User login
Lucas Franki is an associate editor for MDedge News, and has been with the company since 2014. He has a BA in English from Penn State University and is an Eagle Scout.
AAD issues position statement addressing sexual, gender minority health
The individuals.
Some of the unique dermatologic issues faced by SGM individuals include a disproportionate risk for skin cancer in men who have sex with men; higher rates of sexually transmitted infections such as HIV, anogenital dysplasia, and anal cancer; and management of complications such as acne or scarring stemming from medical and/or surgical gender-affirming treatments for transgender individuals. In addition, racial and ethnic minority persons who identify as SGM or LGBTQ face additional stigma and health care disparities.
“While the precise role of dermatology remains controversial regarding anal cancer screening, treatment, and surveillance in these populations, comprehensive skin examinations as well as appropriate counseling and referrals may be linked to earlier detection and improved outcomes,” the statement notes.
The AAD has already taken some steps in advancing the care of SGM individuals, including dedicated educational sessions and workshops at AAD meetings, formation of the AAD LGBTQ/SGM Health Expert Resource Group, incorporation of LGBTQ/SGM content into online AAD basic dermatology curriculum modules, revision of the AAD position statement on isotretinoin, and forthcoming book chapters and CME articles for the Journal of the American Academy of Dermatology.
In order to further commit to the care of diverse populations, the AAD recognized a series of 11 positions in accordance with the association’s “core values of patient-first medicine and visionary leadership,” such as recognizing and affirming the identity and dignity of LGBTQ/SGM individuals, opposing all bias and discrimination, endorsing policies and initiatives that ensure nondiscrimination, and supporting training in cultural humility and structural competency.
“Adequate training of medical professionals regarding the unique health care needs of LGBTQ/SGM people and ongoing research into best care practices are necessary to provide care that facilitates trust and resilience while ensuring the ability of LGBTQ/SGM individuals to thrive,” the statement says.
Find the full position statement on the AAD website.
The individuals.
Some of the unique dermatologic issues faced by SGM individuals include a disproportionate risk for skin cancer in men who have sex with men; higher rates of sexually transmitted infections such as HIV, anogenital dysplasia, and anal cancer; and management of complications such as acne or scarring stemming from medical and/or surgical gender-affirming treatments for transgender individuals. In addition, racial and ethnic minority persons who identify as SGM or LGBTQ face additional stigma and health care disparities.
“While the precise role of dermatology remains controversial regarding anal cancer screening, treatment, and surveillance in these populations, comprehensive skin examinations as well as appropriate counseling and referrals may be linked to earlier detection and improved outcomes,” the statement notes.
The AAD has already taken some steps in advancing the care of SGM individuals, including dedicated educational sessions and workshops at AAD meetings, formation of the AAD LGBTQ/SGM Health Expert Resource Group, incorporation of LGBTQ/SGM content into online AAD basic dermatology curriculum modules, revision of the AAD position statement on isotretinoin, and forthcoming book chapters and CME articles for the Journal of the American Academy of Dermatology.
In order to further commit to the care of diverse populations, the AAD recognized a series of 11 positions in accordance with the association’s “core values of patient-first medicine and visionary leadership,” such as recognizing and affirming the identity and dignity of LGBTQ/SGM individuals, opposing all bias and discrimination, endorsing policies and initiatives that ensure nondiscrimination, and supporting training in cultural humility and structural competency.
“Adequate training of medical professionals regarding the unique health care needs of LGBTQ/SGM people and ongoing research into best care practices are necessary to provide care that facilitates trust and resilience while ensuring the ability of LGBTQ/SGM individuals to thrive,” the statement says.
Find the full position statement on the AAD website.
The individuals.
Some of the unique dermatologic issues faced by SGM individuals include a disproportionate risk for skin cancer in men who have sex with men; higher rates of sexually transmitted infections such as HIV, anogenital dysplasia, and anal cancer; and management of complications such as acne or scarring stemming from medical and/or surgical gender-affirming treatments for transgender individuals. In addition, racial and ethnic minority persons who identify as SGM or LGBTQ face additional stigma and health care disparities.
“While the precise role of dermatology remains controversial regarding anal cancer screening, treatment, and surveillance in these populations, comprehensive skin examinations as well as appropriate counseling and referrals may be linked to earlier detection and improved outcomes,” the statement notes.
The AAD has already taken some steps in advancing the care of SGM individuals, including dedicated educational sessions and workshops at AAD meetings, formation of the AAD LGBTQ/SGM Health Expert Resource Group, incorporation of LGBTQ/SGM content into online AAD basic dermatology curriculum modules, revision of the AAD position statement on isotretinoin, and forthcoming book chapters and CME articles for the Journal of the American Academy of Dermatology.
In order to further commit to the care of diverse populations, the AAD recognized a series of 11 positions in accordance with the association’s “core values of patient-first medicine and visionary leadership,” such as recognizing and affirming the identity and dignity of LGBTQ/SGM individuals, opposing all bias and discrimination, endorsing policies and initiatives that ensure nondiscrimination, and supporting training in cultural humility and structural competency.
“Adequate training of medical professionals regarding the unique health care needs of LGBTQ/SGM people and ongoing research into best care practices are necessary to provide care that facilitates trust and resilience while ensuring the ability of LGBTQ/SGM individuals to thrive,” the statement says.
Find the full position statement on the AAD website.
In memoriam: Dr. Henry T. Lynch
Henry T. Lynch, MD, an eminent researcher and trailblazer in the field of hereditary cancers, died June 2, 2019, at age 91.
Born in 1928, Dr. Lynch joined the military at age 16, becoming a gunner in the U.S. Navy. After a stint as a professional boxer, he obtained his high school equivalent, then attended the University of Oklahoma as an undergraduate. After receiving his master’s degree from the University of Denver, he earned a PhD in human genetics from the University of Texas, Austin, and his medical degree from the University of Texas, Galveston.
In 1967, Dr. Lynch accepted a position at Creighton University, Omaha, Neb., where he would spend the rest of his career. He was the founder and director of the Hereditary Cancer Center at Creighton, served as chair of the institution’s Department of Preventive Medicine and Public Health, and was named the inaugural holder of the Charles F. and Mary C. Heider Endowed Chair in Cancer Research.
It was at Creighton that Dr. Lynch began his work on genetic causes of and familial susceptibility to certain cancers. Dr. Lynch created a hereditary cancer registry, and definitively identified several genetic cancer syndromes that persist through multiple familial generations.
Dr. Lynch is credited with identifying a strain of hereditary nonpolyposis colon cancer that was named after him – Lynch syndrome. He is also credited with the discovery of hereditary breast-ovarian cancer syndrome, which would eventually lead to the discovery of the BRCA gene.
Find the full remembrance on the ASCO website.
Henry T. Lynch, MD, an eminent researcher and trailblazer in the field of hereditary cancers, died June 2, 2019, at age 91.
Born in 1928, Dr. Lynch joined the military at age 16, becoming a gunner in the U.S. Navy. After a stint as a professional boxer, he obtained his high school equivalent, then attended the University of Oklahoma as an undergraduate. After receiving his master’s degree from the University of Denver, he earned a PhD in human genetics from the University of Texas, Austin, and his medical degree from the University of Texas, Galveston.
In 1967, Dr. Lynch accepted a position at Creighton University, Omaha, Neb., where he would spend the rest of his career. He was the founder and director of the Hereditary Cancer Center at Creighton, served as chair of the institution’s Department of Preventive Medicine and Public Health, and was named the inaugural holder of the Charles F. and Mary C. Heider Endowed Chair in Cancer Research.
It was at Creighton that Dr. Lynch began his work on genetic causes of and familial susceptibility to certain cancers. Dr. Lynch created a hereditary cancer registry, and definitively identified several genetic cancer syndromes that persist through multiple familial generations.
Dr. Lynch is credited with identifying a strain of hereditary nonpolyposis colon cancer that was named after him – Lynch syndrome. He is also credited with the discovery of hereditary breast-ovarian cancer syndrome, which would eventually lead to the discovery of the BRCA gene.
Find the full remembrance on the ASCO website.
Henry T. Lynch, MD, an eminent researcher and trailblazer in the field of hereditary cancers, died June 2, 2019, at age 91.
Born in 1928, Dr. Lynch joined the military at age 16, becoming a gunner in the U.S. Navy. After a stint as a professional boxer, he obtained his high school equivalent, then attended the University of Oklahoma as an undergraduate. After receiving his master’s degree from the University of Denver, he earned a PhD in human genetics from the University of Texas, Austin, and his medical degree from the University of Texas, Galveston.
In 1967, Dr. Lynch accepted a position at Creighton University, Omaha, Neb., where he would spend the rest of his career. He was the founder and director of the Hereditary Cancer Center at Creighton, served as chair of the institution’s Department of Preventive Medicine and Public Health, and was named the inaugural holder of the Charles F. and Mary C. Heider Endowed Chair in Cancer Research.
It was at Creighton that Dr. Lynch began his work on genetic causes of and familial susceptibility to certain cancers. Dr. Lynch created a hereditary cancer registry, and definitively identified several genetic cancer syndromes that persist through multiple familial generations.
Dr. Lynch is credited with identifying a strain of hereditary nonpolyposis colon cancer that was named after him – Lynch syndrome. He is also credited with the discovery of hereditary breast-ovarian cancer syndrome, which would eventually lead to the discovery of the BRCA gene.
Find the full remembrance on the ASCO website.
Air-conditioned cognition, brain worm, and six-fingered success
(Women’s) Winter is coming
Summer approaches and the Great Freeze begins to make its way through offices across the country.
Women everywhere start dragging out those cardigans stored in desks long ago. Blankets start appearing draped over the backs of chairs. “God, it’s freezing in here” becomes the oft-repeated refrain. It’s women’s winter … and a recent study has shown it has a marked effect on productivity.
Published last month in PlosOne, the study examined the effect of temperature on cognitive performance in both men and women. After studying participants’ performances on math and verbal tasks at various temperatures, researchers found that the women performed better at higher temps while the men performed worse. However, the performance increase for women was much larger than the decrease for men.
The authors concluded that workplaces with mixed genders might increase productivity (and overall office happiness) by cranking that thermostat a little higher than current standards. Perhaps this will be the beginning of the end of the thermostat war.
Need a hand – or finger?
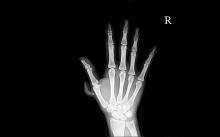
Polydactyly. No, not the flying dinosaur – the congenital condition of having extra fingers or toes. One in every 2,000-3,000 babies is born with polydactyly, and while most doctors quickly remove the extra digits, a German study found that maybe they shouldn’t be so quick to the chopping block.
Researchers found that polydactyl people have more dexterity of movement, and the subjects’ brains showed a distinct representation of the extra digit. The subjects were able to carry out two-handed tasks with just one hand, and the study authors concluded that the subjects’ hand movements “had increased complexity relative to common five-fingered hands.”
Researchers also designed a special video game to test the six-fingered hand vs. using both hands. Video game results were the same with one hand or two, proving that more fingers equals more fun.
Cooking up controversy
If you had a giant spoon, what would you do with it?
Boston artist Domenic Esposito has just such a spoon – being an artist, he made it himself – and he’s been using it to draw attention to the opioid use disorder crisis by placing it “on the doorsteps of corporations and individuals whose recklessness and irresponsibility have fueled the epidemic,” according to the spoon’s website, The Opioid Spoon Project.
Since its creation, the 800-lb, 10.5-foot-long steel heroin spoon has visited Purdue Pharma headquarters in Stamford, Conn. – the spoon was hauled off by the city and taken to a police evidence lot – and Rhodes Pharmaceuticals in Coventry, R.I.
More recently, Mr. Esposito has taken the spoon on a 14-city “Honor Tour.” At each stop, individuals have the opportunity to sign the spoon in memory of “those who have lost their opioid addiction battles.”
The tour, which began in Marlborough, Mass., on May 11 and has been to such cities as Providence, R.I., and Boston, ends on June 7 in Rockville, Md., which happens to be the home of LOTME world headquarters. So, who doesn’t want to see a spoon over 10 feet long?
Worms on (or in) my mind
It sounds like a bad riddle: What looks like a brain tumor, acts like a brain tumor, but isn’t a brain tumor?
Rachel Palma, a 42-year-old woman from Middletown, N.Y., had the joy of finding out the answer.
For months, she’d been having strange symptoms: hallucinations, nightmares, trouble remembering words, memory blackouts, occasional loss of motor control. Her doctors were stumped, until they performed an MRI of her brain, discovering a marble-sized lesion in her brain. Another MRI, performed with contrast, caused the lesion to light up, a surefire sign of a malignant brain tumor. Treatment would require surgery, then chemo and radiation therapy.
But when the surgeons opened her head, they were greeted not with a tumor, but with something they described as looking “like a quail egg.” The object was removed and opened up, and to the delight of the doctors, a tapeworm popped out.
The condition – neurocysticercosis – is common in developing countries but rare in the United States. We won’t share all the details on how tapeworms end up in the brain, but needless to say, poor washing of hands is involved.
On the one hand, Mrs. Palma has made a full recovery with no need for chemo or radiation. On the other hand, she had a tapeworm in her brain. Somehow, she’s managed to make the brain tumor seem like the less unpleasant option. And for that, we salute her.

(Women’s) Winter is coming
Summer approaches and the Great Freeze begins to make its way through offices across the country.
Women everywhere start dragging out those cardigans stored in desks long ago. Blankets start appearing draped over the backs of chairs. “God, it’s freezing in here” becomes the oft-repeated refrain. It’s women’s winter … and a recent study has shown it has a marked effect on productivity.
Published last month in PlosOne, the study examined the effect of temperature on cognitive performance in both men and women. After studying participants’ performances on math and verbal tasks at various temperatures, researchers found that the women performed better at higher temps while the men performed worse. However, the performance increase for women was much larger than the decrease for men.
The authors concluded that workplaces with mixed genders might increase productivity (and overall office happiness) by cranking that thermostat a little higher than current standards. Perhaps this will be the beginning of the end of the thermostat war.
Need a hand – or finger?
Polydactyly. No, not the flying dinosaur – the congenital condition of having extra fingers or toes. One in every 2,000-3,000 babies is born with polydactyly, and while most doctors quickly remove the extra digits, a German study found that maybe they shouldn’t be so quick to the chopping block.
Researchers found that polydactyl people have more dexterity of movement, and the subjects’ brains showed a distinct representation of the extra digit. The subjects were able to carry out two-handed tasks with just one hand, and the study authors concluded that the subjects’ hand movements “had increased complexity relative to common five-fingered hands.”
Researchers also designed a special video game to test the six-fingered hand vs. using both hands. Video game results were the same with one hand or two, proving that more fingers equals more fun.
Cooking up controversy
If you had a giant spoon, what would you do with it?
Boston artist Domenic Esposito has just such a spoon – being an artist, he made it himself – and he’s been using it to draw attention to the opioid use disorder crisis by placing it “on the doorsteps of corporations and individuals whose recklessness and irresponsibility have fueled the epidemic,” according to the spoon’s website, The Opioid Spoon Project.
Since its creation, the 800-lb, 10.5-foot-long steel heroin spoon has visited Purdue Pharma headquarters in Stamford, Conn. – the spoon was hauled off by the city and taken to a police evidence lot – and Rhodes Pharmaceuticals in Coventry, R.I.
More recently, Mr. Esposito has taken the spoon on a 14-city “Honor Tour.” At each stop, individuals have the opportunity to sign the spoon in memory of “those who have lost their opioid addiction battles.”
The tour, which began in Marlborough, Mass., on May 11 and has been to such cities as Providence, R.I., and Boston, ends on June 7 in Rockville, Md., which happens to be the home of LOTME world headquarters. So, who doesn’t want to see a spoon over 10 feet long?
Worms on (or in) my mind
It sounds like a bad riddle: What looks like a brain tumor, acts like a brain tumor, but isn’t a brain tumor?
Rachel Palma, a 42-year-old woman from Middletown, N.Y., had the joy of finding out the answer.
For months, she’d been having strange symptoms: hallucinations, nightmares, trouble remembering words, memory blackouts, occasional loss of motor control. Her doctors were stumped, until they performed an MRI of her brain, discovering a marble-sized lesion in her brain. Another MRI, performed with contrast, caused the lesion to light up, a surefire sign of a malignant brain tumor. Treatment would require surgery, then chemo and radiation therapy.
But when the surgeons opened her head, they were greeted not with a tumor, but with something they described as looking “like a quail egg.” The object was removed and opened up, and to the delight of the doctors, a tapeworm popped out.
The condition – neurocysticercosis – is common in developing countries but rare in the United States. We won’t share all the details on how tapeworms end up in the brain, but needless to say, poor washing of hands is involved.
On the one hand, Mrs. Palma has made a full recovery with no need for chemo or radiation. On the other hand, she had a tapeworm in her brain. Somehow, she’s managed to make the brain tumor seem like the less unpleasant option. And for that, we salute her.

(Women’s) Winter is coming
Summer approaches and the Great Freeze begins to make its way through offices across the country.
Women everywhere start dragging out those cardigans stored in desks long ago. Blankets start appearing draped over the backs of chairs. “God, it’s freezing in here” becomes the oft-repeated refrain. It’s women’s winter … and a recent study has shown it has a marked effect on productivity.
Published last month in PlosOne, the study examined the effect of temperature on cognitive performance in both men and women. After studying participants’ performances on math and verbal tasks at various temperatures, researchers found that the women performed better at higher temps while the men performed worse. However, the performance increase for women was much larger than the decrease for men.
The authors concluded that workplaces with mixed genders might increase productivity (and overall office happiness) by cranking that thermostat a little higher than current standards. Perhaps this will be the beginning of the end of the thermostat war.
Need a hand – or finger?
Polydactyly. No, not the flying dinosaur – the congenital condition of having extra fingers or toes. One in every 2,000-3,000 babies is born with polydactyly, and while most doctors quickly remove the extra digits, a German study found that maybe they shouldn’t be so quick to the chopping block.
Researchers found that polydactyl people have more dexterity of movement, and the subjects’ brains showed a distinct representation of the extra digit. The subjects were able to carry out two-handed tasks with just one hand, and the study authors concluded that the subjects’ hand movements “had increased complexity relative to common five-fingered hands.”
Researchers also designed a special video game to test the six-fingered hand vs. using both hands. Video game results were the same with one hand or two, proving that more fingers equals more fun.
Cooking up controversy
If you had a giant spoon, what would you do with it?
Boston artist Domenic Esposito has just such a spoon – being an artist, he made it himself – and he’s been using it to draw attention to the opioid use disorder crisis by placing it “on the doorsteps of corporations and individuals whose recklessness and irresponsibility have fueled the epidemic,” according to the spoon’s website, The Opioid Spoon Project.
Since its creation, the 800-lb, 10.5-foot-long steel heroin spoon has visited Purdue Pharma headquarters in Stamford, Conn. – the spoon was hauled off by the city and taken to a police evidence lot – and Rhodes Pharmaceuticals in Coventry, R.I.
More recently, Mr. Esposito has taken the spoon on a 14-city “Honor Tour.” At each stop, individuals have the opportunity to sign the spoon in memory of “those who have lost their opioid addiction battles.”
The tour, which began in Marlborough, Mass., on May 11 and has been to such cities as Providence, R.I., and Boston, ends on June 7 in Rockville, Md., which happens to be the home of LOTME world headquarters. So, who doesn’t want to see a spoon over 10 feet long?
Worms on (or in) my mind
It sounds like a bad riddle: What looks like a brain tumor, acts like a brain tumor, but isn’t a brain tumor?
Rachel Palma, a 42-year-old woman from Middletown, N.Y., had the joy of finding out the answer.
For months, she’d been having strange symptoms: hallucinations, nightmares, trouble remembering words, memory blackouts, occasional loss of motor control. Her doctors were stumped, until they performed an MRI of her brain, discovering a marble-sized lesion in her brain. Another MRI, performed with contrast, caused the lesion to light up, a surefire sign of a malignant brain tumor. Treatment would require surgery, then chemo and radiation therapy.
But when the surgeons opened her head, they were greeted not with a tumor, but with something they described as looking “like a quail egg.” The object was removed and opened up, and to the delight of the doctors, a tapeworm popped out.
The condition – neurocysticercosis – is common in developing countries but rare in the United States. We won’t share all the details on how tapeworms end up in the brain, but needless to say, poor washing of hands is involved.
On the one hand, Mrs. Palma has made a full recovery with no need for chemo or radiation. On the other hand, she had a tapeworm in her brain. Somehow, she’s managed to make the brain tumor seem like the less unpleasant option. And for that, we salute her.

FDA approves new treatment for hospital-acquired, ventilator-associated bacterial pneumonia
authorizing it for the treatment of both hospital-acquired and ventilator-associated bacterial pneumonia.
The new indication is for patients 18 years and older. It was based on results of a multinational, double-blind study that compared Zerbaxa with a different antibacterial drug in 726 patients hospitalized with hospital-acquired/ventilator-associated bacterial pneumonia. Mortality and cure rates were similar in the Zerbaxa and comparator groups.
The most common adverse events observed in the trial were elevated liver enzyme levels, renal impairment or failure, and diarrhea. Patients with hypersensitivity to beta-lactam drugs should not be receive Zerbaxa.
“A key global challenge we face as a public health agency is addressing the threat of antimicrobial-resistant infections. Hospital-acquired and ventilator-associated bacterial pneumonia are serious infections that can result in death in some patients. ... That’s why, among our other efforts to address antimicrobial resistance, we’re focused on facilitating the development of safe and effective new treatments to give patients more options to fight life-threatening infections,” said Amy Abernethy, MD, PhD, the FDA’s principal deputy commissioner.
Zerbaxa was initially approved in 2014 for treatment of complicated intra-abdominal and urinary tract infections.
Find the full press release on the FDA website.
authorizing it for the treatment of both hospital-acquired and ventilator-associated bacterial pneumonia.
The new indication is for patients 18 years and older. It was based on results of a multinational, double-blind study that compared Zerbaxa with a different antibacterial drug in 726 patients hospitalized with hospital-acquired/ventilator-associated bacterial pneumonia. Mortality and cure rates were similar in the Zerbaxa and comparator groups.
The most common adverse events observed in the trial were elevated liver enzyme levels, renal impairment or failure, and diarrhea. Patients with hypersensitivity to beta-lactam drugs should not be receive Zerbaxa.
“A key global challenge we face as a public health agency is addressing the threat of antimicrobial-resistant infections. Hospital-acquired and ventilator-associated bacterial pneumonia are serious infections that can result in death in some patients. ... That’s why, among our other efforts to address antimicrobial resistance, we’re focused on facilitating the development of safe and effective new treatments to give patients more options to fight life-threatening infections,” said Amy Abernethy, MD, PhD, the FDA’s principal deputy commissioner.
Zerbaxa was initially approved in 2014 for treatment of complicated intra-abdominal and urinary tract infections.
Find the full press release on the FDA website.
authorizing it for the treatment of both hospital-acquired and ventilator-associated bacterial pneumonia.
The new indication is for patients 18 years and older. It was based on results of a multinational, double-blind study that compared Zerbaxa with a different antibacterial drug in 726 patients hospitalized with hospital-acquired/ventilator-associated bacterial pneumonia. Mortality and cure rates were similar in the Zerbaxa and comparator groups.
The most common adverse events observed in the trial were elevated liver enzyme levels, renal impairment or failure, and diarrhea. Patients with hypersensitivity to beta-lactam drugs should not be receive Zerbaxa.
“A key global challenge we face as a public health agency is addressing the threat of antimicrobial-resistant infections. Hospital-acquired and ventilator-associated bacterial pneumonia are serious infections that can result in death in some patients. ... That’s why, among our other efforts to address antimicrobial resistance, we’re focused on facilitating the development of safe and effective new treatments to give patients more options to fight life-threatening infections,” said Amy Abernethy, MD, PhD, the FDA’s principal deputy commissioner.
Zerbaxa was initially approved in 2014 for treatment of complicated intra-abdominal and urinary tract infections.
Find the full press release on the FDA website.
FDA announces clearance of modified endoscope connector
which was designed to reduce the risk of cross-contamination previously identified by the FDA.
The FDA approval of the modified ERBEFLO port connector is based on a review of the functional and simulated use testing of the modified device design. The effectiveness of the device at reducing the risk of backflow and contamination is also supported by simulated testing.
Revised labeling included with the product identifies compatible endoscopes and accessories and provides warnings to ensure proper usage.
“The clearance of the modified ERBEFLO 24-hour use port connector provides another option for health care facilities whose staff understand and can fully implement the instructions for use to reduce the risk of cross-contamination and infection,” the FDA said in the May 23 update letter.
AGA Center for GI Innovation and Technology will continue to monitor this issue and encourages all GIs to follow the most up-to-date FDA guidance.
which was designed to reduce the risk of cross-contamination previously identified by the FDA.
The FDA approval of the modified ERBEFLO port connector is based on a review of the functional and simulated use testing of the modified device design. The effectiveness of the device at reducing the risk of backflow and contamination is also supported by simulated testing.
Revised labeling included with the product identifies compatible endoscopes and accessories and provides warnings to ensure proper usage.
“The clearance of the modified ERBEFLO 24-hour use port connector provides another option for health care facilities whose staff understand and can fully implement the instructions for use to reduce the risk of cross-contamination and infection,” the FDA said in the May 23 update letter.
AGA Center for GI Innovation and Technology will continue to monitor this issue and encourages all GIs to follow the most up-to-date FDA guidance.
which was designed to reduce the risk of cross-contamination previously identified by the FDA.
The FDA approval of the modified ERBEFLO port connector is based on a review of the functional and simulated use testing of the modified device design. The effectiveness of the device at reducing the risk of backflow and contamination is also supported by simulated testing.
Revised labeling included with the product identifies compatible endoscopes and accessories and provides warnings to ensure proper usage.
“The clearance of the modified ERBEFLO 24-hour use port connector provides another option for health care facilities whose staff understand and can fully implement the instructions for use to reduce the risk of cross-contamination and infection,” the FDA said in the May 23 update letter.
AGA Center for GI Innovation and Technology will continue to monitor this issue and encourages all GIs to follow the most up-to-date FDA guidance.
Weight-based teasing may mean further weight gain in children
according to Natasha A. Schvey, PhD, of the Uniformed Services University of the Health Sciences, Bethesda, Md., and her associates.
The investigators conducted a longitudinal, observational study of 110 children who were either in the 85th body mass index (BMI) percentile or greater or had two parents with a BMI of at least 25 kg/m2. Children were recruited between July 12, 1996, and July 6, 2009, administered the Perception of Teasing Scale at baseline and during follow-up, and followed for up to 15 years. Children were aged a mean of 12 years at baseline, and attended an average of nine visits.
At baseline, 53% of children were overweight, with overweight being more common in girls and in non-Hispanic whites. A total of 62% of children who were overweight at baseline reported at least one incidence of weight-based teasing (WBT), compared with 21% of children at risk. WBT at baseline was associated with BMI throughout the study (P less than .001). In addition, children who reported more WBT showed a steeper gain in BMI (P = .007). Overall, children who reported high levels of WBT had 33% greater gains in BMI per year than those with no WBT.
Fat mass was associated with WBT in a similar manner, but to an increased extent, as children who reported high levels of WBT gained 91% more fat per year than those with no WBT.
“As adolescence marks a critical period for the study of weight gain, it will be important to further explore the effects of WBT and weight‐related pressures on indices of weight and health throughout development and to identify both risk and protective factors. The present findings ... may provide a foundation upon which to initiate clinical pediatric interventions to determine whether reducing WBT affects weight and fat gain trajectory,” the investigators concluded.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The investigators did not report any conflicts of interest.
SOURCE: Schvey NA et al. Pediatr Obes. 2019 May 29. doi: 10.1111/ijpo.12538.
according to Natasha A. Schvey, PhD, of the Uniformed Services University of the Health Sciences, Bethesda, Md., and her associates.
The investigators conducted a longitudinal, observational study of 110 children who were either in the 85th body mass index (BMI) percentile or greater or had two parents with a BMI of at least 25 kg/m2. Children were recruited between July 12, 1996, and July 6, 2009, administered the Perception of Teasing Scale at baseline and during follow-up, and followed for up to 15 years. Children were aged a mean of 12 years at baseline, and attended an average of nine visits.
At baseline, 53% of children were overweight, with overweight being more common in girls and in non-Hispanic whites. A total of 62% of children who were overweight at baseline reported at least one incidence of weight-based teasing (WBT), compared with 21% of children at risk. WBT at baseline was associated with BMI throughout the study (P less than .001). In addition, children who reported more WBT showed a steeper gain in BMI (P = .007). Overall, children who reported high levels of WBT had 33% greater gains in BMI per year than those with no WBT.
Fat mass was associated with WBT in a similar manner, but to an increased extent, as children who reported high levels of WBT gained 91% more fat per year than those with no WBT.
“As adolescence marks a critical period for the study of weight gain, it will be important to further explore the effects of WBT and weight‐related pressures on indices of weight and health throughout development and to identify both risk and protective factors. The present findings ... may provide a foundation upon which to initiate clinical pediatric interventions to determine whether reducing WBT affects weight and fat gain trajectory,” the investigators concluded.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The investigators did not report any conflicts of interest.
SOURCE: Schvey NA et al. Pediatr Obes. 2019 May 29. doi: 10.1111/ijpo.12538.
according to Natasha A. Schvey, PhD, of the Uniformed Services University of the Health Sciences, Bethesda, Md., and her associates.
The investigators conducted a longitudinal, observational study of 110 children who were either in the 85th body mass index (BMI) percentile or greater or had two parents with a BMI of at least 25 kg/m2. Children were recruited between July 12, 1996, and July 6, 2009, administered the Perception of Teasing Scale at baseline and during follow-up, and followed for up to 15 years. Children were aged a mean of 12 years at baseline, and attended an average of nine visits.
At baseline, 53% of children were overweight, with overweight being more common in girls and in non-Hispanic whites. A total of 62% of children who were overweight at baseline reported at least one incidence of weight-based teasing (WBT), compared with 21% of children at risk. WBT at baseline was associated with BMI throughout the study (P less than .001). In addition, children who reported more WBT showed a steeper gain in BMI (P = .007). Overall, children who reported high levels of WBT had 33% greater gains in BMI per year than those with no WBT.
Fat mass was associated with WBT in a similar manner, but to an increased extent, as children who reported high levels of WBT gained 91% more fat per year than those with no WBT.
“As adolescence marks a critical period for the study of weight gain, it will be important to further explore the effects of WBT and weight‐related pressures on indices of weight and health throughout development and to identify both risk and protective factors. The present findings ... may provide a foundation upon which to initiate clinical pediatric interventions to determine whether reducing WBT affects weight and fat gain trajectory,” the investigators concluded.
The study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The investigators did not report any conflicts of interest.
SOURCE: Schvey NA et al. Pediatr Obes. 2019 May 29. doi: 10.1111/ijpo.12538.
FROM PEDIATRIC OBESITY
Reappraisal in emotion regulation unrelated to working memory in bipolar disorder
Working memory may not function properly during the reappraisal process of emotion regulation in patients with bipolar disorder, according to Dong Hun Oh of the department of psychiatry at Yonsei University, Seoul, South Korea, and associates.
For the study, published in the Journal of Affective Disorders, 43 patients with euthymic bipolar I disorder were recruited from a psychiatric outpatient clinic in Seoul and compared with 48 healthy controls. The Korean versions of the operation span task (OSPAN), emotion regulation questionnaire (K-ERQ), ruminative response scale (K-RRS), and Difficulties in Emotion Regulation Scale (K-DERS) were administered to all the patients.
In a between-group comparison of scores on the four scales measured, patients with bipolar disorder had significantly lower scores on OSPAN (P = .031), on the brooding section of the K-RRS (P =.016), and on the nonacceptance section of the K-DERS (P = .039). In a regression analysis of the interaction between working memory and the four components of emotion regulation (reappraisal, expressive suppression, brooding, and reflective pondering), a significant interaction was found in OSPAN scores for reappraisal between healthy controls and the BD group (P = .007). Brooding scores were significantly lower in the control group, but the interaction was not significant.
A simple slope analysis showed that, while working memory was worse in patients with bipolar disorder, a relationship between cognitive capacity and the efficacy of reappraisal was found only in healthy controls, the investigators noted.
“The absence of interaction between [working memory capacity] and reappraisal in euthymic [bipolar disorder] patients that we report may indicate that the positive effects of cognitive remediation or [working memory] training previously reported for healthy people, may not effectively improve [emotion regulation] for patients with [bipolar disorder],” they wrote.
Furthermore, if this is the case, cognitive interventions aimed at improving emotion regulation in patients with bipolar disorder might not work.
The investigators cited several limitations. One limitation was the small sample size; another was the use of self-administered questionnaires.
The study was supported by a faculty research grant from Yonsei University. The authors did not report any conflicts of interest.
SOURCE: Oh DH et al. J Affect Disord. 2019 Apr 8. doi: 10.1016/j.jad.2019.04.042.
Working memory may not function properly during the reappraisal process of emotion regulation in patients with bipolar disorder, according to Dong Hun Oh of the department of psychiatry at Yonsei University, Seoul, South Korea, and associates.
For the study, published in the Journal of Affective Disorders, 43 patients with euthymic bipolar I disorder were recruited from a psychiatric outpatient clinic in Seoul and compared with 48 healthy controls. The Korean versions of the operation span task (OSPAN), emotion regulation questionnaire (K-ERQ), ruminative response scale (K-RRS), and Difficulties in Emotion Regulation Scale (K-DERS) were administered to all the patients.
In a between-group comparison of scores on the four scales measured, patients with bipolar disorder had significantly lower scores on OSPAN (P = .031), on the brooding section of the K-RRS (P =.016), and on the nonacceptance section of the K-DERS (P = .039). In a regression analysis of the interaction between working memory and the four components of emotion regulation (reappraisal, expressive suppression, brooding, and reflective pondering), a significant interaction was found in OSPAN scores for reappraisal between healthy controls and the BD group (P = .007). Brooding scores were significantly lower in the control group, but the interaction was not significant.
A simple slope analysis showed that, while working memory was worse in patients with bipolar disorder, a relationship between cognitive capacity and the efficacy of reappraisal was found only in healthy controls, the investigators noted.
“The absence of interaction between [working memory capacity] and reappraisal in euthymic [bipolar disorder] patients that we report may indicate that the positive effects of cognitive remediation or [working memory] training previously reported for healthy people, may not effectively improve [emotion regulation] for patients with [bipolar disorder],” they wrote.
Furthermore, if this is the case, cognitive interventions aimed at improving emotion regulation in patients with bipolar disorder might not work.
The investigators cited several limitations. One limitation was the small sample size; another was the use of self-administered questionnaires.
The study was supported by a faculty research grant from Yonsei University. The authors did not report any conflicts of interest.
SOURCE: Oh DH et al. J Affect Disord. 2019 Apr 8. doi: 10.1016/j.jad.2019.04.042.
Working memory may not function properly during the reappraisal process of emotion regulation in patients with bipolar disorder, according to Dong Hun Oh of the department of psychiatry at Yonsei University, Seoul, South Korea, and associates.
For the study, published in the Journal of Affective Disorders, 43 patients with euthymic bipolar I disorder were recruited from a psychiatric outpatient clinic in Seoul and compared with 48 healthy controls. The Korean versions of the operation span task (OSPAN), emotion regulation questionnaire (K-ERQ), ruminative response scale (K-RRS), and Difficulties in Emotion Regulation Scale (K-DERS) were administered to all the patients.
In a between-group comparison of scores on the four scales measured, patients with bipolar disorder had significantly lower scores on OSPAN (P = .031), on the brooding section of the K-RRS (P =.016), and on the nonacceptance section of the K-DERS (P = .039). In a regression analysis of the interaction between working memory and the four components of emotion regulation (reappraisal, expressive suppression, brooding, and reflective pondering), a significant interaction was found in OSPAN scores for reappraisal between healthy controls and the BD group (P = .007). Brooding scores were significantly lower in the control group, but the interaction was not significant.
A simple slope analysis showed that, while working memory was worse in patients with bipolar disorder, a relationship between cognitive capacity and the efficacy of reappraisal was found only in healthy controls, the investigators noted.
“The absence of interaction between [working memory capacity] and reappraisal in euthymic [bipolar disorder] patients that we report may indicate that the positive effects of cognitive remediation or [working memory] training previously reported for healthy people, may not effectively improve [emotion regulation] for patients with [bipolar disorder],” they wrote.
Furthermore, if this is the case, cognitive interventions aimed at improving emotion regulation in patients with bipolar disorder might not work.
The investigators cited several limitations. One limitation was the small sample size; another was the use of self-administered questionnaires.
The study was supported by a faculty research grant from Yonsei University. The authors did not report any conflicts of interest.
SOURCE: Oh DH et al. J Affect Disord. 2019 Apr 8. doi: 10.1016/j.jad.2019.04.042.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
CDC creates interactive education module to improve RMSF recognition
The Centers for Disease Control and Prevention has created a first-of-its-kind interactive training module to help physicians both recognize and diagnose Rocky Mountain spotted fever (RMSF).
A record number of cases of RMSF were reported to the CDC in 2017 (6,248, up from 4,269 in 2016), but less than 1% of those cases had sufficient laboratory evidence to be confirmed. The CDC education module includes scenarios based on real cases to aid providers in recognizing RMSF and differentiating it from similar diseases. CME is available for physicians, nurse practitioners, physician assistants, veterinarians, nurses, epidemiologists, public health professionals, educators, and health communicators.
The disease initially presents with nonspecific symptoms such as fever, headache, or rash, but if left untreated, patients may require the amputation of fingers, toes, or limbs because of low blood flow; heart and lung specialty care; and ICU management. About 20% of untreated cases are fatal; half of these deaths occur within 8 days of initial presentation.
“Rocky Mountain spotted fever can be deadly if not treated early – yet cases often go unrecognized because the signs and symptoms are similar to those of many other diseases. With tickborne diseases on the rise in the U.S., this training will better equip health care providers to identify, diagnose, and treat this potentially fatal disease,” said CDC director Robert R. Redfield, MD.
Find the full press release on the CDC website.
The Centers for Disease Control and Prevention has created a first-of-its-kind interactive training module to help physicians both recognize and diagnose Rocky Mountain spotted fever (RMSF).
A record number of cases of RMSF were reported to the CDC in 2017 (6,248, up from 4,269 in 2016), but less than 1% of those cases had sufficient laboratory evidence to be confirmed. The CDC education module includes scenarios based on real cases to aid providers in recognizing RMSF and differentiating it from similar diseases. CME is available for physicians, nurse practitioners, physician assistants, veterinarians, nurses, epidemiologists, public health professionals, educators, and health communicators.
The disease initially presents with nonspecific symptoms such as fever, headache, or rash, but if left untreated, patients may require the amputation of fingers, toes, or limbs because of low blood flow; heart and lung specialty care; and ICU management. About 20% of untreated cases are fatal; half of these deaths occur within 8 days of initial presentation.
“Rocky Mountain spotted fever can be deadly if not treated early – yet cases often go unrecognized because the signs and symptoms are similar to those of many other diseases. With tickborne diseases on the rise in the U.S., this training will better equip health care providers to identify, diagnose, and treat this potentially fatal disease,” said CDC director Robert R. Redfield, MD.
Find the full press release on the CDC website.
The Centers for Disease Control and Prevention has created a first-of-its-kind interactive training module to help physicians both recognize and diagnose Rocky Mountain spotted fever (RMSF).
A record number of cases of RMSF were reported to the CDC in 2017 (6,248, up from 4,269 in 2016), but less than 1% of those cases had sufficient laboratory evidence to be confirmed. The CDC education module includes scenarios based on real cases to aid providers in recognizing RMSF and differentiating it from similar diseases. CME is available for physicians, nurse practitioners, physician assistants, veterinarians, nurses, epidemiologists, public health professionals, educators, and health communicators.
The disease initially presents with nonspecific symptoms such as fever, headache, or rash, but if left untreated, patients may require the amputation of fingers, toes, or limbs because of low blood flow; heart and lung specialty care; and ICU management. About 20% of untreated cases are fatal; half of these deaths occur within 8 days of initial presentation.
“Rocky Mountain spotted fever can be deadly if not treated early – yet cases often go unrecognized because the signs and symptoms are similar to those of many other diseases. With tickborne diseases on the rise in the U.S., this training will better equip health care providers to identify, diagnose, and treat this potentially fatal disease,” said CDC director Robert R. Redfield, MD.
Find the full press release on the CDC website.
FDA grants Priority Review to Vascepa for cardiovascular risk reduction
The Food and Drug Administration has granted a Priority Review to the supplemental new drug application for icosapent ethyl (Vascepa).
If approved, Vascepa – which is produced by Amarin – would be the first drug indicated to reduce residual cardiovascular risk in patients with LDL cholesterol managed by statins who still have persistent elevated triglycerides. The drug is now approved for reducing triglyceride levels in patients with baseline values of 500 mg/dL or greater.
The Priority Review is based on results of REDUCE-IT, a landmark cardiovascular outcomes trial whose primary results were presented at the American Heart Association scientific sessions last November and published in the New England Journal of Medicine. Vascepa achieved the primary study endpoint, reducing the relative risk for the first occurrence of a major adverse cardiovascular event significantly, by 25%.
The drug also met the study’s key secondary endpoint, reducing the incidence of a composite of cardiovascular death, nonfatal heart attack, and nonfatal stroke by 26%. Significant adverse events associated with Vascepa in the trial were peripheral edema, constipation, and atrial fibrillation.
Vascepa is currently indicated as an adjunct to diet to reduce triglyceride in adults with severe hypertriglyceridemia, a significantly smaller population than that represented in REDUCE-IT.
“We expect earlier approval of an expanded indication for Vascepa to lead to faster improvements in care for millions of patients with residual cardiovascular risk after statin therapy,” John F. Thero, president and CEO of Amarin, said in the statement.
The FDA is expected to issue a complete response by the end of September. Find the full press release on the Amarin website.
The Food and Drug Administration has granted a Priority Review to the supplemental new drug application for icosapent ethyl (Vascepa).
If approved, Vascepa – which is produced by Amarin – would be the first drug indicated to reduce residual cardiovascular risk in patients with LDL cholesterol managed by statins who still have persistent elevated triglycerides. The drug is now approved for reducing triglyceride levels in patients with baseline values of 500 mg/dL or greater.
The Priority Review is based on results of REDUCE-IT, a landmark cardiovascular outcomes trial whose primary results were presented at the American Heart Association scientific sessions last November and published in the New England Journal of Medicine. Vascepa achieved the primary study endpoint, reducing the relative risk for the first occurrence of a major adverse cardiovascular event significantly, by 25%.
The drug also met the study’s key secondary endpoint, reducing the incidence of a composite of cardiovascular death, nonfatal heart attack, and nonfatal stroke by 26%. Significant adverse events associated with Vascepa in the trial were peripheral edema, constipation, and atrial fibrillation.
Vascepa is currently indicated as an adjunct to diet to reduce triglyceride in adults with severe hypertriglyceridemia, a significantly smaller population than that represented in REDUCE-IT.
“We expect earlier approval of an expanded indication for Vascepa to lead to faster improvements in care for millions of patients with residual cardiovascular risk after statin therapy,” John F. Thero, president and CEO of Amarin, said in the statement.
The FDA is expected to issue a complete response by the end of September. Find the full press release on the Amarin website.
The Food and Drug Administration has granted a Priority Review to the supplemental new drug application for icosapent ethyl (Vascepa).
If approved, Vascepa – which is produced by Amarin – would be the first drug indicated to reduce residual cardiovascular risk in patients with LDL cholesterol managed by statins who still have persistent elevated triglycerides. The drug is now approved for reducing triglyceride levels in patients with baseline values of 500 mg/dL or greater.
The Priority Review is based on results of REDUCE-IT, a landmark cardiovascular outcomes trial whose primary results were presented at the American Heart Association scientific sessions last November and published in the New England Journal of Medicine. Vascepa achieved the primary study endpoint, reducing the relative risk for the first occurrence of a major adverse cardiovascular event significantly, by 25%.
The drug also met the study’s key secondary endpoint, reducing the incidence of a composite of cardiovascular death, nonfatal heart attack, and nonfatal stroke by 26%. Significant adverse events associated with Vascepa in the trial were peripheral edema, constipation, and atrial fibrillation.
Vascepa is currently indicated as an adjunct to diet to reduce triglyceride in adults with severe hypertriglyceridemia, a significantly smaller population than that represented in REDUCE-IT.
“We expect earlier approval of an expanded indication for Vascepa to lead to faster improvements in care for millions of patients with residual cardiovascular risk after statin therapy,” John F. Thero, president and CEO of Amarin, said in the statement.
The FDA is expected to issue a complete response by the end of September. Find the full press release on the Amarin website.
Children’s book effectively assesses literary skills during well-child visits
reported John S. Hutton, MS, MD, of Cincinnati Children’s Hospital Medical Center, and his associates.
The Reading House (TRH) is a 14-page, full-color, board book with a simple, rhyming narrative and illustrated content showing children of various ethnicities and sexes going about their day. For the study, published in Pediatrics, 278 children aged 36-52 months (mean age, 43.1 months) were recruited from seven pediatric primary care clinics, two of which were affiliated with an academic children’s hospital primarily serving families of lower socioeconomic status. The children’s reading comprehension was measured by way of a 9-item TRH assessment, as well as the 25-item Get Ready to Read! (GRTR) validated measure; parent, child, and provider impressions of TRH also were collected.
The mean TRH assessment score was 4.2, and the mean GRTR score was 11.1. The TRH score was positively associated with GRTR score, female sex, private practice, and child age (Pediatrics 2019 May 30. doi: 10.1542/peds.2018-3843).
Of the 72 clinical providers surveyed on the effectiveness of the TRH assessment, most reported that the assessment was not invasive in patient flow (93% not at all, 6% somewhat, 1% very much), that TRH would be feasible to administer (49% yes, 43% not sure, 8% no), would be clinically useful (67% yes, 31% not sure, 2% no), and would be useful for families (85% yes, 14% not sure, 1% no). Similar results on the effectiveness and enjoyability of TRH were reported by parents and children.
“Although psychometric properties are critical, effective screening should be perceived as useful and not burdensome or invasive. Responses to parent, child, and provider surveys were favorable, which suggests that TRH screening may be an enjoyable and valuable addition to well-child visits,” the investigators wrote.
Dr. Hutton conceived, wrote, and edited the children’s book used in the study, and is the founder of the company that published the book, although he receives no salary or compensation for this role. The book’s intended use is as a screening tool, distributed at low cost to clinical practices and organizations. The other study authors did not report any conflicts of interest.
reported John S. Hutton, MS, MD, of Cincinnati Children’s Hospital Medical Center, and his associates.
The Reading House (TRH) is a 14-page, full-color, board book with a simple, rhyming narrative and illustrated content showing children of various ethnicities and sexes going about their day. For the study, published in Pediatrics, 278 children aged 36-52 months (mean age, 43.1 months) were recruited from seven pediatric primary care clinics, two of which were affiliated with an academic children’s hospital primarily serving families of lower socioeconomic status. The children’s reading comprehension was measured by way of a 9-item TRH assessment, as well as the 25-item Get Ready to Read! (GRTR) validated measure; parent, child, and provider impressions of TRH also were collected.
The mean TRH assessment score was 4.2, and the mean GRTR score was 11.1. The TRH score was positively associated with GRTR score, female sex, private practice, and child age (Pediatrics 2019 May 30. doi: 10.1542/peds.2018-3843).
Of the 72 clinical providers surveyed on the effectiveness of the TRH assessment, most reported that the assessment was not invasive in patient flow (93% not at all, 6% somewhat, 1% very much), that TRH would be feasible to administer (49% yes, 43% not sure, 8% no), would be clinically useful (67% yes, 31% not sure, 2% no), and would be useful for families (85% yes, 14% not sure, 1% no). Similar results on the effectiveness and enjoyability of TRH were reported by parents and children.
“Although psychometric properties are critical, effective screening should be perceived as useful and not burdensome or invasive. Responses to parent, child, and provider surveys were favorable, which suggests that TRH screening may be an enjoyable and valuable addition to well-child visits,” the investigators wrote.
Dr. Hutton conceived, wrote, and edited the children’s book used in the study, and is the founder of the company that published the book, although he receives no salary or compensation for this role. The book’s intended use is as a screening tool, distributed at low cost to clinical practices and organizations. The other study authors did not report any conflicts of interest.
reported John S. Hutton, MS, MD, of Cincinnati Children’s Hospital Medical Center, and his associates.
The Reading House (TRH) is a 14-page, full-color, board book with a simple, rhyming narrative and illustrated content showing children of various ethnicities and sexes going about their day. For the study, published in Pediatrics, 278 children aged 36-52 months (mean age, 43.1 months) were recruited from seven pediatric primary care clinics, two of which were affiliated with an academic children’s hospital primarily serving families of lower socioeconomic status. The children’s reading comprehension was measured by way of a 9-item TRH assessment, as well as the 25-item Get Ready to Read! (GRTR) validated measure; parent, child, and provider impressions of TRH also were collected.
The mean TRH assessment score was 4.2, and the mean GRTR score was 11.1. The TRH score was positively associated with GRTR score, female sex, private practice, and child age (Pediatrics 2019 May 30. doi: 10.1542/peds.2018-3843).
Of the 72 clinical providers surveyed on the effectiveness of the TRH assessment, most reported that the assessment was not invasive in patient flow (93% not at all, 6% somewhat, 1% very much), that TRH would be feasible to administer (49% yes, 43% not sure, 8% no), would be clinically useful (67% yes, 31% not sure, 2% no), and would be useful for families (85% yes, 14% not sure, 1% no). Similar results on the effectiveness and enjoyability of TRH were reported by parents and children.
“Although psychometric properties are critical, effective screening should be perceived as useful and not burdensome or invasive. Responses to parent, child, and provider surveys were favorable, which suggests that TRH screening may be an enjoyable and valuable addition to well-child visits,” the investigators wrote.
Dr. Hutton conceived, wrote, and edited the children’s book used in the study, and is the founder of the company that published the book, although he receives no salary or compensation for this role. The book’s intended use is as a screening tool, distributed at low cost to clinical practices and organizations. The other study authors did not report any conflicts of interest.
FROM PEDIATRICS