User login
Family physicians’ compensation levels stable in pandemic
to $236,000, up from $234,000 last year, even as many practices saw a decrease in hours and patient visits during the pandemic.
Only pediatricians earned less ($221,000) according to the Medscape Family Physician Compensation Report 2021. Plastic surgeons topped this year’s list, at $526,000, followed by orthopedists, at $511,000, and cardiologists, at $459,000.
Family physicians ranked in the middle of specialties in terms of the percentages of physicians who thought they were fairly compensated: 57% of family physicians said they were fairly paid, and 79% of oncologists said they were. Only 44% of infectious disease physicians said they were fairly compensated.
Survey answers indicate, though, that pay isn’t driving family physicians’ satisfaction.
Only 10% of family physicians in the survey said that “making good money at a job I like” was the most rewarding aspect of the job. The top two answers by far were “gratitude/relationships with patients” (chosen by 34%) and “knowing I’m making the world a better place” (27%). Respondents could choose more than one answer.
Despite the small uptick in earnings overall in the specialty, more than one-third of family physicians (36%) reported a decline in compensation in this year’s survey, which included 18,000 responses from physicians in 29 specialties.
Male family physicians continue to be paid much more than their female colleagues, this year 29% more, widening the gap from 26% last year. Overall, men in primary care earned 27% more than their female colleagues, and male specialists earned 33% more.
As for decline in patients seen in some specialties, family physicians are holding their own.
Whereas pediatricians have seen a drop of 18% in patient visits, family physicians saw a decline of just 5%, from an average of 81 to 77 patients per week.
Most expect return to normal pay within 3 years
Most family physicians (83%) who incurred financial losses this year said they expect that income will return to normal within 3 years. More than one-third of that group (38%) said they expect compensation to get back to normal in the next year.
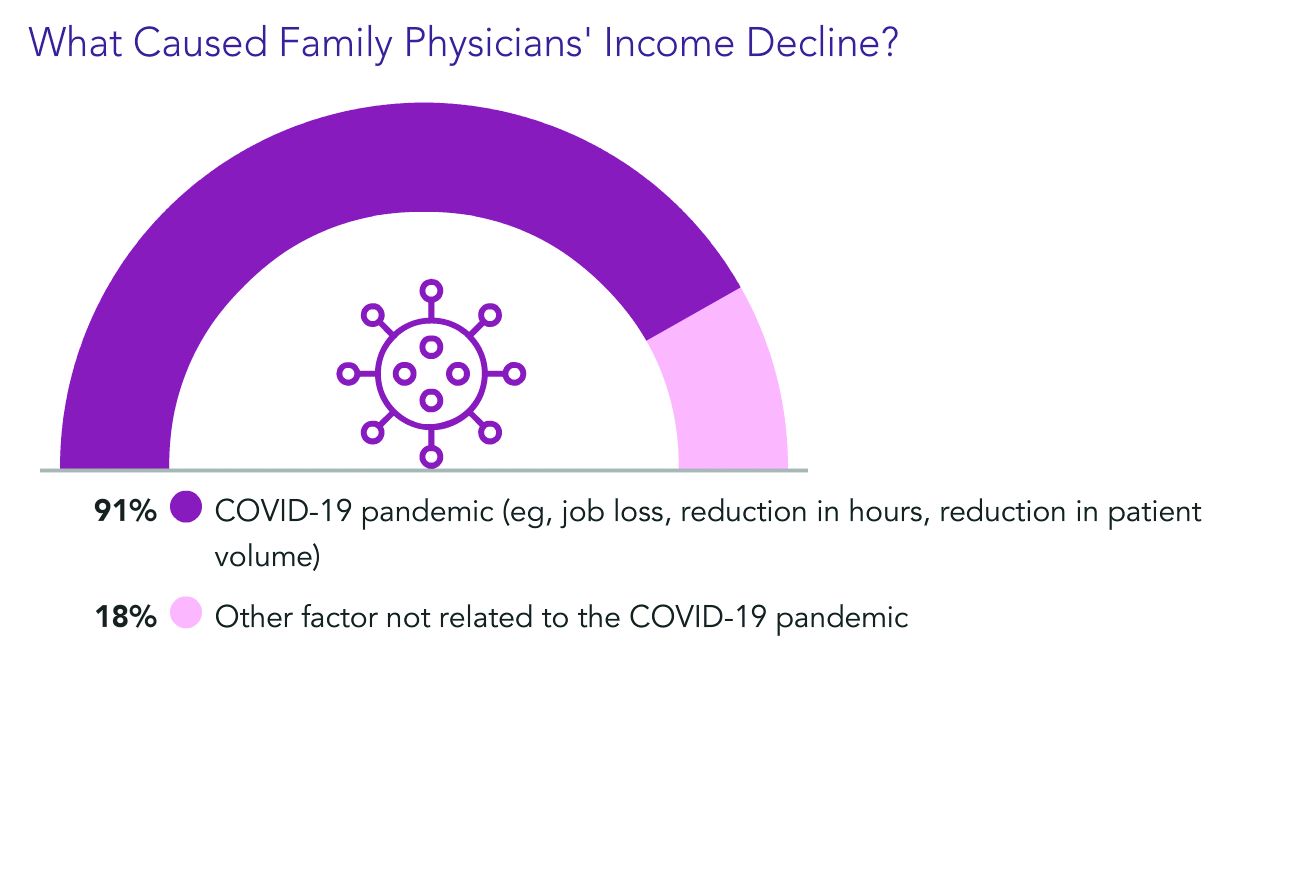
Almost all of the family physicians who lost income (91%) pointed the finger at COVID-19. Respondents could choose more than one answer, and 18% said other factors were also to blame.
Family physicians averaged $27,000 in incentive bonuses, higher than those in internal medicine, pediatrics, and psychiatry. Orthopedists had by far the highest bonuses, at $116,000.
For family physicians who received a bonus this year, the amount equaled about 12% of their salary, up from 10% last year. Bonuses are usually based on productivity but can also be tied to patient satisfaction, clinical processes, and other factors.
The number of family physicians who achieved more than three-quarters of their potential annual bonus rose to 61% this year, up from 55%.
17 hours a week on administrative tasks
The survey also ranked specialties by the amount of time physicians spent on paperwork and administrative tasks, including participation in professional organizations and clinical reading.
Family physicians fell squarely in the middle, with 17 hours per week spent on such tasks. Infectious disease physicians spent the most time, at 24.2 hours a week, and anesthesiologists spent the least, at 10.1.
Work hours declined for many physicians during the pandemic, and some were furloughed.
But, like most physicians, family physicians are once more working normal hours. They average 49 hours per week, which is slightly more than before the pandemic.
Specialists whose weekly hours are above normal are infectious disease physicians, intensivists, and public health and preventive medicine physicians; all are working 6 to 7 hours a week more than usual, according to the survey responses.
Responses also turned up some uncertainty on the future makeup of patient panels.
Most family physicians (69%) said they would continue to take new and current Medicare/Medicaid patients.
However, close to one-third of family physicians said they would stop treating at least some patients they already have and will not take new ones or haven’t decided yet.
A version of this article first appeared on Medscape.com.
to $236,000, up from $234,000 last year, even as many practices saw a decrease in hours and patient visits during the pandemic.

Only pediatricians earned less ($221,000) according to the Medscape Family Physician Compensation Report 2021. Plastic surgeons topped this year’s list, at $526,000, followed by orthopedists, at $511,000, and cardiologists, at $459,000.
Family physicians ranked in the middle of specialties in terms of the percentages of physicians who thought they were fairly compensated: 57% of family physicians said they were fairly paid, and 79% of oncologists said they were. Only 44% of infectious disease physicians said they were fairly compensated.
Survey answers indicate, though, that pay isn’t driving family physicians’ satisfaction.
Only 10% of family physicians in the survey said that “making good money at a job I like” was the most rewarding aspect of the job. The top two answers by far were “gratitude/relationships with patients” (chosen by 34%) and “knowing I’m making the world a better place” (27%). Respondents could choose more than one answer.
Despite the small uptick in earnings overall in the specialty, more than one-third of family physicians (36%) reported a decline in compensation in this year’s survey, which included 18,000 responses from physicians in 29 specialties.
Male family physicians continue to be paid much more than their female colleagues, this year 29% more, widening the gap from 26% last year. Overall, men in primary care earned 27% more than their female colleagues, and male specialists earned 33% more.
As for decline in patients seen in some specialties, family physicians are holding their own.
Whereas pediatricians have seen a drop of 18% in patient visits, family physicians saw a decline of just 5%, from an average of 81 to 77 patients per week.
Most expect return to normal pay within 3 years
Most family physicians (83%) who incurred financial losses this year said they expect that income will return to normal within 3 years. More than one-third of that group (38%) said they expect compensation to get back to normal in the next year.
Almost all of the family physicians who lost income (91%) pointed the finger at COVID-19. Respondents could choose more than one answer, and 18% said other factors were also to blame.
Family physicians averaged $27,000 in incentive bonuses, higher than those in internal medicine, pediatrics, and psychiatry. Orthopedists had by far the highest bonuses, at $116,000.
For family physicians who received a bonus this year, the amount equaled about 12% of their salary, up from 10% last year. Bonuses are usually based on productivity but can also be tied to patient satisfaction, clinical processes, and other factors.
The number of family physicians who achieved more than three-quarters of their potential annual bonus rose to 61% this year, up from 55%.
17 hours a week on administrative tasks
The survey also ranked specialties by the amount of time physicians spent on paperwork and administrative tasks, including participation in professional organizations and clinical reading.
Family physicians fell squarely in the middle, with 17 hours per week spent on such tasks. Infectious disease physicians spent the most time, at 24.2 hours a week, and anesthesiologists spent the least, at 10.1.
Work hours declined for many physicians during the pandemic, and some were furloughed.
But, like most physicians, family physicians are once more working normal hours. They average 49 hours per week, which is slightly more than before the pandemic.
Specialists whose weekly hours are above normal are infectious disease physicians, intensivists, and public health and preventive medicine physicians; all are working 6 to 7 hours a week more than usual, according to the survey responses.
Responses also turned up some uncertainty on the future makeup of patient panels.
Most family physicians (69%) said they would continue to take new and current Medicare/Medicaid patients.
However, close to one-third of family physicians said they would stop treating at least some patients they already have and will not take new ones or haven’t decided yet.
A version of this article first appeared on Medscape.com.
to $236,000, up from $234,000 last year, even as many practices saw a decrease in hours and patient visits during the pandemic.

Only pediatricians earned less ($221,000) according to the Medscape Family Physician Compensation Report 2021. Plastic surgeons topped this year’s list, at $526,000, followed by orthopedists, at $511,000, and cardiologists, at $459,000.
Family physicians ranked in the middle of specialties in terms of the percentages of physicians who thought they were fairly compensated: 57% of family physicians said they were fairly paid, and 79% of oncologists said they were. Only 44% of infectious disease physicians said they were fairly compensated.
Survey answers indicate, though, that pay isn’t driving family physicians’ satisfaction.
Only 10% of family physicians in the survey said that “making good money at a job I like” was the most rewarding aspect of the job. The top two answers by far were “gratitude/relationships with patients” (chosen by 34%) and “knowing I’m making the world a better place” (27%). Respondents could choose more than one answer.
Despite the small uptick in earnings overall in the specialty, more than one-third of family physicians (36%) reported a decline in compensation in this year’s survey, which included 18,000 responses from physicians in 29 specialties.
Male family physicians continue to be paid much more than their female colleagues, this year 29% more, widening the gap from 26% last year. Overall, men in primary care earned 27% more than their female colleagues, and male specialists earned 33% more.
As for decline in patients seen in some specialties, family physicians are holding their own.
Whereas pediatricians have seen a drop of 18% in patient visits, family physicians saw a decline of just 5%, from an average of 81 to 77 patients per week.
Most expect return to normal pay within 3 years
Most family physicians (83%) who incurred financial losses this year said they expect that income will return to normal within 3 years. More than one-third of that group (38%) said they expect compensation to get back to normal in the next year.
Almost all of the family physicians who lost income (91%) pointed the finger at COVID-19. Respondents could choose more than one answer, and 18% said other factors were also to blame.
Family physicians averaged $27,000 in incentive bonuses, higher than those in internal medicine, pediatrics, and psychiatry. Orthopedists had by far the highest bonuses, at $116,000.
For family physicians who received a bonus this year, the amount equaled about 12% of their salary, up from 10% last year. Bonuses are usually based on productivity but can also be tied to patient satisfaction, clinical processes, and other factors.
The number of family physicians who achieved more than three-quarters of their potential annual bonus rose to 61% this year, up from 55%.
17 hours a week on administrative tasks
The survey also ranked specialties by the amount of time physicians spent on paperwork and administrative tasks, including participation in professional organizations and clinical reading.
Family physicians fell squarely in the middle, with 17 hours per week spent on such tasks. Infectious disease physicians spent the most time, at 24.2 hours a week, and anesthesiologists spent the least, at 10.1.
Work hours declined for many physicians during the pandemic, and some were furloughed.
But, like most physicians, family physicians are once more working normal hours. They average 49 hours per week, which is slightly more than before the pandemic.
Specialists whose weekly hours are above normal are infectious disease physicians, intensivists, and public health and preventive medicine physicians; all are working 6 to 7 hours a week more than usual, according to the survey responses.
Responses also turned up some uncertainty on the future makeup of patient panels.
Most family physicians (69%) said they would continue to take new and current Medicare/Medicaid patients.
However, close to one-third of family physicians said they would stop treating at least some patients they already have and will not take new ones or haven’t decided yet.
A version of this article first appeared on Medscape.com.
Pressure on primary care expected to intensify with long-COVID
, experts say.
“It could be as many as 5% to 10% who are still having symptoms at 12 weeks. Those numbers are higher if you’re talking about patients who had been hospitalized with COVID-19,” Russ Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston, said in an interview.
A recent study from the Centers for Disease Control and Prevention and Kaiser Permanente Georgia found that among 3,171 nonhospitalized adult patients with COVID-19, 69% had one or more outpatient visits 28 to 180 days after the diagnosis. Two-thirds had a visit for a new primary diagnosis, and about one-third had a new specialist visit. Symptom diagnoses included cough, shortness of breath, chest or throat pain, and fatigue.
These visits have come while cases of acute COVID continue to occur, and there has been an increase in patients returning to primary care after avoiding it while the pandemic surged. For these patients, delay in seeking care has often led a worsening of chronic conditions.
Dr. Phillips pointed to a shortcoming in primary care that will need to be addressed with regard to long-COVID: “We don’t have good systems to follow patients and their symptoms over time.”
Long-COVID will require that kind of care, but current payment systems don’t support proactively reaching out to patients to track them over time, he noted.
“We do a good job of identifying these issues for patients who come in, but it’s the patients who don’t that we worry about the most,” he said.
Dr. Phillips provided examples of the kind of management plans needed to improve outcomes for patients with long-COVID. In anticoagulation clinics, patients who receive blood thinners are monitored closely, and in mental health care, patients with depression are linked with social workers and are monitored regularly.
“Around COVID, those management plans are in their infancy,” he said.
John Brooks, MD, chief medical officer for the CDC’s COVID-19 response, testified in a congressional hearing at the end of April that interim guidance concerning protocols for long-COVID in primary care are forthcoming. He also noted that the CDC is working closely with the Centers for Medicare & Medicaid Services to develop medical coding for long-COVID.
In the meantime, Dr. Phillips said, one strategy is to have patients self-monitor their condition and relay results to primary care physicians electronically.
As an example, Dr. Phillips described a patient with long-COVID who was receiving supplemental oxygen and who wanted to resume her exercise regimen.
She checked her own oxygen saturation levels before and during exercise and reported the levels every few days through their patient portal.
“Very slowly we were able to cut down on her oxygen and increase her exercise capacity until she no longer needed oxygen and could go back to her usual activities of daily living,” he said.
Nurse practitioners, social workers, and other nonphysician care team members may be increasingly relied upon to provide care for long-COVID patients as well, he said.
Additionally, telehealth, which is currently reimbursed the same way as in-person visits are, enables easier access for checking in with patients, he said.
Empathy and listening needed
Sabrina Assoumou, MD, MPH, assistant professor of medicine at Boston University, told this news organization that it will be crucial to address health care disparities as long-COVID cases mount.
COVID disproportionately affects communities of color, and it stands to reason that this will be the case for long-COVID as well, she said. Diversifying the workforce will be vital, inasmuch as diagnosis may depend on how well a physician listens to patients as they describe their symptoms, continued Dr. Assoumou, whose primary care practice centers on HIV patients.
The symptoms of long-COVID are vague, she explained, and include brain fog, fatigue, and shortness of breath, and it takes longer to diagnose than many conditions.
Dr. Assoumou said some people were never tested for COVID and never received a diagnosis, yet they are now experiencing the extended effects.
“Long-COVID will force us to go back to the basics – like really listening to our patients,” she said. “We’re definitely going to need to be more empathetic.”
No large influx yet
Charles Vega, MD, health sciences clinical professor of family medicine at the University of California, Irvine, said he is skeptical that the primary care system will be overwhelmed with long-COVID cases.
Dr. Vega is a family physician working in the largest safety net clinic in Orange County, California. About 90% of his patients are LatinX, a population disproportionately burdened by COVID, yet he hasn’t seen a surge in long-COVID cases.
He said that may be because patients know there isn’t a treatment for long-COVID. They are well connected through online forums such as Body Politic COVID-19 Support Group and may not feel they need to see a doctor.
“It wasn’t scientists finding [long-COVID], it was patients who developed this disease model themselves,” he said. “That’s where most of the data sharing is.”
Yet, for long-COVID patients who do need care, primary care is the best home for them, Dr. Vega said.
He said the most common symptoms he sees are fatigue and poor activity tolerance. “They get winded going to the bathroom,” he said.
The most difficult symptom is dyspnea, he said. Patients describe being breathless, but it’s not bad enough to qualify for supplemental oxygen.
“Being breathless is a pretty desperate thing and hurts quality of life,” he said.
Most patients describe general malaise.
Care for long-COVID will require medical care and mental health care, Dr. Vega notes. Primary care is already set up to screen and to coordinate care with the appropriate provider.
“I think there’s a role for specialists, but primary care has to be involved,” he said.
Dr. Phillips, Dr. Assoumou, and Dr. Vega report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, experts say.
“It could be as many as 5% to 10% who are still having symptoms at 12 weeks. Those numbers are higher if you’re talking about patients who had been hospitalized with COVID-19,” Russ Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston, said in an interview.
A recent study from the Centers for Disease Control and Prevention and Kaiser Permanente Georgia found that among 3,171 nonhospitalized adult patients with COVID-19, 69% had one or more outpatient visits 28 to 180 days after the diagnosis. Two-thirds had a visit for a new primary diagnosis, and about one-third had a new specialist visit. Symptom diagnoses included cough, shortness of breath, chest or throat pain, and fatigue.
These visits have come while cases of acute COVID continue to occur, and there has been an increase in patients returning to primary care after avoiding it while the pandemic surged. For these patients, delay in seeking care has often led a worsening of chronic conditions.
Dr. Phillips pointed to a shortcoming in primary care that will need to be addressed with regard to long-COVID: “We don’t have good systems to follow patients and their symptoms over time.”
Long-COVID will require that kind of care, but current payment systems don’t support proactively reaching out to patients to track them over time, he noted.
“We do a good job of identifying these issues for patients who come in, but it’s the patients who don’t that we worry about the most,” he said.
Dr. Phillips provided examples of the kind of management plans needed to improve outcomes for patients with long-COVID. In anticoagulation clinics, patients who receive blood thinners are monitored closely, and in mental health care, patients with depression are linked with social workers and are monitored regularly.
“Around COVID, those management plans are in their infancy,” he said.
John Brooks, MD, chief medical officer for the CDC’s COVID-19 response, testified in a congressional hearing at the end of April that interim guidance concerning protocols for long-COVID in primary care are forthcoming. He also noted that the CDC is working closely with the Centers for Medicare & Medicaid Services to develop medical coding for long-COVID.
In the meantime, Dr. Phillips said, one strategy is to have patients self-monitor their condition and relay results to primary care physicians electronically.
As an example, Dr. Phillips described a patient with long-COVID who was receiving supplemental oxygen and who wanted to resume her exercise regimen.
She checked her own oxygen saturation levels before and during exercise and reported the levels every few days through their patient portal.
“Very slowly we were able to cut down on her oxygen and increase her exercise capacity until she no longer needed oxygen and could go back to her usual activities of daily living,” he said.
Nurse practitioners, social workers, and other nonphysician care team members may be increasingly relied upon to provide care for long-COVID patients as well, he said.
Additionally, telehealth, which is currently reimbursed the same way as in-person visits are, enables easier access for checking in with patients, he said.
Empathy and listening needed
Sabrina Assoumou, MD, MPH, assistant professor of medicine at Boston University, told this news organization that it will be crucial to address health care disparities as long-COVID cases mount.
COVID disproportionately affects communities of color, and it stands to reason that this will be the case for long-COVID as well, she said. Diversifying the workforce will be vital, inasmuch as diagnosis may depend on how well a physician listens to patients as they describe their symptoms, continued Dr. Assoumou, whose primary care practice centers on HIV patients.
The symptoms of long-COVID are vague, she explained, and include brain fog, fatigue, and shortness of breath, and it takes longer to diagnose than many conditions.
Dr. Assoumou said some people were never tested for COVID and never received a diagnosis, yet they are now experiencing the extended effects.
“Long-COVID will force us to go back to the basics – like really listening to our patients,” she said. “We’re definitely going to need to be more empathetic.”
No large influx yet
Charles Vega, MD, health sciences clinical professor of family medicine at the University of California, Irvine, said he is skeptical that the primary care system will be overwhelmed with long-COVID cases.
Dr. Vega is a family physician working in the largest safety net clinic in Orange County, California. About 90% of his patients are LatinX, a population disproportionately burdened by COVID, yet he hasn’t seen a surge in long-COVID cases.
He said that may be because patients know there isn’t a treatment for long-COVID. They are well connected through online forums such as Body Politic COVID-19 Support Group and may not feel they need to see a doctor.
“It wasn’t scientists finding [long-COVID], it was patients who developed this disease model themselves,” he said. “That’s where most of the data sharing is.”
Yet, for long-COVID patients who do need care, primary care is the best home for them, Dr. Vega said.
He said the most common symptoms he sees are fatigue and poor activity tolerance. “They get winded going to the bathroom,” he said.
The most difficult symptom is dyspnea, he said. Patients describe being breathless, but it’s not bad enough to qualify for supplemental oxygen.
“Being breathless is a pretty desperate thing and hurts quality of life,” he said.
Most patients describe general malaise.
Care for long-COVID will require medical care and mental health care, Dr. Vega notes. Primary care is already set up to screen and to coordinate care with the appropriate provider.
“I think there’s a role for specialists, but primary care has to be involved,” he said.
Dr. Phillips, Dr. Assoumou, and Dr. Vega report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, experts say.
“It could be as many as 5% to 10% who are still having symptoms at 12 weeks. Those numbers are higher if you’re talking about patients who had been hospitalized with COVID-19,” Russ Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston, said in an interview.
A recent study from the Centers for Disease Control and Prevention and Kaiser Permanente Georgia found that among 3,171 nonhospitalized adult patients with COVID-19, 69% had one or more outpatient visits 28 to 180 days after the diagnosis. Two-thirds had a visit for a new primary diagnosis, and about one-third had a new specialist visit. Symptom diagnoses included cough, shortness of breath, chest or throat pain, and fatigue.
These visits have come while cases of acute COVID continue to occur, and there has been an increase in patients returning to primary care after avoiding it while the pandemic surged. For these patients, delay in seeking care has often led a worsening of chronic conditions.
Dr. Phillips pointed to a shortcoming in primary care that will need to be addressed with regard to long-COVID: “We don’t have good systems to follow patients and their symptoms over time.”
Long-COVID will require that kind of care, but current payment systems don’t support proactively reaching out to patients to track them over time, he noted.
“We do a good job of identifying these issues for patients who come in, but it’s the patients who don’t that we worry about the most,” he said.
Dr. Phillips provided examples of the kind of management plans needed to improve outcomes for patients with long-COVID. In anticoagulation clinics, patients who receive blood thinners are monitored closely, and in mental health care, patients with depression are linked with social workers and are monitored regularly.
“Around COVID, those management plans are in their infancy,” he said.
John Brooks, MD, chief medical officer for the CDC’s COVID-19 response, testified in a congressional hearing at the end of April that interim guidance concerning protocols for long-COVID in primary care are forthcoming. He also noted that the CDC is working closely with the Centers for Medicare & Medicaid Services to develop medical coding for long-COVID.
In the meantime, Dr. Phillips said, one strategy is to have patients self-monitor their condition and relay results to primary care physicians electronically.
As an example, Dr. Phillips described a patient with long-COVID who was receiving supplemental oxygen and who wanted to resume her exercise regimen.
She checked her own oxygen saturation levels before and during exercise and reported the levels every few days through their patient portal.
“Very slowly we were able to cut down on her oxygen and increase her exercise capacity until she no longer needed oxygen and could go back to her usual activities of daily living,” he said.
Nurse practitioners, social workers, and other nonphysician care team members may be increasingly relied upon to provide care for long-COVID patients as well, he said.
Additionally, telehealth, which is currently reimbursed the same way as in-person visits are, enables easier access for checking in with patients, he said.
Empathy and listening needed
Sabrina Assoumou, MD, MPH, assistant professor of medicine at Boston University, told this news organization that it will be crucial to address health care disparities as long-COVID cases mount.
COVID disproportionately affects communities of color, and it stands to reason that this will be the case for long-COVID as well, she said. Diversifying the workforce will be vital, inasmuch as diagnosis may depend on how well a physician listens to patients as they describe their symptoms, continued Dr. Assoumou, whose primary care practice centers on HIV patients.
The symptoms of long-COVID are vague, she explained, and include brain fog, fatigue, and shortness of breath, and it takes longer to diagnose than many conditions.
Dr. Assoumou said some people were never tested for COVID and never received a diagnosis, yet they are now experiencing the extended effects.
“Long-COVID will force us to go back to the basics – like really listening to our patients,” she said. “We’re definitely going to need to be more empathetic.”
No large influx yet
Charles Vega, MD, health sciences clinical professor of family medicine at the University of California, Irvine, said he is skeptical that the primary care system will be overwhelmed with long-COVID cases.
Dr. Vega is a family physician working in the largest safety net clinic in Orange County, California. About 90% of his patients are LatinX, a population disproportionately burdened by COVID, yet he hasn’t seen a surge in long-COVID cases.
He said that may be because patients know there isn’t a treatment for long-COVID. They are well connected through online forums such as Body Politic COVID-19 Support Group and may not feel they need to see a doctor.
“It wasn’t scientists finding [long-COVID], it was patients who developed this disease model themselves,” he said. “That’s where most of the data sharing is.”
Yet, for long-COVID patients who do need care, primary care is the best home for them, Dr. Vega said.
He said the most common symptoms he sees are fatigue and poor activity tolerance. “They get winded going to the bathroom,” he said.
The most difficult symptom is dyspnea, he said. Patients describe being breathless, but it’s not bad enough to qualify for supplemental oxygen.
“Being breathless is a pretty desperate thing and hurts quality of life,” he said.
Most patients describe general malaise.
Care for long-COVID will require medical care and mental health care, Dr. Vega notes. Primary care is already set up to screen and to coordinate care with the appropriate provider.
“I think there’s a role for specialists, but primary care has to be involved,” he said.
Dr. Phillips, Dr. Assoumou, and Dr. Vega report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ID doctors have the most paperwork, administrative demands
Infectious disease physicians are among the doctors carrying the largest burdens in the COVID-19 pandemic.
Perhaps not surprisingly, they were the specialists least likely to feel they were fairly compensated in the Medscape Infectious Diseases Physician Compensation Report 2021.
Only 44% said the pay was fair (down from 51% the prior year) compared with those at the high end – 79% in oncology, 69% in psychiatry, and 68% in plastic surgery who answered that way.
Income, which averaged $245,000, varied little from the previous year overall, according to the survey, but nearly one-third of ID physicians saw a decline in pay.
Again this year, ID physicians ranked near the bottom on the compensation spectrum. Pediatricians were lowest paid at $221,000. Plastic surgeons topped the chart at $526,000, followed by orthopedists at $511,000.
At the same time, the ID specialty is facing increasing shortages, a gap made even more visible in the pandemic. Medscape reported last year that nearly 80% of U.S. counties have no infectious disease specialists.
Thomas File Jr., MD, last year’s president of the Infectious Diseases Society of America, emphasized that COVID-19 is not the only threat that ID specialists have had to deal with or will have. He cited the threats that Zika and SARS posed in past years.
“COVID-19 illustrates the need for more trained ID specialists, because we know we’re going to be seeing more outbreaks in the future,” he said in an interview at the onset of the pandemic in March 2020.
Longer hours in pandemic
ID physicians’ hours generally increased during the pandemic, and they remain inflated by 8 hours per week (60 compared with 52 prepandemic) as the nation struggles to manage continuing COVID-19 infections. Physicians in critical care and public health and preventive medicine are seeing heavier workloads as well, by an average of 6-7 hours per week.
At the same time, ID physicians spent the most time of physicians in all specialties on paperwork and administrative tasks. Those tasks, which include electronic health record entry and clinical reading, took ID doctors 24.2 hours a week, more the twice the hours spent by those in anesthesiology (10.1), ophthalmology (10.3), and radiology (11.6).
The 24.2 hours was a substantial increase from the last report, when ID physicians said they spent 18.5 hours on the tasks.
The survey asked about the most challenging part of the job. ID physicians reported “long hours” as number one followed by “having so many rules and regulations.”
Only 4% said the danger or risk associated with treating COVID-19 patients was the most challenging part.
The top two aspects of their work they deemed most rewarding were “being very good at what I do” (chosen by 33%) and “knowing that I’m making the world a better place” (31%).
Patient volume up 17%
ID physicians reported seeing 78 patients per week in this report compared with 66 prepandemic, a 17% increase. Conversely, pediatricians saw an 18% drop in patient visits, followed by dermatologists, orthopedists and otolaryngologists (all down about 15%).
Despite the challenges and dissatisfaction with pay, the great majority of ID physicians said they would choose both medicine (83%) and their specialty (89%, up from 85% last year) again.
A version of this article first appeared on Medscape.com.
Infectious disease physicians are among the doctors carrying the largest burdens in the COVID-19 pandemic.
Perhaps not surprisingly, they were the specialists least likely to feel they were fairly compensated in the Medscape Infectious Diseases Physician Compensation Report 2021.
Only 44% said the pay was fair (down from 51% the prior year) compared with those at the high end – 79% in oncology, 69% in psychiatry, and 68% in plastic surgery who answered that way.
Income, which averaged $245,000, varied little from the previous year overall, according to the survey, but nearly one-third of ID physicians saw a decline in pay.
Again this year, ID physicians ranked near the bottom on the compensation spectrum. Pediatricians were lowest paid at $221,000. Plastic surgeons topped the chart at $526,000, followed by orthopedists at $511,000.
At the same time, the ID specialty is facing increasing shortages, a gap made even more visible in the pandemic. Medscape reported last year that nearly 80% of U.S. counties have no infectious disease specialists.
Thomas File Jr., MD, last year’s president of the Infectious Diseases Society of America, emphasized that COVID-19 is not the only threat that ID specialists have had to deal with or will have. He cited the threats that Zika and SARS posed in past years.
“COVID-19 illustrates the need for more trained ID specialists, because we know we’re going to be seeing more outbreaks in the future,” he said in an interview at the onset of the pandemic in March 2020.
Longer hours in pandemic
ID physicians’ hours generally increased during the pandemic, and they remain inflated by 8 hours per week (60 compared with 52 prepandemic) as the nation struggles to manage continuing COVID-19 infections. Physicians in critical care and public health and preventive medicine are seeing heavier workloads as well, by an average of 6-7 hours per week.
At the same time, ID physicians spent the most time of physicians in all specialties on paperwork and administrative tasks. Those tasks, which include electronic health record entry and clinical reading, took ID doctors 24.2 hours a week, more the twice the hours spent by those in anesthesiology (10.1), ophthalmology (10.3), and radiology (11.6).
The 24.2 hours was a substantial increase from the last report, when ID physicians said they spent 18.5 hours on the tasks.
The survey asked about the most challenging part of the job. ID physicians reported “long hours” as number one followed by “having so many rules and regulations.”
Only 4% said the danger or risk associated with treating COVID-19 patients was the most challenging part.
The top two aspects of their work they deemed most rewarding were “being very good at what I do” (chosen by 33%) and “knowing that I’m making the world a better place” (31%).
Patient volume up 17%
ID physicians reported seeing 78 patients per week in this report compared with 66 prepandemic, a 17% increase. Conversely, pediatricians saw an 18% drop in patient visits, followed by dermatologists, orthopedists and otolaryngologists (all down about 15%).
Despite the challenges and dissatisfaction with pay, the great majority of ID physicians said they would choose both medicine (83%) and their specialty (89%, up from 85% last year) again.
A version of this article first appeared on Medscape.com.
Infectious disease physicians are among the doctors carrying the largest burdens in the COVID-19 pandemic.
Perhaps not surprisingly, they were the specialists least likely to feel they were fairly compensated in the Medscape Infectious Diseases Physician Compensation Report 2021.
Only 44% said the pay was fair (down from 51% the prior year) compared with those at the high end – 79% in oncology, 69% in psychiatry, and 68% in plastic surgery who answered that way.
Income, which averaged $245,000, varied little from the previous year overall, according to the survey, but nearly one-third of ID physicians saw a decline in pay.
Again this year, ID physicians ranked near the bottom on the compensation spectrum. Pediatricians were lowest paid at $221,000. Plastic surgeons topped the chart at $526,000, followed by orthopedists at $511,000.
At the same time, the ID specialty is facing increasing shortages, a gap made even more visible in the pandemic. Medscape reported last year that nearly 80% of U.S. counties have no infectious disease specialists.
Thomas File Jr., MD, last year’s president of the Infectious Diseases Society of America, emphasized that COVID-19 is not the only threat that ID specialists have had to deal with or will have. He cited the threats that Zika and SARS posed in past years.
“COVID-19 illustrates the need for more trained ID specialists, because we know we’re going to be seeing more outbreaks in the future,” he said in an interview at the onset of the pandemic in March 2020.
Longer hours in pandemic
ID physicians’ hours generally increased during the pandemic, and they remain inflated by 8 hours per week (60 compared with 52 prepandemic) as the nation struggles to manage continuing COVID-19 infections. Physicians in critical care and public health and preventive medicine are seeing heavier workloads as well, by an average of 6-7 hours per week.
At the same time, ID physicians spent the most time of physicians in all specialties on paperwork and administrative tasks. Those tasks, which include electronic health record entry and clinical reading, took ID doctors 24.2 hours a week, more the twice the hours spent by those in anesthesiology (10.1), ophthalmology (10.3), and radiology (11.6).
The 24.2 hours was a substantial increase from the last report, when ID physicians said they spent 18.5 hours on the tasks.
The survey asked about the most challenging part of the job. ID physicians reported “long hours” as number one followed by “having so many rules and regulations.”
Only 4% said the danger or risk associated with treating COVID-19 patients was the most challenging part.
The top two aspects of their work they deemed most rewarding were “being very good at what I do” (chosen by 33%) and “knowing that I’m making the world a better place” (31%).
Patient volume up 17%
ID physicians reported seeing 78 patients per week in this report compared with 66 prepandemic, a 17% increase. Conversely, pediatricians saw an 18% drop in patient visits, followed by dermatologists, orthopedists and otolaryngologists (all down about 15%).
Despite the challenges and dissatisfaction with pay, the great majority of ID physicians said they would choose both medicine (83%) and their specialty (89%, up from 85% last year) again.
A version of this article first appeared on Medscape.com.
Internists’ patient visits rebound to near pre-COVID norms: Pay down slightly from previous year
Internists are seeing only 3% fewer patients than they did before the COVID-19 pandemic (72 per week on average now vs. 74 before the pandemic). Comparatively, for pediatricians, patient volume remains down 18%. Dermatologists, otolaryngologists, and orthopedists report that visits are down by about 15%.
The number of hours worked also rebounded for internists. In fact, some report working slightly more hours now than they did before the pandemic (52 hours a week, up from 50).
Pay for internists continues to hover near the bottom of the scale among specialties. In this year’s Medscape Internist Compensation Report 2021, internists averaged $248,000, down from $251,000 last year. Pediatricians were the lowest paid, at $221,000, followed by family physicians, at $236,000. Plastic surgeons made the most, at $526,000, followed by orthopedists, at $511,000.
It helped to be self-employed. These internists made $276,000 on average, compared with $238,000 for their employed counterparts.
Half say pay is fair
Internists are also near the bottom among specialists who feel they are fairly compensated. As in last year’s survey, just more than half of internists (52%) said they felt that they were fairly paid this year. By comparison, 79% of oncologists reported they were fairly compensated, which is on the high end regarding satisfaction, but only 44% of infectious diseases specialists felt that way.
Some indicators in the survey responses may help explain the dissatisfaction.
Internists are near the top in time spent on paperwork. On average, they spent 19.7 hours on paperwork and administration this year, up slightly from 18.5 last year. Infectious disease physicians spent the most time on those tasks (24.2 hours a week), and anesthesiologists spent the fewest, at 10.1 hours per week.
Administrative work was among many frustrations internists reported. The following are the top five most challenging aspects of the job, according to the respondents:
- Having so many rules and regulations (24%)
- Having to work long hours (16%)
- Dealing with difficult patients (16%)
- Working with electronic health records systems (11%)
- Danger/risk associated with treating COVID-19 patients (10%)
Conversely, the most rewarding aspects were “gratitude/relationships with patients” (31%); “knowing that I’m making the world a better place” (26%); and “being very good at what I do” (20%).
More than one-third lost income
More than one-third of internists (36%) reported that they lost some income during the past year.
Among those who lost income, 81% said they expect income to return to prepandemic levels within 3 years. Half of that group expected the rebound would come within the next year.
Slightly more than one-third of internists said they would participate in the merit-based incentive payment system (MIPS), and 12% said they would participate in advanced alternative payment models. The rest either said they would participate in neither, or they hadn’t decided.
“The stakes for the Quality Payment Program – the program that incorporates MIPS – are high, with a 9% penalty applied to all Medicare reimbursement for failure to participate,” says Elizabeth Woodcock, MBA, CPC, president of the physician practice consulting firm Woodcock and Associates, in Atlanta, Georgia.
“With margins already slim,” she told this news organization, “most physicians can’t afford this massive penalty.”
If they could choose again, most internists (76%) said they would choose medicine, which was almost the same number as physicians overall who would pick medicine again. Oncologists (88%) and ophthalmologists (87%) were the specialists most likely to choose medicine again. Those in physical medicine and rehabilitation were least likely to choose medicine again, at 67%.
But asked about their specialty, internists’ enthusiasm decreased. Only 68% said that they would make that same choice again.
That was up considerably, however, from the 2015 survey: For that year, only 25% said they would choose internal medicine again.
A version of this article first appeared on Medscape.com.
Internists are seeing only 3% fewer patients than they did before the COVID-19 pandemic (72 per week on average now vs. 74 before the pandemic). Comparatively, for pediatricians, patient volume remains down 18%. Dermatologists, otolaryngologists, and orthopedists report that visits are down by about 15%.
The number of hours worked also rebounded for internists. In fact, some report working slightly more hours now than they did before the pandemic (52 hours a week, up from 50).
Pay for internists continues to hover near the bottom of the scale among specialties. In this year’s Medscape Internist Compensation Report 2021, internists averaged $248,000, down from $251,000 last year. Pediatricians were the lowest paid, at $221,000, followed by family physicians, at $236,000. Plastic surgeons made the most, at $526,000, followed by orthopedists, at $511,000.
It helped to be self-employed. These internists made $276,000 on average, compared with $238,000 for their employed counterparts.
Half say pay is fair
Internists are also near the bottom among specialists who feel they are fairly compensated. As in last year’s survey, just more than half of internists (52%) said they felt that they were fairly paid this year. By comparison, 79% of oncologists reported they were fairly compensated, which is on the high end regarding satisfaction, but only 44% of infectious diseases specialists felt that way.
Some indicators in the survey responses may help explain the dissatisfaction.
Internists are near the top in time spent on paperwork. On average, they spent 19.7 hours on paperwork and administration this year, up slightly from 18.5 last year. Infectious disease physicians spent the most time on those tasks (24.2 hours a week), and anesthesiologists spent the fewest, at 10.1 hours per week.
Administrative work was among many frustrations internists reported. The following are the top five most challenging aspects of the job, according to the respondents:
- Having so many rules and regulations (24%)
- Having to work long hours (16%)
- Dealing with difficult patients (16%)
- Working with electronic health records systems (11%)
- Danger/risk associated with treating COVID-19 patients (10%)
Conversely, the most rewarding aspects were “gratitude/relationships with patients” (31%); “knowing that I’m making the world a better place” (26%); and “being very good at what I do” (20%).
More than one-third lost income
More than one-third of internists (36%) reported that they lost some income during the past year.
Among those who lost income, 81% said they expect income to return to prepandemic levels within 3 years. Half of that group expected the rebound would come within the next year.
Slightly more than one-third of internists said they would participate in the merit-based incentive payment system (MIPS), and 12% said they would participate in advanced alternative payment models. The rest either said they would participate in neither, or they hadn’t decided.
“The stakes for the Quality Payment Program – the program that incorporates MIPS – are high, with a 9% penalty applied to all Medicare reimbursement for failure to participate,” says Elizabeth Woodcock, MBA, CPC, president of the physician practice consulting firm Woodcock and Associates, in Atlanta, Georgia.
“With margins already slim,” she told this news organization, “most physicians can’t afford this massive penalty.”
If they could choose again, most internists (76%) said they would choose medicine, which was almost the same number as physicians overall who would pick medicine again. Oncologists (88%) and ophthalmologists (87%) were the specialists most likely to choose medicine again. Those in physical medicine and rehabilitation were least likely to choose medicine again, at 67%.
But asked about their specialty, internists’ enthusiasm decreased. Only 68% said that they would make that same choice again.
That was up considerably, however, from the 2015 survey: For that year, only 25% said they would choose internal medicine again.
A version of this article first appeared on Medscape.com.
Internists are seeing only 3% fewer patients than they did before the COVID-19 pandemic (72 per week on average now vs. 74 before the pandemic). Comparatively, for pediatricians, patient volume remains down 18%. Dermatologists, otolaryngologists, and orthopedists report that visits are down by about 15%.
The number of hours worked also rebounded for internists. In fact, some report working slightly more hours now than they did before the pandemic (52 hours a week, up from 50).
Pay for internists continues to hover near the bottom of the scale among specialties. In this year’s Medscape Internist Compensation Report 2021, internists averaged $248,000, down from $251,000 last year. Pediatricians were the lowest paid, at $221,000, followed by family physicians, at $236,000. Plastic surgeons made the most, at $526,000, followed by orthopedists, at $511,000.
It helped to be self-employed. These internists made $276,000 on average, compared with $238,000 for their employed counterparts.
Half say pay is fair
Internists are also near the bottom among specialists who feel they are fairly compensated. As in last year’s survey, just more than half of internists (52%) said they felt that they were fairly paid this year. By comparison, 79% of oncologists reported they were fairly compensated, which is on the high end regarding satisfaction, but only 44% of infectious diseases specialists felt that way.
Some indicators in the survey responses may help explain the dissatisfaction.
Internists are near the top in time spent on paperwork. On average, they spent 19.7 hours on paperwork and administration this year, up slightly from 18.5 last year. Infectious disease physicians spent the most time on those tasks (24.2 hours a week), and anesthesiologists spent the fewest, at 10.1 hours per week.
Administrative work was among many frustrations internists reported. The following are the top five most challenging aspects of the job, according to the respondents:
- Having so many rules and regulations (24%)
- Having to work long hours (16%)
- Dealing with difficult patients (16%)
- Working with electronic health records systems (11%)
- Danger/risk associated with treating COVID-19 patients (10%)
Conversely, the most rewarding aspects were “gratitude/relationships with patients” (31%); “knowing that I’m making the world a better place” (26%); and “being very good at what I do” (20%).
More than one-third lost income
More than one-third of internists (36%) reported that they lost some income during the past year.
Among those who lost income, 81% said they expect income to return to prepandemic levels within 3 years. Half of that group expected the rebound would come within the next year.
Slightly more than one-third of internists said they would participate in the merit-based incentive payment system (MIPS), and 12% said they would participate in advanced alternative payment models. The rest either said they would participate in neither, or they hadn’t decided.
“The stakes for the Quality Payment Program – the program that incorporates MIPS – are high, with a 9% penalty applied to all Medicare reimbursement for failure to participate,” says Elizabeth Woodcock, MBA, CPC, president of the physician practice consulting firm Woodcock and Associates, in Atlanta, Georgia.
“With margins already slim,” she told this news organization, “most physicians can’t afford this massive penalty.”
If they could choose again, most internists (76%) said they would choose medicine, which was almost the same number as physicians overall who would pick medicine again. Oncologists (88%) and ophthalmologists (87%) were the specialists most likely to choose medicine again. Those in physical medicine and rehabilitation were least likely to choose medicine again, at 67%.
But asked about their specialty, internists’ enthusiasm decreased. Only 68% said that they would make that same choice again.
That was up considerably, however, from the 2015 survey: For that year, only 25% said they would choose internal medicine again.
A version of this article first appeared on Medscape.com.
55 new chemicals found in pregnant women, their newborns
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fifty-five chemicals never before reported in humans were found in pregnant women, according to a study from the University of California, San Francisco. The chemicals likely come from consumer products or industrial sources, researchers say.
Findings were published online in Environmental Science and Technology.
Co-first authors Aolin Wang, PhD, and Dimitri Panagopoulos Abrahamsson, PhD, postdoctoral fellows in UCSF’s obstetrics and gynecology department, and colleagues found 109 chemicals in the blood of pregnant women, including 42 “mystery chemicals” whose sources and uses are not known.
The chemicals were also found in their newborns, tests from umbilical cord blood show, suggesting the chemicals cross through the placenta.
Among the chemicals, 40 are used as plasticizers, 28 are used in cosmetics, another 25 are used in consumer products, 29 as pharmaceuticals, 23 as pesticides, three as flame retardants, and seven are PFAS [per- and polyfluoroalkyl substances] compounds used in multiple applications including carpeting and upholstery, the authors report.
Senior author Tracey Woodruff, PhD, MPH, characterized their discoveries as “disturbing.”
She told this news organization that it’s not only frustrating to know the chemicals are present but to know so little about them.
“We know it’s a chemical registered to be manufactured, and it’s used in commerce, but we don’t know where,” she explained. “That’s very disturbing, that we can’t trace them, and that shows a failure in public policy and government.”
“Exposures are occurring without our consent,” said Ms. Woodruff, a former U.S. Environmental Protection Agency scientist, who directs the Program on Reproductive Health and the Environment (PRHE) and the Environmental Research and Translation for Health (EaRTH) Center, both at UCSF.
She said researchers know from previous studies that when the U.S. government acts to remove harmful chemicals from the marketplace, the levels of those chemicals measured in people drop.
“Examples include lead, certain PFAS, flame retardant chemicals, and certain phthalates,” she said. “So public policies can be effective in preventing exposures that can be harmful.”
Technological advances led to the discoveries
The team used high-resolution mass spectrometry (HRMS) to identify human-made chemicals in people.
Dr. Abrahamsson said in an interview that the technology is relatively new in research and had not previously been used to scan for chemicals in pregnant women and their infants.
Because scientists often study what other scientists have studied, he said, the same chemicals tend to get attention. The wider scope made possible by the new technology helps illumine where to focus future research, he said.
A benefit of the technology is that now researchers don’t have to know which chemicals they are looking for when they scan blood samples, but they can observe whatever appears, he said.
Ms. Woodruff said, “We hope this is further data and evidence that support government policies that require industries to tell us where they are using their chemicals and how we might be exposed to them.”
She said this research will also help identify which chemicals to prioritize for monitoring in the environment.
Average age of the women in the study was 32 years. Nearly half were Hispanic; 37% were non-Hispanic Whites; and 17% were non-Hispanic Asians, Pacific Islanders, and African Americans. Half of the participants were born outside the United States and had lived in the U.S. for an average 22 years.
Sean Palfrey, MD, a professor of clinical pediatrics and public health at Boston University, said more chemical discoveries like these will come as technology continues to evolve.
Dr. Palfrey, who was not involved in the study, agrees with the authors that there is a lack of oversight as to what substances are used in products.
“Our industrial regulations are very poor and therefore our industries get away with using new and untested substances in their products,” he told this news organization.
“This lack of regulation is really important when it results in us not recognizing that known and serious toxins are being put into foods or other products, or when a new class of toxin has been invented which is a serious poison. Most of the toxins, though, are discovered in products in very low levels,” he said.
Dr. Palfrey said, however, that focus should stay on the known and serious toxins that seep into the environment from common products.
“It has taken us decades to ban certain flame retardants from home products,” he said. “TOSCA [the Toxic Substances Control Act passed by Congress in 1976] was too limited when it was passed decades ago and is now fearfully out of date. Unless we discover a COVID among the toxins discovered in studies like this, we should focus on the big stuff.”
The authors and Dr. Palfrey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia? Referral, drugs not usually needed
Too often, medications are the treatment of choice, and when used long term they can perpetuate a problematic cycle, said Dr. Lettieri, professor in pulmonary, critical care, and sleep medicine at Johns Hopkins University, Baltimore.
However, medications alone won’t work without other behavior modifications and they come with potential side effects, he said in his talk. Prescription medications typically don’t treat the cause of the insomnia, just the symptoms.
“In the 15 years I’ve been practicing sleep medicine, I can honestly say I only have a handful of patients that I treat with long-term pharmacotherapy,” Dr. Lettieri said.
He said he typically uses pharmacotherapy only when conservative measures have failed or to help jump-start patients to behavior modifications.
Restricted sleep is a good place to start for chronic insomnia, he continued.
Physicians should ask patients the latest time they can wake up to make it to school, work, etc. If that time is 6 a.m., the goal is to move bedtime back to 10 p.m.–11 p.m. If the patient, however, is unable to sleep until 12:30 a.m., move bedtime there, he said.
Though the 5.5-hour window is not ideal, it’s better to get into bed when ready for sleep. From there, try to get the patient to move bedtime back 15 minutes each week as they train themselves to fall asleep earlier, he said.
“I promise you this works in the majority of patients and doesn’t require any medication. You can also accomplish this with one or two office visits, so it is not a huge drain on resources,” he said.
Sleep specialists in short supply
Cognitive-behavioral therapy (CBT) is “without question the best way to treat chronic insomnia and it’s recommended as first-line therapy by all published guidelines,” Dr. Lettieri said.
He defined chronic insomnia as happening most nights over at least 3 months. It affects twice as many women as men.
CBT offers a formalized way of changing sleep patterns with the help of an expert in sleep behavior disorders. It combines cognitive therapies with education about sleep and stimulus control and uses techniques such as mindfulness and relaxation.
However, most programs take 4-8 sessions with a sleep medicine provider and are usually not covered by insurance. In addition, the number of insomnia specialists is not nearly adequate to meet demand, he added.
Online and mobile-platform CBT programs are widely effective, Dr. Lettieri said. Many are free and all are convenient for patients to use. He said many of his patients use Sleepio, but many other online programs are effective.
“You can provide sufficient therapy for many of your patients and reserve CBT for patients who can’t be fixed with more conservative measures,” he said.
Insomnia among older patients
Interest in helping older patients with insomnia dominated the chat session associated with the talk.
Insomnia increases with age and older patients have often been using prescription or over-the-counter sleep aids for decades.
Additionally, “insomnia is the second-most common reason why people get admitted to long-term care facilities, second only to urinary incontinence,” Dr. Lettieri said.
If physicians use medications with older patients, he said, extra caution is needed. Older people have more neurocognitive impairments than younger adults and may already be taking several other medications. Sleep medications may come with longer elimination half-lives. Polypharmacy may increase risk for falls and have other consequences.
“If you have to go to a medication, try something simple like melatonin,” he said, adding that it should be pharmaceutical grade and extended release.
Also, bright lights during the day, movement throughout the day, and dim lights closer to bedtime are especially important for the elderly, Dr. Lettieri said.
Andrew Corr, MD, a geriatric specialist in primary care with the Riverside (Calif.) Medical Clinic, said in an interview the main message he will take back to his physician group is more CBT and less medication.
He said that, although he has long known CBT is the top first-line treatment, it is difficult to find experts in his area who are trained to do CBT for insomnia, so he was glad to hear online programs and self-directed reading are typically effective.
He also said there’s a common misperception that there’s no harm in prescribing medications such as trazodone (Desyrel), an antidepressant commonly used off label as a sleep aid.
Dr. Lettieri’s talk highlighted his recommendation against using trazodone for sleep. “Despite several recommendations against its use for insomnia, it is still commonly prescribed. You just shouldn’t use it for insomnia,” Dr. Lettieri said.
“It has no measurable effect in a third of patients and at least unacceptable side effects in another third. Right off the bat, it’s not efficacious in two thirds of patients.”
Additionally, priapism, a prolonged erection, has been associated with trazodone, Dr. Lettieri said, “and I have literally never met a patient on trazodone who was counseled about this.”
Trazodone also has a black box warning from the Food and Drug Administration warning about increased risk for suicidal thoughts.
Dr. Lettieri and Dr. Corr disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too often, medications are the treatment of choice, and when used long term they can perpetuate a problematic cycle, said Dr. Lettieri, professor in pulmonary, critical care, and sleep medicine at Johns Hopkins University, Baltimore.
However, medications alone won’t work without other behavior modifications and they come with potential side effects, he said in his talk. Prescription medications typically don’t treat the cause of the insomnia, just the symptoms.
“In the 15 years I’ve been practicing sleep medicine, I can honestly say I only have a handful of patients that I treat with long-term pharmacotherapy,” Dr. Lettieri said.
He said he typically uses pharmacotherapy only when conservative measures have failed or to help jump-start patients to behavior modifications.
Restricted sleep is a good place to start for chronic insomnia, he continued.
Physicians should ask patients the latest time they can wake up to make it to school, work, etc. If that time is 6 a.m., the goal is to move bedtime back to 10 p.m.–11 p.m. If the patient, however, is unable to sleep until 12:30 a.m., move bedtime there, he said.
Though the 5.5-hour window is not ideal, it’s better to get into bed when ready for sleep. From there, try to get the patient to move bedtime back 15 minutes each week as they train themselves to fall asleep earlier, he said.
“I promise you this works in the majority of patients and doesn’t require any medication. You can also accomplish this with one or two office visits, so it is not a huge drain on resources,” he said.
Sleep specialists in short supply
Cognitive-behavioral therapy (CBT) is “without question the best way to treat chronic insomnia and it’s recommended as first-line therapy by all published guidelines,” Dr. Lettieri said.
He defined chronic insomnia as happening most nights over at least 3 months. It affects twice as many women as men.
CBT offers a formalized way of changing sleep patterns with the help of an expert in sleep behavior disorders. It combines cognitive therapies with education about sleep and stimulus control and uses techniques such as mindfulness and relaxation.
However, most programs take 4-8 sessions with a sleep medicine provider and are usually not covered by insurance. In addition, the number of insomnia specialists is not nearly adequate to meet demand, he added.
Online and mobile-platform CBT programs are widely effective, Dr. Lettieri said. Many are free and all are convenient for patients to use. He said many of his patients use Sleepio, but many other online programs are effective.
“You can provide sufficient therapy for many of your patients and reserve CBT for patients who can’t be fixed with more conservative measures,” he said.
Insomnia among older patients
Interest in helping older patients with insomnia dominated the chat session associated with the talk.
Insomnia increases with age and older patients have often been using prescription or over-the-counter sleep aids for decades.
Additionally, “insomnia is the second-most common reason why people get admitted to long-term care facilities, second only to urinary incontinence,” Dr. Lettieri said.
If physicians use medications with older patients, he said, extra caution is needed. Older people have more neurocognitive impairments than younger adults and may already be taking several other medications. Sleep medications may come with longer elimination half-lives. Polypharmacy may increase risk for falls and have other consequences.
“If you have to go to a medication, try something simple like melatonin,” he said, adding that it should be pharmaceutical grade and extended release.
Also, bright lights during the day, movement throughout the day, and dim lights closer to bedtime are especially important for the elderly, Dr. Lettieri said.
Andrew Corr, MD, a geriatric specialist in primary care with the Riverside (Calif.) Medical Clinic, said in an interview the main message he will take back to his physician group is more CBT and less medication.
He said that, although he has long known CBT is the top first-line treatment, it is difficult to find experts in his area who are trained to do CBT for insomnia, so he was glad to hear online programs and self-directed reading are typically effective.
He also said there’s a common misperception that there’s no harm in prescribing medications such as trazodone (Desyrel), an antidepressant commonly used off label as a sleep aid.
Dr. Lettieri’s talk highlighted his recommendation against using trazodone for sleep. “Despite several recommendations against its use for insomnia, it is still commonly prescribed. You just shouldn’t use it for insomnia,” Dr. Lettieri said.
“It has no measurable effect in a third of patients and at least unacceptable side effects in another third. Right off the bat, it’s not efficacious in two thirds of patients.”
Additionally, priapism, a prolonged erection, has been associated with trazodone, Dr. Lettieri said, “and I have literally never met a patient on trazodone who was counseled about this.”
Trazodone also has a black box warning from the Food and Drug Administration warning about increased risk for suicidal thoughts.
Dr. Lettieri and Dr. Corr disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too often, medications are the treatment of choice, and when used long term they can perpetuate a problematic cycle, said Dr. Lettieri, professor in pulmonary, critical care, and sleep medicine at Johns Hopkins University, Baltimore.
However, medications alone won’t work without other behavior modifications and they come with potential side effects, he said in his talk. Prescription medications typically don’t treat the cause of the insomnia, just the symptoms.
“In the 15 years I’ve been practicing sleep medicine, I can honestly say I only have a handful of patients that I treat with long-term pharmacotherapy,” Dr. Lettieri said.
He said he typically uses pharmacotherapy only when conservative measures have failed or to help jump-start patients to behavior modifications.
Restricted sleep is a good place to start for chronic insomnia, he continued.
Physicians should ask patients the latest time they can wake up to make it to school, work, etc. If that time is 6 a.m., the goal is to move bedtime back to 10 p.m.–11 p.m. If the patient, however, is unable to sleep until 12:30 a.m., move bedtime there, he said.
Though the 5.5-hour window is not ideal, it’s better to get into bed when ready for sleep. From there, try to get the patient to move bedtime back 15 minutes each week as they train themselves to fall asleep earlier, he said.
“I promise you this works in the majority of patients and doesn’t require any medication. You can also accomplish this with one or two office visits, so it is not a huge drain on resources,” he said.
Sleep specialists in short supply
Cognitive-behavioral therapy (CBT) is “without question the best way to treat chronic insomnia and it’s recommended as first-line therapy by all published guidelines,” Dr. Lettieri said.
He defined chronic insomnia as happening most nights over at least 3 months. It affects twice as many women as men.
CBT offers a formalized way of changing sleep patterns with the help of an expert in sleep behavior disorders. It combines cognitive therapies with education about sleep and stimulus control and uses techniques such as mindfulness and relaxation.
However, most programs take 4-8 sessions with a sleep medicine provider and are usually not covered by insurance. In addition, the number of insomnia specialists is not nearly adequate to meet demand, he added.
Online and mobile-platform CBT programs are widely effective, Dr. Lettieri said. Many are free and all are convenient for patients to use. He said many of his patients use Sleepio, but many other online programs are effective.
“You can provide sufficient therapy for many of your patients and reserve CBT for patients who can’t be fixed with more conservative measures,” he said.
Insomnia among older patients
Interest in helping older patients with insomnia dominated the chat session associated with the talk.
Insomnia increases with age and older patients have often been using prescription or over-the-counter sleep aids for decades.
Additionally, “insomnia is the second-most common reason why people get admitted to long-term care facilities, second only to urinary incontinence,” Dr. Lettieri said.
If physicians use medications with older patients, he said, extra caution is needed. Older people have more neurocognitive impairments than younger adults and may already be taking several other medications. Sleep medications may come with longer elimination half-lives. Polypharmacy may increase risk for falls and have other consequences.
“If you have to go to a medication, try something simple like melatonin,” he said, adding that it should be pharmaceutical grade and extended release.
Also, bright lights during the day, movement throughout the day, and dim lights closer to bedtime are especially important for the elderly, Dr. Lettieri said.
Andrew Corr, MD, a geriatric specialist in primary care with the Riverside (Calif.) Medical Clinic, said in an interview the main message he will take back to his physician group is more CBT and less medication.
He said that, although he has long known CBT is the top first-line treatment, it is difficult to find experts in his area who are trained to do CBT for insomnia, so he was glad to hear online programs and self-directed reading are typically effective.
He also said there’s a common misperception that there’s no harm in prescribing medications such as trazodone (Desyrel), an antidepressant commonly used off label as a sleep aid.
Dr. Lettieri’s talk highlighted his recommendation against using trazodone for sleep. “Despite several recommendations against its use for insomnia, it is still commonly prescribed. You just shouldn’t use it for insomnia,” Dr. Lettieri said.
“It has no measurable effect in a third of patients and at least unacceptable side effects in another third. Right off the bat, it’s not efficacious in two thirds of patients.”
Additionally, priapism, a prolonged erection, has been associated with trazodone, Dr. Lettieri said, “and I have literally never met a patient on trazodone who was counseled about this.”
Trazodone also has a black box warning from the Food and Drug Administration warning about increased risk for suicidal thoughts.
Dr. Lettieri and Dr. Corr disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2021
When to refer patients with new memory loss
Initial questions should zero in on what the patient is forgetting, said Megan Richie, MD, a neurohospitalist at the University of California, San Francisco, who spoke to a virtual audience at the American College of Physicians (ACP) annual Internal Medicine meeting.
Is the patient forgetting to buy things in a store, having trouble recalling events, forgetting important dates? How often do these incidents occur?
These questions “will help get at how pervasive and how likely the memory loss is affecting their lives, versus a subjective complaint that doesn’t have much impact on the day-to-day function,” she said.
It’s also important to ask whether other neurocognitive symptoms accompany the memory loss, Dr. Richie noted.
Does the patient search for words, struggle with attention, or have problems with executive function? Does the patient have psychiatric symptoms, such as hallucinations or delusions, or other neurologic complaints, including weakness, numbness, vision change, or movement disorders?
“When you know how many neurocognitive symptoms they have, think about how [those symptoms] are affecting their safety and functional status. How are they on their activities of daily living?” Dr. Richie suggests.
Also ask whether the patient is taking medications and whether they drive a vehicle. If they do drive, do they get lost?
“These are all going to help you determine the acuity of the workup,” she said.
After a thorough history, cognitive screening is the next consideration.
Cognitive screening can be performed in minutes
One of the tests Dr. Richie recommends is the Mini-Cog. It takes 3 minutes to administer and has been formally recommended by the Alzheimer’s Association because it can be completed in the time frame of a Medicare wellness visit, she said.
It entails a three-word recall and clock-drawing test.
Dr. Richie said it’s important to eliminate some key causes first: “Certainly if the patient has signs and symptoms of depression, pseudodementia is a very real and treatable disease you do not want to miss and should consider in these patients,” she pointed out.
Systemic medical conditions can also lead to memory loss.
If there’s an acute component to the complaint, a new infection or medication withdrawal or a side effect could be driving it, so that’s key in questioning.
Dr. Richie explained that the American Academy of Neurology recommends a very limited workup.
“It’s really just to check their thyroid, their vitamin B12 levels, and then a one-time picture of their brain, which can be either MRI or a CT, to look for structural problems or vascular dementia or hydrocephalus, etc.”
“You do not routinely need spinal fluid testing or an EEG,” she emphasized.
Signs that a neurologist should be involved include a rapid decline, signs of potential seizures, or that the patient doesn’t seem safe in their condition.
Neuropsychological testing is helpful, but it takes nearly 3 hours and may not be a good choice for restless or aggressive patients, Dr. Richie said.
Such testing is often not available, and if it is, insurance coverage is often a barrier because many plans don’t cover it.
Patients often ask about drugs and supplements they see advertised to help with memory loss. Medications are not helpful for mild cognitive impairment, although there is evidence that some are beneficial for patients with dementia, Dr. Richie said.
Celine Goetz, MD, assistant professor of internal medicine at Rush University Medical Center, Chicago, Illinois, told this news organization that it’s easy to relate to the fear that patients and families feel when cognitive impairment begins to emerge.
“[Dr.] Richie’s talk was right on point for internists like myself who see many patients with memory complaints, cognitive impairment, and dementia. I think we’re all terrified of losing our memory and the social and functional impairment that comes with that,” she said.
Although cognitive impairment and dementia aren’t curable or reversible, Dr. Goetz noted, internists can help patients optimize management of conditions such as diabetes and heart disease, which can affect cognitive function.
Dr. Richie pointed out that some interventions lack evidence for the treatment of mild cognitive impairment, but Dr. Goetz emphasized that resources are plentiful and can be effective in combination.
“Engaging social workers, pharmacists, nutritionists, physical and occupational therapists, and, on the inpatient side, delirium protocols, chaplains, and music therapists make a huge difference in patient care,” she said.
Dr. Richie and Dr. Goetz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initial questions should zero in on what the patient is forgetting, said Megan Richie, MD, a neurohospitalist at the University of California, San Francisco, who spoke to a virtual audience at the American College of Physicians (ACP) annual Internal Medicine meeting.
Is the patient forgetting to buy things in a store, having trouble recalling events, forgetting important dates? How often do these incidents occur?
These questions “will help get at how pervasive and how likely the memory loss is affecting their lives, versus a subjective complaint that doesn’t have much impact on the day-to-day function,” she said.
It’s also important to ask whether other neurocognitive symptoms accompany the memory loss, Dr. Richie noted.
Does the patient search for words, struggle with attention, or have problems with executive function? Does the patient have psychiatric symptoms, such as hallucinations or delusions, or other neurologic complaints, including weakness, numbness, vision change, or movement disorders?
“When you know how many neurocognitive symptoms they have, think about how [those symptoms] are affecting their safety and functional status. How are they on their activities of daily living?” Dr. Richie suggests.
Also ask whether the patient is taking medications and whether they drive a vehicle. If they do drive, do they get lost?
“These are all going to help you determine the acuity of the workup,” she said.
After a thorough history, cognitive screening is the next consideration.
Cognitive screening can be performed in minutes
One of the tests Dr. Richie recommends is the Mini-Cog. It takes 3 minutes to administer and has been formally recommended by the Alzheimer’s Association because it can be completed in the time frame of a Medicare wellness visit, she said.
It entails a three-word recall and clock-drawing test.
Dr. Richie said it’s important to eliminate some key causes first: “Certainly if the patient has signs and symptoms of depression, pseudodementia is a very real and treatable disease you do not want to miss and should consider in these patients,” she pointed out.
Systemic medical conditions can also lead to memory loss.
If there’s an acute component to the complaint, a new infection or medication withdrawal or a side effect could be driving it, so that’s key in questioning.
Dr. Richie explained that the American Academy of Neurology recommends a very limited workup.
“It’s really just to check their thyroid, their vitamin B12 levels, and then a one-time picture of their brain, which can be either MRI or a CT, to look for structural problems or vascular dementia or hydrocephalus, etc.”
“You do not routinely need spinal fluid testing or an EEG,” she emphasized.
Signs that a neurologist should be involved include a rapid decline, signs of potential seizures, or that the patient doesn’t seem safe in their condition.
Neuropsychological testing is helpful, but it takes nearly 3 hours and may not be a good choice for restless or aggressive patients, Dr. Richie said.
Such testing is often not available, and if it is, insurance coverage is often a barrier because many plans don’t cover it.
Patients often ask about drugs and supplements they see advertised to help with memory loss. Medications are not helpful for mild cognitive impairment, although there is evidence that some are beneficial for patients with dementia, Dr. Richie said.
Celine Goetz, MD, assistant professor of internal medicine at Rush University Medical Center, Chicago, Illinois, told this news organization that it’s easy to relate to the fear that patients and families feel when cognitive impairment begins to emerge.
“[Dr.] Richie’s talk was right on point for internists like myself who see many patients with memory complaints, cognitive impairment, and dementia. I think we’re all terrified of losing our memory and the social and functional impairment that comes with that,” she said.
Although cognitive impairment and dementia aren’t curable or reversible, Dr. Goetz noted, internists can help patients optimize management of conditions such as diabetes and heart disease, which can affect cognitive function.
Dr. Richie pointed out that some interventions lack evidence for the treatment of mild cognitive impairment, but Dr. Goetz emphasized that resources are plentiful and can be effective in combination.
“Engaging social workers, pharmacists, nutritionists, physical and occupational therapists, and, on the inpatient side, delirium protocols, chaplains, and music therapists make a huge difference in patient care,” she said.
Dr. Richie and Dr. Goetz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initial questions should zero in on what the patient is forgetting, said Megan Richie, MD, a neurohospitalist at the University of California, San Francisco, who spoke to a virtual audience at the American College of Physicians (ACP) annual Internal Medicine meeting.
Is the patient forgetting to buy things in a store, having trouble recalling events, forgetting important dates? How often do these incidents occur?
These questions “will help get at how pervasive and how likely the memory loss is affecting their lives, versus a subjective complaint that doesn’t have much impact on the day-to-day function,” she said.
It’s also important to ask whether other neurocognitive symptoms accompany the memory loss, Dr. Richie noted.
Does the patient search for words, struggle with attention, or have problems with executive function? Does the patient have psychiatric symptoms, such as hallucinations or delusions, or other neurologic complaints, including weakness, numbness, vision change, or movement disorders?
“When you know how many neurocognitive symptoms they have, think about how [those symptoms] are affecting their safety and functional status. How are they on their activities of daily living?” Dr. Richie suggests.
Also ask whether the patient is taking medications and whether they drive a vehicle. If they do drive, do they get lost?
“These are all going to help you determine the acuity of the workup,” she said.
After a thorough history, cognitive screening is the next consideration.
Cognitive screening can be performed in minutes
One of the tests Dr. Richie recommends is the Mini-Cog. It takes 3 minutes to administer and has been formally recommended by the Alzheimer’s Association because it can be completed in the time frame of a Medicare wellness visit, she said.
It entails a three-word recall and clock-drawing test.
Dr. Richie said it’s important to eliminate some key causes first: “Certainly if the patient has signs and symptoms of depression, pseudodementia is a very real and treatable disease you do not want to miss and should consider in these patients,” she pointed out.
Systemic medical conditions can also lead to memory loss.
If there’s an acute component to the complaint, a new infection or medication withdrawal or a side effect could be driving it, so that’s key in questioning.
Dr. Richie explained that the American Academy of Neurology recommends a very limited workup.
“It’s really just to check their thyroid, their vitamin B12 levels, and then a one-time picture of their brain, which can be either MRI or a CT, to look for structural problems or vascular dementia or hydrocephalus, etc.”
“You do not routinely need spinal fluid testing or an EEG,” she emphasized.
Signs that a neurologist should be involved include a rapid decline, signs of potential seizures, or that the patient doesn’t seem safe in their condition.
Neuropsychological testing is helpful, but it takes nearly 3 hours and may not be a good choice for restless or aggressive patients, Dr. Richie said.
Such testing is often not available, and if it is, insurance coverage is often a barrier because many plans don’t cover it.
Patients often ask about drugs and supplements they see advertised to help with memory loss. Medications are not helpful for mild cognitive impairment, although there is evidence that some are beneficial for patients with dementia, Dr. Richie said.
Celine Goetz, MD, assistant professor of internal medicine at Rush University Medical Center, Chicago, Illinois, told this news organization that it’s easy to relate to the fear that patients and families feel when cognitive impairment begins to emerge.
“[Dr.] Richie’s talk was right on point for internists like myself who see many patients with memory complaints, cognitive impairment, and dementia. I think we’re all terrified of losing our memory and the social and functional impairment that comes with that,” she said.
Although cognitive impairment and dementia aren’t curable or reversible, Dr. Goetz noted, internists can help patients optimize management of conditions such as diabetes and heart disease, which can affect cognitive function.
Dr. Richie pointed out that some interventions lack evidence for the treatment of mild cognitive impairment, but Dr. Goetz emphasized that resources are plentiful and can be effective in combination.
“Engaging social workers, pharmacists, nutritionists, physical and occupational therapists, and, on the inpatient side, delirium protocols, chaplains, and music therapists make a huge difference in patient care,” she said.
Dr. Richie and Dr. Goetz report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2021
Most labeled penicillin-allergic are no longer intolerant
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mislabeling has implications for patient outcomes and efforts to fight antibiotic resistance, said Olajumoke Fadugba, MD, program director for the allergy and immunology fellowship at University of Pennsylvania Health System, Philadelphia.
About 10% of the general population reports a history of penicillin allergy (up to 15% of hospitalized patients), but up to 90% of patients with that label are able to tolerate penicillin, Dr. Fadugba said. The mislabeling comes either because reactions were improperly characterized early on or people have outgrown the allergy.
“There are data that tell us penicillin IgE-mediated wanes over time and that after 10 years of avoidance of a drug, greater than 80% of patients have a resolution of their penicillin IgE.”
Data also show patients outgrow their aminopenicillin reactions (including those from amoxicillin and Ampicillin) faster than parenteral penicillin reactions, she noted.
Josune Iglesias, MD, assistant professor of internal medicine at Rush University Medical Center in Chicago, said in an interview that she often sees patients who said their parents told them when they were kids that they were allergic to penicillin and that information just keeps getting entered into their records.
She said physicians are aware the penicillin-allergic label is not always accurate, but there is hesitancy to challenge those labels.
“We are cautious because of the potential side effects and the harm that we could cause if we unlabel the patient,” she said. “I think having this information will help us unlabel those patients well so we don’t cause harm.”
Also, the threat to antibiotic resistance is real, she said, when penicillin is eliminated as an option unnecessarily.
When a person is labeled allergic to penicillin, the treatment choices often go to broad-spectrum antibiotics that are more costly, have potentially worse side effects, and may contribute to resistance.
“It’s really important, especially with older people, patients sicker with chronic conditions to really make sure we unlabel those patients [who are not truly penicillin allergic],” Dr. Iglesias said.
The label can also cause harm in the hospital setting and worsen outcomes, according to Dr. Fadugba.
She noted that the penicillin allergy label has been linked with longer hospital length of stay, higher rate of readmission, acute kidney injury, multidrug-resistant organisms such as MRSA, and nosocomial infections including Clostridioides difficile.
Getting an effective drug history is an important part of determining who really has a penicillin allergy.
A questionnaire should ask whether the patient was likely to have had an immediate hypersensitivity to penicillin, such as hives or anaphylaxis, which would be more worrisome than a delayed rash.
Knowing the time frame of the reaction helps determine how likely or unlikely people are to still have the allergy, Dr. Fadugba said. “We also want to ask, have they received a penicillin antibiotic since that initial reaction and have they tolerated it?”
She continued: “If a patient received amoxicillin 2 weeks ago, and they tolerated it, you can essentially remove the allergy label and essentially change that patient’s potential hospital course – that immediate course or future outcomes.”
After obtaining the history, there are choices to make.
If a patient is not allergic, she said, the next step is removing the label and documenting why so that in the future another clinician doesn’t see the deleted label and put it back. If a person is deemed allergic by history, clinicians should document the nature of the reaction and if the patient needs a beta-lactam during a hospitalization or in clinic, make a decision based on what kind of beta-lactam they need.
“Generally, for a fourth-generation cephalosporin, for a distant history of penicillin allergy, you can probably give the full dose or – if you’re conservative – give it cautiously, perhaps 10% initially and then monitor because cross-reactivity is known to be low, about 2%,” Dr. Fadugba said.
If the patient needs a penicillin antibiotic specifically, options are guided by the resources.
If a clinician has personnel or an allergy specialist available, skin testing may be an option and “if negative, you can rule out the allergy,” Dr. Fadugba said.
“If that’s not available and the patient really needs a penicillin, you can consider desensitization,” she said.
However, she said, “If the patient is very high risk, then you have no choice but to use an alternative, especially if you can’t desensitize.”
Dr. Fadugba is a consultant for the Health Resources & Services Administration. Dr. Iglesias disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Feds lift pause of J&J COVID vaccine, add new warning
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
CDC panel: Pause of J&J COVID-19 vaccine to remain for now
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.

