User login
Baricitinib’s approval for alopecia areata: Considerations for starting patients on treatment
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
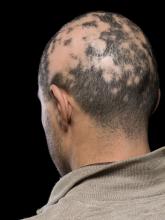
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fertility rates lower in disadvantaged neighborhoods
A new study ties the odds of conception to the advantages of the neighborhood a woman lives in.
In a cohort of more than 6,000 women who were trying to get pregnant without fertility treatments, the probability of conception was reduced 21%-23% per menstrual cycle when comparing the most disadvantaged neighborhoods with the least disadvantaged.
“When disadvantaged neighborhood status was categorized within each state (as opposed to nationally), the results were slightly larger in magnitude,” wrote authors of the study published online in JAMA Network Open.
Among 6,356 participants, 3,725 pregnancies were observed for 27,427 menstrual cycles of follow-up. Average age was 30, and most participants were non-Hispanic White (5,297 [83.3%]) and had not previously given birth (4,179 [65.7%]).
When the researchers compared the top and bottom deciles of disadvantaged neighborhood status, adjusted fecundability ratios (the per-cycle probability of conception) were 0.79 (95% confidence interval [CI], 0.66-0.96) for national-level area deprivation index (ADI) rankings and 0.77 (95% CI, 0.65-0.92) for within-state ADI rankings. ADI score includes population indicators related to educational attainment, housing, employment, and poverty.
“These findings suggest that investments in disadvantaged neighborhoods may yield positive cobenefits for fertility,” the authors wrote.
The researchers used the Pregnancy Study Online, for which baseline data were collected from women in the United States from June 19, 2013, through April 12, 2019.
In the United States, 10%-15% of reproductive-aged couples experience infertility, defined as the inability to conceive after a year of unprotected intercourse.
Reason behind the numbers unclear
Mark Hornstein, MD, director in the reproductive endocrinology division of Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, said in an interview that this study gives the “what” but the “why” is harder to pinpoint.
What is not known, he said, is what kind of access the women had to fertility counseling or treatment.
The association between fertility and neighborhood advantage status is very plausible given the well-established links between disadvantaged regions and poorer health outcomes, he said, adding that the authors make a good case for their conclusions in the paper.
The authors ruled out many potential confounders, such as age of the women, reproductive history, multivitamin use, education level, household income, and frequency of intercourse, and still there was a difference between disadvantaged and advantaged neighborhoods, he noted.
Dr. Hornstein said his own research team has found that lack of knowledge about insurance coverage regarding infertility services may keep women from seeking the services.
“One of the things I worry about it access,” he said. “[The study authors] didn’t really look at that. They just looked at what the chances were that they got pregnant. But they didn’t say how many of those women had a workup, an evaluation, for why they were having difficulty, if they were, or had treatment. So I don’t know if some or all or none of that difference that they saw from the highest neighborhood health score to the most disadvantaged – if that was from inherent problems in the area, access to the best health care, or some combination.”
Discussions have focused on changing personal behaviors
Discussions on improving fertility often center on changing personal behaviors, the authors noted. “However, structural, political, and environmental factors may also play a substantial role,” they wrote.
The findings are in line with previous research on the effect of stress on in vitro outcomes, they pointed out. “Perceived stress has been associated with poorer in vitro fertilization outcomes and reduced fecundability among couples attempting spontaneous conception,” the authors noted.
Studies also have shown that living in a disadvantaged neighborhood is linked with comorbidities during pregnancy, such as increased risks of gestational hypertension (risk ratio for lowest vs. highest quartile: 1.24 [95% CI, 1.14-1.35]) and poor gestational weight gain (relative risk for lowest vs. highest quartile: 1.1 [95% CI, 1.1-1.2]).
In addition, policies such as those that support civil rights, protect the environment, and invest in underresourced communities have been shown to improve health markers such as life expectancy.
Policy decisions can also perpetuate a cycle of stress, they wrote. Disadvantaged communities may have more air pollution, which has been shown to have negative effects on fertility. Unemployment has been linked with decreased population-level fertility rates. Lack of green space may result in fewer areas to reduce stress.
A study coauthor reported grants from the National Institutes of Health during the conduct of the study; nonfinancial support from Swiss Precision Diagnostics GmbH, Labcorp, Kindara.com, and FertilityFriend.com; and consulting for AbbVie outside the submitted work. No other author disclosures were reported. Dr. Hornstein reported no relevant financial relationships.
A new study ties the odds of conception to the advantages of the neighborhood a woman lives in.
In a cohort of more than 6,000 women who were trying to get pregnant without fertility treatments, the probability of conception was reduced 21%-23% per menstrual cycle when comparing the most disadvantaged neighborhoods with the least disadvantaged.
“When disadvantaged neighborhood status was categorized within each state (as opposed to nationally), the results were slightly larger in magnitude,” wrote authors of the study published online in JAMA Network Open.
Among 6,356 participants, 3,725 pregnancies were observed for 27,427 menstrual cycles of follow-up. Average age was 30, and most participants were non-Hispanic White (5,297 [83.3%]) and had not previously given birth (4,179 [65.7%]).
When the researchers compared the top and bottom deciles of disadvantaged neighborhood status, adjusted fecundability ratios (the per-cycle probability of conception) were 0.79 (95% confidence interval [CI], 0.66-0.96) for national-level area deprivation index (ADI) rankings and 0.77 (95% CI, 0.65-0.92) for within-state ADI rankings. ADI score includes population indicators related to educational attainment, housing, employment, and poverty.
“These findings suggest that investments in disadvantaged neighborhoods may yield positive cobenefits for fertility,” the authors wrote.
The researchers used the Pregnancy Study Online, for which baseline data were collected from women in the United States from June 19, 2013, through April 12, 2019.
In the United States, 10%-15% of reproductive-aged couples experience infertility, defined as the inability to conceive after a year of unprotected intercourse.
Reason behind the numbers unclear
Mark Hornstein, MD, director in the reproductive endocrinology division of Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, said in an interview that this study gives the “what” but the “why” is harder to pinpoint.
What is not known, he said, is what kind of access the women had to fertility counseling or treatment.
The association between fertility and neighborhood advantage status is very plausible given the well-established links between disadvantaged regions and poorer health outcomes, he said, adding that the authors make a good case for their conclusions in the paper.
The authors ruled out many potential confounders, such as age of the women, reproductive history, multivitamin use, education level, household income, and frequency of intercourse, and still there was a difference between disadvantaged and advantaged neighborhoods, he noted.
Dr. Hornstein said his own research team has found that lack of knowledge about insurance coverage regarding infertility services may keep women from seeking the services.
“One of the things I worry about it access,” he said. “[The study authors] didn’t really look at that. They just looked at what the chances were that they got pregnant. But they didn’t say how many of those women had a workup, an evaluation, for why they were having difficulty, if they were, or had treatment. So I don’t know if some or all or none of that difference that they saw from the highest neighborhood health score to the most disadvantaged – if that was from inherent problems in the area, access to the best health care, or some combination.”
Discussions have focused on changing personal behaviors
Discussions on improving fertility often center on changing personal behaviors, the authors noted. “However, structural, political, and environmental factors may also play a substantial role,” they wrote.
The findings are in line with previous research on the effect of stress on in vitro outcomes, they pointed out. “Perceived stress has been associated with poorer in vitro fertilization outcomes and reduced fecundability among couples attempting spontaneous conception,” the authors noted.
Studies also have shown that living in a disadvantaged neighborhood is linked with comorbidities during pregnancy, such as increased risks of gestational hypertension (risk ratio for lowest vs. highest quartile: 1.24 [95% CI, 1.14-1.35]) and poor gestational weight gain (relative risk for lowest vs. highest quartile: 1.1 [95% CI, 1.1-1.2]).
In addition, policies such as those that support civil rights, protect the environment, and invest in underresourced communities have been shown to improve health markers such as life expectancy.
Policy decisions can also perpetuate a cycle of stress, they wrote. Disadvantaged communities may have more air pollution, which has been shown to have negative effects on fertility. Unemployment has been linked with decreased population-level fertility rates. Lack of green space may result in fewer areas to reduce stress.
A study coauthor reported grants from the National Institutes of Health during the conduct of the study; nonfinancial support from Swiss Precision Diagnostics GmbH, Labcorp, Kindara.com, and FertilityFriend.com; and consulting for AbbVie outside the submitted work. No other author disclosures were reported. Dr. Hornstein reported no relevant financial relationships.
A new study ties the odds of conception to the advantages of the neighborhood a woman lives in.
In a cohort of more than 6,000 women who were trying to get pregnant without fertility treatments, the probability of conception was reduced 21%-23% per menstrual cycle when comparing the most disadvantaged neighborhoods with the least disadvantaged.
“When disadvantaged neighborhood status was categorized within each state (as opposed to nationally), the results were slightly larger in magnitude,” wrote authors of the study published online in JAMA Network Open.
Among 6,356 participants, 3,725 pregnancies were observed for 27,427 menstrual cycles of follow-up. Average age was 30, and most participants were non-Hispanic White (5,297 [83.3%]) and had not previously given birth (4,179 [65.7%]).
When the researchers compared the top and bottom deciles of disadvantaged neighborhood status, adjusted fecundability ratios (the per-cycle probability of conception) were 0.79 (95% confidence interval [CI], 0.66-0.96) for national-level area deprivation index (ADI) rankings and 0.77 (95% CI, 0.65-0.92) for within-state ADI rankings. ADI score includes population indicators related to educational attainment, housing, employment, and poverty.
“These findings suggest that investments in disadvantaged neighborhoods may yield positive cobenefits for fertility,” the authors wrote.
The researchers used the Pregnancy Study Online, for which baseline data were collected from women in the United States from June 19, 2013, through April 12, 2019.
In the United States, 10%-15% of reproductive-aged couples experience infertility, defined as the inability to conceive after a year of unprotected intercourse.
Reason behind the numbers unclear
Mark Hornstein, MD, director in the reproductive endocrinology division of Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, said in an interview that this study gives the “what” but the “why” is harder to pinpoint.
What is not known, he said, is what kind of access the women had to fertility counseling or treatment.
The association between fertility and neighborhood advantage status is very plausible given the well-established links between disadvantaged regions and poorer health outcomes, he said, adding that the authors make a good case for their conclusions in the paper.
The authors ruled out many potential confounders, such as age of the women, reproductive history, multivitamin use, education level, household income, and frequency of intercourse, and still there was a difference between disadvantaged and advantaged neighborhoods, he noted.
Dr. Hornstein said his own research team has found that lack of knowledge about insurance coverage regarding infertility services may keep women from seeking the services.
“One of the things I worry about it access,” he said. “[The study authors] didn’t really look at that. They just looked at what the chances were that they got pregnant. But they didn’t say how many of those women had a workup, an evaluation, for why they were having difficulty, if they were, or had treatment. So I don’t know if some or all or none of that difference that they saw from the highest neighborhood health score to the most disadvantaged – if that was from inherent problems in the area, access to the best health care, or some combination.”
Discussions have focused on changing personal behaviors
Discussions on improving fertility often center on changing personal behaviors, the authors noted. “However, structural, political, and environmental factors may also play a substantial role,” they wrote.
The findings are in line with previous research on the effect of stress on in vitro outcomes, they pointed out. “Perceived stress has been associated with poorer in vitro fertilization outcomes and reduced fecundability among couples attempting spontaneous conception,” the authors noted.
Studies also have shown that living in a disadvantaged neighborhood is linked with comorbidities during pregnancy, such as increased risks of gestational hypertension (risk ratio for lowest vs. highest quartile: 1.24 [95% CI, 1.14-1.35]) and poor gestational weight gain (relative risk for lowest vs. highest quartile: 1.1 [95% CI, 1.1-1.2]).
In addition, policies such as those that support civil rights, protect the environment, and invest in underresourced communities have been shown to improve health markers such as life expectancy.
Policy decisions can also perpetuate a cycle of stress, they wrote. Disadvantaged communities may have more air pollution, which has been shown to have negative effects on fertility. Unemployment has been linked with decreased population-level fertility rates. Lack of green space may result in fewer areas to reduce stress.
A study coauthor reported grants from the National Institutes of Health during the conduct of the study; nonfinancial support from Swiss Precision Diagnostics GmbH, Labcorp, Kindara.com, and FertilityFriend.com; and consulting for AbbVie outside the submitted work. No other author disclosures were reported. Dr. Hornstein reported no relevant financial relationships.
Typhoid fever bacteria becoming more resistant to antibiotics
Bacteria that cause typhoid fever are becoming increasingly resistant to common antibiotics worldwide, a new analysis indicates.
Resistant strains of Salmonella enterica serovar typhi – almost all originating in South Asia – have spread across borders nearly 200 times since 1990.
Until now, analysis has been limited by small samples. This genome analysis is the largest to date and included 3,489 newly sequenced isolates (collected between 2014 and 2019) from prospective surveillance studies in four of the countries with the highest typhoid burden: Bangladesh, Nepal, Pakistan, and India.
Findings of the study, led by Kesia Esther da Silva, PhD, with the division of infectious diseases and geographic medicine at Stanford (Calif.) University, were published online in The Lancet Microbe.
Global deaths: 100,000 annually
Typhoid fever remains a global public health threat, causing 11 million infections and more than 100,000 deaths each year. Most cases (70%) are in South Asia, but typhoid also has significant presence in sub-Saharan Africa, Southeast Asia, and Oceania.
The findings are further evidence of the need for a global response, the authors write.
Jason Andrews, MD, a coauthor and associate professor in the division of infectious diseases and geographic medicine at Stanford University, said in an interview that the research helps pinpoint where the highest burden is and where the biggest need is for the two highly effective typhoid vaccines.
“We’re seeing higher levels of resistance than we’ve ever seen before against our latest and greatest antibiotics,” he said.
He said so far, strategies for tackling typhoid have involved country-level decisions and local funding and that needs to be shifted to a global priority. “Given contemporary travel migration patterns, what we see is that when antimicrobial resistance develops in one country, it quickly spreads to other countries.”
Dr. Andrews said the United States sees about 300-500 typhoid cases a year. “About 80% of those cases involve people traveling from South Asia,” he said.
Infections also come from people from the United States visiting high-burden countries, especially to see family. Often they don’t perceive the risk and skip vaccination, he said. U.S. clinicians can help with educating patients traveling to typhoid-endemic regions on pretravel vaccination.
Physician awareness is also important when patients have recently returned from such regions. Data from this study show a need to carefully consider which antibiotics will be effective with the growing resistance.
Only one oral option left in Pakistan
“We are running low on treatment options for typhoid,” Dr. Andrews said. The resistance pattern in Pakistan, for example, has left only one oral option, azithromycin, and resistance is building to that.
Without that option, “we’ll have to hospitalize patients and give intravenous antibiotics,” he said. “That’s concerning.”
Moreover, some resistant strains from Pakistan have been turning up in the United States.
“There are actually some cases that have not been tracked at all to travelers going to Pakistan and are thought to be from local transmission in the United States,” he said.
Valida Bajrovic, MD, assistant professor of medicine in infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, said in an interview that, in addition to vaccinating travelers before they head to typhoid-endemic areas, physicians should educate patients on avoiding fecal transmission of typhoid with vigilant hand washing, drinking bottled water, and avoiding foods that may have been prepared in unsanitary conditions.
Dr. Bajrovic, who directs the antimicrobial stewardship efforts at the Mount Sinai Morningside and Mount Sinai West Hospitals, said stricter antimicrobial stewardship efforts are needed, particularly in Europe and South Asia, but also in the United States.
“Restriction of antibiotic use is the way to prevent antibiotic resistance,” she said, adding that such restrictions need to be part of a global effort.
Strains in the study were classified as multidrug resistant (MDR) if they contained genes resistant to ampicillin, chloramphenicol, and trimethoprim/sulfamethoxazole. The authors also traced the presence of genes demonstrating resistance to macrolides and quinolones.
At first, fluoroquinolones were effective against MDR S. typhi and in the 1990s became the primary therapy. By the 2010s, however, the majority of S. typhi in south Asia contained mutations in the quinolone resistance-determining regions.
The authors wrote: “We found evidence of frequent international (n = 138) and intercontinental transfers (n = 59) of antimicrobial-resistant S. typhi.”
According to the analysis, since 2000, MDR S. typhi has declined steadily in Bangladesh and India and remained at less than 5% of typhoid strains in Nepal, though it has increased slightly in Pakistan.
However, these are being replaced “with strains containing ceftriaxone resistance (extensively drug resistant), high-level fluoroquinolone resistance, or azithromycin resistance, which are reversing declines in the effective population size of S. typhi,” the authors wrote.
The analysis supports urgency for prevention measures, including use of typhoid conjugate vaccines in typhoid-endemic countries, the authors said.
But given the rise in international spread of increasingly resistant strains, they said, preventive measures should not be limited to those countries.
The study was funded by the Bill & Melinda Gates Foundation. Dr. Da Silva, Dr. Andrews, and Dr. Bajrovic have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bacteria that cause typhoid fever are becoming increasingly resistant to common antibiotics worldwide, a new analysis indicates.
Resistant strains of Salmonella enterica serovar typhi – almost all originating in South Asia – have spread across borders nearly 200 times since 1990.
Until now, analysis has been limited by small samples. This genome analysis is the largest to date and included 3,489 newly sequenced isolates (collected between 2014 and 2019) from prospective surveillance studies in four of the countries with the highest typhoid burden: Bangladesh, Nepal, Pakistan, and India.
Findings of the study, led by Kesia Esther da Silva, PhD, with the division of infectious diseases and geographic medicine at Stanford (Calif.) University, were published online in The Lancet Microbe.
Global deaths: 100,000 annually
Typhoid fever remains a global public health threat, causing 11 million infections and more than 100,000 deaths each year. Most cases (70%) are in South Asia, but typhoid also has significant presence in sub-Saharan Africa, Southeast Asia, and Oceania.
The findings are further evidence of the need for a global response, the authors write.
Jason Andrews, MD, a coauthor and associate professor in the division of infectious diseases and geographic medicine at Stanford University, said in an interview that the research helps pinpoint where the highest burden is and where the biggest need is for the two highly effective typhoid vaccines.
“We’re seeing higher levels of resistance than we’ve ever seen before against our latest and greatest antibiotics,” he said.
He said so far, strategies for tackling typhoid have involved country-level decisions and local funding and that needs to be shifted to a global priority. “Given contemporary travel migration patterns, what we see is that when antimicrobial resistance develops in one country, it quickly spreads to other countries.”
Dr. Andrews said the United States sees about 300-500 typhoid cases a year. “About 80% of those cases involve people traveling from South Asia,” he said.
Infections also come from people from the United States visiting high-burden countries, especially to see family. Often they don’t perceive the risk and skip vaccination, he said. U.S. clinicians can help with educating patients traveling to typhoid-endemic regions on pretravel vaccination.
Physician awareness is also important when patients have recently returned from such regions. Data from this study show a need to carefully consider which antibiotics will be effective with the growing resistance.
Only one oral option left in Pakistan
“We are running low on treatment options for typhoid,” Dr. Andrews said. The resistance pattern in Pakistan, for example, has left only one oral option, azithromycin, and resistance is building to that.
Without that option, “we’ll have to hospitalize patients and give intravenous antibiotics,” he said. “That’s concerning.”
Moreover, some resistant strains from Pakistan have been turning up in the United States.
“There are actually some cases that have not been tracked at all to travelers going to Pakistan and are thought to be from local transmission in the United States,” he said.
Valida Bajrovic, MD, assistant professor of medicine in infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, said in an interview that, in addition to vaccinating travelers before they head to typhoid-endemic areas, physicians should educate patients on avoiding fecal transmission of typhoid with vigilant hand washing, drinking bottled water, and avoiding foods that may have been prepared in unsanitary conditions.
Dr. Bajrovic, who directs the antimicrobial stewardship efforts at the Mount Sinai Morningside and Mount Sinai West Hospitals, said stricter antimicrobial stewardship efforts are needed, particularly in Europe and South Asia, but also in the United States.
“Restriction of antibiotic use is the way to prevent antibiotic resistance,” she said, adding that such restrictions need to be part of a global effort.
Strains in the study were classified as multidrug resistant (MDR) if they contained genes resistant to ampicillin, chloramphenicol, and trimethoprim/sulfamethoxazole. The authors also traced the presence of genes demonstrating resistance to macrolides and quinolones.
At first, fluoroquinolones were effective against MDR S. typhi and in the 1990s became the primary therapy. By the 2010s, however, the majority of S. typhi in south Asia contained mutations in the quinolone resistance-determining regions.
The authors wrote: “We found evidence of frequent international (n = 138) and intercontinental transfers (n = 59) of antimicrobial-resistant S. typhi.”
According to the analysis, since 2000, MDR S. typhi has declined steadily in Bangladesh and India and remained at less than 5% of typhoid strains in Nepal, though it has increased slightly in Pakistan.
However, these are being replaced “with strains containing ceftriaxone resistance (extensively drug resistant), high-level fluoroquinolone resistance, or azithromycin resistance, which are reversing declines in the effective population size of S. typhi,” the authors wrote.
The analysis supports urgency for prevention measures, including use of typhoid conjugate vaccines in typhoid-endemic countries, the authors said.
But given the rise in international spread of increasingly resistant strains, they said, preventive measures should not be limited to those countries.
The study was funded by the Bill & Melinda Gates Foundation. Dr. Da Silva, Dr. Andrews, and Dr. Bajrovic have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bacteria that cause typhoid fever are becoming increasingly resistant to common antibiotics worldwide, a new analysis indicates.
Resistant strains of Salmonella enterica serovar typhi – almost all originating in South Asia – have spread across borders nearly 200 times since 1990.
Until now, analysis has been limited by small samples. This genome analysis is the largest to date and included 3,489 newly sequenced isolates (collected between 2014 and 2019) from prospective surveillance studies in four of the countries with the highest typhoid burden: Bangladesh, Nepal, Pakistan, and India.
Findings of the study, led by Kesia Esther da Silva, PhD, with the division of infectious diseases and geographic medicine at Stanford (Calif.) University, were published online in The Lancet Microbe.
Global deaths: 100,000 annually
Typhoid fever remains a global public health threat, causing 11 million infections and more than 100,000 deaths each year. Most cases (70%) are in South Asia, but typhoid also has significant presence in sub-Saharan Africa, Southeast Asia, and Oceania.
The findings are further evidence of the need for a global response, the authors write.
Jason Andrews, MD, a coauthor and associate professor in the division of infectious diseases and geographic medicine at Stanford University, said in an interview that the research helps pinpoint where the highest burden is and where the biggest need is for the two highly effective typhoid vaccines.
“We’re seeing higher levels of resistance than we’ve ever seen before against our latest and greatest antibiotics,” he said.
He said so far, strategies for tackling typhoid have involved country-level decisions and local funding and that needs to be shifted to a global priority. “Given contemporary travel migration patterns, what we see is that when antimicrobial resistance develops in one country, it quickly spreads to other countries.”
Dr. Andrews said the United States sees about 300-500 typhoid cases a year. “About 80% of those cases involve people traveling from South Asia,” he said.
Infections also come from people from the United States visiting high-burden countries, especially to see family. Often they don’t perceive the risk and skip vaccination, he said. U.S. clinicians can help with educating patients traveling to typhoid-endemic regions on pretravel vaccination.
Physician awareness is also important when patients have recently returned from such regions. Data from this study show a need to carefully consider which antibiotics will be effective with the growing resistance.
Only one oral option left in Pakistan
“We are running low on treatment options for typhoid,” Dr. Andrews said. The resistance pattern in Pakistan, for example, has left only one oral option, azithromycin, and resistance is building to that.
Without that option, “we’ll have to hospitalize patients and give intravenous antibiotics,” he said. “That’s concerning.”
Moreover, some resistant strains from Pakistan have been turning up in the United States.
“There are actually some cases that have not been tracked at all to travelers going to Pakistan and are thought to be from local transmission in the United States,” he said.
Valida Bajrovic, MD, assistant professor of medicine in infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, said in an interview that, in addition to vaccinating travelers before they head to typhoid-endemic areas, physicians should educate patients on avoiding fecal transmission of typhoid with vigilant hand washing, drinking bottled water, and avoiding foods that may have been prepared in unsanitary conditions.
Dr. Bajrovic, who directs the antimicrobial stewardship efforts at the Mount Sinai Morningside and Mount Sinai West Hospitals, said stricter antimicrobial stewardship efforts are needed, particularly in Europe and South Asia, but also in the United States.
“Restriction of antibiotic use is the way to prevent antibiotic resistance,” she said, adding that such restrictions need to be part of a global effort.
Strains in the study were classified as multidrug resistant (MDR) if they contained genes resistant to ampicillin, chloramphenicol, and trimethoprim/sulfamethoxazole. The authors also traced the presence of genes demonstrating resistance to macrolides and quinolones.
At first, fluoroquinolones were effective against MDR S. typhi and in the 1990s became the primary therapy. By the 2010s, however, the majority of S. typhi in south Asia contained mutations in the quinolone resistance-determining regions.
The authors wrote: “We found evidence of frequent international (n = 138) and intercontinental transfers (n = 59) of antimicrobial-resistant S. typhi.”
According to the analysis, since 2000, MDR S. typhi has declined steadily in Bangladesh and India and remained at less than 5% of typhoid strains in Nepal, though it has increased slightly in Pakistan.
However, these are being replaced “with strains containing ceftriaxone resistance (extensively drug resistant), high-level fluoroquinolone resistance, or azithromycin resistance, which are reversing declines in the effective population size of S. typhi,” the authors wrote.
The analysis supports urgency for prevention measures, including use of typhoid conjugate vaccines in typhoid-endemic countries, the authors said.
But given the rise in international spread of increasingly resistant strains, they said, preventive measures should not be limited to those countries.
The study was funded by the Bill & Melinda Gates Foundation. Dr. Da Silva, Dr. Andrews, and Dr. Bajrovic have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alcohol consumption habits can predict gout tophi
The more years a person drinks alcohol, the kind of alcohol consumed, and the amount consumed can help to predict gout tophi, researchers say in a newly published paper in Arthritis Care and Research.
The study, led by Lin Han, PhD, of the gout laboratory, Shandong provincial clinical research center for immune diseases and gout, Affiliated Hospital of Qingdao (China) University, helps clarify the already-established relationship between alcohol consumption and gout tophi.
Additionally, the effects of drinking alcohol on ultrasound (US)–detected tophi and subcutaneous tophi (subtophi) were evaluated separately for the first time in this work, the authors say.
Tophi may be underdiagnosed because they are hard to find with only a physical exam. US can help with early detection, especially with small clusters of crystals or those found deep in the tissues, and offers good diagnostic accuracy with high specificity.
“Unlike subtophi, which represent long-term subcutaneous MSU [monosodium urate] deposition over many years, US-detected tophi represent the early stage of tophi in both intra- and extra-articular settings,” the authors write.
This cross-sectional study in China included 554 patients with gout who had joint ultrasound and physical exams through the Affiliated Hospital of Qingdao University. Physicians gathered medical histories using the Biobank Information Management System.
Physicians also tracked alcohol consumption patterns through the biobank information, which included answers to a detailed drinking questionnaire.
Patients were classified as either nondrinkers (no history of drinking; n = 141), former drinkers (n = 60), or current regular drinkers (n = 353). Current regular drinkers were asked further questions about their drinking patterns, including how long they have been drinking, type of alcohol they drink, and how much and how often they drink. In China, the average drink is considered to contain 10 g of alcohol, according to the World Health Organization.
Results from US and clinically detected tophi
Compared with nondrinkers, excessive drinkers (more than 70 g/week); long-term drinkers (at least 10 years), and spirits drinkers had a greater proportion, size, and number of US-detected tophi and subtophi (all P < .05).
After adjusting for confounders, the researchers found that excessive drinking was significantly associated with having US-detected tophi (odds ratio, 1.79) and subtophi (OR, 2.00). Similar associations were found for consumption of alcohol for at least 10 years (OR, 1.96 for US-detected tophi; OR, 2.17 for sub-tophi) and drinking spirits (OR, 1.81 for US-detected tophi; OR, 2.10 for subtophi). All comparisons were P < .05.
Among patients who already have US-detected tophi or subtophi, moderate drinking (70 g/week or less) was linked with larger or multiple tophi (all P < .05).
Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham, said in an interview that the results are likely generalizable.
“I wouldn’t expect them to be specific to the Chinese population,” he said.
Most of the 554 patients were male (97.8%) and had no family history of gout (79.8%). The median duration of gout was 4 years, and the average age was 45.1 years.
Dr. Gaffo noted the population age was fairly young and the average duration of gout in these patients was fairly short. He also noted most had small tophi that were detected only by ultrasound and small numbers of tophi overall.
“I would like to see how these results will replicate in a population that has had gout for, say, 10 years on average,” he said.
Dr. Gaffo says he explores alcohol history with his patients with gout. If they are frequent drinkers, he encourages them to cut back.
“At the very least,” he said, “you have to restrict your intake to no more than 1-2 servings per week,” he said. “For some patients, even minimal amounts of alcohol intake can be associated with the development of flares.”
Still, research like this, he says, can help physicians point to evidence in their advice to patients about alcohol use.
He noted that the authors found the association between different types of alcohol and tophi was independent of serum urate level.
“That surprised me,” Dr. Gaffo said. “That’s a very unique finding.”
This work was supported by grants from the National Natural Science Foundation of China, the Natural Science Foundation of Shandong Province, Qingdao applied basic research project, National College Students’ Innovation and Entrepreneurship Training Program, and Shandong Provincial Science Foundation for Outstanding Youth Scholars.
The authors of the study and Dr. Gaffo report no relevant financial relationships.
The more years a person drinks alcohol, the kind of alcohol consumed, and the amount consumed can help to predict gout tophi, researchers say in a newly published paper in Arthritis Care and Research.
The study, led by Lin Han, PhD, of the gout laboratory, Shandong provincial clinical research center for immune diseases and gout, Affiliated Hospital of Qingdao (China) University, helps clarify the already-established relationship between alcohol consumption and gout tophi.
Additionally, the effects of drinking alcohol on ultrasound (US)–detected tophi and subcutaneous tophi (subtophi) were evaluated separately for the first time in this work, the authors say.
Tophi may be underdiagnosed because they are hard to find with only a physical exam. US can help with early detection, especially with small clusters of crystals or those found deep in the tissues, and offers good diagnostic accuracy with high specificity.
“Unlike subtophi, which represent long-term subcutaneous MSU [monosodium urate] deposition over many years, US-detected tophi represent the early stage of tophi in both intra- and extra-articular settings,” the authors write.
This cross-sectional study in China included 554 patients with gout who had joint ultrasound and physical exams through the Affiliated Hospital of Qingdao University. Physicians gathered medical histories using the Biobank Information Management System.
Physicians also tracked alcohol consumption patterns through the biobank information, which included answers to a detailed drinking questionnaire.
Patients were classified as either nondrinkers (no history of drinking; n = 141), former drinkers (n = 60), or current regular drinkers (n = 353). Current regular drinkers were asked further questions about their drinking patterns, including how long they have been drinking, type of alcohol they drink, and how much and how often they drink. In China, the average drink is considered to contain 10 g of alcohol, according to the World Health Organization.
Results from US and clinically detected tophi
Compared with nondrinkers, excessive drinkers (more than 70 g/week); long-term drinkers (at least 10 years), and spirits drinkers had a greater proportion, size, and number of US-detected tophi and subtophi (all P < .05).
After adjusting for confounders, the researchers found that excessive drinking was significantly associated with having US-detected tophi (odds ratio, 1.79) and subtophi (OR, 2.00). Similar associations were found for consumption of alcohol for at least 10 years (OR, 1.96 for US-detected tophi; OR, 2.17 for sub-tophi) and drinking spirits (OR, 1.81 for US-detected tophi; OR, 2.10 for subtophi). All comparisons were P < .05.
Among patients who already have US-detected tophi or subtophi, moderate drinking (70 g/week or less) was linked with larger or multiple tophi (all P < .05).
Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham, said in an interview that the results are likely generalizable.
“I wouldn’t expect them to be specific to the Chinese population,” he said.
Most of the 554 patients were male (97.8%) and had no family history of gout (79.8%). The median duration of gout was 4 years, and the average age was 45.1 years.
Dr. Gaffo noted the population age was fairly young and the average duration of gout in these patients was fairly short. He also noted most had small tophi that were detected only by ultrasound and small numbers of tophi overall.
“I would like to see how these results will replicate in a population that has had gout for, say, 10 years on average,” he said.
Dr. Gaffo says he explores alcohol history with his patients with gout. If they are frequent drinkers, he encourages them to cut back.
“At the very least,” he said, “you have to restrict your intake to no more than 1-2 servings per week,” he said. “For some patients, even minimal amounts of alcohol intake can be associated with the development of flares.”
Still, research like this, he says, can help physicians point to evidence in their advice to patients about alcohol use.
He noted that the authors found the association between different types of alcohol and tophi was independent of serum urate level.
“That surprised me,” Dr. Gaffo said. “That’s a very unique finding.”
This work was supported by grants from the National Natural Science Foundation of China, the Natural Science Foundation of Shandong Province, Qingdao applied basic research project, National College Students’ Innovation and Entrepreneurship Training Program, and Shandong Provincial Science Foundation for Outstanding Youth Scholars.
The authors of the study and Dr. Gaffo report no relevant financial relationships.
The more years a person drinks alcohol, the kind of alcohol consumed, and the amount consumed can help to predict gout tophi, researchers say in a newly published paper in Arthritis Care and Research.
The study, led by Lin Han, PhD, of the gout laboratory, Shandong provincial clinical research center for immune diseases and gout, Affiliated Hospital of Qingdao (China) University, helps clarify the already-established relationship between alcohol consumption and gout tophi.
Additionally, the effects of drinking alcohol on ultrasound (US)–detected tophi and subcutaneous tophi (subtophi) were evaluated separately for the first time in this work, the authors say.
Tophi may be underdiagnosed because they are hard to find with only a physical exam. US can help with early detection, especially with small clusters of crystals or those found deep in the tissues, and offers good diagnostic accuracy with high specificity.
“Unlike subtophi, which represent long-term subcutaneous MSU [monosodium urate] deposition over many years, US-detected tophi represent the early stage of tophi in both intra- and extra-articular settings,” the authors write.
This cross-sectional study in China included 554 patients with gout who had joint ultrasound and physical exams through the Affiliated Hospital of Qingdao University. Physicians gathered medical histories using the Biobank Information Management System.
Physicians also tracked alcohol consumption patterns through the biobank information, which included answers to a detailed drinking questionnaire.
Patients were classified as either nondrinkers (no history of drinking; n = 141), former drinkers (n = 60), or current regular drinkers (n = 353). Current regular drinkers were asked further questions about their drinking patterns, including how long they have been drinking, type of alcohol they drink, and how much and how often they drink. In China, the average drink is considered to contain 10 g of alcohol, according to the World Health Organization.
Results from US and clinically detected tophi
Compared with nondrinkers, excessive drinkers (more than 70 g/week); long-term drinkers (at least 10 years), and spirits drinkers had a greater proportion, size, and number of US-detected tophi and subtophi (all P < .05).
After adjusting for confounders, the researchers found that excessive drinking was significantly associated with having US-detected tophi (odds ratio, 1.79) and subtophi (OR, 2.00). Similar associations were found for consumption of alcohol for at least 10 years (OR, 1.96 for US-detected tophi; OR, 2.17 for sub-tophi) and drinking spirits (OR, 1.81 for US-detected tophi; OR, 2.10 for subtophi). All comparisons were P < .05.
Among patients who already have US-detected tophi or subtophi, moderate drinking (70 g/week or less) was linked with larger or multiple tophi (all P < .05).
Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham, said in an interview that the results are likely generalizable.
“I wouldn’t expect them to be specific to the Chinese population,” he said.
Most of the 554 patients were male (97.8%) and had no family history of gout (79.8%). The median duration of gout was 4 years, and the average age was 45.1 years.
Dr. Gaffo noted the population age was fairly young and the average duration of gout in these patients was fairly short. He also noted most had small tophi that were detected only by ultrasound and small numbers of tophi overall.
“I would like to see how these results will replicate in a population that has had gout for, say, 10 years on average,” he said.
Dr. Gaffo says he explores alcohol history with his patients with gout. If they are frequent drinkers, he encourages them to cut back.
“At the very least,” he said, “you have to restrict your intake to no more than 1-2 servings per week,” he said. “For some patients, even minimal amounts of alcohol intake can be associated with the development of flares.”
Still, research like this, he says, can help physicians point to evidence in their advice to patients about alcohol use.
He noted that the authors found the association between different types of alcohol and tophi was independent of serum urate level.
“That surprised me,” Dr. Gaffo said. “That’s a very unique finding.”
This work was supported by grants from the National Natural Science Foundation of China, the Natural Science Foundation of Shandong Province, Qingdao applied basic research project, National College Students’ Innovation and Entrepreneurship Training Program, and Shandong Provincial Science Foundation for Outstanding Youth Scholars.
The authors of the study and Dr. Gaffo report no relevant financial relationships.
FROM ARTHRITIS CARE AND RESEARCH
Common endoscopic procedure needs quality improvement
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
FROM GASTROINTESTINAL ENDOSCOPY
Why do we treat menopause as a disease?
Menopause gets a bad rap in medical literature and throughout society, say authors of a new analysis. And they argue that the negativity undermines women’s health outlook in the years that should be a natural life transition.
Menopause has been medicalized over centuries and talked about as if it were a disease, they say, and that may increase women’s anxiety and apprehension about the midlife stage.
It’s time to change the narrative, says Martha Hickey, MD, with the department of obstetrics and gynaecology at the Royal Women’s Hospital in Victoria, Australia, and her coauthors. Their analysis was published online in the BMJ.
“The message that menopause signals decay and decline, which can potentially be delayed or reversed by hormonal treatments, persists and is reinforced by the media, medical literature, and information for women, often driven by marketing interests,” they write.
Such messages may chip away at women’s confidence. Dr. Hickey and colleagues cite surveys in the United States and Ireland that found that most women (65%-77%) feel unprepared for menopause.
“Together with limited public discussion and education and shame attached to ageing in women, this may contribute to embarrassment and negative expectations about menopause,” the authors write.
The ‘untold misery of oestrogen-starved women’
These messages have deep roots. Take for instance, gynecologist Robert Wilson’s words in his 1966 book “Feminine Forever.” The authors note he recommended estrogen for all menopausal women “to treat their ‘serious, painful and often crippling disease’ and avoid the ‘untold misery of alcoholism, drug addiction, divorce, and broken
homes caused by these unstable, oestrogen-starved women.’ ”
Women experience menopause in very different ways. Experience with menopause also differs by country, the authors explain. “Women’s experience of menopause is also strongly influenced by social values around reproduction and ageing, with positive or negative ramifications,” they write.
“For example, women tend to have worse experiences of menopause in countries where their value is predicated on youth and reproductive capacity and ageing is associated with decline.”
The authors argue that the medicalization of menopause has condensed the wide range of women’s experiences at a typical age into “a narrowly defined disease requiring treatment.”
Promoting exercise, stopping smoking among positive messages
An editorial by Haitham Hamoda, MD, and Sara Moger, with the British Menopause Society, notes that more than 75% of women experiencing menopause report symptoms, and more than 25% describe severe symptoms.
The editorialists point out that the National Institute of Health and Care Excellence and others recommend an individualized approach to addressing menopause that includes a comprehensive approach – advice on exercise, weight management, stopping smoking, and reducing alcohol as well as options such as hormone therapy (HT).
The literature says the main indication for HT is for severe symptoms and not as a preventive measure. “Evidence does not support use of HT to reduce the risk of dementia,” they point out.
While some women may benefit from HT, that should not be explored to the exclusion of other avenues of help, Dr. Hickey and colleagues write. Risks must also be considered.
Menopause blamed in a difficult time of life
Jennifer Howell, MD, an obstetrician/gynecologist and certified menopause provider at Duke University in Durham, N.C., told this news organization that menopause is often blamed in a time of life when women naturally are experiencing an array of stressful and emotional changes.
It often coincides with children heading to college, navigating midlife challenges in marriage, helping aging parents, managing demanding careers, and health issues.
People want a reason for changes women experience, and too often the finger gets pointed at menopause, Dr. Howell said.
The message women hear has always been, “It’s got to be your hormones. And people want to hear that there’s a hormonal solution.”
Making menopause the target also has led to nonevidence-based “snake-oil” type remedies sold in unregulated powders, creams, and pellets, Dr. Howell noted.
Dr. Howell has treated thousands of menopausal women in her clinic and she says she spends a good deal of time with them explaining a holistic view of the process, much like what the authors describe, with lifestyle changes and treatment options.
Sometimes HT is the solution, Dr. Howell says, but “it’s become a crutch. Hormones are not a panacea.”
She is frustrated with the amount of disinformation circulating online. Groups like the North American Menopause Society put out reliable evidence-based information, but they compete “with a lot of nonsense,” she says.
The message that women should hear, she says is that “[menopause] is a natural part of aging and there may or may not be symptoms that come along with it. If there are, there are things we can do,” she says.
Menopause gets a bad rap in medical literature and throughout society, say authors of a new analysis. And they argue that the negativity undermines women’s health outlook in the years that should be a natural life transition.
Menopause has been medicalized over centuries and talked about as if it were a disease, they say, and that may increase women’s anxiety and apprehension about the midlife stage.
It’s time to change the narrative, says Martha Hickey, MD, with the department of obstetrics and gynaecology at the Royal Women’s Hospital in Victoria, Australia, and her coauthors. Their analysis was published online in the BMJ.
“The message that menopause signals decay and decline, which can potentially be delayed or reversed by hormonal treatments, persists and is reinforced by the media, medical literature, and information for women, often driven by marketing interests,” they write.
Such messages may chip away at women’s confidence. Dr. Hickey and colleagues cite surveys in the United States and Ireland that found that most women (65%-77%) feel unprepared for menopause.
“Together with limited public discussion and education and shame attached to ageing in women, this may contribute to embarrassment and negative expectations about menopause,” the authors write.
The ‘untold misery of oestrogen-starved women’
These messages have deep roots. Take for instance, gynecologist Robert Wilson’s words in his 1966 book “Feminine Forever.” The authors note he recommended estrogen for all menopausal women “to treat their ‘serious, painful and often crippling disease’ and avoid the ‘untold misery of alcoholism, drug addiction, divorce, and broken
homes caused by these unstable, oestrogen-starved women.’ ”
Women experience menopause in very different ways. Experience with menopause also differs by country, the authors explain. “Women’s experience of menopause is also strongly influenced by social values around reproduction and ageing, with positive or negative ramifications,” they write.
“For example, women tend to have worse experiences of menopause in countries where their value is predicated on youth and reproductive capacity and ageing is associated with decline.”
The authors argue that the medicalization of menopause has condensed the wide range of women’s experiences at a typical age into “a narrowly defined disease requiring treatment.”
Promoting exercise, stopping smoking among positive messages
An editorial by Haitham Hamoda, MD, and Sara Moger, with the British Menopause Society, notes that more than 75% of women experiencing menopause report symptoms, and more than 25% describe severe symptoms.
The editorialists point out that the National Institute of Health and Care Excellence and others recommend an individualized approach to addressing menopause that includes a comprehensive approach – advice on exercise, weight management, stopping smoking, and reducing alcohol as well as options such as hormone therapy (HT).
The literature says the main indication for HT is for severe symptoms and not as a preventive measure. “Evidence does not support use of HT to reduce the risk of dementia,” they point out.
While some women may benefit from HT, that should not be explored to the exclusion of other avenues of help, Dr. Hickey and colleagues write. Risks must also be considered.
Menopause blamed in a difficult time of life
Jennifer Howell, MD, an obstetrician/gynecologist and certified menopause provider at Duke University in Durham, N.C., told this news organization that menopause is often blamed in a time of life when women naturally are experiencing an array of stressful and emotional changes.
It often coincides with children heading to college, navigating midlife challenges in marriage, helping aging parents, managing demanding careers, and health issues.
People want a reason for changes women experience, and too often the finger gets pointed at menopause, Dr. Howell said.
The message women hear has always been, “It’s got to be your hormones. And people want to hear that there’s a hormonal solution.”
Making menopause the target also has led to nonevidence-based “snake-oil” type remedies sold in unregulated powders, creams, and pellets, Dr. Howell noted.
Dr. Howell has treated thousands of menopausal women in her clinic and she says she spends a good deal of time with them explaining a holistic view of the process, much like what the authors describe, with lifestyle changes and treatment options.
Sometimes HT is the solution, Dr. Howell says, but “it’s become a crutch. Hormones are not a panacea.”
She is frustrated with the amount of disinformation circulating online. Groups like the North American Menopause Society put out reliable evidence-based information, but they compete “with a lot of nonsense,” she says.
The message that women should hear, she says is that “[menopause] is a natural part of aging and there may or may not be symptoms that come along with it. If there are, there are things we can do,” she says.
Menopause gets a bad rap in medical literature and throughout society, say authors of a new analysis. And they argue that the negativity undermines women’s health outlook in the years that should be a natural life transition.
Menopause has been medicalized over centuries and talked about as if it were a disease, they say, and that may increase women’s anxiety and apprehension about the midlife stage.
It’s time to change the narrative, says Martha Hickey, MD, with the department of obstetrics and gynaecology at the Royal Women’s Hospital in Victoria, Australia, and her coauthors. Their analysis was published online in the BMJ.
“The message that menopause signals decay and decline, which can potentially be delayed or reversed by hormonal treatments, persists and is reinforced by the media, medical literature, and information for women, often driven by marketing interests,” they write.
Such messages may chip away at women’s confidence. Dr. Hickey and colleagues cite surveys in the United States and Ireland that found that most women (65%-77%) feel unprepared for menopause.
“Together with limited public discussion and education and shame attached to ageing in women, this may contribute to embarrassment and negative expectations about menopause,” the authors write.
The ‘untold misery of oestrogen-starved women’
These messages have deep roots. Take for instance, gynecologist Robert Wilson’s words in his 1966 book “Feminine Forever.” The authors note he recommended estrogen for all menopausal women “to treat their ‘serious, painful and often crippling disease’ and avoid the ‘untold misery of alcoholism, drug addiction, divorce, and broken
homes caused by these unstable, oestrogen-starved women.’ ”
Women experience menopause in very different ways. Experience with menopause also differs by country, the authors explain. “Women’s experience of menopause is also strongly influenced by social values around reproduction and ageing, with positive or negative ramifications,” they write.
“For example, women tend to have worse experiences of menopause in countries where their value is predicated on youth and reproductive capacity and ageing is associated with decline.”
The authors argue that the medicalization of menopause has condensed the wide range of women’s experiences at a typical age into “a narrowly defined disease requiring treatment.”
Promoting exercise, stopping smoking among positive messages
An editorial by Haitham Hamoda, MD, and Sara Moger, with the British Menopause Society, notes that more than 75% of women experiencing menopause report symptoms, and more than 25% describe severe symptoms.
The editorialists point out that the National Institute of Health and Care Excellence and others recommend an individualized approach to addressing menopause that includes a comprehensive approach – advice on exercise, weight management, stopping smoking, and reducing alcohol as well as options such as hormone therapy (HT).
The literature says the main indication for HT is for severe symptoms and not as a preventive measure. “Evidence does not support use of HT to reduce the risk of dementia,” they point out.
While some women may benefit from HT, that should not be explored to the exclusion of other avenues of help, Dr. Hickey and colleagues write. Risks must also be considered.
Menopause blamed in a difficult time of life
Jennifer Howell, MD, an obstetrician/gynecologist and certified menopause provider at Duke University in Durham, N.C., told this news organization that menopause is often blamed in a time of life when women naturally are experiencing an array of stressful and emotional changes.
It often coincides with children heading to college, navigating midlife challenges in marriage, helping aging parents, managing demanding careers, and health issues.
People want a reason for changes women experience, and too often the finger gets pointed at menopause, Dr. Howell said.
The message women hear has always been, “It’s got to be your hormones. And people want to hear that there’s a hormonal solution.”
Making menopause the target also has led to nonevidence-based “snake-oil” type remedies sold in unregulated powders, creams, and pellets, Dr. Howell noted.
Dr. Howell has treated thousands of menopausal women in her clinic and she says she spends a good deal of time with them explaining a holistic view of the process, much like what the authors describe, with lifestyle changes and treatment options.
Sometimes HT is the solution, Dr. Howell says, but “it’s become a crutch. Hormones are not a panacea.”
She is frustrated with the amount of disinformation circulating online. Groups like the North American Menopause Society put out reliable evidence-based information, but they compete “with a lot of nonsense,” she says.
The message that women should hear, she says is that “[menopause] is a natural part of aging and there may or may not be symptoms that come along with it. If there are, there are things we can do,” she says.
FROM BMJ
FDA OKs first systemic treatment for alopecia areata
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
Double morning-after pill dose for women with obesity not effective
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
FROM OBSTETRICS & GYNECOLOGY
People with HIV may need an additional COVID vaccine dose
People with HIV have an increased risk of breakthrough SARS-CoV-2 infections, a new study finds, and the authors say an additional primary vaccine dose should be considered for all who are living with the disease.
Currently, an additional primary dose administered 28 days after a second dose of the mRNA (Moderna or Pfizer) vaccines or after the first dose of the Johnson & Johnson (J&J) vaccine is recommended only for those with advanced or untreated HIV.
The Centers for Disease Control and Prevention recommends boosters for all adults with or without HIV.
Sally B. Coburn, PhD, of the department of epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, led the study, which was published online in JAMA Network Open. In their study, the researchers estimate the risk of breakthrough infections among fully vaccinated adults on the basis of HIV status in the United States.
Adults with HIV who were fully vaccinated before June 30, 2021, were matched with adults without HIV with regard to date of full vaccination, age, race/ethnicity, and sex. All were followed through Dec. 31, 2021.
Patients were considered fully vaccinated either 14 days after the second dose of the Pfizer or Moderna shots or 14 days after the single dose of the J&J shot.
Breakthrough risk 28% higher
In the study of 113,994 patients, researchers found that risk of breakthrough SARS-CoV-2 infection was low overall (3.8%) but was 28% higher among people with HIV in comparison with people without HIV (adjusted hazard ratio, 1.28; 95% confidence interval, 1.19-1.37).
The breakthrough rate was also higher in the HIV group (55 cases per 1,000 person-years, vs. 43 cases per 1,000 person-years in people without HIV).
Patients were drawn from the Corona-Infectious-Virus Epidemiology Team (CIVET)–II of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), which is part of the International Epidemiology Databases to Evaluate AIDS (IeDEA) collaboration involving four cohorts.
Among people with HIV, those younger than 45 years (vs. those aged 45-54) and those with a history of COVID-19 were more likely to experience breakthrough infections. Those who did not get any additional shots after the primary vaccination were more likely to have breakthrough infections, amplifying the need to get boosters, the authors wrote.
There was no link between breakthrough infections and HIV viral load suppression, but high CD4 counts (> 500 cells/mm3) were associated with fewer breakthrough cases among people with HIV, they noted.
Monica Gandhi, MD, professor of medicine and associate division chief of HIV, infectious diseases, and global medicine at the University of California, San Francisco, praised the study, noting that until now, large studies have not examined the rate of breakthrough infections among vaccinated people with HIV and people without HIV in the United States.
She told this news organization she agrees with the authors that a third dose for all who are living with HIV is needed because rates of breakthrough infections were high across all populations during the Omicron surge (which largely occurred after the period of this study).
She said she was not convinced the third shot was needed before Omicron, because breakthrough rates in both HIV and non-HIV groups were low.
“However, the most interesting part of this study for me was how well the vaccines worked in people with HIV with generally higher CD4 counts and virologic suppression, again telling us as HIV providers how well the HIV medicines work and how our patients with HIV have relatively normal immune systems if treated,” she said.
One limitation was that the study population was 92% male. Also, those without regular access to health care (who may be at greater risk for COVID-19) were less likely to be included in the study. People engaged in care may seek more frequent COVID-19 testing, which could lead to higher detection of breakthrough infections than in the general population.
“Future analyses should account for testing practices and include a larger proportion of women with HIV,” the authors wrote. “Ultimately, policy makers must determine the appropriate balance between preventing further COVID-19 infections and possibly unnecessary additional vaccinations.”
Coauthor Keri N. Althoff, PhD, told this news organization that there’s one unanswered question that would strengthen the call to action by the CDC: Do people with HIV have more severe postvaccination COVID-19 breakthrough illness?
“We have a second paper that is a preprint and currently under peer review,” she said. “In this paper, we found that people with HIV with a CD4 count less than 350 cells/mm3 were more likely to be hospitalized with postvaccination COVID-19 breakthrough illness compared to similar people without HIV. “
At a minimum, Dr. Althoff said, policymakers should consider including people with HIV with a CD4 less than 350 cells/mm3 (loosening the restriction to less than 200 cells/mm3) in their recommendations for people who are moderately or severely immunocompromised.
The research was funded with supplemental funds to the North American AIDS Cohort Collaboration on Research and Design. Dr. Coburn reports no relevant financial relationships. A coauthor has received grants from the Canadian Institutes of Health Research, Alberta Innovates, and University of Calgary/Alberta Health Services outside the submitted work. One coauthor reports serving as a consultant to Trio Health, Kennedy Dundas, and MedIQ outside the submitted work.
A version of this article first appeared on Medscape.com.
People with HIV have an increased risk of breakthrough SARS-CoV-2 infections, a new study finds, and the authors say an additional primary vaccine dose should be considered for all who are living with the disease.
Currently, an additional primary dose administered 28 days after a second dose of the mRNA (Moderna or Pfizer) vaccines or after the first dose of the Johnson & Johnson (J&J) vaccine is recommended only for those with advanced or untreated HIV.
The Centers for Disease Control and Prevention recommends boosters for all adults with or without HIV.
Sally B. Coburn, PhD, of the department of epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, led the study, which was published online in JAMA Network Open. In their study, the researchers estimate the risk of breakthrough infections among fully vaccinated adults on the basis of HIV status in the United States.
Adults with HIV who were fully vaccinated before June 30, 2021, were matched with adults without HIV with regard to date of full vaccination, age, race/ethnicity, and sex. All were followed through Dec. 31, 2021.
Patients were considered fully vaccinated either 14 days after the second dose of the Pfizer or Moderna shots or 14 days after the single dose of the J&J shot.
Breakthrough risk 28% higher
In the study of 113,994 patients, researchers found that risk of breakthrough SARS-CoV-2 infection was low overall (3.8%) but was 28% higher among people with HIV in comparison with people without HIV (adjusted hazard ratio, 1.28; 95% confidence interval, 1.19-1.37).
The breakthrough rate was also higher in the HIV group (55 cases per 1,000 person-years, vs. 43 cases per 1,000 person-years in people without HIV).
Patients were drawn from the Corona-Infectious-Virus Epidemiology Team (CIVET)–II of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), which is part of the International Epidemiology Databases to Evaluate AIDS (IeDEA) collaboration involving four cohorts.
Among people with HIV, those younger than 45 years (vs. those aged 45-54) and those with a history of COVID-19 were more likely to experience breakthrough infections. Those who did not get any additional shots after the primary vaccination were more likely to have breakthrough infections, amplifying the need to get boosters, the authors wrote.
There was no link between breakthrough infections and HIV viral load suppression, but high CD4 counts (> 500 cells/mm3) were associated with fewer breakthrough cases among people with HIV, they noted.
Monica Gandhi, MD, professor of medicine and associate division chief of HIV, infectious diseases, and global medicine at the University of California, San Francisco, praised the study, noting that until now, large studies have not examined the rate of breakthrough infections among vaccinated people with HIV and people without HIV in the United States.
She told this news organization she agrees with the authors that a third dose for all who are living with HIV is needed because rates of breakthrough infections were high across all populations during the Omicron surge (which largely occurred after the period of this study).
She said she was not convinced the third shot was needed before Omicron, because breakthrough rates in both HIV and non-HIV groups were low.
“However, the most interesting part of this study for me was how well the vaccines worked in people with HIV with generally higher CD4 counts and virologic suppression, again telling us as HIV providers how well the HIV medicines work and how our patients with HIV have relatively normal immune systems if treated,” she said.
One limitation was that the study population was 92% male. Also, those without regular access to health care (who may be at greater risk for COVID-19) were less likely to be included in the study. People engaged in care may seek more frequent COVID-19 testing, which could lead to higher detection of breakthrough infections than in the general population.
“Future analyses should account for testing practices and include a larger proportion of women with HIV,” the authors wrote. “Ultimately, policy makers must determine the appropriate balance between preventing further COVID-19 infections and possibly unnecessary additional vaccinations.”
Coauthor Keri N. Althoff, PhD, told this news organization that there’s one unanswered question that would strengthen the call to action by the CDC: Do people with HIV have more severe postvaccination COVID-19 breakthrough illness?
“We have a second paper that is a preprint and currently under peer review,” she said. “In this paper, we found that people with HIV with a CD4 count less than 350 cells/mm3 were more likely to be hospitalized with postvaccination COVID-19 breakthrough illness compared to similar people without HIV. “
At a minimum, Dr. Althoff said, policymakers should consider including people with HIV with a CD4 less than 350 cells/mm3 (loosening the restriction to less than 200 cells/mm3) in their recommendations for people who are moderately or severely immunocompromised.
The research was funded with supplemental funds to the North American AIDS Cohort Collaboration on Research and Design. Dr. Coburn reports no relevant financial relationships. A coauthor has received grants from the Canadian Institutes of Health Research, Alberta Innovates, and University of Calgary/Alberta Health Services outside the submitted work. One coauthor reports serving as a consultant to Trio Health, Kennedy Dundas, and MedIQ outside the submitted work.
A version of this article first appeared on Medscape.com.
People with HIV have an increased risk of breakthrough SARS-CoV-2 infections, a new study finds, and the authors say an additional primary vaccine dose should be considered for all who are living with the disease.
Currently, an additional primary dose administered 28 days after a second dose of the mRNA (Moderna or Pfizer) vaccines or after the first dose of the Johnson & Johnson (J&J) vaccine is recommended only for those with advanced or untreated HIV.
The Centers for Disease Control and Prevention recommends boosters for all adults with or without HIV.
Sally B. Coburn, PhD, of the department of epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, led the study, which was published online in JAMA Network Open. In their study, the researchers estimate the risk of breakthrough infections among fully vaccinated adults on the basis of HIV status in the United States.
Adults with HIV who were fully vaccinated before June 30, 2021, were matched with adults without HIV with regard to date of full vaccination, age, race/ethnicity, and sex. All were followed through Dec. 31, 2021.
Patients were considered fully vaccinated either 14 days after the second dose of the Pfizer or Moderna shots or 14 days after the single dose of the J&J shot.
Breakthrough risk 28% higher
In the study of 113,994 patients, researchers found that risk of breakthrough SARS-CoV-2 infection was low overall (3.8%) but was 28% higher among people with HIV in comparison with people without HIV (adjusted hazard ratio, 1.28; 95% confidence interval, 1.19-1.37).
The breakthrough rate was also higher in the HIV group (55 cases per 1,000 person-years, vs. 43 cases per 1,000 person-years in people without HIV).
Patients were drawn from the Corona-Infectious-Virus Epidemiology Team (CIVET)–II of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), which is part of the International Epidemiology Databases to Evaluate AIDS (IeDEA) collaboration involving four cohorts.
Among people with HIV, those younger than 45 years (vs. those aged 45-54) and those with a history of COVID-19 were more likely to experience breakthrough infections. Those who did not get any additional shots after the primary vaccination were more likely to have breakthrough infections, amplifying the need to get boosters, the authors wrote.
There was no link between breakthrough infections and HIV viral load suppression, but high CD4 counts (> 500 cells/mm3) were associated with fewer breakthrough cases among people with HIV, they noted.
Monica Gandhi, MD, professor of medicine and associate division chief of HIV, infectious diseases, and global medicine at the University of California, San Francisco, praised the study, noting that until now, large studies have not examined the rate of breakthrough infections among vaccinated people with HIV and people without HIV in the United States.
She told this news organization she agrees with the authors that a third dose for all who are living with HIV is needed because rates of breakthrough infections were high across all populations during the Omicron surge (which largely occurred after the period of this study).
She said she was not convinced the third shot was needed before Omicron, because breakthrough rates in both HIV and non-HIV groups were low.
“However, the most interesting part of this study for me was how well the vaccines worked in people with HIV with generally higher CD4 counts and virologic suppression, again telling us as HIV providers how well the HIV medicines work and how our patients with HIV have relatively normal immune systems if treated,” she said.
One limitation was that the study population was 92% male. Also, those without regular access to health care (who may be at greater risk for COVID-19) were less likely to be included in the study. People engaged in care may seek more frequent COVID-19 testing, which could lead to higher detection of breakthrough infections than in the general population.
“Future analyses should account for testing practices and include a larger proportion of women with HIV,” the authors wrote. “Ultimately, policy makers must determine the appropriate balance between preventing further COVID-19 infections and possibly unnecessary additional vaccinations.”
Coauthor Keri N. Althoff, PhD, told this news organization that there’s one unanswered question that would strengthen the call to action by the CDC: Do people with HIV have more severe postvaccination COVID-19 breakthrough illness?
“We have a second paper that is a preprint and currently under peer review,” she said. “In this paper, we found that people with HIV with a CD4 count less than 350 cells/mm3 were more likely to be hospitalized with postvaccination COVID-19 breakthrough illness compared to similar people without HIV. “
At a minimum, Dr. Althoff said, policymakers should consider including people with HIV with a CD4 less than 350 cells/mm3 (loosening the restriction to less than 200 cells/mm3) in their recommendations for people who are moderately or severely immunocompromised.
The research was funded with supplemental funds to the North American AIDS Cohort Collaboration on Research and Design. Dr. Coburn reports no relevant financial relationships. A coauthor has received grants from the Canadian Institutes of Health Research, Alberta Innovates, and University of Calgary/Alberta Health Services outside the submitted work. One coauthor reports serving as a consultant to Trio Health, Kennedy Dundas, and MedIQ outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
PCOS comes with high morbidity, medication use into late 40s
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
FROM ACTA OBSTETRICIA ET GYNECOLOGICA SCANDINAVICA