phase 3 trial, which will likely speed its path toward use in the United States.
Although clinical trials have started to test combinations and boosters, there’s currently no definitive evidence from human trials on mixing COVID vaccines, Dr. Poland pointed out.
But a study of a mixed-vaccine regimen is currently underway in the United Kingdom.
Participants in that 13-month trial will be given the Oxford/AstraZeneca and Pfizer/BioNTech vaccines in different combinations and at different intervals. The first results from that trial are expected this summer.
And interim results from a trial combining Russia’s Sputnik V and the AstraZeneca vaccines are expected in 2 months, according to a Reuters report.
Mix only in ‘exceptional situations’
The Centers for Disease Control and Prevention has been hesitant to open the door to mixing Pfizer and Moderna vaccinations, noting that the two “are not interchangeable.” But CDC guidance has changed slightly. Now, instead of saying the two vaccines should not be mixed, CDC guidance says they can be mixed in “exceptional situations,” and that the second dose can be administered up to 6 weeks after the first dose.
It is reasonable to assume that mixing COVID-19 vaccines that use the same platform – such as the mRNA platform used by both the Pfizer and Moderna vaccines – will be acceptable, Dr. Poland said, although human trials have not proven that.
However, it is unclear whether vaccines that use different platforms can be mixed. Can the first dose of an mRNA vaccine be followed by an adenovirus-based vaccine, like the Johnson & Johnson product or Novavax, if that vaccine is granted an EUA?
Ross Kedl, PhD, a vaccine researcher and professor of immunology at the University of Colorado at Denver, Aurora, said matching vaccine platforms might not be the preferred vaccination strategy.
He disagreed that there’s a lack of science surrounding the issue, and said all signs point to mixing as not only a good option, but probably a better one.
Researcher says science backs mixing
A mix of two different vaccine platforms likely enhances immunity, Dr. Kedl said. The heterologous prime-boost strategy has been used in animal studies for decades, “and it is well known that this promotes a much better immune response than when immunizing with the same vaccine twice.
“If you think about it in a Venn diagram sort of way, it makes sense,” he said in an interview. “Each vaccine has a number of components in it that influence immunity in various ways, but between the two of them, they only have one component that is similar. In the case of the coronavirus vaccines, the one thing both have in common is the spike protein from SARS-CoV-2. In essence, this gives you two shots at generating immunity against the one thing in each vaccine you care most about, but only one shot for the other vaccine components in each platform, resulting in an amplified response against the common target.”
In fact, the heterologous prime-boost vaccination strategy has proven to be effective in humans in early studies.
For example, an Ebola regimen that consisted of an adenovirus vector, similar to the AstraZeneca COVID vaccine, and a modified vaccinia virus vector showed promise in a phase 1 study. And an HIV regimen that consisted of the combination of a DNA vaccine, similar to the Pfizer and Moderna mRNA vaccines, and another viral vector showed encouraging results in a proof-of-concept study.
In both these cases, the heterologous prime-boost strategy was far better than single-vaccine prime-boost regimens, Dr. Kedl pointed out. And neither study reported any safety issues with the combinations.
For now, it’s best to stick with the same manufacturer for both shots, as the CDC guidance suggests, he said, agreeing with Dr. Poland.
But “I would be very surprised if we didn’t move to a mixing of vaccine platforms for the population,” Dr. Kedl said.
A version of this article first appeared on Medscape.com.
COVID vaccines could lose their punch within a year, experts say
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
2021 match sets records: Who matched and who didn’t?
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
First pill for COVID-19 could be ready by year’s end
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
Variants spur new FDA guidance on COVID vaccines, tests, drugs
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
COVID cuts internists’ happiness in life outside work
Before the pandemic, a large majority of internists reported that they were generally happy with life outside of work, although by specialty, they were near the bottom in happiness.
But this year’s Medscape Internist Lifestyle, Happiness & Burnout Report 2021 shows a sharp drop, with just 55% of respondents saying they are somewhat or very happy in life outside work, compared with 78% last year.
Internists were not alone among the more than 12,000 physicians who responded to the survey. The contrast from last year’s report was clear for physicians in general and reflects COVID-19’s substantial toll on clinicians.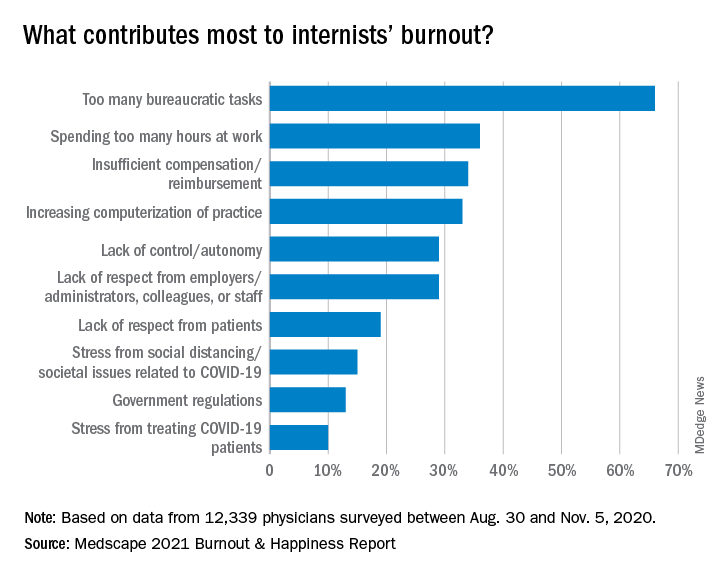
Just 58% of physicians overall reported happy lives outside work, down from 82% last year.
Perhaps not surprising, given the particular demands on certain specialties, physicians in infectious disease were the least happy, at 45%, followed by pulmonologists (47%) and rheumatologists and intensivists, at 49%.
The highest happiness level was reported by those in diabetes and endocrinology, at 73% this year, but that proportion was also substantially lower than the 89% from last year.
Burnout has ‘strong impact on lives’
The percentage of internists who reported burnout or depression, however, has stayed fairly consistent.
More than half (52%) said that burnout had a strong or severe impact on their lives, and nearly 1 in 10 said it was severe enough that they are considering leaving medicine.
One percent of the internists who responded to the survey said they had attempted suicide, and 12% said they had thoughts of suicide but had not attempted it.
Most of those reporting burnout (82%) said it started before the COVID-19 pandemic, but 18% said it began with the pandemic.
Notably, though, physicians ranked problems related to stress from COVID-19 near the bottom among burnout drivers. The top factor, by far, again, was “too many bureaucratic tasks.”
A large majority (78%) of internists work online for up to 10 hours a week, a number that could grow as telemedicine grows.
Exercise is top coping method
Responses gave a peek into how physicians are coping with burnout. Among internists, 49% put exercise at the top. Isolating themselves from others was the next most popular choice, at 45%. Eating junk food and drinking alcohol were further down the list, at 34% and 24%, respectively.
Few internists said they drink alcohol daily, a finding consistent with past years. In fact, 29% said they don’t drink at all, and 26% said they have fewer than one alcoholic drink per week. Only 7% said they had seven or more drinks per week.
The National Institute on Alcohol Abuse and Alcoholism advises that men not have more than 14 alcoholic drinks per week and that women not have more than 7.
Work-life balance topped list of concerns
By far, internists said work-life balance was their top workplace concern. Nearly half (48%) chose that answer, more than twice the percentage who said compensation was the biggest concern (21%).
Asked whether they would take a salary cut for more work-life balance, a similar proportion (46%) said yes.
Forty-three percent of internists manage to take 3-4 weeks of vacation, and 10% take at least 5 weeks, similar to reported vacation time in last year’s survey.
The vast majority are in committed relationships, with 79% reporting that they are married, and 5% reporting that they are living with a partner. Of those who are married, 48% described the marriage as very good; 32%, good; 16%, fair; 2%, poor; and 1%, very poor; 1% preferred not to answer.
One in five internists said their spouse was a physician, and 24% said their spouse worked in the health care field but not as a physician.
Pandemic has increased burnout
Douglas S. Paauw, MD, and Eileen Barrett, MD, two members of the Internal Medicine News editorial advisory board, said they were not surprised by the survey findings.
“There is more burnout since the pandemic,” said Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and third-year medical student clerkship director at the University of Washington. “People may be working more hours, higher stress, but also, some may be working less hours, are socially isolated, taking on a new role of helping their kids in virtual education, andn living in cramped quarters with family that they may not be accustomed to spending so much time with.”
“Also, most physicians love travel, to detress, get back in balance, and that has by and large been taken away by the pandemic,” Dr. Paauw noted. “Unfortunately, bureaucracy did not go away during the pandemic!
Dr. Barrett, an internal medicine hospitalist, said, “It is most concerning to me today that 12% have had thoughts of suicide, and yet 39% are too busy to seek care for depression or burnout, and 17% aren’t seeking due to fear it will be disclosed,”
“Credentialing, medical license applications, and malpractice insurance applications can and must be changed posthaste to support physicians and stop stigmatizing mental health diagnoses and mental health care,” she said. “Removing application questions about physician mental health will be consistent with recommendations from the Federation of State Medical Boards, medical professional societies, and the Americans with Disabilities Act, and is something actionable and achievable for every organization to do in 2021.” “From a public policy perspective, I am deeply concerned about the physician workforce and how patients will be able to receive care from exhausted, burned out physicians who may be reducing their clinical hours to restore their personal happiness – understandably so,” added Dr. Barrett, who is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
She pointed out that there are mental health resources available for physicians that don’t go through their employers or insurance such as www.emotionalppe.org/.
A version of this article first appeared on Medscape.com.
Katie Lennon contributed to this report.
Before the pandemic, a large majority of internists reported that they were generally happy with life outside of work, although by specialty, they were near the bottom in happiness.
But this year’s Medscape Internist Lifestyle, Happiness & Burnout Report 2021 shows a sharp drop, with just 55% of respondents saying they are somewhat or very happy in life outside work, compared with 78% last year.
Internists were not alone among the more than 12,000 physicians who responded to the survey. The contrast from last year’s report was clear for physicians in general and reflects COVID-19’s substantial toll on clinicians.
Just 58% of physicians overall reported happy lives outside work, down from 82% last year.
Perhaps not surprising, given the particular demands on certain specialties, physicians in infectious disease were the least happy, at 45%, followed by pulmonologists (47%) and rheumatologists and intensivists, at 49%.
The highest happiness level was reported by those in diabetes and endocrinology, at 73% this year, but that proportion was also substantially lower than the 89% from last year.
Burnout has ‘strong impact on lives’
The percentage of internists who reported burnout or depression, however, has stayed fairly consistent.
More than half (52%) said that burnout had a strong or severe impact on their lives, and nearly 1 in 10 said it was severe enough that they are considering leaving medicine.
One percent of the internists who responded to the survey said they had attempted suicide, and 12% said they had thoughts of suicide but had not attempted it.
Most of those reporting burnout (82%) said it started before the COVID-19 pandemic, but 18% said it began with the pandemic.
Notably, though, physicians ranked problems related to stress from COVID-19 near the bottom among burnout drivers. The top factor, by far, again, was “too many bureaucratic tasks.”
A large majority (78%) of internists work online for up to 10 hours a week, a number that could grow as telemedicine grows.
Exercise is top coping method
Responses gave a peek into how physicians are coping with burnout. Among internists, 49% put exercise at the top. Isolating themselves from others was the next most popular choice, at 45%. Eating junk food and drinking alcohol were further down the list, at 34% and 24%, respectively.
Few internists said they drink alcohol daily, a finding consistent with past years. In fact, 29% said they don’t drink at all, and 26% said they have fewer than one alcoholic drink per week. Only 7% said they had seven or more drinks per week.
The National Institute on Alcohol Abuse and Alcoholism advises that men not have more than 14 alcoholic drinks per week and that women not have more than 7.
Work-life balance topped list of concerns
By far, internists said work-life balance was their top workplace concern. Nearly half (48%) chose that answer, more than twice the percentage who said compensation was the biggest concern (21%).
Asked whether they would take a salary cut for more work-life balance, a similar proportion (46%) said yes.
Forty-three percent of internists manage to take 3-4 weeks of vacation, and 10% take at least 5 weeks, similar to reported vacation time in last year’s survey.
The vast majority are in committed relationships, with 79% reporting that they are married, and 5% reporting that they are living with a partner. Of those who are married, 48% described the marriage as very good; 32%, good; 16%, fair; 2%, poor; and 1%, very poor; 1% preferred not to answer.
One in five internists said their spouse was a physician, and 24% said their spouse worked in the health care field but not as a physician.
Pandemic has increased burnout
Douglas S. Paauw, MD, and Eileen Barrett, MD, two members of the Internal Medicine News editorial advisory board, said they were not surprised by the survey findings.
“There is more burnout since the pandemic,” said Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and third-year medical student clerkship director at the University of Washington. “People may be working more hours, higher stress, but also, some may be working less hours, are socially isolated, taking on a new role of helping their kids in virtual education, andn living in cramped quarters with family that they may not be accustomed to spending so much time with.”
“Also, most physicians love travel, to detress, get back in balance, and that has by and large been taken away by the pandemic,” Dr. Paauw noted. “Unfortunately, bureaucracy did not go away during the pandemic!
Dr. Barrett, an internal medicine hospitalist, said, “It is most concerning to me today that 12% have had thoughts of suicide, and yet 39% are too busy to seek care for depression or burnout, and 17% aren’t seeking due to fear it will be disclosed,”
“Credentialing, medical license applications, and malpractice insurance applications can and must be changed posthaste to support physicians and stop stigmatizing mental health diagnoses and mental health care,” she said. “Removing application questions about physician mental health will be consistent with recommendations from the Federation of State Medical Boards, medical professional societies, and the Americans with Disabilities Act, and is something actionable and achievable for every organization to do in 2021.” “From a public policy perspective, I am deeply concerned about the physician workforce and how patients will be able to receive care from exhausted, burned out physicians who may be reducing their clinical hours to restore their personal happiness – understandably so,” added Dr. Barrett, who is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
She pointed out that there are mental health resources available for physicians that don’t go through their employers or insurance such as www.emotionalppe.org/.
A version of this article first appeared on Medscape.com.
Katie Lennon contributed to this report.
Before the pandemic, a large majority of internists reported that they were generally happy with life outside of work, although by specialty, they were near the bottom in happiness.
But this year’s Medscape Internist Lifestyle, Happiness & Burnout Report 2021 shows a sharp drop, with just 55% of respondents saying they are somewhat or very happy in life outside work, compared with 78% last year.
Internists were not alone among the more than 12,000 physicians who responded to the survey. The contrast from last year’s report was clear for physicians in general and reflects COVID-19’s substantial toll on clinicians.
Just 58% of physicians overall reported happy lives outside work, down from 82% last year.
Perhaps not surprising, given the particular demands on certain specialties, physicians in infectious disease were the least happy, at 45%, followed by pulmonologists (47%) and rheumatologists and intensivists, at 49%.
The highest happiness level was reported by those in diabetes and endocrinology, at 73% this year, but that proportion was also substantially lower than the 89% from last year.
Burnout has ‘strong impact on lives’
The percentage of internists who reported burnout or depression, however, has stayed fairly consistent.
More than half (52%) said that burnout had a strong or severe impact on their lives, and nearly 1 in 10 said it was severe enough that they are considering leaving medicine.
One percent of the internists who responded to the survey said they had attempted suicide, and 12% said they had thoughts of suicide but had not attempted it.
Most of those reporting burnout (82%) said it started before the COVID-19 pandemic, but 18% said it began with the pandemic.
Notably, though, physicians ranked problems related to stress from COVID-19 near the bottom among burnout drivers. The top factor, by far, again, was “too many bureaucratic tasks.”
A large majority (78%) of internists work online for up to 10 hours a week, a number that could grow as telemedicine grows.
Exercise is top coping method
Responses gave a peek into how physicians are coping with burnout. Among internists, 49% put exercise at the top. Isolating themselves from others was the next most popular choice, at 45%. Eating junk food and drinking alcohol were further down the list, at 34% and 24%, respectively.
Few internists said they drink alcohol daily, a finding consistent with past years. In fact, 29% said they don’t drink at all, and 26% said they have fewer than one alcoholic drink per week. Only 7% said they had seven or more drinks per week.
The National Institute on Alcohol Abuse and Alcoholism advises that men not have more than 14 alcoholic drinks per week and that women not have more than 7.
Work-life balance topped list of concerns
By far, internists said work-life balance was their top workplace concern. Nearly half (48%) chose that answer, more than twice the percentage who said compensation was the biggest concern (21%).
Asked whether they would take a salary cut for more work-life balance, a similar proportion (46%) said yes.
Forty-three percent of internists manage to take 3-4 weeks of vacation, and 10% take at least 5 weeks, similar to reported vacation time in last year’s survey.
The vast majority are in committed relationships, with 79% reporting that they are married, and 5% reporting that they are living with a partner. Of those who are married, 48% described the marriage as very good; 32%, good; 16%, fair; 2%, poor; and 1%, very poor; 1% preferred not to answer.
One in five internists said their spouse was a physician, and 24% said their spouse worked in the health care field but not as a physician.
Pandemic has increased burnout
Douglas S. Paauw, MD, and Eileen Barrett, MD, two members of the Internal Medicine News editorial advisory board, said they were not surprised by the survey findings.
“There is more burnout since the pandemic,” said Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and third-year medical student clerkship director at the University of Washington. “People may be working more hours, higher stress, but also, some may be working less hours, are socially isolated, taking on a new role of helping their kids in virtual education, andn living in cramped quarters with family that they may not be accustomed to spending so much time with.”
“Also, most physicians love travel, to detress, get back in balance, and that has by and large been taken away by the pandemic,” Dr. Paauw noted. “Unfortunately, bureaucracy did not go away during the pandemic!
Dr. Barrett, an internal medicine hospitalist, said, “It is most concerning to me today that 12% have had thoughts of suicide, and yet 39% are too busy to seek care for depression or burnout, and 17% aren’t seeking due to fear it will be disclosed,”
“Credentialing, medical license applications, and malpractice insurance applications can and must be changed posthaste to support physicians and stop stigmatizing mental health diagnoses and mental health care,” she said. “Removing application questions about physician mental health will be consistent with recommendations from the Federation of State Medical Boards, medical professional societies, and the Americans with Disabilities Act, and is something actionable and achievable for every organization to do in 2021.” “From a public policy perspective, I am deeply concerned about the physician workforce and how patients will be able to receive care from exhausted, burned out physicians who may be reducing their clinical hours to restore their personal happiness – understandably so,” added Dr. Barrett, who is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
She pointed out that there are mental health resources available for physicians that don’t go through their employers or insurance such as www.emotionalppe.org/.
A version of this article first appeared on Medscape.com.
Katie Lennon contributed to this report.
One in 10 family docs with burnout consider quitting medicine
and 1 in 10 said it was serious enough to make them consider leaving medicine.
Yet, responses to the Medscape Family Medicine Physician Lifestyle, Happiness & Burnout Report 2021 also indicate that family physicians are in the middle of the pack again this year in rankings by specialty of physician happiness outside work. Overall, more than 12,000 physicians from more than 29 specialties responded to this year’s survey, conducted between Aug. 30 and Nov. 5, 2020.
Happiness levels sink for physicians
In light of the COVID-19 pandemic, happiness levels took a sharp drop among physicians across the board. Last year, for instance, the happiness level was highest for physicians practicing in diabetes and endocrinology, at 89%. They remain the happiest this year, but the proportion saying they were happy dropped to 73%. Infectious disease physicians were the least happy outside work both last year and this year, with the proportion reporting they were happy dropping from 69% to 45%.
For family physicians, happiness levels outside work plunged from 79% last year to 57% this year.
Burnout and depression levels, however, remained steady. The portion saying they were either burned out or burned out and depressed was up only 1 percentage point, rising to 47%.
Fifteen percent of family physicians have had thoughts of suicide, and 1% said they had attempted it, according to the survey responses.
The most common strategy for coping with burnout, reported by 48% of family physicians, is talking with family members and close friends, followed closely by exercise, reported by 46%.
Sixty-eight percent of family physicians say they exercise at least twice a week, and 12% exercise every day.
However, not all coping strategies were as positive: Forty-five percent said they cope by isolating themselves from others; 40% turned to junk food; and 23%-24% said they drank alcohol or were binge-eating to cope. Respondents could choose more than one answer.
Among family physicians, 75% expressed anxiety about their futures, given the pandemic, which is similar to the proportion among physicians overall (77%) who had the same worries.
Work-life balance biggest worry
The survey also asked what workplace issues concern family physicians the most. The biggest concern, by far, was work-life balance, chosen by 51%. Next highest was compensation, at 19%, followed by combining parenthood and work (9%) and relationships with colleagues/staff (6%).
More than half (52%) of family doctors said they would take a cut in pay to have better work-life balance.
A little more than a third (36%) of family physicians – about the same percentage as physicians overall – said they always or most of the time spend enough time on their own health and wellness. One in five said they rarely or never do.
The amount of work required beyond the bedside continues to frustrate family physicians.
Again this year, the top cause of burnout, chosen by 70% of family physicians, was “too many bureaucratic tasks.” That was followed by “spending too many hours at work” (37%) and “increasing computerization of practice” (32%).
A large majority (82%) of family doctors report that they work online up to 10 hours a week, a number that could increase with the rise of telemedicine; 64% are personally online up to 10 hours a week. But even with combined personal and professional Internet time, family doctors don’t come close to the average time spent online among all Internet users, which Hootsuite and We Are Social report is an average of 7 hours per day.
Most in committed, satisfying relationships
Most family medicine physicians are juggling committed relationships with work life. In this survey, 78% said they were married, and another 5% said they were living with a partner.
A little more than half of married family doctors described their marriages as very good (51%). The rest were good (32%); fair (13%); poor (2%); and very poor (2%). Some (15%) had spouses who were also physicians, and 25% said their spouses worked in the health care field but were not physicians.
Almost all family physicians were able to take some vacation time during this reporting period – 43% took 3-4 weeks; 35% took 1-2 weeks; 10% took less than 1 week; 9% took 5-6 weeks; and 4% took more than 6 weeks.
If they drove to vacation destinations, they were likely to be in their favorite make of vehicle, which for family physicians were Toyotas (22%), Hondas (14%) and Fords (11%), according to the survey responses. Physicians overall favored Toyotas, Hondas, and BMWs.
A version of this article first appeared on Medscape.com.
and 1 in 10 said it was serious enough to make them consider leaving medicine.
Yet, responses to the Medscape Family Medicine Physician Lifestyle, Happiness & Burnout Report 2021 also indicate that family physicians are in the middle of the pack again this year in rankings by specialty of physician happiness outside work. Overall, more than 12,000 physicians from more than 29 specialties responded to this year’s survey, conducted between Aug. 30 and Nov. 5, 2020.
Happiness levels sink for physicians
In light of the COVID-19 pandemic, happiness levels took a sharp drop among physicians across the board. Last year, for instance, the happiness level was highest for physicians practicing in diabetes and endocrinology, at 89%. They remain the happiest this year, but the proportion saying they were happy dropped to 73%. Infectious disease physicians were the least happy outside work both last year and this year, with the proportion reporting they were happy dropping from 69% to 45%.
For family physicians, happiness levels outside work plunged from 79% last year to 57% this year.
Burnout and depression levels, however, remained steady. The portion saying they were either burned out or burned out and depressed was up only 1 percentage point, rising to 47%.
Fifteen percent of family physicians have had thoughts of suicide, and 1% said they had attempted it, according to the survey responses.
The most common strategy for coping with burnout, reported by 48% of family physicians, is talking with family members and close friends, followed closely by exercise, reported by 46%.
Sixty-eight percent of family physicians say they exercise at least twice a week, and 12% exercise every day.
However, not all coping strategies were as positive: Forty-five percent said they cope by isolating themselves from others; 40% turned to junk food; and 23%-24% said they drank alcohol or were binge-eating to cope. Respondents could choose more than one answer.
Among family physicians, 75% expressed anxiety about their futures, given the pandemic, which is similar to the proportion among physicians overall (77%) who had the same worries.
Work-life balance biggest worry
The survey also asked what workplace issues concern family physicians the most. The biggest concern, by far, was work-life balance, chosen by 51%. Next highest was compensation, at 19%, followed by combining parenthood and work (9%) and relationships with colleagues/staff (6%).
More than half (52%) of family doctors said they would take a cut in pay to have better work-life balance.
A little more than a third (36%) of family physicians – about the same percentage as physicians overall – said they always or most of the time spend enough time on their own health and wellness. One in five said they rarely or never do.
The amount of work required beyond the bedside continues to frustrate family physicians.
Again this year, the top cause of burnout, chosen by 70% of family physicians, was “too many bureaucratic tasks.” That was followed by “spending too many hours at work” (37%) and “increasing computerization of practice” (32%).
A large majority (82%) of family doctors report that they work online up to 10 hours a week, a number that could increase with the rise of telemedicine; 64% are personally online up to 10 hours a week. But even with combined personal and professional Internet time, family doctors don’t come close to the average time spent online among all Internet users, which Hootsuite and We Are Social report is an average of 7 hours per day.
Most in committed, satisfying relationships
Most family medicine physicians are juggling committed relationships with work life. In this survey, 78% said they were married, and another 5% said they were living with a partner.
A little more than half of married family doctors described their marriages as very good (51%). The rest were good (32%); fair (13%); poor (2%); and very poor (2%). Some (15%) had spouses who were also physicians, and 25% said their spouses worked in the health care field but were not physicians.
Almost all family physicians were able to take some vacation time during this reporting period – 43% took 3-4 weeks; 35% took 1-2 weeks; 10% took less than 1 week; 9% took 5-6 weeks; and 4% took more than 6 weeks.
If they drove to vacation destinations, they were likely to be in their favorite make of vehicle, which for family physicians were Toyotas (22%), Hondas (14%) and Fords (11%), according to the survey responses. Physicians overall favored Toyotas, Hondas, and BMWs.
A version of this article first appeared on Medscape.com.
and 1 in 10 said it was serious enough to make them consider leaving medicine.
Yet, responses to the Medscape Family Medicine Physician Lifestyle, Happiness & Burnout Report 2021 also indicate that family physicians are in the middle of the pack again this year in rankings by specialty of physician happiness outside work. Overall, more than 12,000 physicians from more than 29 specialties responded to this year’s survey, conducted between Aug. 30 and Nov. 5, 2020.
Happiness levels sink for physicians
In light of the COVID-19 pandemic, happiness levels took a sharp drop among physicians across the board. Last year, for instance, the happiness level was highest for physicians practicing in diabetes and endocrinology, at 89%. They remain the happiest this year, but the proportion saying they were happy dropped to 73%. Infectious disease physicians were the least happy outside work both last year and this year, with the proportion reporting they were happy dropping from 69% to 45%.
For family physicians, happiness levels outside work plunged from 79% last year to 57% this year.
Burnout and depression levels, however, remained steady. The portion saying they were either burned out or burned out and depressed was up only 1 percentage point, rising to 47%.
Fifteen percent of family physicians have had thoughts of suicide, and 1% said they had attempted it, according to the survey responses.
The most common strategy for coping with burnout, reported by 48% of family physicians, is talking with family members and close friends, followed closely by exercise, reported by 46%.
Sixty-eight percent of family physicians say they exercise at least twice a week, and 12% exercise every day.
However, not all coping strategies were as positive: Forty-five percent said they cope by isolating themselves from others; 40% turned to junk food; and 23%-24% said they drank alcohol or were binge-eating to cope. Respondents could choose more than one answer.
Among family physicians, 75% expressed anxiety about their futures, given the pandemic, which is similar to the proportion among physicians overall (77%) who had the same worries.
Work-life balance biggest worry
The survey also asked what workplace issues concern family physicians the most. The biggest concern, by far, was work-life balance, chosen by 51%. Next highest was compensation, at 19%, followed by combining parenthood and work (9%) and relationships with colleagues/staff (6%).
More than half (52%) of family doctors said they would take a cut in pay to have better work-life balance.
A little more than a third (36%) of family physicians – about the same percentage as physicians overall – said they always or most of the time spend enough time on their own health and wellness. One in five said they rarely or never do.
The amount of work required beyond the bedside continues to frustrate family physicians.
Again this year, the top cause of burnout, chosen by 70% of family physicians, was “too many bureaucratic tasks.” That was followed by “spending too many hours at work” (37%) and “increasing computerization of practice” (32%).
A large majority (82%) of family doctors report that they work online up to 10 hours a week, a number that could increase with the rise of telemedicine; 64% are personally online up to 10 hours a week. But even with combined personal and professional Internet time, family doctors don’t come close to the average time spent online among all Internet users, which Hootsuite and We Are Social report is an average of 7 hours per day.
Most in committed, satisfying relationships
Most family medicine physicians are juggling committed relationships with work life. In this survey, 78% said they were married, and another 5% said they were living with a partner.
A little more than half of married family doctors described their marriages as very good (51%). The rest were good (32%); fair (13%); poor (2%); and very poor (2%). Some (15%) had spouses who were also physicians, and 25% said their spouses worked in the health care field but were not physicians.
Almost all family physicians were able to take some vacation time during this reporting period – 43% took 3-4 weeks; 35% took 1-2 weeks; 10% took less than 1 week; 9% took 5-6 weeks; and 4% took more than 6 weeks.
If they drove to vacation destinations, they were likely to be in their favorite make of vehicle, which for family physicians were Toyotas (22%), Hondas (14%) and Fords (11%), according to the survey responses. Physicians overall favored Toyotas, Hondas, and BMWs.
A version of this article first appeared on Medscape.com.
CDC chief lays out attack plan for COVID variants
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.